Note: Descriptions are shown in the official language in which they were submitted.
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
STABLE CATHODE MATERIALS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application depends from and claims priority to U.S. Patent
Application No:
62/796,950 filed January 25, 2019, U.S. Patent Application No: 16/728,379
filed December
27, 2019, U.S. Patent Application No: 16/250,615 (now U.S. Patent No:
10,501,335) filed
January 17, 2019, U.S. Patent Application No: 16/250,762 filed January 17,
2019, U.S. Patent
Application No: 16/250,622 filed January 17, 2019, and PCT Application No:
PCT/US2019/057630, filed October 23, 2019, the entire contents of each of
which are
incorporated herein by reference.
FIELD
[0002] This disclosure is directed to electrochemically active materials
for use in a cathode
of a secondary battery.
BACKGROUND
[0003] LiM02 materials based on lithium nickel oxide (LiNi02) in a layered
structure are
desirable as lithium battery cathode materials because they generally provide
lower cost, higher
capacity, and higher rate capability than lithium cobalt oxide (LiCo02).
However, pure LiNi02
materials exhibit poor electrochemical stability and cycling performance. It
has been found
that by substituting varying amounts of other metals for some or much of the
bulk material Ni
in LiNi02, some of the capacity and cost benefits of LiNi02 can be obtained
with improved
electrochemical cycling stability.
1
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0004] It was also found that even such substituted LiNi02 materials may
have inadequate
stability when they are charged to high capacity (e.g., >220 mAh/g). As such,
new materials
are needed that have improved capacity and/or cycle life.
SUMMARY
[0005] The following summary is provided to facilitate an understanding of
some of the
innovative features unique to the present disclosure and is not intended to be
a full description.
A full appreciation of the various aspects of the disclosure can be gained by
taking the entire
specification, claims, drawings, and abstract as a whole.
[0006] Provided are electrochemically active particles that include grain
boundaries with
a higher electrochemical affinity for lithium that exhibit improved cycling
ability with
comparable capacity relative to base materials without stabilized grain
boundaries. By
increasing the electrochemical affinity for Li by selectively increasing the
oxidation potential
of the grain boundary region of the particles, the inventors found they could
increase capacity
retention and reduce impedance growth during cycling without significantly
reducing capacity
relative to that of the active material without grain boundary stabilization.
[0007] As such, provided are electrochemically active particles that may be
used as an
active material in a cathode of an electrochemical cell (or other such
device), that include: a
plurality of crystallites comprising a first composition comprising lithium,
nickel, and oxygen;
and a grain boundary between adjacent crystallites of the plurality of
crystallites and
comprising a second composition comprising lithium, nickel, and oxygen;
wherein the grain
boundary has a higher electrochemical affinity for lithium than the
crystallites.
2
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0008] Also provided are electrochemically active particles that include a
plurality of
crystallites comprising a first composition comprising lithium, nickel, and
oxygen; a grain
boundary between adjacent crystallites of the plurality of crystallites and
comprising a second
composition comprising lithium, nickel, and oxygen; wherein lithium is present
in the grain
boundary at a higher concentration than lithium inside the crystallites when
the particle is at a
state of charge greater than or equal to 10 percent.
[0009] Also provided are electrochemically active particles that include: a
plurality of
crystallites comprising a first composition comprising lithium, nickel, and
oxygen; a grain
boundary between adjacent crystallites of the plurality of crystallites and
comprising a second
composition comprising lithium, nickel, and oxygen; wherein lithium is present
in the grain
boundary at a higher concentration than lithium in the crystallites when an
electrode
incorporating the particles is charged to a potential greater than or equal to
4.1 V versus lithium,
or wherein lithium is present in the grain boundary at a higher concentration
than lithium in the
crystallites at a charge capacity of 40 mAh/g or greater.
[0010] Also provided are electrodes that incorporate one or more of the
electrochemically
active particles as provided herein. Electrochemical cells are also provided
that include in the
cathode one or more of the electrochemically active particles as provided
herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] The aspects set forth in the drawings are illustrative and exemplary
in nature and
not intended to limit the subject matter defined by the claims. The following
detailed
description of the illustrative aspects can be understood when read in
conjunction with the
3
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
following drawings, where like structure is indicated with like reference
numerals and in
which:
[0012] FIG. 1 illustrates that as the amount of Ni substitution in LiM02 by
other metals
increases, the amount of Li extracted from LiM02 upon charging to a given
potential decreases;
[0013] FIG. 2 illustrates that LiM02 materials with lower Ni content than
LiNi02 are
delithiated to a lesser extent than LiNi02 when the materials are charged to
the same potential;
[0014] FIG. 3 shows the locations of EDS line scans in a thin lamella
prepared from a
particle of grain boundary-modified LiM02 material of Example 4;
[0015] FIG. 4 shows results of EDS line scans in a thin lamella prepared
from a particle
of grain boundary-modified LiM02 material of Example 4 with A and C
representative of the
LS1 scan line of FIG. 3, and B and D representative of the LS2 scan line of
FIG. 3;
[0016] FIG. 5 shows XRD results for the charged and fresh cathode
electrodes of the grain
boundary-modified LiM02 material of Example 3 and its uniform-composition
LiM02 base;
[0017] FIG. 6 shows high resolution XRD results for the charged electrodes
of the grain
boundary-modified LiM02 material of Example 3 and its uniform-composition
LiM02 base
with peak position offset and scaled to the (104) peak;
[0018] FIG. 7 illustrates discharge capacity as a function of cycle number
for grain
boundary-modified LiM02 material of Example 1 and its uniform-composition
LiM02
precursor during cycling at 45 C in full coin cells;
[0019] FIG. 8 illustrates a comparison of the impedance increase during
cycle life
measurement at 45 C of materials as illustrated in FIG. 7 in full coin cells;
4
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0020] FIG. 9 illustrates discharge capacity as a function of cycle number
for grain
boundary-modified LiM02 material of Example 2 and its uniform-composition
LiM02
precursor during cycling at 45 C in full coin cells;
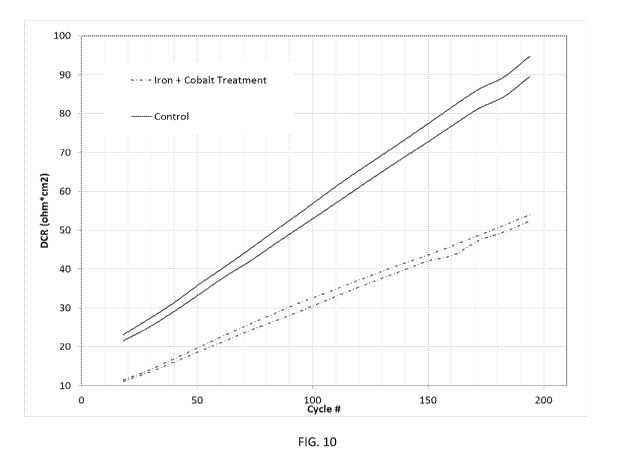
[0021] FIG. 10 illustrates a comparison of the impedance increase during
cycle life
measurement at 45 C of materials as illustrated in FIG. 9 in full coin cells.
[0022] FIG. 11 illustrates discharge capacity as a function of cycle number
for grain
boundary-modified LiM02 material of Examples 3-5 and its uniform-composition
LiM02
precursor during cycling at 45 C in full coin cells;
[0023] FIG. 12 illustrates a comparison of the impedance increase during
cycle life
measurement at 45 C of materials as illustrated in FIG. 11 in full coin
cells;
[0024] FIG. 13 illustrates the increased fracture toughness of particles
with low Ni in the
grain boundaries relative to the adjacent crystallites; and
[0025] FIG. 14 illustrates the reduction in the rate of impedance of
materials as provided
herein according to some aspects wherein the open symbols are bulk materials
and the closed
boxes are selectively reduced in Ni at the grain boundaries.
DETAILED DESCRIPTION
[0026] The inventors of this disclosure found that electrochemically
extracting high levels
of lithium from polycrystalline LiM02-based materials having 2D a-NaFe02-type
layered
structure induces the 2D layers of transition metal atoms within the
crystallite grain boundaries
to reorganize into a NiO-type rock salt structure with associated reduction in
transition metal
oxidation state and loss of oxygen, and introduction of defects like oxygen
vacancies and
intermixing of nickel between nickel and lithium layers in the ceramic. This
restructuring is
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
initiated at grain boundaries and propagates deeper into the crystallites with
repeated cycling.
Sometimes, that restructuring is associated with mechanical damage including
the appearance
of cracks between adjacent crystallites. This loss of material stability at
high levels of lithium
extraction results in decreasing capacity and increasing impedance of the
cathode materials as
they are cycled.
[0027] This disclosure is based on the discovery that the performance of
LiNi02-based
materials can be improved by designing the materials to retain more lithium in
the grain
boundary region at the end of charge by raising the oxidation potential of
this region; thus,
preventing or slowing down grain boundary reconstruction, reducing the likely
formation of
NiO and Li2O, and as is illustrated in this disclosure, reducing the rate of
impedance growth
for these materials. As such, provided in this disclosure are polycrystalline
LiM02-based
materials including 2D a-NaFe02-type layered structure that selectively retain
more Li in the
grain boundaries than in their crystallites' interior structure when the
materials are charged
and/or have increased stability in particular regions of the particles so as
to reduce the rate of
impedance growth during charge/discharge cycling of the battery and optionally
improve cycle
life. Even in the charged state, these materials retain Li in the grain
boundary where it is needed
to stabilize the delithiated crystallite material against structural
reconstruction propagating
from the grain boundaries, while still releasing Li from the bulk crystallite
structure to provide
for high cycling capacity. Retention of higher Li levels in the grain
boundaries of charged
materials relative to the bulk crystallites can be accomplished by selectively
raising the local
oxidation potential of the grain boundary region, thereby preventing grain
boundaries from
being delithiated to as great an extent as the LiM02 bulk crystallites when
the materials are
charged to a given electrochemical potential.
6
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0028] The nature of the role that raising the local oxidation potential in
the composition
of LiM02 grain boundaries plays in promoting Li retention in those grain
boundaries can be
understood by referring to Figure 1, which shows C/20 1" charge curves in Li
metal anode half
cells for cathode materials having varying proportions of Ni as M in LiM02.
These data show
that for a series of conventional LiM02-based Li-ion cathode materials, the
potential at which
Li is extracted upon charge increases, and the amount of Li that is extracted
at a given potential
decreases as the proportion of Ni in LiM02 decreases. Table 1 summarizes the
Figure 1 data
showing that as the Ni content of an LiM02 material decreases, the amount of
Li it retains when
it is charged to 4.3 V vs. Li increases. Data in Figure 1 and Table 1 are
based on coin cell
measurements made in-house with an exception of NM-11, which is based on Kang,
K., et al.,
Science, 311 (17), 2006, p.977 results.
Table 1: x in LixM02 remaining in cathode materials charged to 4.3V vs. Li.
x in LixM02 C/20 Charge to
Abbreviated
Material when charged 4.3V vs. Li
name
to 4.3V vs. Li (mAh/g)
LiNi02 LNO 0.06 258
NCM-811 0.19 223
LiNio.8Coo.15Alo.0502 NCA 0.27 204
LiNio.5Co0.2Mno.3 NCM-523 0.36 177
LiNi0.5Mno.5* NM-11 0.38 174
LiCo02 LCO 0.39 166
[0029] The metals replacing a portion of the Ni in the bulk (not selective
localization) of
the LNO materials of Figure 1 and Table 1 include Co and Mn that are
representative of metals
that effectively lessen the tendency for oxidation of LiM02 relative to Ni
when the LiM02
materials are charged, and Al which is representative of metals that are not
oxidized at all when
7
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
LiM02 materials are charged. The relative amounts of such metal substituents
impact these
bulk materials' extent of oxidation and retention of Li. Therefore, although
both NCM 811
and NCA have Ni composing 80% of M in LiM02, NCA retains more Li when it is
charged to
4.3 V vs. Li because whereas both Co and Mn in NCM 811 can be oxidized, Al in
NCA cannot
be oxidized.
[0030] The differing impacts of raising the local oxidation potential in
the LiM02 structure
can generally be understood by considering simplified concepts such as the
metals' oxidation
potentials and/or their electron count in the context of crystal field theory.
In the LiM02
stoichiometry, the mean oxidation state of M in the structure is +3. In the 2D
a-NaFe02-type
layered structure, the M atoms are in a relatively strong octahedral field,
which will therefore
be stabilized by metals with d6 (q9 crystal field configuration) and d3 (q9
crystal field
configuration) electron counts. Co' is d6, whereas Ni' is d7, and therefore
LiNi02 is more
prone to oxidation than LiCo02 because LiNi02 gains the stable q9 crystal
field configuration
when oxidized, whereas LiCo02 loses the q9 crystal field configuration when
oxidized.
Published X-ray absorption studies have indicated that Mn substitution for Ni
results in Mn
being accommodated in the stable +4 oxidation state (q9 crystal field
configuration), charge-
compensated by Ni in the +2 oxidation state (t.092 crystal field
configuration). Therefore,
oxidation of Ni atoms to the more stable q9 configuration requires
simultaneous transfer of
two electrons and charge-compensating Li + ions, whereas oxidation of Ni atoms
in LiNi02
proceeds by a more facile single electron/single ion process. Al in LiM02 is
already in the 3+
oxidation state and does not have an accessible 4+ oxidation state at
realistic battery cathode
material potentials.
8
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0031] The preceding discussion of oxidation and Li retention in the bulk
LiM02 cathode
materials illustrates how raising the oxidation potential in the grain
boundaries of LiM02
materials can provide for greater retention of Li in the grain boundaries than
in the bulk
crystallites when those materials are charged. Thus, for example, if a
material with LNO bulk
crystallite composition (M in LiM02 is 100% Ni) has grain boundaries with
composition
corresponding to one of the other materials in Figure 1, the reframed Figure 1
data that are
plotted in Figure 2 illustrate how much Li will be retained in the grain
boundaries relative to
the amount of Li retained in the LNO bulk crystallites when the material is
oxidized (charged)
to a given potential. The figure shows that the grain boundaries will retain
more Li than the
bulk crystallites at any potential or state of charge up to 4.3 V vs. Li.
Similarly, these data can
be normalized to the Li content of one of the other materials; for example, to
show that
materials with NCM(811) bulk crystallites and NCM(523) grain boundaries would
also retain
more Li in their grain boundaries than in the bulk crystallites when they were
charged. Note
that the 4.3 V vs. Li is illustrative, and similarly higher amount of Li can
be retained in the
grain boundaries than in the bulk even when the materials are charged to
potentials higher or
lower than 4.3V vs. Li.
[0032] As used herein the term "state of charge" (SoC) is the level of
charge of a battery
relative to its capacity. The units of SoC are percentage points (0% = empty
or discharged;
100% = full or charged). For the lithium nickel oxide materials as provided
herein full charge
is achieved at a potential of 4.1 V or greater, optionally 4.2 V or greater,
optionally 4.3 V or
greater, optionally 4.4 V or greater vs. Li.
[0033] As used herein the term "electrochemical affinity" for lithium is
defined as the
tendency to retain lithium when oxidized to a certain potential or voltage.
Therefore, a material
9
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
with a higher electrochemical affinity will retain more lithium when charged
to a certain
potential than a material with a lower electrochemical affinity for lithium.
The potential can be
a range of relevant potentials. A material with a higher electrochemical
affinity for lithium
implies that the material (or a portion of the material) will have a higher
lithium content
compared to a material with a lower electrochemical affinity when both
materials are at the
same potential.
[0034] As such provided are particles that include a plurality of
crystallites comprising a
first composition comprising lithium, nickel, and oxygen and having a layered
a-NaFe02-type
structure, a grain boundary between adjacent crystallites of the plurality of
crystallites and
comprising a second composition having the layered a-NaFe02-type structure, a
cubic
structure, a spinel structure, a monoclinic structure, or a combination
thereof, wherein the
second composition has either a higher electrochemical affinity for lithium
relative to the first
composition and/or compared to a material with a lower electrochemical
affinity when both
materials are at the same potential; and/or wherein nickel is present in the
second composition
at a lower concentration than nickel in the first composition.
[0035] Also provided according to some aspects of this disclosure are
particles that include
a plurality of crystallites comprising a first composition including layered-
layered
compositions with an overall formula of zLiM02.(1-z)Li2M' 03 where z is in the
range of 0.7
< z < 1.0, optionally 0.8<z<0.95 moles per mole of the composition. The
layered-layered
materials also include a grain boundary between adjacent crystallites of the
plurality of
crystallites and comprising a second composition having a layered a-NaFe02-
type structure, a
cubic structure, a spinel structure, a monoclinic structure, or a combination
thereof In some
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
exemplary aspects, M can include Ni, Co, Mn, or a combination thereof, and M'
can include
one or more of Mn, Ti, or Cr. It is noted that the oxidation potentials and
electrochemical
affinity of the grain boundary regions of the layered-layered materials may be
as described for
other materials as provided herein.
[0036] In the layered-layered materials, M optionally includes Ni alone or
with one or
more of Co, Mn, V, or Fe. The Ni component of M is optionally at or greater
than 0.3 to 0.95
moles per mole of M. Co, if present in M is optionally present at 0 to 0.33
moles per mole of
M. Mn, if present in M, is optionally present at equal to or greater than 0.05
to 0.8 moles per
mole of M. In some aspects M includes Ni at greater than 0.3 to 0.95 moles per
mole of M, Co
at 0 to 0.33 moles per mole of M, and Mn at 0.05 to 0.5 moles per mole of M.
In the layered-
layered materials M' includes Mn alone or in addition to one or more of Ti,
Zr, Ru, Re, and Pt.
[0037] In compositions having a layered a-NaFe02-type structure, hexagonal
metal oxide
layers are separated by planes of the alkali metal (e.g. Li). The metal oxide
layers form metal
centered oxygen octahedra that are separated by alkali metal ions. These metal
oxide layers are
laterally offset to provide a three-layer structure. In a layered a-NaFe02-
type structure, the
alkali metal atoms occupy the so called "3a" sites in the structure (x=0, y=0,
and z=0), the
metal atoms occupy the "3h" sites (x=0, y=0, and z=0.5), and the oxygen atoms
occupy the
"6c" sites (x=0, y=0, and z=0.25). The coordinates of the atoms and the cell
parameters can
vary according to the composition. Compositions having this structure type may
have cell
parameters in which a is about 2.75 to about 2.95 angstroms (A), and c is
about 13.9 to about
14.6 A. By substituting a metal with a higher oxidation potential than Ni in
the 3b sites of the
grain boundary selectively, one can stabilize the particles as a whole
relative to unstabilized
materials of otherwise identical overall composition.
11
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0038] According to some aspects, materials as provided herein include a
particle
comprising a plurality of crystallites each comprising a first composition.
The particle formed
of a plurality of crystallites may be referred to as a secondary particle. The
particles as provided
herein are uniquely tailored to have grain boundaries between the primary
crystallites where
the grain boundaries include a second composition. Stabilizing these grain
boundaries by
increasing the electrochemical affinity for Li relative to the bulk
crystallites, results in particles
that provide improved performance and cycle life, as well as reduced impedance
growth during
cycling, of a cell incorporating the particles as a component of a cathode.
[0039] The particles are appreciated to include a grain boundary formed of
or including a
second composition, wherein the second composition differs from the first
composition in that
the electrochemical affinity for Li in the second composition is increased
relative to a first
composition defining the crystallites of the secondary particle and/or the
second composition
has a lower concentration of Ni relative to the first composition. Optionally,
the particles as
provided herein are capable of maintaining a greater amount of Li in the grain
boundary than
in the crystallites at any particular state of charge greater than zero.
Optionally, the particles as
provided herein are capable of maintaining a greater amount of Li in the grain
boundary than
in the crystallites when the particle is at a given potential, or over a range
of potentials.
Optionally, the provided materials have a lower concentration of Ni in the
grain boundary
region relative to the adjacent crystallites. Optionally, the provided
materials include a further
outer coating layer that may be disposed on an outer surface of the secondary
particle to provide
a coated secondary particle.
[0040] In some aspects provided are electrochemically active particles that
include: a
plurality of crystallites comprising a first composition comprising lithium,
nickel, and oxygen;
12
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
a grain boundary between adjacent crystallites of the plurality of
crystallites and comprising a
second composition comprising lithium, nickel, and oxygen; wherein the grain
boundary has a
higher electrochemical affinity for lithium than the crystallites. Higher
electrochemical affinity
for Li in the grain boundary promotes an increased concentration of Li in the
grain boundary
relative to the crystallites at any particular state of charge.
[0041] Also provided are electrochemically active particles that include: a
plurality of
crystallites comprising a first composition comprising lithium, nickel, and
oxygen; a grain
boundary between adjacent crystallites of the plurality of crystallites and
comprising a second
composition comprising lithium, nickel, and oxygen; wherein nickel is present
in the grain
boundary at a lower concentration than nickel concentration inside the
crystallites.
[0042] Optionally, the molar lithium content per mole of a second
composition defining
the grain boundary relative to the molar Li content per mole of a first
composition defining the
crystallites is found to be higher at a state of charge of 10% or greater,
optionally 20% or
greater, optionally 30% or greater, optionally 40% or greater, optionally 50%
or greater,
optionally 60% or greater, optionally 70% or greater, optionally 80% or
greater, optionally
90% or greater, optionally 95% or greater, optionally 96% or greater,
optionally 97% or greater,
optionally 98% or greater, optionally 99% or greater, optionally 100%.
[0043] In some aspects, provided are electrochemically active particles
that include: a
plurality of crystallites comprising a first composition comprising lithium,
nickel, and oxygen;
a grain boundary between adjacent crystallites of the plurality of
crystallites and comprising a
second composition comprising lithium, nickel, and oxygen; wherein the grain
boundary has a
higher electrochemical affinity for lithium than the crystallites at a state
of charge of 10% or
greater.
13
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0044] In some aspects provided are electrochemically active particles that
include: a
plurality of crystallites comprising a first composition comprising lithium,
nickel, and oxygen;
a grain boundary between adjacent crystallites of the plurality of
crystallites and comprising a
second composition comprising lithium, nickel, and oxygen; wherein the grain
boundary has a
higher electrochemical affinity for lithium than the crystallites at a state
of charge of 80% or
greater.
[0045] In some aspects provided are electrochemically active particles that
include: a
plurality of crystallites comprising a first composition comprising lithium,
nickel, and oxygen;
a grain boundary between adjacent crystallites of the plurality of
crystallites and comprising a
second composition comprising lithium, nickel, and oxygen; wherein the grain
boundary has a
higher electrochemical affinity for lithium than the crystallites at a state
of charge of 100%.
[0046] Also provided are electrochemically active particles that include: a
plurality of
crystallites comprising a first composition comprising lithium, nickel, and
oxygen; a grain
boundary between adjacent crystallites of the plurality of crystallites and
comprising a second
composition comprising lithium, nickel, and oxygen; wherein lithium is present
in the grain
boundary at a higher concentration than lithium inside the crystallites when
the particle is at a
state of charge greater than or equal to 10 percent.
[0047] Optionally, the molar lithium concentration per mole of a second
composition
defining the grain boundary relative to the molar concentration of Li per mole
of the first
composition defining the crystallites is found to be higher at a state of
charge of 10% or greater,
optionally 20% or greater, optionally 30% or greater, optionally 40% or
greater, optionally
50% or greater, optionally 60% or greater, optionally 70% or greater,
optionally 80% or greater,
14
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
optionally 90% or greater, optionally 95% or greater, optionally 96% or
greater, optionally
97% or greater, optionally 98% or greater, optionally 99% or greater,
optionally 100%.
[0048] Also provided are electrochemically active particles that include: a
plurality of
crystallites comprising a first composition comprising lithium, nickel, and
oxygen; a grain
boundary between adjacent crystallites of the plurality of crystallites and
comprising a second
composition comprising lithium, nickel, and oxygen; wherein lithium is present
in the grain
boundary at a higher concentration than lithium inside the crystallites when
the particle is at a
state of charge greater than or equal to 20 percent.
[0049] Also provided are electrochemically active particles that include: a
plurality of
crystallites comprising a first composition comprising lithium, nickel, and
oxygen; a grain
boundary between adjacent crystallites of the plurality of crystallites and
comprising a second
composition comprising lithium, nickel, and oxygen; wherein lithium is present
in the grain
boundary at a higher concentration than lithium inside the crystallites when
the particle is at a
state of charge greater than or equal to 80 percent.
[0050] Also provided are electrochemically active particles that may be
used in a cathode
of an electrochemical cell that may include: a plurality of crystallites
comprising a first
composition comprising lithium, nickel, and oxygen; a grain boundary between
adjacent
crystallites of the plurality of crystallites and comprising a second
composition comprising
lithium, nickel, and oxygen; wherein lithium is present in the grain boundary
at a higher
concentration than lithium in the crystallites when an electrode incorporating
the particle is
charged to a potential greater than or equal to 4.00 V versus lithium.
[0051] In some aspects, the Ni mole fraction within the second region of
less than or equal
to 0.95 results in the improved electrochemical performance of the materials.
It was found that
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
by reducing Ni levels in the grain boundary region(s) of the particles that
enhanced stability to
the overall particle could be obtained. Optionally, the ratio of mole fraction
of Ni in the second
composition relative to the mole fraction of Ni in the first composition is in
the range of 0.95
to 0.5. Optionally, the ratio of the mole fraction of Ni in the second
composition to the mole
fraction of Ni in the first composition is 0.95 or less, optionally 0.90,
optionally 0.85, optionally
0.80, optionally 0.75, optionally 0.7, optionally 0.65, optionally 0.6,
optionally 0.55, or less.
[0052] Optionally, the lithium is present in the grain boundary (second
composition) at a
higher concentration than lithium in the crystallites (first composition) when
an electrode
incorporating the particle is charged to a potential greater than or equal to
4.1 V versus lithium.
Optionally, the lithium is present in the grain boundary at a higher
concentration than lithium
in the crystallites when an electrode incorporating the particle is charged to
a potential greater
than or equal to 4.2 V versus lithium. Optionally, the lithium is present in
the grain boundary
at a higher concentration than lithium in the crystallites when an electrode
incorporating the
particle is charged to a potential greater than or equal to 4.25 V versus
lithium. Optionally, the
lithium is present in the grain boundary at a higher concentration than
lithium in the crystallites
when an electrode incorporating the particle is charged to a potential greater
than or equal to
4.3V versus lithium.
[0053] The increase in the amount of Li retained in the second composition
of the grain
boundary relative to the bulk is optionally 0.02 moles Li per mole of the
second composition
or greater at the potential as provided in the preceding paragraphs.
Optionally, at the potentials
as provided in the preceding paragraphs, the moles of Li per mole of the
second composition
is greater than the moles of Li per mole of the first composition by 0.01,
optionally by 0.02,
16
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
optionally by 0.05, optionally by 0.1, optionally 0.15, optionally 0.2,
optionally 0.25 ,
optionally 0.3.
[0054] In some aspects, the elemental composition of the first composition
and the second
composition are identical with the exception of differing relative amounts of
each of the
individual metal constituents with the proviso that the amount of Ni in the
second composition
is lower than the amount of Ni in the first composition. In other aspects, a
second composition
includes one or more additional metals not present in the first composition
wherein the one or
more additional metals may substitute for the Ni in the crystal lattice of the
second region to
thereby promote increased order to these regions and improved cycle life and
reduced internal
resistance.
[0055] Also provided are electrochemically active particles that may be
used in a cathode
of an electrochemical cell that may include: a plurality of crystallites
comprising a first
composition comprising lithium, nickel, and oxygen; a grain boundary between
adjacent
crystallites of the plurality of crystallites and comprising a second
composition comprising
lithium, nickel, and oxygen; lithium is present in the grain boundary at a
higher concentration
than lithium in the crystallites at a charge capacity of the particles of 40
mAh/g or greater.
[0056] The concentration of Li in the grain boundaries is increased
relative to the
crystallites when the particle is charged to a capacity of optionally 50 mAh/g
or greater,
optionally 60 mAh/g or greater, optionally 70 mAh/g or greater, optionally 80
mAh/g or
greater, optionally 90 mAh/g or greater, optionally 100 mAh/g or greater,
optionally 110
mAh/g or greater, optionally 120 mAh/g or greater, optionally 130 mAh/g or
greater, optionally
140 mAh/g or greater, optionally 150 mAh/g or greater, optionally 160 mAh/g or
greater,
17
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
optionally 170 mAh/g or greater, optionally 180 mAh/g or greater, optionally
190 mAh/g or
greater, optionally 200 mAh/g or greater, optionally 220 mAh/g or greater.
[0057] Optionally, at a given potential or at a charge capacity of 40 mAh/g
or greater, the
moles of Li per mole of the second composition is greater than the moles of Li
per mole of the
first composition by 0.01, optionally by 0.02, optionally by 0.05, optionally
0.1, optionally
0.15, optionally 0.2, optionally 0.25, optionally 0.3. Optionally, the
increase in the amount of
Li retained in the second composition of the grain boundary relative to the
amount of Li
retained in the first composition of the crystallite is 0.01 moles Li per mole
of the second
composition or greater, optionally 0.15 moles Li per mole of the second
composition or greater,
optionally 0.2 moles Li per mole of the second composition or greater,
optionally 0.25 moles
Li per mole of the second composition or greater, optionally 0.3 moles Li per
mole of the
second composition or greater. Optionally, the amount of Li retained in the
second composition
is greater, optionally by the foregoing amount, than the amount of Li retained
in the first
composition at a charge capacity of the particle of 50 mAh/g or greater,
optionally 60 mAh/g
or greater, optionally 70 mAh/g or greater, optionally 80 mAh/g or greater,
optionally 90
mAh/g or greater, optionally 100 mAh/g or greater, optionally 110 mAh/g or
greater, optionally
120 mAh/g or greater, optionally 130 mAh/g or greater, optionally 140 mAh/g or
greater,
optionally 150 mAh/g or greater, optionally 160 mAh/g or greater, optionally
170 mAh/g or
greater, optionally 180 mAh/g or greater, optionally 190 mAh/g or greater,
optionally 200
mAh/g or greater, optionally 220 mAh/g or greater.
[0058] Optionally, a particle with that includes a second composition
present in the grain
boundary regions, optionally exclusively in the grain boundary region wherein
the second
composition has a lower concentration of Ni relative to the concentration of
Ni in the first
18
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
composition of the crystallites leads to increased physical stability of the
particle and increased
fracture toughness or fracture resistance.
[0059] One method of measuring stability of a particle is through
measurement of fracture
toughness. Fracture toughness measurements may be made by placing a known
quantity of
particles as provided herein in a die and applying a suitable amount of
pressure, optionally at
900 MPa, and then measuring the increase in the amount of fines generated in
the material by
the application of the pressure. This serves as a direct measurement of
physical particle stability
that the inventors found corresponds directly with reduced rates of impedance
growth during
cycling. Optionally, the amount of fines increases by less than 50 number
percent in the
provided particles following application of 890 MPa or 900 MPa of pressure.
Measurement of
fines in a sample following application of pressure may be made by particle
size analyses by
recognized techniques. Fines as defined herein are particles (or particle
fragments) that have a
size as measured by particle size analyses of 3.5 i_tm or lower. For example,
in some particle
size analyses, the absolute number of particles is known and the percent pass
is the number of
fines related to the total particle number as determined in the analyses. In
some aspects, particle
surface area correlates with improved performance such as cycle life or
reduction in impedance
growth. In this disclosure, increased surface area may be measured by
techniques such as
Brunauer¨Emmett¨Teller (BET) surface area measurements. Here the methods used
are related
to the number of fines with a size of 3.5 i_tm or lower as a rapid and readily
reproducible method
of determining improved particle performance.
[0060] As an exemplary method of quantifying percent fines, samples of
particle material
may be crushed in a 2 cm diameter die in a hydraulic press to the desired
pressure. A total mass
19
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
of 2.5 g of powder may be placed in the die and crushed to form a pellet. The
pellet may then
be placed in 20 mL of water and subjected to ultrasonic dispersion for 30
minutes. Once
dispersed, a small portion of the dispersed slurry may be added to a Malvern
Mastersizer 3000
laser-light particle size instrument equipped with a liquid cell. To ensure
full dispersion of
fines, the ultrasonic function may be used during the measurement and an
average of five
measurements used to calculate the final result. The distribution may be
calculated using
properties for NiO taken from the instrument library.
[0061] The percent fines generated by application of pressure in a die
decreases with
decreasing relative Ni concentration in the grain boundary relative to the
crystallites.
Optionally, the percent fines generated in by the application of pressure in
the die are equal to
or less than a percentage of 50, 40, 35, 30, 25, 20, 19, 18, 17, 16, 15, 14,
13, 12, 11, 10, 9, 8, 7,
6, 5, 4, 3, or 2 percent. In some aspects, the concentration of nickel in the
second composition
is lower than the concentration of nickel in the first composition, and the
fracture resistance is
such that when the material is pressed to a pressure of 900 MPa, less than 15%
fines are
generated.
[0062] Optionally when the material is an NCA material with 80 atomic
percent Ni or
above in the bulk particle the number of fines is generated is less than 15
percent, optionally
less than 14 percent, optionally less than 13%, optionally less than 12%,
optionally less than
11%, optionally less than 10% optionally less than 9%, optionally less than
8%, optionally less
than 7%, optionally less than 6%. Optionally when the material is an NCA
material with 90
atomic percent Ni or above the number of fines generated is less than 7%,
optionally less than
6 percent, optionally less than 5% optionally less than 4%, optionally less
than 3%. Optionally
when the material is an NCM material with 80 atomic percent Ni or above the
number of fines
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
generated is less than 6 percent, optionally less than 5 percent, optionally
less than 4 percent,
optionally less than 3 percent, optionally less than 2 percent.
[0063] In some aspects of the presently provided particles, the first
composition that
defines the crystallites of the secondary particle includes polycrystalline
layered-structure
lithiated metal oxides defined by composition defined by Formula I:
Li i+xM02+y (I)
and optionally a cell or battery formed therefrom, where ¨0.1<x<0.3 and
¨0.3<y<0.3 when in
a state of discharge or where ¨0.9<x<0.1 and ¨0.3<y<0.3 when in a state of
charge. In some
aspects, x is ¨0.1, optionally 0, optionally 0.1, optionally 0.2, or
optionally 0.3. Optionally, x
is greater than or equal to ¨0.10, ¨0.09, ¨0.08, ¨0.07, ¨0.06, ¨0.05, ¨0.04,
¨0.03, ¨0.02, ¨0.01,
0.00, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.11, 0.12,
0.13, 0.14, 0.15, 0.16,
0.17, 0.18, 0.19, 0.20, 0.21, 0.22, 0.23, 0.24, 0.25, 0.26, 0.27, 0.28, 0.29,
or 0.30. In some
aspects, y is ¨0.3, optionally ¨0.2, optionally ¨0.1, optionally 0, optionally
0.1, optionally 0.2,
or optionally 0.3. Optionally, y is greater than or equal to ¨0.30, ¨0.29,
¨0.28, ¨0.27, ¨0.26,
¨0.25, ¨0.24, ¨0.23, ¨0.22, ¨0.21, ¨0.20, ¨0.19, ¨0.18, ¨0.17, ¨0.16, ¨0.15,
¨0.14, ¨0.13,
¨0.12, ¨0.11, ¨0.10, ¨0.09, ¨0.08, ¨0.07, ¨0.06, ¨0.05, ¨0.04, ¨0.03, ¨0.02,
¨0.01, 0.00, 0.01,
0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.11, 0.12, 0.13, 0.14,
0.15, 0.16, 0.17, 0.18,
0.19, 0.20, 0.21, 0.22, 0.23, 0.24, 0.25, 0.26, 0.27, 0.28, 0.29, or 0.3.
[0064] It is appreciated that in some aspects Li need not be exclusively
Li, but may be
partially substituted with one or more elements selected from the group
consisting of Mg, Sr,
Na, K, and Ca. The one or more elements substituting Li, are optionally
present at 10 atomic %
or less, optionally 5 atomic % or less, optionally 3 atomic % or less,
optionally no greater than
2 atomic percent, where percent is relative to total Li in the material.
21
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0065] M as provided in the first composition includes Ni. The amount of Ni
in the first
composition is optionally from 10 atomic percent to 100 atomic percent (at%)
of total M.
Optionally, the Ni component of M is greater than or equal to 75 at%.
Optionally, the Ni
component of M is greater than or equal to 80 at%. Optionally, the Ni
component of M is
greater than or equal to 85 at%. Optionally, the Ni component of M is greater
than or equal to
90 at%. Optionally, the Ni component of M is greater than or equal to 95 at%.
Optionally, the
Ni component of M is greater than or equal to 75 at%, 76 at%, 77 at%, 78 at%,
79 at%, 80 at%,
81 at%, 82 at%, 83 at%, 84 at%, 85 at%, 86 at%, 87 at%, 88 at%, 89 at%, 90
at%, 91 at%, 92
at%, 93 at%, 94 at%, 95 at%, 96 at%, 97 at%, 98 at%, 99 at%, 99.5 at%, 99.9
at%, or 100 at%.
[0066] In some aspects, M in the first composition is Ni alone or in
combination with one
or more additional elements. The additional elements are optionally metals.
Optionally, an
additional element may include or be one or more of Mg, Sr, Co, Al, Ga, Ca,
Cu, Zn, Mn, V,
Ba, Y, Nb, Zr, Ti, Cr, Fe, Mo, W, B, and any combination thereof. In
particular aspects, the
additional element may include Mg, Co, Al, or a combination thereof.
Optionally, the
additional element may be Mg, Al, V, Ti, B, or Mn, or a combination thereof.
Optionally, the
additional element is selected from the group consisting of Mg, Al, V, Ti, B,
or Mn. Optionally,
the additional element is selected from the group consisting of Mg, Co, and
Al. Optionally, the
additional element selected from the group consisting of Ca, Co, and Al. In
some aspects, the
additional element is Mn or Mg, or both Mn and Mg. Optionally, the additional
element is Mn,
Co, Al, or any combination thereof. Optionally the additional element includes
Co and Mn.
Optionally, the additional element is Co and Al. Optionally the additional
element is Co.
[0067] An additional element of the first composition may be present in an
amount of
about 1 to about 90 at% of total M, specifically about 5 to about 80 at%, more
specifically
22
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
about 10 to about 70 at% of M in the first composition. Optionally, the
additional element may
be present in an amount of about 1 to about 20 at%, specifically about 2 to
about 18 at%, more
specifically about 4 to about 16 at%, of M in the first composition. In some
illustrative
examples, M is about 75-100 at% Ni, 0-15 at% Co, 0-15 at% Mn, and 0-10 at%
additional
elements.
[0068] Within the polycrystalline material, each crystallite may have any
suitable shape,
which can be the same or different within each particle. Further, the shape of
each crystallite
can be the same or different in or within different particles. Because of its
crystalline nature,
the crystallite may be faceted, the crystallite may have a plurality of flat
surfaces, and a shape
of the crystallite may approximate a geometric shape. In some aspects, the
crystallite may be
fused with neighboring crystallites with mismatched crystal planes. The
crystallite may
optionally be a polyhedron. The crystallite may have a rectilinear shape, and
when viewed in
cross-section, a portion of or an entirety of the crystallite may be
rectilinear. The crystallite
may be square, hexagonal, rectangular, triangular, or a combination thereof. A
crystallite is
optionally a single crystal and a particle is optionally an agglomerate of
single crystals.
[0069] The particles include a grain boundary that separates two adjacent
crystallites. A
grain boundary includes a second composition. In some aspects, the grain
boundary includes a
second composition having the a-NaFe02-type structure with the generic Formula
II:
Li l+xM' 02+y (II)
wherein M' is defined as M in the first composition but with a relatively
lower mole Ni per
mole of LiM02. The Ni sites in the crystal structure are substituted with one
or more
substitution elements that increases the electrochemical affinity for lithium
of the structure
relative to the unsubstituted material such that the amount of the
substitution elements are at a
23
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
greater mole per mole concentration in the second composition than in the
first composition,
and optionally at a state of charge of 80% or greater or at a charge capacity
of 150 mAh/g or
greater, ¨0.6 < x < -0.2. In some aspects, x is ¨0.6, optionally -0.65,
optionally -0.7, optionally
-0.75, optionally -0.8, optionally -0.9, optionally -0.95. In some aspects, y
is ¨0.3, optionally
¨0.2, optionally ¨0.1, optionally 0, optionally 0.1, optionally 0.2, or
optionally 0.3. Optionally,
y is greater than or equal to ¨0.30, ¨0.29, ¨0.28, ¨0.27, ¨0.26, ¨0.25, ¨0.24,
¨0.23, ¨0.22,
¨0.21, ¨0.20, ¨0.19, ¨0.18, ¨0.17, ¨0.16, ¨0.15, ¨0.14, ¨0.13, ¨0.12, ¨0.11,
¨0.10, ¨0.09,
¨0.08, ¨0.07, ¨0.06, ¨0.05, ¨0.04, ¨0.03, ¨0.02, ¨0.01, 0.00, 0.01, 0.02,
0.03, 0.04, 0.05, 0.06,
0.07, 0.08, 0.09, 0.10, 0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18, 0.19,
0.20, 0.21, 0.22, 0.23,
0.24, 0.25, 0.26, 0.27, 0.28, 0.29, or 0.3. When the particles with the
stabilized second
composition are incorporated in a cathode electrochemically charged to about
4.3 V (can be
lower or higher based on the specific application) improved performance is
observed resulting
from the increased electrochemical affinity for Li relative to the
crystallites.
[0070] Optionally, Ni in the second composition can be in the range of 0 to
0.99 moles per
mole of M' in the second composition. Optionally, M' in the second composition
is free of Ni.
Optionally, the amount (i.e. relative concentration) of Ni in the second
composition is lower
than the amount of Ni in the first composition in relative atomic percent
(with respect to the
respective composition in which the Ni is present). Optionally, the Ni
component of M' is less
than or equal to 1 moles per mole of M'. Optionally, the Ni component of M' is
less than or
equal to 0.5 moles per mole of M'. Optionally, the Ni component of M' is less
than or equal to
0.10 moles per mole of M'. Optionally, the Ni component of M' is less than or
equal to 0.20
moles per mole of M'. Optionally, the Ni component of M' is less than or equal
to 0.75 moles
per mole of M'. Optionally, the Ni component of M' is less than or equal to
0.80 moles per
24
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
mole of M'. Optionally, the Ni component of M' is less than or equal to 0.85
moles per mole
of M'. Optionally, the Ni component of M' is less than or equal to 0.90 moles
per mole of M'.
Optionally, the Ni component of M' is less than or equal to 0.95 moles per
mole of M'.
Optionally, the Ni component of M' is less than or equal to 0.75 moles per
mole of M', 0.76
moles per mole of M', 0.77 moles per mole of M', 0.78 moles per mole of M',
0.79 moles per
mole of M', 0.80 moles per mole of M', 0.81 moles per mole of M', 0.82 moles
per mole of
M', 0.83 moles per mole of M', 0.84 moles per mole of M', 0.85 moles per mole
of M', 0.86
moles per mole of M', 0.87 moles per mole of M', 0.88 moles per mole of M',
0.89 moles per
mole of M', 0.90 moles per mole of M', 0.91 moles per mole of M', 0.92 moles
per mole of
M', 0.93 moles per mole of M', 0.94 moles per mole of M', 0.95 moles per mole
of M', 0.96
moles per mole of M', 0.97 moles per mole of M', 0.98 moles per mole of M',
0.99 moles per
mole of M', or 0.999 moles per mole of M'.
[0071] For the materials as provided herein, the nominal or overall
formulated
composition of the secondary particles (for example, characterized by
Inductively Coupled
Plasma (ICP)), optionally the first composition, or optionally the second
composition, is
defined by the nominal formula LiM02, wherein M is Ni and optionally one or
more
substitution elements that in the second composition must include at least one
element
substituting for Ni in the crystal structure that imparts greater
electrochemical affinity for Li to
the second composition relative to the first composition. The mole fraction of
the substitution
element in the first composition, if present, as defines the composition of
the crystallites is
lower than the mole fraction of the total substitution element(s)
independently or combined in
the total particle composition as determined by ICP. The mole fraction of the
substitution
element independently or combined in the first composition can be zero. The
mole fraction of
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
the substitution element in the second composition independently or combined
as defines the
grain boundary is higher than the mole fraction of the substitution element(s)
independently or
combined in the total particle as measured by ICP.
[0072] Examples of substitution elements that can potentially be included
in M' in
Formula II and promote the retention of Li in the grain boundaries of charged
high-Ni LiM02
cathode materials include a variety of elements that can substitute for Ni in
the LiM'02
structure. Such doping elements can promote Li retention (i.e. greater
electrochemical affinity
for Li) by being more resistant to oxidation (having higher oxidation
potential) than the Ni
atoms they are substituting for, by stabilizing the structure to oxidation, or
by inductively
raising the oxidation potential of nearby Ni atoms. If trivalent (3+) ions of
doping elements
that can directly substitute for Ni' are less easily oxidized than the Ni ions
when the material
is charged, they will promote Li retention; substitution of Ni(III) by Al(III)
is an example. If
tetravalent (4+) ions substitute for Ni', they are charge-compensated by Ni
ions in the 2+ state
and their inductive effects raise the potential for oxidation of those Ni ions
to the 4+ state;
substitution of Ni(III) by Mn(IV) is an example. Alternatively, if difficult
to oxidize 2+ ions
substitute for Ni, they are charge-compensated by Ni ions in the 4+ state;
substitution of Ni(III)
by Mg (II) is an example. In order to substitute for Ni in the LiM'02
structure, doping ions
must be of size comparable to that of the Ni ions, and in order to facilitate
retention of Li, they
must raise the local oxidation potential. The relative impact of a given ion's
impact on the
oxidation potential can often be estimated from its ionization energy relative
to that of Ni'.
Therefore, ions of size comparable to Ni' and having comparable or higher
ionization energy
can potentially serve to stabilize oxidized cathodes' grain boundaries by
increasing their
retention of Li. The following table provides the ionization energies and
hexacoordinate
26
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
(octahedral environment) ionic radii for examples of ions that might stabilize
the grain
boundaries of charged high-Ni LiM02 cathode materials by increasing the grain
boundaries'
retention of Li.
Table 2: Oxidation potentials and ionic radii for elements*
...............................................................................
...............................................................................
.....................
gnunaimunnum],,,,mnu(4.4f#110.)mummunnumm(gpg trpp.7.0)mg,,,,E,,,A
...............................................................................
.................................
.....................................................................
..................................
...................................................
...............................................................................
................
.....................
.............................................................................
....... ......................................................
Ni 2+ 3395 0.69
Ni 3+ 5300 0.56-0.6
Ni 4+ 7339 0.48
Co 3+ 4950 0.545-0.61
Co 4+ 7670 0.53
A13+ 11577 0.535
Mn 3+ 4940 0.58-0.645
Mn 4+ 6990 0.53
Mg 2+ 7733 0.72
Ti 4+ 9581 0.605
V4+ 6299 0.58
Cr 3+ 4743 0.615
Cr 4+ 6702 0.55
Fe 3+ 5290 0.55-0.645
Fe 4+ 7240 0.585
Cu 2+ 5536 0.73
Cu 3+ 7700 0.54
Zn 2+ 5731 0.74
Ga 3+ 6180 0.62
Zr 4+ 7752 0.72
Mo4+ 5257 0.65
Sn 4+ 7456 0.69
Y3+ 5847 0.9
Y4+ 7430 <0.9
*ionization energy
[0073] In the second composition, M' further includes one or more
substitution elements
that may be selected from a group that oxidize less than nickel when
electrochemically charged
27
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
to 4.3V or higher relative to Li metal anode. In one example, M' can comprise
Ni and a
combination of Co and Mn, which oxidizes less than nickel when charged to
4.3V. In other
aspects, M' may include Ni and one more elements selected from the group
comprising Mn,
Cr, Fe, Ti, V, Co, Cu, Zn, Zr, Nb, Sb, W, Sc, Al, Mo, Y, etc., which oxidize
less than Ni when
charged to 4.3V relative to lithium metal. Optionally, M' excludes the
combination of Ni with
Co alone, Al alone, or a combination of Co and Al, and Co, Al, or both may be
present with
doping of one or more additional substitution elements as provided herein. In
some aspects, M'
may include an element selected from the group of elements that will not
oxidize when charged
to 4.3V relative to lithium such as Y, Sc, Ga, In, Tl, Si, Ge, Sn, Pb, etc.
[0074] Note that 4.3V is chosen as a representative example only. The
voltage can also
be lower (e.g. 4.0, 4.1, 4.2, etc. vs. Li) or higher (4.35, 4.4, 4.5 V, 4.6V,
4.7V vs. Li). Specific
voltage of interest would depend on the operating potential of the battery
when the cathode is
paired with the anode.
[0075] In some aspects, the second composition of the grain boundaries has
greater
electrochemical affinity for Li such that when an electrode incorporating the
particle is charged
to a potential greater than or equal to 4.1 V versus lithium, optionally
greater than or equal to
4.2 V versus lithium, optionally greater than or equal to 4.3V versus lithium,
the grain boundary
retains greater than 0.15 moles of lithium per mole of the second composition
at said potential.
[0076] In some aspects, the second composition of the grain boundaries has
greater
electrochemical affinity for Li such that when an electrode incorporating the
particle is charged
to a capacity of 100 mAh/g or greater, optionally 200 mAh/g or greater, the
grain boundary
retains greater than 0.15 moles of lithium per mole of the second composition
at said potential.
28
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[0077] Optionally, at the potential or capacity as illustrated herein, the
second
composition comprises lithium in an amount of about 0.1 to about 1.3 moles,
specifically about
0.15 to about 1.2 moles, more specifically about 0.3 to about 1.1 moles, per
mole of the second
composition; nickel in an amount of about 0.1 to about 0.999 mole,
specifically about 0.2 to
about 0.90 mole, more specifically about 0.3 to about 0.85 mole, per mole of
the second
composition; manganese in an amount of about 0.02 to about 0.99 mole,
specifically about 0.04
to about 0.90 mole, more specifically about 0.06 to about 0.80 mole, per mole
of the second
composition; and oxygen in an amount of about 1.7 to about 2.3 moles,
specifically about 1.8
to about 2.2 moles, more specifically about 1.9 to about 2.1 moles per mole of
the second
composition.
[0078] The second composition may further comprise an additional metal, and
the
additional metal of the second composition may be present in an amount of
about 0.01 to about
0.9 mole, specifically about 0.05 to about 0.8 mole, more specifically about
0.1 to about 0.7
mole, per mole of the second composition. In an embodiment, the additional
metal of the
second composition may be present in an amount of about 0.01 to about 0.2
mole, specifically
about 0.02 to about 0.18 mole, more specifically about 0.04 to about 0.16
mole, per mole of
the second composition.
[0079] The additional metal of the second composition may include Mg, Sr,
Ca, Cu, Zn,
Mn, Al, V, Ba, Zr, Ti, Cr, Fe, Mo, B, or a combination thereof. Optionally,
the additional metal
of the second composition includes Mg, Al, V, Ti, B, Zr, or Mn, or a
combination thereof.
Optionally, the additional metal of the second composition includes of Mg, Al,
V, Ti, B, Zr, or
Mn. An embodiment in which the additional metal of the second composition is
Mn or Mg is
specifically mentioned. Optionally, the additional metal of the first
composition and the
29
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
additional metal of the second composition are each Mg. Optionally, the first
composition
further comprises Mn, and the Mn is present in the first composition in an
amount of about
0.01 to about 0.6 mole, specifically about 0.02 to about 0.5 mole, per mole of
the first
composition, and the second composition comprises Mn, and the Mn is present in
the second
composition in an amount of about 0.01 to about 0.6 mole, specifically about
0.02 to about 0.5
mole, per mole of the second composition.
[0080] The grain boundary is between adjacent crystallites, is on a surface
of the
crystallite, and comprises or consists of the second composition. The second
composition has
the layered a-NaFe02-type structure, a cubic structure, a monoclinic
structure, or a
combination thereof. As noted above, the grain boundary includes at least one
substitution
element such that the electrochemical affinity for Li of the second
composition is greater than
that of the first composition as found in the bulk crystallites. An embodiment
in which the
grain boundaries include or consist of a layered a-NaFe02-type structure is
specifically
mentioned.
[0081] The shape of the grain boundary is defined by the shape of the
crystallite adjacent
to the grain boundary. The shape of the grain boundary may approximate a
geometric shape.
The grain boundary may have a rectilinear shape, and when viewed in cross-
section the grain
boundary may be rectilinear. The grain boundary may be square, hexagonal,
rectangular,
triangular, or a combination thereof
[0082] A direction of a surface of the grain boundary corresponds to a
direction of a
surface of the adjacent crystallite(s). The surface of the grain boundary and
the surface of the
crystallite may have any of a variety of orientations relative to an outer
surface of the secondary
particle. Thus the direction of the surface of the crystallite and the
direction of the surface of
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
the grain boundary may be parallel to or be different from the direction of a
nearest outer
surface of the secondary particle. In some aspects, a direction of a tangent
of the nearest outer
surface of the particle is different than the direction of the surface of the
grain boundary and
the direction of the surface of the adjacent particle.
[0083] The grain boundaries may intersect to form an angle therebetween. In
some
aspects, disposed on adjacent faces of a crystallite is a first grain boundary
and second grain
boundary. The first grain boundary and the second grain boundary intersect at
an angle E. The
angle E may be defined by the shape of the crystallite on which the first
grain boundary and
the second grain boundary are disposed. Generally, a shape of a crystallite is
influenced by a
crystal structure of the crystallite. While not wanting to be bound by theory,
it is understood
that because the crystal structure of the first composition governs the shape
of the crystallite,
the angle between the first and second grain boundaries is influenced by the
crystal structure
of the first composition. The first and second grain boundaries may intersect
at any angle,
specifically an angle of about 10 to about 170 degrees, specifically about 20
to about 160
degrees, more specifically about 30 to about 150 degrees, so long as the angle
is consistent
with the crystal structure of the first composition.
[0084] The dimensions of the grain boundary are not particularly limited. A
length and a
width of the grain boundary may each independently be about 10 to about 1000
nm, specifically
about 60 to about 900 nm, more specifically about 70 to about 800 nm. The
length and width
of the grain boundaries are perpendicular to each other and are parallel to
the surface of the
adjacent crystallite(s). A thickness of the grain boundary may be about 0.5 to
about 30 nm,
specifically about 1 to about 20 nm, more specifically about 1 to about 10 nm.
The thickness
of the grain boundary is substantially perpendicular to the length and the
width of the grain
31
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
boundary and may be perpendicular to the surface of the adjacent crystallite.
The composition
of the grain boundary may be substantially uniform or may vary along the
thickness.
[0085] An average grain boundary length and an average grain boundary width
of the
plurality of grain boundaries may each independently be about 10 to about 1000
nm,
specifically about 60 to about 900 nm, more specifically about 70 to about 800
nm. Also, an
average grain boundary thickness of the plurality of grain boundaries may be
about 0.1 to about
30 nm, specifically about 1 to about 20 nm, more specifically about 1 to about
10 nm.
[0086] Optionally, a particle as provided herein includes an outer coating
such as a
passivation layer or a protective layer that may be deposited on an outer
surface of the
secondary particle to provide a coated secondary particle. The coating may
fully or partially
cover the secondary particle. The outer coating layer may be amorphous or
crystalline. The
outer coating layer may comprise an oxide, a sulfate, a phosphate, a
pyrophosphate, a
fluorophosphate, a carbonate, a fluoride, an oxyfluoride, or a combination
thereof, of a metal
such as Zr, Al, Ti, Al, B, or Si, or a combination thereof. Optionally, the
outer coating layer
includes a borate, an aluminate, a silicate, a fluoroaluminate, or a
combination thereof.
Optionally, the outer coating layer comprises a carbonate. In an embodiment,
the layer
comprises ZrO2, A1203, TiO2, A1PO4, A1F3, B203, SiO2, Li2O, Li2CO3, or a
combination
thereof. A layer comprising A1PO4 or Li2CO3 is specifically mentioned.
Optionally, an outer
coating layer includes oxide of one or more elements selected from Al, Zr, Y,
Co, Ni, Mg, and
Li. Optionally, an outer coating layer includes a fluoride comprising one or
more elements
selected from Al, Zr, and Li. Optionally, an outer coating layer includes a
carbonate comprising
one or more elements selected from Al, Co, Ni, Mn, and Li. Optionally, an
outer coating
includes a sulfate comprising one or more elements selected from Al, Co, Ni,
Mn, and Li.
32
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
Optionally, an outer coating includes a phosphate comprising one or more
elements selected
from Al and Li. The layer may be deposited by any process or technique that
does not adversely
affect the desirable properties of the secondary particle. Representative
methods include spray
coating and immersion coating, for example.
[0087] A secondary particle may be formed by a multi-step process whereby a
first
composition particle is formed and calcined so as to establish the formation
of defined grain
boundaries optionally with the crystallites having a¨NaFe02 structure with few
defects. The
resulting secondary particles are then subjected to a liquid process that
applies one or more
substitution elements at the desired concentration levels followed by drying
and then a second
calcination so as to move the substitution element precipitated species at the
surface selectively
into the grain boundaries to thereby form the secondary particle with a
stabilized grain
boundary that has a higher electrochemical affinity for Li than the bulk
crystallites. According
to methods of manufacturing a secondary particle that has a base of Ni, Co,
and Mg as provided
herein as an example, formation may include: combining a lithium compound, and
a hydroxide
precursor compound of one or more metals or metalloids (e.g. Ni, Co, and Mg
combined as
previously generated such as by a co-precipitation reaction) to form a
mixture; heat treating the
mixture at about 30 to about 200 C to form a dried mixture; heat treating the
dried mixture at
about 200 to about 500 C for about 0.1 to about 5 hours; then heat treating
at 600 C to less
than about 950 C for about 0.1 to about 20 hours to manufacture the secondary
particle. A first
calcination maximum temperature is relative and specific to the material used
in the hydroxide
precursor. Optionally, in a first calcination, a maximum temperature may be at
or less than 850
degrees Celsius, optionally at or less than 720 degrees Celsius, optionally at
or less than 715
degrees Celsius, optionally at or less than 710 degrees Celsius, optionally at
or less than 705
33
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
degrees Celsius, optionally at or less than 700 degrees Celsius. Optionally,
the maximum
temperature of the first calcination may be about 680 degrees Celsius or less.
Optionally, the
maximum temperature may be about 660 degrees Celsius or less. Optionally, the
maximum
temperature may be about 640 degrees Celsius or less. In yet other aspects,
the maximum
temperature may be less than about 700 degrees Celsius, about 695 degrees
Celsius, about 690
degrees Celsius, about 685 degrees Celsius, about 680 degrees Celsius, about
675 degrees
Celsius, about 670 degrees Celsius, about 665 degree Celsius, about 660
degrees Celsius, about
655 degrees Celsius, about 650 degrees Celsius, about 645 degrees Celsius, or
about 640
degrees Celsius. The dwell time at the maximum temperature is optionally less
than 10 hours.
Optionally, the dwell time at the maximum temperature is less than or equal to
8 hours;
optionally less than or equal to 7 hours; optionally less than or equal to 6
hours; optionally less
than or equal to 5 hours; optionally less than or equal to 4 hours; optionally
less than or equal
to 3 hours; optionally less than or equal to 2 hours.
[0088] After the first calcination, subsequent processing may include
breaking up the
calcined material with a mortar and pestle so that the resulting powder passes
through a desired
sieve, optionally a #35 sieve. The powder is optionally then jar milled in a 1
gallon jar with a
2 cm drum YSZ media for optionally 5 minutes or an adequate time such that the
material may
pass through optionally a #270 sieve.
[0089] The product of the first calcination or milled product may be
subsequently
processed, optionally by a method so as to result in stabilized grain
boundaries following a
second calcination. A process to stabilize grain boundaries and create a
greater grain boundary
electrochemical affinity for Li than the crystallites within a primary
particle may be performed
by suspending the product of the first calcination in an aqueous slurry
comprising one or more
34
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
substitution elements and a lithium compound optionally at a temperature of
about 60 degrees
Celsius whereby the substitution element(s) is present in the aqueous solution
at the
concentrations as desired so as to result in stabilization. The slurry may
then be spray dried to
form a free-flowing powder that is then subjected to a second calcination
optionally with a
heating curve following a two-step ramp/dwell process. Alternatively, a
substitution
element(s) may be dispersed in a non-aqueous solvent along with suspended
polycrystalline
material. The non-aqueous solvent can be removed by evaporation with the
substitution
elements precipitated out on the surface of the polycrystalline material that
is then subjected to
a second calcination optionally with a heating curve following a two-step
ramp/dwell process.
The first of the two ramp/dwell temperature profiles may be from ambient
(about 25 degrees
Celsius) to 450 degrees Celsius and optionally at a rate of 5 degrees Celsius
per minute with a
1 hour hold at 450 degrees Celsius. Subsequently, the second ramp/dwell step
may be from
450 degrees Celsius to a maximum temperature at a rate of 2 degree Celsius per
minute with a
2 hour hold at the maximum temperature. In some aspects, the maximum
temperature is less
than or equal to about 850 degrees Celsius.
[0090] By combining a first calcination with a maximum temperature as
described above
with a process to incorporate one or more substitution elements into the
resulting grain
boundaries by a second calcination also as described above, it was found that
the resulting
particles could be used in a cathode so as to produce significantly improved
cycle life, reduced
capacity fade, and reduced impedance growth, and/or significantly improving
the
electrochemical performance of the material.
[0091] Also provided are cathodes for a lithium-ion battery comprising the
secondary
particle. The cathode comprises the secondary particle disclosed above as an
active material,
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
and may further include a conductive agent and a binder. The conductive agent
may include
any conductive agent that provides suitable properties and may be amorphous,
crystalline, or a
combination thereof. The conductive agent may comprise a carbon black, such as
acetylene
black or lamp black, a mesocarbon, graphite, carbon fiber, carbon nanotubes
such as single
wall carbon nanotubes or multi-wall carbon nanotubes, or a combination
thereof. The binder
may include any binder that provides suitable properties and may comprise
polyvinylidene
fluoride, a copolymer of polyvinylidene fluoride and hexafluoropropylene,
poly(vinyl acetate),
poly(vinyl butyral-co-vinyl alcohol-co vinyl acetate), poly(methylmethacrylate-
co-ethyl
acrylate), polyacrylonitrile, polyvinyl chloride-co-vinyl acetate, polyvinyl
alcohol, poly(1-
vinylpyrrolidone-co-vinyl acetate), cellulose acetate, polyvinylpyrrolidone,
polyacrylate,
polymethacrylate, polyolefin, polyurethane, polyvinyl ether, acrylonitrile-
butadiene rubber,
styrene-butadiene rubber, acrylonitrile-butadiene-styrene, tri-block polymer
of sulfonated
styrene/ethylene-butylene/styrene, polyethylene oxide, or a combination
thereof, for example.
[0092] The cathode may be manufactured by combining the secondary particle,
the
conductive agent, and the binder in a suitable ratio, e.g., about 80 to about
98 weight percent
of the secondary particle, about 2 to about 20 weight percent of the
conductive agent, and about
2 to about 10 weight percent of the binder, based on a total weight of the
secondary particle,
the conductive agent, and the binder. The secondary particle, the conductive
agent, and the
binder may be suspended in a suitable solvent, such as N-methylpyrrolidone,
and disposed on
a suitable substrate, such as aluminum foil, and dried in air.
[0093] Also disclosed is a battery comprising the cathode. The battery may
be a lithium-
ion battery, a lithium-polymer battery, or a lithium battery, for example. The
battery may
include a cathode, an anode, and a separator interposed between the cathode
and the anode.
36
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
The separator may be a microporous membrane, and may include a porous film
including
polypropylene, polyethylene, or a combination thereof, or may be a woven or
non-woven
material such a glass-fiber mat. The anode may include a coating on a current
collector. The
coating may include a suitable carbon, such as graphite, coke, a hard carbon,
or a graphitized
mesocarbon such as a mesocarbon microbead, for example. The anode may also
include
lithium metal or a material capable of alloying (e.g. Si, Ge, etc.) or
conversion reactions (e.g.
metal oxides or sulfides) with lithium. Alternatively, the anode maybe be
titanium containing
materials such as lithium titanate spinel (Li4Ti5012), or titanium niobium
oxide or titanium
niobium tungsten oxide or titanium oxide. The current collector may be copper
foil or nickel
foil or titanium foil or aluminum foil, for example.
[0094] The battery also includes an electrolyte that may contact the
positive electrode
(cathode), the negative electrode (anode), and the separator. The electrolyte
may include an
organic solvent and a lithium salt. The organic solvent may be a linear or
cyclic carbonate.
Representative organic solvents include ethylene carbonate, propylene
carbonate, butylene
carbonate, trifluoropropylene carbonate, y-butyrolactone, sulfolane, 1,2-
dimethoxyethane, 1,2-
diethoxyethane, tetrahydrofuran, 3-methyl-1,3-dioxolane, methyl acetate, ethyl
acetate,
methylpropionate, ethylpropionate, methyl butyrate, dimethyl carbonate,
diethyl carbonate,
ethyl methyl carbonate, dipropyl carbonate, methylpropyl carbonate, propane
sultone, or a
combination thereof. In another aspect the electrolyte is a polymer
electrolyte.
[0095] Representative lithium salts useful in an electrolyte include but
are not limited to
LiPF6, LiBF4, LiAsF6, LiC104, LiCF3S03, Li(CF3502)2N, LiN(502C2F5)2, Li SbF6,
LiC(CF3502)3, LiC4F9S03, and LiA1C14. The lithium salt may be dissolved in the
organic
37
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
solvent. A combination comprising at least one of the foregoing can be used.
The
concentration of the lithium salt can be 0.1 to 2.0 M in the electrolyte.
[0096] The electrolyte may be a solid ceramic electrolyte.
[0097] Various aspects of the present disclosure are illustrated by the
following non-
limiting examples. The examples are for illustrative purposes and are not a
limitation on any
practice of the present invention. It will be understood that variations and
modifications can
be made without departing from the spirit and scope of the invention.
EXAMPLES
Example 1: Preparation and testing of Lii.oiM2o.oiNio.9oCoo.osY0.020/
[0098] A polycrystalline base material with overall composition
Li1.oiMgo.oiNio.92Coo.0802
was synthesized by standard solid-state synthesis techniques. 2.7 grams (g)
yttrium nitrate
hexahydrate (Y(NO3)3 = 6H20) (99.9% Alfa Aesar, Ward Hill, MA) was dissolved
into 30
milliliters (m1) of 40 C methanol in a glass beaker. Once dissolved, 35 grams
(g) of
polycrystalline base material (Li1.oiMgo.oiNio.92Coo.0802) was added to the
solution. The
solution was stirred for 3 minutes to ensure the base was distributed in the
methanol solution.
The methanol was removed from the solution by evaporation on a rotary
evaporator at 40
degrees Celsius ( C) and 20 mmHg.
[0099] The dry powder was placed in an alumina crucible and calcined.
Calcination was
performed by heating at a rate of 5 C per minute to about 130 C and then
holding at about
130 C for about six hours. The temperature was then raised at about 5 C per
minute to about
450 C and then holding for about 1 hour. The temperature was then raised at
about 2 C per
38
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
minute to 700 C and held at about 700 C for two hours. The sample was then
allowed to cool
naturally to room temperature.
[00100] The
material as synthesized above was cast into cathode electrodes. The material
was first mixed with PVdF, conductive carbon, and NMP solvent to prepare an
electrode slurry.
The electrode slurry was coated on to aluminum foil using a doctor blade
approach. The coated
foil was dried at 130 C to drive off the NMP leaving behind a coated
electrode. The electrode
was then pressed, punched, and assembled into coin cells with Li metal anode
(half cells) or
with graphite anode (full cells), and tested.
Comparative Example 1
[00101] The
polycrystalline base material (Li1.oiMgo.oiNio.92Coo.0802) used above, without
additional calcination, was used as a control material. An
electrode with
Li1.oiMgo.oiNio.92Coo.0802 cathode powder control was prepared according to
the method
described in Example 1.
[00102]
Table 3 shows the half-cell results for the Example 1 and Comparative example
1
electrodes tested between 4.3 Volts (V) ¨ 3.0 V, showing that treatment with
yttrium does not
significantly change the discharge capacity as measured in half cells. Figure
7 and Figure 8
show the full cell results of accelerated cycle life measurements carried out
at 45 C. Material
with yttrium exhibits more stable cycling and much reduced impedance increase
compared to
the base material. Presence of yttrium in the grain boundary region enhances
lithium retention
at the end of charge, and thereby reduces the damage to the material resulting
in improved
cycle life at 45 C. Capacity retention and rate of impedance growth during
accelerated cycle
life testing at 45 C are shown in Figure 7 and Figure 8, respectively.
39
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
Table 3: Electrochemical capacity (mAh/g) of powders prepared in Example 1
C/20 C/20
Sample C/10 C/5 C/2 1C 2C 3C 5C
CGH DCH
Yttrium Treated 232 215 210 205 196 190 185 182 178
Control 236 216 212 206 197 192 187 184 179
Cells were charged to 4.3V versus Li and discharged to 3.0V, at the rates
indicated in the top
row. The capacities are normalized to mass of active material in the
electrode.
Example 2: Preparation and testing of Lii.oiMgo.oiNio.87ComiFeo.0202
[00103]
0.61 g iron acetate (Fe(C2H302)2) (Alfa Aesar, Ward Hill, MA) and 3.08 g
cobalt
nitrate hexahydrate (Co(NO3)2 6H20) were dissolved into 30 ml of 40 C
methanol in a glass
beaker. Once dissolved, 35 g of Base (Lii.oiNi0.92Co0.08Mg0.0102) was added to
the solution.
The solution was stirred for 3 minutes to ensure the base was distributed in
the methanol
solution. The methanol was removed from the solution by evaporation on a
rotary evaporator
at 40 C and 20 mmHg.
[00104] The
dry powder was placed in an alumina crucible and calcined. Calcination
was performed by heating at a rate of 5 C per minute to about 130 C, and
held at about 130
C for about six hours. The temperature was then raised at about 5 C per
minute to about 450
C, and held for about 1 hour. The temperature was then raised at about 2 C
per minute to 700
C and held at about 700 C for two hours. The sample was then allowed to cool
naturally to
room temperature.
[00105]
Lii.oiMgo.oiNio.87CoodiFeo.0202 cathode material was electrochemically tested
along with the untreated base material control with Lii.oiNio.92Coo.o8Mgo.o102
composition in
full coin cells opposite a graphite anode, 1M LiPF6 in 1:1:1 EC:DMC:EMC with
1% VC
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
(EDEV1) electrolyte, and a polymer separator. Full coin cells were cycled
between 2.7 and
4.2 V at 45 C using accelerated charge and discharge rates. Figure 9 and
Figure 10 show the
rate of capacity fade and impedance increase, respectively, during the
accelerated cycle life
testing.
Example 3: Preparation of Lii.oiMgo.oiNio.8975Coo.o897Mno.012802.
[00106] To enable higher levels of Li in the grain boundary at 4.3V charge,
a coating with
1/1/1 Ni/Co/Mn composition was formulated for grain boundary enrichment. A
LiM02
material with this composition is expected to have 37% of the lithium retained
when it is
charged to 4.3V vs. Li. The base material used was Li1.oiMgo.oiNio.92Coo.0802.
To this was
applied a 4% grain boundary-enrichment formulation of 1/1/1 Ni/Co/Mn
composition. As such,
1.33% of each Ni, Co and Mn were formulated relative to total transition metal
content of the
base.
[00107] A solution of 200 ml water was made with manganese nitrate
tetrahydrate (6.78 g),
nickel nitrate heptahydrate (8.34 g), cobalt nitrate hexahydrate (7.86 g) and
lithium nitrate (2.85
g) and was heated to 60 C. To this was added 200 g of the base material and
the dispersion
was stirred for two minutes. The dispersion was then spray dried to generate a
free-flowing
powder. This powder was then calcined at 700 C for two hours (NCM111 enriched
- sample
1) and at 715 C for 0.25 hours (NCM111 enriched - sample 2) under a flow of
CO2-free air.
[00108] The samples were then characterized for residual LiOH and average
oxidation state
and compared to the base material. Reduction in residual LiOH while
maintaining oxidation
state is a strong indication that well-ordered materials were made. In
addition, separate phases
of LiM02 material with NCM111 composition were not detected in the XRD
spectrum, also
41
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
suggesting that the coating composition enriched the grain boundary (GB)
region rather than
forming a separate NCM1 11 LiM02 phase
Table 4: Summary of physico-chemical characterization of materials prepared
with grain
boundary region having the composition LiNiCoMn02.
Sample Overall Composition Wt% Average Transition
LiOH Metal Oxidation
State
Base Li 1.01Mgo.o iNio.92Coo.o802 0.100 2.95
NCM1 11 Li 1.01Mg0.0
iNio.8975Coo.o897Mno.oi2802 0.066 2.93
enriched -sample
1
NCM1 11 Li 1.01Mg0.0
iNio.8975Coo.o897Mno.oi2802 0.069 2.94
enriched - sample
2
Example 4: Preparation of Lii.oiMgo.oiNio.8975Coo.o769Alo.onsMno.012802.
[00109] A polycrystalline base cathode material with overall composition
Li1.oiMgo.oiNio.92Co0.0802 (base material) was synthesized by standard solid-
state synthesis
techniques. The grain boundaries of base material were then fortified with
elements which, in
combination with nickel, still form a layered 2D a-NaFe02 structure, but which
exhibit a
significantly reduced degree of oxidation at 4.3V. Incorporation of these
elements, specifically
Al and Mn demonstrated promotion of Li retention. The overall composition of
this Al and
Mn-grain-boundary-enriched material (referred to as NAM1 11 enriched) was
Lii.oiMgo.oiNio.8975Coo.o769Alo.o128Mno.o12802. The material was synthesized
according to the
following procedure.
[00110] A solution of 100 ml water was made with manganese nitrate
tetrahydrate (3.38 g),
nickel nitrate heptahydrate (3.92 g), aluminum nitrate nonahydrate (5.05 g)
and lithium nitrate
42
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
(1.42 g) and was heated to 60 C. To this was added 100 g of the base material
and the
dispersion was stirred for 20 minutes. The dispersion was then spray dried to
generate a free-
flowing powder. This powder was then calcined at 700 C for two hours (NAM111
enriched)
under a flow of CO2-free air.
[00111] The polycrystalline cathode material, NAM111 enriched, was then
analyzed to
confirm that the grain boundaries were indeed enriched in Al and Mn. A 100 nm
thick section
of the polycrystalline particle of NAM111 enriched was prepared using focused
ion beam
milling. EDS line scans were performed across two different grain boundaries
at the indicated
locations in Figure 3. Figure 4 A and B shows the atomic ratios of aluminum,
manganese and
cobalt relative to nickel across these grain boundaries. As can be seen, there
is clear enrichment
of aluminum and manganese at these grain boundaries, but not cobalt. That is,
the concentration
of manganese at the grain boundaries is higher than the concentration of
manganese in the
crystallites. Also, the concentration of aluminum in the grain boundaries is
higher than the
concentration of aluminum in the crystallites.
[00112] Figure 4 C and D illustrate the atomic percent of nickel relative
to the total atomic
content of nickel, aluminum, manganese and cobalt across these grain
boundaries. As can be
seen, the concentration of nickel at the grain boundaries is lower than the
concentration of
nickel in the crystallites.
Example 5: Preparation of Lii.oiMgo.oiNi0.908Coo.o769Mno.019202.
[00113] Using the same polycrystalline base cathode material with overall
composition
Li LoiMgomiNio.92Coo.o802 (base material) of Example 3 and 4 was used for the
synthesis of the
Ni and Mn grain boundary enriched material (referred to as NM11 Enriched),
with an overall
43
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
final composition of Lii.oiMgo.oiNio.9o38Coo.o769Mno.o19202. The material was
synthesized
according to the following procedure.
[00114] A solution of 100 ml water was made with manganese nitrate
tetrahydrate (5.90 g),
nickel nitrate heptahydrate (5.09 g), and lithium nitrate (1.42 g) and was
heated to 60 C. To
this was added 100 g of the base material and the dispersion was stirred for
10 minutes. The
dispersion was then spray dried to generate a free-flowing powder. This powder
was then
calcined at 700 C for two hours under a flow of CO2-free air.
Example 6: Electrochemical testing of NMC111, NAM!!!, and NM!! Grain Boundary
Enriched Materials.
[00115] Cathode electrodes with NCM111 enriched - sample 1 described in
Example 3,
Base Material and NAM111 Enriched material described in Example 4, and NM11
Enriched
Material of Example 5 were prepared and electrochemically tested. Cathode
active materials
were first mixed with PVdF binder, conductive carbon, and NMP solvent to
prepare an
electrode slurry. The electrode slurry was coated onto aluminum foil using a
draw down table.
The coated foil was dried at 130 C to drive off the NMP leaving behind a
coated electrode.
The electrode was then pressed, punched, and assembled into coin cells with Li
metal anode
(half cells) or with graphite anode (full cells), and tested.
[00116] Cycle life of the three materials in full coin cells was tested
using identical
electrochemical procedures. Figure 11 and 12 show capacity retention and
impedance growth,
respectively, during accelerated cycle life testing at 45 C. In addition to
high rate cycle steps,
a 1C continuous discharge step is included after every 10 high rate cycles.
Figure 12 shows
DCR measured with a 10 second, 2C pulse at the end of charge after every 10
high rate cycles.
44
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
The superior performance of the grain boundary enriched materials both in
terms of reduced
capacity fade and reduced impedance growth is confirmed by these measurements.
The
selective enrichment of Al and Mn at the grain boundary promotes additional
retention of Li at
the end of charge.
Example 7: XRD Analysis of the charged grain boundary-enriched materials.
[00117] Figure 5 shows x-ray diffraction data for the homogeneous
Li1.oiMgo.oiNio.92Coo.0802 base material and NCM111 grain boundary enriched
material of
Example 3 with the overall composition of Li
LoiMgo.oiNio.8975Coo.o897Mno.o12802 (Sample 1).
Cathode electrodes coated on Al current collectors were X-rayed either fresh
or charged to
4.3V vs. Li. Electrodes were charged in coin cells opposite a Li metal counter
electrode.
Charged electrodes were harvested, washed, and dried prior to x-ray
diffraction. X-ray
diffraction spectra were collected using a continuous scan between 12 and 120
degrees in 2-
theta at 0.75 degrees/min using an automated Shimadzu XRD-6000 diffractometer
with a Cu
X-ray tube. The two cathode materials have essentially identical
diffractograms, and many of
the peaks for both materials shift to significantly higher 20 values when they
are charged.
[00118] However, comparing the XRD of the two charged materials reveals an
important
difference. The 20 = 19 and 46 peaks on the charged grain boundary-modified
Li1.oiMgo.oiNio.8975Coo.o897Mno.012802 cathode are broader and have a shoulder
at low 20
compared to those for the homogeneous Li LoiMgo.oiNio.92Coo.0802 cathode, as
shown in Figure
6.
[00119] The 20 = 19 and 46 peaks in the LiM02 XRD are associated with the
003 and the
104 crystallographic orientations for the 2D a-NaFe02 crystal structure,
respectively, and are
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
most directly impacted by c-axis inhomogeneity arising from Li distribution
inhomogeneity.
Therefore, the low 20 shoulder for the charged grain boundary-modified
Li1.oiMgo.oiNio.8975Coo.o897Mno.012802 cathode in Figure 6 is an indication
that it retains more Li
in its grain boundaries than in its bulk crystallites when it is charged.
Example 8: Preparation of Lii.oiMgo.oiNio.9oCoom8Ndo.0202.
[00120] A polycrystalline grain boundary enriched material with overall
composition of
Li1.oiMgo.oiNio.9oCoo.o8Ndo.0202 was synthesized as follows: 2.7 grams (g)
neodymium nitrate
hexahydrate (Nd(NO3)3 = 6H20) (99.9% Sigma Aldrich Milwaukee, WI) was
dissolved into
30 milliliters (m1) of 40 C methanol in a glass beaker. Once dissolved, 30
grams (g) of
polycrystalline base material (Li1.oiMgo.oiNio.92Coo.0802) was added to the
solution. The
solution was stirred for 3 minutes to ensure the base was distributed in the
methanol solution.
The methanol was removed from the solution by evaporation on a rotary
evaporator at 40
degrees Celsius ( C) and 20 mmHg.
[00121] The dry powder was placed in an alumina crucible and calcined.
Calcination was
performed by heating at a rate of 5 C per minute to about 130 C and then
holding at about
130 C for about six hours. The temperature was then raised at about 5 C per
minute to about
450 C and then holding for about 1 hour. The temperature was then raised at
about 2 C per
minute to 700 C and held at about 700 C for two hours. The sample was then
allowed to cool
naturally to room temperature.
Example 9: Preparation of Lii.oiMgo.oiNio.9oCoo.o8Gao.0202.
46
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[00122] A polycrystalline grain boundary enriched material with overall
composition of
Li1.oiMgo.oiNio.9oCoo.o8Ga0.0202 was synthesized as follows:. 1.55 grams (g)
gallium nitrate
hydrate (Ga(NO3)3 = H20) (99.999% Sigma Aldrich Milwaukee, WI) was dissolved
into 30
milliliters (m1) of 40 C ethanol in a glass beaker. Once dissolved, 30 grams
(g) of
polycrystalline base material (Li1.oiMgo.oiNio.92Coo.0802) was added to the
solution. The
solution was stirred for 3 minutes to ensure the base was distributed in the
methanol solution.
The methanol was removed from the solution by evaporation on a rotary
evaporator at 40
degrees Celsius ( C) and 20 mmHg.
[00123] The dry powder was placed in an alumina crucible and calcined.
Calcination was
performed by heating at a rate of 5 C per minute to about 130 C and then
holding at about
130 C for about six hours. The temperature was then raised at about 5 C per
minute to about
450 C and held there for about 1 hour. The temperature was then raised at
about 2 C per
minute to 700 C and held at about 700 C for two hours. The sample was then
allowed to cool
naturally to room temperature.
Example 10: Preparation of Lii.oiMgo.oiNio.9oCoo.o813o.0202.
[00124] A polycrystalline grain boundary enriched material with overall
composition of
Li1.oiMgo.oiNio.90Coo.o8Bo.0202 was synthesized as follows:. 0.21 grams (g)
boron trioxide
(B203) (99% Sigma Aldrich Milwaukee, WI) was dissolved into 40 milliliters
(m1) of 40 C
methanol in a glass beaker. Once dissolved, 30 grams (g) of polycrystalline
base material
(Li1.oiMgo.oiNio.92Coo.0802) was added to the solution. The solution was
stirred for 3 minutes
47
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
to ensure the base was distributed in the methanol solution. The methanol was
removed from
the solution by evaporation on a rotary evaporator at 40 degrees Celsius ( C)
and 20 mmHg.
[00125] The dry powder was placed in an alumina crucible and calcined.
Calcination was
performed by heating at a rate of 5 C per minute to about 130 C and then
holding at about
130 C for about six hours. The temperature was then raised at about 5 C per
minute to about
450 C and then held there for about 1 hour. The temperature was then raised
at about 2 C per
minute to 700 C and held at about 700 C for two hours. The sample was then
allowed to cool
naturally to room temperature.
Example 11: Preparation of Lii.oiMgo.oiNio.9oCoo.o8Feo.0202.
[00126] A grain boundary enriched material with overall composition of
Li1.oiMgo.oiNio.90Coo.08Feo.0202 was synthesized as follows:. 1.23 grams (g)
iron acetate
anhydrous (Fe(00CH3)2) (99.9% Alfa Aesar, Ward Hill, MA) was dissolved into 30
milliliters
(m1) of 40 C methanol in a glass beaker. Once dissolved, 35 grams (g) of
polycrystalline base
material (Li1.oiMgo.oiNio.92Coo.0802) was added to the solution. The solution
was stirred for 3
minutes to ensure the base was distributed in the methanol solution. The
methanol was removed
from the solution by evaporation on a rotary evaporator at 40 degrees Celsius
( C) and 20
mmHg.
[00127] The dry powder was placed in an alumina crucible and calcined.
Calcination was
performed by heating at a rate of 5 C per minute to about 130 C and then
holding at about
130 C for about six hours. The temperature was then raised at about 5 C per
minute to about
450 C and then holding for about 1 hour. The temperature was then raised at
about 2 C per
48
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
minute to 700 C and held at about 700 C for two hours. The sample was then
allowed to cool
naturally to room temperature.
Example 12: Electrochemical testing of cathode materials of Examples 1 and 8
to 11.
Cathode active materials of Example 1, 8, 9, 10, and 11 were formulated into
cathode
electrodes. Materials were first mixed with PVdF, conductive carbon, and NMP
solvent to
prepare an electrode slurry. Electrode slurries were then coated on to
aluminum foil using a
doctor blade approach. The coated foils were dried at 130 C to drive off the
NMP leaving
behind coated electrodes. Electrodes were then pressed, punched, and assembled
into coin cells
with Li metal counter electrodes, polymer separator, and carbonate
electrolyte. Half cells were
then tested for capacity and rate capability (charged to 4.3V and discharged
to 3.0V).
Electrochemical performance data for the enriched materials and the un-
enriched
polycrystalline base material are shown in Table 5.
Table 5: Electrochemical performance of materials described in Examples 1, 8,
9, 10, and 11.
Capacity normalized by the weight of active material.
1st C/20 Discharge Capacity
Example Charge (mAh/g)
(mAh/g) C/20 C/5 5C
iNio.9oCoo.o8Y0.0202 233 213 202 176
iNio.9oCoo.o8Ndo.0202 230 219 209 178
iNio.9oCoo.o8Ga0.0202 239 213 205 180
iNio.9oCoo.o8B0.0202 246 232 221 188
iNio.9oCoo.o8Fe0.0202 231 197 ... 187 158
Li i.o iMgo.o iNio.9oCoo.o802 236 213 206 179
49
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[00128]
Without being limited by one particular theory, it is believed the superior
cycling
stability of materials with modified grain boundaries is related to their
selective retention of
more Li in grain boundaries when they are charged, as can be inferred from the
preceding
examples.
Example 13:
[00129] An
NCM base material having first composition LiNio.8Coo.iMno.102 (NCM 811)
was prepared from co-precipitated precursor transition metal hydroxide
containing 10 at% Co
and 10 at% Mn and the balance Ni. An NCA material having a first composition
of various
amounts of Ni, Co, and Al (atomic ratio 86:12:2, 89:8:3, or 93:4:3,
respectively) were prepared
from co-precipitated precursor transition metal hydroxide containing the
appropriate amounts
of Ni, Co and Al. A micronized LiOH powder was made by placing 87.7 g of LiOH
into a
plastic jar with 500 g of Y-stabilized zirconia 1/4" spheres and shaking on a
paint shaker for 45
minutes. This micronized powder was then transferred to another plastic jar
containing 335.7
g of the NCM or NCA transition metal hydroxide precursors and the two were
blended by
shaking on a paint shaker for a further 10 minutes. After blending, the
roughly 440 g of powder
was split between three crucibles and fired in an oxygen atmosphere by first
ramping to 450 C
at 5 C/min and soaking at temperature for 2 hours, and then ramping to 770 C
at 2 C/min
and soaking for 10 hours at 770 C. The furnace was then allowed to cool to
130 C and the
powder was removed and placed into ajar mill. The jar mill contained 3/4" drum
media and was
used to mill the powder for 2 minutes. The powder was then sieved through a
270 mesh sieve.
[00130] The
sieved powder was then divided into base (no further treatment) or grain-
boundary-enriched with Co and Al by making a solution of 200 g water, 11.9 g
cobalt nitrate
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
(2 at% Co relative to the total metal content of the base composition), 3.1 g
aluminum nitrate
(0.4 at% Al relative to the total metal content of the base composition), 3.4
g lithium nitrate
and heating to 60 C. To this was added 200 g of the previously prepared
lithiated precursor
powder (base materials). The slurry was allowed to stir for 10 minutes after
which it was spray
dried to remove the water from the slurry and prepare a dry powder. This
powder was then
fired in an air atmosphere by first ramping to 450 C at 5 C/min and soaking
at temperature
for 1 hour, and then ramping to 770 C at 2 C/min and soaking for 0.25 hour.
The furnace was
then allowed to cool to 130 C and the powder was removed from the furnace and
sieved
through a 270 mesh sieve.
[00131] The resulting base or grain boundary enriched particles were
crushed in a 2 cm
diameter die in a hydraulic press to the desired pressure. A total mass of 2.5
g of powder was
placed in the die and crushed to form a pellet. The pellet was then placed in
20 mL of water
and subjected to ultrasonic dispersion for 30 minutes. Once dispersed, a small
portion of the
dispersed slurry was tested on a Malvern Mastersizer 3000 laser-light particle
size instrument
equipped with a liquid cell. To ensure full dispersion of fines, the
ultrasonic function was used
during the measurement and an average of 5 measurements were used to calculate
the final
result. The particle distribution was calculated using properties for NiO
taken from the
instrument library. The results of this process using the exemplary NCM811
material with or
without Ni poor grain boundary regions are illustrated in FIG. 13.
[00132] The various materials were assembled into cathodes and tested as
presented in
Example 6. As is illustrated in FIG. 13, the presence of relatively lower Ni
concentration in the
grain boundaries results in reduced impedance relative to the base materials.
51
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[00133] The foregoing description of particular aspect(s) is merely
exemplary in nature and
is in no way intended to limit the scope of the disclosure, its application or
uses, which may of
course vary. The materials and processes are described with relation to the
non-limiting
definitions and terminology included herein. These definitions and terminology
are not
designed to function as a limitation on the scope or practice of the
disclosure, but are presented
for illustrative and descriptive purposes only. While the processes or
compositions are
described as an order of individual steps or using specific materials, it is
appreciated that steps
or materials may be interchangeable such that the description of the invention
may include
multiple parts or steps arranged in many ways as is readily appreciated by one
of skill in the
art.
[00134] It will be understood that, although the terms "first," "second,"
"third," etc. may
be used herein to describe various elements, components, regions, layers,
and/or sections, these
elements, components, regions, layers, and/or sections should not be limited
by these terms.
These terms are only used to distinguish one element, component, region,
layer, or section from
another element, component, region, layer, or section. Thus, unless specified
otherwise, "a
first element," "component," "region," "layer," or "section" discussed below
could be termed
a second (or other) element, component, region, layer, or section without
departing from the
teachings herein.
[00135] The terminology used herein is for the purpose of describing
particular aspects only
and is not intended to be limiting. As used herein, the singular forms "a,"
"an," and "the" are
intended to include the plural forms, including "at least one," unless the
content clearly
indicates otherwise. "Or" means "and/or." As used herein, the term "and/or"
includes any and
all combinations of one or more of the associated listed items. It will be
further understood
52
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
that the terms "comprises" and/or "comprising," or "includes" and/or
"including" when used
in this specification, specify the presence of stated features, regions,
integers, steps, operations,
elements, and/or components, but do not preclude the presence or addition of
one or more other
features, regions, integers, steps, operations, elements, components, and/or
groups thereof. The
term "or a combination thereof' means a combination including at least one of
the foregoing
elements.
[00136] Unless otherwise defined, all terms (including technical and
scientific terms) used
herein have the same meaning as commonly understood by one of ordinary skill
in the art to
which this disclosure belongs. It will be further understood that terms such
as those defined in
commonly used dictionaries, should be interpreted as having a meaning that is
consistent with
their meaning in the context of the relevant art and the present disclosure,
and will not be
interpreted in an idealized or overly formal sense unless expressly so defined
herein.
[00137] Various modifications, in addition to those shown and described
herein, will be
apparent to those skilled in the art of the above description. Such
modifications are also
intended to fall within the scope of the disclosure.
[00138] It is appreciated that all reagents are obtainable by sources known
in the art unless
otherwise specified.
[00139] Patents, publications, and applications mentioned in the
specification are indicative
of the levels of those skilled in the art to which the disclosure pertains.
These patents,
publications, and applications are incorporated herein by reference to the
same extent as if each
individual patent, publication, or application was specifically and
individually incorporated
herein by reference.
53
CA 03126008 2021-07-07
WO 2020/150084 PCT/US2020/013038
[00140] The foregoing description is illustrative of particular aspects of
the invention, but
is not meant to be a limitation upon the practice thereof
54