Note: Descriptions are shown in the official language in which they were submitted.
IN THE CANADIAN INTELLECTUAL PROPERTY OFFICE
Patent Application of
Wenhui Jiang
for
Downhole Tools Comprising Degradable Components
DESCRIPTION
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of U.S. Provisional Patent
Application Ser. No. 62/992,591,
filed on Mar. 20, 2020, titled "Dissolvable Materials, Methods of Their
Fabrications, and
Applications", and U.S. Provisional Patent Application Ser. No. 63/091,040,
filed on Oct. 13, 2020,
titled "Downhole Tools Comprising Degradable Components", the disclosures of
which are
incorporated herein in their entireties by reference.
BACKGROUND OF THE INVENTION ¨ PRIOR ART
[0002] The following is a tabulation of some prior arts that presently appear
relevant:
U. S. Patents
Patent Number Kind Code Issue Date Patentee
4175954 - 1979-11-27 Oden, et al.
4184852 - 1980-01-22 Russ
8211248 B2 2012-07-03 Marya
9528343 B2 2016-12-27 Jordan, et al.
9903010 B2 2018-02-27 Doud, et al.
9925589 B2 2018-03-27 Xu
10059092 B2 2018-08-28 Welch, et al.
10240022 B2 2019-03-26 Roy, et al.
10316601 B2 2019-06-11 Walton, et al.
10316616 B2 2019-06-11 Stafford, et al.
1
Date Recue/Date Received 2021-02-22
10329653 B2 2019-06-25 Doud, etal.
10364629 B2 2019-07-30 Jacob, etal.
10472909 B2 2019-11-12 Xu, et al.
10526868 B2 2020-01-07 Walton, et al.
10544304 B2 2020-01-28 Sherman, etal.
10544645 B2 2020-01-28 Xu
10633974 B2 2020-04-28 Fripp, etal.
10655411 B2 2020-05-19 Fripp, etal.
10737321 B2 2020-08-11 Xu
10760151 B2 2020-09-01 Doud, etal.
10865465 B2 2020-12-15 Sherman, etal.
10888926 B2 2021-01-12 Roy, etal.
U. S. Patent Application Publications
Publication Nr. Kind Code Publication Date Applicant
20170073797 Al 2017-03-16 Otsuka, etal.
20180016662 Al 2018-01-08 Qin, etal.
20180086894 Al 2018-03-29 Roy, etal.
20180252070 Al 2018-09-06 Zhu, et al.
20180265682 Al 2018-09-20 Roy, etal.
20180283129 Al 2018-10-04 Roy
20190144753 Al 2019-05-16 Fripp, etal.
20190271061 Al 2019-09-05 Tang, etal.
20200318456 Al 2020-10-08 Kovalchuk, etal.
20200407822 Al 2020-12-31 Sherman, etal.
BACKGROUND OF THE INVENTION
[0003] The present disclosure is related to downhole tools utilized in oil and
natural gas exploration
and production, more particularly, to the downhole tools comprising at least
one component
comprised of inorganic hydrolysable compound-containing materials.
2
Date Recue/Date Received 2021-02-22
[0004] In oil and natural gas exploration and production, various downhole
tools are used to build and
develop a wellbore, so as to extract hydrocarbon resource such as petroleum
and natural gas from
subterranean formation. A downhole tool may consist of various components. The
components are
made from various materials, so as to realize their functions, such as metals
and alloys, ceramics,
polymers, as well as their composites. Some of the components need prolonged
service lives, while
the others may require limited service lives only.
[0005] In oil and natural gas exploration and production such as hydraulic
fracking, some downhole
tools or components are utilized that are only required to have limited
service lives. After a downhole
tool or component service function is complete, it must be removed or disposed
of to recover the
original size of fluid pathway for use, including hydrocarbon production, etc.
Disposal of a downhole
tools or component has conventionally been done by milling or drilling the
downhole tool or
component out of the wellbore. This is time consuming and expensive
operations. In order to eliminate
the need for milling or drilling operations, the removal of tools or
components from a wellbore by
degradation/dissolution of degradable/dissolvable materials has been proposed.
While these materials
are useful, it is also very desirable that these materials have a good
combination of mechanical strength
and degradability/dissolvability in aqueous environment. Commercial products
of
degradable/dissolvable materials for a downhole tool or component include
aluminum alloys,
magnesium alloys, polymers, and their composites. The further improvement of
the
degradable/dissolvable materials to increase their mechanical strength,
degradability/dissolvability,
and manufacturability is very desirable.
SUMMARY OF THE INVENTION
[0006] The present disclosure relates to downhole tools utilized in oil and
natural gas exploration and
production, more particularly, to downhole tools comprising at least one
component comprised of
inorganic hydrolysable compound-containing materials. The component comprised
of inorganic
hydrolysable compound-containing materials is degradable/dissolvable, and it
is designed for limited
service lives. The component comprised of inorganic hydrolysable compound-
containing materials
degrades/dissolves substantially in aqueous environment such as fresh water,
water-containing
solution, or moist air within a wellbore and loses its integrity, when it
completes its designed functions.
[0007] The downhole tools include one or more degradable/dissolvable
components comprised of
inorganic hydrolysable compounds-containing materials. The downhole tools may
be, but are not
limited to, a wellbore isolation device, a perforation tool, a cementing tool,
a completion tool, a drilling
3
Date Recue/Date Received 2021-02-22
tool, a testing tool, a slickline tool, a wireline tool, an autonomous tool, a
tubing conveyed perforating
tool, and any combination thereof.
[0008] The degradable/dissolvable components comprised of inorganic
hydrolysable compound-
containing materials include, but are not limited to, the mandrel of a packer
or plug, a spacer ring, a
slip, a wedge, a retaining ring, an extrusion limiter or backup shoe, a mule
shoe, a ball, a ball seat, a
flapper, a sleeve, a perforation gun housing, a cement dart, a wiper dart, a
sealing element, a slip block,
a logging tool, a housing, a release mechanism, a pump down tool, an inflow
control device plug, an
autonomous inflow control device plug, a coupling, a connector, a support, an
enclosure, a cage, a slip
body, a tapered shoe, or any other components therefor.
The inorganic hydrolysable compounds include, but not limited to, hydrolysable
carbides, nitrides,
sulfide, etc., such as aluminum carbide (A14C3), calcium carbide (CaC2),
magnesium carbide (Mg2C3
or MgC2), manganese carbide (Mn3C), aluminum nitride (A1N), calcium nitride
(Ca3N2), magnesium
nitride (Mg3N2), aluminum sulfide (Al2S3), and aluminum magnesium carbide
(Al2MgC2). The
inorganic hydrolysable compounds may be binary, ternary, or multicomponent.
[0009] The inorganic hydrolysable compound-containing materials are metal-
based materials
comprising inorganic hydrolysable compounds and metallic matrix. The metallic
matrix is a metal or
alloy selected from the group consisting of aluminum and its alloys, magnesium
and its alloys, copper
and its alloys, titanium and its alloys, zirconium and its alloys, nickel and
its alloys, cobalt and its
alloys, zinc and its alloys, and iron and its alloys including steels. The
metal-based materials comprise
no more than 50 wt.% (weight percentage) of the inorganic hydrolysable
compounds.
[0010] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are ceramic-based materials comprising inorganic hydrolysable
compounds and ceramic
matrix. The ceramic matrix is selected from the group consisting of carbides,
nitrides, borides,
silicides, oxides, and any combination thereof. The ceramic-based materials
comprise no more than
50 wt.% of the inorganic hydrolysable compounds.
[0011] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are polymer-based materials comprising inorganic hydrolysable
compounds and polymer
matrix. The polymer matrix is selected from the group consisting of thermoset
plastics, thermoplastic
polymers, elastomers including rubbers, and any combination thereof. The
polymer-based materials
comprise no more than 50 wt.% of the inorganic hydrolysable compounds.
[0012] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are carbon-based materials comprising inorganic hydrolysable
compounds and carbon
matrix. The carbon matrix is selected from the group consisting of carbon and
its composites. The
4
Date Recue/Date Received 2021-02-22
carbon is selected from the group consisting of expanded graphite, expandable
graphite, natural
graphite, synthetic graphite, and any combination thereof. The composites
comprise a carbon matrix
and reinforcements. The reinforcements are selected from the group consisting
of metals/alloys,
carbon, ceramics, or any combination thereof. The carbon-based materials
comprise no more than 50
wt.% of the inorganic hydrolysable compounds.
[0013] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are inorganic hydrolysable compound-based materials. The inorganic
hydrolysable
compound-based materials comprise at least 50 wt.% of inorganic hydrolysable
compounds. The
inorganic hydrolysable compound-based materials may have inorganic
hydrolysable compounds as
exclusive constituents. The inorganic hydrolysable compound-based material may
also comprise other
constituents besides the inorganic hydrolysable compounds. The other
constituents are selected from
the group consisting of metals, alloys, ceramics, polymers, carbon, and their
composites.
[0014] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are gradient-distributed, inorganic hydrolysable compound-
containing materials. The
materials comprise inorganic hydrolysable compounds and a matrix. The
inorganic hydrolysable
compounds have a gradient distribution (including a layered distribution) in
the matrix. The matrix is
selected from the group consisting of metals, alloys, ceramics, polymers,
carbon, and their composites.
[0015] The degradable/dissolvable components comprised of inorganic
hydrolysable compound-
containing materials have a coating. The coating has a degradation/dissolution
rate in water or water-
containing solutions different from that of the inorganic hydrolysable
compound-containing materials.
The coating is selected from the group consisting of metals, alloys, ceramics,
polymers, carbon, and
their composites.
[0016] The fabrication methods of inorganic hydrolysable compound-containing
materials for the
degradable/dissolvable components are selected from the group consisting of
ball milling, powder
metallurgy, mixing, infiltration, melting, deposition, heat treatment, and any
combination thereof. In
these fabrication methods, inorganic hydrolysable compounds form either ex-
situ, in-situ, or in their
combination. The ex-situ formation is that the inorganic hydrolysable
compounds act as one of starting
materials and are dispersed into a matrix and/or bonded by the matrix or a
binder material. The in-situ
formation is that inorganic hydrolysable compounds form via a chemical
reaction in a matrix during
fabrication.
BRIEF DESCRIPTION OF THE DRAWING
Date Recue/Date Received 2021-02-22
[0017] Fig. 1 is a schematic illustration of a well system that employs one or
more principles of the
present disclosure, according to one or more embodiments.
[0018] Fig. 2 is a schematic illustration of an exemplary downhole tool ¨ frac
plug that employs one
or more principles of the present disclosure, according to one or more
embodiments.
DRAWING - REFERENCE NUMERALS
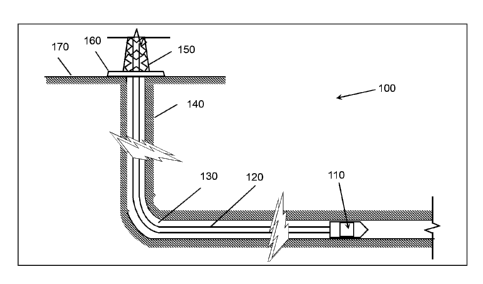
[0019] 100 well system
[0020] 110 downhole tool
[0021] 120 tool string
[0022] 130 wellbore
[0023] 140 subterranean formation
[0024] 150 derrick
[0025] 160 rig floor
[0026] 170 earth's surface
[0027] 200 frac plug
[0028] 210 body
[0029] 220 slip
[0030] 230 mechanical slip body
[0031] 240 seal
[0032] 250 taped shoe
[0033] 260 ball
[0034] 270 cage
[0035] 280 flowbore
DETAILED DESCRIPTION OF THE INVENTION
[0036] The present disclosure relates to downhole tools utilized in oil and
natural gas exploration and
production, more particularly, to downhole tools comprising one or more
degradable/dissolvable
components comprised of inorganic hydrolysable compound-containing materials.
The
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials are designed for limited service lives. They degrade/dissolve
substantially in aqueous
environment of a wellbore and lose their integrity when they complete their
designed functions. The
6
Date Recue/Date Received 2021-02-22
degradable/dissolvable components are made of the inorganic hydrolysable
compound-containing
materials.
[0037] In oil and natural gas exploration and production, downhole tools are
referred as to tools used
in developing and building a wellbore in subterraneous formation. Various
downhole tools are
developed for performing various functions. Some downhole tools or their
components need to be
retrieved from a wellbore when they complete their functions, so as to leave a
path for subsequent
operations or production fluid flow. For example, in hydraulic fracking, after
a downhole tool or
component service function is complete, it must be removed or disposed of in
order to recover the
original size of fluid pathway for use, including hydrocarbon production, etc.
Disposal of downhole
tools or components has conventionally been done by milling or drilling the
downhole tools or
component out of the wellbore. This is time consuming and expensive
operations. In order to eliminate
the need for milling or drilling operations, the removal of tools or
components from the wellbore by
degradation/dissolution of degradable/dissolvable materials has been proposed.
[0038] In some embodiments, the downhole tools described herein include one or
more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The downhole tools are used for various downhole operations. The
downhole operations
may include, but are not limited to, a drilling operation, a completion
operation, a remedial operation,
a stimulation operation, an acidizing operation, an acid-fracturing operation,
a frac-packing operation,
a perforating operation, a near-wellbore consolidation operation, a sand
control operation, and any
combination thereof.
[0039] In some embodiments, the downhole tools described herein include one or
more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The downhole tools may be, but are not limited to, a wellbore
isolation device, a perforation
tool, a cementing tool, a completion tool, a drilling tool, a testing tool, a
slickline tool, a wireline tool,
an autonomous tool, a tubing conveyed perforating tool, and any combination
thereof. The downhole
tools may have one or more components comprised of inorganic hydrolysable
compound-containing
materials including, but are not limited to, the mandrel of a packer or plug,
a spacer ring, a slip, a
wedge, a retaining ring, an extrusion limiter or backup shoe, a mule shoe, a
ball, a ball seat, a flapper,
a sleeve, a perforation gun housing, a cement dart, a wiper dart, a sealing
element, a slip block, a
logging tool, a housing, a release mechanism, a pump down tool, an inflow
control device plug, an
autonomous inflow control device plug, a coupling, a connector, a support, an
enclosure, a cage, a slip
body, a tapered shoe, or any other components.
7
Date Recue/Date Received 2021-02-22
[0040] In some embodiments, the downhole tools described herein include one or
more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The downhole tools may be any type of wellbore isolation device
capable of fluidly sealing
two sections of the wellbore from one another and maintaining differential
pressure. That is, the
wellbore isolation device is used to isolate one pressure zone from another.
The wellbore isolation
device may be used in direct contact with the formation face of the wellbore,
with casing string, with
a screen or wire mesh, and the like. The wellbore isolation device may
include, but are not limited to,
a frac plug, a frac ball, a setting ball, a bridge plug, a wellbore packer, a
wiper plug, a cement plug, a
basepipe plug, a sand screen plug, an inflow control device plug, a tubing
section, a tubing string, and
any other wellbore isolation device therefor.
[0041] FIG. 1 schematically illustrates an exemplary well system 100 for a
downhole tool 110. As
depicted, a derrick 150 with a rig floor 160 is positioned on the earth's
surface 170. A wellbore 130
is positioned below the derrick 150 and the rig floor 160 and extends into
subterranean formation 140.
A tool string 120 extends from the derrick 150 and the rig floor 160
downwardly into the wellbore
130. The tool string 120 may be any mechanical connection to the surface, such
as wireline, slick line,
drill pipe, or coiling tubing. The tool string 120 suspends the downhole tool
110 for placement into
the wellbore 130 at a desired location to perform a specific downhole
operation. Examples of such the
downhole operations may include, but are not limited to, a stimulation
operation, an acidizing
operation, a fracturing operation, a frac packing operation, a remedial
operation, a perforating
operation, a near-wellbore consolidation operation, a drilling operation, a
completion operation, and
any combination thereof.
[0042] In some embodiments, the downhole tool 110 may comprise one or more
components made
of an inorganic hydrolysable compound-containing material. That is, at least
one portion of the
downhole tool 110 may be composed of an inorganic hydrolysable compound-
containing material.
The downhole tool 110 may be any type of wellbore isolation device capable of
fluidly sealing two
sections of the wellbore 130 from one another and maintaining differential
pressure. Examples of
suitable wellbore isolation device may include, but are not limited to a frac
plug, a frac ball, a setting
ball, a bridge plug, a wellbore packer, a wiper packer, a wiper plug, a cement
plug, a basepipe plug, a
sand screen plug, an inflow control device, an autonomous inflow control
device plug, a tubing
section, a tubing string, and any combination thereof.
[0043] In some embodiments, the downhole tool 110 may be a wellbore isolation
device, a perforation
tool, a cementing tool, or a combination tool. The downhole tool 110 may be a
drilling tool, a testing
tool, a slickline tool, a wireline tool, an autonomous tool, a tubing conveyed
perforating tool, and any
8
Date Recue/Date Received 2021-02-22
combination thereof. The downhole tool 110 may have one or more components
made of the inorganic
hydrolysable compound-containing material including, but not limited to, the
mandrel of a packer or
plug, a spacer ring, a slip, a wedge, a retaining ring, an extension limiter
or a backup shoe, a mule
shoe, a ball, a flapper, a ball seat, a sleeve, a perforation gun housing, a
cement dart, a wiper dart, a
sealing element, a wedge, a slip block, a logging tool, a housing, a release
mechanism, a pumpdown
tool, an inflow control device plug, an autonomous inflow control device plug,
a coupling, a
connector, a support, an enclosure, a cage, a slip body, a tapered shoe, or
any other downhole tool or
component therefor.
[0044] The well system 100 as shown in FIG. 1 is just an example of a wide
variety of well systems
in which the principal of the present disclosure may be utilized. Therefore,
the principle of this
disclosure is not necessarily limited to any of the details of the depicted
well system or the various
components thereof, depicted in the drawing or otherwise described herein. For
example, it is not
necessary in keeping with the principle of this disclosure for the wellbore
130 to include a horizontal
section. The well system 100 may be equally employed in vertical and/or
deviated wellbores, without
departing from the scope of the present disclosure. Furthermore, it is not
necessary for a single
downhole tool 110 to be suspended from the tool string 120.
[0045] In addition, it is not necessary for the downhole tool 110 to be
lowered into wellbore 130 using
the derrick 150. Rather, any other type of device suitable for lowering the
downhole tool 110 into the
wellbore 130 for placement at a desired location, or use therein to perform a
downhole operation may
be utilized without departing from the scope of the present disclosure.
Although not depicted, the
downhole tool 110 may alternatively be hydraulically pumped into the wellbore
130 and thus, not
need the tool string 120 for delivery into the wellbore 130.
[0046] Referring to FIG. 2, with continued reference to FIG. 1, one specific
type of downhole tool
110 described herein is a frac plug 200, which is a wellbore isolation device
used during a well
stimulation/fracturing operation. FIG. 2 illustrates an exemplary frac plug
200 being lowered into a
wellbore 130 on a tool string 120. The frac plug 200 generally comprises a
body 210, slips 220 and a
sealing element 240.
[0047] The body 210 of the frac plug 200 comprises an axial flowbore 280
extending therethrough.
A cage 270 is formed at the upper end of the body 210 for retaining a ball 260
that acts as a one-way
check valve. In particular, the ball 210 seals off the flowbore 280 to prevent
flow downwardly
therethrough, but permits flow upwardly through the flowbore 280. One or more
slips 220 are mounted
around the body 210 below the sealing element 240. The slips 220 are guided by
a mechanical slip
9
Date Recue/Date Received 2021-02-22
body 230. A taped shoe 250 is provided at the lower end of the body 210 for
guiding and protecting
the frac plug 200 as it is lowered into the wellbore 130.
[0048] At least one of a body 210, slips 220, and a sealing element 240 may be
composed at least
partially of an inorganic hydrolysable compound-containing material. Moreover,
components of one
or more of a body 210, slips 220, and a sealing element 240 may be composed of
one or more of the
inorganic hydrolysable compound-containing materials. For example, one or more
of the ball 260, the
cage 270, the mechanical slip body 230, or the taped shoe 250 may be formed
from the same or a
different type of the inorganic hydrolysable compound-containing materials,
without departing from
the scope of the present disclosure. Moreover, although components of a
downhole tool 110 (FIG. 1)
are explained herein with reference to a frac plug 200, other downhole tools
and components thereof
may be formed from an inorganic hydrolysable compound-containing material
without departing from
the scope of the present disclosure.
[0049] Inorganic hydrolysable compounds are a kind of inorganic compounds
which can react with
water in aqueous environment. Their hydrolysis reactions would generate
gaseous reaction products
and even solid products with a significant volume expansion. Such
characteristic features of the
hydrolysis reactions would lead to disintegrating of a material or structure,
i.e., substantial cracking
and even pulverizing. As a result, the material or structure would lose its
integrity. This is a
degradation mechanism for the inorganic hydrolysable compound-containing
materials. Only the
inorganic hydrolysable compound constituents in the materials can dissolve and
cause the degradation
of the materials or structures, while other constituents may not be able to
dissolve and are just subject
to mechanical damages caused by the hydrolysis of the inorganic hydrolysable
compound
constituents. This degradation mechanism is different from the dissolution
mechanisms of commercial
dissolvable materials such as Al alloys, Mg alloys, and polymers such as
polyglycolic acid (PGA)
wherein the materials get corroded or hydrolyzed. The present invention is
conceived based on such
degradation mechanism. Throughout this specification, the terms "degradation"
and "di s soluti on" and
their variants refer to disintegration of a material or structure including
cracking, pulverizing, and
dissolving. The inorganic hydrolysable compounds are ideal constituents for
degradable/dissolvable
components of a downhole tool and component with a limited service life.
In some embodiments, the downhole tools described herein include one or more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The inorganic hydrolysable compounds include, but not limited to,
hydrolysable carbides,
borides, nitrides, sulfides, etc., such as aluminum carbide (A14C3), calcium
carbide (CaC2),
magnesium carbide (Mg2C3 or MgC2), manganese carbide (Mn3C), aluminum nitride
(A1N), calcium
Date Recue/Date Received 2021-02-22
nitride (Ca3N2), magnesium nitride (Mg3N2), aluminum sulfide (Al2S3), and
aluminum magnesium
carbide (Al2MgC2). The inorganic hydrolysable compounds may be binary,
ternary, or even
multicomponent.
[0050] In some embodiments, the downhole tools described herein include one or
more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The inorganic hydrolysable compound is aluminum carbide (A14C3).
The aluminum carbide
(A14C3) may be further alloyed using some alloying elements to form a complex
carbide. That is, the
complex carbide comprises other elements besides aluminum (Al) and carbon (C).
For example, the
aluminum carbide (A14C3) is alloyed with zinc (Zn) to form a complex carbide -
aluminum zinc
carbide (A14Zn2C3). But such complex carbide needs to be still hydrolysable.
[0051] A14C3 is a kind of binary compounds consisting of aluminum and carbon.
It is chemically
instable in aqueous environment such as water, water-containing solutions, or
even moist air. The
chemical reaction between A14C3 and water, i.e., a hydrolysis reaction, is as
below:
A14C3 + 12H20 4A1(011)3 + 3CH4
The reaction generates a gaseous phase CH4 and a solid phase Al(OH)3. The
formation of the solid
phase leads to more than 110% volume expansion. The volume expansion would
generate
compression stress and favor materials degradation/disintegration. Besides, a
similar reaction occurs
between A14C3 and other protic reagents, for example,
A14C3 + 12HC1 4A1C13 + 3CH4
[0052] In some embodiments, the downhole tools described herein include one or
more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The inorganic hydrolysable compound is aluminum nitride (A1N). The
aluminum nitride
(A1N) may be further alloyed using some alloying elements to form a complex
nitride. That is, the
complex nitride comprises other elements besides Al and N. But such complex
nitride needs to be still
hydrolysable.
[0053] MN is a kind of binary compounds consisting of aluminum (Al) and
nitrogen (N). It is
chemically instable in aqueous environment such as water, water-containing
solutions, or even moist
air. The chemical reaction between MN and water, i.e., a hydrolysis reaction,
is as below:
MN + 311,0 Al(OH)3 + NH3
The reaction generates a gaseous phase NH3 and a solid phase Al(OH)3. The
formation of the solid
phase leads to more than 150% volume expansion. The volume expansion would
generate
compression stress and favor material degradation/disintegration.
11
Date Recue/Date Received 2021-02-22
[0054] In some embodiments, the downhole tools described herein include one or
more
degradable/dissolvable components comprised of inorganic hydrolysable compound-
containing
materials. The inorganic hydrolysable compound is magnesium nitride (Mg3N2).
The magnesium
nitride (Mg3N2) may be further alloyed using some alloying elements to form a
complex nitride. That
is, the complex nitride comprises other elements besides Mg and N. But such
complex nitride needs
to be still hydrolysable.
[0055] Mg3N2 is a kind of binary compounds consisting of magnesium (Mg) and
nitrogen (N). It is
chemically instable in aqueous environment such as water, water-containing
solutions, or even moist
air. The chemical reaction between Mg3N2 and water, i.e., a hydrolysis
reaction, is as below:
Mg3N2 + 6H20 3Mg(OH)2 + 2NH3
The reaction generates a gaseous phase NH3 and a solid phase Mg(OH)2. The
formation of the solid
phase leads to more than 100% volume expansion. The volume expansion would
generate
compression stress and favor material degradation/disintegration.
[0056] Such instability of the inorganic hydrolysable compounds such as A14C3,
MN, and Mg3N2 in
aqueous environment makes them be good candidate constituents in a
degradable/dissolvable material.
Furthermore, they have a high mechanical strength and hardness. For example,
A14C3 has
approximately the same hardness as topaz, a hardness of 8 on Mohs hardness
scale. It is used as
abrasives in a tool. All the characteristic features make the inorganic
hydrolysable compounds be ideal
candidate constituents in degradable/dissolvable, structural materials for a
downhole tool and its
components that are required to have degradability/dissolution in aqueous
environment of a wellbore.
[0057] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing metal-based materials. The metal-based materials comprise inorganic
hydrolysable
compound phase and metallic matrix. The metal-based materials comprise no more
than 50 wt.% of
the inorganic hydrolysable compound phase. According to the contents of the
inorganic hydrolysable
compounds, the metal-based materials can be further divided into alloys and
metallic matrix
composites. For the materials comprising at least 5 wt.% of the inorganic
hydrolysable compounds,
they can be referred to as inorganic hydrolysable compound-containing metallic
matrix composites.
For the materials comprising no more than 5 wt.% of the inorganic hydrolysable
compounds, they can
be referred to as inorganic hydrolysable compound-containing alloys.
[0058] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing metal matrix composites. The composites comprise inorganic
hydrolysable compounds
12
Date Recue/Date Received 2021-02-22
and metallic matrix. The composite comprises at least 5 wt.% of the inorganic
hydrolysable
compounds. The inorganic hydrolysable compounds have various shapes and sizes
such as spherical,
platelet-like, needle-like, etc., and in millimeters, micrometers, and even
nanometers. The metallic
matrix comprises a metal or alloy selected from the group consisting of
aluminum and its alloys,
magnesium and its alloys, copper and its alloys, titanium and its alloys,
zirconium and its alloys, nickel
and its alloys, cobalt and its alloys, zinc and its alloys, and iron and its
alloys including steels, etc.
The composites may comprise other second phases besides the inorganic
hydrolysable compounds,
such as C, SiC, 134C, TiC, A1203, etc. The other second phases can take
various shapes such as
particulates and fibers, and have various sizes from nanometers, micrometers,
to millimeters.
[0059] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing aluminum matrix composites. The composites comprise inorganic
hydrolysable
compounds and aluminum or its alloy matrix. The composites comprise at least 5
wt.% of the
inorganic hydrolysable compounds. The inorganic hydrolysable compounds have
various shapes and
sizes such as spherical, platelet-like, needle-like, etc., and in millimeters,
micrometers, and even
nanometers. The composites may comprise other second phases besides the
inorganic hydrolysable
compounds, such as C, SiC, 134C, TiC, WC, A1203, etc. The other second phases
can take various
shapes such as particulates and fibers, and have various sizes from
nanometers, micrometers, to
millimeters. Furthermore, the aluminum alloy matrix may contain one or more of
low-melting-point
elements selected from the group consisting of gallium (Ga), tin (Sn), indium
(In), etc.
[0060] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing magnesium matrix composites. The composites comprise inorganic
hydrolysable
compounds and magnesium alloy matrix. The composite comprises at least 5 wt.%
of the inorganic
hydrolysable compounds. The inorganic hydrolysable compounds have various
shapes and sizes such
as spherical, platelet-like, needle-like, etc., and in millimeters,
micrometers, and even nanometers.
The composites can comprise other second phases besides the inorganic
hydrolysable compounds,
such as C, SiC, 134C, TiC, WC, A1203, etc. The second phases can take various
shapes such as
particulates and fibers, and various sizes from nanometers, micrometers, to
millimeters.
[0061] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing alloys. The alloys comprise inorganic hydrolysable compounds and
metallic matrix. The
alloys comprise no more than 5 wt.% of the inorganic hydrolysable compounds.
The inorganic
13
Date Recue/Date Received 2021-02-22
hydrolysable compounds have various shapes and sizes such as spherical,
platelet-like, needle-like,
etc., and in millimeters, micrometers, and even nanometers. The metallic
matrix is selected from the
group consisting of aluminum and its alloys, magnesium and its alloys,
titanium and its alloys,
zirconium and its alloys, nickel and its alloys, cobalt and its alloys, zinc
and its alloys, and iron and
its alloys including steels, etc. The alloys may comprise other second phases
besides the inorganic
hydrolysable compounds. Compared with the inorganic hydrolysable compound-
containing metallic
matrix composites, the inorganic hydrolysable compound-containing alloys have
a lower content of
the inorganic hydrolysable compounds.
[0062] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing aluminum alloys. The alloys comprise inorganic hydrolysable
compounds and aluminum
alloy matrix. The alloys comprise no more than 5 wt.% of the inorganic
hydrolysable compounds. The
inorganic hydrolysable compounds have various shapes and sizes such as
spherical, platelet-like,
needle-like, etc., and in millimeters, micrometers, and even nanometers. The
aluminum alloy matrix
is either pure aluminum or aluminum alloys. The aluminum alloys may contain
one or more alloying
elements selected from the group consisting of silicon (Si), magnesium (Mg),
copper (Cu), zinc (Zn),
manganese (Mn), titanium (Ti), zirconium (Zr), lithium (Li), nitrogen (N),
boron (B), carbon (C),
gallium (Ga), tin (Sn), indium (In), etc.
[0063] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing magnesium alloys. The alloys comprise inorganic hydrolysable
compounds and
magnesium alloy matrix. The inorganic hydrolysable compound-containing
magnesium alloys
comprise no more than 5 wt.% of the inorganic hydrolysable compounds. The
inorganic hydrolysable
compounds have various shapes and sizes such as spherical, platelet-like,
needle, etc., and in
millimeters, micrometers, and even nanometers. The magnesium alloy matrix is
either pure
magnesium or magnesium alloys. The magnesium alloys may contain one or more
alloying elements
selected from the group consisting of silicon (Si), aluminum (Al), copper
(Cu), zinc (Zn), manganese
(Mn), lithium (Li), nickel (Ni), iron (Fe), silver (Ag), calcium (Ca), carbon
(C), rare earth (RE) such
as neodymium (Nd), yttrium (Y), and thorium (Th), etc.
[0064] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing ceramic-based materials. The ceramic-based materials comprise
inorganic hydrolysable
compound phase and ceramic matrix. The ceramic-based materials comprise no
more than 50 wt.%
14
Date Recue/Date Received 2021-02-22
of the inorganic hydrolysable compound phase. According to the contents of the
inorganic
hydrolysable compounds, the ceramic-based materials can be further divided
into ceramics and
ceramic matrix composites. For the materials comprising at least 5 wt.% of the
inorganic hydrolysable
compound phase, they can be referred to as inorganic hydrolysable compound-
containing ceramic
matrix composites. For the materials comprising no more than 5 wt.% of the
inorganic hydrolysable
compound phase, they can be referred to as inorganic hydrolysable compound-
containing ceramics.
[0065] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing ceramic matrix composites. The composites comprise inorganic
hydrolysable compounds
and ceramic matrix. The composites comprise at least 5 wt.% of the inorganic
hydrolysable
compounds. The inorganic hydrolysable compounds have various shapes and sizes
such as spherical,
platelet-like, needlelike, etc., and in millimeters, micrometers, and even
nanometers. The ceramic
matrix is selected from the group consisting of carbides, nitrides, borides,
silicides, oxides, and any
combination thereof. The carbides include, but are not limited to, silicon
carbide (SiC), titanium
carbide (TiC), zirconium carbide (ZrC), and tungsten carbide (WC). The
nitrides include, but are not
limited to, titanium nitride (TiN), and chromium nitride (CrN). The borides
include, but are not limited
to, titanium boride (TiB2), chromium boride (CrB), and zirconium boride
(ZrB2). The oxides include,
but are not limited to, aluminum oxide (A1203), silicon oxide (5i02), and
zirconium oxide (ZrO2).
Furthermore, the ceramics may comprise binder materials. The binder materials
are selected from the
group consisting of metals, alloys, polymers, carbon, and their composites.
[0066] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing ceramics. The ceramics comprise inorganic hydrolysable compounds
and ceramic matrix.
The ceramics comprise no more than 5 wt.% of the inorganic hydrolysable
compounds. The inorganic
hydrolysable compounds have various shapes and sizes such as spherical,
platelet-like, needle-like,
etc., and in millimeters, micrometers, and even nanometers. The ceramic matrix
is selected from the
group consisting of carbides, nitrides, borides, silicides, oxides, and any
combination thereof. The
carbides include, but are not limited to, silicon carbide (SiC), titanium
carbide (TiC), zirconium
carbide (ZrC), and tungsten carbide (WC). The nitrides include, but are not
limited to, titanium nitride
(TiN), and chromium nitride (CrN). The borides include, but are not limited
to, titanium boride (TiB2),
chromium boride (CrB), and zirconium boride (ZrB2). The oxides include, but
are not limited to,
aluminum oxide (A1203), silicon oxide (5i02), and zirconium oxide (ZrO2).
Furthermore, the ceramics
Date Recue/Date Received 2021-02-22
may comprise binder materials. The binder materials are selected from the
group consisting of metals,
alloys, polymers, carbon, and their composites.
[0067] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing polymer-based materials. The polymer-based materials comprise
inorganic hydrolysable
compound phase and polymer matrix. The polymer-based materials comprise no
more than 50 wt.%
of the inorganic hydrolysable compound phase. According to the contents of the
inorganic
hydrolysable compounds, the polymer-based materials can be further divided
into polymers and
polymer matrix composites. For the materials comprising at least 5 wt.% of the
inorganic hydrolysable
compound phase, they can be referred to as inorganic hydrolysable compound-
containing polymer
matrix composites. For the materials comprising no more than 5 wt.% of the
inorganic hydrolysable
compound phase, they can be referred to as inorganic hydrolysable compound-
containing polymers.
[0068] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing polymer matrix composites. The composites comprise inorganic
hydrolysable compounds
and polymer matrix. The composites comprise at least 5 wt.% of the inorganic
hydrolysable
compounds. The inorganic hydrolysable compounds have various shapes and sizes
such as spherical,
platelet-like, needle-like, etc., and in millimeters, micrometers, and even
nanometers. The polymer
matrix is selected from the group consisting of thermoset plastics,
thermoplastic polymers, elastomers
including rubber, and any combination thereof. The thermoset polymers include,
but are not limited
to, polyester resin, duroplast, epoxy resin, silicone resin,
polyurea/polyurethane, and phenolic resin.
The thermoplastic polymers include, but are not limited to, polylactic acid,
polycarbonate, polyether
sulfone, polyoxymethylene, polyetheretherketone, and polyetherimide. The
elastomers include, but
are not limited to, natural rubber, silicone elastomer, fluoroelastomer,
polyurethane elastomer, nitrile
rubber, and polyisoprene. The composites may comprise other constituents
besides the inorganic
hydrolysable compounds and polymer matrix, such as carbon and/or glass fibers.
[0069] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing polymers. The inorganic hydrolysable compound-containing polymers
comprise
dispersive inorganic hydrolysable compounds and polymer matrix. The polymers
comprise no more
than 5 wt.% of the inorganic hydrolysable compounds. The inorganic
hydrolysable compounds have
various shapes and sizes such as spherical, platelet-like, needle-like, etc.,
and in millimeters,
micrometers, and even nanometers. The polymers are selected from the group
consisting of thermoset
16
Date Recue/Date Received 2021-02-22
plastics, thermoplastic polymers, elastomers including rubber, and any
combination thereof. The
thermoset polymers include, but are not limited to, polyester resin,
duroplast, epoxy resin, silicone
resin, polyurea/polyurethane, and phenolic resin. The thermoplastic polymers
include, but are not
limited to, polylactic acid, polycarbonate, polyether sulfone,
polyoxymethylene,
polyetheretherketone, and polyetherimide. The elastomers include, but are not
limited to, natural
rubber, silicone elastomer, fluoroelastomer, polyurethane elastomer, nitrile
rubber, and polyisoprene.
The polymers may comprise other constituents besides the inorganic
hydrolysable compounds and
polymer matrix, such as carbon and/or glass fibers.
[0070] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are carbon-based materials comprising inorganic hydrolysable
compounds and carbon
matrix. The carbon-based materials comprise no more than 50 wt.% of the
inorganic hydrolysable
compounds. The inorganic hydrolysable compounds have various shapes and sizes
such as spherical,
platelet-like, needle-like, etc., and in millimeters, micrometers, and even
nanometers. The carbon
matrix is selected from the group consisting of carbon and its composites. The
carbon is selected from
the group consisting of expanded graphite, expandable graphite, natural
graphite, synthetic graphite,
and any combination thereof. The composites comprise a carbon matrix and
reinforcements. The
reinforcements are selected from the group consisting of metals/alloys,
carbon, ceramics, or any
combination thereof. The metals/alloys are selected from the group consisting
of copper (Cu),
aluminum (Al), titanium (Ti), zirconium (Zr), tungsten (W), nickel (Ni),
cobalt (Co), silicon (Si),
chromium (Cr), iron (Fe), and their alloys. The ceramics are selected from the
group consisting of
silicon carbide (SiC), silicon nitride (Si3N4), silicon oxide (5i02), boron
nitride (BN), and any
combination thereof. The carbon is selected from the group consisting of
amorphous carbon, natural
graphite, carbon fiber, and any combination thereof.
[0071] The inorganic hydrolysable compound-containing materials for the
degradable/dissolvable
components are an inorganic hydrolysable compound-based material. The
inorganic hydrolysable
compound-based material comprises at least 50 wt.% of inorganic hydrolysable
compounds. The
inorganic hydrolysable compounds have various shapes and sizes such as
spherical, platelet-like,
needle-like, etc., and in millimeters, micrometers, and even nanometers. The
inorganic hydrolysable
compound-based materials may have the inorganic hydrolysable compounds as the
exclusive
constituents. Furthermore, the inorganic hydrolysable compound-based materials
may comprise other
constituents besides the inorganic hydrolysable compounds. The other
constituents are selected from
the group consisting of metals, alloys, ceramics, polymers, carbon, and their
composites. The metals
and alloys are selected from the group consisting of aluminum (Al), magnesium
(Mg), zinc (Zn),
17
Date Recue/Date Received 2021-02-22
copper (Cu), titanium (Ti), zirconium (Zr), nickel (Ni), cobalt (Co), iron
(Fe), and their alloys. The
ceramics are selected from the group consisting of carbides, nitrides,
borides, silicides, oxides, and
any combination thereof. The polymers are selected from the group consisting
of thermoset plastics,
thermoplastic polymers, elastomers including rubbers, and any combination
thereof.
[0072] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of gradient-distributed
inorganic hydrolysable
compound-containing materials. The materials comprise inorganic hydrolysable
compounds and a
matrix. The inorganic hydrolysable compounds have a gradient distribution,
including a layered
distribution, in the matrix. For example, the amount of the inorganic
hydrolysable compounds
increases from surface to core of the component, or vice versa, so that a
designed
degradation/dissolution rate can be achieved. The inorganic hydrolysable
compounds have various
shapes and sizes such as spherical, platelet-like, needle-like, etc., and in
millimeters, micrometers, and
even nanometers. The matrix is selected from the group consisting of metals,
alloys, ceramics,
polymers, carbon, and their composites. Furthermore, the matrix may comprise
other constituents
besides the inorganic hydrolysable compounds and the matrix.
[0073] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. The fabrication methods are selected from the
group consisting of
ball milling, powder metallurgy, mixing, infiltration, melting, deposition,
heat treatment, and any
combination thereof. In these fabrication methods, inorganic hydrolysable
compounds form either ex-
situ, in-situ, or in their combination. The ex-situ formation is that
inorganic hydrolysable compounds
act as one of starting materials and are dispersed into a matrix and/or bonded
by the matrix or a binder
material. The in-situ formation is that inorganic hydrolysable compounds form
via a chemical reaction
in a matrix during fabrication. For example, the chemical reaction of A14C3
phase formation is between
aluminum (Al) and carbon (C), or between aluminum (Al) and silicon carbide
(SiC) in an aluminum
melt or an aluminum matrix during heat treatment.
[0074] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Ball milling is used to synthesize inorganic
hydrolysable compound-
containing materials. The inorganic hydrolysable compounds are formed in-situ
during ball milling
via a chemical reaction between pure elements or compounds. Alternatively, the
inorganic
hydrolysable compounds are added as a starting material. Ball milling mixes
the inorganic
hydrolysable compounds with other constituents. Furthermore, the ball milling
may refine the sizes
of the constituents.
18
Date Recue/Date Received 2021-02-22
[0075] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Powder metallurgy is used to synthesize
inorganic hydrolysable
compound-containing materials. It includes mechanically mixing starting powder
materials, forming
a preform compact, and sintering it at elevated temperatures. Ball milling may
be used to mix the
starting powder materials.
[0076] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Mixing is used to synthesize inorganic
hydrolysable compound-
containing polymer materials. It includes mixing starting materials in which
polymer is in a form of
either powder or liquid, forming a preform, and curing it at room temperature
or elevated
temperatures.
[0077] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Infiltration is used to synthesize inorganic
hydrolysable compound-
containing materials. It includes infiltrating an inorganic hydrolysable
compound-containing preform
with a binder material at elevated temperatures. The binder material is
selected from the group
consisting of metals, alloys, polymers, and their composites.
[0078] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Melting method is used to synthesize inorganic
hydrolysable
compound-containing materials. The inorganic hydrolysable compounds are added
as a starting
material into a molten melt, stirred to make their uniformity, and then, the
melt is poured/cast into a
casting mold. Alternatively, the inorganic hydrolysable compounds may form in-
situ and disperse
during melting. The melt materials are either metals, alloys, polymers, or
their composites.
[0079] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Deposition method is used to synthesize
inorganic hydrolysable
compound-containing materials, which includes 3D printing. It includes spray
fusion (Oxy acetylene
flame), laser deposition, plasma transferred arc melting, and electron beam
melting. Inorganic
hydrolysable compound powder and binder materials are taken as starting
materials. During the
processes, the inorganic hydrolysable compounds are bonded together by the
binder materials. A
component comprised of the inorganic hydrolysable compound-containing
materials is built up layer
by layer.
[0080] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Heat treatment method is used to synthesize in-
situ inorganic
hydrolysable compound-containing materials. During heat treatment, a chemical
reaction between
pure elements or compounds occurs forming inorganic hydrolysable compounds.
For example, a
19
Date Recue/Date Received 2021-02-22
chemical reaction for forming Al4C3 phase is between aluminum (Al) and carbon
(C) in an
aluminum/carbon composite, between aluminum (Al) and silicon carbide (SiC) in
an aluminum/SiC
composite, or between aluminum (Al) and boron carbide (B4C) in an aluminum/B4C
composite.
[0081] Embodiments of the disclosure relate to fabrication methods of
inorganic hydrolysable
compound-containing materials. Heat treatment method is used to modify
mechanical properties and
degradability/dissolution of inorganic hydrolysable compound-containing
materials. During heat
treatment, there is a microstructural change in a matrix of an inorganic
hydrolysable compound-
containing material such as aging hardening of an aluminum alloy, with or
without companying
formation/modification of inorganic hydrolysable compounds.
[0082] Some embodiments of the disclosure relate to the downhole tools
described herein include one
or more degradable/dissolvable components comprised of inorganic hydrolysable
compound-
containing materials with coating. The coating has a degradation/dissolution
rate different from that
of the inorganic hydrolysable compound-containing materials. The coating
modifies a
degradation/dissolution rate of the component comprised of the inorganic
hydrolysable compound-
containing materials, so as to meet a designed requirement. The coating is
selected from the group
consisting of metals, alloys, ceramics, polymers, carbon, and their
composites. The coating is applied
by a method selected from the group consisting of cold/thermal spray, physical
vapor deposition,
chemical vapor deposition, spray fusion (Oxy acetylene flame), laser
deposition, plasma transferred
arc deposition, electron beam deposition, plating, painting, impregnation, and
any combination
thereof.
[0083] Examples are provided below to illustrate the working of the
embodiments, but such the
examples are by no means considered restrictive.
[0084] Example 1: In-situ formation of A14C3-containing Al-based material.
[0085] Commercially pure Al, pure iron, and a cast iron with 4.0 wt.% C are
taken as starting
materials. They are weighed with a target composition of 50 wt.% Al, 49.5 wt.%
Fe, and 0.5 wt.% C.
The staring materials are melted in an electric arc furnace in vacuum. Button
samples with the target
composition are prepared. Metallographic observations demonstrate the
formation of needle-like
A14C3 phase distributed in the Al matrix. If all the C atoms react with Al
atoms and forms the A14C3
phase, the content of the A14C3 phase in the Al-based material is calculated
to be 2 wt.%.
[0086] A button sample of the A14C3-containing Al-based material is immersed
in a beaker of fresh
water at room temperature for testing its degradability/dissolution in fresh
water. After 5 days of
immersion, the button sample is cracked and pulverized into powder. The
results show that the A14C3-
containing Al-based material is degradable/dissolvable in water at room
temperature.
Date Recue/Date Received 2021-02-22
[0087] A14C3-containing Al-based material suitable for dissolvable downhole
tools and components
for various wellbores, such as frac plugs, balls, etc.
[0088] Example 2. Ex-situ formation of A14C3-containing polymer matrix
composite.
[0089] A polyurethane casting resin, EasyFlo 60, and A14C3 powder with a size
of 15 gm (D50) are
taken as starting materials. EasyFlo 60 includes liquid part A and liquid part
B, 1:1 by volume, which
is purchased from Brick In The Yard (BITY) (www.brickintheyard.com). EasyFlo
60 has 2-2.5 minute
working time and 15-30 minute demold. Two kinds of EasyFlo 60 castings are
prepared. One is
EasyFlo 60 castings without any fillers, and the other is EasyFlo 60 castings
with additions of the
A14C3 powder at 30 wt.% and 40 wt.%. For those without any fillers, liquid
part A and liquid part B
are mixed with continuous stirring for about 1 minute and poured into a mold.
For those with additions
of the A14C3 powder, firstly, the A14C3 powder and liquid part A are mixed
with continuous stirring,
and then, liquid part B is added with continuous stirring for about 1.5-2
minutes and poured into a
mold. All the castings are cured at room temperature for 24 hours. The blank
polyurethane castings
(EasyFlo 60) without any fillers and the polyurethane castings (EasyFlo 60)
with additions of the
A14C3, that is, A14C3-containing polyurethane matrix composite, are
synthesized.
[0090] The blank polyurethane casting and the A14C3-containing polyurethane
matrix composites are
immersed in a beaker of fresh water at about 95 C for testing its
degradability/dissolution in hot water.
After 6 hours of immersion, the polyurethane matrix composites with additions
of 30 wt.% and 40
wt.% of the A14C3 are cracked extensively and the blank polyurethane casting
does not show any
changes in appearance. The results show that the A14C3-containing polyurethane
matrix composites
are degradable/dissolvable in hot water at 95 C.
[0091] A14C3-containing polymer-based materials are suitable for dissolvable
downhole tools and
components for intermediate- and low-temperature wellbores, such as frac
plugs, seals, etc.
[0092] Advantageously, embodiments of the present disclosure provide a
component of a downhole
tool comprised of inorganic hydrolysable compound-containing materials with
improved combination
of light weight, degradability/dissolvability, manufacturability, and
adaptability. The constituents and
microstructures of the inorganic hydrolysable compound-containing materials
can be tailored in a
wide range, to meet overall property needs for a degradable/dissolvable
downhole tool and
component. Most importantly, their degradability/dissolution is highly
dependent on temperature and
insensitive to chemistry of aqueous solutions, which would benefit their
downhole operations due to
complexity of a wellbore solution. They would improve the efficiency and
performance of downhole
operations in oil & gas exploration and production. Undoubtedly, their
application may include any
21
Date Recue/Date Received 2021-02-22
articles which require such combination, or at least the
degradability/dissolution in water, water-
containing solutions, and even moist air.
[0093] While the foregoing written description of the invention enables one of
ordinary skill to make
and use what is considered presently to be the best mode thereof, those of
ordinary skill will
understand and appreciate the existence of variations, combinations, and
equivalents of the specific
embodiment, method, and examples herein. The invention should therefore not be
limited by the above
described embodiments, methods, and examples, but by all embodiments and
methods within the
scope and spirit of the invention as claimed.
22
Date Recue/Date Received 2021-02-22