Note: Descriptions are shown in the official language in which they were submitted.
PSU004-CANP
PSYCHOACTIVE ALKALOID EXTRACTION AND COMPOSITION WITH CONTROLLED
DEPHOSPHORYLATION
TECHNICAL FIELD
[0001] This application relates to a process of obtaining psychoactive
alkaloid
extracts. More specifically, the present invention relates to controlling
dephosphorylation
during extraction. Further, the present invention relates to psychoactive
alkaloid
compositions with controlled dephosphorylation.
BACKGROUND
[0002] A psychoactive substance is a chemical substance that changes brain
function
and results in alterations in perception, mood, consciousness, cognition, or
behavior. The
psychoactivity of these substances may include sedative, stimulant, euphoric,
deliriant,
and hallucinogenic effects. These substances have been used recreationally, to
purposefully improve performance or alter one's consciousness, as entheogens
for ritual,
spiritual, or shamanic purposes. Some categories of psychoactive compounds
have also
shown therapeutic values and are prescribed by physicians and other healthcare
practitioners.
[0003] The active constituents of the majority of psychoactive plants,
fungi, animals, or
yeasts fall within a class of basic, naturally occurring, nitrogen-containing,
organic
compounds called alkaloids (e.g. nicotine, morphine, cocaine, mescaline,
caffeine,
ephedrine, psilocin). Alkaloids have a wide range of pharmacological
activities including
antimalarial, antiasthma, anticancer, cholinomimetic, vasodilatory,
antiarrhythmic,
analgesic, antibacterial, and antihyperglycemic activities. Many alkaloids
have found use
in traditional or modern medicine, or as starting points for drug discovery.
Recently,
psychotropic and stimulant activities of psychoactive alkaloids have been
gaining interest
from researchers as therapeutic agents for treating various conditions such as
alcoholism,
opioid addiction and pain to name a few.
[0004] Psychoactive alkaloids present in natural sources can be broadly
divided into
two categories, which are phosphorylated psychoactive alkaloids and
dephosphorylated
1
Date Recue/Date Received 2020-12-04
PSU004-CANP
psychoactive alkaloids, although other non-phosphorylatable psychoactive
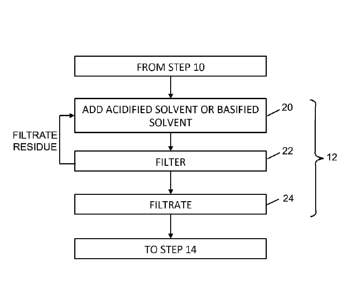
alkaloids may
also be present.
[0005] Phosphorylated psychoactive alkaloids are phosphoric acid esters of
dephosphorylated psychoactive alkaloids. For example psilocybin is a
phosphoric acid
ester of psilocin, at the 4th position. Phosphorylated psychoactive alkaloids
are
biosynthesized in natural sources. Dephosphorylated psychoactive alkaloids are
the
bioactive forms that are converted from phosphorylated alkaloids, through
phosphatase
action or chemical hydrolysis, and released when the natural source is
damaged,
harvested, or eaten. Because of this phenomenon, phosphorylated psychoactive
alkaloids
are often either partially or entirely converted to dephosphorylated
psychoactive alkaloids
during the alkaloid extraction process, which involves harvesting as a
necessary prior
step.
[0006] Although the dephosphorylated psychoactive alkaloids are the
bioactive form of
their counterpart phosphorylated psychoactive alkaloids, dephosphorylated
psychoactive
alkaloids are easily degraded into non-bioactive compounds in the presence of
light, heat,
and oxygen. For example, oxidation of psilocin, the dephosphorylated
counterpart to
psilocybin, a phosphorylated alkaloid produced by biological synthesis in
mushrooms,
begins rapidly when exposed to air, especially in solution, and heat increases
the
oxidation rate. From our own data, the oxidation of psilocin in a moist and/or
high light
environment begins immediately, leading to about 10% decay within 30 minutes,
25%
after 5 hours, and 40-60% at 20 hours when shielded from light. Due to this
instability of
the dephosphorylated psychoactive alkaloids, the bioactivity of the
psychoactive alkaloid
extracts may also be unstable over time.
[0007] Extracts or compositions with an active ingredient made from natural
sources
generally have increased consumer acceptance and lower cost of production
compared to
synthetic compositions. There may be potential benefits of multiple natural
compounds
working synergistically, colloquially known as the "entourage" or "halo"
effect. However,
the availability of psychoactive alkaloid compositions with a desired specific
psychoactive
alkaloid content is a major challenge faced by researchers. The variability in
the content of
the psychoactive alkaloids extracted from their natural sources is a hurdle in
trying to
2
Date Recue/Date Received 2020-12-04
PSU004-CANP
avoid variability in the psychoactive alkaloid concentration in extracted
compositions. It is
even more challenging to produce consistent formulations when the
concentration of
active ingredients being extracted is typically very low in the natural
source. Maintaining
physical and chemical stability is also an issue with these compositions.
Extracts or
compositions containing psychoactive alkaloids are often not amenable to
drying,
processing (due to poor flowability), or packaging methods such as tabulation
or
encapsulation.
[0008] This background information is provided to reveal information
believed by the
applicant to be of possible relevance to the present invention. No admission
is necessarily
intended, nor should be construed, that any of the preceding information
constitutes prior
art against the present invention.
SUMMARY OF INVENTION
[0009] The inventors have realized that there are occasions where it would
be
beneficial to control, either by halting or promoting, the conversion of
phosphorylated
alkaloids such as psilocybin during extraction and any subsequent purification
process.
For example, there may be occasions where extracts with phosphorylated
psychoactive
alkaloids as the only or majority of the total psychoactive alkaloid in the
extract are
required. Likewise, there may be occasions where extracts with
dephosphorylated
psychoactive alkaloids as the only or majority of the total psychoactive
alkaloid in the
extract are required.
[0010] For example, the alkaloid in the psychoactive extract may be
entirely psilocin,
resulting from promotion of the conversion, or all or mostly psilocybin, from
the halting or
inhibition of the conversion. There will likely, but not necessarily, be some
psilocin present
in the process where the conversion is halted, as the harvesting of the
mushrooms can
often cause unavoidable conversion into psilocin.
[0011] Controlling the promotion or inhibition of dephosphorylation of the
aforementioned alkaloids to result in a psychoactive alkaloid extract with a
specific
desired content of both phosphorylated and dephosphorylated psychoactive
alkaloid has
3
Date Recue/Date Received 2020-12-04
PSU004-CANP
not been seen in the industry or academia to date. Likewise, compositions
resulting from
such control have not yet been seen.
[0012] Thus, there is a need in the art of a process for controlling the
dephosphorylation of the aforementioned alkaloids to result in a psychoactive
alkaloid
extract with specific desired amounts of phosphorylated and dephosphorylated
psychoactive alkaloid. Also required in the art is a psychoactive alkaloid
composition
having an accurate psychoactive alkaloid content, with specific desired
amounts of
phosphorylated and dephosphorylated psychoactive alkaloid.
[0013] Disclosed herein is a process for obtaining a psychoactive alkaloid
extract with
a desired amount of a phosphorylated psychoactive alkaloid and a desired
amount of a
dephosphorylated psychoactive alkaloid, the process comprising: drying and
pulverizing a
psychoactive alkaloid source to obtain a dried powdered biomass; extracting a
psychoactive alkaloid from the dried powdered biomass with an acidified
solvent or a
basified solvent to obtain a psychoactive alkaloid liquid with a specific pH,
wherein the
specific pH is lower than 3.5 or greater than 10.5; adjusting the pH of the
psychoactive
alkaloid liquid to a pH ranging from 3.5-4.5; and evaporating the solvent from
the
psychoactive alkaloid liquid to obtain the psychoactive alkaloid extract with
the desired
amount of the phosphorylated psychoactive alkaloid and the desired amount of
the
dephosphorylated psychoactive alkaloid; wherein: the desired amount of the
phosphorylated psychoactive alkaloid is 0-100% by weight of a total
phosphorylatable
psychoactive alkaloid content in the psychoactive alkaloid extract; and the
desired amount
of the dephosphorylated psychoactive alkaloid is the remainder of the total
phosphorylatable psychoactive alkaloid content in the psychoactive alkaloid
extract.
[0014] Also disclosed is a process for obtaining a psychoactive alkaloid
composition
with a specific ratio of a phosphorylated psychoactive alkaloid to a
dephosphorylated
psychoactive alkaloid, the process comprising: extracting a psychoactive
alkaloid from a
dried powdered biomass with a basified solvent to obtain a psychoactive
alkaloid liquid
with a pH greater than 10.5, wherein a majority of a total phosphorylatable
psychoactive
alkaloid content is the phosphorylated alkaloid and a remainder thereof is the
dephosphorylated alkaloid; adjusting the pH of the psychoactive alkaloid
liquid to a pH
4
Date Recue/Date Received 2020-12-04
PSU004-CANP
ranging from 3.5-4.5; extracting another psychoactive alkaloid from another
dried
powdered biomass with an acidified solvent to obtain another psychoactive
alkaloid liquid
with a pH lower than 3.5, wherein all of a total phosphorylatable psychoactive
alkaloid is
the dephosphorylated alkaloid; adjusting the pH of the other psychoactive
alkaloid liquid to
a pH ranging from 3.5-4.5; evaporating a portion of the basified solvent from
the
psychoactive alkaloid liquid and a portion of the acidified solvent from the
other
psychoactive alkaloid liquid to obtain a psychoactive alkaloid extract slurry
and another
psychoactive alkaloid extract slurry respectively; mixing measured portions of
the
psychoactive alkaloid extract slurry and the other psychoactive alkaloid
extract slurry to
obtain a bulk psychoactive alkaloid extract slurry comprising the
phosphorylated
psychoactive alkaloid and the dephosphorylated psychoactive alkaloid in the
specific ratio;
standardizing the bulk psychoactive alkaloid extract slurry by adding thereto
a measured
quantity of one or more excipients to obtain a standardized bulk slurry; and
drying the
standardized bulk psychoactive alkaloid slurry to obtain the psychoactive
alkaloid
composition, wherein the phosphorylated psychoactive alkaloid and the
dephosphorylated
psychoactive alkaloid are in the specific ratio; wherein the specific ratio of
phosphorylated
psychoactive alkaloid to dephosphorylated psychoactive alkaloid ranges from
1:1000 to
1000:1.
[0015] Further disclosed is a psychoactive alkaloid composition comprising:
a
psychoactive alkaloid extract comprising a desired amount of a phosphorylated
psychoactive alkaloid and a desired amount of a dephosphorylated psychoactive
alkaloid,
wherein: the desired amount of the phosphorylated psychoactive alkaloid is 0-
100% by
weight of a total phosphorylatable psychoactive alkaloid content in the
psychoactive
alkaloid extract, and the desired amount of the dephosphorylated psychoactive
alkaloid is
the remainder of the total phosphorylatable psychoactive alkaloid content in
the
psychoactive alkaloid extract; and one or more excipients.
[0016] Still further disclosed is a psychoactive alkaloid composition with
a specific ratio
of a phosphorylated psychoactive alkaloid and a dephosphorylated psychoactive
alkaloid,
the composition comprising: a psychoactive alkaloid extract having a total
phosphorylatable psychoactive alkaloid content of 100% of a phosphorylated
Date Recue/Date Received 2020-12-04
PSU004-CANP
psychoactive alkaloid; another psychoactive alkaloid extract having a total
phosphorylatable psychoactive alkaloid content of 100% of a dephosphorylated
psychoactive alkaloid; and one or more excipients; wherein the psychoactive
alkaloid
extract and the other psychoactive alkaloid extract are present in a
proportion such that
the specific ratio of phosphorylated psychoactive alkaloid to phosphorylated
psychoactive
alkaloid ranges from 1:1000 to 1000:1.
[0017] This summary does not necessarily describe all features of the
invention.
BRIEF DESCRIPTION OF DRAWINGS
[0018] The following drawings illustrate embodiments of the invention,
which should
not be construed as restricting the scope of the invention in any way.
[0019] FIG. 1 illustrates the key steps of a process for obtaining a
psychoactive
alkaloid extract with dephosphorylation control, according to an embodiment of
the
present invention.
[0020] FIG. 2 illustrates in detailed steps of a process for obtaining a
psychoactive
alkaloid extract with dephosphorylation control, according to an embodiment of
the
present invention.
[0021] FIG. 3 illustrates a process for standardizing a psychoactive
alkaloid extract to
obtain a standardized psychoactive alkaloid extract.
[0022] FIG. 4 illustrates a process for obtaining a psychoactive alkaloid
composition
with a specific ratio of a phosphorylated psychoactive alkaloid to a
dephosphorylated
psychoactive alkaloid, according to an embodiment of the present invention.
[0023] FIG. 5 illustrates detailed steps of a process for obtaining a
psychoactive
alkaloid composition with a specific ratio of a phosphorylated psychoactive
alkaloid to a
dephosphorylated psychoactive alkaloid, according to another embodiment of the
present
invention.
[0024] FIG. 6 illustrates a schematic diagram of the apparatus used for
obtaining a
psychoactive alkaloid extract and standardizing the same to result in a
standardized
psychoactive alkaloid extract, according to an embodiment of the present
invention.
6
Date Recue/Date Received 2020-12-04
PSU004-CANP
DESCRIPTION
A. GLOSSARY
[0025] To facilitate the understanding of this invention, a number of terms
are defined
below. Terms defined herein have meanings as commonly understood by a person
of
ordinary skill in the areas relevant to the present invention. Terms such as
"a", "an" and
"the" are not intended to refer to only a singular entity but include the
general class of
which a specific example may be used for illustration. The terminology herein
is used to
describe specific embodiments of the invention, but their usage does not
delimit the
invention, except as outlined in the claims.
[0026] The term "psychoactive alkaloid" used herein refers to alkaloids
that upon
introduction to the human body are capable of changing brain function, for
example
resulting in alterations in perception, mood, consciousness, cognition, or
behavior. The
psychoactive alkaloid to which the present invention applies is either a
phosphorylated
psychoactive alkaloid or a dephosphorylated psychoactive alkaloid, and there
may be
multiple different compounds in each.
[0027] The term "psychoactive alkaloid source" used herein refers to a
fungus, a
mycelium, a spore, a plant, a bacterium, an animal or a yeast, which has in it
a
phosphorylated psychoactive alkaloid, a dephosphorylated psychoactive
alkaloid, or a
combination or both. The source of the psychoactive alkaloid can also be
another extract
or a solution with a phosphorylated psychoactive alkaloid, a dephosphorylated
psychoactive alkaloid, or a combination of both.
[0028] The term "phosphorylatable psychoactive alkaloid" refers to
psychoactive
alkaloids that have phosphorylated derivatives, and includes psychoactive
alkaloids in
both their phosphorylated and dephosphorylated forms.
[0029] The term "psychoactive alkaloid composition" used herein can also be
referred
to as "composition" and describes a mixture of psychoactive alkaloid and one
or more
excipients. The composition can be of pharmaceutical, nutraceutical, or
veterinarian
grade.
7
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0030] The term "psychoactive alkaloid liquid" used herein refers to
psychoactive
alkaloid obtained in liquid form after a dried powdered biomass of a
psychoactive alkaloid
source has been extracted using an acidified solvent or a basified solvent.
The liquid form
can be a solution or a slurry.
[0031] The term "psychoactive alkaloid extract" used herein refers to a
psychoactive
alkaloid extract obtained by an extraction process of the present invention.
The extract
can be in a solid, solid-powdered, semi-solid or a slurry form.
[0032] As used herein, the term "specific amount" when referring to a total
psychoactive alkaloid content means a desired percentage, accurate to one or
two
decimal places or one or two significant figures, of total psychoactive
alkaloid in a
psychoactive alkaloid composition. The specific amount is defined as a
percentage by
weight and can be selected by a person of skill in the art according to
preference.
[0033] The term "specific pH" herein refers to a desired pH value of a
solvent or a
psychoactive alkaloid liquid obtained by adding an acidified solvent or a
basified solvent.
[0034] The term "specific ratio" herein refers to a weight ratio between a
phosphorylated psychoactive alkaloid and a dephosphorylated psychoactive
alkaloid
present in a psychoactive alkaloid composition. The ratio can be altered by a
person of
skill in the art according to preference.
[0035] The term "desired amount" herein refers to an amount of a
phosphorylated
psychoactive alkaloid or a dephosphorylated psychoactive alkaloid in a total
phosphorylatable psychoactive alkaloid content, in the psychoactive alkaloid
liquid, extract
or composition. The amount of each of these alkaloids is controlled by the
process for
making the psychoactive alkaloid extract or psychoactive alkaloid composition.
The
amount can be altered by a person of skill in the art according to preference.
The amount
is usually a percentage ratio by weight that may be accurate up to two
significant figures.
[0036] As used herein, the expression "standardizing" the psychoactive
alkaloid slurry
or bulk psychoactive alkaloid extract slurry refers to adding an excipient to
an extract to
obtain a slurry with a specific, total concentration of alkaloid, by weight.
The slurry may
then be dried to form a powdered composition with a pre-calculated percentage
8
Date Recue/Date Received 2020-12-04
PSU004-CANP
concentration by weight of psychoactive alkaloid. The total amount of alkaloid
content can
be specified to an accuracy of up to three significant figures.
[0037]
The phrase "one or more excipients" is used herein to refer that one excipient
or more than one excipient can be used in any combination. The number of
excipients to
be used will be at the discretion of a person skilled in the art, and they may
be of different
types.
[0038]
The term "therapeutic effects" is intended to qualify the amount of active
ingredients required in the treatment of a disease or disorder or on the
effecting of a
clinical endpoint. Reference to "treatment" of a patient is intended to
include prophylaxis.
Treatment may also be preemptive in nature, i.e., it may include prevention of
disease.
Prevention of a disease may involve complete protection from disease, for
example as in
the case of prevention of infection with a pathogen or may involve prevention
of disease
progression. For example, prevention of a disease may not mean complete
foreclosure of
any effect related to the diseases at any level, but instead may mean
prevention of the
symptoms of a disease to a clinically significant or detectable level.
Prevention of
diseases may also mean prevention of progression of a disease to a later stage
of the
disease.
B. BASIC PROCESS
[0039] In one embodiment, referring to FIG. 1, a basic process for obtaining a
psychoactive alkaloid extract with a desired amount of a phosphorylated
psychoactive
alkaloid and a desired amount of a dephosphorylated psychoactive alkaloid is
shown. The
phosphorylated alkaloid may be psilocybin, baeocystin, norbaeocystin,
aeruginascin, or
any combination selected therefrom. The dephosphorylated alkaloid may be
psilocin,
norpsilocin, 4-hydroxytryptamine, N ,N ,N-
trimethy1-4-hydroxytryptamine, or any
combination selected therefrom. The control aspect of the present invention
relates to
psychoactive alkaloids that have phosphorylated forms, and not to other
psychoactive
alkaloids that may be present in a psychoactive alkaloid source. Depending on
the strain
or harvest, there may be no or substantially no phosphorylatable psychoactive
alkaloids in
9
Date Recue/Date Received 2020-12-04
PSU004-CANP
the psychoactive alkaloid source, or they may represent as much 80-90% of the
total
alkaloid content.
[0040] The process includes step 10 of obtaining powdered biomass from a
psychoactive alkaloid source. The powdered biomass is obtained by drying and
pulverizing a psychoactive alkaloid source. The drying is carried out via
vacuum
desiccation, freeze drying, timed forced air drying, or other suitable drying
method known
to a person of skill in the art, to obtain a dried biomass. The pulverization
is carried out by
milling, grinding, or other method to reduce the particle size of the dried
biomass. In one
embodiment, the drying is carried out in a forced air oven completely shielded
from all
light at 20-30 C for a time period of 5-10 hours. However, there is room for
optimization of
the drying step, using different temperatures (e.g. 10 ¨ 50 C) and different
durations.
[0041] In one embodiment, the drying is carried out in a manner to not promote
the
conversion of phosphorylated psychoactive alkaloid. Taking care not to bruise
while
harvesting the psychoactive alkaloid source, harvesting at the right time of
the fruiting
body life cycle, potentially freeze drying the fruiting body, low heat
desiccation, and gentle
air drying are all ways that reduce the conversion of phosphorylated
psychoactive alkaloid
to dephosphorylated psychoactive alkaloid. It may be feasible to harvest the
fungi in a
basic or methanol environment, or soak the whole mycelium in methanol and
cutting the
fruits while soaked. Once dried, there is negligible conversion, so that
subsequent
pulverization has little effect on it, unless moisture is added back. The
prevention of
conversion of phosphorylated psychoactive alkaloid to dephosphorylated
psychoactive
alkaloid during harvesting allows for the preparation of psychoactive alkaloid
extracts, by
the present process, having a total phosphorylatable psychoactive alkaloid
content that is
up to 100% by weight of phosphorylated psychoactive alkaloid.
[0042] In one embodiment, the psychoactive alkaloid source is a mushroom from
the
genus Conocybe, Copelandia, Galerina, Gymnopilus, lnocybe, Panaeolus,
Pholiotina,
Pluteus or Psilocybe, or any combination of mushrooms selected therefrom. In
one
embodiment, gills, caps, stems, or the whole of the fungi is used as the
alkaloid source.
[0043] Step 12 involves extracting a psychoactive alkaloid from the dried
powdered
biomass with an acidified solvent or a basified solvent to obtain a
psychoactive alkaloid
Date Recue/Date Received 2020-12-04
PSU004-CANP
liquid with a specific pH, wherein the specific pH is lower than 3.5 or
greater than 10.5.
Between pH3.5 and pH10.5, the conditions are such that psilocybin is readily
converted to
psilocin, and psilocin is converted to the quinoid dimer, which is completely
inactive.
[0044]
When used, the acid may be acetic acid, adipic acid, ascorbic acid, phosphoric
acid, ammonium aluminum sulphate, ammonium citrate dibasic, ammonium citrate
monobasic, calcium citrate, calcium fumarate, calcium gluconate, calcium
phosphate
dibasic, calcium phosphate monobasic, hydrochloric acid, sulphuric acid
monobasic,
calcium phosphate tribasic, citric acid, fumaric acid, gluconic acid,
magnesium fumarate,
malic acid, phosphoric acid, potassium acid tartrate, potassium citrate,
potassium
fumarate, sodium citrate, sodium fumarate, sodium gluconate, sodium lactate,
sodium
potassium hexametaphosphate, sodium potassium tartrate, sodium potassium
tripolyphosphate, sodium pyrophosphate tetrabasic, sodium tripolyphosphate,
tartaric
acid, and any combination of one or more of these. In some embodiments, the
acid is
either only hydrochloric acid or only phosphoric acid, for example. It is also
envisaged that
other acids may be used, for example non-food-grade acids that may be used by
pharmaceuticals.
[0045] When used, the base may be ammonium bicarbonate, ammonium carbonate,
ammonium hydroxide, calcium acetate, calcium carbonate, calcium chloride,
calcium
hydroxide, calcium lactate, calcium oxide, calcium phosphate dibasic, calcium
phosphate
monobasic, magnesium carbonate, potassium aluminum sulphate, potassium
bicarbonate, potassium carbonate, potassium hydroxide, potassium lactate,
potassium
phosphate dibasic, potassium pyrophosphate tetrabasic, potassium phosphate
tribasic,
potassium tripolyphosphate, sodium acetate, sodium acid pyrophosphate, sodium
aluminum phosphate, sodium aluminum sulphate, sodium bicarbonate, sodium
bisulphate, sodium carbonate, sodium hexametaphosphate, sodium hydroxide,
sodium
lactate, sodium phosphate dibasic, sodium phosphate monobasic, sodium
phosphate
tribasic or any combination therefrom. In one embodiment, the base is solely
sodium
hydroxide, for example. Other bases may be used in other embodiments, for
example
non-food-grade bases that may be used by pharmaceuticals.
11
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0046] After adding the acidified solvent or the basified solvent, the
psychoactive alkaloid
liquid has a pH ranging from 0.5-3.5 or from 10.5-13.5 respectively. In an
exemplary
embodiment, the pH of the psychoactive alkaloid liquid obtained after addition
of the
basified solvent is 13. In another exemplary embodiment, the pH of the
psychoactive
alkaloid liquid obtained after addition of the acidified solvent is 2.
[0047] The pH is adjusted in the extraction step 12 to halt or promote
conversion of
phosphorylated psychoactive alkaloid to dephosphorylated psychoactive
alkaloid, thus
allowing the preparation of the psychoactive alkaloid liquid with the desired
amount of a
phosphorylated psychoactive alkaloid and a desired amount of a
dephosphorylated
psychoactive alkaloid. A specific pH lower than 3.5 promotes the conversion of
the
phosphorylated psychoactive alkaloid to the dephosphorylated psychoactive
alkaloid. A
specific pH greater than 10.5 halts the conversion of the phosphorylated
psychoactive
alkaloid to the dephosphorylated psychoactive alkaloid.
[0048] In step 14 of the process, the pH of the obtained psychoactive alkaloid
liquid is
adjusted to a pH ranging from 3.5-4.5. The pH is adjusted by adding a base or
an acid.
The pH is adjusted to a value in this range as the psychoactive alkaloid
liquid exhibits a
good anti-microbial stability in this pH range. Also, there is no
dephosphorylation at this
pH after the alkaloids are removed from the biomass, which points to enzymatic
hydrolysis being responsible for conversion in the source of the psychoactive
alkaloids. In
exemplary embodiments, the base is sodium hydroxide and the acid is citric
acid. Any
other appropriate acid or base can be used to adjust the pH, which a person of
skill in the
art may determine. The selection of the acid or the base will depend upon the
nature of
the pH of the psychoactive alkaloid liquid prior to adjusting it to the range
of 3.5-4.5,
according to which a person of skill in the art can make the appropriate acid
or base
selection.
[0049] Step 16 of the process involves evaporating the solvent from the
psychoactive
alkaloid solution to obtain the psychoactive alkaloid extract with the desired
amount of the
phosphorylated psychoactive alkaloid and the desired amount of the
dephosphorylated
psychoactive alkaloid. The solvent is completely or partially evaporated to
result in the
psychoactive alkaloid extract (slurry or powder) with the desired amount of
the
12
Date Recue/Date Received 2020-12-04
PSU004-CANP
phosphorylated psychoactive alkaloid and the desired amount of the
dephosphorylated
psychoactive alkaloid. The evaporation is carried out by methods such as air
drying,
rotary evaporation, or other methods known in the art to suitably evaporate
solvent from
psychoactive alkaloid liquid. At this point in time, away from the biomass,
psilocybin
and/or psilocin are fairly heat resistant, more so under vacuum, and so rotary
evaporation,
for example, is a suitable process. The desired amount of the phosphorylated
psychoactive alkaloid is 0-100% of a phosphorylatable psychoactive alkaloid
content in
the psychoactive alkaloid extract. The desired amount of the dephosphorylated
psychoactive alkaloid is the remainder of the total phosphorylatable
psychoactive alkaloid
content in the psychoactive alkaloid extract.
[0050] In some embodiments, when during the extraction step the psychoactive
alkaloid
liquid has a pH greater than 10.5, the desired amount of the phosphorylated
psychoactive
alkaloid is 50-90% by weight (crude extraction) of the total phosphorylatable
psychoactive
alkaloid content in the psychoactive alkaloid extract, without going to
onerous lengths in
selecting the raw material. The desired amount of the dephosphorylated
psychoactive
alkaloid is the remainder of the total phosphorylatable psychoactive alkaloid
content in the
psychoactive alkaloid extract.
[0051] In another embodiment, when using a psychoactive alkaloid source that
has not
undergone conversion (i.e. no significant conversion) of any phosphorylated
alkaloid to
dephosphorylated alkaloid, and when during the extraction step the
psychoactive alkaloid
liquid has a pH greater than 10.5, the desired amount of the phosphorylated
psychoactive
alkaloid is 100% by weight of the total phosphorylatable psychoactive alkaloid
content in
the psychoactive alkaloid extract. The desired amount of the dephosphorylated
psychoactive alkaloid is 0% by weight of the total phosphorylatable
psychoactive alkaloid
content in the psychoactive alkaloid extract.
[0052] In yet another embodiment, when during the extraction step the
psychoactive
alkaloid liquid has a pH lower than 3.5, the desired amount of the
phosphorylated
psychoactive alkaloid is 0% by weight of the total phosphorylatable
psychoactive alkaloid
content in the psychoactive alkaloid extract. The desired amount of the
dephosphorylated
psychoactive alkaloid is 100% by weight of the total phosphorylatable
psychoactive
13
Date Recue/Date Received 2020-12-04
PSU004-CANP
alkaloid content in the psychoactive alkaloid extract. Even with neutral
hydroethanol
extraction, a large portion of psilocybin may be converted to psilocin.
However, the low pH
environment (<3.5) protects the psilocin from oxidation.
C. EXTRACTION
[0053] In some embodiments, referring to FIG. 2, additional, optional steps in
the
extraction step 12 are shown. Step 20 is performed by adding the acidified
solvent or the
basified solvent to the powered biomass. The obtained psychoactive liquid has
a specific
pH, the specific pH being lower than 3.5 or greater than 10.5. After the
addition of the
acidified solvent or the basified solvent, the powered biomass and the solvent
are mixed,
followed by step 22 of filtration to result in the extracted filtrate of step
24 (i.e.
psychoactive alkaloid liquid). To this obtained filtrate, the acid or the base
of step 14 is
added to adjust the pH to within the range of 3.5-4.5.
[0054] In some embodiments, the extraction step comprises further extracting
the
psychoactive alkaloid by repeating the extraction step. Filtrate residue from
step 22 is
collected and to this filtrate residue, the same or a different acidified
solvent, or the same
or a different basified solvent is added. The resulting mixture is mixed
followed by filtration
to obtain another filtrate. This filtrate and the previous filtrate are mixed
together to result
in a bulk filtrate. To this bulk filtrate the acid or the base is added to
adjust the pH to 3.5-
4.5 according to step 14.
[0055] In some embodiments, further extraction of the filtrate obtained after
extraction
with the acidified or the basified solvent is repeated until a required amount
of the
phosphorylated psychoactive alkaloid and/or the dephosphorylated psychoactive
alkaloid
is extracted. The number of extraction cycles to be repeated will depend on
various
variable factors such as the source of the psychoactive alkaloid and the
solubility of the
psychoactive alkaloid in the acidified or the basified solvent.
[0056] In some embodiments, the extraction is performed at a temperature
ranging from
5-95 C. In other embodiments, the extraction is performed at a temperature
ranging from
50-75 C.
14
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0057] In some embodiments, the extraction is performed for a time period
ranging from
10-720 minutes. For most cases, a time below 10 min would result in a mostly
incomplete
yield, and above 720 min the extraction may be incomplete but would be
continuing at a
negligible rate. In another embodiment, and more usually, the extraction is
performed for
a time period ranging from 30-240 minutes.
[0058] In some embodiments, the extraction is performed at a pressure ranging
from 7
to 20,000 psi. In yet another embodiment, the extraction is performed at a
pressure
ranging from 10 to 20 psi.
[0059] In some embodiments, the extraction is performed with a solvent to
solid ratio in
the range 1L:1kg to 50L:1kg, wherein the solid is the dried powdered biomass.
In one
embodiment, the extraction is performed with a solvent to solid ratio of
20L:1kg.
[0060] The solvent in the evaporation step can be completely or partially
evaporated, to
result in a powdered solid or a slurry. Evaporation may be paused, for
standardization,
and continued after.
D. STANDARDIZATION
[0061] Referring to FIG. 3, the present invention also relates to a process
for obtaining a
psychoactive alkaloid composition with a psychoactive alkaloid extract and one
or more
excipients.
[0062] At step 28 the evaporation step 16 (FIG. 1) is paused when a portion of
the
solvent has been evaporated from the psychoactive alkaloid liquid to obtain a
psychoactive alkaloid slurry. The evaporation of a portion of the solvent,
before collection
of the psychoactive alkaloid slurry for standardization, is done to obtain a
quantity of a
psychoactive alkaloid slurry that is easy to handle in the subsequent steps of
the
standardization process. The quantity of the portion of the solvent to be
evaporated before
pausing the evaporation is not so much as to make it too viscous to handle
well. The
quantity of the portion of the solvent to be evaporated will depend on various
factors, for
example, but not limited to, the contents of the psychoactive alkaloid liquid
and the
quantity of the psychoactive alkaloid liquid present at the beginning of the
evaporation
step.
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0063] Step 30 includes standardizing the obtained psychoactive alkaloid
slurry by
adding thereto a measured quantity of one or more excipients to obtain a
standardized
slurry with a specific amount of psychoactive alkaloid content. The specified
amount of the
total psychoactive alkaloid content in the psychoactive alkaloid composition
is achieved by
first determining the proportion, by weight, of solids in the psychoactive
alkaloid slurry.
The weight proportion of the psychoactive alkaloids in the slurry is also
determined. Then
a measured amount of one or more excipients is added to a measured amount of
the
psychoactive alkaloid slurry. Thus, after evaporation of the remaining
solvent, the
resultant composition is a standardized composition with a specified total
psychoactive
alkaloid content.
[0064] Step 32 includes continuing the evaporating step 16 (FIG. 1) by drying
the
standardized slurry to obtain a psychoactive alkaloid composition with the
psychoactive
alkaloid extract and the one or more excipients.
[0065] The evaporation in the standardization process is carried out by
methods such as
air drying, rotary evaporation, or other methods known in the art to suitably
evaporate
solvent from psychoactive alkaloid slurry.
[0066] The psychoactive alkaloid composition obtained has a specific total
psychoactive
alkaloid content in the composition due to the standardizing step. The
specified amount of
the total psychoactive alkaloid content may be accurate to one or two decimal
places, or
one or two significant figures depending on how accurately the measurements
are made
during the standardization process.
[0067] Thus, the psychoactive alkaloid composition obtained has a specific
amount of a
total psychoactive alkaloid content, which is made up of a desired amount of
phosphorylated psychoactive alkaloid and a desired amount of dephosphorylated
psychoactive alkaloid, and possibly other psychoactive alkaloids that are not
phosphorylatable.
[0068] In one embodiment, the specific amount of the total psychoactive
alkaloid content
in the psychoactive alkaloid composition ranges from 0.1-99% by weight of the
composition, the higher concentrations being obtained when purifying steps are
included.
Purifying may involve treating the extract with a resinous material prior to
the
16
Date Recue/Date Received 2020-12-04
PSU004-CANP
standardization step, to remove impurities such as sugars and proteins. In
another
embodiment, the specific amount of the total psychoactive alkaloid content in
the
psychoactive alkaloid composition ranges from 0.1-10% by weight of the
composition,
which may be achieved without purifying steps. In an exemplary embodiment, the
specific
amount of the total psychoactive alkaloid content in the psychoactive alkaloid
composition
is 0.53% by weight of the composition. In yet another exemplary embodiment,
the specific
amount of the total psychoactive alkaloid content in the psychoactive alkaloid
composition
is 0.501% by weight of the composition.
[0069]
In some embodiments, the desired amount of the phosphorylated psychoactive
alkaloid is 0-100% by weight of a total phosphorylatable psychoactive alkaloid
content in
the psychoactive alkaloid extract, and the desired amount of the
dephosphorylated
psychoactive alkaloid is the remainder of the total phosphorylatable
psychoactive alkaloid
content in the psychoactive alkaloid extract. In one embodiment, the desired
amount of
the phosphorylated psychoactive alkaloid is 10-90% by weight of the total
phosphorylatable psychoactive alkaloid content in the psychoactive alkaloid
extract, and
the desired amount of the dephosphorylated psychoactive alkaloid is the
remainder of the
total phosphorylatable psychoactive alkaloid content in the psychoactive
alkaloid extract.
In another embodiment, the desired amount of the phosphorylated psychoactive
alkaloid
is 0% by weight of the total phosphorylatable psychoactive alkaloid content in
the
psychoactive alkaloid extract, and the desired amount of the dephosphorylated
psychoactive alkaloid is 100% by weight of the total phosphorylatable
psychoactive
alkaloid content in the psychoactive alkaloid extract. In yet another
embodiment, the
desired amount of the phosphorylated psychoactive alkaloid is 100% by weight
of the total
phosphorylatable psychoactive alkaloid content in the psychoactive alkaloid
extract, and
the desired amount of the dephosphorylated psychoactive alkaloid is 0% by
weight of the
total phosphorylatable psychoactive alkaloid content in the psychoactive
alkaloid extract.
E. MIXTURE
[0070] Referring to FIG. 4, in one embodiment, the present invention also
relates to a
process for preparing a psychoactive alkaloid composition by mixing a
phosphorylated
17
Date Recue/Date Received 2020-12-04
PSU004-CANP
psychoactive alkaloid composition and a dephosphorylated psychoactive alkaloid
composition in a specific ratio. In step 34, the psychoactive alkaloid
composition obtained
after step 32 (FIG. 3) is taken. The psychoactive alkaloid composition in step
32 has the
desired amount of the dephosphorylated psychoactive alkaloid of 100% by weight
of the
total phosphorylatable psychoactive alkaloid content in the psychoactive
alkaloid extract.
The aforesaid is achieved by extracting the psychoactive alkaloid from the
dried powdered
biomass with an acidified solvent having a specific pH lower than 3.5.
[0071] In step 36, another psychoactive alkaloid composition is obtained
according to
the process described above. This other psychoactive alkaloid composition has
a desired
amount of phosphorylated psychoactive alkaloid of 100% by weight of the total
phosphorylatable psychoactive alkaloid content. The aforesaid is achieved by
extracting
the psychoactive alkaloid from the dried powdered biomass with a basified
solvent having
a pH greater than 10.5, where the biomass is not dephosphorylated or not
significantly
dephosphorylated.
[0072] In step 38, both psychoactive alkaloid compositions are mixed in a
measured
ratio to obtain a psychoactive alkaloid composition. This composition includes
the
dephosphorylated psychoactive alkaloid of the first psychoactive alkaloid
composition and
the phosphorylated psychoactive alkaloid of the second psychoactive alkaloid
composition
in a specific ratio. In some embodiments, the specific ratio of phosphorylated
psychoactive alkaloid to dephosphorylated psychoactive alkaloid is in the
range from
1000:1 to 1:1000. If the total phosphorylatable psychoactive alkaloid
concentrations are
the same in both starting compositions, then the final ratio of phosphorylated
to
dephosphorylated alkaloids is the same as the ratio in which the starting
compositions are
mixed. However, in other embodiments, the mixing ratio may need to be modified
to take
into account the different starting concentrations of the phosphorylated and
dephosphorylated alkaloids in their respective compositions. In yet other
embodiments,
one of the starting compositions may include both phosphorylated and
dephosphorylated
alkaloids, and the mixing ratio of the two compositions may be adjusted to
take this into
account.
18
Date Recue/Date Received 2020-12-04
PSU004-CANP
F. ALTERNATE PROCESS
[0073] FIG. 5, in another embodiment, describes a process for preparing a
psychoactive
alkaloid composition having a phosphorylated psychoactive alkaloid from one
psychoactive alkaloid extraction and a dephosphorylated psychoactive alkaloid
from
another psychoactive alkaloid extraction in a specific ratio.
[0074] A dried powdered biomass is obtained in step 40. Step 42 includes
extracting a
psychoactive alkaloid with a basified solvent to obtain a psychoactive
alkaloid liquid with a
pH greater than 10.5. A majority, or all, of a total phosphorylatable
psychoactive alkaloid
content is phosphorylated alkaloid and a remainder thereof is dephosphorylated
alkaloid.
[0075] In step 44 the pH of the resulting psychoactive alkaloid liquid is
adjusted to a pH
ranging from 3.5-4.5. The pH is adjusted by adding an acid.
[0076] Another dried powdered biomass is obtained in step 50. Step 52 includes
extracting a psychoactive alkaloid with an acidified solvent to obtain another
psychoactive
alkaloid liquid with a pH lower than 3.5. All of the psychoactive alkaloid
present is
dephosphorylated.
[0077] Step 54 involves adjusting the pH of the second psychoactive alkaloid
liquid to a
pH ranging from 3.5-4.5. The pH is adjusted by adding a base.
[0078]
In steps 46 and 56, a portion of the basified solvent from the first
psychoactive
alkaloid liquid, and a portion of the acidified solvent from the second
psychoactive alkaloid
liquid are evaporated to obtain first and second psychoactive alkaloid
slurries 48, 58
respectively.
[0079] In step 60, measured portions of the psychoactive alkaloid slurries are
mixed to
obtain a bulk psychoactive alkaloid slurry. The bulk psychoactive alkaloid
slurry includes
the phosphorylated psychoactive alkaloid and the dephosphorylated psychoactive
alkaloid
in the specific ratio. The measured portions, by weight, of each of the
psychoactive
alkaloid slurries are chosen in such a manner as to achieve the specific ratio
of the
phosphorylated psychoactive alkaloid and the dephosphorylated psychoactive
alkaloid,
taking into account the percentage by weight of these psychoactive alkaloids
in each of
the initial slurries. In one exemplary embodiment, mixing 100 g of a 1.0%
phosphorylated
19
Date Recue/Date Received 2020-12-04
PSU004-CANP
psychoactive alkaloid slurry, with 50 g of a 0.5% dephosphorylated
psychoactive alkaloid
slurry results in the specific ratio of 4:1 of phosphorylated to
dephosphorylated alkaloid.
[0080] In step 62, the obtained bulk psychoactive alkaloid slurry is
standardized by
adding thereto a measured quantity of one or more excipients to obtain a
standardized
bulk slurry with a specific total amount of psychoactive alkaloid.
[0081] Step 64 includes drying the standardized bulk psychoactive alkaloid
slurry by
evaporation to obtain the psychoactive alkaloid composition. The obtained
psychoactive
alkaloid composition has a specific total amount of psychoactive alkaloid
content, and the
phosphorylated psychoactive alkaloid and dephosphorylated psychoactive
alkaloid are in
the specific ratio. In some embodiments of the psychoactive alkaloid
composition, the
specific ratio of phosphorylated psychoactive alkaloid to dephosphorylated
psychoactive
alkaloid ranges from 1:1000 to 1000:1.
G. PSYCHOACTIVE ALKALOID COMPOSITION
[0082] In one embodiment, the present invention also relates to a psychoactive
alkaloid
composition. The psychoactive alkaloid composition includes a specific amount
of a total
phosphorylatable psychoactive alkaloid content, made up of a desired amount of
a
phosphorylated psychoactive alkaloid and a desired amount of a
dephosphorylated
psychoactive alkaloid, and one or more excipients.
[0083] In one embodiment, the specific amount of the total phosphorylatable
psychoactive alkaloid content in the psychoactive alkaloid composition ranges
from 0.1-
99% by weight of the composition. In another embodiment, the specific amount
of the total
phosphorylatable psychoactive alkaloid content in the psychoactive alkaloid
composition
ranges from 0.1-10% by weight of the composition. In an exemplary embodiment,
the
specific amount of the total phosphorylatable psychoactive alkaloid content in
the
psychoactive alkaloid composition is 0.533% by weight of the composition. In
yet another
exemplary embodiment, the specific amount of the total phosphorylatable
psychoactive
alkaloid content in the psychoactive alkaloid composition is 0.501% by weight
of the
composition.
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0084] In one embodiment, the desired amount of the phosphorylated
psychoactive
alkaloid is 0-100% by weight of a total phosphorylatable psychoactive alkaloid
content in
the psychoactive alkaloid extract, and the desired amount of the
dephosphorylated
psychoactive alkaloid is the remainder of the total phosphorylatable
psychoactive alkaloid
content in the psychoactive alkaloid extract. In one embodiment, the desired
amount of
the phosphorylated psychoactive alkaloid is 10-90% by weight of the total
phosphorylatable psychoactive alkaloid content in the psychoactive alkaloid
extract, and
the desired amount of the dephosphorylated psychoactive alkaloid is the
remainder of the
total phosphorylatable psychoactive alkaloid content in the psychoactive
alkaloid extract.
In another embodiment, the desired amount of the phosphorylated psychoactive
alkaloid
is 0% by weight of the total phosphorylatable psychoactive alkaloid content in
the
psychoactive alkaloid extract, and the desired amount of the dephosphorylated
psychoactive alkaloid is 100% by weight of the total phosphorylatable
psychoactive
alkaloid content in the psychoactive alkaloid extract. In yet another
embodiment, the
desired amount of the phosphorylated psychoactive alkaloid is 100% by weight
of the total
phosphorylatable psychoactive alkaloid content in the psychoactive alkaloid
extract, and
the desired amount of the dephosphorylated psychoactive alkaloid is 0% by
weight of the
total phosphorylatable psychoactive alkaloid content in the psychoactive
alkaloid extract.
[0085] In some embodiments, a maximum desired amount of the phosphorylated
alkaloid is limited by an amount of the dephosphorylated alkaloid in the
psychoactive
alkaloid source.
[0086] In an exemplary embodiment, the desired amount of the phosphorylated
psychoactive alkaloid is 0.503% by weight of the psychoactive alkaloid
composition; and
the desired amount of the dephosphorylated psychoactive alkaloid is by 0.03%
weight of
the psychoactive alkaloid composition. In yet another exemplary embodiment,
the desired
amount of the phosphorylated psychoactive alkaloid is 0.00% by weight of the
psychoactive alkaloid composition, and the desired amount of the
dephosphorylated
psychoactive alkaloid is by 0.501% weight of the psychoactive alkaloid
composition.
21
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0087] In one embodiment, the desired amount of the phosphorylated
psychoactive
alkaloid is 100% by weight of the total psychoactive alkaloid content in the
psychoactive
alkaloid extract.
[0088] In one embodiment, the desired amount of the dephosphorylated
psychoactive
alkaloid is 100% by weight of the total psychoactive alkaloid content in the
psychoactive
alkaloid extract.
[0089] The dephosphorylated psychoactive alkaloids are quicker in becoming
bioavailable than their phosphorylated psychoactive alkaloids counterparts.
Thus, the
psychoactive compositions comprising dephosphorylated psychoactive alkaloids
100% by
weight in the total phosphorylatable psychoactive alkaloid content may exhibit
therapeutic
effects faster than psychoactive alkaloid compositions comprising
phosphorylated
psychoactive alkaloids as the majority alkaloids in the total phosphorylatable
psychoactive
alkaloid content.
[0090] In one embodiment, the psychoactive alkaloid composition is obtained by
the
process for obtaining a psychoactive alkaloid extract with a desired amount of
a
phosphorylated psychoactive alkaloid and a desired amount of a
dephosphorylated
psychoactive alkaloid of the present invention.
[0091] In one embodiment, the present invention also relates to a psychoactive
alkaloid
composition with a specific ratio of a phosphorylated psychoactive alkaloid
and a
dephosphorylated psychoactive alkaloid. The composition includes a
psychoactive
alkaloid extract having a total phosphorylatable psychoactive alkaloid content
of 100% by
weight of a phosphorylated psychoactive alkaloid, another psychoactive
alkaloid extract
having a total phosphorylatable psychoactive alkaloid content of 100% by
weight of a
dephosphorylated psychoactive alkaloid, and one or more excipients.
[0092] In one embodiment, one psychoactive alkaloid extract and another
psychoactive
alkaloid extract are present in a proportion such that the specific ratio of
phosphorylated to
dephosphorylated psychoactive alkaloid ranges from 1:1000; 1000:1. In
exemplary
embodiments, a phosphorylated psychoactive alkaloid extract and a
dephosphorylated
psychoactive alkaloid extract are present in a proportion such that the
specific ratio of
phosphorylated to dephosphorylated psychoactive alkaloid is 1:1, 1:3 and 3:1.
22
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0093] In other embodiments, the psychoactive alkaloid composition is defined
by a
specific total amount of psychoactive alkaloid content, which has a known
ratio of
phosphorylated and dephosphorylated alkaloids.
[0094] In some embodiments, the psychoactive alkaloid composition with a
specific ratio
of a phosphorylated psychoactive alkaloid and a dephosphorylated psychoactive
alkaloid
is made by the process for obtaining a psychoactive alkaloid composition with
a specific
ratio of a phosphorylated psychoactive alkaloid to a dephosphorylated
psychoactive
alkaloid of the present invention.
[0095] The psychoactive alkaloid composition of the present invention is in
powder form.
This free-flowing powder form allows the composition to be easily handled. The
components of the composition are also in powder form.
[0096] The psychoactive alkaloid composition of the present invention can be
used, for
example, in medical research on the use of psychedelic substances in
treatments for
mental illnesses.
H. VARIATIONS
[0097]
In some embodiments, the phosphorylated alkaloid is psilocybin, baeocystin,
norbaeocystin, aeruginascin, or any combination therefrom; and the
dephosphorylated
alkaloid is psilocin, norpsilocin,
4-hydroxytryptamine, N,N,N-trimethy1-4-
hydroxytryptamine, or any combination therefrom. In other embodiments, the
phosphorylated alkaloid is psilocybin and the dephosphorylated alkaloid is
psilocin.
[0098] In some embodiments, the acidified solvent is a mixture of an acid and
a C1-C4
primary aliphatic alcohol, a C3-C4 ketone, water, or any combination selected
therefrom.
The acid may be citric acid, ascorbic acid, formic acid, acetic acid,
hydrochloric acid,
phosphoric acid, sulphuric acid, or any combination selected therefrom. In
other
embodiments, the basified solvent is a mixture of a base and a C1-C4 primary
aliphatic
alcohol, a C3-C4 ketone, water, or any combination selected therefrom. The
base may be
sodium hydroxide, potassium hydroxide, ammonium hydroxide, sodium bicarbonate,
calcium carbonate, or any combination selected therefrom.
23
Date Recue/Date Received 2020-12-04
PSU004-CANP
[0099] The excipients described herein refer to excipients which aid in the
manufacturing and/or administration of the compositions described herein. Non-
limiting
examples of such excipients are well known in the art and include flavorants,
colorants,
palatants, antioxidants, viscosity modifying agents, tonicity agents, drug
carriers,
sustained-release agents, comfort-enhancing agents, emulsifiers, solubilizing
aids,
lubricants, binding agents, stabilizing agents and other agents to aid in the
manufacturing
and/or administration of the compositions. The excipients used in the present
invention
are acceptable for use in pharmaceutical or nutraceutical applications or as
food
ingredients. The amount of excipients will vary depending on the concentration
of
psychoactive alkaloids in the psychoactive alkaloid extract to result in a
flowable
psychoactive alkaloid composition, which will be obvious to a person of skill
in the art.
[0100] In some embodiments, the excipients are selected from silicon
dioxide,
ascorbic acid, maltodextrin from corn, potato or tapioca for example, gum
arabic,
microcrystalline cellulose, sodium benzoate, sodium phosphate, sodium citrate,
rice hulls,
and rice. A combination of any of these excipients may be used.
[0101] Any source that contains phosphorylated psychoactive alkaloids may
be used
as the psychoactive alkaloid source.
[0102] Other water may be used in place of reverse osmosis water, which was
selected for its purity.
[0103] The temperature of extraction may be lowered to reduce conversion,
and the
duration may be in the range of 30-240 minutes.
I. EXAMPLES
[0104] In order to further illustrate the present invention, the following
specific
examples are given with the understanding that these examples are intended
only to be
illustrations without serving as a limitation on the scope of the present
invention. All
parameters, dimensions, materials, quantities and configurations described
herein are
examples only and may be changed depending on the specific embodiment.
Accordingly,
the scope of the invention is to be construed in accordance with the substance
defined by
24
Date Recue/Date Received 2020-12-04
PSU004-CANP
the appended claims. The process may be scaled up using larger quantities and
modified
apparatus.
[0105]
Although the examples of the present invention have been formulated
specifically using psilocybe cubensis as a source to obtain a psychoactive
alkaloid extract,
the extract comprising phosphorylated psychoactive alkaloid (i.e. psilocybi)n
and
dephosphorylated psychoactive alkaloid (i.e. psilocin), other sources are
possible. A
person skilled in the art would appreciate that the psilocybe cubensis can be
readily
substituted by other sources of psychoactive alkaloids to obtain a variety of
psychoactive
alkaloids having similar properties, such psilocybin, baeocystin,
norbaeocystin,
aeruginascin, psilocin, norpsilocin,
4-hydroxytryptamine, N,N,N-trimethy1-4-
hydroxytryptamine, or any combination therefrom, to name a few, to result in
compositions
with similar efficacy and efficiency as well. For example, mushrooms from the
genus
Conocybe, Copelandia, Galerina, Gymnopilus, lnocybe, Panaeolus, Pholiotina,
Pluteus,
Psilocybe, or any combination therefrom may be used.
Example 1.1 Process with prevention of dephosphorylation
[0106]
2.5 kilograms of psilocybe cubensis were dried in a forced air oven at 25 C
for
hours to result in 140 grams of dried biomass. The dried biomass was then
pulverized
to a size of 200 mesh with a mill.
[0107]
A basified solvent, i.e. a pH-adjusted, hydro-ethanol mixture, was prepared.
50
g of sodium hydroxide pellets were placed into a 5 L vessel with 1.25 L of RO
(reverse
osmosis) water followed by addition of 3.75 L of ethanol. The contents were
mixed until
completely dissolved. A basified solvent with 75% Et0H v/v% and a pH of 13 was
obtained.
[0108]
The dried powdered biomass was placed into an agitated, heat-controlled
vessel with 5 L of the basified solvent and mixed for extraction of
psychoactive alkaloid.
The extraction was controlled to a constant 75 C, and the time of extraction
was 1 hour.
The extraction slurry was then filtered. Filtration resulted in a filtrate,
i.e. the psychoactive
alkaloid liquid, and a filter residue. The filter residue was placed back into
the extraction
vessel and extracted with an additional 5 L of the basified solvent. The
temperature of
Date Recue/Date Received 2020-12-04
PSU004-CANP
extraction was again 75 C and the time was again 1 hour. The resulting
extraction slurry
was then filtered. The filtrates from the first and second extractions were
mixed to form 10
L of mixed filtrate. The pH of the mixed filtrate was then reduced with 3 M
citric acid until a
pH of 4.5 was achieved. Immediately after adjusting the pH, the mixed filtrate
was placed
into a rotary evaporator at 50 C and 250 torr, and the solvent was partially
or completely
evaporated to obtain a psychoactive alkaloid extract in a slurry or powdered
form
respectively. Final stages of evaporation were performed using a freeze dryer.
When
dried to a powder, the desired amount of the phosphorylated psychoactive
alkaloid
obtained was 0.80% by weight (measured by HPLC analysis) in the psychoactive
alkaloid
extract. The desired amount of the dephosphorylated psychoactive alkaloid
obtained was
0.05% by weight in the psychoactive alkaloid extract.
Example 1.2 Process for heavily phosphorylated psychoactive alkaloid
composition
[0109] The evaporation of the solvent from the mixed filtrate from example
1.1 was
paused when the final volume of the filtrate was reduced to 2.5 L. The
resulting slurry
contained 236.9 mg/L psilocybin and 15.12 mg/L psilocin. The obtained
psychoactive
alkaloid slurry was standardized by the addition of 38.64 g of maltodextrin,
1.12 g of
ascorbic acid and 2.24 g of silicon dioxide. The standardization was followed
by drying by
freeze drying to yield 112 g of the psychoactive alkaloid composition in free-
flowing
powder form. The composition had a psilocybin content of 0.503% dry wt/wt% and
a
psilocin content of 0.003% dry wt/wt%. Further, the composition had a total
phosphorylatable psychoactive alkaloid content of 0.506% dry wt/wt%.
Example 2.1 Process with promotion of dephosphorylation
[0110] 2.5 kilograms of psilocybe cubensis were dried in a forced air oven
at 25 C for
hours to result in 140 grams of dried biomass. The dried biomass was then
pulverized
to a size of 200 mesh with a hammer mill.
[0111] An acidified solvent, i.e. a pH-adjusted, hydro-ethanol mixture, was
prepared.
144 g of anhydrous citric acid was placed into a 5 L vessel with 1.25 L of RO
water
26
Date Recue/Date Received 2020-12-04
PSU004-CANP
followed by the addition of 3.75 L of ethanol. The contents were mixed until
completely
dissolved. An acidified solvent with a pH of 2 was obtained.
[0112] The dried powdered biomass was placed into an agitated, heat-
controlled
vessel with 5 L of the acidified solvent and mixed for the extraction of
psychoactive
alkaloid. The extraction was controlled to a constant 75 C, and the duration
of extraction
was 1 hour. The extraction slurry was then filtered. Filtration resulted in a
filtrate, i.e. the
psychoactive alkaloid liquid, and a filter residue. The filter residue was
placed back into
the extraction vessel and extracted with an additional 5 L of the acidified
solvent. The
temperature of extraction was again 75 C and the time was 1 hour. The
extraction slurry
was filtered. The filtrates from the first and second extraction were mixed to
form 10 L of
mixed filtrate. The pH of the mixed filtrate was then increased with 5 M
sodium hydroxide
until a pH of 4.5 was achieved. Immediately after adjusting the pH, the mixed
filtrate was
placed into a roto-evaporator at 50 C and 250 torr, and the solvent was
partially or
completely evaporated to obtain a psychoactive alkaloid extract. Final stages
of
evaporation were performed using a freeze dryer. When dried to a powder, the
desired
amount of the phosphorylated psychoactive alkaloid obtained was 0.00% by
weight in the
psychoactive alkaloid extract. The desired amount of the dephosphorylated
psychoactive
alkaloid obtained was 0.86% by weight in the psychoactive alkaloid extract.
Example 2.2 Process for preparation of a psychoactive alkaloid composition
[0113] The evaporation of the solvent from the mixed filtrate from example
2.1 was
paused until the final volume of the filtrate was reduced to 2.5 L. The
obtained
psychoactive alkaloid slurry had a 241.85 mg/L of psilocin and a 0 mg/L of
psilocybin. The
slurry was standardized by the addition of 47.07 g of maltodextrin, 1.21 g of
ascorbic acid
and 2.41 g of silicon dioxide. The standardization was followed by freeze
drying to yield
120.7 g of the psychoactive alkaloid composition in free-flowing powder form.
The
composition had a psilocybin content of 0.00% dry wt/wt% and a psilocin
content of
0.501% dry wt/wt%. Further, the composition had a total phosphorylatable
psychoactive
alkaloid content of 0.501% dry wt/wt%.
27
Date Recue/Date Received 2020-12-04
PSU004-CANP
Example 2.3 Process for preparation of a psychoactive alkaloid composition
containing a
mixture of phosphorylated and dephosphorylated alkaloids
[0114] In an alternate method, the slurry from 1.1 and from 2.1 can be
combined to
form 5 L of slurry containing 118.45 mg/L of psilocybin and 128.49 mg/L of
psilocin. The
slurry was standardized by the addition of 85.70 g of maltodextrin, 2.33 g of
ascorbic acid
and 4.65 g of silicon dioxide. The standardization was followed by
lyophilization to yield
232.70 g of the psychoactive alkaloid composition in the free-flowing powder
form. The
composition had a psilocybin content of 0.255% dry wt/wt% and a psilocin
content of
0.276% dry wt/wt%. Further, the composition had a total phosphorylatable
psychoactive
alkaloid content of 0.502% dry wt/wt%.
J. APPARATUS
[0115] Referring to FIG. 6, an example of the apparatus is shown
schematically. Raw
psilocybe cubensis mushrooms were added to a hopper 100 and were released in
batches into container 102. The raw fungal material was then dried in a forced
air oven
104 to result in the dried biomass. The dried biomass was placed into a
grinder 106 for
grinding to result in dried powdered biomass.
[0116] The dried powdered biomass was placed into a heat-controlled vessel
110 and
acidified/basified solvent (S) was added to the vessel to obtain a specific pH
(lower than
3.5 or greater than 10.5). The vessel 110 was surrounded by an insulating wall
108.
Alternately, an insulating jacket may have been wrapped around the vessel. The
insulating wall 108 or jacket helps to maintain the contents 112 under a
constant
temperature (T) between 5 ¨ 95 C. The pressure (P) inside the extraction
vessel 110 may
be regulated from 7 to 20,000 psi. The extraction was performed with a solvent
to solid
(dried powdered biomass) proportion in the range of 1L:1kg to 50L:1kg.
[0117] After the extraction, the bottom of the extraction vessel 110 was
opened at
outlet 114 and the extraction slurry was collected in a container 120. The
extraction slurry
was then fed into a filter 122 and a first filtrate was collected in container
124. The first
filtrate residue 130 was then fed back (R) into the agitated, heat-controlled
vessel 110 and
28
Date Recue/Date Received 2020-12-04
PSU004-CANP
more solvent (S) was added for a second extraction. After the second
extraction, the
extraction slurry was collected in the container 120 and was then fed into a
filter 132. After
filtration, the obtained second filtrate was collected in container 136.
[0118] After the two filtration stages, the filtrates were mixed in
container 140 to obtain
a mixed filtrate i.e. the psychoactive alkaloid liquid. In other embodiments,
if there is only
a single filtration step, this mixing step is not required. By adding an acid
or a base, the
pH of the psychoactive alkaloid liquid was brought to a pH ranging from 3.5-
4.5.
[0119] The pH-adjusted, mixed filtrate was then placed in a rotary
evaporator 142 and
part of the solvent was evaporated from the mixed filtrate to form the
psychoactive
alkaloid extract, which was here a slurry.
[0120] For obtaining the psychoactive alkaloid composition, the evaporation
in the
rotary evaporator 142 was stopped after a desired portion of solvent was
evaporated. The
resultant slurry was transferred to a container 144 where a measured quantity
of one or
more excipients was added to obtain a standardized slurry. The obtained
standardized
slurry was dried in a freeze dryer 146 to obtain the psychoactive alkaloid
composition.
[0121] In other embodiments, parts of the apparatus may be reused or
duplicated. For
example, if desired, for further extraction the filtrate residue may be
reloaded into the
extraction vessel 110 and the obtained filtrate can be added to mixed filtrate
container of
140 and the steps from thereon can be repeated to obtain the psychoactive
alkaloid
extract.
K. CONCLUSION
[0122] Throughout the description, specific details have been set forth in
order to
provide a more thorough understanding of the invention. However, the invention
may be
practiced without these particulars. In other instances, well known elements
have not
been shown or described in detail and repetitions of steps and features have
been omitted
to avoid unnecessarily obscuring the invention. Accordingly, the specification
and
drawings are to be regarded in an illustrative, rather than a restrictive,
sense.
[0123] All parameters, dimensions, materials, quantities and configurations
described
herein are examples only and may be changed depending on the specific
embodiment.
29
Date Recue/Date Received 2020-12-04
PSU004-CANP
Numbers are given to the nearest significant figure, or to 10%, whichever is
the greater.
Accordingly, the scope of the invention is to be construed in accordance with
the
substance defined by the claims. The process may be scaled up using larger
quantities
and a modified apparatus.
[0124] It will be clear to one having skill in the art that further variations
to the specific
details disclosed herein can be made, resulting in other embodiments that are
within the
scope of the invention disclosed. Steps in the flowchart may be performed in a
different
order, other steps may be added, or one or more may be removed without
altering the
main outcome of the processes.
Date Recue/Date Received 2020-12-04