Note: Descriptions are shown in the official language in which they were submitted.
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
NON-INVASIVE, UNIFORM AND NON-UNIFORM RF METHODS AND SYSTEMS
RELATED APPLICATIONS
[0001] This application is a continuation-in-part of U.S. Patent
Application No. 16/238,483
filed January 2, 2019, which is a continuation-in-part of U.S. Patent
Application No. 15/640,710,
filed on July 3, 2017, which claims the benefit of priority to U.S.
Provisional App. No. 62/357,920,
filed on July 1, 2016, and U.S. Provisional App. No. 62/514,778, filed on June
2, 2017, each of
which is incorporated herein by reference in its entirety.
FIELD
[0002] The present disclosure relates generally to systems and methods for
treating a patient's
skin (e.g., dermis and hypodermis) and other target tissue, including tissue
at a depth below a tissue
surface with radiofrequency (RF) energy.
BACKGROUND
[0003] Electrosurgical devices are known for applying RF energy to tissue
so as to generate a
variety of effects, including invasive procedures (e.g., for ablating or
vaporizing tissue) or less-
invasive procedures (e.g., to gently heat the surface of the skin). However, a
need remains for
improved methods and system for providing uniform and large-area application
of RF energy in
cosmetic and/or aesthetic applications, for example, in order to improve the
appearance of skin so
that it is (or appears) tightened/smoothed and/or to reduce fat present in
subcutaneous tissue (e.g.,
hypodermi s).
SUMMARY
[0004] Systems and methods utilizing RF energy to treat a patient's skin
(e.g., dermis and
hypodermis) or other target tissue at a depth below a tissue surface with RF
energy are described
herein. In various aspects, the present teachings can provide a non-invasive,
cooled (or uncooled)
RF-based treatment to achieve one or more of body sculpting (lipolysis), skin
tightening (laxity
improvement), cellulite treatment apparatus, vaginal laxity treatment or
rejuvenation, urinary
incontinence treatment, fecal incontinence treatment, and treatment of other
genitourinary
conditions, by way of non-limiting examples.
[0005] In various aspects, the non-invasive treatment of unwanted fat, the
improvement in skin
laxity/tightness and the improvement in the appearance of cellulite can be
accomplished by the
application RF energy (e.g., 500kHz, 1Mhz, or other) delivered to the surface
of the patient's tissue
1
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
(e.g., skin, vaginal wall, esophagus) via a water-cooled treatment electrode
or electrode array
operating in either monopolar or bipolar mode, the RF energy propagating from
the tissue surface
into the deeper tissue layers. In accordance with various aspects of the
present teachings, cooling
the superficial layers and selectively controlling the deposition of RF energy
can heat the tissue
below the surface and can help ensure heating uniformity, patient safety and
tolerance, and
consistent clinical results.
[0006] In accordance with various aspects of the present teachings, systems
and methods
described herein can be one or more of:
1. user-friendly and/or hands-free (e.g., after initial set-up);
2. provide patient safety and/or comfort through cooling of the upper
layers of
the tissue and/or modulation of RF energy and/or modulation of cooling to
improve a patient's tolerance; and
3. flexible so as to address a variety of anatomical features.
By way of non-limiting example, various systems and methods in accordance with
the present
teachings can be utilized in a hand's free manner such that an RF applicator
or multiple RF
applicators can be applied to the patient at the start of the treatment,
energized and optionally left
unattended until the completion of the procedure (e.g., patients can be left
largely unattended for
treatments, for example, for at least as long as 5 minutes or at least as long
as 10 minutes following
initial set-up). In various aspects, the methods and systems described herein
can include a cooling
system (e.g., via the circulation of refrigerated, temperature-controlled
water adjacent to the RF
source and/or electrode array) to provide patient safety (e.g., avoid burning
of the skin and/or
nodule formation in the tissue subsequent to heat treatment of the tissue) in
accordance with FDA
and IEC safety recommended safety standards, improve patient comfort, and/or
increase a patient's
tolerance to potentially painful effects of the RF energy during treatment. In
various aspects, the
methods and systems described herein can be sufficiently flexible and/or
adaptable so as to be able
to treat a variety of desired locations (e.g., abdomen, submental region, any
of a number of areas
of the face, arms, legs) on the patient's body despite inter- and intra-
patient anatomical differences,
differing surface areas, and complex curvatures, which can be difficult to
maintain contact with
during the time required to complete the treatment.
2
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[0007] In accordance with various aspects of the present teachings, a
system for treating a
patient's tissue is provided, the system comprising a source of RF energy and
a treatment applicator
having a plurality of treatment electrodes configured to be disposed in
contact with a surface of a
patient's tissue (e.g., a skin surface, a mucosal surface) and to deliver RF
energy thereto and a
return electrode. The plurality of treatment electrodes can comprise at least
two individually-
addressable treatment electrodes to which different treatment RF signals can
be applied, the RF
signals exhibiting one or more of a power, duty cycle, pulse duration, phase,
and RF frequency.
The system can also include a controller configured to determine the impedance
of each of the at
least two individually-addressable treatment electrodes, wherein the
controller is further
configured to adjust the treatment RF signals applied simultaneously to the at
least two
individually-addressable treatment electrodes based on the impedance thereof
so as to maintain
uniformity of heating in a target tissue disposed below the treatment
applicator. Optionally, in
some aspects, the system can also include a cooling mechanism for cooling the
tissue surface in
contact with the plurality of electrodes. In various aspects, the at least one
return electrode can be
disposed on a skin surface or internally (e.g., within the urethra).
[0008] In some aspects, the different RF signals applied simultaneously to
the at least two
individually-addressable treatment electrodes can comprise one or more of
different powers, pulse
widths, duty cycles, phases, and RF frequencies. In some related aspects, the
controller can be
configured to reduce the power of the RF signal to the electrode of the at
least two individually-
addressable treatment electrodes exhibiting a lower impedance.
[0009] In various aspects, the at least two individually-addressable
treatment electrodes can
comprise at least two groups (e.g., clusters) of individually-addressable
treatment electrodes,
wherein each treatment electrode in each of group of individually-addressable
treatment electrodes
have the same RF signal simultaneously applied thereto as the other treatment
electrodes in the
group, and wherein each group of individually-addressable treatment electrodes
are configured to
have different RF signals applied simultaneously thereto.
[0010] In some aspects according to the present teachings, the system can
also include a second
treatment applicator configured to be disposed in contact with a tissue
surface spaced apart from
the tissue surface to which the first treatment applicator is disposed. The
second treatment
applicator can, in some aspects, represent the at least one return electrode,
though a return electrode
3
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
can also be a separate electrode. Optionally, the second treatment applicator
can comprise a
cooling mechanism for cooling the tissue surface in contact with the plurality
of electrodes of the
second treatment applicator. In some aspects, the second treatment applicator
can comprise a
second plurality of treatment electrodes configured to be disposed in contact
with the patient's
tissue surface and to deliver RF energy thereto, wherein the second plurality
of treatment
electrodes comprise at least two individually-addressable treatment electrodes
to which different
RF signals can be applied. In such aspects, the controller can be configured
to activate only one
of the individually-addressable treatment electrodes on each of the first and
second treatment
applicator at a given time, for example. Additionally, the controller can be
configured to determine
the impedance between each of the at least two individually-addressable
treatment electrodes of
the first treatment applicator and each of the at least two individually-
addressable treatment
electrodes of the second treatment applicator (e.g., by polling one electrode
from each applicator
at a time). By way of example, the controller can be configured to determine
the impedance
between each of the at least two individually-addressable treatment electrodes
of the first treatment
applicator and each of the at least two individually-addressable treatment
electrodes of the second
treatment applicator by generating a sub-treatment threshold RF current
therebetween prior to
applying treatment RF signals to the first plurality of electrodes.
Additionally or alternatively, in
some aspects, the controller can be configured to determine the impedance
between each of the at
least two individually-addressable treatment electrodes of the first treatment
applicator and each
of the at least two individually-addressable treatment electrodes of the
second treatment applicator
while applying treatment RF signals to the first plurality of electrodes so as
to determine when to
terminate treatment by terminating the treatment RF signals.
[0011] The return electrode can have a variety of configurations. For
example, the return
electrode can be a passive electrode configured to be disposed in contact with
a tissue surface
spaced apart from the tissue surface to which the first treatment applicator
is disposed. For
example, the passive electrode can be a neutral drain pad. In some related
aspects, a second
treatment applicator can also be provided in addition to the return electrode,
the second treatment
applicator configured to be disposed in contact with a tissue surface spaced
apart from the tissue
surfaces to which the first treatment applicator and the passive electrode are
disposed, wherein the
4
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
second treatment applicator comprises a second plurality of treatment
electrodes configured to be
disposed in contact with the patient's tissue surface and to deliver RF energy
thereto.
[0012] In various aspects, the controller can be configured to separately
poll each of at least
two individually-addressable treatment electrodes of the first treatment
applicator with a low-
power sub-treatment threshold RF signal.
[0013] Methods and systems in accordance with the present teachings can
provide a variety of
treatments. By way of example, the RF treatment signals can be configured to
reduce skin laxity
by stimulating the production of collagen and/or lipolysis in fat tissue below
the tissue surface
(e.g., by bulk heating). By way of example, each electrode can be configured
to deliver RF power
in a range from about 1 W/cm2 to about 5 W/cm2, wherein the RF signal has a
pulse width greater
than about 1 second. Additionally or alternatively, the RF treatment signals
can be configured to
reduce the appearance of cellulite. For example, each electrode can be
configured to deliver RF
pulses exhibiting an energy per pulse in a range from about 10 J/cm2 to about
1000 J/cm2 and
wherein the RF signal has a pulse width less than about 500 ms.
[0014] The cooling mechanism can have a variety of configurations in
accordance with the
present teachings. By way of example, the cooling mechanism can comprise a
circulating fluid,
thermoelectric elements, or a phase change material disposed in the applicator
in thermal contact
with the electrode. In certain aspects, the cooling mechanism can comprise a
circulating fluid,
with the temperature of the circulating fluid being controlled by a
temperature regulator (e.g.,
under the influence of the controller) such that a target tissue region
disposed can comprise below
the tissue surface is maintain at a temperature in a range from about 42 C to
about 47 C during
a treatment time in a range from about 10 minutes to about 30 minutes. In some
aspects, the
circulating fluid can comprise water. In various aspects, at least a portion
of a fluid pathway of
the circulating fluid can be in thermal contact with a side of the electrodes
that is not configured
for contact with the tissue surface. Additionally or alternatively, at least a
portion of a fluid
pathway of the circulating fluid can be in thermal contact with the tissue
surface at a location
between adjacent electrodes of the plurality of treatment electrodes.
[0015] In various aspects, the system can also include one or more
temperature detectors for
detecting a temperature of the tissue surface around the perimeter of the
electrode array, wherein
the controller is further configured to adjust the RF signals (e.g., reduce
the power of the treatment
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
RF signals) applied to electrodes on a side of the applicator exhibiting the
highest temperature.
Additionally or alternatively, the controller can be configured to adjust the
RF signals (e.g.,
increase the power of the treatment RF signals) applied to electrodes on a
side of the applicator
opposed to the side of the applicator exhibiting the lowest temperature.
[0016] In some aspects, the source of RF energy can comprise two or more
individually-
controllable RF energy sources, each of the individually controllable RF
energy sources being
configured to operate at the same fundamental frequency, but the RF signals
generated thereby can
have different phases and amplitudes. In such aspects, the system can comprise
two or more
treatment applicators each associated with one of the RF energy sources,
wherein current amongst
each of the two or more treatment applicators can be shared such that the two
or more applicators
can be disposed on two or more distinct treatment regions of the body of the
subject and each of
the two or more applicators can be configured to deliver a suitable amount of
RF energy to each
of the distinct treatment regions.
[0017] In accordance with various aspects of the present teachings, a
system for treating a
patient's tissue is provided, the system comprising a source of RF energy, a
treatment applicator
comprising a treatment electrode configured to be disposed in contact with a
surface of a patient's
tissue and to deliver RF energy thereto, and at least one return electrode.
The system can also
include a controller configured to provide an RF signal to the treatment
electrode, the RF signal
having a pulse duration that selectively heats septae within fat tissue while
substantially avoiding
conduction of heat into adjacent tissue, and an impedance tracker for
monitoring the patient's
tissue impedance during the pulse duration and for providing information about
the patient's tissue
impedance changes to the controller so that the controller can terminate the
RF signal when the
desired treatment is completed. Optionally, the system can include a cooling
mechanism for
cooling the tissue surface in contact with the electrodes.
[0018] In various related aspects, the treatment electrode can be
configured to deliver RF
pulses exhibiting an energy per pulse in a range from about 10 Fcm2 to about
500 J/cm2, wherein
the RF signal has a pulse width less than about 500 ms. In some aspects, the
controller can be
further configured to adjust the RF signals provided to the plurality of
electrodes such that second
treatment RF signals are simultaneously provided to each of the plurality of
electrodes, wherein
the second RF signals comprise a lower RF power and longer pulse width
relative to the RF
6
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
treatment signals for selectively heating the septae. By way of example, the
second RF treatment
signals can be configured to reduce skin laxity and/or cause lipolysis (e.g.,
after or before the
septae are selectively targeted). In various aspects, the second RF treatment
signals can be
configured such that each electrode simultaneously delivers RF power in a
range from about 1
W/cm2 to about 5 W/cm2, wherein the RF signal has a pulse width greater than
about 1 second.
[0019] In accordance with various aspects of the present teachings, a
system for treating a
patient's tissue is provided, the system comprising a source of RF energy and
a treatment applicator
comprising a plurality of treatment electrodes configured to be disposed in
contact with a surface
of a patient's tissue and to deliver RF energy thereto, wherein the plurality
of treatment electrodes
comprise at least two individually-addressable treatment electrodes to which
treatment RF signals
can be applied. The system can also, in some aspects, include at least one
return electrode and
optionally, a cooling mechanism for cooling the tissue surface in contact with
the plurality of
electrodes. A controller can be provided that is configured to sequentially
provide treatment RF
signals to each of the at least two individually-addressable treatment
electrodes such that each of
the at least two individually-addressable treatment electrodes are configured
to selectively heat
septae within fat tissue while substantially avoiding conduction of heat into
adjacent tissue. In
some aspects, each of the at least two individually-addressable treatment
electrodes can be
configured to deliver RF pulses having an energy in a range from about 10
J/cm2 to about 500
J/cm2 and wherein the RF signal has a pulse width less than about 100 ms.
Additionally, the
controller can be further configured to adjust the RF signals provided to the
plurality of electrodes
such that second treatment RF signals are simultaneously provided to each of
the plurality of
electrodes, wherein the second RF signals comprise a lower RF power and longer
pulse width
relative to the RF treatment signals for selectively heating the septae. By
way of example, the
second RF treatment signals can be configured to reduce skin laxity and/or
cause lipolysis. In
certain aspects, each electrode subject to the second RF treatment signals can
simultaneously
deliver RF power in a range from about 1 W/cm2 to about 5 W/cm2, wherein the
RF signal has a
pulse width greater than about 1 second.
[0020] In accordance with various aspects of the present teachings, an
apparatus for treating a
female genitourinary condition is provided, the apparatus comprising a probe
adapted for vaginal
insertion having a distal end configured to apply heat to at least a portion
of a vaginal wall surface
7
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
and a plurality of radiofrequency (RF) energy radiating therapeutic electrodes
disposed in an array
at the distal end of the probe to heat tissue either in contact with the probe
or in proximity to it. At
least one temperature sensor can also be incorporated into the probe to
monitor the temperature of
the vaginal wall surface and/or the target tissue. In various aspects, the
temperature sensor can be
an infrared (IR) sensor configured to detect black body radiation emitted by
heated tissue or can
be implemented by one or more of the electrodes operating as an impedance
measuring electrode.
Optionally, the probe can further comprise one or more cooling circuits to
avoid overheating of
the vaginal wall surface.
[0021] In some aspects, the electrodes are programmable (e.g., under the
influence of a
controller) such that a subset of the array components can be activated to
deliver heat in a specific
pattern. In various aspects, the apparatus can further comprise one or more
return electrodes to
provide a return path for an RF current from the therapeutic electrode. By way
example, the return
electrode can be a drain pad (e.g., a neutral paddle) adapted to be disposed
on an external surface
a patient's body (e.g., a skin surface). Alternatively, the return electrode
can be disposed in a
urethral catheter. Alternatively, the return electrode can be implemented by
one or more electrodes
in the array serving as a grounding electrode.
[0022] In certain aspects, a fixation device can also be provided to ease
insertion of the probe
and/or for holding the probe in place upon insertion into a patient. For
example, the fixation device
can comprise a locking sleeve or balloon.
[0023] In accordance with various aspects of the present teachings, a
method of treating a
female genitourinary condition is provided. By way of example, in various
aspects a method of
treating stress urinary incontinence (SUI) is provided, the method comprising
delivering a
controlled amount of heat to a vaginal wall surface to remodel tissue in a
target region adjacent to
a patient's bladder neck or urethra. In various aspects, the heating can be
performed by activating
one or more radio frequency (RF) energy emitting therapeutic electrodes in
contact with the
vaginal wall surface to transmit an RF current into the target region. In
certain exemplary aspects,
the therapeutic electrodes can comprise an electrode array carried by a probe,
the method further
comprising inserting the probe into a patient such that at least one
therapeutic electrode contacts
at least a portion of a vaginal wall surface. In certain aspects, the power
delivered by individual
electrodes in the array can be varied to ensure uniform heating of tissue in
the target region. In
8
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
some aspects, the electrode(s) can be configured to contact at least a portion
of the anterior vaginal
wall and/or the method can further comprise delivering RF energy to heat
tissue between the
patient's vaginal wall surface and urethra. By way of example, the method can
further comprise
delivering RF energy to heat tissue in a target region that extends to a
treatment depth of about 2
to 9 cm, preferably about 5 to 8 cm beyond the inner vaginal wall surface.
[0024] In some related aspects, RF energy can be delivered so as to heat
tissue in a target
region for a period of time, preferably less than 30 minutes, or less than 10
minutes or in some
instances less than five minutes. Additionally in certain aspects, the target
tissue can be heated to
about 40 C to 45 C, or from about 41 C to 43 C. Optionally, the method can
comprise cooling
the vaginal wall surface before, after or during heating the tissue in the
target region.
[0025] In various aspects, the method can further comprise mapping the
heating effects of the
RF electrode by thermal imaging or impedance measurements.
[0026] In accordance with various aspects of the present teachings, a
system for treating a
patient's tissue is provided, the system comprising a source of RF energy, a
treatment applicator
comprising a treatment electrode configured to be disposed in contact with a
surface of a patient's
tissue (e.g., a treatment probe configured for insertion into a patient's
vagina having one or more
treatment electrodes) and to deliver RF energy thereto, and at least one
return electrode. The
system can also include a controller configured to provide an RF signal to the
treatment electrode,
the RF signal having a pulse duration and the treatment electrode being sized
so as to apply current
density sufficient to ablate the surface of the tissue in contact with the
treatment electrode.
Optionally, a cooling mechanism for cooling the tissue surface in contact with
the electrode(s). In
various aspects, the pulse duration can be less than about 100 ms. (e.g., in a
range from about 5
ms. to about 35 ms.). In various aspects, the treatment electrode(s) can have
a size that ranges
from about 0.1 mm to about 10 mm, or from about 0.1 mm to about 5 mm.
[0027] In various aspects, the system can also include a second treatment
electrode that can be
disposed adjacent to the treatment electrode, the controller also being
configured to provide the
RF signal to the second treatment electrode, the RF signal having a pulse
duration and the second
treatment electrode being sized so as to apply current density sufficient to
ablate the surface of the
tissue in contact with the treatment electrode. In various aspects, the pitch
between the treatment
electrode and the second electrode can range from about 0.1 mm to about 10 mm
or from about
9
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
0.5 mm to about 5 mm. In some related aspects, the treatment electrode can be
addressed by the
controller simultaneous with the second treatment electrode.
[0028] In various aspects, the treatment electrode can comprise a cluster
of two or more
electrodes, each of the electrodes in the cluster having a size that ranges
from about 0.1 mm to
about 10 mm, or from about 0.1 mm to about 5 mm. In such aspects, each of the
two or more
electrodes in the cluster can be sized so as to apply current density
sufficient to ablate the surface
of the tissue in contact with each treatment electrode of the cluster.
Additionally, in some aspects,
a second cluster of two or more electrodes can be provided, the controller
being configured to
provide the RF signal to the second cluster and the RF signal having a pulse
duration and each of
the two or more electrodes in the second cluster being sized so as to apply
current density sufficient
to ablate the surface of the tissue in contact with each treatment electrode
of the second cluster. In
various aspects, the controller can separately address the cluster and the
second cluster.
[0029] In accordance with various aspects of the present teachings, a
system for treating a
patient's tissue is provided, the system comprising two or more treatment
applicators each adapted
to be disposed on a tissue surface and two or more individually controllable
RF energy sources.
In exemplary aspects, each of the individually controllable RF energy sources
can operate at the
same fundamental frequency, but the phases and the amplitudes of each of the
two or more RF
energy sources can be controllable relative to one another. In such aspects,
each of the two or
more treatment applicators can be associated with its own individually
controllable RF energy
source such that current can be shared amongst the two or more treatment
applicators such that the
two or more applicators can be placed on two or more distinct treatment
regions of the body of the
subject and each of the two or more applicators can be capable of delivering a
suitable amount of
RF energy to each of the distinct treatment regions. In various aspects, the
system can also include
a return electrode. Additionally, in certain aspects, each treatment
applicator can comprise a
plurality of treatment electrodes configured to be disposed in contact with a
surface of a patient's
tissue and to deliver RF energy thereto, wherein the plurality of treatment
electrodes comprise at
least two individually-addressable treatment electrodes to which RF signals
can be applied.
[0030] In accordance with various aspects of the present teachings, a
radiofrequency (RF)-
based treatment system include an RF transmission cable comprising a plurality
of electrical leads
and an RF input port and a first applicator. The first applicator includes a
plurality of electrical
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
contacts in electrical communication with the plurality of electrical leads.
The system can also
include a first support comprising a tissue facing surface and a bottom
surface, the tissue facing
surface defining a first shape and an array of K individually addressable
electrodes disposed in or
on the first support and arranged relative to the tissue facing surface, each
of the K electrodes in
communication with at least one of the electrical contacts. In one embodiment,
K is a positive
integer.
[0031] In various aspects, the system can in include a first support that
can be a first flexible
substrate. In some aspects, the system can include electrical contacts and
each addressable
electrode can be flexible and can be disposed on the first support.
Optionally, in some aspects, the
RF-based treatment system includes a second support, the second support can
include a second
flexible material, where the second support can be disposed on or above the
bottom surface.
[0032] In various aspects, the RF-based treatment system can include a
second flexible
material can be a resilient compressible foam material. Optionally, in some
aspects, the RF-based
treatment system can include a fluid-based cooling device defining one or more
coolant flow
channels, the cooling device disposed below the tissue facing surface. In some
aspects, the RF-
based treatment system can include coolant flow channels sized to reduce
tissue surface heating
when the array is activated during a tissue treatment. In some aspects, the RF-
based treatment
system can include a cooling device including one or more connectors extending
therefrom, the
cooling device sandwiched between the first support and the foam material.
[0033] In many aspects, the RF-based treatment system can include a first
support which can
be a flexible polymer substrate, wherein the plurality of electrical contacts
is disposed on one or
more edges of the polymer substrate. In some aspects, the RF-based treatment
system can include
an applicator kit, the applicator kit including a first applicator and M
applicators, wherein each of
the M applicators can be substantially the same as the first applicator,
wherein the first shape of
each applicator can be selected such that M+1 applicators can tile a tissue
treatment surface when
placed adjacent to each other. M can range from 1 to 1000 in one embodiment.
In one
embodiment, M is a positive integer.
[0034] In various aspects, the RF-based treatment system can include one or
more temperature
sensors arranged in a pattern to measure temperature of tissue during
treatment, wherein one or
more temperature sensors can be in communication with one or more of the
plurality of electrical
11
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
contacts. In some aspects, the RF-based treatment system can include an
adhesive layer disposed
on or near skin facing surface to temporarily attach the applicator to the
skin. In certain aspects,
the RF-based treatment system can include an upper housing portion disposed on
the foam,
wherein the upper housing portion includes an attachment member.
[0035] In many aspects, the RF-based treatment system can include the RF
transmission cable
with a cable length CL between the RF input port and the electrode array,
wherein CL ranges from
about 1 foot to about 40 feet. In some aspects, the RF-based treatment system
can include a first
control node disposed between and in electrical communication with the RF
input port and the
electrode array. In some aspects, the RF-based treatment system can include a
first control node
including a first controller, wherein the first controller generates control
signals to turn individual
electrodes in the electrode array "on" and "off.
[0036] In various aspects, the RF-based treatment system can include a
distance of Y between
output of first control node along RF transmission cable and connection point
of RF transmission
cable with first applicator. In some aspects, the RF-based treatment system
can include Y ranges
from about 0 to about 2 inches. In some aspects, the RF-based treatment system
can include Y
ranges from about 0 to about 6 inches. In various aspects, the RF-based
treatment system can
include Y ranges from about 0 to about 24 inches. In some aspects, the RF-
based treatment system
can include a first control node comprises a first controller, wherein the
first controller generates
control signals to measure individual current flow for one or more electrodes
of the electrode array.
[0037] In many aspects, the RF-based treatment system can include a foam
material and a rigid
support, the foam material sandwiched between the rigid support and the first
support. In various
aspects, the RF-based treatment system can include, as applicable, a
controller configured to
provide an RF signal to the electrode array through the RF cable, the RF
signal having a pulse
duration that selectively heats septae within fat tissue while substantially
avoiding conduction of
heat into adjacent tissue.
[0038] In various aspects, the RF-based treatment system can include an
impedance tracker
for monitoring a patient's tissue impedance during treatment and sensing
patient's tissue
impedance changes and relaying them to controller so that the controller can
terminate the RF
signal in response to occurrence of one or more events. In some aspects, the
RF-based treatment
system a first support that can be a rigid housing. In some aspects, the RF-
based treatment system
12
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
can include the array of K individually addressable electrodes which can be a
first array, and
further including a second array, wherein the first array can be arranged
relative to treatment
regions in a first zone. In various aspects, the RF-based treatment system can
include the second
array which can be arranged relative to treatment regions in a first zone,
wherein the first zone and
second zone can be separate parts of patient's body.
[0039] In various aspects, a method of controlling an RF-based treatment
system can include
connecting a plurality of control nodes, wherein the first control node is
master control of other
nodes, synchronizing second node and third node with first node, wherein first
node, second node
and third node are in electrical communication with a radiofrequency (RF)
transmission line; and
transmitting control signals from first node to second node using serial
communication protocol
during an active treatment session. In some aspects, the method can include an
active treatment
session which can be an impedance mapping performed using an electrode array
in communication
with the RF transmission line. Optionally, in some aspects, the method can
include phasing the
second node using one or more output signals from the first node. In some
aspects, the method
can include measuring current signals at one or more electrodes using the
third node. In various
aspects, the method can include activating and deactivating electrode using
the third node. In
many aspects, the method can include wherein the nodes are connected using a
plurality of
connections, wherein one of the connections is the first node connected to a
plurality of subnodes
of the second node. In some aspects, the method can include wherein the active
treatment session
further includes an impedance mapping performed using the electrode array in
communication
with one or both of a second electrode array and a drain pad.
[0040] In accordance with various aspects of the present teachings, a
radiofrequency (RF)-
based treatment system includes a flexible applicator that includes a
plurality of layers, the
plurality of layers that includes a first dielectric layer, a second
dielectric layer, and a conductive
layer, wherein the first dielectric layer and the second dielectric layer
sandwich the conductive
layer, the plurality of layers define a plurality of kerfs, and an inner
region and N regions extending
from the inner region, wherein the plurality of kerfs divide the applicator
into N regions.
[0041] In some aspects, N ranges from 2 to 12. Optionally, some aspects,
the plurality of
layers define one or more strain relief elements, wherein each strain relief
element is a circular or
elliptical hole in the plurality of layers. Optionally, in some aspects, one
or more of the plurality
13
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
of kerfs terminate at one or more strain relief elements, wherein the inner
region is adjacent the
one or more strain relief elements. Optionally, in some aspects, the inner
region is a kerf-free
region, wherein N is 6. Optionally, in some aspects, the plurality of N
regions include a first region
and a second region, wherein each of the first and second region define one or
more areas, borders,
or kerfs that are substantially the same.
[0042] In some aspects, the plurality of layers include a label, wherein
the label includes N
region identifiers, wherein each of the N region identifiers is disposed on
one of the N regions.
Optionally, in some aspects, the applicator defines an applicator shape,
wherein applicator shape
is selected from the group consisting of elliptical, circular, substantially
elliptical, substantially
circular, pear shaped, substantially pear shaped, submental, and combinations
thereof. Optionally,
in some aspects, the conductive layer includes a patterned region of copper
traces in each of the N
regions, wherein each of the patterned regions has one or more copper traces
in electrical
communication with copper traces arranged along the inner region. Optionally,
in some aspects,
the applicator further includes an electrical connector, the electrical
connector in electrical
communication with one or more addressable regions of conductive layer.
[0043] In some aspects, the system includes an RF treatment system
comprising an RF
generator, the RF generator having an operating frequency that ranges from
about 0.5 MHz to
about 10 MHz, wherein the RF generator is in electrical communication with the
electrical
connector. Optionally, in some aspects, the applicator further includes an
electrical connector, the
electrical connector in electrical communication with one or more addressable
regions of
conductive layer, the electrical connector comprising a plurality of
electrical contacts, wherein the
copper traces arranged along the inner region are in electrical communication
with the electrical
contacts. Optionally, in some aspects, the copper traces arranged along the
inner region are
arranged in a series of three or more adjacent sections that increase in width
in direction towards
the electrical connector. Optionally, in some aspects, the conductive layer
includes a continuous
copper sheet and wherein each of the N regions further includes a first region
of dielectric material
having a first thickness and a first area, and a second region of dielectric
material having a second
thickness and a second area, wherein area of each region is greater than first
area disposed therein,
wherein each first area is greater than each second area.
14
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[0044] In some aspects, the system includes an RF treatment system
comprising an interface
device in communication with the RF treatment system, the interface device
comprising a clamp
and a cable adapter, wherein the clamp opens and closes to releasably connect
and align with
electrical connector, wherein cable adapter is in electrical communication
with electric contacts of
clamp. Optionally, in some aspects, the area of electrode ranges from about 50
cm2 to about 600
cm2. Optionally, in some aspects, the system includes a heat shield layer,
wherein conductive layer
includes arrangements of electrical traces, wherein heat shield layer covers a
portion of inner
region below which adjacent electrical traces span the portion and change in
density along the
portion. Optionally, in some aspects, the system includes one or more
temperature sensors per
each of the N regions.
[0045] In some aspects, the system includes an RF treatment system in
electrical
communication with the applicator and each temperature sensor, further
comprising a control
system, wherein control system selectively addresses each of the N regions to
transmit RF energy
sequentially to facilitate uniform heating according to one or more patterns.
Optionally, in some
aspects, the system includes an RF treatment system in electrical
communication with the
applicator and each temperature sensor, further comprising a control system,
wherein control
system selectively bypasses one or more of the N regions in response to an
operator selection that
the one or more N regions is positioned above a sensitive tissue region.
Optionally, in some
aspects, the plurality of layers further includes one or more adhesive layers,
a polyamide layer,
and an aqueous gel layer.
[0046] In accordance with various aspects of the present teachings, a
method of treating tissue
using an RF-based applicator includes providing a flexible applicator
comprising an elongate inner
spine region and a plurality of regions extending therefrom, wherein each of
the plurality of regions
is bounded by a first kerf and a second kerf; and transmitting RF energy from
each of the plurality
of regions according to an alternating or sequential addressing schemes to
raise tissue below
applicator to a target temperature during an initial heating time period.
Optionally, in some
aspects, the method includes shielding the inner spine region to avoid
unwanted heating of target
tissue below inner spine region of applicator. Optionally, in some aspects,
the method includes
controlling transmission of RF energy such that one or more sensitive regions
below one or more
of the plurality of regions is not interrogated with RF energy. In one
embodiment, the method
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
includes controlling transmission of RF energy such that one or more sensitive
regions below one
or more of the plurality of regions is cosmetically treated to increase or
initiate lipolysis, skin
tightening, and cellulite reduction. The method may be performed over a
treatment time that
ranges from about 10 to about 15 minutes.
[0047] Although, the disclosure relates to different aspects and
embodiments, it is understood
that the different aspects and embodiments disclosed herein can be integrated,
combined, or used
together as a combination system, or in part, as separate components, devices,
and systems, as
appropriate. Thus, each embodiment disclosed herein can be incorporated in
each of the aspects
to varying degrees as appropriate for a given implementation. Further, the
various systems, probes,
control nodes, applicators, controllers, components and parts of the foregoing
can be used with
any suitable tissue surface, cosmetic applications, and medical applications
and other methods and
conjunction with other devices and systems without limitation.
[0048] These and other features of the applicant's teachings are set forth
herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0049] The skilled person in the art will understand that the drawings,
described below, are for
illustration purposes only. The drawings are not intended to limit the scope
of the applicant's
teachings in any way.
[0050] FIG. 1A schematically shows an exemplary system for providing RF
treatment of
various target regions of a patient's body in accordance with various aspects
of the present
teachings.
[0051] FIG. 1B schematically shows additional exemplary aspects of the
system of FIG. 1A
in accordance with various aspects of the present teachings.
[0052] FIG. 1C schematically shows an exemplary system for providing RF
treatment of a
target region of a patient's body utilizing an electrode tip in accordance
with various aspects of the
present teachings.
[0053] FIG. 1D schematically shows another exemplary system for providing
RF treatment of
a target region of a patient's body utilizing an electrode array in accordance
with various aspects
of the present teachings.
16
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[0054] FIG. 1E schematically shows another exemplary system for providing
RF treatment of
a target region of a patient's body utilizing two electrode arrays in
accordance with various aspects
of the present teachings.
[0055] FIG. 1F schematically shows another exemplary system for providing
RF treatment of
a target region of a patient's body utilizing one or more electrodes and a
drain paid in accordance
with various aspects of the present teachings.
[0056] FIG. 1G schematically shows an exemplary system for providing an RF
treatment that
includes various nodes that represent different connections and operative
components, such as
control nodes, of the system in accordance with various aspects of the present
teachings.
[0057] FIG. 1H schematically shows an exemplary arrangement of control
nodes suitable for
use with an RF-based system embodiment in accordance with various aspects of
the present
teachings.
[0058] FIG. 11 schematically shows an exemplary arrangement of nodes
suitable for use with
an RF-based system embodiment in accordance with various aspects of the
present teachings.
[0059] FIG. 1J schematically shows an exemplary arrangement of nodes
suitable for use with
an RF-based system embodiment in accordance with various aspects of the
present teachings.
[0060] FIG. 1K schematically shows an exemplary alternative arrangement of
nodes suitable
for use with an RF-based system embodiment in accordance with various aspects
of the present
teachings.
[0061] FIG. 2A schematically depicts an exemplary, disposable system for
providing RF
treatment of a target region of a patient's body in accordance with various
aspects of the present
teachings.
[0062] FIGS. 2B-D schematically depict various attachable electrode arrays
having different
shapes tailored to cover various regions of a patient's skin for targeted RF-
based treatment in
accordance with various aspects of the present teachings.
[0063] FIGS. 2E-G schematically depict various attachable electrode arrays
having the same
shape tailored to cover or tile various regions of a patient's skin for
targeted RF-based treatment
in accordance with various aspects of the present teachings.
17
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[0064] FIG. 2H schematically depicts a target tissue region that has been
tiled or covered with
multiple tissue attachable electrode arrays having the same shape as part of a
kit or set of
applicators in accordance with various aspects of the present teachings.
[0065] FIG. 3A schematically depicts an exemplary system for cooling a
flexible electrode
array and/or the patient's skin in accordance with various aspects of the
present teachings.
[0066] FIGS. 3B-F schematically depict various exemplary applicator
embodiments suitable
for treating tissue regions of various shapes in accordance with various
aspects of the present
teachings.
[0067] FIGS. 3G-H schematically depict various exemplary applicator
embodiments suitable
for adhering to a tissue surface when placed in contact therewith in
accordance with various aspects
of the present teachings.
[0068] FIGS. 3I-J schematically depict various exemplary separable
applicator embodiments
that include a disposable component and a reusable component in accordance
with various aspects
of the present teachings.
[0069] FIGS. 3K-3Q depict various images of exemplary separable applicator
embodiments
in accordance with various aspects of the present teachings.
[0070] FIGS. 3R-T schematically depict various views of an exemplary rigid
applicator
embodiment in accordance with various aspects of the present teachings.
[0071] FIGS. 3U depicts an electrode array facing image of an exemplary
rigid applicator
embodiment in accordance with various aspects of the present teachings.
[0072] FIG. 4A depicts an exemplary array of electrodes that can be
individually addressed
according to an exemplary method for monitoring and/or controlling the
distribution of RF energy
provided by the electrode arrays in accordance with various aspects of the
present teachings.
[0073] FIG. 4B schematically depicts an exemplary scan of a patient using
one or more RF-
based applicators to generate a tissue assessment or other outputs of interest
in accordance with
various aspects of the present teachings.
[0074] FIG. 4C depicts a patient undergoing RF-based tissue treatment with
regard to two
sections of the patient with multiple tissue regions being treated using RF
applicators contacting
the patient in each such section in accordance with various aspects of the
present teachings.
18
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[0075] FIG. 4D depicts a patient undergoing RF-based tissue treatment with
regard to two
sections of the patient with multiple tissue regions being treated using
multiple applicators
positioned relative to supports relative to each such section in accordance
with various aspects of
the present teachings.
[0076] FIGS. 5A-F schematically depict an exemplary treatment targeting
septae and
exemplary method for monitoring and/or controlling the distribution of RF
energy in accordance
with various aspects of the present teachings.
[0077] FIG. 6A depicts an exemplary plot of tissue temperature for a target
region including a
fat region of relatively uniform thickness during an exemplary treatment in
accordance with
various aspects of the present teachings.
[0078] FIG. 6B depicts an exemplary plot of tissue temperature for a target
region including a
fat region of relatively non-uniform thickness during an exemplary treatment
in accordance with
various aspects of the present teachings.
[0079] FIG. 6C schematically depicts treatment zone shift due to a fat
region exhibiting a
relatively non-uniform thickness during RF treatment.
[0080] FIG. 6D depicts an exemplary plot of tissue temperature due to a
treatment zone shift
of a fat region exhibiting a relatively non-uniform thickness during RF
treatment.
[0081] FIG. 6E depicts an exemplary plot of tissue temperature for a target
region and
correction of treatment zone shift during RF treatment of a fat region
exhibiting a relatively non-
uniform thickness in accordance with various aspects of the present teachings.
[0082] FIG. 7A depicts a plot of RF power and temperature of a target
region at a depth of 1.5
cm during an exemplary treatment in accordance with various aspects of the
present teachings.
[0083] FIG. 7B depicts a plot of tissue impedance during the exemplary
treatment of FIG. 7A,
while utilizing different cooling temperatures.
[0084] FIG. 7C depicts exemplary electronics for an applicator having an
electrode array in
accordance with various aspects of the present teachings.
[0085] FIG. 8 is a schematic perspective view of a system for treating
genitourinary conditions
according to various aspects of the present teachings;
[0086] FIG. 9 is schematic perspective view of a probe and an introducer
according to various
aspects of the present teachings;
19
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[0087] FIG. 10A is a schematic illustration of a female genitourinary
tract;
[0088] FIG. 10B is a schematic illustration of a female genitourinary tract
showing insertion
of a monitoring catheter into the urethra;
[0089] FIG. 10C is a schematic illustration of a female genitourinary tract
showing insertion
of a vaginal treatment probe in accordance with various aspects of the present
teachings;
[0090] FIG. 11 is a schematic illustration of a probe according to
exemplary aspects of the
present teachings for operating in two different modes;
[0091] FIG. 12 is a schematic illustration of a RF system including
exemplary electronics
according to various aspects of the present teachings;
[0092] FIG. 13 depicts an exemplary fractional, ablative treatment in
accordance with various
aspects of the present teachings;
[0093] FIG. 14A-C depict the results of exemplary fractional, ablative
treatments at different
pulse durations in accordance with various aspects of the present teachings;
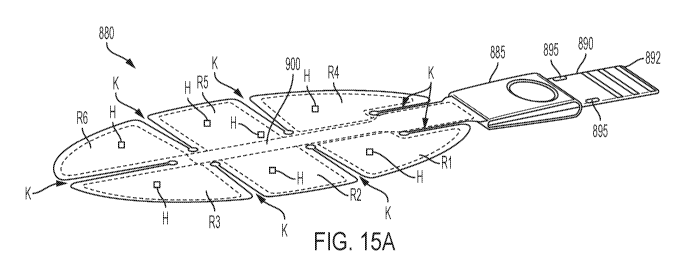
[0094] FIGS. 15A-15C schematically depict various exemplary flexible RF-
based applicator
embodiments suitable for treating tissue in accordance with various aspects of
the present
teachings;
[0095] FIG. 16 schematically depicts an interface device suitable for
quickly connecting and
releasing an applicator according to various aspects of the present teachings;
[0096] FIG. 17 schematically depicts a flexible RF-based applicator
embodiment that includes
dielectric material of varying thicknesses in different regions suitable for
treating tissue in
accordance with various aspects of the present teachings;
[0097] FIG. 18 schematically depicts various layers of a thin flexible RF-
based applicator
embodiment in accordance with various aspects of the present teachings;
[0098] FIG. 19A schematically depicts an exploded view of various layers
and components of
a flexible RF-based applicator embodiment in accordance with various aspects
of the present
teachings;
[0099] FIGS. 19B-19C schematically depict top perspective views of the thin
Flexible RF-
based applicator embodiment of FIG. 19A in accordance with various aspects of
the present
teachings;
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00100] FIG. 20A schematically depicts a top view of an RF-based flexible
applicator in
accordance with various aspects of the present teachings;
[00101] FIG. 20B schematically depicts a bottom view an RF-based flexible
applicator in
accordance with various aspects of the present teachings;
[00102] FIG. 20C schematically depicts a view of one side of an RF-based
flexible applicator
in accordance with various aspects of the present teachings;
[00103] FIG. 20D schematically depicts a view of another side of an RF-based
flexible
applicator in accordance with various aspects of the present teachings;
[00104] FIG. 20E schematically depicts a rear view of an RF-based flexible
applicator in
accordance with various aspects of the present teachings;
[00105] FIG. 20F schematically depicts a front view of an RF-based flexible
applicator in
accordance with various aspects of the present teachings;
[00106] FIGS. 21A-21F schematically depict the views of the flexible
applicator of FIGS. 20A-
20F, without a releasable liner in accordance with various aspects of the
present teachings;
[00107] FIGS. 22A-22B schematically depict components of a flexible RF-based
applicator
showing regions with electrical traces of one or more conductive layers in
accordance with various
aspects of the present teachings;
[00108] FIGS. 23A-23B schematically depict a top and a bottom view,
respectively of a flexible
applicator prior to coupling with an interface device in accordance with
various aspects of the
present teachings;
[00109] FIGS. 23C-23D schematically depict an interface device in and open and
closed
configuration in accordance with various aspects of the present teachings;
[00110] FIG. 24A schematically depicts a top view of an interface device in
accordance with
various aspects of the present teachings;
[00111] FIG. 24B schematically depicts a top side view of one side an
interface device in
accordance with various aspects of the present teachings;
[00112] FIG. 24C schematically depicts a bottom view of an interface device in
accordance
with various aspects of the present teachings;
[00113] FIG. 24D schematically depicts a top side view of another side of an
interface device
in accordance with various aspects of the present teachings
21
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00114] FIG. 24E schematically depicts a front view of an interface device in
accordance with
various aspects of the present teachings;
[00115] FIG. 24F schematically depicts a rear view of an interface device in
accordance with
various aspects of the present teachings;
[00116] FIGS. 25A-25C schematically depict various distribution arrangements
suitable for use
with the flexible RF-based applicators and other applicators disclosed herein
in accordance with
various aspects of the present teachings;
[00117] FIG. 26A schematically depicts a top view of an RF-based flexible
applicator for
submental treatment, in accordance with various aspects of the present
teachings;
[00118] FIG. 26B schematically depicts a bottom view of an RF-based flexible
applicator for
submental treatment, in accordance with various aspects of the present
teachings;
[00119] FIG. 26C schematically depicts a view of one side of an RF-based
flexible applicator
for submental treatment, in accordance with various aspects of the present
teachings;
[00120] FIG. 26D schematically depicts a view of another side of an RF-based
flexible
applicator in accordance with various aspects of the present teachings;
[00121] FIG. 26E schematically depicts a rear view of an RF-based flexible
applicator in
accordance with various aspects of the present teachings;
[00122] FIG. 26F schematically depicts a front view of an RF-based flexible
applicator in
accordance with various aspects of the present teachings;
[00123] FIG. 27A schematically depicts an exploded view of various layers and
components of
a flexible RF-based applicator embodiment for treating the submental region,
in accordance with
various aspects of the present teachings;
[00124] FIGS. 27B-27C schematically depict top perspective views of the
Flexible RF-based
applicator embodiment of FIG. 27A in accordance with various aspects of the
present teachings;
and
[00125] FIGS. 28A and 28B depicts a graphical user interface (GUI) for use
with a treatment
system using an applicator showing different configurations according to
various aspects of the
present teachings.
22
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
DETAILED DESCRIPTION
[00126] It will be appreciated that for clarity, the following discussion
will explicate various
aspects of embodiments of the applicant's teachings, while omitting certain
specific details
wherever convenient or appropriate to do so. For example, discussion of like
or analogous features
in alternative embodiments may be somewhat abbreviated. Well-known ideas or
concepts may
also for brevity not be discussed in any great detail. The skilled person will
recognize that some
embodiments of the applicant's teachings may not require certain of the
specifically described
details in every implementation, which are set forth herein only to provide a
thorough
understanding of the embodiments. Similarly, it will be apparent that the
described embodiments
may be susceptible to alteration or variation according to common general
knowledge without
departing from the scope of the disclosure. The following detailed description
of embodiments is
not to be regarded as limiting the scope of the applicant's teachings in any
manner.
[00127] The terms "about" and "substantially identical" as used herein,
refer to variations in a
numerical quantity that can occur, for example, through measuring or handling
procedures in the
real world; through inadvertent error in these procedures; through
differences/faults in the
manufacture of electrical elements; through electrical losses; as well as
variations that would be
recognized by one skilled in the art as being equivalent so long as such
variations do not encompass
known values practiced by the prior art. Typically, the term "about" means
greater or lesser than
the value or range of values stated by 1/10 of the stated value, e.g., 10%.
For instance, applying
a voltage of about +3V DC to an element can mean a voltage between +2.7V DC
and +3.3V DC.
Likewise, wherein values are said to be "substantially identical," the values
may differ by up to
5%. Whether or not modified by the term "about" or "substantially" identical,
quantitative values
recited in the claims include equivalents to the recited values, e.g.,
variations in the numerical
quantity of such values that can occur, but would be recognized to be
equivalents by a person
skilled in the art.
[00128] As discussed in detail below, systems and methods utilizing RF energy
to treat a patient's
skin (e.g., dermis and hypodermis), the surface of a patient's mucosal tissue
(e.g., surface of
vaginal tissue or surface of esophageal tissue), or other target tissue
including tissue at a depth
below a tissue surface (e.g., skin surface, mucosal surfaces of the vagina or
esophagus) are
23
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
provided and can generally comprise one or more sources of RF energy (e.g., a
RF generator), a
treatment applicator comprising one or more electrode arrays configured to be
disposed in contact
with a tissue surface, and a return electrode (e.g., a neutral pad) coupled to
the tissue surface. The
electrodes may include zones or regions of an applicator that include a
plurality of electrical traces
or patterned / gradient containing regions of materials of differing
dielectric constants / properties
and other combinations and configurations of electrical elements as disclosed
herein. The systems
and methods disclosed herein for delivering RF energy to one or more target
regions can be used
in one or more lumens or cavities of a patient without limitation.
[00129] In general, the methods and systems disclosed herein may be used to
provide various
non-medical treatments such as cosmetic treatments, aesthetic treatments, and
combinations
thereof. Skin tightening, such as by improving skin laxity, and body sculpting
(for example, via
hyperthermic treatment and via lipolysis) are examples of cosmetic and/or
aesthetic treatments that
may be implemented using various RF-based systems and methods such as those
discussed in more
detail below.
[00130] In various aspects, the systems and methods can treat unwanted fat
(e.g., via lipolysis),
improve skin laxity/tightness (e.g., through the stimulation of collagen),
improve the appearance
of cellulite (e.g., by breaking septae), and various genitourinary conditions
through the application
of RF energy (e.g., about 500kHz, about 0.5 MHz, about 1MHz, less than about 1
MHz, greater
than about 1 MHz, about 1.5 MHz, about 2 MHz, about 2.5 MHz, about 3 MHz,
about 3.5 MH,
about 4 MHz, about 4.5 MHz , and 5.5 MHz , or other frequencies include
frequencies ranging
from about 0.5 MHz to about 10 MHz) delivered to the surface of the patient's
tissue (e.g., skin,
vaginal wall, esophagus) via treatment electrode or electrode array, the
treatment electrode or
electrode array is optionally water-cooled, the RF energy propagating from the
surface into deeper
tissue layers and returning to the RF generator via a return electrode (e.g.,
a large surface area
neutral pad) coupled to the tissue surface at a location distant from the
treatment electrode or
electrode array. In accordance with various aspects of the present teachings,
systems and methods
are provided for utilizing RF energy to heat a relatively large area of target
tissue (e.g., greater
than about 24 cm2, greater than about 50 cm2, or greater than about 200 cm2 by
applying (e.g.,
placing, fixing) an applicator to the skin, energizing the device (e.g.,
activating the RF generator),
while cooling the superficial layers and selectively controlling the
deposition of RF energy so as
24
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
to heat the tissue below the surface. In accordance with various aspects of
the present teachings,
the deposition of the RF energy and/or the cooling of the tissue can be
provided such that the tissue
below the surface is heated substantially uniformly. It will be appreciated in
light of the present
teachings that heating uniformity can be required to help provide for safety,
patient tolerance, and
uniform clinical results.
[00131] With reference now to FIGS. 1A and 1B, an exemplary system 100 in
accordance with
various aspects of the present teachings is schematically depicted. As shown,
the system 100
generally includes a console 110 and one or more applicators 130a-d comprising
one or more
electrically-conductive electrodes (e.g., comprised of metal) that are
configured to be disposed in
electrical contact with the patient's tissue (e.g., adjacent a region to be
treated) for applying the
RF energy to the tissue surface, and a return electrode (e.g., a neutral/drain
pad 130e as in FIG. 1A
or an active electrode array 160 as in FIG. 1B). The console 110 can have a
variety of
configurations and can include a display 132 (e.g., enabling reporting and/or
control of various
treatment parameters) and a housing 134 containing one or more RF energy
generators 135,136, a
temperature-controlled water circulator 138 (e.g., including a chiller and/or
a heater), and a power
supply 139 (e.g., a low voltage power supply), all by way of non-limiting
example. The system
100 also comprises a controller 137 (e.g., including a CPU or microprocessor)
for controlling the
operation of the RF energy generators 135, 136, the application of RF energy
to particular
electrodes 162, and/or the water temperature regulator/circulator 138 in
accordance with the
teachings herein. As shown, the console 110 can include a plurality of ports
(e.g., CH1-4) for
electrical and fluid connection of the applicators 130a-d as well as an
additional port for electrical
connection to the drain pad return 130e. As discussed in detail below, for
example, each applicator
130a-d can include cooling water attachments and electrical connections to
support serial
communications between the console 110 and the applicators 130a-d, each
applicator is connected
to the console 110 via its own cable or umbilical 133.
[00132] As discussed in more detail herein with regard to FIG. 1G, the length
of the umbilical
may also be referred to as length X. Each of the ranges recited herein may
apply to the length X
shown in FIG. 1G between control Node 1 and Node 2. In one embodiment, the
length of the
umbilical can range from about 10 feet to about 20 feet. In one embodiment,
the length of the
umbilical can range from about 1 foot to about 10 feet. In one embodiment, the
length of the
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
umbilical can range from about 2 feet to about 8 feet. In one embodiment, the
length of the
umbilical can range from about 20 feet to about 50 feet. In one embodiment,
the length of the
umbilical is greater than about 20 feet.
[00133] The one or more RF generators 135, 136 are generally configured to
produce energy
that is delivered to the applicator(s) 130a-d via one or more transmission
lines extending through
an umbilical 133 for application to the tissue (e.g., as modified by
distribution electronics within
the applicators 130a-d) and can be any known or hereafter-developed source of
RF energy
modified in accordance with the present teachings. Exemplary commercially-
available RF sources
suitable for use to be modified in accordance with the present teachings
include the ForceTriadTm
Energy Platform, marketed by Covidien. In some aspects, a plurality of RF
energy generators can
be provided, with each configured to generate RF energy of different
characteristics from one
another such that one or more of the generators can be utilized alone or in
combination depending
on the desired treatment. As shown in FIG. 1A, the system 100 includes two
generators, one
labeled 135 can generate RF energy of a maximum power of 300W at 1 MHz (and
can be operated
at 100% duty) and the other labeled 136 can generate RF energy of a maximum
power of 1 kW at
1 MHz (and can be operated at 20% duty), by way of non-limiting example.
[00134] It will be appreciated by a person skilled in the art in light of
the present teachings that
the various parameters of the RF energy (maximum power, frequency, duty cycle,
pulse duration,
etc.) can be selected depending on the desired treatment and the treatment
area, as discussed
otherwise herein. By way of example, it will be appreciated that one or more
of the plurality of
RF generators 135, 136 can be modulated to provide various powers including,
for example, 300
W of RF energy that is provided to an applicator (e.g., 130a of FIG. 1B) and a
return electrode
(e.g., 130b if FIG. 1B) configured to cover ¨200cm2 (-100 cm2 x 2) or about
1.5 W/cm2 per
applicator, with each applicators 130a and 130b each providing about 1.5
W/cm2.
[00135] Other suitable RF energy generators can be employed as discussed
otherwise herein,
for example, suitable RF energy generators can provide a wattage range of from
about 0.5 W/cm2
to about 5 W/cm2, by way of non-limiting example. In various aspects, suitable
duty cycles can
vary depending on the targeted tissue type, however, in some exemplary tissue
heating applications
the objective can be to deliver an amount of RF energy so as to cause a
temperature rise, while
maintaining the treatment time as short as possible. Thus, as the duty cycle
decreases, the RF
26
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
energy can be increased to compensate for the reduced amount of "on time" so
as not to extend
the total treatment time. An exemplary duty cycle for heating skin and fat is
from about 30% to
about 80%, for example an about 50% RF duty cycle would be on for 5 seconds
and then off for 5
seconds. Duty cycles can be modulated at varying frequencies that range from
microseconds to
seconds, because in some applications a faster modulation cycle can enable
more precise control
whereas in other application a longer modulation cycle may be desirable. The
duty cycle may also
be adjusted to optimize energy deposition in differing tissue layers or types:
anatomical areas
where large volume, deep, and highly perfused tissue target areas (e.g., fat)
can allow for a
relatively longer duty cycle (e.g., an 80% duty cycle as opposed to a 30% duty
cycle), whereas
shallower, smaller, and poorly perfused tissue (e.g., skin), the tissue can
require a relatively shorter
duty cycle (e.g., a 30% duty cycle is preferred over a 80% duty cycle).
Applications other than
bulk heating that rely on tissue impedance to select the targeted tissue can
benefit strongly from
very short duty cycles, even <1% duty cycle. Such short duty cycles can also
be characterized as
or referred to as pulsed RF.
[00136] As shown in FIGS. 1A and 1B, the exemplary system 100 can include a
plurality of
applicators 130a-d, representing a variously-adaptable, stand-alone system to
heat and/or cool
tissue safely and effectively. In various aspects, reducing and or maintaining
the temperature of
the surface of the patient's skin tissue, for example, by flowing water
adjacent to a relatively rigid
applicator (e.g., applicators 130a and 130b) or a flexible applicator (e.g.,
applicator 130c applied
by adhesive to the skin), can be important in maintaining patient safety and
comfort. As shown
schematically, each applicator 130a-c can comprise a relatively rigid or
flexible applicator body,
distribution electronics, a water bladder or reservoir, an electrode array,
and an adhesive for
helping secure the applicator(s) 130a-c to the patient's skin, all by way of
non-limiting example.
In some additional or alternative aspects, vacuum can be used to help secure
the applicator(s) to
the skin. As discussed in detail below, the applicators 130a-c can have a
variety of configurations
but are generally configured to be coupled to the patient's tissue surface
such that the RF energy
delivered to the applicator 130a-c can be applied to the patient's tissue
through one or more
electrodes disposed in contact with the tissue surface. The applicator(s) 130a-
c can also have a
variety of configurations. Additional exemplary applicator configurations are
described in further
detail herein.
27
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00137] In the exemplary system 100 of FIGS. 1A and 1B, for example,
applicators 130a-b can
be substantially identical to one another, with one of the electrode arrays
serving as the treatment
electrode array and the other completing the circuit as the return electrode.
In various aspects, the
system 100 can be operated in a monopolar mode such that a circuit is formed
by a source electrode
162a of an electrode array 160a from one applicator (e.g., 130a of FIG. 1B)
with a return electrode
162b of another electrode array 160b from the other applicator (e.g., 130b of
FIG. 1B).
Additionally or alternatively, in some aspects, a large area drain pad 130e
(also referred to herein
as a "return electrode") can be attached to the tissue surface at a location
distant from the treatment
applicators 130a-d to disperse and/or return the RF energy applied to the
patient's tissue from one
or more of the "active" applicators 130a-d, as best shown in FIG. 1A. As
discussed otherwise
herein, as the tissue reaches the clinical endpoint for some electrode arrays,
it is possible that the
other arrays will not have delivered a full dose due to anatomical
differences. In such cases, a
power drain to an ancillary return electrode 130e can be used to boost the
relative temperature of
the lagging site. In some alternative aspects, bipolar operation could be
achieved by activating
electrodes within a single applicator array (e.g., array 160a of applicator
130a).
[00138] As shown in FIG. 1A, and discussed otherwise herein, applicator 130c
can also include
an electrode array and can be relatively rigid but have a shape configured to
suit a particular body
area. By way of non-limiting example, applicator 130c can provide an electrode
array disposed
within a concavity that can be configured to receive a patient's submental
region such that contact
is substantially maintained between the skin surface and the electrode surface
when coupled to the
patient's submental area. Alternatively, the applicator 130c can be relatively
flexible such that it
can be conformed to a curved tissue surface (e.g., the submental area, jowls,
neck, and abdomen).
As shown in FIG. 1A, and discussed otherwise herein, an applicator
handpiece130d having one or
more electrodes can be provided that can be operated in a stamping mode. By
way of example,
applicator 130d can be held against a tissue surface of a particular treatment
region while one or
more RF pulses are applied to the tissue surface. In some aspects, the
applicator 130d can be
configured to provide one or more short-duration, high power RF pulses that
can utilize one or
more of impedance mapping, impedance tracking, and temperature monitoring as
otherwise
discussed herein. After treatment of one particular region is performed, the
handpiece applicator
28
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
130d can be moved to another location. It will also be appreciated in light of
the present teachings
that more than two applicators can be used to cover larger areas.
[00139] With reference now to FIGS. 1C-1F, the electrode(s) of other exemplary
applicators
will now be described with reference to an electrosurgery unit (ESU) system
100 having a console
110 known in the art and modified in accordance with the present teachings. As
shown in FIG.
1C, for example, the ESU 100 can be configured to concentrate the RF power and
subsequent
tissue heating at an electrode tip 162d (e.g., comprising a single, small area
electrode) of an
applicator 130d (e.g., configured to be held against the patient's tissue
surface and operate in
stamping mode), while a relatively large area drain pad 130e (e.g., the return
electrode can have a
surface area up to about 5000x the surface area of the delivery tip). In such
a manner, non-
uniformities in the return path can be still sufficiently safe to avoid burns
due to the adequate
distribution and/or dispersion of the RF power.
[00140] Referring now to FIG. 1D, in some alternative aspects the ESU 100 can
instead include
an applicator 130a having an electrode array 160a (e.g., comprising a
plurality of individually-
addressable electrodes 162a) for distributing the power uniformly over a large
area, with the drain
pad 130e representing the return path. As with FIG. 1C, the surface area of
the return electrode
130e relative to the treatment electrode array 160a can help ensure that the
RF energy is sufficiently
distributed to avoid non-desired damage. However, unlike the return pad 130e
shown in FIG. 1C,
the return pad 130e in FIG. 1D is similar in surface area to the electrode
array 130a such that
benefits of large area uniformity in the return pad 130e can diminish. That
is, a return pad having
a larger surface area than the electrode array can generally help avoid
undesirable side effects in
the return pad (e.g., hot spots). With a large area treatment goal with an
electrode array, shown in
FIG. 1D, the size requirement of the return pad may be impractical and not
possible to size to
connect to a non-treated part of the body (e.g., too large to connect to a non-
treated part of the
body).
[00141] Additionally, as discussed in detail below, various mechanisms in
accordance with the
present teachings can be utilized to reduce "hot spots" on the active
treatment electrode and ensure
a more uniform treatment. For example, as discussed in detail below,
distribution electronics of
the applicator(s) 130a can be utilized to provide the same or different RF
signals to the individual
29
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
electrodes 162a of the electrode array 160a so as to provide improved control
of the treatment
procedure.
[00142] As shown in the FIG. 1E, in some aspects, the system 100 can instead
utilize two
electrode arrays disposed on different applicators: a first applicator 130a
having a delivery
treatment electrode array 160a and a second applicator 130b having a return
electrode array 160b
that also functions to delivery treatment energy via an electrode array. In
such aspects, the return
electrode array 160b can mirror the treatment electrode array 160a, likewise
providing treatment
energy, and can help achieve good uniformity for both skin contact areas that
contact the first and
second applicators, 130a and 130b. In some aspects, both treatment pads
measure about ¨100cm2
and each can deliver RF energy so as to provide uniform deep heating, while a
third electrode is
capable of draining power from a site if the two treatment sites in contact
with the first and second
applicators 130a, 130b heat differentially, for example, due to perfusion (as
noted above with
respect to FIG. 1A). RF energy, current, signals or energy 159a can flow
between the applicators
/ electrodes as shown.
[00143] As shown in the FIG. 1F, in some aspects, the system 100 can also
utilize an applicator
130j having an electrode array 160a and another applicator 130k having an
electrode 160b that is
used with a drain pad 130e or other drain device. The applicators 130j, 130k
may attach via an
umbilical 133. The applicators 130j, 130k and drain paid 130e can be used
during an active
treatment session to perform impedance mapping performed using an electrode
array (130k or
130j) in communication with one or both of a second electrode array (130j or
130k) and a drain
pad 130e. RF energy, current, signals or energy 159b can flow between an
applicator 130k and
the drain paid 130e as shown.
[00144] Optionally, in some exemplary aspects, the applicator(s) 130a-d can
include one or
more coupling features (e.g., clips) that allow the applicator to clip into a
frame, the frame being
attached to a belt or the like which would encircle or attach the frame (and
the applicator attached
thereto) to the patient surface so as to provide a hands free connection of
the device to the patient
for the clinician. In another embodiment, the applicator(s) 130a-d can attach
directly to the skin
surface via, for example, adhesive, gel, and/or mild suction.
[00145] Though the applicators of FIGS. 1D-F are generally shown as comprising
generally
planar arrays of electrodes (e.g., rigid or flexible arrays of electrodes) or
individual electrodes in
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
accordance with the embodiments disclosed herein, in some alternative aspects
the applicator can
be configured for insertion into an internal tissue site so as to provide for
the application of RF
energy to a mucosal tissues surface or to reach a depth below a mucosal
surface (e.g., vaginal wall,
esophageal lining). For example, as discussed in detail below with reference
to FIGS. 8-12, the
applicator can comprise a generally tubular probe that can be sized and shaped
to be inserted into
the vagina or esophagus for RF treatment thereof. As will be understood by a
person skilled in the
art in light of the discussion herein, the probe can comprise a plurality of
electrodes (or groups of
electrodes) that can be activated to apply RF energy to the target tissue in
monopolar, bipolar, or
hybrid mode. Additional examples of applicators suitable for applying and
directing RF energy
are also discussed herein and depicted in FIGS. 15A-15C, 17, 19A-21F, 22A,
22B, 23A, 23B,
25A-25C, 26A-26F and 27A-27C.
Operation Mode
[00146] The teachings herein include a variety of electrical configurations,
namely, monopolar,
bipolar, and a hybrid thereof. The monopolar configuration includes an active
electrode (or
electrode array) and an inactive electrode (e.g., a drain pad). The bipolar
configuration includes
two separate, active electrodes (or two separate, active electrode arrays).
The hybrid configuration
includes two separate, active electrodes (or two separate, active electrode
arrays) and an inactive
electrode (e.g., a drain pad). The exemplary electrical configurations shown
in FIGS. 1C and 1D
are monopolar and the electrical configuration shown in FIG. 1E is bipolar.
The electrode
configuration shown in FIG. 1A is hybrid. It will be appreciated that where
only the pulsed
handpiece 130d and the drain pad 130e as shown in FIG. 1 are used, such a
configuration would
be monopolar. On the other hand, using and activating only the electrodes of
the electrode arrays
on the two applicators 130a and 130b would be a bipolar configuration. Still
another subset of the
options shown in FIG. 1A utilizing the two applicators 130a, 130b and the
drain pad 130e would
be a hybrid configuration.
[00147] FIG. 1G is a schematic diagram showing a representation of RF-based
tissue treatment
system 170A according to the disclosure in which various operative and/or
connecting control
nodes are shown. Specifically, Node 0, Node 1, Node 2, and Node 3 are shown in
sequence. As
an introduction to specific roles for a given node type, Node 0 can serve as
the master control node
in some embodiments. Node 1 can control and direct the transmission of various
DC power signals
31
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
and other signals discussed in more detail herein. Further, Node 2 can control
individual activation
and deactivation of electrodes and other functions as described herein. Node 3
is used to designate
the electrode array which receives on or more signals, power, and other
parameters from one or
more of the nodes to which it connects. The nodes can be referred to as
control nodes or nodes
interchangeably. In one embodiment, the nodes have a hierarchical architecture
with Node 0
sending control signals and related signals to the other nodes to control
their operation. The nodes
can be considered as reference points or labels that correspond to various
electronic components
or other components or subassemblies of a given RF-based tissue treatment
embodiment.
Temperature measurements can be made using sensors disposed in the applicator
in the vicinity of
Node 3. Specifically, in one embodiment the temperature measurements are made
using signals
from such sensors and the temperature measurements are obtained at Node 1 or
Node 2.
[00148] Further, each of these nodes (Node 0 - Node 3) can also serve as
categories or grouping
such that each of these four nodes has other nodes grouped with it. For
example, in FIG. 1H, Node
0 can have a plurality of other Node 0's. As shown, Node 0 can have nodes 0 to
n, with node k
being labeled as an example node. In turn, node k, as Node 0 level node, is in
communication with
several Node 1 type nodes labeled as 1.1, 1.2, 1.3 - in. A group of nodes
within one of the higher-
level node categories (Node 0, Node 1, Node 2, and Node 3) can be referenced
as a sub-node in
various embodiments. n is used as an index to identify the number of nodes for
a particular node
type. The set of nodes of type Node 0 has a cardinality of n. In some
embodiments, the number
of nodes per Node type can be greater or less than n. For example, there can
be one Node 0 and
twenty nodes that are of the Node 1, 2, or 3 type. In general, the number of
nodes per node type
can be any positive integer greater than or equal to 1.
[00149] Referring back to FIG. 1G, each node may have nodes within or
connected to another
node. Starting with Node 0, this node corresponds to a RF driver platform for
a given RF treatment
system embodiment. Node 2 is responsible for turning on/off individual
electrodes in the
applicator and taking local measurement of individual current flowing out of
each individual
electrode. The RF driver platform (Node 0) includes one or more components
that provide other
nodes or sub-nodes with individual commands. The RF driver platform (Node 0)
can be
implemented with one or more logical devices, FPGAs, circuits, circuit
elements or combinations
thereof. The nodes can be connected using one or more electrical connections
171, which can
32
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
include one or more cables, buses, or other electrical signal connection and
transmission
mechanism.
[00150] Node 0 includes one or more devices that serve as a master controller
or "brain" such
all of the drivers, controls, signals, parameters, including phase, frequency,
period, and amplitude
values for drive or control signals that are directed to the other nodes and
sub-nodes to which Node
0 connects are regulated and controlled in a consistent manner thereby. Thus,
if Node 0 specifies
a specific phase and amplitude for a signal that will drive a set of
electrodes of an array of
addressable electrodes at nodes of type Node 3, those outputs and other
signals may be clocked
and controlled by Node 0, in whole or in part.
[00151] Still referring to FIG. 1G, in one embodiment, Node 0 can include one
or more control
or timing components 173. The control or timing components 173 can include a
system clock, a
clock generator, or other device for clocking and synchronizing the nodes to
which Node 0
connects such a Node 1, Node 2, and Node 3. The control or timing components
173 can include
one or more of a controller, a feedback loop, a waveform generator, and one or
more filters. Node
0 can include or connect to a DC power supply, an AC power supply, connections
thereto or
combinations thereof. The RF driver platform (Node 0) may include a master
logic device
disposed in a system component, such as console 110 or other consoles
described herein. A coolant
flow path 172 extending from Node 0 is also shown consistent with embodiments
having Node 0
within the console 110. In general, the coolant flow path 172, also shown as a
dotted line, relative
to electrical connection between Node 0, Node 1, and Node 2 shown as a solid
line, can start from
any of the nodes or connect to applicator from a different source.
[00152] Node 0 controls the timing for all of the other nodes in the system
such as Node 1,
Node 2, and Node 3 as well as sub-nodes within each of these node types or
categories. This can
be performed using the one or more timing components 173. As a result, Node 0
can facilitate
serial communication over an active RF treatment line with the other
downstream nodes such as
the type 1, type 2, and type 3 Nodes. In turn, this nodal configuration alone
or together with the
use of serial communication signals facilitates phase control of the various
nodes and sub-nodes
that are in electrical communication with Node 0. In one embodiment, the
system drives the serial
communication length synchronously with the fundamental RF frequency. Given
that phase angle
can be time varying for various waveforms, the timing control afforded by Node
0 and the timing
33
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
components supports specifying a given phase on a per signal basis. In turn,
this facilitates each
addressable electrode, such as those shown in Node 3 to be phase tuned or
adjusted. Further, such
an implementation of timing and phase control using Node 0 supports any
phasing scenario with
regard to Node 1 and other nodes.
[00153] The RF-based treatment systems described and depicted herein including
those
described with regard to FIGS 1H-K including Node 0, and any sub-nodes
thereof, are designed
to phase Node 1 and any sub-nodes of the Node 1 category. Node 0 may output
one or more
signals with a set phase or phase relationship. In one embodiment, Node 1 is
disposed in, near, or
about the RF generator.
[00154] In various embodiments, Node 1 controls one or more (or all) of the DC
voltage; the
RF Drive signal, and the relative phasing of the RF drive signal. In addition,
in various
embodiments, Node 1 specifies frequency and pulse form (Sine, triangle, sq.
wave, chirp,
sawtooth, etc.) of RF drive signal and other signal parameters.
[00155] There could be any of a number of these nodes in the generator (e.g.,
one per applicator,
multiple nodes per applicator, or multiple applicators per node). These Node 1
nodes (or sub-
nodes) are labeled 1.1. 1.2, 1.3...1.n, etc. as shown in FIGS. 1H-K. As was
the case with Node 0,
and the other nodes described and depicted herein, Node 1 can serve as
category or node type for
all nodes that are part of or performing RF generator operations or functions.
The use of sub-
nodes is only to convey the idea that the sub-nodes are one type of node, but
each sub-node is a
node in its own right which can connect to other nodes. The hierarchy of nodes
is without limit
and any given node of a particular function, electrode connections, applicator
connections, etc. can
define another node category to group nodes with similar functions and/or
connections. Thus,
nodes can map to one or more applicators and vice versa, without limitation.
[00156] As noted herein, Node 0 can facilitate serial communication over an
active RF
treatment line with the other control nodes or other components to which it is
connected. In
addition, differential serial communication between Node 1 and Node 2 can be
implemented using
twisted pair conductors as shown in the node-based system 170C depicted in
FIG. 1J. As shown,
exemplary node 1.1 of Node 1 connects to Node 2 through one or more twisted
pair conductors.
In turn, each Node 2 connects to one node in Node 3 as shown. A limited number
of twisted pairs
(e.g., from 1 to 20 twisted pairs) delivering RF power and control signals to
the distribution
34
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
electronics (Node 2) allows for no practical restriction on the length of the
electrical connections
171 between Node 1 and 2 and being twisted improves noise immunity thereby
reducing the
likelihood of contaminating control signal integrity.
[00157] In addition, having the distribution electronics (Node 2) remote from
the platform
(Node 0), and close to the applicator's electrodes (Node3), allows for the
improved fidelity in
controlling and reading the individual electrodes. Long lead lengths from the
distribution
electronics to the individual electrodes will blur the control of individual
electrodes by fostering
cross-talk between individual channels and reducing the performance of the
mapping and
treatment capabilities of this architecture. In this way, controlling the
length of the lead improves
mapping and treatment performance.
[00158] In various embodiments, Node 1 has one or more outputs. This can be
seen in the node
configuration 170B shown In FIG. 11. The outputs of Node 1 include one or more
of the main RF
drive signals to power electrode for treatment; communication signals or data
between node 1 and
node 2; and the DC power to power node 2. In one embodiment, Node 1 provides
patient
isolation/electrical safety to prevent any dangerous currents from getting to
the patient. In one
embodiment, Node 1 is configured to prevent the transmission of any harmful
alternating current.
In one embodiment, the nodal connections shown in FIG. 11 are configure for a
typical treatment
regimen using RF energy.
[00159] Each individual Node 1 sub-node (1.1, 1.2, 1.3, ... 1.m) can be
phased individually
such that one or more or all output signals from Node 1 are set with all of
the same phase, differing
phases, or phase groupings among node subsets prior to such signals being
transmitted to Node 2.
As shown in FIG. 11, each node of the Node 1 type, nodes 1.1, 1.2, 1.3, 1.4,
... 1.m. each connect
to a Node 2 type node on a 1 for 1 basis. In turn, each node in the Node 2
group of nodes (node
1.1.1 .... 1.n.1) branches to multiple node 3 type nodes.
[00160] Specifically, as shown in FIG. 11, each node in the Node 2 grouping
connects or maps
to n nodes in the Node 3 grouping. Accordingly, the node configuration 170B
has each Node 0
connecting to n nodes at Node 1, with each of the n nodes at Node 1 connecting
to a single node
at Node 2, and then finally all of the n nodes at Node 2 each connect to n
nodes at Node 3. This
hierarchical mapping has a network topology of the form 1 to all n, each n to
one n, and each n to
all n moving from left to right from Node 0 to Node 3.
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00161] Node 2 is responsible for turning on/off individual electrodes in the
applicator. In one
embodiment, Node 2 is also responsible for taking local measurement of
individual current flowing
out of each individual electrode. In contrast with FIG. 11, FIG. 1K shows an
alternative configure
of nodes 170D in which each Node 0 connects to n nodes of the Node 1 type. In
turn, all n of the
Node 1 nodes connects to a single node at Node 2, shown as node 1.1.1. The
only Node 2 node,
node 1.1.1, connects to all of then nodes at Node 3. This configuration is of
the form 1 to all n;
all n to only 1 n, and only in to all n.
[00162] Based on the arrangement of node connections for node-based control
system 170D in
FIG. 1K, such local measurements cannot be performed at Node 1, but instead
are measured at
Node 2. This follows because Node 2 has each node at the Node 2 level
connecting to a set of
electrode groupings in the applicator such that all of the Node 2 nodes
interface with all of the
electrode nodes (Node 3) at the applicator. The ability of Node 2 nodes to be
used to measure
individual currents facilitates impedance mapping and individual addressing of
electrodes in the
electrode array.
[00163] The logical elements, such as FPGA's, ASICs, circuits, and
combinations thereof are
selected and arranged such that Node 1 can individually set all of the phase
values for signals being
output from Node 1 to other nodes such as Node 2 and Node 3, and sub-nodes of
each. This is
advantageous because it broadens the range of treatment options and RF
profiles that can be
achieved at the electrode array of Node 3. Specifically, as Node 1 can be
selectively phased in
different configurations based on the nodes selected, the phase of each node,
and the timing of
initiating phasing or waveform propagation from or to each node.
[00164] Various configurations for phasing Node 1 are possible. These may,
include, without
limitation, the following exemplary phasing sequences:
= Phase nodes all in one phase as in: 1.1, 1.2, 1.3, ... .1.n.
= Phase the nodes individually as in: Phase node 1.1 then Phase node 1.7
then Phase node
1.2 then Phase node 1.16 then Phase node 1.1 then Phase node 1.2 then Phase
node 1.3 then
Phase node 1.n.
= Phase the nodes in clusters as in: Phase nodes 1.1-1.6 then phase nodes
1.7-1.12 then phase
nodes 1.13-1.18, then phase nodes 1.1-1.6, etc.
36
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00165] The foregoing are just examples, and any combination or all of the sub-
nodes of Node
1 or subsets thereof can be phased in clusters, individually, collectively, in
different sequences or
orders of phasing, or combinations thereof. This flexibility to phase all
together, individually, in
clusters, etc. enables one to adapt treatment to contours of the patient's
tissue being treated and
enables one to adapt the treatment to the user placement. In general, although
the exemplary
control nodes are labeled or referenced by Node 0, Node 1, Node 2, and Node 3,
each of the
foregoing can be referenced as a first node, a second node, a third node ,or
fourth node without
limitation.
Cable/Umbilical
[00166] As described herein with regard to FIG. 1A, the various conformable,
rigid, semi-rigid,
hybrid, disposable, re-usable, partially re-usable, and other applicators of
RF energy described
herein connect to a console such as console 110. The electrical connections to
the applicator from
a given console embodiment and one or more of the various control nodes
electrical connections
can be routed through an umbilical housing to safe guard the signal
transmitting conductors. In
one embodiment, one or more section of such an umbilical can house a RF
transmission cable,
sections thereof, and tubes or flow paths for circulating coolant to a given
applicator.
[00167] In one embodiment, the lengths of the RF transmission cable, or
sections thereof or
lengths of conductor segments between the various control nodes, or subsets or
combinations of
the foregoing lengths should be selected to support operation of the RF-based
systems described
herein. In one embodiment, the disclosure relates to various operating lengths
for various electrical
conductor lengths used in the system that support its operation. In some
embodiments, if these
lengths are exceeded excessive noise, cross talk, or other deleterious effects
can prevent the RF-
based treatment systems disclosed herein from operating or can prevent the RF-
based treatment
systems from operating with the desired efficiency and effectiveness. Some
examples of these
length parameters can be seen by referring to FIG. 1G.
[00168] As shown in FIG. 1G, the distance between Node 1 and Node 2 is
identified as length
X. This length X may correspond to the length of an umbilical from a given
console embodiment.
Various suitable X length distances are discussed herein with regard to FIG.
1A. In addition, the
distance between Node 2 and Node 3 is identified as length Y. Length Y can
include the length
of one or more conductors such as multiple conductors used in an RF-
transmission cable. Length
37
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
Y can be less than the flow path of coolant from Node 2 through the
applicator. Length Y can be
measured from the output of Node 2 to the input of the applicator. In
addition, length Y can include
traces or wires that extend into the applicator and terminate at a given
electrode. Length Y can
also include the average distance between the various conductors within the
applicator to each
electrode and the entry point for such conductors into the applicator added to
the length of such
conductors between the output of Node 2 and the input of Node 3. The input of
Node 3 can include
the opening or channel at which conductors from Node 2 enter the applicator.
In general, the
distance or length Y is the distance beyond Node 2 from the output of node 2
to the start of the
applicator.
[00169] For the overall system to work well with all of the desired capacity,
the distance
selection of length or distance Y is important for successful operation of a
given applicator-based
RF treatment system. The selection of the length Y maintains Node 2's
electrode activation and
deactivation functions, and Node 2's current measurement functionality, and
thus its contribution
to impedance mapping and other derived functions. As part of the design effort
and the node-
based conductor configurations, it has been determined that in one embodiment
Y ranges from
about 0 inches to about 2 inches. This specification of the Y distances
supports a system
configuration that avoids wires or other conductors and uses a printed circuit
board with
conductive traces to improve impedance mapping and reduce cross coupling below
a threshold
level. In one embodiment, the range of from about 0 to about 2 inches includes
the tracings on
the PCB. In yet another embodiment, the distance Y ranges from about 0 to
about 6 inches. In
still another embodiment, the distances Y ranges from about 0 to about 1 foot.
Alternatively, the
distance Y can range from about 1 foot to 2 feet. In still other embodiments,
Y is less than about
3 feet.
[00170] With the foregoing discussion, of exemplary system and the description
of various
electrode configurations and arrangements, it is informative to consider
additional details relating
to various treatment parameters and other features of the disclosure. As will
be appreciated by a
person skilled in the art in light of the present teachings, exemplary systems
can provide the
following benefits and/or include some or all of the following features:
38
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
Treatment Temperatures and Cooling of Patient Skin
[00171] In various aspects, it can be important to enforce uniformity of
delivered RF energy so
as to safely raise the target tissue to a desired temperature. In particular,
to provide an efficacious
treatment, it can be important to raise target tissues to an intended
temperature range but also
maintain the tissue in the targeted region at that elevated target temperature
for a given duration.
That is, "time at temperature" can be important to confer the desired clinical
benefit. For example,
temperatures may range between about 39 C to about 47 C, or between about 39
C to about 44
C, or between about 42 C to about 47 C within the fat layer, with from about
41 C to about 42
C providing a typical tissue temperature for treating tissue within the fat
layer or within other
similar tissues at a depth. In some aspects, the temperature ranges from about
41 C to about 42
C can be used to preferentially stimulate collagen development. Higher
temperatures up to about
46-47 C can be used to target tissues with more damage, thus providing a more
aggressive
treatment, e.g., in deeper tissue layers. However, the range of 46-47 C may
not be able to be
tolerated directly on the skin surface due to the uncomfortable sensation of
the relatively high
temperature felt by the patient. In some aspects, treatment temperatures of
tissue beneath the
mucosa may be able to tolerate higher temperatures, up to about 70 C, or from
about 40 C to
about 60 C. Treatment time at temperature could range from about 5 minutes to
about 25 minutes
and may vary with, for example, depth or volume of targeted tissue. As such,
it can be important
that the RF energy is actively controlled as otherwise discussed herein to
distribute through
targeted tissues in the targeted treatment zone in a substantially homogenous
fashion, substantially
uniformly, predictably and automatically (without user intervention). In some
embodiments, the
tissue surface temperature (e.g., skin surface and/or mucosal tissue surface)
may be controlled to
be held at a range from about 15 C to about 40 C, or from about 25 C to
about 40 C during the
treatment of the tissue at a depth. Higher temperature ranges at a depth
(e.g., from about 46-47
C) may be tolerated and thereby realized during treatment due to temperature
control ranging
from about 15 C to about 40 C, or from about 25 C to about 40 C at the
skin surface.
[00172] Cooling of patient skin surface can protect the epidermis and also
improve patient
comfort. Adequate surface cooling (e.g., cooling water at a temperature from
about 10 C to about
40 C, or at a temperature from about 25 C to about 40 C or about 25 C to
about 35 C) can
allow the application of higher RF powers safely and comfortably than could be
applied in the
39
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
absence of such cooling. This can be important as most target tissues are
located at some depth
from the surface such that surface cooling acts to protect the intervening
tissue layers which are
not targeted.
[00173] As discussed below, the electrode array can have a variety of
configurations, though in
some exemplary aspects, the electrode array can be attached to an applicator
comprising a metal
coolant housing (e.g., bonded or adhered via an adhesive). An electrical
insulating and thermal
conducting layer (Kapton or ceramic, A102 or the like) can be located between
the cooling housing
(e.g., containing a reservoir or bladder of temperature-controlled cooling
water) and the electrode
array such that the cooling water cools the electrode array and the patient's
skin surface in
accordance with various aspects of the present teachings. As noted above, the
cooling water can
be circulated from the console 110 of FIGS. 1A and 1B via one or more pumps
through one or
more fluid conduits (e.g., via one or more umbilical 133 to the respective
applicator connected
thereto), with the chiller/heater 138 being configured to detect and/or
maintain the temperature of
the cooling water as desired.
RF Pulse duration in view of target tissue selected for treatment
[00174] A variety of treatment regimens can be provided in accordance with
various aspects of
the present teachings. In various aspects, both long duration (e.g., greater
than 1 second, CW),
low power RF energy (e.g., from about 1 W/cm2 to about 5 W/cm2) and short
duration (e.g., less
than 500 ms, or less than 100 ms), high energy RF pulses (e.g., from about 10
to about 1000 J/cm2
per pulse, 10 J/cm2-500 J/cm2, 10 J/cm2-300J/cm2, 10 J/cm2-100 J/cm2) regimens
are envisioned
and can provide different benefits depending on the biological target
selection and biological target
treatment. Without being bound by any particular theory, the method of action
can be thermal in
nature where delivered RF power acts to primarily or preferentially heat (or
even coagulate)
selected tissues. Thermal diffusion or conduction to adjacent tissues is also
envisioned as a
treatment regimen. More precisely, because different tissues have different
electrical impedances
and RF energy tends to propagate through anatomical structures or tissues
exhibiting the lowest
impedance, connective tissues (e.g., fibrous septae tissue that interpenetrate
fat layers) can
represent a relatively-low impedance preferential path through which the RF
will be conducted.
As such, heat will tend to accumulate in the relatively low-impedance RF
conduction path. For
example, the connective fibers of the septae tissue would begin to heat
relative to adjacent tissue.
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
As low impedance tissues accumulate heat (e.g., exhibit a temperature rise),
they also begin to
thermally conduct to nearby adjacent tissues such as fat, for example, which
has a relatively higher
electrical impedance compared to the connective fibers (e.g., the septae
tissue). In light of the
present teachings, it will be appreciated that the pulse duration of the
applied RF can therefore
provide a method to select anatomical target tissues as discussed in detail
below.
[00175] Short duration, high power RF pulses can act to heat or even coagulate
low impedance
tissue (e.g., connective fibers of the septae tissue), whereas long duration,
low power RF energy
tends to heat the low impedance tissues at a sufficiently slow rate that heat
is conducted into
adjacent high electrical impedance tissues (e.g., fat). For example, by
applying RF energy in short
duration, high power pulses, fibrotic structures can be rapidly heated without
being able to conduct
heat away into adjacent higher resistance tissues (e.g., fat) sufficiently
fast enough to dissipate the
rapid buildup of heat within the fibrotic tissue. Short pulse duration, high
magnitude RF power
can thus deposit a temperature increase in tissues having a low electrical
impedance within the
treatment region (beneath the applicator). Short duration (e.g., from about 10
ms to about 500 ms,
preferably <100 ms) and high magnitude RF pulse energies (e.g., from about 10
to 1000 J/cm2)
can be used to selectively treat low impedance tissues such as septae or other
fibrotic structures
within the patient's tissues. Since the preponderance of electrical current
will flow through fibrotic
structures located for example in more resistive, higher impedance fat layers,
rapid delivery of
such short duration RF treatment pulses acts to preferentially accumulate a
temperature rise in the
fibrous connective tissue structures such as septae. Given the short duration
of the RF pulse, the
rapidly heated fibrotic structures are unable to conduct heat away into
adjacent higher resistance
tissues (e.g., fat) fast enough to counter the rapid buildup in fibrotic
tissue temperature rise. This
pulse duration effect can thus act to "select" the fibrous tissues or septae
for treatment, by
accumulation of temperature rise, whereas the surrounding tissues remain
relatively cool. This
method can be useful for selectively heating fibrotic structures such as
septae (a main component
causing the cottage cheese or dimpled appearance of cellulite). This approach
can be useful for
coagulation of fibrotic structures such as septae in the tissue. While the
example of septae and
surrounding fat is used, the ability to target or "select" tissues of distinct
electrical impedances can
be applied to many other tissue types or layers.
41
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00176] On the other hand, relatively long duration, low power RF energy can
be preferred to
heat (more or less uniformly) tissue layers exhibiting differing electrical
impedances. That is,
longer pulse durations or even CW (continuous RF emission) can be used to
treat all tissue types
within the treatment area because the low impedance connective/fibrotic tissue
or septae is heated
slowly enough to allow the heat to transfer via thermal diffusion and/or
conduction into the
surrounding relatively higher impedance tissues. The result can thus be more
or less bulk heating
of all tissue within the treatment area (e.g., beneath the electrode array
applicator). Thus, long
pulse duration or CW emission (from about 1 second to continuous (CW)) with
relatively lower
magnitude RF power (e.g., from about 1 to about 5 W/cm2) can be used to
homogenously treat a
block or zone of tissue regardless of tissue components and their differing
electrical impedances
of the tissues within the zone. Long pulse duration, low magnitude RF power
tends to generate a
temperature increase in all tissues in the target region through thermal
conduction, regardless of
electrical impedance. Because fat cells have a lower damage tolerance
(elevated temperature
tolerance) compared to the connective fibers, the fat cells can therefore be
lysed while the
connective tissue remains largely undamaged. The present teachings thus
provide, for example, a
method to perform lipolysis by providing low magnitude, long pulse duration
(or CW) heating of
connective fibers (septae) which then heat the adjacent fat cells. It will
further be appreciated in
light of the present teachings that pulse durations can be fine-tuned to
optimize temperature
accumulation in desired target tissues, while protecting surrounding tissues
from exposure to
excessive temperature rise.
Electrode Array
[00177] In various aspects, a large electrode face (e.g., an electrode pad)
can be broken into a
mosaic of smaller electrodes (e.g., an array of multiple individual
electrodes). An electrode array
can have a variety of configurations but is generally configured such that the
plurality of the
electrodes comprising the array can be placed in electrical contact with the
tissue so as to provide
RF energy thereto. The individual electrodes that comprise the electrode array
can exhibit a variety
of numbers of electrodes and have a variety of shapes, sizes, and layouts
(e.g., pitch). Suitable
individual electrodes can each have a diameter that ranges from about 3 mm to
about 100 mm,
from about 10 mm to about 70 mm, from about 10 mm to about 30 mm, by way of
non-limiting
example. In one embodiment, for example, each individual electrode of a given
electrode array
42
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
can measure approximately 1 cm in diameter. In some aspects, a group of
electrodes in an
electrode array or multiple electrode arrays can be arranged in a pattern
covering from about 1 cm2
to about 500 cm2. The electrode array(s) can form a shape, for example,
hexagonal, rectangular,
circular, elliptical, rhombus, trapezoid, or other shape suited to target
particular tissue areas for
treatment. The number of individual electrodes in a single electrode array can
also vary. In some
aspects, for example, there can be from about 2 to about 100 individual
electrodes in an electrode
array, while in another embodiment there can be from about 6 to about 20
individual electrodes in
an electrode array. In one non-limiting example, 19 individual electrodes are
arranged in a
hexagonal pattern covering about 20 cm2 of surface area. A larger area of
tissue may be treated
by providing several applicators or groups of electrodes (e.g., several
electrode arrays) that cover
a desired surface area of tissue.
Individually switched electrode array
[00178] As will be appreciated in light of the present teachings,
substantially uniform deposition
of energy can be achieved by breaking the large electrode face into a
plurality of smaller electrodes,
where each electrode within the array can be addressed and activated
individually. In order to
achieve uniform deposition of energy, one or more individual electrodes within
the array can be
individually addressed and activated based on tissue feedback including
temperature and/or
impedance feedback, for example, as discussed further below. In some aspects,
for example, only
one electrode (or a subset of the electrode array) may be activated based on
the tissue feedback to
help provide substantially uniform heating of tissue. In other aspects,
individually controlling the
electrodes can help ensure or control that the heated zone remains centered
within the desired
treatment zone location (e.g., beneath the electrode array applicator) as well
as to maintain
substantial homogeneity and consistency of the temperature rise within the
desired treatment area
despite variations in the patient's underlying tissue electrical impedance or
despite nearby or
adjacent anatomical structures.
[00179] By way of example, distribution electronics of the applicator(s) 130a-
d of the system
100 of FIGS. 1A and 1B can be utilized to provide the same or different RF
signals to the individual
electrodes of the electrode array(s) 160 so as to provide improved control of
the treatment
procedure, for example, by adjusting one or more of power, RF frequency, pulse
width, and/or
duty cycle. In such aspects, each of the individual electrodes in the
electrode array in contact with
43
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
the patient can be independently addressed (e.g., switched to gate the RF
power or duty cycle
applied thereto), with each individual "channel" capable of also providing
current, voltage, and/or
phase angle feedback information useful for calculating power and impedance of
individual
electrodes. In some aspects, the independently-switched electrodes in the
array can be switched
(e.g., via controller 137) to gate RF power simultaneously to each of the
individual electrodes in
the array, or alternatively, the independently-switched patient contact
electrodes in the array may
be switched to gate RF power sequentially first to one of the electrodes in
the array and next to
another electrode in the array until all or substantially all of the
electrodes in the array are addressed
(e.g., during impedance mapping discussed below).
[00180] In some aspects, an individually-controlled RF electrode array can be
employed to
disrupt connective tissues that interpenetrate fat layers (e.g., fibrous
septae tissue present in
cellulite through septae disruption). In such exemplary aspects, the electrode
array can be placed
over the tissue region to be treated, with the septae beneath the electrode
array being targeted for
treatment by individually addressing one of the electrodes (or a subset of the
electrodes) in the
array of multiple electrodes with short duration (e.g., less than 100 ms),
high energy pulse(s) (e.g.,
from about 10 to about 1000 Fcm2). After the short pulse or series of pulses
is completed by the
first electrode (or subset of electrodes), another electrode or subset of
electrodes in the array can
be addressed with a short pulse or series of pulses, with the process being
repeated until multiple
electrodes or all electrodes in the array have been addressed with a short
duration, high power RF
pulse so as to target all of the tissue region below the array. Optionally,
the individual electrodes
are addressed sequentially with short pulses of high power RF energy. In one
embodiment, all or
substantially all of the RF energy available to the entire electrode array is
gated to an individual
electrode so that, due to the relatively low impedance of the septae tissue,
the septae tissue is
preferentially heated by the relatively short pulse. Alternatively, a greater
power supply is
employed that enables the desired and/or required high magnitude energies
(e.g., from about 10 to
about 1000 Fem2) to be gated to an individual electrode to thereby
preferentially target septae
tissue.
[00181] In another embodiment, an individually-controlled RF electrode
array can be
employed to disrupt connective tissues that interpenetrate fat layers as well
as to provide for
treatments of laxity (and/or lipolysis). By way of example, a RF electrode
array can first be
44
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
employed using relatively short pulses of high magnitude power as discussed
above to disrupt
(e.g., break) the septae in the tissue region below the array. That is, after
each short pulse is
completed by an electrode (or subset of electrodes), another electrode or
subset of electrodes in
the array can be addressed with a short pulse, with the process being repeated
to target all of the
tissue region below the array. Thereafter, the same electrode array can be
used to heat the same
tissue region as a whole (including septae tissue and other tissue in the
region including fat, dermis,
hypodermis, the dermal/hypodermal junction) by utilizing relatively long, low
power RF pulses
(e.g., from about 1 to about 5 W/cm2) to provide for relatively bulk heating,
for example, for a
lipolysis and/or laxity treatment. For example, after the septae tissue has
been targeted with the
short pulse, high power RF treatment, the RF electrode array can be used to
treat same tissue region
for laxity by simultaneously addressing all or substantially all of the
electrodes with a relatively
long pulse or series of long pulses (e.g., from about 1 second to continuous
(CW)) and for an
exposure time ranging from about 5 minutes to about 35 minutes, or from about
10 minutes to
about 30 minutes, or for about 25 minutes to maintain the target tissue within
the treatment
temperature range. It will also be appreciated that in some aspects, the
thermal treatment of the
tissue region via long pulses of multiple and/or all RF electrodes in the
array can occur first, with
the targeted treatment of the septae occurring thereafter via a sequential
application of a short pulse
by one (or possibly a few) of the electrodes in the array of multiple
electrodes.
Flexible electrode arrays
[00182] In accordance with various aspects of the present teachings, flexible
electrode arrays
are envisioned wherein the electrode array allows for an improved connection
to curved surfaces
or contours of a patient's body. In such aspects, the applicator array can
include a plurality of
electrodes (e.g., individually-controlled electrodes), with the individual
electrode units each
exhibiting an active area of about 1 cm2, for example, and comprising a thin
metallic surface
integrated on a flexible substrate. In some aspects, the individual electrodes
can also be flexible
(e.g., capable of bending) due to the limited thickness of the electrode's
conductive material (e.g.,
a metal). Alternatively, the electrode can comprise, for example, a woven
metal (e.g., copper)
cloth that itself exhibits flexibility so as to conform to the contours of the
tissue surface. An
electrode array can thus be comprised of rows and columns of the rigid or
flexible electrode units
disposed on a flexible substrate that is scaled so as to provide an applicator
with an area ranging
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
from l's to 100's of cm2. Such flexibility allows treatment uniformity to be
achieved on both a
small and large scale. Custom shaped array patterns are also envisioned such
that any shape
suitable for a given treatment area may be employed, for example, a boomerang
shape, a rectangle
shape, or a trapezoid shape may be useful for sub-mental or chin treatments.
It will be appreciated
in light of the present teachings that many variations in shapes and sizes are
possible. Various
electrode arrays suitable for conforming to and attaching to a tissue surface
having various non-
standard shapes and sets of standard shapes are shown in FIGS. 2B-D and FIGS.
2E-F,
respectively.
Disposable Applicators
[00183] In some aspects, the applicator (e.g., applicator 130a of FIG. 1A)
or a portion thereof
can be provided as a disposable. By way of example, the skin contacting
portion of the applicator
containing the treatment electrodes and a portion of the cooling conduits can
be configured to
couple to a non-disposable umbilical side (which couples the applicator to the
console) containing
relatively more-expensive distribution electronics that can be removably
coupled (e.g., via pins)
to the electrodes in the disposable portion of the applicator. The umbilical
side can also contain
one or more fluid conduits for delivering fluid to the disposable portion of
the applicator (e.g., via
one or more fluid coupling elements). In various aspects, an adhesive gel can
be applied to the
face of the applicator that is covered by a protective sheet. The sheet can be
removed (e.g., torn
off) and the applicator applied to the skin. Optionally, the adhesive gel pad
can be discarded after
one or more treatments, while the remainder of the applicator can be reused.
Alternatively, in
some aspects, the entire applicator can be disposable. In such aspects, the
relatively expensive
fittings and circuitry can be relegated to the umbilical side such that the
cost of the disposable
applicator can be minimized.
[00184] With reference now to FIG. 2A, a portion of another exemplary system
for RF
treatment in accordance with these and other aspects of the present teachings
is shown
schematically. FIG. 2A depicts a cross section of the skin including dermis,
hypodermis (mostly
fat), and muscle layers with an exemplary RF applicator 230 adhered to the
skin's surface. As
discussed otherwise herein, coolant from the console (e.g., console 110 of
FIGS. 1A and 1B having
a temperature-controlled water circulator 138) flows through cooling lines in
the umbilical 233
and the flowing coolant can maintain the surface temperature of the skin while
RF energy is applied
46
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
to an array 260 of electrodes 262 to heat the skin. The ratio of cooling to
heating can regulate the
skin's surface temperature and can be used to adjust the distribution of heat
in the skin so as to
enable selection of a target treatment zone (e.g., treatment depth).
Generally, less cooling for the
same RF power tends to shift the heated zone toward the skin surface (e.g., to
heat the dermis for
tightening and increasing the thickness of the skin). If cooling is increased,
the heated zone will
tend to push down to lower tissue layers. As discussed below, short duration
pulses of RF energy
in combination with cooling will tend to preserve the skin (e.g., prevent bulk
tissue heating), while
preferentially heating those tissue of lowest impedance (e.g., the septae). In
this manner,
regulation of the skin's surface temperature can be used to adjust the
distribution of thermal energy
in the skin.
[00185] In various aspects, the disposable applicator 230 can also be
flexible as discussed
above, and may include a sticky adhesive on the patient facing side of the
electrodes such that the
flexible pad sticks to the patient surface. In certain aspects, contact with
the patient's skin surface
can be made through an adhesive gel. Though in some aspects the gel layer can
be thermally
conductive in order to enable skin cooling, the gel layer need not be
electrically conductive,
because most of the power coupling can be capacitive due to the high RF
frequencies used. As
shown in FIG. 2A, for example, a disposable portion of the flexible applicator
230 can include an
adhesive gel pad 263 that can be disposed between the electrodes 262 to which
the RF signal is
applied and the tissue surface. Additionally, a bladder 264 through which
heated or cooled water
can be flowed can be provided such that the coupling of the disposable portion
(i.e., below the
broken line) to the umbilical side of the applicator allows for a fluid
pathway. As discussed below,
the bladder 264 can be flexible such that the applicator 230 generally adopts
the contours of the
tissue surface when applied (e.g., adhered thereto). Also shown interspersed
within the applicator
230 are electrodes 262 each of which can in some aspects by individually-
addressed via leads, for
example, that can electrically couple to pins of the distribution electronics
provided on the
umbilical side of the applicator 230.
[00186] In certain aspects, these electrodes and the area around them can be
ideally cooled,
though it will be appreciated that cooling only a fraction of the applicator
area can nonetheless be
effective for certain applications. Also shown is that there can be different
amounts of energy that
are applied to the different electrodes depending on the impedance underneath;
where there is
47
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
thicker fat and higher impedance, more energy would be deposited accordingly.
The exemplary
connector concept shown in FIG. 2A is intended to describe at least one non-
limiting disposable
concept, where the expensive components used to accurately distribute RF and
monitor the
electrodes are on the reusable side and a multi-trace array connector and
water lines are formed as
the disposable portion (including the relatively low cost flexible
electrodes).
Electrode and Applicator Geometry and Surface Coverage
[00187] Various geometric shapes can be used for the skin / tissue contacting
or skin/ tissue
facing region of a given electrode array. FIGS. 2B-C show a representation of
a patient 211 that
is a candidate for one or more RF-based cosmetic, medical, or other tissue
surface treatments. The
patient may have treatment regions 275 for which an applicator that includes
an electrode array
has not yet been applied as well as other regions for which various non-
standard shaped tissue
surface contacting applicator-based electrode arrays have been applied such
the half circle
applicators 277a and the elongate flared applicators 277b having slightly
chamfered or rounded
corners. Two half pear shaped applicators 277c can also be used to cover the
stomach or other
treatment regions of interest for a given patient. Complementary applicators
277b, 277e can also
be used to approximate the edges of other specialized non-standard
applicators. The
complementary applicators can be side-piece applicators that are positioned on
either side of larger
primary applicator. Various complementary applicator embodiments 277b, 277e
are shown. As
shown in FIG. 2C, complementary applicators 277e interface closely with the
two half pear-shaped
applicators 277c to cover the majority of the stomach region of patient 211.
[00188] Other non-standard applicator shapes can be used, without limitation,
such as a rounded
wedged shaped applicator 277f and complementary applicators 277e that include
curved
boundaries that mimic the curves of the rounded wedge-shaped applicator 277f.
In one
embodiment, a primary nonstandard electrode is packaged with or otherwise
provided with other
non-standard complimentary applicators that track one or more edges or
boundaries of the primary
nonstandard applicator in order to cover or tile the treatment surface
efficiently with a minimal
amount of uncovered tissue in a given treatment area.
[00189] In general, gaps between electrode arrays of applicators can result in
irregular
treatments and unwanted boundary effects such as ridges or other anomalies
that follow from
uneven lipolysis or other variations in tissue response when gaps are present
between electrode
48
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
arrays. In one embodiment, the primary applicators are sized to cover a larger
surface area relative
to the smaller complementary applicators. In one embodiment, the surface area
covered by a given
primary applicator ranges from about 100 cm2 to about 300 cm2. In one
embodiment, the surface
area covered by a given primary applicator ranges from about 150 cm2 to about
250 cm2. In one
embodiment, the surface area covered by a given complementary applicator or
side piece
applicator ranges from about 50 cm2 to about 100 cm2. In one embodiment, the
surface area
covered by a given complementary applicator or side piece applicator ranges
from about 70 cm2
to about 120 cm2. The foregoing ranges can also be used to specify the area of
a set of standard
applicators wherein each applicator in the set has the same shape.
[00190] FIGS. 2E-G show a representation of a patient 211 that is a candidate
for one or more
RF-based cosmetic, medical, or other tissue surface treatments and various
standard conformable
applicators 280 in position or suitable or placing on an uncovered treatment
region 275. In contrast
with the non-standard applicators described with regard to FIGS. 2B-D, the use
of standard
applicators, such as the rectangular applicators 280 shown in FIGS. 2E-2G has
the advantage of
being able to tile or cover a tissue treatment region without having to use
specialized shapes that
have a higher manufacturing cost. The use of standard applications allows such
applicators to be
sold in a kit or other grouping. These applicators typically include a gel pad
for adhering the
electrode array of the applicator a tissue surface. These applicators
effectively fill the space of a
tissue surface and reduce the number of gaps. Regular polygons, fractal
shapes, pairs of
complementary shapes, and other repeating patterns can be used to specify kits
of standard
applicators that efficiently cover the surface area required for a given
treatment regimen.
[00191] FIG. 2H is a schematic representation of a target region 285 of a
tissue surface of a
patient. For various tissue types or as otherwise constrained by patient
specific parameters and the
desired outcome for a particular treatment regimen, an electrode array that
may be affixed to the
skin surface may often by desirable. These electrodes can be positioned and
remain in place during
treatment. These conformal electrodes are also the type used in the
embodiments described above
relative to FIGs. 2B-G. A specific example of a standard applicator shape
having electrode array
is described and depicted herein with regard to FIG. 2H. The applicators 280a
may be attached to
the tissue surface using various materials having suitable adhesion
properties. Further, the
adhesive material also is tailored to release from the skin in response to
manual manipulation.
49
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00192] In order to affect an RF-based treatment of the target region 285
various electrodes
array geometries can be used that are space filing or otherwise amenable to
efficiently tiling a
surface such that gaps between applicators are reduced. Various specialized
electrode geometries
were introduced with regard to FIGS. 2B-D, although such specific geometric
designs are suitable
for particular procedures and use cases, manufacturing and cost per unit
factors can come into play
when a given electrode is a one-time-use device.
[00193] As shown the target region 285 has an irregular boundary. A custom
electrode for this
boundary would be expensive to make and would likely have limited
applicability to the general
population of candidates for RF treatment. Accordingly, in one aspect, the
disclosure relates to
kits or sets of attachable electrode arrays that include a set of electrode
arrays that may be
positioned and aligned to efficiently fill a two-dimensional region using K
shapes per a given kit.
K can range from 1 shape to about 20 shapes. In other embodiments, K can range
from about 1 to
about 10 shapes. In still other embodiments, K can range from about 1 to about
5 shapes. K can
also be a positive integer greater than or equal to 1 and less than 50. The
shapes are selected so
that they are all the same shape in one embodiment, such as shown by the
square electrodes 280a
having a dotted border. The square shapes of the electrodes 280a effectively
tile the area that
defines treatment region 285. Pairs of shapes such hexagons and pentagons and
other similar
groupings can be used to cover an area while reducing gaps between applicator
edges.
[00194] As noted above, a flexible electrode can be supplied with a cooling
water flow path
which thermally conducts through an electrically insulating layer to the
backside (not patient-
connected side) of the electrodes such that the cooling water controls the
patient's skin surface
temperature during the treatment. For example, FIG. 3A depicts an exemplary
flexible cooling
bladder layer 305a for a flexible applicator that is configured to bend over a
compound curve such
as the submental area or a flank. A multi-layer adhesive pad design can thus
comprise electrodes
made of thin plated copper foil or fine plated copper fabric (e.g., die-cut to
shape) and embedded
in the adhesive laminate. The flexible cooling water manifold / bladder layer
305a depicted in
FIG. 3A can include the top layer of the disposable pad, with the manifold
using two layers of
polymer sheet (e.g., die cut and thermally bonded in a labyrinth pattern at
various locations 310)
so as to define one or more fluid flow paths 312 therebetween. In various
aspects, the electrodes
can be cooled directly with the water rather than relying on conduction
through the flexible
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
substrate. Parallelograms, squares, rectangles, trapezoids, regular polygons,
and other shapes can
be used to provide kits that include one or more applicators sized and shape
to efficiently cover or
tile a tissue surface.
[00195] Electrode layers that may be used in association with the flexible
cooling bladder layer
305a of FIG. 3A can be any electrode array as otherwise discussed herein
including in association
with a rigid, semi-rigid, conformable, hybrid, or flexible electrode array as
described above, for
example, with reference to the system of FIGS. 1A-1F and FIG. 2A and the
applicators of FIGS.
3A-F and 3I-3X. Various cooling bladder embodiments can be integrated with
different applicator
designs. For a given applicator design, the bladder may be sandwiched between
one or more layers
or other assemblies. Various exemplary alternative cooling bladder embodiments
are described
and depicted in more detail. In some embodiments, the cooling bladders are
part of an applicator
that includes a disposable portion and a re-usable portion. In turn, each
disposable portion and re-
usable portion can include various components and subassemblies.
[00196] FIG. 3B shows an applicator 320A that may be implemented as a held in
place
applicator using a fastener or it can be used with adhesive tabs or strips and
be adhered to a given
patient as a conformal applicator. Applicator 320A is a hybrid applicator that
can be combined
with attachment devices or adhesives such as the gel pads disclosed herein. In
addition to the
mechanism of affixing it relative to a target tissue region, applicator 320A
is also a hybrid
applicator in the sense that it may include disposable components and re-
usable components.
When adhesive is not used to attach applicator 320A to a tissue surface,
various mechanical
attachment devices can be used such as belts, clamps, straps, and other
devices.
[00197] In one embodiment, a belt 317 is used in conjunction with an
applicator coupler 319
that includes a slot or other mechanism to receive the belt. The applicator
coupler 319 is shown
as including two slots to receive and slidably affix to the belt 317. This is
but one possible
configuration of the belt 317 and the applicator coupler 319. The belt and
coupler can be of various
shapes and configurations so long as they facilitate securing the applicator
320A to the patient for
a given treatment session. The applicator coupler 319 includes a bottom
surface having a
deformable cylindrical shell having slits 318 spaced along at sides. The slits
facilitate expansion
of the cylindrical shell and gripping of one or more raised structures 340d,
340e on applicator such
that the belt can be selectively secured to remove from the applicator 320a.
51
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00198] In one embodiment, the applicator 320A includes a plurality of
electrodes (not shown)
which are individually addressable and form an array disposed on first surface
340a. Surface or
support 340a is applied to skin or another tissue targeted for treatment.
Although shown as one
surface or structure, support 340a can be formed from multiple layers, for
example, the layers may
include one or more of electrode, electrical insulating material, a thermal
conducting layer,
electrical leads and contacts, and others.
[00199] In one embodiment, the applicator also includes a second support 340b
which is
disposed on or adjacent to the first support 340a. The second support 340b can
include one or
more compressible materials such as a foam or other flexible material that
conforms to the contours
of the patient. The second support can include slots, clips or other
attachment mechanisms so that
it is separable from other components of the applicator to facilitate the
reuse of one or more
components of the application. The applicator 320A also includes a third
support 340c that
includes a rigid or semi-rigid material that forms the upper surface of the
applicator 320A. The
third support can include one or more attachment mechanisms to interface with
a belt or other
apparatus to secure the applicator to the tissue surface such that the
electrodes are in close
proximity thereto or in contact with the tissue surface. The third support
340c and the second
support 340b are designed to slide appear or tear along a pre-scored line or
region to facilitate
reusing the rigid or semi-rigid third support 340c.
[00200] The electrode array of the applicator can be formed in or on flexible
substrate such as
by printing deposition or other manufacturing techniques. Such a flexible
substrate can constitute
one or more layers of the first support 340a. Each electrode is connected to
an electrical trace or
lead that extends and connects to one or more other electrodes or an
electrical contact. For
example, as shown flexible substrate 350a can be part of the first support
340a and extend
therefrom as tab or a flexible ribbon. The portion of the flexible substrate
350a extending from
the rear facing side of the applicator as shown has a plurality electrical
contacts. Each such contact
350b is in electrical communication with one or more electrodes arranged
relative skin contacting
surface applicator on tissue facing surface of support 340a. These contacts
350b can be electrical
traces or printed electrical leads or other electrical leads in various
embodiments.
[00201] The applicator may also include one or more cooling mechanisms 305
such as a closed-
loop bladder having multiple flow channels such as shown in FIG. 3A. As shown
in FIG. 3B, two
52
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
fluid transport ports 351 are shown that support cooling fluid ingress and
egress. During a given
treatment session, water or another suitable coolant circulates in one port
351 and out the other
port 351 after circulating through a series of channels within the applicator
in order to draw out
heat and thereby cool treatment region receiving RF energy from the electrode
array. FIG 3C
shows an alternative view of applicator 320A with the ports and flexible
substrate 350b shown in
front. The two and three-dimensional shape of each of the first, second and
third supports can vary
over a virtually unlimited range of shapes.
[00202] Although the overall shape of the first support 340a is substantially
square or
substantially rectangle, the shape of the support and the surfaces thereof can
be any suitable regular
or irregular shape. For example, in FIG. 3D, an alternative applicator
embodiment 320B is shown
that includes a first support 340g that is round. Similarly, the second
support 340b is also shown
as having around or curved shape. In addition, FIGS. 3E and 3F show two
additional alternative
embodiments of applicators 341a, 341b that are triangular and moon shaped,
respectively. The
applicator and the tissue contacting surface thereof can include any suitable
two-dimensional
shape, including regular and irregular shapes. These shapes are shown as
exemplars and any
suitable shape can be used in various embodiments. These embodiments can
include fluid flow
paths 351 for cooling bladders and also include electrical connectors 350d,
which may be flexible
substrates or include wires or other electrical conductors.
[00203] FIG. 3G shows a flexible applicator embodiment 325A. An exploded view
of the
applicator 325A is shown in FIG. 3H. The applicator 325A includes a plurality
of temperature
sensors 364. Suitable temperature sensors can include thermistors, but may
implemented using
other devices. The applicator also includes a flexible electrode 362. This
electrode 362 can be an
array of electrodes. FIGS. 3G and 3H show an embodiment of an electrode with
no mapping and
no cooling that offers the opportunity to provide substantially uniform
heating and flexibility to
provide good conformance to the target treatment regions. Although one
electrode is shown in
FIGS. 3G and 3H, an array of electrodes can be used in other embodiments for a
given flexible
applicator embodiment 325A and variations thereof. The applicator may include
one or more
regions of adhesive 370, which is typically disposed around the border of the
applicator 325A.
The regions 370 can include gel or other materials. An electrical cable 368
has a terminal contact
or connector 368a. The electrical cable 368, such as an RF transmission cable
attached to the
53
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
applicator at a connection terminal or electrical contact 369. The connector
368a of cable 368
receives the contact 369 in one embodiment.
[00204] As shown in FIG. 3H, the applicator 325A can include one or more
coatings 361 to
facilitate manufacture or contact with patient. These can include gels, gel,
Kapton pads or other
materials. In the exploded view, various electrical tracings or leads 365 are
shown. These are in
electrical communication with the cable 368. The applicator may also include
an electrical
insulating and thermal conducting layer 367 as shown. Various layers of the
applicators and
electrode arrays described herein can include one or more an electrical
insulating and thermal
conducting layer (e.g., Kapton polyimide or a ceramic, such as A102 or the
like) located between
a cooling device and one or more electrodes or electrical connections. In
various embodiments,
different dielectric materials can be used to form portions of a given
electrode array or otherwise
support or be disposed relative to metal layers and conductor traces. Kapton
may be used as
suitable dielectric material in various embodiments, although other
dielectrics suitable for RF
applicators for patient directed applications may be used.
[00205] For various RF delivery applicator embodiments, suitable dielectric
materials can
include Kapton and other polyesters. In these embodiments, dielectric
materials are selected to
have some of the following characteristics: dielectric constant in the range
of about 3 to about 4,
which provides a good balance of capacitance vs. dielectric thickness;
flexibility; ability to
conform to patient tissue geometry such as skin surface geometry and others;
low thermal energy
losses / dissipation factor; tissue safe, skin same, biocompatible, cost
effective, high temperature
tolerant to allow soldering without material destruction; and durable and
tough.
[00206] FIG. 31 shows a first view of another applicator embodiment 327B with
the tissue
facing surface facing downwards and a second view with the tissue facing
surface facing upwards
such that the electrodes 380 are visible. FIG. 3J shows an exploded view of
the applicator 327B
of FIG. 31. The applicator 327B is a type of hybrid applicator that includes a
disposable assembly
and a reusable assembly. A rigid or semi-rigid housing 371 protects some of
the components
nested there within such as a print circuit board stack 372. A rigid substrate
374, which is typically
a hard polymer such as a plastic, sandwiches the PCB stack 372 so that the
stack is secured in the
housing. The rigid substrate 374 may include one or more elongate members
374m, 374n or fins
extending therefrom. These members can be received by slits or grooves 374r,
374s in the PCB
54
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
stack 372 or in the housing 371. These three components, the housing, the
rigid substrate, and the
PCB stack can be treated as a set of reusable elements in one embodiment.
[00207] A compressible or conformal substrate 376 such as a layer of foam may
also be present.
As shown in FIG. 3J and 3N, a cooling bladder 305b is also present in some
embodiments and
used to cool tissue when exposed to RF treatments. The cooling bladder 305b
connects to fluid
transport lines 377. A flexible electrode array 378 is the bottom element
shown in FIG. 3J. An
array of 48 electrodes that are part of the flexible array 378 are shown in
FIG. 31. Flexible or rigid
electrical traces or leads 311 can bend around and connect to electrode array
378 as shown in FIG.
3J. The applicator 327B includes a cable 368 suitable for transmitting RF
signals and receiving
impedance measurements that extends from housing 371. In part, the disclosure
relates to flexible
cooling bladders that include quick release connections such as connections
382a shown in FIG.
3N. These arrays facilitate cooling and with a quick release connector, it is
possible to start a
procedure and end procedure quickly. Various bladder designs also benefit from
being disposable
components in one embodiment.
[00208] FIGS. 3K-S show images of alternative embodiments of the different
components of
applicator 327B shown in FIGS. 31 and 3J. FIG. 3K shows a bottom view of the
PCB stack 374
combined with the foam layer 376 and the electrode array and the cooling
bladder 305b. FIG. 3L
shows a perspective view with the rigid substrate 374 shown as well as the
inputs and outputs 377
for the cooling bladder. Connectors to the fluid inputs and outputs 382a, 382b
are also shown in
FIG. 3L and FIG. 3N. The groove or slits 374s, 374r of the PCT stack 372 are
shown in FIG. 3M,
the slits or grooves receive members 374 n, m. These slits or groove can also
be formed in housing
371 or not used in some embodiments.
[00209] FIG. 30 shows a top perspective view of the electrode array 380
sandwiching foam
layer 376 and the rigid substrate 374 and an exemplary electrode 381A. FIG. 3P
shows the
electrode array 380 and rigid substrate of FIG. 30 relative to the flexible
substrate 381 that forms
the electrode array 380. The flexible substrate includes various individual
electrodes, for example
electrode 381A, that connect to one or more electrical leads or tracings 311.
Various electrical
contacts or traces formed along the flexible substrate 381 are shown. These
traces are also shown
on either side of the foam layer 376 in FIG. 3Q. The arrangement of a flexible
substrate 380 with
electrode 381A as part of the array and the comfortable / compressible layer
376 combined with a
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
substrate 374 offers the benefits of a conformal applicator while facilitating
disposing of the
flexible substrate array and conformable / compressible layer while saving the
expensive electronic
components for re-use.
[00210] FIGS. 3R-3T and 3U show alternative applicator embodiments 333A and
333B that
incorporate a rigid electrode array 395a, 395b with individual electrodes 394.
An outer housing
388a, 388b is used to protect various electronic components such as those on
PCB stack 390. A
cooling bladder 305c is also incorporated with cooling inlets and outlets 377.
These inlets and
outlets extend from housing at one or more channels 393. An electrical cable
368 provides signals
and power to the array and is in electrical communication with various control
nodes as described
herein. These rigid applicators are particularly suitable for scanning
patients and generating before
and after impedance scans and other data derived therefrom. As discussed
herein, various types
of applicators can be to perform a RF-based tissue treatment. In addition,
with regard to each
applicator various electrode sizes can be used in a given single electrode or
electrode array
embodiment.
Electrode size and pitch
[00211] Electrode size and pitch can be manipulated to achieve the desired RF
deposition
uniformity, while maintaining flexibility and reducing electrical complexity.
A rigid portion of
electrode area of approximately 1 cm2 can allow for a sufficient area to
safely couple RF power to
the skin (e.g., without high fluxes) and still allow for flexibility between
adjacent electrodes so as
to contour to most anatomical structures. If the electrode itself is flexible,
such as a woven copper
cloth, size limitations of the electrode may be governed by edge effects in
which high frequencies
are concentrated to the periphery of the electrodes, thereby inducing a non-
uniform deposition of
RF and consequently non-uniform heating. By balancing edge effects with the
thermal properties
of the tissue, the electrode area can be optimized resulting in substantially
uniform heating of the
skin and underlying tissue. The pitch or distance between adjacent electrodes
in an array can also
be optimized to heat the targeted area over the treatment time. Suitable pitch
between adjacent
electrodes may range from about 0.1 mm to about 2 cm, for example, from about
1 mm to about 1
cm. Suitable electrode diameter size may range from about 3 mm to about 20 mm,
or about 10
mm in the case of a resistively-coupled electrode. Suitable electrode diameter
size in the case of
a capacitively-coupled electrode may range from about 3 mm to about 200 mm, or
about 10 mm.
56
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00212] For the case of fractionally ablative RF treatment discussed below,
for example, in an
array of electrodes the size and pitch can be relatively small, ranging from
about 0.1 mm to about
mm, or from about 0.5 mm to about 5 mm, with each electrode in close proximity
to one another
to cover substantially all of the applicator area. Because, for the case of
fractionally ablative RF
treatment, the pulse is so short (e.g., less than about 100 ms, or from about
5 ms to about 35 ms)
that there is no time for thermal diffusion between the particular tissue
addressed by each electrode
and the short pulses at relatively high energies can ablate the tissue.
[00213] In the case of tissue heating and laxity applications, exposures
may be long (e.g., 10-
30 minutes) with the thermal properties of the skin/fat dictating the heat
distribution and allowing
for larger electrodes and greater pitches to accomplish bulk heating to
tissue.
[00214] In the case of septae disruption, short duration, high power RF pulses
are delivered to
targeted tissue, and one can use a single electrode or an array of electrodes,
and the electrode can
be applied to the tissue and used hands free as discussed herein or, due to
the short pulse associated
with septae disruption, the single electrode or the array of electrodes can be
constructed as a
handpiece that is used in a stamping mode.
Electrode Clusters
[00215] In some aspects, electrode clusters (i.e., a node comprising a
plurality of electrodes of
an array sharing common electrical control) may be utilized to reduce
electrical complexity while
still capitalizing on the use of smaller electrodes that help with uniformity,
flexibility, and reducing
edge effects. In the simplest case, instead of driving each individual
electrode of an array of
electrodes, clusters of two, three, or more electrodes could be subject to
similar control (e.g.,
identical RF signals) since the resolution of the thermal effect may not
require more specific
control, though it may nonetheless be preferable to maintain a high number of
electrodes.
Electrode clusters may be used, for example, to treat connective tissues that
interpenetrate fat
layers (e.g., fibrous septae present in cellulite) with short duration, high
power RF pulses to one
electrode cluster followed by short duration high power RF pulses to another
electrode cluster in
the electrode array, and so on, until all or substantially all electrode
clusters in the array have been
addressed. In one embodiment, all or substantially all of the RF energy
available to the entire
electrode array is gated to a single electrode cluster so that, due to the
relatively low impedance of
the septae tissue, the septae tissue is preferentially heated by the
relatively short pulse.
57
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
Alternatively, a greater power supply can be employed that enables the desired
and/or required
high magnitude energies per pulse (e.g., from about 10 to about 1000 J/cm2) to
be gated to an
individual single electrode cluster to preferentially target septae tissue. As
discussed further
below, monitoring and/or knowing the impedance of each electrode (or most of
the electrodes or
substantially all of the electrodes) in real time can enable determination of
contact integrity of each
electrode (or most of the electrodes or substantially all of the electrodes)
to the tissue and thereby
enables avoidance of unintentional over treatment of a smaller area than the
targeted area (e.g.,
burns can be avoided).
Patient Impedance Mapping
[00216] Various detection and/or feedback mechanisms are contemplated to help
provide
improved RF treatments in accordance with various aspects of the present
teachings. RF treatment
uniformity can be assisted by utilizing tissue impedance mapping alone or in
combination with
surface perimeter temperature feedback, as discussed below. In some aspects, a
patient's tissue
impedance may be "mapped" by detecting the impedance of the tissue region to
be treated (or
undergoing treatment) such that impedance differences can be compensated for,
by way of
example, by controlling or modifying the distribution of RF power (or total
treatment time, or duty
cycle) delivered through each individual electrode in an electrode array based
upon the information
gathered via impedance mapping and/or surface perimeter temperature feedback.
Such impedance
mapping can adjust for and/or prevent accumulation of heat in an untargeted
region (e.g., outside
of the applicator perimeter). Such impedance mapping can adjust for and/or
prevent non-
uniformity of the treatment zone whether due to anatomical variation or tissue
layer thickness
variations, and/or unintended non-uniformity of RF deposition.
[00217] In certain aspects, electrical impedance mapping of individual
electrodes in the
electrode array can occur by polling electrodes of the electrode array placed
against the patient's
tissue surface to determine an individual impedance of the tissue between each
electrode pair of
the pair of applicators, and thus, the corresponding tissue impedance beneath
each electrode. By
way of example, a mapping step can be performed at very low RF power (e.g.,
sub-treatment
powers that do not substantially raise the temperature of the tissue) with two
exemplary electrode
arrays being disposed in contact with the tissue surface (or with different
tissue surfaces).
Impedance can then be detected for every combination of one electrode from one
array and one
58
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
electrode from the other array, for example, by selectively activating
individual electrodes. After
tissue impedance for one combination is determined, the electrodes can be
deactivated and other
electrodes "polled" to determine impedance along this particular path, and so
on, until each of the
individual electrodes in the two arrays (e.g., in the left and right arrays)
are addressed.
[00218] Optionally, this process can be repeated so that each of the
individual electrodes in only
one array are addressed. In this manner, the tissue impedance will be measured
in the tissue lying
below each electrode in the array. It will be appreciated that this process
can be repeated at various
RF frequencies and can be performed just before application of RF treatment
power or at various
times during treatment. For example, this initial step of impedance mapping
can be performed in
less than about a minute (e.g., about 30 seconds). Based on these
measurements, it will be
appreciated in light of the present teachings that the relative thickness of
the subcutaneous fat layer
can be calculated due to differences in the impedance between fat and muscle,
for example. A
map of the patient's impedance under each discrete electrode provides a
corresponding map of
tissue impedance throughout the treatment zone.
[00219] In addition to having such a mapping on a per electrode basis, one or
more applicators
can be scanned across a patient's tissue to generate a baseline map or report
which can include
various representations that show impedance values or values derived or
calculated based on such
values including fat layer thickness, muscle regions, variations in tissue
type, and other tissue
specific parameters such a tissue type, hydration level, and others. An
example applicator-based
scan of multiple regions of a patient are shown with regard to FIG. 2A and
discussed in more detail
herein. In addition, the treatment of mucosal tissue, such as vaginal tissue
can also be scanned
using a moving array or a fixed array that is multiplexed. An array can be
selectively addressed
such that a sequence of electrodes are energized to cover different tissue
regions.
[00220] As discussed above in association with FIGS. 1A-1F, distribution
electronics of the
applicator(s) 130a can thus be utilized to provide the same or different RF
signals to the individual
electrodes 162a of the electrode array 160a so as to provide improved control
of the treatment
procedure. In some related aspects, the distribution electronics can also be
controlled such that
each of the electrodes in the electrode array can be independently switched
(e.g., to gate RF power
to individual electrodes), with each individual channel providing current,
voltage, and/or phase
angle feedback information useful for calculating power and impedance of
individual electrodes.
59
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
To map the tissue, for example, the independently-switched contact electrodes
in the electrode
array may be switched to gate RF power sequentially first to one of the
electrodes in the array and
next to another electrode in the array until all or substantially all of the
electrodes in the array are
addressed, during an exemplary impedance mapping step as described below with
reference to
FIG. 4A. It will be noted that though FIG. 4A depicts an impedance mapping
step between two
electrodes in two different applicators, a person skilled in the art will
appreciate that such a
description is equally applicable to any number of applicators and electrode
arrays.
[00221] As shown schematically in FIG. 4A, two applicators 405a, 405b, each of
which
comprises an array 410a, 410b of 16 electrodes 408, can be disposed in contact
with the tissue
surface. With these applicators coupled to the tissue surface at the intended
treatment locations,
an impedance mapping step can be performed before applying treatment RF energy
(i.e., energy
of a sufficient power to effectuate a treatment in the target tissue) in order
to determine the
impedance (tissue resistance to RF energy) for every combination of one
electrode 408 from
applicator 405a and one electrode 408 from applicator 405b. For example, the
electrodes 408 of
the two applicators can be selectively activated so as to run very low RF
current (e.g., sub-
treatment energy) from Al to Bl, Al to B2, Al to B3, and so on, until a 16x16
matrix of resistance
values has been generated such that the tissue resistance is known between
every electrode in
applicator 405a to every electrode in applicator 405b. When applicators 405a,
405b are disposed
on tissue adjacent to one another as depicted (e.g., as opposed to distant
from one another or on
opposed tissue surfaces), it is generally observed that the lowest impedance
would be exhibited
between adjacent edges of applicators 405a, 405b. That is, the resistance
measured between A4
and Bl, A8 and B5, Al2 and A9, and Al6 and Al3 would tend to be among the
lowest impedance
measured (depending on the tissue type, as discussed otherwise herein). Such
an observation
would indicate that the highest RF current and highest heating would also
occur along these low-
impedance pathways during treatment.
[00222] The impedance topography revealed by this method can thereby identify
variations in
patient tissues' electrical impedance and can therefore be used to re-
apportion or adjust the RF
power and/or treatment time delivered to each discrete electrode so as to
improve uniformity of
the deposition of heat (temperature rise) for more effective adipose
destruction, dermal tightening,
collagen heating, or septae targeting, as well as to center the treated zone
beneath the applicator
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
(e.g., electrode array) in order to achieve more uniform tissue temperatures.
For example,
individual electrodes which detect lower impedance relative to the mean
impedance of all
electrodes will tend to deposit more RF energy (and result in a relatively
larger temperature rise)
than electrodes which encounter a higher impedance. Thus, to homogenize the
treated zone for
uniformity and centering within the treated zone, the impedance topography map
can be used to
select individual electrodes at lower impedance locations for a reduction in
RF power and/or to
select individual electrodes at higher impedance locations for an increase in
RF power. The
increase or reduction in RF power delivered via individual electrodes can be
proportional to the
variation in electrode impedance with respect to the mean or average electrode
power. Thus, in
certain aspects, the distribution electronics of the applicator(s) can be
utilized to adjust RF signal
to the individual electrodes of the electrode array to account for the
differences in impedance. For
example, independently-switched contact electrodes 408 in the arrays 410a,
410b can be switched
(e.g., under the influence of controller 137 of FIGS. 1A-1F) to modify the RF
power provided to
each of the individual electrodes 408 to assist in the uniform deposition of
thermal energy within
the treatment region.
[00223] With reference again to FIG. 4A, the data collected during the
impedance mapping step
can be used to adjust the electrode activation pattern (e.g., RF power, pulse
width, total treatment
time, duty cycle) to help maintain uniform heating under the applicators 410a,
410b. For example,
one possible method to mitigate the edge effects between the electrodes of the
adjacent edges is to
alternate between activating electrodes A{1,2,5,6,9,10,13,14} and
B{1,2,5,6,9,10,13,14} for a first
duration (while the other electrodes are inactive), and activating electrodes
At3,4,7,8,11,12,15,161
and B{3,4,7,8,11,12,15,16} during a second duration so as to promote more even
spacing and
more uniform heating. Alternatively, electrodes A {4,8,12,16} and/or
B{1,5,9,13} could have
their RF power substantially reduced and/or permanently disabled, for example,
for the duration
of the treatment. The second-to-adjacent rows of electrodes between the
applicators, namely
electrodes At3,7,11,151 and B{2,6,10,14} would still have a slight proclivity
to send current
laterally to each other, and so would heat the area under the electrodes which
have been turned
off Such a pattern (e.g., generated by distribution electronics under the
effect of a controller)
would allow for more uniform heating under two adjacent electrodes operating
in bipolar mode,
for example.
61
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00224] Further, during a treatment, the RF power applied to each electrode
408 can also be
tracked and controlled, with ongoing impedance monitoring (e.g., sampling)
being employed to
track changes in tissue impedance and with power to each array location being
adjusted
accordingly based on such feedback and/or to determine an endpoint in
treatment. For example,
during a treatment, the distribution electronics can be controlled such that
each of the electrodes
408 in the electrode arrays 410a, 410b can occasionally be sampled (e.g., by
gating RF power to
individual electrodes), with each individual "channel" providing current,
voltage, and/or phase
angle feedback information useful for calculating power and impedance of
individual electrodes.
That is, this impedance mapping can also be done real time during the
treatment (e.g., at intervals
during the treatment). Ideally, this control feedback mechanism can inform a
power-
homogenizing algorithm to monitor and/or adjust treatment conditions. Such
impedance mapping
can be especially useful during the early portion of a treatment, for example,
before temperature
changes have accumulated on the tissue surface adjacent to the target
treatment zone that can be
detected by the temperature detectors as discussed in detail below. Later in
the treatment when
surface temperature rises can be observed, the impedance mapping feedback can
optionally be
summed with the feedback provided by the detection of the tissue surface
temperature (e.g., around
the perimeter of the applicator) so as to provide additional feedback
information. Summing the
two feedback mechanisms together (e.g., take 50% of the RF correction factor
from the impedance
topography map and 50% from RF correction factor indicated by surface
temperature observation)
is one non-limiting exemplary approach. Another feedback approach would be to
shift toward use
of the surface temperature feedback method after detectable differences in
surface temperature
manifest (e.g., a 1/2 to 1 degree C difference or more), by way of non-
limiting example. It is also
possible in accordance with various aspects of the present teachings to rely
entirely on impedance
mapping to re-apportion RF power applied through each electrode to achieve
optimum treatment
placement, optimum homogeneity, the desired uniformity, and to acquire
temperature information
about the target tissue (e.g., the tissue beneath the surface of the skin or
the mucosa).
[00225] FIG. 4B shows a patient 420 that is a candidate for one or more RF-
based treatments.
One or more of the applicators described herein or other RF applicators or
delivery devices can be
used to scan one or more tissue surfaces of a patient to generate an impedance
mapping. In FIG.
4B, the skin of portions of the patient's skin and torso are scanned. The scan
can be performed by
62
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
moving an applicator, such as applicator embodiment 315C, along the surface of
the patient 420
according to various scan patterns such as shown by the exemplary directional
arrows shown. Any
suitable scan pattern can be used.
[00226] As an alternative to moving one or more applicators over a tissue
surface, a set of
applicators or electrode arrays can be disposed on the patient and scanned by
multiplexing /
selectively addressing the different electrodes or by collecting impedance
measurements with
regard to the tissue at one or more instances in time.
[00227] In general, scanning a patient before treatment and then after
treatment over time has
various beneficial outcomes for users of the RF-based treatment systems
herein. The temporal
measurements can be taken over the course of one treatment sessions or
multiple treatments
sessions. In some embodiments, one or more impedance mappings are obtained
with regard to the
candidate tissues areas for treatment before a treatment session begins. The
pattern of left and
right oriented scans and up and down scans shown by the arrows in FIG. 4B are
examples of the
types of scanning that can be performed to identify impedance values
associated with different
locations on the patient and fat distribution relative thereto. The impedance
values can be
correlated with areas of more fat / high fat levels HF as present relative to
same threshold and less
fat LF is present relative to the same or different threshold. The boundaries
of the higher fat levels
HF and less or lower fat levels LF are shown by dotted lines. Other indicia
and representations
can be shown on a representative tissue scan. In light of the results of the
scanning shown, it is
clear that more treatments on the lower torso and upper thighs is likely
warranted given the
increased fat levels HF.
[00228] In this way, a "before" impedance map can be generated and stored
for subsequent
comparison to additional impedance mappings over time. Such a "before" or pre-
treatment
impedance mapping and an "after" treatment impedance mapping can be used to
provide evidence
of treatment efficacy, increase and sustain patient motivation when assessing
benefits of ongoing
treatment, and to also provide diagnostic information with regard to treatment
parameters such as
fat levels and a reduction in fat over time.
[00229] FIG. 4C depicts a patient 425 undergoing RF-based tissue treatment
with regard to two
sections of the patient with multiple tissue regions being treated.
Specifically, multiple RF
applicators 430a, 430b, 430c, 430d, and 430e are used and placed in contact
with the patient.
63
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
Although any suitable applicator can be used, a conformable applicator or a
rigid applicator is
typical for the multizone treatment shown in FIG. 4C. Applicators 430a, 430b
are positioned in a
first treatment zone Zone 1 that includes the upper back torso while
applicators 430c, 430d, and
430e are positioned in a second treatment zone Zone 2 that includes the upper
back part of each
thigh. The applicators shown can be held in place with one or more attachment
mechanisms such
as belts, clips, clamps, straps, fasteners, and other devices suitable for
holding an applicator in a
given position relative to a target treatment region of the patient 425.
[00230] Alternatively, the applicators can be adhered to the skin in each of
the treatment zones
using suitable adhesive strips, gel, gel pads, tabs, or other regions of
adhesives that will hold the
applicator in place but can be removed without patient discomfort at the end
of the treatment. Each
of the applicators is in electronic communication with one or more RF system
components 440a,
440b as shown. These components 440a, 440b may include a console such as
console 110
described herein or one or more components or subsystems of a given console
110. The applicators
are in electrical communication with one or more electronic devices such as
one or more control
nodes as discussed herein. In one embodiment, the RF drive electronics are
disposed in the
applicator.
[00231] Each applicator can have one or more leads and one or more cables to
provide control
signals, power, transmit impedance measurements or for the transmission of
other electronic
signals as disclosed herein. For example, each applicator may include one or
more transmission
cables. In one embodiment, the distance of cable section (or the straight-line
distance) between
output of a control node 444 and a connection point of transmission cable with
an applicator 430e
is S. In one embodiment, control node 444 is a controller that generates
control signals to measure
individual current flow for one or more electrodes of the electrode array of
applicator 430e. Each
applicator used in a given embodiment may connect, directly or indirectly
though other electrical
device, to one or more control nodes, such as Nodes 0, 1, 2, 3 disclosed
herein.
[00232] In one embodiment, the distance S is a target operational range
suitable to maintain
device operation and/or accuracy of applicator outputs relative to expected or
baseline applicator
outputs for a given set of input signals and/or power. In one embodiment, S
ranges from about 0
to about 2 inches. In one embodiment, S ranges from about 0 to about 6 inches.
In one
embodiment, S ranges from about 0 to about 12 inches. In one embodiment, S
ranges from about
64
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
0 to about 24 inches. In one embodiment, control node or node 444 includes a
radio frequency
printed circuit board (RF PCB) stack that is disposed in a housing or other
assembly such within
component 440b. In one embodiment, each of the applicators depicted in FIG. 4C
is in electrical
communication with one or more control nodes such as a node the same as or
similar to node 444.
[00233] In one embodiment, it is desirable to simultaneously treat two or more
sections or zones
of patient in order to reduce the overall amount of time that would be spent
in treatment. As a
result, parallel treatment of different tissue regions in different parts of
the body simultaneously
with RF is one advantage of the disclosure. In some instances, additional
treatment zones can be
treated in addition to those zones. In some embodiments, each tissue zone can
be treated in an
alternating sequence, first zone is active with applicators delivering RF
energy and second zone is
inactive, and vice versa for a certain period of time or number of alternating
iterations. This
approach can be used to the extent such an approach is most comfortable to the
patient or if a
particular treatment regimen benefits from a rest period or alternating RF
exposure during a given
RF treatment.
[00234] FIG. 4D depicts a patient 445 undergoing RF-based tissue treatment
with multiple
tissue regions being treated using multiple applicators positioned relative
thereto. The applicators
are typically conformable applicators that are stuck or otherwise adhered to
the skin. In some
embodiments, other applicators can be used. In more detail, FIG. 4D depicts a
patient 445
undergoing RF-based tissue treatment with multiple tissue regions being
treated in two different
sections or zones of their body Zone A, Zone B.
[00235] Specifically, multiple RF applicators 450a, 450b, 450c, 450d, and
450e are used and
placed in contact with the patient. Two supports such as supports 455a, and
455b are also shown
having extension arms 457a, and 457b which support distribution devices 460a,
460b. The benefit
of such a configuration supports the multi-zone treatment benefits discussed
above with regard to
FIG. 4C. The use of separate distribution devices 457a, 457b in electrical
communication with the
applications 450a, 450b, 450c, 450d, and 450e via cables such as cable 462
facilitate the use of
thin conformable electrodes in a multi-zone treatment setup.
[00236] Although any suitable applicator can be used, a conformable applicator
is typical for
the multizone treatment shown in FIG. 4C. Given that the conformal applicators
can be thin
flexible sheets or stacks of layers, it is advantageous to separate the drive
electronics such that they
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
are located near the electrode array of such an applicator. With this in mind,
the embodiment of
FIG. 4D has conformable applicators having electrode arrays arranged as shown
with the drive
electronics and other electronic components such as one or more control node
disposed in a
distribution devices 460a, 460b as shown.
[00237] Each distribution device 460a, 460b can include multiple arms that
have a connection
port or direct connection to a transmission cable 462 that connects each arm
of the distribution
device to a given applicator. As shown each distribution device has four arms,
with three of them
visible in the figure and the fourth positioned on the other side of the
device opposite the middle
arm for each such device. The drive electronics in the distribution devices
transmits power and
control signals to the electrode array of each applicator that it is connected
to and receives
impedance data when an impedance mapping is performed relative to the various
tissue treatment
regions in Zone A and Zone B.
[00238] Applicators 450a, 450b are positioned in a first treatment zone Zone A
that includes
the upper back and the triceps area of each arm while applicators 450c, 450d,
and 450e are
positioned in a second treatment zone Zone B that includes the lower back and
the upper back part
of each thigh. The simultaneous or alternating treatment zones depicted in
FIG. 4D facilitate the
use of conformal applicators that stay fixed relative to the target treatment
regions. These
configurations can reduce treatment visits and also improve treatment outcomes
with the
placement of conformal applicators on specific tissue regions. Some further
benefits of selective
tissue targeting are discussed in more details as follows in the context of RF
energy selection.
[00239] As noted above, short duration relatively-higher magnitude RF energy
can be used to
"selectively target" tissues such as fibrotic or connective tissue, septae or
even blood or lymphatic
vessels, which structures are found within all tissues and exhibit relatively
lower electrical
impedance compared to bulk tissue. In accordance with various aspects of the
present teachings,
the measured impedance of the tissue region (including septae) can be
monitored and tracked
during the application of the RF pulse(s) to determine changes in tissue
composition in real-time.
For example, during the RF pulse emission, the current, voltage and their
phase relationship can
be monitored so as to calculate the impedance of the tissue to which the RF
energy is being applied.
With reference now to FIGS. 5A-F, in various aspects of the present teachings,
impedance tracking
of the individual electrodes and/or the average impedance of the electrodes of
the array during
66
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
treatment can also be used to determine when to terminate treatment. As
indicated above, certain
tissue types (e.g. fibrotic structures such as septae) generally exhibit lower
impedance relative to
fat tissue, for example. Accordingly, in accordance with certain aspects of
the present teachings,
monitoring of the impedance can indicate when those lower-impedance tissues
have been
sufficiently altered by the application of RF energy to indicate that a
desired outcome has been
achieved.
[00240] FIG. 5A represents a plot of impedance of tissue during the
application of an exemplary
RF signal 205 that is intended to provide a 500 ms pulse of high power RF
energy, starting at time
210B as shown at the top of the figure. By way of example, prior to the
initiation of the pulse at
time 210B, the impedance of the native tissue schematically depicted as FIG.
5B can be determined
utilizing a sub-treatment threshold low RF-power between an active electrode
and a drain pad (or
another active electrode on a second applicator). As discussed otherwise
herein, this relatively
low impedance detected at time 210B would be understood to represent the RF
energy propagating
through the untreated septae 200 depicted in FIG. 5B. However, as shown in
FIG. 5A and 5C, the
application of the treatment RF energy following the initiation of the pulse
at 210B can result in
changes in the impedance of the tissue during the heating. For example,
largely due to the RF
energy propagating within the septae 200 between the initiation of the pulse
at 210B and the time
point 210C (e.g., about 300 ms), the impedance measurements generally indicate
a decrease in
impedance in the tissue between an active electrode and a drain pad (or
another active electrode
on a second applicator) as the septae heat and/or shrink as shown
schematically by the decreased
septae 210 length of FIG. 5C. For example, in some aspects, a small change in
the impedance
during the RF energy pulse, (e.g., the measured impedance drops a discernible
amount, about 3%,
greater than 3%, from about 3% to about 20%, or about 10%) can indicate a
temperature rise in
the septae and/or be indicative of septa shrinking and/or tightening. As heat
continues to
accumulate in the septae, the impedance suddenly increases rapidly between
time 210C and 210D
as shown in FIG. 5A.
[00241] Without being limited by any particular theory, this drastic increase
in impedance can
be attributed to a dramatic shift in the structure and/or composition of the
tissue between the active
electrode and the drain pad (or another active electrode on a second
applicator). With reference to
FIG. 5D, for example, this impedance rise can be attributed to the breaking of
the septae caused
67
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
by the RF energy such that the low-impedance path through the bulk tissue is
no longer present
and the detected impedance increases to a level more in line with bulk tissue
(which includes the
high impedance fat tissue). For example, in some aspects, after the initial
decrease in impedance
noted above, a drastic increase in the impedance during the RF energy pulse,
(e.g., the measured
impedance quickly increases a discernible amount, about 3%, greater than 3%,
from about 3% to
about 20%, about 10%, greater than 10%, or greater than 20% can indicate a
large temperature rise
in the septae causing coagulation, denaturation, breaking, and/or destruction
of the septae. As
shown in FIG. 6A, after time 210D (e.g., about 400 ms) the detected impedance
stays relatively
level though the exemplary RF pulse continues to be applied for its entire
duration of 500 ms (time
210E). It will thus be appreciated in light of the present teachings that by
monitoring the
impedance of the tissue during treatment, it can be determined if a desired
outcome has been
achieved, and in some aspects, such changes can be used to determine the end
point of the
application (terminating treatment) and/or to adjust the treatment parameters
(e.g., increase power,
increase pulse width, apply additional RF pulses). For example, treatment can
be terminated (e.g.,
by ending the pulse or series of pulses) at time 210D when this drastic
increase in impedance is
ob served.
[00242] In various aspects, the sampling rate of the monitored impedance
(e.g., as indicated by
the black dots of FIG. 5A) can be selected to achieve the desired fidelity in
the result. By way of
example, the sampling rate of monitoring can include any of a number of sample
times and
frequencies during the pulse emission, for example, the sampling rate of
monitoring can occur
about 5 times, about 10 times, about 100 time, or about 1000 times during the
RF pulse emission.
[00243] The above description of impedance tracking during RF treatment can be
utilized with
either a pulsed, single electrode applicator (e.g., applicator 130d of FIG.
1A) or an applicator
having a plurality of electrodes (e.g., applicator 130a of FIGS. 1A, 1B, 1D
and 1E). In various
aspects for applicators containing an array of individually-addressable
electrodes, after the
sufficient impedance change of tissue between one electrode 562 (or a cluster
of electrodes) of an
array 560a on one applicator 530a and one electrode 562 (or a cluster of
electrodes) of an array
560b on a second applicator 530b as indicated by FIG. 5E, a similar treatment
can then be
performed utilizing a different combination of electrodes 562 between the two
applicators (or
68
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
between one applicator and a drain pad) to address a different region of
tissue and septae under
the array of electrodes as shown in FIG. 5F.
[00244] In various aspects, electrode monitoring can also be provided to
monitor the electrical
condition of every electrode to satisfy open circuit (no contact) conditions
so as to determine if the
electrode(s) are in sufficient contact with the tissue. In such aspects,
uniform attachment of the
applicator and any drift in its electrical conditions from the point of
application (e.g., the start of
treatment) to the finish of the procedure (e.g., dehydration of the gel
adhesive) can be optimized
to avoid misinterpretation of tissue impedance conditions. Because the
electrode array can be
comprised of many individual electrodes, and the impedance of each array
location can be
continually monitored, a robust method of electrode array monitoring can be
provided for
automatically.
Patient Surface Temperature Feedback: Patient surface temperature perimeter
feedback used for
RF uniformity compensation.
[00245] As discussed above, various detection and/or feedback mechanisms are
contemplated
to help provide improved RF treatments in accordance with various aspects of
the present
teachings. For example, RF treatment uniformity can be assisted by utilizing
surface temperature
feedback alone or in combination with impedance mapping, as discussed above.
For example, by
detecting temperature differences at various portions of the tissue surface
adjacent the target
region, the distribution of RF power (or total treatment time or duty cycle)
delivered through each
individual electrode in an electrode array can be controlled or modified to
adjust for and/or prevent
accumulation of heat in an untargeted region (e.g., outside of the applicator
perimeter) or non-
uniformity of the treatment zone whether due to anatomical variation or tissue
layer thickness
variations.
[00246] In exemplary aspects, the surface temperature of the patient's skin in
areas around the
perimeter of the applicator electrode array can be monitored by IR sensors,
thermocouples, or the
like (by way of non-limiting example) so as to identify uneven heating of skin
surface areas
adjacent to the intended treatment zone. Based on these signals (alone or in
combination with the
impedance mapping), a controller (including a microprocessor and algorithm as
in FIG. IA) can
provide correction factors to the RF power set-point for individual electrodes
so as to optimize
treatment uniformity, homogeneity, and placement of the treatment zone.
69
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00247] As discussed above, skin laxity and other treatments requiring bulk
heating may require
"time at temperature" to be maintained for a given tissue type, anatomical
area, and desired
treatment endpoint, by way of example. A suitable treatment temperature range
can be from about
42-47 C and a suitable total treatment time can range from about 10-35
minutes, by way of non-
limiting example. However, dosimetry methods in which a total energy versus
volume approach
is utilized may fail to recognize wide variations in patient perfusion (e.g.,
the cooling effect) or
variations in the thermal capacity of differing tissue types (e.g., nearby or
adjacent bone, viscera,
and/or thick fat layers all cause variation in temperature deposition when
exposed to a fixed dose
of Joules/volume).
[00248] Predictable RF uniformity is important for efficacy and safety in an
applied RF
treatment and can become a concern in the case of non-uniform fat layers.
However, application
of uniform RF energy (e.g., 1MHz) through a patient's skin and then into
deeper tissues (e.g., a fat
layer) is complicated by various tissue types and differing impedance
variations. For example, as
discussed above, fibrous structures and other connective tissues have a lower
impedance to RF
energy relative to fat tissue. Additionally, tissue layers below the fat layer
including muscle, large
vessels, etc. likewise have a much lower impedance than fat. Consequently, RF
energy will
preferentially travel along these lower-impedance pathways as opposed to the
fat tissue such that
the RF energy tends to preferentially heat (at least initially) these low-
impedance tissues prior to
being diffused into the adjacent fat cells.
[00249] In particular, RF treatment applicators that are placed on a tissue
surface directly over
a fat layer of non-uniform thickness (e.g., one side of the applicator is
above a 20mm thick fat
layer and the opposite side of applicator is over a 40mm thick fat layer) can
cause uneven
distribution of heat and/or the treatment of non-targeted tissue. That is,
because fat cells have a
higher impedance relative to the deeper muscle tissues, for example, the RF
energy uniformly
delivered at the surface will "drift" toward the direction of least impedance,
in this case toward the
muscle. Because RF energy will generally progress to the deeper tissues via
the shortest path
length through the high impedance fat layer, the RF energy will tend to be
delivered through the
20mm thick fat layer such that the temperature of that side of the applicator
increases more than
on the side of the applicator of the 40mm thick fate layer. This causes the
"treated zone" (area of
tissue exposed to temperature rise) to drift toward the shallowest fat layer
such that the actual
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
treated zone is offset from beneath the applicator toward the shallowest fat
layer side, an
undesirable and somewhat difficult to predict effect.
[00250] Such non-uniform energy distribution effects and the benefits provided
by various
aspects of the present teachings can be further understood with reference to
FIGS. 6A-E. First,
with reference to FIG. 6A, the temperature profile of a relatively uniform
thickness of a fat layer
of 40 mm is depicted during a treatment in which the same RF power is
delivered by each of a
plurality of electrodes. In FIG. 6A, the left vertical axis is the distance
from the patient skin surface
measured in meters (e.g., a depth below the skin surface), while the
horizontal axis is the distance
from the applicator center measured in meters. The right vertical axis is the
temperature in degrees
C. As shown, uniformity of temperature aggregated in the treated zone can be
observed, with the
treated zone being symmetric and located directly below the RF energy
applicator.
[00251] FIG. 6B, on the other hand, shows the temperature profile of a non-
uniform fat layer
during the same RF treatment as in FIG. 6A. In particular, the left side of
FIG. 6B exhibits a fat
layer of about 40mm thickness, while the right side of FIG. 6B has a fat layer
about 20mm thick.
Asymmetry and drift of treated zone temperature away from the thicker fat
layer can be observed
such that the treated zone is not directly below the RF energy applicator
(shifted toward the thinner
fat layer on the right side of FIG. 6B), which can be an undesirable result.
FIG. 6C schematically
depicts this treatment zone drift with the vertical axis representing depth
and the horizontal axis
representing distance parallel to the skin surface from the applicator center
(measured in meters).
As shown, the zone exhibiting the target treatment temperature is asymmetric
and shifted away
from the center of the applicator.
[00252] FIG. 6D depicts the exemplary temperature profile of the tissue
surface based on the
simulation of FIGS. 6B and 6C, with the left vertical axis as temperature in
degrees C, while the
horizontal axis is distance from the applicator center (along the skin
surface) measured in meters.
As shown in FIG. 6D, two heated lobes are observed, with each lobe disposed on
a side of the
perimeter of the applicator's cooled surface. In case of a uniform fat layer
thickness, these two
lobes would have been expected to be equal magnitude to each other. In this
case, however, more
RF energy was deposited toward the right side of the depicted treatment area
due to the shallower
fat layer such that the lobes are asymmetric. As such, correcting and/or
preventing non-uniform
treatment zones or treatment zone drift from beneath the applicator is one
object of this disclosure.
71
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00253] In various aspects as discussed otherwise herein, a more uniform
treatment can be
attained by delivering proportionately more RF energy to the thicker fat layer
side and
proportionately less RF energy to the thinner fat layer side of the
applicator. In this exemplary
case, the electrode being an array of a plurality of independently switchable
skin surface contact
electrodes (e.g., 19 electrodes arranged in a hexagonal array for the depicted
example). This
electrode array can be electrically isolated from but thermally bonded to a
water-cooled plate (e.g.,
as discussed with reference to FIGS. 2A and 3A). The switched electrode array
allows for RF
energy to be apportioned non-uniformly to the tissue to counter the tendency
of the treatment zone
to drift toward the thinnest fat layers. Specifically, drift can be prevented
by increasing RF power
delivered to the thick fat layer side of the applicator while simultaneously
decreasing the RF power
switched to independent electrodes on the thinner fat layer side of the
applicator. This non-uniform
applicator power approach forces the treated zone to remain centered beneath
the applicator despite
tissue impedance and/or fat thickness variations.
[00254] FIG. 6E shows improved uniformity on the right side due to the
reapportionment of RF
power to provide a non-uniform RF power input to compensate for the non-
uniform fat layer as
compared to the uniform RF power input approach provided on the left side as
was shown above
in association with FIG. 6C. The left hand image of FIG. 6E providing uniform
RF power input
generates an offset to the right of the highest temperature region that
extends well outside the
applicator's dimensions. The right hand image of FIG. 6E, however, provides
non-uniform RF
applicator power input by which power has been modulated to compensate for the
underlying
tissue thickness variation (e.g., as determined by impedance mapping). The
right hand image in
FIG. 6E depicts the highest temperature region, which is directly adjacent the
applicator's
dimensions and is illustrative of the modulated system's ability to cause the
tissue heating to occur
under the applicator despite the variation in tissue impedance caused by a non-
uniform fat layer in
accordance with various aspects of the present teachings. For example, each of
the independently
switched electrodes can be operated in a closed loop with regard to power.
Additionally, each
electrode may act as a discrete impedance detector by monitoring delivered
amps, volts, phase
angle, etc. This impedance information may be used to derive a "map" of
general tissue layer non-
uniformity proximal to the applicator so as to provide the control system with
a starting RF
applicator power correction term such that individual electrodes located over
high impedance
72
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
regions (e.g., above thicker fat layers) are "corrected" to add a compensating
increase of RF power.
And where individual electrodes are located over a relatively lower impedance
region, the RF
powers are "corrected" so as to generate a uniform thermally-treated zone
which remains centered
beneath the applicator.
[00255] The tissue impedance map approach described above is used for
providing feedback to
the control system for use to reapportion (add a plus or minus correction term
to RF power
command) RF power delivered through each individual electrode for the purpose
of controlling
the treatment to remain within the desired treatment zone, centered beneath
the applicator.
[00256] In addition, in the case of a non-uniform tissue impedance or
thickness (e.g., a fat layer),
where no correction term is applied to individual electrode power or where
insufficient correction
is applied, the resulting skin surface temperature adjacent to the perimeter
of the applicator can be
asymmetric. That is, the skin surface adjacent the perimeter of the applicator
edge located over
the thinner fat layer (of lower impedance) will tend to get hotter than the
skin adjacent the
applicator perimeter side located over the thicker fat layer (of higher
impedance). Thus, even when
the electrode array is uncompensated or undercompensated with regard to the re-
apportionment of
RF power to the individual electrodes based on impedance mapping, monitoring
of the skin surface
temperature rise adjacent the applicator perimeter can provide a useful
control feedback
mechanism to correct for asymmetry or drift of the intended treatment zone. In
some aspects,
monitoring the patient surface adjacent to the perimeter of the electrode (a
few mm's away from
the cooled patient cooling block edge) alone can provide sufficient feedback
for the control
algorithm to re-apportion or correct RF power delivered to individual
electrodes such that the
treated zone is controlled to remain uniform and symmetric with respect to the
applicator center
(e.g., the treated zone is centered beneath the applicator).
[00257] In accordance with various aspects of the present teachings, patient
surface
temperatures outside the perimeter of the actively cooled patient water
cooling block and electrode
array can therefore give an indication of the tissue temperature located
deeper (i.e., below the skin
surface). An asymmetry in surface temperatures around the perimeter of the
applicator, for
example, can indicate an asymmetry in or drift of the resulting "treated zone"
(zone of tissue
temperature rise which reaches the target temperature). Specifically,
individual electrodes closest
to areas of the applicator with the highest skin surface temperature would be
switched to reduce
73
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
RF power whereas the opposite side of the applicator would be switched to
increase the duty cycle
of applied RF power. For example, a side of a given electrode array that is
overheating can be
shut off (or its duty cycle reduced) in favor of another portion of the same
array that has a lower
skin surface temperature. Therefore, the skin surface temperature around the
perimeter of the
applicator electrode array can act to indicate that RF power must be modified
so that the
temperature rise of the skin surface can be controlled to remain consistent
and homogenous around
the perimeter of the applicator.
[00258] Maintaining homogeneity of skin surface temperature rise by monitoring
temperature
of the perimeter of the electrode array and/or impedance mapping by
individually monitoring
impedance of the individual electrodes in the array alone or in combination
can thus provide
feedback to the control system for the purpose of homogenizing and centering
the treated zone
beneath the center of the applicator electrode array.
Impedance Measurements and Temperature Feedback of Subcutaneous Tissue
[00259] One of the primary goals of hyperthermic treatments, including those
applied to adipose
destruction and tissue tightening, is to raise the temperature of tissue
beneath the superficial surface
of the skin to a range of from about 39 C to about 47 C, from about 39 C to
about 44 C, from
about 41 C to about 42 C, from about 42-47 C while preserving the
temperature of the skin
surface to a normal temperature of about 35 C or less. However, temperature
at depth is typically
unknown or requires an invasive method to monitor the temperature beneath the
surface, such that
it has heretofore been difficult to directly infer subcutaneous temperatures
from the surface
temperature due to active cooling of this tissue surface. It has thus been
common to use the
patient's sensation to determine the proper heating rate or dose.
[00260] Applicant has discovered, however, that the measured impedance and
subcutaneous
temperature can be closely related. As discussed above, the impedance of the
area under the
electrode array can be mapped to determine where more or less energy should be
deposited to
compensate for anatomical variations. Through observations of the impedance
mapping during
treatments, a strong correlation was observed between the impedance and the
temperature of the
tissue beneath the surface, which can further be applied to a closed-loop
feedback mechanism
whereby the system can determine the temperature of the subcutaneous volume
under a specific
electrode, under a cluster of electrodes, or under an electrode array. It will
further be appreciated
74
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
that one advantage of knowing the temperature under the tissue surface is that
treatment
temperature variations can be minimized by compensating for changes in
perfusion or regional
anatomical hot spots that might be dictating the overall sensation.
[00261] The plots described below depict exemplary aspects of the identified
correlation
between impedance and subcutaneous tissue temperature. As shown in FIG. 7A,
the tissue
temperature at a depth of 1.5 cm was determined by an invasive temperature
sensor (a fluorophore
tipped optical fiber, which is not influenced by RF like a conventional
thermocouple) during an
exemplary RF treatment. The power in Watts is positioned on the right vertical
axis, the resulting
temperature in degrees Celsius is positioned on the left vertical axis, and
treatment time is on the
horizontal axis. As shown, the plot exhibits a ramp up phase as the tissue
temperature increases
in the first several minutes of the treatment, after which the RF power is
reduced so as to maintain
an approximate plateau at about 45 C. That is, the target tissue can be
raised to the therapeutic
temperature range (e.g., 42-47 C) during an initial heating or build phase in
which the RF power
(or duty cycle) is increased, after which the RF power (or its duty cycle) can
be reduced to maintain
the target tissue in the desired therapeutic temperature range (e.g., at its
plateau of about 45 C).
[00262] The same exposure is plotted another way in FIG. 7B. Instead of
temperature, FIG. 7B
depicts the total impedance of the combined array of electrodes plotted
against exposure time for
two different cooling temperatures, 15 C as indicated by the squares and 28
C as indicated by
diamonds. Based on the plot and in light of the present teachings, a person
skilled in the art would
appreciate a clear relationship between the ramp and sustain phases where the
impedance is
inversely proportional to the tissue temperature depicted in FIG. 7A. In
accordance with the
present teachings, a person skilled in the art would therefore appreciate that
this observation can
be utilized to determine an absolute or relative calibration based on the
impedance measurements
(e.g., relative to the starting point and a recorded delta) that can aid in
maintaining the consistency
and efficacy of RF treatments.
[00263] The plot of FIG. 7B also demonstrates that the detected impedance
generally reflects
an offset between the different cooling settings (e.g., lower temperature
correlates to a higher
impedance). In this case, the 15 C cooling water (as indicated by squares)
conductively cools the
patient surface and adjacent deeper tissue layers more than the 28 C cooling
water (as indicated
by diamonds), thereby resulting in differing offsets or differing nominal
starting impedances.
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
Without being bound by any particular theory, this phenomenon may be because
the cooler tissue
constricts blood vessels, thus resulting in a higher impedance for the cooler
15 C surface
temperature. It can be seen that for the tissue under the 15 C water-cooled
area, the electrode
starts at a higher impedance (resistance) than the tissue under the less-
cooled area (28 C cooling
water), with the impedances differing by about 19-20 ohms. As noted above, the
patient
impedance is inversely proportional to the temperature rise at a given depth
such that when
comparing the FIGS. 7A-B, a person skilled in the art would appreciate in view
of the present
teachings that a tissue temperature rise of about 11-12 C at 1.5 cm depth
corresponds to about a
19-20 ohms decrease in resistance of the patient tissue. Moreover, in both the
28 C and the 15
C curves, a similar delta or decrease in patient tissue resistance (impedance)
is observed,
indicating a similar temperature at depth. In light of this relationship, the
delta in impedance which
occurs during the course of the treatment can be effectively used in
accordance with various
aspects of the present teachings, for example, to determine the treatment
endpoint and in order to
help maintain a consistent treatment temperature at depth, thereby reducing
side-effects due to
overtreatment and improving efficacy. Thus, in accordance with various aspects
of the present
teachings, a control scheme can be provided in which the change in patient
tissue impedance can
be monitored during the treatment and where the energy emission to the patient
is decreased or
increased in order to maintain, for example, a target value decrease in
resistance (e.g.,
approximately 19-20 Ohm). That is, the RF signal can be adjusted or modulated
to approach and
then maintain the impedance at a target value by means of a closed loop
algorithm.
Multiple Treatment Pads
[00264] Multiple treatment pads can be used in accordance with various aspects
of the present
teachings. In its simplest form of treatment pads, one array can be used as
the "source" and another
array as a "return." The two electrode arrays can cover the same area and the
clinical endpoint can
be the same for the two areas. In this case, there is no return electrode
where current is uselessly
completing the circuit, but rather the return current is doing the exact same
tissue heating as the
source current. A multiple electrode array, e.g., two or more electrode arrays
or three or more
electrode arrays, could also be supported by this method.
76
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
Running Multiple Treatment Pads
[00265] In some aspects, two or more treatment pads (e.g., treatment
applicators having an
electrode array) may be run in a bipolar or hybrid configuration as discussed
herein in association
with FIGS. 1A and 1E. In an embodiment where there can be two or more
treatment applicators,
each can have an active electrode array that is given its own DC and RF drive
circuit, which can
be independently controllable, including voltage and phase.
[00266] The two or more treatment applicators each have RF drive circuits that
operate at the
same RF frequency, however, each of the treatment applicators operate at
various phases (e.g., the
phases are not necessarily the same). In some aspects, for the two or more
treatment applicators,
all of the RF transformer secondaries (e.g., the "output" side of each
transformer that is connected
to a subject or patient) are connected together and are referenced to a single
drain electrode.
[00267] In one embodiment, only one active electrode array is utilized and the
drain electrode
serves as the return electrode. In this case, all of the RF current flows
through both the active
electrode array and the drain (return) electrode.
[00268] In another embodiment, two or more active electrode arrays are
utilized and a minimal
amount of current flows through the drain electrode. The RF applied to each of
the two or more
active electrodes can be controlled, in voltage and/or phase, in order to
achieve all or almost all of
the current flowing among and between the two or more active electrode arrays,
with a minimal
amount of current flowing through the drain electrode. This approach can be
employed for any
number of active electrode arrays greater than one, including odd numbers or
even numbers of
active electrode arrays. For example, it is feasible using phasing, to have
three active electrode
arrays sharing all of the current between the three active electrode arrays
with minimal current
flowing through the drain electrode.
[00269] In the case of multiple active electrode arrays, the drain electrode
can be used for two
purposes: (1) to monitor the voltage among the secondaries of the RF
transformers (e.g., the output
side of each transformer that is connected to a subject or patient) and hence
monitor the body
voltage; and/or (2) to act as a "dump" or "drain" of small amounts of RF
energy in the case where
the anatomy underlying all or a portion of an active RF electrode requires
less current than the
other of the two or more active RF electrode arrays. In such cases, the
phasing can be arranged to
divert some of the current to the drain so as to reduce some of the current
flowing through one of
77
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
the multiple active RF electrodes in the array to achieve uniform tissue
heating or uniform tissue
temperature despite varying anatomy.
[00270] Active electrode phasing can also be adjusted to compensate for the
anatomical
placement of the various active electrodes. In the case of four electrodes,
for example, if two are
placed adjacent on the body, the active electrodes can be phased so that the
two adjacent electrodes
are in-phase and do not pass current through the skin between them, but rather
act as one large
electrode array, effectively heating the desired tissue. Phase control of
signal transmitted to
electrode can be used to effectively steer energy, current, RF signals, power
delivery and other
parameters in or near a given target treatment area to facilitate targeted
tissue treatment or other
objectives of a given treatment.
[00271] It will be appreciated that this exemplary architecture can therefore
provide for use of
any number of active electrode arrays to achieve large area tissue heating
without limitations of
return electrode size and also with more flexibility as to the placement of
the active electrodes.
[00272] In one exemplary configuration, three treatment applicators can be
connected in a wye
or a star configuration, wherein each applicator is provided an RF output 120
degrees out of phase
from each other and wherein the RF currents sum to substantially zero on the
neutral pad (e.g.,
drain electrode or return electrode) such that a minimal amount of current
flows through the drain
electrode. Other exemplary configurations would include an even number of
applicators (e.g., two
or four treatment applicators) and where the phase angles of the RF power
signal to each applicator
are 180 degrees out of phase. In the case of four applicators, two of the four
applicators could
have a phase angle of 0 degrees, for example, and the other two could have a
phase angle of 180
degrees, wherein RF power returning via the drain electrode would be
substantially zero, or sum
to zero.
[00273] Any even number of applicators can be applied with a result of
substantially zero
neutral or minimal amount of return current flowing through the return pad by
providing an equal
number of electrodes with phase angle 0 and phase angle 180 degrees. In the
case of an odd
number of applicators, multiples of three may be utilized with a result of
substantially zero neutral
return current or a minimal amount of return current by delivering a phase
angle difference of 120
degrees and where the number of applicators operated at each phase angle is
equal with respect to
each node. In the case of six applicators, for example, a RF signal of phase
angle 0 could be
78
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
applied to two applicators, a different RF signal of phase angle 120 degrees
could be applied to
two other applicators, and a different RF signal of phase angle 240 degrees
could be applied to the
remaining two applicators.
[00274] In the case of an odd number of applicators which are not divisible by
3 (e.g., 5, 7, 11,
13, etc.), the return current will not sum out to substantially zero. However,
the neutral return
current will be substantially equivalent to the RF power provided to a single
applicator and the
remaining applicators all cancel out one another, and as a result, they sum to
substantially zero
return current.
[00275] For such cases of odd number of applicators which are not divisible by
3, there can be
two equal groups at 180 degrees out of phase from each other, and the
remaining electrode can be
operated at any phase angle. Alternatively, the number of applicators can be
divided into three
groups of equal numbers, with each group operated at 120 degrees out of phase,
with the remaining
ungrouped applicator operating at any phase angle. In both of these examples
(two groups 180
degrees out of phase, or three groups 120 degrees out of phase), all
applicators sum to substantially
zero return current except for a single applicator, which would not sum to
zero and where the
neutral return current would be substantially equivalent to that of a single
applicator regardless of
how many odd number of applicators that is not divisible by 3 is used.
[00276] The utility of these approaches where the neutral return currents sum
to zero (except
for one treatment applicator) is that any number of treatment applicators can
be used without
concern of overheating the return pad. As a result, a very large area of the
body can be
simultaneously treated/covered with treatment applicators, while requiring the
use of only a single
return pad. Optionally, multiple return pads can also be used. In this case,
the individual applicator
size could increase since the return current (substantially corresponding to
only one applicator as
described above) will be distributed amongst the multiple return pads. This
can allow the treatment
applicator size and number of treatment applicators/electrode arrays to be
scaled to properly
address a given treatment area. In these examples, substantially zero presumes
a substantially
equivalent amount of energy delivered to each treatment applicator (e.g., a
substantially even
underlying anatomy). However, where small variations in the RF power delivered
to each
treatment applicator or electrode array are observed due to variation in the
anatomy underlying
79
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
each applicator or electrode array, a minimal amount of return current may
flow to the drain
(return) electrode.
[00277] Referring now to FIG 7C, the exemplary electronics for a system 700 in
accordance
with various aspects of the present teachings is depicted, with inset 700'
representing a block
diagram of the single electrode array/applicator. Element 720 represents the
neutral electrode
return circuit. Elements 730, 740, 750, 760 represents four separate RF
amplifiers (e.g., RF energy
sources) which are connected in wye configuration and may be operated at any
phase angle with
respect to each other. Each RF amplifier is connected to a single electrode
array/treatment
applicator. For example, RF amplifier 730 is connected to electrode array
700'. As shown, an
adjustable 48V isolated DC power supply 770 provides electrical power to the
four RF power
amplifiers. A block diagram of the system controller 780 determines the
operating level and phase
angles at which each RF amplifier operates. The isolated communication circuit
785 connects each
applicator to the system controller 780. The applicator/electrode array
controller 790 switches
individual electrodes within a single array and also monitors individual
electrode voltages, currents
and phase, within the single array and this electrical feedback is used to
determine the impedance
of each individual electrode within an electrode array/applicator. As
discussed otherwise herein,
the controller 790 is capable of adjusting the duty cycle of RF energy applied
to each individual
electrode within the electrode array so as to enable the uniform deposition of
thermal energy in
tissue below the array.
[00278] In some exemplary aspects, a system for treating a patient's tissue
can include two or
more treatment applicators that are employed to treat a single region of
patient tissue (e.g., the
abdomen) or to treat differing regions of patient tissue (e.g., the upper arm
and the thigh). To be
capable of both types of treatment, each treatment applicator can have its own
individually-
controllable RF energy source and each of the RF energy sources can operate at
the same
fundamental frequency (e.g., at a single fundamental frequency), but the
phases and the amplitudes
of each of the two or more RF energy sources can be controllable.
Specifically, the phases and the
amplitudes of each of the two or more RF energy sources may be controlled
relative to one another
to enable sharing of current amongst the two or more applicators. In various
aspects, this capability
to share current amongst the two or more applicators can enable the flexible
placement of the
applicators on the body of the subject such that the two or more applicators
may be placed in the
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
same treatment region (e.g., the abdomen) or in two distinct treatment regions
(e.g., one applicator
placed on the upper arm and the other applicator place on the thigh) so that
each distinct treatment
region can have a suitable amount of RF energy delivered thereto. For example,
in an embodiment
where one applicator is placed on the upper arm, any excess current flowing to
the upper arm that
would be unnecessary to treat the targeted tissue can be shared with (e.g.,
diverted to) the other
applicator to treat the thigh tissue, a region of higher tissue density than
the arm. In some
embodiments, a return or drain electrode can additionally be employed. In
various aspects, the
two or more treatment applicators each can have a plurality of treatment
electrodes (e.g., an array
of treatment electrodes) configured to be disposed in contact with a surface
of a patient's tissue
and to deliver RF energy thereto, wherein the plurality of treatment
electrodes comprises at least
two individually-addressable treatment electrodes to which RF signals can be
applied.
Drain Pad
[00279] A drain pad may be used to balance two treatment pads, for example. If
multiple arrays
are used, one may heat up faster than the other requiring that some of the RF
energy be drained off
to a third, non-treatment return electrode.
Water Temperature Changes
[00280] Water temperature changes can be induced by changing the set-point of
the coolant and
thereby changing the heating profile in the skin. Colder temperatures would
drive the heated zone
deeper, and conversely, heating the water will bring the zone closer to the
dermis for tightening.
In various aspects, the circulating water can be configured to maintain the
temperature of the skin
in a range of about 15-35 C during the treatment, with adjustments occurring
to effect the
sensation/patient comfort and/or to control the depth of the heated zone as
discussed otherwise
herein.
RF Modulation
[00281] Modulation of the RF power may be utilized to improve the sensation
(e.g., reduce
patient pain). By way of example, the hyperthermic treatment can be confined
to the target tissue
while keeping temperatures of tissue (e.g., epidermal and/or dermal tissue)
above the targeted
tissue at depth below injury threshold (i.e., lower than about 46-47 C). For
example, the RF
treatment parameters (such as delivery pattern, power, pulse duration, etc.)
can be modulated over
the treatment time, and in some aspects by taking into account the cooling
rate on the skin surface,
81
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
an optimized temperature profile/gradient in the target tissue (e.g., tissue
about or below the
dermal/hypodermal junction such as hypodermal tissue) can be achieved during
the treatment.
Electrode Sampling
[00282] Sampling of each individual electrode for control purposes can be
preferably done at
frequencies that avoid the enervation of muscular nerves. While the
fundamental frequency is
between 0.5-4 MHz (lower frequencies may be preferred to reduce cross talk
between electrodes),
the control loop may operate at frequencies closer to 100 Hz. The modulation
of the duty cycle of
each electrode should be staggered to reduce the effects of nerve enervation.
Exemplary Treatments of Mucosal Tissues
[00283] As noted above, systems and methods in accordance with various aspects
of the present
teachings can also be utilized to provide treatment to various internal
tissues by applying RF
energy to mucosal tissue surfaces via a water-cooled treatment electrode or
electrode array
operating in either monopolar or bipolar mode, the RF energy propagating from
the mucosal tissue
surface into the deeper tissue layers. In such aspects, tissue remodeling, for
example, can be
accomplished by the heat generated within sub-tissue surface regions by tissue-
penetrating RF
energy, while the cooling can protect overlying tissue. In some embodiments,
the RF electrode
array for treatment of mucosal tissues is uncooled. Though described below
with reference to
exemplary treatments of the vagina (e.g., vaginal laxity, rejuvenation,
urinary incontinence, and
other genitourinary conditions), it will be appreciated that the present
teachings can be adapted to
provide a desired treatment to other internal tissue surfaces (e.g.,
esophagus, oral cavity, treatment
of fecal incontinence and digestive tract).
[00284] Stress urinary incontinence (SUI), for example, is a condition
characterized by the
inability to prevent involuntary urination when the body is stressed, e.g.,
during coughing,
sneezing, or vigorous physically activity. It is commonly the result of
weakened muscle strength
at the neck of the bladder and around the urethra. SUI is often reported by
post-menopausal women
and is believed to be associated with vaginal changes that occur during
menopause that weaken
the vaginal wall or the muscles that lay between the vaginal wall and the
urethra. While surgical
interventions are known and sometimes necessary in severe cases of vaginal
laxity, surgery is often
undesirable because of the costs, time-consuming recovery periods, and
potential side effects and
82
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
complications. Non-surgical devices and methods of treating SUI and other
genitourinary
conditions, particularly in women, would therefore meet a long-felt need.
[00285] In various aspects, the methods and systems in accordance with the
present teachings
can deliver a controlled amount of heat through the application of RF energy
to the vaginal wall
to remodel tissue, e.g., the anterior vaginal wall so as to treat SUI. The
tissue can be the vaginal
wall itself or tissue adjacent to the vagina in the vicinity of the urethra.
For example, a target
region for localized heating can be the tissue between the vaginal wall and
the mid-urethra. In
certain aspects, the target tissue can be heated to about 40 C to about 45
C, or from about 41 C
to about 43 C, or to about 42 C (e.g., without surface cooling). The RF
energy can be applied
for a period of time, preferably less than 30 minutes, or less than 10
minutes, or in some instances
less than five minutes. For example, RF energy can be applied for about one
minute to attain the
desired temperature in the target tissue region and continue to be applied to
maintain the desired
temperature for about 5 minutes. Thereafter the heat source can be
deactivated, and the treatment
probe can be allowed to cool and removed from the vagina. In some instances,
the entire procedure
can be completed in less than 10 minutes. Optionally, if surface cooling of
the mucosal tissue
(e.g., vaginal tissue) is utilized, the tissue can be heated to temperatures
higher than in a range
from about 40 C to about 45 C, for example, from about 40 C to about 70 C,
or from about 45
C to about 60 C.
[00286] In certain aspects, the method can include the step of applying RF
energy to the anterior
vaginal wall, to a treatment depth of about 2 to 9 cm, preferably about 5 to 8
cm, or more
specifically to about 7 cm beyond the outer vaginal wall surface. In such
embodiments, the anterior
portion encompasses about 120 degrees of the vaginal wall closest to the
urethra, e.g., from about
to 2 o'clock, from 11 to 1 o'clock, from half past 11 to half past 12 o'clock,
with the 12 o'clock
defined by the portion of vaginal wall closest to the urethra.
[00287] In certain aspects, it can be desirable to uniformly heat the entire
target volume.
Various methods of ensuring uniform heating by varying the power delivered by
individual
electrodes as discussed otherwise herein. However, in some aspects, the
methods of the disclosure
can also include using an array of electrodes to deliver heat to multiple loci
of tissue within a target
region. This fractional heating creates a lattice of hyperthermic islets, with
each islet surrounded
by relatively unaffected tissue. Such "fractional" therapy can be a desirable
method of tissue
83
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
remodeling because damage occurs within smaller sub-volumes or islets within
the larger volume
being treated. Because the resulting islets are surrounded by neighboring
healthy tissue that is
substantially spared from the damage, the healing process can be thorough and
fast.
[00288] Devices and methods of treating female genitourinary conditions, such
as urinary
incontinence, particularly stress urinary incontinence, are disclosed to
remodel tissue in the
anterior region of the vaginal wall and/or in the muscles adjacent to the
vaginal wall in the vicinity
of the urethra.
[00289] The devices can include a probe adapted for vaginal insertion having a
surface
configured to apply heat to the anterior vaginal wall. In certain embodiments,
the probe can take
the form of an elongated tube or wand having one or more therapy pads, e.g.,
RF energy radiating
electrode arrays, to deliver energy to the tissue either in contact with the
probe or in proximity to
it. As described previously, each electrode within the array can be addressed
and activated
individually. The individually programmable electrodes in the array not only
permit delivery of
tailored therapy but can also serve as sensors when not active, thereby
permitting control of the
applied energy to achieve a desired heating regime and homogeneity of
treatment within a target
region regardless of variations in the patient's underlying tissue electrical
impedance or anatomical
structures.
[00290] The probe can also include one or more temperature sensors to monitor
the temperature
of the vaginal wall surface and/or the target tissue. For example, the
temperature sensors can be
thermistors or infrared (IR) sensors configured to detect black body radiation
emitted by heated
tissue. Alternatively, temperature monitoring can be implemented by one or
more of the electrodes
operating as an impedance measuring electrode. The relationship of impedance
changes with
temperature is described in this application. The probes can also include
cooling pads to avoid
overheating of the vaginal wall surface and thereby permit heat to be
primarily delivered to
subsurface target tissue regions.
[00291] In certain embodiments, the probe can include an array of pads or
electrodes,
programmable such that a subset of the array components can be activated to
deliver heat to a
specific region or in a specific pattern. For example, RF electrodes can be
distributed over all or
part of the probe's surface to heat either the entire vaginal vault or section
of the vaginal wall. A
plurality of electrodes allows for not only mono-polar treatments
(characterized by an energy path
84
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
from at least one electrode to a remotely located return pad) but also bi-
polar treatments (with
energy flowing between electrodes). In certain embodiments, the plurality of
electrodes can also
be employed to monitor tissue impedance (or simply resistance) in order to map
the underlying
tissue and/or to further control procedures. For example, adjustment of power
gated to individual
electrodes based on the tissue impedance map can be used to homogenize the
temperature rise
across all treated areas. Controlling individual electrodes output power
(e.g., via gate duty cycle)
also permits the clinician to achieve a controlled and consistent tissue
temperature rise across all
treated tissue ranges. This is especially useful for a device that is fixed to
the anatomy, activated
and subsequently only monitored by the physician or staff member.
[00292] In certain embodiments, the probe can also include one or more
fixation devices. For
example, a locking sleeve or sheath can be provided that can be inserted into
the vagina before the
probe can be employed to fix the probe in place at a desired orientation and
depth for treatment.
The probe can also include one or more inflatable elements which can be
inflated following probe
insertion to force the energy-delivering elements of the probe into proper
contact with the anterior
vaginal wall. The devices of the present disclosed herein can be handheld or
computer-directed.
The probes can include markings to indicate depth of penetration.
[00293] Systems incorporating the devices are also encompassed by the present
teachings
including, for example, controllers, power supplies, coolant reservoirs,
monitors and alarms, all or
some of which can be incorporated into a console providing a graphic user
interface and displaying
various parameters. The systems can also include imaging elements, either
within the probes
themselves or partially within the probes and used in conjunction with an
ancillary transurethral
catheter, to help identify the target tissue region. Alternatively, the probes
can be used in
conjunction with stand-alone imaging systems, such ultrasound, x-ray or
fluoroscopic imagers.
[00294] In other aspects, the devices and methods disclosed herein can be used
to treat other
genitourinary conditions by delivering a controlled pattern of heating or RF
energy to other regions
of the vagina. One or more embodiments of this disclosure can further be used
to rejuvenate
vaginal tissue generally and provide relief from numerous genitourinary
syndromes of menopause
(GSM).
[00295] It is believed that the close proximity of the urethra and the vagina
influences the
improvement in SUI symptoms. Without being bound by any particular theory, it
is further
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
believed that heating of the vaginal wall and adjacent tissue between the
vagina and urethra leads
to tissue remodeling by contraction of the target tissue, collagen
regeneration, enervation or
combination thereof, such that urinary leakage symptoms improve.
[00296] With reference now to FIG. 8, an exemplary system 800 in accordance
with various
aspects of the present teachings is schematically depicted. As shown, the
system 800 includes a
console 810 that houses an RF generator and other electronic components (e.g.,
one or more
microprocessors) and provides a display 832, for example, of the operating
parameters. In one
embodiment, the RF generator is designed to incorporate one or more features
described herein
with regard to Node 1. Node 1 may be disposed in or near console 810 in
various embodiments.
The display 832 can be a touch sensitive screen, for example, that provides a
graphic user interface
(GUI) and/or the console 810 can provide separate user controls 811. As noted
above, though some
exemplary applicators are described herein as being generally planar (rigid or
flexible) arrays of
electrodes, in some exemplary aspects, the applicator can be configured for
insertion into a patient
(e.g., through a lumen or natural body orifice) so as to provide for the
application of RF energy to
a mucosal tissues surface (e.g., vaginal wall, esophageal lining). By way of
example, as shown in
FIG. 8, the applicator can comprise a generally tubular probe 830 (e.g., a
wand-like applicator)
that can be sized and shaped to be inserted into the vagina or esophagus for
RF treatment thereof.
The console 810 can be connected to the intelligent, temperature controlled
probe 830 via a cable
or umbilical 833, for example, for delivery of RF energy from a generator
disposed within the
console 810 to the probe 830. In certain aspects, the console 810 can also
house a coolant source
to provide circulating coolant to the applicator probe 830 via a cable or
umbilical 833, as discussed
otherwise herein. It will be appreciated that in certain aspects, the probe
830 can instead be
wireless and contain its own RF generator, electronics, cooling and power
supply (e.g.,
rechargeable batteries)).
[00297] As shown in FIG. 8, the probe 830 can be used for tissue heating and
can include an
array 860 of electrodes 862 which range from two to several hundred that can
have an individual
area of approximately 1 cm2, by way of non-limiting example. As will be
understood by a person
skilled in the art in light of the discussion herein, the probe 830 can
comprise a plurality of
electrodes (or groups of electrodes or groups of arrays of electrodes) that
can be activated to apply
RF energy to the target tissue in monopolar or bipolar mode. By way of
example, in some aspects,
86
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
one electrode or a group of electrodes of the probe 830 can represent the
"active" electrodes while
another electrode or a group of electrodes can represent the neutral "return"
electrode.
Alternatively, it will be appreciated that a return pad can be placed on the
skin surface (e.g., near
the pubic region, on a portion of a patient's leg) during a vaginal treatment
to provide a return path
for the RF energy provided by the electrodes to the mucosal lining of the
vagina. Further, as
discussed in detail below with respect to FIG. 11 for a separate probe 1130,
the electrode array
1160a can consist of pin-point electrodes 1162a configured to fractionally
ablate the mucosa (e.g.,
50 individual electrodes in a 5 x 5 mm area). With reference again to FIG. 8,
the probe 830 can
further include one or more temperature sensors 842. In various aspects, the
probe 830 can further
include markers 844 to indicate the depth of its penetration into the vagina.
[00298] As shown in FIG. 9, the system 800 can also include a locking sleeve
or sheath 850 (or
introducer) that can be useful for guiding the treatment, for example, to ease
insertion, provide
alignment, and/or to set a depth based on sounding of bladder neck with a
Foley catheter or manual
sounding of the vagina. By way of example, the probe 830 can include a groove
851a to mate with
a corresponding ridge 85 lb on the introducer 850, though other mating or
locking mechanisms
can be substituted as will be appreciated by the person skilled in the art in
light of the teachings
herein.
[00299] With reference now to FIGS. 10A-C, an exemplary method of treating
SUI in
accordance with various aspects of the present teachings is illustrated. In
particular, FIG. 10A
provides a schematic illustration of the female genitourinary tract including
the uterus 802, vagina
804, bladder 806, and urethra 808. At the vaginal opening, the urethra 808 and
vaginal wall are
anatomically close. However, as the urethra 808 nears the bladder neck, the
urethra 808 is
separated from the vaginal wall. In various exemplary aspects, this is the
region targeted for the
RF-based heat therapy (e.g., near the mid urethra).
[00300] With reference now to FIG. 10B, the insertion of a catheter 801 (e.g.,
a Foley catheter)
is shown after insertion into the urethra 808. As shown, the catheter 801 can
be inserted in to the
urethra 808 until its distal end reaches the bladder 806, after which a
balloon 803 can be inflated
to stabilize and fix the catheter 801 in place. The urethra's full length can
be identified by the
external orifice and its termination at the bladder neck. Identification of
the bladder neck is a
87
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
routine clinical practice by inserting a catheter, inflating the balloon, and
retracting it until the
balloon hits the neck.
[00301] In certain aspects, the catheter 801 can include one or more
temperature sensors 805
disposed along its length and configured to measure, for example, a
temperature rise in the urethra
808 and/or to monitor the temperature of tissue remote to the tissue-electrode
interface (e.g., at the
target tissue that is intended to be heated). The catheter 801 can also be
connected to the console
810 of FIG. 8 such that current to the RF probe 830 can be controlled by
monitoring impedance to
ensure contact between the probe electrodes 862 (e.g., individually-monitored
electrodes) and the
vaginal wall, as discussed otherwise herein.
[00302] As noted above, in certain aspects, the target region targeted for the
RF-based heat
therapy (e.g., near the mid urethra) can be located at about the mid-urethra
as the urethra 808
becomes separated (e.g., diverges) from the vaginal wall. In various exemplary
aspects, the target
region 809 can be the tissue that lies beyond the vaginal wall between the
vagina 804 and urethra
808. To heat this region via application of RF energy, it is preferable that
the probe's electrodes
862 should be disposed in contact with the anterior wall of the vaginal vault,
as shown in FIG.
10B.
[00303] With reference now to FIG. 10C, exemplary procedures in accordance
with various
aspects of the present teachings are depicted in which the probe 830 is
disposed in contact with
the desired regions of the vaginal wall such that one or more electrodes 862
can heat the vaginal
wall via the application of RF thereto. It will be appreciated that more than
one electrode 862 can
be used simultaneously to apply RF energy, for example, if it is desired to
heat a larger length or
width of the vaginal tissue with a less tiring hand motion (or to automate the
procedure). In various
exemplary aspects, the probe 830 can further include an inflatable balloon 832
to stabilize the
probe 830 in contact with the vaginal wall surface. As discussed otherwise
herein, the electrodes
862 can be connected to a common node (e.g., one or more electrode clusters)
or can be
individually controlled to only deliver power to those electrodes in contact
with the vaginal wall,
for example. In various aspects, each of the plurality of electrodes 862 (or
groups of electrodes
862) can be activated to apply RF energy to the target tissue in monopolar or
bipolar mode.
Alternatively, it will be appreciated that a return pad (e.g., pad 130e of
FIG. 1C) can be placed on
the skin surface (e.g., near the pubic region, or on a patient's thigh) during
a vaginal treatment to
88
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
provide a return path for the RF energy provided by the electrodes 862 to the
mucosal lining of the
vagina 804. Alternatively, in various aspects, the catheter 801 can serve as a
return path to focus
the energy into the tissue between the vagina 804 and the urethra 808. It will
be appreciated that
configuration can help concentrate tissue heating to the vaginal wall
immediately adjacent to the
urethra 808, any muscle in between, and the urethra 808 itself. In yet another
aspect, the probe
electrodes 862 can be bi-polar such that one electrode 862 in the array 862
(or a group of
electrodes) act as a therapeutic "active" electrode emitting RF energy, while
one or more other
electrodes in the array 860 act as a "return" (grounding) electrode to provide
an electrical return
path for the RF energy. In certain aspects, pulsed RF, concentrating high
energies in short pulses,
can be preferred.
[00304] In certain aspects, a hands free set-up can be preferred. For example,
after sounding
the vagina and bladder neck, a practitioner can adjust the probe 830 to apply
the RF to the correct
zone along the urethra 808, fix the probe 830 into place with a balloon 832 or
other means, and
employ feedback to determine that the probe 830 (and its electrodes 862) are
in contact in order to
initiate the application of RF. In accordance with various aspects of the
present teachings, the
probe 830 can then be operated so as to uniformly deposit RF energy, maintain
a uniform desired
temperature range in the target region, provide consistent dosimetry, and/or
provide surface
cooling as discussed otherwise herein.
[00305] The probe 830, for example, can also include a cooling mechanism 835,
such as one or
more cooling surfaces interspersed with the electrodes 862 that cool the
vaginal wall surface by
circulation of a coolant through the probe 830. Alternatively, in various
aspects, cooling can be
achieved by thermoelectric (Peltier) devices or the use of a phase change
material (e.g., ice) in
thermal contact with a patient contact surface (e.g., via the electrodes). As
discussed otherwise
herein, controlling the temperature of the electrode-tissue interface can be
useful to control the
depth of targeted tissue. Cooling can change the therapeutic goal such that
heating the target tissue
is not limited due to patient tolerance (e.g., in a range from about 40 C to
about 45 C). With
cooling, for example, the target temperature can be increased to a temperature
in a range from
about 40 C to about 70 C, or from about 45 C to about 60 C.
[00306] With reference now to FIG. 11, another exemplary probe 1130 according
to various
aspects of the present teachings is depicted. As shown, the probe 1130 can
include a plurality of
89
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
distinct electrodes 1162 disposed over the entire surface of the probe 1130,
as opposed to the
anterior, distal region of the probe. It will be appreciated in view of the
present teachings that the
advantages of such a probe 1162 includes the ability to treat the entire
vagina 804 by means of
switching differing electrodes on and off. This type of probe (electrodes over
entire surface) could
treat vaginal conditions (rejuvenation) other than SUI, throughout the vagina.
However, to address
the SUI-treatment target tissue region alone, the electrodes 1162 within the
desired anterior vaginal
region (e.g., in the 10 o'clock to 2 o'clock positions) could be energized,
while the other electrodes
remain off Additionally, such a probe can allow the clinician to adjust ranges
greater than the 10
o'clock to 2 o'clock positions, for example, by energizing more electrodes and
thereby treating a
larger area of tissue up to including the entire circumference of the vagina.
Such a probe can
likewise allow the clinician to adjust ranges narrower than the 10 o'clock to
2 o' -clock positions,
for example, by energizing fewer electrodes and thereby treating a smaller
area of tissue. Any
desired region (or the entirety of the vagina) can thus be selected for
treatment.
[00307] Various aspects of the control of the RF therapy can be based on the
feedback from
multiple temperature sensors along the urethra. If the urethra is the target
to be heated for
stimulation of the tissue and surrounding musculature, having a monitor within
the urethra can
help standardize clinical outcomes and significantly improve safety. Thus,
monitoring temperature
at discrete locations along the catheter can be beneficial to enable detection
of any thermal
anomalies, e.g., hot spots. Additionally or alternatively, these discrete
temperature sensors can
inform the treatment endpoint decision. Variations in patient anatomy and
tissue perfusion can
thus be compensated for by monitoring the actual tissue temperature rise
during the application of
the RF energy.
[00308] In various aspects, both long duration, low irradiance (-1-5 W/cm2)
and short duration,
high fluence (-10-1000 J/cm2) regimes discussed previously herein are also
envisioned for tissue
accessed internally and can provide contrasting benefits as to biological
target selection and
treatment. Without being bound by any particular theory, the method of action
can be thermal in
nature where delivered RF power acts to heat or even coagulate selected
tissues. For long,
continuous exposures, uniform heating of structures can be accomplished. For
short bursts of
concentrated energy, foci of ablated tissue can be created. In various
aspects, it can also be
desirable to ensure uniformity of delivered RF energy. To provide efficacious
treatment in some
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
applications, it can be desirable to not only raise the temperature of target
tissues to a temperature
range, but also to hold the target tissue in the targeted region at an
elevated target temperature for
a given duration. That is, maintaining the temperature for a period of time
can confer a desired
clinical benefit. It can also be advantageous to actively control the RF
energy as discussed herein
to distribute energy through targeted tissues in the targeted treatment zone
in a homogenous
fashion, uniformly, predictably and automatically (e.g., without user
intervention). In addition,
RF pulse duration can be used to select and/or target particular tissue. High
magnitude, short
duration RF pulses, when concentrated to small electrode-tissue interface
areas, can generate
sufficient flux or current density to coagulate and vaporize tissue, thereby
resulting in the
"fractional" treatment discussed above.
[00309] With reference now to FIG. 12, another exemplary system 1200 in
accordance with the
present teachings is depicted. As show, system 1200 can include a console
1210, a coolant source
1238, a microprocessor 1237, an RF power source 1235, switching controls 1211
and measurement
circuitry 1213 (which can be separate or housed together in a single console
1210). The system
1200 further includes at least one probe 1230a with an associated array 1260a
of electrodes 1262a.
The individual electrodes 1262a of the array 1260a can be independently
switched by the switching
controls 1211 to gate RF energy to individual electrodes in the array.
Electrodes not actively
receiving RF energy can be monitored by the measurement circuitry 1213 such
that each electrode
serves as a signal channel, providing current, voltage, and/or phase angle
feedback useful for
calculating the power and impedance at each of the individual electrodes
1262a. The system 1200
can further include an optional second probe 1230b to provide an electrical
return path. Probe
1230b can also include an array 1260b to either deliver RF energy or provide
another mechanism
for sensing impedance and/or other electrical parameters and/or temperature.
In some
embodiments, probe 1230b can be incorporated into a catheter for disposition
in the patient's
urethra. Alternatively or in addition, the system 1200 can also include a
return (ground or neutral)
pad 1230e, for example, to be connected to the patient so as to provide a
drain for applied electrical
current. The system 1200 can be used with other applicators disclosed herein
in various
embodiments.
[00310] As discussed otherwise herein, electrode arrays suitable for use on
internal tissue
surfaces can also have a variety of configurations in accordance with the
present teachings. For
91
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
example, the electrode array can be configured as a probe including of a metal
coolant housing,
with an electrical insulating and thermal conducting layer (e.g., Kaptong
polyimide or a ceramic,
such as A102 or the like) located between a coolant circuit and the electrode
array. The electrode
array can be attached to the applicator cooling housing via an adhesive such
that a circulating
coolant, e.g., chilled water from a coolant source, can cool the electrode
array and the patient's
internal tissue surface to which the electrodes are disposed in contact (e.g.,
the vaginal wall).
[00311] In various exemplary aspects, for example, the electrode array
applicator can have 50
individually controlled electrodes arranged in a square, circular, or
hexagonal pattern.
Additionally or alternatively, the surface temperature of the patient's tissue
surface (e.g., the
vaginal wall) in areas around the perimeter of the applicator electrode array
can be monitored by
IR sensors, thermocouples, or the like (by way of non-limiting example) so as
to identify uneven
heating of vaginal wall surface areas adjacent to the intended treatment zone.
Based on these
signals, a microprocessor and algorithm can provide correction factors to the
RF power set-point
for individual electrodes so as to optimize treatment uniformity, homogeneity,
and placement of
the treatment zone. In various aspects, the electrodes can be individually
monitored for impedance
as discussed otherwise herein, which can be used by the microprocessor and
algorithm to define a
map of the patient's impedance topography and to provide correction factors
for changing the RF
power set-point to optimize treatment uniformity, homogeneity, and placement
of the treatment
zone.
[00312] Cooling of patient's internal tissue surface to which the
electrodes are applied (e.g., the
mucosal lining, the vaginal wall) can protect the tissue surface and also
improve patient comfort
during the procedure or minimize discomfort afterwards. Adequate surface
cooling (e.g., via
circulating water within the probe at about 10 C to 35 C) allows the
application of larger
magnitude of RF power safely and comfortably. This can be desirable as most
target tissues are
located at some depth from the internal tissue surface (e.g., the vaginal wall
surface) and therefore
surface cooling acts to protect the intervening tissue layers which are not
targeted and allows the
heat to penetrate deeper into the tissue. Because mucosal tissue tends to have
nerve endings that
are close to the surface, cooling the tissue surface enables higher
temperatures to be tolerated by
the subject at the desired treatment depth below the cooled surface.
92
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00313] As discussed above, individually-switched electrode arrays (e.g.,
individually
controllable electrodes where RF power delivery can be independently
adjusted/controlled) can be
provided to help ensure or control the treated zone to remain centered with
the desired treatment
zone (beneath the electrode array applicator) as well as to remain homogenous
and consistent in
terms of temperature rise within the desired treatment area regardless of
variations in the patient's
underlying tissue electrical impedance or anatomical structures. Each
electrode (or subsets of
electrodes) within the array can be addressed and activated individually. By
way of example, a
map of the impedance can be generated for the entire vaginal vault and, based
on this impedance
information, govern the activation of only certain electrodes to avoid
structures which are not
targeted. Vaginal wall (and/or urethral) surface temperatures can also be
monitored and used for
RF uniformity compensation. Application of uniform RF energy (e.g., at a
frequency of about
1MHz) through the vaginal wall and then into deeper tissues is complicated by
various tissue types
and differing impedance variations. For example, fibrous structures and
connective tissues have a
lower impedance to RF energy relative to fat. Consequently, RF energy will
preferentially travel
along connective fibrous tissues opposed to fat. Heated connective tissues
thereafter thermally
diffuse and/or conduct heat from the fibers into adjacent fat cells and raise
their temperature.
Likewise, muscle tissue can have a much lower impedance than other tissue
types. Because
predictable RF uniformity is important for efficacy and safety in an applied
RF treatment, non-
homogeneous tissue structures in the target region should be taken into
account. Since some tissue
structures have a higher impedance relative to others (e.g., deeper muscle
tissues), RF energy
uniformly delivered at the surface can "drift" toward the direction of least
impedance. The RF
energy will typically progress to the deeper tissues via the shortest path
length through the high
impedance layer closest to the vaginal wall. Thus, as discussed otherwise
herein, only one
electrode (or a subset of the electrode array) can be activated based on
tissue feedback (e.g., based
on impedance and/or temperature feedback) and/or the power, duration, duty
cycle, etc. of the RF
signal provided to the individual electrodes can be individually adjusted to
help provide uniform
heating.
[00314] As discussed above in accordance with various aspects of the present
teachings, the
application of different RF pulse durations can be utilized to provide for the
treatment of selective
tissues and/or a variety of treatments. With respect to internal tissues, both
long duration (e.g.,
93
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
greater than 1 second, CW), low power RF energy (e.g., from about lto about 5
W/cm2) and short
duration (e.g., less than 500 ms, less than 100 ms), high energy RF pulses
(e.g., from about 10 to
about 1000 J/cm2 per pulse, 10 J/cm2-500 J/cm2, 10 J/cm2-300J/cm2, 10 J/cm2-
100 J/cm2) regimens
are envisioned, for example. In some aspects, high-magnitude, short duration
RF energy pulses
can be utilized to generate sufficient flux or current density to ablate,
coagulate, and/or vaporize
tissue. By way of example, the RF pulses can be concentrated (e.g., focused)
to a small-area of
the electrode-tissue interface so as to induce sufficient flux or current
density to coagulate and
vaporize tissue.
[00315] With reference to FIG. 13, the results of an exemplary RF-based
treatment on bovine
liver is depicted in which an array of electrodes are spaced and configured to
deliver heat to
multiple loci of tissue within a target region such that treated portions are
separated by untreated
portions. In particular, the exemplary electrode arrays each comprise 20
electrodes to which an
RF signal was applied while the electrodes were in contact with the liver
surface. The RF signal
comprised a 25 ms pulse, a pulse energy of about 30 mJ per electrode in each
electrode of the array
of 20 electrodes. As shown in FIG. 13, this exemplary treatment can be
utilized to provide damage
(e.g., ablation, coagulation) to separated islets within a larger volume. In
such a "fractional"
treatment, the damaged islets (vaporized tissue) are surrounded by healthy
tissue that was
substantially spared from damage caused by the application of the RF energy.
In various aspects,
the neighboring, undamaged (e.g., healthy) tissue can improve the healing
process of the islets of
damaged tissue.
[00316] The results of another exemplary "fractional" treatment is depicted in
FIGS. 14A-C.
The RF signals applied to the two arrays of electrodes, each array having 20
electrodes for each of
these figures exhibit the same pulse energy of about 30 mJ per electrode in
each array but differ in
the duration of the RF signal. FIG. 14A, for example, shows a plurality of
separated islets of
vaporized tissue on a patient's skin surface caused by the application of an
RF pulse duration of
35 milliseconds and energy to each of the electrodes in the array. FIG. 14B
depicts the separated
islets caused by the use of a pulse duration of 25 milliseconds, and FIG. 14C
depicts the separated
islets caused by the use of a pulse duration of 12 milliseconds. It can be
observed that shorter
pulse durations for the same energy per pulse cause more damage (e.g.,
vaporization of tissue) at
the foci.
94
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00317] As discussed herein the disclosure relates to various embodiments.
These include
various applicator and electrode array designs that sandwich or combine or
connect various
components such as re-usable and disposable components. Flexible electrode
array printed circuit
board-based designs can include any suitable rectangular, arcuate, round,
regular, and/or irregular
shapes and combinations thereof These flexible electrode arrays can be paired
with foams, gels,
and other materials to facilitate conforming to a patient. They can also be
paired with rigid
components that house electronic components designed for re-use. This approach
facilitates an
approach that address the technical problem of creating RF-based treatment
devices that can be
used cost effectively in a timely manner to increase patient comfort.
Uniform Heating Systems and Methods and Additional Applicator Embodiments
[00318] In part, the disclosure describes a flexible non-invasive body
contouring applicator
suitable for directing RF-based energy. The flexible applicator can be
relatively thin. In various
embodiments, the thickness of the applicator ranges from about 3 mil to about
10 mil. In one
embodiment, the thickness of applicator is less than about 10 mil. In one
embodiment, the
thickness of applicator is greater than about 5 mil and less than about 12
mil. In various
embodiments, the flexible applicator includes multiple separate treatment
zones, wherein each
zone may be set to reach a target temperature using one or more control
systems by selectively
energizing regions in a pattern to gradually bring overall region into a
preferred treatment
temperature range. The applicator uses an RF energy source / generator to
generate heat via each
of the treatment zones using RF-heating through conductive traces and/or
dielectric gradients. In
some embodiments, the applicator is connected to an RF generator via an
interface device that
includes an electronic subsystem. The interface device secures and releasably
connects to the
disposable applicator and operates as a quick disconnect / connect device. The
individual separate
zones / regions of the applicator may be energized to achieve uniform heating
for each such zone
/ region. Further, the zones / regions of the applicator are selectively
energized to facilitate uniform
heating of the overall region of tissue in contact with the applicator.
[00319] A given flexible RF applicator may be energized using an RF source
having an
operating frequency. In various embodiments, the operating frequency may range
about 0.5 MHz
to about 10 MHz and as otherwise disclosed herein. In one embodiment, the RF
frequency used
with the flexible applicator is about 3 MHz or about 4 MHz. Further, the zones
or regions of the
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
applicator may be addressed according to various schemes and patterns, with
various delay periods
and changes to the addressing pattern being controllable by operator, for
example, by an individual
operating the device and/or by achieving a predetermined temperature goal for
each zone/region
that temperature goal being held within temperature range for a predetermined
time range. Various
distribution systems may be used to connect and address a given applicator.
Regions or zones of
the applicator can be energized or addressed such that the input electrical
signal to a given region
or zone is conducted zone by zone or region by region using a random, round
robin, alternating,
zig zag, and other addressing / energizing scheme. Heating of one tissue
region below each
interrogated region of an applicator allows for uniform heating without
uncomfortable sensations.
This may be achieved by cycling through different regions and energizing them
while heat from
one region extends to other regions to promote heating of an overall aggregate
tissue section or
region below or extending beyond the applicator.
[00320] In various embodiments, all of the regions of an applicator are
typically not energized
at the same time, although this is possible in some instances. Generally,
applicator regions are
selectively energized according to one of the aforementioned schemes or
patterns to facilitate
uniform heating of the body region underlying the zones/regions of the
applicator being heated.
Further, the applicator includes multiple layers arranged in a stack or
combinations of substacks.
In turn, the applicator has a shape defined in part by an outer boundary or
border. The overall
shape of the applicator is generally curved with sharp edges and straight
lines being avoided as
part of the applicator's border. Elliptical, circular, arcuate contours, and
curved boundaries and
combinations and fractions of the foregoing are preferred to mitigate against
edge effects and non-
uniform heating.
[00321] Various flexible and/or conformable applicators can also be used to
implement the RF-
based treatments and methods of treatment, including cosmetic treatments as
disclosed herein.
FIG. 15A shows a flexible RF-based applicator 880 suitable for directing RF
energy to one or more
tissues and body regions / body volumes. As shown, the applicator 880 has six
regions or zones
R1, R2, R3, R4, R5, and R6 that are divided by seven kerfs K. The kerfs K
facilitate the applicator
880 conforming to contours of patient tissue, such skin generally, abdomen,
and other patient
tissues without limitation. In one embodiment, a kerf is the gap between the
regions implemented
to allow a flat applicator to conform to a compound curved surface. In various
embodiments, the
96
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
shape in which the plurality of layers is cut defines the shape of the kerfs
and circular or elliptical
strain relief elements at the end of kerfs. These strain relief elements /
terminal strain relief
elements may be incorporated in various applicator designs disclosed herein.
[00322] In general, the applicator can include two or more regions in some
embodiments with
one or more kerfs K. In some embodiments, the applicator 880 may have one
region and not
include a kerf. The applicator is flexible and suitable for conforming to one
or more surfaces of a
patient's skin, tissue, muscles, organs, body lumens, organ systems, and other
regions and surfaces
on or within a patient. The applicator 880 include an electrical connector 890
that includes a
plurality of electrical contacts 892. The contacts may connect to an
electrical trace disposed in or
on the applicator.
[00323] In one embodiment, electrical connector 890 is part of applicator 980.
The electrical
contacts 892 connect to electrical traces and other electrical components of
the applicator. The
applicator 880 may also include a strain relief device / element 885 disposed
relative to such as by
surrounding or sandwiching flexible layers of applicator and electrical
connector 890. The
electrical connector 890 may include one or more alignment devices 895
suitable for facilitating
alignment of the electrical contacts 892 with corresponding electrical
contacts of an interface
device. The first surface 900 of the applicator may include one or more
labels. In one embodiment,
an integer N, or other variable may be used to refer to the N conductive
traces such as copper traces
that direct RF to N outer regions of an applicator. A given set of patterned
traces arranged in a
configuration such as spiral, loop, or other concentric or nested
configuration can be used as a RF
transmitter for a given applicator or region thereof.
Uniformity- Flexibility/Contour
[00324] In various embodiments, the applicator features kerfs K. The kerfs are
voids or cut
outs in the flexible layer stacks that functions in a manner akin to a dart in
a garment that enables
the applicator sheet to accept the contour of the body while maintaining
contact. Use of kerfs
enables improved tolerance in sheet thickness. When the applicator size is
above a treatment area
of about 50 cm2 or so then kerfs are used to provide flexibility in the
applicator. Kerfs K enable
the applicator to contour to larger treatment areas and are used for multiple
zones / regions RI-RN
in a single applicator.
97
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00325] In general, the regions or zones of an applicator are separated with
kerfs, channels,
gaps, cavities, voids, or other defined spaces to facilitate the use of one
flexible applicator that can
bend and be adjustable relative to a tissue region such as stomach, abdomen,
submental region,
organs, organ systems, skin, subcutaneous tissue, body tissue, any of a number
of areas of the face,
arms, legs, and other regions, lumens, or volumes of a patient. The selective
and alternating
energizing of regions of a given applicator is performed to facilitate uniform
heating of target
tissue subject to the regions of a given RF-based applicator being separated
along one or more
boundaries, such as kerfs or channels.
[00326] A given applicator includes a stack of layers and has a first side 900
as shown in FIG.
15A. The second side or tissue facing side is the surface opposite the first
side. Additional details
relating to the layer stack are described with regard to FIGS. 18 and 19A and
elsewhere herein.
Generally, each applicator has a first side and a second side. In one
embodiment, the first side 900
corresponds to a labelled side, also referenced to as a vinyl or polymer side
or upper or top side.
Alternatively, the second side refers to gel side, wet side, lower side, or
patient facing side. The
wet side refers to the presence of aqueous material or gel disposed on a side
of the applicator. The
gel or other aqueous material facilitates maintaining skin contact and
positioning of applicator
region for targeted RF energy transmission and subsequent tissue heating.
References to first side
and second side are not limiting and may refer to any of the foregoing sides
of an applicator as
informed by context.
Shapes/Scalability
[00327] In various embodiments, the applicators may have different shapes.
Preferably, the
applicators have a curved boundary, in contrast with straight edges such as
polygonal boundary.
Elliptical, circular, oval, curved and other shapes may be used for specifying
shape of applicator
and/or its outer edge or boundary. For example, in various embodiments, an
applicator is shaped
as an ellipse or football having a surface area tailored for a particular
patient size or tissue of
interest (i.e., about 225 cm2 or about 300 cm2). Larger applicators are
designed for larger treatment
areas, such as, but not limited to: the abdomen and thighs, while smaller
applicators are for smaller
treatment areas, such as the face or arms. The geometry, size and coverage of
an applicator is
scalable based on which potential areas on body will be treated. Further, in
various embodiments,
an applicator can include various number of zones or regions R, such as, but
not limited to, 6 zones
98
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
R1-R6, 12 zones, 48 zones, or another number of zones. Each zone is typically
bounded by one
or more kerfs. The limit on the geometry and size is configurable based on
energy available from
one or more of the systems described and depicted herewith such as systems
100, 800, 1200.
[00328] In various embodiments, an applicator can selectively control and/or
turn on / off one
or more zones. In some embodiments, a zone can be selectively sized to have an
effective
treatment a zone to be smaller than an adjacent zone. Further, in some
embodiments, an applicator
can be shaped to include one or more voids or regions free of RF-transmission
elements or
programmed to not energize certain RF transmission elements or regions to
compensate for
sensitive regions (i.e., belly button, scars, etc.). This can be controlled by
a user interface on a
display connected to system 100. An exemplary portion of an applicator that
may be used to
compensate for the presence of a navel, scar, or other depression is shown in
FIG 15C as region
912. As shown in FIG. 15C, region 912 is a portion of zone R5. In some
embodiments, an entire
region, such as region / zone R5 of applicator 905 may be selectively avoided
such that RF energy
is not transmitted to the navel or other sensitive region. In other
embodiments, the region 912
could be enlarged, made smaller, and/or moved to other regions depending on
the location of
sensitive regions. The deactivation of regions of a user interfaces suitable
for use with treatment
systems and applicators disclosed herein is shown in FIGS. 28A and 28B.
[00329] In various embodiments, unwanted edge effects are avoided by selecting
dielectric /
trace for a given zone, in some embodiments one temperature sensor such as a
thermistor is used
per zone / region to regulate target tissue temperature such as skin
temperature. In some
embodiments, two thermistors are provided per zone for redundancy and to
provide backup should
one fail. For example, in region / zone R5 of applicator 900 in FIG. 15A, two
temperature sensors
H are shown. Each temperature sensor H connects to one or more electrical
conductors that in
turn are in electrical communication with one or more electrical contacts in
the electrical connector
890. In some embodiments, one temperature sensor H is provided per each zone
or region. The
thermistors provide an outer temperature reading such as a tissue or
applicator surface temperature
reading and discerns the internal at depth temperature based on the outer
temperature reading. In
one embodiment, an outer surface of the tissue or the applicator surface
temperature is measured
using one or more applicator temperature sensors, such as thermistors. In
turn, the internal at depth
99
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
temperature is discerned from that reading. This follows from the internal
temperature being
correlated with surface temperature of tissue / applicator contacting the
tissue.
[00330] The target skin surface temperature ranges from about 40 C to about
44 C. This
correlates to a temperature at depth that ranges from about 41-45 C. In some
embodiments, the
temperature range may extend to from about 42 to about 47 C due to thermal
accumulation.
[00331] In at least one embodiment, when applied to skin tissue, the
applicator provides uniform
heating up to a target surface temperature range (about 40 to about 44 C),
which corresponds to a
range of (about 42 to about 47 C) at the depth of fat tissue. In various
embodiments, temperature
is measured at the surface of the skin tissue and the temperature of the
treated skin surface is used
to determine the temperature of underlying tissue, i.e., the fat tissue below
the target surface. One
or more thermal / temperature sensors are incorporated in each region or
section of a given
applicator. In various embodiments, two thermal / temperature sensors such as
for example
thermistors are disposed in each applicator region.
[00332] In various embodiments, an applicator is capable of setting the
temperature of each
separate treatment zone individually. In some embodiments, one or more
individual zones can
have a different target temperature or can be shut off entirely depending on
the prescribed course
of treatment. For example, a treatment zone placed over the belly button
region may be shut off
due to sensitivity. In various embodiments, the amount of zone heating can be
determined by ratio
of change in resistance to resistance (AR/R), which is the change of
impedance. During treatment,
the applicator is configured to maintain a specified temperature in a target
temperature range for
at least about 12 minutes. In one embodiment, an example range for treatment
time is from about
12 to about 15 minutes. In one embodiment, the time each region is energized
is 12 to 15 minutes
divided by number of applicator regions.
[00333] In various embodiments, the applicator is suited for applying energy
to a uniform
volume of tissue. Uniformity or overall uniformity is achieved using zone by
zone uniformity
across each treatment zone of the applicator. In some embodiments, each zone /
region is heated
for a time period of P or about P, wherein the total treatment time TTT is
product of (about P or
P)(number of applicator regions). In one embodiment, about P or P ranges from
about 20 seconds
to about 2 minutes. In one embodiment, about P or P ranges from about 40
seconds to about 3
minutes. In one embodiment, about P or P ranges from about 1 minute to about 2
minutes. In
100
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
one embodiment, about P or P ranges from about 1.5 minutes to about 2.5
minutes. In one
embodiment, about P or P ranges from about 1 minutes to about 10 minutes. In
one embodiment,
about P or P ranges from about 1 minutes to about 3 minutes. In one
embodiment, about P or P
ranges from about 2 minutes to about 8 minutes.
[00334] Outside the boundaries of the applicator, the energy applied by the
applicator may
cause thermal spread. For example, in at least one embodiment, the tissue
under the kerfs
eventually reaches a uniform temperature or within a particular treatment
temperature range as a
result of heat delivered by two or more regions of the applicator spreading
and spanning tissue
under a kerf. In one embodiment, kerfs terminate within boundary of applicator
at a channel, hole
or other opening such opening 910 shown in FIG. 15C. In some embodiments, the
holes or
openings are circular or elliptical. These holes or opening can serve as
strain relief elements.
[00335] As shown in FIG. 15A, the applicator 900 includes zones or regions 1-
6, R1-R6. In
some embodiments, the applicator includes an outer label that shows and
enumerates1-6, R1-R6.
Such a labelled outer layer is shown in applicator 905 in FIG. 15C, with
regions 1-6 shown with a
number printed on the applicator label with each number within a circular
boundary. The printing
of the numbers on the label is white in one embodiment, but may be any
suitable color. In one
embodiment, the label includes vinyl, plastic or another polymer material. The
labelled regions 1-
6 correspond to applicator regions or zones R1-R6. These zones may be
displayed on a user
interface to facilitate deactivating one or more zones prior to starting
treatment.
[00336] In one embodiment, each zone or region is bounded by a two kerfs K
with seven kerfs
K in total. In some embodiments, applicators can use fewer zones, more zones,
and/or have a
different shape and size. The limits on geometry and size of an applicator
depends on whether or
not the RF generator can provide enough energy for heating the desired
treatment zone. During
the initial stages of treatment, each treatment zone of the applicator is
activated sequentially in a
clockwise manner R1 to R2, R2 to R3, R3 to R6, R6 to R5, and R5 to R4. A
counterclockwise
activation scheme or other activation schemes and patterns as described herein
may be used.
Thereafter, the temperature of each zone is measured using a temperature
sensor H and modified
as necessary to maintain a set surface temperature of (40-44 C). Individually
each treatment zone
maintains a temperature in the range of (42-47 C) to achieve the set surface
temperature of (40-
44 C) for the overall region covered by the applicator. In this embodiment,
the applicator covers
101
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
an area 300 cm2. In various embodiments, a size of an applicator can range
from about 50 cm2 to
about 600 cm2. In one embodiment, the label used on applicator surface 900 may
include a
polymer and designed to look like a metallic surface such as brushed chrome or
another gray or
silver metallic visual element. In one embodiment, the applicator includes a
label that includes
one or more pigments arranged to display a metal, metallic, or metal like
appearance.
Flexibility
[00337] In various embodiments, applicator flexibility is enhanced by
kerfs/voids that enable
the applicator be flexible enough to conform to the contours of the body. For
example, as shown
in FIG. 15A, each kerf frees various portions / zones of an applicator to flex
according depending
on where the portion / zone is being applied on the body. The applicator shown
in FIG. 15A has
seven kerfs K in total. In various embodiments, the number kerfs employed
depends on the size
and shape of an applicator and how the applicator is intended to be applied to
various body parts.
In various embodiments, each applicator includes a plurality of kerf-free
region such as an inner
region or spine from which regions or zones extend and before which various
kerfs terminate. An
exemplary inner region 495 is shown within dotted lines in FIG. 22A. The inner
region typically
includes a high density of substantially parallel conductors that branch off
to supply current to
various regions. The density of conductors is high because an increased number
of conductive
traces are positioned next to each other in this region. As a result, the
inner region can generate
excess heat. As a result, the inner region can include one or more heat
shields. The inner region
495 has a spyglass, skyscraper, stepped, or tapered configuration that follows
from the number of
adjacent traces decreasing in direction of dotted arrow AW moving away from
the electrical
connector 890 of the applicator.
[00338] The dielectric of the applicator has a dielectric constant that
ranges about 3 to about 4,
which provides a balance of capacitance vs. dielectric thickness while also
having flexibility
suitable for a patient tissue contacting applicator. As another consideration,
when selecting
dielectric material for an applicator, it is desirable for the material to
have a low heat loss or heat
dissipation factor and also be tolerant of high temperatures to permit
soldering of components
relative to conductors used with the dielectric material. Further, the
selected dielectric of the
applicator is skin safe and biocompatible. For example, in one embodiment,
Kapton is one
102
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
dielectric that fulfills these requirements. However, Kapton could be replaced
by any dielectric
that fulfills these requirements.
Uniform Heating- Applicator Considerations
[00339] In various embodiments, each zone of an applicator includes a
plurality of RF traces to
provide RF energy and/or heating from each of the zones. Without any
management of the heat,
RF traces can result in edge effects which makes providing uniform heating
from an applicator
difficult. A relatively flexible dielectric insulation, such as Kapton, can be
selectively placed to
promote uniform heating in a given treatment zone.
[00340] FIG. 18 shows an exemplary arrangement of materials 940 of an
applicator or a portion
thereof such as a zone of an applicator, in accordance with an embodiment of
the disclosure. These
layers are also combined to form applicator 980 in FIG. 19A. As shown, there
is a first side 900
and a second side 945. In one embodiment, the first side and second side
correspond to a vinyl
side and a wet side, or vice versa. Alternatively, in one embodiment, the
first side and second side
correspond to a patient facing side and an air facing side, or vice versa.
During treatment, the wet
side is placed in contact with patient tissue to be treated. In some
embodiments, an aqueous gel
such as a hydrogel is applied to the tissue facing / wet side to reduce or
avoid air gaps. Further,
the use of such a gel may improve the amount of current that can penetrate the
tissue. In various
embodiments, one or more thermistors are placed within each zone to monitor
temperature during
a given treatment method. Temperature sensor are in communication with a
control system and
can be used to change current levels when higher RF energy and associated
current level may
otherwise cause edge effects or instances of non-uniform heating. In at least
one embodiment, a
six zone applicator includes a thermistor in each of the zones.
[00341] As shown in FIG. 18, optional release liners 946 may be included with
sterile
disposable applicator and are peeled off prior to apply applicator to patient.
The liner protects a
gel layer 960 in some embodiments. Two dielectric material layers 950A, 950B
sandwich a
conductive layer 955 and a polyimide layer 957 using adhesive which may be
disposed as two
layers 953A, 953B. In various, embodiments the dielectric material layers may
include a Kapton
layer such as a cover or overlay. Layers 950A, 950B, 957 may be dielectric
layers, such as first,
second and third dielectric layers. The conductive layer 955 include a metal
or an arrangement of
electrical traces. The conductive layer includes copper or copper traces in
various embodiments.
103
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
The copper layer can include a continuous thin layer of copper metal.
Alternatively, for various
embodiments the copper layer comprises a plurality of copper traces extending
into each region or
zone of the applicator. The extent to which traces are placed closer to each
other and more of them
clustered in a parallel or concentric or other configuration results in
increased trace density. A
patient contacting layer 945, such as a water-based gel layer is also shown.
An arrangement of the
various layers of FIG. 18 are also shown relative to an exploded view of an
applicator in FIG. 19A.
[00342] In FIG. 19A, the releasable liner 946 is adjacent or below the gel
layer 960. A heat
shield layer 975 is also used to protect the inner region or spine of
applicator. The heat shield layer
975 is elongate and optionally flared or tapered in some embodiments. The
flexible applicator 880
includes the conductive layer, multiple dielectric layers, and adhesive layers
discussed above. A
label may be disposed on the applicator as discussed herein. In addition,
another release liner 947
may also be used. A strain relief element 885 may also be used to reinforce
extension region of
applicator that includes electrical connector 890. In one embodiment, an
elongate region 893
extends from applicator like a tail or parallel electrical cable that
terminates in electrical connector
890. FIGS. 19B and 19C show different views of the combination of layers shown
in FIG. 19A.
In turn, FIGS. 20A-20F show different views of the applicator 980 with one or
more release liners.
FIGS. 21A-21F show various views of an applicator 981 that does not include a
release liner.
[00343] As shown in FIGS. 22A and 22B, the copper traces can be arranged in
various patterns
such as various paths. In some embodiments, the copper traces are arranged in
spiral, area filling
curves, nested rectilinear regions, nested curved regions and combinations
thereof. Examples of
conductive traces 490 are shown in FIGS. 22A and 22B. The center of a given
zone includes a
higher concentration of copper trace material, while the outer perimeter of
the same zone shows
thinner copper gradients towards the edges of the perimeter.
[00344] In various embodiments, the copper traces are arranged to have a
greater positional
density or be clustered at the center of the zone or in a particular part of
the applicator. In some
embodiments, there are higher concentration of copper trace material in the
center portions of a
given zone as a result of more copper traces being placed relative to each
other. Thus, in one part
of applicator in a given area more traces are placed next to each other than
others, thereby
increasing density or count of copper traces per unit area. A single set
thickness is typically used
for traces. Although that thickness can vary over suitable ranges such as from
about .01 inches to
104
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
about .3 inches. In some embodiments, a set of traces with a common width or
thickness may be
ore densely clustered in one area and less densely clustered in another area.
As an example, in
the spine area multiple traces are positioned next to each other in parallel,
juxtaposed, adjacently,
or otherwise. This can result in additional heating relative to the region of
increased traces being
positioned relative to each other and benefits from the inclusion of one or
more heat shield layers.
[00345] In various embodiments, the Kapton can be replaced with another
suitable dielectric,
subject to the preferred dielectric properties described herein. Further, in
these embodiments, the
base polyimide can be replaced with another dielectric, i.e., polyester. In
various embodiments,
the thickness of each of the layers of FIG. 18 ranges from about 0.5 mil to
about 1.5 mil. The
optional hydrogel layer has a thickness greater than 1 mil in various
embodiments.
[00346] The applicators are generally designed to be disposable and work in
conjunction with
a suitable interface device 905 as shown in FIG. 16. The interface device 905
is in electrical
communication with a RF-based treatment system such as systems 100, 800, 1200
or other suitable
systems disclosed herein. The interface device supports using sterile
applicators and changing to
applicators having different sizes, shapes and regions that facilitate
alignment of electrical traces.
The interface device 905 also supports quick connection and release of
applicator from the
interface device. The interface device shown is one exemplary device for
connection of an
applicator. In another embodiment, pluggable cable interfaces that releasably
attach to an
applicator's electrical terminus may be used and other interface devices
suitable for making
aligned contact with multiple electrical contacts at the electrical terminus
of the applicator.
[00347] As shown, the interface device 905 of FIG. 16 depicts a clamp / clip
device 920 and a
cable adapter 921. The cable adapter 921 may include one or more electrical
conductors and/or
one or more optical connectors or optical devices such as a light pipe or
optical fiber section. In
various embodiments, a light pipe or optical fiber section is used as part of
an interface device to
support optically showing when applicator is energized or in a particular
state or treatment. The
clamp / clip device 920 may include one or more electrical to optical
conversion devices. The
cable adapter 921 may connect to other extension cables or subsystems. In one
embodiment, cable
adapter 921 connects to one or more of the systems disclosed herein such as
systems 100, 800,
1200/ Additional details of the interface device are discussed in more detail
below.
105
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00348] As edge effects are present in RF traces, the applicators' use of
selective dielectric
insulation creating various gradients of dielectric insulation throughout the
applicator surface and
approach to graduating and/or scaling trace geometries by creating various
gradients of copper
trace throughout the applicator surface (FIGS. 22A, 22B) both manipulate edge
effects to achieve
better uniformity of temperature and flexibility.
[00349] FIG. 17 shows an alternative applicator embodiment 905 that
incorporates nested
dielectric regions. Within the applicator, in one embodiment, a conductive
layer that includes
copper is evenly spread throughout. The surface of the applicator, shown in
FIG. 17, is a gradient
of dielectric with multiple layers of dielectric such that the center of a
given zone features a single
layer of the dielectric (1 mil thick). For each zone, when moving outwards
from the center, each
subsequent gradient has an additional layer. For example, the center has a
single layer of dielectric
DL1, the next gradient outwards has two layers DL2 of the dielectric (1 mil
thick), the next gradient
outwards has three layers of the dielectric (3 mils thick) DL3, and the final
outer perimeter includes
four layers of the dielectric DL4 (4 mils thick). The center region 913 is a
hole defined by layered
components of applicator and suitable for navel placement or over other
sensitive regions.
[00350] In various embodiments, the applicator is a conformable applicator
that applies RF
energy to heat one or more zones. In at least one embodiment, the applicator
is used with a
waveform generator that operates at about 4MHz. The 4 MHz generator
facilitates providing RF
energy uniformly across a dielectric using thin common materials over large
areas. However, in
other embodiments, other high frequency generators can be used. In one
embodiment a 3MHz
generator is used. Suitable waveform generators for use with the flexible
applicators disclosed
herein are typically components of or in electrical communication with one or
more the systems
disclosed herein including systems 100, 800, and 1200.
[00351] In various embodiments, using RF energy and placing current into the
body, for a given
capacitive electrode area, is dependent on various properties. For example, as
dielectric thickness
decreases, RF current and the tendency of any air to ionize increase
proportionally. The relative
permittivity (i.e., the dielectric constant) of the dielectric layer
increases, RF current and the
tendency of any air to ionize increase proportionally. As voltage applied to
the electrode increases,
RF current and the tendency of any air to ionize increase proportionally.
106
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00352] As the frequency of the RF waveform increases, the RF current
increases
proportionally. In contrast, the tendency of any air to ionize does not
increase proportionally.
Thus, RF frequency may be increased to increase RF current per unit area
without increasing the
tendency of forming a corona discharge or a plasma in air in proximity to
tissue undergoing
treatment with an applicator. Accordingly, mitigating corona or plasma
discharge is one advantage
of the RF-based applicators disclosed herein. In various embodiments, the
frequency range of RF
generator is from about 1 to about 5 MHz. In some embodiments, a 4 MHz
generator balances
requirements because at higher frequencies inductance can be problematic and
at lower
frequencies the capacitive gradient required for uniform heating is more
difficult to achieve.
[00353] In various embodiments, the applicator makes use of, or manipulates,
edge effects via
selective dielectric insulation placement and positioning, creating various
gradients of dielectric
insulation such by thickness variation throughout the applicator surface and
graduating and/or
scaling trace geometries by creating various gradients of copper traces
throughout the applicator
surface. Examples of such traces 490 are shown in FIGS. 22A and 22B.
Graduating trace
geometries creates multiple edges so that edge effects are present throughout
the applicator surface
providing a pattern that leads to substantially uniform heating. In addition
to the temperature
uniformity provided by the applicator, the flexibility of the applicator is
also considered when
determining the topology of dielectric gradient and/or copper trace gradients
in the applicator.
[00354] In various embodiments, the pattern of materials sandwiched together
is as follows:
dielectric, copper, dielectric, etc. Various stacks of layers of material can
be used for a given
applicator. In turn, various sandwich constructions are possible in which one
or more layers is
sandwiched between two layers, a layer and a stack of layers, or two stacks of
layers.
[00355] In various embodiments, a given applicator has a skin tissue
contacting side 945. The
skin contacting side includes a substance that is wet enough to conform to the
stratum corneum
therebetween. For example, in at least one embodiment, a thin adhesive that is
micro-conformable
(i.e., "wet") enough to conform to the stratum corneum will work well to allow
coupling into the
skin. The thin adhesive facilitates avoiding air gaps, which can reduce how
much current can be
coupled into the body. The air gap is filled with something water-based, and
optimally water-
based with a current-carrying ion, such as saline as part of various methods
of treatment. For
example, a suitable substances can include a hydrogel. In an alternate
embodiment, an aggressive
107
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
thin adhesive would also work, but should be made in a way to allow it to
"wet" into the stratum
corneum. In various embodiments, the applicator can be pre-wetted such that a
cover is peeled off
prior to application of the wet side to the subject's skin. Various
embodiments include removable
covers such as releasable liners for example such as those shown in FIGS. 19A,
19B, 19C, 20A
and 20B
[00356] For example, a conductive hydrogel, is loaded onto the subject facing
side of the
applicator, the hydrogel compensates for bumps, hairs and it sticks the
applicator to the subject's
body. The hydrogel hydrates the skin and makes it tacky or sticky. An
alternative could be a
conductive adhesive or pressure sensitive adhesive. However, another
embodiment could include
a dry surface and the subject's skin is wetted with the wetting substance
(e.g., Ultrasound gel,
hydrogel) and then the applicator is maintained in place. In some embodiments,
a bandage is
wrapped around the applicator and all or a portion of the subject's body to
ensure the applicator is
maintained in place. This applicator would likely be less convenient because
proper placement
might require two practitioners to apply the applicator and/or a cummerbund or
wrap to maintain
the applicator in place.
Quick Connect Interface Devices
[00357] An applicator may connect to a system, such as RF-based systems shown
in FIGS. 1A-
1F, 8 and 12 using various types of interface devices, connectors, and
adapters. The relative
placement of applicator and clamp 920 are shown in FIGs. 23A-23C. An exemplary
interface unit
is shown in FIG. 16. Additional views of interface device are shown in FIGS.
23A-24F. The
interface unit 918 includes various components. A cable interface device /
cable interface adapter
extends 921 from clamp 920. In some embodiments, the cable is fixedly attached
to the interface
device, while in others it is releasably attached such as by being pluggable
and unpluggable relative
to the interface device. In some embodiments, the applicator includes a
plurality of electrical traces
at its connection interface. In light of the goal of matching up the
electrical traces to corresponding
interface device trace specific connections the interface device is a spring-
biased clamp / clip. In
this way, when interface device is compressed, it opens to receive a terminal
portion of an
applicator a shown in FIG. 23C. The applicator terminal includes one or more
alignment features
to facilitate the proper mating and alignment of terminal electrical
connections in the applicator
such as via alignment device 895. The alignment device 895 can include holes
or other regions or
108
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
shapes defined by connector 890 that are designed to mate with and align with
connectors in
interface device 920, such as pins, grooves, etc. In other embodiments, the
interface device may
be used with the other applicators disclosed herein. FIG.23C shows the clamp
in an open position
ready to receive and electrically connect with electrical connector of a given
applicator. FIGS.
24A-24F show various views of the interface device 918.
[00358] The interface device includes an upper or top housing, a lower or
bottom housing, and
an electrical subsystem that may include a printed circuit board. The top
housing and the bottom
housing sandwich the printed circuit board one or more optical connection
Distributor Function
[00359] The applicator is connected to the RF generator using an interface
device such as a
durable quick disconnect clamp embodiment and others as disclosed herein. The
interface device
is connected to an electrical distribution box that connects to the RF
generator RFG. In one
embodiment, the distribution box includes a printed circuit board and/or a
collection of circuit
elements. In one embodiment, the interface device includes a distribution box
and provides
distribution and/or control functions while also locking and releasing the
disposable applicator. In
a given distribution box / hub implementation, the box/ hub is configured to
monitor and control
the various regions / zones of the applicator with minimal cross talk. Various
distribution
arrangements suitable for use with the flexible RF-based applicators and other
applicators
disclosed herein are shown in FIGS. 25A-25C.
[00360] In one embodiment, the distribution box / hub avoids use of multiple
umbilicals that
can lead to cross talk/signal interference. In one embodiment, a splitter for
two or more umbilicals
can be used simultaneously.
[00361] FIG. 25A shows as distribution system that includes a distribution box
local to the
applicator which performs the task routing RF from the generator and locally
distributing it to the
individual electrodes to improve feedback control.
[00362] In various embodiments, the system shown in FIG. 25B includes a
distribution box
local to each of the applicators. The combination of applicator and
distribution box obtains RF
from the generator through a second distribution box. The architecture shown
in FIG. 25C also
has a distribution box local to each of the applicators. However, in this
embodiment, each
109
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
distribution box is directly connected to the generator. These and other
applicator connection
schemes can be used relative to a treatment system 100.
Exemplary RF-based Treatment methods
[00363] In various embodiments, the multi-zone / multi-region applicator that
includes one or
more kerfs and inner region / spine can be used to facilitate various
treatment methods. These
systems and methods can include various non-medical related cosmetic and/or
aesthetic
treatments, such as for skin tightening and/or body sculpting such as through
causing lipolysis. In
one method, a template is used to characterize one or more patient areas for
treatment such that
the patient and operator can reach consensus regarding the treatment approach
and target regions.
Once treatment is agreed upon, the patient may be marked up and otherwise
measured and
evaluated to determine which applicator to use and various other treatment
parameters such as
treatment time and target temperature range for uniform heating. Typically,
marking up of
patient's body is performed to outline regions of treatment that will receive
one or more RF-based
applicators.
[00364] In one embodiment, patient lies down (after neutral electrode pad or
NEM attached)
and operate prepares subject using one or more of the following use hydrogel
predisposed on
applicator, use pressure sensitive adhesive predisposed on applicator, layer
hydrogel on the patient
then apply it to patient, and /or layer ultrasound gel on patient then
applicator and then bandage to
maintain attachment of applicator. In various embodiments, operator accesses
user interface of
system 100 to identify a region such as belly button or select a small zone
(e.g., zone 5 in FIG.
15C or a subset of a zone such as region 912) on belly button and then turn
off zone 5 in GUI prior
to treatment.
[00365] During initially heating phase, the operator selects a predetermined
round robin region
/ zone activation scheme and then interrogates tissue with RF to reach target
temperature. In one
embodiment, each zone / region is heated for a set time such as a max time,
such as about 10
seconds, and then each zone is sequentially heated for 10 seconds until target
temperature is
reached. In one embodiment, target temperature is reached in about 1 to about
60 seconds. A
threshold of 42 C may be set such that thermistors will cause a cessation of
heating when this
temperature is reached. In various embodiments, each zone / region is
initially heated in a
predetermined sequence (i.e., round robin) until a desired temperature is
achieved. For example,
110
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
in one embodiment, each zone / region is activated for a specified time (i.e.,
10 seconds) to bring
the tissue up to a desired temperature. As treatment progresses, the
applicator zones are
interrogated pursuant to an alternating pattern or scheme such as round robin,
sequential based on
region number, random, or others such that a given zone / region is actively
being heated for a
period of time and then another zone / region is switched to and also heated.
In this way, shifting
between different zones and actively heating them before again switching to
another zone
maintains the desired temperature or temperature range for the underlying
tissue until the overall
treatment time has been reached. In various embodiments, treatment time can be
from about 10
minutes to about 15 minutes. In various embodiments, treatment time can be
from about 12
minutes to about 24 minutes. In one embodiment, tissue is interrogated on a
zone by zone or
region by region basis so that the tissue underlying a given zone or region is
maintained within the
desired temperature region for the required time period, e.g., a zone is
interrogated for 10 seconds
to bring it within the range of about 42 C to about 47 C or from about 42 C
to about 44 C and
interrogated multiple times over the course of treatment such that overall
treatment time of from
about 10 min to about 15 min is achieved.
[00366] In other embodiments, compressible foam is used to bias tissue towards
an applicator
or array for contact to one or more electrodes to facilitate or support a
given RF treatment of the
tissue being contacted. In one embodiment, the electrodes are coupled
electrically to reduce cross
talk by providing a tacky but easy to remove adhesive applicator for pre-
positioning on target
treatment areas. Cross-talk may be reduced by including one or more
insulating, conductive,
semiconducting, materials such as gels or materials or layers. These layers
can be doped with or
formed using various insulating, conducting, and semiconducting materials to
reduce cross-talk or
other electrical interference when performing an RF-based treatment using one
or more electrodes
and/or applicators.
[00367] FIGS. 26A-26F show various different views of an applicator
constructed and
configured for use in the submental region (i.e., the neck and/or chin areas),
in accordance with an
embodiment of the disclosure. In one embodiment, a submental applicator
includes two or more
regions. In one embodiment, a submental applicator may have a shape that is a
fraction of a circle
or an ellipse, such a semicircle, a sector, or half or a third or another
portion of an ellipse. FIG.
26A shows a top view of an RF-based flexible applicator 2600 for treating the
submental region.
111
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
FIG. 26B shows a bottom view of the applicator 2600 shown in FIG. 26A. FIG.
26C and FIG.
26D show side views of the applicator 2600. FIGS. 26E and 26F show a rear view
and a front
view of the applicator 2600 described herein. The submental applicator may
include one or more
release liners although such liners are optional for the applicators disclosed
herein.
[00368] FIG. 27A shows an exploded view of the various layers of an applicator
constructed
and configured for use in the submental region, in accordance with an
embodiment of the
disclosure. Similar to the applicator shown in FIGS. 19A-19C, the applicator
2600 for treating the
submental region (i.e., neck / chin) has multiple layers. As shown, the
releasable liner 2740 is
adjacent or below the gel layer 2735. A heat shield layer 2730 is also used to
protect the inner
region or spine of the applicator. The heat shield layer 2730 is optionally
flared or tapered in some
embodiments. The flexible applicator 2725 includes the conductive layer,
multiple dielectric
layers, and adhesive layers. A label 2720 may be disposed on the applicator as
discussed herein.
In addition, another release liner 2715 may also be used. FIGS. 27B and 27C
show different views
of the applicator with one or more release liners. The applicator electrical
connector 2710 which
is connected to the flexible applicator 2725. The system connects to the
electrical connector 2710
using mated connector 2705 which may also operate to provide strain relief.
[00369] FIGS. 27B and 27C show two different perspective views of the
applicator shown in
FIG. 27A.
[00370] Graphical User Interface Features
[00371] The functioning of the display/GUI as it relates to the zones is
helpful for protecting
sensitive regions and showing temperature information to an operator of an
applicator-based
treatment system. The GUI 2800 of FIGS. 28A and 28B shows treatment
temperature of each
zone (zones 1 to 6 shown), identifies when zone is at treatment temperature
and whether it is
actively delivering RF. In some embodiments, such as applicator embodiment of
FIG. 15C one or
more of the plurality of layers comprises a label, wherein the label comprises
W region identifiers,
wherein each of the W region identifiers is disposed on one of the W regions.
These regions are
labeled 1 to 6 and correspond with regions / zones 1 to 6 shown in the GUI
2800. In some
embodiments, W ranges from 2 to 16. In FIG. 15C W is six as shown by
applicator label.
[00372] Figs. 28A and 28B depicts a graphical user interface (GUI) for use
with a system using
an applicator, such as the applicator shown in Fig. 15C, in accordance with
various aspects of the
112
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
present teachings. Fig. 28A shows a GUI 2800 in communication with an
applicator. The GUI
2800 is displayed on a display of one of the treatment systems100, 800, 1200
and is implemented
using one or more software modules that receive input signals from temperature
sensors of
applicator. Fig. 28A shows the graphics/buttons that are oriented in the same
manner as the zones
on the applicator. Fig. 28B shows one of the buttons grayed out which means
the zone has been
turned off for sensitive regions.
[00373] In this embodiment, the GUI 2800 configures / manages each zone of the
applicator. In
this case, buttons / controls 2805-1, 2805-2, 2805-3, 2805-4, 2805-5, and 2805-
6 (2805 generally)
correspond to six zones within the applicator. In other embodiments, an
applicator can have more
or less than six zones. Each of the buttons / controls 2805 designates a
desired temperature for
each zone. The controls 2810 can be used to modify the desired temperature for
each zone or
adjust the treatment time. Additionally, the GUI 2800 shows the treatment time
in display
2815. As shown in Fig. 28B, button / control 2805-2 is grayed out, which
indicates that zone two
has been turned off due to sensitive regions being in close proximity to zone
two. In various
embodiments, the GUI 2800 is capable of deactivating one or more zones as
needed.
[00374] All of the drawings submitted herewith include one or more ornamental
features and
views, each of which include solid lines any of which also incorporate and
correspond to and
provide support for dotted lines and alternatively, each of which include
dotted lines any of which
also incorporate and correspond to and provide support for solid lines.
[00375] The use of the terms "include," "includes," "including," "have,"
"has," or "having"
should be generally understood as open-ended and non-limiting unless
specifically stated
otherwise.
[00376] The use of the singular herein includes the plural (and vice versa)
unless specifically
stated otherwise. Moreover, the singular forms "a," "an," and "the" include
plural forms unless
the context clearly dictates otherwise. In addition, where the use of the term
"about" is before a
quantitative value, the present teachings also include the specific
quantitative value itself, unless
specifically stated otherwise.
[00377] It should be understood that the order of steps or order for
performing certain actions
is immaterial so long as the present teachings remain operable. Moreover, two
or more steps or
actions may be conducted simultaneously.
113
CA 03125664 2021-06-30
WO 2020/142470 PCT/US2019/069015
[00378] Where a range or list of values is provided, each intervening value
between the upper
and lower limits of that range or list of values is individually contemplated
and is encompassed
within the disclosure as if each value were specifically enumerated herein. In
addition, smaller
ranges between and including the upper and lower limits of a given range are
contemplated and
encompassed within the disclosure. The listing of exemplary values or ranges
is not a disclaimer
of other values or ranges between and including the upper and lower limits of
a given range.
[00379] It should be appreciated that numerous changes can be made to the
disclosed
embodiments without departing from the scope of the present teachings. While
the foregoing
figures and examples refer to specific elements, this is intended to be by way
of example and
illustration only and not by way of limitation. It should be appreciated by
the person skilled in the
art that various changes can be made in form and details to the disclosed
embodiments without
departing from the scope of the teachings encompassed by the appended claims.
114