Note: Descriptions are shown in the official language in which they were submitted.
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
COMPOSITIONS, DEVICES, AND METHODS FOR THE TREATMENT OF
OVERDOSE AND REWARD-BASED DISORDERS
CROSS REFERENCE TO RELATED APPLICATION(S)
[0001] This application claims the benefit of priority under 35 U.S.C.
119(e) of U.S.
Serial No. 62/782,943, filed December 20, 2018 and U.S. Serial No. 16/311,944,
filed
December 20, 2018, the entire disclosure of both are considered part of and
are incorporated
by reference in the disclosure of this application in their entireties. This
application also
incorporates by reference the disclosures of PCT Publication Nos.
W02017/223566 and
W02018/089709 as if written herein in their entireties.
FIELD OF INVENTION
[0002] The invention relates generally to pharmaceutical compositions and
more
particularly to intranasal formulations comprising naltrexone and forms
thereof, and methods
of use thereof in the treatment of and conditions such as opioid overdose and
symptoms thereof,
and disorders such as alcohol use disorder including administering an
intranasal formulation of
the opioid antagonist naltrexone.
BACKGROUND INFORMATION
[0003] Naltrexone was initially developed to treat opioid dependence due to
its effect of
blocking the euphoric effects of opioids. Naltrexone tablet formulations for
oral administration
have been used for treating opioid addiction since 1984. Long-acting depot
forms of naltrexone
to be administered once monthly or longer were developed to improve
compliance. Data from
clinical trials demonstrated that the depot formulations were effective in
reducing relapse to
opioid use. Currently, there is one intramuscular, extended-release
formulation, and one oral
formulation, of naltrexone (Vivitro10) for monthly administration approved by
the FDA.
These formulations aim to maintain a relatively steady state of an amount of
naltrexone
sufficient to prevent naltrexone intoxication at all times.
[0004] Opioid overdose, a related but somewhat different problem, is a
serious public
health issue. In 2017, approximately 72,000 people died from drug overdoses.
Most of these,
around 49,000, involved opioids. Over 19,000 of these deaths involved
prescription opioid
analgesics other than non-methadone synthetics; almost 3,300 involved
methadone; about
16,000 involved heroin; and almost 30,000 of these deaths were attributed to
fentanyl and
related synthetic opioids, a stroking increase over previous years. Taken
together, the number
of opioid-related overdose deaths in 2016 far exceeded both the peak number of
H.I.V. related
1
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
deaths and the peak number of fatalities related to firearms, and have
drastically increased over
the past nine years. A need remains for effective treatments to reverse opioid
overdose.
[0005] Meanwhile, there also remains a need for treatments for "reward-
based" disorders,
often involving opioids or other abusable substances such as alcohol, but also
involving other
activities which stimulate the brain's centers of pleasure, reward, and
reinforcement and lead
to an unhealthy excess of consumption of those substances or engagement in
those behaviors.
[0006] For example, alcohol can stimulate the brain's reward circuitry and
can reinforce
the continued drinking of alcohol. The problematic drinking of alcohol that
becomes
sufficiently severe is given the medical diagnosis of alcohol use disorder
(AUD).
Approximately 6.8 percent (16.3 million adults) in the United States over the
age of 18 had an
AUD in 2014. This includes 10.6 million men and 5.7 million women. In
addition, in 2014, an
estimated 679,000 adolescents between the ages 12-17 (2.7% of this age group)
had an AUD.
In 2012, 3.3 million deaths or 5.9 percent of all global deaths (7.6% for men
and 4.0% for
women) were attributable to alcohol consumption (WHO Global Status Report on
Alcohol and
Health, 2014).
[0007] To be diagnosed with an AUD in the United States, individuals must
meet certain
criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders
(DSM). For
example, under the fifth edition of the DSM, any individual meeting two of the
eleven criteria
during the same 12-month period receives a diagnosis of AUD. The severity of
an AUD¨
mild, moderate, or severe¨is based on the number of criteria met. In Europe,
individuals are
screened using the Alcohol Use Disorders Identification Test (AUDIT). People
with AUD
drink to excess and, consequently, can endanger both themselves and others.
[0008] Alcohol abuse is a drinking pattern that results in significant and
recurrent adverse
consequences. Alcohol abusers may fail to fulfill major school, work, or
family obligations.
People with alcoholism (also known as alcohol dependence) have lost reliable
control of their
alcohol use and are often unable to stop drinking once they start. Alcohol
dependence is
characterized by tolerance (the need to drink more to achieve the same "high")
and withdrawal
symptoms if drinking is suddenly stopped. Withdrawal symptoms may include
nausea,
sweating, restlessness, irritability, tremors, hallucinations and convulsions.
[0009] Problem drinking has multiple causes, with genetic, physiological,
psychological,
and social factors all playing a role. Not every individual is equally
affected by each cause. For
some with AUD, psychological traits such as impulsiveness, low self-esteem and
a need for
approval prompt inappropriate drinking. Genetic factors make some people
especially
vulnerable to alcohol dependence. AUD can cause physiological changes that
make more
2
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
drinking the only way to avoid discomfort and individuals with AUD may drink
partly to
reduce or avoid withdrawal symptoms.
[0010] People with disorders associated with reward-based behavior, such as
AUD, can
seek counseling and psychological therapy from health professionals including
physicians,
nutritionists, psychiatrists, psychologists, clinical social workers or by
attending 12-step
Alcoholic Anonymous meetings. However, for a variety of reasons, access to,
acceptance of,
and success of such resources can be limited.
[0011] Considerable resistance to the use of medications for the treatment
of disorders
associated with reward-based behavior, such as AUD, persists and current
evidence shows that
medications are underused in the treatment of AUD. In Europe, oral nalmefene
has been
approved, and can be taken by a patient while drinking. However, as of January
2015,
disulfiram, acamprosate, and oral or extended release injectable naltrexone
are the only drugs
approved by the Food and Drug Administration in the United States specifically
for the
treatment of AUD. However, all of these medications must be taken in patients
who can abstain
from alcohol before the initiation of treatment or have completed alcohol
withdrawal.
Accordingly, there is still a need for medications which treat subjects with
AUD who are still
drinking alcohol.
[0012] Non-drug reward-based disorders manifest in similar psychological
and behavioral
patterns as substance use disorders. Specifically, craving, impaired control
over the behavior,
tolerance, withdrawal, and high rates of relapse can be seen in subjects who
suffer from
addictive behavior that has negative consequences to the person's physical,
mental, social or
financial well-being. (See, e.g., Marks, 1990; Lejoyeux et al, 2000; National
Institute on Drug
Abuse (NIDA) et al, 2002; Potenza, 2006; and Olsen, 2011). Drugs and non-drug
rewards also
demonstrate similar physiological manifestations. For example, functional
neuroimaging
studies in humans have shown that gambling (Breiter et al, 2001), shopping
(Knutson et al,
2007), orgasm (Komisaruk et al, 2004), playing video games (Koepp et al, 1998;
Hoeft et al,
2008) and the sight of appetizing food (Wang et al, 2004) activate many of the
same brain
regions (i.e., the mesocorticolimbic system and extended amygdala) as drugs of
abuse (Volkow
et al, 2004).
[0013] An intranasal (IN) formulation of naltrexone has the potential to be
used for
reversing opioid overdose, and for treating reward-based disorders without the
use of needles
or an extended-release formulation. While studies have shown that opioid
antagonists, such as
naltrexone, administered in oral or injectable forms, can reverse opioid
overdose, and can
3
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
decrease alcohol drinking and operant responding for it, there remains a
substantial need for a
simple, fast and compliant means of treating such conditions.
SUMMARY
[0014] Disclosed herein are intranasal formulations comprising an aqueous
solution
comprising naltrexone or a pharmaceutically acceptable salt thereof
[0015] Also disclosed herein are methods of treatment of opioid overdose or
a reward-
based disorder such as alcohol use disorder (AUD) in a subject comprising
administering to
the subject an IN formulation comprising a therapeutically effective amount of
naltrexone or a
pharmaceutically acceptable salt thereof
[0016] Also disclosed herein is a device adapted for nasal delivery of a
pharmaceutical
composition to a subject experiencing opioid overdose or having a reward-based
disorder such
as alcohol use disorder (AUD), comprising a therapeutically effective amount
of a
pharmaceutical formulation as disclosed herein. In certain embodiments, the
device is pre-
primed. In certain embodiments, the device can be primed before use. In
certain embodiments,
the device is a single-dose device. In certain embodiments, the device is a
multi-dose device.
BRIEF DESCRIPTION OF THE DRAWINGS
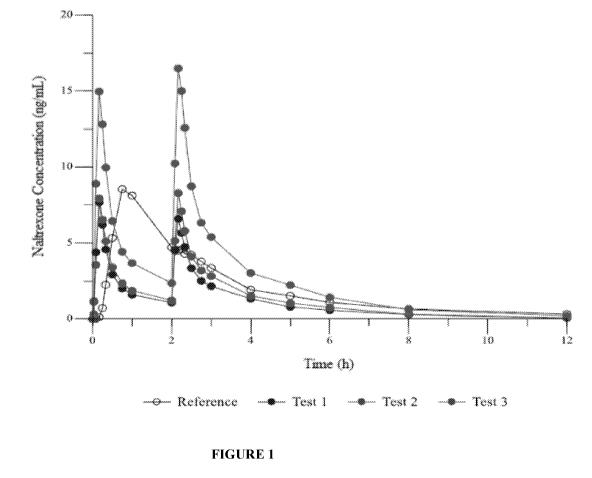
[0017] Figure 1 shows the mean naltrexone plasma concentration versus time
profiles
following the administration of Test Product 1, Test Product 2, Test Product 3
and Reference
Product, in linear scale out to 12 hours.
[0018] Figure 2 shows the mean natlrexone plasma concentration versus time
profiles
following the administration of Test Product 1, Test Product 2, Test Product 3
and Reference
Product, in semi-logarithmic scale out to 12 hours.
[0019] Figure 3 shows the mean 6P-naltrexol (the main naltrexone
metabolite) plasma
concentration versus time (truncated at 12h) profiles following the
administration of Test
Product 1, Test Product 2, Test Product 3 and Reference Product, in linear
scale out to 12 hours.
[0020] Figure 4 shows the mean 6P-naltrexol (the main naltrexone
metabolite) plasma
concentration versus time (truncated at 12h) profiles following the
administration of Test
Product 1, Test Product 2, Test Product 3 and Reference Product, in semi-
logarithmic scale out
to 12 hours.
DETAILED DESCRIPTION
[0021] The following embodiments further illustrate the invention disclosed
herein.
[0022] Provided herein is Embodiment 1: an intranasal formulation
comprising an
aqueous solution comprising between about 1 mg and about 4 mg naltrexone, or a
pharmaceutically acceptable salt thereof
4
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[0023] Also provided herein is Embodiment 2: an intranasal formulation
comprising, in a
volume of about 50 to about 250 pL (preferably about 50 to about 150 p.L), an
aqueous solution
comprising between about 10 mg/mL and about 40 mg/mL (preferably about 10
mg/mL and
about 30 mg/mL) naltrexone, or a pharmaceutically acceptable salt thereof
[0024] The disclosure further provides the following embodiments.
[0025] Embodiment 3: The formulation as recited in either Embodiment 1 or
Embodiment
2, additionally comprising:
an isotonicity agent;
a preservative;
a stabilizing agent;
an absorption enhancer; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
pL (preferably about 50 to about 150 p.L).
[0026] Embodiment 4: The formulation as recited in Embodiment 3,
comprising:
between about 1 mg and about 3 mg naltrexone or a pharmaceutically acceptable
salt thereof;
between about 0.1 mg and about 1.2 mg of the isotonicity agent;
between about 0.001 mg and about 0.1 mg of the preservative;
between about 0.1 mg and about 0.5 mg of the stabilizing agent;
between about 0.05 mg and about 2.5 mg of the absorption enhancer; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
pL (preferably about 50 to about 150 p.L).
[0027] Embodiment 5: The formulation as recited in Embodiment 3,
comprising:
between about 1% and about 3% naltrexone or a pharmaceutically acceptable salt
thereof;
between about 0.1% and about 1.2% of the isotonicity agent;
between about 0.001% and about 0.1% of the preservative;
between about 0.1% and about 0.5% of the stabilizing agent;
between about 0.05% mg and about 2.5% of the absorption enhancer.
[0028] Embodiment 6: The formulation as recited in either Embodiment 4 or
Embodiment
5, wherein:
the isotonicity agent is NaCl;
the preservative is benzalkonium chloride;
the stabilizing agent is disodium edetate; and
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
the absorption enhancer is an alkylsaccharide.
[0029] Embodiment 7: The formulation as recited in Embodiment 6, wherein
the
alkylsaccharide is dodecyl maltoside.
[0030] Embodiment 8: The formulation as recited in Embodiment 7,
comprising:
between about 1 mg and about 3 mg naltrexone or a pharmaceutically acceptable
salt thereof;
between about 0.1 mg and about 1.2 mg of NaCl;
between about 0.001 mg and about 0.1 mg of benzalkonium chloride;
between about 0.15 mg and about 0.5 mg of disodium edetate;
between about 0.05 mg and about 2.5 mg of dodecyl maltoside; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
pL (preferably about 50 to about 150 p.L).
[0031] Embodiment 9: The formulation as recited in Embodiment 8, comprising
between
about 0.1 mg to about 0.5 mg of dodecyl maltoside.
[0032] Embodiment 10: The formulation as recited in Embodiment 9,
comprising about
0.25 mg of dodecyl maltoside.
[0033] Embodiment 11: The formulation as recited in Embodiment 8,
comprising about
0.2 mg and about 0.3 mg of disodium edetate.
[0034] Embodiment 12: The formulation as recited in Embodiment 9,
comprising:
between about 1 mg and about 3 mg naltrexone or a pharmaceutically acceptable
salt thereof;
between about 0.3 mg and about 0.7 mg of NaCl;
about 0.02 mg of benzalkonium chloride;
about 0.3 mg of disodium edetate;
about 0.25 mg of dodecyl maltoside; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
pL (preferably about 50 to about 150 p.L).
[0035] Embodiment 13: The formulation as recited in Embodiment 10, wherein
the
amount of water is sufficient to achieve a final volume of about 80 to about
120 L.
[0036] Embodiment 14: The formulation as recited in Embodiment 11, wherein
the
amount of water is sufficient to achieve a final volume of about 100 L.
[0037] Embodiment 15: The formulation as recited in Embodiment 7,
comprising:
between about 1% and about 3% naltrexone or a pharmaceutically acceptable salt
thereof;
6
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
between about 0.1% and about 1.2% of NaCl;
between about 0.001% and about 0.1% of benzalkonium chloride;
between about 0.15% and about 0.5% of disodium edetate;
between about 0.05% and about 2.5% of dodecyl maltoside; and
water.
[0038] Embodiment 16: The formulation as recited in Embodiment 15,
comprising
between about 0.1% to about 0.5% of dodecyl maltoside.
[0039] Embodiment 17: The formulation as recited in Embodiment 16,
comprising about
0.25% of dodecyl maltoside.
[0040] Embodiment 18: The formulation as recited in Embodiment 15,
comprising about
0.2% and about 0.3% of disodium edetate.
[0041] Embodiment 19: The formulation as recited in Embodiment 17,
comprising:
between about 1% and about 3% naltrexone or a pharmaceutically acceptable salt
thereof;
between about 0.3% and about 0.7% of NaCl;
about 0.02% of benzalkonium chloride;
about 0.3% of disodium edetate;
about 0.25% of dodecyl maltoside; and
water.
[0042] Embodiment 20: The formulation as recited in Embodiment 19, wherein
the
amount of water is sufficient to achieve a final volume of about 50 to about
150 L.
[0043] Embodiment 21: The formulation as recited in Embodiment 20, wherein
the
amount of water is sufficient to achieve a final volume of about 100 L.
[0044] Embodiment 22: The formulation as recited in any of Embodiments 1-
21, wherein
the naltrexone is naltrexone hydrochloride.
[0045] Embodiment 23: The formulation as recited in Embodiment 22,
comprising about
1.2 mg, about 1.6 mg, about 2.0 mg, or about 3.0 mg naltrexone or an
equivalent amount of
naltrexone hydrochloride.
[0046] Also provided herein is Embodiment 24: a method of treatment of
opioid overdose
or a reward-based disorder in a subject, comprising administering to the
subject an intranasal
formulation comprising an aqueous solution comprising between about 1 mg and
about 4 mg
naltrexone or a pharmaceutically acceptable salt thereof.
[0047] Also provided herein is Embodiment 25: a method of treatment of
opioid overdose
or a reward-based disorder in a subject, comprising administering to the
subject a first
7
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
intranasal formulation comprising an aqueous solution comprising between about
1 mg and
about 4 mg naltrexone or a pharmaceutically acceptable salt thereof and
administrating a
second intranasal formulation comprising an aqueous solution comprising
between about 1 mg
and about 4 mg naltrexone or a pharmaceutically acceptable salt thereof.
[0048] Also provided herein is Embodiment 26: a method of treatment of
opioid overdose
or a reward-based disorder in a subject, comprising administering to the
subject an intranasal
formulation comprising, in a volume of about 50 to about 250 pL (preferably
about 50 to about
150 p.L), an aqueous solution comprising between about 10 mg/mL and about 40
mg/mL
(preferably about 10 mg/mL and about 30 mg/mL) naltrexone or a
pharmaceutically acceptable
salt thereof
[0049] Also provided herein is Embodiment 27: a method treatment of opioid
overdose
or a reward-based disorder in a subject, comprising administering to the
subject a first
intranasal formulation comprising, in a volume of about 50 to about 250 ul, an
aqueous solution
comprising between about 10 mg/mL and about 40 mg/mL naltrexone or a
pharmaceutically
acceptable salt thereof and administrating a second intranasal formulation
comprising, in a
volume or about 50 to about 250 p.L, an aqueous solution comprising between
about 10 mg/mL
and about 40 mg/mL naltrexone or a pharmaceutically acceptable salt thereof.
[0050] Embodiment 28: The method as recited in either Embodiment 25 or
claim
Embodiment 27 wherein the second intranasal formulation is administered
between about 1
hour and about 3 hours after the administration of the first intranasal
formulation.
[0051] Embodiment 29: The method as recited in any of Embodiments 24 -28,
wherein
the intranasal formulation additionally comprises:
an isotonicity agent;
a preservative;
a stabilizing agent;
an absorption enhancer; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
pL (preferably about 50 to about 150 p.L).
[0052] Embodiment 30: The method as recited in Embodiment 29, wherein the
intranasal
formulation comprises:
between about 1 mg and about 3 mg naltrexone or a pharmaceutically acceptable
salt thereof;
between about 0.1 mg and about 1.2 mg of the isotonicity agent;
between about 0.001 mg and about 0.1 mg of the preservative;
8
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
between about 0.1 mg and about 0.5 mg of the stabilizing agent;
between about 0.05 mg and about 2.5 mg of the absorption enhancer; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
'IL (preferably about 50 to about 150 p.L).
[0053] Embodiment 31: The method as recited in Embodiment 29, wherein the
intranasal
formulation comprises:
between about 1% and about 3% naltrexone or a pharmaceutically acceptable salt
thereof;
between about 0.1% and about 1.2% of the isotonicity agent;
between about 0.001% and about 0.1% of the preservative;
between about 0.1% and about 0.5% of the stabilizing agent;
between about 0.05% mg and about 2.5% of the absorption enhancer.
[0054] Embodiment 32: The method as recited in either Embodiment 30 or
Embodiment
28, wherein:
the isotonicity agent is NaCl;
the preservative is benzalkonium chloride;
the stabilizing agent is disodium edetate; and
the absorption enhancer is an alkylsaccharide.
[0055] Embodiment 33: The method as recited in Embodiment 32, wherein the
alkylsaccharide is dodecyl maltoside.
[0056] Embodiment 34: The method as recited in Embodiment 33, wherein the
intranasal
formulation comprises:
between about 1 mg and about 3 mg naltrexone or a pharmaceutically acceptable
salt thereof;
between about 0.1 mg and about 1.2 mg of NaCl;
between about 0.001 mg and about 0.1 mg of benzalkonium chloride;
between about 0.15 mg and about 0.5 mg of disodium edetate;
between about 0.05 mg and about 2.5 mg of dodecyl maltoside; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
'IL (preferably about 50 to about 150 p.L).
[0057] Embodiment 35: The method as recited in Embodiment 34, wherein the
intranasal
formulation comprises between about 0.1 mg to about 0.5 mg of dodecyl
maltoside.
[0058] Embodiment 36: The method as recited in Embodiment 35, wherein the
intranasal
formulation comprises about 0.25 mg of dodecyl maltoside.
9
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[0059] Embodiment 37: The method as recited in Embodiment 34, wherein the
intranasal
formulation comprises between about 0.2 mg and about 0.3 mg of disodium
edetate.
[0060] Embodiment 38: The method as recited in Embodiment 36, wherein the
intranasal
formulation comprises:
between about 1 mg and about 3 mg naltrexone or a pharmaceutically acceptable
salt thereof;
between about 0.3 mg and about 0.7 mg of NaCl;
about 0.02 mg of benzalkonium chloride;
about 0.3 mg of disodium edetate;
about 0.25 mg of dodecyl maltoside; and
an amount of water sufficient to achieve a final volume of about 50 to about
250
pL (preferably about 50 to about 150 p.L).
[0061] Embodiment 39: The method as recited in Embodiment 38, wherein the
amount of
water is sufficient to achieve a final volume of about 50 to about 150 L.
[0062] Embodiment 40: The method as recited in Embodiment 39, wherein the
amount of
water is sufficient to achieve a final volume of about 100 L.
[0063] Embodiment 41: The method as recited in Embodiment 33, comprising:
between about 1% and about 3% naltrexone or a pharmaceutically acceptable salt
thereof;
between about 0.1% and about 1.2% of NaCl;
between about 0.001% and about 0.1 % of benzalkonium chloride;
between about 0.1% and about 0.5% of disodium edetate;
between about 0.05% and about 2.5% of dodecyl maltoside; and
water.
[0064] Embodiment 42: The method as recited in Embodiment 41, wherein the
intranasal
formulation comprises between about 0.1% to about 0.5% of dodecyl maltoside.
[0065] Embodiment 43: The method as recited in Embodiment 42, wherein the
intranasal
formulation comprises about 0.25% of dodecyl maltoside.
[0066] Embodiment 44: The method as recited in Embodiment 41, wherein the
intranasal
formulation comprises between about 0.2% and about 0.3% of disodium edetate.
[0067] Embodiment 45: The method as recited in Embodiment 43, wherein the
intranasal
formulation comprises:
between about 1% and about 3% naltrexone or a pharmaceutically acceptable salt
thereof;
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
between about 0.3% and about 0.7% of NaCl;
about 0.02% of benzalkonium chloride;
about 0.3% of disodium edetate;
about 0.25% of dodecyl maltoside; and
water.
[0068] Embodiment 46: The method as recited in Embodiment 45, wherein the
amount of
water is sufficient to achieve a final volume of about 50 to about 150 L.
[0069] Embodiment 47: The method as recited in Embodiment 46, wherein the
amount of
water is sufficient to achieve a final volume of about 100 L.
[0070] Embodiment 48: The method as recited in any of Embodiments 24-47,
wherein the
naltrexone is naltrexone hydrochloride.
[0071] Embodiment 49: The method as recited in Embodiment 48, wherein the
intranasal
formulation comprises about 1.2 mg, about 1.6 mg, about 2.0 mg, or about 3.0
mg naltrexone
or an equivalent amount of naltrexone hydrochloride.
[0072] Embodiment 50: The method as recited in any of Embodiments 24-49,
wherein the
method treats opioid overdose.
[0073] Embodiment 51: The method as recited in any of Embodiments 24-49,
wherein the
method treats a reward-based disorder.
[0074] Embodiment 52: The method as recited in Embodiment 48, wherein the
reward-
based disorder is a substance use disorder.
[0075] Embodiment 53: The method as recited in Embodiment 48, wherein the
substance
use disorder is alcohol use disorder.
[0076] Embodiment 54: The method as recited in Embodiment 53, wherein the
intranasal
formulation comprising naltrexone is administered prior to ingestion of
alcohol.
[0077] Embodiment 55: The method as recited in Embodiment 54, wherein the
intranasal
formulation comprising naltrexone is administered about 1-2 hours prior to
ingestion of
alcohol.
[0078] Embodiment 56: The method as recited in Embodiment 54, wherein the
intranasal
formulation comprising naltrexone is administered about 0.5 to about 1 hours
prior to ingestion
of alcohol.
[0079] Embodiment 57: The method as recited in Embodiment 54, wherein the
intranasal
formulation comprising naltrexone is administered about 10 to about 30 minutes
prior to
ingestion of alcohol.
11
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[0080] Embodiment 58: The method as recited in Embodiment 54, wherein the
intranasal
formulation comprising naltrexone is administered about 5 to about 10 minutes
prior to
ingestion of alcohol.
[0081] Embodiment 59: The method as recited in Embodiment 53, wherein the
intranasal
formulation comprising naltrexone is administered contemporaneously with the
ingestion of
alcohol.
[0082] Embodiment 57: The method as recited in Embodiment 53, wherein the
intranasal
formulation comprising naltrexone is administered within 0.5 hours after
commencement of
ingestion of alcohol.
[0083] Embodiment 58: The method as recited in any of Embodiments 24-57,
wherein the
intranasal formulation comprising naltrexone is administered to a subject from
once to four
times per day.
[0084] Embodiment 59: The method as recited in Embodiment 58, wherein the
intranasal
formulation comprising naltrexone is administered in doses of about 1.2 mg,
about 1.6 mg,
about 2 mg, or about 3 mg throughout the day as needed by the subject.
[0085] Embodiment 60: The method as recited in Embodiment 58, wherein the
intranasal
formulation comprising naltrexone is administered as a first dose of about 1.2
mg, about 1.6
mg, about 2 mg, or about 3 mg in the morning, and subsequent doses of about
1.2 mg, about
1.6 mg, about 2 mg, or about 3 mg as needed prior to consumption of alcohol.
[0086] Also provided herein is Embodiment 61: A multi-dose device adapted
for nasal
delivery of a pharmaceutical formulation to a subject experiencing an opioid
overdose or
having a reward-based disorder, comprising a plurality of doses each
comprising an intranasal
formulation as recited in any of Embodiments 1-23.
[0087] Embodiment 62: The device as recited in Embodiment 61, wherein about
0.05 to
about 0.2 mL (preferably about 0.05 to about 0.15 mL) of said formulation is
delivered to the
subject in each dose.
[0088] Embodiment 63: The device as recited in Embodiment 62, wherein about
0.1 mL
of said formulation is delivered to the subject in each dose.
[0089] Also provided herein is Embodiment 64: The formulation, method, or
device as
recited in any one of the preceding Embodiments, wherein:
where "comprising between about 1 mg and about 4 mg naltrexone, or a
pharmaceutically acceptable salt thereof', the amount comprised is about 3 mg
naltrexone, or
a pharmaceutically acceptable salt thereof; or
12
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
where the method recites "comprising between about 10 mg/mL and about 40
mg/mL (preferably about 10 mg/mL and about 30 mg/mL) naltrexone, or a
pharmaceutically
acceptable salt thereof', the concentration comprised is about 30 mg/mL mg
naltrexone, or a
pharmaceutically acceptable salt thereof
[0090] Embodiment 64:Also provided is a formulation chosen from those
described in the
Examples disclosed herein.
[0091] Also provided are embodiments wherein any embodiment above may be
combined
with any one or more of these embodiments, provided the combination is not
mutually
exclusive.
Definitions
[0092] As use herein, the following terms have the meanings indicated.
[0093] When ranges of values are disclosed, unless otherwise specified,
this notation is
intended to include the numbers themselves and the range between them. This
range may be
integral or continuous between and including the end values. By way of
example, the range
"from 2 to 6 doses" is intended to include two, three, four, five, and six
doses, since doses come
in integer units. Compare, by way of example, the range "from 1 to 3 i.IM
(micromolar),"
which is intended to include 1 M, 3 M, and everything in between to any
number of
significant figures (e.g., 1.255 M, 2.1 M, 2.9999 M, etc.).
[0094] The term "about," as used herein, is intended to qualify the
numerical values which
it modifies, denoting such a value as variable within a range. When no
particular range, such
as a margin of error or a standard deviation to a mean value given in a chart
or table of data, is
recited, the term "about" should be understood to mean the greater of the
range which would
encompass the recited value and the range which would be included by rounding
up or down
to that figure as well, taking into account significant figures, and the range
which would
encompass the recited value plus or minus 20%.
[0095] The term "absorption enhancer," as used herein, refers to a
functional excipient
included in formulations to improve the absorption of a pharmacologically
active drug. This
term usually refers to an agent whose function is to increase absorption by
enhancing
membrane permeation, rather than increasing solubility. As such, such agents
are sometimes
called permeation enhancers. Examples of absorption enhancers include
aprotinin,
benzalkonium chloride, benzyl alcohol, capric acid, ceramides, cetylpyridinium
chloride,
chitosan, cyclodextrins, deoxycholic acid, decanoyl carnitine, dodecyl
maltoside, EDTA,
glycocholic acid, glycodeoxycholic acid, glycofurol, glycosylated
sphingosines, glycyrrhetinic
acids, 2-hydroxypropyl- P-cyclodextrin, laureth-9, lauric acid, lauroyl
carnitine, sodium lauryl
13
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
sulfate, lysophosphatidylcholine, menthol, poloxamer 407 or F68, poly-L-
arginine,
polyoxyethylene-9-lauryl ether, polysorbate 80, propylene glycol, quillaja
saponin, salicylic
acid, sodium salt, P-sitosterol- P-D-glucoside, sucrose cocoate, taurocholic
acid,
taurodeoxycholic acid, taurodihydrofusidic acid, and tetradecyl maltoside.
Alkylsaccharides
(e.g., nonionic alkylsaccharide surfactants such as alkylglycosides and
sucrose esters of fatty
acids that consist of an aliphatic hydrocarbon chain coupled to a sugar moiety
by a glycosidic
or ester bond, respectively), cyclodextrins (cyclic oligosaccharides composed
of six or more
monosaccharide units with a central cavity, which form inclusion complexes
with hydrophobic
molecules and they have primarily been used to increase drug solubility and
dissolution and to
enhance low molecular weight drug absorption), chitosans (linear cationic
polysaccharides
produced from the deacetylation of chitin), and bile salts and their
derivatives (such as sodium
glycocholate, sodium taurocholate, and sodium taurodihydrofusidate) tend to be
amongst the
best-tolerated absorption enhancers. See, e.g., Aungst, AAPS Journal 14(1):10-
8, 2011;
Maggio, J. Excipients and Food Chem. 5(2):100-12, 2014.
[0096] The term "addiction," as used herein, refers to a medical condition
characterized by
compulsive engagement in rewarding stimuli despite adverse consequences. The
term,
"addictive behavior," as used herein, refers to a behavior that is both
rewarding and reinforcing.
[0097] The term "agonist," as used herein, refers to a moiety that
interacts with and
activates a receptor, and thereby initiates a physiological or pharmacological
response
characteristic of that receptor. The term "antagonist," as used herein, refers
to a moiety that
competitively binds to a receptor at the same site as an agonist (for example,
the endogenous
ligand), but which does not activate the intracellular response initiated by
the active form of
the receptor and can thereby inhibit the intracellular responses by an agonist
or partial agonist.
An antagonist does not diminish the baseline intracellular response in the
absence of an agonist
or partial agonist. The term "inverse agonist" refers to a moiety that binds
to the endogenous
form of the receptor or to the constitutively activated form of the receptor
and which inhibits
the baseline intracellular response initiated by the active form of the
receptor below the normal
base level of activity which is observed in the absence of an agonist or
partial agonist.
[0098] The term "alcohol use disorder" is defined by criteria set forth the
Diagnostic and
Statistical Manual of Mental Disorders (DSM, most recent revision, presently
DSM-V) in the
US, or by similar criteria set forth in corresponding well-accepted standards
such as the World
Health Organization's ICD (International Statistical Classification of
Diseases and Related
Health Problems, most recent revision, presently the ICD-10). Related terms
and disorders
14
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
include "alcohol abuse" and "alcohol dependence" (used in DSM-IV), "alcohol
harmful use"
and "alcohol dependence syndrome" (used in the ICD-10), and alcoholism.
[0099] The term "antimicrobial preservative," as used herein, refers to a
pharmaceutically
acceptable excipient with antimicrobial properties which is added to a
pharmaceutical
composition to maintain microbiological stability. Compounds act both as
preservatives and
stabilizers.
[00100] The term "disease" as used herein is intended to be generally
synonymous, and is
used interchangeably with, the terms "disorder," "syndrome," and "condition"
(as in medical
condition), in that all reflect an abnormal condition of the human or animal
body or of one of
its parts that impairs normal functioning, is typically manifested by
distinguishing signs and
symptoms, and causes the human or animal to have a reduced duration or quality
of life.
[00101] The term "pharmaceutical composition" is used herein interchangeably
with the
term "Pharmaceutical formulation," or just "formulation," and denotes an
active
pharmaceutical ingredient (i.e., a drug substance) in combination with at
least one
pharmaceutically acceptable excipient or carrier.
[00102] The term "equivalent," as used herein refers to a weight of the opioid
antagonist
naltrexone and pharmaceutically acceptable salts thereof that is equimolar to
a specified weight
of naltrexone hydrochloride.
[00103] The term "excipient," as used herein refers to a natural or synthetic
substance
formulated alongside the active ingredient of a medication, included for the
purpose of long-
term stabilization, bulking up solid formulations, or to confer a therapeutic
enhancement on the
active ingredient in the final dosage form, such as facilitating drug
absorption, reducing
viscosity, or enhancing solubility.
[00104] The term "therapeutically effective dose," as used herein refers to
a dose that is
effective to treat a disease, to decrease one or more observable symptoms of a
disease, or to
delay onset or progression of or mitigate the symptoms of a more serious
condition that often
follows after the condition that a patient is currently experiencing. A
therapeutically effective
dose may, but need not necessarily, completely eliminate all symptoms of the
disease.
[00105] The term "in need of treatment" and the term "in need thereof' when
referring to
treatment are used interchangeably and refer to a judgment made by a caregiver
(e.g. physician,
nurse, nurse practitioner, that a subject will benefit from treatment.
[00106] As used herein, two embodiments are "mutually exclusive" when one is
defined to
be something which is different than the other. For example, an embodiment
wherein the
amount of naltrexone hydrochloride is specified to be 3 mg is mutually
exclusive with an
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
embodiment wherein the amount of naltrexone hydrochloride is specified to be 2
mg.
However, an embodiment wherein the amount of naltrexone hydrochloride is
specified to be 4
mg is not mutually exclusive with an embodiment in which less than about 10%
of said
pharmaceutical composition leaves the nasal cavity via drainage into the
nasopharynx or
externally.
[00107] The term "naloxone," as used herein, refers to a compound of the
following
structure:
CH2
ri
N
.HO
.=%.
HO CP 0
or a pharmaceutically acceptable salt, hydrate, or solvate thereof. The CAS
registry number for
naloxone is 465-65-6. Other names for naloxone include: 17-ally1-4,5a-epoxy-
3,14-
dihydroxymorphinan-6-one; (¨)-17-ally1-4,5a-epoxy-3,14-dihydroxymorphinan-6-
one; 4,5a-
epoxy-3, 14-dihydroxy-17-(2-prop enyl)morphinan-6-one; and (¨)-12-ally1-
7,7a,8,9-
tetrahydro-3,7a-dihydroxy-4aH-8,9c-iminoethanophenanthro [4,5 -bccl] furan-
5(61/)-one.
Naloxone hydrochloride may be anhydrous (CAS Reg. No. 357-08-4) and also forms
a
dihydrate (CAS No. 51481-60-8). It has been sold under various brand names
including
Narcan , Nalone , Nalossone , Naloxona , Naloxonum , Narcanti , and Narcon .
[00108] The term "naltrexone," as used herein, refers to a compound of the
following
structure:
r-4
N
400H0
.,s.
HO 0% 0
or a pharmaceutically acceptable salt, hydrate, or solvate thereof. The CAS
registry number for
naltrexone is 16590-41-3. Other names for naltrexone include: 17-
(cyclopropylmethyl)-4,5a-
epoxy- 3,14-dihydroxymorphinan-6-one; (5 a)-17-(cyclopropylmethyl)-3,14-
dihydroxy-4,5 -
16
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
epoxymorphinan-6-one; and (1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-
dihydroxy-12-
oxa-4-azapentacyclo [9.6.1.01,13 .05,17.07,18]octadeca-7(18),8,10-trien-14-one
. The term
"naltrexone" includes "naltrexone hydrochloride." Naltrexone hydrochloride
(CAS Reg. No.
16676-29-2) has been marketed under the trade names Antaxone , Depade ,
Nalorex ,
Revia , Trexan , Vivitrex , and Vivitrol .
[00109] The term "methylnaltrexone," as used herein, refers to a
pharmaceutically
acceptable salt comprising the cation (5 a)-17-(cyclopropylmethyl)-3,14-
dihydroxy-17-methyl-
4,5-epoxymorphinanium-17-ium-6-one a compound of the following structure:
N X
.HO
HO 0
wherein X- is a pharmaceutically acceptable anion. Methylnaltrexone bromide
(CAS Reg. No.
75232-52-7) has been marketed under the trade name Relistor .
[00110] The term "nalmefene," as used herein, refers to 17-cyclopropylmethy1-
4,5a-epoxy-
6-methylenemorphinan-3,14-diol, a compound of the following structure:
.HO
HO 0 CH2
Nalmefene hydrochloride (CAS Reg. No. 58895-64-0) has been marketed under the
trade
names Nalmetrene , Cervene , Revex , Arthrene , and Incystene .
[00111] The term "nostril," as used herein, is synonymous with "naris."
[00112] The term "opioid antagonist" includes, in addition to naltrexone and
pharmaceutically acceptable salts thereof: naloxone, methylnaltrexone, and
nalmefene, and
pharmaceutically acceptable salts thereof. In certain embodiments, the opioid
antagonist is
naltrexone hydrochloride. In certain embodiments, the opioid antagonist is
naloxone. In certain
17
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
embodiments, the opioid antagonist is methylnaltrexone bromide. In certain
embodiments, the
nasally administering is accomplished using a device described herein.
[00113] The term "pharmaceutical composition," as used herein, refers to a
composition
comprising at least one active ingredient; including but not limited to,
salts, solvates and
hydrates of the opioid antagonist naltrexone, whereby the composition is
amenable to use for
a specified, efficacious outcome in a mammal (for example, without limitation,
a human).
[00114] The term "reinforcing stimuli," as used herein refers to stimuli
that increase the
probability of repeating behaviors paired with them.
[00115] The term, "rewarding stimuli," as used herein, refers to stimuli
that the brain of a
subject interprets as intrinsically positive or as something to be approached.
A rewarding
stimulus typically results in the release of dopamine in the brain of the
subject.
[00116] As used herein, a "reward-based disorder" is a disorder associated
with reward-
based behavior, wherein the user pursues engagement in and/or engages in
rewarding stimuli
despite adverse consequences. Reward-based disorders include substance use
disorders such
as alcohol use disorder, as well as addictions, as well as disorders of
activity engagement such
as gambling disorder (compulsive gambling), eating disorders (binge eating,
bulimia nervosa),
kleptomania, pyromania, and the like.
[00117] The term "subject," as used herein, is intended to be synonymous with
"patient,"
and refers to any mammal (preferably human) afflicted with a condition likely
to benefit from
a treatment with a therapeutically effective amount of the opioid antagonist
naltrexone or a salt
thereof
[00118] The term "substance use disorder" is defined by criteria set forth the
Diagnostic and
Statistical Manual of Mental Disorders (DSM, most recent revision, presently
DSM-V) in the
US, or by similar criteria set forth in corresponding well-accepted standards
such as the World
Health Organization's ICD (International Statistical Classification of
Diseases and Related
Health Problems, most recent revision, presently the ICD-10). Related terms
and disorders
include "substance abuse" and "substance dependence" (used in DSM-IV).
Substance use
disorders occur when the recurrent use of alcohol and/or drugs causes
clinically and
functionally significant impairment, such as health problems, disability, and
failure to meet
major responsibilities at work, school, or home. According to the DSM-5, a
diagnosis of
substance use disorder is based on evidence of impaired control, social
impairment, risky use,
and pharmacological criteria. Substances which may be the focus of a substance
use disorder
include abusable substances such as opioids, alcohol, tobacco, cannabinoids,
stimulants such
as cocaine and amphetamines, depressants such as benzodiazepines,
hallucinogens, inhalants,
18
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
and the like. Alcohol use disorder is a substance use disorder. Substance use
disorders may be
considered "reward-based disorders."
[00119] The term "tonicity agent," as used herein, refers to a compound which
modifies the
osmolality of a formulation, for example, to render it isotonic. Tonicity
agents include,
dextrose, lactose, sodium chloride, calcium chloride, magnesium chloride,
sorbitol, sucrose,
mannitol, trehalose, raffinose, polyethylene glycol, hydroxyethyl starch,
glycine and the like.
[00120] As used herein, "treating," "treatment," and the like means
ameliorating a disorder,
so as to reduce or eliminate its cause, its progression, its severity, or one
or more of its
symptoms, or otherwise beneficially alter the disease in a subject.
[00121] As used herein, the term "AUC" refers to the area under the drug
plasma
concentration-time curve. As used herein, the term "AUCo-t" refers to the area
under the drug
plasma concentration-time curve from t = 0 to the last measurable
concentration. As used
herein, the term "AUCo_."or "AUCo_inf" refers to the area under the drug
plasma concentration-
time curve extrapolated to Go (infinity).
[00122] As used herein, the term "bioavailability (F)" refers to the fraction
of a dose of drug
that is absorbed from its site of administration and reaches, in an unchanged
form, the systemic
circulation. As used herein, the term "absolute bioavailability" is used when
the fraction of
absorbed drug is related to its IV bioavailability. It may be calculated using
the following
formula:
F = AUC extravas cular X Dose
AUC intravenous Do S eextravascular
[00123] The term relative bioavailability (Frei) is used to compare two
different
extravascular routes of drug administration and it may be calculated using the
following
formula:
AUCextravascul X arl Dose extravascular2
Frel AUCextravascular2 D S e extravas cular 1
[00124] As used herein, the term "clearance (CL)" refers to the rate at which
a drug is
eliminated divided by its plasma concentration, giving a volume of plasma from
which drug is
completely removed per unit of time. CL is equal to the elimination rate
constant (X) multiplied
by the volume of distribution (Vd), wherein "Vd" is the fluid volume that
would be required to
contain the amount of drug present in the body at the same concentration as in
the plasma. As
used herein, the term "apparent clearance (CL/F)" refers to clearance that
does not take into
account the bioavailability of the drug. It is the ratio of the dose over the
AUC.
19
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00125] As used herein, the term "Cmax" refers to the maximum observed plasma
concentration.
[00126] As used herein, the term "ti/2" or "half-life" refers to the amount of
time required
for half of a drug to be eliminated from the body or the time required for a
drug concentration
to decline by half.
[00127] As used herein, the term "coefficient of variation (CV)" refers to the
ratio of the
sample standard deviation to the sample mean. It is often expressed as a
percentage.
[00128] As used herein, the term "confidence interval" refers to a range of
values which will
include the true average value of a parameter a specified percentage of the
time.
[00129] As used herein, the term "elimination rate constant (X)" refers to the
fractional rate
of drug removal from the body. This rate is constant in first-order kinetics
and is independent
of drug concentration in the body. X is the slope of the plasma concentration-
time line (on a
logarithmic y scale). The term "Xz," as used herein, refers to the terminal
phase elimination rate
constant, wherein the "terminal phase" of the drug plasma concentration-time
curve is a straight
line when plotted on a semilogarithmic graph. The terminal phase is often
called the
"elimination phase" because the primary mechanism for decreasing drug
concentration during
the terminal phase is drug elimination from the body. The distinguishing
characteristic of the
terminal elimination phase is that the relative proportion of drug in the
plasma and peripheral
volumes of distribution remains constant. During this "terminal phase" drug
returns from the
rapid and slow distribution volumes to the plasma, and is permanently removed
from the
plasma by metabolism or renal excretion.
Opioid Antagonists
[00130] Opioid receptor antagonists are a well-recognized class of chemical
agents. They
have been described in detail in the scientific and patent literature.
Naltrexone and its active
metabolite 6P-naltrexol are opioid antagonists, with no agonist properties, at
the li-opioid
receptor (MOR), the K-opioid receptor (KOR), and the 6-opioid receptor (DOR).
Naltrexone
operates by reversibly blocking the opioid receptors thereby attenuating the
effects of opioids.
Its mechanism of action in alcohol dependence is not fully understood but,
without being
limited by theory, naltrexone likely modulates the dopaminergic mesolimbic
pathway (one of
the primary centers for risk-reward analysis in the brain, and a tertiary
pleasure center) which
is believed to be a major center of the reward associated with addiction that
all major drugs of
abuse are believed to activate. The mechanism of action may be antagonism to
endogenous
opiates such as tetrahydropapaveroline, whose production is augmented in the
presence of
alcohol.
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00131] Naltrexone is commercially available as a hydrochloride salt.
Naltrexone
hydrochloride (17-(cyclopropylmethyl)-4,5a-epoxy-3,14-dihydroxymorphinan-6-
one) is used
to prevent euphorigenic effects in the treatment of patients addicted to
opioids. It markedly
blocks the physical dependence to intravenously administered opioids and
motivates
withdrawal from opioid dependency, but the patient does not develop tolerance
or dependence
to naltrexone. Naltrexone is also effective in reducing the craving for
alcohol in the treatment
of alcoholism, especially when combined with psychosocial therapy.
[00132] When naltrexone is administered intranasally, rather than orally,
the bioavailability
is significantly higher. When administered orally, despite being nearly
completely absorbed
from the gastrointestinal tract, naltrexone undergoes rapid and extensive
first-pass metabolism
to 6-P-naltrexol. As a result, the amount of naltrexone reaching systemic
circulation is limited.
The oral bioavailability of naltrexone has been reported to be as low as 5%.
Gonzalez and
Brogden, Drugs 35:192-213, 1988. Studies presented herein found the oral
bioavailability of
naltrexone to be similarly low, about 9%.
[00133] Provided herein are methods of treatment employing nasal delivery of a
pharmaceutical composition to a patient, comprising a therapeutically
effective amount of the
opioid antagonist naltrexone. In certain embodiments, the therapeutically
effective amount per
dose is equivalent to about 1 to about 4 mg of naltrexone hydrochloride. In
certain
embodiments, the therapeutically effective amount is equivalent to about 1 to
about 3 mg of
naltrexone hydrochloride. In certain embodiments, the therapeutically
effective amount per
dose is equivalent to about 1.0, about 1.1, about 1.2, about 1.3, about 1.4,
about 1.5, about
1.6, about 1.7, about 1.8, about 1.9, about 2.0, about 2.1, about 2.2, about
2.3, about 2.4,
about 2.5, about 2.6, about 2.7, about 2.8, about 2.9, about 3.0, about 3.1,
about 3.2, about
3.3, about 3.4, about 3.5, about 3.6, about 3.7, about 3.8, about 3.9, or
about 4.0 mg of
naltrexone hydrochloride. In certain embodiments, the therapeutically
effective amount per
dose is equivalent to about 1.2 mg of naltrexone hydrochloride. In certain
embodiments, the
therapeutically effective amount per dose is equivalent to about 1.6 mg of
naltrexone
hydrochloride. In certain embodiments, the therapeutically effective amount
per dose is
equivalent to about 2.0 mg of naltrexone hydrochloride. In certain
embodiments, the
therapeutically effective amount per dose is equivalent to about 3.0 mg of
naltrexone
hydrochloride. Multiple doses in succession may be taken to achieve
therapeutic efficacy. In
certain embodiments, the opioid antagonist is anhydrous naltrexone
hydrochloride.
[00134] While many of the embodiments of the pharmaceutical compositions
described
herein will be described and exemplified with naltrexone, other opioid
antagonists can be
21
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
adapted for nasal delivery based on the teachings of the specification. In
fact, it should be
readily apparent to one of ordinary skill in the art from the teachings herein
that the devices
and pharmaceutical compositions described herein may be suitable for other
opioid antagonists.
The opioid receptor antagonists described herein include li-opioid, ic-opioid,
and 6-opioid
receptor antagonists. Examples of useful opioid receptor antagonists include
naltrexone,
naloxone, methylnaltrexone, and nalmefene. Other useful opioid receptor
antagonists are
known in the art (e.g., U.S. Patent No. 4,987,136).
Pharmaceutical Formulations
[00135] Also provided are pharmaceutical compositions comprising the opioid
antagonist
naltrexone. In certain embodiments the pharmaceutical compositions comprise
the opioid
antagonist naltrexone and a pharmaceutically acceptable carrier. The
carrier(s) must be
"acceptable" in the sense of being compatible with the other ingredients of
the formulation and
not overly deleterious to the recipient thereof. Some embodiments of the
present invention
include a method of producing a pharmaceutical composition comprising admixing
the opioid
antagonist naltrexone and a pharmaceutically acceptable carrier.
Pharmaceutical compositions
are applied directly to the nasal cavity using the devices described herein.
In the case of a spray,
this may be achieved for example by means of a metering atomizing spray pump.
[00136] Liquid preparations include solutions, suspensions and emulsions, for
example,
water or water-propylene glycol solutions. Additional ingredients in liquid
preparations may
include: antimicrobial preservatives, such as benzalkonium chloride,
methylparaben, sodium
benzoate, benzoic acid, phenyl ethyl alcohol, and the like, and mixtures
thereof; surfactants
such as Polysorbate 80 NF, polyoxyethylene 20 sorbitan monolaurate,
polyoxyethylene (4)
sorbitan monolaurate, polyoxyethylene 20 sorbitan monopalmitate,
polyoxyethylene 20
sorbitan monostearate, polyoxyethylene (4) sorbitan monostearate,
polyoxyethylene 20
sorbitan tristearate, polyoxyethylene (5) sorbitan monooleate, polyoxyethylene
20 sorbitan
trioleate, polyoxyethylene 20 sorbitan monoisostearate, sorbitan monooleate,
sorbitan
monolaurate, sorbitan monopalmitate, sorbitan monostearate, sorbitan
trilaurate, sorbitan
trioleate, sorbitan tristearate, and the like, and mixtures thereof; a
tonicity agent such as:
dextrose, lactose, sodium chloride, calcium chloride, magnesium chloride,
sorbitol, sucrose,
mannitol, trehalose, raffinose, polyethylene glycol, hydroxyethyl starch,
glycine, and the like,
and mixtures thereof; and a suspending agent such as microcrystalline
cellulose,
carboxymethylcellulose sodium NF, polyacrylic acid, magnesium aluminum
silicate, xanthan
gum, and the like, and mixtures thereof
22
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00137] Ideally, when an opioid antagonist is administered intranasally prior
to ingestion of
alcohol to treat AUD, the opioid antagonist is absorbed quickly, i.e., within
about fifteen to
about thirty minutes and/or yielding a time to the maximum plasma
concentration (Tmax) of
about 25 to about 40 minutes. For example, in certain embodiments, the opioid
antagonist is
absorbed within the first 15 min after administration and the time to the
maximum plasma
concentration (Tmax) is 25 min or less. Alternatively, the opioid antagonist
is absorbed within
the first 30 min after administration and the Tmax is 40 min or less.
[00138] The use of absorption enhancers, such as alkylsaccharides (also
referred to herein
as alkylglycosides), cyclodextrins, and chitosans may increase the rate at
which naltrexone is
absorbed and decrease the T.. Such absorption enhancers typically operate by
affecting two
primary mechanisms for nasal absorption: paracellular transport via opening of
tight junctions
between cells, and transcellular transport or transcytosis through cells via
vesicle carriers.
[00139] In various aspects, alkylglycosides of the present invention may
include, but not
limited to: alkylglycosides, such as octyl-, nonyl-, decyl-, undecyI-, dodecyl-
, tridecyl-,
tetradecyl-, pentadecyl-,hexadecyl-, heptadecyl-, and octadecyl- a- or (3-D-
maltoside, -
glucoside or -sucroside; alkyl thiomaltosides, such as heptyl, octyl, dodecyl-
, tridecyI-, and
tetradecyl-P-D-thiomaltoside; alkyl thioglucosides, such as heptyl- or octyl 1-
thio a- or (3-D-
glucopyranoside; alkyl thiosucroses; alkyl maltotriosides; long chain
aliphatic carbonic acid
amides of sucrose 3-amino-alkyl ethers; derivatives of palatinose and
isomaltamine linked by
amide linkage to an alkyl chain; derivatives of isomaltamine linked by urea to
an alkyl chain;
long chain aliphatic carbonic acid ureides of sucrose 3-amino-alkyl ethers;
and long chain
aliphatic carbonic acid amides of sucrose 3-amino-alkyl ethers.
[00140] As described above, the hydrophobic alkyl can thus be chosen of any
desired size,
depending on the hydrophobicity desired and the hydrophilicity of the
saccharide moiety. For
example, one preferred range of alkyl chains is from about 9 to about 24
carbon atoms. An
even more preferred range is from about 9 to about 16 or about 14 carbon
atoms. Similarly,
some preferred glycosides include maltose, sucrose, and glucose linked by
glycosidic linkage
to an alkyl chain of 9, 10, 12, 13, 14, 16, 18, 20, 22, or 24 carbon atoms,
e.g., nonyl-, decyl-,
dodecyl- and tetradecyl sucroside, glucoside, and maltoside, etc. These
compositions are
nontoxic, since they are degraded to an alcohol or fatty acid and an
oligosaccharide, and
amphipathic. Additionally, the linkage between the hydrophobic alkyl group and
the
hydrophilic saccharide can include, among other possibilities, a glycosidic,
thioglycosidic,
amide, ureide, or ester linkage.
23
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00141] As use herein, a "saccharide" is inclusive of monosaccharides,
oligosaccharides or
polysaccharides in straight chain or ring forms, or a combination thereof to
form a saccharide
chain. Oligosaccharides are saccharides having two or more monosaccharide
residues.
Accordingly, examples of saccharides include glucose, maltose, maltotriose,
maltotetraose,
sucrose and trehalose.
[00142] In one embodiment, an exemplary alkylsaccharide is an alkylmaltoside.
Alkylmaltosides are glycosides of the disaccharide maltose and alcohols.
Typical
alkylmaltosides are dodecylmaltoside, tetradecylmaltoside and
hexadecylmaltoside which
consist of a 12, 14 and 16 carbon straight chain alcohol respectively,
glycosidically attached to
maltose. In an exemplary embodiment, the alkylglycoside is
tetradecylmaltoside.
[00143] For example, one alkyl saccharide is 1-0-n-dodecyl-P-D-maltopyranoside
(alternately referred to as lauryl-P-D-maltopyranoside, dodecyl
maltopyranoside, dodecyl
maltoside, Intravail0, and DDM; C24H46Q1 1). Alkylsaccharides are used in
commercial food
and personal care products and have been designated Generally Recognized as
Safe (GRAS)
substances for food applications. They are non-irritating enhancers of
transmucosal absorption
that are odorless, tasteless, non-toxic, non-mutagenic, and non-sensitizing in
the Draize test up
to a 25% concentration. Alkylsaccharides increase absorption by increasing
paracellular
permeability, as indicated by a decrease in transepithelial electrical
resistance; they may also
increase transcytosis. The effect is short-lived. Other alkylsaccharides
include tetradecyl
maltoside (TDM) and sucrose dodecanoate.
[00144] In sugar chemistry, an anomer is either of a pair of cyclic
stereoisomers (designated
a or r3) of a sugar or glycoside, differing only in configuration at the
hemiacetal (or hemiketal)
carbon, also called the anomeric carbon or reducing carbon. If the structure
is analogous to
one with the hydroxyl group on the anomeric carbon in the axial position of
glucose, then the
sugar is an alpha anomer. If, however, that hydroxyl is equatorial, the sugar
is a beta anomer.
For example, a-D-glucopyranose and P-D-glucopyranose, the two cyclic forms of
glucose, are
anomers. Likewise, alkylglycosides occur as anomers. For example, dodecyl P-D-
maltoside
and dodecyl a-D-maltoside are two cyclic forms of dodecyl maltoside. The two
different
anomers are two distinct chemical structures, and thus have different physical
and chemical
properties. In one aspect of the invention, the alkylglycoside of the present
invention is a r3
anomer. In an exemplary aspect, the alkylglycoside is a r3 anomer of an
alkylmaltoside, such
as tetradecyl-P-D-maltoside (TDM).
[00145] Thus, in one aspect of the present invention, the alkylglycoside used
is a
substantially pure alkylglycoside. As used herein a "substantially pure"
alkylglycoside refers
24
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
to one anomeric form of the alkylglycoside (either the a or r3 anomeric forms)
with less than
about 2% of the other anomeric form, preferably less than about 1.5% of the
other anomeric
form, and more preferably less than about 1% of the other anomeric form. In
one aspect, a
substantially pure alkylgycoside contains greater than 98% of either the a or
r3 anomer. In
another aspect, a substantially pure alkylgycoside contains greater than 99%
of either the a or
r3 anomer. In another aspect, a substantially pure alkylgycoside contains
greater than 99.5% of
either the a or r3 anomer. In another aspect, a substantially pure
alkylgycoside contains greater
than 99.9% of either the a or r3 anomer.
[00146] In certain embodiments, an intranasal formulation comprises about
0.001% to about
5.0% dodecyl maltoside by weight. In certain embodiments, an intranasal
formulation
comprises about 0.01% to about 2.5% dodecyl maltoside. In certain embodiments,
an
intranasal formulation comprises about 0.05% to about 2.5% dodecyl maltoside.
In certain
embodiments, an intranasal formulation comprises about 0.1% to about 0.5%
dodecyl
maltoside. In certain embodiments, an intranasal formulation comprises about
0.15% to about
0.35% dodecyl maltoside. In certain embodiments, an intranasal formulation
comprises about
0.15% to about 0.2% dodecyl maltoside. In certain embodiments, an intranasal
formulation
comprises about 0.18% dodecyl maltoside. In certain embodiments, an intranasal
formulation
comprises about 0.2% to about 0.3% dodecyl maltoside. In certain embodiments,
an intranasal
formulation comprises about 0.25% dodecyl maltoside.
[00147] When 0.18% dodecyl maltoside was added to an intranasal formulation of
sumatriptan, the maximum plasma concentration increased almost four-fold in
comparison to
Imitrex nasal spray and Tmax was reduced from 1-2 hours to 8-10 minutes. Total
exposure, as
measured by the area under the concentration-time curve (AUC), increased 32%.
An intranasal
formulation of naltrexone has the potential to be used for treating AUD
without the use of
needles or an extended-release formulation. Inclusion of dodecyl maltoside may
improve
pharmacokinetic parameters in some applications.
[00148] Some absorption enhancing excipients can alter the paracellular
and/or transcellular
pathways, others can extend residence time in the nasal cavity or prevent
metabolic changes.
Without an absorption enhancer, the molecular-weight limit for nasal
absorption is about 1
kDa, while administration of drugs in conjunction with absorption enhancers
can enable the
absorption of molecules from 1-30 kDa. Intranasal administration of most
absorption
enhancers, however, can cause nasal mucosa damage. Maggio, J. Excipients and
Food Chem.
5(2):100-12, 2014.
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00149] Examples of absorption enhancers include aprotinin, benzalkonium
chloride,
benzyl alcohol, capric acid, ceramides, cetylpyridinium chloride, chitosan,
cyclodextrins,
deoxycholic acid, decanoyl carnitine, dodecyl maltoside, EDTA, glycocholic
acid,
glycodeoxycholic acid, glycofurol, glycosylated sphingosines, glycyrrhetinic
acids, 2-
hydroxypropyl- P-cyclodextrin, laureth-9, lauric acid, lauroyl carnitine,
lauryl sulfate,
lysophosphatidylcholine, menthol, poloxamer 407, poloxamer F68, poly-L-
arginine,
polyoxyethylene-9-lauryl ether, polysorbate 80, propylene glycol, quillaja
saponin, salicylic
acid, P-Sitosterol- P-D-glucoside, sucrose cocoate, taurocholic acid,
taurodeoxycholic acid,
taurodihydrofusidic acid, and tetradecyl maltoside.
[00150] The opioid antagonist naltrexone described herein can be formulated
into
pharmaceutical compositions using techniques well known to those in the art.
Suitable
pharmaceutically acceptable carriers, outside those mentioned herein, are
known in the art.
[00151] The opioid antagonist naltrexone described herein may optionally exist
as
pharmaceutically acceptable salts including pharmaceutically acceptable acid
addition salts
prepared from pharmaceutically acceptable non-toxic acids including inorganic
and organic
acids. Representative acids include, but are not limited to, acetic,
benzenesulfonic, benzoic,
camphorsulfonic, citric, ethenesulfonic, dichloroacetic, formic, fumaric,
gluconic, glutamic,
hippuric, hydrobromic, hydrochloric, isethionic, lactic, maleic, malic,
mandelic,
methanesulfonic, mucic, nitric, oxalic, pamoic, pantothenic, phosphoric,
succinic, sulfuric,
tartaric, oxalic, p-toluenesulfonic and the like, such as those
pharmaceutically acceptable salts
listed by Berge et al., Journal of Pharmaceutical Sciences, 66:1-19 (1977).
The acid addition
salts may be obtained as the direct products of compound synthesis. In the
alternative, the free
base may be dissolved in a suitable solvent containing the appropriate acid
and the salt isolated
by evaporating the solvent or otherwise separating the salt and solvent. The
opioid antagonist
naltrexone described herein may form solvates with standard low molecular
weight solvents
using methods known to the skilled artisan.
[00152] Accordingly, provided herein are pharmaceutical formulations for
intranasal
administration comprising naltrexone or a salt thereof, e.g., naltrexone
hydrochloride. In
certain embodiments, the formulation is an aqueous solution. In certain
embodiments, the
formulation comprises, per dose, between about 25 and about 200 'AL of the
aqueous solution.
In certain embodiments, the formulation comprises, per dose, between about 50
and about 200
pL of the aqueous solution. In certain embodiments, the formulation comprises,
per dose, not
more than about 140 1AL. In certain embodiments, the formulation comprises,
per dose, not
more than about 10011L.
26
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00153] In certain embodiments, the formulation comprises between about 1%
(w/w) and
about 4% (w/w) of naltrexone hydrochloride. In certain embodiments, the
formulation
comprises between about 1% (w/w) and about 3% (w/w) of naltrexone
hydrochloride. In
certain embodiments, the formulation comprises about 1.0, about 1.1, about
1.2, about 1.3,
about 1.4, about 1.5, about 1.6, about 1.7, about 1.8, about 1.9, about 2.0,
about 2.1, about
2.2, about 2.3, about 2.4, about 2.5, about 2.6, about 2.7, about 2.8, about
2.9, about 3.0,
about 3.1, about 3.2, about 3.3, about 3.4, about 3.5, about 3.6, about 3.7,
about 3.8, about
3.9, or about 4.0 % (w/w) of naltrexone hydrochloride. In certain embodiments,
the
formulation comprises about 1.2% (w/w) of naltrexone hydrochloride. In
certain
embodiments, the formulation comprises about 1.6% (w/w) of naltrexone
hydrochloride. In
certain embodiments, the formulation comprises about 2% (w/w) of naltrexone
hydrochloride.
In certain embodiments, the formulation comprises about 3% (w/w) of naltrexone
hydrochloride.
[00154] In certain embodiments, the formulation comprises between about 1 mg
and about
4 mg of naltrexone hydrochloride. In certain embodiments, the formulation
comprises between
about 1 mg and about 3 mg of naltrexone hydrochloride. In certain embodiments,
the
formulation comprises between about 2 mg and about 4 mg of naltrexone
hydrochloride. In
certain embodiments, the formulation comprises about 1.0, about 1.1, about
1.2, about 1.3,
about 1.4, about 1.5, about 1.6, about 1.7, about 1.8, about 1.9, about 2.0,
about 2.1, about
2.2, about 2.3, about 2.4, about 2.5, about 2.6, about 2.7, about 2.8, about
2.9, about 3.0,
about 3.1, about 3.2, about 3.3, about 3.4, about 3.5, about 3.6, about 3.7,
about 3.8, about
3.9, or about 4.0 mg of naltrexone hydrochloride. In certain embodiments, the
formulation
comprises about 1.2 mg of naltrexone hydrochloride. In certain embodiments,
the formulation
comprises about 1.6 mg of naltrexone hydrochloride. In certain embodiments,
the formulation
comprises about 2 mg of naltrexone hydrochloride. In certain embodiments, the
formulation
comprises about 3 mg of naltrexone hydrochloride.
[00155] Aqueous formulations for intranasal administration disclosed herein
may also
include pharmaceutically acceptable excipients, such as one or more
isotonicity agents, one or
more preservatives, one or more stabilizing agents, one or more absorption
enhancers, and one
or more agents to adjust pH or buffer the solution.
[00156] In certain embodiments, the intranasal formulation additionally
comprises am
isotonicity agent, such as sodium chloride (NaCl).
[00157] In certain embodiments, the intranasal formulation additionally
comprises a
compound which is a preservative and/or surfactant.
27
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00158] In certain embodiments, the preservative and/or surfactant is chosen
from
benzalkonium chloride, methylparaben, sodium benzoate, benzoic acid, phenyl
ethyl alcohol,
and the like, and mixtures thereof; surfactants such as Polysorbate 80 NF,
polyoxyethylene 20
sorbitan monolaurate, polyoxyethylene (4) sorbitan monolaurate,
polyoxyethylene 20 sorbitan
monopalmitate, polyoxyethylene 20 sorbitan monostearate, polyoxyethylene (4)
sorbitan
monostearate, polyoxyethylene 20 sorbitan tristearate, polyoxyethylene (5)
sorbitan
monooleate, polyoxyethylene 20 sorbitan trioleate, polyoxyethylene 20 sorbitan
monoisostearate, sorbitan monooleate, sorbitan monolaurate, sorbitan
monopalmitate, sorbitan
monostearate, sorbitan trilaurate, sorbitan trioleate, sorbitan tristearate,
and the like, and
mixtures thereof.
[00159] In certain embodiments, the intranasal formulation additionally
comprises a
stabilizing agent.
[00160] In certain embodiments, the stabilizing agent is disodium edetate
(EDTA).
[00161] In certain embodiments, the pharmaceutical composition is in an
aqueous solution
of about 100 L.
[00162] In certain embodiments, upon nasal delivery of said pharmaceutical
composition to
said patient, less than about 10% of said pharmaceutical composition leaves
the nasal cavity
via drainage into the nasopharynx or externally.
Nasal Drug Delivery Devices and Kits
[00163] Also provided are pharmaceutical compositions in a device adapted for
nasal
delivery to a subject suffering AUD, comprising a therapeutically effective
amount of the
opioid antagonist naltrexone or pharmaceutically acceptable salt thereof In
certain
embodiments, the device is pre-primed. In certain embodiments, the device can
be primed
before use. In certain embodiments, the device can be actuated with one hand.
[00164] Nasal delivery is considered an attractive route for systemic drug
delivery,
especially when rapid absorption and effect are desired. In addition, nasal
delivery may help
address issues related to unpleasant taste, poor bioavailability, slow
absorption, drug
degradation, adverse events (AEs) in the gastrointestinal tract, and avoids
first-pass metabolism
and the hepatic toxicity associated with long-term oral naltrexone usage.
[00165] Liquid nasal formulations are mainly aqueous solutions, but
suspensions and
emulsions can also be delivered.
[00166] Some emergency medical service (EMS) programs have developed a system
using
existing technologies of an approved drug and an existing medical device to
administer the
opioid antagonist naloxone intranasally, albeit in a non-FDA approved manner.
This has been
28
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
accomplished by using the injectable formulation (1 mg/mL) and administering 1
mL per
nostril via a marketed nasal atomizer/nebulizer device. The system combines an
FDA-approved
naloxone injection product (with a Luer fitted tip, no needles) with a
marketed, medical device
called the Mucosal Atomization Device (MADTm Nasal, Wolfe Tory Medical, Inc.).
This
initiative is consistent with the U.S. Needlestick Safety and Prevention Act
(Public Law 106-
430). The EMS programs recognize limitations of this system, one limitation
being that it is
not assembled and ready-to-use. Although this administration mode appears to
be effective in
reversing narcosis, the formulation is not concentrated for retention in the
nasal cavity. The 1
mL delivery volume per nostril is larger than that generally utilized for
intranasal drug
administration. Therefore, there is loss of drug from the nasal cavity, due
either to drainage
into the nasopharynx or externally from the nasal cavity. The devices
described herein are
improved ready-to-use products specifically optimized, concentrated, and
formulated for nasal
delivery.
[00167] Metered spray pumps have dominated the nasal drug delivery market
since they
were introduced. The pumps typically deliver 1001AL (or other volumes in the
range of 25-200
1AL, and higher) per spray, and they offer high reproducibility of the emitted
dose and plume
geometry in in vitro tests.
[00168] Examples of standard metered spray pumps include those offered by
Aptar Pharma,
Inc., such as the multi-dose "classic technology platform" nasal spray
devices. Such devices
comprise a reservoir which holds multiple doses of the nasal spray formulation
(e.g., 50, 100,
150, 200, 60, or 120 doses), a closure (e.g., screw, crimp, or snap-on), and
an actuator which
delivers anywhere from 45 to 1000 pt (e.g. 50, 100, 140, 150, or 200 p.L) of
fluid per actuation
to comprise a single dose. The actuator may be configured to count doses,
deliver gel
formulations, deliver in an upside-down configuration, etc.
[00169] In traditional spray pump systems, antimicrobial preservatives are
typically
required to maintain microbiological stability in liquid formulations.
However, preservative-
free systems are also available, e.g. the Advanced Preservative Free (APF)
system from Aptar,
which is vented, contains a filter membrane for air flow which prevents
contamination, has a
metal-free fluid path for oxidizing formulations, and can be used in any
orientation. Additional
nasal spray devices from Aptar and others are optimized with dispenser tips
that prevent
clogging (useful for high-viscosity and high-volatile formulations), actuators
that do not need
re-priming after long periods of disuse, etc.
[00170] The particle size and plume geometry can vary within certain limits
and depend on
the properties of the pump, the formulation, the orifice of the actuator, and
the force applied.
29
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
The droplet size distribution of a nasal spray is a critical parameter, since
it significantly
influences the in vivo deposition of the drug in the nasal cavity. The droplet
size is influenced
by the actuation parameters of the device and the formulation. The prevalent
median droplet
size should be between about 30 and about 100 pm. If the droplets are too
large (> about 120
pm), deposition takes place mainly in the anterior parts of the nose, and if
the droplets are too
small (< about 10 pm), they can possibly be inhaled and reach the lungs, which
should be
avoided because of safety reasons. In its capacity as a surfactant,
benzalkonium chloride can
affect the surface tension of droplets from a delivered nasal spray plume,
producing spherical
or substantially spherical particles having a narrow droplet size distribution
(DSD), as well as
the viscosity of a liquid formulation.
[00171] Plume geometry, droplet size and DSD of the delivered plume subsequent
to
spraying may be measured under specified experimental and instrumental
conditions by
appropriate and validated and/or calibrated analytical procedures known in the
art. These
include photography, laser diffraction, and impaction systems (cascade
impaction, NGI).
Plume geometry, droplet size and DSD can affect pharmacokinetic outcomes such
as Cmax,
Tmax, and linear dose proportionality.
[00172] Droplet size distribution can be controlled in terms of ranges for the
D10, D50, D90,
span [(D90-D10)/D50], and percentage of droplets less than 10 mm. In certain
embodiments,
the formulation will have a narrow DSD. In certain embodiments, the
formulation will have a
D(v,50) of 30-70 i.tm and a D(v, 90) < 100
[00173] In certain embodiments, the percent of droplets less than 10 i.tm will
be less than
10%. In certain embodiments, the percent of droplets less than 10 i.tm will be
less than 5%. In
certain embodiments, the percent of droplets less than 10 i.tm will be less
than 2%. In certain
embodiments, the percent of droplets less than 10 i.tm will be less than 1%.
[00174] In certain embodiments, the formulation when dispensed by actuation
from the
device will produce a uniform circular plume with an ovality ratio close to 1.
Ovality ratio is
calculated as the quotient of the maximum diameter (D.) and the minimum
diameter (Dmin)
of a spray pattern taken orthogonal to the direction of spray flow (e.g., from
the "top"). In
certain embodiments, the ovality ratio is less than 2Ø In certain
embodiments, the ovality
ratio is less than 1.5. In certain embodiments, the ovality ratio is less
than 1.3. In certain
embodiments, the ovality ratio is less than 1.2. In certain embodiments, the
ovality ratio is
less than 1.1. In certain embodiments, the ovality ratio is about 1Ø
[00175] The details and mechanical principles of particle generation for
different types of
nasal aerosol devices has been described. Reviewed in Vidgren and Kublik, Adv.
Drug Deliv.
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Rev. 29:157-77, 1998. Traditional spray pumps replace the emitted liquid with
air, and
preservatives are therefore required to prevent contamination. However, driven
by the studies
suggesting possible negative effects of preservatives (e.g., irritation of
nasal mucosa), pump
manufacturers have developed different spray systems that avoid the need for
preservatives.
These systems use a collapsible bag, a movable piston, or a compressed gas to
compensate for
the emitted liquid volume (www.aptar.com and www.rexam.com). The solutions
with a
collapsible bag and a movable piston compensating for the emitted liquid
volume offer the
additional advantage that they can be emitted upside down, without the risk of
sucking air into
the dip tube and compromising the subsequent spray. This may be useful for
some products
where the patients are bedridden and where a head-down application is
recommended. Another
method used for avoiding preservatives is that the air that replaces the
emitted liquid is filtered
through an aseptic air filter. In addition, some systems have a ball valve at
the tip to prevent
contamination of the liquid inside the applicator tip (www.aptar.com). More
recently, pumps
have been designed with side-actuation and introduced for delivery of
fluticasone furoate for
the indication of seasonal and perennial allergic rhinitis. The pump was
designed with a shorter
tip to avoid contact with the sensitive mucosal surfaces. New designs to
reduce the need for
priming and re-priming, and pumps incorporating pressure point features to
improve the dose
reproducibility and dose counters and lock-out mechanisms for enhanced dose
control and
safety are available (www.rexam.com and www.aptar.com).
[00176] Traditional, simple metered-dose spray pumps require priming and some
degree of
overfill to maintain dose conformity for the labeled number of doses. They are
well suited for
drugs to be administered daily over a prolonged duration, but due to the
priming procedure and
limited control of dosing, unless a specialty device is selected, they are
less suited for drugs
with a narrow therapeutic window, particularly if they are not used often. For
expensive drugs
and vaccines intended for single administration or sporadic use and where
tight control of the
dose and formulation is of particular importance, single-dose or bi-dose spray
devices are
preferred (www.aptar.com). A simple variant of a single-dose spray device
(MADTm) is offered
by LMA (LMA, Salt Lake City, UT, USA; www.lmana. com). A nosepiece with a
spray tip is
fitted to a standard syringe. The liquid drug to be delivered is first drawn
into the syringe and
then the spray tip is fitted onto the syringe. This device has been used in
academic studies to
deliver, for example, a topical steroid in patients with chronic
rhinosinusitis and in a vaccine
study. A pre-filled device based on the same principle for one or two doses
(AccusprayTM,
Becton Dickinson Technologies, Research Triangle Park, NC, USA;
www.bdpharma.com) is
used to deliver the influenza vaccine FluMistTm (www.flumist.com), approved
for both adults
31
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
and children in the US market. A similar device for two doses was marketed by
a Swiss
company for delivery of another influenza vaccine a decade ago.
[00177] Pre-primed single- and bi-dose devices are also available, and
consist of a reservoir,
a piston, and a swirl chamber (see, e.g., the UDS UnitDose and BDS BiDoseTM
devices from
Aptar, formerly Pfeiffer). The spray is formed when the liquid is forced out
through the swirl
chamber. These devices are held between the second and the third fingers with
the thumb on
the actuator. A pressure point mechanism incorporated in some devices secures
reproducibility
of the actuation force and emitted plume characteristics. Currently, marketed
nasal migraine
drugs like Imitrex (www.gsk.com) and Zomig (www.az.com; Pfeiffer/Aptar
single-dose
device), the marketed influenza vaccine Flu-Mist (www.flumist.com; Becton
Dickinson single-
dose spray device), and the intranasal formulation of naloxone for opioid
overdose rescue ,
Narcan Nasal (narcan.com; Adapt Pharma) are delivered with this type of
device.
[00178] In certain embodiments, the 90% confidence interval for dose delivered
per
actuation is about 2%. In certain embodiments, the 95% confidence interval
for dose
delivered per actuation is about 2.5%.
[00179] Historically, intranasal administration of drugs in large volume, such
as from
syringes adapted with mucosal atomizer devices, has encountered difficulty due
to the tendency
of some of the formulation to drip back out of the nostril or down the
nasopharynx.
Accordingly, in certain embodiments, upon nasal delivery of said
pharmaceutical composition
to said patient, less than about 20% of said pharmaceutical composition leaves
the nasal cavity
via drainage into the nasopharynx or externally. In certain embodiments, upon
nasal delivery
of said pharmaceutical composition to said patient, less than about 10% of
said pharmaceutical
composition leaves the nasal cavity via drainage into the nasopharynx or
externally. In certain
embodiments, upon nasal delivery of said pharmaceutical composition to said
patient, less than
about 5% of said pharmaceutical composition leaves the nasal cavity via
drainage into the
nasopharynx or externally.
[00180] Current container closure system designs for inhalation spray drug
products include
both pre-metered and device-metered presentations using mechanical or power
assistance
and/or energy from patient inspiration for production of the spray plume. Pre-
metered
presentations contain previously measured doses or a dose fraction in some
type of units (e.g.,
single or multiple blisters or other cavities) that are subsequently inserted
into the device during
manufacture or by the patient before use. Typical device-metered units have a
reservoir
containing formulation sufficient for multiple doses that are delivered as
metered sprays by the
device itself when activated by the patient.
32
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00181] A new nasal drug delivery method, which can be adapted to any type of
dispersion
technology for both liquids and powders, is breath-powered BiDirectionalTM
technology. This
concept exploits natural functional aspects of the upper airways to offer a
delivery method that
may overcome many of the inherent limitations of traditional nasal devices.
Breath-powered
BiDirectionalTM devices consist of a mouthpiece and a sealing nosepiece with
an optimized
frusto-conical shape and comfortable surface that mechanically expands the
first part of the
nasal valve. The user slides a sealing nosepiece into one nostril until it
forms a seal with the
flexible soft tissue of the nostril opening, at which point, it mechanically
expands the narrow
slit-shaped part of the nasal triangular valve. The user then exhales through
an attached
mouthpiece. When exhaling into the mouthpiece against the resistance of the
device, the soft
palate (or velum) is automatically elevated by the positive oropharyngeal
pressure, isolating
the nasal cavity from the rest of the respiratory system. This mechanism
enables release of
liquid or powder particles into an air stream that enters one nostril, passes
entirely around the
nasal septum, and exits through the opposite nostril.
[00182] With sterile filling, the use of preservatives is not required in
devices, but overfill
is required resulting in a waste fraction similar to the metered-dose, multi-
dose sprays. To emit
100 11,1_õ a volume of 125 11,1_, is filled in the device (Pfeiffer/Aptar
single-dose device) used for
the intranasal migraine medications Imitrex0 (sumatriptan) and Zomig0
(zolmitriptan) and
about half of that for a bi-dose design. Sterile drug products may be produced
using aseptic
processing or terminal sterilization. Terminal sterilization usually involves
filling and sealing
product containers under high-quality environmental conditions. Products are
filled and sealed
in this type of environment to minimize the microbial and particulate content
of the in-process
product and to help ensure that the subsequent sterilization process is
successful. In most cases,
the product, container, and closure have low bioburden, but they are not
sterile. The product in
its final container is then subjected to a sterilization process such as heat
or irradiation. In an
aseptic process, the drug product, container, and closure are first subjected
to sterilization
methods separately, as appropriate, and then brought together. Because there
is no process to
sterilize the product in its final container, it is critical that containers
be filled and sealed in an
extremely high-quality environment. Aseptic processing involves more variables
than terminal
sterilization. Before aseptic assembly into a final product, the individual
parts of the final
product are generally subjected to various sterilization processes. For
example, glass containers
are subjected to dry heat; rubber closures are subjected to moist heat; and
liquid dosage forms
are subjected to filtration. Each of these manufacturing processes requires
validation and
control.
33
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Methods of Treatment
[00183] Provided herein are methods of treatment of alcohol use disorder and
related
conditions comprising the intranasal administration of a therapeutically
effective amount of
naltrexone or a salt or hydrate thereof.
[00184] Sinclair Method and Variations
[00185] The Sinclair Method is a treatment for AUD that employs
pharmacological
extinction¨the use of an opioid antagonist, such as naltrexone, to turn the
habit-forming
behavior of drinking alcohol into a habit-erasing behavior. The effect returns
a person's craving
for alcohol to its pre-addiction state.
[00186] The method consists of taking an oral dose of naltrexone about 1,
about 2, about 3,
or about 4 hours before a subject ingests alcohol. This pre-ingestion dose of
oral naltrexone
disrupts the body's behavior and reward cycle thereby causing the person to
want to drink less
instead of more. Most significantly, studies have shown that this methodology
is equally
effective with or without therapy, so subjects can choose whether or not to
combine this
treatment method with other therapies without negatively impacting the actual
physical results.
Importantly, unlike the other currently approved medication treatments for
AUD, the Sinclair
Method calls for the use of oral naltrexone while the individual continues
their normal drinking
behavior. As a result, maintenance of the medication treatment protocol is
expected to be much
higher than abstinence alone.
[00187] Using the Sinclair Method, extinction of AUD can occur within 6
months. However,
the efficacy of oral naltrexone is hampered by slow onset, very low
bioavailability and high
levels of the peripherally selective active metabolite 6-P-naltrexol, and the
injectable form of
naltrexone presents itself with the obvious difficulties associated with
needles including, for
example, the need for administration by a practitioner at regularly scheduled
intervals. Thus,
intranasal administration of naltrexone, and use of absorption enhancers, in a
pre-primed, single
or multi-use nasal spray pump should significantly improve results in the
treatment of AUD.
The timing of administration might also affect efficacy. An intranasal
formulation of
naltrexone absorbs quickly, providing fast onset of action and high
bioavailability without the
use of needles.
[00188] Accordingly, disclosed herein is a method of treatment of alcohol use
disorder, or a
related condition, in a subject, comprising administering to the subject an
intranasal
formulation comprising a therapeutically effective amount of naltrexone or a
pharmaceutically
acceptable salt thereof.
34
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00189] In certain embodiments, the intranasal formulation comprising
naltrexone is
administered prior to ingestion of alcohol.
[00190] In certain embodiments, the intranasal formulation comprising
naltrexone is
administered about 1 to about 2 hours prior to ingestion of alcohol. In
certain embodiments,
the intranasal formulation comprising naltrexone is administered about 1 hour
prior to ingestion
of alcohol. In certain embodiments, the intranasal formulation comprising
naltrexone is
administered about 0.5 to about 1 hours prior to ingestion of alcohol. In
certain embodiments,
the intranasal formulation comprising naltrexone is administered about 10 to
about 30 minutes
prior to ingestion of alcohol. In certain embodiments, the intranasal
formulation comprising
naltrexone is administered about 5 to about 10 minutes prior to ingestion of
alcohol. In certain
embodiments, the intranasal formulation comprising naltrexone is administered
just before
ingestion of alcohol.
[00191] In certain embodiments, the intranasal formulation comprising
naltrexone is
administered contemporaneously with the ingestion of alcohol.
[00192] In certain embodiments, the intranasal formulation comprising
naltrexone is
administered just after ingestion of alcohol. In certain embodiments, the
intranasal formulation
comprising naltrexone is administered within an hour after commencement of
ingestion of
alcohol.
[00193] It is expected that because intranasal naltrexone has a rapid uptake
via the nasal
mucosa and rapid appearance in the plasma, as evidenced by the studies below,
intranasal
administration will permit the subject to dose naltrexone much more
immediately before, and
even contemporaneously with or after, ingestion if alcohol, and experience
benefits such as
extinction, reduction in craving, etc. It is expected that absorption
enhancers will further this
effect.
[00194] In certain embodiments, the alcohol use disorder is alcohol abuse. In
certain
embodiments, the alcohol use disorder is alcohol dependence. In certain
embodiments, the
alcohol use disorder is alcoholism.
[00195] It is also expected that these methods will be effective in the
treatment of other
substance use disorders and reward-based disorders.
[00196] The methods disclosed herein may be achieved by administration of
various
embodiments of the formulations disclosed herein, for example above in the
section
"Pharmaceutical Formulations," the embodiments above, and the Examples below.
The
formulations may be administered using devices known on the art, for example
the devices
disclosed herein in the section entitled "Nasal Drug Delivery Devices and
Kits."
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00197] Also provided herein are embodiments wherein any embodiment described
above
may be combined with any one or more other embodiment(s), provided the
combination is not
mutually exclusive.
EXAMPLES
[00198] The following examples are included to demonstrate preferred
embodiments of the
invention. The following examples are presented only by way of illustration
and to assist one
of ordinary skill in using the invention. The examples are not intended in any
way to otherwise
limit the scope of the invention. Those of skill in the art should, in light
of the present
disclosure, appreciate that many changes can be made in the specific
embodiments which are
disclosed and still obtain a like or similar result without departing from the
spirit and scope of
the invention.
EXAMPLE 1: INTRANASAL NALTREXONE PROTOCOL FOR THE
TREATMENT OF ALCOHOL USE DISORDER
[00199] Individuals with alcohol use disorder (AUD) will be treated with
intranasal
naltrexone and examined for abstinence, reduced consumption of alcohol, and/or
extinguished
consumption of alcohol. Individuals with AUD are believed to release
endogenous opioids
upon the ingestion of alcohol. The binding of these opioids to receptors in
the brain may be
responsible for the positive reinforcing effects of alcohol. Drinking alcohol
while the opioid
antagonist naltrexone blocks the positive reinforcement from alcohol should
extinguish alcohol
drinking and craving.
[00200] In one example of a protocol, subjects (e.g., about 10-20) with AUD
will make be
admitted as in-patients to a study site. An initial visit serves the purpose
of screening, to confirm
the diagnosis and obtain informed consent. During their in-patient stay (e.g.,
one or more
weeks), each subject will receive a placebo or intranasal dose of naltrexone
followed by the
consumption one or more alcoholic beverages. Naltrexone will be administered
at the
designated dose and by the designated method at about 0.25 to about 4 hrs
before consumption
of alcohol. One example of a dosing treatment is an intranasal formulation
delivering about 1
to about 4 mg of naltrexone hydrochloride per administration, delivered by a
single- or multi-
use spray device. Another example of a dosing treatment is an intranasal
formulation delivering
a first dose of 3 mg of naltrexone hydrochloride in the morning, followed by
subsequent doses
of 3 mg of naltrexone hydrochloride throughout the day as needed by the
patient. Yet another
example of a dosing treatment is an intranasal formulation delivering up to 12
mg of naltrexone
hydrochloride per day. The intranasal formulation of naltrexone may or may not
contain an
absorption enhancer, such as Intravail0.
36
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00201] It is expected that intranasal naltrexone will improve post-treatment
suppression of
alcohol intake. It is also expected that intranasal naltrexone will reduce
alcohol cravings and
the amount of time required for a subject to exhibit pharmacological
extinction of alcohol
cravings.
[00202] Approximately 1 hour prior to dosing, ECG, blood pressure, heart rate,
and
respiration rate will be measured and the time will be recorded. At
approximately 1 and 4 hours
after dosing, the ECG will be repeated and the time will be recorded. Vital
signs including
sitting (after 5 minutes) heart rate, blood pressure and respiration rate will
be measured pre-
dose and approximately 1 and 4 hours after each dose. Adverse events (AEs)
will be recorded
and treatment terminated if necessary. The nasal passage will be examined at
pre-dose, 5
minutes, 30 minutes, 60 minutes, 4 hours, and 24 hours post-dose after
intranasal
administration only.
[00203] At screening, admission, and discharge, ECG, and vital signs will be
checked once
per day. Vital signs will also be checked once on the day after naltrexone
administration. AEs
will be assessed by spontaneous reports by subjects, by examination of the
nasal mucosa, by
measuring vital signs, ECG, and clinical laboratory parameters.
EXAMPLE 2: PHARMACOKINETIC DATA ANALYSIS
[00204] The non-compartmental pharmacokinetic (PK) parameters of naltrexone
and 6[3-
naltrexol (Cmax, Tmax, AUCo-t, AUCo-co, t1/2, XZ, and apparent clearance
(CL/F, naltrexone only)
will be determined. PK parameters of various AUD treatment protocols (e.g., 4
mg intranasal
with or without an absorption enhancer such as an alkylsaccharide; 50 mg oral
tablet) will be
compared with a 2 mg intramuscular (IM) dose of naltrexone. Dose-adjusted
values for AUCs
and Cmax will be calculated. The relative extent of intranasal (IN) and oral
absorption (PO)
absorption will be estimated from the dose-corrected AUCs. Within an ANOVA
framework,
comparisons of IN-transformed PK parameters for IN and PO versus IM naltrexone
treatments
will be performed. The 90% confidence interval for the ratio (IN/IM and PO/IM)
of the
geometric least squares means of AUC and Cmax parameters will be constructed
for comparison
of each treatment with IM naltrexone. These 90% confidence intervals will be
obtained by
exponentiation of the 90% confidence intervals for the difference between the
least squares
means based upon a log scale.
[00205] AEs will be coded using the most recent version of the Medical
Dictionary for
Regulatory Activities (MedDRA) preferred terms and will be grouped by system,
organ, class
(SOC) designation. The severity, frequency, and relationship of AEs to study
drug will be
presented by preferred term by SOC grouping. Separate summaries will be
provided for the 4
37
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
study periods: after the administration of each dose of study drug up until
the time of the next
dose of study drug or clinic discharge. Listings of each individual AE
including start date, stop
date, severity, relationship, outcome, and duration will be provided.
[00206] Clinically significant changes in vital signs, ECG, and clinical
laboratory
parameters will be presented as counts and percentages by dosing session.
EXAMPLE 3: DOSE SELECTION AND PHARMACEUTICAL COMPOSITION
WITH ABSORPTION ENHANCERS
[00207] Intranasal naltrexone may optionally be formulated with absorption-
enhancing
excipients.
[00208] One such excipient is the alkylsaccharide Intravail . Concentrations
of Intravail0
in nasal formulations have generally been 0.1 % and 0.2% by weight. The
present study will
use a concentration of 0.25% by weight of an alkylsaccharide. Concentrations
of 25%
Intravail0 were non-irritating in the rabbit eye model. The oral "no
observable effect level"
was approximately 20,000 to 30,000 mg/kg body weight. While there is no
comparable
intranasal data, the essential lack of oral safety suggests that the amount of
an alkylsaccharide
needed for nasal toxicity would be much higher than the amount that will be
administered in
this study.
[00209] In the present study, a single dose of naltrexone was administered 4
ways: a) 4 mg
IN in sterile water for injection; b) 4 mg IN in sterile water for injection
with 0.25% Intravail0;
c) 2 mg as an IM injection; and d) a 50-mg oral tablet. Intranasal
administration is expected to
increase the rate of absorption as compared to oral administration. Addition
of Intravail is
expected to further increase the rate of absorption from the nasal passages.
EXAMPLE 4: PHARMACOKINETIC EVALUATION OF INTRANASAL
NALTREXONE
[00210] Study Goals. The purpose of this clinical study was twofold: to
determine the
pharmacokinetics of two intranasal formulations (4 mg with and without
Intravail0) and one
oral formulation (50 mg tablet) of naltrexone compared to a 2-mg intramuscular
dose of
naltrexone; and to determine the safety of intranasal naltrexone, particularly
with respect to
nasal irritation, such as inflammation (erythema, edema, and erosion) and
bleeding. To that
end, the study's primary endpoints were the pharmacokinetic parameters (C.,
T., AUCo-t,
and AUCo_inf) of the IN and oral naltrexone formulations compared with an IM
dose of 2 mg
of naltrexone. Secondary endpoints included adverse events (AEs), vital signs
(heart rate,
sitting blood pressure, and respiration rate), electrocardiogram (ECG),
clinical laboratory
changes and nasal irritation using the nasal irritation scale.
38
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00211] Study design. Fourteen healthy volunteers were enrolled and completed
all study
drug administrations and blood collections for PK assessments. This was an in-
patient open-
label, crossover study involving approximately 14 healthy volunteers. Each
subject received
each naltrexone treatment: 4 mg IN (one 0.1 mL spray of a 40 mg/mL solution in
one nostril),
4 mg plus Intravail0 IN (one 0.1 mL spray of a 40mg/mL solution containing
0.25% Intravail0
in one nostril), 2 mg IM, and 50 mg oral tablet. Subjects stayed in the in-
patient facility for 13
days to complete the entire study. Subjects were called 3 to 5 days after
discharge to inquire
concerning AEs and concomitant medications since discharge. Informed consent
was obtained
from all subjects, and all were screened for eligibility to participate in the
study including
medical history, physical examination, clinical chemistry, coagulation
markers, hematology,
infectious disease serology, urinalysis, urine drug and alcohol toxicology
screen, vital signs
and ECG.
[00212] On the day after clinic admission, subjects were administered study
drug with a 3-
day washout period between doses until all treatments had been administered.
Blood was
collected for analysis prior to dosing and approximately 2.5, 5, 10, 15, 20,
30, 45, 60 minutes
and 2, 3, 4, 6, 8, 12, 16, 24, 30, 36, and 48 hours after study drug
administration. On days of
study drug administration, a 12-lead ECG was performed approximately 1 hour
prior to dosing
and at approximately 1 and 4 hours post-dose. Vital signs were measured pre-
dose and
approximately 1 and 4 hours post-dose.
[00213] On dosing days, the order of assessments were ECG, vital signs, then
PK blood
collection when scheduled at the same nominal times. The target time of the PK
blood
collection was considered the most critical and if the collection was more
than 1 minute from
the scheduled time for the first 60 minutes of collections or more than 5
minutes for the
scheduled time points thereafter, this was considered a protocol deviation.
ECG and vital signs
were collected within the 10 minute period before the nominal time of blood
collections. At
screening, admission, and discharge, ECG, and vital signs were checked once
per day. Vital
signs were also checked once on the day after naltrexone administration.
Clinical laboratory
measurements were repeated after the last PK blood draw prior to clinic
discharge. AEs were
assessed by spontaneous reports by subjects, by examination of the nasal
mucosa, by measuring
vital signs, ECG, and clinical laboratory parameters.
[00214] Inclusion and exclusion criteria: 1. Males and females 18 to 55 years
of age,
inclusive were included in this study. Written informed consent was required.
Subject had to:
have body mass index (BMI) ranging from 18 to 30 kg/m2, inclusive;
have adequate venous access;
39
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
have no clinically significant concurrent medical conditions determined by
medical
history, physical examination, clinical laboratory examination, vital signs,
and 12-lead ECG;
agree to use an acceptable method of contraception, other than oral
contraceptives,
throughout the study and for 90 days after the last study drug administration
(30 days for
women); and
agree not to ingest alcohol, drinks containing xanthine >500 mg/day (e.g.,
Coca-
Cola , tea, coffee, etc.), or grapefruit/grapefruit juice or participate in
strenuous exercise 72
hours prior to admission through the last blood draw of the study.
[00215] Exclusion criteria included:
any IN conditions including abnormal nasal anatomy, nasal symptoms (i.e.,
blocked
and/or runny nose, nasal polyps, etc.);
having a product sprayed into the nasal cavity prior to screening and drug
administration;
having been administered an investigational drug within 30 days prior to Day -
1;
having taken prescribed or over-the-counter medications, dietary supplements,
herbal products, vitamins, or recent use of opioid analgesics for pain relief
(within 14 days of
last use of any of these products);
a positive urine drug test for alcohol, opioids, cocaine, amphetamine,
methamphetamine, benzodiazepines, tetrahydrocannabinol (THC), barbiturates, or
methadone
at screening or admission;
previous or current opioid, alcohol, or other drug dependence (excluding
nicotine
and caffeine), based on medical history;
consumption of greater than 20 cigarettes per day on average, in the month
prior to
screening, or would be unable to abstain from smoking (or use of any nicotine-
containing
substance) for at least one hour prior to and 2 hours after naltrexone dosing;
systolic blood pressure less than 90 mm Hg or greater than 140 mm Hg;
diastolic
blood pressure less than 55 mmHg or greater than 90 mmHg; respiratory rate
less than 8
respirations per minute or greater than 20 respirations per minute;
on standard 12-lead ECG, a QTcF interval >440 msec for males and >450 msec for
females; significant acute or chronic medical disease (investigator judgment);
a likely need for concomitant medication treatment during the study;
donated or received blood or underwent plasma or platelet apheresis within the
60
days prior to Day -1;
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
female who is pregnant, breast feeding, or plans to become pregnant during the
study period or within 30 days after the last naltrexone administration;
positive test for hepatitis B surface antigen (HBsAg), hepatitis C virus
antibody
(HCVAb) or human immunodeficiency virus antibody (HIVAb) at screening;
current or recent (within 7 days prior to screening) upper respiratory tract
infection;
and
abnormal liver function test (ALT, AST, total bilirubin) > 1.5 times upper
limit of
normal
[00216] Study Drugs and Dosing. Naltrexone hydrochloride (HCI) was obtained
from
Mallinckrodt Pharmaceuticals. The IN (40 mg/mL) formulations were made by the
staff
pharmacist at Vince & Associates; the vehicle for the IN formulations was
sterile water for
injection. The IM formulation (2 mg/mL) was made by the staff pharmacist at
Vince &
Associates; the vehicle was sterile saline for injection. IN naltrexone was
administered using
an Aptar multi-dose device with the subject in a reclined position
(approximately 45 degrees).
The subject was instructed not to breathe through the nose when the IN dose of
naltrexone was
administered. Naltrexone HCI for the IM injection was administered with a 23-g
needle as a
single 1-mL injection into the gluteus maximus muscle. Naltrexone HCI for oral
administration
(50 mg tablet) was sourced from a commercial supplier and administered with
240 mL water.
[00217] Naltrexone was administered on Days 1, 4, 7, and 10, in the following
order: 4 mg
naltrexone IN, 4 mg naltrexone plus Intravail IN, 2 mg IM, and 50 mg oral.
Subjects stayed
in the in-patient facility for 13 days to complete the entire study and were
discharged 2 days
after the fourth dose.
[00218] PK Assessments. Blood (4 mL) was collected in sodium heparin
containing tubes
for PK analysis prior to dosing and 2.5,5, 10, 1.5, 20, 30, 45, 60 minutes and
2, 3, 4, 6, 8, 12,
16, 24, 30, 36, and 48 hours after the start of study drug administration.
Plasma was separated
from whole blood and stored frozen at <20 C until assayed. Naltrexone and 6P-
naltrexol
plasma concentrations were determined by liquid chromatography with tandem
mass
spectrometry at XenoBiotic laboratories, Inc., Plainsboro, New Jersey.
[00219] Safety Assessments. Heart rate, blood pressure, and respiration rate
were recorded
approximately 1 hour before naltrexone dosing and approximately 1 and 4 hours
after dosing.
A 12-lead ECG was obtained approximately 1 hour before and 1 and 4 hours after
each
naltrexone dose. ECG and vital signs was performed within the 10 minute period
before the
nominal time for post-dose blood collections. AEs were recorded from the start
of study drug
administration until clinic discharge. AEs were recorded relative to each
dosing session to
41
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
attempt to establish a relationship between the AE and type of naltrexone dose
administered.
An examination of the nasal passage was conducted at Day -1 to establish
eligibility and at pre-
dose, 5 minutes, 30 minutes, 60 minutes, 4 hours, and 24 hours post IN
naltrexone
administration to evaluate evidence of irritation to the nasal mucosa after IN
administration
only.
[00220] Analysis. Non-compartmental PK parameters of naltrexone and 6P-
naltrexol,
including, T., AUCo-t, and AUCo_ t and apparent clearance (CL/F, naltrexone
only),
was determined. Pharmacokinetic parameters (Cmax, T. and AUCs) for IN and PO
naltrexone
were compared with those for IM naltrexone. Dose-adjusted values for AUCs and
C. were
calculated. The relative extent of IN and PO absorption (IN and PO versus IM)
will be
estimated from the dose-corrected AUCs. Within an ANOVA framework, comparisons
of ln-
transformed PK parameters (Cmax and AUC) for IN and PO versus IM naltrexone
treatments
were performed. The 90% confidence interval for the ratio (IN/IM and PO/IM) of
the geometric
least squares means of AUC and C. parameters were constructed for comparison
of each
treatment with IM naltrexone. These 90% CIs were obtained by exponentiation of
the 90% CIs
for the difference between the least squares means based upon an ln scale.
[00221] Results. Results are shown below in Tables 1-5.
Table 1. Mean (SD) concentrations of naltrexone following a single IN, IM or
oral
administration to healthy subjects.
Treatment
Hour 4 mg IN + 0.25%
4 mg IN 2 mg IN 50 mg Oral
Intravail
0 0 0 0 0 0 0 0 0
0.042 0.117 (0.17) 1.15 (0.919) 0.678 (1.69)
0 0
0.083 1.51 (1.62) 11.9 (9.69) 1.04 (1.26)
0.109 (0.232)
0.17 3.4 (3.86) 12.1 (6.36) 2.97 (2) 0.851 (1.5)
0.25 4.36 (3.71) 10.4 (3.93) 3.45 (1.58) 2.5
(3.54)
0.33 4.46 (3.62) 9.81 (2.41) 3.58 (1.46) 4.75
(4.71)
0.5 4.08 (1.99) 7.19 (2.08) 3.43 (1.06)
7.16 (4.69)
0.75 3.39 (1.46) 5.46 (1.48) 3.02 (0.749) 6.9
(3.54)
1 3.19 (1.51) 4.55 (1.78) 2.73 (0.676)
6.34 (2.78)
2 2.33 (0.832) 3.07 (1.27) 2.35 (0.698)
5.22 (1.73)
3 1.5 (0.633) 2 (0.885) 1.79 (0.491)
3.66 (1.76)
4 1.02 (0.369) 1.25 (0.465) 1.3 (0.341)
2.39 (1.16)
42
CA 03124202 2021-06-17
WO 2020/132263
PCT/US2019/067513
6 0.418 (0.193) 0.536 (0.188) 0.584 (0.185) 1.13
(0.462)
8 0.22
(0.0941) 0.267 (0.105) 0.242 (0.0803) 0.596 (0.426)
12 0.0641 (0.021) 0.0726 (0.028) 0.0626 (0.0269) 0.3 (0.183)
16 0.0214 (0.0131) 0.0226 (0.0165) 0.0101 (0.0132) 0.141 (0.104)
24 0.00462 (0.0117) 0 0 0 0 0.0657
(0.0528)
30 0.00187 (0.00674) 0 0 0 0 0.0345
(0.0224)
36 0 0 0 0 0 0 0.0207
(0.0255)
48 0.0018 (0.0065) 0 0 0 0 0 0
Table 2. Mean CV% PK Parameters for Naltrexone Following Administration to
Healthy
Subjects
4 mg IN plus
PK Parameter 4 mg IN' 0.25% 2 mg IM` 50 mg
Oral
Intravail b
C. (ng/mL) 5.35 (66.8) 15.7 (52.0) 4.10
(34.0) 9.34 (31.8)
C. /Dose (ng/mL/mg) 1.48 (66.8) 4.35 (52.0) 2.27
(34.0) -- 0.206 (31.8)
T h d 0.50 (0.17, 0.17 (0.083, 0.33
(0.17, 0.50 (0.33,
max ()
2.00) 0.33) 1.00) 3.00)
AUCo-t (h=ng/mL) 11.9 (34.1) 18.3 (31.2) 12.1
(25.5) 26.5 (32.3)
AUCo-t /Dose (h=ng/mL/mg) 3.28 (34.1) 5.07 (31.2) 6.71
(25.5) 0.587 (32.3)
AUCo-mf (h=ng/mL) 12.0 (33.7) 18.5 (31.0) 12.3
(25.6) 26.9 (31.8)
AUCo-mf/Dose (h=ng/mL/mg) 3.32 (33.7) 5.10 (31.0) 6.78
(25.6) -- 0.594 (31.8)
AUCextrap (%) 1.09 (57.0) 0.707 (44.0) 1.01
(71.7) 1.38 (70.1)
CL/F (L/h) 330 (28.9) 214 (33.6 154
(19.0) 1890 (41.4)
X, (1/h) 0.281 (15.1) 0.317 (15.1)
0.361 (16.8) 0.122 (38.0)
t1/2 (h) 2.52 (14.9) 2.23 (14.9) 1.97
(15.5) 6.41 (36.6)
0.481 0.783
Fret (17.7)c NA 0.0903 (37.0)
(36.1)c
NA = Not applicable; Frel = Bioavailability relative to IM dose, calculated as
ratio of
AUCinf/Dose for IN or PO route relative to IM route.
a: N=13; b: N=12; c: N=10; d: Median (minimum, maximum)
[00222] Following IN administration of 4 mg naltrexone, the mean concentration
at 2.5
minutes postdose was 0.117 ng/mL. When 0.25% Intravail was added to the
formulation, the
43
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
mean concentration was 10 times greater (1.15 ng/mL) at 2.5 minutes. At 5
minutes postdose,
the mean concentrations of naltrexone with and without Intravail were 11.9
ng/mL and 1.51
ng/mL, respectively, an 8-fold difference. The addition of 0.25% Intravail to
the IN
formulation decreased median Tmax from 30 minutes to 10 minutes and increased
Cmax almost
3-fold (15.7 versus 5.35 ng/mL). Overall exposure as measured by AUCo-mf
increased by 54%,
indicating that the main effect of Intravail was to increase the rate of
absorption more than the
extent.
[00223] The mean plasma concentrations of naltrexone at 2.5 and 5 minutes
after
administration of 2 mg naltrexone IM were 0.678 ng/mL and 1.04 ng/mL,
respectively. The
mean Cmax value of 4.10 ng/mL 20 minutes after the 2 mg IM dose was 23% less
than after the
4 mg IN dose and 74% less compared to when Intravail was part of the IN
formulation.
[00224] The mean Cmax value after the oral dose was 9.34 ng/mL, which was less
than
observed after the IN dose with Intravail even though 50 mg was administered
orally
compared to only 4 mg IN.
[00225] The mean terminal phase half-life (t1/2) of naltrexone was 1.97 hours
to 2.52 hours
after IM and IN administration. The t1/2 was 6.41 hours after the oral dose.
[00226] When AUCo-mf values were corrected for dose, the relative
bioavailability of
naltrexone after the IN doses with and without 0.25% Intravail was 78% and
48%,
respectively, compared to the IM administration. The relative bioavailability
for the oral dose
was only 9%, indicating extensive first pass metabolism by the
gastrointestinal tract and liver.
[00227] Statistical analysis of dose-adjusted PK parameters suggested exposure
for the IN
dose was approximately 48% or 60% of the IM dose on a per mg basis, in terms
of geometric
least-squares mean (GM) dose-adjusted AUC and Cmax, respectively. IN
administration of
naltrexone with 0.25% Intravail resulted in dose-adjusted exposure that was
higher than the
IM route in terms of Cmax (geometric least-squares mean ratio between
treatments [GMR] of
188%) and lower in terms of AUC (GMR of 76%). For the oral route, the GMR for
dose-
adjusted naltrexone exposure was approximately 9% of the IM dose.
44
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Table 3. Mixed-Effects ANOVA Results for Naltrexone Pharmacokinetic Parameters
Following Intranasal or Oral Administration vs. Intramuscular Administration
to Healthy
Subjects
Comparison (2 mg IM
GMR 90% CI of GMR (/0)
PK Parameter (%)
Reference)
Lower
Upper
4 mgINvs 2 mgIM 121 91.1 160
4 mg IN plus Intravail0 vs 2 mg
Cmax (ng/mL) 377 321 442
50 mgPOvs 2 mgIM 231 190 282
4 mgINvs 2 mgIM 96.4 82.6 112
AUCo-t 4 mg IN plus Intravail0 vs 2 mg
152 136 169
(h=ng/mL) IM
50 mgPOvs 2 mgIM 221 182 268
4 mgINvs 2 mgIM 96.4 82.7 112
AUCO-inf 4 mg IN plus Intravail0 vs 2 mg
151 136 168
(h=ng/mL) IM
50 mgPOvs 2 mgIM 221 183 268
4 mg IN vs 2 mg IM 60.4 45.5 80.2
Cmax/Dose 4 mg IN plus Intravail0 vs 2 mg
188 161 221
(ng/mL/mg) IM
50 mgPOvs 2 mgIM 9.3 7.6 11.3
4 mgINvs 2 mgIM 48.2 41.3 56.2
AUCo_t /Dose 4 mg IN plus Intravail0 vs 2 mg
75.9 68.1 84.6
(h=ng/mL/mg) IM
50 mgPOvs 2 mgIM 8.8 7.3 10.7
4 mgINvs 2 mgIM 48.2 41.4 56.2
AUCo-inf/Dose 4 mg IN plus Intravail0 vs 2 mg
75.7 68 84.2
(h=ng/mL/mg) IM
50 mgPOvs 2 mgIM 8.9 7.3 10.7
GMR = Geometric least-squares mean ratio between treatments (expressed as
percentage of
reference)
[00228] The mean Cmax values of 6P-naltrexol were 1.5 ng/mL after the IM
administration
and approximately 3 ng/mL after the IN administration; Cmax was 90.7 ng/mL
after the 50 mg
oral dose (Table 2-3). When adjusted for the administered dose, the Cmax
values were similar
for the IN and IM doses (0.833 and 0.838 ng/mL/mg) but approximately 2-fold
higher (2.00
ng/mL/mg) after oral administration.
[00229] Values of AUCo-mf also were increased considerably after the oral dose
in
comparison to the IN and IM doses (675 h=ng/mL and 44.0 to 27.1 h=ng/mL,
respectively). The
greater extent of first pass metabolism of naltrexone was evident in the ratio
of AUCO-inf for
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
6P-naltrexol compared to that of naltrexone: after the IN and IM doses, the
ratio was
approximately 2.2 to 3.7 while it was 25 after the oral dose.
[00230] The mean t1/2 of the metabolite was 12.4 to 13.9 hours and was
independent of the
route of administration.
Table 4. Mean (SD) concentrations of 6P-naltrexol following a single IN, IM or
oral
administration to healthy subjects.
Treatment
Hour 4 mg IN + 0.25%
4 mg IN 2 mg IN 50 mg Oral
Intravail
0 0 0 0.0682
(0.0257) 0.0661 (0.0256) 0.0454 (0.0141)
0.042 0 0 0.082 (0.0378)
0.0627 (0.0248) 0.0448 (0.0202)
0.083 0.0321 (0.0432) 0.238 (0.146) 0.12 (0.117) 1.4 (3.49)
0.17 0.196 (0.196) 0.994 (0.558) 0.283 (0.281)
14.2 (31)
0.25 0.45 (0.448) 1.86 (0.763) 0.454 (0.293)
31.6 (47.3)
0.33 0.693 (0.624) 2.55 (0.918) 0.677 (0.385)
45.5 (43.7)
0.5 1.11 (0.559) 2.84 (0.748) 0.852 (0.328)
68.7 (41.3)
0.75 1.82 (1.16) 2.93 (0.757) 1.08 (0.452)
60.8 (28.2)
1 1.83 (0.815) 2.73 (0.481) 1.1 (0.404)
58.6 (18.2)
2 2.68 (0.842) 2.9 (0.767) 1.39 (0.462) 54
(16.3)
3 2.61 (0.793) 2.61 (0.708) 1.48 (0.402)
45.8 (15.2)
4 2.37 (0.669) 2.45 (0.598) 1.46 (0.388) 38
(12.2)
6 1.97 (0.554) 2.03 (0.399) 1.3 (0.252)
28.5 (7.52)
8 1.62 (0.418) 1.67 (0.27) 1.08 (0.165)
22.2 (5.51)
12 1.26 (0.299) 1.25 (0.176) 0.81 (0.131)
15.4 (2.85)
16 0.919 (0.229) 0.923 (0.155) 0.595 (0.115)
11.4 (1.78)
24 0.602 (0.194) 0.618 (0.145) 0.365 (0.0808) 8.14 (2.09)
30 0.418 (0.114) 0.457 (0.116) 0.255 (0.0666) 5.93 (1.85)
36 0.292 (0.0868) 0.312 (0.0862) 0.18 (0.0478) 4.35 (1.54)
48 0.184 (0.0645) 0.175 (0.0623) 0.106 (0.0329) 2.43 (0.882)
46
CA 03124202 2021-06-17
WO 2020/132263
PCT/US2019/067513
Table 5. Mean (CV%) PK Parameters for 6P-Naltrexol Following Administration to
Healthy
Subjects
4 mg IN plus
PK Parameter 4 mg IN'
0.25% Intravails b 2 mg 'Mc 50 mg
Oral
C. (ng/mL) 3.01 (33.2) 3.29(23.7) 1.52(26.8)
90.7(30.3)
0.833 0.838
C. /Dose (ng/mL/mg) 0.908 (23.7) 2.00 (30.3)
2.00 (0.7 3.00 (0.75,
0.63 (0.25,
Tmax (1) d 5' 0.75 (025 400)
6.0) . , .
4.00) 3.00)
AUCo_t (h.ng/mL) 40.3 (23.3) 43.0 (17.4) 25.1 (18.3)
614 (19.5)
AUCo-t /Dose
h.ng/mL/mg) 11.1(23.3) 11.9(17.4) 13.9(18.3)
13.6(19.5)
(
AUCo-mf (h.ng/mL) 44.0 (23.1) 46.3 (18.3) 27.1 (19.0)
675 (19.9)
AUCo-mf/Dose
12.2 (23.1) 12.8 (18.3) 15.0 (19.0)
14.9 (19.9)
(h.ng/mL/mg)
AUCextrap (%) 8.57 (46.1) 7.02 (37.3) 7.02 (29.4)
8.79 (57.3)
0.0530 0.0570
X( 1/h) 0.0553 (15.1)
0.0510(15.8)
t1/2 (h) 13.7 (22.7) 12.8 (14.6) 12.4 (13.2)
13.9 (15.9)
*Median (Min, Max) statistics presented for Tmax. All other values presented
as: Mean (Percent
coefficient of variation); a: N=13; b: N=12; c: N=10; d: Median (minimum,
maximum)
1002311 With the exception of the mean Cmax of naltrexone following the 4 mg
IN dose,
which was approximately 2-fold higher in females compared to males, there was
no clinically
meaningful difference between the sexes for the PK parameters of either
naltrexone or 6[3-
naltrexol following IN, IM, or PO administration.
[00232] Safety. In total, 10 of 14 subjects (71%) in the safety population
experienced at
least one AE (any dosing period, any relationship to drug). The most frequent
AEs were of the
Nervous System Disorders SOC (7 subjects, 50%), and dizziness was the most
frequent AE
regardless of severity or attribution (5 subjects, 36%). No severe AEs were
observed, and only
one moderate AE was observed, a case of dizziness after the first dose (4 mg
IN) that was
considered related to the study agent. Three subjects experienced AEs that
were unexpected
(UAE, defined as AEs that were not described with respect to nature, severity,
or frequency in
the current product package insert):two UAEs were considered unrelated to the
study agent and
one treatment-related UAE of mild syncope after administration of the Day 1
dose (4 mg IN).
Three subjects were discontinued from the study due to AEs (hypertension,
syncope, and out-
of-range pre-dose vital signs).
47
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
EXAMPLE 5: FORMULATIONS OF INTRANASAL NALTREXONE
[00233] The following tables set forth examples of formulations of naltrexone
for intranasal
administration for the treatment of disorders. Table 6 sets forth simple
aqueous solution
formulations such as those used in the experiment above, to be dispensed in
increments of
about 100 L.
Table 6.
Naltrexone HCl,Absorption .1_, Conc.,
Ex. per
dose (mg) Enhancer dose mg/mL
1 1.2 Intravail 0.25% 50 24
2 1.2 Intravail 0.25% 100 12
3 1.2 Intravail 0.25% 150 8
4 1.6 Intravail 0.25% 50 32
1.6 Intravail 0.25% 100 16
6 1.6 Intravail 0.25% 150 10.7
7 2 Intravail 0.25% 50 40
8 2 Intravail 0.25% 100 20
9 2 Intravail 0.25% 150 13.3
3 Intravail 0.25% 100 30
11 3 Intravail 0.25% 150 20
12 4 Intravail 0.25% 100 40
13 4 Intravail 0.25% 150 26.7
[00234] Table 7 sets forth formulations for intranasal administration in 100
pi, of an
aqueous solution including excipients such as an isotonicity agent, a
stabilizing agent, and/or a
compound which acts as a preservative or surfactant. EDTA stands for disodium
edetate and
BZK stands for benzalkonium chloride.
Table 7.
E Naltrexone Absorption Isotonicity
Stabilizing Preservative/
x.
HC1 Enhancer Agent Agent Surfactant
NaC1
14 1.2 mg Intravail 0.25% EDTA 0.3% BZK 0.02%
0.74%
NaC1
1.2 mg Intravail 0.25% EDTA 0.3% BZK 0.01%
0.74%
NaC1
16 1.2 mg Intravail 0.25% EDTA 0.2% BZK 0.02%
0.74%
NaC1
17 1.2 mg Intravail 0.25% EDTA 0.2% BZK 0.01%
0.74%
NaC1
18 1.6 mg Intravail 0.25% EDTA 0.3% BZK 0.02%
0.74%
NaC1
19 1.6 mg Intravail 0.25% EDTA 0.3% BZK 0.01%
0.74%
48
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
NaC1
20 1.6 mg Intravail 0.25% EDTA 0.2% BZK
0.02%
0.74%
21 1.6 mg Intravail 0.25% NaC1 0.74% EDTA 0.2% BZK
0.01%
22 2 mg Intravail 0.25% NaC1 0.74% EDTA 0.3%
BZK 0.02%
23 2 mg Intravail 0.25% NaC1 0.74% EDTA
0.3% BZK 0.01%
24 2 mg Intravail 0.25% NaC1 0.74% EDTA 0.2%
BZK 0.02%
25 2 mg Intravail 0.25% NaC1 0.74% EDTA
0.2% BZK 0.01%
26 3 mg Intravail 0.25% NaC1 0.74% EDTA
0.3% BZK 0.02%
27 3 mg Intravail 0.25% NaC1 0.74% EDTA
0.3% BZK 0.01%
28 3 mg Intravail 0.25% NaC1 0.74% EDTA 0.2%
BZK 0.02%
29 3 mg Intravail 0.25% NaC1 0.74% EDTA
0.2% BZK 0.01%
30 1.2 mg Intravail 0.18% NaC1 0.74% EDTA 0.3% BZK
0.02%
31 1.2 mg Intravail 0.18% NaC1 0.74% EDTA 0.3% BZK
0.01%
32 1.2 mg Intravail 0.18% NaC1 0.74% EDTA 0.2% BZK
0.02%
33 1.2 mg Intravail 0.18% NaC1 0.74% EDTA 0.2% BZK
0.01%
34 1.6 mg Intravail 0.18% NaC1 0.74% EDTA 0.3% BZK
0.02%
35 1.6 mg Intravail 0.18% NaC1 0.74% EDTA 0.3% BZK
0.01%
36 1.6 mg Intravail 0.18% NaC1 0.74% EDTA 0.2% BZK
0.02%
37 1.6 mg Intravail 0.18% NaC1 0.74% EDTA 0.2% BZK
0.01%
38 2 mg Intravail 0.18% NaC1 0.74% EDTA
0.3% BZK 0.02%
39 2 mg Intravail 0.18% NaC1 0.74% EDTA
0.3% BZK 0.01%
40 2 mg Intravail 0.18% NaC1 0.74% EDTA
0.2% BZK 0.02%
41 2 mg Intravail 0.18% NaC1 0.74% EDTA
0.2% BZK 0.01%
42 3 mg Intravail 0.18% NaC1 0.74% EDTA
0.3% BZK 0.02%
43 3 mg Intravail 0.18% NaC1 0.74% EDTA
0.3% BZK 0.01%
44 3 mg Intravail 0.18% NaC1 0.74% EDTA
0.2% BZK 0.02%
45 3 mg Intravail 0.18% NaC1 0.74% EDTA
0.2% BZK 0.01%
[00235] Also provided are examples 1A-45A which additionally contain an amount
of
hydrochloric acid sufficient to achieve a pH of 3.5-5.5. The acid should be
pharmaceutically
acceptable, for example, hydrochloric acid.
49
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
EXAMPLE 6: INTRANASAL NALTREXONE FORMULATION EXPERIMENTS
[00236] A series of experiments were performed to identify formulations of
naltrexone
which would make pharmaceutically appropriate intranasal products as disclosed
herein. The
formulations were designed to have at least some, and in certain embodiments,
all, of the
following properties:
containing an efficacious amount of naltrexone;
naltrexone quickly absorbed after administration, yielding a good C. and
relatively short Tmax;
would form a solution at room temperature, and remain a solution at cold
storage
conditions, or revert to solution when removed from cold storage;
would not be contaminated with microorganisms;
would be storage-stable, would not form or acquire undesirable impurities,
and/or
would not discolor over time; and
non-irritating to nasal membrane tissue.
[00237] A range of formulations were tested with the following observations. A
50 mg/mL
preparation of naltrexone HC1 was cloudy at room temperature, indicating
incomplete
dissolution; the solubility limit of naltrexone hydrochloride in solution at
ambient temperature
was determined to be approximately 40 mg/mL. Several preparations were made at
30 mg/mL
thereafter, which additionally contained fixed amounts of (dodecyl maltoside
0.25%) and NaCl
(about 0.74%), and varying amounts of preservative (benzalkonium chloride, 0-
0.02%) and
stabilizing agent (EDTA, 0-0.3%). Crystallization behavior and discoloration
were visually
monitored. All of these formulations developed crystals when stored under
refrigerated
conditions. Naltrexone solutions tend to turn yellow with time, over the
course of 0 to 3
months; formulations, containing 0.2% or 0.3% EDTA, did not form yellow
solutions, whereas
a formulation containing 0% or 0.1% EDTA did begin to yellow. The 0.3% EDTA
formulation
appeared to resist yellowing for the longest period of time. Thereafter, a
range of co-solvents
were assessed for their potential to reduce crystallization of drug out of
solution. Polyethylene
glycol (PEG), propylene glycol, and benzyl alcohol were tested. PEG-containing
formulations
did not prevent crystallization; propylene glycol containing formulations
prevented
crystallization but resulted in high osmolality, which would be expected to
result in irritation
of nasal membrane tissue; benzyl alcohol containing formulations prevented
crystallization at
low concentration. Total solid content was observed to be related to
crystallization, and
reducing the amount of naltrexone hydrochloride to produce the lower
concentrations and the
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
amount of NaCl in order to adjust for osmolality assisted in keeping the
formulation in solution
when stored under refrigerated conditions. Four formulations of naltrexone
suitable for
intranasal administration are given below in Example 7.
EXAMPLE 7: ADDITIONAL FORMULATIONS OF INTRANASAL NALTREXONE
[00238] The following are additional examples of batch formulations of
naltrexone, which
each make 2000 liquid grams of the give formulation for intranasal
administration for the
treatment of disorders, including Alcohol Use Disorder, which may be dispensed
in increments
of, e.g., about 100 L.
[00239] Examples below were prepared as follows. To a tared 4 L batch
container with a
stir bar, 1800.0 grams of water for injection (WFI) was added and mixing
initiated. Either 24.0
g, 32.0 g, 40.0 g or 60.0 g of naltrexone hydrochloride (NH) was added, the NH
container
rinsed with four (4) 5 mL WFI rinses, and mixed until the NH was visually
dissolved. While
mixing, 5.0 g DDM was added and mixed until visually dissolved; then the same
procedure
with 6.0 g EDTA. Next, an initial quantity of 12.6 g, 11.7 g, 10.5 g, or 7.9 g
of NaCl was added
depending on the formulation concentration; NaCl was added intermittently and
mixed
between additions until visually dissolved. Next, while mixing, 40.0 g of BZK
1% stock
solution was added, the BZK 1% stock solution container rinsed with a 10 mL of
rinse, and
mixed until the BZK was visually dissolved. Finally, pH of the solution was
measured using
a calibrated pH meter, and if above 5.5, adjusted accordingly utilizing 10%
HC1 until it was
between 3.5 and 5.5, with a target pH of 5Ø This was done by dropwise
addition and mixing
of 3-5 min between drops, then water added Q.S. to approximately 2000.0 g and
stirred for 5
min.
Formulation 49: 12 mg/mL (0.3% EDTA & 0.02% BZK)
Ingredient w/w % Formula Quantity (g)
Water for Injection 95.62 1912.4
Naltrexone Hydrochloride 1.2 24
Sodium Chloride 0.63 12.6
Disodium EDTA Dihydrate 0.3 6
Benzalkonium Chloride (1%) 2 40
DDM (Dodecyl Maltoside) 0.25 5
Formulation 50: 16 mg/mL (0.3% EDTA & 0.02% BZK)
Ingredient w/w Formula Quantity (g)
Water for Injection 95.265 1905.3
51
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Naltrexone Hydrochloride 1.6 32
Sodium Chloride 0.585 11.7
Disodium EDTA Dihydrate 0.3 6
Benzalkonium Chloride (1%) 2 40
DDM (Dodecyl Maltoside) 0.25 5
Formulation 51: 20 mg/mL (0.3% EDTA & 0.02% BZK)
Ingredient w/w Formula Quantity (g)
Water for Injection 94.912 1898.24
Naltrexone Hydrochloride 2 40
Sodium Chloride 0.538 10.76
Disodium EDTA Dihydrate 0.3 6
Benzalkonium Chloride (1%) 2 40
DDM (Dodecyl Maltoside) 0.25 5
Formulation 52: 30 mg/mL (0.3% EDTA & 0.02% BZK)
Ingredient w/w Formula Quantity (g)
Water for Injection 94.055 1881.1
Naltrexone Hydrochloride 3 60
Sodium Chloride 0.395 7.9
Disodium EDTA Dihydrate 0.3 6
Benzalkonium Chloride (1%) 2 40
DDM (Dodecyl Maltoside) 0.25 5
[00240] The formulations above may be tested according to the procedures above
in
Examples A-E and according to methods known in the art; certain of these
formulations have
been tested in Example 8 below. It is expected that the pharmacokinetic
properties of these
formulations will be consistent with those of an effective medication to treat
opioid overdose
as well as to use in 'as needed' fashion to treat multiple substance use
disorders, exemplified
by alcohol use disorder, and other reward-based disorders. These properties
include a rapid
onset (short Tmax), high plasma concentration, and short half-life relative to
oral administration
that can be achieved with these formulations.
EXAMPLE 8: PHARMACOKINETIC EVALUATION OF INTRANASAL
NALTREXONE
[00241] A single-center, open-label, randomized, four-sequence, four-
treatment, four-
period crossover pilot study was completed in healthy male and nonpregnant
female subjects.
52
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Twenty (20) healthy subjects were enrolled. Twenty-one (20) subjects completed
the study and
have evaluable data for all study periods.
[00242] Endpoints. The primary study endpoint was to determine the
pharmacokinetics of
three different doses (1.2 mg, 1.6 mg, and 3 mg) of naltrexone hydrochloride
nasal spray (Test
Products 1, 2, and 3, respectively, also referred to herein as Ti, T2, and T3)
compared to a 50
mg oral dose of naltrexone hydrochloride (Reference Product, also referred to
herein as R) and
to identify the intranasal dose of Test Product that could achieve naltrexone
systemic exposure
comparable to the 50 mg oral dose. The secondary study endpoint was to assess
the safety and
tolerability of the Test Product, especially nasal irritation (e.g., erythema,
edema and erosion).
[00243] Inclusion and Exclusion Criteria. Inclusion criteria for the study
were: written
informed consent; male or female subject between 18 and 55 years, inclusive,
at the time of
consent; body weight >48 kg and body mass index (BMI) of 18.0 to 30.0 kg/m2,
inclusive;
resting systolic blood pressure between 90 and 140 mmHg, inclusive, and
diastolic blood
pressure between 65 and 90, inclusive; resting heart rate between 40 and 100
bpm, inclusive;
resting respiratory rate between 8 and 20, inclusive; adequate venous access;
no clinically
significant abnormalities on physical examination; no clinically significant
abnormalities on
12-lead ECG, recorded after at least 3 minutes in supine position; no
clinically significant
abnormalities on hematology, biochemistry, coagulation and urinalysis
parameters; negative
test results for anti-HIV-1Ab and anti-HIV-2Ab antibodies, hepatitis B surface
antigen
(HBsAg) and anti-hepatitis C virus antibodies (anti-HCVAb); non-smoker or ex-
smoker (i.e.
someone who abstained from using tobacco- or nicotine-containing products
within the
previous 3 months; (occasional use of tobacco- or nicotine-containing products
is acceptable,
providing that there is no nicotine addiction and the subject agrees to
abstain from smoking
during the study periods); willingness to accept and comply with study
restrictions (e.g. alcohol
consumption, methylxanthines, diet, exercise, contraception and medications);
and if female,
infertile, postmenopausal, or if of childbearing potential, agrees to use an
effective non-
hormonal contraceptive or a hormonal contraceptive method from at least 4
weeks prior to
admission to Period 1, and to continue on a stable continuous regimen until
the end of the study
to ensure stable plasma hormonal levels during the whole study duration.
[00244] Exclusion criteria at screening were: known hypersensitivity/allergy
reaction to the
study drug substance or any of the excipients; known severe hypersensitivity
reaction to any
other drug; any nasal conditions including abnormal nasal anatomy, nasal
symptoms (i.e.
blocked and/or runny nose, nasal polyps, etc.), rhinitis and other conditions
that are known to
impact nasal absorption or having a product sprayed in to the nasal cavity
prior to drug
53
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
administration; previous or current opioid, alcohol, or other drug dependence
(excluding
nicotine and caffeine), based on medical history; concurrent disease
considered by the
investigator to be clinically significant in the context of the study; need
for concomitant
treatment medication during the study; any medical condition (e.g.
gastrointestinal, renal or
hepatic, including peptic ulcer, inflammatory bowel disease or pancreatitis)
or surgical
condition (e.g. cholecystectomy, gastrectomy) that could affect drug
pharmacokinetics
(absorption, distribution, metabolism or excretion) or subject safety; current
or recent (within
7 days prior to screening) upper respiratory tract infection; QTc interval >
450 msec for males
and > 470 msec for females; positive result in urine drugs-of-abuse test, or
ethanol breath test;
use of a depot injection or an implant of any drug (all but contraceptives)
within the previous
3 months; average weekly alcohol consumption of >14 units (12 grams per unit)
for males and
>7 units for females within the previous 6 months; average daily consumption
of
methylxanthines-containing beverages or food (e.g. coffee, tea, cola, sodas,
chocolate)
equivalent to >500 mg methylxanthines (100 mg of methylxanthines is equivalent
to
approximately 150 mL of coffee, 300 mL of tea, 75 mL of hot chocolate, 800 mL
of cola, 300
mL of energy drinks, or 25 g chocolate bar); participation in any clinical
trial within the
previous 2 months, or in more than 2 clinical trials within the previous 12
months; blood
donation or significant blood loss (?450 mL) due to any reason or had
plasmapheresis within
the previous 2 months; difficulty in fasting or any dietary restriction such
as lactose intolerance,
vegan, low-fat, low sodium, etc., that may interfere with the diet served
during the study;
difficulty in donating blood on either arm; difficulty in swallowing capsules
or tablets; if
female, pregnant or breast-feeding; and any other condition that the
Investigator considers
rendering the subject unsuitable for the study.
[00245] Exclusion criteria at admission were: resting systolic blood pressure
between 90
and 140 mmHg, inclusive, and diastolic blood pressure between 65 and 90,
inclusive; resting
heart rate between 40 and 100 bpm, inclusive; resting respiratory rate between
8 and 20,
inclusive; QTcF interval >500 msec; significant arrhythmia defined as > 6
beats of
supraventricular tachycardia or > 3beats of ventricular tachycardia; any
recent disease or
condition or treatment that, according to the Investigator, would put the
subject at undue risk
due to study participation or occurred at a timeframe in which may interfere
with the
pharmacokinetics of study drug; use of prescription or nonprescription
medicinal products,
including vitamins, food supplements, herbal supplements (including St John's
Wort), within
the previous 14 days, unless in the Investigator's opinion the medication
would not interfere
with the pharmacokinetics of study drug or compromise subject safety (the use
of topical
54
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
products without systemic absorption, or recommended contraceptives was
acceptable);
consumption of any alcoholic product within the previous 72 hours; positive
result in urine
drugs-of-abuse test, or ethanol breath test; if female of childbearing
potential, positive result in
urine beta-hCG pregnancy test; and any other condition that the investigator
considered
rendering the subject unsuitable for the study period.
[00246] Clinical Procedure. Subjects were confined at the clinical research
facilities for the
duration of the study, 15 nights. Each subject underwent four treatments
periods during the
study. Each subject received one of the 4 treatments in each of the 4
treatment periods:
Table 8.
One naltrexone hydrochloride (NTX) 50 mg film-coated tablets as a
Reference (R)
single dose.
Two administrations of 1.2 mg naltrexone hydrochloride (NTX)
Test Product 1 intranasal dose (two 0.1 mL spray of a 12 mg/mL solution),
separated by
(Ti) two hours interval. Ti was prepared as set forth above in
Formulation
Example 49.
Two administrations of 1.6 mg naltrexone hydrochloride (NTX)
Test Product 2 intranasal dose (two 0.1 mL spray of a 16 mg/mL solution),
separated by
(T2) two hours interval. T2 was prepared as set forth above in Formulation
Example 50.
Two administrations of 3 mg naltrexone hydrochloride (NTX)
Test Product 3 intranasal dose (two 0.1 mL spray of a 30 mg/mL solution),
separated by
(T3) two hours interval. T3 was prepared as set forth above in Formulation
Example 52.
[00247] According to the randomization schema, subjects were assigned to one
of several
treatment sequences in which investigational products Ti, T2, T3, and R were
administered in
various orders. Investigational products were administered in the morning,
after an overnight
fasting of at least 10 hours. The Reference Product was administered as a
single dose, orally,
with 240 mL of water (swallowed). Each Test Product was administered twice:
the first
administration was performed in one nostril and the second was performed in
the other nostril,
2 hours after, with the subject in an upright position. Subjects were
instructed to hold their
breath during administration of the nasal spray into the scheduled nostril.
Test Product was
primed before use. Following priming, the dosing device was weighed before and
after each
administration to determine the weight of the dose administered. Doses of each
investigational
product were separated by a washout interval of 3 days.
[00248] In each study period, subjects fasted overnight remained fasted until
breakfast,
which occurred approximately 3 hours after the Reference Product dosing and 1
hour after the
second administration of Test Product dosing. No fluids were allowed from 1
hour before dose
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
until 3 hours post-dose (timepoints defined in relation to first
administration of Test product or
ad ministration of Reference product). Water was provided ad libitum at all
other times.
Standardized meals and snacks identical in all periods were provided. Lunch
was served
approximately 3 hours after breakfast, and all other meals were scheduled at
appropriate times
by the clinical site.
[00249] Sample Collection and Analysis. In each study period, 24 venous blood
samples
(volume of 4 mL each) were collected for the determination of plasma
concentrations of
naltrexone and 6P-naltrexol at the following timepoints: pre-dose and at 0:02,
0:05, 0:10, 0:15,
0:20, 0:30, 0:45, 1:00, 2:00, 2:05, 2:10, 2:15, 2:20, 2:30, 2:45, 3:00, 4:00,
5:00, 6:00, 8:00,
12:00, 24:00 and 48:00 hours:minutes following Reference product dose and
first dose of Test
product.
[00250] Naltrexone and 6P-naltrexol plasma concentrations were measured using
a
previously validated liquid chromatography with tandem mass spectrometry (LC-
MS/MS)
analytical method.
[00251] Pharmacokinetic parameters of naltrexone and 6P-naltrexol were
estimated with
Phoenix WinNonlin version 8.1 (Certara USA Inc, Princeton, NJ) or higher, by
using a non-
compartmental approach with a /n-linear terminal phase assumption. Actual
times of blood
sampling was used to estimate pharmacokinetic parameters.
[00252] The following pharmacokinetic parameters were estimated: maximum
observed
plasma concentration (Cmax); time of occurrence of Cmax (tmax); area under the
plasma
concentration versus time curve (AUC) from pre-dose (time zero) to the last
sampling time
with quantifiable concentrations (AUCo-t); AUC from time zero to infinity
(AUCo-.); apparent
terminal elimination rate constant (Xz); apparent terminal elimination half-
life (tp2; though it
should be noted that here, a single value is given for each two-dose
administration) and
apparent total clearance of the drug from plasma (CL/F). Cmax, tmax, AUCo-t
and AUCo-. of
naltrexone and 6P-naltrexol were the primary endpoints.
[00253] Using a mixed effects model, an analysis of variance (ANOVA) was
performed for
comparisons of /n-transformed Cmax, AUCo-t and AUCo-.. Sequence, period and
treatment were
included in the ANOVA model as the independent factors. The Test-to-Reference
geometric
least-square means ratio (GMR) and the corresponding 90% confidence interval
(CI) was
calculated for Cmax, AUCo-t and AUCo-.. The relative extent (Frel) of
intranasal absorption
(Test versus Reference) was estimated from the dose-corrected AUCo-t. Tmax was
compared
using a non-parametric test. The secondary endpoints were Xz, ti/2 and CL/F.
56
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00254] Safety. Safety was evaluated through the assessment of adverse events,
12-lead
ECG, vital signs, nasal cavity examination, weight measurement, smell test and
clinical
laboratory tests (see flow-chart). Adverse events were monitored throughout
the study.
[00255] Results. Results are given below and in the accompanying Figures.
[00256] Table 9 presents the average of naltrexone primary pharmacokinetic
parameters and
respective summary statistics for twenty (20) subjects, following
administration of Test
Product 1, Test Product 2, Test Product 3, and Reference Product.
57
0
t..)
o
Table 9.
t..)
o
,..,
C. (ng/mL) tmax (h) AUCo-
t(ng.h/mL) AUCo-.0 (ng.h/mL) t..)
t..)
o,
Test Product 1 n 20 20 20
20 c,.)
(1.2 mg of NTX) Gmean 8.10 0.58
12.42 12.60
Amean 8.90 1.18
13.35 13.54
SD 3.86 1.05
4.75 4.81
CV(%) 43.3 88.9
35.6 35.5
Test Product 2 n 20 20 20
20
(1.6 mg of NTX) Gmean 9.22 0.60
15.19 15.46 p
Amean 9.68 1.18
16.07 16.35 2
SD 3.03 1.03
5.03 5.09
"
vi
co CV(%) 31.3 88.1
31.3 31.2
Test Product 3 n 20 20 20
20 ,
.
,
(3.0 mg of NTX) G. 19.88 0.80
31.33 31.94
Amean 21.01 1.39
32.57 33.13
SD 7.23 1.01
9.02 8.91
CV(%) 34.4 72.5
27.7 26.9
Reference n 20 20 20
19
Product Gmean 9.31 0.85
23.15 23.81
n
Amean 11.14 0.98
26.14 27.09
SD 6.92 0.65
13.65 14.53
cp
t..)
CV(%) 62.1 66.3
52.2 53.6 o
,o
O-
o,
--.1
u,
c,.)
[00257] Table 10 presents the average of naltrexone secondary pharmacokinetic
parameters and respective summary statistics for twenty (20)
subjects, following administration of Test 1 Product, Test 2 Product, Test 3
Product, and Reference Product. 0
t..)
o
Table 10.
t..)
o
,..,
pAUCo-o 033 pAUCom 083 pAUCom 167 pAUCo-o 250 PAUCO-0 333 PAUCO-0 500 kz
t1/2 (h) CL/F t..)
t..)
(ng.h/mL) (ng.h/mL) (ng.h/mL) (ng.h/mL)
(ng.h/mL) (ng.h/mL) (1/h) (L/h) c4,
Test 1 n 20 20 20 20 20
20 20 20 20
Product G. 0.11 0.56 1.08 1.50
2.09 0.295 2.35 190.45
(1.2 mg of
NTX) Arne an 0.02 0.16 0.66 1.22 1.67
2.28 0.324 2.64 207.51
SD 0.04 0.10 0.34 0.56 0.70
0.88 0.129 1.43 95.66
CV(%) 169.5 66.0 50.9 45.7 42.0
38.6 39.9 54.2 46.1
Test 2 n 19 20 20 20 20
20 20 20 20 P
Product Gmean 0.08 0.50 1.03 1.47 2.11
0.219 3.16 206.97 0.08
,-
r.,
..
(1.6 mg of
" r.,
NTX) Arne 0.01 0.10 0.58 1.18 1.66
2.36 0.267 3.86 222.73
vi
r.,
vz, SD 0.01 0.06 0.29 0.56 0.78
1.04 0.158 2.31 105.01 ,-
,
CV(%) 105.1 60.1 49.2 48.0 46.8
44.1 59.3 59.8 47.1 ,-
-,
Test 3 n 20 20 20 20 20
20 20 20 20
Product Gmean 0.01 0.21 1.09 2.10 2.93
4.14 0.130 5.34 187.87
(3.0 mg of
NTX) Arne an 0.02 0.27 1.26 2.41 3.35
4.69 0.137 6.06 195.47
SD 0.02 0.17 0.67 1.21 1.61
2.12 0.036 4.93 58.78
CV(%) 83.8 63.2 52.9 50.3 48.0
45.2 25.9 81.4 30.1 Iv
n
Reference n 19 20 20 20 20
20 19 19 19
Product Gmean
0.127 5.45 2099.84 cp
t..)
o
1-,
yD
Amean 0.00 0.00 0.00 0.04 0.16
0.79 0.130 5.57 2364.41
-4
SD 0.00 0.00 0.01 0.08 0.27
0.95 0.029 1.21 1121.61 vi
1-,
CV(%) 235.8 208.1 165.6
120.2 22.5 21.8 47.4
[00258] Table 11 presents the average naltrexone pharmacokinetic parameters
and respective summary statistics for twenty (20) subjects,
following first and second administrations of Test 1 Product, Test 2 Product,
Test 3 Product, and Reference Product 0
t..)
o
Table 11.
t..)
o
1-
Pt Administration 2nd Administration
t..)
t..)
c,
Cmax tmax (h) pAUC 0-2 Cmax tmax (h) pAUC2-
t Cmax c,.)
(ng/mL) (ng.h/mL) (ng/mL)
(ng.h/mL) (ng/mL)
Test 1 n 20 20 20 20 20
20
Product Gmean 6.98 0.17 4.34 5.64 2.19
7.69
(1.2 mg of Amean 7.87 0.18 4.64 6.87 2.19
8.67
NTX) SD 3.61 0.06 1.57 3.97 0.06
3.73
CV(%) 45.9 34.9 33.9 57.8 2.7
43.1 P
Test 2 n 20 20 20 20 20
20 2
Product Gmean 7.25 0.18 4.69 7.72 2.19
10.11 rt.
2
cr
(1.6 mg of Amean 8.12 0.18 5.11 8.47 2.19
10.92
"
,
NTX) SD 3.74 0.03 1.95 2.87 0.05
3.66 ,
.
,
CV(%) 46.1 18.6 38.3 33.9 2.2
33.5
Test 3 n 20 20 20 20 20
20
Product Gmean 13.98 0.16 8.89 15.15 2.21
21.36
(3.0 mg of Amean 16.07 0.18 9.99 17.13 2.21
22.50
NTX) SD 8.15 0.08 4.14 8.21 0.06
7.08
CV(%) 50.7 43.4 41.4 47.9 2.6
31.4 1-d
Reference n 19 20 20 20 20
20 n
1-i
Product Gmean 0.127 5.45
2099.84
cp
t..)
Amean 0.00 0.00 0.00 0.04 0.16
0.79 o
r-
,.z
SD 0.00 0.00 0.01 0.08 0.27
0.95 O-
c,
-.1
CV(%) 235.8 208.1 165.6 120.2
22.5 u,
r-
c,.)
[00259] Table 12 presents the average of 613-naltrexol primary pharmacokinetic
parameters and respective summary statistics for twenty (20)
subjects, following administration of Test 1 Product, Test 2 Product, Test 3
Product, and Reference Product. 0
t..)
o
Table 12.
t..)
o
1-
C. (ng/mL) tmax (h) AUCo-t
(ng.h/mL) AUCo-.0 t..)
t..)
c,
(ng.h/mL)
c,.)
Test 1 Product n 20 20
20 20
Gmean 2977.69 2.43
39661.64 44752.81
(1.2 mg of NTX) A mean 4726.08 3.00
43010.42 49049.12
SD 8815.95 1.65
18372.27 22391.88
CV(%) 186.5 55.0
42.7 45.7
Test 2 Product n 20 20
20 20 p
Gmean 3460.35 2.93
45308.65 51158.30 2
(1.6 mg of NTX) A mean 5772.44 3.10
49901.89 57840.89
"
cr SD 11486.17 0.88
24055.17 34255.04 "
0.
2
,
CV(%) 199.0 28.4
48.2 59.2 ,
.
,
Test 2 Product n 20 20
20 20
Gmean 5423.19 3.03
77062.89 86359.93
(3.0 mg of NTX) A mean 5742.45 3.09
80141.96 89628.51
SD 1885.91 0.64
22003.15 24149.84
CV(%) 32.8 20.8
27.5 26.9
Reference n 20 20
20 20 od
n
Product Gmean 111377.95 0.97
727323.16 795385.17
Amean 117199.63 1.13
739175.55 809654.98 cp
t..)
o
SD 37154.79 0.71
129584.07 150316.07 .
,.z
CV(%) 31.7 62.7
17.5 18.6 O-
c,
--.1
u,
c,.)
[00260] Table 13 presents the average of 613-naltrexol secondary
pharmacokinetic parameters and respective summary statistics for twenty (20)
subjects, following administration of Test 1 Product, Test 2 Product, Test 3
Product, and Reference Product. 0
t..)
o
Table 13.
t..)
o
,..,
pAUCo-o 033 pAUCom 083 pAUCom 167 pAUCo-o 250 PAUCO-0 333 PAUCO-0 500 kz
t1/2 (h) CL/F t..)
t..)
(ng.h/mL) (ng.h/mL) (ng.h/mL) (ng.h/mL)
(ng.h/mL) (ng.h/mL) (1/h) (L/h) c4,
Test n 20 20 20 20 20
20 20 20 20
Product 1 Gme, 52.32 121.46 218.83
450.19 0.045 15.43 53.63
(1.2 mg of
NTX) Arne an 17.83 39.49 94.17 174.39 282.58
531.05 0.046 15.75 58.42
SD 26.38 55.90 111.62 170.14 233.62
352.46 0.009 3.41 24.11
CV(%) 147.9 141.5 118.5 97.6 82.7
66.4 19.4 21.6 41.3
Test n 19 20 20 20 20
20 20 20 20 P
Product 2 Gm. 49.63 116.10 208.74
434.59 0.047 14.85 62.55 ,-
r.,
..
cr (1.6 mg of
r..)
r.,
NTX) Arne an 14.45 35.67 87.27 166.76 273.69
530.56 0.048 15.28 70.05
r.,
,-
SD 20.65 50.66 102.20 159.50 225.54
368.09 0.010 4.17 36.83 ,
,
CV(%) 143.0 142.0 117.1 95.6 82.4
69.4 20.9 27.3 52.6 ,-
-,
Test n 20 20 20 20 20
20 20 20 20
Product 3 Gme, 58.40 153.54 294.84
647.77 0.047 14.59 69.48
(3.0 mg of
NTX) Amean 9.93 27.32 82.31 191.08 353.70
750.71 0.048 14.93 72.36
SD 13.68 33.68 69.66 127.72 212.93
392.87 0.009 3.57 22.31
CV(%) 137.8 123.3 84.6 66.8 60.2
52.3 19.0 23.9 30.8 Iv
n
Reference n 19 20 20 20 20
20 20 20 20
Product G. 273.59
2489.10 0.048 14.39 62.86 cp
t..)
o
1-,
yD
Amean 2.11 5.70 49.31 427.56 1769.35
8799.34 0.049 14.67 64.10
-4
SD 2.90 7.39 75.91 713.22 2729.39
9755.51 0.010 2.97 13.57 vi
1-,
CV(%) 137.7 129.6 153.9 166.8 154.3
110.9 19.5 20.2 21.2
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
[00261] In accordance to the protocol, using a mixed effects model, an
analysis of variance
(ANOVA) was performed for comparisons of naltrexone in-transformed Cmax, AUCo-
t and
AUCo-. The Test-to-Reference geometric least-square means ratio (GMR) and the
corresponding 90% confidence interval (CI) was calculated. Results comparing
the three
different Test Products (Test Product 1, Test Product 2, and Test Product
3)with Reference are
summarized in Table 14.
Table 14. Mixed Effects ANOVA results for Naltrexone Pharmacokinetic
Parameters.
Geometric LSmeans1
Test 1-to-
PK Parameter Test 1 Reference Reference 90% CI
GMR (/o)
Cmax 8.10 9.31 86.93 65.69 - 115.04
AUCo-t 12.42 23.15 53.63 41.69 - 69.00
AUCo-. 12.59 23.80 52.92 41.15 - 68.05
Test 2-to-
PK Parameter Test 2 Reference Reference 90% CI
GMR (/o)
Cmax 9.22 9.31 99.00 74.54 - 131.49
AUCo-t 15.19 23.15 65.63 50.98 - 84.49
AUCo-. 15.42 23.80 64.78 50.34 - 83.37
Test 3-to-
PK Parameter Test 3 Reference Reference 90% CI
GMR (/o)
Cmax 19.88 9.31 213.41 162.41 - 280.42
AUCo-t 31.33 23.15 135.34 107.88 - 169.80
AUCo-. 31.85 23.80 133.83 106.74 - 167.80
1LSmeans values are given in ng/mL for C. and ng.h/mL for AUC.
[00262] In accordance to the protocol, using a mixed effects model, an
analysis of variance
(ANOVA) was performed for comparisons of 613-naltrexol in-transformed Cmax,
AUCo-t and
AUCo-.. The Test-to-Reference geometric least-square means ratio (GMR) and the
corresponding 90% confidence interval (CI) was calculated. Results comparing
the three
different Test Products (Test 1, Test 2, and Test 3)with Reference are
summarized in Table 15.
63
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Table 15. Mixed Effects ANOVA results for Naltrexone Pharmacokinetic
Parameters.
Geometric LSmeans
Test 1-to-
PK Parameter Test 1 Reference Reference
90% CI
GMR (/o)
Cmax 2977.69 111377.95 2.67 2.01 -
3.56
AUCo-t 39661.64 727323.16 5.45 4.75 -
6.26
AUCo, 44752.81 795385.17 5.63 4.91 -
6.44
Test 2-to-
PK Parameter Test 2 Reference Reference
90% CI
GMR (/o)
Cmax 3460.35 111377.95 3.11 2.34 -
4.12
AUCo-t 45308.65 727323.16 6.23 5.38 -
7.21
AUCo, 51158.30 795385.17 6.43 5.51 -
7.51
Test 3-to-
PK Parameter Test 3 Reference Reference
90% CI
GMR (/o)
Cmax 5423.19 111377.95 4.87 4.06 -
5.83
AUCo-t 77062.89 727323.16 10.60 9.39 -
11.95
AUCo, 86359.93 795385.17 10.86 9.67 -
12.20
1LSmeans values are given in ng/mL for Cmax and ng.h/mL for AUC.
[00263] In accordance to the protocol, the relative extent (Frei) of
intranasal absorption (Test
versus Reference) of naltrexone was estimated from the dose-corrected AUCo-t
(AUCo-t/D) of
the drug. Table 16 presents the arithmetic means and coefficient of variation
(CV%) for the
estimated parameters.
64
CA 03124202 2021-06-17
WO 2020/132263 PCT/US2019/067513
Table 16. Naltrexone - Relative Extent of Intranasal Absorption
PK Parameter Test 3 (n = Reference (n =
Test 1 (n = 20) Test 2 (n = 20)
(unit) 20) 20)
AUCo-t
(ng.h/mL) 13.35 (35.6%) 16.07(31.3%)
32.57(27.7%) 26.14(52.2%)
AUCo-t/D
(ng.h/mL/mg) 5.56(35.6%) 5.02 (31.3%) 5.43
(27.7%) 0.52 (52.2%)
1328.78 1238.87 1306.38
Frel (IN)
(61.7%) (65.8%) (61.9%)
n - Number of Subjects
AUCo_t/D - Dose normalized AUCO-t. For each Test product, D corresponds to the
total
administered dose of the product
Fret - relative extent of intranasal absorption (Test/Reference)
[00264] In accordance to the protocol, tmax obtained for each Formulation was
compared
using a non-parametric test as shown in Table 17.
Table 17. Naltrexone: tmax Non-parametric Analysis.
Treatment Difference Median 90% CI
Test 1 ¨ Reference 0.29 -0.54 ¨ 0.54
Test 2 ¨ Reference 0.29 -0.46 ¨ 0.64
Test 3 ¨ Reference 0.46 0.17 ¨ 1.17
Other Embodiments
[00265] The detailed description set forth herein is provided to aid those
skilled in the art in
practicing the present disclosure. However, the disclosure described and
claimed herein is not
to be limited in scope by the specific embodiments herein disclosed because
these embodiments
are intended as illustration of several aspects of the disclosure. Any
equivalent embodiments
are intended to be within the scope of this disclosure. Indeed, various
modifications of the
disclosure in addition to those shown and described herein will become
apparent to those
skilled in the art from the foregoing description, which do not depart from
the spirit or scope
of the present inventive discovery. Such modifications are also intended to
fall within the scope
of the appended claims.
[00266] All references, patents or applications, U.S. or foreign, cited in
the application are
hereby incorporated by reference as if written herein in their entireties.
Where any
inconsistencies arise, material literally disclosed herein controls.
[00267] Although the invention has been described with reference to the above
examples,
it will be understood that modifications and variations are encompassed within
the spirit and
scope of the invention. Accordingly, the invention is limited only by the
following claims.