Note: Descriptions are shown in the official language in which they were submitted.
CA 03133881 2021-09-16
=
Device and method for sterilizing medical products by means of X-ray radiation
Description
[1] The invention relates to a device and a method for sterilizing three-
dimensional medical
products using low-energy X-ray radiation
Background of the invention
[2] Sterility is a key requirement for many medical products A medical
product is described as
sterile if the probability that a viable microorganism is on or in the product
is less than or equal to
10-6 (EN 556-1 2001)
[3] A medical product describes an object or a substance that is used for
medically therapeutic
or diagnostic purposes for people In contrast to medicines, the main intended
effect of medical
products is primarily not pharmacological, metabolic or immunological, but
physical or
physicochemical Medical products are therefore all instruments, apparatuses,
devices, software,
substances or other objects used individually or in combination that are
intended by the manufacturer
for people for the following purposes. identifying, preventing, monitoring,
treating or alleviating
disease, identifying, monitoring, treating, alleviating or compensating for an
injury or disability,
investigating, replacing or modifying the anatomical structure or a
physiological process, conception
control and its intended main effect Likewise, "products that are specifically
intended for cleaning,
disinfecting or sterilizing" medical products are considered to be medical
products
[4] One possibility for sterilizing medical products is that of irradiation
with ionizing radiation
(radiation sterilization) Methods used on a large scale for the irradiation of
medical products are
sterilization using gamma radiation (gamma sterilization), sterilization using
accelerated electrons (e-
beam sterilization, electron beam sterilization, beta sterilization) and
sterilization using high-energy
X-ray radiation (X-ray sterilization)
Gamma sterilization
[5] Gamma radiation is a particularly penetrating electromagnetic radiation
that arises from
spontaneous transformations ("decay") of the atomic nuclei of many naturally
occurring or artificially
produced radioactive nuclides Gamma radiation is the term used to describe
short-wave photons
that are created by nuclear reactions, while X-ray radiation results from the
change In speed of
charged particles. Gamma radiation is often used to sterilize single-use
medical devices such as
syringes, needles, cannulas and IV sets, as well as food, since its
penetration depth is usually more
than 50 cm Radioisotopes, mostly cobalt-60 (6000) or cesium-137 (137Cs),
emitted with photon
energies of up to 1 3 or 0 66 MeV, have a technical application
CA 03133881 2021-09-16
[6] Gamma rays are electromagnetic waves (as well as light, infrared, X-ray
or UV rays)
However, gamma rays have a shorter wavelength (less than 0 005 nm) and
therefore have more
energy During irradiation, this energy is transferred to the electrons of the
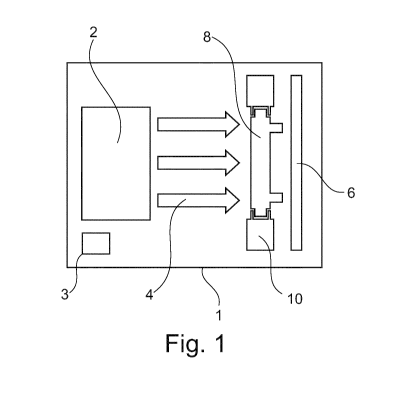
molecules of the products
and generates highly reactive radicals in the process This is therefore also
referred to as ionizing
radiation These free radicals now break the DNA of the existing microorganisms
such that they can
no longer multiply and die The irradiated product is therefore sterile Since
gamma radiation only
affects the electron shell of the molecules, it is physically impossible for
the irradiated product itself
to become radioactive
[7] The irradiation process takes place in a special facility The gamma
rays required therefor
result from the decay of the radioactive isotope cobalt-60. Said isotope is
stored in stainless steel
cylinders within the facility and constitutes the radiation source During the
irradiation operation, the
radiation source is surrounded by the products to be irradiated on a conveyor
system In order to be
able to enter the facility safely, the radiation source can be lowered into a
water basin, the water
column of which shields the rays A great advantage is the good penetration
capacity of the gamma
radiation, which makes it possible to sterilize the products in the final
packaging. This simplifies the
production process and ensures that the products are not contaminated again by
subsequent
packaging work
[8] The energy absorbed by the product or the irradiated object during
irradiation is measured
in kilogray (kGy) The energy absorbed by the product or the irradiated object
depends on various
factors (including exposure time, radiation intensity of the source, density
of the material, packing
density and packing size of the products, packaging material) and is checked
using one or more
dosimeters It can thus be determined that every product receives the specified
radiation dose
Electron beam sterilization
[9] Electrons emitted by an electron source (cathode) are accelerated in an
electric field (direct
voltage or alternating field) in a vacuum vessel to almost the speed of light,
either on curved paths
(e.g. Rhodotron, cyclotron, betatron) or linearly (cathode ray tube, linear
accelerator, Cockcroft¨
Walton accelerator, Van de Graaff accelerator) The accelerated electrons are
then optionally
deflected (scanned) by an alternating magnetic field in order to be able to
expose a defined area,
optionally additionally deflected by a static magnetic field in order to
achieve product exposure
deviating from the direction of acceleration, and then directed through a
suitable exit window from
the vacuum to the ambient atmosphere and then to the product The actual
sterilization process takes
place under ambient conditions Electron energies of 70 keV to 10 MeV are used
for electron beam
sterilization
[10] In the e-beam irradiation process, the beam generation begins with
electrons generated in
a hot cathode, which electrons are introduced into the acceleration unit,
which is referred to as the
CA 03133881 2021-09-16
3
cavity With the Rhodotron principle, said electrons pass through the cavity
several times with the
aid of magnetic deflection systems until they have reached the intended energy
In electron beam
treatment of medical products, the electrons are channeled out of the cavity
with a maximum energy
of 10 MeV The generated electrons are caused to move in a horizontally
oscHlating manner by a
scanning magnet, as a result of which the electrons or the X-ray photons sweep
over the entire article
to be sterilized
X-ray sterilization
[11] The spectrum of X-ray radiation begins below extreme UV radiation at a
wavelength of
around 10 nm (super-soft X-ray radiation) and extends down to less than 1 pm
(super-hard or high-
energy X-ray radiation) The energy ranges of the gamma radiation and X-ray
radiation overlap over
a wide range Both types of radiation are electromagnetic radiation and
therefore have the same
effects with the same energy The distinguishing criterion is the origin in
contrast to gamma radiation,
X-ray radiation does not arise from processes in the atomic nucleus, but
rather from high-energy
electron processes The radiation spectrum generated in X-ray tubes is a
superposition of a
continuous spectrum (bremsstrahlung) with a discrete (characteristic X-ray
radiation) spectrum
Photons from X-ray tubes have an energy of approximately 1 keV to 250 keV
[12] For electron beam sterilization and X-ray sterilization, high-energy
electrons are generated
by an electron accelerator For electron beam sterilization, the electrons are
used directly for
sterilization During product treatment using X-ray technology, the electrons
do not leave the vacuum
vessel, but are accelerated onto a metal plate, which is referred to as the
target. When interacting
with this target, part of its energy is converted and emitted in the form of X-
rays, which are used for
product sterilization. Facilities with electron energies of 5-7 MeV are used
for X-ray sterilization.
[13] X-rays, like gamma radiation, are a very penetrating type of radiation
that allows larger
volumes and higher densities to be sterilized than when using e-beam
technology Gamma and X-
ray sterilization are suitable for sterilizing palletized articles due to the
high penetration depth of the
photons
Disadvantages of radiation sterilization
[14] The three sterilization methods explained have specific disadvantages
Facilities for gamma
sterilization are dependent on a radioactive isotope Both the production of Co-
60 by neutron
activation of 00-59 in nuclear reactors and the transport and disposal of the
decay products are
associated with safety risks and high costs. In addition, long-term
availability cannot be ensured
[15] Electron beam sterilization is only suitable for medical products of
small dimensions and
densities due to its low penetration capacity compared with gamma or X-ray
radiation of the same
energy
CA 03133881 2021-09-16
4
[16] For X-ray sterilization, there is the difficulty that a large part of
the electrical energy used is
not inherently converted into X-ray radiation, but instead releases as heat on
the X-ray target, and
this results in a low degree of efficiency A cost-intensive high-energy
electron accelerator is also
necessary for electron beam and X-ray sterilization facilities
[17] Complex shielding measures are necessary for all 3 methods in order to
ensure sufficient
radiation protection Gamma, electron beam and X-ray sterilization facilities
are therefore operated
in a radiation protection bunker With the explained conventional sterilization
methods, the
construction of compact sterilization units that can be integrated directly
into the manufacturing
process of medical products is difficult and very time-consuming
Low-enerqy X-ray radiation
[18] One possibility for implementing a sterilization method that is based
on the sterilizing effect
of ionizing radiation and that can be integrated into the continuous
production process of many
medical products is to use lower-energy ionizing radiation This has two main
advantages the
measures for shielding the radiation are reduced, since the depth of
penetration of the radiation
decreases with decreasing energy, in addition, no high-energy electron
accelerators are necessary
to generate low-energy X-ray radiation, but compact electron guns or X-ray
tubes can be used Using
these devices, electron energies of up to approximately 800 keV can be
generated In the following,
the term "low-energy" or "soft" X-ray radiation denotes the energy range up to
this limit
[19] Low-energy X-ray radiation can be generated without the use of a high-
energy electron
accelerator and requires less effort for radiation shielding, which allows a
sterilization method to be
implemented that can be integrated into the continuous production process of
many medical
products
Penetration depth
[20] In order to sterilize a medical product using ionizing radiation, the
radiation has to be able to
penetrate sufficiently deeply Accelerated electrons (in electron beam
sterilization) have a high
probability of interaction with matter due to their particle properties Their
depth of penetration is
therefore low For example, electrons having an energy of 600 keV have a
penetration depth of
approx 2 mm in polyethylene and are therefore only suitable for sterilizing
two-dimensional, i e , very
thin, medical products or for sterilizing surfaces Low-energy electrons are
therefore not suitable for
sterilizing three-dimensional medical products, i e , medical products of
which the height/thickness
is on the same order of magnitude as the length and width thereof and is in
the range of centimeters
or greater
CA 03133881 2021-09-16
,
[21] In contrast to electrons, photons (gamma radiation/X-ray radiation)
have neither a charge
nor a mass The interaction probability of photons when penetrating matter is
therefore much lower
than for electrons Gamma radiation or X-ray radiation can therefore penetrate
much more deeply
into matter than electron radiation of the same energy Photon energies in the
low two-digit keV range
are sufficient to penetrate many three-dimensional medical products such as
dialyzers with photon
radiation An increase in the energy of the photon radiation leads to an
increase in the dose
homogeneity If the homogeneity of the input absorbed dose is too low, high
doses that cause
material damage can occur at points at which dose maxima form These can affect
the performance
characteristics of the medical product, for example reducing biocompatibility
Prior art
[22] WO 2014/132049 A2 (Apparatus for the generation of low-energy X-rays)
discloses a device
that is used to generate X-ray radiation with low energy, as well as a method
for sterilizing products
using this device The field of application for sterilization using this device
includes, inter elle, medical
products and pharmaceutical products. The device differs in some respects from
a classic X-ray tube
(for example the X-ray radiation generated at the anode (X-ray target) is
scattered back to the
cathode and penetrates said cathode, whereas in an X-ray tube the anode has a
defined angle and
the X-ray radiation is emitted at an angle)
[23] GB 2 440 310 A (Surface sterilization) discloses a device which
generates X-ray radiation
with an energy of less than 50 keV. The apparatus can be used to sterilize
surfaces and thin
materials
[24] EP 2 668 963 Al (Device for sterilizing containers with sterilization
checking) discloses a
device for sterilizing containers, which are guided, by means of a transport
device, past a sterilization
means, where they are sterilized by means of radiation The containers are then
moved past another
means that checks the success of the sterilization. Electron radiation is
mentioned as the preferred
type of radiation for the sterilization and it is explained that X-ray
radiation or UV radiation can also
be used to sterilize the containers Other than containers, no further
application examples are
mentioned The purpose of the method is exclusively to sterilize surfaces
[25] WO 2008/129397 A2 (Sterilization system for PET containers and
bottles) discloses a
system for sterilizing containers made of PET Electron radiation is used for
the sterilization The
sterilization effect of the electron radiation is facilitated by X-ray targets
being arranged within the
system, which targets convert the incident electron radiation into X-ray
radiation
[26] WO 93/17446 Al (A microwave X-ray source and methods of sterilization)
discloses a device
which generates X-ray radiation by means of a cyclotron resonance plasma Among
other things, the
sterilization of medical equipment and instruments is disclosed as an
application
CA 03133881 2021-09-16
6
Disadvantages in the prior art
[27] X-ray sources in the low-energy range are mainly used for analytical
purposes They are
therefore designed to achieve the highest possible image quality
Sterilization, in contrast, requires
the generation of a high radiation power in order to achieve the required
sterility assurance level
(SAL) in the shortest possible time The use of commercial X-ray tubes for
sterilizing medical
products is therefore not expedient
[28] Conventional sterilization methods for medical products that are based
on the sterilizing
effect of ionizing radiation (gamma, electron beam and high-energy X-ray
sterilization) can be
integrated into the production process of medical products only with great
effort The main reason
for this is the high radiation energy that occurs, which requires complex
radiation protection
measures Electron beam and high-energy X-ray sterilization also require the
use of a high-energy
electron accelerator of high radiation energy and power, which takes up a lot
of space and is cost-
intensive
Object of the invention
[29] The object of the present invention is therefore that of providing a
device and a method for
sterilizing medical products, which device is compact, avoids the use of
radioactive substances, is
easy to control in an open-loop and closed-loop manner, has a high level of
sterilization efficiency,
allows a high penetration depth and achieves a homogeneous dose in the product
to be sterilized
[30] As explained above, low X-ray energies lead to reduced homogeneity of
the dose input into
a three-dimensional medical product, and this can lead to material damage in
these regions if a local
overdose is then necessary Furthermore, many medical products are
inhomogeneous in their
geometric shape and material composition, i e , there are regions in which the
medical product has
a greater thickness and/or density than in other regions, and this can also
cause high local overdoses
and thus material damage
[31] Thus, it is preferably also an object of the invention to irradiate an
inhomogeneous medical
product/three-dimensional medical product as homogeneously as possible even
with low X-ray
energies or to reduce the overdose factor (max locally applied dose in the
product/target dose).
Brief description of the invention
[32] The object or objects of the invention is/are achieved by a method for
sterilizing medical
products according to claim 1 and a device for sterilizing medical products
according to claim 9
[33] The device for sterilizing at least one medical product has at least
one radiation source,
preferably at least one detector for detecting a radiation intensity, at least
one holder for holding a
CA 03133881 2021-09-16
,
7
medical product in front of the radiation source, preferably between the
radiation source and the
detector, and at least one control unit for controlling the radiation source
and preferably the holder in
an open-loop or closed-loop manner The intensity of the radiation from the
radiation source can be
controlled by the control unit, preferably continuously or cyclically, in a
closed-loop manner by means
of feedback and/or in an open-loop manner by means of feedforward control such
that the radiation
intensity assumes a predetermined or predeterminable value that is minimally
necessary for
sterilization at every position of the medical product. In other words, the
intensity of the radiation from
the radiation source can be controlled by the control unit, preferably
continuously or cyclically, in a
closed-loop manner by means of feedback and/or in an open-loop manner by means
of feedforward
control such that predetermined optimal intensity distribution of the X-ray
radiation is achieved which
leads to achieving the required sterilization dose at every point of the
medical product, more
homogeneous dose distribution in the medical product (minimization of the
overdose factor) and a
reduced irradiation time (= time to achieve the required sterilization dose)
Predetermined optimal
intensity distribution of the X-ray radiation is preferably determined
experimentally by means of dose
mapping or using a simulation Every position of the medical product means at
every position in/on
the three-dimensional body of the medical product
[34] The device for sterilizing at least one medical product can also be
referred to as a sterilization
device or sterilization unit or can be provided and adapted, in the form of a
sterilization device or
sterilization unit, for the sterilization of medical products
[35] The radiation source is preferably a directional radiation source,
preferably an
electromagnetic radiation source, preferably an X-ray radiation source and
particularly preferably a
low-energy X-ray radiation source, which is provided and adapted to
provide/generate primary
electrons having an energy of 100 to 800 keV The radiation source is also
provided and adapted to
individually set the radiation intensity/the absorbed dose of the radiation
locally/in a spatially resolved
manner/in a locally determined manner/individually, that is to say cyclically
or continuously within an
exposed irradiated region The absorbed dose input into the medical product can
thus be locally
controlled in an open-loop or closed-loop manner, generally resulting in an
inhomogeneous/controllable irradiation intensity within the medical product
[36] The detector is preferably provided and adapted to detect the
radiation from the radiation
source The detector is also preferably an area detector (detector having a
large-area sensor),
preferably an X-ray detector or an area X-ray detector The detector is further
preferably a digital
detector which generates data signals and forwards said signals to a control
device The radiation
source emits radiation and emits the radiation directionally, the detector is
preferably introduced in
the directional radiation/in the beam path This means that the detector is
irradiated by the radiation
source The detector preferably has at least the size required to be able to
detect the smallest
dimension of the shaded area, preferably at least the size required to detect
the entire area shaded
CA 03133881 2021-09-16
=
8
by the medical product, in order to be able to draw conclusions about the
absorbed dose in the entire
medical product If the detector is moved in the direction of the other
dimension, or if the medical
product moves in this direction, the same statement can be obtained
[37] A medical product is generally known and is defined in the
introductory part The method
and the device are provided and adapted to sterilize at least one medical
product at a time
[38] The device has a holder/clamping device/holding device/medical product
holder, which is
preferably provided and adapted to hold at least one medical product,
particularly preferably between
the radiation source and the detector The holder further preferably has a
transport device by means
of which the medical product can be transported between the radiation source
and the detector In
other words, the transport device moves the medical product into the beam path
for a certain period
and then out again Furthermore, the holder preferably has a movement
device/rotation device which
rotates the medical product about at least one axis or causes said product to
wobble In other words,
the at least one medical product can be secured/stabilized/held in the holder
and, preferably, can be
rotated about the longitudinal axis The holder is introduced in the
directional radiation/in the beam
path of the radiation source, preferably an X-ray radiation beam path The
holder and thus the
medical product are arranged between the radiation source and the detector In
one variant, the
holder can only introduce part of the medical product into the beam path if
the size thereof exceeds
the irradiated region in the beam path, but it can also introduce a single
medical product or a plurality
of medical products into the beam path at the same time and thus sterilize
said product(s)
Furthermore, the holder holds the medical product in such a way that the
irradiation of the product is
not hindered or any hindrance is minimized The holder preferably holds the
medical product or the
medical product is clamped in the holder in such a way that the holder does
not overlap the medical
product in the direction of irradiation. In other words, the medical product
and the holder are not
arranged one behind the other in the direction of radiation, but rather in
parallel therewith The
medical product is preferably held or clamped by the holder on the outer
surfaces of said product
[39] In a further aspect of the invention, the holder has a transport
device by means of which the
at least one medical product can be transported through a beam path between
the X-ray radiation
source and the X-ray detector In other words, the at least one medical product
can be transported
mechanically/electromechanically through the beam path, preferably by means of
a conveyor belt or
the like
[40] The device can thus consist of a radiation source and a detector,
between which the medical
product is introduced, but may also consist of a plurality of radiation-
source¨detector pairs, the
medical product being arranged therebetween The device preferably has at least
two, preferably
three, X-ray radiation sources and X-ray detectors
CA 03133881 2021-09-16
9
[41] The method for sterilizing medical products has the following steps
a introducing a medical product into a sterilization device,
locally irradiating the medical product with a radiation source of the
sterilization device,
locally determining the radiation intensity by means of (dose mapping) or
using
simulations, and
controlling the radiation source in an open-loop or closed-loop manner by
means of a
control unit such that at least one radiation intensity that is minimally
necessary for
sterilization is achieved at every position of the medical product In other
words,
predetermined optimal intensity distribution of the X-ray radiation is
achieved, which
leads to achieving the required sterilization dose at every point of the
medical product,
more homogeneous dose distribution in the medical product, and a reduced
irradiation
time.
[42] The medical product can be introduced into the sterilization
device/irradiation device/device
for sterilizing medical products manually and/or mechanically, preferably
between the radiation
source and the detector or the sensor of a detector Further preferably, the
medical product can be
introduced/subsequently changed automatically, preferably in a computer-
controlled manner In
addition, the medical product is further preferably held/secured/supported by
a holder/clamping
device between the radiation source and the detector The holder has a movement
device and/or
rotation device and/or a transport device The rotation device of the holder
rotates the medical
product about at least one axis and the transport device changes the medical
product or transports
said product
[43] The local/individual irradiation, preferably stepwise and/or
continuous, of the medical product
(along the medical product) with a radiation source of the sterilization
device is preferably carried out
by means of a directional radiation source or electromagnetic radiation source
or X-ray radiation
source or low-energy X-ray radiation source or low-energy X-ray radiation
source which is provided
and adapted to use a primary electron having an energy of 100 to 800 keV Local
irradiation is to be
understood as irradiation having a local intensity resolution that can
irradiate different
surfaces/points/locations/positions of an object or medical product with a
relatively/mutually different
radiation/radiation intensity/radiation dose/dose/absorbed dose/photon energy
This means that the
medical product can be irradiated with a different intensity at every
point/individual points/other
points
[44] The determination of the radiation intensity at every position of the
medical product shows
how much of the radiation emitted by the radiation source is absorbed by the
medical product
[45] The radiation source is controlled in an open-loop or closed-loop
manner such that at every
position of the medical product a radiation intensity that is minimally
necessary for sterilization is
CA 03133881 2021-09-16
achieved in the medical product In other words, previously determined optimal
intensity distribution
of the X-ray radiation is achieved, which leads to the required sterilization
dose being achieved at
every point of the medical product, more homogeneous dose distribution in the
medical product, and
a reduced radiation time In other words again, a(n) (intensity) model for
irradiation for the medical
product can be set up in advance and loaded onto a storage unit of the control
unit/CPU such that
the control unit controls the spatial resolution of the radiation source The
(intensity) model can be
determined by a simulation/calculation or reference measurement
[46] In a further aspect of the invention, the medical product is
irradiated from a plurality of sides
and/or rotates about at least one axis, preferably in/on/with the holder This
means that the medical
product is introduced into a sterilization device having a plurality of
radiation sources and/or rotates
on/in/with the holder, preferably about its own axis. In addition to the
plurality of radiation sources,
the device can also have a plurality of detectors
[47] In the variant with a plurality of radiation sources, the medical
product is irradiated from two,
three or more sides at the same time The means for generating the low-energy X-
ray radiation is
accordingly designed such that there is a plurality, and these means are
arranged around the medical
product so as to be uniformly offset. Irradiation from multiple sides has the
advantage that the use of
a plurality of X-ray sources with the same power as with irradiation from one
side shortens the
sterilization time Alternatively, by reducing the power of the individual X-
ray sources, the thermal
load on the targets can be reduced and their service life can thus be
increased Since the medical
product does not have to be rotated, the holder can be constructed in a
structurally more simple way
The dose homogeneity increases with an increasing number of X-ray sources that
are arranged
around the medical product The rotation of the medical product during the
irradiation is comparable
to the arrangement of an infinite number of X-ray sources around the medical
product and therefore
provides the best dose homogeneity.
[48] In a further alternative embodiment variant, the design of irradiation
from two or more sides
is selected and arranged two or more times one behind the other This results
in a sterilization tunnel
through which a plurality of medical products can be transported by means of a
transport device and,
in the process, can be irradiated and thus sterilized For a given target dose,
the transport speed and
thus the achievable throughput are dependent on the intensity of the radiation
sources and on the
number of radiation sources arranged one behind the other in the transport
direction
[49] Further design variants can be obtained by combining the design
variants explained above
For example, the rotating irradiation can also take place from two or more
sides in order to achieve
a high level of dose homogeneity with a reduced irradiation time or reduced
thermal load of the target
CA 03133881 2021-09-16
1 i
[50] In a further aspect of the invention, the medical product is
irradiated in such a way that the
radiation intensity of the X-ray radiation varies locally and is set such that
the dose is distributed as
homogeneously/evenly as possible in the medical product This means that at
least the minimum
dose occurs at every point/every position of the medical product In this case,
specific intensity
distribution of the transmitted X-ray radiation is preferably established,
which can be measured by a
detector located behind the product in the beam path in order to readjust the
radiation source
accordingly if necessary This distribution of the radiation intensity is
referred to as the optimal
intensity distribution
[51] As already explained above, the radiation source can be controlled in
an open-loop or
closed-loop manner by the control unit, such that the optimal intensity
distribution is achieved at
every position of the medical product The control is carried out by the
position and the shape of the
medical product in the sterilization device being stored on the control device
and the medical product
being irradiated with a previously determined intensity by the radiation
source in a spatially resolved
manner A(n) (intensity) model (a model for spatially resolved irradiation with
a predetermined
intensity) for the particular medical product is thus set up in advance The
(intensity) model is
determined by a reference measurement or by means of a simulation The
reference measurement
includes, inter alia, dose mapping
[52] With dose mapping, a test sample is equipped with dosimeters (e g ,
alanine dosimeters).
The dosimeters are placed wherever minima and maxima of the dose are expected
The medical
product is then irradiated and the dosimeter is evaluated
[53] To determine the optimal intensity distribution, the medical product
is divided over its length
into a plurality of regions that differ significantly in terms of their
geometry and/or material
composition For each region', a factor k_i is determined by which the
intensity of the X-ray radiation
in the corresponding region is multiplied in order to achieve the optimal
intensity for this region
is selected in each case such that the minimum dose in the region under
consideration corresponds
to the required sterilization dose To determine the factor the
occurring minimum dose D_min,i
has to be determined for each region, either by means of dose mapping or by
means of simulations
The harmonic mean D_min,HM is calculated from the dose minima D_rnin,i of each
region
1/
Dmin,f/M (Dnun,t 0)
1
1 Dmin,1
[54] The
factor for each region results from the harmonic mean of the minimum doses
divided
by the dose minimum of the particular region
CA 03133881 2021-09-16
1
kt Dmin,f/M/Dmin,t
The data obtained in this way for the intensity distribution are valid for all
medical products of this
type and can be used as long as the geometry and materials of the medical
product as well as the
parameters of the sterilization apparatus (radiation energy, distance between
target and medical
product, etc) remain unchanged
[55] The simulation can be a Monte Carlo simulation or a simulation based
on the law of
attenuation or the like.
[56] The detector can be used to check the dose introduced into the medical
product, in order to
release the medical product immediately after irradiation For this purpose,
the determined intensity
distribution is set in a pre-test and a medical product equipped with
dosimeters is irradiated Since
the medical product is located in the beam path, "shadowing" occurs on a
detection surface of the
detector The doses measured at the detector during the irradiation are
recorded The dosimeters in
the medical product are then evaluated and checked to determine whether the
required sterilization
dose has been achieved at every point. If this is the case, the data recorded
by the detector can be
used for all subsequent irradiations of medical products of the same type or
the same size During
each sterilization process, the doses determined at the detector are compared
with the recorded
doses If the deviations do not exceed a specified limit, the irradiated
medical products can be
designated as sterile and released
[57] The variation of the intensity of the radiation source or of an
electron beam impinging on an
X-ray target over the surface of the target allows an X-ray radiation field to
be adapted to the medical
product in a spatially resolved manner For at least one medical product, the
holder allows the
medical product to move in space, preferably to rotate axially along an axis
The detector allows the
X-ray radiation absorbed by the medical product to be measured Shielding the
sterilization device
protects the operator The method for using this sterilization unit for
sterilizing medical products is
carried out using the above device The X-ray radiation field is, so to speak,
adapted to the medical
product to be sterilized, and is also adapted continuously over time if the
medical product is rotating
in order to adapt the absorbed dose. The intensity distribution is preferably
determined before the
irradiation in series on test samples of the medical product to be irradiated.
For this purpose, either
dose mapping or computer simulations (e g , Monte Carlo simulation) are
carried out
[58] The shielding of the sterilization unit is designed in such a way that
the production staff and
the environment are protected from the effects of radiation, and the
applicable laws, regulations and
standards are complied with
CA 03133881 2021-09-16
I
,
13
[59] The holder is designed in such a way that it can hold at least one or
more medical products
at the same time and does not hinder the desired radiation exposure of the
product In one
embodiment variant, the holder makes it possible to move the medical product
during the irradiation,
and in the preferred embodiment for the example product makes it possible to
rotate said product
With this system, the dose inhomogeneity due to the depth dose distribution
that occurs at low
energies because of the limited penetration depth of the X-ray radiation can
be improved The dose
homogeneity can be increased by increasing the number of X-ray sources that
are arranged around
the medical product The rotation of the medical product corresponds to an
infinite number of X-ray
sources and thus represents the best possible case in terms of achieving a
high level of dose
homogeneity The holder is preferably designed in such a way that fully
automatic loading and
unloading of the medical product(s) is made possible
[60] The invention makes it possible to achieve a high level of dose
homogeneity despite the low
energy of the X-ray radiation Furthermore, due to the low radiation energy and
the associated lower
required shielding measures, for example in comparison with Co-60 gamma
irradiation facilities or
MeV e-beam irradiation facilities, the invention allows integration into the
continuous production
process of medical products There is no longer any dependency on service
providers who perform
sterilization using gamma radiation, high-energy electron radiation or high-
energy X-ray radiation
The system is easily scalable depending on the throughput of the production
system, the necessary
number of sterilization units is purchased A high level of production
reliability can be achieved by
operating a number of sterilization units redundantly Sterilization using low-
energy X-ray radiation
also has the known advantages of methods that are based on the sterilizing
effect of ionizing
radiation This includes avoiding the use of toxic substances such as ethylene
oxide, the possibility
of sterilization in the final packaging and parametric product release based
on the applied absorbed
dose
[61] Process observation is preferably provided for monitoring the
sterilization process This
consists of at least one X-ray radiation detector which is arranged in such a
way that it is possible to
draw conclusions about the absorbed dose in the medical product For this
purpose, electronically
readable detector plates are preferably arranged in such a way that the
medical product to be
sterilized is located between the X-ray source and the detector plates The
size of the detector plate
is selected such that it fully detects the X-ray radiation shadowed by the
medical product and also
covers a region in which the X-ray radiation was not attenuated by the medical
product From the
difference in the intensity of the X-ray radiation attenuated by the medical
product and the
unattenuated X-ray radiation, conclusions can be drawn about the energy
absorbed by the medical
product A method for sterilizing three-dimensional medical products using low-
energy X-ray
radiation can be carried out in the following steps
CA 03133881 2021-09-16
14
[62] For the sake of simplicity, the specific sequence of the method is
explained only for a single
medical product
= providing and introducing the medical product in a radiation-resistant
sterile barrier
system/packaging suitable for sterilization using ionizing radiation
(optionally removing
oxygen from the packaging for medical products for which irradiation in the
presence of
oxygen can cause material damage),
= equipping the sterilization unit with the packaged medical product,
preferably by means
of an automatic handling system,
= initiating all necessary measures to ensure the radiation safety of the
arrangement,
= irradiating the medical product with locally differing radiation
intensities according to the
above description over a defined irradiation time in order to achieve the
required
irradiation dose, which is required, for example. according to national
standardswithdrawing the sterile medical product, preferably by means of an
automatic
handling system,
= evaluating the data from the X-ray detectors and dosimetrically releasing
the medical
product
[63] The device has a radiation source and preferably a detector, between
which a medical
product is introduced, the radiation source being controllable by means of an
open-loop and/or
closed-loop control device in a closed-loop manner by means of feedback from
the detector or in an
open-loop manner by means of a result of dose mapping or a simulation
Preferably, during closed-
loop control of the radiation source using the spatially resolved intensity,
the closed-loop control is
carried out in such a way that the setpoints of the spatially resolved
detector are reached The method
for sterilizing medical products comprises the following steps introducing a
medical product into a
sterilization device, irradiating the medical product with a radiation source,
preferably an X-ray
radiation source, of the sterilization device, determining the radiation
intensity at every position of the
medical product, controlling and/or readjusting the radiation source according
to the relationship,
[determined in a reference measurement or simulation and] stored in the
control device, between the
radiation intensity at the detector and the minimum dose in the medical
product at the corresponding
point, such that the medical product is homogeneously irradiated and thus
sterilized
[64] Description of the figures
Fig 1 shows the structure of the device in an abstract form
Fig 2 shows an X-ray radiation source with a solid target in a vacuum
(classic X-ray tube)
Fig 3 shows an X-ray radiation source with a transmission target
Fig 4 shows a simplified model for operating adapted intensity distribution
CA 03133881 2021-09-16
=
1
Fig 5 shows the targeted change in the intensity distribution of the X-ray
radiation field in
accordance with the geometry and material composition of a medical product
(here by way
of example for a dialyzer)
Fig 6 shows the increase in dose homogeneity by increasing the number of X-
ray sources
Fig. 7 shows a second embodiment of the invention, irradiation from three
sides (holder and
shield not shown)
Fig 8 shows a third embodiment of the invention, a two-sided arrangement of
a plurality of X-ray
modules to form a sterilization tunnel (X-ray detector, holder and shield not
shown)
[65] Fig. 1 shows, in an abstract form, the structure of the device for
sterilizing medical products
according to a preferred embodiment of the invention An X-ray radiation source
2 (radiation source)
is introduced into a sterilization device 1. The X-ray radiation source is
controlled by a CPU/control
unit 3. The representation in Fig. 1 is schematic and the CPU 3 is actually
located outside the
radiation space The radiation source 2 emits directional radiation 4 with a
locally determined
absorbed dose or intensity A detector 6 is located in the direction of the
directional radiation 4 A
medical product 8, for example a dialyzer, is introduced in front of the
detector 6 in the directional
radiation 4, i e., between the radiation source 2 and the detector 6 The
medical product 8 is held by
a holder 10 and can also be rotated by said holder
[66] Fig 2 shows an X-ray radiation source with a solid target in a vacuum
(classic X-ray tube)
The radiation source consists of an electron source 12 which directionally
accelerates electron
radiation 14 The electron radiation 14 hits an X-ray target 16 and generates
directional X-ray
radiation 4 at said target The X-ray radiation emerges from the vacuum through
the exit window 18
[67] Fig. 3 shows an X-ray radiation source with a transmission target The
structure of the X-ray
source in Fig. 3 is analogous to that in Fig 2, with the exception that the
electron radiation does not
hit a solid X-ray target, the X-ray radiation being generated at said target,
but rather the electron
radiation 14 hits a very thin X-ray target 22 that simultaneously serves as an
exit window, in which
target the directional X-ray radiation 4 is generated in the direction of the
primary electron radiation
[68] In other words, the arrangement of the X-ray target 16 and 22 can be
possible in two variants
the X-ray target can be designed as a solid target (thick target) 16, which is
located within the vacuum
vessel of the electron accelerator (this structure corresponds to the classic
X-ray tube) The X-ray
target can, however, also be designed as a transmission-type target (thin
target) 22
CA 03133881 2021-09-16
,
16
[69] The electron source 12 subsequently has,ie , between the electron
source 12 and the target
16, 22, a system for the spatially resolved increase or decrease in the
intensity of the electron current
impinging on the X-ray target 16, 22 in defined regions The X-ray target then
converts the kinetic
energy of the accelerated electrons into X-ray radiation with a spatially
resolved increase or decrease
in intensity
[70] The X-ray target 16, 22 preferably consists of a metal with a high
atomic number One
embodiment is tungsten because of its high X-ray yield and very good heat
resistance. Another
embodiment is silver, since its emission lines of the characteristic X-ray
radiation are in a lower
energy range than for tungsten In this lower energy range, the mass energy
absorption coefficient
pen/p of the materials of the medical product is greater than at higher
energies, as a result of which
the absorbed dose input into the medical product is greater, and this can lead
to increased efficiency
of the irradiation process The X-ray target 16, 22 preferably has a means for
cooling said target
[71] Fig 4 shows the absorbed doses that occur, greatly simplified, by
means of two individual,
one-dimensional, monoenergetic X-rays 24 and 26 extending in parallel The two
X-rays penetrate a
medical product 8 consisting of a homogeneous material, the thickness of the
material which is
penetrated by the ray 24 being only half as great as the thickness of the
material which is penetrated
by the ray 26 A detector 6 is shown in the beam direction behind the medical
product 8 In the high-
density region, a longer irradiation time is required to reach the
sterilization dose than in the low-
density region However, since the medical product 8 is irradiated as a whole,
every region
experiences the same irradiation time The low-density region is thus
irradiated for a longer time than
would be necessary to achieve the sterilization dose
[72] Fig 5 shows the targeted change in the intensity distribution of the X-
ray radiation field in
accordance with the geometry and material composition of the medical product
8, here by way of
example for a dialyzer (top picture schematic representation of the medical
product, bottom picture
location-dependent radiation intensity) In the region of the PUR potting
compound (9), a dialyzer
has a higher density at the two ends of the dialyzer than in the middle region
In order to achieve
more homogeneous dose input, the intensity of the radiation field is increased
in the high-density
region and reduced in the low-density region (total intensity or power remains
constant) As an
alternative or in addition to this, homogeneous dose input can also be
achieved by estimating a
longer irradiation time and/or rotating the dialyzer during irradiation This
results in more
homogeneous dose distribution overall The irradiation time across the entire
medical product is
reduced This reduced irradiation time in combination with the reduced
radiation intensity in the low-
density region leads to a lower maximum dose in the low-density region, and
this reduces potentially
harmful radiation-induced material changes. In the high-density region, in
contrast, the maximum
dose remains unchanged, since the reduced irradiation time and the increased
radiation intensity
balance each other out. The reduced irradiation time results in increased
efficiency of the process
CA 03133881 2021-09-16
I
1 7
In Fig 5, the dialyzer ports of the dialyzer are drawn leading upward (leading
away from the image
of the radiation intensity), while the radiation is radiated onto the drawing
in the image plane
[73] Fig 6 shows the increase in dose homogeneity by increase in the number
of X-ray sources,
with a rotation of the medical product in front of an X-ray source being the
best case (here simulated
with 16 sources) Simulation parameters solid tungsten target, target angle
450, electron energy
400 keV, 1 mm Al filter, distance from the X-ray source(s) to the center of
the dialyzer 12 cm, the
dose absorbed in water is shown A shows the absorbed dose in the case of one
source on the left,
B shows the absorbed dose in the case of two sources, on the left and right,
respectively, and C
shows the absorbed dose in the case of 16 sources evenly distributed around
the medical product
[74] Fig 7 shows a second embodiment of the invention, more precisely
irradiation of the medical
product 8 from three sides (holder and shield not shown) The medical product 8
is irradlated with
directional X-ray radiation 4 from three radiation sources 2 that distributed
uniformly on one plane at
an angular spacing (at a circular angle of approx 120 ) A detector is located
in each case behind
the medical product 8 in the radiation direction of the X-ray radiation
[75] Fig 8 shows a third embodiment of the invention, more precisely an
embodiment in which a
two-sided arrangement of a plurality of X-ray modules to form a sterilization
tunnel is shown (X-ray
detector, holder and shield not shown) The medical products 8 are irradiated
from two opposite
sides by radiation sources 2 and transported in a transport direction 28
(shown schematically) by
means of a transport device (not shown) The medical products 8 are thus
conveyed through an
"irradiation tunnel" A different arrangement of the radiation sources 2, for
example as shown in
Fig 7, would also be possible here
CA 03133881 2021-09-16
,
,
18
List of reference signs
1 sterilization device
2 radiation source
3 CPU
4 directional X-ray radiation
6 detector
8 medical product
holder
12 electron source
14 electron radiation
16 X-ray target
18 exit window
vacuum
22 exit window with integrated X-ray target
24 low-intensity X-ray
26 high-intensity X-ray
28 transport direction