Note: Descriptions are shown in the official language in which they were submitted.
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
CELL SEPARATION APPARATUS AND METHODS OF USE
RELATED APPLICATION INFORMATION
None.
BACKGROUND
Cell therapy and tissue engineering is developing toward clinical applications
for the
repair and restoration of damaged or diseased tissues and organs. In
particular, the
development of tissue grafts promotes developments in surgeries, including
cardiac and
peripheral vascular surgery, limb tissue repair, dental applications, as well
as veterinary
surgeries. Grafts and other cell-based products may be formed by isolating
and/or
culturing cells from human or animal tissue.
A potential source for endothelial cell seeding is microvascular endothelial
cells
(MVEC). Williams et al. pioneered both freshly isolated and cultured human,
canine,
rabbit, rat, bovine and pig endothelial cells, specifically MVEC, in their
laboratory to
study cellular function. The source for human MVEC was aspirated tissue from
cosmetic
liposuction. Two separate protocols for human fat MVEC isolation were used
depending
on the end use of the cell population. The protocols differed in isolation
complexity from
zo a simple, operating room-compatible procedure for immediate sodding of
human or
animal grafts to a more elaborate procedure if the MVEC will be subsequently
cultured.
Endothelial cells are of critical importance in establishing a non-
thrombogenic cell
lining. A need still exists for an efficient and reliable method for producing
endothelial
1
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
cell linings on a synthetic graft in an operating room setting. It is
desirable to achieve
rapid cell adhesion in or on a permeable matrix, scaffold or other permeable
cell
substrate material in a matter of minutes or hours with an instrument that
lends itself to
the operating room environment, maintains a sterile barrier, is easy to use,
produces
consistent results, and is inexpensive.
LISTING OF THE FIGURES
FIG. 1 is a partial isometric view of a cell separation system 100 according
to certain
embodiments of the present disclosure.
FIG. 2 is a partial exploded view of a single-walled bowl according to certain
embodiments of the present disclosure.
FIG. 3A is a partial top view of a single-walled bowl according to certain
embodiments
of the present disclosure.
FIG. 3B is a front view of a single-walled bowl according to certain
embodiments of the
present disclosure.
FIG. 3C is a partial side view of a single-walled bowl according to certain
embodiments
of the present disclosure.
FIG. 4A is a partial cross-sectional view of a single-walled bowl taken across
the bowl
through the pellet concentration areas according to certain embodiments of the
present
disclosure.
zo .. FIG. 4B is a partial cross-sectional view of a single-walled bowl taken
across the bowl
perpendicular through the pellet concentration areas according to certain
embodiments
of the present disclosure.
2
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
FIG. 5A is a partial exploded view of a of a single walled bowl assembly 102
according
to certain embodiments of the present disclosure.
FIG. 5B is a partial isometric view of a single walled bowl assembly 102
according to
certain embodiments of the present disclosure.
FIG. 6 is partial exploded view of a mounting plate 504 and a centrifuge
assembly 132
according to certain embodiments of the present disclosure.
FIG. 7 is a method of separating endothelial cells from biologic material such
as adipose
tissue according to certain embodiments of the present disclosure.
SUMMARY
An embodiment of the present disclosure describes a method of separating
cells,
comprising: agitating, in a digestion area of a cell separation system, a
volume of
biologic material and a volume digestion media to form a digested volume of
biologic
material; centrifuging the digested volume of biologic material at a force
from about 500
G to about 1000 G from about 5 minutes to about 10 minutes to separate the
digested
volume into a plurality of concentrated cell volumes and a plurality of waste;
collecting
the plurality of concentrated cell volumes in a product reservoir wherein the
concentrated cell volume comprises material with a smaller diameter than the
pores of a
filter such that the material can pass through the filter into a cell
concentration area;
isolating the plurality of waste in a waste reservoir wherein the waste
reservoir is
zo partitioned in the bowl from the digestion area and wherein the
plurality of waste
comprises waste with a larger diameter than the pores of the filter and is not
in contact
with the concentrated cell volumes; and removing the concentrated cell volumes
from
the product reservoir.
3
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
An embodiment of the present disclosure describes a cell separation system,
comprising: a non-transitory storage device comprising a plurality of logic
associated
with centrifugation programs, wherein execution of a centrifugation program
separates a
cell volume from a biologic material volume; a heating mechanism electrically
coupled
to a power supply; a containment mechanism in proximity of the heating
mechanism; an
assembly removably coupled to the containment mechanism, wherein the assembly
comprises: a single-walled bowl comprising a product reservoir, plurality of
cell
concentration areas contained within the product reservoir, a digestion area,
a waste
reservoir, and a center column comprising a access tube removably coupled to
the
product reservoir, a mounting plate, wherein the single-walled bowl is
removably
coupled to the mounting plate, an alignment mechanism coupled to the center
column
to restrict movement, wherein, in a first state of a centrifugation program,
the assembly
is configured to rotate around a central axis in a first direction and in a
second direction
in an alternating fashion and the heating mechanism is activated, and wherein,
in a
.. second state of a centrifugation program, the heating mechanism is
deactivated and the
single-walled bowl is configured to rotate in a single direction around the
central axis to
separate a plurality of waste from a plurality of cells.
An embodiment of the present disclosure describes a cell separation system
comprising: a non-transitory storage device comprising a plurality of logic
associated
zo with a plurality of different centrifugation programs, wherein, when
executed by a
processor; agitates in a digestion area a volume of biologic material and a
volume of
digestion media to form a digested volume of biologic material; separates, via
centrifugation, the digested volume of biologic material at a force from about
500 G to
4
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
about 1000 G from about 5 minutes to about 10 minutes to separate the digested
volume into a plurality of concentrated cell volumes and a plurality of waste;
collects the
plurality of concentrated cell volumes in a product reservoir wherein the
concentrated
cell volume comprises material with a smaller diameter than the pores of a
filter such
that the material can pass through the filter into a cell concentration area;
isolates the
plurality of waste in a waste reservoir wherein the waste reservoir is
partitioned in the
bowl from the digestion area and wherein the plurality of waste comprises
waste with a
larger diameter than the pores of the filter and is not in contact with the
concentrated
cell volumes; and removes the concentrated cell volumes from the product
reservoir.
DETAILED DESCRIPTION
Endothelial cells are used to establish non-thrombogenic cell lining within
synthetic
grafts. Thus, it is desirable to achieve rapid cellular adhesion in or on a
permeable
matrix, scaffold, or other permeable cell substrate material in a matter of
minutes or
hours with an instrument that lends itself to the operating room environment,
maintains
a sterile barrier, is easy to use, and produces consistent graft results.
Currently, there are various approaches for meeting these requirements, but
with limited
success: (i) the use of decellularized tissue materials; (ii) the use of a
self-assembly
mechanism, wherein cells are cultured on tissue culture plastic in a medium
that
induces extracellular matrix (ECM) synthesis; (iii) the use of synthetic
biodegradable
zo polymers, onto which cells are subsequently seeded and cultured in a
simulated
physiological environment; and (iv) the use of biopolymers, such as a
reconstituted type
I collagen gel, which is formed and compacted with tissue cells by the
application of
mechanical forces to simulate a physiological environment.
5
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
Embodiments of this disclosure describe systems and methods that enable the
isolation
of large quantities of endothelial cells from fat tissue and the rapid cell
sodding of
synthetic grafts, and that enable the automation and adhesion of cells in a
turn-key,
operating room ready instrument for the rapid sodding of the graft.
Embodiments of this
disclosure likely have other applications in addition to the lining of grafts
for
implantation.
The systems and method discussed herein are of a cell separation system that
comprises a single-walled bowl that may be formed as a single piece (no seams
or
welds) or which may be formed as a plurality of pieces. The single-walled bowl
("bowl")
may comprise various areas including a center column that houses a single
access tube
connected to a product reservoir, permitting direct access to the cell
concentration
areas containing the desired end product. The bowl further comprises a
digestion area
and a waste reservoir separated by a plurality of internal walls which may be
formed
integrally with the bowl or which are separable components. The internal walls
permit
the separation of the digestion area and waste area such that when the bowl is
centrifuged, the cell separation process occurs faster and results in a more
pure end
product. The physical separation of the digestion area and waste area further
permits
the digestion area to be directly heated, further assisting in the cell
separation process.
The single-walled bowl further comprises a plurality of cell concentration
areas disposed
zo circumferentially around a central axis of the bowl such that, during
centrifugation, the
gravitational forces separate the cells from a biological material volume.
Waste is
collected in the waste reservoir and cells are collected in the cell
concentration areas.
The bowl further comprises one or more filters or screens connected to a
product
6
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
reservoir that extends from one end of the bowl to the other end, from one
cell
concentration area to another. The product reservoir is connected to the
access tube in
the center column, thereby providing access to the concentrated cells. The
product
reservoir further comprises one or more screen holders that house screens or
filters that
prevent waste product from contaminating the concentrated cell product. The
bowl
couples to a mounting plate which couples to a containment mechanism via a
spring
locking mechanism. An alignment mechanism is coupled to the bowl such that it
engages with the mounting plate on which the assembly of the bowl is disposed.
In one example of cell separation using the cell separation system discussed
herein, a
plurality of logic is stored in a non-transitory storage device (memory) and
comprises a
plurality of centrifugation programs. Each program of the plurality of
programs
comprises instructions that may be based upon a plurality of factors including
media
type and biological material volume and/or target cell volume or target cell
concentration. When executed by a processor, each program initiates cell
separation
through a plurality of states as discussed herein, resulting in the automated
removal of
the separated cells and the trapping and/or removal of waste. The programs may
differ
and/or overlap in various aspects, including temperatures, times, forces, and
overall
program length (time) from disposal of the biologic material volume and the
media until
the removal of the separated cell volume.
zo In one example of the cell separation system, a plurality of digestion
media and a
plurality of biologic material (a volume) are disposed in the bowl, in
particular in a mixing
and digestion area of the bowl. The bowl is then agitated, rotated in each
direction
around the central axis to break up the biologic material volume to enable the
7
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
separation during centrifugation. The bowl may be heated prior to and/or
during the
agitation via a heating mechanism, the heat generated by the heating mechanism
causes the air in a gap between the bowl and the mounting plate and/or the
containment mechanism to heat up and circulate. The bowl may be heated from
about
25 C to about 45 C, and the heating mechanism may be shut off subsequent to
completion of agitation such that the remainder of the cell separation occurs
at room
temperature (from about 20 C to about 25 C).
Subsequent to the agitation, centrifugation is performed. During
centrifugation, the bowl
rotates freely with respect to the containment mechanism, an alignment
mechanism
coupled to the bowl and to the mounting plate, and in some embodiments further
coupled to the containment mechanism, prevents a portion of the bowl from
rotating.
The centrifugation may be performed at a force from about 500G to about 1000G
for
from 5 minutes to 20 minutes, or from 1 minute to 10 minutes, or other ranges
of time in
various embodiments. Cells are separated from the mixture in the mixing and
digestion
area and are gravitationally forced into a plurality of cell concentration
areas of the bowl.
After the centrifugation, the speed of the bowl is reduced and waste materials
collect in
the waste reservoir. As the speed of the bowl is reduced, a vacuum is created
in the
product reservoir through the access tube and the separated cells are
collected during
operation. Cells and media that are of a smaller diameter than the holes in
the
zo filters/screens pass through the screens and into the cell concentration
areas while
waste and other materials with a diameter larger than the filter/screen holes
are
prevented from passing through the screens. This waste is maintained in the
waste
reservoir and does not contact the separated cell volume.
8
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
In an embodiment, after centrifugation, the rotation may be stopped, and the
collected
cells remain in the product reservoir. The cells are removed via the access
tube using a
vacuum. By using a single-walled bowl, the heat transfer may be more
effective, thus
increasing the efficiency of the system. A single walled bowl system reduces
the cost
associated with cell separation. Direct access to the cell concentration areas
permits the
capture of a more pure and more concentrated cell product. The access to the
product
reservoir further permits the cell product to be captured while the assembly
is still
rotating.
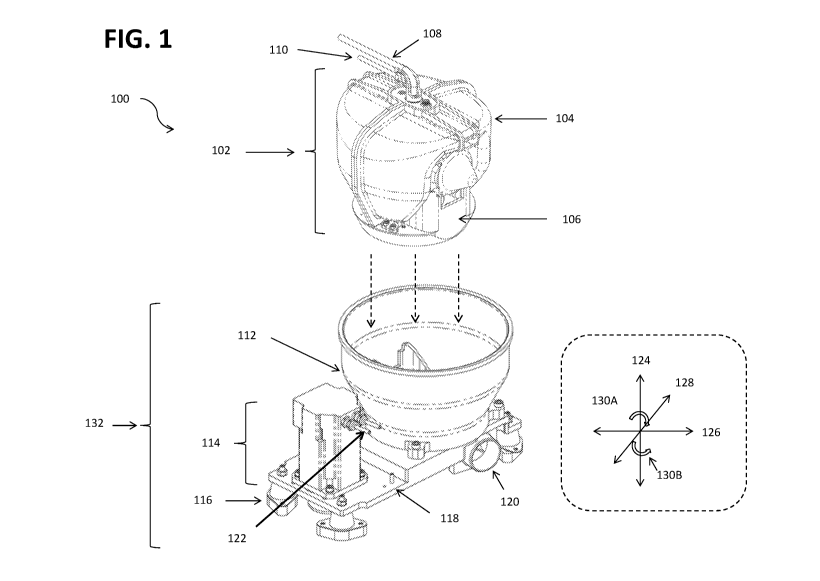
FIG. 1 is a partial isometric view of a cell separation system 100 according
to certain
embodiments of the present disclosure. The cell separation system 100
comprises a
centrifuge assembly 132 and an assembly 102 of a single-walled bowl 104
coupled to a
mounting plate 106 via a spring locking mechanism. The mounting plate 106 is
coupled
to the drive shaft of a centrifuge (not shown). The assembly 102 rotates
freely while the
alignment rod 110 (and single access tube 108) remain stationary. During
agitation and
centrifugation, the assembly 102 spins in either direction 130A or 130B around
a central
axis 124, shown in a coordinate system in FIG. 1 and referenced throughout.
The
coordinate system further comprises a second axis 126 that is perpendicular to
the
central axis 124, and a third axis 128 perpendicular to both 124 and 128.
In an embodiment, the assembly 102 is agitated by rotating less than 360
degrees in
zo each direction 130A and 130B around central axis 124. The bowl 104 may be
heated
during agitation and/or during centrifugation. During agitation and
centrifugation, the
biological material is separated and the cells are concentrated in the product
reservoir
206 and waste is collected in the waste reservoir 202.
9
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
In an embodiment, the mounting plate 106 is seated in and removably coupled to
a
containment mechanism 112, which acts to direct the heat and hot air generated
by the
activation of the heating unit 114. Containment mechanism 112 is also
removably
coupled to a centrifuge that operates to rotate the assembly 102. The coupling
of the
containment mechanism 112 to the assembly 102 creates an air gap between the
two
through which hot air may be circulated via a plurality of apertures (not
shown) on the
bottom of the mounting plate 106. In an embodiment the single walled bowl
assembly
102 and associated tubing are disposable.
In an embodiment, the heating unit 114 is employed to elevate a temperature of
the
io system 100 during at least agitation. The heating unit 114 is coupled to
a base 118
comprising a plurality of feet 116 configured to prohibit movement of the base
118 and
system 100 during execution of a plurality of centrifugation programs. The
heating unit
114 may be powered by a direct wired connection via 122, or may comprise a
portable
power source, such as a rechargeable battery (or batteries). The heating unit
114
further comprises a plurality of heating elements configured to elevate a
temperature of
the containment mechanism 112 and thus the assembly 102 coupled to the
mechanism
112. The containment mechanism 112 may also be coupled or removably coupled to
the base 118 as well.
In an embodiment, the cell separation assembly 102 is able to spin relative to
the
zo containment mechanism 112 because the alignment rod 110 is coupled to the
center
column 402 and the bowl 104 is secured to the mounting plate 106 by a spring
locking
mechanism. The assembly is removably coupled to containment mechanism 112,
enabling free rotation of the assembly 102.
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
In an embodiment, the system 100 may comprise at least one storage device (not
shown) comprising a plurality of centrifugation programs and a processor, as
well as a
plurality of controls (not shown) activated by the execution of a
centrifugation program
via the processor. The storage device and/or the plurality of controls that
may be
located on the system 100 or located remotely and accessed via a tablet,
mobile phone,
wearable technology, kiosk, laptop computer, or desktop computer. Each
centrifugation
program may comprise a plurality of parameters employed in the centrifugation
cycle,
and may be selected manually or dynamically and in an automated fashion based
upon
inputs such as the biologic material volume used and/or the volume and/or type
of
.. digestion enzyme(s) employed.
FIG. 2 is a partial exploded view 200 of a single-walled bowl 104 according to
certain
embodiments of the present disclosure. The single-walled bowl 104 may be
fabricated
as a single, seamless piece or as multiple pieces assembled into the bowl 104.
The
"single-wall" of the bowl is in contrast to a bowl that has at least two
nested walls.
In an embodiment, the bowl 104 comprises one or more cell concentration areas
212
removably coupled to a product reservoir 206. Cell concentration areas 212
comprise
smooth internal geometries and act to isolate and concentrate, via the removal
of fluid
and solids, the cells separated during centrifugation. A center portion of the
bowl 104
houses the alignment rod 110 and a single access tube 108. The alignment rod
110 is
zo coupled to the bowl 104 and further to the product reservoir 206 by way
of rotary seals
214. The bowl 104 further comprises a waste reservoir 202 that is separated
from a
mixing and digestion area 204 by a plurality of internal walls which may be
formed
integrally with the bowl 104 or which are separable components.
11
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
In an embodiment, the bowl 104 further comprises one or more filter holders
208
removably coupled to product reservoir 206 and which hold one or more filters
210. The
one or more filters 210 prevent waste product, including undigested material,
from
entering into the product reservoir 206 and contaminating the concentrated
cell product.
The one or more filters 210 may be as large as a 500 micron filter size. The
one or
more filters may be of a size between 250 microns and 500 microns. The one or
more
filters may be of a size between 100 microns and 250 microns. The one or more
filters
201 may be less than 100 microns in size.
The one or more filters 201 may be
configured in a serial assembly with a range of larger filter sizes to smaller
filter sizes.
In an embodiment, the bowl 104 further comprises one or more feet 216 that
couple to a
spring loaded locking mechanism (not shown) and act to secure the bowl 104 in
the
mounting plate 106 during operation. Once secured, the bowl 104 can rotate
freely with
respect to the containment mechanism 112, which does not rotate and acts at
least in
part to direct and circulate heated air towards the bowl 104 during agitation
(and
breakdown) of the biologic material volume.
FIGS. 3A-C are various views of a single-walled bowl 104 according to certain
embodiments of the disclosure. FIG. 3A is a partial top view 300 of a single-
walled bowl
104. Bowl 104 may comprise an at least one seam 306 along a centerline of the
bowl
104. The cell concentration areas 212 and single access tube 108 are also
shown. FIG.
zo 3B is a partial front view 302 of a single-walled bowl 104 according to
certain
embodiments of the disclosure. The seam 306, cell concentration areas 212,
single
access tube 108, and alignment rod 110 are also shown. FIG. 3C is a partial
side view
12
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
304 of a single-walled bowl according to certain embodiments of the
disclosure. The
cell concentration areas 212, single access tube 108, and feet 216 are shown.
FIG. 4A is a partial cross-sectional view 400 of a single-walled bowl
according to certain
embodiments of the present disclosure. The view 400 of the bowl 104 is taken
across
the bowl through the pellet concentration areas 212. The product reservoir 206
extends
across the bowl 104 from one pellet concentration area 212 to a diametrically
opposite
pellet concentration area 212. Filters 210 in filter holders 208 are shown
removably
coupled to product reservoir 206.
FIG. 4A further discloses a center column or cavity 402 that contains single
access tube
io 108. Single access tube 108 is configured to permit the introduction of
sterile media, to
permit access to concentrated cell product, and to permit the removal of the
concentrated cell product including during operation. Single tube 108 remains
stationary
during rotation, thus enabling access to the concentrated cell product during
rotation of
the assembly 102. This configuration also reduces contamination of the
separated cell
product. FIG. 4A further comprises an access port 404 which is configured to
permit
access to the interior of the bowl only and permits the introduction of raw
material into
the mixing and digestion area 204 of bowl 104 while stationary. The material
may be
biological material, media, digestion enzymes, or other material necessary to
assist in
the cell separation.
zo .. FIG. 4B a partial cross-sectional view 406 of a single-walled bowl 104
according to
certain embodiments of the present disclosure. View 406 is taken across the
bowl 104
perpendicular through the cell concentration areas 212. FIG. 4B shows features
illustrated in FIGS. 2-3, including waste reservoir 202 and the mixing and
digestion area
13
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
204. Also shown in FIG. 4B are internal walls 408 that form a partial barrier
between
the mixing and digestion area 204 and the waste reservoir 202. During the
digestion
and mixing process, the assembly 102 rotates in each direction 130A and 130B
around
central axis 124. During centrifugation, the assembly 102 spins in either
direction 130A
or 130B around a central axis 124. The cells are separated and collected in
the product
reservoir 206. As the bowl is agitated or centrifuged the waste material is
spun over the
internal walls 408 and collected in the waste reservoir 202.
In an embodiment, the waste reservoir 202 is configured to hold and isolate
large and
undesired solids as well as used media and other liquids. By collecting in the
waste
io .. reservoir, these particles are prohibited from clogging the system. An
additional
measure to prevent clogging are the filters 210 (held in filter holders 208)
which are
removably coupled to the outer edges of the product reservoir 206 (seen in
FIG. 4A).
FIG. 5A is a partial exploded view of a of a single walled bowl assembly 102
according
to certain embodiments of the present disclosure. FIG. 5A shows that the
single-walled
bowl 104 fits into the spring loaded locking mechanism of mounting plate 504
by way of
feet 216 being inserted into apertures 506 and locking into place. FIG. 5B
depicts the
assembly 508 when the locking mechanism is engaged and bowl 104 is coupled to
mounting plate 504.
FIG. 6 is partial exploded view 600 of mounting plate 504 and centrifuge
assembly 132
zo according to certain embodiments of the present disclosure. FIG. 6 shows
where the
mounting plate 504 is located within and removably coupled to the containment
mechanism 112 of the centrifuge assembly 132.
14
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
FIG. 7 is a method of separating endothelial cells from biologic material
according to
certain embodiments of the present disclosure. The method 700 is an automated
mechanism for the washing, separation, concentration, and removal/harvesting
of viable
cells. The harvested cells may be employed for grafts or otherwise employed
for use in
healthcare (e.g., FDA approved implantation or further processing) and/or
healthcare
research (trials to approve cell separation methods and cell-derivative
products for use).
In an embodiment, at block 702 of the method 700, a biologic material volume
is
disposed in a mixing and digestion area 204 (FIG. 2) of a single-walled bowl
104 of a
cell separation system such as the system 100 in FIG 1. At block 704, a
plurality of
media and a collagenase enzyme or enzymes may be introduced into the bowl 104
in
particular into the mixing and digestion area 204 via access port 404.
Examples of
media may be Lactated Ringers (LR), Hartmans, Water For Injection (WFI) or any
other
similar media. In some examples, the mixing and digestion area 204 may be pre-
heated
to from about 30 C to about 40 C prior to disposal of the biologic material
volume
and/or media in the area. In some examples, block 702 may occur prior to block
704,
and in other examples, block 704 may occur prior to block 702. In still other
examples,
blocks 702 and 704 may occur near-simultaneously such that the biologic
material
volume and media are disposed in the mixing and digestion area 204 at
approximately
the same time.
zo In an example where the mixing and digestion area 204 is heated, it may
be heated to
and maintained at a temperature from about 30 C to about 40 C prior to block
702,
block 704, or after either or both of the biologic material volume and/or
media are
disposed in the mixing and digestion area 204. This heating may be referred to
as a
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
pre-heating, which may take from about 10 minutes to about 45 minutes, or less
than 10
minutes depending upon a target temperature or temperature range.
Further in the method 700 at block 706, the biologic material volume disposed
at block
702 is completely or partially digested via the enzyme media disposed at block
704.
The digestion at block 706 of the biologic material volume disposed at block
702 may
occur via heating of the mixing and digestion area 204, or via the agitation
of the single-
walled bowl 104, or by a combination of both. This digestion may occur over
various
time periods from 5 minutes to 1 hour, from 15 minutes to 45 minutes, or other
periods
of time depending upon a type of enzyme(s) used, a number of enzyme(s) used,
and/or
a volume of each enzyme used at block 704, and the volume of biologic material
disposed at block 702.
Further in method 700 at block 706, the agitation at block 706 may occur by
the partial
or complete rotation of the bowl in alternating directions around a central
axis, and is not
the same as, nor does it generate the force of, the centrifugation discussed
herein. The
agitation employed to promote digestion at block 706 may comprise whole or
partial
rotations in different directions around a central axis 124 (FIG. 1), and may
occur for a
predetermined period of time. The digestion at block 706 may be referred to as
a first
state of the apparatus, wherein centrifugal forces are not applied and the
media and
biologic material volume are agitated in the chamber for a predetermined
period of time
zo or for a predetermined number of agitation cycles. In one example, the
temperature of
the mixing and digestion area 204 is maintained at about 37 C during the
digestion at
block 706, this first state is maintained for a predetermined time period
based upon a
type of enzyme(s) used, a number of enzyme(s) used, and/or a volume of each
enzyme
16
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
used at block 704, and the volume of biologic material disposed at block 702.
The
systems discussed herein may comprise a plurality of stored programs
comprising
parameters for the digestion at block 706 as well as for other states, blocks,
and phases
discussed herein.
Further in method 700 at block 708, in response to and subsequent to the
completion of
digestion at block 706, centrifugation occurs. As discussed herein, the
"completion" of
the digestion at block 706 refers to when the digestion has progressed to a
point where
the cells are still viable but the biologic volume has been broken down such
that it is
capable of centrifugation at block 708. This centrifugation at block 708 may
be
characterized by the separation of cells from the digested volume formed at
block 606.
In an embodiment, the configuration at block 708 comprises a g-force from 600
G to
1000 G for a time period from about 5 minutes to about 10 minutes. The g-
forces or "G"
referred to herein is the force of gravity applied to a body, in this case,
the force applied
to the cells collected in the cell concentration areas which continue to have
fluid
.. removed (thus becoming a more concentrated cell volume with the reduced
fluid/waste)
and remain isolated from the mixing and digestion area 204 during at least the
centrifugation at block 708.
The centrifugation at block 708 may be referred to as a second state of the
cell
separation system. The centrifugation at block 708 may comprise programs of
various
zo RPM speeds and times for cycles. These cycles may increase in RPM and/or in
duration until cessation at block 712 as discussed below. In an embodiment, a
single
centrifugation cycle may be employed from 500 G to 1000 G for a time period
from
about 5 minutes to about 10 minutes and in still other examples, multiple
centrifugation
17
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
cycles may be employed that first increase in the G-force applied and then
decrease the
force applied, leading into the slowed rotation discussed in detail in block
710 below.
In an embodiment, the digestion at block 706 separates a plurality of cells
from adipose
tissue and fluids, the centrifugation cycle(s) at block 708 acts to force the
separation of
the cells and the movement of those separated cells into the cell
concentration areas
212.
In an embodiment, the heating mechanism used to preheat the mixing and
digestion
area 204 during digestion at block 706 is shut off subsequent to block 706 and
prior to
the initiation of block 708, such that the centrifugation at block 708 may
proceed
between room temperature (from about 20 C to about 25 C) and the temperature
employed at block 706. Further at block 708, during separation of the cells
into the
product reservoir 206 and cell concentration areas 212, after a predetermined
time
period of centrifugation at block 708, clean media may be introduced into the
chamber
of the single walled bowl 104 via single access tube 108 (block 716). This
media further
displaces a plurality of materials including fat and other tissues and liquids
from the
mixing and digestion area 204 such that those materials are removed from the
mixing
and digestion area 204 as the volume increases and captured into the waste
reservoir
202.
In some examples, a speed of from 500 G to 800 G may be used at block 708 in
order
zo to separate cell volume in the concentration areas 212, and in other
examples, a speed
from 700 G to 900 G may be used for centrifugation at block 708. The clean
media of
block 716 may be added at block 708 to increase the total volume inside the
bowl 104
to promote the expulsion of waste product into the waste reservoir 202. The
introduction
18
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
of media at block 716 may occur depending upon the rotation speeds,
centrifugation
programs (cycles), and geometry of the system and collection regions.
In an
embodiment where wash media is introduced at block 716, the amount employed
may
double or triple the total volume of all fluids thus far introduced into the
bowl 102.
Block 710 is a slowed rotation phase and may also be referred to as the third
state of
the cell separation apparatus. During this phase, which may be from 3 minutes
to 45
minutes, a lower centrifugal force is applied at block 710. In one example, at
block 710,
the rotation of the single-walled bowl 104 is slowed to a predetermined speed
or range
of speeds, and a temperature of the bowl may be from room temperature to the
maximum temperature employed at block 706. The parameters such as speed (force
generated) and temperature may be employed at block 710 to enable the
separated cell
volume to remain in the cell concentration areas 212 while the remaining fluid
from the
media and biological volume drains down the interior walls of the bowl to the
waste
reservoir 202.
Waste may be collected in waste reservoir 202 in whole or in part while the
cell volumes
are retained in the cell concentration areas 212 by the centrifugal force
applied at block
710. Some waste may remain trapped in the mixing and digestion area 204, where
it is
isolated from the separated cells. In some examples, the slowed spin of block
710 may
be iterative, for example, a first slowed rotation at block 710 may be at 90%
of an
zo average speed of block 708, a second, subsequent slowed rotation at
block 710 may be
at 80% of an average speed of block 708, and subsequent slowed rotations may
be at
lesser and lesser speeds until a predetermined period has expired or until a
predetermined amount of fluid and/or solids has been removed to the waste
reservoir
19
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
202, as determined by volume and/or optic sensors. During block 710, material
that is
too large to pass through filters 210 is retained and isolated in waste
reservoir 202 such
that the separated cell volume is not contacted by this material.
A vacuum is employed to draw cells in from the cell concentration area 212
into the
center of the product reservoir 206. Thus, the cells collected in 212 move
from those
collection areas to an area of the product reservoir 206 wherein, at block
714, the
concentrated cells (separated cell volume) may be removed using vacuum and via
the
single access tube 108. In some embodiments, this removal is automated and
occurs in
response to completion of blocks 710 and 712. In some embodiments, this
removal is
io automated and occurs before block 712. Blocks 712 and 714 may be
collectively
referred to as a fourth state of the cell separation apparatus when the bowl
is no longer
rotating relative to the containment mechanism 112.
The blocks discussed in the method 700 are associated with an automated,
dynamic
method of cell separation and collection, such that the loading of the bowl
104 at blocks
702 and 704 proceeds through the removal and collection at block 712 without
manual
intervention. In one example, a non-transitory memory stored on a storage
device and
coupled to the cell separation apparatus comprises a plurality of code
executable by a
processor. This plurality of code comprises centrifugation programs for
samples of
varying properties, each program may comprise a flow rate for blocks 702
and/or 704,
zo as well as cell volume and/or concentration targets, flow rates, times,
and ranges for
forces generated (rotation rate/RPM) at blocks 706, 708, 710, 714, and 716 as
appropriate for the actions occurring at each block. Each program may be
associated
CA 03123386 2021-06-14
WO 2020/122916
PCT/US2018/065405
with an overall time to completion from the deposition of the media and
biologic material
volume to the removal of the separated cells.
In another example, the plurality of code contains programs that automatically
detect
when the system has clogged and shuts down the separation process. The program
then initiates either media flush of the system or agitates the assembly 102
such that
the clog is dispersed and the separation procedure is then reinitiated.
While preferred embodiments have been shown and described, modifications
thereof
can be made by one skilled in the art without departing from the scope or
teachings
herein. The embodiments described herein are exemplary only and are not
limiting.
Many variations and modifications of the systems, apparatus, and processes
described
herein are possible and are within the scope of the invention. For example,
the relative
dimensions of various parts, the materials from which the various parts are
made, and
other parameters can be varied. Accordingly, the scope of protection is not
limited to the
embodiments described herein, but is only limited by the claims that follow,
the scope of
which shall include all equivalents of the subject matter of the claims.
Unless expressly
stated otherwise, the steps in a method claim may be performed in any order.
The
recitation of identifiers such as (a), (b), (c) or (1), (2), (3) before steps
in a method claim
are not intended to and do not specify a particular order to the steps, but
rather are
used to simplify subsequent reference to such steps.
21