Note: Descriptions are shown in the official language in which they were submitted.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
CANNABIS ROOT EXTRACT, METHOD OF MANUFACTURE, METHOD OF USE
[0001] Field of the Invention
[0002] The invention relates to a method of manufacture for an oil soluble
fraction of cannabis
root, and its use in a variety of pharmaceutical and cosmetic formulations.
[0003] Background of the Invention
[0004] Cannabis Root Oil
[0005] Although cannabis plant has numerous known pharmaceutical and
recreational uses, the
largest concentration of desirable active ingredients, and historically, the
most desirable parts of
the plant are found above ground, most particularly, the stem, leaves,
flowers, and
seeds. Cannabis root has been generally overlooked as a pharmaceutical source.
[0006] Cannabis root has relatively small concentrations of THC as compared to
other portions
of the plant. Historically, traditional Chinese medicine dating from about
2700 BC appears to
have used cannabis root as a medicinal ingredient, in a dried and ground form,
to reduce pain,
heal broken bones and recover from surgical wounds. It was also crushed to
extract juice, or
boiled to make a decoction, used as a diuretic, an anti-haemorrhagic to stop
bleeding, to ease
difficult childbirth and to reduce pain and swelling from bruises. In Roman
times, cannabis root
was boiled in water to make a preparation that relieved joint cramps, gout and
acute pain. Boiled
cannabis poultices were also used to treat inflammation, gout and other
ailments. In the early
18th century, in England, physicians used cannabis root, mixed with barley
flour as a poultice,
whereas in America, decoctions were used to treat inflammation, incontinence
and venereal
disease.
1
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0007] Cannabis root is believed to contain a complex mixture of
pharmaceutically active
ingredients, including terpenes such as antioxidant friedelin
((4R,4aS,6aS,6aS,6bR,8aR,12aR,14aS,14bS)-4,4a,6a,6b,8a,11,11,14a-Octamethyl-
2,4,5,6,6a,7,8,9,10,12,12a,13,14,14b-tetradecahydro-1H-picen-3-one), anti-
tumor epifriedelanol,
pentacyclic triterpene ketones, alkaloids, ketones, atropine, and choline.
Interestingly, cannabis
root has relatively very low amounts of the cannabinoids generally known for
cannabis' active
properties, such as cannabidiol and tertrahydrocannabinol (THC), through a
complex mixture of
literally dozens of active cannabinoids is present in small quantities.
Friedelin is thought to have
liver protecting and anti-tumour effects, while certain ketones found in
cannabis root are thought
to cause apoptosis, with certain studies suggesting they also reduce pain,
inflammation, and
bacterial infection.
0
:
_
H =,,,,
H
:
_
_
Friedelin
2
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
=-
,
,
el 77
H H H
HO
Epifriedelanol
H
-,...,.
HO
Cannabidiol
lo
CH3
OH
.
H
H3C 0 CH3
H3C
Tetrahydrocannabinol
3
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
NN
0 OH
0
Atropine
[0008] To the inventor's knowledge, cannabis root oil, or a purified oil
soluble fraction of
cannabis root, has not been used or known as a therapeutic. Typically the
cannabis root, if used,
is pulverized or powdered.
[0009] Radiation Recall
[0010] Radiation recall is a skin reaction that is a side effect of radiation
therapy when it is
combined with certain chemotherapy regimens. Radiation recall is an acute,
though often severe,
inflammatory reaction, and its cause and management is not well understood.
Radiation recall is
often presented as a rash or appearing like a severe sun burn. Current
treatment options are
limited, and are generally based on traditional anti-inflammatory drugs, such
as corticosteroids.
In certain instances, radiation recall has been known to be so severe as to
require delay of
chemotherapy and/or radiation therapy until the skin clears and can better
withstand it, which is
not desirable, since delay of chemotherapy, while good for the radiation
recall, is often bad for
the cancer treatment.
[0011] Even without radiation treatment, minor skin changes and/or skin
irritation often occurs
during, and for some time after, chemotherapy. Skin can become very itchy,
sensitive, irritated
4
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
and red, either in a localized manner (such as the forearms), or the entire
body. Localized or
entire body rashes, dryness, redness, and/or acne are also common.
[0012] Cannabis has known healing properties for skin itching. Neff et al.
reported a
preliminary observation that pruritus due to cholestatic liver disease can be
alleviated with
ingestion of 5 mg marinol (delta-9-tetrahydrocannabinol, "THC") administration
daily
(American Journal of Gastroenterology, 97, 2117-2119 (2002). Schlosburg et al.
reported that
systemic THC administration reduced scratching response in mice in a pruritus
model involving
subcutaneous administration of the mast cell degranulator compound 48/80,
which evoked an
intense, concentration-dependent scratching response (Journal of Pharmacology
and
Experimental Therapeutics, 329(1) 314-323, 2009). More recently, a study at
the University of
Colorado School of Medicine found that eight of twenty-one patients who
applied a cannabinoid
cream twice a day for three weeks completely eliminated severe itching or
pruritus. The same
group found that injection of THC in mice reduced inflammation and swelling,
as well as
significant inhibition of melanoma tumor growth
(https://www.eurekalert.org/pub releases/2017-
04/uoca-cms041717.php). Ironically, the smoking of cannabis plant has been
linked to skin
irritation, acne, rashes and redness in a small but not unsignificant
population.
[0013] Fibromyalgia
[0014] Fibromyalgia is a devastating condition characterized by chronic
widespread pain and a
heightened pain response to tactile pressure. Though the cause of fibromyalgia
is not known, it
is frequently associated with post-traumatic stress disorder, depression, and
anxiety. There is no
satisfactory treatment for fibromyalgia; serotonin-norepinephrine reuptake
inhibitors such as
duloxetine and milnacipran, and selective calcium channel inhibitors such as
pregabalin are used
to varying effect. In some cases, opioids are used to treat the pain.
5
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0015] Although the cause of fibromyalgia is unknown, most hypotheses are to a
systemic,
nervous system pain processing dysfunction, which comes as a result of hyper-
excitability of
pain processing pathways and/or under-activity of inhibitory pain pathways.
Pain-sensitive nerve
cells in the spinal cord or brain may have increased reactivity. Another
hypothesis relates to
impairments in proinflammatory cytokine signalling, or opioid signalling.
Because of this, most
treatments for fibromyalgia have focused on systemic administration of small
molecules that can
cross the blood/brain barrier, either through ingestion or injection.
[0016] Interestingly, fibromyalgia has been correlated with increased levels
of pro-inflammatory
cytokines, specifically, increased levels of interleukin-1RA, interleukin 6
and interleukin 8,
suggesting that there may be an inflammatory aspect to the disease.
[0017] Multiple Sclerosis
[0018] Multiple Sclerosis (MS) is a nervous tissue disease, involving the
demyelination of nerve
cells in the brain and spinal cord. Symptoms include motor or sensory
problems, optic neuritis,
brainstem dysfunction, either as episodes or as a gradual worsening.
Spasticity, centrally
mediated pain and painful spasms are some of its symptoms. It is believed to
be an immune-
mediated disorder, with no known cure and very few treatments. In some
isolated studies, oral
cannabis extract was suggested as effective treatment against the spasticity,
centrally mediated
pain and painful spasms associated with MS. To the knowledge of the inventor,
topical forms of
cannabis have not been used for MS, and would be counter-intuitive because MS
is known to be
disease primarily associated with nervous tissue in the brain and spinal cord.
[0019] Dysmenorrhea
6
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0020] Dysmenorrhea, also known as painful periods or menstrual cramps, are
typically a pain
in the pelvis or lower abdomen which occurs around the time that menstruation
begins,
sometimes associated with bloating, headache, mood variation, and other
symptoms. It is the
most common menstrual disorder, with some studies suggesting it affects as
many as 90% of
women. Dysmenorrhea often occurs without an underlying problem, especially in
women with
heavy periods, irregular periods, or women with low body weight. Dysmenorrhea
is also often
due to, or exacerbated by, an underlying problem such as uterine fibroids,
adenomyosis, or
endometriosis.
[0021] Likely the most often used medications for treatment of dysmenorrhea
are non-steroidal
anti-inflammatory drugs such as acetylsalicylic acid, isobutylphenylpropionic
acid, and
naproxen; or acetaminophen. Hormonal birth control often mitigates the
symptoms of
dysmenorrhea. It has also been suggested that vitamin B or magnesium
supplements may help
with the symptoms.
[0022] Dysmenorrhea is often associated with other symptoms, such as nausea,
vomiting,
headaches, dizziness, disorientation, diarrhea, constipation, hypersensitivity
to sound, light, smell
or touch, and fatigue.
[0023] It would be desirable to have novel, effective treatments for
dysmenorrhea, specifically,
menstrual pain associated with uterine fibroids, adenomyosis, or
endometriosis.
[0024] Cancer
[0025] Cancer is a large family of diseases related to abnormal and/or
unregulated cell growth,
which may spread to other parts of the body. It is one of the most important
diseases of our time,
effecting an estimated 90 million people, with approximately 14 million new
cases every year.
7
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
Cancers have a wide variety of causes, epidemiologies, and physiologies, and
are typically
classified by the type of cell from which the tumor/cancerous cell originates.
[0026] Cancer treatments can be quite severe and invasive, and include surgery
to remove the
cancerous cells; chemotherapy utilizing a variety of agents, which typically
act either by killing
all rapidly dividing cells, or in a more targeted manner by acting at
specific, known, molecular
differences between cancer cells and non-cancerous cells; immunotherapy, which
is loosely
based on triggering or heightening the body's immune response to the cancerous
cells; and
radiation therapy, which utilizes localized ionizing radiation in the region
of the tumor.
Unfortunately, most treatments err on the side of broadness, and end up
killing many healthy
cells, either surrounding the tumor tissue, or resembling the tumor tissue in
some way. For
example, many chemotherapeutic drugs act by killing cells during the
reproductive cycle, leading
to the killing of the rapidly dividing cells in the tumor, but also killing
other rapidly dividing
cells, such as stem cells.
[0027] Certain types of cancers, such as non-Hodgkin lymphoma, have been
observed to express
higher levels of cannabinoid receptors type 1 and type 2, suggesting that
therapies using
cannabinoid receptor ligands may have efficiency in reducing tumor burden in
certain types of
malignant lymphomas. Cannabinoid receptor type 2 has been associated with cell
growth and
reproduction, with one study suggesting that cannabinoid receptor type 2
agonists may be able to
inhibit cell growth and arrest cell reproduction at the GO/G1 stage.
[0028] Cancer treatments are wrought with often debilitating side effects,
which can include
pain, nausea, vomiting, and appetite suppression.
[0029] It would be desirable to have novel, effective treatments for cancer,
either to be used
alone, or in combination, preferably synergistic combination, with
conventional cancer
8
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
treatments. It would also be desirable to have novel, effective ways to
decrease the side effects
of conventional cancer drugs.
[0030] Vaginal Yeast Infections
[0031] Vaginal yeast infection, or candida vulvovaginitis, is a common
affliction resulting from
an excessive growth of yeast, typically Candida alb/cans but sometimes other
Candida, or
Saccaromyces cerevisiae. Symptoms of vaginal yeast infection include vaginal
itching, burning
or pain while urinating, vaginal discharge, pain or discomfort during sexual
intercourse,
localized inflammation, and redness.
[0032] Vaginal yeast infection is usually treated with intravaginally
administered anti- fungal
agents, such as clotrimazole, nystatin, butoconazole, miconazole, or
terconazole; or using an oral
dose of fluconazole. It has been reported that up to 40% of women with vaginal
yeast infections
seek alternative treatments, such as herbal preparations, probiotics,
cleansing diets, and gentian
violet. The majority of studies of such alternative treatments are
inconclusive or negative as to
benefits.
[0033] Bacterial vaginosis, sometimes called vaginal bacteriosis, is another
common affliction,
resulting from excessive growth of bacteria in the vagina. Symptoms of
bacterial vaginosis
include increased vaginal discharge, and pain or burning while urinating.
Bacterial vaginosis can
increase the risk of infection by sexually transmitted diseases, and can
increase the risk of early
delivery among pregnant women.
[0034] Bacterial vaginosis is usually treated with an antibiotic, such as
clindamycin or
metronidazole, either orally or intravaginally. Recurrence rates after
antibiotic treatment are
extremely high.
9
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0035] Vaginitis is a broad term describing any inflammation of the vagina. It
is typically a
symptom of an infection of the vagina, such as a vaginal yeast infection or
bacterial vaginosis. It
may also be caused by trichomoniasis.
[0036] It would be desirable to have novel, effective treatments for vaginal
yeast infection,
bacterial vaginosis and vaginitis.
[0037] Menopause
[0038] Menopause occurs in most women between 49 and 52 years of age. It is
generally
defined as having occurred when a woman has not had any vaginal bleeding for a
year. For a
time leading up to menopause, a woman's periods may become irregular, either
lighter or
heavier, or longer or shorter. During this time, women often experience hot
flashes, which
typically last from 30 seconds to 10 minutes, and may be associated with
shivering, sweating,
and reddening of the skin. Other symptoms occurring either in the time leading
up to
menopause, or post-menopause, include mood changes, vaginal dryness, and
trouble sleeping.
Menopause is associated with, and likely triggered by, a decrease in the
ovaries' production of
estrogen and progesterone.
[0039] Likely the most often used medications for treatment of the symptoms of
menopause or
pre-menopause include menopausal hormone therapy, clonidine, SSR1s, or
gabapentin. Non-
medicinal, behavioural changes that may improve these symptoms include
avoiding smoking,
caffeine and alcohol, and increasing exercise.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0040] It would be desirable to have novel, effective treatments for certain
effects of menopause
and pre-menopause, such as for improvement of mood, relieving of dryness,
aiding in sleep, and
calming of hot flashes.
[0041] Skin Care
[0042] Skin care is a billion dollar industry. A wide range of skin
moisturizing formulations are
known. Literally thousands of such products are sold, for moisturizing dry
skin, removing fine
lines and wrinkles, and obtaining fresh and youthful looking skin. A wide
range of anti-aging
serum formulations are known. These are intended for maintaining the firmness,
suppleness,
plumpness and pliability of skin, as well as decreasing the appearance of
wrinkles. The total skin
care market is estimated to be $116.3 billion in 2015, with some estimating
the total market to be
$180 billion by 2024. In one survey in 2016, 57 percent of U.S. women said it
was important to
buy all-natural skin care products. The U.S. anti-aging skin care market was
estimated to
generate about 2.11 billion U.S. dollars in 2013. Some of the manufacturers
and suppliers of
skin moisturizing formulations include Dow Chemicals, L'Oreal SA, Unilever
PLC, Beiseidorf
AG, Colgate Palmolive, Procter & Gamble, Estee Lauder, Johnson and Johnson,
and Avon
Product Inc. Face moisturizing products represent more than 50% of the skin
moisturizing
products sold.
[0043] Female Sexual Dysfunction
[0044] Sexual dysfunction has been a persistent and relatively unaddressed
problem, especially
in an aging, sexually active population. Female sexual dysfunction, in
particular, has not
received as much attention or treatment as male sexual dysfunction, for which
a variety of
treatments currently exist.
11
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0045] Female sexual behaviour is typically described in a sexual response
cycle consisting of
desire, excitement, orgasm, and resolution. Female sexual arousal disorder
(FSAD) is the
persistent or recurrent inability to attain, or to maintain, sufficient sexual
excitement, a lack of
genital response (such as lubrication and swelling), and/or lack of other
somatic responses.
Female sexual arousal disorder is one form of female sexual dysfunction, and
is associated with
the excitement phase, though it typically also results in an inability to
reach orgasm and
resolution.
[0046] It is believed that a certain amount of female sexual dysfunction is
related to vascular
insufficiency which results in insufficient swelling, specifically, in the
smooth muscle of the
muscularis portion. Certain vasodilators have been implicated in the mediation
of vaginal
vasodilation and the formation of lubricating fluid during sexual arousal. It
may also be a
nervous dysfunction: the vagina has significant nerve fibers, with
significantly more nerve fibers
in the distal areas of the vagina compared to the more proximal parts, with
the anterior wall
showing a denser innervation than the posterior wall.
[0047] Female sexual dysfunction may also be related to insufficient
lubrication: the vaginal
canal is lubricated primarily from a transudate originating from the
subepithelial vascular bed,
passively transported through intercellular channels. Additional moistening
during intercourse
comes from secretion from Bartholin's glands.
[0048] Estrogen is also believed to effect maintenance and function of female
genitalia, with
estriol stimulation, vaginal lubrication, and thickness of the vaginal wall
all being estrogen-
dependent. Vaginal dryness can be controlled by estrogen replacement therapy.
[0049] The clitoris is also believed to play a major role during sexual
activity in that it is not only
part of what makes the sexual act enjoyable for the woman but also enhances
her response to
12
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
coitus upon clitoral stimulation. Clitoral stimulation may induce local
autonomic and somatic
reflexes causing vaginal vasocongestion, engorgement, and subsequent
transudation, lubricating
the introital canal making the sexual act easier, more comfortable, and more
pleasurable. The
more stimulation, the higher the level of arousal and the easier it is to
further increase
stimulations.
[0050] It is believed that a multisynaptic circuit of neurons may be involved
in clitoral
neurological control. Autonomic innervation of the clitoris passes from the
pelvic and
hypogastric nerves to the clitoris through the urogenital diaphragm; pelvic
nerve stimulation
results in clitoral smooth muscle relaxation and arterial smooth muscle
dilation.
[0051] Female sexual dysfunction can included disorders of desire/libido,
disorders of arousal,
pelvic pain disorders, and inhibited orgasm. Patient surveys estimate that 18-
76% of adult
women have such complaints during sexual activity.
[0052] THC
[0053] THC, or Delta-9-tetrahydrocannabinol, is the principal psychoactive
constituent of
cannabis. It is a partial agonist of cannabinoid receptors CB1 and CB2.
Because THC is an
illegal drug in many countries, medical research has been limited and often
anecedotal. The
American Cancer Society has reported that patients with kidney cancer have
required less pain
medication when it was combined with cannabis extracts containing THC. Smoking
cannabis
has been found to alleviate nausea and vomiting in chemotherapy patients.
Certain patients with
suppressed appetite, including patients taking HIV drugs, have reported that
smoking cannabis
.. has improved and promoted food intake. Cannabis has also been documented as
a pain relief
agent, when inhaled.
13
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0054] Surprisingly, recent studies have suggested that cannabinoids may
inhibit cancer cell
proliferation and induce cancer cell apoptosis. A 2013 Korean study has
suggested that THC
may be useful in treating stomach cancer, even drug resistant stomach cancer,
by inducing
apoptosis in the cancer cells. There are studies suggesting cannabinoids
reduce expression of
vascular endothelial growth factor (VEGF) in cancer cells, reducing the tumor
blood supply. It
appears THC and CBD may have anti-metastatic properties, though the mechanism
of action is
not completely understood.
[0055] THC has known anti-inflammatory properties for skin itching. Neff et
al. reported a
preliminary observation that pruritus due to cholestatic liver disease can be
alleviated with
ingestion of 5 mg marinol (THC) administration daily (American Journal of
Gastroenterology,
97, 2117-2119 (2002). Schlosburg et al. reported that systemic THC
administration reduced
scratching response in mice in a pruritus model involving subcutaneous
administration of the
mast cell degranulator compound 48/80, which evoked an intense, concentration-
dependent
scratching response (Journal of Pharmacology and Experimental Therapeutics,
329(1) 314-323,
2009). More recently, a study at the University of Colorado School of Medicine
found that eight
of twenty-one patients who applied a cannabinoid cream twice a day for three
weeks completely
eliminated severe itching or pruritus. The same group found that injection of
THC in mice
reduced inflammation and swelling, as well as significant inhibition of
melanoma tumor growth
(https://www.eurekalert.org/pub releases/2017-04/uoca-cms041717.php).
Ironically, the
smoking of cannabis plant has been linked to skin irritation, acne, rashes and
redness in a small
but not unsignificant population.
[0056] THC-containing topical medications, including lotions, balms and oils,
are known and
utilized for localized relief of pain, soreness and inflammation.
[0057] CBD
14
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0058] Cannabidiol is one of the active ingredients found in Cannabis. It is
believed to have
antioxidant, anti-inflammatory, anti-anxiety and anti-epileptic properties.
[0059] Ashwagandha Oil
[0060] Ashwagandha oil is an essential oil made from the root of the
ashwagandha plant, also
known as Withania Somnifera. The root is also known as Indian Ginseng, Winter
Cherry, or
poison gooseberry. Ashwagandha oil is a steam distilled extract of the root of
the plant.
[0061] Ashwagandha oil has been used and known in the Ayurvedic system of
medicine for
centuries, and has been used to increase immunity to infection and disease,
for treatment of
arthritis, cramps and pains, and for its analgesic effects. The oil has been
used as an anti-
depressive, and in massage oil for relief of stress and anxiety. As a member
of the nightshade
family, oil of Ashwagandha has also been used in the treatment of insomnia,
and is known for its
potential to promote sleep. Made into a tonic, it has been used for
rheumatism, insomnia,
arthritis, impotence, fatigue, and depression. It is rumored to be useful as
an anti-stress,
aphrodisiac, astringent, antioxidant, diuretic, stimulant, antibacterial, anti-
tumor, anti-
inflammatory, anti-carcinogenic, cardio-protective, immunomodulatory and anti-
depressant.
Ashwaganda root extract has been seen to have anticonvulasant effects;
pretreatment with root
extract is believed to have an anti-parkinsonian effect. It is claimed to be
the most powerful
aphrodisiac known to humanity; believed to be useful for treating loss of
libido, impotence,
premature ejaculation, erectile dysfunction, as well as, in women, to
strengthen the uterine walls,
ovaries and ligaments to support pregnancy. Ashwagandha has been found to
stimulate nitric
oxide production. Nitric oxide is believed to be involved in the mediation of
clitoral and arterial
smooth muscle dilation.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0062] Ashwaganda oil contains alkaloids, including anaferine, isopelletierine
(1-(1-Methy1-2-
piperidinyl)acetone), anahygrine, cuscohygrine, psudeotropine, tropanol, and
pellertierine,
steroidal lactones, including withaferins and withanolides, as well as
saponins. It is
commercially available.
0
N N
Anaferine
0
Lie
Isopelletierine
y ,
=
H
Anahygrine
0
\ /1
ri
CH,
C H-
Cuscohygrine
16
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
I /
Psudeotropine
--
H
=
-
Tropanol
,
_ 11
0
H
Pellertierine
[0063] Anaferine is known as an immunomodulatory which induces interferon
alpha.
Suscohygrine is known as a CNS stimulant.
[0064] To the inventor's knowledge, THC has not been used topically in a
female sexual
lubricant, or as a treatment of female sexual dysfunction. To the inventor's
knowledge
ashwagandha oil (an extract of the root of the ashwagandha plant) has not been
used topically in
17
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
a female sexual lubricant, or as a topical treatment of sexual dysfunction.
The combination of
THC and ashwagandha oil has accordingly also never been used. The combination
of THC,
ashwagandha oil, and cannabis root oil has accordingly also never been used.
[0065] Hyaluronic Acid
[0066] Hyaluronic acid (HA) is found in the basal layer of the epidermis,
where proliferating
keratinocytes are found. It appears to play an important role in the normal
epidermis, and may
function in the re-epithelization process. It serves as an integral part of
the extracellular matrix
of basal keratinocytes and may have both a free-radical scavenging function
and a role in
keratinocyte proliferation and migration.
[0067] HA has water retention properties and certain formulations of HA are
commercially
available as Restylane 0, an injectable formulation used for lip volume
enhancement, to
diminish wrinkles and aging lines, and for filling aging-related facial
hollows, such as those
under the eyes) and for contouring of the chin, forehead, and nose.
[0068] HA is used in anti-aging serums.
[0069] Arnica Montana
[0070] Extract can be made from arnica montana flowers, using supercritical
fluid extraction
with natural carbon dioxide. The extract can be made into an essential oil by
adding a lipid.
Arnica montana extract is believed to have analgetic, anti-arthritic,
antibacterial, anti-
inflammatory, anti-septic properties. It is used for muscle soreness,
fibrositis, relief of muscle
and joint pain.
[0071] Menthol
18
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0072] Menthol is a known compound, either made synthetically, or extracted
from mentha
arvensis (wild mint). Its most common production method is by freezing
peppermint oil, then
separating the resultant crystal of menthol by filtration. Natural menthol is
most abundantly
.. found in the L configuration (-)-menthol. It provides a cooling sensation
when inhaled, eaten or
applied to the skin, and provides analgesic properties through selective
activation of K-opioid
receptors. Menthol has been used as a pain reliever.
[0073] Clary Sage
[0074] Clary sage (Salvia sclarea) seeds provide an essential oil widely used
in perfumes and as
a muscatel flavoring for vermouth, wine and liqueur. It has use in traditional
medicine, and is
believed to maintain good health of the uterus. Some literature has suggested
it may be useful in
treatment of uterine and ovarian cancers, by regulating certain hormones such
as estrogen. Some
traditional medicine use includes stimulating the opening of obstructed
menses, curing dizziness
and mental irritation during menses, and alleviation of symptoms of post-
menopausal syndrome.
[0075] It is believed that clary sage essential oil may reduce the symptoms
and negative effects
associated with menstruation like cramping, bloating, mood swings, cravings
for food. As usual
with 'traditional' medicines, there is varied and largely unsupported
literature suggesting clary
sage essential oil may have antidepressant, anticonvulsant, antispasmodic,
antiseptic,
aphrodisiac, astringent, bactericidal, carminative, deodorant, digestive,
emmenagogue, euphoric,
hypotensive, nervine, sedative, stomachic, and uterine effects.
[0076] Geranium
[0077] Geranium essential oil is an essential oil obtained from Pelargonium
roseum. It is
primarily cultivated for its scent and flavoring agent. However, it has a
history in traditional
19
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
medicine use, and believed to have astringent, hemostatic, cicatrizant,
cytophylactic, diuretic,
deodorant, styptic, tonic, vermifuge, and vulnerary properties. It is
believed, as an aromatherapy,
to aid in skin care, balance hormones, relieve stress and depression, reduce
inflammation and
irritation, alleviate the effects of menopause, improve circulation, benefit
dental health, boost
kidney health, and reduce blood pressure. Sometimes referred to as "women's
oil", geranium
essential oil has a traditional use in treating feminine problems like
excessive menstrual flow,
vaginal infections, and discharges during menopause. For example, it is
believed that massaging
the lower abdomen and back with geranium essential oil helps in treating
painful menstration,
excessive bleeding, mood fluctuations, fatigue and anxiety during menses and
menopause. It is
believed to be provide calming, soothing, tonic and stimulant effect,
relieving pain, stress and
mood fluctuations. It is also used as a flavouring agent for food and pipe
tobacco, and in massage
oil. It is believed to improve blood circulation just below the surface of the
skin and aids in
distribution of melanin. Geranium essential oil may have astringent
properties.
[0078] Chamomile
[0079] Chamomile essential oil is an essential oil obtained from Chamaemelum
nobile . It is
used as a scent, a flavor, and is believed to reduce stress and aid in sleep
when used as an
aromatherapy. Chamomile essential oil is believed to be useful in the
treatment of cracked
nipples that develop during breastfeeding; it may also be a treatment to
candida vulvovaginitis.
[0080] Lavender
[0081] Lavender oil is the essential oil of Lavandula angustifolia. It is
believed to have
antiseptic, antifungal, and anti-inflammatory properties, but is most used as
a fragrance. It
consists primarily of monoterpeneoids and sesquiterpeneoids, and can include:
the
monoterpenols linalool, alpha-terpineol, gamma-terpineol, borneol, isoborneol,
terpinen-4-ol,
nerol, and lavandulol; the terpene esters linalyl acetate, geranyl acetate,
neryl acetate, octane-3-y1
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
acetate, and lavandulyl acetate; the monoterpenes (E)-beta-ocimene, (z)-beta-
ocimene,
camphene, beta-pinene, alpha-pinene, myrcene, and beta-phellandrene; the
terpene oxide 1,8-
cineol; the sesquiterpenes beta-caryophyllene, beta-farnesene, germacrene, and
alpha-humulene;
and the keytones camphor, 3-octanone, and cryptone. Lavender oil is used in
aromatherapy to
.. decrease stress and anxiety levels in patients. It is believed to be useful
in treatment of Candida
vulvovaginnis (vaginal yeast infection) and vaginal discharges. Lavendere is
used in
commercially available mosquito repellants, skin cleansers, toners, serums and
creams.
[0082] Rosemary
[0083] Rosemary essential oil is an extract from rosmarinus officinalis, an
herb known primarily
for its culinary purposes but containing a number of phytochemicals such as
rosmarinic acid,
about 10-20% camphor, caffeic acid, ursolic acid, betunlinic acid, carnosic
acid, and carnosol.
.. [0084] Basil
[0085] Basil, or Saint-Joseph's-wort, is most frequently used as a culinary
herb. An essential
oil of European basil is reported to contain high concentrations of linalool
and methyl chavicol,
as well as other ingredients such as 1,8-cineole, eugenol and myrcene. Basil
has deep roots in
many religious and cultural ceremonies, used to sprinkle or make holy water,
and is even thought
to protect against scorpions. It is also thought to be a mental stimulant,
which aids in
concentration; it is believed to have anti-depressive properties.
[0086] Basil oil is also believed to have anti-inflammatory properties, making
it useful as a skin
remedy for irritations,small wounds and sores. It has a soothing and relaxing
effect that helps
while dealing with eczema. Basil oil contains vitamin C that boosts skin cell
metabolism; it also
is believed to maintain skin collagen. It can be useful as a remedy for a
number of chronic skin
conditions such as acne. Acne can result from overactive sebaceous glands.
Accumulation of
21
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
excess sebum and dirt manifests the growth of bacteria and thereby produce
painful red swellings
on your face. Applying a face pack using basil oil on a daily basis is
beneficial for acne-inflamed
skin due to its anti-inflammatory and antibacterial properties.
[0087] Frankincense
[0088] Frankincense is an aromatic resin used in incense and perfumes, and is
obtained from
trees of Boswellia burseraceae. It contains an acid resin having the formula
C20H3204, a gum,
3-acetyl-beta-boswellic acid, alpha-boswellic acid, 4-0-methyl-glucuronic
acid, incensole
acetate, phellandrene, and (+) cis and trans olibanic acids. Frankincense
essential oil is obtained
by steam distillation of the dry resin, and contains monoterpenes,
sesquiterpenes,
monoterpenoles, sesquiterpenolsõ and ketones, such as alpha-pinene, limonene,
alpha-thujene,
and beta-pinene. Frankincense essential oil and/or resin appears to have anti-
mutagenic and
apoptotic properties in vitro and in cell culture, and is thought to
potentially have anti-
inflammatory and anti-cancer potential. One conference paper (Salmani, 2015)
has suggested
that frankincense, particularly its primarily biologically active component, 3-
0-acety1-11-keto-
beta-boswellic acid, may have cytotoxicity towards high grade serous ovarian
cancer cell lines.
[0089] Frankincense oil is also believed to be a cytophylactic, promoting
regeneration of healthy
cells and keeping existing cells and tissues healthy. Frankincense oil is also
believed to have
astringent capabilities, and may be useful as an anti-aging agent for skin. It
may be helpful in
elimination of sun spots, removing micro-wrinkles around the eyes and cheeks,
and generally
tone and tighten skin.
[0090] Carrot Seed
22
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0091] Carrot seed oil is believed to be high in antioxidants. It also
contains the sesquiterpene
alcohol carotol, vitamin E and vitamin C. Carotol is believed to have
antifungal, herbicidal and
insecticidal properties.
OH
Carotol
[0092] Cypress
[0093] Essential oil of the Mediterranean cypress (Cupressus sempervirens L.)
has been used in
traditional medicine as a treatment of stomach pain, diabetes, inflammation,
toothache, and
laryngitis. It has also been used as a contraceptive. Such uses, while
documented, are neither
well understood nor known through rigorous scientific methodology to be
effective. The main
components of cypress essential oil appear to be alpha-pinene, 6-3-carene,
limonene, and alpha-
terpinolene. In vitro studies suggest that cypress essential oil may have
antimicrobial and
antibiofilm activity.
[0094] Tea Tree
[0095] Tea tree oil, also known as melaleuca oil or ti tree oil, is an
essential oil from the leaves
of the tea tree, Melaleuca alternifolia. It contains a wide variety of
compounds, including
terpinen-4-ol, its major component, as well as gamma-terpinene, alpha-
terpinene, terpinolene,
alpha-terpineol, alpha-pinene, and p-cymene. It may also contain 1,8-cineole.
Though toxic
when taken by mouth, it is used topically in certain folk medicines, and is
claimed to be useful in
treatment of skin conditions such as lice, herpes, scabies, insect bites,
dandruff, acne, and skin
23
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
fungal or bacterial infections such as athlete's foot. As with many
traditional medicines, there is
little evidence to support these uses. There have been traditional medicine
studies suggesting it
may be useful in treatment of bacterial vaginosis.
[0096] Thyme
[0097] Thyme essential oil contains 20-54% thymol, an antiseptic used in
various commercially
produced mouthwashes and hand sanitizers. Thyme oil also contains p-cymene,
myrcene,
borneol, and linalool. Before modern antibiotics, thyme essential oil was used
to medicate
bandages and to treat toenail fungal infections. There have been some studies
suggesting that
thyme oil may be useful for treating bacterial vaginosis, and that thymol may
prevent the growth
and biofilm formation of Gardnerella vaginal/s.
[0098] Manuka
[0099] Manuka essential oil is obtained from the leaves of Leptospermum
scoparium, a plant
native to Australia and New Zealand. It has been used in traditional Maori
medicine for treating
head colds, to soothe stiff muscles and aching joints, and for urinary
complaints. It is believed to
have antimicrobial efficacy, especially against gram-positive bacteria, and
against certain
antibiotic-resistant strains.
[0100] Helichrysum
[0101] Helichrysum oil is an essential oil of the blossoms of Helichrysum
nalicum, often also
called immortelle or curry plant. Often used in perfumes, it has an intense
fragrance, and anti-
inflammatory, fungicidal and astringent properties. It has been used to soothe
burns and raw
chapped skin. As with many traditional herbal remedies and medicines, there is
little evidence,
but some suggestions of usefulness for a wide variety of conditions: it is
believed to have anti-
24
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
fungal, anti-viral, and anti-inflammatory properties, and is used to treat
bruises, burns, acne,
allergies, exzema, broken veins, stretch marks, inflammation, spots, warts,
wounds, chronic
dermatitis, muscular aches and pains, rheumatism, sprains, whooping cough,
headaches,
pulmonary spasms, stress-related conditions, depression, psoriasis, stomach
cramps, sinus
infection, phlebitis, hematoma, viral colitis, gallbladder infection,
sciatica, and sunburns.
[0102] Vitex Berry
[0103] Vitex berry essential oil is an essential oil of the fruit of the Vitex
agnus-castus, or
chasteberry plant. It has long been believed to be an aphrodisiac, and there
have been
occasional, largely non-clinical studies suggesting it may be useful for
management of
premenstrual stress syndrome, including premenstrual dysphoric disorder and
latent
hyperprolactinaemia. The berries are the most popular part of the plant used
and contain a wide
range of potentially active constituents, including essential oils, iridoids,
and flavonoids. In
humans, it has been shown (at doses of 120 mg/d) to reduce levels of FSH and
increase LH
resulting in decreased estrogen and increased progesterone and prolactin
levels. One proposed
mechanism of action is that this herb (doses of approximately 480 mg/d) causes
a decrease in
prolactin, which leads to a reversal of LH suppression allowing full
development of the corpus
luteum, increasing progesterone levels, and reducing symptoms of PMS. However,
chaste berry
also appears to have dopamine-agonistic properties at higher doses. A double-
blind study
involving 217 women tested Vitex against placebo for the treatment of
premenstrual syndrome.
For a period of 3 months, 105 women took chaste berry 300 mg tablets three
times daily,
whereas 112 women took placebos. The results of the study showed a dramatic
improvement at
the end of the first cycle for both groups with relative stability over the
remaining two cycles.
Vitex improved the symptom "feel jittery or restless," but there was a
difference observed
between placebo and the herb for other symptoms. A study compared the efficacy
of chaste berry
to pyridoxine (B6) in the treatment of PMS over three menstrual cycles. Ninety
participants were
given one capsule of Agnolyt (each capsule containing 3.5 to 4.2 mg of dried
chaste berry), and
one placebo capsule daily. The other 85 participants were given placebo twice
daily from day 1
to 15 and then 100 mg bid of pyridoxine from days 16 to 35. When assessed by
patients and the
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
investigators, the chaste tree group achieved a significantly greater
improvement in typical PMS
complaints such as breast tenderness, edema, inner tension, headache,
constipation, and
depression compared with pyridoxine. Both preparations were well tolerated
with only mild
reactions reported in a few patients. Five patients from the chaste tree group
became pregnant.
Another double-blind, placebo controlled study examined the tolerability and
efficacy of chaste
berry extract for premenstrual mastalgia. The treatment or placebo was given
over three
menstrual cycles. Mastalgia during at least 5 days of the cycle before the
treatment was the strict
inclusion criteria. The results showed the intensity of the cyclical breast
pain diminished in the
chaste berry group and the herb was well tolerated. In a prospective,
multicenter trial the
efficacy of a chaste berry was investigated in 43 patients with PMS. The
patients took 20 mg
Vitex extract daily for three menstrual cycles. Symptoms in three
posttreatment cycles were
compared with baseline cycles before administration of the herb. A menstrual
distress
questionnaire was the tool used for self-assessment. At the end of the study,
symptoms were
reduced in the late luteal phase by 47.2%. Although symptoms gradually
returned after treatment
.. cessation, a difference from baseline remained for up to three cycles. A
multicentric open trial
investigated the efficacy and tolerance of Vitex in 1634 patients suffering
from PMS. A specific
questionnaire was developed for determining the effect of chaste berry on the
four characteristic
PMS symptom complexes: depression, anxiety, craving, and fluid retention.
After three
menstrual cycles, 93% of patients reported a decrease in the number of
symptoms or symptom
complexes or even cessation of PMS complaints and 85% of physicians rated the
treatment as
good or very good. The severity and frequency of breast pain reduced after 3
months. The
majority of patients assessed the tolerance of Vitex as good or very good.
Adverse drug reactions
were suspected in only 1.2% of patients, but none were serious. A randomized,
double-blind,
placebo-controlled trial compared chaste berry with placebo in 170 women with
premenstrual
syndrome over three menstrual cycles. Women undertook self-assessment of
irritability, mood
alteration, headache, breast fullness, and other menstrual symptoms and were
also assessed for
changes in clinical global impression. The study showed that chaste berry was
an effective and
well-tolerated treatment for the relief of PMS symptoms in 52% of the trial
participants
compared with 24% placebo. As with all herbal remedies, a variety of chaste
berry preparations
are used that can differ substantially in terms of, for instance,
concentration of active ingredients
or bioavailability. Surveys of members of the National Institute of Medical
Herbalists, and the
American Herbalists Guild, showed that the tincture is the most popular
preparation among
26
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
herbalists, in a dose of 3 to 5 mL qd¨bid, but fluid extracts and powdered
herb preparations are
also used. Chaste berry is approved by the German Commission E for
irregularities of the
menstrual cycle, PMS, and mastodynia.
[0104] Jojoba
[0105] Jojoba oil, a liquid or wax produced in the seed of the Simmondsia
chinensis
(jojoba)plant, is known to contain Vitamin E and B, as well as antioxidants
and minerals like
chromium, copper, and zinc, all of which are believed to nourish and protect
the skin. Jojoba oil
is known to be similar to human skin oil, and is excellent as penetrating skin
and smooths easily.
It is commonly used as a replacement for whale oil in the cosmetics industry.
[0106] Avocado
[0107] Avocado oil is an edible oil pressed from the fruit of the Persea
Americana (avocado). It
is most used as a cooking oil and as a food ingredient. It is believed to be
an excellent skin
nourishing product, boosting collagen production in skin and promoting soft,
youthful skin and
delaying aging.
[0108] Apricot
[0109] Apricot oil, obtained from the kernel of the Prunus armeniaca
(apricot), contains vitamin
E, A, unsaturated fatty acids, and vitamin C, which promotes collagen
production. Vitamin A is
believed to repair UV-related skin damage and smooths skin to reduce wrinkles,
fine lines and
rough skin. Vitamin E is believed to combat free-radical damage and
inflammation.
Unsaturated fatty acids are believed to enhance skin elasticity.
[0110] Tumeric
27
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0111] Tumeric (Curcuma longa) is a plant of the ginger family. Turmeric oil,
containing
curcumin, have received a fair amount of press recently as a potential
pharmaceutical. It is
typically ingested. It has been used in Ayurvedic practices to treat internal
disorders, such as
indigestion, throat infections, common colds, or liver ailments, as well as
topically to cleanse
wounds or treat skin sores.
[0112] Juniper Berry
[0113] Juniper berry is the female seed cone produced by Juniperus communis.
Its oil is
believed to have antibacterial, antiseptic, astringent and detoxifying
properties, and is believed
useful in the treatment of acne, weeping eczema, dermatitis and psoriasis.
[0114] Rose oil
.. [0115] Rose oil, the essential oil extracted form the petals of various
types of rose, is widely
used for its scent. It is believed to have antioxidant effect.
[0116] Jasmine
[0117] Jasmine oil also has antioxidant effect.
[0118] Sandalwood
[0119] Sandalwood oil is believed to have antiseptic and antimicrobial
properties, and is often
used to provide relief to itching and redness caused by exzema, rosacea and
psoriasis.
[0120] Copaiba
28
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0121] Copaiba oil is a steam distilled extract of an oleoresin exudate
obtained from the trunk of
Copaifera tree. It has been traditionally used in folk medicine to impart
knowledge and ward off
hexes, it is believed to have anti-inflammatory, anti-tumor, anti-tetanus,
antiseptic, and
antihemorrhagic properties.
[0122] Pomegranate
[0123] Pomegranate seed oil comprises over 65% punicic acid, as well as
palmitic acid, stearic
acid, oleic acid, and linoleic acid. Punicic acid is believed to reduce skin
inflammation,
enhances skin repair, and hydrates the skin. Pomegranate oil is believed to
stimulate
keratinocyte proliferation, helping skin retain its youthful appearance by
creating new skin cells.
Pomegranate seed oil also contains a human compatible form of pro-estrogen
which is believed
to enhance skin texture by supporting hormonal balance.
[0124] Sweet almond
[0125] Sweet almond oil, derived from the seeds of Prunus dulcis var. dulcis
is a rich source of
of minerals like calcium, zinc, manganese, phosphorus, magnesium, and
potassium. Almonds
contain L-carnitine and phenylalanine, the chemicals that improve cognitive
function and
enhance mood. The phenylalanine in this quick-absorbing oil is carried through
the skin to
support the secretion of mood-boosting hormones, including dopamine and
adrenaline. Sweet
almond oil is believed to provide topical and systemic anti-inflammatory
effects, and is believed
to be useful in treatment of eczema, psoriasis and rosacea.
[0126] Ginger
[0127] Ginger oil or ginger paste is often often topically massaged on aching
muscles to remove
muscle strain. It is further believed that regular use of ginger leads to the
reduction of
29
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
prostaglandins, which are the compounds associated with pain. Therefore,
ginger helps in pain
relief Extract of ginger is also used in certain traditional medicines to aid
in the reduction of
inflammation.
[0128] Cinnamon
[0129] Cinnamon oil is believed to have anti-bacterial and anti-fungal
properties, possibly due
to its o-methoxycinnamaldehyde content. It is believed to be effective in
fighting multi-drug
resistant bacteria in particular.
[0130] Clove
[0131] Clove bud oil is believed to have analgesic, antiseptic, antispasmodic,
anti-neuralgic,
carminative, anti-infectious, disinfectant, insecticidal, stimulant,
stomachic, uterine, and tonic
properties. It has been shown to be effective against planktonic cells and
biofilms of
Staphylococcus aureus and is therefore useful in the topical treatment of
acne.
[0132] Cardamom
[0133] Cardamom oil is an oil extracted from the aromatic pod of cardamom, a
seed from the
Zingiberaceae Elettaria and Zingiberaceae Amomum plants. It is believed to be
useful in
treating muscular spasm (cramps and pulls in muscle fibers) and respiratory
spasms (whoopinc
cough or pertussis, asthma, etc.). The rich glutinous extract comprises a
number of essential
volatile oils like pinene, methyl eugenol, sabinene, geraniol, linalyl
acetate, myrcene, nerol,
phellandrene, citronellol, linalool, limonene, a-terpineol acetate, 1, 8-
cineole, terpinene, a-
terpineol, p-cymene, terpinen-4-oil, terpinolene, and trans-nerolidol. It has
numerous benefits for
our health, skin and hair.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0134] Bergamot
[0135] Bergamot oil is believed to increase hormone secretions, and lessen the
sensitivity of
nerves that create pain. It is used to reduce symptoms of sprains, muscle
aches and headaches,
and to relieve tension.
[0135] Fennel
[0136] Fennel oil is believed to have anti-fungal properties, and can be used
to treat yeast and
fungal infections.
[0137] Lactobacillus
[0138] Leucidal Liquid SF (Active Micro Technologies, NC) is a commercially
available
Lactobacillus ferment, marketed as a natural preservative. It is derived from
radishes fermented
with Leuconostoc kimchi, a lactic acid bacteria that has been traditionally
used to make kimchi.
[0139] Fermented Coconut Fruit
[0140] AMTicide 0 Coconut (Active Micro Technologies, NC) is a commercially
available
moisturizer and conditioner for hair and skin care applications, with anti-
fungus activity. It is
developed by fermenting coconut fruit with Lactobacillus.
[0141] Lysolecithin
[0142] ECOGEL0 (Lucas Meyer Cosmetics, a division of International Flavors &
Fragrances
Inc, NY) is a commercially available gelling-emulsifying agent made from
lysolecithin,
sclerotium gum, xanthan gum and pullulan.
31
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0143] Suppositories
[0144] Suppositories are a known, effective dosage form for the administration
of medicinal
ingredients. They are a solid dosage form of drug, inserted into the rectum,
vagina, or urethra.
Suppositories are formulated such that they dissolve or melt at body
temperature, and
accordingly, once inserted, they provide local or, by diffusion into the
tissue or bloodstream,
systemic, effects.
[0145] Typically, a suppository utilizes as a base an ingredient which has a
melting point above
and below 38 degrees Celcius. Cacao butter, polyethylene glycol, glycerinated
gelatin are
often used as a base ingredient, as are hydrogel formulations. The active
ingredients are
typically blended into the base.
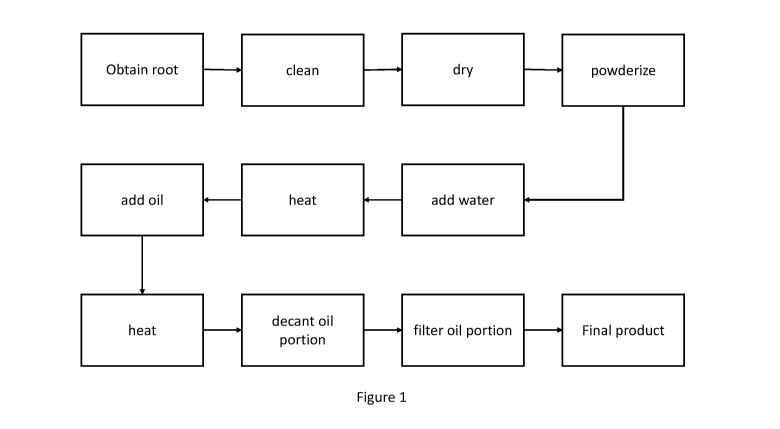
15 [0146] Brief Description of the Drawings
[0147] Figure 1 is a schematic process diagram of the process for making
cannabis root oil (a
purified, oil soluble fraction of cannabis root) as hereindescribed.
20 [0148] Summary of the Invention
[0149] According to a certain aspect of the invention is provided a method for
manufacturing a
root oil from cannabis, comprising: obtaining a root from a cannabis plant;
cleaning the root;
drying the root; chopping the root; heating the chopped, dry root in an
aqueous solvent; adding a
lipid solvent to form a heterogeneous solution of macerated root in aqueous
and lipid solvent;
heating the heterogeneous solution; separating the lipid portion; and
filtering the lipid portion to
obtain a cannabis root oil.
32
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0150] In certain embodiments, the method further comprises wet milling and/or
sonicating the
chopped, dry root in aqueous solvent prior to addition of the lipid solvent.
[0151] According to a certain aspect of the present invention is provided a
method for
manufacturing a cannabis root emulsion, comprising: manufacturing the root oil
utilizing the
method according to claim 1; and ultrasonically emulsifying the root oil with
water and
surfactant.
[0152] In certain embodiments, the cannabis is cannabis sativa.
[0153] In certain embodiments, the chopped pieces are between 100 microns and
8 cm in size.
[0154] In certain embodiments, the aqueous solvent is purified water.
[0155] In certain embodiments, the lipid solvent comprises vegetable oil, for
example, a mixture
of fractionated coconut oil and refined castor oil, such as about 50%
fractionated coconut oil and
about 50% refined castor oil, by volume.
[0156] In certain embodiments, the ratio of aqueous solvent to lipid solvent
in the heterogeneous
solution is about 6:8 (vol/vol).
[0157] In certain embodiments, the ratio of dry chopped form to lipid solvent
in the
heterogeneous solution is about 1:16 (wt/vol).
[0158] In certain embodiments, the filtration is through a filter with a pore
size of 25 microns.
33
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0159] According to a further aspect of the present invention is a cannabis
root oil, for example,
a cannabis root oil manufactured by the hereindisclosed method.
[0160] According to a further aspect of the present invention is a method of
treatment selected
from the group consisting of anti-inflammatory, pain relief, burn treatment,
blister prevention,
bruise reduction, skin rash reduction, and acne treatment, comprising
administering an effective
amount of cannabis root oil.
[0161] According to a further aspect of the present invention is a composition
for reducing
.. inflammation, pain relief, burn treatment, blister prevention, bruise
reduction, skin rash
reduction, or acne treatment, comprising an effective amount of the cannabis
root oil in a
pharmaceutically effective carrier. The pharmaceutically effective carrier
may, for example, be
pharmaceutically effective for topical administration, inhaled administration,
oral administration,
or vaginal administration.
[0162] According to a further aspect of the present invention is provided an
anti-microbial, anti-
biotic, anti-fungal, anti-neuralgic, anti-pruritic, anti-rheumatic, anti-
sclerotic, anti-septic,
emollient, hemostatic, resolvent, and/or styptic composition, comprising an
effective amount of
cannabis root oil. The carrier may be for topical, inhaled, oral or vaginal
administration.
[0163] According to one aspect of the present invention is described a
pharmaceutical
preparation for topical administration, comprising: delta-9-
tetrahydrocannabinol and a cannabis
root oil.
[0164] In certain embodiments, the pharmaceutical preparation is in the form
of a cream, an
ointment or a lotion.
34
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0165] In certain embodiments, the cannabis root oil is prepared by: obtaining
a root from a
cannabis plant; cleaning the root; drying the root; chopping the root; heating
chopped, dry root in
aqueous solvent; adding a lipid solvent to form a heterogeneous solution of
macerated root in
aqueous and lipid solvent; heating the heterogeneous solution; decanting the
lipid portion; and
filtering the lipid portion to obtain a cannabis root oil.
[0166] In certain embodiments, the delta-9-tetrahydrocannabinol is obtained
from a THC resin.
[0167] In certain embodiments, the pharmaceutical preparation further
comprises aloe vera gel.
io
[0168] In certain embodiments, the pharmaceutical preparation further
comprises lavender
essential oil.
[0169] In certain embodiments, the pharmaceutical preparation further
comprises turmeric
essential oil.
[0170] In certain embodiments, the pharmaceutical preparation further
comprises coriander
essential oil.
[0171] In certain embodiments, the pharmaceutical preparation comprises
cannabis root oil, aloe
vera gel, lavender essential oil, cannabis THC resin, turmeric essential oil,
coriander essential oil,
calendula officinalis extract, rosehip seed extract, and sea buckthorn berry
extract.
[0172] In certain embodiments, the cannabis root oil and the THC resin are
present in a weight
ratio of 20:1.
[0173] In certain embodiments, the cannabis root oil and the aloe vera gel are
present in a
weight ratio of 1:3.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0174] According to a certain embodiment, the pharmaceutical preparation
comprises, by
weight, about 200 parts cannabis root oil, about 600 parts aloe vera gel,
about 20 parts lavender
essential oil, about 10 parts cannabis THC resin, about 2 parts turmeric
essential oil, and about 2
parts coriander essential oil.
[0175] According to a further aspect of the present invention is provided a
method of treatment
of an ailment selected from the group consisting of inflammation, skin rash,
acne, and skin
irritation, comprising administering, topically, an effective amount of the
pharmaceutical
preparation as hereindescribed.
[0176] In certain embodiments, the inflammation or skin irritation is
associated with radiation
recall.
[0177] In certain embodiments, the inflammation or skin irritation is a side-
effect of
chemotherapy.
[0178] In certain embodiments, the inflammation or skin irritation is
associated with skin
cancer.
[0179] According to one aspect of the present invention is provided a
pharmaceutical
preparation for topical administration, comprising: delta-9-
tetrahydrocannabinol; cannabidiol;
and a cannabis root oil or emulsion.
[0180] In certain embodiments, the pharmaceutical preparation is in the form
of a cream, an
ointment or a lotion.
[0181] In certain embodiments, the cannabis root oil is prepared by: obtaining
a root from a
cannabis plant; cleaning the root; drying the root; chopping the root; heating
chopped, dry root in
36
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
aqueous solvent; adding a lipid solvent to form a heterogeneous solution of
macerated root in
aqueous and lipid solvent; heating the heterogeneous solution; decanting the
lipid portion; and
filtering the lipid portion to obtain a cannabis root oil.
[0182] In certain embodiments, the delta-9-tetrahydrocannabinol is obtained
from a THC resin.
[0183] In certain embodiments, the cannabidiol is obtained from a THC resin.
[0184] In certain embodiments, the pharmaceutical preparation further
comprises aloe vera gel.
io
[0185] In certain embodiments, the pharmaceutical preparation further
comprises lavender
essential oil.
[0186] In certain embodiments, the pharmaceutical preparation further
comprises menthol.
[0187] In certain embodiments, the pharmaceutical preparation further
comprises rosemary
essential oil.
[0188] In certain embodiments, the pharmaceutical preparation further
comprises arnica
Montana essential oil.
[0189] According to one aspect of the present invention, the pharmaceutical
preparation
comprises cannabis root oil, cannabis THC resin, cannabis CBD resin, aloe vera
gel, menthol,
lavender essential oil, rosemary essential oil, and arnica Montana essential
oil.
[0190] In certain embodiments, the cannabis root oil and the THC are present
in a weight ratio
of about 50:1.
37
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0191] In certain embodiments, the cannabis root oil and the CBD are present
in a weight ratio
of about 50:1.
[0192] In certain embodiments, the CBD and the THC are present in a weight
ratio of about 1:1.
[0193] In certain embodiments, the cannabis root oil and the aloe vera gel are
present in a
weight ratio of about 1:18.
[0194] In certain embodiments, the pharmaceutical preparation comprises, by
weight, about 50
parts cannabis root oil, about 900 parts aloe vera gel, about 1 part cannabis
CBD resin, about 1
part cannabis cannabidiol resin, about 35 parts of menthol crystals, about 10
parts lavender
essential oil, about 5 parts rosemary essential oil, and about 5 parts of
arnica Montana essential
oil.
[0195] In certain embodiments, the cannabis root oil and the THC are present
in a weight ratio
of about 10:1.
[0196] In certain embodiments, the cannabis root oil and the CBD are present
in a weight ratio
of about 10:1.
[0197] In certain embodiments, the CBD and the THC are present in a weight
ratio of about 1:1.
[0198] In certain embodiments, the cannabis root oil and the aloe vera gel are
present in a
weight ratio of about 1:18.
[0199] In certain embodiments, the pharmaceutical preparation comprises, by
weight, about 50
parts cannabis root oil, about 900 parts aloe vera gel, about 5 part cannabis
CBD resin, about 5
part cannabis cannabidiol resin, about 35 parts of menthol crystals, about 10
parts lavender
38
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
essential oil, about 5 parts rosemary essential oil, and about 50 parts of
arnica Montana essential
oil.
[0200] According to a further aspect of the present invention is provided a
method of treatment
of pain, comprising administering, topically, an effective amount of the
pharmaceutical
preparation as hereindescribed.
[0201] In certain embodiments, the pain is associated with multiple sclerosis.
[0202] In certain embodiments, the pain is associated with fibromyalgia.
[0203] According to one aspect of the present invention is provided a
suppository formulation
comprising cannabis root oil. The suppository formulation may also contain THC
and/or CBD.
[0204] According to a further aspect of the present invention is provided a
suppository
formulation comprising THC and/or CBD.
[0205] In certain embodiments, the suppository formulation further comprises
any one or more
of lavender oil, clary sage oil, geranium oil, and chamomile oil.
[0206] In certain embodiments, the suppository formulation comprises cannabis
root oil, THC,
CBD, lavender oil, clary sage oil, geranium oil, and chamomile oil.
[0207] In certain embodiments, the suppository formulation further comprises
cacao butter.
[0208] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, about 200 mg of 1:1
THC/CBD resin, about
39
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
2.5 ml lavender oil, about 0.25 ml of clary sage oil, about 0.25 ml of
geranium oil, about 0.25 ml
of chamomile oil.
[0209] According to a further aspect of the present invention, is provided a
suppository
formulation as hereindescribed, for treatment of pain and other effects of
dysmenorrhea.
[0210] According to a further aspect of the present invention is provided a
method of treating
the effects of dysmenorrhea, comprising applying an effective amount of the
suppository.
[0211] In certain embodiments, the dysmenorrhea is associated with uterine
fibrosis.
[0212] In certain embodiments, the dysmenorrhea is associated with
endometriosis.
[0213] In certain embodiments, the effects treated are selected from the group
consisting of pain
and cramping.
[0214] In certain embodiments, the suppository formulation further comprises
any one or more
of lavender oil, frankincense essential oil, cypress essential oil, clary sage
essential oil, and basil
essential oil.
[0215] In certain embodiments, the suppository formulation comprises cannabis
root oil, THC,
CBD, lavender oil, frankincense essential oil, cypress essential oil, clary
sage essential oil, and
basil essential oil.
[0216] In certain embodiments, the suppository formulation further comprises
cacao butter.
[0217] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, between 200 mg and 10 g
of 1:1 THC/CBD
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
resin, about 1 ml lavender oil, about 1 ml of frankincense essential oil,
about 0.5 ml of cypress
essential oil, about 0.2 ml of clary sage oil, and about 0.15 ml of basil
essential oil.
[0218] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, about 2 g of 1:1 THC/CBD
resin, about 1 ml
lavender oil, about 1 ml of frankincense essential oil, about 0.5 ml of
cypress essential oil, about
0.2 ml of clary sage oil, and about 0.15 ml of basil essential oil.
[0219] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, about 5 g of 1:1 THC/CBD
resin, about 1 ml
lavender oil, about 1 ml of frankincense essential oil, about 0.5 ml of
cypress essential oil, about
0.2 ml of clary sage oil, and about 0.15 ml of basil essential oil.
[0220] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, about 10 g of 1:1 THC/CBD
resin, about 1
ml lavender oil, about 1 ml of frankincense essential oil, about 0.5 ml of
cypress essential oil,
about 0.2 ml of clary sage oil, and about 0.15 ml of basil essential oil.
[0221] According to a further aspect of the present invention, is provided a
suppository
formulation as hereindescribed, for treatment for cancer, either to be used
alone, or in
combination, preferably synergistic combination, with conventional cancer
treatments.
[0222] According to a further aspect of the present invention, is provided a
suppository
formulation as hereindescribed, for use to alleviate certain side effects of
conventional cancer
drugs, for example, to alleviate pain, to reduce nausea, to prevent vomiting,
and/or to decrease
appetite suppression.
[0223] According to a further aspect of the present invention is provided a
method of treating
cancer, comprising administering an effective amount of the suppository,
either alone or in
41
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
combination, preferably synergistic combination, with conventional cancer
treatments. In certain
embodiments, the cancer is stomach cancer, non-Hodgkin lymphoma, prostate
cancer, or kidney
cancer.
[0224] According to a further aspect of the present invention is provided a
method of treating
the side effects of conventional chemotherapy or radiation therapy, comprising
administering an
effective amount of the suppository as hereindescribed. In certain
embodiments, the side effects
are pain, nausea, vomiting, and/or loss of appetite.
[0225] In certain embodiments, the suppository formulation further comprises
any one or more
of lavender oil, tea tree oil, clary sage oil, geranium oil, basil essential
oil, thyme essential oil,
Manuka essential oil, and helichrysum essential oil.
[0226] In certain embodiments, the suppository formulation comprises cannabis
root oil, THC,
CBD, lavender oil, tea tree oil, clary sage oil, geranium oil, basil essential
oil, thyme essential
oil, Manuka essential oil, and helichrysum essential oil.
[0227] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, about 200 mg of 1:1
THC/CBD resin, about
1.25 ml lavender oil, about 1.25 ml of tea tree essential oil, about 0.5 ml of
geranium oil, about
0.25 ml of clary sage oil, about 0.25 ml of basil essential oil, about 0.25 ml
of thyme essential
oil, about 0.25 ml of Manuka essential oil, and about 0.15 ml of helichrysum
essential oil.
[0228] According to a further aspect of the present invention, is provided a
suppository
formulation as hereindescribed, for treatment of vaginal yeast infection,
bacterial vaginosis
and/or vaginitis.
42
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0229] According to a further aspect of the present invention is provided a
method of treating
vaginal yeast infection, bacterial vaginosis and/or vaginitis, comprising
administering the
suppository.
[0230] According to a further aspect of the present invention is provided a
suppository
formulation comprising THC and/or CBD.
[0231] In certain embodiments, the suppository formulation further comprises
any one or more
of lavender oil, vitex berry essential oil, clary sage oil, geranium oil,
basil essential oil, and
chamomile oil.
[0232] In certain embodiments, the suppository formulation comprises cannabis
root oil, THC,
CBD, lavender oil, vitex berry essential oil, clary sage oil, geranium oil,
basil essential oil, and
chamomile oil.
[0233] In certain embodiments, the suppository formulation further comprises
cacao butter.
[0234] In certain embodiments, the suppository formulation comprises a base of
cacao butter,
and, per 100g of base, about 1 ml cannabis root oil, about 200 mg of 1:1
THC/CBD resin, about
1.25 ml lavender oil, about 1.25 ml of vitex berry essential oil, about 0.25
ml of clary sage oil,
about 0.25 ml of geranium oil, about 0.25 ml of chamomile oil, and about 0.15
ml of basil
essential oil.
[0235] According to a further aspect of the present invention, is provided a
suppository
formulation as hereindescribed, for treatment of pre-menopausal and menopausal
symptoms such
as hot flashes, shivering, sweating and reddening of the skin, mood changes,
vaginal dryness,
and trouble sleeping.
43
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0236] According to a further aspect of the present invention is provided a
method of treating
the pre-menopausal and menopausal symptoms such as hot flashes, shivering,
sweating and
reddening of the skin, mood changes, vaginal dryness, and trouble sleeping,
comprising applying
an effective amount of the suppository.
[0237] According to one aspect of the present invention is provided a skin
moisturizer
composition comprising cannabis root oil.
[0238] According to another aspect of the present invention is provided a skin
moisturizer
composition comprising cannabidiol.
[0239] According to another aspect of the present invention is provided a skin
moisturizer
composition comprising cannabis root oil and cannabidiol.
[0240] In certain embodiments, the skin moisturizer composition comprises 1 to
20% (wt)
cannabis root oil, for example, 4 to 16.5% (wt) cannabis root oil, or about 4%
(wt) cannabis root
oil, or about 4.5% (wt) cannabis root oil, or about 16.5% (wt) cannabis root
oil.
[0241] In certain embodiments, the skin moisturizer composition comprises
about 0.003%
cannabidiol.
[0242] In certain embodiments, the skin moisturizer composition further
comprises any one or
more of: Simmondsia chinensis (jojoba) seed oil; Persea gratissima (Avocado)
oil; Prunus
armeniaca (apricot) kernel oil; Citrus grandis (Grapefruit) seed extract;
Cetyl-stearyl alcohol
(emulsifying wax); Stearic acid; Curcuma longa (Tumeric) root oil; Juniperus
communis
(juniper) berry oil; Rosa damascene (rose) flower oil; Jasminium sambac
(Jasmine) flower oil;
Santalum spicatum (Sandalwood) wood oil; Copaifera spp (copaiba) oil; Punica
Granatum
(Pomegranate) seed oil; Zingiber Officinale (Ginger) root oil; Lavandula
Angustifoila
(Lavender) flower oil; Cinamomum Zeylanicum (cinnamon) bark oil; Syzygium
Aromaticum
44
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
(clove) bud oil; Elettaria Cardamomom (Cardamom) seed oil; Prunus amygdalus
(sweet almond)
oil; Goat milk powder; Ocimum basilicum (basil) oil; Citrus bergamia
(Bergamot) peel oil;
Foeniculum (Fennel) seed oil; and Boswellia Serrata (Frankincense) oil.
.. [0243] In certain embodiments, the skin moisturizer composition is in the
form of an
emulsification comprising an aqueous component and a lipid soluble component.
The aqueous
component may comprise water, honey, Chamaemelum Nobile (Chamomile) water,
and/or rose
damascene (Rose) water.
[0244] In certain embodiments is provided a skin moisturizer composition
comprising: Cannabis
root oil; Cannabidiol oil; Simmondsia chinensis (jojoba) seed oil; Persea
gratissima (Avocado)
oil; Prunus armeniaca (apricot) kernel oil; Rose damascene (Rose) water;
Honey; Citrus grandis
(Grapefruit) seed extract; Cetyl-stearyl alcohol (emulsifying wax); Stearic
acid; Curcuma longa
(Tumeric) root oil; Juniperus communis (juniper) berry oil; Rosa damascene
(rose) flower oil;
Jasminium sambac (Jasmine) flower oil; Santalum spicatum (Sandalwood) wood
oil; and
Copaifera spp (copaiba) oil.
[0245] In certain embodiments is provided a skin moisturizer composition
comprising, by
weight: 4.5% Cannabis root oil; 0.003% Cannabidiol oil; 13% Simmondsia
chinensis (jojoba)
seed oil; 8% Persea gratissima (Avocado) oil; 8% Prunus armeniaca (apricot)
kernel oil; 56%
Rose damascene (Rose) water; 0.6% Honey; 0.3% Citrus grandis (Grapefruit) seed
extract;
4.75% Cetyl-stearyl alcohol (emulsifying wax); 4.75% Stearic acid; 0.014%
Curcuma longa
(Tumeric) root oil; 0.014% Juniperus communis (juniper) berry oil; 0.014% Rosa
damascene
(rose) flower oil; 0.014% Jasminium sambac (Jasmine) flower oil; 0.014%
Santalum spicatum
(Sandalwood) wood oil; and 0.027% Copaifera spp (copaiba) oil.
[0246] In certain embodiments is provided a skin moisturizer composition
comprising:
Cannabis root oil; Cannabidiol oil; Simmondsia chinensis (jojoba) seed oil;
Persea gratissima
(Avocado) oil; Honey; Citrus grandis (Grapefruit) seed extract; Cetyl-stearyl
alcohol
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
(emulsifying wax); Stearic acid; Rosa damascene (rose) flower oil; Copaifera
spp (copaiba) oil;
Punica Granatum (Pomegranate) seed oil; Chamaemelum Nobile (Chamomile) water;
Zingiber
Officinale (Ginger) root oil; Lavandula Angustifoila (Lavender) flower oil;
Cinamomum
Zeylanicum (cinnamon) bark oil; Syzygium Aromaticum (clove) bud oil; and
Elettaria
Cardamomom (Cardamom) seed oil.
[0247] In certain embodiments is provided a skin moisturizer composition
comprising, by
weight: 4% Cannabis root oil; 0.003% Cannabidiol oil; 13% Simmondsia chinensis
(jojoba)
seed oil; 10% Persea gratissima (Avocado) oil; 0.6% Honey; 0.3% Citrus grandis
(Grapefruit)
seed extract; 4.75% Cetyl-stearyl alcohol (emulsifying wax); 4.75% Stearic
acid; 0.014% Rosa
damascene (rose) flower oil; 0.016% Copaifera spp (copaiba) oil; 6.5% Punica
Granatum
(Pomegranate) seed oil; 55.997% Chamaemelum Nobile (Chamomile) water; 0.014%
Zingiber
Officinale (Ginger) root oil; 0.014% Lavandula Angustifoila (Lavender) flower
oil; 0.014%
Cinamomum Zeylanicum (cinnamon) bark oil; 0.014% Syzygium Aromaticum (clove)
bud oil;
and 0.014% Elettaria Cardamomom (Cardamom) seed oil.
[0248] In certain embodiments is provided a skin moisturizer composition
comprising:
Cannabis root oil; Cannabidiol oil; Simmondsia chinensis (jojoba) seed oil;
Prunus amygdalus
(sweet almond) oil; Honey; Citrus grandis (Grapefruit) seed extract; Cetyl-
stearyl alcohol
(emulsifying wax); Stearic acid; Copaifera spp (copaiba) oil; Water; Lavandula
Angustifoila
(Lavender) flower oil; Syzygium Aromaticum (clove) bud oil; Goat milk powder;
Ocimum
basilicum (basil) oil; Citrus bergamia (Bergamot) peel oil; Foeniculum
(Fennel) seed oil; and
Boswellia Serrata (Frankincense) oil.
[0249] In certain embodiments is provided a skin moisturizer composition
comprising: 16.5%
Cannabis root oil; 0.003% Cannabidiol oil; 10% Simmondsia chinensis (jojoba)
seed oil; 6.5%
Prunus amygdalus (sweet almond) oil; 0.6% Honey; 0.3% Citrus grandis
(Grapefruit) seed
extract; 4.75% Cetyl-stearyl alcohol (emulsifying wax); 4.75% Stearic acid;
0.02% Copaifera
46
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
spp (copaiba) oil; 55.893% Water; 0.014% Lavandula Angustifoila (Lavender)
flower oil;
0.014% Syzygium Aromaticum (clove) bud oil; 0.6% Goat milk powder; 0.014%
Ocimum
basilicum (basil) oil; 0.014% Citrus bergamia (Bergamot) peel oil; 0.014%
Foeniculum (Fennel)
seed oil; and 0.014% Boswellia Serrata (Frankincense) oil.
[0250] According to a further embodiment of the present invention is provided
a method of
moisturizing dry skin, comprising applying an effective amount of the skin
moisturizer
composition as hereindescribed.
[0251] According to a certain aspect of the present invention is provided a
pharmaceutical
preparation for topical administration as a female sexual lubricant,
comprising ashwagandha oil,
THC, and/or cannabis root oil.
[0252] In certain embodiments, the pharmaceutical preparation further
comprises avocado oil,
menthol, and/or black pepper oil.
[0253] In certain embodiments, the pharmaceutical preparation is for topical
administration as a
female sexual lubricant and comprises hemp root oil, avocado oil, ashwaganda
oil, THC,
menthol crystals and black pepper oil.
[0254] In certain embodiments, the pharmaceutical composition comprises, by
weight, about
20% hemp root oil, about 60% avocado oil, about 20% ashwaganda oil, about 0.2%
THC, about
1% menthol, and about 0.8% black pepper oil.
[0255] In certain embodiments, the pharmaceutical composition comprises, by
weight, 19.65%
cannabis sativa hemp root oil, 58.73% avocado oil, 19.65% ashwaganda oil, 0.2%
THC, 0.98%
menthol crystals, and 0.79% black pepper oil.
47
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0256] In certain embodiments, the cannabis root oil is prepared by the
hereindescribed method.
[0257] In certain embodiments, the delta-9-tetrahydrocannabinol is obtained
from a THC resin.
[0258] According to a certain aspect of the present invention is provided a
female sexual
lubricant comprising the pharmaceutical composition or preparation as
hereindescribed.
[0259] According to a certain aspect of the present invention is provided a
method of enhancing
a sexual experience of a human female, comprising topical, transdermal and/or
transmucosal
administration of the female sexual lubricant as hereindescribed.
[0260] In certain embodiments, the female suffers from female sexual
dysfunction, such as
F SAD.
[0261] According to one aspect of the present invention is provided a skin
anti-aging serum
comprising a unique combination of cannabidiol (CBD) and hyaluronic acid (HA).
In certain
embodiments, the skin anti-aging serum also comprises hemp root oil or
emulsion, for example,
a hemp root oil or emulsion made as hereindescribed.
[0262] In certain embodiments, the skin anti-aging serum composition also
comprises any one
or more of lavender essential oil, copaiba essential oil, frankincense
essential oil, carrot seed
essential oil, geranium essential oil, and sea buckthorn berry essential oil.
[0263] In certain embodiments, the skin anti-aging serum composition comprises
CBD, HA,
lavender essential oil, copaiba essential oil, frankincense essential oil,
carrot seed essential oil,
geranium essential oil, and sea buckthorn berry essential oil.
48
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0264] In certain embodiments, the skin anti-aging serum composition also
comprises a gelling
agent, an emulsifying agent, additional moisturizing ingredients, and/or a
preservative.
[0265] In certain embodiments, the gelling agent or emulsifying agent is
ECOGEL.
[0266] In certain embodiments, the additional moisturizing ingredient is
AMTicide Coconut.
[0267] In certain embodiments, the preservative is Leucidal Liquid SF.
[0268] In certain embodiments, the skin anti-aging serum comprises about 1 wt%
HA and about
0.3 wt% of CBD, in an aqueous solution or emulsion.
[0269] In certain embodiments, the skin anti-aging serum comprises about 1%
lavender oil,
about 1% copaiba oil, about .4% frankincense oil, about .25% carrot seed oil,
about 0.3%
geranium oil, about .25% sea buckthorn berry oil, about .3% CBD, in an aqueous
solution or
emulsion.
[0270] In certain embodiments, the skin anti-aging serum comprises .93% HA,
0.93% lavender
oil, 0.93% copaiba oil, .37% frankincense oil, .23% carrot seed oil, 0.27%
geranium oil, .23%
sea buckthorn berry oil, .3% CBD, in an aqueous solution or emulsion.
[0271] In certain embodiments, and as presently measured and used, the CBD is
in crystalline or
isolate form. In other embodiments, a CBD oil is used, with the weight
concentration of the
CBD oil in the skin anti-aging serum adjusted according to the concentration
of the CBD oil.
[0272] In certain embodiments, the skin anti-aging serum comprises about 2wt%
Ecogel, for
example, 1.9% Ecogel.
49
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0273] In certain embodiments, the skin anti-aging serum comprises about 2wt%
AMTicide, for
example, 1.9% AMTicide.
[0274] In certain embodiments, the anti-aging serum comprises about 2wt%
Leucidal liquid SF,
for example, 1.9% Leucidal liquid SF.
[0275] In certain embodiments, the aqueous solution or emulsion is a water-
based emulsion.
[0276] According to a further embodiment of the present invention is provided
a method of
treating skin to prevent signs of aging, comprising applying an effective
amount of the skin anti-
aging serum composition as hereindescribed.
[0277] In certain embodiments, the skin anti-aging serum further comprises by
weight about 1%
lavender oil, about 1% copaiba oil, about .4% frankincense oil, about .25%
carrot seed oil, about
0.3% geranium oil, and about .25% sea buckthorn berry oil.
[0278] In certain embodiments, the skin anti-aging serum further comprises by
weight 0.93%
HA, 0.93% lavender oil, 0.93% copaiba oil, .37% frankincense oil, .23% carrot
seed oil, 0.27%
geranium oil, .23% sea buckthorn berry oil.
[0279] In certain embodiments, the skin anti-aging serum further comprises
about 2wt%
ECOGEL, for example, 1.9wt% ECOGEL, about 2wt% AMTicide coconut, for example,
1.9wt% AMTicide coconut, about 2wt% Leucidal liquid SF, for example, 1.9wt%
Leucidal
liquid SF and/or wherein the aqueous emulsion or solution is a water-based
emulsion.
[0280] In certain embodiments, the skin anti-aging serum composition further
comprises
lactobacillus ferment, lactobacillus, coconut fruit extract, hyaluronic acid,
lavender oil, copaiba
resin oil, Franckinsence gum oil, Geranium flower oil, Carrot seed oil, Sea
buckthorn oil,
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
Cannabis seed oil, Cannabis root oil, and optionally one or more of
lysolecithin, sclerotium gum,
xanthan gum, and pullulan.
[0281] In certain embodiments, the skin anti-aging serum composition
comprises, by weight,
91.220% water, 2% lactobacillus ferment, 2% lactobacillus, coconut fruit
extract, 1 hyaluronic
acid, 1% lavender oil, 1% balsam copaiba resin oil, 0.5% mixture of
lysolecithin, sclerotium
gum, xanthan gum and pullulan, 0.4% Frankincense gum oil, 0.3% Geranium flower
oil, .25%
carrot seed oil, 0.25% sea buckthorn oil, 0.04% hemp seed oil, and 0.04% Hemp
root oil.
[0282] Detailed Description
[0283] Example 1: Manufacture of a Cannabis Root Oil Extract and Nano Emulsion
of Same
[0284] Disclosed herein is a unique cannabis root oil with medicinal
properties, a method of
manufacturing the cannabis root oil from cannabis root, for example, Cannabis
sativa root, and
formulations containing said cannabis root oil.
[0285] The cannabis root oil produced by this method can be used
advantageously in therapeutic
products, for a variety of purposes, and a variety of administration methods,
as detailed and
exemplified further below.
[0286] The cannabis root oil is made as follows.
[0287] The root structure of one or more cannabis plants is obtained and/or
separated from the
rest of the plant. Preferably, the entire cannabis root is used. A cannabis
root is defined as the
portion of the plant that would ordinarily reside underground in a naturally
growing, healthy
plant, or underwater in a hydroponically-grown plant.
51
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0288] The cannabis root is thoroughly cleaned, to remove soil, pesticide,
fertilizer, or other
impurities, by dry shaking, mechanical manipulation, and cold or room
temperature water spray.
In certain embodiments, for example, if the root is obtained straight out of
the ground, a high
pressure room temperature or cold water washer can be used to wash away most
of the dirt and
impurities. In certain other embodiments, the cannabis root may be obtained
from the
grower/manufacturer in a dry, washed form.
[0289] In certain embodiments, the cannabis root may be washed and sterilized
in a vegetable
washing machine, such as a modified Sormac GWM 2500, wherein the root is
intensely washed
in the machine's wash tank in a solution of 1.5% H202 in water, and then mixed
and transported
along the length of the wash tank as a result of the turbulent water flow. The
root is then over-
flumed out of the wash tank, onto a vibratory de-watering screen where it is
sprayed off with
fresh water, then gently transported forward, draining the excess water.
Remaining water and
moisture is then removed, using an air knife dryer at the end of the wash and
drainage cycle,
which uses forced air to dry the root.
[0290] In certain embodiments, the cannabis root is then thoroughly dried,
preferably at room
temperature and pressure, but alternatively, if a more rapid drying is
desired, a dehydrator
utilizing air circulation, vacuum and/or heat can be used. A heat of up to 60
degrees Celsius may
.. be used without affecting the desired chemical properties of the extract,
with even higher heat
potentially possible. Preferably, the cannabis root is dried to a moisture
content of less than 5%.
[0291] After drying, the cannabis root is roughly chopped, to pieces with
widely varying size up
to about 8 cm. Preferably, the root is cut to about 1.5mm, which may be done
in a commercial
food processor such as the Hobart FP400i, which offers a continuous use hopper
feeding system
for high volume production.
52
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0292] In certain embodiments, the dried cannabis root is then wet milled
and/or (preferably)
sonicated into a slurry mixture in water. For example, an Ultrasonic UP400St,
with a Sonotrode
S24d7 with a 7mm tip, or a Sonotrode S24d22 with a 22mm tip, can be utilized
for simultaneous
wet milling and sonication (sonic cavitation). For commercial applications,
the Ultrasonic can
be replaced with a Digital Ultrasonic Device UIP1000hd (Hielscher), utilizing
for example a
Sonotrode BS2d22 (22 mm tip) or a Sonotrode BS2d34 (34mm tip). In one
embodiment, 250 g
of dessicated hemp root is combined with 500 ml of distilled water, and the
cells in the hemp
root are disrupted with the ultrasound for a period of about 5 minutes. The
use of sonication and
the resultant cell disruption has been found to increase the quantity of
recoverable desirable
active compounds recovered from the root.
[0293] Water is added to the dry, chopped material in a ratio of about 6
litres of water for about
500 grams of dry cannabis chopped root, and the mixture is then heated to 100C
for 6 hours,
preferably without agitation. A lipid solvent, for example, a vegetable oil,
is added; preferably
an oil blend of fractionated coconut oil and refined castor oil (preferably
about 50/50 by
volume), in an amount of about 4 litres of each for about 500 grams of dry
cannabis root powder.
The solution is then heated again to about 100 Celsius for about 48 hours,
with stirring about
every hour. During this stage, the oil-soluble elements of the hemp root are
solubilized into the
oil.
[0294] In (preferred) embodiments where sonication is utilized, the sonication
slurry is heated in
a kettle and simmered for about an hour, left to cool and settle, and the
water hemp root mixture
is decanted. A lipid solvent, for example, a vegetable oil, is added to the
remaining water,
preferably an oil blend of fractionated coconut oil and refined castor oil
(preferably about 50/50
by volume), in an amount of about 2 litres of each for about 500 ml of the
sonication slurry.
The solution is then heated again to about 100 Celsius for about 48 hours,
with stirring about
every hour. During this stage, the oil-soluble elements of the hemp root are
solubilized into the
oil.
53
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0295] The oil/water mixture is cooled and the oil portion is decanted,
optionally further dried,
and filtered through 25 microns to produce the cannabis root oil product.
[0296] In certain embodiments, the cannabis root oil product is nanoized with
the addition of a
surfactant, to produce a water in oil emulsion, utilizing an ultrasonic
process to produce an
emulsion measurable in the 20 ¨ 40 nanometer range.
[0297] The cannabis root oil produced by this method has unique and desirable
pharmaceutical
properties, and can be combined with other ingredients for additional
benefits, and with known
pharmaceutically acceptable additives, carriers, fillers and excipients for
oral, inhaled,
subcutaneous, intravenous, intramuscular, vaginal, rectal, or topical
administration. In particular,
the cannabis root oil produced by this method can be used advantageously in a
topical
therapeutic, for pain, anti-inflammation, as well as relief of arthritis,
endometriosis, menstrual
cramping, and rheumatism.
[0298] The cannabis root oil and/or emulsion product exhibits advantageous
therapeutic
properties, which may include anti-inflammatory, pain relieving, burn
treating, blister
prevention, bruise reducing, skin rash reducing and anti-acne properties. It
also exhibits further
.. advantageous properties, including anti-microbial, anti-biotic, anti-
fungal, anti-neuralgic, anti-
pruritic, anti-rheumatic, anti-sclerotic, anti-septic, emollient, hemostatic,
resolvent, and styptic
properties.
[0299] The cannabis root oil and/or emulsion can be ingested, either alone or
in an edible
formulation, inhaled, for example in a vaporized form, or used as a topical
cream, ointment,
balm, gel, or spray. Alternatively, the cannabis root oil can be injected in a
suitable carrier,
either intravenously, subcutaneously or intramuscularly; the cannabis root oil
may be
administered using known skin patch technologies, rectally for example in a
suppository,
54
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
intravaginally, or through other high permeability areas such as the nasal
cavity, the inside of the
cheek, or sublingually.
[0300] Example 2 ¨ Method and Formulation for Treating Skin Inflammation
[0301] The cannabis root oil and/or emulsion as described in Example 1 is
particularly useful as
an ingredient in a topical formulation for the treatment of skin inflammation,
particularly,
inflammation, irritation and rash associated with radiation recall, from skin
cancer, and/or from
chemotherapy. The formulation contains the oil soluble fraction of cannabis
root, as well as
THC. It is believed topical, oil soluble fractions of cannabis root have never
previously been
used medicinally. It is also believed that the combination of an oil soluble
fraction of cannabis
root, with THC, provides advantageous effects, and possibly additive and/or
synergistic effects.
[0302] The cannabis root oil is combined with a THC active, for example,
marinol, but more
preferably, a cannabis THC resin, and blended or mixed into a topical
formulation. In certain
preferred embodiments, aloe vera gel is used as a carrier/base for the
formulation, because of its
known cancer inhibiting and skin nourishing qualities, which can act in
combination with the
cannabis root oil and THC active ingredients. Optionally, other active
ingredients, such as
ingredients known to have anti-inflammatory or irritation soothing effects, or
cancer fighting
properties, can also be added. Such ingredients may include lavender essential
oil, turmeric
essential oil, coriander essential oil, extract of calendula officinalis,
extract of rosehip seed, and
extract of sea buckthorn berry.
[0303] According to one example of a topical cream of the present invention,
100 grams of
cannabis root oil prepared as described above is mixed into 300 grams of aloe
vera gel, to obtain
an oil/gel emulsion. 5 grams of cannabis THC resin is added, along with one
gram of turmeric
essential oil, one gram of coriander essential oil, 10 grams of lavender
essential oil, and 2.5
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
grams of CO2 Combination extract, an extract of calendula officinalis, rosehip
seed and sea
buckthorn berry available from Rae Dunphy Aromatics Ltd (Canada)
(www.raedunphy.ca).
[0304] The mixture provides a smooth emoliant cream which is easy to apply
onto skin. As
would be understood by a person of skill in the art, with the proper added
ingredients, the
mixture can also be made into an ointment or (for example) by adding more
water soluble
ingredients or more aloe vera gel, the mixture can be made into a lotion. The
cream provides
excellent treatment for inflammation caused by radiation recall, as well as
for skin irritation
resulting from chemotherapy.
io
[0305] Example 3 ¨ Method and Formulation for Pain Management
[0306] The cannabis root oil and/or emulsion as described in Example 1 is
particularly useful in
a topical formulation for pain management, especially useful for the treatment
of chronic pain
presented with or manifesting in association with inflammation. The
formulation is particularly
useful for alleviation of pain and/or inflammation related to fibromyalgia
and/or multiple
sclerosis. The formulation comprises the oil soluble fraction of cannabis root
manufactured as
described in Claim 1, THC, and CBD. It is believed topical, oil soluble
fractions of cannabis
root have never been used medicinally. It is also believed that the
combination of an oil soluble
fraction of cannabis root, when combined with THC and CBD, provides
advantageous effects,
and possibly additive and/or synergistic effects, especially for the treatment
or alleviation of pain
with inflammation, for example, the pain associated with fibromyalgia and/or
multiple sclerosis.
[0307] The cannabis root oil or emulsion produced as hereindescribed can be
used
advantageously in a topical therapeutic, in combination with THC and CBD, for
pain.
[0308] The cannabis root oil is combined with a THC active, for example,
marinol, but more
preferably, a cannabis THC resin, as well as CBD, preferably a CBD resin, and
blended or mixed
56
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
into a topical formulation. In certain preferred embodiments, aloe vera gel is
used as a
carrier/base for the formulation, because of its known skin nourishing
qualities. Optionally,
other active ingredients, such as ingredients known to have anti-inflammatory
or pain relief
effects, can also be added. Such ingredients may include menthol, which
provides a soothing,
cooling sensation, lavender essential oil (essential oil derived from
lavandula angustifolia),
which is known to have disinfectant and skin soothing properties, rosemary
essential oil
(essential oil derived from Rosmarinus officinalis), which is known for its
pain reduction
properties, and essential oil derived from arnica Montana, a flower known to
have pain reduction
properties.
[0309] According to one example of a topical cream of the present invention,
50 parts by weight
of cannabis root oil prepared as described above is mixed into about 900 parts
of aloe vera gel, to
obtain an oil/gel emulsion. About 1 part cannabis THC resin and about 1 part
cannabis CBD
resin is added. Alternatively, about 2 parts of an approximately 50:50 mixture
of THC and CBD,
for example, Sativex, can be added. In preferred embodiments, about 35 parts
of menthol
crystals (L-menthol), about 10 parts of lavender essential oil, about 5 parts
of rosemary essential
oil, and about 5 parts of arnica montana essential oil are also added.
[0310] The mixture provides a smooth emoliant cream which is easy to apply
onto skin. As
would be understood by a person of skill in the art, with the proper added
ingredients, the
mixture can also be made into an ointment or (for example) by adding more
water soluble
ingredients or more aloe vera gel, the mixture can be made into a lotion. The
cream provides
excellent treatment for the pain caused by MS. The cream also provides
excellent treatment of
pain caused by fibromyalgia.
[0311] Example 4 ¨ Method and Formulation for Management of Chronic Intense
Pain
57
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0312] The cannabis root oil and/or emulsion as described in Example 1 is
particularly useful in
a topical formulation for pain management, especially useful for the treatment
of chronic pain
presented with or manifesting in association with inflammation. The
formulation is particularly
useful for alleviation of pain and/or inflammation related to fibromyalgia
and/or multiple
sclerosis. The formulation comprises an oil soluble fraction of cannabis root,
THC, and CBD. It
is believed topical, oil soluble fractions of cannabis root have never been
used medicinally. It is
also believed that the combination of an oil soluble fraction of cannabis
root, when combined
with THC and CBD, provides advantageous effects, and possibly additive and/or
synergistic
effects, especially for the treatment or alleviation of pain with
inflammation, for example, the
pain associated with fibromyalgia and/or multiple sclerosis.
[0313] The cannabis root oil produced by this method can be used
advantageously in a topical
therapeutic, in combination with THC and CBD, for pain.
[0314] The cannabis root oil is combined with a THC active, for example,
marinol, but more
preferably, a cannabis THC resin, as well as CBD, preferably a CBD resin, and
blended [mixed]
into a topical formulation. In certain preferred embodiments, aloe vera gel is
used as a
carrier/base for the formulation, because of its known skin nourishing
qualities. Optionally,
other active ingredients, such as ingredients known to have anti-inflammatory
or pain relief
.. effects, can also be added. Such ingredients may include menthol, which
provides a soothing,
cooling sensation, lavender essential oil (essential oil derived from
lavandula angustifolia),
which is known to have disinfectant and skin soothing properties, rosemary
essential oil
(essential oil derived from Rosmarinus officinalis), which is known for its
pain reduction
properties, and essential oil derived from arnica Montana, a flower known to
have pain reduction
properties.
[0315] According to one example of a topical cream of the present invention,
50 parts by weight
of cannabis root oil prepared as described above is mixed into about 900 parts
of aloe vera gel, to
58
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
obtain an oil/gel emulsion. About 5 part cannabis THC resin and about 5 part
cannabis CBD
resin is added. Alternatively, about 10 parts of an approximately 50:50
mixture of THC and
CBD, for example, Sativex, can be added. In preferred embodiments, about 35
parts of menthol
crystals (L-menthol), about 10 parts of lavender essential oil, about 5 parts
of rosemary essential
oil, and about 50 parts of arnica montana essential oil are also added.
[0316] The mixture provides a smooth emoliant cream which is easy to apply
onto skin. As
would be understood by a person of skill in the art, with the proper added
ingredients, the
mixture can also be made into an ointment or (for example) by adding more
water soluble
ingredients or more aloe vera gel, the mixture can be made into a lotion. The
cream provides
excellent treatment for the pain caused by MS. The cream also provides
excellent treatment of
pain caused by fibromyalgia.
[0317] Example 5 ¨ Method and Formulation for Treatment of Dysmenorrhea
[0318] The cannabis root oil and/or emulsion as described in Example 1 is
particularly useful as
a remedy for dysmenorrhea, in particular, in treating menstrual pain
associated with uterine
fibroids, adenomyosis, or endometriosis, when administered in a suppository
formulation. It has
also been discovered that tetrahydrocannabinol and/or cannabidiol are also
useful in alleviating
menstrual pain. We have obtained excellent therapeutic effect, which may be
additive or even
synergistic, when administering combinations of cannabis root oil,
tetrahydrocannabinol and
cannabidiol.
[0319] Disclosed are a variety of suppositories highly effective for treating
menstrual pain,
containing such cannabis root oil, either alone, or combined with THC and/or
CBD. Other
therapeutics may also be used in the formulation. For example, lavender
essential oil, clary sage
essential oil, geranium essential oil, and/or chamomile essential oil. We have
created a
59
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
combination particularly useful and effective in treating dysmenorrhea,
comprising cannabis root
oil, CBD, THC, lavender oil, clary sage oil, geranium oil, and chamomile oil.
[0320] Any known base can be used for the suppository; we have found that
cacao butter can be
effectively used.
[0321] Example 5A: Suppository formulation utilizing cannabis root oil
[0322] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil is added, and the liquid is stirred until the oil appears
completely blended.
The composition is poured into about 50 suppository moulds, each containing
about 2g of
composition; the suppository moulds are sealed and cooled.
[0323] The suppository is administered vaginally and/or rectally to women
suffering from
dysmenorrhea associated with a wide variety of conditions, including
endometriosis and uterine
fibroids, and results in a noticeable decrease in pain.
[0324] Example 5B: Suppository formulation utilizing cannabis root oil, THC
and CBD
[0325] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, and about 200 mg of a 2 mg active/gram, 1:1 ratio
THC/CBD resin is
added, and the liquid is stirred until homogeneous. The composition is poured
into about 50
suppository moulds, each containing about 2g of composition; the suppository
moulds are sealed
and cooled.
[0326] The suppository is administered vaginally and/or rectally to women
suffering from
dysmenorrhea associated with a wide variety of conditions, including
endometriosis and uterine
fibroids, and results in a noticeable decrease in pain.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0327] Example 5C: Suppository formulation
[0328] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 200 mg of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 2.5 ml
lavender oil, and about 0.25 ml of each of clary sage oil, geranium oil, and
chamomile oil was
added, and the liquid was stirred until homogeneous. The composition was
poured into about 50
suppository moulds, each containing about 2g of composition; the suppository
moulds were
sealed and cooled.
io
[0329] The suppository was administered vaginally to women suffering from
dysmenorrhea
associated with a wide variety of conditions, including endometriosis and
uterine fibroids, and
resulted in a noticeable decrease in pain.
[0330] Example 6 ¨ Method and Formulation for Treatment of Cancer
The cannabis root oil and/or emulsion as described in Example 1 may also be
highly effective in
treating certain forms of cancer, including stomach cancer, non-Hodgkin
lymphoma, prostate
cancer, and kidney cancer, both on its own, or in synergistic combination with
known cancer
therapies. The formulations may also be useful in reducing the side effects of
conventional
cancer therapies, including pain, nausea, vomiting, and/or loss of appetite.
[0331] In particular, it is possible that the cannabis root oil produced by
this method can be used
advantageously in a suppository, for treating certain forms of cancer,
including stomach cancer,
non-Hodgkin lymphoma, prostate cancer, and kidney cancer, both on its own, or
in synergistic
combination with known cancer therapies. The formulations may also be useful
in reducing the
side effects of conventional cancer therapies, including pain, nausea,
vomiting, and/or loss of
appetite.
61
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0332] The cannabis root oil product exhibits advantageous therapeutic
properties, which may
include treating certain forms of cancer, including stomach cancer, non-
Hodgkin lymphoma,
prostate cancer, and kidney cancer, either on its own, formulated in
combination with other
ingredients, and/or in synergistic combination with known cancer therapies.
The cannabis root
oil may also be useful in reducing the side effects of conventional cancer
therapies, including
pain, nausea, vomiting, and/or loss of appetite. It also exhibits further
advantageous properties,
including anti-microbial, anti-biotic, anti-fungal, anti-neuralgic, anti-
pruritic, anti-rheumatic,
anti-sclerotic, anti-septic, emollient, hemostatic, resolvent, and styptic
properties.
[0333] The cannabis root oil can be ingested, either alone or in an edible
formulation, inhaled,
for example in a vaporized form, or used as a topical cream, ointment, balm,
gel, or spray.
Alternatively, the cannabis root oil can be injected in a suitable carrier,
either intravenously,
subcutaneously or intramuscularly; the cannabis root oil may be administered
using known skin
patch technologies, rectally for example in a suppository, intravaginally, or
through other high
permeability areas such as the nasal cavity, the inside of the cheek, or
sublingually.
[0334] It has been discovered that the cannabis root oil as hereindescribed
may be useful for
treating certain forms of cancer, including stomach cancer, non-Hodgkin
lymphoma, prostate
cancer, and kidney cancer, both on its own, or in synergistic combination with
known cancer
therapies. The cannabis root oil may also be useful in reducing the side
effects of conventional
cancer therapies, including pain, nausea, vomiting, and/or loss of appetite,
when administered in
a suppository formulation. It has also been discovered that
tetrahydrocannabinol and/or
cannabidiol are also useful in these treatments. We believe we will obtain
excellent therapeutic
effect, which may be additive or even synergistic, when administering
combinations of cannabis
root oil, tetrahydrocannabinol and cannabidiol.
[0335] Disclosed are a variety of suppositories believed to be highly
effective for treating
certain forms of cancer, including stomach cancer, non-Hodgkin lymphoma,
prostate cancer, and
62
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
kidney cancer, both on their own, or in synergistic combination with known
cancer therapies.
The suppositories may also be useful in reducing the side effects of
conventional cancer
therapies, including pain, nausea, vomiting, and/or loss of appetite. The
suppositories contain
cannabis root oil as hereindescribed, either alone, or combined with THC
and/or CBD. Other
therapeutics may also be used in the formulation. For example, lavender oil,
frankincense
essential oil, cypress essential oil, clary sage oil, and/or basil essential
oil may form part of the
suppository formulation.
[0336] We have created a combination particularly useful and effective in such
cancer treatment
and/or relief of side effects, comprising cannabis root oil, CBD, THC,
lavender oil, frankincense
essential oil, cypress essential oil, clary sage oil, and basil essential oil.
[0337] Any known base can be used for the suppository; we have found that
cacao butter can be
effectively used.
103381 Example 6A: Suppository formulation utilizing cannabis root oil
[0339] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil is added, and the liquid is stirred until the oil appears
completely blended.
The composition is poured into about 50 suppository moulds, each containing
about 2g of
composition; the suppository moulds are sealed and cooled.
[0340] The suppository is administered vaginally and/or rectally to
individuals suffering from
cancer, either alone or in combination with conventional cancer therapy, and
results in a
reduction of tumor growth. The suppository is also administered vaginally
and/or rectally to
individuals afflicted with side effects resulting from conventional cancer
therapy, and reduces
the incidences of such side effects.
63
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
103411 Example 6B: Suppository formulation utilizing cannabis root oil, THC
and CBD
[0342] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, and about 2 g of a 2 mg active/gram, 1:1 ratio THC/CBD
resin is added,
and the liquid is stirred until homogeneous. The composition is poured into
about 50 suppository
moulds, each containing about 2g of composition; the suppository moulds are
sealed and cooled.
[0343] The suppository is administered vaginally and/or rectally to
individuals suffering from
cancer, either alone or in combination with conventional cancer therapy, and
results in a
reduction of tumor growth. The suppository is also administered vaginally
and/or rectally to
individuals afflicted with side effects resulting from conventional cancer
therapy, and reduces
the incidences of such side effects.
10344] Example 6C: Suppository formulation
[0345] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 200 mg of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 1 ml
lavender oil, about 1 ml of frankincense essential oil, about 0.5 ml of
cypress essential oil, about
0.2 ml of clary sage oil, and about 0.15 ml of basil essential oil were added,
and the liquid was
stirred until homogeneous. The composition was poured into about 50
suppository moulds, each
containing about 2g of composition; the suppository moulds were sealed and
cooled.
[0346] The suppository is administered vaginally and/or rectally to
individuals suffering from
cancer, either alone or in combination with conventional cancer therapy, and
results in a
reduction of tumor growth. The suppository is also administered vaginally
and/or rectally to
individuals afflicted with side effects resulting from conventional cancer
therapy, and reduces
the incidences of such side effects.
64
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
10347] Example 6D: Suppository formulation
[0348] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 2 g of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 1 ml lavender
oil, about 1 ml of frankincense essential oil, about 0.5 ml of cypress
essential oil, about 0.2 ml of
clary sage oil, and about 0.15 ml of basil essential oil were added, and the
liquid was stirred until
homogeneous. The composition was poured into about 50 suppository moulds, each
containing
about 2g of composition; the suppository moulds were sealed and cooled.
[0349] The suppository is administered vaginally and/or rectally to
individuals suffering from
cancer, either alone or in combination with conventional cancer therapy, and
results in a
reduction of tumor growth. The suppository is also administered vaginally
and/or rectally to
individuals afflicted with side effects resulting from conventional cancer
therapy, and reduces
the incidences of such side effects.
10350] Example 6E: Suppository formulation
[0351] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 5 g of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 1 ml lavender
oil, about 1 ml of frankincense essential oil, about 0.5 ml of cypress
essential oil, about 0.2 ml of
clary sage oil, and about 0.15 ml of basil essential oil were added, and the
liquid was stirred until
homogeneous. The composition was poured into about 50 suppository moulds, each
containing
about 2g of composition; the suppository moulds were sealed and cooled.
[0352] The suppository is administered vaginally and/or rectally to
individuals suffering from
cancer, either alone or in combination with conventional cancer therapy, and
results in a
reduction of tumor growth. The suppository is also administered vaginally
and/or rectally to
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
individuals afflicted with side effects resulting from conventional cancer
therapy, and reduces
the incidences of such side effects.
10353] Example 6F: Suppository formulation
[0354] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 10 g of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 1 ml
lavender oil, about 1 ml of frankincense essential oil, about 0.5 ml of
cypress essential oil, about
0.2 ml of clary sage oil, and about 0.15 ml of basil essential oil were added,
and the liquid was
stirred until homogeneous. The composition was poured into about 50
suppository moulds, each
containing about 2g of composition; the suppository moulds were sealed and
cooled.
[0355] The suppository is administered vaginally and/or rectally to
individuals suffering from
cancer, either alone or in combination with conventional cancer therapy, and
results in a
reduction of tumor growth. The suppository is also administered vaginally
and/or rectally to
individuals afflicted with side effects resulting from conventional cancer
therapy, and reduces
the incidences of such side effects.
[0356] Example 7 ¨ Method and Formulation for Treatment of Vaginal Yeast
Infection,
Bacteriosis and Vaginitis
[0357] The cannabis root oil and/or emulsion as described in Example 1 is
particularly useful as
a treatment of vaginal yeast infection, bacterial vaginosis and vaginitis. In
particular, the
cannabis root oil produced by this method can be used advantageously in a
suppository, for
treatment of vaginal yeast infection, bacterial vaginosis and vaginitis.
[0358] It has been discovered that the cannabis root oil and/or emulsion as
hereindescribed is a
useful remedy for treatment of vaginal yeast infection, bacterial vaginosis
and vaginitis, when
66
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
administered in a suppository formulation. It has also been discovered that
tetrahydrocannabinol
and/or cannabidiol are also useful in alleviating these symptoms. We have
obtained excellent
therapeutic effect, which may be additive or even synergistic, when
administering combinations
of cannabis root oil, tetrahydrocannabinol and cannabidiol.
[0359] Disclosed are a variety of suppositories highly effective for treatment
of vaginal yeast
infection, bacterial vaginosis and vaginitis, containing such cannabis root
oil, either alone, or
combined with THC and/or CBD. Other therapeutics may also be used in the
formulation. For
example, tea tree essential oil, lavender essential oil, geranium essential
oil, clary sage essential
oil, basil essential oil, thyme essential oil, Manuka essential oil, and/or
helichrysum essential oil.
We have created a combination particularly useful and effective in treatment
of vaginal yeast
infection, bacterial vaginosis and vaginitis, comprising cannabis root oil,
CBD, THC, tea tree
essential oil, lavender essential oil, geranium essential oil, clary sage
essential oil, basil essential
oil, thyme essential oil, Manuka essential oil, and helichrysum essential oil.
[0360] Any known base can be used for the suppository; we have found that
cacao butter can be
effectively used.
10361] Example 7A: Suppository formulation utilizing cannabis root oil
[0362] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil is added, and the liquid is stirred until the oil appears
completely blended.
The composition is poured into about 50 suppository moulds, each containing
about 2g of
composition; the suppository moulds are sealed and cooled.
[0363] The suppository is administered vaginally and/or rectally to women
suffering from
vaginal yeast infection, bacterial vaginosis and/or vaginitis, and results in
a noticeable decrease
in those ailments.
67
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
103641 Example 7B: Suppository formulation utilizing cannabis root oil, THC
and CBD
[0365] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, and about 200 mg of a 2 mg active/gram, 1:1 ratio
THC/CBD resin is
added, and the liquid is stirred until homogeneous. The composition is poured
into about 50
suppository moulds, each containing about 2g of composition; the suppository
moulds are sealed
and cooled.
[0366] The suppository is administered vaginally and/or rectally to women
suffering from
vaginal yeast infection, bacterial vaginosis and/or vaginitis, and results in
a noticeable decrease
in those ailments.
103671 Example 7C: Suppository formulation
[0368] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 200 mg of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 1.25 ml
lavender oil, about 1.25 ml of tea tree essential oil, about 0.5 ml of
geranium oil, about 0.25 ml
of clary sage oil, about 0.25 ml of basil essential oil, about 0.25 ml of
thyme essential oil, about
0.25 ml of Manuka essential oil, and about 0.15 ml of helichrysum essential
oil was added, and
the liquid was stirred until homogeneous. The composition was poured into
about 50
suppository moulds, each containing about 2g of composition; the suppository
moulds were
sealed and cooled.
[0369] The suppository was administered vaginally to women suffering from
vaginal yeast
infection, bacterial vaginosis and/or vaginitis, and resulted in a noticeable
decrease in at least one
or more of those ailments.
68
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0370] Example 8 ¨ Method and Formulation for the Treatment of Effects of
Menopause
[0371] The cannabis root oil and/or emulsion as described in Example 1 is
particularly effective
when used in a formulation for treatment of pre-menopausal and menopausal
symptoms such as
hot flashes, shivering, sweating and reddening of the skin, mood changes,
vaginal dryness, and
trouble sleeping. In particular, the cannabis root oil produced by this method
can be used
advantageously in a suppository, for treatment of pre-menopausal and
menopausal symptoms
such as hot flashes, shivering, sweating and reddening of the skin, mood
changes, vaginal
dryness, and trouble sleeping.
[0372] It has been discovered that the cannabis root oil as hereindescribed is
a useful remedy for
treatment of pre-menopausal and menopausal symptoms such as hot flashes,
shivering, sweating
and reddening of the skin, mood changes, vaginal dryness, and trouble
sleeping, when
administered in a suppository formulation. It has also been discovered that
tetrahydrocannabinol
and/or cannabidiol are also useful in alleviating these symptoms. We have
obtained excellent
therapeutic effect, which may be additive or even synergistic, when
administering combinations
of cannabis root oil/emulsion, tetrahydrocannabinol and cannabidiol.
[0373] Disclosed are a variety of suppositories highly effective for treatment
of pre-menopausal
and menopausal symptoms such as hot flashes, shivering, sweating and reddening
of the skin,
mood changes, vaginal dryness, and trouble sleeping, containing such cannabis
root oil, either
alone, or combined with THC and/or CBD. Other therapeutics may also be used in
the
formulation. For example, vitex berry essential oil, lavender essential oil,
clary sage essential
oil, geranium essential oil, basil essential oil and/or chamomile essential
oil. We have created a
combination particularly useful and effective in treatment of pre-menopausal
and menopausal
symptoms such as hot flashes, shivering, sweating and reddening of the skin,
mood changes,
vaginal dryness, and trouble sleeping, comprising cannabis root oil, CBD, THC,
vitex berry
essential oil, lavender oil, clary sage oil, basil oil, geranium oil, and
chamomile oil.
69
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0374] Any known base can be used for the suppository; we have found that
cacao butter can be
effectively used.
10375] Example 8A: Suppository formulation utilizing cannabis root oil
[0376] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil is added, and the liquid is stirred until the oil appears
completely blended.
The composition is poured into about 50 suppository moulds, each containing
about 2g of
composition; the suppository moulds are sealed and cooled.
io
[0377] The suppository is administered vaginally and/or rectally to women
suffering from pre-
menopausal and menopausal symptoms such as hot flashes, shivering, sweating
and reddening of
the skin, mood changes, vaginal dryness, and trouble sleeping, and results in
a noticeable
decrease in those symptoms.
103781 Example 8B: Suppository formulation utilizing cannabis root oil, THC
and CBD
[0379] 100 g of cacao butter is melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, and about 200 mg of a 2 mg active/gram, 1:1 ratio
THC/CBD resin is
added, and the liquid is stirred until homogeneous. The composition is poured
into about 50
suppository moulds, each containing about 2g of composition; the suppository
moulds are sealed
and cooled.
[0380] The suppository is administered vaginally and/or rectally to women
suffering from pre-
menopausal and menopausal symptoms such as hot flashes, shivering, sweating
and reddening of
the skin, mood changes, vaginal dryness, and trouble sleeping, and results in
a noticeable
decrease in those symptoms.
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
103811 Example 8C: Suppository formulation
[0382] 100 g of cacao butter was melted in a stainless steel pot, to about 102
degrees F. About 1
ml cannabis root oil, about 200 mg of a 2mg/g active, 1:1 ratio THC/CBD resin,
about 1.25 ml
lavender oil, about 1.25 ml vitex berry essential oil, about 0.25 ml of each
of clary sage oil,
geranium oil, and chamomile oil, and about 0.15 ml of basil essential oil was
added, and the
liquid was stirred until homogeneous. The composition was poured into about 50
suppository
moulds, each containing about 2g of composition; the suppository moulds were
sealed and
cooled.
[0383] The suppository was administered vaginally to women suffering from pre-
menopausal
and menopausal symptoms such as hot flashes, shivering, sweating and reddening
of the skin,
mood changes, vaginal dryness, and trouble sleeping, and resulted in a
noticeable decrease in at
least one or more of those symptoms.
[0384] Example 9 ¨ Skin Moisturizer Formulations
[0385] The cannabis root oil and/or emulsion as described in Example 1 is
highly effective as
skin moisturizers when used in topical formulations, containing such cannabis
root oil.
Unexpectedly, cannabis root oil has been found to have excellent skin
moisturizing properties.
Also unexpectedly, CBD oil has been found to have excellent skin moisturizing
properties. We
have found that a combination of cannabis root oil and CBD oil to be
particularly effective as a
skin moisturizer. In particular, one or more of CBD oil and cannabis root oil
with other oils have
resulted in formulations highly effective as skin moisturizers. For example,
one or more of CBD
oil and cannabis root oil may be combined with one or more of jojoba seed oil,
avocado oil,
apricot kernel oil, rose water, honey, grapefruit seed extract, cetyl stearyl
alcohol, stearic acid,
turmeric root oil, juniper berry oil, jasmine flower oil, sandalwood oil,
copaiba oil, pomegranate
seed oil, chamomile water, stearic acid, ginger root oil, cinnamon bark oil,
clove bud oil,
71
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
cardamom seed oil, lavender flower oil, rose flower oil, sweet almond oil,
goat milk powder,
basil oil, bergamot peel oil, fennel seed oil, frankincense oil, and lavender
flower oil.
[0386] Example 9A: Balance moisturizer
[0387] A skin moisturizer was made by blending and emulsifying the following
ingredients in
the following wt. proportions:
Cannabis root oil prepared according to the 4.5%
above method
Cannabidiol oil 0.003%
Simmondsia chinensis (jojoba) seed oil 13%
Persea gratissima (Avocado) oil 8%
Prunus armeniaca (apricot) kernel oil 8%
Rose damascene (Rose) water 56%
Honey 0.6%
Citrus grandis (Grapefruit) seed extract 0.3%
Cetyl-stearyl alcohol (emulsifying wax) 4.75%
Stearic acid 4.75%
Curcuma longa (Tumeric) root oil 0.014%
Juniperus communis (juniper) berry oil 0.014%
Rosa damascene (rose) flower oil 0.014%
Jasminium sambac (Jasmine) flower oil 0.014%
Santalum spicatum (Sandalwood) wood oil 0.014%
Copaifera spp (copaiba) oil 0.027%
72
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0388] The skin moisturizer was found to have excellent hydrating effect, that
provided a
superior benefit and would last all day. The skin moisturizer composition was
found to work
especially well for sensitive skin types. The moisturizer composition was
tested against a
known, commercially available, highly rated skin moisturizer, by applying the
known
moisturizer on half the face of an individual, and applying similar amounts of
the moisturizer of
the present invention on the other half The moisturizer of the present
invention provided
immediate difference in skin texture when applied, and the skin felt more
robust and elastic, and
appeared more lustrous and youthful. The skin felt thicker to the touch, yet
softer at the same
time. The skin looked "uplifted", and these qualities remained throughout the
day. These
differences were noticeable and improved both against the non-treated faces,
as well as the face
treated with known moisturizer.
[0389] Example 9B: Vitalize moisturizer
[0390] A skin moisturizer was made by blending and emulsifying the following
ingredients in
the following wt. proportions:
Cannabis root oil prepared according to the 4%
above method
Cannabidiol oil 0.003%
Simmondsia chinensis (jojoba) seed oil 13%
Persea gratissima (Avocado) oil 10%
Honey 0.6%
Citrus grandis (Grapefruit) seed extract 0.3%
Cetyl-stearyl alcohol (emulsifying wax) 4.75%
Stearic acid 4.75%
Rosa damascene (rose) flower oil 0.014%
73
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
Copaifera spp (copaiba) oil 0.016%
Punica Granatum (Pomegranate) seed oil 6.5%
Chamaemelum Nobile (Chamomile) water 55.997%
Zingiber Officinale (Ginger) root oil 0.014%
Lavandula Angustifoila (Lavender) flower oil 0.014%
Cinamomum Zeylanicum (cinnamon) bark oil 0.014%
Syzygium Aromaticum (clove) bud oil 0.014%
Elettaria Cardamomom (Cardamom) seed oil 0.014%
[0391] The skin moisturizer was found to have excellent hydrating effect, that
provided a
superior benefit and would last all day. The skin moisturizer composition was
found to work
especially well for dry skin types. The moisturizer composition was tested
against a known,
commercially available, highly rated skin moisturizer, by applying the known
moisturizer on half
the face of an individual, and applying similar amounts of the moisturizer of
the present
invention on the other half The moisturizer of the present invention provided
immediate
difference in skin texture when applied, and the skin felt more robust and
elastic, and appeared
more lustrous and youthful. The skin felt thicker to the touch, yet softer at
the same time. The
.. skin looked "uplifted", and these qualities remained throughout the day.
These differences were
noticeable and improved both against the non-treated faces, as well as the
face treated with
known moisturizer.
[0392] Example 9C: Align moisturizer
[0393] A skin moisturizer was made by blending and emulsifying the following
ingredients in
the following wt. proportions:
74
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
Cannabis root oil prepared according to the 16.5%
above method
Cannabidiol oil 0.003%
Simmondsia chinensis (jojoba) seed oil 10%
Prunus amygdalus (sweet almond) oil 6.5%
Honey 0.6%
Citrus grandis (Grapefruit) seed extract 0.3%
Cetyl-stearyl alcohol (emulsifying wax) 4.75%
Stearic acid 4.75%
Copaifera spp (copaiba) oil 0.02%
Water 55.893%
Lavandula Angustifoila (Lavender) flower oil 0.014%
Syzygium Aromaticum (clove) bud oil 0.014%
Goat milk powder 0.6%
Ocimum basilicum (basil) oil 0.014%
Citrus bergamia (Bergamot) peel oil 0.014%
Foeniculum (Fennel) seed oil 0.014%
Boswellia Serrata (Frankincense) oil 0.014%
[0394] The skin moisturizer was found to have excellent hydrating effect, that
provided a
superior benefit and would last all day. The skin moisturizer composition was
found to work
especially well for oily skin types. The moisturizer composition was tested
against a known,
commercially available, highly rated skin moisturizer, by applying the known
moisturizer on half
the face of an individual, and applying similar amounts of the moisturizer of
the present
invention on the other half The moisturizer of the present invention provided
immediate
difference in skin texture when applied, and the skin felt more robust and
elastic, and appeared
more lustrous and youthful. The skin felt thicker to the touch, yet softer at
the same time. The
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
skin looked "uplifted", and these qualities remained throughout the day. These
differences were
noticeable and improved both against the non-treated faces, as well as the
face treated with
known moisturizer.
[0395] Example 9D: Testimonials
[0396] Users of the skin moisturizers of the present invention provided the
following
testimonials:
= The serum and vitalize moisturizer feel like a fountain of youth on my
face when used
together - I am truly amazed at how it works literally as soon as they are
applied!
= I wanted to reach out and let you know that your moisturizer is amazing!
I held off on
using it however I ran out of my usual moisturizer and this was a game changer
for my
skin.
= I am very prone to hormonal breakouts and this past month and a half I
have not
experienced breakouts, my skin is a lot brighter and so much softer.
= I get stopped on the street by people who don't even know me asking what
products I use.
My skin looks hydrated, healthy and alive.
= This product is unreal! My Mom and I both are using it and we love it. It
smells gorgeous
and it hydrates my dry skin in the prairies. No negative reactions to my
sensitive skin. I
love it.
= I suffer from inflammation daily. This line has completely helped my skin
to calm the
inflammation. I will continue to purchase it.
= The moisturizing face cream really helps hydrate my skin. Not greasy.
= I suffer from psoriasis. These products have totally saved my hands and
feet.
= My skin is SO sensitive. This line totally calms my skin and makes it feel
so good. I'm no
longer as red in the face. Use it twice a day. My new favourite line.
76
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
= I never use product normally but my girlfriend had these products and she
had me try
them. I'm hooked.
= I found the products to be surprisingly hydrating with just a small
amount.
= I live in Saskatchewan where it is very dry. I have been receiving
numerous comments
from my staff at the bank telling me how great my skin feels. It is due to
these products!
= This has been so amazing for my dry skin in the wintertime!!! I've been
applying the
moisturizer right after washing my face in the morning (and under my make up),
and I've
also been utilizing it at night time after washing my make up off I love the
fact that it has
such a soothing smell; it doesn't leave any oily residue behind, and it
doesn't seem to
affect my make up application/wear either (which is a total plus).
= I don't have sensitive skin by any means, but I'm usually pretty hesitant
when it comes to
trying out new serums. I've been using this at night time only, and I've
noticed a
significant difference in the appearance of my skin - it's brighter, and
there's a lot less
discoloration. It's also made my skin a lot softer, and it's removed the dry
patches/spots
that I had gotten from the drying winter months. I've also gotten my mom to
test it, who
has much oily skin than me, and she's had good results as well.
[0397] Example 10 ¨ Method and Formulation for use as a Female Sexual
Lubricant
[0398] Disclosed is a topical formulation for use as a female sexual
lubricant. The topical
formulation is useful in healthy individuals as an aid or enhancer of the
sexual experience. It
promotes lubrication, provides a desirable "tingling" sensation, and
encourages and/or enhances
orgasm. In addition, it is useful in individuals suffering from sexual
dysfunction, providing the
same benefits. Informal studies have shown that the topical formulation has
allowed or
facilitated certain women suffering from sexual dysfunction to experience
orgasm for the first
time.
77
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0399] The topical formulation contains THC, for example in the form of a THC
resin, and/or
ashwagandha oil, and/or cannabis root oil. Without being limited by theory, it
is believed that the
THC provides a desirable "tingling" sensation in the sexual lubricant, the
cannabis root oil has
promoted vascularization and relaxation, and the ashwagandha oil enhances the
sexual
experience and promotes excitement and/or orgasm, by relaxing muscle and skin,
and by
stimulating natural vaginal lubrication. The topical formulation has also been
found to promote
lubrication; it is believed that all three active ingredients may contribute
to this, as well as some
of the other ingredients in the formulation. It is believed that the three
active ingredients, or any
two of the three active ingredients, may act synergistically or additively to
greatly enhance the
sexual experience in both healthy women and women suffering from sexual
dysfunction.
[0400] In certain embodiments, the pharmaceutical formulation is in the form
of a cream, an
ointment, or a lotion. Preferably, the pharmaceutical formulation is in the
form of an oil.
[0401] The topical formulation is prepared in the form of an oil. The topical
formulation
comprises ashwaganda oil, cannabis root oil, and/or THC.
[0402] In certain embodiments, the topical formulation comprises ashwaganda
oil and THC. In
other embodiments, the topical formulation comprises ashwaganda oil and
cannabis root oil. In a
preferred embodiment, the topical formulation comprises ashwaganda oil,
cannabis root oil, and
THC.
[0403] It is believed that the hemp root oil provides anti-bacterial, anti-
microbial, and pain
relieving qualities. It may also act as a relaxant, which can act to aid in
the sexual experience.
[0404] It has been found that the addition of the THC, preferably in the form
of a THC resin,
amplified the sexual experience. Interestingly, users of the topical
formulation reported a
pleasurable "tingling" sensation when the topical formulation contained THC.
78
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0405] The ashwagandha oil was found to enhance the sexual experience and
promotes
excitement and/or orgasm, by relaxing muscle and skin, and by stimulating
natural vaginal
lubrication.
[0406] The three active ingredients can be placed in a carrier/diluent, which
may be any
pharmaceutically acceptable oil. Preferably, avocado oil can be used as a
carrier and diluant; we
have found that the specific viscosity of the avocado oil to be especially
suitable for use as a
sexual lubricant. Avocado oil was also particularly advantageous due to its
skin nourishing
properties. Preferably, a "natural" oil is utilized, i.e. an oil extracted
from a fruit or vegetable
source, or a grain or plant.
[0407] Other, optional ingredients may be added, as follows.
[0408] Menthol crystals, can be added to the formulation to enhance
stimulation, and provide a
unique sensation.
[0409] Black pepper oil can be added to the formulation, for its energizing
and warming
(stimulating) properties, as well as for its anti-oxidant and antimicrobial
qualities.
[0410] A topical formulation was prepared comprising (by weight) about 20%
hemp root oil,
about 60% avocado oil (Persea gratissima), and about 20% ashwaganda oil. About
0.2% by
weight of THC, about 1% by weight of menthol crystals, and about 0.8% by
weight of black
pepper oil was added.
[0411] The topical formulation was provided to women pre-coitus, and was
applied liberally,
intervaginally. The topical formulation was found to enhance and improve the
sexual
79
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
experience. In many cases, orgasm was induced, notably, in some cases, in
individuals who had
never previously experienced the sensation.
[0412] Example 11: Method and Formulation for Use as an Anti-Aging Serum
[0413] Disclosed are a variety of topical formulations highly effective as
anti-aging serum. A
combination of CBD and HA has been found to have unexpectedly high
effectiveness at
reducing the signs of aging, including wrinkles, loss of firmness, and
diminished appearance of
plumpness, suppleness, and elasticity. The formulations are effective in
smoothing skin,
increasing suppleness and elasticity, and giving skin a more youthful and
desirable appearance.
It is believed that the combination of CBD and HA provide results that are
better than either
ingredient alone, and may provide additive or even synergistic results.
[0414] CBD and HA can be combined with other ingredients to form formulations
with even
greater effect. For example, it has been found that CBD and HA may be combined
with one or
more of a lactobacillus and coconut fruit extract such as AMTicide Coconut,
lavender essential
oil, copaiba essential oil, frankincense essential oil, carrot seed essential
oil, geranium essential
oil, and sea buckthorn berry essential oil. CBD and/or HA can also be combined
with the hemp
root oil or emulsion as hereindescribed for excellent results.
[0415] The formulations may also contain an gel or emulsifying agent, or both;
for example, the
formulations may contain ECOGELTM, a gelling-emulsifying agent that increases
the viscosity
and stability of formulations. As well, the formulations may contain
preservative agents, such as
for example, Leucidal Liquid SF.
[0416] Example 11A: Anti-aging Serum
[0417] An anti-aging serum was made by blending and emulsifying the following
ingredients in
the following wt. proportions:
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
Hyaluranic acid EIMW 1.0-1.5 Mil Daltons .93
Leucidal Liquid SF 1.86
AMTicide Coconut 1.86
Ecogel 1.86
Water 90.2
Lavender essential oil .93
Copaiba essential oil .93
Frankincense essential oil .37
Carrot seed essential oil .23
Geranium essential oil .28
Sea buckthorne essential oil .23
CBD .30
[0418] The anti-aging serum was found to have excellent and unexpectedly high
effectiveness at
reducing the signs of aging, including wrinkles, loss of firmness, and
diminished appearance of
plumpness, suppleness, and elasticity. The formulations were effective in
smoothing skin,
increasing suppleness and elasticity, and giving skin a more youthful and
desirable appearance.
The anti-aging serum composition was tested against a known, commercially
available, highly
rated anti-aging serum, by applying the known anti-aging serum on half the
face of an individual,
and applying similar amounts of the anti-aging serum of the present invention
on the other half
The anti-aging serum of the present invention provided immediate difference in
skin texture
when applied, and the skin felt more robust and elastic, and appeared more
lustrous and youthful.
The skin felt thicker to the touch, yet softer at the same time. The skin
looked "uplifted", and
these qualities remained throughout the day. These differences were noticeable
and improved
both against the non-treated faces, as well as the face treated with known
anti-aging serum.
81
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
[0419] Example 11B: Anti-aging serum
[0420] An anti-aging serum can be made by blending and emulsifying the
following ingredients
in the following wt. proportions:
Hyaluranic acid UMW 1.0-1.5 Mil Daltons .90
Water 98.8
CBD .30
[0421] The anti-aging serum has excellent and unexpectedly high effectiveness
at reducing the
signs of aging, including wrinkles, loss of firmness, and diminished
appearance of plumpness,
suppleness, and elasticity. The formulation is effective in smoothing skin,
increasing suppleness
and elasticity, and giving skin a more youthful and desirable appearance. The
anti-aging serum
composition can be tested against a known, commercially available, highly
rated anti-aging
serum, by applying the known anti-aging serum on half the face of an
individual, and applying
similar amounts of the anti-aging serum of the present invention on the other
half The anti-
aging serum of the present invention can provide immediate difference in skin
texture when
applied, with the skin feeling more robust and elastic, and more lustrous and
youthful. The skin
will feel thicker to the touch, yet softer at the same time. The skin looks
"uplifted", and these
qualities remain throughout the day. These differences are noticeable and
improved both against
the non-treated faces, as well as the face treated with known anti-aging
serum.
[0422] Example 11C: Anti-Aging Serum
[0423] An anti-aging serum can be made by blending and emulsifying the
following ingredients
in the following wt. proportions:
Ingredient - INCI name Formula %
Aqua 91.220
82
CA 03096547 2020-10-08
WO 2019/195943
PCT/CA2019/050451
Lactobacillus Ferment 2.000
Lactobacillus, Cocos Nucifera (Coconut) Fruit Extract 2.000
Hyaluronic Acid 1.000
Lavandula Angustifolia (Lavender) Oil 1.000
Copaifera Officinalis (Balsam Copaiba) Resin Oil 1.000
Lysolecithin, Sclerotium Gum, Xanthan Gum, Pullulan 0.500
Boswellia Carterii (Frankincense) Gum Oil 0.400
Pelargonium Graveolens (Geranium) Flower Oil 0.300
Daucus Carota Sativa (Carrot) Seed Oil 0.250
Hippophae Rhamnoides (Sea Buckthorn) Oil 0.250
Cannabis Sativa (Hemp) Seed Oil 0.040
Hemp Root Oil 0.040
[0424] The anti-aging serum has an off-white slightly viscous serum form, and
a pH of 4.5-5.5.
It has excellent and unexpectedly high effectiveness at reducing the signs of
aging, including
wrinkles, loss of firmness, and diminished appearance of plumpness,
suppleness, and elasticity.
The formulation is effective in smoothing skin, increasing suppleness and
elasticity, and giving
skin a more youthful and desirable appearance. The anti-aging serum
composition can be tested
against a known, commercially available, highly rated anti-aging serum, by
applying the known
anti-aging serum on half the face of an individual, and applying similar
amounts of the anti-aging
serum of the present invention on the other half The anti-aging serum of the
present invention
can provide immediate difference in skin texture when applied, with the skin
feeling more robust
and elastic, and more lustrous and youthful. The skin will feel thicker to the
touch, yet softer at
the same time. The skin looks "uplifted", and these qualities remain
throughout the day. These
differences are noticeable and improved both against the non-treated faces, as
well as the face
treated with known anti-aging serum.
83