Note: Descriptions are shown in the official language in which they were submitted.
METHOD OF USING PHOTON MODULATION FOR REGULATION OF HORMONES
IN MAMMALS
CROSS REFERENCE TO RELATED APPLICATION
[0001] The present application claims priority to U.S. Application No.
62/480,685, as filed on
April 3, 2017, entitled "PHOTON MODULATION MANAGEMENT SYSTEM FOR
STIMULATION OF A DESIRED RESPONSE IN MAMMALS AND FISH", the entire contents
of which are incorporated herein by reference for all purposes.
SUMMARY
[0002] The following embodiments and aspects thereof are described and
illustrated in
conjunction with systems, tools and methods, which are meant to be exemplary
and illustrative,
not limiting in scope.
[0003] An embodiment of the present invention comprises a method of
regulation of hormones
in mammals, the method comprising: providing a system for pulsing photon
signals toward a
mammal comprising: at least one photon emitter; at least one photon emission
modulation
controller in communication with the photon emitter; where the at least one
photon emitter is
configured to produce a photon signal directed toward the mammal, where the
photon signal
comprises two or more independent components, where the two or more
independent components
comprise: a first independent component of a repetitive first modulated photon
pulse group, where
the first modulated photon pulse group has one or more first photon pulse ON
durations with one
or more first intensities, has one or more first photon pulse OFF durations,
and a first wavelength
color; where the one or more durations of the first photon pulse ON is between
0.01 microseconds
and 5000 milliseconds and where the one or more durations of the first photon
OFF is between is
between 0.1 microseconds and 24 hours; and a second independent component of a
repetitive
second modulated photon pulse group, where the second modulated photon pulse
group has one
1
Date Recue/Date Received 2021-06-11
or more second photon pulse ON durations with one or more second intensities,
has one or more
second photon pulse OFF durations, and a second wavelength color; where the
one or more
durations of the second photon pulse ON is between 0.01 microseconds and 5000
milliseconds and
where the one or more durations of the second photon OFF is between is between
0.1 microseconds
and 24 hours; where the first independent component and the second independent
component are
produced within the signal simultaneously; where the second modulated photon
pulse group is
different from the first modulated photon pulse group; and emitting the signal
toward the mammal;
where the combined effect of the signal is regulation of hormone levels in the
mammal when
compared to the established baseline hormone level of the mammal and/or, the
modification of
behavior, reproduction cycling, hair growth, calming or metabolism rates.
[0004]
An embodiment of the present invention further comprises a system for
regulating
hormone production in a mammal, comprising: at least one photon emitter; at
least one photon
emission modulation controller in communication with the at least one photon
emitter; where the
at least one photon emitter is configured to produce a photon signal to the
mammal, where the
where the photon signal comprises two or more independent components, where
the two or more
independent components comprise: a first independent component of a repetitive
first modulated
photon pulse group, where the first modulated photon pulse group has one or
more first photon
pulse ON durations with one or more first intensities, has one or more first
photon pulse OFF
durations, and a first wavelength color; where the one or more durations of
the first photon pulse
ON is between 0.01 microseconds and 5000 milliseconds and where the one or
more durations of
the first photon OFF is between is between 0.1 microseconds and 24 hours; and
a second
independent component of a repetitive second modulated photon pulse group,
where the second
modulated photon pulse group has one or more second photon pulse ON durations
with one or
2
Date Recue/Date Received 2021-06-11
more second intensities, has one or more second photon pulse OFF durations,
and a second
wavelength color; where the one or more durations of the second photon pulse
ON is between
0.01 microseconds and 5000 milliseconds and where the one or more durations of
the second
photon OFF is between is between 0.1 microseconds and 24 hours; where the
first independent
component and the second independent component are produced within the signal
simultaneously;
where the second modulated photon pulse group is different from the first
modulated photon pulse
group; and where the signal toward the mammal has the combined effect of the
first photon pulse
group and the second photon pulse group regulates hormone production in the
mammal and/or,
the modification of behavior, reproduction cycling, hair growth, calming or
metabolism rates.
BRIEF DESCRIPTION OF THE FIGURES
[0005] The accompanying drawings, which are incorporated herein and form a
part of the
specification, illustrate some, but not the only or exclusive, example
embodiments and/or features.
It is intended that the embodiments and figures disclosed herein are to be
considered illustrative
rather than limiting.
[0006] Figure 1 is a diagram showing an example of a photon modulation
growth system for
regulation of hormones in a mammal.
[0007] Figure 2 is a diagram showing an example of an individual color
photon modulation
growth system pulsing different specific wavelengths of light to regulate
hormone production in a
mammal.
[0008] Figure 3 is a diagram showing a photon emission modulation
controller in
communication with a plurality of photon emitters with sample LED arrays.
[0009] Figure 4 is a diagram showing photon emission modulation through a
master/slave
LED array.
3
Date Recue/Date Received 2021-06-11
100101 Figure 5 is a diagram showing a master logic controller in
communication and control
of a series of photon emitters.
[0011] Figure 6 is a diagram showing a photon modulation management system
in
communication with a series of mammal sensors.
[0012] Figure 7 is a diagram showing a sample LED array in communication
with various
SSRs (Solid State Relays), BITs or FETS.
[0013] Figure 8a is a photo showing the power converter, SPI, and
microcontroller of a
multiple colored die within a single LED.
[0014] Figure 8b is a photo showing the backside of the multiple colored
die within a single
LED of Figure 8a.
[0015] Figure 8c is a photo showing the high-speed switching circuitry for
flashing of the
multiple colored die within a single LED of Figure 8a.
[0016] Figure 8d is a photo showing the backside of the LED array of Figure
8c with a
replaceable multicolor die LED.
[0017] Figure 9 is an example layout of LEDs within a LED array.
[0018] Figure 10 is a flow diagram showing a method of regulation of
hormones in a mammal
through pulsing of various wavelengths to stimulate specific opsins in an
organism.
[0019] Figure 11 is a flow diagram showing a method of regulation of
hormones, behavior,
reproduction cycling, hair growth, calming or metabolism rates. in a mammal
through the use of
mammal sensors.
[0020] Figure 12 is a graph showing an example of a photon signal with a
photon pulse of near
red, with the photon signal having a repetitive rate of 400 iis for the
regulation of hormone
production in mammals.
4
Date Recue/Date Received 2021-06-11
[0021] Figure 13 is a graph showing an example of a photon signal with a
photon pulse of near
red and a photon pulse of far red, with the photon signal having a repetitive
rate of 600 i.is for the
regulation of hormone production in mammals.
[0022] Figure 14 is a second graph showing an example of a photon signal
with a photon pulse
of near red and a photon pulse of far red, where the two photon pulses have a
different duration
ON and duration OFF from the example shown in Figure 13, with the photon
signal having a
repetitive rate of 600 i.is for the regulation of hormone production in
mammals.
[0023] Figure 15 is a graph showing an example of a photon signal with a
photon pulse of blue
and a photon pulse of green, with the photon signal having a repetitive rate
of 600 i.is for the
regulation of hormone production in mammals.
[0024] Figure 16 is a graph showing an example of a photon signal with a
photon pulse of
blue, a photon pulse of green, and a pulse of near red with the photon signal
having a repetitive
rate of 800 i.is for the regulation of hormone production in mammals.
[0025] Figure 17 is a graph showing an example of a photon signal with a
photon pulse of
blue, a photon pulse of ultraviolet, a photon pulse of orange, a photon pulse
of green, and a pulse
of near red with the photon signal having a repetitive rate of 600 i.is for
the regulation of hormone
production in mammals.
[0026] Figure 18 is a third graph showing an example of a photon signal
with a photon pulse
of near red and a photon pulse of far red, where the two photon pulses have a
different duration
ON and duration OFF from the examples shown in Figure 13 and Figure 14, with
the photon signal
having a repetitive rate of 400 i.is for the regulation of hormone production
in mammals.
[0027] Figure 19 is a fourth graph showing an example of a photon signal
with a photon pulse
of near red and a photon pulse of far red, where the two photon pulses have a
different duration
Date Recue/Date Received 2021-06-11
ON with different intensities and duration OFF from the examples shown in
Figure 13 and Figure
14, with the photon signal having a repetitive rate of 400 jis for the
regulation of hormone
production in mammals.
[0028] Figure 20 is a Melatonin Elisa Kit Standard Curve showing the
concentrations ranging
from 0.04 ng/mL to 50 ng/mL. The reading of blank is not show on the plot
because of the log-
scale of X axis.
[0029] Figure 21 is a Graph of melatonin concentrations in ng/mL. The
control light is shown
in the "Subject 1 w/o" and lights as described herein is shown in "Subject 1,
w". All concentrations
were calculated based on the standards shown in Figure 20.
[0030] Figure 22 is a Melatonin Elisa Kit Standard Curve showing the
concentrations ranging
from 0.04 ng/mL to 50 ng/mL. The reading of blank is not show on the plot
because of the log-
scale of X axis.
[0031] Figure 23 is a Graph of bovine melatonin concentrations in ng/mL
with and without
lighting. The control light is shown in the "Bull 1 w/o" and lights as
described herein is shown in
"Bull 1, w". All concentrations shown are averages taken from replicate
samples. All
concentrations were calculated based on the standards shown in Figure 22.
DETAILED DESCRIPTION
[0032] Embodiments of the present disclosure provide systems, apparatuses
and methods for
regulation of hormone production in mammals, where the hormones to be
regulated may include,
but are not limited to, hypothalamic hormones, such as corticotropin-releasing
hormone, prolactin-
releasing factors (serotonin, acetylcholine, opiates, & estrogens),
somatostatin, prolactin-
inhibiting factors (dopamine), pituitary hormones such as adrenocorticotropic
hormone (ACTH),
melanocyte-stimulating hormone, endorphins, growth hormone, luteinizing
hormone (LH) and
6
Date Recue/Date Received 2021-06-11
follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH),
prolactin, epinephrine,
melatonin, leukotrienes, follicle-stimulating hormone, growth hormones,
insulin, insulin-like
growth factor, oxytocin, parathyroid hormone, thyrotropin-releasing hormone,
testosterone,
estradiol and progesterone. The systems and methods described herein include,
but are not limited
to, creating electro-magnetic wave emission pulse trains (photons) of
individual color spectrums
in sufficient intensity to drive hormone production in a mammal, as well as
using a characteristic
frequency or pattern to minimize the required input power necessary to
regulate hormone
production, while also allowing for the monitoring of the power consumption
and other variables
of the system. As will be discussed in further detail, by controlling the duty
cycle, intensity,
wavelength band and frequency of photon signals to a mammal, production of
specific hormones
can be regulated through the cycling between blue, green, yellow, near-red,
far-red, infrared and
ultra violet photon modulation.
[0033] Specifically, by combining multiple repetitive wavelengths of
photons pulses into
photon signals at specific combination of pulse rates, hormone production by
mammals can be
regulated and optimized, including allowing for an increase in the production
of specific hormones
from 0.1%, 1.0%, 5%, 7.5, 10%, 12.2%, 20%, 33.3%, 50%, 81.7%, 100%, 143.9%,
150%, 181.4%,
200%, 250%, 444.2%, 500% and 1000% or more and all integers in between, over
the baseline
hormone level of a mammal, or a decrease in the production of specific
hormones from 0.1%,
1.2%, 7.7%, 10%, 15.6, 20%, 47.2%, 50%, 74.5%, 100%, 150%, 200%, 250%, 500%
and 1000%
or less and all integers in between, under the baseline hormone level of a
mammal as in the
mammal, along with regulation or control of a mammal's mood by reducing stress
or calming the
mammal.
[0034] The embodiments of the present disclosure provided herein regulate
the production of
7
Date Recue/Date Received 2021-06-11
specific hormones. Each light "recipe" or option (a photon signal having one
or more repetitive
modulated photon pulse groups with one or more first photon pulse ON durations
with one or more
first intensities, one or more first photon pulse OFF durations, and a first
wavelength color) can be
optimized for each hormone to be regulated to each species of mammal.
[0035] An additional example embodiment to the methods, systems and
apparatuses described
herein may include less heat creation: LED lighting intrinsically creates less
heat than conventional
lights. When LED lights are used in a dosing application, they are ON less
than they are OFF.
This creates an environment with nominal heat production from the LED lights.
This is not only
beneficial in terms of not having to use energy to evacuate the heat from the
system but is beneficial
to the mammal because lighting may also be used to reduce animal stress or
calm the animal.
Regulation of Hormones
[0036] The hypothalamus functions as the coordinating center of the
endocrine system. Inputs
from the somatic and autonomic nervous system, peripheral endocrine feedback,
and
environmental cues such as light and temperature are processed in the
hypothalamus. The
hypothalamus then affects the function of multiple endocrinologic systems via
hypothalamic-
pineal interaction (via the suprachiasmatic nucleus) and the hypothalamic-
pituitary axis. The
hypothalamus is responsible for control of the circadian rhythm, temperature
regulation, and
metabolism. Hypothalamic hormones also affect pituitary hormone production.
Pituitary
hormones control adrenal, thyroid, and gonadal function in addition to water
balance, growth,
modification of behavior, reproduction cycling, hair growth, calming or
metabolism rates. and
milk production.
[0037] The hypothalamus is located in the middle of the head. It is
posterior to the eyes and
sits just below the third ventricle and above the optic chiasm and pituitary
gland. Afferent inputs
8
Date Recue/Date Received 2021-06-11
to the hypothalamus originate from the brainstem, thalamus, basal ganglia,
cerebral cortex,
olfactory areas, and the optic nerve. Efferent pathways go to the brainstem
reticular centers,
autonomic nervous system, thalamus, pineal gland, median eminence, and the
hypothalamo-
neurohypophysial tract which connects the paraventricular and supraoptic
nuclei to nerve terminal
in the posterior pituitary.
[0038] In mammals, the eye functions as the primary source of
photoreceptors and
subsequently light input. This primarily occurs through the rods/cones in the
retina that utilize
opsin-based proteins (chromophores). Rhodopsin in the best known of these
photoreceptors in
mammals. A novel photopigment, melanopsin, has also been identified in retinal
ganglion cells
named ipRGCs (intrinsically photosensitive retinal ganglion cells), but do not
have classic
photoreceptive tasks. Opsins are known to be widely expressed in other
mammalian tissues but
the utility and function of these is not as well documented. OPN3 is one
example of an extraocular
opsin. OPN3 is expressed in the brain, testis, liver, placenta, heart, lung,
muscle, kidney, pancreas,
scrotum and skin.
[0039] Visual photoreceptors take light input from the eye and turn this
into an electrical
impulse that is then sent through the optic nerve. Many of these cells
continue to the visual center
of the brain in the occipital lobe but some of the neurons traverse to the
Suprachiasmatic nucleus
(SCN) within the hypothalamus. The SCN serves as the main controller of the
circadian rhythm
in humans through the expression of "clock genes". These "clock genes"
transcribe various
proteins that result in control of multiple behavioral and physiological
rhythms including
locomotion, sleep-wake cycles, thermoregulation, cardiovascular function, and
many endocrine
processes.
[0040] Additional hypocretin-producing neurons in the lateral hypothalamus
respond to the
9
Date Recue/Date Received 2021-06-11
nutritional status of the organism and light cues from the SCN to stimulate
alertness, appetite, and
feeding behaviors. Disturbances of these cycles can result in abnormalities of
metabolism that
lead to obesity and metabolic syndrome (diabetes type II, hyperlipidemia, and
hypertension).
[0041] A multi-synaptic pathway utilizing the sympathetic nervous system
from the SCN to
the pineal gland controls release of melatonin from the pineal gland.
Melatonin is derived from
serotonin which itself is derived from the amino acid tryptophan. Melatonin is
directly involved
in the regulation of the circadian rhythm but also has a key role in the
reproductive physiology of
mammals. Specific effects include changes in sperm count, changes in
progesterone, estradiol,
luteinizing hormone, and thyroid levels. Melatonin can also inhibit sex drive
and alter
menstruation. Photoperiod directly correlates to melatonin release and the
resulting timing of
breeding season in mammals. Melatonin also affects the sleep-wake cycle, can
decrease motor
activity, lower body temperature, and induce fatigue.
[0042] Regulation and release of other hormones from the hypothalamus and
pituitary can also
be affected by complex pathways that involve the SCN. The hypothalamus
releases hormones that
travel down the pituitary stalk to the pituitary gland. These hormones then
cause release or
inhibition of pituitary hormones. Pituitary hormones then express their effect
widely throughout
the body. Examples of hypothalamic and pituitary hormones are shown in Table 1
below:
Date Recue/Date Received 2021-06-11
Table 1
Hypothalamic Hormones Pituitary Hormones
Corticotropin-releasing hormone Adrenocorticotropic hormone (ACTH)
Corticotropin-releasing hormone Melanocyte-stimulating hormone
Corticotropin-releasing hormone Endorphins
Growth hormone releasing hormone Growth hormone
Gonadotropin-releasing hormone Luteinizing hormone (LH) and
follicle-
stimulating hormone (FSH)
Thyrotropin-releasing hormone Thyroid-stimulating hormone (TSH)
Prolactin-releasing factors (serotonin, Prolactin
acetylcholine, opiates, & estrogens)
Somatostatin Inhibits release of growth hormone
Prolactin-inhibiting factors (dopamine) Inhibits release of prolactin
[0043] Table 2 below describes the effects of the hormones listed in Table
1:
Table 2
Hormone Effect
Stimulates cortisol which increases blood
ACTH sugar, suppress the immune system, and
affects metabolism of fat, protein, and
carbohydrates
Stimulates production and release of melanin
Melanocyte-stimulating hormone in skin and hair, suppresses appetite,
contributes to sexual arousal
Inhibits transmission of pain signals, produces
Endorphins
feeling of euphoria
Promotes cell growth and reproduction, cell
Growth hormone regeneration, raises glucose and fatty
acids,
stimulates production of IGF-1
Triggers ovulation, stimulates production of
LH & FSH testosterone, regulation of menstrual
cycle,
production of sperm
Stimulates release of thyroid hormone from
the thyroid gland which affects basal
TSH metabolic rate, impacts body temp and
vascular dilatation, affects growth and brain
development, sexual function, sleepy, thought
patterns
Milk production in females, also plays a role
Prolactin in metabolism, immune system
regulation, and
pancreatic development
Somatostatin Inhibits release of growth hormone
11
Date Recue/Date Received 2021-06-11
[0044]
Melatonin (N-acetyl-5-methoxytryptamine) is a major regulatory component of
the
circadian rhythm produced in the pineal gland by the amino acid, tryptophan,
via a series of
hydroxylation and methylation reactions. In response to reduced light, by
night-time, a melatonin
secretion signal is sent by the optic nerve to the pineal gland which boosts
melatonin production.
Upon production, melatonin is secreted into the bloodstream and carried
throughout the body. See
Cassone, V M, et al. "Melatonin, the Pineal Gland, and Circadian Rhythms."
Journal ofBiological
Rhythms., U.S. National Library of Medicine, 1993,
www.ncbi.nlm.nih.gov/pubmed/8274765.
"The Human Suprachiasmatic Nucleus HHMI's BioInteractive." HHMI
BioInteractive,
www.hhmi.org/biointeractive/human-suprachiasmatic-nucleus. Mure, L S, et al.
"Melanopsin-
Dependent Nonvisual Responses: Evidence for Photopigment Bistability in Vivo."
Journal of
Biological Rhythms., U.S. National Library of Medicine, Oct. 2007,
www.ncbi.nlm.nih.gov/pubmed/17876062. Musio, Carlo. "NON-VISUAL PHOTORECEPTION
in INVERTEBRATES." Non-Visual Photoreception in
Invertebrates,
photobiology.info/Musio.html. The Pineal Gland and Melatonin, Richard Bowen,
www.vivo.colostate.edu/hbooks/pathphys/endocrine/otherendo/pineal.html.
Sargis, Robert M.
"An Overview of the Pineal Gland."
Endocrine Web,
www.endocrineweb.com/endocrinology/overview-pineal-gland. Srour, Marc.
"Photoreception in
Animals." Teaching Biology, 23 Jan. 2018, bioteaching.com/photoreception-in-
animals/. Welt,
Corrine. "Hypothalamic - Pituitary Axis." UpToDate,
Apr. 2017,
www.uptodate.c om/c ontents/hypothal ami c-pituitary-axi s .
[0045]
Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein
polypeptide
petuitary hormone. The hormone is synthesized and secreted by the gonadotropic
cells of the
anterior pituitary gland, and has been found to regulate the development,
growth, pubertal
12
Date Recue/Date Received 2021-06-11
maturation, and reproductive processes of the body. See "Follicle-Stimulating
Hormone".
WebMD.
[0046] Luteinizing hormone is a pituitary hormone produced by gonadotropic
cells in the
anterior pituitary gland. In females, a rise in the hormone has been found to
trigger ovulation as
well as the development of the corpus luteum. In males, the hormone has been
found to stimulate
1 production of testosterone.https://en.wikipedia.org/wiki/Luteinizing hormone
- cite note-
Georgia-2 See ^ Ujihara M, Yamamoto K, Nomura K, Toyoshima 5, Demura H,
Nakamura Y,
Ohmura K, Osawa T (June 1992). "Subunit-specific sulphation of
oligosaccharides relating to
charge-heterogeneity in porcine lutrophin isoforms". Glycobiology. 2 (3): 225-
31.
doi:10.1093/glycob/2.3.225. PMID 1498420.
[0047] Corticotropin-releasing hormone (CRH) is a 41-amino acid peptide
derived from a 196-
amino acid preprohormone. CRH is secreted by the hypothalamus in response to
stress. Increased
CRH production has been observed to be associated with Alzheimer's disease and
major
depression, and autosomal recessive hypothalamic corticotropin deficiency has
multiple and
potentially fatal metabolic consequences including hypoglycemia. In addition
to being produced
in the hypothalamus, CRH is also synthesized in peripheral tissues, such as T
lymphocytes, and is
highly expressed in the placenta. In the placenta, CRH is a marker that
determines the length of
gestation and the timing of parturition and delivery. A rapid increase in
circulating levels of CRH occurs
at the onset of parturition, suggesting that, in addition to its metabolic
functions, CRH may act as a trigger
for parturition. See Entrez Gene: CRH corticotropin releasing hormone".
[0048] The posterior pituitary also functions by releasing hormones
synthesized in the
hypothalamus. These hypothalamic neurons produce hormones that are mobilized
down the axon
of the cell and terminate in the posterior pituitary. The main
neurohypophysial hormones and their
effect are shown in Table 3:
13
Date Recue/Date Received 2021-06-11
Table 3
Vasopression Anti-diuretic action on the kidney,
mediates
vasoconstriction of the peripheral vessels
Oxytocin
Mediates contraction of the smooth muscle of
the uterus and mammary glands
[0049] Given the photoreceptive pathways discussed above, extraocular
photoreceptors, as
well as the many complex interactions that involve the hypothalamus
(pituitary, brain stem,
autonomic nervous system, and peripheral endocrine feedback), a number of
hormones, including
those in Tables 1, 2 and 3 as well as those listed below, may be regulated by
the methods and
systems described herein through the use of pulsed photon inputs.
[0050] In addition to the hormones provided above, number of additional
hormones may be
regulated in mammals using the methods and systems provided herein, including
but not limited
to:
[0051] A. Amino acid derived hormones such as epinephrine, triidothuyronine
and thyroxine.
[0052] B. Eicosanoid hormones such as but not limited to leukotrienes.
[0053] C. Peptide hormones such as but not limited to amylin, insulin,
insulin-like growth
factor, and parathyroid hormone.
[0054] D. Steroid hormones such as testosterone, estradiol and
progesterone.
Determination of hormone concentrations in mammals
[0055] There are various analytical techniques used to determine hormone
concentration in
mammals, such as melatonin, including but not limited to enzyme immunoassay
(ELISA), high-
performance liquid chromatography (HPLC) and gas chromatography mass
spectrometry (GC-
MS).
[0056] Enzyme immunoassay (ELISA) kits have been developed to determine
melatonin
14
Date Recue/Date Received 2021-06-11
concentrations for many biological samples including Homo sapiens. ELISA
involves detection of
an analyte which is a specific substance whose presence is being
quantitatively analyzed. In
ELISA, a sample is added onto a stationary phase that contain specific binding
properties. Multiple
liquid reagents are sequentially added, incubated and washed followed by an
enzymatic reaction
that produces an optical change in the final liquid in the well from which the
concentration of the
analyte is measured. The samples are qualitatively measured with the detection
through light
transmittance by spectrophotometry. This involves quantifiable transmission of
some specific
wavelength of light through the sample and well plate. The detection
sensitivity depends on the
signal amplification during the chemical reactions. Enzymes that are linked to
the detection
reagents generate the signal which allow accurate quantification.
[0057]
High-performance liquid chromatography or HPLC may also be used to determine
hormone concentrations in mammals. HPLC is a technique in analytical chemistry
used to
separate, identify, and quantify each component in a mixture. It relies on
pumps to pass a
pressurized liquid solvent containing the sample mixture through a column
filled with a solid
adsorbent material. Each component in the sample interacts slightly
differently with the adsorbent
material, causing different flow rates for the different components and
leading to the separation of
the components as they flow out the column. HPLC has been used for
manufacturing (e.g. during
the production process of pharmaceutical and biological products), legal (e.g.
detecting
performance enhancement drugs in urine), research (e.g. separating the
components of a complex
biological sample, or of similar synthetic chemicals from each other), and
medical (e.g. detecting
vitamin D levels in blood serum) purposes. See Gerber, F.; Krummen, M;
Potgeter, H.; Roth, A.;
Siffi-in, C.; Spoendlin, C. (2004). "Practical aspects of fast reversed-phase
high-performance
liquid chromatography using 3,um particle packed columns and monolithic
columns in
Date Recue/Date Received 2021-06-11
pharmaceutical development and production working under current good
manufacturing
practice". Journal of Chromatography A. 1036 (2): 127-133. doi:
10.1016/j.chroma.2004.02.056.
PMID 15146913.
[0058] Gas chromatography¨mass spectrometry (GC-MS) is an analytical method
that
combines the features of gas-chromatography and mass spectrometry to identify
different
substances within a test sample. See 0. David Sparkman; Zelda Penton; Fulton
G. Kitson (17 May
2011). Gas Chromatography and Mass Spectrometg: A Practical Guide. Academic
Press.
ISBN 978-0-08-092015-3.
Hormone regulation in mammals through stimulation of opsins
[0059] An embodiment herein includes the regulation of hormones in a
mammals through the
emission of one or more repetitive modulated photon pulse groups within a
photon signal to the
mammal, where each repetitive pulse group has individual color spectrums or
ranges of color
spectrums, including blue, green and/or red spectrums, at a frequency,
intensity and duty cycle,
which can be customized, monitored and optimized for the specific homione to
be regulated in the
mammal while minimizing energy used in the system. By supplying control over
the rates and
efficiencies of modulated photon energy to the mammal, different parts of the
photostimulation of
the mammal's opsins located in the hypothalamus and the retina (such as red
opsins and green
opsins) photo receptors are maximized allowing for regulation of hormones,
including an increase
in the production of specific hormones from 0.1% 10%, 20%, 50%, 100%, 150%,
200%, 250%,
500% and 1000% or greater and all integers in between, over the base line
hormone level of a
mammal, a decrease in the production of specific hormones from 0.1% 10%, 20%,
50%, 100%,
150%, 200%, 250%, 500% and 1000% or less and all integers in between, under
the base line
hormone level of a mammal as in the mammal, as well as regulation or control
of a mammal mood
16
Date Recue/Date Received 2021-06-11
by reducing stress or calming the mammal.
[0060] Opsins are a type of membrane bound phytochrome receptors found in
the retina and
the hypothalamus region of the brain of mammals. Opsins mediate a variety of
functions in
mammals, including hormone production, through the conversion of photons of
light into an
electrochemical signal.
[0061] In dairy cattle, the pineal gland is involved in synthesizing and
secreting the hormone
melatonin. This synthesis is initiated in mammals via light information
received in the
suprachiasmatic nuclei via the retinohypothalmic tract. Melanopsin, which is a
photopigment, is
thought to play an important role in this light signaling cascade. Melanopsin
is in ganglion cells
such as rods and cones and is also found throughout many of the structures in
the
brain. Melanopsin photoreceptors have a peak light absorption at 480
nanometers. Additionally,
studies have shown that when melanopsin is pre-stimulated with 620 nm light
responses to 480
nm light is enhanced. This efficiency has also been proven to be wavelength,
irradiance and
duration dependent.
[0062] Melanopsin stimulation is thought to inhibit melatonin production by
the pineal
gland. Melatonin production is directly related to milk production in dairy
cows as it is an inhibitor
to prolactin, the hormone responsible for milk production. Studies have shown
that cows which
are between milk production cycles which have higher melatonin levels will
produce more milk
when brought back into a production cycle. Low melatonin levels are also
important during the
milk production cycle as it allows for maximum prolactin levels.
[0063] In an embodiment of the current disclosure, by regulating the
melatonin levels in dairy
cattle via alternating wavelengths of light, such as the simultaneous pulsing
of near-red and far red
wavelength, in an off-set pattern within a signal (such as the signal pattern
shown in Figure 13,
17
Date Recue/Date Received 2021-06-11
Figure 14 or Figure 18), milk production in cattle can be directly controlled.
This same mechanism
is thought to exist in all mammalian species.
[0064] Melatonin is also an important element of a mammal's sense of
photoperiod which is
directly hormonally tied to the ovulation cycle of the animal. By regulating
melatonin levels in
mammals via alternating wavelengths of light (such as the signal pattern shown
in Figure 13,
Figure 14 and Figure 18) mammalian ovulation may be regulated.
[0065] Photons are massless, elementary particles with no electric charge.
Photons are emitted
from a variety of sources such as molecular and nuclear processes, the quantum
of light and all
other forms of electromagnetic radiation. Photon energy can be absorbed by
phytochromes in
living mammals and convert it into an electrochemical signal which manipulates
a metabolite.
[0066] This phenomenon can be seen in the vision opsin chromophore in
humans. The
absorption of a photon of light results in the photoisomerisation of the
chromophore from the 11-
cis to an all-trans conformation. The photoisomerization induces a
conformational change in the
opsin protein, causing the activation of the phototransduction cascade. The
result is the conversion
of rhodopsin into prelumirhodopsin with an all-trans chromophore. The opsin
remains insensitive
to light in the trans form. The change is followed by several rapid shifts in
the structure of the
opsin and also changes in the relation of the chromophore to the opsin. It is
regenerated by the
replacement of the all-trans retinal by a newly synthesized 11-cis-retinal
provided from the retinal
epithelial cells. This reversible and rapid chemical cycle is responsible for
the identification and
reception to color in humans. Similar biochemical processes exist in mammals.
Phytochromes and
pheophytins behave very similarly to opsins in that they can be rapidly
regulated to switch between
the cis and trans configurations by dosing with differing wavelengths of
light.
[0067] The responses of mammals to the variations in the length of day and
night involve
18
Date Recue/Date Received 2021-06-11
photon absorption molecular changes that closely parallel those involved in
the vision cycle in
humans.
[0068] Mammal responses to a photon signal with one or more specific photon
modulations
may be monitored depending upon the desired hormone to be regulated. When the
desired
hormone is the production of melatonin, the mammal may be monitored for the
stimulation of the
pineal gland for the expression or release of melatonin or the release of
luteinizing hormones, a
heterodimeric glycoprotein to indicate impending ovulation in female mammals.
Melatonin or
luteinizing hormones may be monitored via blood or urinary samples. Samples
may be taken daily
or at various times during the day to identify the mammal reaction to the
photon modulation to
ensure efficient ovulation, or milk production.
[0069] The present disclosure also provides methods and systems for the
amount of electric
power used in the process of mammal hormone production, where the amount of
energy delivered
can be defined by calculating the total area under the graph of power over
time. The present
disclosure further provides methods and systems that allow for the monitoring,
reporting and
control of the amount of electric power used to regulate a desired hormone in
a mammal, allowing
an end user or energy provider to identify trends in energy use.
[0070] An embodiment of the system of the present disclosure comprises at
least one photon
emitter with at least one photon source, such as an LED or array of LEDs in
communication with
a photon emission modulation controller, including but not limited to a
digital output signal, a
solid-state relay or field effect transistor BJT, or FET, or power converter.
Photon emitters are
modulated to send a repetitive pulse of photons, where each individual pulse
comprises at least
one color spectrum, wavelength or multiple color spectrums or wavelengths and
is capable varying
intensities. Each photon pulse is directed toward a mammal for a duration of
time ON, such as
19
Date Recue/Date Received 2021-06-11
two milliseconds with one or more intensities, with a duration of delay or
time OFF between
photon pulses, such as two hundred milliseconds or up to 24 hours.
[0071] As used herein, "mammal" includes warm-blooded, vertebrates
possessing hair and
mammary glands, including but not limited to mammals from the orders primates
including but
not limited to humans, ungulates, including but not limited to cattle, horses,
camels, pigs, deer,
elk, alpacas, lamas, and moose, carnivores, including but not limited to
bears, the weasel family,
dogs, cats, wolves, lions, tigers, skunks, rodents, including but not limited
to rats, mice, and beaver,
chiropteras, including but not limited to bats, marsupials, including but not
limited to kangaroos
and opossums and cetacean, including, whales and dolphins.
[0072] As used herein, "duty cycle" is the length of time it takes for a
device to go through a
complete ON/OFF cycle or photon signal. Duty cycle is the percent of time that
an entity spends
in an active state as a fraction of the total time under consideration. The
term duty cycle is often
used pertaining to electrical devices, such as switching power supplies. In an
electrical device, a
60% duty cycle means the power is on 60% of the time and off 40% of the time.
An example duty
cycle of the present disclosure may range from 0.01% to 90% including all
integers in between.
[0073] As used herein "frequency" is the number of occurrences of a
repeating event per unit
time and any frequency may be used in the system of the present disclosure.
Frequency may also
refer to a temporal frequency. The repeated period is the duration of one
cycle in a repeating event,
so the period is the reciprocal of the frequency.
[0074] As used herein, the term "waveform" refers to the shape of a graph
of the varying
quantity against time or distance.
[0075] As used herein, the term "pulse wave" or "pulse train" is a kind of
non-sinusoidal
waveform that is similar to a square wave, but does not have the symmetrical
shape associated
Date Recue/Date Received 2021-06-11
with a perfect square wave. It is a term common to synthesizer programming and
is a typical
waveform available on many synthesizers. The exact shape of the wave is
determined by the duty
cycle of the oscillator. In many synthesizers, the duty cycle can be modulated
(sometimes called
pulse-width modulation) for a more dynamic timbre. The pulse wave is also
known as the
rectangular wave, the periodic version of the rectangular function.
[0076] In an embodiment of the present disclosure and as will be described
in further detail
below, the emission of one or more repetitive photon pulses within a photon
signal from the growth
system described herein where each repetitive photon pulse has a duration ON
with one or more
intensities and a duration OFF, a wavelength band and duty cycle induce a gain
efficiency greater
than 1 where Gain = Amplitude out / Amplitude in.
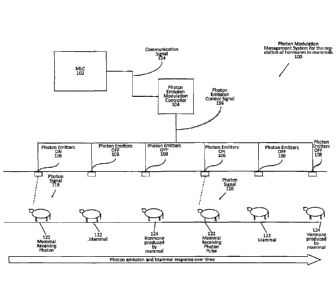
[0077] Figure 1 provides a block diagram showing an example of a photon
modulation
management system 100 for use in the regulation of hormones in mammals. As
shown in Figure
1, a photon emitter 106, 108, 110, 112, 114 and 116 is shown over a period of
time in
communication with a photon emission modulation controller 104 for the purpose
of modulating
the emission of photons to a mammal for stimulating opsins in order to
regulate hormone
production as well as to control the animals stress and mood. The modulated
application of
photons to a mammal by providing photon pulses of one or more frequencies
followed by pulses
of one or more other frequencies for a duration along with a delay between
pulses, allows for peak
stimulation/modulation of a mammals biological components (opsins receptors)
and biological
responses, including hormone production such as the pulsing of one or more
specific spectrums of
light to induce a specific electrochemical signal for the production of a
specific hormone or the
pulsing of two or more specific wavelengths within a signal, (such as the
signal pattern shown in
Figures 13-19) to produce a specific hormone, allowing for an increase in the
production of specific
21
Date Recue/Date Received 2021-06-11
hormones from 0.1%, 1.0%, 5%, 7.5, 10%, 12.2%, 20%, 33.3%, 50%, 81.7%, 100%,
143.9%,
150%, 181.4%, 200%, 250%, 444.2%, 500% and 5000% or greater and all integers
in between,
over the baseline hormone level of a mammal, or a decrease in the production
of specific hormones
from 0.1%, 1.2%, 7.7%, 10%, 15.6, 20%, 47.2%, 50%, 74.5%, 100%, 150%, 200%,
250%, 500%
and 5000% or less and all integers in between, under the baseline hormone
level of a mammal as
in the mammal, along with regulation or control of a mammal's mood by reducing
stress or
calming the mammal. Further the modulation of photons to a mammal allows for
the optimization
of photon absorption by opsin receptors without oversaturation of the mammal's
receptors. As
described below, the modulation of the photon pulses increases energy and heat
efficiency of
current dairy production lighting systems by reducing the overall power draw
by the system of the
present disclosure as much as 99% or more of the photon source when compared
to conventional
beef or dairy production lighting systems, such as a 60 watt light, thereby
reducing the amount of
power and cost used to facilitate hormone production in a mammal. In an
example of the energy
saving potential of the system of the present disclosure, the system pulses
49.2 watts of photons
for two microseconds per 200 microseconds creating an effective power
consumption of 0.49 watt-
hrs/hr on the power payment meter or 0.82% of the power in a 60-watt standard
incandescent bulb.
In addition, because the photon emitter is not continuously emitting photons,
the amount of heat
produced from the photon emitter will be significantly reduced, thereby
significantly reducing the
cost of cooling a facility to compensate for the increased heat from lighting.
The system of the
present disclosure may be customized based upon mammal specific requirements
for photon
intensity, pulse ON duration, pulse OFF (or duty cycle), the light spectrum of
the pulse including
but not limited to white, near-red, yellow, green, and blue, orange, far-red,
infrared, and ultra-
violet to encourage optimal hormone production as well as the control of the
animal's stress and
22
Date Recue/Date Received 2021-06-11
mood.
[0078] As shown in Figure 1, a master logic controller (MLC) 102, such as
solid-state circuit
with digital output control or a central processing unit (CPU) is in
communication with a photon
emission modulation controller 104 by means of a communication signal 134. The
MLC 102
provides the system of the present disclosure with input/output of the
parameters and the
appropriate instructions or the specialized functions for the modulation of
photons within a signal
from a photon emitter 106 and 108.
[0079] In a further embodiment, the MLC 102 may be hard wired or wireless
to an external
source such as a host, allowing external access to the MLC 102 by a host. This
allows remote
access by a user to monitor the input and output of the MLC 102, provide
instructions or control
to the systems while also allowing for remote programming and monitoring of
the MLC 102.
[0080] In a further embodiment, a power measurement or power consumption
sensor may be
integrated or embedded into the MLC 102 in the form of an integrated circuit
allowing for the
measurement and reporting of the power consumption of the system based on the
voltage and the
current draw of the system of the present disclosure. The power consumption of
the system can
then be communicated either wirelessly or by hardwire from the MLC 102 to a
host. Data,
including power consumption may also be sent to an outside receiver such as a
database that is not
connected to the system.
[0081] The photon emission modulation controller 104 receives commands and
instructions
from the MLC 102 including but not limited to the duration ON and intensity,
duration OFF duty
cycle, intensity, wavelength band and frequency of each repetitive photon
pulse within a photon
signal 118 from a photon emitter 106. The photon emission modulation
controller 104 may be any
device that modulates the quanta and provides the control and command for the
duration ON and
23
Date Recue/Date Received 2021-06-11
intensity, duration OFF, wavelength band and frequency of each repetitive
photon pulse from a
photon emitter 106 and 108. A variety of devices may be used as the photon
emission modulation
controller 104, including but not limited to a solid-state relay (SSR), such
as the Magnacraft 70S2
3V solid-state relay from Magnacraft Inc., optical choppers, power converters
and other devices
that induce modulation of a photon pulse. A variety of photon emitters 106 and
108 may be used,
including but not limited to, an incandescent (Tungsten-halogen and Xenon),
Fluorescent (CFL's),
high intensity discharge (Metal Halide, High-Pressure Sodium, Low-Pressure
Sodium, Mercury
Vapor), sunlight, light emitting diodes (LEDs). It should be understood that
this description is
applicable to any such system with other types of photon emission modulation
controllers,
including other methods to cycle a light or photon source ON and OFF, cycling
one or more colors
or spectrums of light at different times, durations and intensities, such as
near-red, green, blue and
far-red, allowing multiple pulses of one spectrum before pulsing another
spectrum, as will be
understood by one skilled in the art, once they understand the principles of
the embodiments.
[0082]
As shown in Figure 1, based on the instructions from the MLC 102, the photon
emission
modulation controller 104 sends a photon emission control signal 136 to a
photon emitter 106.
When the photon emission control signal 136 is sent to the photon emitter 106
goes ON, the photon
emitter 106 emits at least one photon signal 118 where each photon signal
comprises one or more
repetitive photon pulses, where each repetitive photon pulse has separate
duration ON with one or
more intensities, a wavelength band and frequency, which is transmitted to a
mammal 122. Then
based on the instructions from the MLC 102, when the photon emitter control
signal 136 sent to
the photon emitter 108 goes OFF, the photon emitter 108 will not emit a photon
pulse, and
therefore no photons are transmitted to a mammal 122. As shown in Figure 1,
starting from the
left side of Figure 1, the emission of photons 118, such as a pulse of near-
red photons, and mammal
24
Date Recue/Date Received 2021-06-11
122 hormone production 124 is shown over a period of time 120. The example of
Figure 1 provides
a photon signal 118, such as ultraviolet, violet, near-red, green, yellow,
orange, blue and far-red,
allowing multiple pulses of one spectrum before pulsing another spectrum or in
combination, as
will be understood by one skilled in the art, once they understand the
principles of the
embodiments. It should also be understood that this ON and OFF cycling can be
in the form of a
digital pulse, pulse train, or varying waveform.
[0083] As will be understood by one skilled in art, in an additional
embodiment, the system
for use in the regulation of hormones as described in Figure 1 may be
completely housed in a
single unit comprising multiple photon emitters creating an array (shown in
Figure 3, Figure 7,
Figures 8a, 8b, 8c, 8d, and Figure 9), allowing each individual single unit to
be self-sufficient,
without the need for an external control or logic unit. An example self-
sufficient unit with multiple
photon emitters may be in the form of a unit that may be connected to a light
socket, or light
fixtures that may be suspended above one or more mammals and connected to a
power source.
[0084] The systems as shown in Figure 1 may also take the form of a
master/slave system, as
will be discussed in Figure 4, where by example, a master photon emitter
containing all logic and
controls for the emission of photon from master photon emitter as well as any
additional photon
emitters in communication with the master photon emitter.
[0085] The systems as shown in Figure 1 and Figure 2 may also take the form
of a
synchronized series of lights or daisy chain of lights, where by example, two
of more photon
emitters are in communication with each other to synchronize the emission of
signals with two or
more components. To clarify, each photon emitter will individually emit a
signal comprising at
least two components, however the system, by example, through commands from a
master logic
controller, will allow for the emission of signals from the series of emitters
to be synchronized.
Date Recue/Date Received 2021-06-11
[0086] A variety of power supplies may be used in the present disclosure.
These sources of
power may include but are not limited to battery, converters for line power,
solar and/or wind
power. The intensity of the photon pulse may be static with distinct ON/OFF
cycles or the intensity
may be changes of 5% or larger of the quanta of the photon pulse. The
intensity of the photon
pulse from the photon emitter can be controlled through the variance of
voltage and/or current
from the power supplies and delivered to the light source. It will also be
appreciated by one skilled
in the art as to the support circuitry that will be required for the system of
the present disclosure,
including the photon emitter control unit and the photon emitters. Further, it
will be appreciated
that the configuration, installation and operation of the required components
and support circuitry
are well known in the art. The program code, if a program code is utilized,
for performing the
operations disclosed herein will be dependent upon the particular processor
and programming
language utilized in the system of the present disclosure. Consequently, it
will be appreciated that
the generation of a program code from the disclosure presented herein would be
within the skill of
an ordinary artisan.
[0087] Figure 2 provides a block diagrams showing an example of a photon
modulation
management system 200 for the regulation of hormones in a mammal. As shown in
Figure 2 and
repeated from Figure 1, a photon emitter 106 and 108 is shown over a period of
time in
communication with a photon emission modulation controller 104 for the purpose
of modulating
individual pulses of photons comprising individual color spectrums to a mammal
(not shown),
including but not limited to white, green, near-red, blue, yellow orange, far-
red, infrared, and ultra-
violet color spectrums, wavelength between 0.1 nm and 1 cm. As will be
understood by one skilled
in the art, the present disclosure may include color spectrums of specific,
individual wavelengths
between 0.1 nm and 1.0 cm, or may include a range or band of wavelengths 0.1
to 200 nm in width,
26
Date Recue/Date Received 2021-06-11
herein "wavelength band."
[0088] The modulation of individual color spectrums of photons to a mammal
by providing
specific color spectrum pulses for a duration along with a delay between
pulses (examples are
shown in Figures 13-19), allows for peak stimulation of a mammal's biological
components and
responses, such as a mammal's retina opsins and hypothalamus opsins for
ovulation, pineal gland
to regulation hormone production. This peak stimulation allows for the
regulation of hormones by
increasing the production of specific hormones from 0.1%, 1.0%, 5%, 7.5, 10%,
12.2%, 20%,
33.3%, 50%, 81.7%, 100%, 143.9%, 150%, 181.4%, 200%, 250%, 444.2%, 500% and
1000% and
all integers in between, over the baseline hormone level of a mammal, or
decreasing the production
of specific hormones from 0.1%, 1.2%, 7.7%, 10%, 15.6, 20%, 47.2%, 50%, 74.5%,
100%, 150%,
200%, 250%, 500% and 1000% and all integers in between, under the baseline
hormone level of
a mammal as in the mammal, along with regulation or control of a mammal's mood
by reducing
stress or calming the mammal.
[0089] Examples of the ability to control specific aspects of a mammal
biological components
or responses through the pulsing of individual color spectrums, specific color
wavelength or a
range of color wavelengths may include but are not limited to:
a. milk production in mammals through the modulation of pulses when
melanopsin
is pre-stimulated with 620 nm light responses to 480 nm light is enhanced;
b. use of blue spectrum between 390 to 470 nm to treat jaundice in prenatal
mammals, such as human premature babies;
c. ovulation through the modulation of pulses of a specific far-red
wavelength
(such as 730 nm, an example wavelength range may include 710 to 850 nm) for
a period of time;
27
Date Recue/Date Received 2021-06-11
d. hunger, growth, sexual development as well as helps to control the mood
of the
mammals by pulses of blue light, as well as the regulation of circadian
rhythms
(an example range may include with a range of 450 to 495 nm);
e. ultraviolet or violet light (by example 10 nm to 450 nm) may be used to
influence
social behavior and mood as well as to facilitate nutrient update such as
calcium;
and
f. additional orange light (590 nm to 620 nm) and/or yellow light (570 nm
to 590
nm) may also be used to influence mammal responses.
[0090] The modulation of individual color spectrums, specific wavelength
and a range of
wavelengths of photons to a mammal by providing specific color spectrum pulses
for a duration
along with a delay between pulses also allows for the control hormone
production for mood,
growth, ovulation, sexual maturity, and hunger in mammal. An example may
include one light or
through the combination of many lights, cycling the lights on and off to
control ovulation, milk
production and growth in a mammal.
[0091] As shown in Figure 2 and repeated from Figure 1, a master logic
controller (MLC) 102
is in communication with a photon emission modulation controller 104 by means
of a
communication signal 134. The MLC 102 provides the system of the present
disclosure with
input/output of the parameters and the appropriate instructions or the
specialized functions for the
modulation of a specific individual color spectrum of photons from a photon
emitter 106 and 108.
[0092] The photon emission modulation controller 104 receives commands and
instructions
from the MLC 102 including but not limited to the duration ON and intensity,
duration OFF,
wavelength band and frequency of each repetitive photon pulse 202 and 204
within a photon signal
118 or a plurality of pulses of a specific color spectrum from a photon
emitter 106 and 108 within
28
Date Recue/Date Received 2021-06-11
a photon signal. The photon emission modulation controller 104 provides the
control and
command for the duration ON and intensity, duration OFF, wavelength band and
frequency of
each repetitive photon pulse 202 and 204 within a photon signal 118 or
plurality of pulses from a
photon emitter 106, and 108.
[0093]
As shown in Figure 2, based on the instructions from the MLC 102, the photon
emission
modulation controller 104 sends a photon emission control signal 136 to a
photon emitter 106 and
108. When the photon emission control signal 136 sent to the photon emitter
106 ON, the photon
emitter 106 emits one or more repetitive photon pulses of a specific color
spectrum 202 or 204,
comprising the photon signal 118, which is transmitted to a mammal 122. Then
based on the
instructions from the MLC 102, when the photon emitter control signal 136 sent
to the photon
emitter 108 goes OFF, the photon emitter 108 will not emit a photon signal,
and therefore no
photons are transmitted to a mammal 122. As shown in Figure 2, starting from
the left side of
Figure 2, the emission of a photon signal 118 comprising repetitive photon
pulses of a specific
color spectrum 202 (green) and 204 (far-red) and mammal 122 hormone production
is shown over
a period of time 120. The example of Figure 2 provides a photon signal 118
with photon pulse or
plurality of pulses of a green color spectrum 202 emitted from a photon
emitter 106 for two (2)
milliseconds, followed by a photon pulse or plurality of pulses of a far-red
color spectrum 204 for
a duration of two (2) milliseconds with a duration of delay of two hundred
(200) milliseconds of
each pulse before the photon signal repeats with a photon pulse or plurality
of pulses 202 emitted
from the same photon emitter 106 for two milliseconds followed by a second
photon pulse or
plurality of pulses of a far-red color spectrum 204 for a duration of two
milliseconds from the same
photon emitter 114 @lease note that Figure 2 is a descriptive example of
photon pulses emitted
over time. Figure 2 is not drawn to scale and the amount of hormone production
by the mammal
29
Date Recue/Date Received 2021-06-11
between pulses in Figure 2 is not necessarily to scale). Please note that the
two pulses (green and
far-red) within the signal 118 are pulsed simultaneously but with their
durations ON and OFF
offset in this example. While two photon pulses are shown in Figure 2, as one
skilled in the art
will understand once they understand the invention, any number of pulses, from
1 to 15 or even
more, may be within a photon signal directed to an organism.
[0094] The system of the present disclosure as described in Figures 1 and 2
allows for the
regulation and control of the production of various hormones in a mammal
through the cycling of
one or more colors or spectrums of light at different times, durations and
intensities, such as near-
red, green, blue and far-red, allowing single pulses or multiple pulses of one
spectrum with a delay
before pulsing another spectrum (examples shown in Figures 13-19). The pulsing
of individual
color spectrums in unison or individually offset for a duration with a delay
between pulses within
a signal allows for increased efficiency in the stimulation of opsins for
hormone regulation and
production.
[0095] A variety of sources or devices may be used to produce photons from
the photon
emitters, many of which are known in the art. However, an example of a device
or sources suitable
for the emission or production of photons from a photon emitter include an
LED, which may be
packaged within an LED array designed to create a desired spectrum of photons.
While LEDs are
shown in this example, it will be understood by one skilled in the art that a
variety of sources may
be used for the emission of photons including but not limited to metal halide
light, fluorescent
light, high-pressure sodium light, incandescent light and LEDs. Please note
that if a metal halide
light, fluorescent light, high-pressure sodium light, incandescent light is
used with the methods,
systems and apparatuses described herein, the proper use of these forms of
photon emitters would
Date Recue/Date Received 2021-06-11
be to modulate and then filter the light to control what wavelength for what
duration is passed
through.
[0096] Embodiments of the present disclosure can apply to LEDs having
various durations of
photon emissions, including durations of photon emissions of specific color
spectrums and
intensity. The pulsed photon emissions of specific color spectrums within a
photon signal may be
longer or shorter depending on the mammal in question, the age of the mammal
and how the
emission will be used in facilitating the regulation of hormones and control
of stress or mood.
[0097] The use of an array of LEDs may be controlled to provide the optimal
photon pulse of
one or more color spectrums for specific mammal ovulation, milk production and
growth such as
in beef. The user may simply select the photon pulse intensity, color
spectrum, frequency and duty
cycle for a particular type of mammal to encourage efficient biological
responses in mammals.
LED packages can be customized to meet each mammal's specific requirements. By
using
packaged LED arrays with the customized pulsed photon emission, as discussed
above,
embodiments described herein may be used to control light to alter the mammal
weight, and sexual
maturity within the target mammal.
[0098] Figure 3 is a diagram of an example of a plurality of photon
emitters with LED arrays
300 as the source of photons from the photon emitter. As shown in Figure 3, a
photon emission
modulation controller 104 is in communication by means of a plurality of
photon emitter control
signals 136 with a plurality of photon emitters. As further shown in Figure 3,
each photon emitter
comprises an array of LEDs 302, 304, 306 and 308. Each array of LEDs 302, 304,
306 and 308
and the circuitry to allow for the array of LEDs to communicate with the
photon emission
modulation controller 104 are contained in an LED array housing 310, 312, 314
and 316.
31
Date Recue/Date Received 2021-06-11
[0099] As shown in Figure 3, the shape of LED array is a circle, however as
will be understood
by one skilled in the art, the shape of the array may take a variety of forms
based upon the needed
biological response of the mammal. The shape of the array may include but is
not limited to,
circular, square, linear, rectangular, triangular, octagonal, pentagonal and a
variety of other shapes.
1001001 The LED array housing 310, 312, 314 and 316 for each photon emitter
may be made
of a variety of suitable materials including, but are not limited to, plastic,
thermoplastic, and other
types of polymeric materials. Composite materials or other engineered
materials may also be used.
In some embodiments, the housing may be made by a plastic, aluminum, aluminum
alloy, zinc,
zinc alloy, zinc, casting or injection molding manufacturing process. In some
embodiments, the
housing may be transparent or semi-transparent and in any color.
1001011 Figure 4 is a diagram of an example of a plurality of photon
emitters with a master
photon emitter in communication and control of one or more slave photon
emitters, 400. As shown
in Figure 4, a master photon emitter 402 is in communication by means of a
photon control signal
136 with a series of slave photon emitters 404, 406, and 408. The master
photon emitter 402
contains a controller, such as the MLC (102 of Figure 1 and 2), as well as
photon emission
modulation controller (shown as 104 Figures 1 and 2) which controls the
duration ON and
intensity, duration OFF, and frequency of each specific color spectrum photon
pulse within each
photon signal from an array of LEDs housed within the master photon emitter
402 while also
allowing the master photon emitter to control the duration ON and intensity,
duration OFF, and
frequency of each specific color spectrum photon pulse within each photon
signal from each slave
photon emitters 404, 406, and 408.
[00102] Conversely, each slave photon emitter 404, 406, and 408 contains
the circuitry to
receive command signals 136 from the master photon emitter 402 and the
circuitry necessary to
32
Date Recue/Date Received 2021-06-11
emit a photon pulse of a specific spectrum from an array of LEDs (such as near-
red, far-red, blue,
green or orange) housed within each slave photon emitter 404, 406, and 408.
For clarity, each
slave photon emitter does not contain a controller such as the MLC nor does
the slave photon
emitter 404, 406, and 408 contain a photon emission modulation controller. All
commands and
controls for the slave photon emitter 404, 406, and 408 are received from the
master photon emitter
402. This master/slave system allows for sharing of a single power supply and
microcontroller.
Master has the power supply and that power is also transferred to the slaves.
Additionally, the
master/slave system can be utilized to pulse photons in patterns to help
regulate the production of
hormones in other mammals.
[00103] A bus system (wired or wireless) may be included in MLC of the
master photon emitter
402 or in each slave photon emitter 404, 406 and 408 to allow for the specific
control by the master
photon emitter 402 of each individual slave photon emitter 404, 406 and 408.
By way of example,
the master photon emitter 402 may send a signal 136 to a specific slave photon
emitter 404
commanding the slave photon emitter 404 to emit photon signal with a far-red
pulse for a specific
duration, while the master photon emitter 402 simultaneously sends a command
signal 136 to a
second slave photon emitter 406 to emit a photon signal with green pulse for a
specific duration.
While this descriptive example shows an array, plurality or chain of three
slave photon emitters
404, 406 and 408 in with a master photon emitter 402, it should be understood
that this description
is applicable to any such system with any number of slave photon emitters in
communication and
under the control of a master photon emitter, as will be understood by one
skilled in the art, once
they understand the principles of the embodiments.
[00104] In a further embodiment, the master photon emitter 402 may be hard
wired or wireless
to allow external access to the master photon emitter 402 by a host, allowing
remote access to
33
Date Recue/Date Received 2021-06-11
monitor the input and output of the master photon emitter 402 while also
allowing for remote
programming of the master photon emitter.
[00105] Figure 5 is a diagram of an example of a master logic controller in
communication and
control of one or more photon emitters, 500. As shown in Figure 5, a master
logic controller 102
is in communication by means of a photon emission control signal 136 with a
series of photon
emitters 106, 502, 504 and 506 located above four different mammals 512, 514,
516 or 518. In
this example, the master logic controller or MLC 102 (as previously discussed
in Figures 1, 2 and
3) also contains a photon emission modulation controller 104 (shown discussed
in Figures 1, 2 and
3) which allows the MLC 102 to control the duration ON and intensity, duration
OFF, and
frequency of each specific color spectrum photon pulse within a photon signal
from an array of
LEDs housed within each photon emitter 106, 502, 504 and 506.
[00106] Through the photon emission modulation controller 104, the MLC 102
communicates
commands and instructions to each photon emitter 106, 502, 504 and 506
including but not limited
to the duration ON, intensity, duration OFF and frequency of each specific
color spectrum photon
pulse within each photon signal 508 and 510 from each photon emitter 106, 502,
504 and 506. The
MLC 102 also maintains control of the power supply to the system and control
the transfer of
power to each individual photon emitter 106, 502, 504 and 506.
[00107] As shown in Figure 5, based on the instructions from the MLC 102,
the photon emission
modulation controller 104 sends a photon emission control signal 136 to each
individual photon
emitter 106, 502, 504 and 506. Based on the specific instructions sent to each
photon emitter 106,
502, 504 and 506, individual photon emitters 106 or 506 may a photon signal
comprising repetitive
photon pulses of one or more specific color spectrums 508 and 510 to a mammal
512, 514, 516 or
518 (such as a photon signal with a far-red pulse and a near-red pulse 508 at
various durations ON
34
Date Recue/Date Received 2021-06-11
and OFF or a photon signal with pulse of far-red, a pulse of near-red and a
pulse of blue at various
durations ON and OFF 510). As further shown in Figure 5, based on the
instructions from the
MLC 102, other individual photon emitters 502 or 504 may not emit a photon
signal toward a
mammal 122 for a duration.
[00108] The ability of the MLC 102 to control the photon output or emitted
from each individual
photon emitter 106, 502, 504 and 506 allows the system of the present
disclosure to modify the
photon emission to a mammal based on the specific needs or requirements for a
mammal. As
discussed in association with Figure 2, by way of example, the MLC may be
programmed to issue
a signal to a specific emitter for modulation of pulses of far-red light for a
period of time followed
by pulses of blue light in combination with near-red light for the control of
a biological responses
in mammals and mood/hunger.
[00109] In the example shown in Figure 5, all commands and controls for
each photon emitter
106, 502, 504 and 506 are received externally from the MLC 102. However, as
will be understood
by one skilled in the art, the logic and hardware associated with the MLC 102
and photon emission
modulation controller 104 may also be housed within each individual photon
emitter, allowing
each individual photon emitter to be self-sufficient, without the need for an
external control or
logic unit.
1001101 In a further embodiment, the MLC 102 may be hard wired or wireless,
allowing
external access to the MLC 102 by a user. This allows remote access by a user
to monitor the
input and output of the MLC 102 while also allowing for remote programming of
the MLC 102.
1001111 Figure 6 provides an example of a further embodiment, showing the
photon modulation
system of the present disclosure where one or more sensors are used to monitor
a mammal's
environmental conditions as well as the mammal's responses 600 to the photon
system provided
Date Recue/Date Received 2021-06-11
herein. As shown in Figure 6, one or more sensors 602, 604, 606 and 608 are
associated with each
mammal 618, 620, 622, and 624 in order to monitor various conditions
associated with the
mammal 618, 620, 622, and 624. The conditions associated with the mammal,
which may be
monitored include but are not limited to, humidity, air temperature, volume,
movement, 02, CO2,
CO, pH, and weight. As will be understood by one skilled in the art, the
sensors may include but
are not limited to temperature sensor, an infrared sensor, motion sensor,
microphones, gas sensors,
cameras, and scales.
[00112] The sensors 602, 604, 606 and 608 monitor one or more conditions
associated with the
mammal 618, 620, 622, and 624 and then transmit the data 610, 612, 614 or 616
to the MLC 102.
Transferring the data from the one or more sensors 602, 604, 606 and 608 to
the MLC 102 can be
accomplished in a number of ways, either wirelessly or hard wired. As will be
understood by one
skilled in art, a variety of communication systems may be used for the
delivery of sensor-derived
information from the mammal 618, 620, 622, and 624 to the MLC 102.
[00113] The data from the one or more sensors 602, 604, 606 and 608 is
analyzed by the MLC
102. Based on the information from the sensors, the MLC 102, through the
photon emission
modulation controller 104, the MLC 102 is able to adjust the duration ON,
intensity, duration OFF,
duty cycle and frequency of each specific color spectrum photon pulse 608 and
610 of each photon
signal 118 of each individual photon emitter 106, 602, 604 and 606, or to
adjust the duration ON,
intensity, duration OFF, duty cycle and frequency of a group of photon
emitters based on the needs
of the individual mammals 618, 620, 622, and 624 associated with a specific
sensor 602, 604, 606
and 608 or the needs of the mammals as a whole. An example may include
adjusting a signal to
comprise both blue and far-red 608 at various durations or adjusting duration
of a pulse of far-red,
green and blue 610.
36
Date Recue/Date Received 2021-06-11
[00114] In additional embodiments, the system of the present disclosure may
also include a
watering system, feeding systems, environmental as well as health system (not
shown in Figure 6)
in communication and under the control of the MLC 102 or a separate logic
controller. Based on
information from the sensors 602, 604, 606 and 608 associated with each
mammal, the MLC 102
is able to communicate with a watering system, feeding system, heating and
cooling systems,
medication systems based upon the needs of the mammals. Data, including power
can be sent to
an outside receiver such as a database that is not connected to the system.
[00115] Figure 7 provides an example of one embodiment of an array of LEDs
in
communication with a series of solid-state relays or SSRs 700. As shown in
Figure 7 and repeated
from Figure 1, a MLC 102 is in communication by means of a communication
signal 134 with a
photon emission modulation controller 104. The photon emission modulation
controller 104 of
this example contains three SSRs. The MLC 102 outputs a signal to control the
SSRs. The first
SSR controls an array of near-red LEDs 702, the second SSR controls an array
of far-red LEDs
704 and the third SSR to controls an array of blue LEDs 706. Each SSR 702, 704
and 706 is in
communication with an array of LEDs, 714, 716 and 718 by means of a photon
emission signal
136. As shown in Figure 7, the near-red SSR 702 sends a photon emission signal
136 to initiate a
photon pulse of the near-red LEDS 714 comprising a near-red voltage 708 to an
array of near-red
LEDs 714. The near-red voltage 708 is then transmitted from the array of near-
red LEDs 714 to a
series of resistors 720, 742, 738, such as a 68-ohm resistor, with each
resistor 720, 742 and 738
connected to a ground 744.
[00116] As further shown in Figure 7, the far-red SSR 704 sends a photon
emission signal 136
to initiate a photon pulse of far-red LEDs comprising a far-red voltage 710 to
an array of red LEDs
718. The red voltage 710 is then transmitted from the red LED array 718 and a
series of resistors
37
Date Recue/Date Received 2021-06-11
724, 728, 732 and 734, such as 390-ohm resistor with each resistor 724, 728,
732 and 734
connected to a ground 744. Figure 7 also shows the blue SSR 706 sending a
photon emission
signal 136 to initiate a photon pulse of blue LEDs comprising a blue voltage
712 to an array of
blue LEDs 716. The blue voltage 712 is then transmitted from the array of blue
LEDs 716 and
transmitted to a series of resistors 722, 726, 730, 736 and 740, such as a 150-
ohm resistor, with
each resistor 722, 726, 730, 736 and 740 connected to a ground 744.
[00117] Figures 8a to 8d show various aspects of an example light assembly
for the emission
of photons within a signal for use in systems and methods described herein.
Figure 8a is a photo
showing a power converter, serial peripheral interface (SPI), and
microcontroller of a multiple
colored die within a light assembly. Figure 8b is a photo showing the backside
of the multiple
colored die within the light assembly of Figure 8a. Figure 8c is a photo
showing the high-speed
switching circuitry for flashing of the multiple colored die within the light
assembly of Figure 8a.
Figure 8d is a photo showing the backside of the light assembly of Figure 8c
with a replaceable
multicolor die LED.
[00118] The light assembly of Figures 8a to 8d may be used in several
embodiments described
herein, including a master/slave system, where a master photon emitter
contains all logic and
controls for the emission of photons and signals from the master photon
emitter as well as any
additional photon emitters in communication with the master photon emitter.
The light assembly
of Figures 8a ¨ 8d may also be used in a controller system. As discussed
above, controller is in
communication with two or more photon emitters
[00119] Figure 9 provides an example layout of LEDs within a LED array 900.
As shown in
Figure 9, twelve LEDs form an array of photon emitters 302 in a photon emitter
housing 310. The
sample layout includes 400 nm (violet) 902, 436 nm (deep blue) 904, 450 nm
(royal blue) 906,
38
Date Recue/Date Received 2021-06-11
460 nm (dental blue) 908, 490 nm (cyan) 910, 525 nm (green) 912, 590 nm
(amber) 914, 625 nm
(red) 916, 660 nm (deep red) 918, and 740 nm (far red) 920.
[00120]
Figure 10 is a flow diagram showing the method of modulation of individual
color
spectrums pulsed for mammal hormone production 1000. As shown in Figure 10, in
step 1002,
the master logic controller receives instructions regarding each individual
color spectrum to be
pulsed within in a signal, the duration of each pulse of each color spectrum
within a signal, the
combination of colors to be pulsed and duration of delay between each color
spectrum pulse.
Instructions and information sent to the master logic controller may relate to
the photon pulse
duration of each color to be pulsed, photon pulse delay, intensity, frequency,
duty cycle, mammal
type, state of maturity of the mammal and the type of hormone to be produced.
In step 1004, the
master logic controller sends instructions to the photon emission modulation
controller the
regarding each color spectrum to be pulsed, the duration of each pulse of each
color spectrum,
combination of colors pulse and duration of delay between different color
spectrums. In step 1006,
the photon emission modulation controller sends at least one signal to one or
more photon emitters
capable of emitting pulses of one or more individual color spectrums toward a
mammal, such as
green LEDs, far-red LEDs, blue LEDs and orange LEDs. In step 1008, one or more
photon emitters
emit one or more photon pulses of individual color spectrums directed to a
mammal allowing for
specific opsins within the mammal to be stimulated to regulate hormone
production. The methods
for regulation of hormone production allow for hormones in a mammal to in in
production levels
by 0.1%, 1.0%, 5%, 7.5, 10%, 12.2%, 20%, 33.3%, 50%, 81.7%, 100%, 143.9%,
150%, 181.4%,
200%, 250%, 444.2%, 500% and 1000% and all integers in between, over the
baseline hormone
level of a mammal. Conversely, the methods described herein also allow for the
production of
hormone levels to decrease from 0.1%, 1.2%, 7.7%, 10%, 15.6, 20%, 47.2%, 50%,
74.5%, 100%,
39
Date Recue/Date Received 2021-06-11
150%, 200%, 250%, 500% and 1000% and all integers in between, under the
baseline hormone
level of a mammal as in the mammal, as will be understood by one skilled in
the art, once they
understand the disclosure provided herein.
[00121]
Figure 11 provides an additional embodiment of the present disclosure, showing
a
flowing diagram of the regulation of hormones in a mammal based on information
from mammal
sensors 1100. As shown in step 1102, a mammal sensor monitors one or more
conditions
associated with the environment of a mammal. The conditions to be monitored by
include but is
not limited to the air temperature, humidity, the mammal's body temperature,
weight, sound,
movement of the mammal, infrared, 02, CO2 and CO. In step 1104, the mammal
sensor sends data
regarding the environmental or physical conditions associated with a mammal to
the MLC. The
MLC then analyzes the data sent from the mammal sensor or the analysis may be
done by a third-
party software program that is remote to the system. In step 1106, based on
the information from
the mammal sensor, the MLC sends instructions to change an embodiment of the
environment
such as air temperature or humidity. In step 1108, the environmental system
initiates an event to
one or more animals based on the analysis of the data from the sensor. As will
be understood by
one skilled in the art, the adjustment of the event can be on a micro level,
such as an adjustment to
the environment of one specific mammal or the adjustment can be on a macro
level such as an
entire growth chamber or operation. In step 1110, based on the information
from the mammal
sensor the MLC sends instructions to a feeding system, nutrient system or
nutrient source, such as
a drip, nutrient film or nutrient injection system, regarding the timing
and/or concentration of the
nutrient to be distributed to a mammal during a nutrient event. In step 1112,
nutrient system
initiates a nutrient event where nutrients are directed to a mammal based on
the analysis of the
data from the mammal sensor. As will be understood by one skilled in the art,
the adjustment of
Date Recue/Date Received 2021-06-11
the nutrient event can be on a micro level, such as an adjustment to the
nutrients to one specific
mammal or the adjustment can be on a macro level such as an entire growth
chamber or operation.
In step 1114, based on the analysis of the data from the mammal sensor, the
MLC sends
instructions to the photon emission modulation controller adjusting the
duration, intensity, color
spectrum and/or duty cycle of each photon pulse between different pulses of
color spectrums to a
specific an animal or to a group of animals. In step 1116, the photon emission
modulation
controller sends a signal to one or more photon emitters adjusting the
duration, intensity, color
spectrum and/or duty cycle of each photon pulse between different pulses of
color spectrums to a
specific an animal or to a group of animals. In step 1118, based on the signal
received from the
photon emission modulation controller, one or more photon emitters emit one or
more photon
pulses of individual color spectrums directed to an animal or to a group of
animals.
[00122]
Figure 12 is a graph showing an example photon signal with a repetitive photon
pulse
of near-red, showing a duration ON and a duration OFF for the controlled
regulation of hormones
in mammals. As shown in Figure 12 and previously described in Figures 1-11, an
example of the
cycling of a photon signal with repetitive photon pulses of one color
spectrums within the photon
signal is provided where a photon signal having a repetitive near-red photon
pulse is emitted from
a photon emitter. As shown in the graph near-red spectrum is pulsed first
followed by a delay.
Next, a second pulse comprising of near-red spectrum is again pulsed followed
by a delay. This
photon signal may be repeated indefinitely or until the mammal hormone
production under and
receiving the photon pulses has reached its desired production amount. While
in this descriptive
example of a photon signal having a repetitive photon pulse set comprising
offset pulsing of one
color spectrum, it should be understood that this description is applicable to
any such system with
other emissions of photon pulses over a period of time, as various
combinations of pulses of color
41
Date Recue/Date Received 2021-06-11
spectrums including but not limited to near-red, far-red, infra-red, green
blue, yellow, orange and
ultraviolet excluding the standard analog frequency lighting emission
standards of the United
States of 60 Hz and Europe of 50 Hz. Examples of the photon pulse duration
between pulses of
each individual color spectrum or color spectrum combinations may include but
is not limited to,
0.01 microseconds to 5000 milliseconds and all integers in between. The system
of the present
disclosure also allows for other durations between pulses of each individual
color spectrum or
color spectrum combinations including but not limited to 0.1 microsecond to 24
hours, and all
integers in between. The system of the present disclosure may be programmed to
allow for
variations of photon emission as well as variations of photon emission delay
to allow for events
such as extended dark cycles.
[00123]
Figure 13 is a graph showing an example photon signal containing photon pulses
of
two color spectrums, near-red and far red. The time scale on this chart is not
to scale but serves as
an example embodiment exhibiting the variation of color spectrum, duration ON,
duration OFF
frequency and duty cycle within a photon signal that may be utilized to
regulation hormone
production. As shown in Figure 13 and previously described in Figures 1-11,
another example of
a signal producing simultaneously and cycling photon pulses of various color
spectrum of the
present disclosure is provided where photon signal comprising photon pulses of
two color
spectrums are emitted from a photon emitter. As shown in the graph a signal
provides a far-red
spectrum that is pulsed first followed by a delay and then a pulse of a near-
red spectrum and then
followed by a delay. Next, a second pulse of near red is initiated followed by
a delay followed by
an individual pulse of far-red. This photon signal may be repeated
indefinitely or until the desired
mammal response has been initiated under and receiving the photon pulses. As
discussed above,
this example may also be used to stimulate hormones for ovulation or to reset
the mammal's
42
Date Recue/Date Received 2021-06-11
circadian rhythm. While in this descriptive example of a photon pulse set
comprising offset
pulsing of two color spectrum, it should be understood that this description
is applicable to any
such system with other emissions of photon pulses over a period of time, as
various combinations
of pulses of color spectrums including but not limited to near-red, far-red,
infra-red, green, blue,
yellow, orange and ultraviolet excluding the standard analog frequency
lighting emission standards
of the United States of 60 Hz and Europe of 50 Hz. Examples of the photon
pulse duration between
pulses of each individual color spectrum or color spectrum combinations may
include but is not
limited to, 0.01 microseconds to 5000 milliseconds and all integers in
between. The system of the
present disclosure also allows for other durations between pulses of each
individual color spectrum
or color spectrum combinations including but not limited to 0.1 microsecond to
24 hours, and all
integers in between. The system of the present disclosure may be programmed to
allow for
variations of photon emission as well as variations of photon emission delay
to allow for events
such as extended dark cycles.
[00124]
Figure 14 is a graph showing a second example photon signal containing photon
pulses
of two color spectrums, near-red and far red. Again, the time scale on this
chart is not to scale but
serves as an example embodiment exhibiting the variation of color spectrum,
duration ON,
duration OFF frequency and duty cycle within a photon signal that may be
utilized to regulate
hormone production. As shown in Figure 14 and previously described in Figures
1-11, another
example of the cycling of photon pulses of various color spectrum of the
present disclosure is
provided where photon signal comprising photon pulses of two color spectrums
are emitted from
a photon emitter. As shown in the graph, a far-red spectrum is pulsed in a
series or pulse train of
five pulses followed by a pulse of a near-red spectrum and then followed by a
delay. This photon
signal may be repeated indefinitely or until the desired mammal hormone level
has been achieved.
43
Date Recue/Date Received 2021-06-11
As discussed above, this example may also be used to regulation hormone
production for the
stimulate ovulation or to reset the mammal's circadian rhythm. While in this
descriptive example
of a photon pulse set comprising offset pulsing of two color spectrum, it
should be understood that
this description is applicable to any such system with other emissions of
photon pulses over a
period of time, as various combinations of pulses of color spectrums including
but not limited to
near-red, far-red, infra-red, green, blue, yellow, orange and ultraviolet
excluding the standard
analog frequency lighting emission standards of the United States of 60 Hz and
Europe of 50 Hz.
Examples of the photon pulse duration between pulses of each individual color
spectrum or color
spectrum combinations may include but is not limited to, 0.01 microseconds to
5000 milliseconds
and all integers in between. The system of the present disclosure also allows
for other durations
between pulses of each individual color spectrum or color spectrum
combinations including but
not limited to 0.1 microsecond to 24 hours, and all integers in between. The
system of the present
disclosure may be programmed to allow for variations of photon emission as
well as variations of
photon emission delay to allow for events such as extended dark cycles.
[00125]
Figure 15 is a graph showing an example photon signal containing photon pulses
of
two color spectrums, blue and green. The time scale on this chart is not to
scale but serves as an
example embodiment exhibiting the variation of color spectrum, frequency and
duty cycle that
may be utilized to stimulate hunger or a specific mood and to reset the
circadian rhythm of the
mammal. As shown in Figure 15 and previously described in Figures 1-11,
another example of the
cycling of photon pulses of various color spectrum of the present disclosure
is provided where
photon pulses of two color spectrums are emitted from a photon emitter. As
shown in the graph
pulses of blue and green are pulsed first followed by a delay. Next, a second
pulse of blue is
initiated followed by a delay followed by an individual pulse of green. This
cycle may be repeated
44
Date Recue/Date Received 2021-06-11
indefinitely or until the desired mammal response has been initiated under and
receiving the photon
pulses. As discussed above, this example may also be used to regulate
hormones, hunger, mood
or even to reset the mammal's circadian rhythm. While in this descriptive
example of a photon
pulse set comprising offset pulsing of two color spectrum, it should be
understood that this
description is applicable to any such system with other emissions of photon
pulses over a period
of time, as various combinations of pulses of color spectrums including but
not limited to near-
red, far-red, infra-red, green, blue, yellow, orange and ultraviolet excluding
the standard analog
frequency lighting emission standards of the United States of 60 Hz and Europe
of 50 Hz.
Examples of the photon pulse duration between pulses of each individual color
spectrum or color
spectrum combinations may include but is not limited to, 0.01 microseconds to
5000 milliseconds
and all integers in between. The system of the present disclosure also allows
for other durations
between pulses of each individual color spectrum or color spectrum
combinations including but
not limited to 0.1 microsecond to 24 hours, and all integers in between. The
system of the present
disclosure may be programmed to allow for variations of photon emission as
well as variations of
photon emission delay to allow for events such as extended dark cycles.
[00126]
Figure 16 graph showing an example photon signal containing photon pulses of
three
color spectrums, near-red, blue and green. The time scale on this chart is not
to scale but serves as
an example embodiment exhibiting the variation of color spectrum, frequency
and duty cycle that
may be utilized to stimulate ovulation, hunger or a specific mood and to reset
the circadian rhythm
of the mammal. As shown in Figure 16 and previously described in Figures 1-11,
another example
of the cycling of photon pulses of various color spectrum of the present
disclosure is provided
where photon pulses of three color spectrums are emitted from a photon
emitter. As shown in the
graph, a pulse of near red is provided followed by a delay. Next, a pulse of
blue is initiated
Date Recue/Date Received 2021-06-11
followed by a delay followed by an individual pulse of green. This signal and
cycle may be
repeated indefinitely or until the desired mammal response has been initiated
under and receiving
the photon pulses. As discussed above, this example may also be used to
regulate hormones,
ovulation, hunger, mood or even to reset the mammal's circadian rhythm. While
in this descriptive
example of a photon pulse set comprising offset pulsing of three color
spectrums, it should be
understood that this description is applicable to any such system with other
emissions of photon
pulses over a period of time, as various combinations of pulses of color
spectrums including but
not limited to near-red, far-red, infra-red, green, blue, yellow, orange and
ultraviolet excluding the
standard analog frequency lighting emission standards of the United States of
60 Hz and Europe
of 50 Hz. Examples of the photon pulse duration between pulses of each
individual color spectrum
or color spectrum combinations may include but is not limited to, 0.01
microseconds to 5000
milliseconds and all integers in between. The system of the present disclosure
also allows for
other durations between pulses of each individual color spectrum or color
spectrum combinations
including but not limited to 0.1 microsecond to 24 hours, and all integers in
between. The system
of the present disclosure may be programmed to allow for variations of photon
emission as well
as variations of photon emission delay to allow for events such as extended
dark cycles.
[00127]
Figure 17 graph showing an example photon signal containing photon pulses of
five
color spectrums, green, ultra-violet, orange, near-red, and blue. The time
scale on this chart is not
to scale but serves as an example embodiment exhibiting the variation of color
spectrum, frequency
and duty cycle that may be utilized to regulate hormones, ovulation, hunger or
a specific mood
and to reset the circadian rhythm of the mammal. As shown in Figure 17 and
previously described
in Figures 1-11, another example of the cycling of photon pulses of various
color spectrum of the
present disclosure is provided where photon pulses of five color spectrums are
emitted from a
46
Date Recue/Date Received 2021-06-11
photon emitter. As shown in the graph, pulses of green and ultraviolet are
provided followed by a
delay. Next, a pulse of near red is initiated followed by a delay followed
pulses of green and
ultraviolet. This cycle may be repeated with five pulses of green and
ultraviolet and three pulses
of near red and then a single pulse of blue and orange. This pulse signal may
be repeated
indefinitely or until the desired mammal response has been initiated under and
receiving the photon
pulses. As discussed above, this example may also be used to regulate
hormones, ovulation,
hunger, mood or even to reset the mammal's circadian rhythm. While in this
descriptive example
of a photon pulse set comprising offset pulsing of three color spectrums, it
should be understood
that this description is applicable to any such system with other emissions of
photon pulses over a
period of time, as various combinations of pulses of color spectrums including
but not limited to
near-red, far-red, infra-red, green, blue, yellow, orange and ultraviolet
excluding the standard
analog frequency lighting emission standards of the United States of 60 Hz and
Europe of 50 Hz.
Examples of the photon pulse duration between pulses of each individual color
spectrum or color
spectrum combinations may include but is not limited to, 0.01 microseconds to
5000 milliseconds
and all integers in between. The system of the present disclosure also allows
for other durations
between pulses of each individual color spectrum or color spectrum
combinations including but
not limited to 0.1 microsecond to 24 hours, and all integers in between. The
system of the present
disclosure may be programmed to allow for variations of photon emission as
well as variations of
photon emission delay to allow for events such as extended dark cycles.
[00128]
Figure 18 is a graph showing a third example photon signal containing photon
pulses
of two color spectrums, near-red and far red. The time scale on this chart is
not to scale but serves
as an example embodiment exhibiting the variation of color spectrum, duration
ON, duration OFF
frequency and duty cycle within a photon signal that may be utilized to
regulate hormones. As
47
Date Recue/Date Received 2021-06-11
shown in Figure 18 and previously described in Figures 1-11, another example
of the cycling of
photon pulses of various color spectrum of the present disclosure is provided
where photon signal
comprising photon pulses of two color spectrums are emitted from a photon
emitter. As shown in
the graph a far-red spectrum is pulsed first followed by a delay and then a
pulse of a near-red
spectrum and then followed by a delay. Next, a second pulse of near red is
initiated followed by
a delay followed by an individual pulse of far-red. This photon signal may be
repeated indefinitely
or until the desired mammal response has been initiated under and receiving
the photon pulses. As
discussed above, this example may also be used to regulate hormone, ovulation
or to reset the
mammal's circadian rhythm. While in this descriptive example of a photon pulse
set comprising
offset pulsing of two color spectrum, it should be understood that this
description is applicable to
any such system with other emissions of photon pulses over a period of time,
as various
combinations of pulses of color spectrums including but not limited to near-
red, far-red, infra-red,
green, blue, yellow, orange and ultraviolet excluding the standard analog
frequency lighting
emission standards of the United States of 60 Hz and Europe of 50 Hz. Examples
of the photon
pulse duration between pulses of each individual color spectrum or color
spectrum combinations
may include but is not limited to, 0.01 microseconds to 5000 milliseconds and
all integers in
between. The system of the present disclosure also allows for other durations
between pulses of
each individual color spectrum or color spectrum combinations including but
not limited to 0.1
microsecond to 24 hours, and all integers in between. The system of the
present disclosure may
be programmed to allow for variations of photon emission as well as variations
of photon emission
delay to allow for events such as extended dark cycles.
[00129]
Figure 19 is a graph showing an example photon signal containing photon pulses
of
two color spectrums, near-red and far red. The time scale on this chart is not
to scale but serves as
48
Date Recue/Date Received 2021-06-11
an example embodiment exhibiting the variation of color spectrum, duration ON
with varying
intensities, duration OFF frequency and duty cycle within a photon signal that
may be utilized to
regulate hormones. As shown in Figure 19 and previously described in Figures 1-
11, another
example of the cycling of photon pulses of various color spectrum of the
present disclosure is
provided where photon signal comprising photon pulses of two color spectrums
are emitted from
a photon emitter. As shown in the graph a far-red spectrum is pulsed first
followed by a delay and
then a pulse of a near-red spectrum and then followed by a delay. Next, a
second pulse of near red
is initiated followed by a delay followed by an individual pulse of far-red.
This photon signal may
be repeated indefinitely or until the desired mammal response has been
initiated under and
receiving the photon pulses. As discussed above, this example may also be used
to stimulate
ovulation or to reset the mammal's circadian rhythm. While in this descriptive
example of a
photon pulse set comprising offset pulsing of two color spectrums with varying
intensities, it
should be understood that this description is applicable to any such system
with other emissions
of photon pulses over a period of time, as various combinations of pulses of
color spectrums
including but not limited to near-red, far-red, infra-red, green, blue,
yellow, orange and ultraviolet
excluding the standard analog frequency lighting emission standards of the
United States of 60 Hz
and Europe of 50 Hz. Examples of the photon pulse duration between pulses of
each individual
color spectrum or color spectrum combinations may include but is not limited
to, 0.01
microseconds to 5000 milliseconds and all integers in between. The system of
the present
disclosure also allows for other durations between pulses of each individual
color spectrum or
color spectrum combinations including but not limited to 0.1 microsecond to 24
hours, and all
integers in between. The system of the present disclosure may be programmed to
allow for
49
Date Recue/Date Received 2021-06-11
variations of photon emission as well as variations of photon emission delay
to allow for events
such as extended dark cycles.
[00130] Table 4 below provides a table of lighting options. As shown in
Table 4, column one
provided the name or designation of the lighting option or pulse signal,
column two provide the
colors pulses in the lighting option, column three is the duration ON of each
pulse within the pulse
signal, column four is the duration OFF of each pulse within the pulse signal,
column five provides
the time from ON to OFF.
Table 4
Lighting Duration Duration . . Ma of each
Colors Tuning from t-O
Option ON OFF color
Near red 1 ON ¨ 0 600
Option 1 50us 200us
Near red 2 OFF- SOUS
Near red 1 ON ¨ 0 600
50us 50us
Near red 2 OFF- SOUS
Option 2
ON ¨ 100 us 900
Far Red 50us 100us
OFF- 150us
Near red 1 ON ¨ 0 600
50us 100us
Near red 2 OFF- SOUS
Option 3
ON ¨ 100 us 900
Far Red 50us 100us
OFF- 150us
Near red 1 ON ¨ 0 600
Option 4 50us 200us
Near red 2 OFF- SOUS
Near red 1 ON ¨ 0 600
50us 100us
Near red 2 OFF- SOUS
Option 5
ON ¨ 150 us 900
Far Red 50us 500us
OFF- 200us
Near red 1 ON ¨ 0 600
50us 50us
Near red 2 OFF- SOUS
Option 6
ON ¨ 100 us 900
Far Red 50us 100us
OFF- 150us
ON ¨ 0 600
Green 50us 50us
OFF- SOUS
Option 7
ON ¨ 100 us 900
Far Red 50us 100us
OFF- 150us
ON ¨ 0 600
Blue 50us 50us
OFF- SOUS
Option 8
ON ¨ 100 us 900
Far Red 50us 100us
OFF- 150us
Near red 1 ON ¨ 0 600
50us 100us
Near red 2 OFF- SOUS
Option 9
ON ¨ 150 us 600
Green 50us 500us
OFF- 200us
Near red 1 ON ¨ 0 600
Option 10 50us 100us
Near red 2 OFF- SOUS
Date Recue/Date Received 2021-06-11
Table 4
Lighting Duration Duration . Ma of each
Colors Tun ing from t-O
Option ON OFF color
ON ¨ 150 us 600
Blue 50us 500us
OFF- 200us
ON ¨ 0 600
Near red 1 50us 100us
OFF- SOUS
ON ¨ 150 us 600
Option 11 Blue 50us 500us
OFF- SOUS
ON ¨ 0 600
Green 50us 50us
OFF- SOUS
ON ¨ 0 600
Near red 1 50us 100us
OFF- SOUS
Blue ON ¨ 150 us 600
Option 12 50us 500us
Orange OFF- SOUS
Green ON ¨ 0 600
50us 50us
Ultraviolet OFF- SOUS
ON ¨ 150 us 600
Blue 50us 50us
OFF- SOUS
Option 13
ON ¨ 0 600
Green 50us 50us
OFF- SOUS
ON ¨ 150 us 600
Option 14 Blue 50us 50us
OFF- SOUS
ON ¨ 0 600 to 1100
Near red 50-100us 50-1500us
OFF- SOUS
Option 15
ON ¨ 100 us 900 to 1100
Far Red 50-100us 50-1500us
OFF- 150us
EXAMPLES
[00131] The following examples are provided to illustrate further the
various applications and
are not intended to limit the invention beyond the limitations set forth in
the appended claims.
Example 1- Regulation of melatonin in human
[00132] An adult male human (Homo sapiens), was exposed on March 22, 2018 and
March 23,
2018 in Greeley, Colorado to supplemental pulsed lighting (Option 15 in Table
4 at 600 Ma for
near red and 900 Ma for far red) approximately six hours during the night and
eight hours during
the day within a 24-hour period to assess melatonin levels under typical daily
activities.
Supplemental lighting was additive to normal environmental lighting, such as
computers,
television, etc.
51
Date Recue/Date Received 2021-06-11
[00133] Blood was collected from the Caucasian male human in his mid-40s.
The first two
samples were collected under ambient lighting conditions at 9am and 5pm. The
subject was then
exposed to supplemental pulsed lighting (Option 15 in Table 4) for 14 hours,
including sleep, over
the course of the next 24 hours and his blood was drawn at 9am and 5pm. A
total of eight samples
were drawn. The samples were taken from the antecubital area of the arm. The
blood was collected
using 25-gauge needles with 3cc syringes. The samples were immediately
transferred to a lithium-
heparin tube and inverted a total of ten times. The blood cells were
centrifuged for 10 min at 3200
rpm using a Cole-Parmer centrifuge to isolate the plasma. The plasma samples
were poured into
1.5 mL centrifuge tubes and placed into the freezer at -17 C. The samples
were prepared using
the ab213978 melatonin ELISA kit from Abcam Labs. The samples were analyzed
using a
Varioskan LUX from Thermo Scientific.
[00134] All precipitates and solids were removed via centrifugation. Equal
volumes (500 jiL)
of cold ethyl acetate and plasma sample were placed into an Eppendorf tube and
gently vortexed.
The layers were allowed to separate over ice. The sample was vortexed again
and incubated over
ice for two minutes. After, the samples were centrifuged at 1000 g for 10 min.
The organic layer
was carefully pipetted into a new tube. It was then dried over a stream of
inert gas (Argon). Next,
the pellet was suspended in 100 - 200 jiL of lx stabilizer. The sample was
then kept on ice after
the suspension and the assay was performed immediately.
[00135] The ELISA kit was purchased as a 96-well plate and ready to use
upon arrival. The
immunoassay was stored in a sealed pouch with desiccant in the refrigerator at
8 C until the day
of use.
[00136] All kit components were brought to room temperature. Plasma samples
were used
directly without any dilution. Next, 100 jiL of sample was added to each well
of a pre-coated well
52
Date Recue/Date Received 2021-06-11
plate along with 100 jiL of lx stabilizer added to the blank wells. Then, 50
jiL of 1X melatonin
tracer and 50 jiL of lx melatonin antibody were added to each sample well
except to the blank
wells, respectively. The plate was sealed and incubated at room temperature
(RT) on a shaker
plate for 1 hour at about 500 rpm. After incubation, the samples were washed
with the wash buffer
a total of three times with 400 jiL per well. After the last wash, the plate
was emptied, and the
contents were aspirated, and the plate was blot dried by tapping on a paper
towel to remove any
remaining wash buffer. Next, 200 jiL of melatonin conjugate solution was added
to each well
expect to the blank wells. Again, the plate was sealed and was incubated at RT
on a plate shaker
for 30 minutes at about 500 rpm. The plate was washed again in the same manner
as before and
all the wash buffer was removed. At this point, 200 jiL of TMB substrate
solution was added to
each well, and the plate was incubated for 30 minutes at RT on a shaker plate
at the same rate as
previously performed. Then, 50 jiL of the stop solution was added to each
well. Optical Density
(OD) readings were recorded at a 450 nm wavelength by a plate reader.
[00137] All data is presented as means using curve fitting programs (4-
parameters) from the
plate reader software (Skanit Software 5.0 for microplate readers). All the
plots were created in
excel. Known concentrations of melatonin antibody were pre-immobilized onto
the plates. Figure
20 shows the dilution curve for each pre-immobilized dilution (0, 50, 100,
250, 500, 1000 pg/mL)
of melatonin antibody in the well plates.
[00138] With known standards, the change in melatonin concentrations in
ng/mL were obtained
under lights (Option 15 in Table 4) as described herein and compared to a
control light (Figure
21). Blood was collected from a human subject over a two-day period. The first
set of samples
were collected approximately eight hours apart under standard light
conditions. The second set of
samples were collected under lights as described herein (Option 15 in Table 4)
at the same time of
53
Date Recue/Date Received 2021-06-11
day as the first set of samples, respectively. The samples were placed into
1.5 mL Eppendorf tubes
and stored in the freezer at -17 C until the day of use. All the standards,
blanks and samples were
taken in replicate and averages were obtained.
[00139] Melatonin is a major factor in the circadian rhythm in mammals.
Extensive research
has shown that different light cycles effect melatonin production. This trial
was conducted to
determine the effect of lights as described herein on human melatonin levels.
[00140] The data in Figure 21 shows that human melatonin levels increased
by 24.79% after
the first and second eight-hour timepoints. There was a greater increase in
the melatonin level
after a longer exposure to the lights (Option 15 in Table 4 at 600 Ma and 900
Ma) as described
herein. The data would indicate that pulsing of lighting as described herein
results in direct
regulation of melatonin levels in humans.
Example 2 - Regulation of melatonin in cattle
[00141] The 10-month-old black angus bull, raised in Yuma, Arizona was
placed in a 12 x 12
ft agricultural panel pen under normal lighting. After blood samples were
collected for the first 3
timepoints (1400 hours, 2200 hours and 700 hours), the bull was housed in a
tarped enclosure
framed in by the agricultural panels and the only light source was a specific
set of lights as
described herein (Option 15 in Table 4 at 1100 Ma). Supplemental air into the
tent was provided
via an HVAC fan and was fed ad libitum grass hay and 5 pounds of sweet grain a
day consistent
with normal rations. Light intensity under lights as described herein (Option
15 in Table 4 at 1100
Ma) within the enclosure ranged from 52 to 1012 mW/m2. If required, the bull
was moved into a
squeeze chute for blood collection and then returned to the enclosure.
[00142] Blood was collected from the bull at approximately eight (8) hour
intervals. The first
three samples were collected under ambient lighting conditions at 1400 hours,
2200 hours and
54
Date Recue/Date Received 2021-06-11
700hours followed by a 74-hour exposure to a specific pulsed lighting recipe.
Three additional
samples were taken after the light exposure at approximately the same time of
day as the initial
blood collection (1400 hours, 2200 hours and 700hours). The samples were taken
from the
coccygeal (tail) vein. The blood was collected using 23-gauge needles with 3cc
syringes. The
samples were immediately transferred to a lithium-heparin tube and inverted a
total of ten times.
The blood samples were centrifuged for 10 min at 3200 rpm using a Cole-Parmer
centrifuge to
isolate the plasma. The plasma samples were poured into 1.5 mL centrifuge
tubes and placed into
the freezer at -17 C. The samples were prepared using the ab213978 melatonin
ELISA kit from
Abcam Labs. The samples were analyzed using a Varioskan LUX from Thermo
Scientific.
[00143] All precipitates and solids were removed via centrifugation. Equal
volumes (500 [IL)
of cold ethyl acetate and plasma sample were placed into an Eppendorf tube and
gently vortexed.
The layers were allowed to separate over ice. The sample was vortexed again
and incubated over
ice for two minutes. After, the samples were centrifuged at 1000 g for 10 min.
The organic layer
was carefully pipetted into a new tube. It was then dried over a stream of
inert gas (Argon). Next,
the pellet was suspended in 100 - 200 [IL of lx stabilizer. The sample was
then kept on ice after
the suspension and the assay was performed immediately.
[00144] The ELISA kit was purchased as a 96-well plate and ready to use
upon arrival. The
immunoassay was stored in a sealed pouch with desiccant in the refrigerator at
8 C until the day
of use.
[00145] All kit components were brought to room temperature. Plasma samples
were used
directly without any dilution. Next, 100 [IL of sample was added to each well
of a pre-coated well
plate along with 100 [IL of lx stabilizer added to the blank wells. Then, 50
[IL of 1X melatonin
tracer and 50 [IL of lx melatonin antibody were added to each sample well
except to the blank
Date Recue/Date Received 2021-06-11
wells, respectively. The plate was sealed and incubated at room temperature
(RT) on a shaker
plate for 1 hour at about 500 rpm. After incubation, the samples were washed
with the wash
buffer a total of three times with 400 jiL per well. After the last wash, the
plate was emptied, and
the contents were aspirated, and the plate was blot dried by tapping on a
paper towel to remove
any remaining wash buffer. Next, 200 jiL of melatonin conjugate solution was
added to each well
except to the blank wells. Again, the plate was sealed and was incubated at
room temperature on
a plate shaker for 30 minutes at about 500 rpm. The plate was washed again in
the same manner
as before and all the wash buffer was removed. At this point, 200 jiL of TMB
substrate solution
was added to each well, and the plate was incubated for 30 minutes at room
temperature on a
shaker plate at the same rate as previously performed. Then, 50 jiL of the
stop solution was added
to each well. Optical Density (OD) readings were recorded at a 450 nm
wavelength by a plate
reader.
[00146] All data is presented as means using curve fitting programs (4-
parameters) from the
plate reader software (Skanit Software 5.0 for microplate readers). All the
plots were created in
excel. Known concentrations of melatonin antibody were pre-immobilized onto
the plates. Figure
22 shows the standard curve for each pre-immobilized dilution (50, 10, 2, 0.4,
0.08 ng/mL) of
melatonin antibody in the well plates.
[00147] With known standards, the change in melatonin concentrations in
ng/mL were obtained
under lights as described herein and compared to a control light (shown in
Figure 23). Blood was
collected from a bull over a five-day period. The first set of samples were
collected every eight
hours for a total of three times under the control light. The second set of
samples were collected
lights as described herein at the same time of day as the first set of
samples, respectively. The
56
Date Recue/Date Received 2021-06-11
samples were placed into 1.5 mL Eppendorf tubes and stored in the freezer at -
17 C until the day
of use. All the standards, blanks and samples were taken in replicate and
averages were obtained.
[00148] Melatonin is a major factor in the circadian rhythm in mammals.
Extensive research
has shown that different light cycles effect melatonin production. This trial
was conducted to
determine the effect of lights as described herein on bovine melatonin levels.
1001491 The data in Figure 23 shows that the bovine melatonin levels
increased by 20.79% with
longer exposure to lights as described herein. After exposure to lights as
described herein (Option
15 in Table 4 at 1100 Ma) for approximately 92 hours a significant increase of
20.79% was
observed. The preliminary data would indicate that different lighting recipes
can result in direct
regulation over melatonin levels in bovine.
Example 3 - genetic expression and hormonal excretion found in pigs
[00150] In another example, the light inputs of the systems and methods
described herein affect
genetic expression and hormonal excretion found in pigs. In both gilts and
sows, seasonal
infertility has many important economic impacts. Reduced farrowing rates are a
result of increased
numbers of gilts and sows returning to oestrus and insemination and a higher
proportion of
spontaneous abortions occurring from breedings completed during late summer
and early autumn.
This results in inefficient use of facilities and a decreased number of
piglets being produced.
Additionally, smaller litter sizes, increased time from weaning to oestrus and
delayed puberty in
gilts expected to mature between August and November in the norther hemisphere
has been
associated with long days. All of these factors contribute to the animal's non-
productive days.
Example 4 - Regulation of circadian rhythm in mammals
[00151] In yet another example of light inputs and circadian rhythms
affecting human genetic
expression and hormonal excretion can be found in the spring forward effects
from daylight
57
Date Recue/Date Received 2021-06-11
savings time (DST). These affects are widespread and from modern research show
effects ranging
from a 10% increased myocardial infarction risk, 8% increased risk of
cerebrovascular accidents,
increase in suicides, and decreased in-vitro fertilization successes.
Example 5 - Regulation of circadian rhythm in mammals
[00152] In another example, dairy cattle, calves raised under long day
photoperiods yield larger
and leaner bodies at maturity with greater mammary parenchymal growth. Long
day photoperiod
exposed lactating cattle produced higher milk yield due to lower melatonin
concentrations and
higher prolactin concentration, whereas short day photoperiod during the dry
period of multiparous
cows enhances milk production in the following lactation. These items signify
the importance of
light exposure in dairy cattle for optimized production. Bovine somatotropins
are a naturally
occurring substance in cattle in order to maximize postpartum milk production.
In the 1970's,
rBST was created in using e-coli in order to create an artificial growth
hormone in dairy cattle.
Unfortunately, studies have found that this artificial hormone causes many
health effects in cattle
including, 24% increase in cases of mastitis ($1.4 to $2.0 billion dollars per
year economic impact),
40% reduced fertility, 55% increase in lameness. These side effects are not
seen in naturally
occurring BST which is created using the photo cues of existing light.
[00153] The foregoing description of the invention has been presented for
purposes of
illustration and description. It is not intended to be exhaustive or to limit
the invention to the
precise form disclosed, and other modifications and variations may be possible
in light of the above
teachings. The embodiment was chosen and described in order to best explain
the principles of
the invention and its practical application to thereby enable others skilled
in the art to best utilize
the invention in various embodiments and various modifications as are suited
to the particular use
58
Date Recue/Date Received 2021-06-11
contemplated. It is intended that the appended claims be construed to include
other alternative
embodiments of the invention except insofar as limited by the prior art.
59
Date Recue/Date Received 2021-06-11