Note: Descriptions are shown in the official language in which they were submitted.
EVERTING TRANSCATHETER VALVE AND METHODS
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application is claims priority to provisional application Serial
No.
61/675,744 filed July 25, 2012.
FIELD
[002] The present disclosure relates generally to prosthetic valves and
more
specifically leaflet-type prosthetic valve devices, systems, and methods for
transcatheter delivery.
BACKGROUND
[003] A transcatheter prosthetic valve that can be delivered endovascularly
via a catheter can help to minimize patient trauma as compared with an open-
heart,
surgical procedure. Open heart surgery involves extensive trauma to the
patient, with
attendant morbidity and extended recovery. A valve delivered to the recipient
site via
a catheter avoids the trauma of open heart surgery and may be performed on
patients too ill or feeble to survive the open heart surgery.
[004] Transcatheter valve implantation with currently available
transcatheter
valves and associated delivery catheters, together referred herein as delivery
systems, present several procedural-related complications. Trauma to the
peripheral
vasculature as well as dissection of the ascending and descending aorta has
been
observed. This trauma is associated, in part, with the relatively large
diameter of the
delivery systems. Minimizing such trauma can be facilitated by minimizing the
diameter of the delivery system which is determined, in part, by the profile
of the
valve on the associated delivery catheter.
[005] Reducing the profile of the prosthetic heart valve on the delivery
catheter is technically challenging. For example, a 23 mm diameter aortic
prosthetic
valve might have to be advanced through 10 mm diameter vasculature to reach
the
deployment site. This requires that the valve be compressed to a smaller
diameter
1
Date Recue/Date Received 2020-11-04
upon the delivery catheter such that it and the delivery catheter present a
diameter
somewhat smaller than 10 mm.
[006] The profile of the valve is dependent, in part, on the valve
components.
Some transcatheter valve devices comprise a valve having flexible leaflets
mounted
inside a tubular metal frame. The metal frame may be self expanding or balloon-
expanded from a pre-deployed compressed diameter to the deployed functional
diameter. The diameter of the delivery system is dependent, in part, on the
resulting
thickness of the compressed valve leaflets within the frame as it is mounted
on the
delivery catheter.
[007] The transcatheter valve must be capable of being securely coupled to
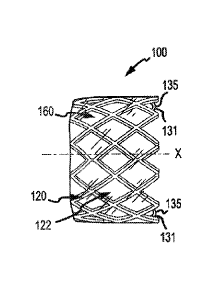
the tissue orifice of the implantation site after endovascular placement so as
to
avoid, for example, dislodgement or migration of the valve after placement.
The
coupling of the valve to the implantation site is commonly facilitated by
relatively high
hoop strength of the frame placed in urging engagement with the tissue
orifice.
[008] Bioprosthetic valves have been developed that attempt to mimic the
function and performance of a native valve. Flexible leaflets are fabricated
from
biological tissue such as bovine pericardium. In some valve designs the
biological
tissue is sewn onto a relatively rigid frame that supports the leaflets and
provides
dimensional stability when implanted. Although bioprosthetic valves can
provide
excellent hemodynamic and biomechanical performance in the short term, they
are
prone to calcification and cusp tears, among other failure modes, requiring
reoperation and replacement.
[009] Attempts have been made to use synthetic materials, such as
polyurethane, among others, as a substitute for the biological tissue, to
provide a
more durable flexible leaflet prosthetic valve, herein referred to as a
synthetic leaflet
valve (SLV). However, synthetic leaflet valves have not become a valid valve
replacement option since they suffer premature failure, due to, among other
things,
suboptimal design and lack of a durable synthetic material.
[0010] A number of fabrication techniques have been used to couple the
leaflets to a frame, including sewing individual leaflets to the frame
(biological and
synthetic), and for synthetic leaflets only, injection molding and dip coating
a polymer
onto the frame. In each case, the resulting leaflet is supported on the frame
and
defines a flap having a mounting edge where the leaflet is coupled to the
frame and
a free edge that allows the flap to move. The flap moves under the influence
of fluids
2
Date Recue/Date Received 2020-11-04
pressure. In operation, the leaflets open when the upstream fluid pressure
exceeds
the downstream fluid pressure and close when the downstream fluid pressure
exceeds the upstream fluid pressure. The free edges of the leaflets coapt
under the
influence of downstream fluid pressure closing the valve to prevent downstream
blood from flowing retrograde through the valve.
[0011] Valve durability under the repetitive loads of the leaflets opening and
closing is dependent, in part, on the load distribution between the leaflet
and the
frame. Further, substantial load is encountered on the leaflet when in the
closed
position. Mechanical failure of the leaflet can arise, for example, at the
mounting
edge, where the flexible leaflet is supported by the relatively rigid frame.
The
repetitive loads of leaflet opening and closing leads to material failure by
fatigue,
creep or other mechanism, depending in part on the leaflet material.
Mechanical
failure at the mounting edge is especially prevalent with synthetic leaflets.
[0012] There exists a need for a durable transcatheter prosthetic valve that
is
compressible to a small diameter and capable of being delivered
endovascularly.
SUMMARY
[0013] Described embodiments are directed to an apparatus, system, and
methods for valve replacement, such as cardiac valve replacement. More
specifically, described embodiments are directed toward flexible leaflet valve
devices
and systems having a multi-part support member or frame, and methods of making
and delivering the valve devices.
[0014] According to an embodiment, a valve comprises a leaflet frame, a body
frame, and any number of leaflets suitable for the size and function of the
valve,
having a collapsed configuration and an expanded configuration. In a further
embodiment, the valve can comprise an everted configuration and a non-everted
configuration.
[0015] According to an embodiment, a transcatheter valve comprising a body
frame and a leaflet frame coupled by a film is provided. The body frame has a
generally tubular shape defining a body frame lumen. The leaflet frame has a
generally annular shape defining a plurality of U-shaped portions each
defining a
base and a plurality of posts. The body frame extends coaxially, adjacent to
and
spaced apart from the leaflet frame. The base of each U-shaped portion being
3
Date Recue/Date Received 2020-11-04
located proximate to but not in contact with a body frame first end of the
body frame
with the U-shaped portions of the leaflet frame extending away from the body
frame
and the posts extending away from body frame, the posts being distal from the
body
frame first end. The film extends across and between the U-shaped portions and
the
body frame. The film that extends between the body frame and the leaflet frame
defines a fold region. The film that extends across each of the U-shaped
portions
defines a leaflet. The leaflet frame is operable to evert to an everted
position by
rotating about the fold region to a position in which the leaflet frame is at
least
partially coaxially disposed at least partially within the body frame lumen,
wherein
each leaflet is moveable between an open and closed position.
[0016] According to an embodiment, a transcatheter valve comprising a body
frame and a leaflet frame coupled by a film is provided. The body frame
defines a
generally tubular shape. The leaflet frame defines a generally annular shape.
The
leaflet frame is coaxially disposed relative to the body frame, extending away
and
spaced apart from the body frame defining a fold region therebetween. The
leaflet
frame defines a plurality of U-shaped portions each defining a base and a
plurality of
posts. The base of each U-shaped portion being located proximate to but not in
contact with a body frame first end of the body frame with the U-shaped
portions of
the leaflet frame extending away from the body frame and the posts extending
away
from body frame, the posts being distal from the body frame first end. The
film
extends across and between the body frame and leaflet frame bridging the fold
region and coupling the body frame to the leaflet frame. The leaflet frame and
film
defines a plurality of leaflets disposed within each U-shaped portion, each
leaflet
having a leaflet free edge. The leaflet frame is operable to evert along the
fold
region so as to dispose the leaflet frame at least partially within the body
frame and
defining a valve wherein the leaflet free edges abut adjacent leaflet free
edges and
are moveable between an open and closed position.
[0017] According to an embodiment, a transcatheter valve delivery system
comprising a delivery catheter, and a transcatheter valve having a body frame
and a
leaflet frame coupled by a film is provided. The body frame has a generally
tubular
shape defining a body frame lumen. The leaflet frame has a generally annular
shape defining a plurality of U-shaped portions each defining a base and a
plurality
of posts. The body frame extends coaxially, adjacent to and spaced apart from
the
leaflet frame. The base of each U-shaped portion being located proximate to
but not
4
Date Recue/Date Received 2020-11-04
in contact with a body frame first end of the body frame with the U-shaped
portions
of the leaflet frame extending away from the body frame and the posts
extending
away from body frame, the posts being distal from the body frame first end.
The film
extends across and between the U-shaped portions and the body frame. The film
that extends between the body frame and the leaflet frame defines a fold
region.
The film that extends across each of the U-shaped portions defines a leaflet.
The
leaflet frame is operable to evert to an everted position by rotating about
the fold
region to a position in which the leaflet frame is at least partially
coaxially disposed at
least partially within the body frame lumen, wherein each leaflet is moveable
between an open and closed position. The transcatheter valve comprises a
collapsed configuration and an expanded configuration. The delivery catheter
is
operable to advance the transcatheter valve to an implantation site.
[0018] According to another embodiment, a transcatheter valve replacement
system comprises a valve having a leaflet frame, a body frame, and any number
of
leaflets, wherein the valve comprises a collapsed configuration and an
expanded
configuration, and a catheter. The system can further comprise an everting
device to
transition the valve from an everted configuration to a non-everted
configuration.
[0019] According to another embodiment, a method of making a transcatheter
valve comprises the steps of coupling a leaflet frame and a body frame with a
biocompatible material as described herein, either simultaneously or
sequentially,
and thereby also forming leaflets.
[0020] Other methods can comprise delivering, via an intravascular
procedure, a transcatheter valve comprising a leaflet frame, a body frame, and
any
number of leaflets and having a collapsed configuration and an expanded
configuration. The method can comprise everting the valve once the
transcatheter
valve is at its implantation site.
[0021] According to another embodiment, a method of delivery of a
transcatheter valve comprises loading a transcatheter valve in a collapsed
configuration onto a distal section of an elongated flexible catheter having
proximal
and distal ends, delivering the transcatheter valve to a native valve orifice
intravascularly, expanding the transcatheter valve into a native orifice, and
everting
the leaflet frame into the body frame lumen of the transcatheter valve. The
transcatheter valve comprises a body frame and a leaflet frame coupled by a
film is
provided. The body frame has a generally tubular shape defining a body frame
Date Recue/Date Received 2020-11-04
lumen. The leaflet frame has a generally annular shape defining a plurality of
U-
shaped portions each defining a base and a plurality of posts. The body frame
extends coaxially, adjacent to and spaced apart from the leaflet frame. The
base of
each U-shaped portion being located proximate to but not in contact with a
body
frame first end of the body frame with the U-shaped portions of the leaflet
frame
extending away from the body frame and the posts extending away from body
frame,
the posts being distal from the body frame first end. The film extends across
and
between the U-shaped portions and the body frame. The film that extends
between
the body frame and the leaflet frame defines a fold region. The film that
extends
across each of the U-shaped portions defines a leaflet. The leaflet frame is
operable
to evert to an everted position by rotating about the fold region to a
position in which
the leaflet frame is at least partially coaxially disposed at least partially
within the
body frame lumen, wherein each leaflet is moveable between an open and closed
position.
BRIEF DESCRIPTION OF THE DRAWINGS
[0022] The accompanying drawings are included to provide a further
understanding of the present disclosure and are incorporated in and constitute
a part
of this specification, illustrate embodiments described herein, and together
with the
description serve to explain the principles discussed in this disclosure.
[0023] FIG. 1A is a side view of an embodiment of a two piece valve in a non-
everted configuration;
[0024] FIG. 1B is a side view of the embodiment of the two piece valve of FIG.
1A in an everted configuration;
[0025] FIG. 1C is a perspective view of the embodiment of the two piece valve
of FIG. 1A in an everted configuration;
[0026] FIG. 1D is a representation of a valve in an expanded configuration;
[002/ FIG. 1E is a representation of a valve in a compressed configuration;
[0028] FIG. 2 is a representation of the embodiment of the two piece valve of
FIG. 1A unrolled to a flat orientation;
[0029] FIG. 3A is an axial view of the embodiment of the two piece valve of
FIG. 1A in an open configuration;
6
Date Recue/Date Received 2020-11-04
[0030] FIG. 3B is a side view of the embodiment of the two piece valve of FIG.
1A in a closed configuration;
[0031] FIG. 3C is a side cross-sectional view of the embodiment of the
everted two piece valve of FIG. 1B;
[0032] FIG. 4 is a side view of an embodiment of a delivery system within
anatomy;
[0033] FIG. 5A is a cross-sectional view of an embodiment of the two piece
valve as mounted on a delivery catheter;
[0034] FIG. 5B is a side view of an embodiment of an everter;
[0035] FIG. 5C is a side view of the embodiment of the everter of FIG. 5B;
[0036] FIG. 6 is a perspective view of an embodiment of a winding jig for
forming a wire into a leaflet frame;
[0037] FIG. 7 is a side view of valve components on an assembly mandrel, in
accordance with an embodiment;
[0038] FIG. 8A is a side view of valve components on a two-piece mandrel for
forming leaflets, in accordance with an embodiment;
[0039] FIG. 8B is a side view of the two-piece mandrel for forming leaflets of
the embodiment of FIG. 8A;
[0040] FIG. 9A is a scanning electron micrograph image of ePTFE, in
accordance with an embodiment;
[0041] FIG. 9B is a scanning electron micrograph image of ePTFE, in
accordance with another embodiment; and
[0042] FIG. 9C is a higher magnification of the scanning electron micrograph
image of ePTFE of FIG. 9B.
DETAILED DESCRIPTION
[0043] Persons skilled in the art will readily appreciate that various aspects
of
the present disclosure can be realized by any number of methods and apparatus
configured to perform the intended functions. Stated differently, other
methods and
apparatuses can be incorporated herein to perform the intended functions. It
should
also be noted that the accompanying drawing figures referred to herein are not
necessarily drawn to scale, but may be exaggerated to illustrate various
aspects of
the present disclosure, and in that regard, the drawing figures should not be
construed as limiting.
7
Date Recue/Date Received 2020-11-04
[0044] Although the embodiments herein may be described in connection with
various principles and beliefs, the described embodiments should not be bound
by
theory. For example, embodiments are described herein in connection with
prosthetic valves, more specifically cardiac prosthetic valves. However,
embodiments within the scope of this disclosure can be applied toward any
valve or
mechanism of similar structure and/or function. Furthermore, embodiments
within
the scope of this disclosure can be applied in non-cardiac applications.
[0045] The term leaflet as used herein in the context of prosthetic valves is
a
component of a one-way valve wherein the leaflet is operable to move between
an
open and closed position under the influence of a pressure differential. In an
open
position, the leaflet allows blood to flow through the valve. In a closed
position, the
leaflet substantially blocks retrograde flow through the valve. In embodiments
comprising multiple leaflets, each leaflet cooperates with at least one
neighboring
leaflet to block the retrograde flow of blood. The pressure differential in
the blood is
caused, for example, by the contraction of a ventricle or atrium of the heart,
such
pressure differential typically resulting from a fluid pressure building up on
one side
of the leaflets when closed. As the pressure on an inflow side of the valve
rises
above the pressure on the outflow side of the valve, the leaflets opens and
blood
flows therethrough. As blood flows through the valve into a neighboring
chamber or
blood vessel, the pressure on the inflow side equalizes with the pressure on
the
outflow side. As the pressure on the outflow side of the valve raises above
the blood
pressure on the inflow side of the valve, the leaflet returns to the closed
position
generally preventing retrograde flow of blood through the valve.
[0046] The term membrane as used herein refers to a sheet of material
comprising a single composition, such as, but not limited to, expanded
fluoropolymer.
[0047] The term composite material as used herein refers to a combination of
a membrane, such as, but not limited to, expanded fluoropolymer, and an
elastomer,
such as, but not limited to, a fluoroelastomer. The elastomer may be imbibed
within a
porous structure of the membrane, coated on one or both sides of the membrane,
or
a combination of coated on and imbibed within the membrane.
[0048] The term laminate as used herein refers to multiple layers of
membrane, composite material, or other materials, such as elastomer, and
combinations thereof.
8
Date Recue/Date Received 2020-11-04
[0049] The term film as used herein generically refers to one or more of the
membrane, composite material, or laminate.
[0050] The term biocompatible material as used herein generically refers to a
film or a biological material, such as, but not limited to, bovine
pericardium.
[0051] The terms evert, everting, everted, eversion, and evertable as used
herein refer to the act, condition, or ability of being turned inside out by
folding
inward. As used herein, a leaflet frame extends away from a body frame in a
non-
everted condition, wherein the leaflet frame may be everted by folding the
leaflet
frame inward such that it extends at least partially into the body frame.
[0052] The terms native valve orifice and tissue orifice refers to an
anatomical
structure into which a prosthetic valve may be placed. Such anatomical
structure
includes, but is not limited to, a location wherein a cardiac valve may or may
not
have been surgically removed. It is understood that other anatomical
structures that
may receive a prosthetic valve include, but are not limited to, veins,
arteries, ducts
and shunts. Although reference is made herein to replacing a native valve with
a
prosthetic valve, it is understood and appreciated that a valve orifice or
implant site
may also refer to a location in a synthetic or biological conduit that may
receive a
valve for a particular purpose, and therefore the scope of the embodiments
provided
herein is not limited to valve replacement.
[0053] As used herein, "couple" means to join, couple, connect, attach,
adhere, affix, or bond, whether directly or indirectly, and whether
permanently or
temporarily.
[0054] Embodiments herein include various apparatus, systems, and methods
for a prosthetic valve suitable for transcatheter placement, such as, but not
limited to,
cardiac valve replacement. The valve is operable as a one-way valve wherein
the
valve defines a valve orifice into which leaflets open to permit flow and
close so as to
occlude the valve orifice and prevent flow in response to differential fluid
pressure.
[0055] In accordance with embodiments the valve is operable to have a pre-
deployed configuration where the valve leaflets are carried by a leaflet frame
that is
external to a body frame and a post-deployed configuration wherein the leaflet
frame
is everted into the body frame presenting the leaflets inside the body frame.
This
allows for greater radial compression of the valve to a smaller diameter
during
delivery as compared to a configuration wherein the leaflet frame and leaflets
are
within the body frame.
9
=
Date Recue/Date Received 2020-11-04
[0056] Further, each of the body frame and leaflet frame may have different
physical properties suitable for a particular purpose. In accordance with
embodiments, the body frame may be relatively stiff so as to abut and fixedly
engage
the tissue orifice as well as provide dimensional stability to the valve. The
leaflet
frame may be relatively less stiff relative to the body frame. The benefit of
the leaflet
frame being relatively less stiff relative to the body frame may be to slow
down the
rate of loading on the leaflets to reduce the stress levels on the leaflets
whereby
improving valve durability. Stiff and stiffness, as used herein and as is
commonly
used in engineering, is a measure of the resistance to deformation given by a
body.
Stiff and stiffness is a function of, among other things, material properties,
the shape
of the object, and the boundary conditions on the object. Stiffness of the
leaflet
frame 130 (see FIG. 1C) may be measured by any number of methods known in the
art. In accordance with one method, cables may be coupled to each of the three
posts 131 and brought together so as to allow the cables to be pulled
simultaneously
along the axis of the leaflet frame, with the leaflet frame restrained about
the flex
points 136 or as held by the body frame 120. The amount of force on the cables
required to deflect the three posts toward the axis provides a measure of
stiffness.
The same may be done with the body frame 120 with the cables coupled to three
equally spaced points on the body frame 120, such as an apex of the diamond-
shaped apertures 120 opposite from the fold region 144. The stiffness
measurement
may be performed in the un-everted configuration (see FIG. 1A) or everted
configuration (see FIG. 1B).
[0057] In accordance with embodiments the valve comprises means for
ensuring that the leaflet frame is accurately and reliably indexed and aligned
within
the body frame. This is accomplished by virtue of elements that provide for
the
capability of everting the leaflet frame into the body frame as well and in
addition to
alignment elements.
The Valve
[0058] FIGS. 1A-1B are side views of a valve 100 in a non-everted and
everted configuration, respectively, in accordance with an embodiment. FIG. 1C
is a
perspective view of the embodiment of FIG. 1B. FIG. 2 illustrates the
embodiment of
FIG. 1A wherein the valve 100 has been longitudinally cut and laid open to
better
illustrate the elements of the generally tubular-shaped valve 100. FIGs. 3A
and 3B
Date Recue/Date Received 2020-11-04
are axial views of the valve 100 in an open and closed configuration,
respectively.
The valve 100 comprises a body frame 120, a leaflet frame 130, and a film 160
covering the body frame 120 and leaflet frame 130, coupling the body frame 120
to
the leaflet frame 130, and defining leaflets 140.
The Film
[0059] The film 160 is generally any sheet-like material that is biologically
compatible and configured to couple to the body frame 120 and the leaflet
frame
130. The leaflets 140 are also comprised of the film 160. It is understood
that the
film 160 is used generically for one or more biocompatible materials suitable
for a
particular purpose. It is also understood that the film 160 coupled to the
body frame
120 may not be the same film 160 coupled to the leaflet frame 130. Details of
various types of film are discussed below. In an embodiment, the film 160 may
be
formed from a generally tubular material to at least partially cover the body
frame
120 and the leaflet frame 130. The film 160 can comprise one or more of a
membrane, composite material, or laminate. Details of various types of film
160 are
discussed below.
The Body Frame
[0060] The body frame 120 is a generally tubular member defining a body
frame lumen 123 having a body frame inner surface 129, as shown in FIGs. 1A,
1C,
and 3A. The body frame 120 defines a generally open pattern of apertures 122
operable to allow the body frame 120 to be compressed and expanded between
different diameters. The body frame 120 may comprise a structure known in the
art
as a stent. A stent is a tubular member that may have a small diameter
suitable for
percutaneous transcatheter delivery into the anatomy, and may be expanded to a
larger diameter when deployed into the anatomy. Stents having various designs
and
material properties are well known in the art.
[0061] By way of example, and as illustrated in the embodiments of FIGs. 1A-
1C and 2, the valve 100 includes the body frame 120 that defines a stent
having
apertures 122 having a generally square diamond-shape when in a large diameter
configuration, as shown in FIG. 1D. Upon compression to a smaller diameter,
the
apertures 122 deform to generally define an elongated diamond shape, as shown
in
11
Date Recue/Date Received 2020-11-04
FIG. 1E. Upon re-expansion to a larger diameter, the apertures 122 re-expand
to
again define a generally square diamond shape.
[0062] An open framework of the stent can define any number of features,
repeatable or otherwise, such as geometric shapes and/or linear or meandering
series of sinusoids. Geometric shapes can comprise any shape that facilitates
substantially uniform circumferential compression and expansion. An open
framework can be etched, cut, laser cut, or stamped into a tube or a sheet of
material, with the sheet then formed into a substantially cylindrical
structure.
Alternatively, an elongated material, such as a wire, bendable strip, or a
series
thereof, can be bent or braided and formed into a substantially cylindrical
structure
wherein the walls of the cylinder comprise an open framework that is
compressible to
a smaller diameter in a generally uniform and circumferential manner and
expandable to a larger diameter.
[0063] It is known that stents of various designs may be elastically
deformable
so as to be self-expanding under spring loads. It is also known that stents of
various
designs may be plastically deformable so as to be mechanically expanded such
as
with a balloon. It is also known that stents of various designs may be
plastically
deformable as well as elastically deformable. The embodiments of the body
frame
120 presented herein are not to be limited to a specific stent design or mode
of
expansion.
[0064] The body frame 120 can comprise any metallic or polymeric material.
For example, the body frame 120 can comprise a material, such as, but not
limited to
nitinol, cobalt-nickel alloy, stainless steel, or polypropylene, acetyl
homopolymer,
acetyl copolymer, ePTFE, other alloys or polymers, or any other material that
is
generally biocompatible having adequate physical and mechanical properties to
function as described herein.
[0065] In accordance with embodiments, the body frame 120 can be
configured to provide positive engagement with an implant site to firmly
anchor the
valve 100 to the site, as shown in FIG. 4. In accordance with an embodiment,
the
body frame 120 can comprise a sufficiently rigid frame having small elastic
recoil so
as to maintain sufficient apposition against a tissue orifice 150 to maintain
position.
In accordance with another embodiment, the body frame 120 can be configured to
expand to a diameter that is larger than a tissue orifice 150 so that when
valve 100
expands into the tissue orifice 150, it can be firmly seated therein. In
accordance
12
Date Recue/Date Received 2020-11-04
with another embodiment, the body frame 120 can comprise one or more anchors
(not shown) configured to engage the implant site, such as a tissue orifice
150, to
secure the valve 100 to the implant site.
[0066] It is appreciated that other elements or means for coupling the valve
100 to an implant site are anticipated. By way of example, but not limited
thereto,
other means, such as mechanical and adhesive means may be used to couple the
valve 100 to a synthetic or biological conduit.
Leaflet Frame
[0067] The leaflet frame 130 comprises a generally annular member defining
a predetermined repeating pattern as shown in FIGs. 1A and 2. The leaflet
frame
130 may comprise a wire, ribbon, cut tube, or any other element suitable for
the
particular purpose. As shown in FIG. 2, the leaflet frame 130 comprises three
interconnected U-shaped portions 132. Each of the U-shaped portions 132
defines
two sides 133 that define a base 134, with each side 133 having a free end
135. In
this embodiment, the base 134 defines a flex point 136 which will be described
further below. The free end 135 of one U-shaped portion 132 is interconnected
with
a free end 135 of an adjacent U-shaped portion 132 which define a post 131.
[0068] As shown in FIG. 2, the three posts 131 extend away from body frame
when in the non-everted configuration.
[0069] The leaflet frame 130 is elastically compressible to obtain a
relatively
small diameter to accommodate percutaneous transcatheter mounting and
delivery.
In accordance with an embodiment as shown in FIG. 2, the leaflet frame 130 may
comprise one or more flex points 136 so as to provide a preferential flexing
location
for the leaflet frame 130 to flex when compressed to a smaller diameter. A
flex point
136 comprises a site on the leaflet frame 130 that undergoes the highest
degree of
bending when transitioning from an expanded state to collapsed state and visa
versa. In accordance with an embodiment, at least one flex point 136 is
proximate
the post 131, and at least one flex point 136 is proximate the base 134 of the
U-
shaped portion 132. The flex point 136 can comprise a structural modification
or
material modification that biases the leaflet frame 130 to bend at the flex
point 136
when compressed.
[0070] The leaflet frame 130 is elastically deformable so as to allow the
leaflet
frame 130 to flex when everted from the non-everted extended position, shown
in
13
Date Recue/Date Received 2020-11-04
FIG. 1A, to the everted configuration shown in FIG. 3C. In addition, a
relatively less
stiff leaflet frame 130 supporting the leaflets 140 is more likely to reduce
the loading
encountered by the opening and closing leaflets 140 as compared to a more
stiff
leaflet frame 130. The leaflet frame 130 having a relatively less stiff
property may
reduce leaflet accelerations and reduce the closing stresses on the leaflets
140.
[0071] The leaflet frame 130 may comprise, such as, but not limited to, any
elastically deformable metallic or polymeric material that is biocompatible.
The leaflet
frame 130 may comprise a shape-memory material, such as nitinol, a nickel-
titanium
alloy. Other materials suitable for the leaflet frame 130 include, but are not
limited to,
other titanium alloys, stainless steel, cobalt-nickel alloy, polypropylene,
acetyl
homopolymer, acetyl copolymer, other alloys or polymers, or any other material
that
is generally biocompatible having adequate physical and mechanical properties
to
function as a leaflet frame 130 as described herein.
[0072] In accordance with an embodiment, the leaflet frame 130 comprises a
shape memory material operable to flex under load and retain its original
shape
when the load is removed, thus allowing the leaflet frame 130 to self-expand
from a
compressed shape to a predetermined shape. The leaflet frame 130 and the body
frame 120 may comprise the same or different materials. In accordance with an
embodiment, the body frame 120 is plastically deformable to be expanded by a
balloon and the leaflet frame 130 is elastically deformable so as to be self-
expanding.
Leaflet
[0073] Each of the U-shaped portions 132 of the leaflet frame 130 defines an
inner region 137. Each inner region 137 is provided with a biocompatible
material,
such as film 160, which is coupled to the sides 133 and base 134 of the
leaflet frame
130 with the film 160 defining a leaflet 140. Each leaflet 140 defines a
leaflet free
edge 142.
[0074] In accordance with an embodiment, the biocompatible material that
makes up the leaflet 140 comprises a biological tissue, such as, but not
limited to,
bovine pericardium. In accordance with other embodiments, the biocompatible
material is a film 160 that is not of a biological source and that is
sufficiently
compliant and strong for the particular purpose, such as a biocompatible
polymer. In
14
Date Recue/Date Received 2020-11-04
an embodiment, the leaflet 140 comprises a biocompatible polymer that is
combined
with an elastomer, referred to as a composite.
[0075] The shape of the leaflets 140 are defined in part by the shape of the
leaflet frame 130 and the leaflet free edge 142. As will be discussed below in
accordance with an embodiment, the shape of the leaflets 140 also depends in
part
on molding the leaflets 140 using a molding process to impart a predetermined
shape to the leaflet 140.
[0076] In accordance with an embodiment, in the everted configuration,
substantially the entire leaflet frame 130 lies adjacent to the body frame
inner
surface 129. As such, when the leaflets 140 are in a fully open position, the
valve
100 presents a substantially circular valve orifice 102 as shown in FIG. 3A,
where
the leaflet frame 130 minimally extends into the flow orifice. Fluid flow is
permitted
through the valve orifice 102 when the leaflets 140 are in an open position.
[0077] The leaflets 140 generally flex about the base 134 of the U-shaped
portion 132 as the leaflets 140 open and close. When the valve 100 is closed,
generally about half of each leaflet free edge 142 abuts an adjacent half of a
leaflet
free edge 142 of an adjacent leaflet 140, as shown in FIG. 3B. The three
leaflets 140
of the embodiment of FIG. 3B meet at a triple point 148. The valve orifice 102
is
occluded when the leaflets 140 are in the closed position stopping fluid flow.
[0078] The leaflet 140 can be configured to actuate at a pressure differential
in
the blood caused, for example, by the contraction of a ventricle or atrium of
the
heart, such pressure differential typically resulting from a fluid pressure
building up
on one side of the valve 100 when closed. As the pressure on an inflow side of
the
valve 100 rises above the pressure on the outflow side of the valve 100, the
leaflet
140 opens and blood flows therethrough. As blood flows through the valve 100
into
a neighboring chamber or blood vessel, the pressure equalizes. As the pressure
on
the outflow side of the valve 100 rises above the blood pressure on the inflow
side of
the valve 100, the leaflet 140 returns to the closed position generally
preventing the
retrograde flow of blood through the inflow side of the valve 140.
[0079] It is understood that the leaflet frame 130 may comprise any number of
U-shaped portions 132, and thus leaflets 140, suitable for a particular
purpose.
Leaflet frames 130 comprising one, two, three or more U-shaped portions 132
and
corresponding leaflets 140 are anticipated.
Date Recue/Date Received 2020-11-04
Valve Film
[0080] As shown in FIG. 1A, the body frame 120 is located coaxially, laterally
adjacent to and spaced apart from the leaflet frame 130 and, as shown in FIG.
2,
coplanar therewith in the unwrapped view of the valve 100. The base 134 of the
U-
shaped portion 132 is located proximate to a body frame first end 127 of the
body
frame 120 with the U-shaped portions 132 of the leaflet frame 130 extending
away
from the body frame 120. The space between the body frame 120 and the leaflet
frame 130 defines a fold region 144 of the valve 100 when bridged with film
160. The
valve 100 further comprises a film 160 which is coupled to the body frame 120
and
the leaflet frame 130 which couples the body frame 120 to the leaflet frame
130
across at least the fold region 144. As will be discussed below, in the
everted
configuration, the film 160 is folded along a generally circumferential line
146 in the
fold region 144. The film 160 in the fold region 144 provides a hinge about
which the
leaflet frame 130 may evert into the body frame 120.
[0081] It is anticipated that the film 160 may be coupled to the leaflet frame
130 and the body frame 120 in many ways suitable for a particular purpose. By
way
of example, and not limited thereto, the body frame 120 may be wrapped with
overlapping layers of a film 160 having a first composition. The leaflet frame
130 may
be wrapped with overlapping layers of a film 160 having a second composition.
The
wrapped leaflet frame 130 and the wrapped body frame 120 may both be wrapped
with overlapping layers of a film 160 having a third composition bridging the
fold
region 144 between the leaflet frame 130 and the body frame 120.
[0082] In another embodiment, the film 160 may be coupled to the inside or
outside surface of the leaflet frame 130 and body frame 120. In another
embodiment, the film 160 may be coupled to the inside and outside surface of
the
leaflet frame 130 and body frame 120 sandwiching the leaflet frame 130 and
body
frame 120 between the film 160.
[0083] The film 160 is configured to prevent blood from traveling through or
across the valve 100 other than through the valve orifice 102 when the
leaflets 140
are in an open position. As such, the film 160 creates a barrier to blood flow
in any
interstitial space(s) of the body frame 120 and leaflet frame 130, and
therebetween,
that the film 160 covers.
[0084] The film 160 is fixedly secured or otherwise coupled at a single or a
plurality of locations of the inside surface or outside surface of the body
frame 120
16
Date Recue/Date Received 2020-11-04
and leaflet frame 130, for example, using one or more of taping, heat
shrinking,
adhesion and other processes known in the art. In some embodiments, a
plurality of
membrane/composite layers, i.e., a laminate, are used and can be coupled to
both
the inner and outer surfaces of the body frame 120 and the leaflet frame 130
to form
at least a portion of the film 160.
[0085] The film 160 comprises any material(s) that have the suitable physical
and mechanical properties to perform the functions described herein. The film
160
may comprise the same material that the leaflet 140 comprises, as described
above,
or a different material. Similarly, the film 160 may or may not be homogenous
in
material composition. Different portions of the film 160 can comprise
different
materials which can give it different physical and mechanical properties.
[0086] Referring again to FIG. 1A, in the non-everted configuration, the body
frame 120 is located coaxially, laterally adjacent to and spaced apart from
the leaflet
frame 130, in accordance with an embodiment. The base 134 of the U-shaped
portion 132 is located proximate to but not in contact with a body frame first
end 127
of the body frame 120 with the U-shaped portions 132 of the leaflet frame 130
extending away from the body frame 120 and the posts 131 extending away from
body frame 120 when in the non-everted configuration, as shown in FIG. 2. Note
that the posts 131 are distal from the body frame first end 127 of the body
frame 120.
The film 160 extends across and between the U-shaped portions 132. The film
160
that extends between the U-shaped portions 132 prevents blood flow between the
body frame 120 and the leaflet frame 130 when in the everted configuration.
The
film 160 that extends across the U-shaped portions 132 defines the leaflets
140.
Catheter Loading Profile
[0087] In the non-everted configuration the leaflet frame 130 is located
coaxial
with and extending away from the body frame 120, as shown in FIG. 1A. In the
everted configuration the leaflet frame 130 is everted into the body frame 120
by
folding about the fold region 144 to become disposed within body frame 120
while
remaining coaxial therewith, as shown in FIG. 3C. The transition from a non-
everted
configuration to an everted configuration may be made in situ endovascularly
generally at the time of deployment.
[0088] With reference to FIGS. 1D-1E, the valve 100 may be compressed into
a collapsed configuration having a smaller diameter and expanded into an
expanded
17
Date Recue/Date Received 2020-11-04
configuration so that the valve 100 can be endovascularly delivered in the
collapsed
configuration and expanded upon deployment within the tissue orifice 150 as
shown
in FIG. 4. The leaflet frame 130 and the body frame 120 can be operable to
recover
circumferential uniformity when transitioning from the collapsed configuration
to the
expanded configuration.
[0089] The valve 100 may be mounted onto a delivery catheter either in the
everted or non-everted configuration, suitable for a particular purpose. In
accordance with an embodiment, the valve 100 is mounted onto a delivery
catheter
in the everted configuration. The valve 100 in the everted configuration has a
shorter
length as compared with the non-everted configuration although the profile of
the
valve 100 in the collapsed configuration may be determined in part by the
thickness
of the leaflet frame 130 being within the body frame 120.
[0090] In accordance with another embodiment, the valve 100 is mounted
onto a delivery catheter in the non-everted configuration. The valve 100 being
in the
non-everted configuration may have a longer length as compared with the
everted
configuration although the profile of the valve 100 in the collapsed
configuration is no
longer determined in part by the thickness of the leaflet frame 130 which
resides
outside of the body frame 120. Therefore, the valve 100 in the non-everted
configuration may have a smaller profile when mounted and compressed onto a
delivery catheter. In other words, the valve 100 in the non-everted
configuration can
collapse to a smaller diameter onto a delivery catheter in comparison to the
valve
100 that is in the everted configuration.
[0091] Referring again to FIG. 1A, in the non-everted configuration, the body
frame 120 is located coaxially, laterally adjacent to and spaced apart from
the leaflet
frame 130, in accordance with an embodiment. The base 134 of the U-shaped
portion 132 is located proximate to but not in contact with a body frame first
end 127
of the body frame 120 with the U-shaped portions 132 of the leaflet frame 130
extending away from the body frame 120 and the posts 131 extending away from
body frame 120 when in the non-everted configuration, as shown in FIG. 2.
[0092] It is noted that the leaflet frame 130 does not touch the body frame
120. The space between the body frame 120 and the leaflet frame 130 defines a
fold region 144 of the valve 100 when bridged with film 160. The fold region
144 in
combination with the non-contact between the body frame 120 and the leaflet
frame
130, among other things, allows for articulation (as in a joint) of the valve
100 about
18
Date Recue/Date Received 2020-11-04
the fold region when the valve 100 is mounted onto a delivery catheter and
during
delivery to the implantation site in the non-everted configuration.
Everted Leaflet Frame Engagement
[0093] In accordance with an embodiment, after the leaflet frame 130 is
everted into the body frame 120, the leaflet frame 130 may be urged against
the
body frame inner surface 129 to achieve a final operational configuration. In
accordance with an embodiment, the leaflet frame 130 has a spring bias towards
the
everted configuration wherein the leaflet frame 130 engages the body frame 120
in
biased urging engagement.
[0094] In accordance with an embodiment, in the everted configuration the
posts 131 abut the body frame inner surface 129 of the body frame 120, as
shown in
FIG. 1C. In accordance with an embodiment, the posts 131 are held adjacent to
the
body frame inner surface 129 by a spring bias of the leaflet frame 130. In
accordance with another embodiment, the posts 131 are held in urging
engagement
with the body frame inner surface 129 by a spring bias of the leaflet frame
130. In
accordance with yet another embodiment, the posts 131 are coupled with the
body
frame inner surface 129 by an engagement element (not shown) defined by the
body
frame 120.
[0095] In accordance with an embodiment, as shown in FIG. 1C and 3C, the
posts 131 are held adjacent to the body frame inner surface 129 by a spring
bias of
the leaflet frame 130 and further aligned by the engagement of the posts 131
lying
within a valley 128 defined by the body frame 120. The valley 128 is operable
to
direct the post 131 towards the apex of the valley 128 so as to preferentially
position
the post 131 with respect to the body frame 120. It is understood that the
posts may
lie entirely within the body frame 120, or at least partially extending from
and outside
of the body frame 120.
[0096] The engagement of the posts 131 of the leaflet frame 130 with the
body frame 120 provides support to the leaflet frame 130 to a greater extent
than
wherein the leaflet frame 130 is unsupported by the body frame 120. The
engagement of the posts 131 with the body frame 120 allows for the transfer of
loading on the leaflet 140 to the leaflet frame 130 and then to the body frame
120. In
accordance with an embodiment, substantially the entire leaflet frame 130 is
in
urging engagement with the body frame inner surface 129. It is anticipated
that the
19
Date Recue/Date Received 2020-11-04
degree of engagement of the leaflet frame 130 with the body frame 120 will
determine the degree of support provided on the leaflet frame 130 by the body
frame
120, which may be predetermined for a particular purpose.
[0097] In other embodiments, the posts 131 are not held in engagement with
the body frame inner surface 129 so as to allow inward flexing of the posts
131
under the loading of the leaflet 140 during valve operation, particularly when
closing
or closed. Flexing of the posts 131 may ensure that the leaflet free edges 142
coapt
to form a tight seal when closed.
[0098] In embodiments of the valve 100, the inclusion of a body frame 120
and a leaflet frame 130 provides a means for providing different physical
properties
for each of the body frame 120 and the leaflet frame 130 suitable for a
particular
purpose. In accordance with an embodiment, the body frame 120 is generally
inelastic as compared with the leaflet frame 130. The body frame 120, when
expanded to engage the tissue orifice 150, as shown in FIG. 4, is rigid enough
to
remain in urging engagement with the tissue orifice 150 and to not
significantly recoil
to a smaller diameter or deform under physiological loading.
[0099] The physical properties of the body frame 120 and the leaflet frame
130 depends, in part, on the size, shape, thickness, material property of the
body
frame 120 and the leaflet frame 130 as well as the different physical
properties and
number of layers or wrappings of the film 160.
Clasp and/or Engagement Element
[00100] In accordance with an embodiment, one or more clasps (not shown) or
some other similar engagement mechanism can secure the post 131 to the body
frame 120 and add a predetermined amount of structural rigidity to the leaflet
frame
130. As such, forces on the leaflet frame 130 may at least partially be
transferred or
distributed to the body frame 120. In this regard, the clasp comprises any
structure
configured to interlock, connect, fasten, or otherwise hold the leaflet frame
130 and
body frame 120 together. The clasp connecting the leaflet frame 130 to the
body
frame 120 is operable to transfer at least some of the forces on the leaflet
frame 130
to the body frame 120.
Date Recue/Date Received 2020-11-04
Leaflet Film
[00101] The biocompatible material that makes up the leaflet 140 can comprise
any biological tissue or synthetic, biocompatible materials sufficiently
compliant and
flexible, such as a biocompatible polymer. In an embodiment, the leaflet 140
comprises a biocompatible polymer that is combined with an elastomer, referred
to
as a composite. A material according to one embodiment includes a composite
material comprising an expanded fluoropolymer membrane, which comprises a
plurality of spaces within a matrix of fibrils, and an elastomeric material.
It should be
appreciated that multiple types of fluoropolymer membranes and multiple types
of
elastomeric materials can be combined to form a laminate while remaining
within the
scope of the present disclosure. It should also be appreciated that the
elastomeric
material can include multiple elastomers, multiple types of non-elastomeric
components, such as inorganic fillers, therapeutic agents, radiopaque markers,
and
the like while remaining within the scope of the present disclosure.
[00102] In accordance with an embodiment, the composite material includes an
expanded fluoropolymer material made from porous ePTFE membrane, for instance
as generally described in U.S. Patent No. 7,306,729 to Bacino.
[00103] The expandable fluoropolymer, used to form the expanded
fluoropolymer material described, may comprise PTFE homopolymer. In
alternative
embodiments, blends of PTFE, expandable modified PTFE and/or expanded
copolymers of PTFE may be used.. Non-limiting examples of suitable
fluoropolymer
materials are described in, for example, U.S. Patent No. 5,708,044, to Branca,
U.S.
Patent No. 6,541,589, to Baillie, U.S. Patent No. 7,531,611, to Sabol et al.,
U.S.
Patent Application No. 11/906,877, to Ford, and U.S. Patent Application No.
12/410,050, to Xu et al.
[00104] The expanded fluoropolymer membrane can comprise any suitable
microstructure for achieving the desired leaflet performance. In accordance
with an
embodiment, the expanded fluoropolymer comprises a microstructure of nodes
interconnected by fibrils, such as described in U.S. Patent No. 3,953,566 to
Gore, as
shown in the scanning electron micrograph image in Figure 9A, in accordance
with
an embodiment. The fibrils radially extend from the nodes in a plurality of
directions,
and the membrane has a generally homogeneous structure. Membranes having this
microstructure may typically exhibit a ratio of matrix tensile strength in two
orthogonal directions of less than 2, and possibly less than 1.5. Embodiments
of
21
Date Recue/Date Received 2020-11-04
expanded fluoropolymer membrane provided herein contain a majority of fibrils
having a diameter that is less than about 1 pm. Other embodiments of expanded
fluoropolymer membrane provided herein contain a majority of fibrils having a
diameter that is less than 0.1 pm. The embodiments provided herein recognize
that
a membrane comprising fibrils the majority of which are less than about 1 to
beyond
less than about 0.1 pm provide a significant improvement to, at least, but not
limited
to, the durability and lifetime of the heart valve when used as leaflet
material.
Embodiments of expanded fluoropolymer membrane provided herein may have a
mean flow pore sizes of less than about 5 pm, less than about 1 pm, and less
than
about 0.10 pm, in accordance with embodiments.
[00105] In another embodiment, the expanded fluoropolymer membrane has a
microstructure of substantially only fibrils, as is generally taught by U.S.
Patent No.
7,306,729, to Bacino, as shown in the scanning electron micrograph image in
Figure
9B, in accordance with an embodiment. Figure 9C is a higher magnification of
the
scanning electron micrograph image in Figure 9B and more clearly shows the
homogeneous microstructure having substantially only fibrils. The expanded
fluoropolymer membrane having substantially only fibrils, can possess a high
surface
area, such as greater than 20m2/g, or greater than 25m2/g, and in some
embodiments can provide a highly balanced strength material having a product
of
matrix tensile strengths in two orthogonal directions of at least 1.5 x 105
MPa2,
and/or a ratio of matrix tensile strengths in two orthogonal directions of
less than 4,
and possibly less than 1.5. Embodiments of expanded fluoropolymer membrane
provided herein contain a majority of fibrils having a diameter that is less
than about
1 pm. Other embodiments of expanded fluoropolymer membrane provided herein
contain a majority of fibrils having a diameter that is less than about 0.1
pm. The
embodiments provided herein recognize that a membrane comprising fibrils the
majority of which are less than about 1 to beyond less than about 0.1 pm
provide a
significant improvement to, at least, but not limited to, the durability and
lifetime of
the heart valve when used as leaflet material. Embodiments of expanded
fluoropolymer membrane provided herein may have a mean flow pore sizes of less
than about 5 pm, less than about 1 pm, and less than about 0.10 pm, in
accordance
with embodiments.
[00106] The expanded fluoropolymer membrane can be tailored to have any
suitable thickness and mass to achieve the desired leaflet performance. By way
of
22
Date Recue/Date Received 2020-11-04
example, but not limited thereto, the leaflet 140 comprises an expanded
fluoropolymer membrane having a thickness of about 0.1 pm. The expanded
fluoropolymer membrane can possess a mass per area of about 1.15 g/m2.
Membranes according to an embodiment of the invention can have matrix tensile
strengths of about 411 MPa in the longitudinal direction and 315 MPa in the
transverse direction.
[00107] Additional materials may be incorporated into the pores or within the
material of the membranes or in between layers of membranes to enhance desired
properties of the leaflet. Composite materials described herein can be
tailored to
have any suitable thickness and mass to achieve the desired leaflet
performance.
Composite materials according to embodiments can include fluoropolymer
membranes and have a thickness of about 1.9 pm and a mass per area of about
4.1
gim2.
[00108] The expanded fluoropolymer membrane combined with elastomer to
form a composite material provides the elements of the present disclosure with
the
performance attributes required for use in high-cycle flexural implant
applications,
such as heart valve leaflets, in various ways. For example, the addition of
the
elastomer can improve the fatigue performance of the leaflet by eliminating or
reducing the stiffening observed with ePTFE-only materials. In addition, it
may
reduce the likelihood that the material will undergo permanent set
deformation, such
as wrinkling or creasing, that could result in compromised performance. In one
embodiment, the elastomer occupies substantially all of the pore volume or
space
within the porous structure of the expanded fluoropolymer membrane. In another
embodiment the elastomer is present in substantially all of the pores of the
at least
one fluoropolymer layer. Having elastomer filling the pore volume or present
in
substantially all of the pores reduces the space in which foreign materials
can be
undesirably incorporated into the composite. An example of such foreign
material is
calcium that may be drawn into the membrane from contact with the blood. If
calcium
becomes incorporated into the composite material, as used in a heart valve
leaflet,
for example, mechanical damage can occur during cycling open and closed, thus
leading to the formation of holes in the leaflet and degradation in
hemodynamics.
[00109] In an embodiment, the elastomer that is combined with the ePTFE is a
thermoplastic copolymer of tetrafluoroethylene (TFE) and perfluoromethyl vinyl
ether
(PMVE), such as described in U.S. Patent No. 7,462,675 to Chang et al. As
23
Date Recue/Date Received 2020-11-04
discussed above, the elastomer is combined with the expanded fluoropolymer
membrane such that the elastomer occupies substantially all of the void space
or
pores within the expanded fluoropolymer membrane to form a composite material.
This filling of the pores of the expanded fluoropolymer membrane with
elastomer can
be performed by a variety of methods. In one embodiment, a method of filling
the
pores of the expanded fluoropolymer membrane includes the steps of dissolving
the
elastomer in a solvent suitable to create a solution with a viscosity and
surface
tension that is appropriate to partially or fully flow into the pores of the
expanded
fluoropolymer membrane and allow the solvent to evaporate, leaving the filler
behind.
[00110] In one embodiment, the composite material comprises three layers:
two outer layers of ePTFE and an inner layer of a fluoroelastomer disposed
therebetween. Additional fluoroelastomers can be suitable and are described in
U.S.
Publication No. 2004/0024448 to Chang.
[00111] In another embodiment, a method of filling the pores of the expanded
fluoropolymer membrane includes the steps of delivering the filler via a
dispersion to
partially or fully fill the pores of the expanded fluoropolymer membrane.
[00112] In another embodiment, a method of filling the pores of the expanded
fluoropolymer membrane includes the steps of bringing the porous expanded
fluoropolymer membrane into contact with a sheet of the elastomer under
conditions
of heat and/or pressure that allow elastomer to flow into the pores of the
expanded
fluoropolymer membrane.
[00113] In another embodiment, a method of filling the pores of the expanded
fluoropolymer membrane includes the steps of polymerizing the elastomer within
the
pores of the expanded fluoropolymer membrane by first filling the pores with a
prepolymer of the elastomer and then at least partially curing the elastomer.
[00114] After reaching a minimum percent by weight of elastomer, the leaflets
constructed from fluoropolymer materials or ePTFE generally performed better
with
increasing percentages of elastomer resulting in significantly increased cycle
lives.
In one embodiment, the elastomer combined with the ePTFE is a thermoplastic
copolymer of tetrafluoroethylene and perfluoromethyl vinyl ether, such as
described
in U.S. Patent No. 7,462,675 to Chang et al., and other references that would
be
known to those of skill in the art. Other biocompatible polymers which can be
suitable for use in leaflet 140 include but are not limited to the groups of
urethanes,
24
Date Recue/Date Received 2020-11-04
silicones(organopolysiloxanes), copolymers of silicon-urethane,
styrene/isobutylene
copolymers, polyisobutylene, polyethylene-co-poly(vinyl acetate), polyester
copolymers, nylon copolymers, fluorinated hydrocarbon polymers and copolymers
or
mixtures of each of the foregoing.
Other Considerations
[00115] In accordance with an embodiment, the valve 100 can be configured to
prevent interference with a heart conduction system by not covering the bundle
branch in the left ventricle when implanted, such as might be encountered with
an
aortic valve replacement procedure. For example, the valve 100 can comprise a
length of less than about 25 mm or less than about 18 mm. The valve 100 can
also
comprise an aspect ratio of less than one, wherein the ratio describes the
relationship between the length of the valve 100 to the expanded, functional
diameter. However, the valve 100 can be constructed at any length and, more
generally, any desirable dimension.
[00116] In a collapsed state, the valve 100 can have a collapsed profile that
is
less than about 35% of the expanded profile. For example, the valve 100
comprising
a 26 mm expanded diameter can have a collapsed diameter of less than about 8
mm, or less than about 6 mm. The percent difference in diameter is dependent
on
dimensions and materials of the valve 100 and its various applications, and
therefore, the actual percent difference is not limited by this disclosure.
[00117] The valve 100 can further comprise a bio-active agent. Bio-active
agents can be coated onto a portion or the entirety of the film 160 for
controlled
release of the agents once the valve 100 is implanted. The bio-active agents
can
include, but are not limited to, vasodilator, anti-coagulants, anti-platelet,
anti-
thrombogenic agents such as, but not limited to, heparin. Other bio-active
agents
can also include, but are not limited to agents such as, for example, anti-
proliferative/antimitotic agents including natural products such as vinca
alkaloids (i.e.
vinblastine, vincristine, and vinorelbine), paclitaxel, epidipodophyllotoxins
(i.e.
etoposide, teniposide), antibiotics (dactinomycin (actinomycin D)
daunorubicin,
doxorubicin and idarubicin), anthracyclines, mitoxantrone, bleomycins,
plicamycin
(mithramycin) and mitomycin, enzymes (L-asparaginase which systemically
metabolizes L-asparagine and deprives cells which do not have the capacity to
synthesize their own asparagine); antiplatelet agents such as G(GP) Ilb/Illa
inhibitors
Date Recue/Date Received 2020-11-04
and vitronectin receptor antagonists; anti-proliferative/antimitotic
alkylating agents
such as nitrogen mustards (mechlorethamine, cyclophosphamide and analogs,
melphalan, chlorambucil), ethylenimines and methylmelamines
(hexamethylmelamine and thiotepa), alkyl sulfonates-busulfan, nitrosoure,as
(carmustine (BCNU) and analogs, streptozocin), trazenes-dacarbazinine (DTIC);
anti-proliferative/antimitotic antimetabolites such as folic add analogs
(methotrexate), pyrimidine analogs (fluorouracil, floxuridine, and
cytarabine), purine
analogs and related inhibitors (mercaptopurine, thioguanine, pentostatin and 2-
chlorodeoxyadenosine (cladribine}); platinum coordination complexes
(cisplatin,
carboplatin), procarbazine, hydroxyurea, mitotane, aminoglutethimide; hormones
(i.e. estrogen); anti-coagulants (heparin, synthetic heparin salts and other
inhibitors
of thrombin); fibrinolytic agents (such as tissue plasminogen activator,
streptokinase
and urokinase), aspirin, dipyridamole, tidopidine, clopidogrel, abcixirnab;
antimigratory; antisecretory (breveldin); anti-inflammatory: such as
adrenocortical
steroids (cortisol, cortisone, fludrocortisone, prednisone, prednisolone, 6a-
methylprednisolone, triamcinolone, betamethasone, and dexamethasone), non-
steroidal agents (salicylic acid derivatives i.e. aspirin; para-aminophenol
derivatives
i.e. acetominophen; indole and indene acetic acids (indomethacin, sulindac,
and
etodalac), heteroaryl acetic acids (tolmetin, diclofenac, and ketorolac),
arylpropionic
acids (ibuprofen and derivatives), anthranilic acids (mefenamic acid, and
meclofenamic acid), enolic acids (piroxicam, tenoxicam, phenylbutazone, and
oxyphenthatrazone), nabumetone, gold compounds (auranofin, aurothioglucose,
gold sodium thiomalate); immunosuppressives: (cyclosporine, tacrolimus (FK-
506),
sirolimus (rapamycin), azathioprine, mycophenolate mofetil); angiogenic
agents:
vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF);
angiotensin receptor blockers; nitric oxide donors; anti-sense
oligionucleotides and
combinations thereof; cell cycle inhibitors, mTOR inhibitors, and growth
factor
receptor signal transduction kinase inhibitors; retenoids; cyclin/CDK
inhibitors; HMG
co-enzyme reductase inhibitors (statins); and protease inhibitors.
Delivery System
[00118] In an embodiment, with reference to FIGs. 4, 5A-5C, a valve delivery
system 500 comprises a valve 100 having a collapsed configuration and an
expanded configuration as previously described and an elongated flexible
catheter
26
Date Recue/Date Received 2020-11-04
480, such as a balloon catheter, configured to endovascularly deploy the valve
100.
The catheter 480 can comprise a balloon to expand the valve 100 and/or if
required,
to touch up the valve 100 to ensure proper seating. The valve 100 can be
mounted
to the distal section of the catheter 480 for delivery through the
vasculature. In order
to hold the valve in a collapsed configuration on the catheter 480, the valve
delivery
system may further comprise a removable sheath 482 to closely fit over the
transcatheter valve 100.
[00119] A valve delivery system 500 is operable to endovascularly transition
the valve 100 from a non-everted configuration to an everted configuration.
For
example, delivery system 500 comprises an everter 485 as shown in FIGs. 5B-5C.
The everter 485 comprises any mechanism to facilitate the transition from a
non-
everted configuration to an everted configuration. In one embodiment, the
everter
485 is configured to fit over the posts 131 of the body frame 120 while in a
non-
everted configuration. The everter 485 is moveable between a distal position,
shown
in FIG. 5B, to a proximal position, shown in FIG. 5C, relative to the valve
100, which
thereby moves the leaflet frame 130 from the extended position to the everted
position. The everter 485 can comprise an annular or funnel-shaped structure
that
radially compresses the posts 131. The everter 485 can be tethered to an
elongate
member that extends through the valve orifice 102 of the valve 100 and is
accessible
by a clinician to facilitate eversion. The above describes one embodiment;
however,
any device of any configuration can be used to facilitate eversion.
[00120] A method of delivery can comprise the steps of radially compressing an
everted valve into its collapsed configuration onto the distal end of an
elongate
flexible catheter having proximal and distal ends; delivering the valve to a
tissue
orifice, such as a native aortic valve orifice, via a transfemoral or
transapical route,
and expanding the valve into the tissue orifice. The valve can be expanded by
inflating a balloon.
[00121] A method of delivery can comprise the steps of radially compressing an
evertable valve, while in a non-everted configuration, into its collapsed
configuration,
onto the distal section of an elongated flexible catheter having proximal and
distal
ends. A restraint, which can be connected to a tether that passes through the
orifice
of valve and the lumen of the catheter, is fitted around the posts of the
valve. The
valve is then delivered to a native valve orifice, such as a native aortic
valve orifice,
via a route of delivery and expanded into the native orifice. The route of
delivery can
27
Date Recue/Date Received 2020-11-04
comprise a transfemoral or transapical route. The valve can be expanded by
inflating a balloon. Next, a clinician will evert a leaflet frame of the valve
by axially
displacing the restraint in a distal to proximal location. The leaflet frame
can then be
connected to the body frame by securing the posts into the clasps on the body
frame.
Surgical Embodiments
[00122] It is appreciated that the embodiments of the valve 100 may be
surgically implanted rather than using transcatheter techniques. Embodiments
of a
surgically implanted valve 100 may be substantially the same as those
described
above, with the addition of a sewing cuff about a body frame outer surface 127
in
accordance with an embodiment. The sewing cuff, which is well known in the
art, is
operable to provide structure that receives suture for coupling the valve 100
to an
implant site, such as the tissue orifice. The sewing cuff may comprise any
suitable
material, such as, but not limited to, double velour polyester. The sewing
cuff may
be located circumferentially around the body frame 120 or perivalvular
depending
from the base frame. The leaflet frame 130 may be everted into the body frame
120
before or after the body frame 120 is secured to the implant site.
Method of making
[00123] Embodiments described herein also pertain to a method of making the
valve embodiments as described herein. In order to make the various
embodiments,
a winding jig and a two-piece leaflet mandrel can be used. With reference to
FIG. 6,
winding jig 590 comprises a structural form defining the valve orifice of the
valve and
a leaflet frame guide 591 configured to facilitate the shaping of a wire into
a desired
leaflet frame shape. With reference to FIG. 8A-8B, two-piece mandrel 595
comprises a leaflet clamp 596 and a base mold 597 which together form the
mandrel
to mold a tubular membrane or composite to form the leaflets. Leaflet clamp
596
can comprise contoured grooves 594 along the seams of leaflet clamp 596
wherein
the posts 131 will be placed into in order to define the desired curvature or
bend in
the leaflet frame 130.
[00124] With reference to FIG. 6, a method of making the leaflet frame can
comprise the step of shaping a wire to form leaflet frame 130. Winding jig 590
can
28
Date Recue/Date Received 2020-11-04
be used to form the leaflet frame 130 wherein wire is bent around posts and
guides
and then heat set.
[00125] With reference to FIGS. 7 and 8A-8B, an embodiment of a method of
making valve 100 in the non-everted configuration comprises the steps of
wrapping a
first layer of biocompatible material such as film 160, e.g., a composite as
described
herein, into a tubular form about a first mandrel 710; placing the leaflet
frame 130
and body frame 120 over the first layer of film 160, as shown in FIG. 7;
forming a
second layer of film 160 over the leaflet frame 130 and the body frame 120;
thermally setting the assembly; removing the assembly from the first mandrel
710
and inserting the assembly into a two-piece mandrel 596; molding the leaflets
140
with the leaflet clamp 696 placing the leaflet clamp 696 in urging engagement
with
the leaflets 140; and thermal setting the leaflets 140.
EXAMPLE
[00126] By way of example, one embodiment of an evertable valve can be
made as follows.
[00127] A leaflet frame was constructed by winding a nitinol wire (0.020"
diameter) onto a winding jig as illustrated in FIG. 6. Once the pattern as
shown in
FIG. 2 was obtained, the frame was shape set in an oven set to 450 C for 10
minutes. The leaflet frame was then exposed to a surface roughening step to
improve adherence of the membrane to the frame. The frame was submersed in an
ultrasonic bath of acetone for approximately five minutes. The frame surface
was
then subjected to a plasma treatment with methods commonly known to those
having ordinary skill in the art.
[00128] FEP powder (Daikin America, Orangeburg N.Y.) was applied to the
frame. The leaflet frame was then heated in a forced air oven set to 320 C for
approximately three minutes. In this way, the powder was melted and adhered as
a
thin coating to the entire frame. The leaflet frame was removed from the oven
and
left to cool to room temperature.
[00129] A body frame was laser cut from a tube of 316 stainless steel having a
wall thickness of about 0.5 mm (0.02"), a diameter of about 2.5 cm (1.0"), and
a
length of 2 cm. A diamond-shaped pattern was cut into the tube to form an
annular-
shaped body frame shown in FIG. 2. The same surface treatment and FEP powder
coating steps as described above were applied to the body frame.
29
Date Recue/Date Received 2020-11-04
[00130] A leaflet material was obtained. A membrane of ePTFE can be
manufactured according to the general teachings described in US Patent
7,306,729
to Bacino et al. The ePTFE membrane had a mass per area of about 1.15 g/m2, a
bubble point of about 79.7MPa, a thickness of about 1.016 pm, a matrix tensile
strength of about 410.9 MPa in the longitudinal direction and about 315.4 MPa
in the
transverse direction.
[00131] A fluoroelastomer that is a copolymer comprising tetrafluoroethylene
and perfluoro(methyl vinylether) as described in U.S Pat. No. 7,462,675 to
Chang, et
al. was obtained. The copolymer consisted essentially of between about 65 and
70
weight percent perfluoromethyl vinyl ether and complementally about 35 and 30
weight percent tetrafluoroethylene.
[00132] This copolymer was dissolved in Novec HFE7500 (3M, St Paul, MN) in
a 2.5% concentration. The ePTFE membrane (while being supported by a
polypropylene release film) was coated with the prepared solution using a
mayer bar
and dried in a convection oven set to 145 C for 30 seconds thereby creating an
imbibed composite material. After two coating steps, the final
ePTFE/fluoroelastomer
or composite material had a mass per area of approximately 4.08 g/m2, 28.22 %
fluoropolymer by weight, a dome burst strength of 15.9 KPa, and a thickness of
1.89
pm.
[00133] Fifteen layers of the composite material were wrapped around the
combined 25mm diameter aluminum mandrel assembly shown in FIG. 7 with the
elastomer rich side facing away from the mandrel. The fifteen layers of
composite
material were each circumferentially wrapped around the mandrel so as to
orient the
transverse direction of the composite along the longitudinal axis of the
mandrel. The
leaflet frame was everted from its wire wound condition, then coaxially
positioned on
the mandrel, as illustrated in FIG. 8A. The body frame was then positioned
onto the
mandrel as shown in FIG. 7.
[00134] Five additional layers of composite material were wrapped around the
leaflet frame and body frame with the elastomer rich side of each layer facing
toward
the leaflet frame and the body frame.
[00135] The assembly was then circumferentially wrapped with a polyimide
release film sacrificial layer. The assembly was heated in a forced air oven
set to
about 280 C for about 30 minutes. The assembly was removed from the oven and
water quenched. The sacrificial layer was removed thereby exposing the valve.
Date Recue/Date Received 2020-11-04
Excess leaflet material was trimmed to form the free edge with scissors from
the top
of the frame posts to the common triple point of each leaflet as shown in FIG.
1A and
8A to create three commissures or coapting surface regions. The non-everted
frame
assembly was removed from the tooling.
[00136] The leaflets were then formed to a predetermined shape by positioning
the leaflet clamp 596 as shown in FIG. 8A and 8B and subsequently closing the
leaflet clamp 596 against the leaflets. The combined mandrel assembly were
then
thermal treated to set the leaflet shape.
Testing Methods
[00137] It should be understood that although certain methods and equipment
are described below, any method or equipment determined suitable by one of
ordinary skill in the art may be alternatively utilized.
Bubble Point and Mean Flow Pore Size
[00138] Bubble point and mean flow pore size were measured according to the
general teachings of ASTM F31 6-03 using a capillary flow Porometer, Model CFP
1500AEXL from Porous Materials, Inc., Ithaca NY, USA. The sample membrane was
placed into the sample chamber and wet with SilWick Silicone Fluid (available
from
Porous Materials Inc.) having a surface tension of about 20.1 dynes/cm. The
bottom
clamp of the sample chamber had an about 2.54 cm diameter hole. The test fluid
was isopropyl alcohol. Using the Capwin software version 7.73.012 the
following
parameters were set as specified in the table below. As used herein, mean flow
pore size and pore size are used interchangeably.
Parameter Set Point
Maxflow (cm3/m) 200000
Bublflow(cm3/m) 100
F/PT (old bubltime) 50
Minbpress (PSI) 0
Zerotime (sec) 1
V2incr(cts) 10
Preginc (cts) 1
Pulse delay(sec) 2
31
Date Recue/Date Received 2020-11-04
Maxpre (PSI) 500
Pulse width (sec) 0.2
Mineqtime (sec) 30
Presslew (cts) 10
Flowslew (cts) 50
Eqiter 3
Aveiter 20
Maxpdif (PSI) 0.1
Maxfdif (PSI) 50
Sartp(PSI) 1
Sartf (cm3/m) 500
Presence of Elastomer within the Pores
[00139] The presence of elastomer within the pores can be determined by
several methods known to those having ordinary skill in the art, such as
surface
and/or cross section visual, or other analyses. These analyses can be
performed
prior to and after the removal of elastomer from the composite.
Diameter of Fibrils
[00140] The average diameter of the fibrils was estimated by examining
micrographs that were obtained having at a magnification suitable for showing
numerous fibrils, such as the scanning electron microscopy (SEM) micrographs
of
FIGs. 9A-C. In the case of a composite material, it may be necessary to
extract the
elastomer or other material that may be filling the pores, by any suitable
means, to
expose the fibrils.
Mass, Thickness, and Density of ePTFE Membranes
[00141] Membrane thickness was measured by placing the membrane between
the two plates of a Kafer FZ1000/30 thickness snap gauge 'cater
Messuhrenfabrik
GmbH, Villingen-Schwenningen, Germany. The average of the three measurements
was reported.
[00142] Membrane samples were die cut to form rectangular sections about
2.54 cm by about 15.24 cm to measure the weight (using a Mettler-Toledo
analytical
balance model AG204) and thickness (using a Kafer Fz1000/30 snap gauge). Using
32
Date Recue/Date Received 2020-11-04
these data, density was calculated with the following formula: p = m/(w*I1),
in which:
p = density (g/cm3), m = mass (g), w = width (cm), I = length (cm), and t =
thickness
(cm). The average of three measurements was reported.
Matrix Tensile Strength (MTS) of ePTFE Membranes
[00143] Tensile break load was measured using an INSTRON 122 tensile test
machine equipped with flat-faced grips and a 0.445 kN load cell. The gauge
length
was about 5.08 cm and the cross-head speed was about 50.8 cm/min. The sample
dimensions were about 2.54 cm by about 15.24 cm. For highest strength
measurements, the longer dimension of the sample was oriented in the highest
strength direction. For the orthogonal MTS measurements, the larger dimension
of
the sample was oriented perpendicular to the highest strength direction. Each
sample was weighed using a Mettler Toledo Scale Model AG204, then the
thickness
was measured using the Kafer FZ1000/30 snap gauge; alternatively, any suitable
means for measuring thickness may be used. The samples were then tested
individually on the tensile tester. Three different sections of each sample
were
measured. The average of the three maximum loads (i.e., peak force)
measurements was reported. The longitudinal and transverse matrix tensile
strengths (MTS) were calculated using the following equation: MTS= (maximum
load/cross-section area)*(bulk density of PTFE)/ (density of the porous
membrane),
where the bulk density of the PTFE was taken to be about 2.2 g/cm3.
[00144] Numerous characteristics and advantages have been set forth in the
preceding description, including various alternatives together with details of
the
structure and function of the devices and/or methods. The disclosure is
intended as
illustrative only and as such is not intended to be exhaustive. It will be
evident to
those skilled in the art that various modifications can be made, especially in
matters
of structure, materials, elements, components, shape, size and arrangement of
parts
including combinations within the principles of the disclosure, to the full
extent
indicated by the broad, general meaning of the terms in which the appended
claims
are expressed. To the extent that these various modifications do not depart
from the
spirit and scope of the appended daims, they are intended to be encompassed
therein.
33
Date Recue/Date Received 2020-11-04