Note: Descriptions are shown in the official language in which they were submitted.
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
COATING FOR INTRALUMINAL EXPANDABLE CATHETER PROVIDING
CONTACT TRANSFER OF DRUG MICRO-RESERVOIRS
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] This disclosure is related to the field of drug delivery via
expandable
catheters.
Description of the Related Art
[0002] Balloon angioplasty is an established method for the treatment
of vascular
disease by physically dilating an area of atherosclerosis, stenosis or
reduction in luminal
diameter in a diseased blood vessel. Angioplasty is typically performed with a
catheter which
may be advanced within the circulatory system to the diseased area. The
catheter has a
balloon at the distal end that is inflated to dilate and expand the area of
stenosis. In many
cases, such as in the coronary arteries, a stent is also mounted on the
exterior of the balloon.
The balloon is expanded at the area of atherosclerosis, and the stent is left
in place after
deflation and removal of the balloon to maintain the patency of the expanded
lumen.
[0003] In order to achieve the physical enlargement of the treated
area of the
vessel, large forces are exerted upon the tissues of the vessel wall during
high pressure
balloon inflation. This physical dilatation results in injury to the vessel,
including endothelial
disruption, fragmentation of the inner elastic lamina, and dissection of the
vessel tunica
media. Injury often extends into the outer adventitia as well. The biological
response of the
vessel progresses through a thrombotic phase during days 0 to 3, involving
platelet activation
and adhesion and thrombus formation. The thrombotic phase is followed by a
cell
recruitment phase during days 3 to 8 involving the infiltration of
inflammatory cells,
macrophages and lymphocytes, into the site of vessel damage. The release of
growth factors
and cytokines from the inflammatory cells lead to the proliferative phase
during days 8 to 14,
in which the dormant smooth muscle cells in the tunica media of the vessel are
stimulated to
proliferate. Subsequently, the migration of the proliferating smooth muscle
cells into the
tunica intima and injury-derived thrombus in the lumen results in neointimal
hyperplasia, a
primary component of restenosis. Although cell proliferation ceases after 14
days, continued
-1-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
production of extracellular matrix by the smooth muscle cells in the area of
injury continues
to increase the extent of neointimal hyperplasia and restenosis. The
restenosis effectively
reverses the dilatation treatment and potentially creates a critical threat to
the patient. Human
clinical studies have demonstrated that restenosis generally occurs from 1 to
3 months after
balloon angioplasty and the restenosis typically peaks at approximately 3
months.
[0004] Although balloon angioplasty provides a much needed acute
increase in
blood flow in diseased vessels, restenosis is inherent due to the extent of
associated
mechanical injury. One strategy for reducing the restenosis response is to
release drugs into
the vessel in combination with the balloon dilatation treatment to counteract
the
inflammation and healing response. Approaches include the coating of the
balloon with
drugs, such as paclitaxel and sirolimus (rapamycin), which limit cellular
proliferation. During
contact of the balloon onto the luminal surface of the vessel it is believed
that use of an
excipient coating facilitates transfer of the drug to the vessel injury site.
These methods
attempt to provide a drug concentration in the vessel wall after balloon
expansion which is
sufficient to reduce restenosis caused by cell proliferation and at the same
time is low enough
to minimize toxicity to the vessel that may result in damage or impairment of
the vessel. It is
believed that it is desirable to maintain an effective drug concentration for
a sufficient time to
minimize restenosis.
[0005] In practice, drug delivery to the tissues of the vessel wall by
drug coated
balloons as described in the art is limited by the short period of time during
which the balloon
may be placed in contact with the vessel. Typically, the balloon inflation
during angioplasty
is performed for approximately 30 to approximately 120 seconds to limit
cardiac ischemia
and potential patient complications and discomfort. These short balloon
inflation and drug
delivery times may be sufficient for the antineoplastic drug paclitaxel which
has
demonstrated inhibition of neointimal formation in animals after a few minutes
of exposure
time. However, to provide maximum therapeutic effect and minimize potential
high dose
toxicity to the vessel, it would be desirable to provide delivery of drugs to
the vessel over an
extended period of time, ideally longer than the duration of balloon
inflation. Additionally,
drugs such as sirolimus and its analogues have both anti-proliferative and
anti-inflammatory
-2-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
activity that may provide benefit beyond the acute period for restenosis if
delivered over an
extended time.
[0006] Many of the drug coated balloons described in the prior art use
high initial
levels of active agent and multiple treatments to create a high initial
concentration, but then
the concentration rapidly falls off. This is undesirable because most of the
active agent on
the device is lost as possible embolic particulate into the bloodstream, or by
diffusion away
from the treatment site.
[0007] Many of the drug coatings described in the prior art include
hydrophilic
polymers and excipients or excipients that are liquid at body temperature.
Such hydrophilic
coating formulations provide a hydrophilic matrix for the hydrophobic drug
particles and may
be effective at transferring the drug to the vessel wall. However, such
coatings do not
provide significant resistance to wash off from blood either during
maneuvering of the
balloon to the treatment site or after transfer of the drug coating to the
vessel surface.
SUMMARY OF THE INVENTION
[0008] Some embodiments provide a coating for an expandable portion of
a
catheter comprising a hydrophobic matrix and a dispersed phase, wherein the
dispersed phase
comprises a plurality of micro-reservoirs dispersed in the hydrophobic matrix,
wherein the
plurality of micro-reservoirs comprises a first active agent intermixed with
or dispersed in a
first biodegradable or bioerodable polymer. Some embodiments provide a coating
wherein
the dispersed phase comprises a plurality of micro-reservoirs dispersed in the
hydrophobic
matrix wherein some of the plurality of micro-reservoirs comprises a first
active agent and a
first biodegradable or bioerodable polymer.
[0009] Some embodiments provide a catheter comprising an expandable
portion
on an elongated body and a coating over the expandable portion. The coating
comprises a
lipophilic matrix, wherein the lipophilic matrix comprises at least one lipid,
a plurality of
micro-reservoirs dispersed in the lipophilic matrix, wherein the plurality of
micro-reservoirs
comprises an active agent, and wherein the lipophilic matrix is configured to
adhere to a
luminal surface when the expandable portion is expanded, and transfer at least
a portion of
the plurality of micro-reservoirs to the luminal surface.
-3-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0010] Some embodiments provide a catheter comprising an expandable
portion
on an elongated body and a coating as described herein over the expandable
portion. In some
embodiments, the catheter further comprises a release layer between the
expandable portion
and the coating, wherein the release layer is configured to release the
coating from the
expandable portion. In some embodiments, the catheter further comprises a
protective
coating over the coating.
[0011] Some embodiments provide a coating formulation for an
expandable
portion of a catheter comprising a solid portion and a fluid. The solid
portion comprises a
plurality of micro-reservoirs and at least one hydrophobic compound. The
plurality of micro-
reservoirs comprises a first active agent and a first biodegradable or
bioerodable polymer.
[0012] Some embodiments provide a coating formulation for an
expandable
portion of a catheter comprising a plurality of micro-reservoirs comprising an
active agent
and at least one lipid.
[0013] Some embodiments provide a method for coating an expandable
portion of
a catheter comprising disposing a coating formulation described herein over
the surface of an
expanded expandable portion of a catheter, evaporating the fluid, and
collapsing the
expandable portion.
[0014] Some embodiments provide a method for treating or preventing a
condition at a treatment site comprising advancing a catheter comprising an
expandable
portion to the treatment site, wherein the expandable portion is coated with a
coating
described herein, expanding the expandable portion to allow contact between
the coating and
a tissue at the treatment site, collapsing the expandable portion, and
removing the catheter.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] Features and aspects, and advantages of the embodiments of the
present
disclosure are described in detail below with reference to the drawings of
various
embodiments, which are intended to illustrate and not to limit the invention.
These drawings
depict only several embodiments in accordance with the disclosure and are not
to be
considered limiting of its scope.
[0016] FIG. 1 depicts one embodiment of a balloon catheter with a
coating on the
expandable portion of the catheter.
-4-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0017] FIG. 2 depicts one embodiment of a balloon catheter with a
release layer
between the coating and the expandable portion of the catheter.
[0018] FIG. 3 depicts one embodiment of a balloon catheter with a
protective
layer over the coating.
[0019] FIG. 4 is a photomicrograph of the luminal surface of a vessel
treated with
one embodiment of the balloon catheter.
[0020] FIG. 5 is a photomicrograph of the luminal surface of a vessel
treated with
one embodiment of the balloon catheter.
[0021] FIG. 6 is a photomicrograph of the coated balloon surface at
100X
magnification showing a coating containing a crystalline sirolimus micro-
reservoir.
[0022] FIG. 7 is a photomicrograph of the artery surface at 50X
magnification
showing adhered material.
[0023] FIG. 8 is a photomicrograph of the artery surface at 1000X
magnification
showing adhered material.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
[0024] To overcome the limitations of the prior art, the embodiments
disclosed
herein provide coatings for an expandable portion of a catheter that have time-
release micro-
reservoirs of drug intermixed with or dispersed within a coating on a balloon
that can be
transferred to the luminal surface of the vessel during the 30 to about 120
seconds balloon
inflation time. This approach enables an extended and controlled release of
drug over a
longer period of time that may be tailored by the design of the micro-
reservoirs for the
characteristics of a particular drug or the pathology of the diseased vessel.
In addition to
providing sustained release, the coating disclosed herein can also resist
blood wash off, which
both increases drug transfer efficiency and patient safety from excessive
particulate.
Coating
[0025] Disclosed herein is a coating for an expandable portion of a
catheter or a
catheter system. The catheter is designed for insertion into a living body for
delivering at
least one active agent locally. The coating is formulated and constructed for
minimal
solubilization and dispersion into the blood stream while the catheter is
being positioned into
the target vessel for treatment, or after transfer of the coating to the
tissues of the vessel wall.
-5-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
In some embodiments, the active agent or drug is delivered to the vessel for
preventing or
minimizing restenosis after balloon angioplasty. In some embodiments, the
expandable
portion may be a balloon of a balloon catheter.
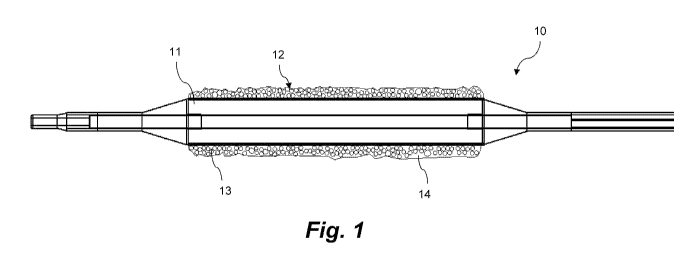
[0026] With reference to Fig. 1, in some embodiments, the coating 12
for an
expandable portion 11 of a catheter 10 includes two phases, a hydrophobic
matrix 14 and a
dispersed phase 13. The dispersed phase 13 is dispersed in the hydrophobic
matrix 14. The
dispersed phase 13 includes a plurality of micro-reservoirs, and the plurality
of micro-
reservoirs include a first active agent and a first biodegradable or
bioerodable polymer. In
some embodiments, the first active agent is intermixed with or dispersed in
the first
biodegradable or bioerodable polymer. In some embodiments, some micro-
reservoirs may
comprise a first active agent and a biodegradable or bioerodable polymer. In
some
embodiments, the plurality of micro-reservoirs also include a second active
agent. In some
embodiments, the plurality of micro-reservoirs may further include a second
biodegradable or
bioerodable polymer. In some embodiments, the first and the second
biodegradable or
bioerodable polymer may be the same or different. In some embodiments, the
plurality of
micro-reservoirs may contain only one type of micro-reservoirs. In some
embodiments, the
coating 12 includes about 10% to about 75%, about 20% to about 65%, or about
30 % to
about 55% by weight of the plurality of micro-reservoirs. In some embodiments,
the coating
12 has a surface concentration of about 1 [tg/mm2 to about 10 [tg/mm2, about 2
[tg/mm2 to
about 9 [tg/mm2, or about 3 [tg/mm2 to about 8 [tg/mm2 on the expendable
portion of the
catheter 10.
[0027] The hydrophobic matrix 14 comprises a combination of materials
selected
for its desired adhesive properties to the luminal surface. Preferred
hydrophobic matrix 14
includes a combination of hydrophobic compounds that are resistant to
dissolution into blood
but provide for uniform distribution of the formulation including the micro-
reservoirs when
applied to the surface of the balloon. In some embodiments, the hydrophobic
matrix 14
includes at least one hydrophobic compound selected from the group consisting
of sterols,
lipids, phospholipids, fats, fatty acids, surfactants, and their derivatives.
Particularly useful
formulations are a combination of a sterol and a fatty acid or phospholipid.
The sterol may
be a sterol which utilizes the body's natural clearance mechanism such as by
forming
-6-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
complexes with serum lipids or aggregates with serum apolipoproteins to
provide transport to
the liver for metabolic processing. In some embodiments, the sterol may be
cholesterol. Due
to the natural compatibility of cholesterol and fatty acids or phospholipids,
such combinations
may provide a homogenous mixture for coating 12 and a resulting homogenous
coating on
the balloon surface. The coating 12 formed by such combinations are homogenous
without
the formation of micelles or liposomes in the hydrophobic matrix 14.
[0028] In
some embodiments, the hydrophobic matrix 14 includes a cholesterol
and a fatty acid. In some embodiments, the weight ratio of cholesterol to
fatty acid is in the
range of about 1:2 to about 3:1, about 1:1.5 to about 2.5:1, or about 1:1 to
about 2:1. The
cholesterol component of the formulation may comprise cholesterol, chemically
modified
cholesterol or a cholesterol conjugate. In
some embodiments, the cholesterol is
dimethylaminoethane-carbamoyl cholesterol (DC-Cholesterol). For
physiological
compatibility, preferred fatty acids are fatty acids normally found in serum
or cell
membranes. In some embodiments, the fatty acid is selected from the group
consisting of
lauric acid, lauroleic acid, tetradeadienoic acid, octanoic acid, myristic
acid, myristoleic acid,
decenoic acid, decanoic acid, hexadecenoic acid, palmitoleic acid, palmitic
acid, linolenic
acid, linoleic acid, oleic acid, vaccenic acid, stearic acid, eicosapentaenoic
acid, arachadonic
acid, mead acid, arachidic acid, docosahexaenoic acid, docosapentaenoic acid,
docosatetraenoic acid, docosenoic acid, tetracosanoic acid, hexacosenoic acid,
pristanic acid,
phytanic acid, and nervonic acid.
[0029] In
some embodiments, the hydrophobic matrix 14 includes a cholesterol
and a phospholipid. In some embodiments, the weight ratio of cholesterol to
phospholipid is
in the range of about 1:2 to about 3:1, about 1:1.5 to about 2.5:1, or about
1:1 to about 2:1.
The cholesterol component of the formulation may comprise cholesterol,
chemically
modified cholesterol or a cholesterol conjugate. In some embodiments, the
cholesterol is
DC-Cholesterol. Preferred phospholipids are phospholipids normally found in
serum or cell
membranes. In some embodiments, the phospholipid is selected from the group
consisting of
phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, or
phosphatidylinositol.
In some embodiments, the phospholipid comprises an acyl chain length of about
20 to about
34 carbons. In some embodiments, the hydrophobic matrix 14 may further include
a third
-7-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
active agent, which can be the same or different from the first active agent
in the plurality of
micro-reservoirs.
[0030] In
some embodiments of the disclosure, the hydrophobic matrix 14
comprises only hydrophobic components such as lipids, sterols and fatty acids.
In other
words, in some embodiments, the hydrophobic matrix contains no hydrophilic
polymers or
hydrophilic excipients. In some embodiments of the disclosure, the hydrophobic
matrix 14
comprises only hydrophobic components such as lipids, sterols and fatty acids,
and no
amphiphilic constituents are present. Preferably, the coating 12 and its
components have a
limited solubility in blood or analogues such as plasma or phosphate buffered
saline. The use
of cationic cholesterol or a cationic phospholipid in the formulation may
provide additional
chemical attraction of the hydrophobic matrix 14 to the vessel surface and
potentially to the
surface of the micro-reservoirs to increase the transfer of the coating 12 and
resistance to
dissolution into blood after transfer. Suitable cationic forms of cholesterol
are modified at
the 3 carbon position to attach a pendant tertiary or quaternary amine and
include DC-
Cholesterol.
Suitable cationic forms of phospholipids include naturally occurring
phospholipids and synthetic modifications of phospholipids such as
phosphatidylethanolamine, dioleoylphosphatidylethanolamine (DOPE), and amine
derivatives of phosphatidylcholine such as ethylphosphatidylcholine.
[0031] In
some embodiments, the acyl chain length and degree of unsaturation of
the phospholipid component of the hydrophobic matrix 14 can be used for
tailoring the
physical and chemical properties of the hydrophobic matrix 14. In some
embodiments, long
acyl chain lengths are selected to increase hydrophobicity of the phospholipid
for adhesion to
the vessel surface and to decrease solubility and wash-off due to blood flow
exposure. The
acyl chain length of fatty acids and fatty acid portion of phospholipids are
described by
shorthand notation with the number of carbons followed by a colon with the
number of
carbon-carbon double bonds. In the following description of phospholipids,
both the generic
or trivial name, the stereo specific numbering and shorthand notation is used
for the first
description of the compound. Acyl chain lengths of 20 to 34 carbons (C20 to
C34) are
suitable for use as a coating 12 component, with acyl chain lengths of 20 to
24 carbons (C20
to C24) particularly preferred. Although the present invention will also work
with saturated
-8-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
acyl chains, one or more sites of unsaturation may provide an increased chain
flexibility.
Examples of preferred phospholipids include dieicosenoyl phosphatidylcholine
(1,2-
dieicosenoyl-sn-glycero-3-phosphocholine, C20:1 PC), diarachidonoyl
phosphatidylcholine
(1,2-diarachidoyl- sn-glycero-3 -pho sphocholine, C20:0 PC), dierucoyl
phosphatidylcholine
(1,2-dierucoyl- sn-glycero-3 -pho sphocholine, C22:1
PC), didocosahexaenoyl
phosphatidylcholine (1,2-didocosahexaenoyl- sn-glycero-3 -pho sphocholine,
C22:6 PC),
heneicosenoyl phosphatidylcholine (1,2-heneicosenoyl- sn-glycero-3 -pho
sphocholine, C21:1
PC) and dinervonyl phosphatidylcholine (1,2-dinervonoyl-sn-glycero-3-
phosphocholine,
C24:1 PC) In some embodiments, the phospholipids have a transition temperature
at or
above ambient temperature (20 C) such that the hydrophobic matrix 14
constitutes a solid
during storage.
[0032] The
plurality of micro-reservoirs comprises an active agent and a polymer.
The active agent may be referred to as a first active agent or a second active
agent. The
active agent is associated with the polymer in a way to provide slow or
extended release of
the active agent from the micro-reservoirs. In some embodiments, the active
agent is
intermixed with or dispersed in the biodegradable or bioerodable polymer. In
some
embodiments, the active agent may be encapsulated by the biodegradable or
bioerodable
polymer. In some embodiments, the plurality of micro-reservoirs may include a
first active
agent. In some embodiments, the plurality of micro-reservoirs may further
include a second
active agent. Suitable active agent may include antiproliferative or anti-
inflammatory agents
such as paclitaxel, sirolimus (rapamycin) and their chemical derivatives or
analogues which
are mTOR inhibitors, inhibitory RNA, inhibitory DNA, steroids and complement
inhibitors.
In some embodiments, the active agent is selected from the group consisting of
paclitaxel,
sirolimus, paclitaxel derivative, sirolimus derivative, paclitaxel analogues,
sirolimus
analogues, inhibitory RNA, inhibitory DNA, steroids, and complement
inhibitors. In some
embodiments, the active agent is about 10% to about 50%, about 15% to about
45%, about
20% to about 40%, or about 25% to about 35% by weight of the plurality of
micro-reservoirs.
The micro-reservoirs may include microparticles or microspheres. In some
embodiments,
polylactic-co-glycolic acid (PLGA) microspheres are well suited for
incorporation of the
-9-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
active agent for sustained release up to approximately 50% by weight of the
active agent in
the micro sphere.
[0033] In some embodiments, the hydrophobic matrix 14 may be a
lipophilic
matrix, and the dispersed phase 13 is dispersed in the lipophilic matrix. In
some
embodiments, the lipophilic matrix may include at least one lipid. In some
embodiments, the
lipid may be a phospholipid, sphingolipids, ceramides, terpenes, terpenoids,
monoglycerides,
diglycerides, triglycerides, phytosterols, prostaglandins, vegetable oils
(e.g., amaranth, apricot
stone, argan, almond, avocado, coconut, grape seed, palm, safflower, sesame,
soybean,
sunflower, and wheat germ oils), vegetable waxes (e.g., beeswax, jojoba, and
shea butter),
paraffin wax, fat soluble vitamins and pro-vitamins (e.g., carotenes and
vitamins A, D, E, K),
steroids, squalene. In some embodiments, the phospholipid is a cationic
phospholipid. In
some embodiments, the lipophilic matrix may further include a sterol, such as
cholesterol.
The lipophilic matrix as described is designed to adhere to a luminal surface
when the
expandable portion of the catheter is expanded in a lumen, such as blood
vessel. When the
expandable portion of the catheter is expanded in a lumen, at least a portion
of the plurality of
micro-reservoirs are transferred to the luminal surface along with at least a
portion of the
lipophilic matrix.
[0034] The dispersed phase 13 includes a plurality of micro-
reservoirs. In some
embodiments, the plurality of micro-reservoirs include a first active agent.
In some
embodiments, the plurality of micro-reservoirs include a first active agent
and a first
biodegradable or bioerodable polymer. In some embodiments, the first active
agent is
intermixed with or dispersed in the first biodegradable or bioerodable
polymer. In some
embodiments, some micro-reservoirs may include the first active agent alone,
and some
micro-reservoirs may include the first active agent intermixed with or
dispersed in the first
biodegradable or bioerodable polymer. In other embodiments, the first active
agent may be
crystalline. In some embodiments, the plurality of micro-reservoirs may
contain only one type
of micro-reservoirs.
[0035] In some embodiments, the coating 12 includes about 10% to about
75%,
about 20% to about 65%, or about 30 % to about 55% by weight of the plurality
of micro-
reservoirs. In some embodiments, the coating 12 has a surface concentration of
about 1
-10-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
1.tg/mm2 to about 10 1.tg/mm2, about 2 1.tg/mm2 to about 9 1.tg/mm2, or about
3 1.tg/mm2 to
about 81.tg/mm2 on the expendable portion of the catheter 10.
[0036] In some embodiments, the micro-reservoirs comprise active agent
microparticles. In some embodiments, the active agent, such as sirolimus, can
be crystalized
powder from the manufacturer or recrystallized through a controlled process.
For examples,
sirolimus microparticles may be prepared by grinding the crystalline powder
for 2 hours in
Novec 7100 hydrofluorcarbon solvent. Through selection of grinding ball size
and hardness,
as well as grinding speed and time, crystalline sirolimus can be reduced to
micron sized
particles or smaller. Grinding can be done dry or wet in an anti-solvent for
sirolimus such as
water, hexane, or hydroflurocarbons, which are then subsequently removed with
drying or
vacuum. Alternative methods of mechanical size reduction include miniature
hammer mills,
automatic mortar and pestle, ultrasonic homogenization, electrohydraulic (arc
cavitation)
homogenization or any mechanical process which leaves the crystals intact
without
dissolving them in a solvent.
[0037] In some embodiments, ground crystalline sirolimus can then be
sieved to
remove large particles. For example, an ASTM E-11 sieve number 100 (150iim
openings)
could be used on this sirolimus sample and particles that did not pass through
were returned
to the planetary ball mill for additional grinding.
[0038] In some embodiments, a specific size range microparticles can
be selected
using any particle size sorting techniques. For example, flowing the particles
in an anti-
solvent through progressively smaller sieves. In some embodiments, optional
further size
reduction may be provided by an ultrasonic homogenization probe,
electrohydraulic
lithotripsy or other sources of high shear cavitation known in the art. In
some embodiments,
a recirculating loop can be constructed to continue to break particles down to
sub- red blood
cell sizing.
[0039] In some embodiments, once the maximum size of the particles has
been
reduced to less than about 10 microns, the uniformity of the particles can be
further improved
via flow sorting such as winnowing to remove finer particles that could give
too much of a
burst effect. In some embodiments, particles can be circulated in an anti-
solvent (water,
-11-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
heptane, hydrofluorocarbon) and by controlling geometry and flow rate,
particles of desired
size can be collected via sedimentation.
[0040] In some embodiments, the plurality of micro-reservoirs has an
average
diameter of about 0.5 microns to about 10 microns, about 1 micron to about 10
microns,
about 0.5 microns to about 8 microns, about 1.8 micron to about 8 microns,
about 2 microns
to about 6 microns, or about 3 microns to about 5 microns. In some
embodiments, the micro-
reservoirs are desired to have a size large enough to provide a sustained
release of the active
agent, approximately 1.5 micron or greater in diameter or average cross-
sectional dimension
for microparticles of non-uniform size. Smaller sizes of micro-reservoirs
typically have an
increased surface area to volume ratio and reduced diffusional pathway for the
active agent
that does not provide sufficient extended release. The maximum size of the
micro-reservoirs
is approximately the size of a red blood cell, about 6 microns to about 8
microns, to prevent
embolization into capillaries due to any micro-reservoirs released into the
blood stream
during or subsequent to treatment. In some embodiments, the plurality of micro-
reservoirs
does not contain nano-sized particles. In some embodiments, less than about
5%, less than
about 8%, less than about 10%, less than about 15%, less than about 20%, less
than about
25%, less than about 30%, less than about 40%, less than about 50% of the
plurality of
micro-reservoirs have a diameter of 1.5 micron or less. In some embodiments,
the less than
about 5%, less than about 8%, less than about 10%, less than about 15%, less
than about
20%, less than about 25%, less than about 30%, less than about 40%, less than
about 50% of
the plurality of micro-reservoirs have a diameter of 1 micron or less. In some
embodiments,
the micro-reservoirs do not necessarily have affinity or adhesion to the
vessel surface.
[0041] The biodegradable or bioerodable polymer can provide controlled
and
extended release of the active agent. The biodegradable or bioerodable polymer
may be
referred to as a first biodegradable or bioerodable polymer or a second
biodegradable or
bioerodable polymer. The polymer acts as a barrier to drug diffusion thereby
providing a
release profile tailored for the pharmacokinetics of the active agent acting
on the treated
vessel. For example, the active agent may be intermixed and distributed into a
polymer in a
solid solution. The polymer may provide controlled release by reducing active
agent
diffusion or by coupling drug release to biodegradation, dissolution or
bioerosion of the
-12-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
polymer. In some embodiments, the biodegradable or bioerodable polymer is
selected from
the group consisting of polylactic acid, polyglycolic acid and their
copolymers,
polydioxanone, polycaprolactone, polyphosphazine, collagen, gelatin, chitosan,
glycosoaminoglycans, and combination thereof. In some embodiments, the micro-
reservoirs
may also be microspheres or microparticles containing at least one active
agent which treats
the inflammation or healing response. In some embodiments, the plurality of
micro-
reservoirs may include a first biodegradable or bioerodable polymer. In some
embodiments,
the plurality of micro-reservoirs may include a second biodegradable or
bioerodable polymer.
[0042]
After contact of the coating 12 with the vessel wall, the kinetics of active
agent release is controlled by the release of active agent from the micro-
reservoirs into the
surrounding medium, thereby making available a sustained elution of active
agent to
penetrate into the vessel wall. To provide significant active agent during the
initial high risk
period for restenosis following dilation, it is preferred that the active
agent in the coating 12
be continuously released with a half-life release kinetics of about 2 weeks to
about 6 weeks or
greater. In some embodiments, the plurality of micro-reservoirs has active
agent release
kinetics with a half-life of at least 14 days.
[0043] The
active agent release kinetics may be tailored by the characteristics of
the micro-reservoirs. Two or more types of micro-reservoirs with different
active agents or
different release kinetics for the same active agent may be formulated into
the coating 12 to
tailor the treatment effect. In some embodiments, some active agent may be
incorporated
into the coating formulation outside of the micro-reservoirs to provide a
rapid initial release
of active agent to the vessel walls, allowing the micro-reservoirs to provide
sufficient active
agent to maintain effective tissue concentration of active agent for a
prolonged period of
time. Since the healing and resolution of inflammation in the region of
dilation typically
takes 4-12 weeks, it is desirable to have micro-reservoirs and coating 12 to
elute active agent
to provide therapeutic tissue levels for at least about 4 weeks to about 12
weeks following the
treatment. In
certain applications, such as very long, extensively diseased vessels,
maintenance of active agent levels for longer than 4 to 12 weeks may be
desirable to provide
additional protection from the effects of less common late restenosis.
-13-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0044] The release of active agent intermixed with or dispersed in a
solid has
been shown to follow Higuchi kinetics with decreasing active agent release
over time. For
spherical particles with active agent dispersed in a polymer, the active agent
release kinetics
also follows a power law of decreasing release rate, Korsmeyer-Peppas kinetic
model, similar
to the Higuchi equation. (J. Siepmanna J, Peppas NA, Modeling of active agent
release from
delivery systems based on hydroxypropyl methylcellulose (HPMC), Advanced Drug
Delivery
Reviews 48 (2001) 139-157). The release kinetics of active agent from such
micro-
reservoirs is well suited for treatment of the vessel wall post dilatation.
The design and
selection of micro-reservoirs with the appropriate release constant provides
for rapid initial
release of active agent with sustained active agent release and extended
active agent residence
in the vessel wall over longer time periods compared to devices of the prior
art. The active
agent release rate may be tailored by the solubility of the active agent in
the micro-reservoir
material and by adjusting microporosity of the micro-reservoir. The length of
effective active
agent delivery may be tailored by the selection of micro-reservoir size,
active agent solubility
in the micro-reservoir material, and amount of active agent loaded in the
micro-reservoirs.
The total amount of active agent to be delivered is determined by the amount
of micro-
reservoirs in the coating formulation and their level of active agent loading.
As a result, the
coating 12 is able to be formulated to have a concentration of active agent in
the range of
about 0.3 to about 3 i.t.g per mm2 of expandable portion 11 surface. The
desired kinetics of
active agent release from the coating 12 may be provided by a single type of
micro-reservoir
or alternatively by a mixture of micro-reservoirs with different size or
release characteristics
to provide the desired release profile to the vessel wall.
[0045] In some embodiments, the coating 12 further includes a PEG-
lipid for
increased hemocompatiblity. Since the coating 12 disclosed herein is designed
to be
transferred to the surface of a blood vessel and to remain there to release
drug during the
vessel healing period, hemocompatiblity of the coating 12 is desired. In
addition to
preventing dissolution of the coating 12 into the blood stream prior to
healing of the vessel, it
is desired to prevent initiation of significant clotting and the attachment of
fibrin and platelets
to the coating surface exposed to blood after transfer. The addition of a PEG-
lipid to the
composition of cholesterol and a phospholipid or fatty acid may be used to
provide increased
-14-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
hemocompatiblity of the formulation. PEG grafted polymer surfaces have shown
improved
blood contact characteristics primarily by lowering the interfacial free
energy and by the
steric hindrance of the hydrated PEG chains on the surface. While not wishing
to be bound
to a particular theory of operation, it is believed that a small amount of PEG-
lipid conjugate
added to the composition may migrate to the blood interface surface after
transfer, especially
for PEG-lipids of relatively low molecular weight. The PEG chains are thereby
able to lower
the interfacial free energy at the blood interfacing surface. Since the
coating material at the
blood interface is a small portion of the total coating, a relatively small
amount of PEG-lipid
is required.
[0046] In
some embodiments, the PEG-lipid is selected from the group consisting
of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene
glycol)-350
(DSPE-mPEG350), 1,2-
dip almitoyl- sn-glycero-3 -pho sphoethanolamine-
methoxy(polyethylene glycol)-350 (DPPE-mPEG350), 1,2-dioleoyl-sn-glycero-3-
phosphoethanolamine-N-methoxy(polyethylene glycol)-350 (DOPE-mPEG350), 1,2-
distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol)-550
(DSPE-
mPEG550), 1,2-
dip almitoyl- sn-glycero-3 -pho sphoethanolamine-N-methoxy(polyethylene
glycol)-550 (DPPE-mPEG550), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-
N-
methoxy(polyethylene glycol)-500 (DOPE-mPEG550). In some embodiments, the PEG-
lipid
is about 1% to about 30% by weight of the hydrophobic matrix 14 consisting of
the
combination of the cholesterol, the fatty acid or phospholipid and the PEG-
lipid. In other
embodiments, the PEG-lipid is about 2% to about 25%, about 3% to about 20%, or
about 5%
to about 10% by weight of the hydrophobic matrix 14. In some embodiments, the
amount of
PEG-lipid is about 12% or less.
[0047] In
some embodiments, the coating 12 further includes one or more
additives. In some embodiments, the one or more additives are independently
selected from
penetration enhancers and stabilizers. For example, the coating 12 may further
include
additives to enhance performance, such as penetration enhancers. The
penetration enhancer
can aid diffusion of the active agent into the vessel wall and maximize tissue
delivery of the
active agent. Suitable penetration enhancers may include surfactants, cationic
excipients and
cationic lipids. In some embodiments, the additive may be added to the
hydrophobic matrix,
-15-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
the micro-reservoirs, or both. In some embodiments, stabilizers may be added
to protect the
drug during sterilization of the balloon catheter system and its subsequent
storage before use.
Stabilizers may include antioxidants and free radical scavengers. Examples of
stabilizers
include gallic acid, propylgallate, tocopherols and tocotrienols (Vitamin E),
butylatedhydroxytoluene, butylatedhydroxyanisole, ascorbic acid, thioglycolic
acid, ascorbyl
palmitate, and EDTA.
[0048] In some embodiments, the coating 12 further comprises a third
active
agent, wherein the third active agent is outside of the micro-reservoirs or in
the hydrophobic
matrix 14. The third active agent may be the same or different from the first
or the second
active agent in the plurality of micro-reservoirs. However, since the active
agent(s) are
primarily contained in the micro-reservoirs and not in direct contact with the
hydrophobic
matrix 14, the need to solubilize or emulsify the active agent in the
hydrophobic matrix14
itself is obviated. Since the active agent(s) are primarily contained in the
micro-reservoirs
and not in contact with the hydrophobic matrix 14, the need to include an
amphiphilic
constituent or constituent with active agent affinity in the hydrophobic
matrix 14 itself is
obviated. The hydrophobic matrix 14 can therefore be optimized toward suitable
properties
for resistance to blood wash-off and adhesion to the vessel surface for
coating 12 transfer.
Catheter
[0049] With reference to Fig. 2, disclosed herein is also a catheter
10 that includes
an expandable portion 11 on an elongated body 17, a coating 12 as described
above over the
expandable portion 11, and a release layer 15 between the expandable portion
11 and the
coating 12. In some embodiments, the release layer 15 is configured to release
the coating 12
from the expandable portion 11. A release layer 15 which is immiscible with
the coating 12
is preferred to maintain distinct layers. In some embodiments, PEG conjugated
lipids are
used as a release layer 15 as the degree of hydrophilicity and miscibility
with the active agent
coating 12 may be tailored by the selection of the lipid and the PEG chain
length. In some
embodiments, the release layer 15 is 1,2-distearoyl-sn-glycero-3-
phosphoethanolamine-N-
(methoxy(polyethylene glycol)-350) (DSPE-mPEG350) or 1,2-distearoyl-sn-glycero-
3-
phosphoethanolamine-N-(methoxy(polyethylene glycol)-550) (DSPE-mPEG550). In
some
-16-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
embodiments, the release layer 15 has a surface concentration of about 0.1
[tg/mm2 to about 5
[tg/mm2, 0.25 [tg/mm2 to about 3 [tg/mm2, or 0.5 [tg/mm2 to about 2 [tg/mm2.
[0050] With reference to Fig. 3, in some embodiments, the catheter 10
further
includes a protective layer 16 over the coating 12 as a top coat. In some
embodiments, the
protective layer 16 includes a hydrophilic polymer, a carbohydrate, or an
amphiphilic
polymer. In some embodiments, the protective layer 16 is a glycosaminoglycan
or a
crystalized sugar. Examples of glycosaminoglycans include dextran sulfate,
chondroitin
sulfate, heparan sulfate, and hyaluronic acid. Examples of crystalized sugars
include
mannitol, sorbitol, erythritol, and xylitol. The crystalline nature of these
sugars provides a
hard surface that protects the underlying micro-reservoirs. The thickness of
the protective
layer 16 can be adjusted such that the protective layer 16 washes away during
the transit time
required to advance the catheter 10 to the target site. In some embodiments,
the protective
layer 16 has a surface concentration of about 0.1 [tg/mm2 to about 5 [tg/mm2,
about 0.2
[tg/mm2 to about 4 [tg/mm2, or about 0.3 [tg/mm2 to about 3 [tg/mm2.
[0051] The expandable portion 11 of the catheter 10 may be a balloon,
which acts
as a substrate for the coating 12. In some embodiments, the balloon may be of
a low pressure
design using an elastomeric material such as polyisoprene, polystyrene
copolymers,
polysiloxane, or polyurethane. In some embodiments, the balloon may also be of
a high
pressure design using high tensile strength polymers such as
polyvinylchloride, polyethylene,
polyethylene terephthalate, or nylon. In some embodiments, the expandable
portion 11 may
be made of Nylon 12. The coating 12 may be sufficiently adhered to the
expandable portion
11, but is readily transferred to the tissues of the vessel lumen upon
contact. In such cases, a
release layer may be omitted. In addition, Nylon 12 has sufficient strength
such that the
balloon may further act as a post-dilatation balloon (if needed) in a
subsequent procedure
after transfer of the coating 12.
[0052] In some embodiments, the expandable portion 11 underneath the
coating
12 may be used to dilate the target vessel. In some embodiments, the vessel
may be pre-
dilated with another balloon catheter 10 prior to treatment with the coated
balloon of the
present embodiments.
Coating Formulation
-17-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0053] Disclosed herein is also a coating formulation for an
expandable portion
11 of a catheter 10. The formulation includes a solid portion and a fluid. The
solid portion
includes a plurality of micro-reservoirs and at least one hydrophobic
compound. The fluid
acts to disperse or solubilize the at least one hydrophobic compound. In some
embodiments,
the fluid may disperse some hydrophobic compounds and solubilize other
hydrophobic
compounds. The micro-reservoirs are dispersed and suspended in the resultant
fluid mixture
to form the coating formulation. The fluid mixture is formulated to form a
homogenous
mixture of the hydrophobic compounds that does not separate during drying to
result in a
uniform, conformal coating of the hydrophobic matrix 14. The coating
formulation is
characterized by weight of the solid portion, which refers to all the non-
volatile components
of the coating formulation, but excludes the fluid that is subsequently
evaporated during
drying of the coating.
[0054] The micro-reservoirs include an active agent and a polymer. The
active
agent may be referred to as a first active agent or a second active agent as
described herein.
The polymer may be a first biodegradable or bioerodable polymer or a second
biodegradable
or bioerodable polymer described herein. In some embodiments, the active agent
is
intermixed with or dispersed in the biodegradable or bioerodable polymer
described herein.
In some embodiments, the formulation may include more than one type of micro-
reservoirs.
For example, the plurality of micro-reservoirs may include a first active
agent and a first
biodegradable or bioerodable polymer. In some embodiments, the plurality of
micro-
reservoirs may further include a second active agent. In some embodiments, the
plurality of
micro-reservoirs may also include a second biodegradable or bioerodable
polymer.
[0055] The micro-reservoirs may be fabricated by any of the known
means for
particle manufacture, including spray drying, coacervation, micromolding, and
milling. All
such processes begin by dissolving the active agent and the polymer together
in a suitable
solvent such as acetonitrile or dichloromethane, then removing the solvent in
a controlled
manner that creates uniform particles. The particles may be further shaped by
mechanical
means. Processes that produce particles with size distributions with
coefficients of variation
of 10% or less are particularly useful for providing more consistent active
agent release rates.
Methods for producing microspheres of uniform size are described by forming an
emulsion
-18-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
of the microsphere material and extruding the emulsion through a substrate
with through-
holes of controlled size as described in US 7,972,543 and US 8,100,348.
Alternatively,
microspheres may be produced by spray-drying solutions of polymers as
described in US
6,560,897 and US 20080206349.
[0056] The fluid of the coating formulation may comprise water,
organic solvent,
perfluorocarbon fluids, or a mixture of such fluids. In some embodiments, the
fluid is
selected from the group consisting of pentane, hexane, heptane, heptane and
fluorocarbon
mixture, alcohol and fluorocarbon mixture, and alcohol and water mixture.
Fluids which
readily solubilize the active agent or the polymer of the micro-reservoirs are
not preferred
since they may extract the active agent from the micro-reservoirs. Such non-
preferred fluids
include acetic acid, acetonitrile, acetone, dichloromethane, ethyl formate,
cyclohexanone,
DMSO, and chloroform. Optionally, the fluid/fluid blend may be selected to
saturate at the
desired level of extracted active agent. Additional active agent that is the
same as the one in
the micro-reservoirs may be added to the fluid in advance to pre-saturate the
solution, thereby
reducing extraction from the micro-reservoirs during processing of the
coating.
[0057] In some embodiments, the at least one hydrophobic compound is
selected
from the group consisting of sterols, lipids, phospholipids, fats, fatty
acids, and surfactants,
and their derivatives. In some embodiments, the at least one hydrophobic
compound
comprises a cholesterol and a fatty acid as described herein. In other
embodiments, the at
least one hydrophobic compound comprises a cholesterol and a phospholipid as
described
herein. In some embodiments, the formulation can also include a PEG-lipid as
described
herein. In some embodiments, the formulation can further include additives
like penetration
enhancers and stabilizers.
[0058] In some embodiments, the solid portion further includes a third
active
agent outside of the plurality of micro-reservoirs. In other words, the
coating formulation can
lead to a hydrophobic matrix 14 that further comprises the third active agent.
The active agent
outside of the micro-reservoirs may be the same or different from the active
agent(s) in the
micro-reservoirs. In some embodiments, the solid portion may further comprise
a PEG-lipid.
In some embodiments, the solid portion may also further comprise an additive
described
herein.
-19-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0059] In some embodiments, the concentration of the solid portion by
percent
weight in the coating formulation is approximately 1% to approximately 90%. In
some
embodiments, the solids content of the coating formulation has a concentration
of about 2%
to about 80% by weight, about 3% to about 70% by weight, or about 4% to about
60% by
weight. In some embodiments for spray coating, the solid portion of the
coating formulation
has a concentration of about 2% to about 7% by weight. The solid portion of
the coating
formulation comprises about 10% to about 75%, about 20% to about 65%, or about
30 % to
about 55% by weight of the plurality of micro-reservoirs.
Method for Coating
[0060] Disclosed herein is also a method for coating an expandable
portion 11 of
a catheter 10. The steps include, disposing a formulation described herein
over the surface of
an expanded expandable portion 11 of a catheter 10, evaporating the fluid
constituents of the
coating formulation, and collapsing the expandable portion 11. Disposing a
formulation over
the surface of an expanded expandable portion 11 includes disposing the
formulation on the
surface of an expanded expandable portion 11. In some embodiments, the
formulation can be
disposed on or over the expanded expandable portion 11 by spray coating, dip
coating, roll
coating, electrostatic deposition, printing, pipetting, or dispensing.
[0061] The coating formulation is prepared by mixing the coating
components in
a fluid as disclosed herein. In some embodiments, the micro-reservoirs are
dispersed into the
fluid formulation. Once fully mixed, the coating formulation may be applied to
the surface of
the expanded expandable portion 11 such as a balloon and let dry to form the
coating 12. The
application of the coating formulation may be repeated as necessary to deposit
the desired
amount of coating 12, usually in the range of about 5 mg to about 9 mg of
coating 12 per
mm2 of the balloon surface. The coating 12 is allowed to dry and the balloon
deflated and
folded to allow introduction into the vascular system.
[0062] In some embodiments, the method may further comprise disposing
a
release layer on the surface of an expanded expandable portion 11. As such,
the coating
formulation would be disposed on the release layer, while the release layer is
disposed onto
the surface of the expanded expandable portion 11. The release layer is
described above.
Method for Treating or Preventing a Condition
-20-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0063] Disclosed herein is also a method for treating or preventing a
condition at
a treatment site. The method involves the steps of advancing a catheter 10
comprising an
expandable portion 11 to the treatment site, expanding the expandable portion
11 to allow
contact between the coating 12 and a tissue at the treatment site, collapsing
the expandable
portion 11, and removing the catheter 10. The expandable portion 11 is coated
with a coating
described herein. In some embodiments, the contact between the tissue and the
coating 12
results in a transfer of at least a portion of a coating on the expandable
portion 11 to the
treatment site during contact for a period of from about 30 to about 120
seconds.
[0064] A catheter 10 with expandable portion 11 such as a coated
balloon catheter
is used here to demonstrate the concept of delivering an active agent or a
combination of
active agents to a vessel. The coated balloon catheter is introduced into a
vessel with the
expandable portion 11 folded to provide a small cross-sectional profile and to
facilitate
percutaneous insertion of the catheter 10, for example by the well-known
Seldinger
technique. After the expandable portion 11 of the catheter 10 is advanced to
the diseased
area of the vessel for treatment, the balloon is inflated, and the coating 12
makes firm contact
with the vessel lumen. The coating is formulated to have affinity to the
luminal tissue
surface, resulting in adhesion of a layer of the coating on the vessel lumen.
The expandable
portion 11 may be inflated or expanded for a period of 30 seconds up to 2
minutes to promote
adhesion and provide for initial active agent penetration into the vessel. The
expandable
portion 11 may be deflated and inflation repeated as desired for treatment to
manage the time
period and risks of vessel occlusion or tissue ischemia. The coating is
adhesively transferred
to the lumen of the vessel upon balloon inflation and firm contact of the
balloon surface to
the vessel luminal surface. The adhesion of the coating to the vessel surface
thereby carries
the micro-reservoirs and transfers them to the vessel surface.
[0065] In some embodiments, the condition is selected from the group
consisting
of atherosclerosis, stenosis or reduction in luminal diameter in a diseased
blood vessel,
restenosis, and in-stent restenosis. In some embodiments, an additional
release layer as
described herein is disposed between the expandable portion 11 and the coating
12.
[0066] While the present disclosure is directed at the treatment of
restenosis
associated with balloon dilatation of blood vessels, the invention may be used
to deliver
-21-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
drugs to various other lumens and hollow structures of the body such as the
structures of the
respiratory system, gastrointestinal system, urinary system, reproductive
system, and
lymphatic system. The coated device may be an inflatable balloon or other
inflatable device.
Alternatively the device delivering the coating of the present invention may
be a non-
inflatable device or any other type of expandable device that is used for
treatment of a living
body.
EXAMPLES
Example 1
[0067] Drug Containing Micro-Reservoirs (Micro spheres) fabricated by
coacervation of polylactic-co-glycolic acid copolymer incorporating sirolimus
(rapamycin)
were obtained.
[0068] Microsphere sample 1: 50% DL-lactide / 50% glycolide copolymer,
average diameter 3.1 p.m, SD 0.44 p.m, 39% rapamycin by weight
[0069] Microsphere sample 2: 75% DL-lactide / 25% glycolide copolymer,
average diameter 3.2 p.m, SD 0.76 p.m, 40% rapamycin by weight
[0070] Microsphere sample 3: 50% DL-lactide / 50% glycolide copolymer,
average diameter 2.7 p.m, SD 0.8 p.m, 45% rapamycin by weight
[0071] Microsphere sample 4: 75% DL-lactide / 25% glycolide copolymer,
average diameter 3.3 p.m, SD 1.2 p.m, 46% rapamycin by weight
[0072] Microsphere sample 5: 75% DL-lactide / 25% glycolide copolymer,
average diameter 4.1 p.m, SD 0.61 p.m, 25% rapamycin by weight
[0073] Microsphere sample 6: 75% DL-lactide / 25% glycolide copolymer,
average diameter 3.78 p.m, SD 0.44 p.m, 28.8% rapamycin by weight
[0074] Microsphere sample 7: 75% DL-lactide / 25% glycolide copolymer,
average diameter 3.8 p.m, SD 0.34 p.m, 27.7% rapamycin by weight
[0075] Microsphere sample 8: 75% DL-lactide / 25% glycolide copolymer,
average diameter 3.79 p.m, SD 0.39 p.m, 29.4% rapamycin by weight
[0076] Drug content of these micro-reservoirs was verified by HPLC
quantitation
method. Typically, micro-reservoirs (1 to 5 mg) were weighed and dissolved in
1 ml
acetonitrile, agitated gently at room temperature for several hours or 37 C
for 1 hour, and
-22-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
diluted 50- to 200-fold with acetonitrile. Absorbance at 278 nm was monitored,
and content
was determined from linear calibration curves.
Example 2: Sustained drug release from micro-reservoirs under physiological
conditions
[0077] Micro-reservoirs from Example 1 were tested for sustained
release of drug.
Micro-reservoir samples of 2 to 5 mg weight were placed in 1.6 ml Eppendorf
tubes with 1.2
ml of phosphate buffered saline (PBS) to simulate a physiological environment.
After an
initial wash to remove any drug not incorporated in the micro-reservoirs, the
tubes were
incubated at 37 C with gentle mixing at 250 rpm. The PBS was sampled at time
intervals
and the released drug quantitated by reverse phase HPLC using a C18 column.
[0078] Micro-reservoirs were assayed for drug elution over 5 hours.
The resultant
drug release was fit to the Korsmeyer-Peppas kinetic equation for drug release
from a
polymer with dispersed drug. The results of the Korsmeyer-Peppas model are
listed in Table
1.
Table 1. Korsmeyer-Peppas Modeling of 5 Hour Drug Release
Q=a*x^b Microsphere 1 Microsphere 2 Microsphere 3 Microsphere 4
R (correlation
0.9061 0.8778 0.8579 0.9016
coefficient)
SE of estimate 0.0026 0.0025 0.0021 0.0033
a 0.0450 0.0382 0.0305 0.0506
b 0.5241 0.5204 0.5167 0.4502
[0079] The short term delivery results demonstrate Korsmeyer-Peppas
drug
release constants typical for drug dispersed in a spherical polymer particle
with likely a small
contribution from polymer erosion or degradation for Microsphere samples 1, 2,
and 3.
[0080] Extended drug release study: Microspheres were assayed for drug
elution
over 7 days using the methods described for testing over 5 hours. The
resulting drug release
is listed in Table 2.
Table 2. Testing of 7 Day Drug Release
Cumulative Drug Release, % of Total Drug
Time [days] Microsphere 1 Microsphere 2 Microsphere 3 Microsphere 4
0 0.9% 1.5% 2.3% 2.2%
1 1.8% 2.8% 3.3% 3.9%
2 2.3% 4.1% 4.0% 5.0%
3 4.2% 6.1% 4.6% 5.9%
4 5.7% 13.4% 5.2% 6.9%
-23-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
7.5% 19.6% 5.8% 7.7%
6 10.0% 26.2% 6.4% 8.7%
7 11.9% 30.7% 7.0% 9.5%
[0081] The release rates from the 7 day delivery results were fit to
the Higuchi
equation:
Q = A [D(2C - Cs)Cs t]1/2
Q = Kb (t)1/2
where Q is the amount of drug released in time t per unit area A, C is the
drug initial
concentration, Cs is the drug solubility in the polymer media and D is the
diffusion
coefficient for the drug in the microsphere polymer. In the generalized
equation, Kb is the
Higuchi constant incorporating the area, diffusion coefficient and drug
concentration
coefficients.
[0082] The Higuchi equation was used to determine the release half-
life of the
micro-reservoirs and to also to estimate the half-life as a function of the
microsphere size.
The resultant release half-lives are presented in Table 3.
Table 3. Drug Release Half-Life from Higuchi Modeling
Micro sphere t1/2 [days]
Diameter
Micro sphere 1 Micro sphere 2 Micro sphere 3 Micro sphere 4
[microns]
0.5 0.14 0.02 0.42 0.11
1 2.29 0.34 6.65 1.70
1.5 11.58 1.71 33.66 8.61
2 36.60 5.42 106.38 27.22
3 185.29 27.43 538.53 137.81
4 585.62 86.71 1702.01 435.55
5 1429.74 211.69 4155.29 1063.36
6 2964.70 438.96 8616.42 2204.98
7 5492.48 813.22 15962.98 4084.99
8 9369.93 1387.32 27232.13 6968.81
[0083] The results demonstrate that the delivery half-life of drug
from the micro-
reservoirs may be tailored by the formulation and size of the micro-
reservoirs. For a delivery
half-life of at least 14 days, a microsphere size of 1.5 micron diameter or
greater is estimated
to be required.
-24-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0084] Verification of extended release: Microsphere Sample 4 was
assayed for
drug release over 8 weeks using the methods previously described. Due to the
relatively long
time intervals between sampling as compared to the previous release
experiments, the micro-
reservoirs may not have released into sink conditions at later time points,
potentially slowing
effective release rate. The resultant drug release is listed in Table 4.
Table 4. Testing of Extended Drug Release Over 56 Days
Time Cumulative
[days] Eluted Drug [%]
0 0
7 1.00
14 3.00
31 7.50
56 15.50
[0085] The results verify sustained release of drug from the micro-
reservoirs.
Micro-reservoirs may be tailored or selected with a half¨life to provide drug
through the
healing period of the dilated vessel.
Example 3: Formulations of micro-reservoirs in coating formulation of
cholesterol and fatty
acid with PEG-lipid
[0086] A coating formulation was prepared with 107 mg of stearic acid,
105 mg
of cholesterol, and 50 mg of DPPE-mPEG350 mixed with 14 mL of heptane and
heated to
60 C such that a clear solution was obtained. The solution was then vortex
mixed for 30
seconds and allowed to cool. Next, 200 mg of sirolimus loaded microspheres of
sample #6
was added, and the formulation was placed in an ultrasonic bath for 4 minutes
to disperse and
suspend the microspheres. [Formulation 1023E]
[0087] A coating formulation was prepared with 58 mg of erucic acid,
43 mg of
DC-Cholesterol, and 6.25 mg of DOPE-mPEG350 mixed with 7 mL of heptane and
heated to
60 C such that a clear solution was obtained. The solution was then vortex
mixed for 30
seconds and allowed to cool. Next, 100 mg of sirolimus loaded microspheres of
sample #8
was added, and the formulation was placed in an ultrasonic bath for 5 minutes
to disperse and
suspend the microspheres. [Formulation 0424A]
[0088] A coating formulation was prepared with 25 mg of nervonic acid,
75 mg
of DC-Cholesterol, and 6.25 mg of DOPE-mPEG350 mixed with 7 mL of heptane and
heated
-25-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
to 60 C such that a clear solution was obtained. The solution was then vortex
mixed for 30
seconds and allowed to cool. Next, 97 mg of sirolimus loaded microspheres of
sample #8
was added, and the formulation was placed in an ultrasonic bath for 5 minutes
to disperse and
suspend the microspheres. [Formulation 0422E]
Example 4: Formulation of micro-reservoirs in coating formulation of
cholesterol, fatty acid,
PEG-lipid and stabilizing additive
[0089] A coating formulation was prepared with 77 mg of stearic acid,
40 mg
cholesterol, 50 mg DPPE-mPEG350, and 58 mg of alpha-tocopherol mixed with 7 mL
of
heptane and heated to 60 C until a clear solution was obtained. The solution
was vortex
mixed for 1 minute and allowed to cool to room temperature. Next, 100 mg of
sirolimus
loaded microspheres of sample #5 was added. The formulation was placed in an
ultrasonic
bath for 5 minutes to disperse and suspend the microspheres. [Formulation
1009A]
Example 5: Formulation of micro-reservoirs in coating formulation of
cholesterol and
phospholipid
[0090] A coating formulation was prepared with 43 mg cholesterol and
42 mg L-
alpha-phosphatidylcholine mixed with 7 mL of heptane and heated to 60 C. The
solution
was vortex mixed for 30 seconds and then allowed to cool to room temperature.
Next, 100
mg of sirolimus loaded microspheres from sample #5 were added to the vial
which was then
placed in an ultrasonic bath for 8 minutes to disperse and suspend the
microspheres.
[Formulation 0311A]
Example 6: Formulation of micro-reservoirs in coating formulation of
cholesterol and long
acyl chain phospholipid with and without PEG-lipid
[0091] A coating formulation was prepared with 51 mg DC-Cholesterol,
6.25 mg
DOPE-mPEG350 and 51 mg dierucoyl phosphatidylcholine (DEPC) mixed with 7 mL of
heptane and heated to 60 C. The solution was vortex mixed for 30 seconds and
then allowed
to cool to room temperature. Next, 100 mg of sirolimus loaded microspheres
from sample #7
were added to the vial which was then placed in an ultrasonic bath for 5
minutes to disperse
and suspend the microspheres. [Formulation 0410A]
[0092] A coating formulation was prepared with 20 mg DC-Cholesterol,
26 mg
cholesterol, 6.25 mg DOPE-mPEG350 and 75 mg dinervonyl phosphatidylcholine
(DNPC)
-26-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
mixed with 7 mL of heptane and heated to 60 C. The formulation had a weight
ratio of
DNPC to DC-Cholesterol of 1.6:1. The solution was allowed to cool to room
temperature.
Next, 97 mg of sirolimus loaded microspheres from sample #7 were added to the
vial which
was then vortex mixed for 30 seconds and then placed in an ultrasonic bath for
5 minutes to
disperse and suspend the microspheres. [Formulation 0421A]
[0093] A coating formulation was prepared with 28 mg DC-Cholesterol,
26 mg
cholesterol, 6.25 mg DOPE-mPEG350 and 50 mg dinervonyl phosphatidylcholine
(DNPC)
mixed with 7 mL of heptane and heated to 60 C. The solution was vortex mixed
for 30
seconds and then allowed to cool to room temperature. Next, 97 mg of sirolimus
loaded
microspheres from sample #7 were added to the vial which was then placed in an
ultrasonic
bath for 5 minutes to disperse and suspend the microspheres. [Formulation
0421B]
[0094] A coating formulation was prepared with 50 mg DC-Cholesterol
and 50
mg dinervonyl phosphatidylcholine (DNPC) mixed with 7 mL of heptane and heated
to 60 C.
The formulation had a weight ratio of DNPC to DC-Cholesterol of 1:1. The
solution was
vortex mixed for 30 seconds and then allowed to cool to room temperature.
Next, 100 mg of
sirolimus loaded microspheres from sample #7 were added to the vial which was
then placed
in an ultrasonic bath for 4 minutes to disperse and suspend the microspheres.
[Formulation
1205A]
[0095] A coating formulation was prepared with 49 mg DC-Cholesterol,
6.25 mg
DOPE-mPEG350 and 50 mg dinervonyl phosphatidylcholine (DNPC) mixed with 7 mL
of
heptane and heated to 60 C. The formulation had a weight ratio of DNPC to DC-
Cholesterol
of 1:1. The solution was vortex mixed for 30 seconds and then allowed to cool
to room
temperature. Next, 100 mg of sirolimus loaded microspheres from sample #7 were
added to
the vial which was then placed in an ultrasonic bath for 2 minutes to disperse
and suspend the
microspheres. [Formulation 1209A]
[0096] A coating formulation was prepared with 76 mg DC-Cholesterol,
6.25 mg
DOPE-mPEG350 and 25 mg dinervonyl phosphatidylcholine (DNPC) mixed with 7 mL
of
heptane and heated to 60 C. The formulation had a weight ratio of DNPC to DC-
Cholesterol
of 1:3. The solution was allowed to cool to room temperature. Next, 100.7 mg
of sirolimus
loaded microspheres from sample #8 were added to the vial, vortex mixed for 30
seconds and
-27-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
then then placed in an ultrasonic bath for 5 minutes to disperse and suspend
the microspheres.
[Formulation 0513A]
Example 7: Formulation of micro-reservoirs in coating formulation of DC-
Cholesterol with
varying PEG-lipid content
[0097] A coating formulation was prepared with 12.5 mg of DOPE-
mPEG350, 44
mg of DC-Cholesterol and 44 mg of dinervonoyl phosphatidylcholine (DNPC) mixed
with 7
mL of heptane heated to 60 C. The clear solution was allowed to cool to room
temperature,
then 97 mg of sirolimus loaded microspheres from microsphere from sample #8
were added.
The formulation was then placed in an ultrasonic bath and sonicated for 5
minutes to disperse
and suspend the microspheres. [Formulation 0422A]
[0098] A coating formulation was prepared with 25 mg of DOPE-mPEG350,
37.5
mg of DC-Cholesterol and 37.5 mg of dinervonoyl phosphatidylcholine (DNPC)
mixed with
7 mL of heptane heated to 60 C. The clear solution was allowed to cool to room
temperature
then 97 mg of sirolimus loaded microspheres from microsphere sample #8 were
added. The
formulation was then placed in an ultrasonic bath and sonicated for 5 minutes
to disperse and
suspend the microspheres. [Formulation 0422B]
Example 8: Coating with additional drug
[0099] A coating formulation was prepared with 72.9 mg DC-cholesterol
in 7 mL
of heptane and heated to 60C until the DC-cholesterol was solubilized to
produce a clear
solution. To the solution was added 15.5 mg of sirolimus and vortex mixed for
30 seconds.
The solution was heated for 40 minutes, vortexing 10 seconds every 10 minutes
and
sonicated for 5 minutes while cooling to room temperature. To the solution was
added 50 mg
of DNPC. When at room temperature, the solution was filtered through a 0.2
micron PTFE
filter to remove large drug particles. The solution was left overnight with no
observed
particulates formed overnight. The solution was assayed, and the sirolimus
content was
found to be 0.96 mg per ml. To the solution was added 98 mg of sirolimus
loaded
microspheres from microsphere sample #8, vortex mixed for 30 seconds and
sonicated for 8
minutes to disperse and suspend the microspheres. The resulting coating
formulation
contained 0.71 % by weight sirolimus of which 19.1% of the drug was in the DC-
cholesterol
and DNPC hydrophobic matrix with the remainder in the microspheres.
[Formulation 0512A]
-28-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0100] The weight percentage compositions of the coating formulations
described
in Examples 3, 4, 5, 6, 7 and 8 are presented in Table 5.
Table 5. Weight percentage compositions of coating formulations
Coating Fatty Acid or PEG- Micro-
Cholesterol Other Heptane
Sirolimus
Formulation Phospholipid Lipid spheres
ifki ifki ifki ifki ifki ifki [%1
DPPE-
Stearic Acid Cholesterol
1023E mPEG350
1.07% 1.05% 0.50% 2.01% 95.37% 0.58%
DOPE-
Erucic Acid DC Cholesterol
0424A mPEG350
1.17% 0.87% 0.13% 2.01% 95.82% 0.59%
DOPE-
Nervonic Acid DC Cholesterol
0422E mPEG350
0.50% 1.51% 0.13% 1.96% 95.90% 0.57%
DPPE- alpha
Stearic Acid Cholesterol
1009A mPEG350 Tocopherol
1.52% 0.79% 0.98% 1.97% 1.14% 93.60% 0.45%
L-alpha
Phosphatidyl- Cholesterol
0311A choine
0.85% 0.87% 2.02% 96.26% 0.47%
DOPE-
DEPC DC Cholesterol
0410A mPEG350
1.03% 1.03% 0.13% 2.01% 95.81% 0.56%
Cholesterol/DC DOPE-
DNPC
0421A Cholesterol mPEG350
1.51% 0.52%/0.40% 0.13% 1.95% 95.50% 0.54%
Cholesterol/DC DOPE-
DNPC
0421B Cholesterol mPEG350
1.01% 0.52%/0.56% 0.13% 1.95% 95.82% 0.54%
DNPC DC Cholesterol
1205A
1.01% 1.01% 2.02% 95.96% 0.56%
DOPE-
DNPC DC Cholesterol
1209A mPEG350
1.01% 0.99% 0.13% 2.02% 95.86% 0.56%
DOPE-
DNPC DC Cholesterol
0513A mPEG350
0.50% 1.53% 0.13% 2.03% 95.81% 0.60%
DOPE-
DNPC DC Cholesterol
0422A mPEG350
0.89% 0.89% 0.25% 1.96% 96.01% 0.58%
DOPE-
DNPC DC Cholesterol
0422B mPEG350
0.76% 0.76% 0.50% 1.96% 96.02% 0.58%
-29-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
Sirolimus in
DNPC DC Cholesterol Hydrophobic
0512A Matrix
1.00% 1.46%
1.97% 0.13% 95.43% 0.71%
Example 9: Application of coating formulation to balloon catheter
[0101] The
stearic acid coating formulation of Example 3 (Formulation 1023E)
was sprayed onto the balloon surface of 5.0 mm diameter X 20 mm length Nylon
angioplasty
balloons. Seven ml of the coating formulation was loaded into a 25 mL gas-
tight syringe
with an integrated magnetic stir bar system. The formulation was continuously
stirred during
spraying to keep the drug micro-reservoirs well suspended. A syringe pump
delivered the
coating formulation at a rate of 0.11 mL/min through a 120 kHz ultrasonic
nozzle being
activated with 5.5 watts of power [Sonotek DES1000]. To verify process
parameters, a 5.0
mm diameter x 20 mm length cylinder of balloon material was cut, weighed and
placed over
the same size balloon. This sleeve of balloon material was then coated and
weighed to verify
approximately 2.2 mg total coating was applied, corresponding to 7 1g/mm2 of
coating
density. Of this 7 1g/mm2 of the formulation from Example 3, stearic acid
comprised
approximately 1.6 1g/mm2, cholesterol comprised 1.6 1g/mm2 , DPPE-mPEG350 0.8
1g/mm2 and sirolimus loaded microspheres from microsphere sample #5 at 3
1g/mm2
resulting in a drug density of 0.87 p.g /mm2. Once sleeve weights confirmed
target weight had
been reached, full balloons were coated. A 5.0 mm diameter x 20mm length
balloon was
inflated, positioned underneath the spray and then rotated constantly while
moving back and
forth 5 times. The balloon was then removed and allowed to dry. The process
was repeated
until 6 balloons were coated. This same process was repeated to spray the
coating
formulation of Example 6 (Formulation 0513A) on 3.0mm diameter x 20mm length
balloons.
The sleeve coating target weight for a 3.0mm diameter x 20mm length balloon
with the
formulation of Example 6 (Formulation 0513A) was 1.4 mg to achieve a coating
density of
7.6 p.g/mm2. Of this 7.6 p.g/mm2, dinervonoyl phosphatidylcholine comprised
0.9 p.g/mm2,
DC-cholesterol 2.7 p.g/mm2, DOPE-mPEG350 0.23 p.g/mm2, and the sirolimus
loaded
microspheres of sample #5 comprised 3.7 p.g/mm2 resulting in a drug density of
1.08 p.g
/mm2.
-30-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0102] The
coating formulations of Examples 4, 5, 6, 7 and 8 were also sprayed
onto the surface of 20 mm length balloons in the manner of spraying the
formulation of
Example 3 previously described. The resultant coating weights and coating
densities are
presented in Table 6.
Table 6. Coating of Balloon Catheters
Fatty
Coating
h. C oleste- Acid or PEG- Micro-
Exam- Formu- Balloon Formulation Coating Coating Drug
rol Phospho- lipid sphere
ple lation Diameter % Solids Weight
Density . Density
( / ) Density lipid Density Density
w w
Density
[mm] Fel [mg] [ug/mm2] [ug/mm2] [ug/mm2] [ug/mm2]
[ug/mm2] [ug/mm2]
3 1023E 5 4.86% 2.19 6.97 1.58 1.61 0.75
3.02 0.87
3 424A 5 4.36% 2.05 6.53 1.35 1.83 0.20
3.15 0.93
3 0422E 5 4.27% 1.82 5.79 2.14 0.71 0.18
2.76 0.81
109A/
4 5 6.83% 2.54 8.09 1.00 1.92 1.24
2.49 0.57
1010D
0311A 5 3.89% 1.7 5.41 1.26 1.23 0.00 2.93
0.67
6 410A 5 4.38% 2.31 7.35 1.80 1.80 0.22
3.53 0.98
6 0421A 5 4.71% 1.88 5.98 1.23 2.00 0.17
2.59 0.72
6 0421B 5 4.36% 1.83 5.83 1.52 1.41 0.18
2.73 0.76
6 1205A 5 4.20% 1.78 5.67 1.42 1.42 0.00
2.83 0.78
6 1209A 5 4.32% 2.24 7.13 1.70 1.74 0.22
3.47 0.96
6 513A 3 4.37% 1.43 7.59 2.77 0.91 0.23
3.67 1.08
7 0422A 5 4.15% 1.8 5.73 1.28 1.28 0.36
2.81 0.83
7 0422B 5 4.14% 1.83 5.83 1.11 1.11 0.74
2.87 0.84
8 512A 3 4.79% 1.51 8.01 2.57 1.76 0.00
3.45 1.25
[0103] For
the balloons coated with the formulation of Example 4, each balloon
was sprayed with an additional top coat formulation (1010D) consisting of 1 mg
of
cholesterol and cholesterol-PEG600 coating to cover the micro-reservoir layer.
To make this
top coating, 23 mg of cholesterol-PEG600 and 224 mg of cholesterol were
dissolved in 7 mL
of isopropanol. The target coating weight of 1 mg on a 5.0 mm diameter x 20 mm
long
balloon corresponds to 3.2 1g/mm2 of total top coating comprised of 0.3 1g/mm2
cholesterol-
PEG600 and 2.9 1g/mm2 cholesterol.
Example 10: Adhesion of coatings to vessel luminal surface
[0104] Ex-
vivo porcine arteries were flushed with 37 C Lactated Ringer's
solution at 50 mL/min pulsatile flow (approximately 72 BPM) for 5 minutes. The
balloons
coated with the formulation of Example 3 were inflated in the lumen of ex-vivo
porcine
-31-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
arteries to an approximate overstretch of 1:1.2 to transfer the drug
containing coating to the
vessel lumen. The solution that passed through the arteries prior to and after
inflation (pre
and post flush), the balloon used for the arteries, and the section of artery
contacting the
inflated balloon were subsequently assayed for drug after 5 minutes of post
inflation flush.
The vessels treated with formulations 1205A and 1209A were flushed for a total
of 60
minutes to evaluate extended stability of the transferred coating. The amount
of drug
measured from all sources in the assay was totaled and compared to the
estimated drug
content of the balloon based on coating weight. The proportion of drug
transferred to the
artery based on the estimated drug content of the balloon by coating weight
was used as a
measure of transfer efficiency.
Table 7. Stearic Acid - Cholesterol Formulation [Formulation 1023E]
% of Total
Sirolimus Recovered [ug] Total
Sirolimus on
Balloon Sirolimus
1 min 1 min 2 min Balloon
Sample
Pre Post Post Balloon
Artery Recovered
Transferred
Residual [1.1g]
Flush Flush Flush to Artery
53 12 110 10 9 66 207 16
54 27 120 10 19 90 266 22
55 30 87 7 26 136 286 33
56 23 177 10 6 53 269 13
57 37 186 9 6 99 337 24
58 16 148 10 0 38 212 9
Average 24.2 138.0 9.3 11.0 80.3 262.8 19.5
SD 9.2 39.17 1.2 9.6 35.5 48.6 9
Table 8. Erucic Acid - DC-Cholesterol Formulation [Formulation 0424A]
Sirolimus Recovered [ug] Total % of Total
Sirolimus on
Balloon Sirolimus
1 min 1 min 2 min Balloon
Sample Pre Post Post Balloon
Artery Recovered
Transferred
Residual
Flush Flush Flush [Idgl to Artery
0424A-1 1 4 1 160 8 185 3
0424A-2 2 5 2 214 12 247 5
0424A-3 2 9 1 253 7 290 3
Average 1.8 5.9 1.5 209.2 8.8 240.8 3.9
SD 0.4 2.7 0.8 46.7 2.9 53.0 1.3
Table 9. Nervonic Acid - DC-Cholesterol Formulation [Formulation 0422E]
Balloon Sirolimus Recovered [jig] Total % of Total
-32-
CA 03114461 2021-03-25
WO 2020/081455
PCT/US2019/056127
Sample 1 mm 1 mm 2 m Sirolimus Sirolimus on
in n n
Balloon Recovered Balloon
Pre Post Post Artery
Residual [i.t.g]
Transferred to
Flush Flush Flush
Artery
0422E-1 5 28 6 128 62 229 22
0422E-2 3 39 4 90 35 171 12
0422E-3 16 8 4 76 84 187 29
Average 8 25 4 98 61 196 21.2
SD 7 16 1 27 25 30 8.6
[0105] The balloons coated with the formulation of Example 4 were also
tested in
ex-vivo porcine arteries.
Table 10. Stearic Acid ¨ Cholesterol-alpha Tocopherol Formulation [Formulation
1009A/1010D]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus
Balloon Sirolimus
on Balloon
Sample 1 min 1 min 2 min Recovered
Balloon , Transferred
Pre Post Post Artery [iig]
Residual to Artery
Flush Flush Flush
40 N/A 78 3 354 28 463 6
41 12 120 4 301 31 468 6
[0106] The balloons coated with the formulation of Example 5 were also
tested in
ex-vivo porcine arteries.
Table 11. L-alpha-Phosphatidylcholine ¨ Cholesterol Formulation [Formulation
0311A]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus on
Balloon Sirolimus
1 min Balloon
Sample 1 min 2 min Balloon
Artery Recovered
Pre Transferred
post post residual [Idg]
Flush to Artery
0311A-1 51 60 4 12 26 153 9
0311A-2 100 74 4 25 7 210 2
0311A-3 44 92 5 26 45 212 15
Average 65.0 75.3 4.3 21.0 26.0 191.7 8.7
SD 30.5 16.0 0.6 7.8 19.0 33.5 6.5
[0107] The balloons coated with the formulation of Example 6 were also
tested in
ex-vivo porcine arteries.
Table 12. DEPC - DC-Cholesterol [Formulation 0410A]
-33-
CA 03114461 2021-03-25
WO 2020/081455
PCT/US2019/056127
% of Total
Sirolimus Recovered kig] Total
Sirolimus
Balloon Sirolimus
1 min on Balloon
Sample 1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual hdgl
Flush to Artery
0410A-1 17 21 3 12 196 249 52
0410A-2 34 12 2 15 228 290 60
0410A-3 17 30 1 14 137 199 53
Average 65 75 4 21 26 192 55
SD 31 16 1 8 19 34 6.3
Table 13. DNPC - DC-Cholesterol Formulation [Formulation 0421A]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus
Balloon Sirolimus
1 min on Balloon
Sample 1 min 2 min Balloon Recovere
Pre Artery Transferred
post post residual d [jig]
Flush to Artery
0421A-1 16 6 1 32 127 259 40%
0421A-2 18 13 3 29 114 240 35%
0421A-3 21 9 7 24 138 264 43%
Average 18.4 9.4 3.6 28.4 126.4 254.4 39.4%
SD 2.7 3.6 2.7 4.2 12.2 12.5 3.8%
Table 14. DNPC - DC-Cholesterol - Cholesterol Formulation [Formulation 0421B]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus on
Balloon Sirolimus
1 min Balloon
Sample 1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual [Idg]
Flush to Artery
0421B-1 8 16 1 120 131 276 45
0421B-2 5 21 2 196 108 331 37
0421B-3 4 22 5 137 83 250 28
Average 5.3 19.7 2.7 151.2 107.1 286.0 36.7
SD 2.1 2.9 2.0 39.9 23.9 41.3 8.2
Table 15. DNPC - DC-Cholesterol (no PEG-Lipid) Formulation [Formulation 1205A]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus
Balloon Sirolimus
1 min on Balloon
Sample 1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual [jig]
Flush to Artery
106 14 47 3 94 168 326 47
105 10 84 5 142 165 406 46
-34-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
107 8 68 4 100 147 327 41
108 9 43 4 121 144 321 41
109 4 66 9 62 158 299 45
110 3 52 1 128 126 310 35
Average 8.0 60.0 4.3 107.8 151.3 331.5 42.5
SD 4.0 15.5 2.7 28.6 15.6 38.0 4.5
Table 16. [DNPC - DC-Cholesterol (PEG-Lipid) Formulation [Formulation 1209A]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus
Balloon Sirolimus
1 min on Balloon
Sample 1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual [Idg]
Flush to Artery
124 5 64 1 30 148 248 38
125 5 79 4 88 158 334 41
126 4 45 9 144 152 354 39
127 8 73 5 135 124 345 32
128 2 49 5 98 190 344 49
129 4 89 5 90 149 337 38
Average 4.7 66.5 4.8 97.5 153.5 327.0 39.5
SD 2.0 17.2 2.6 40.7 21.3 39.3 5.5
Table 17. DNPC - DC-Cholesterol (PEG-Lipid) Formulation [Formulation 0513A]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus
Balloon Sirolimus
1 min on Balloon
Sample 1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual h-Igl
Flush to Artery
0513A-1 6 4 1 134 67 212 30%
0513A-2 5 12 2 150 85 254 38%
0513A-3 5 2 1 152 88 248 39%
Average 5.3 6.0 1.3 145.3 80.0 238.0 35.4%
SD 0.6 5.3 0.6 9.9 11.4 22.7 5.0%
[0108] The luminal surface of the artery after inflation of the
balloon coated with
Formulation 1209A and after one hour of post inflation fluid flush was viewed
under
darkfield microscopy. Fig. 4 is a photomicrograph of the luminal surface at
200X
magnification showing adhered material. Fig. 5 is a photomicrograph of the
surface at 1000X
magnification showing the adhered material to be a layer of spherical micro-
reservoirs
surrounded by coating material.
-35-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
Example 11: Adhesion of Coatings to Vessel Luminal Surface of Formulations
with Varying
PEG-lipid Content
[0109] The samples from Example 7 were tested for coating transfer and
resistance to wash-off using the methods of Example 10. The results have been
tabulated to
compare the coatings with DNPC and DC-Cholesterol in equal weight proportion
with
varying amounts of DOPE-mPEG350. [Formulations 1205A, 1209A, 0422A, 0422B]
Table 18. Coating Transfer and Resistance to Wash-Off for Various Coating
Formulations
% of Total
Sirolimus Recovered hug] Total
Sirolimus on
Sirolimus
Formulation 1 min Balloon
1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual [tig]
Flush to Artery
No PEG- 8.0 60.0 4.3 107.8 151.3 331.5
42.5
lipid 4.0 15.5 2.7 28.6 15.6 38.0 4.5
5.9% mPEG 4.7 66.5 4.8 97.5 153.5 327.0 39.5
350 2.0 17.2 2.6 40.7 21.3 39.3 5.5
12.4% 6.5 38.9 4.5 107.4 90.4 247.6 30.0
mPEG 350 3.2 21.0 0.6 35.5 29.2 55.1
9.7%
25% mPEG 25.0 68.3 6.2 17.9 106.7 224.1
36.0
350 26.1 36.7 3.2 12.0 19.8 27.8
6.7%
[0110] The results demonstrate significant transfer of drug coating to
the vessel
lumen. Drug coating loss during pre-flush was increased for coating
formulation with 25%
PEG-lipid.
Example 12: Adhesion of coating with additional rapamycin to vessel luminal
surface
[0111] The formulation of Example 8 was tested for coating transfer
and
resistance to wash-off using the methods of Example 10.
Table 19. DNPC - DC-Cholesterol Formulation with Additional Drug [Formulation
0512A]
% of Total
Sirolimus Recovered [jig] Total
Sirolimus
Balloon Sirolimus
1 min on Balloon
Sample 1 min 2 min Balloon Recovered
Pre Artery Transferred
post post residual [Idg]
Flush to Artery
0512A-1 3 43 2 155 76 279 29%
0512A-2 5 9 10 51 39 114 15%
0512A-3 6 8 2 135 47 198 18%
Average 4.7 20.0 4.7 113.7 54.0 197.0 20.9%
SD 1.5 19.9 4.6 55.2 19.5 82.5 7.5%
-36-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0112] The results demonstrate significant transfer of drug to the
vessel lumen
from a coating with additional drug added to the phospholipid and cholesterol
components of
the coating formulation.
Example 13: Drug release into treated vessel in-vivo
[0113] To prepare balloon catheters coated with drug micro-reservoir
containing
formulation, 100 mg of DNPC, 103 mg DC-Cholesterol and 12.5 mg DOPE-mPEG350
was
mixed into 14 mL of heptane. The mixture was heated to 60 C to dissolve the
solid
components and cooled to room temperature. Next, 195 mg of microsphere sample
#6 were
added and stirred to suspend the microspheres. Balloon catheters with balloons
of 3.0 mm
diameter x 20 mm length were coated with the formulation using the methods
described in
Example 9. The coated balloon catheters were allowed to dry. An average of
1.28 mg 0.12
mg of dried coating was applied to the balloons, resulting in a coating
density of 6.80 p.g
/mm2 and a drug density of 1.06 p.g /mm2. The balloons were deflated and
folded to a pre-
deployment configuration with a smaller cross-section and packaged in a sleeve
to retain the
folded configuration. The balloon catheters were packaged and sterilized by
ionizing
radiation at a dose of 25 kiloGray minimum.
[0114] The iliofemoral artery of rabbits was used to assess the in-
vivo transfer of
the drug coating to an arterial vessel. The iliofermoral artery segment for
treatment was first
denuded of endothelium to reproduce post-angioplasty tissue damage. A
dissection was
made to the common carotid artery, and a size 5F balloon wedge catheter was
inserted into
the artery and directed under fluoroscopic guidance to the treatment site of
the iliofemoral
artery. Contrast agent was injected through the catheter and angiograms of the
iliofemoral
arteries recorded. The balloon wedge catheter was exchanged for a 3.0 mm
diameter x 8 mm
length standard angioplasty balloon catheter under fluoroscopic guidance,
inflated, and
withdrawn proximally in its inflated state approximately to the level of the
iliac bifurcation to
denude the section of the artery. The angioplasty balloon catheter was
exchanged for a drug
coated balloon catheter. The catheter was advanced to the denuded vessel
segment and
inflated for 120 seconds. The balloon was deflated and withdrawn. Both the
right and left
iliac arteries of each animal were treated.
-37-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0115] A total of eleven animals were treated. One animal (2 iliac
arteries
treated) was euthanized 1 hour after treatment and vessel segments recovered
for microscopic
examination. Another animal (2 iliac arteries treated) was euthanized 24 hours
after
treatment and vessel segments recovered for microscopic examination. Three
animals (6 iliac
arteries) were recovered at each time point of 1 hour, 7 days and 28 days.
Blood samples
were taken from these animals prior to surgery, at 0.5, 1, 4 hours post
treatment and at
sacrifice. The vessel segments were recovered and assayed for drug content by
HPLC/MS
quantitation.
[0116] Assay of the blood samples showed a rapid decline of drug in
circulating
blood with a concentration of 4.75 ng/ml at 30 minutes, 2.63 ng/ml at 1 hour
and 0.82 ng/ml
at 4 hours. The blood concentration of drug collected at sacrifice for the 7
day and 28 day
time points were below the limit of detection for the quantitation assay. The
blood levels
were fit to an exponential decay curve with a half-life of 0.77 hours,
indicating rapid dilution
and clearance of drug from the bloodstream
[0117] Scanning electron microscopy and light microscopy of the tissue
samples
collected 1 hour and 24 hours after treatment showed a layer of material on
the vessel lumen
surface with spherical drug micro-reservoirs observed within the layer. Patchy
areas of fibrin
were observed on the luminal surface but no large fibrin deposits indicative
of blood
incompatibility were observed to be associated with the coating.
[0118] Assay of the treated vessel segments demonstrated tissue drug
levels of
261 [tg/g 116.5 [tg/g at 1 hour after treatment, 43.8 [tg/g 34.2 [tg/g at
7 days after
treatment and 21.5 [tg/g 17.3 [tg/g at 28 days after treatment. The results
indicate adhesion
of the drug containing micro-reservoir coating to the luminal surface of an
artery with
sustained presence of drug associated with the tissues of the treated vessel
through 28 days.
The tissue associated levels of drug demonstrated a rapid initial decline
which slowed
between 7 to 28 days. The tissue associated drug levels from 7 and 28 days
were fit to an
exponential decay, indicating a half-life of approximately 20.4 days.
Example 14: Adhesion of coatings to vessel luminal surface for coating
formulation
comprising sirolimus microparticles
-38-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0119] Crystalline sirolimus powder was ground, and 100mgwas selected
and
added to about 75mg of phospholipid excipient formulation (about 15% DOPE-
mPEG350,
35% DNPC, 50% DC-Chol). Ground Sirolimus microparticles were dispersed and
suspended
in the formulation via magnetic stirring and then sprayed on 4x30mm balloon
catheters using
the Sonotek PSI Ultrasonic spray system. Ultrasonic spraying formulation flow
rate was set
at 0.210 ml/min and used 4 passes to build up to a target coating weight of 2
milligrams
corresponding to approximately 3i.tg of Sirolimus per mm2 of balloon surface
area. Fig. 6 is a
photomicrograph of the coated balloon surface at 100X magnification showing
the coating
containing crystalline sironlimus micro-reservoirs.
[0120] Several 4mm diameter porcine carotid arteries were connected to
a 72
BPM pulsatile flow system of lactated ringers solution at approximately
100m1/min. Coated
balloon catheters were inserted in the artery and left deflated while fluid
was pumped through
the artery for 1 minute and collected to simulate wash-off during tracking to
the lesion. The
balloon was then inflated for one minute, deflated, removed and the artery
flushed and the
fluid collected for an additional minute. A second minute of wash-off was
collected
separately before allowing 3 more minutes of flow for a total of 5 minutes.
After 5 minutes
the artery was cut down the length, visually inspected, then assayed for
sirolimus. Three
coated catheters of the same formulation were tested in arteries. White
residue coating is
visible on dried arteries indicating significant transfer has occurred. Fig. 7
is a
photomicrograph of the artery surface at 50X magnification showing adhered
material, and
Fig. 8 is a photomicrograph of the artery surface at 1000X magnification
showing adhered
material.
[0121] After visual inspection, the 3 treated arteries were dissolved
in acetonitrile
and assayed for Sirolimus. The balloon catheters were assayed for residual
sirolimus. 1
minute pre, post, and 2 minute post wash-off samples were filtered with 0.2um
PTFE filters
and dissolved with acetonitrile. The amount of sirolimus recovered from each
group is
presented in the Table 20. Of the total drug mass tracked, an average of 42%
was found
adhered to a porcine artery after 5 minutes of flushing. This demonstrates
that such a ground
microcrystalline sirolimus coating is capable of transferring to arteries.
Table 20. Coating Transfer and Resistance to Wash-Off
-39-
CA 03114461 2021-03-25
WO 2020/081455
PCT/US2019/056127
Sirolimus Recovered [14]
1 min 1 min 2min Balloon Total
Sirolimus
ID Pre flush Post Post Residue Artery
Recovered [pg]
FR 4-1 24.537 71.876 3.5756 120.77 167.28 --
388.04
FR 4-2 1.3316 65.114 3.1056 140.61 212.24 --
422.40
FR 4-3 4.2115 68.768 5.7644 191.6 130.43
400.77
Average 10.03 68.59 4.15 150.99 169.98
403.74
Percent 2.5% 17.0% 1.0% 37.4% 42.1%
100.0%
Additional Embodiments
[0122] Although this invention has been disclosed in the context of
certain
preferred embodiments and examples, it will be understood by those skilled in
the art that the
present invention extends beyond the specifically disclosed embodiments to
other alternative
embodiments and/or uses of the invention and obvious modifications and
equivalents thereof.
Additionally, it is contemplated that various aspects and features of the
invention described
can be practiced separately, combined together, or substituted for one
another, and that a
variety of combination and sub-combinations of the features and aspects can be
made and
still fall within the scope of the invention. Further, the disclosure herein
of any particular
feature, aspect, method, property, characteristic, quality, attribute,
element, or the like in
connection with an embodiment can be used in all other embodiments set forth
herein. Thus,
it is intended that the scope of the present invention herein disclosed should
not be limited by
the particular disclosed embodiments described above, but should be determined
only by a
fair reading of the claims.
[0123] Conditional language, such as, among others, "could," "might,"
or "may,"
unless specifically stated otherwise, or otherwise understood within the
context as used, is
generally intended to convey that certain embodiments include while other
embodiments do
not include, certain features or elements. Thus, such conditional language is
not generally
intended to imply that features or elements are in any way required for one or
more
embodiments.
Summary of Embodiments
[0124] A coating for an expandable portion of a catheter comprising a
hydrophobic matrix and a dispersed phase comprising a plurality of micro-
reservoirs
-40-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
dispersed in the hydrophobic matrix, wherein the plurality of micro-reservoirs
comprises a
first active agent and a first biodegradable or bioerodable polymer.
[0125] In embodiments of the coating as described above, the first
active agent is
intermixed with or dispersed in the first biodegradable or bioerodable
polymer.
[0126] In embodiments of the coating as described above, the plurality
of micro-
reservoirs further comprises a second active agent. The second active agent is
selected from
the group consisting of paclitaxel, sirolimus, paclitaxel derivative,
sirolimus derivative,
paclitaxel analogues, sirolimus analogues, inhibitory RNA, inhibitory DNA,
steroids, and
complement inhibitors.
[0127] In embodiments of the coating as described above, the plurality
of micro-
reservoirs further comprises a second biodegradable or bioerodable polymer.
The second
biodegradable or bioerodable polymer is selected from the group consisting of
polylactic
acid, polyglycolic acid and their copolymers, polydioxanone, polycaprolactone,
polyphosphazine, collagen, gelatin, chitosan, glycosoaminoglycans, and
combination thereof.
[0128] In embodiments of the coating as described above, the
hydrophobic matrix
comprises at least one hydrophobic compound selected from the group consisting
of sterols,
lipids, phospholipids, fats, fatty acids, surfactants, and their derivatives.
[0129] In some embodiments of the coating described above, wherein the
hydrophobic matrix comprises a cholesterol and a fatty acid. In some
embodiments, the
weight ratio of cholesterol to fatty acid is in the range of about 1:2 to
about 3:1.
[0130] In embodiments of the coating as described above, the fatty
acid is
selected from the group consisting of lauric acid, lauroleic acid,
tetradeadienoic acid,
octanoic acid, myristic acid, myristoleic acid, decenoic acid, decanoic acid,
hexadecenoic
acid, palmitoleic acid, palmitic acid, linolenic acid, linoleic acid, oleic
acid, vaccenic acid,
stearic acid, eicosapentaenoic acid, arachadonic acid, mead acid, arachidic
acid,
docosahexaenoic acid, docosapentaenoic acid, docosatetraenoic acid, docosenoic
acid,
tetracosanoic acid, hexacosenoic acid, pristanic acid, phytanic acid, and
nervonic acid..
[0131] In other embodiments of the coating described above, wherein
the
hydrophobic matrix comprises a cholesterol and a phospholipid. In some
embodiments, the
weight ratio of cholesterol to phospholipid is in the range of about 1:2 to
about 3:1.
-41-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0132] In
some embodiments, the phospholipid is selected from the group
consisting of phosphatidylcholine, phosphatidylethanolamine,
phosphatidylserine, and
phosphatidylinositol.
[0133] In
some embodiments, the phospholipid is a cationic phospholipid. In
some embodiments, the cationic phospholipid is phosphatidylethanolamine,
dioleoylphosphatidylethanolamine (DOPE), or an amine derivative of
phosphatidylcholine.
[0134] In
some embodiments, the phospholipid comprises an acyl chain length of
about 20 to about 34 carbons. In some embodiments, the phospholipid is
selected from the
group consisting of dieicosenoyl phosphatidylcholine (1,2-dieicosenoyl-sn-
glycero-3-
phosphocholine, C20:1 PC), diarachidonoyl phosphatidylcholine (1,2-
diarachidoyl-sn-
glycero-3-phosphocholine, C20:0 PC), dierucoyl phosphatidylcholine (1,2-
dierucoyl- sn-
glycero-3-phosphocholine, C22:1 PC), didocosahexaenoyl phosphatidylcholine
(1,2-
didoco s ahex aenoyl- sn-glycero-3 -pho sphocholine, C22:6
PC), heneicosenoyl
phosphatidylcholine (1,2-heneicosenoyl- sn-glycero-3 -pho sphocholine, C21:1
PC) and
dinervonyl phosphatidylcholine (1,2-dinervonoyl- sn-glycero-3 -pho
sphocholine, C24:1 PC).
[0135] In
embodiments of the coating as described above, the cholesterol is DC-
Cholesterol.
[0136] In
embodiments of the coating as described above, the plurality of micro-
reservoirs is about 10% to about 75% by weight of the coating.
[0137] In
embodiments of the coating as described above, the plurality of micro-
reservoirs has an average diameter of about 1.5 microns to about 8 microns. In
some
embodiments, the plurality of micro-reservoirs has an average diameter of
about 2 microns to
about 6 microns. In some embodiments, the plurality of micro-reservoirs has an
average
diameter of about 3 microns to about 5 microns.
[0138] In
embodiments of the coating as described above, the plurality of micro-
reservoirs has an active ingredient release kinetics with a half-life of at
least 14 days.
[0139] In
embodiments of the coating as described above, the first biodegradable
or bioerodable polymer is selected from the group consisting of polylactic
acid, polyglycolic
acid and their copolymers, polydioxanone, polycaprolactone, polyphosphazine,
collagen,
gelatin, chitosan, glycosoaminoglycans, and combination thereof.
-42-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0140] In
embodiments of the coating as described above, the first active agent is
selected from the group consisting of paclitaxel, sirolimus, paclitaxel
derivative, sirolimus
derivative, paclitaxel analogues, sirolimus analogues, inhibitory RNA,
inhibitory DNA,
steroids, and complement inhibitors.
[0141] In
embodiments of the coating as described above, the first active agent is
about 10% to about 50% by weight of the plurality of micro-reservoirs.
[0142] In
embodiments of the coating as described above, the coating further
comprises a third active agent outside of the plurality of micro-reservoirs.
In some
embodiments, the third active agent is selected from the group consisting of
paclitaxel,
sirolimus, paclitaxel derivative, sirolimus derivative, paclitaxel analogues,
sirolimus
analogues, inhibitory RNA, inhibitory DNA, steroids, and complement
inhibitors. In some
embodiments, the third active agent is the same as the first active agent.
[0143] In
embodiments of the coating as described above, the hydrophobic matrix
further comprises a PEG-lipid. In some embodiments, the PEG-lipid is selected
from the
group consisting of 1,2-
distearoyl- sn-glycero-3-phosphoethanolamine-N-
methoxy(polyethylene glycol)-350 (DS PE-mPEG350), 1,2-
dip almitoyl- sn-glycero-3 -
phosphoethanolamine-methoxy(polyethylene glycol)-350 (DPPE-mPEG350), 1,2-
dioleoyl-
sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene
glycol)-350 (DOPE-
mPEG350), 1,2-
distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene
glycol)-550 (DS PE-mPEG550), 1,2-
dip almitoyl- sn-glycero-3-phosphoethanolamine-N-
methoxy(polyethylene glycol)-550 (DPPE-mPEG550), and 1,2-dioleoyl-sn-glycero-3-
phosphoethanolamine-N-methoxy(polyethylene glycol)-500 (DOPE-mPEG550). In some
embodiments, the PEG-lipid is about 1% to about 30% by weight of the
hydrophobic matrix.
In some embodiments, the PEG-lipid is about 12% or less by weight of the
hydrophobic
matrix.
[0144] In
embodiments of the coating as described above, the coating further
comprises one or more additives independently selected from penetrating
enhancers and
stabilizers.
[0145] In
embodiments of the coating as described above, wherein the coating has
a surface concentration of about 11.tg/mm2 to about 101.tg/mm2.
-43-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0146] A catheter comprising an expandable portion on an elongated
body, and
any embodiment of the coating described above over the expandable portion. In
some
embodiments, the catheter further comprises a release layer between the
expandable portion
and the coating, wherein the release layer is configure to release the coating
from the
expandable portion. In some embodiments, the release layer comprises DSPE-
mPEG350 or
DSPE-mPEG500. In some embodiments, the release layer has a surface
concentration of
about 0.11.tg/mm2 to about 5 1.tg/mm2.
[0147] In embodiments of the catheter as described above, the catheter
further
comprises a protective coating over the coating. In some embodiments, the
protective
coating comprises a hydrophilic polymer, a carbohydrate, or an amphiphilic
polymer. In
some embodiments, the protective coating is a glycosaminoglycan or a
crystalized sugar. In
some embodiments, the protective coating has a surface concentration of about
0.11.tg/mm2 to
about 5 1.tg/mm2.
[0148] A coating formulation for an expandable portion of a catheter
comprising
a solid portion and a fluid. The solid portion comprises a plurality of micro-
reservoirs and at
least one hydrophobic compound, wherein the plurality of micro-reservoirs
comprises a first
active agent and a first biodegradable or bioerodable polymer. In some
embodiments, the
first active agent is intermixed with or dispersed in the first biodegradable
or bioerodable
polymer.
[0149] In some embodiments, the plurality of micro-reservoirs further
comprises
a second active agent. In some embodiments, the second active agent is
selected from the
group consisting of paclitaxel, sirolimus, paclitaxel derivative, sirolimus
derivative,
paclitaxel analogues, sirolimus analogues, inhibitory RNA, inhibitory DNA,
steroids, and
complement inhibitors. In some embodiments, the plurality of micro-reservoirs
further
comprises a second biodegradable or bioerodable polymer. In some embodiments,
the
second biodegradable or bioerodable polymer is selected from the group
consisting of
polylactic acid, polyglycolic acid and their copolymers, polydioxanone,
polycaprolactone,
polyphosphazine, collagen, gelatin, chitosan, glycosoaminoglycans, and
combination thereof.
-44-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0150] In some embodiments of the coating formulation described above,
the
fluid is selected from the group consisting of pentane, hexane, heptane,
heptane and
fluorocarbon mixture, alcohol and fluorocarbon mixture, and alcohol and water
mixture.
[0151] In some embodiments of the coating formulation described above,
wherein
the solid portion further comprises a third active agent outside of the
plurality of micro-
reservoirs. In some embodiments, the third active agent is selected from the
group consisting
of paclitaxel, sirolimus, paclitaxel derivative, sirolimus derivative,
paclitaxel analogues,
sirolimus analogues, inhibitory RNA, inhibitory DNA, steroids, and complement
inhibitors.
[0152] In some embodiments of the coating formulation described above,
wherein
the first active agent is selected from the group consisting of paclitaxel,
sirolimus, paclitaxel
derivative, sirolimus derivative, paclitaxel analogues, sirolimus analogues,
inhibitory RNA,
inhibitory DNA, steroids, and complement inhibitors.
[0153] In some embodiments of the coating formulation described above,
wherein
the at least one hydrophobic compound is selected from the group consisting of
sterols,
lipids, phospholipids, fats, fatty acids, surfactants, and their derivatives.
[0154] In some embodiments of the coating formulation described above,
wherein
the at least one hydrophobic compound comprises a cholesterol and a fatty
acid. In some
embodiments, the weight ratio of cholesterol to fatty acid is in the range of
about 1:2 to about
3:1. In some embodiments, the fatty acid is selected from the group consisting
of lauric acid,
lauroleic acid, tetradeadienoic acid, octanoic acid, myristic acid,
myristoleic acid, decenoic
acid, decanoic acid, hexadecenoic acid, palmitoleic acid, palmitic acid,
linolenic acid, linoleic
acid, oleic acid, vaccenic acid, stearic acid, eicosapentaenoic acid,
arachadonic acid, mead
acid, arachidic acid, docosahexaenoic acid, docosapentaenoic acid,
docosatetraenoic acid,
docosenoic acid, tetracosanoic acid, hexacosenoic acid, pristanic acid,
phytanic acid, and
nervonic acid.
[0155] In some embodiments of the coating formulation described above,
wherein
the at least one hydrophobic compound comprises a cholesterol and a
phospholipid. In some
embodiments, the weight ratio of cholesterol to phospholipid is in the range
of about 1:2 to
about 3:1. In some embodiments, the phospholipid is selected from the group
consisting of
-45-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine,
and
phosphatidylinositol.
[0156] In
some embodiments, the phospholipid is a cationic phospholipid. In
some embodiments, the cationic phospholipid is phosphatidylethanolamine,
dioleoylphosphatidylethanolamine (DOPE), or an amine derivative of
phosphatidylcholine.
[0157] In
some embodiments, the phospholipid comprises an acyl chain length of
about 20 to about 34 carbons. In some embodiments, the phospholipid is
selected from the
group consisting of dieicosenoyl phosphatidylcholine (1,2-dieicosenoyl-sn-
glycero-3-
phosphocholine, C20:1 PC), diarachidonoyl phosphatidylcholine (1,2-
diarachidoyl-sn-
glycero-3-phosphocholine, C20:0 PC), dierucoyl phosphatidylcholine (1,2-
dierucoyl-sn-
glycero-3-phosphocholine, C22:1 PC), didocosahexaenoyl phosphatidylcholine
(1,2-
didoco sahexaenoyl- sn-glycero-3 -pho sphocholine, C22:6
PC), heneicosenoyl
phosphatidylcholine (1,2-heneicosenoyl- sn-glycero-3 -pho sphocholine, C21:1
PC) and
dinervonyl phosphatidylcholine (1,2-dinervonoyl- sn-glycero-3 -pho
sphocholine, C24:1 PC).
[0158] In
some embodiments of the coating formulation described above, the
cholesterol is DC-Cholesterol.
[0159] In
some embodiments of the coating formulation described above, the
solid portion further comprising a PEG-lipid, and/or an additive. In some
embodiments, the
PEG-lipid is selected from the group consisting of 1,2-distearoyl-sn-glycero-3-
phosphoethanolamine-N-methoxy(polyethylene glycol)-350 (DS PE-mPEG350), 1,2-
dip almitoyl- sn-glycero-3-phosphoethanolamine-methoxy(polyethylene glycol)-
350 (DPPE-
mPEG350), 1,2-
dioleoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene
glycol)-350 (DOPE-mPEG350), 1,2-
di stearoyl- sn-glycero-3-phosphoethanolamine-N-
methoxy(polyethylene glycol)-550 (DSPE-mPEG550), 1,2-dipalmitoyl-sn-glycero-3-
phosphoethanolamine-N-methoxy(polyethylene glycol)-550 (DPPE-mPEG550), and 1,2-
dioleoyl- sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol)-500
(DOPE-
mPEG550).
[0160] In
some embodiments of the coating formulation described above, the
plurality of micro-reservoirs is about 10% to about 75% by weight of the solid
portion.
-46-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0161] In some embodiments of the coating formulation described above,
the
solid portion is about 2 to about 7% by weight of the coating formulation.
[0162] A method for coating an expandable portion of a catheter
comprising
disposing a coating formulation of any embodiments described above over the
surface of an
expanded expandable portion of a catheter, evaporating the fluid, and
collapsing the
expandable portion. In some embodiments, disposing the coating formulation
comprises
spray coating, dip coating, roll coating, electrostatic deposition, printing,
pipetting, or
dispensing.
[0163] In some embodiments of the method described above, the method
further
comprises disposing a release layer on the expandable portion. In some
embodiments, the
release layer comprises DSPE-mPEG350 or DSPE-mPEG500.
[0164] A method for treating or preventing a condition at a treatment
site
comprising advancing a catheter comprising an expandable portion to the
treatment site,
wherein the expandable portion is coated with a coating of any embodiments
described
above, expanding the expandable portion to allow contact between the coating
and a tissue at
the treatment site, collapsing the expandable portion, and removing the
catheter.
[0165] In embodiments of the method described above, the contact
between the
tissue and the coating results in a transfer of at least a portion of a
coating on the expandable
portion to the treatment site. In some embodiments, the method further
comprises
maintaining the contact between the coating and the tissue for a period of
from about 30 to
about 120 seconds.
[0166] In embodiments of any of the method described above, the
condition is
selected from the group consisting of atherosclerosis, stenosis or reduction
in luminal
diameter in a diseased blood vessel, restenosis, in-stent restenosis, and
combinations thereof.
[0167] In embodiments of any of the method described above, wherein an
additional release layer is disposed between the expandable portion and the
coating.
[0168] In some embodiments, a catheter comprising an expandable
portion on an
elongated body; and a coating over an outer surface of the expandable portion,
wherein the
coating comprises: a lipophilic matrix, wherein the lipophilic matrix
comprises at least one
lipid; a plurality of micro-reservoirs dispersed in the lipophilic matrix,
wherein the plurality
-47-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
of micro-reservoirs comprises an active agent; and wherein the lipophilic
matrix is
configured to adhere to a luminal surface when the expandable portion is
expanded, and
transfer at least a portion of the plurality of micro-reservoirs to the
luminal surface.
[0169] In
some embodiments of the catheter described above, wherein the active
agent is crystalline.
[0170] In
some embodiments of the catheter described above, wherein the
plurality of micro-reservoirs further comprises a biodegradable or bioerodable
polymer. In
some embodiments, the biodegradable or bioerodable polymer is selected from
the group
consisting of polylactic acid, polyglycolic acid and their copolymers,
polydioxanone,
polycarpolactone, polyphosphazine, collagen, gelatin, chitosan, and
glycosoaminoglycans. In
some embodiments, the active agent is about 10% to about 50% by weight of the
micro-
reservoirs.
[0171] In
some embodiments of the catheter described above, wherein the at least
one lipid comprises phospholipid. In some embodiments, the phospholipid
comprises an acyl
chain length of about 20 to about 34 carbons. In some embodiments, the
phospholipid is
selected from the group consisting of phosphatidylcholine,
phosphatidylethanolamine,
phosphatidylserine, and phosphatidylinositol. In some embodiments, the
phospholipid is
selected from the group consisting of dieicosenoyl phosphatidylcholine (1,2-
dieicosenoyl-sn-
glycero-3-phosphocholine, C20:1 PC), diarachidonoyl phosphatidylcholine (1,2-
diarachidoyl-
sn-glycero-3-phosphocholine, C20:0 PC), dierucoyl phosphatidylcholine (1,2-
dierucoyl-sn-
glycero-3-phosphocholine, C22:1 PC), didocosahexaenoyl phosphatidylcholine
(1,2-
didoco s ahex aenoyl- sn-glycero-3 -pho sphocholine, C22:6
PC), heneicosenoyl
phosphatidylcholine (1,2-heneicosenoyl- sn-glycero-3 -pho sphocholine, C21:1
PC), and
dinervonyl phosphatidylcholine (1,2-dinervonoyl- sn-glycero-3 -pho
sphocholine, C24:1 PC).
[0172] In
some embodiments, the phospholipid comprises cationic phospholipid.
In some embodiments, the cationic phospholipid is phosphatidylethanolamine,
dioleoylphosphatidylethanolamine, or an amine derivative of
phosphatidylcholine. In some
embodiments, the lipophilic matrix further comprises a sterol. In some
embodiments,
wherein the sterol is selected from the group consisting of cholesterol,
stigmasterol,
-48-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
lanosterol, sitosterol, DHEA, N4-Cholesteryl-Spermine, Guanidium-
Cholesterol/BGTC, and
DC-Cholesterol.
[0173] In
some embodiments of the catheter, wherein the coating has a melting
point between room temperature and body temperature. In some embodiments of
the catheter,
the coating comprises about 10% to about 75% by weight of the plurality of
micro-reservoirs.
[0174] In
some embodiments of the catheter, the plurality of micro-reservoirs has
an average diameter of about 1.5 microns to about 8 microns. In some
embodiments, the
plurality of micro-reservoirs has an average diameter of about 2.0 microns to
about 6
microns.
[0175] In
some embodiments of the catheter, wherein the active agent is selected
from the group consisting of paclitaxel, sirolimus, paclitaxel derivative,
sirolimus derivative,
paclitaxel analogues, sirolimus analogues, inhibitory RNA, inhibitory DNA,
steroids, and
complement inhibitors.
[0176] In
some embodiments of the catheter, wherein the coating further
comprising a polyethylene glycol-lipid (PEG-lipid). In some embodiments, the
PEG-lipid is
selected from the group consisting of 1,2-distearoyl-sn-glycero-3-
phosphoethanolamine-N-
methoxy(polyethylene glycol)-350 (DS PE-mPEG350), 1,2-
dip almitoyl- sn-glycero-3 -
phosphoethanolamine-methoxy(polyethylene glycol)-350 (DPPE-mPEG350), 1,2-
dioleoyl-
sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene
glycol)-350 (DOPE-
mPEG350), 1,2-
distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene
glycol)-550 (DS PE-mPEG550), 1,2-
dip almitoyl- sn-glycero-3-phosphoethanolamine-N-
methoxy(polyethylene glycol)-550 (DPPE-mPEG550), and 1,2-dioleoyl-sn-glycero-3-
phosphoethanolamine-N-methoxy(polyethylene glycol)-500 (DOPE-mPEG550). In some
embodiments, the PEG-lipid is about 1% to about 10% by weight of the
hydrophobic matrix.
[0177] In
some embodiments of the catheter, the coating further comprising one
or more additives independently selected from penetrating enhancers and
stabilizers.
[0178] In
some embodiments of the catheter, the coating has a surface
concentration of about 11.tg/mm2 to about 101.tg/mm2.
[0179] In
some embodiments, a catheter comprising: an expandable portion on an
elongated body; a coating over an outer surface of the expandable portion,
wherein the
-49-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
coating comprises: a lipophilic matrix, wherein the lipophilic matrix
comprises at least one
lipid; a plurality of micro-reservoirs dispersed in the lipophilic matrix,
wherein the plurality
of micro-reservoirs comprises an active agent; and wherein the lipophilic
matrix is
configured to adhere to a luminal surface when the expandable portion is
expanded, and
transfer at least a portion of the plurality of micro-reservoirs to the
luminal surface; and a
release layer between the expandable portion and the coating, wherein the
release layer is
configured to release the coating from the expandable portion.
[0180] In some embodiments, the release layer comprises DSPE-mPEG350
or
DSPE-mPEG500. In some embodiments, the release layer has a surface
concentration of
about 0.11.tg/mm2 to about 5 1.tg/mm2.
[0181] In some embodiments, the catheter further comprises a
protective coating
over the first coating. In some embodiments, the protective coating comprises
a hydrophilic
polymer, a carbohydrate, or an amphiphilic polymer. In some embodiments, the
protective
coating is a glycosaminoglycan or a crystalized sugar. In some embodiments,
the protective
coating has a surface concentration of about 0.11.tg/mm2 to about 5 1.tg/mm2.
[0182] In some embodiments, a method for coating an expandable portion
of a
catheter comprising: disposing a coating formulation over the surface of an
expanded
expandable portion of a catheter wherein the coating formulation comprises: a
plurality of
micro-reservoirs comprising an active agent; at least one lipid; and a fluid,
wherein the fluid
is selected from the group consisting of pentane, hexane, heptane, heptane,
and fluorocarbon
mixture, alcohol and fluorocarbon mixture, and alcohol and water mixture;
evaporating the
fluid; and collapsing the expandable portion. In some embodiments, the coating
formulation
has a solid content comprising the plurality of micro-reservoirs and at least
one lipid, and the
plurality of micro-reservoirs is about 10% to about 75% by weight of the solid
content.
[0183] In some embodiments of the method, the plurality of micro-
reservoirs
further comprises a biodegradable or bioerodable polymer. In some embodiments,
the active
agent is selected from the group consisting of paclitaxel, sirolimus,
paclitaxel derivative,
sirolimus derivative, paclitaxel analogues, sirolimus analogues, inhibitory
RNA, inhibitory
DNA, steroids, and complement inhibitors.
-50-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0184] In
some embodiments of the method described above, the active agent is
crystalline.
[0185] In
some embodiments of the method described above, the at least one lipid
comprises phospholipid. In some embodiments, the phospholipid is selected from
the group
consisting of phosphatidylcholine, phosphatidylethanolamine,
phosphatidylserine, and
phosphatidylinositol.
[0186] In
some embodiments, the phospholipid comprises a phospholipid with an
acyl chain length of about 20 to about 34 carbons. In some embodiments, the
phospholipid is
selected from the group consisting of dieicosenoyl phosphatidylcholine (1,2-
dieicosenoyl-sn-
glycero-3-phosphocholine, C20:1 PC), diarachidonoyl phosphatidylcholine (1,2-
diarachidoyl-
sn-glycero-3-phosphocholine, C20:0 PC), dierucoyl phosphatidylcholine (1,2-
dierucoyl-sn-
glycero-3-phosphocholine, C22:1 PC), didocosahexaenoyl phosphatidylcholine
(1,2-
didoco s ahex aenoyl- sn-glycero-3 -pho sphocholine, C22:6
PC), heneicosenoyl
phosphatidylcholine (1,2-heneicosenoyl- sn-glycero-3 -pho sphocholine, C21:1
PC) and
dinervonyl phosphatidylcholine (1,2-dinervonoyl- sn-glycero-3 -pho
sphocholine, C24:1 PC).
[0187] In
some embodiments of the method described above, the phospholipid
comprises cationic phospholipid. In some embodiments, the cationic
phospholipid is
phosphatidylethanolamine, dioleoylphosphatidylethanolamine, or an amine
derivative of
phosphatidylcholine.
[0188] In
some embodiments of the method described above, the coating
formulation further comprises a sterol. In some embodiments, the sterol is
selected from the
group consisting of cholesterol, stigmasterol, lanosterol, sitosterol, DHEA,
N4-Cholesteryl-
Spermine, Guanidium-Cholesterol/BGTC, and DC-Cholesterol.
[0189] In
some embodiments of the method described above, the coating
formulation has a solid content of about 2% to about 7% by weight, wherein the
solid content
comprises a plurality of micro-reservoirs and at least one lipid.
[0190] In
some embodiments of the method described above, the coating
formulation further comprising a polyethylene glycol-lipid (PEG-lipid).
-51-
CA 03114461 2021-03-25
WO 2020/081455 PCT/US2019/056127
[0191] In some embodiments of the method described above, disposing
the
coating formulation comprises spray coating, dip coating, roll coating,
electrostatic
deposition, printing, pipetting, or dispensing.
[0192] In some embodiments of the method described above further
comprising
disposing a release layer over the surface of the expanded expandable portion
before
disposing the coating formulation.
[0193] In some embodiments, a method for treating or preventing a
condition at a
treatment site comprising advancing a catheter of Claim 1 to the treatment
site; expanding the
expandable portion to allow contact between the coating and a tissue at the
treatment site;
collapsing the expandable portion; and removing the catheter is described.
[0194] In some embodiments of the method described above, the contact
between
the tissue and the coating results in a transfer of at least a portion of a
coating on the
expandable portion to the treatment site.
[0195] In some embodiments of the method described above further
comprising
maintaining the contact between the expandable portion and the coating for a
period of from
about 30 to about 120 seconds.
[0196] In some embodiments of the method described above, the
condition is
selected from the group consisting of atherosclerosis, stenosis or reduction
in luminal
diameter in a diseased blood vessel, restenosis, and in-stent restenosis.
-52-