Note: Descriptions are shown in the official language in which they were submitted.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
1
PATIENT-SPECIFIC ARTHROPLASTY SYSTEM
COPYRIGHT NOTICE
[0001] A portion of the disclosure of this patent document contains
material, which is
subject to copyright protection. The copyright owner has no objection to the
facsimile
reproduction by anyone of the patent document or the patent disclosure, as it
appears in the
Patent and Trademark Office patent files or records, but otherwise reserves
all copyright rights
whatsoever.
CROSS REFERENCE TO RELATED APPLICATION
[0002] This application claims the priority of U.S. Patent Application
No. 15/880,955,
entitled "DYNAMIC LIGAMENT BALANCING SYSTEM WITH PIN POSITIONING
BLOCK," filed on January 26, 2018, the disclosure of which is hereby
incorporated by reference
in its entirety.
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
[0003] This application generally relates to soft tissue testing systems,
and in particular,
apparatus and methods for measuring tension, pressure and distance in the soft
tissue of a
patient's knee in connection with endoprosthetic surgery.
DESCRIPTION OF THE RELATED ART
[0004] The knee is very stable and can reach up to one and a half short-
term ton load, and
it is a commonly injured joint for athletes. However, knee problems can occur
at almost all ages
even if injury though sports are avoided. In addition to acute damage due to
sports-related
overloads such as ligament tears, meniscus injuries, or knee-disk
dislocations, may also occur.
Risk factors such as overweight, congenital or acquired postural impairment,
also untreated
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
2
injuries of the knee in addition to the natural aging process can contribute
to knee joint damage.
Smaller injuries in the joint may be severe enough to cause medium to long
term joint damage
when they are not treated properly or at all.
[0005] In the case of advanced knee joint wear, knee joint arthrosis, the
insertion of an
implant may be a final step that can be taken by patients as a solution for
the permanent relief of
his pain to restore joint function and improvement of mobility. To restore the
patient to a healthy
and active lifestyle, it is necessary by means of endoprosthetic surgery, to
use an artificial knee
joint prosthesis. Endoprosthesis is a surgical procedure, in which permanent
implants remain in
the body that completely or partially replace the damaged joint. The procedure
is generally
considered safe, but only if it is carried out by experienced specialists. The
prospect of
alleviating pain and more life-joy and quality of life for the patient is
therefore very sensitive to
the surgeon's experience.
[0006] When asked how the quality of life has changed after surgery, 14%
of patients
answered with negative or very negative, and only less than 80% are satisfied
with the result of
the operation or very satisfied. The reason is mainly the lack of
standardization and
standardization of the fabrication of tendon and tendon tension in the
patient. Surgery can cause
pain in the patient by incorrectly adjusted soft tensile stresses due to
loosely or tightly adjusted
tendons, which are equally unpleasant and stressful for the patient. These
patients are often
mistreated for many years and often a final remedy is another surgery, where
the cause of the
pain, the wrong compliance of the soft tissue stress is eliminated. Doctors
are constantly
confronted with such cases in their practices and has therefore dealt
extensively with the idea of
how the endoprosthetics in the area of the knee can be simplified in that the
patient can be
replaced in a standardized surgical method where a long process of suffering
with a new
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
3
operation is spared, long convalescence times are avoided and patients briefly
after surgery can
again live an active and healthy lifestyle with high quality of life and joy
of life.
[0007] The knee joint is the largest joint in the human body and connects
the thigh bones,
knee, and shin bone. As so-called twist and hinge gels, allows diffraction and
bending stretching
the leg, as well as a slight turning in and out in the flexed state. The knee
joint is secured and
stabilized by complex system of ligaments, in cooperation with the function of
tendons, muscles
connective tissue, the articular cartilage and the intervertebral discs, the
menisci. The knee must
be able to withstand heavy loads during its daily use and also guarantee
sufficient mobility. The
contact surfaces of the knee joint bones are several millimeters thick, very
smooth and elastic
cartilage layer. Cartilage cells and matrix tissue function as a shock
absorber and allows for a
painless and undisturbed mobility of the knee joint. The two menisci, which
consist of
connective tissue and elastic cartilage and extending between the femur and
the rail header,
enlarge the joint surface of the knee and thus distributing the pressure or
the weight on the joint
optimally affects that overall knee. The joint is encapsulated by a capsule,
which is used for the
nutrition of the articular cartilage.
[0008] Endoprosthetics has made great strides in the past two years,
therefore a large
number is now available as a replacement for damaged joints. Prosthesis models
are made by
different manufacturers but are generally comprised of three main components ¨
a femoral part
also called a femoral part, a lower leg part of the tibial part, and knee
arthroplasty or patellar
replacement. The femoral part and the lower part are made of a chromium,
cobalt, molybdenum
metal alloy or different metal alloys, and the knee portion consists of the
plastic polyethylene.
The choice of the appropriate type of prosthesis for the patient depends on
the quality ¨ the knee-
deep bones, the stability of the sidebands, and the axial deformity of the
knee joint (X, 0-legs).
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
4
[0009] There are two types of prosthesis which can be used. A prosthesis
for surface
replacement, and a second type, a steered axle endoprosthesis. Surface
replacement can be used
when there is sufficient bone strength and a stable sideband. This type offers
the advantage of
minimal bone loss. The stability of the artificial joint is determined mainly
by the intact and
stable sidebands. The upper part of the thigh has the shape of a shell which
fits the right fit thigh
roller and is placed after it has been form-fitted. The tibial part has the
form of a plate, which is
connected to a stem. This plate is placed on the previously prepared lower leg
plateau. The stem
optimizes the connection between the implant and the lower margin mark. On
this, an inlay
made of an abrasion - resistant plastic is placed, which has an inlay of the
artificial thigh
replacement corresponding to a concave depression as the actual joint surface.
Patellar
replacement is performed by replacing the back surface of the knee disc with a
plastic disc.
[0010] Axle-guided endoprosthesis may be used for soft bones, loose side
bands or shear
axis deformities. The axis-guided prosthesis is implanted. In doing so, more
bones have to be
sacrificed, but the artificial joint offers a very high stability, the reduced
function of the loosened
sidebands compensated. Again, femur and tibia are appropriately prepared so
that the individual
prosthesis parts fit into the seat. The anchoring of the prosthesis parts is
achieved by means of
the long stems, which allow an extremely stable attachment. The stability
itself is achieved by a
hinge joint present in the prosthesis. For each part of the prosthesis,
whether it is a surface
replacement or a guided endoprosthesis, they are available in different sizes,
all of which are
compatible with each other. Due to this modular design of the prostheses, it
is possible to
intraoperatively compensate for the dimensions of the patient's personal knee
joint.
[0011] In order to replace the diseased knee joint with an implant, the
surgeon makes a
curved skin incision that is approximately 20 cm long at the front of the
joint. The joint capsule
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
is then opened. The knee joint is angled about 90 and the anterior cruciate
ligament and the
remains of internal and external ligaments and external meniscus can be
removed. Subsequently,
the thigh, then the lower plateau, and finally the knee arthroplasty surface
is prepared by exactly
predetermined bone sections, such that the prosthesis not only comes to an
optimal seat, but also
leads to a sufficient degree of movement for the patient. After insertion of
the three prosthesis
parts, with or without cement, plastic inlays, the joint is implanted and
flexibility is tested.
During the operation, it is ensured that not only the normal leg axis is
restored, but also that the
leg is fully stretched and over a right angle. This is necessary especially
for everyday
movements such as climbing stairs.
[0012] For performing the surgery and placing the implants correctly,
different tools and
gauges from the individual implant manufacturers are available, which provide
a precise
resection of the bone and an exact positioning of the implants. However, a
measuring instrument
or a precision tool for recording the values of the soft tissue tension (the
ligament balancing) of
the patient before surgery, and for adjusting the soft tissue tension during
the operation, in order
to verify and document the values after completion of the procedure, does not
currently exist.
Existing instruments in the area of endoprosthetics of the knee are only on
the maintenance of
the articular line but not the preservation of the joint line tension in the
soft tissues. The soft
tissue tension is still adjusted by means of a "simple clamping tool" and is
dependent on the
experience of the surgeon. Decisions for the maintenance of the correct
tension condition after
the intervention is at the subjective assessment of the surgeon, which is very
sensitive with
experience in carrying out such interventions, and is not linked to objective
evaluation criteria.
If this soft tissue tension is applied by the surgeon on the basis of a false
judgment (e.g., is too
tight, or too loose), this has a dramatic negative-effect impact on the
patient's well-being.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
6
[0013] Before the operation, the patient was greatly restricted in
movement due to the
severe wear of the cartilage and the missing damping function in the knee.
With surgery, pain in
the joints may have disappeared, but may now experience new pain in the region
of the muscles
and the tendon muscles. Inaccurate implantation results in the new pain in the
sense of an
overloading of the muscles, negatively influencing the success of the
operation. As a result, the
mobility as well as the quality of life of patients can be severely hampered.
The resulting
increased need for therapies and drug administration causes considerable
additional costs in the
health care sector, which are avoidable. The consequences are often lengthy
and expensive after
treatments of the patient, such as, for example, different movement therapies,
pain therapies (as
potent as morphine patches) and are all too often long periods of suffering
for the patient.
[0014] The remedy is, however, only a new intervention on the already
operated knee to
correct the previous error. In summary, the patient experiences a long process
and painful
suffering if the operation is poor. Unsatisfactory operation results also
cause the insurer
enormous extra costs. Accordingly, there is a need for an objective measuring
and control
system for the reproduction of the original muscle and tendon tension during
the implantation of
the knee implant of the patient.
[0015] Stryker Medical is a global group with more than 26,000 employees
with its
product range is mainly focused on the development and distribution of medical
and orthopedic
articles in the field of endoprosthetics, the traumatology and endoscopy.
Stryker Medical
distributes a software product under the name OrthoMap that provides automatic
dimensioning
and positioning of the implants on the basis of the unique anatomy of the
patient. The software
solution is primarily aimed at the mechanical axis of the patient.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
7
[0016] Corin Group PLC, headquartered in England, develops and
manufactures
worldwide, products in the field of endoprosthesis hip, knee, and ankle
joints. Corin draws on
many years of experience in the field of bone-conservation and gentle implant
technology. The
Corin implants are characterized by their optimized longevity, significant
abrasion reduction and
reduction of the contact stress of knee implants compared to "single radius
designs" from other
manufacturers. The design additionally takes anthropometric female and male
features based on
global data to ensure optimized performance in implant seating. An instrument
offered by Corin
in combination with the design of the Implant - Unity TM provides intro-
operative flexibility.
Stems and augmentations, in the case of primarily complicated interventions is
made possible by
means of the instrument. Here, as with Stryker, the main focus is on
preserving the joint line.
[0017] As is also the case with the two competitors above, Zimmer Biomet
has a
comprehensive portfolio of innovative knee products and instruments. Their
instrument portfolio
includes a tool for optimum axial alignment and the alignment of the implants,
however a tool
for measuring the soft tissue tension of the patient before implant placement
and adjustment of
the optimal soft tissue tension before fixation of the implant is completely
absent.
[0018] A system for measuring and restoring the soft tissue tension in
the leg of the
patient is neither present nor thought of. Searches for further large implant
and instrument
manufacturers in the field of endoprosthesis, as well as in the examples given
above companies,
has provided neither a corresponding tool to the objective measurement of the
soft tissue tension,
nor the reproduction of this soft tissue tension after the insertion of the
implant, has been
developed. In addition, manufacturers are striving only to sell their own
products, therefore
mainly instruments and devices that are only offered in connection with their
own implants.
These instruments are usually not combined or can be used with products from
other companies.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
8
[0019] In summary, therefore, it can be stated that there is a need for a
medical tool in the
field of endoprosthesis for an objective adjustment of the dynamic soft tissue
tension in the leg of
patients during the course of the implantation of an artificial knee joint,
which is not related to
any specific implant ¨ it should be platform independent.
SUMMARY OF THE INVENTION
[0020] The present invention provides a patient-specific arthroplasty
system comprising
a database comprising preoperative data, ligament balancing tool data, and
postoperative data
associated with a plurality of patients, a preoperative evaluation module that
receives
preoperative data for a given patient, an analysis engine that analyzes the
database, receives the
preoperative data, and generates a surgical recommendation based on the
preoperative data of the
given patient and the analysis of the database, and a pin positioning block
module that receives
the surgical recommendation and determines a pin positioning block based on
the surgical
recommendation.
[0021] The preoperative data may include scans, testing, physiological
analysis, and
surveys. The preoperative data may also include anatomical situations,
kinematic situations,
requests, and demands. In one embodiment, the preoperative data includes long
leg X-rays of
anterior-posterior views, side views, and a patella sunrise view. The
preoperative data may also
include a computed tomography scan for bony landmarks and three-dimensional
impression.
The preoperative data may also include magnetic resonance imaging to obtain
cartilage thickness
and cartilage wear. The preoperative data may also include electromyography
testing of
muscles. The preoperative data may also include gait analysis.
[0022] In one embodiment, the analysis engine further generates an avatar
from the
preoperative data, the avatar including a three-dimensional movement of a knee
and adjacent
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
9
joints. The analysis engine may further store the preoperative data to the
database. The ligament
balancing tool data may include tension data at various knee angles. The
analysis engine may
further determine success and failure of prior surgical recommendations based
on the
postoperative data. In another embodiment, the analysis engine further
modifies the surgical
recommendation based on the analysis of the database. The surgical
recommendation may
include parameters for the pin positioning block. In yet another embodiment,
the analysis engine
further determines optimal configurations of an implant for the given patient
based on a
comparison to the plurality patients according to sex, age, weight, height,
and physical factors.
The pin positioning block module may also further produce the produce pin
positioning block
using a three-dimensional printer.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] The invention is illustrated in the figures of the accompanying
drawings which are
meant to be exemplary and not limiting, in which like references are intended
to refer to like or
corresponding parts.
[0024] Fig. 1 illustrates a prospective view of a ligament balancing tool
according to an
embodiment of the present invention.
[0025] Fig. 2 illustrates a right side view of the ligament balancing
tool according to an
embodiment of the present invention.
[0026] Fig. 3 illustrates a top view of the ligament balancing tool
according to an
embodiment of the present invention.
[0027] Fig. 4 illustrates a left perspective cross-sectional view of the
ligament balancing
tool according to an embodiment of the present invention.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
[0028] Fig. 5 illustrates a left cross-sectional view of the ligament
balancing tool
according to an embodiment of the present invention.
[0029] Fig. 6 and Fig. 7 illustrate schematic top view diagrams of the
ligament balancing
tool according to an embodiment of the present invention.
[0030] Figs. 8A and 8B illustrates an exemplary user interface for
displaying the
initiating of the ligament balancing tool according to an embodiment of the
present invention.
[0031] Figs. 9A, 9B, 10A, and 10B illustrate an exemplary user interface
for recording a
tension profile according to an embodiment of the present invention.
[0032] Fig. 11 illustrates a prospective view of a ligament balancing
tool according to an
embodiment of the present invention.
[0033] Fig. 12 illustrates a rear side view of the ligament balancing
tool according to an
embodiment of the present invention.
[0034] Fig. 13 illustrates a right-side cross-sectional view of the
ligament balancing tool
according to an embodiment of the present invention.
[0035] Fig. 14 illustrates a bottom view of the ligament balancing tool
according to an
embodiment of the present invention.
[0036] Figs. 15A and 15B illustrate a surgical pin positioning block
according to an
embodiment of the present invention.
[0037] Figs. 16A and 16B illustrate a surgical pin positioning block
according to another
embodiment of the present invention.
[0038] Fig. 17 illustrates a data flow diagram of a patient-specific
arthroplasty system
according to an embodiment of the present invention.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
11
DETAILED DESCRIPTION OF THE INVENTION
[0039] Subject matter will now be described more fully hereinafter with
reference to the
accompanying drawings, which form a part hereof, and which show, by way of
illustration,
exemplary embodiments in which the invention may be practiced. Subject matter
may, however,
be embodied in a variety of different forms and, therefore, covered or claimed
subject matter is
intended to be construed as not being limited to any example embodiments set
forth herein;
example embodiments are provided merely to be illustrative. It is to be
understood that other
embodiments may be utilized and structural changes may be made without
departing from the
scope of the present invention. Likewise, a reasonably broad scope for claimed
or covered
subject matter is intended. Among other things, for example, subject matter
may be embodied as
methods, devices, components, or systems. Accordingly, embodiments may, for
example, take
the form of hardware, software, firmware or any combination thereof (other
than software per
se). The following detailed description is, therefore, not intended to be
taken in a limiting sense.
[0040] Throughout the specification and claims, terms may have nuanced
meanings
suggested or implied in context beyond an explicitly stated meaning. Likewise,
the phrase "in
one embodiment" as used herein does not necessarily refer to the same
embodiment and the
phrase "in another embodiment" as used herein does not necessarily refer to a
different
embodiment. It is intended, for example, that claimed subject matter include
combinations of
exemplary embodiments in whole or in part.
[0041] The disclosed systems and methods provide for a platform to help
surgeons
reproduce the natural kinematics of the patient in endoprosthetic surgery.
Accordingly,
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
12
objectives associated with one or more of the embodiments described in the
present disclosure
may include:
= Measurement of the soft tissue or tendon tension of the patient before
installation of the implant,
= Measurement of the joint and rotational angle in connection with the
pressure
load on the device,
= Objectification and standardization of the operating method in the field
of
fabrication of the band and soft tissue tension of the patient during the
endoprosthetic surgery,
= Preparation of the muscle condition of the patient ¨ the original
condition before
the surgical procedure,
= Manufacturer-independent measuring instrument, suitable for all knee
prostheses from different manufacturers,
= Automatic logging of the measured values, the tension state and before
and after
implantation/surgery,
= Patient-related unalterable protocol to traceability and legal certainty
for critical
surgical results for the doctor as well as the patient, and
= Easy-to-use measuring instrument.
[0042] Through the development of a measuring instrument(s) disclosed
herein, for
recording the soft tissue tension of the patient in the region of the knee
before the implantation of
the prosthesis and use of this measuring instrument for the reproduction of
the natural kinematics
of the patient during and/or after insertion of the implant, a revolutionary
step is provided
towards an objectification and standardization of a surgical method during
endoprosthetic
procedures with the aid of the measuring instrument. Exact measurements of the
tension profile
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
13
of the soft parts and ligaments of the affected person for the purposes of
comparison during the
operation, as well as for the subsequent complete documentation of the
operation results are
made possible with the measuring instrument(s) disclosed herein.
[0043] The measurement of the tendon tension itself can take place in
contrast to only
when preparation of the tibia was previously available. In doing so, the
medial and lateral forces
during the rolling of the knee (0 to 90 ) can be captured exactly by the
sensors of the disclosed
measuring instrument to record a reference profile produced by tension signals
from the
measuring instrument. Through the dynamic measurement over the entire movement
space, the
existing kinematics are measured, the correct incision planes are obtained for
the femur in
flexion and stretching. This is an approximation, or ideal achievement of the
original state after
the operation ensured.
[0044] After successful slices on the femur, measurement of the tendon
tension is
repeated with a femur trial and compared with previous measurements. After
insertion of the
knee implants, before the fixation, present practice in the case of the
stretching and bending of
the joint is manually and optically checked by the surgeon to determine
appropriate function, the
result for the patient therefore is based on a subjective impression. However,
according to
embodiments of the present invention, previously recorded values by the
measuring instrument
can be dynamically compared over the entire radius of movement of the knee.
Data from the
measuring instrument may be shown on a monitor to show a doctor how far the
profile of the
tension progress after the final placement of the prosthesis in the knee
deviates from the previous
recorded reference profile, during the unrolling of the stretched into the
angled state and back
again.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
14
[0045] In doing so, a user interface may be displayed on the monitor
including a color
bar display indicating "in range" and "out of range" values. The color
provides a visual
depiction of how far the profile deviates from the previously recorded
reference profile to adjust
the implant accordingly, that the tension profile measured from the device are
within defined
boundaries, and ideally, to show a uniform matching to the previously recorded
reference profile
after the setting of the implant. The comparison of the tension profile may be
provided by an
application that provides a traffic light comparison of the band tension by a
color. For example,
green can mean correct band tension, yellow that the tension measured by the
device is within a
certain tolerance range and red that the deviation is too strong, i.e., the
tension has been set too
tightly or too loosely. The user interface may be displayed on a display
device such as a
computer, laptop, tablet, or mobile device for the medical field.
[0046] For the patient, the disclosed procedure can be used to ensure
that the ligament
balancing in the patient's leg after surgery closely matches that of the
previously recorded
reference values. An improvement in rehabilitation of the patient from the
operation may result
by the use of the measuring instrument to produce the natural kinematics of
the patient, in the
ideal case the same muscle tension state of the patient prior to the surgical
procedure is restored.
[0047] Automatic logging
[0048] A further feature of the disclosed measuring instrument lies in
the automatic
recording of the recorded reference values of the patient before the start of
the operation, as well
as the measured values after successful application of the implant in the
patient's joint.
Measurement data can be recorded into a program from the measuring device and
unambiguously assigned to a corresponding patient where the recorded patient-
specific
measurement and reference values may be stored in an immutable file. The
tremendous
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
advantage of this is that in case of post-surgery issues, a more accurate
diagnosis can be made
using the patient-specific data to determine a source of complications. In the
long term, due to
the evaluation of this data by the orthopedic surgeon can result in improved
reliability of
operations and increase patient satisfaction. In addition to these advantages,
the recording of the
data may also provide legal certainty after operations, both for the surgeon,
as well as for the
patient, due to accurate logging and unchangeable documentation of the values
and their
traceability through the operation.
[0049] Manufacturer-independent measuring instrument
[0050] On the market, there are among some big players like the
aforementioned
companies Stryker, Corin, Mathis, and Zimmer Biomet that are in the field of
the manufacture of
endoprosthesis. The presently disclosed measuring instrument is distinguished
by the fact that it
is developed in such a way that it may be used independently of the
endoprosthetic product used.
A limitation of the applicability and thus the dependency of one or a few
manufacturers of
implants for the replacement of knee joints are thus eliminated and the market
for the application
is broadly diversified.
[0051] The platform independence of the disclosed device may be achieved
via an
incision adapter for the femur to the respective prosthesis.
[0052] Advantages of one or more of embodiments of the disclosed system
and method
include:
= Objectification and standardization of an operating method,
= Manufacturer independence,
= Measurement of the soft tissue tension and the tension profile of the
medial and
lateral acting forces over the entire range of motion of the joint,
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
16
= Easy operation and visually clear display to show deviations from an
ideal state,
= Production of the patient's natural kinematics,
= Reduction of the proportion of dissatisfied patients with problems after
the
surgery,
= Faster rehabilitation,
= Reduced follow-up,
= Significantly reduced follow-up costs,
= Recording and logging of the measurement results documentation,
= Storage in unchangeable file format - traceability for patients and
doctors, and
= Connection to common hospital software interfaces.
[0053] Technical Aspects
[0054] Ligament Balancing
[0055] An emphasis for the disclosed system and method is to ensure the
correct
measurement of the original tension of the tendons and weighing, as well as
the corresponding
transmission of these measured values in the connection with an artificial
knee joint in the
patient's leg. An existing problem is that measurements for preparing tendon
tension on the
opened knee of the patient should not be taken on the "intact" knee since
values obtained with an
intact knee are not transferable. The corresponding solution disclosed herein
includes recording
the original tension condition of the soft parts and tendons in the open state
of the knee and
transferring the original condition accordingly.
[0056] Validation
[0057] Closely linked to the previously identified problem of correct
measurement, is the
topic of the validation of the tension history recordings of the measurement
of the soft tissue
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
17
tension. Given the novelty of the disclosed method of operation and production
of the correct
tendon tension using a completely new approach, neither reference values, nor
preliminary
studies dealing with of the problem exist. Therefore, performance of detailed
examinations and
measurements are disclosed herein.
[0058] Material selection and sensor technology
[0059] In the area of the right material selection for the manufacture of
the disclosed
measurement instrument of tendon tension at the opened knee, the following
factors are
considered, where according to one embodiment, the measurement instrument
(hereinafter
referred to as a "ligament balancing tool") may be a "medical disposable
product." A sterile
packaged ligament balancing tool suitable for use in the open knees may be
produced from cost-
effective materials. Alternatively, it is possible for the tool to be made
from completely inert and
biologically "safe" materials such as titanium, or gold, for disposable use,
albeit cost-intensive.
Therefore, in the selection process, a corresponding use is a decisive factor
in determining
suitable materials for the ligament balancing tool. The following points for
material selection are
from a cost-effective point of view.
[0060] = Biocompatibility of the material
[0061] In terms of biocompatibility, it is important that materials or
assemblies used for
the ligament balancing tool do not have any negatives effect on the patient.
In particular, for
sensors that are embedded within the ligament balancing tool, there are two
aspects that are
disclosed in detail herein ¨ the equipment of the sensors in the ligament
balancing tool, e.g.,
methods and materials for embedding the sensors to the ligament balancing
tool, and the sensors
themselves. Capable sensors that are biocompatible and certified for the
presently disclosed
usages are described herewith along with sensor development.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
18
[0062] = Resistance of the material - biological corrosion
[0063] In addition to being safe for the patient when using the material,
resistance of the
material itself by means of the body or body tissue fluids is of crucial
importance. These
thematic issues arise especially with regard to the consideration of using
sensors in the opened
knee, as well as the data transmission required by the sensors to a recording
device. The highly
corrosive effect of tissue fluids on the formation of biotically formed acids
or salts, can have a
negative effect on the connections and contacts of sensors. Due to the
aggressiveness of these
fluids, rapid progression of corrosion in the sensors and materials used in
the ligament balancing
tool is to be expected.
[0064] = Sensor technology - ensuring measurement cycles
[0065] In addition to the previously mentioned biocompatibility and the
stability of the
materials against the aggressive environment in the area of the open knee,
that is, the medical
fitness of the materials used, the sensitivity, signal recording, signal
interpretation, and sensor
position(s) in the ligament balancing tool is of crucial importance. It is
important that the
necessary measuring cycles for the recording as well as the setting of the
soft tissue tension can
be processed without appreciable changes in the characteristics of the
recording and transmission
of the data.
[0066] = Sterilization
[0067] The field of sterilization of tools, are described herein. The
preferred way of
sterilization of disposable medical devices in industrial sterilization is
carried out with ionizing
radiation. X-ray radiation, gamma radiation or electron bombardment are
predominantly used.
Typical radiation doses that are to be used are in the range of 25 kGy, mostly
from gamma
radiation from cobalt-60 sources.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
19
[0068] Usability for all standard implants
[0069] A feature of the disclosed ligament balancing tool includes a
broadest possible
applicability for nearly all common implants from different manufacturers. The
success of the
disclosed system is ensured by applicability to at least the implants produced
by the largest
manufacturers on the market.
[0070] Data recording, data storage
[0071] Issues addressed in this section are related to ensuring personal
data, the
immutability of recorded data, as well as the reading of the data via the
interface.
[0072] = Interface issues
[0073] The data recorded by the ligament balancing tool can be archived
accordingly via
an interface from the tool to a data acquisition system connected to a
hospital. In the field of
medicine, there are internationally standardized interfaces for data
transfers, but not all hospitals
support these interface. Additionally, it is to be considered whether there
are country-specific or
regional regulations are to be followed.
[0074] = Personal data recording
[0075] The disclosed system records sensitive personal data ¨ that is,
health-related
information, it is ensured that such information is not viewed by unauthorized
persons, and a
misuse of the personal information is prevented.
[0076] = Immutability of data
[0077] Another feature is the immutability of the data recorded by the
disclosed system.
Data recorded during the operation, neither during data recording, nor
transmission of the data to
a system of a hospital, in the course of archiving the data in the system, can
it be changed. In
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
this manner can a complete documentation ensure, in case of a complaint from
the patient is
received, an objective source of information for both the patient and the
surgeon.
[0078] Technical Solutions
[0079] Ligament Balancing and Validation
[0080] A measurement of soft tissue tensions in an open knee, as close as
possible to the
original conditions, is described herewith. To get validated measurement
results, it is important
that the tibia is prepared accordingly. A ligament balancing tool may be
fitted to the tibial
plateau at an angle of 90 to the axis of the tibia. After mounting and fixing
the tool on the tibial
plateau, the soft tissue tension in the leg of the patient stretched from 0
to 90 can be
measured. The measured values characterized the normal state of the knee and
the values
themselves can be validated. If needed, the measurement range can be extended
to a full range
of motion.
[0081] The ligament balancing tool may comprise a knee endo-prosthetic
inlay that is
integrated with sensors to record forces and/or tensions. The inlay may be
produced according to
varying sizes of the human knee. The inlay may be equipped with two or more
pressure sensors
to record a tension profile of both medially and laterally acting forces
during unrolling of the
knee in the bended 90 angled position. A reference tension profile can be
recorded
(measurements may be repeated to eliminate errors) and used for comparison to
a final
arrangement of the prosthesis in the knee. In this case, the doctor is
informed of the range of
motion of the leg of the patient, by recording a second tension profile after
successful
implantation of the artificial joint to determine deviations from the
reference profile. A visual
display may be presented via a color bar display where a quick overview (e.g.,
in range, out of
range) can be represented by a color coding.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
21
[0082] According to one embodiment, the inlay may include two or more
platforms
where each platform is supported by a scissor arm structure and an underlying
coil spring
overlying a sliding surface. One leg of the scissor arm structure may be in a
fixed position while
a second leg of the scissor arm structure is capable of moving along the
sliding surface upon
downward pressure or tension on the platform. Force exerted on the platform
may be transferred
to the underlying coil spring where a pressure sensor may be positioned below
the coil spring to
measure a degree of the tension. In a default or initial position, the
platforms may be supported
in an up-right position by the springs. After inserting the inlay in the
opened knee, the sliding
surfaces are able to be depressed a given displacement through a 90 range of
motion of the leg,
which may record the tension produced on the inlay at certain angles
throughout the range of
motion. Measurement may be activated by pressing a start button beginning at
the 0 start
position of the knee and pressing an end button to confirm reaching an end
position (90 ) of the
knee. Data or signals from the sensors in the inlay may be transmitted by
either a wired or
wireless communication channel (e.g., Bluetooth) to a computing device over a
network.
[0083] By evaluating the results in relation to a previously recorded
reference tension
profile, complex calibration steps in the approval (e.g., for tension forces)
of setting soft tissue
tension and implant can be avoided. A relatively linear tension profile for
the knee in the range
of 0 to 90 may be expected and any extreme variation may be noted. In one
embodiment, the
ligament balancing tool may include sensors for measuring the joint cavity as
well as an inertial
measurement unit to measure the tilt of the tibia in space.
[0084] In another embodiment, a femoral sensor may be used in conjunction
with the
ligament balancing tool to calculate flexion or extension of the knee. The
femoral sensor may
include electronics, batteries, a housing and a fixation device such as a
strap. The femoral sensor
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
22
may be worn and positioned on the thigh or blood arrest of a same leg with the
ligament
balancing tool. The femoral sensor may further include an inertial measuring
unit that measures
the tilt of the device that corresponds to the tilt of the femur in space. The
measured tilt angle of
the femoral sensor may be compared in relation to the ligament balancing tool
and used to
calculate flexion of the leg. The femoral sensor may be connected with a
tablet or computing
device via wired or wireless communication protocols. The tablet/computing
device may
connect wirelessly with both the ligament balancing tool and the femoral
sensor. According to
one embodiment, a measurement program may be programmed to automatically
trigger
measurements with the ligament balancing tool at certain flexion angles (e.g.,
between the range
of 0 to 90 ) measured by an inertial measurement unit or the femoral
sensor.
[0085] The disclosed ligament balancing tool and femoral sensor may also
be applied in
other joint applications such as, elbows, hips, and shoulders.
[0086] Material and sensor selection
[0087] A special purpose plastic may be envisioned for producing the
inlay (including
the platforms, scissor arm structure, and sliding surface) as a packaged
sterile single-use part.
For example, PMFP (polymer medical flexible plastic), similar to
polytetrafluoroethylene
(PTFE), has a smooth surface such that foreign substances (e.g., wound
secretions) do not adhere
to it, and may be used for the inlay. PMFP has high elasticity and temperature
resistance and is a
biocompatible material. Alternatively, one or more components of the inlay may
be high quality
surgical steel (316L stainless steel), in addition to the coil springs. The
sensors may be
comprised of biocompatible materials that is capable of temporarily remaining
in the body for a
short duration of time e.g., less than 60 minutes. According to one
embodiment, the sensors may
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
23
be encapsulated within the inlays such that the sensors would not be in direct
contact with patient
tissue.
[0088] Sterilization is of the utmost importance to comply with the
safety of the patient.
Sterilization of the instrument may be ensured by selecting appropriate
materials for the
manufacture of the ligament balancing tool. A preferred method of gamma
radiation
sterilization, the doses of radiation in the impact areas are tolerable for
most materials, however,
having a higher radiation resistance on the outer sides of the product as
compared to the product
core is advantageous because the doses are significantly higher. Especially
with plastics,
damage can be difficult to avoid because the polymer structure is changed by
irradiation. The
consequences can reduce tensile, breakage, or impact strength of the
components. The
sterilization process may be taken into account in the consideration of the
design and selection of
material for the inlays comprised in the ligament balancing tool.
[0089] Platform independence plays a significant role in the design
process of the
ligament balancing tool. To achieve platform independence, product-specific
adapters may be
provided to operate the ligament balancing tool with products from various
global manufacturers
of knee implants.
[0090] Data Recording and Storage
[0091] Data may be recorded to a computer program that generates a
tension profile
according to a start and end point. Such data may be processed in a program
that supports
international data interfaces in the medical or hospital sector. Additionally,
paper prints of the
data collected by the computer program are possible. The data may be tamper-
proof and
unchangeable according to international standards, thus providing clear
traceability. The
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
24
computer program may be further coupled to an access system to provide secure
access to users
by entering a user name and password
[0092] Fig. 1 presents a ligament balancing tool according to an
embodiment of the
present invention. The ligament balancing tool 100 may comprise an inlay 102
including
integrated sensors below each of left platform 104 and right platform 106 to
measure medial and
lateral forces of the knee when placed between the tibia and femur bone, and a
pin positioning
block 130. Inlay 102 may be produced in different sizes in accordance to
variation in sizes of the
human knee. The pin positioning block 130 may be a detachable component
capable of
providing a guide for pin positioning/drilling to assist a surgeon to perform
steps required to
prepare the femur and tibia for receiving the implant. According to one
embodiment, the
ligament balancing tool 100 may be produced as a sterile packaged single-use
part and combined
into a product set (e.g., including a vernier caliper). Additionally, the
ligament balancing tool
100 may be either platform-dependent (e.g., designed for specific products
from different
manufacturers) or platform independent (universally compatible, e.g., via an
adapter).
[0093] In one embodiment, the medial and lateral forces may be captured
from the
sensors to create a representative tension profile of the knee during 00 to 90
flexion of the knee.
The sensors may create voltage or signals representative of the amount of
force or tension
produced on the left platform 104 and right platform 106, individually.
Voltage or signals from
the sensors may be coupled to connecting cable 120 for transmission of the
voltage or signals to
a computing device via an interface. The computing device may include software
for signal
acquisition and processing of the voltage or signals from the sensors to
provide a visualization of
the measured data, which is described in further detail with respect to the
description of Figs. 9A,
9B, 10A, and 10B. The computing device may comprise computing devices (e.g.,
desktop
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
computers, terminals, laptops, personal digital assistants (PDA), cell phones,
smartphones, tablet
computers, or any computing device having a central processing unit and memory
unit capable
of connecting to a network). The computing device may also comprise a
graphical user interface
(GUI) or a browser application provided on a display (e.g., monitor screen,
LCD or LED display,
projector, etc.).
[0094] Each of left platform 104 and right platform 106 may be supported
by a scissor
arm structure 112 and 108, respectively, and an underlying coil spring
overlying (and attached to
inlay 102 at) respective recessed sliding surfaces 116 and 118. A given
platform may be
supported in an up-right position by an elastic material such as coil spring.
For example, Fig. 2
presents a right side view of the ligament balancing tool according to an
embodiment of the
present invention. Right platform 106 may be fixed above scissor arm structure
108 and coil
spring 110. Pressure may be individually applied to each platform causing the
platforms to move
from an extended (or fully upright) position to a depressed position.
[0095] Fig. 3 presents a top view of the ligament balancing tool
according to an
embodiment of the present invention. The ligament balancing tool 100 may be
placed between
the femur and the tibia such that the left and right platforms 104 and 106 are
beneath the femur
(or femoral component) and inlay 102 is above the tibia (or tibial baseplate).
Left platform 104
and right platform 106 further includes indentation 130 and indentation 132,
respectively.
Indentation 130 and 132 may be provided to accommodate the femur bone. The
indentations are
generally mirror images of each other, as shown. Accordingly, the femur bone
is able to fit in
indentation 130 and 132 without slippage when pressed against left and right
platforms 104 and
106.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
26
[0096] Referring back to Fig. 1, each scissor arm structure may include a
fixed leg (122
and 126) that is configured in a fixed position and a sliding leg (124 and
128) that is capable of
moving vertically along the recessed sliding surfaces 116 and 118 upon
downward pressure or
tension placed on the left and right platforms (104 and 106). Fig. 4 presents
an exposed view of
the coil spring 114 and recessed sliding surface 116 including fixed leg 122
and sliding leg 124.
Pressure applied on left platform 104 and right platform 106 may be
transferred to pressure
sensors beneath coil springs 110 and 114. An additional exposed view and
exemplary
dimensions of inlay 102 are presented in Fig. 5.
[0097] Fig. 6 and Fig. 7 present schematic top view diagrams of the
ligament balancing
tool. Pressure sensors may be positioned below each coil spring to measure a
degree of tension.
Sensor 202 may be beneath coil spring 114 and sensor 204 may be beneath coil
spring 110. In
alternative embodiments, the sensors may be embedded within the coil springs,
scissor arm
structures, and/or the platforms. Exemplary width of device from sensor 202 to
sensor 204 as
illustrated is 46.37 mm. Sensors 202 and 204 may have of a height of
approximately one mm
and a diameter of about eight mm.
[0098] The sensors 202 and 204 may be comprised of pressure or force
measurement
devices such as piezo or force-sensitive resistor (FSR) sensors that are
commercially available.
However, capacitive sensors and other strain gauges may also be used
accordingly to their
durability and reliability. The sensors 202 and 204 may be connected to an
electrical or signal
bus comprised in connecting cable 120. Connecting cable 120 may be adapted
from inlay 102 to
a connector (e.g., via a wired connection) for communication with external
electronics that are
able to receive and convert the voltages or signals from sensors 202 and 204
into data for display
and recording.
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
27
[0099] Fig. 8A presents an exemplary user interface for displaying data
from the
ligament balancing tool according to an embodiment of the present invention.
The illustrated
screen includes patient data 320, implant type 322, left/right selector 324,
and joint angulation
326. Patient data 320 may include the patient's first name, last name, date of
birth, patient ID,
admission number, and insurance number. Various modes of operation may be
selected by
toggling implant type 322. For example, implant type 322 may select between
knee, hip, or
shoulder. A left/right selector 324 may be selected to indicate whether a
current measurement is
of a patient's anatomical left or right body part (e.g., left knee or right
knee). Joint angulation
326 may be used to specify a patient's joint angulation such as, varus,
neutral, or valgus.
[00100] Fig. 8B presents an exemplary user interface for displaying data
from the
ligament balancing tool according to another embodiment of the present
invention. An initiation
screen may include fields for patient name 302, patient ID 304, patient number
306 (e.g., an
independent patient number that can be provided to individual identification
systems), knee
identifier 308, and doctor identifier 310. A size of an inlay may also be
selected using the inlay
identifier 312. Upon population and verification of the data, a user may
proceed to
measurements by selecting the accept data button 314.
[00101] Fig. 9A presents an exemplary user interface for recording
reference data for
distance and pressure according to an embodiment of the present invention. The
illustrated user
interface includes a chart of reference data for distance and pressure
comprising columns for
medial data 420, angle 422, and lateral data 424. Medial data 420 and lateral
data 424 includes
distance (measured in millimeters(mm)) and pressure (measured in Newtons(N))
sub-columns.
The medial data 420, angle 422, and lateral data 424 may correspond to
distance and pressure
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
28
measurements detected by a ligament balancing tool as depicted by the ligament
balancing tool
diagram 434.
[00102] Joint diagram 426 displays a representative diagram of a measured
joint. Current
flexion indicator 428 may represent a detected flexion position of the joint
corresponding to
current flexion angle 232 which displays the detected flexion angle of the
joint. Target flexion
indicator 430 may represent an indicated position to move the joint for a next
measurement, e.g.,
30 . Trigger toggle 436 may allow the operator to select between automatic or
manual
measurement triggers. According to one embodiment, an automatic measurement
trigger may
automatically record measurements at certain flexion angles when moving the
joint through the
flexion angle. By contrast, a manual measurement trigger may allow an operator
to manually
record measurements.
[00103] Fig. 9B presents an exemplary user interface for recording a
reference tension
profile according to an embodiment of the present invention. The disclosed
ligament balancing
tool may transmit measurement signals to software for calculating pressure,
angles and distances.
A reference tension profile of a "pre-prosthetic" knee may be created and
recorded from the
signals for comparison to a second tension profile, with the final assembly of
a prosthesis in the
knee. The software may be activated via a start button to start capturing
tension data from a
reference point when the knee is at a 0 position. When the knee has reached a
90 position, the
measuring process may be terminated via an end button.
[00104] Signals from the ligament balancing tool may be populated to "pre"
medial height
402 and "pre" lateral height 404 measurements on the left region of the user
interface. The
heights may correspond to measured displacements of the left and right
platforms (e.g., medial
and lateral on the left knee) when placed between the femur and tibia during
flexion of the knee
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
29
at reference angles 410. The heights may provide information about necessary
thickness of bone
cuts parallel to the tibial baseplate and in flexion about rotation. Reference
angles 410 include
angles of 0 , 30 , and 60 that may be influenced by distal femoral cuts, and
90 influenced by
dorsal cuts (condyles rotation).
[00105] Measurements may be repeated on a "post-prosthetic" knee to create
the second
tension profile for comparison with the reference tension profile. As
illustrated in Fig. 10A,
measurement signals from the ligament balancing tool may be populated to
"post" medial height
406 and "post" lateral height 408 for reference angles 410 on the right region
of the user
interface. The "pre" and "post" measurements at the reference angles 410 may
be compared to
determine a degree of difference between the tension profiles. By comparing
the "pre" and
"post" measurements, a surgeon may be able to determine or adjust medial and
lateral heights for
the knee to achieve appropriate stability and tension state. According to the
illustrated example,
each box in reference angles 410 may be shaded in a color that corresponds to
an indication of
"in-range" and "out-of-range" knee angles after "post" or operation
incisions." A knee is
preferably operated such that the length between the medial and lateral
distance is within a
certain range to avoid instability and pain. For example, green may be shown
to indicate that
there is not more than a three-millimeter difference between the medial and
lateral heights.
Yellow may indicate that there is more than a three-millimeter difference
between the medial and
lateral heights which may be borderline acceptable depending on general
condition and
anatomical condition, and recuts may be necessary according to the measured
results. Red may
indicate that there is more than five mm difference between the medial and
lateral heights which
may be out of a required range, and recuts are necessary. Accordingly,
reference angles 410 may
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
indicate certain knee angles that require recuts and an amount to recut based
on the color
indications.
[00106] Fig. 10B presents an exemplary interface for comparing reference
data to post-
surgery data according to an embodiment of the present invention. The measured
reference data
may be compared with post-prosthetic measurements from the ligament balancing
tool. In the
illustrated embodiment, the distance and pressure measurements along the
various flexion angles
are verified to be identical in both pre- and post- surgery.
[00107] Fig. 11-14 present views of a ligament balancing tool according to
an alternative
embodiment of the present invention. Fig. 11 shows a ligament balancing tool
1100 comprising
an inlay 1102 that includes a left platform 1104 and a right platform 1106.
Referring to Fig. 12,
left platform 1104 is attached to scissor arm structure 1108 which is
comprised of a fixed leg
1122 and a sliding leg 1124. Right platform 1106 is attached to scissor arm
structure 1112 which
is comprised of a fixed leg 1126 and a sliding leg 1128. Referring back to
Fig. 11, fixed leg
1122 and sliding leg 1124 are attached to inlay 1102 at recessed sliding
surface 1116, and
similarly, fixed leg 1126 and sliding leg 1128 are attached to inlay 1102 at
recessed sliding
surface 1118. The sliding legs (1124 and 1128) are capable of moving
vertically along the
recessed sliding surfaces 1116 and 1118 upon downward pressure or tension
placed on the left
platform 1104 and right platform 1106.
[00108] Fig. 12 further illustrates left platform 1104 and right platform
1106 positioned
above coil spring device 1110 and coil spring device 1114. Force sensing
sensors may be placed
underneath coil spring device 1110 and coil spring device 1114. For example,
Fig. 13 presents a
right-side cross-sectional view of ligament balancing tool 1100 where coil
spring device 1110 is
positioned above sensor 1130 such that coil spring device 1110 makes contact
(direct or indirect)
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
31
with sensor 1130 when right platform 1106 is depressed. Inlay 1102 further
includes a cable
1120 that is electronically connected to the sensors beneath coil spring
device 1110 and coil
spring device 1114 for transmission of signals or data from the sensors
(associated with a
tension, pressure, or displacement applied to left platform 1104 and right
platform 1106) to a
computing device. Fig. 14 presents a bottom view of the ligament balancing
tool where inlay
102 further includes a sensor cover 1132. Sensor cover 1132 may be placed over
circuitry
associated the sensors and cable 1120.
[00109] Fig. 15A presents a front view a pin positioning block according
to an
embodiment of the present invention. The illustrated pin positioning block
1500 comprises a top
portion 1520 that includes pin position dial 1502, dial position notches 1504A
and 1504B, pin
position dial indicator 1506, stabilizing bolt 1508, sliding member 1516 and
sliding member
1518. Pin position dial 1502 includes pin hole guides 1524 and 1526 on
opposite distal ends of
pin position dial 1502. Each of pin hole guides 1524 and 1526 may include an
orifice for drilling
of pin holes. The pin positioning block 1500 may be used to drill pin holes
into the femur bone
through pin hole guides 1524 and 1526. The pin hole guides 1524 and 1526 may
be and 'X'
distance from each other, depending on the size of the femur and/or tibia
bones. Pin positioning
block 1500 further comprises a bottom portion 1522 that includes a ligament
balancing tool
adapter 1514, as illustrated in Fig. 15B. Ligament balancing tool adapter 1514
is configurable
for mating with a ligament balancing tool or prosthetic inlay device to
stabilize and position pin
positioning block 1500 with a femur. The ligament balancing tool adapter 1514
may comprise
one or more prongs or inserts that may be inserted, attached or coupled to the
ligament balancing
tool or prosthetic inlay device. The distance between the center of pin
position dial 1502 and
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
32
ligament balancing tool adapter 1514 may be a distance 'A'. Bottom portion
1522 may have a
thickness of '13'.
[00110] Pin position dial 1502 may be turned either clockwise or counter-
clockwise to
select an angular position for both pin hole guides 1524 and 1526. The angular
positions of pin
hole guides 1524 and 1526 may be inversely related to each other. For example,
pin hole guides
1524 and 1526 remains 180 from each other such that an increase in an angular
position of one
pin hole guide results in a corresponding decrease in an angular position of
the second pin hole
guide. Dial position notches 1504A and 1504B may secure the pin position dial
1502 in a given
position. Pin position dial indicator 1506 may indicate an angular position of
the pin position
dial 1502. The distance between the middle of stabilizing bolt 1508 and the
bottom of bottom
portion 1522 may be varied by a distance 'Y'. Top portion 1520 may be
removably coupled to
bottom portion 1522 by sliding member 1516 and sliding member 1518. Sliding
members 1516
and 1518 can be inserted into their respective receptacles in bottom portion
1522. The top
portion 1520 may be extended from bottom portion 1522 via sliding member 1516
and sliding
member 1518 to adjust the horizontal position or height of pin hole guides
1524 and 1526. The
horizontal position or height may be secured by locking mechanism 1512.
Stabilizing bolt 1508
may be secured to the femur or a femoral component to provide additional
stability at the top
portion 1520. Bottom portion 1522 further includes caliper 1510 that slides
along a scale and
may be configured as a tibial plate sizer.
[00111] 16A presents a front view a pin positioning block according to an
embodiment of
the present invention. Pin positioning block 1600 comprises a top portion 1614
that includes pin
position dial 1602, dial position notch 1604, stabilizing bolt 1622, sliding
member 1610 and
sliding member 1612. Pin position dial 1602 includes pin hole guides 1618 and
1620. Each of
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
33
pin hole guides 1618 and 1620 may include an orifice for drilling of pin
holes. The pin
positioning block 1600 may be used to drill pin holes into the femur bone
through pin hole
guides 1618 and 1620. The pin hole guides 1618 and 1620 may be and 'X'
distance from each
other, depending on the size of the femur and/or tibia bones. Pin positioning
block 1600 further
comprises a bottom portion 1616 that includes a ligament balancing tool
adapter 1624, as
illustrated in Fig. 16B. Ligament balancing tool adapter 1624 is configurable
for mating with a
ligament balancing tool or a prosthetic inlay device to stabilize and position
pin positioning
block 1600 with a femur. The ligament balancing tool adapter 1624 may comprise
one or more
prongs or inserts that may be inserted, attached or coupled to the ligament
balancing tool or
prosthetic inlay device. The distance between the center of pin position dial
1602 and ligament
balancing tool adapter 1624 may be a distance 'A'. Bottom portion 1616 may
have a thickness
of 'B'. The distance between the top of top portion 1614 and bottom portion
1616 may be a
distance 'C'.
[00112] Pin position dial 1602 may be raised or lowered to adjust the
angular position of
pin hole guide 1618. In the present embodiment, pin hole guide 1620 may
comprise a pivot
point of pin position dial 1602 and remains in a constant angular position.
Dial position notch
1604 may secure the pin position dial 1602 in a given position. The distance
between the middle
of stabilizing bolt 1622 and the bottom of bottom portion 1616 may be varied
by a distance 'Y'.
Top portion 1614 may be removably coupled to bottom portion 1616 by sliding
member 1610
and sliding member 1612. Sliding members 1610 and 1612 can be inserted into
their respective
receptacles in bottom portion 1616. The top portion 1614 may be extended from
bottom portion
1616 via sliding member 1610 and sliding member 1612 to adjust the horizontal
position or
height of pin hole guides 1618 and 1620. The horizontal position or height may
be secured by
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
34
locking mechanism 1606. Stabilizing bolt 1622 may be secured to the femur or a
femoral
component to provide additional stability at the top portion 1614. Bottom
portion 1616 further
includes caliper 1608 that slides along a scale and may be configured as a
tibial plate sizer.
[00113] According to one embodiment of the present invention, a patient-
specific
arthroplasty (PSA) system for individualized knee implant configurations may
be used in
conjunction with the disclosed ligament balancing tool and pin positioning
block. In particular,
knee implants may be adjusted according to specific and individual anatomical
situations (e.g.,
alignment, planned implant and procedure), individual kinematic situations
(e.g., demands and
physiological condition), and individual requests and demands (e.g., to
perform certain physical
activities, such as rock climbing, running, biking, etc.). The PSA system may
collect
preoperative measurements and dynamic evaluation of the kinematics of the knee
to evaluate
correct cuts, and produce or configure the disclosed pin positioning blocks.
The PSA system
used in conjunction with the ligament balancing tool may facilitate optimal
fitting of the implant
with an adequate soft tissue balancing.
[00114] A set of preoperative evaluations may be performed to determine
appropriate
configuration of pin positioning blocks. The evaluation may include obtaining
information from
long leg X-rays anterior-posterior (longitudinal alignment frontal plane),
from the side
(longitudinal alignment sagittal plane), and patella sunrise view (alignment
of the patellofemoral
joint); computed tomography (CT) scan (for bony landmarks and three-
dimensional impression)
or magnetic resonance imaging (MM) (to obtain cartilage thickness and
cartilage wear);
electromyography (EMG) testing (preoperative status of the following muscles:
musculus
quadriceps femoris (musculus rectus femoris, musculus vastus medialis),
musculus
semitendinosus, and musculus biceps femoris, compared to a healthy side and/or
control cohort
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
of healthy people), gait analysis (e.g., shows the walking procedure and
pressure and load
distribution on the healthy and affected side), and clinical examination and
scoring. An
evaluation may further include a survey for preferences and lifestyles (e.g.,
current activities and
capabilities) and demands (e.g., desired kinds of activities and
capabilities). A CT or MM scan
may be performed to obtain the proper shape of the femoral and tibial surface.
An EMG testing
may also be performed to evaluate the muscular strength for kinematic
evaluation. Long leg x-
rays can be performed to achieve an imagination of the individual kinematics.
An avatar may be
generated from the testing and scans to show three-dimensional movement of the
knee and the
adjacent joints. Additionally, when combined with gait analysis, a complete
set of data may be
used to model an individual movement.
[00115] Data from the preoperative measurements can provide a surgical
recommendation
to a surgeon according to the general physical condition of the knee and
identify the soft tissue
situation to achieve an optimally balanced knee. The preoperative measurements
and evaluation
data may be stored in a database and compared with data from other patients to
improve the
recommendation. The recommendation may be determined using an algorithm to
determine
optimal configurations of an implant for a particular patient according to
their preoperative
measurements and evaluations in addition to their sex, age, weight, height,
and other physical
factors to be compared with other patients. The recommendation may be used to
produce pin
positioning blocks, for example, printed by a three-dimensional or 3-D printer
on site according
to a suitable implant determined from the preoperative measurements and
survey. During the
surgical procedure, the ligament balancing tool may be used to additionally
improve the balance
situation of the soft tissue. The results of the ligament balancing tool can
give an imagination to
change the soft tissues situation intraoperatively by re-cutting or releasing.
Progress of the
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
36
treatment can be surveilled by regular postoperative checkups by re-performing
one or more of
the preoperative evaluations. Such postoperative data may be used to further
improve the
recommendations for other patients
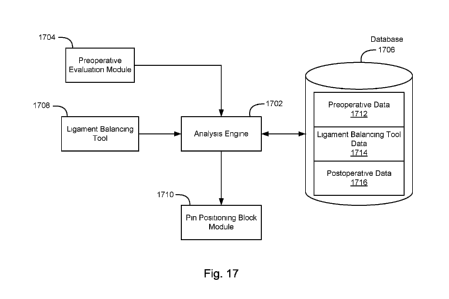
[00116] Fig. 17 illustrates a data flow diagram of a patient-specific
arthroplasty system
according to an embodiment of the present invention. Analysis engine 1702 is
operable to
generate surgical (e.g., implant type, pin positioning block, and/or cutting)
recommendations
based on data from preoperative evaluation module 1704 and database 1706.
Database 1706
may comprise a storage device containing data from a plurality of patients.
The database 1706
includes preoperative data 1712, ligament balancing tool data 1714, and
postoperative data 1716.
[00117] Preoperative evaluation module 1704 may gather preoperative
measurements and
evaluations data of a given patient. The preoperative measurements and
evaluations data may
include scans, testing, physiological analysis, and surveys to provide
anatomical situations,
kinematic situations, requests, and demands. The analysis engine 1702 may
generate an avatar
from the preoperative measurements and evaluations data to show three-
dimensional movement
of the knee and the adjacent joints. Data gathered by preoperative evaluation
module 1704 may
be stored to database 1706 in preoperative data 1712. Ligament balancing tool
1708 may record
and transmit tension data at various knee angles (as disclosed above) and use
the tension data
during surgical intervention to aid in adjusting the implant. The tension data
may be collected by
the analysis engine 1702 and transmitted to database 1706 for storage in
ligament balancing tool
data 1714.
[00118] Postoperative data 1716 may include postoperative measurements and
evaluations
data of a given patient after surgical implant. The postoperative measurements
and evaluations
data may be similar in type to the preoperative measurements and evaluations
data but is instead
CA 03092912 2020-09-02
WO 2020/008270 PCT/IB2019/001069
37
recorded after certain periods following surgery. The data in database 1706
may be analyzed to
determine trends and patterns in comparison with certain surgical
recommendations. For
example, analysis engine 1702 may analyze the postoperative data 1716 to
determine the success
and/or failure of certain recommendations. According to one embodiment,
analysis engine 1702
may generate a surgical recommendation by using data from preoperative
evaluation module
1704. Analysis engine 1702 may further modify or improve the surgical
recommendation using
the analysis of data of other patients from database 1706. For example, the
analysis engine 1702
may determine optimal configurations of an implant for a particular patient
according to their
preoperative measurements and evaluations in addition to their sex, age,
weight, height, and
other physical factors to be compared with other patients.
[00119] The recommendations may include parameters or configuration for a
type of
implant and a pin positioning block. Analysis engine 1702 may receive an
acceptance of a
recommendation to produce or configure the pin positioning block. The analysis
engine 1702
may transmit data associated with the recommendation to pin positioning block
module 1710.
The pin positioning block module 1710 may use the data to produce or determine
a pin
positioning block according to parameters or configurations specified in the
recommendation.
[00120] Figures 1 through 17 are conceptual illustrations allowing for an
explanation of
the present invention. Notably, the figures and examples above are not meant
to limit the scope
of the present invention to a single embodiment, as other embodiments are
possible by way of
interchange of some or all of the described or illustrated elements. Moreover,
where certain
elements of the present invention can be partially or fully implemented using
known
components, only those portions of such known components that are necessary
for an
understanding of the present invention are described, and detailed
descriptions of other portions
CA 03092912 2020-09-02
WO 2020/008270
PCT/IB2019/001069
38
of such known components are omitted so as not to obscure the invention. In
the present
specification, an embodiment showing a singular component should not
necessarily be limited to
other embodiments including a plurality of the same component, and vice-versa,
unless explicitly
stated otherwise herein. Moreover, applicants do not intend for any term in
the specification or
claims to be ascribed an uncommon or special meaning unless explicitly set
forth as
such. Further, the present invention encompasses present and future known
equivalents to the
known components referred to herein by way of illustration.
[00121] It
should be understood that various aspects of the embodiments of the present
invention could be implemented in hardware, firmware, software, or
combinations thereof. In
such embodiments, the various components and/or steps would be implemented in
hardware,
firmware, and/or software to perform the functions of the present invention.
That is, the same
piece of hardware, firmware, or module of software could perform one or more
of the illustrated
blocks (e.g., components or steps). In software implementations, computer
software (e.g.,
programs or other instructions) and/or data is stored on a machine-readable
medium as part of a
computer program product, and is loaded into a computer system or other device
or machine via
a removable storage drive, hard drive, or communications interface. Computer
programs (also
called computer control logic or computer readable program code) are stored in
a main and/or
secondary memory, and executed by one or more processors (controllers, or the
like) to cause the
one or more processors to perform the functions of the invention as described
herein. In this
document, the terms "machine readable medium," "computer readable medium,"
"computer
program medium," and "computer usable medium" are used to generally refer to
media such as a
random access memory (RAM); a read only memory (ROM); a removable storage unit
(e.g., a
magnetic or optical disc, flash memory device, or the like); a hard disk; or
the like.
CA 03092912 2020-09-02
WO 2020/008270
PCT/IB2019/001069
39
[00122] The
foregoing description of the specific embodiments will so fully reveal the
general nature of the invention that others can, by applying knowledge within
the skill of the
relevant art(s) (including the contents of the documents cited and
incorporated by reference
herein), readily modify and/or adapt for various applications such specific
embodiments, without
undue experimentation, without departing from the general concept of the
present
invention. Such adaptations and modifications are therefore intended to be
within the meaning
and range of equivalents of the disclosed embodiments, based on the teaching
and guidance
presented herein. It is to be understood that the phraseology or terminology
herein is for the
purpose of description and not of limitation, such that the terminology or
phraseology of the
present specification is to be interpreted by the skilled artisan in light of
the teachings and
guidance presented herein, in combination with the knowledge of one skilled in
the relevant
art(s).