Note: Descriptions are shown in the official language in which they were submitted.
SYSTEM AND Ammon FOR PRODUCING STATISTICALLY VALID
ASSAY MEANS AND RAIN GES FOR QUALITY CONTROL
MATERIALS
CROSS REFERENCES TO RELATED APPLICATIONS
This Patent Cooperation Treaty application claims priority to US Patent
Application Number
13/074,649, filed March 29, 2011, entitled "System and Method for Producing
Statistically
Valid Assay Means and Ranges for Quality Control Materials."
BACKGROUND OF TfIE INVENTION
Certain medical tests have become highly automated. For example common tests
such as
measuring a patient's cholesterol levels or blood sugar, testing for the
presence of drugs in a
subject's blood or urine, or measuring other aspects of a patient's blood
chemistry can now
be performed by automated testing machines at rates up to thousands of tests
per hour. In a
typical test, a sample such as a quantity of a patient's blood is reacted with
a reagent, and the
resulting product studied to determine the presence or amount of a particular
analyte in the
sample. The reagent may be specifically designed for the performance of the
particular test.
Because important medical decisions may be made on the basis of the test
results, it is highly
desirable that the testing machines be qualified periodically to maintain
confidence that the
machines are operating properly, or to try to detect when the machines are not
operating
properly. In fact, U.S. government regulations require such periodic
validation. By
regulation, each testing machine must be qualified at least once per day for
any day that
patient testing is performed.
Qualification may involve testing a sample having known characteristics, and
checking
whether the machine produces a test result that agrees with the known
characteristics. If so,
the machine may be assumed to be operating properly, and if not, the operation
of the
machine may be suspect.
Machine qualification thus requires the ready availability of test samples
having known
characteristics. These test samples used for machine qualification may be
called "quality
control materials". Because. a single testing machine may be able to perform a
large number
of different tests, including testing for an analyte using different reagents,
and because testing
Date Recue/Date Received 2021-06-15
machines front different manufacturers may perform tests differently, the
number of quality
control materials that must be readily available is very large, in order to
qualify every kind of
machine in the performance of every test it is capable of. For at least some
tests, for example
tests that test for unusual conditions in human tissue or unstable analytes,
it may be
impracticable to maintain a reserve of actual biological samples having known
characteristics, so the quality control material may mimic the behavior of an
actual biological
sample. Quality control materials are preferably stabilized, so that they can
be stored for long
periods. For example, some quality control materials are lyophilized at the
time of
manufacture, and reconstituted for use.
Because of natural variability in the process of manufacturing quality control
materials, each
new lot of a particular quality control material is characterized at the time
of its manufacture,
to determine a range of test result values within which a result from a test
of a sample from
the lot is expected, with a specified probability, to fall when the quality
control material is
tested using a testing instrument that is operating properly. A mean value is
also typically
published. These means and ranges, known as assay means and ranges, are
published for the
use of testing laboratories in the qualification of their machines. Means and
ranges may be
published for each possible combination of testing machine model and quality
control
material, or testing method and quality control material.
Recent guidance from the U.S. Food and Drug Administration requires that
published assay
means and ranges for quality control materials be statistically valid.
BRIEF SUMMARY OF THE INVENTION
According to one aspect, a method of establishing a statistically valid assay
mean and assay
range for a particular lot of a quality control material comprises testing a
number of samples
from the particular lot of the quality control material and obtaining a test
result from each
sample, and computing a mean of the test results. The uncertainty in the
estimate of the mean
is also computed. The method further comprises accessing a database of
historical test results
obtained from tests performed on prior lots of the quality control material,
and computing at
least in part from the historical test results a variability estimate that is
an estimate of the
variability of test results obtained from tests performed on at least one
prior lot of the quality
control material. A target probability is specified, which a new qualification
test result
performed on a sample of the new lot of quality control material will fall
within the assay
range. The method further comprises computing, based at least in part on the
mean, the
uncertainty in the estimate of the mean, and the variability estimate, a range
of test result
2
Date Recue/Date Received 2021-06-15
values within which a result from a qualification test of a sample from the
particular lot of the
quality control material is expected to fall, with the target probability. The
method may
further comprise testing the samples from the particular lot of the quality
control material to
obtain the test results. The method may further comprise outputting the range.
In some
embodiments, computing the variability estimate comprises computing at least
in part from
the historical test results an estimate of the uncertainty of the mean,
computing at least in part
from the historical test results a within-instrument variability estimate that
is an estimate of
the variability of test results obtained using a single testing instrument on
at least one prior lot
of the quality control material, and computing at least in part from the
historical test results a
between-instrument variability estimate that is an estimate of the variability
of test results
obtained using different testing instruments on at least one prior lot of the
quality control
material; and computing the range based at least in part on the mean and the
variability
estimate comprises computing the range based at least in part on the mean, the
estimate of the
uncertainty of the mean, the within-instrument variability estimate, and the
between-
instrument variability estimate. In some embodiments, the method further
comprises setting
an upper limit for the percentage of the assay range that is due to
uncertainty in estimating the
mean, and establishing a sampling plan, based at least in part on the within-
and between-
instrument variability estimates, to achieve an assay range having a
percentage of its width
due to uncertainty in estimating the mean, the percentage being at or below
the upper limit.
In some embodiments, the within-instrument variability estimate is derived
from an average
of variances, each variance being the variance of test results from a
respective one of a
plurality of instruments. In some embodiments, the between-instrument
variability estimate
is derived from a variance of averages, each average being an average test
result from a
respective one of a plurality of instruments. In some embodiments, the
estimate of the
uncertainty of the mean is a standard error of the mean, and the standard
error of the mean is
computed based at least in part on estimates of variability derived from the
database of
historical test results. In some embodiments, computing the variability
estimate at least in
part from the historical test results comprises accounting for an observed
relationship
between the mean and the variability of test results. In some embodiments,
computing the
variability estimate at least in part from the historical test results
comprises performing a
regression using the historical test results to characterize the relationship
between the mean
and at least one component of the variability, and adjusting the variability
estimate based on
the mean and the relationship. In some embodiments, computing the variability
estimate at
least in part from the historical test results comprises identifying a
specific prior lot of the
quality control material that has a mean test result comparable to the mean
computed from
3
Date Recue/Date Received 2021-06-15
the test results from the particular lot of quality control material,
computing a variability of
test results obtained from tests of the specific prior lot of quality control
material, and
assigning to the particular lot of quality control material a variability
estimate that is equal to
the computed variability of test results from. the specific prior lot of
quality control material.
In some embodiments, computing the variability estimate comprises computing at
least in
part from the historical test results an estimate of the uncertainty of the
mean, computing at
least in part from the historical test results a within-laboratory variability
estimate that is an
estimate of the variability of test results obtained within a single
laboratory on at least one
prior lot of the quality control material, and computing at least in part from
the historical test
.. results a between-laboratory variability estimate that is an estimate of
the variability of test
results obtained from different laboratories on at least one prior lot of the
quality control
material; and computing the range based at least in part on the mean and the
variability
estimate comprises computing the range based at least in part on the mean, the
estimate of the
uncertainty of the mean, the within-laboratory variability estimate, and the
between-
laboratory variability estimate. The method may further comprise removing from
consideration anomalous test results found in the database of historical test
results.
According to another aspect, a method of establishing a sampling plan for
assigning an assay
mean and range to a particular lot of a quality control material comprises
setting an upper
limit for the percentage of the assay range that is due to uncertainty in
estimating the mean,
and accessing a database of historical test results obtained from tests
performed on prior lots
of the quality control material. A within-instrument variability estimate is
computed at least
in part from the historical test results, and is an estimate of the
variability of test results
obtained using a single testing instrument on at least one prior lot of the
quality control
material. A between-instrument variability estimate is computed at least in
part from the
historical test results, and is an estimate of the variability of test results
obtained using
different testing instruments on at least one prior lot of the quality control
material. The
method further comprises establishing a sampling plan, based at least in part
on the within-
and between-instrument variability estimates, to achieve an assay range having
a percentage
of its width due to uncertainty in estimating the mean, the percentage being
at or below the
upper limit.
According to another aspect, a system for establishing a statistically valid
assay mean and
assay range for a particular lot of a quality control material comprises a
processor, a database
holding historical test results obtained from tests performed on prior lots of
the quality
control material, and a memory readable by the processor. The memory holds
processor
4
Date Recue/Date Received 2021-06-15
instructions that when executed by the processor cause the system to obtain a
number of test
results obtained from tests on a number of samples from the particular lot of
the quality
control material, compute a mean of the test results, and access the database
of historical test
results obtained from tests performed on prior lots of the quality control
material. The
instructions further cause the system to compute at least in part from the
historical test results
a variability estimate that is an estimate of the variability of test results
obtained from tests
performed on at least one prior lot of the quality control material, and
receive a specification
of a target probability with which a new qualification test result performed
on a sample of the
new lot of quality control material will fall within the assay range. The
instructions further
cause the system to compute, based at least in part on the mean and the
variability estimate, a
range of test result values within which a result from a qualification test of
a sample from the
particular lot of the quality control material is expected to fall, with the
target probability. In
some embodiments, the instructions, when executed by the processor to compute
the
variability estimate, further cause the processor to compute at least in part
from the historical
test results an estimate of the uncertainty of the mean; compute at least in
part from the
historical test results a within-instrument variability estimate that is an
estimate of the
variability of test results obtained using a single testing instrument on at
least one prior lot of
the quality control material; and compute at least in part from the historical
test results a
between-instrument variability estimate that is an estimate of the variability
of test results
obtained using different testing instruments on at least one prior lot of the
quality control
material
According to another aspect, an assay mean and range assignment system for
establishing a
statistically valid assay mean and assay range for a particular lot of a
quality control material
comprises a database holding historical test results obtained from tests
performed on prior
lots of a quality control material, and a mean determination module that
receives a number of
test results obtained from tests on a number of samples from a new lot of the
quality control
material and computes a mean of the test results. The system further comprises
a variability
estimation module that computes at least in part from the historical test
results a variability
estimate that is an estimate of the variability of test results obtained from
tests performed on
at least one prior lot of the quality control material, and a range
establishment module that
establishes, based at least in part on the mean and the variability estimate,
a range of test
result values within which a result from a qualification test of a sample from
the new lot of
the quality control material is expected to fall, with a target probability.
In some
embodiments, the the variability estimate comprises a within-instrument
variability estimate
that is an estimate of the variability of test results obtained using a single
testing instrument
5
Date Recue/Date Received 2021-06-15
on at least one prior lot of the quality control material; and the variability
estimate comprises
a between-instrument variability estimate that is an estimate of the
variability of test results
obtained using different testing instrtunents on at least one prior lot of the
quality control
material; and the system further comprises a sampling plan establishment
module that
establishes a sampling plan based at least in part on the within-instrument
variability estimate
and the between-instrument variability estimate. In some embodiments, the
sampling plan
establishment module receives a specification of an upper limit for the
percentage of the
assay range that is due to uncertainty in estimating the mean, and establishes
the sampling
plan based at least in part on the within-instrument variability estimate, the
between-
instrument variability estimate, and the upper limit for the percentage of the
assay range that
is due to uncertainty in estimating the mean. The range establishment module
may account
for an observed relationship between the mean and variability of historical
test results. In
some embodiments, the variability estimate comprises an estimate the
uncertainty of the
mean; the variability estimate comprises a within-instrument variability
estimate that is an
estimate of the variability of test results obtained using a single testing
instrument on at least
one prior lot of the quality control material; the variability estimate
comprises a between-
instrument variability estimate that is an estimate of the variability of test
results obtained
using different testing instruments on at least one prior lot of the quality
control material; and
the range establishment module establishes the range based at least in part on
the mean, the
estimate of the uncertainty of the mean, the within-instrument variability
estimate, and the
between-instrument variability estimate.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates in flowchart form an overview of steps of a method for
establishing a
statistically valid assay mean and assay range for a particular lot of a
quality- control material,
in accordance with embodiments of the invention.
Figure 2 illustrates a simplified block diagram of the interactions of various
systems involved
in the assignment of assay means and ranges, in accordance with embodiments of
the
invention.
Figure 3 illustrates a system according to embodiments of the invention.
Figure 4 illustrates in more detail data flows involved in the assignment of
assay means and
ranges, in accordance with embodiments of the invention.
6
Date Recue/Date Received 2021-06-15
Figure 5 is a block diagram illustrating an exemplary computer system in which
embodiments of the present invention may be implemented.
DETAILED DESCRIPTION OF THE INVENTION
Table I below is an extract of an example table of assay means and ranges, as
may be
published by a manufacturer of quality control materials.
Table 1. Example Assay Means and Ranges
Instrument Analyte Units Level 1 Level 2
Mean Range Mean Range
Brand A Model X Glucose 92 82-102 270 253-287
Brand A Model X Cholesterol (Tot) mg/dL 253 214-293 129 99-
159
Brand B Model Y Glucose mg/dL 89 75-103 288 242-
334
Brand B Model Y cho.lestf..191 (Tot) mg/dL 268 230-305 128 110-
146
Method Analyte Units Level 1 Level 2
Mean Range Mean Range
Method A Glucose m gldl.. 87.4 73.4-101 274
230-318
Method B Cholesterol (Tot) mg/dL 286 229-
343 141 113-169
Method C Glucose m.g/dL 89 74.8-103 277 222-
321
Method D Cholesterol (Tot) mg/dL 262 225-
298 128 110-145
The first part of Table 1 is organized according to particular tests performed
on particular
testing instruments. In the "Instrument" section of Table 1, there are eight
sets of means and
ranges given -- two kinds of tests (glucose and cholesterol) performed on two
different
models of testing machine (Brand A model X and Brand B model Y), and for two
different
concentrations of analyte in the quality control materials (Level 1 and Level
2). For example,
an owner of a Brand B Model Y testing machine may refer to the third line of
the table to
determine the range within which a test result would be expected to fall for a
qualification
test performed with the quality control material for which the table is
produced. According to
the third line of the table, a user of a Brand B Model Y testing machine may
expect a test of
the particular Level 1 quality control material for testing glucose to produce
a test result
between 75 and 103 mg/dL, with a confidence established by the statistical
methods used to
assign the range. In some embodiments, the range may be established such that
the machine
user would expect the test result to fall within the specified range about
99.7% of the time
when the testing machine being qualified is operating properly. A test result
outside the
range may therefore raise a doubt about whether the machine is operating
properly or the
quality control material has been compromised, prompting further
investigation.
7
Date Recue/Date Received 2021-06-15
The "Method" part of Table 1 is organized according to testing method. A
laboratory using a
testing machine that is not represented in the "Instrument" section of the
table may use the
"Method" section to determine the expected range for qualification tests
according to the
method used by its particular testing machine.
.. The abbreviated listing of Table 1 includes 16 different mean and range
entries. For the
purposes of this disclosure, each entry will be said to correspond to a "test
condition", which
may be a particular combination of factors such as testing machine make and
model, analyte,
reagent, quality control material, quality control material concentration, and
test method. A
complete table may include entries for many different test conditions,
encompassing dozens
of different testing machine models and dozens of diffetent tests to be
performed by any of
several different methods. A single product line of quality control materials
may require that
assay means and ranges be characterized at over 1,500 test conditions. A
manufacturer of
quality control materials must establish each of those means and ranges by
statistically valid
methods, and the means and ranges must be re-established for each new lot of
each quality
control material.
One technique for establishing the means and ranges would be to simply, for
each new lot of
a particular quality control material, perform tests on samples of the new lot
on several
different testing machines in several different laboratories, and to
statistically characterize the
results. However, this approach may be very expensive, as many tests of each
material/machine combination may be needed to account for several sources of
uncertainty in
the testing process.
For example, as is well known, estimating a mean of a population by sampling
involves
uncertainty, as different sample sets will yield different estimates for the
population mean.
This uncertainty is often expressed as a "standard error of the mean", which
is one estimate
of the uncertainty in the mean.
Other sources of uncertainty arise as well. Repeated tests on a single testing
machine will
vary somewhat. This variation is referred to as "within-instrument"
variability. Additionally,
tests performed using different testing machines of the same make and model
will vary. This
variation is referred to as "between-instrument" variability. And tests
performed in different
laboratories using testing machines of the same make and model will vary. This
variation
will be referred to as "between-laboratory" variability. In some embodiments,
it may be
assumed that between-instrument and between-laboratory variabilities are
interchangeable
terms. This assumption would be perfectly valid, for example, if each
laboratory used only
8
Date Recue/Date Received 2021-06-15
one instrument of any particular make and model. In practice, any error
introduced by
equating between-instrument and between-laboratory variability may be
negligible. For
example, all results from a particular laboratory for a particular machine
make and model
may be assumed to be performed on the same testing instrument.
If mean and range values were to be assigned based only on tests of a new lot
of the material,
multiple tests would be performed using each of multiple testing machines at
multiple
laboratories, and enough test results would be collected to account for
uncertainty in
establishing the mean, for within-instrument variability, and for between-
instrument or
between-laboratory variability. This approach may require a large number of
tests for
statistical validity, to establish the range with confidence.
Embodiments of the invention exploit two observations to dramatically reduce
the number of
tests that must be performed to establish statistically valid means and ranges
for new quality
control material lots. First, it is observed that a sample mean may be
estimated using a
relatively small number of samples, as compared with the number of samples
required to
estimate the sample variability with similar confidence. Second, it has also
been realized that
while the mean test result expected from a particular quality control material
varies between
lots (resulting in the need to reassign means and ranges for each new lot),
the variability of
the test results tends to remain relatively stable between lots.
Embodiments of the invention establish the assay means for a new lot of
quality control
material using tests performed on the new lot of material, but utilize the
database of historical
test results derived from prior lots to establish the assay ranges, which may
be based on the
uncertainty in the estimate of the mean, the within-instrument variability,
and the between-
instrument or between-laboratory variability. Thus, relatively few new tests
must be
performed to establish the assay means and ranges for the new lot of material.
In some
embodiments, the estimates of variability may be further adjusted based on the
newly-
established assay mean. The assay mean and range can then be specified for
each test
condition.
Historical Results
Nearly every qualification test performed on the many testing machines by the
many testing
laboratories throughout the world is recorded, and the results communicated
back to the
manufacturer of the quality control material used in the qualification test.
These qualification
test results are accumulated in a database of historical results, and may be
used in various
ways by the quality control material manufacturer. Such a database may include
millions of
9
Date Recue/Date Received 2021-06-15
individual qualification test results, spanning years of data gathering. Each
recorded record
typically indicates the test condition at which the result was taken, and may
include such
information as the laboratory where the qualification test was run, the type
of quality control
material used in the test, the make and model of testing instrument on which
the qualification
test was run, the analyte being tested, the reagent used, the test method
used, and the test
result. Other kinds of information could be included as well. The database may
therefore
contain the results of many tests performed using prior lots of each quality
control material at
each test condition of interest.
Example Assay Mean Computation
In some embodiments, the assay mean is computed as an average of test results
obtained from
tests of the new lot of quality control material, performed under like test
conditions. The like
test conditions may include using testing machines of like make and model in
the different
laboratories, for example. In some eases, tests perfirmed on a single testing
instrument may
be sufficient. The mean may be computed as follows:
Let y be the jth test result reported by the eh lab, i 1, ...,L,,j= ni
is the number of reported results for each reporting lab. Note that it is not
necessary that all of the laboratories perform the same number of tests.
Let yi be the mean of the reported results for the ith lab.
n,
= n.
.1=1
The assay mean is computed as the average of all the lab means:
L
Mean = -E
Example Within-instrument (lab) Variability Computation
According to embodiments, the within-instrument variability is estimated using
historical
data from qualification tests performed on tpjQr lots of the particular
quality control material,
across multiple testing machines, which likely reside in multiple
laboratories. Depending on
the make and model of testing machine corresponding to the test condition of
interest, there
may be many test results, possibly many thousands of results, in the database
that are usable
for estimating the within-instrument variability. In some embodiments, the
within-instrument
Date Recue/Date Received 2021-06-15
variability may be computed as an average variance of test results obtained
from individual
testing machines (an average of variabilities), or otherwise derived from the
variances of test
results from the individual testing machines. The estimate may be corrected
for bias. The
within-instrument variability estimate may be computed as follows:
For each reporting lab, compute the sum of the reported results: n,
=
.1=1
For each reporting lab, compute the sum of reported results squared:
SS; = 11
Compute the sum of all reported results from all laboratories:
S =ESi
i=1
Compute the total number of reported results from all laboratories: N = En,
From the above quantities, compute the sum square within, S2
SSiv E SS; ¨E
n,
and compute the within-lab variance, V 7-- SS AN -1.)
w w
The within-lab standard deviation can then be computed as SD
w -v w
Note that these computations make the assumption that all results from a
particular laboratory
are obtained using the same instrument. Of course, separate instruments could
be tracked
separately. The within lab standard deviation SD, is an example of an estimate
of within-
instrument or within-laboratory variability. As is explained in more detail
below, the
estimate may be further adjusted based on the mean determined above before
computing the
limits of the assay range.
Example Between-instrument (Laboratory) Variability Computation
According to embodiments, the between-instrument variability is also estimated
using
historical data from qualification tests performed on prior lots of the
particular quality control
material, across multiple testing machines, which likely reside in multiple
laboratories. Each
particular testing machine may produce results that differ from results
obtained from other
machines of like make and model. The differences may result from differences
in calibration
or operational techniques. Depending on the make and model of testing machine
corresponding to the test condition of interest, there may be many
laboratories, possibly
11
Date Recue/Date Received 2021-06-15
hundreds, that use the testing machine, and whose qualification test results
are in the
historical database and are usable for estimating the between-instrument
variability. In some
embodiments, the between-instrument variability may be computed as a
variability between
average test results obtained from different individual testing machines (a
variability of
averages). The between-instrument variability estimate may be computed as
follows,
utilizing the quantities Si, SSi, and N defined above:
Compute sum square between: L s2 s2
SS 8 = ..............................
I N
Compute \
En/2
n N __
= Compute between-lab variance,
SS AL ¨1.)¨ Vw)
Vs = Max( 0,
Compute between-lab standard deviation, 50 B 1 B
The between-laboratory standard deviation SDB is an example of an estimate of
between-
laboratory or between-instrument variability. As is explained in more detail
below, the
estimate may be further adjusted based on the mean determined above before
computing the
limits of the assay range.
On the assumption that the within- and between-instrument variabilities are
the two sources
of variation that contribute to the total variability SDT, then SDT = AD; +
SD.
Adjustment of Variability Estimates Based on Mean
In some embodiments, the estimates of within-instrument, between-instrument,
and total
variability may be adjusted before proceeding further. For example, it has
been observed that
the within-instrument variability, such as SW, varies as a function of the
mean test result for
the particular quality control material of interest. (The mean test result
will vary as a function
of the concentration of the analyte in the quality control material, which
varies between lots.)
In one hypothetical scenario, a first lot of the particular quality control
material may produce
slightly higher mean test results than a second lot, and the results may also
vary more within
a particular testing instrument for the first lot than for the second. That
is, in this hypothetical
example, within-instrument variability is positively correlated with the mean
test result.
Because the estimate of the mean computed for a new lot of the quality control
material may
12
Date Recue/Date Received 2021-06-15
differ from the average of the means from prior lots, it may be desirable to
adjust the
variability estimates, in accordance with the observed relationship of
variability and mean.
To characterize the relationship of mean and variability, a regression
analysis may be
performed on data from the database of historical test results, for each test
condition of
interest. Once the relationship is lcnown, the variability estimates may be
adjusted.
Preferably, separate correlations and adjustments are performed for the
separate within-
instrument, between-instrument, and total variability estimates. If regression
analysis is
performed to establish the relationship between mean and variability for any
two of the three
variabilities (within-instrument, between-instrument, and total), then the
third relationship
can be established directly from the equation relating total variability to
between-instrument
and within-instrument variability.
Other techniques may be utilized for accounting for an observed relationship
between
variability and mean test result. For example, another way to establish the
variability
estimate would be to identify, in the database of historical test results, a
specific prior lot of
the quality control material that has a mean test result comparable to the
mean test result
computed for the new lot of quality control material. In this embodiment, the
variability of
test results from the specific prior lot is computed, and the variability
estimate for the new lot
of quality control material is then assigned to be equal to the computed
variability of test
results from the specific prior lot of quality control material. Whether the
mean of a prior lot
is comparable to the mean computed for the new lot of material may be
established in any
suitable way. For example, a prior lot may be considered to have a comparable
mean if its
mean is within 1%, 2%, 5%, or another suitable percentage of the mean of test
results from
the new lot. Or a prior lot may be considered to have a comparable mean if its
mean is within
the standard error of the mean computed for the new lot of quality control
material, as
described below.
Example Error of the Mean Estimation
The range of test results within which a new test result, resulting from a
test performed on
any instrument in any laboratory, is expected to fall is widened by several
factors, including
the uncertainty of determining the sample mean, the within-instrument
variability, and the
between-instrument variability. The uncertainty in the estimate of the mean
depends on the
number of instruments and samples used to compute the estimate of the sample
mean, and on
the between-instrument and within-instrument variability. In embodiments of
the invention,
very few samples may be used to estimate the assay mean, for example as few as
four
13
Date Recue/Date Received 2021-06-15
samples. If the estimate of the standard error of the mean were to be computed
based on the
same small number of samples, the assay range may be undesirably unreliable.
Thus according to embodiments of the invention, the uncertainty in the
estimate of the mean
is computed using variability data from the database of historical results,
because a much
larger sample size is available with which to estimate the variability. In
some embodiments,
the standard error of the mean is used as the uncertainty estimate of the
mean, according to
ti-V V I- 1
SEM= Bi- wE
L L2
In this formula for the standard error of the mean (SEM), the terms Vw and VE
are the within-
and between-instrument or between-laboratory variances computed above, L is
the number of
laboratories, and ni is the number of reported results for each reporting lab
used to estimate
the new quality control lot mean.
Example Assay Range Determination
Once the error of the mean and the within- and between-instrument
variabilities have been
estimated, the assay range can be determined. The expected distribution of
test results, taken
using any testing instrument in any laboratory, will be centered on the assay
mean, and have a
width that is influenced by the uncertainty in the estimate of the mean, as
well as the within-
and between-instrument variabilities. Using the estimates computed above, a
standard
deviation of the distribution of expected test results may be computed as:
SDR = -viSD82 +SDI2,v + SEM2
For the purposes of this disclosure, an estimate of population variability
including the
estimate of the standard error of the mean and the within- and between-
instrument variability
estimates will be referred to as an estimate of the "overall" variability
(which is different than
the "total" variability discussed above). SDR as computed above is an example
of an overall
variability estimate. The assignment of the assay range is then made to
encompass a
specified portion of the expected test result distribution. For example, if it
is desired that the
assay range encompass 99.7% of all expected test results from testing machines
operating
under statistical control, the range may be set at Mean 3 SDR. In that case,
then a test on
a sample of the new lot of quality control material would be expected to fall
within the
specified range with probability of 99.7%, regardless of which instrument is
used to perform
the test, and regardless of which laboratory the instrument resides in. In
another example, if
14
Date Recue/Date Received 2021-06-15
it is desired that the assay range encompass 95.4% of all expected test
results, the range may
be set at Mean 2* SPR , in which case a test on a sample of the new lot of
quality control
material would be expected to fall within the specified range with a
probability of 95.4%, for
any normally-operating test instrument in any lab.
Curation of Data
Preferably, all of the data used in the determination of assay means and
ranges, including the
data in the database of historical results and the results of tests performed
on a new lot of
quality control material, is "curated" before use. That is, the data is
examined and clearly
erroneous or anomalous data is either corrected or removed from consideration.
Anomalous data may arise from many causes. For example, different laboratories
report their
qualification test results to the quality control material manufacturers in
different ways.
Some testing machines may be automated and connected to a computer network, so
that
qualification test results are transmitted automatically and electronically
with little
opportunity for human error, although collection and transmission errors are
possible. Some
laboratories may record qualification test results manually, so that many
other opportunities
for error are present For example, digits may be transposed or mistyped, or
the units of
measure for a particular test may be entered incorrectly. In the latter case,
a test whose
results are properly reported in milligrams per liter could mistakenly be
reported in grains per
liter, so that the reported results differ from the actual results by a factor
of 1000. Many,
many other error sources are possible.
It is important, however, not to remove data that are likely to be within the
normal
distribution of test results, but merely in the tails of the distribution.
Examples of criteria by
which a test result may be excluded include:
= test result is outside the range of possible results for the test of
interest;
= test result differs from the average test result by more than a specified
number
of standard deviations of the population of similar tests, for example four,
five,
or six standard deviations;
= test result is a negative number for a quantity that can only be measured
in
positive numbers;
= test result exceeds the average result of similar tests by more than. a
specified
factor, for example a factor of 100 or more; or
= reported units of a test result are inconsistent with the nature of the
test itself.
Many other criteria for excluding data may be envisioned and used within the
scope of the
appended claims. In some embodiments, data may not be excluded automatically,
but an
Date Recue/Date Received 2021-06-15
automated system may flag suspicious data for review by a skilled operator,
who may then
decide which data are to be excluded, if any.
Sampling Plan Specification
The database of historical test results may be exploited for other uses as
well. For example,
as is explained above, the uncertainty in the estimate of the mean. is
influenced by the number
of samples tested to establish the assay mean, the within-instrument
variability, and the
between-instrument (laboratory) variability. It is desirable that the error of
the mean
contribute only a small portion of the width of the assay range, for example
no more than
12% of the range width. However, without prior knowledge of the within- and
between-
instrument variabilities, it is difficult or impossible to know how the assay
range width will
be influenced by tests conducted on different instruments and at different
laboratories. If the
number of samples used to establish the assay mean and the allocation of tests
to different
testing instruments and laboratories are chosen blindly at the beginning of a
mean and range
assignment process, it may be discovered at the end of the process that the
range is
unnecessarily wide due to an excessive contribution from the uncertainty in
the mean.
The database of historical test results can be utilized for designing a
sampling plan, to
minimize the number of tests that must be done in order to establish an assay
mean and range
for which the uncertainty in the mean contributes an acceptably small portion
of the assay
range. For example, if it is known a priori that for a particular test
condition, the between-
instrument or between-laboratory variability is very small in relation to the
within-instrument
variability, it may be possible to establish the assay mean using tests
conducted on only a
single testing machine, as tests conducted on other machines would likely
merely duplicate
those of the first machine. However, if the between-instrument variability is
large in relation
to the within-instrument variability, it would be very important to test using
multiple
machines, to account for the between-instrument variability. In this case,
relatively few tests
may be required on each machine, as a small within-instrument variability
indicates that such
tests may quickly become redundant. When the within- and between-instrument
variabilities
are closely balanced, then it may be necessary to perform multiple tests on
each of several
testing instruments.
Table 2 below illustrates the effect on a sampling plan of different
relationships of within-
and between instrument variabilities. The effects are given in terms of the
ratio k of the
between-lab (instrument) variability to the within-lab (instrument)
variability, that is
k = SDB I SD, , and the portion of the assay range that is attributable to the
error of the mean
16
Date Recue/Date Received 2021-06-15
for different sampling plans. As can be seen from Table 2, if the between-lab
variability is
very small in relation to the within-lab variability (k , it may
be necessary to test only on
a single instrument to establish the mean with sufficient confidence that only
a small portion
of the assay range will be attributable to the uncertainty in the mean. Using
a 12%
contribution as an example, it can be seen that in the "k=0.1" column of Table
2, that four or
more tests performed on a single testing machine are sufficient.
Alternatively, if two or three
instruments are used, then two or more tests on each machine are sufficient,
and if four
instruments are used, then only one test on each machine is needed.
If k=2, then at least four testing machines would have to be utilized to
achieve a 12% or less
contribution to the assay range width, as can be seen in the "k=2" column of
Table 2.
If k-4).5, then any of several sampling plans may suffice, including four or
more tests on each
to two instruments, two or more tests on each of three instruments, or one or
more tests on
each of four instruments.
By estimating SDB and S.Div beforehand using the historical test data, a
tester may be able to
safely design an efficient sampling plan for the determination of a neve assay
range.
I 7
Date Recue/Date Received 2021-06-15
Table 2. % Increase in Range due to Uncertainty of Mean Estimate
Between-Lab Si) is Between-Lab SD is
N of Between-
Lab SD Between-Lab SD is 2
# Reps 1/10 as Large as 1/2 as Large as
# Labs. n Lab Data Equals
Within- Times Larger Than
Within-Lab SD Within-Lab SD
Points Lab SD
(k=1) Within-Lab SD (k=2)
(k41.1) (k4.5)
1 1 1 41% 410/ 41% 41%
1 2 2 23% 26% 32% 38%
1 3 3 16% 11% 29% 37%
1 4 4 12% 18% 27% 36%
I 5 5 10% 17% 26% 36%
1 ..... 6 6 8% 15% 26% 35%
I ¨7----3-----13A; 15% 25-67; I5-67,,
1 8 8 6% 14% 25% 35%
I 9 9 6% 14% 25% 35%
1 10 10 5% 13% 24% 35%
- 1 I 1 22% 22% 22% 22%
=
1 1 4 12% 14% 17% 20%
2 3 6 8% 11% 15% 20P/0
2 I 4 -----/F--- -6% 10% 15% 19%
2 ' 5 10 5% 9% 14% 19%
2 6 12 4% 8% 14% 19%
2 7 14 4% 8% 13% 19%
2 8 16 3% 7% 13% 19%
=
2 9 18 3% 7% 13% 19%
2 10 20 3% 7% 13% 19%
3 1 3 15% 15% 15% 15%
i
-
, .. 6 8% 10% 12% 14%
3 3 9 6% 7% 11% 14%
3 4 12 4% 6% 10 /0 13%
3 5 15 3% 6% 10% 13%
3 6 18 3% 5% 9% 13%
3 7 21 2% 5% 9% 13%
3 8 24 2% 5% 9% 13% ,
3 9 27 2% 5% 9% 13%
3 10 30 2% 5% 9% 13%
4 1 4 12% 12% 12% 12%
4 2 8 6% 7% 9% 11%
4 3 11 4% 6% 8% 10%
4 4 16 3% 5% 8% 10%
4 5 20 3% 4% 7% 10%
4 6 24 2% 4% 7% 10%
4 7 28 2% 4% 7% 10%
4 8 32 2% 4% 7% 10%
4 9 36 1% 4% 7% 10%
........ .... _________
4 10 40 1% 3% 7% 10%
Figure 1 illustrates in flowchart form an overview of steps of a method 100
for establishing a
statistically valid assay mean and assay range for a particular lot of a
quality control material,
in accordance with embodiments of the invention. It will be recognized that
some steps
illustrated in Figure 1 are optional or may not be performed in some
embodiments. In
18
Date Recue/Date Received 2021-06-15
addition, the order of steps may be rearranged when appropriate. While the
flow chart of
Figure 1 is shown as sequential, some steps may be performed in parallel with
other steps.
In step 101, the database of historical test results is collected, as
described above. That is,
testing laboratories record and report the results of qualification tests, and
report the results
.. along with information identifying the test condition for each result. The
results may be
reported to the manufacturer of the quality control material used in each
qualification test In
step 102, the data in the database is curated to remove clearly erroneous or
anomalous entries.
The database preferably includes the results of many qualification tests,
encompassing prior
lots of the quality control material for which a new mean and assay range are
desired.
In step 103, an upper limit is set for the percentage of the assay range that
will be due to the
uncertainty in estimating the mean. In some embodiments, this limit may be set
at 6%, 8%,
10%, 12%, 15%, 20%, 25%, or another suitable value. The limit may be selected
by the
entity performing the method, and may be selected arbitrarily, or for reasons
chosen by the
entity establishing the limit.
In step 104, the database of historical results is accessed and used to
estimate the within- and
between-instrument variabilities of prior lots of the quality control
material. As is explained
above, the variability estimates may be expressed as standard deviations SDw
and SDB. Other
kinds of variability estimates may be used in embodiments, for example maximum
likelihood
or restricted maximum likelihood estimates of variances.
In step 105, a sampling plan is determined, based at least in part on the
within- and between-
instrument variability estimates. The sampling plan may indicate how many
samples of the
new lot of quality control material need be tested on how many different
testing machines in
order that the mean can be estimated precisely enough that the uncertainty in
the mean
contributes no more to the width of the assay range than the upper limit set
in step 103. If the
estimates of within- and between-instrument variability are expressed as
standard deviations
SDw and SDB, Table 2 may be used to establish the sampling plan.
In step 106, samples are tested of the new lot of quality control material for
which a new
assay mean and range are desired. The samples may be tested according to the
sampling plan
established in step 105.
In step 107, the estimates of between- and within-instrument variability may
be adjusted
based on the mean computed in step 106, according to historical relationships
between
variabilities and means for the test condition of interest. For example,
regressions may be
performed on data from the database of historical results, to establish the
expected
19
Date Recue/Date Received 2021-06-15
relationships between the mean and the within-instrument variability and
between the mean
and the between-instrument variability.
In step 108, an estimate of the overall variability is computed from the
within- and between-
instrument variability estimates, and an estimate of the uncertainty in the
mean. As is
explained above, the standard error of the mean may be used as the estimate of
the
uncertainty in the mean. The standard deviation SDR may be used as the
estimate of the
overall variability, although embodiments of the invention may use other
reassures of overall
variability.
In step 109, a target probability is set for the probability that a new
qualification test result,
performed on a properly-functioning testing machine in a laboratory that is
operating under
statistical control, will fall within the assay range to be established. For
example, the target
probability may be set at 75%, 80%, 90%, 95.4%, 99%, 99.7%, 99.9%, or any
other suitable
value.
And in step 110, the limits of the assay range are established. Preferably,
the range is
centered on the calculated mean, and has a width such that new qualification
test results will
fall within the range with the target probability established in step 109.
When the overall
variability is estimated using a standard deviation SDR , the range may be
established using
the number of standard deviations that are required to encompass the desired
proportion of
qualification test results.
At least steps 104-110 may be repeated for each new test condition of
interest, for example a
new combination of testing instrument make and model, test method, particular
quality
control material, and quality control material concentration.
Figure 2 illustrates a simplified block diagram of the interactions of various
systems
involved in the assignment of assay means and ranges, in accordance with
embodiments of
the invention. A number of laboratories 201 a-h record the results of
qualification tests.
Those results are collected in a database of historical results 202, and may
be curated as
described above. In Figure 2, eight laboratories utilizing three different
testing machine
models are depicted, but it will be recognized that many more laboratories and
testing
machine models may be present. An assay mean and range assignment system 203
accesses
the database 202, and performs the calculations for assigning the mean and
range. Assay
mean and range assignment system 203 may first utilize data from database 202
to formulate
a sampling plan 204, and samples of a new lot of quality control material may
be tested 205
in accordance with the sampling plan. The results of those tests are
communicated back to
Date Recue/Date Received 2021-06-15
assay mean and range assignment system 203, which computes and outputs the
assay mean
and range 206.
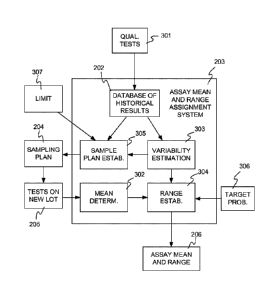
Figure 3 illustrates assay mean and range assignment system 203 in more
detail. Assay
mean and range assignment system 203 includes database 202, which receives
results from
qualification tests 301 performed on a number of different testing
instruments, as described
above. The system further includes a mean determination module 302 that
receives the
results of tests performed on samples of the new lot of quality control
material and computes
the mean of the test results. System 203 also includes a variability
estimation module 303
that computes an estimate of the variability of test results obtained from
tests performed on at
least one prior lot of the quality control material, using data from database
202. A range
establishment module 304 receives the mean and the variability estimate, and
also a target
probability 306 with which a new qualification test result performed on a
sample of the new
lot of quality control material should fall within the assay range, and
computes the assay
mean and range 206. System 203 may also include a sample plan establishment
module 305
that formulates sampling plan 204 based on data from database 202 and one or
more other
inputs, for example a specification 307 of an upper limit for the percentage
of the assay range
that is due to uncertainty in estimating the mean. Modules 302-305 may be
implemented in
hardware, software, firmware, or a combination of these, for example using a
computer
system as described in more detail below.
.. A more detailed description of various data flows, in accordance with
embodiments, is shown
in Figure 4. For the purposes of this disclosure, "peer" data from the
database are data that
can be used in establishing an assay mean and range for a particular test
condition.
Figure 5 is a block diagram illustrating an exemplary computer system 500 in
which
embodiments of the present invention may be implemented. This example
illustrates a
computer system. 500 such as may be used, in whole, in part, or with various
modifications, to
provide the functions of assay mean and range assignment system 203 and/or
other
components of the invention. For example, various functions of assay mean and
range
assignment system 203 may be controlled or performed by computer system 500,
including,
merely by way of example, the estimation of the within- and between-instrument
variabilities,
the computation of the mean and the uncertainty in the estimate of the mean,
regressions and
the adjustment of the within- and between-instrument variability estimates,
the computation
of an overall variability estimate, and the computation of the assay range.
21
Date Recue/Date Received 2021-06-15
Computer system 500 is shown comprising hardware elements that may be
electrically
coupled via a bus 590. The hardware elements may include one or more central
processing
units 510, one or more input devices 520 (e.g., a mouse, a keyboard, etc.),
and one or more
output devices 530 (e.g., a display device, a printer, etc.). Computer system
500 may also
include one or more storage devices 540. By way of example, storage device(s)
540 may be
disk drives, optical storage devices, solid-state storage device such as a
random access
memory ("RAM") and/or a read-only memory ("ROM"), which can be programmable,
flash-
updateable and/or the like.
Computer system 500 may additionally include a computer-readable storage media
reader
550, a communications system 560 (e.g., a modern, a network card (wireless or
wired), an
infra-red communication device, BluetoothTm device, cellular communication
device, etc.),
and working memory 580, which may include RAM and ROM devices as described
above.
In some embodiments, computer system 500 may also include a processing
acceleration unit
570, which can include a digital signal processor, a special-purpose processor
and/or the like.
.. Computer-readable storage media reader 550 can further be connected to a
computer-
readable storage medium, together (and, optionally, in combination with
storage device(s)
540) comprehensively representing remote, local, fixed, and/or removable
storage devices
plus storage media for temporarily and/or more permanently containing computer-
readable
information. Communications system 560 may permit data to be exchanged with a
network,
system, computer and/or other component described above.
Computer system 500 may also comprise software elements, shown as being
currently
located within a working memory 580, including an operating system 584 and/or
other code
588. It will be appreciated that alternate embodiments of computer system 500
may have
numerous variations from that described above. For example, customized
hardware might
also be used and/or particular elements might be implemented in hardware,
software
(including portable software, such as applets), or both. Furthermore,
connection to other
computing devices such as network input/output and data acquisition devices
may also occur.
Software of computer system 500 may include code 588 for implementing any or
all of the
function of the various elements of the architecture as described herein. For
example,
software, stored on and/or executed by a computer system such as system 500,
can provide
the functions of assay mean and range assignment system 203 and/or other
components of the
invention such as those discussed above. Methods implementable by software on
some of
these components have been discussed above in more detail.
22
Date Recue/Date Received 2021-06-15
The invention has now been described in detail for the purposes of clarity and
understanding.
However, it will be appreciated that certain changes and modifications may be
practiced
within the scope of the appended claims.
23
Date Recue/Date Received 2021-06-15