Note: Descriptions are shown in the official language in which they were submitted.
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
METHOD FOR FRACTURING IN HYDROCARBON RESERVOIRS
CLAIM OF PRIORITY
[0001] This application claims priority to U.S. Provisional Patent
Application
No. 62/652,733, filed April 4, 2018, the contents of which are hereby
incorporated by
reference.
TECHNICAL FIELD
[0002] This disclosure relates to hydraulic fracturing hydrocarbon
reservoirs,
and more specifically, in tight gas reservoirs.
BACKGROUND
[0003] Low permeability reservoirs that produce mainly dry natural gas are
commonly called tight gas reservoirs. On an individual well bases, a well in a
tight gas
reservoir will typically produce less gas over a longer period of time than
one would
expect from a well completed in a higher permeability, conventional reservoir.
In many
cases, hydrocarbon production from low permeability reservoirs rapidly decline
during
the first year of production. Hydraulic fracturing processes have been used to
stimulate
such tight gas reservoirs and improve hydrocarbon production.
SUMMARY
[0004] This disclosure describes technologies relating to hydraulic
fracturing in
hydrocarbon reservoirs, and more specifically, in tight gas reservoirs (for
example,
carbonate reservoirs).
[0005] Certain aspects of the subject matter described here can be
implemented
as a method for treating a subterranean zone. An acid-generating material and
a
proppant is introduced to the subterranean zone. Fractures are created in the
subterranean zone using the acid-generating material. The proppant is
positioned within
the created fractures to keep the fractures open.
[0006] This, and other aspects, can include one or more of the
following
features. The acid-generating material and the proppant can be mixed to form a
mixture,
and the mixture can be introduced to the subterranean zone.
[0007] The mixture can include multiple layers, and each of the layers
can
include the acid-generating material and the proppant.
[0008] The subterranean zone can include carbonate mineral.
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[0009] An acid can be generated in the subterranean zone with the acid-
generating material. The carbonate mineral can be reacted with the generated
acid.
[0010] The acid-generating material can include a degradable ester.
[0011] The degradable ester can include polylactic acid, polyglycolic
acid, or
combinations thereof
[0012] The proppant can have a maximum dimension less than or equal to
100
micrometers (p.m).
[0013] The proppant can have a maximum dimension less than or equal to
1 p.m.
[0014] The proppant can have a maximum dimension less than or equal to
100
nanometers (nm).
[0015] The proppant can be coated with the acid-generating material to
form
coated proppant, and the coated proppant can be introduced to the subterranean
zone.
[0016] The details of one or more implementations of the subject
matter of this
disclosure are set forth in the accompanying drawings and the description.
Other
features, aspects, and advantages of the subject matter will become apparent
from the
description, the drawings, and the claims.
DESCRIPTION OF DRAWINGS
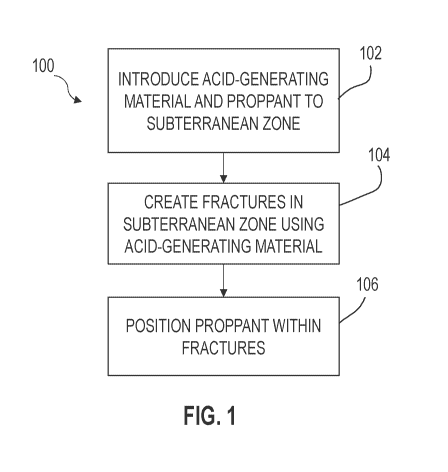
[0017] FIG. 1 is a flow chart of a method for treating a subterranean
zone.
[0018] FIGs. 2A & 2B are schematics showing stages of treating a
subterranean
zone using a mixture of acid-generating material and proppant in multiple
layer packing.
[0019] FIGs. 3A & 3B are schematics showing stages of treating a
subterranean
zone using a mixture of acid-generating material and proppant in single layer
packing.
[0020] FIGs. 4A & 4B are schematics showing stages of treating a
subterranean
zone using proppant coated with an acid generating material in a single layer.
[0021] FIG. 5 is a schematic of a fracture without proppant.
[0022] FIG. 6 is a schematic of laminar flow within a fracture at
steady state.
[0023] FIG. 7 is a schematic of fluid flow within a fracture packed
with proppant
at steady state.
[0024] FIG. 8A is a schematic of a fracture packed with proppant.
[0025] FIG. 8B is a schematic of fluid flow within the fracture of FIG. 8A
at
steady state with some of the proppant removed.
[0026] FIG. 9 is a schematic showing an example composite core
assembly.
2
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[0027] FIG. 10A is a photograph of sand and an acid-generating
material packed
on a rock sample.
[0028] FIG. 10B is a surface scan of the rock sample of FIG. 10A prior
to being
packed with the sand and the acid-generating material.
[0029] FIG. 10C is a magnified scan of the area in the rectangle of FIG.
10B.
[0030] FIG. 10D is a magnified scan of the area in the rectangle of
FIG. 10C.
[0031] FIG. 10E is a height profile along the dashed line of FIG. 10C.
[0032] FIG. 1OF is a height profile along the dashed line of FIG. 10D.
[0033] FIG. 11A is a surface scan of the rock sample of FIG. 10A after
io undergoing core flooding and subsequent removal of the sand.
[0034] FIG. 11B is a magnified scan of the area in the rectangle of
FIG. 11A.
[0035] FIG. 11C is a magnified scan of the area in the rectangle of
FIG. 11B.
[0036] FIG. 11D is a height profile along the dashed line of FIG. 11B.
[0037] FIG. 11E is a height profile along the dashed line of FIG. 11C.
[0038] FIG. 12A is a photograph of the rock sample of FIG. 10A after
undergoing core flooding and subsequent removal of the sand and undissolved
acid-
generating material.
[0039] FIG. 12B is a surface scan of the rock sample of FIG. 12A.
[0040] FIG. 12C is a magnified scan of the area in the rectangle of
FIG. 12B.
[0041] FIG. 12D is a magnified scan of the area in the rectangle of FIG.
12C.
[0042] FIG. 12E is a height profile along the dashed line of FIG. 12C.
[0043] FIG. 12F is a height profile along the dashed line of FIG. 12D.
[0044] FIG. 13A is a photograph of an acid-generating material on a
rock
sample.
[0045] FIG. 13B is a surface scan of the rock sample of FIG. 13A prior to
being
treated with the acid-generating material.
[0046] FIG. 13C is a magnified scan of the area in the rectangle of
FIG. 13B.
[0047] FIG. 13D is a magnified scan of the area in the rectangle of
FIG. 13C.
[0048] FIG. 13E is a height profile along the dashed line of FIG. 13C.
[0049] FIG. 13F is a height profile along the dashed line of FIG. 13D.
[0050] FIG. 14A is a photograph of the rock sample of FIG. 13A after
undergoing core flooding and subsequent removal of undissolved acid-generating
material.
3
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[0051] FIG. 14B is a surface scan of the rock sample of FIG. 14A.
[0052] FIG. 14C is a magnified scan of the area in a rectangle of FIG.
14B.
[0053] FIG. 14D is a magnified scan of the area in another rectangle
of FIG.
14B.
[0054] FIG. 14E is a height profile along the dashed line of FIG. 14C.
[0055] FIG. 14F is a height profile along the dashed line of FIG. 14D.
[0056] FIG. 15A is a photograph of sand and an acid-generating
material on a
rock sample.
[0057] FIG. 15B is a surface scan of the rock sample of FIG. 15A prior
to being
treated with the sand and the acid-generating material.
[0058] FIG. 15C is a magnified scan of the area in the rectangle of
FIG. 15B.
[0059] FIG. 15D is a magnified scan of the area in the rectangle of
FIG. 15C.
[0060] FIG. 15E is a height profile along the dashed line of FIG. 15C.
[0061] FIG. 15F is a height profile along the dashed line of FIG. 15D.
[0062] FIG. 16A is a photograph of the rock sample of FIG. 15A, after
undergoing core flooding and subsequent removal of the sand and undissolved
acid-
generating material.
[0063] FIG. 16B is a surface scan of the rock sample of FIG. 16A.
[0064] FIG. 16C is a magnified scan of the area in a rectangle of FIG.
16B.
[0065] FIG. 16D is a magnified scan of the area in another rectangle of
FIG.
16B.
[0066] FIG. 16E is a height profile along the dashed line of FIG. 16C.
[0067] FIG. 16F is a height profile along the dashed line of FIG. 16D.
[0068] FIG. 17A is a schematic illustrating fluid flow in a fracture
without
proppant.
[0069] FIG. 17B is a schematic illustrating fluid flow in a fracture
with proppant.
[0070] FIG. 17C is a schematic illustrating fluid flow in a fracture
with proppant
and void space generated by an acid-generating material.
[0071] FIG. 18 is a three-dimensional schematic illustrating fluid
flow in a
fracture packed with single layer of proppant.
DETAILED DESCRIPTION
4
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[0072] Carbonate reservoirs make up approximately 70% of oil
reservoirs and
approximately 90% of gas reservoirs in the Middle East region. Hydraulic
fracturing
processes have been used to stimulate reservoirs to improve hydrocarbon
production. In
a typical hydraulic fracturing process, multi-million gallons of water-based
fracturing
fluids are used as carrying fluids to transport proppants into the
hydraulically-induced
fractures. It has been estimated that for some hydraulic fracturing processes,
only about
10% to 35% of the fracturing fluids flow back to the well, while the rest of
the fluids are
retained within the formation. The imbibition of fracturing fluids into the
rock matrix
has been considered to be one of the main mechanisms that cause fracturing
fluid loss
to and reservoir damage. Fracturing fluids imbibed into the rock matrix can
invade the
permeability of the gas/oil phase, thereby decreasing the productivity of a
well.
[0073] Dissolution of sulfate and carbonate minerals within carbonate
reservoirs
can increase permeability of the reservoir rock. The subject matter described
in this
disclosure utilize acid-generating material and proppant together (for
example, in pad or
pre-pad fluids) to increase the permeability of tight gas reservoirs, for
example, by
improving fluid flow in induced or naturally existing far-field micro-
fractures. The acid-
generating material and the proppant can be used to further increase the
matrix
permeability by improving mineral dissolution ability of the imbibed fluid to
the
formation. The materials described in this disclosure can dissolve minerals on
fracture
(and micro-fracture) surfaces and can penetrate into the rock matrix, so that
hydrocarbon
production from the formation can be increased. The materials described in
this
disclosure can generate additional fractures (and micro-fractures) by reacting
with
minerals that make up the formation. The proppants can more easily occupy the
induced
micro-fractures and in some cases, even the natural micro-fractures within the
formation.
[0074] As used in this disclosure, the term "subterranean material" or
"subterranean zone" refers to any material under the surface of the earth,
including under
the surface of the bottom of the ocean. For example, a subterranean zone or
material
can be any section of a wellbore and any section of a subterranean hydrocarbon-
or
water-producing formation or region in fluid contact with the wellbore.
Placing a
material in a subterranean zone can include contacting the material with any
section of
a wellbore or with any subterranean region in fluid contact the material.
Subterranean
materials can include any materials placed into the wellbore such as cement,
drill shafts,
5
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
liners, tubing, casing, or screens; placing a material in a subterranean zone
can include
contacting with such subterranean materials. In some examples, a subterranean
zone or
material can be any downhole region that can produce liquid or gaseous
hydrocarbon
materials, water, or any downhole section in fluid contact with liquid or
gaseous
hydrocarbon materials, or water. For example, a subterranean zone or material
can be
at least one of an area desired to be fractured, a fracture or an area
surrounding a fracture,
and a flow pathway or an area surrounding a flow pathway, in which a fracture
or a flow
pathway can be optionally fluidly connected to a subterranean hydrocarbon- or
water-
producing region, directly or through one or more fractures or flow pathways.
[0075] As used in this disclosure, "treatment of a subterranean zone" can
include
any activity directed to extraction of water or hydrocarbon materials from a
subterranean
hydrocarbon- or water-producing formation or region, for example, including
drilling,
stimulation, hydraulic fracturing, clean-up, acidizing, completion, cementing,
remedial
treatment, abandonment, aquifer remediation, identifying oil rich regions via
imaging
techniques, and the like.
[0076] As used
in this disclosure, the term "about" or "approximately" can allow
for a degree of variability in a value or range, for example, within 10%,
within 5%, or
within 1% of a stated value or of a stated limit of a range.
[0077] As used
in this disclosure, the term "substantially" refers to a majority
of, or mostly, as in at least about 50%, 60%, 70%, 80%, 90%, 95%, 96%, 97%,
98%,
99%, 99.5%, 99.9%, 99.99%, or at least about 99.999% or more.
[0078] As used
in this disclosure, the term "solvent" refers to a liquid that can
dissolve a solid, another liquid, or a gas to form a solution. Non-limiting
examples of
solvents are silicones, organic compounds, water, alcohols, ionic liquids, and
supercritical fluids.
[0079] As used
in this disclosure, the term "drilling fluid" refers to fluids,
slurries, or muds used in drilling operations downhole, such as during the
formation of
the wellbore.
[0080] As used
in this disclosure, the term "stimulation fluid" refers to fluids or
slurries used downhole during stimulation activities of the well that can
increase the
production of a well, including perforation activities. In some examples, a
stimulation
fluid can include a fracturing fluid or an acidizing fluid.
6
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[0081] As used in this disclosure, the term "fracturing fluid" refers
to fluids or
slurries used downhole during fracturing operations.
[0082] As used in this disclosure, the term "remedial treatment fluid"
refers to
fluids or slurries used downhole for remedial treatment of a well. Remedial
treatments
can include treatments designed to increase or maintain the production rate of
a well,
such as stimulation or clean-up treatments.
[0083] As used in this disclosure, the term "fluid" refers to liquids
and gels,
unless otherwise indicated.
[0084] As used in this disclosure, a "flow pathway" downhole can
include any
suitable subterranean flow pathway through which two subterranean locations
are in
fluid connection. The flow pathway can be sufficient for hydrocarbons or water
to flow
from one subterranean location to the wellbore or vice-versa. A flow pathway
can
include at least one of a hydraulic fracture, and a fluid connection across a
screen, across
gravel pack, across proppant, including across resin-bonded proppant or
proppant
deposited in a fracture, and across sand. A flow pathway can include a natural
subterranean passageway through which fluids can flow. In some
implementations, a
flow pathway can be a water source and can include water. In some
implementations, a
flow pathway can be a hydrocarbon source and can include hydrocarbons. In some
implementations, a flow pathway can be sufficient to divert water, a downhole
fluid, or
a produced hydrocarbon from a wellbore, fracture, or flow pathway connected to
the
pathway.
[0085] FIG. 1 is a flow chart of a method 100 for treating a
subterranean zone.
The subterranean zone can include a tight gas reservoir. For example, the
subterranean
zone can include a carbonate rock formation, which can include carbonate
mineral. At
step 102, an acid-generating material and a proppant is introduced to the
subterranean
zone. The acid-generating material can be, for example, pumped downhole
through a
tubing into the subterranean zone. The acid-generating material and the
proppant can
be pumped together into the subterranean zone. The acid-generating material
and the
proppant can be mixed to form a mixture, and the mixture can be introduced to
the
subterranean zone. For example, the acid-generating material and the proppant
can be
mixed to form a mixture before being introduced to the subterranean zone, and
then the
mixture can be pumped into the subterranean zone. In some implementations, the
mixture of acid-generating material and proppant includes multiple layers, and
each of
7
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
the layers includes acid-generating material and proppant. In some
implementations,
the mixture of acid-generating material and proppants forms a single layer
that includes
acid-generating material and proppant. In some implementations, the proppant
is coated
with the acid-generating material to form coated proppant, and the coated
proppant is
introduced to the subterranean zone.
[0086] The acid-
generating material and the proppant can be introduced to the
subterranean zone with another fluid. For example, the acid-generating
material and the
proppant can be introduced to the subterranean zone with a drilling fluid, a
stimulation
fluid, a fracturing fluid, a remedial treatment fluid, a pad fluid, a pre-pad
fluid, or
combinations thereof The acid-generating material can be a delayed acid-
generating
material. For example, the acid-generating material does not generate acid
until after
the acid-generating material has been introduced to the subterranean zone. The
acid-
generating material can be an acid precursor, that is, a compound that
participates in a
chemical reaction that produces an acid. The acid-generating material can be a
material
that can degrade to produce an acid as a degradation product. The acid-
generating
material can be in the form of a solid. The acid-generating material can
include an ester,
such as a degradable polyester (for example, polylactic acid, polyglycolic
acid, and
copolymers thereof). Esters have hydrolysable ester bonds that can be cleaved
to
produce acid. For example, polyesters can undergo hydrolysis under high
pressure and
temperature (as is usually the case in subterranean zones) to produce an acid.
Some
additional non-limiting examples of acid-generating material include
polycaprolactone,
polyhydroxybutyrate (such as poly(3-hydroxybutyrate) or poly(4-
hydroxybutyrate)),
poly(3-hydroxy valerate), poly(ethylene succinate), poly(propylene succinate),
poly(butylene succinate), polyhydroxyalkanoate, and copolymers thereof
[0087] In some implementations, a surfactant is introduced to the
subterranean
zone before introducing the acid-generating material and the proppant to the
subterranean zone. The surfactant can coat a portion of the subterranean zone.
The
surfactant can be anionic or non-ionic. The acid-generating material and the
proppant
can be introduced to the subterranean zone, and acid generated from the acid-
generating
material can react with the carbonate mineral at the portion of the
subterranean zone that
is not coated with surfactant.
[0088] At step
104, fractures are created in the subterranean zone using the acid-
generating material. Creating the fractures in the subterranean zone using the
acid-
8
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
generating material can involve generating an acid in the subterranean zone
with the
acid-generating material and reacting the carbonate mineral in the
subterranean zone
with the generated acid. The reaction between the carbonate mineral and the
generated
acid can etch the formation in the subterranean zone and create additional
fractures and
micro-fractures (that is, fractures on the micrometer scale). The fractures
created at step
104 can include micro-fractures, etched fracture surfaces, or a combination of
these. In
some implementations, the acid-generating material increases the size of
existing
fractures in the subterranean zone at step 104. In some implementations, the
acid-
generating material etches the surface of existing fractures in the
subterranean zone at
step 104.
[0089] At step 106, the proppant is positioned within the created
fractures to
keep the fractures open. The proppant can also be positioned within natural
fractures
(that is, fractures already present in the subterranean zone before creating
the fractures
at step 104, enlarged at step 104, or etched at step 104) to keep the natural
fractures
open. The proppant is permeable to gas under high pressures (such as pressures
encountered in subterranean zones), and interstitial space between individual
particles
of proppants can be sufficiently large, yet have the mechanical strength to
withstand
closure stresses to hold fractures open. The proppant can therefore be used to
form
conductive pathways for hydrocarbons (such as oil and gas) to flow. The
proppant can
be made of, for example, sand, treated sand, man-made ceramic materials,
silica, or
combinations thereof In some implementations, individual particles of the
proppant
have a maximum dimension that is less than or equal to 1 millimeter. In some
implementations, individual particles of the proppant have a maximum dimension
that
is less than or equal to 100 micrometers (p.m). In some implementations,
individual
particles of the proppant have a maximum dimension that is less than or equal
to 1 p.m.
In some implementations, individual particles of the proppant have a maximum
dimension less than or equal to 100 nanometers (nm). The individual particles
of the
proppant can have a maximum dimension in a range between approximately 1 nm to
approximately 1 millimeter (mm), in a range between approximately 1 nm to
approximately 100 p.m, in a range between approximately 1 nm to approximately
1 p.m,
and in a range between approximately 1 nm to 100 nm. In cases where the
proppant is
coated with the acid-generating material, the individual particles of the
coated proppant
can have a maximum dimension that is less than or equal to 100 p.m. In some
9
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
implementations, individual particles of the coated proppant have a maximum
dimension that is less than or equal to 1 p.m. In some implementations,
individual
particles of the coated proppant have a maximum dimension less than or equal
to 100
nm. The individual particles of the coated proppant can have a maximum
dimension in
a range between approximately 1 nm to approximately 1 millimeter (mm), in a
range
between approximately 1 nm to approximately 100 p.m, in a range between
approximately 1 nm to approximately 1 pm, and in a range between approximately
1 nm
to 100 nm.
[0090] FIGs. 2A and 2B are schematics showing stages of treating a
subterranean zone, for example, by method 100. The subterranean zone 250 can
include
a flow pathway, a fracture, a channel, or combinations thereof through which
fluids and
solids can flow. As shown in FIG. 2A, a composition 200 including an acid-
generating
material 201 and a proppant 203 is introduced to the subterranean zone 250.
The
composition 200 can include multiple layers of acid-generating material 201
and
proppant 203. As described earlier, the acid-generating material 201 can
generate acid,
which can react with carbonate in the subterranean zone 250. As shown in FIG.
2B, the
reaction between the acid and the carbonate can etch a surface of the
subterranean zone
250 and create fractures 251. The proppant 203 can keep the fractures 251
open.
[0091] FIGs. 3A and 3B are schematics showing stages of treating a
subterranean zone, for example, by method 100. As shown in FIG. 3A, a
composition
300 including an acid-generating material 301 and a proppant 303 is introduced
to the
subterranean zone 250. The acid-generating material 301 can be substantially
the same
as the acid-generating material 201, and the proppant 303 can be substantially
the same
as the proppant 203. The composition 300 can include a single layer of acid-
generating
material 301 and proppant 303. As described earlier, the acid-generating
material 301
can generate acid, which can react with carbonate in the subterranean zone
250. As
shown in FIG. 3B, the reaction between the acid and the carbonate can etch a
surface of
the subterranean zone 250 and create fractures 251. The proppant 303 can keep
the
fractures 251 open.
[0092] FIGs. 4A and 4B are schematics showing stages of treating a
subterranean zone, for example, by method 100. As shown in FIG. 4A, a
composition
400 including an acid-generating material 401 and a proppant 403 is introduced
to the
subterranean zone 250. The acid-generating material 401 can be substantially
the same
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
as the acid-generating material 201, and the proppant 403 can be substantially
the same
as the proppant 203. The proppant 403 can be coated with the acid-generating
material
401. Although the composition 400 shown in FIG. 4A includes a single layer of
proppant 403 coated in acid-generating material 401, the composition 400 can
include
multiple layers of proppant 403 coated in acid-generating material 401. As
described
earlier, the acid-generating material 401 can generate acid, which can react
with
carbonate in the subterranean zone 250. As shown in FIG. 4B, the reaction
between the
acid and the carbonate can etch a surface of the subterranean zone 250 and
create
fractures 251. The proppant 403 can keep the fractures 251 open.
EXAMPLE 1
[0093] The permeability of natural fractures for three scenarios
(without
proppant, filled with proppant, and partially filled with proppant) were
simulated using
combined discrete element method and lattice Boltzmann method (DEM-LBM)
simulations. In the DEM-LBM coupling system, a proppant pack was modeled by an
assembly of spherical particles in PFC3D (Itasca Consulting Group, Inc.), and
the fluid
flow in the pore space was computed by LBM. The interaction between the pore
fluids
and proppants was handled by an immersed boundary scheme. Additional details
about
the DEM-LBM coupling system can be found in "LBM-DEM modeling of fluid-solid
interaction in porous media" by Han and Cundall (International Journal for
Numerical
and Analytical Methods in Geomechanics 37.10 (2013): 1391-1407).
[0094] FIG. 5 is a schematic of a fracture, for example, in a
subterranean zone,
such as a carbonate reservoir. For this example, the fracture was assumed to
be planar
with an aperture of height a. In relation to the direction of the aperture,
the extensions
of the fracture were treated as being infinite (that is, boundless) in the
other two
directions. In the DEM-LBM coupling simulation, a cubic model with edge length
a
was adopted to represent the fracture, with a periodic condition enforced in
the two
infinite extension directions.
[0095] Scenario 1: Fracture without proppant
[0096] For Scenario 1, the fracture aperture height a was assumed to
be 1.35
mm. Water having a density of 1,000 kilograms per cubic meter (kg/m') and a
viscosity
of 0.89 Pascal-second (Pa-s) was used as flooding fluid in the permeability
measurement. The simulation was driven with gravity of 0.1 meter per square
second
(m/s2) in the x-direction. Because the Reynolds number was small, laminar flow
11
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
condition applied. The flux, that is, the discharge per unit area, with units
of length per
time was measured when fluid flow reached steady state. FIG. 6 shows a
schematic of
the laminar flow at steady state within the fracture.
[0097] The permeability was then calculated using Darcy's law shown in
Equation (1).
(1)
Vp
where k is mobility coefficient (which is permeability divided by viscosity)
in square
meters per Pascal-second (m2/Pa-s), q is flux in meter per second (m/s), and
Vp is the
pressure gradient vector in Pascal per meter (Palm). In this case, the
pressure gradient
vector was equal to pgr, where p is density in kg/m', and g, is gravity in the
x-direction
in m/s2. The mobility coefficient is equal to permeability in square meters
(m2) divided
by viscosity in Pa-s, which means that the permeability is proportional to the
mobility
coefficient.
[0098] Scenario 2: Fracture filled with proppant
[0099] The proppant was modeled as spherical particles having a diameter of
0.45 mm. Three layers of proppants fit within the aperture of the fracture.
FIG. 7 shows
a schematic of the fluid flow within the fracture filled with proppant at
steady state. The
mobility coefficient was calculated using Darcy's law shown in Equation (1).
[00100] Scenario 3: Fracture partially filled with proppant
[00101] Some proppant can be removed by chemical treatment. As such, the
permeability of the proppant pack can increase. To illustrate this effect,
several
proppants (the lighter spherical particles in FIG. 8A) were removed from the
model of
Scenario 2. FIG. 8A shows a schematic of the fracture packed with proppant.
The
darker proppant particles are the proppant particles that will stay within the
fracture,
while the lighter proppant particles are the proppant particles that are to be
removed
from the fracture. FIG. 8B shows a schematic of the fluid flow within the
fracture at
steady state after some of the proppant particles (the lighter proppant
particles in FIG.
8A) have been removed. The mobility coefficient was calculated using Darcy's
law
shown in Equation (1).
[00102] The mobility coefficients measured from the DEM-LBM simulations are
summarized in Table 1.
12
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
Scenario Mobility coefficient (m2/Pa-s)
1: Fracture without proppant 1.36 X 10-10
2: Fracture filled with proppant 1.09 X 10-12
3: Fracture partially filled with proppant 1.84 X 10-12
TABLE 1
EXAMPLE 2
[00103] A study
was performed on tight organic-rich carbonate source rock
samples obtained from an outcrop from Eagle Ford shale. 100 mesh (about 150
micrometers in diameter) white sand was used in this study. The acid-
generating
material used in this study was polyglycolic acid (PGA) with an average size
of 200
micrometers. Half-core Eagle Ford outcrop plugs were obtained by splitting a
full core
of 1.0 inch in diameter by 1.0 inch in length using a trim saw in the
longitudinal
direction. The cut rock surfaces where then finely trimmed using a target
surface
trimmer.
[00104] FIG. 9 is
a schematic showing an example composite core assembly 900.
The composite core assembly 900 included a half-core spacer 901 made of
hastelloy and
the half-core Eagle Ford outcrop plug 903 (described in the previous paragraph
and also
referred as the half-core sample). The aperture between the two halves 901 and
903 was
used to simulate the width of a filled microfracture in the shale. The width
of the
aperture was about 150 micrometers. Acid-generating material 201 (PGA),
proppant
203 (100 mesh white sand), or both (depending on the test) were placed in this
aperture.
The composite core assembly 900 was placed into a hastelloy core holder for
testing
high pressure and high temperature. The confining pressure was set at 2,000
pounds per
square inch gauge (psig) and backpressure was maintained at 1,000 psig
throughout each
core flooding test. For each core flooding test, 2% potassium chloride (KC1)
solution
was used as the flow media. Differential pressure across the composite core
assembly
900 was measured throughout each core flooding test.
[00105] After
thermal treatment, the half-core sample 903 was removed from the
core holder, and the etched surface was analyzed to identify the change in
morphology
caused by any chemical reactions (for example, due to the acid-generating
material 201).
The texture and surface profile of the half-core sample 903 was analyzed using
a
Nanovea PS50 profilometer. The profilometer measured a physical wavelength
that was
directly related to a specific height and did not require the use of complex
algorithms.
The surface characterization was conducted for each of the half-core samples
903 before
13
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
and after chemical treatment in order to identify the change in morphology
caused by
the chemical reaction(s).
[00106] Sample 1: Acid-generating material and sand intermixed
[00107] FIG. 10A is a photograph of an acid-generating material 201 and
sand
203 (intermixed with one another) on a half-core sample 903. 23.9 milligrams
(mg) of
PGA 201 and 31.9 mg of 100 mesh white sand 203 were placed on the surface of
the
half-core sample 903. The testing temperature for the core flooding test was
180 degrees
Fahrenheit ( F). 2% KC1 was flowed through the composite core assembly 900
during
the core flooding test at a rate of 1 milliliter per minute (mL/min) for 3
hours. The
composite core assembly 900 was shut-in within the core holder overnight. No
further
fluid flow was conducted, and the composite core assembly 900 was then removed
from
the core holder.
[00108] FIG. 10B is a surface scan (15 millimeters by 10 millimeters)
of the half-
core sample 903 of FIG. 10A prior to being treated with the acid-generating
material
201 and the sand 203. The surface of the half-core sample 903 was relatively
uniform,
and the small grooves generated during the core preparation process were
measured to
be in a range from about 1 micrometer to about 5 micrometers. FIG. 10C is a
magnified
scan of the area in the rectangle (15 millimeters by 5 millimeters) of FIG.
10B, and FIG.
10E is a height profile along the dashed line of FIG. 10C. FIG. 10D is a
magnified scan
of the area in the rectangle (4 millimeters by 5 millimeters) of FIG. 10C, and
FIG. 1OF
is a height profile along the dashed line of FIG. 10D.
[00109] FIG. 11A is a surface scan (15 millimeters by 10 millimeters)
of the half-
core sample 903 of FIG. 10A after undergoing core flooding and subsequent
removal of
the sand 203. FIG. 11B is a magnified scan of the area in the rectangle (15
millimeters
by 5 millimeters) of FIG. 11A, and FIG. 11D is a height profile along the
dashed line of
FIG. 11B. The height of the solids coverage in FIG. 11D was about 100
micrometers to
about 150 micrometers, which was similar to the aperture size of the composite
core
assembly 900. FIG. 11C is a magnified scan of the area in the rectangle (4
millimeters
by 5 millimeters) of FIG. 11B, and FIG. 11E is a height profile along the
dashed line of
FIG. 11C. The height of the solids coverage in FIG. 11E was about 100
micrometers to
about 150 micrometers, which was in the same range of the aperture of the
composite
core assembly 900 (about 150 micrometers, similar to the diameter of the 100
mesh sand
203).
14
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[00110] FIG. 12A is a photograph of the half-core sample 903 of FIG.
10A after
undergoing core flooding and subsequent removal of the acid-generating
material 201
and the sand 203. The acid-generating material 201 and the sand 203 were
removed
from the half-core sample 903 by blowing the half-core sample 903 with an air
nozzle.
The surface of the half-core sample 903 was rougher than the original surface
(compare
with FIG. 10B). The depth of the etched areas ranged from about 5 micrometers
to about
40 micrometers.
[00111] FIG. 12B is a surface scan (15 millimeters by 10 millimeters)
of the half-
core sample 903 of FIG. 12A. FIG. 12C is a magnified scan of the area in the
rectangle
(15 millimeters by 5 millimeters) of FIG. 12B, and FIG. 12E is a height
profile along
the dashed line of FIG. 12C. FIG. 12D is a magnified scan of the area in the
rectangle
(5.5 millimeters by 5 millimeters) of FIG. 12C, and FIG. 12F is a height
profile along
the dashed line of FIG. 12D.
[00112] Sample 2: Acid-generating material without sand
[00113] FIG. 13A is a photograph of an acid-generating material 201 on a
half-
core sample 903. 51.9 mg of PGA 201 was placed on the surface of the half-core
sample
903. No sand was placed on this half-core sample 903. The testing temperature
for the
core flooding test was 180 F. 2% KC1 was flowed through the composite core
assembly
900 during the core flooding test at a rate of 0.001 mL/min for 90 hours. The
composite
core assembly 900 was then removed from the core holder.
[00114] FIG. 13B is a surface scan (15 millimeters by 10 millimeters)
of the half-
core sample 903 of FIG. 13A prior to being treated with the acid-generating
material
201. Similar to that of the half-core sample 903 of FIG. 10B, the surface of
the half-
core sample 903 of FIG. 13A was relatively uniform, and the small grooves
generated
during the core preparation process were measured to be in a range from about
2
micrometers to about 5 micrometers. FIG. 13C is a magnified scan of the area
in the
rectangle (15 millimeters by 5 millimeters) of FIG. 13B, and FIG. 13E is a
height profile
along the dashed line of FIG. 13C. FIG. 13D is a magnified scan of the area in
the
rectangle (3 millimeters by 5 millimeters) of FIG. 13C, and FIG. 13F is a
height profile
along the dashed line of FIG. 13D.
[00115] After conducting the core flooding test and retrieving the
composite core
assembly 900 from the core holder, it was found that some acid-generating
material 201
remained on the surface of the half-core sample 903. This could have been a
result of
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
the slower flow rate of the 2% KC1 aqueous solution which might have
preferentially
flowed around the two columns of the acid-generating material 201, thereby
resulting in
not exposing the acid-generating material 201 to enough water for full
degradation of
the acid-generating material 201.
[00116] FIG. 14A is a photograph of the half-core sample 903 of FIG. 13A
after
undergoing core flooding and subsequent removal of the acid-generating
material 201.
Two rough patches with irregularly shaped pockets were observed at the areas
where
the acid-generating material 201 was previously packed on the surface of the
half-core
sample 903. The depth of the irregularly shaped pockets was determined to be
from
about 10 micrometers to about 50 micrometers, similar to the range observed
with
Sample 1 (see, for example, FIGs. 12A through 12F).
[00117] FIG. 14B
is a surface scan (15 millimeters by 20 millimeters) of the half-
core sample 903 of FIG. 14A. FIG. 14C is a magnified scan of the area in a
rectangle
(3.5 millimeters by 5 millimeters) of FIG. 14B, and FIG. 14E is a height
profile along
the dashed line of FIG. 14C. FIG. 14D is a magnified scan of the area in
another
rectangle (15 millimeters by 5 millimeters) of FIG. 14B, and FIG. 14F is a
height profile
along the dashed line of FIG. 14D.
[00118] Sample 3:
Acid-generating material and sand, separated and intermixed
[00119] FIG. 15A
is a photograph of an acid-generating material 201 and sand
203 on a half-core sample 903. 23.0 milligrams (mg) of PGA 201 and 40.7 mg of
100
mesh white sand 203 were placed on the surface of the half-core sample 903.
The PGA
201 and sand 203 were arranged in four arrays (rows) across the half-core
sample 903.
In order from top to bottom (referring to FIG. 15A), the placed materials were
1) sand
203; 2) acid-generating material 201; 3) sand 203; and 4) intermixed acid-
generating
material 201 and sand 203. The testing temperature for the core flooding test
was 250 F.
2% KC1 was flowed through the composite core assembly 900 during the core
flooding
test at a rate of 0.02 mL/min for 48 hours. Effluent from the core flooding
setup was
collected using auto-collectors for further analysis. The composite core
assembly 900
was then removed from the core holder.
[00120] FIG. 15B is a surface scan (15 millimeters by 10 millimeters) of
the half-
core sample 903 of FIG. 15A prior to being treated with the acid-generating
material
201 and the sand 203. Before the acid-generating material 201 and the sand 203
were
placed on the surface of the half-core sample 903, the surface of the half-
core sample
16
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
903 was rubbed with 60 grit sandpaper in order to reduce the potential of
solid particle
movement during the core flooding test. The grooves generated during the core
preparation process were measured to be in a range from about 10 micrometers
to about
30 micrometers (slightly deeper than those for Samples 1 and 2). FIG. 15C is a
magnified scan of the area in the rectangle (15 millimeters by 5 millimeters)
of FIG.
15B, and FIG. 15E is a height profile along the dashed line of FIG. 15C. FIG.
15D is a
magnified scan of the area in the rectangle (2 millimeters by 5 millimeters)
of FIG. 15C,
and FIG. 15F is a height profile along the dashed line of FIG. 15D.
[00121] FIG. 16A is a photograph of the half-core sample 903 of FIG.
15A, after
undergoing core flooding and subsequent removal of acid-generating material
201 and
the sand 203. Two rough patches with irregularly shaped pockets were observed
at the
areas where the acid-generating material 201 was previously packed on the
surface of
the half-core sample 903. The depth of the irregularly shaped pockets was
determined
to be from about 50 micrometers to about 150 micrometers, which was deeper in
comparison to the range observed with Samples 1 and 2 (see, for example, FIGs.
12A
through 12F and FIGs.14A through 14F). This increase in depth profile could
have been
the result of faster reaction kinetics at the increased temperature in
comparison to the
temperatures implemented for Samples 1 and 2.
[00122] FIG. 16B is a surface scan (15 millimeters by 20 millimeters)
of the half-
core sample 903 of FIG. 16A. FIG. 16C is a magnified scan of the area in a
rectangle
(5.5 millimeters by 15 millimeters) of FIG. 16B, and FIG. 16E is a height
profile along
the dashed line of FIG. 16C. FIG. 16D is a magnified scan of the area in
another
rectangle (3 millimeters by 5 millimeters) of FIG. 16B, and FIG. 16F is a
height profile
along the dashed line of FIG. 16D.
[00123] The experiments conducted on Samples 1, 2, and 3 prove that the
acid-
generating material 201 was capable of creating voids (for example, dimples)
along the
flow-path of microfractures by nature of its degradation under the operating
conditions
(for example, increased temperature) and the chemical reaction between the
acid
generated by the acid-generating material 201 and the calcite present in the
half-core
sample 903.
[00124] Numerical modeling
[00125] Numerical modeling was employed to predict the permeability
change
based on the fracture width and the quantified ranges of etched fracture
surfaces. The
17
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
DEM-LBM coupling model proved capable of precisely and accurately capturing
the
fluid flow in pore space along with the interaction among the pore fluid,
solid particles,
and confining walls. The model was employed to verify the results of etching
with acid-
generating material 201 and quantify the change in permeabilities and
hydraulic
conductivities in the various scenarios explored. The model was employed also
to
quantify the changes in conductivity due to the placement of proppant 203 in
the
microfractures and etches formed by the interaction of the acid-generating
material 201
and the fracture face (for example, formed on the surface of the half-core
sample 903).
[00126] The following Table 1 is applicable to FIGs. 17A, 17B, 17C, and
18. The
units of permeability in Table 1 are square meters per Pascal-second (m2/Pa-
s). The
units of fracture conductivity in Table 1 are cubic meters per Pascal-second
(m3/Pa-s).
Fracture Particle gap Etched depth, Fracture
width (micrometers) dc Permeability conductivity
Case (micrometers) cl,:dz (micrometers) (m2/Pa-s)
(m3/Pa-s)
1 5 - - 2.09x10-9 1.05x10-1-
4
2 150 0:0 - 1.90x10-8 2.85x10-1-
2
3 150 37.5:37.5 - 9.42x10-8
1.41x10-13
4 150 75:75 - 2.87x10-7 4.30x10-13
5 150 150:150 - 6.00x10-7 9.00x10-13
6 150 75:0 - 2.75x10-8 4.13x10-1-
2
7 150 150:0 - 3.41x10-8 5.12x10-12
8 150 75:0 50 4.81x10-8 7.21x10-12
9 150 75:0 100 4.81x10-8 7.21x10-12
10 150 75:0 150 4.86x10-8 7.28x10-12
11 150 150:0 50 6.03x10-8 9.04x10-12
12 150 150:0 100 6.03x10-8 9.05x10-12
13 150 150:0 150 6.10x10-8 9.15x10-12
TABLE 1 - Permeabilities of various scenarios measured by DEM-LBM simulations
[00127] FIG. 17A is a schematic illustrating fluid flow in a fracture
without
proppant. The fracture was assumed to be 5 micrometers wide. This scenario is
applicable to Case 1 in Table 1.
18
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
[00128] FIG. 17B is a schematic illustrating fluid flow in a fracture
with proppant
203. The fracture was assumed to be 150 micrometers wide with the support of
the
proppant 203. This scenario is applicable to Cases 2-7 in Table 1. Cases 2-7
indicated
that the fracture permeability can be increased by a factor of tens to
hundreds with
proppant 203 support (in comparison to an unsupported fracture, such as Case
1),
depending on the particle-to-particle gaps between the proppant 203.
[00129] FIG. 17C is a schematic illustrating fluid flow in a fracture
with proppant
203 and void space generated by an acid-generating material 201. The fracture
was
assumed to be 150 micrometers wide with the support of the proppant 203. The
corroded
depth (d c) in the fracture walls (for example, due to the interaction of the
acid-generating
material 201 and the fracture face) varied from 50 micrometers to 150
micrometers.
This scenario is applicable to Cases 8-13 in Table 1. Cases 8-13 indicated
that the
fracture permeability can be further increased by the voids created by acid
erosion (for
example, through the interaction between the acid-generating material 201 and
the
fracture face).
[00130] FIG. 18 is a three-dimensional schematic illustrating fluid
flow in a
fracture with proppant 203. The fracture walls were assumed to be
perpendicular to the
y-direction, and therefore the proppant 203 was distributed across an x-z
plane. The
particle-to-particle gaps between the proppant 203 in the x-direction were
uniform (d x).
The particle-to-particle gaps between the proppant 203 in the z-direction were
uniform
(d i) . The ratio of dx to dz varied across the various cases. The arrows
signify the
direction of fluid flow. In the simulations, the fluid flow was in the general
x-direction.
[00131] It is noted that fluid transport capacity of a fracture depends
not only on
the permeability of the material inside the fracture (for example, proppant
203) but also
on the width of the fracture aperture. The product of the fracture aperture
and its
permeability is equal to the fracture conductivity (provided in the last
column of Table
1). The fracture conductivity in the cases supported by proppant 203 (cases 2-
13) is
increased by a factor of hundreds to thousands in relation to that of an
unsupported
fracture (case 1).
[00132] While this disclosure contains many specific implementation
details,
these should not be construed as limitations on the scope of the subject
matter or on the
scope of what may be claimed, but rather as descriptions of features that may
be specific
to particular implementations. Certain features that are described in this
disclosure in
19
CA 03096028 2020-10-02
WO 2019/195368
PCT/US2019/025504
the context of separate implementations can also be implemented, in
combination, in a
single implementation. Conversely, various features that are described in the
context of
a single implementation can also be implemented in multiple implementations,
separately, or in any suitable sub-combination. Moreover, although previously
described features may be described as acting in certain combinations and even
initially
claimed as such, one or more features from a claimed combination can, in some
cases,
be excised from the combination, and the claimed combination may be directed
to a sub-
combination or variation of a sub-combination.
[00133] Particular implementations of the subject matter have been
described.
Other implementations, alterations, and permutations of the described
implementations
are within the scope of the following claims as will be apparent to those
skilled in the
art. While operations are depicted in the drawings or claims in a particular
order, this
should not be understood as requiring that such operations be performed in the
particular
order shown or in sequential order, or that all illustrated operations be
performed (some
operations may be considered optional), to achieve desirable results.
[00134] Accordingly, the previously described example implementations
do not
define or constrain this disclosure. Other changes, substitutions, and
alterations are also
possible without departing from the spirit and scope of this disclosure.