Note: Descriptions are shown in the official language in which they were submitted.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
1
SORBENT COMPOSITION FOR AN ELECTROSTATIC PRECIPITATOR
Technical field
The present invention relates to a calcium-magnesium
compound and to a sorbent composition for use in flue gas streams equipped
with an electrostatic precipitator, a method for obtaining such sorbent
composition and a process of flue gas treatment using an electrostatic
precipitator which comprises a step of injecting such a sorbent composition.
In another aspect, the present invention is related to a flue gas treatment
installation using the sorbent composition according to the invention.
State of the art
Fuel combustion in industrial processes or energy production
generates particulate matter (e.g. fly ashes) and acid gases for which their
release in the atmosphere has to be minimized. The removal of fly ash from
flue gas streams can be performed by an electrostatic precipitator (ESP). Some
examples of electrostatic precipitators are described in US patent 4,502,872,
US patent 8,328,902 or US patent 6,797,035. An electrostatic precipitator
generally comprises a shell with a flue gas inlet and a flue gas outlet, the
shell
enclosing a plurality of collection electrodes, and discharge electrodes
spaced
from each other and a plurality of hoppers positioned under the collecting
plates. A voltage is applied between the discharge electrodes and the
collection electrodes such as to create an electrostatic field charging the
particulate material in the flue gas to obtain charged particulate material.
The
charged particulate material is collected by the collecting electrodes. The
electrostatic precipitator further comprises rappers which provide mechanical
shocks or vibrations to the collecting electrodes to remove the collected
particles from the collecting electrodes. The collected particles fall down
into
hoppers arranged at the bottom of the shell and which are periodically or
continuously emptied. The collecting electrodes can be planar or in a form of
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
2
tubular or honeycomb structure and the discharge electrodes, are generally
under the form of a wire or a rod.
Generally, the flue gas treatment installations including
electrostatic precipitators are provided with an air preheater, which may be
included in a boiler and/or otherwise provided as an additional element of the
flue gas installation. The air preheater comprises a heat exchanger
transferring the heat from the flue gas stream produced by the boiler to heat
the combustion air to the boiler to increase the thermal efficiency of the
boiler. In some embodiments, the flue gas treatment comprises multiple
electrostatic precipitators. Cold-side electrostatic precipitators are located
downstream the air preheater, thereby operate at lower temperatures
generally less than 200 C (392 F). Hot side electrostatic precipitators are
located upstream the air preheater and operate at higher temperatures,
generally more than 250 C (482 F).
Sometimes for existing plants, the electrostatic precipitator
units already operate at the boundary of their design capability due to more
stringent particulate matter emission limits that have been introduced over
the years and/or changes to plant operating conditions such as fuel switching.
The equation of Deutsch-Anderson describes with some
approximations the collection efficiency of an electrostatic precipitator as:
-ri = 1 ¨ exp (¨ l'I'Acv )
Q /
Wherein n is the fractional collection efficiency, Ac is the area of
the collection electrode, Vpm is the particle migration velocity and Q is the
volumetric flow rate of gas. The properties of the particles that influence
collection efficiency are primarily the particle size distribution and their
resistivity. The resistivity of the particles influences the particle
migration
velocity as described previously in the Deutsch-Anderson equation.
Various attempts have been tried to reduce the resistivity of
particles. It is known for example from US patent 4,439,351 that for an
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
3
electrostatic precipitator to work efficiently, the electrical resistivity of
the fly
ash must be within 1E7 (1x107) to 2E10 (2x101 ) ohms=cm. Another document,
Mastropietro, R. A. Impact of Hydrated Lime Injection on Electrostatic
Percipitator Performance in ASTM Symposium on Lime Utilization; 2012; pp
2-10, states that the resistivity of fly ash should be within 1E8 (1x108) to
1E11
(1x1011) ohms=cm. However, the electrical resistivity of fly ash is generally
higher and chemical additives were used such as SO3, HCI, NH3, Na2CO3,
Na2SO4 and NH(CH2CH2OH) to lower the resistivity of fly ash. However, those
additives are susceptible to release of undesired compounds. The same
document discloses the use of polymers for lowering the resistivity of fly
ash.
However, polymer additives generally degrade at high temperatures and must
be injected to the flue gas stream at low temperatures.
Document US patent 6,126,910 discloses the removal of acid
gas from a flue gas with an electrostatic precipitator by spraying a solution
of
sodium bisulfite, calcium bisulfite, magnesium bisulfite potassium bisulfite
or
ammonium bisulfite or a combination thereof into a stream of gas upstream
to the electrostatic precipitator unit. Such bisulfite salts selectively
remove
the acidic gases such as HCI, HF and SO3 but they don't remove sulfur dioxide.
Sulfur dioxide in the flue gas has to be removed afterwards with a reagent
such as hydrated lime. Document US patent 6,803,025 discloses a similar
process using a reaction compound selected from the group consisting of
sodium carbonate, sodium bicarbonate, sodium hydroxide, ammonium
hydroxide, potassium hydroxide, potassium hydroxide, potassium carbonate
and potassium bicarbonate to remove acidic gases such as HCI, HF, SO3 and
partially SO2 from the flue gas. However, remaining SO2 still has to be
removed by using another reagent such as hydrated lime. For the treatment
of flue gas released by power plants, the amounts of chloride released by
burning fuel or coal are generally very low relative to SO2, therefore the
flue
gas treatment process can be simplified by using only hydrated lime as a
sorbent.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
4
The document W02015/119880 relates to the drawbacks of
trona or hydrated lime as sorbents for flue gas treatment process with
electrostatic precipitator units. Sodium based sorbents are known to decrease
the resistivity of particulate matter; however, a main drawback of the use of
sodium sorbents is the leaching of heavy metals from the fly ash is enhanced
leading to potential environmental contamination. Calcium hydroxide based
sorbents do not present the problem of heavy metal leaching from fly ash, but
they are known to increase resistivity of the particulate matter (fly ash)
entrained in the flue gas stream so that the efficiency of the electrostatic
precipitator unit may be lowered when calcium based sorbents are used. The
same document discloses a composition for reducing particulate resistivity in
a flue gas and for capturing acid gases, wherein the composition comprises an
alkali metal/alkali earth particulate having a formula (Lii_a_p Nac,
1<p)w(Mg1_6
Ca6)x(OH)y(CO3),=nH20, more specifically a formula NawCax(OH)y(CO3)z.r1H20
wherein a ratio of W to x is about 1/3 to about 3/1. Therefore the
composition still presents a high amount of sodium which would be likely to
not only leach itself, but sodium is also know to increase the leaching of
heavy
metals contained in the fly ash.
US 6,797,035 discloses a process for reducing the resistivity of
fly ash by spraying an aqueous solution of potassium nitrate or potassium
nitrite on the stream of flue gas or by injecting powder of potassium nitrate
or
potassium nitrite into the duct through which the flue gas flows. A drawback
of using those powders of nitrate or nitrite salts is that they react with
other
species than fly ash and results in less reactive chemical reaching the
collection plates of the electrostatic precipitator. Therefore, it is
suggested to
inject those nitrate salts as finely divided powders to reduce the exposed
reactive surface area and inhibit reactions with nitrous oxides and sulfur
oxides.
US 7,744,678 B2 discloses a method where addition of an alkali
metal species, comprising sodium, between 0.2 and 3.5 wt%, to calcium
hydroxide sorbents provides an improved reactivity towards SO2 capture.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
Addition of the alkaline metal species is carried out in such a way that the
BET
specific surface area (SSA) by nitrogen adsorption remains high at 30 < SSA <
40 (m2/g).
The combination of sodium salts and hydrated lime beyond
5 concentrations mentioned in US 7,744,678 B2 is undesired because of two
adverse effects: (1) increase of the sodium content will lead to increased
leaching of heavy metals from the fly ash residue, (2) addition of sodium in
aqueous form to hydrated lime reduces the BET specific surface area of the
hydrated lime thus reducing the reactivity towards acidic gases.
In the paper #49 presented at the power plant pollutant control
and carbon management "MEGA" symposium, August 16-19, 2016, Baltimore,
MD, Foo et al. present a successful industrial application of SO2 removal with
an enhanced hydrated lime sorbent used in a cold side electrostatic
precipitator. Laboratory resistivity measurements of fly ash mixtures with
hydrated lime and enhanced hydrated lime have been performed with CaSO4,
wherein CaSO4 was added to simulate typical fly ash residues. Enhanced
hydrated lime of this paper has a surface area greater than 40 m2/g, a pore
volume greater than 0.2 cm3/g and a median particle size d50 comprised
between 6 and 12 micrometers and has been found to present acceptable
maximum resistivity of 1E11 (1x1011) Ohms=cm.
However, there is still a need to provide calcium-magnesium
compound which can be advantageously used in flue gas treatment
installations highly compatible with electrostatic precipitators.
The object of the present invention is to provide calcium-
magnesium compound and sorbent composition comprising said calcium-
magnesium compound removing the intrinsic drawback of these sorbents in
their application in electrostatic precipitator units.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
6
Summary of the invention
According to a first aspect, the present invention is related to
powdery calcium-magnesium compound comprising at least a calcium-
magnesium hydroxide content greater or equal to 80 weight %, with respect
to the total weight of the powdery calcium-magnesium compound, further
presenting a resistivity at 300 C (372 F) R300 lower than 1E11 (1x1011)
Ohms=cm and higher than 1E7 (1x107) Ohms=cm, advantageously lower than
1E10 (1x1019) Ohms=cm and higher than 5E7 (5x107) Ohms=cm, preferably
lower than 5E9 (5x109) Ohms=cm, more preferably lower than 1E9 (1x109)
Ohms=cm, even more preferably lower than 5E8 (5x108) Ohms=cm and the
calcium-magnesium compound is doped with calcium nitrate an amount
greater than or equal to 0.05 weight % and lower or equal to 5 weight % with
respect to the total weight of the powdery calcium-magnesium compound.
It was surprisingly observed that a powdery calcium-
magnesium compound can be successfully used in flue gas treatment using
electrostatic precipitators when the resistivity at 300 C (372 F) is still
lower
than 1E11 (1x1011) Ohms=cm, preferably lower than 1E10 (1x1019) Ohms=cm,
meaning that the powdery calcium-magnesium compound is robust and does
not decompose at relatively high temperature. Accordingly, this powdery
calcium-magnesium compound is able to positively modify the resistivity of air
pollution control residue without impacting negatively the operation of the
electrostatic precipitator.
If the powdery calcium-magnesium compound is a calcium-
magnesium compound comprising at least a calcium-magnesium hydroxide
content greater than or equal to 80 weight %, preferably greater than or equal
to 82 weight %, more preferably greater than or equal to 85 weight %,
advantageously greater or equal to 88 weight %, with respect to the total
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
7
weight of the powdery calcium-magnesium compound, it will be preferably
injected at a location near upstream of the preheater as in that location of
the
flue gas flow inside which the calcium-magnesium compound is to be injected,
the temperature is favorable for capture of pollutant compounds in the flue
gas by the high hydroxide content. In this case, as the product does not
decompose at typical temperatures upstream or near upstream of the air
preheater, the resistivity of the calcium-magnesium compound after exposure
at such typical temperatures, for example 370 C (700 F) is still low enough at
typical temperatures of cold side ESP installations or hot side ESP
installations
to modify the resistivity of the mixture of the fly ashes present in the flue
gas
and the calcium-magnesium compound injected.
By the terms calcium-magnesium compound with a calcium-
magnesium hydroxide content greater than or equal to 80 weight %,
preferably greater than or equal to 82 weight %, more preferably greater than
or equal to 85 weight %, advantageously greater or equal to 88 weight %, with
respect to the total weight of the powdery calcium-magnesium compound, it
is meant within the meaning of the present invention that at least one
calcium-magnesium compound according to the present invention is
therefore at least formed with (calcitic) slaked lime, slaked dolomitic lime
(or
dolime), magnesium slaked lime.
The molar proportion of calcium to magnesium in dolomitic
lime (also called dolime) can vary from 0.8 to 1.2. In the calcium-magnesium
compound, the proportion of calcium to magnesium can be also higher or
lower up to 0.01 to 10 or even 100. Indeed, natural limestone which is
calcined to form quicklime, which latter being further slaked to provide
hydrated lime comprises magnesium carbonate at a level which can vary from
1 to 10 weight % with respect to the total weight of the powdery calcium-
magnesium compound. If the compound in question is a magnesium
carbonate which is calcined to form magnesium oxide, which latter being
further slaked to provide magnesium hydroxide, its content in calcium
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
8
carbonate can also vary from 1 to 10 weight %. It should be noted that a part
of the magnesium oxide might remain unslaked.
The calcium-magnesium compound can also contain impurities.
The impurities notably comprise all those which are encountered in natural
limestones and dolomites, such as clays of the silico-aluminate type, silica,
impurities based on common transition metal such as iron or manganese. The
CaCO3, MgCO3, Ca(OH)2 and Mg(OH)2 contents in calcium-magnesium
compounds may easily be determined with conventional methods. For
example, they may be determined by X-ray fluorescence analysis, the
procedure of which is described in the EN 15309 standard, coupled with a
measurement of the loss on ignition and a measurement of the CO2 volume
according to the EN 459-2:2010 E standard.
Preferably, the calcium-magnesium compound according to the
present invention presents a maximum resistivity Rmax lower than 5E11
(5x1011) Ohms=cm, preferably lower than 1E11 (1x1011) Ohms=cm and more
preferably lower than 5E10 (5x101 )Ohms=cm.
In a preferred embodiment of the calcium-magnesium
compound according to the present invention, the total weight of said calcium
nitrate is greater than or equal to 0.1 weight % and lower than or equal to 5
weight %, preferably between 0.3 and 3 weight %, with respect to the total
weight of the powdery calcium-magnesium compound.
In yet another preferred embodiment, the calcium-magnesium
compound of the invention further comprises a sodium based compound in
an amount up to 3.5 weight % with respect to the total weight of the powdery
calcium-magnesium compound and expressed as sodium equivalent.
Preferably, sodium is in a minimum amount of 0.2 wt.% with respect to the
total weight of the powdery calcium-magnesium compound and expressed as
sodium equivalent.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
9
Sodium under the form of a sodium based additive in such
amounts is known to have a slight effect on decreasing the resistivity of the
sorbent, as presented by Foo et al. (2016) document previously mentioned.
The applicant found that sodium based additive in such amounts in
combination with the presence as described hereunder of calcium nitrate
further provides an additional effect on the decreasing of the resistivity of
the
sorbent composition. The use of sodium additive in combination with the
presence as described hereunder of calcium nitrate decreases the resistivity
of sorbent composition more than when presence as described hereunder of
calcium nitrate is used alone in the calcium-magnesium compound and more
than when sodium additive is used alone in the calcium-magnesium
compound.
In a preferred embodiment, the powdery calcium-magnesium
comprises particles having a d50 comprised between 5 and 25 um, preferably
between 5 and 20um, more preferably between 5 and 16 um.
The notation dx represents a diameter expressed in um, as
measured by laser granulometry in methanol optionally after sonication,
relatively to which X % by mass of the measured particles are smaller or
equal.
Preferably, in particular if the powdery calcium-magnesium
compound is a calcium-magnesium compound comprising at least a calcium-
magnesium hydroxide content greater than or equal to 80 weight %, the
calcium-magnesium compound according to the invention has a BET specific
surface area of at least 20 m2/g, preferably of at least 25 m2/g, preferably
of
at least 30 m2/g, more preferably of at least 35 m2/g. The BET surface area is
determined by manometry with adsorption of nitrogen after degassing in
vacuum at 190 C (374 F) for at least 2 hours and calculated according to the
multipoint BET method as described in the ISO 9277/2010E standard.
Preferably, in particular if the powdery calcium-magnesium
compound is a calcium-magnesium compound comprising at least a calcium-
magnesium hydroxide content greater than or equal to 80 weight %, the
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
sorbent composition according to the invention has a BJH pore volume of at
least 0.1 cm3/g, preferably of at least 0.15 cm3/g, preferably of at least
0.17
cm3/g, more preferably of at least 0.2 cm3/g. The BJH pore volume is
determined by manometry with desorption of nitrogen after degassing in
5 vacuum at 190 C (374 F) for at least 2 hours and calculated according to
the
BJH method as described in the ISO 9277/2010E standard.
Other embodiments of the calcium-magnesium compound
according to the present invention are mentioned in the appended claims
According to a second aspect, the present invention also
10 relates to a sorbent composition for flue gas treatment installation
including
an electrostatic precipitator comprising said calcium-magnesium compound
according to the present invention.
Preferably, the sorbent composition according to the invention
further comprises activated charcoal, lignite coke, halloysite, sepiolite,
clays
such as bentonite, kaolin, vermiculite or any other sorbent such as fire clay,
aerated cement dust, perlite, expanded clay, lime sandstone dust, trass dust,
Yali rock dust, trass lime, fuller's earth, cement, calcium aluminate, sodium
aluminate, calcium sulphide, organic sulphide, calcium sulfate, open-hearth
coke, lignite dust, fly ash, or water glass.
In a preferred embodiment, the sorbent composition according
to the present invention comprises sodium based additive in an amount up to
3.5 weight % with respect to the total weight of the powdery calcium-
magnesium compound and expressed as sodium equivalent. In particular, the
amount of sodium in the composition would be higher than 0.2 weight % with
respect to the total weight of the powdery sorbent composition.
In a preferred embodiment, the sorbent composition according
to the present invention comprises said calcium nitrate at an amount greater
than or equal to 0.05 weight % and lower or equal to 5 weight % with respect
to the total weight of the powdery calcium-magnesium compound and
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
11
wherein preferably the total weight of said calcium nitrate is greater than or
equal to 0.1 weight % and lower than or equal to 5 weight %, preferably
between 0.3 and 3 weight %, with respect to the total weight of the dry
sorbent composition.
In a preferred embodiment of the sorbent composition
according to the invention, the said calcium-magnesium compound is
hydrated lime.
Other embodiments of the sorbent composition according to
the present invention are mentioned in the appended claims
According to a third aspect, the present invention is related to
a process for manufacturing a sorbent composition for a flue gas treatment
installation including an electrostatic precipitator, the process comprising
the
steps of :
a) providing a calcium-
magnesium compound to a
reactor;
b) adding
calcium nitrate or nitric acid or a
combination thereof in an amount calculated to obtain between 0.1 weight %
and 5 weight %, preferably between 0.3 weight % to 3 weight % of calcium
nitrate in weight of dry sorbent composition.
In a preferred embodiment, the sorbent composition comprises
particles having a d50 comprised between 5 and 25 um, preferably between 5
and 20um, more preferably between 5 and 16 um.
In another preferred embodiment of the process according to
the present invention, said calcium-magnesium compound comprises a
calcium-magnesium hydroxide content greater or equal to 80 weight %, with
respect to the total weight of the dry calcium-magnesium compound.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
12
Preferably the process of manufacturing said sorbent
composition comprises a step of adding a sodium based additive expressed as
sodium equivalent in an amount calculated to obtain up to 3.5% of sodium
equivalent in weight of the dry sorbent composition.
In an embodiment of the process of manufacturing according
to the invention, the step of providing a calcium-magnesium compound to a
reactor comprises the step of providing a quicklime to said reactor, slaking
said quicklime with a predetermined amount of water to obtain said calcium-
magnesium compound comprising at least a calcium hydroxide content
greater or equal to 80 weight %, with respect to the total weight of the dry
calcium-magnesium compound with an predetermined amount of moisture.
More advantageously, said step of slaking is performed in
conditions such as to obtain hydrated lime with a BET specific surface area by
nitrogen adsorption of at least 20m2/g, preferably of at least 25 m2/g,
preferably of at least 30 m2/g, more preferably of at least 35 m2/g.
In further preferred embodiment, said step of slaking is
performed in conditions such as to obtain hydrated lime with a BJH pore
volume for pores having a diameter lower or equal to 1000 A by nitrogen
desorption of at least 0.1 cm3/g, 0.15 cm3/g, preferably of at least 0.17
cm3/g,
more preferably of at least 0.2 cm3/g.
Preferably, said step of slaking is performed in the same
conditions as the ones described in US patent 6,322,769 of the applicant and
incorporated by reference.
In an alternative embodiment of the process of manufacturing
according to the invention, the said step of slaking is performed in the same
conditions as the ones described in the US patent 7,744,678 of the applicant
and incorporated by reference.
In an embodiment of the process of manufacturing said sorbent
according to the invention, the step of adding an additive or a mixture of
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
13
additives, comprising at least calcium nitrate or nitric acid or a combination
thereof is performed before said step of slaking quicklime.
In another embodiment of the process of manufacturing said
sorbent composition, the said step of adding an additive or a mixture of
additives, comprising at least calcium nitrate or nitric acid or a combination
thereof is performed during said step of slaking quicklime.
Alternatively, in the process of manufacturing said sorbent
composition, the said step of adding an additive or a mixture of additives,
comprising at least calcium nitrate or nitric acid or a combination thereof is
performed after the said step of slaking quicklime.
It has been found by the applicant that the step of adding an
additive or a mixture of additives, comprising at least calcium nitrate or
nitric
acid or a combination thereof performed before, during or after the said step
of slaking, in the amounts mentioned hereinabove, does not substantially
change the pore volume of the calcium-magnesium compound. Also, the
specific surface area in any case remains above 20m2/g. In particular, the
specific surface area of the sorbent composition according to the present
invention is substantially the same as for calcium hydroxide sorbent prepared
by the known methods such as the one described in US patents 6,322,769 and
7,744,678 incorporated by reference, provided that addition of calcium
nitrate or nitric acid or a combination thereof is performed after the step of
slaking and preferably before the step of drying. Therefore, the properties of
the sorbent ensuring the efficiency of SO2 removal are preserved.
Preferably, the said process of manufacturing is characterized
in that it further comprises a step of adding activated charcoal, lignite
coke,
halloysite, sepiolite, clays, bentonite, kaolin, vermiculite, fire clay,
aerated
cement dust, perlite, expanded clay, lime sandstone dust, trass dust, Yali
rock
dust, trass lime, fuller's earth, cement, calcium aluminate, sodium aluminate,
calcium sulphide, organic sulphide, calcium sulfate, open-hearth coke, lignite
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
14
dust, fly ash, or water glass, preferably performed after the said step of
slaking.
Other embodiments of the process for manufacturing a sorbent
composition according to the present invention are mentioned in the
appended claims
In a fourth aspect, the present invention is related to a flue gas
treatment process using an installation comprising an injection zone arranged
upstream of an electrostatic precipitator, characterized in that it comprises
a
step of injecting in said injection zone a sorbent composition as disclosed
herein according to the present invention.
More particularly, the flue gas treatment process using an
installation including an electrostatic precipitator, and an injection zone
arranged upstream of said electrostatic precipitator and through which flue
gas is flowing towards said electrostatic precipitator is characterized in
that
the said process comprises a step of injection of a sorbent composition in
said
injection zone, said sorbent composition comprising a calcium-magnesium
sorbent, calcium nitrate, the total amount of said calcium nitrate being
comprised between 0.1 % and 5 %, preferably 0.3 to 3.5% in weight of the dry
composition.
According to the present invention, the said sorbent
composition has a lower resistivity compared to calcium-magnesium sorbents
of prior art, for example at 200 C or lower after exposure to a temperature of
300 C (572 F). Injection of the sorbent composition according to the invention
in an injection zone to mix with flue gas is effective for the removal of SO2
and
other gaseous acids and the lower resistivity of such sorbent composition
improves the collection of particulate matter on the collecting electrodes of
the electrostatic precipitator.
In another preferred embodiment of the process according to
the present invention, the sorbent composition comprises a calcium-
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
magnesium compound at least a calcium-magnesium hydroxide, and said
sorbent composition is injected in said injection zone wherein said flue gas
has a temperature greater than or equal to 180 C (356 F), preferably greater
than 200 C (392 F), more preferably comprised between 300 C (572 F) and
5 425 C (797 F).
The said sorbent composition can be used in the flue gas
treatment process according to the present invention under a broad range of
temperatures, for example between 100 C (212 F) and 425 C (797 F).
Advantageously, the said additives of the sorbent composition according to
10 the present invention do not encounter degradation at temperatures
higher
than 180 C (356 F) so that said sorbent composition can be injected in the
said injection zone wherein the temperature is greater than or equal to 180 C
(356 F), preferably greater than or equal to 300 C (572 F). As the injection
zone is located upstream of the air preheater, temperatures at the injection
15 zone can range between 300 C (372 F) to 425 C (797 F), preferably 350 C
(662 F) to 380 C (716 F).
Preferably, in the flue gas treatment process according to the
invention, the said injection zone is located upstream of an air preheater
itself
located upstream of said electrostatic precipitator.
Preferably, in the flue gas treatment process of the invention,
the said sorbent composition comprises another sodium based additive in an
amount up to 3.5% in weight of the dry composition and expressed as sodium
equivalent.
Preferably, in the flue gas treatment process of the invention,
the said sorbent composition has a BET specific surface area of at least 20
m 2/g.
Preferably, in the flue gas treatment process of the invention,
the said sorbent composition has a BJH pore volume obtained from nitrogen
desorption of at least 0.1 cm3/g.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
16
Preferably, in the flue gas treatment process of the invention,
the said sorbent composition has a BJH pore volume obtained from nitrogen
desorption of at least 0.15 cm3/g, preferably of at least 0.17 cm3/g, more
preferably of at least 0.2 cm3/g.
Preferably, in the flue gas treatment process of the invention,
the said sorbent composition further comprises activated charcoal, lignite
coke, halloysite, sepiolite, clays, bentonite, kaolin, vermiculite, fire clay,
aerated cement dust, perlite, expanded clay, lime sandstone dust, trass dust,
Yali rock dust, trass lime, fuller's earth, cement, calcium aluminate, sodium
aluminate, calcium sulphide, organic sulphide, calcium sulfate, open-hearth
coke, lignite dust, fly ash, or water glass.
In an embodiment of the flue gas treatment process of the
invention, the said sorbent composition is injected as a dry powder in a dry
injection system or as an atomized slurry in a spray dryer absorber.
Other embodiments of the flue gas treatment process
according to the present invention are mentioned in the appended claims.
In a fifth aspect, the present invention is related to a flue gas
treatment device comprising an electrostatic precipitator downstream of an
air preheater, said air preheater being connected to said electrostatic
precipitator by a duct, characterized in that it further comprises an
injection
zone for injecting a sorbent composition according to the present invention
arranged upstream of said air preheater.
Other embodiments of the flue gas treatment device according to the present
invention are mentioned in the appended claims.
Preferably the said flue gas treatment device or installation is
used for treating flue gas of a plant, in particular a power plant, using coal
or
fuel containing sulfur species or other acid gas precursors.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
17
Preferably the said flue gas treatment installation further
comprises a reservoir comprising said sorbent composition to provide said
sorbent composition to the said injection zone through a sorbent inlet.
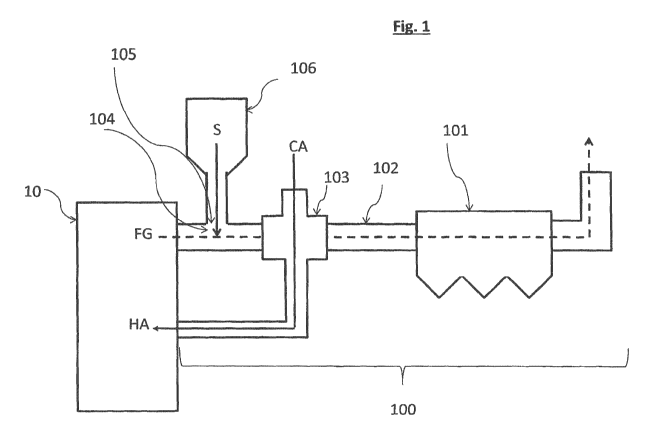
Brief description of the drawings
Figure 1 presents a schematic embodiment of a
flue gas treatment installation carrying out the flue gas treatment process
with the sorbent composition according to the present invention.
Detailed description of the invention
According to a first aspect, the present invention is related to a
sorbent composition for flue gas treatment installation including an
electrostatic precipitator, said sorbent composition comprising calcium-
magnesium compound, characterized in that it further comprises an additive
or a mixture of additives in an amount comprised between 0.1% and 5%,
preferably 0.3% to 3% in weight of the dry composition, said additive or
additives containing at least calcium nitrate.
In a preferred embodiment, the calcium-magnesium compound
is based on hydrated lime.
Calcium hydroxide sorbents are manufactured by reacting (or
slaking) calcium oxide, CaO or quick lime, with water in a so called hydrator,
also called slaking unit. Alternatively, calcium magnesium hydroxide sorbents
are manufactured by reacting dolomitic lime (also called dolime) or
magnesium lime with water in a hydrator. Alternatively, quick lime and
dolomitic lime can be mixed together and slaked with water in a hydrator to
provide a mixture of calcium hydroxide and calcium magnesium hydroxide. In
the following, the process of manufacturing of the sorbent composition will
refer to quick lime but the process of manufacturing is not limited to quick
lime as a starting material and dolomitic lime or a combination of dolomitic
lime and/or magnesium lime and quick lime can also be used as starting
materials.
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
18
The process of manufacturing of the said sorbent composition
according to the invention comprises a step of slaking quicklime with a
predetermined amount of water to obtain hydrated lime with an
predetermined amount of moisture, and is characterized in that it comprises a
step of adding an additive or a mixture of additives to dope the sorbent
composition in an amount calculated to obtain between 0.1% and 5 %,
preferably between 0.3 and 3.5% of said additive or mixture of additives in
weight of the dry sorbent composition, said additive or additives containing
at
least calcium nitrate or nitric acid or a combination thereof.
In an embodiment of the process of manufacturing the said
sorbent composition, the predetermined amount of water in the said step of
slaking is in a water to lime ratio 2:1 by weight or higher.
In an embodiment of the process of manufacturing the said
sorbent composition, the amount of water in the slaking step can be adapted
to obtain a hydrated lime with a moisture less than or equal to 10 wt.%,
preferably less than or equal to 5 wt.%, preferably less than or equal to 2
w%,
more preferably less than or equal to 1 w% with respect to the total weight of
the sorbent composition at a powdery state.
In another embodiment, the amount of water in the slaking
step can be adapted to obtain a hydrated lime with a moisture content
comprised between 5 wt.% and 20 wt.%. The amount of water in the slaking
step can also be higher such as to obtain a hydrated lime with a moisture
content above 20 wt.%, all % being expressed with respect to the total weight
of the sorbent composition at a powdery state.
In an embodiment, the hydrated lime obtained after the slaking
step is dried in a further step.
In an embodiment of the process of manufacturing of the
sorbent composition according to the invention, the said additive containing
calcium nitrate is used to dope the sorbent composition by adding the
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
19
additive containing calcium nitrate as an aqueous solution or as a suspension
or as a powder before or during the said step of slaking of calcium oxide or
calcium magnesium oxide or a combination thereof.
In another embodiment of the process of manufacturing of the
sorbent composition according to the invention, calcium nitrate is added as
aqueous solution or as a suspension or as a powder after the said step of
slaking. Preferably, a step of drying is performed after the step of slaking
and
after the step of adding calcium nitrate. Calcium nitrate is preferably added
to
calcium hydroxide or calcium magnesium hydroxide before injection in an
injection zone of the flue gas treatment installation.
In a preferred embodiment of the process of manufacturing of
the sorbent composition, the said step of slaking quicklime is performed in
the
conditions such as to obtain hydrated lime with a BET specific surface area
from nitrogen adsorption of at least 20m2/g and a BJH pore volume obtained
from nitrogen desorption of at least 0.1 cm3/g. Various processes are
available
to the man skilled in the art to obtain an hydrated lime with such properties,
and are disclosed for example in documents US patent 6,322,769 and US
patent 7,744,678 of the applicant and incorporated by reference.
In the process of manufacturing the sorbent composition
according to the invention, particles of quicklime are advantageously used
having a particle size distribution of less than 5 mm, in particular quicklime
particles of particle size distribution 0-2 mm.
Other processes for obtaining hydrated lime with high specific
area and/or high pore volume can be found for example in US patent
5,492,685 wherein an amount of alcohol such methanol or ethanol is added
prior and/or the step of slaking quicklime and is removed after drying, in
patent DE3620024 wherein sugar is added in the step of slaking for increasing
the specific surface area and wherein glycols or amines are added to increase
the flowability, in US patent 5,277,837 and US patent 5,705,141 wherein
additives such as ethylene glycol, diethylene glycol, tri ethylene glycol,
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
monoethanolamine, diethanolamine, triethanolamine or a combination
thereof is added in the step of slaking for increasing the surface area of
hydrated lime.
In the process of manufacturing the sorbent composition,
5 calcium nitrate can be added in certain amounts according to the
invention as
disclosed herein before the said step of slaking, during the step of slaking
or
after the step of slaking without substantially changing the BJH pore volume
for pores having a diameter lower than or equal to 1000 A of the sorbent
composition. Moreover the BJH pore volume of the sorbent composition
10 according to the present invention is substantially the same as for
calcium
hydroxide sorbent prepared by the known methods such as the one described
in US patents 6,322,769 and 7,744,678 incorporated by reference. Also, the
BET specific surface area of the sorbent composition is above 20 m2/g.
Therefore, the properties of the sorbent ensuring the efficiency of SO2
15 removal are preserved. Alternatively, nitric acid or calcium nitrate
and nitric
acid can be added before, during or after the step of slaking. Preferably, a
higher BET specific surface area is obtained when calcium nitrate or nitric
acid
or a combination thereof is added after the step of slaking, and preferably
before a drying step.
20 In the
said process of manufacturing the sorbent composition
according to the invention, if a hydrated lime composition is prepared
according to the method described in US patent 7,744,678, such method
comprises a step of adding a quantity of an alkali metal, preferably sodium in
an quantity to the quicklime or to the slaking water or to the hydrated lime,
sufficient to obtain in the hydrated lime an alkali metal content that is
equal
to or greater than 0.2 % and equal or less than 3.5 % by weight based on the
total weight of the dry sorbent composition. The sodium can be added, for
example, as Na2CO3. According to this embodiment, calcium nitrate or nitric
acid or a combination thereof is further added after the step of slaking, and
preferably before a drying step with an amount such as to obtain a content in
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
21
calcium nitrate between 0.1 % and 5%, preferably 0.3% to 3% in weight of the
dry sorbent composition.
Various sorbent compositions have been prepared according to
the method of the present invention and measurements of the resistivity of
dry powders of said sorbent compositions have been carried out in following
the procedure outlined by IEEE (Estcourt, 1984). Basically, a resistivity cell
of a
determined volume is filled by a dry powder of sorbent composition and the
powder is then compacted with a weight such as to obtain a flat surface. An
electrode with a guard is placed over the surface of the powder and the
resistivity of the powder is measured in an oven under a stream of air
comprising 10% of humidity at various temperatures comprised between
150 C (302 F) and 300 C (372 F). The resistivity of comparatives examples
have been measured in the same conditions. For each measurement, a
maximum resistivity Rmax and a resistivity at 300 C (572 F) has been
determined. The resistivity measurements are presented herein after:
Example set A
Example 1 is a comparative sample of calcium hydroxide
sorbent designed for the removal of acid gas pollutants manufactured
according to US 6,322,769 B1. This sample was obtained from an industrial
installation. No sodium nor calcium nitrate nor nitric acid has been added.
Example 2 is a comparative sample of a calcium hydroxide
sorbent designed for the removal of acid gas pollutants manufactured
according to US 7,744,678 B2. This sample has a content of Ca(OH)2> 90 w%,
CaCO3 < 8 w%, and of Na2CO3 of about 0.8 w% and the rest of impurities. No
further sodium or calcium nitrate or nitric acid has been added. This sample
was obtained from an industrial installation.
Example 3 is another sample of a calcium hydroxide sorbent
designed for the removal of acid gas pollutants manufactured according to US
7,744,678 B2 and wherein the lime comes from another source. This sample
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
22
has a content of Ca(OH)2> 90 w%, of CaCO3 <7 w%, and 2.1 w% of Na2CO3 and
the rest of impurities. No further sodium or calcium nitrate nor nitric acid
has
been added. This sample was obtained from an industrial installation.
Example 4 is a calcium hydroxide sorbent manufactured
according to the present invention using same source of lime as for the
example 3 and using calcium nitrate as dopant in an amount of 1% relative to
the dry product. This sample was obtained from an industrial installation.
Example 5 is a calcium hydroxide sorbent manufactured
according to the present invention using same source of lime as for the
example 3 and using calcium nitrate as dopant in an amount of 2% relative to
the dry product. This sample was obtained from an industrial installation.
Example 6 is a calcium hydroxide sorbent manufactured
according to the present invention, at laboratory scale by mixing (slaking),
in a
mixer with paddles, quicklime with stoichiometric amount of water and a
quantity of NaCO3 such as to obtain a sodium content of 2% by weight based
on the total weight of the dried powdered composition obtained. The
quicklime was obtained by calcination of lime from the same source of lime as
for the example 3. After reaction in the mixer, the hydrated lime (calcium
hydroxide) was discharged, dried and submitted to post treatment with 1% of
HNO3 by weight of the dry product.
Table 1 shows the measured resistivity parameters Rmax and
R300 for those examples. All the measurements of resistivity parameters have
been performed by measuring the resistivity of samples under increasing
temperatures.
Table 1 : Resistivity parameters of calcium hydroxide sorbents
of examples 1 to 6.
Example Rmax (O=cm) R300 (O=cm)
CA 03103171 2020-12-09
WO 2020/011953 PCT/EP2019/068758
23
Ex. 1 8E12 3E12
Ex. 2 4E11 1E11
Ex. 3 9 E 10 4E09
Ex. 4 9E09 1E08
Ex. 5 6 E 09 4E07
Ex. 6 4E10 1E08
From Table 1, it is clear that the both the Rmax value and the
R300 value of Example 1 are high at and above the preferred range of
resistivity
values comprised between 10E7 ohms.cm and 2E10 ohms.cm.
The presence of 0.8 wt.% of Na2CO3 in the sorbent composition
of the Example 2 reduces the Rmax and R300 values by more than one order of
magnitude respect to the Rmax and R300 values of the composition of example
1. The presence of 2.1 w% of Na2CO3 in the sorbent composition of example 3
reduces the Rmax and R300 values by more than two orders of magnitude
respect to the Rmax and R300 values of the composition of example 1.
Surprisingly the presence of a small amount of calcium nitrate in an amount of
1 wt% in the composition of example 4 reduces the Rmax value by nearly three
order of magnitude and the R300 value by nearly four orders of magnitude
respect to the Rmax and R300 values of the composition of example 1. The
presence of 2 w% of calcium nitrate in the composition of example 5
decreases even more the values of Rmax and R300 relative to the composition of
example 1. Therefore, surprisingly the addition of calcium nitrate or nitric
acid
is more effective for lowering the resistivity than the addition of sodium.
Despite some differences due to the different process conditions (industrial
scale and laboratory scale), the presence of calcium nitrate in the
composition
of example 6 by addition of HNO3 instead of by addition of calcium nitrate has
the same tendency of the lowering the resistivity of the sorbent as the
addition of Ca(NO3)2.
Examples set B
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
24
Example 7 is a sample of fly ash obtained from a coal power station.
Example 8 is a blend of 80 w% of fly ash of example 7 with 20 w% of a sorbent
according to example 3.
Example 9 is a blend of 80 w% of fly ash of example 7 with 20 w% of a sorbent
according to example 4.
Example 10 is a blend of 80 w% of fly ash of example 7 with 20 w% of a
sorbent according to example 5.
Table 2 shows the measurement of resistivity parameters of Rmax and R300
for those examples 7 to 10. One set of measurements of Rmax and R300 has
been performed by measuring the resistivity of the samples under increasing
temperatures and one set of measurements of Rmax has been performed by
measuring the resistivity of the samples under decreasing temperatures.
Table 2.
Rmax under R300 under Rmax under
increasing increasing decreasing
temperature temperature temperature
(Q=cm) (Q=cm) (Q=cm)
Example 7 3E10 5E09 3E10
Example 8 2E12 3E10 1E12
Example 9 1E11 1E09 2E10
Example 10 4E10 7E07 2E09
The results presented in table 2 shows that for the same
proportions of fly ash and calcium based sorbent, the blend of fly ash with a
calcium based sorbent without calcium nitrate additive presents higher
resistivity parameters Rmax and R300 than fly ash without calcium based
sorbent, whereas the presence of only 1 w%, preferably 2 w% of CaNO3
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
additive in the calcium based sorbent has an positive influence on the
resistivity parameters Rmax and R300 of the blend.
It is to be mentioned that the examples of sorbent
5 compositions presented herein above are not !imitative for the
present
invention, and other additives in the amounts comprised between 0.1 and 5 %
in weight of the dry sorbent composition can be used to decrease the
resistivity of sorbent compositions destined to be used in flue gas treatment
processes using an electrostatic precipitator.
10 It is to
be mentioned that improvements of particulate matter
collection on collecting electrodes of an electrostatic precipitators can be
observed with the use of the sorbent according to the present invention.
According to another aspect, the present invention is related to
a flue gas treatment installation. Figure 1 shows a schematic embodiment of a
15 flue gas treatment installation 100 comprising an electrostatic
precipitator
101 arranged downstream a first duct portion 102 arranged downstream an
air preheater 103, characterized in that an injection zone 104 is arranged
upstream said air preheater 103 and comprises a sorbent inlet 105. The said
flue gas treatment installation 100 further comprises a reservoir 106
20 comprising said sorbent composition S to provide said sorbent
composition to
the said injection zone through the said sorbent inlet. The hot flue gas FG
produced by a boiler 10 is flown through the injection zone wherein the
sorbent S according to the invention is injected to react with SO2 and other
acidic gases from the flue gas, then the hot flue gas crosses the air
preheater
25 through which cold air CA is flown to absorb the heat of the hot
flue gas and
to be injected as hot air HA in the boiler. Then the flue gas flows through
the
electrostatic precipitator 101 wherein charged collecting electrodes collects
the particulate matter including the sorbent composition according to the
invention that has reacted with undesired acidic gases. The flue gas treatment
CA 03103171 2020-12-09
WO 2020/011953
PCT/EP2019/068758
26
installation described herein is relatively simple and is well adapted for the
use of the sorbent composition according to the present invention.
Preferably the said flue gas treatment installation is used for
treating flue gas of a power plant using coal or fuel containing sulfur
species
or other acid gas precursors.
It should be understood that the present invention is not
limited to the described embodiments and that variations can be applied
without going outside of the scope of the appended claims.
For example, in the preferred embodiment, the installation for
flue gas treatment was described with an electrostatic precipitator
downstream of an air preheater, said air preheater being connected to said
electrostatic precipitator by a duct with an injection zone for injecting a
sorbent composition according to the present invention arranged upstream of
said air preheater. An alternative within the scope of the present may
comprises a particulate collection device upstream of said preheater.
Another alternative of the flue gas treatment device according
to the present invention comprises in sequence an electrostatic precipitator,
a
preheater followed by optionally a particulate collection device, before
reaching the chimney.
The particulate collection device can be another electrostatic
precipitator or any kind of filter, such as a bag house filter.
In all of those embodiments, the sorbent composition
according to the present invention is injected in an injection zone located
upstream of said electrostatic precipitator, before or after the preheater,
depending on the on-site configuration.