Note: Descriptions are shown in the official language in which they were submitted.
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
1
SYSTEMS, DEVICES, AND METHODS
FOR ADJUSTING BLOOD FLOW IN A BODY LUMEN
RELATED APPLICATIONS
[0001] This application claims benefit of and priority to U.S. provisional
patent application
no. 62/777,788, filed 11 December 2018, the entire disclosure of which is
herein incorporated
by reference.
FIELD OF THE DISCLOSURE
[0002] Embodiments of the present disclosure generally relate to systems,
devices, and
methods for any and all of controlling venous pressure, renal function
alteration, and urine
output (e.g., such as in acute heart failure patients).
BACKGROUND OF THE DISCLOSURE
[0003] Congestive heart failure (CHF) is characterized by a progressive loss
in the heart's
ability to pump blood. Causes range from valvular disease to infection. The
affected heart has
difficulty in supplying blood to body organs with each contraction. Congestive
heart failure
symptoms typically include shortness of breath, fluid retention and general
fatigue. Most
patients with CHF require additional treatments to help manage their disease,
typically oral
diuretics, inotropes, vasodilators and beta-blockers.
[0004] Diuretics help the kidneys rid the body of excess fluid, thereby
reducing blood
volume and the heart's workload. Inotropes strengthen the heart's pumping
action.
Vasodilators, such as ACE (angiotension conversion enzyme) inhibitors, cause
the peripheral
arteries to dilate, making it easier for blood to flow. Beta-blockers slow the
heart rate and
reduce blood pressure by blocking the effects of adrenaline.
[0005] Many congestive heart failure patients eventually experience a rapid
deterioration and
worsening of symptoms. This sudden worsening of symptoms is called Acute Heart
Failure
(AHF), and refers to rapid onset or worsening of signs and symptoms of chronic
heart failure.
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
2
It is a life-threatening medical condition requiring urgent treatment that
often leads to urgent
hospital admission (1M admissions due to AHF annually in the US). Fluid
decongestion is
the key treatment for AHF, namely high doses of diuretics, a family of drugs
designed to
increase urine output and sodium secretion. Acutely decompensated heart
failure resulting in
hospitalization marks a fundamental change in the natural history of the
progression of
congestive heart failure. Mortality rates in the year following
hospitalization for acute heart
failure patients are significantly higher than in non-hospitalized patients.
Moreover, these
patients are particularly prone to readmission, with recurrent hospitalization
rates of 50%
within 6 months of discharge.
[0006] A certain percentage of patients (20-30%) admitted to the hospital for
AHF suffer
from insufficient response to diuretics and do not achieve rapid fluid removal
or complete
decongestion. These numbers are supported by published literature and real-
world analysis of
consecutive admissions. As of today, there are no viable therapies for AHF
patients resistant
to diuretics. These patients face risks of increased in-hospital mortality,
longer hospital stays
and high rates of 30-day readmission (23-26%).
SUMMARY OF AT LEAST SOME OF
THE EMBODIMENTS OF THE DISCLOSURE
[0007] Embodiments of the present disclosure provide systems, devices, and
methods for
controlling venous pressure, renal function alteration, and urine output, such
as in acute heart
failure patients, through controlling blood flow in blood vessels (which may
also be referred
to as body lumen) associated with the kidneys.
[0008] In some embodiments, a renal function alteration system is provided and
comprises a
blood-flow regulation device, which includes an inner tube, an expandable,
adjustable
member (EAM) coupled to the inner tube, a downstream tip member coupled to a
distal
portion of the inner tube which is distal to the EAM, the tip member being
coupled to a first
fluid pressure sensor, and a second fluid pressure sensor coupled to a portion
of the inner tube
proximal to the EAM. The system can also include a proximal control handle
including a
controller operatively coupled to the EAM and configured to control expansion
of the EAM,
a fluid connector coupled to the control handle, and a supply of a therapeutic
agent in fluid
communication with the fluid connector. The fluid connector can be coupled to
the control
handle, and upon delivering the EAM to a site near a kidney (for example), the
EAM is
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
3
adjustably expanded so as to effect a desired reduction in blood-flow at the
site, and an
amount of therapeutic agent is delivered to the implantation site via the
fluid connector.
[0009] Such embodiments, can further include one and/or another of the
following (and in
some embodiments, at least two of the following, and in some further
embodiments, all of the
following) features, structure, functionality, or clarification, leading to
yet further
embodiments of the present disclosure:
- the therapeutic agent is selected from the group consisting of: a
diuretic, a blood
thinning substance, and an antiplatelet substance, where the blood thinning
substance
comprises an anticoagulant substance;
- the tip member can be conical with a rounded distal-most open tip;
- the tip member can be concentric with a distal opening of the EAM;
- the first fluid pressure sensor can be coupled to the tip member via a
guidewire fluid
connector located on a proximal portion of the inner tube;
- the first fluid pressure sensor can be disposed in or on the tip member;
- the first fluid pressure sensor can comprise a left/first sensor and a
right/second
sensor;
- the tip member and the inner tube can be movable axially with respect to
the EAM;
- the inner tube can be disposed in an intermediate tube, which can be
disposed in a
removable outer sheath, and the EAM can be initially disposed in the outer
sheath;
and
- a cover impervious to blood flow, which can be arranged to cover at least
a portion of
the EAM.
[0010] In some embodiments, a renal function alteration system is provided
comprising a
blood-flow adjustment device which comprises an inner tube, an expandable,
adjustable
member (EAM) coupled to the inner tube, a downstream tip member coupled to a
distal
portion of the inner tube which is distal to the EAM, the tip member being
coupled to a first
fluid pressure sensor, and a second fluid pressure sensor coupled to a portion
of the inner tube
proximal to the EAM. One of skill in the art will appreciate that the blood-
flow adjustment
device, can be a standalone embodiment (i.e., separate from the system).
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
4
[0011] Such embodiments (including the stand alone blood-flow adjustment
device), can
further include one and/or another of the following (and in some embodiments,
at least two of
the following, and in some further embodiments, all of the following)
features, structure,
functionality, or clarification, leading to yet further embodiments of the
present disclosure:
- a proximal control handle including a controller operatively coupled to
the EAM and
configured to control expansion of the EAM;
- a fluid connector coupled to the control handle;
- a supply of a therapeutic agent in fluid communication with the fluid
connector;
- the fluid connector can be coupled to the control handle; and
- upon delivering the EAM to a site near a kidney, the EAM can be
adjustably
expanded so as to effect a desired reduction in blood-flow at the site, and
optionally,
an amount of therapeutic agent can be delivered to the implantation site via
the fluid
connector.
[0012] In some embodiments, a renal function alteration method is provided and
comprises
providing a blood-flow adjustment system/device according to any disclosed
embodiments
(e.g., see above), and providing a fluid connector coupled to the control
handle. The method
also includes delivering the EAM to a site near a kidney, expanding the EAM to
a desired
amount so as to effect a reduction in blood-flow at the site; and delivering a
therapeutic agent
to the site via sad fluid connector.
[0013] These and other embodiments, advantages, and objects thereof are made
even more
apparent by the detailed description which follows, and accompanying figures,
a brief
description of which is set out below.
BRIEF DESCRIPTION OF THE DRAWINGS
[0014] At least some of the embodiments of the present disclosure will be
understood and
appreciated more fully from the following detailed description, taken in
conjunction with the
drawings, a brief description of which follows.
[0015] Fig. 1 is an illustration of an adjustable blood-flow device (BFD),
according to some
embodiments, which includes a multiple shaft or tube construction, an
expanding frame, an
impervious cover and a distal tip (for example);
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
[0016] Fig. 2 is an illustration of a distal expanding element of the device
of Fig. 1;
[0017] Fig. 3 is an illustration of an external control handle for the device
of Fig. 1,
according to some embodiments, which includes a control knob, pressure
measurement fluid
connectors (e.g., luers for upstream and/or downstream) and a therapeutic
agent introduction
fluid connector (e.g., luer);
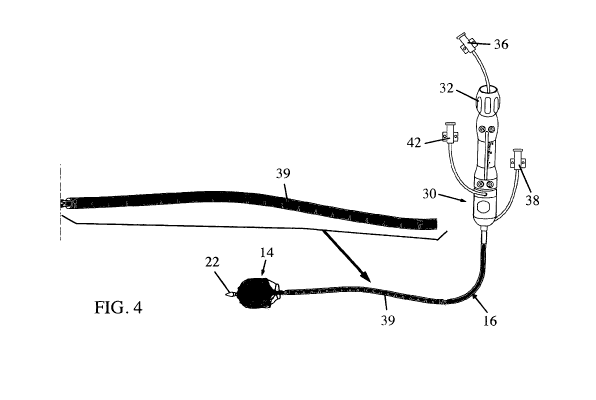
[0018] Fig. 4 is an illustration of the device of Fig. 1, including the
expanded distal element
and the external control handle;
[0019] Fig. 5 is an illustration of the device of Fig. 1 after introduction
into the patient and
coupled to one or more therapeutic agents and pressure sensors;
[0020] Fig. 6 is an illustration of the introduction of the device of Fig. 1
through a body
lumen (e.g., shown in this example with the catheter system is introduced in
the common iliac
vein);
[0021] Fig. 7 is an illustration of a distal end of the device of Fig. 1 being
located at a
treatment location/site (e.g., shown in this example in the inferior vena cava
(IVC) near the
junction to the left and right renal veins);
[0022] Fig. 8 is an illustration of the starting of unsheathing of an outer
shaft of the device of
Fig. 1;
[0023] Fig. 9 is an illustration of the device of Fig. 1 in its fully radially
expanded
orientation, in which a distal element is not yet effected an adjustment of
blood flow in the
body lumen;
[0024] Fig. 10 is an illustration of a control knob being rotated on a control
handle, according
to some embodiments, associated with the device of Fig. 1, which causes
foldable leaflets in
the distal end of the device (not shown here) to be pulled radially inward,
causing an
adjustment of flow in the body lumen, by partially blocking a portion of the
flow; and
[0025] Figs. 11A and 11B are illustrations of the distal element of the device
of Fig. 1,
respectively in partially and fully closed positions, with foldable leaflets
pulled radially
inward, thereby respectively adjusting blood flow therein, up to and including
substantially
blocking flow of the body lumen (i.e., obstructing a substantial portion
thereof, in some
embodiments).
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
6
DETAILED DESCRIPTION OF AT LEAST SOME OF THE EMBODIMENTS
[0026] Reference is now made to Figs. 1 and 2, which illustrate an adjustable
blood-flow
device 10, in accordance with some embodiments of the present disclosure.
Accordingly, the
device 10 can include an inner tube 12, such as a hollow flexible catheter
tube. Inner tube 12
can be disposed in an intermediate tube 13, which in turn can be disposed in
an outer sheath
16. An expandable member 14 (which, in some embodiments, is adjustable) is
coupled to
inner tube 12 and can be initially disposed in outer sheath 16. Member 14 can
be deployed by
retracting outer sheath 16, which uncovers member 14 and allows it to expand
radially
outwards.
[0027] In some embodiments, member 14 is self-expanding (e.g., constructed of
a shape
memory material, such as but not limited to, nitinol), or expandable by
mechanical means
(e.g., wires that push/pull expandable elements), or expandable by fluid means
(e.g.,
hydraulic or pneumatic inflation/deflation of flexible members, such as but
not limited to,
balloons).
[0028] In some embodiments, member 14 expands radially (and/or axially) and
conforms to
the shape of the body lumen/vessel in which it is deployed. Accordingly,
member 14 can be
generally cylindrical in shape (although other shapes are within the scope of
the disclosure).
[0029] In some embodiments, member 14 is constructed of foldable leaflets or
struts 18, such
as wires or other slender elements, which can be coupled to a flexible frame
19 which
includes loops interconnected at different junctures (for example). Such a
structure of
interconnecting struts 18 and flexible frame 19 can be easily compressed and
subsequently
expanded to a predetermined shape.
[0030] In some embodiments, member 14 can be formed with a distal opening 15
(Fig. 2),
which may be referred to as an orifice, through which inner tube 12 protrudes.
The foldable
leaflets or struts 18 can be coupled to a guidewire 34 (connected to a control
knob shown
below in Fig. 3), whereas a proximal outer portion of flexible frame 19 of
member 14 can be
coupled to intermediate tube 13. Similar functionality can be found in
associated published
PCT no. W02017081561A1, the entire disclosure of which is herein incorporated
by
reference.
[0031] In some embodiments, the foldable leaflets (which, in some embodiments,
include
struts 18 or can be separate) can be retracted proximally (by suitable
movement of the
guidewire) to expand the member 14 from a radially closed position for
transition through the
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
7
body lumen to a radially open position at the target site. The radially open
position has a
larger diameter that the radially closed state. Alternatively, in some
embodiments, the device
can be designed such that distal advancement of the foldable leaflets or
struts 18 expands
member 14 from the radially closed position to the radially open position.
[0032] In some embodiments, a covering 20 can cover a portion of member 14,
and can
comprise a membrane which is impervious to blood flow.
[0033] In some embodiments, a downstream tip member 22 can be coupled to a
distal portion
of inner tube 12. The tip member 22 can be conical with a rounded distal-most
open tip 24
that provides little fluid resistance so as not to disturb fluid flow in the
body lumen and not
cause local turbulences. The downstream tip member 22 can be concentric with
the distal
opening 15 of member 14, which further ensures that no local turbulences or
eddy currents
are created and ensure very little pressure drop at the area of the tip member
22.
[0034] Reference is now made to Fig. 3, which illustrates an external control
handle for the
device according to some embodiments. To this end, all the components at the
distal end of
device 10 (according to some embodiments) are operatively coupled to a
proximal control
handle 30, which is located externally from the patient's body during
treatment. The distal
end of control handle 30 can be coupled to outer sheath 16 in order to move
outer sheath 16
proximally to allow member 14 to deploy (expand) radially outwards, or to move
outer
sheath 16 distally to contract member 14 from its radially open state to its
radially closed
state.
[0035] In some embodiments, device 10 can be introduced using an over-the-wire
percutaneous approach (as is known in the field). As mentioned above,
guidewire 34 can pass
through inner tube 12, where the distal end thereof can reach the distal tip
22 (Fig. 1) and the
proximal end of guidewire 34 can exit through a guidewire fluid connector 36
(e.g., luer) at
the proximal end of the control handle 30.
[0036] A controller 32 (also, in some embodiments, referred to as a control
knob, but could
also be automated, motorized controller) which can be located on control
handle 30 (e.g.,
proximal end) and can control the degree of flow (i.e., size of the opening
upon which blood
flows out member 14) of member 14 by controlling the extent of the radial
expansion of
member 14. In some embodiments, control knob 32 is coupled to guidewire 34
which is
coupled to leaflets or struts 18 (Fig. 1). Accordingly, by turning control
knob 32, the user can
move the foldable leaflets 18 to expand member 14 at the site of the body
lumen, thus,
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
8
adjusting blood flow in the vessel and causing a localized pressure-drop near
the location in
which member 14 is positioned. The user can control the degree of size of the
opening in
which blood exits member 14 by suitable turning of control knob 32 (please
note, in other
embodiments, other kinds of controllers can be employed, mechanical or
electrical, instead of
the control knob).
[0037] In some embodiments, once the distal end of the device is in place in
the body lumen,
the guidewire 34 can be removed, and a first fluid pressure sensor 26 can be
connected to the
guidewire fluid connector 36 in the control handle 30. The pressure sensor(s)
26 senses the
fluid (blood) pressure at the vicinity of the open tip 24 (Fig. 2), which is
the pressure in the
blood vessel downstream of the location of the device. The fluid pressure
sensor 26 can
communicate blood pressure information to external controller 28 for data
processing and
display.
[0038] In some embodiments:
- intermediate tube 13 can couple the flexible frame 19 of member 14 for
expanding or
contracting member 14 (and as mentioned above);
- the flexible frame 19, can be configured to ensure contact between member
14 and the
inner vessel wall;
- the gap between the intermediate tube 13 and the inner (guidewire) tube
12 can be
used to measure the pressure upstream of the location of member 14;
- a fluid connector 38 (e.g., luer) can be coupled to control handle 30 and
is in fluid
communication with the intermediate tube 13 via pathways in control handle 30;
- a second fluid pressure sensor 40 can be connected to fluid connector 38
to
continuously monitor the upstream pressure near the location of member 14
within the
blood vessel. The pressure difference between the upstream and downstream
sites can
be used to control the degree of flow according to the patient's needs (as
before,
alternatively, second fluid pressure sensor 40 can be disposed in or on other
portions
of the device);
- depth or distance markers 39 can be provided, in some embodiments, on
device 10 for
helping the user in the procedure (see Fig. 4); and/or
- the outer sheath 16, as noted above, is coupled to the distal end of
control handle 30.
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
9
[0039] In some embodiments, a third fluid connector 42 (e.g., luer) can be
coupled to the
outer sheath hub on control handle 30. The third fluid connector 42 can be
used to introduce a
therapeutic agent through the outer sheath 16, which, without limitation, the
therapeutic agent
can be a diuretic and/or a blood thinning substance for reducing the risk of
thrombi forming
on the distal expanding end during blood flow regulation.
[0040] Reference is now made to Fig. 5, which illustrates the device 10 after
being delivered
to a site near the renal vasculature, according to some embodiments (Fig. 5
shows the option
of bedside delivery). Accordingly, control handle 30 is shown external to
device 10 and is
coupled to the fluid pressure sensor 26 (and/or circuitry) for continuously
monitoring the
pressure in the blood vessel downstream of the site and to second fluid
pressure sensor 40
(and/or circuitry) for continuously monitoring the upstream pressure near the
site. The control
handle 30 is in fluid communication with one or more therapeutic agents, such
as a blood
thinning substance 43 and/or a diuretic 45.
[0041] In some embodiments, a therapeutic agent 43 (e.g., a blood thinning
substance) can be
an antiplatelet substance. Antiplatelet therapy has been shown to reduce
clinical ischemic
events and improve outcomes for acute coronary syndrome patients. Non-limiting
examples
of antiplatelet substances include GPIIb/IIIa inhibitors, dipyridamole, (low-
dose) aspirin
(acetylsalicylic acid), a selective COX-2 or nonselective COX-1/COX-2
inhibitor, or a ADP
receptor inhibitor, such as a thienopyridine (e.g., clopidogrel, ticlopidine
or prasugrel),
elinogrel or ticagrelor, or a thrombin receptor antagonist such as vorapaxar,
or analogs or
derivatives or combinations thereof. Thienopyridines such as clopidogrel
irreversibly inhibit
P2Y12 receptors, which play an active role in platelet activation. In the
normal state, when
blood vessels are damaged, platelet activation mediated by P2Y12receptors play
an important
role to arrest bleeding at the site of injury. In a diseased state, platelet
activation leads to
vascular obstruction and ischemic damage. Thus, P2Y12receptor antagonists play
a key role
in antiplatelet therapy in assisting to prevent coronary artery disease and
for the immediate
treatment of acute coronary syndrome and percutaneous coronary intervention.
[0042] In some embodiments:
- the therapeutic agent 43 (blood thinning substance) can be an
anticoagulant substance,
such as but not limited to, heparin, warfarin, rivaroxaban, dabigatran,
apixaban,
edoxaban, enoxaparin, or fondaparinux; and
- the diuretic 45 can include, without limitation, thiazide diuretics
(e.g., chlorthalidone,
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
hydrochlorothiazide, metolazone, indapamide), loop diuretics (e.g., torsemide,
furosemide, bumetanide), and/or potassium-sparing diuretics (e.g., amiloride,
triamterene, spironolactone eplerenone).
[0043] In some embodiments, the device can also include a controllable device
having
continuous pressure measurement across the device. For example, as shown in
broken lines in
Fig. 2, alternatively fluid pressure sensor 26 can be disposed in or on tip
member 22. The
fluid pressure sensor 26 can be a wireless blood pressure sensor, available
from many
manufacturers, which wirelessly communicates blood pressure information to
circuitry 27
and/or external controller 28 (Fig. 3). Two fluid pressure sensors 26 can be
used: a left sensor
and a right sensor (and even in some embodiments, also a central censor) for
measuring the
blood pressure in the vicinity of the left and right renal veins, respectively
(see Fig. 2). The
pressure sensors can be at left, central, and/or right portions of the tip
member 22;
alternatively, a bifurcated tip member (shown in broken lines) can be
employed. In another
alternative, tip member 22 with inner tube 12 can be movable axially with
respect to member
14. In this manner, the user can adjust the distance tip member 22 protrudes
from member 14
to suit different anatomical geometries.
[0044] Reference is now made to Fig. 6, which illustrates delivering the
device 10 via the
common iliac vein towards the inferior vena cava (IVC), according to some
embodiments. In
Fig. 7, a distal end of device 10 is being located at a site; in this example,
in the IVC near the
junction to the left and right renal veins.
[0045] Reference is now made to Fig. 8, which illustrates the starting of the
unsheathing of
the outer sheath 16 of device 10, according to some embodiments.
[0046] Reference is now made to Fig. 9, which illustrates device 10 in its
fully radially
expanded orientation, according to some embodiments. The distal member 14 is
not yet
affecting flow in the body lumen.
[0047] Reference is now made to Fig. 10, which illustrates control knob 32
being rotated on
control handle 30, which causes radially-outward expansion of the member to
eventually
cause a change in flow in the body lumen, according to some embodiments.
[0048] Reference is now made to Fig. 11A, which illustrates the member 14 in a
partially
expanded position and Fig. 11B which illustrates the member 14 in a fully
expanded position,
thereby substantially closing the flow in the body lumen, according to some
embodiments
(and in some embodiments, closing the flow).
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
11
[0049] In some embodiments, placement of device 10 in the inferior vena cava,
caudal to the
inlet of the renal veins, effects a change in flow and creates a lower
pressure zone in the area
thereof, thereby increasing the pressure gradient on the kidneys and
increasing urine output.
The controllable device catheter can be designed for bedside delivery, which
minimizes
treatment time and recovery. Once a desired change in flow is achieved,
diuretics can be
given to the patient. The combination of the change in flow and the diuretics
can improve
renal function, increase patient urine output and help extract extraneous
fluid from the
interstitial tissue.
[0050] While various inventive embodiments have been described and illustrated
herein,
those of ordinary skill in the art will readily envision a variety of other
means and/or
structures for performing the function and/or obtaining the results and/or one
or more of the
advantages described herein, and each of such variations and/or modifications
is deemed to
be within the scope of the inventive embodiments described herein. More
generally, those
skilled in the art will readily appreciate that all parameters, dimensions,
materials, and
configurations described herein are meant to be an example and that the actual
parameters,
dimensions, materials, and/or configurations will depend upon the specific
application or
applications for which the inventive teachings is/are used. Those skilled in
the art will
recognize, or be able to ascertain using no more than routine experimentation,
many
equivalents to the specific inventive embodiments described herein. It is,
therefore, to be
understood that the foregoing embodiments are presented by way of example only
and that,
within the scope of claims supported by the subject disclosure and equivalents
thereto, and
inventive embodiments may be practiced otherwise than as specifically
described and
claimed. Inventive embodiments of the present disclosure are directed to each
individual
feature, system, article, material, kit, step, and/or method described herein.
In addition, any
combination of two or more such features, systems, articles, materials, kits,
steps, and/or
methods, if such features, systems, articles, materials, kits, steps, and/or
methods are not
mutually inconsistent, is included within the inventive scope of the present
disclosure.
[0051] Embodiments disclosed herein may also be combined with one or more
features, as
well as complete systems, devices and/or methods, to yield yet other
embodiments and
inventions. Moreover, some embodiments, may be distinguishable from the prior
art by
specifically lacking one and/or another feature disclosed in the particular
prior art
reference(s); i.e., claims to some embodiments may be distinguishable from the
prior art by
including one or more negative limitations.
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
12
[0052] Also, various inventive concepts may be embodied as one or more
methods, of which
an example has been provided. The acts performed as part of the method may be
ordered in
any suitable way. Accordingly, embodiments may be constructed in which acts
are performed
in an order different than illustrated, which may include performing some acts
simultaneously, even though shown as sequential acts in illustrative
embodiments.
[0053] Any and all references to publications or other documents, including
but not limited
to, patents, patent applications, articles, webpages, books, etc., presented
anywhere in the
present application, are herein incorporated by reference in their entirety.
Moreover, all
definitions, as defined and used herein, should be understood to control over
dictionary
definitions, definitions in documents incorporated by reference, and/or
ordinary meanings of
the defined terms.
[0054] The indefinite articles "a" and "an," as used herein in the
specification and in the
claims, unless clearly indicated to the contrary, should be understood to mean
"at least one."
[0055] The phrase "and/or," as used herein in the specification and in the
claims, should be
understood to mean "either or both" of the elements so conjoined, i.e.,
elements that are
conjunctively present in some cases and disjunctively present in other cases.
Multiple
elements listed with "and/or" should be construed in the same fashion, i.e.,
"one or more" of
the elements so conjoined. Other elements may optionally be present other than
the elements
specifically identified by the "and/or" clause, whether related or unrelated
to those elements
specifically identified. Thus, as a non-limiting example, a reference to "A
and/or B", when
used in conjunction with open-ended language such as "comprising" can refer,
in one
embodiment, to A only (optionally including elements other than B); in another
embodiment,
to B only (optionally including elements other than A); in yet another
embodiment, to both A
and B (optionally including other elements); etc.
[0056] As used herein in the specification and in the claims, "or" should be
understood to
have the same meaning as "and/or" as defined above. For example, when
separating items
in a list, "or" or "and/or" shall be interpreted as being inclusive, i.e., the
inclusion of at least
one, but also including more than one, of a number or list of elements, and,
optionally,
additional unlisted items. Only terms clearly indicated to the contrary, such
as "only one of
or "exactly one of," or, when used in the claims, "consisting of," will refer
to the inclusion of
exactly one element of a number or list of elements. In general, the term "or"
as used herein
shall only be interpreted as indicating exclusive alternatives (i.e. "one or
the other but not
CA 03119843 2021-05-11
WO 2020/121309 PCT/IL2019/051360
13
both") when preceded by terms of exclusivity, such as "either," "one of,"
"only one of," or
"exactly one of" "Consisting essentially of," when used in the claims, shall
have its ordinary
meaning as used in the field of patent law.
[0057] As used herein in the specification and in the claims, the phrase "at
least one," in
reference to a list of one or more elements, should be understood to mean at
least one element
selected from any one or more of the elements in the list of elements, but not
necessarily
including at least one of each and every element specifically listed within
the list of elements
and not excluding any combinations of elements in the list of elements. This
definition also
allows that elements may optionally be present other than the elements
specifically identified
within the list of elements to which the phrase "at least one" refers, whether
related or
unrelated to those elements specifically identified. Thus, as a non-limiting
example, "at least
one of A and B" (or, equivalently, "at least one of A or B," or, equivalently
"at least one of A
and/or B") can refer, in one embodiment, to at least one, optionally including
more than one,
A, with no B present (and optionally including elements other than B); in
another
embodiment, to at least one, optionally including more than one, B, with no A
present (and
optionally including elements other than A); in yet another embodiment, to at
least one,
optionally including more than one, A, and at least one, optionally including
more than one,
B (and optionally including other elements); etc.
[0058] In the claims, as well as in the specification above, all transitional
phrases such as
"comprising," "including," "carrying," "having," "containing," "involving,"
"holding,"
"composed of," and the like are to be understood to be open-ended, i.e., to
mean including
but not limited to. Only the transitional phrases "consisting of and
"consisting essentially of'
shall be closed or semi-closed transitional phrases, respectively, as set
forth in the United
States Patent Office Manual of Patent Examining Procedures, Section 2111.03.