Note: Descriptions are shown in the official language in which they were submitted.
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
CATALYTIC ACTIVATION OF ISOPENTANE-ENRICHED MIXTURES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application is a PCT International application which claims the
benefit of and
priority to U.S. Provisional Application Serial No. 62/648,570 filed March 27,
2018 and U.S.
Application Serial No. 16/364,737 filed March 26, 2019, titled "Catalytic
Activation of
Isopentane-Enriched Mixtures", U.S. Provisional Application Serial No.
62/648,557 filed March
27, 2018, titled "Catalytic Activation of Isopentane-Enriched Mixtures" and
U.S. Application
Serial No. 16/364,764 filed March 26, 2019, titled "Catalytic Activation and
Oligomerization of
Isopentane-Enriched Mixtures", U.S. Provisional Application Serial No.
62/648,584 filed March
27, 2018, titled "Catalytic Activation of Isopentane-Enriched Mixtures" and
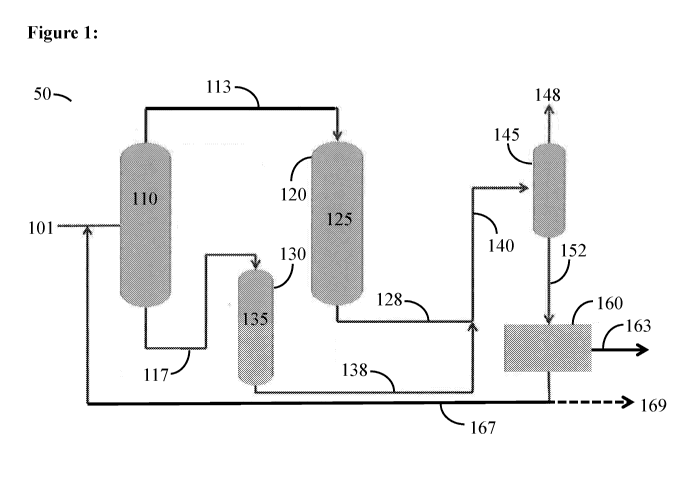
U.S. Application
Serial No. 16/364,799 filed March 26, 2019, titled "Catalytic Activation and
Alkylation of
Isopentane-Enriched Mixtures", U.S. Provisional Application Serial No.
62/648,592 filed March
27, 2018, titled "Catalytic Activation of Isopentane-Enriched Mixtures", and
U.S. Application
Serial No. 16/364,836 filed March 26, 2019, titled "Systems for Catalytic
Activation of
Isopentane-Enriched Mixtures" which are hereby incorporated by reference in
its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
[0002] None.
FIELD OF THE INVENTION
[0003] The present disclosure relates to processes and systems for
separating isopentane
from a feed stream comprising both isopentane and n-pentane, separately
activating each isomer
to maximize the yield of olefins and aromatics, then optionally further
converting to chemicals
or liquid hydrocarbon transportation fuels in a subsequent step by alkylation
or oligomerization.
BACKGROUND
[0004] A large surplus of pentanes are available in the petroleum refining
industry, arising
predominantly from the increased production of light hydrocarbons from U.S.
shale formations,
and also from limits on the quantity of volatile components that can be
blended into finished
transportation fuels, which must adhere to regulations on minimum vapor
pressure.
1
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
Unfortunately, conventional processes for upgrading light alkanes to value-
added products are
not well-suited for hydrocarbon feed streams that primarily comprise pentanes
(i.e., isopentane
and n-pentane). Therefore, it would be beneficial to find improved processes
and systems for
efficiently converting pentanes to more valuable products, including
transportation fuels and
chemicals, while minimizing the production of C1-C4 light paraffins.
[0005] The inventive processes disclosed herein provide an improved
upgrading route for
pentane-rich mixtures, such as fuel blend stocks and other pentane-rich
streams that do not meet
government fuel specifications. The inventive processes and systems provide
enhanced yields of
upgraded products that may be suitable for use as transportation fuels or
other value-added
chemical products.
BRIEF SUMMARY OF THE DISCLOSURE
[0006] The present inventive disclosure relates to methods and systems for
converting a
mixture predominantly comprising isopentane (i-05) and n-pentane (n-05)
isomers into products
that can be used as a liquid transportation fuel, or a blend component
thereof. Certain
embodiments comprise a method for converting a feed stream comprising pentanes
to produce a
liquid transportation fuel, comprising: a) providing a hydrocarbon feed stream
comprising at
least 50 wt.% pentanes, including both n-pentane and isopentane, wherein the
hydrocarbon feed
stream further comprises less than 10 wt.% of hydrocarbons containing four or
fewer carbons; b)
at least partially separating various constituents in the hydrocarbon feed
stream according to
each constituent's characteristic vapor pressure, to produce: a first fraction
comprising at least
80% of the isopentane present in the feed stream and at least 90% of
hydrocarbons present in the
hydrocarbon feed stream that are characterized by a vapor pressure equal to or
greater than the
vapor pressure of isopentane; a second fraction that comprises at least 80% of
the n-pentane
present in the hydrocarbon feed stream and at least 90% of any hydrocarbons
containing six or
more carbons that were present in the hydrocarbon feed stream; c) contacting
the first fraction
with a first activation catalyst at conditions comprising a first temperature
and first pressure that
facilitate conversion of at least a portion of the first fraction by the first
activation catalyst to a
first effluent comprising olefins containing from two to five carbon atoms,
monocyclic aromatics
and unconverted alkanes containing from two to five carbon atoms; d)
contacting at least a
2
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
portion of the second fraction with a second activation catalyst at a second
temperature and
second pressure that facilitate conversion of at least a portion of the second
fraction by the
second activation catalyst to produce a second effluent comprising olefins
containing from two
to five carbon atoms, monocyclic aromatics and unconverted alkanes containing
from two to five
carbon atoms, wherein the first temperature is lower than the second
temperature, optionally at
least 25 C lower, wherein the first activation catalyst optionally differs
from the second
activation catalyst in at least one of chemical composition and structural
composition, wherein
the weight hourly space velocity of the first fraction as it contacts the
first activation catalyst is
optionally higher than the weight hourly space velocity of the second fraction
as it contacts the
second activation catalyst; e) combining at least a portion of the of the
first effluent and the
second effluent to produce a mixed effluent; and f) at least partially
condensing the mixed
effluent to produce a mixed liquid hydrocarbons fraction and an uncondensed
light hydrocarbons
fraction, wherein at least 80 wt.% of the the mixed liquid hydrocarbons
fraction comprises
pentene, single-ring aromatics and unreacted alkanes containing at least five
carbon atoms,
wherein the light hydrocarbons fraction comprises hydrogen and at least 80
wt.% of
hydrocarbons containing four or fewer carbon atoms.
[0007] Certain embodiments further comprise separating the mixed liquid
hydrocarbons into
an aromatics fraction and an unreacted C5/C6 hydrocarbons fraction, wherein
the aromatics
fraction comprises monocyclic aromatics and the unreacted C5/C6 hydrocarbons
fraction
comprises alkanes and olefins containing from five to six carbons that is
optionally mixed with
the hydrocarbon feed stream.
[0008] In some embodiments, at least a portion of the mixed effluent is
contacted with an
alkylation catalyst at conditions of temperature and pressure that facilitate
the conversion of the
mixed effluent to an alkylation effluent comprising an increased mol% of mono-
alkylated
aromatics containing from seven to nine carbon atoms, wherein the alkylation
effluent is
separated to produce a heavy hydrocarbons fraction comprising at least 80
wt.%, wherein the
heavy hydrocarbons fraction is separated to produce an aromatics fraction
comprising aromatic
hydrocarbons containing at least seven carbon atoms, and a recycle fraction
comprising benzene,
alkanes and olefins containing from five to six carbons hydrocarbons that
contain at least five
carbons, and a light hydrocarbons fraction comprising at least 80 wt.%
hydrocarbons that
contain four or fewer carbons.
3
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
[0009] In some embodiments, at least a portion of the mixed effluent is
contacted with an
oligomerization catalyst at conditions of temperature and pressure that
facilitate the conversion
of the mixed effluent to an oligomerization effluent comprising an increased
mol% of aliphatic
hydrocarbons containing from six to nine carbon atoms, wherein the
oligomerization effluent is
separated into a condensable liquid hydrocarbons comprising at least 80 wt.%
hydrocarbons that
contain at least five carbons, and a light hydrocarbons fraction comprising at
least 80 wt.%
hydrocarbons that contain four or fewer carbons, wherein the condensable
liquid hydrocarbons is
separated to produce an aliphatic hydrocarbon fraction predominantly
comprising aliphatic
hydrocarbons containing from six to nine carbon atoms, and a recycle fraction
predominantly
comprising alkanes and olefins containing from four to six carbon atoms.
[0010] Some embodiments further comprise adding a diluent to at least one
of: the first
fraction prior to the contacting of c) and the second fraction prior to the
contacting of d), wherein
the first activation catalyst is less likely to react with the diluent than
the first fraction at the
conditions of temperature and pressure that are maintained in the first
reactor, wherein the
second activation catalyst is less likely to chemically react with the diluent
than the second
fraction at the conditions of temperature and pressure that are maintained in
the second reactor.
[0011] In some embodiments, the diluent is added in an amount that is
effective to produce a
mixed effluent comprising a ratio of olefins to aromatics in the range from
0.5 to 2.0, wherein
the diluent is optionally selected from methane, ethane, propane, butanes,
benzene, toluene,
xylenes, alkyl- or dialkyl-benzenes, naphthenes, C2 - C5 olefins, and
combinations thereof.
[0012] Certain embodiments comprise a system operable to convert a
hydrocarbon feed
stream comprising pentanes to produce a liquid transportation fuel, the system
comprising: a) a
hydrocarbon feed stream comprising at least 50 wt.% pentanes, including both n-
pentane and
isopentane, wherein the hydrocarbon feed stream further comprises less than 10
wt.% of
hydrocarbons containing four or fewer carbons; b) a first separator operable
to at least partially
separate constituents in the hydrocarbon feed stream according to the
characteristic vapor
pressure of each constituent to produce: a first fraction comprising at least
80% of the isopentane
present in the feed stream, and at least 90% of hydrocarbons present in the
hydrocarbon feed
stream that are characterized by a vapor pressure equal to or greater than the
vapor pressure of
isopentane; a second fraction that comprises at least 80% of the n-pentane
present in the
hydrocarbon feed stream and at least 90% of any hydrocarbons containing six or
more carbon
4
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
atoms that were present in the hydrocarbon feed stream; c) a first reactor
containing a first
activation catalyst, the first reactor operable to receive the first fraction
and facilitate contact
between the first fraction and the first activation catalyst, wherein the
first reactor is further
operable to maintain an internal temperature and an internal pressure that
facilitate the catalytic
conversion of at least a portion of the first fraction by the first activation
catalyst to produce a
first effluent comprising containing from two to five carbon atoms, monocyclic
aromatics and
unconverted alkanes containing from two to five carbon atoms; d) a second
reactor containing a
second activation catalyst, the second reactor operable to receive the second
fraction and
facilitate contact between the second fraction and the second activation
catalyst, wherein the
second reactor is further operable to maintain an internal temperature and an
internal pressure
that facilitate the catalytic conversion of at least a portion of the second
fraction by the second
activation catalyst to produce a second effluent comprising containing from
two to five carbon
atoms, monocyclic aromatics and unconverted alkanes containing from two to
five carbon
atoms, wherein the system is further operable to maintain the first reactor at
a temperature that is
lower than the temperature maintained in the second reactor; e) a condenser
operable to receive
and mix at least a portion of the of the first effluent with at least a
portion of the second effluent,
the condenser further operable to condense at least a portion of the resulting
mixed activation
effluent to produce a mixed liquid hydrocarbons and a gas-phase mixed
effluent, the condenser
comprising a first outlet operable to allow egress of the mixed liquid
hydrocarbons and a second
outlet to allow egress of the mixed effluent; f) a second separator operable
to receive and split
the mixed effluent into a heavy hydrocarbons fraction comprising at least 50
wt.% hydrocarbons
containing at least five carbon atoms, including olefins, monocyclic aromatics
and unreacted
molecules from the hydrocarbon feed stream and a light hydrocarbons fraction
comprising
hydrogen and at least 80 wt.% hydrocarbons containing from one to four carbon
atoms; g) a
third separator operable to separate the heavy hydrocarbons fraction to
produce a liquid
hydrocarbon product fraction comprising the liquid hydrocarbon product
aromatics fraction
comprising aromatic hydrocarbons containing at least six carbon atoms, and an
olefins fraction
comprising alkanes and olefins containing from five to six carbon atoms.
[0013] Certain embodiments of the system also comprise a third reactor
containing at least
one oligomerization catalyst, the third reactor operable to receive the mixed
effluent of part e)
and facilitate contact between the mixed effluent and the oligomerization
catalyst at a
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
temperature and a pressure that are suitable to facilitate catalytic
conversion of the mixed
effluent to a third reactor effluent comprising an increased weight percentage
of hydrocarbons
containing at least five carbon atoms (C5+), wherein the second separator is
operable to receive
the third reactor effluent instead of the mixed effluent.
[0014] Certain alterative embodiments of the system also comprise a third
reactor containing
at least one alkylation catalyst, the third reactor operable to receive the
mixed effluent of part e)
and facilitate contact between the mixed effluent and the alkylation catalyst
at a temperature and
a pressure that facilitate catalytic conversion of the mixed effluent to a
third reactor effluent
comprising an increased mol% of mono-alkylated aromatics containing from seven
to nine
carbon atoms, wherein the second separator is operable to receive the third
reactor effluent
instead of the mixed effluent.
[0015] In some embodiments of the system, the second separator separates
the third reactor
effluent into a light hydrocarbons fraction comprising at least 80 wt.%
hydrocarbons that contain
from one to four carbon atoms and hydrogen, and a heavy hydrocarbons fraction
comprising 80
wt. % hydrocarbons that contain at least five carbon atoms.
[0016] Some embodiments of the system further comprise a fourth separator
operable to
receive and separate the light hydrocarbons fraction to produce hydrogen gas
and a light
paraffins stream predominantly comprising paraffins containing from one to
four carbon atoms.
[0017] Some embodiments of the system further comprise a conduit operable
to receive and
convey at least a portion of the light paraffins stream to mix with at least
one of the first fraction
and the second fraction at a location that is upstream from at least one of
the first activation
catalyst and the second activation catalyst.
[0018] In certain embodiments, adding the diluent alters the specificity of
at least one of the
first activation catalyst and the second activation catalyst to increase the
production of olefins,
decrease the production of aromatics, or combinations thereof, thereby
increasing the ratio of
olefins to aromatics in the mixed effluent. In certain embodiments, the
diluent is added in an
amount that is effective to produce a mixed effluent comprising an olefins to
aromatics ratio in
the range of 0.5 to 2.0, optionally in the range from 0.5 to 1.5, optionally
in the range from 1.0 to
1.5, optionally in the range of 0.5 to 1Ø In certain embodiments, the
diluent is selected from
6
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
methane, ethane, propane, butanes, benzene, toluene, xylenes, alkyl- or
dialkyl-benzenes,
naphthenes, C2 - C5 olefins, and combinations thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
[0019] A more complete understanding of the present invention and benefits
thereof may be
acquired by referring to the follow description taken in conjunction with the
accompanying
drawings in which:
[0020] Figure 1 is a diagram depicting a first embodiment of the inventive
processes and
systems.
[0021] Figure 2 is a diagram depicting a second embodiment of the inventive
processes and
systems.
[0022] Figure 3 is a diagram depicting a third embodiment of the inventive
processes and
systems.
[0023] Figure 4 is a bar graph depicting product selectivity resulting from
catalytic
activation of either n-pentane or iso-pentane at two different temperatures.
[0024] Figure 5 is a bar graph showing the effect of methane diluent on the
total conversion
of the feed stream, as well as the selectivity of the conversion toward light
olefins, aromatics,
and byproduct C1-C4 light paraffins.
[0025] Figure 6 is a bar graph showing the effect of methane diluent on the
total conversion
of the feed stream, as well as the selectivity of the conversion toward light
olefins, aromatics,
and byproduct C1-C4 light paraffins.
[0026] The invention is susceptible to various modifications and
alternative forms, specific
embodiments thereof are shown by way of example in the drawings. The drawings
may not be to
scale. It should be understood that the drawings are not intended to limit the
scope of the
invention to the particular embodiment illustrated.
DETAILED DESCRIPTION
[0027] The present disclosure provides processes to convert a mixture of
light hydrocarbons
to liquid transportation fuels. The process and systems described herein
relate primarily to the
conversion of any hydrocarbon mixture that predominantly comprises pentanes to
generate
7
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
upgraded products that may be sold as a value-added chemical or utilized as a
blend component
of a liquid transportation fuel.
[0028] Generally speaking, the inventive processes and systems described
herein utilize a
feed stream comprising both isopentane and n-pentane, and perform at least a
partial separation
of these pentane isomers to generate a first fraction that is enriched in
isopentane and smaller
hydrocarbons, and a second fraction that largely retains n-pentane and any C6
or larger (C6+)
hydrocarbons that were present in the original feed stream. The two streams
are catalytically
activated in separate activation reactors. For each fraction, the temperature
and/or pressure of the
fraction (measured at the inlet of its respective activation reactor) is
maintained at a value that
maximizes the catalytic conversion of that fraction to olefins and aromatics,
while minimizing
the production of C1-C4 light paraffins. In certain embodiments, the
temperature and/or pressure
that is maintained in each of the two reactors may be different, based upon
the relative reactivity
of each fraction to catalytic activation. The resulting first effluent and
second effluent are
combined, then optionally further upgraded by in a third reactor by
oligomerization and/or
alkylation to produce value-added chemicals and/or products suitable for use
as a liquid
transportation fuel blend component.
[0029] The present inventive processes and systems take advantage of the
differing
reactivity of pentane isomers to catalytic activation. Isopentane (i-05)
exhibits catalyst-
dependent reactivity that is typically different from n-pentane (n-05), and
the optimal reactor
conditions for the two isomers are therefore distinct. Experimentally,
isopentane (i-05) is more
reactive than n-pentane (n-05), and thus, can be activated at lower
temperatures while
maintaining high yields of desired products (such as olefins and aromatics)
and decreasing the
yield of C1-C4 paraffins. The inventive system allows activation conditions to
be individually
tailored for each pentane isomer in a way that cannot be achieved via
processes that upgrade a
mixed pentane stream. Additional advantages will become evident from the
detailed disclosure
provided below.
[0030] As mentioned, the feed stream generally comprises a stream of light
hydrocarbons
that comprises a mixture of pentane isomers (C5), although certain embodiments
may
additionally comprise C1-C4 hydrocarbons, C6-C7 hydrocarbons, or both. In
certain
embodiments, the feed stream comprises at least 10 wt.% of a mixture of
pentane isomers;
optionally, at least 20 wt. %, at least 30 wt.%, at least 40 wt.%, at least 50
wt.%, at least 60
8
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
wt.%, or at least 70 wt.%. of a mixture of pentane isomers. In certain
embodiments, the feed
stream may be obtained by processing a stream of natural gas liquids to remove
lighter
components (i.e., C1-C4) by way of conventional natural gas processing
technologies that are
well-characterized, such as de-methanizer, de-ethanizer, de-propanizer and de-
butanizer
fractionation columns. A typical result of such processes is commonly
characterized as natural
gasoline, comprising about 72 wt.% pentanes, with the remainder mostly
comprising C6.
[0031] A first embodiment of the inventive processes and systems is
illustrated by the
process flow-diagram of Figure 1. A pentane-rich feed stream 101 comprising
both n-pentane
and isopentane is converted in a system 50. The feed stream 101 is received by
a first separation
unit 110 that operates to separate the feed stream 101 into a first fraction
113 that comprises an
increased wt. % of isopentane (i-05) relative to the feed stream 101, and a
second fraction 117
that comprises an increased wt. % of n-pentane relative to the feed stream
101. The second
fraction further comprises a large majority of any C6 and larger (C6+)
hydrocarbons that were
originally present in the feed stream 101. The first separation unit 110 may
operate using
conventional separation technology to separate the i-05 isomer from the
remaining compounds
present within the CS-rich hydrocarbon feed stream. Alternatively, any other
conventional
separation technology may be used to assist in separating i-05 from n-05 to
produce the first
fraction and the second fraction.
[0032] Following separation, the first fraction 113 is conveyed to a first
activation reactor
120 containing a first activation catalyst 125, while the second fraction 117
is introduced into a
second reactor 130 containing a second activation catalyst 135. Each of the
first and second
rectors, respectively, is operable to maintain a temperature and pressure that
is suitable to
facilitate conversion of the first fraction 113 to a first effluent 128, and
the second fraction 117
to a second effluent 138. Speaking generally, the first and second effluent
each comprise
products that may be utilized as a commodity chemical, an intermediate
amenable to further
catalytic upgrading, or a transportation fuel (or a component thereof). Each
activation catalyst
may comprise a single catalyst, or a mixture of different catalysts that
contact the alkanes present
in a given feed stream and facilitates at least one of dehydrogenation,
cracking, and
aromatization of the alkanes, thereby producing upgraded products including
olefins and
aromatics.
9
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
[0033] Speaking generally, each activation catalyst may comprise any
catalyst capable of
cracking and/or aromatizing hydrocarbons. Favored catalysts include supported
or unsupported
solid acids, metals, metal chalcogenides, or metal pnictogenides, including
(but not limited to)
structured and amorphous silica-aluminas, structured and amorphous solid
phosphoric acids,
clays, other metal oxides, metal sulfates, or metal phosphates, and graphite-
supported materials.
In certain embodiments, ZSM-5 zeolite catalysts are utilized that are
characterized by Si/A1
ratios ranging from 12-80, optionally ranging from 35 to 50. Optionally, one
or more elements
may be impregnated on the zeolite catalyst, including one or more of Ga, Pt,
Ni, Mn, Mg, Fe, Cr,
P, Cu, La, Sr and F.
[0034] Optionally, the activation catalyst utilized in the first catalytic
activation zone is
different from the activation catalyst utilized in the second catalytic
activation zone. Generally
speaking, dehydrogenation catalysts are not a prerequisite of paraffin
activation in the inventive
process. A sufficient concentration of intermediate olefins can be generated
through a
combination of thermal dehydrogenation and catalytic cracking such that
typical
dehydrogenation metals (such as platinum, zinc, molybdenum, or gallium) can be
avoided
without significantly decreasing product yield. Known dehydrogenation
catalysts are prone to
fouling by sulfur and nitrogen contaminants that are often present in
hydrocarbon feed streams
derived from petroleum, so the ability to operate in the absence of sensitive
catalytic materials is
highly advantageous.
[0035] The inventive process generally takes advantage of the large
difference in catalytic
reactivity between n-05 and i-05. For example, over a solid acid catalyst at
temperature in
excess of 550 C, the measured activation rates differ by up to 4 fold in favor
of i-05, when each
isomer is contacted with the same catalyst under identical conditions (even in
the same reactor
simultaneously). Thus, an at least partial separation of the mixed pentanes
feed stream to form a
first fraction that largely comprises i-05 and a second fraction that retains
a large majority of the
n-05 in the feed stream, followed by separately activating each fraction in a
distinct catalytic
activation zone, allows each pentane isomer to be upgraded with an optimal
catalyst and
conditions of temperature and pressure that maximize the yield of value-added,
upgraded
products (such as olefins and/or aromatics). Separation of the isomers also
helps to minimize
selectivity to C1-C4 light paraffins.
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
[0036] Table 1 (below) illustrates the difference in the activation
reactivity of i-05 versus n-
05 over a microporous silica-alumina catalyst. Feed streams comprising either
100 wt.% i-05 or
100 wt.% n-05 were each catalytically activated in separate experiments
utilizing temperatures
of either 600 C or 550 C. The conversion and product distribution for i-05 are
shown in Table
1, columns 2 and 3, while similar results for the activation of n-05 are shown
in Table 1,
columns 4 and 5. The data shows that when comparing the activation of pentane
isomers,
conversion of i-05 to olefins and aromatics is possible at a temperature about
50 C less than is
required for equivalent conversion of n-05. To be clear, we observed that
activation of the i-05
feed stream at 550 C converted about the same weight percentage of the feed
stream as did
activation of n-05 at 600 C using the same WHSV. Further, utilizing a
decreased temperature of
550 C for activation of the i-05 feed stream advantageously decreased the
production of C1-C4
light paraffins from 21.0% to 19.4% by increasing the product distribution
toward olefins rather
than aromatic products. Thus, the ability to separate the i-05 isomer from n-
05 isomer (and any
C6+ hydrocarbons) and activate the i-05 enriched mixture at relatively reduced
temperature,
results in approximately equivalent total conversion of the overall feed
stream, while decreasing
the formation of undesired C1-C4 light paraffins.
[0037] Referring again to the embodiment illustrated in Figure 1, the first
fraction 113 enters
an inlet of the first activation reactor 125. The temperature within the first
activation reactor 125
(typically measured at the inlet of the first activation reactor) is
maintained in the range from
500 C to 650 C; optionally, within the range from 525 C to 625 C; optionally,
within the range
from 525 C to 600 C; optionally, within the range from 550 C to 600 C;
optionally, within the
range from 550 C to 575 C.
[0038] The second fraction 117 enters an inlet of the second reactor 135.
The temperature
within the second reactor 135 (typically measured at the inlet of the second
reactor) is
maintained in the range from 500 C to 650 C. Optionally, the temperature is
maintained within
the range from 525 C to 650 C. Optionally, the temperature is maintained
within the range from
550 C to 625 C. Optionally, the temperature is maintained within the range
between 575 C and
600 C. Lower pressure favors the conversion of light alkanes to light olefins.
Thus, the first
activation reactor 125 and second reactor 135 are generally maintained at a
pressure ranging
11
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
from 15 psia to 165 psia. In certain embodiments, the pressure is maintained
in the range from
15 psia to 50 psia.
[0039] In certain embodiments, the temperature maintained within the first
activation reactor
(first temperature, typically measured at the inlet of the first activation
reactor) is lower than the
temperature maintained at the inlet of the second activation reactor (second
temperature,
typically measured at the inlet of the second activation reactor). Optionally,
the first temperature
may be at least 10 F lower, at least 20 F lower, at least 25 F lower, at least
30 F lower, at least
35 F lower, at least 40 F lower, at least 45 F lower or at least 50 F lower
than the second
temperature. Utilizing a lower temperature for the conversion of the first
fraction 113 allows
approximately equivalent conversion to upgraded products that may be blended
into liquid
transportation fuel or sold as chemical feed streams, while minimizing the
production of
undesirable light hydrocarbons containing four or fewer carbons.
[0040] Referring again to the embodiment depicted in Figure 1, the first
effluent 128 is
combined with the second effluent 138 to form a mixed activation effluent 140
that
predominantly comprises C2-05 olefins, single-ring aromatics, hydrogen and
unreacted alkanes
that were present in hydrocarbon feed stream 101. Speaking generally, Table 1
details the
molecular profile for examples of either a first effluent (comprising i-05)
and a second effluent
(comprising n-05) each being activated at temperatures of 550 C and 600 C,
respectively.
12
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
Table 1. Product distributions for i-05 or n-05 isomer feed streams following
conversion by a
1/8" extrudate consisting of 50 wt.% alumina binder and 50 wt.% ZSM-5 zeolite.
Activation was
performed by contacting the ZSM-5 catalyst with a feed stream comprising
either 100 wt.% of i-
05 or 100 wt % of n-05. Results were time-averaged over 16 hours and all
reactions were
performed at 1 atm with a WHSV = 4.0 hfl.
Feed Isomer: i-05 i-05 n-05 n-05
Inlet Temperature: 600 C 550 C 600 C 550 C
Conversion (wt.%): 94.5 82.4 78.5 48.3
Product Distribution (wt.%)
Hydrogen 2.4% 1.6% 1.1% 0.4%
Methane 9.8% 7.8% 5.3% 2.3%
Ethane 3.0% 2.6% 11.5% 6.6%
Ethylene 17.4% 15.6% 14.3% 7.7%
Propane 5.3% 4.8% 10.6% 9.9%
Propylene 21.2% 22.1% 16.4% 10.3%
Butane 2.9% 4.2% 0.8% 1.2%
Butene 8.5% 9.9% 5.8% 5.5%
Isopentane 5.5% 17.6% 0.1% 0%
n-Pentane 0% 0% 21.5% 51.7%
Pentene 1.2% 1.8% 0.8% 1.2%
C6+ alkanes 0.0% 0.3% 0.0% 0.0%
Benzene 4.8% 3.3% 4.9% 1.0%
Toluene 11.1% 6.0% 5.3% 1.6%
Xylene 6.4% 2.2% 1.3% 0.6%
EB 0.3% 0.1% 0.1% 0.0%
Coke 0.2% 0.1% 0.2% 0.1%
[0041] Referring again to the embodiment depicted in Figure 1, the mixed
activation effluent
140 is conveyed into a second separator 145 that separates hydrogen and light
hydrocarbons 148
containing four or fewer carbons from a mixed liquid hydrocarbons 152 that
predominantly
comprises C5 olefins, single-ring aromatics as well as unreacted pentanes and
larger C6+
components present in the hydrocarbon feed stock 101. In certain embodiments,
the second
separator 145 is a two-phase splitter and separation of the mixed activation
effluent 140 is
achieved by partial condensation. The light hydrocarbons 148 can be either
combusted for heat
generation or diverted to other upgrading processes that are outside the scope
of this disclosure.
13
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
The mixed liquid hydrocarbons 152 is next conveyed to a conventional naphtha
stabilizer 160
that separates the mixed liquid hydrocarbons 152 into an aromatics fraction
163 (predominantly
comprising aromatics) and an unreacted C5/C6 components fraction 167 that
predominantly
comprises unreacted pentanes and larger C6+ components. The unreacted C5/C6
components
fraction 167 may optionally be recycled and reintroduced upstream from the
first separator 110
or utilized directly as a gasoline blend component 169.
[0042] Certain embodiments of the inventive processes and systems convey a
mixed effluent
(derived by mixing at least a portion of the effluent from the first and
second activation reactors)
to an oligomerization reactor containing at least one oligomerization
catalyst. The mixed effluent
contacts the oligomerization catalyst and is converted to larger hydrocarbon
products that can be
utilized as a component of a liquid transportation fuel, such as, but not
limited to: gasoline,
diesel and jet fuel.
[0043] A second embodiment of the inventive processes and systems that
includes an
oligomerization reactor and additional inventive features is illustrated by
the process flow-
diagram of Figure 2. A feed stream 202 comprising both n-05 and i-05 is
converted in a system
200. The feed stream 202 is received by a first separation unit 210 that is
operable to separate the
feed stream 202 into a first fraction 213 that comprises an increased wt.% of
isopentane (i-05)
relative to the feed stream 202, and a second fraction (i.e., n-05/C6+
fraction) 217 that
comprises an increased wt.% of n-pentane (n-05) relative to the feed stream
202. The second
fraction 217 additionally comprises a large majority of any hydrocarbons
containing six or more
carbon atoms that were originally present in the feed stream 202.
[0044] The first separation unit 210 uses conventional separation
technology understood by
those having experience in petroleum refining. In one embodiment, separation
unit 210 may be a
deisopentanizer that is operable to separate the i-05 isomer from the
remaining compounds
present within the feed stream 202. Alternatively, any other conventional
separation technology
may be used to assist in separating i-05 from n-05 to produce the first
fraction and the second
fraction. Such technology is conventional, and thus, outside the scope of the
present disclosure.
[0045] Upon leaving the first separation unit 210, the first fraction 213
is conveyed into a
first activation reactor 220 containing a first catalyst 225, while the second
fraction 217 is
conveyed into a storage vessel 227 comprising at least two outlets: a first
outlet that allows a
first portion of the second fraction 218 to be directed to a second activation
reactor 230
14
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
containing a second activation catalyst, and a second outlet that controls the
flow rate of a
second portion of the second fraction 219 that is diverted and blended with
the final liquid
hydrocarbon product of the inventive process and system (discussed in greater
detail below). The
second activation reactor 230 contains a second catalyst 235. Certain
embodiments do not
require a storage vessel. In such embodiments, any excess second fraction that
is not directed to
the second activation reactor is diverted and conveyed for blending with the
final liquid
hydrocarbon product of the inventive process and system (discussed in greater
detail below).
[0046] Speaking generally, the first activation reactor and second
activation reactor are
operable to maintain a temperature and a pressure that are suitable to
facilitate either catalytic
conversion of the first fraction by the first catalyst, or catalytic
conversion of the second fraction
by the second catalyst to produce products that may be utilized as a commodity
chemical, an
intermediate amenable to further catalytic upgrading, or liquid transportation
fuel (or a blend
component thereof).
[0047] Referring again to the embodiment outlined in Figure 2, the first
fraction 213 enters
an inlet of the first activation reactor 225. The temperature within the first
activation reactor
(termed the first temperature, typically measured within the inlet of the
first activation reactor) is
maintained in the range from 500 C to 650 C; optionally, the temperature is
maintained in the
range from 525 C to 625 C; optionally, the temperature is maintained in the
range from 525 C
to 600 C; optionally, the temperature is maintained in the range from 550 C to
600 C;
optionally, the temperature is maintained in the range from 550 C to 575 C.
[0048] The second fraction 217 enters an inlet of the second activation
reactor 235. The
temperature within the second activation reactor 235 (termed the second
temperature, typically
measured within the inlet of the second activation reactor) is maintained in
the range from 500 C
to 650 C. Optionally, the temperature is maintained in the range from 525 C to
650 C.
Optionally, the temperature is maintained in the range from 550 C to 625 C.
Optionally, the
temperature is maintained in the range between 575 C and 600 C. Lower pressure
favors the
conversion of light alkanes to light olefins. Thus, the first activation
reactor 225 and second
activation reactor 235 are generally maintained at a pressure ranging from 15
psia to 165 psia. In
certain embodiments, the pressure is maintained in the range from 15 psia to
50 psia.
[0049] In certain embodiments, the first temperature is lower than the
second temperature.
Optionally, the first temperature may be at least 10 F lower, at least 20 F
lower, at least 25 F
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
lower, at least 30 F lower, at least 35 F lower, at least 40 F lower, at least
45 F lower or at least
50 F lower than the second temperature.
[0050] Each of the first and second activation catalysts may comprise a
single catalyst, or a
mixture of chemically- and/or structurally-different catalysts that contact
the alkanes present in a
given feed stream and facilitate at least one of dehydrogenation, cracking,
and aromatization of
the alkanes, thereby producing an effluent that predominantly comprises light
paraffins that
contain from one to four carbons (C1-C4 paraffins), C2-05 olefins and single
ring aromatic
compounds (see Table 1).
[0051] A first effluent 236 leaves the first activation reactor 220, while
a second effluent 237
leaves the second activation reactor 230. Each effluent is conveyed to
condenser 240, which may
comprise one or more functions including a condenser, splitter, compressor and
pump.
Condenser 240 is operable to receive and combine the first effluent 236 and
the second effluent
237 and condense liquids from the mixture to produce a mixed liquid
hydrocarbons and a gas-
phase mixed effluent 245 (that is gaseous). The mixed liquid hydrocarbons 242
are removed
while the mixed effluent 245 is then compressed either within the condenser
240 or by a separate
compressor located immediately downstream (not depicted), and then conveyed to
a third reactor
that in the embodiment depicted is an oligomerization reactor 250 that
contains an
oligomerization catalyst 255.
[0052] Speaking generally, the oligomerization catalyst may comprise any
solid catalyst (or
mixture of catalysts) characterized as possessing either Bronsted or Lewis
acidic properties. In
certain embodiments, the oligomerization catalyst is a zeolite or mixture of
zeolites, or a reactive
transition metal oxide. In certain embodiments, the oligomerization catalyst
is ZSM-5, although
many zeolites are well-characterized as possessing oligomerization properties
and may be
suitable for use (either alone or in combination) with the inventive processes
and systems
described herein. Other well-characterized oligomerization catalysts include,
but are not limited
to: nickel oxides, aluminum alkyls, aluminum halides, perfluoroaryl boranes,
oligomeric methyl
aluminoxanes (including supported), perfluoroaryl boranes, fluoroarylanes,
trityl borate,
ammonium borate (and aluminate salts thereof), supported PhNMe2H+B(C6F5)4- and
borate
anions and superacidic solid Bronsted acids, among others.
[0053] Speaking generally, the oligomerization reactor is maintained at a
temperature and
pressure suitable to facilitate oligomerization of olefins present in the
mixed effluent, thereby
16
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
producing larger hydrocarbons comprising at least six carbons that are
preferably characterized
by a boiling point that is in the boiling point range of a liquid
transportation fuel (e.g., gasoline
or diesel). The oligomerization reactor is generally maintained at a total
pressure in a range from
14 psia to 800 psia, optionally in the range from 50 psia to 300 psia. The
oligomerization reactor
is typically maintained at a temperature (measured within the oligomerization
reactor inlet) in
the range from 200 C to 420 C, optionally in the range from 200 C to 350 C.
Typically, flow
thorough the oligomerization reactor is maintained at a weight hourly space
velocity (WHSV) in
the range from 0.5 hr-1 to 10 hr-1. Optionally, the WHSV is in the range from
0.5 hr-1 to 2.0 hr-1.
While higher overall throughput is desirable, ideally the chosen WHSV allows
for conversion of
at least 85% of hydrocarbons present in the mixed effluent at the selected
operating temperature
and pressure.
[0054] The catalytic conversion occurring in the oligomerization reactor
produces an
oligomerization effluent that typically comprises an increased quantity of
hydrocarbon
molecules that are characterized by a boiling-point in the range of gasoline.
Preferably, the
combination of and subsequent oligomerization converts at least 30 wt % of the
original feed
stream to hydrocarbon molecules that are characterized by a boiling point that
is in the range of
gasoline.
[0055] Referring again to the embodiment depicted in Figure 2, the
oligomerization effluent
257 is conveyed to a second separator 260 that separates the oligomerization
effluent 257 into
two fractions: a light hydrocarbons fraction 265 comprising C1-C4 hydrocarbons
and hydrogen,
and a condensable liquid hydrocarbons fraction 268 comprising hydrocarbons
containing at least
five carbon atoms (C5+) that may be utilized directly as a blend component of
a liquid
transportation fuel or an intermediate product that may be additionally
processed prior to
blending into a liquid transportation fuel.
[0056] In certain embodiments, the condensed liquid hydrocarbons 268 may be
conveyed to
a third separator 270 that in certain embodiments is a naphtha stabilizer. In
certain embodiments
the third separator 270 is operable to remove a recycle fraction 272
(comprising predominantly
alkanes and olefins containing four to six carbon atoms) from the condensed
liquid hydrocarbons
268 in order to decrease Reid vapor pressure and increase octane rating. The
resulting liquid
hydrocarbon product 274 predominantly comprises hydrocarbon molecules that are
characterized by a boiling-point in the range of a liquid transportation fuel,
such as, but not
17
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
limited to, gasoline, diesel and jet fuel. The recycle fraction 272 is
combined with feed stream
202 at a point that is upstream from the first separation unit 210.
Optionally, a portion of the
second fraction that is stored in storage vessel 227 may be directed via
excess second fraction
conduit 219 to be combined with liquid hydrocarbon product 274.
[0057] Speaking more generally, in certain embodiments the liquid
hydrocarbon product
may be hydrotreated in a hydrotreating reactor containing a hydrotreating
catalyst in order to
reduce olefin and aromatic content in the liquid hydrocarbon product, as well
as to remove
nitrogen-containing and sulfur-containing compounds. The hydrotreating reactor
contains at
least one hydrotreating catalyst (such as, for example, NiMo, CoMo, etc.) or a
precious metal
catalyst (such as Pt/A1203, Pd/A1203, or Pd/C, etc) and is maintained at a
pressure and
temperature suitable for facilitating hydrotreating catalytic reactions. Such
processes are
conventional in nature and therefore will not be described in greater detail
here.
[0058] Again, referring to the embodiment depicted in Figure 2, light
hydrocarbons fraction
265 predominantly comprises hydrogen as well as Cl - C4 hydrocarbons that were
not converted
in the oligomerization reactor 255. Light hydrocarbons fraction 265 leaves the
second separator
260 and is optionally conveyed to a fourth separator 266 that utilizes a
conventional separation
technology (such as, but not limited to, pressure swing adsorption technology,
membrane
separation technology, etc.) to separate hydrogen from light hydrocarbons to
produce a hydrogen
stream 267 and a C1-C4 light paraffins stream 269 that may be combusted to
provide at least a
portion of the heat required for the process, or recycled to serve as a
diluent that is mixed with at
least one of: the first fraction 213 and the second fraction 218.
[0059] Certain embodiments of the inventive processes and systems combine
the effluent
from each of the first and second activation reactors, then convey the
resulting mixture to an
aromatic alkylation reactor containing at least one alkylation catalyst. The
mixed effluent
contacts the alkylation catalyst and is converted to larger hydrocarbon
products that can be
utilized as either gasoline or diesel transportation fuel, or a component
thereof.
[0060] A third embodiment of the inventive processes and systems that
includes an
alkylation reactor and additional inventive features is illustrated by the
process flow-diagram of
Figure 3. A feed stream 302 comprising both n-05 and i-05 is converted in a
system 300. The
feed stream 302 is received by a first separation unit 310 that is operable to
separate the feed
stream 302 into a first fraction 313 that comprises an increased wt % of
isopentane (i-05)
18
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
relative to the feed stream 302, and an second fraction (i.e., n-05/C6+
fraction) 317 that
comprises an increased wt.% of n-pentane (n-05) relative to the feed stream
302. The second
fraction 317 additionally comprises a large majority of any hydrocarbons
containing six or more
carbon atoms that were originally present in the feed stream 302.
[0061] The first separation unit 310 uses conventional separation
technology understood by
those having experience in petroleum refining. In one embodiment, separation
unit 310 may be a
deisopentanizer that is operable to separate the i-05 isomer from the
remaining compounds
present within the feed stream 302. Alternatively, any other conventional
separation technology
may be used to assist in separating i-05 from n-05 to produce the first
fraction and the second
fraction.
[0062] Upon leaving the first separation unit 310, the first fraction 313
is conveyed into a
first reactor 320 containing a first activation catalyst 325, while the second
fraction 317 is
conveyed into a storage vessel 327 comprising at least two outlets: a first
outlet that allows a
first portion of the second fraction 318 to be conveyed to a second activation
reactor 330
containing a second activation catalyst 335, and a second outlet that controls
the flow rate of a
second portion of the second fraction 319 that is diverted and blended with
the final liquid
hydrocarbon product 374 of the inventive process and system (discussed in
greater detail below).
Certain embodiments do not require a storage vessel. In such embodiments, any
excess second
fraction that is not directed to the second activation reactor is diverted and
conveyed for
blending with the final liquid hydrocarbon product of the inventive process
and system
(discussed in greater detail below).
[0063] Speaking generally, the first activation reactor and second
activation reactor are
operable to maintain a temperature and a pressure that are suitable to
facilitate either catalytic
conversion of the first fraction by the first catalyst, or catalytic
conversion of the second fraction
by the second catalyst to produce products that may be utilized as a commodity
chemical, an
intermediate amenable to further catalytic upgrading, or liquid transportation
fuel (or a blend
component thereof).
[0064] Referring again to the embodiment outlined in Figure 3, the first
fraction 313 enters
an inlet of the first activation reactor 325. The temperature within the first
activation reactor
(termed the second temperature, typically measured at the inlet of the first
activation reactor) is
maintained in the range from 500 C to 650 C; optionally, the temperature is
maintained in the
19
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
range from 525 C to 625 C; optionally, the temperature is maintained in the
range from 525 C
to 600 C; optionally, the temperature is maintained in the range from 550 C to
600 C;
optionally, the temperature is maintained in the range from 550 C to 575 C.
[0065] The second fraction 317 enters an inlet of the second activation
reactor 335. The
temperature within the second activation reactor (termed the second
temperature, typically
measured within the inlet of the second activation reactor) is maintained in
the range from 500 C
to 650 C. Optionally, the temperature is maintained in the range from 525 C to
650 C.
Optionally, the temperature is maintained in the range from 550 C to 625 C.
Optionally, the
temperature is maintained in the range between 575 C and 600 C. Lower pressure
favors the
conversion of light alkanes to light olefins. Thus, the first activation
reactor 325 and second
activation reactor 335 are generally maintained at a pressure ranging from 15
psia to 165 psia. In
certain embodiments, the pressure is maintained in the range from 15 psia to
50 psia.
[0066] In certain embodiments, the first temperature is lower than the
second temperature.
Optionally, the first temperature may be at least 10 F lower, at least 20 F
lower, at least 25 F
lower, at least 30 F lower, at least 35 F lower, at least 40 F lower, at least
45 F lower or at least
50 F lower than the second temperature.
[0067] A first effluent 336 leaves the first activation reactor 320, while
a second effluent 337
leaves the second activation reactor 330. Each effluent is conveyed to
condenser 340, which may
comprise one or more functions including a condenser, splitter, compressor and
pump.
Condenser 340 is operable to receive and combine the first effluent 336 and
the second effluent
337 and condense liquids from the mixture to produce a mixed liquid
hydrocarbons and a mixed
effluent 345 (that is gaseous). The mixed liquid hydrocarbons 342 are removed
while the mixed
effluent 345 is then compressed either within the condenser 340 or by a
separate compressor
located immediately downstream (not depicted), and then conveyed to a third
reactor that in the
embodiment depicted is an aromatic alkylation reactor 350 containing aromatic
alkylation
catalyst 355.
[0068] Speaking generally, the alkylation reactor is maintained at a feed
inlet temperature
and pressure that facilitate the catalytic alkylation of aromatics present in
the mixed effluent.
The aromatics that are alkylated may be produced by aromatization that takes
place in the first or
second activation reactor, may be present in the hydrocarbon feed stream for
the process, or a
combination of these possibilities. These aromatics are alkylated by olefins
that are largely
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
produced by the activation of alkanes in the first and second activation
reactors. Alkylation of
aromatics in the alkylation reactor produces larger hydrocarbons comprising at
least seven
carbons that are preferably characterized by a boiling point that is in the
boiling point range of a
liquid transportation fuel (e.g., gasoline or diesel) or a blend component
thereof. Typically, the
alkylation effluent comprises an increased percentage of alkylated aromatic
compounds
comprising from seven to nine carbon atoms. Optionally, the larger
hydrocarbons also are
characterized by a lower Reid vapor pressure and an increased octane rating.
[0069] The alkylation reactor is generally maintained at a pressure in a
range from 14 psia to
800 psia, optionally in the range from 50 psia to 600 psia. The alkylation
reactor is typically
maintained at a temperature (measured within the alkylation reactor inlet) in
a range from 150 C
to 350 C, optionally between 200 C to 350 C. Typically, flow thorough the
alkylation reactor is
maintained at a weighted hourly space velocity (WHSV) in the range from 0.5 hr-
1- to 10 hr-1- on
an olefin basis. Optionally, the WHSV is in the range from 0.5 hr-1- to 2.0 hr-
1. While higher
overall throughput is desirable, ideally the chosen WHSV allows for conversion
of at least 85 %
of hydrocarbons present in the mixed effluent at the selected operating
temperature and pressure.
The catalytic conversion occurring in the alkylation reactor produces an
aromatic alkylation
reactor effluent that typically comprises at least 30 wt.% (preferably, at
least 40 wt %) of
hydrocarbon molecules that are characterized by a boiling-point in the range
of a liquid
transportation fuel.
[0070] Speaking generally, the alkylation catalyst may comprise any
catalyst characterized
as either Bronsted or Lewis acidic. A wide variety of catalysts have been
found to promote
aromatic alkylation including, but not limited to, aluminum chloride,
phosphoric acid, sulfuric
acid, hydrofluoric acid, silica, alumina, sulfated zirconia, zeolites
(including, for example, ZSM-
5, ZSM-3, ZSM-4, ZSM-18, ZSM-20, zeolite-beta, H-Y, MCM-22, MCM-36 and MCM-
49). In
certain embodiments, the alkylation catalyst simultaneously promotes
alkylation of aromatics
and oligomerization of olefins present in the mixed effluent.
[0071] Referring again to the embodiment depicted in Figure 3, the
alkylation reactor
effluent 357 is conveyed to a second separator 360 that separates the
alkylation effluent 357 into
two fractions: a light hydrocarbons fraction 365 comprising C1-C4 hydrocarbons
and Hz, and a
condensed liquid hydrocarbons 368 comprising hydrocarbons containing at least
five carbon
atoms (C5+) that may be utilized directly as a blend component of a liquid
transportation fuel or
21
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
additionally processed prior to blending into a liquid transportation fuel.
Preferably, the
alkylation effluent comprises an increased quantity (or increased wt%) of
alkylated aromatics
containing from seven to nine carbon atoms. Preferably, these alkylated
aromatics are
monocyclic aromatic hydrocarbons.
[0072] In the embodiment depicted in Figure 3, the condensed liquid
hydrocarbons 368 is
conveyed to a third separator 370 that is optionally a naphtha stabilizer. The
third separator 370
is operable to remove a recycle fraction 372 (comprising predominantly alkanes
and olefins
containing four to six carbon atoms) from the condensed liquid hydrocarbons
368 in order to
decrease Reid vapor pressure and increase octane rating. The resulting liquid
hydrocarbon
product 374 predominantly comprises hydrocarbon molecules that are
characterized by a
boiling-point in the range of a liquid transportation fuel, such as, but not
limited to, gasoline,
diesel and jet fuel. The recycle fraction 372 is mixed with feed stream 302 at
a point that is
upstream from the first separation unit 310. This recycling also serves to
indirectly recycle any
benzene present in the recycled fraction 372 to the alkylation reactor 350, as
the benzene would
be relatively unreactive in the second activation reactor 330. Optionally, a
portion of the second
fraction that is stored in storage vessel 327 may be directed via excess
second fraction conduit
319 to be combined with the liquid hydrocarbon product 374.
[0073] Speaking more generally, in certain embodiments the liquid
hydrocarbon product
may be hydrotreated in a hydrotreating reactor containing a hydrotreating
catalyst in order to
reduce olefin and aromatic content in the liquid hydrocarbon product, as well
as to remove
nitrogen-containing and sulfur-containing compounds. The hydrotreating reactor
contains at
least one hydrotreating catalyst (such as, for example, NiMo, CoMo, etc.) or a
precious metal
catalyst (such as Pt/A1203, Pd/A1203, or Pd/C, etc) and is maintained at a
pressure and
temperature suitable for facilitating hydrotreating catalytic reactions. Such
processes are
conventional in nature and therefore will not be described in greater detail
here.
[0074] Again, referring to the embodiment depicted in Figure 3, light
hydrocarbons fraction
365 predominantly comprises hydrogen as well as Cl - C4 hydrocarbons that were
not converted
in the alkylation reactor 355. Light hydrocarbons gas 365 leaves the second
separator 360 and is
optionally conveyed to a fourth separator 366 that utilizes a conventional
separation technology
(such as, but not limited to, pressure swing adsorption technology, membrane
separation
technology, etc.) to separate hydrogen from light hydrocarbons to produce a
hydrogen stream
22
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
367 and a C1-C4 light paraffins stream 369 that is optionally combusted to
provide at least a
portion of the heat required for the process (not depicted), or recycled to
serve as a diluent that is
mixed with at least one of: the first fraction 313 and the second fraction
318.
[0075] Certain embodiments comprise mixing a diluent with the first
fraction and/or the
second fraction prior to contacting with an activation catalyst. The diluent
may be added in a
ratio ranging from 10:1 to 1:10 molar ratio relative to the quantity of
pentanes feed stream fed to
each activation reactor. The diluent may be added at any point that is
upstream from, or within,
the first or second activation reactors.
[0076] The diluent may comprise any substance that is less chemically-
reactive than the
constituents present in the first fraction or the second fraction at the
conditions of temperature
and pressure that are maintained within the first and second activation
reactors. This is intended
to prevent the diluent from reacting with the first and second activation
catalysts. Such
properties are found in a large number of substances that are fully within the
grasp of one having
experience in the art. In certain embodiments, the diluent may comprise a C1-
C4 light paraffins,
including recycling C1-C4 light paraffins produced by the processes and
systems described
herein. In certain embodiments, the diluent may comprise any of methane,
ethane, propane,
butanes, benzene, toluene, xylenes, alkyl- or dialkyl-benzenes, naphthenes, C2-
05 olefins, and
combinations thereof
[0077] The presence of diluent during catalytic activation (i.e.,
activation) provides
numerous advantages. First, it effectively decreases the concentration of the
first fraction within
the first activation reactor, decreases the concentration of the second
fraction within the second
activation reactor, or both. This results in a small increase in the total
conversion of pentanes
(typically approximately 5-6 wt.%) to olefins or aromatics within each
activation reactor.
However, it increases the selectivity toward the production of olefins in both
the first and second
effluent, while slightly decreasing the selectivity toward aromatics.
Adjusting the ratio of diluent
to feed stream changes the ratio of olefins to aromatics exiting the reactor,
thereby providing a
valuable point of operational control for downstream processes. Typically, the
optimal molar
production ratio of olefins to aromatics ranges from about 0.5:1 to about
1.5:1, in order to
maximize the value captured in the olefin intermediates during the alkylation
in the alkylation
reactor. Mono-alkylated aromatics exhibit beneficial (increased) octane rating
and vapor
pressure for application as blending components in certain transportation
fuels such as gasoline.
23
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
In contrast, di-alkyl and tri-alkyl aromatics comprising more than nine carbon
atoms are not
well-suited for blending into gasoline, and exhibit nonoptimal cetane number
for blending into
diesel.
[0078] Addition of a diluent also advantageously favors the production of
value-added
olefins relative to C1-C4 light paraffins, and also mitigates dimerization of
C5 hydrocarbons to
form durene (1,2,4,5-tetramethylbenzene), a byproduct notorious for
precipitating as a solid out
of gasoline blends.
EXAMPLES
[0079] The following examples are representative of one embodiment of the
inventive
processes and systems disclosed herein, and the scope of the invention is not
intended to be
limited to the embodiment specifically disclosed. Rather, the scope is
intended to be as broad as
is supported by the complete disclosure and the appending claims.
EXAMPLE 1:
[0080] The graphs below illustrate differences in activation reactivity for
n-05 and i-05.
Feed streams were utilized that comprised either 100 wt.% i-05 (i-05) or 100
wt.% of n-05 (n-
05). Feed streams comprising essentially 100 % of a single pentane isomer are
representative of
separated pentanes streams due to preliminary data showing that a mixed
pentanes stream can be
separated into i-05 and n-05 fractions of approximately 93% purity when
separating is
performed according to the methods disclosed herein. The catalyst was 1/8"
extrudate consisting
of 50 wt.% alumina binder and 50 wt.% ZSM-5 zeolite, and experiments were
conducted at a
WHSV of 1.3 hr-1 at 1 atm. Results were averaged over the total time on stream
of 16 hr.
[0081] Figure 4 is a bar graph depicting the results of catalytically
activating each fraction at
either 550 C or 600 C. The graph depicts, as percentages, the total catalytic
conversion of each
feed stream (first column), the selectivity to light olefins as product
(second column), the
selectivity to aromatics as product (third column) and the selectivity to C1-
C4 light paraffins
(defined as non-olefin hydrocarbons containing from one to four carbon atoms),
fourth column.
Selectivity was calculated on a % carbon basis, relative to the portion of the
feed stream fraction
that was converted.
24
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
[0082] The results demonstrate that total conversion of a 100% n-05
fraction at 600 C was
79%, and a similar 82% conversion was observed when activating a 100% i-05
feed stream at
50 C cooler (i.e., 550 C). Activating the first fraction at 550 C (instead of
600 C) also increased
the selectivity towards the production of olefins while decreasing the
selectivity of conversion
toward aromatics. Lastly, these changes in selectivity caused no significant
increase in the
production of byproduct C1-C4 light paraffins. However, activation of n-05 at
550 C was
generally unsuitable, and resulted in a 31% decrease in total conversion, and
a noticeable
increase in the production of C1-C4 light paraffins.
EXAMPLE 2:
[0083] A 100% i-05 feed stream was upgraded by first contacting it with a
zeolite activation
catalyst, followed by contacting a zeolite oligomerization catalyst.
Activation was conducted by
contacting the feed stream with 1/8 in. diameter catalyst extrudate consisting
of 50 wt.% alumina
binder and 50 wt.% ZSM-5 zeolite catalyst at a temperature of 579 C, and a
WHSV of 2.6 hr1 at
1 atm. Oligomerization was conducted by contacting the activation effluent
with a ZSM-5
catalyst in a reactor where the inlet temperature for the feed stream was
maintained at 250 C, the
pressure was 1 atm, and the WHSV for the feed stream was 1.3 hfl. Results were
time-averaged
over 16 hours. The table shows the product distribution following conversion
along with the
selectivity to olefins and liquid product. The term "selectivity" indicates
the percentage of the
catalytically converted feed stream that was converted to a particular
product.
Table 2. Upgrading pentanes by activation alone or activation plus
oligomerization.
Activation Activation +
Oligomerization
Total Conversion (wt.%) 88 87
C1-C4 Light paraffins Yield 32 32
Upgraded Product Yield (wt.%) 55 54
Total Coke Yield (wt.%) 0.1 0.1
Light Olefin Yield (wt.%) 42 16
Light Olefin Selectivity (wt.%) 48 19
Liq. Yield (wt.%) 13 38
Liq. Product Selectivity (wt.%) 15 44
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
[0084] The data in Table 2 show that the subjecting the effluent from the
first activation
reactor to a subsequent oligomerization step in a second activation reactor
increased the liquid
product yield from 13 wt.% to 38 wt.%. This liquid product yield represents a
liquid product
suitable for blending into a liquid transportation fuel such as gasoline (up
from 13 wt.% prior to
oligomerization), and that selectivity to liquid product for the portion of
the feed stream that was
converted was 44 wt.%. Undesirable C1-C4 light paraffins production was
limited to 32 wt.% of
the original feed stream, which in a commercial embodiment would preferably be
recycled to
process to be either activated or to serve as a diluent in at least one of the
activation reactors.
Further, the final product only comprised 16 wt.% of light olefins, (primarily
ethylene), which
may be recycled to the process, or diverted to be utilized in any of a variety
of conventional
processes.
EXAMPLE 3:
[0085] A 100 wt.% i-05 feed stream was upgraded by first contacting it with
a zeolite
activation catalyst, followed by contacting the effluent with a zeolite
alkylation catalyst.
Activation was conducted by contacting the feed stream with a 1/8 in. diameter
catalyst extrudate
consisting of 50 wt.% alumina binder and 50 wt.% ZSM-5 zeolite catalyst in an
activation
reactor. The temperature of the activation reactor at the inlet for the feed
stream was 579 C, the
pressure was 1 atm, and the WHSV for the feed stream was 2.6 hr-'. Alkylation
was then
conducted by contacting the effluent with a ZSM-5 catalyst in a reactor where
the temperature at
the inlet for the feed stream was 230 C and the WHSV of the feed stream was
1.3 hr' at 1 atm.
Results were time-averaged over 16 hours. The table shows the product
distribution following
conversion along with the selectivity to olefins and liquid product. The term
"selectivity"
indicates the percentage of the catalytically converted feed stream that was
converted to a
particular product.
Table 3. Upgrading pentanes by activation only or activation followed by
alkylation.
Activation Activation +
Alkylation
Total Conversion (wt.%) 87 87
Light paraffins Yield (wt.%) 32 32
Upgraded Product Yield (wt.%) 55 55
Total Coke Yield (wt.%) 0.1 0.2
Light Olefin Yield (wt.%) 42 12
26
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
Light Olefin Selectivity (wt.%) 48 14
Liquid Yield (wt.%) 13 42
Liquid Product Selectivity (wt.%) 15 48
[0086] The data in Table 3 show that subjecting the effluent to a
subsequent alkylation step
increased the liquid product yield from 13 wt.% to 42 wt.%. This liquid
product is suitable for
blending into a liquid transportation fuel such as gasoline, and possesses an
increased research
octane number, a suitable distillation T50 and endpoint, and low vapor
pressure. Selectivity to
liquid product for the portion of the feed stream that was converted increased
from 15 wt.% to
48 wt.%. Undesirable C1-C4 light paraffins production was limited to 32 wt.%
of the original
feed stream. Further, the final product only comprised 14 wt.% of light
olefins. These olefins
may be recycled to the process or diverted to be utilized in any of a variety
of conventional
processes.
[0087] Note that the results shown in the above table may underestimate the
total percentage
of a mixed pentanes feed stream that would be available for blending into a
liquid transportation
fuel, as a mixed pentanes feed stream (or other light hydrocarbon feed stream,
such as natural
gasoline) may also include an excess quantity of C5/C6+ that would not be
either catalytically
cracked or introduced into the alkylation reactor. This excess quantity of
C5/C6+ is suitable for
direct blending into the liquid hydrocarbon product. In certain embodiments, a
portion of the
nC5/C6+ fraction is diverted when necessary to achieve the desired 0.5:1 to
1.5:1 olefin to
aromatic ratio that maximizes production of mono-alkylated aromatics in the
alkylation reactor.
EXAMPLE 4:
[0088] The following table shows the viability of a product produced by one
embodiment of
the inventive processes and systems described herein. The composition of a
conventional
commercial reformate product (Comparative Example) following processing to
remove light
components (including benzene), is compared with the composition of an
unprocessed product
produced by an embodiment that included activation in a first reactor followed
by alkylation in a
second reactor. The temperature of the first reactor at the inlet for the feed
stream was
maintained at 600 C and a WHSV = 2.6 hr', while the temperature of the
alkylation reactor (at
the inlet for the feed stream) was maintained at 230 C and a WHSV = 1.3 hr-1.
Results shown
represent average wt.% for each product over 16 hours, and all reactions were
performed at 1
atm. RON = road octane number; MON = motor octane number.
27
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
Table 4. Product distributions of identical iC5 isomer streams subjected to
catalytic activation
alone (Column 1) or catalytic activation followed by catalytic alkylation
(Column 2).
Component / Property Comparative Example (A Inventive Example
Commercial Reformate Composition) (Activation Alkyl ati on)
n-Paraffins (wt.%) 4.7 2.9
Iso-paraffins (wt.%) 15,9 9,5
Aromatics (wt.%) 77.9 67.6
Mono-alkyl aromatics 75.0 64.4
(wt.%)
n-Olefins (wt.%) 0.1 1.2
'so-Olefins (wt.%) 0,2 4,4
Estimated RON 96.84 98.3
Estimated MON 91.46 89.9
Avg. Molecular Weight 107.62 103.50
Avg, Specific Gravity 0.82 0.80
Avg. API Gravity @,15.5 40.6 41.5
oc
Reid Vapor Pressure (kPa) 19.2 44.8
Total Hydrogen 11.01 10.80
Carbon: Hydrogen Ratio 8,08 7,90
EXAMPLE 5:
[0089] A feed stream comprising 100 wt.% of i-05 was fed at a WHSV of 1.3
hr-1 to a
reactor containing 1/8" extrudate consisting of 50 wt.% alumina binder and 50
wt.% ZSM-5
zeolite activation catalyst to produce an effluent comprising light olefins,
aromatics and light
paraffins. The temperature of the reactor (at the inlet for the feed stream)
was maintained at
600 C and 20 psig (2.4 Bar) and results were time-averaged for 16.5 hr. For
certain reactions,
methane diluent was co-fed along with i-05 at a methane:i-05 molar ratio of
2:1 and 4:1.
[0090] Figure 5 is a bar graph that shows the effect of the diluent on the
total conversion of
the feed stream, as well as the selectivity of the conversion toward light
olefins, aromatics, and
byproduct C 1-C4 light paraffins. Increasing the ratio of diluent did not
significantly decrease
conversion but correlated with a large increase in selectivity to light olefin
production and
greatly diminished selectivity to production of C1-C4 light paraffins.
Meanwhile, only a small
drop in selectivity to aromatics production were observed. All of these
results are advantageous
28
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
to the process, particularly in certain embodiments where the mixed effluent
is immediately
utilized as feed stream for either an oligomerization or alkylation process.
In certain
embodiments that comprise an oligomerization process, diluent is added to the
feed stream at a
ratio that maximizes light olefin production, providing an advantageous feed
stream for the
oligomerization catalyst. In certain embodiments that comprise an aromatic
alkylation process,
diluent can be added to the feed stream at a ratio that produces a first
effluent comprising olefins
and aromatics at a ratio (typically between 0.5:1 and 1.5:1 by mole) that
provides an
advantageous feed stream for an aromatic alkylation process.
EXAMPLE 6:
[0091] A feed stream comprising 100 wt.% of i-05 was fed at a WHSV of 1.3
hr' to an
activation reactor containing 1/8" extrudate consisting of 50 wt.% alumina
binder and 50 wt.%
ZSM-5 zeolite activation catalyst to produce an effluent comprising light
olefins, aromatics and
C1-C4 light paraffins. The temperature of the reactor (at the inlet for the
feed stream) was
maintained at 550 C and 20 psig (2.4 Bar) and results were time-averaged for
16 hr. For certain
reactions, a diluent comprising methane was co-fed along with i-05 at a
methane:i-05 molar
ratio of 4:1 and 8:1.
[0092] Figure 6 is a bar graph that shows the effect of the diluent on the
total conversion of
the feed stream, as well as the selectivity of the conversion toward light
olefins, aromatics, and
byproduct C1-C4 light paraffins. As in EXAMPLE 5, increasing the ratio of
inert diluent did not
significantly decrease conversion, but correlated with a large increase in
selectivity to light
olefin production and greatly diminished selectivity to production of C1-C4
light paraffins.
Meanwhile, only a small drop in selectivity to aromatics production were
observed. All of these
results are advantageous to the process, particularly in certain embodiments
where the mixed
effluent is immediately utilized as feed stream for either an oligomerization
or alkylation
process.
[0093] In closing, it should be noted that the discussion of any reference
is not an admission
that it is prior art to the present disclosure, in particular, any reference
that may have a
publication date after the priority date of this application. Although the
systems and processes
described herein have been described in detail, it is understood that various
changes, substitutions,
29
CA 03107128 2020-09-25
WO 2019/191113 PCT/US2019/024095
and alterations can be made without departing from the spirit and scope of the
invention as defined
by the following claims.
Definitions:
[0094] In the present disclosure, the term "conversion" is defined as any
of the chemical
reactions that occur during upgrading of hydrocarbons to liquid transportation
fuels. Examples
of such reactions include, but are not limited to: oligomerization,
aromatization,
dehydrogenation, alkylation, hydrogenation and cracking.