Note: Descriptions are shown in the official language in which they were submitted.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
1
AN ORAL NICOTINE PRODUCT COMPRISING A pH ADJUSTING AGENT
TECHNICAL FIELD
The present disclosure relates to an oral pouched nicotine product comprising
no tobacco
or a small amount of tobacco, a method for manufacturing thereof and use of a
pH
adjuster for counteracting an increase in pH when said product is stored.
BACKGROUND
Traditionally, oral smokeless tobacco products are used in the oral cavity of
a consumer
to provide nicotine satisfaction from the tobacco in the product. In addition
to the tobacco,
the oral smokeless tobacco product generally comprises water, salt, pH
adjuster(s) and
additional ingredients such as flavours and humectants. Commonly, these
products are
called snuff. The snuff may be dry or moist, and may be provided in loose form
or in
pouched form. Moist snuff is divided into two types, namely American snuff and
Scandinavian snuff. American moist snuff is commonly produced through a
fermentation
process of moisturized ground or cut tobacco. Scandinavian-type moist snuff
(snus) is
commonly produced using a heat-treatment process (pasteurization) instead of
fermentation. The heat treatment is carried out in order to degrade, destroy
or denature at
least a portion of the organisms within the tobacco preparation.
Oral pouched nicotine products comprising no tobacco or a small amount of
tobacco are
now becoming increasingly popular among consumers due to inter alia their
appealing
appearance, freshness and taste. Moreover, this kind of product allows a user
to enjoy
nicotine without being exposed to tobacco.
WO 2012/134380 discloses a pouch containing nicotine in free salt form, i.e.
an oral
pouched nicotine-containing non-tobacco snuff product. The product comprises a
powder
of at least one free nicotine salt, at least one pH adjusting agent and at
least one filler,
and a water insoluble pouch, wherein said pouch is permeable for saliva and
therein
dissolved parts of the powder, wherein said product upon contact with purified
water gives
a pH of at least 6.
EP 3 087 852 discloses an oral pouched product having a rectangular shape. The
oral
pouched product may be an oral pouched non-tobacco nicotine-containing snuff
product.
The filling material of the product may be a particulate material comprising
nicotine or a
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
2
salt thereof and one or more fillers, such as polysaccharides and/or
microcrystalline
cellulose. It is stated that salt, such as sodium chloride, potassium
chloride, magnesium
chloride, calcium chloride and any combinations thereof, may be added mainly
for its
effect on taste but it also has a preservative action which contributes to
improved shelf life
of the product. Salt, such as sodium chloride, lowers the water activity of
the products,
thus preventing microorganisms from growing.
pH is known to contribute to regulating the uptake of nicotine through the
mucous
membranes in the oral cavity of a consumer, such as a human. A pH adjusting
agent is
needed to increase the amount of nicotine present in the form of a free base,
which may
be absorbed through the mucous membranes in the oral cavity of a consumer.
However,
an alkaline pH reduces nicotine extraction from the product. In particular, a
very high pH
has a negative effect on the taste of the product and is also detrimental for
the oral
mucous membranes. On the other hand, an acidic pH improves nicotine mobility
and
extraction from the product, but diminishes nicotine uptake in the oral cavity
and is bad for
oral health. Consequently, it is frequently desired to adjust the pH of oral
pouched nicotine
products to be neutral or slightly alkaline such as from pH 7 to 10. Commonly,
this is
achieved using pH adjusters comprising sodium carbonate and/or sodium
bicarbonate.
Unfortunately, the pH of snuff products such as oral smokeless tobacco snuff
products
has been found to drop upon storage. In order to counteract the pH drop the
product may
be kept refrigerated and/or the amount of sodium carbonate and/or sodium
bicarbonate
may be increased. However, this makes handling of the product less convenient,
and may
affect the taste of the product in an undesired way.
WO 2009/082331 discloses a tobacco or non-tobacco product comprising magnesium
carbonate for conferring pH stability to the product and preventing growth of
bacteria and
fungi therein.
WO 2015/193379 discloses a tobacco or non-tobacco product comprising magnesium
carbonate. The composition is enclosed by a wrapping material, which comprises
magnesium carbonate. In this way, the oral pH is stabilized during use of said
product.
Figure 1 shows that the oral pH during use of oral smokeless tobacco products
drops
during use.
WO 2018/233795 discloses a nicotine pouch containing a matrix composition
comprising
a combination of nicotine and a water-soluble composition.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
3
Thus, there is a need for oral pouched nicotine products comprising no tobacco
or a small
amount of tobacco, in which products the pH upon storage does not change,
changes to a
limited extent and/or remains within a range from about 7 to about 10.
An object of the present disclosure is to alleviate at least one of the
problems
discussed above, and to provide advantages and aspects not provided by
hitherto
known technique. Further, it is an object of the present disclosure to
prevent, reduce
and/or counteract a change in pH such as an increase in pH in an oral pouched
nicotine product comprising no tobacco or a small amount of tobacco.
SUMMARY
The present disclosure provides an oral pouched nicotine product comprising a
filling material and a saliva-permeable pouch of a packaging material
enclosing the
filling material, the filling material comprising or consisting of:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total
weight of the filling material, and
- a pH adjusting agent comprising or consisting of:
(i) Na2003, K2003, NaHCO3 and/or KHCO3, and
(ii) a salt of Formula (I):
Formula I
or a hydrate of said salt,
wherein
M2+ is selected from the group consisting of Ca2+, Mg2+, Mn2+, Zn2+ and Fe2+,
An- is an anion selected from the group consisting of chloride, lactate,
malate,
succinate, citrate, ascorbate, tartrate, acetate, phosphate, sulphate,
nitrate,
gluconate, alginate, ammonium, oxalate, aspartate, glycinate, picolinate,
oxide,
cysteinate, glutamate, hydroxide, laurate, stearate, palmitate, undecylenate,
gluceptate, glucerophosphate, glubionate, glucoheptonate, guanylate,
inosinate,
propionate, sorbate, salicylate, benzoate, erythorbate, formate, iodide,
pangamate
and any combination thereof,
n is 1 0r2,
m is 1 or 2, and
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
4
-(n x m)= -2,
said salt being present in an amount within the range of from 0.05 wt% to 5
wt%,
based on the total weight of the filling material,
said salt having a water solubility equal to or above about 0.04 M and/or
equal to or
above 1 g/L, and
- optionally a tobacco material in an amount within the range of from 0.05
wt% to 10
wt % based on the total weight of the filling material.
The present disclosure also provides a use of a salt of Formula I, or a
hydrate of said salt,
as described herein for controlling pH in an oral pouched nicotine product
comprising a
filling material, said filling material comprising:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total weight
of the filling material, and
- a pH adjusting agent comprising or consisting of Na2003, K2003, NaHCO3
and/or
KHCO3, and
- optionally a tobacco material within the range of from 0.05 wt% to 10 wt
% based on the
total weight of the filling material,
when said product is stored, such as stored
at a relative humidity from 60% to 75%,
at a temperature from 22 C to 30 C, and/or
for a time of 15 weeks.
BRIEF DESCRIPTION OF THE DRAWINGS
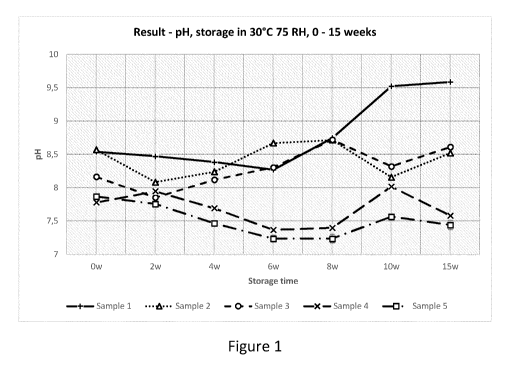
Figure 1 shows the pH value (pH units) as a function of storage time (weeks)
for dry oral
pouched nicotine products comprising no CaCl2 or increasing amounts of CaCl2,
when
storage was performed at a temperature of 30 C, and a relative humidity of
75%.
Figure 2 shows the pH value (pH units) as a function of storage time (weeks)
for two
samples of snus when storage is performed at a temperature of 22 C and a
relative
humidity of 60 %.
Figure 3 shows the pH value (pH units) as a function of storage time (weeks)
for moist
oral pouched nicotine products comprising no CaCl2 or increasing amounts of
CaCl2,
when storage was performed at a temperature of 22 C and a relative humidity of
60%.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
DESCRIPTION
The term "tobacco material" is used herein for tobacco extract and/or fibrous
material of tobacco leaves or parts of tobacco leaves, such as lamina and
stem. The
leaves and parts of leaves may be finely divided (disintegrated), such as
ground,
5 cut, shredded or threshed, and the parts of leaves may be blended in defined
proportions in the tobacco material.
By "tobacco" as used herein is meant any part, e.g., leaves, stems, and
stalks, of any
member of the genus Nicotiana. The tobacco may be whole, shredded, threshed,
cut,
ground, cured, aged, fermented, or treated otherwise, e.g., granulated or
encapsulated.
"Oral" and "oral use" is in all contexts used herein as a description for use
in the oral
cavity of a human, such as buccal placement.
As used herein, the term "moisture content" refers to the total amount of oven
volatile
ingredients, such as water and other oven volatiles (e.g. propylene glycol) in
the
preparation, composition or product referred to. The moisture content is given
herein as
percent by weight (wt%) of the total weight of the preparation, composition or
product
referred to. Some fibrous materials may exhibit hygroscopic properties.
Hygroscopic
materials maintain equilibrium moisture content depending on the ambient
moisture and
temperature. The moisture content as referred to herein may be determined by
using a
method based on literature references Federal Register/ vol.74, no. 4/712-
719/Wednesday, January 7, 2009/Notices "Total moisture determination" and AOAC
(Association of Official Analytical Chemics), Official Methods of Analysis
966.02: "Moisture
in Tobacco" (1990), Fifth Edition, K. He!rich (ed). In this method, the
moisture content is
determined gravimetrically by taking 2.5 0.25 g sample and weighing the sample
at
ambient conditions, herein defined as being at a temperature of 22 C and a
relative
humidity (RH) of 60%, before evaporation of moisture and after completion of
dehydration.
Mettler Toledo's Moisture Analyzer HB43, a balance with halogen heating
technology, is
used (instead of an oven and a balance as in the mentioned literature
references) in the
experiments described herein. The sample is heated to 105 C (instead of 99.5
0.5 C as
in the mentioned literature references). The measurement is stopped when the
weight
change is less than 1 mg during a 90 seconds time frame. The moisture content
as weight
percent of the sample is then calculated automatically by the Moisture
Analyzer HB43.
"Flavour" or "flavouring agent" is used herein for a substance used to
influence the aroma
and/or taste of the nicotine product, including, but not limited to, essential
oils, single
flavour compounds, compounded flavourings, and extracts.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
6
As used herein "% w/w", "w/w %", "wt%", "weight c/0" or "% by weight" refers
to the weight
percent of the ingredient referred to of the total weight of the preparation,
composition or
product referred to.
As used herein, reference to "dry weight percent", "% by weight, based on dry
weight" and
the like refers to the weight percent of the ingredient referred to on the
basis of the total
weight of the dry ingredients, i.e. all ingredients of the preparation,
composition or product
referred to excluding the moisture content.
As used herein, reference to "wet weight percent", "% by weight, based on wet
weight"
and the like refers to the weight percent of the ingredient referred to on the
basis of the
total weight of the ingredients, i.e. all ingredients of the preparation,
composition or
product referred to including the moisture content. Thus, "% by weight, based
on total
weight" as used herein is the same as "% by weight, based on wet weight".
As used herein the terms "pouched nicotine product for oral use" or "oral
pouched nicotine
product" refer to a portion of nicotine-containing filling material packed in
a saliva-
permeable pouch material intended for oral use. Two examples of oral pouched
nicotine
products are oral pouched nicotine non-tobacco products and oral pouched low
tobacco
nicotine products.
As used herein the terms "oral pouched nicotine non-tobacco product", "oral
pouched
tobacco free nicotine product" or "oral pouched nicotine product free from
tobacco" refer
to a portion of nicotine-containing filling material packed in a saliva-
permeable pouch
material intended for oral use wherein no tobacco is included in said product.
As used herein the term "oral pouched low tobacco nicotine product" refers to
a portion of
nicotine-containing filling material packed in a saliva-permeable pouch
material intended
for oral use wherein an amount of tobacco material within the range of from
about 0.1% to
about 10% by weight or from about 0.1% to about 5% by weight, based on the
total weight
of the filling material, is included in said product.
As used herein, the term "non-particulate" refers to a component which is not
in
particulate form. For instance, the flavouring agent described herein may be a
non-
particulate flavouring agent such as a liquid, an oil or a mixture thereof.
As used herein, the term "particulate non-tobacco material" refers to a non-
tobacco
material comprising particles. The particles may have an average particle size
within the
range of from 50 to 500 pm.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
7
The oral pouched nicotine product as disclosed herein is intended for use in
the oral
cavity, such as by buccal placement (e.g. by placing the pouched product
between the
upper or lower gum and the lip or cheek), and may therefore be referred to as
portion-
packed (pouched) product for oral use. The oral pouched nicotine product is
sized and
configured to fit comfortably and discreetly in a user's mouth between the
upper or lower
gum and the lip or cheek.
The oral pouched nicotine product as disclosed herein may have an oblong
shape, such
as a substantially rectangular shape (as seen from above when the product is
placed on a
planar surface). In such case, the longitudinal direction of the product
corresponds to the
length of the substantially rectangular product and the transverse direction
of the product
corresponds to the width of the substantially rectangular product.
The total weight of the oral pouched nicotine product (including filling
material and
packaging material) may be within the range of from about 0.3 to about 1.5 g.
The filling material of the oral pouched nicotine product described herein may
be provided
as a powder or granulate. Thus, the filling material enclosed by the saliva-
permeable
pouch of the packaging material may be provided in a non-compressed form.
The pouch of the oral pouched nicotine product may be made of any suitable
saliva-
permeable (and preferably non-dissolvable) packaging material, such as a non-
woven
material. The packaging material (herein also called pouch material) may be a
nonwoven
material comprising staple fibres of regenerated cellulose, such as viscose
rayon staple
fibres, and a binder, such as a polyacrylate.
The oral pouched (i.e. portion-packed) nicotine products may be positioned
randomly in a
container or in a pattern, for instance as described in WO 2012/069505.
Alternatively or
additionally, each oral pouched nicotine product may be placed in a sachet.
The present disclosure provides an oral pouched nicotine product comprising a
filling
material and a saliva-permeable pouch of a packaging material enclosing the
filling
material, the filling material comprising or consisting of:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from about 1 wt% to about 50 wt%
based on the
total weight of the filling material, and
- a pH adjusting agent comprising or consisting of:
(i) Na2003, K2003, NaHCO3 and/or KHCO3, and
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
8
(ii) a salt of Formula (I):
m2,-(An-)m
Formula I
or a hydrate of said salt,
wherein
M2+ is selected from the group consisting of Ca2+, Mg2+, Mn2+, Zn2+ and Fe2+,
An- is an anion selected from the group consisting of chloride, lactate,
malate, succinate,
citrate, ascorbate, tartrate, acetate, phosphate, sulphate, nitrate,
gluconate, alginate,
ammonium, oxalate, aspartate, glycinate, picolinate, oxide, cysteinate,
glutamate,
hydroxide, laurate, stearate, palmitate, undecylenate, gluceptate,
glucerophosphate,
glubionate, glucoheptonate, guanylate, inosinate, propionate, sorbate,
salicylate,
benzoate, erythorbate, formate, iodide, pangamate and any combination thereof,
n is 1 or 2,
m is 1 or 2, and
-(n x m)= -2,
said salt being present in an amount within the range of from about 0.05 wt%
to about 5
wt%, based on the total weight of the filling material,
said salt having a water solubility equal to or above about 0.04 M and/or
equal to or above
1 g/L, and
- optionally a tobacco material in an amount within the range of from 0.05 wt%
to 10 wt %
based on the total weight of the filling material.
It will be appreciated that in the salt of Formula I as described herein, n
may have the
value 1 and m may have the value 2. Alternatively, n may have the value 2 and
m may
have the value 1. Therefore, - (n x m)= -2.
The anion An- of the oral pouched nicotine product described herein may be
selected from
one or more of the following: chloride, lactate, malate, citrate, ascorbate,
acetate,
sulphate, nitrate, gluconate, glutamate, guanylate, inosinate, propionate,
sorbate,
benzoate, formate, pangamate. For instance, M2+ may then be Ca2+.
Further, the anion An- of the oral pouched nicotine product described herein
may be
selected from one or more of the following: chloride, lactate, citrate,
ascorbate, acetate,
sulphate, nitrate, propionate, benzoate, formate, pangamate. For instance, M2+
may then
be Mg2+.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
9
Moreover, the anion An- of the oral pouched nicotine product described herein
may be
selected from one or more of the following: chloride, lactate, citrate,
acetate, sulphate,
nitrate, gluconate, benzoate, formate. For instance, M2+ may then be Mn2+.
In an example, the anion An- of the oral pouched nicotine product described
herein may be
selected from one or more of the following: chloride, acetate, sulphate,
nitrate, formate. In
a further example, the anion An- of the oral pouched nicotine product
described herein
may be selected from one or more of the following: chloride, acetate,
sulphate, nitrate,
gluconate, propionate, formate. For instance, M2+ may then be Zn2+.
In still a further example, the anion An- of the oral pouched nicotine product
described
herein may be selected from one or more of the following: chloride, acetate,
sulphate,
nitrate, gluconate, propionate, formate. In another example, the anion An- of
the oral
pouched nicotine product described herein may be selected from one or more of
the
following: chloride, lactate, sulphate, nitrate. For instance, M2+ may then be
Fe2+.
In still another example, the anion An- of the oral pouched nicotine product
described
herein may be selected from one or more of the following: chloride, lactate,
malate,
citrate, ascorbate, tartrate, acetate, gluconate, aspartate, glycinate. . For
instance, M2+
may then be Ca2+.
The pH adjusting agent of the oral pouched nicotine product as described
herein allows
the pH to be within the range of from about 7 to about 10. This pH may be
achieved after
manufacture of the product, such as immediately after manufacture of the
product.
Further, this pH is provided upon storage of the product such as upon storage
as
described herein. As described herein, achieving and/or maintaining a pH
within the range
of about 7 to about 10, such as from about 7 to about 9.5, such as from about
7 to about
9.2, such as from about 7 to about 9, such as from about 8 to about 9, is a
significant
advantage since it allows for good nicotine extraction and taste while not
impacting oral
mucous membranes negatively.
Storage of smokeless tobacco products comprising Na2003, K2003, NaHCO3 and/or
KHCO3 as pH adjuster, leads to a decrease in the product pH. In contrast, the
present
inventors have found an increase in pH upon storage of oral pouched tobacco
free
nicotine products and oral pouched low tobacco nicotine products comprising
Na2003,
K2003, NaHCO3 and/or KHCO3 as pH adjuster. Surprisingly, it has been found
that this
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
increase in pH is counteracted by combining the Na2003, K2003, NaHCO3 and/or
KHCO3
with a salt of Formula I as described herein.
While not wishing to be bound by any specific theory, it is believed that the
salt of Formula
I acts by preventing formation of hydroxide ions from water and the carbonate
ions of the
5 pH adjusting agent. Instead, it is believed that the salt of Formula I
reacts with the
carbonate ions of the pH adjusting agent to form a carbonate salt having low
water
solubility. For instance, when the salt of Formula I is CaCl2 a carbonate salt
of formula
CaCO3 may be formed. As a result, the pH of or in the oral pouched nicotine
product into
which the pH adjusting agent is incorporated increases less as compared to a
10 corresponding product lacking the salt of Formula I.
The salt of Formula I may have a water solubility equal to or above about 0.04
M, such as
above about 0.045 M, such as above about 0.05 M. Additionally or
alternatively, the
Formula I may have a water solubility equal to or above one or more of the
following:
about 0.045 M, such as equal to or above about 0.05 M, such as equal to or
above about
0.06 M, such as equal to or above about 0.07 M, such as equal to or above
about 0.8 M,
such as equal to or above about 0.9 M, such as equal to or above about 1.0 M.
Further,
the salt of Formula I may have a water solubility equal to or less than about
10 M, such as
less than about 5 M, such as less than about 3 M.
Moreover, the salt of Formula I may have a water solubility equal to or above
about 1 g/L
such as equal to or above about 2 g/L, such as equal to or above about 3 g/L,
such as
equal to or above about 4 g/L, such as equal to or above about 5 g/L, such as
equal to or
above about 10 g/L, such as equal to or above about 50 g/L, such as equal to
or above
about 100 g/I, such as equal to or above about 150 g/L, such as equal to or
above about
200 g/L, such as equal to or above about 250 g/L, such as equal to or above
about 300
g/L, such as equal to or above about 350 g/L such as equal to or above about
400 g/L,
such as equal to or above about 450 g/L, such as equal to or above about 500
g/L, such
as equal to or above about 600 g/L, such as equal to or above about 700 g/L.
Further, the
salt of Formula I may have a water solubility equal to or less than about 1
kilogram/L,
such as about 900 g/L, such as about 800 g/L. In this document, "g" stands for
gram(s)
and L stands for liter.
In this document, "M" stands for molar, i.e. mole per liter (i.e. mole/L or
mol/L). Thus, the
solubility may be measured in moles per liter of the solution. The solution
may be the
solution resulting from mixing such as dissolving the salt of Formula I with
the water.
CA 03127725 2021-07-23
WO 2020/157280 PC T/EP2020/052437
11
Additionally or alternatively, the solution may be measured in g/L, i.e.
gram(s) per liter of
solution. The may be the solution resulting from dissolving the salt of
Formula I with the
water. Additionally or alternatively, the solution may be the solution to
which the salt of
Formula I is added.
The water solubility described herein such as the water solubility of the salt
of Formula I
described herein may be measured at a temperature from about 10 C to about 40
C,
such as from about 20 C to about 25 C. Further, the water solubility may be
measured at
atmospheric pressure such as at a pressure of about 101,325 Pa, i.e. 101,325
Pascal.
The oral pouched nicotine product may be free from tobacco, i.e. an oral
pouched nicotine
non-tobacco product.
Alternatively, the oral pouched nicotine product may comprise a low amount of
tobacco
material thereby providing an oral pouched low tobacco nicotine product. The
amount of
tobacco material of the oral pouched low tobacco nicotine product may be
within the
range of from about 0.1% to about 10% by weight such as from about 0.1% to
about 5%
by weight, such as from about 0.1 wt% to about 1 wt%, based on the total
weight of the
filling material. The presence of this small amount of tobacco will not impact
the pH of the
product to be substantially different from that exhibited by the oral pouched
tobacco free
products described herein.
The tobacco material may be provided in a form as described herein.
Further, the tobacco material may be a purified tobacco material, such as a
bleached
tobacco material or a tobacco extract.
The tobacco material described herein may comprise one, two or more
particulate non-
tobacco materials.
The amount of water of the filling material of the oral pouched nicotine
product described
herein may be present in an amount within the range of from about 0.5 wt% to
about 12
wt% such as from about 0.5 wt% to about 5 wt%, such as from about 0.5 wt% to
about 3
wt%, such as about 3 wt%, based on the total weight of the filling material.
When the
amount of water is within the range of from about 0.5 wt % to about 12 wt% or
from about
0.5 wt% to about 3 wt% as described herein the oral pouched nicotine product
may be
considered dry, i.e. a dry oral pouched nicotine product.
Alternatively, the water content of the filling material of the oral pouched
nicotine product
described herein may be within the range of from about 20 wt% to about 50 wt%,
such as
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
12
from 20 wt% to 45 wt%, based on the total weight of the filling material. When
the amount
of water is within the range of from about 20 wt% to about 45 wt% as described
herein the
oral pouched nicotine product may be considered moist, i.e. a moist oral
pouched nicotine
product.
Surprisingly, it has been found that the pH adjusting agent described herein
reduces,
prevents and/or counteracts an increase in pH in an oral pouched nicotine
product when it
is stored. The oral pouched nicotine product may be dry or moist. The storage
may be as
described herein.
The salt of Formula I, or the hydrate of said salt, of the pH adjusting agent
described
herein may be present in an amount within the range of from about 0.05 wt% to
about 10
wrk,such as from about 0.05 wt% to about 7 wt%, such as from about 0.05 wt% to
about
5 wt%, such as from about 0.05 wt% to about 3 wt%, such as from about 0.05 wt%
to
about 2 wt%, such as from about 0.05 wt% to about 1 wt%, based on the total
weight of
the filling material. For example, the salt of Formula I or hydrate of the
salt may be
present in an amount within the range of from about 0.05 wt% to about 0.3 wt%
or from
about 0.1 wt% to about 0.3 wt%, based on the total weight of the filling
material.
The Na2003, K2003, NaHCO3 and/or KHCO3 of the pH adjusting agent described
herein
may be present in an amount within the range of about 1 wt% to about 10 wt%,
such as
from about 1.5 wt% to about 4 wt% or from about 4 wt% to about 9 wt%, based on
the
total weight of the filling material of the oral pouched nicotine product.
The divalent metal ion M2+ of the salt of Formula I may be Ca2+ or Mg2+.
Alternatively, the
divalent metal ion M2+ may be Mn2+, Zn2+ or Fe2+. For instance, the divalent
metal ion M2+
of the salt of Formula may be Mn2+. In a further example. the divalent metal
ion M2+ of the
salt of Formula may be Zn2+. In still a further example, the divalent metal
ion M2+ of the
salt of Formula may be Fe2+.
The salt of Formula I described herein may comprise or consist of CaCl2 or a
hydrate
thereof. Additionally or alternatively, the salt of Formula I described herein
may comprise
or consist of MgCl2 and/or ZnCl2 or a hydrate of any of the aforementioned
salts. Further,
the salt of Formula I described herein may comprise or consist of MnCl2 and/or
FeCl2 or a
hydrate of the aforementioned salts.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
13
The anion An- of the salt of Formula I described herein may be lactate. Thus,
there is
described a salt of Formula I as described herein which comprises or consists
of calcium
lactate and/or zinc lactate. Further, the salt of Formula I may comprise or
consist of
magnesium lactate and/or iron lactate. It will be appreciated that any of the
aforementioned salts may be provided in the form of a hydrate.
The anion An- of the salt of Formula I described herein may be acetate. Thus,
there is
described a salt of Formula I as described herein which comprises or consists
of calcium
acetate and/or zinc acetate. Further, the salt of Formula I may comprise or
consist of
magnesium acetate and/or iron acetate. It will be appreciated that any of the
aforementioned salts may be provided in the form of a hydrate.
In an example, the pH adjusting agent comprises or consists of Na2003 and
CaCl2. In a
further example the pH adjusting agent comprises or consists of Na2003, NaHCO3
and
CaCl2. In a further example, the pH adjusting agent comprises or consists of
K2003 and
CaCl2. In yet a further example, the pH adjusting agent comprises or consists
of NaHCO3
and CaCl2. In still a further example, the pH adjusting agent comprises or
consists of
KHCO3 and CaCl2.
In an example, the pH adjusting agent comprises or consists of (i) Na2003 and
NaHCO3
and (ii) CaCl2 or a hydrate thereof.
The pH adjusting agent as described hereinmay be present in an amount from
about 4
wt% to about 9 wt% based on the total weight of the filling material of the
oral pouched
nicotine product. Further, the amount of water may be within the range of from
about 0.5
wt% to about 12 wt% or from about 0.5 wt% to about 3 wt%, based on the total
weight of
the filling material of the oral pouched nicotine product, and/or the amount
of filling
material may be within the range of from about 60 wt% to about 90 wt% or from
about 30
wt% to about 85 wt%, based on the total weight of the filling material of the
oral pouched
nicotine product. Further, the non-particulate non-tobacco material may
comprise or
consist of maltitol and/or microcrystalline cellulose. In an example, the non-
particulate
non-tobacco material comprises or consists of microcrystalline cellulose and
optionally
maltitol.
The pH adjusting agent described herein may comprise or consist of (i) Na2003
and (ii)
CaCl2 or a hydrate thereof. The pH adjusting agent may be present in an amount
from
about 1.5 wt% to about 4 wt% based on the total weight of the filling material
of the oral
pouched nicotine product. Further, the amount of water may be within the range
of from
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
14
about 20 wt% to about 45 wt%, based on the total weight of the filling
material of the oral
pouched nicotine product, and/or the amount of filling material may be within
the range of
from about 30 wt% to about 90 wt% or from about 30 wt% to about 85 wt%, based
on the
total weight of the filling material of the oral pouched nicotine product.
Further, the non-
particulate non-tobacco material may comprise or consist of cellulose and/or
microcrystalline cellulose.
The oral pouched nicotine product described herein may be free from sodium
chloride, i.e.
NaCI. Alternatively, it may comprise NaCI in an amount within the range of
from about 0.1
wt% to about 5 wt% such as from about 0.1 wt% to about 3 wt%, based on the
total
weight of the filling material.
The oral pouched nicotine product described herein may further comprise MgCO3.
Additionally or alternatively, the oral pouched nicotine product described
herein may
comprise CaCO3 and/or dolomite.
The oral pouched nicotine product described herein may comprise no further
salt and/or
no further pH adjuster.
The filling material of the oral pouched nicotine product described herein may
comprise
particulate non-tobacco material within the range of from about 30 wt% to
about 90 wt%,
such as from about 30 wt% to about 85 wt%, such as from about 30 wt% to about
80 wt%,
such as from about 60 wt% to about 90 wt%, based on the total weight of the
filling
material. Further, the filling material described herein may be water-
insoluble optionally
further including water soluble filling material. Thus, at least part of the
filling material of
the oral pouched nicotine product described herein may be water-insoluble such
as water-
insoluble at room temperature and/or at atmospheric pressure.
The particulate non-tobacco material may be water-insoluble, water-soluble or
a
combination thereof.
The particulate non-tobacco material may comprise or consist of a sugar
alcohol such as
maltitol, and/or of cellulose such as microcrystalline cellulose and/or
powdered cellulose.
For instance, the particulate non-tobacco material may comprise maltitol
and/or
microcrystalline cellulose.
Additionally or alternatively, the filling material of the oral pouched
nicotine product
described herein may comprise one or more water-insoluble fibers selected from
the
group consisting of maize fibers, oat fibers, tomato fibers, barley fibers,
rye fibers, sugar
beet fibers, buck wheat fibers, wheat fibers, pea fibers, potato fibers, apple
fibers, cocoa
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
fibers, bamboo fibers, citrus fibers, and any combination thereof. In an
example, the
water-insoluble fibers may form part of the non-tobacco particulate material.
The filling material may comprise one, two or more nicotine sources.
The filling material of the oral pouched nicotine product as described herein
may comprise
5 within the range of from about 1.0 % to about 10 % by weight of the nicotine
source,
based on the total weight of the filling material.
The nicotine source may be a nicotine salt and/or nicotine base. The nicotine
source such
as nicotine base may be bound to an ion exchange resin, such as polacrilex,
e.g. via a
salt bridge. Alternatively or additionally, the ion exchange resin may
function as a solid
10 support for the nicotine source such as nicotine base.
Nicotine base, such as in the form of an oily liquid, may be synthetically
produced or
extracted from tobacco.
The nicotine source described herein may comprise or consist of one or more of
the
following: nicotine hydrochloride, nicotine dihydrochloride, nicotine
monotartrate, nicotine
15 bitartrate, nicotine bitartrate dihydrate, nicotine sulphate, nicotine zinc
chloride
monohydrate, nicotine salicylate, nicotine polacrilex.
The nicotine source may be a nicotine salt such as a nicotine salt selected
from the group
consisting of nicotine hydrochloride, nicotine dihydrochloride, nicotine
monotartrate,
nicotine bitartrate, nicotine bitartrate dihydrate, nicotine sulphate,
nicotine zinc chloride
monohydrate and nicotine salicylate, and any combination thereof.
In particular, the filling material may comprise nicotine bitartrate and/or
nicotine bitartrate
dihydrate.
The amount of nicotine source such as nicotine salt and/or nicotine base per
pouched
product may be within the range from about 0.1 mg to about 20 mg of nicotine
calculated
as nicotine base, such as about 0.5 mg, about 1.0 mg, about 1.5 mg, about 2.0
mg, about
2.5 mg, about 3.0 mg, about 3.5 mg, about 4.0 mg, about 4.5 mg, about 5.0 mg,
about 6.0
mg, about 7.0 mg, about 8.0 mg, about 9.0 mg, about 10 mg, about 12 mg, about
14 mg,
about 16 mg, about 18 mg, or about 20 mg of nicotine.
The nicotine salt of the filling material in the oral pouched nicotine product
as disclosed
herein may be a nicotine salt present in solid form and/or dissolved form.
The nicotine source as disclosed herein may be adsorbed or non-adsorbed onto
the
particulate non-tobacco material as disclosed herein. It will be appreciated
that the
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
16
expression "adsorbed onto" means that the nicotine source adheres to an outer
surface of
the non-tobacco particulate material. When the nicotine source is adsorbed
onto the non-
tobacco particulate material it adheres to the outer surface of said non-
tobacco particulate
material without substantially penetrating into any void(s) of said non-
tobacco particulate
material.
Alternatively or additionally, the nicotine source as disclosed herein may be
absorbed into
and/or adsorbed onto the tobacco material described herein.
The filling material of the oral pouched nicotine product described herein may
further
comprise a flavouring agent. The filling material may comprise one, two or
more flavouring
agents. For example the flavouring agent may be a non-encapsulated agent.
Additionally
or alternatively, the flavouring agent may be encapsulated. The non-
encapsulated
flavouring agent and the encapsulated flavouring agent may be the same or
different. As
used herein, an encapsulated flavouring agent is a flavouring agent contained
within a
capsule. Accordingly, a non-encapsuled flavouring agent is not contained
within a
capsule.
The flavouring agent of the filling material in the oral pouched nicotine
product as
disclosed herein may be a hydrophobic flavouring agent.
The flavouring agent of the filling material of the oral pouched nicotine
product described
herein may be an oil, a liquid, a lyophilized material, a spray-dried
material, or a mixture
thereof. In an example, the flavouring agent(s) is/are an oil and/or a liquid.
The filling material of the oral pouched nicotine product described herein may
comprise
within the range of from about 0.5 % to about 3 % by weight of the flavouring
agent,
based on the total weight of the filling material.
The filling material of the oral pouched nicotine product described may
comprise a
humectant such as polypropylene glycol.
In the oral pouched nicotine product as described herein, the particulate non-
tobacco
material, the nicotine source, the water, the pH adjusting agent, optionally
the tobacco
material, optionally the flavouring agent and optionally the humectant may be
homogeneously mixed.
Anatabine is one of the minor alkaloids found in plants in the Solanaceae
family, which
inter alia includes the tobacco plant. The oral pouched nicotine non-tobacco
product
described herein may be free from tobacco, i.e. may contain no tobacco
material, and
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
17
may then consequently be free from anatabine. Alternatively, the oral pouched
nicotine
product described herein may contain a small amount of tobacco material, such
as from
about 0.1% to about 10% by weight as described herein, and may then not
comprise any
anatabine except for the anatabine present in said tobacco material.
The oral pouched nicotine product described herein may be free from glycerides
such
triglycerides. Alternatively, the oral pouched nicotine product described
herein may
comprise glycerides such as triglycerides.
The filling material of the oral pouched nicotine product as described herein
may be
manufactured using a method comprising the step(s) of:
- providing a mixture comprising a particulate non-tobacco material, a
nicotine source
such as a nicotine salt, and water, said mixture being provided by mixing the
aforementioned components in any order,
wherein a pH adjusting agent as defined herein is added before, in and/or
after any of the
foregoing step(s), optionally a flavouring agent is added before, in and/or
after any of the
foregoing step(s), optionally a humectant is added before, in and/or after any
of the
foregoing step(s), and optionally a tobacco material is added in and/or after
any of the
foregoing step(s). The amount of the particulate non-tobacco material, the
nicotine
source, the water, the optional flavouring agent, the optional humectant and
the optional
tobacco material may be as described herein.
Further, the filling material of the oral pouched nicotine product as
described herein may
be manufactured using a method comprising the steps of:
- providing a powder comprising or consisting of a mixture comprising a
particulate non-
tobacco material, a nicotine source, a pH adjusting agent, water and
optionally a tobacco
material, and
- granulating said powder.
The method may further comprise a step of enclosing the filling material in a
saliva-
permeable pouch of a packaging material.
There is also provided a filling material and/or an oral pouched nicotine
product obtained
or obtainable by the method described herein.
The pH of the oral pouched nicotine product, such as the filling material of
said oral
pouched nicotine product, may be within the range of from about 7.0 to about
10.0, such
as a pH within the range of from about 7 to about 9, such as from about 7 to
about 9.5,
such as from about 7 to about 9.2, such as from about 8 to about 9, such as
about 8, such
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
18
as about 8.3, such as about 8.5 or such as about 8.8, when it is dispersed in
purified
water. As described herein, these pH values are favourable for nicotine
extraction and
taste while not negatively affecting oral mucous membranes. The pH may be
measured
as described herein. Further, the oral pouched nicotine product may exhibit
these pH
values upon manufacture and/or also upon storage.
For example, the pH of the filling material may be measured by adding 100
milliliters (ml)
of water such as distilled water to 5.0 grams of filling material to provide a
mixture,
followed by stirring of said mixture such as stirring at 100 rpm for about 5
minutes and
then measuring the pH on at least part of said mixture. The pH of the filling
material
described herein may be measured at room temperature such as room temperature
at
atmospheric pressure. A pH meter may be used for the measurement. In this
document,
"rpm" stands for revolutions per minute. In this document, room temperature
intends a
temperature from about 20 C to about 25 C, such as about 22 C. The
atmospheric
pressure may be a pressure of about 101,325 Pa, i.e. 101,325 Pascal.
Thus, there is provided an oral pouched nicotine product as described herein,
wherein the
pH of said product does not exceed about 10, does not exceed about 9.5 or does
not
exceed about 9 upon storage. Additionally or alternatively, the pH of the oral
pouched
nicotine product changes by no more than 0.5 pH units upon storage. The
storage may
take place at a relative humidity from about 60% to about 75%, at a
temperature from
about 22 C to about 30 C and/or for a time of about 15 weeks. In an example,
the storage
takes place at a relative humidity at about 75%, at a temperature at about 30
C and/or for
a time of about 15 weeks. In a further example, the storage takes place at
about 30 C
and/or for a time of about 15 weeks.
The storage described herein may take place in a container such as a packaging
and/or
consumer container suitable for oral pouched nicotine products. Thus, the
container
described herein may be adapted for being conveniently carried in a consumer
pocket or
handbag, and/or may also be used for packaging any known type of snuff product
such as
oral pouched nicotine products as described herein. The container may be made
of
plastics and/or metal. Further, the container may have any desired shape or
geometrical
form. For example, the container may have the form of a cylinder. The
container may
comprise a top and a base defining an interior space. The base may comprise a
base
surface and surrounding walls extending from said base surface. The top may be
in the
form of a lid that is detachable from the base of the container, or in the
form of a lid that is
hinged or otherwise attached to the base of the container. For example, the
lid may be
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
19
attached by to the base by snap-fit. The container may be tamper resistant. In
an
example, the container may be as described in WO 2017/125405 which is
incorporated
herein in its entirety. In a further example, the container may be as shown in
the Design
Registration in Norway No. 085548.
Component (i) of the pH adjusting agent of the oral pouched nicotine product
described
herein may comprise or consist of one or more of the following: Na2CO3, K2CO3,
NaHCO3,
KHCO3. Further, the pH adjusting agent of the oral pouched nicotine product
described
herein may consist of:
(i) one or more of the following: Na2CO3, K2CO3, NaHCO3, KHCO3, and
(ii) a salt of Formula I as described herein.
For example, the pH adjusting agent of or in the oral pouched nicotine product
as
described herein may comprise or consist of:
(i) Na2CO3, K2CO3, NaHCO3 and/or KHCO3, and
(ii) a salt of Formula (I):
m2,-(An-)m
Formula I
or a hydrate of said salt,
wherein
M2+ is selected from the group consisting of Ca2+, Mg2+, Mn2+, Zn2+ and Fe2+,
An- is an anion selected from the group consisting of chloride, lactate,
malate, succinate,
citrate, ascorbate, tartrate, acetate, phosphate, sulphate, nitrate,
gluconate, alginate,
ammonium, oxalate, aspartate, glycinate, picolinate, oxide, cysteinate,
glutamate,
hydroxide, laurate, stearate, palmitate, undecylenate, gluceptate,
glucerophosphate,
glubionate, glucoheptonate, guanylate, inosinate, propionate, sorbate,
salicylate,
benzoate, erythorbate, formate, iodide, pangamate and any combination thereof,
n is 1 0r2,
m is 1 or 2, and
-(n x m)= -2.
In an example, there is provided a pH adjusting agent as described herein in
which:
M2+ is selected from the group consisting of Ca2+ and Mg2+; and/or
An- is selected from the group consisting of chloride, lactate, malate,
citrate, ascorbate,
tartrate, acetate, gluconate, aspartate and glycinate.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
In an example, there is provided a pH adjusting agent as described herein in
which:
M2+ is selected from the group consisting of Mn2+, Zn2+ and Fe2+, and/or
An- is selected from the group consisting of chloride, lactate, malate,
citrate, ascorbate,
tartrate, acetate, gluconate, aspartate and glycinate.
5 In one particular example, the pH adjusting agent described herein comprises
or consists
of (i) Na2003 and/or NaHCO3, and (ii) CaCl2. In further example, the pH
adjusting agent
may comprise or consist of (i) K2003 and/or KHCO3, and (ii) CaCl2. The CaCl2
may be
present in the form of a hydrate such as a monohydrate.
As described herein, an oral pouched nicotine product lacking the salt of
Formula I
10 increases its pH when it is stored. Unexpectedly, when the salt of Formula
I is combined
with Na2003, K2003, NaHCO3 and/or KHCO3, such as in amounts as described
herein,
this results in a pH adjusting agent that allows for:
(i) controlling, such as mitigating, reducing, preventing and/or counteracting
an increase in
pH of an oral pouched nicotine product when said product is stored,
15 (ii) preventing pH of an oral pouched nicotine product from exceeding a
value of about 9,
of about 9.2 or of about 9.5 when said product is stored, and/or
(iii) preventing pH of an oral pouched nicotine product from changing by more
than 0.5
pH units, such as changing by more than 0.5 pH units from an initial pH
value, when
said product is stored. Thus, the pH adjusting agent allows for one or more of
the
20 following:
(i) controlling, such as mitigating, reducing, preventing and/or counteracting
an increase in
pH of an oral pouched nicotine product when said product is stored,
(ii) preventing pH of an oral pouched nicotine product from exceeding a value
of about 9,
of about 9.2 or of about 9.5 when said product is stored,
(iii) preventing pH of an oral pouched nicotine product from changing by more
than 0.5
pH units, such as changing by more than 0.5 pH units from an initial pH
value, when
said product is stored.
It will be appreciated that the expression " 0.5 pH units" used herein may
intend + 0.5 pH
units and/or ¨ 0.5 pH units.
Thus, there is provided a use of a salt of Formula I, or a hydrate of said
salt, as described
herein for controlling pH in or of an oral pouched nicotine product comprising
a filling
material, said filling material comprising said salt of Formula I, or a
hydrate thereof, and
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
21
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total weight
of the filling material, and
- a pH adjusting agent comprising or consisting of Na2003, K2003, NaHCO3
and/or
KHCO3, and
- optionally a tobacco material within the range of from 0.05 wt% to 10 wt
% based on the
total weight of the filling material,
when said product is stored, such as stored
at a relative humidity from 60% to 75%,
at a temperature from 22 C to 30 C, and/or
for a time of 15 weeks.
The salt of Formula I, or a hydrate thereof, may be mixed with the other
components of
the filling material. For instance, the salt of Formula I, or a hydrate
thereof, may be
substantially homogenously mixed with the other components of the filling
material.
It will be appreciated that the amount of the filling material components may
be as
described in this document. For instance, the salt of Formula I such as CaCl2,
Na2CO3,
K2CO3, NaHCO3 and/or KHCO3 may be present in amounts as described herein. In
an
example, the salt of Formula I such as CaCl2, Na2CO3, K2CO3, NaHCO3 and/or
KHCO3
may be present in an amount such as a total amount from 1 wt% to 15 wt%, such
as from
1 wt% to 10 wt%, such as from 1 wt% to 9 wt%, such as from 1 to 7 wt% or such
as from
1 wt% to 5 wt%, based on the total weight of the filling material of said oral
pouched
nicotine product.
The controlling may comprise or consist of:
(i) mitigating an increase in pH in or of said product when said product is
stored,
(ii) preventing pH in or of said product from exceeding a value of about 9, of
about 9.2 or
of about 9.5 when said product is stored, and/or
(iii) preventing pH in or of said product from changing by more than 0.5 pH
units, such
as changing by more than 0.5 pH units as compared to pH of a corresponding
freshly
prepared product, when said product is stored. Thus, the controlling may
comprise or
consist of one or more of the following:
(i) mitigating an increase in pH in or of said product when said product is
stored,
(ii) preventing pH in or of said product from exceeding a value of about 9, of
about 9.2 or
of about 9.5 when said product is stored,
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
22
(iii) preventing pH in or of said product from changing by more than 0.5 pH
units, such
as changing by more than 0.5 pH units as compared to pH of a corresponding
freshly
prepared product, when said product is stored.
The storage described herein may take place at a relative humidity from about
60% to
about 75%, at a temperature from about 22 C to about 30 C and/or for a time of
about 15
weeks. In an example, the storage takes place at a relative humidity at about
75%, at a
temperature at about 30 C and/or for a time of about 15 weeks. In a further
example, the
storage takes place at about 30 C and/or for a time of about 15 weeks.
The present disclosure also provides a method for controlling pH in or of an
oral pouched
nicotine product, such as an oral pouched nicotine product as described
herein, when
said product is stored. The controlling may be as described herein. The method
involves
use of a pH adjusting agent as described herein. The method may comprise or
consist of
preparing an oral pouched nicotine product as described herein followed by
storage of
said product. The storage may be as described herein.
The controlling of pH as described herein may impart an improved storage
stability such
as improved storage stability of an oral pouched nicotine product as described
herein. In
particular, the storage stability may be improved with respect to a
corresponding oral
pouched nicotine product lacking the salt of Formula I.
It will be appreciated that the oral pouched nicotine product described herein
may be
considered a portion packed oral nicotine product. The filling material may be
in the form
of a particles, granules, beads, a powder such as a dry powder or a moist
powder, a dry
mixture or a moist mixture. Further, the present disclosure is also directed
to the filling
material as described herein, i.e. the filling material as such excluding any
packaging such
as a pouch.
EXAMPLES
Example 1: Substantially dry oral pouched tobacco free nicotine product
Five granulates were prepared by mixing anhydrous calcium chloride (supplied
by Merck
GmbH, Germany), nicotine bitartrate dihydrate (supplied by Siegfried AG,
Switzerland),
sodium bicarbonate (supplied by TATA Chemicals, UK) and sodium carbonate
(supplied
by Novacarb, France) with fillers such as sugar alcohol and cellulose fiber
according to
Table 1B below.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
23
Table 1A
Sample Calcium Nicotine Sodium Sodium Fillers Start
chloride bitartrate bicarbonate carbonate (w/w%) moisture
(w/w%) dihydrate (w/w%) (w/w%) (w/w%)
(w/w%)
1 0 2.51 2.33 1.91 93.25 1.08
2 0.1 2.51 2.33 1.91 93.15 1.02
3 1 2.51 2.33 1.91 92.25 1.38
4 5 2.51 2.33 1.91 88.25 2.00
10 2.51 2.33 1.91 83.25 3.86
The moisture content in Table 1A was determined as described herein using the
method
in literature references Federal Register/ vol.74, no. 4/712-719/Wednesday,
January 7,
5 2009/Notices "Total moisture determination" and AOAC (Association of
Official Analytical
Chemics), Official Methods of Analysis 966.02: "Moisture in Tobacco" (1990),
Fifth
Edition, K. He!rich (ed).
The granulates were packed into portion pouches of 0.4 g each using a water
permeable
nonwoven pouch material. The pouches were packed into plastic cans suitable
for
containment of oral pouches. The plastic cans were as described in WO
2017/125405.
The pH was analysed for the fresh samples before storage, using the herein
described
method. The plastic cans were thereafter transferred to a climate cabinet
(VC0100,
\kitsch lndustrietechnik) for storage. The cabinet conditions were set at 35
C and 75 %
RH. Storage was done for 15 weeks. After 2, 4, 6, 8, 10 and 15 weeks cans from
each
sample were taken out of the climate cabinet and the pH of the pouches was
analyzed,
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
24
using the herein defined method. In this document, "g" stands for gram(s) and
"RH"
stands for relative humidity.
The results are shown in Figure 1 and Table 1B. It can be seen that the
reference without
calcium chloride (Sample 1) increased by more than 1 pH unit after storage for
15 weeks.
Thus, the pH of the oral pouched tobacco free nicotine product lacking CaCl2
increased
upon storage. In contrast, the pH of samples 2-5, which contained a
combination of
calcium chloride, sodium bicarbonate and sodium carbonate, increased by 0.45
pH units
or less upon storage. Further, after storage the pH of samples 2-5 was from
7.40 to 8.61.
Thus, pH increased less in the presence of a salt such as CaCl2.
Table 1B
Sample pH at 0 weeks pH at 15 weeks pH change at
weeks
1 8.54 9.58 1.04
2 8.57 8.52 -0.05
3 8.16 8.61 0.45
4 7.78 7.58 -0.20
5 7.86 7.44 -0.42
Example 2: Snus products
This is a comparative example in which it is shown how the pH of snus changes
upon
storage.
15 Two batches of snus (GR One and General One) were prepared in a standard
snus
production procedure using production scale equipment and the composition
disclosed in
Table 2. Tobacco, fibre and water were mixed in a blender. The blend was
heated to
100 C and the temperature kept between 85-100 C for 4.5 hours. Before
cooling sodium
chloride, sodium carbonate, propylene glycol and additional water was added
and mixed
into the snus blend. After cooling a final addition of flavour and additional
sodium chloride
were added and mixed into the snus blend. The snus was packed into water
permeable
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
nonwoven pouch material and the pouches packed into plastic cans suitable for
containment of oral pouches. The plastic cans were as shown in the Design
Registration
in Norway No. 085548.
The pH was analyzed for the fresh samples before storage, using the herein
described
5 method. The plastic cans were thereafter transferred to a climate cabinet
for storage. The
cabinet conditions were set at 22 C and 60 % RH. Storage was done for 17
weeks. After
1, 3, 5, 10 and 17 weeks cans from each sample were taken out of the climate
cabinet
and the pH of the pouches was analyzed, using the herein defined method.
The results are shown in Figure 2. It can be seen that in both snus samples
the pH-value
10 follows a typical pH-development for snus during storage. This means that
the pH
decreased steadily. After storage for 17 weeks the pH decreased approximately
by 0.6
pH-units, as compared to the initial value. Thus, the pH of tobacco containing
snus
decreases upon storage.
Table 2
Sample Tobacco Water Sodium Sodium Fibre,
flour (w/w /0) (w/w /0) chloride carbonate propylene
(w/w%) (w/w%) glycol,
flavour,
pouch paper
(w/w%)
GR One 44.7 39.1 3.4 3.2 9.6
General One 44.7 38.8 3.4 3.2 9.9
Example 3: Moist oral tobacco free nicotine product
Two batches (Ang-124 and Ang-125) of a moist oral nicotine product were
prepared in lab
scale (500 g per batch) according to the composition in Table 3A. Dry
ingredients
(nicotine bitartrate, sodium carbonate, calcium chloride (only in Ang-124),
cellulose,
sodium chloride and sweetener were mixed in a lab mixer (Kenwood) to a
homogenous
powder blend. Water, glycerol and vegetable oil were added followed by
continued
mixing. The water was present in an amount of about 30-40 wt% based on the
total
amount of the composition.
Thereafter flavour and sweetener were added and the blend mixed to a final
oral nicotine
blend. The pH was analyzed for the fresh samples before storage, using the
herein
described method.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
26
Table 3A
Sample Nicotine Sodium Calcium Water, Cellulose,
bitartrate carbonate chloride glycerol, sodium
(w/w%) (w/w%) (w/w%) vegetable chloride,
oil (w/w%) flavour,
sweetener
(w/w%)
Ang-124 4.6 2.3 1.5 46.6 45.0
Ang-125 4.6 2.5 0 46.7 46.2
The two samples of oral nicotine blend were distributed in plastic cans
suitable for oral
pouched nicotine products. The plastic cans were thereafter sealed with a side
label and
transferred to a climate cabinet for storage. The plastic cans were as shown
in the Design
Registration in Norway No. 085548. The cabinet conditions were set at 22 C
and 60 %
RH. Storage was done for 15 weeks. After 2, 4, 8, 10 and 15 weeks cans from
each
sample were taken out of the climate cabinet and the pH of the pouches was
analyzed,
using the herein defined method.
The results are shown in Figure 3 and Table 3B. It can be seen that the pH of
the
reference without calcium chloride (Ang-125) increased by 0.86 pH unit upon
storage for
weeks. In contrast, the pH of the sample comprising calcium chloride (Ang-124)
increased only by 0.40 pH units upon storage. The pH after storage for the
sample Ang-
124 was 9.04. Thus, pH increased less in the presence of a salt such as CaCl2.
15 Table 3B
Sample pH at 0 weeks pH at 15 weeks pH change at
15 weeks
Ang-124 8.63 9.04 0.4
Ang-125 8.81 9.67 0.86
Method for measurement of pH
The pH of the filling material of the above-mentioned examples was measured by
adding
100 ml of distilled water to 5.0 gram of filling material, in a 125 ml beaker,
stirring the
resulting mixture at room temperature with a magnetic stirrer at 100 rpm for
about 5
minutes, and then measuring the pH of an extract obtained therefrom with a
calibrated
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
27
(according to the manufacturer's instructions) pH meter. For correctness of
readings, the
sample solutions were analyzed within one hour. In this document, "rpm" stands
for
revolutions per minute. In this document, room temperature intends a
temperature from
about 20 C to about 25 C, such as about 22 C.
ITEMS
1. An oral pouched nicotine product comprising a filling material and a saliva-
permeable pouch of a packaging material enclosing the filling material, the
filling
material comprising:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total
weight of the filling material, and
- a pH adjusting agent comprising:
(i) Na2003, K2003, NaHCO3 and/or KHCO3, and
(ii) a salt of Formula (I):
Formula I
wherein
M2+ is selected from the group consisting of Ca2+, Mg2+, Mn2+, Zn2+ and Fe2+,
An- is an anion, wherein said anion is not 0032- or H003-,
n is 1 or 2,
m is 1 or 2, and
-(n x m)= -2,
said salt having a water solubility, such as a water solubility at a
temperature from
about 20 C to about 25 C, equal to or above 0.04 M,
-optionally a tobacco material within the range of from 0.05 wt% to 10 wt%,
based
on the total weight of the filling material.
2. An oral pouched nicotine product comprising a filling material and a saliva-
permeable pouch of a packaging material enclosing the filling material, the
filling
material comprising:
- a particulate non-tobacco material,
- a nicotine source,
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
28
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total
weight of the filling material, and
- a pH adjusting agent comprising:
(i) Na2003, K2003, NaHCO3 and/or KHCO3, and
(ii) a salt of Formula (I):
m2,-(An-)m
Formula I
wherein
M2+ is selected from the group consisting of Ca2+, Mg2+, Mn2+, Zn2+ and Fe2+,
An- is an anion selected from or, Br, 1, F, and conjugate bases of organic
acids
having a pKa equal to or above 4.7,
n is 1 or 2,
m is 1 or 2, and
-(n x m)= -2,
said salt having a water solubility, such as a water solubility at a
temperature from
about 20 C to about 25 C, equal to or above 0.04 M and/or being present in an
amount within the range of from 0.05 wt% to 7 wt%, such as in the range of
from
0.05 wt% to 5 wt%, based on the total weight of the filling material,
-optionally a tobacco material within the range of from 0.05 wt% to 10 wt%,
based
on the total weight of the filling material.
3. An oral pouched nicotine product comprising a filling material and a saliva-
permeable pouch of a packaging material enclosing the filling material, the
filling
material comprising:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 50 wt% based on the
total
weight of the filling material, and
- a pH adjusting agent comprising:
(i) Na2003, K2003, NaHCO3 and/or KHCO3, and
(ii) a salt of Formula (I):
m2,-(An-)m
Formula I
or a hydrate of said salt,
wherein
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
29
M2+ is selected from the group consisting of Ca2+, Mg2+, Mn2+, Zn2+ and Fe2+,
An- is an anion selected from the group consisting of chloride, lactate,
malate,
succinate, citrate, ascorbate, tartrate, acetate, phosphate, sulphate,
nitrate,
gluconate, alginate, ammonium, oxalate, aspartate, glycinate, picolinate,
oxide,
cysteinate, glutamate, hydroxide, laurate, stearate, palmitate, undecylenate,
gluceptate, glucerophosphate, glubionate, glucoheptonate, guanylate,
inosinate,
propionate, sorbate, salicylate, benzoate, erythorbate, formate, iodide,
pangamate
and any combination thereof,
n is 1 or 2,
m is 1 or 2, and
-(n x m)= -2
said salt being present in an amount within the range of from 0.05 wt% to 5
wt%,
based on the total weight of the filling material,
-optionally a tobacco material within the range of from 0.05 wt% to 10 wt%,
based
on the total weight of the filling material.
4. An oral pouched nicotine product according to any one of the preceding
items,
wherein said pH adjusting agent comprises or consists of
(i) Na2003 and optionally NaHCO3, and
(ii) a salt of Formula (I) or a hydrate thereof.
5. An oral pouched nicotine product according to any one of the preceding
items,
wherein An- is selected from the group consisting of chloride, lactate,
malate,
citrate, ascorbate, tartrate, acetate, gluconate, aspartate and glycinate.
6. An oral pouched nicotine product according to any one of the preceding
items,
wherein said salt of Formula (I), or hydrate thereof, has a water solubility,
such as
a water solubility at a temperature from about 20 C to about 25 C, equal to or
above about 0.04 M, such as above about 0.045 M, such as above about 0.05 M.
7. An oral pouched nicotine product according to any one of the preceding
items,
wherein the filling material does not comprise a tobacco material.
8. An oral pouched nicotine product according to any one of items 1-6, wherein
the
tobacco material is present in an amount within the range of from about 0.1
wt% to
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
about 10 wt%, such as from about 0.1 wt% to about 5 wt%, such as from about
0.1
wt% to about 1 wt%, based on the total weight of the filling material.
9. An oral pouched nicotine product according to any one of items 1-6 or 8,
wherein
5 the tobacco material is provided as tobacco fibers, ground tobacco and/or
snuff
such as snus.
10. An oral pouched nicotine product according to any one of items 1-6 or 8-9,
wherein the tobacco material comprises or consists of purified tobacco
material,
10 such as bleached tobacco material.
11. An oral pouched nicotine product according to any one of the preceding
items,
wherein the amount of water is within the range of from about 0.5 wt% to about
12 wt% such as from about 0.5 wt% to about 5 wt%, such as about 3 wt%, based
15 on the total weight of the filling material.
12. An oral pouched nicotine product according to any one of items 1-10,
wherein the
amount of water is within the range of from about 20 wt% to about 50 wt%, such
as from 20 wt% to 45 wt%, based on the total weight of the filling material.
13. An oral pouched nicotine product according to any one of the preceding
items,
wherein the salt of Formula (I), or a hydrate of said salt, is present in an
amount
within the range of from about 0.05 wt% to about 5 wt%, such as from about
0.05
wt% to about 3 wt%, such as from about 0.05 wt% to about 2 wt%, such as from
about 0.05 wt% to about 1 wt%, based on the total weight of the filling
material.
14. An oral pouched nicotine product according to any one of the preceding
items,
wherein the salt of Formula (I) or a hydrate of said salt is present in an
amount
within the range of from about 0.05 wt% to about 0.3 wt%, based on the total
weight of the filling material.
15. An oral pouched nicotine product according to any one of the preceding
items,
wherein the Na2003, K2003, NaHCO3 and/or KHCO3 of the pH adjusting agent
is/are present in an amount within the range of from about 1 wt% to about 10
wt%,
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
31
such as from about 1.5 wt% to about 4 wt% or such as from about 4 wt% to about
9 wt%, based on the total amount of the filling material.
16. An oral pouched nicotine product according to any one of the preceding
items,
wherein M2+ is Ca2+ or Mg2+.
17. An oral pouched nicotine product according to any one of items 1-15,
wherein M2+
is Mn2+, Zn2+ or Fe2+.
18. An oral pouched nicotine product according to any one of items 1-16,
wherein the
salt of Formula I or hydrate thereof comprises or consists of CaCl2 or a
hydrate
thereof.
19. An oral pouched nicotine product according to any one of the preceding
items,
wherein the filling material further comprises NaCI such as NaCI in an amount
within the range of from about 0.1 wt% to about 5 wt% such as from about 0.1
wt%
to about 3 wt%, based on the total weight of the filling material.
20. An oral pouched nicotine product according to any one of the preceding
items,
wherein the filling material further comprises CaCO3, MgCO3 and/or dolomite.
21. An oral pouched nicotine product according to any one of the preceding
items,
wherein said product comprises no further salt and/or no further pH adjusting
agent.
22. An oral pouched nicotine product according to any one of the preceding
items,
wherein said oral pouched nicotine product has a pH from about 7 to about 10,
such as from about 7 to about 9.5, such as from about 7 to about 9.2, such as
from about 7 to about 9, such as from about 8 to about 9, such as about 8,
such as
about 8.3, such as about 8.5 or such as about 8.8, when it is dispersed in
purified
water.
23. An oral pouched nicotine product according to any one of the preceding
items,
wherein the pH of said oral pouched nicotine product does not exceed a value
of
about 9; or about 9.2 or about 9.5 upon storage and/or changes by no more than
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
32
0.5 pH units upon storage,
such as storage taking place
at a relative humidity from about 60% to about 75 %,
at a temperature from about 22 C to about 30 C, and/or
for a time of about 15 weeks.
24. An oral pouched nicotine product according to any one of the preceding
items,
wherein the particulate non-tobacco material is present in an amount within
the
range of from about 30 wt% to about 90 wt%, such as from about 30 wt% to about
85 wt%, such as from about 30 wt% to about 80 wt%, based on the total weight
of
the filling material.
25. An oral pouched nicotine product according to any one of the preceding
items,
wherein the particulate non-tobacco material comprises a sugar alcohol such as
maltitol and/or cellulose such as microcrystalline cellulose and/or powdered
cellulose.
26. An oral pouched nicotine product according to any one of the preceding
items,
wherein the particulate non-tobacco material comprises maltitol and/or
microcrystalline cellulose.
27. An oral pouched nicotine product according to any one of the preceding
items,
wherein the filling material comprises one or more water-insoluble fibers
selected
from the group consisting of maize fibers, oat fibers, tomato fibers, barley
fibers,
rye fibers, sugar beet fibers, buck wheat fibers, wheat fibers, pea fibers,
potato
fibers, apple fibers, cocoa fibers, bamboo fibers, citrus fibers, and any
combination
thereof.
28. An oral pouched nicotine product according to item 27, wherein said water-
insoluble fibers form part of said particulate non-tobacco material.
29. An oral pouched nicotine product according to any one of the preceding
items,
wherein the filling material comprises within the range of from about 1.0 % to
about 10 % by weight of the nicotine source, based on the total weight of the
filling
material.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
33
30. An oral pouched nicotine product according to any one of the preceding
items,
wherein the nicotine source is a nicotine salt, nicotine base and/or nicotine
bound
to an ion exchange resin such as nicotine polacrilex.
31. An oral pouched nicotine product according to according to item 30,
wherein the
nicotine salt is selected from the group consisting of nicotine hydrochloride,
nicotine dihydrochloride, nicotine monotartrate, nicotine bitartrate, nicotine
bitartrate dihydrate, nicotine sulphate, nicotine zinc chloride monohydrate
and
nicotine salicylate, and any combination thereof.
32. An oral pouched nicotine product according to item 30 or item 31, wherein
the
amount of nicotine salt per pouched product may be within the range from about
0.1 mg to about 20 mg of nicotine calculated as nicotine base, such as about
0.5
mg, about 1.0 mg, about 1.5 mg, about 2.0 mg, about 2.5 mg, about 3.0 mg,
about
3.5 mg, about 4.0 mg, about 4.5 mg, about 5.0 mg, about 6.0 mg, about 7.0 mg,
about 8.0 mg, about 9.0 mg, about 10 mg, about 12 mg, about 14 mg, about 16
mg, about 18 mg, or about 20 mg of nicotine.
33. An oral pouched nicotine product according to any one of the preceding
items,
wherein the nicotine source is a nicotine salt present in solid form and/or
dissolved
form.
34. An oral pouched nicotine product according to any one of the preceding
items,
wherein the nicotine source is adsorbed onto the particulate non-tobacco
material.
35. An oral pouched nicotine product according to any one of the preceding
items,
wherein the filling material further comprises a flavouring agent.
36. An oral pouched nicotine product according to item 35, wherein the
flavouring
agent is a hydrophobic flavouring agent.
37. An oral pouched nicotine product according to any one of items 35 or 36,
wherein
the flavouring agent is an oil, a liquid, a lyophilized material, a spray-
dried material,
or a mixture thereof.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
34
38. An oral pouched nicotine product according to any one of items 35-37,
wherein the
filling material comprises within the range of from about 0.5 % to about 3 %
by
weight of the flavouring agent, based on the total weight of the filling
material.
39. An oral pouched nicotine product according to any one of the preceding
items,
wherein the flavouring agent is encapsulated and/or non-encapsulated.
40. An oral pouched nicotine product according to item 39, wherein the
encapsulated
flavouring agent is the same or different from the non-encapsulated flavouring
agent.
41. An oral pouched nicotine product according to any one of the preceding
items,
further comprising a humectant such as polypropylene glycol and/or glycerol.
42. An oral pouched nicotine product according to any one of the preceding
items,
wherein the particulate non-tobacco material, the nicotine source, the water,
the
pH adjusting agent, optionally the tobacco material, optionally the flavouring
agent
and optionally the humectant, are homogeneously mixed.
43. An oral pouched nicotine product according to any one of items 1-7 or 11-
42,
which does not comprise anatabine.
44. An oral pouched nicotine product according to any one of items 1-6 or 8-
42, which
does not comprise anatabine in addition to the anatabine present in the
tobacco
material.
45. A packaging container comprising the oral pouched nicotine product
according to
any one of the preceding items.
46. A method for manufacturing a filling material as defined in any one items
1-45,
said method comprising the steps of:
- providing a mixture comprising a particulate non-tobacco material, a
nicotine
source such as a nicotine salt, and water, said mixture being provided by
mixing
the the particulate non-tobacco material, the nicotine source and the water in
any
order,
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
wherein a pH adjusting agent as defined in any one of items 1-41 is added
before,
in and/or after any of the foregoing steps, optionally a flavouring agent is
added
before, in and/or after any of the foregoing steps, optionally a humectant is
added
before, in and/or after any of the foregoing steps, and optionally a tobacco
material
5 is added before, in and/or after any of the foregoing steps.
47. A method for manufacturing a filling material as defined in any one items
1-46,
said method comprising the steps of:
- providing a powder comprising or consisting of a mixture comprising a
particulate
10 non-tobacco material, a nicotine source, a pH adjusting agent, water and
optionally a tobacco material, and
- granulating said powder.
48. A method according to item 46 or 47, further comprising a step of
enclosing the
15 filling material in a saliva-permeable pouch of a packaging material.
49. A filling material obtained or obtainable by the method of item 46 or 47.
50. An oral pouched nicotine product obtained or obtainable by a method
according to
20 item 48.
51. Use of a salt of Formula (I) as defined in any one of the preceding items
for
controlling pH in or of an oral pouched nicotine tobacco free product, i.e. an
oral
pouched nicotine product lacking tobacco, when said product is stored such as
25 stored in a packaging container as described herein.
52. Use according to item 51, wherein said controlling comprises or consists
of
(i) mitigating an increase in said pH,
(ii) preventing said pH from exceeding a value of about 9, or a value of about
9.2,
30 or a value of about 9.2, and/or
(iii) preventing said pH from changing by more than 0.5 pH units.
53. Use of a salt of Formula (I) as defined in any one of the preceding items
for
mitigating an increase in pH in an oral pouched nicotine tobacco free product
35 when said product is stored.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
36
54. Use of a salt of Formula (I) as defined in any one of the preceding items
for
preventing pH in an oral pouched nicotine tobacco free product from exceeding
a
value of about 9, or a value of about 9.2, or a value of about 9.5, when said
product is stored.
55. Use of a salt of Formula (I) as defined in any one of the preceding items
for
preventing pH in an oral pouched nicotine tobacco free product changing by
more
than 0.5 pH units when said product is stored.
56. Use according to any one of items 51-55, wherein the oral pouched nicotine
tobacco free product comprises Na2003, NaHCO3, K2003 and/or KHCO3.
57. Use according to any one of items 51-56, wherein said product is stored
at a relative humidity from about 60% to about 75 %,
at a temperature from about 22 C to about 30 C, and/or
for a time of about 15 weeks.
58. Use according to any one of items 51-57, wherein the oral pouched nicotine
tobacco free product comprises a filling material as described herein such as
a
filling material comprising:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total
weight of the filling material, and
- a pH adjusting agent comprising or consisting of Na2CO3, K2CO3, NaHCO3
and/or KHCO3.
59. Use of a salt of Formula (I) as defined in any one of the preceding items
for
controlling pH in an oral pouched nicotine low tobacco product, i.e. an oral
pouched nicotine product comprising a tobacco material in an amount as
described herein, when said product is stored such as stored in a packaging
container as described herein.
60. Use according to item 59, wherein said controlling comprises or consists
of
(i) mitigating an increase in pH in or of said product,
(ii) preventing pH in or of said product from exceeding a value of about 9, or
a
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
37
value of about 9.2, or a value of about 9.5, and/or
(iii) preventing pH in or of said product from changing by more than 0.5 pH
units.
61. Use of a salt of Formula (I) as defined in any one of the preceding items
for
mitigating an increase in or of pH in an oral pouched nicotine low tobacco
product
when said product is stored.
62. Use of a salt of Formula (I) as defined in any one of the preceding items
for
preventing pH in or of an oral pouched nicotine low tobacco product from
exceeding a value of about 9, or a value of about 9.2, or a value of about
9.5,
when said product is stored.
63. Use of a salt of Formula (I) as defined in any one of the preceding items
for
preventing pH in or of an oral pouched nicotine low tobacco product from
changing
by more than 0.5 pH units when said product is stored.
64. Use according to any one of items 59-63, wherein said oral pouched low
tobacco
nicotine product comprises a filling material and optionally a saliva-
permeable
pouch enclosing said filling material, the filling material comprising a
tobacco
material in an amount within the range of from 0.05 wt% to 10 wt%, such as
from
from 0.1 wt% to 10 wt%, such as from from 0.1 wt% to 5 wt%, such as from 0.1
wt% to 3 wt%, such as from 0.1 wt% to 2 wt%, such as from 0.1 wt% to 1 wt%,
based on the total weight of said filling material.
65. Use according to any one of items 59-64, wherein the oral pouched nicotine
low
tobacco product comprises a filling material as described herein such as a
filling
material comprising:
- a particulate non-tobacco material,
- a nicotine source,
- water in an amount within the range of from 1 wt% to 45 wt% based on the
total
weight of the filling material, and
- a pH adjusting agent comprising or consisting of Na2003, K2003, NaHCO3
and/or KHCO3, and
- a tobacco material within the range of from 0.05 wt% to 10 wt % based on
the
total weight of the filling material.
CA 03127725 2021-07-23
WO 2020/157280 PCT/EP2020/052437
38
66. Use according to any one of items 59-65, wherein the said product is
stored
at a relative humidity from about 60% to about 75 %,
at a temperature from about 22 C to about 30 C, and/or
for a time of about 15 weeks.
67. Use according to any one of items 51-66, wherein the salt of Formula (I),
Na2003,
NaHCO3, K2003 and/or KHCO3 is/are present in a total amount from 1 wt% to 15
wt%, such as from 1 wt% to 10 wt%, such as from 1 wt% to 9 wt%, such as from 1
to 7 wt% or such as from 1 wt% to 5 wt%, based on the total weight of the
filling
material.
68. A filling material as described herein, such as a filling material defined
in any one
of items 1-44.