Note: Descriptions are shown in the official language in which they were submitted.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
1
TREATMENT OF TAILINGS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional
Application No. 62/699,335,
filed 17 July 2018, the entire disclosure of which are hereby incorporated by
reference herein.
TECHNICAL FIELD
[0002] The present disclosure relates to dewatering and consolidating
aqueous
compositions including solids such as tailings. Such tailings result from
processing ore such as
metal, phosphate and coal-based ores.
BACKGROUND
[0003] Various mining and extraction processes produce a tailings stream
characterized as
a slurry of particulate matter in water. These tailings often contain
components that are hazardous
and cannot be discharged directly into rivers and streams. A common practice
is to store tailings
in ponds, which can be very large or encompass numerous sites. For example, it
has recently been
estimated that Canadian oil-sands tailings ponds cover an area of about 200
square kilometers. In
the U.S., the Environmental Protection Agency has identified more than 500 ash
and coal slurry
ponds, mostly in the Appalachian coal mining region. In Florida, phosphate
mining results in the
production of approximately 100,000 tons a day of phosphatic clays in the form
of a slurry that is
also stored in ponds. It is very difficult to dewater and the phosphate
industry leaves about 40% of
mined land in unstable clay settling areas. The processes for mining and
extracting of ores of
aluminum, copper, zinc, lead, gold, silver, etc., also create tailing streams.
It is also of particular
interest in processing ores to recycle water, but such recycling is hindered
by particulate matter
suspended in waste water.
[0004] The management and sustainability of tailings ponds pose
significant and growing
problems. The dams or impoundments used to form the ponds are often
constructed from local
material and are a significant potential danger. The failure of a coal slurry
dam in West Virginia
resulted in the Buffalo Creek flood, which killed more than 125 people.
Various other, more recent,
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
2
dam failures in tailings containment ponds have resulted in significant if not
catastrophic
environmental damage. For example, in 2016 the failure of a tailings dam in
Henan province,
China, released about 2 million cubic meters of red mud, totally immersing a
nearby village. In
2015, a waste heap from Jade mining failed in Myanmar, killing at least 113
people.
[0005] In the oil sands industry, fines are defined as particles having a
diameter equal to
or less than 44 [tm. They are part of a waste stream that settles much more
slowly than coarse sand,
leaving a layer of water with some entrained fines near the surface of the
ponds. This water is
reused in the bitumen extraction process. Initially, most of the fines (mainly
silica and clay
particles) form an intermediate layer of so-called fluid fine tailings (FFT).
This fluid has a low
solids content, between 15% and 30% and is also referred to as thin fine
tailings (TFT). Over time,
additional settling occurs, but the negative surface charge of the mineral
particles limits
aggregation and a distinct layer of so-called mature fine tailings (MFT) is
formed. The solids
content of the MFT is on average about 30%, but varies with depth. It has gel-
like properties that
make it difficult to handle and dewater. It has been estimated that under the
action of gravity alone,
this tailings component could take decades to centuries to consolidate and
settle and thus allow for
land reclamation. The tailings from phosphate mining in Florida form a similar
gel-like structure.
Beneath a surface crust, these tailings have about a 25% solids content with a
fluid-like
consistency.
[0006] So-called impoundment ponds are used to store two types of waste
from coal
handling and combustion. Coal ash that is a residue of combustion is one such
material and
includes several components (fly ash, bottom ash, etc.). The EPA estimated
that 100 million tons
of coal ash was generated in the U.S. in 2012. There are dry methods of
disposal and coal ash can
also be recycled into building material, but for economic reasons the wet
disposal of ash into ash
ponds has been common practice. The EPA estimated that there are more than 500
units,
presumably ash ponds, at more than 200 power plants. There are increasing
environmental
concerns regarding leachate from these ponds.
[0007] The second type of impoundment pond for coal processing wastes
stores material
that is a product of coal preparation plants, where soil and rock are removed
from run-of-mine coal
to lower its ash content and increase its value. This is accomplished by
washing. However, this
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
3
coal cleaning process produces a reject stream in the form of a sludge or
slurry. This slurry contains
very fine coal particles together with other material (such as clays) and, as
with the tailings streams
mentioned above, is very difficult to dewater economically using standard
methods. There are now
about 600 so-called slurry impoundments in the U.S. where this waste material
is stored, mostly
in the Appalachian coal mining region. The impoundments can be as large as 50
acres in size and
contain billions of gallons of toxic sludge. This material represents both an
economic cost in terms
of the loss of a valuable resource (in the form of coal fines) and a major
environmental hazard.
The Washington Post (April 24, 2013) reported that a study by the Office of
Surface Mining
Reclamation and Enforcement found that many sludge impoundment walls are weak
and are
known to leak. Historically, a number of catastrophic failures of ash and
sludge ponds have
occurred, resulting in significant loss of life and environmental devastation.
With the coal industry
in decline and mining companies filing for bankruptcy, the impoundment ponds,
both those that
remain in use and those that have been abandoned, are a significant and
growing problem.
[0008] The production of alumina from bauxite also results in the
generation of a large
tailings stream. Approximately 77 million tons of a highly alkaline waste
product composed
mainly of iron oxide and known as red sludge or red mud is generated every
year. This poses a
significant disposal problem and a tailings dam failure led to catastrophic
consequences, as
described above.
[0009] There is a continuing need to manage and treat aqueous
compositions including
suspended solids, e.g., tailings, to reduce the volume of such tailings and/or
to dewater and
consolidate solids in such tailings and in a manner preferable for land
reclamation, remediation
and/or reclaiming water for use in mining operations.
SUMMARY OF THE DISCLOSURE
[0010] Advantages of the present disclosure include processes to dewater
aqueous
compositions including suspended solids, e.g., tailings, to produce high
solids content materials.
[0011] These and other advantages are satisfied, at least in part, by a
process of
consolidating solids in tailings. The process comprises treating tailings with
a highly water soluble
salt. Advantageously, the process can include treating tailings with at least
one highly water
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
4
soluble salt or solution thereof and can optionally include either or both of
(i) at least one polymer
flocculant or solution thereof and/or (ii) optionally coarse particles, e.g.,
sand, to form a treated
tailings. The treated tailings can include a consolidated material in the
process water, which can
then advantageously be separated from the consolidated material.
[0012] Implementations of the process of the present disclosure include,
for example, (i)
treating tailings with at least one highly water soluble salt to form a
treated tailings including a
consolidated material in the process water, (ii) treating tailings with at
least one highly water
soluble salt and at least one polymer flocculant to form a treated tailings
including a consolidated
material in the process water, (iii) treating tailings with at least one
highly water soluble salt and
coarse particles to form a treated tailings including a consolidated material
in the process water,
and (iv) treating tailings with at least one highly water soluble salt, at
least one polymer flocculant
and coarse particles to form a treated tailings including a consolidated
material in the process
water. Each of these implementations can include aqueous solutions of the salt
and/or polymer
flocculant to treat tailings. Each of these implementations can include
separating the process water
from the consolidated material. Advantageously, the consolidated materials can
have a density
greater than the process water.
[0013] Embodiments of the processes include one or more of the following
features
individually or combined. For example, the tailings subject to treatment can
resulting from
processing a metal-based ore, phosphate-based ore, or coal based ore. In some
embodiments, the
at least one highly water soluble salt can have a solubility in water (a
salt/water solubility) of at
least about 5 g/100 g at 20 C, e.g., at least about 10 g/100 g at 20 C. In
other embodiments, the
at least one highly water soluble salt is a non-hydrolyzing salt. In still
further embodiments, the
at least one highly water soluble salt can have a monovalent cation and can
include an ammonium
based salt, a phosphate based salt, or a sulfate based salt or combinations
thereof.
[0014] In certain embodiments, the treated tailings can have a salt-
tailings concentration
of at least 0.5 wt% of the at least one highly water soluble salt and
preferably no less than about
0.70 wt%, such as at least about 1 wt%, 1.25 wt %, 1.5 wt%, 1.75 wt%, 2 wt%
and even at least
about 2.5 wt%, 3 wt%, 4 wt%, 5 wt%, etc. of the at least one highly water
soluble salt. In some
embodiments, the at least one polymer flocculant is a polyacrylamide or co-
polymer thereof The
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
treated tailings can have a polymer-tailings concentration of the at least one
polymer flocculant of
not less than zero and up to about 0.001 wt%, e.g., up to about 0.003 wt%,
0.005 wt%, 0.01 wt%
or 0.04 wt%. In other embodiments, tailings are treated with coarse particles,
e.g., sand, at a sand
to fines ratio of less than 4:1, e.g., including about 2.5:1.0 to about 0.5:1
or including about 2.25:1
to about 0.75:1. Advantageously, when added, the polymer flocculant can form
high density flocs,
e.g., having a density greater than the process water, which facilitates
separation and dewatering
of the consolidated materials.
[0015] In various embodiments, treating tailings can include combining
tailings with a
solution including the at least one highly water soluble salt and the at least
one polymer flocculant.
In some embodiments, treating tailings can include combining a stream of
tailings, e.g., tailings
from processing metal ore such as copper ore, with a stream of a solution
including the at least one
highly water soluble salt and a separate stream of a solution including the at
least one polymer
flocculant. Alternatively, or in combination, treating tailings can include
combining a stream of
tailings with a stream of a solution including both the at least one highly
water soluble salt and the
at least one polymer flocculant. Coarse particles (e.g., sand) can also be
added to tailings or stream
thereof and/or to any or all of the solution streams. Advantageously, the
streams can be mixed
inline and/or with the aid of an inline mixer. In certain embodiments,
treating tailings can be
carried out at ambient temperature, e.g., no more than about 2 C to about 5
C above ambient. In
other embodiments, treating tailings can be carried out a temperature of no
more than about 50 C,
e.g., no more than about 40 C or 30 C. In still further embodiments,
treating tailings includes
using a solution of one or more highly soluble salts sourced from a natural or
existing source such
as seawater or a body of hypersaline water or sourced from a brine waste
stream.
[0016] In still further embodiments, the process water can be separated
from the
consolidated material by any one or more of decanting, filtering, vacuuming,
gravity draining,
electrofiltering, etc. or combinations thereof In various embodiments,
separating the process
water from the consolidated material can include mechanically dewatering the
consolidated
material, e.g., mechanically dewatering the consolidated material by a
dewatering screw, industrial
filter, etc.. Once separated, the consolidated material can be transferred for
further dewatering or
disposal.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
6
[0017] In practicing aspects of the processes of the present disclosure
and the various
embodiments thereof, the separated process water can include the at least one
highly water soluble
salt and the process can further comprise one or more of: (i) recovering at
least a portion of the
separated process water; (ii) recycling at least a portion of recovered
separated process water to
treat additional tailings; (iii) purifying at least a portion of recovered
process water; or (iv)
concentrating the at least one highly water soluble salt in recovered process
water to form a brine
and using the brine to treat additional tailings.
[0018] Yet another aspect of the present disclosure includes recovering
valuable materials
from the aqueous composition of fines, e.g., tailings. The valuable materials
can include rare earth
elements (REE) associated with solids such as clays in tailings from various
types of aqueous fines
such as tailings stream. Therefore, in practicing certain aspects of the
processes of the present
disclosure and the various embodiments thereof, the aqueous compositions can
further include rare
earth element materials which can be recovered by treating tailings with at
least one highly water
soluble salt, e.g., an ammonium based salt such as ammonium sulfate, to form a
treated tailings
including REE in the process water and/or in the consolidated materials. In
some embodiments,
the process further includes separating the process water from the
consolidated material and
recovering the REE from the separated process water and/or the consolidated
materials.
[0019] Advantageously, the processes of the present disclosure can
consolidate the solids
of tailings to produce a consolidated material having a solids content in
excess of about 45% by
weight, e.g., a solids content of greater than about 50% and higher than about
60%, 65%, 70% and
75% by weight.
[0020] In practicing certain aspects of the processes of the present
disclosure and the
various embodiments thereof, the consolidated material formed in the treated
tailings according to
certain embodiments can result in a high solids content after mixing and/or
dewatering the treated
tailings in a short period of time. In some embodiments, the consolidated
material can have a
solids content of greater than about 50% and at least about 60%, 65%, 70%, 75%
and 80% by
weight after mixing and/or dewatering.
[0021] Another aspect of the present disclosure includes an aqueous
solution for treating
aqueous fines. The aqueous solution includes a highly water soluble ammonium
based salt and a
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
7
polymer flocculant, e.g., a water soluble polymer. Embodiments include,
together or individually,
an aqueous solution of one or more of the highly water soluble salt(s) and
having a concentration
of no less than about 1 wt%, e.g., at least about 2 wt%, 5 wt%, 10 wt%, 20
wt%, 30 wt% and even
as great as a 40 wt% or as an aqueous salt slurry. The aqueous solution can
also include one or
more of the polymer flocculant(s) and having a concentration of not less than
zero and up to about
0.005 wt%, e.g., up to about 0.01 wt%, 0.04 wt%, 0.05 wt %, 0.1 wt%, 0.2 wt%,
0.4 wt%, for
example.
[0022] Additional advantages of the present invention will become readily
apparent to
those skilled in this art from the following detailed description, wherein
only the preferred
embodiment of the invention is shown and described, simply by way of
illustration of the best
mode contemplated of carrying out the invention. As will be realized, the
invention is capable of
other and different embodiments, and its several details are capable of
modifications in various
obvious respects, all without departing from the invention. Accordingly, the
drawings and
description are to be regarded as illustrative in nature, and not as
restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] Reference is made to the attached drawings, wherein elements
having the same
reference numeral designations represent similar elements throughout and
wherein:
[0024] Figure 1A schematically illustrates a process of consolidating a
tailings stream in
accordance with aspects of the present disclosure.
[0025] Figure 1B schematically illustrates another process of
consolidating a tailings
stream in accordance with aspects of the present disclosure.
[0026] Figure 2 are pictures of vials containing waste coal slurry
treated according to an
embodiment of the present disclosure. The pictures show coal slurry after
adding an ionic solution
(left), then centrifuging (middle) and after removal of supernatant solution
(right).
[0027] Figure 3 are pictures of the dewatered coal slurry from Figure 2
after removal from
the vial (left) and subsequent hand-pressing between paper towels.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
8
[0028] Figure 4 shows pictures of vials containing mature fine tailings
from oil sands
processing treated with an ammonium salt solution including a polyacrylamide
flocculant at the
concentrations indicated in the figure.
[0029] Figure 5 shows pictures of vials containing mature fine tailings
treated with an
ammonium salt and a polyacrylamide flocculant and illustrate effects of
increasing salt
concentration and reducing polymer concentration under the conditions tested.
[0030] Figure 6 shows a picture of vials containing mature fine tailings
from oil sands
processing treated with seawater which included varying amounts of a
polyacrylamide flocculant.
[0031] Figures 7A, 7B and 7C show picture of treating tailings generated
from processing
copper ore.
DETAILED DESCRIPTION OF THE DISCLOSURE
[0032] The present disclosure relates to treating tailings and other
aqueous compositions
which include solids to consolidate and dewater the tailings. Tailings are
typically produced when
mining and processing ores such as metal-based ores, e.g., aluminum, copper,
zinc, lead, iron,
gold, silver, molybdenum, lithium, etc., non-metal based ores, e.g., phosphate
ore, nitrate ore,
iodine ore, oil sands, etc. Aqueous compositions of fines can also be produced
when processing
coal. For example, certain processes finely grind coal prior to combustion to
more readily liberate
pyrite (a sulfur based compound) and hence reduce sulfur emissions upon
combustion of the
ground coal. Such processes can produce fine coal particles as well as other
fine mineral or mineral
matter in an aqueous composition that are difficult to recapture and reuse.
[0033] Particulate solids in the tailings or aqueous compositions of the
present disclosure
can be minerals and mineral like materials, i.e., mineral matter, clays, slit,
and in sizes ranging
from fines to coarse solids. The term fines as used herein is consistent with
the Canadian oil sands
classification system and means solid particles with sizes equal to or less
than 44 microns (pm).
Sand is considered solid particles with sizes greater than 44 i_tm. The
composition of the fines
depends on the source of the materials, but generally fines are comprised
mostly of silt and clay
material and sometimes minerals or mineral matter, depending on the ore.
Tailings can have
various solids contents and various amounts of fines as its solids content.
The tailings treated
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
9
according to embodiments of the present disclosure can include a significant
amount of fines by
weight (>5 wt%) as their solids content. Such tailings can include at least
about 10 wt%, 20 wt%,
30 wt%, 40 wt%, 50 wt%, 60 wt%, 70 wt% or higher fines as their solids
content.
[0034] Advantageously, the process of the present disclosure can
consolidate the solids of
tailings to produce consolidated material having a solids content in excess of
about 45% by weight,
e.g., a solids content of greater than about 50% and higher than about 60%,
65%, 70% and 75%
by weight.
[0035] The terms coagulation and flocculation are often used
interchangeably in the
literature. As used herein, however, coagulation means particle aggregation
brought about by the
addition of hydrolyzing salts, whereas flocculation means particle aggregation
induced by
flocculating polymers. Hydrolyzing salts undergo hydrolysis when added to
water to form metal
hydroxides, which precipitate from the solution, trapping fines and other
minerals in the
coagulating mass. Hydrolyzing salts typically have low solubility in water and
are used as
coagulants. Aggregation induced by flocculation, in contrast, is believed to
be the result of the
polymer binding to the particles thereby tying the particles together into a
so called floc causing
aggregation of the particles.
[0036] In practicing aspects of the present disclosure, tailings, and
other an aqueous
composition of solids and process water, can be consolidated by treating the
tailings with one or
more highly water soluble salt(s) or an aqueous solution thereof to
destabilize and consolidate
solids in the tailings, e.g., to destabilize and consolidate coarse solids and
fines in tailings.
Aggregation induced by the addition of salts is believed to be the result of
destabilizing the
particles suspended in the fluid by an alteration or a shielding of the
surface electrical charge of
the particles to reduce the inter-particle repulsive forces that prevent
aggregation. In certain
embodiments, tailings, e.g., a suspension of particulate solids, which can
include fines, in process
water are treated. Such tailings that can be treated include tailings streams
from processing metal-
based ores, non-metal based ores, or a coal slurry. The process includes
treating the tailings with
a highly water soluble salt(s) or an aqueous solution thereof to form a
treated tailings including a
consolidated material, e.g., consolidated solids and/or fines, in process
water. The process water
can then be separated from the consolidated material. Advantageously, the
consolidated material
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
has a solids content of at least 45% by weight, e.g., a solids content of
greater than about 50% and
higher than about 60%, 65%, 70% and 75% by weight.
[0037] Salts that are useful in practicing the present disclosure include
salts that are highly
soluble in water. A highly water soluble salt as used herein is one that has a
solubility in water of
greater than 2 g of salt per 100 g of water (i.e., a salt/water solubility of
2g/100g) at 20 C.
Preferably the highly water soluble salt has a water solubility of at least
about 5 g/100 g at 20 C,
e.g., at least about 10 g/100 g of salt/water at 20 C.
[0038] In addition, the highly water soluble salts used in the processes
of the present
disclosure are preferably non-hydrolyzing. Hydrolyzing salts undergo
hydrolysis when added to
water to form metal hydroxides, which precipitate from the solution. Such
hydrolyzing salts are
believed to form open flocs with inferior solids content and cannot be readily
recycled for use with
additional tailings in continuous or semi-continuous processes. In addition,
hydrolyzing salts
typically have low solubility in water and are used at elevated temperatures
to ensure sufficient
solubility for aggregation, which is an energy intensive process. See US
4,225,433 which discloses
the use of lime as a coagulating agent at a temperature of 75 C.
[0039] Further, the highly water soluble salts are preferably not
carboxylate salts since
such organic acid salts tend to be more expensive than inorganic salts and can
be deleterious to
plant and/or animal life.
[0040] Highly water soluble salts that are not hydrolyzing and useful in
practicing
processes of the present disclosure include salts having a monovalent cation,
e.g., alkali halide
salts such as sodium chloride, potassium chloride; also salts with monovalent
cations such as
sodium nitrate, potassium nitrate, sodium and potassium phosphates, sodium and
potassium
sulfates, etc. are useful in practicing processes of the present disclosure.
Other monovalent
cationic salts useful in practicing processes of the present disclosure
include ammonium based
salts such as ammonium acetate (NH4C2H302), ammonium chloride (NH4C1),
ammonium bromide
(NH4Br), ammonium carbonate ((NH4)2CO3), ammonium bicarbonate (NH4HCO3),
ammonium
nitrate (NH4NO3), ammonium sulfate ((NH4)2504), ammonium hydrogen sulfate
(NH4HSO4),
ammonium dihydrogen phosphate (NH4H2PO4), ammonium hydrogen phosphate
((NH4)2HPO4),
ammonium phosphate ((NH4)3PO4), etc. Mixtures of such salts can also be used.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
11
[0041] Ammonium based salts are useful for practicing the present
disclosure since
residual ammonium based salts on the consolidated material after combining the
salt with the
aqueous fines, e.g., tailings, can be beneficial to plant life. In fact, many
of the ammonium based
salts are useful as fertilizers, e.g., ammonium chloride, ammonium nitrate,
ammonium sulfate, etc.
Many of the monovalent sulfate and phosphate salts are also useful as
fertilizers. In certain
embodiments of the present disclosure, the highly water soluble salt or salts
used in the processes
of the present disclosure can preferably be non-toxic and beneficial to plant
life to aid in
environmental remediation and the restoration of mine sites.
[0042] Highly water soluble salts that can be used in practicing the
present process can
also include salts having multivalent cations. Such salts include, for
example, divalent cation salts
such as calcium and magnesium cation salts, such as calcium chloride (CaCl2),
calcium bromide
(CaBr2), calcium nitrate (Ca(NO3)2), magnesium chloride (MgCl2), magnesium
bromide (MgBr2),
magnesium nitrate (Mg(NO3)2), magnesium sulfate (Mg504); and trivalent cation
salts such as
aluminum and iron (III) cation salts, e.g., aluminum chloride (A1C13),
aluminum nitrate
(Al(NO3)3), aluminum sulfate (Al2(504)3), iron (III) chloride (FeCl3), iron
(III) nitrate (Fe(NO3)3),
iron (III) sulfate (Fe2(504)3, etc. As explained above, the highly water
soluble salts used in the
processes of the present disclosure are preferably non-hydrolyzing. Many of
the multivalent cation
salts are hydrolyzing and thus less preferred for the reasons stated above.
Moreover,
experimentation with multivalent salts showed increased fouling of containers
and formation of
less cohesive consolidated materials as compared to highly water soluble salts
having monovalent
cations. In addition, some multivalent salts, such as FeCl3 and Fe2(504)3, are
particularly corrosive
and Fe2(504)3 is formed from oxidizing pyrite and results in acid mine run-
off, which make such
salts less preferable for use in processes of the present disclosure.
[0043] When a sufficiently high concentration of the highly water soluble
salt is included
in the treated tailings, the salt can destabilize and consolidate solids in
tailings. For a relatively
short process times with a relatively low energy input, the salt-tailings
concentration of the at least
one highly water soluble salt should preferably be at least 0.5 wt% and
preferably no less than
about 0.70 wt%, such as at least about 1 wt%, 1.25 wt %, 1.5 wt%, 1.75 wt%, 2
wt% and even at
least about 2.5 wt%, 3 wt%, 4 wt%, 5 wt%, etc. The term "salt-tailings
concentration" as used
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
12
herein refers to the concentration of the highly water soluble salt(s) in the
treated tailings and is
determined by taking the percentage of the mass of highly water soluble
salt(s) divided by the
combined mass of the salt(s) plus the tailings and any water used to dilute
the salt(s). For example,
combining 1 part undiluted (i.e., neat) salt to 99 parts tailings by weight
results in a salt-tailings
concentration of 1 wt%. Alternatively, treating tailings with an equal weight
of a 2 wt% solution
of the salt also results in a salt-tailings concentration of 1 wt% in the
treated tailings.
[0044] The highly water soluble salt(s) can be used to treat compositions
of the present
disclosure as a solid, e.g., combining the salt as a powder with tailings.
Alternatively, the salt can
be used to treat as a solution, e.g., combining an aqueous salt solution with
tailings. In some
aspects of the present disclosure, an aqueous solution of the highly water
soluble salt can be
prepared having a concentration of no less than about 1 wt%, e.g., greater
than about 2 wt%, 5
wt%, 10 wt%, 20 wt%, 30 wt% and even as great as a 40 wt% or as an aqueous
salt slurry. The
tailings and salt solution or slurry should be mixed at a ratio sufficient to
destabilize the tailings to
cause consolidation of the solids therein. In one aspect of the present
disclosure, the tailings and
the salt solution can be mixed at a ratio of tailings to salt solution at a
range of about 80:1 to 1:1,
e.g., 70:1 to 1:1, 50:1 to 1:1, 30:1 to 1:1, 20:1 to 1:1, 15:1 to 1:1, 10:1 to
1:1, 5:1 to 1:1, and/or
about 2:1 to 1:1 tailings to salt solution.
[0045] In some embodiments of the present processes, it can be more
advantageous to use
a natural source of a highly soluble salt or salts such as in a natural body
of water including such
salts in sufficiently high concentration such as at least about 2 wt% and even
at least about 3 wt%
or greater. For example, ocean or seawater can be used as a source of highly
soluble salts, which
can significantly improve the economics of the process under certain
conditions. The vast majority
of seawater has a salinity of between 31 g/kg and 38 g/kg, that is, 3.1-3.8%.
On average, seawater
in the world's oceans has a salinity of about 3.5% (35 g/L, 599 mM). Seawater
includes of a
mixture of salts, containing not only sodium cations and chlorine anions
(together totaling about
85% of the dissolved salts present), but also sulfate anions and calcium,
potassium and magnesium
cations. There are other ions present (such as bicarbonate), but these are the
main components.
Another natural source of highly soluble salts that can be used as a source of
highly soluble salts
includes a hypersaline body of water, e.g., a hypersaline lake, pond, or
reservoir. A hypersaline
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
13
body of water is a body of water that has a high concentration of sodium
chloride and other highly
soluble salts with saline levels surpassing ocean water, e.g., greater than
3.8 wt% and typically
greater than about 10 wt%. Such hypersaline bodies of water are located on the
surface of the
earth and also subsurface, which can be brought to the surface as a result of
ore mining operations.
[0046] In other embodiments of the present processes, it can be
advantageous to use a brine
produced in desalinization of salt water as a source of a highly soluble
salt(s). The brine can be
used alone as a source of a highly soluble salt(s) or in combination with
another source of a highly
soluble salt(s) such as seawater. Seawater has been used in grinding and
flotation processes of
mining copper ore. See Moreno et al., "The use of seawater as process water at
Las Luces copper-
molybdenum beneficiation plant in Taltal (Chile)", Minerals Engineering
2011:24:852-858.
However, use of seawater requires increased capital and maintenance costs to
combat the corrosive
effects of seawater. Id. Seawater can also adversely affect yield of producing
certain copper
minerals. See Jeldres et al., "Effect of seawater on sulfide ore flotation: a
review", Mineral
Processing and Extractive Metallurgy Review 2016:37(6):369-384. To offset
adverse effects of
seawater, some mining operations desalinate the seawater to produce desalted
water for their
mining operations. Desalinating seawater, however, produces a waste brine
stream. See Galvez
et al., "Innovative Solutions for Seawater Use in Mining Operations, Case
Study of Innovative
Projects Bernardo Llamas, IntechOpen", DOT: 10.5772/intechopen.68191,
Published August 30,
2017. It is believed that neither seawater has nor waste brine has been used
to treat tailings. Hence,
in some implementations the present processes, tailings from ore processes,
such as metal or
processes, can be treated with a waste brine from desalinization as a source
of the at least one
highly water soluble salt, with or without other sources of highly soluble
salt(s) such as seawater.
[0047] After treating the tailings with at least one highly water soluble
salt, the solids in
the tailings can be consolidated such as by mixing followed by gravity
sedimentation in a settling
tank or by mechanically consolidation such as by pressing or centrifugation to
increase the rate of
forming a consolidated material in the treated tailings. The consolidated
material can be separated
from the process water by decanting, filtering, electrofiltration, cross-flow
filtering, vacuuming,
and/or by mechanical dewatering, i.e., applying an external force to the
consolidated material.
Once separated, the consolidated material can be transferred for further
dewatering or disposal.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
14
[0048] Although highly water soluble salts can destabilize and
consolidate solids in the
tailings, it was found that the process could be significantly improved by
adding one or more
polymer flocculant(s). The addition of a polymer flocculant to the treated
tailings reduced the time
for forming a consolidated material.
[0049] The one or more polymer flocculants(s) can be added concurrent
with or subsequent
to treating the tailings with the at least one highly water soluble salt to
form the treated tailings.
The one or more polymer flocculants(s) can also be added prior to treating the
tailings with the at
least one highly water soluble salt but it appeared more effective to add
flocculant, even with
tailings already containing polymer flocculant such as thickener underflow
tailings, concurrent
with or subsequent with the at least one highly water soluble salt to form the
treated tailings.
[0050] In addition, the processes of the present disclosure can also
include treating aqueous
fines with coarse particles, e.g., particles with sizes greater than 44 um,
such as sand, to
significantly increase the solids content. Mixing with sand is appropriate for
aqueous fines that
have solids mostly as fines, as the fine particles can sit in the voids
between the coarse particles,
enhancing packing and solids content. It was found, however, that for certain
compositions such
as coal slurry and metal ore tailings, the addition of sand was not needed to
achieve a high solids
content, as there were sufficient coarse particles present in the tailings to
give a high solids content
material within a short period of time.
[0051] Hence, implementations of the process of the present disclosure
include, for
example, (i) treating tailings with at least one highly water soluble salt to
form a treated tailings
including a consolidated material in the process water, (ii) treating tailings
with at least one highly
water soluble salt and at least one polymer flocculant to form a treated
tailings including a
consolidated material in the process water, (iii) treating tailings with at
least one highly water
soluble salt and coarse particles to form a treated tailings including a
consolidated material in the
process water, and (iv) treating tailings with at least one highly water
soluble salt, at least one
polymer flocculant and coarse particles to form a treated tailings including a
consolidated material
in the process water. Each of these implementations can include aqueous
solutions of the salt
and/or polymer flocculant to treat the tailings. Each of these implementations
can include
separating the process water from the consolidated material. Advantageously,
the consolidated
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
material has a density greater than the process water. The process water can
then be readily
separated from the consolidated material as, for example, by one or more of
decanting, filtering,
gravity draining, electrofiltering, cross-flow filtering, vacuuming and other
evaporating
techniques, etc. and/or by one or more of a device for dewatering consolidated
material such as a
centrifuge, decanting centrifuge, dewatering screw, hydrocyclone, vacuum belt
filter, filter press
or pressing devices, etc. In addition, the separated consolidated material can
be disposed or
deposited in a containment structure which allows removal of released water
from the consolidated
material. In addition, the process water separated from the treated tailings
can be cycled back to
treat additional tailings.
[0052] Polymers that are useful in practicing the present disclosure
include water soluble
flocculating polymers such as polyacrylamides or copolymers thereof such as a
nonionic
polyacrylamide, an anionic polyacrylamide (APAM) such as a polyacrylamide-co-
acrylic acid,
and a cationic polyacrylamide (CPAM), which can contain co-monomers such as
acryloxyethyltrimethyl ammonium chloride, methacryloxyethyltrimethyl ammonium
chloride,
dimethyldiallyammonium chloride (DMDAAC), etc. Other water soluble
flocculating polymers
useful for practicing the present disclosure include a polyamine, such as a
polyamine or
quaternized form thereof, e.g., polyacrylamide-co-dimethylaminoethylacrylate
in quaternized
form, a polyethyleneimine, a polydiallyldimethyl ammonium chloride, a
polydicyandiamide, or
their copolymers, a polyamide-co-amine, polyelectrolytes such as a sulfonated
polystyrenes can
also be used. Other water soluble polymers such as polyethylene oxide and its
copolymers can
also be used. The polymer flocculants can be synthesized in the form of a
variety of molecular
weights (MW), electric charge types and charge density to suit specific
requirements.
Advantageously, the flocculating polymer used in practicing processes of the
present disclosure
do not include use of activated polysaccharides or activated starches, i.e.,
polysaccharides and
starches that have been heat treated, in sufficient amounts to lower the
density of the floc to below
the density of the process water from which they are separated. Such activated
polysaccharides
and activated starches when used in sufficiently high dosages tend to form low
density flocs which
rise to the surface of an aqueous composition, which can hinder removal of
solids in large scale
operations involving high solids content and can also hinder dewatering of
consolidated material.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
16
[0053] The amount of polymer(s) used to treat tailings should preferably
be sufficient to
flocculate the solids in tailings and any added coarse particles, e.g., sand.
The amount of
polymer(s) used to treat tailings can be characterized as a concentration
based on the total weight
of tailings or as a dosage based on the weight percent of the solids in
tailings.
[0054] In some embodiments of the present disclosure, the concentration
of the one or
more polymer flocculant(s) in the treated tailings has a polymer-tailings
concentration of up to
about 0.001 wt%, e.g., up to about 0.003 wt%, 0.005 wt% or up to about 0.01
wt%. For relatively
short processing times, consolidation of the solids can be obtained at polymer-
tailings
concentrations of no less than about 0.04 wt%. The term "polymer-tailings
concentration" as used
herein refers to the concentration of the flocculating polymer(s) in the
treated tailings and is
determined by taking the percentage of the mass of the polymer(s) divided by
the combined mass
of the polymer(s) plus the tailings and any water used to dissolve the
polymer(s). For example,
combining 1 part undiluted (i.e., neat) polymer to 9999 parts tailings by
weight results in a
polymer-tailings concentration of 0.01 wt%. Alternatively, treating tailings
with an equal weight
of a 0.02 wt% solution of the polymer also results in a tailings concentration
of 0.01 wt%. In
certain embodiments, tailings are treated with at least one polymer flocculant
to yield a polymer-
tailings concentration of up to about 0.02 wt%, such as up to about 0.03 wt%,
0.04 wt%, 0.05 wt%,
and even up to about 0.07 wt%, 0.09 wt%, 0.1 wt%, 0.2 wt%, etc. The amount of
polymer
flocculant can be used in greater concentrations. However, at high
concentrations it becomes
difficult to dissolve the flocculant, the solution becomes too viscous, and
the process is less
economical.
[0055] In some embodiments of the present disclosure, the concentration
of the one or
more polymer flocculant(s) in the treated tailings, has a dosage (weight of
the flocculant(s) to
weight of the solids in the tailings) of no less than zero and up to about
0.005 wt%, e.g., up to
about 0.01 wt% and in some implementations up to about 0.015 wt%, 0.020 wt%,
0.025 wt%, 0.03
wt%, or 0.04 wt%.
[0056] The amount of polymer flocculant can be reduced if the salt-
tailings concentration
is increased. While the reason for this effect is not clear, a very low
polymer-tailings concentration
of up to about 0.001 wt%, e.g., up to about 0.003 wt%, 0.005 wt%, 0.01 wt%,
0.02 wt%, 0.03 wt%,
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
17
0.04 wt%, 0.05 wt %, for example, can achieve reasonably fast consolidation of
solids in tailings,
if the salt-tailings concentration is increased.
[0057] Coarse particles useful for practicing certain processes according
to the present
disclosure are preferably sand and when used in treating compositions the
amount of such particles
are preferably in a sand to fines ratio (SFR ratio) of less than 4:1, e.g.,
including about 2.5:1.0 to
about 0.5:1 or including about 2.25:1 to about 0.75:1. The SFR ratio is
calculated by determining
the amount of sand added to an estimated amount of solid fines in the aqueous
fines on a weight
basis. It is believed that the use of coarse particles facilitates packing of
the consolidated fines
which advantageously increases the solids content and can even form a jammed
structure of
consolidated solids, i.e. a structure in which generally individual particles
of the consolidated solid
can no longer move freely relative to other particles.
[0058] Treating tailings, e.g., tailings from metal ore and non-metal ore
processes, with at
least one highly water soluble salt and optionally with either or both of at
least one polymer
flocculant and/or optionally sand can be carried out in a number of ways. In
certain embodiments,
treating the tailings includes combining and/or mixing the various components.
In addition, the at
least one salt can be added directly to the tailings either as an undiluted
solid in powder form or as
a solution; the at least one polymer flocculant can be added directly to the
tailings either as an
undiluted material or as a solution, and optionally coarse particles (e.g.,
sand) can be added to the
tailings directly or with the salt and/or polymer or solutions thereof. The
salt and polymer can be
combined in a single solution, with or without sand, and combined with the
tailings. The order of
combining the salt, polymer, and optionally sand, to the tailings can give
equivalent results and
optimization of the process will depend on the nature of tailings, and the
scale and equipment used
in the process.
[0059] However, it tends to be more convenient to use one or more
solutions including the
one or more highly water soluble salt(s) and the one or more polymer
flocculant(s) followed by
combining the one or more solutions with the tailings. In certain embodiments,
an aqueous
solution of one or more highly water soluble salt(s) can be used having a
concentration of no less
than about 0.5 wt% or 1 wt%, e.g., at least about 2 wt%, 3 wt%, 4 wt%, 5 wt%,
6 wt%, 7 wt%, 8
wt%, 10 wt%, 15 wt%, 20 wt%, 30 wt% and even as great as a 40 wt% or as an
aqueous salt slurry
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
18
for use in treating the tailings. The one or more polymer flocculant(s) can
also be included in the
aqueous solution of the salt(s) and can have a concentration of up to about
0.005 wt%, e.g., up to
about 0.01 wt%, 0.04 wt%, 0.05 wt %, 0.1 wt%, 0.2 wt%, 0.4 wt%, for example.
The aqueous
solution of the highly water soluble salt(s) and polymer flocculant(s) can be
used to treat the
tailings and can be combined with such tailings at a ratio of tailings to salt
solution at a range of
about 80:1 to 1:1, e.g., 70:1 to 1:1, 50:1 to 1:1, 30:1 to 1:1, 20:1 to 1:1,
15:1 to 1:1, 10:1 to 1:1,
5:1 to 1:1, and/or about 2:1 to 1:1 tailings to salt solution. Optionally,
sand can be combined with
the tailings before, during, or after combining the tailings with the aqueous
solution of salt and/or
polymer flocculant.
[0060] Because highly water soluble salts and polymer flocculants that
are preferably
water soluble are used in the process of the present disclosure, the
temperature of the treated
tailings need not be elevated above ambient temperature to practice the
process. In certain
embodiments, treating the tailings according to the various embodiments herein
can be carried out
at about ambient temperature or no more about 2 to about 5 C above ambient
temperature. In
other embodiments, treating the aqueous coal waste composition can be carried
out at a
temperature of no more than about 50 C, e.g., no more than about 40 C or 30
C.
[0061] In practicing aspects of the present disclosure, tailings, e.g.,
tailings from metal ore
and non-metal ore processes, can be consolidated by treating such tailings
with at least one highly
water soluble salt or aqueous solutions thereof and can optionally include
either or both of at least
one polymer flocculant, e.g., a water-soluble flocculating polymer, or aqueous
solutions thereof,
and/or optionally coarse particles, e.g., sand to form a treated tailings.
Treating tailings in this
manner can cause destabilization and consolidation of the solids, e.g., fines
and coarse solids, in
the treated tailings to form a consolidated material, which can aggregate
relatively quickly, in the
process water.
[0062] The treated tailings and/or consolidated material can be further
dewatered to further
separate the process water from the consolidated material and, in some
instances, further
consolidate the solids. In some embodiments, the consolidated material formed
in the treated
tailings can be separated from the process water by any one or more of
decanting, filtering, e.g.,
electrofiltering, cross-flow filtering, gravity draining, vacuuming and other
evaporating
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
19
techniques, etc. and/or by any one or more of a mechanical dewatering, i.e.,
applying an external
force to the consolidated material, with a device for dewatering consolidated
material such as by
applying a centrifuge, decanting centrifuge, dewatering screw, hydrocyclone,
filter press, pressing
device, etc. or combinations thereof. In one aspect of the processes of the
present disclosure, the
process water can be separated from the consolidated material by passing a
stream of treated
tailings through a cross-flow filter, e.g., a porous or slotted pipe, which
filters and dewaters the
treated tailings stream to separate the process water from the consolidated
material. The process
water can then be readily separated from the consolidated material. In another
aspect of the
processes of the present disclosure, the process water can be separated from
the consolidated
material by gravity draining to achieve a solids content of at least about 70%
within about a month
after treating the tailings, e.g., within about two weeks or within about one
week of gravity draining
after treating the tailings. In still further aspect of the processes of the
present disclosure, the
consolidated material can be further dewatered after separating from the
treated tailings by
depositing the separated consolidated material in a thin lift deposition.
[0063] The consolidated material formed in the treated tailings can
advantageously have a
high solids content, e.g., a solids content of greater than about 50% and at
least about 60%, 65%,
70% and 75% by weight. In addition, the consolidated material formed in the
treated tailings
according to certain embodiments can result in a high solids content after
mixing and/or
dewatering the treated tailings in a short period. In embodiments of the
present disclosure, the
consolidated material can have a solids content of greater than about 50% and
at least about 60%,
65%, 70%, 75% and 80% by weight after mixing and/or dewatering. In certain
embodiments a
solids content of at least about 70 % is achieved within about one month of
gravity draining after
treating tailings, e.g., within about two weeks or within about one week of
gravity draining after
treating tailings.
[0064] In an embodiment of the present disclosure, the process includes
mixing the tailings
with a highly water soluble salt, e.g., an ammonium based salt, a water
soluble polymer, e.g., a
polyacrylamide, and optionally sand, such as in a sand to fines ratio of
between about 2.25:1 to
about 0.75:1 to form a treated tailings including a consolidated material
having a high solids
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
content, e.g., a solids content of greater than about 50% by weight, e.g., at
least about 60%, 65%,
70 wt% or higher in less than 10 minutes, depending on the dewatering method
used.
[0065] Another advantage of the processes of the present disclosure is
the recovery of
materials from tailings that include rare earth elements. For example, certain
tailings can include
valuable minerals that include rare earth elements. A rare earth element
(REE), as defined by
IUPAC, is one of a set of seventeen chemical elements in the periodic table,
specifically the fifteen
lanthanides, as well as scandium and yttrium. Scandium and yttrium are
considered rare earth
elements because they tend to occur in the same ore deposits as the
lanthanides and exhibit similar
chemical properties. Many of the REE are used in electronic devices, magnets,
high performance
coatings. Such REE include cerium (Ce), dysprosium (Dy), erbium (Er), europium
(Eu),
gadolinium (Gd), holmium (Ho), lanthanum (La), lutetium (Lu), neodymium (Nd),
praseodymium
(Pr), promethium (Pm), samarium (Sm), scandium (Sc), terbium (Tb), thulium
(Tm), ytterbium
(Yb) and yttrium (Y).
[0066] REE in aqueous fines are typically in the form of an ion or oxide.
For example,
zirconium can be present as zircon, ZrSiO4, titanium can be present as the
minerals ilmenite,
leucoxene and rutile. Coal ash and coal cleaning wastes contain rare earth
elements. Fire clay
coal ash has unusually high concentrations of Yttrium and zirconium. Oil sands
tailings also
contain REE.
[0067] The processes of the present disclosure are useful in recovering
REE. It is believed
that in some tailings, REEs absorb on the surface of clays in tailings. In
other tailings, REEs are
predominately included among the solids of the tailings but can also be in the
process water.
Absorbed REEs can be exchanged with the highly water soluble salts of the
present disclosure,
e.g., ammonium based salts due to an exchange of ammonium ions for the REE
ions. REEs from
the solids of the tailings can be obtained by leaching the solids with acid
followed by extraction
and precipitation or by caustic decomposition followed by acid leaching.
[0068] Another aspect of processes of the present disclosure includes
consolidating
tailings, which include REE materials, by treating the tailings with at least
one highly water soluble
salt, e.g., an ammonium based salt such as ammonium sulfate, to form a treated
tailings including
a consolidated material in process water which includes the REE materials in
the process water
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
21
and/or among the consolidated materials. In one aspect of the present
disclosure, the treated
tailings consolidate the fines and also separates REE materials from the
solids and into the process
water. The process water can then be separated from the consolidated material
and the REE
materials can be recovered from the separated process water. The REE materials
can be recovered
from the process water by precipitation, e.g., using oxalic acid, or
extraction. Other methods for
recovering REE from the process water include mineral processing and physical
beneficiation,
deep eutectic solvents/ionic liquids extraction, acid dissolution, high
temperature phase
separations, use of REE selective sorbents, photophoresis, in-situ brine
injection and extraction,
reactive grinding, etc. In other aspect of the present disclosure, the treated
tailings consolidate the
fines and REEs are among the consolidated materials. The process water can
then be separated
from the consolidated material. The consolidated material can then be leached
with acid, e.g.,
nitric acid, sulfuric acid, etc., followed by extraction with solvent and/or
ion exchange resins and
precipitated. Alternatively, the consolidated material can then be treated
with a caustic reagent
such as sodium hydroxide to decompose certain of the materials to form
hydroxides of the REEs
followed by leaching in acid, e.g., HC1.
[0069] In addition, the tailings which include REE materials can be
treated with at least
one polymer flocculant and optionally sand to form the treated tailings. The
treated tailings can
have a salt-tailings concentration of at least 0.5 wt% of the at least one
highly water soluble salt
and preferably no less than about 0.70 wt%, such as at least about 1 wt%, 1.25
wt %, 1.5 wt%,
1.75 wt%, 2 wt% and even at least about 2.5 wt%, 3 wt%, 4 wt%, 5 wt%, etc. of
the at least one
highly water soluble salt.
[0070] The process of the present disclosure allows for large scale
treatment of tailings in
a continuous or semi-continuous process with further recovering, recycling and
purifying at least
a portion of the process water in the tailings and optionally recovering REE
materials. When non-
hydrolyzing, highly water soluble salts are used in the processes of the
present disclosure, the
process water separated from an initial treated tailings can advantageously
include a significant
amount of the one or more highly water soluble salt(s) initially used to treat
the tailings.
[0071] In practicing aspects of the processes of the present disclosure
and the various
embodiments thereof, the separated process water can include the at least one
highly water soluble
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
22
salt and the process can further comprise one or more of: (i) recovering at
least a portion of the
separated process water; (ii) recycling at least a portion of recovered
separated process water to
treat additional tailings; and/or (iii) purifying at least a portion of
recovered process water. In
some implementations, the recovered separated process water, which includes
the highly soluble
salts(s), can be processed to concentrate the highly soluble salts(s) in the
water. For example, a
reserve osmosis system, which generates desalted water and a waste brine, can
be used to generate
a brine including the highly soluble salts(s) from recovered separated process
water from the
treated tailings.
[0072] In other embodiments, the separated process water includes REE
materials salt and
the process further includes recovering at least a portion of the separated
process water and
recovering the REE materials and/or purifying at least a portion of the
recovered process water.
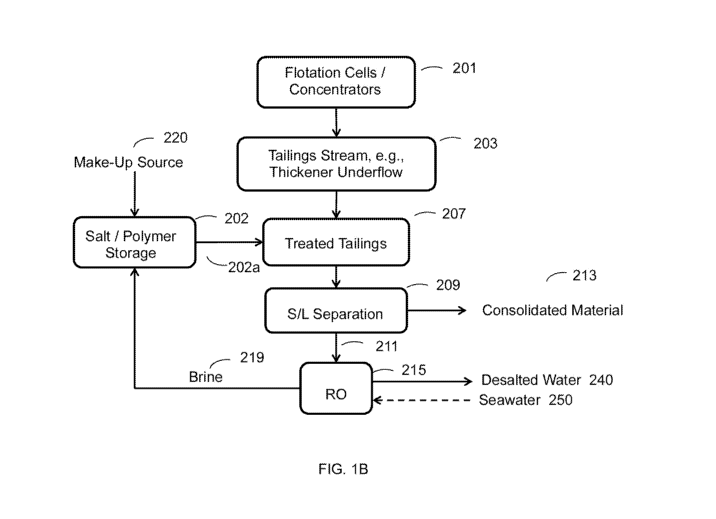
[0073] Figure 1A schematically illustrates an exemplary continuous or
semi-continuous
process. As shown in the figure, tailings are treated with one or more highly
water soluble salt(s),
and optionally one or more polymer flocculant(s) and optionally coarse
particles (sand) by
combining a stream of the salt(s) (101a), which can be an aqueous solution
with a stream of the
tailings (103a). Optionally, tailings can also be treated with one or more
polymer flocculant(s) by
combining a stream of the flocculants(s) (102a), which can be as an aqueous
solution, with the
tailings stream (103a). Alternatively, the salts(s) and flocculant(s) can be
combined together as a
solution to treat the tailings as a stream thereof. Coarse particles (sand)
can also be added to the
tailings or stream thereof and/or to any or all of the solution streams.
[0074] The solution streams of salt(s) and polymer(s) can be sourced from
holding tanks
101 and 102 and the streams of tailings and sand can be sourced from holding
tanks or ponds 103
and 105, respectively. Alternatively, the tailings can be sourced directly
from an ore extraction
process.
[0075] For this embodiment, the stream of salt(s) (101a) and polymer(s)
(102a) and tailings
stream (103a) are carried to mixing device 107 and the combination mixed. (A
stream of sand
(105a) can be optionally added). Mixing device 107 can be an inline mixer, a
mixing tank, ribbon
mixer or other mixing device that can mix streams 101a, 102a, 103a and,
optionally 105a. For this
embodiment, the tailings are combined with the salt(s) followed by polymer(s)
and as solutions.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
23
However, the tailings can be treated with an aqueous solution including both
the salt(s) and
polymer(s). In some embodiments, the combination of the streams in a line can
cause sufficient
mixing to eliminate the need for a separate mixing device, e.g., inline
mixing, and the combined
streams can be carried directly to a tailings pond or a mechanical dewatering
device to separate
consolidated material from process water.
[0076] As shown in the embodiment of Figure 1A, after mixer 107, the
treated tailings,
which include a consolidated material and process water, is transferred to
Solid/Liquid separator
109 to separate the process water from the consolidated material. Such devices
include, for
example, one or more of a decanting, filtering, electrofiltering, cross-flow
filtering, gravity
draining, or vacuuming device or combination thereof and/or by one or more of
a device for
dewatering consolidated material such as a centrifuge, decanting centrifuge,
dewatering screw,
hydrocyclone, vacuum belt filter, filter press or pressing devices, etc. or
combinations thereof.
[0077] Separated process water can be recovered and collected in a tank
or pond (111) and
separated consolidated material can be recovered and collected or transported
(113). For this
embodiment, a stream of recovered process water (111) includes the process
water from the
tailings diluted with stream 101a and thus includes residual salt(s) from the
one or more highly
water soluble salt(s) and can possibly include residual polymer(s) from the
one or more polymer
flocculant(s) as well as components from tailings. If the tailings include REE
materials, the
recovered stream of process water (111) and/or the consolidated material (113)
can also include
REE materials. There can also be highly water soluble salts that are
constituents of the original
treated tailings and these become part of the recovered process water (111).
The recovered process
water (111) can be transferred to a water purifying system (115) to purify at
least a portion of the
recovered process water 117 which can be recycled in the mining process. Water
purifying
systems that can be used for embodiments of the processes of the present
disclosure include reverse
osmosis systems, vacuum distillation systems, electrodialysis, filtration
systems, etc. The
remaining, non-purified recovered process water or brine, which includes the
highly water soluble
salts from stream 101a and potentially highly water soluble salt(s) that are
constituents of the
original tailings, (119) can be recycled back to the treatment process. For
this embodiment, at
least a portion of the non-purified recovered process water can be recycled
back to holding tank
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
24
101 and deficiency in the concentration of the salt(s) or polymer(s) can be
corrected by adding
additional highly water soluble salt(s) or polymer flocculant(s) from one or
more make-up tanks
such as make-up containers 121 and 122.
[0078] The process of the present disclosure can also include recovering
REE materials
from recycled separated process water or from the consolidated solids. The REE
materials can be
recovered from the process water by precipitation, e.g., using oxalic acid, or
extraction. Other
methods for recovering REE from the process water include mineral processing
and physical
beneficiation, deep eutectic solvents/ionic liquids extraction, acid
dissolution, high temperature
phase separations, use of REE selective sorbents, photophoresis, in-situ brine
injection and
extraction, reactive grinding, etc. The process of the present disclosure can
also include recovering
REE materials from the consolidated solids by acid leaching or caustic
decomposition.
[0079] In addition, the consolidated solids can be recovered. The
recovered consolidated
solids can include residual highly water soluble salt(s) from the treatment of
the tailings. When
the salt used in treating the tailings are beneficial to plant life, such as
an ammonium based salt or
sulfate based salt or phosphate based salt, the residual salt can act as a
fertilizer with the
consolidated solids. The recovered consolidated solids can include REE
materials which can be
separated from the consolidated solids as explained elsewhere.
[0080] Figure 1B schematically illustrates another exemplary continuous
or semi-
continuous process. For this embodiment, tailings from a metal ore, such as
tailings from copper
ore processing are illustrated. As shown in the figure, flotation cells or
concentrator (201)
generates tailings 203. Many processes of metal and even non-metal ore include
a concentration
step in which valuable minerals are concentrated by flotation in an aqueous
mixture including
various agents. The valuable minerals are separated and tailings stream is
produced. In this
particular example, the tailings can be a thickener underflow tailings stream.
Such thickener
underflow streams can still benefit from treatment with a highly water soluble
salt and optionally
additional polymer flocculant to further consolidate solids in accordance with
processes of the
present disclosure. In accordance with aspects of the present processes, the
tailings stream is
treated with a stream of an aqueous solution including at least one highly
water soluble salt. For
this embodiment, the aqueous solution also includes the at least one polymer
flocculant. As shown
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
in Figure 1B, tailings stream 203 is combined with an aqueous solution stream
including the at
least one highly water soluble salt and the at least one polymer flocculant
(202a) to produce a
treated tailings stream 207. For this embodiment, aqueous solution stream 202a
and tailings 203
are mixed in-line to produce treated tailings 207. Combining the streams (202a
and 203) produces
treated tailings that include a consolidated material in process water.
[0081] Although not shown, the tailings could have been treated with
separate streams of
the salt(s) and flocculant(s). The aqueous streams of salt(s) and polymer
flocculant(s) can be
sourced from storage 202. In certain embodiments, seawater is used as the
source of the highly
water soluble salt as a make-up source of the salt in 220. In other,
embodiments, brine from a
reverse osmosis system is used as the source of the highly water soluble salt
as a make-up source
of the salt in 220 and in still further embodiments, both seawater and brine
are used as the source
of the highly water soluble salt as a make-up source of the salt in 220.
[0082] For this embodiment, the treated tailings are carried to a
solids/liquid separator
(209). The S/L separator separates the process water of the treated tailings
from the consolidated
material. Such S/L separators include, for example, one or more of a
centrifuge, decanting
centrifuge, dewatering screw, hydrocyclone, vacuum belt filter, filter press
or pressing devices,
etc. or combinations thereof. S/L separator 209 generated a stream of
consolidated material 213
and a stream of separated process water 211. Process water stream 211 includes
the process water
from tailings stream 203 diluted with aqueous solution stream 202a and thus
includes residual
salt(s) from the one or more highly water soluble salt(s) and can possibly
include residual
polymer(s) from the one or more polymer flocculant(s). At least a portion, if
not all, of process
water stream 211 can be recovered and purified with a reverse osmosis system
215.
[0083] Reverse osmosis system 215 can concentrate the at least one highly
soluble salt in
the recovered portion of separated process water 211 to form brine 219. At
least a portion, if not
all, of the brine 219 can be cycled back to salt / polymer flocculant storage
202 to treat additional
tailings 203. Reverse osmosis system 215 can concentrate the at least one
highly soluble salt to a
concentration of greater than 2 wt% such as greater than 4 wt%, 5 wt%, 6 wt%,
7 wt%, 8 wt%, 9
wt%, 10 wt% and higher such that the salt-composition concentration in salt
/polymer flocculant
storage can be at an equilibrium of about 2 wt% to about 10 wt%, and values
therebetween, or
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
26
higher. The aqueous solution stream including the at least one highly water
soluble salt and the at
least one polymer flocculant (202a) can be combined with the tailings stream
203 stream at a ratio
range of tailings to salt solution including about 30:1 to 1:1, 20:1 to 1:1,
15:1 to 1:1, 10:1 to 1:1,
5:1 to 1:1, and/or about 2:1 to 1:1 tailings to salt solution.
[0084] Reverse osmosis system 215 can also be used to generate desalted
water 240 from
seawater 250 in which desalted water 240 is used in other processes of the
mining operation. Waste
brine from system 215 can be used as a source of the highly soluble salt to
treat tailings thereby
improving efficiency of the overall operation and reducing the adverse
environmental impact of
discharging brine into the environment. In addition, seawater can be used as a
source of the highly
water soluble salt to treat tailings such as providing seawater in make-up
source 250.
EXAMPLES
[0085] The following examples are intended to further illustrate certain
preferred
embodiments of the invention and are not limiting in nature. Those skilled in
the art will recognize,
or be able to ascertain, using no more than routine experimentation, numerous
equivalents to the
specific substances and procedures described herein.
[0086] Coal Ash Slurry Consolidation
[0087] An initial sample of a coal ash slurry was analyzed by infrared
spectroscopy to
determine the content of solids content. Further, the sample was estimated to
have 30% or more
coal fines present, i.e., a mixture of fine coal particles and fine mineral
particles. Approximately
g of the coal slurry was placed in a vial and an equal weight of an aqueous
ionic solution was
added and the diluted slurry shaken to mix the components. The aqueous ionic
solution was
composed of water, 10 wt% ammonium sulfate and 0.1 wt% polyacrylamide (PAM).
Settling
started immediately, as can be seen in the picture on the left in Figure 2.
The vial was then
centrifuged for 30 seconds at 3000 rpm and the particles consolidated into a
compact mass, as
shown in the picture in the center of Figure 2. The supernatant liquid
appeared to be clear, with no
visible suspended particles. Upon removal of the liquid it was found that the
compacted solids
have enough cohesive strength to hold their shape when the vial was inverted,
as can be seen in
the picture on the right in Figure 2.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
27
[0088] The material was removed from the vial (Figure 3, left) and a
portion dried. The
consolidated material had an initial solids content of 54%. Some of the
remainder was pressed (by
hand) between paper towels (Figure 3, right). This pressed material had a
solids content of 74%.
[0089] Varying Salt and Salt Concentration in Treating Oil Sands Tailings
[0090] Additional experiments were carried out with various highly water
soluble salts and
in different concentrations and with and without sand to treat oil sands
tailings. A series of
salt/polymer solutions were prepared. All of the salt/polymer solutions
included 0.1 wt% of
polyacrylamide (PAM) but varied the type and concentration of the salt. For
example, a series of
wt%, 5 wt% and 2 wt% calcium chloride solutions each with 0.1 wt% of PAM were
prepared
and used to treat MFT. Other 10 wt%, 5 wt% and 2 wt% salt solutions of
ammonium sulfate,
potassium chloride, etc. were prepared each with 0.1 wt% of PAM. An equal
weight of a particular
salt/polymer solution was then combined with NWT, with or without sand, in a
vial followed by
vigorous mixing. The vials were then centrifuged at 3000 rpm on a LW
Scientific laboratory
centrifuge for 30 seconds to form a consolidated material in the form of a
slurry. After
centrifugation, the supernatant liquid was separated from the consolidated
material by a pipette.
The consolidated material was then weighed, dried and reweighed to determine a
solids content of
the consolidated material. The various salts and their concentrations which
were used to treat MFT
and the resultant solids content data are summarized in Tables 1 and 2 below.
Table 1: Solids content of NWT treated with an equal weight of a salt/PAM
solution without the
addition of sand and after centrifugation.
Salt 10% Concentration' 5% Concentration2 2%
Concentration3
(+ 0.1 wt% PAM)
No Sand
Ferric Chloride (FeCl3) 34.9% 3 5 .6%
Aluminum Sulfate 3 3 . 1% 34.1%
(Al2(504)3)
Calcium Chloride 36.8% 3 7. 1% 3 5 .8%
(CaCl2)
Ammonium Sulfate 33.1% 3 1 . 8% 3 1 .4%
(NH4 SO4)
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
28
Potassium Chloride 35.4% 32.4% 33.5%
(KC1)
Table 2: Solids content of MFT treated with an equal weight of a salt/PAM
solution with the
addition of sand (SFR ratio 1:1) after centrifugation.
Salt 10% Concentration' 5% Concentration2 2%
Concentration3
(+ 0.1 wt% PAM)
With Sand
Ferric Chloride 45.7% 52.8%
(FeCl3)
Aluminum Sulfate 51.4% 53.7%
(Al2(504)3)
Calcium Chloride 58% 56.8% 56.1%
(CaCl2)
Ammonium Sulfate 53.6% 52.3% 53.5%
(NH4 SO4)
Potassium Chloride 53.4% 52.5% 53.9%
(KC1)
1. The salt-tailings concentration was about 5 wt%.
2. The salt-tailings concentration was about 2.5 wt%.
3. The salt-tailings concentration was about 1 wt%.
[0091] Table 1 reports the solids content of dried consolidated material
following treating
of MFT with the various salt/polymer solutions without sand. After
centrifugation for just 30
seconds, the highly water soluble salts gave solids contents for the
consolidated materials in a
range between about 31%-37%. However, the use of highly water soluble salts
having a
multivalent cation such as the aluminum and ferric cations appeared to cause
fouling of the vial
walls and gave a less cohesive consolidated material as compared to highly
water soluble salts
having a monovalent cation under the tested conditions. In some tests using
salt concentrations of
10%, the clarified water sitting on top of the consolidated materials were
removed using a pipette
and the wet solids pressed between paper towels. It was found that the salts
with multivalent
cations, aluminum chloride (A1C13), ferric chloride (FeCl3) and calcium
chloride (CaCl2), which
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
29
all gave significant deposits of a slimy material on the vial walls, were less
cohesive than the
pressed solids obtained using salts with monovalent cations, such as the
ammonium salts NH4C1
and (NH4)2SO4.
[0092] Table 2 reports the solids content of dried consolidated material
following treating
MFT with the various salt/polymer solutions and sand. Sand was added with a
1:1 sand to fines
ratio (i.e., 1.5 g of sand was added to the 5 gm of MFT having 30% solids to
give a 1:1 ratio of the
weight of sand to that of the solids in the MFT). After centrifugation for
just 30 seconds, the highly
water soluble salts gave solids contents for the consolidated materials in a
range between about
46%-58%, which was significantly higher than the range of solids contents
without use of sand.
Although the solids content of the vials containing added sand is twice those
without sand, the
volume of the centrifuged slurry is about the same.
[0093] The data in Tables 1 and 2 show that addition of 2 wt% salt
solution to treat MFT
was as effective as a 10 wt% salt solution. That is, a 1 wt% salt-tailings
concentration was as
effective as a 5 wt% salt-tailings concentration. Since an equal weight of the
salt/polymer solution
was used to treat MFT, the salt concentration of the added salt in the treated
tailings is one-half of
the concentration in the salt/polymer solution, i.e., the added 2 wt% salt
solution provided a 1 wt%
salt-tailings concentration and the 10 wt% salt solution provided a 5 wt% salt-
tailings
concentration. The salt-tailings concentration in treated MFT can be achieved
in a number of
ways. For ease of handling in the foregoing vial tests, it was convenient to
combine equal weights
of salt/polymer solutions to MFT. However, smaller amounts of salt/polymer
solutions with higher
concentrations thereof to give the same salt-tailings concentration give
equivalent results of
consolidated materials.
[0094] Centrifuging in flat-bottomed vials is not as effective in terms
of producing a high
solids material as using centrifuge tubes. It should be kept in mind that for
all sets of laboratory
vial and tube tests, there is always solution remaining in the voids between
the particles. It will be
shown later that the solids content of the consolidated material can easily be
increased from the
46%-58% range by simply draining or the use of mechanical dewatering methods
known to the
art, such as filter presses, belt filters, cross-flow filtering, dewatering
sand screws, decanting
centrifuges, hydrocyclones, etc.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
[0095] Varying Salt Concentration and Polymer Concentration in Treating
Oil Sands
Tailings
[0096] When salt, polymer and sand are used together, salt-tailings
concentrations in
excess of 0.5 wt% and preferably no less than about 0.70 wt%, such as at least
about 1 wt%, should
be used to achieve reasonably fast consolidation of the solids in the
tailings. In addition, although
a degree of consolidation of the fines/sand mixture is obtained at polymer-
tailings concentrations
as low as 0.01 wt% for relatively short processing times, superior results are
obtained at polymer-
tailings concentrations of 0.05% and higher. These preferences were determined
by a set of vial
experiments. The top set of vials in Figure 4 shows results obtained by adding
5 g of a 2 wt%
ammonium sulfate ((NH4)2504) solution containing PAM to 5 g of MFT. Sand was
also added to
give a sand-to-fines ratio of 1:1 (i.e., 1.5 g of sand was added). The amount
of PAM in the solutions
was varied between 0.1% (by weight) and 0.02% (by weight). The bottom set of
vials show what
is observed when a 1 wt% of the ammonium sulfate was used. The vials were
centrifuged at 3000
rpm for 30 seconds to accelerate settling.
[0097] It can be seen that for all the vials treated with the 1 wt%
(NH4)2504 solutions,
there is a degree of settling of the fines and sand, but the supernatant
liquid contains a significant
amount of suspended particles. In addition, visually there appears to be a
degree of segregation of
the sand and fines. In contrast, the MFT treated with a 2 wt% (NH4)2504
solution containing 0.1
wt% PAM showed settled and compacted solids in contact with a clear
supernatant. As the amount
of polymer in the solution is reduced from vial A4 to E4, the clarity of the
supernatant decreases,
as more suspended particles remain in the liquid phase. Greater clarity of the
supernatant liquid
should be achievable at longer centrifuge times, but for short processing
times, treating MFT to
result in a salt-tailings concentration of no less than about 0.5 wt% and a
polymer-tailings
concentration of no less than about 0.04 wt% are preferable.
[0098] The solids contents of the consolidated materials in each of the
vials shown in
Figure 4 was determined by drying, i.e., the centrifuged consolidated material
was separated from
its supernatant liquid, the wet mass weighed, dried and reweighed to determine
a solids content.
The solids content of the consolidated materials for the sets of vials are
summarized in Table 3.
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
31
Table 3: The solids content of centrifuged ammonium sulfate/PAM treated NWT as
determined
by separating and drying consolidated material.
0.1% PAM 0.08% PAM 0.06% PAM 0.04% PAM 0.02% PAM
% Solids % Solids % Solids % Solids % Solids
2% (NH4)2504 60.3% 58.8% 58.1% 52.0% 48.5%
1% (NH4)2504 54.4% 57.2% 58.1% 56.3% 44.6%
[0099] It can be seen that for the 2 wt% (NH4)2SO4 solution containing
0.1 wt% PAM, a
solids content of just over 60% was achieved. This decreased only slightly
when treating MFT
with solutions including PAM concentrations of 0.08 wt% and 0.06 wt%, but
significantly at lower
PAM concentrated solutions. Treating MFT with an equal weight of the (NH4)2504
/ polymer
solutions resulted in a salt-tailings concentration of about 1 wt% for each of
vials A4-E4, and for
vial A4, a polymer-tailings concentration of about 0.05 wt% PAM, for vial B4 a
polymer-tailings
concentration of about 0.04 wt% PAM, for vial C4 a polymer-tailings
concentration of about 0.03
wt% PAM, for vial D4 a polymer-tailings concentration of about 0.02 wt% PAM,
and for vial E4
a polymer-tailings concentration of about 0.01% PAM. For the 1 wt% (NH4)2504
solutions, the
solids content was very variable, reflecting the problems with segregation of
coarse and fine
particles in the consolidated materials in these experiments.
[00100] Increased Salt Concentration Allows for Lower Polymer
Concentration
[00101] When salt, polymer and sand are used together to treat tailings,
it was observed that
the polymer-tailings concentration can be reduced if the salt-tailings
concentration is increased
under certain circumstances. Thus, very low polymer-tailings concentration can
achieve
reasonably fast consolidation of solids in the tailings if the salt-tailings
concentration is increased.
Figure 5 illustrates that as the salt concentration increases, less polymer
flocculant is needed to
obtain clear supernatant solutions. For these tests, the polymer-tailings
concentration increases
from 0.01% to 0.05% in 0.01% increments from right to left while the salt-
tailings concentration
increases from 1% to 2% from top to bottom.
[00102] Varying Polymer Concentration in Treating Oil Sands Tailings with
Seawater
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
32
[00103] For these experiments, solutions of seawater (sourced from the
U.S. eastern shore
of the Atlantic Ocean) were prepared with various concentrations of a nonionic
polyacrylamide
(available from SNF as FA920) between 0.1% (by weight) and 0.02% (by weight).
The
concentration of highly soluble salts in the seawater is believed to be
greater than 3 wt%. The
seawater-polymer solutions were used to treat MFT from oil sands processing.
An equal amount
of seawater ¨polymer solution was used to treat MFT (about 5 g of seawater
¨polymer solution to
about 5 g of MFT) in a vial. The treated mixtures were first stirred and then
the vials were
centrifuged at 3000 rpm for 30 seconds to accelerate settling. The results are
shown in the picture
of Figure 6. From left to right, the seawater used to treat the MFT included
about 0.1 wt%, 0.08
wt%, 0.06 wt%, 0.04 wt% and 0.02 wt% of the polymer flocculant, respectively.
These
experiments show that a mixture of highly soluble salts sourced from an ocean
can be used in the
process of the present disclosure.
[00104] Treating Tailings from Copper Ore processing
[00105] A sample of tailings generated from processing copper ore was
combined with an
approximate equal amount of an aqueous ionic solution in a 500 ml beaker. The
aqueous ionic
solution comprised water, a highly water soluble salt and flocculant
(polyacrylamide (PAM)).
Upon combining and slightly mixing the tailings with the aqueous ionic
solution, the solids started
to consolidate almost immediately. After standing for just a few minutes, the
sold aggregated and
settled to the bottom with a clarified water layer above the consolidated
solids. See Figure 7A.
The consolidated solids were also cohesive. Consolidated material taken from
the beaker was
readily compressed by hand to form a ball (see Figure 7B). A step test, in
which the hand
compacted ball shown in Figure 7B was placed between paper towels and stepped
on by a tester,
showed that the consolidated material does not foul paper towels (See Figure
7C). Such a test is
an indication of how readily the consolidated material can be filtered and
mechanically dewatered
in which a low fouling indicates good filterability and mechanical dewatering.
The solids content
of the pressed tailings was measured to be about 75%.
[00106] Only the preferred embodiment of the present invention and
examples of its
versatility are shown and described in the present disclosure. It is to be
understood that the present
invention is capable of use in various other combinations and environments and
is capable of
CA 03106360 2021-01-12
WO 2020/018397 PCT/US2019/041751
33
changes or modifications within the scope of the inventive concept as
expressed herein. Thus, for
example, those skilled in the art will recognize, or be able to ascertain,
using no more than routine
experimentation, numerous equivalents to the specific substances, procedures
and arrangements
described herein. Such equivalents are considered to be within the scope of
this invention, and are
covered by the following claims.