Note: Descriptions are shown in the official language in which they were submitted.
CA 03135512 2021-09-29
DESCRIPTION
METHOD FOR PREPARING 2-ARYLMALONIC ACID DERIVATIVE AND
INTERMEDIATE, AND USE THEREOF
TECHNICAL FIELD
This application relates to organic synthesis, and more particularly to a
method
for preparing a 2-arylmalonic acid derivative, and an intermediate, and use
thereof.
BACKGROUND
2-Arylmalonic acid derivatives, as an important class of organic compounds,
are
widely used in the preparation of materials, medicines and pesticides. For
example,
2-phenylmalonate is a significant raw material for the preparation of polymer
stabilizers (CN 102617450B); and 2-(2,6-diethyl-4-methylphenyl) malonate
diester
and 2-(2,6-diethyl-4-methylphenyl) malononitrile are crucial intermediates for
preparing highly-effective herbicide Pinoxaden (WO 00/78881).
Currently, the reported strategies for preparing the 2-arylmalonic acid
derivatives
may be divided into three categories according to the construction of C-C
bond.
The first type of strategy is characterized by constructing a skeleton of a
target
compound through the C-C coupling of a halogenated aromatic hydrocarbon and a
malonic acid derivative under the action of a catalyst (Journal of the
Chemical Society,
Chemical Communications (1984), (14), 932-3 , WO
00/78712 and WO
2004/050607). This method usually requires an expensive organometallic
catalyst,
and the catalyst is difficult to recycle, leading to a high cost. In addition,
active
halogenated aromatic hydrocarbons such as brominated or iodized aromatic
hydrocarbons are generally required as raw materials. However, the halogenated
aromatic hydrocarbons, especially those with different substituents at
specific
positions, are difficult to synthesize, and are usually prepared from the
corresponding
aniline by diazo-halogenation reaction. The diazo-halogenation reaction not
only
involves the generation of a large quantity of wastes, but also carries
problems of
safety concerns and halogen corrosion.
With respect to the second type of strategy, a phenylacetic acid derivative is
used
1
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
as a raw material and undergoes a condensation reaction with a dialkyl
carbonate in
the presence of a strong base (i.e., sodium hydride) to construct the skeleton
of the
target compound (Zi, W. and Toste, F.D. Gold(I)-Catalyzed Enantioselective
Desymmetrization of 1,3-Diols through Intramolecular Hydroalkoxylation of
Allenes.
Angew. Chem. Int. Ed., (2015), 54(48), 14447-14451). However, the phenylacetic
acid derivatives, especially the multi-substituted phenylacetic acid
derivatives, are
difficult to prepare. In addition, the strong base such as sodium hydride
(needing
anhydrous and oxygen-free operation) and the hydrogen produced by the reaction
will
cause major safety hazards. Therefore, this method is not suitable for
industrial
production.
The third type of strategy is to use a 2-(cyclohexenylidene)malonic acid
derivative as a raw material to obtain the target product through
dehydrogenation
reaction at 180-200 C in the presence of a metal catalyst (generally a noble
metal,
such as palladium) (WO 2018/120094). This method has high cost and high
reaction
temperature, and thus is not conducive to the industrial production.
SUMMARY
Aiming at the shortcomings of the prior art, the present disclosure provides a
method for preparing a 2-arylmalonic acid derivative, which is safe and
economical,
and thus suitable for industrial production.
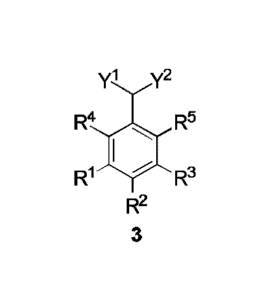
A method for preparing a 2-arylmalonic acid derivative of formula (3),
comprising:
(1) subjecting a compound (1) to an isomerization reaction to obtain an
intermediate (2), as shown in the following reaction scheme:
y1 y2 yl y2
R4 I R5 lsomerization R4 I R5
_>
R1 R3 R1 R3
R2 R2
1 2 ;and
(2) subjecting the intermediate (2) to a halogenation reaction in the presence
of a
halogenating agent and a dehydrohalogenation-aromatization reaction to obtain
the
2
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
2-arylmalonic acid derivative (3), as shown in the following reaction scheme:
yi y2 yl y2
R4 I R5 Halogenation R4 R5
R1
________________________________________________ ..-
Dehydrohalogenation-aromatization R1
R3 R3
R2 R2
2 3
,
wherein le, R2, R3, R4 and R5 each are independently hydrogen, a Ci-Cio alkyl
group, a C6-C12 aryl group or a heteroaryl group containing one or two atoms
selected
from nitrogen, oxygen and sulfur;
Yl and Y2 are each independently cyano or -COR6 where R6 is hydrogen, a
Ci-Cio alkyl group, a Ci-Cio alkoxy group, a C6-C12 aryloxy group, amino, a Ci-
Cio
alkylamino group, a C6-C12 arylamino group, a di-(Ci-Cio alkyl)-amino group, a
(Ci-Cio alkyl)-(C6-C12 aryl)-amino group, a di-(C6-C12 aryl) amino group, a C6-
C12
aryl group or a heteroaryl group containing one or two atoms selected from
nitrogen,
oxygen and sulfur.
In some embodiments, le, R2, R3, R4 and R5 are each independently hydrogen, a
Ci-C4 alkyl group or a C6-02 aryl.
In some embodiments, Yl and Y2 are each independently cyano, -COOMe,
-COOEt or -CONH2.
In some embodiments, in step (1), the isomerization reaction is carried out in
the
presence of a base; and the base is selected from the group consisting of an
alkali
metal hydroxide, an alkali metal alcoholate, an alkaline earth metal
hydroxide, an
alkaline earth metal alcoholate and a combination thereof, preferably sodium
hydroxide or sodium methoxide;
a molar ratio of the base to the compound (1) is (O.8-2.4):1, preferably
(1.O-1.2):1; and
in step (2), the halogenating reagent is selected from the group consisting of
an
elemental halogen (such as chlorine gas and liquid bromine), a hypohalous acid
(such
as hypochlorous acid and hypobromous acid), a sulfonyl halide (such as a
sulfuryl
chloride), a thionyl halide (such as thionyl chloride) and a combination
thereof,
preferably chlorine gas, sulfonyl chloride or liquid bromine.
3
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
In some embodiments, in step (2), the dehydrohalogenation-aromatization
reaction is carried out under an action of a catalyst; and the catalyst is
selected from
the group consisting of an alkali metal halide, an alkaline earth metal halide
and a
combination thereof, preferably lithium chloride or sodium chloride.
In some embodiments, a molar ratio of the catalyst to the intermediate (2) is
(0.005-2.4):1, preferably (0.02-0.1):1.
In some embodiments, in step (2), the dehydrohalogenation-aromatization
reaction is carried out at 0-150 C, preferably 110-150 C.
In some embodiments, the above preparation method of the 2-arylmalonic acid
derivative (3) is carried out in a one-pot manner.
The 2-arylmalonic acid derivative (3) prepared by the method mentioned above,
for example, 2-(2,6-diethyl-4-methylphenyl)malononitrile, can be used to
prepare
8-(2,6-diethyl-4-methylpheny1)-7-oxo-1,2,4,5-tetrahydro-7H-pyrazole[1,2-d]
[1,4,5]
oxadiazepine-9-pyrivalate (Pinoxaden) through further transformation and
reaction.
The beneficial effects of the present disclosure are described as follows.
(1) The reaction does not require expensive metal catalysts.
(2) The reaction avoids the use of strong bases with potential safety hazards.
(3) The reactions are safety, the conditions are mild, yield is high and cost
is low,
making it suitable for the industrial production.
DETAILED DESCRIPTION OF EMBODIMENTS
The technical solutions of the present disclosure will be further described
below
with reference to the embodiments, and the embodiments are not intended to
limit the
scope of the present disclosure.
The raw material 1 is prepared by a method known in the prior art (for
example,
W02018/120094).
Example 1 Preparation of 2-(2,6-diethyl-4-methyl-3-ene-1-cyclohexylidene)
malononitrile
To a 250 mL three-necked flask equipped with a magnetic stirrer and a
4
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
thermometer were sequentially added 85.0 g of methanol and 42.9 g of
2-(2,6-diethy1-4-methy1-2-ene-1-cyclohexylidene) malononitrile. The reaction
mixture
was stirred and heated to 50 C, and 10.8 g of sodium methoxide was added. The
reaction was stirred for 5 min. The reaction mixture was cooled, acidificated,
extracted, concentrated and separated to give 39.0 g of 2-(2,6-diethyl-4-
methyl-3-ene
-1-cyclohexylidene) malononitrile (91% yield).
1H NMR (CDC13, 500 MHz, TMS): 6 41 (m, 1H), 3.23 (m, 1H), 3.12 (q, J= 7.5
Hz, 1H), 2.40-2.35 (m, 1H), 2.15 (d, J = 17.5 Hz, 1H), 1.73 (d, J = 1.5 Hz,
3H),
1.68-1.59 (m, 4H), 1.13 (t, J= 7.5 Hz, 3H), 0.95 (t, J = 7.6 Hz, 3H).
13C NMR (CDC13, 125 MHz): 6 189.5, 131.7, 119.0, 111.9, 111.7, 84.8, 44.0,
43.0, 35.9, 30.5, 27.4, 23.3, 12.8, 12.2.
Example 2 Preparation of 2-(2,6-diethyl-4-methyl-3-ene-1-cyclohexylidene)
malononitrile
To a 250 mL three-necked flask equipped with a magnetic stirrer and a
thermometer were sequentially added 85.0 g of tetrahydrofuran and 42.9 g of
2-(2,6-diethy1-4-methy1-2-ene-1-cyclohexylidene) malononitrile. The reaction
mixture
was stirred and heated to 50 C, and 11.22 g of potassium hydroxide was added.
The
reaction was stirred for 30 min. The reaction mixture was cooled,
acidificated,
extracted, concentrated and separated to give 36.9 g
of
2-(2,6-diethy1-4-methy1-3-ene-1- cyclohexylidene) malononitrile (86% yield).
Example 3 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 250 mL three-necked flask equipped with a magnetic stirrer, a thermometer
and a reflux condenser were sequentially added 125 g of chlorobenzene and 53.5
g of
2-(2,6-diethy1-4-methy1-3-ene-1-cyclohexylidene) malononitrile prepared in
Example
1. The reaction mixture was stirred, cooled to 0 C, and introduced with
chlorine gas
until the reaction was complete. The reaction mixture was then concentrated,
and 200
mL of N,N-dimethylformamide and 0.42 g of LiC1 were sequentially added, and
refluxed until the reaction was complete. After that, the reaction mixture was
cooled,
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
acidificated, extracted, concentrated and separated to give 47.8 g of 2-(2,6-
diethy1-4-
methylphenyl) malononitrile (90% yield).
Example 4 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 500 mL three-necked flask equipped with a magnetic stirrer and a
thermometer were sequentially added 125.0 g of N,N-dimethylformamide and 64.4
g
of 2-(2,6-diethy1-4-methy1-3-ene-1-cyclohexylidene) malononitrile prepared in
Example 1. The reaction mixture was stirred, cooled to 0 C, and introduced
with
chlorine gas until the reaction was complete. The reaction mixture was then
concentrated, 300 mL of N-methylpyrrolidone was added and heated to 130 C
until
the reaction was complete. The reaction mixture was cooled to room
temperature, and
extracted, washed, concentrated and separated to give 51.0 g of 2-(2,6-diethy1-
4-
methylphenyl) malononitrile (80% yield).
Example 5 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 250 mL three-necked flask equipped with a magnetic stirrer, a thermometer
and a reflux condenser were sequentially added 85.0 g of acetic acid and 21.5
g of
2-(2,6-diethy1-4-methy1-3-ene-1-cyclohexylidene) malononitrile prepared in
Example
1. The reaction mixture was stirred, heated to 45 C, 60 g of an acetic acid
solution
containing 17.6 g of liquid bromine were added and reacted at 45 C for 2 h.
The
reaction solution was then concentrated, 100 mL of N,N-dimethylformamide and
0.95
g of LiBr sequentially were added and refluxed until the reaction was
complete. After
that, the reaction mixture was cooled, extracted, washed, concentrated and
separated
to give 10.6 g of 2-(2,6-diethyl-4-methylphenyl) malononitrile (50% yield).
Example 6 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 500 mL three-necked flask equipped with a magnetic stirrer and a
thermometer were sequentially added 170.0 g of acetic acid and 42.9 g of
2-(2,6-diethy1-4-methy1-3-ene-1-cyclohexylidene) malononitrile prepared in
Example
1. The reaction mixture was stirred, heated to 45 C, 29.8 g of sulfonyl
chloride was
6
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
dropwise added and reacted at 45 C for 1 h. The reaction mixture was then
concentrated, 200 mL of N,N-dimethylformamide was added and heated to 130 C
until the reaction was complete. After that, the reaction mixture was cooled
to room
temperature, and extracted, washed, concentrated and separated to give 30.0 g
of
2-(2,6-diethyl-4-methylphenyl) malononitrile (70% yield).
Example 7 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 250 mL three-necked flask equipped with a magnetic stirrer and a
thermometer were sequentially added 125.0 g of chlorobenzene and 64.4 g of
2-(2,6-diethy1-4-methy1-2-ene-1-cyclohexylidene) malononitrile. The reaction
mixture
was stirred, heated to 50 C, 16.2 g of sodium methoxide was added. The
reaction was
stirred for 5 min. The reaction mixture was then cooled to 0 C, and introduced
with
chlorine gas until the reaction was complete. After that, the reaction mixture
was
concentrated, 300 mL of N-methylpyrrolidone was added and heated to 110 C
until
the reaction was complete. The reaction mixture was cooled, extracted, washed,
concentrated and separated to give 49.0 g of 2-(2,6-diethyl-4-methylphenyl)
malononitrile (77% yield).
Example 8 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 500 mL three-necked flask equipped with a magnetic stirrer, a thermometer
and a reflux condenser were sequentially added 125.0 g of chlorobenzene and
53.5 g
of 2-(2,6-diethy1-4-methy1-2-ene-1-cyclohexylidene) malononitrile. The
reaction
mixture was stirred, heated to 50 C, 13.4 g of sodium methoxide added. The
reaction
was stirred for 5 min. The reaction mixture was cooled to 0 C, and introduced
with
chlorine gas until the reaction was complete. Subsequently, the reaction
mixture was
desolventized, 200 g of N,N-dimethylformamide and 0.85 g of LiC1 were
sequentially
added and refluxed until the reaction was completed. After that, the reaction
mixture
was concentrated, washed and separated to give 47.2 g of 2-(2,6-diethy1-4-
methylphenyl) malononitrile (89% yield).
7
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
Example 9 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 500 mL three-necked flask equipped with a magnetic stirrer, a thermometer
and a reflux condenser were sequentially added 125.0 g of chlorobenzene and
53.5 g
of 2-(2,6-diethy1-4-methy1-2-ene-1-cyclohexylidene) malononitrile. The
reaction
mixture was stirred, heated to 50 C, 13.4 g of sodium methoxide was added. The
reaction was stirred for 5 min. The reaction mixture was cooled to 0 C, and
introduced with chlorine gas until the reaction was complete. Subsequently,
the
reaction mixture was desolventized, 200 g of N,N-dimethylformamide and 1.17 g
of
NaCl were sequentially added and refluxed until the reaction was completed.
The
reaction mixture was concentrated, washed and separated to give 45.6 g of
2-(2,6-diethyl-4-methylphenyl) malononitrile (86% yield).
Example 10 Preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
To a 250 mL three-necked flask equipped with a magnetic stirrer and a
thermometer were sequentially added 85.0 g of tetrahydrofuran and 42.9 g of
2-(2,6-diethy1-4-methy1-2-ene-1-cyclohexylidene) malononitrile. The reaction
mixture
was stirred, heated to 50 C, 8.0 g of sodium hydroxide was added. The reaction
was
stirred for 5 min. After being cooled to room temperature, the reaction
mixture was
added with 32.7 g of a 5% sodium hypochlorite solution, adjusted to pH 3-4
with 10%
hydrochloric acid, reacted at room temperature under stirring for 30 min and
added
with ethyl acetate for extraction. The organic phase was collected, washed,
dried and
concentrated, and then 200 mL of N,N-dimethylformamide was added. The reaction
mixture was refluxed until the reaction was complete, and cooled,
acidificated,
washed, concentrated and separated to give 29.5 g of 2-(2,6-diethyl-4-
methylphenyl)
malononitrile (70% yield).
Example 11 Preparation of methyl 2-cyano-2-(2,6-diethyl-4-methylphenyl)
acetate
To a 250 mL three-necked flask equipped with a magnetic stirrer, a thermometer
and a reflux condenser were sequentially added 60.0 g of ethyl acetate and
30.0 g of
8
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
methyl 2-cyano-2-(2,6-di ethy1-4 -methy1-2-ene-l-cyclohexy li
dene)acetate. The
reaction mixture was stirred, heated to 50 C, 6.8 g of sodium methoxide was
added .
The reaction was stirred for 5 min. The reaction mixture was cooled to 5 C,
and was
introduced with chlorine gas until the reaction was complete. The reaction
mixture
was then desolventized, 100 mL of N,N-dimethylformamide and 0.22 g of LiC1
were
sequentially added and refluxed until the reaction was complete. After that,
the
reaction mixture was concentrated, washed and separated to give 23.1 g of
methyl
2-cyano-2-(2,6-diethyl-4-methylphenyl) acetate (81% yield).
1H NMR (CDC13, 500 MHz, TMS): 6 6.95 (s, 2H), 3.80 (s, 3H), 2.76-2.59 (m,
4H), 2.32 (s, 3H), 1.24 (t, J= 9.5 Hz, 6H).
13C NMR (CDC13, 125 MHz): 6 166.5, 142.8, 139.2, 128.2, 123.9, 115.9, 53.7,
36.8, 26.3, 21.1, 15Ø
Example 12 Preparation of 2-(2,6-diethyl-4-methylphenyl) malonamide
To a 100 mL three-necked flask equipped with a magnetic stirrer and a
thermometer were sequentially added 3.6 g of water and 50.0 g of concentrated
sulfuric acid. The reaction mixture was stirred, heated to 45 C, 21.2 g of
2-(2,6-diethyl-4-methylphenyl) malononitrile was slowly added. The reaction
was
stirred for 5 h at 50 C. Then the reaction mixture was cooled, poured into ice
water,
and extracted with ethyl acetate. The organic phases were combined, dried and
concentrated to give 24.1 g of 2-(2,6-diethyl-4-methylphenyl) malonamide (97%
yield).
Example 13 Synthesis of Pinoxaden
To a 250 mL three-necked flask equipped with a magnetic stirrer, a thermometer
and a reflux condenser were sequentially added 24.8 g of
2-(2,6-diethyl-4-methylphenyl) malonamide prepared in Example 12, 21.0 g of
[1,4,51-oxydiazepine dihydrochloride, 125.0 g of chlorobenzene and 40.4 g of
triethylamine. The reaction mixture was refluxed for reaction. After the
reaction was
completed, the reaction mixture was cooled to room temperature, 21.6 g of
pivaloyl
9
Date Recue/Date Received 2021-09-29
CA 03135512 2021-09-29
DESCRIPTION
chloride was slowly added and reacted at room temperature under stirring for 2
h. The
reaction mixture was then adjusted to pH 3-4 with diluted hydrochloric acid,
and
extracted with ethyl acetate. The organic phases were combined, dried,
concentrated
and crystallized with hexane to give 29.6 g of Pinoxaden (74% yield).
1-11 NMR (CDC13, 500 MHz, TMS): 6 8.88 (s, 2H), 4.28-4.26 (m, 2H), 3.94-3.93
(m, 2H), 3.89-3.83 (m, 4H), 2.56-2.47 (m, 2H), 2.45-2.40 (m, 2H), 2.39 (s,
3H), 1.12
(t, J = 9.0 Hz, 3H), 1.23 (s, 9H).
Date Recue/Date Received 2021-09-29