Note: Descriptions are shown in the official language in which they were submitted.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 1 -
BARRIER COATING RESIN FORMULATIONS, BARRIER COATINGS FORMED
FROM THE BARRIER COATING RESIN FORMULATIONS, ARTICLES
COMPRISING THE BARRIER COATINGS, ROCKET MOTORS COMPRISING
THE BARRIER COATINGS, AND RELATED METHODS
PRIORITY CLAIM
This application claims the benefit of the filing date of United States Patent
Application Serial No. 16/113,257, filed August 27, 2018, for "BARRIER COATING
RESIN FORMULATIONS, BARRIER COATINGS FORMED FROM THE BARRIER
COATING RESIN FORMULATIONS, ARTICLES COMPRISING THE BARRIER
COATINGS, ROCKET MOTORS COMPRISING THE BARRIER COATINGS, AND
RELATED METHODS."
STATEMENT REGARDING FEDERALLY SPONSORED
RESEARCH OR DEVELOPMENT
This invention was made with government support under Contract Number
FA8811-16-9-0002 awarded by the United States Department of Defense (Air
Force). The
government has certain rights in the invention.
TECHNICAL FIELD
Embodiments of the disclosure relate generally to barrier coating resin
formulations
that are resistant to heat, moisture, and oxidation. More particularly,
embodiments of the
disclosure relate to barrier coating resin formulations that include at least
one polycarbosilane
preceramic polymer, at least one organically modified silicon dioxide
preceramic polymer, at
least one filler, and at least one solvent, barrier coatings including a
reaction product of the at
least one polycarbosilane preceramic polymer and the at least one organically
modified silicon
dioxide preceramic polymer, articles including the barrier coating, rocket
motors including the
barrier coating, and related methods.
BACKGROUND
Barrier coatings, such as environmental barrier coatings (EBCs), are used to
protect
silicon-based materials, such as silicon-based ceramic materials, from high
temperatures,
moisture, and oxidation. The EBC is placed on locations of the silicon-based
ceramic material
that are exposed to high temperatures and pressures, such as high pressure,
high temperature
fluids including high temperature, high pressure water. The EBC prevents the
reaction of
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 2 -
water and other reactive species with the silicon, which can form reaction
products (e.g., silicon
hydroxide) that volatilize and cause erosion of the silicon-based ceramic
material.
Conventional barrier coatings have been formed of silicates, such as
barium-strontium-aluminosilicates, other alkaline earth aluminosilicates, or
lutetium
silicates, yttria-stabilized zirconia (YSZ), etc., which are expensive
materials. Multi-layer
barrier coatings have also been used, with each layer providing a different
function or functions,
such as an adhesion (e.g., bond) layer, an oxygen barrier layer, a temperature
resistant layer, etc.
Silicon carbide (SiC) and other silicon-based ceramic materials are used to
produce
ceramic matrix composites (CMCs) having high structural and mechanical
strength at a
temperature above 1200 C (2200 F). The CMCs are commonly used in aerospace and
other
industries where resistance to heat (e.g., high temperatures) is desired.
Conventional barrier
coatings are applied by plasma spray processes, such as atmospheric plasma
spray (APS)
processes, that utilize specialized equipment in a vacuum environment.
Multiple layers of the
barrier coating are typically needed to protect the CMCs. Forming the barrier
coatings are,
therefore, expensive and time consuming. In addition, these barrier coatings
are not able to
withstand temperatures above 1482 C (2700 F). Therefore, even with the barrier
coating, the
CMCs may decompose when exposed to high temperature water.
DISCLOSURE
In accordance with some embodiments described herein, a barrier coating resin
formulation is disclosed. The barrier coating resin formulation comprises at
least one
polycarbosilane preceramic polymer, at least one organically modified silicon
dioxide
preceramic polymer, at least one filler, and at least one solvent.
In accordance with other embodiments, a barrier coating is disclosed and
comprises a
reaction product of at least one polycarbosilane preceramic precursor and at
least one
organically modified silicon dioxide preceramic polymer. At least one filler
is in the reaction
product and comprises from about 30 volume percent to about 90 volume percent
of the barrier
coating.
In additional embodiments, an article is disclosed. The article comprises a
silicon-based ceramic material and a barrier coating on the silicon-based
ceramic material.
The barrier coating comprises a reaction product of at least one
polycarbosilane preceramic
precursor and at least one organically modified silicon dioxide preceramic
polymer, and at least
one filler.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 3 -
In accordance with other embodiments, a rocket motor is disclosed. The rocket
motor
comprises a casing and a nozzle secured to the casing, at least a portion of
the nozzle
comprising a barrier coating comprising a reaction product of at least one
polycarbosilane
preceramic precursor and at least one organically modified silicon dioxide
preceramic polymer
and at least one filler.
In accordance with other embodiments, a method of forming an article is
disclosed.
The method comprises applying a barrier coating resin formulation to a
substrate and curing the
barrier coating resin formulation to form a cured barrier coating. The barrier
coating resin
formulation comprises at least one polycarbosilane preceramic polymer, at
least one
organically modified silicon dioxide preceramic polymer, at least one filler,
and at least one
solvent.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a simplified cross-sectional view of an article including a barrier
coating in
accordance with embodiments of the disclosure, the barrier coating formed on a
substrate;
FIG. 2 is a simplified cross-sectional view of a rocket motor including one or
more
components that include a barrier coating in accordance with embodiments of
the disclosure;
and
FIGS. 3A-3C illustrate erosion testing and results for a coated nozzle
extension in
accordance with embodiments of the disclosure.
MODE(S) FOR CARRYING OUT THE INVENTION
Barrier coating resin formulations comprising at least one silicon carbide
precursor
resin, at least one silicon dioxide precursor resin, at least one filler, and
at least one solvent are
disclosed, as are barrier coatings (e.g., environmental barrier coatings),
articles comprising the
barrier coating, and methods of forming the articles. The filler constitutes a
majority of the
barrier coating resin formulation relative to the silicon carbide precursor
resin and silicon
dioxide precursor resin, providing the articles with increased resistance to
heat, moisture, and
oxidation. The barrier coating resin formulation may also include at least one
catalyst. The
barrier coating resin formulation is applied to a substrate and cured (e.g.,
crosslinked) to form a
cured barrier coating over the substrate. The cured barrier coating may be
ceramified to form a
ceramified barrier coating over the substrate. The barrier coating resin
formulation is applied
to the substrate by a spray coating process that is conducted under ambient
conditions, enabling
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 4 -
the barrier coating to be formed using a low complexity, low cost process. The
barrier coating
may be utilized on any substrate in which protection from heat, moisture, and
oxidation at a
temperature of greater than or equal to about 2000 F (about 1093 C) is
desired, such as
between about 2000 F (about 1093 C) and about 5000 F (about 2760 C). The
barrier coating
according to embodiments of the disclosure provides a high temperature
capability and low
mass loss to the article having the barrier coating incorporated therein
compared to an uncoated
article or to an article having a conventional barrier coating. Since the
barrier coating may be
formed on the substrate by a low cost process, the cost of fabricating the
article including the
barrier coating is low since the article may be formed without utilizing
specialized equipment
or facilities.
As used herein, the singular forms "a," "an," and "the" are intended to
include the plural
forms as well, unless the context clearly indicates otherwise.
As used herein, the term "about" or "approximately" in reference to a
numerical value
for a given parameter is inclusive of the numerical value and has the meaning
dictated by the
context (e.g., it includes the degree of error associated with measurement of
the given
parameter). For example, "about" or "approximately" in reference to a
numerical value may
include additional numerical values within a range of from 90.0% to 110.0% of
the numerical
value, such as within a range of from 95.0% to 105.0% of the numerical value,
within a range of
from 97.5% to 102.5% of the numerical value, within a range of from 99.0% to
101.0% of the
numerical value, within a range of from 99.5% to 100.5% of the numerical
value, or within a
range of from 99.9% to 100.1% of the numerical value.
As used herein, the term "and/or" includes any and all combinations of one or
more of
the associated listed items.
As used herein, the term "barrier coating resin formulation" means and
includes a
formulation including the silicon carbide precursor resin (e.g., a
polycarbosilane preceramic
polymer), the silicon dioxide precursor resin (e.g., an organically modified
silicon dioxide
preceramic polymer), the filler, and the solvent before cure and
ceramification of the barrier
coating resin formulation.
As used herein, the term "barrier coating" or "environmental barrier coating"
means
and includes a reaction product of the silicon carbide precursor resin and
silicon dioxide
precursor resin and is used to refer generally to the reaction product whether
cured or cured and
ceramified.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 5 -
As used herein, spatially relative terms, such as "beneath," "below," "lower,"
"bottom,"
"above," "upper," "top," "front," "rear," "left," "right," and the like, may
be used for ease of
description to describe one element's or feature's relationship to another
element(s) or
feature(s) as illustrated in the figures. Unless otherwise specified, the
spatially relative terms
are intended to encompass different orientations of the materials in addition
to the orientation
depicted in the figures. For example, if materials in the figures are
inverted, elements
described as "below" or "beneath" or "under" or "on bottom of' other elements
or features
would then be oriented "above" or "on top of' the other elements or features.
Thus, the term
"below" can encompass both an orientation of above and below, depending on the
context in
which the term is used, which will be evident to one of ordinary skill in the
art. The materials
may be otherwise oriented (e.g., rotated 90 degrees, inverted, flipped, etc.)
and the spatially
relative descriptors used herein interpreted accordingly.
As used herein, the term "ceramic yield" means and includes a residual mass of
the
reaction product of the silicon carbide precursor resin and silicon dioxide
precursor resin
remaining after cure at from about 0 C to about 400 C and/or ceramification of
the barrier
coating resin formulation at a temperature of about 1200 C or greater.
As used herein, the term "ceramified barrier coating" means and includes a
reaction
product of the silicon carbide precursor resin and silicon dioxide precursor
resin following cure
and ceramification of the barrier coating resin formulation.
As used herein, the terms "comprising," "including," "containing,"
"characterized by,"
and grammatical equivalents thereof are inclusive or open-ended terms that do
not exclude
additional, unrecited elements or method steps, but also include the more
restrictive terms
"consisting of' and "consisting essentially of' and grammatical equivalents
thereof
As used herein, the term "configured" refers to a size, shape, material
composition, and
arrangement of one or more of at least one structure and at least one
apparatus facilitating
operation of one or more of the structure and the apparatus in a pre-
determined way.
As used herein, the term "cured barrier coating" means and includes a reaction
product
of the silicon carbide precursor resin and silicon dioxide precursor resin
following cure of the
barrier coating resin formulation and before ceramification of the barrier
coating resin
formulation.
As used herein, the term "may" with respect to a material, structure, feature
or method
act indicates that such is contemplated for use in implementation of an
embodiment of the
disclosure and such term is used in preference to the more restrictive term
"is" so as to avoid
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 6 -
any implication that other, compatible materials, structures, features and
methods usable in
combination therewith should or must be excluded.
As used herein, the term "preceramic" means and includes a polymer material
that is
converted to a ceramic material when heated to a temperature of greater than
about 649 C
(greater than about 1200 F).
As used herein, the term "substantially," in reference to a given parameter,
property, or
condition, means to a degree that one of ordinary skill in the art would
understand that the given
parameter, property, or condition is met with a small degree of variance, such
as within
acceptable manufacturing tolerances. By way of example, depending on the
particular
parameter, property, or condition that is substantially met, the parameter,
property, or condition
may be at least 90.0% met, at least 95.0% met, at least 99.0% met, or even at
least 99.9% met.
As used herein, the term "substrate" refers to a silicon-based ceramic
material, a
carbon-carbon material, a ceramic matrix composite (CMC), or a metal material.
The
silicon-based ceramic material may be a silicon carbide material, a silicon
nitride material, a
silicon oxynitride material, a metal silicide, or a combination thereof and
may be monolithic.
The CMC may include a matrix material and a reinforcement material, with the
matrix material
including a ceramic material or a ceramic material and a metal or a metalloid.
By way of
example only, the substrate may be a silicon-based ceramic material, a CMC, or
a metal
material configured for use in the aerospace industry, automotive industry, or
aviation industry,
or other industry where substrates resistant to heat, moisture, and oxidation
are used.
The following description provides specific details, such as materials,
material
thicknesses, and processing conditions in order to provide a thorough
description of
embodiments of the disclosure. However, a person of ordinary skill in the art
will understand
that the embodiments of the disclosure may be practiced without employing
these specific
details. Indeed, the embodiments of the disclosure may be practiced in
conjunction with
conventional fabrication techniques employed in the industry. In addition, the
description
provided below does not form a complete process flow for manufacturing the
article from the
barrier coating resin formulation. Only those process acts and structures
necessary to
understand the embodiments of the disclosure are described in detail below.
Additional acts to
form the article from the barrier coating resin formulation may be performed
by conventional
techniques. Also note, any drawings accompanying the application are for
illustrative
purposes only, and are thus not drawn to scale. Additionally, elements common
between
figures may retain the same numerical designation.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 7 -
The barrier coating resin formulation includes the at least one silicon
carbide precursor
resin, the at least one silicon dioxide precursor resin, the at least one
filler, and the at least one
solvent. The barrier coating resin formulation is formulated to exhibit a
viscosity at room
temperature that is suitable (e.g., formulated) for applying the barrier
coating resin formulation
by spray coating, such as by air assisted spray coating or by airless spray
coating. The barrier
coating formed from the barrier coating resin formulation may exhibit desired
material
properties (e.g., rheological properties, mechanical properties, physical
properties, chemical
properties, thermal properties), such as resistance to heat, moisture, and
oxidation at
temperatures between about 2000 F (about 1093 C) and about 5000 F (about 2760
C).
To apply the barrier coating resin formulation by spray coating, the barrier
coating resin
formulation may be formulated to exhibit a viscosity of less than or equal to
about 20,000 cP at
a temperature of about 25 C, such as less than or equal to about 10,000 cP at
a temperature of
about 25 C, such as less than or equal to about 5,000 cP at a temperature of
about 25 C, less
than or equal to about 2,000 cP at a temperature of about 25 C, less than or
equal to
about 1,000 cP at a temperature of about 25 C, less than or equal to about 500
cP at a
temperature of about 25 C, less than or equal to about 300 cP at a temperature
of about 25 C,
less than or equal to about 200 cP at a temperature of about 25 C, less than
or equal to
about 150 cP at a temperature of about 25 C, or less than or equal to about
100 cP at a
temperature of about 25 C. By way of example only, the viscosity of the
barrier coating resin
formulation may be between about 50 cP and about 150 cP at a temperature of
about 25 C. In
some embodiments, the barrier coating resin formulation is formulated to
exhibit a viscosity of
less than or equal to about 100 cP at a temperature of about 25 C. The
viscosities of the silicon
carbide precursor resin and silicon dioxide precursor resin at least partially
contribute to the
overall viscosity of the barrier coating resin formulation, with the filler
and the solvent also
contributing to the viscosity.
The silicon carbide precursor resin may have a viscosity of less than or equal
to
about 250 cP at a temperature of about 25 C. The silicon carbide precursor
resin is a
polycarbosilane preceramic polymer formed of monomers having the following
chemical
structure:
R2
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 8 -
where Ri and R2 of each monomer is independently a hydrogen (H) group, a
methyl (CH3)
group, or a vinyl group (CH2=CH) and n is an integer from 2 to 10,000 (e.g.,
from 100
to 5,000). When vinyl groups are present, the vinyl group may be directly
bonded to the
silicon atom or may be bonded to the silicon atom by an alkyl group or other
linker. By way of
example only, the alkyl group may include from one carbon atom to six carbon
atoms. At least
a portion of the monomers in the polycarbosilane preceramic polymer include
the vinyl group
as Ri or R2 to enable crosslinking with the organically modified silicon
dioxide preceramic
polymer during cure of the barrier coating resin formulation. The amount of
vinyl groups in
the polycarbosilane preceramic polymer may be sufficient to crosslink the
barrier coating resin
formulation. The polycarbosilane preceramic polymer may include at least
about 0.01 vinyl eq/kg, such as from about 0.2 vinyl eq/kg to about 5.0 vinyl
eq/kg. The
polycarbosilane preceramic polymer may also include at least about 0.01
hydride eq/kg, such
as from about 0.2 hydride eq/kg to about 10 hydride eq/kg. The polycarbosilane
preceramic
polymer may be photocurable, chemically curable, or thermally curable.
By selecting the Ri and R2 groups of each monomer and the degree of
polymerization
(i.e., the number of monomer repeat units), a desired viscosity of the
polycarbosilane
preceramic polymer may be achieved. The polycarbosilane preceramic polymer is
formulated
to exhibit a viscosity of less than or equal to about 250 cP at a temperature
of about 25 C, such
as from about 1 cP to about 250 cP at about 25 C, from about 1 cP to about 200
cP at
about 25 C, from about 1 cP to about 100 cP at about 25 C, from about 10 cP to
about 250 cP at
about 25 C, from about 10 cP to about 200 cP at about 25 C, from about 40 cP
to about 250 cP
at about 25 C, from about 40 cP to about 200 cP at about 25 C, from about 40
cP to
about 120 cP at about 25 C, from about 40 cP to about 100 cP at about 25 C,
from about 5 cP
to 8 cP at about 25 C, from about 4 cP to about 7 cP at about 25 C, from about
8 cP to
about 12 cP at about 25 C, from about 8 cP to about 15 cP at about 25 C, or
from about 200 cP
to about 250 cP at about 25 C. In some embodiments, the polycarbosilane
preceramic
polymer has a viscosity of from about 40 cP to about 120 cP at about 25 C.
Such polycarbosilane preceramic polymers are commercially available from
numerous
sources including, but not limited to, EEMS, LLC (Saratoga Springs, NY),
Starfire Systems,
Inc. (Schenectady, NY), or Matech (Westlake Village, CA). The polycarbosilane
preceramic
polymer may include, but is not limited to, SMP-10, STARPCSO SMP-500, or
STARPCSO
SMP-877 silicon carbide precursor resin from Starfire Systems, Inc. (Malta,
NY). Additional
polycarbosilane preceramic polymers are commercially available from EEMS, LLC
as
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 9 -
MS 208, MS 272, MS 250, MS 440, CSO 110, or CSO 116. The polycarbosilane
preceramic
polymer may also include a combination of polycarbosilane preceramic polymers
or a
combination of the polycarbosilane preceramic polymer with at least one other
polymer, such
as a polysiloxane or other compatible polymer. The polycarbosilane preceramic
polymer may
be available at a relatively low cost, such as less than about $100/pound.
Commercially
available polycarbosilane preceramic polymers may also include a combination
of the
polycarbosilane preceramic polymer.
The silicon dioxide precursor resin may have a viscosity of greater than or
equal to
about 2,500 cP at a temperature of about 25 C. The silicon dioxide precursor
resin is an
organically modified silicon dioxide preceramic polymer formed of monomers
having the
following chemical structure:
0
¨Si
0
Si
0
where each of R3 and R4 is independently a methyl (CH3) group or a vinyl group
(CH2=CH)
and n is an integer from 2 to 10,000 (e.g., from 100 to 5,000). When vinyl
groups are present,
the vinyl group may be directly bonded to the silicon atom or may be bonded to
the silicon atom
by an alkyl group or other linker. By way of example only, the alkyl group may
include from
one carbon atom to six carbon atoms. The organically modified silicon dioxide
preceramic
polymer includes a quaternary coordinated (QC) oxygen to silicon atom and may
also be
referred to as a QC silicon dioxide preceramic polymer. At least a portion of
the monomers in
the organically modified silicon dioxide preceramic polymer may, optionally,
include the vinyl
group as R3 or R4 to enable crosslinking with the polycarbosilane preceramic
polymer during
cure of the barrier coating resin formulation. The organically modified
silicon dioxide
preceramic polymer may include from about 0 vinyl eq/kg to about 5.0 vinyl
eq/kg, such as
from about 0.18 vinyl eq/kg to about 0.3 vinyl eq/kg. The organically modified
silicon dioxide
preceramic polymer may be photocurable, chemically curable, or thermally
curable.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 10 -
R3 and R4 of each monomer of the organically modified silicon dioxide
preceramic
polymer and the degree of polymerization are selected to provide the desired
viscosity to the
organically modified silicon dioxide preceramic polymer. The organically
modified silicon
dioxide preceramic polymer also has a low carbon content and a high degree of
quaternary
coordinated oxygen to the silicon atoms in the polymer chain. The organically
modified
silicon dioxide preceramic polymer is formulated to exhibit a viscosity
greater than
about 200 cP at a temperature of about 25 C, such as greater than about 2,500
cP at a
temperature of about 25 C, from about 3,000 cP to about 100,000 cP at about 25
C, from
about 4,000 cP to about 100,000 cP at about 25 C, from about 5,000 cP to about
100,000 cP at
about 25 C, from about 6,000 cP to about 100,000 cP at about 25 C, from about
4,500 cP to
about 7,000 cP at about 25 C, from about 40,000 cP to about 80,000 cP at about
25 C, from
about 45,000 cP to about 75,000 cP at about 25 C, from about 50,000 cP to
about 70,000 cP at
about 25 C, or from about 50,000 cP to about 60,000 cP at about 25 C. In some
embodiments,
the organically modified silicon dioxide preceramic polymer has a viscosity of
from
about 50,000 cP to about 60,000 cP at a temperature of about 25 C. In other
embodiments, the
organically modified silicon dioxide preceramic polymer has a viscosity of
from
about 4,500 cP to about 7,000 cP at about 25 C.
Such organically modified silicon dioxide preceramic polymers are commercially
available from numerous sources including, but not limited to, Gelest, Inc.
(Morrisville, PA).
The organically modified silicon dioxide preceramic polymer may include, but
is not limited to,
VQM 135, VQM 135R, VQM 146, HQM 105, HQM 107, or combinations thereof
Relative amounts of the polycarbosilane preceramic polymer and the organically
modified silicon dioxide preceramic polymer may be adjusted to tailor the
mechanical
properties and performance properties of the barrier coating. The barrier
coating resin
formulation may include from about 10% by weight (wt%) to about 90 wt% of the
polycarbosilane preceramic polymer and from about 10 wt% to about 90 wt% of
the
organically modified silicon dioxide preceramic polymer, such as from about 20
wt% to
about 80 wt% of the polycarbosilane preceramic polymer and from about 20 wt%
to
about 80 wt% of the organically modified silicon dioxide preceramic polymer.
In some
embodiments, the barrier coating resin formulation includes 80 wt% of the
polycarbosilane
preceramic polymer and 20 wt% of the organically modified silicon dioxide
preceramic
polymer.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 11 -
The filler in the barrier coating resin formulation may be a ceramic material
that is
resistant to temperatures to which the barrier coating is exposed during use
and operation of the
article including the barrier coating. The filler may have a melting point of
between
about 1,800 C and about 4,000 C, such as between about 2,000 C and about 3,900
C. The
filler is also thermally stable at a temperature above about 2000 F (about
1093 C) and does not
degrade at processing temperatures. The filler also exhibits a low density,
minimizing the
overall mass of the barrier coating. The density of the filler may be
between 1.8 g/ml (1.8 g/cm3) and 13.0 g/ml (13.0 g/cm3), such as between about
2.0 g/ml (2.0 g/cm3) and about 12.5 g/ml (12.5 g/cm3), or between about 2.1
g/ml (2.1 g/cm3)
and about 12.2 g/ml (12.2 g/cm3). The filler also exhibits a low effect on the
viscosity of the
barrier coating resin formulation, even at high filler loading.
The filler may include, but is not limited to, silicon carbide, hafnium
carbide, tantalum
carbide, niobium carbide, zirconium carbide, tungsten carbide, molybdenum
carbide,
zirconium oxide, aluminum oxide, hafnium oxide, magnesium oxide, thorium
oxide, boron
nitride, hafnium nitride, tantalum nitride, zirconium nitride, titanium
nitride, titanium diboride,
hafnium diboride, tantalum diboride, zirconium diboride, tungsten boride, or
combinations
thereof The filler is commercially available from various sources, such as
Momentive
Performance Materials Inc. (Waterford, NY) or Panadyne Inc. (Montgomeryville,
PA). In
some embodiments, the filler includes zirconium oxide and titanium diboride.
In other
embodiments, the filler includes zirconium oxide, titanium diboride, and
silicon carbide. In
yet other embodiments, the filler includes silicon carbide.
Alternatively or in addition to, the filler may be a density, thermal
conductivity, or
ablation aid filler. The filler may include, but is not limited to, hollow
glass microspheres,
hollow polymeric microspheres, hollow ceramic microspheres, hollow polymeric
fibers,
hollow glass fibers, other hollow fibers, or combinations thereof The fibers
may include, but
are not limited to, glass fibers, ceramic fibers, carbon fibers, KEVLARO
fibers, metal fibers, or
combinations thereof The fiber filler may include chopped fibers, such as
chopped carbon
fibers; chopped glass fiber, chopped aramid fibers, or combinations thereof
Such fillers are
commercially available from numerous sources, such as from 3M (Maplewood, MN).
At high temperatures, such as above about 3000 F (about 1649 C) or between
about 3000 F (about 1649 C) and 5000 F (about 2760 C), the filler may be
present and
selected to enable a high filler loading while having a minimal effect on the
viscosity of the
barrier coating resin formulation, a minimal effect on mechanical properties
of the barrier
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 12 -
coating, and a minimal mass loss of the barrier coating. In high temperature
environments, the
filler may have a melting point of greater than about 3000 F (about 1649 C).
In general, as the
amount of filler in the barrier coating resin formulation increases, the
viscosity of the barrier
coating resin formulation increases. Therefore, the amount of filler used in
the barrier coating
resin formulation is a balance between achieving high filler loading and the
desired viscosity of
the barrier coating resin formulation. The filler may be present in the
barrier coating resin
formulation at up to about 90 volume percent (vol%), such as from about 1
volume percent to
about 90 volume percent, from about 5 volume percent to about 80 volume
percent, from about
volume percent to about 80 volume percent, from about 20 volume percent to
about 80
10 volume percent, from about 30 volume percent to about 80 volume percent,
from about 30
volume percent to about 70 volume percent, from about 30 volume percent to
about 60 volume
percent, from about 30 volume percent to about 50 volume percent, from about
30 volume
percent to about 40 volume percent, from about 40 volume percent to about 80
volume percent,
from about 40 volume percent to about 70 volume percent, from about 40 volume
percent to
about 60 volume percent, or from about 40 volume percent to about 50 volume
percent. Any
increased viscosity contributed by the filler may be counteracted by
increasing the amount of
solvent present in the barrier coating resin formulation.
The filler loading of the barrier coating resin formulation enables the
barrier coating to
be resistant to a temperature between about 2000 F (about 1093 C) and
about 5000 F (about 2760 C). If the article is to be used at a temperature
between
about 3000 F (about 1649 C) and about 5000 F (about 2760 C), the filler
loading may be at
the upper end of the range mentioned above. If the article is to be used at a
temperature
between about 2000 F (about 1093 C) and about 3000 F (about 1649 C), the
filler loading
may be at the lower end or middle end of the range mentioned above. If the
article is to be used
at a temperature below about 2000 F (about 1093 C), no filler may be utilized
in the barrier
coating resin formulation or a lower amount of the filler may be sufficient to
provide the desired
properties. In other words, the filler may be optional if the barrier coating
is to be used in a low
temperature (e.g., below about 1093 C (about 2000 F) or below about 1649 C
(about 3000 F))
environment. By way of example only, if the article is to be used at a
temperature below
about 3000 F (about 1649 C), the filler may be present at from about 1 volume
percent to
about 50 volume percent. If the article is to be used at a temperature above
about 3000 F (about 1649 C), the filler may be present at from about 30 volume
percent to
about 90 volume percent.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 13 -
Particle size of the filler may also affect the viscosity and filler loading
of the barrier
coating resin formulation. The filler may have an average mean diameter of
from
about 0.1 m to about 150 m, such as from about 0.1 m to about 50 m, from
about 0.1 m
to about 40 m, from about 0.1 m to about 30 m, from about 0.1 m to about
20 m, from
about 0.1 m to about 10 m, from about 0.1 m to about 5 m, from about 0.5
m to
about 40 m, from about 0.5 m to about 30 m, from about 0.5 m to about 20
m, from
about 0.5 m to about 10 m, from about 0.5 m to about 5 m, from about 0.5
m to
about 1 m, from about 1 m to about 50 m, from about 1 m to about 40 m,
from
about 1 m to about 30 m, from about 1 m to about 20 m, from about 1 m to
about 10 m, from about 2 m to about 8 m, or from about 2 m to about 5 m.
To achieve
a desired balance between the viscosity and filler loading, the filler may be
present in two or
more particle sizes or particle size ranges, where the particle sizes are
selected to improve
packing efficiency and loading of the filler in the barrier coating resin
formulation. For
instance, a small particle size filler may have an average mean diameter of
less than
about 1.0 m and a large particle size filler may have an average mean
diameter of from
about 1.5 m to about 5 m. In some embodiments, the small particle size
filler has an
average mean diameter of from about 0.5 m to about 0.6 m and the large
particle size filler
has an average mean diameter of from about 2 m to about 5 m. The filler may
also include
two or more fillers, with each filler having a different particle size or
particle size range. If, for
example, two fillers are used, the two fillers may be present at a ratio
of 1:1, 1:2, 1:3, 1:4, 2:1, 3:1, or 4:1. In some embodiments, the two fillers
are present at a ratio
of 2:1. The amount of small particle size filler present in the barrier
coating resin formulation
may be limited by the effect on viscosity, which increases at higher filler
loading. Using the
large particle size filler may enable a higher filler loading with a smaller
effect on the viscosity
of the barrier coating resin formulation. By using the different particle size
fillers, a desired
amount of filler may be present in the barrier coating resin formulation
without negatively
affecting its viscosity.
In some embodiments, zirconium oxide and titanium diboride are used as fillers
in the
barrier coating resin formulation. The zirconium oxide is present at an
average mean diameter
of about 0.6 m and the titanium diboride is present at an average mean
diameter of about 3 m
and the ratio of zirconium oxide:titanium diboride is 2:1. The zirconium oxide
and titanium
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 14 -
diboride are present at 65 wt% with respect to the polycarbosilane preceramic
polymer and the
organically modified silicon dioxide preceramic polymer.
The solvent functions as a carrier fluid and may be present in an amount
sufficient to
formulate the barrier coating resin formulation at the desired viscosity
(i.e., the viscosity
suitable for spray coating), enabling the barrier coating resin formulation to
be applied to the
substrate at a low temperature and low pressure, such as at ambient
conditions. The solvent
may also be volatile. The solvent is selected to provide solubility of the
polycarbosilane
preceramic polymer, the organically modified silicon dioxide preceramic
polymer, and the
filler in the barrier coating resin formulation, in addition to enabling the
high filler loading and
the desired viscosity. The solvent may be an aprotic organic solvent
including, but not limited
to, acetone, acetonitrile (MeCN), benzene, dichloromethane (DCM),
dimethylformamide
(DMF), dimethylsulfoxide (DMSO), ethyl acetate (Et0Ac),
hexamethylphosphoramide
(HMPA), methyl ethyl ketone (MEK), tetrahydrofuran (THF), toluene, xylene, or
combinations thereof The solvent may also include the aprotic organic solvent
in
combination with an alcohol, such as methanol, ethanol, propanol, or a
combination thereof
The barrier coating resin formulation may include a solids content of from
about 1 wt%
to about 20 wt% solids, such as from about 5 wt% to about 20 wt% solids, from
about 10 wt%
to about 20 wt% solids, or from about 15 wt% to about 20 wt% solids, with the
solvent, the
silicon carbide precursor resin, and the silicon dioxide precursor resin
accounting for the
remainder of the barrier coating resin formulation. By way of example only,
the barrier
coating resin formulation may be a 10 wt% solids coating solution. The solids
content may be
adjusted to achieve the desired viscosity of the barrier coating resin
formulation. By way of
example only, if the barrier coating resin formulation is to be applied by a
HVLP spray process,
the solids content may be adjusted so that the viscosity is below about 500 cP
at a temperature
of about 25 C. If the barrier coating resin formulation is to be applied by an
airless spray
process, the solids content may be adjusted so that the viscosity is below
about 20,000 cP at a
temperature of about 25 C.
The barrier coating resin formulation may also include a crosslinking agent,
such as a
radical initiator, a cationic initiator, or a catalyst, such as a
hydrosilylation catalyst. The
crosslinking agent initiates crosslinking of the polycarbosilane preceramic
polymer and the
organically modified silicon dioxide preceramic polymer by reacting the vinyl
groups with
silicon-hydrogen groups in the barrier coating resin formulation. The radical
initiator may be a
peroxide compound or an azo compound used to cure (e.g., crosslink) the
polycarbosilane
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 15 -
preceramic polymer and the organically modified silicon dioxide preceramic
polymer. The
peroxide compound may include, but is not limited to, benzoyl peroxide,
dicumyl peroxide,
bis-(2,4-dichlorobenzoy1)-peroxide, or combinations thereof The azo compound
may
include, but is not limited to, azobisisobutyronitrile. The cationic initiator
may include a
protonic acid, a Lewis acid/Friedel-Crafts catalyst (e.g., SnC14, A1C13, BF3,
and TiC14),
carbenium ion salts (e.g., with trityl or tropylium cations), or through
ionizing radiation. The
hydrosilylation catalyst may be a transition metal catalyst, such as platinum,
rhodium,
ruthenium iridium, palladium, nickel, cobalt, iron, manganese, or combinations
thereof In
some embodiments, the crosslinking agent is a platinum catalyst and HQM 105 or
HQM 107 is
used as the crosslinking agent. The crosslinking agent may be present at an
amount sufficient
to react (e.g., crosslink) the polycarbosilane preceramic polymer and
organically modified
silicon dioxide preceramic polymer and selection of the crosslinking agent at
least partially
depends on the polycarbosilane preceramic polymer and organically modified
silicon dioxide
preceramic polymer used, as well as on the desired cure time of the barrier
coating resin
formulation. The crosslinking agent may, for example, be present in the
preceramic resin
formulation at from about 0.01 parts per hundred parts of resin (phr) to about
10 phr, such as
from about 0.5 phr to about 5.0 phr, from about 0.5 phr to about 2.5 phr, or
about 1.0 phr.
The barrier coating resin formulation may include optional components (e.g.,
additives) to provide desirable properties to the barrier coating formed from
the barrier
coating resin formulation. If present, the additive may be at least one
compound that
enhances at least one material property (e.g., ceramic yield, extent of
cracking) of the barrier
coating to be formed from the barrier coating resin formulation. By way of
example only, the
additive may be a cure accelerator, an adhesion promoter, a lubricant, a
filler, a pigment, or
combinations thereof Such additives are known in the art and are not described
in detail
herein. In some embodiments, the barrier coating resin formulation is
substantially free of
additives other than the crosslinking agent (e.g., catalyst). Thus, the
barrier coating resin
formulation consists essentially of or consists of the polycarbosilane
preceramic polymer, the
organically modified silicon dioxide preceramic polymer, the crosslinking
agent, the filler, and
the solvent.
In some embodiments, the barrier coating resin formulation includes a 80:20
ratio of the
polycarbosilane preceramic polymer to the organically modified silicon dioxide
preceramic
polymer, the filler at about 65 wt% of the resin (e.g., the polycarbosilane
preceramic polymer
and the organically modified silicon dioxide preceramic polymer), and about
1.0 phr of the
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 16 -
crosslinking agent. The polycarbosilane preceramic polymer and the organically
modified
silicon dioxide preceramic polymer are blended with the zirconium oxide and
titanium diboride
at a 4:1 ratio. In some embodiments, the polycarbosilane preceramic polymer is
CSO-110 from EEMS, LLC, the organically modified silicon dioxide preceramic
polymer is
VQM-146 from Gelest, Inc., the filler is zirconium oxide and titanium
diboride, the
crosslinking agent is a platinum catalyst, and the solvent is toluene.
The barrier coating resin formulation may be formed by mixing the
polycarbosilane
preceramic polymer, the organically modified silicon dioxide preceramic
polymer, the filler,
the catalyst if present, and the solvent, along with any optional additives.
The polycarbosilane
preceramic polymer, organically modified silicon dioxide preceramic polymer,
filler, catalyst if
present, and solvent may be mixed by conventional techniques, such as by hand,
using a high
shear mixer, or using a planetary mixer. Mixing the components under vacuum
may remove
gases from the barrier coating resin formulation, which inhibits the formation
of voids or pores
during curing. The components may be mixed under inert conditions, such as
under argon.
The polycarbosilane preceramic polymer, organically modified silicon dioxide
preceramic
polymer, filler, catalyst if present, and solvent may be mixed for an amount
of time sufficient to
form a substantially homogeneous barrier coating resin formulation (e.g., the
polycarbosilane
preceramic polymer, organically modified silicon dioxide preceramic polymer,
filler, and
catalyst if present may be uniformly dispersed throughout the barrier coating
resin
formulation), or may be heterogeneous (e.g., at least one of the
polycarbosilane preceramic
polymer, organically modified silicon dioxide preceramic polymer, filler, and
catalyst if present
may be non-uniformly dispersed throughout the barrier coating resin
formulation). The
solvent may be added with the other components to achieve the desired
viscosity. In some
embodiments, the barrier coating resin formulation is substantially
homogeneous as formed.
During mixing, the barrier coating resin formulation may be maintained at a
temperature below
the lowest cure temperature of each of the components. In one embodiment, the
polycarbosilane preceramic polymer, organically modified silicon dioxide
preceramic polymer,
filler, catalyst if present, and solvent are maintained at room temperature
(from about 20 C to
about 25 C) during mixing. A water-cooled jacket may be used, as needed, to
maintain the
barrier coating resin formulation at or near room temperature to inhibit
potential reactions from
occurring during the mixing.
If the barrier coating resin formulation is to be applied by an air assisted
spray coating
technique, the barrier coating resin formulation may exhibit a viscosity
within a range of from
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 17 -
about 50 cP to about 500 cP at a temperature of about 25 C, such as from about
50 cP to
about 150 cP at a temperature of about 25 C, or from about 50 cP to about 100
cP at a
temperature of about 25 C. The barrier coating resin formulation also exhibits
a room
temperature pot life. If the barrier coating resin formulation is to be
applied by an airless spray
coating technique, the barrier coating resin formulation may exhibit a
viscosity within a range
of from about 200 cP to about 20,000 cP at a temperature of about 25 C, from
about 500 cP to
about 20,000 cP at a temperature of about 25 C, or from about 500 cP to about
5,000 cP at a
temperature of about 25 C.
The barrier coating resin formulation may be applied to the substrate by spray
coating.
The spray coating may be conducted at ambient conditions, such as at room
temperature
(between about 20 C and about 25 C) and atmospheric pressure. The spray
coating process
may be an air assisted spray coating technique or an airless spray coating
technique. The
spray coating process may be conducted using a conventional spray apparatus,
such as a high
volume low pressure (HVLP) spray apparatus. The barrier coating resin
formulation may be
applied as a single application or as multiple applications until a desired
thickness of the
barrier coating is achieved. Since the barrier coating resin formulation may
be applied by
spray coating at ambient conditions, the barrier coating resin formulation may
be easily and
quickly applied to the substrate. In addition, no specialized equipment or
building
infrastructure is required.
The barrier coating resin formulation may be applied at a thickness of from
about 1 im to about 13,000 m, such as from about 15 im to about 3000 m, from
about 20 Into about 3500 tin, from about 100 In to about 3500 m, from about
500 Into
about 3500 tin, from about 1000 wn to about 2000 tin, from about 1000 im to
about 3000 tin, from about 1500 wn to about 3000 tin, or from about 2000 im to
about 3000 tin. The thickness of the barrier coating may depend on the
intended
application for the barrier coating and the temperature and pressure
conditions to which the
barrier coating may be subjected. The barrier coating may exhibit a
substantially uniform
(e.g., homogeneous) composition throughout its thickness.
Once applied, the barrier coating resin formulation may be subjected to one or
more
heat treatments to form a cured barrier coating on the substrate. The heat
treatment may also
remove the solvent, which is volatile. If the barrier coating resin
formulation is applied in a
single application, the heat treatment may be conducted after the barrier
coating resin
formulation is applied to the desired thickness. If the barrier coating resin
formulation is
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 18 -
applied by conducting multiple applications, the heat treatment may be
conducted between
each application or after the last application. The polycarbosilane preceramic
polymer and
organically modified silicon dioxide preceramic polymer may be reacted (e.g.,
crosslinked,
cured) to form the cured barrier coating as a hardened coating. The cured
barrier coating on
the substrate may be referred to herein as the article or a coated substrate.
The conditions used
to cure the barrier coating resin formulation may depend on the
polycarbosilane preceramic
polymer, organically modified silicon dioxide preceramic polymer, and
crosslinking agent (if
present) used in the barrier coating resin formulation. The barrier coating
resin formulation
may be cured by exposure to a temperature with a range from about 0 C (about
32 F) to
about 400 C (about 752 F), such as from about 20 C (about 68 F) to about 371 C
(700 F),
from about 121 C (about 250 F) to about 371 C (700 F), or from about 20 C
(about 68 F) to
about 121 C (about 250 F). Depending on the cure temperature used, the barrier
coating resin
formulation may be cured in an amount of time ranging from a few seconds
(e.g., photoinitiated
cure) to a few days. The barrier coating resin formulation may be cured in
hours, such as from
about one hour to about thirty hours, from about four hours to about twenty
hours, or from
about six hours to about ten hours. By increasing the cure temperature, a
shorter amount of
time may be needed to cure the barrier coating resin formulation. Conversely,
by decreasing
the cure temperature, a longer amount of time may be needed to cure the
barrier coating resin
formulation. The curing may be conducted using conventional processing
equipment, which
is not described in detail herein.
During curing, the polycarbosilane preceramic polymer and organically modified
silicon dioxide preceramic polymer in the barrier coating resin formulation
react (e.g.,
crosslink), forming a hardened material. Without being bound by any theory, it
is believe that
during the cure, the barrier coating resin formulation is converted into an
amorphous
silicon-oxy-carbide material having the filler dispersed therein. The barrier
coating, therefore,
includes a reaction product of the polycarbosilane preceramic polymer and the
organically
modified silicon dioxide preceramic polymer, with the filler dispersed
therein. By way of
example only, the vinyl groups of the barrier coating resin formulation react
with
silicon-hydrogen bonds during the cure act. The curing may be conducted in a
low oxygen
environment (e.g., an inert atmosphere environment), such as below 100 ppm of
oxygen, to
reduce oxidation of the polycarbosilane preceramic polymer and the organically
modified
silicon dioxide preceramic polymer. If gases are produced from the barrier
coating resin
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 19 -
formulation during the cure, the cured barrier coating may be porous and the
filler may enter
pores in the cured barrier coating.
The cured barrier coating of the coated substrate may exhibit sufficient
mechanical
properties after curing to provide the desired heat, oxidation, and moisture
resistance to the
coated substrate. The cured barrier coating may prevent oxidation of the
underlying substrate.
The cured barrier coating may be stable to temperatures of up to 5000 F (about
2760 C). The
barrier coating (e.g., the cured barrier coating) may provide the coated
substrate with
resistance to a temperature of up to about 5000 F (about 2760 C), such as from
about 2000 F
(about 1093 C) to about 5000 F (about 2760 C), from about 2000 F (about 1093
C) to
about 3000 F (about 1649 C), from about 3000 F (about 1649 C) to about 4000 F
(about 2204 C), from about 4000 F (about 2204 C) to about 5000 F (about 2760
C), from
about 2000 F (about 1093 C) to about 4000 F (about 2204 C), or from about 3000
F
(about 1649 C) to about 5000 F (about 2760 C). The coated substrate may also
exhibit
reduced mass loss and reduced corrosion.
The cured barrier coating according to embodiments of the disclosure may be
configured as a single barrier coating that exhibits the desired resistance
properties and
mechanical properties in addition to providing sufficient adhesion to the
substrate. The cured
barrier coating provides the desired resistance properties, mechanical
properties, and adhesion
properties in a coating having a single chemical composition across a
thickness thereof In
other words, the cured barrier coating has a homogeneous composition across
its thickness.
The cured barrier coating is, therefore, a single composition coating that
provides the desired
properties along with the desired adherence to the substrate. In comparison,
conventional
environmental barrier coatings include coatings of different, multiple
chemical compositions to
provide different functions, such as adhesion, oxygen barrier layer,
temperature resistant layer,
etc. The conventional environmental barrier coatings include coatings of
separate and
different compositions to provide the desired overall properties.
The cured barrier coating may then be ceramified to further harden the cured
barrier
coating and to convert the crosslinked material to a ceramified barrier
coating. Thus, the
ceramified barrier coating includes a reaction product of the polycarbosilane
preceramic
polymer and the organically modified silicon dioxide preceramic polymer with
the filler
dispersed throughout the reaction product. Without being bound by any theory,
it is believed
that during the cure and ceramification, the barrier coating resin formulation
is converted into
an amorphous silicon-oxy-carbide material with the filler dispersed therein.
The cured barrier
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 20 -
coating may be exposed to a temperature of greater than about 649 C (greater
than
about 1,200 F), such as a temperature of greater than about 816 C (greater
than about 1,500 F)
or greater than about 1,093 C (greater than about 2,000 F) to ceramify the
cured barrier coating.
By way of example only, the ceramification temperature may range from about
816 C to
about 1,093 C or from about 816 C to about 1,200 C or greater. The ceramic
yield of the
ceramified barrier coating may be greater than about 50%, such as greater than
about 70%,
greater than about 75%, greater than about 80%, greater than about 90%, or
greater than
about 95% when ceramified at these temperatures. Without being bound by any
theory, it is
believed that the high degree of quaternary coordinate oxygen in the
organically modified
silicon dioxide preceramic polymer results in the high ceramic yield. When
silicon atoms are
fully coordinated with oxygen atoms, SiO2 is maintained during the cure and
ceramification.
The organically modified silicon dioxide preceramic polymer has sufficient
organic groups
bonded to the silicon atoms to keep the barrier coating resin formulation in a
polymeric state,
which enables ease of blending with other materials. It is also believed that
at a temperature of
about 1,093 C (about 2,000 F), the barrier coating resin formulation may be
characterized as a
semi-amorphous silicon-oxy-carbide material.
The ceramification may be conducted during use and operation (e.g., in situ)
of the
article including the cured barrier coating. By way of example only, if the
article including the
cured barrier coating is configured as a rocket motor nozzle, the cured
barrier coating may be
converted to the ceramified barrier coating during use and operation of the
rocket motor nozzle.
By ceramifying the cured barrier coating in situ, fewer process acts are
conducted to produce
the article. Since the barrier coating resin formulation is applied at ambient
conditions, the
article according to embodiments of the disclosure may also be formed by a
lower cost spray
process compared to conventional spray processes, such as a plasma spray
process. Therefore,
the overall cost of the articles formed according to embodiments of the
disclosure may be lower
than the cost of conventional coated articles formed by plasma spray
processes. By
conducting the ceramification in situ, the risk of damage to the article is
also reduced because
the high temperature ceramification is conducted just before use and operation
of the article.
Forming the article according to embodiments of the disclosure may also be
more efficient
because less damage occurs to the article.
Alternatively, the ceramification may be conducted before use and operation of
the
coated substrate. The cured barrier coating may be exposed to a temperature
sufficient to
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 21 -
ceramify the cured barrier coating, and the coated substrate having the
ceramified barrier
coating may then be incorporated into or configured as the desired article.
The barrier coating (e.g., the ceramified barrier coating) may provide the
coated
substrate with resistance to a temperature of up to about 5000 F (about 2760
C), such as
from about 2000 F (about 1093 C) to about 5000 F (about 2760 C), from
about 2000 F (about 1093 C) to about 3000 F (about 1649 C), from
about 3000 F (about 1649 C) to about 4000 F (about 2204 C), from
about 4000 F (about 2204 C) to about 5000 F (about 2760 C), from about 2000 F
(about 1093 C) to about 4000 F (about 2204 C), or from about 3000 F (about
1649 C) to
about 5000 F (about 2760 C). For instance, the barrier coating may be used to
provide a
solid rocket motor with resistance to a temperature of up to about 5000 F
(about 2760 C).
The coated substrate may also exhibit reduced mass loss and reduced corrosion.
Without being bound by any theory, it is believed that the high degree of
quaternary
coordinate oxygen in the organically modified silicon dioxide preceramic
polymer results in the
high ceramic yield. When silicon atoms are fully coordinated with oxygen
atoms, SiO2 is
maintained during the cure and ceramification. The organically modified
silicon dioxide
preceramic polymer has sufficient organic groups bonded to the silicon atoms
to keep the
preceramic resin formulation in a polymeric state, which enables ease of
blending with other
materials. It is also believed that at a temperature of about 1,093 C (about
2,000 F), the
preceramic resin formulation may be characterized as a semi-amorphous silicon-
oxy-carbide
material.
With its resistance to high temperature, moisture, and oxidation, the barrier
coating may
be used in a variety of cost sensitive, high temperature applications. For
instance, the barrier
coating on the substrate (e.g., the coated substrate) may be incorporated into
articles for use in
aerospace, automotive, aviation, or other industries needing temperature,
water, and oxidation
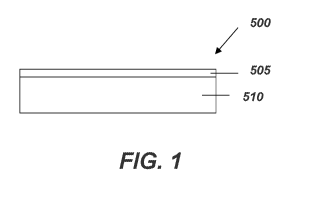
protection. An article 500 including the barrier coating 505 on the substrate
510 is shown in
FIG. 1. The barrier coating 505 may include the cured barrier coating or the
ceramified barrier
coating. The article 500 according to embodiments of the disclosure may be
configured for
use in applications having a short functional life, such as less than about 30
minutes, with
exposure to high temperatures and high pressure during that time. In some
embodiments, the
article 500 is configured as a rocket motor nozzle. The article 500 according
to other
embodiments of the disclosure may be configured for applications having a
longer functional
life, such as, for example, turbine components having a functional life of
greater than
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 22 -
about 10,000 hours and needing to withstand thermal cycling with little
decrease in mechanical
properties.
The article 500 according to embodiments of the disclosure may be used as a
structural
component of a rocket motor, a hypersonic vehicle, or other aerostructure,
which is expected to
function for less than about 30 minutes during use and operation. The rocket
motor may
include a solid rocket motor or a liquid rocket motor. The aerostructure may
include, but is not
limited to, a turbine, a turbine blade, a turbine housing, a turbine engine
vane, an insulating tile,
a rotor blade, an insulation blanket, insulation, a compressor blade, a wing
component, a
fuselage skin, a landing gear, a shroud, an exhaust nozzle, an engine exhaust
duct, a nose cone,
a re-entry shield, or a heat shield. By way of example only, the article 500
may be used as a
structural component of a nozzle of the rocket motor or of a casing of the
rocket motor. In
addition to being used as an oxidation resistant coating on a rocket motor
nozzle or other high
temperature aerostructure, the article 500 may be used as a high temperature
adhesive, a mortar
material for filling cracks or gaps, an insulation, a thermal protection
material, or a thermal
ablation material. In some embodiments, the article 500 is configured as a
rocket motor
nozzle. In other embodiments, the article 500 is configured as a turbine
component.
FIG. 2 is a simplified cross-sectional view of a rocket motor 1000 (e.g., a
solid rocket
motor, a liquid rocket motor), in accordance with embodiments of the
disclosure. The
rocket motor 1000 may, for example, be configured to be a component (e.g.,
stage) of a larger
assembly (e.g., a multi-stage rocket motor assembly). As shown in FIG. 2, the
rocket
motor 1000 includes a casing 1002, a propellant structure 1004 disposed within
the
casing 1002, and a nozzle assembly 1006 connected to an aft end of the casing
1002. The
rocket motor 1000 may also include one or more of a liner structure 1008 and
an insulation
structure 1010 between the propellant structure 1004 and the casing 1002. For
example, the
liner structure 1008 may be located on or over the propellant structure 1004,
and the insulation
structure 1010 may be located on and between the liner structure 1008 and an
inner surface of
the casing 1002. The components of the rocket motor 1000 may be formed using
conventional processes and equipment, which are not described in detail
herein. The
article 500 according to embodiments of the disclosure may be incorporated in
(e.g.,
incorporated as) one or more components of the rocket motor 1000. By way of
example only,
the article 500 may be configured as at least a portion of the nozzle assembly
1006 or the
casing 1002. For instance, the barrier coating may be used to provide a solid
rocket motor
with resistance to a temperature of up to about 5000 F (about 2760 C).
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 23 -
While embodiments described herein refer to preceramic precursors of silicon
carbide
and silicon dioxide, the preceramic precursor of silicon dioxide may also be
used with
preceramic precursors of other ceramics, such as preceramic precursors of
silicon carbide,
preceramic precursors of silicon nitride, preceramic precursors of silicon
hexaboride,
preceramic precursors of aluminum nitride, preceramic precursors of boron
nitride, preceramic
precursors of boron carbide, preceramic precursors of titanium boride,
preceramic precursors of
titanium carbide, and preceramic precursors of hafnium carbide.
The following examples serve to explain embodiments of the disclosure in more
detail.
These examples are not to be construed as being exhaustive or exclusive as to
the scope of this
disclosure.
EXAMPLES
Example 1
Barrier Coating Resin Formulation 1
A barrier coating resin formulation including 15.97 mass percent of a
polycarbosilane
preceramic polymer, 3.99 mass percent of an organically modified silicon
dioxide preceramic
polymer, 65.07 mass percent zirconium dioxide, 14.97 mass percent of titanium
diboride,
and 0.40 mass percent of a platinum catalyst was prepared. The polycarbosilane
preceramic
polymer was commercially available from EEMS, LLC as CSO-110. The organically
modified silicon dioxide preceramic polymer was commercially available from
Gelest, Inc. as
VQM-146. The zirconium dioxide had an average mean diameter of 0.6 n) and was
commercially available from Panadyne Inc. (Montgomeryville, PA) as HCTF. The
titanium
diboride had an average mean diameter of 3 n) and was commercially available
from
Momentive Performance Materials Inc. (Waterford, NY) as PGZ-06. The platinum
catalyst
was commercially available from EEMS as CLC-PL005. The polycarbosilane
preceramic
polymer, organically modified silicon dioxide preceramic polymer, zirconium
dioxide,
titanium diboride, and platinum catalyst were combined with mixing and diluted
with toluene
to produce the barrier coating resin formulation as a solution that was 10% by
weight solids.
The barrier coating resin formulation is referred to herein as Formulation
HTR48 and was a
Zr02/TiB2/SiOC coating.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 24 -
Example 2
Barrier Coating Resin Formulation 2
A barrier coating resin formulation including 15.97 mass percent of a
polycarbosilane
preceramic polymer, 3.99 mass percent of an organically modified silicon
dioxide preceramic
polymer, 65.07 mass percent zirconia, 14.97 mass percent of silicon carbide,
and 0.40 mass
percent of a platinum catalyst was prepared. The polycarbosilane preceramic
polymer was
commercially available from EEMS, LLC as CSO-110. The organically modified
silicon
dioxide preceramic polymer was commercially available from Gelest, Inc. as VQM-
146. The
zirconium dioxide had an average mean diameter of 0.6 m and was commercially
available
from Panadyne Inc. (Montgomeryville, PA) as HCTF. The silicon carbide had an
average
mean diameter of 0.5 m and was commercially available from Panadyne Inc.
(Montgomeryville, PA) as Green SiC. The platinum catalyst was commercially
available
from EEMS as CLC-PL005. The polycarbosilane preceramic polymer, organically
modified
silicon dioxide preceramic polymer, zirconia, silicon carbide, and platinum
catalyst were
combined with mixing and diluted with toluene to produce the barrier coating
resin formulation
as a 10% by weight solids coating solution. The barrier coating resin
formulation is referred to
herein as Formulation HTR49 and was a Zr02/SiC/SiOC formulation.
Example 3
Barrier Coating Resin Formulation 3
A barrier coating resin formulation including 34.0 mass percent of the
organically
modified silicon dioxide preceramic polymer, 0.9 mass percent of a
crosslinking agent
(HQM-105 from Gelest), 65.0 mass percent of silicon carbide, and 0.1 mass
percent of a
platinum catalyst was produced. The organically modified silicon dioxide
preceramic
polymer was commercially available from Gelest, Inc. as VQM-146. The silicon
carbide had
an average mean diameter of 0.5 m and was commercially available from
Panadyne Inc.
(Montgomeryville, PA) as Green SiC. The platinum catalyst was commercially
available
from EEMS as CLC-PL005. The organically modified silicon dioxide preceramic
polymer,
silicon carbide, crosslinking agent, and platinum catalyst were combined with
mixing and
diluted with toluene to produce the barrier coating resin formulation as a
solution that was 10%
by weight solids. The barrier coating resin formulation is referred to herein
as Formulation
RC12 and was a SiC/SiOC formulation.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 25 -
Example 4
Coating Process
The formulations in Examples 1, 2, and 3 were applied to carbon substrates by
HVLP
spray coating processes to determine their respective effectiveness as a
barrier coating. One of
the substrates was a carbon-carbon panel of a carbon filled phenolic with low
fired stretch
broken and spun carbon commercially available from Barrday Advanced Material
Solutions
(Ontario, Canada) as LR1406 and had a laminate thickness of 0.25 inch (6350
um). The
substrate is referred to herein as "LR1406." The other substrate was a chopped
carbon-carbon
panel that is a low cost carbon substrate having a thickness of 0.125 inch
(3175 um) and a
density of 1.7 g/m1(1.7 g/cm3) and was commercially available from
CeraMaterials (Port
Jervis, NY) as PC70. The substrate is referred to herein as "Commercial."
The formulations in Examples 1, 2, and 3 were sprayed onto the substrates at
room
temperature and atmospheric pressure. The substrates were placed a distance
of 10-12 inches (25.4-30.48 cm) away from the spray apparatus. The spray
apparatus was a
conventional HVLP spray gun. The formulations in Examples 1, 2, and 3 were
added to a
reservoir of the spray apparatus and the spray process was conducted at a
compressed air
pressure of 15 psi (103421 Pa), a spray volume of 0.25 to 0.5 turns, and a
spray shape of a cone.
The substrates were weighed before and after coating to determine the coating
thickness.
The coating thicknesses ranged between 0.050 inch (about 1270 um)
and 0.100 inch (about 2540 um) on the substrates. The coated substrates were
dried and cured
at a temperature of about 121 C (250 F) for 4 hours.
Conventional silicon carbide (SiC), SiC/YSZ, or tungsten/YSZ formulations were
applied to substrates by atmospheric plasma spray (APS) processes and used as
controls.
Example 5
Scorch Testing
The effectiveness of the HTR48 formulation (Example 1) and the RC12
formulation
(Example 3) as barrier coatings was evaluated by exposing the coated
substrates to a nozzle fire
environment and determining the ability of the barrier coatings to protect the
substrates from
oxidation. Surfaces of the coated substrates was subjected to various surface
temperatures
using an oxygen-propane torch. The coated substrates were exposed to
temperatures typically
seen in rocket motor nozzle sections and carbon-carbon panels during use and
operation of a
rocket motor. The coated substrates were subjected to an over-test condition
where the torch
CA 03110326 2021-02-22
WO 2020/055579
PCT/US2019/048080
- 26 -
fuel included an oxygen: propane ratio of about 2.5:1 and at temperatures
of 2200 F (about 1204 C) and 3000 F (about 1649 C). The torch was placed a
distance
of 9 inches (22.86 cm) from the coated substrates for the 2200 F (about 1204
C) testing and a
distance of 7 inches (17.78 cm) from the coated substrates for the 3000 F
(about 1649 C)
testing. The carbon-carbon panels were exposed for 600 seconds at 2200 F
(about 1204 C) or
for 300 seconds at 3000 F (about 1649 C). The nozzle sections were exposed
for 724 seconds at 2200 F (about 1204 C) or for 900 seconds at 3000 F (about
1649 C). The
effectiveness of the coated substrates against oxidation was determined by
mass loss and visual
inspection.
Mass loss to the coated substrates was calculated by comparing the initial
mass to the
percent mass post test. Surface damage to the coated substrates was determined
by visual
inspection. The barrier coating resin formulations of Examples 1 and 3 were
compared to the
SiC, SiC/YSZ, or tungsten/YSZ formulations applied by APS. The mass loss and
visual
results are shown in Table 1, with spalling and/or melting visual results only
reported when
observed:
Table 1: Mass Loss and Visual Inspection of Coated Substrates.
C-C Coating Surface Temp Coating
Heat Flux Start/End (BTU/ft2-sec) Mass Visua
Substrate ("F) Thickness Loss 1
(inches) (0/0)
Commercial None 2200 NA 12.41
(about 1204 C)
Commercial None 2200 NA 15.62
(about 1204 C)
LR1406 None 2200 NA 5.79
(about 1204 C)
LR1406 None 2200 NA 5.84
(about 1204 C)
Commercial SiC 2200 Not 4.33
(about 1204 C) measured
Commercial SiC 2200 Not 4.56
(about 1204 C) measured
Commercial SiC/YS 2200 Not 446.6 (-/529214.7 W/m2) 5.05
(about 1204 C) measured
Commercial SiC/YS 2200 Not 4.75
(about 1204 C) measured
Commercial W/YSZ 2200 Not 56.8/60.2 (645051.4/683663.62 W/m2)
1.46 Oxida
(40 (about 1204 C)
measured tion of
passes)
Wand
YSZ
spallin
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 27 -
C-C Coating Surface Temp Coating Heat Flux
Start/End (BTU/ft2-sec) Mass Visua
Substrate ("F) Thickness Loss 1
(inches) (0/0)
Commercial W/YSZ 2200 Not 28.4/32.6 (322525.7/370223.1 W/m2)
1.13 Oxida
(20 (about 1204 C)
measured tion of
passes) Wand
YSZ
spallin
Commercial RC12 2200 0.056 24.7/67.2 (280506.5/763159.4 W/m2)
7.70
(about 1204 C) (0.142 cm)
Commercial RC12 2200 0.062 72.2/61.7 (819942.1/700698.4 W/m2)
9.44
(about 1204 C) (0.157 cm)
Commercial HTR48 2200 0.076 50.4/61(572369.5/692748.8 W/m2)
5.83
(about 1204 C) (0.193 cm)
Commercial HTR48 2200 0.085 57.2/60.1 (649594.0/682527.9 W/m2)
7.38
(about 1204 C) (0.215 cm)
LR1406 HTR48 2200 0.109 60.9/58.4 (691613.2/663221.8 W/m2)
3.59
(about 1204 C) (0.276 cm)
LR1406 None 3000 NA 86.9/92.2 (986883.2/1047072.8 W/m2)
17.22
(about 1649 C)
Commercial SiC 3000 Not 8.78 Meltin
(about 1649 C) measured g and
spallin
visible
Commercial SiC/YS 3000 Not 479.7 (-/275850.3 W/m2) 7.13
Spalli
(about 1649 C) measured ng
visible
LR1406 HTR48 3000 0.149 28.7/100.2 (325932.7/1137925.2 W/m2)
2.38
(about 1649 C) (0.378 cm)
As shown in Table 1, the substrates having the HTR48 coating exhibited the
lowest
mass loss at both 2200 F (about 1204 C) and 3000 F (about 1649 C). The low
mass loss with
the HTR48 coating indicated that a minimal amount of the substrate was
consumed (e.g.,
combusted) and that the barrier coating protected the substrate. In the 2200 F
(about 1204 C)
testing environment, the HTR48 coating performed better than the SiC and
SiC/YSZ
conventional coatings applied by APS. In the 3000 F (about 1649 C) testing
environment, the
HTR48 coating performed better than the SiC/YSZ conventional coating applied
by APS. The
W/YSZ conventional coating applied by APS and subjected to 2000 F (about 1093
C) was
damaged due to spalling or melting. The SiC and SiC/YSZ conventional coatings
applied by
APS and subjected to 3000 F (about 1649 C) were damaged due to spalling or
melting. The
damage observed with the conventional coatings indicated a risk of premature
failure during
firing in a nozzle environment.
CA 03110326 2021-02-22
WO 2020/055579 PCT/US2019/048080
- 28 -
Example 6
Erosion Testing
The HTR49 formulation (Example 2) was applied by an HVLP spray coating process
to
a liquid rocket engine carbon-carbon nozzle extension. The liquid rocket
engine
carbon-carbon nozzle extension was coated with 2.7 g of the HTR49 formulation
and cured at a
temperature of about 121 C (250 F) for 4 hours. The liquid rocket engine
carbon-carbon
nozzle extension was tested for 9 minutes at 2300 F (1260 C). Nozzle erosion
was tested at
different locations within the nozzle extension, which locations are shown in
FIG. 3A. The
nozzle erosion at each location was measured and is shown in FIG. 3B.
Photographs of the
nozzle extension at different times during the erosion testing are shown in
FIG. 3C. The
HTR49 formulation applied to the liquid rocket engine carbon-carbon nozzle
extension
prevented oxidation of the carbon-carbon substrate during operation of the
liquid rocket engine
and erosion was observed to be below 0.005 inch (0.0127 cm) during the
testing.
While the disclosure is susceptible to various modifications and alternative
forms,
specific embodiments have been shown by way of example in the drawings and
have been
described in detail herein. However, the disclosure is not intended to be
limited to the
particular forms disclosed. Rather, the disclosure encompasses all
modifications, equivalents,
and alternatives falling within the scope of the disclosure as defined by the
following appended
claims and their legal equivalents.