Note: Descriptions are shown in the official language in which they were submitted.
CA 03134253 2021-09-20
STICK-ON BIOSENSOR
TECHNICAL FIELD
[0001] The present invention relates to a stick-on
biosensor.
BACKGROUND ART
[0002] Conventionally, a biosensor which uses a
biocompatible polymer substrate comprising a plate-
like first polymer layer and second polymer layer, an
electrode, and a data acquisition module has been
known (see, for example, Patent Document 1).
PRIOR ART DOCUMENT(S)
[0003] Patent Document 1
Japan Patent Application Laid-open
Publication No. 2012-010978
SUMMARY OF THE INVENTION
TECHNICAL PROBLEM TO BE SOLVED
[0004] A biosensor measures various biological
information such as electrocardiographic waveforms or
brain waves, while being attached onto a living body.
If the skin is crinkled during the measurement of
biological information by a biosensor, the electrodes
may peel off from the living body, or a gap may be
produced between the skin and the sensor. In such a
case, noise is contained in the biological
information, and it may be difficult to acquire
satisfactory biological information.
[0005] Therefore, an objective is to provide a
stick-on biosensor capable of acquiring satisfactory
1
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
biological information.
TECHNICAL SOLUTION (5)
[0006] In one aspect of the invention, a stick-on
biosensor is provided, which includes a pressure-
sensitive adhesive layer and an electrode part, said
pressure-sensitive adhesive layer having a stick-on
surface to be attached to a subject; a base material
layer provided on the side opposite the stick-on
surface of the pressure-sensitive adhesive layer; and
an electronic device provided on the base material
layer and configured to process a biological signal
acquired through the electrode part. A structure
including the pressure-sensitive adhesive layer, the
electrode part, and the base material layer has a
flexural rigidity of 0.010 [MPa.mm3/mm] or higher, and
the adhesive strength of the electrode part to adhere
to the subject is greater than 0.6 [N/cm2] and less
than or equal to 5.0 [N/cm2].
[0007] In another aspect of the disclosure, the
stick-on biosensor is provided, which includes a
pressure-sensitive adhesive layer and an electrode
part, said pressure-sensitive adhesive layer having a
stick-on surface to be attached to a subject; a base
material layer provided on the side opposite the
stick-on surface of the pressure-sensitive adhesive
layer; and an electronic device provided on the base
material layer and configured to process a biological
signal acquired through the electrode part. A
structure including the pressure-sensitive adhesive
layer, the electrode part, and the base material layer
has a flexural rigidity of 0.034 [MPa.mm3/mm] or
higher, and the adhesive strength of the electrode
part to adhere to the subject is 1.3 [N/cm2] or more.
ADVANTAGEOUS EFFECT OF THE INVENTION
2
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
[0008] A stick-on biosensor capable of acquiring
satisfactory biological information can be achieved.
BRIEF DESCRIPTION OF DRAWINGS
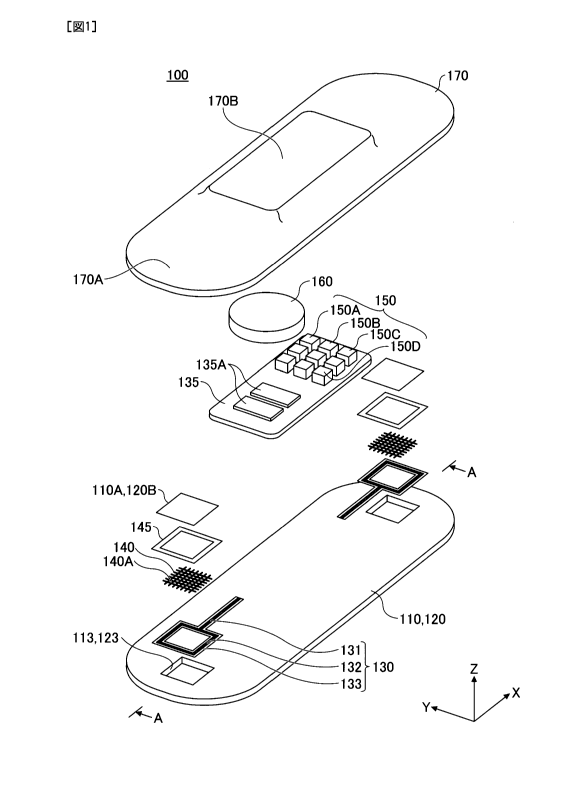
[0009] FIG. 1 is an exploded view of a stick-on
biosensor 100 according to an embodiment;
FIG. 2 is a cross-sectional view of the biosensor
in the assembled state, taken along the A-A line of
FIG. 1;
FIG. 3 shows a circuit configuration of the
stick-on biosensor 100;
FIG. 4A shows the evaluation result of a
plurality of samples;
FIG. 4B shows the evaluation result of a
plurality of samples;
FIG. 4C shows the evaluation result of a
plurality of samples;
FIG. 5A shows how the baseline fluctuation is
evaluated;
FIG. 5B shows how the baseline fluctuation is
evaluated;
FIG. 6 is a chart summarizing the evaluation
results of FIG. 4A to FIG. 4C; and
FIG. 7 is a chart summarizing the evaluation
results of FIG. 4A to FIG. 4C.
EMBODIMENT(S) FOR IMPLEMENTING THE INVENTION
[0010] Preferred embodiments for implementing the
invention are described below.
<EMBODIMENTS>
[0011] FIG. 1 is an exploded view of a stick-on
biosensor 100 according to an embodiment. FIG. 2 is a
cross-sectional view of the stick-on biosensor 100 in
3
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
the assembled state, taken along the A-A line of FIG.
1. The stick-on biosensor 100 includes a pressure-
sensitive adhesive layer 110, a base material layer
120, a circuit unit 130, a substrate 135, a probe 140,
a fixing tape 145, an electronic device 150, a battery
160, and a cover 170 as the main components. Of these,
the pressure-sensitive adhesive layer 110, the base
material layer 120, and a probe part 143 including the
probe 140 constitute a structure 101 (shown in FIG.
2).
[0012] In the following, the XYZ-coordinate system
will be defined to explain the configuration. For
convenience of description, the negative direction of
the Z axis is referred to as the lower or the bottom
side, and the positive direction of the Z axis is
referred to as the upper or the top side; however,
such directional terms do not mean absolute positional
relations.
[0013] In the embodiment, the stick-on biosensor
100 is attached onto a living body, i.e., a subject
whose biological information is to be measured. A
living body may be either a human body or a lifeform
other than a human being. The stick-on biosensor 100
can be attached to the skin, the scalp, the forehead,
etc. of the living body. The respective components
constituting the stick-on biosensor 100 will be
described in detail below.
[0014] In the following, the electrode brought into
contact with a living body (i.e., a subject) is called
a probe 140, and a region in which the probe 140 is
provided is named a probe part 143. The fixing tape
145 is used as an example of a bonding means. The
probe part 143 is an example of an electrode part.
[0015] The stick-on biosensor 100 is a sheet-like
sensor having a shape of an elongated ellipse in a
plan view. The top surface of the stick-on biosensor
100 is covered with a cover 170, which is located on
4
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
the side opposite the bottom surface (positioned in
the -Z direction) of the stick-on biosensor 100 that
is attached to the skin 10 of a living body. The
bottom surface of the stick-on biosensor 100 is a
stick-on surface.
[0016] The circuit unit 130 and the substrate 135
are mounted on the upper surface of the base material
layer 120. The probe 140 is embedded in the pressure-
sensitive adhesive layer 110 so as to be exposed at
the lower surface 112 of the pressure-sensitive
adhesive layer 110. The lower surface 112 of the
pressure-sensitive layer 110 is the bottom surface,
and therefore, the stick-on surface of the stick-on
biosensor 100 described above.
[0017] The pressure-sensitive adhesive layer 110 is
a flat adhesive layer, and has a longitudinal axis
extending in the X direction, while having a width in
the Y direction. The pressure-sensitive adhesive layer
110 is supported by the base material layer 120, and
is bonded to the lower surface 121 of the base
material layer 120.
[0018] The pressure-sensitive adhesive layer 110
has an upper surface 111 and a lower surface 112, as
shown in FIG. 2. The upper surface 111 and the lower
surface 112 are flat surfaces. The pressure-sensitive
adhesive layer 110 is the layer that allows the stick-
on biosensor 100 to adhere to a living body. The lower
surface 112 sticks to the skin 10 of the living body
owing to the adhesiveness. The lower surface 112
serves as the bottom surface of the stick-on
biological sensor 100, and is attached to the surface
(such as the skin 10) of a living body.
[0019] The pressure-sensitive adhesive layer 110
has a through hole 113. The size and the position of
the through hole 113 are the same as those of a
through hole 123 formed in the base material layer 120
in the plan view, such that the through holes 113 and
5
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
123 communicate with each other.
[0020] The material of the pressure-sensitive
adhesive layer 110 is not particularly limited as long
as it has an adhesiveness. For example, a
biocompatible adherent material may be used. Examples
of the material of the pressure-sensitive adhesive
layer 110 include, but are not limited to acrylic
pressure-sensitive adhesives and silicone-based
pressure-sensitive adhesives. Preferably, an acrylic
pressure-sensitive adhesive is used.
[0021] Acrylic pressure-sensitive adhesive contains
an acrylic polymer as the main component.
[0022] Acrylic polymer serves as a pressure-
sensitive adhesive component. One example of the
acrylic polymer is a polymer produced by polymerizing
a monomer component which contains (meth)acrylic acid
ester such as isononyl acrylate or methoxyethyl
acrylate as a main material, and contains a monomer
capable of copolymerization with the (meth)acrylic
acid ester as an optional material. The content of the
main material in the monomer component is 70 mass% to
99 mass%, and the content of the optional material in
the monomer component is 1 mass% to 30 mass%. As the
acrylic polymer, (meth)acrylic acid ester-based
polymer described in JP-A-2003-342541 may be used.
[0023] The acrylic pressure-sensitive adhesive may
further contain a carboxylic acid ester.
[0024] The carboxylic acid ester contained in the
acrylic pressure-sensitive adhesive serves as a
modifier for reducing the pressure-sensitive
adhesiveness of the acrylic polymer to adjust the
adhesive strength of the pressure-sensitive adhesive
layer 110. The carboxylic acid ester is miscible or
compatible with the acrylic polymer.
[0025] An example of the carboxylic acid ester is
glyceryl tri-fatty acid ester.
[0026] The content of the carboxylic acid ester is
6
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
preferably 30 to 100 parts by mass, and more
preferably 50 to 70 parts by mass, with respect to
100 parts by mass of the acrylic polymer.
[0027] The acrylic pressure-sensitive adhesive may
contain a cross-linking agent, as necessary. The
cross-linking agent is a cross-linker for cross-
linking the acrylic polymer. Examples of the cross-
linking agent include, but are not limited to
polyisocyanate compounds, epoxy compounds, melamine
compounds, peroxide compounds, urea compounds, metal
alkoxide compounds, metal chelate compounds, metal
salt compounds, carbodiimide compounds, oxazoline
compounds, aziridine compounds, and amine compounds.
These cross-linking agents may be used alone or in
combination. The cross-linking agent is preferably a
polyisocyanate compound (polyfunctional isocyanate
compound).
[0028] The content of the cross-linking agent is
preferably, for example, 0.001 to 10 parts by mass,
and more preferably 0.01 to 1 part by mass with
respect to 100 parts by weight of the acrylic polymer.
[0029] Preferably, the pressure-sensitive adhesive
layer 110 has satisfactory biocompatibility. For
example, according to the keratin peeling test
performed on the pressure-sensitive adhesive layer
110, the keratin peeled area ratio is preferably 0% to
50%, more preferably 1% to 15%. Within the range of 0%
to 50% of the keratin peeled area ratio, the load on
the skin 10 (see FIG. 2) can be suppressed when the
pressure-sensitive adhesive layer 110 is attached to
the skin 10. The keratin peeling test may be performed
by the measuring method described in JP-A-2004-83425.
[0030] The moisture permeability of the pressure-
sensitive adhesive layer 110 is 300 (g/m2/day) or
higher, preferably 600 (g/m2/day) or higher, and more
preferably 1000 (g/m2/day) or higher. With the
moisture permeability of the pressure-sensitive
7
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
adhesive layer 110 of 300 (g/m2/day) or higher, the
load on the skin 10 (see FIG. 2) can be suppressed
when the pressure-sensitive adhesive layer 110 is
attached to the skin 10.
[0031] The pressure-sensitive adhesive layer 110
can be biocompatible when it satisfies at least one of
the following conditions; the condition that the
keratin peeled area ratio measured by the keratin
peeling test is 50% or less, and the condition that
the moisture permeability is 300 (g/m2/day) or higher.
It is more preferable for the material of the
pressure-sensitive adhesive layer 110 to satisfy both
of the conditions. In this case, the pressure-
sensitive adhesive layer 110 is more biocompatible in
a stable manner.
[0032] The thickness of the pressure-sensitive
adhesive layer 110 between the upper surface 111 and
the lower surface 112 is preferably 10 pm to 300 pm.
With the thickness of the pressure-sensitive adhesive
layer 110 of 10 pm to 300 pm, the stick-on biosensor
100 can be made thinner, and in particular, the stick-
on biosensor 100 except for the electronic device 150
can be made thinner.
[0033] The base material layer 120 is a support
layer configured to support the pressure-sensitive
adhesive layer 110. The pressure-sensitive adhesive
layer 110 is bonded to the lower surface 121 of the
base material layer 120. The circuit unit 130 and the
substrate 135 are provided on the upper surface side
of the base material layer 120.
[0034] The base material layer 120 is a flat plate-
like (or sheet-like) member made of an insulator. The
shape of the base material layer 120 in a plan view is
the same as the shape of the pressure-sensitive
adhesive layer 110, and they are aligned to and
stacked with each other.
[0035] The base material layer 120 has a lower
8
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
surface 121 and an upper surface 122, both of which
are flat surfaces. The lower surface 121 is in contact
with the upper surface 111 of the pressure-sensitive
adhesive layer 110 by pressure-sensitive adhesion. The
base material layer 120 may be made of a flexible
resin having appropriate elasticity, flexibility and
toughness. For example, a thermoplastic resin such as
polyurethane resin, silicone resin, acrylic resin,
polystyrene resin, vinyl chloride resin or polyester
resin may be used. The thickness of the base material
layer 120 is 1 to 300 pm, preferably 5 to 100 pm, and
more preferably 10 to 50 pm.
[0036] The circuit unit 130 has a wiring 131, a
frame 132, and a substrate 133. The circuit unit 130
is connected to the electrode via the frame 132, and
connected to the electronic device 150 via the wiring
131. The stick-on biosensor 100 has two circuit units
130. The wiring 131 and the frame 132 are integrally
formed on the upper surface of the substrate 133. The
wiring 131 connects the frame 132 to the electronic
device 150 and the battery 160.
[0037] The wiring 131 and the frame 132 may be made
of copper, nickel, gold, or alloys thereof. The
thickness of the wiring 131 and the frame 132 is 1 to
100 pm, preferably 1 to 50 pm, and more preferably 5
to 30 pm.
[0038] The two circuit units 130 are provided
corresponding to two through holes 113 of the
pressure-sensitive adhesive layer 110 and two through
holes 123 of the base material layer 120. The wiring
131 is connected to the electronic device 150 and the
terminal 135A of the battery 160 via the wiring on the
substrate 135. The frame 132 is a conductive member
shaped in a rectangular loop, which is larger than the
through hole 123 of the base material layer 120.
[0039] The substrate 133 has the same shape as the
wiring 131 and the frame 132 in a plan view. A part of
9
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
the substrate 133 in which the frame 132 is provided
has a rectangular frame area larger than the opening
of the through hole 123 of the base material layer
120. The frame 132 and the rectangular frame area of
the substrate 133 in which the frame 132 is formed,
are placed on the upper surface of the base material
layer 120 so as to surround the through hole 123. The
substrate 133 is made of an insulator, and a polyimide
substrate or film may be used as the substrate 133.
[0040] The substrate 135 is an insulative substrate
on which the electronic device 150 and the battery 160
are mounted, and it is provided on the upper surface
122 of the base material layer 120. For the substrate
135, a polyimide substrate or film may be used. Wiring
and the terminal 135A for the battery 160 are provided
on the upper surface of the substrate 135. The wiring
of the substrate 135 is connected to the electronic
device 150 and the terminal 135A, and is also
connected to the wiring 131 of the circuit unit 130.
[0041] The probe 140 is an electrode which comes
into contact with a subject, specifically, with the
skin 10, and configured to detect a biological signal
when the pressure-sensitive adhesive layer 110 is
attached to the skin 10. The biological signal is an
electric signal representing, for example,
electrocardiographic waveforms, brain waves
(electroencephalogram), pulsing, or the like.
[0042] The probe 140 is embedded in the pressure-
sensitive adhesive layer 110 so as to be exposed at
the lower surface 112 of the pressure-sensitive
adhesive layer 110. The probe 140 is not limited to
such a form exposed at the lower surface 112 of the
pressure-sensitive adhesive layer 110, and it may be
fabricated in any suitable forms capable of being in
contact with the skin 10. The probe may be integrated
with at least a part of the lower surface 112 of the
pressure-sensitive adhesive layer 110.
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
[0043] The electrode used as the probe 140 is
fabricated using a conductive composition containing
at least a conductive polymer and a binder resin, as
will be described later. Further, the electrode is
fabricated by punching the sheet-like member formed of
the conductive composition with a mold or the like, so
as to be used as the probe.
[0044] The probe 140 has a rectangular shape in a
plan view, which is larger than the through holes 113
of the pressure-sensitive adhesive layer 110 and the
through holes 123 of the base material layer 120. The
probe 140 has holes 140A arranged in a matrix. Along
the edges (i.e., the four sides) of the probe 140,
ladder-like protrusions extending in the X direction
and the Y direction may be formed. The electrode used
as the probe 140 may have a predetermined pattern.
Examples of the electrode pattern include a mesh
pattern, a stripe pattern, or such a pattern that
multiple electrodes are exposed at the sticking
surface.
[0045] The fixing tape 145 is an example of the
bonding part of the present embodiment. The fixing
tape 145 is, for example, a copper tape shaped into a
rectangular loop in a plan view. An adhesive is
applied to the lower surface of the fixing tape 145.
The fixing tape 145 is provided over the frame 132
along the four sides of the probe 140 so as to
surround the openings of the through holes 113 and 123
in a plan view to fix the probe 140 to the frame 132.
The fixing tape 145 may be a metal tape other than
copper.
[0046] The fixing tape 145 may be a non-conductive
tape such as a resin tape composed of a non-conductive
resin base and an adhesive, in place of a metal tape
such as a copper tape. However, a conductive or metal
tape may be preferable because the probe 140 can be
electrically connected to the frame 132 of the circuit
11
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
unit 130, while securing the probe 140 onto the frame
132.
[0047] The probe 140 is secured to the frame 132
such that the four sides overlap the frame 132, using
the fixing tape 145 which is applied so as to cover
the periphery of the probe 40. The fixing tape 145
adheres to the frame 132 through the holes 140A of the
probe 140.
[0048] The pressure-sensitive adhesive layer 110A
and the base material layer 120A are placed over the
fixing tape 145 and the probe 140, while keeping the
four sides of the probe 140 secured to the frame 132
by the fixing tape 145, and pressed downward into the
through holes 113 and 123. The probe 140 is pushed
into the through holes 113 and 123 along the inner
walls thereof, and the pressure-sensitive adhesive
layer 110A penetrates through the holes 140A of the
probe 140.
[0049] The probe 140 is pushed downward until the
center area of the probe 140 substantially aligns with
the lower surface 112 of the pressure-sensitive
adhesive layer 110, while keeping the four edges fixed
to the frame 132 by the fixing tape 145. Accordingly,
when the probe 140 is pressed against the skin 10 (see
FIG. 2) of a living body, the pressure-sensitive
adhesive layer 110A adheres to the skin 10 to keep the
probe 140 in tight contact with the skin 10.
[0050] The thickness of the probe 140 is preferably
less than that of the pressure-sensitive adhesive
layer 110. The thickness of the probe 140 is
preferably 0.1 to 100 pm, more preferably 1 to 50 pm.
[0051] The peripheral area of the pressure-
sensitive adhesive layer 110A, which forms a
rectangular frame area surrounding the center area in
the plan view, is located on the fixing tape 145.
Although in FIG. 2, the upper surface of the pressure-
sensitive adhesive layer 110A is substantially flat,
12
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
the center area may be slightly indented lower than
the peripheral area. In either case, the base material
layer 120A is superposed on the upper surface of the
pressure sensitive adhesive layer 110A.
[0052] The pressure-sensitive adhesive layer 110A
may be made of the same materials as the pressure-
sensitive adhesive layer 110, and the base material
layer 120A may be made of the same materials as the
base material layer 120. In an alternative, the
pressure-sensitive adhesive layer 110A may be made of
a different material from the pressure-sensitive
adhesive layer 110, or the base material layer 120A
may be made of a different material from the base
material layer 120.
[0053] Although in FIG. 2 the thicknesses of the
respective parts are exaggerated for clarifying the
structure, the thickness of the pressure-sensitive
adhesive layers 110 and 110A is 10 to 300 pm, and the
thickness of the base material layers 120 and 120A is
1 to 300 pm in the actual configuration. The thickness
of the wiring 131 may be 0.1 to 100 pm, the thickness
of the substrate 133 may be about several hundred
microns, and the thickness of the fixing tape 145 may
be 10 to 300 pm.
[0054] With the configuration of FIG. 2, in which
the probe 140 and the frame 132 are in direct contact
with each other to ensure electrical connection, the
fixing tape 145 may be a non-conductive resin tape or
the like.
[0055] In FIG. 2, the fixing tape 145 covers the
edges of the frame 132 and the substrate 133, together
with the sides of the probe 140, and it reaches the
upper surface of the base material layer 120. However,
the fixing tape 145 may not reach the upper surface of
the base material layer 120, or may not cover the
edges of the substrate 133 and the frame 132, because
it is sufficient for the fixing tape 145 to be able to
13
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
bond the probe 140 and the frame 132.
[0056] The substrate 133 and the two substrates 135
may be monolithically formed. In this case, wiring
131, two frames 132, and the terminal 135A are formed
on the surface of the monolithic substrate, on which
the electronic device 150 and the battery 160 are
mounted.
[0057] The electrode serving as the probe 140 may
be fabricated by thermosetting and molding a
conductive composition described below. The conductive
composition contains a conductive polymer, a binder
resin, and at least one of a cross-linking agent or a
plasticizer.
[0058] As the conductive polymer, for example,
polythiophene, polyacetylene, polypyrrole,
polyaniline, polyphenylene vinylene, or the like can
be used. Any one, or combinations of two or more of
these materials may be used. It is preferable to use,
among these, a polythiophene compound. From the
viewpoint of lower contact impedance with the living
body and higher conductivity, it is preferable to use
PEDOT/PSS obtained by doping polystyrene sulfonic acid
(poly 4-styrene sulfonate abbreviated as PSS) to poly
3,4-ethylenedioxythiophene (PEDOT).
[0059] The content of the conductive polymer is
preferably 0.20 to 20 parts by mass with respect to
100 parts by mass of the conductive composition. With
the above range of the conductive polymer,
satisfactory conductivity, toughness, and flexibility
can be imparted to the conductive composition. The
content of the conductive polymer is more preferably
2.5 to 15 parts by mass, and further preferably 3.0 to
12 parts by mass with respect to the conductive
composition.
[0060] As the binder resin, either a water-soluble
polymer or a water-insoluble polymer can be used. From
the viewpoint of compatibility with other components
14
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
contained in the conductive composition, it is
preferable to use a water-soluble polymer for the
binder resin. The water-soluble polymer includes a
hydrophilic polymer which may not be completely
soluble in water, but has hydrophilicity.
[0061] For the water-soluble polymer, a hydroxyl
group-containing polymer or the like can be used. The
hydroxyl group-containing polymer includes saccharides
such as agarose, polyvinyl alcohol (PVA), modified
polyvinyl alcohol, and a copolymer of acrylic acid and
sodium acrylate. Any one or a combination of two or
more of these materials may be used. Among these,
polyvinyl alcohol and modified polyvinyl alcohol are
preferable, and modified polyvinyl alcohol is more
preferable.
[0062] Examples of the modified polyvinyl alcohol
include acetacetyl group-containing polyvinyl alcohol,
and diacetone acrylamide modified polyvinyl alcohol.
As the diacetone acrylamide-modified polyvinyl
alcohol, a diacetone acrylamide-modified polyvinyl
alcohol-based resin (DA-modified PVA-based resin)
described in JP-A-2016-166436 can be used, for
example.
[0063] The content of the binder resin is
preferably 5 to 140 parts by mass, with respect to 100
parts by mass of the conductive composition. With this
content range of the binder resin, conductivity,
toughness, and flexibility can be satisfactorily
imparted to the conductive composition. The content of
the binder resin is more preferably 10 to 100 parts by
mass, and further preferably 20 to 70 parts by mass
with respect to the conductive composition.
[0064] The cross-linking agent and the plasticizer
have properties to impart toughness and flexibility to
the conductive composition. By imparting flexibility
to the molded product of the conductive composition,
an elastic electrode can be obtained. Thus, the probe
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
140 having elasticity is fabricated.
[0065] Toughness is a property that achieves both
strength and elongation (extensibility). Regarding the
toughness, one of the strength and the elongation may
be remarkably high, while the other is not remarkably
low, such that the strength and the elongation are
well balanced.
[0066] Flexibility is a property that can suppress
damage or breakage even when the molded body (i.e.,
the electrode sheet) of the conductive composition is
bent.
[0067] The cross-linking agent crosslinks the
binder resin. By mixing the cross-linking agent in the
binder resin, the toughness of the conductive
composition can be enhanced. Preferably, the cross-
linking agent has reactivity with a hydroxyl group.
Using such a cross-linking agent, the cross-linking
agent can react with the hydroxyl group contained in a
binder resin formed of a hydroxyl group-containing
polymer.
[0068] Examples of the cross-linking agent include
zirconium compounds such as zirconium salts; titanium
compounds such as titanium salts; borates such as
boric acid; isocyanate compounds such as blocked
isocyanate; aldehyde compounds such as dialdehyde
(e.g., glyoxal); alkoxyl group-containing compounds,
and methylol group-containing compounds. Any one or a
combination of two or more of these may be used. From
the viewpoint of reactivity and safety, a zirconium
compound, an isocyanate compound, and an aldehyde
compound are preferable.
[0069] The content of the cross-linking agent is
preferably 0.2 to 80 parts by mass, with respect to
100 parts by mass of the conductive composition. With
this content range of the cross-linking agent,
satisfactory toughness and flexibility can be imparted
to the conductive composition. The content of the
16
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
cross-linking agent is more preferably 1 to 40 parts
by mass, and more preferably 3.0 to 20 parts by mass.
[0070] The plasticizer improves the tensile
elongation and flexibility of the conductive
composition. Examples of the plasticizer include
glycerin, ethylene glycol, propylene glycol, sorbitol,
polyol compounds with these polymers, and aprotonic
compounds such as N-methylpyrrolidone (NMP),
dimethylformamide (DMF), NN'-dimethylacetamide (DMAc),
or dimethyl sulfoxide (DMSO). Any one or a combination
of two or more of these materials may be used. Among
these, glycerin is preferable from the viewpoint of
compatibility with other components.
[0071] The content of the plasticizer is preferably
0.2 to 150 parts by mass, with respect to 100 parts by
mass of the conductive composition. With this content
range of the plasticizer, satisfactory toughness and
flexibility can be imparted to the conductive
composition. The content of the plasticizer is more
preferably 1.0 to 90 parts by mass, and further
preferably 10 to 70 parts by mass with respect to 100
parts by mass of the conductive polymer.
[0072] Adding either the cross-linking agent or the
plasticizer to the conductive composition is
sufficient. By adding the cross-linking agent or the
plasticizer in the conductive composition, the
toughness and flexibility of the molded product of the
conductive composition can be improved.
[0073] If the conductive composition contains a
cross-linking agent, without containing a plasticizer,
the molded product of the conductive composition can
have further improved toughness, that is, both tensile
strength and tensile elongation, as well as improved
flexibility.
[0074] If the conductive composition contains a
plasticizer, without containing a cross-linking agent,
the tensile elongation of the molded product of the
17
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
conductive composition can be improved, and therefore,
toughness is imparted to the molded product of the
conductive composition as a whole. The flexibility of
the molded product of the conductive composition can
also be improved.
[0075] It may be preferable that both the cross-
linking agent and the plasticizer are contained in the
conductive composition. By adding both the cross-
linking agent and the plasticizer to the conductive
composition, the molded product of the conductive
composition has toughness more satisfactorily.
[0076] In addition to the above-described
components, the conductive composition may contain a
surfactant, a softener, a stabilizer, a leveling
agent, an antioxidant, an anti-hydrolysis agent, a
swelling agent, a thickener, a colorant, a filler, or
other known additives, at an appropriate ratio.
Examples of the surfactant include silicone-based
surfactants.
[0077] The conductive composition is prepared by
mixing the above-described components in the above-
described ratios.
[0078] The conductive composition may contain a
solvent in an appropriate ratio, as necessary. In this
case, an aqueous solution of the conductive
composition is appropriately prepared.
[0079] The solvent may be an organic solvent or an
aqueous solvent. Examples of the organic solvent
include ketones such as acetone or methyl ethyl ketone
(MEK); esters such as ethyl acetate; ethers such as
propylene glycol monomethyl ether; and amides such as
N, N-dimethylformamide. Examples of the aqueous
solvent include water; and alcohol for methanol,
ethanol, propanol, isopropanol, etc. Among these, an
aqueous solvent may be preferably used.
[0080] At least one of the conductive polymer, the
binder resin, and the cross-linking agent may be used
18
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
in a form of an aqueous solution dissolved in a
solvent. In this case, the above-described aqueous
solvent can be preferably used.
[0081] The electronic device 150 is provided on the
upper surface 122 of the base material layer 120 and
is electrically connected to the wiring 131. The
electronic device 150 processes a biological signal
acquired via the electrode used as the probe 140. The
electronic device 150 has a rectangular shape in the
cross-sectional view. Terminals are provided on the
lower surface (-Z direction) of the electronic device
150. Examples of the material for the terminals of the
electronic device 150 include solder, conductive
paste, or the like.
[0082] As shown in FIG. 1, the electronic device
150 may include an application-specific integrated
circuit (ASIC) 150A, a micro processing unit (MPU)
150B, a memory 150C, and a wireless communication unit
150D. The electronic device 150 is connected to the
probe 140 and the battery 160 via circuit unit 130.
[0083] The ASIC 150A includes an analog to digital
(A/D) converter. The electronic device 150 is driven
by the electric power supplied from the battery 160
and acquires the biological signal measured by the
probe 140. The electronic device 150 performs
processing such as filtering or digital conversion on
the biological signal. The MPU 150B may determine
arithmetic means by averaging the biological signals
acquired over multiple times, and it stores the
biological signals in the memory 150C. The electronic
device 150 can continuously acquire biological signals
over 24 hours, for example. Because the electronic
device 150 may be used for a long period of time to
measure biological signals, a configuration for
reducing power consumption is employed.
[0084] The wireless communication unit 150D is a
transceiver used when the biological signals are read
19
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
out from the memory 150C through radio communication
with a test device for evaluation test, at a frequency
of, for example, 2.4 GHz. The evaluation test is, for
example, a JIS 60601-2-47 standard test. The
evaluation test is performed after the assembling of
the biological sensor to confirm the performance of
the biological sensor which serves as a medical device
to detect biological signals. The evaluation test
requires that the attenuation ratio of the biological
signal output from the biological sensor with respect
to the input signal to the biological sensor is less
than 5%. This evaluation test is performed on all of
the final products.
[0085] As shown in FIG. 2, the battery 160 is
provided on the upper surface 122 of the base material
layer 120. A lead-acid battery, a lithium ion
secondary battery, or the like can be used as the
battery 160. The battery 160 may be a button cell or a
coin battery. The battery 160 has electrical terminals
provided on its bottom surface. Two terminals of the
battery 160 are connected to the probe 140 and the
electronic device 150 via the circuit unit 130. The
capacity of the battery 160 is determined so that the
electronic device 150 can measure the biological
signals for 24 hours or longer, for example.
[0086] The cover 170 covers the entirety of the
base material layer 120, the circuit unit 130, the
substrate 135, the probe 140, the fixing tape 145, the
electronic device 150, and the battery 160. The cover
170 has a base 170A and a protrusion 170B protruding
from the center of the base 170A in the +Z direction.
The base 170A shapes a basic form of the cover 170 in
a plan view, and extends in a plane lower than the
protrusion 170B. A recess 170C is formed on the bottom
side of the protrusion 170B. The bottom surface of the
base 170A of the cover 170 is adhered to the upper
surface 122 of the base material layer 120. The
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
substrate 135, the electronic device 150, and the
battery 160 are housed in the recess 170C. Thus, the
cover 170 is provided to the upper surface 122 of the
base material layer 120 with the electronic device 150
and the battery 160 accommodated in the recess 170C.
[0087] The cover 170 serves not only as a cover
protecting the circuit unit 130, the electronic device
150, and the battery 160 provided on the base material
layer 120, but also as a shock absorber to protect the
internal components from an impact applied from the
above to the stick-on biosensor 100. The cover 170 is
formed of, for example, silicone rubber, soft resin,
urethane, or the like.
[0088] FIG. 3 is a diagram showing a circuit
configuration of the stick-on biosensor 100. Each of
the probes 140 is connected to the electronic device
150 and the battery 160 via the wiring 131 and the
wiring 135B of the substrate 135. The two probes 140
are connected in parallel to the electronic device 150
and the battery 160.
[0089] Next, the flexural rigidity of the structure
101, and the adhesive strength of the probe part 143
will be described. The flexural rigidity of the
structure 101 is the bending rigidity of the sheet-
like structure 101 comprised of the pressure-sensitive
adhesive layer 110, the base material layer 120, and
the probe 140, which represents the flexural rigidity
per unit width (e.g., 1 mm) expressed in MPa.mm3/mm.
[0090] The adhesive strength of the probe part 143
is represented as the adhesive strength per unit area,
and expressed in newton per centimeters squared
[N/cm2]. The adhesiveness of the probe part 143 may be
imparted by the pressure-sensitive adhesive layer
110A. If the probe 140 itself has an adhesiveness,
then the adhesive strength of the probe part 143 is
determined by the total adhesiveness of the probe 140
and the pressure-sensitive adhesive layer 110A.
21
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
[0091] Accordingly, the adhesive strength of the
probe part 143 is represented as a force acting from
the pressure-sensitive adhesive layer 110A to a unit
area of the probe part 143 expressed in [N/cm2], or a
force acting from both the pressure-sensitive adhesive
layer 110A and the probe 140 to a unit area of the
probe part 143 expressed in [N/cm2].
[0092] The surface of the skin 10 to which the
stick-on biosensor 100 is attached may become uneven
due to the movements of the living body.
[0093] Even if the stick-on biosensor 100 is
attached onto a flat skin 10 of the living body in the
stationary state, the skin 10 may become wavy or
uneven afterward when the living body moves. If the
rigidity of the structure 101 and the adhesive
strength of the probe part 143 are appropriately high,
then the stick-on biosensor 100 strongly sticks to the
skin 10, and the skin 10 under the stick-on biosensor
100 will be maintained in the flat state.
[0094] This is because the stick-on biosensor 100
which strongly sticks to the skin 10 stretches on the
skin 10, while being pressed against the skin 10,
thereby preventing the skin 10 from being uneven. In
such a case, the probe 140 is in tight contact with
the skin 10, and biological signals can be measured in
a satisfactory state.
[0095] In contrast, if the rigidity of the
structure 101 and the adhesive strength of the probe
part 143 are insufficient, the skin 10 becomes uneven
as the living body moves. The stick-on biosensor 100
is pulled by the unevenness of the skin, and warps on
the skin. The stick-on sensor may partially come off
from the skin 10. In this case, the probe 140 peels
from the skin 10, and biological signals cannot be
acquired in satisfactory conditions.
[0096] Furthermore, a stick-on biosensor 100 having
a certain weight tends to come off from the skin 10 as
22
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
the living body moves, and there is a risk that
biological signals may not be acquired in satisfactory
conditions.
[0097] Therefore, in the embodiment, a stick-on
biosensor 100 is devised such that a satisfactory
biological signal can be acquired.
[0098] FIG. 4A to FIG. 4C show evaluation results
of the stick-on biosensor 100, which are acquired
while changing the values of the material, the
thickness [mm] of the upper part of the electrode, the
elastic modulus [MPa], the area moment of inertia
[mm3], the flexural rigidity [MPa=mm3/mm], and the
adhesive strength [N/cm2] of the base material layer
120.
[0099] The thickness of the upper part of the
electrode represents the total thickness of the
pressure-sensitive adhesive layer 110A and the base
material layer 120 positioned above the probe 140 in
the structure 101 (namely, the thickness determined by
subtracting the thickness of the probe 140 from the
thickness of the structure 101). The elastic modulus
is that of the structure 101. The area moment of
inertia is the area moment of inertia per unit width
of the structure 101, which indicates the difficulty
of deformation with respect to the bending moment. The
flexural rigidity is the flexural rigidity per unit
width of the structure 101. The adhesive strength is
the adhesive strength of the probe part 143 as
described above.
[0100] Thirty five (35) samples of the stick-on
biosensor 100 were prepared and evaluated, while
changing the values of the above-described parameters.
Seven items, namely, baseline fluctuation and noise of
ECG waveforms measured by the stick-on biosensor 100
attached to the chest during running, baseline
fluctuation and noise of ECG waveforms due to friction
23
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
from the clothes of the subject who wears the stick-on
biosensor 100 on the chest, signal evaluation, wearing
comfort, and pain in peeling off are evaluated. Based
on the total evaluation results of the seven items,
the thirty five samples are grouped into the first
group (samples 1 to 12) suitably used as the stick-on
sensor 100, and the second group (samples 2-1 to 2-23)
unsuitable for practical use.
[0101] Among the evaluation items, "during running"
refers to the state in which the subject with the
stick-on biosensor 100 attached to the chest is
running. "Friction from fabric" refers to the state in
which the neckline of the clothes the subject is
wearing is shaken up and down such that the clothes
are rubbed against the stick-on biosensor 100 attached
to the chest of the subject.
[0102] The baseline fluctuation is observed from
the electrocardiographic waveform obtained from the
stick-on biosensor 100, as shown in FIG. 5A and FIG.
5B. As in FIG. 5A, the case where the fluctuation of
the baseline is small and the electrocardiographic
data can be stably obtained is evaluated as good and
marked with an open circle (C)). The case where the
baseline fluctuates within a certain range as shown in
FIG. 5B is evaluated as acceptable and marked with an
open triangle (A). The case where the baseline
fluctuates beyond the range shown in FIG. 5B is
evaluated as not good and marked with a cross mark
(x) .
[0103] The signal evaluation includes assessment of
degree of baseline fluctuation, whether the
electrocardiographic waveform can be confirmed, and
whether the electrocardiographic waveform is observed
without being buried in noise. Wearing comfort is a
subjective evaluation as to how the subject feels when
wearing the stick-on biosensor 100. The pain in
peeling off indicates whether the subject feels pain
24
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
when the stick-on biosensor 100 is peeled off from the
skin after the measurement.
[0104] The pain in peeling off is evaluated by
verbal rating scale (VRS). VRS is assessment using
terms describing three levels of pain together with
scores, and choosing one of "no pain", "slightly
painful", and "painful". In this embodiment, "no pain"
and "slightly painful" are assessed as satisfactory
and marked with an open circle good (C)), and "painful"
is assessed as poor and marked with a cross mark (x).
The "painful" case is assessed as poor because the
skin is pulled when the probe electrode is peeled off,
which causes pain.
[0105] As to the material of the base material
layer 120, silicone rubber, polyethylene terephthalate
(PET), acrylic resin, and urethane rubber are used.
The thickness [mm] of the upper part electrode, the
elastic modulus [MPa], the flexural rigidity
[MPa.mm3/mm], and the adhesive strength [N/cm2] are set
to various values, as shown in FIG. 4A to FIG. 4C.
[0106] The evaluation results, except for the pain
assessment, are indicated as three different levels,
namely, good (marked with a circle), acceptable
(marked with triangle), and poor (marked with cross
mark). These samples marked with circles or triangles
(evaluated as good or acceptable) for all the seven
items successfully pass the evaluation test of the
stick-on biosensor 100. Samples 1-12 of the first
group have passed the test. A sample having a cross
mark in any one of the seven items is inappropriate
for the stick-on biosensor 100. Samples 2-1 to 2-23 of
the second group do not pass the test.
[0107] FIG. 6 is a chart summarizing the evaluation
results of FIG. 4A to FIG. 4C. The horizontal axis
represents the flexural rigidity, and the vertical
axis represents the adhesive strength of the probe
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
part 143. In FIG. 6, the acceptable samples 1 to 10
among the 35 samples are indicated by circles, and the
unacceptable samples 2-1 to 2-9 and 2-11 to 2-20 are
indicated by cross marks.
[0108] It is derived from FIG. 6 that the flexural
rigidity of the samples 1 to 10 is 0.010 or higher,
more preferably 0.034 or higher, and that the adhesive
strength of the probe part 143 is greater than 0.6 and
less than or equal to 3.5. Samples 2-1 to 2-6, which
are evaluated as unacceptable, have a flexural
rigidity of 0.034 or higher, and the adhesive strength
of the probe part 143 is within the above-described
range (greater than 0.6 and less than or equal to
3.5). However, these samples have poor wearing comfort
and are marked with cross marks. The reason for the
poor wearing comfort is that the flexural rigidity is
too strong and a strong tensile stress is applied to
the skin 10.
[0109] Therefore, the upper limit of the flexural
rigidity is 1.898, which is the maximum value among
the samples 1 to 9. Because the evaluation of the
wearing comfort is subjective, samples 2-1 to 2-6 may
be grouped in a feasible group as quasi-acceptable
samples.
[0110] Focusing on samples 1 to 10, 2-1 to 2-9, and
2-11 to 2-20, in order to allow the stick-on biosensor
100 to acquire satisfactory biological information in
spite of movements of the living body, the flexural
rigidity of the structure 101 is set to 0.034 or
higher, and the adhesive strength of the probe part
143 is set to the range greater than 0.6 and less than
or equal to 3.5, preferably set to the range from 1.0
to 2.5, and more preferably set in the vicinity of
1.3. If the flexural rigidity of the structure 101 is
within the range greater than 0.034 and less than or
equal to 1.898, the wearing comfort will be
satisfactory.
26
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
[0111] FIG. 7 is another chart summarizing the
evaluation results of FIG. 4A to FIG. 4C. The
horizontal axis represents the area moment of inertia
per unit width, and the vertical axis represents the
adhesive strength of the probe part 143. Among the 35
samples, samples 1 to 12 of the first group are
indicated by squares, and samples 2-1 to 2-23 of the
second group are indicated by black circles. The total
number of the symbols appears to be less than 35
because there are several samples having the same or
very close measurements.
[0112] In FIG. 7, the three samples having an
adhesive strength exceeding 5.0 [N/cm2] are samples 2-
21 to 2-23. The adhesive strength of these samples is
too strong, which causes pain when the stick-on
biosensor 100 is peeled off. It is desirable that the
adhesive strength is 5.0 [N/cm2] or less.
[0113] On the other hand, the samples having an
adhesive strength of 0.6 [N/cm2] are samples 2-11 to
2-20. The adhesive strength of these samples is too
low, causing a high noise, which makes it difficult to
acquire accurate electrocardiographic data. Hence, the
adhesive strength is preferably in the range greater
than 0.6 [N/cm2] and less than or equal to 5.0 [N/cm2].
More preferably, as described above, the adhesive
strength ranges from 1.0 [N/cm2] to 3.5 [N/cm2], and
still more preferably ranges from 1.3 [N/cm2] to 3.3
[N/cm2].
[0114] From FIG. 7, the area moment of inertia is
preferably in the range excluding the second group,
that is, in the range from 0.0001 [mm3] to 0.7000
[mm3], and more preferably from 0.0003 [mm3] to 0.2300
[mm3].
[0115] From the foregoing, the stick-on biosensor
100 of the embodiment can acquire a satisfactory
biological signal (or biological information), even if
the living body moves during measurement, by setting
27
Date Recue/Date Received 2021-09-20
CA 03134253 2021-09-20
the flexural rigidity of the structure 101 and the
adhesive force of the probe part 143 to the above-
described values.
[0116] Thus, a stick-on biosensor 100 capable of
acquiring satisfactory biological information can be
provided.
[0117] Although the stick-on biosensor has been
described above based on particular examples, the
present invention is not limited to the above-
described specific embodiments, and various
modification and substitutions are available without
departing from the scope of the appended claims.
[0118] The present application is based upon and
claims priority to earlier filed Japanese Patent
Application No. 2019-058328 filed March 26, 2019 and
to earlier filed Japanese Patent Application No. 2020-
036712 filed March 4, 2020. The entirety of both
earlier-filed Japanese patent applications identified
above are herein incorporated.
[0119] Listing of Symbols
100: stick-on biosensor
110: pressure-sensitive adhesive layer
120: base material layer
130: circuit unit
140: probe
145: fixing tape
150: electronic device
160: battery
170: cover
28
Date Recue/Date Received 2021-09-20