Note: Descriptions are shown in the official language in which they were submitted.
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
STABILITY OF VITAMIN D IN I3-HYDROXY- I3-METHYLBUTYRATE (HMB)
Background of the Invention
This application claims priority to United States Provisional Patent
Application No.
62/689,305 filed June 25, 2018 and herein incorporates the provisional
application by reference.
1. Field
The present invention relates to a composition comprising P-hydroxy-P-
methylbutyrate
(HMB) and vitamin D. In particular, the present invention relates to
compositions containing
HMB and vitamin D and methods of stabilizing vitamin D when vitamin D is
combined with
acidic compositions.
2. Background
Vitamin D has classically been associated with calcium and phosphorous
metabolism and
bone strength. Until recently, an adequate vitamin D level has been defined
using the vitamin D
deficiency disease rickets. In recent years vitamin D has been found to have
more widespread
effects on metabolism other than on calcium and bone structure. Low vitamin D
status has been
associated with the following: osteoporosis, falls, Type I diabetes, cancer,
autoimmune disease,
hypertension, periodontal disease, multiple sclerosis, susceptibility/poor
response to infectionl.
Vitamin D deficiency is a problem worldwide, as sun exposure has decreased and
the use
of sunscreen has increased the problem has only been exacerbated. Diet and
health can also be
mitigating factors on vitamin D levels. Subjects with fat malabsorption
syndromes, chronic
diseases, on certain medications, or obesity have a higher risk for vitamin D
deficiency2. Skin
tone can also have an effect, as the darker the skin tone, the more time
required to produce the
1
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
same amount of vitamin D. Therefore supplementation is the best way to
increase vitamin D and
decrease deficiency2. Cholecalciferol (Vitamin D3) can be synthesized by
humans thru
irradiation of 7-dehydrochloesterol in the skin or extracted from lanolin.
Very few naturally occurring foods contain Vitamin D but the flesh of fatty
fish and fish
liver oils are the best sources. Small amounts of Vitamin D are found in beef
liver, dairy
products, and each yolk. Some mushrooms provide Vitamin D2 but the amounts are
variable.
Because Vitamin D is only contained in few foods and often in small amounts,
fortification of
foods or the supplementation through dietary supplements is necessary. Since
the 1930, food
and beverage manufactures started fortify foods such as, milk, bread, hot
dogs, sodas and even
beer. Vitamin D is also available in dietary supplements.
Vitamin D3 is a large and hydrophobic sterol molecule with poor water
solubility that is
easily degraded by light and oxygen. The stability of vitamin D is excellent
in the absence of
water, light, acidity, and at low temperatures3. The 5,6-trans-isomer and
isotachysterol can form
when exposed to acid and light4. Vitamin is less susceptible to oxidation than
vitamin A but
oxidation of Vitamin D can be the predominant route for decomposition at the
conjugated double
bond system at 5,6 and 7,8 position of the secosteroid structure3. Vitamin D
is quite stable in
processes used for fluid milk production or in the production of non-fat dry
milk4. No significant
loss were observed with steam injected at 95 C5. More recently, Vitamin D has
been found to be
stable in orange juice which has a pH of approximately 46. The bioavailability
was equally
available as when consumed in capsule forms. However, long-term stability of
Vitamin D3 at
pH of 4 and lower had not been determined.
HMB
2
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
Alpha-ketoisocaproate (KIC) is the first major and active metabolite of
leucine. A minor
product of KIC metabolism is P-hydroxy-P-methylbutyrate (HMB). HMB has been
found to be
useful within the context of a variety of applications. Specifically, in U.S.
Patent No. 5,360,613
(Nissen), HMB is described as useful for reducing blood levels of total
cholesterol and low-
.. density lipoprotein cholesterol. In U.S. Patent No. 5,348,979 (Nissen et
al.), HMB is described
as useful for promoting nitrogen retention in humans. U.S. Patent No.
5,028,440 (Nissen)
discusses the usefulness of HMB to increase lean tissue development in
animals. Also, in U.S.
Patent No. 4,992,470 (Nissen), HMB is described as effective in enhancing the
immune response
of mammals. U.S. Patent No. 6,031,000 (Nissen et al.) describes use of HMB and
at least one
.. amino acid to treat disease-associated wasting.
The use of HMB to suppress proteolysis originates from the observations that
leucine has
protein-sparing characteristics. The essential amino acid leucine can either
be used for protein
synthesis or transaminated to the a-ketoacid (a-ketoisocaproate, KIC). In one
pathway, KIC can
be oxidized to HMB and this accounts for approximately 5% of leucine
oxidation. HMB is
superior to leucine in enhancing muscle mass and strength. The optimal effects
of HMB can be
achieved at 3.0 grams per day when given as calcium salt of HMB, or 0.038g/kg
of body weight
per day, while those of leucine require over 30.0 grams per day.
Once produced or ingested, HMB appears to have two fates. The first fate is
simple
excretion in urine. After HMB is fed, urine concentrations increase, resulting
in an approximate
20-50% loss of HMB to urine. Another fate relates to the activation of HMB to
HMB-CoA.
Once converted to HMB-CoA, further metabolism may occur, either dehydration of
HMB-CoA
to MC-CoA, or a direct conversion of HMB-CoA to HMG-CoA, which provides
substrates for
3
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
intracellular cholesterol synthesis. Several studies have shown that HMB is
incorporated into the
cholesterol synthetic pathway and could be a source for new cell membranes
that are used for the
regeneration of damaged cell membranes. Human studies have shown that muscle
damage
following intense exercise, measured by elevated plasma CPK (creatine
phosphokinase), is
reduced with HMB supplementation within the first 48 hrs. The protective
effect of HMB lasts
up to three weeks with continued daily use. Numerous studies have shown an
effective dose of
HMB to be 3.0 grams per day as CaHMB (calcium HMB) (-38 mg =kg body weight-
1=day-1).
HMB has been tested for safety, showing no side effects in healthy young or
old adults. HMB in
combination with L-arginine and L-glutamine has also been shown to be safe
when
supplemented to AIDS and cancer patients.
HMB free acid ("HMBFA"), a delivery form of HMB, has been developed. This
delivery
form has been shown to be absorbed quicker and have greater tissue clearance
than CaHMB.
This delivery form is described in U.S. Patent Publication Serial No.
20120053240 which is
herein incorporated by reference in its entirety.
HMB has been shown to be effective for increasing muscle mass, increasing
strength, and
improving muscle function. These effects are most significant when vitamin D
levels in the
blood are sufficient, which has been further defined to be around at least 25
ng/ml or higher.
Thus, it is desirable to combine HMB with vitamin D in order to maximize the
effects of
HMB on muscle mass, strength and function. It has been found, however, that
vitamin D, and
vitamin D3 in particular, is not stable when combined with acidic
compositions, including HMB
in the free acid form (HMBFA). Vitamin D levels in compositions the solely
comprise HMBFA
and Vitamin D degrade significantly over time. As HMBFA may be a more
effective delivery
4
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
form of HMB in terms of effect on muscle, it is desirable to combine HMBFA
with Vitamin D in
order to maximize the efficacy of HMB. Thus, the need exists to develop a
stable formulation
containing HMBFA and vitamin D. To that end, it has been discovered that
Vitamin D is
stabilized against degradation in acidic formulations, including formulations
containing HMB
with the inclusion of at least one stabilization excipient.
Summary of the Invention
One object of the present invention is to provide a composition containing
HMBFA and
vitamin D that is stable.
Another object of the present invention is to provide a composition containing
HMBFA
and vitamin D, wherein the vitamin D does not decrease significantly over
time.
These and other objects of the present invention will become apparent to those
skilled in
the art upon reference to the following specification, drawings, and claims.
The present invention intends to overcome the difficulties encountered
heretofore. To
that end, a composition comprising HMBFA and vitamin D is provided.
Brief Description of the Drawings
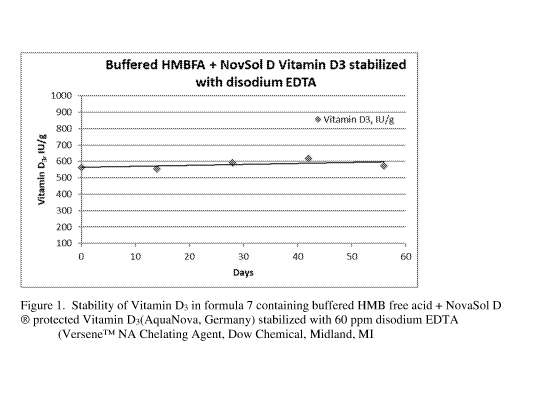
Figure 1 depicts the stability of Vitamin D in Formula 7.
Figure 2 depicts the stability of Vitamin D in Formula 10.
Figure 3 depicts the stability of Vitamin D in Formula 11.
Detailed Description of the Invention
5
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
There is disclosed herein formulations for improving the stability of Vitamin
D when
Vitamin D is combined with HMB. It has been discovered that inclusion of
additional
components that act as a stabilizing excipient in formulations of HMB and
Vitamin D improves
the stability of Vitamin D. Representative examples of the stabilizing
excipients include but are
not limited to EDTA, butylated hydroxytoluene (BHT), carnitine, rosemary
extract, carnosolic
acid, and/or sorbic acid. The inclusion of at least one of these additives in
formulations
containing HMB and Vitamin D results in minimal loss of Vitamin D in the
formulation over
time. The addition of an additive to a formulation containing Vitamin D
combined with an
acidic components (such as HMBFA), stabilizes the Vitamin D against
degradation over time.
Vitamin D is intended to include vitamins D2, D3, and D4 and related analogs.
Stabilizing excipients that are encompassed by this invention include EDTA or
antioxidants including BHT, BHA, ascorbyl palmitate, propyl gallate, vitamin
E, ascorbic acid,
cysteine, methionine, monothiogylcerol, sodium thiosulphate, or sulfites.
Vitamin D has been found to degrade significantly over time when Vitamin D is
included
in acidic formulations. As it is desirable to combine Vitamin D with HMBFA in
low pH, acidic
formulations, the need exists for stable formulations of HMB and Vitamin D. It
was
unexpectedly discovered that including a stabilizing excipient, such as EDTA,
BHT, carnitine,
rosemary extract, carnosolic acid, and/or sorbic acid results in a formulation
in which Vitamin D
is stabilized against degradation over time.
In most instances, the HMB utilized in clinical studies and marketed as an
ergogenic aid
has been in the calcium salt form. Recent advances have allowed the HMB to be
manufactured in
a free acid form for use human and animal uses, and preferably as a
nutritional supplement or
6
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
incorporated into foodstuffs. HMB in the free acid form (HMBFA) has been
developed, which
was shown to be more rapidly absorbed than CaHMB, resulting in quicker and
higher peak
serum HMB levels and improved serum clearance to the tissues. The pH of HMBFA
is <3.
HMB free acid may therefore be a more efficacious method of administering HMB
than
the calcium salt form, particularly when administered directly preceding
intense exercise. One
of ordinary skill in the art, however, will recognize that this current
invention encompasses HMB
in any form.
HMB in any form may be incorporated into the delivery and/or administration
form in a
fashion so as to result in a typical dosage range of about 0.5 grams HMB to
about 30 grams
HMB.
Any suitable dose of HMB can be used within the context of the present
invention.
Methods of calculating proper doses are well known in the art.
When the composition is administered orally in an edible form, the composition
is
preferably in the form of a dietary supplement, foodstuff or pharmaceutical
medium, more
preferably in the form of a dietary supplement or foodstuff. Any suitable
dietary supplement or
foodstuff comprising the composition can be utilized within the context of the
present invention.
One of ordinary skill in the art will understand that the composition,
regardless of the form (such
as a dietary supplement, foodstuff or a pharmaceutical medium), may include
amino acids,
vitamins, proteins, peptides, carbohydrates, fats, sugars, minerals and/or
trace elements.
In order to prepare the composition as a dietary supplement or foodstuff, the
composition
will normally be combined or mixed in such a way that the composition is
substantially
7
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
uniformly distributed in the dietary supplement or foodstuff. Alternatively,
the composition can
be dissolved in a liquid, such as water.
The composition of the dietary supplement may be a powder, a gel, a liquid or
may be tabulated or encapsulated, for instance in a softgel formulation. The
following examples
.. examined the stability of Vitamin D when combined with HMB in a softgel or
capsule delivery
form. The invention is not limited to any particular delivery form, and can
include combinations
of HMB and Vitamin D in liquid formulations, gels and foodstuffs.
Although any suitable pharmaceutical medium comprising the composition can be
utilized within the context of the present invention, preferably, the
composition is combined with
a suitable pharmaceutical carrier, such as dextrose or sucrose.
Furthermore, the composition of the pharmaceutical medium can be intravenously
administered in any suitable manner. For administration via intravenous
infusion, the
composition is preferably in a water-soluble non-toxic form. Intravenous
administration is
particularly suitable for hospitalized patients that are undergoing
intravenous (IV) therapy. For
example, the composition can be dissolved in an IV solution (e.g., a saline or
glucose solution)
being administered to the patient. Also, the composition can be added to
nutritional IV solutions,
which may include amino acids, glucose, peptides, proteins and/or lipids. The
amounts of the
composition to be administered intravenously can be similar to levels used in
oral administration.
Intravenous infusion may be more controlled and accurate than oral
administration.
Methods of calculating the frequency by which the composition is administered
are well-
known in the art and any suitable frequency of administration can be used
within the context of
8
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
the present invention (e.g., one 6 g dose per day or two 3 g doses per day)
and over any suitable
time period (e.g., a single dose can be administered over a five minute time
period or over a one
hour time period, or, alternatively, multiple doses can be administered over
an extended time
period). The composition can be administered over an extended period of time,
such as weeks,
months or years.
Any suitable dose of HMB and vitamin D can be used within the context of the
present
invention. Methods of calculating proper doses are well known in the art.
The term administering or administration includes providing a composition to a
mammal,
consuming the composition and combinations thereof.
Experimental Example
The following examples will illustrate the invention in further detail. It
will be readily
understood that the composition of the present invention, as generally
described and illustrated in
the Examples herein, could be synthesized in a variety of formulations and
dosage forms. Thus,
the following more detailed description of the presently preferred embodiments
of the methods,
formulations and compositions of the present invention are not intended to
limit the scope of the
invention, as claimed, but it is merely representative of the presently
preferred embodiments of
the invention.
The objective of the following experiment was to test the stability of Vitamin
D3 in
HMBFA in different formulas. A loss of Vitamin D3 less than 10% was considered
acceptable
Methods: Eleven different HMBFA/Vitamin D3 formulas were tested. These
formulas were
tested for the stability of Vitamin D3 over time. The formulas were as
follows:
9
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
1. HMBFA + Vitamin D3 (100 HP, BASF, Denmark)
2. Vitamin D3 (100 HP, BASF, Denmark) in HMB FA plus Choline Chloride (Acros
Organics)
3. Vitamin D3(100 HP, BASF, Denmark) in HMB FA plus Betaine (Sigma-Aldrich)
4. Buffered HMBFA + Vitamin D3(100 HP, BASF, Denmark)
5. Buffered HMBFA + BASF protected Vitamin D3 (100GFP/HP, BASF, Denmark)
6. HMBFA + NovaSol DC), protected Vitamin D3 (Aquallova , Germany)
7. Buffered HMB free acid + NovaSol D protected Vitamin D3(Aquallova,
Germany)
stabilized with 60 ppm disodium EDTA (VerseneTM NA Chelating Agent, Dow
Chemical, Midland, MI),
8. HMBFA + NovaSol D Vitamin D3 (Aquallova, Germany) stabilized with 60 ppm
disodium EDTA(VerseneTm NA Chelating Agent, Dow Chemical, Midland, MI)
9. Buffered HMBFA + Vitamin D3 (Sigma-Aldrich) stabilized with 60 ppm disodium
EDTA (VerseneTM NA Chelating Agent, Dow Chemical, Midland, MI)
10. Buffered HMBFA + NovaSol D Vitamin D3(Aquallova, Germany) + L-carnitine
stabilized with BHT (Butylated hydroxytoluene, Sigma-Aldrich)
11. Buffered HMBFA+ NovaSol D Vitamin D3 (Aquallova, Germany) + L-carnitine
stabilized with NovaSol Rosemary , NovaSol DS/4 , and BHT (Butylated
hydroxytoluene, Sigma-Aldrich)
Vitamin D3 was measured by HPLC by Heartland assays.
Results: Of the 11 formula tested, formula 7 containing buffered HMBFA plus
NovaSol D
protected Vitamin D3 stabilized with 60 ppm disodium EDTA (VerseneTM NA),
formula 10
containing HMBFA plus NovaSol D protected Vitamin D3 stabilized with BHT, and
formula 11
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
Buffered HMBFA + NovaSol D Vitamin D3 stabilized with NovaSol Rosmary, NovaSol
DS/4,
and BHT were stable. The percent chance in Vitamin D3 is presented in the
following table:
Table 1
Vit.
Formula D3, %
No. days change
1 HMBFA + vitamin D3 28 -57%
Vitamin D3 in HMBFA plus Choline Chloride
2 14 -28%
Vitamin D3 in HMBFA plus Betaine
3 14 -28%
4 Buffered HMBFA acid + Vitamin D3 28 -61%
Buffered HMBFA + BASF protected Vitamin D3
35 -35%
6 HMB FA + NovaSol D protected Vitamin D3 35 -32%
Buffered HMB free acid + NovaSol Dprotected
7 Vitamin D3 stabilized with 60 ppm disodium EDTA 56 2%
HMBFA + NovaSol D protected Vitamin D3
8 stabilized with 60 ppm disodium EDTA 14 -32%
Buffered HMBFA + Sigma Vitamin D3 stabilized
9 with 60 ppm disodiumEDTA 28 -11%
Buffered HMBFA + NovaSol D protected Vitamin
D3 + L-carnitine stabilized with BHT 56 14.9
Buffered HMBFA + NovaSol D protected Vitamin
D4+ L- carnitine stabilized with NovaSol Rosmary,
11 NovaSol DS/4, and BHT 56 -7.9
5
Figures 1, 2, and 3 illustrate the stability of Vitamin D3 in formula 7, 10,
and 11, respectively.
In conclusion, these studies demonstrate that vitamin D3 is stable when
formulated with buffered
HMBFA and disodium EDTA or when BHT is substituted for disodium EDTA. A
protected
vitamin D3 such as NovaSol D is also necessary.
11
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
Preparation of Buffered HMB in the free acid form (HMBFA)
Briefly, 400 g HMBFA (TSI lot 12111036) was weigh into a one liter beaker and
then 37.8 g of
with potassium carbonate (K2CO3) was added to the HMBFA. A stir bar was added
and the
mixture was stirred for 60 h. The resulting pH was approximately 4.5.
Alternatively, the
reaction may be accelerated by mixing water (35 g) with potassium carbonate
(37) g and then
adding HMBFA (400 g).
The vitamin D3 in formula 7 was from Aquallova and is described as NovaSOL D.
This
vitamin D3 product is protected with Polysorbate 80. The product is water and
oil soluble,
resulting in a clear solution. To calculate the amount of the component's
needed, the following
was used: to use per capsule 250 IU Vitamin D3 (Calculation 11.tg = 40 IU),
meaning 6.25 1.tg
based on this 0.25% NovaSOL D product requires 2.5mg per capsule of NovaSOL D.
The EDTA used in formula 7 and formula 8 was Dow's disodiumEDTA (VerseneTM
NA Chelating Agent, Dow Chemical, Midland, MI. In formula, 10 and 11 disodium
EDTA was
replaced with the preservative BHT. In addition, formula 11 also contained the
antioxidant
NovaSol rosemary extract which is high in carnosolic acid and the preservative
NovaSol DS/4
(sorbic acid).
The foregoing description and drawings comprise illustrative embodiments of
the present
inventions. The foregoing embodiments and the methods described herein may
vary based on
the ability, experience, and preference of those skilled in the art. Merely
listing the steps of the
method in a certain order does not constitute any limitation on the order of
the steps of the
method. The foregoing description and drawings merely explain and illustrate
the invention, and
the invention is not limited thereto, except insofar as the claims are so
limited. Those skilled in
12
CA 03105039 2020-12-23
WO 2020/263229
PCT/US2019/038948
the art who have the disclosure before them will be able to make modifications
and variations
therein without departing from the scope of the invention.
References
1 Heaney, R. P. Vitamin D in Health and Disease. Clin. J. Am. Soc. Nephrol
3, 1535-1541 (2008).
2 Holick, M. F. et al. Evaluation, treatment, and prevention of
vitamin D deficiency: an Endocrine
Society clinical practice guideline. J Clin Endocrinol Metab 96, 1911-1930,
doi:10.1210/jc.2011-
0385 (2011).
3 Eitenmiller, R. R. L. J., W.O.; . in Vitamin Analysis for the Health
of Food Sciences 77-107 (CRC
Press, 1998).
4 Renken, S. A. W., J.J. Vitamin stability in milk. Food Sci 58, 552-
556 (1993).
5 Indyk, H. E. L., V.; Woollard, d.C. Solubility of vitamin D3 during
spray-dying of milk. Food Chem
57, 283-286 (1996).
6 Biancuzzo, R. M. et al. Fortification of orange juice with vitamin
D(2) or vitamin D(3) is as
effective as an oral supplement in maintaining vitamin D status in adults. Am
J Clin Nutr 91,
1621-1626, doi:10.3945/ajcn.2009.27972 (2010).
13