Note: Descriptions are shown in the official language in which they were submitted.
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
OPHTHALMIC COMPOSITIONS AND METHODS OF USE
FIELD OF THE INVENTION
[0001] The present invention relates to an ophthalmic composition
comprising at least
two active pharmaceutical ingredients. In particular, the active
pharmaceutical ingredients are
selected from the group consisting of: an alpha 2 adrenergic receptor agonist;
a beta-adrenergic
receptor agonist; an immunosuppressant; a lymphocyte associated antigen
antagonist; an anti-
inflammatory; a beta-blocker; a prostaglandin analog; a histamine receptor
antagonist; a carbonic
anhydrase inhibitor; and an antibiotic. In some embodiments, the composition
of the invention is
a nanoemulsion formulation. The present invention also provides a method for
treating various
clinical conditions associated with an eye disorder or eye disease using the
composition of the
invention.
BACKGROUND OF THE INVENTION
[0002] Dry eye syndrome is a multifactorial disease. Dry eye syndromes
engender
inflammation and ocular surface irritation. Thus, the goals for the treatment
of dry eye syndrome
are to improve the patient's ocular comfort and to return the ocular surface
and tear composition
to their basal and healthy states.
[0003] Conventional treatments for dry eye syndrome include (i)
instillation of artificial
tears for tear supplementation and stimulation and (ii) the use of anti-
inflammatory drugs to
reduce ocular surface inflammation. Generally, current dry eye treatment
involves topical
application of artificial tear products/lubricants, tear retention management,
stimulation of tear
secretion, topical application of antibiotics (e.g., erythromycin or
bacitracin ointments), oral
administration of tetracyclines (e.g., tetracycline, doxycycline, or
minocycline), application of a
calcineurin inhibitor immunosuppressant, such as cyclosporine, and
corticosteroids. These
treatments are often time consuming, frustrating, and frequently ineffective
or variably effective.
[0004] One currently available formulation for treating dry eye syndrome
is cyclosporine
(commercially available as Restasis (Allergan, Irvine, CA)). While
cyclosporine reduces
symptoms of dry eye syndrome to some extent, it has many undesired side
effects, such as
burning and stinging sensations. To decrease local side effects and to enhance
the patient's
- 1 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
comfort is one of the objectives of the present invention. Another product for
treating dry eye
syndrome is lifitegrast (commercially available as Xiidrag ophthalmic solution
5%, Shire US
Inc., Lexington, MA). Lifitegrast (chemical name: N-{[2-(1-Benzofuran-6-
ylcarbony1)-5,7-
di chl oro-1,2,3,4-tetrahydro-64 soquinolinyl] carbonyl } -3 -(methyl
sulfony1)-L-phenyl al anine)
ophthalmic solution 5.0% has been reported to improve symptoms of ocular
discomfort and eye
dryness compared with placebo when administered twice daily (Sheppard et al.,
Ophthalmology,
2014, 121(2), pp. 475-483).
[0005] Dry eye disease ("DED") is a multifactorial disease of ocular
surface leading to
discomfort, visual disturbance, and tear film instability with damage to the
ocular surface. DED
is typically categorized into two groups: 1) aqueous tear deficient DED and 2)
evaporative DED.
DED is often a result of changes to the lacrimal functional unit, or LFU. The
LFU is composed
of the lacrimal glands, cornea, eyelids, meibomian glands, conjunctiva, goblet
cells and ocular
nerves. The LFU is responsible for the sustained production of adequate tear
film to consistently
lubricate the ocular surface. Structural changes to the LFU can induce tear
film instability and
insufficiency, which in turn can lead to tear hyperosmolarity. Various
stresses to the ocular
surface such as environmental factors, infection, endogenous stress, antigens,
genetic factors are
identified as primary pathogenic triggering cause. Chronic osmotic stress from
tear film can
activate stress-associated pathways in ocular surface epithelial cells,
thereby triggering a pro-
inflammatory response that involves a mix of chemokines, cytokines, and matrix
metalloproteinases. The subsequent maturation of antigen-presenting cells on
the ocular surface
leads to the migration, activation and expansion of autoreactive T cell
lymphocytes as well as
other leukocytic classes in the LFU. The constant recruitment of pro-
inflammatory leukocytes
onto the ocular surface may inflict epithelium damage in the form of small
abrasions and
epithelium barrier defects. These abrasions can eventually progress to
superficial punctuate
keratitis, squamous metaplasia, extracellular matrix ("ECM") deposits,
decreased goblet cell
differentiation, increased epithelial cell turnover (epitheliopathy) and
significant ocular surface
nerve damage and neuropathy. Thus, the involvement of various cellular and
physiological
processes leading to inflammation, pain, tissue damage, hyperactive immune
responses are
associated with pathophysiology of DED and suggests the role of different
molecular pathways.
- 2 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
[0006] Conventional therapies for treating DED are focused on using a
single active
pharmaceutical ingredient. This significantly limits modes of action and
provides suboptimal
benefit to the patients. Therefore, there is an unmet need for the treatment
of DED to address
multiple pathophysiological processes involving different modes of action.
[0007] Another significant deficiency in current treatment of DED is that
there are no
conventional ophthalmic formulations that allow the preferential distribution
of active
pharmaceutical ingredients into target lacrimal gland tissues. This inability
to target lacrimal
gland tissues significantly reduces the efficacy of conventional ophthalmic
formulations.
[0008] Accordingly, there is an ongoing need for compositions and methods
for effective
treatment of dry eye syndrome as well as other eye disorders and/or diseases.
SUMMARY OF THE INVENTION
[0009] The current invention in directed to a combination formulation
consisting two or
more APIs with different molecular targets for the pathogenesis of DED.
Combination of two or
more APIs with different mechanism of action in single formulation provides
regulations of
more than one molecular pathway and provides a significant benefit to DED
patients. In
particular, some embodiments of the present invention is directed to a
selection of APIs in
combination formulations targeting multiple molecular pathways involved in
pathophysiology
ocular surface diseases, anterior segment of eye diseases as well as pain and
inflammation
associated with ocular surgery.
[0010] One particular aspect of the present invention provides an
ophthalmic
composition comprising at least two active pharmaceutical ingredients. In one
embodiment, the
at least two active pharmaceutical ingredients are selected from a different
classification. In one
particular embodiment, the active pharmaceutical ingredients are selected from
the following
classification of active pharmaceutical ingredients: an alpha 2 adrenergic
receptor agonist; a
beta-adrenergic receptor agonist; an immunosuppressant; a lymphocyte
associated antigen
antagonist; an anti-inflammatory; a beta-blocker; a prostaglandin analog; a
histamine receptor
antagonist; a carbonic anhydrase inhibitor; and an antibiotic.
[0011] In some embodiments, the composition of the invention is a
nanoemulsion
formulation. Compared to non-nanoemulsion formulations, a nanoemulsion
formulation of the
- 3 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
invention provides a wide variety of surprising an unexpected benefits
including, but not limited
to, extended release of active pharmaceutical ingredients, better penetration
profile of the active
pharmaceutical ingredient(s) to the desired cells, increased resident time in
the cornea, etc.
[0012] In one particular embodiment, the composition of the invention is
an ophthalmic
nanoemulsion formulation. In some embodiments, a first active pharmaceutical
ingredient is
said alpha 2 adrenergic agonist, and a second active pharmaceutical ingredient
is selected from
the group consisting of: said immunosuppressant, said lymphocyte associated
antigen agonist,
said corticosteroid, said beta-blocker, said prostaglandins analog, said
carbonic anhydrase
inhibitor, and a combination thereof. In one particular embodiment, the alpha
2 adrenergic
agonist comprises brimonidine, a pharmaceutical salt thereof, or a combination
thereof
[0013] In some embodiments, the immunosuppressant is selected from the
group
consisting of cyclosporine, tacrolimus, and a combination thereof Yet in other
embodiments,
the lymphocyte associated antigen agonist comprises Lifitegrast. Still in
other embodiments, the
corticosteroid is selected from the group consisting of prednisolone,
methylprednisolone,
difluprednate, prednisone acetate, prednisolone sodium phosphate,
triamcinolone, fluocinolone;
fluorometholone, betamethasone, medrysone, and a combination thereof. In other
embodiments,
the anti-inflammatory is selected from a group consisting of a corticosteroid,
a non-steroidal anti-
inflammatory drug ("NSAID"), thymosin beta 4, and a combination thereof. In
one particular
instances, the NSAID is selected from the group consisting of diclofenac,
flubiprofen, ketorolac,
ketorolac thromethamine, bromfenac, nepafenac, flurbiprofen, and a combination
thereof. In yet
another embodiment, the beta-adrenergic receptor agonist is selected from the
group consisting
of Dopexamine, Epinephrine, Isoprenaline, isoproterenol, levalbuterol,
Salbutamol, albuterol,
and a combination thereof Still in other embodiments, the beta-blocker is
selected from the
group consisting of Timolol, Propranolo, Sotalol, nadolol, and a combination
thereof. Yet still in
other embodiments, the prostaglandins analog is selected from the group
consisting of
latanoprost, bimatoprost, travoprost, tafluprost, and a combination therof.
Yet in other
embodiments, the carbonic anhydrase inhibitor is selected from the group
consisting of
dorzolamide, methazolamide, brinzolamide, dichlorphenamide, and a combination
thereof
- 4 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
[0014] In other embodiments, the active pharmaceutical ingredients
consist of (i)
brimonidine, a pharmaceutically acceptable salt thereof, or a combination
thereof (ii)
cyclosporine; and (iii) Lifitegrast or Loteprednol.
[0015] One particular aspect of the invention provides an aqueous
ophthalmic solution
comprising (i) brimonidine, a pharmaceutically acceptable salt thereof, or a
combination thereof
and (ii) cyclosporine. In some embodiments, the aqueous ophthalmic solution is
a nanoemulsion
solution.
[0016] Compositions of the invention can also include a pharmaceutically
acceptable
excipient. In one particular embodiment, the pharmaceutically acceptable
excipient comprises:
an emulsion stabilizing polymer, a water-soluble polymer, a surfactant, a
tonicity modifier or a
stabilizer, a viscosity modifier, or a combination thereof In one particular
embodiment, the
pharmaceutically acceptable excipient comprises (i) an emulsion stabilizing
polymer; (ii) a
surfactant; (iii) a tonicity modifier or a stabilizer selected from the group
consisting of a polyol, a
non-reducing disaccharide and a combination thereof or (iv) a combination
thereof. In other
embodiments, the pharmaceutically acceptable excipient comprises polysorbate
80, Pemuleng,
carbomer copolymer type A, a polyol or a combination thereof. Yet in other
embodiments, the
pharmaceutically acceptable excipient comprises polysorbate 80, Pemuleng,
carbomer
copolymer type B, a polyol or a combination thereof. Still in other
embodiments, the tonicity
modifier or a stabilizer is selected from the group consisting of a polyol, a
non-reducing
disaccharide, and a combination thereof. Yet still in other embodiments, the
viscosity modifier
is selected from the group consisting of carbomer homopolymer type A, carbomer
homopolymer
type B, carbomer homopolymer type C, and a combination thereof. Still yet in
other
embodiments, the surfactant is selected from the group consisting of: (i) a
nonionic surfactant,
such as glyceryl laurate, polysorbate, spans, poloxamers, Nonoxyno1-9; (ii) a
cationic surfactant
such as benzalkonium chloride, benzethonium chloride, benzododecinium bromide,
cetrimonium
bromide, cetrimonium chloride, tetramethylammonium hydroxide, lauralkonium
chloride; (iii) a
zwitterionic surfactant such as lecithin; and (iv) a combination thereof.
Still in other
embodiments, the pharmaceutically acceptable excipient comprises (i) an
emulsion stabilizing
polymer, (ii) a surfactant, (iii) a tonicity modifier or a stabilizer selected
from the group
- 5 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
consisting of a polyol, a non-reducing disaccharide, and a combination
thereof, or (iv) a
combination thereof.
[0017] Still another aspect of the invention provides a method for
treating a clinical
condition associated with eye, such as an eye disorder or an eye disease. The
method includes
administering to a subject in need of such a treatment a therapeutically
effective amount of a
composition of the invention. In some embodiments, the clinical condition
associated with eye is
selected from the group consisting of dry eye syndrome (e.g.,
keratoconjunctivitis sicca),
sjogren's syndrome, congenital alacrima, xerophthalmia (dry eye from vitamin A
deficiency),
keratomalacia, thyroid eye disease, ocular rosacea, eyelid disorders,
meibomian gland disease,
meibomian gland dysfunction, ectropion, blepharitis, blepharochalasis,
sarcoidosis, stye,
hordeolum, chalazion, ptosis, pterygium, eyelid edema, eyelid dermatitis,
trichiasis, madarosis,
dacryoadenitis, stevens-johnson syndrome, ocular graft versus host disease,
dacryocystitis,
conjunctivitis, keratoconjunctivitis, blepharoconjunctivitis,
blepharokeratoconjunctivitis, allergic
conjunctivitis, vernal conjunctivitis, conjunctival suffusion,
conjunctivochalasis, subconjunctival
hemorrhage, pterygium, pinguecula, chemosis, iritis, iridocyclitis, anterior
uveitis, glaucoma,
ocular hypertension, red eye, keratitis, scleritis, episcleritis, peripheral
ulcerative keratitis,
neurotrophic keratitis, neurotrophic eye disease, corneal ulcer, ulcerative
keratitis, corneal
abrasion, photokeratitis, ultraviolet keratitis, exposure keratitis,
superficial punctuate keratitis,
thygeson's superficial punctuate keratopathy, herpes zoster keratitis, acne
rosacea, corneal
neovascularization, corneal dystrophy, epithelial basement membrane dystrophy,
fuch's
dystrophy, posterior polymorphous corneal dystrophy, macular corneal
dystrophy, cyclitis,
uveitis, iritis, post-operative inflammation following ocular surgery (i.e.
eyelid surgery, cataract
surgery, corneal surgery, refractive surgery including photorefractive
keratectomy, glaucoma
surgery, lacrimal gland surgery, conjunctival surgery, eye muscle surgery),
ocular surface
conditions caused by chemical burns, thermal burns or physical trauma, ocular
conditions caused
by the following autoimmune or vascular disorders: rheumatoid arthritis,
juvenile rheumatoid
arthritis, ankulosing spondylitis, reiter's syndrome, enteropathic arthritis,
psoriatic arthritis,
discoid and systemic lupus erythematosus, multiple sclerosis, graves' disease,
antiphospholipid
syndrome, sarcoidosis, wegner's granulomatosis, behcet's syndrome,
polyarteritis nodosa,
takayasu's arteritis, dermatomyositis, psoriasis, relapsing polychondritis,
vasculitis, sickle cell-
anemia, type II diabetes, diabetic retinopathy., and a combination thereof.
- 6 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
[0018] In some embodiments, the eye disorder is selected from the group
consisting of:
(i) a dry eye syndrome; (ii) ocular graft-versus-host-disease; (iii) ocular
rosacea; (iv) allergic
conjunctivitis; (v) autoimmune ocular surface disease; (vi) thygeson's
superficial punctuate
keratopathy; (vii) herpes zoster keratitis; (viii) Stevens-Johnson syndrome;
(ix) keratitis; (x)
conjunctivitis; (xi) blepharitis; (xii) blepharochalasis; (xiii)
conjunctivochalasis; (xiv)
blepharoconjunctivitis; (xv) blepharokeratoconjunctivitis; (xvi) post-
operative inflammation or
pain from ocular surgery; (xvii) scleritis; (xviii) episcleritis; (xix)
anterior uveitis; (xx) iritis;
(xxi) cyclitis; (xxii) ocular surface vascular disorder; (xxiii) ulcerative
keratitis; (xxiv)
photokeratitis; (xxv) dacryocystitis; (xxvi) eyelid disorder; (xxvii)
congenital alacrima; (xxviii)
xerophthalmia; (xxix) dacryoadenitis; and (xxx) ocular surface disorder
induced by chemical
burns, thermal burns, use of contact lenses, or physical insult to the ocular
surface.
[0019] Yet in some embodiments, the dry eye syndrome is selected from the
group
consisting of sjogren's syndrome, meibomian gland dysfunction and
keratoconjunctivitis. Still
in other embodiments, the eyelid disorder comprises eyelid inflammation, pain
and/or edema.
[0020] In some embodiments, the composition is administered topically to
an eye of said
subject. In one embodiment, the composition is formulated as a homogeneous
ophthalmic
aqueous formulation. Yet in another embodiment, the composition is formulated
as a
heterogeneous ophthalmic aqueous solution. In some instances, the
heterogeneous ophthalmic
aqueous solution comprises emulsion, suspension or a combination thereof
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] Figure 1 is a table showing measured median diameter of particle
size distribution
[Dx(50)] of some of the ophthalmic formulations of the invention.
[0022] Figure 2 is a table showing median diameter of particle size
distribution [Dx(50)]
of an ophthalmic nanoemulsion formulation of brimonidine tartrate and
loteprednol etabonate at
different temperatures (RT is room temperature) at days 0, 36 and 66.
[0023] Figure 3 is a table shows measured median diameter of particle
size distribution
[Dx(50)] of (i) brimonidine tartrate (0.2 % w/w) and cyclosporine (0.05% w/w)
combination of
ophthalmic nanoemulsion formulation and (ii) brimonidine tartrate (0.2% w/w)
and cyclosporine
(0.1% w/w) ophthalmic nanoemulsion formulation at different temperatures at
days 0 and 14.
- 7 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
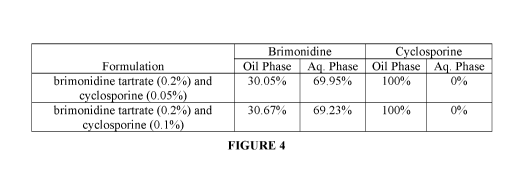
[0024] Figure 4 is a table showing partitioning of brimonidine tartrate
and cyclosporine
in various phases, oil and aqueous, of (i) combination of brimonidine tartrate
(0.2 % w/w) and
0.05% w/w cyclosporine ophthalmic nanoemulsion formulation and (ii)
combination of
brimonidine tartrate (0.2% w/w) and 0.1% w/w cyclosporine ophthalmic
nanoemulsion
formulation.
[0025] Figure 5 shows stability data at different temperatures of an
ophthalmic
nanoemulsion formulation of the present invention consisting of active
pharmaceutical
ingredients brimonidine tartrate and cyclosporine.
[0026] Figure 6A is a graph showing particle size distribution [Dx(50)]
of an ophthalmic
nanoemulsion formulation of the present invention having active pharmaceutical
ingredients
brimonidine tartrate and loteprednol etabonate.
[0027] Figure 6B is a graph showing particle size distribution [Dx(50)]
of an ophthalmic
nanoemulsion formulation of the present invention having active pharmaceutical
ingredients
brimonidine tartrate (0.2 % w/w) and 0.05% w/w cyclosporine.
[0028] Figure 6C is a graph showing particle size distribution [Dx(50)]
of an ophthalmic
nanoemulsion formulation of the present invention having active pharmaceutical
ingredients (B)
brimonidine tartrate (0.2% w/w) and 0.1% w/w cyclosporine.
DETAILED DESCRIPTION OF THE INVENTION
[0029] One aspect of the invention provides a nanoemulsion ophthalmic
composition
comprising at least two active pharmaceutical ingredients. As used herein, the
term
"nanoemulsion" refers to emulsion having a median emulsion droplet particle
size (i.e., Dx(50))
of about 250 nm or less, typically about 220 nm or less, often about 200 nm or
less, and most
often about 100 nm or less.
[0030] In one particular embodiment, the active pharmaceutical
ingredients are selected
from the following classification of active pharmaceutical ingredients: an
alpha 2 adrenergic
receptor agonist; a beta-adrenergic receptor agonist; an immunosuppressant; a
lymphocyte
associated antigen antagonist; an anti-inflammatory; a beta-blocker; a
prostaglandin analog; a
histamine receptor antagonist; a carbonic anhydrase inhibitor; and an
antibiotic. In one particular
- 8 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
embodiment, the composition of the invention comprises an alpha 2 adrenergic
receptor agonist
in combination with one or more of a calcineurin inhibitor (e.g.,
cyclosporine) and a lymphocyte
function-associated antigen antagonist (e.g., lifitegrast). In another
embodiment, the composition
of the invention comprises an alpha 2 adrenergic receptor agonist in
combination with a
corticosteroid. Still another aspect of the invention provides a composition
comprising an alpha
2 adrenergic receptor agonist in combination with one or more of the following
components: (i) a
calcineurin inhibitor; (ii) a lymphocyte function-associated antigen
antagonist; (iii) an anti-
inflammatory (e.g., corticosteroid comprising loteprednol, thymosin beta 4,
etc.); (iv) a sodium
channel blocker; (v) a non-steroidal anti-inflammatory drug (i.e., NSAID); and
(vi) an
antihistamine.
[0031] In some embodiments, the anti-inflammatory is a corticosteroid.
Still in other
embodiments, the anti-inflammatory is an NSAID. Yet in other embodiments, the
anti-
inflammatory is thymosin beta 4.
[0032] Yet in some embodiments, compositions of the invention are
heterogeneous
solution formulations, containing a combination of therapeutically effective
amount of active
pharmaceutical components in the formulation. In other embodiments,
compositions of the
invention are homogeneous aqueous formulations, containing a combination of
therapeutically
effective amount of active pharmaceutical components in the formulation.
[0033] The compositions or formulations of the invention can contain just
two active
ingredients or more than two active ingredients. In some embodiments, at least
one of the active
ingredient is an alpha 2 adrenergic receptor agonist such as brimonidine, or a
pharmaceutically
acceptable salt there of or a combination thereof.
[0034] Compositions of the invention are useful for treatment of various
eye disorders or
eye diseases including, but not limited to, dry eye syndrome
(keratoconjunctivitis sicca),
sjogren's syndrome, congenital alacrima, xerophthalmia (dry eye from vitamin A
deficiency),
keratomalacia, thyroid eye disease, ocular rosacea, eyelid disorders,
meibomian gland disease,
meibomian gland dysfunction, ectropion, blepharitis, blepharochalasis,
sarcoidosis, stye,
hordeolum, chalazion, ptosis, pterygium, eyelid edema, eyelid dermatitis,
trichiasis, madarosis,
dacryoadenitis, stevens-johnson syndrome, ocular graft versus host disease,
dacryocystitis,
conjunctivitis, keratoconjunctivitis, blepharoconjunctivitis,
blepharokeratoconjunctivitis, allergic
- 9 -
CA 03115664 2021-03-29
WO 2020/047197
PCT/US2019/048721
conjunctivitis, vernal conjunctivitis, conjunctival suffusion,
conjunctivochalasis, subconjunctival
hemorrhage, pterygium, pinguecula, chemosis, iritis, iridocyclitis, anterior
uveitis, glaucoma,
ocular hypertension, red eye, keratitis, scleritis, episcleritis, peripheral
ulcerative keratitis,
neurotrophic keratitis, neurotrophic eye disease, corneal ulcer, ulcerative
keratitis, corneal
abrasion, photokeratitis, ultraviolet keratitis, exposure keratitis,
superficial punctuate keratitis,
thygeson's superficial punctuate keratopathy, herpes zoster keratitis, acne
rosacea, corneal
neovascularization, corneal dystrophy, epithelial basement membrane dystrophy,
fuch's
dystrophy, posterior polymorphous corneal dystrophy, macular corneal
dystrophy, cyclitis,
uveitis, iritis, post-operative inflammation following ocular surgery (i.e.
eyelid surgery, cataract
surgery, corneal surgery, refractive surgery including photorefractive
keratectomy, glaucoma
surgery, lacrimal gland surgery, conjunctival surgery, eye muscle surgery),
ocular surface
conditions caused by chemical burns, thermal burns or physical trauma, ocular
conditions caused
by the following autoimmune or vascular disorders: rheumatoid arthritis,
juvenile rheumatoid
arthritis, ankulosing spondylitis, reiter's syndrome, enteropathic arthritis,
psoriatic arthritis,
discoid and systemic lupus erythematosus, multiple sclerosis, graves' disease,
antiphospholipid
syndrome, sarcoidosis, wegner's granulomatosis, behcet's syndrome,
polyarteritis nodosa,
takayasu's arteritis, dermatomyositis, psoriasis, relapsing polychondritis,
vasculitis, sickle cell-
anemia, type II diabetes, diabetic retinopathy., and a combination thereof.
[0035] Some
aspects of the invention provide a method for treating dry eye syndrome
using the compositions disclosed herein. There are two major classes of dry
eye syndrome: (i)
aqueous tear-deficient dry eye (ADDE) and (ii) evaporative dry eye (EDE).
There are also cases
of mixed mechanism dry eye (i.e., both ADDE and EDE). ADDE is primarily due to
failure of
lacrimal tear secretion. ADDE can be further subdivided into Sjogren syndrome
dry eye (where
the lacrimal and salivary glands are targeted by an autoimmune process, e.g.,
rheumatoid
arthritis) and non-Sjogren's syndrome dry eye (lacrimal dysfunction, but the
systemic
autoimmune features of Sjogren's syndrome are excluded, e.g., age-related dry
eye). In contrast,
EDE is primarily due to excessive water loss from the exposed ocular surface
in the presence of
normal lacrimal secretory function. Its causes can be extrinsic (e.g., ocular
surface disorder due
to some extrinsic exposure, contact lens wear or vitamin A deficiency) or
intrinsic (e.g.,
Meibomian gland dysfunction and disorders of eyelid aperture). Meibomian
glands secrete a
mixture of lipids and other components that form the outer layer of the
preocular tear film. This
- 10 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
lipid layer functions to decrease tear film evaporation. Meibomian gland
dysfunction (MGD)
leads to evaporative dry eye disease. One of the most well recognized clinic
finding in MGD is
the presence of numerous telangiectatic blood vessels coursing across the
eyelid margin. MGD
can also accompany tear deficient dry eye disease, as seen in ocular graft-
versus-host-disease
(oGVHD). Other specific dry eye syndromes that can be treated using
compositions of the
invention include keratoconjunctivitis, dry eye caused by conjunctivitis, dry
eye caused by
allergic conjunctivitis, dry eye caused by blepharitis, dry eye caused by
keratitis, dry eye caused
by dacryoadenitis, dry eye caused by ocular rosacea, dry eye caused by boehm
syndrome, dry
eye caused by conjunctivochalasis, dry eye caused by blepharoconjunctivitis,
dry eye caused by
blepharokeratoconjunctivitis, dry eye caused by superficial punctuate
keratitis, dry eye caused by
thygeson's superficial punctuate keratopathy, dry eye caused by oGVHD,
Sjogren's dry eye
syndrome, dry eye caused by Stevens-Johnson syndrome, MGD, dry eye caused by
meibomian
gland disease, vitamin A deficiency induced dry eye, pharmacological induced
dry eye (i.e.
hormone replacement therapy, blood pressure medication, antihistamine,
antidepressants,
anticholinergic medications, glaucoma medication, antihypertensives,
diuretics, sedatives,
isotretinoin, nasal decongestants, oral contraceptives, beta-blockers,
phenothiazines, atropine,
pain relieving opiates), pregnancy induced dry eye, LASIK surgery or
refractive surgery induced
dry eye, dry eye induced by collagen vascular diseases (i.e. systemic lupus
erythematosus,
Wegener's granulomatosis, rheumatoid arthritis, relapsing polychondritis), dry
eye caused by the
infiltration of the lacrimal glands by tumors or sarcoidosis, dry eye caused
by postradiation
fibrosis of tear producing glands, dry eye caused by lacrimal gland, meibomian
gland, or goblet
cell ablation, dry eye caused by sensory denervation, dry eye caused by
thermal or chemical
burns, dry eye caused by underlying diabetic conditions, dry eye caused by
viral, fungal, or
bacterial infection, dry eye caused by prolonged contact lens use, dry eye
caused by eyelid
disorders or injury to the eyelid (i.e. bulging eyes, drooping eyelid), dry
eye caused by corneal
dystrophy, dry eye caused by autoimmune disorders, age-induced dry eye, and a
combination
thereof.
[0036] In some embodiments, methods for treating dry eye syndrome
comprise treating a
patient in need of a treatment for Meibomian gland dysfunction (MGD). In other
embodiments,
methods for treating dry eye syndrome comprise treating a patient in need of a
treatment for
aqueous tear-deficient dry eye (ADDE). In some instances, methods for treating
ADDE
- 11 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
comprise treating a patient in need of a treatment for Sjogren dry eye
syndrome, ocular Graft-
Versus-Host-Disease (oGVHD) or non-Sjogren dry eye syndrome. Yet in other
embodiments,
methods for treating dry eye syndrome comprise treating a patient in need of a
treatment of
evaporative dry eye (EDE). Still in other embodiments, methods of the
invention include
treating a patient in need of a treatment for mixed mechanism dry eye
consisting of ADDE and
EDE. Yet still in other embodiments, methods of the invention include treating
a patient
suffering from dry eye syndrome due to a complication of refractive eye
surgery,or is attributable
to one or more of the following causes: vitamin A deficiency, ocular surface
disorders, allergy,
aging, contact lens usage, medication usage or eyelid disorders.
[0037] In some embodiments, compositions of the invention include an
alpha 2 (a2)
adrenergic receptor agonists. Exemplary alpha 2 adrenergic receptor agonists
include, but are
not limited to, brimonidine, 4-NEMD, 7-Me-marsanidine, agmatine,
apraclonidine, cannabigerol,
clonidine, detomidine, dexmedetomidine, fadolmidine, guanabenz, guanfacine,
lofexidine,
marsanidine, medetomidine, methamphetamine, mivazerol, rilmenidine,
romifidine, talipexole,
tizanidine, tolonidine, xylazine, xylometazoline, and the like including
pharmaceutically
acceptable salts thereof In one particular embodiment, the alpha 2 adrenergic
receptor agonist is
brimonidine (5-Bromo-N-(4,5-dihydro-1H-imidazol-2-y1) quinoxalin-6-amine), a
pharmaceutically acceptable salt thereof or a combination thereof.
[0038] "Pharmaceutically acceptable salt" of a compound means a salt that
is
pharmaceutically acceptable and that possesses the desired pharmacological
activity of the parent
compound. Such salts include: (1) acid addition salts, formed with inorganic
acids such as
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid, and the like; or
formed with organic acids such as acetic acid, trifluoroacetic acid, propionic
acid, hexanoic acid,
cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic
acid, succinic acid,
malic acid, maleic acid, fumaric acid, tartaric acid, citric acid, benzoic
acid, 3-(4-
hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, methanesulfonic
acid,
ethanesulfonic acid, 1,2-ethane-disulfonic acid, 2-hydroxyethanesulfonic acid,
benzenesulfonic
acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic acid, 4-
toluenesulfonic acid,
camphorsulfonic acid, 4-methylbicyclo[2.2.2]-oct-2-ene-1carboxylic acid,
glucoheptonic acid, 3-
phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid, lauryl
sulfuric acid, gluconic
- 12 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid,
muconic acid, and the
like; or (2) salts formed when an acidic proton present in the parent compound
either is replaced
by a metal ion, e.g., an alkali metal ion, an alkaline earth ion, or an
aluminum ion; or coordinates
with an organic base such as ethanolamine, diethanolamine, triethanolamine,
tromethamine, N-
methylglucamine, and the like. Particular examples of pharmaceutically
acceptable salts of
brimonidine include, but not limited to, tartrate salt, trifluoroacetate salt,
hydrochloric salt,
acetate salt, oxalic acid salt, as well as others disclosed herein and/or
known to one skilled in the
art.
[0039] Alpha-2 adrenergic receptor agonists are those compounds that
activate alpha-2
adrenergic receptors. There are three subtypes of this receptor, designated A,
B, and C. An
alpha-2 adrenergic receptor agonist that can activate any or all of these
receptor subtypes can be
used in the present invention. However, in some embodiments of the invention,
an alpha 2
adrenergic receptor agonist has a higher activity or efficacy at the alpha-2A
adrenergic receptor
subtype compared to its activity at the alpha-2B receptor subtype (e.g.,
brimonidine and its salts).
In some embodiments, the alpha 2 adrenergic agonist in compositions of the
invention has a
higher alpha 2A agonist activity compared to alpha 2B agonist activity. In
some instances, the
alpha 2A agonist activity of the alpha 2 adrenergic agonist is at least about
10% greater, typically
at least about 20% greater and often at least about 30% greater than its alpha
2B agonist activity.
As used herein, the term "about" when referring to a numeric value means -
20%, typically
- 10%, often - 5% and most often - 2% of the numeric value.
[0040] In one embodiment, the second pharmaceutically active compound
comprises a
calcineurin inhibitor, a lymphocyte function-associated antigen antagonist, or
a combination
thereof. Calcineurin (CaN) is a calmodulin and calcium dependent
serine/threonine protein
phosphatase (also known as protein phosphatase 3, and calcium-dependent serine-
threonine
phosphatase). It activates the T cells of the immune system and can be blocked
by a class of
drugs called calcineurin inhibitors, which includes cyclosporine, tacrolimus,
pimecrolimus,
voclosporin, as well as others known to one skilled in the art. Any known
calcineurin inhibitors
(e.g., cyclosporine) or those developed by one skilled in the art can be used
in compositions of
the invention. Lymphocyte function-associated antigen (LFA)-1/intercellular
adhesion molecule
(ICAM)-1 interactions mediate several important steps in the evolution of an
immune response.
- 13 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
Exemplary lymphocyte function-associated antigen antagonists include, but are
not limited to,
lifitegrast (i.e., (S)-2-(2-(benzofuran-6-carbony1)-5,7-dichloro-1,2,3,4-
tetrahydroisoquinoline-6-
carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoic acid), which is a water-
soluble drug that
blocks LFA-1 from binding to ICAM-1, and other lymphocyte function-associated
antigen
antagonists that are known to one skilled in the art. Any lymphocyte function-
associated antigen
can be used in compositions of the invention.
[0041] Yet in another embodiment, the composition of the invention is
formulated as an
aqueous solution, which can be a homogeneous or heterogeneous solution. In
such
embodiments, compositions of the invention include at least two active
ingredients. In one
particular embodiment, the formulation contains one active ingredient that is
water soluble and
the another active ingredient that is a lipophilic. In another particular
embodiment, the
formulation contains two or more active ingredients that are water soluble. In
another particular
embodiment, the formulation contains two or more active ingredients that are
lipophilic. Still in
other embodiments, compositions of the invention are formulated as an aqueous
ophthalmic
solution. As stated above, the aqueous ophthalmic solution can be homogenous
or
heterogeneous and can include aqueous suspension or dispersion, where at least
some of the
active ingredients are present as suspension or dispersion in aqueous
solution. The aqueous
ophthalmic solution can be a substantially homogeneous aqueous solution, where
substantially
all (i.e., 85%, typically 90%, often 95%, and most often 97%) of the active
ingredients
are dissolved in the aqueous solution.
[0042] In one particular embodiment, one of the active ingredients is
brimonidine, its
pharmaceutically acceptable salt thereof or a combination thereof. The
composition includes a
second active ingredient that can be cyclosporine, lifitegrast or a
combination thereof.
[0043] As used herein, the terms "active ingredient" and "active
pharmaceutical
ingredient" are used interchangeably herein and refer to a compound that is
used or known to one
skilled in the art in treating an eye disorder, such as dry eye syndrome.
Thus, while water and oil
can be present in some formulations, they are not used primarily for the
purpose of treating eye
disorder but are used as a vehicle to carry active ingredients. Generally, an
active ingredient
works on a particular receptor or cells or has been approved for treating an
eye disorder by the
U.S. Food and Drug Administration ("FDA").
- 14 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
[0044] In one particular embodiment, the term "active ingredient" refers
to an alpha 2
adrenergic receptor agonist; a beta-adrenergic receptor agonist; an
immunosuppressant; a
lymphocyte associated antigen antagonist; an anti-inflammatory (e.g.,
corticosteroid, NSAID,
thymosin beta 4, etc.); a beta-blocker; a prostaglandin analog; a histamine
receptor antagonist; a
carbonic anhydrase inhibitor; and an antibiotic. Yet in another embodiment,
the term "active
ingredient" refers to: an alpha 2 adrenergic agonist; a calcineurin inhibitor;
a lymphocyte
function-associated antigen antagonist; a corticosteroid; CRGP receptor
antagonists; anti-CGRP
receptor monoclonal antibodies; an inhibitor of adrenomedullin, serotonin,
cathelicidin or
neuropeptides; a sodium channel blocker; an antihistamine; and/or a non-
steroidal anti-
inflammatory drug. Other ingredients that may be present in
formulations/compositions of the
invention are used primarily as pharmaceutically acceptable excipients or
vehicles, such as a pH
adjusting agent, a tonicity modifier or a stabilizer, a surfactant, an
emulsion stabilizer, etc.
[0045] A therapeutically effective amount of an active ingredient in the
composition of
the invention can be readily determined by one skilled in the art. In some
embodiments, the
composition of the invention is formulated as a heterogeneous aqueous
solution. In one
particular embodiment, the composition of the invention include from about
0.01 to about 5
mg/mL (about 0.001% to about 0.5% w/v) typically about 0.2% w/v or less (e.g.,
0.05-0.2%
often 0.07-0.15%) of brimonidine or a salt thereof (e.g., brimonidine tartrate
and hydroxy
brimonidine trifluoroacetate). The ingredient amounts are presented in units
of either %
weight/volume (% w/v) or weight/weight (% w/w). In one specific embodiment,
brimonidine
tartrate is used as an alpha 2 adrenergic agonist. In one embodiment, the
amount of brimonidine
tartrate present in the composition is from about 0.01% w/w to about 1% w/w,
typically from
0.01% w/w to about 0.7% w/w, and often from about 0.02% to about 0.5% w/w.
[0046] In some embodiments, the second therapeutically active compound
comprises
cyclosporine. In one particular embodiment, the second therapeutically active
compound
comprises cyclosporine A. A typical amount of cyclosporine A present in the
composition of the
invention is from about 0.005% w/w to about 0.5% w/w, often from about 0.01%
w/w to about
0.3% w/w.
[0047] Still in another embodiment, the second therapeutically active
compound
comprises lifitegrast . In one particular embodiment, the amount of
lifitegrast present in
- 15 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
compositions of the invention is from about 0.1% w/w to about 20% w/w,
typically from about
0.2% w/w to about 15% w/w, and often from about 0.3% w/w to about 10% w/w.
[0048] Yet in some embodiments, the second therapeutically active
compound comprises
a corticosteroid. Exemplary corticosteroids include, but are not limited to,
methylprednisolone,
hydrocortisone, betamethasone, dexamethasone and loteprednol etabonate. In one
particular
embodiment, the corticosteroid used in the composition of the invention is
loteprednol etabonate.
In some embodiments, the amount of loteprednol etabonate present in the
compositions of the
invention is from about 0.01% w/w to 2% w/w; typically from about 0.05% w/w to
1%, and
often from about 0.1% to about 0.3%.
[0049] Still in another embodiment, the second therapeutically active
compound
comprises a sodium channel blocker and/or mucolytic agents. Suitable sodium
channel blockers
and/or mucolytic agents for treatment of eye disorder are known to one skilled
in the art and
include those disclosed, for example, in U.S. Patent Nos. 9,586,911,
9,346,753, 8,980,898,
8,673,340, 8,058,278, 7,875,619, 7,868,010, 7,842,697, 7,820,678, 7,410,968,
7,399,766,
7,388,013, 7,375,107, 7,368,451, 7,368,450, 7,368,447, 7,375,107, 7,368,451,
7,368,447,
7,345,044, 7,332,496, 7,317,013, 7,247,637, 7,247,636, 7,241,766, 7,192,959,
7,192,958,
7,189,719, 7,186,833, 7,064,129, 7,030,177, 7,026,325, 6,995,160, 6,903,105,
6,858,615, and
6,858,614 which are incorporated herein by reference in their entirety.
Specific examples of
suitable sodium channel blockers for the present invention include, but are
not limited to
amiloride, benzamil, phenamil, amiloride analogues, as well as those disclosed
in US Patent Nos.
9,586,911, 9,346,753, 8,980,898, 8,673,340, 8,058,278, 7,875,619, 7,868,010,
7,842,697,
7,820,678, 7,410,968, 7,399,766, 7,388,013, 7,375,107, 7,368,451, 7,368,450,
7,368,447,
7,375,107, 7,368,451, 7,368,447, 7,345,044, 7,332,496, 7,317,013, 7,247,637,
7,247,636,
7,241,766, 7,192,959, 7,192,958, 7,189,719, 7,186,833, 7,064,129, 7,030,177,
7,026,325,
6,995,160, 6,903,105, 6,858,615, and 6,858,614.
[0050] Yet still in another embodiment, the second therapeutically active
compound
comprises a non-steroidal anti-inflammatory drug (i.e., NSAID). Suitable
NSAIDs that are
useful in treating eye disorder include ketorolac (0.05 to 0.3%), diclofenac
(0.01 to 1%),
flurbiprofen (0.01 to 1%), bromfenac (0.01 to 0.5%), nepafenac (0.05 to 0.5%),
etc. Some of
these are commercially available as Acular, Acular PF, and Acular LS
(ketorolac tromethamine,
- 16 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
Allergan), Ocufen (flurbiprofen sodium, Allergan), Voltaren (diclofenac
sodium, Novartis),
Xibrom (bromfenac ophthalmic solution, Ista Pharmaceuticals), Prolensa
(bromfenac ophthalmic
solution, Bausch & Lomb) and Nevanac (nepafenac, Alcon).
[0051] In still further embodiments, the second therapeutically active
compound
comprises an antihistamine. Suitable antihistamines that are useful in
treating eye disorder
include alcaftadine (0.01 to 0.5%), azelastine (0.001% to 0.2%), bepotastine
(0.1% to 3%),
emedastine (0.001% to 0.2%), epinastine (0.001% to 0.2%), ketotifen (0.001% to
0.2%), and
olopatadine (0.01% to 1.5%). Some of these are commercially available as
Lastacaft (alcaftadine,
Allergan), Optivar (zelastine hydrochloride, Meda Pharmaceuticals), Bepreve
(bepotastine
besilate, Bausch & Lomb), Emadine (emedastine difumarate, Alcon), Elestat
(epinastine
hydrochloride, Allergan), Alaway (ketotifen fumarate, Baush & Lomb), Zaditor
(ketotifen
fumarate, Alcon), Pazeo (olopatadine hydrochloride, Alcon) Pataday
(olopatadine hydrochloride,
Alcon), and Patanol (olopatadine hydrochloride, Alcon).
[0052] Still in other embodiments, the second therapeutically active
compound comprises
thymosin beta 4. In some embodiments, the amount of thymosin beta 4 present in
the
compositions of the invention is from about 0.01% w/w to 2% w/w; typically
from about 0.05%
w/w to 1%, and often from about 0Ø05% to about 0.3%.
[0053] In other embodiments, the second therapeutically active compound
comprises a
prostaglandin analog. Exemplary prostaglandin analogs that are useful in
compositions of the
invention include, but are not limited to, latanoprost, bimatoprost,
travoprost, and tafluprost.
When present, the amount of prostaglandin analog in the compositions of the
invention is from
about 0.001% w/w to 1% w/w; typically from about 0.005% w/w to 0.5%, and often
from about
0.005% to about 0.1%.
[0054] In some embodiments, compositions of the invention are used as an
ophthalmic
formulation. Such ophthalmic formulations can be homogeneous or heterogeneous
formulations.
In such embodiments, the formulated composition contains an oil or a fatty
acid ester. A fatty
acid ester has the meaning commonly understood in the art, being an ester
formed between an
alcohol and a fatty acid. Exemplary fatty acid esters that are useful in
formulations of the
invention include, but are not limited to, triglyceride esters commonly known
as vegetable oils,
mono and diglyceride esters of fatty acids, fatty acid methyl esters, as well
as other fatty acid
- 17 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
esters that are known to one skilled in the art. It should be appreciated the
fatty acid ester can be
a mixture of several chemical compounds or an essentially pure compound.
Typically, the fatty
acid ester is a vegetable oil. Particular examples of vegetable oils that can
be used include, but
are not limited to, castor oil, sesame oil, soybean oil, cottonseed oil, olive
oil, peanut oil,
safflower oil, sunflower oil, palm oil, palm kernel oil, canola oil, and
Miglyol oil . In one
particular embodiment, the fatty acid ester is castor oil.
[0055] Various vehicles can be used in the ophthalmic formulations of the
present
invention. These vehicles include, but are not limited to, purified water
(water), polyvinyl
alcohol, povidone, hydroxypropyl methyl cellulose, poloxamers, carboxymethyl
cellulose,
hydroxyethyl cellulose, cyclodextrin and a mixture of two or more thereof The
vehicle is used
in the formulation in amounts as needed to provide the concentration of the
active compound(s)
disclosed herein. In one particular embodiment, the vehicle comprises water.
[0056] In some embodiments of this invention, an emulsion stabilizing
polymer is used.
While not intending to limit the scope of the invention, emulsion stabilizing
polymers generally
contain hydrophilic groups such as cellulose, sugars, ethylene oxide,
hydroxide, carboxylic acids
or other polyelectrolytes. Without being bound by any theory, it is believed
that these polymers
help to stabilize emulsions by increasing the viscosity of the formulation as
well as by reducing
the interfacial tension. Some examples of emulsion stabilizing polymers useful
in this invention
include, but are not limited to, carbomers, Pemulen , sodium
carboxymethylcellulose,
hydroxypropylmethylcellulose, povidone, polyvinyl alcohol, polyethylene glycol
and a mixture
of two or more thereof.
[0057] In one particular embodiment, Pemulen (B.F. Goodrich, Cleveland,
OH) is used
as the polymeric based stabilizer. Pemulen are Acrylates/C10-30 Alkyl
Acrylate Cross-Polymers.
[0058] In another embodiment of this invention, the formulation comprises
a surfactant.
Without being bound by any theory, a surfactant is used to help facilitate the
formation of the
emulsion and improve its stability. Any type of surfactant can be used
including, anionic,
cationic, amphoteric, zwitterionic, nonionic, as well as a mixture of two or
more thereof. In one
particular embodiment, the formulation of the invention comprises a nonionic
surfactant.
Exemplary nonionic surfactants include, but are not limited to, polysorbates,
poloxamers, alcohol
ethoxylates, ethylene glycol-propylene glycol block copolymers, fatty acid
amides, alkylphenol
- 18 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
ethoxylates, phospholipids, and two or mixture thereof In one particular
embodiment, the
surfactant is Polysorbate 80 (ICI Americas, Inc., Wilmington, DE).
[0059] Various buffers and means for adjusting pH can be used so long as
the resulting
preparation is ophthalmically acceptable. Accordingly, useful buffers include,
but are not limited
to, acetate buffers, citrate buffers, phosphate buffers and borate buffers. In
one particular
embodiment, a buffering agent is used to maintain the pH in the
therapeutically useful range of
pH 4-10, typically about pH 5-8, often a pH range of 6.5 -8.0, more often a pH
range of 7.0-8.0,
and most often a pH range of 7.2 -7.6. It should be appreciated, however, that
the scope of the
invention is not limited to these particular pH ranges. In general, any pH
range that provides
suitable penetration of the active ingredient(s) through the eye can be used.
Typically, a
buffering agent known to those skilled in the art is used including, but not
limited to, acetate,
borate, tris, carbonate, citrate, histidine, succinate, and phosphate. In one
particular embodiment,
the buffering agent comprises boric acid. In another embodiment, the buffering
agent comprises
sodium citrate.
[0060] To provide the ophthalmic formulations with a pH substantially
corresponding to
the pH of the fluids of the eye or at an acceptable physiological pH, as
described above, the pH
of the ophthalmic formulation can be adjusted by addition of an acid or a base
in quantity
sufficient to achieve the desired pH. The pH adjustment can be achieved
through use of various
chemicals such as hydrochloric acid, sodium hydroxide, citric acid, sodium
citrate, acetic acid,
sodium acetate, ammonium acetate, succinic acid, lactic acid, calcium lactate,
sodium lactate,
sodium fumarate, sodium propionate, boric acid, tris base, ammonium borate,
maleic acid,
phosphoric acid, sulfuric acid and aluminum potassium sulfate and the like. A
specific example
of an acid that can be used to adjust the pH of the aqueous buffered
ophthalmic formulation is 1
N hydrochloric acid. A specific example of a base that can be used to adjust
the pH of the
aqueous buffered ophthalmic formulation is 1 N sodium hydroxide. However, it
should be
appreciated that the scope of the invention is not limited to this particular
acid and base. In
general, any pharmaceutically acceptable acids and bases can be used to adjust
the pH. In one
particular embodiment, the ophthalmic formulations of the present invention
contain a
combination of dibasic and monobasic phosphate or boric acid and sodium borate
- as buffering
agents. For example, the formulations contain an amount of boric acid and
sodium borate
- 19 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
sufficient to buffer the formulation in a pH range of 6.5-8.0 or 7.5-8.0 or
dibasic and monobasic
phosphate sufficient to buffer the formulation in a pH range of 6.5-8.0 or 7.0-
8.0 or 7.5-8Ø
[0061] In another embodiment, a tonicity agent (tonicity-adjusting agent)
is used to
adjust the composition of the formulation to the desired isotonic range. The
tonicity-adjusting
agent can be a polyol or a disaccharide including non-reducing disaccharides.
Such tonicity
agents are known to one skilled in the art, and include, but are not limited
to, glycerin, mannitol,
sorbitol, trehalose, xylitol, sodium chloride, and other electrolytes. In one
particular
embodiment, the tonicity agent is glycerin.
[0062] If desired, gum and/or resin can be included in the formulations
of the invention,
including for example, sodium polyacrylate, cellulose ether, calcium alginate,
carboxyvinyl
polymer, ethylene-acrylic acid copolymer, vinyl pyrrolidone polymer, vinyl
alcohol-vinyl
pyrrolidone copolymer, nitrogen-substituted acrylamide polymer,
polyacrylamide, cationic
polymer such as cationic guar gum, dimethylacrylic ammonium polymer, acrylic
acid-
methacrylic acid copolymer, polyoxyethylene-polypropylene copolymer, polyvinyl
alcohol,
pullulan, agar, gelatine, chitosan, polysaccharide from tamarindo seed,
xanthan gum, carageenan,
high-methoxyl pectin, low-methoxyl pectin, guar gum, acacia gum,
microcrystalline cellulose,
arabinogalactan, karaya gum, tragacanth gum, alginate, albumin, casein,
curdlan, gellan gum,
dextran, cellulose, polyethyleneimine, high polymerized polyethylene glycol,
cationic silicone
polymer, synthetic latex, acrylic silicone, trimethylsiloxysilicate and
fluorinated silicone resin.
[0063] In some embodiments, the formulations are preservative-free. In
other
embodiments, a preservative is used. Preservatives are used, for example, to
prevent bacterial
contamination in multiple-use ophthalmic preparations. Exemplary preservatives
include, but
are not limited to, benzalkonium chloride, stabilized oxychloro complexes
(otherwise known as
Purite ), phenylmercuric acetate, chlorobutanol, benzyl alcohol, parabens, and
thimerosal. In one
particular embodiment, the preservative is Purite .
[0064] Other excipient components or ingredients that can also be
included in the
ophthalmic formulations of the present invention are chelating agents and
antibiotics. Suitable
chelating agents are known in the art. Particular examples of useful chelating
agents include, but
are not limited to, edetate salts like edetate disodium, edetate calcium
disodium, edetate sodium,
edetate trisodium, and edetate dipotassium. In one particular embodiment, the
chelating agent is
- 20 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
edentate disodium. It should be appreciated that other chelating agents may
also be used in place
of or in conjunction with edentate disodium. Some examples of antibiotics that
can be included
in formulations of the invention include, but are not limited to, trimethoprim
sulfate/polymyxin
B sulfate, gatifloxacin, moxifloxacin hydrochloride, tobramycin, teicoplanin,
vancomycin,
azithromycin, clarithromycin, amoxicillin, penicillin, ampicillin,
carbenicillin, ciprofloxacin,
levofloxacin, amikacin, gentamicin, kanamycin, neomycin and streptomycin.
[0065] The formulations of the present invention can be packaged in
various package
forms known in the field of topical ophthalmics. In one embodiment, the
formulation is
packaged in sterile, preservative-free single-use packs or vials or containers
(i.e., the unit dose
vials). Each vial, for example as small as a 0.9 mL, may be made of low
density polyethylene so
as to contain a small quantity of the formulation, e.g., 0.4 mL for a single
use. This way, where
the pharmaceutical composition is sterilized and contained in disposable
single-dose containers
for topical use in drop form, multiple vials in the form of a set of 30 vials,
60 vials and so on can
be packaged in a tray with a lid, for example, a polypropylene tray with an
aluminum peelable
lid. The entire contents of each tray can be dispensed intact, and one vial or
pack is used each
time and immediately discarded after each use. For example, plastic ampules or
vials or
containers can be manufactured using blow¨fill¨seal (BF S) technology. The BF
S processes may
involve plastic extrusion, molding, aseptic filling, and hermetic sealing in
one sequential
operation and those processes are known in the art. In another embodiment, the
formulation is
packaged in multi-dose vials such that the materials can be dispensed as
sterile at each time using
specialized container/ closure maintaining the sterility integrity. In yet
another embodiment, the
formulation is packed in conventional vials! containers as sterile product.
[0066] In some embodiments, the dosage form of the invention is eye drops
of
heterogeneous aqueous solution, eye drop formulations containing two or more
active
ingredients in which the first active ingredient is an alpha 2 adrenergic
receptor agonist and a
second active ingredient is selected from the group consisting of a
calcineurin inhibitor, a
lymphocyte function-associated antigen antagonist, a corticosteroid, NSAID, a
sodium channel
blocker, an anti-histamine, and a combination of two or more thereof. For
example, an eye drop
formulations can contain bromonidine or brimonidine tartrate and cyclosporine,
or bromonidine
or brimonidine tartrate and lifitegrast or cyclosporine and lifitegrast
combination or brimonidine
-21 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
or brimonidine tartrate, cyclosporine and lifitegrast. Eye drops typically
contain, according to
the invention, aqueous / oily suspensions of the active ingredients in
pharmaceutically acceptable
carriers and/or excipients. In some embodiments, the particle size of the
active ingredient
employed is about 10 p.m or less, typically 5 p.m or less, often 1 m or less,
more often 0.5 m or
less, still more often 0.2 p.m or less and most often 0.15 p.m or less
[0067] In another aspect, the invention relates to methods of treating a
subject or human
patient suffering from an eye disorder (e.g., dry eye syndrome) by
administering to the eye of the
patient an ophthalmic formulation disclosed herein. For example, in some
embodiments,
formulations used in treating an eye disorder comprise (i) brimonidine or a
pharmaceutically
acceptable salt thereof (e.g., brimonidine tartrate) and cyclosporine, (ii)
brimonidine or a
pharmaceutically acceptable salt thereof and lifitegrast, (iii) cyclosporine
and lifitegrast
combination or (iv) all three actives, i.e., brimonidine or a pharmaceutically
acceptable salt
thereof, cyclosporine and lifitegrast. Still other examples of compositions of
the invention
include brimonidine or a pharmaceutically acceptable salt thereof and
loteprednol; a combination
of brimonidine a pharmaceutically acceptable salt thereof and an NSAID; and a
combination of
brimonidine a pharmaceutically acceptable salt thereof and a sodium channel
blocker and
brimonidine or a pharmaceutically acceptable salt there of and an
antihistamine.
[0068] The active ingredients are present in an amount effective to
provide a desired
therapeutic benefit to a patient suffering from an eye disorder to whom the
composition is
administered. The therapeutically effective amount should be sufficient to
realize relief from the
eye disorder after the treatment. The eye of a subject or human patient can be
the entire eye
structure or a tissue or gland in or around the eye such as the ocular tissue,
eyelids, margin of the
eyelid of the subject, ocular surface. The ophthalmological pharmaceutical
formulation is
topically administrable and/or is administered in, on or around the eye. The
dry eye syndrome
can be aqueous tear-deficient dry eye (ADDE) or evaporative dry eye (EDE) or
consists of both
ADDE and EDE (mixed mechanism dry eye). ADDE may be Sjogren syndrome dry eye
(where
the lacrimal and salivary glands are targeted by an autoimmune process, e.g.,
rheumatoid
arthritis) and non-Sjogren's syndrome dry eye (lacrimal dysfunction, but the
systemic
autoimmune features of Sjogren's syndrome are excluded, e.g., age-related dry
eye). The actual
dose of the active compounds of the present invention depends on the specific
compound, and on
- 22 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
the condition to be treated; the selection of the appropriate dose is well
within the knowledge of
the skilled artisan.
[0069] Additional objects, advantages, and novel features of this
invention will become
apparent to those skilled in the art upon examination of the following
examples thereof, which
are not intended to be limiting. In the Examples, procedures that are
constructively reduced to
practice are described in the present tense, and procedures that have been
carried out in the
laboratory are set forth in the past tense.
EXAMPLES
[0070] An example of a topical heterogeneous ophthalmic solution with its
various
components (w/w) useful for treating an eye disorder (e.g., dry eye syndrome)
is as follows:
brimonidine tartrate in the amount of 0.02% to 0.2% by weight, preferably
about 0.075% and
cyclosporine 0.01 to 0.1% by weight, surfactant such as Polysorbate 80 at
about 0.02% - 2% by
weight or poloxamer/tyloxapol at about 0.1% and 0.25% by weight; carbomer
copolymer (type
A or type B) about 0.05% by weight; tonicity agent (glycerine or includes
glycerine about 2.2%
by weight; phosphate (combination of dibasic and monobasic) buffer (or other
buffers such as
Tris or sodium citrate buffer) of pH 6.0¨ 8.0; sodium EDTA in the amount of
about 0.02% or
less by weight; an oil (e.g., castor oil) in the amount of about 1.25% by
weight. Alternatively,
the oil for the oil phase is a medium chain triglyceride in the range from 0.5
¨ 4%, typically at
about 2%. To prepare this formulation, all water soluble components can be
added and heated
(about 60-70 C) to make water the phase with buffer. A lipophilic solution is
prepared using a
lipophilic solvent (e.g., castor oil) and heating to about 60-70 C.
Heterogeneous solution is
formed by rapid addition of lipophilic solution into water phase followed by
high shear mixing.
The final solution is sterilized via 0.22 micron filter. Alternatively,
sterilization can also be done
by autoclaving at about 121 C for 20 min. The sterilized heterogeneous
solution is filled into
single dose disposable tubes by BFS technology or the like.
[0071] Another general method for preparing a heterogeneous aqueous
solution
comprising a composition of the invention is as follows:
1. Mix Oil phase: Mix appropriate amounts of castor oil and polysorbate 80
until
uniformity is obtained;
- 23 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
2. Mix Aqueous phase: Mix required amounts of Pemulen, water and glycerin
until
uniformity is obtained
3. Perform primary mixing of oil and aqueous phase mixtures from steps 1 and
2;
4. Perform high shear mixing and homogenization of mixture from step 3;
5. Confirm the ophthalmic solution properties via in process testing
[0072] Another example of a topical ophthalmic nanoemulsion formulation
with its
various components (w/w) useful for treating an eye disorder (e.g., dry eye
syndrome) is as
follows: it contains colloid particles with an average particle size of equal
to or less than 0.2 p.m
and greater than 0.02 p.m and has an oily core surrounded by an interfacial
film. The size
population distribution of the colloidal particles may be monomodal. The
solution contains
anywhere from 0.05% to 0.5% (e.g., 0.2%) alpha 2 adrenergic receptor agonist,
cyclosporine
from 0.01% to 0.3% (e.g., 0.075%), and 0.5 to 4% w/w (e.g., 1.25% w/w) castor
oil or medium
chain glycerides. It contains surfactants, preferably 0.5-4% by weight,
polysorbate 80 (e.g. about
1.0% by weight); acrylate/C10-30 alkyl acrylate cross-polymer (about 0.05% by
weight). Topical
ophthalmic solution contains a tonicity agent or a demulcent component (e.g.,
glycerine, which
can be in an amount of about 2.2% by weight), a buffer, such sodium citrate,
tris-base to adjust
the pH. The pH of this topical ophthalmic solution may be in the range of
about 6.0 to about 8Ø
The topical ophthalmic solution is therapeutically effective in increasing
tear production.
[0073] Another example of a topical ophthalmic aqueous solution with its
various
components (w/w) useful for treating an eye disorder (e.g., dry eye syndrome)
is as follows: it
contains colloid particles with an average particle size of equal to or less
than 0.2 p.m and greater
than 0.02 p.m and has an oily core surrounded by an interfacial film. The size
population
distribution of the colloidal particles may be monomodal. The solution
contains anywhere from
0.05% to 0.2% (e.g., 0.075%) alpha 2 adrenergic receptor agonist (e.g.,
brimonidine or a salt
thereof) in weight to the total weight (w/w) of the oil phase, cyclosporine
from 0.01% to 0.3%
(e.g., 0.1%), and 0.5 to 4% w/w (e.g., 2% w/w) medium-chain triglycerides,
0.02% w/w
benzalkonium chloride or no benzalkonium (preservative-free) for single dose
sterile containers,
and surfactants. The surfactants, for example, consist of a mixture of
tyloxapol in an amount of
0.3% w/w and poloxamer in an amount of 0.1% w/w. The ophthalmic solution can
include one
or more oils selected from, castor, olive, soy, corn, mineral, cottonseed,
safflower and sesame.
The solution does not contain any significant amount (< 1%, typically < 0.5%,
often < 0.1% and
- 24 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
most often < 0.01%) of substances capable of generating a negative charge
and/or phospholipids.
The ophthalmic heterogeneous solution can be used for treating a dry eye
syndrome.
[0074] Yet another example of a topical ophthalmic nanoemulsion
formulation with its
various components (w/w) useful for treating an eye disorder is as follows: It
contains
brimonidine tartrate in an amount of about 0.2%; lifitegrast from 0.3% to 10%
by weight,
preferably 4% by weight, polysorbate 80 (e.g. about 1.0% by weight);
acrylate/C10-30 alkyl
acrylate cross-polymer (about 0.05% by weight); water q.s.; and castor oil in
an amount of about
1.25% by weight. Topical ophthalmic solution contains a tonicity agent or a
demulcent
component (e.g., glycerine, which can be in an amount of about 2.2% by
weight), a buffer, such
sodium citrate, tris-base to adjust the pH. The pH of this topical ophthalmic
solution may be in
the range of about 6.0 to about 8Ø The topical ophthalmic solution is
therapeutically effective
in increasing tear production.
[0075] Yet another example of a topical ophthalmic aqueous solution with
its various
components (w/w) useful for treating an eye disorder is as follows: It
contains brimonidine
tartrate in an amount of about 0.02%; lifitegrast from 0.3% to 10% by weight,
preferably 3% by
weight, polysorbate 80 (e.g. about 1.0% by weight); acrylate/C10-30 alkyl
acrylate cross-
polymer (about 0.05% by weight); water q.s.; and castor oil in an amount of
about 1.25% by
weight. The alpha 2 adrenergic receptor agonist lifitegrast are the only
active agents present in
the topical ophthalmic solution but contains a tonicity agent or a demulcent
component (e.g.,
glycerine, which can be in an amount of about 2.2% by weight), a buffer. The
pH of this topical
ophthalmic solution may be in the range of about 6.0 to about 8Ø The topical
ophthalmic
solution is therapeutically effective in increasing tear production.
[0076] Yet another example of a topical ophthalmic solution with its
various components
(w/w) is as follows: It contains brimonidine tartrate in an amount ranging
from about 0.01% to
about 0.5%, typically in an amount of about 0.2% by weight; cyclosporine from
0.01% to about
0.2%, typically in an amount of about 0.075% by weight; lifitegrast from 0.3%
to 10% by
weight, typically 4% by weight, carbomer homopolymer type B in an amount
ranging from about
0.2 to about 0.6%, typically in an amount of about 0.4% or about 0.25%, and/or
carbomer
homopolymer type C in an amount ranging from about 0.4 to about 5% typically
in an amount of
about 4% or about 2.5%, and/or polycarbophil in an amount ranging from about
0.2% to about
- 25 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
0.5% typically in an amount of about 0.4% or about 0.2%; glycerin in an amount
ranging from
about 0.5% to about 1% typically in an amount of about 0.9%; benzalkonium
chloride in an
amount ranging from about 0.003% to about 0.01% typically in an amount of
about 0.007%;
edetate sodium in an amount ranging from about 0.03% to about 0.07 % typically
in an amount
of about 0.05%; sodium chloride in an amount of up to about 0.09%, typically
in an amount of
about 0.06% or q.s. to isotonicity, or mannitol q.s. to isotonicity, or
without isotonicity adjustors
sodium chloride and mannitol; propylene glycol in an amount ranging from about
0.3% to about
0.6% typically in an amount of about 0.5%; water q.s., to 100 gms and sodium
hydroxide or
hydrochloric acid q.s., to adjust pH ranging from pH 6.0 to about 8Ø The
topical ophthalmic
solution is therapeutically effective for treating dry eye syndrome. Although
preservatives such
as benzalkonium chloride can be used in the formulations of the present
invention as described in
the non-limiting examples, typically the formulations are preservative-free.
[0077] Another example of a topical ophthalmic formulation with its
various components
(w/w) useful for treating an eye disorder (e.g., dry eye syndrome) is as
follows: brimonidine
tartrate in the amount of 0.02% to 0.3% by weight, typically about 0.1 to 0.2%
and loteprednol
etabonate 0.02 to 0.6% by weight, typically about 0.1 to 0.3%, surfactant such
as Polysorbate 80
at about 0.02% - 2% by weight or poloxamer/tyloxapol at about 0.1% and 0.25%
by weight;
carbomer copolymer (type A or type B) about 0.05% by weight; tonicity agent
(glycerine or
includes glycerine about 2.2% by weight; sodium citrate and tris buffer of pH
6.0¨ 8.0, sodium
EDTA in the amount of about 0.02% or less by weight; an oil (e.g., castor oil)
in the amount of
about 1.25% by weight. Alternatively, the oil for the oil phase is a medium
chain triglyceride in
the range from 0.5 ¨ 4%, typically at about 2%. To prepare this formulation,
all water soluble
components can be added and heated (about 60-70 C). Oil phase: the oil (e.g.,
castor oil) is
heated to about 60-70 C. Coarse heterogeneous solution is formed by rapid
addition of oil into
water phase followed by high shear mixing. The final heterogeneous solution is
sterilized via
0.22 micron filter. Alternatively, sterilization can also be done by
autoclaving at about 121 C
for 20 min. The sterilized heterogeneous solution is filled into single dose
disposable tubes by
BF S technology or the like.
[0078] Yet another example of a topical ophthalmic combination
formulation with its
various components (w/w) useful for treating an eye disorder (e.g., dry eye
syndrome) is as
- 26 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
follows: brimonidine tartartrate in the amount of 0.02% to 0.3% by weight,
preferably about 0.1
to 0.2% and loteprednol etabonate 0.02 to 0.6% by weight, preferably about 0.1
to 0.3%,
povidone at about 0.6% by weight, poloxamer/tyloxapol at about 0.1% and 0.25%
by weight;
tonicity agent (glycerine or includes glycerine about 1 to 3% by weight;
sodium citrate and tris
buffer of pH 6.0¨ 8.0; sodium EDTA in the amount of about 0.02% or less by
weight.
[0079] Formulation Examples: Ophthalmic pharmaceutical compositions can be
formulated with the compositions shown in Table below. Heterogeneous solution
formulation
can be prepared according to the process described below where the water
insoluble active(s) are
added to the oil phase (e.g., castor oil) before introducing the oil phase to
the aqueous phase.
[0080] Heterogeneous solution Formulation ¨ Process Flow:
1. Oil phase: Mix appropriate amounts of castor oil and polysorbate 80 until
uniformity
is obtained;
2. Aqueous phase: Mix required amounts of Pemulen, water and glycerin until
uniformity is obtained
3. Perform primary mixing of oil and aqueous phase mixtures from steps 1 and
2;
4. Perform high shear mixing and form heterogeneous solution from step 3;
5. Confirm heterogeneous solution properties via in process testing
The above steps of the process flow need not be carried out in the same order.
Ingredient Range per Per mL Other substitutes Function
mL
Brimonidine 0.2 to 5 mg 2 mg Tacrolimus, Active Pharmaceutical
Tartrate pimecrolimus Ingredient*
Cyclosporin 0.1 to 3 mg 1 mg voclosporin
Lifitegrast 3 to 100 mg 30 mg
Loteprednol 0.1 to 20 mg 2 mg
etabonate
Castor Oil 5-100 mg 12.5 mg Olive oil, Oleic Oil Phase
acid
Polysorbate 80 0.1 to 40 mg 10 mg Polysorbate-20, A component to both
Poloxamer 188 help facilitate the
formation of the
heterogeneous mixture
and improve its stability.
Pemulen TR-2 0.1 to 2 mg 0.5 mg N/A Emulsion Stabilizer
Glycerin 0-100 mg 22 mg Trehalose, sorbitol, Tonicity-Adjusting
mannitol, xylitol Agent
- 27 -
CA 03115664 2021-03-29
WO 2020/047197
PCT/US2019/048721
Sodium Citrate 0-20 mg 1.47 mg Phosphate, tris, Maintain pH
Dihydrate histidine, acetate,
succinate
Preservative Optional Optional benzalkonium For multidose and non-
chloride (BAK), sterile products
stabilized
oxychloro complex
(Purite)
Base for pH pH 5 to 8 Maintain pH
Acid for pH pH 5 to 8 Maintain pH
Water for QS Vehicle
Inj ecti on
*. For example, formulations containing different combinations of active
ingredients are:
bromonidine or brimonidine tartrate + cyclosporine; bromonidine or brimonidine
tartrate +
lifitegrast; cyclosporine + lifitegrast combination; bromonidine or
brimonidine tartrate +
cyclosporine + lifitegrast; brimonidine or brimonidine tartrate + loteprednol
etabonate, etc.
Ingredient Range per Per mL Other substitutes Function
mL
Brimonidine 0.2 to 5 mg 2 mg Active Pharmaceutical
Tartrate Ingredient*
Cyclosporin 0.1 to 3 mg 0.75 mg
Castor Oil 5-100 mg 12.5 mg Olive oil, Oleic Oil Phase
acid
Polysorbate 80 0.1 to 40 mg 10 mg Polysorbate-20, A component to both
Poloxamer 188 help facilitate the
formation of the
heterogeneous mixture
and improve its stability.
Pemulen TR-2/ 0.1 to 2 mg 0.5 mg N/A Emulsion
Stabilizer
carbomer
copolymer type
Glycerin 0-100 mg 22 mg Trehalose, sorbitol, Tonicity-Adjusting
mannitol, xylitol Agent
Sodium Citrate 0-20 mg 1.5 mg Phosphate, tris, Maintain pH
Dihydrate histidine, acetate,
succinate
Preservative Optional Optional benzalkonium For multidose and non-
chloride (BAK), sterile products
stabilized
oxychloro complex
(Purite)
Base for pH, pH 6 to 8 Maintain pH
- 28 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
Tr is-base
Acid for pH pH 6 to 8 Maintain pH
Water for QS Vehicle
Inj ecti on
Ingredient Range per Per mL Other substitutes Function
mL
Brimonidine 0.2 to 5 mg 2 mg Active Pharmaceutical
Tartrate Ingredient*
Lifitegrast 3 to 100 mg 40 mg
Castor Oil 5-100 mg 12.5 mg Olive oil, Oleic Oil Phase
acid
Polysorbate 80 0.1 to 40 mg 10 mg Polysorbate-20, A component to both
Poloxamer 188 help facilitate the
formation of the
heterogeneous mixture
and improve its stability.
Pemulen TR-2/ 0.1 to 2 mg 0.5 mg N/A Emulsion
Stabilizer
carbomer
copolymer type
Glycerin 0-100 mg 22 mg Trehalose, sorbitol, Tonicity-Adjusting
mannitol, xylitol Agent
Sodium Citrate 0-20 mg 1.5 mg Phosphate, tris, Maintain pH
Dihydrate histidine, acetate,
succinate
Preservative Optional Optional benzalkonium For multidose and non-
chloride (BAK), sterile products
stabilized
oxychloro complex
(Purite)
Base for pH, pH 6 to 8 Maintain pH
Tr is-base
Acid for pH pH 6 to 8 Maintain pH
Water for QS Vehicle
Inj ecti on
The physical stability of these exemplary heterogeneous formulations can be
monitored. For
example, the heterogeneous solutions are allowed to stand for a period of time
(e.g., 6 months) at
20 to 25 C, and the heterogeneity sizes are measured. The heterogeneity sizes
within
experimental error, should be identical at end of the test period to those
measured right after the
- 29 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
heterogeneous solution is prepared there by suggesting that there is no
significant coalescence of
the heterogeneity. Additionally, there should be no precipitation of the
actives. Such results
demonstrate that the heterogeneous formulations so prepared have superior
physical stability.
[0081] Aqueous formulation (homogeneous and heterogeneous solutions):
General
process for producing aqueous formulation of the compositions of the invention
is provided
below. Briefly, for X Volume (V) of final formulation ¨ complete following
steps:
1(a). Mix Carboxymethycellulose-Na in X/4 V of water
1(b). Mix Polysorbate, API (Brimonidine Tartrate and liftegrast) and
stabilizer
(Trehalose/Mannitol) in X/2 V of water
2. Mix mixtures from 1(a) and 1(b) together until formation of homogenous
or
heterogeneous solution.
3. Add 10 X Stock of buffer (X/10 V) to final mixture from Step 2.
4. Adjust osmomolarity by adding NaCl Stocks to mixture from Step 3.
5. Adjust pH by adding HC1/NaOH to mixture from Step 4.
6. Make final volume to V by adding water to mixture from Step 5.
7. Perform filter sterilization.
8. Filling (BF S).
[0082] Combined Aqueous Formulations: Example of combination product of
aqueous
formulation of Brimonidine Tartrate and Liftegrast:
Aqueous formulation Range Substitutes
Carboxymethylcellulose-Na 0.1 to 0.5% Povidone, PEG 400, dextran,
gelatin,
hydroxy propyl methyl cellulose,
vinyl polymers
Trehalose 1 to 12% Glycerol, sucrose, mannitol
Polysorbate- 80 0.01 to 4% Tyloxapol, pluronic F-68,
polaxomer
Citrate, Tris 1 mM to 100mM Phosphate, acetate, borate,
histidine,
succinate
Sodium Chloride 0-140 mM Magnesium chloride, calcium
chloride, potassium chloride etc
Na0H/HCL as per requirement N/A N/A
pH pH 5 to 8 N/A
Osmolality 200-400 mOSm N/A
[0083] Treatment Example: Several drops of a given formulation
exemplified herein are
administered to the eye(s) of a patient suffering from dry eye syndrome.
Reduction of the
- 30 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
symptoms becomes noticeable within a reasonable period. The treatment is
repeated one or more
times daily while the condition persists.
[0084] Combination Formulation: Various combinations of active
pharmaceutical
ingredient combinations were prepared using the processes disclosed herein.
The particle or
globule size distribution of these exemplary ophthalmic nanoemulsion
formulations (e.g.,
homogeneous/heterogeneous solution; or suspensions) were determined using
particle size
analyzer, Mastersizer 3000Tm (Malvern Panalytical, Malvern, UK). Formulations
were dispersed
in water during measurement and data were plotted as volume density (%) of
particle v. size.
Median size of globules/particles in formulation reported as Dx (50) as
presented in tables in
Figures 1-3. These data indicated that nanosized globule can be manufactured
using process
described in this application for nanoemulsion formulations (Figure 3).
[0085] The physical stability nanoemulsion/solution/suspension
formulations were also
monitored using size distribution analysis over the extended time at various
temperatures. For
example, formulations are incubated for a period (e.g., 1-3 months) at storage
temperature (e.g.,
20 C to 25 C), and accelerated (e.g., 40 C), and harsh (e.g., 60 C) and
the heterogeneity in
sizes were measured. The heterogeneity in sizes, within experimental error,
should be identical
at end of the test period to those measured right after the
nanoemulsion/suspension/solutions
were prepared. Such consistency in the particle size would indicate that there
is no significant
coalescence of the heterogeneity at storage temperature. Even under
accelerated stability
temperature, there was no significant change in median size of
globule/particles (Figures 2 and
3). These results demonstrate that the nanoemulsion/suspension formulations so
prepared have
superior physical stability.
[0086] The quantity of active pharmaceutical ingredients in various
exemplary
nanoemulsion formulations (e.g., homogeneous/heterogeneous solution; or
suspensions) were
determined using RP-HPLC methods. For combination formulation of brimonidine
tartrate and
cyclosporine, the HPLC method utilized a mixture of acetonitrile and a low pH
buffer as an
eluent. The chemical stability of APIs in nanoemulsion/solution/suspension
formulations was
determined using HPLC method after incubation for an extended time at various
temperatures.
For example, formulations are incubated at storage temperature (e.g., 20 to 25
C), and
accelerated (e.g., 40 C), and harsh (e.g., 60 C) and the purity was measured
using HPLC
- 31 -
CA 03115664 2021-03-29
WO 2020/047197 PCT/US2019/048721
method. The purity was substantially identical at the end of the 2 week test
period compares to
those measured right after the nanoemulsion formulation (Figure 5) thereby
suggesting that there
is no significant degradation at storage temperature, accelerated and even at
harsh temperature.
[0087] The partitioning of active pharmaceutical ingredients in oil and
aqueous phases of
these exemplary nanoemulsion formulations were determined using RP-HPLC
methods. For
combination formulation of brimonidine tartrate and cyclosporine, the HPLC
method utilized a
mixture of acetonitrile and a low pH buffer as eluent. In nanoemulsion
formulation, oil and
aqueous phase were separated using ultrafiltration unit by centrifugation
process. Quantity of
APIs in these oil and aqueous phase was quantified using HPLC method. Analyses
showed that
brimonidine partitioned in both phases of nanoemulsion formulation (-30% in
oil and ¨70% in
aqueous phase) while cyclosporine partitioned substantially exclusively in oil
phase. These
results establish the unique differential characteristics of nanoemulsion
formulation in
partitioning of drug of different physicochemical characteristics in different
phases of
homogeneous or heterogeneous nanoemulsion formulations.
[0088] The foregoing discussion of the invention has been presented for
purposes of
illustration and description. The foregoing is not intended to limit the
invention to the form or
forms disclosed herein. Although the description of the invention has included
description of
one or more embodiments and certain variations and modifications, other
variations and
modifications are within the scope of the invention, e.g., as may be within
the skill and
knowledge of those in the art, after understanding the present disclosure. It
is intended to obtain
rights which include alternative embodiments to the extent permitted,
including alternate,
interchangeable and/or equivalent structures, functions, ranges or steps to
those claimed, whether
or not such alternate, interchangeable and/or equivalent structures,
functions, ranges or steps are
disclosed herein, and without intending to publicly dedicate any patentable
subject matter. All
references cited herein are incorporated by reference in their entirety.
- 32 -