Note: Descriptions are shown in the official language in which they were submitted.
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
SATIN COPPER BATH AND METHOD OF DEPOSITING A SATIN COPPER
LAYER
RELATED APPLICATION
[0001] This application claims priority from U.S. Provisional Application
No.
62/756,787, filed November 7, 2018, the subject matter of which is
incorporated herein by
reference in its entirety.
BACKGROUND
[0002] Satin nickel coatings can be used as an intermediate layer or
underlayer in
decorative multilayer coating systems. Nickel, however, is an allergen, and
nickel salts can
be carcinogenic, mutagenic and reprotoxic. This makes the use of nickel more
restricted,
especially for fashion and jewellery applications. Several attempts have been
made to
develop a nickel-free electrolytic deposit for obtaining a satin layer.
[0003] U.S. Patent Application Publication No. 2005/0178668 Al discloses a
method
for depositing nickel and chromium (VI)-free metal matte layers. The first
matte layer is free
of nickel and comprises at least one metal selected from the group consisting
of copper,
silver, tin, zinc or an alloy, which does not contain nickel. This method
allows one to obtain
a "matte" layer defined as a dull, non-glare or pearl surface finish. However,
the matte layer
obtained is not characterized and the additive used is a commercial additive
without any
indication about the chemistry of the molecule used. Additionally, there is no
indication
about the stability of the satin emulsion, which should be maintained to
obtain a constant and
uniform satin finish.
[0004] U.S. Patent Application Publication No. 2013/0341199 Al discloses a
copper
electroplating additive and copper electroplating bath to provide a glossy
copper plating film.
To obtain a uniform plating film in a range from a low current density to high
current density
and provide a good glossiness, a block copolymer compound R-0-(PO)m-(E0)n-H is
used in
an acidic copper electroplating bath. The additive has a structure in which
one end includes
an oxypropylene group capped with an alkyl group or an alkenyl group. This
allows one to
obtain a portion of R-0-(PO)m, which act together as a hydrophobic group,
whereby high
hydrophobicity is provided.
[0005] U.S. Patent Application Publication No. 2015/0014177A1 discloses a
method
for deposition of a matte copper coating comprising two layers to obtain a
homogeneous
matte appearance for decorative applications. However, the matte layers
obtained are not
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-2-
characterized and the copper electroplating bath used to form the copper
coating only
includes additives, such as polyethylene glycol and a sulfur-containing
additive.
SUMMARY
[0006] Embodiments described herein relate to an aqueous acidic copper
electroplating
bath that can be used to provide a satin layer of copper, i.e., a copper
coating with a satin
appearance, on a surface of a substrate or work piece including a work piece
with a complex
shape. Advantageously, the copper electroplating bath can be use for
decorative applications
to replace satin nickel. The copper electroplating bath is substantially non-
allergenic and
eco-friendly as result of being nickel-free and includes a source of copper
ions, an acid, a
satin additive, and optionally one or more acidic copper electroplating bath
additive(s). The
satin additive includes a block copolymer, which has a fixed sequence of an
oxyethylene
group (EO) and an oxypropylene group (PO), with the structure of
RO(E0)m(P0)õH, wherein
R represents an alkyl group or alkenyl group having a linear or branched chain
structure and
having a carbon number of 5 to 20, m is an integer of 3 to 7, and n is an
integer of 3 to 6.
[0007] In some embodiments, the acidic copper electroplating bath
additive(s) can
include at least one additive selected from the group consisting of reaction
products of
bisphenol A and ethylene oxide; polyether compounds; organic divalent sulfur
compounds;
organo-propyl sulfonic acids; an adduct of an alkyl amine and
polyepichlorohydrin; reaction
products of polyethyleneimines and an alkylating agent; organic sulfonates;
high protein
polymers; animal glue; alkoxythio compounds; organic carboxylates;
dithiocarbamic acids;
disulfides; reaction products of a disulfide, a halohydroxy sulfonic acid, and
an aliphatic
aldehyde; polyalkylene glycols; block copolymers with the structure of
OH(E0)x(PO)y(E0)zH where x, y, and z are integers between 1 and 10; urea;
thiourea;
organic thiourea compounds; acetamides; sulfurized, sulfonated organic
compounds; reaction
products of dialkylaminothioxomethylthioalkane sulfonic acids; hydroquinones;
ethoxylated
alkylphenols; polyethylene oxides; disubstituted ethane sulfonic compounds;
sodium lauryl
sulfate; tosyl and mesyl sulfonic acids; alkoxylated lactam amides; glycerin;
alkylarylenes;
sulfurized hydrocarbons; alkylated polyalkyleneimines; phenolphthalein;
epihalohydrins;
sulfoalkylsulfide compounds; arylamines, substituted phenylphenazinium
compounds and
substituted benzothiazole compounds.
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-3-
[0008] In some embodiments, the satin additive can be provided in the
acidic copper
electroplating bath at a concentration of about 1 mg/L to about 1 g/L, for
example, about
50 mg/L to about 500 mg/L or about 100 mg/L to about 300 mg/L.
[0009] In other embodiments, the source of copper ions can be a copper
salt, such as
copper sulfate pentahydrate. The copper salt can be provided in the acidic
copper
electroplating bath at a concentration of about 50 g/L to about 260 g/L, for
example, about
100 g/L to about 200 g/L or about 130 g/L to about 180 g/L.
[00010] In some embodiments, the acid can be selected from sulfuric acid,
fluoboric
acid, phosphoric acid, an alkane sulfonic acid, an alkanol sulfonic acid or
combinations
thereof. For example, the acid can include sulfuric acid at a concentration of
about 50 g/L to
about 260 g/L, about 80 g/L to about 170 g/L, or about 110 g/L to about 150
g/L.
[00011] In still other embodiments, the aqueous acidic copper
electroplating bath can
further include halide ions. The halide ions can be provided in the acidic
copper
electroplating bath at a concentration of about 20 mg/1 to about 200 mg/l.
[00012] Other embodiments described herein relate to a method for
deposition of copper
onto a substrate. The method can include providing a substrate and contacting
the substrate
with an aqueous acidic copper electroplating bath as described herein. An
electrical current
can be applied between the substrate and at least one anode to deposit copper
onto the
substrate.
BRIEF DESCRIPTION OF THE DRAWINGS
[00013] The present invention and advantages thereof will become more
apparent upon
consideration of the following specification with reference to the
accompanying drawings in
which:
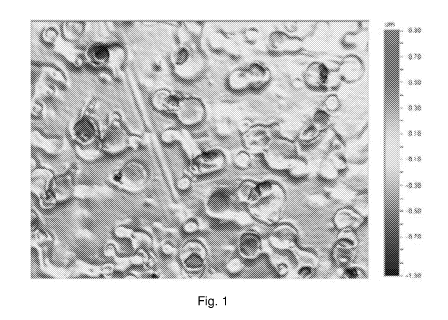
[00014] Fig. 1 illustrates an image showing a profile of a satin nickel
deposit (Ra = 164
nm).
[00015] Fig. 2 illustrates an image showing a profile of a satin copper
deposit (Ra = 261
nm).
[00016] Fig. 3 illustrates an SEM image of a satin nickel deposit (20 kV ¨
x5000).
[00017] Fig. 4 illustrates an SEM image of a satin copper deposit (20 kV ¨
x5000).
[00018] Figs. 5(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener; and (B) a
satin copper
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-4-
deposit electroplated using a conventional copper plating bath with a CUBRAC
2900
brightener and a block copolymer having a structure of R-0-(E0),(P0).-H (satin
additive).
[00019] Figs. 6(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and
POLYGLYKOL
B11/30 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with CUBRAC 2900 brightener, POLYGLYKOL B11/30 surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00020] Figs. 7(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and
POLYGLYKOL
B11/100 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with CUBRAC 2900 brightener, POLYGLYKOL B11/100 surfactant, and a
block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H
[00021] Figs. 8(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and
POLYGLYKOL
D21/150 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, POLYGLYKOL D21/150 surfactant, and
a
block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00022] Figs. 9(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and PLURONIC
PE
6200 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, PLURONIC PE 6200 surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00023] Figs. 10(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
PLURONIC
PE 6800 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, PLURONIC PE 6800 surfactant, and a
block
copolymer having a structure of R-0-(E0),(P0).-H (satin additive).
[00024] Figs. 11(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
PLURONIC
RPE 1740 surfactant; and (B) a satin copper deposit electroplated using a
conventional
copper plating bath with a CUBRAC 2900 brightener, PLURONIC RPE 1740
surfactant, and
a block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-5-
[00025] Figs. 12(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
DEHYPON
GRA surfactant, and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, DEHYPON GRA surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00026] Figs. 13(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
DEHYPON
WET surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, DEHYPON WET surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00027] Figs. 14(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and PEG
4000
surfactant; and (B) a satin copper deposit electroplated using a conventional
copper plating
bath with a CUBRAC 2900 brightener, PEG 4000 surfactant, and a block copolymer
having a
structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00028] Figs. 15(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and PEG
12000
surfactant; and (B) a satin copper deposit electroplated using a conventional
copper plating
bath with a CUBRAC 2900 brightener, PEG 12000 surfactant, and a block
copolymer having
a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
DETAILED DESCRIPTION
[00029] Embodiments described herein relate to an aqueous acidic copper
electroplating
bath that can be used to provide a satin layer of copper, i.e., a copper
coating with a satin
appearance, on a surface of a substrate or work piece including a work piece
with a complex
shape. Advantageously, the copper electroplating bath can be used for
decorative
applications to replace satin nickel. The copper electroplating bath is
substantially non-
allergenic and eco-friendly as result of being nickel-free.
[00030] The copper electroplating bath includes a satin additive that
allows a
conventional copper electroplating bath to form a substantially uniform and
homogenous
satin copper plating layer on the substrate or work piece at a low current
density (e.g., about
0.1 A/dm2) to high current density (e.g., about 8 A/dm2 or more). The copper
electroplating
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-6-
bath including the satin additive can produce a scratch resistant copper layer
having a
homogenous satin aspect at a high total yield.
[00031] The satin additive is a non-ionic surfactant that includes a block
copolymer or
fatty alcohol oxyethylene (E0)/oxypropylene (PO) derivative that has a fixed
sequence of an
EO group and a PO group. In some embodiments, the satin additive has a
structure of R-0-
(E0)õ,-(P0)õ-H, wherein R represents an alkyl group or alkenyl group having a
linear or
branched chain structure and having a carbon number of 5 to 20 (e.g., C8-C16
alkyl or C12-C14
alkyl), m is an integer of about 3 to about 7 (e.g., 4 to 6), and n is an
integer of about 3 to
about 6 (e.g., 4 to 5). In some embodiments, m is greater than n. In other
embodiments, m is
less than n. In still other embodiments, m is equal to n.
[00032] In one example, the satin additive can comprise a block copolymer
having the
general formula (I):
RO-(CH2-CH2-p)-(CH-CH2-0)-H
CH3
(I)
[00033] wherein R represents an alkyl group or alkenyl group having a
linear or
branched chain structure and having a carbon number of 5 to 20 (e.g., C12-C14
alkyl), m is an
integer of about 3 to about 7 (e.g., 4 to 6), and n is an integer of about 3
to about 6 (e.g., 4 to
5). In some embodiments, R is a linear or branched C12-C14 alkyl. In other
embodiments, m
is greater than n. or m is less than n. In still other embodiments, m is equal
to n.
[00034] The satin additive having the formula R-0-(E0),(P0).-H can be
provided in
the copper electroplating bath at a concentration of about 1 mg/L to about 1
g/L, for example,
about 50 mg/L to about 500 mg/L or about 100 mg/L to about 300 mg/L. By adding
the satin
additive to an acidic copper electroplating bath, a uniform satin copper layer
can be obtained
in a wide range of current density (e.g., about 0.1 A/dm2 to about 8 A/dm2 or
more). The
concentration of the satin additive in the copper electroplating bath can be
readily managed
by removing the additive by active carbon filtration. Moreover, the satin
additive is not
consumed when the copper electroplating bath is not in use.
[00035] The acidic copper electroplating bath comprises an aqueous
solution. However,
the copper plating bath may optionally contain one or more co-solvents. Such
co-solvents
include water-miscible solvents, such as alcohols, glycols, alkoxy alkanols,
ketones, and
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-7-
various other aprotic solvents. Specific examples of co-solvents include
methanol, ethanol,
propanol, ethylene glycol, 2-ethoxy ethanol, acetone, dimethyl formamide,
dimethyl
sulfoxide, acetonitrile, and the like.
[00036] Copper is generally present in the copper electroplating bath in an
ionic state
(Cu2 ). The copper can be provided in the bath by adding a copper source, such
as one or
more copper salts, to the electroplating bath. For example, copper may be
obtained from a
copper salt such as copper sulfate, copper polyphosphate, copper sulfamate,
copper chloride,
copper formate, copper fluoride, copper nitrate, copper oxide, copper
tetrafluoroborate,
copper trifluoromethanesulfonate, copper trifluoroacetate, or hydrates
thereof, such as copper
sulfate pentahydrate.
[00037] In some embodiments, the copper electroplating bath can include a
copper salt,
such as copper sulphate pentahydrate (CuSO4.5H20), at a concentration of about
70 g/1 to
about 250 g/L, for example, about 100 to about 200 g/L or about 130 g/L to
about 180 g/L.
[00038] The copper electroplating bath can also include at least one acid.
In some
embodiments, the acid can be sulfuric acid, fluoboric acid, phosphoric acid,
alkane sulfonic
acid, an alkanol sulfonic acid or combinations thereof. In one example, the
copper
electroplating bath includes sulfuric acid and an optional supplemental acid.
The optional
supplemental acid can include, for example, fluoboric acid, alkane sulfonic
acids, and alkanol
sulfonic acids.
[00039] In some embodiments, the copper electroplating bath can include
about 50 g/L
to about 180 g/L of concentrated sulfuric acid, for example, about 80 g/L to
about 170 g/L or
about 110 g/L to about 150 g/L sulfuric acid. When the copper electroplating
bath contains
sulfuric acid and one or more supplemental acids, the ratio of sulfuric acid
to supplemental
acid (total amount of sulfuric acid to the total amount of supplemental acids)
is maintained to
promote the efficient plating of copper on a substrate. Typically, in most
embodiments, more
sulfuric acid is employed compared to supplemental acid.
[00040] The copper electroplating bath can also include chloride ions. In
many instances,
the presence of a small amount of chloride ions in the copper electroplating
bath can facilitate
the plating process and improves the characteristics of the resultant layer of
copper. Chloride
ions can be introduced into the copper electroplating bath by the addition of
hydrochloric acid
or a chloride salt, such as sodium chloride.
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-8-
[00041] In one embodiment, the copper electroplating bath can include about
20 to about
120 mg/L of NaCl, for example, about 50 mg/L to about 70 mg/L NaCl or about 30
to about
60 mg/L chloride ions.
[00042] In some embodiments, the acidic copper electroplating bath has a
composition
comprising, consisting essentially, or consisting of about 70 g/L to about 250
g/L of copper
sulphate pentahydrate (CuSO4.5H20) (e.g., about 100 g/L to about 200 g/L or
about 130 g/L
to about 180 g/L), about 50 g/L to about 180 g/L of concentrated sulfuric acid
(e.g., about 80
g/L to about 170 g/L or about 110 g/L to about 150 g/L), about 20 mg/L to
about 120 mg/L of
NaCl, (e.g., about 40 mg/L to about 80 mg/L or about 50 mg/L to about 70
mg/L), and aboutl
mg/L to about 1 g/L of the satin additive (e.g., about 50 mg/L to about 500
mg/L or about 100
mg/L to about 300 mg/L).
[00043] The copper electroplating bath can optionally include one or more
conventional
acidic copper electroplating bath additives to facilitate the plating process
(e.g., to provide
good throwing power) and/or improve the characteristics of the resultant layer
of copper
(e.g., to enhance uniformity of the deposit). Conventional acidic copper
electroplating bath
additives include, for example, brighteners, carriers, leveling agents,
surfactants, wetting
agents, complexing agents, chelating agents, reducing agents, and promoters.
[00044] In some embodiments, the copper electroplating bath can include 10
ppb or
more and about 5 g/1 or less of one or more additives/brighteners. In another
embodiment, the
copper plating bath contains about 100 ppb or more and about 2 g/1 or less of
one or more
additives/brighteners. In yet another embodiment, the copper plating bath
contains about
300 ppb or more and about 1 g/1 or less of one or more additives/brighteners.
[00045] Brighteners contribute to the ability of the copper plating bath to
provide copper
deposits on substrates. Examples of brighteners that can be used in the copper
electroplating
bath are sulfate bath brighteners, fluoborate, cyanide, and pyrophosphate
plating bath
brighteners. Sulfate plating bath brighteners are described in U.S. Pat. Nos.
5,433,840;
5,431,803; 5,417,841; 5,403,465; 5,215,645; 5,174,886; 5,151,170; 5,145,572;
5,068,013;
5,024,736; 4,990,224; 4,954,226; 4,948,474; 4,897,165; 4,781,801; 4,673,467;
4,551,212;
4,540,473; 4,490,220; 4,430,173; 4,334,966; 4,242,181; and 2,424,887, which
are herein
incorporated by reference in their entirety.
[00046] Examples of sulfate bath brighteners that can used in the acid
copper
electroplating baths include one or more of the reaction products of bisphenol
A and ethylene
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-9-
oxide; polyether compounds; organic divalent sulfur compounds; organo-propyl
sulfonic
acids; an adduct of an alkyl amine and polyepichlorohydrin; reaction products
of
polyethyleneimines and an alkylating agent; organic sulfonates; high protein
polymers;
gelatine or animal glue; alkoxythio compounds; organic carboxylates;
dithiocarbamic acids;
disulfides; reaction products of a disulfide, a halohydroxy sulfonic acid, and
an aliphatic
aldehyde; polyalkylene glycols, such as polypropylene glycols, and especially
those having a
molecular weight from about 1,000 to about 90,000; urea; thiourea; organic
thiourea
compounds; acetamides; sulfurized, sulfonated organic compounds, such as
sulfurized,
sulfonated benzenes; reaction products of dialkylaminothioxomethyl thioalkane
sulfonic
acids; hydroquinones; ethoxylated alkylphenols; polyethylene oxides;
disubstituted ethane
sulfonic compounds; sodium lauryl sulfate; tosyl and mesyl sulfonic acids;
alkoxylated
lactam amides; glycerin; alkylarylenes; sulfurized hydrocarbons, such as
sulfurized benzenes;
alkylated polyalkyleneimines; phenolphthalein; epihalohydrins;
sulfoalkylsulfide compounds;
arylamines; polysulfides; polymeric phenazonium compounds; and sulfonium
compounds
[00047] Fluoborate, cyanide, and pyrophosphate brighteners generally
include
mercaptothiadiazoles, iminodiacetic acid, acetylenic alcohols, sulfamic acids,
glucoheptonic
acid, and phosphonates. In one embodiment, the plating baths of the present
invention do not
contain fluoborate, cyanide, and pyrophosphate brighteners.
[00048] Leveling agents promote the formation of a flat, planar surface of
the plated
copper layer, even if the surface on which the copper layer is formed is not
smooth.
Examples of leveling agents include the condensation products of thiourea and
aliphatic
aldehydes; thiazolidinethiones; imidazolidinethiones; and quatemized
polyamines; and the
like.
[00049] Wetting agents promote leveling and brightening, as well as
promoting bath
stability. Examples of wetting agent include polyoxyalkylated naphthols;
ethylene
oxide/polyglycol compounds; sulfonated wetting agents; carbowax type wetting
agents; and
the like.
[00050] Surfactants contribute to the overall stability of the bath and
improve various
properties in the resultant copper layer. General examples of surfactants
include one or more
of a nonionic surfactant, cationic surfactant, anionic surfactant, and
amphoteric surfactant.
Specific examples of surfactants include nonionic polyoxyethylene surfactants;
alkoxylated
amine surfactants; ethylene oxide-fatty acid condensation products;
polyalkoxylated glycols
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-10-
and phenols; betaines and sulfobetaines; amine ethoxylate surfactants;
quatemary ammonium
salts; pyridinium salts; imidazolinium salts; sulfated alkyl alcohols; and
sulfated lower
ethoxylated alkyl alcohols; and the like.
[00051] Chelating agents facilitate the displacement of metal in the
plating bath.
Examples of chelating agents include polyamines; aminocarboxylic acids;
hydroxycarboxylic
acids.
[00052] In some embodiments, the conventional acidic copper electroplating
bath
additive(s) can include at least one additive selected from the group
consisting of reaction
products of bisphenol A and ethylene oxide; polyether compounds; organic
divalent sulfur
compounds; organo-propyl sulfonic acids; an adduct of an alkyl amine and
polyepichlorohydrin; reaction products of polyethyleneimines and an alkylating
agent;
organic sulfonates; high protein polymers; animal glue; alkoxythio compounds;
organic
carboxylates; dithiocarbamic acids; disulfides; reaction products of a
disulfide, a halohydroxy
sulfonic acid, and an aliphatic aldehyde; polyalkylene glycols; block
copolymers with the
structure of OH(E0)x(PO)y(E0)zH where x, y, and z are integers between 1 and
10; urea;
thiourea; organic thiourea compounds; acetamides; sulfurized, sulfonated
organic
compounds; reaction products of dialkylaminothioxomethylthioalkane sulfonic
acids;
hydroquinones; ethoxylated alkylphenols; polyethylene oxides; disubstituted
ethane sulfonic
compounds; sodium lauryl sulfate; tosyl and mesyl sulfonic acids; alkoxylated
lactam
amides; glycerin; alkylarylenes; sulfurized hydrocarbons; alkylated
polyalkyleneimines;
phenolphthalein; epihalohydrins; sulfoalkylsulfide compounds; arylamines,
substituted
phenylphenazinium compounds and substituted benzothiazole compounds.
[00053] In other embodiments, the aqueous acidic copper electroplating bath
does not
include or is free of a block copolymer having the formula: R-0-(P0)õ,-(E0)õ-H
, wherein
EO is an oxyethylene group and PO is an oxypropylene group, R is an alkyl
group or an
alkenyl group having linear-chain or branched-chain structure and has a carbon
number from
1 to 15, m is an integer from 1 to 30, and n is an integer from 1 to 40. Such
block copolymers
are described in, for example, U.S. Patent Application Publication No.
2013/0341199, which
is herein incorporated by reference in its entirety.
[00054] The pH of the plating bath can be maintained to promote the
efficient plating of
copper on a substrate or work piece. In one embodiment, the pH is about 3 or
less. In another
embodiment, the pH is about 2 or less. In yet another embodiment, the pH is
about 1 or less.
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-11-
The pH of the plating bath may be adjusted using an acid or a basic compound.
For example,
sodium hydroxide and/or sulfuric acid may be used to adjust the pH of the
bath.
[00055] During electroplating a substrate, the temperature of the plating
bath is
maintained to promote the efficient plating of copper on a substrate or work
piece. In one
embodiment, the temperature of the copper plating bath, during plating, is
about 15 C to
about 40 C, for example, about 19 C to about 32 C.
[00056] The current density can be imposed from an energy source through
the
electrodes causing copper ions from the copper electroplating bath to migrate
towards and
attach to the substrate or work piece forming a layer of copper thereon. Due,
in part, to the
components present in the copper electroplating bath, a wide range of current
densities may
be employed. In one embodiment, current densities of about 0.1 to about 8
A/dm2 (e.g., about
0.5 to 5 A/dm2) can be used.
[00057] Any anode, cathode, power source, bath container, agitator, etc.
suitable for
plating metal, such as copper on a substrate or work piece may be employed. In
some
embodiments, a copper plate can be used as the anode. Advantageously, a strong
air agitation
can be maintained during electroplating to obtain a uniform satin deposit.
[00058] Any suitable source of power is connected to the electrodes, such
as direct
current or alternating current. Once the electrodes and the substrate or work
piece are in
contact or immersed in the copper electroplating bath, a current can be
applied. Once a
copper layer of desired thickness is deposited on the substrate, the plated
substrate is removed
from the electroplating bath and optionally rinsed with water. The plated
substrate or work
piece may then be subjected to further processing.
[00059] The length of time that the substrate or work piece is in contact
with the plating
bath under a specified current density depends upon the desired thickness of
the resultant
copper layer and the concentrations of the bath components. In one embodiment,
the
substrate or work piece is in contact with the plating bath under a specified
current density for
a time of about 5 seconds or longer and about 360 minutes or shorter. In
another
embodiment, the substrate or work piece is in contact with the plating bath
under a specified
current density for a time of about 10 seconds or longer and about 180 minutes
or shorter. In
yet another embodiment, the substrate or work piece is in contact with the
plating bath under
a specified current density for a time of about 30 seconds or longer and about
30 minutes or
shorter.
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-12-
[00060] In one embodiment, the thickness of the resultant copper layer
electroplated as
described herein can be about 0.1 micron or more and about 1,000 microns or
less. In another
embodiment, the thickness of the resultant copper layer electroplated as
described herein can
be about 1 micron or more and about 100 microns or less.
[00061] The present invention is further illustrated by the following
examples. These
examples are provided for illustration and are not to be construed as limiting
the scope or
content of the invention in any way.
Example 1
[00062] The copper electroplating bath described herein was used in a
method of
electrodepositing a satin layer of copper on the surface of a work piece.
Experiments were
conducted on brass items with a surface comprising from 0.1 dm2 to 0.7 dm2.
The brass items
were submitted to the preparation sequence as described below:
= Alkaline cathodic cleaner (PRESOL 7073 ¨4 V ¨ 50 C ¨ 1 minute)
= Acidic activation (PICKLANE 33 ¨ room temperature ¨ 30 seconds)
[00063] The items were then plated with an acidic copper electrolyte CUBRAC
660
from the company COVENTYA using the following parameters:
= 3 A/dm2 - room temperature
= Time to reach sufficient copper thickness (10 ¨ 15 um)
[00064] The copper plated items were then plated with a satin copper layer
using the
following electrolyte:
= 130 ¨ 180 g/L CuSO4.5H20
= 110 ¨ 150 g/L H2SO4
= 50 ¨ 70 mg/L NaC1
= 20 ¨ 200 mg/L non-ionic surfactant
= 2 ¨ 50 mg/L sulfur compound
= 100 ¨ 300 mg/L block copolymer R-0-(E0)m-(PO)n-H (satin additive)
[00065] The operating conditions to obtain the satin copper layer were:
= Current density 2 A/dm2
= Temperature 27 C
= Time of deposition: 10 minutes to reach 5 um
= Air agitation
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-13-
[00066] The satin copper layer obtained is characterized by a roughness of
about 200 nm
to about 1 um, depending on the satin aspect desired. The roughness is
measured with a
Wyko NT 1100 profilometer with an objective of 50X ¨ FOV: 1.0X. Fig. 1 and
Fig. 2
illustrate profiles of a satin nickel deposit and satin copper deposit to
compare the two
aspects.
[00067] The satin copper layer obtained has a specific microstructure with
drops, which
are characteristics of the deposit, such as in satin nickel. Fig. 3 and Fig. 4
are SEM pictures
of the two microstructures. The apparatus used to make the measurement is a
FEI quanta 250
FEG with an ETDetector with high vacuum.
Example 2
[00068] Copper layers were electrodeposited on substrate under similar
conditions as
Example 1 using: (A) an acidic copper electroplating baths with CUBRAC 2900
brightener
from the company COVENTYA and a conventional non-ionic surfactant at a similar
concentration as CUBRAC 2900; and (B) an acidic copper electroplating baths
with
CUBRAC 2900 brightener, a conventional non-ionic surfactant at a similar
concentration as
CUBRAC 2900, and a block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H
(satin
additive)). The results are shown in Figs. 5-18.
[00069] Figs. 5(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener; and (B) a
satin copper
deposit electroplated using a conventional copper plating bath with a CUBRAC
2900
brightener and a block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H
(satin additive).
[00070] Figs. 6(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and
POLYGLYKOL
B11/30 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with CUBRAC 2900 brightener, POLYGLYKOL B11/30 surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00071] Figs. 7(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and
POLYGLYKOL
B11/100 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with CUBRAC 2900 brightener, POLYGLYKOL B11/100 surfactant, and a
block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-14-
[00072] Figs. 8(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and
POLYGLYKOL
D21/150 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, POLYGLYKOL D21/150 surfactant, and
a
block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00073] Figs. 9(A-B) illustrate images of: (A) a bright copper deposit
electroplated using
a conventional copper plating bath with a CUBRAC 2900 brightener and PLURONIC
PE
6200 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, PLURONIC PE 6200 surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00074] Figs. 10(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
PLURONIC
PE 6800 surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, PLURONIC PE 6800 surfactant, and a
block
copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00075] Figs. 11(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
PLURONIC
RPE 1740 surfactant; and (B) a satin copper deposit electroplated using a
conventional
copper plating bath with a CUBRAC 2900 brightener, PLURONIC RPE 1740
surfactant, and
a block copolymer having a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00076] Figs. 12(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
DEHYPON
GRA surfactant, and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, DEHYPON GRA surfactant, and a
block
copolymer having a structure of R-0-(E0),(P0).-H (satin additive).
[00077] Figs. 13(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and
DEHYPON
WET surfactant; and (B) a satin copper deposit electroplated using a
conventional copper
plating bath with a CUBRAC 2900 brightener, DEHYPON WET surfactant, and a
block
copolymer having a structure of R-0-(E0),(P0).-H (satin additive).
[00078] Figs. 14(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and PEG
4000
CA 03119028 2021-05-06
WO 2020/096906
PCT/US2019/059496
-15-
surfactant; and (B) a satin copper deposit electroplated using a conventional
copper plating
bath with a CUBRAC 2900 brightener, PEG 4000 surfactant, and a block copolymer
having a
structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00079] Figs. 15(A-B) illustrate images of: (A) a bright copper deposit
electroplated
using a conventional copper plating bath with a CUBRAC 2900 brightener and PEG
12000
surfactant; and (B) a satin copper deposit electroplated using a conventional
copper plating
bath with a CUBRAC 2900 brightener, PEG 12000 surfactant, and a block
copolymer having
a structure of R-0-(E0)õ,-(P0)õ-H (satin additive).
[00080] As shown in the foregoing Figures, the inclusion of the satin
additive described
herein in the acidic copper electroplating bath provided the electrodeposited
copper layers
with a substantially uniform satin appearance.
[00081] From the above description of the invention, those skilled in the
art will perceive
improvements, changes and modifications. Such improvements, changes and
modifications
within the skill of the art are intended to be covered by the appended claims.
All patents and
publications cited herein are incorporated by reference in their entirety.