Note: Descriptions are shown in the official language in which they were submitted.
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
MEDICAL FLUID DELIVERY SYSTEM INCLUDING A MOBILE PLATFORM FOR
PATIENT ENGAGEMENT AND TREATMENT COMPLIANCE
BACKGROUND
[0001] Engaging a patient outside of a medical environment for an extended
period is
currently a virtually impossible task. Similar to beginning a gym membership
or buying a
treadmill, many patients typically begin strong. For example, at first
patients are readily engaged
with a medical treatment (e.g., a medical fluid delivery treatment) that is
self-administered in the
patients' homes. For treatment, patients have to connect themselves to a
medical fluid delivery
machine (or containers that contain a renal failure treatment fluid) to
cleanse their blood from a
build-up of toxins. Part of the treatment may include tasks that the patients
have to perform such
as weighing themselves, taking their blood pressure, and/or recording
information related to their
treatment. The information recorded by patients is oftentimes reviewed by
clinicians to ensure the
treatment is progressing as prescribed. Clinicians also review the recorded
data to determine
whether an adjustment to the treatment is needed.
[0002] Overtime, many patients become less enthusiastic with the treatments as
they lose
their novelty and become just another obligation. As one can imagine, patients
would rather
engage in more exciting, relaxing, or stimulating activities compared to a
self-administered
medical treatment. While patients continue the treatments, they sometimes
begin to omit
performing the additional tasks that go along with the treatments. Omitting
the additional tasks
and becoming less enthused with treatments has the potential to create gaps in
clinical oversight
for the ongoing treatment. As patients become further disengaged from the
treatments, they may
begin to skip treatments or forgo them altogether, risking their health in the
process.
SUMMARY
[0003] A medical fluid data transfer system including a mobile platform is
disclosed
herein. The medical fluid data transfer system is configured to improve
engagement and/or
treatment compliance with a patient through interactions provided through a
patient's portable
device (e.g., a cellular phone, smartphone, tablet computer, etc.).
Specifically, the medical fluid
1
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
data transfer system provides a patient increased feedback and control
(without feeling
overwhelmed), which causes the patient to feel as though they are in control
of their own treatment
rather than following instructions from a clinician. For example, a personal
mobile communication
device of the medical fluid data transfer system may display treatment
information and/or a
patient's vital sign data. The personal mobile communication device may also
enable a patient to
switch between different prescribed treatments or programs without having to
directly program a
medical fluid delivery machine. Patients that feel in control are more likely
to stay engaged with
their treatment.
[0004] The example medical fluid data transfer system also reduces data
gathering burdens
on the patient by automating the process. For example, a personal mobile
communication device
enables a patient to capture treatment data or vital sign data for automatic
transmission to a
centralized clinician database. Patients may enter data directly into a
personal mobile
communication device, receive the data electronically from a connected
machine, or record the
data using a camera. The data is transmitted within the medical fluid data
transfer system to a
database that stores the data in a patient medical record.
[0005] Additionally, the example medical fluid data transfer system provides a
gateway to
clinicians to enable a patient to communicate with a clinician in real-time
regarding any concerns
or questions about a medical fluid delivery treatment. In some embodiments,
the medical fluid
data transfer system also provides patient access to educational or training
material. As such, the
example medical fluid data transfer system is configured to connect a patient
to needed or
requested assistance for staying engaged and/or compliant with a treatment.
[0006] The medical fluid data transfer system and methodology of the present
disclosure
is applicable, for example, to fluid delivery for: plasmapherisis,
hemodialysis ("HD"),
hemofiltration ("HF") hemodiafiltration ("HDF"), and continuous renal
replacement therapy
("CRRT") treatments. The medical fluid data transfer system described herein
is also applicable
to peritoneal dialysis ("PD"), intravenous drug delivery, and nutritional
fluid delivery. These
modalities may be referred to herein collectively or generally individually as
medical fluid delivery
or treatment.
[0007] The above modalities may be provided by a medical fluid delivery
machine that
houses components needed to deliver medical fluid, such as one or more pumps,
valves, heaters if
needed, online medical fluid generation equipment if needed, sensors, such as
any one, or more,
2
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
or all of pressure sensors, conductivity sensors, temperature sensors, air
detectors, blood leak
detectors, and the like, user interfaces, and control units, which may employ
one or more
processors and memory to control the above-described equipment. The medical
fluid delivery
machine may also include one or more filters, such as a dialyzer or hemofilter
for cleansing blood
and/or an ultrafilter for purifying water, dialysis fluid, or other fluid.
[0008] The medical fluid delivery machine and the medical fluid data transfer
system and
methodology described herein may be used with home-based machines. For
example, the systems
may be used with home HD, HF or HDF machines, which are operated at the
patient's
convenience. One such home system is described in U.S. Patent No. 8,029,454
("the '454 Patent"),
issued October 4, 2011, entitled "High Convection Home
Hemodialysis/Hemofiltration And
Sorbent System", filed November 4, 2004, assigned to the assignees of the
present application.
Other such home systems are described in U.S. Patent No. 8,393,690 ("the '690
Patent"), issued
March 12, 2013, entitled "Enclosure for a Portable Hemodialysis System", filed
August 27, 2008.
The entire contents of each of the above references are incorporated herein by
reference and relied
upon.
[0009] As described in detail below, the medical fluid data transfer system
and
methodology of the present disclosure may operate within an encompassing
platform system that
may include many machines comprising many different types of devices,
patients, clinicians,
doctors, service personnel, electronic medical records ("EMR") databases, a
website, a resource
planning system handling data generated via the patient and clinician
communications, and
business intelligence. The medical fluid data transfer system and methodology
of the present
disclosure operates seamlessly within the overall system and without
contravening its rules and
protocols.
[0010] In light of the disclosure herein and without limiting the disclosure
in any way, in
a first aspect of the present disclosure, which may be combined with any other
aspect listed herein
unless specified otherwise, a machine-accessible device having instructions
stored thereon that are
configured, when executed, to cause a machine to populate patient medical
records of a clinical
system by operating a camera to record images, operating a display interface,
operating a
connection interface configured to connect to a clinician database, the
clinician database
configured to store patient medical records, and operating a processor to
obtain medical
information. The processor is operated to obtain medical information by
displaying, via the
3
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
display interface, a user interface with fields to be populated with medical
information, and after
selection of a data field of the user interface, providing graphically via the
display interface, a first
option to enter medical information from an image and a second option to enter
medical
information via text entry. If the first option is selected, the processor
receives a recorded image
from the camera, the recorded image including a medical device or a screen of
a medical device,
extracts text from the image, enables a selection, via the display interface,
of at least a portion of
the text from the image, and writes the selected text from the image into the
data field of the user
interface as the medical information. If the second option is selected, the
processor enables text
entry of the data field, via the display interface, as the medical
information. The processor is also
operated to obtain medical information by, after a send instruction is
received, transmitting the
medical information written to the data field to a patient medical record
stored in the clinician
database.
[0011] In accordance with a second aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, the
machine-accessible
device further comprises instructions stored thereon that are configured, when
executed, to cause
the machine to operate the processor to determine a data template for the
extracted text on the
image, process the extracted text using the data template to group the
extracted text into fields, and
enable a selection of at least one of the fields to write the selected text
from the image into the data
field of the user interface.
[0012] In accordance with a third aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, the
data template is
configured to specify a context for the extracted text in relation to text
positions within the image.
[0013] In accordance with a fourth aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, the
machine-accessible
device further comprises instructions stored thereon that are configured, when
executed, to cause
the machine to operate the processor to determine the data template based on
at least one of (i) a
selection, via the user interface, of a medical device type, (ii) information
scanned from an
identifier located on the medical device, and (iii) a label within the
extracted text, or (iv) a relative
placement of the extracted text.
[0014] In accordance with a fifth aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, the
identifier located on
4
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
the medical device includes at least one of a quick-response ("QR") code, a
barcode, a serial
number, or a hardware number located on a housing of the medical device or the
screen of the
medical device.
[0015] In accordance with a sixth aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, the
medical device
includes at least a renal failure therapy machine, an infusion pump, an oxygen
sensor, a respiratory
monitor, a glucose meter, a blood pressure monitor, an ECG monitor, a weight
scale, and a heart
rate monitor.
[0016] In accordance with a seventh aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the data field of the user
interface is configured to receive at least one of blood pressure measurement
data, pulse data,
weight data, glucose data, temperature data, renal failure manual exchange
data, subjective data,
or consumable data regarding a consumable item.
[0017] In accordance with an eighth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the consumable item
includes at least one of a filter, a blood line set, a dialysate concentrate
container, a blood
anticoagulant container, a medication container, a disposable cassette, a
sorbent cartridge, and a
water purification container.
[0018] In accordance with a ninth aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, the
machine is a personal
mobile communication apparatus.
[0019] In accordance with a tenth aspect of the present disclosure, which may
be used in
combination with any other aspect listed herein unless stated otherwise, a
method for populating a
patient medical record of a clinical database using recorded images includes
transmitting to a
personal device, a first message prompting a patient to use a camera of the
personal device to
record a first image of a medical device, and receiving, from the personal
device, the first image.
The method also includes determining from the first image, via a processor,
first medical
information indicative of a type or model of the medical device, determining,
via the processor, a
data template and a second message associated with the determined type or the
model of the
medical device, and transmitting, via the processor, the second message to the
personal device
prompting the patient to use the camera to record a second image of a screen
of the medical device.
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
The method further includes receiving, via the processor from the personal
device, the second
image, extracting, via the processor, text from the second image, and
processing, via the processor,
the extracted text using the data template to group the extracted text into
fields. The method
moreover includes writing, via the processor, at least some of the text from
at least one of the fields
to the patient medical record.
[0020] In accordance with an eleventh aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the method further
includes determining, via the processor, a correspondence between one of the
fields to a record
field in the patient medical record, and writing, via the processor, the at
least some of the text from
the field to the record field in the patient medical record.
[0021] In accordance with a twelfth aspect of the present disclosure, which
may be used in
combination with any other aspect listed herein unless stated otherwise, the
method further
includes determining, via the processor, a treatment time from the patient
medical record, and
transmitting, via the processor, the first message before the treatment time.
[0022] In accordance with a thirteenth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the method further
includes receiving, in the processor from the personal device, a treatment
message indicative that
a patient is to begin a treatment, and transmitting, via the processor, the
first message before the
treatment is to begin.
[0023] In accordance with a fourteenth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the first image is
received in the processor via a first text message or first Short Messaging
Service ("SMS")
message and the second image is received in the processor via a second text
message or second
SMS message.
[0024] In accordance with a fifteenth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the method further
includes converting, via the processor, the at least some of the text from at
least one of the fields
to a Health-Level-7 ("HL7") format before writing to the patient medical
record.
[0025] In accordance with a sixteenth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the method further
includes comparing, via the processor, the at least some of the text from at
least one of the fields
6
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
to a predetermined range, and writing, via the processor, the at least some of
the text from at least
one of the fields if the at least some of the text from at least one of the
fields is within the
predetermined range.
[0026] In accordance with a seventeenth aspect of the present disclosure,
which may be
used in combination with any other aspect listed herein unless stated
otherwise, a system for
transmitting information to a patient includes a home therapy machine of the
patient configured to
transmit treatment information, and a clinician database configured to store
the medical record and
a registration file identifying the home therapy machine and a personal device
of the patient as
registered devices and identifying whether the personal device installed an
application for viewing
the treatment information and the medical information. The system also
includes a clinician server
communicatively coupled to the clinician database, the home therapy machine,
and the personal
device. The clinician server is configured to store the treatment information
and the medical
information to the medical record, receive an indication that at least some of
the treatment
information and the medical information is to be displayed at the personal
device, and determine
from the registration file if the application is installed on the personal
device. If the application is
installed, the clinician server is configured to convert the at least some of
the treatment information
and the medical information to an application format for display within the
application, and
transmit the converted at least some of the treatment information and the
medical information to
the personal device. If the application is not installed, the clinician server
is configured to convert
the at least some of the treatment information and the medical information to
text message or Short
Messaging Service ("SMS") format, and transmit the converted at least some of
the treatment
information and the medical information to the personal device via one or more
text message or
SMS message.
[0027] In accordance with an eighteenth aspect of the present disclosure,
which may be
used in combination with any other aspect listed herein unless stated
otherwise, the application
format includes at least one of an Extensible Markup Language ("XML") format
or an HyperText
Markup Language ("HTML") format.
[0028] In accordance with a nineteenth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the indication that at
least some of the treatment information and the medical information is to be
displayed at the
7
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
personal device includes at least one of a message from the application or a
text/SMS message
from the personal device.
[0029] In accordance with a twentieth aspect of the present disclosure, which
may be used
in combination with any other aspect listed herein unless stated otherwise,
the registration file is
included within the medical record.
[0030] In accordance with a twenty-first aspect of the present disclosure,
which may be
used in combination with any other aspect listed herein unless stated
otherwise, the home therapy
machine is configured to store a prescription with two programs, each of the
programs providing
parameters to operate the home therapy machine to perform a treatment, and the
clinician server
is configured to receive a program message from the personal device indicative
of a change to a
second program from a first program, and transmit a program instruction to the
home therapy
machine to change to the second program from the first program.
[0031] In a twenty-second aspect of the present disclosure, any of the
structure and
functionality disclosed in connection with Figs. 1 to 33 may be combined with
any other structure
and functionality disclosed in connection with Figs. 1 to 33.
[0032] In light of the present disclosure and the above aspects, it is
therefore an advantage
of the present disclosure to provide an improved medical fluid delivery
system.
[0033] It is another advantage of the present disclosure to provide improved
patient
lifestyle.
[0034] It is a further advantage of the present disclosure to provide improved
clinician or
caregiver efficiency.
[0035] It is still another advantage of the present disclosure to provide
improved machine
efficiency.
[0036] It is still a further advantage of the present disclosure to provide
improved patient
compliance.
[0037] It is yet another advantage of the present disclosure to provide a
medical fluid data
transfer system and methodology that may be applied to different types of
medical fluid delivery
machines.
[0038] It is yet a further advantage of the present disclosure to provide a
medical fluid data
transfer system and methodology that enables communication between a medical
fluid delivery
machine and multiple people, such as a patient and clinician or patient and
primary caregiver.
8
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[0039] Moreover, it is an advantage of the present disclosure to reduce waste
of disposable
sets and other ancillary soft goods due to discards, which occur often when
machine timers expire.
[0040] The advantages discussed herein may be found in one, or some, and
perhaps not all
of the embodiments disclosed herein. Additional features and advantages are
described herein,
and will be apparent from, the following Detailed Description and the figures.
BRIEF DESCRIPTION OF THE FIGURES
[0041] Fig. 1 is a schematic view illustrating one embodiment for a medical
fluid data
transfer system that incorporates the medical fluid delivery machines of the
present disclosure, so
that data may be transferred to and from such machines, according to an
embodiment of the present
disclosure.
[0042] Fig. 2 is a schematic illustration of one embodiment of a medical fluid
delivery
machine of the present disclosure.
[0043] Fig. 3 is a perspective view illustrating a blood set for use with one
embodiment of
the medical fluid delivery machine of Fig. 2, according to an embodiment of
the present disclosure.
[0044] Fig. 4 is a schematic view of one embodiment for a medical fluid
delivery machine
and data transfer system and method of the present disclosure.
[0045] Fig. 5 is a schematic view of a second embodiment for a medical fluid
delivery
machine and data transfer system and method of the present disclosure.
[0046] Fig 6. is a schematic view of a third embodiment for a medical fluid
delivery
machine and data transfer system and method of the present disclosure.
[0047] Fig. 7 is a schematic view of one embodiment of a hospital or clinical
version of a
medical fluid delivery device and data transfer system and method of the
present disclosure having
a mobile communication device application in a first state.
[0048] Fig. 8 is a schematic view of one embodiment of a hospital or clinical
version of a
medical fluid delivery device and data transfer system and method of the
present disclosure having
a mobile communication device application in a second state.
[0049] Fig. 9 is a schematic view of one embodiment of a hospital or clinical
version of a
medical fluid delivery device and data transfer system and method of the
present disclosure having
a mobile communication device application in a third state.
[0050] Fig. 10 shows a second embodiment of a medical fluid data transfer
system.
9
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[0051] Fig. 11 shows a diagram illustrative of operational modules of an
application at a
clinician server of the medical fluid data transfer system of Fig. 10,
according to an embodiment
of the present disclosure.
[0052] Fig. 12 shows a diagram illustrative of communications between a home
therapy
machine, a personal mobile communication device, and the clinician server of
Figs. 10 and 11,
according to an example embodiment of the present disclosure.
[0053] Fig. 13 illustrates an example patient data structure stored on a
clinician database
of the medical fluid data transfer system of Fig. 10, according to an example
embodiment of the
present disclosure.
[0054] Figs. 14 and 15 show diagrams illustrative of user interfaces of the
application of
Fig. 11, according to example embodiments of the present disclosure.
[0055] Fig. 16 is a schematic diagram of the personal mobile communication
device of
Figs. 10 and 11, according to an example embodiment of the present disclosure.
[0056] Fig. 17 illustrates a schematic diagram of a data template, according
to an example
embodiment of the present disclosure.
[0057] Fig. 18 shows a diagram illustrative of a writing to data fields of the
user interface
of Fig. 14, according to an example embodiment of the present disclosure.
[0058] Fig. 19 is a flow diagram of an example procedure for entering medical
information
from an image using the application of the personal mobile communication
device of Figs. 10 and
11, according to an example embodiment of the present disclosure.
[0059] Fig. 20 shows an example of a user interface displayable by the
application on the
personal mobile communication device of Figs. 10 and 11 that enables a patient
to provide a
recorded image, according to an example embodiment of the present disclosure.
[0060] Fig. 21 shows a schematic diagram of a patient medical template used by
the
clinician server of Figs. 10 and 11 to populate data fields of a patient's
medical record, according
to an example embodiment of the present disclosure.
[0061] Fig. 22 is a schematic diagram of a data acquisition module of the
clinician server
of Fig. 11, according to an example embodiment of the present disclosure.
[0062] Figs. 23 and 24 are flow diagrams of example procedures to populate the
medical
device template of Fig. 21 using images recorded by (and/or text messages
received from) the
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
personal mobile communication device of Figs. 10 and 11, according to an
example embodiment
of the present disclosure.
[0063] Fig. 25 shows a diagram of the clinician server hosting a web site or
file transfer site
to receive medical information via an image from the personal mobile
communication device of
Figs. 10 and 11, according to an example embodiment of the present disclosure.
[0064] Figs. 26 to 29 show diagrams of the personal mobile communication
device
displaying medical information provided by the clinician server of Figs. 10
and 11, according to
example embodiments of the present disclosure.
[0065] Fig. 30 shows a diagram of the personal mobile communication device of
Figs. 10
and 11 prompting a patient for medical information, according to an example
embodiment of the
present disclosure.
[0066] Fig. 31 shows a diagram of the personal mobile communication device of
Figs. 10
and 11 providing a patient to select a program for a medical treatment,
according to an example
embodiment of the present disclosure.
[0067] Fig. 32 shows a diagram of the personal mobile communication device of
Figs. 10
and 11 providing educational content a patient, according to an example
embodiment of the present
disclosure.
[0068] Fig. 33 shows a diagram of the personal mobile communication device of
Figs. 10
and 11 providing a video chat session between a patient and a clinician,
according to an example
embodiment of the present disclosure.
DETAILED DESCRIPTION
[0069] A medical fluid delivery system is disclosed herein. The example
medical fluid
delivery system is configured to improve a patient's engagement with a medical
treatment, such
as a medical fluid delivery treatment. The medical fluid delivery system is
configured to provide
a patient more transparency regarding their treatment and at least some
control to direct their
treatment while providing resources to educate or otherwise assist the patient
throughout the
treatment process. The example medical fluid delivery system is configured to
provide patient
engagement regardless of a type or feature sets of medical device(s) related
to the treatment or
personal mobile communication device(s) of the patient. Such a configuration
enables the
disclosed medical fluid delivery system to be compatible with virtually any
patient situation.
11
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[0070] In some embodiments, the medical fluid delivery system includes a
personal mobile
communication device, a medical fluid delivery machine, and a clinician
server/database. The
example personal mobile communication device and the medical fluid delivery
machine are
located at a patient's home and/or located at a self-service medical facility.
The clinician server is
communicatively coupled to the personal mobile communication device and/or the
medical fluid
delivery machine via a wide area network (i.e., the Internet). The clinician
server is configured as
a hub that enables the personal mobile communication device to provide
features designed to
engage a patient with a medical fluid delivery treatment.
[0071] As disclosed herein, the example clinician server receives medical
fluid delivery
data (e.g., treatment information) from the medical fluid delivery machine,
which is stored to one
or more patient records in a clinician database. The clinician server provides
the personal mobile
communication device access to the medical fluid delivery data to enable a
patient to track
treatment progress. In addition, a patient may use the personal mobile
communication device to
change a treatment program (or request to change a treatment program). The
request is routed
through the clinician server to verify the change is appropriate before being
transmitted to the
medical fluid delivery machine.
[0072] The example clinician server operates in connection with the personal
mobile
communication device to enable a patient to effortlessly provide medical
information, such as vital
sign data, medical device data, etc. The personal mobile communication device
is configured to
enable a patient to manually enter data, to record pictures that include data,
and/or wirelessly
receive data from connected medical devices, such as weight scales, blood
pressure monitors,
thermometers, glucose meters, etc.. The collected data is stored to a
patient's medical record. In
some embodiments, the clinician server and/or the personal mobile
communication device may
use templates to determine how received data is to be automatically populated
into a patient's
medical record.
[0073] As disclosed herein, the personal mobile communication device may
include a
feature-rich smart-device, such as a smartphone or tablet computer. The smart-
device may operate
a specialized application (e.g., an "app") that is defined by instructions
stored in a memory.
Execution of the stored instructions causes a process of the smart-device to
operate the application,
which is configured to increase a patient's engagement with a medical
treatment. In some
instances, the personal mobile communication device may include a feature-lite
traditional cellular
12
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
phone that contains considerably less processing power and features compared
to a smart-device.
The cellular device may operate using text messages and/or a specialized
application (e.g., an App)
that is defined by instructions stored in a memory. The application for the
feature-lite device may
have fewer features compared with the application for the smart-device.
[0074] The disclosure below is separated into two sections. A first section
discloses an
embodiment of a medical fluid delivery system. A second section discloses
features that enable
the medical fluid delivery system to increase a patient's engagement and/or
compliance with a
medical treatment.
I. Medical Fluid Delivery System Embodiment
[0075] The example medical fluid delivery system includes one or more medical
fluid
delivery machines. One example of a medical fluid delivery machine is a renal
failure therapy
machine. Regarding renal failure therapy machines, due to various causes, a
patient's renal system
can fail. Renal failure produces several physiological derangements. It is no
longer possible to
balance water and minerals or to excrete daily metabolic load. Toxic end
products of nitrogen
metabolism (urea, creatinine, uric acid, and others) can accumulate in blood
and tissue.
[0076] Kidney failure and reduced kidney function have been treated with
dialysis.
Dialysis removes waste, toxins and excess water from the body that normal
functioning kidneys
would otherwise remove. Dialysis treatment for replacement of kidney functions
is critical to
many people because the treatment is life saving.
[0077] One type of kidney failure therapy is Hemodialysis ("HD"), which in
general uses
diffusion to remove waste products from a patient's blood. A diffusive
gradient occurs across the
semi-permeable dialyzer between the blood and an electrolyte solution called
dialysate or dialysis
fluid to cause diffusion.
[0078] Hemofiltration ("HF") is an alternative renal replacement therapy that
relies on a
convective transport of toxins from the patient's blood. HF is accomplished by
adding substitution
or replacement fluid to the extracorporeal circuit during treatment (typically
ten to ninety liters of
such fluid). The substitution fluid and the fluid accumulated by the patient
in between treatments
is ultrafiltered over the course of the HF treatment, providing a convective
transport mechanism
that is particularly beneficial in removing middle and large molecules (in
hemodialysis there is a
13
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
small amount of waste removed along with the fluid gained between dialysis
sessions, however,
the solute drag from the removal of that ultrafiltrate is not enough to
provide convective clearance).
[0079] Hemodiafiltration ("HDF") is a treatment modality that combines
convective and
diffusive clearances. HDF uses dialysis fluid flowing through a dialyzer,
similar to standard
hemodialysis, to provide diffusive clearance. In addition, substitution
solution is provided directly
to the extracorporeal circuit, providing convective clearance.
[0080] Most HD (HF, HDF) treatments occur in centers. A trend towards home
hemodialysis ("HHD") exists today in part because HHD can be performed daily,
offering
therapeutic benefits over in-center hemodialysis treatments, which occur
typically bi- or tri-
weekly. Studies have shown that frequent treatments remove more toxins and
waste products than
a patient receiving less frequent but perhaps longer treatments. A patient
receiving treatments
more frequently does not experience as much of a down cycle as does an in-
center patient, who
has built-up two or three days' worth of toxins prior to treatment. In certain
areas, the closest
dialysis center can be many miles from the patient's home causing door-to-door
treatment time to
consume a large portion of the day. HHD may take place overnight or during the
day while the
patient relaxes, works or is otherwise productive.
[0081] Another type of kidney failure therapy is peritoneal dialysis, which
infuses a
dialysis solution, also called dialysis fluid, into a patient's peritoneal
cavity via a catheter. The
dialysis fluid contacts the peritoneal membrane of the peritoneal cavity.
Waste, toxins and excess
water pass from the patient's bloodstream, through the peritoneal membrane and
into the dialysis
fluid due to diffusion and osmosis, i.e., an osmotic gradient occurs across
the membrane. An
osmotic agent in dialysis provides the osmotic gradient. The used or spent
dialysis fluid is drained
from the patient, removing waste, toxins and excess water from the patient.
This cycle is repeated,
e.g., multiple times.
[0082] There are various types of peritoneal dialysis therapies, including
continuous
ambulatory peritoneal dialysis ("CAPD"), automated peritoneal dialysis
("APD"), and tidal flow
dialysis and continuous flow peritoneal dialysis ("CFPD"). CAPD is a manual
dialysis treatment.
Here, the patient manually connects an implanted catheter to a drain to allow
used or spent
dialysate fluid to drain from the peritoneal cavity. The patient then connects
the catheter to a bag
of fresh dialysis fluid to infuse fresh dialysis fluid through the catheter
and into the patient. The
patient disconnects the catheter from the fresh dialysis fluid bag and allows
the dialysis fluid to
14
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
dwell within the peritoneal cavity, wherein the transfer of waste, toxins and
excess water takes
place. After a dwell period, the patient repeats the manual dialysis
procedure, for example, four
times per day, each treatment lasting about an hour. Manual peritoneal
dialysis requires a
significant amount of time and effort from the patient, leaving ample room for
improvement.
[0083] Automated peritoneal dialysis ("APD") is similar to CAPD in that the
dialysis
treatment includes drain, fill and dwell cycles. APD machines, however,
perform the cycles
automatically, typically while the patient sleeps. APD machines free patients
from having to
perform the treatment cycles manually and from having to transport supplies
during the day. APD
machines connect fluidly to an implanted catheter, to a source or bag of fresh
dialysis fluid and to
a fluid drain. APD machines pump fresh dialysis fluid from a dialysis fluid
source, through the
catheter and into the patient's peritoneal cavity. APD machines also allow for
the dialysis fluid to
dwell within the cavity and for the transfer of waste, toxins and excess water
to take place. The
source may include multiple sterile dialysis fluid bags.
[0084] APD machines pump used or spent dialysate from the peritoneal cavity,
though the
catheter, and to the drain. As with the manual process, several drain, fill
and dwell cycles occur
during dialysis. A "last fill" occurs at the end of APD and remains in the
peritoneal cavity of the
patient until the next treatment.
[0085] Any of the above modalities performed by a machine may be run on a
scheduled
basis and may require a start-up procedure. For example, dialysis patients
typically perform
treatment on a scheduled basis, such as every other day, daily, etc. Blood
treatment machines
typically require a certain amount of time before treatment for setup, for
example, to run a
disinfection procedure. Patients for the above modalities may lead busy lives
and have projects to
perform or errands to run on a day scheduled for treatment.
[0086] Much of the appeal of a home treatment for the patient revolves around
the lifestyle
flexibility provided by allowing the patient to perform treatment in his or
her home largely
according to his or her own schedule. The home medical fluid delivery machine
may however
include software timers that dictate to and constrain the user or patient. A
home hemodialysis
system may for example require the patient to be in immediate proximity to the
home hemodialysis
machine to initiate pre-treatment, during treatment, and post-treatment
sequences.
[0087] In one particular example, a home therapy machine may reuse certain
components
by disinfecting them in between treatments. The machine may employ one or more
disinfection
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
timer that requires the patient or caregiver to start a treatment using the
machine before the
disinfection timer expires. Otherwise, the patient will have to wait until
another disinfection
procedure is completed before starting treatment. The home therapy machine in
an embodiment
communicates the treatment start time deadlines via the machine's graphical
user interface, which
requires the patient to be in the proximity of the machine to access the start
time deadlines and
react accordingly.
[0088] It should be appreciated that the present disclosure applies to any
type of
disinfection, such as, hot water disinfection and chemical disinfection. In
this regard, the present
disclosure is not limited to home therapy machines, for example, in-center
machines are typically
chemically disinfected and may set a treatment start deadline after such
disinfection. Further
additionally, the present disclosure is not limited to start time deadlines
based upon disinfection
but may also be applied to other start time deadlines, e.g., ones based upon
the completion of
priming. Still further, the present disclosure is not limited to initial start
time deadlines. For
example, most machines will allow the patient to temporarily stop treatment
and disconnect from
the machine to perform some type of necessary action away from the machine.
For a blood
treatment, the machine will typically rinse blood back to the patient and may
or may not circulate
the dialysis fluid for a period of time. In either case, the time that the
patient may be temporarily
disconnected from the machine is not unlimited, and it is contemplated that
the present disclosure
also applies to the return time limit.
[0089] In one embodiment, the system of the present disclosure provides a
software
application ("app") that is installed on the patient's and/or caregiver's
personal mobile
communication device, e.g., smartphone. The app is provided in one embodiment
via a
middleware software application, an example of which is discussed in detail
below. In an
alternative embodiment, the software is configured to communicate with the
patient's and/or
caregiver's personal mobile communication device, e.g., smartphone, directly
using a text
messaging feature through a middleware software application. In either case,
the app or text
message is structured in one embodiment to remind the patient of any impending
deadline and to
allow the patient and/or caregiver to keep track of when a treatment needs to
start without tethering
the patient to the machine.
[0090] It is contemplated to alternatively or additionally structure the
communication
software to program reminders automatically on the user's mobile communication
device, for
16
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
example, on the device's native task tracking features, such as a calendar
application. Most
smartphones are provided with a calendar that separates each day into time
segments, such as
hours. The software of the system and methodology of the present disclosure
may be programmed
to access the smartphone calendars of authorized patients and/or caregivers
and to populate the
appropriate time segment(s) of the appropriate day with the appropriate
information, for example,
that the machine is to begin or complete disinfection within that time
segment.
[0091] In one embodiment, communication from the software system and
methodology of
the present disclosure is one-way. For example, communication may be from the
medical fluid
delivery machine, which may be a home machine, to a patient or caregiver's
mobile
communication device. In an alternative embodiment, the software system and
methodology of
the present disclosure enables two-directional communication between the
medical fluid delivery
machine and the patient or caregiver's mobile communication device. In one
example, the two-
way communication may allow for certain machine routines to be started
remotely by the patient
or caregiver using their mobile communication device. One example routine is
an automated self-
test routine, which may be performed without any user interaction with the
system other than
initiating or starting the sequence. Starting the sequence remotely may
benefit the patient or
caregiver, e.g., by providing additional time that the patient or caregiver
may be away from the
machine performing other tasks. The communication becomes two-way when the
machine
initiates the communication by indicating that the machine is ready to perform
the self-test routine.
The patient or caregiver at a desired time responds back to the machine via
the software system
and methodology of the present disclosure to initiate the sequence.
[0092] It is contemplated for the software of the system and methodology of
the present
disclosure to disable communication between the patient and/or caregiver and
the machine
whenever the machine is in a "patient connected" software state. For example,
if a clinician tries
to send a command to a machine currently treating a patient, the command may
be intercepted by
the middleware software application so that the command is not transferred to
the machine. The
middleware software application may then communicate back to the clinician
informing that the
machine is busy and not accepting communication.
[0093] The examples described herein are applicable to any medical fluid
delivery system
that delivers a medical fluid, such as blood, dialysis fluid, substitution
fluid or and intravenous
drug ("IV"). The examples are particularly well suited for kidney failure
therapies, such as all
17
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
forms of hemodialysis ("HD"), hemofiltration ("HF"), hemodiafiltration
("HDF"), continuous
renal replacement therapies ("CRRT") and peritoneal dialysis ("PD"), referred
to herein
collectively or generally individually as renal failure therapy. The medical
fluid delivery machines
may alternatively be a drug delivery or nutritional fluid delivery device,
such as a large volume
peristaltic type pump or a syringe pump. The machines described herein may be
used in home
settings. For example, a machine operating with the data transfer regime of
the present disclosure
may be employed with a home HD machine, which can for example be run at night
while the
patient is sleeping. The medical fluid data transfer system and methodology of
the present
disclosure may alternatively be used to help clinicians or nurses in hospitals
and/or clinics.
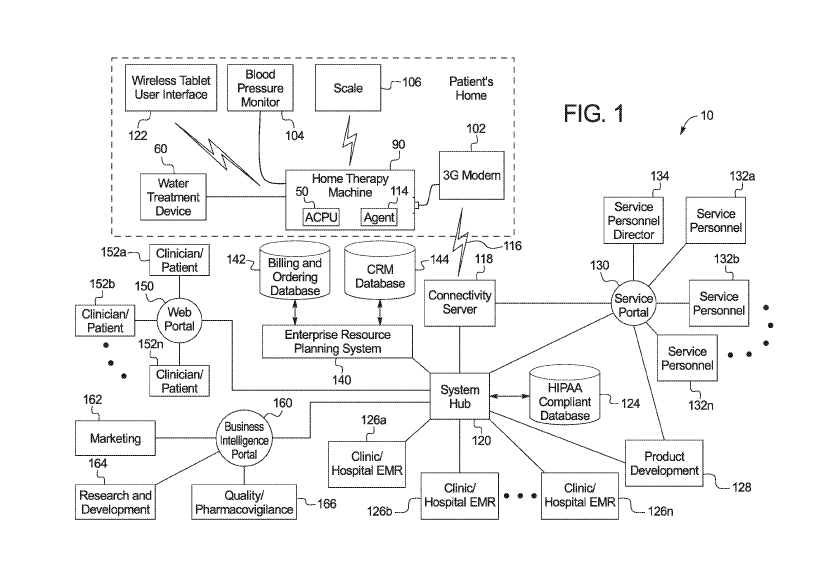
[0094] Referring now to the drawings and in particular to Fig. 1, a medical
fluid data
transfer system 10 is illustrated operating within a medical fluid delivery
machine 90. System 10
incorporates many medical fluid delivery machines 90 (one type of which is
discussed in detail
below). Machines 90 of data transfer system 10 may be of a same type (e.g.,
all HD machines) or
be of different types (e.g., a mix of HD, PD, CRRT, and medical or nutritional
fluid delivery).
[0095] While a single medical fluid delivery 90 is illustrated as
communicating with a
connectivity server 118, system 10 oversees the operation of a plurality of
medical fluid delivery
systems and machines, of the same type or of different types listed above. For
example, there may
be M number of hemodialysis machines 90, N number of hemofiltration machines
90, 0 number
of CRRT machines 90, P number of peritoneal dialysis machines 90, Q number of
home drug
delivery machines 90, and R number of nutritional or drug delivery machines 90
connected to
server 118 and operating with system 10. The numbers M through R may be the
same or different
numbers, and may be zero, one, or more than one. In Fig. 1, medical fluid
delivery machine 90 is
illustrated as a home therapy machine 90 (the home indicated by dashed lines).
[0096] Home therapy machine 90 may receive at its front end purified water
from a water
treatment device 60 as discussed above. Water treatment device 60 connects to
home therapy
machine 90 via an Ethernet cable in an embodiment. Home therapy machines 90 in
the illustrated
embodiment operate with other devices besides water treatment device 60, such
as a blood pressure
monitor 104, a weigh scale, e.g., wireless weigh scale 106, and a user
interface such as a wireless
tablet user interface 122. Home therapy machine 90 connects to server 118
wirelessly in one
embodiment via a modem 102. Each of these components may (but does not have to
be) located
within the patient's home, as demarcated by the dashed lines in Fig. 1. Any
one, or more, or all of
18
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
components 60, 104, 106 and 122 may communicate wired or wirelessly with home
therapy
machine 90. Wireless communication may be via BluetoothTM, WiFiTM, Zigbee , Z-
Wave ,
wireless Universal Serial Bus ("USB"), infrared, or any other suitable
wireless communication
technology. Alternatively, any one, or more or all of components 60, 104, 106
and 122 may
communicate with home therapy machine 90 via wired communication.
[0097] Connectivity server 118 communicates with medical fluid delivery
machine 90 via
a medical device system hub 120. System hub 120 enables data and information
concerning each
home therapy machine 90 and its peripherals to travel back and forth via
connectivity server 118
between machines 90 and the other clients connected to server 118. In the
illustrated embodiment,
system hub 120 is connected to a service portal 130, an enterprise resource
planning system 140,
a web portal 150, a business intelligence portal 160, a HIPAA compliant
database 124, a product
development team 128 and electronic medical records databases maintained for
example at clinics
or hospitals 126a to 126n.
[0098] Electronic medical records ("EMR") databases at clinics or hospitals
126a to 126n
store electronic information concerning patients. System hub 120 may send the
data collected
from log files of machine 90 to hospital or clinic databases 126a to 126n to
merge or supplement
that patient's medical records. Databases at clinics or hospitals 126a to 126n
may contain patient-
specific treatment and prescription data and therefore access to such
databases may be highly
restricted. Enterprise resource planning system 140 obtains and compiles data
generated via the
patient and clinician website access, such as complaints, billing information
and life cycle
management information. Web portal 150 enables patients and clinics 152a to
152n treating the
patients to access a website publicly available for users of medical fluid
delivery machines 90.
Business intelligence portal 160 collects data from system hub 120 and
provides data to marketing
162, research and development 164, and quality/pharmacovigilance 166.
[0099] It should be appreciated that the systems, methods and procedures
described herein
may be implemented using one or more computer program or component. The
programs of
components may be provided as a series of computer instructions on any
conventional computer-
readable medium, including random access memory ("RAM"), read only memory
("ROM"), flash
memory, magnetic or optical disks, optical memory, or other storage media. The
instructions may
be configured to be executed by a processor, which when executing the series
of computer
19
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
instructions performs or facilitates the performance of all or part of the
disclosed methods and
procedures.
[00100] In one embodiment, home therapy machine 90 performs a home
treatment,
such as home hemodialysis on a patient at the patient's home and then reports
the results of that
treatment to clinicians, doctors and nurses who are responsible for managing
the health and well-
being of that patient.
[00101] Home therapy machines 90 in an embodiment write log files
using, e.g., a
LinuxTM operating system. The log files document pertinent home therapy
machine 90 data,
including peripheral device data. The log files may include any one or more of
Extensible Markup
Language ("XML"), comma-separated values ("CSV") or text files. The log files
are placed into
a file server box of the software of home therapy machine 90. It is also
contemplated to store data
at a peripheral device, e.g., water treatment device 60, which is not sent to
machine 90. Such data
may otherwise be obtained via the wired or wireless connection to the
peripheral device or
downloaded through other data connections or storage media. For example, a
service person can
access additional data via a laptop connected to water treatment device 60 or
wireless weigh scale
106, e.g., via an Ethernet connection. Or, the additional data may be
retrieved remotely from the
peripheral devices, with home therapy machine 90 serving as the data transfer
liaison between the
peripheral device and authorized clients of medical fluid data transfer
system.
[00102] In one embodiment, home therapy machine 90, e.g., via the
internet, uses a
connectivity service to transfer data between modem 102 and system hub 120.
Here, a dedicated
line may be provided at each patient's home for connecting the home therapy
machine 90 to the
connectivity server 118 via modem 102. Home therapy machine 90 in one
embodiment accesses
the internet using a separate, e.g., 3G, 4G or 5G, modem 102. Modem 102 may
use an internet
Service Provider ("ISP"), such as VodafoneTM. In one implementation, a
connectivity agent 114
developed by a connectivity service provider (e.g., provider of connectivity
server 118) is installed
onto the home therapy machine 90 and run on ACPU 50 of the machine. One
suitable connectivity
service is provided by AxedaTM, which provides a secure managed connection 116
between
medical devices and the connectivity server 118.
[00103] Connectivity agent 114 allows the home therapy machine 90 to
connect to
connectivity server 118 and transfer data to and from the connectivity server
118. The connectivity
service operating via agent 114 and server 118 ensures that the connection
with machine 90 is
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
secure, ensures that the data correctly passes through machine 90's firewalls,
checks whether there
has been a data or system crash, and ensures that connectivity server 118 is
communicating with
the correct home therapy machine 90.
[00104] In one embodiment, home therapy machine 90 may only connect
to
connectivity server 118 when connectivity agent 114 is turned on or activated.
During treatment
and post-treatment disinfection, while machine 90 and its peripherals are
functioning, connectivity
agent 114 is turned off if one embodiment, which prevents home therapy machine
90 from
communicating with any entity and sending or receiving data during treatment
and disinfection or
when machine 90 is live or running. When home therapy machine 90 is idle,
e.g., after treatment
and post-disinfection is complete, ACPU 50 turns connectivity agent 114 on in
one embodiment.
In an embodiment, connectivity agent 114 is off during treatment and possibly
pretreatment. After
treatment, connectivity agent 114 retrieves the log files from the home
therapy machine 90 and
transfers data to the connectivity server 118 using the connectivity service.
The connectivity
service routes data packets to their proper destination but in one embodiment
does not modify,
access, or encrypt the data.
[00105] In medical fluid data transfer system 10 system of Fig. 1,
the connectivity
service via connectivity server 118 may communicate data to various places via
a system hub 120,
such as a service portal 130, clinics or hospitals 126a to 126n, and a web
portal 150. Connectivity
server 118 allows service personnel 132a to 132n and/or clinicians to track
and retrieve various
assets across the network, such as appropriate home therapy machines 90 and
3G, 4G or 5G modem
102, and their associated information, including machine or modem serial
numbers. Connectivity
server 118 may also be used to receive and provide firmware upgrades, approved
by a director of
service personnel 134 and obtained remotely via service portal 130, to
authorized home therapy
machines 90 and associated peripherals, such as water treatment devices 60.
A. Example Medical Fluid Delivery Machine
[00106] Referring now to Fig. 2, an example of an HD flow schematic
for medical
fluid delivery machine 90 is illustrated. Because the HD system of Fig. 2 is
relatively complicated,
Fig. 2 and its discussion also provide support for any of the renal failure
therapy modalities
discussed above and for an IV, drug delivery, or nutritional fluid delivery
machine. Generally,
medical fluid delivery machine 90 is shown having a simplified version of a
dialysis fluid or
21
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
process fluid delivery circuit. The blood circuit is also simplified but not
to the degree that the
dialysis fluid circuit is simplified. It should be appreciated that the
circuits have been simplified
to make the description of the present disclosure easier, and that the systems
if implemented would
have additional structure and functionality, such as is found in the
publications incorporated by
reference above.
[00107] Medical fluid delivery machine 90 of Fig 2 includes a blood
circuit 20.
Blood circuit 20 pulls blood from and returns blood to a patient 12. Blood is
pulled from patient
12 via an arterial line 14, and is returned to the patient via a venous line
16. Arterial line 14
includes an arterial line connector 14a that connects to an arterial needle
14b, which is in blood
draw communication with patient 12. Venous line 16 includes a venous line
connector 16a that
connects to a venous needle 16b, which is in blood return communication with
the patient. Arterial
and venous lines 14 and 16 also include line clamps 18a and 18v, which can be
spring-loaded, fail-
safe mechanical pinch clamps. Line clamps 18a and 18v are closed automatically
in an emergency
situation in one embodiment.
[00108] Arterial and venous lines 14 and 16 also include air or
bubble detectors 22a
and 22v, respectively, which can be ultrasonic air detectors. Air or bubble
detectors 22a and 22v
look for air in the arterial and venous lines 14 and 16, respectively. If air
is detected by one of air
detectors 22a and 22v, system 10 closes line clamps 18a and 18v, pauses the
blood and dialysis
fluid pumps, and provides instructions to the patient to clear the air so that
treatment can resume.
[00109] A blood pump 30 is located in arterial line 14 in the
illustrated embodiment.
In the illustrated embodiment, blood pump 30 includes a first blood pump pod
30a and a second
blood pump pod 30b. Blood pump pod 30a operates with an inlet valve 32i and an
outlet valve
32o. Blood pump pod 30b operates with an inlet valve 34i and an outlet valve
34o. In an
embodiment, blood pump pods 30a and 30b are each blood receptacles that
include a hard outer
shell, e.g., spherical, with a flexible diaphragm located within the shell,
forming a diaphragm
pump. One side of each diaphragm receives blood, while the other side of each
diaphragm is
operated by negative and positive air pressure. Blood pump 30 is alternatively
a peristaltic pump
operating with the arterial line 14 or multiple peristaltic pumps operating
with arterial line 14 and
venous line 16.
[00110] A heparin vial 24 and heparin pump 26 are located between
blood pump 30
and blood filter 40 (e.g., dialyzer) in the illustrated embodiment. Heparin
pump 26 may be a
22
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
pneumatic pump or a syringe pump (e.g., stepper motor driven syringe pump).
Supplying heparin
upstream of blood filter 40 helps to prevent clotting of the filter's
membranes.
[00111] A primary control processor ("ACPU") or control unit control
unit 50
includes one or more processor and memory. Control unit 50 receives air
detection signals from
air detectors 22a and 22v (and other sensors of system 10, such as temperature
sensors, blood leak
detectors, conductivity sensors, pressure sensors, and access disconnection
transducers 86, 88),
and controls components such as line clamps 18a and 18v, blood pump 30,
heparin pump 26,
dialysis fluid pumps 64 and 96, and valves 32i, 32o, 34i, 34o, 68i, 68o, 98i
and 980. Blood exiting
blood filter 40 via venous line 16 flows through an airtrap 28. Airtrap 28
removes air from the
blood before the dialyzed blood is returned to patient 12 via venous line 16.
[00112] With the hemodialysis version of medical fluid delivery
machine 90 of Fig.
2, dialysis fluid is pumped along the outside of the membranes of blood filter
40, while blood is
pumped through the insides of the blood filter membranes. Dialysis fluid is
prepared beginning
with the purification of water via a water purification unit 60. One suitable
water purification unit
is set forth in U.S. Patent Publication No. 2011/0197971, entitled, "Water
Purification System and
Method", filed Apr. 25, 2011, the entire contents of which are incorporated
herein by reference
and relied upon. In one embodiment, water purification unit includes filters
and other structures
to purify tap water (e.g., remove pathogens and ions such as chlorine), so
that the water is in one
implementation below 0.03 endotoxin units/ml ("EU/m1") and below 0.1 colony
forming units/ml
("CFU/m1"). Water purification unit 60 may be provided in a housing separate
from the housing
or chassis of the hemodialysis machine 90, which includes blood circuit 20 and
dialysis fluid circuit
70.
[00113] Dialysis fluid circuit 70 is again highly simplified in Fig.
2 to ease
illustration. Dialysis fluid circuit 70 in actuality may include all of the
relevant structure and
functionality set forth in the publications incorporated by reference above.
Certain features of
dialysis fluid circuit 70 are illustrated in Fig. 2. In the illustrated
embodiment, dialysis fluid circuit
70 includes a to-blood filter dialysis fluid pump 64. Pump 64 is in one
embodiment configured
the same as blood pump 30. Pump 64, like pump 30, includes a pair of pump pods
66 each having
inlet valves 68i and outlet valves 68o, which again may be spherically
configured. The two pump
pods, like with blood pump 30, are operated alternatingly so that one pump pod
is filling with HD
dialysis fluid, while the other pump pod is expelling HD dialysis fluid.
23
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00114]
Pump 64 is a to-blood filter dialysis fluid pump. There is another dual pod
pump chamber 96 operating with valves 98i and 98o located in drain line 82 to
push used dialysis
fluid to drain. There is a third pod pump (not illustrated) for pumping pump
purified water through
a bicarbonate cartridge 72. There is a fourth pod pump (not illustrated) used
to pump acid from
acid container 74 into mixing line 62. The third and fourth pumps, the
concentrate pumps, may
be single pod pumps because continuous pumping is not as important in mixing
line 62 due to a
buffering dialysis fluid tank (not illustrated) between mixing line 62 and to-
blood filter dialysis
fluid pump 64 in one embodiment.
[00115]
A fifth pod pump (not illustrated) provided in drain line 82 is used to remove
a known amount of ultrafiltration ("UF") when an HD therapy is provided.
System 10 keeps track
of the UF pump to control and know how much ultrafiltrate has been removed
from the patient.
System 10 ensures that the necessary amount of ultrafiltrate is removed from
the patient by the
end of treatment.
[00116]
Each of the above-described pumps may alternatively be a peristaltic pump
operating with a pumping tube. If so, the system valves may still be actuated
pneumatically
according to the features of the present disclosure.
[00117]
In one embodiment, purified water from water purification unit 60 is
pumped along mixing line 62 though bicarbonate cartridge 72. Acid from
container 74 is pumped
along mixing line 62 into the bicarbonated water flowing from bicarbonate
cartridge 72 to form an
electrolytically and physiologically compatible dialysis fluid solution.
The pumps and
temperature-compensated conductivity sensors used to properly mix the purified
water with the
bicarbonate and acid are not illustrated but are disclosed in detail in the
publications incorporated
by reference above.
[00118]
Fig. 2 also illustrates that dialysis fluid is pumped along a fresh dialysis
fluid line 76, through a heater 78 and an ultrafilter 80, before reaching
blood filter 40, after which
used dialysis fluid is pumped to drain via drain line 82. Heater 78 heats the
dialysis fluid to body
temperature or about 37 C. Ultrafilter 80 further cleans and purifies the
dialysis fluid before
reaching blood filter 40, filtering foreign matter and/or contaminants
introduced for example via
bicarbonate cartridge 72 or acid container 74 from the dialysis fluid.
[00119]
Dialysis fluid circuit 70 also includes a sample port 84 in the illustrated
embodiment. Dialysis fluid circuit 70 will further include a blood leak
detector (not illustrated but
24
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
used to detect if a blood filter 40 fiber is torn) and other components that
are not illustrated, such
as balance chambers, plural dialysis fluid valves, and a dialysis fluid
holding tank, all illustrated
and described in detail in the publications incorporated by reference above.
[00120] In the illustrated embodiment, medical fluid delivery
machine 90 is an
online, pass-through system that pumps dialysis fluid through blood filter one
time and then pumps
the used dialysis fluid to drain. Both blood circuit 20 and dialysis fluid
circuit 70 may be hot water
disinfected after each treatment, such that blood circuit 20 and dialysis
fluid circuit 70 may be
reused. In one implementation, blood circuit 20 including blood filter 40 is
hot water disinfected
and reused daily for about one month, while dialysis fluid circuit 70 is hot
water disinfected and
reused for about six months.
[00121] In alternative embodiments, for CRRT for example, multiple
bags of
sterilized dialysis fluid or infusate are ganged together and used one after
another. In such a case,
the emptied supply bags can serve as drain or spent fluid bags.
[00122] Medical fluid delivery machine 90 includes an enclosure as
indicated by the
dashed line of Fig. 2. The enclosure of machine 90 varies depending upon the
type of treatment,
whether the treatment is in-center or a home treatment, and whether the
dialysis fluid/infusate
supply is a batch-type (e.g., bagged) or on-line.
[00123] Fig. 3 illustrates that machine 90 of Fig. 2 may operate
with a blood set 100.
Blood set 100 includes arterial line 14, venous line 16, heparin vial 24,
heparin pump 26/blood
pump 30 and blood filter 40 (e.g., dialyzer). An airtrap 28 may be located in
venous line 16 to
remove air from the blood before being returned to patient 12. Air detectors
22a and 22v contact
arterial and venous lines 14 and 16, respectively, for operation.
[00124] In Figs. 2 and 3, any of pumps 26, 30 (30a and 30b), 64, 96
(and other pumps
not illustrated) and any of the valves, such as valves 32i, 32o, 34i, 34o,
68i, 68o, 98i, and 98o may
be pneumatically actuated. In an embodiment, each of the pumps and valves has
a fluid side and
an air side, separated by a flexible membrane. Negative pneumatic pressure may
be applied to the
air side of the membrane to draw fluid into a pump chamber or to open a valve
(or the pump or
valve could be opened by venting positive closing pressure to atmosphere and
allowing fluid
pressure to open). Positive pneumatic pressure is applied to the air side of
the membrane to expel
fluid from a pump chamber or to close a valve.
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
B. Connectivity Embodiments of the Example Medical Fluid Delivery System
[00125] Referring now to Fig. 4, a system 110a of the present
disclosure is
illustrated. System 110a in the illustrated embodiment operates with system 10
described above,
including connectivity server 118, system hub 120, service portal 130,
enterprise resource planning
system 140, web portal 150, and business intelligence portal 160, which are
illustrated in Fig. 4 as
being part of a cloud environment. Connectivity server 118, system hub 120,
service portal 130,
enterprise resource planning system 140, web portal 150, and business
intelligence portal 160 may
each be part of a cloud environment or be located at one or more dedicated
server.
[00126] Other components of system 10 not illustrated in Fig. 4 may
also be part of
system 110a. For instance, medical fluid delivery machines 90a and 90b may
reside separately in
the homes of patients 12a and 12b (who are illustrated as being outside the
home). Alternatively,
medical fluid delivery machines 90a and 90b may reside in the same clinic 126a
to 126n or in
different ones of clinics 126a to 126n. Clinicians 112a and 112b may reside
inside or outside of
the clinics.
[00127] Medical fluid delivery machines 90a and 90b are connected to
connectivity
server 118 via secure managed connections 116 as described above. To do so,
machines 90a and
90b connect to internet 52, e.g., via modems 102 discussed above. System hub
120 in one
embodiment stores middleware software that may be accessed by mobile
communication devices
200a and 200b (referred to herein collectively as devices 200 or generally
individually as device
200). Mobile communication devices 200a and 200b may be smartphones, for
example, running
on AndroidTM, iOSTM, Windows PhoneTM, BlackBerryTM, Sailfish OSTM, TizenTm, or
Ubuntu
TouchTm operating systems. Mobile communication devices 200a and 200b may
belong to patients
12a and 12b, respectively, and/or clinicians 112a and 112b, respectively.
Mobile communication
devices 200a and 200b as illustrated in Fig. 4 are also connected to interne
52.
[00128] In one embodiment, mobile communication devices 200a and
200b
download application software ("app") from middleware software stored on
system hub 120 via
their connection to interne 52. The app is updated whenever there is a change
of state of the
corresponding machine 90a or 90b. For example, medical fluid delivery machine
90a may have
just completed its automated self-test routine and is now ready to run a
disinfection procedure.
Machine 90a may generate a code identifying this state and send it to
middleware software stored
on system hub 120. Middleware software then translates the code into a
message, e.g., using a
26
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
look-up table, such as, "self-test completed, ready for disinfection" and
cause the app downloaded
onto mobile communication device 200a of patient 12a or clinician 112a to
display the message.
The app may be programmed to provide a visual identifier along with the
message, such as, an
icon that is associated with the particular state in which machine 90a
resides. The app may also
provide any one or more of an audio alert, such as a "ding" sound, and/or a
haptic alert, such as a
vibration, which prompt patient 12a or clinician 112a to view the app and see
the sate change of
machine 90.
[00129] In another example, medical fluid delivery machine 90b may
have been
preprogrammed to begin treatment at 3:00 PM. Medical fluid delivery machine
90b may need
three hours for self-test and disinfection. Patient 12a or clinician 112a
therefore needs to be at
machine 90b by noon to start pre-treatment. In an embodiment, patient 12a or
clinician 112a
makes a setting on machine 90b as to how soon before the three hour
preparation time that the
patient 12a or clinician 112a should be notified or alerted, e.g., two hours.
So in this example,
machine 90b may generate a code at 10:00 AM and send the code to middleware
software stored
on system hub 120. Middleware software then translates the code into a
message, e.g., using a
look-up table, such as, "treatment preparation needs to start in two hours"
and cause the app
downloaded onto mobile communication device 200b of patient 12b or clinician
112b to display
the message. The app may again be programmed to provide a visual identifier
along with the
message, such as, a countdown timer that counts down from one-hundred-twenty
minutes to a
timeout at zero. The app may also provide any one or more of an audio alert,
such as a "ding"
sound, and/or a haptic alert, such as a vibration, which prompt patient 12b or
clinician 112b to
view the app and see the treatment preparation notification. The app may also
be programmed to
repeat the "ding" sound and/or haptic feedback at preprogrammed intervals
during the countdown
period, e.g., at an hour and at thirty minutes.
[00130] In addition or alternatively to providing the app on the
user's
communication device 200b, it is contemplated for the middleware software at
system hub 120 to
convert the code from machine 90b into a message that is lodged onto device
200's native task
tracking feature, such as its calendar application. Most smartphone devices
200, for example, are
provided with a calendar that separates each day into time segments, such as
hours. Here, the
message converted by middleware software of system hub 120 may be programmed
to access the
calendar of authorized communication device 200b and to populate the
appropriate time segment
27
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
of the appropriate day with the appropriate information. In the above example,
for the appropriate
day, the native calendar software application will have its 10:0:00 AM
timeslot filled with a
message, such as, "treatment preparation needs to start in two hours". An
audio and/or haptic
feedback signal may be provided to notify patient 12 or clinician 112 about
the calendar entry.
[00131] It should be appreciated that machines 90a and 90b,
middleware software
at central server 120, and communication devices 200a and 200b, may be
programmed and
operated as described above to provide any desired message to patients 12a,12b
and/or clinicians
112a, 112b and are not limited to the messages described herein. For example,
patients 12a, 12b
and/or clinicians 112a, 112b may be likewise informed at the end of
disinfection with an
accompanying countdown timer that treatment needs to start within the
countdown time to avoid
having to re-disinfect machine 90a, 90b.
[00132] Referring now to Fig. 5, a system 110b of the present
disclosure is
illustrated. System 110b in the illustrated embodiment operates with system 10
described above,
including connectivity server 118, system hub 120, service portal 130,
enterprise resource planning
system 140, web portal 150, and business intelligence portal 160, which are
illustrated in Fig. 5 as
being part of a cloud environment, but may be located alternatively at one or
more dedicated server.
Other components of system 10 not illustrated in Fig. 5 may also be part of
system 110a. A single
medical fluid delivery machine 90 is illustrated for ease of description,
however, multiple medical
fluid delivery machines 90 may be likewise connected to system 110b. Medical
fluid delivery
machine 90 may reside in the home of patient 12 (illustrated as being outside
the home) or in a
clinic 126a to 126n for clinician 112. Medical fluid delivery machine 90 is
connected again to
connectivity server 118 via secure managed connection 116 and an internet 52
connection using,
e.g., modem 102 in the illustrated embodiment.
[00133] System hub 120 in one embodiment stores middleware software
that may
be accessed by mobile communication device 200 (shown as single device for
ease, but multiple
devices 200 may be likewise connected to system 110b). Mobile communication
devices 200 in
Fig. 5 include all of the structure, functionality and alternatives disclosed
for devices 200a and
200b illustrated in Fig. 4, including being connected to internet 52. In Fig.
5, mobile
communication device 200 may, but does not have to, download a software
application ("app")
from middleware software stored on system hub 120 via their connection to
internet 52. The app
may be operated exactly as described above in connection with Fig. 4,
including middleware
28
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
software converting a coded message from machine 90 into a format presentable
on the app.
Alternatively or additionally, middleware software stored on system hub 120
may be able to
convert the code from machine 90 into a message that is lodged onto mobile
communication device
200's native task tracking feature, such as its calendar application, in any
of the ways described in
Fig 4.
[00134] Further alternatively or additionally, system 110b includes
a cellular
network 210 that interfaces between middleware software, e.g., stored at
system hub 120, and
mobile communication device 200. Cellular network 210 may include a network of
cellular phone
towers operating using radio waves and/or employ a satellite. Communication
protocols suitable
for use with cellular network 210 of system 110b may be long range protocols,
such as (i) the
"worldwide interoperability for microwave access" ("WiMAX") protocol; and (ii)
the "global
system for mobile communications" ("GSM") protocol, which is a widespread long-
range wireless
protocol enabling data communication to the many of the world's cellular
telephones. Network
210 may alternatively or additionally employ a medium range protocol, such as
a wireless local
area network ("WLAN"), which can be a protocol that is part of the Institute
of Electrical &
Electronics Engineers ("IEEE") 802.11 standard, such as (i) IEEE 802.11a, (ii)
IEEE 802.11b, (iii)
WEE 802.11g, or (iv) 802.11n. Other suitable cellular technologies may include
CDMA, AMPS
(analog), General Packet Radio Service ("GPRS"), cdmaOne, CDMA2000, Evolution-
Data
Optimized ("EV-DO"), Enhanced Data Rates for GSM Evolution ("EDGE"), Universal
Mobile
Telecommunications System ("UMT 5"), Digital Enhanced Cordless
Telecommunications
("DECT"), Digital AMPS ("IS-136/TDMA"), and Integrated Digital Enhanced
Network
("iDEN").
[00135] Mobile communication devices 200 communicate with cellular
network
210 via any of the ways known to those of skill, e.g., via Short Messaging
Service ("SMS") or
Multimedia Messaging Service ("MIMS") protocols. Middleware software at system
hub 120 may
communicate with cellular network 210 in a number of ways. In one example, the
phone numbers
and carriers of users 12, 112 (any or all of patient 12, patient's at home
care partner, patient's
clinician 112) are associated, e.g., via a look-up table at middleware
software, with a specific
machine 90. When a message/code from a specific machine 90 is received by
middleware,
middleware software may be programmed to send an email to [user phone
number]@[carrier].net.
For example, if patient 001's phone number is (555) 555-5555 and patient 001's
carrier is
29
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
AT&TTm, when patient 001's machine 90 sends a message to middleware software
of system hub
120, upon receipt, middleware software 120 is programmed to relay an email to
5555555555@att.net, which is received by patient 001's mobile communication
device 200 as a
text message. Those of skill in the art understand that there are multiple
websites devoted to
informing how to email to a text message, outlining the specifics required by
different carriers.
[00136] Middleware software stores each of the telephone numbers of
each of
mobile communication devices 200 and matches each of those numbers with a
machine 90. When
an event code is sent from a machine 90 to middleware software as has been
described above,
middleware software locates the telephone number of the mobile communication
device 200
associated with that machine, converts the code to an appropriate message,
e.g., using a look-up
table as described above, and sends the converted message to the recalled
telephone number. It is
contemplated that multiple communication devices 200 may be associated with
the same medical
fluid delivery machine 90. For example, in any of clinics 126a to 126n,
multiple doctor, nurse
and/or clinician telephone numbers may be associated with the same machine 90.
In a home
environment, the telephone numbers for patient 12 and his or her clinician
and/or caregiver
assistant may be associated with the same machine 90.
[00137] Likewise, a telephone number for a mobile communication
device 200 may
be associated with multiple medical fluid delivery machines 90. For example,
in any of clinics
126a to 126n, a single nurse may monitor multiple machines 90. If an event
occurs to any of those
machines during the nurse's shift, the nurse may be notified via a cellular
message sent to the
nurse's mobile communication device 200. This scenario is described in detail
below in
connection with Figs. 7 to 9.
[00138] The cellular messages may convey in formation concerning any
of the same
events discussed above for the software app and calendar updating modes of
populating mobile
communication devices 200 with information. For example, medical fluid
delivery machine 90
may have just completed its automated self-test routine and is now ready to
run a disinfection
procedure. Machine 90 may generate a code identifying this state and send it
to middleware
software stored on system hub 120. Middleware software then translates the
code into a message,
e.g., using a look-up table, such as, "self-test completed, ready for
disinfection" and cause the
cellular output routine discussed above for example to send a text message to
mobile
communication device 200 of patient 12 or clinician 112 to display the
message. In an alternative
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
embodiment, a code is not needed and machine 90 instead sends an actual text
string, which
middleware software forwards on to the mobile communication device 200 as a
text message via
the cellular output routine discussed above for example. As is known, the
receipt of the text
message on communication device 200 may be accompanied with an audio, e.g.,
"ding" sound,
and/or a haptic alert, such as a vibration, which prompt patient 12 or
clinician 112 to view the
message.
[00139] In another example, medical fluid delivery machine 90 may
have been
preprogrammed to begin treatment at 3:00 PM. Medical fluid delivery machine 90
may again need
three hours for self-test and disinfection. Patient 12 or clinician 112
therefore needs to be at
machine 90 by noon to start pre-treatment. In an embodiment, patient 12 or
clinician 112 makes
a setting on machine 90 as to how soon before the three hour preparation time
that patient 12 or
clinician 112 should be notified or alerted, e.g., two hours. Here, machine 90
generates a code at
10:00 AM and sends the code to middleware software stored on system hub 120.
Middleware
software then translates the code into a message, e.g., using a look-up table,
such as, "treatment
preparation needs to start in two hours" and cause the cellular output routine
discussed above for
example to send a text message to mobile communication device 200 of patient
12 or clinician 112
to display the message, e.g., along with an audio alert, such as a "ding"
sound, and/or a haptic
alert, such as a vibration, which prompt patient 12 or clinician 112 to view
the notification.
[00140] It should be appreciated that machine 90, middleware
software at central
server 120, and communication device 200 may be programmed and operated as
described above
to provide any desired message to patients 12 and/or clinicians 112 using
cellular network 210
alternatively or additionally. For example, patients 12 and/or clinicians 112
may be likewise
informed at the end of disinfection that treatment needs to start within the
countdown time to avoid
having to re-disinfect machine 90. It should also be appreciated that the
updating of the native
task tracking features, such as the calendar application of communication
device 200 may be done
over an internet connection or via cellular network 210 illustrated in Fig. 5.
[00141] Referring now to Fig. 6, a system 110c of the present
disclosure is
illustrated. System 110c in the illustrated embodiment operates with system 10
described above,
including connectivity server 118, system hub 120, service portal 130,
enterprise resource planning
system 140, web portal 150, and business intelligence portal 160, which are
illustrated in Fig. 6 as
being part of a cloud environment, but may be located alternatively at one or
more dedicated server.
31
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
Other components of system 10 not illustrated in Fig. 6 may also be part of
system 110a. A single
medical fluid delivery machine 90 is illustrated for ease of description,
however, multiple medical
fluid delivery machines 90 may be likewise connected to system 110b. Medical
fluid delivery
machine 90 may reside in the home of patient 12 (illustrated as being outside
the home) or in a
clinic 126a to 126n for clinician 112. Medical fluid delivery machine 90 is
connected again to
connectivity server 118 via secure managed connection 116 and an internet 52
connection using,
e.g., modem 102 in the illustrated embodiment. In Fig. 6, connectivity server
118 and secure
managed connection 116 are used for two-way communication.
[00142] System hub 120 in one embodiment stores middleware software
that may
be accessed by mobile communication device 200 (shown as single device for
ease, but multiple
devices 200 may be likewise connected to system 110b). Mobile communication
devices 200 in
Fig. 6 include all of the structure, functionality and alternatives disclosed
for devices 200a and
200b illustrated in Fig. 4, including being connected to internet 52. In Fig.
6, mobile
communication device 200 may, but does not have to, download a software
application ("app")
from middleware software stored on system hub 120 via their connection to
internet 52. The app
may be operated exactly as described above in connection with Fig. 4,
including middleware
software converting a coded message from machine 90 into a format presentable
on the app.
Alternatively or additionally, middleware software stored on system hub 120
may be able to
convert the code from machine 90 into a message that is lodged onto mobile
communication device
200's native task tracking feature, such as its calendar application, in any
of the ways described in
Fig 4. The calendar application may alternatively be updated via a cellular
network 210 (illustrated
as an alternative via dashed lead lines in Fig. 6) discussed above in
connection with Fig. 5.
[00143] Fig. 6 illustrates that communication may be two-way between
medical
fluid delivery machines 90 and mobile communication devices 210. Communication
between
mobile communication devices 210 and middleware software at server computer
120 may be via
internet 52 and/or cellular network 210. Communication between middleware
software at server
computer 120 may be via connectivity server 118 via secure managed connection
116 as described
in detail above.
[00144] As discussed above, home therapy machine 90 connects to
connectivity
server 118 via its onboard connectivity agent 114, which in one embodiment is
turned off during
treatment (may or may not be turned off during post-treatment disinfection),
e.g., while machine
32
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
90 and its peripherals are functioning. This prevents home therapy machine 90
from
communicating with any entity and sending or receiving data during treatment
and disinfection or
when machine 90 is live or running. It is contemplated that the communication
via systems 110a
to 110c be protected in the same way. For instance, suppose that a particular
machine 90 is set via
the middleware software to communicate with both patient 12 and clinician 112.
Here, if patient
is being treated by machine 90, it is contemplated that connectivity agent 114
be shut off so that
clinician 112 at that time cannot receive notifications from or send commands
to that machine 90.
In an alternative embodiment, clinician 112 may be able to receive
notifications machine 90 during
treatment.
[00145] Determining when to disconnect connectivity agent 114 (no
communication) may be dependent upon what or how many machine states that
systems 110a to
110c desire to communicate to mobile communication devices 200. For instance,
suppose that it
is only desired to inform patient 12 or clinician 112 two hours before
treatment preparation that
the patient 12 or clinician 112 needs to return to machine 90 to start
treatment preparation. Here,
connectivity agent 114 may be turned off as soon as patient 12 or clinician
112 begins the first
treatment preparation step, e.g., running self-test routine.
[00146] In another example, it may be desired for machine 90 to run
the self-test
routine automatically at some preset time before treatment is set to start.
Machine 90 notifies
patient 12 or clinician 112 when it is time to begin disinfection. Here,
connectivity agent 114 may
be disconnected once patient 12 or clinician 112 begins the machine
disinfection. In a further
example, it may be desired for machine 90 to notify patient 12 when
disinfection is complete so
that the patient begins treatment within a certain amount of time from the end
of disinfection, so
that disinfection does not need to be repeated. Here, connectivity agent 114
may be disconnected
once patient 12 or clinician 112 begins treatment, e.g., upon the beginning of
prime in which the
patient is still yet to be connected to treatment lines, e.g., to arterial
line 14 or venous line 16.
[00147] System 110c allows patient 12 or clinician 112 to begin any
of the above
actions (and others not expressly described herein) remotely. Patient 12 or
clinician 112 may for
example select an icon on the app displayed on mobile communication device 200
to begin, e.g.,
the self-test routine or a disinfection procedure. The selection of the icon
is transmitted over
internet 52 to middleware software. Middleware software may then for example
translate, e.g.,
via a look-up table, the icon selection into an action code that is sent via
connectivity server 118
33
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
and secure managed connection 116 to machine 90 whose connectivity agent 114
is on, allowing
the action code for the selected action to be sent to the machine's ACPU 50,
which begins the
performance of the selected action.
[00148] In an alternative embodiment, patient 12 or clinician 112
may for example
enters a known code in a text message selecting a particular action to be
performed at machine 90,
e.g., the self-test routine or a disinfection procedure. The code may be a
suggestive code, such as
"self-test" or "disinfection". The text message is sent via cellular network
210 to middleware
software at system hub 120. Middleware software converts, e.g., via a look-up
table, the texted
code into an action code for the selected action. Or, the code entered by
patient 12 or clinician
112 may be the action code, so that no conversion is needed. In either case,
the action code is sent
via connectivity server 118 and secure managed connection 116 to machine 90
whose connectivity
agent 114 is on, allowing the action code for the selected action to be sent
to the machine's ACPU
50, which begins the performance of the selected action.
[00149] Fig. 6 illustrates the following example seven step
sequence. In step 1,
medical fluid delivery machine 90 sends a message to middleware software
application at system
hub 120 indicating that the machine is ready for patient 12 to initiate the
start of machine 90's,
e.g., two hour, automated self-test routine. In step 2, the middleware
software application at
system hub 120 sends a corresponding, e.g., translated, message to the
patient's mobile
communication device 200 indicating that machine 90 is ready for patient 12 to
initiate the start of
the automated self-test routine.
[00150] In step 3, a custom app downloaded to the patient's mobile
communication
device 200 alerts patient 12 via an audio, visual and/or haptic alert and
associated message that
patient 12's machine 90 is ready for the patient to initiate the start of the,
e.g., two hour, automated
self-test routine. In step 4, patient 12 uses the custom app on mobile
communication device 200
to confirm that machine 90 should begin its automated self-test routine.
[00151] In step 5, the patient's mobile communication device 200
sends a message
to middleware software application at system hub 120 confirming that it is
desired for the patient's
machine 90 to begin its automated self-test routine. In step 6, middleware
software application at
system hub 120 sends (e.g., converts and sends) a message to machine 90
indicating that patient
12 has confirmed that machine 90 is to begin its automated self-test routine.
In step 7, machine
90 begins and performs its automated self-test routine.
34
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00152] Once the self-test is performed, it is contemplated for
system 110c to
perform the same steps 1 to seven discussed above, except that the action is
now a disinfection
procedure instead of the automated self-test routine. Here, the custom app
downloaded to the
patient's mobile communication device 200 may display a countdown timer to
patient 12
reminding the patient how much time the patient has to return to machine 90 to
begin treatment.
It should be appreciated that different types of medical fluid delivery
machines may have different
one, two, three or more actions that patient 12 or clinician 112 may perform
before treatment
begins.
[00153] Regarding systems 110a to 110c, it is contemplated to
program the app on
mobile communication device 200 to be configurable by the user to select which
type of
notification that the user would like to receive on their device 200, e.g.,
via the app itself, via text
message, and/or via calendar notification. System hub 120 may in one
embodiment send all
notification types, where mobile communication device 200 ignores the
communication types that
the user has disabled. System hub 120 in another embodiment stores the user's
preferences and
only sends information in selected notification types.
C. Clinician Device/Server Embodiments
[00154] Referring now to Figs. 7 to 9, one embodiment of a system
110d having a
clinician-based downloadable software application ("app") 230 for a doctor's,
clinician's or
nurse's mobile communication device or server 200 is illustrated on screens
232 to 236. As
discussed above, mobile communication device 200 may be that of a patient 12
or that of a
doctor/nurse/clinician 112. Screens 232 to 236 of Figs. 7 to 9 illustrate that
app 230 may be used
in a clinic or hospital 126a to 126n, where a nurse, for example, is
responsible for multiple
machines 90a to 90n. Machines 90a to 90n may again be hemodialysis machines,
peritoneal
dialysis machines, CRRT machines, drug and/or nutritional fluid delivery
machines and
combinations thereof
[00155] Screen 232 illustrates that app 230 may monitor and, if
desired, control
multiple machines 90. In the illustrated embodiment, machines 90a to 90n are
each represented
by a dedicated icon 190a to 190n displayed on screen 232 of app 230. Icons
190a to 190n in the
illustrated embodiment are ordered the same on screens 232 to 236 as machines
90a to 90n are
ordered in clinic 126a to 126c to help orient doctor/nurse/clinician 112.
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00156] It is contemplated that app 230 operates with system hub 120
as has been
discussed herein, where system hub 120 is remote from clinic or hospital 126a
to 126n and is
maintained for example by a manufacturer of one or more of machines 90a to
90n. App 230 may
for example be developed initially at product development 128 illustrated in
Fig. 1. App 230 may
then be sent from product development 128 to system hub 120 via service portal
130 as illustrated
in Figs. 1 and 7. Any nurse, clinician or doctor 112 authorized to download
app 230 may do so
from system hub 120. Thereafter, system hub 120 maintains middleware software
to operate with
app 230 in the manners described above in systems 110a to 110c.
[00157] In an alternative embodiment, clinics 126a to 126n may
maintain their own
local area networks, each operating with a local system hub 220. App 230 may
again be developed
by product development 128 (Fig. 1) and delivered via service portal 130 to a
local system hub
220 of a clinic 126a to 126n operating with overall system 10. Each nurse,
clinician or doctor 112
authorized to download app 230 does so from local system hub 220. Thereafter,
local system hub
220 maintains middleware software to operate with app 230 in the manners
described above for
system hub 120 in systems 110a to 110c. In a further alternative embodiment,
app 230 may be
developed by clinic 126a to 126n and stored on its local system hub 220.
[00158] Middleware software of system hub 120 or local system hub
220 updates
the status of each machine 90a to 90n. Nurse, clinician or doctor 112 may
select an icon 190a to
190n at any time to see the current status of each machine 90a to 90n, e.g.,
"at rest", "self-test,
"disinfect", or "treating patient" as illustrated in screen 234 of Fig. 8.
Other status markers are
contemplated and may be different for different types of machines. Nurse,
clinician or doctor 112
may then select any of "at rest", "self-test, "disinfect", or "treating
patient" to return to the home
icons 190a to 190n as illustrated in Fig. 9.
[00159] As discussed above, it is contemplated to turn connectivity
agent 114 of
each machine 90 off when the machine is running and in particular when a
patient 12 is connected
to the machine. It is also contemplated however to allow connectivity agent
114 of each machine
90a to 90n of clinics 126a to 126n to remain on until the end of disinfection,
so that middleware
software at system hub 120 or local system hub 220 may receive from each
machine 90a to 90n a
status change to "treating patient". In addition, because each machine 90a to
90n knows its
scheduled treatment duration, the machines may also send to middleware
software the scheduled
duration, which then sends the duration in the form of a countdown timer along
with the status
36
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
change for "treating patient". Here then, when nurse, clinician or doctor 112
selects "treating
patient" in Fig. 8, they are able to see a countdown timer showing the time of
treatment remaining
as illustrated in Fig. 9.
[00160] It is contemplated that for the countdown timers,
connectivity agent 114
allows machines 90a to 90n to send time remaining data to system hub 120, so
that app 230 may
display the actual time remaining for each machine 90 undergoing a timed
process. App 230 takes
into account alarms or other delays that machines 90 may experience. During an
alarm situation,
the corresponding icon 190a to 190f may display a message such as "alarm" or
"safe mode".
Nurse, clinician or doctor 112 may then select the countdown time in Fig. 9 to
return to the home
icons 190c, 190d, and 190h illustrated in Fig. 7.
[00161] Nurse, clinician or doctor 112 may also toggle an alert
on/off icon 238 to
either allow or not allow status changes for machines 90a to 90n to be alerted
visually, audibly
and/or haptically. If alert on/off icon 238 is switched to "on", app 230 of
mobile communication
device 200 will provide a visual, audible and/or haptic alert each time a
machine's status changes,
e.g., (i) self-test started, (ii) self-test completed, (iii) disinfection
started, (iv) disinfection
completed, (v) treatment started, (vi) treatment completed. In an embodiment,
codes for (i) to (v)
are sent via machines 90a to 90n though secure managed connection 116,
connectivity server 118
and system hub 120 or local system hub 220 to be translated by middleware
software and
forwarded to app 230, which updates the appropriate icon 190. In various
embodiments, "(vi)
treatment completed" may be (a) sent via machines 90a to 90n with connectivity
agent 114
activated or (b) inferred when the countdown timer of the appropriate icon
190a to 190n expires,
and where connectivity agent 114 may still be off
[00162] If alert on/off icon 238 is switched to off, e.g., if nurse,
clinician or doctor
112 does not want to be interrupted at a given moment, icons 190a to 190n are
still updated as
described above but audible and/or haptic alerts are not provided. Nurse,
clinician or doctor 112
may still actively view the status of each machine 90a to 90n, however, by
selecting the associated
icon 190a to 190n.
[00163] Screens 232 to 236 illustrate action buttons 240a and 240b
(referred to
herein collectively to action buttons 240 or generally individually as action
button 240). Any
number of action buttons 240 may be provided for any type of pretreatment
action needed for any
modality, e.g., hemodialysis, peritoneal dialysis, CRRT, drug and/or
nutritional fluid delivery. In
37
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
the illustrated embodiment, action buttons 240a is for starting a self-test
for machines 90, while
action button 240b is for starting a disinfection sequence for machines 90.
[00164] In one embodiment, when self-test button 240a is selected,
any machine 90a
to 90n capable at that time of performing a self-test has its corresponding
icon 190a to 190n
highlighted. Nurse, clinician or doctor 112 selects whichever icon(s) 190 for
the machine(s) 90
that the nurse, clinician or doctor 112 wishes to perform a self-test. That
selected icon(s) 190 may
then turn into a "confirm" button, which the nurse, clinician or doctor 112
has to press again to
cause the selected machine(s) 90 to perform its self-test. App 230 of mobile
communication device
200 then sends a corresponding self-test code to middleware software at system
hub 120 or local
system hub 220, which converts, if needed, the self-test code into a self-test
initiation command,
which is sent via connectivity server 118 over secure managed connection 116
to the connectivity
agent 114 of the selected machine 90, which transfers the command to the
machine's ACPU 50,
which in turn initiates the self-test.
[00165] In the illustrated embodiment, when disinfection button 240b
is selected,
any machine 90a to 90n capable at that time of performing disinfection has its
corresponding icon
190a to 190n highlighted. Nurse, clinician or doctor 112 selects whichever
icon(s) 190 for the
machine(s) 90 that the nurse, clinician or doctor 112 wishes to perform
disinfection. That selected
icon(s) 190 may again turn into a "confirm" button, which the nurse, clinician
or doctor 112 has
to press again to cause the selected machine(s) 90 to perform its
disinfection. App 230 of mobile
communication device 200 then sends a corresponding disinfection code to
middleware software
at system hub 120 or local system hub 220, which converts, if needed, the
disinfection code into a
disinfection initiation command, which is sent via connectivity server 118
over secure managed
connection 116 to the connectivity agent 114 of the selected machine 90, which
transfers the
command to the machine's ACPU 50, which in turn initiates disinfection.
[00166] The procedure just described for action buttons 240 may also
be
implemented in system 110c and be implemented for other machine commands,
which may vary
depending on the type of machine 90. It is also contemplated that a clinic
126a may decide that it
is safe enough with one or more nurse, clinician or doctor 112 present at the
clinic to leave
connectivity agent 114 on during treatment or a portion of treatment. In such
case, nurse, clinician
or doctor 112 may control in-treatment activities for machines 90. For
example, nurse, clinician
or doctor 112 may receive and respond to alarms/alerts via app 230 at mobile
connection device
38
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
200, start and stop pumps and other facets of treatment, start and stop
disinfection, start and stop
priming, and the like.
[00167] Each of systems 110a to 110d operates with some form of
addressing. As
discussed above, connectivity server 118 is provided in one embodiment to
ensure that data is
delivered in the proper form to the proper machine 90, and that data from a
machine 90 is delivered
in its proper form to the proper destination. In one embodiment, when a
machine 90 sends data to
system hub 120 or local system hub 220 for delivery to a mobile communication
device 200, the
data is provided with a machine identifier that identifies the machine 90 from
which the data was
sent. Connectivity server 118 knows each mobile communication device 200 to
which a particular
machine's data belongs and tells system hub 120 or local system hub 220 which
communication
devices 200 are to receive the data. System hub 120 or local system hub 220
may then convert the
data as has been discussed herein. When sending the, e.g., converted, data,
system hub 120 or
local system hub 220 may strip the machine identifier from the data since it
is not needed anymore.
In system 110d, however, the machine identifier may be delivered along with
the, e.g., converted,
data so that app 230 knows which icon 190a to 190n to populate with the new
data. Here, app 230
may strip the machine identifier once it is not needed anymore.
[00168] In one embodiment, when a mobile communication device 200
sends data
to system hub 120 or local system hub 220 for delivery to a machine 90, the
data is provided with
a mobile communication device 200 identifier that identifies the mobile
communication device
200 from which the data was sent. System hub 120 or local system hub 220 may
or may not
convert the data from mobile communication device 200 as discussed above, but
in either case,
the mobile communication device 200 identifier is maintained for connectivity
server 118.
Connectivity server 118 knows which machine 90 is to receive the, e.g.,
converted, data for each
mobile communication device 200, and sends the, e.g., converted, data to each
associated
communication device 200. Connectivity server 118 may strip the mobile
communication device
200 identifier from the data once delivered to machine 90 since it is no
longer needed.
[00169] App 230 as described above allows nurse, clinician or doctor
112 to setup,
monitor and perhaps control treatment at a medical fluid delivery machine 90.
It is contemplated
to provide similar functionality via an app to patient 12 or a caregiver for
patient 12 at the patient's
home (dashed box in Fig. 1). Connectivity may be the same as shown in Figs. 7
to 9. However,
the setting is not a clinic 126a to 126n, but is instead the home or other non-
clinical location such
39
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
as a business or vacation location. In addition, there is typically only a
single machine 90, not
multiple machines 90a to 90n. It is possible however that a single patient 12
may be treated via
multiple machines 90, which could each be supported by the app as described
herein. If patient
12 is at home but away from machine 90, the app may provide valuable
information, such as
amount of time left for starting or completing a start-up procedure task, a
disinfection procedure
or a self-test routine. When the patient is being treated by machine 90,
he/she can see information
on its user interface 122, which may itself be a tablet as illustrated in Fig.
1. But there may also
be a caregiver that helps patient 12 at home during treatment, such as a
spouse, friend, or in-home
nurse. The caregiver benefits from the home app by receiving status updates,
start-up procedure
time remaining, disinfection time remaining, priming time remaining, treatment
time remaining,
information regarding whether or not patient 12 is connected to machine 90,
alerts, alarms, and the
like. The app in one embodiment requires a login and password associated with
the patient to be
entered before it can be downloaded to the caregiver's mobile communication
device 200, so that
only authorized people can view patient treatment data or information.
Patient Engagement Embodiments
[00170] The example medical fluid data transfer system 10 disclosed
above in
connection with Figs. 1 to 9 is configured to improve a patient's engagement
with a medical fluid
delivery treatment, such as a dialysis treatment, a renal failure treatment,
and/or a peritoneal
treatment. Fig. 10 shows an example medical fluid data transfer system 1000
that is similar to the
medical fluid data transfer system 10 discussed in connection with Figs. 1 to
9, accordingly to an
example embodiment of the present disclosure. The example medical fluid data
transfer system
1000 (e.g., a mobile platform) is configured to improve a patient's engagement
and/or compliance
with a treatment by increasing treatment transparency, providing a patient
features to control and
report information related to the treatment, and/or providing access to
educational material and/or
real-time assistance from a clinician.
[00171] The example medical fluid data transfer system 1000
includes, for example,
a personal mobile communication device 200a that is operated by a patient. The
medical fluid
data transfer system 1000 also includes a blood pressure monitor 104, a scale
106, and a home
therapy machine 90, which are similar to the respective devices discussed
above in connection
with Figs. 1 to 9. The personal mobile communication device 200a, the home
therapy machine
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
90, the blood pressure monitor 104, and the scale 106 may be located, for
example, at a patient's
home, a self-service clinic, and/or a serviced medical clinic.
[00172] The home therapy machine 90 may include any type of
hemodialysis
machine, peritoneal dialysis machine, CRRT machine, drug and/or nutritional
fluid delivery
machine, and combinations thereof. The home therapy machine 90 may provide,
for example
continuous cycling peritoneal dialysis ("CCPD"), tidal flow automated
peritoneal dialysis
("APD"), and continuous flow peritoneal dialysis ("CFPD"). The home therapy
machine 90 may
perform drain, fill, and dwell cycles automatically, typically while a patient
sleeps.
[00173] Peritoneal dialysis dialysate may include a solution or
mixture that includes
between 0.5% and 10% dextrose (or more generally glucose), preferably between
1.5% and 4.25%.
Peritoneal dialysis dialysate may include, for example, Dianeal , Physioneal ,
Nutrineal , and
Extraneal dialysates marketed by the assignee of the present disclosure. The
dialysate may
additionally or alternatively include a percentage of icodextrin.
[00174] In both hemodialysis and peritoneal dialysis, "sorbent"
technology can be
used to remove uremic toxins from waste dialysate, re-inject therapeutic
agents (such as ions
and/or glucose) into the treated fluid, and reuse that fluid to continue the
dialysis of the patient.
One commonly used sorbent is made from zirconium phosphate, which is used to
remove ammonia
generated from the hydrolysis of urea. Typically, a large quantity of sorbent
is necessary to remove
the ammonia generated during dialysis treatments.
[00175] The example weight scale 106 includes any device configured
to measure a
mass of a patient or treatment component. For example, the weight scale 106
may measure a
patient weight before, during, and/or after a renal failure therapy treatment.
Additionally or
alternatively, the weight scale 106 may measure a supply or drain bag for
tracking a renal failure
therapy. Specifically, weight scale 106 may be used to measure an amount of UF
removed or an
amount of fluid provided to a patient. The weight scale 106 may display a
digital value indicative
of weight. Alternatively, the weight scale 106 may display a physical scale or
dial that aligns with
a marker to indicate a measured weight. In some embodiments, the weight scale
106 may store
weight values before, during, and/or after treatment in separate windows such
that patient input is
required to view all the values when medical information is recorded.
[00176] The example blood pressure monitor 104 includes any device
configured to
measure a blood pressure and/or pulse of a patient. For example, the blood
pressure monitor 104
41
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
may measure a patient blood pressure before, during, and/or after a renal
failure therapy treatment.
The blood pressure monitor 104 may display a digital value indicative of a
patient's blood pressure.
Alternatively, blood pressure monitor 104 may display a physical scale with a
dial that aligns with
a numerical value to indicate a measured blood pressure. In some embodiments,
the blood pressure
monitor 104 may store blood pressure values before, during, and/or after
treatment in separate
windows such that patient input is required to view all the values when
medical information is
recorded. The blood pressure monitor 104 may be integrated with the home
therapy machine 90.
In another embodiment, the blood pressure monitor 104 may include a wearable
sensor, such as a
smartwatch or fitness-tracking device.
[00177] In addition to obtaining medical information (e.g., medical
device data)
from the medical devices 90, 104, and 106, example personal mobile
communication device 200a
may also obtain medical information from a patient and/or therapy consumable
item 1006. Fig.
shows an example of a consumable item 1006, which includes, for example, a
renal failure
therapy medical device filter, a disposable cassette, a blood line set, a drug
delivery line set, and a
container (e.g., a dialysis solution concentrate container, a blood
anticoagulant container, a
medication container, and/or a water purification container). The consumable
item 1006 may also
include a sorbent cartridge or any other disposable or material supply for a
medical treatment. The
consumable item may include an identifier 1008, which is configured to provide
medical
information in the form of consumable information or consumable data. For
example, the
identifier 1008 may include information identifying a type of consumable item,
a serial number,
and/or properties of the consumable item. In some instances, the consumable
item 1006 may also
include a label containing medical information such as chemical composition
properties. A patient
or clinician uses the personal mobile communication device 200a to record
images of the identifier
1008, images of labels on the consumable items 1006, and/or images of the
consumable item 1006
itself to document materials used during a treatment.
[00178] The personal mobile communication device 200a may be
communicatively
coupled to the home therapy machine 90, the blood pressure monitor 104, and/or
the scale 106 via
a wired connection (e.g., a USB connection) or a wireless connection (e.g.,
BluetoothTM, WiFiTM,
Zigbeeg, Z-Wave , wireless USB, or a wireless local area network ("WLAN")). In
other
examples, the personal mobile communication device 200a is not communicatively
coupled to any
one of the home therapy machine 90, the blood pressure monitor 104, and/or the
scale 106. In
42
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
these other examples, a patient may manually enter data displayed by the home
therapy machine
90, the blood pressure monitor 104, and/or the scale 106 into the personal
mobile communication
device 200a. Additionally or alternatively, in these other examples, a patient
may record one or
more images of data displayed by (or an identifier placed on) the home therapy
machine 90, the
blood pressure monitor 104, and/or the scale 106 using a camera 1004 of the
personal mobile
communication device 200a.
[00179] Collectively, the blood pressure monitor 104, and the scale
106 are referred
to as medical devices. It should be appreciated that the medical fluid data
transfer system 1000
may include additional medical devices such as an infusion pump (e.g., a
syringe pump, a linear
peristaltic pump, a large volume pump ("LVP"), an ambulatory pump, multi-
channel pump), an
oxygen sensor, a respiratory monitor, a glucose meter, a blood pressure
monitor, an
electrocardiogram ("ECG") monitor, a weight scale, and/or a heart rate
monitor. In other
examples, the medical fluid data transfer system 1000 may include fewer
medical devices and/or
medical devices integrated together (e.g., a blood pressure monitor 104
integrated with the home
therapy machine 90).
[00180] In some embodiments, the personal mobile communication
device 200a is
configured to record, display, and/or populate a patient medical record with
medical information
or data. As disclosed herein, medical information or data includes medical
device data and patient
data, which may refer to data or information created by, generated by, or
otherwise related to
medical devices, patients, and/or consumable items used by medical devices.
For instance, the
medical information includes prescription or programming information used by a
medical device
to administer a treatment. Medical information also includes sensed data such
as fluid pressure,
flowrate, conductivity, concentration, temperature and patient data. As
provided herein, patient
data (e.g., vital sign data) includes sensed patient physiological information
such as patient blood
pressure, weight, heart rate, etc. The medical information may also include
subjective information,
such as information regarding how a patient is feeling (e.g., tired, fatigued,
happy, excited, etc.).
The medical information may be displayable on a screen of a medical device,
provided by a
physical dial or display of a medical device, or printed on a label attached
or a medical device.
Accordingly, medical device data or medical information includes a medical
device setting, a
medical device reading, and/or a patient reading.
43
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00181] Medical information also refers to information contained on
an identifier
attached to a patient or treatment consumable item. Specifically, the medical
information may
include information conveyed by an identifier of a patient provided on a
patient wristband for
identifying a patient. Medical information also includes information regarding
a consumable item,
which may identify a consumable item type, a consumable item model, and/or
properties of a
consumable item, such as a level of dextrose in a supply bag of renal failure
therapy solution.
[00182] Treatment data or treatment information refers to data
generated and/or
transmitted by the home therapy machine 90 of Figs. 1 to 10. Treatment
information may include
a volume of fluid infused, an amount of ultrafiltration UF removed from a
patient, and/or a
treatment time. The treatment information may also include alarms, alerts,
and/or diagnostic
information generated by the home therapy machine 90. Generally, the treatment
information is
transmitted from a home therapy machine 90 to a clinician server 200b. In some
embodiments,
the treatment information may be recorded in a personal mobile communication
device 200a. In
these embodiments, the treatment information may be referred to as and/or be
included/processed
as medical information.
[00183] As shown in Fig. 10, some of all of the medical devices 90,
104, and 106
may include an identifier 1012 configured to store a unique identification
number. Identifier 1012
may code, for example, an assigned device number, a serial number, a hardware
number, a model
number, and/or a device type of the respective medical devices 90, 104, and
106. For example,
identifier 1012b of the home therapy machine 90 may store an assigned device
number. The
personal mobile communication device 200a reads identifier 1012b to determine,
for example, a
medical device type for subsequent analysis and identification of medical
information in images
recorded from a screen of the machine 90. In some embodiments, the identifier
1012 may more
generally indicate a model or type of a medical device. For example,
identifier 1012c may indicate
that device 106 is a weight scale and/or indicate a model number of a weight
scale.
[00184] The identifier 1012 may include machine readable markings
such as, for
example, a barcode or a quick-response ("QR") code. The identifier 1012 may
also include
human-readable text, such as a serial number, asset number, or hardware
number. In some
embodiments, identifier 1012 may be printed to an article physically attached
to a housing of the
respective medical devices 90, 104, and 106, such as identifier 1012b shown
for the home therapy
machine 90. Additionally or alternatively, the identifier 1012 may be
displayed on a screen of
44
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
some of all of the medical devices 90, 104, and 106. For example, a clinician
may select a control
interface to cause the home therapy machine 90 to display a window with the
identifier 1012b on
a screen. In yet other embodiments, the identifier 1012 may be included within
a radio frequency
("RF") microchip, such as an RFID chip or NFC chip.
[00185] The example medical devices 90, 104, and 106 may also
include one or
more control interface for providing control instructions. For example, the
home therapy machine
90 includes a control interface 1014. Control interfaces may include buttons,
a control panel, or a
touchscreen. A control interface may be configured to enable a user to
navigate to a certain
window or user interface on a screen of the respective medical devices 90,
104, and 106. Control
interfaces may also provide instructions for operating or controlling the
respective medical devices
90, 104, and 106.
[00186] As illustrated in Fig. 10, the example fluid data transfer
system 1000
includes a web portal 150, a system hub 120, and a clinician server 200b
(similar to the respective
devices discussed in connection with Figs. 1 to 9) to communicate with the
personal mobile
communication device 200a. The web portal 150, system hub 120, and/or the
clinician server 200b
transmit medical information from a patient's medical record (stored in a
clinician database 1010)
to the personal mobile communication device 200a. In addition, the web portal
150, system hub
120, and/or the clinician server 200b may provide the personal mobile
communication device 200a
access to educational material or a real-time help session with a clinician.
The example web portal
150, system hub 120, and/or the clinician server 200b is also configured to
enable the personal
mobile communication device 200a to provide medical information for populating
a patient's
medical record.
[00187] The example fluid data transfer system 1000 of Fig. 10 also
includes a
connectivity server 118, which is communicatively coupled to the home therapy
machine 90. As
discussed above in connection with Figs. 1 to 9, the connectivity server 118
provides bidirectional
communication between the home therapy machine 90 and the clinician server
200b and/or the
clinician database 1010. The home therapy machine 90 may connect to the
connectivity server
118 via the Internet.
[00188] In the illustrated example of Fig. 10, the example personal
mobile
communication device 200a includes a processor 1016 in communication with a
memory 1018
storing instructions. At least some of the instructions define or specify an
application 1002, that,
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
when executed by the processor 1016, cause the processor 1016 to provide
features that improve
a patient's engagement and/or compliance with a treatment. The processor 1016
may comprise
digital and analog circuity structured as a microprocessor, application
specific integrated circuit
("ASIC"), controller, etc. The memory 1018 includes a volatile or non-volatile
storage medium.
Further, the memory 1018 may include any solid state or disk storage medium.
[00189] As discussed in more detail below, the features of the
application 1002
include displays of treatment progress information, control of which treatment
programs are run
by the home therapy machine 90, recording of medical information, displays of
educational
material, and/or initiating a help session with a clinician. The application
1002 may be feature-
rich or feature-lite. A feature-rich application is configured for a
smartphone and utilizes native
graphics, touchscreen, and processing power of the personal mobile
communication device 200a.
A feature-lite application is configured to operate on a cellular phone that
has relatively less
sophisticated graphics and reduced processing power. The cellular phone may
also not include a
touchscreen or instead have a touchscreen with limited capability.
[00190] In some embodiments, the personal mobile communication
device 200a
may not include the application 1002. Instead, the personal mobile
communication device 200a
may use native or other installed applications to communicate with the
clinician server 200b. For
example, the personal mobile communication device 200a may use text, SMS, or
an email program
to communicate with the clinician server 1020.
[00191] The example clinician server 200b includes an application
1020, such as the
application 230 described in connection with Figs. 1 to 9. The application
1020 is configured to
communicate with the personal mobile communication device 200a to provide
improved patient
engagement and/or compliance. In some embodiments, the example application
1020 is
configured to facilitate the storage of medical information (recorded by the
personal mobile
communication device 200a) to one or more patient records in the database
1010. The application
1020 also may include one or more interfaces or application programming
interface ("API") to
provide treatment progress data and/or medical information to the application
1002 for display on
a display interface 1022 (e.g., a touchscreen) on the personal mobile
communication device 200a.
The application 1020 at the clinician server 200b may also provide educational
material upon
request from the application 1002 and/or facilitate a communication session
between the personal
mobile communication device 200a and a clinician's device 152.
46
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00192] In some instances, the application 1002 on the personal
mobile
communication device 200a and/or the application 1020 on the clinician server
200b is configured
to convert or otherwise provide medical information to the clinician database
1010 of other devices
in the medical fluid data transfer system 1000 that conforms to a Health-Level-
7 ("HL7") standard
(e.g., a medical standard). This conversion enables medical information to be
stored in an HL7
format regardless of a format when entered at the personal mobile
communication device 200a.
The application 1002 and/or the application 1020 may operate as a network
conduit to seamlessly
propagate relevant medical information from a medical device to a patient
medical record when
gaps in network or device connectivity exist.
[00193] Fig. 11 shows a diagram illustrative of operational modules
of the
application 1020 at the clinician server 200b of Fig. 10, according to an
embodiment of the present
disclosure. The application 1020 may include a registration module 1102
configured to register a
home therapy machine 90 and/or a personal mobile communication device 200a
with a particular
patient and/or patient medical records stored in the clinician database 1010.
As described in more
detail in connection with Fig. 12, registration may include determining a type
of the personal
mobile communication device 200a for the formatting of subsequent
communications.
[00194] The application 1020 may also include a data acquisition
module 1104
configured to receive treatment and/or medical information from the registered
home therapy
machine 90 and/or personal mobile communication device 200a. In addition, the
application 1020
may include a data access module 1106 to transmit or otherwise provide access
to medical
information stored in one or more patient records associated with a patient.
The application 1020
may further include an education module 1108 configured to provide access to
educational
material or help-documentation for a patient regarding their treatment and/or
operation of the home
therapy machine 90. Moreover, the application 1020 may include a treatment
control module 1110
that enables a patient (or clinician) to change (or modify) a treatment
program performed by the
home therapy machine 90. The application 1020 may additionally include an
assistance module
1112 that creates a real-time communication session between the personal
mobile communication
device 200a and a clinician device 152.
[00195] Each of the example modules 1102 to 1112 are configured to
improve a
patient's engagement with a medical fluid delivery treatment, thereby
increasing a patient's
compliance with a prescribed treatment. It should be appreciated that while
modules 1102 to 1112
47
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
are shown, the example application 1020 may include fewer or additional
modules. For instance,
in some embodiments, the education module 1108 and the assistance module 1112
may be omitted.
The sections below provide further description regarding each of the modules
1102 to 1112.
[00196] As discussed below, each of the modules 1102 to 1112 are
configured to
communicate with personal mobile communication device(s) 200a and/or clinician
device(s) 152.
In some embodiments, communications from personal mobile communication
device(s) 200a may
be addressed generally to the clinician server 200b, web portal 150,
connectivity server 118, and/or
system hub 120. The messages may be routed internally to the different modules
1102 to 1112
based on content and/or identifiers. For example, the application 1002 may
provide an identifier
in a header to specify which of the modules 1102 to 1112 is to receive the
message. For text-based
messages, a router at the clinician server 200b may determine a destination
module 1102 to 1112
based on message content or previous messages. For example, the router may
determine that a
received message corresponds to a message previously transmitted by the data
acquisition module
1104. Based on this correspondence, the router sends the received message for
processing via the
data acquisition module 1104.
A. Registration Module Embodiment
[00197] The example registration module 1102 is configured to
register the personal
mobile communication device 200a and/or the home therapy machine 90 with the
clinician server
200b and/or with a patient's medical records stored in the database 1010. The
example registration
module 1102 is configured to provide different types of registration, which is
used by the data
access module 1106 to determine how information is to be displayed to a
patient based on which
types of devices are registered. In addition, the data acquisition module 1104
determines how
treatment and/or medical information is to be acquired based on registration
information.
[00198] The registration module 1102 is configured to store
registration information
to a registration file stored in the clinician database 1010. The registration
file may specify, for
each patient or patient activation code ("PAC"), information indicative of a
registered a personal
mobile communication device 200a, information indicative of a registered home
therapy machine
90, and/or an indication as to whether the application 1002 is installed on
the personal mobile
communication device 200a. In some embodiments, the registration file (or
information from the
registration file) may be included within a patient's medical record.
48
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00199] In some embodiments, there are different registration
scenarios. For
example, a patient may register a personal mobile communication device 200a by
downloading
application 1002 while also registering a home therapy machine 90. In this
scenario, the
registration module 1102 records that the patient installed the application
1002 on the personal
mobile communication device 200a and (separately or through the application
1002) registered the
home therapy machine 90. Based on this registration, the data acquisition
module 1104 determines
that the application 1002 installed on the registered personal mobile
communication device 200a
is to guide or otherwise prompt the patient for acquiring medical information.
In addition, the data
acquisition module 1104 determines that treatment information is to be
received from the home
therapy machine 90 rather than via the personal mobile communication device
200a. Accordingly,
the data acquisition module 1104 may send a message to the application 1002
disabling a user
interface for acquiring treatment information from the home therapy machine 90
(but still enabling
a user interface for a manual exchange if one is prescribed for the patient).
If a home therapy
machine 90 is not registered, the data acquisition module 1104 causes the
application 1002 to
display a user interface to acquire treatment information from the home
therapy machine 90 in the
application 1002. Through registration, the data access module 1106 determines
that information
is to be displayed through the application 1002 and accordingly uses APIs
and/or other data read
components that are compatible or configured for the application 1002.
[00200] If a patient registers without downloading and/or installing
the application
1002, the data acquisition module 1104 determines that data acquisition is to
be prompted or
guided. For example, the data acquisition module 1104 may transmit text
messages and/or emails
to the registered personal mobile communication device 200a prompting a
patient for certain
medical information and/or treatment information (if a home therapy machine 90
has not been
registered). Information in the messages specifies which data is needed from
the patient. While
this remote guidance may be used for feature-lite personal mobile
communication devices 200a, it
may also be used for smartphones where a patient does not wish to download or
install the
application 1002 on a feature-rich device.
[00201] The data access module 1106 may also be configured to
display information
from a patient's medical record differently if the application 1002 is not
installed. For example,
instead of sending data that plugs into a well-defined feature-rich user
interface, the data access
module 1106 may render data in pictures that are transmitted via text to the
personal mobile
49
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
communication device 200a for viewing. Additionally or alternatively, the data
access module
1106 may transmit the stored medical record information as text to the
personal mobile
communication device 200a. Thus, even without the application 1002 installed,
the application
1020 at the clinician server 200b is configured to provide a patient access to
data using native
applications on the personal mobile communication device 200a.
[00202] Fig. 12 shows a diagram illustrative of communications
between the home
therapy machine 90, the personal mobile communication device 200a, and the
clinician server
200b of Figs. 10 and 11, according to an example embodiment of the present
disclosure. Initially,
the home therapy machine 90 registers with the clinician server 200b via the
registration module
1102 of Fig. 11. Otherwise, the clinician server 200b will not have
information as to which patient
record data from the home therapy machine 90 is to be stored. In some
embodiments, the home
therapy machine 90 is preprogrammed with destination address information for
the connectivity
server 118, the system hub 120, and/or the clinician server 200b. The
destination address may
include an interne protocol ("IP") address and/or a Hypertext Transfer (or
Transport) Protocol
("HTTP") address.
[00203] During setup, a patient (or clinician) enters a patient
activation code
("PAC") into the home therapy machine 90. The PAC is originally generated and
stored at the
clinician server 200b and is provided to the patient when the patient receives
the home therapy
machine 90. The PAC may include a patient identifier or other code that is
unique to the patient.
The registration module 1102 at the clinician server 200b stores the generated
PAC to one or more
patient records and/or registration file associated with the patient. After
entering the PAC, the
home therapy machine 90 generates and transmits a message 1202 that includes
the PAC. The
message may also include a hardware identifier of the home therapy machine 90
and/or an IP
address assigned to the home therapy machine 90. The home therapy machine 90
transmits the
message 1202 to the preprogrammed address, in this case, the connectivity
server 118. The
example connectivity server 118 relays the message 1202 to the system hub 120,
which routes the
message to the clinician server 200b. The registration module 1102 at the
clinician server 200b
registers the home therapy machine 90 with the patient using the PAC.
Registration includes, for
example, associating an identifier of the home therapy machine 90 with patient
medical records of
the patient. Registration may also include the clinician server 200b storing
the IP address of the
home therapy machine 90 to enable messages to be transmitted to the home
therapy machine 90.
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
After registration, the data acquisition module 1104 of the clinician server
200b stores treatment
information received from the home therapy machine 90 to one or more medical
records associated
with the patient.
[00204] The example personal mobile communication device 200a is
also
configured to register with the clinician server 200b via the registration
module 1102. To register,
the personal mobile communication device 200a may download or otherwise
receive application
1002 via one or more message 1204 from the clinician server 200b (or a third-
party application
store). In an embodiment, during registration of the home therapy machine 90,
the patient (or
clinician) may provide a phone number of the personal mobile communication
device 200a. The
registration module 1102 uses the phone number to transmit the message 1204 as
a text message.
The message may include a hyperlink to a location (e.g., the database 1010 or
a third-party website)
that provides the application 1002 for download to the personal mobile
communication device
200a. In other instances, the message 1204 may include an attached file, that
when executed,
installs the application 1002 on the personal mobile communication device
200a. Instead of
providing a phone number, the patient may instead provide an email address
during registration of
the home therapy machine 90. Accordingly, the message 1204 includes an email
message with a
file or hyperlink for installing the application 1002 on the personal mobile
communication device
200a.
[00205] In another embodiment, the message 1204 may enable a patient
to register
two different ways depending on a capability of their personal mobile
communication device 200a
and/or personal preference. The message 1204 includes text prompting a patient
to respond to the
text if they desire to register their personal mobile communication device
200a as a feature-lite
device. A reply to the message 1204 is provided via text message 1206, which
is routed to the
registration module 1102. In turn, the registration module 1102 registers the
personal mobile
communication device 200a with an indication the application 1002 is not
installed. In some
instances, the message 1204 may also include text or a hyperlink prompting the
patient to select
the hyperlink or otherwise obtain the application 1002 if desired. The message
1204 may also
provide a prompt or an option to select a device type, such as an Apple
device or an Android
device. During the registration process, the registration module 1102
registers the personal mobile
communication device 200a based on information provided by a patient and/or
read from the
personal mobile communication device 200a.
51
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00206] The patient may register the personal mobile communication
device 200a
after the application 1002 is installed. In an embodiment, to register, the
patient completes a
registration form or fields provided by the application 1002. The form or
fields are configured to
accept the patient's PAC. The form or fields may also include prompts for a
patient's name, email
address, home address, phone number, etc. Information from the form or fields
are sent in one or
more message 1206 to the web portal 150, which transmits the message 1206 to
the system hub
120 for routing to the clinician server 200b. The message 1206 may also
include device type
information of the personal mobile communication device 200a (as determined by
the application
1002).
[00207] In some instances, the application 1002 may be configured
with a
destination address of the web portal 150, which is used for transmitting the
messages. In other
instances, the messages 1206 may be provided to an API at the web portal 150
for registering the
application 1002 at the clinician server 200b. In yet other instances, the
application 1002 transmits
the message 1206 as one or more text messages or email messages to the web
portal 150 (where
the web portal 150 is assigned a telephone number, IP address, email address,
or other address).
The example registration module 1102 uses the PAC within the message 1206 to
register the
personal mobile communication device 200a with the medical records and/or
registration file
stored in the database 1010. At this point, both the home therapy machine 90
and the personal
mobile communication device 200a are registered to the same patient at the
clinician server 200b.
[00208] As mentioned above, in some embodiments, the personal mobile
communication device 200a may not include an application. In these
embodiments, the personal
mobile communication device 200a may communicate with the clinician server
200b via text
messages or through a web browser. Accordingly, the registration module 1102
of the clinician
server 200b may transmit, to the personal mobile communication device 200a, a
text message with
a hyperlink to a database or webpage for completing a registration.
Alternatively, the text message
may include a prompt for a patient to respond with their PAC. Providing the
PAC via either of
these registration processes enables the registration module 1102 to associate
the personal mobile
communication device 200a (e.g., a phone number, hardware address, or IP
address) with the
appropriate patient medical records and/or registration file.
52
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
B. Data Acquisition Module Embodiment
[00209] Fig. 12 also illustrates data communication between the home
therapy
machine 90, the personal mobile communication device 200a, and the clinician
server 200b.
During operation, the home therapy machine 90 generates treatment information
and status
information (e.g., medical information). As discussed above in connection with
Figs. 1 to 9, the
treatment information includes a volume of fluid infused, an amount of
ultrafiltration ("UF")
removed from a patient, and/or a treatment time. The status information
includes alarms, alerts,
or diagnostic information. Before or after a treatment, the home therapy
machine 90 generates
one or more message 1208 with the medical information, which is transmitted to
the connectivity
server 118. In turn, the connectivity server 118 routes the message 1208 to
the system hub 120,
which routes the message 1208 to the data acquisition module 1104 of the
clinician server 200b.
After receipt, the data acquisition module 1104 stores the medical information
to a designated
section of a patient's medical record (e.g., patient medical record 1302 of
Fig. 13). The message
1208 may include an identifier or address of the home therapy machine 90,
which is used by the
data acquisition module 1104 to locate the appropriate medical record in the
database 1010.
[00210] The example home therapy machine 90 may be configured to
code, label,
or otherwise identify, using metadata or other data identification technique,
the medical
information being transmitted. The coding or labeling enables the data
acquisition module 1104
(or interfaces at the server 200b) to determine a context of the medical
information for writing the
medical information to the appropriate fields of a patient record.
Additionally or alternatively, the
coding or labeling may also be stored to the record, which is later used for
searching and displaying
the medical information.
[00211] Fig. 12 also illustrates that the personal mobile
communication device 200a
transmits medical information or medical information to the clinician server
200b. As described
in more detail below, the personal mobile communication device 200a is
configured to acquire
medical information from medical devices, including devices 90, 104, and 106.
Acquiring medical
information may include receiving information manually entered by a patient,
via a wired or
wireless connection, and/or processing images recorded by camera 1004 of the
personal mobile
communication device 200a. The acquired medical information is packaged into
one or more
message 1210 and transmitted by the personal mobile communication device 200a
to the web
portal 150. The message 1210 may be a text message, an email message, or a web-
based message
53
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
(e.g., a HTTP message, an Extensible Markup Language ("XML") message, a
JavaScript Object
Notation ("JSON") payload, etc.). In some embodiments, the personal mobile
communication
device 200a may format the acquired medical information into one more data
fields prior to
transmission. Generally, the medical information acquired in the personal
mobile communication
device 200a is less structured than the medical information generated by the
home therapy machine
90. Accordingly, the personal mobile communication device 200a and/or the data
acquisition
module of the clinician server 200b performs at least some processing of the
medical information
to provide an appropriate context or structure for inclusion within a patient
medical record.
Examples of the processing performed are described below in further detail.
[00212] Fig. 13 illustrates an example patient data structure 1300
stored on the
clinician database 1010 of Fig. 10, according to an example embodiment of the
present disclosure.
The patient data structure 1300 includes separate patient records for
different patient. In the
illustrated example, the data structure 1300 includes a patient medical record
1302 for a patient
associated with identifier "DCM31913" and a patient medical record 1304 for a
patient associated
with identifier "GA1V141215". The data structure 1300 may include additional
patient medical
records.
[00213] As shown in Fig. 13, each of the medical records 1302 and
1304 includes
data fields that identify a patient, personal mobile communication device
200a, and home therapy
machine 90. For example, the records 1302 and 1304 include data fields for a
patient identifier,
personal mobile communication device 200a type, home therapy machine 90 type,
and home
therapy machine identifier (received via registration). The patient identifier
may correspond to the
PAC assigned to the patient. The records 1302 and 1304 may include additional
fields for a patient
name, address, gender, birthdate, etc. The records 1302 and 1304 may further
include fields for
network addresses of the personal mobile communication device 200a and/or the
home therapy
machine 90. In some embodiments, the data access module 1106 of the
application 1020 uses the
information in the device type field to determine how the treatment
information and medical
information is to be presented and/or transmitted to the personal mobile
communication device
200a.
[00214] Also as shown in Fig. 13, the medical records 1302 and 1304
include fields
for treatment and medical information. The data acquisition module 1104 stores
treatment
information to the records 1302 and 1304 received from the respective home
therapy machines 90.
54
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
In addition, the data acquisition module 1104 stores medical information to
the records 1302 and
1304 received from the respective personal mobile communication device 200a.
In some
embodiments, the data fields are further partitioned into individual fields
for data type. For
example, the records 1302 and 1304 may include treatment information fields
for a volume of fluid
infused, an amount of ultrafiltration ("UF") removed from a patient, and/or a
treatment time. The
records 1302 and 1304 may also include data fields for alerts or alarms
generated during a
treatment and/or alarms or alerts determined at the clinician server 200b
based on the treatment
information and/or medical information. The records 1302 and 1304 may further
include medical
information for a patient's weight and/or blood pressure recorded before,
during, and/or after a
treatment.
[00215] The treatment and/or medical device may be organized by
treatment,
date/time generated, and/or date/time received. In some embodiments, the
medical records 1302
and 1304 may store communications from a patient. The communications may
include pictures
or video recorded by the personal mobile communication device 200a related to
a treatment or a
question about a treatment. The communications may also include text messages,
emails, etc. sent
from the personal mobile communication device 200a regarding treatment
assistance. Moreover,
the communications may include information related to a request to change or
modify a treatment
from the personal mobile communication device 200a and/or a clinician device
152. Generally,
the medical records 1302 and 1304 are configured to store information to
improve a patient's
engagement with a treatment in addition to documenting results of a treatment
and information
related to a patient's engagement with the clinician server 200b for the
treatments.
[00216] The data received by the example data acquisition module
1104 varies based
on the source. For instance, treatment information received from the home
therapy machine 90 is
generally structured. In other words, the home therapy machine 90 is
configured to transmit
treatment information that is formatted for direct entry into one or more
fields of a patient's
medical records. In some embodiments, the home therapy machine 90 formats
messages for
transmission through an API (or otherwise accesses one or more API) of the
data acquisition
module 1104 for population of treatment information into the appropriate data
field. In contrast,
medical information recorded by a patient's personal mobile communication
device 200a may not
initially be structured for inclusion into the patient's medical record.
Different techniques may be
used by the application 1002 of the personal mobile communication device 200a
and/or the data
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
acquisition module 1104 of the clinician server 200b to process and/or format
medical information
for population to a medical record, as described in more detail below.
1. Manual Entry of Medical Information into an Application Embodiment
[00217] To receive structured information, the example application
1002 of the
personal mobile communication device 200a, in some embodiments, is configured
to display
prompts that indicate which medical information is needed for population, by a
patient, into patient
medical records. The example application 1002 may include a routine or
algorithm that specifies
which fields are to be displayed to prompt medical information from a patient.
In some
embodiments, the fields or screens in which the fields are displayed are
arranged and/or ordered
in relation to a medical fluid delivery treatment. The display of the fields
informs a patient
regarding which medical information is needed for a patient record.
[00218] Figs. 14 and 15 show diagrams illustrative of user
interfaces of the
application 1002 that enable a patient to enter medical information for
transmission to the data
acquisition module 1104. Specifically, user interface 1400 of Fig. 14 prompts
a patient for blood
pressure information while user interface 1500 of Fig. 15 prompts a patient
for medical fluid
delivery information (e.g., treatment information). It should be appreciated
that the application
1002 of the personal mobile communication device 200a may display other user
interface screens
that enable a patient to manually enter medical information. For example, the
application 1002
may display user interface screens for a blood glucose level, patient
temperature, patient weight,
etc.
[00219] In some embodiments, the patient may enter some of the
medical
information in the user interfaces 1400 or 1500 manually while obtaining other
medical
information wirelessly or recording some medical information via an image. In
these
configurations, the application 1002 may be configured to enable a patient to
select an information
entry source. A patient may enter medical information manually after selecting
a manual source
from a menu of available data entry methods or by default.
[00220] After receiving medical information manually entered by a
patient, the
example application 1002 transmits the medical information to the data
acquisition module 1104
for population into a patient's medical record. The application 1002 and user
interfaces 1400 and
1500 are configured such that the data fields for receiving medical
information are aligned with
56
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
data fields in one or more medical record. In some examples, the data fields
may correspond to
one or more APIs at the data acquisition module 1104 for writing medical
information directly into
the designed data field of a patient's medical record. The user interfaces
1400 and 1500 of the
application accordingly prompt a patient for medical information in a
structured manner such that
identification or formatting of the medical information is not needed or
necessary.
[00221] In some embodiments, the application 1002 may be configured
to display
the user interfaces 1400 and 1500 at designed times. For example, the
application 1002 may
include a calendar that includes times/days in which a treatment is scheduled.
At a designed time
before treatment (e.g., 5 minutes, 15 minutes, 30 minutes, etc.), the example
application 1002 is
configured to cause the personal mobile communication device 200a to display
the user interface
1400, prompting a patient for blood pressure medical information. The prompt
informs a patient
that a blood pressure measurement is needed before treatment begins. The
patient accordingly
uses blood pressure monitor 104 to take a blood pressure measurement. The
patient then enters
value(s) of the measurement into the user interface 1400 before treatment
begins.
[00222] In the illustrated embodiment, the user interface 1400
includes a systolic
blood pressure data field 1402, a diastolic blood pressure data field 1404,
and a pulse data field
1406. A patient may select the respective data field 1402, 1404, or 1406
causing the application
1002 to display a text entry feature to enter a value. The user interface 1400
also includes a field
1408 that enables a patient to specify if the blood pressure measurement was
performed standing
or sitting. Further, the user interface 1400 includes a data field 1410 that
enables a user to specify
a date/time the blood pressure measurement was performed.
[00223] In some examples, a patient may select any one of the fields
1402 to 1410
to receive more information about performing the corresponding measurement.
For example, a
patient may select the systolic blood pressure data field 1402, which causes
the application 1002
to display a tutorial instructing a patient how to perform a blood pressure
measurement and identify
a systolic blood pressure measurement value. The tutorial may include text,
text/pictures, an audio
recording, an animation, and/or a video. The tutorial may be stored locally as
part of the
application 1002 or be stored at the clinician server 200b for remote access
or streaming.
[00224] The user interface 1500 of Fig. 15 is configured to be
displayed by the
application 1002 when a patient is scheduled to perform a manual exchange
treatment and/or if a
home therapy machine 90 is not registered with the clinician server 200b. A
manual renal failure
57
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
exchange treatment requires that a patient connect containers of dialysis
fluid to their peritoneal
cavity for a dwell duration before allowing spent dialysis fluid to drain, all
without the assistance
of a machine. For a manual treatment, a patient needs to enter their manual
exchange medical
information to enable their records to reflect accurate treatment information.
[00225] The example application 1002 may also be configured to
display the user
interface 1500 when a patient does not register a home therapy machine 90.
During registration,
the registration module 1102 may transmit a message to the application 1002
indicative of whether
a home therapy machine 90 has been registered. If a home therapy machine has
not been
registered, the application 1002 may be configured to display the user
interface 1500 and/or other
user interfaces for acquiring treatment information that may normally be
transmitted by the home
therapy machine 90.
[00226] In the illustrated example, the user interface 1500 includes
a progression
through a treatment, including a solution phase, a drain phase, a fill phase,
a UF or dwell phase,
and a summary phase. The application 1002 may display a separate user
interface for each phase
prompting a patient to enter corresponding or requested treatment information.
The example user
interface 1500 corresponds to a UF phase, as shown by a highlighted box 1502.
The user interface
1500 includes a drain volume field 1504, a UF field 1506, and a fill volume
field 1508. In the
illustrated example, the user interface 1500 prompts a patient to enter a
drain volume into data
field 1504 during a drain phase and a fill volume into a data field 1508
during a fill phase. The
user interface 1500 may calculate the UF value for the UF data field 1506 or
prompt a patient for
a value.
[00227] Instead of displaying the user interfaces 1400 and 1500 at
designed times,
the application 1002 may instead cause an alarm to be displayed on the
personal mobile
communication device 200a. The alarm may identify which medical information is
needed or
include a link to either of the user interfaces 1400 or 1500. The alarm may
include a popup window
displayed by the personal mobile communication device 200a. The alarm may also
include an
icon displayed adjacent to an icon for the application 1002 on a home screen
of the personal mobile
communication device 200a. In yet other embodiments, the application 1002 may
not notify a
patient about which medical information is needed. Instead, the application
1002 may enable a
patient to navigate to the desired user interface to enter medical device data
and/or treatment
information.
58
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00228] In some embodiments, the application 1002 may identify at
least some of
the data fields of one or more user interface as being required for entry
which other data fields are
optional. The application 1002 may outline or otherwise graphically indicate
while of the data
fields are required (e.g., displaying a red box around the systolic blood
pressure data field 1402).
The application 1002 may cause an alert message to be displayed by the
personal mobile
communication device 200a if a required data field is not completed or not
completed by a certain
time. In some embodiments, the application 1002 may prevent a patient from
navigating to another
user interface until all required fields are populated for a currently viewed
user interface.
[00229] After medical information is entered by a patient into one
or more of the
interface 1400 and 1500, the application 1002 transmits the medical
information to the data
acquisition module 1104 of the clinician server 200b for entry into the
patient's medical record.
The application 1002 is configured to transmit the medical information after a
patient competes
the data fields in the user interface or otherwise presses a send button on
the interface. In other
instances, the application 1002 may transmit the medical information at
predetermined times, such
as before or after a treatment.
2. Wireless Entry of Medical Information into an Application Embodiment
[00230] In some embodiments, a patient may select to enter medical
information
wirelessly in their personal mobile communication device 200a. The medical
information may be
received in the personal mobile communication device 200a via, for example, a
Bluetooth
connection, a Zigbee connection, a Z-Wave connection, a wireless USB
connection, a
wirelessly RF connection, an NFC connection, an infrared connection, or any
other suitable
wireless communication technology. In some instances, a patient may have to
wirelessly pair the
personal mobile communication device 200a with the medical device, such as the
blood pressure
monitor 104 and/or scale 106. In other embodiments, a patient activates a
connection application
(e.g., an NFC application) that wirelessly reads medical information from a
medical device.
[00231] In an example, a patient may pair their personal mobile
communication
device 200a with a blood pressure monitor 104. To enter the blood pressure
medical information
into the user interface 1400 of Fig. 14, the user selects, for instance, the
systolic blood pressure
data field 1402. Selection of the field 1402 causes the application 1002 to
display a prompt asking
the patient to select a data entry method (e.g., manual, wireless, photo,
etc.). After selecting the
59
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
wireless option, the application 1002 displays a menu of available wireless
connection options
and/or a list of paired Bluetooth devices. The patient selects the blood
pressure monitor 104
from the list, which causes a menu to be displayed of available medical
information. The patient
selects the systolic blood pressure, which causes the systolic blood pressure
value to be populated
into the systolic blood pressure data field 1402. In some embodiments, the
application 1002 is
configured to read the medical information from the blood pressure monitor 104
after the device
is selected by the patient. The application 1002 may use data labels or
metadata to determine
which of the fields 1402 to 1410 is to be populated with medical information
from the blood
pressure monitor 104.
[00232] In some embodiments, the application 1002 is configured to
enable a patient
to establish a permeant or semi-permanent connection with a medical device.
During a setup
operation, the application 1002 prompts the patient to select a data field
from the user interface
1400. After selection, the application 1002 prompts the patient to select a
paired wireless medical
device (and/or select a data type from a menu related the medical device). The
selections by the
patient configure the application 1002 to automatically read medical
information from the blood
pressure monitor 104, for example, when new medical information is available.
In other words,
the application 1002 is configured to access a remote medical device
wirelessly automatically to
read certain medical information to populate one or more data field. For
instance, after a patient
uses the blood pressure monitor 104 to take a blood pressure measurement, the
monitor 104
transmits a ping or status message to the application 1002 indicative that new
data is available.
Alternatively, the application 1002 may poll or otherwise ping the blood
pressure monitor 104 to
determine if new data is available. The application 1002 then reads the new
data into one or more
of the data field 1402 to 1410 to automatically populate the user interface
1400. The application
1002 then sends the medical information to the data acquisition module 1104 of
the clinician server
200b to automatically update the patient's medical record.
3. Image Entry of Medical Information into an Application
Embodiment
[00233] The example application 1002 on the personal mobile
communication
device 200a is configured to enable a patient to enter medical information
and/or treatment
information by recording an image of a medical device or screen of a medical
device. The personal
mobile communication device 200a uses a camera 1004 to record one or more
image. The
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
application 1002 uses optical character recognition ("OCR") to extract text
from the recorded
image. The extracted text is populated in one or more data field of a user
interface of the
application 1002. The use of images may reduce data entry errors from patients
or clinicians.
[00234] In some embodiments, the application 1002 uses rules or data
templates to
identify which text from an image may be selectable as relevant medical
information for a data
field. Otherwise, simply using an OCR feature makes all of the displayed text
selectable. As such,
a patient still has to copy and past the text from the image into a data
field. By comparison, rules
or data templates may group or identify text from an image as a field, which
enables easy selection
by a patient or automatic selection for population into a data field of a user
interface.
[00235] The data templates or rules of the application 1002 are
configured to
organize or otherwise decipher extracted text from an image. The application
1002 determines or
otherwise selects one or more data template for establishing a context for
medical information
based, for example, on a position of the medical information within the image
and/or
labels/keywords included within the medical information. To determine a data
template, the
example application 1002 may prompt a patient to specify a medical device type
from which an
image was recorded. Additionally or alternatively, the application 1002
enables a patient to select
a medical device template. In yet other embodiments, a patient may first
record an image of an
identifier 1012 (e.g., an identifier image), which is used by the application
1002 to determine a
type, model, etc. of the corresponding medical device. The application 1002
then selects a data
template or rule that corresponds to the type, model, screen, etc. of the
medical device.
[00236] The data templates define or specify data fields of certain
medical
information contained within an image. Generally, images include extracted
text comprising
medical information. In some instances, not all of the extracted medical
information is needed or
necessary. Instead, only certain medical information in a recorded image is
needed for entry into
a data field of a user interface or patient medical record. Relevant medical
information or relevant
medical information refers to medical device data or medical information that
is identified or
selected for population into a data field of a user interface of the
application 1002, or more
generally, for population into a patient's medical record.
[00237] Fig. 16 illustrates a schematic diagram of the personal
mobile
communication device 200a for recording and processing images for medical
information
extraction, according to an example embodiment of the present disclosure. The
illustration of the
61
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
personal mobile communication device 200a is exemplary and some of the blocks
may be
combined, further partitioned, or removed. In addition, in some embodiments,
the personal mobile
communication device 200a may include additional blocks, such as a memory 1018
storing
instructions, which when executed by a data processor 1602 (or more generally,
processor 1016),
cause the application 1002 to process images to extract medical information.
[00238] The example data processor 1602 may be configured to manage
the
acquisition of medical information from one or more image. Such management
includes
displaying, via the screen 1002, one or more camera message that provides
information prompting
a clinician or a patient (e.g., an operator) to record certain images.
Alternatively, the data processor
1602 may initiate an image processing mode after a patient has selected to
input medical
information into one or more data field of the application 1002 using an image
entry method. After
selection of an image entry method, the data processor 1602 is configured to
acquire one or more
image.
[00239] In some embodiments, the data processor 1602 causes the
personal mobile
communication device 200a to open a camera application to enable a patient to
record one or more
images. A patient may select which of the recorded images are to be further
processed or
discarded. The selected images are analyzed to identify text (as medical
information) for potential
population into data fields of one or more user interface of the application
1002.
[00240] In other embodiments, the data processor 1602 may guide the
patient
through one or more steps for acquiring the images. The data processor 1602
may execute a
workflow or routine based on which data field(s) was selected by the patient.
For example, the
data processor 1602 executes a blood pressure workflow for obtaining an image
from a blood
pressure monitor based on a patient selecting the systolic blood pressure data
field 1402.
[00241] In an example, the data processor 1602 of Fig. 16 is
configured to display
camera messages that identify a medical device, a user interface window of a
medical device,
and/or an identifier on a medical device that is to be recorded. The data
processor 1602 may also
display navigation messages that specify a user interface window of a medical
device for imaging.
Moreover, the data processor 1602 may display reminder messages if an image is
not recorded
within a predetermined time period (e.g., five minutes). The messages may
include text that
provides instructions and/or identifying the intended target for imaging. The
messages may also
include instructions regarding how to navigate to a certain user interface
window of a medical
62
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
device using control interfaces of the medical device. The messages may
further include graphical
elements, such as an exemplary illustration of a medical device, a consumable
items, identifier
1012, and/or user interface window for which an image is to be recorded.
[00242] It should be appreciated that in some embodiments, the data
processor 1602
does not display messages. Instead, the data processor 1602 is reactive to
images recorded by a
patient to determine relevant medical information. For example, upon receiving
an indication that
an image is recorded, the data processor 1602 operates a workflow in which the
application 1002
prompts a clinician to identify a medical device from which the image has been
recorded. The
prompt may include a pull-down menu of available or common medical devices. In
other
examples, the data processor 1602 automatically identifies medical information
using one or more
data templates or data labels to determine which extracted medical information
is to be populated
or written into a data field of a user interface or medical record.
[00243] To record an image, the example data processor 1602 and an
image
processor 1604 (more generally processor 1016) provide operations for the
application 1002 in
connection with the camera 1004. A patient provides an indication via a camera
user interface
1606 (e.g., a touchscreen or button on the personal mobile communication
device 200a) to record
an image. The patient actuates the camera user interface 1606 when, for
example, a camera is
focused on a medical device user interface window or identifier 1012. The data
processor 1602
receives the indication and instructs the camera 1004 to record an image. The
recorded image is
transmitted from the camera 1004 to the image processor 1604. In addition, a
copy of the image
is displayed by data processor 1602 on the display interface 1022 of the
personal mobile
communication device 200a.
[00244] In some embodiments, the data processor 1602 may cause a
ghost image to
appear on the display interface 1022 that is illustrative of an image to be
recorded. The ghost
image is provided on top of a stream of images provided by camera 1004 in a
preview mode. The
purpose of the ghost image is to provide assistance to a clinician or patient
confirming that the
image to be recorded contains the desired medical information and is recorded
at an appropriate
distance. For example, the data processor 1602 may display a ghost image of a
given identifier on
a given medical device. The patient aligns the personal mobile communication
device 200a such
that a stream of images of identifier 1012a is aligned positionally with the
ghost image. The patient
may then record the image of identifier 1012a. In some instances, the data
processor 1602 uses
63
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
image analysis to determine deltas between the ghost image and the stream of
images. The data
processor 1602 may cause instructions to be displayed that prompt a patient to
move the personal
mobile communication device 200a in a certain direction to reduce the
determined deltas. The
data processor 1602 may determine when the deltas are below a threshold,
indicating that the
images are aligned. Once the images are substantially aligned, the data
processor 1602 may
provide a graphical indication on display interface 1022 indicative that an
image can be recorded
or automatically cause the image to be recorded without input from a patient.
[00245]
The data processor 1602 may provide a prompt asking the patient to accept
the image. After receiving an indication via camera user interface 1606 of
acceptance, image
processor 1604 may analyze the image to identify or otherwise extract text. In
some instances, the
data processor 1602 may not prompt a clinician to accept an image. Instead, a
patient may provide
an indication via the camera user interface 1606 to delete an image. Until an
image is deleted, the
image processor 1604 performs an analysis to identify text.
[00246]
To identify text, the image processor 1604 uses, for example, OCR. In
addition, the image processor 1604 may determine a location or position of the
text with respect
to a center or origin of the image. In some instances, the image processor
1604 may assign two-
dimensional coordinates to each character or group of characters. The
positional text information
may be stored to an image file of the image as metadata. The image processor
1604 may also use
a clock of the personal mobile communication device 200a to attach a date/time
(corresponding to
a time when the image was recorded) to metadata associated with the image.
[00247]
In addition to performing OCR to identify text, the image processor 1604
may also be configured to identify patients, medical devices, and/or
consumable items using image
analysis. For example, the image processor 1604 may access a library of
medical device images
to identify a medical device within an image. In this example, the image
processor 104 may use
image matching routines to determine a match. Such a comparison may be made in
lieu of the
medical device having an identifier 1012. The image processor 1604 may use
similar routines
and/or algorithms for identifying consumable items 1006.
[00248]
The example data processor 1602 is configured to decode identifiers 1012
to determine a type of medical device of consumable item. Decoding may include
correlating
positions and thicknesses of lines and/or rectangles into relevant medical
information. The coded
lines and rectangles may correspond to a sequence of letters and/or numbers.
For example, the
64
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
data processor 1602 may use the lines or rectangles of the identifier 1012 to
determine a device
model number, medical device type, asset code, etc. The decoded identifiers
1012 may provide
relevant medical information for population into one or more data field of a
user interface of the
application 1002. Additionally or alternatively, the decoded identifiers may
be used by the data
processor 1602 for selecting a workflow for acquiring images from a medical
device or
consumable and/or for determining a data template.
[00249] The example image processor 1604 of Fig. 16 transmits the
images with the
extracted or otherwise identified text and/or medical information to data
processor 1602. The
example data processor 1602 uses, for example, one or more data templates from
a data template
database 1608 to identify relevant medical information from the extracted
text. In some examples,
data processor 1602 receives data templates from the clinician server 200b,
which may then be
stored in database 1608. In other examples, the data processor 1602 maintains
the database 1608
with data templates.
[00250] Fig. 17 illustrates a schematic diagram of a data template
1700, according
to an example embodiment of the present disclosure. The example data template
1700 is used by
the image processor 1604 (e.g., the application 1002) to identify extracted
text as relevant medical
information. Generally, screens of medical devices display medical
information. Some of the
information is relevant for inclusion in a data field of a user interface of
the application 1002.
Other of the data may be less relevant or not relevant. Further, depending on
a model of medical
device, the medical information may be in different locations or have
different labels. The data
template 1700 is configured to specify locations and names of relevant medical
information for a
particular home therapy machine 90.
[00251] The data template 1700 is stored in the database 1608 with a
plurality of
other data templates for different types and/or models of medical devices
and/or consumable items.
After receiving an identifier 1012 of a medical device (or specification of a
medical device or data
field by a patient), the image processor 1604 selects a corresponding data
template 1700.
Additionally or alternatively, the image processor 1604 may use image
processing to select a data
template that best matches a recorded image using, for example, information
labels or text position.
[00252] The example data template 1700 of Fig. 17 includes device
data (or text)
fields 1702, 1704, 1706, and 1708 that specify where certain medical
information is located on a
particular user interface window of a medical device. In some examples, the
data template 1700
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
is graphical such that an image analysis is performed to align fields 1702 to
1708 with extracted
text in an image. In other examples, the data template 1700 includes a file
(or other data structure)
having coordinates or positions for each of device data fields 1702 to 1708
relative to an origin.
The image processor 1604 identifies an origin in an image with extracted text
and identifies text
for each of data fields 1702 to 1708 based on substantial matches to locations
in the data template
1700. In some examples, image processor 1604 may scale the image to match a
size or coordinate
space of data template 1700.
[00253] Some of the illustrated data fields 1702 to 1708 may include
label text in
addition to coordinates and/or locations. For example, device data field 1702
includes label text
"Ultrafiltration Window", while device data field 1704a includes label text
"UF Vol.". The image
processor 1604 matches the label text to similar text extracted from an image.
In some instances,
matches between label text are used exclusively for identifying device data
fields, rather than using
positional or image analysis.
[00254] Matches between the label text, including label text for non-
relevant device
data fields, may be used to confirm that the image is from the correct window
or screen of a medical
device. For example, the image processor 1604 may match label text
"Ultrafiltration Window" to
corresponding extracted text in relatively the same location of a recorded
image. The match
confirms that the image has been recorded from an ultrafiltration window of a
renal failure therapy
medical device. However, the extracted text is not relevant medical
information for a patient's
medical record. If the label text does not match the extracted text, the image
processor 1604 may
display a message prompting a patient to record an image of the
Ultrafiltration Window of renal
failure therapy medical device.
[00255] The label text associated with device data fields 1706 and
1708 may be used
to confirm a recorded image is current, or recorded within a determined time
period. For example,
some windows of medical devices display a current date and time. This
information may be
extracted by image processor 1604 and identified using device data fields 1706
and 1708. The
image processor 1604 compares the extracted date/time to date/time rules or
limits associated with
the device data fields 1706 to 1708 to determine whether the recorded image is
current. For
example, the image processor 1604 may determine another image is required to
be recorded if the
date does not match or the time is not within a predetermined threshold (e.g.,
five minutes, 15
66
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
minutes, 60 minutes, 3 hours, etc.) of a current time on the personal mobile
communication device
200a.
[00256] The example device data fields 1704a and 1704b of Fig. 17
are used by the
application 1002 to identify relevant medical information. In some instances,
the application 1002
may display a flag or metadata indicative that device data fields 1704a and
1704b are relevant to
a selected data field of a user interface (e.g., the user interface 1400 or
1500). By comparison,
device data fields 1702, 1706, and 1708 may include a flag or metadata
indicative that the
corresponding extracted data is not relevant. In the example illustrated in
Fig. 17, the image
processor 1604 uses the label text of the data field 1704a to locate
corresponding extracted text in
an image. The image processor 1604 then uses a positional relationship between
device data fields
1704a and 1704b or text value markers to identify extracted medical
information from the image
that corresponds to the numerical value of the ultrafiltration volume. The
image processor 1604
copies the extracted medical information related to field 1704b from the image
to populate, for
example, the systolic blood pressure data field 1402 of Fig. 14.
[00257] As discussed above in connection with Fig. 17, the data
processor 1602 of
Fig. 16 may use known positional relationships of text in an image and text
label(s) to determine
which of the extracted text corresponds to relevant medical information. In
some embodiments,
data processor 1602 selects a data template based on an indication of the
model or type of medical
device and/or consumable item. The indication may be determined from a
previous image of an
identifier 1012, received from a patient via the camera user interface 1606,
and/or specified
through a selection of a data field of a user interface for the application
1002 to which the medical
information is to be populated. In other examples, the data processor 1602
compares the data
templates in the database 1608 to the image with the extracted text to find a
match. In these other
examples, the data processor 1602 uses text labels and a position of the text
between the image
and data templates to determine a match.
[00258] The example data processor 1602 is configured, after
identifying relevant
medical information, to write or otherwise populate the relevant medical
information into one or
more data field of a user interface of the application 1002. In some
embodiments, the data
processor 1602 is configured to automatically populate the data fields of a
user interface of the
application 1002 using a data template. To automatically populate medical
information, the data
processor 1602 compares text, labels, and/or metadata associated with fields
of a data template to
67
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
the text, labels, metadata, and/or other data field information of one or more
user interface of the
application 1002. If at least some of the text, labels, and/or metadata match,
the data processor
1602 identifies certain text (e.g., numerical values) corresponding to the
matching field of the data
template. The identified text is written to the corresponding matching data
field of the user
interface of the application 1002.
[00259] In other embodiments, the data processor 1602 is configured
to prompt a
patient to select one or more field of a data template to populate one or more
data field of a user
interface of the application 1002. Fig. 18 shows a diagram illustrative of a
patient populating data
fields 1402 to 1406 of the user interface 1400, according to an example
embodiment of the present
disclosure. In the illustrated example, the user interface 1400 is opened on
the personal mobile
communication device 200a. To enter medical information into the data fields
1402 to 1406, a
patient selects each of the data fields sequentially or together. For each
selection, the application
1002 displays a prompt asking the patient how the data is to be entered. After
a patient selects a
photo entry method, the application 1002 opens a camera application to enable
a patient to record
an image 1800 of a screen of the blood pressure monitor 104 using the personal
mobile
communication device 200a. In some embodiments, the application 1002 may
display text or
graphics to assist the patient in acquiring the image, as described above.
[00260] After the image 1800 is recorded, the application 1002
performs an OCR
operation to extract text. The application 1002 then determines a data
template 1801 that
corresponds to the extracted text. In some embodiments, the application 1002
matches locations
of the text and/or labels within the image to text and/or label locations in
data templates to find a
matching data template. In other examples, the patient may specify an
indication of the blood
pressure monitor 104, which enables the application 1002 to locate the
corresponding data
template 1801. In yet other examples, the application 1002 may first prompt a
patient to record an
image of the identifier 1012a of the blood pressure monitor 104. Data from the
identifier is used
by the application 1002 to select the data template. Regardless of how the
data template 1801 is
identified, the application 1002 applies the data template 1801 to the
extracted text in the image
1800. This process includes the application 1002 identifying groups or fields
of similar text. In
some instances, the application 1002 may identify groups or fields of similar
text based on spacing
between letters and/or words.
68
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00261] In the illustrated example of Fig. 18, the application 1002
provides fields
1802, 1804a, 1804b, 1806, 1808 and 1810 for the different sets of text. The
patient selects the
field from which data is to be populated into the selected data field of the
user interface 1400. For
example, to populate the systolic blood pressure data field 1402, the patient
selects the field 1804a.
The selection by the patient causes the application 1002 to copy at least some
of the data from the
field 1804a to the data field 1402. The data field 1402 may include a rule
that specifies only a
numerical integer may be accepted. The application 1002 reads the text from
the selected field
1804a for numeric integers (i.e., "145"). The application 1002 then populates
the identified
numeric integers into the systolic blood pressure data field 1402. The patient
may continue for the
other fields 1404 to 1410 of the user interface 1400 using the image 1800. In
each instance, the
patient may select between entering the data manually, wirelessly, or via an
image. If a second
image is needed (e.g., prompted by the application 1002 or determined by the
patient), the
application 1002 enables the patient to record and view multiple images. The
application 1002
applies the appropriate data template to each of the recorded images. The
application 1002
accordingly enables a patient to enter information into the user interface
1400 using one or more
recorded image as though the patient is manually entering the values.
[00262] In some embodiments, the application 1002, and more
specifically, the data
processor 1602 performs a check on the extracted data selected by a user to
ensure the values are
within a predetermined range and/or of a specified type. The application 1002
may perform similar
checks for data manually entered by a patient or data received wirelessly from
a medical device.
Each data template and/or data field of a user interface may include metadata
or rules for certain
fields that provide thresholds or acceptable ranges of values. For example,
metadata or rules for a
weight data field may specify that a predetermined acceptable range of values
is between 20 kg
and 200 kg. For values outside the predetermined range, the application 1002
may display an error
on the display interface 1022 or prompt a patient to record another image or
amend the value.
[00263] Fig. 19 illustrates a flow diagram of an example procedure
1900 for entering
medical information from an image using the application 1002 of the personal
mobile
communication device 200a, according to an example embodiment of the present
disclosure.
Although procedure 1900 is described with reference to the flow diagram
illustrated in Fig. 19, it
should be appreciated that many other methods of performing the steps
associated with the
procedure 1900 may be used. For example, the order of many of the blocks may
be changed,
69
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
certain blocks may be combined with other blocks, and many of the blocks
described may be
optional. In addition, example procedure 1900 may include optical blocks for
prompting a patient
to record an image of a screen and/or identifier 1012 of a medical device.
[00264]
Example procedure 1900 begins in one embodiment when the application
1002 is launched on the personal mobile communication device 200a and operates
with processor
1016 to establish a wired and/or wireless connection with the clinician server
200b (block 1902).
Establishing a connection may include, for example, transmitting and/or
receiving one or more
message 1903 that provides a device address, patient identifier, device
identifier, network address,
and/or protocol information for accessing and/or writing to a patient's
medical records. After
opening the application 1002, the patient navigates or otherwise accesses a
user interface (e.g., the
user interfaces 1400 or 1500 of Figs 14 and 15) for entering medical
information (block 1904).
[00265]
Before block 1906, a patient uses the application 1002 to select a data field
and select that medical information is to be provided via photo entry. At
block 1906, the personal
mobile communication device 200a records an image 1907 of a medical device,
consumable,
patient, etc. The image may include an identifier and/or a screen of a medical
device. The personal
mobile communication device 200a then determines or identifies text within the
recorded image
(block 1908). For example, the personal mobile communication device 200a may
perform an OCR
routine on the image. In instances in which the image includes a barcode
and/or a QR code, the
personal mobile communication device 200a decodes the barcode and/or QR code.
The imaged
or coded data is converted into textural or American Standard Code for
Information Interchange
("ASCII") characters. In some embodiments, the personal mobile communication
device 200a
may also determine data fields for the identified text (block 1910). The data
fields may be
determined using, for example, a data template. As described above in
connection with Figs. 17
and 18, the data template may specify locations of certain text and/or specify
labels of certain text
used for placing data fields operationally over identified text within the
recorded image. In some
instances, the data fields and/or data template may be selected by a patient
and/or determined from
an identifier 1012 of the medical device. Alternatively, instead of using
templates, the procedure
1900 may instead provide an indication that the recently recorded image has
relevant medical
information for extraction, selection, and/or transfer.
[00266]
The example procedure 1900 continues in one embodiment by
determining if there are additional images to record (decision block 1912). In
an example, the
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
procedure 1900 may access a list of images needed to be recorded for a
specified therapy or
treatment. The procedure 1900, via the personal mobile communication device
200a, may guide
a patient through a sequence to obtain all needed images or provide prompts to
obtain images
containing medical information that is determined to be needed or missing. In
other instances, the
patient may determine which images are needed. If additional images are to be
recorded, the
procedure 1900 returns to block 1906.
[00267] If no additional images are needed, as determined at
decision block 1912, a
patient begins the process to populate relevant medical information into data
fields of a user
interface. The process in the illustrated embodiment includes enabling the
patient to select an
image from which relevant medical information is to be transferred. The
selection of the image
causes the personal mobile communication device 200a to display the image on
the display
interface 1022 (block 1914). It should be appreciated that the selected image
includes identified
text, and optionally data fields. The patient may also specify the data field
(or location in the data
field) of the user interface to which the data is to be populated.
[00268] The personal mobile communication device 200a receives a
selection of the
relevant medical information and/or data fields with relevant medical
information for population
into a designed data field of a user interface of the application 1002.
Selection may be performed
by the patient pressing on the area of the touch screen 1002 of the personal
mobile communication
device 200a corresponding to the medical information to be populated.
Selection of the relevant
medical information and/or fields causes the personal mobile communication
device 200a, in an
embodiment, to automatically populate at least a portion of the selected text
(block 1916).
[00269] After the selected text has been populated, the personal
mobile
communication device 200a determines if additional relevant medical
information is to be
populated based on a selection of additional data fields of a user interface
(decision block 1918).
In some examples, the personal mobile communication device 200a operates a
sequence or routine
that provides prompts for a patient to select the appropriate text and/or
images for populating into
data fields. If there is additional relevant medical information, as
determined at decision block
1918, the procedure 1900 returns to blocks 1914 and 1916, where the patient
specifies the image
and/or relevant medical information for data field population. If there is no
additional relevant
medical information for population, as determined at decision block 1918, the
example procedure
ends 1900.
71
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
4. Image/Text Attachment Application Embodiment
[00270] The example application 1002 and clinician server 200b in
some
embodiments are configured to enable patients to provide images or text as
attachments or
addendums to their medical records. In the above sections, the application
1002 provides data
fields of user interfaces as prompts for desired information. However, in some
instances, a patient
or clinician may want to provide additional information. Additionally or
alternatively, one or more
user interfaces of the application 1002 may include data fields for free text
entry, prompts for a
patient to enter text (e.g., "how are you feeling today"), or features to
enable a photo or image to
be incorporated as part of the record. For example, the user interface 1400
may include a photo
icon adjacent to a prompt for an image of a fluid connection to the patient's
abdomen, which when
selected, opens a camera application to enable a patient to attach an image
for transmission with
the medical information. The images may be stored to designed fields in the
appropriate patient
medical record.
[00271] The example application 1002 on the personal mobile
communication
device 200a is configured to accept images and/or text provided by a patient.
In an embodiment,
the application 1002 is configured to enable a patient to select between text
or photo entry. If a
patient selects text entry, the application 1002 displays a text box. A
patient enters information
into the text box, which is stored by the application 1002. The application
1002 may then transmit
the information in one or more message to the clinician server 200b. The
application 1020 at the
clinician server 200b determines the appropriate patient medical record in the
database 1010 and
locates a "note" or free text field. The application 1020 stores the patient
provided text into the
field. In some instances, the application 1020 may also include a time/date
stamp with the entered
text. This information provides a clinician with additional information from a
patient, including
feedback regarding the treatment.
[00272] Fig. 20 shows an example of a user interface 2000
displayable by the
application 1002 on the personal mobile communication device 200a that enables
a patient to
provide a recorded image, according to an example embodiment of the present
disclosure. The
example user interface 2000 includes an image title field 2002, which may be
editable by a patient.
The user interface 2000 also includes one or more recorded image 2004, or a
preview of recorded
image. The application 1002 is configured to enable a patient to browse a
gallery folder of
recorded images to select which images are to be transmitted. A date field
2006 and a time field
72
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
2008 provide information in the user interface 2000 indicative of a date/time
the displayed image
was recorded. A patient may select a "Trash" button to discard the image 2004
or a "Upload"
button to transmit the image 2004 from the application 1002 to the clinician
server 200b. The
example photo capture feature of the application 1002 enables a patient to
document fluid line
connectivity with the home therapy machine 90 or physiological conditions
related to a treatment.
The application 1020 at the clinician server 200b is configured to attach or
otherwise link the
received image 2004 to the patient's medical record.
5. Manual/Wireless/Image Entry Medical Information via Text Embodiment
[00273] In some embodiments, the personal mobile communication
device 200a of
Figs. 10 to 12 may not be capable of installing or operating the application
1002, or a patient may
decide not to install the application 1002. However, the clinician server 200b
still registers the
personal mobile communication device 200a during a registration process.
Instead of receiving
medical information via the application 1002, the data acquisition module 1104
of the clinician
server 200b determines that a routine or algorithm is to be executed for
prompting or otherwise
obtaining medical information from a patient via the personal mobile
communication device 200a.
The example data acquisition module 1104 may read a registration file and/or
medical record of
the patient to determine the personal mobile communication device 200a is
registered but the
application 1002 is not installed.
[00274] In some embodiments, the data acquisition module 1104 is
configured to
obtain medical information from a patient through one or more text messages.
For instance, at
designed times, corresponding to times of prescribed treatments, the data
acquisition module 1104
may transmit one or more text messages prompting a patient to respond with
medical information
using the registered personal mobile communication device 200a. In other
instances, the data
acquisition module 1104 is configured to respond to a text from a patient to
begin a sequence or
routine for acquiring medical information. In yet other instances, the patient
may send a text
message with medical information and/or images to the data acquisition module
without a prompt
message.
[00275] The example data acquisition module 1104 of Fig. 11 is
configured to
format or otherwise structure the received information for population into one
or more data field
of a patient's medical record. Generally, medical information received from a
text message is
73
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
unstructured. In other words, the text message does not provide a clear
correlation or reference to
a designed field of a patient's medical record. Instead, the data acquisition
module 1104 is
configured to determine appropriate fields for the medical information
received in the text
message.
[00276] In some embodiments, the data acquisition module 1104 uses a
context of
the received text message from the personal mobile communication device 200a
to determine a
data field. For example, a patient may send a message that includes the text
"systolic blood
pressure 145". The data acquisition module 1104 compares at least some of the
text (e.g., one or
more words in a string) to field data labels, keywords, and/or metadata. If at
least some of the
words match, the data acquisition module 1104 is configured to identify a
numeric value in the
contents of the text message and write the identified numeric value in a data
field of a patient's
medical record corresponding to the matching text. In some embodiments, the
data acquisition
module 1104 may compare the value to an acceptable range of values before
writing the value. If
the value is outside the acceptable range, the data acquisition module 1104
may transmit, to the
personal mobile communication device 200a, a message indicative of the error
or a message
prompting a patient to reenter the value.
[00277] In these embodiments, the data acquisition module 1104 may
be configured
to send follow-up texts to a patient if text from a received text message
cannot be properly placed
into a data field. For example, the data acquisition module 1104 may receive a
message with text
comprising "blood pressure 145". The data acquisition module 1104 determines
the message may
correspond to "systolic" or "diastolic" blood pressure data fields based on
matching text.
Accordingly, the data acquisition module 1104 transmits a message to the
personal mobile
communication device 200a with text asking a patient if the value is
"systolic" or "diastolic".
[00278] In other embodiments, the data acquisition module 1104
prompts a patient
through a sequence of messages (that may be defined by a routine or algorithm)
to provide certain
medical information. The order of the sequence is known or defined and
corresponds to data fields
in a medical record. As such, the data acquisition module 1104 automatically
determines that a
response to a certain prompt corresponds to the data field of a medical record
that is related to the
prompt. For example, the data acquisition module 1104 transmits a text message
prompting a
patient for a "systolic blood pressure measurement". The data acquisition
module 1104 determines
that a response to the text contains a value for the "systolic blood pressure
measurement". The
74
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
data acquisition module 1104 may analyze the received message to identify the
numeric value
among other text and/or compare the value to an acceptable range. After
confirming the patient
provided an acceptable response with medical information, the data acquisition
module 1104
determines a subsequent message to send to the personal mobile communication
device 200a based
on the sequence of messages.
[00279] Instead of receiving messages with text, the data
acquisition module 1104
may also receive messages with a photo attachment. Similar to the process
described above in
connection with Figs. 16 to 19, the data acquisition module 1104 is configured
to process the image
to extract text and identify relevant medical device data for one or more data
field of a medical
record. Further, while the above-disclosure describes the use of text
messages, the data acquisition
module 1104 may additionally or alternatively receive the medical information
and/or photos via
other communication mediums, such as emails, instant or web-based messages,
social media posts,
etc.
[00280] Fig. 21 shows a schematic diagram of a patient medical
template 2100 that
may be used by the data acquisition module 1104 of the clinician server 200b
to populate data
fields of a patient's medical record, according to an example embodiment of
the present disclosure.
Fields of the template 2100 may correspond to or otherwise be linked or
referenced to data fields
of a patient's medical record. In other embodiments, a copy of a completed
template 2100 may be
stored to a medical record or as a medical record itself In some embodiments,
the patient medical
template 2100 may be part of or otherwise integrated with a patient's medical
record.
[00281] The example template 2100 includes predefined fields 2102,
2104, 2106,
2108, 2110, 2112, 2114, 2116, 2118, and 2120 that correspond to a renal
failure therapy treatment
("RFT"). The example data acquisition processor 1104 may select the template
2100 based on the
treatment prescribed for a patient. While an RFT template 2100 is illustrated,
the example data
acquisition module 1104 may select other templates configured specifically for
pre-treatment data
acquisition, post-treatment data acquisition, etc. For example, a pre-
treatment acquisition template
may include data fields for blood pressure, pulse, and weight.
[00282] It should be appreciated that in other embodiments, the
patient medical
template 2100 may include additional or fewer fields. For example, the
template 2100 may
additionally include data fields for pre-treatment patient weight and post-
treatment patient weight,
patient glucose level, and/or patient birth date. In another example, the
template 2100 may include
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
fields for a fill rate, a dwell time, a drain or fluid removal rate, a blood
flow rate, an effluent dose,
an ultrafiltration removal rate, a dialysis solution removal rate, a total
dialysis solution infused,
dialysis solution flow, replacement pre-flow, replacement post-flow, patient
weight balance, return
pressure, excess patient fluid sign, filtration fraction, a time remaining,
dialysis solution
concentration, dialysis solution name, a patient identifier, a room
identifier, a care area identifier,
a timestamp indicative of when the data was generated, an alarm condition, an
alert condition,
and/or an event.
[00283] In some embodiments, the data acquisition module 1104 is
configured to
select the template 2100 based on a prompt from a patient. For example, a
patient may send the
data acquisition module 1104 a message including text of "manual exchange". In
response, the
data acquisition module 1104 identifies and selects the template 2100 for a
manual exchange. In
another example, a patient may send the data acquisition module 1104 a
message, via the personal
mobile communication device 200a, including text of "pre-treatment" or "begin
treatment". In
response, the data acquisition module 1104 identifies and selects a template
for acquiring medical
information needed before a treatment begins.
[00284] In the illustrated example of Fig. 21, the example data
fields include a field
2102 for a patient's name, a field 2104 for a patient identifier, a field 2106
for a patient's weight,
a field 2108 for a patient's blood pressure, a field 2110 for a date of
treatment, a field 2112 for an
amount of UF removed, a field 2114 for an amount of total fluid provided to a
patient, a field 2116
for a dextrose level, a field 2118 for a treatment prescription identifier,
and a field 2120 for a
disposable cassette identifier. In instances where the renal therapy machine
90 is registered with
the clinician server 200b, the data acquisition module 1104 may remove at
least fields 2112 to
2120 (or select a separate template with the fields 2112 to 2120 omitted)
because such information
is already provided by the machine 90. However, the fields 2112 to 2180 may be
used in the
template if a patient is reporting a manual exchange. Further, the template
2100 may omit fields
2102 and 2104 based on at least some of the patient information already having
been registered.
[00285] The example data fields of the patient medical template 2100
may be
populated from one or more different sources of medical information including,
device
information, patient information, and/or consumable item information. For
example, the patient
name field 2102 and patient identifier field 2104 may be populated from
image(s) recorded by the
personal mobile communication device 200a of an identifier 1012 of a patient
(or an identifier
76
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
1012 worn by the patient). The blood pressure field 2108 may be populated from
an image
recorded by the personal mobile communication device 200a of a screen of the
blood pressure
monitor 104, while the weight field 2106 may be populated from an image
recorded by the personal
mobile communication device 200a of a screen of the scale 106. The date field
2110, the UF
removed field 2112, and the fluid fill field 2114 may be populated from an
image recorded by the
personal mobile communication device 200a of a screen (showing a treatment
status window) of
the home therapy machine 90. Similarly, the dextrose level field 2116 may be
populated from an
image recorded by the personal mobile communication device 200a of a screen
(showing a setup
window) of the home therapy machine 90, while the prescription identifier
field 2118 may be
populated from an image recorded by the personal mobile communication device
200a of a screen
(showing a prescription window) of the home therapy machine 90. Finally, the
cassette identifier
field 2120 may be populated from an image recorded by the personal mobile
communication
device 200a of an identifier 1008 of a disposable cassette consumable item
1006. As discussed
above, the data acquisition module 1104 may receive one or more message with
text for the fields
2102 to 2120 instead of receiving images containing the medical information.
[00286] The patient medical template 2100 illustrated in Fig. 21 is
configured to be
stored on the clinician database 1010 and accessed by the clinician server
200b illustrated in Figs.
and 11. Upon receiving a request to populate a template for a patient, the
clinician server 200b
is configured to make a copy or create an instance of template 2100. Medical
information received
from the personal mobile communication device 200a is inputted by the data
acquisition module
1104 into the appropriate data fields 2102 to 2120 of the copy or instance of
the template 2100.
Once complete, the copy or instance is stored in the clinician database 1010
repository as a medical
record of the patient.
[00287] In some embodiments, the data acquisition module 1104
operates a routine
2150 in conjunction with or alternatively to the example patient medical
template 2100. In some
embodiments, the routine 2150 may be programmed as metadata or computer
executable code for
the respective data fields 2102 to 2120. In other examples, the routine 2150
may be stored in the
clinician database 1010 in relation to the patient medical template 2100.
Further, in these other
examples, selection of the template 2100 causes the routine 2150 to be
executed. The example
routine 2150 contains routine modules 2152 to 2164 that provide associations
between data fields
2102 to 2120 and corresponding medical devices, identifiers 1012, and/or
consumable items 1006.
77
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00288] At least some of the routine modules 2152 to 2164 may be
associated with
or otherwise related to data templates (e.g., the data template 1700 of Fig.
17). The data acquisition
module 1104 is configured to access a data template for a corresponding
routine module 2152 to
2164 when a patient provides an image and/or text in a message. For example,
while executing
the routine module 2156 for blood pressure, the data acquisition module 1104
transmits a message
that prompts the personal mobile communication device 200a for "a systolic
blood pressure
measurement". A patient responds with a text message containing an image of a
screen or dial of
the blood pressure monitor 104. The data acquisition module 1104 is configured
to access a data
template corresponding to or referenced to the routine module 1156 for blood
pressure
measurements. The data acquisition module 1104 then applies the data template
to extract text
from the image, using the procedure described above in connection with Figs.
16 to 19, to identify
the relevant systolic blood pressure medical information for population into
the blood pressure
data field 2108 of the template 2100.
[00289] The example routine 2150 may be configured to begin, upon
request from
a patient, based on one or more messages received by the data acquisition
module 1104. For
example, the data acquisition module 1104 may receive a message with text
including "begin",
"start", and/or "pre-treatment". Reception of the message causes the data
acquisition module 1104
to determine and start the routine 2150 to populate the data fields 2102 to
2120 of the template
2100. In other examples, the data acquisition module 1104 transmits a first
message specified by
the routine 2150 to a patient at a predetermined time relative to a treatment.
The data acquisition
module 1104 sequentially sends the messages specified by the routine 2150
after receiving
response message(s) from the personal mobile communication device 200a with
the appropriate
medical information. In this manner, the data acquisition module 1104 controls
an acquisition of
medical information from a patient according to a predetermined sequence. The
data acquisition
module 1104 may not transmit a next message in a sequence defined by the
routine 2150 until an
appropriate response message is received (and medical information is populated
into the
designated data field 2102 to 2120 of the template 2100) from the personal
mobile communication
device 200a.
[00290] In the illustrated example, the patient band module 2152 may
include
metadata or preformatted messages instructing a patient to record an image of
a patient's
wristband. The patient band module 2152 may also include character
verification checks to ensure
78
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
the received medical information conforms to text requirements for a patient's
name and/or patient
identifier. For example, the patient band module 2152 may reject or discard
medical information
for a patient's name that includes numbers.
[00291]
The weight scale module 2154 may include metadata or preformatted
messages instructing a patient to record an image of identifier 1012c and a
screen of the scale 106
of Fig. 10.
The weight scale module 2154 may also include character verification checks to
ensure the received medical information is within an acceptable range of
values and/or of the
correct unit type. In some instances, the weight scale module 2154 may use
medical information
from the identifier 1012c to confirm that the medical information from the
screen of the scale 106
is patient weight medical information. In other instances, medical information
from the identifier
1012c is used for selecting a data template based, for example, on a model or
type of the weight
scale 106. The data template is used by the data acquisition module 1104 to
identify relevant
weight scale medical information that was extracted from images of the screen
of the scale 106.
[00292]
The blood pressure module 2156 is similar to weight scale module 2154
with respect to the blood pressure monitor 104. The renal failure therapy
("RFT") modules 2158
to 2162 are also similar to the weight scale module 2154. However, multiple
modules 2158 to
2162 are used for the home therapy machine 90 (or a manual exchange) for each
of the different
windows from which medical information is needed. For example, the module 2158
provides
messages for acquiring images of the identifier 1012 of the home therapy
machine 90 and a first
window showing a treatment status window, while the module 2160 provides one
or more message
for acquiring an image of a setup window of the home therapy machine 90, and
the module 2162
provides one or more message for acquiring an image of a prescription window
of the home
therapy machine 90.
[00293]
The cassette module 2164 may include metadata or preformatted messages
instructing a clinician or patient to record an image of identifier 1008, a
disposable cassette
consumable item 1006, and/or a label on packaging or the cassette consumable
itself It should
be appreciated that routine 2150 may include additional modules if the patient
medical template
2100 includes additional data fields or fewer modules if the template 2100
includes fewer fields.
[00294]
Once the medical information is received using the routine 2150, the data
acquisition module 1104 of the application 1020 uses data verification checks
to ensure the data is
within an acceptable range, formatted correctly, and/or in the appropriate
units. In some instances,
79
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
the modules of the routine 2150 may include conversion or formatting
instructions, which are used
by the data acquisition module 1104 to prepare the medical information for
inclusion in the
respective field of the template 2100 and/or the patient's medical record.
Once the data is in the
appropriate format and unit, the data acquisition module 1104 writes the
medical information to
the respective field of the template 2100 and/or the patient's medical record.
[00295] In alternative embodiments, the patent medical template 2100
may not have
an associated routine. Instead, the data acquisition module 1104 of Fig. 11 is
configured to read
data fields 2102 to 2120 of patient medical template 2100 to determine, for
example, incomplete
data fields. In these alternative embodiments, the data acquisition module
1104 identifies missing
data and transmits one or more message to the personal mobile communication
device 200a,
prompting a patient for the missing data.
[00296] To populate the data fields, the data acquisition module
1104, in some
embodiments, may read a name of the data field 2102 to 2120 (and any
corresponding metadata)
to create and send a message to a patient prompting the recording of certain
images. In an example,
the weight data field 2106 includes metadata identifying a weight scale as a
related medical device.
The data acquisition module 1104 determines that data field 2106 is unfilled,
reads the
corresponding metadata, and constructs a message instructing a patient of the
personal mobile
communication device 200a to record an image of a screen of a weight scale. In
the illustrated
embodiments, the data acquisition module 1104 may progress sequentially
through template 2100
(or a patient's medical record) searching for unfilled data fields and
accordingly request medical
information from a patient via message(s) displayed by the personal mobile
communication device
200a. Alternatively, the data acquisition module 1104 may progress through
template 2100
according to a predetermined order or sequence. For example, the data
acquisition module 1104
may search first for data fields associated with a patient wristband, followed
by data fields for a
weight scale medical device, data fields for blood pressure medical device,
and data fields for a
renal failure therapy medical device.
[00297] Fig. 22 is a schematic diagram of the data acquisition
module 1104 of the
clinician server 200b of Fig. 11, according to an example embodiment of the
present disclosure.
It should be appreciated that the illustration of the data acquisition module
1104 is exemplary and
that some of the blocks may be combined, further partitioned, or removed. In
addition, in some
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
embodiments, the data acquisition module 1104 may include additional blocks,
such as a block for
a user interface.
[00298] The example data acquisition module 1104 (and more generally
the
clinician server 200b) includes an interface 2202 that provides connectivity
with the personal
mobile communication device 200a. The interface 2202 may include, for example,
an Internet
port or connection. The interface 2202 is configured, in one embodiment, to
receive and convert
messages from the personal mobile communication device 200a into a format
compatible for
internal processing. For example, the interface 2202 may convert SMS or text
messages into an
HL7 or ASCII format. The example interface 2202 is also configured to format
or convert
messages for transmission to the personal mobile communication device 200a. In
some instances,
the interface 2202 may encrypt messages for transmission and/or decrypt
received messages.
[00299] The example data acquisition module 1104 includes a template
processor
2204 configured to manage the writing or population of medical information
from the personal
mobile communication device 200a to a patient medical template 2100 or record.
For example,
upon request from a patient or clinician, or automatically, the template
processor 2204 selects a
template from a patient medical template database 2206. The selected template
(e.g., the template
2100 of Fig. 21) is used by the template processor 2204 to operate a routine
(e.g., the routine 2150),
if available or configured, to acquire medical information from the personal
mobile
communication device 200a.
[00300] The example template processor 2204 is configured to
identify messages
from modules of a routine (e.g., the routine 2150) for transmission to the
personal mobile
communication device 200a. In some instances, the messages may be transmitted
in a
predetermined sequence to direct or guide a clinician or a patient through a
process to populate a
patient medical template. For example, the template processor 2204 may read
module 2156 of
routine 2150 of Fig. 21 and determine a message is to be transmitted that
prompts a patient to
record an image of the identifier 1012c of the scale 106. The template
processor 2204 may be
configured to wait until medical information related to the identifier 1012c
is received (for
populating the data field 2106 of Fig. 21) before identifying messages from
routine module 2156
that are to be transmitted. In other instances, the template processor 2204
selects messages for
transmission based on messages received from the personal mobile communication
device 200a.
For example, the processor 2204 may receive medical information associated
with the identifier
81
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
1012c of the weight scale 106. In response to the received medical
information, the template
processor 2204 may determine that module 2154 corresponds to the received data
and accordingly
selects messages prompting a patient to record an image of a screen of the
weight scale 104. As
illustrated in Fig. 22, the personal mobile communication device 200a displays
a text message
from the template processor 2204 instructing a patient to record an image of a
screen 2220 of the
scale 106.
[00301] In addition to sending messages, the template processor 2204
may be
configured to select data templates. The data templates are stored in a data
template database 2208
in the illustrated embodiment. The template processor 2204 selects data
templates based on a type
or model of a medical device, which is indicated in medical information
corresponding to identifier
1012. In some instances, a patient may specify the model and/or type of
medical device type to
the template processor 2204 via a message. Further, as discussed above, the
template processor
2204 may receive image(s) from a personal mobile communication device 200a,
extract text from
the images, and select the appropriate data template from the database 2208
for identifying relevant
medical information from the image(s), as described above.
[00302] In some embodiments, the template processor 2204 may receive
a stream of
messages from a personal mobile communication device 200a containing
substantially all the
medical information for the patient medical template 2100 or a patient's
medical record. In these
embodiments, the template processor 2204 reads labels, metadata, and/or device
data field
information provided with the data to determine a data field of the template
2100 to which the data
is to be populated or written. The template processor 2204, for example,
matches metadata or
information of the modules (or data fields 2102 to 2120 themselves) to the
labels, metadata, and/or
device data field information provided with the medical information to
determine the appropriate
data field of template 2100.
[00303] After populating or otherwise completing a patient medical
template, the
template processor 2204 of Fig. 22 is configured to store the completed
template to the clinician
database 1010. This may include populating a patient's medical record using
the template 2100,
where fields in the template correspond to, are linked to, or reference fields
in the patient's medical
record. In other examples, the writing to a medical record may include storing
a populated
template to a patient's medical record or storing the template 2100 as a
medical record itself
82
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00304] Figs. 23 and 24 are flow diagrams of example procedures 2300
and 2350 to
populate the medical device template 2100 of Fig. 21 using images recorded by
(and/or text
messages received from) the personal mobile communication device 200a of Figs.
10 to 12 and
22, according to an example embodiment of the present disclosure. Although the
procedures 2300
and 2350 are described with reference to the flow diagrams illustrated in
Figs. 23 and 24, it should
be appreciated that many other methods of performing the steps associated with
the procedures
2300 and 2350 may be used. For example, the order of many of the blocks may be
changed, certain
blocks may be combined with other blocks, and many of the blocks described may
be optional. In
an embodiment, the order of the blocks may be modified if a persistent
connection does not exist
between the clinician server 200b and the personal mobile communication device
200a. Instead,
in the embodiment, the personal mobile communication device 200a may first
acquire and queue
substantially all relevant medical information for a patient medical template
until a connection
with the clinician server 200b is available (or by design). The actions
described in the procedures
2300 and 2350 may be performed among multiple devices including, for example
the personal
mobile communication device 200a and the clinician server 200b.
[00305] The example procedure 2300 begins in Fig. 23 when the
clinician server
200b of Figs. 10 to 12 and 22 receives a message 2301 from the personal mobile
communication
device 200a (block 2302). The message 2301 is indicative of a treatment to be
performed on a
patient or a request to begin sending medical information. The clinician
server 200b then
determines a patient medical template (e.g., the patient medical template 2100
of Fig. 21) based
on the treatment type specified in the message 2301 or a prescribed treatment
specified in a
patient's medical record (block 2304). In instances where the clinician server
200b only provides
for the completion of one type of template (e.g., a template for a renal
failure therapy), the message
2301 may indicate a request to start populating a blank template. In response
to the message 2301,
the clinician server 200b creates a copy of the patient medical template for
population.
[00306] After providing a patient medical template for population,
the clinician
server 200b determines at least one medical device from which medical
information is needed
(using a routine associated with the template or reading the template itself)
and accordingly
transmits a first camera message 2305 to the personal mobile communication
device 200a (block
2306). The first camera message 2305 includes instructions indicating that an
image is to be
recorded of, for example, an identifier 1012 of a medical device. Some time
later, the clinician
83
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
server 200b receives a message 2307 that includes medical information
indicative of a type of
medical device (e.g., medical information from an identifier 1012a) (block
2308). The clinician
server 200b then determines a device data template (e.g., the device data
template 1700 of Fig. 17)
based on information included within the message 2307 or specified in a
patient's medical record
(block 2310). For example, after determining that the message 2307 identifies
a blood pressure
monitor 104 (type and/or model), the clinician server 200b determines or
locates a device data
template for the blood pressure monitor. The clinician server 200b loads the
device data template
for identifying relevant medical device data from an image received of a
screen of the blood
pressure monitor 104 (block 2312).
[00307] Example procedure 2300 continues in Fig 24 where the
clinician server
200b transmits a second camera message 2313 to the personal mobile
communication device 200a
(block 2314). The second camera message 2313 may be determined based on a type
of medical
device specified by the message 2307. Further, the second camera message 2313
may include
information for displaying a certain window (or relevant medical information)
on a medical device
for recording an image. Some time later, the clinician server 200b receives a
message 2315 that
includes text from or an image of the window of the medical device (block
2316). The example
clinician server 200b uses the identified data template to extract relevant
medical information from
an image and determines or otherwise identifies the data fields of the patient
medical template that
correspond to the relevant medical information (block 2318). If the message
2315 includes text,
the clinician server 200b determines or otherwise identifies the data fields
of the patient medical
template that correspond to the relevant medical information. The clinician
server 200b next
populates the determined and/or identified data fields of the template with
the relevant received
medical information (block 2320).
[00308] After populating the relevant data fields, the example
clinician server 200b
determines if additional relevant medical information is needed from the
medical device associated
with the received relevant medical information (decision block 2322). For
example, the clinician
server 200b may determine that the current medical device may include
additional windows or
operating displays from which relevant medical information is still needed. If
additional medical
information is needed, the example clinician server 200b returns to block 2314
and transmits a
camera message 2313 for another window for which relevant medical information
is needed.
However, if no additional medical information is needed for the current
medical device, the
84
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
clinician server 200b determines if medical information is needed from other
medical devices (or
consumable item 1006) (decision block 2324). If additional medical information
is needed, the
clinician server 200b returns to block 2306 and transmits a camera message
2305 identifying
another medical device for imaging or from which to receive related medical
information. If no
additional medical information is needed for completion of the patient medical
template 2100, the
example clinician server 200b stores the completed patient medical template
2100 to the clinician
database 1010 as a patient's medical record and procedure 2300 ends.
[00309] The example procedure 2350 begins on Fig. 23 by the personal
mobile
communication device 200a transmitting a message 2301 that is indicative of a
treatment to be
performed on a patient or a request to begin entering medical information
(2352). The personal
mobile communication device 200a next receives a camera message 2305 from the
clinician server
200b. Information from the message 2305 is used by the personal mobile
communication device
200a to display a prompt to a patient (block 2354). The prompt may specify,
for example, that an
identifier 1012 of a medical device is to be imaged. The personal mobile
communication device
200a then records an image of identifier 1012 of a medical device (block 2356)
based on input
from the patient. In some embodiments, the patient may enter text specifying a
medical device
type/model or select from a drop-down menu if an identifier is not available
or the patient does not
want to (or cannot) record an image.
[00310] After recording an image of the identifier 1012, the
personal mobile
communication device 200a extracts or otherwise determines medical information
encoded in the
identifier (block 2358). The personal mobile communication device 200a sends
the extracted
medical information in a message 2307 to the clinician server 200b. In some
embodiments, the
personal mobile communication device 200a transmits instead the recorded image
within the
message 2307.
[00311] Afterwards, the personal mobile communication device 200a
receives a
camera message 2313 from the server 200b with information for displaying a
prompt to a patient
for recording an image of a screen (or other specified area) of a medical
device (block 2360). The
personal mobile communication device 200a accordingly displays a prompt to the
patient with
information needed for imaging a medical device. Responsive to the prompt, the
patient uses the
personal mobile communication device 200a to record an image of a screen (or
other specified
area) of medical device (block 2362).
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00312] In Fig. 24, the example procedure 2350 continues by the
personal mobile
communication device 200a creating a text message with the recorded image or
text entered by the
patient (block 2364). In some examples, the patient may modify or specify the
medical
information. The personal mobile communication device 200a then transmits a
message 2315 that
includes the medical information or image thereof (block 2366).
[00313] The example the personal mobile communication device 200a
next
determines if additional relevant medical information is needed from the
medical device associated
with the extracted relevant medical information (decision block 2368). The
determination may
include, for example, checking to see if additional camera messages related to
the current medical
device are received from the clinician server (block 2360). If additional
medical information is
needed, the example the personal mobile communication device 200a returns to
block 2360 and
processes a camera message 2313 for another window (or portion of the medical
device/consumable item) for which relevant medical information is needed.
However, if no
additional medical information is needed for the current medical device, the
personal mobile
communication device 200a determines if medical information is needed from
other medical
devices (or consumable item 1006) (decision block 2370). If additional medical
information is
needed, the personal mobile communication device 200a returns to block 2354
and processes a
camera message 2305 identifying another medical device for imaging. If no
additional medical
information is needed for completion of the patient medical template, the
example personal mobile
communication device 200a ends the session, thereby ending the procedure 2350.
6. Manual/Wireless/Image Medical Information Entry via A Web Browser or File
Transfer
[00314] In some embodiments, the data acquisition module 1104 of
Fig. 11 is
configured to enable a patient to use their personal mobile communication
device 200a to enter
medical information or provide images via a web browser or file transfer
program. The web
browser or file transfer program enables the personal mobile communication
device 200a to
provide medical information and/or images without using the application 1002.
In this
embodiment, the clinician server 200b hosts a website, API(s), and/or a file
transfer site that is
assigned an address or Uniform Resource Locator ("URL"). A web browser or file
transfer
86
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
program on the personal mobile communication device 200a is configured to
access the hosted
website, API(s), and/or file transfer site to transmit medical information.
[00315] In some embodiments, the data acquisition module 1104 is
configured to
prompt a patient to enter a username and password to access the website or
file transfer site. In
other embodiments, the data acquisition module 1104 determines the personal
mobile
communication device 200a is previously registered and provides access. In yet
other
embodiments, the data acquisition module 1104 provides a mailbox or other
interface (e.g., an
API) to receive medical information or images without providing the personal
mobile
communication device 200a access to a more secure location.
[00316] Once a patient gains access via the personal mobile
communication device
200a, the data acquisition module 1104, in some embodiments, provides a
graphical user interface
that prompts a patient for medical information and/or images. The user
interface may be similar
to the user interfaces 1400 and 1500 described in connection with Figs. 14 and
15. Similar to the
personal mobile communication device 200a containing the application 1002, the
clinician server
200b provides the user interfaces and data fields to receive medical
information. The data
acquisition module 1104 may also enable a patient to select a data entry
method, such as text or an
image.
[00317] In some embodiments, the clinician server 200b may provide a
menu to
enable a patient to select the appropriate user interface. In other
embodiments, the clinician server
200b may select the user interface(s) for which medical information is needed
based on a
prescribed treatment or treatment information provided by a patient. In yet
other embodiments,
the application 1020 of the clinician server 200b may operate a routine 2150
that provides
graphical prompts for medical information.
[00318] Fig. 25 shows a diagram of the clinician server 200b hosting
a website or
file transfer site to receive medical information via an image from the
personal mobile
communication device 200a, according to an example embodiment of the present
disclosure. In
the illustrated example, the personal mobile communication device 200a is
operating a web
browser application 2500 that is directed to a website 2502 that is hosted by
the clinician server
200b. The website 2502 includes a graphical user interface for entering
medical information. In
this example, the personal mobile communication device 200a records an image
1800 of a screen
of the blood pressure monitor 104 of Fig. 10. The clinician server 200b
applies a data template
87
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
1801 to the image to extract sets of text, as shown in fields 1802, 804, 1806,
1808, and 1810. The
patient selects the field(s) 1802, 804, 1806, 1808, and 1810 for which text is
to be populated into
one or more selected data fields of the website 2502. In this manner, the
clinician server 200b
enables a patient to provide medical information via a website, file transfer
program, or API(s).
C. Data Access Module Embodiment
[00319] Returning to Fig. 11, the example data access module 1106 is
configured to
provide patients and clinicians access to medical information stored in
medical records at the
clinician database 1010. As described above in connection with the data
acquisition module 1104,
there are different possible configurations of the personal mobile
communication device 200a
where the application 1002 may or may not be installed. The data access module
1106 is
configured to provide access or otherwise display data based on the
configurations. To determine
which configuration is in use by a particular patient, the example data access
module 1106 is
configured to access a registration table or the patient's medical records to
identify the registered
personal mobile communication device 200a and/or whether the application 1002
is installed.
[00320] In some embodiments, the data access module 1106 is
configured to provide
the medical information differently based on an operating system of the
personal mobile
communication device 200a and/or a capability of the personal mobile
communication device
200a. For example, a first subset of medical information may be specified for
feature-rich personal
mobile communication devices 200a while a second subset of medical information
is provided for
feature-lite personal mobile communication devices 200a. Based on registration
information, the
data access module 1104 determines if the application 1002 is operating on a
feature-rich or
feature-lite device and selects the corresponding first or second subset of
medical information.
[00321] The example data access module 1106 may also determine if
the medical
and/or treatment information in a patient's medical record is to be converted
to a different format
prior to transmission. For example, medical information and/or treatment
information may be
stored in a medical record in an HL7 format. However, this format is not
conducive for text
messages and/or applications. The example data access module 1106 determines
capabilities of
the personal mobile communication device 200a to which the data is to be
transmitted. The data
access module 1106 determines capabilities based on registration information
that indicates
whether the application 1002 is installed and/or if the personal mobile
communication device 200a
88
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
is a feature-rich device. For viewing medical information on the application
1002, the data access
module 1106 uses one or more APIs to convert the medical information and/or
treatment
information from an HL7 format to, for example, an HyperText Markup Language
("HTML")
format, a JavaScript Object Notation ("JSON") format, or XML format (e.g., an
application
format). If the data access module 1106 determines that the application 1002
is not installed, the
data access module 1106 converts the information from an HL7 format, to a text
message or SMS
message format via, for example, one or more APIs. The example data access
module 1106
accordingly enables patient medical record information to be viewed by a
patient regardless of the
capabilities of their personal mobile communication devices 200a.
1. Information Display via Application Embodiment
[00322] In some embodiments, the data access module 1106 is
configured to display
medical and/or treatment information via the application 1002 that is
installed on the personal
mobile communication device 200a. In these examples, the data access module
1106 provides
medical information from one or more patient's records for display in
specified fields, graphs, etc.
of one or more user interfaces that are provided by the application 1002. In
some instances, the
fields from a medical record are referenced to fields of a user interface of
the application 1002.
[00323] Figs. 26 to 29 show diagrams of the application 1002 on the
personal mobile
communication device 200a displaying medical information provided by the
access module 1006,
according to example embodiments of the present disclosure. The application
1002 may be
configured to display different user interfaces 2600, 2700, 2800, and 2900
upon selection by a
patient. In some instances, the application 1002 provides a menu or other
selectable graphical
feature listing the different user interfaces available. The application 1002
may be configured to
receive the medical information via one or more APIs linked to data fields of
a patient's medical
record(s). In other words, the medical information from a patient's records is
plugged into blank
templates that define a graphical user interface for displaying medical
information.
[00324] The user interface 2600 of Fig. 26 provides a first graph
2602 that illustrates
a total UF removed per day and a second graph 2604 that illustrates an average
UF removed per
separate day/night exchanges. It should be appreciated that the medical
information displayed
within the graphs 2602 and 2604 may have originated from the home therapy
machine 90 and/or
one or more medical devices. The data access module 1106 of the clinician
server 200b provides
89
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
access to medical information from different sources as long as the
information is already stored
to a patient's medical record, thereby providing informational transparency to
the patient.
[00325] The user interface 2600 is configured to enable a patient to
select data points
on the graphs 2602 and 2604 to provide additional treatment information. The
user interface 2600
also enables a patient to select a time-range for the graphs 2602 and 2604.
After receiving a
selection, the application 1002 transmits a message to the data access module
1106 indicative of
the selection. In return, the data access module 1106 provides the requested
medical information.
[00326] The example user interface 2700 of Fig. 27 illustrates an
average drain time
for each UF exchange. The drain time is provided in a graph 2702. Between the
user interfaces
2600 and 2700, a patient can gauge how a treatment is progressing over time.
Any deviations in
treatment should be readily apparent and help convince the patient to adhere
to the prescribed
therapy.
[00327] The example user interface 2800 includes a calendar that
indicates the days
in which a patient adhered to a treatment or therapy compared to days in which
a patient did not
adhere to a therapy. A patient may select one of the days to view additional
medical information.
For example, the user interface 2900 shows treatment or medical information
for April 26. The
information includes a total amount of UF removed during the day, in addition
to a breakdown of
UF removed during a manual exchange compared to UF removed via the home
therapy machine
90. In the illustrated embodiment, the machine treatment information includes
a program name, a
prescribed therapy time, an actual therapy time, and an amount of UF removed.
The data access
module 1106 may determine if a treatment was adhered to based on the actual
treatment time being
within a threshold of the prescribed treatment time. In some embodiments, the
data access module
1106 and/or the data acquisition module 1104 may determine adherence at a time
when the medical
or treatment information is received and set a corresponding flag or other
indication in the patient's
medical record to reflect adherence or lack thereof.
[00328] In some examples, the treatment information for the manual
exchange is
entered by the patient via the application 1002 on the personal mobile
communication device 200a
while the machine treatment information is separately received from the home
therapy machine
90. In other examples, the user interface 2900 may display treatments that
occurred at a patient's
home and treatments that occurred at a clinic. The information may be
organized based on which
machine the information was received from, a program name, treatment type,
prescription, etc.
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
The user interface 2900 in the illustrated example accordingly provides for a
single display of UF
removed for different exchanges, thereby providing patient information
regarding an effectiveness
of a manual exchange compared to a machine-operated exchange. The example
system 100 of
Fig. 10 enables information from both exchanges to be stored together (based
on a day of
treatment) for subsequent display and/or analysis.
2. Information Display via Web Browser Embodiment
[00329] In some embodiments, the personal mobile communication
device 200a
may not have the application 1002 installed. Instead, a patient may use a web
browsing application
to access a website or interface hosted by the clinician server 200b. In these
embodiments, the
data access module 1006 is configured to display the medical information
and/or treatment
information in one or more webpage, similar to the user interfaces 2600 to
2900 of Figs. 26 to 29.
In this embodiment, the personal mobile communication device 200a accesses the
data access
module 1106 via a website. Selection of a user interface or feature via the
personal mobile
communication device 200a causes the data access module 1106 to display the
medical
information within the webpage. The data access module 1106 may be configured
to format or
render the medical information and/or treatment information based on a web
browser type and/or
operating system of the personal mobile communication device 200a. In some
instances, the data
access module 1106 may request a username and password from a patient prior to
providing access
to the website.
3. Information Display via Text Embodiment
[00330] In some embodiments, the data access module 1106 is
configured to provide
medical information and/or treatment information to the personal mobile
communication device
200a via text messages. In these examples, the data access module 1106 may
provide the
information in response to a text received from the personal mobile
communication device 200a.
In other examples, the data access module 1106 may transmit the medical and/or
treatment
information periodically (e.g., daily, weekly, etc.) to a patient's personal
mobile communication
device 200a.
[00331] In an example, the data access module 1106 may receive a
text message
including text of "send 7 day UF data". In response, the data access module
1106 identifies a
91
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
patient record that corresponds to the phone number of the personal mobile
communication device
200a from which the text message was received. The data access module 1106 may
then use a
look-up table or keyword search to identify fields in a patient's medical
record that includes
matching sets of characters or text related to UF data (e.g., "7 day UF"). The
data access module
1106 copies the matching text and creates a reply message with a value of UF
for the previous
seven days, which is transmitted to the personal mobile communication device
200a. Additionally
or alternatively, the data access module 1106 creates and renders a seven-day
graph, similar to the
graph 2602 in Fig. 26. The data access module 1106 creates an image of the
graph, which is
transmitted to the personal mobile communication device 200a as an image in a
text message. The
image may be stored a .jpeg, .gif, .png, etc. file. A patient of the personal
mobile communication
device 200a may view the graph via the text message. In this manner, the
example data access
module 1106 is configured to provide patient access to medical and treatment
information
regardless of the capabilities and/or operating system of their personal
mobile communication
device 200a. In addition, the data access module 1106 determines how data is
to be transmitted
based on registration information provided by a patient and/or an indication
of whether the
application 1002 is installed on the patient's personal mobile communication
device 200a.
4. Alarm/Alert Embodiments
[00332] In addition to providing a display of medical and/or
treatment information,
the example data access module 1106 of Fig. 11 is configured to determine
and/or generate alarms
and/or alerts for patients and/or clinicians. The data access module 1106 may
send the alarms
and/or alerts to a personal mobile communication device 200a of a patient or a
clinician device
152. In some embodiments, the data access module 1106 may include a rules
table that specifies
to which devices certain alarms/alerts are to be transmitted. A clinician may
use the clinician
device 152 to subscribe to certain alarms/alerts and/or patients.
[00333] The data access module 1106 is configured to provide
alarms/alerts based
on how the personal mobile communication device 200a is configured to display
medical and/or
treatment information. For instance, if the application 1002 is installed, the
data access module
1106 provides the alarms/alerts through the appropriate user interface of the
application 1002. In
some instances, the application 1002 is configured to display a notification
indicative of the
alarm/alert. If the application 1002 is not installed, the data access module
1106 determines that
92
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
an alarm and/or alert is to be transmitted via an SMS or other text message to
the personal mobile
communication device 200a.
[00334] In some embodiments, the data access module 1106 includes or
has access
to a data structure or list that specifies certain conditions under which an
alarm or alert is to be
generated. In an example, a condition may compare a single data value or a
trend (e.g., a 30-day
moving average) to a range of acceptable values and/or a threshold (which may
comprise hard
and/or soft limits). In other examples, alarms or alerts may be generated
based on multiple
conditions based on comparing different types of information to respective
limits. In some
instances, the data access module 1106 determines derived information
calculated from treatment
and/or medical information, which is then compared to an acceptable range. The
following
provides examples of alarms/alerts that may be transmitted by the data access
module 1106 via
text message and/or displayed within the application 1002.
[00335] In an example, the data access module 1106 may generate an
alarm or alert
if it is detected that a patient is beginning to deviate from a prescribed
treatment. For example,
after detecting that two days have passed since treatment information has been
received, the data
access module 1106 (operating according to an alert rule) transmits an alert
message to the personal
mobile communication device 200a. The alert message specifies, for example,
that the patient
should perform a treatment. The alert message may also include information (or
a link to
information) that describes why the treatment is important or describes to the
patient what happens
to their body if a treatment is not performed in a timely manner. If the data
access module 1106
detects, for example, four days without treatment information being received,
the data access
module 1106 promotes the alert to an alarm (operating according to an alarm
rule). The data access
module 1106 transmits the alarm to the personal mobile communication device
200a and/or the
clinician device 152 to provide an indication of the severity of not adhering
to a treatment. As
mentioned above, the alarms and/or alerts may be transmitted by the data
access module 1106
based on whether the application 1002 is installed. Even if the application
1002 is installed, the
data access module 1106 is configured to transmit a text message to the
personal mobile
communication device 200a to flag the attention of the patient.
[00336] In some examples, a patient is prescribed a manual exchange.
Accordingly,
the patient has to provide treatment information indicative of the manual
exchange. The data
acquisition module 1106 is configured to transmit an alarm or alert if the
manual exchange
93
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
information is not received within a predetermined time period (e.g., within 2
days after a
scheduled treatment). Further, if a manual exchange was not completed properly
(e.g., a short fill
or dwell time), the data access module 1106 sends an alert and/or an alarm
regarding the
insufficient exchange. To determine an insufficient exchange, the data access
module 1106 may
compare fill, dwell, and/or drain times to pre-established acceptable ranges
and/or thresholds. In
some instances, the data access module 1106 may request that the patient
perform a full exchange.
[00337] In an example, an alarm and/or alert may be generated to
indicate that a
patient should select a different treatment program from a plurality of
prescribed treatment
programs or make an adjustment to a treatment program. In this example, an
alarm or alert rule
may specify that the data access module 1106 is to compare a patient's blood
pressure or weight
to respective change thresholds while calculating and comparing an accumulated
fluid value to a
threshold. The accumulated fluid value may be determined from individual fluid
fill and drain
volumes, which is indicative of fluid left in a patient's peritoneal cavity.
If the blood pressure or
weight, and accumulated fluid values (or trends) are outside of acceptable
ranges, the data access
module 1106 generates an alarm. The alarm may indicate that the patient has
accumulated fluid
and that a treatment program with a longer drain duration (or a shorter fill
duration) should be
selected (or a treatment program should be adjusted to provide a longer drain
duration or shorter
fill duration). The data access module 1106 transmits the alarm for display on
the personal mobile
communication device 200a. A patient may respond via the application 1002 or a
text message,
causing the clinician server 200b to make the appropriate change to the
patient's prescription or
therapy program.
[00338] In some embodiments, alarms and/or alerts may specify
conditions under
which additional information is to be prompted from a patient. In these
embodiments, the data
access module 1106 uses treatment information from the home therapy machine 90
as a basis for
determining if a patient is to provide medical information via the personal
mobile communication
device 200a. In an example, the data acquisition module 1106 may access alarm
and/or alert rules
that specify conditions under which a prompt or text message is sent to the
personal mobile
communication device 200a of the patient prompting for an additional weight or
blood pressure
measurement. For example, an alarm or alert may specify that a prompt for a
new blood pressure
measurement is needed if a blood pressure value (or trend) exceeds a
predetermined threshold. In
other examples, an alarm or alert may specify that a prompt for a new blood
pressure measurement
94
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
is needed if an accumulated fluid value or UF removed value (or trend) is
outside of an acceptable
range. In response, the data access module 1106 transmits a text message or a
notification via the
application 1002 for the patient to perform and record a blood pressure
measurement. In some
instances, the application 1002 may open a user interface (e.g., the user
interface 3000 of Fig. 30)
prompting the patient to enter blood pressure information. If the additional
data received from the
personal mobile communication device 200a is not within an acceptable range,
the data access
module 1106 may promote an alert to an alarm that is transmitted to the
clinician device 152 and/or
the personal mobile communication device 200a. Thus, the data access module
1106 is configured
to operate rules that determine borderline patient conditions, which seek
additional information
from a patient before deciding whether further attention or action is required
from a clinician or
the patient.
[00339] In further embodiments, rules of the data access module 1106
may instruct
a patient to inspect and/or make a change to the home therapy machine 90. In
an example, a rule
may specify that the data access module 1106 is to transmit an alert to a
patient if treatment
information has not been received from the home therapy machine 90 within a
defined time period
(e.g., two days). Under this situation, a patient may have provided medical
information indicative
that a treatment occurred, which the data access module 1106 uses to determine
that an alert
regarding adhering to a treatment is not necessary. Instead, the data access
nodule 1106 determines
that an alert is to be transmitted to the patient to check a network
connection of the home therapy
machine 90 so that the treatment information stored on the machine 90 can be
retrieved. A patient
may respond to the alert using the personal mobile communication device 200a
to indicate that the
connection was checked. After the response from the patient is received, the
data acquisition
module 1104 may send a ping message to the home therapy machine 90 for the
missing treatment
information. If a connection still cannot be made, the data access module 1106
may send an alert
to the patient, a clinician, and/or a network administrator with more specific
instructions for
activating the home therapy machine 90 and/or overcoming the network
connectivity issue.
[00340] In another example, the data access module 1106 may include
one or more
rules that specify an alert is to be generated responsive to treatment
information being out of range.
For example, a large difference between fluid fill and drain volumes may be
indicative of a leak
in dialysis tubing or a connection to a patient. In response, the data access
module 1106 transmits
an alert to the patient prompting the patient to verify fluid connections. A
patient may provide a
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
response indicating whether a leak was detected. In response, the data access
module 1106
determines if the leak was corrected in a subsequent treatment cycle (or prime
sequence) or if
tubing should be replaced. The data access module 1106 may determine similar
consumable issues
for cassettes, cartridges, etc. based on treatment and/or medical information.
For example, a low
volume of UF removed may prompt the data access module 1106 to transmit an
alert for a patient
to verify a concentration of dialysis fluid and/or concentrate being connected
to the home therapy
machine 90.
D. Treatment Control Module Embodiment
[00341] The example treatment control module 1110 of Fig. 11 is
configured to
enable a patient and/or clinician to change a prescription and/or program on a
home therapy
machine 90. To operate, the home therapy machine 90 is assigned one or more
prescriptions for a
patient. A prescription may specify a treatment type (e.g., automated
peritoneal dialysis treatment,
manual exchange treatment, hemodialysis treatment, etc.) a time period in
which a patient is to
receive treatments, a dextrose level or other concentrate level of treatment
fluid, a daily amount of
UF to remove, and/or a number of times per day or duration range for each
treatment. Each
prescription may include one or more programs. A program may specify a
treatment duration, a
total volume of fluid to be provided to a patient, a number of fill, dwell,
drain cycles to be repeated,
and/or an indication whether the treatment comprises a tidal therapy. The
differences in programs
within a prescription enable a patient or clinician to change certain
treatment parameters based on
a condition or activity of a patient.
[00342] A prescription and associated programs are stored to an
electronic
prescription in the clinician database 1010. In some embodiments, the
prescription may be stored
to a patient's medical record. The home therapy machine 90 is programmed with
one or more
prescriptions. The programming may be performed locally via a clinician or
patient, or remotely
from the clinician server 200b. For example, the clinician server 200b (e.g.,
the treatment control
module 1110) may send a copy of a prescription from the clinician database
1010 to the home
therapy machine 90 after registration.
[00343] In some embodiments, the example treatment control module
1110 is
configured to operate in connection with the application 1002 on the personal
mobile
communication device 200a and/or an application on the clinician device 152 to
enable a patient
96
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
and/or clinician to select a different prescription program and/or
prescription. Fig. 31 shows a
diagram of a user interface 3100 of the application 1002 that enables a
patient to select between
three different programs: a Short Program, a Long Program, and a Weekend
Program. A patient
may select a program based on their activity and/or circumstances. In some
embodiments, the
application 1002 may display an alert from the data access module 1106 that
provides a
recommendation to change a program based on a detected accumulation of fluid,
weight gain, or
higher blood pressure.
[00344] After a patient selects a different program via the user
interface 3100, the
application 1002 sends a message to the treatment control module 1110
indicative of the selection.
In response, the treatment control module 1110 updates the patient's medical
record to reflect the
changed program, including a time/date of the change. Further, the treatment
control module 1110
transmits a message to the home therapy machine 90 providing an instruction to
change to the
selected program. In some instances, the message may include treatment
parameters for the newly
selected program. The home therapy machine 90 accordingly performs a next
treatment based on
the newly selected program. In some instances, the home therapy machine 90 may
display a
prompt for a patient to confirm the new program before operating according to
the program.
[00345] In some embodiments, the treatment control module 1110 may
perform a
check to verify that the patient is authorized to make a change to a program
and/or whether the
change is permitted. For example, the treatment control module 1110 receives
an indication that
a patient desires to change from the Short Program to the Long Program. The
treatment control
module 1110 compares to patient's medical information to one or more
thresholds to ensure the
change will not negatively affect the patient. For instance, the treatment
control module 1110 may
not approve the change from the Short to the Long Program if the patient has
already accumulated
a relatively large volume of fluid. If a change cannot be made, the treatment
control module 1110
sends a message to the personal mobile communication device 200a indicative as
to why the
change cannot be made.
[00346] In an embodiment, the treatment control module 1110 may send
a
notification to the clinician device 152 indicative that the patient desires
to change a program. The
treatment control module 1110 may not communicate the change to the home
therapy machine 90
until a confirmation is received from the clinician device 152. In some
embodiments, a clinician
may change a program and/or prescription via the treatment control module 1110
using their
97
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
clinician device 152. In these embodiments, the treatment control module 1110
may send a
message for display in the application 1002 of the personal mobile
communication device 200a
indicative of the change in program and/or indicative that a clinician made
the change.
[00347] In instances where the personal mobile communication device
200a does
not include the application 1002, the treatment control module 1110 is
configured to permit
changes to a prescription. For example, the treatment control module 1110 may
host a website
accessible by a web browser on the personal mobile communication device 200a.
The website
may include features similar to the user interface 3100 of Fig. 31 to enable a
patient to remotely
select a different program and/or prescription.
[00348] Additionally or alternatively, the treatment control module
1110 is
configured to enable treatment changes via text messages. In an example, a
patient may send a
message from the personal mobile communication device 200a including text of
"change
treatment". In response, the treatment control module 1110 is configured to
send a reply message
with different treatment program options and a corresponding code or
designator next to each
option. A patient may enter the designator or code in a response message to
select a desired
program. In another example, a patient may send a message with text consisting
of, for example,
"Long Program" to cause the treatment control module 1110 to change programs
to the Long
Program.
E. Education Module Embodiment
[00349] The example education module 1108 of Fig. 11 is configured
to provide
educational material and/or encouragement to a patient via the personal mobile
communication
device 200a. Similar to the other modules 1102 to 1106 and 1110 of Fig. 11,
the education module
1108 is configured to provide content/information based on a configuration of
the personal mobile
communication device 200a. For example, if the application 1002 is installed,
the education
module 1108 is configured to select and provide educational material through
the application 1002.
If the application is not installed, the education module 1108 is configured
to provide educational
material via a website and/or text messages. In instances of text messages,
the education module
1108 may be configured to structure educational material to fit within a text
message or provide a
link to educational material at the clinician database 1010 or hosted by a
third-party server.
98
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00350] As described herein, educational material may include text-
based articles,
audio, video, multimedia presentations, etc. The educational material may
provide general
information about a patient's condition, information about the application
1002, and/or
information regarding the home therapy machine 90. The educational material
may also be
targeted based on detected patient conditions (determined from their medical
record) and/or
feedback regarding a patient's use of the application 1002 and/or the home
therapy machine 90.
[00351] Also, as described herein, encouragement includes text,
audio, video, or
multimedia presentations designed to improve a patient's mood or help a
patient adhere to a
program. Encouragement may also include rewards or badges. In some
embodiments, the
education module 1108 may be configured to provide encouragement based on
detected
conditions. In some instances, the encouragement may be provided in
combination with an alert
generated by the data access module 1106. In an example of encouragement, a
different status
level may be provided to a patient based on a rate of adherence (e.g.,
"Dialysis SuperStar" for
near-perfect adherence).
[00352] The educational material and/or encouragement may be stored
in the
clinician database 1010. In addition, the education module 1108 may have
access to third-party
material, such as from the National Institute of Health or the Cleveland
Clinic . The education
module 1108 may include a rules data structure that specifies conditions under
which certain
educational material and/or encouragement is to be provided to the personal
mobile
communication device 200a.
[00353] In an example, the data access module 1106 determines a
patient is not
adhering to a treatment. In addition to the data access module 1106 sending an
alert, the education
module 1108 may recommend or provide a link to a video regarding the
importance of adherence.
Fig. 32 shows an example user interface 3200 of the application 1002
displaying an educational
video related to adherence provided by the education module 1108. The example
user interface
3200 also provides a list of recommended educational material based on
detected conditions of the
patient. For example, after detecting the patient has high blood pressure from
a medical record or
received as medical information, the education module 1108 is configured to
recommend
educational content about lowing blood pressure. Further, after detecting that
a patient has not
changed programs of a prescription, the education module 1108 determines that
educational
content explaining the program options should be recommended.
99
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
[00354] The example education module 1108 may also detect how a
patient interacts
with the application 1002 and recommend educational content. For example, the
education module
1108 receives feedback from the application 1002 indicative that a patient has
made multiple
attempts in populating a data field of a user interface (e.g., the user
interface 14 of Fig. 14) using
a recorded image. In response, the education module 1108 may provide a
notification via the
application 1002 recommending that the patient view a tutorial regarding
information entry using
a recorded image.
[00355] The example education module 1108 may store to a patient's
medical record
an indication of any educational content or encouragement displayed by the
personal mobile
communication device 200a. Such information may be useful to a clinician to
determine how
engaged a patient is with a treatment. The stored information may also provide
an indication of a
patient's awareness of a treatment.
F. Assistance Module Embodiment
[00356] The example assistance module 1112 of Fig. 12 is configured
to create a
communication session between a patient and a clinician. Specifically, the
assistance module 1112
is configured to create a communication session between a clinician device 152
and the personal
mobile communication device 200a. The assistance module 1112 may use a
capability of the
personal mobile communication device 200a to determine an appropriate
communication session.
[00357] A communication session may include a video session, an
audio call, a
conference call, an SMS session, or a web-messaging session. If the
application 1002 is installed,
the assistance module 1112 may access an option for selecting a video session,
a conference
session, or a web-messaging session for connecting a patient to a clinician
through the application
1002. If the application 1002 is not installed, the assistance module 1112 may
be limited to options
related to the native communication capabilities of the personal mobile
communication device
200a, such as text messaging and audio calls.
[00358] In some embodiments, a patient may initiate a session. The
application
1002 is configured to enable a patient to request to contact a clinician,
including specifying a
preferred communication method. Using a text feature of the application 1002,
a patient may send
a text message including text of "help" to the assistance module 1112 to begin
a session. After a
patient requests to begin a session, the assistance module 1112 is configured
to identify an
100
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
available clinician. In some embodiments, the assistance module 1112 may
locate a record of
clinicians associated with the patient and send a ping/request message to
their devices. A received
response from one of the devices 152 provides an indication that the clinician
is available and
willing to communicate with the patient. In some instances, the response may
indicate the
clinician's method of communication. In response to a received message, the
assistance module
1112 begins a communication session. This may include establishing a web call,
a messaging
session, or audio call between a personal mobile communication device 200a and
a clinician device
152. In some embodiments, instead of broadcasting a ping message to a group of
clinicians, the
assistance module 1112 may sequentially send ping/request messages to
clinicians according to a
predetermined order (e.g., primacy clinician: first, on-call clinicians of the
same office: second,
on-call clinicians of a different office: third, etc.). Fig. 33 shows a
diagram of a user interface
3300 of the application 1002 providing a video session within a clinician. The
video session
enables a patient to address their concerns regarding their treatment. The
video session also
enables a clinician to view a real-time setup of the treatment or assist a
patient setting up a
treatment.
[00359] In some embodiments, the assistance module 1112 may
determine that a
communication session is to be opened or recommends opening a communication
session. The
assistance module 1112 may provide a recommendation in conjunction with the
data access
module 1106 generating an alarm and/or alert. The assistance module 1112 may,
after detecting
conditions for an alert and/or alarm, identify a clinician device 152 that is
available to participate
in a session and then initiate a call to the personal mobile communication
device 200a to address
an alert and/or alarm. In an example, the assistance module 1112 may attempt
to create a
communication session after detecting a patient's blood pressure, weight,
accumulated fluid, etc.
is above a predetermined threshold or has changed significantly within a
predetermined period of
time.
[00360] The example assistance module 1112 may determine a type of
assistance
being sought to identify the correct individual for connection. In addition to
a clinician, the
assistance module 1112 may be able to connect to information technology
specialists and home
therapy machine specialists. Before initiating a session, the application 1002
may prompt a patient
to identify an assistance type (e.g., application help, machine help, clinical
help, etc.). In other
instances, the assistance module 1112 may determine an assistance type based
on a context in
101
CA 03110999 2021-02-25
WO 2020/051325 PCT/US2019/049737
which a request for a session was received. For example, after reviewing an
educational training
program about the home therapy machine 90, the assistance module 1112
determines that a
subsequent request for assistance is in regards to operating the home therapy
machine 90. In
another example, while the application 1002 is displaying a user interface
regarding UF trending,
the assistance module 1112 determines that a subsequent request for assistance
is in regards to a
clinical matter.
[00361] The example assistance module 1112 may be configured to
record a log of
a communication session to a patient's medical record. The assistance module
1112 may record a
date/time of a communication session and an indication of how the session was
initiated. The
record may also include participants of the communication session as well as a
transcript of the
communications. For text messages, this may include a copy of the messages.
For audio or video,
this may include a recording of the call or a transcription of the call.
III. Conclusion
[00362] It should be understood that various changes and
modifications to the
presently preferred embodiments described herein will be apparent to those
skilled in the art. Such
changes and modifications can be made without departing from the spirit and
scope of the present
subject matter and without diminishing its intended advantages. It is
therefore intended that such
changes and modifications be covered by the appended claims.
102