Note: Descriptions are shown in the official language in which they were submitted.
MULTILAYER SYSTEMS AND METHODS OF MAKING MULTILAYER SYSTEMS
[1] This application claims the benefit of U.S. Provisional Application No.
62/803,727 filed on February
11,2019.
FIELD
[2] The disclosure relates to methods of making multilayer systems
comprising at least one sealant layer,
multilayer systems made using the methods, and uses of multilayer systems.
Each individual layer of a
multilayer system can be designed to have a desired property. Individual
layers of a multilayer system can
also have an inhomogeneous concentration of one or more constituents within an
individual layer. The
multilayer systems can be made using extrusion methods such as three-
dimensional printing. The multilayer
systems can be used as sealants.
BACKGROUND
[31 Sealants are typically provided as homogeneous compositions that are
applied to a substrate. In one-
part systems the sealant is applied to a substrate and curing is initiated by
application of energy such as by
exposure to ultraviolet radiation. In two-part systems, the individual parts
are combined and mixed prior to
use and the curing reaction proceeds when the reactive components are
combined. Performance attributes for
cured sealants can include, for example, one or more of chemical resistance,
low-temperature flexibility,
hydrolytic stability, high temperature resistance, tensile strength,
%elongation, substrate adhesion, adhesion
to an adjoining layer, tack-free time, time to Shore 10A hardness, electrical
conductivity, EMI/REI shielding,
static dissipation, thermal conductivity, low density, corrosion resistance,
surface hardness, fire retardanc,e,
UV resistance, and rain erosion resistance. Multilayer systems having at least
one sealant and methods for
making the sealant systems having one or more of these attributes are
desirable.
SUMMARY
[4] According to the present invention, methods of making a multilayer
system comprising two or more
layers, wherein one or more of the layers comprises a sealant layer, comprise:
(a) mixing a first component
and a second component to form a coreactive sealant composition, wherein, the
coreactive sealant
composition comprises a first reactive compound and a second reactive
compound; and the first reactive
compound is reactive with the second reactive compound; (b) extruding the
coreactive sealant composition
to form an extrudate; and (c) depositing the extrudate to form the sealant
layer.
BRIEF DESCRIPTION OF THE DRAWINGS
[5] The drawings described herein are for illustration purposes only. The
drawings are not intended to
limit the scope of the present disclosure.
1
Date Regue/Date Received 2022-10-28
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[6] FIGS. 1A-ID show cross-sectional views of multilayer systems comprising
at least one sealant
layer provided by the present disclosure.
[7] FIG. 2A show cross-sectional views of a layer of a multilayer system
provided by the present
disclosure in which the concentration of a constituent varies within the
thickness of the layer.
[8] FIG. 2B show cross-sectional views of a layer of a multilayer system
provided by the present
disclosure in which the concentration of a constituent varies within a lateral
dimension of the layer.
[9] FIG. 3 shows a cross-sectional view of a multilayer system provided by
the present disclosure
that includes a coating.
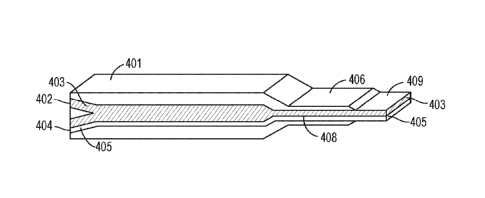
[10] FIG. 4 shows a cross-sectional view of an example of a coextruder.
DETAILED DESCRIPTION
[11] For purposes of the following detailed description, it is to be
undeistood that embodiments
provided by the present disclosure may assume various alternative variations
and step sequences, except
where expressly specified to the contrary. Moreover, other than in any
operating examples, or where
otherwise indicated, all numbers expressing, for example, quantities of
ingredients used in the
specification and claims are to be understood as being modified in all
instances by the term "about."
Accordingly, unless indicated to the contrary, the numerical parameters set
forth in the following
specification and attached claims are approximations that may vary depending
upon the desired properties
to be obtained by the present invention. At the very least, and not as an
attempt to limit the application of
the doctrine of equivalents to the scope of the claims, each numerical
parameter should at least be
construed in light of the number of reported significant digits and by
applying ordinary rounding
techniques.
[12] Notwithstanding that the numerical ranges and parameters setting forth
the broad scope of the
invention are approximations, the numerical values set forth in the specific
examples are reported as
precisely as possible. Any numerical value, however, inherently contains
certain errors necessarily
resulting from the standard variation found in their respective testing
measurements.
[13] Also, it should be understood that any numerical range recited herein
is intended to include all
sub-ranges subsumed therein. For example, a range of "1 to 10" is intended to
include all sub-ranges
between (and including) the recited minimum value of 1 and the recited maximum
value of 10, that is,
having a minimum value equal to or greater than 1 and a maximum value of equal
to or less than 10.
[14] "Allcanearene" refers to a hydrocarbon group having one or more aryl
groups and/or arenediyl
groups and one or more alkyl and/or alkanediyl groups, where aryl, arenediyl,
alkyl, and alkanediyl are
defined here. Each aryl and/or arenediyl group(s) can be C6-I2, C6-10, phenyl
or benzenediyl. Each alkyl
and/or alkanediylgroup(s) can be C1-6, C1-4, C1_3, methyl, methanediyl, ethyl,
or ethane-1,2-diyl. An
alkanearene group can be C4-18 alkanearene, Co alkanearene, C4-I2 alkanearene,
C4_8 alkanearene, C6-12
2
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
alkanearene, C6_10 alkanearene, or C6_9 alkanearene. Examples of alkanearene
groups include diphenyl
methane.
[15] "Alkanearenediyl" refers to a diradical of an alkanearene group. An
alkanearenediyl group can
be C6_18 alkanearenediyl, C6_16 alkanearenediyl, C6_12 alkanearenediyl, C68
alkanearenediyl, C6-12
alkanearenediyl, C6_10 alkanearenediyl, or C6-9 alkanearenediyl. Examples of
alkanearenediyl groups
include diphenyl methane-4,4'-diyl.
[16] "Alkanecycloalkane" refers to a saturated hydrocarbon group having one
or more cycloalkyl
and/or cycloalkanediyl groups and one or more alkyl and/or alkanediyl groups,
where cycloalkyl,
cycloalkanediyl, alkyl, and alkanediyl are defined herein. Each cycloalkyl
and/or cycloalkanediyl
group(s) can be C3-6, C68, cyclohexyl or cyclohexanediyl. Each alkyl and/or
alkanediyl group(s) can be
C1-6, C1-4, C1-3, methyl, methanediyl, ethyl, or ethane-1,2-diyl. An
alkanecycloalkane group can be C6-18
alkanecycloalkane, C6-16 alkanecycloalkane, C6-12 alkanecycloalkane, C6-8
alkanecycloalkane, C6-12
alkanecycloalkane, C6-10 alkanecycloalkane, or C6-9 alkanecycloalkane.
Examples of alkanecycloalkane
groups include 1,1,3,3-tetrarnethylcyclohexane and cyclohexylmethane.
[17] "Alkanecycloalkanediy1" refers to a diradical of an alkanecycloalkane
group. An
alkanecycloalkanediyl group can be C4_18 alkanecycloalkanediyl, C4-16
alkanecycloalkanediyl, C4-12
alkanecycloalkanediyl, C68 alkanecycloalkanediyl, C6-12 alkanecycloalkanediyl,
C6_10
alkanecycloalkanediyl, or C6-9 alkanecycloalkanediyl. Examples of
alkanecycloalkanediyl groups include
1, 1,3,3-tetratnethylcyclohexane-1,5-diy1 and cyclohexylmethane-4,4'-diyl.
[18] "Allcanediy1" refers to a diradical of a saturated, branched or
straight-chain, acyclic hydrocarbon
group, having, for example, from 1 to 18 carbon atoms (C1_18), from 1 to 14
carbon atoms (C1_14), from 1
to 6 carbon atoms (C14, from 1 to 4 carbon atoms (C14), or from 1 to 3
hydrocarbon atoms (Ci_3). It will
be appreciated that a branched alkanediyl has a minimum of three carbon atoms.
An alkanediyl can be
C2-14 alkanediyl, C2-10 alkanediyl, C2-8 alkanediyl, C2-6 alkanediyl, C2-4
alkanediyl, or C2-3 alkanediyl,
Examples of alkanediyl groups include methane-diyl (-CH2-), ethane-1,2-diy1 (-
CH2CH2-), propane-1,3-
diyl and iso-propane-1,2-diy1 (e.g., -CH2CH2CH2- and -CH(CH3)CH2-), butane-1,4-
diy1 (-
CH2CH2CH2CH2-), pentane-1,5-diy1 (-CH2CH2CH2CH2CH2-), hexane-1,6-diy1 (-
CH2CH2CH2CH2CH2CH2-), heptane-1,7-diyl, octane-1,8-diyl, nonane-1,9-diyl,
decane-1,1 0-diyl, and
dodecane-1,12-diyl. Alkanediyl groups can include single, double, and/or
triple bonds between carbon
atoms.
[19] "Alkenyl" group refers to the structure -CR=C(R)2 where the alkenyl
group is a group and is
bonded to a larger molecule. In such embodiments, each R may independently
comprise, for example,
hydrogen and C1-3 alkyl. Each R can be hydrogen and an alkenyl group can have
the structure -C1-1H.2.
3
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[20] "Alkoxy" refers to a ¨OR group where R is alkyl as defined herein.
Examples of alkoxy groups
include methoxy, ethoxy, n-propoxy, isopropoxy, and n-butoxy. An alkoxy group
can be C1_8 alkoxy, C1-6
alkoxy, C1-4 alkoxy, or C1-3 alkoxy.
[21] "Alkyl" refers to a monoradical of a saturated, branched or straight-
chain, acyclic hydrocarbon
group having, for example, from 1 to 20 carbon atoms, from 1 to 10 carbon
atoms, from 1 to 6 carbon
atoms, from 1 to 4 carbon atoms, or from 1 to 3 carbon atoms. It will be
appreciated that a branched alkyl
has a minimum of three carbon atoms. An alkyl group can be C1-6 alkyl, C1_4
alkyl, or C1-3 alkyl.
Examples of alkyl groups include methyl, ethyl, n-propyl, iso-propyl, n-butyl,
iso-butyl, tert-butyl, n-
hexyl, n-decyl, and tetradecyl. An alkyl group is C1_6 alkyl, C1_4 alkyl, and
C1-3 alkyl.
[22] "Arenediyl" refers to diradical monocyclic or polycyclic aromatic
group. Examples of arenediyl
groups include benzene-diyl and naphthalene-diyl. An arenediyl group can be
C6_12 arenediyl, C6-10
arenediyl, C6-9 arenediyl, or benzene-diyl.
[23] "Catalyst" refers to a substance that increases the rate of a reaction
without modifying the overall
standard Gibbs energy change in the reaction.
[24] When reference is made to a chemical group defined, for example, by a
number of carbon atoms,
the chemical group is intended to include all sub-ranges of carbon atoms as
well as a specific number of
carbon atoms. For example, a C2_10 alkanediyl includes a C2-4 alkanediyl, C5-7
alkanediyl, and other sub-
ranges, a C2 alkanediyl, a C6 alkanediyl, and alkanediyls having other
specific number(s) of carbon atoms
from 2 to 10.
[25] "Coating" refers to a thin film such as a film having an applied and
dried thickness less than 500
pm, less than 100 gm, or less than 50 pm. A coating can have a thickness less
than that of a layer
forming a multilayer system.
[26] "Component" refers to a composition in which the constituents of the
component are not
coreactive until combined and mixed with another component to form a
coreactive composition.
[27] A compound having a reactive functionality refers to a compound that
has functional group
capable of reacting with a complimentary reactive functional group of another
compound. The reactive
functional group can be bonded to the ends of the compound, may be bonded to
the backbone of the
compound.
[28] "Constituent" refers to an organic compound or an inorganic compound.
A composition and a
component can comprise one or more constituents. Examples of constituents
include prepolymers,
monomers, polyfunctionalizing agents, and additives as disclosed herein.
[29] A "core" of a polyfunctionalizing agent B(¨V)z refers to the moiety B.
[30] A "core" of a compound or a polymer refers to the segment between
reactive groups. For
example, the core of a polythiol HS¨R¨SH is ¨R¨. A core of a compound or
prepolymer can also be
4
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
referred to as a backbone of a compound or a backbone of a prepolymer. A core
of a polyfunctionalizing
agent can be an atom or a structure such as a cycloalkane, a substituted
cycloalkane, heterocycloalkane,
substituted heterocycloalkane, arene, substituted arene, heteroarene, or
substituted heteroarene from
which moieties having a reactive functional are bonded.
[31] "Coreactive composition" refers to a composition comprising at least
two reactive compounds
capable of reacting with each other. A coreactive composition refers to a
composition comprising two or
more coreactive compounds capable of reacting at a temperature, for example,
less than 50 C, less than
40 C, less than 30 C, or less than 20 C. The reaction between the two or more
reactive compounds may
be initiated by combining and mixing the two or more coreactive compounds, by
adding a catalyst to a
coreactive composition comprising two or more coreactive compounds and/or by
activating a
polymerization initiator in a coreactive composition comprising the two or
more coreactive compounds.
A coreactive composition can be formed, for example, by combining and mixing a
first reactive
component comprising a first reactive compound with a second reactive
component comprising a second
reactive compound, wherein the first reactive compound can react with the
second reactive compound. A
coreactive composition can be a thermosetting composition and when cured forms
a thennoset.
[32] "Coreactive non-sealant composition" refers to a coreactive
composition that is not formulated as
a sealant. Although a cured coreactive non-sealant composition can exhibit
some properties of a sealant,
the primary function of a cured coreactive non-sealant composition is not to
act as a sealant.
[33] "Coreactive sealant composition" refers to a coreactive composition
formulated as a sealant.
[34] "Coreactive three-dimensional printing" refers to a method as
disclosed herein in which a
coreactive composition is extruded through a nozzle or extrusion in successive
layers to form a part.
[35] "Cycloalkanediyl" refers to a dirailical saturated monocyclic or
polycyclic hydrocarbon group. A
cycloalkanediyl group can be C3_12 cycloalkanediyl, C3_8 cycloalkanediyl, C3_6
cycloalkanediyl, or C5-6
cycloalkanediyl. Examples of cycloalkanediyl groups include cyclohexane-1,4-
diyl, cyclohexane-1,3-diy1
and cyclohexane-1,2-diyl.
[36] "Cycloalkyl" refers to a saturated monocyclic or polycyclic
hydrocarbon mono-radical group. A
cycloalkyl group can be C3-12 cycloalkyl, C3-8 cycloalkyl, C3-6 cycloalkyl, or
C5-6 cycloalkyl.
[37] "Heteroalkanediyl" refers to an alkanediy1 group in which one or more
of the carbon atoms are
replaced with a heteroatom, such as N, 0, S, or P. In a heteroalkanediyl, the
one or more heteroatoms can
comprise N or 0.
[38] "Cure time" refers to the duration from when the curing reaction of a
coreactive composition is
first initiated, for example, by combining and mixing to coreactive components
to form the coreactive
composition and/or by exposing a coreactive composition to actinic radiation,
until a layer prepared from
the coreactive composition exhibits a hardness of Shore 30A at conditions of
25 C and 50%RH. For an
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
actinic radiation-curable composition the cure time refers to the duration
from when the coreactive
composition is first exposed to actinic radiation to the time when a layer
prepared from the exposed
coreactive composition exhibits a hardness of Shore 30A at conditions of 25 C
and 50%RH.
[39] A dash ("¨") that is not between two letters or symbols is used to
indicate a point of bonding for a
substituent or between two atoms. For example, ¨CONH2 is attached through the
carbon atom.
[40] "Derived from" as in "a moiety derived from a compound" refers to a
moiety that is generated
upon reaction of a parent compound with a reactant. For example, a
bis(alkenyl) compound CH2=CH¨R¨
CH=CH2 can react with another compound such as a compound having thiol groups
to produce the
moiety ¨(CH2)2¨R¨(CH2)2¨, which is derived from the reaction of the alkenyl
groups with the thiol
groups. As another example, for a parent diisocyanate having the structure
0=C=N¨R¨N=C=O, a moiety
derived from the diisocyanate has the structure ¨C(0)¨NII¨R¨NH¨C(0)¨.
[41] "Derived from the reaction of ¨R with a thiol" refers to a moiety ¨IC¨
that results from the
reaction of a thiol group with a moiety comprising a group reactive with a
thiol group. For example, a
group R¨ can comprise CH2=CH¨CH2-0¨, where the alkenyl group CH2=CH¨ is
reactive with a thiol
group ¨SH. Upon reaction with a thiol group, the moiety ¨R'¨ is ¨CH2¨CH2¨CH2-
0¨.
[42] "Extrudate" refers to a coreactive composition that have been extruded
through a nozzle or
extrusion die. A coextrudate refers to two or more coreactive compositions
that have been simultaneously
extruded through a nozzle or coextrusion die.
[43] "Formed from" or "prepared from" denotes open, e.g., comprising, claim
language. As such, it is
intended that a composition "formed from" or "prepared from" a list of recited
components be a
composition comprising at least the recited components or the reaction product
of at least the recited
components, and can further comprise other, non-recited components used to
form or prepare the
composition.
[44] "Fracture energy" is determined according to ASTM D7313.
[45] Glass transition temperature Tg is determined by dynamic mechanical
analysis (DMA) using a
TA Instruments Q800 apparatus with a frequency of 1 Hz, an amplitude of 20
microns, and a temperature
ramp of -80 C to 25 C, with the Tg identified as the peak of the tan 5 curve.
[46] "Heterocycloalkanediyl" refers to a cycloalkanediyl group in which one
or more of the carbon
atoms are replaced with a heteroatom, such as N, 0, S, or P. In a
heterocycloalkanediyl, the one or more
heteroatoms can comprise N or 0.
[47] A monomer refers to a low molecular weight compound and can have a
molecular weight, for
example, less than 1,000 Da, less than 800 Da less than 600 Da, less than 500
Da, less than 400 Da, or
less than 300 Da. A monomer can have a molecular weight, for example, from 100
Da to 1,000 Da., from
100 Da to 800 Da, from 100 Da to 600 Da, from 150 Da, to 550 Da, or from 200
Da to 500 Da. A
6
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
monomer can have a molecular weight greater than 100 Da, greater than 200 Da,
greater than 300 Da,
greater than 400 Da, greater than 500 Da, greater than 600 Da, or greater than
800 Da. A monomer can
have a reactive functionality of two or more, for example, from 2 to 6, from 2
to 5, or from 2 to 4. A
monomer can have a functionality of 2, 3, 4, 5, 6, or a combination of any of
the foregoing. A monomer
can have an average reactive functionality, for example, from 2 to 6, from 2
to 5, from 2 to 4, from 2 to 3,
from 2.1 to 2.8, or from 2.2 to 2.6. Reactive functionality refers to the
number of reactive functional
groups per molecule. A combination of molecules having a different number of
reactive functional
groups can have a non-integer average number of reactive functional groups.
[48] A "polyalkenyl" refers to a compound having at least two alkenyl
groups. The at least two
alkenyl groups can be alkenyl groups and such polyalkenyls can be referred to
as alkenyl-terminated
compounds. Alkenyl groups can also be pendent alkenyl groups. A polyalkenyl
can be a dialkenyl,
having two alkenyl groups. A polyalkenyl can have more than two alkenyl groups
such as from three to
six alkenyl groups. A polyalkenyl can comprise a single type of polyalkenyl,
can be a combination of
polyalkenyls having the same alkenyl functionality, or can be a combination of
polyalkenyls having
different alkenyl functionalities.
[49] A polyfunctionalizing agent can have the structure:
B(¨V)z
where B is the core of the polyfunctionalizing agent, each V is a moiety
terminated in a reactive
functional group such as a thiol group, an alkenyl group, an epoxy group, an
isocyanate group, or a
Michael acceptor group, and z is an integer from 3 to 6, such as 3, 4, 5, or
6. In polyfunctionalizing
agents, each ¨V can have the structure, for example, ¨R¨SH or ¨R¨CH=CH2, where
R can be, for
example, C2_10 alkanediyl, C2_10 heteroalkanediyl, substituted C2_10
alkanediyl, or substituted C2-I0
heteroalkanediyl. When the moiety V is reacted with another compound the
moiety ¨NO¨ results and is
said to be derived from the reaction with the other compound. For example,
when V is ¨R¨CH=CH2 and
is reacted, for example, with a thiol group, the moiety V' is ¨R¨CH2¨CH2¨ is
derived from the reaction.
[50] "Polymerization initiator" refers to a compound capable of initiating
a polymerization reaction
following activation of the polymerization initiator. A polymerization
initiator can be activated, for
example, upon exposure to actinic radiation, heat, and/or shear forces.
[51] "Prepolymer" refers to homopolymers, and copolymers. For thiol-
terminated prepolymers,
molecular weights are number average molecular weights "Mn" as determined by
end group analysis
using iodine titration. For prepolymers that are not thiol-terminated, the
number average molecular
weights are determined by gel permeation chromatography using polystyrene
standards. A prepolymer
comprises a backbone and reactive groups capable of reacting with another
compound such as a curing
agent or crosslinker to form a cured polymer. A prepolymer includes multiple
repeating subunits bonded
7
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
to each other than can be the same or different. The multiple repeating
subunits make up the backbone of
the prepolymer.
[52] "Prepolymer backbone" refers to a segment between the reactive
functional groups of the
prepolymer. A prepolymer backbone typically includes repeating subunits. For
example, the backbone
of a polythiol having the structure HS¨(R)11¨SH is ¨(R)n¨.
[53] "Reaction product or means a chemical reaction product(s) of at least
the recited reactants and
can include partial reaction products as well as fully reacted products and
other reaction products that are
present in a lesser amount. For example, a "prepolymer comprising the reaction
product of reactants"
refers to a prepolymer or combination of prepolymers that are the reaction
product of at least the recited
reactants. The reactants can further comprise additional reactants.
[54] "Reactive compound" refers to a compound that is reactive with another
compound. A reactive
compound can comprise one or more functional groups that are reactive with
functional groups of another
compound.
[55] "Sealant layer" refers to a layer that when cured functions as a
sealant. A sealant layer can be
prepared from a coreactive sealant composition.
[56] Shore A hardness is measured using a Type A durometer in accordance
with ASTM D2240.
[57] Specific gravity and density of particles is determined according to
ISO 787-11.
[58] A "sulfur-containing prepolymer" refers to a prepolymer in which the
backbone comprises one or
more thioether ¨Sn¨ groups, where n can be, for example, 1 to 6, in the
backbone of the prepolymer.
Prepolymers that contain only thiol or other sulfur-containing groups either
as groups or as pendent
groups of the prepolymer are not encompassed by sulfur-containing prepolymers.
The prepolymer
backbone refers to the portion of the prepolymer having repeating segments.
Thus, a prepolymer having
the structure HS¨R¨R(¨CH2¨SH)¨[¨R¨(CH2)2¨S(0)2¨(CH2)¨S(0)21.¨CH=CH2 where each
R is a moiety
that does not contain a sulfur atom in the prepolymer backbone, is not
encompassed by a sulfur-
containing prepolymer. A prepolymer having the structure)
HS¨R¨R(¨CH2¨SH)¨[¨R¨(CH2)2¨S(0)2¨
(CH2)¨S(0)2]¨CH=CH2 where at least one R is a moiety that contains a sulfur
atom, such as a thioether
group, is encompassed by a sulfur-containing prepolymer. Examples of sulfur-
containing prepolymers
include polythioether prepolymers, polysulfide prepolymers, sulfur-containing
polyformal prepolymers,
and monosulfide prepolymers.
[59] "Substituted" refers to a group in which one or more hydrogen atoms
are each independently
replaced with the same or different substituent(s). A substituent can
comprise, for example, halogen, ¨
S(0)20H, ¨S(0)2, ¨SH, ¨SR where R is C1_10 alkyl, ¨COOH, ¨NO2, ¨NR2 where each
R independently
comprises hydrogen and C1-10 alkyl, ¨CN, =0, Ci-loalkyl, ¨CF3, ¨OH, phenyl,
C2_10 heteroalkyl, C5-6
8
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
heteroaryl, C1_10 alkoxy, or ¨COR where R is Ci_io alkyl. A substituent can
be, for example, ¨OH, ¨NH2,
or C1_3 alkyl.
[60] "Tack free time" refers to the duration from the time when the curing
reaction of a coreactive
composition is initiated, for example, by mixing two coreactive components or
by exposing a coreactive
composition to energy such as UV radiation, until the time when the coreactive
composition is no longer
tack free. The property of being tack free is determined by applying a
polyethylene sheet to the surface of
the layer with hand pressure and observing whether the sealant adheres to the
surface of the polyethylene
sheet, where the layer is considered to be tack free if the polyethylene sheet
separates easily from the
layer. For an actinic radiation-curable coreactive composition, the tack free
time refers to the time from
when the coreactive composition is exposed to actinic radiation to the time
when a layer prepared from
the coreactive composition is no longer tack free.
[61] Tensile strength and elongation are measured according to AMS 3279.
[62] "Thermoset" refers to a cured thermosetting polymer composition.
[63] "Thermosetting composition" refers to a composition comprising
coreactive compounds that
change irreversibly into an infusible, insoluble polymer network by curing.
Curing is the chemical
process of converting a prepolymer and curing agents into a polymer of higher
molecular weight and then
into a polymer network. Curing results in chemical reactions that create
extensive cross-linking between
A polymer network is a highly ramified structure in which essentially each
constitutional unit is
connected to each other constitutional unit and to the macroscopic phase
boundary by many paths through
the structure, the number of such paths increasing with the average number of
intervening constitutional
units; the paths must on average co-extensive with the structure.
[64] Reference is now made to certain compounds, compositions, and methods
of the present
invention. The disclosed compounds, compositions, and methods are not intended
to be limiting of the
claims. To the contrary, the claims are intended to cover all alternatives,
modifications, and equivalents.
[65] Methods of making a multilayer system comprising two or more layers,
wherein one or more of
the layers comprises a sealant layer, comprise (a) mixing a first component
and a second component to
form a coreactive sealant composition, wherein, the coreactive sealant
composition comprises a first
reactive compound and a second reactive compound; and the first reactive
compound is reactive with the
second reactive compound; (b) extruding the coreactive sealant composition to
form an extrudate; and (c)
depositing the extrudate to form the sealant layer.
[66] A sealant composition refers to a material that has the ability, when
cured, to form a sealant
capable of resisting at least one of an atmospheric condition, such as
moisture and/or temperature and at
least partially block the transmission of materials, such as water, solvent,
fuel, hydraulic fluid and other
liquids and gasses. A sealant can exhibit chemical resistance such as
resistance to fuels, hydraulic fluids,
9
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
solvents, greases, lubricants, salt spray, gases, oils, and/or cleaning
fluids. A chemically resistant material
can exhibit, for example, a % swell less than 25%, less than 20%, less than
15%, or less than 10%
following immersion in the chemical for 7 days at 70 C as determined according
to EN ISO 10563. A
multilayer system prepared using methods provided by the present disclosure
can meet or exceed the
requirements for aerospace sealants as set forth in AMS 3277. A sealant is
designed to minimize the
penetration of gases and liquids to a surface during in the use environment of
the part being sealed.
[67] A multilayer system can comprise two or more layers in which each of
the layers can be designed
to optimize one or more properties of the multilayer system. At least one of
the layers can comprise a
sealant layer. In a multilayer system an exterior layer or an outermost layer
can comprise a sealant and
can be designed, for example, to exhibit chemical resistance and an internal
layer can be designed to
exhibit, for example, low-density, substrate adhesion, and/or high tensile
strength and %elongation. As
another example, the exterior layer of a multilayer system can exhibit a fast
cure rate to facilitate handling
and manufacturability and underlying layers can have slower cure rates that
can facilitate, for example,
enhanced adhesion and/or enhanced mechanical properties. A multilayer system
also can have the
potential to reduce costs. Expensive material can be used in only those layers
where they are desired for
their properties, and other layers can use alternative materials.
[68] A multilayer sealant system can comprise any suitable number of layers
such as 2, 3, 4, 5, or 6
layers, where each layer is formed from a different material and can exhibit
different properties. A
multilayer sealant system can comprise one sealant layer, can comprise more
than one sealant layer, or
each layer can comprise a sealant layer. A multilayer system can comprise at
least one sealant layer, and
each of the other layers can independently comprise a sealant layer or a non-
sealant layer. A non-sealant
layer is a layer that is not intended to primarily function as a sealant in
the multilayer sealant system,
although a non-sealant layer can have some ability to restrict penetration of
gases and liquids.
[69] An example of a multilayer system is shown in FIGS. lA and 1B. The
multilayer system shown
in FIG. IA includes an inner first layer 101 underlying an intermediate second
layer 102, which underlies
an exterior third layer 103. FIG. 1B shows a multilayer system overlying a
fastener 105 mounted to
substrate 104, and includes an inner first layer 101, an overlying
intermediate second layer 102, and an
overlying exterior third layer 103. Only the exterior third layer 103 can
comprise a sealant or all layers
101/102/103 can comprise a sealant. For example, inner layer 101 can comprise
a composition
configured to promote adhesion to a surface, and intermediate layer 102 can
comprise a composition
having high tensile strength and/or %elongation.
[70] Another example of a multilayer system is shown in FIG. IC in which a
first layer 106 is adjacent
a second layer 107. FIG. ID shows a multilayer system in which a first layer
106 is adjacent a second
layer 107, and a third layer 108 overlies both the first and second layers
106/107. In FIG. 1C first layer
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
106 and/or second layer 107 can be a sealant. In FIG. ID, exterior layer 108
can be a sealant layer and
layers 106 and 107 can be non-sealant layers. In FIG 1D, each of layers
106/107/108 can independently
be selected from a sealant layer and anon-sealant layer, where at least one of
layers 106/107/108 is a
sealant layer. Other configurations of the various layers of a multilayer
system are possible.
[71] At least one of the layers of a multilayer system can be different
than another layer of the
multilayer system. For example, the layers can differ in the type and/or
amount of the constituents such
as the prepolymers, monomers, and/or additives in the layers. The differences
in the type and/or amount
of the constituents can result in the various layers of the multilayer system
having different properties.
Each of the layers can independently comprise, for example, reactive
compounds, catalysts,
polymerization initiators, adhesion promoters, filler, reactive diluents,
colorants, rheological control
agents, and/or photochromic agents that can be the same or different or be
present in a different wt% or
vol% than another layer of the multilayer system.
[72] The constituents of a layer can be different than those in another
layer, for example, in terms of
composition, curing chemistries, molecular weights of constituents, sizes of
constituents, wt% of
constituents, and/or vol% of constituents.
[73] For example, each layer can independently be configured to provide a
cured layer, for example,
that exhibits one or more of chemical resistance, low-temperature flexibility,
hydrolytic stability, high
temperature resistance, high tensile/elongation, bonding to the substrate,
bonding to a primer coating,
adhesion to an adjoining layer, fast tack-free time, cure time to a hardness
of Shore 10A, time to complete
cure, electrical conductivity, EMI/RFI shielding, static dissipation,
corrosion resistance, cured hardness,
low-density, and/or sound damping.
[74] Each of the layers of a multilayer system can have the same or
different curing chemistry than
another layer of the multilayer system and/or than an adjoining layer of the
multilayer system. To provide
a robust interface between adjacent layers it can be desirable that adjacent
layers are chemically or
physically bonded. The formation of chemical or physical bonding between
layers can be facilitated by
using coreactive compositions for the adjacent layers that have the same
curing chemistry and/or that
contain compounds capable of coreacting with compounds in adjacent layers.
[75] Adjoining layers of a coreactive composition can chemically bond
and/or physical bond to
create a mechanically strong interlayer interface. The strength of the
interlayer interface can be
determined by measuring the fracture energy according to ASTM D7313.
Chemically resistant multilayer
sealants made using methods provided by the present disclosure can have a
fracture energy that is
substantially the same as the fracture energy of an individual layer. For
example, the fracture energy of
the multilayer sealant and the fracture energy of an individual cured layer of
the coreactive composition
can be, for example, within less than 10%, less than 5%, less than 2% or less
1%.
11
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[76] Each layer of a multilayer system can be selected to enhance a desired
property or properties of
individual cured layers. For example, an innermost layer can provide enhanced
surface adhesion to a
substrate, but not necessarily have a low density. For example, an outermost
layer can be formulated to
provide enhanced chemical resistance and/or be able to dissipate static
charge. An intermediate layer
between the inner and outer layers can be low density and be formulated to
exhibit enhanced mechanical
properties. In this way each layer of a multilayer system can be configured to
optimize a different
property or combination of properties without compromising other properties of
a layer, and where the
other overall properties of the multilayer s system can be imparted by other
layers.
[77] A layer of a multilayer system can be inhomogeneous within the
horizontal plane of the layer
and/or perpendicular to the horizontal plane of the layer. The inhomogeneity
can be discrete or
continuous. FIG. 2A shows a cross-section of a layer comprising, for example,
an additive such as a filler
in which the concentration of the filler, identified by the stripling, varies
within the dimension
perpendicular to the horizontal plane of the layer. FIG. 2B shows a cross-
section of a layer in which the
concentration of the filler varies both within the horizontal plane of the
layer and in certain regions,
within the dimension perpendicular to the horizontal plane of the layer.
[78] The composition within a layer of a multilayer system can also vary
within the layer. The
composition can vary across the thickness of a layer, i.e., the cross-
sectional dimension, and/or within a
lateral dimension of a layer, i.e., the longitudinal dimension. For example, a
concentration of a
constituent such as a coreactive compound and/or an additive can vary across
the thickness of a layer such
that, for example, the concentration is higher toward one side of the layer
than toward the opposite side of
the layer, or the concentration can be higher in the middle of a layer than on
either of the two sides. The
concentration of one or more constituents can vary linearly, non-linearly,
continuously, discontinuously,
and/or discretely across the thickness of a layer. Similarly, a concentration
of one or more constituents
such as a coreactive compound and/or an additive can vary within a lateral
dimension of a layer such as in
a dimension orthogonal to the thickness of the layer. For example, the
concentration of a constituent such
as a compound and/or an additive can be higher on one side of a layer than on
another side of the layer.
The concentration or a constituent can vary within certain regions of the
layer. The concentration of a
constituent can vary linearly, non-linearly, continuously, discontinuously,
and/or discretely across a
lateral dimension of a layer.
[79] Each of the layers forming a multilayer system provided by the present
disclosure can
independently comprise an internal compositional structure. For example, the
composition can be
substantially uniform throughout the thickness of a layer or can vary across
the thickness of a layer. By
uniform is meant that the concentration of each of the constituents forming a
layer is within 10%, within
5%, or within 1%, or within 0.1% of a nominal concentration throughout the
layer, where the nominal
12
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
concentration refers to an average concentration of the constituent within the
layer. For example, the
composition can be uniform within the thickness dimension of a layer or can
vary within the lateral
dimension, i.e., orthogonal to the thickness dimension, of the layer.
[80] A coreactive composition used to form a layer of a multilayer system
can comprise at least two
coreactive compounds and one or more additives. Within a layer the
concentration of a coreactive
compound and/or the one or more additives can be substantially the same such
as within +/-5%, within
+/-1%, or within +1-0.5%. Alternatively, within a layer the concentration of a
coreactive compound
and/or the one or more additives can vary. The concentration can vary across
the thickness of a layer
and/or in the longitudinal dimension of a layer. Also, the concentration of a
coreactive compound and/or
the one or more additives can vary in a portion of the thickness and/or a
portion of the longitudinal
dimension of a layer.
[81] These layers can be referred to as structured layers to indicate that
the layers are characterized by
an internal compositional structure and that the composition is not uniform
throughout the layer.
[82] The composition within a structured layer can vary discretely,
continuously, discontinuously,
linearly, non-linearly or variably.
[83] A concentration of a constituent within a layer can vary discretely
across the thickness of a layer.
For example, an electrically conductive filler can be present in an outer
portion of a layer to a certain
depth and be absent in the inner portion of the layer.
[84] A concentration of a constituent of a layer composition can vary, for
example, linearly, or non-
linearly across the thickness or a portion of the thickness of a layer.
[85] A concentration of one or more constituents of a layer can be, for
example, greater toward one
surface, greater toward both surfaces, or greater toward the center of the
layer.
[86] A multilayer system can have any suitable physical structure as
appropriate to seal a part intended
to be sealed. For example, to seal a two-dimensional continuous surface, a
multilayer system can be in
the form of a multilayer sheet. To seal a small part, a multilayer system can
be in the form of a cap, shell,
or any other suitable shape.
[87] Each layer of a multilayer system can independently have a
substantially uniform thickness or can
have a variable thickness. The thickness of each layer can be substantially
the same or can be different
than another layer forming a multilayer system. For example, a thickness of a
layer can be substantially
the same and can be within 10%, within 5%, or within 1% of another layer. For
example, a thickness of a
layer can be different than the thickness of another layer can differ by more
than 10%, for than 20%, more
than 50%, or more than 100% the thickness of another layer.
[88] For example, a multilayer system used to seal a two-dimensional
continuous surface can include
multiple layers with each layer has a substantially uniform thickness and
where the thickness of an
13
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
individual layer may be the same or may be different than the thickness of
another layer. For example, a
layer having a substantially uniform thickness can have a thickness that does
not vary by more than 10%,
more than 5%, or by more than 1% across the surface.
[89] A multilayer system can have a total thickness, for example, greater
than 2 mm, greater than 4
mm, greater than 6 mm, greater than 8 mm, greater than 10 mm, greater than 12
mm, or greater than 14
mm. A multilayer system can have a total thickness, for example, from 2 mm to
15 mm, from 3 mm to
14 mm, from 3 mm to 12 mm, from 4 mm to 10 mm, or from 6 mm to 8 mm.
[90] Each layer of a multilayer system can independently have a thickness,
for example, from 0.1 mm
to 25 mm, from 0.5 mm to 25 mm, from 1 mm to 20 mm, from 2 mm to 15 mm, or
from 3 mm to 10 mm.
Each layer of a multilayer system can independently have a thickness, for
example, greater than 0.1 mm,
greater than 0.5 mm, greater than 1 mm, greater than 5 mm, greater than 10 mm,
greater than 15 mm, or
greater than 20 mm. Each layer of a multilayer system can independently have a
thickness, for example,
less than 25 mm, less than 20 mm, less than 15 mm, less than 10 mm, less than
5 mm, or less than 1 mm.
[91] An outermost layer of a multilayer system can have a thickness that is
greater than a thickness of
each of the underlying layers, either individually or combined. An outermost
layer can have a thickness
that is less than a thickness of each of the underlying layers, either
individually or combined.
[92] An interior layer can have a thickness that is greater than a
thickness of each of the overlying
layers, either individually or combined. An interior layer can have a
thickness that is less than a thickness
of each of the overlying layers, either individually or combined.
[93] For sealing a three-dimensional part, an inner layer can have a
variable cross-sectional thickness
such as to cover and conform to a complex shape of the part and to provide a
smooth, continuous outer
surface. Overlying layers can have a substantially uniform thickness.
[94] A multilayer system can be formed by extruding a coreactive sealant
composition to form an
extrudate and depositing the extrudate onto a substrate or onto a previously
deposited layer to form a
sealant layer. The previously deposited layer can be a sealant layer or a non-
sealant layer. The
previously deposited layer can be the outermost layer of a multilayer system.
One or more layers can be
deposited onto the deposited sealant layer to form a multilayer system.
[95] A multilayer system can be applied to a substrate using additive
manufacturing technology such
as three-dimensional printing. Additive manufacturing methods facilitate the
ability to apply a multilayer
system in a consistent and reproducible manner. Furthermore, in part because
the temporal constraints
associated with manual sealant application methods are avoided, additive
manufacturing enables the use
of alternative curing chemistries such as fast curing chemistries.
[96] A coreactive sealant composition can comprise a first reactive
compound and a second reactive
compound, where the first reactive compound is reactive with the second
reactive compound. The first
14
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
and second reactive compounds can react at a temperature less than 50 C, such
as less than 40 C, less
than 30 C, less than 25 C, less than 20 C, or less than 15 C. The first and
second reactive compounds
can react in the absence of a catalyst and/or activated polymerization
initiator. The first and second
reactive compounds can react in the presence of a catalyst or combination of
catalysts. The first and
second reactive compounds can react in the presence of an activated
polymerization initiator such as an
activated photoinitiator. The catalyst and polymerization initiator can be
suitable for catalyzing or
initiating a chemical reaction between the fist reactive compound and the
second reactive compound.
[97] A coreactive sealant composition can be a thermosetting composition
such that the cured
coreactive sealant composition can be a thermoset. Each of the layers of a
multilayer sealant system can
comprise a thermoset.
[98] A coreactive sealant composition con be formed by combining and mixing
a first component and
a second component. The first component can comprise a first reactive compound
and a second reactive
compound; and the second component can comprise a catalyst and/or
polymerization initiator. The first
component can comprise the first reactive compound and the second component
can comprise the second
reactive compound, and the first and/or second component can comprise a
catalyst and/or a
polymerization initiator. In addition to a first component and a second
component, a coreactive sealant
composition can be formed by combining and mixing one or more additional
components.
[99] A coreactive sealant composition can be formed, for example, by pumping a
first component and
a second component into a mixer and mixing the first and second components to
form a coreactive sealant
composition.
[100] A deposition system can include an in-line static and/or dynamic mixer
as well as separate
pressurized pumping compartments to hold the at least two components and feed
the coreactive
components into the static and/or dynamic mixer. A mixer such as an active
mixer can comprise a
variable speed central impeller having high shear blades within a conical
nozzle. A range of conical
nozzles may be used which have an exit orifice dimension, for example, from
0.2 mm to 50 mm, from 0.5
mm to 40 mm, from 1 mm to 30 mm, or from 5 mm to 20 mm.
[101] A range of static and/or dynamic mixing nozzles may be used which have,
for example, an exit
orifice dimension from 0.6 mm to 2.5 mm, and a length from 30 mm to 150 mm.
For example, an exit
orifice diameter can be from 0.2 mm to 4.0 mm, from 0.4 mm to 3.0 mm, from 0.6
mm to 2.5 mm, from
0.8 mm to 2 mm, or from 1.0 mm to 1.6 mm. A static mixer and/or dynamic can
have a length, for
example, from 10 mm to 200 mm, from 20 mm to 175 mm, from 30 mm to 150 mm, or
from 50 mm to
100 mm. A mixing nozzle can include a static and/or dynamic mixing section and
a dispensing section
coupled to the static and/or dynamic mixing section. The static and/or dynamic
mixing section can be
configured to combine and mix the components. The dispensing section can be,
for example, a straight
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
tube having any of the above orifice diameters. The length of the dispensing
section can be configured to
provide a region in which the components can begin to react and build
viscosity before being deposited.
The length of the dispensing section can be selected, for example, based on
the speed of deposition, the
rate of reaction of the co-reactants, and the desired viscosity. A coreactive
composition can have a
residence time in the static and/or dynamic mixing nozzle, for example, from
0.25 seconds to 5 seconds,
from 0.3 seconds to 4 seconds, from 0.5 seconds to 3 seconds, or from 1
seconds to 3 seconds. Other
residence times can be used as appropriate based on the curing chemistries and
curing rates. The flow
rate can be, for example, from 1 mL/min to 20 mL/min, from 2 mL/min to 15
mL/min, from 3 mL/min to
mL/min, or from 4 mL/min to 8 mL/min, through a nozzle having a diameter, for
example, from 0.8
mm to 1 mm. In general, a suitable residence time is less than the gel time of
a coreactive composition.
A suitable gel time can be less than 10 min, less than 8 min, less than 6 mm,
less than 5 min, less than 4
mm, less than 3 min, less than 2 min, or less than 1 mm. A gel time of the
coreactive composition can be,
for example, from 0.5 min to 10 mm, from 1 mm to 7 min, from 2 min to 6 mm, or
from 3 min to 5 min.
[102] A coreactive composition for fabricating a multilayer sealant can have a
gel time, for example, of
less than 12 hours, less than 8 hours, less than 4 hours, less than 1 hour,
less than 30 minutes, less than 10
minutes, or less than 1 at 23C/50%RH. A coreactive composition for fabricating
a multilayer sealant can
have a gel time, for example, from 10 seconds to 12 hours, from 1 minute to 8
hours, from 30 minutes to
4 hours, or from 1 hour to 3 hours at 23C/50% RH. A coreactive composition for
fabricating a multilayer
sealant can have a gel time, for example, of greater than 10 seconds, greater
than 1 minute, greater than
30 minutes, greater than 1 hour, greater than 4 hours, or greater than 8
hours. Gel time refers the duration
from the time when curing of the coreactive composition is initiated, for
example, either by mixing of
coreactive components or by exposure to energy such as UV radiation, to when
the coreactive
composition is no longer stirrable by hand.
[103] A static and/or dynamic mixing nozzle can be heated or cooled to
control, for example, the rate of
reaction between the coreactive compounds and/or the viscosity of the
coreactive composition. An orifice
of a deposition nozzle can have any suitable shape and dimensions. A system
can comprise multiple
deposition nozzles. The nozzles can have a fixed orifice dimension and shape,
or the nozzle orifice can
be controllably adjusted. The mixer and/or the nozzle may be cooled to control
an exotherm generated by
the reaction of the coreactive compounds.
[104] The one or more additional layers of a multilayer system can be
deposited by methods other than
extrusion. For example, each layer underlying and/or overlying a sealant layer
can be deposited using any
suitable method such as by spraying, brushing, roller coating, and/or
spreading. Each of the one or more
underlying and/or overlying layers can independently comprise a sealant layer
or a non-sealant layer.
16
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[105] In addition to a sealant layer of a multilayer system, other layers of
the multilayer system can be
formed by extruding a suitable coreactive composition. The one or more
additional layers can be formed
by combining and mixing a first component and a second component to form a
coreactive composition
comprising a first reactive compound and a second reactive compound. Each of
the one or more
additional coreactive compositions can be merged with a coreactive sealant
composition to form a
coextrudate, which can be deposited along with the other layers of the
multilayer system. Each of the
additional coreactive compositions can independently be selected from an
additional coreactive sealant
composition or a coreactive non-sealant composition.
[106] The one or more additional layers can be formed by depositing the
respective extrudates
sequentially. By sequentially depositing means that an extrudate comprising a
first coreactive
composition is deposited, then a second extrudate comprising a second
coreactive composition is
deposited and so forth. In this way a multilayer system is built up layer by
layer.
[107] Alternatively, one or more of the additional coreactive compositions can
be coextruded with the
coreactive sealant composition to form a coextrudate, which can then be
deposited to simultaneously form
all or a portion of the multilayer system. As with the coreactive sealant
composition, each of the
additional coextruded coreactive compositions can be formed by combining and
mixing a first component
and a second component to form the respective additional coreactive
composition. Each of the additional
coreactive compositions can be merged with the flow of the coreactive sealant
composition and
coextruded through a coextrusion die to form a coextrudate. The coextrudate
can be deposited to form a
multilayer system in which at least one of the layers is a sealant.
[108] Each of the additional coextruded coreactive compositions can
independently comprise a
coreactive sealant composition or a coreactive non-sealant composition and the
respective layers comprise
sealants or non-sealants. Each of the additional coextruded reactive
compositions comprises a
thermosetting material, which when cured forms a thermoset.
[109] Adjoining coreactive compositions forming the extrudate can comprise the
same or different
curing chemistries and/or can comprise reactive compounds capable of reacting
with reactive compounds
in an adjoining coreactive composition. This allows bonding between adjoining
coreactive compositions
which provides a cured multilayer system in which the adjacent layers are
integrally bonded and have a
high cohesive strength.
[110] Each coreactive composition of a multilayer system, such as a coreactive
sealant composition or a
coreactive non-sealant composition, can independently comprise a first
compound having a first
functional group and a second compound comprising a second functional group,
where the first functional
group is capable of reacting with the second functional group.
17
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[1111 The first and second functional groups can be capable of reacting, for
example, at a temperature
less than 50 C, less than 40 C, less than 30 C, less than 20C, or less than 15
C. The first and second
functional groups can be capable of reacting, for example, at a temperature
from 10 C to 50 C, from
15 C to 40 C, or from 20 C to 30 C. The first and second functional groups can
be capable of reacting,
for example, at a temperature greater than 10 C, greater than 20 C, greater
than 30 C, or greater than
40 C.
[112] A coreactive composition can be a one-part composition in which the
curing reaction is initiated
upon the application of energy such as by exposing the one-part coreactive
composition to actinic
radiation such as UV radiation. A coreactive composition can be a two-part
composition in which two
coreactive components are combined and mixed to initiate the curing reaction.
For example, a first
coreactive component comprising a first compound comprising a first functional
group can be combined
and mixed with a second coreactive component comprising a second compound
comprising a second
functional group to form a coreactive composition, where the first and second
functional groups are
coreactive. The first and second coreactive components can be combined and
mixed before being
introduced into the coextruder or can be combined and mixed within the
coextruder to form a coreactive
composition that is merged with the flow of another coreactive composition.
[113] Properties of the multilayer system and the layers fonning the
multilayer system such as the
viscosity and cure rate of the coreactive compositions, can be selected to
facilitate the ability of an
extrudate or coextrudate to retain an intended shape following deposition onto
a surface.
[114] A coreactive composition can have an initial, as deposited, viscosity,
for example, from 1E2
poise to 1E7 poise, from 5E2 poise to 5E6 poise, from 1E3 poise to 1E5 poise,
or from 5E3 poise to 5E4
poise, where viscosity is determined using a Brookfield rheometer fitted with
a #7 paddle at 2 rpm and
25 C. A coreactive composition can have an initial viscosity, for example,
greater than 1E2 poise, greater
than 5E2 poise, greater than 1E3 poise, greater than 5E3 poise, greater than
1E4 poise, greater than 1E5
poise, or greater than 1E6 poise. A coreactive composition can have an initial
viscosity, for example, less
than 1E7 poise, less than 1E6 poise, less than 1E5 poise, less than 1E4 poise,
or less than 1E3 poise.
[115] A coreactive composition can have a tack free, for example, of less than
24 hours, less than 10
hours, less than 1 hour, less than 30 minutes, less than 10 minutes or less
than 5 minutes at 23 C/50%RH
A coreactive composition for fabricating a multilayer sealant can have a tack
free time, for example,
greater than 10 seconds, greater than 1 minute, greater than 1 hour, greater
than 6 hours, or greater than
12 hours at 23 C/50%RH A coreactive composition can have a tack free, for
example, from 30 seconds
to 24 hours, from 1 minute to 12 hours, from 1 hour to 10 hours, or from 2
hours to 8 hours at
23 C/50%RH. Tack free time" refers to the duration from the time when curing
of the coreactive
composition is initiated, for example, either by mixing of coreactive
components or by exposure to energy
18
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
such as UV radiation, to the time when a layer prepared from the coreactive
composition is no longer
tack free, where tack free is determined by applying a polyethylene sheet to
the surface of the layer with
hand pressure and observing whether sealant adheres to the surface of the
polyethylene sheet, where
[116] A coreactive composition can have a time to a hardness of Shore 10A, for
example, of less than 2
minutes, less than 5 minutes, less than 30 minutes, less than 1 hour, less
than 5 hours, less than 10 hours,
or less than 20 hours. at 23 C/50%RH. A coreactive composition can have a time
to a hardness of Shore
10A, for example, of greater than 30 seconds, greater than 1 minute, greater
than 1 hour, greater than 5
hours, or greater than 10 hours at 23 C/50%RH. A coreactive composition can
have a time to a hardness
of Shore 10A, for example, from 30 seconds to 20 hours, from minute to 12
hours, or from 1 hour to 10
hours, at 23 C/50%RH.
[117] A coreactive composition can have a cure time such as the time to a
hardness of Shore 30A of
from 1 day to 7 days at 23 C/50%RH.
[118] A coreactive composition can have along working time and following the
end of the working
time, can have a fast time to cure. Working time refers to the time from when
the coreactive compounds
are first combined and mixed to form the coreactive composition until the time
the coreactive
composition is no longer stirrable by hand; or the time from when a catalyst
is added and/or a
polymerization initiator is activated to cause the coreactive compounds to
react until the time the
coreactive composition is no longer stirrable by hand.
[119] Each coreactive composition used to form a multilayer system can
independently comprise one or
more prepolymers, one or more monomers, and one or more additives.
[120] A coreactive composition can be a thermosetting composition and when
cured can form a
thermoset.
[121] A coreactive composition can be substantially free of solvent. For
example, a coreactive
composition can comprise less than 5 wt% solvent, less than 2 wt%, less than 1
wt%, or less than 0.1 wt%
solvent, where wt% is based on the total weight of the coreactive composition.
[122] A coreactive composition can comprise coreactive compounds that coreact
and cure at room
temperature, where room temperature refers to a temperature from 20 C to 25 C,
from 20 C to 22 C, or
about 20 C.
[123] A prepolymer can comprise any suitable backbone. A prepolymer backbone
can be selected, for
example, based on the end use requirements of a multilayer system and the
desired attributes of a
particular layer.
[124] A coreactive composition can comprise a prepolymer or combination of
prepolymers.
Prepolymers can influence, for example, the tensile strength, %elongation,
hydrolytic stability, and/or
chemical resistance, as well as other properties of the cured sealant.
19
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[125] A prepolymer can have a number average molecular weight, for example,
less than 20,000 Da,
less than 15,000 Da, less than 10,000 Da, less than 8,000 Da, less than 6,000
Da, less than 4,000 Da, or
less than 2,000 Da. A prepolymer can have a number average molecular weight,
for example, greater
than 2,000 Da, greater than 4,000 Da, greater than 6,000 Da, greater than
8,000 Da, greater than 10,000
Da, or greater than 15,000 Da. A prepolymer can have a number average
molecular weight, for example,
from 1,000 Da to 20,000 Da, from 2,000 Da to 10,000 Da, from 3,000 Da to 9,000
Da, from 4,000 Da to
8,000 Da, or from 5,000 Da to 7,000 Da.
[126] A prepolymer can be liquid at 25 C and can have a glass transition
temperature Tg, for example,
less than -20 C, less than -30 C, or less than -40 C.
[127] A prepolymer can exhibit a viscosity, for example, within a range from
20 poise to 500 poise (2
Pa-sec to 50 Pa-sec), from 20 poise to 200 poise (2 Pa-sec to 20 Pa-sec) or
from 40 poise to 120 poise (4
Pa-sec to 12 Pa-sec), measured using a Brookfield CAP 2000 viscometer, with a
No. 6 spindle, at speed
of 300 rpm, and a temperature of 25 C.
[128] A prepolymer can have a reactive functionality, for example, less than
12, less than 10, less than
8, less than 6, or less than 4. Each of the first reactive compound and the
second reactive compound can
comprise a prepolymer having respective reactive functionality, for example,
from 2 to 12, from 2 to 8,
from 2 to 6, from 2 to 4, or from 2 to 3. Each of the first reactive compound
and the second reactive
compound can independently have a functionality, for example, of 2, 3, 4, 5,
or 6.
[129] A coreactive composition can comprise a prepolymer or combination of
prepolymers having any
suitable polymeric backbone. A polymeric backbone can be selected, for
example, to impart solvent
resistance to the cured coreactive composition, to impart desired physical
properties such as tensile
strength, %elongation, Young's modulus, impact resistance, or other
application-relevant property. A
prepolymer backbone can be terminated in one or more suitable functional
groups as appropriate for a
particular curing chemistry.
[130] A prepolymer can comprise segments having different chemical structure
and properties within
the prepolymer backbone. The segments can be distributed randomly, in a
regular distribution, or in
blocks. The segments can be used to impart certain properties to the
prepolymer backbone. For example,
the segments can comprise flexible linkages such as thioether linkages into
the polymer backbone.
Segments having pendent groups can be incorporated into the prepolymer
backbone.
[131] For example, a prepolymer backbone can comprise a polythioether, a
polysulfide, a polyformal, a
polyisocyanate, a polyurea, polycarbonate, polyphenylene sulfide, polyethylene
oxide, polystyrene,
acrylonitrile-butadiene-styrene, polycarbonate, styrene acrylonitrile,
poly(methylmethacrylate),
polyvinylchloridc, polybutarlicne, polybutylenc terephthalatc, poly(p-
phenylencoxide), polysulfone,
polyethersulfone, polyethylenimine, polyphenylsulfone, acrylonitrile styrene
acrylate, polyethylene,
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
syndiotactic or isotactic polypropylene, polylactic acid, polyamide, ethyl-
vinyl acetate homopolymer or
copolymer, polyurethane, copolymers of ethylene, copolymers of propylene,
impact copolymers of
propylene, polyetheretherketone, polyoxymethylene, syndiotactic polystyrene
(SPS), polyphenylene
sulfide (PPS), liquid crystalline polymer (LCP), homo- and copolymer of
butene, homo- and copolymers
of hexene; and combinations of any of the foregoing.
[132] Examples of other suitable prepolymer backbones include polyolefins
(such as polyethylene,
linear low density polyethylene (LLDPE), low density polyethylene (LDPE), high
density polyethylene,
polypropylene, and olefin copolymers), styrene/butadiene rubbers (SBR),
styrene/ethylene/butadiene/styrene copolymers (SEBS), butyl rubbers,
ethylene/propylene copolymers
(EPR), ethylene/propylene/diene monomer copolymers (EPDM), polystyrene
(including high impact
polystyrene), poly(vinyl acetates), ethylene/vinyl acetate copolymers (EVA),
poly(vinyl alcohols),
ethylene/vinyl alcohol copolymers (EVOH), poly(vinyl butyral), poly(methyl
methacrylate) and other
acrylate polymers and copolymers (including such as methyl methacrylate
polymers, methacrylate
copolymers, polymers derived from one or more acrylates, methacrylates, ethyl
acrylates, ethyl
methacrylates, butyl acrylates, butyl methacrylates and the like), olefin and
styrene copolymers,
acrylonitrile/butadiene/styrene (ABS), styrene/acrylonitrile polymers (SAN),
styrene/maleic anhydride
copolymers, isobutylene/maleic anhydride copolymers, ethylene/acrylic acid
copolymers,
poly(acrylonitrile), polycarbonates (PC), polyamides, polyesters, liquid
crystalline polymers (LCPs),
poly(lactic acid), poly(phenylene oxide) (PPO), PPO-polyamide alloys,
polysulfone (PSU),
polyetherketone (PEK), polyetheretherketone (PEEK), polyimides,
polyoxymethylene (POM) homo- and
copolymers, polyetherimides, fluorinated ethylene propylene polymers (FEP),
poly(vinyl fluoride),
poly(vinylidene fluoride), poly(vinylidene chloride), and poly(vinyl
chloride), polyurethanes
(thermoplastic and thermosetting), aramides (such as Kevlart and Nomex ),
polytetrafluoroethylene
(PTFE), polysiloxanes (including polydimethylenesiloxane,
dimethylsiloxane/vinylmethylsiloxane
copolymers, vinyldimethylsiloxane terminated poly(dimethylsiloxane)),
elastomers, epoxy polymers,
polyureas, alkyds, cellulosic polymers (such as ethyl cellulose, ethyl
hydroxyethyl cellulose,
carboxymethyl cellulose, cellulose acetate, cellulose acetate propionates, and
cellulose acetate butyrates),
polyethers and glycols such as poly(ethylene oxide)s (also known as
poly(ethylene glycol)s,
poly(propylene oxide)s (also known as poly(propylene glycol)s, and ethylene
oxide/propylene oxide
copolymers, acrylic latex polymers, polyester acrylate oligomers and polymers,
polyester diol diacrylate
polymers, and UV-curable resins.
[133] A coreactive composition can comprise a prepolymer comprising an
elastomeric backbone.
"Elastomer," "elastomeric' and similar terms refer to materials with "rubber-
like" properties and
generally having a low Young's modulus and a high tensile strain. For example,
elastomers can have a
21
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
Young's modulus/tensile strength from about 4 MPa to about 30 MPa. Elastomers
can have a tensile
strain (elongation at break) from about 100% to about 2,000%. The Young's
modulus/tensile strength
and tensile stiain can be determined according to ASTM D412.4893. Elastomers
can exhibit a tear
strength, for example, from 50 IcN/m to 200 IcN/m. Tear strength of an
elastomer can be determined
according to ASTM D624. The Young's modulus of an elastomer can range from 0.5
MPa to 6 MPa as
determined according to ASTM D412.4893.
[134] Examples of suitable prepolymers having an elastomeric backbone include
polyethers,
polybutadienes, fluoroelastomers, perfluoroelastomers, ethylene/acrylic
copolymers, ethylene propylene
diene terpolymers, nitriles, polythiolarnines, polysiloxanes, chlorosulfonated
polyethylene rubbers,
isoprenes, neoprenes, polysulfides, polythioethers, silicones, styrene
butadienes, and combinations of any
of the foregoing. An elastomeric prepolymer can comprise a polysiloxane, such
as, for example, a
polymethylhydrosiloxane, polydimethylsiloxane, polyhydrodiethylsiloxane,
polydiethylsiloxane, or a
combination of any of the foregoing. The elastomeric prepolymer can comprise
terminal functional
groups that have a low reactivity with amine and isocyanate groups such as
silanol groups.
[135] Examples of prepolymers that exhibit high solvent resistance include
fluoropolymers, ethylene
propylene diene terpolymer (EPDM), and other chemically resistant prepolymers
disclosed herein, cured
polymeric matrices having a high crosslinlcing density, chemically resistant
organic filler such as
polyamides, polyphenylene sulfides, and polyethylenes, or a combination of any
of the foregoing.
[136] Examples of prepolymers having a chemically resistant backbone include
polytetrafluorethylene,
polyvinylidene difluoride, polyethylenetetrafluoroethylene, fluorinated
ethylene propylene,
perfluoroalkoxy, ethylene chlorotrifluorethylene, polychlorotrifluoroethylene,
fluorinated ethylene
propylene polymers polyamide, polyethylene, polypropylene, ethylene-propylene,
fluorinated ethylene-
propylene, polysulfone, polyarylether sulfone, polyether sulfone, polyimide,
polyethylene terephthalate,
polyetherketone, polyetherether ketone, polyetherimide, polyphenylene sulfide,
polyarylsulfone,
polybenzimidazole, polyamideimide, liquid crystal polymers, and combinations
of any of the foregoing.
[137] Examples of prepolymers that exhibit low temperature flexibility include
silicones,
polytetrafluoroethylenes, polythioethers, polysulfides, polyformals,
polybutadienes, certain elastomers,
and combinations of any of the foregoing.
[138] Examples of prepolymers that exhibit hydrolytic stability include
silicones,
polytetrafluoroethylenes, polythioethers, polysulfides, polyformals,
polybutadienes, certain elastomers,
and combinations of any of the foregoing, and compositions having a high cros
slinking density.
[139] Examples of prepolymers that exhibit high temperature resistance can
comprise, for example,
prepolymers such as silicones, polytetrafluoroethylenes, polythioethers,
polysulfides, polyformals,
22
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
polybutadienes, certain elastomer, and combinations of any of the foregoing;
and compositions having a
high crosslinking density.
[140] Examples of prepolymers that exhibit high tensile include silicones and
polybutadiene,
compositions having high crosslinking density, inorganic filler, and
combinations of any of the foregoing.
[141] A coreactive sealant composition can comprise a sulfur-containing
prepolymer or combination of
sulfur-containing prepolymers. Sulfur-containing monomers and prepolymers can
impart solvent
resistance to a cured sealant.
[142] For applications where chemical resistance is required, prepolymers
having a sulfur-containing
backbone can be used. The chemical resistance can be with respect to cleaning
solvents, fuels, hydraulic
fluids, lubricants, oils, and/or salt spray. Chemical resistance refers to the
ability of a part to maintain
acceptable physical and mechanical properties following exposure to
atmospheric conditions such as
moisture and temperature and following exposure to chemicals such as cleaning
solvents, fuels, hydraulic
fluid, lubricants, and/or oils. In general, a chemically resistant part has
exhibits a % swell less than 25%,
less than 20%, less than 15%, or less than 10%, following immersion in a
chemical for 7 days at 70 C,
where % swell is determined according to EN ISO 10563.
[143] A sulfur-containing prepolymer refers to a prepolymer that has one or
more thioether ¨Sn¨
groups, where n can be, for example, 1 to 6, in the backbone of the
prepolymer. Prepolymers that contain
only thiol or other sulfur-containing groups either as terminal groups or as
pendent groups of the
prepolymer are not encompassed by sulfur-containing prepolymers. The
prepolymer backbone refers to
the portion of the prepolymer having repeating segments. Thus, a prepolymer
having the structure of HS¨
R¨R(¨CH2¨SH)¨[¨R¨(CH2)2¨S(0)2¨(CH2)¨S(0)2]5¨CH=CH2 where each R is a moiety
that does not
contain a sulfur atom in the prepolymer backbone, is not encompassed by a
sulfur-containing prepolymer.
A prepolymer having the structure
HS¨R¨R(¨CH2¨SH)¨[¨R¨(CH2)2¨S(0)2¨(CH2)¨S(0)21¨CH=CH2
where at least one R is a moiety that contains a sulfur atom, such as a
thioether group, is encompassed by
a sulfur-containing prepolymer.
[144] Sulfur-containing prepolymers having a high sulfur content can impart
chemical resistance to a
cured coreactive composition. For example, a sulfur-containing prepolymer
backbone can have a sulfur
content greater than 10 wt%, greater than 12 wt%, greater than 15 wt%, greater
than 18 wt%, greater than
20 wt%, or greater than 25 wt%, where wt% is based on the total weight of the
prepolymer backbone. A
chemically resistant prepolymer backbone can have a sulfur content, for
example, from 10 wt % to 25 wt
%, from 12 wt % to 23 wt %, from 13 wt % to 20 wt %, or from 14 wt % to 18 wt
%, where wt% is based
on the total weight of the prepolymer backbone. Sulfur content can be
determined according to ASTM
D297.
23
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[145] Examples of prepolymers having a sulfur-containing backbone include
polythioether
prepolymers, polysulfide prepolymers, sulfur-containing polyformal
prepolymers, monosulfide
prepolymers, and a combination of any of the foregoing.
[146] A coreactive sealant composition can comprise, for example, from 40 wt%
to 80 wt%, from 40
wt% to 75 wt%, from 45 wt% to 70 wt%, or from 50 wt% to 70 wt% of a sulfur-
containing prepolymer or
combination of sulfur-containing prepolymers, where wt% is based on the total
weight of the coreactive
composition. A coreactive sealant composition can comprise, for example,
greater than 40 wt%, greater
than 50 wt%, greater than 60 wt%, greater than 70 wt%, greater than 80 wt%, or
greater than 90 wt% of a
sulfur-containing prepolymer or combination of sulfur-containing prepolymer,
where wt% is based on the
total weight of the coreactive sealant composition. A coreactive sealant
composition can comprise, for
example, less than 90 wt%, less than 80 wt%, less than 70 wt%, less than 60
wt%, less than 50 wt%, or
less than 40 wt% of a sulfur-containing prepolymer or combination of sulfur-
containing prepolymers,
where wt% is based on the total weight of the coreactive sealant composition.
[147] A coreactive sealant composition for forming a cured sealant layer that
exhibits fuel resistance
can comprise, for example, prepolymers having a sulfur content greater than 10
wt%, where wt% is based
on the total weight of the prepolymer, rubber such as polybutadiene and
ethylene propylene diene
terpolymer (EPDM), a high crosslinking density, chemically resistant organic
filler such as polyamides,
polyphenylene sulfides, and polyethylenes, or a combination of any of the
foregoing.
[148] A sulfur-containing prepolymer can comprise a polythioether prepolymer
or a combination of
polythioether prepolymers.
[149] A polythioether prepolymer can comprise a polythioether prepolymer
comprising at least one
moiety having the structure of Formula (1), a thiol-terminated polythioether
prepolymer of Formula (la),
a terminal-modified polythioether of Formula (lb), or a combination of any of
the foregoing:
(1)
(la)
(lb)
wherein,
n can be an integer from 1 to 60;
each 12.' can independently be selected from C2-10 alkanediyl, C6-8
cycloalkanediyl, C644
alkanecycloalkanediyl, C5-8 heterocycloalkanediyl, and ¨[(CHR)p¨X¨]q(CHR),¨,
where,
p can be an integer from 2 to 6;
q can be an integer from 1 to 5;
r can be an integer from 2 to 10;
24
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
each R can independently be selected from hydrogen and methyl; and
each X can independently be selected from 0, S. and S-S; and
each A can independently be a moiety derived from a polyvinyl ether of Formula
(2) or a
polyalkenyl polyfunctionalizing agent of Formula (3):
CH2=CH-0-(R2-0)16-CH=CH2
(2)
B(-124-CH=CH2).
(3)
wherein,
m can be an integer from 0 to 50;
each 122 can independently be selected from C1_10 alkanediyl, C6-8
cycloalkanediyl, C6-14 alkanecycloalkanediyl, and -[(CHR)p-X-]q(CHR),--,
wherein p, q,
r, R, and X are as defined as for IV;
B represents a core of a z-valent, polyalkenyl polyfunctionalizing agent B(-R4-
CH=CH2)1 wherein,
z can be an integer from 3 to 6;
each 124 can independently be selected from Chio alkanediyl, Ci-io
heteroalkanediyl, substituted C1-10 alkanediyl, and substituted Ci-io
heteroalkanediyl; and
each le can independently be moiety comprising a terminal reactive group;
[150] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), IV
can be C2_10 alkanediyl.
[151] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), 10
can be -[ (CHR)p-X-
]q(CHR)r-.
[152] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), X
can be selected from 0
and S, and thus -[(CHR)p-X-]q(CHR),- can be -[(CHR)p-0-1q(CHR)r- or -[(CHR)p-S-
]q(CHR)r-. P
and r can be equal, such as where p and r can both be two.
[153] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), can
be selected from C2-
6 alkanediyl and -[(CHR)p-X-]q(CHR),-.
[154] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), can
be -[(CHR)p-X-
]q(CHR),-, and X can be 0, or X can be S.
[155] In moieties of Formula (1) and prepolymers of Formula (la) and (lb),
where IV can be -
[(CHR)p-X-]q(CHR)r-, p can be 2, r can be 2, q can be 1, and X can be S; or p
can be 2, q can be 2, r can
be 2, and X can be 0; or p can be 2, r can be 2, q can be 1, and X can be 0.
[156] In moieties of Formula (1) and prepolymers of Formula (la) and (1b), R1
can be -[(CHR)p-X-
]q(CHR)r-, each R can be hydrogen, or at least one R can be methyl.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[157] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), IV
can be -RCH2)p-X-
UCH2),- wherein each X can independently be selected from 0 and S.
[158] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), IV
can be -RCH2)p-X-
MCH2),- wherein each X can be 0 or each X can be S.
[159] In moieties of Formula (1) and prepolymers of Formula (la) and (lb), IV
can be -[(CH2)p-X-
]q(CH2),-, where p can be 2, X can be 0, q can be 2, r can be 2, R2 can be
ethanediyl, m can be 2, and n
can be 9.
[160] In moieties of Formula (1) and prepolymers of Formula (la) and (lb),
each 10 can be derived
from 1,8-dimercapto-3,6-dioxaoctane (DMDO; 2,2-(ethane-1,2-
diylbis(sulfany1))bis(ethan-l-thiol)), or
each IV can be derived from dimercaptodiethylsulfide (DMDS; 2,2'-thiobis(ethan-
l-thiol)), and
combinations thereof.
[161] In moieties of Formula (1) and prepolymers of Formula (la) and (lb),
each p can independently
be selected from 2, 3, 4, 5, and 6. Each p can be the same and can be 2, 3, 4,
5, or 6.
[162] In moieties of Formula (1) and prepolymers of Formula (la) and (lb),
each q can independently
be 1, 2, 3, 4, or 5. Each q can be the same and can be 1, 2, 3, 4, or 5.
[163] In moieties of Formula (1) and prepolymers of Formula (la) and (lb),
each r can independently
be 2, 3, 4, 5, 6, 7, 8, 9, or 10. Each r can be the same and can be 2, 3, 4,
5, 6, 7, 8, 9, or 10.
[164] In moieties of Formula (1) and prepolymers of Formula (la) and (lb),
each r can independently
be an integer from 2 to 4, from 2 to 6, or from 2 to 8.
[165] In divinyl ethers of Formula (2), m can be an integer from 0 to 50, such
as from 0 to 40, from 0 to
20, from 0 to 10, from 1 to 50, from 1 to 40, from 1 to 20, from 1 to 10, from
2 to 50, from 2 to 40, from 2
to 20, or from 2 to 10.
[166] In divinyl ethers of Formula (2), each R2 can independently be selected
from a C2_10 n-alkanediyl
group, a C3-6 branched alkanediyl group, and a -[(CH2)p-X-]q(C1-12),-- group.
[167] In divinyl ethers of Formula (2), each R2 can independently be a C2_10 n-
alkanediyl group, such as
methanediyl, ethanediyl, n-propanediyl, or n-butanediyl.
[168] In divinyl ethers of Formula (2), each R2 can independently comprise a -
[(CH2)p--X-]q(CH2)r-
group, where each X can be 0 or S.
[169] In divinyl ethers of Formula (2), each R2 can independently comprise a -
[(CH2)p-X-]q(CH2),-
group.
[170] In divinyl ethers of Formula (2), each m can be independently an integer
from 1 to 3. Each m can
be the same and can be 1, 2, or 3.
[171] In divinyl ethers of Formula (2), each R2 can independently be selected
from a C2-10 n-alkanediyl
group, a C3-6 branched alkanediyl group, and a -[(CH2)p-X-1q(CH2),- group.
26
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[172] In divinyl ethers of Formula (2), each R2 can independently be a C2_10 n-
alkanediyl group.
[173] In divinyl ethers of Formula (2), each R2 can independently be a -
[(CH2)p-X-]q(CH2),- group,
where each X can be 0 or S.
[174] In divinyl ethers of Formula (2), each R2 can independently be a -
[(CH2)p-X-]q(CH2)r- group,
where each X can be 0 or S, and each p can independently be 2, 3, 4, 5, and 6.
[175] In divinyl ethers of Formula (2), each p can be the same and can be 2,
3, 4, 5, or 6.
[176] In divinyl ethers of Formula (2), each R2 can independently be a -
[(CH2)p-X-]q(CH2),- group,
where each X can be 0 or S, and each q can independently be 1, 2, 3, 4, or 5.
[177] In divinyl ethers of Formula (2), each q can be the same and can be 1,
2, 3, 4, or 5.
[178] In divinyl ethers of Formula (2), each R2 can independently be a -
[(CH2)p-X-]q(CH2),- group,
where each X can be 0 or S, and each r can independently be 2, 3, 4, 5, 6, 7,
8, 9, or 10.
[179] In divinyl ethers of Formula (2), each r can be the same and can be 2,
3,4, 5, 6, 7, 8, 9, or 10. In
divinyl ethers of Formula (2), each r can independently be an integer from 2
to 4, from 2 to 6, or from 2 to
8.
[180] Examples of suitable divinyl ethers include ethylene glycol divinyl
ether (EG-DVE), butanediol
divinyl ether (BD-DVE) hexanediol divinyl ether (HD-DVE), diethylene glycol
divinyl ether (DEG-
DVE), Methylene glycol divinyl ether, tetraethylene glycol divinyl ether,
polytetrahydrofuryl divinyl
ether, cyclohexane dimethanol divinyl ether, and combinations of any of the
foregoing.
[181] A divinyl ether can comprise a sulfur-containing divinyl ether. Examples
of suitable sulfur-
containing divinyl ethers are disclosed, for example, in PCT Publication No.
WO 2018/085650.
[182] In moieties of Formula (1) each A can independently be derived from a
polyalkenyl
polyfunctionalizing agent. A polyalkenyl polyfunctionalizing agent can have
the structure of Formula
(3), where z can be 3, 4, 5, or 6.
[183] In polyalkenyl polyfunctionalizing agents of Formula (3), each It' can
independently be selected
from CIA alkanediyl, C1_10 heteroalkanediyl, substituted Cl_10 alkanediyl, or
substituted C1_10
heteroalkanediyl. The one or more substituent groups can be selected from, for
example, -OH, =0, C1-4
alkyl, and C1-4 alkoxy. The one or more heteroatoms can be selected from, for
example, 0, S, and a
combination thereof.
[184] Examples of suitable polyalkenyl polyfunctionalizing agents include
triallyl cyanurate (TAC),
triallylisocyanurate (TAIC), 1,3,5-trially1-1,3,5-triazinane-2,4,6-trione),
1,3,5-trially1-1,3,5-triazinane-
2,4,6-trione), 1,3-bis(2-methylally1)-6-methylene-5-(2-oxopropy1)-1,3,5-
triazinone-2,4-dione,
tris(allyloxy)methane, pentaerythritol triallyl ether, 1-(allyloxy)-2,2-
bis((allyloxy)methyl)butane, 2-prop-
2-ethoxy-1,3,5-tris(prop-2-enyl)benzene, 1,3,5-tris(prop-2-eny1)-1,3,5-
triazinane-2,4-dione, and 1,3,5-
27
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
tris(2-methylally1)-1,3,5-triazinane-2,4,6-trione, 1,2,4 -trivinylcyclohexane,
trimethylolpropane trivinyl
ether, and combinations of any of the foregoing.
[185] In moieties of Formula (1) and prepolymers of Formula (la)-(lb), the
molar ratio of moieties
derived from a divinyl ether to moieties derived from a polyalkenyl
polyfunctionalizing agent can be, for
example, from 0.9 mol% to 0.999 mol%, from 0.95 mol% to 0.99 mol%, or from
0.96 mol% to 0.99
mol%.
[186] In moieties of Formula (1) and prepolymers of Formula (1a)-(1b),each IV
can be -(CH2)2-0-
(CH2)2-0-(CH2)2-; each R2 can be -(CH2)2-; and m can be an integer from 1 to
4.
[187] In moieties of Formula (1) and prepolymers of Formula (1a)-(1b), each R2
can be derived from a
divinyl ether such a diethylene glycol divinyl ether, a polyalkenyl
polyfunctionalizing agent such as
triallyl cyanurate, or a combination thereof.
[188] In moieties of Formula (1) and prepolymers of Formula ( la)-( lb), each
A can independently be
selected from a moiety of Formula (2a) and a moiety of Formula (3a):
-(CH2)2-0-(R2-0)10-(CH02--
(2a)
B 1-R44012)2-1 2 {-R4-(CH2)2-S-[-RI-S-A-S-R1-] n-SH} z-2
(3a)
where m, R2, R4, A, B, m, n, and z are defined as in Formula (1), Formula
(2), and Formula (3).
[189] In moieties of Formula (1) and prepolymers of Formula (la)-(lb), each IV
can be -(CH2)2-0-
(CH2)2-0-(CH2)2-; each R2 can be -(CH2)2-; m can be an integer from 1 to 4;
and the
polyfunctionalizing agent B(-It4-CHH2)2 comprises trially1 cyanurate where z
is 3 and each R4 can be
-0-CH2-CH=CH2.
[190] Methods of synthesizing sulfur-containing polythioethers are disclosed,
for example, in U.S.
Patent No. 6,172,179.
[191] The backbone of a thiol-terminated polythioether prepolymer can be
modified to increase one or
more properties such as adhesion, tensile sliength, elongation, UV resistance,
hardness, and/or flexibility
of sealants prepared using polythioether prepolymers. For example, adhesion
promoting groups,
antioxidants, metal ligands, and/or urethane linkages can be incorporated into
the backbone of a
polythioether prepolymer to improve one or more performance attributes.
Examples of backbone-
modified polythioether prepolymers are disclosed, for example, in U.S. Patent
No. 8,138,273 (urethane
containing), U.S. Patent No. 9,540,540 (sulfone-containing), U.S. Patent No.
8,952,124
(bis(sulfonypalkanol-containing), U.S. Patent No. 9,382,642 (metal-ligand
containing), U.S. Application
Publication No. 2017/0114208 (antioxidant-containing), PCT International
Publication No. WO
2018/085650 (sulfur-containing divinyl ether), and PCT International
Publication No. WO 2018/031532
28
(urethane-containing). Polythioether prepolymers include prepolymers described
in U.S.
Application Publication Nos. 2017/0369737 and 2016/0090507.
[192] Examples of suitable thiol-terminated polythioether prepolymers are
disclosed, for example, in
U.S. Patent No. 6,172,179. A thiol-terminated polythioether prepolymer can
comprise Permapole P3.1E,
Permapole P3.1E-2.8, Permapol L56086, or a combination of any of the
foregoing, each of which is
available from PPG Aerospace. These PermapobD products are encompassed by the
thiol -terminated
polythioether prepolymers of Formula (1), (la), and (lb). Thiol-terminated
polythioethers include
prepolymers described in U.S. Patent No. 7,390,859 and urethane-containing
polythiols described in U.S.
Application Publication Nos. 2017/0369757 and 2016/0090507.
[193] A sulfur-containing prepolymer can comprise a polysulfide prepolymer or
a combination of
poly sulfide prepolymers.
[1941 A polysulfide prepolymer refers to a prepolymer that contains one or
more polysulfide linkages,
i.e., ¨So linkages, where x is from 2 to 4, in the prepolymer backbone. A
polysulfide prepolymer can
have two or more sulfur-sulfur linkages. Suitable thiol-terminatecl
polysulfide prepolymers are
commercially available, for example, from AkzoNobel and Toray Industries, Inc.
under the tradenames
Thioplast and from Thiokol-LP , respectively.
[195] Examples of suitable poly sulfide prepolymers are disclosed, for
example, in U.S. Patent Nos.
4,623,711; 6,172,179; 6,509,418; 7,009,032; and 7,879,955.
[196] Examples of suitable thiol-terminated poly sulfide prepolymers include
Thioplast G poly sulfides
such as Thioplasto GI, Thioplast '1 G4, Thioplast G10, Thioplast '1 G12,
Thioplast ') G21, Thioplast
G22, Thioplast G44, Thioplast ' G122, and Thioplasti 6131, which are
commercially available from
AkzoNobel. Suitable thiol-terminated polysulfide prepolymers such as Thioplast
G resins are liquid
thiol-terminated polysulfide prepolymers that are blends of di- and tri-
functional molecules where the
difunctional thiol-terminated poly sulfide prepolymers have the structure of
Formula (4) and the
trifunctional thiol-terminated poly sulfide polymers can have the structure of
Formula (5):
(4)
HS-(-R5-S-S-).-CH2-CH{-C112-(-S-S-R5-)b-SH} {-(-S-S-R5-)c-SH) (5)
where each IV is ¨(CH2)2-0-CH2-0 (CH2)2¨, and n = a + b + c, where the
value for n may be from 7
to 38 depending on the amount of the trifunctional cross-linking agent (1,2,3-
trichloropropane; TCP) used
during synthesis of the poly sulfide prepolymer. Thioplast G polysulfides can
have a number average
29
Date Regue/Date Received 2022-10-28
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
molecular weight from less than 1,000 Da to 6,500 Da, a ¨SH content from 1 wt%
to greater than 5.5
wt%, and a cross-linking density from 0 wt% to 2.0 wt%.
[197] A polysulfide prepolymer can further comprise a terminal-modified
polysulfide prepolymer
having the structure of Formula (4a), a terminal-modified polysulfide
prepolymer having the structure of
Formula (5a), or a combination thereof:
R3¨S¨(¨R5¨S¨S¨)n¨R5¨S¨R3
(4a)
R3¨S¨(-1V¨S¨S¨)n¨CH2¨CH{¨CH2¨(¨S¨S¨R5¨)b¨S¨} {¨(¨S¨S¨R5¨)0¨S¨R3}
(5a)
where n, a, b, c, and R5 are defmed as for Formula (4) and Formula (5), and R3
is a moiety comprising a
terminal reactive group.
[198] Examples of suitable thiol-terminated polysulfide prepolymers also
include Thiokol LP
polysulfides available from Toray Industries, Inc. such as Thiokol LP2,
Thiokol LP3, Thiokol LP12,
Thiokol LP23, Thiokol LP33, and Thiokol LP55. Thiokol LP polysulfides have
a number average
molecular weight from 1,000 Da to 7,500 Da, a ¨SH content from 0.8% to 7.7%,
and a cross-linking
density from 0% to 2%. Thiokol LP polysulfide prepolymers have the structure
of Formula (6) and
terminal-modified polysulfide prepolymers can have the structure of Formula
(6a):
HS¨RCH2)2-0-0-12-0¨(CH2)2¨S¨S¨]5¨(CH2)2-0¨CH2-0¨(CH2)2¨SH
(6)
R3¨S¨[(CH02-0¨CH2-0¨(CH2)2¨S¨S¨b¨(CH2)2-0¨CH2-0¨(CH2)2¨S¨R3
(6a)
where n can be such that the number average molecular weight from 1,000 Da to
7,500 Da, such as, for
example an integer from 8 to 80, and each R3 is a moiety comprising a terminal
reactive functional group.
[199] A thiol-terminated sulfur-containing prepolymer can comprise a Thiokol-
LP polysulfide, a
Thioplast G polysulfide, or a combination thereof.
[200] A polysulfide prepolymer can comprise a polysulfide prepolymer
comprising a moiety of
Formula (7), a thiol-terminated polysulfide prepolymer of Formula (7a), a
terminal-modified polysulfide
prepolymer of Formula (7b), or a combination of any of the foregoing:
¨R6¨(S3,¨R6)1¨
(7)
HS¨R6¨(Sy¨R6)1¨SH
(7a)
R3¨S-1V¨(Sy¨R6)1¨S-R3
(7b)
where,
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
t can be an integer from 1 to 60;
each R6 can independently be selected from branched alkanediyl, branched
arenediyl, and
a moiety having the structure -(CH2)p-0-(CH2)q-0-(CH2)r-;
q can be an integer from 1 to 8;
p can be an integer from 1 to 10;
r can be an integer from 1 to 10;
y can have an average value within a range from 1.0 to 1.5; and
each R3 is a moiety comprising a terminal reactive functional group.
[201] In moieties of Formula (7) and prepolymers of Formula (7a)-(7b), 0% to
20% of the 126 groups
can comprise branched alkanediyl or branched arenediyl, and 80% to 100% of the
R6 groups can be -
(CH2)p-0-(CH2)q-0-(CH2),,-.
[202] In moieties of Formula (7) and prepolymers of Formula (7a)-(7b),a
branched alkanediyl or a
branched arenediyl can be -R(-A).- where R is a hydrocarbon group, n is 1 or
2, and A is a branching
point. A branched alkanediyl can have the structure -CH2(-CH(-CH2-)-)-.
[203] Examples of thiol-terminated polysulfide prepolymers of Formula (7a) and
(7b) are disclosed, for
example, in U.S. Application Publication No. 2016/0152775, in U.S. Patent No.
9,079,833, and in U.S.
Patent No. 9,663,619.
[204] A polysulfide prepolymer can comprise a polysulfide prepolymer
comprising a moiety of
Formula (8), a thiol-terminated polysulfide prepolymer of Formula (8a), a
terminal-modified polysulfide
prepolymer of Formula (8b), or a combination of any of the foregoing:
(8)
HS-(1V-0-CH2-0-R7-Strr-)n-1-R7-0-CH2-0-R7-SH
(8a)
R3-S-(R7-0-C1-12-0-R7-Sm-)n-i-R7-0-CH2-0-R7-S-R3
(8b)
where R7 is C24 alkanediyl, m is an integer from 2 to 8, and n is an integer
from 2 to 370; and each R3 is
independently a moiety comprising a terminal reactive functional group.
[205] Moieties of Formula (8) and prepolymers of Formula (8a)-(8b), are
disclosed, for example, in JP
62-53354.
[206] A sulfur-containing prepolymer can comprise a sulfur-containing
polyformal prepolymer or a
combination of sulfur-containing polyformal prepolymers. Sulfur-containing
polyformal prepolymers
useful in sealant applications are disclosed, for example, in U.S. Patent No.
8,729,216 and in U.S. Patent
No. 8,541,513.
31
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[207] A sulfur-containing polyformal prepolymer can comprise a moiety of
Formula (9), a thiol-
terminated sulfur-containing polyformal prepolymer of Formula (9a), a terminal-
modified sulfur-
containing polyformal prepolymer of Formula (9b), a thiol-terminated sulfur-
containing polyformal
prepolymer of Formula (9c), a terminal-modified sulfur-containing polyformal
prepolymer of Formula
(9d), or a combination of any of the foregoing:
-R8-(S)p-R810-C(R9)2-0-R8-(S)p-R8-15-
(9)
R' -R8-(S)p-R840-C(R9)2-0-R8-(S)p-R8-]n-RIO
(9a)
R3-R8-(S)p-R8-40-C(R9)2-0-R8-(S)p-R8-].-R3
(9b)
{ Rw--R8-(S).-R8- [0-C (R9)2-0-R8-(S)p-R8-] õ--0-C(R9)2-0-1.2
(9c)
{R3-R8-(S)p-R840-C(R9)2-0-R8-(S)p-R8-].-0-C(R9)2-0-}.2
(9d)
where n can be an integer from 1 to 50; each p can independently be selected
from 1 and 2; each R8 can
be C2-6 alkanediyl; and each R9 can independently be selected from hydrogen,
C1_6 alkyl, C7-12
phenylalkyl, substituted C7-12 phenylallcyl, C6-12 cycloalkylalkyl,
substituted C6-12 cycloalkylalkyl, C3-12
cycloalkyl, substituted C3-12 cycloalkyl, C6-I2 aryl, and substituted C6-12
aryl; each IV is a moiety
comprising a terminal thiol group; and each R3 is independently a moiety
comprising a terminal reactive
functional group other than a thiol group; and Z can be derived from the core
of an m-valent parent polyol
Z(OH)m.
[208] A sulfur-containing prepolymer can comprise a monosulfide prepolymer or
a combination of
monosulfide prepolymers.
[209] A monosulfide prepolymer can comprise a moiety of Formula (10), a thiol-
terminated
monosulfide prepolymer of Formula (10a), a thiol-terminated monosulfide
prepolymer of Formula (10b),
a terminal-modified monosulfide prepolymer of Formula (10c), a terminal-
modified monosulfide
prepolymer of Formula (10d), or a combination of any of the foregoing:
_R13_[_s_(Rii_x)p4R12_x)q_m_b_s_
(10)
HS-Ri3--Hs_(Rii_x)p_(z12_x)q_RHH.-SH
(10a)
{HS-Ri3-[-S-(R"-X)p-(R'2-X)q-RJ3-],r-S-Nr-I.B
(10b)
R3-S-12.13-Fs_(t11-x)p_ozi2_xxi_RI3_,].-S-R3
(10c)
{R3-S-R13-[-S-(Rii-X)p-(R12-X)q-R13-1.-S-V'-}7,13
(10d)
wherein,
each RI' can independently be selected from C2-10 alkanediyl, such as C2-6
alkanediyl; C2-
branched alkanediyl, such as C3-6 branched alkanediyl or a C3-6 branched
alkanediyl having one
32
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
or more pendant groups which can be, for example, alkyl groups, such as methyl
or ethyl groups;
C6-8 cycloalkanediyl; C6-14 alkylcycloalkyanediyl, such as C6_10
alkylcycloalkanediyl; and C8_10
alkylarenediyl;
each IV-2 can independently be selected from C1_10 n-alkanediyl, such as C1-6
n-alkanediyl,
C2_10 branched alkanediyl, such as C3-6 branched alkanediyl having one or more
pendant groups
which can be, for example, alkyl groups, such as methyl or ethyl groups; C6-8
cycloalkanediyl; C6-
14 alkylcycloalkanediyl, such as C6_10 alkylcycloalkanediyl; and C8_10
alkylarenediyl;
each RP can independently be selected from C1_10 n-alkanediyl, such as C1_6 n-
alkanediyl,
C2_10 branched alkanediyl, such as C3-6 branched alkanediyl having one or more
pendant groups
which can be, for example, alkyl groups, such as methyl or ethyl groups; C6_8
cycloalkanediyl
group; C644 alkylcycloalkanediyl, such as a C6_10 alkylcycloalkanediyl; and
C8_10 alkylarenediyl;
each X can independently be selected from 0 and S;
p can be an integer from 1 to 5;
q can be an integer from 0 to 5; and
n can be an integer from 1 to 60, such as from 2 to 60, from 3 to 60, or from
25 to 35;
each IV is independently selected from a reactive functional group;
B represents a core of a z-valent polyfunctionalizing agent B(¨V)z, wherein:
z can be an integer from 3 to 6; and
each V can be a moiety comprising a terminal group reactive with a thiol
group;
each ¨V'¨ can be derived from the reaction of ¨V with a thiol.
[210] Methods of synthesizing thiol-terminated monosulfide comprising moieties
of Formula (10) or
prepolymers of Formula (10b)-(10c) are disclosed, for example, in U.S. Patent
No. 7,875,666.
[211] A monosulfide prepolymer can comprise a moiety of Formula (11), a thiol-
terminated
monosulfide prepolymer comprising a moiety of Formula (11a), comprise a thiol-
terminated monosulfide
prepolymer of Formula (1 lb), a thiol-terminated monosulfide prepolymer of
Formula (11c), a thiol-
terminated monosulfide prepolymer of Formula (11d), or a combination of any of
the foregoing:
¨[¨S¨(104¨X)p¨C(R15)2¨(X¨RH)q-1.¨S¨
(11)
H¨[¨S¨(R.14¨X)p¨C(R15)2¨(X¨Ri4)q-1.¨SH
(11a)
R3_[_s_(Ri.4_x)p_c(Ri5)24X¨R in¨S¨R3
(1 lb)
{H4¨S¨(1t14¨X)p¨C(R15)2¨(X¨RH)q¨b¨S¨V'¨}zB
(11c)
(R3¨[¨S¨(104¨X)p¨C(R15)2¨(X¨R14),1¨],¨S¨V¨}z13
(11d)
wherein,
33
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
each It' can independently be selected from C2-10 alkanediyl, such as C2_6
alkanediyl; a C3-10
branched alkanediyl, such as a C3-6 branched alkanediyl or a C3-6 branched
alkanediyl having one or more
pendant groups which can be, for example, alkyl groups, such as methyl or
ethyl groups; a C6-8
cycloalicanediy1; a C6-14 alkylcycloalkyanediyl, such as a C6-10
alkylcycloalkanediyl; and a C8_10
alkylarenediyl;
each 111-5 can independently be selected from hydrogen, C1-10 n-alkanediyl,
such as a C1-6 n-
alkanediyl, C3-10 branched alkanediyl, such as a C3-6 branched alkanediyl
having one or more pendant
groups which can be, for example, alkyl groups, such as methyl or ethyl
groups; a C6_8 cycloalkanediyl
group; a C6-14 alkylcycloalkanediyl, such as a C6_10 alkylcycloalkanediyl; and
a Cs_10 alkylarenediyl;
each X can independently be selected from 0 and S;
p can be an integer from 1 to 5;
q can be an integer from 1 to 5;
n can be an integer from 1 to 60, such as from 2 to 60, from 3 to 60, or from
25 to 35;
each 113 is a moiety comprising a terminal functional group;
B represents a core of a z-valent polyfunctionalizing agent B(¨V)2 wherein:
z can be an integer from 3 to 6; and
each V can be a moiety comprising a terminal group reactive with a thiol
group;
each ¨V'¨ can be derived from the reaction of ¨V with a thiol.
[212] Methods of synthesizing monosulfides of Formula (11)-(11d) are
disclosed, for example, in U.S.
Patent No. 8,466,220.
[213] A prepolymer can comprise a terminal-modified prepolymer such as a
terminal-modified sulfur-
containing prepolymer. Terminal-modified sulfur-containing prepolymers refer
to sulfur-containing
prepolymers comprising terminal reactive functional groups other than thiol
groups.
[214] A terminal reactive functional group such as R3 can be selected from,
for example, an alkenyl,
alkynyl, epoxy, isocyanate, hydroxyl, amine, Michael acceptor, Michael donor,
or another reactive
functional group. A terminal-modified sulfur-containing prepolymer can be
prepared, for example, by
reacting a thiol-terminated sulfur-containing prepolymer with a compound
comprising a terminal
functional group and a group reactive with a thiol group.
[215] Examples of suitable groups reactive with thiol groups include alkenyl
groups, alkynyl groups,
epoxy groups, Michael acceptor groups, and isocyanate groups.
[216] For example, an alkenyl-terminated sulfur-containing prepolymer can be
prepared by reacting a
polyalkenyl compound with a thiol-terminated sulfur-containing prepolymer, an
epoxy-terminated sulfur-
containing prepolymer can be prepared by reacting a polycpoxide with a thiol-
terminated sulfur-
containing prepolymer, an isocyanate-terminated sulfur-containing prepolymer
can be prepared by
34
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
reacting a polyisocyanate with a thiol-terminated sulfur-containing
prepolymer, and a Michael acceptor-
terminated sulfur-containing prepolymer can be prepared by reacting a
polyfunctional Michael acceptor
with a thiol-terminated sulfur-containing prepolymer.
[217] A coreactive composition can comprise a reactive monomer or a
combination of reactive
monomers. A coreactive monomer can comprise functional groups reactive with a
prepolymer and/or
another monomer.
[218] A reactive monomer can have a molecular weight, for example, less than
1,000 Da, less than 800
Da less than 600 Da, less than 500 Da, less than 400 Da, or less than 300 Da.
A monomer can have a
molecular weight, for example, from 100 Da to 1,000 Da, from 100 Da to 800 Da,
from 100 Da to 600
Da, from 150 Da, to 550 Da, or from 200 Da to 500 Da. A monomer can have a
molecular weight greater
than 100 Da, greater than 200 Da, greater than 300 Da, greater than 400 Da,
greater than 500 Da, greater
than 600 Da, or greater than 800 Da.
[219] A reactive monomer can have a reactive functionality of two or more, for
example, from 2 to 6,
from 2 to 5, or from 2 to 4. A reactive monomer can have a functionality of 2,
3, 4, 5, or 6. A reactive
monomer can have an average reactive functionality, for example, from 2 to 6,
from 2 to 5, from 2 to 4,
from 2 to 3, from 2.1 to 2.8, or from 2.2 to 2.6.
[220] A reactive monomer can comprise any suitable functional group such as,
for example, a thiol,
alkenyl, alkynyl, epoxy, isocyanate, Michael acceptor, Michael donor,
hydroxyl, amine, silanol,
polyalkoxysilyl, or other suitable reactive functional group.
[221] A reactive monomer can comprise, for example, a polythiol, a
polyalkenyl, a polyalkynyl, a
polyepoxide, a polyfunctional Michael acceptor, a polyfunctional Michael
donor, a polyisocyanate, a
polyol, a polyamine, a polyfunctional silanol, a polyfunctional
polyalkoxysilyl, or a combination of any
of the foregoing.
[222] A monomer can comprise a sulfur-containing monomer.
[223] A sulfur-containing monomer can have a sulfur content, for example, from
0 wt% to 80 wt%,
from 2 wt% to 75 wt%, from 5 wt% to 70 wt%, from 10 wt% to 65 wt%, from 15 wt%
to 60 wt%, or
from 20 wt% to 50 wt%%, where wt% is based on the total molecular weight of
the monomer. A
monomer can have a sulfur content, for example, greater than 0 wt%, greater
than 10 wt%, greater than
greater than 20 wt%, greater than 30 wt%, greater than 40 wt%, greater than 50
wt%, greater than 60
wt%, greater than 70 wt% or greater than 80 wt%, where wt% is based on the
total molecular weight of
the monomer. A monomer can have a sulfur content, for example, less than 80
wt%, less than 70 wt%,
less than 60 wt%, less than 50 wt%, less than 40 wt%, less than 30 wt%, less
than 20 wt%, less than 10
wt%, or less than 5 wt%, where wt% is based on the total molecular weight of
the monomer.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[224] A reactive monomer can comprise a polyfunctionalizing agent or a
combination of
polyfunctionalizing agents.
[225] Polyfunctionalizing agents can have a functionality of three or more
functional groups that can be
included in a composition to increase the cross-linking density of a cured
polymer matrix. A
polyfunctionalizing agent can comprise functional groups reactive with
reactive prepolymers and/or
reactive monomers.
[226] A polyfunctionalizing agent can comprise an average functionality, for
example, from 3 to 6,
such as from 3 to 5, or from 3 to 4. A polyfunctionalizing agent can have a
functionality of 3, 4, 5, or 6.
[227] A polyfunctionalizing agent can comprise, for example, a polythiol, a
polyalkenyl, a polyalkynyl,
a polyepoxide, a polyfunctional Michael acceptor, a polyfunctional Michael
donor, a polyisocyanate, a
polyol, a polyamine, a polyfunctional silanol, a polyfunctional
polyalkoxysilyl, or a combination of any
of the foregoing.
[228] Each of a first reactive compound and a second reactive compound can
independently comprise at
least two first functional groups and the second compound can comprise at
least two second functional
groups, where the second functional groups are reactive with the first
functional groups.
[229] For example, the first functional group can be a thiol group, and the
second functional group can
be an alkenyl group, an alkynyl group, an epoxy group, a Michael acceptor
group, an isocyanate group, or
a combination of any of the foregoing.
[230] The particular functional groups and curing chemistries can be selected
to provide a desired
curing rate and/or to impart a desired property to a cured layer of a
multilayer system.
[231] Examples of useful curing chemistries include hydroxyl/isocyanate,
amine/isocyanate,
epoxy/epoxy, and Michael acceptor/Michael donor reactions.
[232] Thus, a first functional group can comprise an isocyanate and a second
functional group can
comprise a hydroxyl group, an amine group, or a combination thereof.
[233] A first functional group can comprise an amine group and a second
functional group can
comprise an epoxy group.
[234] A first functional group can comprise an epoxy group and a second
functional group can
comprise an epoxy group.
[235] A first functional group can comprise a Michael acceptor group and a
second functional group
can comprise a Michael donor group.
[236] A first functional group can be a saturated functional group and the
second functional group can
be an unsaturated group. Each of the first functional group and the second
functional can comprise a
saturated functional group. Each of the first functional group and the second
functional can comprise an
unsaturated functional group. A saturated functional group refers to a
functional group and not having a
36
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
double bond. Examples of saturated functional groups include thiol, hydroxyl,
primary amine, secondary
amine, and epoxy groups. An unsaturated functional group refers to a group
having a reactive double
bond. Examples of unsaturated functional groups include alkenyl groups,
Michael acceptor groups,
isocyanate groups, acyclic carbonate groups, acetoacetate groups, carboxylic
acid groups, vinyl ether
groups, (meth)acrylate groups, and malonate groups.
[237] The first functional group can be a carboxylic acid group and the second
functional group can be
an epoxy group.
[238] The first functional group can be a Michael acceptor group such as a
(meth)acrylate group, a
maleic group, or a fumaric group, and the second functional group can be a
primary amine group or a
secondary amine group.
[239] The first functional group can be an isocyanate group and the second
functional group can be a
primary amine group, a secondary amine group, a hydroxyl group, or a thiol
group.
[240] The first functional group can be a cyclic carbonate group, an
acetoacetate group, or an epoxy
group; and the second functional group can be a primary amine group, or a
secondary amine group.
[241] The first functional group can be a thiol group, and the second
functional group can be an alkenyl
group, a vinyl ether group, a (meth)acrylate group.
[242] The first functional group can be a Michael acceptor group such as
(meth)acrylate group, a
cyanoacrylate, a vinylether a vinylpyridine, or an a,I3-unsaturated carbonyl
group and the second
functional group can be a malonate group, an acetylacetonate, a nitroalkane,
or other active alkenyl group.
[243] The first functional group can be a thiol group, and the second
functional group can be an alkenyl
group, an epoxy group, an isocyanate group, an alkynyl group, or a Michael
acceptor group.
[244] The first functional group can be a Michael donor group, and the second
functional group can be
a Michael acceptor group.
[245] Both the first functional group and the second functional group can be
thiol groups.
[246] Both the first functional group and the second functional group can be
alkenyl groups.
[247] Both the first functional group and the second functional group can be
Michael acceptor groups
such as (meth)acrylate groups.
[248] For example, the first reactive compound can comprise a polyamine and/or
a polyol and the
second reactive compound can comprise a polyisocyanate; the first reactive
compound can comprise a
Michael acceptor and the second reactive compound can comprise a Michael
donor; or the first reactive
compound can comprise a polythiol and the second reactive compound can
comprise a polythiol, a
polyisocyanate, a polyalkenyl, a polyalkynyl, a polyepoxide, a Michael
acceptor, or a combination of any
of the foregoing.
37
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[249] Functional groups can be selected to coreact at temperatures, for
example, less than 50 C, less
than 40 C, less than 30 C, less than 20 C, or less than 10 C. Functional
groups can be selected to coreact
at temperatures, for example, greater than 5 C, greater than 10 C, greater
than 20 C, greater than 30 C, or
greater than 40 C. Functional groups can be selected to coreact, for example,
at temperatures from 5 C to
50 C, from 10 C to 40 C, from 15 C, to 35 C, or from 20 C to 30 C.
[250] The cure rate for any of these coreactive chemistries can be modified by
including an appropriate
catalyst or combination of catalysts in a coreactive composition. The cure
rate for any of these coreactive
chemistries can be modified by increasing or decreasing the temperature of the
coreactive composition.
For example, although a coreactive composition can cure at temperatures less
than 30 C such as less than
25 C or less than 20 C, heating the coreactive composition can accelerate the
reaction rate, which can be
desirable under certain circumstances such as to accommodate an increased
printing speed. Increasing
the temperature of the coreactive components and/or the coreactive composition
can also serve to adjust
the viscosity to facilitate mixing the coreactive components and/or depositing
the coreactive composition.
[251] To form a multilayer system, it can be desirable that certain layers
cure faster than other layers.
For example, it can be desirable that an exterior layer cure fast to
facilitate the ability of an applied
multilayer system to retain an intended shape, and an interior layer to cure
slowly to develop adhesion
and/or desirable physical properties overtime.
[252] Each of the coreactive compositions used to prepare a layer of a
multilayer system can
independently comprise, for example, one or more additives such as, for
example, catalysts,
polymerization initiators, adhesion promoters, reactive diluents,
plasticizers, filler, colorants,
photochromic agents, rheology modifiers, cure activators and accelerators,
corrosion inhibitors, fire
retardants, UV stabilizers, rain erosion inhibitors, or a combination of any
of the foregoing.
[253] A coreactive composition can comprise one or more additives selected to
impart one or more
desired properties to a cured layer of a multilayer system. Example of
properties of a cured layer and
additives for providing the properties to a layer are provided in the
following paragraphs.
[254] A coreactive composition can include a catalyst or a combination of
catalysts.
[255] A catalyst or combination of catalysts can be selected to catalyze the
reaction of co-reactants in
the coreactive composition such as the reaction of the first reactive compound
and the second reactive
compound. The appropriate catalyst will depend on the curing chemistry. For
example, a thiol-ene or
thiol epoxy can comprise an amine catalyst.
[256] A coreactive composition can comprise, for example, from 0.1 wt% to 1
wt%, from 0.2 wt% to
0.9 wt%, from 0.3 wt% to 0.7 wt%, or from 0.4 wt% to 0.6 wt% of a catalyst or
combination of catalysts,
where wt% is based on the total weight of the coreactive composition.
38
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[257] A catalyst can include a latent catalyst or combination of latent
catalysts. Latent catalysts include
catalysts that have little or no activity until released or activated, for
example, by physical and/or
chemical mechanisms. Latent catalysts may be contained within a structure or
may be chemically
blocked. A controlled release catalyst may release a catalyst upon exposure to
ultraviolet radiation, heat,
ultrasonication, or moisture. A latent catalyst can be sequestered within a
core-shell structure or trapped
within a matrix of a crystalline or semi-crystalline polymer where the
catalyst can diffuse from the
encapsulant with time or upon activation such as by the application of thermal
or mechanical energy.
[258] A coreactive composition can comprise a dark cure catalyst or a
combination of dark cure
catalysts. A dark cure catalyst refers to a catalyst capable of generating
free radicals without being
exposed to electromagnetic energy.
[259] Dark cure catalysts include, for example, combinations of metal
complexes and organic
peroxides, tialkylborane complexes, and peroxide-amine redox initiators. A
dark cure catalyst can be
used in conjunction with a photopolymerization initiator or independent of a
photopolymerization
initiator.
[260] A coreactive composition based on thiol/thiol curing chemistries can
comprise a cure activator or
a combination of cure activators to initiate the thiol/thiol polymerization
reaction. Cure activators can be
used for example in a coreactive composition in which both the first reactive
compound and the second
reactive compound comprise thiol-terminated sulfur-containing prepolymers,
such as thiol-terminated
polysulfide prepolymers.
[261] A cure activator can comprise an oxidizing agent capable of oxidizing
mercaptan groups to form
disulfide bonds. Examples of suitable oxidizing agents include lead dioxide,
manganese dioxide, calcium
dioxide, sodium perborate monohydrate, calcium peroxide, zinc peroxide, and
dichromate.
[262] A cure activator can comprise an inorganic activator, an organic
activator, or a combination
thereof.
[263] Examples of suitable inorganic activators include metal oxides. Examples
of suitable metal oxide
activators include zinc oxide (Zn0), lead oxide (Pb0), lead peroxide (Pb03),
manganese dioxide (Mn02),
sodium perborate (NaB03 = H20), potassium permanganate (KMn04), calcium
peroxide (CaCO3), barium
peroxide (Ba03), cumene hydroperoxide, and combinations of any of the
foregoing. A cure activator can
be Mn02.
[264] A coreactive composition based on thiol/thiol curing chemistries can
comprise, for example, from
1 wt% to 10 wt% of a cure activator or combination of cure activators, wherein
wt% is based on the total
weight of the coreactive composition. For example, a coreactive composition
can comprise from 1 wt%
to 9 wt%, from 2 wt% to 8 wt%, from 3 wt% to 7 wt%, or from 4 wt% to 6 wt% of
an activator or a
combination of cure activators, wherein wt% is based on the total weight of
the coreactive composition.
39
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
For example, a coreactive composition can comprise greater than 1 wt% of a
cure activator or a
combination of cure activators, greater than 2 wt%, greater than 3 wt%,
greater than 4 wt%, greater than 5
wt%, or greater than 6 wt% of a cure actuator or combination of cure
activators, wherein wt% is based on
the total weight of the coreactive composition.
[265] A coreactive composition based on thiol/thiol curing chemistries can
include a cure accelerator or
combination of cure accelerators.
[266] Cure accelerators can act as sulfur donors to generate active sulfur
fragments capable of reacting
with the thiol groups of a thiol-terminated polysulfide prepolymer.
[267] Examples of suitable cure accelerators include thiazoles, thiurams,
sulfenamides, guanidines,
dithiocarbamates, xanthates, thioureas, aldehydeamines, and combinations of
any of the foregoing.
[268] A cure accelerator can comprise thiuram polysulfide, a thiuram
disulfide, or a combination
thereof.
[269] Examples of other suitable cure accelerators also include triazines and
sulfides or metallic
and amine salts of dialkyldithiophosphoric acids and dithiophosphates such as
triazines and sulfides or
metallic and amine salts of dialkyldithiophosphoric acids, and combinations of
any of the foregoing.
Examples of non-sulfur-containing cure accelerators include tetramethyl
guanidine (TMG), di-o-tolyl
guanidine (DOTG), sodium hydroxide (NaOH), water and bases.
[270] A coreactive composition can comprise, for example, from 0.01 wt% to 2
wt% of a cure
accelerator or combination of cure accelerators, from 0.05 wt% to 1.8 wt%,
from 0.1 wt% to 1.6 wt%, or
from 0.5 wt% to 1.5 wt% of a cure accelerator or combination of cure
accelerators, where wt% is based
on the total weight of the coreactive composition. A coreactive composition
can comprise, for example,
less than 2 wt%, less than 1.8 wt%, less than 1.6 wt%, less than 1.4 wt%, less
than 1.2 wt%, less than 1
wt%, less than 0.5 wt%, less than 0.1 wt%, or less than 0.05 wt% of a cure
accelerator or combination of
cure accelerators, where wt% is based on the total weight of the coreactive
composition.
[271] A coreactive composition can comprise one or more free radial initiators
such as thermally-
activated free radical initiators or free radical initiators activated by
actinic radiation.
[272] A coreactive composition can be curable by actinic radiation such as a
sealant composition based
on thiol/alkenyl, thiol/alkynyl and alkenyl/alkenyl curing chemistries. A
coreactive composition that are
curable by visible or ultraviolet radiation can comprise a photopolymerization
initiator or combination of
photopolymerization initiators.
[273] A coreactive composition can include a photoinitiator or combination of
photoinitiators. The
radiation can be actinic radiation that can apply energy effective in
generating an initiating species from a
photopolymerization initiator upon irradiation therewith, and widely includes
a.-rays, y-rays, X-rays,
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
ultraviolet (UV) light including UVA, UVA, and UVC spectra), visible light,
blue light, infrared, near-
infrared, or an electron beam. For example, the photoinitiator can be a UV
photoinitiator.
[274] Examples of suitable UV photoinitiators include a-hydroxyketones,
benzophenone, a, a.-
diethoxyacetophenone, 4,4-diethylaminobenzophenone, 2,2-dimethoxy-2-
phenylacetophenone, 4-
isopropylphenyl 2-hydroxy-2-propyl ketone, 1-hydroxycyclohexyl phenyl ketone,
isoamyl p-
dimethylaminobenzoate, methyl 4-dimethylaminobenzoate, methyl O-
benzoylbenzoate, benzoin, benzoin
ethyl ether, benzoin isopropyl ether, benzoin isobutyl ether, 2-hydroxy-2-
methyl-1-phenylpropan-1-one,
2-isopropylthioxanthone, dibenzosuberone, 2,4,6-
trimethylbenzoyldiphenylphosphine oxide,
bisacyclophosphine oxide, benzophenone photoinitiators, oxiune
photoinitiators, phosphine oxide
photoinitiators, and combinations of any of the foregoing.
[275] A coreactive composition can comprise from 0.05 wt% to 5 wt%, from
0.1 wt% to 4.0 wt%,
from 0.25 wt% to 3.0 wt%, from 0.5 wt% to 1.5 wt% of a photoinitiator or
combination of photoinitiators,
where wt% is based on the total weight of the coreactive composition.
[276] A coreactive composition can comprise a thermally active free radical
initiator. A thermally
activated free radical initiator can become active at elevated temperature,
such as at a temperature greater
than 25 C.
[277] Examples of suitable thermally activated free radical initiators include
organic peroxy
compounds, azobis(organonitrile) compounds, N-acyloxyamine compounds, 0-imino-
isourea
compounds, and combinations of any of the foregoing. Examples of suitable
organic peroxy compounds,
that may be used as thermal polymerization initiators include
peroxymonocarbonate esters, such as
tertiarybutylperoxy 2-ethylhexyl carbonate and tertiarybutylperoxy isopropyl
carbonate; peroxyketals,
such as 1,1-di-(tert-butyl peroxy)-3,3,5-trimethylcyclohexane;
peroxydicarbonate esters, such as di(2-
ethylhexyl)peroxydicarbonate, di(secondary butyl)peroxydicarbonate and
diisopropylperoxydicarbonate;
diacyperoxides such as 2,4-dichlorobenzoyl peroxide, isobutyryl peroxide,
decanoyl peroxide, lauryl
peroxide, propionyl peroxide, acetyl peroxide, benzoyl peroxide, and p-
chlorobenzoyl peroxide;
peroxyesters such as tert-butylperoxy pivalate, tert-butylperoxy octylate, and
tert-butylperoxyisobutyrate;
methylethylketone peroxide, acetylcyclohexane sulfonyl peroxide, and
combinations of any of the
foregoing. Other examples of suitable peroxy compounds include 2,5-dimethy1-
2,5-di(2-
ethylhexanoylperoxy)hexane, and/or 1,1-bis(tert-butylperoxy)-3,3,5-
trimethylcyclohexane. Examples of
suitable azobis(organonitrile) compounds that may be used as thermal
polymerization initiators include
azobis(isobutyronitrile), 2,2'-azobis(2-methyl-butanenitrile), and/or
azobis(2,4-dimethylvaleronitrile). A
thermally activated free radical initiator can comprise 1-acetoxy-2,2,6,6-
tetramethylpiperidine and/or 1,3-
dicyclohexy1-0-(N-cyclohcxylidencamino)-isourea.
41
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[278] A coreactive composition can comprise an adhesion promoter or
combination of adhesion
promoters. Adhesion promoters can enhance the adhesion of a sealant to an
underlying substrate such as
a metal, composite, polymeric, or a ceramic surface, or to a coating such as a
primer coating or other
coating layer. Adhesion promoters can enhance adhesion to filler and to other
layers of a multilayer
system.
[279] An adhesion promoter can include a phenolic adhesion promoter, a
combination of phenolic
adhesion promoters, an organo-functional silane, a combination of organo-
functional silanes, or a
combination of any of the foregoing. An organo-functional alkoxysilane can be
an amine-functional
alkoxysilane. The organo group can be selected from, for example, a thiol
group, an amine group, an
epoxy group, an alkenyl group, an isocyanate group, or a Michael acceptor
group.
[280] A phenolic adhesion promoter can comprise a cooked phenolic resin, an un-
cooked phenolic
resin, or a combination thereof. Examples of suitable adhesion promoters
include phenolic resins such as
Methylon phenolic resin, and organosilanes, such as epoxy-, mercapto- or
amine-functional silanes,
such as Silquest orgariosilanes. A cooked phenolic resin refers to a phenolic
resin that has been
coreacted with a monomer or prepolymer.
[281] A phenolic adhesion promoter can comprise the reaction product of a
condensation reaction of a
phenolic resin with one or more thiol-terminated polysulfides. Phenolic
adhesion promoters can be thiol-
terminated.
[282] Examples of suitable phenolic resins include those synthesized from 2-
(hydroxymethyl)phenol,
(4-hydroxy-1,3-phenylene)dimethanol, (2-hydroxybenzene-1,3,4-triy1)
trimethanol, 2-benzy1-6-
(hydroxymethyl)phenol, (4-hydroxy-5-((2 -hydroxy-5 -(hydroxymethyl)cyclohexa-
2,4-dien-1-yl)methyl)-
1,3-phenylene)dimethanol, (4-hydroxy-5-((2-hydroxy-3,5-
bis(hydroxymethypcyclohexa-2,4-dien-1-
yOmethyl)-1,3-phenylene)dimethanol, and a combination of any of the foregoing.
[283] Suitable phenolic resins can be synthesized by the base-catalyzed
reaction of phenol with
formaldehyde.
[284] Phenolic adhesion promoters can comprise the reaction product of a
condensation reaction of a
Methylon resin, a Varcum resin, or a Durez resin available from Durez
Corporation with a thiol-
terminated polysulfide such as a Thioplast resin.
[285] Examples of Methylon resins include Methylon 75108 (allyl ether of
methylol phenol, see
U.S. Patent No. 3,517,082) and Methylon 75202.
[286] Examples of Varcum resins include Varcum 29101, Varcum 29108, Varcum
29112,
Varcum 29116, Varcum 29008, Varcum 29202, Varcum 29401, Varcum 74 29159,
Varcum
29181, Varcum 92600, Varcum 94635, Varcum 94879, and Varcum 94917.
[287] An example of a Durez resin is Durez 34071.
42
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[288] A coreactive composition can comprise an organo-functional alkoxysilane
adhesion promoter
such as an organo-functional alkoxysilane. An organo-functional alkoxysilane
can comprise hydrolysable
groups bonded to a silicon atom and at least one organofunctional group. An
organo-functional
alkoxysilane can have the structure R20¨(CH2)11¨Si(-0R)3_nR. , where R" is an
organofunctional group, n
is 0, 1, or 2, and R is alkyl such as methyl or ethyl. Examples of
organofunctional groups include epoxy,
amino, methacryloxy, or sulfide groups. An organo-functional alkoxysilane can
be a dipodal alkoxysilane
having two or more alkoxysilane groups, a functional dipodal alkoxysilane, a
non-functional dipodal
alkoxysilane or a combination of any of the foregoing. An organofunctional
alkoxysilane can be a
combination of a monoalkoxysilane and a dipodal alkoxysilane. For amino
functional alkoxysilanes, It2
can be ¨NH2,
[289] Examples of suitable amino-functional alkoxysilanes under the Silquest
tradename include y-
aminopropyltriethoxysilane (Silquest A-1100), y-aminopropylsilsesquioxane
(Silquest A-1108), y-
aminopropyltrimethoxysilane (Silquest A-1110), N-0-(aminoethyl)-y-
aminopropyltrimethoxysi1ane
(Silquest 1120), benzylamino-silane (Silquest 1128), triaminofunctional
silane (Silquest A-1130),
bis-(y-triethoxysilylpropyl)amine (Silquest Y-11699), bis-(y-
trimethoxysilylpropyl)amine (Silquest A-
1170), polyazamide (Silquest A-1387), ethoxy based polyazamide (Silquest Y-
19139), and N-13-
(aminoethyl)-y-aminopropylmethyldimethoxysilane (Silquest A-2120).
[290] Suitable amine-functional alkoxysilanes are commercially available, for
example, from Gelest
Inc, from Dow Corning Corporation, and Momentive.
[291] A coreactive composition can comprise, for example, from 1 wt% to 16 wt%
of an adhesion
promoter, from 3 wt% to 14 wt%, from 5 wt% to 12 wt%, or from 7 wt% to 10 wt%
of an adhesion
promoter or combination of adhesion promoters, where wt% is based on the total
weight of the coreactive
composition.
[292] A coreactive composition can comprise less than 16 wt% of an adhesion
promoter, less than 14
wt%, less than 12 wt%, less than 10 wt%, less than 8 wt%, less than 6 wt%,
less than 4 wt% or less than 2
wt% of an adhesion promoter or combination of adhesion promoters, where wt% is
based on the total
weight of the coreactive composition.
[293] A coreactive composition for forming a layer of a multilayer system can
comprise a filler or
combination of filler. A filler can comprise, for example, inorganic filler,
organic filler, low-density filler
such as a filler having a specific gravity less than 1, conductive filler, or
a combination of any of the
foregoing.
[294] A coreactive composition for forming a multilayer system can comprise an
inorganic filler or
combination of inorganic filler.
43
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
12951 An inorganic filler can be included, for example, to provide mechanical
reinforcement and to
control the theological properties of the composition. Inorganic filler may be
added to compositions to
impart desirable physical properties such as, for example, to increase the
impact strength, to control the
viscosity, or to modify the electiical properties of a cured coreactive
composition.
[296] Inorganic filler useful in a sealant composition include carbon black,
calcium carbonate,
precipitated calcium carbonate, calcium hydroxide, hydrated alumina (aluminum
hydroxide), talc, mica,
titanium dioxide, alumina silicate, carbonates, chalk, silicates, glass, metal
oxides, graphite, and
combinations of any of the foregoing.
1297] Suitable calcium carbonate filler can include products such as Socal
31, Socal 312, Socal
U1S1, Socal UaS2, Socal N2R, Winnofil SPM, and Winnofil SPT available from
Solvay Special
Chemicals. A calcium carbonate filler can include a combination of
precipitated calcium carbonates.
[298] Inorganic filler can be surface treated to provide hydrophobic or
hydrophilic surfaces that can
facilitate dispersion and compatibility of the inorganic filler with other
components of a coreactive
composition. An inorganic filler can include surface-modified particles such
as, for example, surface
modified silica. The surface of silica particles can be modified, for example,
to be tailor the
hydrophobicity or hydrophilicity of the surface of the silica particle. The
surface modification can affect
the dispensability of the particles, the viscosity, the curing rate, and/or
the adhesion.
[299] A coreactive composition can comprise an organic filler or a combination
of organic filler.
[300] Organic filler can be selected to have a low specific gravity and to be
resistant to solvents such as
JRF Type I and/or to reduce the density of a layer. Suitable organic filler
can also have acceptable
adhesion to the sulfur-containing polymer matrix. An organic filler can
include solid powders or
particles, hollow powders or particles, or a combination thereof.
[301] An organic filler can have a specific gravity, for example, less than
1.15, less than 1.1, less than
1.05, less than 1, less than 0.95, less than 0.9, less than 0.8, or less than
0.7. Organic filler can have a
specific gravity, for example, within a range from 0.85 to 1.15, within a
range from 0.9 to 1.1, within a
range from 0.9 to 1.05, or from 0.85 to 1.05.
[302] Organic filler can comprise thermoplastics, thermosets, or a combination
thereof. Examples of
suitable thermoplastics and thermosets include epoxies, epoxy-amides, ethylene
tetrafluoroethylene
copolymers, nylons, polyethylenes, polypropylenes, polyethylene oxides,
polypropylene oxides,
polyvinylidene chlorides, polyvinylfluorides, tetrafluoroethylene, polyamides,
polyimides, ethylene
propylenes, perfluorohydrocarbons, fluoroethylenes, polycarbonates,
polyetheretherketones,
polyetherketones, polyphenylene oxides, polyphenylene sulfides, polystyrenes,
polyvinyl chlorides,
melamines, polyesters, phenolics, epichlorohydrins, fluorinated hydrocarbons,
polycyclics,
44
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
polybutadienes, polychloroprenes, polyisoprenes, polysulfides, polyurethanes,
isobutylene isoprenes,
silicones, styrene butadienes, liquid crystal polymers, and combinations of
any of the foregoing.
[303] Examples of suitable polyamide 6 and polyamide 12 particles are
available from Toray Plastics as
grades SP-500, SP-10, TR-1, and TR-2. Suitable polyamide powders are also
available from the Arkema
Group under the tradename OrgasolO, and from Evonik Industries under the
tradename Vestosin .
[304] An organic filler can have any suitable shape. For example, an organic
filler can comprise
fractions of crushed polymer that has been filtered to select a desired size
range. An organic filler can
comprise substantially spherical particles. Particles can be solid or can be
porous.
[305] An organic filler can have an average particle size, for example, within
a range from 1 gm to 100
gm, 2 pm to 40 gm, from 2 gm to 30 pm, from 4 gm to 25 gm, from 4 gm to 20 gm,
from 2 gm to 12
gm, or from 5 gm to 15 gm. An organic filler can have an average particle
size, for example, less than
100 gm, less than 75 gm, less than 50 gm, less than 40 gm, or less than 20 pm.
Particle size distribution
can be determined using a Fischer Sub-Sieve Sizer or by optical inspection.
[306] An organic filler can include a low-density filler such as a modified,
expanded thermoplastic
microcapsules. Suitable modified expanded thermoplastic microcapsules can
include an exterior coating
of a melamine or urea/formaldehyde resin.
[307] A coreactive composition can comprise low density microcapsules. A low-
density microcapsule
can comprise a thermally expandable microcapsule.
[308] A thermally expandable microcapsule refers to a hollow shell comprising
a volatile material that
expands at a predetermined temperature. Thermally expandable thermoplastic
microcapsules can have an
average initial particle size of 5 gm to 70 gm, in some cases 10 gm to 24 gm,
or from 10 gm to 17 gm.
The term "average initial particle size" refers to the average particle size
(numerical weighted average of
the particle size distribution) of the microcapsules prior to any expansion.
The particle size distribution
can be determined using a Fischer Sub-Sieve Sizer or by optical inspection.
[309] Examples of materials suitable for forming the wall of a thermally
expandable microcapsule
include polymers of vinylidene chloride, acrylonitrile, styrene,
polycarbonate, methyl methacrylate, ethyl
acrylate, and vinyl acetate, copolymers of these monomers, and combinations of
the polymers and
copolymers. A crosslinking agent may be included with the materials forming
the wall of a thermally
expandable microcapsule.
[310] Examples of suitable thermoplastic microcapsules include ExpancelTM
microcapsules such as
Expance1TM DE microspheres available from AkzoNobel. Examples of suitable
ExpancelTM DE
microspheres include ExpancelTm 920 DE 40 and ExpancelTM 920 DE 80. Suitable
low-density
microcapsules are also available from Kureha Corporation.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[311] Low density filler such as low density microcapsules can be
characterized by a specific gravity
within a range from 0.01 to 0.09, from 0.04 to 0.09, within a range from 0.04
to 0.08, within a range from
0.01 to 0.07, within a range from 0.02 to 0.06, within a range from 0.03 to
0.05, within a range from 0.05
to 0.09, from 0.06 to 0.09, or within a range from 0.07 to 0.09, wherein the
specific gravity is determined
according to ISO 787-11. Low density filler such as low-density microcapsules
can be characterized by a
specific gravity less than 0.1, less than 0.09, less than 0.08, less than
0.07, less than 0.06, less than 0.05,
less than 0.04, less than 0.03, or less than 0.02, wherein the specific
gravity is determined according to
ISO 787-11.
[312] Low density filler such as low microcapsules can be characterized by a
average particle diameter
from 1 pm to 100 gm and can have a substantially spherical shape. A
substantially spherical particle can
have a maximum cross-sectional dimension that is less than the minimum cross-
sectional dimension of a
particle Low density filler such as low-density microcapsules can be
characterized, for example, by a
average particle diameter from 10 gm to 100 gm, from 10 gm to 60 gm, from 10
gm to 40 gm, or from
gm to 30 gm, as determined according to ASTM D6913.
[313] Low density filler such as low-density microcapsules can comprise
expanded microcapsules or
microballoons having a coating of an aminoplast resin such as a melamine
resin. Aminoplast resin-coated
particles are described, for example, in U.S. Patent No. 8,993,691. Such
microcapsules can be formed by
heating a microcapsule comprising a blowing agent surrounded by a
thermoplastic shell. Uncoated low-
density microcapsules can be reacted with an aminoplast resin such as a
urea/formaldehyde resin to
provide a coating of a thermoset resin on the outer surface of the particle.
[314] With the coating of an aminoplast resin, an aminoplast-coated
microcapsule can be characterized
by a specific gravity, for example, within a range from 0.02 to 0.08, within a
range from 0.02 to 0.07,
within a range from 0.02 to 0.06, within a range from 0.03 to 0.07, within a
range from 0.03 to 0.065,
within a range from 0.04 to 0.065, within a range from 0.045 to 0.06, or
within a range from 0.05 to 0.06,
wherein the specific gravity is determined according to ISO 787-11.
[315] A coreactive composition can comprise micronized oxidized polyethylene
homopolymer. An
organic filler can include a polyethylenes, such as an oxidized polyethylene
powder. Suitable
polyethylenes are available, for example, from Honeywell International, Inc.
under the tradename
ACumistk, from INEOS under the tradename Eltrex , and Mitsui Chemicals
America, Inc. under the
tradename Mipelon .
[316] A coreactive composition can comprise, for example, from 1 wt% to 90 wt%
of low-density filler,
from 1 wt% to 60 wt%, from 1 wt% to 40 wt%, from 1 wt% to 20 wt%, from 1 wt%
to 10 wt%, or from 1
wt% to 5 wt% of low-density filler, where wt% is based on the total weight of
the coreactive composition.
46
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[317] A coreactive composition can comprise greater than 1 wt% low density
filler, greater than 1 wt%,
greater than 2 wt%, greater than 3 wt%, greater than 4 wt%, greater than 6
wt%, or greater than 10 wt%
low-density filler, where wt% is based on the total weight of the coreactive
composition.
[318] A coreactive composition can comprise from 1 vol% to 90 vol% low-density
filler, from 5 vol%
to 70 vol%, from 10 vol% to 60 vol%, from 20 vol% to 50 vol%, or from 30 vol%
to 40 vol% low density
filler, where vol% is based on the total volume of the coreactive composition.
[319] A coreactive composition can comprise greater than 1 vol% low-density
filler, greater than 5
vol%, greater than 10 vol%, greater than 20 vol%, greater than 30 vol%,
greater than 40 vol%, greater
than 50 vol%, greater than 60 vol%, greater than 70 vol%, or greater than 80
vol% low-density filler,
where vol% is based on the total volume of the coreactive composition.
[320] A coreactive composition can include a conductive filler or a
combination of conductive filler. A
conductive filler can include electrically conductive filler, semiconductive
filler, thermally conductive
filler, magnetic filler, EMI/RFI shielding filler, static dissipative filler,
electroactive filler, or a
combination of any of the foregoing. EMI/RFI shielding effectiveness can be
determined according to
ASTM D4935
[321] A coreactive composition can comprise an electrically conductive filler
or combination of
electrically conductive filler.
[322] To render a cured layer electrically conductive, the concentration of an
electrically conductive
filler can be above the electrical percolation threshold, where a conductive
network of electrically
conductive particles is formed. Once the electrical percolation threshold is
achieved, the increase in
conductivity as function of filler loading can be modeled by a simple power-
law expression:
EQN. 1
where 9 is the filler volume fraction, (pc is the percolation threshold, of is
the filler conductivity, 9 is the
composite conductivity, and t is a scaling component. The filler need not be
in direct contact for current
flow and conduction can take place via tunneling between thin layers of binder
surrounding the
electrically conductive filler particles, and this tunneling resistance can be
the limiting factor in the
conductivity of an electrically conductive layer.
[323] A conductive filler can have any suitable shape and/or dimensions. For
example, an electrically
conductive filler can be in form of particles, powders, flakes, platelets,
filaments, fiber, crystals, or a
combination of any of the foregoing.
[324] A conductive filler can comprise a combination of conductive filler
having different shapes,
different dimensions, different properties such as, for example, different
thermal conduction, electrical
conduction, magnetic permittivity, electromagnetic properties, or a
combination of any of the foregoing.
47
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[325] A conductive filler can be a solid or can be in the form of a substrate
such as a particle having a
coating of a conductive material. For example, a conductive filler can be a
low-density microcapsule
having an exterior conductive coating.
[326] Examples of suitable conductive filler such as electrically conductive
filler include metals, metal
alloys, conductive oxides, semiconductors, carbon, carbon fiber, and
combinations of any of the
foregoing.
[327] Other examples of electrically conductive filler include electrically
conductive noble metal-based
filler such as pure silver; noble metal-plated noble metals such as silver-
plated gold; noble metal-plated
non-noble metals such as silver plated cooper, nickel or aluminum, for
example, silver-plated aluminum
core particles or platinum-plated copper particles; noble-metal plated glass,
plastic or ceramics such as
silver-plated glass microspheres, noble-metal plated aluminum or noble-metal
plated plastic
microspheres; noble-metal plated mica; and other such noble-metal conductive
filler. Non-noble metal-
based materials can also be used and include, for example, non-noble metal-
plated non-noble metals such
as copper-coated iron particles or nickel-plated copper; non-noble metals,
e.g., copper, aluminum, nickel,
cobalt; non-noble-metal-plated-non-metals, e.g., nickel-plated graphite and
non-metal materials such as
carbon black and graphite. Combinations of electrically conductive filler and
shapes of electrically
conductive filler can be used to achieve a desired conductivity, EMI/RFI
shielding effectiveness,
hardness, and other properties suitable for a particular application.
[328] The amount and type of electrically conductive filler can be selected to
produce a coreactive
composition which, when cured, exhibits a sheet resistance (four-point
resistance) of less than 0.50
0,/cm2, or a sheet resistance less than 0.15 Cl/cm2. The amount and type of
filler can also be selected to
provide effective EMI/RFI shielding over a frequency range of from 1 MHz to 18
GHz for an aperture
sealed using a multilayer composition provided by the present disclosure.
[329] Organic filler, inorganic filler, and low-density filler can be coated
with a metal to provide
conductive filler.
[330] An electrically conductive filler can include graphene. Graphene
comprises a densely packed
honeycomb crystal lattice made of carbon atoms having a thickness equal to the
atomic size of one carbon
atom, i.e., a monolayer of sp2 hybridized carbon atoms arranged in a two-
dimensional lattice.
[331] Graphene can comprise graphenic carbon particles. Graphenic carbon
particles refer to carbon
particles having structures comprising one or more layers of one-atom-thick
planar sheets of sp2-bonded
carbon atoms that are densely packed in a honeycomb crystal lattice. An
average number of stacked
layers can be less than 100, for example, less than 50. An average number of
stacked layers can be 30 or
less, such as 20 or less, 10 or less, or, in some cases, 5 or less. Graphenic
carbon particles can be
substantially flat, however, at least a portion of the planar sheets may be
substantially curved, curled,
48
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
creased or buckled. Graphenic carbon particles typically do not have a
spheroidal or equiaxed
morphology.
[332] Graphenic carbon particles can have a thickness, measured in a direction
perpendicular to the
carbon atom layers, for example, of no more than 10 rim, no more than 5 rim,
or no more than 4 or 3 or 2
or 1 nm, such as no more than 3.6 nm. Graphenic carbon particles can be from 1
atom layer up to 3, 6, 9,
12, 20 or 30 atom layers thick, or more. Graphenic carbon particles can have a
width and length,
measured in a direction parallel to the carbon atoms layers, of at least 50
nm, such as more than 100 nm,
more than 100 nm up to 500 nm, or more than 100 nm up to 200 nm. Graphenic
carbon particles can be
provided in the form of ultrathin flakes, platelets or sheets having
relatively high aspect ratios, where the
aspect ratio is the ratio of the longest dimension of a particle to the
shortest dimension of the particle, of
greater than 3:1, such as greater than 10:1.
[333] Graphenic carbon particles can comprise exfoliated graphite and have
different characteristics in
comparison with the thermally produced graphenic carbon particles, such as
different size distributions,
thicknesses, aspect ratios, structural morphologies, oxygen contents, and
chemical functionalities at the
basal planes/edges.
[334] Graphenic carbon particles can be functionalized. Functionalized
graphenic carbon particles
refers to graphenic carbon particles where organic groups are covalently
bonded to the graphenic carbon
particles. The graphenic carbon particles can be functionalized through the
formation of covalent bonds
between the carbon atoms of a particle and other chemical moieties such as
carboxylic acid groups,
sulfonic acid groups, hydroxyl groups, halogen atoms, nitro groups, amine
groups, aliphatic hydrocarbon
groups, phenyl groups and the like. For example, functionalization with
carbonaceous materials may
result in the formation of carboxylic acid groups on the graphenic carbon
particles. Graphenic carbon
particles may also be functionalized by other reactions such as Diels-Alder
addition reactions, 1,3-dipolar
cycloaddition reactions, free radical addition reactions and diazonium
addition reactions. Hydrocarbon
and phenyl groups may be further functionalized. For graphenic carbon
particles having a hydroxyl
functionality, the hydroxyl functionality can be modified and extended by
reacting these groups with, for
example, an organic isocyanate.
[335] Different types of graphenic carbon particles may be used in a
coreactive composition.
[336] A coreactive composition can comprise, for example, from 2 wt% to 50
wt%, from 4 wt% to 40
wt%, from 6 wt% to 35 wt%, or from 10 wt% to 30 wt% thermally produced
graphenic carbon particles.
[337] Filler used to impart electrical conductivity and EMI/RFI shielding
effectiveness can be used in
combination with graphene.
49
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[338] Electrically conductive non-metal filler, such as carbon nanotubes,
carbon fibers such as
graphitized carbon fibers, and electrically conductive carbon black, can also
be used in a coreactive
composition in combination with gmphene.
[339] Conductive filler can comprise magnetic filler or combination of
magnetic filler.
[340] The magnetic filler can include a soft magnetic metal. This can enhance
permeability of the
magnetic mold resin. As a main component of the soft magnetic metal, at least
one magnetic material
selected from Fe, Fe¨Co, Fe¨Ni, Fe¨Al, and Fe¨Si may be used. A magnetic
filler can be a soft magnetic
metal having a high bulk permeability. As the soft magnetic metal, at least
one magnetic material
selected can be Fe, FeCo, FeNi, FeAl, and FeSi may be used. Specific examples
include a Permalloy
(FeNi alloy), a super Permalloy (FeNiMo alloy), a sendust (FeSiAl alloy), an
FeSi alloy, an FeCo alloy,
an FeCr alloy, an FeCrSi alloy, FeNiCo alloy, and Fe. Other examples of
magnetic filler include iron-
based powder, iron-nickel based powder, iron powder, ferrite powder, Alnico
powder, Sm2Co17 powder,
Nd-B-Fe powder, barium ferrite BaFe204, bismuth ferrite BiFe03, chromium
dioxide Cr02, SmFeN,
NdFeB, and SmCo.
[341] A surface of the magnetic filler can be insulation-coated or can have a
film thickness of the
insulation coating equal to or larger than 10 nm.
[342] A surface of the magnetic filler can be insulation-coated with a metal
oxide such as Si, Al, Ti, Mg
or an organic material for enhancing fluidity, adhesion, and insulation
performance.
[343] Examples of suitable carbonaceous materials for use as conductive filler
other than graphene and
graphite include, for example, graphitized carbon black, carbon fibers and
fibrils, vapor-grown carbon
nanofibers, metal coated carbon fibers, carbon nanotubes including single- and
multi-walled nanotubes,
fullerenes, activated carbon, carbon fibers, expanded graphite, expandable
graphite, graphite oxide,
hollow carbon spheres, and carbon foams.
[344] Conductive filler can include semiconductors or combinations of
semiconductors.
[345] Examples of suitable semiconductive materials include semiconducting
nanomaterials such as
nanoparticles, nanorods, nanowires, nanotubes, and nanosheets, semiconducting
metal oxides such as tin
oxide, antimony oxide, and indium oxide, semiconducting polymers such as
PEDOT:PSS,
polythiophenes, poly(p-phenylene sulfide), polyanilines, poly(pyrrole)s,
poly(acetylene)s, poly(p-
phenylene vinylene), polyparaphenylene, any other conjugated polymer, and
semiconducting small
molecules, for example, having a molecular mass less than 5,000 Da, such as
rubrene, pentacene,
anthracene, and aromatic hydrocarbons. Examples of semiconducting
nanomaterials include quantum
dots, III-V or II-VI semiconductors, Si, Ge, transition metal dichalcogenides
such as WS2, WSe2, and
MoSes, graphenc nanoribbons, semiconducting carbon nanotubes, and fullerencs
and fullercne derivatives.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[346] A filler can include metal fiber such as steel, titanium, aluminum,
gold, silver, and alloys of any
of the foregoing.
[347] Examples of suitable ceramic fiber include metal oxide such as alumina
fibers, aluminasilicate
fibers, boron nitride fibers, silicon carbide fibers, and combinations of any
of the foregoing.
[348] Examples of suitable inorganic fiber include carbon, alumina, basalt,
calcium silicate, and rock
wool.
[349] A fiber can be a glass fiber such as S-glass fibers, E-glass fibers,
soda-lime-silica fibers, basalt
fibers, or quartz fibers. Glass fibers may be in the form of woven and/or
braided glass fibers, or non-
woven glass fibers.
[350] A fiber can include carbon such as graphite fibers, glass fibers,
ceramic fibers, silicon carbide
fibers, polyimide fibers, polyamide fibers, or polyethylene fibers. Continuous
fibers can comprise
titanium, tungsten, boron, shape memory alloy, graphite, silicon carbide,
boron, aramid, poly(p-
phenylene-2,6-benzobisoxazole), and combinations of any of the foregoing.
[351] Fiber capable of withstanding high temperature include, for example,
carbon fiber, high-strength
glass (SiO2) fiber, oxide fiber, alumina fiber, ceramic fiber, metal fiber,
and fibers of high temperature
thermoplastics or thermosets.
[352] A filler can include carbon nanotubes. Suitable carbon nanotubes can be
characterized by a
thickness or length, for example, from 1 nm to 5,000 nm. Suitable carbon
nanotubes can be cylindrical in
shape and structurally related to fullerenes. Suitable carbon nanotubes can be
open or capped at their
ends. Suitable carbon nanotubes can comprise, for example, more than 90 wt%,
more than 95 wt%, more
than 99 wt%, or more than 99.9 wt% carbon, where wt% is based on the total
weight of the carbon
nanotube.
[353] Carbon nanotubes can be provided as single-walled nanotubes (SWNT) and
multi-walled
nanotubes (MWNT), for example, as nanotubes having one single wall and
nanotubes having more than
one wall, respectively. In single-walled nanotubes a one atom thick sheet of
atoms, for example, a one
atom thick sheet of graphite, i.e., graphene, is rolled seamlessly to form a
cylinder. Multi-walled
nanotubes consist of a number of such cylinders arranged concentrically.
[354] A multi-walled carbon nanotube can have, for example, on average from 5
to 15 walls.
[355] Single-walled nanotubes can be characterized by a diameter of at least
0.5 nm, such as at least 1
nm, or at least 2 nm. A SWNT can have a diameter less than 50 nm, such as less
than 30 nm, or less than
nm. A length of single-walled nanotubes can be at least 0.05 gm, at least 0.1
run, or at least 1 gm. A
length can be less than 50 mm, such as less than 25 mm.
[356] Multi-walled nanotubes can be characterized by an outer diameter of at
least 1 nm, such as at
least 2 nm, 4 nm, 6 nm, 8 nm, or at least 9 nm. An outer diameter can be less
than 100 nm, less than 80
51
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
nm, 60 nm, 40 nm, or less than 20 mn. The outer diameter can be from 9 run to
20 nm. A length of a
multi-walled nanotube can be less than 50 nm, less than 75 nm, or less than
100 nm. A length can be less
than 500 1.1M, or less than 100 gin. A length can be from 100 nm to 10 pm. A
multi-walled carbon
nanotube can have an average outer diameter from 9 nm to 20 nm and/or an
average length from 100 nm
to 10 gm.
[357] Carbon nanotubes can have a BET surface area, for example, from 200 m2/g
to 400 m2/g. Carbon
nanotubes can have a mean number of from 5 walls to 15 walls. BET surface area
can be determined
according to ASTM D6556
[358] A coreactive composition can comprise a thermally-conductive filler or
combination of
thermally-conductive filler.
[359] A thermally conductive filler can include, for example, metal nitrides
such as boron nitride,
silicon nitride, aluminum nitride, boron arsenide, carbon compounds such as
diamond, graphite, carbon
black, carbon fibers, graphene, and graphenic carbon particles, metal oxides
such as aluminum oxide,
magnesium oxide, beryllium oxide, silicon dioxide, titanium oxide, nickel
oxide, zinc oxide, copper
oxide, tin oxide, metal hydroxides such as aluminum hydroxide or magnesium
hydroxide, carbides such
as silicon carbide, minerals such as agate and emery, ceramics such as ceramic
microspheres, mullite,
silica, silicon carbide, carbonyl iron, cerium (III) molybdate, copper, zinc,
or combinations of any of the
foregoing.
[360] A coreactive composition can comprise greater than 5 wt% of a conductive
filler, greater than 10
wt%, greater than 20 wt%, greater than 30 wt%, greater than 40 wt%, greater
than 50 wt%, greater than
60 wt%, greater than 70 wt%, greater than 80 wt%, greater than 90 wt%, or
greater than 95 wt% of a
conductive filler, where wt% is based on the total weight of the coreactive
composition. A coreactive
composition can comprise less than 5 wt% of a conductive filler, less than 10
wt%, less than 20 wt%, less
than 30 wt%, less than 40 wt%, less than 50 wt%, less than 60 wt%, less than
70 wt%, less than 80 wt%,
less than 90 wt%, or less than 95 wt% of a conductive filler, where wt% is
based on the total weight of the
coreactive composition. A coreactive composition provided by the present
disclosure can have from 1
wt% to 95 wt% of a conductive filler, from 5 wt% to 75 wt%, from 10 wt% to 60
wt%, or from 20 wt% to
50 wt% of a conductive filler, where wt% is based on the total weight of the
coreactive composition.
[361] A coreactive composition can comprise greater than 5 vol% of a
conductive filler, greater than 10
vol%, greater than 20 vol%, greater than 30 vol%, greater than 40 vol%,
greater than 50 vol%, greater
than 60 vol%, greater than 70 vol%, greater than 80 vol%, greater than 90
vol%, or greater than 95 vol%
of a conductive filler, where vol% is based on the total volume of the
coreactive composition. A
coreactive composition can comprise less than 5 vol% of a conductive filler,
less than 10 vol%, less than
20 vol%, less than 30 vol%, less than 40 vol%, less than 50 vol%, less than 60
vol%, less than 70 vol%,
52
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
less than 80 vol%, less than 90 vol%, or less than 95 vol% of a conductive
filler, where vol% is based on
the total volume of the coreactive composition. A coreactive composition
provided by the present
disclosure can have from 1 vol% to 95 vol% of a conductive filler, from 5 vol%
to 75 vol%, from 10
vol% to 60 vol%, or from 20 vol% to 50 vol% of a conductive filler, where vol%
is based on the total
volume of the coreactive composition.
[362] A coreactive composition can comprise a reactive diluent or combination
of reactive diluents. A
reactive diluent can be used to reduce the initial viscosity of the coreactive
composition. A reactive
diluent can be a compound having at least one functional group capable of
reacting with at least one of
the major reactants of the composition and become part of the cross-linked
network. A reactive diluent
can have, for example, one functional group, or two functional group. A
reactive dilute can be used to
control the viscosity of a composition or improve the wetting of filler in a
coreactive composition.
[363] A coreactive composition can comprise a hydroxyl-functional vinyl ether
or combination of
hydroxyl-functional vinyl ethers as reactive diluents.
[364] A hydroxyl-functional vinyl ether can have the structure of Formula
(12):
CH2---CH-0¨(CH2),¨OH
(12)
where w is an integer from 2 to 10. In hydroxyl-functional vinyl ethers of
Formula (12), w can be 2, 3, 4,
5, or w can be 6. Examples of suitable hydroxyl-functional vinyl ethers
include 1-methy1-3-
hydroxypropyl vinyl ether, 4-hydroxybutyl vinyl ether, and a combination
thereof. A hydroxyl-functional
vinyl ether can be 4-hydroxybutyl vinyl ethers as reactive diluents.
[365] A coreactive composition can comprise, for example, from 0.1 wt% to 10
wt% of a hydroxyl-
functional vinyl ether, from 0.2 wt% to 9 wt%, from 0.3 wt% to 0.7 wt% and
from 0.4 wt% to 0.7 wt%,
where wt% is based on the total weight of the coreactive composition.
[366] A coreactive composition can comprise an amino-functional vinyl ether or
combination of amino-
functional vinyl ethers.
[367] An amino-functional vinyl ether can have the structure of Formula (13):
CH2=CH-0¨(CH2)i¨NH2
(13)
where t is an integer from 2 to 10. In amino-functional vinyl ethers of
Formula (13), t can be 2, 3, 4, 5, or
t can be 6. Examples of suitable amino-functional vinyl ethers include 1-
methy1-3-aminopropyl vinyl
ether, 4-aminobutyl vinyl ether, and a combination of any of the foregoing. An
amino-functional vinyl
ether can be 4-aminobutyl vinyl ether.
[368] A coreactive composition can comprise an epoxy-functional vinyl ether or
combination of epoxy-
functional vinyl ethers.
[369] A hydroxyl-functional vinyl ether can have the structure of Formula
(14):
CH2=CH-0¨(CH2)¨R
(14)
53
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
where w is an integer from 2 to 10, and R is an epoxy group. In epoxy-
functional vinyl ethers of Formula
(14), w can be 2, 3, 4, 5, or w can be 6. An epoxy-functional vinyl ether can
be 2-
(4(vinyloxy)butyl)oxirane.
[370] A coreactive composition can comprise, for example, from 0.1 wt% to 10
wt% of an amino-
functional vinyl ether, from 0.2 wt% to 9 wt%, from 0.3 wt% to 0.7 wt% and
from 0.4 wt% to 0.7 wt%,
where wt% is based on the total weight of the coreactive composition.
[371] A coreactive composition can comprise vinyl-based diluents such as
styrene, a-methyl styrene
and para-vinyl toluene; vinyl acetate; and/or n-vinyl pyrrolidone as a
reactive diluent.
[372] A coreactive composition can contain a plasticizer or a combination of
plasticizers. Plasticizers
can be included to adjust the initial viscosity of the coreactive composition
and to facilitate application.
[373] Examples of suitable plasticizers include a combination of phthalates,
terephathlic, isophathalic,
hydrogenated terphenyls, quateiphenyls and higher or polyphenyls, phthalate
esters, chlorinated paraffins,
modified polyphenyl, tung oil, benzoates, dibenzoates, thermoplastic
polyurethane plasticizers, phthalate
esters, naphthalene sulfonate, trimellitates, adipates, sebacates, maleates,
sulfonamides,
organophosphates, polybutene, butyl acetate, butyl cellosolve, butyl carbitol
acetate, dipentene, tributyl
phosphate, hexadecanol, diallyl phthalate, sucrose acetate isobutyrate, epoxy
ester of iso-octyl tallate,
benzophenone and combinations of any of the foregoing.
[374] A coreactive composition can comprise from 0.5 wt% to 7 wt% of a
plasticizer or combination of
plasticizers from 1 wt% to 6 wt%, from 2 wt% to 5 wt% or from 2 wt% to 4 wt%
of a plasticizer or
combination of plasticizers, where wt% is based on the total weight of the
coreactive composition. A
coreactive composition can comprise less than 8 wt% plasticizer, less than 6
wt%, less than 4 wt%, or less
than 2 wt% of a plasticizer or combination of plasticizers, where wt% is based
on the total weight of the
coreactive composition.
[375] A coreactive composition can comprise a corrosion inhibitor or
combination of corrosion
inhibitors.
[376] Examples of suitable corrosion inhibitors include zinc phosphate-based
corrosion inhibitors, a
lithium silicate corrosion inhibitor such as lithium orthosilicate (Li4SiO4)
and lithium metasilicate
(Li2SiO3), MgO, an azole, a monomeric amino acid, a dimeric amino acid, an
oligomeric amino acid, a
nitrogen-containing heterocyclic compound such as an azole, oxazole, thiazole,
thiazolines, imidazole,
diazole, pyridine, indolizine, and triazine, tetrazole, and/or tolyltriazole,
corrosion resistant particles such
as inorganic oxide particles, including for example, zinc oxide (Zn0),
magnesium oxide (MgO), cerium
oxide (Ce02), molybdenum oxide (Mo03), and/or silicon dioxide (SiO2), and
combinations of any of the
foregoing.
54
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[377] A coreactive composition can comprise less than 5 wt% of a corrosion
inhibitor or combination
of corrosion inhibitors, less than 3 wt%, less than 2 wt%, less than 1 wt%, or
less than 0.5 wt% of a
corrosion inhibitor or combination of a corrosion inhibitors, where wt% is
based on the total weight of the
coreactive composition. A coreactive composition can comprise, for example,
greater than 0.1 wt% of a
corrosion inhibitor, greater than 0.5 wt%, greater than 1 wt%, or greater than
2 wt% of a corrosion
inhibitor, where wt% is based on the total weight of the coreactive
composition.
[378] A coreactive composition can comprise a fire retardant or combination of
fire retardants.
[379] A fire retardant can include an inorganic fire retardant, an organic
fire retardant, or a combination
thereof.
[380] Examples of suitable inorganic fire retardants include aluminum
hydroxide, magnesium
hydroxide, zinc borate, antimony oxides, hydromagnesite, aluminum trihydroxide
(ATH), calcium
phosphate, titanium oxide, zinc oxide, magnesium carbonate, barium sulfate,
barium borate, kaolinite,
silica, antimony oxides, and combinations of any of the foregoing.
[381] Examples of suitable organic fire retardants include halocarbons,
halogenated esters, halogenated
ethers, chlorinated and/or brominated flame retardants, halogen free compounds
such as
organophosphorus compounds, organonitrogen compounds, and combinations of any
of the foregoing.
[382] A coreactive composition can comprise, for example, from 1 wt% to 30
wt%, such as from 1 wt%
to 20 wt%, or from 1 wt% to 10 wt% of a flame retardant or combination of
flame retardants based on the
total weight of the coreactive composition. For example, a coreactive
composition can comprise less than
30 wt%, less than 20 wt%, less than 10 wt%, less than 5 wt%, or less than 2
wt%, of a flame retardant or
combination of flame retardants based on the total weight of the coreactive
composition.
[383] A coreactive composition can comprise a moisture control additive or
combination of moisture
control additives.
[384] Examples of suitable moisture control additives include synthetic
zeolite, activated alumina, silica
gel, calcium oxide, magnesium oxide, molecular sieve, anhydrous sodium
sulphate, anhydrous
magnesium sulphate, alkoxysilanes, and combinations of any of the foregoing.
[385] A coreactive composition can comprise less than 5 wt% of a moisture
control additive or
combination of moisture control additives, less than 3 wt%, less than 2 wt%,
less than 1 wt%, or less than
0.5 wt% of a moisture control additive or combination of a moisture control
additives, where wt% is
based on the total weight of the coreactive composition.
[386] A coreactive composition can comprise a UV stabilizer or a combination
of LTV stabilizers. UV
stabilizers include UV absorbers and hindered amine light stabilizers.
Examples of suitable UV
stabilizers include products under the tradenamcs Cyasorb (Solvay), Uvinul
(BASF), and Tinuvino
(BASF).
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[387] A coreactive composition can comprise colorants such as pigments and/or
dyes.
[388] Examples of suitable inorganic pigments include metal-containing
inorganic pigments such as
those containing cadmium, carbon, chromium, cobalt, copper, iron oxide, lead,
mercury, titanium,
tungsten, and zinc. Examples further include ultramarine blue, ultramarine
violet, reduced tungsten
oxide, cobalt aluminate, cobalt phosphate, manganese ammonium pyrophosphate
and/or metal-free
inorganic pigments. An inorganic pigment nanoparticle can comprise ultramarine
blue, ultramarine
violet, Prussian blue, cobalt blue and/or reduced tungsten oxide. Examples of
specific organic pigments
include indanthrone, quinacridone, phthalocyanine blue, copper phthalocyanine
blue, and perylene
anthraquinone.
[389] Additional examples of suitable pigments include iron oxide pigments, in
all shades of yellow,
brown, red and black; in all their physical forms and grain categories;
titanium oxide pigments in all the
different inorganic surface treatments; chromium oxide pigments also co-
precipitated with nickel and
nickel titanates; black pigments from organic combustion (e.g., carbon black);
blue and green pigments
derived from copper phthalocyanine, also chlorinated and brominated, in the
various a, J3 and E crystalline
forms; yellow pigments derived from lead sulfochromate; yellow pigments
derived from lead bismuth
vanadate; orange pigments derived from lead sulfochromate molybdate; yellow
pigments of an organic
nature based on arylamides; orange pigments of an organic nature based on
naphthol; orange pigments of
an organic nature based on diketo-pyrrolo-pyrrole; red pigments based on
manganese salts of azo dyes;
red pigments based on manganese salts of beta-oxynaphthoic acid; red organic
quinacridone pigments;
and red organic anthraquinone pigments.
[390] Examples of suitable dyes include acridines, anthraquinones, arylmethane
dyes, azo dyes,
phthalocyanine dyes, quinone-imine dyes including azin dyes, indamins,
indophenyls, oxazins, oxazones,
and thiazines, thiazole dyes, saffranin dyes, xanthene dyes including fluorene
dyes. Examples of suitable
dyes include Alcian blue, Alcian yellow, Alizarin, Alizarin red, Alizarin
yellow, Azophloxin, Bismarck
brown R, Bismarck brown Y, Brilliant cresyl blue, Chrysoidine R, Crisoidine Y,
Congo red, Crystal
violet, Ethyl green, Fuchsin acid, Gentian violet, Janus green, Lissamine fast
yellow, Malachite green,
Martius yellow, Meldola blude, Metanil yellow, Methyl orange, Methyl red,
Naphthalene black, Naphthol
green, Naphthol yellow, Orange G, Purpurin, Rose bengal, Sudan II, Titan
yellow, Tropaeolin 0,
Tropaeolin 00, Tropaeolin 000, Victoria blue, and Xylene cyanol.
[391] A coreactive composition can comprise a photochromic material or a
combination of
photochromic materials.
[392] A photochromic material can be a reversible photochromic material or a
non-reversable
photochromic material. A photochromic material can be a thermally reversible
photochromic material or
a thermally non-reversable photochromic material.
56
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[393] A photochromic material can be a compound that is activated by absorbing
actinic radiation
having a particular wavelength, such as UV radiation, which causes a change in
a feature of the
photochromic material. A feature change is an identifiable change in a feature
of the photochromic
material which can be detected using an instrument or visually. Examples of
feature changes include a
change of color or color intensity and a change in structure or other
interactions with energy in the visible
UV, infrared (IR), near IR or far IR portions of the electromagnetic spectrum
such as absorption and/or
reflectance. A color change at visible wavelengths refers to a color change at
wavelengths within a range
from 400 nm to 800 nm.
[394] A photochromic material can be activated by absorbing radiation energy
(visible and non-visible
light) having a particular wavelength, such as UV light, to undergo a feature
change such as a color
change. The feature change can be a change of feature of the photochromic
material alone or it can be a
change of feature of a coreactive composition. Examples of suitable
photochromic materials include
spiropyrans, spiropyrimidines, spirooxazines, diarylethenes, photochromic
quinones, azobenzenes, other
photochromic dyes and combinations thereof. These photochromic materials can
undergo a reversible or
irreversible feature change when exposed to radiation where the first and
second states can be different
colors or different intensities of the same color.
[395] A coreactive composition can comprise a photochromic agent sensitive to
the degree of cure or
the extent of exposure to actinic radiation. A cure indicator can change color
upon exposure to actinic
radiation, which can be permanent or reversible. A cure indicator can be
initially transparent and become
colored upon exposure to actinic radiation or can be initially colored and
become transparent upon
exposure to actinic radiation.
[396] A layer of a multilayer system provided by the present disclosure that
exhibits low temperature
flexibility can comprise, for example, prepolymers such as silicones,
polytetrafluoroethylenes,
polythioethers, polysulfides, polyformals, polybutadienes, certain elastomers,
and combinations of any of
the foregoing.
[397] A layer of a multilayer system provided by the present disclosure that
exhibits hydrolytic stability
can comprise, for example, prepolymers such as silicones,
polytetrafluoroethylenes, polythioethers,
polysulfides, polyformals, polybutadienes, certain elastomers, and
combinations of any of the foregoing,
or compositions having a high crosslinking density and/or can comprise an
elastomer.
[398] A layer of a multilayer system provided by the present disclosure that
exhibits high temperature
resistance can comprise, for example, prepolymers such as silicones,
polytetrafluoroethylenes,
polythioethers, polysulfides, polyfomials, polybutadienes, certain elastomer,
and combinations of any of
the foregoing; or compositions having a high crosslinking density.
57
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[399] A layer of a multilayer system provided by the present disclosure that
exhibits a high tensile
strength can comprise, for example, elastomeric prepolymers such a silicones
and polybutadiene,
compositions having high crosslinking density, inorganic filler, and
combinations of any of the foregoing.
[400] A layer of a multilayer system provided by the present disclosure that
exhibits a high
%elongation can comprise, for example, elastomeric prepolymers such a
silicones and polybutadiene,
compositions having high crosslinking density, inorganic filler, and
combinations of any of the foregoing.
[401] A layer of a multilayer system provided by the present disclosure that
exhibits substrate bonding
or bonding to a primer coating can comprise, for example, adhesion promoters
such as organo-functional
alkoxysilanes, phenolic resins, cooked phenolic resins, and combinations of
any of the foregoing,
titanates, partially hydrolyzed alkoxysilanes, or combinations thereof
[402] A layer of a multilayer system provided by the present disclosure that
exhibits interlayer adhesion
can comprise, for example, adhesion promoters, unreacted functional groups
that are reactive with
compounds in the adjoining layer, and combinations thereof.
[403] A layer of a multilayer system provided by the present disclosure that
exhibits a fast tack free
time can comprise, for example, coreactants having a fast cure chemistry,
systems curable by actinic
radiation, catalysts, and combinations of any of the foregoing.
[404] A layer of a multilayer system provided by the present disclosure can
exhibit, for example, a tack
free time less than 5 minutes where tack free time from when the coreactants
are first mixed to the time a
cotton ball no longer adheres to the surface of the curing sealant.
[405] A layer of a multilayer system provided by the present disclosure that
exhibits a fast time to a
hardness of Shore 10A can comprise, for example, coreactants having a fast
cure chemistry, systems
curable by actinic radiation, catalysts, and combinations of any of the
foregoing.
[406] A layer of a multilayer system provided by the present disclosure that
exhibits electrical
conductivity, EMURFI shielding, and/or static dissipation can comprise, for
example, electrically
conductive filler or a combination of electrically conductive filler.
[407] A layer of a multilayer system provided by the present disclosure that
exhibits a low-density can
comprise, for example, low-density filler such as low-density organic filler,
hollow microspheres, coated
microspheres, or combinations of any of the foregoing.
[408] A layer of a multilayer system provided by the present disclosure that
exhibits corrosion
resistance can comprise, for example, one or more corrosion inhibitors.
[409] A layer of a multilayer system provided by the present disclosure that
exhibits corrosion
resistance can comprise, for example, one or more inorganic filler.
[410] When cured, a multilayer system provided by the present disclosure can
exhibit, for example, one
or more of a desired solvent resistance, low-temperature flexibility,
hydrolytic stability, high temperature
58
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
resistance, high tensile/elongation, bonding to the substrate, bonding to a
primer coating, adhesion to an
adjoining layer, fast tack-free time, fast time to Shore 10A hardness,
electrical conductivity, EMI/RFI
shielding, static dissipation, corrosion resistance, sound damping, or a
combination of any of the
foregoing.
[411] For example, following exposure to Jet Reference Fluid (JRF Type 1)
according to ISO 1817 for
168 hours at 60 C, a cured multilayer provided by the present disclosure
system provided by the present
disclosure can exhibit a tensile strength greater than 1.4 MPa determined
according to ISO 37, a tensile
elongation greater than 150% determined according to ISO 37, and a hardness
greater than Shore 30A
determined according to ISO 868, where the tests are performed at a
temperature of 23 C, and a humidity
of 55%RH.
[412] Following exposure to de-icing fluid according to ISO 11075 Type 1 for
168 hours at 60 C, a
cured multilayer system provided by the present disclosure can exhibit a
tensile strength greater than 1
MPa determined according to ISO 37, and a tensile elongation greater than 150%
determined according to
ISO 37, where the tests are performed at a temperature of 23 C, and a humidity
of 55%RH.
[413] A chemically resistant multilayer system provided by the present
disclosure can exhibit a % swell
less than 25%, less than 20%, less than 15%, or less than 10%, following
immersion in a chemical for 7
days at 70 C, where % swell is determined according to EN ISO 10563.
[414] A multilayer t system provided by the present disclosure that exhibits
low % swell can comprise,
for example, a high cross-linking density. The % swell can be determined by
immersion a cured
composition in a particular solvent for 7 days at a 70 C according to EN ISO
10563.
[415] A multilayer system provided by the present disclosure can exhibit, for
example, an as-cured
tensile strength of at least 1 MPa as determined according to ISO 37 at 23
C/55%RH.
[416] A multilayer system provided by the present disclosure can exhibit, for
example, an as-cured %
elongation of at least 150% as determined according to ISO 37 at 23 C/55%RH.
[417] A multilayer system provided by the present disclosure can exhibit a
fast time to a hardness of
Shore 10A of less than 10 minutes where hardness is determined according to
ISO 868 at 23 C/55%RH.
[418] An electrically conductive multilayer system or a layer of a multilayer
system provided by the
present disclosure can exhibit a surface resistivity, for example, less than
106 Ohm/square, less than 105
Ohm/square, less than 10 Ohm/square, less than 10 Ohm/square, less than 102
Ohm/square, less than 10
Ohm/square, less than 104 Ohm/square, or less than 10' Ohm/square. A surface
of an electrically
conductive multilayer system or a layer of a multilayer system provided by the
present disclosure can
have a surface resistivity, for example, from 10' to 102, from 102 Ohm/square
to 106 Ohm/square, or from
1030hm/square to 105 Ohm/square. Surface resistivity can be determined
according to ASTM D257 at
23 C/55%RI-I.
59
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[419] A multilayer system or a layer of a multilayer system provided by the
present disclosure can have
a volume resistivity, for example, less than 106 Ohm/cm, less than 10 Ohm/cm,
less than 10 Ohm/cm,
less than 1030hm/cm, less than 1020Iun/cm, less than 10 Ohm/cm, less than 104
Ohm/cm, or less than
10' Ohm/cm. An electrically conductive multilayer system or a layer of a
multilayers system can have a
volume resistivity, for example, from 10' Ohm/cm to 101 Ohm/cm, from 101
Ohm/cm to 1060hm/cm, or
from 103 Ohm/cm to 10 Ohm/cm. Volume resistivity can be determined according
to ASTM D257 at
23 C/55 AIM.
[420] A multilayer system or a layer of a multilayer system provided by the
present disclosure can have
an electrical conductivity, for example, greater than 1 S cm-1, greater than
10 S cm-1, greater than 100 S
cm', greater than 1,000 S cm', or greater than 10,000 S cm4. An electrically
conductive multilayer
system can have an electrical conductivity from 1 S cm4 to 10,000 S cm', from
10 S ein4 to 1,000 cm'
or from 10 S cm' to 500 S cm'. Electrical conductivity is determined according
to ASTM D257 at
23 C/556/AH.
[421] A multilayer system or a layer of a multilayer system provided by the
present disclosure can
exhibit an attenuation at frequencies within a range from 10 KHz to 20 GHz,
for example, of greater than
dB, greater than 30 dB, greater than 60 dB, greater than 90 dB, or greater
than 120 dB. An electrically
conductive multilayer system provided by the present disclosure can exhibit an
attenuation at frequencies
within a range from 10 KHz to 20 GHz, for example, of from 10 dB to 120 dB,
from 20 dB to 100 dB,
from 30 dB to 90 dB, or from 40 dB to 70 dB. Shielding effectiveness is
determined according to ASTM
D4935 at 23 C/55%RH.
[422] A multilayer system or a layer of a multilayer system provided by the
present disclosure exhibit a
thermal conductivity from 0.1 to 50 W/(m-K), from 0.5 to 30 W/(m-K), from 1 to
30 W/(m-K), from 1 to
W/(m-K), from 1 to 10 W/(m-K), from 1 to 5 W/(m-K), from 2 to 25 W/(m-K), or
from 5 to 25 W/(m-
K). Thermal conductivity is determined according to ASTM D1461 at 23 C/55%RH.
[423] A multilayer system or a layer of a multilayer system provided by the
present disclosure can
exhibit a specific gravity, for example, less than 1.1, less than 1.0, less
than 0.9, less than 0.8, or less than
0.7, where specific gravity is determined according to ISO 2781 at 23 C/55%RH.
[424] A multilayer system or a layer of a multilayer system provided by the
present disclosure can
exhibit a hardness, for example, greater than Shore 20A, greater than Shore
30A, greater than Shore 40A,
greater than Shore 50A, or greater than Shore 60A, where hardness is
determined according to ISO 868 at
23 C/55 %RH.
[425] A cured multilayer system can have properties acceptable for use in
vehicle and aerospace sealant
applications. In general, it is desirable that sealants used in aviation and
aerospace applications exhibit
the following properties: peel strength greater than 20 pounds per linear inch
(pli) on Aerospace Material
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
Specification (AMS) 3265B substrates determined under dry conditions,
following immersion in JRF
Type I for 7 days, and following immersion in an aqueous solution of 3% NaC1
according to AMS 3265B
test specifications; tensile strength between 300 pounds per square inch (psi)
and 400 psi (2.75 MPa); tear
strength greater than 50 pounds per linear inch (ph) (8.75 N/mm); elongation
between 250% and 300%;
and hardness greater than 40 Durometer A. These and other properties
appropriate for aviation and
aerospace applications are disclosed in AMS 3265B. It is also desirable that,
when cured, multilayer
systems of the present disclosure used in aviation and aircraft applications
exhibit a percent volume swell
not greater than 25% following immersion for one week at 60 C and ambient
pressure in Jet Reference
Fluid (JRF) Type I. Other properties, ranges, and/or thresholds may be
appropriate for other sealant
applications.
[426] A multilayer system provided by the present disclosure can be fuel-
resistant. The term "fuel
resistant" can mean that a composition, when applied to a substrate and cured,
can provide a cured
product, such as a sealant, that exhibits a percent volume swell of not
greater than 40%, in some cases not
greater than 25%, in some cases not greater than 20%, and in other cases not
more than 10%, after
immersion for one week at 60 C and ambient pressure in JRF Type I according to
methods similar to
those described in ASTM D792 (American Society for Testing and Materials) or
AMS 3269 (Aerospace
Material Specification). JRF Type I, as employed for determination of fuel
resistance, has the following
composition: toluene: 28 1% by volume; cyclohexane (technical): 34 1% by
volume; isooctane: 38
1% by volume; and tertiary dibutyl disulfide: 1 0.005% by volume (see AMS
2629, issued July 1, 1989
3.1.1 etc., available from SAE (Society of Automotive Engineers)).
[427] A chemically resistant multilayer system provided by the present
disclosure can exhibit a tensile
elongation of at least 200% and a tensile strength of at least 200 psi when
measured in accordance with
the procedure described in AMS 3279 3.3.17.1, test procedure AS5127/1 7.7.
[428] A multilayer system provided by the present disclosure can exhibit a lap
shear strength of greater
than 200 psi (1.38 MPa), such as at least 220 psi (1.52 MPa), at least 250 psi
(1.72 MPa), and, in some
cases, at least 400 psi (2.76 MPa), when measured according to the procedure
described in SAE
ASS127/1 paragraph 7.8.
[429] A multilayer system provided by the present disclosure can meet or
exceed the requirements for
aerospace sealants as set forth in AMS 3277.
[430] A layer of a multilayer system provided by the present disclosure that
imparts sound damping
properties can comprise an epoxy-containing compound where the epoxy-
containing compound
comprises an epoxy/polyol adduct, a polythiol, and a curing agent.
[431] A multilayer system provided by the present disclosure can impart sound
damping properties to a
structure. For example, when a multilayer system having sound damping
properties is applied to a
61
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
substrate, the substrate can exhibit a sound damping loss factor of at least
0.06 at 800 Hz, at least 0.04 at
400 Hz, or at least 0.02 at 200 Hz at 10 C, 2.5 mm sealant thickness measured
according to SAE test
method J1637 and ASTM E756 on 240 mm long, 10 mm wide, and 1 mm thick steel
panels coated along
215 mm of the length.
[432] A multilayer system can comprise one or more coatings. A coating can be
provided on the
exterior surface of the inner layer of the multilayer system, on the exterior
surface of the outer layer of the
multilayer system, and/or between one or more layers of the multilayer system.
A coating refers to a
layer that has a thickness less than that of a layer of the multilayer system.
[433] A multilayer system provided by the present disclosure can comprise an
intermediate coating
between the layers, an interior coating, an exterior coating, or a combination
of any of the foregoing. An
intermediate coating refers to a coating between adjoining layers; an interior
coating refers to a coating
that that is adjacent a surface; and an exterior coating is on the outside
surface of the multilayer system.
Examples of coatings are shown in FIG. 3. FIG. 3 shows a cross sectional view
of a multilayer system
having a first layer 302 overlying a substrate 305, and a second layer 301
overlying the first layer 302. A
first coating 304 is disposed between the first layer 302 and the substrate
305 to enhance, for example,
adhesion and/or corrosion resistance. A second coating 303 is disposed between
the first and second
layers 301/302 to enhance, for example, interlayer adhesion. A third coating
306 can overly the outer
surface of the multilayer system and can be configured to enhance, for
example, chemical resistance,
abrasion resistance, or electrical conductivity of one or more layers of a
multilayer system
[434] An intermediate, interior or exterior coating can have a thickness, for
example, from 0.001 to 2
mm, from 0.01 mm to 1 mm, from 0.05 mm to 0.5 mm, or from 0.1 mm to 0.4 mm. A
coating can have a
thickness, for example, less than 2 mm, less than 1 mm, less than 0.5 mm, less
than 0.1 mm, or less than
0.05 mm. The thickness of the coating can be less than the thickness of the
layers forming the multilayer
system. An intermediate coating can be used to enhance or to provide certain
desired properties to the
multilayer system such as, for example, interlayer adhesion, electrical
conductivity, EMI/RFI shielding,
or a combination of any of the foregoing.
[435] An intermediate coating can comprise compounds comprising functional
groups reactive with the
reactive compounds of the underlying and/or overlying layers. For example,
when the overlying and/or
underlying layers comprise coreactive compounds having thiol functional
groups, an intermediate coating
layer can comprise compounds having groups reactive with thiol groups such as
alkenyl groups, alkynyl
groups, isocyanate groups, thiol groups or epoxy groups.
[436] An interior coating can provide adhesion to a substrate, provide
corrosion resistance, or a
combination thereof. For example, an interior coating can comprise, for
example, adhesion promoters,
62
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
corrosion inhibitors, partially hydrolyzed/condensed organo-functional
alkoxysilanes, and combinations
of any of the foregoing.
[437] An exterior coating can be configured to provide aesthetics, static
dissipation, electrical
conductivity. EMI/RFI shielding, or a combination of any of the foregoing. For
example, an exterior
coating can comprise, for example, a colorant, electrically conductive filler,
or a combination thereof.
[438] A multilayer system can include an exterior coating such as a clear
coat, an abrasion-resistant
coating, a color coating, a textured coating, a solvent resistant coating, a
UV-protective coating, a haptic,
or a combination of any of the foregoing, overlying the multilayer system._The
surface coatings can be
used to impart a desired surface property such as, for example, electrical
conductivity, reflectivity such as
IR reflectivity, color, wavelength-dependent absorption, wavelength-dependent
reflectivity, scratch
resistance, abrasion resistance, stain resistance, fingerprint resistance,
resistance to cleaning fluids, impart
aesthetic qualities, and/or impart tactile properties. The coating can
comprise a multilayer coating. A
coating can be a haptic coating such as a soft-touch coating. The coating can
be applied to an extrudate
using an extrusion coating die.
[439] A multilayer system provided by the present disclosure can be prepared
by depositing an
extrudate comprising a coreactive sealant composition or a coextrudate
comprising a coreactive sealant
composition and one or more additional coreactive compositions onto a
substrate.
[440] A multilayer system can be applied using additive manufacturing methods.
Additive
manufacturing is broadly used to encompass robotic manufacturing methods.
Additive manufacturing
includes, for example, three-dimensional printing, extrusion, and coextrusion.
[441] Using additive manufacturing methods, a multilayer system comprising
individual layers of
coreactive compositions can be applied directly to a substrate and
subsequently cured and/or allowed to
cure to provide a cured multilayer system.
[442] A multilayer system can be applied by coextrusion. Coextrusion is
broadly used to refer to
methods in which a multilayer system is applied to a substrate using pressure.
Pressure can be applied
manually or automatically. Co-extrusion includes processes involving extrusion
through a coextrusion
die or merging parallel flows of coreactive compositions.
[443] Coextrusion facilitates the ability of a multilayer system to be applied
to a substrate in a single
process. By simultaneously applying the layers of a multilayer system, the
ability to maintain the
consistency, the reproducibility, and the integrity of the multilayer system
can be facilitated.
[444] A first and second coreactive composition can be coextruded through a
coextrusion die having a
suitable shape to provide a coextrudate.
[445] A schematic of an example of a coextruder is shown in FIG. 4. The
coextrudcr includes a barrel
401, a first inlet 402 for a first coreactive composition 403, a second inlet
404 for a second coreactive
63
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
composition 405, and an exit nozzle 406. The inlets can be coupled to pumps
that control the flow of the
coreactive compositions into the coextruder. The coextruder barrel can
comprise sensors coupled to the
pumps to control the flow of the coreactive compositions into the extruder
barrel. The flow of the
coreactive compositions can be controlled such that the flows merge but do not
mix. At the exit nozzle
406 the merged coreactive compositions 408 can be shaped to provide a
coextrudate 409. As shown in
FIG. 4 the coextrudate is in the form of a sheet in which the first coreactive
composition 403 overlies the
second coreactive composition 405. The coextrudate can be applied to a part or
surface to be sealed.
[446] A coextruder can comprise pressure controls, extrusion dies, coextrusion
dies, coating
applicators, temperature control elements, elements for irradiating a
coreactive sealant composition, or
combinations of any of the foregoing.
[447] For automated manufacturing, the coextruder can be mounted on an
apparatus for moving a
nozzle with respect to a surface. The automated manufacturing apparatus
including the coextruder can be
controlled by a processor.
[448] Co-extrusion methods are sufficiently versatile that a wide range of co-
extrudate structures can be
fabricated.
[449] For example, for a three-dimensional multilayer system, the coextrusion
can have a core-shell
configuration comprising an inner layer comprising a first composition and an
outer layer comprising a
second composition. The core-shell coextrusion can be applied directly over a
three-dimensional surface
as a single unit.
[450] For a two-dimensional multilayer system, the coextrusion can be in the
shape of a sheet having
two or more overlying layers. A surface can be sealed by applying successive
sheets of the multilayer
system adjacent to a previously deposited sheet of the multilayer system.
[451] A multilayer system can be fabricated as a separate component that can
subsequently be applied
to a surface to be sealed. For example, a multilayer system can be fabricated
as a sheet or a preform
having a desired shape and partially cured or fully cured. The partially cured
or fully cured multilayer
system component can then be applied to a surface.
[452] A multilayer system can be applied directly to a surface to be sealed.
For example, individual
layers of a multilayer system can be sequentially be applied to a surface
where one or more of the layers
is applied using the coreactive three-dimensional printing methods provided by
the present disclosure. A
multilayer system provided by the present disclosure can be coextruded
directly onto a surface to be
sealed.
[453] As a consequence of having multiple layers, there can be interfaces
between each of the layers of
a multilayer system. The integrity of the layer interfaces can be maintained
in view of the overall
performance requirement of the multilayer system.
64
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[454] Interlayer adhesion between adjoining layers of a multilayer system can
be enhanced in several
ways. For example, an adhesion-promoting coating can be applied between the
adjoining layers. An
adhesion-promoting coating can include adhesion promoters and/or reactive
groups capable of non-
covalently bonding or covalently bonding to one or more constituents of the
adjoining layers.
[455] Adhesion between adjoining strips of a multilayer system can be enhanced
by facilitating the
ability of adjoining layers to chemically and/or physically bond. This can be
accomplished, for example,
by including coreactive compositions in adjoining strips of the multilayer
system having reactive
compounds that can chemically react with compounds in an adjoining coreactive
composition. For
example, for layers based on thiol-ene chemistry, an adhesion-promoting
interlayer coating can include
compounds having unreacted groups capable of reacting with the thiol and/or
the alkenyl groups of the
overlying and underlying layers.
[456] The rate of interlayer crosslinking between adjoining layers of a
multilayer system can be
controlled to facilitate interlayer reaction and thereby improve the
interlayer strength. For example, it can
be desirable that adjoining layers be chemically bond to each other. To
accomplish this, a second layer
can be deposited onto a first layer before the first layer is fully cured such
that the first layer has unreacted
functional groups capable of reacting with functional groups of the second
layer. The rate of interlayer
crosslinking can be controlled, for example, by adjusting the time between
deposition of successive
layers, adjusting the temperature, adjusting the concentration of a catalyst,
and/or adjusting the
components of the composition such as the amount of monomer and prepolymer.
[457] A layer may be homogeneous, or a layer may be inhomogeneous. For an
inhomogeneous layer,
a cross-section of the layer may have different chemical compositions across
the profile. For example, to
improve interlayer adhesion, a portion of a layer may have an excess of a
certain coreactive functionality
that can then react with an excess of a coreactive functionality of an
overlying layer. Similarly, to
improve interlayer adhesion, a lower portion of a layer may have an excess of
a certain coreactive
functionality that can then react with an excess of a coreactive functionality
of an underlying layer. To
improve interlayer bonding and/or adhesion, a tie coating, film, or other
treatment may be applied or
deposited over a deposited layer prior to or during deposition of an overlying
layer. The interlayer tie
layer can include, for example, compounds reactive with the adjoining layers,
catalysts, and/or adhesion
promoters. An interlayer lie coat can be applied to a surface of the extrudate
by coextrusion.
[458] A layer can be applied to at least a portion of a surface of a
coreactive composition and/or
coextrudate. A layer can be applied, for example, by passing a coextrudate
through a liquid composition
to provide a coating on the exterior surface or a portion of the exterior
surface of the coextrudate. The
coating can comprise materials that enhance adhesion between adjoining strips
of the multilayer system.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
For example, a thin film coating can comprise compounds having groups reactive
with functional groups
of coreactive compositions forming the multilayer system.
[459] Coextruded multilayer systems can also be configured to facilitate
adhesion to multiple
substrates. For example, an adhesion package can be optimized for bonding the
inner layer of a
multilayer system to a particular substrate. However, the adhesion package may
not be optimal for
facilitating bonding to a different substrate. For example, different adhesion
packages can be optimized
for bonding to different metals such as aluminum and titanium, or to
composites and to metals. An
innermost layer of a multilayer system can include two or more portions having
a different adhesion
packages, and the other constituents of the coreactive composition forming the
inner layer can be
substantially the same or different. In this way, bonding of a multilayer
system to a substrate comprising
different materials can be enhanced.
[460] An extrudate or coextrudate can be deposited in any orientation. For
example, the nozzle can be
directed downwards, upwards, sideways, or at any angle in between. In this way
a multilayer system can
be deposited as a vertical wall or as an overhang. An extrudate or coextrudate
can be deposited on a
vertical wall, the lower surface of a tilted wall, or on the bottom of a
horizontal surface. The use of an
extrudate or coextrudate with a fast curing chemistry can facilitate the
ability of an overlying layer to be
deposited adjoining an underlying layer such that an angled surface can be
fabricated. The be angled
surface can be angled upward with respect to horizontal or downward with
respect to horizontal.
[461] A coreactive composition can have a volume flow rate, for example, from
0.1 mL/min to 20,000
mL/min, such as from 1 mL/min to 12,000 mL/min, from 5 mL/min to 8,000 mL/min,
or from 10 mL/min
to 6,000 mL/min. The volume flow rate can depend, for example, on the
viscosity of a coreactive
composition, the extrusion pressure, the nozzle diameter, and the reaction
rate of the coreactive
compounds.
[462] A coreactive composition can be used at a print speed, for example, from
1 mm/sec to 400
mm/sec, such as from 5 mm/sec to 300 mm/sec, from 10 mm/sec to 200 nun/sec, or
from 15 mm/sec to
150 mm/sec. The print speed can depend, for example, on the viscosity of the
coreactive composition, the
extrusion pressure, the nozzle diameter, and the reaction rate of the
coreactive components. The print
speed refers to the speed at which a nozzle used to extrude a coreactive
composition moves with respect
to a surface onto which the coreactive composition is being deposited.
[463] A multilayer system comprising a sealant layer provided by the present
disclosure can be used in
any application where a sealant is used to protect a surface from a use
environment. A multilayer can be
used, for example, to seal parts and surfaces of automotive vehicles and
aerospace vehicles.
[464] A multilayer system can be applied directly onto or deposited onto the
surface of a substrate or
over a coating such as a primer coating or an adhesion-promoting coating.
66
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[465] A multilayer system provided by the present disclosure can be applied to
or deposited onto any of
a variety of substrates. Examples of substrates to which a multilayer system
can be applied include
metals such as titanium, stainless steel, steel alloy, aluminum, and aluminum
alloy, any of which may be
anodized, primed, organic-coated or chromate-coated; or can include epoxy,
urethane, graphite, fiberglass
composite, Kevlar , acrylics, polycarbonates, and combinations of any of the
foregoing.
[466] A cured multilayer system provided by the present disclosure can exhibit
properties acceptable
for use in vehicle applications such as automotive and aerospace sealant
applications. In general, it is
desirable that sealants used in aviation and aerospace applications exhibit
the following properties: peel
strength greater than 20 pounds per linear inch (ph) on Aerospace Material
Specification (AMS) 3265B
substrates determined under dry conditions, following immersion in JRF Type I
for 7 days, and following
immersion in a solution of 3% NaCl according to AMS 3265B test specifications;
tensile strength
between 300 pounds per square inch (psi) and 400 psi (2.75 MPa); tear strength
greater than 50 pounds
per linear inch (ph) (8.75 N/mm); elongation between 250% and 300%; and
hardness greater than 40
Durometer A. These and other cured properties of a multilayer system
appropriate for aviation and
aerospace applications are disclosed in AMS 3265B. It is also desirable that,
when cured, a multilayer
system used in aviation and aircraft applications exhibit a percent volume
swell not greater than 25%
following immersion for one week at 60 C and ambient pressure in Jet Reference
Fluid (JRF) Type 1.
Other properties, ranges, and/or thresholds may be appropriate for other
sealant applications such as
automotive applications.
[467] A multilayer system provided by the present disclosure can exhibit a
tensile elongation of at least
200% and a tensile strength of at least 200 psi when measured in accordance
with the procedure described
in AMS 3279, 3.3.17.1, test procedure AS5127/1, 7.7.
[468] A multilayer system provided by the present disclosure can exhibit a lap
shear strength of greater
than 200 psi (1.38 MPa), such as at least 220 psi (1.52 MPa), at least 250 psi
(1.72 MPa), and, in some
cases, at least 400 psi (2.76 MPa), when measured according to the procedure
described in SAE
AS5127/1 paragraph 7.8.
[469] A multilayer system prepared using methods provided by the present
disclosure can meet or
exceed the requirements for aerospace sealants as set forth in AMS 3277.
[470] Prior to environmental exposure a multilayer system provided by the
present disclosure exhibit a
density less than 1.2 g/cm3 (specific gravity less than 1.2) as determined
according to ISO 2781, a tensile
strength greater than 1 MPa determined according to ISO 37, a tensile
elongation greater than 150%
determined according to ISO 37, and a hardness greater than 40 Shore A
determined according to ISO
868, where the tests are performed at a temperature within a range of 21 C to
25 C, and a humidity from
45%RH to 55%RH.
67
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[471] Following exposure to aviation fuel (JRF Type 1) according to ISO 1817
for 168 hours at 60 C, a
multilayer system can exhibit a tensile strength greater than 1.4 MPa
determined according to ISO 37, a
tensile elongation greater than 150% determined according to ISO 37, and a
hardness greater than 30
Shore A determined according to ISO 868, where the tests are performed at a
temperature within a range
of 21 C to 25 C, and a humidity from 45%RH to 55%RH.
[472] Following exposure to 3% aqueous NaCl for 168 hours at 60 C, a
multilayer system can exhibit a
tensile strength greater than 1.4 MPa determined according to ISO 37, a
tensile elongation greater than
150% determined according to ISO 37, and a hardness greater than 30 Shore A
determined according to
ISO 868, where the tests are performed at a temperature within a range of 21 C
to 25 C, and a humidity
from 45%RH to 55%RH.
[473] Following exposure to de-icing fluid according to ISO 11075 Type 1 for
168 hours at 60 C, a
multilayer system provided by the present disclosure can exhibit a tensile
strength greater than 1 MPa
determined according to ISO 37, and a tensile elongation greater than 150%
determined according to ISO
37, where the tests are performed at a temperature within a range of 21 C to
25 C, and a humidity from
45%RH to 55%RH.
[474] Following exposure to phosphate ester hydraulic fluid (Skydrol LD-4)
for 1,000 hours at 70 C,
a multilayer system provided by the present disclosure can exhibit a tensile
strength greater than 1 MPa
determined according to ISO 37, a tensile elongation greater than 150%
determined according to ISO 37,
and a hardness greater than Shore 30A determined according to ISO 868, where
the tests are performed at
a temperature within a range of 21 C to 25 C, and a humidity from 45%RH to
55%RH.
[475] A multilayer system provided by the present disclosure can have a glass
transition temperature,
for example, of less than -10 C, less than -20 C, less than -30 C, less than -
40 C, less than -50 C, or less
than -60 C.
[476] Methods of making multilayer systems and multilayer systems made using
the methods can be
used to seal any suitable part such as, for example, a part or surface of a
vehicle.
[477] The term "vehicle" is used in its broadest sense and includes all types
of aerospace vehicles,
watercraft, and ground vehicles. For example, a vehicle can include, aerospace
vehicles such as airplanes
including private aircraft, and small, medium, or large commercial passenger,
freight, and military
aircraft; helicopters, including private, commercial, and military
helicopters; rockets and spacecraft. A
vehicle can include a ground vehicle such as, for example, automobiles,
trailers, trucks, buses, vans,
construction vehicles, golf carts, motorcycles, bicycles, trains, and railroad
cars. A vehicle can also
include watercraft such as, for example, ships, boats, and hovercraft.
[478] A multilayer system can be used in a F/A-18 jet or related aircraft such
as the F/A-18E Super
Hornet and F/A-18F; in the Boeing 787 Dreamliner, 737, 747, 717 passenger jet
aircraft, a related aircraft
68
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
(produced by Boeing Commercial Airplanes); in the V-22 Osprey; VH-92, S-92,
and related aircraft
(produced by NAVAIR and Sikorsky); in the G650, G600, G550, G500, G450, and
related aircraft
(produced by Gulfstream); and in the A350, A320, A330, and related aircraft
(produced by Airbus). A
multilayer system can be used in any suitable commercial, military, or general
aviation aircraft such as,
for example, those produced by Bombardier Inc. and/or Bombardier Aerospace
such as the Canadair
Regional Jet (CRJ) and related aircraft; produced by Lockheed Martin such as
the F-22 Raptor, the F-35
Lightning, and related aircraft; produced by Northrop Grumman such as the B-2
Spirit and related
aircraft; produced by Pilatus Aircraft Ltd.; produced by Eclipse Aviation
Corporation; or produced by
Eclipse Aerospace (Kestrel Aircraft).
[479] Multilayer systems provided by the present disclosure can be used to
seal parts and surfaces of
vehicles such as fuel tank surfaces and other surfaces exposed to or
potentially exposed to solvents,
hydraulic fluids, lubricants, oils, and fuels.
[480] The present invention includes parts sealed with a multilayer system
provided by the present
disclosure, and assemblies and apparatus comprising a part sealed with a
multilayer system provided by
the present disclosure. Apertures, surfaces, joints, fillets, fay surfaces
including apertures, surfaces,
fillets, joints, and fay surfaces of vehicles, sealed with the multilayer
system are included within the scope
of the invention. Parts, such as vehicle parts, including automotive vehicle
parts and aerospace vehicle
parts sealed using methods provided by the present disclosure are included
within the scope of the
invention.
[481] The present invention includes vehicles comprising a part such as a
surface sealed with a
multilayer system provided by the present disclosure. For example, an aircraft
comprising a fuel tank or
portion of a fuel tank sealed with a multilayer system is included within the
scope of the invention.
Vehicles such as automotive vehicles and aerospace vehicles sealed comprising
parts sealed using
methods provided by the present disclosure are included within the scope of
the invention.
[482] A multilayer system provided by the present disclosure can be used to
seal fasteners. A fastener
can be a fastener on the surface of a vehicle including, for example, motor
vehicles, automobiles, trucks,
buses, vans, motorcycles, scooters, recreational motor vehicles; railed
vehicles trains, trams, bicycles,
aerospace vehicles, airplanes, rockets, spacecraft, jets, helicopters,
military vehicles including jeeps,
transports, combat support vehicles, personnel carriers, infantry fighting
vehicles, mine-protected
vehicles, light armored vehicles, light utility vehicles, military trucks,
watercraft including ships, boats,
and recreational watercraft. Fasteners sealed using a multilayer system
provided by the present disclosure
are included within the scope of the invention.
ASPECTS OF THE INVENTION
[483] The invention can be further defined by one or more of the following
aspects.
69
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[484] Aspect 1. A method of making a multilayer system comprising two or
more layers, wherein
one or more of the layers comprises a sealant layer, comprising: (a) mixing a
first component and a
second component to form a coreactive sealant composition, wherein, the
coreactive sealant composition
comprises a first reactive compound and a second reactive compound; and the
first reactive compound is
reactive with the second reactive compound; (b) extruding the coreactive
sealant composition to form an
extrudate; and (c) depositing the extrudate to form the sealant layer.
[485] Aspect 2. The method of aspect 1, wherein depositing comprises three-
dimensional
printing.
[486] Aspect 3. The method of any one of aspects 1 to 2, wherein depositing
comprises
depositing the extrudate onto an underlying layer of the multilayer system.
[487] Aspect 4. The method of any one of aspects 1 to 3, further
comprising: depositing a first
coreactive composition underlying the deposited sealant layer to form an
underlying layer; and/or
depositing a second coreactive composition overlying the sealant layer to form
an overlying layer,
wherein the first coreactive composition and the second coreactive composition
comprise a different
composition than the sealant layer.
[488] Aspect 5. The method of aspect 4, wherein the first coreactive
composition is different than
the second coreactive composition.
[489] Aspect 6. The method of any one of aspects 1 to 5, wherein any of the
coreactive sealant
compositions comprise a thermosetting composition.
[490] Aspect 7. The method of any one of aspects 1 to 6, wherein each of
the layers of the
multilayer system comprises a thermoset.
[491] Aspect 8. The method of any one of aspects 1 to 7, wherein a cross-
sectional profile of the
extrudate has a homogeneous composition across the cross-sectional profile.
[492] Aspect 9. The method of any one of aspects 1 to 7, wherein a cross-
sectional profile of the
extrudate has an inhomogeneous composition across the cross-sectional profile.
[493] Aspect 10. The method of any one of aspects 1 to 9, wherein an
outermost layer of the two
or more layers of the multilayer system comprises a sealant layer.
[494] Aspect 11. The method of any one of aspects 1 to 10, wherein each of
the layers of the
multilayer system independently comprises a sealant layer or a non-sealant
layer.
[495] Aspect 12. The method of any one of aspects 1 to 11, wherein the
coreactive sealant
composition comprises a prepolymer comprising a chemically resistant backbone.
[496] Aspect 13. The method of any one of aspects 1 to 12, wherein the
coreactive sealant
composition comprises a sulfur content greater than 10 wt%, wherein wt% is
based on the total weight of
the organic constituents of the coreactive sealant composition.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[497] Aspect 14. The method of any one of aspects 1 to 13, wherein the
coreactive sealant
composition comprises a sulfur-containing prepolymer.
[498] Aspect 15. The method of aspect 14, wherein the sulfur-containing
prepolymer comprises a
polythioether, a polysulfide, a sulfur-containing polyformal, a monosulfide,
or a combination of any of
the foregoing.
[499] Aspect 16. The method of any one of aspects 14 to 15, wherein the
sulfur-containing
prepolymer comprises a sulfur content greater than 10 wt%. wherein wt% is
based on the total weight of
the sulfur-containing prepolymer.
[500] Aspect 17. The method of any one of aspects 1 to 16, wherein the
first reactive compound is
reactive with the second reactive compound at a temperature less than 50 C.
[501] Aspect 18. The method of any one of aspects 1 to 17, wherein, the
first reactive compound is
reactive with the second reactive compound in the presence of a catalyst
and/or a polymerization initiator;
and the catalyst and/or polymerization initiator is capable of catalyzing
and/or initiating a reaction
between the first reactive compound and the second reactive compound.
[502] Aspect 19. The method of any one of aspect 18, further comprising
activating the
polymerization initiator before depositing, during deposition, and/or after
depositing the extrudate.
[503] Aspect 20. The method of any one of aspects 1 to 19, wherein, the
first component
comprises the first reactive compound and the second reactive compound; and
the second component
comprises a catalyst, a cure activator, and/or a polymerization initiator for
the reaction between the first
reactive compound and the second reactive compound.
[504] Aspect 21. The method of any one of aspects 1 to 19, wherein the
first component comprises
the first reactive compound and the second component comprises the second
reactive compound.
[505] Aspect 22. The method of any one of aspects 1 to 21, wherein, the
first reactive compound
comprises a polyamine and/or a polyol and the second reactive compound
comprises a polyisocyanate;
the first reactive compound comprises a polyamine and the second reactive
compounds comprises a
polyepoxide; the first reactive compound comprises a Michael acceptor and the
second reactive
compound comprises a Michael donor; or the first reactive compound comprises a
polythiol and the
second reactive compound comprises a polythiol, a polyisocyanate, a
polyalkenyl, a polyalkynyl, a
polyepoxide, a Michael acceptor, or a combination of any of the foregoing.
[506] Aspect 23. The method of any one of aspects 1 to 22, further
comprising: pumping the first
component into a mixer using a first pump; and pumping the second component
into the mixer using a
second pump.
[507] Aspect 24. The method of any one of aspects 1 to 23, further
comprising, after depositing the
extrudate, curing the deposited extrudate.
71
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[508] Aspect 25. The method of aspect 24, wherein curing comprises allowing
the deposited
extrudate to cure at a temperature less than 30 C.
[509] Aspect 26. The method of any one of aspects 1 to 25, further
comprising merging one or
more additional coreactive compositions with the coreactive sealant
composition, wherein extruding
comprises coextruding the coreactive sealant composition and the one or more
additional coreactive
compositions to form a coextrudate; and depositing comprises depositing the
coextrudate to form a
multilayer system comprising one or more sealant layers.
[510] Aspect 27. The method of aspect 26, wherein each of the one or more
additional coreactive
compositions independently comprises an additional coreactive sealant
composition or a coreactive non-
sealant composition.
[511] Aspect 28. The method of any one of aspects 26 to 27, wherein the
coreactive sealant
composition and an adjoining additional coreactive composition comprise the
same curing chemistry.
[512] Aspect 29. The method of any one of aspects 26 to 28, wherein the
coreactive sealant
composition and an adjoining additional coreactive composition comprise
different curing chemistries.
[513] Aspect 30. The method of any one of aspects 26 to 29, wherein the
coreactive sealant
composition is reactive with an adjoining additional coreactive composition.
[514] Aspect 31. The method of any one of aspects 26 to 30, further
comprising: combining an
additive-containing composition with a portion of an additional coreactive
composition to form an
additive-modified additional coreactive composition, wherein the additive-
containing composition
comprises an additive; and extruding comprises coextruding the coreactive
sealant composition and the
additive-modified additional coreactive composition to form a coextrudate.
[515] Aspect 32. The method of aspect 31, wherein a cross-sectional profile
of the coextrudate has
an inhomogeneous concentration of the additive.
[516] Aspect 33. The method of any one of aspects 31 to 32, wherein the
extrudate is characterized
by an inhomogeneous concentration of the additive within a longitudinal
dimension of the coextrudate.
[517] Aspect 34. The method of any one of aspects 26 to 33, further
comprising mixing a third
component with a fourth component to form the one or more additional
coreactive compositions.
[518] Aspect 35. The method of any one of aspects 1 to 34, further
comprising: combining an
additive-containing composition with a portion of the coreactive sealant
composition to form an additive-
modified coreactive sealant composition, wherein the additive-containing
composition comprises an
additive; and extruding the additive-modified coreactive sealant composition
to form the extrudate.
[519] Aspect 36. The method of aspect 35, wherein a cross-sectional profile
of the extrudate has an
inhomogeneous concentration of the additive.
72
[520] Aspect 37. The method of any one of aspects 35 to 36, wherein the
extrudate is characterized by an
inhomogeneous concentration of the additive within a longitudinal dimension of
the extrudate.
[521] Aspect 38. The method of any one of aspects 1 to 37, further comprising
merging an adhesion-
promoting composition with the coreactive sealant composition; and extruding
comprises coextruding the
first coreactive composition and the adhesion-promoting composition.
[522] Aspect 39. The method of any one of aspects 1 to 38, further comprising
applying an adhesion-
promoting layer to the extrudate before depositing the extrudate.
[523] Aspect 40. A multilayer system comprising a sealant layer made by the
method of any one of
aspects 1 to 39.
[524] Aspect 41. The multilayer system of aspect 40, wherein adjoining layers
are chemically and/or
physically bound.
[525] Aspect 42. The multilayer system of any one of aspects 40 to 41, wherein
a fracture energy of the
fully cured multilayer sealant is substantially the same as the fracture
energy of an individual layer, wherein
the fracture energy is determined according to ASTM D7313.
[526] Aspect 43. The multilayer system of any one of aspects 40 to 42, wherein
each of the layers
comprises a thermoset material.
[527] Aspect 44. The multilayer system of any one of aspects 40 to 43, wherein
each of the layers
comprises a different thermoset material.
[528] Aspect 45. The multilayer system of any one of aspects 40 to 44, wherein
the multilayer system
meets or exceeds the requirements for aerospace sealants as set forth in AMS
3277.
[529] Aspect 46. The multilayer system of any one of aspects 40 to 45, wherein
one or more of the
layers of the multilayer sealant has an inhomogeneous composition cross-
sectional profile.
[530] Aspect 47. The multilayer system of any one of aspects 40 to 46, wherein
one or more of the
layers of the multilayer sealant has an inhomogeneous composition in the
longitudinal dimension.
[531] Aspect 48. A part comprising the multilayer system of any one of aspects
40 to 47.
[532] Aspect 49. The part of aspect 48, wherein the part comprises an
automotive vehicle part or an
aerospace vehicle part.
[533] Aspect 50. A vehicle comprising the multilayer system of any one of
aspects 40 to 47.
[534] Aspect 51. The vehicle of aspect 50, wherein the vehicle comprises an
aerospace vehicle or
an automotive vehicle.
73
Date Regue/Date Received 2022-10-28
FURTHER ITEMS
1534a1 The invention can be further defined by one or more of the following
items.
1. A method of making a multilayer system comprising two or more layers,
wherein one or
more of the layers comprises a sealant layer, comprising:
(a) mixing a first component and a second component to form a first
coreactive sealant
composition, wherein,
the coreactive sealant composition comprises a first reactive compound and a
second
reactive compound; and
the first reactive compound is reactive with the second reactive compound;
(b) extruding the first coreactive sealant composition to form an
extrudate;
(c) depositing the extrudate to form the sealant layer, and
(d) depositing a second coreactive composition underlying the deposited
sealant layer to form
an underlying layer; and/or
depositing a third coreactive composition overlying the sealant layer to form
an overlying
layer, wherein the second coreactive composition and the third coreactive
composition comprise a
different composition than the sealant layer,
wherein depositing comprises three-dimensional printing co-extruding.
2. The method of item 1, wherein the first coreactive composition is
different than the
second coreactive composition.
3. The method of item 1 or 2, wherein each of the layers of the multilayer
system comprises
a thermoset.
4. The method of any one of items 1 to 3, wherein a cross-sectional profile
of the extrudate
has a homogeneous composition across the cross-sectional profile.
5. The method of any one of items Ito 3, wherein a cross-sectional profile
of the extrudate
has an inhomogeneous composition across the cross-sectional profile.
6. The method of any one of items 1 to 5, wherein an outermost layer of the
two or more
layers of the multilayer system comprises a sealant layer.
7. The method of any one of items 1 to 6, wherein the coreactive sealant
composition
comprises a sulfur-containing prepolymer.
73a
Date Recue/Date Received 2023-05-31
8. The method of any one of items 1 to 7, wherein the first reactive
compound is reactive
with the second reactive compound at a temperature less than 50 C.
9. The method of any one of items 1 to 8, further comprising merging one or
more
additional coreactive compositions with the coreactive sealant composition,
wherein,
extruding comprises coextruding the coreactive sealant composition and the one
or more
additional coreactive compositions to form a coextrudate; and
depositing comprises depositing the coextrudate to form a multilayer system
comprising one or
more sealant layers.
10. The method of item 9, wherein each of the coreactive sealant
compositions is reactive
with an adjoining coreactive composition.
11. The method of any one of items 1 to 10, further comprising:
combining an additive-containing composition with a portion of the coreactive
sealant
composition to form an additive-modified coreactive sealant composition,
wherein the additive-containing
composition comprises an additive; and
extruding the additive-modified coreactive sealant composition to form the
extrudate,
wherein a cross-sectional profile of the extrudate has an inhomogeneous
concentration of the
additive; and/or
wherein the extrudate is characterized by an inhomogeneous concentration of
the additive within a
longitudinal dimension of the extrudate.
12. The method of any one of items 1 to 11,
further comprising merging an adhesion-promoting composition with the
coreactive sealant
composition; and
extruding comprises coextruding the first coreactive composition and the
adhesion-promoting
composition.
13. A multilayer system comprising a sealant layer made by the method of
any one of items 1
to 12.
14. The multilayer system of item 13, wherein adjoining layers are
chemically and/or
physically bound.
15. The multilayer system of item 13 or 14, wherein each of the layers
comprises a thermoset
material.
73b
Date Recue/Date Received 2023-05-31
16. The multilayer system of any one of items 13 to 15, wherein the
multilayer system meets
or exceeds the requirements for aerospace sealants as set forth in AMS 3277.
17. The multilayer system of any one of items 13 to 16, wherein one or more
of the layers of
the multilayer sealant has an inhomogeneous composition cross-sectional
profile.
18. The multilayer system of any one of items 13 to 17, wherein one or more
of the layers of
the multilayer sealant has an inhomogeneous composition in the longitudinal
dimension.
19. A part comprising the multilayer system of any one of items 13 to 18.
20. The part of item 19, wherein the part comprises an automotive vehicle
part or an
aerospace vehicle part.
21. A method of making a multilayer system comprising two or more layers,
wherein one or
more of the layers comprises a sealant layer, comprising:
(a) mixing a first component and a second component to form a coreactive
sealant
composition, wherein,
the coreactive sealant composition comprises a first reactive compound and a
second
reactive compound; and
the first reactive compound is reactive with the second reactive compound;
(b) extruding the coreactive sealant composition to form an extrudate;
(c) repeatedly depositing by extrusion individual layers of the extrudate
to form the sealant
layer; and
(d) curing the deposited extrudate;
wherein each of the individual layers comprise a thermosetting composition
that cures at
temperatures less than 50 C.
22. The method of item 21, wherein depositing comprises three-dimensional
printing.
23. The method of item 21 or 22, wherein depositing comprises depositing
the extrudate onto
an underlying layer of the multilayer system.
24. The method of any one of items 21 to 23, further comprising:
depositing a first coreactive composition underlying the deposited sealant
layer to form an
underlying layer; and/or
73c
Date Recue/Date Received 2023-05-31
depositing a second coreactive composition overlying the sealant layer to form
an overlying layer,
wherein the first coreactive composition and the second coreactive composition
comprise a
different composition than the sealant layer.
25. The method of item 24, wherein the first coreactive composition is
different than the
second coreactive composition.
26. The method of any one of items 21 to 25, wherein each of the layers of
the multilayer
system comprises a thermoset.
27. The method of any one of items 21 to 26, wherein a cross-sectional
profile of the
extrudate has a homogeneous composition across the cross-sectional profile.
28. The method of any one of items 21 to 26, wherein a cross-sectional
profile of the
extrudate has an inhomogeneous composition across the cross-sectional profile.
29. The method of any one of items 21 to 28, wherein an outermost layer of
the two or more
layers of the multilayer system comprises a sealant layer.
30. The method of any one of items 21 to 29, wherein each of the layers of
the multilayer
system independently comprises a sealant layer or a non-sealant layer.
31. The method of any one of items 21 to 30, wherein the coreactive sealant
composition
comprises a prepolymer comprising a chemically resistant backbone.
32. The method of any one of items 21 to 31, wherein the coreactive sealant
composition
comprises a sulfur content greater than 10 wt%, wherein wt% is based on the
total weight of the organic
constituents of the coreactive sealant composition.
33. The method of any one of items 21 to 32, wherein the coreactive sealant
composition
comprises a sulfur-containing prepolymer.
34. The method of item 33, wherein the sulfur-containing prepolymer
comprises a
polythioether, a poly sulfide, a sulfur-containing polyformal, a monosulfide,
or a combination of any of the
foregoing.
73d
Date Recue/Date Received 2023-05-31
35. The method of item 33 or 34, wherein the sulfur-containing prepolymer
comprises a
sulfur content greater than 10 wt%. wherein wt% is based on the total weight
of the sulfur-containing
prepolymer.
36. The method of any one of items 21 to 35, wherein,
the first reactive compound is reactive with the second reactive compound in
the presence of a
catalyst and/or a polymerization initiator; and
the catalyst and/or polymerization initiator is capable of catalyzing and/or
initiating a reaction
between the first reactive compound and the second reactive compound.
37. The method of any one of item 36, further comprising activating the
polymerization
initiator before depositing, during deposition, and/or after depositing the
extrudate.
38. The method of any one of items 21 to 37, wherein,
the first component comprises the first reactive compound and the second
reactive compound; and
the second component comprises a catalyst, a cure activator, and/or a
polymerization initiator for
the reaction between the first reactive compound and the second reactive
compound.
39. The method of any one of items 21 to 37, wherein the first component
comprises the first
reactive compound and the second component comprises the second reactive
compound.
40. The method of any one of items 21 to 39, wherein,
the first reactive compound comprises a polyamine and/or a polyol and the
second reactive
compound comprises a polyisocyanate;
the first reactive compound comprises a polyamine and the second reactive
compounds comprises
a polyepoxide;
the first reactive compound comprises a Michael acceptor and the second
reactive compound
comprises a Michael donor; or
the first reactive compound comprises a polythiol and the second reactive
compound comprises a
polythiol, a polyisocyanate, a poly alkenyl, a polyalkynyl, a polyepoxide, a
Michael acceptor, or a
combination of any of the foregoing.
41. The method of any one of items 21 to 40, further comprising:
pumping the first component into a mixer using a first pump; and
pumping the second component into the mixer using a second pump.
73e
Date Recue/Date Received 2023-05-31
42. The method of any one of items 21 to 41, wherein curing comprises
allowing the
deposited extrudate to cure at a temperature less than 30 C.
43. The method of any one of items 21 to 42, further comprising merging one
or more
additional coreactive compositions with the coreactive sealant composition,
wherein,
extruding comprises coextruding the coreactive sealant composition and the one
or more
additional coreactive compositions to form a coextrudate; and
depositing comprises depositing the coextrudate to form a multilayer system
comprising one or
more sealant layers.
44. The method of item 43, wherein each of the one or more additional
coreactive
compositions independently comprises an additional coreactive sealant
composition or a coreactive non-
sealant composition.
45. The method of item 43 or 44, wherein the coreactive sealant composition
and an
adjoining additional coreactive composition comprise the same curing
chemistry.
46. The method of any one of items 43 to 44, wherein the coreactive sealant
composition and
an adjoining additional coreactive composition comprise different curing
chemistries.
47. The method of any one of items 43 to 44, wherein the coreactive sealant
composition is
reactive with an adjoining additional coreactive composition.
48. The method of any one of items 21 to 42, further comprising:
combining an additive-containing composition with a portion of an additional
coreactive
composition to form an additive-modified additional coreactive composition,
wherein the additive-
containing composition comprises an additive; and
extruding comprises coextruding the coreactive sealant composition and the
additive-modified
additional coreactive composition to form a coextrudate.
49. The method of item 48, wherein a cross-sectional profile of the
coextrudate has an
inhomogeneous concentration of the additive.
50. The method of item 48 or 49, wherein the extrudate is characterized by
an
inhomogeneous concentration of the additive within a longitudinal dimension of
the coextrudate.
73f
Date Recue/Date Received 2023-05-31
51. The method of any one of items 43 to 50, further comprising mixing a
third component
with a fourth component to form the one or more additional coreactive
compositions.
52. The method of any one of items 21 to 42, further comprising:
combining an additive-containing composition with a portion of the coreactive
sealant
composition to form an additive-modified coreactive sealant composition,
wherein the additive-containing
composition comprises an additive; and
extruding the additive-modified coreactive sealant composition to form the
extrudate.
53. The method of item 52, wherein a cross-sectional profile of the
extrudate has an
inhomogeneous concentration of the additive.
54. The method of item 52 or 53, wherein the extrudate is characterized by
an
inhomogeneous concentration of the additive within a longitudinal dimension of
the extrudate.
55. The method of any one of items 21 to 54,
further comprising merging an adhesion-promoting composition with the
coreactive sealant
composition; and
extruding comprises coextruding the first coreactive composition and the
adhesion-promoting
composition.
56. The method of any one of items 21 to 55, further comprising applying an
adhesion-
promoting layer to the extrudate before depositing the extrudate.
57. A multilayer system comprising a sealant layer made by the method of
any one of items
21 to 56.
58. The multilayer system of item 57, wherein adjoining layers are
chemically and/or
physically bound.
59. The multilayer system of item 57 or 58, wherein a fracture energy of
the fully cured
multilayer sealant is substantially the same as the fracture energy of an
individual layer, wherein the
fracture energy is determined according to ASTM D7313.
60. The multilayer system of any one of items 57 to 59, wherein each of the
layers comprises
a thermoset material.
73g
Date Recue/Date Received 2023-05-31
61. The multilayer system of any one of items 57 to 60, wherein each of the
layers comprises
a different thermoset material.
62. The multilayer system of any one of items 57 to 61, wherein the
multilayer system meets
or exceeds the requirements for aerospace sealants as set forth in AMS 3277.
63. The multilayer system of any one of items 57 to 62, wherein one or more
of the layers of
the multilayer sealant has an inhomogeneous composition cross-sectional
profile.
64. The multilayer system of item 57 or 63, wherein one or more of the
layers of the
multilayer sealant has an inhomogeneous composition in the longitudinal
dimension.
65. A part comprising the multilayer system of any one of items 57 to 64.
66. The part of item 65, wherein the part comprises an automotive vehicle
part or an
aerospace vehicle part.
EXAMPLES
[535] Embodiments provided by the present disclosure are further illustrated
by reference to the
following examples, which describe methods of making multilayer systems and
properties of the
73h
Date Recue/Date Received 2023-05-31
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
multilayer systems. It will be apparent to those skilled in the art that many
modifications, both to
materials, and methods, may be practiced without departing from the scope of
the disclosure.
Example 1
Synthesis of Thiol-terminated Polyepoxy-Extended Copolymer
[536] A thiol-terminated polythioether polymer, Permapol P3.1E, (384.32 g,
commercially available
from PPG Aerospace, mercaptan equivalent weight 1650) and a polyepoxide, DEN
431 (8.45 g,
available from the Dow Chemical) were combined in a plastic cup. The
components were combined
using a mixer (Hauschild Speed Mixer, 2,300 rpm, 45 sec). An amine, Dabco0 33-
LV, (5.38 g, available
from Air Products & Chemicals) was added to the mixture and combined using a
high-speed mixer
(Hauschild SpeedMixer , 30 sec at 2,300 rpm and 5 min at 800 rpm). The
resulting thiol-terminated
polyepoxy-extended polythioether prepolymer was then left at 23 C for 24 h
before combining with other
constituents to prepare a coreactive sealant composition. The thiol-terminated
polyepoxy-extended
polythioether prepolymer had a number average molecular weight of 4,716 Da,
and a thiol equivalent
weight of 2,069 Da.
Example 2
Preparation of SkydrolO-Resistant Polythioether Sealant
[537] A Base coreactive component (Part B) comprising the thiol-terminated
polyepoxy-extended
polythioether prepolymer of Example 1 was prepared. The constituents shown in
Table 1 were combined
and mixed to form the Base component (Part B).
Table 1. Base coreactive component (Part B).
Constituent Material Amount (wt%)
Thiol-terminated chain- Thiol-terminated polyepoxy-extended
61.62
extended prepolymer polythioether prepolymer of Example 1
Organic filler Micronized polyolefin, ACumistO A-6 1 6.08
Organic filler Ganzpearl 2 24.65
Inorganic filler Calcium carbonate, Socal 31 3 2.50
Inorganic filler Fumed silica, Aerosil R202 4 3.08
Adhesion promoter Cooked Phenolic, T-3920 5 0.42
Adhesion promoter Cooked Phenolic, T-3921 5 0.33
Adhesion promoter Silquest A-1110 Alkoxysilane 6 0.5
Adhesion promoter Phenolic resin, Methylon 75108 0.83
I Commercially available from Honeywell, Morris Plains, NJ.
74
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
2 Commercially available from Sakai Trading, New York, NY,
3 Commercially available from Solvay.
4 Commercially available from Cabot Corp.
Commercially available from PPG Aerospace, Sylmar, CA.
6 Commercially available from Momentive.
7 Commercially available Durez Corp.
[538] An Accelerator component (Part A) comprising a polyepoxide curing agent
was prepared. The
constituents of the Accelerator component (Part A) are shown in Table 2.
Table 2. Accelerator (Part A) composition.
Constituents Material Amount (wt %)
Polyepoxide DER 331 1 21.42
Polyepoxide EPU-73B 2 7.14
Polyepoxide Epoxy-terminated polythioether 3 21.91
Polyepoxide Epoxy novolac DEN 4314 9.09
Inorganic filler Calcium carbonate, Winnodil, SPM 40.37
Pigment Sunfast Blue dye 6 0.07
Commercially available from the Dow Chemical.
2 Polyurethane polyepoxide; epoxy eq. wt. 245; commercially available from
Adeka Corporation, Tokyo, Japan.
Difunctional epoxy-terminated polythioether; epoxy eq. wt. 584; commercially
available from PPG Aerospace,
Syhnar, CA.
4 Commercially available from the Dow Chemical.
5 Commercially available from Solvay.
6 Commercially available from Sun Chemical Corp.
[539] A Skydrol LD-4-resistant coreactive sealant composition was prepared by
mixing 100 parts of
the Base component (Part B) with 15.3 parts of the Accelerator component (Part
A).
Example 3
Multilayer System
[540] A multilayer system was made by first preparing a first inner layer
comprising a sealant and then
applying a second, outer layer over the first inner layer. The material used
to prepare the first inner layer
was not Skydrol LD-4-resistant.
[541] An inner layer was prepared by cutting samples of cured PR-2001 or PR-
1776M sealant into 2-
inch x 2-inch x 0.25-inch blocks. Both PR-2001 and PR-1776M are commercially
available from PPG
Aerospace. PR-2001 is a Class B two-part, epoxy-cured Permapol 3.1 thiol-
terminated polythioether
prepolymer-based sealant. PR-1776M is a Class B, two-part, manganese dioxide-
cured Permapol P-5
modified polysulfide-based sealant.
CA 03129262 2021-08-05
WO 2020/167626 PCT/US2020/017428
[542] The blocks of inner sealant were then coated with a layer of the Skydrol
-resistant- coreactive
sealant composition of Example 2 having a variable thickness from 1 mm to 4 mm
to provide an outer
layer. The outer layer covered the top, bottom and sides of the blocks and was
then cured.
[543] The multilayer systems (Multilayer Systems 1-4) were immersed in Skydrol
LD-4 for from 1
day to 5 days at 70 C and the Shore A hardness of the blocks was periodically
measured.
[544] Skydrol LD-4 is a fire-resistant hydraulic fluid based on phosphate
ester chemistry available
from Eastman Chemical Company. Skydrol LD-4 has a concentration of about 58.2
wt% tributyl
phosphate, from about 20 wt% to 30 wt% dibutyl phenyl phosphate, from about 5
wt% to 10 wt% butyl
diphenyl phosphate, less than about 10 wt% 2-ethylhexy1-7-oxabicyclo[4.1.0]
heptane-3-carboxylate, and
from about 1 wt% to 5 wt% 2,6-di-tert-butyl-p-cresol.
[545] Hardness was determined according to ASTM D2240. The hardness of control
sealants
consisting of the PR-2001 (Sealant Cl) or PR-1776M (Sealant C2) sealant blocks
without the layer of the
Skydrol -resistant sealant of Example 2 was also measured at intervals during
immersion in Skydrol
LD-4 at 70 C.
[546] The results are shown in Table 3. The results demonstrate that a
multilayer system comprising a
sealant layer exhibited greater Skydrol LD-4 resistance than a single-layer
sealant.
Table 3. Multilayer system Shore A hardness following immersion in Skydrol LD-
4 at 70 C.
Shore A Hardness
Thickness
Multilayer Inner
Sealant Sealant Second Layer Initial After Skydrol e LD-4
Immersion at 70 C
(mm)
1 day 2 days 3 days 5 days
1 PR-2001 1 64A 43A 37A 33A 32A
2 PR-1776M 1 63A 44A 36A 31A 28A
3 PR-2001 4 64A 42A 37A 36A 33A
4 PR-1776M 4 60A 38A 38A 34A 31A
Cl PR-2001 0 56A 34A 26A 26A 25A
C2 PR-1776M 0 50A 17A 2A
too soft to too soft to
measure measure
[547] Finally, it should be noted that there are alternative ways of
implementing the embodiments
disclosed herein. Accordingly, the present embodiments are to be considered as
illustrative and not
restrictive. Furthermore, the claims are not to be limited to the details
given herein and are entitled to
their full scope and equivalents thereof.
76