Note: Descriptions are shown in the official language in which they were submitted.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
WATER-SOLUBLE FORMULATIONS, METHODS OF MAKING AND USE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of and priority to United
States Patent
Application No. 62/773,644 filed on November 30, 2018; United States Patent
Application
No. 62/773,652 filed on November 30, 2018; and United States Patent
Application No.
62/926,885 filed on October 28, 2019, each of which are hereby incorporated by
reference
in their entirety.
TECHNICAL FIELD
[0002] The present disclosure relates generally to the field of water-
soluble
formulations, and more particularly to water-soluble formulations comprising
cannabinoids
or cannabis-derived compounds for use in beverages, topicals and foodstuffs.
BACKGROUND
[0003] In the cannabis industry, an important aspect of preparing a
commercial
product is the ability to formulate cannabinoids and other cannabis-derived
compounds in a
desirable form for human consumption.
[0004] Smoking is not typically acceptable to non-smokers, as it can be
aesthetically unpleasant and can involve health risks such as irritation to at
least the mouth,
esophagus and lungs. Cigarette smoking has been linked to devastating health
risks
thought to result from the formation of harmful combustion products. In some
jurisdictions,
legislation exists which prohibits smoking in various locations and cannabis
smoking itself is
the target of regulation due to so-called "second hand smoke" risks, as well
as what is said
to be unpleasant smells for some people. Methods for consuming cannabis, and
more
particularly cannabinoids, which do not involve smoking or other vaporous
means of
ingestion may therefore be advantageous as such methods do not involve these
and other
unwanted effects.
[0005] Oral consumption comprises a significant percentage of total
cannabis use in
federally legal jurisdictions as well as on a state, province, or the like,
basis globally. Many
orally consumable products, however, contain unhealthy amounts of substances
other than
cannabis or cannabinoids. Such ingredients include various sugars, caffeine
and a variety
1
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
of non-sugar stimulants, ethanol, and plant-based substances thought to be
nutritional
supplements, but which have not been the subject of extensive safety testing
in complex
formulations including cannabis and cannabinoid-containing compositions.
Further, many
known oral products use expensive gums, which are cost prohibitive and may
also have
unpredictable supply.
[0006] As hydrophobic compounds, cannabinoids and other cannabis-derived
compounds present challenges for preparing desirable consumer products, such
as
beverages and other foodstuffs. Cannabinoids, including many cannabinoid
extracts and
oils, are insoluble in water thereby making many food products and beverages
difficult to
produce, including difficulties in obtaining desirable concentrations of
cannabinoids in these
products.
[0007] A need therefore exists for improved water-soluble formulations of
cannabinoids that may be used in the preparation of consumer products, and in
particular
aqueous-based products such as beverages. There further exists a need that
these
formulations have wide-range applicability in preparing consumer products.
SU MARRY
[0008] The present disclosure provides a convenient water-soluble
formulation of
cannabinoids or cannabis-derived compounds that may be used in beverages and
foodstuffs. More particularly, in select embodiments, the present disclosure
provides a
formulation of cannabinoids for use in liquid or dispersible powder forms that
is soluble in
water, and capable of improving the dispersibility and stability of the
cannabinoids to
provide for acceptable shelf-life of the formulations and products produced
therefrom (e.g.
beverages).
[0009] In some embodiments, the formulation is of natural origin and
calorie-free
(i.e., less than 5 kcal per serving). In some embodiments, the formulation may
advantageously have little or no taste and odor. In particular, in some
embodiments, the
water-soluble formulations may be used to prepare products that are of clean
taste in that
the water-soluble formulations do not impart an unpleasant or undesirable
taste to the
products.
2
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[0010] In some aspects, as an alternative or in addition to the
cannabinoids, the
formulations of the present disclosure may include other cannabis-derived
compounds
(e.g., cannabis extract, terpenes, etc.), non-cannabis-derived compounds
(e.g., non-cannabis terpenes), and/or nutritional supplements (e.g., vitamins)
in a single
convenient formulation or dosage form.
[0011] The present disclosure is directed to water-soluble formulations
including
cannabinoids or cannabis-derived compounds for use in beverages, foods and
other
products, and to methods of preparing the formulations. The present disclosure
is also
directed to foodstuffs and beverages comprising said formulations (e.g.
produced using the
water-soluble formulations). In particular, the water-soluble formulations
comprise a
cannabinoid or a cannabis-derived compound, an emulsifier, and a glycerin-
based carrier
surfactant. In select embodiments, the water-soluble formulations further
comprise a carrier
oil. The water-soluble formulations may be a liquid or a dispersible powder.
[0012] Most suitably and in select embodiments, the formulations are
physically and
chemically stable; transparent or translucent in colour; calorie-free; and
have minimal
flavour. Advantageously, in select embodiments, the water-soluble formulations
are also
transparent or translucent when mixed into an aqueous product, such as a
beverage. As
used herein, "transparent" is defined by transmittance instruments as known in
the art.
"Translucent" is defined by either transmittance or reflectance measurement
modes (see
HunterLab definition, which is available at www.hunterlab.com/transluceent-
beverage-color-
measurement).
[0013] Further, in select embodiments, the formulations include favorable
pharmacokinetics, for example, rapid onset, shorter duration, and minimal food
effect as
described more fully herein.
[0014] The present disclosure is also directed to methods of preparing
the
compositions that are commercially-viable, efficient, and produce shelf-stable
formulations
and products.
[0015] According to one aspect of the present disclosure, there is
provided a
water-soluble formulation comprising a cannabinoid or a cannabis-derived
compound; an
emulsifier; and a glycerin-based carrier surfactant. In select embodiments,
the water-
soluble formulations further comprise a carrier oil. In some embodiments, the
carrier oil is
3
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
comprised of monoglycerides. In some embodiments, the emulsifier comprises a
soy
lecithin. In some embodiments, the glycerin-based carrier surfactant is a
vegetable
glycerin.
[0016] In some embodiments, the water-soluble formulation comprises a
cannabinoid distillate or a cannabinoid isolate; monoglycerides; and a soy
lecithin; a
vegetable glycerin.
[0017] In some embodiments, the water-soluble formulation comprises up to
10%
by weight of the cannabinoid or cannabis-derived compound; up to 10% by weight
of the
carrier oil, and up to 10% by weight of the emulsifier. In some embodiments,
the
water-soluble formulation comprises the cannabinoid or cannabis-derived
compound; the
carrier oil, and the emulsifier at an about equivalent amount by weight. In
some
embodiments, the water-soluble formulation comprises between about 60% and
about 97%
by weight of the glycerin-based carrier surfactant.
[0018] In some embodiments, the water-soluble formulation comprises a
cannabinoid distillate or a cannabinoid isolate; monoglycerides; a soy
lecithin; and a
sucrose monoester; in a vegetable glycerin. In some embodiments, the water-
soluble
formulation comprises an about equivalent amount by weight of the soy lecithin
and the
sucrose monoester.
[0019] In some embodiments, the water-soluble formulation is an emulsion.
In
some embodiments, the water-soluble formulation is clear. In some embodiments,
the
water-soluble formulation is transparent, translucent, or pearlescent when
mixed with an
aqueous solution, including when mixed in an aqueous solution.
[0020] In some embodiments, the water-soluble formulations comprises a
cannabinoid and the cannabinoid is THC (A9-THC), A8-THC, trans-A10-THC, cis-
L,10-THC,
THCA, THCV, A8-THCA, A9-THCA, A8-THCV, A9-THCV, THCVA, CBD, CBDA, CBDV,
CBDVA, CBC, CBCA, CBCV, CBCVA, CBG, CBGA, CBGV, CBGVA, CBN, CBNA, CBNV,
CBNVA, CBND, CBNDA, CBNDV, CBNDVA, CBE, CBEA, CBEV, CBEVA, CBL, CBLA,
CBLV, CBLVA, CBT, or any combination thereof. In select embodiments, the
cannabinoid
is CBD, THC or a combination thereof. In select embodiments, the cannabinoid
is THC
alone or CBD alone.
4
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[0021] In some embodiments, the water-soluble formulations comprise a
cannabis-derived compound and the cannabis-derived compound is a cannabis-
derived
cannabinoid, a cannabinoid distillate, a cannabinoid isolate, a terpene, or
any combination
thereof.
[0022] Advantageously, in some embodiments, the water-soluble formulation
is
shelf-stable at room temperature, including for at least 55 days. In some
embodiments, the
water-soluble formulation loses less than 20% by weight of the cannabinoid or
cannabis
derived compound in 3 months.
[0023] In some embodiments, when mixed with the aqueous solution, the
water-soluble formulation provides a product in which at least 80% by weight
of the
cannabinoid or cannabis-derived compound remains present after about 2 months
at a
temperature between about 17 C and about 40 C, more particularly at least 90%.
In some
embodiments, when mixed with the aqueous solution, the water-soluble
formulation
provides a product in which at least 84.89% by weight of the cannabinoid or
cannabis
derived compound remains present after about 3 months at a temperature of
about 40 C.
[0024] In some embodiments, the water-soluble formulations may further
comprise
one or more additives selected from the group consisting of terpenes,
terpenoids,
flavonoids, viscosity modifiers, natural emulsifiers, oils, thickening agents,
minerals, acids,
bases, vitamins, flavours, colourants, sweeteners, and combinations thereof.
In an
embodiment, the water-soluble formulation comprises a terpene having
antimicrobial
properties.
[0025] In another aspect of the present disclosure, there is provided a
water-soluble formulation comprising a cannabinoid or a cannabis-derived
compound, a
carrier oil, a surfactant, and an emulsifier, wherein the water-soluble
cannabis formulation is
transparent, translucent, or pearlescent when mixed with an aqueous solution.
[0026] According to another aspect of the present disclosure, there is
provided a
powder formulation prepared by drying the water-soluble formulation as
described herein.
In an embodiment, the powder formulation comprises less than 10 kcal per 250
mg of the
powder formulation. More particularly, in an embodiment the present disclosure
is directed
to a powder formulation prepared by drying a water-soluble formulation as
described
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
herein, the water-soluble formulation comprising a cannabinoid or a cannabis-
derived
compound, a carrier oil, an emulsifier, and a glycerin-based carrier
surfactant.
[0027] According to another aspect of the present disclosure, there is
provided a
product comprising the water-soluble formulation as described herein. In an
embodiment,
the product is a foodstuff. In an embodiment, the product is a beverage and
comprises an
aqueous solution. In an embodiment, the water-soluble formulation comprises a
cannabinoid distillate, monoglycerides, a soy lecithin, and a sucrose
monoester, in a
vegetable glycerin, and the aqueous solution comprises a stabilizer, for
example a chelating
agent.
[0028] Advantageously, in some embodiments, the product is shelf-stable
at room
temperature, including for at least 55 days. In some embodiments, the product
loses less
than 20% by weight of the cannabinoid or cannabis-derived compound in 3
months. In
select aspects of these embodiments, the product is a beverage.
[0029] In some embodiments, the product is stable in that at least 80% by
weight of
the cannabinoid or cannabis-derived compound remains present after about 2
months at a
temperature between about 17 C and about 40 C, more particularly at least 90%.
In some
embodiments, the product is stable in that at least 84.89% by weight of the
cannabinoid or
cannabis-derived compound remains present after about 3 months at a
temperature of
about 40 C. In an embodiment, the product has an oxygen content of between
about 0
ppm and about 500 ppm.
[0030] According to another aspect, the present disclosure is directed to
a method
for preparing a water-soluble formulation of the present disclosure, the
method comprising
mixing, in any order, a cannabinoid or a cannabis-derived compound with a
glycerin-based
carrier surfactant and an emulsifier to prepare the water-soluble formulation.
[0031] According to another aspect, the present disclosure is directed to
a method
of preparing a water-soluble formulation of the present disclosure, the method
comprising:
mixing a cannabinoid or a cannabis-derived compound and a carrier oil until a
homogenous
mixture is formed; and mixing a glycerin-based carrier surfactant and
emulsifier into the
homogenous mixture. In an embodiment, the method further comprises mixing a
sucrose
monoester into the homogenous mixture.
6
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[0032] According to another aspect, the present disclosure is directed to
a method
for preparing a powder formulation, the method comprising: mixing a
cannabinoid or a
cannabis-derived compound and a carrier oil until a homogenous mixture is
formed; mixing
the a glycerin-based carrier surfactant and emulsifier into the homogenous
mixture to
prepare the water-soluble formulation; and drying the water-soluble
formulation.
[0033] According to another aspect, the present disclosure is directed to
a method
for preparing a product comprising a water-soluble formulation of the present
disclosure,
the method comprising: mixing, in any order, a cannabinoid or a cannabis-
derived
compound with a glycerin-based carrier surfactant and an emulsifier to prepare
the
water-soluble formulation; and mixing the water-soluble formulation with an
aqueous
solution
[0034] According to another aspect, the present disclosure is directed to
a method
of preparing a product comprising a water-soluble cannabis formulation of the
present
disclosure, the method comprising: mixing a cannabinoid or a cannabis-derived
compound
and a carrier oil until a homogenous mixture is formed; mixing a glycerin-
based carrier
surfactant and emulsifier into the homogenous mixture to prepare the water-
soluble
formulation; and mixing the water-soluble formulation with an aqueous
solution. In an
embodiment, the product is a beverage. In an embodiment, the method further
comprises
mixing a sucrose monoester into the homogenous mixture.
[0035] In yet another aspect, the present disclosure is directed to a
method for
preparing a solid product, the method comprising: mixing a cannabinoid or a
cannabis-
derived compound and a carrier oil until a homogenous mixture is formed;
mixing the
surfactant and emulsifier into the homogenous mixture to prepare the water-
soluble
formulation; and absorbing the water-soluble cannabis formulation into a solid
material
(e.g. tea bag).
[0036] Other aspects and features of the water-soluble formulations,
methods and
products (e.g. dosage forms, beverages and foodstuffs) of the present
disclosure will
become apparent to those ordinarily skilled in the art upon review of the
following
description of specific embodiments. Without being bound by any particular
theory, the
water-soluble formulations of the present disclosure may improve the ability
to formulate
cannabinoids into aqueous mediums (e.g. beverages and foodstuffs).
7
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
BRIEF DESCRIPTON OF THE DRAWINGS
[0037] These and other features of the present disclosure will become
more
apparent in the following detailed description in which reference is made to
the appended
drawings. The appended drawings illustrate one or more embodiments of the
present
disclosure by way of example only and are not to be construed as limiting the
scope of the
present disclosure.
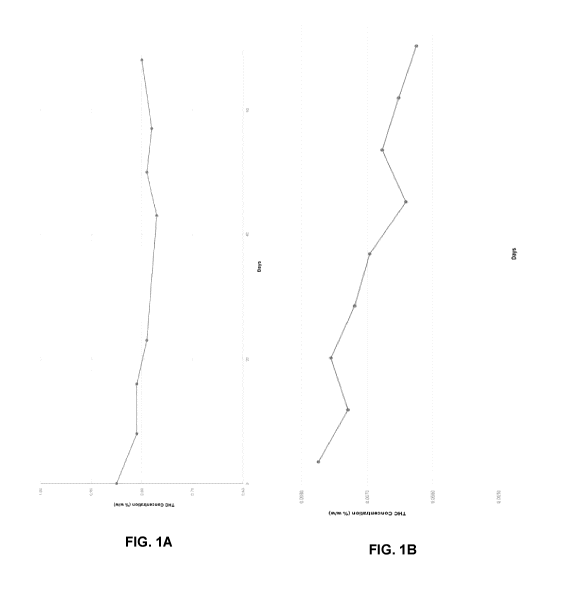
[0038] FIGS. 1A & 1B depict stability data for water-soluble formulations
(FIG. 1A)
an end use beverages including the water-soluble formulations (FIG. 1B).
[0039] FIGS. 2A-2C are graphs depicting mood experience at 0 hour (FIG.
2A),
after 1 hour (FIG. 2B), and after 2 hours (FIG. 2C).
[0040] FIGS. 3A-3B are polar area charts to show the complete experience
in each
category for a Formulation A (FIG. 3A) and a Formulation C (FIG. 3B).
[0041] FIG. 4A is a graph depicting intoxication responses for the
cannabis
formulations.
[0042] FIG. 4B is a graph depicting the best fit for intoxication model
for the
cannabis formulations.
[0043] FIG. 4C is a graph depicting blood alcohol concentration over time
for
comparison to intoxication by cannabis formulations.
[0044] FIG. 5 is a graph of intoxication level over time for a
participant with
cannabis tolerance administered a 6 mg dose of a formulation using Bakerstreet
strain
without terpenes.
[0045] FIG. 6 is a graph of intoxication level over time for a
participant with
cannabis tolerance administered a 12 mg dose of a formulation using Penelope
strain with
terpenes.
[0046] FIG. 7 is a graph of intoxication level over time for a
participant with
cannabis tolerance administered a 16 mg dose of a formulation using Penelope
strain with
terpenes and esters.
8
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[0047] FIG. 8 is a graph of intoxication level over time for a
participant without
cannabis tolerance administered a 12 mg dose of a formulation using Penelope
strain with
terpenes and esters.
[0048] FIG. 9A is a polar area chart showing the sober level of
intoxication for a
6 mg dose of a formulation using Bakerstreet strain without terpenes.
[0049] FIG. 9B is a polar area chart showing the elevated intoxicated
level of
intoxication for a 6 mg dose of a formulation using Bakerstreet strain without
terpenes.
[0050] FIG. 9C is a polar area chart showing the intoxicated level of
intoxication
for a 6 mg dose of a formulation using Bakerstreet strain without terpenes.
[0051] FIG. 10A is a polar area chart showing the sober level of
intoxication for a
12 mg dose of a formulation using Penelope with terpenes and esters.
[0052] FIG. 10B is a polar area chart showing the elevated intoxicated
level of
intoxication for a 12 mg dose of a formulation using Penelope with terpenes
and esters.
[0053] FIG. 10C is a polar area chart showing the intoxicated level of
intoxication for
a 12 mg dose of a formulation using Penelope with terpenes and esters.
[0054] FIG. 11 is a graph showing the loss of cannabinoids for aqueous
solutions
made using water-soluble formulations of the present disclosure with various
different
antioxidants, surfactants and emulsifiers.
[0055] FIG. 12A is a graph showing the amount of THC remaining in a first
beverage
prepared using a water-soluble formulation either having or not having sucrose
monoester.
[0056] FIG. 12B is a graph showing the amount of THC remaining in a first
beverage
prepared using a water-soluble formulation either having or not having sucrose
monoester.
[0057] FIG. 13A is a graph showing THC stability data for beverages with
varying
oxygen concentrations prepared using water-soluble formulations of the present
disclosure.
[0058] FIG. 13B is a graph showing CBD stability data for beverages with
varying
oxygen concentrations prepared using water-soluble formulations of the present
disclosure.
9
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[0059] FIG. 14 is an image showing the clarity/turbidity of aqueous
solutions
prepared with water-soluble formulations of the present disclosure with and
without sucrose
monoester.
[0060] FIG. 15 is an image showing a gummy product containing a water-
soluble
formulation of the present disclosure.
[0061] FIG. 16 is an image showing a gummy product containing a water-
soluble
formulation of the present disclosure after 1 week at 25 C.
[0062] FIG. 17A is a graph showing the THC and CBD concentration a black
tea
beverage at 0, 20, 40, 60, 120 and 240 seconds after steeping.
[0063] FIG. 17B is a graph showing the THC and CBD concentration a white
tea
beverage at 0, 20, 40, 60, 120 and 240 seconds after steeping.
[0064] FIG. 17C is a graph showing the THC and CBD concentration a herbal
tea
beverage at 0, 20, 40, 60, 120 and 240 seconds after steeping.
[0065] FIG. 17D is a graph showing the THC and CBD concentration a green
tea
beverage at 0, 20, 40, 60, 120, 240 and 320 seconds after steeping.
DETAILED DESCRIPTION
[0066] Unless defined otherwise, all technical and scientific terms used
herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which the
disclosure belongs. Although any methods and materials similar to or
equivalent to those
described herein can be used in the practice or testing of the present
disclosure, the
suitable methods and materials are described below.
[0067] The present disclosure is generally directed to water-soluble
formulations,
methods for their preparation, and use thereof. The formulations are suitably
in a nontoxic
consumable liquid form or a dispersible powder form. The formulations may also
be
absorbed, sprayed or otherwise applied into or onto a solid material (e.g. a
tea bag).
Suitably, embodiments of the formulations disclosed herein provide stability,
solubility in
water, have minimal flavour and odor, are calorie-free, and are natural in
origin. In some
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
embodiments, the formulations can contain flavour, odor, and/or calories if
desired,
particularly when comprised in or used for the preparation of a beverage or
foodstuff
[0068] The formulations of the present disclosure include a cannabinoid
or a
cannabis-derived compound, and in particular, may include a cannabinoid
distillate and/or a
cannabinoid isolate. Cannabis has been used in beverage preparations for
years. Most of
the historical cannabis beverages were prepared by boiling or grinding
cannabis leaves,
combining with water, milk, alcohol, or another biocompatible matrix or
beverage liquid and,
optionally, mixing with herbal or other plant-based compositions to form the
final
consumable.
[0069] The present disclosure provides improved formulations for
cannabinoids and
cannabis-derived compounds (e.g. cannabis concentrate, terpenes, etc.). As
shown herein,
the formulations of the present disclosure comprising cannabinoids, a carrier
oil, an
emulsifier, and a glycerin-based carrier surfactant are highly soluble in
water or a beverage
(e.g. Examples 1-7 and 9-10). Thus, the present disclosure provides convenient
water-soluble formulations of cannabinoids that may be readily used in the
preparation of
beverages and foodstuffs (see further Example 8 relating to a gummy product).
[0070] The water-soluble formulations of the present disclosure show high
emulsion
stability evidenced, for example, by the clarity of water-soluble formulations
and resultant
products such as beverages (e.g. Example 1-2, 5 and 7), as well as the
stability of the
products and cannabinoids therein (e.g. Examples 2, 6 and 10).
[0071] The water-soluble formulations of the present disclosure, and in
particular
the water-soluble formulations comprising a sucrose monoester, were found
suitable for
addition to foodstuffs and beverages. In particular, the water-soluble
formulations of the
present disclosure were capable of preparing beverages that are shelf stable
for extended
periods of time (e.g. 3 months accelerated stability at 40 C is roughly
representative of
1-year stability at room temperature (e.g. Example 10). The calculated THC and
CBD loss
at 3-months accelerated testing for beverages 1, 2 and 3 in Example 10 was
only 3.64%,
15.11% and 8.71%, respectively. Thus, the water-soluble formulations of the
present
disclosure are capable of providing excellent cannabinoid stability in
beverages.
[0072] The water-soluble formulations of the present disclosure, and in
particular
the water-soluble formulations comprising a sucrose monoester, were also
advantageous in
11
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
preparing transparent, translucent and/or clear beverages over a broad range
of different
flavour, colour and ionic conditions. For example, as shown in Example 7,
across all
beverages studied, water-soluble formulations of the present disclosure
comprising both
soy lecithin and sucrose monoester exhibited a turbidity of less than 5.0 NTU.
[0073] The water-soluble formulations of the present disclosure are also
advantageous in respect of their preparation. For example, traditional methods
of
emulsification make use of high shear homogenizers or sonication-based methods
that are
unable to produce highly uniform particle sizes leading to cloudy, unstable
emulsions.
Further, while spontaneous formation of cannabinoid containing microemulsions
is reported
in the literature, these previous methods necessarily used very high
concentrations of
artificial emulsifiers and were not always fully dilutable, leading to soapy,
cloudy, expensive
and unpalatable formulations. Additionally, water-soluble cannabinoids may
also be
produced by molecular encapsulation (i.e., in cyclodextrins and modified
starches), but
these formulations are usually cloudy and use unnatural ingredients. The
presently
disclosed water-soluble formulations are generally capable of avoiding such
formulation
challenges.
[0074] Accordingly, the present disclosure provides convenient water-
soluble
formulations of cannabinoids that may be readily used in the preparation of
beverages and
foodstuffs, which are capable of producing beverage products that are
transparent,
translucent and/or clear and that are shelf stable for extended periods of
time.
[0075] Individually and separately, these exemplary improvements produce
advantageous formulations and dosage forms, and, at times, the combinations of
ingredients can provide synergistic beneficial effects on preparation,
storage, distribution
and/or end use of the formulations. Further improvements are described herein
or will
become evident from the present disclosure.
[0076] WATER-SOLUBLE FORMULATIONS
[0077] Generally, the water-soluble formulations of the present
disclosure include a
cannabinoid or cannabis-derived compound (e.g., cannabinoid distillate and/or
cannabinoid
isolate), a surfactant, and an emulsifier. In a more particular embodiment,
the water-soluble
formulations further comprise a carrier. Also in a more particular embodiment,
the
surfactant in the water-soluble formulations is a glycerin-based carrier
surfactant.
12
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[0078] The water-soluble formulations herein serve as a convenient water-
soluble
dosage form of cannabinoids for use in beverages, topicals and foods. The
formations are
suitably in a nontoxic consumable liquid or solid form. Suitably, the
formulations provide
stability, solubility in water, have minimal flavour and odor, are calorie-
free, and are natural
in origin.
[0079] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or a cannabis-derived compound; an
emulsifier; and
a glycerin-based carrier surfactant. In an embodiment, the water-soluble
formulation further
comprises a carrier oil.
[0080] In another embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or a cannabis-derived compound, a carrier
oil, a
surfactant, and an emulsifier, wherein the water-soluble formulation is
transparent,
translucent, or pearlescent when mixed with an aqueous solution. In a
particular
embodiment, the water-soluble formulation is transparent and/or clear.
[0081] As used herein, "water-soluble" has its ordinary meaning in
referring to the
ability of a formulation or component thereof to dissolve when the object is
placed in water.
For example, when the object is mixed with water at room temperature or
slightly above
(e.g. about 25 C to about 50 C).
[0082] As used herein, "transparent" has its ordinary meaning of having
the
property of allowing light to pass through without appreciable scattering.
Transparency may
be measured by a transparency meter (also called a clarity meter) and is
identified by an
object's total transmittance, which is the ratio of transmitted light to the
incident light. In an
embodiment herein, transparent means a total transmittance of between about
80% and
100%. In an embodiment, transparent means a total transmittance of about 90%,
about
91%, about 92%, about 93%, about 94%, about 95%, about 96%, about 97%, about
98%,
or about 99%. In an embodiment, transparent means a total transmittance of at
least 94%.
Visually, the skilled person will appreciate that an object is transparent if
it is easy to see
through without significant distortion.
[0083] Transparency may be equated with clarity (e.g. "clear" or
"substantially
clear"). As used herein, the term "substantially clear" means that the visible
turbidity or
cloudiness is very slight (e.g. barely visible to the naked-eye). Turbidity or
cloudiness may
13
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
be measured by a number of means known to one of ordinary skill in the art,
including by
refractometry, nephelometry, chromatography or spectrometry. In an embodiment,
turbidity
may be measured by a Nephelometer to determine the Nephelometric Turbidity
Units
(NTU). In an embodiment, "substantially clear" means that the liquid has an
NTU of less
than 50. In an embodiment, "substantially clear" means that the liquid has an
NTU of
or '10.
[0084] As used herein, the term "clear" means that there is no visible
turbidity or
cloudiness to the naked-eye. In an embodiment, "clear" means that the liquid
has an NTU
of In an embodiment, "clear" means that the liquid has an NTU of about 5,
about 4,
about 3, about 2, about 1 or less (e.g. zero).
[0085] As opposed to transparent objects and liquids which generally
appear clear,
as used herein the term "translucent" means that the objects permits the
passage of light,
but does not appear clear. Translucent objects typically diffuse light such
that objects
cannot be observed clearly on the opposite side. "Translucent" is defined by
either
transmittance or reflectance measurement modes (see HunterLab definition,
which is
available at www.hunterlab.com/transluceent-beverage-color-measurement).
[0086] As used herein, "pearlescent" has its ordinary meaning of having a
pearly
lustre or sheen. Pearlescent may, for example, be used herein to describe a
water-soluble
formulation that is a powder. In other embodiments, pearlescent may describe
the
water-soluble formulation as an emulsion, having a shiny lustre when placed in
an aqueous
formulation.
[0087] Cannabis
[0088] Cannabis is a genus of flowering plant in the family Cannabaceae.
The
number of species within the genus is disputed. Three species may be
recognized,
Cannabis sativa, Cannabis indica and Cannabis ruderalis. C. ruderalis may be
included
within C. sativa; or all three may be treated as subspecies of a single
species, C. sativa.
The genus is indigenous to central Asia and the Indian subcontinent.
[0089] Cannabis has long been used for hemp fiber, hemp oils, medicinal
purposes,
and as a recreational drug. Industrial hemp products are made from cannabis
plants
selected to produce an abundance of fiber. To satisfy the UN Narcotics
Convention, some
14
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
cannabis strains have been bred to produce minimal levels of
tetrahydrocannabinol (THC),
the principal psychoactive constituent. Many additional plants have been
selectively bred to
produce a maximum level of THC. Various compounds, including hashish and hash
oil, may
be extracted from the plant.
[0090] Within naturally occurring and man-made hybrids, cannabis contains
a vast
array of compounds. Three compound classes are of interest within the context
of the
present disclosure, although other compounds can be present or added to the
compositions
to augment the experience of a given recreational consumer and medical or
medicinal
patient or patient population. Those classes include cannabinoids, terpenes
and
flavonoids.
[0091] There are many ways of growing cannabis, some of which are
natural, and
some are carefully designed by humans, and they will not be recited here.
However, one of
ordinary skill in the art of cannabis production will typically place a
cannabis seed or cutting
into a growth media such as soil, manufactured soil designed for cannabis
growth or one of
many hydroponic growth media. The cannabis seed or cutting is then provided
with water,
light and, optionally, a nutrient supplement. t times, the atmosphere and
temperature are
manipulated to aid in the growth process. Typically, the humidity, air to
carbon dioxide gas
ratio and elevated temperature, either by use of a heat source or waste heat
produced by
artificial light, are used. On many occasions ventilation is carefully
controlled to maintain
the conditions described above within an optimal range to both increase the
rate of growth
and, optionally, maximize the plant's production of the compounds, which
comprise the
compositions of the disclosure. It is possible to control lighting cycles to
optimize various
growth parameters of the plant.
[0092] Given the number of variables and the complex interaction of the
variables, it
is possible to develop highly specific formulas for production of cannabis
which lead to a
variety of desired plant characteristics. The present disclosure is applicable
to use with
such inventive means for growing cannabis as well as any of the variety of
conventional
methods.
[0093] Cannabis sativa is an annual herbaceous plant in the Cannabis
genus. It is a
member of a small, but diverse family of flowering plants of the Cannabaceae
family. It has
been cultivated throughout recorded history, used as a source of industrial
fiber, seed oil,
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
food, recreation, religious and spiritual moods and medicine. Each part of the
plant is
harvested differently, depending on the purpose of its use. The species was
first classified
by Carl Linnaeus in 1753.
[0094] Cannabis indica, formally known as Cannabis sativa forma indica,
is an
annual plant in the Cannabaceae family. A putative species of the genus
Cannabis.
[0095] Cannabis ruderalis is a low-THC species of Cannabis, which is
native to
Central and Eastern Europe and Russia. It is widely debated as to whether C.
ruderalis is a
sub-species of Cannabis sativa. Many scholars accept Cannabis ruderalis as its
own
species due to its unique traits and phenotypes that distinguish it from
Cannabis indica and
Cannabis sativa.
[0096] Cannabis-Derived Compounds
[0097] As used herein, the term "cannabis-derived compound" refers to a
compound found in a cannabis plant, such as for example a compound that has
been
obtained and/or extracted from cannabis. The method of conversion typically
involves
harvesting and, optionally, one of the extraction, fractionation, or
purification steps
described herein. More typically a combination of two or more such steps, more
typically
yet 2, 3, 4, 5, 6, 7, 8, 9, or 10 individual steps described herein. More
typically still a
combination of separating the cannabis from the media in which it is grown,
drying to
reduce the water content, grinding to form a power, extraction and,
optionally, a
fractionation or purification step is performed.
[0098] More typically, the process comprises separation of the cannabis-
derived
compound from the media in which it is grown followed by 2, 3, 4, or 5 steps
as described
above are performed, more typically yet, 2, 3, or 4 steps are performed.
[0099] Suitably, the cannabis-derived compound is separated from the
media in
which it is grown and first dried and then ground. Once in the ground state,
it is, optionally,
sieved and finally the resins of the plant are extracted. These resins
comprise the
cannabis-derived compounds used in the formulations of the disclosure.
Remembering that
optional fractionation and purification steps are possible, the formulations
of the disclosure
may have compounds removed from the resin.
16
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00100] Some steps that can optionally be performed to improve the utility
of the
compositions include addition, removal or control of the absolute
concentrations of
compounds comprising the compositions, direct breeding of cannabis strains,
genetic
manipulation by methods known in the field of molecular biology such as gene
insertion or
deletion, lyophilization and the development of polyploid variants by use of
compounds
such as colicine. For example, it is particularly suitable to further refine
the resin by
molecular distillation to produce a highly concentrated distillate and/or
isolate that is
substantially free from impurities that might alter taste or smell. As used
herein,
"substantially free" refers to a compound or composition including less than
1% by weight
impurities, suitably less than 0.5% by weight, more suitably less than 0.1% by
weight, and
even more suitably 0% by weight of an ingredient or component.
[00101] Suitable cannabis-derived compounds include, for example and
without
limitation, cannabis concentrate, cannabis extract, cannabis resin,
cannabinoid distillate,
cannabinoid isolate, cannabinoids, terpenes, and combinations thereof. Herein,
the term
"cannabinoid distillate" is used interchangeably with "cannabis distillate".
Also, herein,
"cannabinoid isolate" is used interchangeably with "cannabis isolate". Both a
cannabinoid
distillate and a cannabinoid isolate comprise one or more cannabinoids. In
contrast, in
select embodiments, a "cannabis concentrate" or "cannabis extract" may not
contain
cannabinoids (e.g. a terpene distillate).
[00102] In an embodiment, the cannabis-derived compound is a cannabinoid.
[00103] In an embodiment, the cannabis-derived compound is a terpene.
[00104] In an embodiment, the cannabis-derived compound is a cannabinoid
distillate or a cannabinoid isolate. In select embodiments, suitable
cannabinoid distillates
and isolates for use in the formulations of the present disclosure include
distillates and
isolates of one or more of the following cannabinoids: A9-
tetrahydrocannabinolic acid, A8-
tetrahydrocannabinoilic acid, A8-tetrahydrocannabinol, cannabidiolic aicd,
cannabichromenic acid, A9-tetrahydrocannabivarinic acid, A9-
tetrahydrocannabivarin,
cannabigerivarin, cannabidivarin, cannabichromevarin, 11-hydroxy-A9-
tetrahydrocannabinol, and 11-nor-9-carboxy-A9-tetrhydrocannabinol).
17
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00105] Cannabinoids
[00106] The compositions of the present disclosure comprise a cannabinoid
or a
cannabis-derived compound. The cannabis-derived compound may be a cannabinoid,
or
may be an alternative compound derived from cannabis, such as a terpene.
[00107] In an embodiment, the compositions comprise a cannabinoid. The
compositions may comprise a single cannabinoid (e.g. THC, CBD or another
cannabinoid)
or may comprise any combination of two or more cannabinoids (e.g. CBD and
THC).
[00108] As used herein, the term "cannabinoid" refers to a compound
belonging to a
class of secondary compounds commonly found in plants of genus cannabis, but
also
encompasses synthetic and semi-synthetic cannabinoids.
[00109] In an embodiment, a cannabinoid is one of a class of diverse
chemical
compounds that acts on cannabinoid receptors such as CB1 and CB2 in cells that
alter
neurotransmitter release in the brain. Ligands for these receptor proteins
include the
endocannabinoids (produced naturally in the body by animals), the
phytocannabinoids
(found in cannabis and some other plants), and synthetic cannabinoids
(manufactured
artificially as set forth above). The most notable cannabinoid of the
phytocannabinoids is
tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis.
Cannabidiol
(CBD) is another cannabinoid that is a major constituent of the plant. There
are at least
113 different cannabinoids isolated from cannabis, exhibiting varied effects.
[00110] In one embodiment, the cannabinoid is a compound found in a plant,
e.g., a
plant of genus cannabis, and is sometimes referred to as a phytocannabinoid.
In one
embodiment, the cannabinoid is a compound found in a mammal, sometimes called
an
endocannabinoid. In one embodiment, the cannabinoid is made in a laboratory
setting,
sometimes called a synthetic cannabinoid. In one embodiment, the cannabinoid
is derived
or obtained from a natural source (e.g. plant) but is subsequently modified or
derivatized in
one or more different ways in a laboratory setting, sometimes called a semi-
synthetic
cannabinoid.
[00111] Synthetic cannabinoids and semisynthetic cannabinoids encompass a
variety of distinct chemical classes, for example and without limitation: the
classical
cannabinoids structurally related to THC, the non-classical cannabinoids
(cannabimimetics)
18
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
including the aminoalkylindoles, 1,5-diarylpyrazoles, quinolines, and
arylsulfonamides as
well as eicosanoids related to endocannabinoids.
[00112] In many cases, a cannabinoid can be identified because its
chemical name
will include the text string "*cannabi*". However, there are a number of
cannabinoids that
do not use this nomenclature, such as for example those described herein.
[00113] Within the context of this disclosure, where reference is made to
a particular
cannabinoid, each of the acid and/or decarboxylated forms are contemplated as
both single
molecules and mixtures. In addition, salts of cannabinoids are also
encompassed, such as
salts of cannabinoid carboxylic acids.
[00114] As well, any and all isomeric, enantiomeric, or optically active
derivatives are
also encompassed. In particular, where appropriate, reference to a particular
cannabinoid
incudes both the "A Form" and the "B Form". For example, it is known that THCA
has two
isomers, THCA-A in which the carboxylic acid group is in the 1 position
between the
hydroxyl group and the carbon chain (A Form) and THCA-B in which the
carboxylic acid
group is in the 3 position following the carbon chain (B Form).
[00115] Examples of cannabinoids include, but are not limited to,
Cannabigerolic
Acid (CBGA), Cannabigerolic Acid monomethylether (CBGAM), Cannabigerol (CBG),
Cannabigerol monomethylether (CBGM), Cannabigerovarinic Acid (CBGVA),
Cannabigerovarin (CBGV), Cannabichromenic Acid (CBCA), Cannabichromene (CBC),
Cannabichromevarinic Acid (CBCVA), Cannabichromevarin (CBCV), Cannabidiolic
Acid
(CBDA), Cannabidiol (CBD), A6-Cannabidiol (A6-CBD), Cannabidiol
monomethylether
(CBDM), Cannabidiol-C4 (CBD-C4), Cannabidivarinic Acid (CBDVA), Cannabidivarin
(CBDV), Cannabidiorcol (CBD-C1), Tetrahydrocannabinolic acid A (THCA-A),
Tetrahydrocannabinolic acid B (THCA-B), Tetrahydrocannabinol (THC or A9-THC),
A8-tetrahydrocannabinol (A8-THC), trans-A10-tetrahydrocannabinol (trans-L,10-
THC),
cis-A10-tetrahydrocannabinol (cis-L,10-THC), Tetrahydrocannabinolic acid C4
(THCA-C4),
Tetrahydrocannbinol C4 (THC C4), Tetrahydrocannabivarinic acid (THCVA),
Tetrahydrocannabivarin (THCV), M-Tetrahydrocannabivarin (A8-THCV),
A9-Tetrahydrocannabivarin (A9-THCV), Tetrahydrocannabiorcolic acid (THCA-C1),
Tetrahydrocannabiorcol (THC-C1), M-cis-iso-tetrahydrocannabivarin,
A8-tetrahydrocannabinolic acid (A8-THCA), A9-tetrahydrocannabinolic acid (A9-
THCA),
19
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
Cannabicyclolic acid (CBLA), Cannabicyclol (CBL), Cannabicyclovarin (CBLV),
Cannabielsoic acid A (CBEA-A), Cannabielsoic acid B (CBEA-B), Cannabielsoin
(CBE),
Cannabinolic acid (CBNA), Cannabinol (CBN), Cannabinol methylether (CBNM),
Cannabinol-C4 (CBN-C4), Cannabivarin (CBV), Cannabino-C2 (CBN-C2),
Cannabiorcol
(CBN-C1), Cannabinodiol (CBND), Cannabinodivarin (CBDV), Cannabitriol (CBT),
11-
hydroxy-A9-tetrahydrocannabinol (11-0H-THC), 11-nor-9-carboxy-A9-
tetrahydrocannabinol, Ethoxy-cannabitriolvarin (CBTVE), 10-Ethoxy-9-hydroxy-
A6a-
tetrahydrocannabinol, Cannabitriolvarin (CBTV), 8,9-Dihydroxy-A6a(10a)-
tetrahydrocannabinol (8,9-Di-OH-CBT-05), Dehydrocannabifuran (DCBF),
Cannbifuran
(CBF), Cannabichromanon (CBCN), Cannabicitran (CBT), 10-Oxo-A6a(10a)-
tetrahydrocannabinol (OTHC), A9-cis-tetrahydrocannabinol (cis-THC),
Cannabiripsol
(CBR), 3,4,5,6-tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propy1-2,6-
methano-2H-1-
benzoxocin-5-methanol (OH-iso-HHCV), Trihydroxy-delta-9-tetrahydrocannabinol
(tri0H-
THC), Yangonin, Epigallocatechin gallate, Dodeca-2E, 4E, 8Z, 10Z-tetraenoic
acid
isobutylamide, hexahydrocannibinol, and Dodeca-2E,4E-dienoic acid
isobutylamide.
[00116] In an embodiment, the cannabinoid is a cannabinoid dimer. The
cannabinoid may be a dimer of the same cannabinoid (e.g. THC¨THC) or different
cannabinoids. In an embodiment, the cannabinoid may be a dimer of THC,
including for
example cannabisol.
[00117] As used herein, the term "THC" refers to tetrahydrocannabinol.
"THC" refers
to and is used interchangeably herein with "A9-THC".
[00118] In an embodiment, the cannabinoid is THC (A9-THC), A8-THC, trans-
A10-
THC, cis- A1O-THC, THCA, THCV, 8 THCA, A9-THCA, A8-THCV, A9-THCV, THCVA,
CBD, CBDA, CBDV, CBDVA, CBC, CBCA, CBCV, CBCVA, CBG, CBGA, CBGV, CBGVA,
CBN, CBNA, CBNV, CBNVA, CBND, CBNDA, CBNDV, CBNDVA, CBE, CBEA, CBEV,
CBEVA, CBL, CBLA, CBLV, CBLVA, CBT, or any combination thereof, each having
the
following exemplary structural formula:
THC THCA THCV
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
.....o
. . '-.....'"%........-2.-'`,H
H 1
________ 0
r ,r
c...:
,7.D....¨..,...,,,,_,......õ..... ...,,1 1
THCVA A8-THC A8-THCV
__________ I: t
,,.. ow
= ....i...,,
C"' / ....."--,,-' '''..... ----=-.../....."'", ..,;,,c,
,..F''"N....""'''',/''''', ........-'''...
0.,
A9-THCV CBD CBDA
[ 1
i 1
HO HO
CBDV CBDVA CBC
---,.._.c..-----õ--->( -----,"----------">,
0
(....:5L
CBCA CBCV CBCVA
CH OH 0
',....
CBG CBGA
21
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
OH 0
OH
II _.....õ
HO
CBGV CBGVA
--,.....,
OH
6. .. 1
,....
._ )...,_ , ..... ..
1 1
/4õ, , ....0 .., ......., 0
CBN CBNA CBNV (or CBV)
--.,
OH 0 nõ OH 0
I
I'
OH OH
.....'= -
,.............F.,......
0
HO FIO
CBNVA CBND CBN DA
J.,
i
......."'"i-----r"--k)Lon
I l ........[.. j..,,,..
,..,..
HD 11.3
CBNDV CBNDVA CBL
e
õ ,...... ...._........, ,...µ ..
5 OF 0
-41-'-
,,,,a.f.,,,,,......,.....i."'",,
CBLA CBLV CBLVA
22
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
11,
I
CBE CBEA CBEV
Q.
II
0 0
CBEVA trans-A10-THC cis- A10- THC
OH
OH
CBT
[00119] In an embodiment, the cannabinoid is THC, CBD, CBN, CBG, CBGA, or
any
combination thereof.
[00120] Tetrahydrocannabinol (THC) refers to a psychotropic cannabinoid
and is the
principal psychoactive constituent of cannabis. Its chemical name is (¨)-trans-
A9-
tetrahydrocannabinol and the term "THC" is used to refer to isomers as well.
[00121] Can nabidiol (CBD) is one of the active cannabinoids identified
in cannabis.
It is a major phytocannabinoid, by some accounts making up to 40% of the
plant's extract.
CBD does not appear to have any intoxicating effects such as those caused by
THC in
marijuana, but may have effects on anxiety, depression and have an anti-
psychotic effect,
and have effects on other comorbidities. In some instances, the comorbidities
are related
23
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
to disorders described herein such as pain and post-traumatic stress disorders
commonly
referred to as "PTSD."
[00122] Can nabinol (CBN) is thought to be a non-psychoactive cannabinoid
found
only in trace amounts in Cannabis and can be produced via oxidative
degradation of THCA
and THC. Pharmacologically relevant quantities are formed as a metabolite of
tetrahydrocannabinol (THC). CBN acts as a partial agonist at the CB1
receptors, but has a
higher affinity to CB2 receptors, however; with lower affinities in comparison
to THC.
Degraded or oxidized cannabis products, such as low-quality baled cannabis and
traditionally produced hashish, are high in CBN, but modern production
processes have
been alleged to minimize the formation of CBN. Cannabinol has been shown to
have
analgesic properties. Unlike other cannabinoids, CBN does not stem from
cannabigerol
(CBG).
[00123] Can nabigerol (CBG) is thought to be a non-intoxicating
cannabinoid found in
the Cannabis genus of plants. CBG is the non-acidic form of cannabigerolic
acid (CBGA),
the parent molecule ("mother cannabinoid") from which many other cannabinoids
are
obtained. CBG has been found to act as a high affinity a2-adrenergic receptor
agonist,
moderate affinity 5-HT1A receptor antagonist, and low affinity CB1 receptor
antagonist. It
also binds to the CB2 receptor as an antagonist.
[00124] Can nabigerolic Acid (CBGA or CBG-A) is the alleged primordial
phyto-
cannabinoid. It is the alleged compound in cannabis from which all the plant's
other
naturally occurring cannabinoids are formed; without CBGA, the cannabis plant
cannot
produce its most useful compounds.
[00125] In an embodiment, the cannabinoid is THC (A9-THC), A8-THC, trans-
1O-
THC, cis-L,10-THC, CBD, CBC, CBG, CBL, CBN, CBT, or any combination thereof.
[00126] In an embodiment, the cannabinoid is THC or CBD, or a combination
thereof.
[00127] In an embodiment, the cannabinoid is THC.
[00128] In an embodiment, the cannabinoid is CBD.
24
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00129] In an embodiment, the water-soluble formulation may include up to
10% by
weight cannabinoid or cannabis-derived compound (e.g., cannabinoid distillate
and/or
isolate). In select embodiments, the water-soluble formulation may include
from about
0.01% by weight to about 10% by weight, more particularly from about 0.1% by
weight to
about 8% by weight, even more particularly from about 0.5% by weight to about
5% by
weight, and even more particularly still from about 1.0% by weight to about 3%
by weight
of cannabinoid or cannabis-derived compound. In select embodiments, the water-
soluble
formulation may include about 1%, about 2%, about 3%, about 4%, about 5%,
about 6%,
about 7%, about 8%, about 9%, about 10% by weight of cannabinoid or cannabis-
derived
compound. In one particularly suitable embodiment, the water-soluble
formulation
includes about 10 mg/mL total cannabinoids.
[00130] In select embodiments, the cannabis-derived compound includes one
or
more cannabinoid distillates and isolates, and in particular, the cannabis-
derived compound
includes CBD distillates and/or isolates; THC distillates and/or isolates; or
a combination of
THC and CBD distillates and/or isolates. In some embodiments, the cannabis-
derived
compounds include THC distillates and/or isolates.
[00131] In select embodiments of the water-soluble formulations disclosed
herein,
the cannabinoids may be introduced in the form of pure cannabinoids or as a
cannabis
concentrate. As used herein, "pure cannabinoids" is meant to refer to a single
cannabinoid
or a mixture of different cannabinoids that is free of other compounds. The
pure
cannabinoids may be contained in solution in a diluent or other medium, or may
be a liquid
or solid form of the pure cannabinoids absent any diluent. In an embodiment,
the pure
cannabinoids are synthetic or semi-synthetic cannabinoids. As used herein,
"cannabis
concentrate" is meant to refer a concentrated composition of cannabinoids,
such a
cannabinoid extract from a plant. Non-limiting exemplary embodiments of a
cannabis
concentrate include a cannabinoid distillate, a cannabinoid isolate, a
cannabis oil, or any
other type of extract containing one or more cannabinoids
[00132] As described in greater detail elsewhere herein, in addition to
the
cannabinoids or cannabis-derived compounds, the formulations of the present
disclosure
may also include additives, such as for example terpenes, terpenoids,
flavonoids, and the
like and combinations thereof.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00133] In an embodiment, the additives (e.g. terpenes and/or flavonoids)
are
independently or in combination derived from natural sources and are selected
to be stable
in the selected formulations, dosage forms, beverages or foodstuffs herein.
More suitably
still, in some embodiments, the water-soluble formulation or beverage of the
present
disclosure with additives is clear, stable at room temperature and capable of
being provided
in both bulk and unit dose forms. More suitably yet, in some embodiments, the
additives
may act synergistically in the formulations to provide desirable production,
storage,
distribution or end use.
[00134] Another suitable embodiment of the water-soluble formulations,
dosage
forms, beverages or foodstuffs of the present disclosure provides fast onset
of biological
effects of the cannabinoids in human or animal consumers or subjects.
[00135] Carrier Oils
[00136] In select embodiments, the water-soluble formulations of the
present
disclosure include at least one carrier oil to reduce the viscosity of the
cannabinoids or
cannabis-derived compounds and/or provide other suitable properties. Further,
at least in
the case of solid cannabinoids or cannabis-derived compounds (e.g.,
crystalline CBD), the
carrier oil aids in its dissolution and allows for emulsification of the
cannabinoid and
cannabis-derived compounds.
[00137] Thus, in an embodiment, the present disclosure provides a water-
soluble
formulation comprising a cannabinoid or a cannabis-derived compound; a carrier
oil; an
emulsifier; and a glycerin-based carrier surfactant. Water-soluble
formulations comprising a
carrier oil may represent preferred embodiments, for example having regard to
the
disclosure herein.
[00138] In an embodiment, the carrier oil is an "oily medium". By "oily
medium" it is
meant to refer to a medium capable of dissolving lipophilic or hydrophobic
compounds,
such as cannabinoids. Particularly suitable carrier oils include natural oils
as known in the
art, for example, edible vegetable oils. In some alternative embodiments, the
carrier oils
can include synthetic edible oils, for example, hydrogenated vegetable oils,
medium chain
triglyceride (MCT) oils, and the like and combinations thereof.
26
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00139] A non-limiting list of exemplary carrier oils includes ethanol,
isopropanol,
dimethyl sulfoxide, acetone, ethyl acetate, pentane, heptane, diethyl ether,
medium-chain
triglycerides (MCT oil), medium-chain fatty acids (e.g., caproic acid,
caprylic acid, capric
acid, lauric acid), long-chain triglycerides (LCT oil), long-chain fatty acids
(e.g., myristic
acid, palmitic acid, stearic acid, arachidic acid, linoleic acid),
monoglycerides
(e.g. glyceryl monostearate, glyceryl hydroxystearate, glyceryl monoleate,
winterized
glyceryl monoleate, monolaurin, glyceryl monolinoleate, MaisineO CC,
PeceolTm), coconut
oil, corn oil, canola oil, olive oil, avocado oil, vegetable oil, flaxseed
oil, palm oil, palm
kernel oil, peanut oil, sunflower oil, rice bran oil, safflower oil, jojoba
oil, argan oil,
grapeseed oil, castor oil, wheat germ oil, peppermint oil, hemp oil, sesame
oil, terpenes,
terpenoids, beta-myrcene, linalool, a-pinene, beta-pinene, beta-caryophyllene,
caryophyllene oxide, a-humulene, nerolidol, D-limonene, L-limonene, para-
cymene,
eugenol, farnesol, geraniol, phytol, menthol, terpineol, a-terpineol,
benzaldehyde, hexyl
acetate, methyl salicylate, eucalyptol, ocimene, terpinolene, a-terpinene,
isopulegol,
guaiol, a-bisabolol and combinations thereof. Other suitable carrier oils
include Labrasol,
Labrafac Lipophile WL 1349, Labrafil M1944, Peceol, Plurol Oliqiue CC 497,
Transcutol
HP, Tween 80, Gelucire 48/16, Vitamin E TPGS, and combinations thereof. In a
particularly suitable embodiment, the carrier oil is MaisineO CC.
[00140] In an embodiment, a combination of carrier oils may be used in the
water-soluble formulations. When more than one carrier is used, they may be
used at
any amount relative to the other. In an embodiment, the first carrier oil and
the second
carrier oil may be used at a ratio between 10:1 and 1:10 by weight to each
other. In an
embodiment, the two carrier oils may be used at about a ratio of 3:1, 2:1,
1:1, 1:2 or 1:3
by weight to each other. In an embodiment, the two carrier oils may be used at
about a
1:1 by weight ratio to each other.
[00141] In an embodiment, the water-soluble formulations of the present
disclosure
may include a ratio of carrier oil(s):cannabinoid or cannabis-derived compound
of
between 10:1 and 1:10 by weight. In an embodiment, the ratio of carrier
oil(s):cannabinoid or cannabis-derived may be about 3:1, 2:1, 1:1, 1:2 or 1:3
by weight.
In an embodiment, the ratio of carrier oil(s):cannabinoid or cannabis-derived
compound
may be about 1:1 by weight.
27
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00142] In an embodiment, the carrier oil may improve the stability of the
emulsion,
for example by preventing Ostwald ripening of the water-soluble formulation.
[00143] In an embodiment, the carrier oil may contribute to providing
rapid onset of
the cannabinoid or cannabis-derived compound in the water-soluble formulation
or a
beverage prepared therefrom. The carrier oil may improve the rate of
absorption and/or
onset of a medicinal, therapeutic and/or recreational effect of the
cannabinoids. In an
embodiment, the rapid onset occurs within 60 minutes, within 30 minutes, with
15 minutes, or less from administration of the water-soluble formulation to a
subject
(e.g. in the form of a beverage). The carrier oil may also improve the rate of
release of
the cannabinoids into a beverage to provide an improved medicinal, therapeutic
or
recreational effect.
[00144] In an embodiment, the carrier oil is comprised of monoglycerides.
The
monoglycerides may be of a single type (e.g. glyceryl monolinoleate) or may be
a mixture
of different types. The monoglycerides may include only the monoglyceride
ester, or may
include one or both of di- and triglycerides. In some embodiments, the
monoglyceride
fraction is predominant over the di- and triglyceride components. In some
embodiments,
the di- or triglyceride fractions may be predominant over the monoglycerides,
such as for
example in MaisineO CC. In an embodiment, the carrier oil is MaisineO CC. In
an
embodiment, the MaisineO CC contributes to rapid onset of the cannabinoids or
cannabis-derived compounds.
[00145] In select embodiments, the water-soluble formulations may include
up to
20% by weight carrier oil. In an embodiment, the formulations include from
about 0.01% by
weight to 10% by weight, more particularly from about 0.1% by weight to about
8% by
weight, even more particularly from about 0.5% by weight to about 5% by
weight, and even
more particularly still from about 1.0% by weight to about 3% by weight
carrier oil. In select
embodiments, the water-soluble formulation may include about 1%, about 2%,
about 3%,
about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, about 10% by
weight
carrier oil.
[00146] Emulsifiers
[00147] The water-soluble formations include one or more emulsifiers to
stabilize the
mixture of emulsified cannabinoids in the carrier oils described above, to
reduce the particle
28
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
size of the particles in the mixture (e.g. to about 50 nm) and/or to provide
other suitable
properties.
[00148] Any suitable emulsifier may be used. In an embodiment, the
emulsifier is an
ionic emulsifier. In an embodiment, the emulsifier is a non-ionic emulsifier.
In an
embodiment, the water-soluble formulations comprise two emulsifiers, where one
is an ionic
emulsifier and the other is a non-ionic emulsifier.
[00149] Without limitation, phospholipids can act as emulsifiers, enabling
oils to form
a colloid with water. Phospholipids are one of the components of lecithin,
which is found in
egg-yolks, as well as being extracted from soy beans (i.e., soy lecithin), and
is used as a
food additive in many products, and can be purchased as a dietary supplement.
Lysolecithins are typically used for water-oil emulsions like margarine, due
to their higher
HLB ratio.
[00150] Other particularly suitable emulsifiers include, for example,
members of the
ALCOLEC family of lecithins (e.g. ALCOLEC F-100, ALCOLEC EM, ALCOLEC S,
ALCOLEC BS, ALCOLEC HL, ALCOLEC EXTRA-A, ALCOLEC E 35, ALCOLEC E
60 or ALCOLEC HR), including deoiled soy lecithin, sucrose monoesters (e.g.
Habo
Monoesters P90, SE-50, SE-70, SE-110 or SE-150), GELUCIREO 48/16,
rhamnolipids,
LABRASOLO, PLUROLO Oliquie CC, alpha-tocopherol, and combinations thereof.
[00151] Other embodiments of emulsifiers may include, for example and
without
limitation, Vitamin E TPGS, Quillaja extract, PURITY GUM ULTRA, pectin (e.g.
citrus
pectin, sugar beet pectin, apple pectin, etc.), chitosan, Q-NATURALE TM, and
other like
compounds.
[00152] In an embodiment, the water-soluble formulations of the present
disclosure
comprise a soy lecithin as an emulsifier, alone or in combination with other
emulsifiers. In
an embodiment, the soy lecithin is ALCOLEC F-100 or ALCOLEC EM,
[00153] In an embodiment, the water-soluble formulations of the present
disclosure
comprise a sucrose monoester as an emulsifier, alone or in combination with
other
emulsifiers. The sucrose monoester may be sucrose monopalmitate, sucrose
monolaurate, sucrose monostearate, or any combination thereof. For any one of
these
29
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
embodiments, the sucrose monoester may comprise a small quantity of diester.
In a
particular embodiment, the sucrose monoester is sucrose monopalmitate.
[00154] In an embodiment, a combination of emulsifiers may be used in the
water-soluble formulations. When more than one emulsifier is used, they may be
used at
any amount relative to the other. In an embodiment, the first emulsifier and
the second
emulsifier may be used at a ratio between 20:1 and 1:20 by weight to each
other. In an
embodiment, the two emulsifiers may be used at about a 3:1, 2:1, 1:1, 1:2 or
1:3 by
weight ratio to each other. In an embodiment, the two emulsifiers may be used
at about a
1:1 by weight ratio to each other.
[00155] For example, a combination of emulsifiers may be used to
strengthen the
emulsion as compared to when one emulsifier is used alone. This may be
particularly
suitable for certain beverages or aqueous solutions, for example where
incompatibilities
arise between the ingredients of the aqueous solution and the emulsion system.
Emulsion
instability may arise, for example, due to incompatibilities of a single
emulsifier with different
pH conditions, different ionic conditions, different oxygen levels, and
different packaging
materials.
[00156] In an embodiment, the water-soluble formulation of the present
disclosure
includes two or more emulsifiers. In an embodiment, the emulsifiers are
selected from
lecithins and sucrose monoesters. In an embodiment, the water-soluble
formulations
comprise two emulsifiers, one selected from a lecithin and one selected from a
sucrose
monoester. In an embodiment, the two emulsifiers are a soy lecithin and a
sucrose
monopalmitate. In select embodiments, the lecithin and sucrose monoester are
combined
in a ratio of between 10:1 and 1:10 (w/w). In an embodiment, the lecithin and
sucrose
monoester are combined in a ratio of about 2:1 (w/w), about 1.5:1 (w/w), about
1:1 (w/w),
about (w/w), about 1:1.5 (w/w), or about 1:2 (w/w) of lecithin to SME. In an
embodiment,
the lecithin and sucrose monoester are combined in a ratio of about 1:1 (w/w).
[00157] As shown in Example 5, having two emulsifiers can improve
cannabinoid
stability and beverage clarity. In addition, particular combinations of
emulsifiers can
strengthen the emulsion and provide other benefits, such as wider ingredient
compatibility.
Thus, a single water-soluble formulation may be capable of being used in a
broader range
of products.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00158] Selection of an emulsifier may affect the shelf-life and
physicochemical
properties of the water-soluble formulation. Formulations stabilized by
surfactants or other
types of stabilizing agents such as phospholipids, amphiphilic proteins, or
polysaccharides,
have been developed to provide controlled release, improved entrapment
efficiency, and
protection from degradation.
[00159] In select embodiments, the water-soluble formulations may include
up to
10% by weight emulsifier. In an embodiment, the formulations include from
about 0.01% by
weight to 10% by weight, more particularly from about 0.1% by weight to about
8% by
weight, even more particularly from about 0.5% by weight to about 5% by
weight, and even
more particularly still from about 1.0% by weight to about 3% by weight
emulsifier. In select
embodiments, the water-soluble formulation may include about 1%, about 2%,
about 3%,
about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, about 10% by
weight
emulsifier.
[00160] In an embodiment, the water-soluble formulations of the present
disclosure
may include a ratio of emulsifier(s):cannabinoid or cannabis-derived compound
of
between 10:1 and 1:10 by weight. In an embodiment, the ratio of
emulsifier(s):cannabinoid or cannabis-derived may be about 3:1, 2:1, 1:1, 1:2
or 1:3 by
weight. In an embodiment, the ratio of emulsifier(s):cannabinoid or cannabis-
derived may
be about 1:1 by weight.
[00161] Surfactant
[00162] The water-soluble formulations of the present disclosure include a
surfactant.
[00163] Glycerin-Based Carrier Surfactant
[00164] In one particularly suitable embodiment, the surfactant is a
glycerin-based
carrier surfactant. By "carrier surfactant", it is intended to refer to the
feature that the
surfactant is the continuous phase (carrier) in which the other components of
the
water-soluble formulation are dispersed (e.g. the cannabinoids, carrier oil,
and emulsifier).
It further acts as a surfactant in enabling the formulations of the present
disclosure in being
water-soluble. By "glycerin-based", it is meant that the majority component of
the surfactant
is glycerin. It is envisioned that the glycerin may have other compounds
dissolved or
31
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
suspended therein. Alternatively, the glycerin-based surfactant may be
comprised solely of
glycerin.
[00165] In an embodiment, the glycerin-based carrier surfactant may be
present in
the water-soluble formulation in an amount between about 60% and about 97% by
weight.
In select embodiments, the glycerin-based carrier surfactant may be present in
the water-
soluble formulation in an amount between about 70% and about 97% by weight,
more
particularly between about 80% and about 97% by weight, and even more
particularly
between about 90% and about 97% by weight. In an embodiment, the glycerin-
based
carrier surfactant may be present in the water-soluble formulation in an
amount of about
75%, about 76%, about 77%, about 78%, about 79%, about 80%, about 81%, about
82%,
about 83%, about 84%, about 85%, about 86%, about 87%, about 88%, about 89%,
about
90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%, or
about 97%
by weight.
[00166] In an embodiment, the glycerin-based carrier surfactant is
glycerin. In an
embodiment, the glycerin is a natural or synthetic glycerin. In an embodiment,
the glycerin
is a vegetable glycerin. As used herein, "vegetable glycerin" refers to the
glycerin being
made from plant oils. In an embodiment, the vegetable glycerin is made from
soybean,
coconut or palm oils.
[00167] The use of higher amounts of glycerin in the water-soluble
formulations of
the present disclosure is unusual as typically cannabis-derived formulations
are made as
oil-in-water emulsions having water as the main component. It was found,
however, in the
water-soluble formulations of the present disclosure that even when small
amounts of water
were used as an alternative to glycerin, the resulting formulation was opaque
suggesting
larger average particle sizes of emulsified cannabinoids within the
formulation.
[00168] Other Surfactants
[00169] In an embodiment, the water-soluble formulations include a
surfactant other
than a glycerin-based carrier surfactant. The other surfactant may be used as
an
alternative to the glycerin-based carrier surfactant or in addition to the
glycerin-based
carrier surfactant.
32
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00170] In an embodiment, the other surfactant is used as an alternative
to the
glycerin-based carrier surfactant. In such embodiments, most suitably the
alternate
surfactant is likewise a carrier surfactant. In an embodiment, the other
surfactant has
chemical, physical and/or functional properties similar to glycerin.
[00171] In an embodiment, the other surfactant is used in addition to the
glycerin-
based carrier surfactant. In an embodiment, the glycerin-based carrier
surfactant may be
the predominant surfactant by weight (e.g. >50% by weight) or by volume (e.g.
>50% by
volume). In other embodiments, the glycerin-based carrier surfactant may be
the minority
surfactant by weight (e.g. <50% by weight) or by volume (e.g. <50% by volume).
In an
embodiment, there may be 2, 3, 4, 5 or more other surfactants in addition or
in alternative to
a glycerin-based carrier surfactant.
[00172] Other suitable surfactants that can be used as alternatives or in
addition to a
glycerin-based carrier surfactant include, for example and without limitation,
propylene
glycol, class 3 solvents (e.g., ethanol, isopropanol), long chain alcohols,
terpenes (found in
cannabis or not), other poly-alcohols, and the like and combinations thereof.
[00173] In an embodiment, where the water-soluble formulations include
other
surfactants in addition to or in alternative to a glycerin-based carrier
solvent, the total
amount of surfactant in the water-soluble formulation may be between about 60%
and
about 97% by weight. In select embodiments, the total amount of surfactant in
the
water-soluble formulation may be between about 70% and about 97% by weight,
more
particularly between about 80% and about 97% by weight, and even more
particularly
between about 90% and about 97% by weight. In an embodiment, the total amount
of
surfactant in the water-soluble formulation may be about 75%, about 76%, about
77%,
about 78%, about 79%, about 80%, about 81%, about 82%, about 83%, about 84%,
about
85%, about 86%, about 87%, about 88%, about 89%, about 90%, about 91%, about
92%,
about 93%, about 94%, about 95%, about 96%, or about 97% by weight.
[00174] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or cannabis-derived compound, a carrier
oil, an
emulsifier, and a glycerin-based carrier surfactant.
[00175] In select embodiments, the water-soluble formulations of the
present
disclosure comprise the cannabinoid or cannabis-derived compound; the carrier
oil; and the
33
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
emulsifier at an about equivalent amount by weight. In an embodiment, the
water-soluble
formulations comprise up to 10% by weight of the cannabinoid or cannabis-
derived
compound; up to 10% by weight of the carrier oil, and up to 10% by weight of
the emulsifier.
In an embodiment, the water-soluble formulations comprise about 1%, about 2%,
about 3%,
about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, or about 10% by
weight of
the cannabinoid or cannabis-derived compound, the carrier oil, and the
emulsifier.
[00176] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or cannabis-derived compound, a carrier
oil, a first
emulsifier, a second emulsifier, and a glycerin-based carrier surfactant.
[00177] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or a cannabis-derived compound,
monoglycerides,
soy lecithin, sucrose monoester, all in a vegetable glycerin.
[00178] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid distillate or a cannabinoid isolate;
monoglycerides; a
soy lecithin; and a sucrose monoester; in a vegetable glycerin
[00179] In an embodiment, the water-soluble formulations of the present
disclosure
comprise an about equivalent amount by weight of the soy lecithin and the
sucrose
monoester.
[00180] In an embodiment, the water-soluble formulations comprise up to
10% by
weight of each of the cannabinoid or cannabis-derived compound; the carrier
oil, the soy
lecithin; and the sucrose monoester. In an embodiment, the water-soluble
formulations
comprise an about equivalent amount by weight of each of the cannabinoid or
cannabis-derived compound; the monoglycerides; the soy lecithin; and the
sucrose
monoester. In an embodiment, the equivalent amount is about 1%, about 2%,
about 3%,
about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, or about 10% by
weight of
water-soluble formulation.
[00181] In an embodiment, the water-soluble formulations of the present
disclosure
are liquid, such as an emulsion. The term "emulsion" is well known in the art
and refers to a
mixture of two or more liquids that are normally immiscible (unmixable or
unblendable),
34
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
where a first liquid is dispersed in small globules (internal or discontinuous
phase)
throughout a second liquid (external or continuous phase).
[00182] In an embodiment, the water-soluble formulations of the present
disclosure
are a powder. The powder may, for example, be prepared by drying the liquid
water-
soluble formulations of the present disclosure. In an embodiment, the powder
is of low
calorie (e.g. less than 10 kcal per 250 mg of the powder formulation).
[00183] Stabilizers
[00184] In select embodiments, the water-soluble formulations of the
present
disclosure may be used in combination with a stabilizer. The stabilizer may be
added to the
water-soluble formulation or to a product (e.g. aqueous solution, beverage,
topical or food)
that is produced using the water-soluble formulations.
[00185] Thus, in an embodiment, the water-soluble formulations of the
present
disclosure comprise a stabilizer. In an embodiment, a product produced from
the
water-soluble formulations comprises a stabilizer. The stabilizer may be added
to the
product before, during or after admixture with the water-soluble formulation.
[00186] As used herein, a stabilizer is any substance used to prevent an
unwanted
change in state in the water-soluble formulation or product produced therefrom
(e.g.
prevent degradation). The stabilizer may be used to improve or maintain the
stability of the
water-soluble formulation itself (e.g. the emulsion) or to improve or maintain
the stability of
individual components of the water-soluble formulation or product (e.g. the
cannabinoids).
For example, cannabinoids or cannabis-derived compounds within the water-
soluble
formulation or product produced therefrom may be susceptible to degradation,
such as
oxidative degradation. Thus, in an embodiment, the stabilizer protects the
cannabinoids or
cannabis-derived compounds from degradation.
[00187] Non-limiting examples of stabilizers include hydrocolloids (such
as alginate,
agar, carrageenan, cellulose and cellulose derivatives, gelatin, guar gum, gum
Arabic,
locust bean gum, pectin, starch and xanthan gum), antioxidants (water-soluble
and/or oil-
soluble), and chelating agents.
[00188] Water-soluble antioxidants may enhance the stability of the water-
soluble
formulation and/or products containing the water-soluble formulation by
reacting with
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
reactive species in the polar (e.g. aqueous) phase. Oil-soluble antioxidants
may enhance
the chemical stability of the water-soluble formulation and/or products
containing the
water-soluble formulation by reacting with reactive species in the oil phase.
Non-limiting
examples of reactive species include peroxides, free radicals and oxygen.
[00189] Non-limiting examples of antioxidants include ascorbic acid,
ascorbic acid-6
palmitate (ascorbyl palmitate), ascorbyl stearate, alpha-tocopherol, beta-
carotene, butylated
hydroxyaniline (BHA), butylated hydroxytolulene (BHT), delta-tocopherol,
dodecyl gallate,
erythorbic acid, gamma-tocopherol, glutathione, lipoic acid, octyl gallate,
propyl gallate,
mixed tocopherols (e.g. FortiumO), vitamin E (e.g. TocobiolO Plus CP60),
TocobiolO Plus
L-70, TocobiolO Plus GP, TocobiolO Plus PV, Nutrabiol T, sodium
ascorbate,sodium
erythorbate, and Extract of Rosemary (OxiKanO CL).
[00190] In an embodiment, the water-soluble formulations of the present
disclosure
are used in combination with an antioxidant stabilizer. In an embodiment, the
antioxidant
stabilizer is ascorbic acid-6 palmitate (E-304) or a tocopherol.
[00191] Chelating agents may enhance the chemical stability of the water-
soluble
formulation and/or products containing the water-soluble formulation by
binding dissolved
metal ions. Dissolved metal ions, for example copper ions or iron ions, may
catalyze
oxidation-reduction reactions (redox) between dissolved oxygen and the
components of the
water-soluble formulation or product. In particular, cannabinoids may be
susceptible to
oxidation catalyzed by dissolved metal ions. Non-exclusive examples of
chelating agents
include: aminopolycarboxylic acids including ethylenediaminetetraacetic acid
(EDTA) and
its various salts, calixarenes, porphyrins, bipyridines, citric acid,
iminodisuccinic acid, and
polyaspartic acid.
[00192] In an embodiment, the water-soluble formulations of the present
disclosure
are used in combination with a chelating agent as a stabilizer. In an
embodiment, the
chelating agent is ethylenediaminetetraacetic acid (EDTA). In an embodiment,
the EDTA is
disodium EDTA, calcium disodium EDTA, or tetrasodium EDTA. In a particular
embodiment, the EDTA is calcium disodium EDTA.
[00193] The stabilizer may be added to the water-soluble formulation or to
the
product produced therewith. In an embodiment, the stabilizer is added to the
product
36
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
separately from the water-soluble formulation. In an embodiment, the
stabilizer is added to
the product within 30 minutes before or after the water-soluble formulation is
added.
[00194] In an embodiment, the stabilizer is added to the water-soluble
formulation.
In an embodiment, the stabilizer is added to the water-soluble formulation
within 30 minutes
before the water-soluble formulation is added to the product. In an
embodiment, the
stabilizer is added to the water-soluble formulation within 1 minute before
the water-soluble
formulation is added to the product.
[00195] The stabilizer may be added to the water-soluble formulation or to
the
product produced therewith at any suitable concentration. In an embodiment,
the stabilizer
is added in a minor amount. As used herein, by "in a minor amount", it is
meant that the
stabilizer is added to the water-soluble formulation or to the resultant
product at a
concentration of between 1 ppm and 100 ppm, between 10 ppm and 50 ppm, or
between
20 ppm and 30 ppm.
[00196] The use of a stabilizing agent is sometimes to the detriment of
other
important characteristics of a consumer product, e.g. a beverage. For example,
additional
components such as stabilizers may promote turbidity, cloudiness or an
undesired taste
profile in the final product. Also, stabilization of one component (e.g. the
emulsion) may
have a negative effect on the stability of another component (e.g. the active
ingredient).
This may be particularly so for emulsification products where the development
of a
water-soluble formulation that is clear in appearance, easy to drink ("clean"
taste profile),
shelf stable, and quick acting are all relevant considerations.
[00197] In an embodiment, the water-soluble formulations of the present
disclosure
are used in combination with a stabilizer. In an embodiment, the stabilizer is
one that
complements one or more components of the water-soluble formulation to provide
a
product that is clear in appearance, chemically stable, shelf stable, and/or
suitable for use
in a broad range of product having different characteristics (e.g. pH, high or
low ionic
conditions, wide array of ingredients, etc.). By "chemically stable", it is
meant that the
stability of the active ingredient is improved.
[00198] In an embodiment, a chelating agent is used in combination with a
water-soluble formulation of the present disclosure. In an embodiment, the
water-soluble
formulation is one that comprises one or both of an emulsifier selected from
lecithin and
37
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
sucrose monoester. In an embodiment, a chelating agent is used in combination
with a
water-soluble formulation of the present disclosure that comprises both
lecithin and sucrose
monoester as emulsifiers. In an embodiment, the chelating agent is EDTA.
[00199] As shown herein, sucrose monoester and a chelating agent appear to
complement each other in that the sucrose monoester strengthens the emulsion
(e.g. stabilizes the emulsion) as evidenced by improved clarity, while the
chelating agent
stabilizes the cannabinoids. This is an advantageous result since it was found
that a
chelating agent renders the aqueous product more turbid. Combined with a
sucrose
monoester, the clarity of the product improves (see e.g. Example 5).
[00200] The combination of sucrose monoester and lecithin provide better
clarity and
stability then either alone, and the inclusion of a chelating agent in the
aqueous solution
appears to provide even greater protection to the cannabinoid, without
sacrificing other key
characteristics of the product. As seen in Example 4, the chelating agent was
found to
provide significant protection of THC and CBD from degradation.
[00201] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or cannabis-derived compound, a carrier
oil, an
emulsifier, and a glycerin-based carrier surfactant, which is used in
combination with a
stabilizer to prepare a beverage, topical or food.
[00202] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or cannabis-derived compound, a carrier
oil, a first
emulsifier, a second emulsifier, and a glycerin-based carrier surfactant,
which is used in
combination with a stabilizer to prepare a beverage, topical or food.
[00203] In an embodiment, the present disclosure relates to a water-
soluble
formulation comprising a cannabinoid or a cannabis-derived compound,
monoglycerides,
soy lecithin, sucrose monoester, all in a vegetable glycerin, which is used in
combination
with a chelating agent to prepare a beverage, topical or food.
[00204] The water-soluble formulation and/or the products containing the
water-soluble formulations may be treated to reduce the oxygen content as this
may further
enhance their chemical stability. For example, it was found that the rate of
oxidative
degradation of cannabinoids depends on the oxygen concentration in the product
38
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
containing the water-soluble formulation. Reducing the oxygen concentration
may thereby
reduce the rate of oxidative degradation and enhance the chemical stability of
the
water-soluble formulation and/or the products containing the water-soluble
formulation.
Non-limiting examples of treatments to reduce oxygen concentration include:
sparging (with
nitrogen and/or other inert gases), freeze-pump-thaw cycling, and treatment
with reducing
agents (e.g. potassium metabisulphite).
[00205] In an embodiment, products made with the water-soluble formulation
of the
present disclosure are sparged after the water-soluble formulation is added.
In an
embodiment, the products are sparged within at least 60 minutes, at least 45
minutes, at
least 30 minutes, at least 10 minutes, at least 5 minutes or less, after the
water-soluble
formulation is added. In an embodiment, the sparged products are also treated
with
reducing agents.
[00206] The water-soluble formulation and/or the products containing the
water-
soluble formulations may be treated with agents to adjust the pH. In an
embodiment, the
pH of the water-soluble formulation and/or the products containing the water-
soluble
formulation is adjusted to less than 4.5. In an embodiment, the pH of the
water-soluble
formulation and/or the products containing the water-soluble formulation is
adjusted to
between 2.5 and 4.5. In an embodiment, the pH of the water-soluble formulation
and/or the
products containing the water-soluble formulation is adjusted to between 3.8
and 4.3. In an
embodiment, the water-soluble formulation and/or products containing the water-
soluble
formulation may contain buffers to maintain a constant pH.
[00207] Additives
[00208] In some embodiments, the water-soluble formulations or products
(e.g.
beverages, foodstuffs, etc.) of the present disclosure may further include
additives, such as
for example and without limitation terpenes, terpenoids, flavonoids, or any
combination
thereof. Such additives may be used to enhance flavour, viscosity, aroma and
the like.
[00209] In an embodiment, the additives may be derived from cannabis
plants. In an
embodiment, the additives may be derived from natural sources other than a
cannabis
plant, such as a plant of a different species. Alternatively, in some
embodiments, the
additives may be synthetic or semi-synthetic compounds.
39
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00210] Terpenes and Terpenoids
[00211] In an embodiment, the water-soluble formulations herein may
comprise one
or more terpenes and/or terpenoids. In an embodiment, the product containing
the
water-soluble formulations may comprise one or more terpenes and/or
terpenoids.
[00212] Within the context of this disclosure, the term "terpene" includes
cannabis
derived terpenes and non-cannabis derived terpenes.
[00213] Terpenes are a large and diverse class of organic compounds,
produced by
a variety of plants, particularly conifers, and by some insects such as
termites or swallowtail
butterflies, which emit terpenes from their osmeteria. Terpenes are also major
constituents
of Cannabis sativa plants. They often have a strong odor and may protect the
plants that
produce them by deterring herbivores and by attracting predators and parasites
of
herbivores. The difference between terpenes and terpenoids is that terpenes
are
hydrocarbons, whereas terpenoids contain additional functional groups.
[00214] They are the major components of resin, and of turpentine produced
from
resin. The name "terpene" is derived from the word "turpentine". In addition
to their roles
as end-products in many organisms, terpenes are major biosynthetic building
blocks within
nearly every living creature. Steroids, for example, are derivatives of the
triterpene
squalene.
[00215] When terpenes are modified chemically, such as by oxidation or
rearrangement of the carbon skeleton, the resulting compounds are generally
referred to as
terpenoids. Some authors will use the term terpene to include all terpenoids.
Terpenoids
are also known as isoprenoids.
[00216] Within the context of this disclosure, the term "terpene" includes
hemiterpenes, monoterpenols, terpene esters, diterpenes, monoterpenes,
polyterpenes,
tetraterpenes, terpenoid oxides, sesterterpenes, sesquiterpenes,
norisoprenoids, or their
derivatives, as well as isomeric, enantiomeric, or optically active
derivatives.
[00217] Derivatives of terpenes include terpenoids, hemiterpenoids,
monoterpenoids,
sesquiterpenoids, sesterterpenoid, sesquarterpenoids, tetraterpenoids,
triterpenoids,
tetraterpenoids, polyterpenoids, isoprenoids, and steroids. These derivatives
are
encompassed herein by the term "terpene", unless specifically stated
otherwise.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00218] Within the context of this disclosure, the term terpene includes
the
a- (alpha), 13- (beta), y- (gamma), oxo-, isomers, or any combinations thereof
[00219] Terpenes are the primary constituents of the essential oils of
many types of
plants and flowers. Essential oils are used widely as fragrances in perfumery,
and in
medicine and alternative medicines such as aromatherapy. Synthetic variations
and
derivatives of natural terpenes also greatly expand the variety of aromas used
in
perfumery and flavours used in food additives.
[00220] Higher amounts of terpenes are released by trees in warmer
weather, acting
as a natural form of cloud seeding. The clouds reflect sunlight, allowing the
forest to
regulate its temperature. The aroma and flavour of hops comes, in part, from
sesquiterpenes (mainly alpha-humulene and beta-caryophyllene), which affect
beer quality.
Accordingly, in some embodiments, the water-soluble formulations of the
present disclosure
include hop-derived terpenes such as hop-derived terpene blends available as
Aramis,
Brewer's Gold, Bravo and the like, and combinations thereof.
[00221] Plant terpenes are used extensively for their aromatic qualities
and play a
role in traditional herbal remedies. Terpenes contribute to the scent of
eucalyptus, the
flavours of cinnamon, cloves, and ginger, the yellow colour in sunflowers, and
the red
colour in tomatoes.
[00222] Non-limiting examples of terpenes within the context of this
disclosure
include: 7,8-dihydro-alpha-ionone, 7,8-dihydro-beta-ionone, Acetanisole,
Acetic Acid, Acetyl
Cedrene, Anethole, Anisole, Benzaldehyde, Bergamotene (Alpha-cis-Bergamotene)
(Alpha-
trans-Bergamotene), Bisabolol (Beta-Bisabolol), Alpha Bisabolol, Borneol,
Bornyl Acetate,
Butanoic/ Butyric Acid, Cadinene (Alpha-Cadinene) (Gamma-Cadinene), Cafestol,
Caffeic
acid, Camphene, Camphor, Capsaicin, Carene (Delta-3-Carene), Carotene,
Carvacrol,
Dextro-Carvone, Laevo-Carvone, Alpha-Caryophyllene, Beta-Caryophyllene,
Caryophyllene
oxide, Cedrene (Alpha-Cedrene) (Beta-Cedrene), Cedrene Epoxide (Alpha-Cedrene
Epoxide), Cedrol, Cembrene, Chlorogenic Acid, Cinnamaldehyde, Alpha-amyl-
Cinnamaldehyde, Alpha-hexyl-Cinnamaldehyde, Cinnamic Acid, Cinnamyl Alcohol,
Citronellal, Citronellol, Cryptone, Curcumene (Alpha-Curcumene) (Gamma-
Curcumene),
Decanal, Dehydrovomifoliol, Diallyl Disulfide, Dihydroactinidiolide, Dimethyl
Disulfide,
Eicosane/lcosane, Elemene (Beta-Elemene), Estragole, Ethyl acetate, Ethyl
Cinnamate,
41
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
Ethyl maltol, Eucalypto1/1,8-Cineole, Eudesmol (Alpha-Eudesmol) (Beta-
Eudesmol)
(Gamma-Eudesmol), Eugenol, Euphol, Farnesene, Farnesol, Fenchol (Beta-
Fenchol),
Fenchone, Geraniol, Geranyl acetate, Germacrenes, Germacrene B, Guaia-1(10),11-
diene,
Guaiacol, Guaiene (Alpha-Guaiene), Gurjunene (Alpha-Gurjunene), Herniarin,
Hexanaldehyde, Hexanoic Acid, Humulene (Alpha-Humulene) (Beta-Humulene), lanai
(3-
oxo-alpha-ionol) (Beta-lanai), lonone (Alpha-lonone) (Beta-lonone), Ipsdienol,
Isoamyl
Acetate, Isoamyl Alcohol, Isoamyl Formate, Isoborneol, Isomyrcenol,
Isopulegol, Isovaleric
Acid, Isoprene, Kahweol, Lavandulol, Limonene, Gamma-Linolenic Acid, Linalool,
Longifolene, Alpha-Langipinene, Lycopene, Menthol, Methyl butyrate, 3-Mercapto-
2-
Methylpentanal, Mercaptan/Thiols, Beta-Mercaptoethanol, Mercaptoacetic Acid,
Allyl
Mercaptan, Benzyl Mercaptan, Butyl Mercaptan, Ethyl Mercaptan, Methyl
Mercaptan,
Furfuryl Mercaptan, Ethylene Mercaptan, Propyl Mercaptan, Thenyl Mercaptan,
Methyl
Sal icylate, Methylbutenol, Methyl-2-Methylvalerate, Methyl Thiobutyrate,
Myrcene (Beta-
Myrcene), Gamma-Muurolene, Nepetalactone, Nerol, Nerolidol, Neryl acetate,
Nonanaldehyde, Nonanoic Acid, Ocimene, Octanal, Octanoic Acid, P-Cymene,
Pentyl
butyrate, Phellandrene, Phenylacetaldehyde, Phenylethanethiol, Phenylacetic
Acid, Phytol,
Pinene, Beta-Pinene, Propanethiol, Pristimerin, Pulegone, Quercetin, Retinal,
Rutin,
Sabinene, Sabinene Hydrate, cis-Sabinene Hydrate, trans-Sabinene Hydrate,
Safranal,
Alpha-Selinene, Alpha-Sinensal, Beta-Sinensal, Beta-Sitosterol, Squalene,
Taxadiene,
Terpin hydrate, Terpineol, Terpine-4-al, Alpha-Terpinene, Gamma-Terpinene,
Terpinolene,
Thiophenol, Thujone, Thymol, Alpha-Tocopherol, Tonka Undecanone, Undecanal,
Valeraldehyde/Pentanal, Verdoxan, Alpha-Ylangene, Umbelliferone, or Vanillin.
[00223] In select embodiments, the water-soluble formulations disclosed
herein
comprise a terpene selected from 8-caryophyllene, caryophyllene oxide,
borneol,
1,8-cineole, camphene, humulene (e.g., a-humulene), limonene (e.g., D-
limonene,
L-limonene), linalool, hexyl acetate, myrcene (e.g., 3-myrcene), nerolidol,
pulegone,
isopulegol, a-pinene, 3-pinene, para-cymene, eugenol, farnesol, geraniol,
phytol, terpinene
(e.g., gamma-terpinene), terpineol (e.g., a-terpineol) and terpinolene, or any
combination
thereof.
[00224] In particularly suitable embodiments, the water-soluble
formulations include
terpenes and/or terpenoids having antimicrobial properties. Exemplary
antimicrobial
terpenes include, for example, Ocimum basilicum (basil), Laurus nobilis (bay),
Cinnamomum verum (Ceylon cinnamon), Capsicum annuum (paprika), Syzygium
42
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
aromaticum (clove), Mentha piperita (peppermint), Tanacetum vulgare (tansy),
Artemisia
dracunculus (Tarragon), and the like as known in the art. This allows for a
more stable
water-soluble formulation. Particularly, it has been found that the water-
soluble
formulations show no bacterial growth for a time period of up to 50 days or
more, even
without the use of a preservative.
[00225] Furthermore, in some embodiments, the water-soluble formulations
include a
total terpene concentration (relative to the concentration of cannabinoids)
beyond what
would normally be found in the cannabis flower, allowing for the potential of
entourage
effects. As used herein, the "entourage-effect" refers to the residual effect
of one or more
compounds (e.g., cannabinoids, terpenes) of the water-soluble formulations in
the
sequentially administered end products including the water-soluble
formulations.
[00226] Exemplary terpene blends for use in the water-soluble formulations
are
provided below.
Exemplary Terpene Blends:
Beta- . Hexyl Beta-
Limonene Terpinolene
Strain Formulation Pinene Acetate Caryophyllene
/0(w/w) /0(w/w)
/0(w/w) /0(w/w) /0(w/w)
Penelope 1 0.0 0.0 30.0 40.0 30.0
2 0.0 20.0 10.0 40.0 30.0
3 0.0 25.0 5.0 40.0 30.0
4 0.0 25.0 5.0 30.0 40.0
5 0.0 27.5 2.5 40.0 30.0
6 0.0 20.0 2.5 40.0 37.5
7 10.0 20.0 2.5 40.0 27.5
8 10.0 30.0 2.5 30.0 27.5
Beta-
u. E genol p-Cymeme Humulene Terpinolene Limonene
Strain Formulation Caryophyllene
% (w/w) /0(w/w) /0(w/w) /0(w/w) %
(w/w)
/0(w/w)
Houndstooth 1 20.0 10.0 10.0 30.0 30.0
2 5.0 10.0 15.0 40.0 30.0
3 5.0 10.0 15.0 20.0 30.0 20.0
4 5.0 20.0 15.0 20.0 10.0 30.0
43
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
B-pinene Nerolido! Isopulegol y-Terpinene
Strain Formulation % (of terpene % (of terpene % (of terpene % (of
terpene
total weight) total weight) total weight) total
weight)
Houndstooth 1 40.0 20.0 20.0 20.0
2 70.0 10.0 10.0 10.0
3 60.0 0.0 20.0 20.0
4 10.0 10.0 40.0 40.0
20.0 40.0 0.0 40.0
6 30.0 35.0 0.0 35.0
[00227] Flavonoids
[00228] In some embodiments, the water-soluble formulations may further
include
additives such as one or more flavonoids.
[00229] As used herein, the term "flavonoid" refers to any compound of a
large class
of plant pigments having a structure based on or similar to that of flavone.
Chemically,
flavonoids have the general structure of a 15-carbon skeleton, which consists
of two phenyl
rings and a heterocyclic ring.
[00230] Within the context of this disclosure, the term "flavonoids"
includes
bioflavonoids, isoflavonoids and neoflavonoids. Isoflavones use the 3-
phenylchromen-4-
one skeleton (with no hydroxyl group substitution on carbon at position 2).
Examples
include: Genistein, Daidzein, Glycitein, Isoflavanes, Isoflavandiols,
Isoflavenes,
Coumestans, and Pterocarpans.
[00231] Within the context of this disclosure, the term "flavonoids" also
includes
anthocyanidins, anthoxanthins, flavanones, flavanonols and flavens.
[00232] Flavonoids are widely distributed in plants, fulfilling many
functions.
Flavonoids are the most important plant pigments for flower colouration,
producing yellow
or red/blue pigmentation in petals designed to attract pollinator animals. In
higher plants,
flavonoids are involved in UV filtration, symbiotic nitrogen fixation and
floral pigmentation.
They may also act as chemical messengers, physiological regulators, and cell
cycle
inhibitors. Some flavonoids have inhibitory activity against organisms that
cause plant
diseases, e.g. Fusarium oxysporum.
44
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00233] Sources of flavonoids include, without limitation, cannabis,
parsley,
blueberries, black tea, citrus, wine, cocoa and peanut.
[00234] Additional exemplary flavonoids for use in the formulations
include
Apigenin, beta-sitosterol, cannaflavin A, kaempferol, luteolin, orientin,
quercetin and
combinations thereof.
[00235] In an embodiment, the flavonoid is cannaflavin.
[00236] Other Additives
[00237] In some embodiments, the water-soluble formulations or products
(e.g. beverages, foodstuffs, etc.) of the present disclosure may include any
number of other
additives, including without limitation a solvent, a bulking agent, an
antioxidant, or a
nutritional supplement. These components may be used either alone or in
combination to
improve, for example, the chemical and/or physical properties, stability,
nutritional profile,
taste, colour and/or viscosity, of the water-soluble formulations disclosed
herein or a
beverage or foodstuff produced therefrom. In an embodiment, the antioxidant
may be
ascorbyl palm itate or a-tocopherol.
[00238] Yet other suitable types of modifiers and additives that may be
used in the
water-soluble formulations or products (e.g. beverages, foodstuffs, etc.)
disclosed herein
include viscosity modifiers, natural emulsifiers, oils, thickening agents,
minerals, acids,
bases, vitamins, flavours, colourants, sweeteners (e.g. liquid sweeteners),
and the like and
combinations thereof, as known in the beverage and food arts, to provide
improved
solubility, stability, bioavailability, colour and taste.
[00239] Nutritional supplements comprise substances useful to the consumer
of the
formulations disclosed herein, or beverages or foodstuffs prepared therewith,
for
maintenance of normal body health. Suitable nutritional supplements may
comprise, for
example, essential nutrients including vitamins, dietary minerals, amino acids
and fatty
acids. Exemplary nutritional supplements may include vitamin A, vitamin Bl,
vitamin B2,
vitamin B3, vitamin B5, vitamin B6, vitamin B7, vitamin B9, vitamin B12,
vitamin C, vitamin
D, vitamin E, vitamin K calcium, phosphorus, potassium, sulfur, sodium,
chlorine,
magnesium, iron, cobalt, copper, zinc, molybdenum, iodine, selenium,
manganese, nickel,
chromium, fluorine, boron, strontium histidine, isoleucine, leucine, lysine,
methionine,
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
cysteine, phenylalanine, tyrosine, threonine, tryptophan, valine, alpha-
linoleic acid, and
linoleic acid.
[00240] Viscosity modifiers include any compound or agent capable of
altering the
viscosity of the formulations disclosed herein, or a beverage or foodstuff
produced
therewith. Exemplary embodiments of viscosity modifiers include anticaking
agents,
antifoaming agents, bulking agents, coagulation agents, gelling agents,
glazing agents,
humectants, leavening agents, tenderizers, and thickeners. In an embodiment,
the
viscosity modifying agent may be an unmodified starch, pregelatinized starch,
cross-linked
starches, gums (e.g. guar gum, xanthum gum, acacia), polyvinyl pyrrolidone
(PVP),
polyethylene oxide, waxes (e.g. beeswax), and mixtures thereof.
[00241] Sweeteners include any compound or agent that is capable of
sweetening
the taste of the formulations disclosed herein, or a beverage or foodstuff
produced
therewith. The sweetener may be a natural sweetener or an artificial
sweetener. The
sweetener may be a solid, liquid or semi-liquid. Exemplary embodiments of
sweeteners
include sugars and sugar alcohols, and more particularly stevia, erythritol,
and xylitol. In an
embodiment, the sweetener may be a liquid sugar solution, such as without
limitation those
having a Brix value of about 67.5 Bx. In an embodiment, the sweetener may be
an
Isocane 67.5 Bx liquid sugar solution.
[00242] In particularly suitable embodiments, the water-soluble
formulations of the
present disclosure are shelf-stable. As used herein, "shelf-stable" refers to
the formulation
maintaining a homogeneous mixture (i.e., no phase separation) for a period of
at least 30
days, more suitably, at least 40 days, even more suitably, at least 45 days,
and more
suitably, at least 50 days, and even more suitably, at least 55 days or
longer.
[00243] In particularly suitable embodiments, the water-soluble
formulations of the
present disclosure enhance or maintain the stability of the cannabinoids or
cannabis-derived compounds in the water-soluble formulation, in a product
produced
therefrom (e.g. beverage), or both. In an embodiment, loss of cannabinoids or
cannabis-derived compounds in the water-soluble formulations of the present
disclosure is
less than 35% by weight in 3 months, more particularly less than 25% by weight
in
3 months, and more particularly still less than 20% by weight in 3 months. In
an
embodiment, loss of cannabinoids or cannabis-derived compounds in the water-
soluble
46
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
formulations of the present disclosure is about 25%, about 20%, about 15%,
about 10%,
about 5%, or less, by weight in 3 months. In an embodiment, loss of
cannabinoids or
cannabis-derived compounds in the water-soluble formulations of the present
disclosure is
less than 16% by weight THC content in 70 days.
[00244] In particularly suitable embodiments, the water-soluble
formulations of the
present disclosure, when mixed with an aqueous solution, provide a product
which is
stable. By "stable", it is meant that the water-soluble formulation remains
free from one or
more deleterious changes over a period of time, for example at least or longer
than 1 day,
1 week, 1 month, 3 months, 6 months, 1 year, or more. For example, stable may
be in
reference to a lack of degradation of cannabinoids or cannabis-derived
compounds; a
maintenance of clarity; or a maintenance of any other property desirable for
consumption.
[00245] In an embodiment, the water-soluble formulation, when mixed with
an
aqueous solution, provides a product in which at least 60%, at least 65%, at
least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, or at least 95% by weight
of the
cannabinoid or cannabis-derived compound remains present after about 2 months
at a
temperature between about 17 C and about 40 C. In an embodiment, at least 80%
by
weight of the cannabinoid or cannabis-derived compound remains present after
about 2
months at a temperature between about 17 C and about 40 C. In an embodiment,
at least
90% by weight of the cannabinoid or cannabis-derived compound remains present
after
about 2 months at a temperature between about 17 C and about 40 C.
[00246] In an embodiment, the water-soluble formulation, when mixed with
an
aqueous solution, provides a product in which at least 60%, at least 65%, at
least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, or at least 95% by weight
of the
cannabinoid or cannabis-derived compound remains present after about 3 months
at a
temperature between about 17 C and about 40 C. In an embodiment, at least 80%
by
weight of the cannabinoid or cannabis-derived compound remains present after
about
3 months at a temperature between about 17 C and about 40 C. In an embodiment,
at
least 90% by weight of the cannabinoid or cannabis-derived compound remains
present
after about 3 months at a temperature between about 17 C and about 40 C.
[00247] In an embodiment, at least 80% by weight of the cannabinoid or
cannabis-derived compound remains present after about 2 months at about 40 C.
In an
47
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
embodiment, at least 90% by weight of the cannabinoid or cannabis-derived
compound
remains present after about 2 months at about 40 C.
[00248] In an embodiment, at least 80% by weight of the cannabinoid or
cannabis-derived compound remains present after about 3 months at about 40 C.
In an
embodiment, at least 90% by weight of the cannabinoid or cannabis-derived
compound
remains present after about 3 months at about 40 C.
[00249] In an embodiment, at least 84.89% by weight of the cannabinoid or
cannabis-derived compound remains present after about 3 months at a
temperature of
about 40 C.
[00250] In particularly suitable embodiments, the water-soluble
formulations of the
present disclosure are substantially free of cyclodextrins and modified
starches, thereby
reducing unnatural ingredients from end use products including the
formulations.
[00251] Additionally, the water-soluble formulations of the present
disclosure are
suitably prepared to be low calorie. Particularly, in some embodiments, a 250
mL serving
will provide less than 25 kilocalories (Kcal), more suitably less than 10
Kcal, and even more
suitably less than 5 Kcal.
[00252] METHODS OF PREPARING THE WATER-SOLUBLE FORMULATIONS
[00253] It has been found that by mixing the above-described components of
the
water solution formulations in a particular order, a cloudy pre-emulsion can
be formed that,
when mixed with an aqueous solution, can form a transparent or translucent
microemulsion
having favorable pharmacokinetics, for example, rapid onset, shorter duration,
and minimal
food effect. In select embodiments, the water-soluble formulations produced by
the
disclosed methods may also be clear, rather than cloudy.
[00254] In one aspect, to prepare the water-soluble formulations of the
present
disclosure, a cannabinoid or a cannabis-derived compound, a glycerin-based
carrier
surfactant and an emulsifier are mixed, in any order.
[00255] In select embodiments, the water-soluble formulations comprise a
carrier oil.
Accordingly, in another aspect, to prepare the water-soluble formulations of
the present
disclosure, the cannabinoid or cannabis-derived compound (e.g., cannabinoid
distillate
48
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
and/or isolate) and the carrier oil are first mixed to form a homogenous
mixture; and then
the surfactant (e.g. glycerin-based carrier surfactant) and emulsifier are
mixed into the
homogenous mixture.
[00256] In a particular embodiment, the method comprises: mixing a
cannabinoid or
a cannabis-derived compound and a carrier oil until a homogenous mixture is
formed; and
mixing a glycerin-based carrier surfactant and an emulsifier into the
homogenous mixture to
prepare the water-soluble formulation. In an embodiment, the cannabis-derived
compound
is a cannabinoid distillate or isolate; the carrier oil is monoglycerides; the
emulsifier is a soy
lecithin; and the glycerin-based carrier surfactant is a vegetable glycerin.
[00257] During the step of mixing the cannabinoid or cannabis-derived
compound
with the carrier oil, heat may be applied. In an embodiment, the mixing is
performed under
heated conditions of between about 40 C and about 50 C.
[00258] As discussed herein, the water-soluble formulation may comprise
more than
one emulsifier. When two or more emulsifiers are used, they may be mixed into
the
homogenous mixture together or in any order, including consecutively or
simultaneously. In
an embodiment a single emulsifier is used, such as a soy lecithin. In an
embodiment, two
emulsifiers are used such as a soy lecithin and a sucrose monoester.
[00259] When a carrier oil is used, the cannabinoid or cannabis-derived
compound
(e.g. cannabinoid distillate and/or isolate) and carrier oil may be mixed
using any methods
known in the art to reduce the size of the cannabinoid particles in the oil to
form a
homogenous mixture. Suitable methods include, for example, homogenization
methods as
known in the art (e.g., high-pressure homogenization (HPH), high-shear
homogenization,
microfluidization). In some embodiments, the cannabinoid or cannabis-derived
compound
and carrier oil are mixed under heated conditions such as by mixing in a
microwave.
[00260] Small droplet sizes lead to transparent emulsions. In an
embodiment,
droplet sizes of between about 30 nm and about 100 nm are desirable for the
homogenous
mixture. In an embodiment, droplet sizes about 100, 90, 80, 70, 60, 50 or 40
nm are
desirable for the homogenous mixture. Suitably, the droplet sizes for
homogenous
emulsions are in the range of 40 to 60 nm, more suitably they are 45 to 55 nm,
more
suitably yet, 50 nm.
49
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00261] Once the homogenous mixture is formed, the surfactant and
emulsifier can
be mixed into the homogenous mixture. Suitable methods for mixing the
surfactant and
emulsifier into the homogenous mixture include any known methods for mixing
components
into a formulation. It has been found, however, that by first adding the
surfactant to the
homogenous mixture, yet not mixing in the surfactant, with the mixture and
then
subsequently slowing adding the emulsifier to the surfactant-containing
mixture, gelatinous
clumps can be avoided. In embodiments in which more than one emulsifier is
used, they
may be added separately at different times, added separately at the same time,
or mixed
together and then added together. In a particular embodiment, two emulsifiers
are added
separately at the same time.
[00262] This mixture can then be homogenized, such as by using a bench top
homogenizer to mix all the ingredients thoroughly. After this, the mix can be
put into a
microfluidizer where between about 2,500 and about 40,000 psi, more
particularly between
about 10,000 and about 40,000 psi, and more particularly still between about
20,000 and
about 40,000 psi, of pressure is applied to create an emulsion system with
very small
particles (< 100 nm). In an embodiment, the microfluidizing provides a
particle size of
about 40 nm. In an embodiment, the resulting water-soluble formulation is
completely
clear.
[00263] Further, if any additives as described above are to be included in
the
water-soluble formulation, it is suitable to mix the additives into the
homogenous mixture
prior to mixing the surfactant and emulsifier into the homogenous mixture.
[00264] A product may be prepared by mixing the water-soluble formulation
with an
aqueous solution. In an embodiment, the product is a beverage. In an
embodiment, the
method further comprises mixing a sucrose monoester into the homogenous
mixture.
[00265] Further, if any additives as described herein are to be included
in the product,
it is suitable to mix the additives into the product at the appropriate stage.
For example, in
an embodiment, the product may comprise one or more of: terpenes, terpenoids,
flavonoids,
viscosity modifiers, natural emulsifiers, oils, thickening agents, minerals,
acids, bases,
vitamins, flavours, colourants, sweeteners, and combinations thereof. In a
particular
embodiment, the method further comprises mixing a chelating agent into the
aqueous
solution. In an embodiment, the chelating agent is EDTA.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00266] A solid product may also be prepared. An exemplary method for
preparing a
solid product comprises: mixing a cannabinoid or a cannabis-derived compound
and a
carrier oil until a homogenous mixture is formed, as described above; mixing
the surfactant
and emulsifier into the homogenous mixture to prepare the water-soluble
formulation as
described above; and absorbing the water-soluble cannabis formulation into or
onto a solid
material (e.g. tea bag).
[00267] PRODUCTS INCLUDING THE WATER-SOLUBLE FORMULATIONS
[00268] The present disclosure is further directed to using the water-
soluble
formulations to form end use products such as ingestibles, topical solids and
liquids. The
ingestibles can include, for example, beverages, liquids and foodstuffs.
[00269] Thus, the water-soluble formulations of the present disclosure may
be used
in the preparation of foodstuffs and beverages. As used herein, a beverage is
any drink
that may be consumed by a subject. A foodstuff is any substance suitable for
consumption
as a food.
[00270] The compositions may be combined with any beverage-compatible or
food-compatible ingredient. For example, water-soluble formulations of the
present
disclosure may be used directly in the preparation of foodstuffs and
beverages, e.g. as an
additive or ingredient. Powder formulations may be used either directly, e.g.
as an additive
or ingredient, or indirectly e.g. by first dissolving the powder in a solvent
(e.g. water) to form
a liquid composition prior to use. In some embodiments, the powder
compositions may be
added to beverage or foodstuff directly. In other embodiments, the powder
formulations are
diluted with a bulking agent. The pre-bulked and/or bulked powder compositions
can be
packaged for individual servings (e.g. sachets/packets), packages in bulk
within a single
container, or a combination thereof.
[00271] When used in beverages, the water-soluble formulations of the
present
disclosure further comprise a beverage liquid. Generally, beverage liquids are
liquids
meeting the common meaning of the term "biocompatible", which include
materials that are
not harmful to living tissue. Suitably, such beverage liquids comprise water,
oil, alcohol;
with or without additives or modifiers or both. Such beverage liquids can be
divided into
various groups such as plain water, alcohol, non-alcoholic drink, soft drink,
fruit juice,
vegetable juice, tea, coffee, milk, or other hot, room temperature or cold
liquids used in
51
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
drinks. Beverages can be caffeinated or non-caffeinated and may contain
calories or not.
Such beverages may be produced in ready to use form or be produced in a form
suitable
for preparation in final consumable form at or proximate to the time of
ingestion.
[00272] Typically, beverage liquids will make up between about 50% and
99.99% by
weight or by volume of the beverage. In an embodiment, the beverage liquid
will make up
between about 80% and about 99.99% by weight of the beverage. In an
embodiment, the
beverage liquid will make up between about 80% and about 99.9% by weight of
the
beverage. In an embodiment, the beverage liquid will make up between about 95%
and
about 99.9% by weight of the beverage. In an embodiment, the beverage liquid
will make
up about 80%, about 81%, about 82%, about 83%, about 84%, about 85%, about
86%,
about 87%, about 88%, about 89%, about 90%, about 91%, about 92%, about 93%,
about
94%, about 95%, about 96%, about 97%, about 98%, about 99%, or more by weight
of the
beverage. In an embodiment, the beverage liquid will make up about 99.0%,
about 99.1%,
about 99.2%, about 99.3%, about 99.4%, about 99.5%, about 99.6%, about 99.7%,
about
99.8%, or about 99.9% by weight of the beverage. In an embodiment, the
beverage liquid
is water. In an embodiment, additives may be present in addition to the
quantity of
beverage liquid. In an embodiment, a liquid additive (e.g. sweetener) may be
present in
addition to the quantity of beverage liquid.
[00273] In a particular embodiment, the beverage liquid will make up
between about
80% and about 95% by weight of the beverage, and a liquid additive (e.g.
sweetener) will
make up between about 4.9% and about 14.9% by weight of the beverage. In
select
embodiments, the beverage liquid will make up about 80%, about 81%, about 82%,
about
83%, about 84%, about 85%, about 86%, about 87%, about 88%, about 89%, about
90%,
about 91%, about 92%, about 93%, about 94%, about 95% by weight of the
beverage. In
select embodiments, a liquid additive (e.g. sweetener) will make up about 18%,
about 17%,
about 16%, about 15%, about 14%, about 13%, about 12%, about 11%, about 10%,
about
9%, about 8%, about 7%, about 6%, about 5%, about 4%, about 3% or less by
weight of
the beverage. In an embodiment, the beverage liquid is water and the liquid
additive is a
liquid sweetener, such as for example a 67.5 Bx (Brix) sugar solution.
[00274] Non-limiting examples of beverages that may be prepared with the
water-soluble formulations of the present disclosure include but are not
limited to: hot and
cold beverages including water, fruit juice, vegetable juice, tea, coffee,
softs drinks, energy
52
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
drinks, alcohol, flavoured water, or single-serve beverage cartridges. Non-
limiting
examples of foodstuffs include baked goods (e.g. cookies, brownies, cake, pie,
biscuits and
pastries), candies (e.g. hard candy, soft candy, gummies, etc.), chocolates,
lozenges, gum,
mints, dried fruits, nuts, granola, truffles, caramels, chews, taffy, prepared
meals, cooking
ingredients (e.g. food additives, dry spices, honey, sugar, sweeteners, etc.),
ground coffee,
instant coffee and tea leaves.
[00275] The amount of the water-soluble formulation of the present
disclosure added
to beverages or foodstuffs will vary depending on the desired dosage of
cannabinoids
(e.g. THC and CBD) or cannabis-derived compound. For example, in some
embodiments
each serving, unit or item of foodstuff or beverage will contain about 0.5 mg
to about 100
mg of cannabinoids. In an embodiment, the foodstuff or beverage will contain
about 2.0 mg
to about 10 mg of cannabinoids. In an embodiment, the foodstuff or beverage
will contain
about 0.5 mg, about 1.0 mg, about 1.5 mg, about 2.0 mg, about 2.5 mg, about
3.0 mg,
about 3.5 mg, about 4.0 mg, about 4.5 mg, about 5.0 mg, about 5.5 mg, about
6.0 mg,
about 6.5 mg, about 7.0 mg, about 7.5 mg, about 8.0 mg, about 8.5 mg, about
9.0 mg,
about 9.5 mg, or about 10.0 mg of cannabinoids. In an embodiment, the
cannabinoid is
THC. In an embodiment, the cannabinoid is CBD.
[00276] In an embodiment, the product (e.g. beverage or foodstuff) may
comprise
between about 0.5% and about 25% by weight of the water-soluble formulation,
more
particularly between about 1% and about 10% by weight of the water-soluble
formulation,
and more particularly still between about 1% and about 5% by weight of the
water-soluble
formulation. In an embodiment, the product may comprise between about 0.5% and
about
3% by weight of the water-soluble formulation. In some embodiments, low
quantities by
weight of the water-soluble formulation may be used due to advantageous
properties of the
water-soluble formulations of the present disclosure in stably formulating
cannabinoids or
cannabis-based compounds.
[00277] In one embodiment, the water-soluble formulations are mixed with
an
aqueous solution to prepare an end use product (e.g. beverage). The aqueous
solution
can include pure water alone, or an aqueous solution including water and
additives such as
the additives described above to improve end use product stability,
bioavailability, colour,
aroma and taste. Particularly, additives may include terpenes, terpenoids,
flavonoids,
viscosity modifiers, natural emulsifiers, oils, thickening agents, minerals,
acids, bases,
53
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
vitamins, flavours, colourants, sweeteners (liquid and/or solid), and the like
and
combinations thereof, as known in the beverage and food arts.
[00278] In an embodiment, the aqueous solution comprises a stabilizer as
described
herein. In an embodiment, the aqueous solution comprises a chelating agent.
The
chelating agent may be added before or after the water-soluble formulation is
mixed with
the aqueous solution. In an embodiment, the chelating agent is EDTA.
[00279] In some embodiments, the end products incorporating the water-
soluble
formulations of the present disclosure include less than 20% by weight
glycerin, including
less than less than 15% by weight, less than 10% by weight, less than 5% by
weight, and
less than 1% by weight glycerin.
[00280] Beverages may be are packaged as individual packages, suitably
single
use packages, and multiple packages. The packaging can be in air tight
containers.
Packaging may be comprised of paper, plastic, metal, and glass. Beverages may
include
bubble containing or producing liquids with dissolved gas or liquids capable
of producing
gas proximately in time of consumption. In one embodiment of the disclosure,
the
beverages, optionally comprising additives, modifiers or both, are convenient
to
consumers, and are manufactured at modest expense. Beverages with dissolved
gas may
be created by a method comprising addition of carbon dioxide, ozone, oxygen,
and
nitrogen. For beverages with dissolved gas, dissolved gas may be added to the
beverage
by methods comprising application of pressure, and adding water with the
dissolved gas.
The dissolved gas is released from the beverage when pressure is reduced as
effervescence.
[00281] In another embodiment, the water-soluble formulations are absorbed
into a
solid material for use as an end use product. By way of example, the water-
soluble
formulations may be absorbed onto one or more of blotter paper, tea leaves,
coffee
grounds, spices and the like to allow for a convenient water-soluble edible or
tea bag.
[00282] The compositions of the present disclosure are suitably low
calorie, and can
be used to prepare beverages and foodstuffs that are low calorie.
Particularly, in some
embodiments, a 250 mL or 2-5 g serving will provide less than 25 kilocalories
(Kcal), more
suitably less than 10 Kcal, and even more suitably less than 5 Kcal.
54
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00283] In some further embodiments, the water-soluble formulations are
further
dried to form a powder formulation for use in liquid beverages and foods. The
above
described formulations may be dried using any method as known in the drying
arts to
evaporate the water phase of the emulsion, and possibly none, some or
essentially all of
the carrier solvent. For example, in one embodiment, the formulations are
spray dried to
form the powder formulation. Alternative methods of preparing the dried powder
formulation include, but are not limited to, pan coating, air-suspension
coating, centrifugal
extrusion, vibrational nozzle technique, freeze-drying or using a food
dehydrator.
[00284] In some embodiments, the powder formulation can be diluted with a
bulking
agent or a mixture of bulking agents. Suitable bulking agents include, for
example, gum
arabic, waxy maize starch, dextrin, maltodextrin, polydextrose, inulin,
fructooligosaccharide,
sucrose, glucose, fructose, galactose, lactose, maltose, trehalose,
cellobiose, lactulose,
ribose, arabinose, xylose, lyxose, allose, altrose, mannose, gulose, talose,
erythritol,
threitol, arabitol, xylitol, mannitol, ribitol, galactitol, fucitol, inositol,
maltitol, sorbitol, isomalt,
lactitol, polyglycitol, iditol, volemitol, maltotriitol, maltotetraitol,
maltol, stevia, stevio side,
rebaudio side, neotame, sucralose, saccharin, sodium cyclamate, aspartame,
acesulfame
potassium, chitin, and chitosan. In an embodiment, the bulking agent is
erythritol. In an
embodiment, the bulking agent is sucrose. In an embodiment, the bulking agent
is inositol.
In an embodiment, the bulking agent is myo-inositol.
[00285] In some aspects, the bulking material may comprise a sweetener, pH
modifier, pH stabilizer, antimicrobial preservative, antioxidant, texture
modifier, colourant or
combinations thereof.
[00286] In some embodiments, the bulked powder formulations comprise at
least
0.001% by weight, and suitable from 0.001% by weight to about 3% by weight, of
a
cannabinoid or a cannabis-derived compound. More suitably, a dosage form for
an
exemplary product includes 10 milligram of tetrahydrocannabinol (THC) per
serving.
Assuming a 3.5 gram serving size, the bulk powder formulation would contain
approximately 0.3% by weight of the primary cannabinoid (e.g. THC and/or CBD).
Assuming a 5 gram sample size, the bulk powder formulation would contain
approximately
0.2% by weight of the primary cannabinoid.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00287] Once prepared, the powder formulation may be mixed directly in a
liquid
beverage or food or may first be dissolved in a solution to then be added to a
liquid
beverage or food. The powder formulation may be prepared and packaged using
any
packaging known in the art. For example, in one embodiment, the powder
formulation may
packaged as a single serving or multiple servings in a metal, glass, or
plastic container. In
another embodiment, the powder formulation may be packaged as a single serving
stick
pack.
[00288] Thus, in various embodiments, the present disclosure relates to a
product
comprising and/or produced using the water-soluble formulation described
herein. In an
embodiment, the product is a beverage further comprising an aqueous solution.
In an
embodiment, the product comprises a cannabinoid distillate or a cannabinoid
isolate;
monoglycerides; a soy lecithin; a sucrose monoester; and a vegetable glycerin.
In an
embodiment, the beverage comprises a stabilizer, such as for example any
stabilizer
described herein and for example a chelating agent.
[00289] In some embodiments, the water-soluble formulations, beverages
and/or
foodstuffs disclosed herein provide a desired intoxication effect as measured
by a standard
British unit of alcohol. As used herein, "one British unit of alcohol" is
defined as 10 mL (8 g)
of pure alcohol. That is the number of units of alcohol can be determined by
multiplying the
volume of the drink (in milliliters) by percentage ABV, and dividing by 1000.
[00290] Suitably, in some aspects, the beverages or foodstuffs are formed
and
administered to provide a subjective or objective intoxicating effect
equivalent to a standard
British unit of alcohol. More particularly, from about 25 mL to 500 mL of the
beverage,
more particularly, from about 35 ml to about 250 ml, and even more
particularly, from about
60 ml to about 120 ml of the beverage, are formed and administered to provide
an
intoxicating effect equivalent to a standard British unit of alcohol. By
further way of
example, in one aspect, consuming about 35 mL to about 60 mL of the beverage
causes
either a subjective or objective intoxicating effect equivalent to a standard
British unit of
alcohol. In another aspect, consuming about 60 mL to about 120 mL of the
beverage
causes either a subjective or objective intoxicating effect equivalent to a
standard British
unit of alcohol. In yet another aspect, consuming about 120 mL to about 250 mL
of the
beverage causes either a subjective or objective intoxicating effect
equivalent to a standard
British unit of alcohol. In yet another aspect, consuming about 250 mL to
about 500 mL of
56
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
the beverage causes either a subjective or objective intoxicating effect
equivalent to a
standard British unit of alcohol.
[00291] It will further be appreciated that in certain embodiments the
beverage or
foodstuff should provide the human or non-human subject an intoxicating effect
at the
desired time. For example, in some embodiments, the beverage or foodstuff
provides for
an onset of intoxication in a time period of from about 10 minutes to about
120 minutes,
including from about 20 minutes to about 90 minutes, and including from about
30 minutes
to about 60 minutes, after consumption of the beverage or foodstuff. By way of
further
example, in certain embodiments the beverage or foodstuff can be formed and
administered to provide for an onset of the intoxication of about 10 minutes,
or about 15
minutes, or about 20 minutes, or about 25 minutes, or about 30 minutes, 40
minutes, 60
minutes, 90 minutes, or even 120 minutes. In further examples and embodiments,
the
beverage or foodstuff can be formed and administered to provide for an onset
of the
intoxication of about 180 minutes, or even about 240 minutes, or even still
about 300
minutes.
[00292] Advantageously, embodiments of the products (e.g. beverages and/or
foodstuffs) comprising or produced using the water-soluble formulations of the
present
disclosure are shelf-stable.
[00293] As used in the context of the products herein, "shelf-stable"
refers to the
water-soluble formulation maintaining its water-soluble nature in an aqueous
product at
least in respect of the cannabinoid or cannabis-derived compound (e.g., no
precipitation of
these compounds) for a period of at least 30 days, more suitably, at least 40
days, even
more suitably, at least 45 days, and more suitably, at least 50 days, and even
more
suitably, at least 55 days or longer.
[00294] In particularly suitable embodiments, the products disclosed
herein enhance
or maintain the stability of the cannabinoids or cannabis-derived compounds.
In an
embodiment, loss of cannabinoids or cannabis-derived compounds in the products
disclosed herein is less than 35% by weight in 3 months, more particularly
less than 25%
by weight in 3 months, and more particularly still less than 20% by weight in
3 months. In
an embodiment, loss of cannabinoids or cannabis-derived compounds in the
products
disclosed herein is about 25%, about 20%, about 15%, about 10%, about 5%, or
less, by
57
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
weight in 3 months. In an embodiment, loss of cannabinoids or cannabis-derived
compounds in the products disclosed herein is less than 16% by weight THC
content in 70
days.
[00295] In particularly suitable embodiments, the products disclosed
herein are
stable. By "stable", it is meant that the products remain free from one or
more deleterious
changes over a period of time, for example at least or longer than 1 day, 1
week, 1 month,
3 months, 6 months, 1 year, or more. For example, stable may be in reference
to a lack of
degradation of cannabinoids or cannabis-derived compounds; a maintenance of
clarity; or
a maintenance of any other property desirable for consumption.
[00296] In an embodiment, at least 60%, at least 65%, at least 70%, at
least 75%, at
least 80%, at least 85%, at least 90%, or at least 95% by weight of the
original or time 0
quantity of the cannabinoid or cannabis-derived compound remains present in
the product
after about 2 months at a temperature between about 17 C and about 40 C. In an
embodiment, at least 80% by weight of the original or time 0 quantity of the
cannabinoid or
cannabis-derived compound remains present in the product after about 2 months
at a
temperature between about 17 C and about 40 C. In an embodiment, at least 90%
by
weight of the original or time 0 quantity of the cannabinoid or cannabis-
derived compound
remains present in the product after about 2 months at a temperature between
about 17 C
and about 40 C.
[00297] In an embodiment, at least 60%, at least 65%, at least 70%, at
least 75%, at
least 80%, at least 85%, at least 90%, or at least 95% by weight of the
original or time 0
quantity of the cannabinoid or cannabis-derived compound remains present in
the product
after about 3 months at a temperature between about 17 C and about 40 C. In an
embodiment, at least 80% by weight of the original or time 0 quantity of the
cannabinoid or
cannabis-derived compound remains present in the product after about 3 months
at a
temperature between about 17 C and about 40 C. In an embodiment, at least 90%
by
weight of the original or time 0 quantity of the cannabinoid or cannabis-
derived compound
remains present in the product after about 3 months at a temperature between
about 17 C
and about 40 C.
[00298] In an embodiment, at least 80% by weight of the original or time 0
quantity
of the cannabinoid or cannabis-derived compound remains present in the product
after
58
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
about 2 months at about 40 C. In an embodiment, at least 90% by weight of the
original or
time 0 quantity of the cannabinoid or cannabis-derived compound remains
present in the
product after about 2 months at about 40 C.
[00299] In an embodiment, at least 80% by weight of the original or time 0
quantity of
the cannabinoid or cannabis-derived compound remains present in the product
after about
3 months at about 40 C. In an embodiment, at least 90% by weight of the
original or time 0
quantity of the cannabinoid or cannabis-derived compound remains present in
the product
after about 3 months at about 40 C.
[00300] In an embodiment, at least 84.89% by weight of the original or
time 0
quantity of the cannabinoid or cannabis-derived compound remains present in
the product
after about 3 months at a temperature of about 40 C.
[00301] In any of the embodiments described herein, the product may have a
reduced oxygen content, such as by removing the oxygen by means of equipment
designed
to perform this function or by chemical removal (e.g. N2 purge and/or
potassium disulfite).
In an embodiment, the oxygen content of the product is between about 0 ppm and
about
500 ppm. In an embodiment, the product is sealed until use in order to
maintain the
reduced oxygen content.
[00302] DOSAGE FORMS
[00303] A dosage form is that object delivered to a subject human or non-
human
organism for testing, placebo, recreational, therapeutic or other use. In an
embodiment, the
compositions of the present disclosure may be formulated as dosage forms for
administration to a subject (e.g. the liquid or powder formulation within a
soft gel capsule; a
tablet comprising the powder formulation; the liquid or powder formulation
absorbed onto or
into a solid material).
[00304] Thus, in some embodiments, the dried powder formulation can be
formulated into pharmaceutical dosage forms comprising an effective amount of
particles.
Although mainly pharmaceutical dosage forms for oral administration such as
tablets and
capsules are envisaged, the particles of the present disclosure can also be
used to prepare
pharmaceutical dosage forms e.g., for rectal administration. Preferred dosage
forms are
those adapted for oral administration shaped as a tablet. They can be produced
by
59
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
conventional tabletting techniques with conventional ingredients or excipients
and with
conventional tabletting machines.
[00305] As known in the art, tablet blends (including the powder
formulations
disclosed herein and any other conventional tablet ingredient or excipient)
may be
dry-granulated or wet-granulated before tabletting. The tabletting process
itself is
otherwise standard and readily practised by moulding a tablet from a desired
blend or
mixture of ingredients into the appropriate shape using a conventional tablet
press.
[00306] Tablets may further be film-coated to improve taste or provide
ease of
swallowing and an elegant appearance. Many suitable polymeric film-coating
materials
are known in the art. A preferred film-coating material is hydroxypropyl
methylcellulose
HPMC, especially HPMC 2910 5 mPas. Other suitable film-forming polymers also
may
be used herein, including hydroxypropylcellulose and acrylate-methacrylate
copolymers.
Besides a film-forming polymer, the film coat may further comprise a
plasticizer
(e.g. propylene glycol) and, optionally, a pigment (e.g. titanium dioxide).
The film-coating
suspension also may contain talc as an anti-adhesive.
[00307] As noted above, embodiments of end use products including the
water-soluble formulations of the present disclosure show improved
pharmacokinetics, for
example, rapid onset, shorter duration, consistent experience and minimal food
effect.
[00308] Perceived onset is driven by total dosage consumed in one unit of
time and
how quickly the cannabinoids are absorbed after ingestion. With the use of the
water-
soluble formulations having a nanometer average particle size of components,
increased
surface area for absorption is achieved, allowing for improved onset. Further,
the use of
the carrier oils including the emulsified cannabinoids or cannabis-derived
compounds,
lymphatic absorption is encouraged, thereby bypassing first pass metabolism
and food
effects. Finally, the use of the biocompatible surfactant (e.g. glycerin-based
carrier
surfactant) in the water-soluble formulation increases uptake of the
cannabinoids within the
dosage forms by mimicking natural metabolic processes in the gut.
[00309] Consistency is driven by the stability of the water-soluble
formulation used in
the end use product and dosage form, which as described above is improved as
compared
to conventional cannabis formulations.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00310] Suitable dosages of the end use products and dosage forms will
depend
upon many factors including, for example, age and weight of an individual, at
least one
precise event requiring professional consultation, severity of an event,
specific
water-soluble formulation to be used in the end product, route of
administration and
combinations thereof. Ultimately, a suitable dosage can be readily determined
by one
skilled in the art such as, for example, a physician, a veterinarian, a
scientist, and other
medical and research professionals. For example, one skilled in the art can
begin with a
low dosage that can be increased until reaching the desired treatment outcome
or result.
Alternatively, one skilled in the art can begin with a high dosage that can be
decreased until
reaching a minimum dosage needed to achieve the desired treatment outcome or
result.
[00311] In some embodiments, the end use products and dosage forms are
prepared
with water-soluble formulations in a dosage form and administration regime to
provide a
desired intoxication effect as measured by a standard British unit of alcohol,
as described
elsewhere herein in respect of products. This disclosure is equally applicable
in respect of
dosage forms.
[00312] EXEMPLARY EMBODIMENTS
[00313] The following are non-limiting and exemplary embodiments of the
present
disclosure:
[00314] (1) A water-soluble formulation comprising a cannabinoid or a
cannabis-derived compound; an emulsifier; and a glycerin-based carrier
surfactant.
[00315] (2) The water-soluble formulation of (1), further comprising a
carrier oil.
[00316] (3) The water-soluble formulation of (2), wherein the carrier oil
is comprised
of monoglycerides.
[00317] (4) The water-soluble formulation of (3), wherein the
monoglycerides
comprise glyceryl monostearate, glyceryl hydroxystearate, glyceryl monoleate,
winterized
glyceryl monoleate, monolaurin, glyceryl monolinoleate, or any combination
thereof.
[00318] (5) The water-soluble formulation of any one of (2) to (4), which
comprises
up to 10% by weight of the cannabinoid or cannabis-derived compound; up to 10%
by
weight of the carrier oil, and up to 10% by weight of the emulsifier.
61
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00319] (6) The water-soluble formulation of any one of (2) to (5), which
comprises
the cannabinoid or cannabis-derived compound; the carrier oil, and the
emulsifier at an
about equivalent amount by weight.
[00320] (7) The water-soluble formulation of any one of (1) to (6), which
is an
emulsion.
[00321] (8) The water-soluble formulation of any one of (1) to (7), which
is clear.
[00322] (9) The water-soluble formulation of any one of (1) to (8), which
is
transparent, translucent, or pearlescent when mixed with an aqueous solution.
[00323] (10) The water-soluble formulation of any one of (1) to (9),
wherein the
cannabinoid is Cannabigerolic Acid (CBGA), Cannabigerolic Acid monomethylether
(CBGAM), Cannabigerol (CBG), Cannabigerol monomethylether (CBGM),
Cannabigerovarinic Acid (CBGVA), Cannabigerovarin (CBGV), Cannabichromenic
Acid
(CBCA), Cannabichromene (CBC), Cannabichromevarinic Acid (CBCVA),
Cannabichromevarin (CBCV), Cannabidiolic Acid (CBDA), Cannabidiol (CBD),
A6-Cannabidiol (A6-CBD), Cannabidiol monomethylether (CBDM), Cannabidiol-C4
(CBD-C4), Cannabidivarinic Acid (CBDVA), Cannabidivarin (CBDV), Cannabidiorcol
(CBD-C1), Tetrahydrocannabinolic acid A (THCA-A), Tetrahydrocannabinolic acid
B
(THCA-B), Tetrahydrocannabinol (THC or A9-THC), A8-tetrahydrocannabinol (A8-
THC),
A10-tetrahydrocannabinol (Al 0-THC), Tetrahydrocannabinolic acid C4 (THCA-C4),
Tetrahydrocannbinol C4 (THC C4), Tetrahydrocannabivarinic acid (THCVA),
Tetrahydrocannabivarin (THCV), A8-Tetrahydrocannabivarin (A8-THCV),
Tetrahydrocannabivarin (A9-THCV), Tetrahydrocannabiorcolic acid (THCA-C1),
Tetrahydrocannabiorcol (THC-C1), Delta 7 cis iso tetrahydrocannabivarin,
tetrahydrocannabinolic acid (A8-THCA), 9 tetrahydrocannabinolic acid (A9-
THCA),
Cannabicyclolic acid (CBLA), Cannabicyclol (CBL), Cannabicyclovarin (CBLV),
Cannabielsoic acid A (CBEA-A), Cannabielsoic acid B (CBEA-B), Cannabielsoin
(CBE),
Cannabinolic acid (CBNA), Cannabinol (CBN), Cannabinol methylether (CBNM),
Cannabinol-C4 (CBN-C4), Cannabivarin (CBV), Cannabino-C2 (CBN-C2),
Cannabiorcol
(CBN-C1), Cannabinodiol (CBND), Cannabinodivarin (CBDV), Cannabitriol (CBT),
11-hydroxy-A9-tetrahydrocannabinol (11-0H-THC), 11 nor 9-carboxy-A9-
tetrahydrocannabinol, Ethoxy-cannabitriolvarin (CBTVE), 10 Ethoxy-9-hydroxy-
A6a-
62
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
tetrahydrocannabinol, Cannabitriolvarin (CBTV), 8,9 Dihydroxy-A6a(10a)-
tetrahydrocannabinol (8,9-Di-OH-CBT-05), Dehydrocannabifuran (DCBF),
Cannbifuran
(CBF), Cannabichromanon (CBCN), Cannabicitran (CBT), 10 Oxo-A6a(10a)-
tetrahydrocannabinol (OTHC), A9-cis-tetrahydrocannabinol (cis-THC),
Cannabiripsol
(CBR), 3,4,5,6-tetrahydro-7-hydroxy-alpha-alpha-2-trimethyl-9-n-propy1-2,6-
methano-2H-1-
benzoxocin-5-methanol (OH-iso-HHCV), Trihydroxy-delta-9-tetrahydrocannabinol
(tri0H-
THC), Yangonin, Epigallocatechin gallate, Dodeca-2E, 4E, 8Z, 10Z-tetraenoic
acid
isobutylamide, hexahydrocannibinol, Dodeca-2E,4E-dienoic acid isobutylamide,
or any
combination thereof.
[00324] (11) The water-soluble formulation of any one of (1) to (9),
wherein the
cannabinoid is cannabidiol (CBD), tetrahydrocannabinol (THC), or a combination
thereof.
[00325] (12) The water-soluble formulation of any one of (1) to (9),
wherein the
cannabis-derived compound is a cannabis-derived cannabinoid, a cannabinoid
distillate, a
cannabinoid isolate, a terpene, or any combination thereof.
[00326] (13) The water-soluble formulation of any one of (1) to (12),
wherein the
emulsifier comprises a soy lecithin.
[00327] (14) The water-soluble formulation of any one of (1) to (12),
wherein the
emulsifier comprises a sucrose monoester.
[00328] (15) The water-soluble formulation of any one of (1) to (12),
wherein the
emulsifier comprises a soy lecithin and a sucrose monoester.
[00329] (16) The water-soluble formulation of (15), wherein the sucrose
monoester
is sucrose monopalmitate, sucrose monolaurate, sucrose monostearate, or any
combination thereof.
[00330] (17) The water-soluble formulation of (16), wherein the sucrose
monoester
is sucrose monopalmitate.
[00331] (18) The water-soluble formulation of any one of (15) to (17),
which
comprises an about equivalent amount by weight of the soy lecithin and the
sucrose
monoester.
63
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00332] (19) The water-soluble formulation of any one of (1) to (18),
wherein the
glycerin-based carrier surfactant is a vegetable glycerin.
[00333] (20) The water-soluble formulation of any one of (1) to (19),
which comprises
between about 60% and about 97% by weight of the glycerin-based carrier
surfactant.
[00334] (21) The water-soluble formulation of any one of (1) to (20),
wherein the
water-soluble formulation is shelf-stable at room temperature.
[00335] (22) The water-soluble formulation of (21), which is shelf-stable
for at least
55 days.
[00336] (23) The water-soluble formulation of any one of (1) to (22),
wherein the
water-soluble formulation loses less than 20% by weight of the cannabinoid or
cannabis-derived compound in 3 months.
[00337] (24) The water-soluble formulation of (23), which loses less than
16% by
weight THC content in 70 days.
[00338] (25) The water-soluble formulation of any one of (1) to (24),
wherein, when
mixed with an aqueous solution, provides a product which is stable.
[00339] (26) The water-soluble formulation of any one of (1) to (25),
wherein, when
mixed with the aqueous solution, provides a product in which at least 80% by
weight of the
cannabinoid or cannabis-derived compound remains present after about 2 months
at a
temperature between about 17 C and about 40 C.
[00340] (27) The water-soluble formulation of (26), wherein, at least 90%
by weight
of the cannabinoid or cannabis-derived compound remains present in the product
after
about 2 months at a temperature between about 17 C and about 40 C.
[00341] (28) The water-soluble formulation of any one of (1) to (25),
wherein, when
mixed with the aqueous solution, provides a product in which at least 84.89%
by weight of
the cannabinoid or cannabis-derived compound remains present after about 3
months at a
temperature of about 40 C.
[00342] (29) The water-soluble formulation of any one of (25) to (28),
wherein the
product has an oxygen content of between about 0 ppm and about 500 ppm.
64
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00343] (30) The water-soluble formulation of any one of (1) to (29),
comprising less
than 10 kcal per 250 mL of formulation.
[00344] (31) The water-soluble formulation of any one of (1) to (30),
which is
substantially free of cyclodextrins and modified starches.
[00345] (32) The water-soluble formulation of any one of (1) to (31)
further
comprising one or more additives selected from the group consisting of
terpenes,
terpenoids, flavonoids, viscosity modifiers, natural emulsifiers, oils,
thickening agents,
minerals, acids, bases, vitamins, flavours, colourants, and combinations
thereof\
[00346] (33) The water-soluble formulation of (32) comprising a terpene
having
antimicrobial properties.
[00347] (34) The water-soluble formulation of (32) or (33) comprising a
hop-derived
terpene blend selected from the group consisting of Aramis, Brewer's Gold,
Bravo and
combinations thereof.
[00348] (35) A powder formulation prepared by drying the water-soluble
formulation
of any one of (1) to (34).
[00349] (36) The powder formulation of (35) comprising less than 10 kcal
per 250
mg of the powder formulation.
[00350] (37) A product comprising the water-soluble formulation of any one
of (1) to
(34).
[00351] (38) The product of (37), which is a beverage and further
comprises an
aqueous solution.
[00352] (39) The product of (37) or (38), comprising a cannabinoid
distillate or a
cannabinoid isolate; monoglycerides; a soy lecithin; a sucrose monoester; and
a vegetable
glycerin.
[00353] (40) The product of claim any one of (37) to (39), further
comprising a
stabilizer.
[00354] (41) The product of (40), wherein the stabilizer is a chelating
agent.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00355] (42) The product of (41), wherein the chelating agent is calcium
disodium
EDTA.
[00356] (43) The product of any one of (37) to (42), which comprises
between about
0.5% and about 25% by weight of the water-soluble formulation.
[00357] (44) The product of (43), which comprises between about 1.0% and
about
5% by weight of the water-soluble formulation.
[00358] (45) The product of any one of (37) to (44), which further
comprises one or
more of: terpenes, terpenoids, flavonoids, viscosity modifiers, natural
emulsifiers, oils,
thickening agents, minerals, acids, bases, vitamins, flavours, colourants,
sweeteners, and
combinations thereof.
[00359] (46) The product of any one of (37) to (45), wherein from about 25
mL to
about 500 mL of the product provides an intoxicating effect equivalent to a
standard British
unit of alcohol.
[00360] (47) The product of (46), which provides for the intoxicating
effect in from
about 10 minutes to about 120 minutes after use.
[00361] (48) The product of (47), wherein the intoxicating effect lasts
for a time
period of from about 30 minutes to about 300 minutes after use.
[00362] (49) The product of any one of (37) to (48), which is shelf-stable
for at least
55 days.
[00363] (50) The product of any one of (37) to (49), which loses less than
20% by
weight of the cannabinoid or cannabis-derived compound in 3 months.
[00364] (51) The product of (50), which loses less than 16% by weight THC
content
in 70 days.
[00365] (52) The product of any one of (37) to (51), wherein the
cannabinoid or
cannabis-derived compound is stable.
66
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00366] (53) The product of any one of (37) to (52), wherein at least 80%
by weight
of the cannabinoid or cannabis-derived compound remains present after about 2
months at
a temperature between about 17 C and about 40 C.
[00367] (54) The product of (53), wherein at least 90% by weight of the
cannabinoid
or cannabis-derived compound remains present after about 2 months at a
temperature
between about 17 C and about 40 C.
[00368] (55) The product of any one of (37) to (52), wherein at least
84.89% by
weight of the cannabinoid or cannabis-derived compound remains present after
about 3
months at a temperature of about 40 C.
[00369] (56) The product of any one of (37) to (55), which has an oxygen
content of
between about 0 ppm and about 500 ppm.
[00370] (57) A method for preparing the water-soluble formulation of any
one of (1)
to (34), the method comprising mixing, in any order, a cannabinoid or a
cannabis-derived
compound with a glycerin-based carrier surfactant and an emulsifier to prepare
the
water-soluble formulation.
[00371] (58) The method according to (57), comprising: mixing the
cannabinoid or a
cannabis-derived compound with a carrier oil until a homogenous mixture is
formed; and
mixing the glycerin-based carrier surfactant and the emulsifier into the
homogenous mixture
to prepare the water-soluble formulation.
[00372] (59) The method according to (58), comprising mixing the
cannabinoid or
cannabis-derived compound and carrier oil in heated conditions.
[00373] (60) The method according to (59), wherein the heated conditions
are a
temperature between about 40 C and about 50 C.
[00374] (61) The method according to any one of (57) to (60), further
comprising
mixing a sucrose monoester into the homogenous mixture.
[00375] (62) The method according to any one of (57) to (61), further
comprising
microfluidizing the water-soluble formulation to obtain a particle size of
between about
30 nm and about 100 nm.
67
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00376] (63) The method according to (62), wherein the microfluidizing
provides a
particle size of about 40 nm.
[00377] (64) A method for preparing a product comprising the water-soluble
formulation of any one of (1) to (34), the method comprising: mixing, in any
order, a
cannabinoid or a cannabis-derived compound with a glycerin-based carrier
surfactant and
an emulsifier to prepare the water-soluble formulation; and mixing the water-
soluble
formulation with an aqueous solution.
[00378] (65) The method according to (64), comprising: mixing the
cannabinoid or
the cannabis-derived compound and a carrier oil until a homogenous mixture is
formed;
mixing the glycerin-based carrier surfactant and the emulsifier into the
homogenous mixture
to prepare the water-soluble formulation; and mixing the water-soluble
formulation with the
aqueous solution.
[00379] (66) The method according to (65), wherein the aqueous solution
comprises
one or more of: terpenes, terpenoids, flavonoids, viscosity modifiers, natural
emulsifiers,
oils, thickening agents, minerals, acids, bases, vitamins, flavours,
colourants, sweeteners,
and combinations thereof.
[00380] (67) The method according to any one of (65) to (66), further
comprising
mixing a sucrose monoester into the homogenous mixture.
[00381] (68) The method according to any one of (64) to (67), further
comprising
adding a chelating agent to the aqueous solution.
[00382] (69) The method according to (68), wherein the chelating agent is
calcium
disodium EDTA.
[00383] (70) A water-soluble formulation comprising a cannabinoid or a
cannabis-derived compound, a carrier oil, a surfactant, and an emulsifier,
wherein the
water-soluble cannabis formulation is transparent, translucent, or pearlescent
when mixed
with an aqueous solution.
[00384] (71) The water-soluble formulation of (70), which is as further
defined in any
one of (1) to (34).
68
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
EXAMPLES
[00385] The following examples are included to demonstrate various
embodiments of
the present disclosure. It should be appreciated by those of skill in the art
that the techniques
disclosed in the examples that follow represent techniques discovered by the
inventors to
function well in the practice of the present disclosure, and thus may be
considered to
constitute preferred modes for its practice. However, those of skill in the
art should, in light of
the present disclosure, appreciate that many changes can be made in the
specific
embodiments which are disclosed and still obtain a like or similar result
without departing
from the scope of the present disclosure.
[00386] Example 1
[00387] In this Example, water-soluble formulations including various
cannabinoid
distillates for use in a beverage were prepared.
[00388] Initially, 1.5 g of cannabinoid distillate was mixed with 2.0 g of
Maisine CC
and the mixture was then warmed using a microwave oven. Aliquots of 0.05 g of
beta-
pinene, 0.15g of limonene, 0.0125g of hexyl acetate, 0.15g of terpinolene, and
0.1375g of
beta-caryophyllene were then added to the cannabinoid/Maisine CC mixture and
the
mixture was stirred to incorporate these ingredients. Once the above
components were
thoroughly mixed, 194 g of glycerin was added, and 2.0 g ALCOLECO F-100 was
added
slowly with stirring. This mixture was then homogenized and put into a
microfluidizer to
form an emulsion with very small particles (< 100 nm). The resulting water-
soluble
formulation was optically clear.
[00389] The various cannabinoid distillates were: Bakerstreet (pure 100%
Indica
strain, commercially available from Canadian LP Tweed), which includes THC
only;
Houndstooth (a sativa-dominant strain, commercially available from Canadian LP
Tweed),
which includes THC only; and Penelope (a hybrid strain, commercially available
from Lift &
Co.), which includes both THC and CBD in ratios of 1.5:1, THC:CBD.
[00390] Example 2
[00391] In this Example, water-soluble formulations for use in a beverage
were
prepared and analyzed for stability.
69
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00392] The following formulations were prepared using the methods of
Example 1.
The formulations were then mixed with purified water to prepare a beverage
product.
Beverage Product with Formulation A
Water-Soluble Formulation A (100g)
(30 mL)
Component Amount Unit Component Amount Unit
0-Pinene 90 mg 0-Pinene 0.27 mg
Nerolidol 45 mg Nerolidol 0.13 mg
Isopulegol 45 mg Isopulegol 0.13 mg
gamma-
gamma-Terpinene 45 mg 0.13 mg
Terpinene
Bakerstreet
Bakerstreet Terpenes 25 Mg 0.07 mg
Terpenes
Cannabis
Cannabis Distilate
750 Mg Distilate 2.22 mg
(Bakerstreet)
(Bakerstreet)
ALCOLEC
ALCOLEC F100 1000 Mg 2.96 mg
F100
Maisine CC 1000 Mg Maisine CC 2.96 mg
Glycerol 97 G Glycerol 0.29 g
Purified Water 29.70 g
Beverage Product with Formulation B
Water-Soluble Formulation B (100g)
(30 mL)
Component Amount Unit Component
Amount Unit
Eugenol 45 mg Eugenol 0.13 mg
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
p-Cymeme 22.5 mg p-Cymeme 0.07 mg
Humulene 22.5 mg Humulene 0.07 mg
Terpinolene 67.5 mg Terpinolene 0.20 mg
6-Caryophyllene 67.5 mg R- 0.20 mg
Caryophyllene
Houndstooth Houndstooth
25 mg 0.07 mg
Terpenes Terpenes
Cannabis
Cannabis Distilate
750 mg Distilate 2.22 mg
(Houndstooth)
(Houndstooth)
ALCOLEC
ALCOLEC F100 1000 mg 2.96 mg
F100
Maisine CC 1000 mg Maisine CC 2.96 mg
Glycerol 97 g Glycerol 0.29 g
Purified Water 29.70 g
Beverage Product with Formulation
Water-Soluble Formulation C( 100g)
C (30 mL)
Component Amount Unit Component Amount Unit
Limonene 56.25 mg Limonene 0.33 mg
Hexyl Acetate 11.25 mg Hexyl Acetate 0.07 mg
Terpinolene 90 mg Terpinolene 0.53 mg
6-Caryophyllene 67.5 mg 6-
Caryophyllene 0.40 mg
Penelope
Penelope Terpenes 25 mg 0.15 mg
Terpenes
71
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
Cannabis
Cannabis Distilate
750 mg Distilate 4.44 mg
(Penelope)
(Penelope)
ALCOLECC, F100 1000 mg ALCOLECC, 5.93 mg
F100
Maisine CC 1000 mg Maisine CC 5.93 mg
Glycerol 97 g Glycerol 0.57
Purified Water 29.41
[00393] When the water-
soluble formulation was mixed into the purified water, it
resulted in a fully transparent, optically clear mixture.
[00394] Stability of the water-soluble formulation and end use beverage
for
Formulation A were monitored for 71 days. The formulations and beverages were
kept at
17 C and sampled once per week. The results are listed in the Table below and
the data in
the table is illustrated in FIGS. 1A & 1B.
THC Concentration (`)/0 w/w)
. Beverage
Product with
Days Water-Soluble Formulation
Formulation
0 0.8100 0.0077
8 0.8500 0.0073
16 0.8100 0.0076
23 0.8100 0.0072
43 0.7900 0.0070
50 0.7700 0.0064
72
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
57 0.7900 0.0068
68 0.7800 0.0065
71 0.8000 0.0063
[00395] As shown in the table and FIGS. 1A & 1B, the THC concentration
remained
relatively stable over a period of 71 days. That is the formulation lost less
than 16% by
weight THC content in 70 days.
[00396] Example 3
[00397] In this Example, the experiential effect and organoleptic
properties of
formulations of THC distillate was determined.
[00398] 500 grams of cannabis from Bakerstreet (Relax), Penelope
(Enhance/Do),
and Houndstooth (socialize) was cold ethanol extracted to produce a resin. The
resin was
processed by short path distillation to produce a distillate free from
volatile organic
compounds and other impurities. Each drink trial used 1.0 gram of distillate
for formulation
to be prepared. Formulation of the drink syrup was done two days prior to each
session to
allow time for testing and CoA generation. The drink syrup was diluted the day
of the trial to
2 mg/30 mL in MilliQ water. One strain was tested per session and each strain
was tested a
minimum of two times. Trials took place on the same day and the same time for
6 weeks to
12 weeks. Participants were required to remain onsite for one hour following
dose
administration to ensure safety, as well as timely and accurate recording of
experience.
[00399] For organoleptic testing, drink formulations were compared against
a
placebo to confirm that the tastes were indistinguishable. Participants did
not consume the
drinks to minimize THC ingestion. A binary preference based test similar to a
paired-
comparison test was used. Each participant sampled one placebo and one
cannabis drink
and rated which they thought contained cannabis. Results were compared across
the
entire study group and analyzed by binomial test. The 95% confidence interval
was
calculated.
73
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00400] For experiential testing, dosages of 6 mg/person THC (or 3x30 mL
volumes
of 2 mg per 30 mL volume) were taken at once. Tombstone data (gender, weight,
tolerance,
experience, etc.) was collected for each participant. Self-reporting of
intoxication was
obtained by journal entry and cognitive impairment was obtained using a mobile
app
(Otorize). Journal entry and cognitive impairment were collected in parallel
to track
experience at 15 minute intervals with a baseline established directly before
testing.
Cognitive impairment testing measuring reaction time, decision making, time
estimation,
motor tracking, and balance were obtained using Druid (www.druidapp.com). For
self-
reporting, categories were created to match marketing expectations and divided
in the
"positive" and "negative" words (see, Table). The sum of each rating for each
domain was
calculated for both positive and negative words for each participant in order
to minimize
reporting bias. Each week, participants completed a self-reporting survey at
different set
time points beginning at baseline and followed for 5 hours after consumption.
74
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
HabO', Sad
G G
r.lerr, Serio
Mood
fu
Re a. ed An=lous
c Dep=es.s.ed
E.hL arated Fa7 gued
Ale Groggy.
Energet:c Tired
Energy
L-yely Letnarg,c
Hyper Lazy
PeoP,
Ta ,at Zoned DJ':
Art cue Incche'e.n!
Voca al, et
Social
Do nh,o led ParanOid
Open Gaurded
Dul
gh Eu.n-. Out
Drun: Dicomented
Stoned Sober
Intoxication
Into- cated Sc
Buzzed C earbeaded
NaLicea!ed
Product ve Forget'.11
Focused Scartered
Creat ve Slc
Work
Mot vated cored
ma,-;.native Conf...ed
Inventive Aoatnetc
Cold
T.rg
Numb Sore
Physical
Comfortable Rest ess
Heavy J
Ccz, ir= :ated
Table: intoxication trial designs
[00401] Participants rated how they felt in each category (mood, energy,
social,
intoxication level, work, and physical) on a scale of -3 to +3. Three stages
of intoxication
were analyzed including sober, elevated, and intoxicated. Intoxication levels
over time were
reported on a scale from 1(Sober) to 10 (the most intoxicated one could
imagine). Ratings
were then summed across the negative and positive words for a category to
create an
average minimizing bias. The average was then plotted in each category for
each member
of the test group following a modified diamond of opposites test (see, FIGS.
2A-2C). The
resulting point (x, y) was plotted for each participant and domain to create 6
distinct scatter-
plot matrices where the x- axis and y-axis represented the positive words and
negative
words respectfully associated with the given domain. Modified diamond of
opposites test
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
was done on the average scores for each category to track how the experience
changed
over time. Data that was contradictory was discarded from the analysis. Data
points
(excluding contradictory points for each domain) were also summed together to
create a
vector with some magnitude that was used to generate polar area charts to
compare gestalt
results across all categories. The magnitude and direction of this vector was
calculated and
used to create polar area or radar charts to show the complete experience in
each category
for each trial across the study and to compare the differences between the 3
stages of
intoxication studied. These charts were compared over time to see how the
experience
evolved or between formulations to identify ensemble effects. Individual word
pairs were
also analyzed and used to generate polar area and radar charts (see, FIGS. 3A-
3B). The
direction of these vectors indicated a positive or negative association
between the THC-
infused drinks and the domains.
[00402] Intoxication as measured using the mobile app or by self-reporting
was used
to create a model for intoxication by the formulations and compared to
preexisting models
for alcohol intoxication (see, FIGS. 4A-4C). This data was used to develop
additional
formulations.
[00403] As shown in FIG. 2A, the box contains the normal baseline
variability in
experience without intoxication. Values outside this box were considered "of
interest" for
analysis and/or possible follow up. As shown in FIG. 2B, the boxes contained
contradictions
(self reports of conflicting subjective experience). Values outside these
boxes were
considered "of interest" for analysis and/or possible follow up. As shown in
FIG. 2C, the box
in the upper left contains negative results (bad outcomes in some category).
The box in the
lower right of FIG. 2C contains positive results (good outcomes in some
category).
[00404] Formulation A provided primarily mood, energy, and social
experiences
(FIG. 3A). Formulation C provided primarily mood, physical, intoxication, and
social
experiences (FIG. 3B).
[00405] FIG. 5 shows the intoxication level over time for a participant
with cannabis
tolerance administered a 6 mg dose of a formulation using Bakerstreet strain
without
terpenes. FIG. 6 shows the intoxication level over time for a participant with
cannabis
tolerance administered a 12 mg dose of a formulation using Penelope strain
with terpenes.
FIG. 7 shows the intoxication level over time for a participant with cannabis
tolerance
76
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
administered a 16 mg dose of a formulation using Penelope strain with terpenes
and esters.
FIG. 8 shows the intoxication level over time for a participant without
cannabis tolerance
administered a 12 mg dose of a formulation using Penelope strain with terpenes
and esters.
FIGS. 9A-9C are polar area charts showing the three levels of intoxication for
6 mg doses
of a formulation using Bakerstreet strain without terpenes. FIGS. 10A-10C are
polar area
charts showing the three levels of intoxication for 12 mg doses of a
formulation using
Penelope strain with terpenes and esters.
[00406] These results demonstrated that the resultant vectors calculated
from
modified diamond of opposites tests could be used to indicate positive or
negative
association between the THC- infused drinks and the domains studied. For the 6
mg dose
of Bakerstreet without terpenes the data indicated a potential positive
correlation between
intoxication level and mood, energy, social, and intoxication domain. A
potential negative
correlation between intoxication level and work, and a varying correlation
between
intoxication level and physical depended on the level of intoxication. For the
12 mg dose
of Penelope with terpenes and esters the data indicated a potential positive
correlation
between intoxication level and mood, energy, intoxication domain, and
physical. A
potential negative correlation between intoxication level and social and a
varying
correlation between intoxication level and work depended on the level of
intoxication.
77
[00407]
The following are additional exemplary water-soluble
formulations of the present disclosure: 0
n.)
o
n.)
o
1-,
o
Concentration of -4
1-,
Carrier Emulsifier
Antioxidant in
Distillate Terpenes Carrier Oil Oil
Emulsifier Glycerin Antioxidant .6.
Amount Water (g)
Formulation Ethanol (g)
(g) (g) Type
Amount Type
(g) (g)
Used
(PPM)
2.0 0.0 Maisine CC 2.0 Alcolec F100
2.0 194.0 0.0 N/A 0.0 0.0
2.0 0.0 Maisine CC 4.0 Alcolec F100
2.0 192.0 0.0 N/A 0.0 0.0
P
2.0 0.0 Maisine CC 2.0 Alcolec F100
4.0 192.1 0.0 N/A 0.0 0.0
,
N)
.
N)
,
co 2.0 0.0 Maisine CC 4.0 Alcolec F100
4.0 190.1 0.0 N/A 0.0 0.0
.
N)
'7
.
,
4.0 0.0 Maisine CC 2.0 Alcolec F100
2.0 192.0 0.0 N/A 0.0 0.0 ,
,
4.0 0.0 Maisine CC 4.0 Alcolec F100
2.0 190.0 0.0 N/A 0.0 0.0
4.0 0.0 Maisine CC 2.0 Alcolec F100
4.0 190.0 0.0 N/A 0.0 0.0
IV
4.0 0.0 Maisine CC 4.0 Alcolec F100
4.0 188.1 0.0 N/A 0.0 0.0 n
,-i
n
t."..,
u,
oo
C
n.)
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0 o
n.)
o
1-,
o
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 190.0 4.0
N/A 0.0 0.0 --.1
1-,
1-,
.6.
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 184.0 10.0
N/A 0.0 0.0
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 174.0 20.0
N/A 0.0 0.0
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 154.0 40.1
N/A 0.0 0.0
P
.
Alpha
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 190.0
4.1 200.0 0.0 ,
r.,
Tocopherol
0
r.,
--A
,
co
r.,
0
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 190.0 4.1
Alpha 100.0 0.0
Tocopherol
,
,
0
,
,
ha
...]
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 190.0 4.1
Alp 50.0 0.0
Tocopherol
2.0 0.0 Labrasol 2.0 Alcolec F100 2.0 194.0 0.0 N/A
0.0 0.0
Labrafac
2.0 0.0 Lipophile 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0
WL 1349
IV
n
,-i
n
t."..,
u,
oe
C
n.)
o
n.)
o
1--,
Labrafil
=
2.0 0.0 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0 --.1
M1944
1--,
1--,
.6.
2.0 0.0 Peceol 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0
Plurol
2.0 0.0 Oliqiue CC 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0
497
No Carrier
2.0 0.0 0.0 Alcolec F100 2.0 196.0 0.0
N/A 0.0 0.0
Oil used
P
.
,
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0 "
i.,
co
,
i,
i.,
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 193.0 0.0
N/A 0.0 1.0 ,
i
i
,
...i
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 189.7 5.0
N/A 0.0 0.0
Alpha-
2.0 0.0 Maisine CC 2.0 Tocopherol 2.0 194.0 0.0
N/A 0.0 0.0
Conjugate
Alpha-
2.0 0.0 Maisine CC 2.0 Tocopherol 2.0 0.0 194.0
N/A 0.0 0.0 IV
Conjugate
n
,-i
n
0.5 0.0 Maisine CC 2.0 Alcolec F100 2.0 195.5 0.0
N/A 0.0 0.0
tµ...)
-1
un
1--,
o
o
oo
0
(1:1)
n.)
Solution of
2.0 0.0 2.0 Alcolec F100 2.0 194.0 0.0 N/A
0.0 0.0 2
Maisine CC
o
1-,
and Labrasol
o
--.1
1-,
(9:1)
.6.
Solution of
2.0 0.0 2.0 Alcolec F100 2.0 194.0 0.0 N/A
0.0 0.0
Labrasol and
Maisine CC
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0
Labrafil
2.0 0.0 2.0 Alcolec F100 2.0 194.0 0.0 N/A
0.0 0.0
M1944
P
(1:1.5)
0
i,
Solution of
,
N)
2.0 0.0 2.0 Alcolec F100 2.0 194.0 0.0 N/A
0.0 0.0 0
Maisine CC
"
,
co
i,
-, and Labrasol
.
N)
,
i
2.0 2.0 N/A 0.0 Alcolec F100 2.0 194.0 0.0 N/A
0.0 0.0 .
i
,
..]
1.5 0.5 Maisine CC 2.0 Alcolec F100 2.0 154.0 0.0
N/A 0.0 0.0
(6:1) Mixture of
Labrasol and
0.0 0.0 Maisine CC 20.0 Plurol Olique CC 80.0 0.0 100.4
N/A 0.0 0.0
497
IV
n
,-i
n
t."..,
u,
oo
C
n.)
(6:1) Mixture of
o
0.0 0.0 Maisine CC 2.0 Labrasol and Plurol 42.0
0.0 56.1 N/A 0.0 0.0 n.)
o
Olique CC 497
o
--.1
1-,
1-,
.6.
0.0 4.0 Maisine CC 4.0 Alcolec F100 4.0 188.0 0.0
N/A 0.0 0.0
3.0 0.0 Maisine CC 3.1 Alcolec F100 3.1 291.0 0.0
N/A 0.0 0.0
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
Alpha 413.0 0.0
Tocopherol
P
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
Alpha 206.0 0.0 ,
Tocopherol
"
r.,
co
,
m
r.,
Al
0
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
pha 106.0 0.0 "
,
Tocopherol
1
,
,
...]
2.0 0.0 Maisine CC 2.0 Alcolec F100 2.0 194.0 0.0
N/A 0.0 0.0
Labrafac
2.0 0.0 Lipophile 2.0 Alcolec F100 2.0 194.1 0.0
N/A 0.0 0.0
WL 1349
4.00 0.00 Maisine CC 4.00 Alcolec F100 3.90 191.28
0.00 N/A 0.00 0.00 IV
n
,-i
n
4.01 0.00 Maisine CC 4.01 Alcolec F100 3.80 188.27
0.00 N/A 0.00 0.00 tµ...)
-1
un
1-,
o
o
oo
C
4.05 0.00 Maisine CC 4.03 Alcolec F100 3.70 188.35
0.00 N/A 0.00 0.00
2.00 0.00 Maisine CC 2.00 Alcolec F100 2.00 194.05
0.00 N/A 0.00 0.00
4.04 0.00 Maisine CC 4.04 Alcolec F100 3.60 188.41
0.00 N/A 0.00 0.00
4.04 0.00 Maisine CC 4.02 Alcolec F100 3.53 188.53
0.00 N/A 0.00 0.00
4.03 0.00 Maisine CC 4.08 Alcolec F100 3.41 188.60
0.00 N/A 0.00 0.00
4.01 0.00 Maisine CC 4.06 Alcolec F100 3.29 188.76
0.00 N/A 0.00 0.00
4.08 0.00 Maisine CC 4.01 Alcolec F100 3.19 188.83
0.00 N/A 0.00 0.00
co
4.05 0.00 Maisine CC 4.02 Alcolec F100 3.18 188.89
0.00 N/A 0.00 0.00
4.00 0.00 Maisine CC 4.01 Alcolec F100 2.98 188.96
0.00 N/A 0.00 0.00
4.00 0.00 Maisine CC 4.01 N/A 0.00 192.00 0.00 N/A
0.00 0.00
oo
C
3.98 0.00 Maisine CC 4.02 Alcolec F100 2.84 189.18
0.00 N/A 0.00 0.00
4.06 0.00 Maisine CC 4.03 Alcolec F100 2.70 189.53
0.00 N/A 0.00 0.00
2.00 0.00 Maisine CC 2.03 Alcolec F100 1.99 193.97
0.00 Quercetin 110.40 0.00
2.05 0.00 Maisine CC 2.02 Alcolec F100 2.00 193.99
0.00 Quercetin 208.90 0.00
2.03 0.00 Maisine CC 1.98 Alcolec F100 2.02 194.01
0.00 Quercetin 405.90 0.00
co
4.07 0.00 Maisine CC 4.06 Alcolec F100 2.56 189.45
0.00 N/A 0.00 0.00
4.01 0.00 Maisine CC 4.06 Alcolec F100 2.39 189.58
0.00 N/A 0.00 0.00
4.03 0.00 Maisine CC 4.01 Alcolec F100 2.24 189.76
0.00 N/A 0.00 0.00
4.01 0.00 Maisine CC 4.02 Alcolec F100 2.11 189.90
0.00 N/A 0.00 0.00
4.00 0.00 Maisine CC 4.00 Alcolec F100 1.95 190.04
0.00 N/A 0.00 0.00 1-3
oo
C
4.01 0.00 Maisine CC 4.00 Alcolec F100 1.77 190.24
0.00 N/A 0.00 0.00 n.)
o
n.)
o
1-,
o
4.01 0.00 Maisine CC 4.00 Alcolec F100 1.50 190.52
0.00 N/A 0.00 0.00 --.1
1-,
1-,
.6.
4.01 0.00 Maisine CC 4.01 Alcolec F100 1.25 190.74
0.00 N/A 0.00 0.00
4.01 0.00 Maisine CC 4.02 Alcolec F100 1.00 191.00
0.00 N/A 0.00 0.00
4.01 0.00 Maisine CC 3.98 Alcolec F100 5.01 187.00
0.00 N/A 0.00 0.00
P
4.02 0.00 Maisine CC 4.00 Alcolec F100 6.01 186.00
0.00 N/A 0.00 0.00 .
,
r.,
r.,
co
,
oi 3.00 1.00 Maisine CC 4.00 Alcolec F100
3.99 187.97 0.00 N/A 0.00 0.00 "
N,
'7
,
4.03 0.00 Tween 80 4.02 Alcolec F100 4.00 188.00 0.00
N/A 0.00 0.00 ,
...]
4.02 0.00 Maisine CC 4.05 Rhamnolipids 1.00 187.01
4.01 N/A 0.00 0.00
3.03 1.01 Maisine CC 4.05 Alcolec F100 4.01 188.01
0.00 N/A 0.00 0.00
IV
n
,-i
n
t."..,
u,
oo
C
Alpha
2.04 0.00 Maisine CC 2.00 Alcolec F100 2.03 194.10
0.00 Tocopherol / 400 / 100 0.00
Quercetin
Tocoblend
2.00 0.00 Maisine CC 2.01 Alcolec F100 2.03
194.01 0.00 200.00 0.00
GT-10
Tocoblend
2.04 0.00 Maisine CC 2.06 Alcolec F100 2.01
194.03 0.00 200.00 0.00
AR
Tocoblend
2.00 0.00 Maisine CC 2.00 Alcolec F100 2.00
194.00 0.00 200.00 0.00
ATR
2.00 0.00 Maisine CC 1.99 Alcolec F100 1.99 194.03
0.00 Parolox 200.00 0.00
2.00 0.00 Maisine CC 2 Alcolec FH20 2 194
0 N/A N/A N/A
co
oo
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
[00408] Example 4
[00409] In the present example, aqueous solutions were prepared using
water-soluble formulations of the present disclosure. The aqueous solutions
were analyzed
for stability under accelerated temperature conditions (40 C). The purpose of
this example
was to determine the effectiveness of surfactants, emulsifiers, and
stabilizers
(e.g. antioxidants, chelating agents, etc.) in preventing loss of cannabinoids
(e.g. due to
oxidation) in aqueous solutions containing the water-soluble formulations. The
turbidity of
the solutions was also determined.
[00410] Water-soluble formulations were prepared in accordance with Table 1
below.
The control formulation contained approximately equivalent by weight amounts
of
cannabinoid distillate, monoglycerides (carrier oil) and soy lecithin
(emulsifier), in a
vegetable glycerin (carrier surfactant). The pH studies were conducted by
adding suitable
pH adjusters (e.g. potassium citrate to adjust pH between 4.0 and 4.5). Other
modifications
to the control formulation were made as noted as "Difference from Control".
The
formulations were then mixed with purified water to prepare the aqueous
solution with a
target THC concentration of about 0.007% w/w and a target CBD concentration of
about
0.0059% w/w. The resulting aqueous solutions were all sparged with nitrogen
gas unless
otherwise indicated (i.e. "No N2").
[00411] The cannabinoid content of each aqueous solution was determined
after two
weeks and the loss of cannabinoid content after two weeks relative to the
initial cannabinoid
content was calculated. The results are listed in Table 1 below and shown in
FIG. 11.
Table 1:
Water-Soluble Turbidity THC CBD
Difference from Control pH
Formulation (NTU) loss (%) loss (%)
Control None 15.35 4.23 8.39 3.47
No N2 Not sparged with N2 16.48 4.28 22.88 5.53
pH=6.9 pH 5.67 6.92 14.50 6.27
pH=4.2 pH 5.32 4.20 8.54 13.33
pH=3.5 pH 5.84 3.49 11.69 4.65
Blend of Tocopherols added to w-s
Antioxidant: Mixed
emulsion at 20:1 w/w ratio to soy lecithin 17.88 4.27 15.12 3.81
Tocopherols
emulsifier
Blend of Tocopherols added to w-s
Mixed Tocopherols emulsion at 20:1 w/w ratio to soy lecithin
(Antioxidant)! emulsifier.
21.8 4.46 2.23 3.17
Chelating Agent
(EDTA) EDTA added to aqueous solution in minor
amount
87
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
Antioxidant added to w-s emulsion at 1:1
Antioxidant: E-306 17.29 4.14 10.46 7.04
w/w ratio to soy lecithin emulsifier
Antioxidant: Antioxidant added to w-s emulsion at
15.55 4.25 9.07 5.12
ascorbyl palmitate 0.06:1 w/w ratio to soy lecithin emulsifier
Enzyme Modified Replaced soy lecithin emulsifier with
3.43 4.38 7.95 3.91
Soy Lecithin enzyme modified lyso lecithin
Increased quantity of soy lecithin
Soy Lecithin emulsifier to 2:1 w/w to monoglyceride 24.5 4.38
17.85 15.72
carrier oil
Replaced monoglyceride carrier oil with
MCT 21.0 4.15 19.59 15.38
MCT oil
Antioxidant added to w-s emulsion at
Antioxidant: BHA 8.99 4.20 13.35 11.66
0.02:1 w/w ratio to soy lecithin emulsifier
Sucrose monopalmitate added to w-s
Sucrose monoester
(SME emulsion at 1:1 w/w ratio to soy lecithin 5.34 4.3
20.84 14.56
)
emulsifier
Chelating Agent EDTA added to aqueous solution in minor
16.11 4.38 -0.68 -1.39
(EDTA) amount
Antioxidant: Antioxidant added to w-s emulsion at
Extract of 1.6:1 w/w ratio to soy lecithin emulsifier 16.02 4.41
12.59 7.52
Rosemary
[00412] As seen in Table 1 above, the presence of oxygen (no N2 purge)
increased
the loss of THC and CBD relative to the control sample. Surprisingly, the
addition of
antioxidants either had a negligible effect or increased the loss of THC and
CBD relative to
the control sample. Also surprisingly, a chelating agent (EDTA), in the
absence of
antioxidant, significantly decreased the loss of both THC and CBD. The
combination of a
chelating agent and an antioxidant (mixed tocopherols) somewhat decreased the
amount of
THC lost after two weeks, however the effect is small compared to the effect
of a chelating
agent alone. Also, as compared to the effect of the mixed tocopherol
antioxidant alone,
which increased the loss of THC, the addition of a chelating agent was able to
counteract
the negative effect of the antioxidant and actually prevented the loss of THC.
[00413] From this, it can be concluded that oxygen contributes to the
degradation of
cannabinoids in aqueous solutions containing the water-soluble formulation.
Furthermore,
it appears that antioxidants are less effective than a chelating agent, which
had a drastic
effect in preventing the degradation of cannabinoids in aqueous solutions
containing the
water-soluble formulation of the present disclosure.
[00414] Development of stable emulsions is complex. It is noteworthy in
this
example that the combination of soy lecithin emulsifier and sucrose monoester
provided
significantly improved clarity to the aqueous solution, having a turbidity of
only 5.34. This
suggests that sucrose monoesters may be useful in strengthening the emulsion.
88
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00415] Thus, the example shows that a chelating agent and sucrose
monoester aid
in strengthening the emulsion and preventing cannabinoid loss (e.g. due to
oxidation).
Other components tested were not as effective.
[00416] Example 5
[00417] In the present example, actual beverage formulations (e.g. flavour
and
carbonation) were used to test the effectiveness of water-soluble formulations
of the
present disclosure. The purpose of this example was to determine the effect of
sucrose
monoester (SME) in strengthening the emulsion properties of water-soluble
formulations of
the present disclosure, when used in beverages in which a chelating agent
(EDTA) was
added.
[00418] The water-soluble formulations contained approximately equivalent
by
weight amounts of cannabinoid distillate, monoglyceride (carrier oil) and soy
lecithin
(emulsifier), in vegetable glycerin (carrier surfactant). Sucrose monoester
(SME; sucrose
monopalmitate) at about a 1:1 ratio with the soy lecithin was included in the
indicated
formulations as shown in Table 2 below (i.e. Beverages la and 2a). The water-
soluble
formulations, both with and without SME, were optically clear (translucent).
[00419] Beverages were prepared by mixing in the water-soluble
formulations.
Beverage 1 was formulated with a target THC concentration of 0.005% w/w using
either the
water-soluble formulation with SME ("la") or without SME ("lb"). Beverage 2
was
formulated with a target THC concentration of 0.0004% w/w using either the
water-soluble
formulation with SME ("2a") or without SME ("2b"). Chelating agent was added
at a minor
amount to the aqueous beverage solution, and the beverages were carbonated.
When the
water-soluble formulations were mixed into the beverage liquid, it resulted in
a fully
transparent mixture having the colour of the beverage liquid.
[00420] The actual cannabinoid content of each beverage was determined
prior to
and after pasteurization, and then weekly thereafter for 1 week (T1w), 2 weeks
(T2w) and 3
weeks (T3w), and at 3 months (T3M). The results are listed in Table 2 below
and shown in
FIGS. 12A and 12B.
89
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
Table 2:
% THC (w/w)
SME TO- TO-
Beverage Tlw T2w T3w T3m
Included prepast postpast
la Yes 0.00592 0.00579 0.00578 0.00570 0.00570 0.00550
lb No 0.00584 0.00584 0.00573 0.00567 0.00569 0.00546
2a Yes 0.000410 0.000415 0.000429 0.000349 0.000318 0.000282
2b No 0.000488 0.000369 0.000317 0.000150 0.000156 0.0001345
[00421] As seen in Table 2 above, in the beverage containing the higher
THC
concentration, the use of a chelating agent (EDTA) with the water-soluble
formulation was
sufficient at protecting against THC degradation as the results for beverages
la and lb are
similar. This suggests that the chelating agent was effective in preventing
degradation of
the THC. However, in the beverage with the lower THC concentration, the
inclusion of SME
in the water-soluble formulation greatly enhanced the protection against THC
degradation.
This suggests that the combination of soy lecithin and SME has a role in
strengthening the
emulsion and preventing undesired release of cannabinoids from the emulsion.
[00422] Turbidity of the beverages was also measured and is shown in Table
3
below.
Table 3:
% THC (w/w
SME
Turbidity
Turbidity at Turbidity at Turbidity at
Turbidity at
at
Beverage TO-postpast Tlw T2w T3w
(Included) TO-prepast
(NTU) (NTU) (NTU) (NTU)
(NTU)
la Yes 13.17 11.92 10.88 10.06 10.48
lb No 17.46 16.66 15.08 16.90 15.48
2a Yes 0.51 0.59 0.39 0.40 0.41
2b No 2.28 2.25 3.62 2.19 3.52
[00423] As can be seen from Table 3 above, all of the beverages had decent
clarity,
with the highest measured turbidity being below 20. Notably however, the
beverages made
using the water-soluble formulation containing the combination of soy lecithin
and SME
improved the clarity of the beverages over the water-soluble formulations
containing soy
lecithin alone.
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00424] Thus, this example shows that a water-soluble formulation
comprising both
SME and soy lecithin, in a beverage with a chelating agent added thereto,
promotes
stability of the cannabinoids in the beverage.
[00425] Notably, the beverages prepared herein using the water-soluble
formulations
of the present disclosure also had a 'clean' taste in that they did not taste
like a cannabis
plant, but rather had the desired added flavouring. Also, the addition of SME
surprisingly
did not cause the beverages to have an excessively undesirable bitter taste.
[00426] Example 6
[00427] In the present example, aqueous solutions containing water-soluble
formulations of the present disclosure were prepared and analyzed for
stability over varying
oxygen concentrations. The purpose of this example was to determine the
effectiveness of
water-soluble formulations comprising soy lecithin and sucrose monoester
(SME), with
beverages containing a chelating agent, in maintaining the stability of
cannabinoids
(e.g. THC and CBD) over varying oxygen concentrations in aqueous environments.
[00428] The water-soluble formulations contained approximately equivalent
by
weight amounts of cannabinoid distillate, monoglyceride (carrier oil), soy
lecithin (emulsifier)
and SME (emulsifier), in vegetable glycerin (carrier surfactant). Beverages
were prepared
by mixing the water-soluble formulations into an aqueous beverage medium.
[00429] Each of the beverages contained between 5.6 and 6.2 ppm THC and
between 4.3 and 5.2 ppm CBD. Beverage 1 was subjected to lab-scale removal of
oxygen.
Beverages 2-4 were sparged with nitrogen, with beverages 3 and 4 further
containing
175 ppm potassium disulfite to consume excess oxygen. All of the beverages
were
adjusted to a pH of approximately 4.4 using citric acid/potassium citrate, and
a chelating
agent was added as in previous examples to beverages 1-3 at a minor amount.
[00430] The cannabinoid content of each beverage was determined prior to
and after
pasteurization, and then weekly thereafter. Samples were held at accelerated
temperatures (40 C) and stored in amber glass bottles (355 mL capacity). The
results are
listed in Table 4 below and shown in FIG. 13A (THC) and FIG. 13B (CBD).
91
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
Table 4:
% THC Remaining % CBD Remaining
Max Dissolved
Beverage 02 (ppb) TO T1w T2w T3w TO T1w T2w T3w
1 8014 100.0 91.6 86.1 82.3 100.0 95.2 91.5 91.5
2 2666 100.0 98.3 92.8 93.0 100.0 101.8 96.4 98.2
3 442 100.0 96.1 96.1 98.6 100.0 101.4 97.8 101.5
4 688 100.0 96.8 95.8 92.8 100.0 96.8 96.3 93.7
[00431] Although it was thought that the presence of water might be
detrimental to
cannabinoid stability, the water-soluble formulations of the present
disclosure maintained
the cannabinoids in the aqueous environment, indicating excellent stability in
an aqueous
environment. Rather, stability was more closely related to oxygen
concentration.
[00432] As can be seen in Table 4 above, oxygen contributes directly to
the rate of
cannabinoid degradation, particularly for THC. Beverage 2, which was not
sparged with
nitrogen and held the highest concentration of oxygen, showed the greatest
loss of
cannabinoids over time. Beverages 3 and 4, which contained potassium
metabisulfite to
scavenge residual oxygen, had the lowest concentration of oxygen and the
slowest rate of
cannabinoid degradation, with beverage 3 that additionally contained the
chelating agent
showing the best results for both THC and CBD.
[00433] Thus, the example shows that limiting oxygen concentrations in
beverages,
in combination with using a water-soluble formulation of the present
disclosure comprising
soy lecithin and SME, provides excellent stability of cannabinoids. Even with
significant
quantities of oxygen present (Beverage 2), the water-soluble formulation still
performed
exceptionally well, retaining over 80% of the THC and over 90% of the CBD
after nearly a
month.
[00434] Example 7
[00435] In the present example, the clarity of beverages prepared using
the water-
soluble formulations of the present disclosure was observed under different pH
and ionic
conditions.
92
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00436] Aqueous solutions were prepared having different pH and ionic
conditions:
(1) pH 4.85, (2) pH 3.85, (3) Na + citrate (4 g/L), and (4) K+ citrate (4
g/L). To these aqueous
solutions was added an equivalent amount of either:
(A) a water-soluble formulation containing approximately equivalent by
weight amounts of cannabinoid distillate, monoglyceride (carrier oil), and soy
lecithin (emulsifier), in vegetable glycerin (carrier surfactant); or
(B) a water-soluble formulation containing approximately equivalent by
weight amounts of cannabinoid distillate, monoglyceride (carrier oil), soy
lecithin (emulsifier) and SME (emulsifier), in vegetable glycerin (carrier
surfactant).
[00437] The aqueous solutions were observed over the course of 24 hours.
Images
of the aqueous solutions at time 0 ("TO"), 1 hour ("Ti") and 24 hours ("T24")
can be seen in
FIG. 14. Both water-soluble formulations exhibited decent performance at 1
hour at both
pH 4.85 and pH 3.85. However, the soy lecithin and SME combination provided
better
clarity performance at high ionic conditions and over longer periods of time.
Across all
beverages, water-soluble formulations comprising both soy lecithin and SME
exhibited a
turbidity of less than 5.0 NTU.
[00438] Example 8
[00439] In the present example, a gummy product was prepared using a
water-soluble formulation of the present disclosure. The purpose of the
example was to
confirm that the water-soluble formulations could be successfully incorporated
into a
gummy product.
[00440] The gummy base was prepared with the following ingredients:
Ingredient wt%
Pectin 1.91
Cream of tartar 1.72
Citric Acid (Anhydrous) 1.05
Sugar 57.20
Glucose syrup 9.53
Water 28.60
Total 100.0
93
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00441] The gummy base was found to provide both a good gummy set and
texture.
The gummies held up well after sitting at ambient temperatures (e.g. 17-18 C)
for 1 week,
and could be readily separated from each other. It was found to be a suitable
gummy base.
[00442] To prepare gummies containing cannabinoids, the water-soluble
formulation
was added to the gummy base and a gummy product was prepared therefrom. The
water-soluble formulation contained approximately equivalent by weight amounts
of THC
cannabinoid distillate, monoglyceride (carrier oil), soy lecithin (emulsifier)
and SME
(emulsifier), in vegetable glycerin (carrier surfactant). The water-soluble
formulation was
added to the gummy base at a quantity of about 2.5 mg THC / 3.5 g gummy.
[00443] The gummies set well after preparation. An image of the resulting
gummy
product is shown in FIG. 15. After 1 week at 25 C, the gummies had retained
their shape
(see FIG. 16). It is notable that the gummies prepared with the water-soluble
formulations
produced a qualitatively similar set to gummy base without the water-soluble
formulation,
even with the water-soluble formulation containing a high level of glycerin
carrier surfactant.
From this it may be concluded that the water-soluble formulation may be
successfully
incorporated into a gummy product.
[00444] Example 9
[00445] In this example, water-soluble formulations prepared according to
the
present disclosure were added to standard tea bags comprising black tea, white
tea, herbal
tea and green tea leaves. The water-soluble formulations contained
approximately
equivalent by weight amounts of a cannabis concentrate, monoglyceride (carrier
oil), and
soy lecithin (emulsifier), in vegetable glycerin (carrier surfactant). The
cannabis
concentrate was a cannabis distillate having a 1.5:1 ratio of THC:CBD). The
water-soluble
formulation was added to the tea leaves in a tea bag at about a 10 mg quantity
of THC or a
7.5 mg quantity of CBD. One tea bag for each type of tea was placed in boiling
water and
the beverage was left to steep (brew).
[00446] The brewed tea was observed visually and the overall THC
concentration
was assessed over time. Samples were taken at 0, 20, 40, 60, 120 and 240
seconds (and
320 seconds for green tea) after addition of boiling water. 2 duplicates were
performed.
The THC and CBD concentration in the different types of tea are shown in FIGs.
17A-17D
94
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
for black tea (FIG. 17A), white tea (FIG. 17B), herbal tea (FIG. 17C) and
green tea (FIG.
17D).
[00447] Overall, the tea bags dosed with a water-soluble formulation of
the present
disclosure showed good dispersibility of THC and CBD in the steeped tea
beverage. After
40 seconds, over 80% of THC had dispersed in the tea beverage and remained (or
increased further) throughout the trial. Herbal tea was a slight outlier,
achieving 80% after
60 seconds. This may be due to plant interactions with the herbal tea. CBD
also showed
good dispersibility, but with slightly less of the CBD being dispersed in the
tea (about 65-
85%).
[00448] Example 10
[00449] Certain beverages, such as those having a pH <5.0, can sometimes
be more
difficult to maintain stability of active ingredients, such as cannabinoids.
In the present
example, beverages containing water-soluble formulations of the present
disclosure were
prepared and analyzed for stability under accelerated conditions (i.e. at 40
C).
[00450] Beverage #1 was a THC beverage having a target THC quantity of 2
mg, a
lime/ginger flavour profile, and a pH of about 4.2.
[00451] Beverage #2 was a THC beverage having a target THC quantity of 10
mg, a
bold and dark flavour/colour profile, and a pH of about 4.3.
[00452] Beverage #3 was a CBD beverage having a target CBD quantity of 20
mg, a
cucumber/mint flavour profile, and a pH of about 4.26.
[00453] Beverage #4 was a THC beverage having a target THC quantity of 2
mg, a
cucumber/mint flavour profile, and a pH of about 4.28.
[00454] The beverages were prepared using the water-soluble formulations
of the
present disclosure. Water-soluble formulations prepared according to the
present
disclosure were added to the beverages after the flavour and pH adjustments,
by mixing the
water-soluble formulation into each respective beverage. For beverages #1-3,
the
water-soluble formulations contained approximately equivalent by weight
amounts of a
cannabis concentrate, monoglyceride (carrier oil), soy lecithin (emulsifier),
and SME
(emulsifier), in vegetable glycerin (carrier surfactant). The cannabis
concentrate was either
CA 03120213 2021-05-17
WO 2020/107114 PCT/CA2019/051698
a THC distillate or a CBD isolate. For beverage #4, the water-soluble
formulation contained
approximately equivalent by weight amounts of a THC distillate, monoglyceride
(carrier oil),
and soy lecithin (emulsifier), in vegetable glycerin (carrier surfactant).
Beverage #4 did not
contain SME.
[00455] One experiment was performed under laboratory conditions in which
each
beverage was subjected to lab-scale removal of oxygen, and packaged in cans
for the
duration of the experiment. It was determined experimentally that the
dissolved oxygen
content of these beverages was typically around about 229-1438 ppm. To
simulate a
commercial product, a second experiment was performed in which the beverages
were
bottled under conditions that removed oxygen, and sealed to prevent any
exchange of
gases between the inside and outside of the bottles.
[00456] The results of Experiment 1 are shown in Table 5 below, where the
amount
of THC and CBD in the beverage are represented as a percent difference from
the target
amount (% off spec). A positive value indicates the percentage of cannabinoid
loss or
percentage less than target. A negative value indicates the percentage above
target. A
problem occurred with the beverage 2, T2M preparation and this time point
sample was
discarded.
Table 5:
Beverage 1 Beverage 2 Beverage 3 Beverage 4
Target: 2 mg THC 10 THC 20 CBD 2 THC
TO (40 C; % off spec) -0.02 -5.72 6.98 -8.28
T2w (40 C; % off spec) 7.52 16.23 8.19 -3.17
TIM (40 C; % off spec) 15.95 32.47 10.56 -0.33
T2M (40 C; % off spec) 13.38 14.58 19.55
T3M (40 C; % off spec) 20.88 47.98 37.48 19.81
[00457] Stability testing at 40 C represents accelerated stability
testing. Generally,
the 2-month data is roughly representative of 8-month stability at room
temperature.
3-month data is roughly representative of 1-year stability at room
temperature.
[00458] As can be seen in Table 5 above, beverages 1, 3 and 4 show very
good
stability at 2 months accelerated testing, with THC and CBD only being about
13%, 14%
and 19% off spec, respectively. Factoring in the percentage off spec at TO,
beverages 1, 3
and 4 show a loss of THC or CBD at 2-month accelerated testing of about
13.40%, 7.6%
96
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
and 27.83%, respectively (e.g. T2M value ¨ TO value). Loss of THC and CBD
appears to
rise at the 3-month test point, particularly for beverages 1 and 3, perhaps
due to the
presence of oxygen in this lab-scale experiment. Beverage 2 shows a lesser
degree of
cannabinoid stability under these experimental conditions, which Experiment 2
suggests is
due to the presence of oxygen in the lab-scale preparations.
[00459] The results of Experiment 2 are shown in Table 6 below, where
again the
amount of THC and CBD in the beverage are represented as a percent difference
from the
target amount (% off spec) as described above.
Table 6:
Beverage 1 Beverage 2 Beverage 3 Beverage 4
Spec: 2 mg THC 10 mg THC 20 mg CBD 2 mg THC
TO (40 C; % off spec) -2.51 -10.11 4.79 -9.70
T2w (40 C; % off spec) -4.67 -4.67 5.74 -0.55
TIM (40 C; % off spec) 3.17 -2.06 8.42 8.23
T2M (40 C; % off spec) 2.73 12.55 21.46
T3M (40 C; % off spec) 1.13 5.00 13.50 26.51
[00460] As can be seen in Table 6 above, under bottled conditions
representative of
commercial packaging, the beverages showed excellent stability of
cannabinoids. Most
notably, the calculated THC and CBD loss at 3-months accelerated testing for
beverages
1, 2 and 3 was only 3.64%, 15.11% and 8.71%, respectively (i.e. T3M value ¨ TO
value).
As representative of 1-year stability at room temperature, the water-soluble
formulations of
the present disclosure were found to provide significant cannabinoid stability
in beverages
under commercial conditions.
[00461] The outlier was beverage #4, which was about 21% and 26% off spec
at
T2M and T3M, respectively. Taking into account the TO off spec value, beverage
4 showed
THC loss of about 31.16% and 36.21% at T2M and T3M, respectively. Notably,
beverage
#4 did not contain the SME. Experimentally it was observed that this beverage
became
very turbid overtime, and this may have been due to instabilities within the
flavour syrup
that did not contain SME, which was found to help strengthen emulsions for
certain flavour
syrups. It is believed that this instability may have impacted the overall THC
content by
destabilizing the emulsification system leading to less protection of THC.
97
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
[00462] In the present disclosure, all terms referred to in singular form
are meant to
encompass plural forms of the same. Likewise, all terms referred to in plural
form are meant
to encompass singular forms of the same. Unless defined otherwise, all
technical and
scientific terms used herein have the same meaning as commonly understood by
one of
ordinary skill in the art to which this disclosure pertains.
[00463] As used herein, the term "about" refers to an approximately +/-10%
variation
from a given value. It is to be understood that such a variation is always
included in any
given value provided herein, whether or not it is specifically referred to.
[00464] It should be understood that the compositions and methods are
described in
terms of "comprising," "containing," or "including" various components or
steps, the
compositions and methods can also "consist essentially of or "consist of the
various
components and steps. Moreover, the indefinite articles "a" or "an," as used
in the claims,
are defined herein to mean one or more than one of the element that it
introduces.
[00465] For the sake of brevity, only certain ranges are explicitly
disclosed herein.
However, ranges from any lower limit may be combined with any upper limit to
recite a
range not explicitly recited, as well as, ranges from any lower limit may be
combined with
any other lower limit to recite a range not explicitly recited, in the same
way, ranges from
any upper limit may be combined with any other upper limit to recite a range
not explicitly
recited. Additionally, whenever a numerical range with a lower limit and an
upper limit is
disclosed, any number and any included range falling within the range are
specifically
disclosed. In particular, every range of values (of the form, "from about a to
about b," or,
equivalently, "from approximately a to b," or, equivalently, "from
approximately a-b")
disclosed herein is to be understood to set forth every number and range
encompassed
within the broader range of values even if not explicitly recited. Thus, every
point or
individual value may serve as its own lower or upper limit combined with any
other point or
individual value or any other lower or upper limit, to recite a range not
explicitly recited.
[00466] Therefore, the present disclosure is well adapted to attain the
ends and
advantages mentioned as well as those that are inherent therein. The
particular
embodiments disclosed above are illustrative only, as the present disclosure
may be
modified and practiced in different but equivalent manners apparent to those
skilled in the
art having the benefit of the teachings herein. Although individual
embodiments are dis-
98
CA 03120213 2021-05-17
WO 2020/107114
PCT/CA2019/051698
cussed, the disclosure covers all combinations of all those embodiments.
Furthermore, no
limitations are intended to the details of construction or design herein
shown, other than as
described in the claims below. Also, the terms in the claims have their plain,
ordinary
meaning unless otherwise explicitly and clearly defined by the patentee. It is
therefore
evident that the particular illustrative embodiments disclosed above may be
altered or
modified and all such variations are considered within the scope and spirit of
the present
disclosure. If there is any conflict in the usages of a word or term in this
specification and
one or more patent(s) or other documents that may be incorporated herein by
reference,
the definitions that are consistent with this specification should be adopted.
[00467] Many obvious variations of the embodiments set out herein will
suggest
themselves to those skilled in the art in light of the present disclosure.
Such obvious
variations are within the full intended scope of the appended claims.
99