Note: Descriptions are shown in the official language in which they were submitted.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
1
TITLE OF THE INVENTION
PROCESS FOR REMOVING METALS, SULFUR AND OTHER IMPURITIES IN CRUDE OIL
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims benefit of U.S. Provisional Patent Application
No. 62/753,071,
filed on October 31, 2018, the content of which is incorporated herein in its
entirety by
reference.
FIELD OF THE INVENTION
[0002] The present invention relates generally to processes for treating crude
oil. More
specifically, the invention relates to a process for removing metals, sulfur
and other impurities
in a crude oil material. The process according to the invention uses a metal
and sulfur
removing agent which comprises a phosphoric acid ester. The process according
to the
invention may be readily scaled up and integrated to industrial facilities.
BACKGROUND OF THE INVENTION
[0003] During the preceding few decades, considerable attention has been
focused on
petroleum heavy crude oil for use in several applications. The demand for the
oil, however,
has declined due to serious concerns, one of which is the unsatisfactory level
of contamination
by both metals and sulfur. The issue has an adverse effect throughout the
refinery operations
as a result of severe damage to the contact surfaces. Furthermore, it gives
rise to diverse
environmental issues that affect the surrounding area and society. Therefore,
refining heavy
crude oil is required to avoid the challenges facing crude oil and expand its
commercial scale
in various industrial applications.
[0004] Substantial numbers of metal contamination exist in oil, and their
concentrations vary
from a few parts per million (ppm) to higher than 1000 ppm. Some of the most
distinct
elements that have been identified in oil are cobalt (Co), nickel (Ni), copper
(Cu), vanadium
(V), silver (Ag), sodium (Na), potassium (K), lithium (Li), calcium (Ca),
strontium (Sr) and
arsenic [1,2]. Ni and V are the most abundant, and they reside predominantly
in residual fuel
oil fractions in an oil soluble form. The concentration of both Ni and V vary
from a few ppm to
150 ppm and from less than 1 ppm to 1200 ppm, respectively [1-3].
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
2
[0005] Recently, all the attention has been directed to study the extraction
of both Ni and V
from crude oil. These elements exist in the core of stable organometallic
compounds in the
form of metalloporphyrinic and nonporphyrinc structures [4,5]. The high
stability of these
compounds increases the difficulty of elimination of the above metals from
heavy crude oil.
The subsistence of such elements in crude oil produces high volumes of coke
and dry gases
and decreases the liquid output [3]. In addition, the two elements poison the
catalyst in a
short time, ranging from 10 years for light oil to approximately one year for
heavy crude oil
[3,6], which is mainly due to the deposition of the two metal oxides formed on
the pore of the
catalyst and blocking the access site. Thus, the removal of both Ni and V from
heavy oil
becomes a vital objective of the crude oil industry.
[0006] Sulfur exists in crude oil as well in different forms, such as
mercaptans, sulfides,
disulfides, and thiophenes outlined in Erreur ! Source du renvoi introuvable.
[8]. The detected
percentage of sulfur in petroleum oil ranges from 0.1 wt.% to 15 wt.% and
depends on several
parameters, most importantly the origin of the extracted oil [7]. Sulfur is
emitted to the
atmosphere in the form of S0x, causing acid rain formation through its
reaction with water,
oxygen, and other chemicals exist in the atmosphere. The presence of sulfur in
oil increases
corrosion issues during the refinery process and helps in the deactivation of
the catalyst in
minimal time. Removal of dibenzothiophene and its alkyl derivatives is a big
challenge, as
the compounds cannot be transferred into H25 due to the steric hindrance
adsorption on the
surface of the catalyst. The existence of sulfur in various forms,
specifically the thiophinic
form, is a considerable challenge due to the difficlte removal of the element
from its complex
structure.
[0007] Although a large number of techniques have been developed to lessen or
remove the
metals and sulfur from crude oil, only a few of them have been industrially
applied, the main
reason being the disadvantages associated with each method. For instance, the
process
relies on solvent extraction, which leads to removing the whole fraction
containing metals,
thus reducing the yield of the end-product. The distillation technique
enhances the production
of two grades of oils: (1) light oil, which is the primary product and
contains a shallow
concentration of metals; and (2) heavy oil, which includes a much higher level
of contaminants
that must undergo a further upgrading process. The fast catalyst deactivation,
the need for
an emulsification process, the high cost and long processing time are
additional aspects that
limit the performance of the developed demetallization and desulfurization
techniques.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
3
[0008] Ultrafiltration is a costly, value-intensive technique due to the fast
fouling of the
membrane used in the procedure. The bio-demetallization and bio-
desulfurization processes
are novel techniques for metals and sulfur removal from crude oil by the
action of certain
microorganisms. The process is applied directly after the burning of the fuel
oil with keeping
the calorific value without change. The main disadvantages of the process are
the
degradation of the crude oil which destroys the main skeleton of the oil.
Also, the long
processing time of the technique is considered a big challenge. The
hydrotreatment process
for both metals and sulfur removal (HDM-HDS) is the only process widely used
in the industrial
sector. This technique can remove around 90% of the metals and sulfur content
from the oil.
Although the HDM-HDS process effectively removes a large portion of the metals
and sulfur,
it suffers from several drawbacks. For example, they include the fast catalyst
deactivation,
high hydrogen consumption, which reaches Hz/Oil, Scf/bbl 300-2000 for light
oil and 2000-
10,000 for heavy residual oil, an elevated temperature that ranges from 300 to
400 C for light
distillate and from 340 to 425 C for heavy residual, and elevated pressure,
which ranges from
30 t0130 atmosphere.
[0009] Several studies and efforts were performed on the removal of metals and
sulfur from
crude oil. Gould et al. developed a method for the demetallization of cold
lake asphaltenes,
Arabian heavy asphaltenes, and cold lake vacuum residuum [9]. Various
oxidizing agents
were tested to be demetallization agents, such as air at 100 C, NaOH/air,
sodium
hypochlorite, and peroxyacetic acid. It was found that the demetallization
process is
proportional to the amount of oxidant used. Air at 100 C and NaOH/air do not
show any
visible demetallization activity. Sodium hypochlorite and Peroxyacetic have
high
demetallization activity coupled with the ability to remove or destroy
petroporphyrins and
cause chlorine incorporation into the feed. D.A. Young proposed a new
technique using
ZnClz and TiC14 for the demetallization of different hydrocarbon feedstocks
[10]. The raw
materials were blended with ZnClz and TiC14 (2.0-4.5 lb/bbl oil), and the
resulting mixtures
were treated with hydrogen at approximately 288-482 C. A heavy Iranian residue
was
hydrogenated at 343 C, 1034-2500 kPa for 2 hours and a 7000 rpm stirring rate
in the
presence of ZnClz (4.2 ppb). Seventy percent of the Ni and V contaminants were
converted
to oil-insoluble forms with coke formation of less than 3 wt.%. Young [11],
Siskin [12],
Michlmayr [13], Nametkin, Gubin et al. [14], and Gleim [15] have patented
techniques using
different demetallization agents, such as chlorinating compounds (Clz, 50Cl2),
inorganic salts
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
4
(FeCl2, SnCl2, ZnCl2, TiC14, RuC13, Cr0I3, 00012) or their aqueous solutions.
The reaction
temperature ranged from 40 C to 300 C, and metals were successfully converted
into
insoluble constituents and removed by filtration. It was reported that the
concentration of both
Ni and V decreased by up to 70 wt.%. The main disadvantage of this process is
the
incorporation of chlorine and metal into the production and the degradation of
the quality of
the oil.
[0010] A novel technique has been applied by Greaney et al. [16] for
demetallization and
desulfurization by electrolysis using an electrochemical cell. The
commercially available
coulometry cell consisting of a mercury pool cathode, a platinum wire anode, a
standard
calomel reference electrode and a glass stirring paddle. The applied potential
was set at 2.5
V. for 18 hours stirring. In the process, toluene was added to an aqueous
solution of 40 wt.%
tetra-butylammonium hydroxide (20 mL). It was demonstrated that the removal
percentages
reached to 53% of V, 50% of Ni and 65% of Fe. Yan [17] has reported a
demetallization
method using an aqueous solution containing a chelating agent (EDTA, N-
(hydroxyethyl)
ethylenediamido triacetic acid, N-[2-(bi(carboxymethyl)amino)-ethyl]-N-(2-
hydroxyethyl)
glycine, diethylenetriamine pentaacetic acid or its salts as a demetallization
agent. A vacuum
distillation residual oil was treated with 27% EDTA aqueous solution at pH
4.5. It was found
that the removal percentages reached up to 99% of Ca, 35% of Fe, 4% of Ni, and
3% of V.
Aldridge, R. Bearden, K. Riley [18] used a vanadium oxide supported by
activated carbon for
the demetallization of Arabian heavy vacuum residue. The treatment process was
done at
5268 kPa and 290 C. It was noted that the activity of vanadium removal could
be increased
by increasing the percentage of vanadium on the activated carbon support. The
reaction is
highly selective with minimal occurrence of other reactions.
[0011] As can be seen, the removal of metals and sulfur from crude oil has
received
considerable attention.
[0012] There is still a need for processes for lowering the metals and sulfur
contents of the
crude oil. There is a need for such processes which are environmentally
friendly, efficient,
cost-effective and which can be readily scaled-up for industrial applications.
Also, there is a
need for such processes that can performed at industrial level.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
SUMMARY OF THE INVENTION
[0013] The inventors have designed and conducted a process for removing metals
and sulfur
(S) and S-containing compounds from a crude oil material. The process uses a
removing
agent which is both a demetallization (DM) agent and a desulfurization (DS)
agent. The
demetallization and desulfurization agent (DM-DSA) according to the invention
comprises a
phosphoric acid ester.
[0014] In embodiments of the invention, the DM-DSA is suitable for also
removing other
impurities present in the crude oil. In embodiments of the invention, the DM-
DSA is miscible
to the crude oil. In embodiments of the invention, the DM-DSA comprises a
phosphoric acid
ester.
[0015] In embodiments of the invention, the reacted DM-DSA agent may be
further treated
such as to recover DM-DSA which is re-used in the process. Also, any unreacted
DM-DSA
may be recovered and re-used in the process.
[0016] The process of the invention can be readily scaled up and integrated in
an industrial
facility.
[0017] The invention thus provides the following in accordance with aspects
thereof:
(1) A process for removing metals and sulfur (S)-containing compounds in a
crude oil material,
comprising causing the crude oil material to react with a removing agent which
comprises a
phosphoric acid ester.
(2) A process for removing metals and sulfur (S)-containing compounds in a
crude oil material,
comprising the steps of: (a) mixing the crude oil material with a removing
agent which
comprises a phosphoric acid ester, and subjecting the reaction mixture to
stirring for a first
period of time, at a temperature which is lower than the boiling point of the
removing agent;
(b) adding a first mixture of solvents including water to the reaction
mixture, and subjecting
the aqueous reaction mixture to stirring for a second period of time, at a
temperature which is
less than about 100 C; (c) allowing the aqueous reaction mixture to stand for
a third period of
time, thereby obtaining an oil phase comprising a treated oil and one or more
phases including
an aqueous phase; and (d) subjecting the aqueous reaction mixture to
separation thereby
yielding the treated oil.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
6
(3) A process according to (2), further comprising the steps of: (e) washing
the treated oil
using a second mixture of solvents including water; and (f) retrieving a
washed treated oil,
optionally steps (e) and (f) is repeated one time or more.
(4) A process according to (2), wherein the treated oil is further subjected
to steps (b) to (d),
one time or more.
(5) A process according to (2), wherein the treated oil is further subjected
to steps (a) to (d),
one time or more.
(6) A process according to (3), wherein a composition of the first mixture of
solvents at step
(b) and the second mixture of solvents at step (d) is the same or is
different; optionally the first
and second mixtures of solvent each independently comprises an organic
solvent; optionally
the organic solvent is an alcohol such as ethanol, or benzene, or hexane, or 4-
methy1-2-
pentanone.
(7) A process according to (3), wherein step (f) is conducted at ambient
temperature.
(8) A process according to (2) or (3), wherein steps (d) and (f) each
independently comprises
use of a reflux system; optionally steps (d) and (f) each independently
comprises decantation,
centrifugation, filtration or a combination thereof.
(9) A process according to any one of (2) to (8), wherein a length of the
first period of time at
step (a) and the second period of time at step (b) is the same or is
different.
(10) A process according to any one of (2) to (9), wherein the aqueous phase
obtained at step
(c) comprises reacted removing agent, and wherein the reacted removing agent
is further
subjected to a regeneration treatment to yield the removing agent; optionally
the regenerated
removing agent is re-used at step (a); optionally the reacted removing agent
comprises metal
salts of the removing reacted agent.
(11) A process according to (10), wherein the regeneration treatment of the
reacted removing
agent comprises causing the treated reacted removing agent to react with an
acid; optionally
the acid is HCI.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
7
(12) A process according to any one of (2) to (11), wherein the one or more
phases obtained
at step (c) comprise at least one phase comprising unreacted removing agent in
an organic
solvent, and wherein the unreacted removing agent is re-used at step (a).
(13) A process according to any one of (2) to (12), wherein the aqueous phase
obtained at
any of the steps is re-used in the process.
(14) A process according to any one of (1) to (13), wherein an amount of the
removing agent
is: between about 1 vol.% to about 5 vol.% an amount of the crude oil, or
between about 1
vol.% to about 4 vol.% an amount of the crude oil, or between about 1 vol.% to
about 3 vol.%
an amount of the crude oil, or between about 1 vol.% to about 2 vol.% an
amount of the crude
oil, or about 2 vol.% an amount of the crude oil; or about 1 vol.% an amount
of the crude oil.
(15) A process according to any one of (1) to (13), wherein an amount of the
removing agent
is: between about 1 wt.% to about 5 wt.% an amount of the crude oil, or
between about 1 wt.%
to about 4 wt.% an amount of the crude oil, or between about 1 wt.% to about 3
wt.% an
amount of the crude oil, or between about 1 wt.% to about 2 wt.% an amount of
the crude oil,
or about 2 wt.% an amount of the crude oil; or about 1 wt.% an amount of the
crude oil.
(16) A process according any one of (1) to (15), wherein other impurities in
the crude oil are
also removed.
(17) A process according to (16), wherein the metals and other impurities, as
measured by
Neuron Activation Analysis, comprise at least one of: Cd, U, Ca, V, Ti, Sn,
Sr, Ag, Mn, Si, Al,
Mg, Na, Fe, K, Zn, Cr, Cl, V, Co, Ni, Cu, As, Se, Br, Rb, Zr, Mo, In, Sn, Sb,
I, Cs, Ba, La, Hf,
W, Hg, Th, Sc and S.
(18) A process according to (16), wherein the metals and other impurities, as
measured by
Neuron Activation Analysis, comprise at least one of: Ti, Mn, Al, Mg, Na, V,
Ni, Cl, I, Br, Ca
and S.
(19) A process according to any one of (1) to (18), wherein the metals, as
measured by Neuron
Activation Analysis, comprise at least one of: V and Ni.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
8
(20) A process according to any one of (1) to (19), wherein sulfur in the
crude oil is in a form
selected from the group consisting of: thiol, sulfide, disulfide, thiolanes,
thiophene,
benzothiophene, dibenzothiophene and benzonaphtothiophene.
(21) A process according to any one of (1) to (20), wherein the removing agent
is a phosphoric
acid ester of general formula I below
0
R1O PO R2
OH
wherein R1 and R2 are each independently Ci to 020 a linear or branched,
cyclic or
non-cyclic, saturated or unsaturated alkyl group, optionally comprising a
heteroatom which is
0, S or N.
(22) A process according to (21), wherein R1 and R2 are each independently a
08 to 020 or a
08 to 018 or a 018 a linear or branched, cyclic or non-cyclic, saturated or
unsaturated alkyl
group, optionally comprising a heteroatom which is 0, S or N.
(23) A process according to any one of (1) to (22), wherein the metal removing
agent
comprises di-(2-ethylhexyl)phosphoric acid (DEHPA or HDEHP) outlined below
0
O¨P-0
OH
DEHPA or HDEHP
(24) A process according to (23), wherein the temperature at step (a) is up to
about 250 C.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
9
(25) A process according to any one of (1) to (24), wherein the metal removing
agent is
selected from the group consisting of: di-(2-ethylhexyl) phosphoric acid,
bis(2-ethylhexyl)
hydrophosphoric acid, di-(2-ethylhexyl)
orthophosphoric .. acid, .. 0,0-bis(2-
ethylhexyl)phosphoric acid, orthophosphoric acid 2-ethylhexyl alcohol,
phosphoric acid di(2-
ethylhexyl) ester and Hostarex PA 216TM.
(26) A process according to any one of (1) to (25), wherein the removing agent
is miscible to
the crude oil.
(27) A treated oil obtained by the process as defined in any one of (1) to
(26).
(28) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein a
content of the metals in the treated oil is about 75 to 90% or 80 to 90% lower
than in the crude
oil.
(29) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein a
content of S and S-containing compounds in the treated oil is about 50 to 55%
or about 53%
lower than in the crude oil.
(30) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein: a
content of the metals in the treated oil is about 75 to 90% or 80 to 90% lower
than in the crude
oil; and a content of S and S-containing compounds in the treated oil is about
50 to 55% or
about 53% lower than in the crude oil.
(31) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein a
content of V in the treated oil is about 90% lower than in the crude oil.
(32) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein a
content of Ni in the treated oil is about 79% lower than in the crude oil.
(33) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein a
content of S and S-containing compounds in the treated oil is about 53% lower
than in the
crude oil.
(34) A treated oil obtained by the process as defined in any one of (1) to
(26), wherein: a
content of V in the treated oil is about 90% lower than in the crude oil; a
content of Ni in the
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
treated oil is about 79% lower than in the crude oil; a content of S and S-
containing compounds
in the treated oil is about 53% lower than in the crude oil.
(35) A system for treating crude oil, which is adapted for conducting the
process as defined in
any one of (1) to (26).
(36) An oil treatment facility, comprising the system as defined in (35);
optionally the facility is
an industrial facility
[0018] Other objects, advantages and features of the present invention will
become more
apparent upon reading of the following non-restrictive description of specific
embodiments
thereof, given by way of example only with reference to the accompanying
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0019] In the appended drawings:
[0020] Figure 1: Forms of sulfur in petroleum crude oil.
[0021] Figure 2: Experimental setup of the process according to the invention.
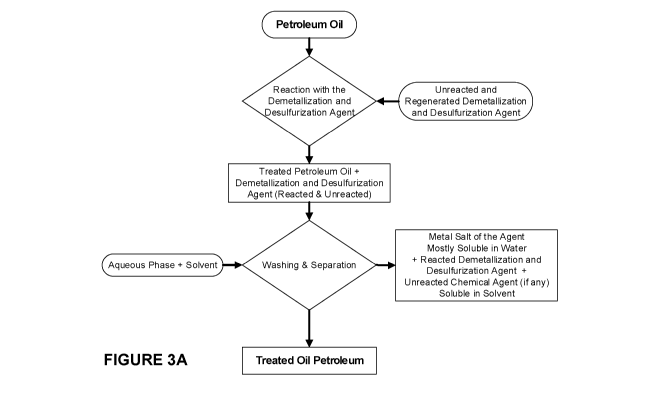
[0022] Figure 3: Flowchart of the process according to the invention.
[0023] Figure 4: FTIR spectra of the raw heavy aromatic naphthenic crude oil
(dotted line)
and the treated oil using 2 vol.c/o DM-DSA (continuous line).
[0024] Figure 5: FTIR spectra of raw light naphthenic crude oil (dotted line)
and the treated
oil using 2 vol.c/o DM-DSA (continuous line).
[0025] Figure 6: FTIR spectra of the treated heavy aromatic naphthenic crude
oil using 2
vol.c/o (continuous line) and using 5 vol.c/o DM-DSA (dotted line).
[0026] Figure 7: FTIR spectra of the treated light naphthenic crude oil using
2 vol.c/o DM-DSA
(dotted line) and using 5 vol.c/o DM-DSA (continuous line).
[0027] Figure 8: Sulfur detection in the raw heavy aromatic naphthenic crude
oil by elemental
analysis technique.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
11
[0028] Figure 9: Sulfur detection in the treated heavy aromatic naphthenic
crude oil using 2
vol.% DM-DSA, by elemental analysis technique.
[0029] Figure 10: Sulfur detection in the raw light naphthenic crude oil by
elemental analysis
technique.
[0030] Figure 11: Sulfur detection in the treated light naphthenic crude oil
using 2 vol.% DM-
DSA, by elemental analysis technique.
[0031] Figure 12: Removal efficiency of Pennsylvania oil treated with 2 vol.%
DM-DSA.
[0032] Figure 13: Removal efficiency of heavy aromatic naphthenic oil treated
with 2 vol.%
DM-DSA.
[0033] Figure 14: Removal Efficiency of light naphthenic oil treated with 2
vol.% DM-DSA.
[0034] Figure 15: Removal Efficiency of Iran oil treated with 2 vol.% DM-DSA.
[0035] Figure 16: Removal efficiency of Basra oil treated with 2 vol.% DM-DSA.
[0036] Figure 17: Removal Efficiency with 2 vol.% (continuous line) and 5
vol.% (dotted line)
DM-DSA for heavy aromatic naphthenic oil.
[0037] Figure 18: Removal Efficiency with 2 vol.% (continuous line) and 5
vol.% (dotted line)
DM-DSA for light naphthenic oil.
[0038] Figure 19: Removal efficiency of treated Iran oil with 2 vol.% DM-DSA
(continuous
line) against that only washed with aqueous solution (dotted line).
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0039] Before the present invention is further described, it is to be
understood that the
invention is not limited to the particular embodiments described below, as
variations of these
embodiments may be made and still fall within the scope of the appended
claims. It is also to
be understood that the terminology employed is for the purpose of describing
particular
embodiments; and is not intended to be limiting. Instead, the scope of the
present invention
will be established by the appended claims.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
12
[0040] In order to provide a clear and consistent understanding of the terms
used in the
present specification, a number of definitions are provided below. Moreover,
unless defined
otherwise, all technical and scientific terms as used herein have the same
meaning as
commonly understood to one of ordinary skill in the art to which this
disclosure pertains.
[0041] Use of the word "a" or "an" when used in conjunction with the term
"comprising" in the
claims and/or the specification may mean "one", but it is also consistent with
the meaning of
"one or more", "at least one", and "one or more than one". Similarly, the word
"another" may
mean at least a second or more.
[0042] As used in this specification and claim(s), the words "comprising" (and
any form of
comprising, such as "comprise" and "comprises"), "having" (and any form of
having, such as
"have" and "has"), "including" (and any form of including, such as "include"
and "includes") or
"containing" (and any form of containing, such as "contain" and "contains"),
are inclusive or
open-ended and do not exclude additional, unrecited elements or process steps.
[0043] As used herein when referring to numerical values or percentages, the
term "about"
includes variations due to the methods used to determine the values or
percentages, statistical
variance and human error. Moreover, each numerical parameter in this
application should at
least be construed in light of the number of reported significant digits and
by applying ordinary
rounding techniques.
[0044] As used herein, the term "removing agent" or "demetallization-
desulfurization agent
(DM-DSA)" refers to a suitable agent that mixes with the crude oil and is
adapted to removing
both metals and sulfur (S)-containing compounds from the crude oil. Such agent
may also be
adapted to removing S in free form. Such agent is also adapted to removing
other impurities
in the crude oil. Such agent comprises a phosphoric acid ester.
[0045] As used herein, the term "sulfur (S)-containing compounds" refers to
any compound
in the crude that comprises a sulfur atom. The term also refers to S in free
form.
[0046] As used herein, the term "demetallization agent" refers to a suitable
agent that mixes
with the crude oil and is adapted to removing metals from the crude oil. Such
agent is also
adapted to removing other impurities in the crude oil. Such agent comprises a
phosphoric
acid ester.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
13
[0047] As used herein, the term "desulfurization agent" refers to a suitable a
suitable agent
that mixes with the crude oil and is adapted to removing sulfur (S)-containing
compounds.
Such agent may also be adapted to removing S in free form. Such agent is also
adapted to
removing other impurities in the crude oil. Such agent comprises a phosphoric
acid ester.
[0048] The inventors have designed and conducted a process for removing metals
and sulfur
(S) and S-containing compounds from a crude oil material. The process uses a
removing
agent which is both a demetallization (DM) agent and a desulfurization (DS)
agent. The
demetallization and desulfurization agent (DM-DSA) according to the invention
comprises a
phosphoric acid ester.
[0049] The present invention is illustrated in further details in the
Experiment Work section
below. The section includes non-limiting examples.
Experimental work conducted
[0050] Materials: The demetallization-desulfurization (DM-DS) process
according to the
invention has been implemented on a number of petroleum crudes obtained from
several
countries. Iran and Basra oils were obtained directly from the tanks of the
TOTAL refinery
station in France with a high concentration of V, Ni and S. Heavy aromatic
naphthenic crude
oil, light naphthenic crude oil, and Pennsylvania crude oil were purchased
from the ONTA
company, in Ontario. The concentrations of V, Ni and S varied from low to high
in the ONTA
samples, as it is desired to gain deeper insight into the removal efficiency
using the DM-DSA
for the high and low metal concentrations. Other chemical agents, such as the
DM-DSA and
the solvents, were purchased from Sigma-Aldrich, Canada; di-(2-
ethylhexyl)phosphoric acid
(DEHPA or HDEHP) outlined below was generally used as DM-DSA in the
experiments
conducted.
0
0 ¨P-0
OH
DEHPA or HDEHP
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
14
[0051] The concentration of metals and sulfur in the oil samples was
determined by the
neutron activation analysis technique (NAA) using the SLOWPOKE reactor at
Polytechnique
Montreal, QC, Canada. The other required information archived directly from
the supplier,
Iran and Basra oils, are presented in Table 1 below.
[0052] Table 1 ¨ Characteristic of the processed oils.
Light Heavy
Property Unit Basra Oil Iran Heavy naphtha aromatic
naphtha
Density
kg/m3 886.8 881.2 873.5 953.0
API 27.98 28.99 30.0 16.4
Viscosity
mm /5 10 C) /5 23.8 18.4
(
Viscosity
mm /5 (37 8 C) /5 9.32 10.6
,
Viscosity
mm /5 (50 C) /5 6.79 8.67
Stotal mg/kg 26354 22795 33557 18385
Ni mg/kg 12 25 61.1 35.3
V mg/kg 40 85 331 383.1
CCR %W 7.845 6.88 H/C= 1.668 H/C= 1.558
[0053] Experimental setup: The experimental setup shown in Figure 2 was
employed to carry
out the experimental work. The reference numerals in Figure 2 are as follows:
batch reactor
(1), conventional heating source (2), heating zone (3), electric stirrer (4),
heat reflux (5), water
cooler (6), treated oil tank (7), washing liquids tank (8), analytical
techniques (9), thermometer
(10) and three-ways valve (11). Also, on Figure 2 "NAA" denotes Neutron
activation analysis
and "FTIR" denotes Fourier transform infrared.
[0054] The mixture of the crude oil and the reactants is poured into the batch
reactor equipped
with a stirring technique. The reactor is attached to a water-cooled Liebig or
Vigreux
condenser fitted onto the top. The condensation system, in other words,
reflux, works at a
temperature of -5 C and ambient pressure. The central role of the reflux is to
condense the
lower molecular weight compounds that might be vaporized during the reaction
time due to
increasing temperature. The reactor is heated conventionally to a temperature
lower than the
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
boiling point of the DM-DSA yet enough to perform the removal reaction. The
temperature
was controlled not only based on the electric oven temperature, but also as a
direct
measurement by using a thermometer immersed inside the oil. The experiment was
carried
out utilizing the conventional heating mechanism to control the reaction
temperature and other
aspects.
[0055] Experiment procedures: A flowchart illustrating the process according
to the invention
is presented in Figure 3A and Figure 3B, also showing the regenarations of
various
components of the process. The process comprises: treatment of the crude oil
with the DM-
DSA (reaction); separation of the treated oil from the reacted and/or
unreacted DM-DSA (in
aqueous phase); and washing the treated oil. More details on each of the steps
of the process
are outlined herein below.
[0056] Treatment of crude oil with the DM-DSA (reaction): A weight/volume
amount of the
crude oil was mixed with the DM-DSA. An amount of DM-DSA between about 2 wt.%
and 5
wt.% of the amount of the crude oil was generally used. The mixture of crude
oil and DM-
DSA was poured into the reactor as outlined in Figure 2. It is worth
mentioning that the
described process does not need an emulsification process, which is contrary
to most of the
existing chemical demetallization techniques. The principal reason for this
aspect is the good
miscibility of the DM-DSA according to the invention with crude oil. Electric
stirring is applied
during the reaction for mixing the reactants and for properly distributing the
heat inside the
reactor. This enhances the replacement reaction taking place between the
treated oil and the
DM-DSA. The mixture is heated for about 1 hour at a temperature of up to 250 C
under stirring
conditions of up to 700 rpm.
[0057] Separation: Efforts were made to separate the treated oil from the
reacted DM-DAS in
the form of metal salts thereof and any unreacted DM-DSA. The challenge is due
to the fact
that all the components involved, namely, the treated oil, DM-DSA in the form
of salts thereof
and any unreacted DM-DSA are all present in the same vessel. The separation
process was
performed using a mixture of solvents comprising organic solvents and water.
In
embodiments of the invention, an organic solvent such as an alcohol was used
together with
water. A first solvent was used mainly to dissolve any unreacted DM-DSA and
separate it
from the treated oil. A second solvent, preferably in aqueous phase, was used
to dilute the
metal salts of DM-DSA. In embodiments of the invention ethanol and water were
used. The
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
16
mixture of the first and second solvents and the treated oil was then
subjected to heating at a
temperature of less than about 100 C under stirring conditions of up to 700
rpm for about 1
hour. The separation is generally performed in a reflux system to avoid the
evaporation of the
solvent which would allow for the precipitation of the dissolved compounds
back into the oil.
After the separation time, a mixture of three phases could be observed in the
reactor. The
upper phase comprising the treated oil, the lower phase comprising both the
metal salts of
DM-DSA dissolved in the aqueous phase and unreacted DM-DSA dissolved in
ethanol.
Eventually, the two obtained phases were separated by decantation and, then,
centrifugation.
The organic solvent phase comprising the unreacted dissolved DM-DSA was
further
separated from the aqueous phase to regenerate the unreacted DM-DSA.
[0058] Washing the treated oil: After the separation, the collected oil phase
was subjected to
washing in order to ensure a complete removal of the metal salts of DM-DSA and
any
unreacted DM-DSA. More than one washing was performed, generally about three
washings
were performed. In embodiments of the invention, the first and second solvents
used in the
separation step were also used in the washings. Washing was performed at room
temperature with vigorous stirring or shaking for approximately a few minutes.
The mixture
was then poured into a separation system where it was left to stand until
complete detachment
of the two phases. A centrifugal separation system was eventually used for the
aqueous
phase/oil phase separation; then the treated oil was sent for the analytical
techniques.
[0059] Analytical techniques: Various analytical techniques were performed to
validate the
performance of the DM-DSA according to the invention as well as to gain a
better
understanding of the process efficiency. The following three analytical
techniques were
applied: (1) Fourier transform infrared (FTIR), (2) Neutron activation
analysis (NAA) and (3)
Elemental analysis C, H, N, and S.
[0060] FTIR technique: Fourier transform infrared spectroscopy analysis was
undertaken
using a Perkin Elmer 65 FTIR-ATR instrument (Perkin Elmer, Woodbridge, ON,
Canada). A
sum of 128 scans was accumulated for the signal averaging of each IR spectral
measurement
with a 4 cm-1 resolution. The spectra of the samples were recorded over a
wavenumber range
of 4000-650 cm-1 to detect the transformation of N-M bond to the N-H bond.
FTIR can detect
the characteristic vibration frequencies for each bond, functional group, side
chain, and cross-
link inside the molecule. The demetallization reaction is primarily founded on
the conversion
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
17
of N-M bonds into the N-H bond. The FTIR technique is a good candidate to
observe the
changes taking place in the N-M bond. Unfortunately, the FTIR instrument used
in the
detection process could not detect peaks lower than 600 cm-1; consequently,
the peaks of N-
M bonds at less than 400 cm-1 have not been recorded.
[0061] Elemental analysis technique: It is desirable to detect the sulfur
concentration in the
crude oil to record the change that occurs after the demetallization-
desulfurization process.
To observe the change, the elemental analysis technique is applied to find out
the variation in
sulfur concentration before and after the extraction process. The sulfur
detection was
performed using the Elemental Analyzer EA3000 (EuroVector) instrument. The
data were
processed using Callidus software interface version 5.1. Callidus. The 5.1
software fully
controls the device, integrates peaks, reprocesses sample data, and reports
result selecting
one of the pre-arranged formats. The Callidus is based on the autorun concept
as the
samples cannot analyze in isolation but must be in association with standards
(for calibration
purposes), blanks (for the determination of potential trace contaminants) and
bypasses (for
conditioning purposes).
[0062] Neutron activation analysis: Neutron activation analysis is a nuclear
technique used to
determine the compactness of each element existing in vast numbers of chemical
compounds. The analysis was performed in a slowpoke lab at Polytechnique
Montreal, QC,
Canada. In this technique, a neutron source is required for bombarding the
sample with
neutrons. Due to this bombardment, the element transfers to its isotopic form.
According to
the radioactive emission and decay data known for each element, the spectra of
emission of
gamma rays for all the elements can be easily studied. Quantifying various
metals in crude
oil is indeed a challenge, due to the complex matrix of crude oil, which
includes vast numbers
of metals and different elements. In addition, the depressed concentration of
each metal
remains a considerable issue to be determined by most of the analytical
techniques. Many of
the metals and elements are interfering as well, which affects the accuracy of
the
measurements. The NAA technique is characterized by high accuracy in
quantifying a wide
assortment of metal elements in the complex matrix of crude oil. Its
proficiency is indirectly
dealing with the oil itself without any digestion process or dilution, such as
the ICP-MS
technique, which has several factors for error production in the measurements.
The
drawbacks, the uncertainty, and the limitations were determined for the NAA
measurements
to heighten the accuracy of the technique. An optimum method that can be used
for metals
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
18
quantification using the NAA technique is the kO-Neutron Activation analysis
(kO-NAA). This
method is a single-comparator standardized method used for high accuracy
quantification of
elements in any type of materials. By applying this method, the calibration of
each element
by changing the matrix or the detector is not required.
[0063] Quantification method: For the quantification of the metals and sulfur
in the crude and
treated oil, the extraction efficiencies in the case of each oil were
calculated. The extraction
efficacy percentage was determined using the following equation:
((C crude - C treated)
Extraction efficacy (%) - * 100,
C crude
where the Cm& is the concentration of an element in the crude oil before the
treatment process, Ctreated is the concentration of the element in the treated
oil after the
treatment process.
[0064] Results and discussion: The DM-DSA according to the invention has the
ability to form
an ionic liquid while it is present in oil at a lower temperature. The DM-DSA
is not miscible
with water, but it forms salts that are soluble in water at low and high
temperatures. Thus, the
unreacted part of the DM-DSA can be recovered and recycled. The immiscibility
of the agent
with water may be attributed to the presence of long side chains in the agent
(Ri and R2 in
formula I are between about 08 and Cio chains), which reduces its polarity.
[0065] The FTIR of the DM-DSA indicates that the DM-DSA has peaks appearing in
regions
that are different from what appeared in the treated oil, in particular,
between 4000cm-1 and
3000 cm-1. This allows us to conclude that peaks appearing in these regions
after the process
do indeed relate to the treatment process.
[0066] The FTIR shows a difference in the absorption frequencies between the
treated and
untreated oil. It is supposed to transfer the metalloporphyrin ring into free
based porphyrin;
accordingly, new frequencies for the N-H bonds should appear instead of the N-
M bonds.
[0067] Figure 5 shows the deviation in the IR absorption frequencies between
the untreated
and treated heavy aromatic naphthenic crude oil. There is a peak located at
3320 cm-1, which
is attributed to the N-H bond stretching frequency. The peak located around
1600 cm-1 is
related to another vibrational mode of N-H [19]. There are two other peaks
appearing at
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
19
around 1110 cm-1 and 740 cm-1 in the treated oil, which are relative to in-
plane N-H and out-
of plane bending N-H, respectively [20]. It should be noted that these two
peaks do not exist
in the crude oil or in the DM-DSA. The bands around 2922 cm-1 were assigned to
the C-H
bond of the benzene ring and pyrrole ring. Bands at -1458 and -1379 cm-1 are
attributed to
the C=C stretching mode and the C=N stretching vibration, respectively. The
bands at around
800 cm-1 and 750 cm-1 were respectively assigned to the C-H bond bending
vibration of the
para-substituted and molecule ortho-substituted phenyl ring.
[0068] Figure 5 shows the same peaks appearing in Figure 4 but for light
naphthenic crude
oil. The FTIR results confirm that new peaks appeared in the treated oil which
related to the
N-H bond. This finding, in turn, confirms that the treatment process using the
DM-DSA
according to the invention was successfully implemented and the N-M bond
transferred to N-
H bond. Similar results were obtained in all processed oil samples.
[0069] An increased concentration of DM-DSA, to about 5 vol.%, was also
applied during the
treatment process of both heavy aromatic naphthenic crude oil and light
naphthenic crude oil.
This experiment performed to determine the variation in the removal efficiency
when a higher
amount of the DM-DSA was used. The difference can be determined by comparing
the
intensity of the peak for both 2 vol.% and 5 vol.% using the FTIR technique.
It was found that
there is no significant increase in the removal efficiency by applying 5 vol.%
of DM-DSA during
the treatment process, as presented in Figure 6 and Figure 7.
[0070] The elemental analysis was carried out to detect the variation in
sulfur concentration
before and after the treatment process, and to record the effect of the DM-DSA
on sulfur
removal. Figure 8 depicts a clear peak for sulfur in the region between 144
and 180 cm' on
the heavy aromatic naphthenic crude oil and Figure 9 shows the change
performed for the
sulfur peak after the process. The strength of the peak was reduced, which
indicates the
decrease in the concentration of sulfur in the treated oil.
[0071] This shows that the DM-DSA eliminated a portion of the sulfur compounds
from the
crude oil during the demetallization-desulfurization process. The same
decrease in the sulfur
content was obtained in the light naphthenic crude oil. Figure 10 and Figure
11 also confirm
the reduction in sulfur concentration that took place after the
demetallization-desulfurization
process.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
[0072] To accurately determine the metal content in the oils, the NAA
technique was
performed. The analysis was carried out on the crude oil, the treated oil and
the aqueous
phase produced after the washings. A difference in the metal content between
the crude oil
and the treated oil was detected. In addition, the analysis of the aqueous
phase showed the
presence of metals in the aqueous phase after the washing step. It is worth
mentioning that
almost all the metals concentrations have been reduced compared to the crude
oils,
specifically, V and Ni, which are known in the art to be difficult to
eliminate. Pennsylvanian
crude oil is a light oil with low metals concentrations. Thus, it was used to
test the removal
efficiency of the DM-DSA in the case of the lower levels of metals in crude
oil. The NAA
results are presented in Table 2 below and Figure 12 confirming that the DM-
DSA has a high
removal efficiency in the case of Ti, I, Al and Ca, which reaches more than 90
wt.%. The
removal efficiency for Br, Mg, V and Cl was detected at higher than 70 wt.%.
The treatment
process also included removal of sulfur alongside metals, which reached 27%.
[0073] Table 2 ¨ NAA results of Pennsylvania oil treated with 2 vol.% DM-DSA.
Element Raw oil (ppm) Treated oil (ppm)
Ti 7 0.3
0.6 0.03
Br 0.5 0.06
Mn 0.02 0.01
Al 12 0.50
Mg 15 1.6
Na 6 2.6
V 0.03 0.01
Cl 12 3.2
Ca 44 2.7
839 612
[0074] The NAA results of the heavy aromatic naphthenic oil are tabulated in
Table 3 below.
Figure 13 displays the removal efficiency of the treated heavy aromatic
naphthenic crude oil
using 2 vol.% DM-DSA. All three of Ti, Mg, and Na have a higher removal
efficiency, which
reached more than 80%, followed by Cl and Ca with more than 65%. The
concentration of S
decreased as well with a removal efficiency of around 15%.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
21
[0075] Table 3 ¨ NAA results of heavy aromatic naphthenic oil treated with 2
vol.% DM-DSA.
Element Raw oil (ppm) Treated oil (ppm)
Ti 20.4 2.05
Mn 0.23 0.15
Mg 41 3.37
Na 5.88 0.85
V 383.1 233.6
Cl 100.8 27.02
Ca 54.16 16.66
18385 15513.8
[0076] Figure 14 and Table 4 below demonstrate the results of the light
aromatic naphthenic
oil.
[0077] Table 4 ¨ NAA results of light naphthenic oil treated with 2 vol.% DM-
DSA.
Element Raw oil (ppm) Treated oil (ppm)
Ti 34 3
Mn 0.2 0.1
Mg 74 10
Na 9 7
V 331 307
Cl 106 18
Ca 55 17
33557 23812
[0078] The NAA analysis shows a high removal efficiency of both Iran and Basra
oils in spite
of the high complexity of the two oils. In the case of Iran oil, the removal
efficiency presented
in Figure 15 and Table 5 below.
[0079] Table 5 ¨ NAA results of Iran oil treated with 2 vol.% DM-DSA.
Metals Raw oil (ppm) Treated oil (ppm)
Ti 6 0.5
Mn 0.02 0.01
Mg 17 6
Na 13 0.1
V 88 59
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
22
Ni 30 8
Cl 22 17
Ca 5 0
22795 14816
[0080] V, Ni and S reached 33%, 73% and 35%, respectively. For Basra oil, the
removal
efficiency was more prominent than in Iran oil, as all of V, Ni and S have
been eliminated with
90%, 79% and 63% efficiency, respectively; see Table 6 below and Figure 16.
[0081] Table 6 ¨ NAA results of Basra oil treated with 2 vol.% DM-DSA.
Metals Raw oil (ppm) Treated oil (ppm)
Ti 4 0.6
Mn 0.05 0.008
Mg 5 1
Na 14 5.5
V 37 3.6
Ni 10 2
Cl 25 10
Ca 8 2.6
26354 9751
[0082] It is essential to distinguish the variation that could take place for
the metals and sulfur
concentrations if the dosage of the DM-DSA rose to more than 2 vol.%.
Therefore, another
batch of experiments was carried out on the heavy aromatic naphthenic crude
oil and light
naphthenic crude oil applying 5 vol.% DM-DSA, Figure 17, Table 7 below, Figure
18 and
Table 8 below. It was found from the NAA analysis that there is an adequate
discrepancy in
the removal efficiency of some metals when raising the DM-DSA dosage,
specifically Na and
Mn. This reduction could be due to the simple structure of the metals in the
oil, which make
them much smoother to attach to the DM-DSA than the other metals complexes.
Regarding
S, it is reasonable to enhance the removal efficiency of S by increasing the
DM-DSA dosage,
because S compounds will encounter more ionic liquids with which to connect.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
23
[0083] Table 7 - comparison between heavy aromatic naphthenic crude oil and
treated oils
with 2 vol.% and 5 vol.% DM-DSA.
Treated oil Treated oil
Metal Raw oil (ppm)
2 vol.% (ppm) 5 vol.% (ppm)
Ti 20.4 2.05 3.06
Mn 0.23 0.15 0.1
Mg 41 3.37 2.5
Na 5.88 0.85 2.24
V 383.1 233.6 222.6
Cl 100.8 27.02 26.76
Ca 54.16 16.66 14.72
18385 15514 10090
[0084] Table 8 - NAA analysis of light naphthenic crude oil and treated oils
with 2 vol.% and
vol.% of DM-DSA.
Treated oil Treated oil
Metal Raw oil (ppm)
2 vol.% (ppm) 5 vol.% (ppm)
Ti 33.66 2.7 1
Mn 0.169 0.105 0.03
Mg 73.99 10.33 0.45
Na 9.4 7.82 2.85
V 331 307.26 300.15
Cl 100.8 18.46 44.68
Ca 54.955 17.48 8.88
33557 23812.9 23602.6
[0085] It was desired to confirm that the decrease in metals concentrations
after the
treatment process was due to the reaction of the metals with the DM-DSA and
not because
of the washing step. A comparison between the treated oil with the process
according to the
invention and the same oil exposed only to the washing step was therefore
carried out. Iran
oil was used, and the results were analyzed by the NAA technique. Figure 19
illustrates the
comparison in removal efficiency between the treated and washed Iran oil. It
was noted that
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
24
all the metals decreased due to the high removal efficiency reaching up to
100% for some
metals, whereas employing just the washing step did not show any drop in the
metals except
those that can be extracted just by washing, such as Ca and Ba. This confirmed
that the
treatment process is indeed responsible for the decline that took place for
the metal
concentrations.
[0086] The DM-DSA according to the invention acts as a proton donor and, thus,
provides
the porphyrin ring with the needed hydrogen ions to occupy the vacancies
created during the
metal extraction reactions. The DM-DSA also helps to extract the metals from
the porphyrin
ring forming the metal salt of the agent. Behind the demetallization and
desulfurization
processes, there are a set of complex chemical reactions taking place. Effort
was made to
better understand these chemical reactions.
[0087] The main idea behind the demetallization reaction is the ion exchange
mechanism that
happens very quickly in homogeneous solutions. In the reaction (cation
exchanger), the agent
acts as a proton donor for the metalloporphyrin ring after the four nitrogen
atoms extract the
electron bonds between the metals, due to the high electronegativity of the
nitrogen atoms,
which reaches up to 3.04 compared to 1.63 for the V atom. The high
electronegativity of the
four nitrogen atoms plays an essential role in leaving the metal atom as a
positive metal ion
after the electron bond extraction. This, in turn, forms four negative
nitrogen ions, which
directly bond with the hydrogen protons from the agent forming the pure
porphyrin ring. At
the same time, the negative side of the agent robustly extracts the positive
metal ion from the
media creating the metal salt of the agent.
[0088] The DM-DSA used for the demetallization process is one of the most
famous cations
that can form ionic liquids with several anions. Both anion and cation species
can interact
forming an ionic liquid, or the cation can interact with a chloride anion from
the oil medium
forming another ionic liquid. The ionic liquid is formed by stirring both of
the DM-DSA with the
crude oil [21]. It was demonstrated that there are some factors affected by
the melting point
and the physical state in general of the ionic liquid at room temperature,
importantly, the
lengths of the alkyl chain and the central atom of the cation [22]. Platzer et
al. have indicated
that larger side chain cations tend to reduce the melting point of the ionic
liquid [23].
[0089] When the ionic liquid is formed, it bonds with the thiophen compounds
[24] in the oil
through two different bonds: (1) through the H-bond between the S and the H
from the agent,
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
and (2) through the electrostatic force between the different charges of the
high molecular
weight part of both the agent and the thiophen compounds.
[0090] The process according to the invention allows for the removal of
metals, sulfur and
other impurities in a crude material. The removing agent or DM-DSA used in the
process is
a phosphoric acid ester such as di-(2-ethylhexyl)phosphoric acid (DEHPA or
HDEHP) outlined
above. As will be understood by a skilled person, the DM-DSA may be any
suitable
phosphoric acid ester, for example of general formula I below. In embodiments
of the
invention, the DM-DSA is miscible with the crude oil.
0
R1O PO R2
OH
wherein R1 and R2 are each independently Ci to 020 a linear or branched,
cyclic or
non-cyclic, saturated or unsaturated alkyl group, optionally comprising a
heteroatom which is
0, S or N; optionally R1 and R2 are each independently a 08 to 020 or a 08 to
016 or a 016
linear or branched, cyclic or non-cyclic, saturated or unsaturated alkyl
group, optionally
comprising a heteroatom which is 0, S or N.
[0091] The process according to the invetntion comprises: at least one
reaction step, at least
one separation step, and at least one washing step. As will be understood by a
skilled person
these steps may invoved other steps such as decantation, centrifugation,
filtration.
[0092] The process according to the invention allows for the regeneration of
the DM-DSA
from the reacted DM-DSA. This is performed by causing the reacted DM-DSA to
react with
an acid such as HCI. The regenerated DM-DSA is re-used in the process. Also,
any
unreacted DM-DSA is recovered and re-used in the process. Moreover, the
aqueous phases
steming from the separations are recovered and re-used in the process.
[0093] A content of metals in an oil treated by the process of the invention
may be between
about 75 to 90% or between about 80 to 90% lower than in the crude oil. A
content of S and
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
26
S-containing compounds in an oil treated by the process of the invention may
be between
about 50 to 55% or about 53% lower than in the crude oil. As will be
understood by a skilled
person, such treated oils are with the scope of the present invention.
[0094] The process according to the invention embodies a system and may be
readily scaled
up and integrated in an industrial facility. As will be understood by a
skilled person, such
system and facility are within the scope of the present invention.
[0095] The scope of the claims should not be limited by the preferred
embodiments set forth
in the examples; but should be given the broadest interpretation consistent
with the description
as a whole.
[0096] The present description refers to a number of documents, the content of
which is
herein incorporated by reference in their entirety.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
27
REFERENCES
1. Jenifer AC, Sharon P, Prakash A, Sande PC. A Review of the Unconventional
Methods
Used for the Demetallization of Petroleum Fractions over the Past Decade.
Energ. Fuel.
2015;29(12):7743-52.
2. Ali MF, Abbas S. A review of methods for the demetallization of residual
fuel oils. Fuel
Processing Technology. 2006;87(7):573-84.
3. Shang H, Liu Y, Shi J-C, Shi Q, Zhang W-H. Microwave-assisted nickel and
vanadium
removal from crude oil. Fuel Processing Technology. 2016;142:250-7.
4. Valkovic V. Trace elements in petroleum1978. Medium: X; Size: Pages: 279 p.
5. Yen TF. Role of trace metals in petroleum. 1975.
6. Dedeles GR, Abe A, Saito K, Asano K, Saito K, Yokota A, et al. Microbial
demetallization
of crude oil: nickel protoporphyrin disodium as a model organo-metallic
substrate. Journal of
bioscience and bioengineering. 2000;90(5):515-21.
7. Miadonye A, Snow S, Irwin D, Khan MR, Britten A. Desulfurization of heavy
crude oil by
microwave irradiation. Computational Methods in Multiphase Flow V.
2009;63:455.
8. Hosseini H, Hamidi A, editors. Sulfur Removal of Crude Oil by Ultrasound-
Assisted
Oxidative Method. International Conference on Biologi-cal, Civil and
Environmental
Engineering (BCEE-2014) March; 2014.
9. Gould KA. Oxidative demetallization of petroleum asphaltenes and residua.
Fuel.
1980;59(10):733-6.
10. Young DA, inventor; Union Oil Co., USA, assignee. Demetalization of
petroleum
feedstocks with zinc chloride and titanium tetrachloride catalysts. United
States 1979.
11. Young DA. Demetallization of petroleum feedstocks with zinc chloride and
titanium
tetrachloride catalysts. U.S. 4,148,717.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
28
12. Siskin M. Hydrodesulfurization with a metal halide-hydrogen halide
catalyst. U.S.
4,043,900.
13. Michlmayr MJ. Upgrading metal-contaminated petroleum oils containing
vanadium and/or
nickel. U.S. 4,039,432.
14. Nametkin NS, Gubin SP, Tjurin VD, Fedorov VV, Larionov LI, Kozin VA, et
al. Method of
purifying crude petroleum and primary refining products. U.S. 3,996,130.
15. Gleim WK. Hydrorefining of metal-containing black oils with a molten Lewis
acid and a
molybdenum halide. U.S. 3,483,117.
16. Greaney MA, Kerby Jr MC, Olmstead WN, VViehe IA. Method for demetallating
refinery
feedstreams. CA 2,208,565.
17. Yan TY. Demetalation of heavy hydrocarbon oils. U.S. 4,379,747.
18. Aldridge CL, Bearden Jr R, Riley KL. Removal of metallic contaminants from
a
hydrocarbonaceous liquid. EP 0 433 026 B1.
19. Castro KA, Silva S, Pereira PM, Simoes MM, Neves MdGP, Cavaleiro JA, et
al.
Galactodendritic porphyrinic conjugates as new biomimetic catalysts for
oxidation reactions.
Inorganic Chemistry. 2015;54(9):4382-93.
20. Sen P, Hirel C, Andraud C, Aronica C, Bretonniere Y, Mohammed A, et al.
Fluorescence
and FTIR Spectra Analysis of Trans-A (2) B (2)-Substituted Di-and Tetra-Phenyl
Porphyrins.
Materials. 2010;3(8):4446-75.
21. Kogelnig D, Stojanovic A, Galanski M, Groessl M, Jirsa F, Krachler R, et
al. Greener
synthesis of new ammonium ionic liquids and their potential as extracting
agents. Tetrahedron
Letters. 2008;49(17):2782-5.
22. Pirkwieser P, Lopez-Lopez JA, Kandioller W, Keppler BK, Moreno C, Jirsa F.
Novel 3-
Hydroxy-2-Naphthoate-Based Task-Specific Ionic Liquids for an Efficient
Extraction of Heavy
Metals. Frontiers in Chemistry. 2018;6:172.
CA 03110269 2021-02-22
WO 2020/087172 PCT/CA2019/051542
29
23. Platzer S, Kar M, Leyma R, Chib S, Roller A, Jirsa F, et al. Task-specific
thioglycolate
ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and
recycling
properties. Journal of Hazardous Materials. 2017;324:241-9.
24. Dharaskar SA, Wasewar KL, Varma MN, Shende DZ, Tadi KK, Yoo OK. Synthesis,
characterization, and application of novel trihexyl tetradecyl phosphonium bis
(2,4,4-
trimethylpentyl) phosphinate for extractive desulfurization of liquid fuel.
Fuel Processing
Technology. 2014;123:1-10.