Note: Descriptions are shown in the official language in which they were submitted.
GO1N27
DEVICE FOR ELECTROPHORETIC ANALYSIS OF MULTICOMPONENT LIQUID SAMPLES
Technical Field
The present disclosure relates to analytical chemistry in general and in
particular to a capillary
electrophoresis (CE) method for use in analysis of multicomponent liquid
samples.
Background of the Invention
Composition of multicomponent liquid samples can be determined with separation
methods.
There are several approaches to electrophoretic analysis of multicomponent
solutions based on
high voltage electric field application to a mixture of components for their
separation in a
quartz capillary. Capillary electrophoresis (CE) systems provide effective
applications for food
industry, environmental applications, pharmaceutical and chemical industries,
biochemistry
and forensic science.
Capillary electrophoretic analysis involves the injection of analyzed sample
into a narrow quartz
capillary that is first washed and filled with an electrolyte solution (known
as a buffer solution).
High voltage is applied on both ends of the capillary and mixture components
of
multicomponent liquid sample start migrating at different velocity, driven by
the electrical field.
When a migrating analyte reaches a detector (such as an optical detector based
on direct or
indirect ultraviolet light (UV) absorbance) located at a specified distance
from capillary inlet,
analytical signals related to the analyte's quantity and times of the
analyte's migration are
measured. Migration time is calculated as an interval between an onset of
electrophoretic
separation and a time point when a component is detected.
The detector response that is recorded when a single analyte passes through
the detector zone
is known as electrophoretic peak. The detector response recorded as a function
of time is
known as an electropherogram, which can be used for qualitative identification
and
quantitative determination of analytes that correspond to different peaks.
Different
components of a sample can be identified by comparing each detected
component's migration
time with reference migration time of analytes in a calibration (standard)
solution. Quantitative
1
Date Recue/Date Received 2021-03-25
determination of the components in a sample can be done by comparing the
detector response
with reference electrophoretic peaks or electropherograms for analytes in a
calibration
solution.
Migration times depend on migration velocity of components of the buffer and
the buffer
velocity in the capillary. The latter depends on buffer composition and
condition of capillary
walls. If the previous runs contaminate the capillary walls, it affects
electroosmotic flow and
changes migration times.
Charged particle migration rate in the capillary during electrophoretic
separation is equal to
algebraic sum of particle electromigration rate and electroosmotic flow (EOF)
rate. Particle
electromigration rate depends on properties of the particle (its charge, mass
and structure),
while EOF rate depends on capillary features. At specified values of
composition and
concentration of buffer electrolyte, EOF rate is determined by equation:
7 EE
Aeof = L)-47.õ7 (1)
where Aeof - EOF rate, - electrokinetic surface potential, ¨ dielectric
constant of solution, E -
electric-field intensity, q - solution viscosity of buffer electrolyte.
The equation (1) shows that EOF rate is proportional to electrokinetic surface
potential
magnitude (K -potential), determined by composition and structure of the
electrical double
layer formed between the inner capillary surface and electrolyte. Under given
conditions the
equilibrium value of c-potential for clean capillary surface is maximal, while
EOF rate is maximal
and component migration time is minimal. For sequential sample injections the
component
migration time increases if the procedure of capillary washing is not carried
out after the
previous analysis. This is attributed to decrease of c-potential magnitude
because of capillary
inner surface contamination by the adsorbed admixtures, which change the
electrical double
layer composition. The substantial change of component migration time due to
capillary
contamination may cause the errors of identification of multicomponent mixture
components.
To compensate for uncontrolled changes of electroosmotic flow rate,
electroosmotic flow
markers (hereinafter referred to as EOF markers) have been used. Components
with zero or
2
Date Recue/Date Received 2021-03-25
low electrophoretic mobility (electromigration rate) in the buffer are added
to a sample. The
migration time measured for these EOF markers allows for an estimation of the
electroosmotic
flow rate and computation of corrected migration times of the detected
analytes. However, use
of EOF markers substantially increases the cost of analysis. Furthermore, the
EOF marker
.. method is not versatile, because different types of buffer solution require
different EOF
markers. Another disadvantage of the EOF marker method is that it is not
useful for negative
electroosmotic flow rate, since the EOF markers would not reach detection zone
at all.
During the capillary washing with electrolyte solution, the value of the
streaming potential can
be determined by measuring the potential difference between the capillary ends
at a certain
pressure difference between the capillary ends. Streaming potential magnitude
is directly
related with electroosmotic flow rate value Aeo. Streaming potential Uõ
measured during
capillary washing with electrolyte solution by a means for measuring potential
difference
between capillary ends at definite pressure difference P between the said
capillary ends, is
proportional to electrokinetic surface potential (K- potential) in a similar
way as electroosmotic
.. flow rate Aeof,:
7 Ep
Us = (,¨ (2)
4777
Thus, the streaming potential can be measured during preparation when the
capillary is washed
with electrolyte solution to monitor the condition of the capillary walls.
To increase analysis sample throughput, an autosampler is used to
automatically introduce
samples into the CE system. Use of an autosampler also increases the
reproducibility of
multicomponent solutions analyses.
Reproducibility of CE analysis, especially during long automated multisample
analyses, is based
on both stable condition of capillary walls and stable composition of buffers
and samples.
Evaporation of buffer solution or change of capillary walls condition can
change migration
times. Evaporation of samples can change concentrations. Contamination of
samples or buffer
can pollute samples and change properties of the buffer which may affect
reproducibility. Even
when using an autosampler, each electrophoretic analysis may last for 15
minutes, so it may
take several hours to analyze 50 or more samples. During this time, liquid
multicomponent
3
Date Recue/Date Received 2021-03-25
samples can evaporate, causing a change of sample and buffer composition.
Furthermore, most
known CE electrophoretic devices work with opened vials. This can cause
evaporation of
volatile components from samples or from the buffer.
To address this problem, some CE electrophoretic devices have an autosampler
capable of
operating with vials sealed with a film or membrane that is pierced by a
special needle
containing the capillary and the electrode. Keeping vials sealed prevents
evaporation, however
the needle piercing the membrane can transfer contaminants (e.g. dust
particles) from an
external surface of the membrane into the vial. In addition, these systems do
not control
changes of migration times due to changes of capillary walls conditions.
Therefore, in these
devices the objective of solution stability is not fully achieved as the
stability is affected not only
by evaporation but by cross-contamination as well.
Brief Description of the Drawings
In the accompanying drawings which illustrate by way of example only
embodiments of the
present invention,
FIG. 1 is a schematic of a CE device comprising an autosampler and lid
removing means.
FIG. 2 is a schematic of the CE device with a lid orientation means and the
lid removing means.
FIG. 3 is a vial holder.
FIG. 4 is a vial holder mounted in a carousel.
FIG. 5 is a side view of the lid removing means in an initial position, with a
lid gripper driver
moving a kinematic chain to start operation.
FIG. 6 is a side view of the lid removing means of FIG. 5 with a lid-catching
hook pulling the
edge of a sample vial lid upwards.
FIG. 7 is a side view of the lid removing means of FIG. 5 in a terminal
position, when the lid is
removed from the vial.
FIG. 8 is a schematic of an embodiment of a CE device.
FIG. 9 is a schematic of the CE device of FIG. 8 with measuring electrodes and
means for moving
vials and for disconnecting of measurement circuit.
4
Date Recue/Date Received 2021-03-25
Detailed Description of the Invention
The present disclosure provides an improved CE device with increased or
improved sample
throughput and reproducibility of electrophoretic analyses of multicomponent
liquid samples
by reducing the risks of contamination and evaporation. The examples and
embodiments
described herein may be implemented in an electrophoretic multicomponent
analysis device
such as that described and shown in United States Patent No. 8,298,393. The
device of the
present disclosure includes an autosampler, means for immersing the ends of a
capillary into
an electrolyte vial and a sample vial, means for generating electrolyte flow,
means for applying
voltage across the capillary ends, a detector connected to the capillary, and
a control and signal
processing system.
The autosampler holds vials sealed with removable lids and comprises a lid-
removing means for
opening vials to access multicomponent liquid samples by removing the lids
from the vials. This
reduces evaporation prior to analysis. Since the lids are not pierced, the
risk of contamination
from piercing needles is avoided.
In one embodiment, the removable lids are asymmetric, said lids have a tip on
one side of their
edge. The autosampler comprises lid orientation elements to position the vial
lids in a
predefined position relative to the lid-removing means.
The lid-removing means includes a lid gripper and a lid gripper driver, while
the lid gripper
comprises a lid-catching hook and a supporting heel while the lid gripper
driver comprises a
tilting means (e.g. a pivoting joint or a cam mechanism) allowing the lid
gripper to tilt around
the supporting heel so that the lid-catching hook pulls an edge of the lid
upwards whereas the
supporting heel contacting with another part of the lid prevents the vial to
be pulled from the
autosampler.
The autosampler comprises a lid position sensor that detects if the lid was
removed to prevent
damaging the capillary and the electrode. The lid removal means removes the
lids from the vial
such that potential contaminants (e.g. dust particles) on the external lid
surface drop outside of
5
Date Recue/Date Received 2021-03-25
the vial to further reduce the risk of contamination. Removed lids are dropped
into a collecting
container.
To further improve of the reproducibility of analyses, the condition of the
capillary walls can
also be monitored. The CE device can include a system for monitoring the
capillary walls for
degradation or contamination. The monitoring system includes means for
generating
electrolyte flow in the capillary that builds up and maintains a preset
differential pressure
between the capillary ends during rinsing, and streaming potential measurement
means that
make electrical contact with the capillary ends, thus forming a closed
electric measurement
circuit with the electrolyte in the capillary so that the potential difference
between the capillary
ends can be measured.
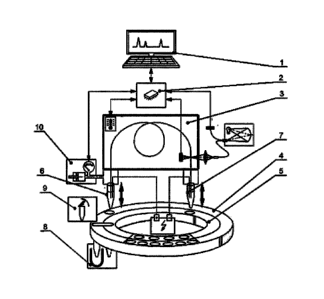
FIG. 1 schematically shows the CE device. Device control and data processing
may be executed
by an external computer 1, which is connected with the device through a
communication port.
Several controllers 2 provide diagnostics and control of the device. All units
are functioning
mainly to provide the reliable work of the main device unit ¨ a capillary
cassette 3.
The device autosampler comprises two carousels, an external carousel 4 and an
internal
carousel 5. These carousels hold vials for both inlet 6 and outlet 7 ends of
the capillary.
Conventional polymer vials can be used in both carousels.
To increase the capacity of the autosampler there is a transferring unit 8,
which is able to
transfer any vial from the internal carousel 5 to the capillary inlet. Lid-
removing means 9 is
integrated into the autosampler to remove lids before the analysis.
FIG. 2 schematically shows the layout of the means in the autosampler. The
present invention
enables to use the conventional polymer vials in both carousels of the
autosampler, such as
Eppendorf-type polypropylene vials, sealed with the proper lids, which
altogether reduces time
for the sample preparation and analysis.
FIGS. 2-4 shows a way to mount vials into the carousel by means of a vial
holder 46. The vial is
inserted into the vial holder 46. The top of the vial holder 46 is shaped with
protruding flanges
48 that limit the placement of the vial to the proper orientation. With a
standard Eppendorf-
type vial and lid, the lid typically has a protruding lip 49 to facilitate lid
removal. When placed
6
Date Recue/Date Received 2021-03-25
correctly in the vial holder, the lip 49 of the vial lid 50 is positioned
between the flanges, as can
be seen in FIG 4. The exterior of the vial holder 46 is contoured so it can
only fit into the
carousel one way, to reduce the likelihood of operator error. In the example
of FIG. 4, the vial
holder 46 has a cross-sectional profile that can generally be described as a
truncated circle, and
fits into a corresponding aperture in the carousel so that a portion of the
vial holder 46
protrudes from the top of the carousel.
A lid-removing means 13 is integrated into the autosampler 11 to open vials
and access
multicomponent liquid samples just prior to the analysis. The autosampler
comprises container
for collecting the lids removed.
10 The autosampler also comprises the lid orientation elements 12 to keep
the vials lids in a
predefined position relative to the lid-removing means and a system for lid
position control 14
and 14' for the external 4 and internal 5 carousels. The lid orientation
elements comprise the
vial holder 46 and a limiter 47. FIG. 3 depicts a non-limiting example of a
the vial holder 46. The
vial holder 46 is shaped to receive a vial and is further provided with a
flange which restricts the
15 orientation of the vial within the vial holder 46 to a predetermined
position.
Referring again to FIG. 2, a limiter 47 prevents the improperly installed vial
from entering the
work area. When a vial is improperly installed, the lid will be oriented in
the wrong direction
and the lid tip will enter an area in which it should not be located, where it
will engage the
limiter 47. The limiter 47 may be optical or mechanical. An optical limiter
may comprise a light
emitter and light receiver that detects a presence of a light beam. The
optical limiter will throw
a signal to the controllers 2 when the beam is interrupted by the lid tip. A
mechanical limiter
will throw a signal to the controllers 2 if the tip touches a special stop
provided on the
mechanical limiter. Consequently, the system for lid position control will
throw an error and
stop the carousel if the lid 50 is oriented incorrectly.
FIG. 5 shows the initial position of the lid removing means. This position as
a standby mode of
said means. The lid-removing means includes a stationary part and a movable
part. The
stationary part comprises a lid gripper driver 16, a standby mode sensor 17, a
pivot ring 18, and
a spring 19. The movable part of the lid removing means comprises a drive
mechanism, a
7
Date Recue/Date Received 2021-03-25
linkage 21 and a lid gripper 22. The drive mechanism is affixed on a driver
axis. In one
embodiment the lid gripper driver 16 is a mechanism that pivots around the
axis 27.
The lid gripper 22 is biased by the spring 19 against the pivot axis 27 of the
linkage. The lid
gripper 22 comprises a lid-catching hook 28 and a supporting heel 29. The lid
gripper driver 16
comprises tilting means allowing the lid gripper 22 to tilt around the
supporting heel 29. The
lid-catching hook 28 pulls a lid edge 32 upwards whereas the supporting heel
29 contacting
with another part of the lid prevents a vial 30 to be pulled from a mounting
set 31.
The lid gripper driver 16 and the pivot ring 18 are affixed on the stationary
part.
The vial 30 is positioned in the mounting set 31 in the way to keep the lid
edge 32 in a
predefined position relative to the lid-removing means.
To prevent capillary degradation, the capillary should be washed after each
analysis (FIG. 8 and
FIG. 9). The required cleaning efficiency of capillary walls from adsorbed
admixtures can be
determined by those skilled in the art through an empirical choice of
compositions of washing
solution and washing time. Cleaning efficiency is determined after analysis
based on the
migration time reproducibility for analytes.
FIG. 8 illustrates an apparatus for electrophoretic analysis of multicomponent
solutions.
The apparatus contains a capillary 33, placed into a means for capillary
installation 34, vials for
electrolyte 35 and 35', vials for analyzed samples 36, and a means for moving
said vials 37,
which may immerse capillary ends to the vials.
A means for generating the electrolyte flow through capillary 38 is connected
with the vial 35,
where the input end of the capillary 33 is located (in FIG. 8 and FIG. 9). A
means for applying
voltage between ends of capillary 39 is electrically connected with the vials
35, 35', where ends
of capillary 33 are located.
A means for streaming potential measurements 41 is made with possibility of
electric
connection with the ends of the capillary 33 during washing. The device
contains a detector 40,
which may be connected to the capillary 33. A control and signal processing
system 42 is
electrically connected to the detector 40, the means for applying voltage 39,
as well as the
8
Date Recue/Date Received 2021-03-25
means for creation of electrolyte flow through capillary 38, and the means for
streaming
potential measurement 41.
The means for moving vials 37 is a device for moving vials with solutions,
placing selected vial in
position at the selected capillary end (operating position of vial). The means
for moving vials 37
thus positions the vials in what may be referred to as their operating
position, as shown in FIGS.
6 and 7.
The means for capillary installation 34 permits immersion of the ends of the
capillary 33 into
the vials 35 and 35' in operating position. In at least one of the vials when
in operating position,
the vial interior is sealed from ambient air but is connected with the means
for creation of
electrolyte flow through capillary 38.
The means for generation of electrolyte flow through capillary 38 is a device
for creating and
maintaining a defined pressure difference between the ends of the capillary 33
submerged in
the vials 35, 35'.
The means for applying voltage 39 is equipped with electrodes 43, installed so
to submerge
their ends to the vials 35 and 35', when in operating position.
The means for streaming potential measurement 41 includes a means for pressure
differential
measurement between the ends of capillary 44.
The means for streaming potential measurements 41, electrolyte in vials 35 and
35', and the
capillary 33 filled with electrolyte form an electric measurement circuit.
The means for streaming potential measurements 41 also includes a means for
opening the
streaming potential measurements circuit 45, which can be used to prevent high
voltage effects
on the means for streaming potential measurement 41.
The means for opening streaming potential measurement electric circuit
includes measurement
electrodes 46 (FIG. 9), electrically connected with the means for pressure
differential
measurement 44 and with the means for streaming potential measurement 41, and
which are
inserted into the vials 35 and 35'. Before applying high voltage to the
electrodes 43, the vials 36
and 36' with analyzed samples are installed in operating position and the
vials 35 and 35' with
9
Date Recue/Date Received 2021-03-25
measurement electrodes 46 are taken out of operating position, and the
measurement electric
circuit appeared open.
The operation of the device according to the present invention is shown in
FIGS. 1, 4, 5, 6, and
7.
When the inlet vial is in its upper position (FIG. 1) the entire inlet
compartment is pressurized.
Capillary rinse or sample injection can be thus done by applying high (for the
rinse) or low (for
the sample injection) pressure mode from a compressor 10. The compressor can
also provide
low pressure in the inlet compartment to receiving an injection at the outlet
end of the capillary
33 (FIG. 1).
Separation of the injected multicomponent solutions is done by applying high
voltage between
the ends of the capillary 33. High voltage originates from a high voltage
power supply unit,
which is connected with two electrodes, immersed in the inlet and outlet
vials.
Right before the vial is lifted to a capillary end the lid-removing means 13
open the vial and
throw the lid into a collecting container (FIG. 2). This reduces the risk of
cross-contamination
between samples.
The operation of the lid-removing means of the invention is shown in FIG. 6.
and FIG. 7.
The autosampler lifts the vial 30 to the operation area of the lid removing
means and the lid
removing means starts the operation process from a standby mode.
The lid gripper driver 16 with the lever 25 affixed on it operates the tilting
means, e.g. a
pivoting joint or a cam mechanism, which executes reciprocating cycles. This
causes the bearing
24 to slide in the slot 23.
In one embodiment, during the operation cycle, the movable part moves so that
lid catching
hook moves down and then left to catch the edge of the lid 32 (FIG. 6). After
that, the movable
part continues forward motion left and causes the lid gripper to slide on the
lid. In the
meantime, the supporting heel 29 contacts with another part of the lid.
The lid gripper 22 tilts around the axis 27 and changes its angular position
relative to the
stationary vial. The lid-catching hook 28 pulls an edge of the lid 32 upwards
(FIG. 7) and pulls it
up to fully open the vial.
Date Recue/Date Received 2021-03-25
To prevent the vial to be pulled from the mounting set 31 of the autosampler
the linkage 21
with the lid gripper 22 are pressed to the vial by the spring 19.
Continuing reciprocating motion the lid gripper 22 drops the lid into a
collecting container. Said
collecting container can hold lids from several analysis cycles and can be
emptied anytime.
The said lid-removing means removes lids outwards to the container so that no
possible
contaminants from the lid reach the vial during opening process (FIG. 7).
If the lid has not been removed by the lid-removing means, the system for lid
position control
14 (FIG. 2) may repeat the opening procedure. In case the lid has not been
removed for the
second time, the external computer may display an error message to operator
and continue the
analysis for the next sample.
At the terminal stage, the lid gripper driver restores the device position to
standby mode.
Capillary washing (FIG. 8 and FIG. 9) is carried out as follows: the selected
vial with solution is
installed in operating position, where the end of capillary 33 is submerged
into washing
solution of the selected vial, and internal chamber of the selected vial is
sealed from ambient
.. air and pneumatically connected with the means for generating electrolyte
flow through
capillary 38, mean increasing pressure in the selected vial, displacing
washing solution through
capillary 33 to a collecting vial (not shown in the figure).
At the final washing stage in process of capillary conditioning a streaming
potential magnitude
is determined. For this purpose, vials 35 and 35' with buffer electrolyte are
installed in
operating position so that input and output ends of capillary 33 are immersed
into the buffer
electrolyte. Electrodes 43 ends are also inserted into the vials with the
buffer electrolyte. A
means for applying voltage 39 is switched off the electrodes by means for
opening streaming
potential measurements circuit 45, being part of the streaming potential
measurement means.
The streaming potential measurement means 41 closes measurement electric
circuit, which
includes the electrodes 43 and buffer electrolyte in both the vials 35, 35'
and the capillary 33.
The flow generation means 38 provides zero flow through capillary by creation
of zero pressure
difference between the ends of capillary. Thus, the streaming potential
measurement
apparatus measures background potential difference between the ends of the
capillary 33. The
11
Date Recue/Date Received 2021-03-25
means for flow generation 38 then increases pressure difference between the
ends of capillary
from zero to a selected value, providing an increasing flow rate of buffer
electrolyte through
the capillary 33. When the selected pressure difference is reached, the means
for flow
generation 38 maintains the mentioned selected pressure difference between
capillary ends
and provides the selected flow rate of buffer electrolyte through the
capillary 33. A steady flow
rate corresponds to a steady (working) potential difference between the
capillary ends. Thus,
the streaming potential measurement means 41 measures the magnitude of working
potential
difference between the ends of the capillary 33, maintaining the selected
pressure difference
between them.
High reproducibility of the analysis can be achieved even with a large number
of samples and
during a long analysis cycle. The risk of evaporation or cross-contamination
of samples is
reduced, and, and degradation of the capillary walls can be detected.
This technical solution is also cost-effective due to the low price and
availability of supplies.
One skilled in the art will understand that the foregoing description and
accompanying
drawings depict only one example of the CE device, and are not meant to limit
the scope of the
subject matter described herein. Variations of the foregoing example will be
apparent to those
skilled in the art and are considered to be within the scope of the subject
matter described
herein.
12
Date Recue/Date Received 2021-03-25