Note: Descriptions are shown in the official language in which they were submitted.
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
MICROALGAE-BASED SOIL INOCULATING SYSTEM AND METHODS OF USE
RELATED APPLICATIONS
[0001] This
application claims the benefit of and priority to U.S. Provisional Application
No. 62/806,543, filed February 15, 2019, entitled "Method of Isolation,
Selection, and Use of
Endemic Microbes for Agriculture Production Areas". This application claims
priority to U.S.
Application No. 16/534,907, filed August 7,2019, entitled "Microalgae-Based
Soil Inoculating
System and Methods of Use", which is a continuation-in-part application of
U.S. Patent
Application No. 16/207,528, filed December 3, 2018, entitled "Microalgae-Based
Soil
Inoculating System and Methods of Use", which is a continuation of U.S. Patent
Application
No. 14/069,932, filed November 1, 2013, entitled "Microalgae-Based Soil
Inoculating System
and Methods of Use", now issued as U.S. Patent No. 10,172,304, which is a
continuation of
International Patent Application No. PCT/U512/36293, filed May 3, 2012,
entitled
"Microalgae-Based Soil Inoculating System and Methods of Use", which claims
the benefit of
and priority to U.S. Provisional Application No. 61/481,998, filed May 3,
2011, entitled
"Microalgae-Based Soil Inoculating System and Methods of Use", and further is
a
continuation-in-part of U.S. Patent Application No. 15/647,005, filed July 11,
2017, entitled
"Soil Enrichment Systems and Methods", this application incorporates the
disclosure of all
such priority applications by reference. To the extent that the present
disclosure conflicts with
any referenced application, however, the present disclosure is to be given
priority.
BACKGROUND
[0002] Microbes
in soil have many well-known beneficial effects. While there are many
references to algae herein, such references are used solely as helpful
examples, and do not limit
the scope of the inventions described and claimed herein, which are directed
to microbes
generally as well.
[0003] Algae
have the ability to adapt to their environment. For instance, algae found in
soil in the Southwestern deserts have adapted to elevated temperatures,
alkaline pH levels, and
periods of desiccation, while algae in northern climates have adapted to much
lower
temperatures, freeze-thaw cycles, higher soil moisture levels, and more acidic
soil pH levels,
etc.
1
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0004] Endemic
algae fill a niche in the field ecosystem. Within the soil ecosystem, a
symbiosis with other organisms has developed resulting in a biochemical
environment where
compounds produced by the endemic algae may augment the growth of other
desirable
microbes and depress the growth of undesirable or non-beneficial organisms.
For example,
algae are known to produce biochemicals such as amino acids, hormones,
peptides, and fatty
acids that augment the growth of beneficial microorganisms. These beneficial
biochemicals
also directly help the crop plants. The beneficial microorganisms produce
biochemicals that
the algae and crop can utilize to grow (e.g., sugars and vitamins) resulting
in continued algal
and crop growth. At the same time, algae may produce compounds that are
antibacterial,
antifungal, algicidal, and/or antiprotozoal which prevent the growth of
unwanted microbes in
soil and surface waters.
[0005] When
soil algae die, cellular biochemicals are released which can directly feed the
soil biome and any crop plants growing in the soil. These biochemicals are
large molecules
(e.g., such as proteins, fats, dyes, peptides, nucleic acids, etc.), some or
all of which can be
absorbed by the crop plant, resulting in crops with greater nutritional value.
[0006] If live,
foreign algae are introduced into the soil, the ecosystem is forced to
rebalance. This imbalance can lead to the production of one or more unwanted
biochemicals
(such as a toxin), or the absence of an important biochemical which may be
required by the
crop plant.
[0007] When
algae are introduced to the soil, the metabolic activity in the soil
increases,
resulting in greater CO2 production. This is particularly true for live algae
whose metabolic
activities continue after introduction to the soil. This CO2 production lowers
the pH of the soil
resulting in the dissolution of calcium and magnesium carbonate bonds, thereby
opening the
soil for greater root penetration and increased water and fertilizer movement.
This increased
water movement carries more salts out of the root zone, thereby reducing the
osmolarity within
the root zone, and increasing the bioavailability of macro and micronutrients
to the crop. The
lower pH also frees up bound potassium and phosphorus making it available to
the plants.
Algae excrete extracellular phosphatases almost immediately upon the onset of
phosphorus
limited conditions. These compounds release the phosphates from soil particles
and make them
available to the plants. Green algae also produce polysaccharides which hold
onto water until
it is needed.
2
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0008]
Substantially constant or periodic addition of algae can result in a desirable
buildup
of organic matter (humus) within the soil which also has the property of
holding water and
nutrients which can be released to the plants as needed. Other methods of
introducing humus
to the soil generally require tilling-in of organic matter (compost, various
plant cuttings,
manures, etc.), which can best be performed when a field is between plantings.
Humus aids in
the formation of natural iron chelates (fulvic acids-Fe), which prevents soil
from being blocked
by calcium and magnesium carbonates, thus avoiding chlorosis problems induced
by low
bioavailability of these nutrients. Chlorosis is the reduction in the green
color of plants due to
a reduction in the amount of chlorophyll in the leaves brought on by a lack of
bioavailable
macro and micronutrients such as nitrogen (N), magnesium (Mg), calcium (Ca),
and iron (Fe),
even when these nutrients are present in the soils.
[0009] Ion
exchange capacity is a quantitative means for describing the binding of
fertilizer
elements to soil particles for storage and release. Humus ion exchange
capacity (e.g., 400 to
600 meq/100g) is 5 to 10 times higher than that of clays (e.g., 50 to 150
meq/100g). It is this
capacity which allows the retention of fertilizers within the soil for use by
the plants as needed.
As plants utilize the nitrogen (N), phosphorus (P), and potassium (K) in the
soil, the stored
elements are released from the humus as needed. By combining with humic
substances, copper
and other trace elements become less toxic and more readily available to the
plants.
[0010]
Fertilizers are more effective if combined with microalgae. Algae cells
process
fertilizers by breaking down certain molecules into more bioavailable forms
that plants can
more readily use. The nutrients are then more efficiently and completely
absorbable by the root
system of the plants. For example, ammonium nitrate, an excellent source of
nitrogen, is one
of the most common bulk fertilizers used to grow crops. While plants can
immediately absorb
the nitrate in this fertilizer, the ammonium component is less accessible to
the plant.
Microalgae cells will absorb the ammonium, naturally convert it to nitrogenous
biochemicals,
and upon their death, will release these valuable biochemicals to the plant
for easy
consumption. Additionally, the nutrients from fertilizers can bind to the
microalgae cells or
their organic remains and are less likely to be lost in run-off water during
rains or irrigation.
Upon their death, the algae can also feed bacteria in the soil, which can
convert the ammonium
ion into nitrate ions.
[0011] Algae
produce growth regulators (e.g., gibberellic acid) that improve salt
tolerance,
induce seed germination and increase plant growth rate and fruit production.
Artificial or
3
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
concentrated growth regulators are expensive, especially when applied in
substantial amounts,
making it impractical for growers to replicate this effect by use of other
products.
[0012] Algae
play a role in controlling agricultural pests by directly producing
antibiotics
and antifungal compounds, and by feeding the beneficial microbes in the soil
which produce
other pest fighting compounds. These compounds give the plants the ability to
prevent the
invasion of pathogenic species. Disease and pests are also resisted due to the
improved vigor
of the plants.
[0013] As
discussed above, live microalgae cells can function as a catalyst to tap and
utilize
all of the benefits available from standard fertilizers; and also, to provide
a natural supply of
essential compounds and phytochemicals, while supporting the overall efficacy
of the growing
environment. These potent attributes work in concert to stimulate plants to
grow heartier and
more quickly; and to consistently produce a more abundant, higher quality and
more nutrient
rich end-product such as a crop. The benefits from an additive of microalgae
cells are available
when the algae cells that are delivered to the soil are in healthy living form
and in great
concentration. The selection and formulation of the algae additive is critical
to its overall
impact. When correctly instituted, a microalgae additive program is simple to
manage, and
offers breakthrough potential in agricultural production. The impact may be
greatest in the
most depleted soils such as arid soils that have significant salt and caliche
buildup with minimal
organic matter. Further, by selecting endemic algae for propagation and
delivery to an
agricultural production area, there is a higher survival rate, and a greater
and faster impact on
soil health.
SUMMARY OF THE INVENTION
[0014] Some
embodiments include a culturing system comprising a bioreactor adapted to
propagate microalgae in a culture solution using in combination natural and/or
artificial light,
and at least one nutrient comprising at least a carbon source, where the
microalgae are freely
suspended in and form part of the culture solution. Some embodiments include
an algae
nutrient supply coupled to the bioreactor and a first controller between a
water conditioning
assembly and the bioreactor. In some embodiments, the water conditioning
assembly is
coupled as an input of supply water to the bioreactor, and configured to
condition the supply
water to a specified purity that enables substantially unhindered growth of
the microalgae in
the culture solution to a specified concentration. Further, in some
embodiments, the first
4
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
controller can be configured to control the delivery of the algae nutrient
supply to the
bioreactor. In some embodiments, a carbon dioxide source is coupled to the
bioreactor, where
the carbon dioxide is injected into the culture solution as the carbon source.
[0015] Some
further embodiments include a second controller coupled to a probe and
configured to regulate release of carbon dioxide from the carbon dioxide
source to the
bioreactor based at least in part on one or more measurements from the probe,
where the carbon
dioxide is injected into the culture solution as the carbon source.
[0016] In some
embodiments, the probe is a pH probe configured to measure a pH of the
culture solution. In some embodiments, the water conditioning assembly
includes an ozone
generator coupled to an ozone contactor, where the ozone generator is
configured to generate
ozone and deliver the ozone to at least partially ozonate the supply water.
[0017] Some
embodiments include a solids filter downstream from an outlet of the ozone
contactor, where the solids filter is configured to remove solids from
ozonated supply water
exiting the ozone contactor. Some embodiments include a carbon filter and/or a
UV light
system positioned downstream from the solids filter, where the carbon filter
and/or the UV
light system can at least partially de-ozonate the ozonated supply water.
[0018] Some
embodiments include at least one pressurized air supply coupled to the
bioreactor, where the at least one pressurized air supply can generate gas
bubbles to at least
partially aerate and/or agitate the culture solution. In some embodiments, the
gas bubbles
include CO2, N2, and/or 02. Some embodiments further comprise at least one
water reservoir
or tank providing or coupled to the input of supply water.
[0019] Some
further embodiments comprise a mobile trailer supporting at least the
bioreactor, the water conditioning assembly, and the carbon dioxide source. In
some
embodiments, the microalgae feed source comprises a fertilizer, a macro-
nutrient, a micro-
nutrient, and at least two different microalgae species.
[0020] In some
embodiments, the macro-nutrient is selected from the group consisting of
phosphorus, nitrogen, carbon, silicon, calcium salt, magnesium salt, sodium
salt, potassium
salt, and sulfur; and the one or more micronutrients is selected from the
group consisting of
manganese, copper, zinc, cobalt, molybdenum, vitamins and trace elements.
Further, in some
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
embodiments, the micro-nutrient comprises a vitamin and a mineral added to the
conditioned
supply water.
[0021] Some
embodiments comprise a telemetry system configured for a remote
monitoring and/or controlling operation of one or more of the first
controller, the second
controller, the bioreactor, and at least one component or assembly of the
water conditioning
assembly.
[0022] In some
embodiments, the artificial light comprises LED lights positioned within
the bioreactor and/or proximate to a surface of the bioreactor and exposing
the microalgae to
light.
[0023] In some
embodiments, the carbon dioxide source comprises a tank comprising
carbon dioxide gas, and/or a carbon dioxide generator, and/or a carbon dioxide-
sequester that
sequesters and temporarily stores atmospheric carbon dioxide.
[0024] In some
further embodiments, the microalgae feed source comprises a first algae
type, and/or a second algae type, and/or bacteria, and/or fungi. Some
embodiments further
comprise a flow-imaging device coupled to an output of the bioreactor, where
the flow imaging
device is configured to create images of algae, predators, and contaminants in
the culture
solution for quality control monitoring. Some embodiments further comprise a
microorganism
mixer configured to blend algae, and/or bacteria, and/or fungi, with any of
the culture solution
exiting the bioreactor.
[0025] Some
embodiments include a method comprising preparing one or more microbe-
containing samples from at least one location of a current or planned plant
growth area, and
preparing at least one cultured sample by culturing microbes from the sample.
Further, some
embodiments include selecting at least one target species of microbe from the
at least one
cultured sample, and propagating the at least one selected target species of
microbe to increase
the concentration of the at least one target species of microbe in the at
least one cultured sample.
Some embodiments include providing a bioreactor adapted to propagate the at
least one
selected target species in a culture solution, where the at least one selected
target species being
freely suspended in and forming part of the culture solution. Further, some
embodiments
include coupling a feed source to the bioreactor and a first controller
between a water
conditioning assembly and the bioreactor, where the water conditioning
assembly is coupled
6
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
as an input of supply water to the bioreactor, and configured to condition the
supply water to a
specified purity that enables substantially unhindered growth of the at least
one selected target
species in the culture solution to a specified concentration. Further, in some
embodiments, the
first controller is configured to control supply of the feed source to the
bioreactor. Further, in
some embodiments of the method, a carbon dioxide source is coupled to the
bioreactor.
[0026] Some
embodiments include a second controller coupled to the probe and configured
to regulate release of carbon dioxide from the carbon dioxide source to the
bioreactor based at
least in part on one or more measurements from the probe, and further, where
the carbon
dioxide is injected into the culture solution a carbon source enabling
propagation of the at least
one selected target species of microbe. Some embodiments include delivering at
least a portion
of the at least one target species of microbe to at least a portion of the at
least one location,
where at least a portion of the at least one target species of microbe being
delivered comprises
at least one live microbe. In some embodiments, the at least one live microbe
is selected to be
a well-adapted endemic species.
[0027] In some
embodiments of the method, the water conditioning assembly includes an
ozone generator coupled to an ozone contactor, where the ozone generator is
configured to
generate ozone and deliver the ozone to at least partially ozonate the supply
water.
[0028] In some
embodiments of the method, a solids filter is positioned upstream from an
inlet of the ozone contactor.
[0029] In some
embodiments of the method, a carbon filter and/or a UV light system are
positioned immediately downstream from the solids filter, where the carbon
filter and/or the
UV light system are configured and arranged to at least partially de-ozonate
the ozonated
supply water. Further, at least one pressurized air supply is coupled to the
bioreactor, where
the at least one pressurized air supply can generate gas bubbles to at least
partially aerate and/or
agitate the culture solution in the bioreactor.
[0030] Some
embodiments of the method further comprise delivering at least a portion of
the at least one target species of microbe to at least a portion of the at
least one location. In
some embodiments, at least a portion of the at least one target species of
microbe delivered
comprises at least one live microbe. In some embodiments, the at least one
live microbe is an
endemic species of algae to the delivery location. In some further
embodiments, the at least
7
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
one live microbe is a live species selected to restore a normal soil flora mix
of a cropland. In
some other embodiments, the live species of algae is selected for its specific
desired properties
for improving the soil in the delivery location.
[0031] Some
embodiments include a method comprising sampling the algal flora from an
agricultural location, and selecting at least one desired algae species for
propagation, where the
at least one desired algae species is present in the agricultural location as
an initial
concentration. Some embodiments include propagating the at least one desired
algae species
in at least one bioreactor, and delivering the at least one desired species to
the agricultural
location to increase the concentration of the algae species to a concentration
greater than the
initial concentration.
[0032] In some
embodiments of the method, the at least one bioreactor is adapted to
propagate at least one desired species in a culture solution using in
combination at least one of
natural and artificial light, and at least one nutrient comprising at least a
carbon source, where
at least one desired species are freely suspended in and form part of the
culture solution.
[0033] In some
embodiments of the method, an algae nutrient supply is coupled to the at
least one bioreactor and a controller for controlling flow between a water
conditioning
assembly and the at least one bioreactor. In some embodiments, the water
conditioning
assembly is coupled as an input of supply water to the at least one bioreactor
to condition the
supply water to a specified purity that enables substantially unhindered
growth of the
microalgae in the culture solution to a specified concentration. Further, in
some embodiments,
the controller is configured to control supply of the algae nutrient supply to
the at least one
bioreactor.
[0034] In some
embodiments of the method, a carbon dioxide source coupled to the at least
one bioreactor, where the carbon dioxide is injected into the culture solution
as the carbon
source. In some further embodiments of the method, a second controller is
coupled to a probe,
the second controller configured to regulate release of carbon dioxide from
the carbon dioxide
source to the bioreactor based at least in part on one or more measurements
from the probe.
[0035] In some
embodiments of the method, the water conditioning assembly includes an
ozone generator coupled to an ozone contactor, where the ozone generator
generates ozone and
8
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
delivers the ozone to at least partially ozonate the supply water. In some
further embodiments
of the method, a solids filter is positioned upstream from an inlet of the
ozone contactor.
[0036] In some
embodiments of the method, the carbon filter and/or a UV light system are
positioned downstream from the solids filter, where the at least one of the
carbon filter and the
UV light system at least partially de-ozonates the ozonated supply water. In
some other
embodiments of the method, the pressurized air supply is coupled to the
bioreactor, where the
pressurized air supply generates gas bubbles to at least partially aerate
and/or agitate the culture
solution in the at least one bioreactor.
DESCRIPTION OF THE DRAWINGS
[0037] FIG. 1
depicts a first embodiment of the microalgae-based soil inoculating system
of the invention.
[0038] FIG. 2
depicts a front-perspective view of a second embodiment of the microalgae-
based soil inoculating system of the invention.
[0039] FIG. 3
depicts a side elevation view of a third embodiment of the microalgae-based
soil inoculating system of the invention.
[0040] FIG. 4A
depicts afield, five weeks after a crop of melons were planted and treated
according to the method and with the system of the invention.
[0041] FIG. 4B
depicts the same field of FIG. 4A at nine weeks after a crop of melons were
planted and treated according to the method and with the system of the
invention.
[0042] FIG. 5A
depicts a melon plant in a section of field not treated according to the
invention.
[0043] FIG. 5B
depicts melon plants in a section of field treated according to the invention.
[0044] FIG. 6A
depicts a melon growing in plant after nine weeks in a section of field not
treated according to the invention.
[0045] FIG. 6B
depicts a melon growing in plant after nine weeks in a section of field
treated according to the invention.
9
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0046] FIG. 7
depicts a fourth embodiment of the microalgae-based soil inoculating system
of the invention.
[0047] FIG. 8
depicts a fifth embodiment of the microalgae-based soil inoculating system
of the invention.
[0048] FIG. 9
illustrates a soil enrichment system in accordance with some further
embodiments of the invention.
DETAILED DESCRIPTION
[0049] Before
any embodiments of the invention are explained in detail, it is to be
understood that the invention is not limited in its application to the details
of construction and
the arrangement of components set forth in the following description or
illustrated in the
following drawings. The invention is capable of other embodiments and of being
practiced or
of being carried out in various ways. Also, it is to be understood that the
phraseology and
terminology used herein is for the purpose of description and should not be
regarded as
limiting. The use of "including," "comprising," or "having" and variations
thereof herein is
meant to encompass the items listed thereafter and equivalents thereof as well
as additional
items. Unless specified or limited otherwise, the terms "mounted,"
"connected," "supported,"
and "coupled" and variations thereof are used broadly and encompass both
direct and indirect
mountings, connections, supports, and couplings. Further, "connected" and
"coupled" are not
restricted to physical or mechanical connections or couplings.
[0050] The
following discussion is presented to enable a person skilled in the art to
make
and use embodiments of the invention. Various modifications to the illustrated
embodiments
will be readily apparent to those skilled in the art, and the generic
principles herein can be
applied to other embodiments and applications without departing from
embodiments of the
invention. Thus, embodiments of the invention are not intended to be limited
to embodiments
shown, but are to be accorded the widest scope consistent with the principles
and features
disclosed herein. The following detailed description is to be read with
reference to the figures,
in which like elements in different figures have like reference numerals. The
figures, which
are not necessarily to scale, depict selected embodiments and are not intended
to limit the scope
of embodiments of the invention. Skilled artisans will recognize the examples
provided herein
have many useful alternatives that fall within the scope of embodiments of the
invention.
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0051] Some
embodiments of the invention include a system capable of delivering a full
range of micronutrients within microalgae to soil. In some embodiments,
microalgae
containing water (effluent) can be inoculated into soil thereby making the
micronutrients
immediately bioavailable to crops grown in the soil. In some embodiments, the
system can be
placed within an irrigation system between the water source and the water
ports, through which
irrigation water can be applied to crops. In some embodiments, the system can
produce
biofertilizers that are immediately bioavailable to crops, such that
negligible runoff pollution
occurs. Using this system, inorganic agricultural chemicals can be used more
efficiently after
being converted into a bioavailable form by the algae; therefore, the amount
of chemicals
needed is reduced.
[0052] In some
embodiments, the system can be used to build soil organics with nutrient-
rich algae biomass to recover depleted (nutrient poor) soils. In some
embodiments, the system
can facilitate and accelerate the transformation of a chemicals-based farm to
an organic farm.
In some embodiments, the system can deliver microalgae to the soil that
dissolve soil
carbonates, build polysaccharide content in the topsoil, and improve soil
porosity up to 500%
or more. In some embodiments, the system also provides for use of specific
algal biotoxins in
place of conventional chemical fungicides and other chemical poisons/toxins to
manage
nematodes and other harmful pests.
[0053] Some
embodiments include a system that can comprise one or more bioreactors. In
some embodiments, the system can comprise plural bioreactors. In some
embodiments, when
plural bioreactors are present, the bioreactors can be the same or different.
Likewise, in some
embodiments, the contents of the bioreactor can be the same or different. In
some
embodiments, the culture medium in a bioreactor of the system can comprise one
or more types
of microalgae. Some embodiments of the invention include those wherein: a) all
of the
microalgae are of the same type; b) two or more different types of microalgae
are present;
and/or c) one or more bioreactors contain one or more types of microalgae, and
one or more
other bioreactors contain one or more other types of microalgae.
[0054] In some
embodiments, the microalgae in the bioreactor can propagate so an initial
microalgae inoculant placed into the bioreactor can provide an endless supply
of microalgae.
In this instance, microalgae feed and water can be loaded into the bioreactor
and a sufficient
amount of microalgae biomass can be removed from the bioreactor periodically
so as to keep
the conditions within the bioreactor suitable for microalgae culture.
11
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0055] In some
embodiments, the system and its method of use can improve overall crop
production 5% to 30% or higher as compared to untreated crops. In some
embodiments, the
system and method of use can improve the texture, taste, size, nutrient
content and/or yield of
a crop as compared to untreated crop. In terms of agriculture use, in some
embodiments, the
system and its method of use can reduce total energy consumption, and/or
reduce ecological
pollution, and/or reduce greenhouse gas emission, and/or increase
bioavailability of
micronutrients and macronutrients, and/or reduce the use of chemical
fertilizers, and/or reduce
overall crop production cost, and/or reduce tillage cost, and/or reduce the
need for and use of
fungicides, herbicides and/or pesticides, and/or reduce soil compaction,
and/or improve soil
porosity, and/or increase microbial content of soil, and/or increase the
organics content of soil,
and/or reduce the amount of irrigation water needed to grow a crop, and/or
reduce the
occurrence of over fertilization, and/or reduce run-off and soil erosion,
and/or improve plant
characteristics and/or improve water/moisture retention by soil, all as
compared to untreated
crop and croplands.
[0056] In some
embodiments, the system can be used to reduce or eliminate the buildup of
carbonates in irrigation equipment by flowing microalgae-containing water
through the
irrigation equipment. In some embodiments, the system can also be used to
reduce or eliminate
buildup of carbonates in soil by inoculating the soil with microalgae-
containing water.
[0057] Many
different species and strains of microalgae can be used according to the crop
needs. Algae may be collected and cultivated from the field where crops are to
be grown or
from commercial sources. Microalgae samples can be obtained from repositories
at Arizona
State University, University of California at Berkeley, University of Texas at
Austin, Woods
Hole Oceanographic Research Institute, Scripps Institute of Oceanography or
other
repositories.
[0058]
Different species and strains of microalgae grow best under different
conditions.
The culture conditions within the bioreactor will be varied according to the
particular species
of microalgae present in the bioreactor. Conditions for culturing many
different types of
microalgae can be found in The Handbook of Microalgal Culture: Biotechnology
and Applied
Phycology (ed. Amos Richmond, Blackwell Publishing, Oxford, U.K., 2004), Algal
Culturing
Techniques: A Book for All Phycologists (ed. Robert A. Andersen, Elsevier
Academic Press,
2005), and Microalgae:
Biotechnology and Microbiology Cambridge Studies in
12
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
Biotechnology (ed. E. W. Becker. Press Syndicate of the University of
Cambridge, 1994), the
disclosures of which are hereby incorporated in their entirety by reference.
[0059] In some
embodiments, indigenous microalgae species can possess properties that
make it optimal for growth under the environmental conditions of the target
geographic
location. In some embodiments, algae from non-indigenous locations or algal
collections may
be used to inoculate the soil of the target geographic location in order to
maximize specific
bioavailable compounds. Some embodiments include a method of inoculating soil
that can
comprise: obtaining a sample of soil from a target geographic location, and/or
isolating a robust
indigenous microalgae species from the sample, and/or culturing the microalgae
to form a first
inoculate. Further, the method can include inoculating a portable microalgae-
based soil
inoculating system with the first inoculate, and/or culturing the microalgae
in the inoculating
system to form a second inoculate, and/or inoculating soil of the target
geographic location one
or more times with the second inoculate. Further details are disclosed below.
[0060] In some
embodiments, the system of the invention can employ various different
types of water as the water source, including, but not limited to, wastewater,
and/or well water,
and/or lake water, and/or creek water, and/or pond water, and/or rainwater,
and/or river water
and/or freshwater. Since the water is intended for crop growth, it is
preferred that the water
source has low salinity and is free from heavy metals. In some embodiments,
after exiting the
micro-algae inoculating system, the inoculate-containing water can be
delivered to a crop by
any conventional irrigation means or system used in agriculture, for example,
by flood,
sprinklers or drip type of irrigation systems or by sprayer or aerial
application. If applied by
sprayer or aerial application, the treatment can be followed by sufficient
water to drive the
algae into the soil.
[0061] In some
embodiments, the system and method can provide for continuous, semi-
continuous, repeated or periodic treatment of soil with microalgae-containing
inoculate. For
example, in some embodiments, the soil can be treated with microalgae-
containing inoculate
daily, or every other day, or every third day, or semi-weekly, or every fourth
day, or every fifth
day, or every sixth day, or weekly, or biweekly, or every third week, or every
fourth week, or
monthly, or bimonthly, or quarterly, each trimester, or semiannually, or
annually. In some
embodiments, the soil can be treated with water not containing the microalgae
and then with
water containing microalgae inoculate, or vice versa. Some embodiments include
a dilute,
semi-concentrated and concentrated algal cultures with a single algal species
or two or more
13
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
different algal species. In some embodiments, the although it is optional,
additional crop
nutrients (macronutrients and/or micronutrients), aside from microalgae feed,
can be included
in the irrigation water. For example, in some embodiments, the nutrients such
as calcium may
be incorporated into the algal species for transport and uptake by the crops.
The following
table includes example macronutrients and micronutrients.
Macronutrients Micronutrients
Nitrogen (N) Boron (13)
Phosphorus (P) Sulfur (S)
Potassium (K) Copper (Cu)
Carbon (C) Chloride (Cl)
Oxygen (0) Iron (Fe)
Magnesium (Mg) Molybdenum (Mo)
Calcium (Ca) Manganese (Mn)
Nickel (Ni)
Zinc (Zn)
Selenium (Se)
Chromium (Cr)
Cobalt (Co)
Biotin
Thiamin
Vitamin B12
Vitamin B6
14
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0062] Algae
operate symbiotically with other organisms, both microorganisms and
macro-organisms. While the primary object of the invention focuses on
culturing algae,
culturing algae in a diverse community of multiple microorganisms may offer
useful solutions.
Nitrogen-fixing microbes, called diazotrophs, fall into two main groups, free-
living and
symbiotic. Aerobic diazotrophs, of which there are over 50 genera, including
Azotobacter,
methane-oxidizing bacteria, and cyanobacteria, require oxygen for growth and
fix nitrogen into
soil when oxygen is present. Azotobacter, some related bacteria, and some
cyanobacteria fix
nitrogen in ordinary air, but most members of this group fix nitrogen only
when the oxygen
concentration is low. Aphanizomenon flosaquae reduces acetylene and fixes
nitrogen in algal
cultures. Some symbiotic bacteria belong to the genus Rhizobium such as
Bradyrhizobium
and Sinorhizobium, which colonize the roots of leguminous plants and stimulate
the formation
of nodules within which they fix nitrogen micro-aerobically. Green microalgae
provide
nitrogen, phosphorous, potassium, calcium and various other micronutrients.
Accordingly,
some embodiments include embodiments wherein one or more microalgae are co-
cultured with
or are inoculated into soil along with one or more diazotrophs.
[0063] In some
embodiments, suitable microorganisms that can be co-cultured with or
inoculated into soil along with the microalgae and/or algae can include
actinomycetes, bacteria,
fungi, and/or mycorrhizae. For example, some embodiments include
actinomycetes, which are
thread-like bacteria that look like fungi. While not as numerous as bacteria,
they perform vital
roles in the soil, where they help decompose organic matter into humus, which
slowly releases
nutrients. They also produce antibiotics to fight root diseases. The same
antibiotics can be
used to treat human diseases. Actinomycetes create the sweet, earthy smell of
biologically
active soil when a field is tilled.
[0064] Some
embodiments can include the use of bacteria which can break down complex
molecules and enable plants to take up nutrients. Some species release N, S, P
and trace
elements from organic matter. Others break down soil minerals and release K,
P, Mg, Ca and
Fe. Other species make and release natural plant growth hormones, which
stimulate root
growth. A few bacteria fix N in the roots of legumes while others fix N
independently of plant
association. Bacteria are responsible for converting N from ammonium to
nitrate and back
again depending on soil conditions. Various bacteria species increase the
solubility of
nutrients, improve soil structure, fight root diseases, and detoxify soil. In
some embodiments,
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
bacteria suitable for co-culture with the microalgae and for use in the system
of the invention
are disclosed in United States Patent No. 7,736,508 to Limcaco (Jun. 15,
2010), the relevant
disclosure of which is hereby incorporated by reference.
[0065] Some
embodiments can include the use of fungi, some species of which can appear
as thread-like colonies, while others are one-celled yeasts. Slime molds and
mushrooms are
also fungi. Many fungi aid plants by breaking down organic matter or by
releasing nutrients
from soil minerals. Fungi are generally early to colonize larger pieces of
organic matter and
begin the decomposition process. Some fungi produce plant hormones, while
others produce
antibiotics including penicillin. Several fungi species trap harmful plant-
parasitic nematodes.
[0066] Some
embodiments can include the use of mycorrhizae, a group of fungi that lives
either on or in plant roots and act to extend the reach of root hairs into the
soil. Mycorrhizae
increase the uptake of water and nutrients especially in less fertile soils.
Roots colonized by
mycorrihizae are less likely to be penetrated by root-feeding nematodes since
the pest cannot
pierce the thick fungal network. Mycorrhizae also produce hormones and
antibiotics, which
enhance root growth and provide disease suppression. The fungi benefit from
plant association
by taking nutrients and carbohydrates from the plant roots where they live.
[0067] Aside
from revitalization or nutrient supplementation of soil, some embodiments of
the system and method can also be used in place of or to reduce the need for
conventional
herbicides, pesticides, fungicides and nematocides. For example, in some
embodiments, after
harvest, an algal species with specially selected toxins may be applied to
manage nematodes
and other soil predators. The algae with toxins are naturally occurring and
typically die out
after killing the nematodes. While it is possible for algae to mutate,
indigenous algae will be
far more robust and quickly crowd out any remaining toxic algae. Microalgae
suitable for use
as pesticides include algae from the genera Nostoc, Scytonema, and
Hapalosiphon. Some
embodiments can include the use of the system and methods in places such as
soil-based farms,
parks, hydroponic farms, aquaponics, nurseries, golf-courses, sporting fields,
orchards,
gardens, zoos and other such places where crops or plants are grown. Some
embodiments can
include the use of additional phytotoxins obtainable from microbes are
described by Duke et
al. ("Chemicals from Nature for Weed Management", Weed Science, (2002) vol.
50, pg. 138-
151). Some non-limiting example phytotoxins include actinonin, brefeldin,
carbocyclic
coformycin, cerulenin cochlioquinone, coronatine, 1,4-cineole, fischerellin,
fumosin,
fusicoccin, gabaculin, gostatin, grandinol, hydantocidin, leptospermone,
phaseolotoxin,
16
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
phosphinothricin, podophyllotoxin, prehelminthosporol, pyridazocidin,
quassinoid,
rhizobitoxin, tagetitoxin, sorgoleone syringotoxin, tentoxin, tricolorin A,
thiolactomycin and
usnic acid.
[0068] Some
embodiments can include the use of a bioreactor adapted to receive and use
natural and/or artificial light. As such, in some embodiments, the bioreactor
can be adapted to
permit exposure of microalgae to a light source. In some embodiments, the wall
of the
bioreactor can comprise a light-permeable material to permit exposure of the
microalgae to
light. If an artificial light source is used, the light source can be placed
within or at the exterior
of the bioreactor, e.g. according to United States Patent No. 8,033,047, the
entire disclosure of
which is hereby incorporated by reference. Alternatively, in some embodiments,
the system
can comprise water conduit having through which microalgae-containing water in
the
bioreactor can be circulated to expose the microalgae to light. Some
embodiments can include
the use of a water conduit adapted to employ sunlight, reflected, bent, fiber
optic or artificial
light.
[0069] In some
embodiments, the system can be run continuously, semi-continuously or in
a batch-type operation.
[0070] In some
embodiments, the system can further comprise one or more monitors or
sensors adapted to monitor: a) growing conditions within the bioreactor;
and/or b) microalgae
cell titer/cell count in the water; and/or c) pH of the water; and/or d)
salinity of the water; and/or
e) the presence of undesired microbes in the bioreactor; and/or 0 water level;
and/or g) water
pressure; and/or h) level of microalgae nutrients; and/or i) level of solids
in the filtered water;
and/or j) the level of undesired compounds in the water; and/or k) oxygen,
ozone and/or CO2
content in the water; and/or 1) level of nitrogen compounds in the water;
and/or m) clarity or
opacity of the water; and/or n) level of desired compound(s) in the water;
and/or o) water flow-
rate; and/or p) weed algae; and/or q) algal predators; and/or) other
contaminants.
[0071] In some
embodiments, the monitor or sensors can be used to control operation of
the system, such as by feedback regulation. In some embodiments, a monitor may
generate
one or more signals to controllers, which control the flow of materials into
and/or out of the
system. For example, in some embodiments, a microalgae cell titer monitor may
send one or
more signals to one or more flow controllers that the flow of source water or
microalgae-
containing water into and/or out of the system. In some embodiments, a pH
monitor may send
17
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
one or more signals to a CO2 flow controller that controls the amount of, or
rate at which, CO2
is added to the system. In some further embodiments, a water level monitor may
send one or
more signals to a water flow controller that controls the amount of or rate of
water flow into
and/or out of the system. In some embodiments, a pH monitor may send one or
more signals
to an acid or base titrating unit that controls the amount of or rate of acid
or base flowing into
and/or out of the system.
[0072] In some
embodiments, a water pressure monitor may send one or more signals to a
water pressure regulator that controls the amount of or rate of water flow
into and/or out of the
system. In some embodiments, an ozone monitor may send one or more signals to
an ozone
flow controller that controls the amount of or rate at which ozone is added to
the system. In
some further embodiments, a clarity monitor may send one or more signals to a
water clarity
controller that controls the efficiency of filtration of water in the system.
In some other
embodiments, a nutrient monitor may send one or more signals to a nutrient
source flow
controller that controls the amount of or rate at which nutrient for the
microalgae is added to
the system.
[0073] In order
to grow, plants and microalgae need nutrients such oxygen, carbon,
nitrogen, phosphorus, potassium, magnesium, sulfur, boron, copper, chloride,
iron, silicon,
sodium, manganese, molybdenum, zinc, cobalt, vanadium, bismuth, iodine, water,
carbon
dioxide, air and/or others.
[0074] The
profile of macronutrients and micronutrients provided by the microalgae will
depend upon the strain or species of microalgae used. Plants may require a
different spectrum
of micronutrients and macronutrients during the different stages of the life
cycle of the plant.
Some embodiments provide a method of growing crops where the macronutrient and
micronutrient profile of microalgae is matched with particular phases in the
life cycle of a plant.
In some embodiments, a field may receive regular nutrient feedings during crop
growth and
development with different species used depending on the needs of the crop.
For example,
microalgae A provides a nutrient profile A, microalgae B provides a nutrient
profile B, and a
target crop requires a nutrient profile A during the early stages of growth
and a nutrient profile
B ring of the latter stages of growth. In such a situation, the soil in which
the crop is planted
will be inoculated first with microalgae A during the early stages of growth
of the target crop
and will be inoculated then with microalgae B during the latter stages of
growth of the target
crop.
18
CA 03130211 2021-08-12
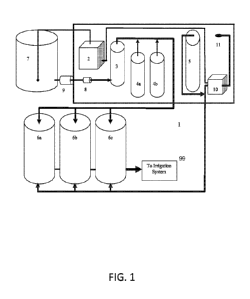
WO 2020/168203
PCT/US2020/018306
[0075] Some
embodiments include a method of producing a crop comprising: planting a
crop into soil and inoculating the soil with a first microalgae that provides
a first nutrient
profile; and/or allowing the plant to pass from a first stage of growth into a
second stage of
growth; and/or inoculating the soil with a second microalgae that provides a
different second
nutrient profile. In some embodiments, the first nutrient profile will be
optimal for plant growth
during the first stage, and the second nutrient profile will be optimal for
plant growth during
the second stage.
[0076] FIG. 1
depicts a first embodiment of a portable microalgae-based soil-inoculating
system 1 of the invention. In some embodiments, the system comprises a water
source 7, an
ozone source 2, a carbon filter/UV light system, 3, a water pump 8, a solids
filter 9, microalgae
nutrient source 4a, 4b, bioreactors 6a, 6b, 6c, a carbon dioxide source 5, a
pressurized air
supply/air pump 10 and various and water conduits. In some embodiments, the
pressurized air
supply may be a blower, and/or air compressor, and/or rocker pump, and/or any
other
conventional producer or source of pressurized air. In some embodiments, air
is taken from
the atmosphere or a tank via the inlet 11, which optionally includes an air
filter. In some
embodiments, the air passes through the air pump 10 to an ozone source 2,
whereby ozone-
treated air is formed and conducted into a water source 7 to form ozone-
treated water. In some
embodiments, the air is also injected with a carbon dioxide source 5 to form
carbon dioxide-
treated air that is conducted into the bioreactors 6a-6c or into water
entering the bioreactors.
In some embodiments, the ozone treated water is filtered through a solids
filter 9 a carbon filter
and/or a UV light system 3 to form filtered water to which microalgae feed is
added by the
microalgae feed source 4a, 4b to form feed water, which is conducted into the
bioreactor. In
some embodiments, during initial startup, the bioreactors 6a, 6b, 6c are
filled with water
containing microalgae nutrients and are then inoculated with a first inoculate
containing
microalgae. In some embodiments, the carbon dioxide-containing air is injected
into the
microalgae-containing water in the bioreactors 6a, 6b, 6c. In some
embodiments, the water in
the bioreactors 6a, 6b, 6c is recirculated for a period of time until the
microalgae cell titer/cell
count has reached a target level suitable for use as an inoculant. In some
embodiments, the
water from the system 1 is then flowed into irrigation water to form a
microalgae-containing
inoculate as the effluent, which is applied to the soil from an irrigation
system 99.
[0077] Various
different operation parameters can be controlled. For example, in some
embodiments, one or more heaters are optionally included in the system to heat
water
19
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
conducted through the system and/or heat the culture medium in the bioreactor,
thereby
permitting culture of microalgae and use of the system even during cold
weather.
[0078] In some
embodiments, the volume of system water and its flow rate into the
irrigation water of the irrigation system 99 can be adjusted as needed to
provide the appropriate
level of inoculation and water penetration into the soil. For example, in some
embodiments, a
200-acre field might receive a total daily volume of 500 to 1 thousand gallons
of water at a
delivery rate of about 21 gallons/hour to 42 gallons/hour. In some
embodiments, the inoculate
obtained from the bioreactor (e.g., such as one or more of the bioreactors 6a,
6b, 6c) can be
applied to soil with or without further dilution. For example, in some
embodiments, the system
1 can be operated such that all water used for irrigation flows through the
bioreactor.
Otherwise, in some embodiments, the system 1 can be operated such that the
inoculate, the
effluent of the bioreactors 6a, 6b, 6c, is diluted with additional irrigation
water prior to
application to the soil.
[0079] In some
embodiments, the microalgae cell titer (the cell count) in a bioreactor
fluctuates over time; therefore, the cell titer of the effluent varies as
well. The titer provides
important metrics regarding the unit's health and productivity. Generally, the
titer in the
effluent can be at least 1,000,000 cells per ml up to 30,000,000 cells per ml.
The titer is also
species specific, and can be higher or lower than the range stated above.
[0080] In some
embodiments, the ozone can be used to destroy unwanted microbes present
in the irrigation water prior to entering the bioreactor. Any organic
contaminants present in
the system can be removed by ozonolysis as described in United States Patent
No. 5,947,057
and United States Patent No. 5,732,654 to Perez et al. Organic contaminants
include
herbicides, pesticides, and fungicides among other things. In some
embodiments, the ozone
source can be an ozone generator. Ozone generators may include the model 01 by
Pacific
Ozone, the Nano by Absolute Ozone, and the OZ8PC20 by Ozotech. In some
embodiments,
the water is treated with ozone as required according to the quality of the
water entering the
system. In some embodiments, the concentration of ozone in the water and prior
to filtration
through a carbon filter will vary with water quality but have an ozone level
sufficient to sterilize
the water. In some embodiments, the treatment of the water with ozone may be
improved by
employing a mixer that mixes the water and ozone.
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0081] In some
embodiments, the carbon filters and UV light systems are used to remove
ozone from the irrigation water prior to entering the bioreactor. In some
embodiments, the
carbon filter generally employs a minimum of 0.75 ft3 of activated carbon. In
some
embodiments, the carbon filter and UV light systems are flow-through systems.
In some
embodiments, the suitable carbon filters include the 0.75 ft3 "Upflow Carbon
Filter System"
from Affordable Water (www.affordablewater.us). UV systems may include the
"CSL Series"
by Aquafine, and the "UVS3XX Series" by UV Sciences (www.aquaneuv.com;
Valencia,
Calif). In some embodiments, the UV light system can be used to disinfect
water prior to
entering the bioreactor, and/or to destroy ozone, destroy chlorine or
chloramines prior to
entering the bioreactor. In some embodiments, the UV light system can
disinfect by
inactivating or killing microorganisms in the water.
[0082] In some
embodiments, when a solids filter is present, it can be used to remove solids
from the irrigation water prior to entering the bioreactor. In some
embodiments, the solids
filter can be a flow-through filter. In some embodiments, suitable solids and
filters can include
the "X100" bag filter from www.filterbag.com or the "FV1" bag filter from
www . a( uaticeco.com
[0083] In some
embodiments, suitable carbon filters and/or solids filters can include, but
not be limited to, media filters, disk filters, screen filters, microporous
ceramic filters, carbon-
block resin filters, membrane filters, ion-exchange filters, microporous media
filters, reverse
osmosis filters, slow-sand filter beds, rapid-sand filter beds, cloth filters,
and/or any other
conventional filter.
[0084] In some
embodiments, carbon dioxide can be used as a carbon source for
microalgae. In some embodiments, the carbon dioxide can be added directly or
indirectly to
the bioreactor. In some embodiments, carbon dioxide source can be a tank
containing carbon
dioxide, a carbon dioxide generator, a carbon dioxide sequestering device that
sequesters
carbon dioxide from the atmosphere, or a combination thereof Alternatively,
carbon dioxide
captured from air can be used, e.g. United States Patent No. 8,083,836, the
entire disclosure of
which is hereby incorporated by reference. In other embodiments, the carbon
dioxide can be
sourced from acetic acid and/or calcium carbonate.
[0085]
Atmospheric air contains approximately 0.035-0.04% wt., of carbon dioxide.
While atmospheric air can serve as a source of carbon dioxide for the
microalgae, the
21
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
concentration of carbon dioxide is generally too low to sustain the rapid
proliferation of
microalgae in the bioreactor. Accordingly, in some embodiments, carbon dioxide
can be added
to the air that is fed into the culture medium. In some embodiments, the
concentration of carbon
dioxide in the air added to the culture medium can be generally in the range
of about 1-3% wt.,
1.5-2.5% wt., 1.8-2.2% wt., or about 2% wt.
[0086] In some
embodiments, a water pump can be included in the system. In some
embodiments, when present, the water pump can facilitate the flow of water
through the water
conduits and/or bioreactors of the system. In some embodiments, if a water
pump is not
included, the pressure of the irrigation water entering can be sufficient to
drive water through
the system.
[0087] In some
embodiments, an air pump or blower (the terms are used interchangeably
herein) can be included in the system. In some embodiments, air pump can
facilitate the flow
of air, which may or may not include carbon dioxide or ozone, through the air
conduits, water
source and/or bioreactors of the system.
[0088] The size
or operating capacity of each piece of equipment comprising the system
can be varied as needed. For example, in some embodiments, a portable system
comprising a
total bioreactor capacity of 500 gallons of culture medium can support 200
acres of land and
will generally require the following minimum operating capacities for the
indicated
components: a) ozone source-1.5 g/hr; (dry air); b) solids filter-40 g/min
maximum flow with
a minimum 2 ft2 surface area; c) carbon filter-0.75 ft3 minimum; d) water pump-
10 gal/min
minimum; e) air pressurized air supply/air pump-25 cfm at 60" H20 minimum; f)
microalgae
feed source-1.0 x 106 cells/ml minimum; g) liquid carbon dioxide source-80
1/week.
[0089] FIG. 2
depicts another embodiment comprising a portable system 51, where the
components of the system 51 are mounted on a trailer. In some embodiments, the
system 51
comprises a water tank 52, a plurality of bioreactors 53, an ozone generator
54, a clarifier 55,
a combination filter/UV light system 56, nutrient feed supply 57, CO2 source
58, a pressurized
air supply 59 and a trailer 60. As shown, any of the water tank 52, plurality
of bioreactors 53,
ozone generator 54, clarifier 55, combination filter/UV light system 56,
nutrient feed supply
57, CO2 source 58, and pressurized air supply 59 can be mounted onto the
trailer 60.
22
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0090] In some
embodiments, the system 51 can accommodate a flow-through capacity of
about 0.35-0.7 gal/min and can be used to support a field in the range of 200-
1000 acres. In
some embodiments, the water tank 52 can receive water from the on-site water
source of a
farm. In some embodiments, the system 51 can comprise eight bioreactors (500-
gallon total
capacity), a water tank, air filter, solids filter, carbon filter, UV light
system, ozone source,
carbon dioxide source, microalgae nutrient source, pressurized air supply and
water pump (not
shown). In some embodiments, the bioreactors 53 can have light-permeable walls
such that
sunlight is used as the light source. In some embodiments, carbon dioxide and
air can be
bubbled into the lower part of the bioreactor 53 so the bubbles agitate the
culture medium as
they rise. In some embodiments, the system 51 optionally comprises a
mechanical agitator. In
some embodiments, the system 51 can provide a minimum of about 800,000
microalgae cells
per second via the effluent, assuming a water flow rate of about 0.35 gal/min.
[0091] FIG. 3
depicts a side elevation view of another system 65 of the invention
comprising an elevated portable platform 66, water tank 67, pressurized air
supply 68, ozone
source 69, clarifier 70, water filter 71, nutrient source 72, carbon dioxide
source 73 and
bioreactors 74. In some embodiments, one or more components can be mounted on
the
platform and one or more components can be placed on the ground or onto one or
more other
platforms.
[0092] Although
FIGS. 2 and 3 depict a water tank 52, 67 as the water supply, in other
embodiments, a flowing water source can be used instead; therefore, in some
embodiments,
the system of the invention optionally includes one or more water tanks as the
water supply or
excludes a water tank as the water supply. Although not depicted in FIGS. 2
and 3, in some
embodiments, the effluent of one or more bioreactors can be fed into the water
flow of an
irrigation system. In some embodiments, the systems described herein can be
placed within a
partial or full enclosure even though the systems are portable.
[0093] In some
embodiments, the performance of the system of FIG. 2 was evaluated in a
crop study where melon crops were planted in 200 acres of land. The land was
divided into
control and sample sections (e.g., see FIGs. 5A-5B). The control sections only
received
irrigation water and were not treated with microalgae supplement. The sample
sections
received only irrigation water containing the microalgae supplement. Melon
seeds were
planted before irrigating with the algae supplement in the soil. The control
plants were irrigated
about every fourth day, depending on the heat. The sample plants were
irrigated on the same
23
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
schedule as the controls. Various aspects of plant and fruit growth were
evaluated five weeks
(shown in FIG. 4A) and nine weeks (shown in FIG. 4B) after planting.
[0094] Briefly,
the crop grown according to the systems and methods disclosed herein
produced larger and hardier plants. For example, compare FIG. 5A (showing a
control plant)
to FIG. 5B (showing a sample plant). Further, compare the larger melons of
FIG. 6B to the
control plant shown in FIG. 6A. Moreover, the sample plants produced more
flowers per vine,
had improved fruit texture and taste, improved sugar content, improved
nutritional content,
improved appearance, and improved Vitamin A content. The specific details and
results are
described in Example 1.
[0095] In some
embodiments, the system can further comprise one or more monitoring
devices for performing functions, including, but not limited to, measuring CO2
flow rate, CO2
content in the culture, 02 content in the culture, pH, cell density and
temperature in the culture,
measuring macronutrient content in the culture or effluent, measuring
micronutrient content in
the culture or effluent, or measuring the microalgae titer in the culture or
effluent.
[0096] FIG. 7
depicts an alternate embodiment of the system of the invention. In some
embodiments, the system 11 is suitable for low, medium and high-volume
irrigation
applications. In some embodiments, the system 11 comprises an optional pump 18
adapted to
receive water from a pressurized or unpressurized water source 11 a. In some
embodiments,
the water received from the water source 11 a is ozonated within an ozone
contactor 12 that
receives ozone from an ozone generator 27 and conducted to a clarifier/filter
19 that removes
precipitated solids from the water. In some embodiments, after clarification,
the water is
conducted to a carbon filter or UV light system 13, that removes the ozone,
and through to a
mixer 22 that mixes the water with algae feed material obtained from the algae
nutrient supply
14. In some embodiments, the algae/water mixture is mixed by use of air
bubbles, which are
produced by a pressurized air supply 30, which conducts air to an air diffuser
in the base of the
bioreactor 16. In some embodiments, the water containing nutrient material is
conducted into
the bioreactor 16, wherein microalgae are cultured. In some embodiments, the
effluent
containing the microalgae exits the bioreactor 16 and passes through a valve
26 that regulates
the ratio of flow of water between the by-pass water source line 28 and the
bioreactor effluent.
In some embodiments, the controller 29 controls the valve 26 to achieve the
desired ratio of
volume of flow between untreated source water (from by-pass line 28) and the
effluent to
provide an inoculant containing a desired or target microalgae titer.
24
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[0097] In some
embodiments, the system 11 can include one or more different controllers.
For example, in some embodiments, the controller 20 can comprise an optional
feedback loop
where water that has been improperly ozonated can be fed back into the ozone
contactor 12 for
proper treatment. In some embodiments, the controller 21 can comprise an
optional feedback
loop such that water that has been insufficiently clarified can be fed back
into the clarifier 19
for proper clarification. In some embodiments, the controller 23 can provide
control over the
algae nutrient supply 14 to regulate the amount of feed material that is
charged into the water.
In some embodiments, the controller 25, by use of a pH probe 24, can provide
control over the
carbon dioxide source 15 that charges carbon dioxide into the bioreactor 16 to
regulate the
concentration of carbon dioxide in the water and ensure the water has the
proper carbon dioxide
concentration. In some embodiments, the algae/water mixture can be mixed by
use of air
bubbles, which are produced by a pressurized air supply 30, which conducts air
to an air
diffuser in the base of the bioreactor.
[0098] In some
embodiments, the system 11 can comprise a portable platform (or body or
frame, not shown) onto which plural components of the system are mounted. In
some
embodiments, the each of the individual components of the system can be
individually
replaceable. Although the components are indicated as single components, each
of the
components can be present in plurality independently of other components of
the system.
[0099] FIG. 8
depicts an alternate embodiment of the system of the invention. In some
embodiments, the system 41 as shown can be suitable for low, medium and high-
volume
irrigation applications or flowing to a distribution tank 37. In some
embodiments, the
distribution tank 37 may sit on a trailer for portability. In some
embodiments, the system 41
comprises an optional pump 18 adapted to receive water from a pressurized or
unpressurized
water source 11 a. In some embodiments, the water from the water source 11 a
is ozonated
within an ozone contactor 12 that receives ozone from an ozone generator 17.
In some
embodiments, the ozonated water is conducted to a clarifier/filter 19 that
removes precipitated
solids from the water. In some embodiments, after clarification, the water is
conducted to a
carbon filter or UV light system 13, that removes the ozone, and through to a
mixer 22 that
mixes the water with algae fertilizer/additives obtained from the algae
nutrient supply 14. In
some embodiments, the water containing nutrient material can be conducted into
the bioreactor
16, where microalgae can be cultured. In some embodiments, the algae/water
mixture can be
mixed by use of air bubbles, which are produced by the pressurized air supply
30, which
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
conducts air to an air diffuser in the base of the bioreactor as discussed
earlier with respect to
the system 11 of FIG. 7. In some embodiments, one or more probes 33 can be
placed in the
culture to measure the critical parameters including pH, temperature, cell
density, water mixing
velocity, dissolved gasses and nutrients. In some embodiments, an optional
telemetry device
34 can send the metrics from the probes (monitoring devices or controllers) to
a computer
server for remote monitoring. In some embodiments, an optional telemetry
capable microscope
can assist the remote culture monitoring. In some embodiments, the optional
telemetry device
34 comprises the optional telemetry capable microscope.
[00100] As used herein, telemetry device 34 can be any device capable of
facilitating
communication between the system of the invention and a communications and/or
control
center remote from or at a different geographic locale than the system of the
invention. In
some embodiments, the telemetry device 34 can employ any type of wireless
communication
system and can employ any frequency of light waves, radio waves, sound waves,
infrared
waves, hypersonic waves, ultraviolet waves, other such wavelengths/frequencies
and
combinations thereof In some embodiments, the telemetry device 34 employ an IP
network
(such as the Internet), GSM (global system for mobile communications) network,
SMS (short
message service) network, other such systems and combinations thereof
[00101] In some embodiments, a flow imaging device 32 can create images of the
algae,
predators and contaminants in the culture for quality control (QC) purposes,
and can send this
data to the telemetry device 34. In some embodiments, the effluent containing
the microalgae
can exit the bioreactor and pass through a valve 31 that regulates the flow of
the bioreactor
effluent. In some embodiments, the optional dewatering device 35 can
concentrate the algae
into slurry of the desired density, which may flow to irrigation or portable
containers 37. In
some embodiments, an optional microorganism mixer 36 can enable the user to
blend the final
product with, in addition to algae, beneficial bacteria, fungi or other
organisms 38 that work
symbiotically with algae.
[00102] In some embodiments, the system 41 can include one or more different
controllers.
In some embodiments, the controller 20 can comprise an optional feedback loop
such that water
that has been improperly ozonated can be fed back into the ozone contactor 12
for proper
treatment. In some embodiments, the controller 21 comprises an optional
feedback loop such
that water that has been insufficiently clarified can be fed back into the
clarifier 19 for proper
clarification. In some embodiments, the controller 23 provides control over
the algae nutrient
26
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
supply 14 in order to regulate the amount of feed material that is charged
into the water. In
some embodiments, the controller 25, by use of a pH probe 24, can provide
control over the
carbon dioxide source 15 that charges carbon dioxide into the bioreactor in
order to regulate
the concentration of carbon dioxide in the water and ensure the water has the
proper carbon
dioxide concentration. In some embodiments, the system 11 can comprise a
portable platform
(or body or frame, not shown) onto which plural components of the system are
mounted. In
some embodiments, each of the individual components of the system can be
individually
replaceable. Although the components are indicated as single components, each
of the
components can be present in plurality independently of other components of
the system.
[00103] In some embodiments, a system similar to the system 41 of FIG. 8 can
be used to
reclaim degraded or abandoned soil. In some embodiments, an algae and
microorganism
mixture produced by the system may be applied though irrigation or spaying on
the soil surface
to restore vital nutrients. Algae and the other microorganisms continue to
flourish in the soil
as long as soil moisture is available. Algae deliver micronutrients, attract
other microorganisms
and add organic matter (humus) to the soil. In some embodiments, the process
can rehabilitate
degraded or abandoned soil.
[00104] In some further embodiments, a system similar to the system 41 of FIG.
8 can
culture other microorganisms in the same culture or separate containers for
blending before the
culture flows into the irrigation or portable containers.
[00105] FIG. 9 illustrates a soil enrichment system 900 in accordance with
some further
embodiments of the invention. Some embodiments include a solids filter 919, a
water storage
tank 912, a sterilization system 917, and a neutralization system 915. A
growth priming system
may comprise one or more nutrient solution feeds, such as first and second
nutrient solution
containers 920, 962 to add nutrient solutions to the treated water. A
bioreactor system may
comprise one or more bioreactors 916 to facilitate inoculation with and growth
of the
microorganism. The systems and methods may include various additional systems
and
subsystems, such as one or more nutrient solution containers, refrigerators,
light sources,
blowers (e.g., at least one pressurized air supply), carbon dioxide sources,
pumps, valves, fluid
conduits, air conduits, gas conduits, air filters, gas filters, control
systems, sensors, air
conditioning units, exhaust systems, portable housings, and/or exterior
holding tanks.
27
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[00106] In some embodiments, one or more pumps 918, such as peristaltic pumps,
may
propel irrigation water from the water source 95 through fluid conduits 910.
The water source
95 supplies water to the soil enrichment system 900. Water flowing from the
water source 95
may be referred to as "irrigation water." The water source 95 may comprise any
suitable source
of irrigation water appropriate for irrigation of plants. In some embodiments,
the water source
95 may be under pressure, such as water from a well or a public utility in a
city, town, or
municipality. In some embodiments, the water source 95 may be substantially
unpressurized.
For example, the water source 5 may comprise a stationary water reservoir,
reclaimed
wastewater, well water, lake water, creek water, pond water, rainwater, river
water, and/or
freshwater.
[00107] Some embodiments of the soil enrichment system 900 may comprise an
automated
cleaning system 970 controlled by a control system. The automated cleaning
system 970 may
comprise a cleaning solution container 968 for holding the cleaning solution
and a pump 918
for pumping the cleaning solution from the cleaning solution container 968
into the fluid
conduit and/or the one or more bioreactors 916. In some embodiments, each of
the one or more
bioreactors 916 may comprise a dedicated valve for connection of a fluid
conduit leading to
the cleaning solution container 968 for the cleaning solution.
[00108] In some embodiments, one or more bioreactors 916 may be inoculated
with the
microorganism inoculant by any suitable method, such as manual inoculation
through a port
935 in the bioreactor 916. In some further embodiments, the neutralized
irrigation water
containing nutrient solution may be conducted into any one or more of the
bioreactors 916 until
it reaches a preselected fill level 940.
[00109] In some embodiments, a light source 945/950 may be configured to
project light
onto and/or into each of the one or more bioreactors 916. In some embodiments,
the light source
945/950 may comprise LED lights in any suitable configuration to provide light
to the
microorganism culture. For example, in one embodiment, a first light source
945 may be
positioned within the one or more bioreactors 916. In another embodiment, the
first light source
945 may overlay an exterior surface of the one or more bioreactors 16. In
another embodiment,
a second light source 950 may be outside of and adjacent to an exterior
surface of the one or
more bioreactors 916.
28
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[00110] In some embodiments, a control system suitable for implementing one or
more of
the present embodiments may include a computer system communicatively linked
to a PLC
system 934. The PLC system 934 may be communicatively linked to the one or
more sensors
933 and may provide measurements obtained by the one or more sensors 933 to a
processor
and/or database for remote monitoring, remote data access, and/or remote
control of the soil
enrichment system 900. The PLC system 934 may similarly be communicatively
linked and
configured to control pumps 918, valves, sterilization system 917,
neutralization system 915,
at least one pressurized air supply 930, lights 950, and/or any carbon dioxide
source.
[00111] In some embodiments, a carbon dioxide source 966 can be used to supply
a carbon
source to the microorganism culture. Carbon dioxide may be added directly
and/or indirectly
to the one or more bioreactors. The carbon dioxide source 966 may be a tank
containing carbon
dioxide gas, a carbon dioxide generator, a carbon dioxide-sequester for
sequestering and
temporarily storing atmospheric carbon dioxide or a combination thereof
[00112] In some embodiments, the microorganism culture may be released from
the one or
more bioreactors 916 through the outlets, flow through the one or more fluid
conduits, and flow
into the external holding tank 937 for storage. In various embodiments, the
external holding
tank 937 may comprise an at least partially transparent material such as high
or low-density
polyethylene, polycarbonate, acrylic, and/or PVC to allow natural or
artificial light to penetrate
through the external holding tank 937 and into the microorganism culture. In
some
embodiments, the external holding tank 937 may comprise a sterile aeration
system to support
the health of the microorganism culture. In some embodiments, the external
holding tank 937
may comprise a cone-shaped base to ensure complete drainage of the
microorganism culture
when it is released onto a target field 955.
[00113] In some embodiments, the exterior holding tank 937 may comprise a
cooling system
such as a refrigerator to cool the microorganism culture during storage. The
refrigerated
exterior holding tank may be configured to receive the microorganism culture
and/or
microorganism slurry, maintain its sterility, and store it at any suitable
temperature.
[00114] In some embodiments, the dewatering device 964 may be configured to
deliver the
concentrated microorganism slurry to the target field 955 and/or the exterior
holding tank 937.
The dewatering device 964 may concentrate the microorganism culture through
any suitable
process such as, but not limited to: 1) flocculation and sedimentation; 2)
flotation and
29
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
collection; and/or 3) centrifugation. Further details and operational
characteristics of the soil
enrichment system 900 are described in United States Patent Application Serial
No.
15/647,005, the entire contents of which are incorporated by reference.
[00115] Some embodiments include methods of isolation, selection, and use of
endemic
microbes for agriculture production areas using any of the systems described
herein. For
example, some embodiments of the invention include methods of selecting,
collecting, and
growing algae for delivery to an agricultural production area. Specifically,
in some
embodiments, the methods focus on collecting, isolating, and/or propagating
endemic
microbes, primarily algae, for mass delivery to the same biome from which the
algae was
collected. In some embodiments, the agricultural production area comprising
the biome may
be a farm field, and/or a raised bed, and/or a greenhouse, and/or a golf
course, and/or degraded
land, and/or an indoor growing facility. Some further embodiments include
collecting,
isolating, and/or propagating, and delivering other endemic microbes in
addition to, or
separately from algae. For example, some embodiments include collecting,
isolating, and/or
propagating, and delivering a bacterial species. Other embodiments include
collecting,
isolating, and/or propagating, and delivering a fungal species.
[00116] In some embodiments of the invention, the algae may be delivered
through a variety
of means including, but not limited to, canal irrigation, flood irrigation,
and/or drip irrigation,
and/or various conventional overhead spray techniques, and/or various
conventional
hydroponic cultivation techniques. In some embodiments of the invention, the
effects of
delivering algae to the agricultural production area may be an increase in
soil organic matter,
and/or improvement in soil structure, and/or reduction in water and fertilizer
utilization, and/or
increase in crop yield and the nutrient value of the product, and/or an
overall improvement in
soil health, and/or reduction in water and chemical runoff, and/or an increase
in carbon dioxide
sequestered from the air by the soil.
[00117] Some embodiments of the invention include a method of obtaining a soil
and/or
water sample from an agricultural production area, and/or culturing microbes
from the soil
sample, and/or selecting a desirable species from the soil sample, and/or
propagating the
selected desirable species in greater numbers and concentration, and/or
delivering live
microbes back to the agricultural production area (e.g., such as dispersing
the live microbes in
solution over a soil area of a farm, or biome area).
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[00118] The following steps constitute a non-limiting embodiment of a method
for
collecting, selecting, and propagating endemic algae from an agricultural
production area (e.g.,
such as a farm or other plant propagation facility):
[00119] Some embodiments include a step of collecting one or more quantities
of soil from
one or more locations on the agricultural production area. In some
embodiments, each quantity
or a total quantity of collected soil can be about 100 grams. In some other
embodiments, the
quantity can be less than 100 grams or more than 100 grams.
[00120] Some embodiments include a step of collecting one or more quantities
of water
from one or more locations on the agricultural production area (e.g., such as
from a surface
water source). In some embodiments, each quantity or a total quantity of
collected water can
be about 50 grams. In some other embodiments, the quantity can be less than 50
grams or more
than 50 grams. In some other embodiments, at least some of the water can be
collected from a
sub-surface source, a run-off source, or a spring or well source.
[00121] In some embodiments, one or more of the water and/or the soil
quantities can be
refrigerated to 35 F to 40 F prior to subsequent processing locations,
including, without
limitation, a laboratory or facility.
[00122] In some embodiments, about 10 grams of soil or 10 ml of water from
each sample
can be added to a 100 ml culture jar containing 75 ml of AF6 (Watanabe) media.
In some
embodiments, more or less soil and/or water can be added to the culture jar.
In some further
embodiments, more or less AF6 (Watanabe) media can be used. In some
embodiments, the
soil and/or water can be incubated in the culture jar. In some embodiments,
the incubation can
occur overnight while being exposed to a 100 to 200 PAR light source. In some
embodiments,
the light source can comprise or emit wavelengths of about 450 nm to 485 nm
and/or about
625 nm to 740 nm. In some embodiments, exposure can be approximately 12 to 24
hours per
day.
[00123] In some embodiments, a portion of the incubated samples can be
propagated in
Agar-coated petri dishes. For example, in one non-limiting embodiment, samples
can be
plated-out onto four 100 x 15 mm petri dishes with AF6 agar with 10 [11
samples with loop
sterilization in-between each streak to dilute the sample. In some embodiments
of the
invention, the petri dishes can be at least partially closed (e.g., taped to
75% closed) and placed
31
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
upside down in front of a 100 to 200 PAR light source for one to two weeks. In
some
embodiments, the light source can comprise or emit wavelengths of about 450 nm
to 485 nm
and about 625 nm to 740 nm. In some embodiments, exposure can be about 12 to
24 hours per
day.
[00124] In some embodiments, when isolated axenic algae colonies have grown to
a specific
size, the algae colonies can be harvested aseptically, and placed into a
sterile test tube with
sterile AF6 media. For example, in some embodiments, when isolated axenic
algae colonies
have grown to about 3 mm in diameter, the algae colonies can be harvested
aseptically and
placed into a sterile test tube with sterile AF6 media.
[00125] Some embodiments can include an incubation time of one to two weeks,
followed
by selecting the tubes with the highest biomass. In some embodiments, the
incubation can
occur while being exposed to a 100 to 200 PAR light source. In some further
embodiments,
the light source can contain wavelengths of about 450 nm to 485 nm and about
625 nm to 740
nm. In some embodiments, exposure can be about 12 to 24 hours per day. In some
further
embodiments, temperatures can range between about 70 F and 80 F.
[00126] Some embodiments include sub-culturing each tube into a new tube,
followed by
placing the contents of the original tube into a sterile 500 ml bottle with
AF6 media outfitted
with sterile air injection. In some embodiments, the sub-culturing tubes can
be exposed to a
100 to 200 PAR light source. In some embodiments, the light source can contain
wavelengths
of about 450 nm to 485 nm and 625 nm to 740 nm. In some embodiments, exposure
can be
about 12 to 24 hours per day.
[00127] Some embodiments include incubating the bottle for 3-5 days, and
selecting the
bottles with the fastest growth rate and highest biomass, and identifying with
a new strain ID.
In some embodiments, the incubation can occur while being exposed to a 100 to
200 PAR light
source. In some embodiments, the light source can contain wavelengths of about
450 nm to
485 nm and about 625 nm to 740 nm. In some embodiments, exposure can be about
12 to 24
hours per day. In some embodiments, temperatures can range between about 70 F
and 80 F.
[00128] In some embodiments of the invention, the strain IDs of the incubated
samples can
be recorded in the strain ID database with date time and location of
collection along with any
32
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
additional algal characteristics. Further, in some embodiments, new test tubes
can be
inoculated with each newly identified strain and place in algal library.
[00129] In some embodiments of the invention, a further step can include an
artificial
selection process to improve, growth rate, maximum density and other desired
characteristics.
In some embodiments, the artificial selection process can contain algae
strains that are exposed
to preferred culture conditions. In some embodiments, algae strains that have
an improved
growth rate, higher maximum density, or other desired characteristics can be
selected over the
inferior strains for future use. In some embodiments, inferior algae strains
may be put through
the artificial selection process to further improve the growth rate, maximum
density or other
desired characteristics.
[00130] In some embodiments of the invention, one or more the steps can be
performed in
a laboratory or facility that is remote from the agricultural production area.
In some
embodiments of the invention, one or more the steps can be performed in a
laboratory or facility
that is proximate to or part of the agricultural production area. In some
embodiments, all of
the steps can be performed in the same location. In other embodiments, at
least some of the
steps can be performed in one location, and one or more other steps can be
performed in another
location.
[00131] In view of the above description and the examples below, one of
ordinary skill in
the art will be able to practice the invention as claimed without undue
experimentation. The
foregoing will be better understood with reference to the following examples.
All references
made to these examples are for the purposes of illustration. The following
examples should
not be considered exhaustive, but merely illustrative of only a few of the
many embodiments
contemplated by the present invention.
Example 1
Evaluation of the System for Melon Growth
[00132] The system of the invention was used to grow the Yosemite variety of
cantaloupe
melons. About 200 acres were infused with microalgae-containing irrigation
water. The crop
was watered every five days during afternoons due to high ambient temperatures
(120 F).
Microalgae were added to the irrigation water continuously with each watering.
Algae from
the phylum Chlorophyta and Cyanophyta were added to the irrigation water at a
combined
33
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
density of 6 billion cells per minute. The algae were cultured in media shown
in the table
below.
FW Media
Final Conc. Stock Solutions Use Rate
(g/L) (g/L) (ml/L)
N & P Solution
NaNO3 0.344 34.4 10
KC1 0.303 30.3
NaH2PO4 0.03 2.91
Missing element solutions
CaCl2-2H20 0.11 11 10
MgSO4=7H20 0.246 24.6
Trace Element Solution
Na2EDTA-2H20 0.0045 4.5000 1
FeC13=6H20 0.00289 2.8910
MnC12-4H20 0.00098 0.9800
ZnSO4=7H20 0.000036 0.0360
CoC12=6H20 0.000011 0.0110
Na2Mo04-2H20 0.00012 0.1200
34
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
Cr03 0.000075 0.0750
Se02 0.000005 0.0050
CuSO4=5H20 0.000012 0.0120
Vitamins
Biotin 0.000025 0.025 1
Thiamine HC1 0.0000175 0.017
B12 0.000015 0.015
[00133] The melons were harvested and the following observations were made
when
comparing melons grown according to the invention to melons not grown
according to the
invention.
Metric Description
Productivity Improved melon production 20% by weight.
Size Fruit increased in diameter by 22%.
Texture Texture of meat of the fruit held or improved.
Shelf-life The shelf-life was extended by 4 days.
Taste Taste of the fruit held or improved.
Sugar Sweetness of the fruit improved by 20%.
Appearance Appearance, color, of the fruit held or improved.
Vitamin A Vitamin content improved by 20%.
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[00134] Various different dimensions of the melon plants were measured at 9-
weeks after
planting both for control plants and plants grown with the system of the
invention. The
observed dimensions are detailed below.
Parameter Control Sample Fold increase
Trunk Diameter 0.129 in 0.38 in 2.9
Stem diameter 0.05 in 0.125 in 2.5
Average Leaf length 2.5 in 4 in 1.6
Largest Leaf length 3.5 in 7 in 2.0
Overall plant radius 37.8 in 87.12 in 2.3
Overall plant height 5.7 in 15 in 2.6
Flower size width 0.9 in 2.3 in 2.6
Melon diameter 2.3 in 5.5 in 2.4
[00135] The algae infused melon fields required 50% less N inorganic
fertilizer and 40%
less P and K. Micronutrient savings were on the order of 70%. The farmer
reported a 5-fold
improvement in soil porosity, looseness, which enabled deeper crop roots.
Higher soil porosity
also enabled symbiotic macro and microorganisms to enter the field such as
earthworms. The
farmer reported that the melon fields needed over 50% less pesticide
application, because the
algae infused crops seemed to make their own biopesticides that discouraged
invaders, such as
white flies that destroyed neighboring fields. The farmer used 70% less
fungicide as the algae
enabled longer roots that were more resistant to nematodes and other soil
pests. Accordingly,
the system of the invention provides substantial improvements in
characteristics of plants and
fruits grown with the system of the invention.
Example 2
Crop Growth Employing Two Different Microalgae
36
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
[00136] Prior to
planting the seeds of a crop in soil, the soil is irrigated repeatedly with an
inoculate containing a first species from the phylum Chlorophyta of microalgae
until the soil
has achieved the desired properties of increased organics with polysaccharides
in the soil to
increase water retention Seeds are planted in the treated soil and irrigated
repeatedly with an
inoculate containing a different second species from the phylum Cyanophyta of
microalgae to
infuse the soil with nitrogen sequestered from the atmosphere until the crop
has reached
maturity. The crop is then harvested using known methods. At this point a
third species also
from the phylum Cyanophyta is introduced into the irrigation water and
delivered to the soil
where it produces a biological toxin to kill unwanted pests in the soil. The
first species of the
phylum Chlorophyta of microalgae is used to enhance the fertility and other
properties of the
soil by increasing the organics in the soil which enhances the colonization by
other micro and
macro organisms which further enhance the soil by converting nutrients into
forms more
available to the crop and by increasing the porosity of the soil. The second
species from the
phylum Cyanophyta of microalgae is used to add nitrogen to the soil thereby
reducing the
amount of nitrogen fertilizer needed by the crop. The third species from the
phylum
Cyanophyta is used to eliminate or reduce the number of pests in the soil.
Example 3
System Employing Co-Culture of Two Different Microalgae
[00137] A system containing a co-culture of two different microalgae strains
are prepared
by preparing a culture medium in one or more bioreactors and inoculating it
with one or more
blue-green algae (cyanobacteria or Cyanophyta) and one or more green algae
(Chlorophyta).
Both algae can be independently unicellular or colonial; however, unicellular
species are
preferred. Some Chlorophyta include those of the class Chlorophyceae, which
includes those
of the order Chaetopeltidales, Chaetophorales, Chlamydomonadales,
Chlorococcales,
Chlorocystidales, Dunaliella, Microsporales, Oedogoniales, Phaeophilales,
Sphaeropleales,
Tetrasporales or Volvocales. Some Chlorophyta species include Chlorella fusca,
Chlorella
zofingiensis, Chlorella spp., Chlorococcum citriforme, Chlorella
stigmataphora, Chlorella
vulgaris, Chlorella pyrenoidosa and others. Some Cyanophyta include those of
the order
Chroococcales, Gloeobaterales, Nostocales, Oscillatoriales, Pseudanabaenales,
and
Synechococcales. The algae are co-cultured with natural and/or artificial
light. The titer of
algae in the culture medium is allowed to increase to a target level of about
1 MM to 100 MM
37
CA 03130211 2021-08-12
WO 2020/168203
PCT/US2020/018306
cells per ml. The culture medium is discharged from the bioreactor and mixed
in with water
for irrigation.
[00138] As used herein and unless otherwise specified, the term "about" or
"approximately"
are taken to mean +-.10%, +-.5%, +-.2.5% or. +-.1% of a specified valued. As
used herein and
unless otherwise specified, the term "substantially" is taken to mean "to a
large degree", "at
least a majority of, greater than 70%, greater than 85%, greater than 90%,
greater than 95%,
greater than 98% or greater than 99%.
[00139] The above is a detailed description of particular embodiments of the
invention. It
will be appreciated that, although specific embodiments of the invention have
been described
herein for purposes of illustration, various modifications may be made without
departing from
the spirit and scope of the invention. Accordingly, the invention is not
limited except as by the
appended claims. All of the embodiments disclosed and claimed herein can be
made and
executed without undue experimentation in light of the present disclosure.
[00140] It will be appreciated by those skilled in the art that while the
invention has been
described above in connection with particular embodiments and examples, the
invention is not
necessarily so limited, and that numerous other embodiments, examples, uses,
modifications
and departures from the embodiments, examples and uses are intended to be
encompassed by
the claims attached hereto. The entire disclosure of each patent and
publication cited herein is
incorporated by reference, as if each such patent or publication were
individually incorporated
by reference herein. Various features and advantages of the invention are set
forth in the
following claims.
38