Note: Descriptions are shown in the official language in which they were submitted.
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Method for Treating Pancreatic Cancer
Introduction
A method for treating pancreatic cancer in a subject in need thereof
comprising,
administering to the subject an effective amount of a tubulin polymerization
inhibitor
compound is described herein. More particularly, a method for treating
pancreatic
ductal adenocarcinoma in a subject in need thereof comprising, administering
to the
subject an effective amount of a substituted reverse pyrimidine tubulin
polymerization
inhibitor compound alone or in combination with other chemo-therapeutic agents
is
described herein.
Background
An estimated 55,440 Americans will be diagnosed with pancreatic cancer in
2018, the fifth most common cause of cancer-related mortality; these figures
are
predicted to rise over the next decade. Pancreatic ductal adenocarcinoma (PDA)
is a
highly chemo-resistant cancer responsible for more than 45,000 deaths
annually,
accounting for about 93% of pancreatic tumors. Despite some measurable
progress in
recent years, PDA remains a largely intractable cancer, with a median survival
of less
than six months and a 5-year survival rate of just 8.7%.
Several factors contribute to this poor prognosis. Most patients (85%) present
with advanced disease, precluding them from the one effective intervention:
surgical
resection. However, even among those with locally confined disease who have
had
surgery, the 5-year and 10-year survival rates are just 25% and 8%
respectively, due to
high recurrence rates (A. Richter et al., World J Surg 27, 324, Mar 2003). For
these
remaining 85% of patients, as well as those with recurrent disease, there are
few
treatments available. The national standard-of-care therapy, gemcitabine
modestly
extends survival by a few weeks, approved primarily for improving quality-of-
life
indicators (H. A. Burris, 3rd et al., J Clin Oncol 15, 2403, Jun 1997). The
only other
FDA approved agent for advanced PDA is Tarceva, which provides an average of
just
10 days additional benefit when combined with gemcitabine. Despite over 60
clinical
trials of different agents and combinations (H. Q. Xiong, K. Carr, J. L.
Abbruzzese,
1
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Drugs 66, 1059, 2006), no other effective therapies have been identified.
Among the most remarkable features of FDA is the presence of an expansive
desmoplastic stroma that conditions the local microenvironment, generating
high
interstitial fluid pressure, poor vascularity, and diminished tissue perfusion
and
diffusion. Consequently, drug delivery to pancreatic tumors is less efficient
than in
normal tissues, contributing to the broad primary chemo-resistance that
characterizes
this disease. The broad resistance of FDA to cytotoxic therapy arises in part
from this
biophysical barrier to drug delivery.
Preclinical testing of chemo-therapeutic agents in the KPC (KrasLSL.G12D/+;
p53R172H/+; PdxCretg/+) genetically engineered mouse model suggest that drug
development efforts for FDA should focus on agents with a long half-life and a
large
therapeutic index (the range of concentrations between efficacy and toxicity)
as a
means of improving drug exposure. As well, drug stability and retention within
tumor
cells should also be considered in the design of new regimens for FDA.
Unfortunately, most traditional cytotoxic agents are rapidly cleared from
circulation, are acutely toxic to normal proliferating tissues, are quickly
metabolized, or
are actively exported from tumor cells. An informative counterexample is nab-
paclitaxel
(Abraxane, Celgene), an albumin-bound form of the microtubule stabilizing
agent
paclitaxel that is FDA approved in combination with gemcitabine for metastatic
pancreatic cancer patients. Nab-paclitaxel has a terminal half-life of 27
hours in
circulation and is less toxic than unmodified paclitaxel. The success of this
regimen
helped to validate the importance of pharmacology in pancreatic cancer drug
development and demonstrated the benefit of combining gemcitabine (a
deoxycytidine
analog) with a microtubule targeted agent.
Accordingly, there remains a need for chemo-therapeutic agents that overcome
the FDA biophysical barrier to produce effective biodistribution into tumor
tissues, have
the requisite pharmacodynamic properties and combine synergistically with
other
chemo-therapeutic agents for the treatment of pancreatic cancer.
2
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Summary
Compound 1 is a small molecule anticancer agent, useful for inhibiting tubulin
polymerization and BMI-1 protein function (see W02014/081906), referred to as
5-
fluoro-2-(6-fluoro-2-methyl-1H-benzo[d]imidazol-1-y1)-
N4[4-(trifluoromethyl)phenyl]pyrimidine-4,6-diamine, having the structure of
Formula
(I):
F H2N F
F FAN
F 1
401 N7N N 40
H )--------N1
Formula (I),
or a pharmaceutically acceptable salt or pharmaceutical composition thereof.
Compound 1 has demonstrated pharmacological properties, including a long
circulating half-life and lack of P-glycoprotein (PGP) substrate activity and
effective
biodistribution into tumor tissues. Further, Compound 1 had been shown to
induce
mitotic arrest and apoptosis in multiple FDA cell lines.
Through mechanistic studies, without being limited to any one particular
theory,
Compound 1 has been demonstrated to function as a microtubule polymerization
inhibitor. In addition, Compound 1 combines synergistically with standard
clinical
regimens such as either or both gemcitabine and nab-paclitaxel to improve
efficacy in
patient derived xenograft models (PDX) yielding potent and durable cancer
regression.
Further, Compound 1 has demonstrated efficacy in combination with gemcitabine
in
the highly chemo-resistant genetically engineered KPC FDA mouse model.
These data and a demonstrated safety profile as an anti-cancer agent in
clinical
development demonstrate clear rationale for the development of Compound 1 in
combination with standard-of-care chemotherapy for FDA.
3
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Description
One aspect described herein includes a method for treating pancreatic cancer
in
a subject in need thereof comprising, administering to the subject an
effective amount
of Compound 1, 5-fluoro-2-(6-fluoro-2-methyl-1 H-benzo[d]imidazol-1-y1)-
N4[4-(trifluoromethyl)phenyl]pyrimidine-4,6-diamine, having the structure of
Formula
(I):
F H2N F
F F
.A1 N
F
401 40
N N N
H )---------N
Formula (I),
or a pharmaceutically acceptable salt or pharmaceutical composition thereof.
Another aspect includes a method for treating pancreatic ductal
adenocarcinoma in a subject in need thereof comprising, administering to the
subject
an effective amount of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof.
One aspect described herein includes a method for treating pancreatic cancer
in
a subject in need thereof comprising, administering to the subject an
effective amount
of Compound 1 or a pharmaceutically acceptable salt or pharmaceutical
composition
thereof in combination with an effective amount of one or more chemo-
therapeutic
agents.
Another aspect includes a method for treating pancreatic ductal
adenocarcinoma in a subject in need thereof comprising, administering to the
subject
an effective amount of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof in combination with an effective amount of
one or
more chemo-therapeutic agents.
One aspect described herein includes a method for treating pancreatic ductal
adenocarcinoma in a subject in need thereof comprising, administering to the
subject
4
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
an effective amount of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof in combination with an effective amount of
either
or both gemcitabine and nab-paclitaxel.
Another aspect includes a method for treating pancreatic ductal
adenocarcinoma in a subject in need thereof comprising, administering to the
subject
an effective amount of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof in combination with an effective amount of
either
or both gemcitabine and nab-paclitaxel.
Brief Description of the Drawings
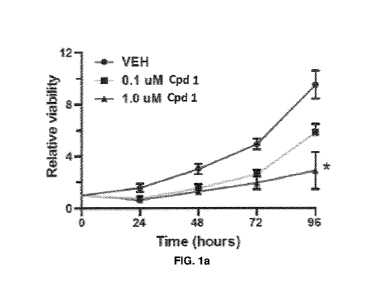
Figure la is a graph of the relative Aspc1 cell viability over time following
treatment with vehicle or Compound 1 (0.1 pM or 1.0 pM). The results show
relative to
vehicle that Compound 1 demonstrates a dose and time-dependent decrease in
human FDA cell viability.
Figure lb depicts representative histograms of DNA content, measured by flow
cytometry for 7-AAD fluorescence, from Aspc1 cells treated with vehicle or
Compound
1(0.1 pM or 1.0 pM).
Figure lc is a graph of the percent of Aspc1 cells in GO/G1 phase, S phase,
and
G2/M phase following 24 hours after treatment with vehicle (DMSO), Compound 1
(0.1
pM, 1.0 pM), or 0.1 pM nocodazole (NOC, positive control).
Figure ld is a plot of the percent of Aspc1 cells in mitosis (PH3-14N DNA
content) after treatment for 24 hours with DMSO, Compound 1 (0.1 pM or 1.0
pM), or
0.1pM nocodazole (NOC). Figures lb, 1c and 1d show that Compound 1
demonstrates a dose and time-dependent effect in cell cycle arrest in human
FDA
cells.
Figure 2a depicts representative histograms of DNA content measured by DAPI
fluorescence in Aspc1 cells treated with vehicle or Compound 1 (1.0 pM) at 24
hour, 48
hour, and 72 hour timepoints.
Figure 2b is a graph of the percent of Aspc1 cells with DNA content >4N after
treatment with DMSO or Compound 1 (0.1 pM or 1.0 pM) at 24 hour, 48 hour, 72
hour
timepoints. Figures 2a and 2b show that Compound 1 demonstrates a dose and
time-
5
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
dependent increase in the amount of human FDA cells affected by polyploidy.
Figure 3a depicts representative flow cytometry scatter plots showing
induction
of apoptosis in Aspc1 cells following treatment with DMSO for 24 hours, or 1.0
pM
Compound 1 at 72 hours. Unfixed cells were stained for active caspase 3 and
DAPI to
distinguish viable cells (lower left) from early apoptosis (top left), late
apoptosis (top
right), and necrosis (lower right).
Figure 3b is a graph of total apoptotic Aspc1 cells (active caspase 3+)
quantified
for DMSO or Compound at 24 hour, 48 hour, and 72 hour timepoints. Figures 3a
and
3b show relative to vehicle that Compound 1 demonstrates a time-dependent
increase
in cell biomarkers that indicate a corresponding increase in cell apoptosis.
Figure 4a is a graph of plasma levels of Compound 1 in mice measured by
mass spectrometry at baseline, or 2, 4, 7, 24, or 48 hours following a single
oral dose
of Compound 1 (10 mg/kg). Figure 4a demonstrates that Compound 1 has a long
plasma half-life and penetrates the CNS and distributes into brain tissues.
Figure 4b is a plot of the ratio of CC50 values for treatment of MDCK-P-gp vs
MDCK-WT cells with Compound 1, Compound 2, chlorpromazine, puromycin,
vinblastine, doxorubicin, paclitaxel, and vincristine. Figure 4b shows that,
compared to
other chemotherapeutic agents that cause cell cycle arrest, Compound 1,
Compound 2
and chlorpromazine do not function as a PGP substrate
Figure 5a is a plot of Compound 1 concentrations in plasma, quadriceps, and
FDA tissues from KPC mice, 24 hours following a single oral dose of Compound 1
(10mg/kg), alone or in combination with gemcitabine (100 mg/kg). Figure 5a
shows that
the improved efficacy of the combination is not due to a pharmacokinetic drug-
drug
interaction.
Figure 5b is a western blot for CYCLIN B1 on tumors from KPC mice treated
with 10 mg/kg of Compound 1. Tumor biopsy samples (Bx) were acquired 48 hours
prior to first dose, and compared to samples acquired at necropsy (Nx) 24
hours after
the third dose.
Figure 5c is a graph quantifying CYCLIN B1 (CycB1) from Figure 5b, normalized
to VINCULIN (Vinc). Figure 5a shows that Compound 1 distributes into FDA
tissues;
6
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
and, Figures 5b and 5c show that Compound 1 demonstrates consistent reduction
in
Cyclin B1 relative to Vinculin suggesting a decrease in FDA cell mitotic
progression
induced by reduction in Cyclin B1 relative to Vinculin induced apoptosis.
Figure 6a is a plot of body weights over time of KPC mice treated with a
combination of Compound 1 and gemcitabine.
Figure 6b is a plot of survival over time of KPC mice treated with vehicle
(VEH),
gemcitabine (GEM, 100mg/kg b.i.w.), Compound 1 (Cpd 1, 17mg/kg b.i.w.), or a
combination of Compound 1 and gemcitabine (Cpd/GEM).
Figure 6c is a plot of tumor growth rate calculations calculated from
longitudinal
tumor volumes in KPC mice treated with vehicle (VEH), gemcitabine (GEM),
Compound 1 (Cpd 1), or a combination of Compound 1 and gemcitabine (Cpd/GEM).
Figure 6d is a plot of quantification of immunohistochemistry for
phosphorylated
Histone H3 (PH3) cells showing average positive cells per 40X field over 10
fields per
tumor following necropsy in KPC mice treated with vehicle (VEH), gemcitabine
(GEM),
Compound 1 (Cpd 1), or a combination of Compound 1 and gemcitabine (Cpd/GEM).
Figure 6e is a plot of quantification of immunohistochemistry for
phosphorylated
cleaved Caspase-3 (CC3) cells showing average positive cells per 40X field
over 10
fields per tumor following necropsy in KPC mice treated with vehicle (VEH),
gemcitabine (GEM), Compound 1 (Cpd 1), or a combination of Compound 1 and
gemcitabine (Cpd 1/GEM). Figures 6a, 6b, 6c, 6d, and 6e show that Compound 1
in
combination with gemcitabine synergistically increases overall survival in the
KPC
mouse model; wherein, Figure 6a shows that Compound 1 in combination with
gemcitabine maintains relative KPC mouse body weight; Figure 6b shows relative
to
vehicle, gemcitabine alone or Compound 1 alone, that Compound 1 in combination
with gemcitabine simultaneously decreases tumor volume while synergistically
increasing overall survival relative to each agent alone; Figures 6c, 6d, and
6e show
relative to vehicle, gemcitabine alone or Compound 1 alone, that Compound 1 in
combination with gemcitabine demonstrates an overall reduction in growth rate
of FDA
cells and certain cell biomarkers.
Figure 7a is a plot of tumor volumes measured by high resolution 3D ultrasound
7
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
for KPC mice treated with vehicle (veh) or Compound 1 (Cpd 1).
Figure 7b is a plot of tumor volumes measured by high resolution 3D ultrasound
for KPC mice treated with vehicle (veh) or a combination of Compound 1 and
gemcitabine (Cpd 1/gem). Figure 7a shows relative to vehicle that Compound 1
demonstrates a time-dependent decrease in KPC mouse model FDA tumor volume;
and, Figure 7b shows, relative to gemcitabine alone or Compound 1 alone, that
Compound 1 in combination with gemcitabine demonstrates an additive time-
dependent decrease in KPC mouse FDA tumor volume.
Figure 8a is a plot of tumor growth measured by percent volume change over 7
days in KPC mice treated with vehicle (VEH), Compound 1 (Cpd 1), gemcitabine
(GEM), or a combination of Compound 1 and gemcitabine (Cpd 1/GEM).
Figure 8b is a plot of the percent of mice having liver metastases in KPC mice
treated with vehicle (V), Compound 1 (Cpd 1+V), gemcitabine (G), or a
combination of
Compound 1 and gemcitabine (Cpd 1+G). Figure 8a shows relative to vehicle,
gemcitabine alone or Compound 1 alone, that Compound 1 in combination with
gemcitabine demonstrates a reduction in KPC mouse FDA tumor growth; and,
Figure
8b shows, relative to vehicle and gemcitabine alone, that Compound 1 in
combination
with gemcitabine demonstrates a reduction in KPC mouse FDA-driven liver
metastases.
Figure 9a is a stained tumor section from KPC mice treated with gemcitabine
(GEM).
Figure 9a is an image of a stained tumor section from KPC mice treated with a
combination of Compound 1 and gemcitabine (Cpd 1/GEM). Figure 9a shows
gemcitabine induced apoptotic cell population; and, Figure 9b shows apoptotic
cell
population induced by Compound 1 in combination with gemcitabine; wherein
Compound 1 in combination with gemcitabine demonstrates a markedly increased
apoptotic cell population, suggesting a synergistic increase in apoptotic
induction.
Figure 10a is a series of western blots showing BMI-1 levels in Aspc1,
MiaPaCa-2, and Panc1 cells treated for 24 hours with vehicle (VEH), 0.1 uM
nocodazole (NOC), 0.1 M Compound 1 (0.1), or 1.0 M Compound 1(1.0) relative
to
8
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
vinculin (VINC) loading control. Figure 10a shows relative vehicle and
Vinculin
expression that Compound 1 demonstrates a consistent reduction in BMI-1
protein
expression in various cell types.
Figure 10b is a graph of relative viability of J1002VEH and J1002TAM cells
treated with vehicle (VEH), 0.1 pM Compound 1 (Cpd 1), or 1.0 pM Compound 1
(Cpd
1) over 96 hours. Figure 10b shows relative to vehicle and the presence of
J1002 that
Compound 1 demonstrates a dose and time-dependent decrease in viability of
cells
dependent on BMI-1 protein expression.
Figure 10c is a graph of dose response curves for J1002VEH and J1002TAM
cells treated with Compound 1 for 72 hours. Figure 10c shows relative to
vehicle
treated cells that Compound 1 demonstrates subliM activity toward reduction in
BMI-1
protein expression in the presence of J1002.
Figure 10d depicts representative DNA histograms for J1002VEH cells treated
for 24 hours with vehicle (VEH) or 1.0 pM Compound 1 (Cpd 1).
Figure 10e is a graph of the percent of J1002VEH cells in GO/G1 phase, S
phase, and G2/M phase following 24 hours after treatment with vehicle (VEH) or
1.0
pM Compound 1 (Cpd 1).
Figure 10f depicts representative DNA histograms for J1002TAM cells treated
for 24 hours with vehicle (VEH) or 1.0 pM Compound 1 (Cpd 1).
Figure lOg is a graph of the percent of J1002TAM cells in GO/G1 phase, S
phase, and G2/M phase following 24 hours after treatment with vehicle (VEH) or
1.0
pM Compound 1 (Cpd 1). Figures 10d, 10e, 10f, and lOg show relative to vehicle
and
the presence of J1002 that Compound 1 demonstrates a dose and time-dependent
increase in the amount of BMI-1 dependent cells affected by polyploidy;
wherein, the
data taken as a whole suggest that Compound 1 induces BMI-1
hyperphosphorylation
indirectly as a result of mitotic arrest.
Figure lla is a plot of differentially expressed genes measured by RNA-seq on
Aspc1 cells treated with DMSO or 1pM Compound 1 (Cpd 1), integrated over 8,
16,
and 24 hour timepoints.
Figure llb is a western blot showing free tubulin from Aspc1 cells treated
with
9
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
vehicle (VEH), 3.0 M Compound 1 (Cpd 1), 1.0 M colchicine (COL), or 1.0 M
paclitaxel (TAX) for 2 hours. Following treatment, cell lysates were
fractionated by
centrifugation in order to separate free tubulin from microtubules. LSP = Low
Speed
Pellet (1,000 x g, 5 min), HSP = High Speed Pellet (100,000 x g, 1 hour), HSS
= High
Speed Supernatant (100,000 x g, 1 hour). Figures lla and llb show relative to
vehicle, COL (carboplatin) and TAX (tamoxifen) that Compound 1 demonstrates a
significant fold-change increase in prevention of tubulin formation.
Figure 11c is an image of tubulin and DAPI in Aspc1 cells treated with vehicle
(VEH) for 24 hours.
Figure 11d is an image of tubulin and DAPI in Aspc1 cells treated with
Compound 1 (Cpd 1) for 24 hours. Figures 11c and lld show relative to vehicle
that
Compound 1 demonstrates a significant reduction in tubulin formation.
Figure lle is a graph of tubulin polymerization assay over time for cells
treated
with vehicle (DMS), tamoxifen (TAX), carboplatin (COL), and Compound 1(0.12
M,
0.37 M, 1.11 M, 3.33 M, and 10 M).
Figure llf is a graph of fluorescence units/minute represented in Figure ii e.
Figures lle and llf show relative to vehicle, COL (carboplatin) and TAX
(tamoxifen)
that Compound 1 demonstrates a dose-dependent decrease in tubulin
polymerization;
wherein, the data taken as a whole suggest that Compound 1 directly inhibits
microtubule formation.
Figure 12a a is a graph of average tumor volumes over time of subcutaneous
patient-derived xenografts derived from a human PDA treated with vehicle
(veh),
gemcitabine (gem), nab-paclitaxel (nabP), a combination of gemcitabine and nab-
paclitaxel (gm/nabP), Compound 1 (Cpd 1), a combination of Compound 1 and
gemcitabine (Cpd 1/gem), a combination of Compound 1 and nab-paclitaxel (Cpd
1/nabP), and a combination of Compound 1, gemcitabine, and nab-paclitaxel (Cpd
1/gem/nabP).
Figure 12b is a graph of the body weight over time of mice treated with
vehicle
(veh), gemcitabine (gem), nab-paclitaxel (nabP), a combination of gemcitabine
and
nab-paclitaxel (gm/nabP), Compound 1 (Cpd 1), a combination of Compound 1 and
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
gemcitabine (Cpd 1/gem), a combination of Compound 1 and nab-paclitaxel (Cpd
1/nabP), and a combination of Compound 1, gemcitabine, and nab-paclitaxel (Cpd
1/gem/nabP).
Figure 12c depicts growth and decay constants for tumors treated with
gemcitabine (gem), nab-paclitaxel (nabP), and Compound 1 (Cpd 1).
Figure 12d is a plot of tumor response of each mouse treated with vehicle
(veh),
gemcitabine (gem), nab-paclitaxel (nabP), a combination of gemcitabine and nab-
paclitaxel (gm/nabP), Compound 1 (Cpd 1), a combination of Compound 1 and
gemcitabine (Cpd 1/gem), a combination of Compound 1 and nab-paclitaxel (Cpd
1/nabP), and a combination of Compound 1, gemcitabine, and nab-paclitaxel (Cpd
1/gem/nabP). Figures 12a, 12b, 12c, and 12d show that Compound 1 in either or
both
dual combination with nab-paclitaxel and triple combination with gemcitabine
and nab-
paclitaxel synergistically decreases tumor volume and overall tumor growth in
a
human-derived xenograft model of FDA; wherein, Figure 12a shows relative to
vehicle,
gemcitabine alone, Compound 1 alone, nab-paclitaxel alone, gemcitabine in
combination with nab-paclitaxel and gemcitabine in combination with Compound 1
that
Compound 1 in either or both dual combination with nab-paclitaxel and triple
combination with gemcitabine and nab-paclitaxel synergistically decrease tumor
volume; Figure 12b shows relative to vehicle, gemcitabine alone, Compound 1
alone,
nab-paclitaxel alone, gemcitabine in combination with nab-paclitaxel and
gemcitabine
in combination with Compound 1, Compound 1 in combination with nab-paclitaxel
and
Compound 1 in combination with both gemcitabine and nab-paclitaxel
synergistically
that all combinations maintain relative KPC mouse body weight; Figure 12c
shows
relative to the presence or absence of any of gemcitabine, Compound 1 and nab-
paclitaxel that Compound 1 in combination with either or both gemcitabine and
nab-
paclitaxel significantly decreases the overall rate of tumor growth while
increasing the
decay rate of tumor volume; and, Figure 12d shows relative to the presence or
absence of any of gemcitabine, Compound 1 and nab-paclitaxel that Compound 1
in
combination with either or both gemcitabine and nab-paclitaxel significantly
decreases
.. initial tumor volume.
11
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Definitions
As used herein, the term "about" means a range around a given value wherein
the resulting value is substantially the same as the expressly recited value.
In one
aspect, "about" means within 25% of a given value or range. For example, the
phrase
"about 70% by weight" comprises at least all values from 52% to 88% by weight.
In
another aspect, the term "about" means within 10% of a given value or range.
For
example, the phrase "about 70% by weight" comprises at least all values from
63% to
77% by weight. In another aspect, the term "about" means within 7% of a given
value
or range. For example, the phrase "about 70% by weight" comprises at least all
values
from 65% to 75% by weight.
Concentrations, amounts, cell counts, percentages and other numerical values
may be presented herein in a range format. It is to be understood that such
range
format is used merely for convenience and brevity and should be interpreted
flexibly to
include not only the numerical values explicitly recited as the limits of the
range but
also to include all the individual numerical values or sub-ranges encompassed
within
that range as if each numerical value and sub-range was explicitly recited.
As used herein, the terms "therapies" and "therapy" can refer to any
protocol(s),
method(s), compositions, formulations, and/or agent(s) that can be used in the
prevention, treatment, management, or amelioration of a condition or disorder
or one or
more symptoms thereof (e.g., pancreatic cancer or one or more symptoms or one
or
more conditions associated therewith).
In certain aspects, the terms "therapies" and "therapy" refer to drug therapy
such
as chemotherapy, adjuvant therapy, radiation, surgery, biological therapy,
supportive
therapy, antiviral therapy and/or other therapies useful in treatment,
management,
prevention, or amelioration of a condition or disorder or one or more symptoms
thereof
(e.g., pancreatic cancer or one or more symptoms or one or more conditions
associated therewith). In certain aspects, the term "therapy" refers to a
therapy other
than Compound 1 or a pharmaceutically acceptable salt or pharmaceutical
composition
thereof. In specific aspects, an "additional therapy" and "additional
therapies" refer to a
therapy other than a treatment using Compound 1 or a pharmaceutically
acceptable
12
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
salt or pharmaceutical composition thereof. In a specific aspect, a therapy
includes the
use of Compound 1 as an adjuvant therapy. For example, using Compound 1 in
conjunction with a drug therapy such as chemotherapy, biological therapy,
surgery,
supportive therapy, antiviral therapy and/or other therapies useful in
treatment,
management, prevention, or amelioration of a condition or disorder or one or
more
symptoms thereof (e.g., pancreatic cancer or one or more symptoms or one or
more
conditions associated therewith).
As used herein, the term "human infant" refers to a newborn to 1 year old year
human.
As used herein, the term "human toddler" refers to a human that is 1 year to 3
years old.
As used herein, the term "human child" refers to a human that is 1 year to 18
years old.
As used herein, the term "human adult" refers to a human that is 18 years or
older.
As used herein, the term "middle-aged human" refers to a human between the
ages of 30 and 64.
As used herein, the term "elderly human" refers to a human 65 years or older.
As used herein, the term "subject" refers to an individual being administered
a
therapy as described herein. In a specific aspect, the individual is a human.
As used herein, the term "pancreatic cancer" refers to pancreatic cancer
generally as described herein. In a specific aspect, the general term
pancreatic cancer
may refer to a pancreatic ductal adenocarcinoma (FDA) without specifically
using the
term.
As used herein, the term "effective amount" in the context of administering
Compound 1 to a subject having a pancreatic cancer refers to the dose of
Compound 1
that results in a beneficial or therapeutic effect. In specific aspects, an
"effective
amount" of Compound 1 refers to an amount of Compound 1 which is sufficient to
achieve at least one, two, three, four or more of the following beneficial or
therapeutic
effects: (i) inhibition of a pancreatic cancer; (ii) regression of the
pancreatic cancer;
13
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
(iii) eradication, removal, or complete remission of the pancreatic cancer;
(iv)
prevention of the development or onset of one or more symptoms associated with
the
pancreatic cancer; (v) reduction or amelioration of the severity of one or
more
symptoms associated with the pancreatic cancer; (vi) the reduction in the
number of
one or more symptoms associated with the pancreatic cancer; (vii) amelioration
of the
severity of one or more symptoms associated with the pancreatic cancer; (viii)
reduction in the duration of one or more symptoms associated with the
pancreatic
cancer; (ix) prevention in the recurrence of proliferation or one or more
symptoms
associated with the pancreatic cancer; (x) a reduction in mortality; (xi) an
increase in
survival rate of subjects; (xii) an increase in relapse free survival; (xiii)
an increase in
the number of pancreatic cancer subjects in remission; (xiv) reduction in
hospitalization
of a subject; (xv) reduction in hospitalization length; (xvi) a decrease in
hospitalization
rate; (xvii) an increase in the survival of a subject; (xviii) an increase in
symptom-free
survival of a pancreatic cancer subject; (xix) an increase in the length of a
period of
remission of a pancreatic cancer in a subject; (xx) improvement in quality of
life (QOL)
as assessed by methods well known in the art, e.g., QOL questionnaires and the
like;
(xxi) a reduction in proliferation from administration of Compound 1 before
treatment
with another chemotherapeutic agent; (xxii) a reduction in proliferation from
administration of Compound 1 after treatment with another chemotherapeutic
agent;
(xxiii) a reduction in proliferation in a combination therapy from
administration of
Compound 1 with another chemotherapeutic agent; (xxiv) an additive
antiproliferative
effect in a combination therapy from administration of Compound 1 with another
chemotherapeutic agent; (xxv) a synergistic antiproliferative effect in a
combination
therapy from administration of Compound 1 with another chemotherapeutic agent;
(xxvi) a reduction in proliferation from administration of Compound 1 before
therapy
with radiation; (xxvii) a reduction in proliferation from administration of
Compound 1
after therapy with radiation; (xxviii) a reduction in proliferation from
administration of
Compound 1 in a combination therapy with radiation; (xxix) a reduction in
proliferation
from administration of Compound 1 before treatment with surgery; (xxx) a
reduction in
proliferation from administration of Compound 1 in a combination treatment
with
14
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
surgery; (xxxi) enhancement of or improvement of the therapeutic effect from
administration of Compound 1 with a palliative therapy; (xxxii) a decrease in
the
plasma concentration of BMI-1 in a subject having a pancreatic cancer;
(xxxiii) a
decrease in circulating proliferative cells in the plasma of a subject having
a pancreatic
.. cancer; (xxxiv) an alteration (e.g., a decrease or increase) in the plasma
concentration
of a pancreatic cancer biomarker in a subject having a pancreatic cancer
(e.g., BMI-1,
tubulin polymerization, apoptotic markers or tissue and the like); (xxxv)
reduction in the
concentration of BMI-1 in a biological specimen (e.g., plasma, serum, urine,
or any
other biofluids) from a subject having a pancreatic cancer; (xxxvi)
proliferative cell
count is maintained after administration of a therapy as described herein as
measured
by conventional methods available to one skilled in the art, such as magnetic
resonance imaging (MRI), dynamic contrast-enhanced MRI (DCE-MRI), X-ray,
computed tomography (CT) scan, positron emission tomography (PET) scan,
7-AAD fluorescence, or DAPI fluorescence; (xxxvii) proliferative cell count is
reduced
.. after administration of a therapy as described herein as measured by
conventional
methods available to one skilled in the art, such as magnetic resonance
imaging (MRI),
dynamic contrast-enhanced MRI (DCE-MRI), X-ray, computed tomography (CT) scan,
positron emission tomography (PET) scan, 7-AAD fluorescence, or DAPI
fluorescence;
or, (xxxviii) proliferative cell count does not increase or increases by less
than expected
after administration of a therapy as described herein as measured by
conventional
methods available to one skilled in the art, such as magnetic resonance
imaging (MRI),
dynamic contrast-enhanced MRI (DCE-MRI), X-ray, computed tomography (CT) scan,
or a positron emission tomography (PET) scan, 7-AAD fluorescence, or
DAPI fluorescence.
As used herein, the term "in a 24 hour period" refers to a period of time over
which a condition is maintained; for example, the effective amount of Compound
1 is
identified when the mean plasma concentration of Compound 1 is achieved and
maintained for a plurality of 24 hour periods. In other words, the mean plasma
concentration of Compound 1 may be reached in a suitable time, which may be
more
.. or less than 24 hours.
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
As used herein, the term "a therapy as described herein" refers to a method of
use for Compound 1 or a pharmaceutically acceptable salt or pharmaceutical
composition thereof as an inhibitor of BMI-1 function by targeting inhibition
of tubulin
polymerization in treating or ameliorating a pancreatic cancer in a subject in
need
thereof comprising, administering to the subject an effective amount of
Compound 1.
In one aspect, the pancreatic cancer is a pancreatic ductal adenocarcinoma. In
another aspect of the therapy described herein, the method of use for Compound
1 or
a pharmaceutically acceptable salt or pharmaceutical composition thereof
comprises a
combination with other chemotherapeutic agents having synergistic
antiproliferative
activity. In one aspect, the other chemotherapeutic agent inhibits BMI-1
functional
activity. In another aspect, the other chemotherapeutic agent inhibits tubulin
polymerization.
As used herein, the term "pharmaceutically acceptable salt(s)" refers to a
salt
prepared from a pharmaceutically acceptable non-toxic acid or base including
an
.. inorganic acid and base and an organic acid and base; see, for example,
Remington's
Pharmaceutical Sciences, 18th eds., Mack Publishing, Easton PA (1990) or
Remington:
The Science and Practice of Pharmacy, 19th eds., Mack Publishing, Easton PA
(1995).
As used herein, the term "Compound 1" generally refers to a 5-fluoro-2-(6-
fluoro-2-methyl-1H-benzo[d]imidazol-1-y1)-N444-
(trifluoromethyl)phenyl]pyrimidine-4,6-
diamine compound and pharmaceutically acceptable salts thereof. "Compound 1"
may
be substantially pure (e.g., about 90%, about 95%, about 98%, about 99%, or
about
99.9% pure). In various aspects, the term "Compound 1" refers to Compound 109
disclosed in International Publication No. W02014/081906, which is
incorporated in its
entirety by reference herein.
Method of Use
As demonstrated herein, Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof is an inhibitor of tubulin polymerization
and BMI-1
function for use in treating or ameliorating a pancreatic cancer in a subject
in need
thereof comprising, administering to the subject an effective amount of
Compound 1 or
a pharmaceutically acceptable salt or pharmaceutical composition thereof.
16
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
In one aspect, the pancreatic cancer is a pancreatic ductal adenocarcinoma.
In another aspect, the method of use for Compound 1 or a pharmaceutically
acceptable salt or pharmaceutical composition thereof comprises, a combination
of
Compound 1 or a pharmaceutically acceptable salt or pharmaceutical composition
thereof with one or more other chemotherapeutic agents, wherein the
combination
demonstrates synergistic antiproliferative activity. In one aspect, the other
chemotherapeutic agent inhibits BMI-1 functional activity. In another aspect,
the other
chemotherapeutic agent inhibits tubulin polymerization. However, there are no
known
or approved inhibitors of either or both BMI-1 functional activity or tubulin
polymerization for use in treating pancreatic cancers. Accordingly, potent and
selective
activity, favorable pharmaceutical properties and extensive clinical
experience suggest
that Compound 1 is a useful agent for treatment of a pancreatic cancer.
In one aspect, methods for inhibiting or reducing tubulin polymerization and
BMI-1 function to induce cell-cycle arrest in a proliferating cell or cell
line are described
herein.
In another aspect, a method for inhibiting or reducing tubulin polymerization
and
BMI-1 function to induce cell-cycle arrest in a proliferating cell or cell
line comprises,
contacting Compound 1 or a pharmaceutically acceptable salt or pharmaceutical
composition thereof with a proliferating cell or cell line, which
proliferating cell or cell
line may be naïve or has been shown to be affected by the inhibition or a
reduction of
tubulin polymerization and BMI-1 function.
In another aspect, non-limiting examples of such cells or cell lines are
selected
from HL-60, HeLa, HT1080, HCT116, HEK293, NCI H460, U-87MG, ASPC-1, PL-45,
HPAF-2, PC-3, MDA-MB-231, MDA-MB-468, A431, SNU-1, AGS, Kato III, A549, Calu-
6, A375, SY5Y, SKOV3, Capan-1, sNF96.2, TIVE-L1, TIVE-L2, LNCaP cells and the
like. In a more specific aspect, the cell or cell line may be a pancreatic
cancer cell.
In one aspect, a method for inhibiting or reducing tubulin polymerization and
BMI-1 function in a subject having a pancreatic cancer in need thereof
comprises,
administering an effective amount of Compound 1 or a pharmaceutically
acceptable
salt or pharmaceutical composition thereof to the subject as described herein.
17
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
In a specific aspect, the subject is diagnosed with a pancreatic cancer
capable
of being treated by inhibiting or reducing tubulin polymerization and BMI-1
function.
In a specific aspect, a method for inhibiting or reducing tubulin
polymerization
and BMI-1 function as described herein inhibits or reduces tubulin
polymerization and
BMI-1 function by about 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%,
60%, 65%, 80%, 85%, 90%, 95%, or 100% relative to tubulin polymerization and
BMI-
1 function prior to administration of Compound 1 to the subject, as assessed
by
methods well known in the art.
In a specific aspect, a method for inhibiting or reducing tubulin
polymerization
and BMI-1 function as described herein inhibits or reduces tubulin
polymerization and
BMI-1 function in a range of from about 5% to about 20%, 10% to 30%, 15% to
40%,
15% to 50%, 20% to 30%, 20% to 40%, 20% to 50%, 30% to 60%, 30% to 70%, 30%
to 80%, 30% to 90%, 30% to 95%, 30% to 99%, or from about 40% to about 100%,
or
any range in between, relative to tubulin polymerization and BMI-1 function
prior to
administration of Compound 1 to the subject, as assessed by methods well known
in
the art.
In a specific aspect, a method for inhibiting or reducing tubulin
polymerization
and BMI-1 function as described herein inhibits proliferation or reduces an in
vitro or in
vivo proliferating cell or cell line population by about 5%, 10%, 15%, 20%,
25%, 30%,
35%, 40%, 45%, 50%, 55%, 60%, 65%, 80%, 85%, 90%, 95 %, or 100%, relative to
the in vitro or in vivo proliferating cell or cell line population prior to
administration of
Compound 1 to the subject, as assessed by methods well known in the art.
In a specific aspect, a method for inhibiting or reducing tubulin
polymerization
and BMI-1 function as described herein inhibits proliferation or reduces an in
vitro or in
vivo proliferating cell or cell line population in a range of from about 5% to
about 20%,
10% to 30%, 15% to 40%, 15% to 50%, 20% to 30%, 20% to 40%, 20% to 50%, 30%
to 60%, 30% to 70%, 30% to 80%, 30% to 90%, 30% to 95%, 30% to 99%, or from
about 40% to about 100%, or any range in between, relative to the in vitro or
in vivo
proliferating cell or cell line population prior to administration of Compound
1 to the
subject, as assessed by methods well known in the art.
18
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
In various aspects, a method for inhibiting or reducing tubulin polymerization
and BMI-1 function as described herein reduces the plasma concentration of BMI-
1 in
a subject as assessed by methods well known in the art, e.g., ELISA.
In one aspect, a method for preventing, treating or ameliorating a pancreatic
cancer in a subject in need thereof comprises, administering an amount of
Compound 1 effective to inhibit or reduce tubulin polymerization and BMI-1
function in
the subject is described herein.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof as described herein inhibits or
reduces
tubulin polymerization and BMI-1 function by about 5%, 10%, 15%, 20%, 25%,
30%,
35%, 40%, 45%, 50%, 55%, 60%, 65%, 80%, 85%, 90%, 95 %, or 100% relative to
tubulin polymerization and BMI-1 function prior to administration of Compound
1 to the
subject, as assessed by methods well known in the art.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof as described herein inhibits or
reduces
tubulin polymerization and BMI-1 function in a range of from about 5% to about
20%,
10% to 30%, 15% to 40%, 15% to 50%, 20% to 30%, 20% to 40%, 20% to 50%, 30%
to 60%, 30% to 70%, 30% to 80%, 30% to 90%, 30% to 95%, 30% to 99%, or from
about 40% to about 100%, or any range in between, relative to tubulin
polymerization
and BMI-1 function prior to administration of Compound 1 to the subject, as
assessed
by methods well known in the art.
In various aspects, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof as described herein reduces the
concentration of BMI-1 in a subject as assessed by methods well known in the
art, e.g.,
ELISA.
In one aspect, a method for preventing, treating or ameliorating a pancreatic
cancer in a subject in need thereof comprises, administering an amount of
Compound 1 effective to inhibit proliferation or reduce an in vitro or in vivo
proliferating
cell or cell line population in the subject is described herein.
In a specific aspect, a method for preventing, treating or ameliorating a
19
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
pancreatic cancer in a subject in need thereof as described herein inhibits
proliferation
or reduces an in vitro or in vivo proliferating cell or cell line population
in the subject by
abOUt 5O/0, 10 /0, 15 /0, 20 /0, 25 /0, 30 /0, 35 /0, 40 /0, 45 /0, 50 /0, 55
/0, 60 /0, 65 /0, 80 /0,
85%, 90%, 95 %, or 100% relative to proliferation or in vitro or in vivo
proliferating cell
or cell line population in the subject prior to administration of Compound 1
to the
subject, as assessed by methods well known in the art.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof as described herein inhibits
proliferation
or reduces an in vitro or in vivo proliferating cell or cell line population
in the subject in
a range of from about 5% to about 20%, 10% to 30%, 15% to 40%, 15% to 50%, 20%
to 30%, 20% to 40%, 20% to 50%, 30% to 60%, 30% to 70%, 30% to 80%, 30% to
90%, 30% to 95%, 30% to 99%, or from about 40% to about 100%, or any range in
between, relative to proliferation or in vitro or in vivo proliferating cell
or cell line
population in the subject prior to administration of Compound 1 to the
subject, as
assessed by methods well known in the art.
In various aspects, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof as described herein inhibits
proliferation
or reduces an in vitro or in vivo proliferating cell or cell line population
in a subject as
assessed by methods well known in the art, e.g., ELISA.
In one aspect, a method for preventing, treating or ameliorating a pancreatic
cancer in a subject in need thereof comprises, administering an amount of
Compound 1 effective to inhibit proliferation or reduce an in vitro or in vivo
proliferating
cell or cell line population in the subject in combination with another
therapy (e.g., one
or more additional therapies that do not comprise Compound 1, or that comprise
a
different anti-proliferative agent) to a subject in need thereof is described
herein.
Such methods may involve administering Compound 1 prior to, concurrent with,
or subsequent to administration of the additional therapy. In certain aspects,
such
methods have an additive or synergistic effect.
In a specific aspect, presented herein is a method for preventing, treating or
ameliorating a pancreatic cancer in a subject in need thereof comprising,
administering
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
to a subject in need thereof an effective amount of Compound 1 and an
effective
amount of another therapy.
Specific examples of cancers that can be prevented, treated or ameliorated in
accordance with the methods provided herein include, but are not limited to,
pancreatic
cancers such as but not limited to, pancreatic ductal adenocarcinoma.
In certain aspects, pancreatic cancers that can be prevented, treated or
ameliorated in accordance with the methods provided herein are selected from
pancreatic ductal adenocarcinoma.
In one aspect, presented herein is a method for preventing, treating or
ameliorating a pancreatic cancer, comprising: (a) administering to a subject
in need
thereof one or more doses of Compound 1 or a pharmaceutically acceptable salt
or
pharmaceutical composition thereof a pharmaceutical composition thereof; and
(b)
monitoring the concentration of certain biomarkers, before and/or after step
(a).
In a specific aspect, the monitoring step (b) is carried out before and/or
after a
certain number of doses (e.g., 1, 2, 4, 6, 8, 10, 12, 14, 15, or 29 doses, or
more doses;
2 to 4, 2 to 8, 2 to 20 or 2 to 30 doses) or a certain time period (e.g., 1,
2, 3, 4, 5, 6, or
7 days; or 1, 2, 3, 4, 5, 10, 15, 20, 30, 40, 45, 48, or 50 weeks) of
administering
Compound 1 or a pharmaceutically acceptable salt or pharmaceutical composition
thereof.
In a specific aspect, one or more of these monitoring parameters are detected
prior to administration of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof to the subject.
In a specific aspect, a decrease in the proliferation of an in vitro or in
vivo
proliferating cell or cell line population following administration of
Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof
indicates that
the course of treatment is effective for preventing, treating or ameliorating
the
pancreatic cancer.
In a specific aspect, a change in the proliferation of an in vitro or in vivo
proliferating cell or cell line population following administration of
Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof may
indicate
21
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
that the dosage, frequency and/or length of administration of Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof may be
adjusted (e.g., increased, reduced or maintained).
In a specific aspect, the concentration of certain biomarkers in biological
specimens of a subject is monitored before, during and/or after a course of
treatment
for a pancreatic cancer involving the administration of Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof to the
subject.
The dosage, frequency and/or length of administration of Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof to a
subject
might be modified as a result of the proliferation of an in vitro or in vivo
proliferating cell
or cell line population. Alternatively, the changes in these monitoring
parameters (e.g.,
concentration of certain biomarkers) might indicate that the course of
treatment
involving the administration of the Compound 1 or a pharmaceutically
acceptable salt
or pharmaceutical composition thereof is effective in preventing, treating or
ameliorating the pancreatic cancer.
The concentration of certain biomarkers in a subject may be detected by any
technique known to one of skill in the art. In certain aspects, the method for
detecting
the concentration of certain biomarkers of a subject comprises obtaining a
biological
sample (e.g., tissue or fluid sample) from the subject and detecting the
concentration of
the biomarkers in the biological sample (e.g., from plasma, serum, urine, or
any other
biofluids), that has been subjected to certain types of treatment (e.g.,
centrifugation),
and detection by use of immunological techniques, such as ELISA.
In a specific aspect, an ELISA assay, as described herein, may be used to
detect the concentration of the biomarkers in a biological sample (e.g., from
plasma,
.. serum, urine, or any other biofluids) that has been subjected to certain
types of
treatment (e.g., centrifugation). Other techniques known in the art that may
be used to
detect the concentration of the biomarkers in a biological sample include
multiplex or
proteomic assays.
In specific aspects, the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein alleviate or manage one, two or more
symptoms
22
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
associated with the pancreatic cancer. Alleviating or managing one, two or
more
symptoms of the pancreatic cancer may be used as a clinical endpoint for
efficacy of
Compound 1 or a pharmaceutically acceptable salt or pharmaceutical composition
thereof for preventing, treating or ameliorating the pancreatic cancer. In
some aspects,
the methods for preventing, treating or ameliorating the pancreatic cancer
provided
herein reduce the duration and/or severity of one or more symptoms associated
with
the pancreatic cancer. In some aspects, the methods for preventing, treating
or
ameliorating the pancreatic cancer provided herein inhibit the onset,
progression
and/or recurrence of one or more symptoms associated with the pancreatic
cancer. In
some aspects, the methods for treating the pancreatic cancer provided herein
reduce
the number of symptoms associated with the pancreatic cancer.
In certain aspects, the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein prolong or delay the G1/S or late G1/S phase
of the
cell cycle (i.e., the period between the late checkpoint (resting or pre-DNA
synthesis
phase), and the early DNA synthesis phase). In other aspects, the methods for
preventing, treating or ameliorating a pancreatic cancer provided herein
prolong or
delay the S or G2/M phase of the cell cycle (i.e., the period between DNA
synthesis
and the early division phase).
In some aspects, the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein reduce, ameliorate, or alleviate the
severity of the
pancreatic cancer and/or one or more symptoms thereof. In other aspects, the
methods for preventing, treating or ameliorating a pancreatic cancer provided
herein
reduce hospitalization (e.g., the frequency or duration of hospitalization) of
a subject
diagnosed with the pancreatic cancer.
In certain aspects, the methods provided herein increase the survival of a
subject diagnosed with a pancreatic cancer. In specific aspects, the methods
provided
herein increase the survival of a subject diagnosed with a pancreatic cancer
by about 6
months or more, about 7 months or more, about 8 months or more, about 9 months
or
more, or about 12 months or more.
In particular aspects, the methods for preventing, treating or ameliorating a
23
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
pancreatic cancer provided herein inhibit or reduce the progression of the
pancreatic
cancer, or one or more symptoms associated therewith. In specific aspects, the
methods for preventing, treating or ameliorating a pancreatic cancer provided
herein
enhance or improve the therapeutic effect of another therapy (e.g., an anti-
cancer
agent, radiation, drug therapy, such as chemotherapy, anti-androgen therapy,
or
surgery). In certain aspects, the methods for preventing, treating or
ameliorating a
pancreatic cancer provided herein involve the use of Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof as an
adjuvant
therapy.
In particular aspects, the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein reduce the mortality of subjects diagnosed
with the
pancreatic cancer. In certain aspects, the methods for preventing, treating or
ameliorating a pancreatic cancer provided herein increase the number of
subjects in
remission or decrease the hospitalization rate. In other aspects, the methods
for
preventing, treating or ameliorating a pancreatic cancer provided herein
prevent the
development, onset or progression of one or more symptoms associated with the
pancreatic cancer.
In particular aspects, the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein increase symptom-free survival of pancreatic
cancer
subjects. In some aspects, the methods for preventing, treating or
ameliorating a
pancreatic cancer provided herein do not cure the pancreatic cancer in
subjects, but
prevent the progression or worsening of the disease. In some aspects, the
methods
for preventing, treating or ameliorating a pancreatic cancer provided herein
improve the
subject's quality of life.
In certain aspects, the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein increase the cancer-free survival rate of
subjects
diagnosed with the cancer. In some aspects, the methods for preventing,
treating or
ameliorating a pancreatic cancer provided herein increase relapse-free
survival. In
certain aspects, the methods for preventing, treating or ameliorating a
pancreatic
cancer provided herein increase the number of subjects in remission. In other
aspects,
24
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
the methods for preventing, treating or ameliorating a pancreatic cancer
provided
herein increase the length of remission in subjects.
Treatment Population
In some aspects, a subject treated for a pancreatic cancer in accordance with
the methods provided herein is a human who has or is diagnosed with a
pancreatic
cancer. In other aspects, a subject treated for a pancreatic cancer in
accordance with
the methods provided herein is a human predisposed or susceptible to a
pancreatic
cancer. In some aspects, a subject treated for a pancreatic cancer in
accordance with
the methods provided herein is a human at risk of developing a pancreatic
cancer.
In one aspect, a subject treated for a pancreatic cancer in accordance with
the
methods provided herein is a human infant. In another aspect, a subject
treated for a
pancreatic cancer in accordance with the methods provided herein is a human
toddler.
In another aspect, a subject treated for a pancreatic cancer in accordance
with the
methods provided herein is a human child. In another aspect, a subject treated
for a
pancreatic cancer in accordance with the methods provided herein is a human
adult.
In another aspect, a subject treated for a pancreatic cancer in accordance
with the
methods provided herein is a middle-aged human. In another aspect, a subject
treated
for a pancreatic cancer in accordance with the methods provided herein is an
elderly
human.
In certain aspects, a subject treated for cancer in accordance with the
methods
provided herein has a pancreatic cancer metastasized to other areas of the
body, such
as the bones, lung and liver. In certain aspects, a subject treated for
pancreatic cancer
in accordance with the methods provided herein is in remission from the
pancreatic
cancer. In some aspects, the subject treated for pancreatic cancer in
accordance with
the methods provided herein had a recurrence of the pancreatic cancer. In
certain
aspects, a subject treated in accordance with the methods provided herein is
experiencing recurrence of one or more symptoms associated with the pancreatic
cancer.
In certain aspects, a subject treated for a pancreatic cancer in accordance
with
the methods provided herein is a human that is about 1 to about 5 years old,
about 5 to
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
years old, about 10 to about 18 years old, about 18 to about 30 years old,
about 25
to about 35 years old, about 35 to about 45 years old, about 40 to about 55
years old,
about 50 to about 65 years old, about 60 to about 75 years old, about 70 to
about 85
years old, about 80 to about 90 years old, about 90 to about 95 years old or
about 95
5 to about 100 years old, or any age in between.
In a specific aspect, a subject treated for a pancreatic cancer in accordance
with
the methods provided herein is a human that is 18 years old or older. In a
particular
aspect, a subject treated for a pancreatic cancer in accordance with the
methods
provided herein is a human child that is between the age of 1 year old to 18
years old.
10 In a certain aspect, a subject treated for a pancreatic cancer in
accordance with the
methods provided herein is a human that is between the age of 12 years old and
18
years old. In a certain aspect, the subject is a male human. In another
aspect, the
subject is a female human. In one aspect, the subject is a female human that
is not
pregnant or is not breastfeeding. In one aspect, the subject is a female that
is
pregnant or will/might become pregnant, or is breast feeding.
In particular aspects, a subject treated for a pancreatic cancer in accordance
with the methods provided herein is a human that is in an immunocompromised
state
or immunosuppressed state. In certain aspects, a subject treated for a
pancreatic
cancer in accordance with the methods provided herein is a human receiving or
recovering from immunosuppressive therapy. In certain aspects, a subject
treated for
a pancreatic cancer in accordance with the methods provided herein is a human
that
has or is at risk of getting a pancreatic cancer. In certain aspects, a
subject treated for
a pancreatic cancer in accordance with the methods provided herein is a human
who
is, will or has undergone surgery, drug therapy, such as chemotherapy,
hormonal
therapy and/or radiation therapy.
In some aspects, a subject treated for a pancreatic cancer in accordance with
the methods provided herein is administered Compound 1 or a pharmaceutically
acceptable salt or pharmaceutical composition thereof, or a combination
therapy before
any adverse effects or intolerance to therapies other than Compound 1
develops. In
some aspects, a subject treated for a pancreatic cancer in accordance with the
26
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
methods provided herein is a refractory subject. In certain aspects, a
refractory subject
is a subject refractory to a standard therapy (e.g., surgery, radiation and/or
drug
therapy such as chemotherapy). In certain aspects, a subject with a pancreatic
cancer
is refractory to a therapy when the pancreatic cancer has not significantly
been
eradicated and/or the one or more symptoms have not been significantly
alleviated.
The determination of whether a subject refractory can be made either in vivo
or in vitro
by any method known in the art for assaying the effectiveness of a treatment
of a
pancreatic cancer, using art-accepted meanings of "refractory" in such a
context.
In some aspects, a subject treated for a pancreatic cancer in accordance with
the methods provided herein is a human that has proven refractory to therapies
other
than treatment with Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof, but is no longer on these therapies. In
certain
aspects, a subject treated for a pancreatic cancer in accordance with the
methods
provided herein is a human already receiving one or more conventional anti-
cancer
therapies, such as surgery, drug therapy such as chemotherapy, anti-androgen
therapy
or radiation. Among these subjects are refractory subjects, subjects who are
too young
for conventional therapies, and subjects with recurring pancreatic cancers
despite
treatment with existing therapies.
In some aspects, a subject treated for a pancreatic cancer in accordance with
the methods provided herein is a human susceptible to adverse reactions to
conventional therapies. In some aspects, a subject treated for a pancreatic
cancer in
accordance with the methods provided herein is a human that has not received a
therapy, e.g., drug therapy such as chemotherapy, surgery, anti-androgen
therapy or
radiation therapy, prior to the administration of Compound 1 or a
pharmaceutically
acceptable salt or pharmaceutical composition thereof. In other aspects, a
subject
treated for a pancreatic cancer in accordance with the methods provided herein
is a
human that has received a therapy prior to administration of Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof. In
some
aspects, a subject treated for a pancreatic cancer in accordance with the
methods
provided herein is a human that has experienced adverse side effects to the
prior
27
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
therapy or the prior therapy was discontinued due to unacceptable levels of
toxicity to
the human.
Dosage and Administration
In accordance with the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein, Compound 1 or a pharmaceutically acceptable
salt
or pharmaceutical composition thereof can be administered to a subject in need
thereof
by a variety of routes in amounts which result in a beneficial or therapeutic
effect.
Compound 1 or a pharmaceutically acceptable salt or pharmaceutical composition
thereof may be orally administered to a subject in need thereof in accordance
with the
methods for preventing, treating or ameliorating a pancreatic cancer provided
herein.
The oral administration of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof may facilitate subjects in need of such
treatment
complying with a regimen for taking Compound 1 or a pharmaceutically
acceptable salt
or pharmaceutical composition thereof. Thus, in a specific aspect, Compound 1
or a
pharmaceutically acceptable salt or pharmaceutical composition thereof is
administered orally to a subject in need thereof. In another aspect, Compound
1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof
provided
herein can be administered orally, with or without food or water.
Other routes of administration include, but are not limited to, intravenous,
intradermal, intrathecal, intramuscular, subcutaneous, intranasal, inhalation,
transdermal, topical, transmucosal, intracranial, epidural and intra-synovial.
In one
aspect, Compound 1 or a pharmaceutically acceptable salt or pharmaceutical
composition thereof is administered systemically (e.g., parenterally) to a
subject in
need thereof. In one aspect, Compound 1 or a pharmaceutically acceptable salt
or
pharmaceutical composition thereof is administered via a route that permits
Compound 1 or a pharmaceutically acceptable salt or pharmaceutical composition
thereof to cross the blood-brain barrier (e.g., orally).
In accordance with the methods for preventing, treating or ameliorating a
pancreatic cancer provided herein that involve administration of Compound 1 or
a
pharmaceutically acceptable salt or pharmaceutical composition thereof in
combination
28
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
with one or more additional therapies, Compound 1 or a pharmaceutically
acceptable
salt or pharmaceutical composition thereof and one or more additional
therapies may
be administered by the same route or a different route of administration.
The dosage and frequency of administration of Compound 1 or a
pharmaceutically acceptable salt or pharmaceutical composition thereof is
administered to a subject in need thereof in accordance with the methods for
preventing, treating or ameliorating a pancreatic cancer provided herein will
be
efficacious while minimizing any side effects. The exact dosage and frequency
of
administration of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical
composition thereof can be determined by a practitioner, in light of factors
related to
the subject that requires treatment.
Factors which may be taken into account include the severity of the disease
state, general health of the subject, age, weight, and gender of the subject,
diet, time
and frequency of administration, drug combination(s), reaction sensitivities,
and
tolerance/response to therapy. The dosage and frequency of administration of
Compound 1 or a pharmaceutically acceptable salt or pharmaceutical composition
thereof may be adjusted over time to provide an effective amount of Compound 1
or a
pharmaceutically acceptable salt or pharmaceutical composition thereof or to
maintain
the desired effect.
As described herein, the methods for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof presented herein comprises,
administering to the subject an effective amount of Compound 1 or a
pharmaceutically
acceptable salt or pharmaceutical composition thereof, wherein the effective
amount is
a dose administered to the subject twice per week on different days, wherein
the
second dose in a week follows the first by three days, and wherein the first
dose in a
following week follows the second dose in a preceding week by four days.
In a specific aspect, the effective amount is a dose administered to the
subject
that may be increased or decreased depending on subject response.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
29
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
effective amount of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof to the subject, wherein the effective
amount is a
dose selected from a dose in a range of from about 50 mg to about 200 mg, from
about
100 mg to about 200 mg, from about 150 mg to about 200 mg, and the like, or
any
range in between, administered orally twice per week.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
effective amount of Compound 1 or a pharmaceutically acceptable salt or
pharmaceutical composition thereof to the subject, wherein the effective
amount is a
dose selected from about 50 mg, about 100 mg, about 150 mg or about 200 mg,
and
the like, or any range in between, administered orally twice per week.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
effective amount of Compound 1 or a pharmaceutically acceptable salt or
.. pharmaceutical composition thereof to the subject, wherein the effective
amount is a
dose of about 50 mg administered orally twice per week.
In some aspects, a method for preventing, treating or ameliorating a
pancreatic
cancer in a subject in need thereof comprises the administration of an
effective amount
of Compound 1 or a pharmaceutical composition thereof to the subject, wherein
the
effective amount is a dosage that is expressed as mg per meter squared
(mg/m2). The
mg/m2 for Compound 1 may be determined, for example, by multiplying a
conversion
factor for an animal by an animal dose in mg per kilogram (mg/kg) to obtain
the dose in
mg/m2 for human dose equivalent. For regulatory purposes, the following
conversion
factors may be used: Mouse = 3, Hamster = 4.1, Rat = 6, Guinea Pig = 7.7.
(based on
Freireich etal., Cancer Chemother. Rep. 50(4):219-244 (1966)). The height and
weight of a human may be used to calculate a human body surface area applying
Boyd's Formula of Body Surface Area. In specific aspects, a method for
preventing,
treating or ameliorating a pancreatic cancer in a subject in need thereof
comprises the
administration of an effective amount of Compound 1 or a pharmaceutical
composition
thereof to the subject, wherein the effective amount is an amount in the range
of from
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
about 0.1 mg/m2 to about 1000 mg/m2, or any range in between.
In one aspect, a method for preventing, treating or ameliorating a pancreatic
cancer in a subject in need thereof comprises the administration of an
effective amount
of Compound 1 or a pharmaceutical composition thereof to the subject, wherein
the
effective amount is a dosage that achieves a target mean plasma concentration
of
Compound 1 in a subject with a pancreatic cancer or an animal model with a pre-
established pancreatic cancer.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
effective amount of Compound 1 or a pharmaceutical composition thereof to the
subject, wherein the effective amount is a dosage that achieves a mean plasma
concentration of Compound 1 in a 24 hour period in a range of from
approximately 3
hr=pg/mL to approximately 70 hr=pg/mL, from approximately 3 hr=pg/mL to
approximately 60 hr=pg/mL, from approximately 3 hr=pg/mL to approximately
50 hr=pg/mL, from approximately 3 hr=pg/mL to approximately 40 hr=pg/mL, from
approximately 3 hr=pg/mL to approximately 30 hr=pg/mL, from approximately
3 hr=pg/mL to approximately 20 hr=pg/mL, from approximately 3 hr=pg/mL to
approximately 10 hr=pg/mL, and the like, or any range in between, in a subject
with the
pancreatic cancer or an animal model with a pre-established pancreatic cancer.
In a specific aspect, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
effective amount of Compound 1 or a pharmaceutical composition thereof to the
subject, wherein the effective amount is a dosage that achieves a mean plasma
concentration of Compound 1 in a 24 hour period of approximately 3 hr=pg/mL,
approximately 10 hr=pg/mL, approximately 20 hr=pg/mL, approximately 30
hr=pg/mL,
approximately 40 hr=pg/mL, approximately 50 hr=pg/mL, approximately 60
hr=pg/mL,
approximately 70 hr=pg/mL, and the like, or any range in between, in a subject
with the
pancreatic cancer or an animal model with a pre-established pancreatic cancer.
To achieve such plasma concentrations, a dose described herein of
Compound 1 or a pharmaceutical composition thereof may be administered. In
certain
31
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
aspects, subsequent doses of Compound 1 or a pharmaceutical composition
thereof
may be adjusted accordingly based on the mean plasma concentrations of
Compound 1 achieved with a dose of Compound 1 or a pharmaceutical composition
thereof administered to the subject.
In specific aspects, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
effective amount of Compound 1 or a pharmaceutical composition thereof to the
subject, wherein the effective amount is a dosage that achieves a reduced
target mean
plasma concentration of one or more biomarkers in a subject with the
pancreatic
cancer or an animal model with a pre-established pancreatic cancer.
In particular aspects, a method for preventing, treating or ameliorating a
pancreatic cancer in a subject in need thereof comprises the administration of
an
effective amount of Compound 1 or a pharmaceutical composition thereof to the
subject, wherein the effective amount is a dosage that achieves the desired
tissue to
mean plasma concentration ratios of Compound 1 or a pharmaceutical composition
thereof as determined, e.g., by any imaging techniques known in the art, in a
subject
with the pancreatic cancer or an animal model with a pre-established
pancreatic
cancer.
In some aspects, a method for preventing, treating or ameliorating a
pancreatic
cancer in a subject in need thereof comprises the administration of an
effective amount
of Compound 1 or a pharmaceutical composition thereof to the subject, wherein
the
effective amount may or may not be the same for each dose. In particular
aspects, a
first (i.e., initial) dose of Compound 1 or a pharmaceutical composition
thereof is
administered to a subject in need thereof for a first period of time, followed
by a second
(i.e., loading) dose of Compound 1 or a pharmaceutical composition thereof is
administered to the subject for a second period of time and, subsequently, a
third (i.e.,
maintenance) dose of Compound 1 or a pharmaceutical composition thereof is
administered to the subject for a second period of time. The first dose may be
more
than the second dose, or the first dose may be less than the second dose. In
similar
fashion, the third dose of Compound 1 or a pharmaceutical composition thereof
may be
32
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
more or less than the second dose and more or less than the first dose.
In some aspects, the dosage amounts described herein refer to total amounts
administered; that is, if more than one Compound is administered, then, in
some
aspects, the dosages correspond to the total amount administered. In a
specific
.. aspect, oral compositions contain about 5% to about 95% of Compound 1 by
weight.
The length of time that a subject in need thereof is administered Compound 1
or
a pharmaceutical composition thereof in accordance with a method for
preventing,
treating or ameliorating a pancreatic cancer in a subject in need thereof will
be the time
period that is determined by cancer free survival or freedom from symptoms. In
certain
aspects, a method for treating a pancreatic cancer presented herein comprises
the
administration of Compound 1 or a pharmaceutical composition thereof for a
period of
time until the severity and/or number of one or more symptoms associated with
the
pancreatic cancer decreases.
In some aspects, a method for preventing, treating or ameliorating a
pancreatic
cancer in a subject in need thereof comprises the administration of Compound 1
or a
pharmaceutical composition thereof for up to 48 weeks. In other aspects, a
method for
preventing, treating or ameliorating a pancreatic cancer in a subject in need
thereof
comprises the administration of Compound 1 or a pharmaceutical composition
thereof
for up to 4 weeks, 8 weeks, 12 weeks, 16 weeks, 20 weeks, 24 weeks, 26 weeks
(0.5
.. year), 52 weeks (1 year), 78 weeks (1.5 years), 104 weeks (2 years), or 130
weeks
(2.5 years) or more.
In certain aspects, a method for preventing, treating or ameliorating a
pancreatic
cancer in a subject in need thereof comprises the administration of Compound 1
or a
pharmaceutical composition thereof for an indefinite period of time. In some
aspects, a
.. method for treating a pancreatic cancer presented herein comprises the
administration
of Compound 1 or a pharmaceutical composition thereof for a period of time
followed
by a period of rest (i.e., a period wherein Compound 1 or a pharmaceutical
composition
thereof is not administered) before the administration of Compound 1 or a
pharmaceutical composition thereof is resumed.
In specific aspects, a method for preventing, treating or ameliorating a
33
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
pancreatic cancer in a subject in need thereof comprises the administration of
Compound 1 or a pharmaceutical composition thereof in cycles, e.g., 1 week
cycles, 2
week cycles, 3 week cycles, 4 week cycles, 5 week cycles, 6 week cycles, 8
week
cycles, 9 week cycles, 10 week cycles, 11 week cycles, or 12 week cycles. In
such
cycles, Compound 1 or a pharmaceutical composition thereof may be administered
once or twice per week. In a specific aspect of a weekly cycle, Compound 1 or
a
pharmaceutical composition thereof may be administered twice per week. In a
specific
aspect of such a weekly cycle, Compound 1 or a pharmaceutical composition
thereof
may be administered once per day.
In specific aspects, the period of time of administration of Compound 1 or a
pharmaceutical composition thereof may be dictated by one or more monitoring
parameters, e.g., concentration of certain biomarkers.
In particular aspects, the period of time of administration of Compound 1 or a
pharmaceutical composition thereof may be adjusted based on one or more
monitoring
parameters, e.g., concentration of biomarkers.
In certain aspects, in accordance with a method for preventing, treating or
ameliorating a pancreatic cancer in a subject in need thereof, Compound 1 or a
pharmaceutical composition thereof is administered to a subject in need
thereof prior
to, concurrently with, or after a meal (e.g., breakfast, lunch, or dinner). In
specific
aspects, in accordance with the methods for treating a pancreatic cancer
presented
herein, Compound 1 or a pharmaceutical composition thereof is administered to
a
subject in need thereof in the morning (e.g., between 5 am and 12 pm).
In certain aspects, in accordance with a method for preventing, treating or
ameliorating a pancreatic cancer in a subject in need thereof, Compound 1 or a
pharmaceutical composition thereof is administered to a subject in need
thereof at
noon (i.e., 12 pm). In particular aspects, in accordance with the methods for
treating a
pancreatic cancer presented herein, Compound 1 or a pharmaceutical composition
thereof is administered to a subject in need thereof in the afternoon (e.g.,
between 12
pm and 5 pm), evening (e.g., between 5 pm and bedtime), and/or before bedtime.
In a specific aspect, a dose of Compound 1 or a pharmaceutical composition
34
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
thereof is administered to a subject once per day and twice per week.
Combination Therapies
Presented herein are combination therapies for the treatment of a pancreatic
cancer which involve the administration of Compound 1 or a pharmaceutical
composition thereof in combination with one or more additional therapies to a
subject in
need thereof. In a specific aspect, presented herein are combination therapies
for the
treatment of a pancreatic cancer which involve the administration of an
effective
amount of Compound 1 or a pharmaceutical composition thereof in combination
with
an effective amount of another therapy to a subject in need thereof.
As used herein, the term "in combination," refers, in the context of the
administration of Compound 1 or a pharmaceutical composition thereof, to the
administration of Compound 1 or a pharmaceutical composition thereof prior to,
concurrently with, or subsequent to the administration of one or more
additional
therapies (e.g., agents, surgery, or radiation) for use in treating a
pancreatic cancer.
The use of the term "in combination" does not restrict the order in which one
or more
therapeutic agents and one or more additional therapies are administered to a
subject.
In specific aspects, the interval of time between the administration of
Compound 1 or a
pharmaceutical composition thereof and the administration of one or more
additional
therapies may be about 1-5 minutes, 1-30 minutes, 30 minutes to 60 minutes, 1
hour,
1-2 hours, 2-6 hours, 2-12 hours, 12-24 hours, 1-2 days, 2 days, 3 days, 4
days, 5
days, 6 days, 7 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 7
weeks,
8 weeks, 9 weeks, 10 weeks, 15 weeks, 20 weeks, 26 weeks, 52 weeks, 11-15
weeks,
15-20 weeks, 20-30 weeks, 30-40 weeks, 40-50 weeks, 1 month, 2 months, 3
months,
4 months 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11
months,
12 months, 1 year, 2 years, or any period of time in between. In certain
aspects,
Compound 1 or a pharmaceutical composition thereof and one or more additional
therapies are administered less than 1 day, 1 week, 2 weeks, 3 weeks, 4 weeks,
one
month, 2 months, 3 months, 6 months, 1 year, 2 years, or 5 years apart.
In some aspects, the combination therapies provided herein involve
administering Compound 1 or a pharmaceutical composition thereof daily, and
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
administering one or more additional therapies once a week, once every 2
weeks, once
every 3 weeks, once every 4 weeks, once every month, once every 2 months
(e.g.,
approximately 8 weeks), once every 3 months (e.g., approximately 12 weeks), or
once
every 4 months (e.g., approximately 16 weeks). In certain aspects, Compound 1
or a
.. pharmaceutical composition thereof and one or more additional therapies are
cyclically
administered to a subject. Cycling therapy comprises the administration of
Compound 1 or a pharmaceutical composition thereof for a period of time,
followed by
the administration of one or more additional therapies for a period of time,
and
repeating this sequential administration. In certain aspects, cycling therapy
may also
include a period of rest where Compound 1 or a pharmaceutical composition
thereof or
the additional therapy is not administered for a period of time (e.g., 2 days,
3 days, 4
days, 5 days, 6 days, 7 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 10
weeks,
weeks, 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8
months, 9 months, 10 months, 11 months, 12 months, 2 years, or 3 years). In an
15 aspect, the number of cycles administered is from 1 to 12 cycles, from 2
to 10 cycles,
or from 2 to 8 cycles.
In some aspects, a method for preventing, treating or ameliorating a
pancreatic
cancer in a subject in need thereof comprises administering Compound 1 or a
pharmaceutical composition thereof as a single agent for a period of time
prior to
20 administering Compound 1 or a pharmaceutical composition thereof in
combination
with an additional therapy. In certain aspects, the methods for treating a
pancreatic
cancer provided herein comprise administering an additional therapy alone for
a period
of time prior to administering Compound 1 or a pharmaceutical composition
thereof in
combination with the additional therapy.
In some aspects, the administration of Compound 1 or a pharmaceutical
composition thereof and one or more additional therapies in accordance with
the
methods presented herein have an additive effect relative the administration
of
Compound 1 or a pharmaceutical composition thereof or said one or more
additional
therapies alone. In some aspects, the administration of Compound 1 or a
pharmaceutical composition thereof and one or more additional therapies in
36
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
accordance with the methods presented herein have a synergistic effect
relative to the
administration of Compound 1 or a pharmaceutical composition thereof or said
one or
more additional therapies alone.
As used herein, the term "synergistic," refers to the effect of the
administration of
Compound 1 or a pharmaceutical composition thereof in combination with one or
more
additional therapies (e.g., agents), which combination is more effective than
the
additive effects of any two or more single therapies (e.g., agents).
In a specific aspect, a synergistic effect of a combination therapy permits
the
use of lower dosages (i.e., sub-optimal doses) of Compound 1 or a
pharmaceutical
composition thereof or an additional therapy and/or less frequent
administration of
Compound 1 or a pharmaceutical composition thereof or an additional therapy to
a
subject.
In certain aspects, the ability to utilize lower dosages of Compound 1 or a
pharmaceutical composition thereof or of an additional therapy and/or to
administer
Compound 1 or a pharmaceutical composition thereof or said additional therapy
less
frequently reduces the toxicity associated with the administration of Compound
1 or a
pharmaceutical composition thereof or of said additional therapy,
respectively, to a
subject without reducing the efficacy of Compound 1 or a pharmaceutical
composition
thereof or of said additional therapy, respectively, in the treatment of a
pancreatic
cancer.
In some aspects, a synergistic effect results in improved efficacy of Compound
1
or a pharmaceutical composition thereof and each of said additional therapies
in
treating a pancreatic cancer. In some aspects, a synergistic effect of a
combination of
Compound 1 or a pharmaceutical composition thereof and one or more additional
therapies avoids or reduces adverse or unwanted side effects associated with
the use
of any single therapy.
The combination of Compound 1 or a pharmaceutical composition thereof and
one or more additional therapies can be administered to a subject in the same
pharmaceutical composition. Alternatively, Compound 1 or a pharmaceutical
composition thereof and one or more additional therapies can be administered
37
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
concurrently to a subject in separate pharmaceutical compositions. Compound 1
or a
pharmaceutical composition thereof and one or more additional therapies can be
administered sequentially to a subject in separate pharmaceutical
compositions.
Compound 1 or a pharmaceutical composition thereof and one or more additional
therapies may also be administered to a subject by the same or different
routes of
administration.
The combination therapies provided herein involve administering to a subject
to
in need thereof Compound 1 or a pharmaceutical composition thereof in
combination
with conventional, or known, therapies for treating a pancreatic cancer. Other
therapies for a pancreatic cancer or a condition associated therewith are
aimed at
controlling or relieving one or more symptoms. Accordingly, in some aspects,
the
combination therapies provided herein involve administering to a subject to in
need
thereof a pain reliever, or other therapies aimed at alleviating or
controlling one or more
symptoms associated with a pancreatic cancer or a condition associated
therewith.
Specific examples of anti-cancer agents that may be used in combination with
Compound 1 or a pharmaceutical composition thereof for treating a pancreatic
cancer
include: a hormonal agent (e.g., aromatase inhibitor, selective estrogen
receptor
modulator (SERM), and estrogen receptor antagonist), chemotherapeutic agent
(e.g.,
microtubule dissembly blocker, antimetabolite, topisomerase inhibitor, and DNA
crosslinker or damaging agent), anti-angiogenic agent (e.g., VEGF antagonist,
receptor
antagonist, integrin antagonist, vascular targeting agent (VTA)/vascular
disrupting
agent (VDA)), radiation therapy, and conventional surgery.
Non-limiting examples of hormonal agents that may be used in combination with
Compound 1 or a pharmaceutical composition thereof for treating a pancreatic
cancer
include aromatase inhibitors, SERMs, and estrogen receptor antagonists.
Hormonal
agents that are aromatase inhibitors may be steroidal or nonsteroidal. Non-
limiting
examples of nonsteroidal hormonal agents include letrozole, anastrozole,
aminoglutethimide, fadrozole, and vorozole. Non-limiting examples of steroidal
hormonal agents include aromasin (exemestane), formestane, and testolactone.
Non-
.. limiting examples of hormonal agents that are SERMs include tamoxifen
38
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
(branded/marketed as Nolvadexe), afimoxifene, arzoxifene, bazedoxifene,
clomifene,
femarelle, lasofoxifene, ormeloxifene, raloxifene, and toremifene. Non-
limiting
examples of hormonal agents that are estrogen receptor antagonists include
fulvestrant. Other hormonal agents include but are not limited to abiraterone
and
lonaprisan.
Non-limiting examples of chemotherapeutic agents that may be used in
combination with Compound 1 or a pharmaceutical composition thereof for
treating
cancer include microtubule disassembly blocker, antimetabolite, topoisomerase
inhibitor, and DNA crosslinker or damaging agent.
Chemotherapeutic agents that are microtubule disassembly blockers include,
but are not limited to, taxenes (e.g., paclitaxel (branded/marketed as TAX00),
docetaxel, nab-paclitaxel (nanoparticle albumin¨bound paclitaxel,
branded/marketed
as ABRAXANE6), larotaxel, ortataxel, and tesetaxel); epothilones (e.g.,
ixabepilone);
and vincalkaloids (e.g., vinorelbine, vinblastine, vindesine, and vincristine
(branded/marketed as ONCOVIN )).
Chemotherapeutic agents that are antimetabolites include, but are not limited
to,
folate antimetabolites (e.g., methotrexate, aminopterin, pemetrexed,
raltitrexed); purine
antimetabolites (e.g., cladribine, clofarabine, fludarabine, mercaptopurine,
pentostatin,
thioguanine); pyrimidine antimetabolites (e.g., 5-fluorouracil, capcitabine,
gemcitabine
(GEMZAR8), cytarabine, decitabine, floxuridine, tegafur); and
deoxyribonucleotide
antimetabolites (e.g., hydroxyurea).
Chemotherapeutic agents that are topoisomerase inhibitors include, but are not
limited to, class I (camptotheca) topoisomerase inhibitors (e.g., topotecan
(branded/marketed as HYCAMTIN ) irinotecan, rubitecan, and belotecan); class
II
(podophyllum) topoisomerase inhibitors (e.g., etoposide or VP-16, and
teniposide);
anthracyclines (e.g., doxorubicin, epirubicin, Doxil, aclarubicin, amrubicin,
daunorubicin, idarubicin, pirarubicin, valrubicin, and zorubicin); and
anthracenediones
(e.g., mitoxantrone, and pixantrone).
Chemotherapeutic agents that are DNA crosslinkers (or DNA damaging agents)
include, but are not limited to, alkylating agents (e.g., cyclophosphamide,
39
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
mechlorethamine, ifosfamide (branded/marketed as IFEX()), trofosfamide,
chlorambucil, melphalan, prednimustine, bendamustine, uramustine,
estramustine,
carmustine (branded/marketed as BiCNIP), lomustine, semustine, fotemustine,
nimustine, ranimustine, streptozocin, busulfan, mannosulfan, treosulfan,
carboquone,
N,N'N'-triethylenethiophosphoramide, triaziquone, triethylenemelamine);
alkylating-like
agents (e.g., carboplatin (branded/marketed as PARAPLATINe), cisplatin,
oxaliplatin,
nedaplatin, triplatin tetranitrate, satraplatin, picoplatin); nonclassical DNA
crosslinkers
(e.g., procarbazine, dacarbazine, temozolomide (branded/marketed as TEMODARe),
altretamine, mitobronitol); and intercalating agents (e.g., actinomycin,
bleomycin,
mitomycin, and plicamycin).
Non-limiting examples of anti-angiogenic agents that may be used in
combination with Compound 1 or a pharmaceutical composition thereof for
treating a
pancreatic cancer include VEGF antagonists, receptor antagonists, integrin
antagonists
(e.g., vitaxin, cilengitide, and S247), and VTAs/VDAs (e.g., fosbretabulin).
VEGF
antagonists include, but are not to, anti-VEGF antibodies (e.g., bevacizumab
(branded/marketed as AVASTIN ) and ranibizumab (branded/marketed as
LUCENTIS6)), VEGF traps (e.g., aflibercept), VEGF antisense or siRNA or miRNA,
and aptamers (e.g., pegaptanib (branded/marketed as MACUGEN8)). Anti-
angiogenic
agents that are receptor antagonists include, but are not limited to,
antibodies (e.g.,
ramucirumab) and kinase inhibitors (e.g., sunitinib, sorafenib, cediranib,
panzopanib,
vandetanib, axitinib, and AG-013958) such as tyrosine kinase inhibitors. Other
non-
limiting examples of anti-angiogenic agents include ATN-224, anecortave
acetate
(branded/marketed as RETAANE8), microtubule depolymerization inhibitor such as
combretastatin A4 prodrug, and protein or protein fragment such as collagen 18
(endostatin).
Non-limiting examples of other therapies that may be administered to a subject
in combination with Compound 1 or a pharmaceutical composition thereof for
treating a
pancreatic cancer include:
(1) a statin such as lovostatin (e.g., branded/marketed as
MEVACOR8);
(2) an mTOR inhibitor such as sirolimus which is also known as Rapamycin
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
(e.g., branded/marketed as RAPAMUNE9, temsirolimus (e.g., branded/marketed as
TORISEL9, evorolimus (e.g., branded/marketed as AFINITOR9, and deforolimus;
(3) a farnesyltransferase inhibitor agent such as tipifarnib
(e.g.,
branded/marketed as ZARNESTRA6);
(4) an antifibrotic agent such as pirfenidone;
(5) a pegylated interferon such as PEG-interferon alfa-2b;
(6) a CNS stimulant such as methylphenidate (branded/marketed as
RITALIN8);
(7) a HER-2 antagonist such as anti-HER-2 antibody (e.g., trastuzumab) and
kinase inhibitor (e.g., lapatinib);
(8) an IGF-1 antagonist such as an anti-IGF-1 antibody (e.g., AVE1642 and
IMC-A11) or an IGF-1 kinase inhibitor;
(9) EGFR/HER-1 antagonist such as an anti-EGFR antibody (e.g.,
cetuximab, panitumamab) or EGFR kinase inhibitor (e.g., erlotinib (e.g.,
branded/marketed as TARCEVA8), gefitinib);
(10) SRC antagonist such as bosutinib;
(11) cyclin dependent kinase (CDK) inhibitor such as seliciclib;
(12) Janus kinase 2 inhibitor such as lestaurtinib;
(13) proteasome inhibitor such as bortezomib;
(14) phosphodiesterase inhibitor such as anagrelide;
(15) inosine monophosphate dehydrogenase inhibitor such as tiazofurine;
(16) lipoxygenase inhibitor such as masoprocol;
(17) endothelin antagonist;
(18) retinoid receptor antagonist such as tretinoin or alitretinoin;
(19) immune modulator such as lenalidomide, pomalidomide, or thalidomide
(e.g., branded/marketed as THALIDOMID8);
(20) kinase (eg, tyrosine kinase) inhibitor such as imatinib (e.g.,
branded/marketed as GLEEVEC8), dasatinib, erlotinib, nilotinib, gefitinib,
sorafenib,
sunitinib (e.g., branded/marketed as SUTENT8), lapatinib, AEE788, or TG100801;
(21) non-steroidal anti-inflammatory agent such as celecoxib
41
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
(branded/marketed as CELEBREX6);
(22) human granulocyte colony-stimulating factor (G-CSF) such as filgrastim
(branded/marketed as NEUPOGEN );
(23) folinic acid or leucovorin calcium;
(24) integrin antagonist such as an integrin a5[31-antagonist (e.g., JSM6427);
(25) nuclear factor kappa beta (NF-K(3) antagonist such as OT-551, which is
also an anti-oxidant;
(26) hedgehog inhibitor such as CUR61414, cyclopamine, GDC-0449, or anti-
hedgehog antibody;
(27) histone deacetylase (HDAC) inhibitor such as SAHA (also known as
vorinostat (branded/marketed as ZOLINZA8)), PCI-24781, SB939, CHR-3996, CRA-
024781, ITF2357, JNJ-26481585, or PCI-24781;
(28) retinoid such as isotretinoin (e.g., branded/marketed as ACCUTANE8);
(29) hepatocyte growth factor/scatter factor (HGF/SF) antagonist such as
HGF/SF monoclonal antibody (e.g., AMG 102);
(30) synthetic chemical such as antineoplaston;
(31) anti-diabetic such as rosiglitazone maleate (e.g., branded/marketed as
AVANDIA8);
(32) antimalarial and amebicidal drug such as chloroquine (e.g.,
branded/marketed as ARALEN8);
(33) synthetic bradykinin such as RMP-7;
(34) platelet-derived growth factor receptor inhibitor such as SU-101;
(35) receptor tyrosine kinase inhibitorsof Flk-1/KDR/VEGFR2, FGFR1 and
PDGFR beta such as SU5416 and SU6668;
(36) anti-inflammatory agent such as sulfasalazine (e.g., branded/marketed as
AZULFIDINE8); and
(37) TGF-beta antisense therapy.
Non-limiting examples of other therapies that may be administered to a subject
in combination with Compound 1 or a pharmaceutical composition thereof for
treating a
pancreatic cancer include: a synthetic nonapeptide analog of naturally
occurring
42
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
gonadotropin releasing hormone such as leuprolide acetate (branded/marketed as
LUPRON ); a nonsteroidal, anti-androgen such as flutamide (branded/marketed as
EULEXIN ) or nilutamide (branded/marketed as NILANDRON8); a non-steroidal
androgen receptor inhibitor such as bicalutamide (branded/marketed as
CASODEX8);
steroid hormone such as progesterone; anti-fungal agent such as Ketoconazole
(branded/marketed as NIZORAL8); glucocorticoid such as prednisone;
estramustine
phosphate sodium (branded/marketed as EMCY-18); and bisphosphonate such as
pamidronate, alendronate, and risedronate.
Additional specific examples of therapies that may be used in combination with
Compound 1 or a pharmaceutical composition thereof for treating a pancreatic
cancer
include, but are not limited to, agents associated with cancer immunotherapy
(e.g.,
cytokines, interleukins, and cancer vaccines).
Specific examples of agents alleviating side-effects associated with a
pancreatic
cancer that can be used as therapies in combination with Compound 1 or a
pharmaceutical composition thereof, include, but are not limited to:
antiemetics, e.g.,
Ondansetron hydrochloride (branded/marketed as ZOFRAN8), Granisetron
hydrochloride (branded/marketed as KYTRIL8), Lorazepam (branded/marketed as
ATIVAN9 and Dexamethasone (branded/marketed as DECADRON8).
In certain aspects, combination therapies provided herein for treating a
pancreatic cancer comprise administering Compound 1 or a pharmaceutical
composition thereof in combination with one or more agents used to treat
and/or
manage a side effect, such as, bleeding (usually transient, low-grade
epistaxis), arterial
and venous thrombosis, hypertension, delayed wound healing, asymptomatic
proteinuria, nasal septal perforation, reversible posterior
leukoencephalopathy
syndrome in association with hypertension, light-headedness, ataxia, headache,
hoarseness, nausea, vomiting, diarrhea, rash, subungual hemorrhage,
myelodysplastic
syndromes, myelosuppression, fatigue, hypothyroidism, QT interval
prolongation, or
heart failure.
In certain aspects, Compound 1 or a pharmaceutical composition thereof is not
used in combination with a drug that is primarily metabolized by CYP2D6 (such
as an
43
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
antidepressant (e.g, a atricyclic antidepressant, a selective serotonin
reuptake inhibitor,
and the like), an antipsychotic, a beta-adrenergic receptor blocker, or
certain types of
anti-arrhythmics) to treat a pancreatic cancer.
Kits
Provided herein is a pharmaceutical pack or kit comprising one or more
containers filled with Compound 1 or a pharmaceutical composition thereof.
Additionally, one or more other therapies useful for the treatment of a
pancreatic
cancer, or other relevant agents can also be included in the pharmaceutical
pack or kit.
Also provided herein is a pharmaceutical pack or kit comprising one or more
containers
filled with one or more of the ingredients of the pharmaceutical compositions
described
herein. Optionally associated with such kits can be a notice in the form
prescribed by a
governmental agency regulating the manufacture, use or sale of pharmaceuticals
or
biological products, which notice reflects approval by the agency of
manufacture, use
or sale for human administration.
Without regard to whether a document cited herein was specifically and
individually indicated as being incorporated by reference, all documents
referred to
herein are incorporated by reference into the present application for any and
all
purposes to the same extent as if each individual reference was fully set
forth herein.
Having now fully described the subject matter of the claims, it will be
understood
by those having ordinary skill in the art that the same can be performed
within a wide
range of equivalents without affecting the scope of the subject matter or
aspects
described herein. It is intended that the appended claims be interpreted to
include all
such equivalents.
Examples
The following examples are intended to illustrate the various methods of the
invention, but in no way limit the scope of the invention.
The materials and methods used in the following examples were either available
from commercial sources or were obtained by methods known to those skilled in
the art
following known procedures or procedures described in the indicated
references.
44
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Compound 1 was prepared according to the procedure described in
International Publication No. W02014/081906.
Compound 2 refers to 6-(5,6-difluoro-2-methyl-1H-benzo[d]imidazol-1-y1)-N-(4-
(trifluoromethyl)phenyl)pyrazin-2-amine, a known inhibitor of BMI-1 function,
prepared
according to the procedure described in International Publication No.
W02015/076800.
Cell lines, cell culture, and viability assays
Authenticated human cell lines were purchased from ATCC, utilized within 30
cumulative passages of initial purchase. Murine KPC lines were generated and
used
within 30 cumulative passages. J1002 cells were generated from KPBBR mice and
used within 15 cumulative passages. All cell lines consistently tested
negative for
mycoplasma throughout the period of experimentation using the MycoAlert
Mycoplasma Detection Kit (Lonza; LT07-318). Cells were maintained under
standard
conditions at 37 C and 5% CO2 and were grown in DMEM (Life Technologies,12430-
054) supplemented with penicillin and streptomycin (Corning, 30-003-CI), and
10%
FBS (Life Technologies, 10438-034).
For proliferation curves, cells were plated in 96-well plates (Corning, 3603)
and
allowed to seed overnight. Replicate plates were plated for each time point
since
viability measurements were an endpoint procedure. The following day, cells
were
treated with Compound at indicated concentrations and treated with DMSO at the
highest used concentration (always below 0.003%). Cells were treated for 96
hours,
and viability was determined every 24 hours using AlamarBlue reagent (BioRad,
BUF012B). Briefly, 10 1_ of AlamarBlue was added to each well (100 4), mixed
briefly, and allowed to incubate at 37 C and 5% CO2 for 4 hours. Following
incubation,
fluorescence was measured on a Promega multimode microplate reader. For
analysis,
background levels were subtracted from raw results, which were then normalized
to be
represented as a fold change compared to DMSO-treated cells on Day 0. All
assays
were carried out in at least triplicate with 4-8 technical replicates per
treatment group,
per experiment. (FIG. 1a and FIG. 10b)
For dose response curves, cells were plated in 96-well plates (Corning, 3603)
and allowed to seed overnight. The following day, cells were treated with
Compound 1.
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Dose response curves began at 10 M, were decreased in 3-fold increments, and
ended with DMSO at the highest used concentration (always below 0.03%). Cells
were
treated for 72 hours and then viability was determined using AlamarBlue
reagent
(BioRad, BUF012B). Briefly, 10 1_ of AlamarBlue was added to each well (100
4),
mixed briefly, and allowed to incubate at 372C and 5% CO2 for 4 hours.
Following
incubation, fluorescence was measured on a Promega multimode microplate
reader.
For analysis, background levels were subtracted from raw results, which were
then
normalized to be represented as a percent of DMSO-treated cells. All assays
were
carried out in at least triplicate with 4-8 technical replicates per
experiment. (FIG. 10c)
Cell cycle analysis
Cells were seeded into 12-well or 6-well plates such that 16 hours later they
would be approximately 30-50% confluent. Cells were then treated for 24 hours
with
either DMSO (<0.003%), Compound 1(100 nM or 1 M), or 100 nM nocodazole
(Sigma, M1404). Following treatment, cells were harvested and fixed in ice-
cold 70%
ethanol for a minimum of one hour. Following fixation, cells were re-suspended
in PBS
+ 2% FBS + 3 M DAPI (BioLegend, 422801) or 0.25 i_ig 7-AAD (BD Pharmingen,
559925), and analyzed on a MACSQuant Analyzer 10 (Miltenyi Biotec) or BD LSR
IITM. For cell cycle analysis time course experiments, cells were prepared as
described
above, except treated for 24, 48, or 72 hours. Data was analyzed using FlowJo
software. Percent populations in G2/M were identified using the univariate
cell cycle
platform offered by FlowJo software. Percent populations >4N were determined
by
gating above the 4N population of DMSO-treated cells. (FIG. lb, FIG. lc, FIG.
2a, FIG.
2b, FIG. 10e, and FIG. 10g)
Flow cytometry for intracellular proteins
For phospho-Histone H3 (PH3) staining, cells were fixed and permeabilized
according to manufacturer's instructions using the eBioscience
Foxp3/Transcription
Factor Staining Buffer Set (ThermoFisher, 00-5523-00). Cells were blocked with
Fc
Block (BD Biosciences, 564220) and stained using a PH3 (Serb 0) antibody
(BioLegend, 650805) with 5 uL per million cells (45 min, room temp, dark).
Samples
46
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
were analyzed on a BD FortessaTM and analyzed by FlowJo software. (FIG. 1d and
FIG. 3a)
For active Caspase-3 (ActCasp3) staining, the Caspase 3 (active) FITC Staining
Kit (Abcam, ab65613) was used according to manufacturer's instructions. Cells
were
seeded in 12-well plates such that -16 hours later they would be approximately
50-
60% confluent. Cells were then treated with drugs at the indicated
concentrations, or
DMSO vehicle, for 24 hours, at which point they were harvested, stained with
FITC-
DEVD-FMK (1 L/sample, 1 hour, room temp, dark), washed, and then stained with
DAPI for analysis. Samples were analyzed on a BD LSR IITM and analyzed by
FlowJo
software. (FIG. 3a and FIG. 3b)
Western blotting
Western blotting was carried out on lysates isolated from treated cells. Cells
were lysed using RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCI, 1% Nonidet P-
40,
0.1% SDS, 0.5% deoxycholate) supplemented with cOmpleteTM EDTA-free Protease
Inhibitor Cocktail (Sigma, 11836170001) and HaltTM Phosphatase Inhibitor
Cocktail
(ThermoFisher, 78420). Lysates were quantified using the Pierce BCA Protein
Assay
Kit (ThermoFisher, 23227), diluted in SDS sample buffer, resolved by SDS-PAGE
(15-
30 g/sample), and transferred onto PVDF membranes. Membranes were blocked
with
5% w/v nonfat dry milk in TBS-T (0.1% Tween20) and primary antibodies were
incubated overnight at 42C, diluted in 5% BSA in TBS-T at the indicated
concentrations. HRP-conjugated secondary antibodies were incubated in blocking
buffer for 1 hour at room temperature before detection using Super Digital-
ECLTM
substrate solution (Kindle Biosciences, R1002). (FIG. 5b, FIG. 10a and FIG.
11b)
Western blot antibodies:
Bmi1: Cell Signaling, #5856, 1:1000
Vinculin: Cell Signaling, #4650S, 1:1000
Beta-tubulin: Cell Signaling, #2146S, 1:1000
P-glycoprotein substrate activity experiment
MDCKII-mdr1 and MDCKII-wt cells were purchased from the Netherlands Cancer
47
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Institute, NKI-AVL. The cells were cultivated in Dulbecco's modified essential
medium
(DMEM), supplemented with 10% FBS and 1% Penn-Strep, and used between passage
3-9. Cells were seeded at a density of 3 X 103 cells/well in 96-well tissue
culture treated
plates. After 4 hours to allow cells to attach, cells were treated with test
compound in
the presence or absence of the P-glycoprotein inhibitor Valspodar. The plates
were
incubated at 37 C, 5% CO2 for 72 hours and ATP was measured via luminescence
using
CellTiter-Glo reagent (Promega). (FIG. 4b)
RNA sequencing
Approximately 1.75 i_ig total RNA per sample underwent poly-A pull-down for
mRNA enrichment, which then was used as input for the IIlumina TruSeq RNA prep
kit.
Samples were prepared for the IIlumina HiSeq 4000 platform using a Beckmann-
Coulter Roboter and the SPRIworks Fragment Library Kit I. PCR using the KAPA
PCR
Amplification Kit was carried out. The libraries were then sequenced by the
Columbia
Genome Center to generate 30 million single-end reads of 100 bp length. (FIG.
11a)
RNA-Seq analysis
Reads were mapped to the human reference genome (NCBI/build 37.2) using
the STAR aligner (version 2.4.2) (Dobin A, Davis CA, Schlesinger F, et al.
STAR:
Ultrafast Universal RNA-Seq Aligner, Bioinformatics 2013;29:15-21) and were
quantified at the gene level using the summarizeOverlaps function from the R
package
`GenomicAlignments' (Lawrence M, Huber W, Pages H, et al. Software for
computing
and annotating genomic ranges, PLoS Computational Biology 2013;9:e1003118)
with
information on gene annotations from the R package
`TxDb.Hsapiens.UCSC.hg19.knownGene' (Carlson M, Maintainer BP.
TxDb.Hsapiens.UCSC.hg19.knownGene:Annotation Package for TxDb Object(s).
2015). RNA-seq data were deposited to GEO (ID: GSE118441). (FIG. 11a)
Differential gene expression (DEG)
Differential gene expression analysis between the indicated conditions was
carried out using the voom-limma frameworks implemented the R package limma'
(Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression
analyses for
48
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
RNA-sequencing and microarray studies, Nucleic Acids Research 2015;43:e47).
The
overall effect of Compound 1 treatment as compared to DMSO was assessed using
a
multivariate design accounting for both treatment and time point.
Gene set enrichment
The R implementation of single sample GSEA: gene set variation analysis with
default parameters was used (Hanzelmann S, Castelo R, Guinney J. GSVA: gene
set
variation analysis for microarray and RNA-seq data, BMC Bioinformatics
2013;14:7).
Raw counts from RNA sequencing were normalized to account for different
library
sizes, and the variance was stabilized by fitting the dispersion to a negative-
binomial
distribution as implemented in the DESeq2 R package (Love MI, Huber W, Anders
S.
Moderated estimation of fold change and dispersion for RNA-seq data with
DESeq2,
Genome Biol 2014;15:550). Gene sets were retrieved from the MSigDb v6.0
modules
HALLMARK, C2 canonical pathways and C6 oncogenic signatures (Subramanian A,
Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based
approach for interpreting genome-wide expression profiles, Proc Natl Acad Sci
U S A
2005;102:15545-50 and Liberzon A, Subramanian A, Pinchback R, et al. Molecular
signatures database (MSigDB) 3.0, Bioinformatics 2011;27:1739-40) .
Differential
enrichment analysis of these gene sets between DMSO and Compound 1 treatment
was carried out using the limma R package (Ritchie ME, Phipson B, Wu D, et al.
limma
powers differential expression analyses for RNA-sequencing and microarray
studies,
Nucleic Acids Res 2015;43:e47) and a false discovery rate (FDR) <0.1 was
considered significant. Single sample enrichment results for select pathways
were
depicted in a heatmap using the pheatmap R package (http://CRAN R-project
org/package= pheatmap R package version 2017;1:2). (FIG. 11a)
Gene set signatures:
Abbreviation Full Name
APC/c deg. of REACTOME_APC_C_CDC2O_MEDIATED_DEGRADATION_OF_CY
Cyc B CLIN B
APC/c late REACTOME CONVERSION FROM APC C CDC20 TO APC C _ _ _ _
anaphase CDH1 JNLATEANAPHASE
phosphorylati
on of APC/c REACTOME PHOSPHORYLATION OF THE APC C
49
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
APC/c deg. of REACTOME_APC_CDC2O_MEDIATED_DEGRADATION_OF_NEK2
NEK2A A
Reg. of
mitosis REACTOME REGULATION OF MITOTIC CELL CYCLE
POL
switching REACTOME POL SWITCHING
DNA
elongation REACTOME DNA STRAND ELONGATION
G1/S Phases SA G1 AND S PHASES
G1/S
Transcription REACTOME_G1_S_SPECIFIC_TRANSCRIPTION
DNA
unwinding REACTOME UNWINDING OF DNA
Cell-free tubulin polymerization assay
Assays were carried out according to manufacturer's recommendations using
the fluorescence-based tubulin polymerization assay kit from Cytoskeleton
(#BK011P)
which uses >99% pure tubulin from porcine brain. All drugs used were dissolved
in
DMSO and then diluted in ultrapure water to 10X final concentration. Final
percentage
of DMSO was kept constant for all samples. Tubulin was prepared by
resuspension at
2 mg/mL in 80 mM PIPES pH 6.9, 2.0 mM MgCl2, 0.5 mM EGTA, 1.0 mM GTP, 15%
glycerol, and 10 M fluorescent reporter. All drug samples (10X) were added to
a pre-
warmed half-area 96-well plate (Corning Costar, 3686) in duplicate, and then
warmed
to 372C for 1 min. Tubulin (50 4/well) was then added to all wells, and the
plate was
placed in a plate reader pre-warmed to 372C. The plate was mixed by medium,
orbital
shaking for 5 seconds, and fluorescence measured every 60 seconds for 90
minutes
(Ex: 340-360 +/- 20 nm, Em: 410-460 nm +/- 20 nm) in a BioTek Synergy 2 plate
reader. Polymerization rates (fluorescence units/minute) were determined by
measuring the maximal slope (by linear regression) of the linear portion of
the growth
phase of the polymerization curve. (FIG. 11e and FIG. 11f)
Analysis of free tubulin content
Free heterodimer tubulin was separated from tubulin incorporated into
microtubules according to manufacturer's recommendations using the
Microtubule/Tubulin In Vivo Assay Biochem Kit (Cytoskeleton, #BK038). Cells
were
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
seeded in 12-well plates such that -16 hours later they would be approximately
50-
60% confluent. Cells were treated with drugs at the indicated concentrations,
or DMSO
vehicle, for 2 hours, at which point they were washed once with PBS and
harvested. All
steps of this assay were carried out at 37 C in order to preserve microtubule
mass.
Cells were lysed in Cytoskeleton's Lysis and Microtubule Stabilization Buffer
(LMS01)
supplemented with 0.1 mM GTP, 1.0 mM ATP, and 1X protease inhibitor cocktail
(#PICO2). Samples were centrifuged at 1,000 x g for 5 minutes at 37 C. The
supernatant was carefully collected for ultracentifugation and the low speed
pellet
(LSP) was suspended in SDS sample buffer (SDS01) and frozen at -20 C for later
analysis by western blot. The low speed supernatant was centrifuged at 100,000
x g for
60 minutes at 37 C. The supernatant (high speed supernatant, HSS) was
carefully
collected, resuspended in SDS sample buffer, and frozen at -20 C for later
analysis by
western blot. The pellet (high speed pellet, HSP) was first dissolved in 1X
Microtubule
Depolymerization Buffer (BUF01) for 15 minutes, resuspended in SDS sample
buffer,
and frozen at -20 C for later analysis by western blot. (FIG. 11b)
lmmunocytochemistry
Cells were seeded onto glass coverslips in 12-well plates. Coverslips were
prepared by soaking in 1N HCI (60 C, 6 hours), washing in 70% ethanol, and
coating
with poly-1-lysine (Sigma, P5899) according to manufacturer's instructions.
Cells were
seeded such that they would be -50% confluent the following day. Cells were
then
treated with vehicle (DMSO, <0.003%) or 1 M Compound 1 and incubated for 24
hours. Following treatment, cells were washed with TBS and free tubulin was
extracted
with Brinkley Buffer 1980 (80 mM PIPES pH 6.8, 1 mM MgCl2, 1mM EGTA)
supplemented with 4mM EGTA and 0.5% Triton-X (30 seconds). After extraction,
cells
.. were fixed for 20 minutes in 4% formaldehyde diluted in Cytoskeleton Buffer
(10 mM
MES pH 6.1, 138 mM KCI, 3 mM MgCl2, 2 mM EGTA) supplemented with 0.32M
sucrose. Following fixation, cells were washed with TBS and then blocked in
TBS-T
(0.1% Triton-X) + 2% BSA + 22.52 mg/mL glycine for 30 minutes. The beta-
tubulin
antibody was incubated overnight at 4 C (Abcam179513, 1:1000), followed by
incubation with secondary antibody for one hour at room temperature using a
goat anti-
51
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
rabbit 594 secondary antibody (Life Technologies, A11012) at 1:500. All washes
were
done with TBS-T and all blocking and antibody incubations were done in TBS-T
(0.1%
Triton-X) + 2% BSA + 22.52 mg/mL glycine. After staining, cells were incubated
with
DAPI (BioLegend, 422801) at 300 nM for 5 min, washed, and mounted with ProLong
Diamond Antifade Mountant (Invitrogen, P36965). Slides were left to dry for 24
hours at
room temperature and then stored at 4 C in the dark until image acquisition.
Confocal imaging was performed using an Al laser scanning confocal
attachment on an Eclipse Ti microscope stand using a 60x/1.49 ApoTIRF oil-
immersion
objective and standard lasers and filter sets (Nikon Instruments, Melville,
NY). The
pinhole diameter was set to 1 Airy unit, and single optical sections were
acquired near
the basal surface of the cells to maximize detection of microtubules. All
imaging
conditions were kept constant. Images were visualized, analyzed, and prepared
for
publication using the Fiji distribution of ImageJ (Schindelin J, Arganda-
Carreras I, Frise
E, et al. Fiji: an open-source platform for biological-image analysis. Nature
Methods
2012;9:676-682). (FIG. 10d and FIG. 10f)
Animal breeding and genotyping
Genetically engineered models were generated by intercrossing the following
engineered strains, of mixed genetic backgrounds:
KrasLSL.G12D _ Reported in Tuveson DA, Shaw AT, Willis NA, et al. Endogenous
oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and
developmental defects, Cancer Cell 2004;5:375-87.
Tp53LSL.R172H _ Reported in Olive KP, Tuveson DA, Ruhe ZC, et al. Mutant p53
gain of function in two mouse models of Li-Fraumeni syndrome, Cell
2004;119:847-60.
Pdxl-Cretg ¨ Reported in Hingorani SR, Petricoin EF, Maitra A, et al.
Preinvasive and invasive ductal pancreatic cancer and its early detection in
the mouse,
Cancer Cell 2003;4:437-50.
Tp53Fl0x _ Reported in Jonkers J, Meuwissen R, van der Gulden H, et al.
Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse
model
for breast cancer, Nat Genet 2001;29:418-25.
KrasFSF.G12D ¨Jackson Laboratory, Stock No. 008653.
52
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
Tp53R172H _ This strain was generated by crossing the Tp53LSL.R172H strain
with a
Deleter-Cre to induce germline recombination, and then crossing out the Cre
allele.
This induced recombination of the Lox-STOP-LOX cassette in the Tp53LSL.R172H
allele,
resulting in a germline Tp53R1721-1 allele with a single exogenous LoxP site
in intron 1.
Pdx1-FlpOtg - The proximal 6kb promoter of the Pdx1-Cre transgene was fused
to the start codon of mammalian codon-optimized, thermostable Flp recombinase
(Flp0), with subsequent fusion of the 5' end of the Flp0 open reading frame to
the
hGH polyadenylation signal sequence.
Bmii Flax _ Described in Mich JK, Signer RA, Nakada D, et al. Prospective
identification of functionally distinct stem cells and neurosphere-initiating
cells in adult
mouse forebrain, Elife 2014;3:e02669.
Rosa26Cre-ERT2 _ Described in Ventura A, Kirsch DG, McLaughlin ME, et al.
Restoration of p53 function leads to tumour regression in vivo, Nature
2007;445:661-5.
KrasLSL.G12D/+; p53LSL.G12D/ ; Pdx1-Cretgi+ (KPC) mice and related models were
utilized as described in Hingorani SR, Wang L, Multani AS, et al. Trp53R172H
and
KrasG12D cooperate to promote chromosomal instability and widely metastatic
pancreatic ductal adenocarcinoma in mice, Cancer Cell 2005;7:469-83. J1002
cells
were derived from an autochthonous pancreatic tumor in a KrasFSF.G12D/+;
p53R172H/+;
Pdx1-FlpOtg/ ; BmilF1/Fl=
, Rosa26CreERT2/+ (KPFBR) mouse that arose following cerulean
treatment (to accelerate tumor development), but prior to tamoxifen treatment.
Genotyping was performed by Transnetyx (Cordova, TN). Studies utilized both
male
and female animals. Animals were provided standard chow and housed under a 12-
hour light/dark cycle.
Generation of KPFBR primary tumor cell lines
A small piece of tumor (-30 mg) was mechanically dissociated by chopping with
sterile scissors for a minimum of five minutes in ice-cold tumor digestion
buffer (5
mL/sample). Tumor digestion buffer consisted of 75 g/mL DNase I
(ThermoScientific,
EN0521), 80 ug/mL Dispase II (ThermoFisher, 17105041), and 1 mg/mL Collagenase
V (Sigma Aldrich, C9263) diluted in sterile PBS. After chopping, the
dissociated tumor
was incubated in digestion buffer at 37 C for 20 minutes. Following digestion,
40 mL of
53
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
ice cold PBS was used to dilute the digested tumor and the entire 45 mL was
filtered
through a 70 pm sterile mesh filter. The single cell suspension was spun down
(300 x
g, 5 min), washed once with PBS, resuspended in serum-free ductal media (SFDM,
recipe below) and plated in 1-2 wells of a collagen-coated 6-well plate
(Corning,
354400). Cells were expanded in SFDM on collagen-coated plates, and
transitioned to
standard DMEM (10% FBS, 1% Pen-Strep, 1% L-glutamine) and standard tissue
culture plates (no collagen).
SFDM Recipe:
DMEM/F-12 media (ThermoFisher, 12634010)
1.22 mg/mL nicotinamide (Sigma, N3376)
5 mg/mL glucose (Sigma, G6152)
5% ITS+ (BD Biosciences, 354352)
2.5 ug/mL Amphotericin B (ThermoFisher, 15290018)
5% Nu-serum IV (Corning, 392-0321)
25 ug/mL Bovine Pituitary Extract (Sigma, 1476)
ng/mL EGF (ThermoFisher, PMG8041)
50 nM 3,3'5-Triiodo-L-thyronine (Sigma, 564605)
1uM Dexamethasone (Sigma, D1756)
100 ng/mL cholera toxin (Quadratech, 100)
20 Assessment of Bmil deletion
In order to assess recombination of the conditional Bmil allele in J1002
cells,
PCR was performed on DNA isolated by phenol-chloroform-isoamyl alcohol
extraction.
Cells were digested overnight at 55 C in lysis buffer (10 mM Tris pH 7.5, 10
mM EDTA,
10 mM NaCI, and 0.5% sarkosyl) supplemented with 1 mg/mL Proteinase K (New
England BioLabs, P8107S). The following day, DNA was isolated by combining the
lysates with a mixture of 50% phenol, 48% chloroform, and 2% isoamyl alcohol
and
centrifuging at 16,000 x g for 30 minutes. The DNA-containing top layer was
removed,
and precipitated and washed with 2.5 volumes of 100% ethanol. DNA
concentrations
were measured using a ThermoScientificTm NanoDrop 2000 spectrophotometer. PCR
reactions were carried out using GoTag Green Master Mix (Promega, M712B), 1
ng of
54
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
DNA, and 250 nM of each primer. Three primers were used (DN437, DN438, DN946)
which amplify wildtype Bmil (BmilwT), Bmil with inserted loxP sites (Bmi11m),
and
recombined Bmil (Bmil) all in a single reaction. Following PCR, amplified DNA
was
resolved on a 2% agarose gel supplemented with SYBRTM Safe DNA Gel Stain
(ThermoFisher, S33102) and visualized using a UV light.
Primers:
DN437: gctagcattcctggttttgc
DN438: ggcacagtgatgaggtgttg
DN946: cacgaggtgcttctttcctc
Cycling Conditions:
1. 95 C, 2 min
2. 95 C, 15 sec
3. 51.4 C, 15 sec
4. 68 C, 45 sec
(Repeat steps 2-4 30 times)
5. 68 C, 5 min
6. 4 C, forever
Expected amplicons:
Wildtype Bmil (BmilwT)= 400 bp
Floxed Bmil (Bmi1filf1)= 500 bp
Recombined Bmil (Bmil ) = 300 bp
PDX model intervention study
Early passage tumor fragments from Champions model CTG-1462 were
implanted subcutaneously in nu/nu mice and then randomized to a treatment arm
once
tumors reached 150 ¨ 300mm3. Mice (n= 5/group) were treated on one of seven
arms:
vehicle, gemcitabine (50mg/kg, q.w., IP), nab-paclitaxel (10mg/kg, IV, q.w.
x3),
Compound 1 (12.5mg/kg, b.i.w. x4, PO), or full dose combinations of
gemcitabine +
nab-paclitaxel, gemcitabine + Compound 1, nab-paclitaxel + Compound 1, or
gemcitabine + nab-paclitaxel + Compound 1. Endpoints were when a tumor reached
1500mm3 or after 90 days on study. Length (L) and width (W) diameters of
tumors
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
were measured using digital calipers and mice were weighed twice per week.
Tumor
volume was calculated as (Lx W2)/2. (FIG. 12a and FIG. 12b)
KPC intervention/survival study
Tumor formation in KPC mice was monitored by weekly palpation until the
detection of a mass. Upon positive palpation, the mass was monitored by twice
weekly
ultrasound until the tumor reached an enrollable size of 4-7 mm average
diameter.
Once enrollable, KPC mice were randomly enrolled into a treatment arm of the
intervention study. Mice were treated with vehicle, Compound 1 (17 mg/kg, PO,
b.i.w.),
gemcitabine alone (100 mg/kg, IP, b.i.w.), or Compound 1 + gemcitabine. Mice
receiving Compound 1 also received gemcitabine vehicle (saline, IP, b.i.w.) at
a
volume ( L) of 50X body weight (g). Mice receiving gemcitabine also received
Compound 1 vehicle (0.5% hydroxypropylmethylcellulose with 0.1% Tween80 (w/v),
PO, b.i.w.) at a volume ( L) of 5.7X body weight (g). All drug and vehicle
combinations
were administered simultaneously.
Measurement of Compound 1 levels in plasma and tissue samples
Blood plasma and tissue samples to be used for analysis were immediately
frozen on dry ice and stored at -80 C until analysis. Concentrations of
Compound 1 in
samples were quantified using high-performance liquid chromatography with
tandem
mass spectrometry (LC-MS/MS). Compound 1 and its internal standard (deuterated
Compound 1) were recovered by protein precipitation extraction from samples.
For the plasma pharmacokinetic time course study, a standard curve was made
to cover concentrations between 0.001 ug/mL and 3.0 g/mL. The lower limit of
quantification (LLOQ) for Compound 1 (either version) in plasma was 0.001
g/mL.
(FIG. 4a)
For the pharmacokinetic study that assessed plasma, quadriceps muscle, and
tumor tissue following a single dose of Compound 1 the assay conditions were
as
follows (FIG. 5a):
Plasma: A standard curve was made to cover concentrations between 0.002
ug/mL and 6.0 ug/mL. the LLOQ for Compound 1 (either version) in plasma was
0.001
56
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
g/mL
Quadriceps muscle: A standard curve was made to cover concentrations
between 0.001 g/g wet tissue and 3.0 g/g wet tissue. The LLOQ for Compound 1
(either version) was 0.01 g/g wet tissue.
Tumor tissue: A standard curve was made to cover concentrations between
0.001 g/g wet tissue and 3.0 g/g wet tissue. The LLOQ for Compound 1 (either
version) was 0.02 g/g wet tissue.
Ultrasound
Tumor ultrasonography and volume quantification were carried out as described
in Sastra SA, Olive KP, Quantification of murine pancreatic tumors by high-
resolution
ultrasound, Methods Mol Biol 2013;980:249-66. (FIG. 7a and FIG. 7b)
Immunohistochemistry
To prepare samples for immunohistochemistry, tissues were first fixed
overnight
in 10% phosphate-buffered formalin and then stored in 70% ethanol for long-
term
storage. Fixed tissues then underwent a standard dehydration protocol and were
embedded in paraffin wax blocks. Tissues were sectioned to 5 pm thickness
using a
Leica RM 2235 microtome, mounted on positively-charged glass slides, and baked
at
60 C for 30 minutes. To prepare for staining, slides were first deparaffinized
in xylene
and then re-hydrated in a series of ethanol steps, before rinsing in distilled
water. Next,
antigen retrieval was carried out in an experimentally determined, antibody-
specific
antigen retrieval buffer (usually 10 mM citrate, pH 6.0 or 10 mM Tris, pH
10.0). Antigen
retrieval buffer was heated to boiling in a pressure-cooker, at which point
slides were
introduced for 5 minutes. After cooling to room temperature, slides were
immersed in
3% hydrogen peroxide in PBS for 20 minutes in order to quench endogenous
peroxidases.
Slides were then blocked for one hour at room temperature in TBS-T (0.1%
Tween20) + 1.5% normal horse serum (Vector Laboratories, S-2000) + 2% Animal
Free Blocker (Vector Laboratories, SP-5030). Primary antibodies were diluted
in
blocking solution and incubated overnight at 4 C at the indicated
concentrations. The
57
CA 03109386 2021-02-10
WO 2020/055544
PCT/US2019/046972
following day, secondary antibody incubation was carried out at room
temperature for
30 minutes using the ImmPRESS HRP Anti-Rabbit IgG (Peroxidase) Polymer
Detection Kit (Vector Laboratories, MP-7401). Detection was carried out with
ImmPACT DAB Peroxidase (HRP) Substrate (Vector Laboratories, SK-4105) and
slides were subsequently counterstained with hematoxylin, dehydrated to
xylene, and
coverslipped with Permount (Fisher, S70104). (FIG. 6d and FIG. 6e)
IHC antibodies:
PH3: Cell Signaling, #9701, 1:100
CC3: Cell Signaling, #9664S, 1:100
58