Note: Descriptions are shown in the official language in which they were submitted.
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
PROCESS FOR PRODUCING HYDROGEN-LEAN SYNGAS FOR SYNTHESIS PROCESSES
TECHNICAL FIELD
[0001] The present disclosure relates to methods of producing hydrogen-lean
synthesis gas (e.g., having
a molar ratio of hydrogen to carbon monoxide (142/C0) in a range of from about
0.8 to 1.6); more
specifically, the present disclosure methods of producing hydrogen-lean
synthesis gas via catalytic partial
oxidation (CPO); still more specifically, the present disclosure relates to
methods of producing hydrogen-
lean synthesis gas via catalytic partial oxidation (CPO) of a CPO reactant
mixture comprising hydrocarbons
and oxygen, wherein the hydrocarbons comprise greater than or equal to about 3
mole percent (mol%) of
higher hydrocarbons (e.g., alkanes comprising 2 or more carbons, C2+).
BACKGROUND
[0002] Synthesis gas (syngas) is a mixture comprising carbon monoxide (CO) and
hydrogen (112), as
well as small amounts of carbon dioxide (CO2), water (1120), and unreacted
methane (CH4). Syngas is
generally used as an intermediate in a variety of synthesis processes,
including, without limitation, dimethyl
ether (DME), alcohols, such as methanol, ethanol, oxoalcohols (e.g., n-butanol
etc.), ethylene glycol,
aldehydes, and the like. Syngas is produced conventionally by steam reforming
of natural gas (steam
methane reforming or SMR), although other hydrocarbon sources can be used for
syngas production, such
as refinery off-gases, naphtha feedstocks, heavy hydrocarbons, coal, biomass,
etc. SMR is an endothermic
process and requires significant energy input to drive the reaction forward.
Conventional endothermic
technologies such as SMR produce syngas with a hydrogen content greater than
the required for a variety
of downstream chemical syntheses.
[0003] In an autothermal reforming (ATR) process, a portion of the natural gas
is burned as fuel to drive
the conversion of natural gas to syngas resulting in relatively low hydrogen
and high CO2 concentrations.
Conventional combined reforming (CR) technology pairs SMR with autothermal
reforming (ATR) to
reduce the amount of hydrogen present in syngas. ATR produces a syngas with a
lower hydrogen content.
CR syngas generally has a hydrogen content greater than needed for many
downstream synthesis processes.
Furthermore, SMR is a highly endothermic process, and the endothermicity of
the SMR technology
requires burning fuel to drive the syngas synthesis. Consequently, the SMR
technology reduces the energy
efficiency of the downstream chemical synthesis process.
[0004] Syngas can also be produced (non-commercially) by catalytic partial
oxidation (CPO or CP0x)
of natural gas. CPO processes employ partial oxidation of hydrocarbon feeds to
syngas comprising CO and
H2. The CPO process is exothermic, thus eliminating the need for external heat
supply. Conventional
partial oxidation processes do not produce hydrogen-lean synthesis suitable
for use in downstream
syntheses requiring molar ratios of hydrogen to carbon monoxide less than
about 1.6. Thus, there is an
ongoing need for the development of methods for syngas production via CPO
processes that provide a
hydrogen-lean synthesis gas for a variety of downstream syntheses.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] For a detailed description of the preferred embodiments of the
disclosed methods, reference will
now be made to the accompanying drawing in which:
1
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
[0006] Figure 1 is a schematic of a chemical production system I for the
production of hydrogen-lean
synthesis gas via catalytic partial oxidation, according to embodiments of
this disclosure;
[0007] Figure 2 is a plot of the molar ratio of carbon monoxide to hydrogen
(CO/H2) in syngas from
CPO as a function of reactor temperature without CO2 injection in the reactant
feed;
[0008] Figure 3 is a plot of the molar ratio of carbon monoxide to hydrogen
(CO/H2) in syngas from
CPO as a function of reactor temperature with CO2 injection for a reactant
feed comprising a molar ratio of
carbon dioxide to methane (CO2/CI-I4) of 0.5;
[0009] Figure 4 is a plot of the molar ratio of carbon monoxide to hydrogen
(CO/H2) in syngas from
CPO as a function of reactor temperature with CO2 injection for a reactant
feed comprising a molar ratio of
carbon dioxide to methane (CO2/CI-I4) of 1;
[0010] Figure 5 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of carbon
dioxide to carbon (CO2/C) in
the reactant feed (in legend) at a pressure of 30 bar and an oxygen to carbon
molar ratio (02/C) of 0.55;
[0011] Figure 6 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of carbon
dioxide to carbon (CO2/C) in
the reactant feed (in legend) at a pressure of 75 bar and an oxygen to carbon
molar ratio (02/C) of 0.55;
[0012] Figure 7 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having three carbons
(C3) to carbon (C3/C) in the reactant feed (in legend) at a pressure of 75
bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and a carbon dioxide to carbon (CO2/C) molar ratio of
0.25;
[0013] Figure 8 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having three carbons
(C3) to carbon (C3/C) in the reactant feed (in legend) at a pressure of 75
bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and without CO2 in the reactant feed;
[0014] Figure 9 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having two carbons (C2)
to carbon (C2/C) in the reactant feed (in legend) at a pressure of 75 bar, an
oxygen to carbon molar ratio
(02/C) of 0.55, and a carbon dioxide to carbon (CO2/C) molar ratio of 0.25;
[0015] Figure 10 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having two carbons (C2)
to carbon (C2/C) in the reactant feed (in legend) at a pressure of 75 bar, an
oxygen to carbon molar ratio
(02/C) of 0.55, and without CO2 in the reactant feed;
[0016] Figure 11 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having four carbons
(C4) to carbon (C4/C) in the reactant feed (in legend) at a pressure of 75
bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and a carbon dioxide to carbon (CO2/C) molar ratio of
0.25; and
[0017] Figure 12 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having four carbons
2
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
(C4) to carbon (C4/C) in the reactant feed (in legend) at a pressure of 75
bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and without CO2 in the reactant feed.
DETAILED DESCRIPTION
[0018] The synthesis gas feeds for a variety of chemical synthesis processes
require hydrogen-lean
synthesis gas having a molar ratio of hydrogen to carbon monoxide (142/C0) of
about 1:1. When the
synthesis gas is produced from conventional reforming processes that provide
synthesis gas having a
higher molar ratio (e.g., about 2:1), the synthesis gas has to be pretreated,
for example via a hydrogen
removal unit (e.g., a pressure swing adsorption PSA unit), to reduce the molar
ratio of 142/C0 of the
synthesis gas. Conventional partial oxidation (P0x) processes do not provide
syngas having a 142/C0
molar ratio of about 1:1. The use of an intermediate hydrogen removal (e.g.,
PSA) step increases energy
and capital cost requirements.
[0019] According to this disclosure, hydrogen-lean syngas (e.g., syngas having
a molar ratio of 142/C0
in the range of from about 0.8 to about 1.6) can be produced via a catalytic
partial oxidation (CPO)
process. Via embodiments of the herein disclosed system and method, a CPO
process can be tailored to
provide a hydrogen-lean syngas having a desired composition (e.g., a reduced
142/C0 molar ratio relative
to that of a syngas produced by a conventional POx process). Accordingly, the
herein disclosed systems
and methods can reduce the size of or eliminate hydrogen removal apparatus,
thus reducing the number
of unit operations, and thus can, in embodiments, also reduce energy
requirements for the process.
[0020] In embodiments, CPO is utilized to produce a hydrogen-lean synthesis
gas by utilizing a CPO
reactant feed mixture that comprises higher hydrocarbons and/or carbon dioxide
(CO2). The use of
reactant feed mixtures comprising higher hydrocarbons can allow for a
reduction of the amount of CO2
required to reach an 142/C0 molar ratio of about 1, and at the same time
enable for production of
hydrogen-lean syngas having the desired 142/C0 molar ratio of about 1 at a
higher conversion of
hydrocarbon to syngas.
[0021] Other than in the operating examples or where otherwise indicated, all
numbers or expressions
referring to quantities of ingredients, reaction conditions, and the like,
used in the specification and claims
are to be understood as modified in all instances by the term "about." Various
numerical ranges are
disclosed herein. Because these ranges are continuous, they include every
value between the minimum and
maximum values. The endpoints of all ranges reciting the same characteristic
or component are
independently combinable and inclusive of the recited endpoint. Unless
expressly indicated otherwise, the
various numerical ranges specified in this application are approximations. The
endpoints of all ranges
directed to the same component or property are inclusive of the endpoint and
independently combinable.
The term "from more than 0 to an amount" means that the named component is
present in some amount
more than 0, and up to and including the higher named amount.
[0022] The terms "a," "an," and "the" do not denote a limitation of quantity,
but rather denote the
presence of at least one of the referenced item. As used herein the singular
forms "a," "an," and "the"
include plural referents.
3
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
[0023] As used herein, "combinations thereof' is inclusive of one or more of
the recited elements,
optionally together with a like element not recited, e.g., inclusive of a
combination of one or more of the
named components, optionally with one or more other components not
specifically named that have
essentially the same function. As used herein, the term "combination" is
inclusive of blends, mixtures,
alloys, reaction products, and the like.
[0024] Reference throughout the specification to "an embodiment," "another
embodiment," "other
embodiments," "some embodiments," and so forth, means that a particular
element (e.g., feature, structure,
property, and/or characteristic) described in connection with the embodiment
is included in at least an
embodiment described herein, and may or may not be present in other
embodiments. In addition, it is to be
understood that the described element(s) can be combined in any suitable
manner in the various
embodiments.
[0025] As used herein, the terms "inhibiting" or "reducing" or "preventing" or
"avoiding" or any
variation of these terms, include any measurable decrease or complete
inhibition to achieve a desired result.
[0026] As used herein, the term "effective," means adequate to accomplish a
desired, expected, or
intended result. As used herein, the terms "comprising" (and any form of
comprising, such as "comprise"
and "comprises"), "having" (and any form of having, such as "have" and "has"),
"including" (and any form
of including, such as "include" and "includes") or "containing" (and any form
of containing, such as
"contain" and "contains") are inclusive or open-ended and do not exclude
additional, unrecited elements or
method steps.
[0027] Unless defined otherwise, technical and scientific terms used herein
have the same meaning as is
commonly understood by one of skill in the art. Compounds are described herein
using standard
nomenclature. For example, any position not substituted by any indicated group
is understood to have its
valency filled by a bond as indicated, or a hydrogen atom. A dash ("-") that
is not between two letters or
symbols is used to indicate a point of attachment for a substituent. For
example, -Cl-JO is attached through
the carbon of the carbonyl group. As used herein, the terms "Cõ hydrocarbons"
and "Cs" are
interchangeable and refer to any hydrocarbon having x number of carbon atoms
(C). For example, the
terms "C4 hydrocarbons" and "C4s" both refer to any hydrocarbons having
exactly 4 carbon atoms, such as
n-butane, iso-butane, cyclobutane, 1-butene, 2-butene, isobutylene, butadiene,
and the like, or combinations
thereof.
[0028] As used herein, the term "Cõ, hydrocarbons" refers to any hydrocarbon
having greater than or
equal to x carbon atoms (C). For example, the term "C2, hydrocarbons" refers
to any hydrocarbons having
2 or more carbon atoms, such as ethane, ethylene, C3s, C4s, C5s, etc.
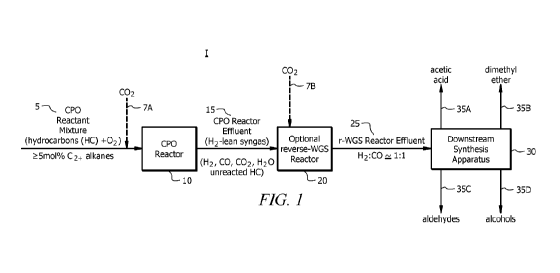
[0029] Referring to Figure 1, a chemical production system I is disclosed.
The chemical production
system I generally comprises a catalytic partial oxidation (CPO or CP0x)
reactor 10 and a downstream
synthesis apparatus 30. Chemical production system I can further comprise a
reverse water gas shift (r-
WGS) reactor 20, in embodiments. As will be appreciated by one of skill in the
art, and with the help of
this disclosure, chemical production system components shown in Figure 1 can
be in fluid communication
4
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
with each other (as represented by the connecting lines indicating a direction
of fluid flow) through any
suitable conduits (e.g., pipes, streams, etc.).
[0030] In embodiments, a process as disclosed herein can comprise a step of
reacting, via a catalytic
partial oxidation (CPO) reaction, a CPO reactant mixture 5 in a CPO reactor 10
to produce a hydrogen-
lean syngas; wherein the CPO reactant mixture comprises hydrocarbons and
oxygen and optionally
carbon dioxide (CO2); wherein the hydrocarbons comprise greater than or equal
to about 3 mol% C2+
alkanes; wherein the CPO reactor comprises a CPO catalyst; wherein the
hydrogen-lean syngas
comprises hydrogen, carbon monoxide, carbon dioxide, water, and unreacted
hydrocarbons; and wherein
the hydrogen-lean syngas is characterized by a hydrogen to carbon monoxide
(142/C0) molar ratio of
from about 0.8 to about 1.6.
[0031] Generally, the CPO reaction is based on partial combustion of fuels,
such as various
hydrocarbons, and in the case of methane, CPO can be represented by equation
(1):
CH4 + 1/2 02 ¨) CO -1 2 H2 (1)
Without wishing to be limited by theory, side reactions can take place along
with the CPO reaction depicted
in equation (1); and such side reactions can produce carbon dioxide (CO2) and
water (H20), for example via
hydrocarbon combustion, which is an exothermic reaction. As will be
appreciated by one of skill in the art,
and with the help of this disclosure, and without wishing to be limited by
theory, the CPO reaction as
represented by equation (1) can yield a syngas with a hydrogen to carbon
monoxide (H2/C0) molar ratio
having the theoretical stoichiometric limit of 2Ø Without wishing to be
limited by theory, the theoretical
stoichiometric limit of 2.0 for the H2/C0 molar ratio means that the CPO
reaction as represented by
equation (1) yields 2 moles of H2 for every 1 mole of CO, i.e., H2/C0 molar
ratio of (2 moles H2/1 mole
CO) = 2. As will be appreciated by one of skill in the art, and with the help
of this disclosure, the
theoretical stoichiometric limit of 2.0 for the H2/C0 molar ratio in a CPO
reaction cannot be achieved
practically because reactants (e.g., hydrocarbons, oxygen) as well as products
(e.g., H2, CO) undergo side
reactions at the conditions used for the CPO reaction. As will be appreciated
by one of skill in the art, and
with the help of this disclosure, and without wishing to be limited by theory,
in the presence of oxygen, CO
and H2 can be oxidized to CO2 and H20, respectively. The relative amounts
(e.g., composition) of CO, H2,
CO2 and H20 can be further altered by the equilibrium of the water-gas shift
(WGS) reaction, which will be
discussed in more detail later herein. The side reactions that can take place
in the CPO reactor 10 can have
a direct impact on the composition of the produced syngas in the CPO reactor
effluent 15 which, according
to this disclosure can comprise the hydrogen-lean syngas. In the absence of
any side reaction
(theoretically), the CPO reaction as represented by equation (1) results in a
syngas with an H2/C0 molar
ratio of 2Ø However, the presence of side reactions can (practically) reduce
H2 (and increase CO2),
thereby resulting in a syngas with a \molar ratio of H2/C0 that is not equal
to 2.
[0032] Further, without wishing to be limited by theory, the CPO reaction as
depicted in equation (1) is
an exothermic heterogeneous catalytic reaction (i.e., a mildly exothermic
reaction) and it occurs in a single
reactor unit, such as the CPO reactor 10 (as opposed to more than one reactor
unit as is the case in
conventional processes for syngas production, such as steam methane reforming
(SMR) - autothermal
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
reforming (ATR) combinations). While it is possible to conduct partial
oxidation of hydrocarbons as a
homogeneous reaction, in the absence of a catalyst, homogeneous partial
oxidation of hydrocarbons process
entails excessive temperatures, long residence times, as well as excessive
coke formation, which strongly
reduce the controllability of the partial oxidation reaction, and may not
produce syngas of the desired
quality in a single reactor unit.
[0033] Furthermore, without wishing to be limited by theory, the CPO reaction
is fairly resistant to
chemical poisoning, and as such it allows for the use of a wide variety of
hydrocarbon feedstocks, including
some sulfur containing hydrocarbon feedstocks; which, in some cases, can
enhance catalyst life-time and
productivity. By contrast, conventional ATR processes have more restrictive
feed requirements, for
example in terms of content of impurities in the feed (e.g., feed to ATR is
desulfurized), as well as
hydrocarbon composition (e.g., ATR primarily uses a CT-I4-rich feed).
[0034] In embodiments, the hydrocarbons suitable for use in a CPO reaction as
disclosed herein can
include methane, natural gas, natural gas liquids, liquefied petroleum gas
(LPG), associated gas, well head
gas, enriched gas, paraffins, shale gas, shale liquids, fluid catalytic
cracking (FCC) off gas, refinery process
gases, refinery off gases, stack gases, fuel gas from a fuel gas header, or
combinations thereof. In
embodiments, an amount of CO2 and/or CO in the reactant mixture 5 can be
increased by diluting a feed
with gases (e.g., stack gases) containing CO2 and/or CO. Such gases containing
CO and/or CO2 include,
without limitation, stack gases, reducing gases, off gases rich in CO, such as
used in the metal industry,
crackers, and the like. For example, dedicated coking reactors can be utilized
which, when injected with
steam supply, air, and CO2 deliver a continuous CO stream to CPO reactor 10.
[0035] In embodiments, reactant mixture 5 comprises fuel gases from a steam
cracker and CPO reactor
is operated at a high CI-I4/U2 molar ratio by providing an autothermal mode of
operation. In
embodiments, a hydrogen content of the reactant mixture 5 can be adjusted to
maintain an appropriate
adiabatic rise.
[0036] The hydrocarbons can include any suitable hydrocarbons source, and can
contain C1-C6
hydrocarbons, as well some heavier hydrocarbons. In embodiments, the CPO
reactant mixture 5 can
comprise natural gas. Generally, natural gas is composed primarily of methane,
but can also contain ethane,
propane and heavier hydrocarbons (e.g., iso-butane, n-butane, iso-pentane, n-
pentane, hexanes, etc.), as well
as very small quantities of nitrogen, oxygen, carbon dioxide, sulfur
compounds, and/or water. The natural
gas can be provided from a variety of sources including, but not limited to,
gas fields, oil fields, coal fields,
fracking of shale fields, biomass, landfill gas, and the like, or combinations
thereof. In some embodiments,
the CPO reactant mixture 5 can comprise primarily Cl-I4 and 02, which can be
introduced separately into
CPO reactor 10, in embodiments.
[0037] The natural gas can comprise any suitable amount of methane. In some
embodiments, the
natural gas can comprise biogas. For example, the natural gas can comprise
from about 45 mol% to
about 80 mol% methane, from about 20 mol% to about 55 mol% carbon dioxide, and
less than about 15
mol% nitrogen.
6
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
[0038] In embodiments, natural gas can comprise Cl-I4 in an amount of greater
than or equal to about
45 mol%, about 50 mol%, about 55 mol%, about 60 mol%, about 65 mol%, about 70
mol%, about 75
mol%, about 80 mol%, about 82 mol%, about 84 mol%, about 86 mol%, about 88
mol%, about 90 mol%,
about 91 mol%, about 92 mol%, about 93 mol%, about 94 mol%, about 95 mol%,
about 96 mol%, or
about 97 mol%.
[0039] According to this disclosure, the hydrocarbons in the reactant mixture
5 comprise greater than
or equal to about 3, 4, 5, 6, 7, 8, 9, or 10 mol% of heavier hydrocarbons
comprising hydrocarbons having
two or more carbons (e.g., C2+ hydrocarbons). In embodiments, the hydrocarbons
in the reactant
mixture 5 comprise greater than or equal to about 3, 4, 5, 6, 7, 8, 9, or 10
mol% of C2+ alkanes. In
embodiments, the hydrocarbons in reactant mixture 5 comprise ethane in an
amount of greater than or
equal to about 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15 mol%. In
embodiments, the hydrocarbons in the
reactant mixture 5 comprise propane in an amount of greater than or equal to
about 4, 5, 6, 7, 8, 9, 10, 11,
12, 13, 14, or 15 mol%. In embodiments, hydrocarbons comprise butanes in an
amount of greater than or
equal to about 3, 4, 5, 6, 7, or 8 mol%. In embodiments, the hydrocarbons in
reactant mixture 5 comprise
ethane in an amount of greater than or equal to about 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, or 15 mol%,
propane in an amount of greater than or equal to about 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, or 15 mol%,
butanes in an amount of greater than or equal to about 3, 4, 5, 6, 7, or 8
mol%, or a combination thereof.
[0040] In embodiments, the CPO reactant mixture 5 further comprises carbon
dioxide (CO2), and the
CPO reactant mixture 5 is characterized by a CO2 to carbon (CO2/C) and/or a
CO2/Cl-I4 molar ratio in the
CPO reactant mixture 5 of greater than or equal to about 0.5:1, 0.25:1, or
0:1, wherein the CO2/C molar
ratio refers to the total moles of CO2 in the reactant mixture divided by the
total moles of carbon (C) in
the hydrocarbons in the reactant mixture 5. In embodiments, the CPO reactant
mixture 5 further
comprises carbon dioxide (CO2), and the CPO reactant mixture 5 is
characterized by a CO2 to carbon
(CO2/C) molar ratio in the CPO reactant mixture 5 of less than or equal to
about 10:1, 5:1, or 2:1. All or
a portion of the CO2 in reactant mixture 5 can be introduced into the reactant
mixture 5 via CO2 stream
7A, in embodiments. In embodiments, CPO reactor 10 is operated in autothermal
mode with CO2
injection or addition via 7A.
[0041] In embodiments, the amount of CO2 in the CPO reactant mixture 5 is
lower than the amount of
CO2 in a CPO reactant mixture in an otherwise similar process that produces a
hydrogen-lean syngas
from a reactant mixture comprising a lower quantity of C2+ alkanes
hydrocarbons (e.g., wherein the
hydrocarbons in the reactant mixture 5 comprise less than about 3 mol% C2+
alkanes). In embodiments, a
portion of the carbon dioxide in the CPO reactor 10 undergoes a reverse water-
gas shift (r-WGS) reaction
within CPO reactor 10 (and/or in a r-WGS reactor 20 downstream of CPO reactor
10, as described
hereinbelow), thereby decreasing the amount of hydrogen in the hydrogen-lean
syngas.
[0042] In some embodiments, the hydrocarbons suitable for use in a CPO
reaction as disclosed herein
can comprise C1-C6 hydrocarbons (e.g., including C2, C3, and/or C4 as
described above), nitrogen (e.g.,
from about 0.1 mol% to about 15 mol%, alternatively from about 0.5 mol% to
about 11 mol%,
alternatively from about 1 mol% to about 7.5 mol%, or alternatively from about
1.3 mol% to about 5.5
7
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
mol%), and carbon dioxide (e.g., from about 0.1 mol% to about 2 mol%,
alternatively from about 0.2
mol% to about 1 mol%, or alternatively from about 0.3 mol% to about 0.6 mol%).
For example, the
hydrocarbons suitable for use in a CPO reaction as disclosed herein can
comprise C1 hydrocarbon (about
89 mol% to about 92 mol%); C2 hydrocarbons (greater than or equal to about 4,
5, 6, 7, 8, 9, 10, 11, 12,
13, 14, or 15 mol%); C3 hydrocarbons (greater than or equal to about 4, 5, 6,
7, 8, 9, 10, 11, 12, 13, 14, or
15 mol%); C4 hydrocarbons (greater than or equal to about 3, 4, 5, 6, 7, or 8
mol%); C5 hydrocarbons
(about 0.06 mol%); and C6 hydrocarbons (about 0.02 mol%); and optionally
nitrogen (about 0.1 mol% to
about 15 mol%), carbon dioxide (about 0.1 mol% to about 2 mol%), or both
nitrogen (about 0.1 mol% to
about 15 mol%) and carbon dioxide (about 0.1 mol% to about 2 mol%).
[0043] The oxygen used in the CPO reactant mixture 5 can comprise 100% oxygen
(substantially pure
02), oxygen gas (which may be obtained via a membrane separation process),
technical oxygen (which
may contain some air), air, oxygen enriched air, oxygen-containing gaseous
compounds (e.g., NO),
oxygen-containing mixtures (e.g., 02/CO2, 02/H20, 02/H202/H20), oxy radical
generators (e.g., CI430H,
CI-I20), hydroxyl radical generators, and the like, or combinations thereof.
[0044] In embodiments, the CPO reactant mixture 5 can be characterized by a
carbon to oxygen (C/O) or
CI-I4/U2 molar ratio of less than about 3:1, alternatively less than about
2.6:1, alternatively less than about
2.4:1, alternatively less than about 2.2:1, alternatively less than about 2:1,
alternatively less than about
1.8:1, alternatively greater than or equal to about 0.1:1, alternatively
greater than or equal to about 0.2:1,
alternatively greater than or equal to about 0.3:1, alternatively greater than
or equal to about 0.4:1,
alternatively greater than or equal to about 0.5:1, alternatively from about
0.5:1 to about 0.6:1, alternatively
from about 0.55:1 to about 0.6:1, alternatively from about 0.5:1 to about 3:1,
alternatively from about 0.7:1
to about 2.5:1, alternatively from about 0.9:1 to about 2.2:1, alternatively
from about 1:1 to about 2:1,
alternatively from about 1.5:1 to about 1.9:1, wherein the C/O molar ratio
refers to the total moles of carbon
(C) of hydrocarbons in the reactant mixture divided by the total moles of 02
in the reactant mixture.
[0045] As the CPO reactant mixture 5 of this disclosure contains other carbon
sources besides CH4, such
as ethane (C2I16), propane (C3I18), butanes (C4I110), etc., the C/O molar
ratio accounts for the moles of
carbon in each compound (e.g., 2 moles of C in 1 mole of C2I16, 3 moles of C
in 1 mole of C3I18, 4 moles of
C in 1 mole of C4I110, etc.). As will be appreciated by one of skill in the
art, and with the help of this
disclosure, the C/O molar ratio in the CPO reactant mixture 5 can be adjusted
along with other reactor
process parameters (e.g., temperature, pressure, flow velocity, etc.) to
provide for a hydrogen-lean syngas
as described herein. The C/O molar ratio in the CPO reactant mixture 5 can be
adjusted to provide for a
decreased amount of unconverted hydrocarbons in the syngas. The C/O molar
ratio in the CPO reactant
mixture 5 can be adjusted based on the CPO reactor temperature in order to
decrease (e.g., minimize) the
unconverted hydrocarbons content of the CPO reactor effluent 15 comprising the
hydrogen-lean syngas.
[0046] In embodiments, a CPO reactor suitable for use in the present
disclosure (e.g., CPO reactor 10)
can comprise a tubular reactor, a continuous flow reactor, a fixed bed
reactor, a fluidized bed reactor, a
moving bed reactor, a circulating fluidized bed reactor (e.g., a riser type
reactor), a bubbling bed reactor, an
8
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
ebullated bed reactor, a rotary kiln reactor, and the like, or combinations
thereof. In some embodiments, the
CPO reactor can comprise a circulating fluidized bed reactor, such as a riser
type reactor.
[0047] In some embodiments, the CPO reactor 10 can be characterized by at
least one CPO operational
parameter selected from the group consisting of a CPO reactor temperature
(e.g., CPO catalyst bed
temperature); CPO feed temperature (e.g., temperature of CPO reactant mixture
5; target temperature of
CPO reactor effluent 15; a CPO pressure (e.g., pressure of CPO reactor 10); a
CPO contact time (e.g., CPO
reactor 10 contact time); a C/O molar ratio in the CPO reactant mixture 5; a
steam to carbon (S/C) molar
ratio in the CPO reactant mixture 5, wherein the S/C molar ratio refers to the
total moles of water (1420) in
the reactant mixture 5 divided by the total moles of carbon (C) of
hydrocarbons in the reactant mixture 5;
and combinations thereof. For purposes of the disclosure herein, the CPO
effluent temperature is the
temperature of the syngas (e.g., hydrogen-lean syngas or CPO reactor effluent
15) measured at the point
where the syngas exits the CPO reactor (e.g., CPO reactor 10), e.g., a
temperature of the syngas measured at
a CPO reactor outlet, a temperature of the syngas reactor effluent, a
temperature of the exit syngas effluent.
For purposes of the disclosure herein, the CPO effluent temperature (e.g.,
target CPO effluent temperature)
is considered an operational parameter. As will be appreciated by one of skill
in the art, and with the help
of this disclosure, the choice of operational parameters for the CPO reactor
such as CPO feed temperature;
CPO pressure; CPO contact time; C/O molar ratio in the CPO reactant mixture;
S/C molar ratio in the CPO
reactant mixture; etc. determines the temperature of the syngas effluent
(e.g., CPO reactor effluent 15), as
well as the composition of the syngas effluent (e.g., CPO reactor effluent
15). Further, and as will be
appreciated by one of skill in the art, and with the help of this disclosure,
monitoring the CPO effluent
temperature can provide feedback for changing other operational parameters
(e.g., CPO feed temperature;
CPO pressure; CPO contact time; C/O molar ratio in the CPO reactant mixture;
S/C molar ratio in the CPO
reactant mixture; etc.) as necessary for the CPO effluent temperature to match
the target CPO effluent
temperature. Furthermore, and as will be appreciated by one of skill in the
art, and with the help of this
disclosure, the target CPO effluent temperature is the desired CPO effluent
temperature, and the CPO
effluent temperature (e.g., measured CPO effluent temperature, actual CPO
effluent temperature) may or
may not coincide with the target CPO effluent temperature. In embodiments
where the CPO effluent
temperature is different from the target CPO effluent temperature, one or more
CPO operational parameters
(e.g., CPO feed temperature; CPO pressure; CPO contact time; C/O molar ratio
in the CPO reactant
mixture; S/C molar ratio in the CPO reactant mixture; etc.) can be adjusted
(e.g., modified) in order for the
CPO effluent temperature to match (e.g., be the same with, coincide with) the
target CPO effluent
temperature. The CPO reactor 10 can be operated under any suitable operational
parameters as described
herein that can provide for a hydrogen-lean syngas as described herein with a
142/C0 molar ratio in a range
of from about 0.8 to 1.6, from about 0.8 to about 1.2, from about 0.9 to about
1.1, or equal to about 1.
[0048] The CPO reactor 10 can be characterized by a CPO reactant mixture
temperature of from about
25 C to about 600 C, alternatively from about 25 C to about 500 C,
alternatively from about 25 C to
about 400 C, alternatively from about 50 C to about 400 C, alternatively
from about 100 C to about
9
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
400 C, or alternatively from about 100 C to about 500 C. In embodiments,
the CPO reactor 10 can be
characterized by a CPO reactor temperature of less than 1200, 1100, or 1000 C.
[0049] The CPO reactor 10 can be characterized by a CPO effluent temperature
(e.g., target CPO
effluent 15 temperature) of greater than or equal to about 300 C, greater
than or equal to about 600 C,
alternatively greater than or equal to about 700 C, alternatively greater
than or equal to about 750 C,
alternatively greater than or equal to about 800 C, alternatively greater
than or equal to about 850 C,
alternatively from about 300 C to about 1,600 C, alternatively from about
600 C to about 1,400 C,
alternatively from about 600 C to about 1,300 C, alternatively from about
700 C to about 1,200 C,
alternatively from about 750 C to about 1,150 C, alternatively from about
800 C to about 1,125 C, or
alternatively from about 850 C to about 1,100 C.
[0050] In embodiments, the CPO reactor 10 can be characterized by any suitable
reactor temperature
and/or catalyst bed temperature. For example, the CPO reactor 10 can be
characterized by a reactor
temperature and/or catalyst bed temperature of greater than or equal to about
300 C, alternatively greater
than or equal to about 600 C, alternatively greater than or equal to about
700 C, alternatively greater than
or equal to about 750 C, alternatively greater than or equal to about 800 C,
alternatively greater than or
equal to about 850 C, alternatively from about 300 C to about 1,600 Cõ
alternatively from about 600 C
to about 1,400 C, alternatively from about 600 C to about 1,300 C,
alternatively from about 700 C to
about 1,200 C, alternatively from about 750 C to about 1,150 C,
alternatively from about 800 C to about
1,125 C, or alternatively from about 850 C to about 1,100 C.
[0051] The CPO reactor 10 can be operated under any suitable temperature
profile that can provide for a
hydrogen-lean syngas as described herein. The CPO reactor 10 can be operated
under adiabatic conditions,
non-adiabatic conditions, isothermal conditions, near-isothermal conditions,
autothermal conditions, etc.
For purposes of the disclosure herein, the term "non-adiabatic conditions"
refers to process conditions
wherein a reactor is subjected to external heat exchange or transfer (e.g.,
the reactor is heated; or the reactor
is cooled), which can be direct heat exchange and/or indirect heat exchange.
As will be appreciated by one
of skill in the art, and with the help of this disclosure, the terms "direct
heat exchange" and "indirect heat
exchange" are known to one of skill in the art. By contrast, the term
"adiabatic conditions" refers to process
conditions wherein a reactor is not subjected to external heat exchange (e.g.,
the reactor is not heated; or the
reactor is not cooled). Generally, external heat exchange implies an external
heat exchange system (e.g., a
cooling system; a heating system) that requires energy input and/or output.
External heat transfer can also
result from heat loss from the catalyst bed (or reactor) due to radiation,
conduction or convection. For
example, this heat exchange from the catalyst bed can be to the external
environment or to the reactor zones
before and after the catalyst bed.
[0052] For purposes of the disclosure herein, the term "isothermal conditions"
refers to process
conditions (e.g., CPO operational parameters) that allow for a substantially
constant temperature of the
reactor and/or catalyst bed (e.g., isothermal temperature) that can be defined
as a temperature that varies by
less than about + 10 C, alternatively less than about + 9 C, alternatively
less than about + 8 C,
alternatively less than about + 7 C, alternatively less than about 6 C,
alternatively less than about + 5 C,
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
alternatively less than about 4 C, alternatively less than about 3 C,
alternatively less than about 2 C,
or alternatively less than about + 1 C across the reactor and/or catalyst
bed, respectively. Further, for
purposes of the disclosure herein, the term "isothermal conditions" comprise a
temperature variation of less
than about + 10 C across the reactor and/or catalyst bed. In embodiments, the
CPO reactor 10 can be
operated under any suitable operational parameters that can provide for
isothermal conditions.
[0053] For purposes of the disclosure herein, the term "near-isothermal
conditions" refers to process
conditions (e.g., CPO operational parameters) that allow for a fairly constant
temperature of the reactor
and/or catalyst bed (e.g., near-isothermal temperature), which can be defined
as a temperature that varies by
less than about + 100 C, alternatively less than about + 90 C, alternatively
less than about + 80 C,
alternatively less than about + 70 C, alternatively less than about + 60 C,
alternatively less than about +
50 C, alternatively less than about + 40 C, alternatively less than about +
30 C, alternatively less than
about + 20 C, alternatively less than about + 10 C, alternatively less than
about + 9 C, alternatively less
than about + 8 C, alternatively less than about + 7 C, alternatively less
than about 6 C, alternatively less
than about + 5 C, alternatively less than about + 4 C, alternatively less
than about + 3 C, alternatively less
than about + 2 C, or alternatively less than about + 1 C across the reactor
and/or catalyst bed, respectively.
In some embodiments, near-isothermal conditions allow for a temperature
variation of less than about +
50 C, alternatively less than about + 25 C, or alternatively less than about
+ 10 C across the reactor and/or
catalyst bed. Further, for purposes of the disclosure herein, the term "near-
isothermal conditions" is
understood to include "isothermal" conditions.
[0054] Furthermore, for purposes of the disclosure herein, the term "near-
isothermal conditions" refers to
process conditions that comprise a temperature variation of less than about +
100 C across the reactor
and/or catalyst bed. In embodiments, a process as disclosed herein can
comprise conducting the CPO
reaction under near-isothermal conditions to produce the hydrogen-lean syngas,
wherein the near-
isothermal conditions comprise a temperature variation of less than about +
100 C across the reactor and/or
catalyst bed. In embodiments, the CPO reactor 10 can be operated under any
suitable operational
parameters that can provide for near-isothermal conditions.
[0055] Near-isothermal conditions can be provided by a variety of process and
catalyst variables, such as
temperature (e.g., heat exchange or heat transfer), pressure, gas flow rates,
reactor configuration, catalyst
bed configuration, catalyst bed composition, reactor cross sectional area,
feed gas staging, feed gas
injection, feed gas composition, and the like, or combinations thereof.
Generally, and without wishing to be
limited by theory, the terms "heat transfer" or "heat exchange" refer to
thermal energy being exchanged or
transferred between two systems (e.g., two reactors, such as a CPO reactor and
a cracking reactor), and the
terms "heat transfer" or "heat exchange" are used interchangeably for purposes
of the disclosure herein.
[0056] In some embodiments, achieving a target CPO effluent temperature and/or
near-isothermal
conditions can be provided by heat exchange or heat transfer. The heat
exchange can comprise heating the
reactor; or cooling the reactor. In embodiments, achieving a target CPO
effluent temperature and/or near-
isothermal conditions can be provided by cooling the reactor. In another
embodiment, achieving a target
CPO effluent temperature and/or near-isothermal conditions can be provided by
heating the reactor.
11
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
[0057] In some embodiments, achieving a target CPO effluent temperature and/or
near-isothermal
conditions can be provided by direct heat exchange and/or indirect heat
exchange. As will be appreciated
by one of skill in the art, and with the help of this disclosure, the terms
"direct heat exchange" and "indirect
heat exchange" are known to one of skill in the art.
[0058] The heat exchange can comprise external heat exchange, external coolant
fluid cooling, reactive
cooling, liquid nitrogen cooling, cryogenic cooling, electric heating,
electric arc heating, microwave
heating, radiant heating, natural gas combustion, solar heating, infrared
heating, use of a diluent in the CPO
reactant mixture, and the like, or combinations thereof. For example, reactive
cooling can be effected by
carrying out an endothermic reaction in a cooling coil/jacket associated with
(e.g., located in) the reactor.
[0059] In some embodiments, achieving a target CPO effluent temperature and/or
near-isothermal
conditions can be provided by removal of process heat from the CPO reactor. In
other embodiments,
achieving a target CPO effluent temperature and/or near-isothermal conditions
can be provided by
supplying heat to the CPO reactor. As will be appreciated by one of skill in
the art, and with the help of this
disclosure, a CPO reactor may need to undergo both heating and cooling in
order to achieve a target CPO
effluent temperature and/or near-isothermal conditions.
[0060] In embodiments, the heat exchange or heat transfer can comprise
introducing a cooling agent,
such as a diluent, into the reactor (e.g., CPO reactor 10), to decrease the
reactor temperature and/or the
catalyst bed temperature, while increasing a temperature of the cooling agent
and/or changing the phase of
the cooling agent. The cooling agent can be reactive or non-reactive. The
cooling agent can be in liquid
state and/or in vapor state. As will be appreciated by one of skill in the
art, and with the help of this
disclosure, the cooling agent can act as a flammability retardant; for example
by reducing the temperature
inside the reactor, by changing the gas mixture composition, by reducing the
combustion of hydrocarbons
to carbon dioxide; etc.
[0061] In some embodiments, the CPO reactant mixture 5 can further comprise a
diluent, wherein the
diluent contributes to achieving a target CPO effluent temperature and/or near-
isothermal conditions via
heat exchange, as disclosed herein. The diluent can comprise water, steam,
inert gases (e.g., argon),
nitrogen, carbon dioxide, and the like, or combinations thereof. Generally,
the diluent is inert with respect
to the CPO reaction, e.g., the diluent does not participate in the CPO
reaction. However, and as will be
appreciated by one of skill in the art, and with the help of this disclosure,
some diluents (e.g., water, steam,
carbon dioxide, etc.) might undergo chemical reactions other than the CPO
reaction within the reactor, and
can change the composition of the resulting syngas, as will be described in
more detail later herein; while
other diluents (e.g., nitrogen (N2), argon (Ar)) might not participate in
reactions that change the composition
of the resulting syngas. As will be appreciated by one of skill in the art,
and with the help of this disclosure,
the diluent can be used to vary the composition of the resulting syngas. The
diluent can be present in the
CPO reactant mixture 5 in any suitable amount.
[0062] The CPO reactor 10 can be characterized by a CPO pressure (e.g.,
reactor pressure measured at
the reactor exit or outlet) of greater than or equal to about 1 barg,
alternatively greater than or equal to about
barg, alternatively greater than or equal to about 20 barg, alternatively
greater than or equal to about 25
12
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
barg, alternatively greater than or equal to about 30 barg, alternatively
greater than or equal to about 35
barg, alternatively greater than or equal to about 40 barg, alternatively
greater than or equal to about 50
barg, alternatively less than about 30 barg, alternatively less than about 25
barg, alternatively less than
about 20 barg, alternatively less than about 10 barg, alternatively from about
1 barg to about 90 barg,
alternatively from about 1 barg to about 70 barg, alternatively from about 1
barg to about 40 barg,
alternatively from about 1 barg to about 30 barg, alternatively from about 1
barg to about 25 barg,
alternatively from about 1 barg to about 20 barg, alternatively from about 1
barg to about 10 barg,
alternatively from about 20 barg to about 90 barg, alternatively from about 25
barg to about 85 barg, or
alternatively from about 20 barg to about 60 barg.
[0063] The CPO reactor 10 can be characterized by a CPO contact time of from
about 0.001
milliseconds (ms) to about 5 seconds (s), alternatively from about 0.001 ms to
about 1 s, alternatively from
about 0.001 ms to about 100 ms, alternatively from about 0.001 ms to about 10
ms, alternatively from about
0.001 ms to about 5 ms, or alternatively from about 0.01 ms to about 1.2 ms.
Generally, the contact time of
a reactor comprising a catalyst refers to the average amount of time that a
compound (e.g., a molecule of
that compound) spends in contact with the catalyst (e.g., within the catalyst
bed), e.g., the average amount
of time that it takes for a compound (e.g., a molecule of that compound) to
travel through the catalyst bed.
In some embodiments, the CPO reactor 10 can be characterized by a contact time
of from about 0.001 ms to
about 5 ms, or alternatively from about 0.01 ms to about 1.2 ms.
[0064] All of the CPO operational parameters disclosed herein are applicable
throughout all of the
embodiments disclosed herein, unless otherwise specified. As will be
appreciated by one of skill in the art,
and with the help of this disclosure, each CPO operational parameter can be
adjusted to provide for a
hydrogen-lean syngas as described herein. For example, the CPO operational
parameters can be adjusted to
provide for an increased H2 content of the syngas, so long as the 142/C0 molar
ratio remains in the desired
range (e.g., from about 0.8 to about 1.6). As another example, the CPO
operational parameters can be
adjusted to provide for a decreased CO2 content of the syngas in the CPO
reactor effluent 15. As yet
another example, the CPO operational parameters can be adjusted to provide for
a decreased unreacted
hydrocarbons (e.g., unreacted Cl-I4) content of the syngas in the CPO reactor
effluent 15.
[0065] In embodiments, the CPO reactor 10 is characterized by at least one CPO
operational parameter
selected from the group consisting of a CPO reactant temperature of from about
100 C to about 500 C;
a CPO pressure of from about 20 barg to about 80 barg; a CPO contact time of
from about 0.001
milliseconds (ms) to about 5 seconds (s); a carbon to oxygen (C/O) molar ratio
in the CPO reactant
mixture of from about 0.5:1 to about 3:1, wherein the C/O molar ratio refers
to the total moles of carbon
(C) in the hydrocarbons in the reactant mixture divided by the total moles of
oxygen (02) in the reactant
mixture; a steam to carbon (S/C) molar ratio in the CPO reactant mixture of
less than about 0.6:1,
wherein the S/C molar ratio refers to the total moles of water (H20) in the
reactant mixture divided by the
total moles of carbon (C) in the hydrocarbons in the reactant mixture; and
combinations thereof.
[0066] In embodiments, the CPO reactor 10 is characterized by at least one CPO
operational parameter
selected from the group consisting of a CPO reactant mixture temperature of
from about 100 C to about
13
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
500 C; a CPO pressure of from about 25 barg to about 80 barg; a CPO contact
time of from about 0.001
milliseconds (ms) to about 5 seconds (s); a carbon to oxygen (C/O) molar ratio
in the CPO reactant
mixture of from about 0.5:1 to about 2:1, wherein the C/O molar ratio refers
to the total moles of carbon
(C) in the hydrocarbons in the reactant mixture divided by the total moles of
oxygen (02) in the reactant
mixture; a steam to carbon (S/C) molar ratio in the CPO reactant mixture of
less than about 0.25:1,
wherein the S/C molar ratio refers to the total moles of water (1420) in the
reactant mixture divided by the
total moles of carbon (C) in the hydrocarbons in the reactant mixture; and
combinations thereof.
[0067] The CPO reaction is an exothermic reaction (e.g., heterogeneous
catalytic reaction; exothermic
heterogeneous catalytic reaction) that is generally conducted in the presence
of a CPO catalyst comprising a
catalytically active metal, i.e., a metal active for catalyzing the CPO
reaction. The catalytically active metal
can comprise a noble metal (e.g., Pt, Rh, Ir, Pd, Ru, Ag, and the like, or
combinations thereof); a non-noble
metal (e.g., Ni, Co, V, Mo, P, Fe, Cu, and the like, or combinations thereof);
rare earth elements (e.g., La,
Ce, Nd, Eu, and the like, or combinations thereof); oxides thereof; and the
like; or combinations thereof.
Generally, a noble metal is a metal that resists corrosion and oxidation in a
water-containing environment.
As will be appreciated by one of skill in the art, and with the help of this
disclosure, the components of the
CPO catalyst (e.g., metals such as noble metals, non-noble metals, rare earth
elements) can be either phase
segregated or combined within the same phase.
[0068] In embodiments, the CPO catalysts suitable for use in the present
disclosure can be supported
catalysts and/or unsupported catalysts. In some embodiments, the supported
catalysts can comprise a
support, wherein the support can be catalytically active (e.g., the support
can catalyze a CPO reaction). For
example, the catalytically active support can comprise a metal gauze or wire
mesh (e.g., Pt gauze or wire
mesh); a catalytically active metal monolithic catalyst; etc. In other
embodiments, the supported catalysts
can comprise a support, wherein the support can be catalytically inactive
(e.g., the support cannot catalyze a
CPO reaction), such as Si02; silicon carbide (SiC); alumina; a catalytically
inactive monolithic support; etc.
In yet other embodiments, the supported catalysts can comprise a catalytically
active support and a
catalytically inactive support.
[0069] In some embodiments, a CPO catalyst can be wash coated onto a support,
wherein the support
can be catalytically active or inactive, and wherein the support can be a
monolith, a foam, an irregular
catalyst particle, etc.
[0070] In some embodiments, the CPO catalyst can be a monolith, a foam, a
powder, a particle, etc.
Nonlimiting examples of CPO catalyst particle shapes suitable for use in the
present disclosure include
cylindrical, discoidal, spherical, tabular, ellipsoidal, equant, irregular,
cubic, acicular, and the like, or
combinations thereof.
[0071] In some embodiments, the support comprises an inorganic oxide, alpha,
beta or theta alumina
(A1203), activated A1203, silicon dioxide (Si02), titanium dioxide (Ti02),
magnesium oxide (Mg0),
zirconium oxide (Zr02), lanthanum (III) oxide (La203), yttrium (III) oxide
(Y203), cerium (IV) oxide
(Ce02), zeolites, ZSM-5, perovskite oxides, hydrotalcite oxides, and the like,
or combinations thereof.
14
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
[0072] Without limitation, CPO processes, CPO reactors, CPO catalysts, and CPO
catalyst bed
configurations suitable for use in the present disclosure are described in
more detail in U.S. Provisional
Patent Application No. 62/522,910 filed June 21, 2017 (International
Application No. PCT/IB2018/054475
filed June 18, 2018) and entitled "Improved Reactor Designs for Heterogeneous
Catalytic Reactions;" and
U.S. Provisional Patent Application No. 62/521,831 filed June 19, 2017
(International Application No.
PCT/IB2018/054470 filed June 18, 2018) and entitled "An Improved Process for
Syngas Production for
Petrochemical Applications;" each of which is hereby incorporated herein by
reference in its entirety for
purposes not contrary to this disclosure.
[0073] In embodiments, the CPO catalyst can be characterized by a catalyst
productivity variation within
about + 20%, alternatively within about + 17.5%, alternatively within about +
15%, alternatively within
about + 12.5%, alternatively within about + 10%, alternatively within about +
7.5%, alternatively within
about + 5%, alternatively within about + 2.5%, or alternatively within about +
1% of a target catalyst
productivity over a time period of equal to or greater than about 500 hours
(h), alternatively equal to or
greater than about 1,000 h, alternatively equal to or greater than about 2,500
h, alternatively equal to or
greater than about 5,000 h, alternatively equal to or greater than about 7,500
h, or alternatively equal to or
greater than about 10,000 h; wherein catalyst productivity is defined as the
amount of syngas in CPO
reactor effluent 15 recovered from the CPO reactor 10 divided by the amount of
hydrocarbons introduced to
the CPO reactor 10 in CPO reactant mixture 5. As will be appreciated by one of
skill in the art, and with
the help of this disclosure, and without wishing to be limited by theory,
catalyst productivity is a
quantitative measure of catalyst activity, wherein the catalyst activity
refers to the ability of a catalyst (e.g.,
CPO catalyst) to increase the rate of a chemical reaction (e.g., CPO reaction)
under a given set of reaction
conditions (e.g., CPO operational parameters). For purposes of the disclosure
herein, a CPO catalyst having
a productivity variation greater than about + 20% can be referred to as a
"spent CPO catalyst" (as opposed
to an active CPO catalyst). As used herein, the target catalyst productivity
is associated with an active CPO
catalyst (e.g., fresh CPO catalyst and/or regenerated CPO catalyst). For
purposes of the disclosure herein,
the term "fresh CPO catalyst" refers to a CPO catalyst that has not been used
in a CPO process. As will be
appreciated by one of skill in the art, and with the help of this disclosure,
an active CPO catalyst displays
optimum (e.g., maximum) catalyst activity with respect to a chemical reaction
(e.g., CPO reaction) under a
given set of reaction conditions (e.g., CPO operational parameters). Further,
and as will be appreciated by
one of skill in the art, and with the help of this disclosure, the target
catalyst productivity is the maximum
catalyst productivity of an active CPO catalyst (e.g., fresh CPO catalyst
and/or regenerated CPO catalyst)
under a given set of reaction conditions (e.g., CPO operational parameters).
Furthermore, and as will be
appreciated by one of skill in the art, and with the help of this disclosure,
the terms "catalyst productivity"
and "target catalyst productivity" are used in the context of steady-state
operation of the CPO reactor (e.g.,
CPO reactor 10).
[0074] As will be appreciated by one of skill in the art, and with the help of
this disclosure, catalyst
activity (e.g., CPO catalyst activity) can vary (e.g., decay, decrease) over
time, for a variety of reasons, such
as poisoning (e.g., feed contaminants), fouling (e.g., coking by carbon
produced by
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
cracking/condensation/decomposition reactions of hydrocarbon reactants,
intermediates, and/or products),
thermal degradation (e.g., collapse of support structure, solid-state
reactions, attrition), active component
leaching, migration of active components within and/or outside catalyst
particles, side reactions,
attrition/crushing, and the like, or combinations thereof. Decay in catalyst
activity leads to spent catalysts
(e.g., spent CPO catalysts). In embodiments, spent catalysts can be
regenerated and returned to a
production process, as will be described in more detail later herein.
[0075] In embodiments, a portion of the hydrocarbons (e.g., methane) in the
CPO reactant mixture 5 can
undergo a thermal decomposition reaction to carbon (C) and H2, for example as
represented by eqn. (2):
Cl-I4 ¨> C + 2 112 (2)
[0076] The decomposition reaction of hydrocarbons, such as methane, is
facilitated by elevated
temperatures, and increases the hydrogen content in the syngas in CPO reactor
effluent 15. However, the
carbon produced by the decomposition reaction of hydrocarbons (e.g., a
decomposition reaction as
represented by equation (2)) can lead to coking of the CPO catalyst via carbon
deposition onto the CPO
catalyst, thereby producing a spent CPO catalyst. As will be appreciated by
one of skill in the art, and with
the help of this disclosure, and without wishing to be limited by theory,
while the percentage of
hydrocarbons in the CPO reactant mixture 5 that undergoes a decomposition
reaction (e.g., a decomposition
reaction as represented by equation (2)) increases with increasing the C/O
molar ratio in the CPO reactant
mixture 5, a portion of hydrocarbons can undergo a decomposition reaction to C
and H2 even at relatively
low C/O molar ratios in the CPO reactant mixture 5 (e.g., a C/O molar ratio in
the CPO reactant mixture 5
of less than about 1:1). Further, and as will be appreciated by one of skill
in the art, and with the help of
this disclosure, the quality of the hydrocarbon feed to the CPO reactor 10 can
influence coking. For
example, higher hydrocarbons (e.g., hydrocarbons having equal to or greater
than 2 C atoms, C2+) can
produce more coke than methane, owing to having a higher carbon content than
methane.
[0077] In an aspect, the CPO reactant mixture 5 can further comprise a
diluent, such as water and/or
steam, and CO2. The CPO reactor 10 can be operated under any suitable
operational conditions (e.g., CPO
operational parameters) that can provide for a syngas with a desired
composition (e.g., a desired H2/C0
molar ratio; a desired CO2 content; etc.); for example, the CPO reactor 10 can
be operated with introducing
water and/or steam, and CO2 to the CPO reactor 10.
[0078] When carbon is present in the reactor (e.g., coke; C produced as a
result of a decomposition
reaction as represented by equation (2)), water and/or steam diluent can react
with the carbon and
generate additional CO and H2, for example as represented by equation (3):
C + H20 ,=s CO + H2 (3)
As will be appreciated by one of skill in the art, and with the help of this
disclosure, the presence of water
and/or steam in the CPO reactor 10 can decrease the amount of coke in the CPO
reactor 10 (e.g., the
amount of coke deposited on the CPO catalyst, the amount of spent CPO catalyst
present in the CPO reactor
10), thereby providing for maintaining the catalyst productivity.
16
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
[0079] Further, and as will be appreciated by one of skill in the art, and
with the help of this disclosure,
water and/or steam can be used to vary the composition of the resulting syngas
in CPO reactor effluent 15.
Steam can react with methane, for example as represented by equation (4):
CH4 + H20 N=s CO + 3 H2 (4)
[0080] In an aspect, a diluent comprising water and/or steam can increase a
hydrogen content of the
resulting syngas in CPO reactor effluent 15. For example, in aspects where the
CPO reactant mixture 5
comprises water and/or steam diluent, the resulting syngas in CPO reactor
effluent 15 can be
characterized by a hydrogen to carbon monoxide molar ratio that is increased
when compared to a
hydrogen to carbon monoxide molar ratio of a syngas produced by an otherwise
similar process
conducted with a reactant mixture comprising hydrocarbons and oxygen without
the water and/or steam
diluent. Without wishing to be limited by theory, the reforming reaction
(e.g., as represented by equation
(4)) is an endothermic reaction. The reforming reaction as represented by
equation (4) can remove a
portion of the process heat (e.g., heat produced by the exothermic CPO
reaction, for example as
represented by equation (1)).
[0081] In the presence of water and/or steam in the CPO reactor 10, carbon
monoxide can react with
the water and/or steam to form carbon dioxide and hydrogen via a water-gas
shift (WGS) reaction, for
example as represented by equation (5):
CO + H20 N=" CO2 + H2 (5)
While the WGS reaction can increase the 142/C0 molar ratio of the syngas
produced by the CPO reactor
10, it also produces CO2.
[0082] Injection of steam and/or water can help maintain CPO catalyst
activity. In embodiments, the
CPO reactant mixture 5 can be characterized by a steam to carbon (S/C) and/or
a steam to Cl-I4 (S/Cl-I4)
molar ratio of less than or equal to 1.0, 0.5, 0.4, 0.3, 0.2, or in a range of
from about 0.1 to 01.0, 0.2 to 0.6,
or 0.2 to 0.5. In embodiments, the CPO reactor 10 can be operated at an S/C
molar ratio ) and/or a steam to
Cl-I4 (S/Cl-I4) molar ratio in the CPO reactant mixture 5 of less than about
0.6:1, alternatively less than about
0.5:1, alternatively less than about 0.4:1, alternatively less than about
0.3:1, alternatively less than about
0.2:1, alternatively less than about 0.1:1, alternatively from about 0.01:1 to
less than about 0.6:1,
alternatively from about 0.05:1 to about 0.6:1, alternatively from about 0.1:1
to about 0.5:1, alternatively
from about 0.15:1 to about 0.6:1, or alternatively from about 0.2:1 to about
0.6:1. As will be appreciated by
one of skill in the art, and with the help of this disclosure, the steam that
is introduced to the CPO reactor
for use as a diluent in a CPO reaction as disclosed herein is present in
significantly smaller amounts than the
amounts of steam utilized in steam reforming (e.g., SMR) processes, and as
such, a process for producing
syngas as disclosed herein can yield a (e.g., hydrogen-lean) syngas with lower
amounts of hydrogen when
compared to the amounts of hydrogen in a syngas produced by steam reforming.
[0083] The S/C molar ratio in the CPO reactant mixture 10 can be adjusted
based on the desired CPO
effluent temperature (e.g., target CPO effluent temperature) in order to
adjust the H2 content of the
produced syngas (e.g., syngas 15). As will be appreciated by one of skill in
the art, and with the help of
this disclosure, the reaction (4) that consumes steam in the CPO reactor may
be less preferable over the
17
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
water-gas shift (WGS) reaction (5) in the CPO reactor 10, as reaction (4)
allows for increasing the H2
content of the produced syngas (e.g., syngas 15), as well as the M ratio of
the produced syngas (e.g.,
syngas 15), wherein the M ratio is a molar ratio defined as (TI2-
0O2)/(CO+CO2). Further, and as will be
appreciated by one of skill in the art, and with the help of this disclosure,
reaction (5) converts water and
CO to both H2 and CO2.
[0084] Without wishing to be limited by theory, the presence of water and/or
steam in the CPO reactor
changes the flammability of the CPO reactant mixture 10, thereby providing for
a wider practical
range of C/O molar ratios in the CPO reactant mixture 10. Further, and without
wishing to be limited by
theory, the presence of water and/or steam in the CPO reactor 10 allows for
the use of lower C/O molar
ratios in the CPO reactant mixture 10. Furthermore, and without wishing to be
limited by theory, the
presence of water and/or steam in the CPO reactor 10 allows for operating the
CPO reactor 10 at
relatively high pressures.
[0085] As will be appreciated by one of skill in the art, and with the help of
this disclosure, the
introduction of water and/or steam in the CPO reactor 10 can lead to
increasing the amount of unreacted
hydrocarbons in the syngas 15. Further, as will be appreciated by one of skill
in the art, and with the help
of this disclosure, some downstream chemical synthesis processes tolerate
limited amounts of unreacted
hydrocarbons in the syngas.
[0086] In some aspects, the syngas 15 can comprise less than about 7.5 mol%,
alternatively less than
about 5 mol%, or alternatively less than about 2.5 mol% hydrocarbons (e.g.,
unreacted hydrocarbons,
unreacted CH4). In such aspects, the syngas 15 can be produced in a CPO
process that employs water
and/or steam.
[0087] In embodiments, CO2 is introduced into the CPO reactor 10 (e.g., via
line 7A). Since oxygen is
present in the CPO reactant mixture 5, the carbon present in the reactor
(e.g., coke; C produced as a result
of a decomposition reaction as represented by equation (2)) can also react
with oxygen, for example as
represented by equation (6):
C + 02 ¨> CO2 (6)
[0088] When carbon is present in the reactor (e.g., coke; C produced as a
result of a decomposition
reaction as represented by equation (2)), CO2 (e.g., introduced to the CPO
reactor 10 as part of the CPO
reactant mixture 5 and/or produced by the reaction represented by equation
(6)) can react with the carbon,
for example as represented by equation (7):
C + CO2 ,=s 2 CO (7)
thereby decreasing the amount of CO2 and increasing the amount of CO in the
resulting syngas in the CPO
reactor effluent 15. The use of reactant mixtures 5 comprising higher
hydrocarbons (e.g., C2+) can lead to
the formation of a greater amount of coke, and thus lead to an enrichment of
CO and a reduced H2/C0
molar ratio in the syngas in CPO reactor effluent 15. As will be appreciated
by one of skill in the art, and
with the help of this disclosure, the presence of CO2 in the CPO reactor 10
can decrease the amount of coke
in the CPO reactor 10 (e.g., the amount of coke deposited on the CPO catalyst,
the amount of spent CPO
catalyst present in the CPO reactor 10), thereby providing for maintaining the
catalyst productivity.
18
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
Injection of CO2 also provides for an enhancement in carbon efficiency,
because the carbon in the CO2 is
converted to additional CO. As a result, more CO will be produced per MMBTU of
reactant feed (e.g.,
natural gas) according to embodiments of this disclosure. This additional CO
can contribute to an increase
in chemical product throughput (e.g., from downstream synthesis 30) at the
same flowrate of reactant feed
(e.g., natural gas).
[0089] Furthermore, CO2 can react with methane in a dry reforming reaction,
for example as represented
by equation (8):
CH4 + CO2 ,=s 2 CO + 2 H2 (8)
thereby decreasing the amount of CO2 in the resulting syngas in CPO reactor
effluent 15. Without wishing
to be limited by theory, the dry reforming reaction (e.g., as represented by
eqn. (8)) is an endothermic
reaction (e.g., highly endothermic reaction). The dry reforming reaction can
remove a portion of the
process heat (e.g., heat produced by the exothermic CPO reaction, for example
as represented by eqn. (1)).
[0090] In
embodiments, a diluent comprising carbon dioxide can increase a carbon
monoxide content
of the resulting syngas in the CPO reactor effluent 15. For example, in
embodiments where the CPO
reactant mixture 5 comprises carbon dioxide, the syngas in CPO reactor
effluent 15 can be characterized by
a hydrogen to carbon monoxide molar ratio that is decreased when compared to a
hydrogen to carbon
monoxide molar ratio of a syngas produced by an otherwise similar process
conducted with a reactant
mixture comprising hydrocarbons and oxygen without the carbon dioxide diluent.
Without wishing to be
limited by theory, carbon dioxide can react with coke inside the CPO reactor
10 and generate additional
CO, for example as represented by equation (7). Further, and without wishing
to be limited by theory,
carbon dioxide can participate in a dry reforming of methane reaction, thereby
generating additional CO
and H2, for example as represented by equation (8). Dry reforming of methane
is generally accompanied by
a reaction between carbon dioxide and hydrogen which results in the formation
of additional CO and water.
[0091] In embodiments, the CPO reactant mixture 5 can comprise carbon dioxide
in an amount effective
to provide for less than about 7 mol%, alternatively less than about 6 mol%,
alternatively less than about 5
mol%, alternatively from about 0.1 mol% to about 7 mol%, alternatively from
about 0.25 mol% to about 6
mol%, or alternatively from about 0.5 mol% to about 5 mol% carbon dioxide in
the syngas in CPO reactor
effluent 15, based on the total mol% of the syngas. The carbon dioxide of the
CPO reactant mixture 5 can
be CO2 from natural gas sources, wherein the CO2 is introduced to the CPO
reactor 10 with the
hydrocarbons; and/or additional or supplemental CO2, for example CO2 recovered
as a process stream and
recycled to the CPO reactor 10 (e.g., CO2 stream 7A).
[0092] In embodiments, the conversion of hydrocarbons in the CPO reactor 10 is
greater than the
conversion of hydrocarbons in a CPO reactor in an otherwise similar process
that produces a hydrogen-
lean syngas from hydrocarbons comprising a reduced amount of higher
hydrocarbons (e.g., C2+
hydrocarbons). For example, in embodiments, the conversion of hydrocarbons in
the CPO reactor 10 of
a reactant feed mixture 5 comprising greater than or equal to about 5, 4, or 3
mol% C2+ alkanes is greater
than the conversion of hydrocarbons in a CPO reactor in an otherwise similar
process that produces a
19
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
hydrogen-lean syngas from a reactant mixture 5 comprising less than about 5,
4, or 3 mol% C2+ alkanes,
respectively.
[0093] In embodiments, CPO reactor effluent 15 comprises the hydrogen-lean
syngas, and no further
adjustment of the molar ratio of 142/C0 is provided prior to downstream
synthesis reactor of downstream
synthesis apparatus 30. In embodiments, a process as disclosed herein can
further comprise: (i) recovering
a CPO reactor effluent 15 from the CPO reactor 10, wherein the CPO reactor
effluent 15 comprises
hydrogen, carbon monoxide, carbon dioxide, water, and unreacted hydrocarbons,
and wherein the CPO
reactor effluent 15 is characterized by a 142/C0 molar ratio of greater than
about 1.6õ1.5, 1.4, 1.3, or 1.2;
and (ii) feeding at least a portion of the CPO reactor effluent 15 to a
reverse water-gas shift (r-WGS) reactor
20, wherein a portion of the hydrogen of the CPO reactor effluent 15 reacts
with carbon dioxide via a r-
WGS reaction to produce water and carbon monoxide, to produce the hydrogen-
lean syngas, which can be
removed from the r-WGS reactor 20 via r-WGS reactor effluent 25.
[0094] The r-WGS reaction is depicted in Equation (9):
CO2 + 1-12 <--> CO + H20 (9)
[0095] In embodiments, a process as disclosed herein can further comprise
introducing additional carbon
dioxide 7B to the r-WGS reactor 20 to drive the r-WGS reaction toward the
production of carbon monoxide
(i.e., to drive the WGS reaction of Equation 5 toward the production of carbon
monoxide).
[0096] In embodiments, a process as disclosed herein can further comprise: (a)
recovering a r-WGS
reactor effluent 25 from the r-WGS reactor, wherein the r-WGS reactor effluent
comprises hydrogen,
carbon monoxide, carbon dioxide, water, and unreacted hydrocarbons; and (b)
removing at least a portion
of the water from the r-WGS reactor effluent 25 to yield the hydrogen-lean
syngas, wherein the amount of
water in the r-WGS reactor effluent is greater than the amount of water in the
hydrogen-lean syngas.
[0097] In embodiments, a process as disclosed herein further comprises: (1)
contacting a portion of the
CPO reactor effluent 15 with at least a portion of the r-WGS reactor effluent
25 to produce a combined
effluent stream; and (2) removing at least a portion of the water from the
combined effluent stream to yield
the hydrogen-lean syngas, wherein the amount of water in the combined effluent
stream is greater than the
amount of water in the hydrogen-lean syngas.
[0098] In embodiments, a process as disclosed herein excludes a step of
introducing at least a portion
of the CPO reactor effluent 15 and/or at least a portion of the hydrogen-lean
syngas in r-WGS reactor
effluent 25 to a hydrogen recovery unit to decrease the amount of hydrogen in
the CPO reactor effluent
15 and/or the hydrogen-lean syngas in r-WGS reactor effluent 25, respectively.
[0099] In embodiments, the hydrogen-lean syngas (e.g., in the CPO reactor
effluent 15 and/or the r-
WGS reactor effluent 25) can have a CO2 content of less than about 10 mol%,
less than about 9 mol%,
less than about 8 mol%, less than about 7 mol%, alternatively less than about
6 mol%, alternatively less
than about 5 mol%, alternatively less than about 4 mol%, alternatively less
than about 3 mol%,
alternatively less than about 2 mol%, alternatively less than about 1 mol%,
alternatively greater than
about 0.1 mol%, alternatively greater than about 0.25 mol%, alternatively
greater than about 0.5 mol%,
alternatively from about 0.1 mol% to about 7 mol%, alternatively from about
0.25 mol% to about 6
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
mol%, or alternatively from about 0.5 mol% to about 5 mol%. As noted
hereinabove, the CO2
concentration in the hydrogen-lean syngas (e.g., in CPO reactor effluent 15
and/or r-WGS reactor
effluent 25) can be controlled via CO2 injection (e.g., via CO2 7A and/or 7B,
respectively) and/or by
changing the operating conditions of CPO reactor 10. The amount of CO2 in the
hydrogen-lean syngas
can be adjusted depending on the downstream synthesis 30. For example, when
small amounts of CO2
are desirable in the hydrogen-lean syngas feed to downstream synthesis
apparatus 30 (e.g., for
downstream synthesis of DME, wherein small amounts of CO2 in the hydrogen-lean
syngas feed are
desirable to increase the production of methanol intermediate and thus enhance
DME synthesis), the CO2
amount in the hydrogen-lean synthesis gas can be adjusted as provided
hereinabove.
[00100] In embodiments, the CPO reactor effluent 15 and/or the r-WGS reactor
effluent 25 can be
subjected to processing, such as the recovery of unreacted hydrocarbons,
diluent, water, etc. In
embodiments, water can be condensed and separated from the CPO reactor
effluent 15 and/or the r-WGS
reactor effluent 25, e.g., in a condenser. As understood, such processing for
the removal of hydrocarbons,
diluent, water, etc. will not alter an I-12/CO molar ratio of the stream. In
embodiments, a process as
disclosed herein can further comprise (i) recovering at least a portion of the
unreacted hydrocarbons from
the CPO reactor effluent 15 and/or the r-WGS reactor effluent 25 to yield
recovered hydrocarbons, and (ii)
recycling at least a portion of the recovered hydrocarbons to the CPO reactor
10. As will be appreciated by
one of skill in the art, and with the help of this disclosure, although fairly
high conversions can be achieved
in CPO processes (e.g., conversions of greater than or equal to about 90%),
the unconverted hydrocarbons
could be recovered and recycled back to the CPO reactor 10.
[00101] In embodiments, a CPO reactor with CO2 injection as described
hereinabove is utilized to
produce the hydrogen-lean syngas for downstream chemical synthesis.
[00102] In embodiments, a process as disclosed herein further comprises using
at least a portion of the
hydrogen-lean syngas (e.g., in CPO reactor effluent 15 and/or r-WGS reactor
effluent 25) in a
downstream synthesis process comprising downstream synthesis apparatus 30.
[00103] The downstream synthesis process can be any process for which a
hydrogen-lean syngas is
utilized to produce at least one chemical product 35. For example, in
embodiments and without
limitation, in embodiments, the downstream process is selected from the group
consisting of acetic acid
synthesis process; dimethyl ether synthesis process; oxo-synthesis of
aliphatic aldehydes and/or alcohols;
and combinations thereof, and downstream synthesis apparatus 30 comprise
apparatus operable for the
synthesis of acetic acid 35A, the synthesis of dimethyl ether (DME) 35B, oxo-
synthesis of aliphatic
aldehydes 35C and/or alcohols 35D, or a combination thereof.
[00104] In embodiments, a process as disclosed herein does not comprise
altering the I-12/CO molar ratio
of the hydrogen-lean syngas (e.g., the CPO reactor effluent 15 and/or the r-
WGS reactor effluent 25)
between the CPO reactor 10 and a downstream synthesis reactor of the
downstream synthesis apparatus
30. Thus, in embodiments, a chemical synthesis system as disclosed herein does
not comprise apparatus
(e.g., a hydrogen removal unit, PSA) for altering the I-12/CO molar ratio of
the hydrogen-lean syngas
(e.g., the CPO reactor effluent 15 and/or the r-WGS reactor effluent 25)
between the CPO reactor 10 and
21
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
the downstream synthesis reactor of downstream synthesis apparatus 30. In
embodiments, a chemical
synthesis system as disclosed herein comprises a reduced size apparatus
(relative to conventional) for
altering the I-12/CO molar ratio of the hydrogen-lean syngas (e.g., the CPO
reactor effluent 15 and/or the
r-WGS reactor effluent 25) between the CPO reactor 10 and the downstream
synthesis reactor of
downstream synthesis apparatus 30.
[00105] In embodiments, a process as disclosed herein comprises no adjusting
of the I-12/CO molar ratio
of the hydrogen-lean syngas other than optionally subjecting the CPO reactor
effluent 15 to reverse water
gas shift prior to the utilizing the hydrogen-lean syngas in a downstream
chemical synthesis reactor of
downstream chemical synthesis apparatus 30. Thus, in embodiments, a chemical
synthesis system as
disclosed herein comprises no apparatus for adjusting the I-12/CO molar ratio
of the hydrogen-lean syngas
other than an optional reverse water gas shift apparatus prior to a downstream
synthesis reactor of the
downstream synthesis apparatus 30.
[00106] In embodiments, a process as disclosed herein does not comprise
removing a hydrogen stream
from the hydrogen-lean syngas (e.g., the CPO reactor effluent 15 and/or the r-
WGS reactor effluent 25)
prior to utilizing the hydrogen-lean syngas in downstream chemical synthesis.
Thus, in embodiments, a
chemical synthesis system as disclosed herein comprises no apparatus
configured to remove a hydrogen
stream from the hydrogen-lean syngas between the CPO reactor and a downstream
synthesis reactor of
the downstream synthesis apparatus 30. In embodiments, a chemical synthesis
system as disclosed
herein comprises a reduced size apparatus (relative to conventional)
configured to remove a hydrogen
stream from the hydrogen-lean syngas between the CPO reactor and a downstream
synthesis reactor of
the downstream synthesis apparatus 30.
[00107] In embodiments, the CPO reactor 10 can produce the hydrogen lean
syngas at high pressures
(e.g., greater than or equal to about 20, 25, 30, 35, 40, 45, 50 bar) that are
required for downstream
chemical (e.g., acetic acid, DME) synthesis. Accordingly, the herein disclosed
system and method for
producing hydrogen-lean syngas via CPO can, in embodiments, further reduce
energy requirements for
production of the chemical produced in downstream synthesis 30.
[00108] In embodiments, a process as disclosed herein can advantageously
display improvements in one
or more process characteristics when compared to conventional processes.
[00109] As will be appreciated by one of skill in the art, and with the help
of this disclosure, since the
CPO reaction is exothermic, very little heat supply in the form of fuel
combustion is needed (e.g., for pre-
heating reactants in the reaction mixture 5 that is supplied to the CPO syngas
generation section), when
compared to conventional steam reforming. As such, the process for chemical
synthesis utilizing CPO
hydrogen-lean syngas as disclosed herein can advantageously generate less CO2
through fuel burning, when
compared to steam reforming.
[00110] The use of CPO reactant mixtures comprising higher hydrocarbons and/or
CO2 as described
herein provides a high selectivity and thus increases the overall carbon
efficiency of hydrogen-lean syngas
synthesis relative to conventional processes. Because CPO can be operated at
higher pressures than
conventional syngas syntheses (e.g., dry reforming) utilized to produce
hydrogen-lean syngas, compression
22
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
requirements of the hydrogen-lean syngas prior to downstream chemical
synthesis therefrom can be
reduced (and/or such compression eliminated) relative to the conventional
processes.
[00111] Additional advantages of the processes for the production of chemicals
as disclosed herein can be
apparent to one of skill in the art viewing this disclosure.
EXAMPLES
[00112] The embodiments having been generally described, the following
examples are given as
particular embodiments of the disclosure and to demonstrate the practice and
advantages thereof. It is
understood that the examples are given by way of illustration and are not
intended to limit the specification
or the claims in any manner.
[00113] Example 1. A syngas process was simulated as an equilibrium reactor in
ASPEN. Figure 2 is a
plot of the molar ratio of carbon monoxide to hydrogen (CO/H2) in syngas from
CPO as a function of
reactor temperature without CO2 injection in the reactant feed for CI-I4/02
molar ratios of 2.2 and 1.7, and
pressures of 40 and 100 bar, which shows the CO/H2 molar ratios which can be
obtained in CPO subject to
thermodynamic constraints at different temperatures of CPO reactor 10. Figure
3 is a plot of the molar ratio
of carbon monoxide to hydrogen (CO/H2) in syngas from CPO as a function of
reactor temperature with
CO2 injection for a reactant feed comprising a molar ratio of carbon dioxide
to methane (CO2/CI-I4) of 0.5,
CI-I4/U2 molar ratios of 2.2 and 1.7, and pressures of 40 and 100 bar. Figure
4 is a plot of the molar ratio of
carbon monoxide to hydrogen (CO/H2) in syngas from CPO as a function of
reactor temperature with CO2
injection for a reactant feed comprising a molar ratio of carbon dioxide to
methane (CO2/CI-I4) of 1, CI-I4/U2
molar ratios of 2.2 and 1.7, and pressures of 40 and 100 bar.
[00114] As seen in Figure 2, above 900 C and at a low CI-I4/U2 molar ratio
synthesis gas having 142/C0
molar ratios less than 2 can be produced. From Figure 3 and Figure 4, it is
apparent that injecting CO2 in
the reactant feed expands the operability window of CPO to lower temperatures
and higher CI-I4/U2 molar
ratios. As noted herein, injection of CO2 also provides for an enhancement in
carbon efficiency, because
the carbon in the CO2 is converted to additional CO. As a result more CO will
be produced per MMBTU of
reactant feed (e.g., natural gas) according to embodiments of this disclosure.
This additional CO can
contribute to an increase in chemical product throughput at the same flowrate
of reactant feed (e.g., natural
gas). A similar effect can be obtained by subjecting all or a portion of the
synthesis gas in the CPO reactor
effluent 15 to reverse WGS in r-WGS reactor 20 by injection of CO2 7B in a
separate r-WGS reactor 20
downstream of CPO reactor 10. As seen in Figures 2-4, the CPO reactor can
produce the hydrogen-lean
syngas at high pressures (e.g., greater than or equal to about 25, 30, 35, 40,
45, 50 bar) that are required for
downstream chemical (e.g., acetic acid, DME) synthesis, thus reducing or
eliminating the need for
compression of the CPO reactor effluent prior to the downstream synthesis 30.
[00115] Figure 5 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of carbon
dioxide to carbon (CO2/C) in
the reactant feed (in legend) at a pressure of 30 bar and an oxygen to carbon
molar ratio (02/C) of 0.55.
Figure 6 is a plot showing the molar ratio of carbon monoxide to hydrogen
(142/C0) in syngas from CPO as
a function of the conversion (%) and the molar ratio of carbon dioxide to
carbon (CO2/C) in the reactant
23
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
feed (in legend) at a pressure of 75 bar and an oxygen to carbon molar ratio
(02/C) of 0.55. As can be seen
from Figures 5 and 6, the molar ratio of CO2/C needed to provide a hydrogen-
lean CPO syngas having a
142/C0 molar ratio of 1 is reduced as the pressure increases.
[00116] Figure 7 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having three carbons
(C3) to carbon (C3/C) in the reactant feed (in legend) at a pressure of 75
bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and a carbon dioxide to carbon (CO2/C) molar ratio of
0.25. Figure 8 is a plot showing
the molar ratio of carbon monoxide to hydrogen (142/C0) in syngas from CPO as
a function of the
conversion (%) and the molar ratio of hydrocarbons having three carbons (C3)
to carbon (C3/C) in the
reactant feed (in legend) at a pressure of 75 bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and
without CO2 in the reactant feed.
[00117] Figure 9 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having two carbons (C2)
to carbon (C2/C) in the reactant feed (in legend) at a pressure of 75 bar, an
oxygen to carbon molar ratio
(02/C) of 0.55, and a CO2/C molar ratio of 0.25. Figure 10 is a plot the molar
ratio of carbon monoxide to
hydrogen (142/C0) in syngas from CPO as a function of the conversion (%) and
the molar ratio of
hydrocarbons having two carbons (C2) to carbon (C2/C) in the reactant feed (in
legend) at a pressure of 75
bar, an oxygen to carbon molar ratio (02/C) of 0.55, and without CO2 in the
reactant feed.
[00118] Figure 11 is a plot showing the molar ratio of carbon monoxide to
hydrogen (142/C0) in syngas
from CPO as a function of the conversion (%) and the molar ratio of
hydrocarbons having four carbons
(C4) to carbon (C4/C) in the reactant feed (in legend) at a pressure of 75
bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and a carbon dioxide to carbon (CO2/C) molar ratio of
0.25. Figure 12 is a plot
showing the molar ratio of carbon monoxide to hydrogen (142/C0) in syngas from
CPO as a function of the
conversion (%) and the molar ratio of hydrocarbons having four carbons (C4) to
carbon (C4/C) in the
reactant feed (in legend) at a pressure of 75 bar, an oxygen to carbon molar
ratio (02/C) of 0.55, and
without CO2 in the reactant feed.
[00119] As seen in Figures 7 through 12, using reactant feeds 5 comprising
higher hydrocarbons (e.g.,
C2, C3, and/or C4) allows a reduction in the amount of CO2 utilized to reach a
molar ratio of hydrogen to
carbon monoxide (142/C0) of about 1, and enables production of hydrogen-lean
syngas having an 142/C0
molar ratio of about 1 at a higher hydrocarbon conversion to syngas.
[00120] While various embodiments have been shown and described, modifications
thereof can be made
by one skilled in the art without departing from the spirit and teachings of
the disclosure. The embodiments
described herein are exemplary only, and are not intended to be limiting. Many
variations and
modifications of the subject matter disclosed herein are possible and are
within the scope of the disclosure.
Where numerical ranges or limitations are expressly stated, such express
ranges or limitations should be
understood to include iterative ranges or limitations of like magnitude
falling within the expressly stated
ranges or limitations (e.g., from about 1 to about 10 includes, 2, 3, 4, etc.;
greater than 0.10 includes 0.11,
0.12, 0.13, etc.). For example, whenever a numerical range with a lower limit,
RL and an upper limit, Ru is
24
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
disclosed, any number falling within the range is specifically disclosed. In
particular, the following
numbers within the range are specifically disclosed: R=RL+k*(Ru-RL), wherein k
is a variable ranging
from 1 percent to 100 percent with a 1 percent increment, i.e., k is 1
percent, 2 percent, 3 percent, 4 percent,
percent, ... 50 percent, 51 percent, 52 percent, ... , 95 percent, 96 percent,
97 percent, 98 percent, 99
percent, or 100 percent. Moreover, any numerical range defined by two R
numbers as defined in the above
is also specifically disclosed. Use of the term "optionally" with respect to
any element of a claim is intended
to mean that the subject element is required, or alternatively, is not
required. Both alternatives are intended
to be within the scope of the claim. Use of broader terms such as comprises,
includes, having, etc. should
be understood to provide support for narrower terms such as consisting of,
consisting essentially of,
comprised substantially of, etc.
[00121] Accordingly, the scope of protection is not limited by the description
set out above but is only
limited by the claims which follow, that scope including all equivalents of
the subject matter of the claims.
Each and every claim is incorporated into the specification as an embodiment
of the present disclosure.
Thus, the claims are a further description and are an addition to the
embodiments of the present disclosure.
The discussion of a reference is not an admission that it is prior art to the
present disclosure, especially any
reference that may have a publication date after the priority date of this
application. The disclosures of all
patents, patent applications, and publications cited herein are hereby
incorporated by reference, to the extent
that they provide exemplary, procedural, or other details supplementary to
those set forth herein.
ADDITIONAL DESCRIPTION
[00122] The particular embodiments disclosed above are illustrative only, as
the present disclosure may
be modified and practiced in different but equivalent manners apparent to
those skilled in the art having the
benefit of the teachings herein. Furthermore, no limitations are intended to
the details of construction or
design herein shown, other than as described in the claims below. It is
therefore evident that the particular
illustrative embodiments disclosed above may be altered or modified and all
such variations are considered
within the scope and spirit of the present disclosure. Alternative embodiments
that result from combining,
integrating, and/or omitting features of the embodiment(s) are also within the
scope of the disclosure.
While compositions and methods are described in broader terms of "having",
"comprising," "containing,"
or "including" various components or steps, the compositions and methods can
also "consist essentially of'
or "consist of' the various components and steps. Use of the term "optionally"
with respect to any element
of a claim means that the element is required, or alternatively, the element
is not required, both alternatives
being within the scope of the claim.
[00123] Numbers and ranges disclosed above may vary by some amount. Whenever a
numerical range
with a lower limit and an upper limit is disclosed, any number and any
included range falling within the
range are specifically disclosed. In particular, every range of values (of the
form, "from about a to about b,"
or, equivalently, "from approximately a to b," or, equivalently, "from
approximately a-b") disclosed herein
is to be understood to set forth every number and range encompassed within the
broader range of values.
Also, the terms in the claims have their plain, ordinary meaning unless
otherwise explicitly and clearly
defined by the patentee. Moreover, the indefinite articles "a" or "an", as
used in the claims, are defined
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
herein to mean one or more than one of the element that it introduces. If
there is any conflict in the usages
of a word or term in this specification and one or more patent or other
documents, the definitions that are
consistent with this specification should be adopted.
[00124] Embodiments disclosed herein include:
[00125] A: A process for producing hydrogen-lean syngas comprising reacting,
via a catalytic partial
oxidation (CPO) reaction, a CPO reactant mixture in a CPO reactor to produce
the hydrogen-lean syngas;
wherein the CPO reactant mixture comprises hydrocarbons and oxygen; wherein
the hydrocarbons
comprise greater than or equal to about 3 mol% C2+ alkanes; wherein the CPO
reactor comprises a CPO
catalyst; wherein the hydrogen-lean syngas comprises hydrogen, carbon
monoxide, carbon dioxide, water,
and unreacted hydrocarbons; and wherein the hydrogen-lean syngas is
characterized by a hydrogen to
carbon monoxide (142/C0) molar ratio of from about 0.8 to about 1.6.
[00126] B: A process comprising: (a) reacting, via a catalytic partial
oxidation (CPO) reaction, a CPO
reactant mixture in a CPO reactor to produce a hydrogen-lean syngas; wherein
the CPO reactant mixture
comprises hydrocarbons and oxygen; wherein the hydrocarbons comprise greater
than or equal to about 3
mol% C2+ alkanes; wherein the CPO reactor comprises a CPO catalyst; wherein
the hydrogen-lean syngas
comprises hydrogen, carbon monoxide, carbon dioxide (CO2), water, and
unreacted hydrocarbons; and
wherein the hydrogen-lean syngas is characterized by a hydrogen to carbon
monoxide (142/C0) molar ratio
of from about 0.8 to about 1.6; (b) optionally introducing CO2 to the CPO
reactor, wherein the CPO
reactant mixture is characterized by a CO2 to carbon (CO2/C) molar ratio in
the CPO reactant mixture of
greater than or equal to about 0.5:1, wherein the CO2/C molar ratio refers to
the total moles of CO2 in the
reactant mixture divided by the total moles of C in the hydrocarbons in the
reactant mixture; and (c) using
at least a portion of the hydrogen-lean syngas in a downstream synthesis
process, wherein the downstream
synthesis process is selected from the group consisting of acetic acid
synthesis process; dimethyl ether
synthesis process; oxo-synthesis of aliphatic aldehydes and/or alcohols; and
combinations thereof.
[00127] C: A chemical synthesis system comprising: (a) a catalytic partial
oxidation (CPO) reactor
comprising a CPO catalyst, and operable to produce a hydrogen-lean syngas from
a CPO reactant mixture;
wherein the CPO reactant mixture comprises hydrocarbons and oxygen; wherein
the hydrocarbons
comprise greater than or equal to about 3 mol% C2+ alkanes; wherein the
hydrogen-lean syngas comprises
hydrogen, carbon monoxide, carbon dioxide (CO2), water, and unreacted
hydrocarbons; and wherein the
hydrogen-lean syngas is characterized by a hydrogen to carbon monoxide
(142/C0) molar ratio of from
about 0.8 to about 1.6; and (b) a downstream synthesis apparatus configured to
produce a chemical product
from at least a portion of the hydrogen-lean syngas, wherein the downstream
synthesis process is selected
from the group consisting of acetic acid synthesis process; dimethyl ether
synthesis process; oxo-synthesis
of aliphatic aldehydes and/or alcohols; and combinations thereof.
[00128] Each of embodiments A, B, and C may have one or more of the following
additional elements:
Element 1: wherein the hydrocarbons comprise methane, natural gas, natural gas
liquids, liquefied
petroleum gas (LPG), associated gas, well head gas, enriched gas, paraffins,
shale gas, shale liquids, fluid
catalytic cracking (FCC) off gas, refinery process gases, refinery off gases,
stack gases, fuel gas from a fuel
26
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
gas header, or combinations thereof. Element 2: wherein the hydrocarbons
comprise ethane in an amount
of greater than or equal to about 4 mol%. Element 3: wherein the hydrocarbons
comprise propane in an
amount of greater than or equal to about 4 mol%. Element 4: wherein the
hydrocarbons comprise butanes
in an amount of greater than or equal to about 3 mol%. Element 5: wherein the
hydrocarbons conversion
in the CPO reactor is greater than the hydrocarbons conversion in a CPO
reactor in an otherwise similar
process that produces a hydrogen-lean syngas from hydrocarbons comprising less
than about 3 mol% C2+
alkanes. Element 6: wherein the CPO reactant mixture further comprises carbon
dioxide (CO2); and
wherein the CPO reactant mixture is characterized by a CO2 to carbon (CO2/C)
molar ratio in the CPO
reactant mixture of greater than or equal to about 0.5:1, wherein the CO2/C
molar ratio refers to the total
moles of CO2 in the reactant mixture divided by the total moles of carbon (C)
in the hydrocarbons in the
reactant mixture. Element 7: wherein the amount of CO2 in the CPO reactant
mixture is lower than the
amount of CO2 in a CPO reactant mixture in an otherwise similar process that
produces a hydrogen-lean
syngas from hydrocarbons comprising less than about 3 mol% C2+ alkanes.
Element 8: wherein the CPO
reactor is characterized by at least one CPO operational parameter selected
from the group consisting of a
CPO reactant temperature of from about 100 C to about 500 C; a CPO pressure
of from about 20 barg to
about 80 barg; a CPO contact time of from about 0.001 milliseconds (ms) to
about 5 seconds (s); a carbon
to oxygen (C/O) molar ratio in the CPO reactant mixture of from about 0.5:1 to
about 3:1, wherein the C/O
molar ratio refers to the total moles of carbon (C) in the hydrocarbons in the
reactant mixture divided by the
total moles of oxygen (02) in the reactant mixture; a steam to carbon (S/C)
molar ratio in the CPO reactant
mixture of less than about 0.6:1, wherein the S/C molar ratio refers to the
total moles of water (1420) in the
reactant mixture divided by the total moles of carbon (C) in the hydrocarbons
in the reactant mixture; and
combinations thereof. Element 9: further comprising: (i) recovering a CPO
reactor effluent from the CPO
reactor, wherein the CPO reactor effluent comprises hydrogen, carbon monoxide,
carbon dioxide, water,
and unreacted hydrocarbons, and wherein the CPO reactor effluent is
characterized by a 142/C0 molar ratio
of greater than about 1.6; and (ii) feeding at least a portion of the CPO
reactor effluent to a reverse water-
gas shift (r-WGS) reactor to produce the hydrogen-lean syngas, wherein a
portion of the hydrogen of the
CPO reactor effluent reacts with carbon dioxide via a r-WGS reaction to
produce water and carbon
monoxide. Element 10: further comprising introducing additional carbon dioxide
to the r-WGS reactor.
Element 11: further comprising: (a) recovering a r-WGS reactor effluent from
the r-WGS reactor, wherein
the r-WGS reactor effluent comprises hydrogen, carbon monoxide, carbon
dioxide, water, and unreacted
hydrocarbons; and (b) removing at least a portion of the water from the r-WGS
reactor effluent to yield the
hydrogen-lean syngas, wherein the amount of water in the r-WGS reactor
effluent is greater than the
amount of water in the hydrogen-lean syngas. Element 12: further comprising:
(1) contacting a portion of
the CPO reactor effluent with at least a portion of the r-WGS reactor effluent
to produce a combined
effluent stream; and (2) removing at least a portion of the water from the
combined effluent stream to yield
the hydrogen-lean syngas, wherein the amount of water in the combined effluent
stream is greater than the
amount of water in the hydrogen-lean syngas. Element 13: excluding a step of
introducing at least a
portion of the CPO reactor effluent and/or at least a portion of the hydrogen-
lean syngas to a hydrogen
27
CA 03127023 2021-07-16
WO 2020/157586
PCT/IB2020/050014
recovery unit to decrease the amount of hydrogen in the CPO reactor effluent
and/or the hydrogen-lean
syngas, respectively. Element 14: wherein a portion of the carbon dioxide in
the CPO reactor undergoes a
reverse water-gas shift (r-WGS) reaction, thereby decreasing the amount of
hydrogen in the hydrogen-lean
syngas. Element 15: further comprising using at least a portion of the
hydrogen-lean syngas in a
downstream synthesis process. Element 16: wherein the downstream synthesis
process is selected from the
group consisting of acetic acid synthesis process; dimethyl ether synthesis
process; oxo-synthesis of
aliphatic aldehydes and/or alcohols; and combinations thereof. Element 17:
wherein (i) the hydrocarbons
conversion in the CPO reactor is greater than the hydrocarbons conversion in a
CPO reactor in an otherwise
similar process that produces a hydrogen-lean syngas from hydrocarbons
comprising less than about 3
mol% C2+ alkanes; and/or (ii) the amount of CO2 in the CPO reactant mixture is
lower than the amount of
CO2 in a CPO reactant mixture in an otherwise similar process that produces a
hydrogen-lean syngas from
hydrocarbons comprising less than about 3 mol% C2+ alkanes. Element 18:
wherein the CPO reactor is
characterized by at least one CPO operational parameter selected from the
group consisting of a CPO
reactant mixture temperature of from about 100 C to about 500 C; a CPO
pressure of from about 25 barg
to about 80 barg; a CPO contact time of from about 0.001 milliseconds (ms) to
about 5 seconds (s); a
carbon to oxygen (C/O) molar ratio in the CPO reactant mixture of from about
0.5:1 to about 2:1, wherein
the C/O molar ratio refers to the total moles of carbon (C) in the
hydrocarbons in the reactant mixture
divided by the total moles of oxygen (02) in the reactant mixture; a steam to
carbon (S/C) molar ratio in the
CPO reactant mixture of less than about 0.25:1, wherein the S/C molar ratio
refers to the total moles of
water (1420) in the reactant mixture divided by the total moles of carbon (C)
in the hydrocarbons in the
reactant mixture; and combinations thereof. Element 19: (i) comprising no
apparatus for altering the
112/C0 molar ratio of the hydrogen-lean syngas between the CPO reactor and the
downstream synthesis
apparatus; (ii) comprising a reverse water gas shift apparatus as a sole
apparatus for altering the 142/C0
molar ratio of the hydrogen-lean syngas prior to the downstream synthesis
apparatus; or (iii) comprising no
apparatus configured to remove a hydrogen stream from the hydrogen-lean syngas
between the CPO reactor
and the downstream synthesis apparatus.
[00129] While preferred embodiments of the invention have been shown and
described, modifications
thereof can be made by one skilled in the art without departing from the
teachings of this disclosure. The
embodiments described herein are exemplary only, and are not intended to be
limiting. Many variations
and modifications of the invention disclosed herein are possible and are
within the scope of the invention.
[00130] Numerous other modifications, equivalents, and alternatives, will
become apparent to those
skilled in the art once the above disclosure is fully appreciated. It is
intended that the following claims be
interpreted to embrace all such modifications, equivalents, and alternatives
where applicable. Accordingly,
the scope of protection is not limited by the description set out above but is
only limited by the claims
which follow, that scope including all equivalents of the subject matter of
the claims. Each and every claim
is incorporated into the specification as an embodiment of the present
invention. Thus, the claims are a
further description and are an addition to the detailed description of the
present invention. The disclosures
of all patents, patent applications, and publications cited herein are hereby
incorporated by reference.
28