Note: Descriptions are shown in the official language in which they were submitted.
CA 03090050 2020-07-29
1
METHOD AND DEVICE FOR THE PRODUCTION OF A SYNTHETIC GASOLINE
The invention relates to a method and a device for producing a synthetic
gasoline, in particular
a high-octane gasoline for use as gasoline and made from alcohols.
Methods for producing gasoline from alcohols are known. Thus, the synthesis of
high-octane
gasoline from methanol was developed in the 1970's via the Mobil Oil
Corporation's MTG
process ("methanol to gasoline"), which, among others, is described in US
3,894,102 A. In the
first stage, a product is synthesised from a mixture of carbon monoxide and
hydrogen over a
catalyst mixture consisting of a methanol synthesis catalyst and an acidic
dehydration catalyst
which mainly contains dimethyl ether (DME). After product cooling and
separation, the dimethyl
ether thus produced is contacted in the second stage with a crystalline
aluminosilicate zeolite
catalyst, and a product is formed which mainly consists of liquid aromatic
hydrocarbons in the
gasoline boiling range (C5 to 400 F).
The conversion of methanol into a hydrocarbon product can be carried out in
fixed bed reactors
(see, e.g., US 3,998,899 A, US 3,931,349 A and US 4,035,430 A) and in reactors
with catalyst
fluid beds (see, e.g., US 4,071,573 A and US 4,138,440 A). In the MTG process,
the methanol
is dehydrated, usually an y alumina catalyst is used, and an equilibrium
mixture of methanol,
dimethyl ether, and water is formed. This mixture is then converted to a
hydrocarbon product
in a boiling range from light hydrocarbons to gasoline and water in a ZSM-5
(zeolite) catalyst
bed.
The main problem to be solved with regard to the MTG process is the problem of
temperature
control in the reactor. As a result of the strongly exothermic reaction,
approx. 1740 kJ per
kilogram of converted methanol are released when methanol is converted to
hydrocarbons. In
an adiabatic reactor, this would lead to a temperature rise of approximately
650 C, which
would cause extremely rapid catalyst ageing or even destruction thereof. In
addition, the
adiabatic temperature rise would lead to the formation of undesirable by-
products.
A method for avoiding an excessive temperature rise in the catalyst bed is
described in US
3,931,349. The strong exothermic reaction from methanol to gasoline
hydrocarbons is
controlled in a system of parallel catalyst beds. The feed methanol is
evaporated and in the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
2
first stage is converted by contact with an y alumina catalyst into a mixture
of dimethyl ether,
methanol and water, wherein 15 to 20 % of the total heat of the reaction from
methanol to
gasoline hydrocarbons are released.
The reaction product of the dehydration of methanol is diluted with the light
hydrocarbon gases
<C5 from the second reaction stage of the conversion. These gases are
separated from the
reaction product of the hydrocarbon synthesis after it has cooled, they are
compressed and
preheated and then mixed with the feed stream of the reactor. The circulating
gas cools the
catalyst bed during the exothermic reaction of the oxygenates to hydrocarbons
on a crystalline
ZSM-5. The reaction of the hydrocarbon synthesis is carried out at the lowest
possible pressure
in order to minimise the undesirable formation of high molecular weight
aromatic compounds,
such as durene. The space velocity, based on pure methanol, is 1 h-1 and the
partial pressure
of the methanol is 1 bar. The low partial pressure of the reactant limits the
formation of
undesirable heavy aromatics.
US 3,998,899 A describes a process for converting Ci to C3 alcohols,
preferably methanol,
into hydrocarbons in the gasoline boiling range. In a first catalytic stage,
ether products are
formed from the alcohols and, in a second catalytic stage, zeolite components
in the gasoline
boiling range are formed on a crystalline ZSM-5 zeolite.
US 4,814,535 A describes a method which makes it possible to keep the degree
of conversion
constant in a reaction period, despite the gradual deactivation of the
catalyst by coke deposits.
For this purpose, the inlet temperature into the reactor for hydrocarbon
synthesis is gradually
increased by 3 to 9 C, in which case the octane number remains constant.
The methods described above require a very high recycle ratio (circulating gas
to feed stream),
which makes the process complex due to the necessary cooling, compression, and
subsequent heating of the circulating gas.
If the ratio of circulating gas to feed stream is reduced in order to increase
the effectiveness of
the process, this causes an increase in the temperature difference across the
catalyst bed,
which is reflected not only in a faster ageing rate of the catalyst, but also
in the increase in the
water vapour partial pressure (due to the greater proportion of water vapour
during reduction
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
3
of the proportion of circulating gas). A high water vapour partial pressure
has a negative effect
on the life of the catalyst, especially when the reaction temperatures are
high.
US 4,404,414 A therefore describes a method of heat dissipation, by means of
which the
.. desired temperatures in the catalyst bed can be maintained with reduced
recycling ratios. The
arrangement of the reactors is designed so that the reactors for the oxygenate
feed are
arranged in parallel, but for the circulating gas they are arranged in series.
The special reactor
arrangement means that the total amount of recycled gas can be reduced by half
with the
same temperature differences across the catalyst beds.
In US 4,788,369 A, the LPG fraction (propane, n-butane and isobutane, and
small amounts of
light olefins) is separated from the reaction product and returned to the
reactor in order to
dissipate the heat of reaction. A major advantage of using the LPG fraction as
a recycle stream
over the otherwise customary recycling of the light hydrocarbon gases is the
improvement in
the economics of the process. There was a slight increase in the gasoline
yield (by
approximately 1 %), since the olefins contained in the recycled LPG stream
have been
converted to gasoline hydrocarbons, but the normal paraffins have not been
converted.
In US 4,035,430 A, in order to limit the temperature rise over the catalyst
bed of the
hydrocarbon synthesis, the feed material (methanol or the product of the DME
synthesis) is
diluted with light hydrocarbons <C5 and sometimes also with durene, which has
been
separated out of the C10, hydrocarbon fraction by crystallisation. The
recycling of durene
reduced the aromatics content in the product (aromatics except for durene)
compared to the
process without durene recycling in a lower T range (<430 C), while it
increased in a higher
temperature range (>430 C). By increasing the aromatics content in the
product at higher
temperatures (>430 C), the gasoline selectivity could be increased in this
way.
WO 2011/061198 Al describes a two-stage method and a corresponding device for
the
production of hydrocarbons. Starting with synthesis gas, in the first step
methanolis generated,
which is then converted to gasoline hydrocarbons and water in the second step.
The method
is characterised in particular by purification of the water obtained.
Unconverted methanol
contained therein is catalytically converted to synthesis gas. Furthermore,
recycling of both the
synthesis gas thus obtained and of the unconverted synthesis gas is provided.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
4
US 4,058,576 A describes a method for more efficient dissipation of the heat
released in the
conversion of methanol to gasoline by dividing the intermediate product
reactions into 3
successive process steps. The reaction stages of the methanol conversion to
dimethyl ether
(DME), the conversion of the DME into olefins, and the olefin conversion into
gasoline
hydrocarbons are carried out in separate catalyst beds.
US 4,052,479 A also describes a multi-stage process for converting alcohols
into gasoline and
olefin hydrocarbons. For this purpose, DME is first isolated in an
intermediate step and, with
very short contact times, this is preferably converted to olefin hydrocarbons
and gasoline. Short
contact times are achieved at very high loads with a space velocity in a range
from 10 to 2000
h-1 (Liquid Hourly Space Velocity - LHSV), preferably from 50 to 1000 h-1. At
the same time,
the high load is a disadvantage of the process.
US 4,387,263 A therefore describes a process for the production of gasoline
and C2 to C4
olefins from a mixture of methanol and optionally water vapour, in which high
olefin yields are
achieved even at significantly lower LHSV.
DE 10 2005 048 931 Al also discloses a method for the preparation of C2-C4
olefins from
methanol or dimethyl ether. An educt mixture of alcohol, dimethyl ether and
water is passed
over a catalyst bed in a reactor and is converted to a product mixture which
is separated in the
first step into a Cs_ olefin-rich mixture, a C5+ hydrocarbon-rich mixture and
an aqueous phase.
A high mass fraction of water in the educt mixture of 20 to 91%, measured on
the total mass
of the alcohol-water mixture, enables a high proportion of lower olefins to be
generated.
The object of the invention is to develop a method and a corresponding device
for producing
synthetic gasoline from oxygenates, in particular from alcohols, in particular
with a small
proportion of olefins, in which the composition of the gasoline is variable
and can be controlled
in a targeted manner.
This object is achieved by a method for the production of a synthetic gasoline
(in the form of a
stable gasoline fraction) comprising the following steps:
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
a. catalytic conversion of feed alcohol into a product mixture containing
water and a
hydrocarbon mixture of olefins, n-paraffins, isoparaffins, aromatics, and
naphthenes in a reactor containing a catalyst, wherein the feed alcohol has a
water
content of less than 20 mass%.
5 b. separation of the product mixture obtained in step I) into:
- a liquid hydrocarbon phase,
- an aqueous phase containing the unconverted alcohol,
and
- a gas phase containing Ci to C5 hydrocarbons
c. recycling of the gas phase obtained in step II) to step I)
d. separation of the liquid hydrocarbon phase obtained in step II) into
- a C3 to C4 hydrocarbon fraction and
- a gasoline hydrocarbon fraction (= C5+ fraction) containing a durene-
containing
heavy aromatics fraction
e. separation of the gasoline hydrocarbon fraction obtained in step IV) into a
durene-
containing heavy aromatics fraction and a stable gasoline fraction.
The raw feed alcohol in the method is referred to as the feed alcohol. It is
known to the person
skilled in the art that alcohol, in particular alcohol of technical quality,
can still have a proportion
of water.
In one embodiment, the feed alcohol is an alcohol-water mixture.
According to the invention, the feed alcohol has a water content of less than
20 mass%,
preferably less than 15 mass%, in particular less than 5 mass%.
Mass% denotes the mass fraction of the total mass, in %.
In one embodiment, the mass fraction of water in the feed alcohol is at least
1 % of the total
mass of the feed alcohol.
In one embodiment, the mass fraction of water in the feed alcohol is 1 to <20
%, preferably 1
to <15% of the total mass of the feed alcohol.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
6
In one embodiment, the feed alcohol is anhydrous.
The feed alcohol is fed into the circulating gas in liquid or gaseous form.
In one embodiment, the circulating gas is the gas phase recycled to the
reactor in step I) in
step III).
The circulating gas is heated together with the feed alcohol to the reaction
temperature. The
mixture obtained is fed into the reactor in gaseous form. This mixture is
designated as feed
gas.
In one embodiment, the catalytic conversion of feed alcohol in a feed gas into
a product mixture
containing water and a hydrocarbon mixture of olefins, n-paraffins,
isoparaffins, aromatics, and
naphthenes takes place in a reactor containing a catalyst, in which case the
feed gas contains
a feed alcohol having a water content of less than 20 mass% (related to the
total mass of the
feed alcohol).
In one embodiment, the mole fraction of the alcohol (pure alcohol without
water) in the total
feed gas at the reactor inlet is between 20 and 90 mol% (related to the total
amount of feed
gas).
In one embodiment, the feed gas contains inert gas and feed alcohol.
In one embodiment, the feed gas contains feed alcohol and recycled components.
The method according to the invention in step I) advantageously gives a
hydrocarbon fraction
with a small proportion of olefins. In one embodiment, the mass fraction of
the olefins in the
hydrocarbon mixture formed is less than 20 %, preferably less than 10 %,
particularly
preferably less than 5 %, and in particular less than 2 % of the total mass of
the hydrocarbon
mixture formed.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
7
In variant VI, the method contains the following steps:
a) catalytic conversion of alcohol into a product mixture containing water and
a
hydrocarbon mixture of olefins, n-paraffins, isoparaffins, aromatics, and
naphthenes, in
a reactor containing a catalyst
b) separation of the product mixture obtained in step a) into:
- a liquid hydrocarbon phase,
- an aqueous phase containing the unconverted alcohol, and
- a gas phase containing Ci to C5 hydrocarbons
c) separation of the unconverted alcohol from the aqueous phase obtained in
step b)
1.0 d) recycling of the alcohol separated off in step c) as feed material
to step a)
e) division of the gas phase from step b) into a first and a second part and
recycling of the
first part of the gas phase obtained in step b) to step a)
f) removal of C3 to C5 hydrocarbons from the second part of the gas phase
from step e)
g) combination of the C3 to C5 hydrocarbons obtained in step f) with the
liquid hydrocarbon
phase obtained in step b)
h) separation of the liquid hydrocarbon phase obtained in step g) into
- a C3 to C4 hydrocarbon fraction and
- a gasoline hydrocarbon fraction (= C5+ fraction) containing a durene-
containing heavy
aromatics fraction
i) recycling the C3 to C4 fraction separated off in step h) as feed material
to step a)
j) separation of the gasoline hydrocarbon fraction obtained in step h) into a
durene-
containing heavy aromatics fraction and a stable gasoline fraction.
According to the invention, in step I) or in step a) of the variant of method
VI, the catalytic
conversion of feed alcohol into a product mixture containing water and a
hydrocarbon mixture
of olefins, n-paraffins, isoparaffins, aromatics, and naphthenes takes place
in one reactor,
preferably an isothermal reactor.
For the purposes of the invention, the term "alcohol" includes organic
compounds R-OH which
.. contain at least one OH group (hydroxy group) which is bonded to an alkyl
residue (R). For the
purposes of the invention, "alcohol" is understood to mean both a single
alcohol and also a
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
8
mixture of several alcohols. In the following, the feed alcohol or the mixture
of several alcohols
is called "feed alcohol" for better understanding.
Water may be contained in the feed alcohol.
The catalytic conversion takes place in several reaction steps. Produced from
the alcohol is
an ether, from which olefins are subsequently produced. In subsequent
reactions, various
hydrocarbons are formed, consisting of olefins, n-paraffins, isoparaffins,
aromatics, and
naphthenes. If the conversion is not complete, intermediate products of the
reaction, such as
ethers and lower olefins (ethylene, propylene, butylene), are also present in
the product in
addition to the feed alcohol.
For the purposes of the invention, ethers are organic compounds which contain
an ether group
R-O-R ¨ an oxygen atom (0) which is substituted by alkyl residue (R). Ethers
are formed from
alcohols, preferably on an acidic catalyst, by dehydrating them (elimination
of water).
Depending on the feed alcohol, different ethers are formed as intermediate
products. Dimethyl
ether is produced from methanol, diethyl ether is produced from ethanol, and
dipropyl ether is
produced from propanol.
The alcohol is expediently and preferably selected from compounds containing
at least one
OH group which is bonded to an alkyl residue having 1 to 3 carbon atoms. The
alkyl residue
can be either branched or unbranched.
The alcohol is preferably selected from the following: methanol, ethanol,
isopropyl alcohol (2-
propanol), and n-propanol (1-propanol), with the alcohol particularly
preferably being methanol.
The feed alcohol can first be synthesised according to the prior art.
Advantageously, the water
contained in the reaction product (e.g., in the raw methanol) does not have to
be separated off
in the method according to the invention before it is fed into the reactor,
but can be fed into the
process together with the alcohol. Thus, a distillation stage can
advantageously be omitted.
As a rule, the feed alcohol is an alcohol-water mixture with a water content
of less than 20 %
of the total mass of the feed alcohol. In one embodiment, the feed alcohol
contains 1 to 20
mass% of water (based on the total mass of the alcohol-water mixture). In one
embodiment,
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
9
the feed alcohol contains less than 20 mass% of water (related to the total
mass of the alcohol-
water mixture), preferably less than 15 mass%, and particularly preferably
less than 10 mass%.
In step a) of variant V1, it is advantageously possible to generate a
hydrocarbon mixture with
a mass fraction of olefins of less than 20% of the total mass of the
hydrocarbon mixture formed.
The presence of a higher proportion of water (in the form of water vapour) in
the reactor would,
on the one hand, promote the formation of lower olefins and, on the other
hand, would reduce
the formation of heavy hydrocarbons.
Furthermore, the presence of water also leads to a certain shortening of the
service life of the
catalyst by deactivation (partly irreversible) of the active acid centres.
The control of the water content of the feed gas into the reactor is therefore
not insignificant,
and a low water content in the feed alcohol of less than 20 % is advantageous
both for the
product formed as well as in economic terms.
The proportion of olefins in the end product is therefore advantageously
lower. A gasoline with
a low olefin content is characterised by a very high storage stability. In
particular, the octane
number of the gasoline remains constant over a long time.
In one embodiment, the alcohol is produced from renewable raw materials, which
can be used
to produce sustainable, CO2-neutral gasoline.
For the catalytic conversion, the alcohol, for example methanol, is fed into a
reaction zone in
a reactor containing a catalyst and is completely or incompletely converted
there.
In one embodiment, the feeding takes place at a pressure between 5 and 15 bar.
In the embodiment for producing a low-aromatics gasoline, the inlet
temperatures into the
reactor are between 300 and 350 C.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
In one embodiment for producing a gasoline high in aromatic compounds, the
inlet
temperatures into the reactor are between 350 and 460 C.
The catalyst usually contains zeolites as the catalytically active component.
In one
5 embodiment, the catalyst contains ZSM-5 zeolites. In one embodiment, a
ZSM-5 catalyst with
an SiO2 to A1203 ratio of the zeolite of at least 30:1, preferably higher, is
used.
The reaction of methanol to hydrocarbons on a zeolite-containing catalyst is
highly exothermic.
The reaction temperature has a very large influence on the reaction rate and
thus the
10 composition of the reaction product and the selectivity of the process.
If the temperatures rise
too much due to the exothermic reaction, this can lead to a rapid deactivation
of the active
centres of the catalyst, which has a negative effect on the reaction time
between the
regenerations and the service life of the catalyst.
A prerequisite for targeted control of the process (with regard to the
reaction product
composition and selectivity of the main product) and economic process
management (service
life of the catalyst, frequency of regeneration, catalyst load) is mastery of
the exothermic nature
of the reaction through internal cooling of the catalyst bed.
After filling the reactor with fresh catalyst or after a catalyst
regeneration, all of the feed alcohol
impinges on the first catalyst layer and reacts at the beginning of the
method, which results in
a sharp rise in temperature in this region and a weakening of the active
centres due to their
partial deactivation. The rise in temperature in the subsequent catalyst
regions gradually
decreases, since less and less of the feed alcohol remains and therefore the
amount converted
gradually decreases.
The next time alcohol is fed in, the temperature in the first catalyst region
no longer rises so
much, since the activity of this catalyst region has been weakened due to the
high
temperatures that have already occurred. The temperature front therefore moves
further into
the next, even more active, catalyst layer.
An efficient removal of the heat of reaction is essential.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
11
In one embodiment of the method for producing a low-aromatics product, the
temperature
difference in the catalyst bed in the region of the temperature front is a
maximum of 40 K at
the beginning of a reaction period to a maximum of 20 K at the end of a
reaction period.
In one embodiment for the production of a low-aromatics product, the
temperature in the
catalyst bed is between 300 and 370 C, depending on the degree of
deactivation of the
catalyst.
In the embodiment of a process for producing a product high in aromatic
compounds, the
temperature difference in the catalyst bed in the region of the temperature
front is a maximum
of 70 K at the beginning of a reaction period up to a maximum of 30 K at the
end of a reaction
period.
In one embodiment for producing a product high in aromatic compounds, the
temperature in
the catalyst bed is between 350 and 490 C, depending on the degree of
deactivation of the
catalyst.
The heat of reaction is removed from the reaction region of the reactor by
heat exchange.
In one embodiment, the reactor contains internal fittings in the form of
plates, tubes, and other
elements for heat exchange, in which case the catalyst is located in the
elements and the
cooling medium is located around the elements or vice versa. The flow of the
reaction gas can
take place in the axial direction (from top to bottom or from bottom to top)
or in the radial
direction (from an inner central tube to the outside) to avoid excessive
pressure losses.
The heat transfer medium is located in the heat exchange elements or also
outside these
elements. Heat transfer media which may be used include all suitable media
which evaporate
or condense in the temperature range of reaction and regeneration or which
maintain the
physical state and dissipate the heat of reaction by convection.
The heat transfer medium for dissipating the heat of reaction goes through a
constant cycle. It
absorbs the heat from the reaction zone, flows outward (outside the catalyst
bed), transfers
the heat to a second heat transfer medium, preferably boiling water, either in
the interior of the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
12
reactor or outside the reactor in a separate heat exchanger, and then returns
to the reaction
zone for heat absorption.
The cooling of the heat transfer medium takes place outside the reactor in a
heat exchanger,
in which preferably saturated steam is generated at a pressure of at least 20
bar, particularly
preferably of at least 40 bar.
The heat transfer medium can also be conducted to the reaction zone in several
zones, each
with a different temperature. This means that the temperature of the heat
transfer medium can
be set lower in the region of high reaction temperature than in regions with a
lower reaction
temperature. The number of heat transfer zones with different temperatures is
only limited by
the economy of the reactor.
A reactor in which the heat of reaction is removed according to the heat pipe
concept can also
be used as the reactor. In this concept, the heat transfer medium is located
in the interior of
heat exchange elements, where it evaporates by absorbing the heat from the
reaction system
and condenses again by releasing heat to a second heat transfer medium
(preferably boiling
water), which is preferably located in the upper part of the reactor outside
the catalyst bed and
cools the heat exchange elements. Condensation and evaporation in the interior
of the heat
exchange elements take place in a closed system. The boiling water evaporates
when the heat
is absorbed by the heat transfer medium. The resulting saturated steam
preferably has a
pressure of at least 20 bar, particularly preferably at least 40 bar. In this
type of reactor, the
reaction gas can either flow axially (from top to bottom or from bottom to
top) through the
catalyst bed or in the radial direction to avoid excessive pressure losses
(from an inner central
tube to the outside).
In one embodiment, the reactor (R) is an isothermal tubular reactor.
According to the invention, in step I) or a) in Vi, the alcohol, for example
the methanol, is
converted into a product mixture containing water and a hydrocarbon mixture
containing
olefins, n-paraffins, isoparaffins, aromatics, and naphthenes.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
13
The mass fraction of the olefins in the hydrocarbon mixture formed is
advantageously less than
20 %, preferably less than 10 %, particularly preferably less than 5%, and in
particular less
than 2 % of the total mass of the hydrocarbon mixture formed.
In one embodiment, the reaction mixture is subsequently cooled to 45 C,
preferably to 35 C,
in heat exchangers, air coolers, and cooling water coolers. In one embodiment,
cooling takes
place using a further coolant down to 5 C.
During cooling, water, alcohol, and the condensable hydrocarbons condense. In
the next step,
this product mixture is separated into three fractions.
According to the invention, in step II) or b) in V1, the product mixture
obtained in step I) or a)
in V1 is separated into:
- a liquid hydrocarbon phase,
- an aqueous phase containing the unconverted alcohol,
and
- a gas phase containing C1 to C5 hydrocarbons.
In one embodiment, the product mixture is separated in a three-phase separator
(S).
The aqueous phase from step II) or b) in V1 contains the alcohols which have
not been
converted during the reaction, for example methanol.
After the separation in step II) or b) in V1, unconverted intermediate
products may be contained
both in the gas phase (e.g., dimethyl ether, diethyl ether, ethylene) and in
the liquid
hydrocarbon phase (e.g., dimethyl ether, diethyl ether, dipropyl ether,
propylene, butylene).
In one embodiment, the unconverted alcohol is separated from the aqueous
phase. In variant
V1, this is step c).
In one embodiment, the aqueous phase obtained in step II) or b) in V1 is
separated in a
separation device into water, and unconverted alcohol and the unconverted
alcohol is recycled
to step I) or a) in V1 .
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
14
In one embodiment, the separation takes place in a separation device K3. In
one embodiment,
the separation device K3 is a column.
In one embodiment, the separation takes place at 2-3 bar. The separation
device K3 is
equipped with a top product cooler and a bottom heater. In one embodiment, the
top
temperature is 70 to 90 C, preferably 80 to 85 C, and the bottom temperature
is 110 to 150
C, preferably 120 to 130 C.
In one embodiment, the separated alcohol is returned to the reactor as feed
material. In variant
V1, this is step d).
In one embodiment, it is liquid and, together with the feed alcohol, it is fed
into the gas circuit
on the pressure side of the compressor and, together with the circulating gas
(i.e., with the gas
phase obtained in step II) or b) in V1), is heated in heat exchangers by
cooling the hot reaction
product in counterflow and is evaporated. After final heating to the reaction
temperature, the
mixture enters the reactor in gaseous form.
Since the feed alcohol is preferably methanol, this circuit is also called the
methanol circuit.
According to the invention, the gas phase obtained in step II) or b) in V1 is
returned to step I)
or a) in V1.
In one embodiment, the gas phase from step II) or b) in variant V1 contains
light gaseous
hydrocarbons which do not condense on cooling of the reaction product. These
are both inert
components that no longer react in the reaction zone of the reactor (e.g.,
methane, ethane,
CO2, nitrogen) and also intermediate reaction products (e.g., dimethyl ether,
ethylene) which,
when used again in the reactor, react further and are converted to the desired
gasoline
hydrocarbons.
In one embodiment, a small proportion of the gas phase from step II) is
discharged from the
gas circuit in order to avoid the accumulation of inert components in the gas
circuit.
A predetermined target pressure of the feed gas should expediently be
maintained at the
reactor inlet. The pressure setting at the inlet into the reactor can
advantageously be regulated
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
by regulating the discharge of gas from the gas circuit when the pressure
rises at the reactor
inlet.
The main part of the recirculating gas is returned to the reactor.
5
In one embodiment, the gas phase obtained in step II) or b) in variant V1 is
divided, and the
first part (= main part) is returned to the reactor R. The division takes
place by opening of a
valve in the gas line from the three-phase separator to the separation device
K4, which opens
in the event of excess pressure at the reactor inlet. The other, second part
of the gas phase
10 flows to the compressor, which compresses the gas and conveys it to the
reactor. In variant
V1, this is step e). In one embodiment, the separation device K4 is a column.
With the aid of a compressor the main part of the gas phase (first part) is
conveyed back as a
circulating gas to the reactor inlet (to step I) or a) in V1), where it causes
a dilution of the feed
15 material. By means of the compressor, the pressure losses in the gas
circuit are compensated
for, and the amount of circulating gas can be set (according to the target
volume flow).
The other part of the gas phase (second part) is removed from the device by
regulating the
pressure at the inlet of the reactor.
The second part of the gas phase is separated in a further step. In variant
V1, this is step f).
In one embodiment, the division of the gas phase is not carried out and,
therefore, no
circulating gas flow (i.e., part of the gas phase from step II) or b) in V1)
is returned to the
reactor. The entire gas phase is discharged from the device after separation
in the three-phase
separator. In this embodiment, the desired temperatures in the reactor can be
maintained
solely by cooling the catalyst bed inside the reactor, so there is no need to
dilute the starting
materials with circulating gas.
In one embodiment, the second part of the gas phase is separated in a
separation device K4,
for example in a separation gas column. The gas phase is separated at a
pressure of 10 to 20
bar, preferably at 15-16 bar. It is necessary to compress the gas to this
pressure before it
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
16
enters the separation device. The separation gas column is equipped with a top
product cooler.
The top temperature is -30 to -10 C, preferably -20 C to -25 C.
This separates off the C3 to C5 hydrocarbons.
In a subsequent step, these C3 to C5 hydrocarbons that are separated off are
combined with
the liquid hydrocarbon phase obtained in step II) or b) in V1. In variant V1,
this is step g).
In one embodiment, a part of the gas phase obtained in step II) orb) in V1 is
separated off in
a separation device in a C3 to C5 hydrocarbon fraction, and this fraction is
combined with the
liquid hydrocarbon phase obtained in step II).
In one embodiment, this hydrocarbon phase is then separated in step IV) into a
C3 to C4 fraction
and a gasoline hydrocarbon fraction including a durene-containing heavy
aromatics fraction.
Depending on the reaction conditions, the liquid hydrocarbon phase obtained in
step II) or b)
in variant V1 contains more or fewer C3 and C4 hydrocarbons. If the methanol
conversion is
incomplete, the intermediate product dimethyl ether (DME) is also a
constituent of the reaction
product. Part of the DME is contained in the liquid hydrocarbon phase.
Primarily C5+
compounds are contained in the liquid hydrocarbon phase.
C3 and C4 hydrocarbons may be the paraffins propane, butane, and isobutane.
Under certain
reaction conditions in which the feed material is not fully converted,
olefinic intermediate
products such as propylene, butylene, and isobutylene can also emerge from the
reactor and
can be contained in the liquid hydrocarbon phase.
In one embodiment, the liquid hydrocarbon phase obtained in step II) or b) in
variant V1 is fed
together with the C3 to C5 fraction into a separation device Kl.
In step IV) or h) in V1, the C3 to C5+ hydrocarbons are separated into
- a C3 and C4 hydrocarbon fraction and
- a gasoline hydrocarbon fraction (= C5+ fraction) containing a durene-
containing
heavy aromatics fraction.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
17
In the context of the invention, a heavy aromatics fraction means a fraction
containing aromatic
hydrocarbons having a higher molecular weight. These are aromatic hydrocarbon
compounds
containing 9 to 11 carbon atoms. The benzene ring can be methylated several
times.
In one embodiment, the C3 to C4 fraction is separated off from the liquid
hydrocarbon phase
as a top product in a separation device Kl, which can be a column. The
separation in the
column takes place at a pressure of 10 to 20 bar, preferably 13 to 17 bar. The
column is
equipped with a top product cooler and a bottom heater. The top temperature is
10 to 90 C,
preferably 20-70 C, and the bottom temperature 150 to 250 C, preferably 180-
225 C. The
parameters strongly depend on the composition of the mixture to be separated.
In one embodiment, the top product consists of the paraffins propane, butane
and isobutane.
At least part of this C3 to C4 fraction of such a composition can be withdrawn
as a saleable
"liquefied petroleum gas (LPG)" product.
In one embodiment, the C3 to C4 fraction contains olefins, and/or dimethyl
ether, and/or
saturated C3 to C4 hydrocarbons.
In one embodiment, at least a portion of the C3 to C4 hydrocarbon fraction
separated off in step
.. IV) is recycled in the circuit to the inlet of the reactor as feed material
to step I) or a) in variant
V1.
According to the invention, the gasoline hydrocarbon fraction (C5+ fraction)
separated off in the
separation device contains a stable gasoline fraction and a durene-containing
heavy aromatics
fraction containing durene, iso-durene, and other multiply-methylated
aromatics.
Durene (1,2,4,5-tetramethylbenzene) and iso-durene (1,2,3,5-
tetramethylbenzene) are heavy
methylated aromatics that have the property of solidifying in the temperature
range in which
gasoline is used (ambient temperature before the fuel enters the engine). The
solidification
temperature of durene is 79.2 C, and that of iso-durene is -20 C. For this
reason, these
tetramethylbenzenes should not be contained in gasoline.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
18
In the process according to the invention, they are separated from the liquid
portion of the
hydrocarbon product, for example by distillation.
The gasoline hydrocarbon fraction from step IV) or h) in variant V1,
containing C5+
hydrocarbons, is further separated into a durene-containing heavy aromatics
fraction and a
stabilised gasoline fraction in step V) or j) in V1.
In one embodiment, the durene-containing heavy aromatics fraction contains
durene, iso-
durene, and multiply methylated aromatics.
In one embodiment, the gasoline hydrocarbon fraction is separated in a
separation device K2.
The separation device K2 can be a column which is equipped with a top product
cooler and a
bottom heater.
In one embodiment, the separation takes place at a pressure of 1 to 3 bar,
preferably 1.2 to
2.5 bar, at a top temperature of 50 to 90 C, preferably 60-75 C, and a
bottom temperature in
the range from 200 to 260 C, preferably 230-240 C.
In this case, a stable gasoline fraction and a durene-containing heavy
aromatics fraction are
obtained. The stable gasoline fraction is the desired gasoline product with a
high octane
number of >90 RON, preferably >1= 95 RON.
In one embodiment, durene is crystallised out of the durene-containing heavy
aromatics
fraction.
In one embodiment, the heavy aromatics fraction is placed in a crystalliser
KR1, in which the
durene which crystallises out at about 79-80 C is separated off by
crystallisation.
In one embodiment, the crystallised durene is dissolved in alcohol and/or in
gasoline
hydrocarbons and, together with the alcohols used and recycled, is recycled to
the reactor.
In one embodiment, it is not pure durene, but rather the bottom product of the
separation device
K2 (heavy durene-containing aromatics fraction) which is completely or partly
returned to the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
19
reactor without the durene being separated off. In this case, no crystalliser
KR1 and no
dissolving device V1 for releasing the durene are required.
In one embodiment, the gasoline hydrocarbon fraction from step IV) or h) in
variant V1 is further
separated in the separation device K2. If K2 is a column, for example, iso-
pentane at a
pressure of 1.1 to 3 bar, preferably 1.2 to 2.5 bar, is separated off in the
top of the column at
30-50 C, the boiling fraction of the C6-C7 paraffin hydrocarbons is separated
off in a side outlet
at 50-100 C, and the stable gasoline fraction is separated off in a further
side outlet.
Isopentane and the stable gasoline fraction are then mixed. The mixture
corresponds to the
.. desired stable gasoline product. The durene-containing heavy aromatics
fraction is obtained
in the bottom at a bottom temperature of 220 to 250 C, preferably 230-240 C.
In one embodiment, the fraction of the C6 - C7 hydrocarbons, or a part
thereof, is recycled to
the reactor to step I) or a) in V1.
The method according to the invention is advantageously characterised in that
the linking of
several material circuits to/from the reactor during the synthesis of
hydrocarbons enables the
setting of variable reaction conditions in wide ranges.
A special feature of the method is the high flexibility of the product
composition by variation of
the process parameters. The method is intended to make it possible to respond
to special
requirements for the gasoline composition. In this way, a gasoline or a
gasoline blend of a
composition can be produced in accordance with economic and
ecological/political
requirements. In particular, the method makes it possible to produce a
gasoline with low
emissions during combustion with reduced CO2 emissions and reduced particle
formation
potential. If required, a gasoline blend with a high octane number can be
produced which is
characterised by a high proportion of high-octane isoparaffinic and/or
aromatic components.
For high-performance engines, gasolines with a very high octane number (RON 98
and higher)
are necessary. Very high octane numbers can no longer be achieved solely
through a high
isoparaffin content in gasoline. In addition to the isoparaffins, a certain
proportion of high-
octane aromatics in the gasoline is necessary for this. Aromatics can reach
octane numbers
of up to approx. RON 120 to 150 (e.g., toluene - RON 124; p-xylene - RON 146).
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
Due to the higher aromatics content, premium gasoline cannot achieve the
ecologically
advantageous high H:C ratio, but has very good combustion properties due to
the high octane
number, which reduces fuel consumption. Despite the higher carbon content,
this gasoline can
also contribute to the reduction of CO2 emissions by producing it in a PtL
process ("power to
5 liquid") and, if possible, from raw materials made from a "renewable"
(biomass for CO2
generation) or "regenerative" (water for hydrogen production) source or from
industrial waste
gases (CO2 or CO2 and H2).
The method according to the invention and the device according to the
invention can
3.0 advantageously not only be integrated into the PtL process chain, but
can also be combined
with conventional processes for the production of synthesis gas from a fossil
source (e.g., from
natural gas) and subsequent methanol synthesis from the synthesis gas.
In the method according to the invention, stabilised crude methanol can be
used
15 advantageously as the feed material without the need for further
processing of the crude
methanol by costly separation off of the water contained therein as well as
other alcohols and
oxygenates.
The gasolines produced according to the invention can be used not only
directly in the engine,
20 but also as blended gasolines in refineries for admixture into the
gasoline pool. As blended
gasolines, they serve, on the one hand, to increase the octane number and, on
the other hand,
to reduce the CO2 footprint of the refinery gasoline.
When a gasoline with a high isoparaffin content and low aromatics content is
used as blended
gasoline, the carbon content in the mixed finished gasoline is reduced, and
the CO2 content in
the exhaust gas and the particle formation potential during combustion is
reduced in this
manner.
The method according to the invention can advantageously be used to produce
gasolines with
a variable composition by adapting process parameters.
In the method according to the invention, process parameters such as catalyst
load,
temperature, degree of conversion, and recycling of certain intermediate
products can
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
21
advantageously be set in a targeted manner in such a way that a gasoline with
a high
isoparaffin content/low aromatics content or with a high aromatics content is
obtained.
Two variants for the extreme cases "high isoparaffin content and low aromatics
content of
gasoline" and "high aromatics content of gasoline" are described below.
For the purposes of the invention, a gasoline with a high isoparaffin content
and low aromatics
content (low-aromatics) contains 50-65 mass% of isoparaffins and 20-35 mass%
of aromatics.
A gasoline with a high aromatic content (high in aromatic compounds) contains
35 - 70 mass%
of aromatics and 25 - 45 mass% of isoparaffins.
In one embodiment, a gasoline with a high isoparaffin content and low
aromatics content (low-
aromatics) contains 50-60 mass% of isoparaffins and 25-35 mass% of aromatics.
A gasoline
with a high aromatics content (high in aromatic compounds) contains 40 - 60
mass% of
aromatics and 25 - 45 mass% of isoparaffins.
Mass% means the mass fraction of the total mass, in %.
Using the process according to the invention, however, it is also possible to
produce gasolines
with compositions that cover ranges between the two extreme cases.
Process parameters that can be set individually are, for example, catalyst
load, contact time
with the catalyst, reaction temperature, flow rate, total pressure in the
reactor, and the partial
pressures of the individual components.
The catalyst load is specified via the parameter of space velocity (LHSV -
Liquid Hourly Space
Velocity). This results from the quotient of the liquid volume flow of the
feed alcohol and the
catalyst volume.
In the method according to the invention, the LHSV is defined as the amount of
liquid alcohol
(at room temperature and normal pressure), based on 1 m3 of catalyst, in m
....3alcohdh n13cat.
The catalyst load indicates the amount of pure feed alcohol (without water)
per hour and
catalyst volume.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
22
According to the reaction mechanism of the MTH reaction described above, DME
and olefins
are formed when methanol is converted. These first reaction steps take place
relatively slowly,
while the subsequent steps of reacting the olefins with the methanol to form
further olefins or
CH2 fragments and the subsequent reactions are very fast reactions.
Generation of gasoline with high isoparaffin/low aromatics content (low-
aromatics and
isoparaffin-rich gasoline)
The decisive factor for the formation of a high proportion of isoparaffins in
the product mixture
is initially the incomplete conversion of the starting materials, for example
methanol. The
following explanation is shown with the example of using methanol.
A high loading of the catalyst with methanol leads to an incomplete reaction
or only to a partial
conversion of the methanol fed into the reactor. Unconverted methanol and
intermediate
products such as dimethyl ether and olefins emerge from the reactor. As the
load increases,
the aromatics content in the product drops. Recycling the olefin-containing C3-
C4 fraction
promotes the formation of isoparaffins during the reaction.
In one embodiment, the alcohol load (for example methanol load) of the
catalyst for producing
an isoparaffin-rich gasoline is between 2 and 5 m3meoH/h m3cat., preferably
between 2.5 and 4
M3Me01-1/11 M3cat. (based on the total volume of catalyst contained in the
reactor).
The high loading of the catalyst with methanol causes an increase in the
proportion of olefin
hydrocarbons in the reaction product.
The contact time of the feed materials in the catalyst bed also influences the
product
composition. In the method according to the invention, the contact time in the
catalyst bed in
the flow direction of the reaction gas can advantageously be adapted to the
requirements of
the described sub-steps of the MTH reaction.
In one embodiment, the catalyst load and contact time are set by changing the
flow cross-
section by means of design measures, for example by changing the diameter of
the catalyst
tubes. An increase in the flow cross-section in the catalyst bed causes an
increase in the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
23
contact time and a reduction in the catalyst load. The opposite effect is
brought about by
narrowing the flow cross-section.
In one embodiment, the catalyst is partially diluted with inert material to
produce an isoparaffin-
rich gasoline. The catalyst volume is influenced in a targeted manner by the
addition of inert
material. By diluting the catalyst with inactive material, the catalyst volume
in the affected
reactor section is reduced, but the flow conditions remain the same (provided
that the inert
material has the same particle shape as the catalyst and the gap volume
corresponds to that
of the catalyst bed without inert material). The contact time is reduced, and
the load of the
catalyst is increased. In one embodiment, when zeolites are used as catalysts,
aluminium
oxide materials (ceramics) are used as the inert material.
The slow reactions of dehydration with the formation of dimethyl ether and
water and the
formation of olefins or CH2 fragments from the oxygenates methanol and DME
take place in
the entry region of the methanol into the catalyst bed.
In order to produce gasoline with a high isoparaffin content, loads and
contact times are set in
the first section of the catalyst bed in the flow direction, which ensure the
incomplete
conversion of the methanol to DME.
In the following section of the catalyst bed in the flow direction, very short
contact times and
very high catalyst loads are required in order to ensure that the MTH reaction
is terminated
when the olefin is formed.
It is only in the last, lower section of the catalyst bed that longer contact
times and lower catalyst
loads have to be set so that the desired gasoline hydrocarbons with a low
aromatics content
are formed.
It is advantageous if the load in this area is so high that the intermediate
products cannot react
further to the end products and components which have not yet been converted -
e.g.,
methanol, DME and olefins - are present in the reaction product.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
24
In one embodiment, the C3 to C4 fraction separated off in step IV) or h) in
variant VI is recycled
to the reactor inlet as starting material to step I) or a) in variant Vi. This
stimulates the formation
of isoparaffins. At the entry into the catalyst bed, the olefin hydrocarbons
or the resulting CH2
fragments can react with the alcohol, for example methanol, and thus lead to
an acceleration
of the MTH (methanol to hydrocarbons) reaction. In the further course of the
reaction, the CH2
fragments methylate the other hydrocarbons formed - such as n-paraffins,
naphthenes, and
aromatics. Since the formation of aromatics is inhibited under the reaction
conditions described
above, isoparaffins are mainly formed by the addition of methyl groups to the
n-paraffins
formed.
In one embodiment, in order to increase the degree of branching of the
isoparaffins in the last
section of the catalyst bed, for example in the lower third, a zeolite
catalyst of the type ZSM-5
is used which is modified with an active component for activating
isomerisation reactions, such
as, for example, a metallic component, e.g. nickel.
The product composition can advantageously be adjusted by varying the degree
of conversion
of the oxygenates, for example the methanol.
As already described above, the degree of conversion of, for example, methanol
is adjusted
by the reaction temperature and the load of the catalyst. A high load (large
amount of methanol
per catalyst volume >3 h-1) of the catalyst with feed methanol and at low
reaction temperatures
(300-350 C) can ensure that not all of the methanol fed in is converted on
the catalyst, and
also that the gasoline hydrocarbons produced in the reaction only partially
react further to the
end product of the aromatics.
In addition to the unconverted methanol, the intermediate product dimethyl
ether, which is
formed by dehydration of the methanol, further reacts only incompletely to
hydrocarbons under
these conditions.
In one embodiment, the degree of conversion of the methanol to produce a
petrol rich in
isoparaffin is between 70 and 90 %, preferably between 70 and 80 %.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
A further process parameter for setting the product composition is the
methanol partial
pressure.
The methanol partial pressure describes the proportion of methanol in the
total feed material
5 into the reactor. It is defined as the molar fraction of methanol in the
feed gas multiplied by the
total pressure at the inlet into the reactor. The methanol partial pressure
reflects the degree of
dilution of the methanol with inert components and influences the degree of
conversion of the
methanol. Low methanol partial pressures indicate a high dilution of the
educt, the degree of
methanol conversion increases with the degree of dilution of the educt.
By the circulation of certain components (for example: circulating gas - Ci to
C4 hydrocarbons
and DME) or recycling of the C3/C4 liquid gas components, a certain volume
flow through the
catalyst bed is set, which, depending on the inflow cross-section of the
catalyst bed and the
parameters pressure and temperature, sets a specific flow rate of the reaction
gas in the
reactor.
A high flow rate of the reaction mixture through the catalyst bed is required
for good heat
transfer from the reaction zone to the heat transfer medium. Flow rates that
are too low result
in a low heat transfer coefficient on the gas side. Flow rates that are too
high cause high
pressure losses across the catalyst bed, in particular if the flow path
through the catalyst bed
is several metres long.
A medium range of the flow rate, which provides both good heat transfer and
low pressure
losses, should therefore be used.
In one embodiment, the flow rate of the reaction mixture is 0.8 to 3.0 m/s,
preferably 1.5 to 2.5
m/s.
In one embodiment for producing an isoparaffin-rich gasoline, the mole
fraction of alcohol, for
example methanol, in the total amount of feed gas at the reactor inlet is 25
to 50 mol%,
preferably 25 to 40 mol%, in which case the feed gas comprises the feed gas
and the recycled
components.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
26
In one embodiment, "recycled components" are understood to be the hydrocarbon
fractions
which are referred to in the individual method steps as "recycled to step 1)".
The recycled components include at least the gas phase (circulating gas)
obtained in step II).
In one embodiment, the recycled components additionally contain a C3 to C4
fraction separated
off in step IV).
In a further embodiment, the recycled components additionally contain alcohol
which has been
separated off from the aqueous phase obtained in step II).
In a further embodiment, the recycled components preferably also contain a C3
to C4 fraction
separated in step IV).
These embodiments can be combined with one another as desired.
To produce a gasoline with a high aromatics content, the recycled components
preferably also
contain a C6-C7 hydrocarbon fraction separated off in step IV).
In order to produce an isoparaffin-rich gasoline, the recycled components
preferably also
contain part of the heavy durene-containing aromatics fraction obtained in
step V).
In order to produce a gasoline with a low aromatics content and a high
isoparaffin content, the
reaction temperature in the catalyst bed must be as low as possible, but must
be above the
initiation temperature of the reaction.
A high increase in the reaction temperature due to the exothermic nature of
the reaction must
be avoided. In particular in the entry region of the methanol into the
catalyst bed, high
temperature increases must be avoided, which can be achieved by dilution of
the catalyst with
inert material.
In one embodiment for producing an isoparaffin-rich gasoline, the catalyst is
partially diluted
with inert material.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
27
In one embodiment, the first catalyst region, on which the greatest amount of
the feed alcohol
impinges at the beginning of the reaction phase (about 1/5 to 1/6 of the bed),
is diluted with
inert material with a mass fraction of 5 to 30 %, particularly preferably 10
to 25 % (based on
the total mass of catalyst and inert material) in order to avoid high
temperature rises.
In one embodiment, aluminium oxide materials (ceramics) are used as the inert
material.
In one embodiment, the reaction temperatures for producing a low-aromatics and
isoparaffin-
rich gasoline are between 300 and 370 C, preferably between 300 and 350 C.
In one embodiment, the total pressure in the reactor for producing a low-
aromatics and
isoparaffin-rich gasoline is <15 bar, preferably <11 bar, particularly
preferably <10 bar.
In one embodiment, durene is crystallised out of the durene-containing heavy
aromatics
fraction.
In one embodiment, the heavy aromatics fraction is placed in a crystalliser
KR1, in which the
durene which crystallises out at about 79-80 C is separated off by
crystallisation.
In one embodiment, the durene which has crystallised out is dissolved in the
feed alcohol or in
the recycled alcohol from the separation device K3 and/or in gasoline
hydrocarbons, for
example the stable gasoline fraction from step V), in order to produce a low-
aromatics and
isoparaffin-rich gasoline. The durene solution is then recycled to step I) or
a) in variant V1 into
the reactor in a circuit.
In one embodiment, in order to produce a isoparaffin-rich gasoline, part of
the heavy durene-
containing aromatics fraction obtained in step V) is returned to step I).
In this way, the aromatisation activity of the catalyst can be influenced. By
recycling of durene
to the reactor, the formation of the other aromatics can be reduced, with the
result that this
method can be used to produce a low-aromatics gasoline. Additional positive
effects include
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
28
increasing the intermediate product regeneration time or reducing the
frequency of necessary
catalyst regenerations and reducing the ageing of the catalyst and increasing
its service life.
In one embodiment, hydrogen is additionally fed into the methanol circuit
and/or into the reactor
in order to produce a gasoline product with a high isoparaffin content and a
low aromatics
content.
The hydrogen serves to saturate the olefinic and aromatic components which are
low in
hydrogen, to convert these components to n-paraffins and isoparaffins and
naphthenes, and
to avoid or reduce the formation of coke on the catalyst surface. Reduced coke
formation
increases the intermediate product regeneration time and the service life of
the catalyst.
The addition of hydrogen enables the isoparaffin content in the reaction
product to be
increased. Increasing the isoparaffin content/reducing the aromatics content
increases the H/C
ratio of the product such that, beginning at a certain ratio of the
isoparaffins to the aromatics
in the product, the stoichiometrically required amount of hydrogen is greater
than that present
in the feed alcohol.
Starting with methanol as the preferred feed material, the maximum isoparaffin
content of the
gasoline of 75 mass% (mass percentage = mass fraction of the total mass, in %)
with an
aromatics content in the gasoline of approximately 11 mass% can be achieved by
the addition
of hydrogen. The amount of hydrogen required is approximately 1 to 5 mass%,
preferably 1 to
2 mass%, based on the alcohol to be converted, for example methanol.
The minimum feed pressure of the hydrogen must be above the system pressure in
the gas
circuit.
In one embodiment, the hydrogen is fed in at a pressure of 8 to 15 bar,
preferably 9 to 12 bar,
particularly preferably 10 bar.
In one embodiment, in order to reduce the formation of aromatics and/or to
produce an
isoparaffin-rich gasoline, in particular at the beginning of a reaction cycle,
part of the durene-
containing heavy aromatics fraction, for example from step j) in variant V1
without separation
off of the durene, is recycled to the reactor to step I) or a) in V1. This
method promotes the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
29
build-up of a carbon pool on the active catalyst surface, so that its activity
is weakened and
fewer aromatics are formed. The ageing of the catalyst will be reduced and its
service life
increased.
Production of gasolines with a high aromatics content (high-aromatics)
The process parameters have to be adjusted accordingly in order to produce
gasolines with a
high aromatics content.
It is important to convert the starting materials as completely as possible at
the beginning of
the reaction.
If the catalyst has a low load of methanol/DME, the starting material can be
completely
converted. Intermediate products such as dimethyl ether and olefins are
converted into
secondary products such as paraffins and aromatics.
The separation off of unconverted oxygenates, such as methanol, from the water
is also
important for the production of high-aromatics gasolines.
Particularly when changing between the reaction and regeneration phases, it is
necessary to
separate the unconverted methanol from the reaction water.
It is necessary to carry out the catalyst regeneration periodically because
the catalyst,
preferably consisting of zeolites, loses activity in the course of the
reaction phase due to coke
deposits on the surface of the active centres, which can be compensated for by
the gradual
increase in the temperature in the reactor (which increases the reaction
rate). In this way, the
degree of conversion of the feed material can be kept constant to a certain
degree.
Above a certain degree of deactivation, however, the conversion rate drops,
and the activity of
the catalyst can only be restored by burning the coke off from the catalyst
surface (=
regeneration). For this reason, the phases of the reaction and the
regeneration alternate
periodically during the entire period of catalyst use.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
In one embodiment, the duration of the reaction phase is 3 to 5 weeks, and the
duration of the
regeneration phase is 3 to 10 days, preferably 1 week.
In the starting phase of the reaction regime or when changing from the
reaction regime to the
5 regeneration regime, the conversion of the feed alcohol is incomplete
and, in one embodiment,
the unconverted alcohol is separated from the water using the separation
device K3 and is
returned to the reactor in the circuit. On the one hand, a high product
selectivity is achieved
and, on the other hand, the water is purified to a residual alcohol content.
10 The preferred formation of aromatics from the intermediate products is
promoted by a high
level of catalyst activity. The reaction temperature is crucial for high
activity. High reaction
temperatures must be set in order to produce a high-aromatics gasoline.
In one embodiment, the reaction temperature for producing a gasoline with a
high aromatics
15 content is between 350 and 490 C.
The reaction temperatures are limited towards the top of the range in order to
avoid irreversible
deactivation of the catalyst.
20 A high reaction temperature can be achieved by setting a high inlet
temperature of the feed
gas into the reactor and a correspondingly higher temperature of the heat
transfer medium at
the inlet into the reactor and by dispensing with or using limited inert
material for dilution of the
catalyst.
25 In one embodiment, the catalyst is mixed with 5-30 % inert material,
preferably 10-25 %, only
in the inlet region of the alcohol (about 20 to 100 mm catalyst bed height).
This leads to the
avoidance of catalyst deactivation due to excessive temperatures, since the
amount of alcohol
converted is greatest in this range.
30 The return of the liquid gas (mainly C3-C4 paraffin hydrocarbons) to the
reactor in step i) has,
in this reaction mode, the purpose of additionally diluting the educt in order
to avoid excessively
high reaction temperatures and the resulting rapid catalyst deactivation.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
31
As already described above, in one embodiment, a C6-C7 hydrocarbon fraction
containing C6-
C7 paraffins is additionally separated from the gasoline hydrocarbon fraction
and returned to
the reactor to step I) or a) in V1. In one embodiment, the separation takes
place via a side
outlet of separation device K2.
The C6-C7 paraffin hydrocarbons contained therein can be partially converted
to aromatics
under the reaction conditions described below.
In one embodiment, the degree of conversion of the alcohol to produce a
gasoline with a high
aromatic content is between 95 and 100 %, preferably between 98 and 100 %. The
degree of
conversion of the oxygenate, for example the feed alcohol at the reactor
inlet, is adjusted using
the reaction temperature. An almost complete degree of conversion of the
oxygenate is set by
a high reactor temperature. With a high degree of conversion and low catalyst
load, the
intermediate products react to a large extent up to the aromatic hydrocarbons.
In one embodiment, the loading of the catalyst with the feed alcohol (for
example feed
methanol) in order to produce a high-aromatics gasoline is low: preferably,
the LHSV = 0.2 to
2.0 m3meoH/h m3õt., particularly preferably, the LHSV = 0.5 to 1.5 m3meoH/h
m'cat. (based on the
total volume of catalyst contained in the reactor).
In one embodiment for producing a high-aromatics gasoline, the mole fraction
of the alcohol,
for example methanol, of the feed gas at the reactor inlet is 40 to 90 mol%,
preferably 50 to 90
mol%, wherein the feed gas comprises the feed alcohol and recycled components.
The dilution
of the alcohol with inert gas is correspondingly low, which worsens the heat
transfer from the
reaction region to the heat transfer medium. This leads to higher reaction
temperatures which
promote the formation of aromatics.
In one embodiment, the feed alcohol is not diluted in the reactor, i.e., it is
not mixed with
hydrocarbon gases. This means that no circulating gas is returned to the
reactor; the gas circuit
in the method is omitted. In this embodiment, the gas phase at the separator
outlet serves as
heating gas after the C3-05 hydrocarbons have been separated off.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
32
If the oxygenates (e.g., the feed methanol and the intermediate product DME
that is formed)
are completely converted and the said conditions (low catalyst load, high
reaction temperature)
are set, the reaction product will contain no or only a few olefin
hydrocarbons.
Device:
The invention also relates to a device for the synthesis of a synthetic
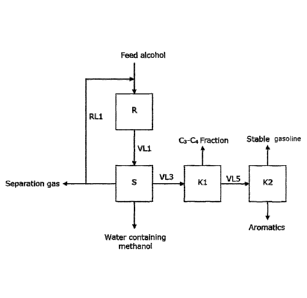
gasoline (Fig. 2a block
flow diagram - Device for the production of gasoline from feed alcohol with
recycling of
circulating gas), comprising the following components:
I. a reactor (R) for the catalytic conversion of alcohols into a product
mixture that contains
a hydrocarbon mixture and water,
II. a three-phase separator (S) for separating the product mixture obtained
in the reactor
(R) into a liquid hydrocarbon phase, an aqueous phase that contains
unconverted
alcohol, and a gas phase,
III. a first separation device (K1) which is suitable for separating a
liquid hydrocarbon
phase that contains hydrocarbons having 3 to 11 carbon atoms into a C3-C4
fraction
and a C5+ fraction,
IV. a second separation device (K2), which is suitable for separating a
hydrocarbon phase
containing hydrocarbons with 5 to 11 carbon atoms into a durene-containing
heavy
aromatics fraction and a stable gasoline fraction,
V. a connecting line (VL1) from the reactor R to the three-phase separator
S for
transporting the reaction product that contains Ci-Cii hydrocarbons, water and
alcohol,
VI. a connecting line (VL3) from the three-phase separator (S) to the first
separation device
(K1), which is suitable for transporting a liquid hydrocarbon phase that
contains
hydrocarbons with 3 to 11 carbon atoms,
VII. a connecting line (VL5) from the first separation device (K1) to the
second separation
device (K2), which is suitable for transporting a C5+ hydrocarbon fraction,
VIII. a return line (RL1) from the three-phase separator (S) to the reactor
(R) for recycling
the gas phase separated off in the three-phase separator (S).
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
33
In one variant (Figs. la, 2b), the device comprises the following components:
a. a reactor (R) for the catalytic conversion of alcohols into a
product mixture that contains
a hydrocarbon mixture and water,
b. a three-phase separator (S) for separating the product mixture obtained in
the reactor
(R) into a liquid hydrocarbon phase, an aqueous phase that contains
unconverted
alcohol, and a gas phase,
c. a first separation device (K1) which is suitable for separating a liquid
hydrocarbon
phase that contains hydrocarbons having 3 to 11 carbon atoms into a C3-C4
fraction
and a C5+ fraction,
d. a second separation device (K2) which is suitable for separating a
hydrocarbon fraction
that contains hydrocarbons having 5 to 11 carbon atoms into a durene-
containing
heavy aromatics fraction and a stable gasoline fraction,
e. a third separation device (K3) which is suitable for separating an aqueous
phase that
contains alcohol into water and alcohol, in which case the alcohols preferably
contain
1 to 3 carbon atoms,
f. a fourth separation device (K4) which is suitable for separating a gas
phase that
contains Ci-05 hydrocarbons into a Ci-C2 hydrocarbon fraction and a C3-05
hydrocarbon fraction,
g. a first connecting line (VL1) from the reactor R to the three-phase
separator S for
transporting the reaction product that contains Ci-Cii hydrocarbons, water,
and
alcohol,
h. a second connecting line (VL2) from the three-phase separator (S) to the
third
separation device (K3), which is suitable for transporting an aqueous phase
that
contains alcohol,
i. a third connecting line (VL3) from the three-phase separator (S) to the
first separation
device (K1), which is suitable for transporting a liquid hydrocarbon phase
that contains
hydrocarbons having 3 to 11 carbon atoms,
j. a fourth connecting line (VL4) from the fourth separation device (K4) to
the first
separation device (K1), which is suitable for transporting C3-05 hydrocarbons,
k. a fifth connecting line (VL5) from the first separation device (K1) to the
second
separation device (K2), which is suitable for transporting a liquid
hydrocarbon phase
that contains hydrocarbons having 5 to 11 carbon atoms,
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
34
I. a sixth connecting line (VL6) from the three-phase separator (S) to
the fourth separation
device (K4), which is suitable for transporting a hydrocarbon gas that
contains
hydrocarbons having 1 to 5 carbon atoms,
m. a first return line (RL1) from the three-phase separator (S) to the reactor
(R) for
recycling part of the gas phase separated in the three-phase separator (S),
n. a second return line (RL2) from the third separation device (K3) to the
reactor (R) for
recycling the unconverted alcohol,
o. a third return line (RL3) from the first separation device (K1) to the
reactor (R) for
recycling the C3-C4 hydrocarbon fraction.
The embodiment is shown by way of example in Fig. 2b (1a), which shows a block
flow diagram
of a device for the production of gasoline from feed alcohol, with recycling
of circulating gas,
the C3 C4 fraction and the unconverted alcohol:
In one embodiment, the components reactor R, three-phase separator S, first
separation
device K1, and second separation device K2 are connected in series.
In one embodiment, at least the separation devices K1 and K2 are distillation
columns. In a
further embodiment, all separation devices K1 to K4 are distillation columns.
The reactor R is connected via the reaction product in the connecting line VL1
to the separator
S, the separator S is connected via the liquid hydrocarbon phase in the
connecting line VL3 to
the first separation device K1, for example, a gasoline stabilisation column,
and the separation
device K1 is connected via the liquid fraction C3-C4 (top product of the
separation device K1)
in a return line RL3 to the reactor R and is directly connected via its bottom
product via a
connecting line VL5 to the second separation device K2, for example a gasoline
separation
column.
In one embodiment, the device contains a return line (RL3) from the first
separation device
(K1) to the reactor (R) for recycling the C3-C4 hydrocarbon fraction.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
In one embodiment, the separator S is directly connected via the aqueous phase
in the
connecting line VL2 to a third separation device K3, for example to a methanol
column, the
top product (alcohol) of which is connected to the reactor R in the return
line RL2.
5 In one embodiment, the three-phase separator S is connected via the Ci-05
hydrocarbon gas
in the connecting line VL6 to a separation device K4, for example to a
separation gas column.
According to the invention, the three-phase S is connected via the liquid
hydrocarbon phase
from the separator S in the connecting line VL3 to the separation device Kl.
The separation device K4 is directly connected to the separation device K1 via
the C3 to C5
hydrocarbon fraction in the connecting line VL4.
In one embodiment (Figs. 2c, 1 b, 1c), the device additionally contains a
crystalliser KR1, which
is suitable for crystallising out durene from a durene-containing fraction or
heavy aromatics
fraction.
In one embodiment, the separation device K2 is connected directly to the
crystalliser KR1 via
a connecting line VL7.
In one embodiment (Figs. 2c, 1 b, 1c), the device additionally contains a
dissolving device V1
for dissolving the durene separated off in the crystalliser KR1 and a fourth
return line RL4 from
the crystalliser KR1 with the dissolving device Vito the reactor R, which is
suitable for
transporting dissolved durene. The crystalliser KR1 is connected to the
reactor R in a return
line RL4 via the durene dissolved in the dissolving device Vi.
In one embodiment (Figs. 2e, le), the second separation device K2, for example
a gasoline
separation column, is equipped with a side outlet for separating off the C6-C7
paraffin
hydrocarbon fraction from the bottom product from the separation device K1 .
In this
embodiment, the second separation device K2 is connected to the reactor R via
the C6-C7
paraffin hydrocarbon fraction in a return line RL6.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
36
In one embodiment (Figs. 2c, 2d, lc, 1d), in particular when a gasoline
product is produced
with a high isoparaffin content and a low aromatics content, the device is
optionally equipped
with an additional external hydrogen source and supply of hydrogen in a line
Ll to the reactor
R. The additional supply of hydrogen serves to saturate the low-hydrogen
olefinic and aromatic
components with hydrogen and to convert them to n-paraffins and isoparaffins
and naphthenes
and to reduce the coke formation on the catalyst surface. Reduced coke
formation increases
the intermediate product regeneration time and the service life of the
catalyst.
In one embodiment (Figs. 2d, 1d) in order to reduce the formation of
aromatics, in particular at
the beginning of a reaction cycle, part of the methylated aromatics fraction
of the separation
device K2, for example a gasoline separation column, can be returned to the
reactor without
the durene having been separated off beforehand. The second separation device
K2 is
connected to the reactor R in a return line RL5 via its bottom product, the
heavy methylated
aromatics fraction (or a part thereof). This method promotes the build-up of a
carbon pool on
the active catalyst surface, whereby its activity is weakened and fewer
aromatics C6 to Cg are
formed. The ageing of the catalyst will be reduced and its service life
increased.
In order to implement the invention, it is also expedient to combine the above-
described
embodiments and features of the claims.
Exemplary embodiments
The invention will be explained in greater detail in the following with
reference to some
exemplary embodiments and accompanying drawings. The embodiments are intended
to
describe the invention without limiting it.
Figures la to le show block flow diagrams of the method according to the
invention for
producing isoparaffin-rich gasoline (Figs. la, lb, lc and 1d) and high-
aromatics gasoline (Figs.
la and le) from alcohol, for example from methanol.
Fig. la: Device for the production of gasoline of variable composition
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
37
Fig. lb: Device with durene recycling for the production of isoparaffin-rich
gasoline without
the addition of hydrogen
Fig. lc: Device with durene recycling for the production of isoparaffin-rich
gasoline with the
addition of hydrogen
Fig. 1d: Device with recycling of aromatics for the production of isoparaffin-
rich gasoline and
with the addition of hydrogen
Fig. le: Device with recycling of the C6-C7 paraffin hydrocarbons for the
production of high-
aromatics gasoline
Furthermore, Figures 2a to 2e show simplified block flow diagrams of the
device.
Figures 2b, 2c and 2d show methods of producing isoparaffin-rich gasoline, and
Figures 2a
and 2e show methods of producing high-aromatics gasoline from alcohol, for
example from
methanol.
Fig. 2a - block flow diagram of the device for the production of gasoline from
feed alcohol
Recycling of circulating gas
Fig. 2b - block flow diagram of the device for the production of gasoline from
feed alcohol
with recycling of circulating gas, the C3-C4 fraction and the unconverted
alcohol
Fig. 2c - block flow diagram of the device for the production of gasoline from
feed alcohol
with recycling of circulating gas, the C3-C4 fraction, the unconverted
alcohol, and
dissolved durene and, optionally, the addition of hydrogen
Fig. 2d - block flow diagram of the device for the production of gasoline from
feed alcohol
with recycling of circulating gas, the C3-C4 fraction, the unconverted
alcohol, and
aromatics and, optionally, the addition of hydrogen
Fig. 2e - block flow diagram of the device for the production of gasoline from
feed alcohol
with recycling of circulating gas, the unconverted alcohol and C6 - C7
paraffins
Exemplary embodiment 1:
Figs. lc, lb/2c show a block flow diagram of the method according to the
invention for the
production of high-octane isoparaffin-rich gasoline from feed alcohol (in this
exemplary
embodiment = methanol) with durene recycling.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
38
The separation devices K1 to K4 are columns in this case.
For the production of a high-octane gasoline, methanol feed (100% Me0H) is
mixed with the
recycled (RL2) unconverted methanol (from the separation device K3), in the
synthesis reactor
R, and is fed into the gas circuit in liquid form. At the same time, the
olefin-containing liquid
gas fraction having a composition of 2-4 mass% of ethylene, 15-25 mass% of
propylene, 30-
50 mass% of butylene, 5-15 mass% of butane, 2-4 mass% of pentene, 2-6 mass% of
i-
pentane, 5-15 mass% of DME, and 2-7 mass% of methanol from the separation
device K1 in
RL3 and the durene dissolved in the methanol (about 3 - 5 mass%, based on the
amount of
methanol entering the reactor) from the crystalliser KR1 and the dissolving
device V1 in RL4
fed into the gas circuit (only at the beginning of each reaction phase).
The said liquids methanol, C3-C4 fraction, and the durene dissolved in the
methanol are
evaporated in countercurrent to the hot reaction product in a heat exchanger.
Then the total
stream - consisting of recirculating gas and the gaseous streams of methanol,
the C3-C4
fraction and the durene - is heated in an furnace up to the required reactor
inlet temperature
of 340 C . The furnace is heated with heating gas, which is a constituent of
the reaction product
and was previously separated off in a distillation column K4 as the top
product.
The gaseous mixture enters the reactor R. With the aid of a compressor which
conveys the
circulating gas to the reactor, the mass flow of the circulating gas can be
adjusted in such a
way that a molar fraction of the methanol in the feed gas in the reactor is 40
to 45 mol%.
In the gasoline synthesis reactor R there is an exothermic reaction of the
conversion of the
methanol and certain components in the feed gas - such as DME and olefins -
into
hydrocarbons, which are mainly in the boiling range of gasoline. Of the
methanol, 95 % is
converted under the set conditions. The heat released is dissipated in the
reactor by an internal
heat exchanger, which contains a suitable heat transfer medium. The heat
transfer medium is
cooled in a separate heat exchanger. Steam at a pressure of 30 bar is
generated from
condensate and used in the separation devices K1, K2 and K3, which serve to
separate the
product, as the heating medium for the bottom heaters. The condensate from the
bottom
heaters of the separators flows back to the cooler of the heat carrier of the
reactor.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
39
The mixture of feed materials is fed into the reactor at a pressure of 7 barg.
The conversion of
the methanol takes place in an isothermal tubular reactor R with a catalyst
bed consisting of a
ZSM-5 catalyst, which was diluted with 10-25 % inert material in the inlet
region for the feed
materials. The other catalyst regions are diluted with inert material to
different degrees of
dilution.
The method steps according to the invention are explained below using
exemplary
embodiments.
a. The 340 C hot reaction product from the gasoline synthesis reactor R is
cooled in
countercurrent to the 50 C cold feed product in heat exchangers to 75 C ,
then the
reaction product is further cooled in a cooler, which can be a water cooler
and a cold
water cooler or a combination of air cooler and water cooler and cold water
cooler, to
5 C and is fed to the three-phase separator S in the connecting line VL1,
whereby the
water, the unconverted methanol and the condensable hydrocarbons condense out.
b. The separator S separates the cooled reaction product into 3 phases - an
aqueous
phase, a liquid hydrocarbon phase, and a gaseous hydrocarbon phase.
c. The aqueous phase from the separator S is led in the connecting line VL2 to
the third
column K3 and separated into methanol and water. The top product of column K3
is
the methanol which has not been converted in the reactor R and has a residual
water
content which is partly returned as reflux to the top of the column. The
column K3
separates the methanol-water at a top pressure of 1.2 barg, a top temperature
of 85
C, and a bottom temperature of 130 C.
d. The amount of methanol not converted in the reactor R is removed from the
column K3
as the top product (water content: approximately 7 mass%), then flows in the
return
line RL2 to the gas circuit and is mixed with the feed alcohol (100 %
methanol) before
both are added to the gas cycle.
The bottom product of the separation device K3 is the water purified from the
methanol.
The degree of purification depends on the separation performance of the
separation
device K3. The methanol content in the purified water is <5 ppm.
e. Part of the gas phase is returned to the reactor R with the aid of a
compressor in the
circuit in the return line RL1 (first part of the gas phase). The compressor
conveys the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
gas to the reactor R with an outlet pressure in the range of 9 to 11 barg and
compensates for the pressure losses in the gas circuit.
f. A partial flow of the gas phase from the separator (second part of the gas
phase) is
discharged from the gas circuit and conveyed to the fourth separation device,
a
5 separation gas column K4, with the aid of a further compressor in the
connecting line
VL6.
The pressure in column K4 is preferably approximately 15 bar. The separation
gas
column K4 has no bottom heater. The hydrocarbon gas emerging from the top of
the
column (mainly Ci-C2 hydrocarbon and small amounts of C3-C4 hydrocarbon) is
cooled,
10 preferably down to -20 C. A conventional cooling medium from a chiller
is used for
cooling in the top product cooler.
The gaseous product (C1-C2 hydrocarbons) emerging from the top product
separator is
used as the heating gas in the furnace for preheating the feed material of the
reactor
R.
15 The liquid product from the top product separator of the separation
device K4 partly
flows back as reflux to the separation device K4, and the other part is
removed as a
liquid product and mixed with the bottom product obtained from the separation
device
K4.
g. The mixture consists of C3-05 hydrocarbons and is added in the connecting
line VL4 to
20 the separation device K1, a stabilising column. The main feed material
of the stabilising
column K1 is the liquid hydrocarbon phase of the reaction product which flows
in the
connecting line VL3 from the separator S to the column K1. The pressure in the
separator S corresponds to the reactor inlet pressure minus the pressure
losses in the
reactor and in the heat exchangers for cooling the reaction product. It is
approximately
25 2.2 barg.
h. The liquid phase from the separator S is heated to 120 C and fed into the
stabilising
column K1.
The column K1 serves to stabilise the liquid hydrocarbons, which means that
the low-
boiling components C3-C4 and partly C5 are separated off at the top of the
column. The
30 aim of the separation in the column K1 is to set the vapour pressure of
the gasoline
according to the specification.
The column K1 separates the liquid hydrocarbons into a liquid gas fraction
(mainly C3-
C4 and DME) and a C5+ fraction at a pressure of 15-16 barg. The temperature of
the
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
41
bottom heater is approximately 220 C. The C5+ hydrocarbons from the bottom of
the
column are fed via the connecting line VL5 into the separation device K2, a
gasoline
separation column.
i. The top product cooler of the stabilisation column K1 cools the top
product (liquefied
gas fraction - mainly C3-C4 and DME) to approximately 20 C. The liquid top
product
obtained is fed into the gas circuit with the aid of the return line RL3, and
part is
removed as product.
j. The gasoline separation column K2 has a top pressure of 0.2 barg and a top
temperature of 70 C. The temperature of the top heater is approximately 240
C. In
the separation device K2, stable gasoline is obtained as the top product and a
heavy
hydrocarbon fraction with a high durene content (approximately 90 mass%) and
other
alkyl aromatics with carbon numbers of 10 and 11 is obtained as the bottom
product.
Durene (1,2,4,5-tetramethylbenzene) is an alkyl aromatic with a low
solidification
temperature of approximately 79 C and can be separated from the other
aromatic
compounds by crystallisation. For this purpose, the bottom product of the
separation
device K2 is fed in the connecting line VL7 to a crystalliser KR1.
In the crystalliser KR1, the durene is separated from the other components by
cooling
of the bottom product to a temperature of <79 C, preferably 60 C. The solid
durene
is separated in the crystallisation unit KR1 and part of the durene is then
dissolved in
methanol in the dissolving device V1. The methanol-durene mixture in the
return line
RL4 is then fed into the gas circuit as a liquid solution.
The gasoline product has the following composition:
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
42
Table 1: Composition of the gasoline
mass% vol%
n-paraffins 1.5 1.8
Isoparaffins 61.6 64.2
Olefins 7.8 9.0
Naphthenes 3.3 3.2
Aromatics 25.2 21.3
Oxygenates 0.6 0.5
100.0 100.0
The vapour pressure of the gasoline is 45 kPa.
Exemplary embodiment 2 - Production of a high-aromatics gasoline:
Fig. 2a shows a block flow diagram of the method according to the invention
for producing
gasoline with circulating gas. If suitable parameters are set, this method can
be used to
produce a high-aromatics high-octane gasoline from methanol, as the following
example
shows.
The device contains the following main equipment: a reactor R with cooling of
the reaction
zone for the synthesis of hydrocarbons from methanol, a separator S for
separating the cooled
reaction product into liquid hydrocarbons, water with methanol and a gas
phase, a separation
device K1 for separating the liquid gas components from the liquid
hydrocarbons and a
separation device K2 for separating the liquid hydrocarbons from the bottom of
the separation
device K1 into a stable gasoline fraction and a fraction of heavy methylated
aromatics.
In the device, part of the gas phase (main part) is returned from the
separator S in the return
line RL1 to the reactor.
Furthermore, the device contains a compressor for maintaining the gas circuit
to/from the
reactor as well as tanks and heat exchangers, inter alia.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
43
In the exemplary embodiment 2, feed alcohol in the form of 100 mass% of
methanol is fed in.
A zeolite-containing catalyst of the ZSM-5 type is used in the gasoline
synthesis reactor.
The conditions at the entry into the reactor are 375 C and 6 barg. The LHSV
of the methanol
is 1.0 h-1. The mole fraction of the methanol in the feed gas into the reactor
is 55.1 mol%. The
degree of conversion of the methanol in the reactor is 100.0 %.
Table 2 shows the material balance of the entire device.
Table 2: Material balance of the device
Inlet Outlet
mass% mass% mass%
Methanol 100.0 Heating gas 7.5 17.2
Liquid gas 3.5 8.0
Gasoline 29.6 67.6
Heavy gasoline 3.2 7.2
Total 43.8 100.0
Water 56.2
Total 100.0 Total 100.0
The gasoline selectivity (based on the mass of the hydrocarbons produced) is
67.6 %.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
44
Table 3 shows the composition of the gasoline produced.
Table 3: Composition of the gasoline
mass% vol%
n-paraffins 5.5 6.4
Isoparaffins 42.5 48.0
Olefins 2.4 2.7
Naphthenes 8.4 8.1
Aromatics 41.2 34.8
Oxygenates 0 0
100.0 100.0
The octane number of the gasoline is RON = 100.
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
Reference signs or list of abbreviations
R Reactor for the catalytic conversion of alcohols
S Three-phase separator
5 K1 First separation device, e.g., gasoline stabilisation column
K2 Second separation device, e.g., gasoline separation column
K3 Third separation device, e.g., methanol column
K4 Separation gas separation device, separation gas column
KR1 Crystalliser
10 V1 Dissolving device for dissolving durene
VL1 Connection line from the reactor R to the three-phase separator S
for transporting the
reaction product containing C1-C11 hydrocarbons, water and alcohol,
VL2 Connection line from the three-phase separator S to the separation
device K3 for
transporting the aqueous phase containing alcohol
15 VL3 Connection line from the three-phase separator S to the separation
device K1 for
transporting the liquid C3-C11 hydrocarbons
VL4 Connection line from the separation device K4 to the separation device K1
for
transporting the liquid C3-05 hydrocarbons
VL5 Connection line from the separation device K1 to the separation
device K2 for
20 transporting the liquid C5-C11 hydrocarbons
VL6 Connection line from the three-phase separator S to the separation
device K4 for
transporting the hydrocarbon gases
VL7 Connection line from the separation device K2 to the crystalliser
KR1 for the transport
of the heavy aromatic fraction
25 .. RL1 Return line from the three-phase separator S to the reactor R for
recycling the
hydrocarbon gas
RL2 Return line from the separation device K3 to the reactor R for
recycling the
unconverted alcohol
RL3 Return line from the separation device K1 to the reactor R for
recycling the C3-C4
30 hydrocarbons
RL4 Return line from the dissolving device for dissolving durene 1/1 to
the reactor R for
recycling the durene
Date Recue/Date Received 2020-07-29
CA 03090050 2020-07-29
46
RL5 Return line from the separation device K2 to the reactor R for
recycling the heavy
aromatics fraction
RL6 Return line from the separation device K2 to the reactor R for
recycling the C6-C7
paraffin hydrocarbons
Date Recue/Date Received 2020-07-29