Note: Descriptions are shown in the official language in which they were submitted.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
1
HMOX1 INDUCERS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
62/833,031,
filed on April 12, 2019 and U.S. Provisional Application No. 62/932,629, filed
on November
8, 2019. The entire teachings of the aforementioned applications are
incorporated herein by
reference.
FIELD OF THE INVENTION
This application is directed to HMOX1 (heme oxygenase 1) inducers, and methods
for their use, such as to control the activity or the amount, or both the
activity and the
amount, of heme-oxygenase in a mammalian subject.
BACKGROUND OF THE INVENTION
Oxidative stress represents an imbalance between cellular reactive oxygen
species
(ROS) production and cellular responses to ROS such as degrading ROS species
and
producing endogenous anti-oxidant molecules.
ROS serve critical cellular signaling needs, but can have deleterious effects
if
overproduced or left unchecked. Increased ROS levels in a cell can result in
damage to
components such as lipids, proteins, polysaccharides, and DNA. Prolonged
oxidative stress
is also linked to chronic diseases that affect nearly every major organ
system. For example,
prolonged oxidative stress is implicated in the onset or progression of
disease states such as
neurodegenerative diseases, lung diseases, cardiovascular diseases, renal
diseases, diabetes,
inflammatory pain, and cancer. Accordingly, strategies to mitigate oxidative
stress are
desirable for a number of therapeutic settings.
Under normal physiological conditions, production of ROS is counterbalanced by
a
well-defined and conserved set of cellular pathways that respond to, limit,
and repair the
damage due to ROS. This adaptive set of genes are called the phase II system.
They encode
enzymes that degrade ROS directly as well as increase levels of cells'
endogenous
antioxidant molecules, including glutathione and bilirubin.
Of the phase II enzyme system, HMOX1, a human gene that encodes for the enzyme
heme oxygenase 1, has been found to be a key component. The role of HMOX1 is
to
metabolize heme into bilirubin, carbon monoxide, and free iron by a two-step
process. The
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
2
first and rate-limiting step is the production of biliverdin and carbon
monoxide from heme by
HMOX1. The second step is the production of bilirubin from biliverdin by
biliverdin
reductase. Both bilirubin and carbon monoxide have been shown to scavenge ROS
and to
have potent anti-oxidant and anti-inflammatory activities.
Agents that induce production of HMOX1 have been shown to have beneficial
activity in models of diabetes, cardiovascular disease, hypertension, and
pulmonary function.
Heme, heavy metal ions (e.g., arsenite, cadmium, iron, lead, chromium and
mercury), and
electrophiles (e.g., natural products such as sulforaphane and curcumin) can
all induce
production of HMOX1. Induction of HMOX1 and other phase II genes are
controlled by a
number of transcription factors that are responsive to heavy metals, heme, and
electrophiles.
The transcription factors Nrf2, Bachl, and small Maf proteins are particularly
important in
this process. For example, a common sequence called antioxidant responsive
element (ARE)
is present in a promoter of each gene of the phase II enzymes, and its
expression is induced
by the transcription factor Nrf2 (NF-E2 related factor 2).
HMOX1 is also induced as part of a generalized stress response to stimuli such
as
thermal shock, oxidative stress and cytokines such as interleukin-1 (IL-1),
tumor necrosis
factor and interleukin-6 (IL-6).This stress response is seen as beneficial in
that it results in
protection of vulnerable cells from multiple insults.
It has been reported that HMOX1 can be induced by small molecules that bind to
the
transcription factor Bachl. Heme binding to Bachl has been shown to reduce DNA
binding
activity of Bachl and induce gene transcription. See Ogawa K et al. EMB 0 J
(2001)
20:2835-284. Additionally, a small molecule has been reported to induce HMOX1
through
binding to Bachl. See Attucks OC et al.
PLOS ONE (2014) 9(7): e101044,
W02011/103018 and W02012/094580.
As such, there is a need for new HMOX1 inducers and/or Bach 1 binders/
inhibitors
for the above referenced therapeutic indications.
SUMMARY OF THE INVENTION
The applicant has discovered novel compounds which are effective HMOX1
inducers
(see Examples 1-142). In particular, it has been demonstrated that certain
compounds of the
present invention effectively induced production of HMOX1 (see Example 143).
Also,
certain compounds of the invention have a combination of desirable properties,
including
potent HMOX1 induction activity (Example 143), significantly reduced hERG
inhibition
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
3
compared to certain comparator compounds (see Example 145), good solubility
(Example
144) and a strong impact on HMOX1 protein expression in vivo (Example 151).
Moreover,
the applicant has also discovered that the compounds disclosed herein bind to
Bach 1 (see
Example 146).
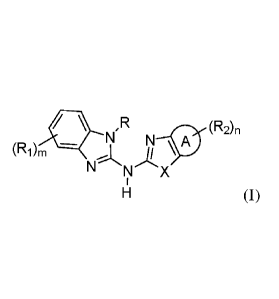
In one embodiment, provided herein is a compound represented by the following
structural Formula (I):
(R2)n
(Ri)m N
.)...., N 10
A \
NNX
1
H (I);
or a pharmaceutically acceptable salt thereof, the definition of each variable
is provided
below.
Pharmaceutical compositions of the compounds of the invention are also
disclosed
herein. Particular embodiments comprise a pharmaceutically acceptable carrier
or diluent
and one or more of the compounds of the invention, or a pharmaceutically
acceptable salt
thereof.
The compounds of Formula (I) and pharmaceutically acceptable salts thereof are
useful
as agents that induce the production of and/or increase the activity of HMOX1,
and thus may
be useful to treat various chronic diseases that are associated, at least in
part, with oxidative
stress including, but not limited to: fibrotic diseases, neurodegenerative
disease,
cardiovascular disease, renal disease, inflammatory disease, liver disease,
eye disease, thyroid
disease, viral infection, osteoporosis, pregnancy disorders, endometriosis,
diabetes, cancers,
skin diseases, mitochondrial diseases, hematological disorders, and muscle
diseases.
Another embodiment of the present invention comprises treating the above-
referenced
diseases or conditions in a subject by administering to the subject an
effective amount of one
or more compounds of the invention, or a pharmaceutically acceptable salt
thereof, or a
pharmaceutical composition comprising the disclosed compound(s).
Also provided herein is the use of one or more compounds of the invention, or
a
pharmaceutically acceptable salt thereof, or a pharmaceutical composition
comprising one or
more compounds of the invention, for the preparation of a medicament for the
treatment of the
above-referenced diseases or conditions.
In another embodiment, provided herein is the compounds of the invention, or a
pharmaceutically acceptable salt thereof, or a pharmaceutical composition
comprising one or
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
4
more of the disclosed compounds for use in treating the above-referenced
diseases or
conditions.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. lA and 1B depict graphs showing significant fold change of Gene-Specific
mRNA in HepG2 cells treated with the compound of Example 17 or Nrf2 activator
DMF.
FIG. lA depicts that the compound of Example 17 induces HMOX1 expression much
more
strongly than DMF. FIG. 1B depicts that the compound of Example 17 and DMF
induce
FTH1 expression with comparable fold increases.
FIG. 2 depicts a graph showing that the compound of Example 17 dose
responsively
inhibit microvascular stasis in Townes-SS sickle mice. Townes-SS mice were
gavaged once
daily with Vehicle or the compound of Example 17 for 8 days. After the last
gavage dorsal
skin-fold chambers were implanted on the mice and 20-22 flowing venules were
selected and
mapped in the subcutaneous skin using intravital microscopy. Mice were then
infused via the
tail vein with Panhematin (3.2 mols heme/kg body weight). One hour after
infusion each
venule was re-examined for microvascular stasis (no flow) and data are
expressed as %
stasis. Stasis values are means + SD. One-Way ANOVA with Dunnett's Multiple
comparison
to Vehicle ****P<0.0001
FIG. 3 depicts a graph showing the percentage of F-cells increase in Townes-SS
mice
treated with the compound of Example 17. Blood smears were made using
heparinized
whole blood for F-cells staining. Stained F-cells and total red blood cells
were counted on the
blood smears in four fields; mean = 77 red blood cells/field. F-cells are
expressed as a
percentage of total red blood cells. Values are means + SD. One-Way ANOVA with
Dunnett's Multiple comparison to Vehicle **p < 0.01, ***p < 0.001, ****p <
0.0001
FIG. 4 depicts a graph showing the glutathione (GSH) levels in primary human
endothelial cells. Two-Tailed Unpaired t test compared to DMSO p <
0.0001, One-
Way ANOVA with Dunnett's Multiple comparison to Hemin (GSH Graph) ****p <
0.0001
FIG. 5 depicts a graph showing fold change of gene expression in primary human
endothelial cells. p < 0.0001, One-Way ANOVA with Dunnett's Multiple
comparison
to TNFa (VCAM graph) ****p < 0.0001
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
DETAILED DESCRIPTION
In response to elevated reactive oxygen species (ROS) levels, cells induce
expression
of oxidative stress-responsive genes, such as genes encoding proteins that
degrade ROS or
increase levels of the cell's endogenous antioxidant molecules. One such gene
is HMOX1.
Induction of expression of HMOX1 and other oxidative stress-responsive genes
is regulated
in part by the transcription factor Nrf2. Under basal conditions, the adaptor
protein Keapl
forms a heterodimer with Nrf2, targeting Nrf2 for proteolysis and suppressing
Nrf2-mediated
transcription. Upon exposure of cells to chemical electrophiles or agents that
elevate ROS,
the interaction of Keapl with Nrf2 is weakened and Nrf2 levels in the cell
increase, which in
turn increases Nrf2 levels in the nucleus and leads to induction of oxidative
stress-responsive
genes. Nrf2 activity is also regulated by the transcriptional repressor Bachl,
which occludes
binding of Nrf2 to the promoter region of oxidative stress-responsive genes.
In order to mitigate the effects of oxidative stress in a cell, e.g., in
disease settings, it
is therefore desirable to identify compounds that promote the induction of
expression of
oxidative stress-responsive genes, for example, compounds that modulate the
interaction of
Nrf2 with Keapl or the interaction of Bachl with the Maf recognition element
(MARE) to
increase cytoprotective gene transcription. However, it is also desirable that
such compounds
not act as electrophiles or otherwise incite a stress response in the cell.
Compounds of the Invention
Disclosed herein are embodiments of compounds having a general structure of
Formula (I).
In a first embodiment, the invention provides a compound represented by the
following structural formula (I):
4* N'R N
(R2)n
(R1)m 0
A
N
- N X
1
H (I);
or a pharmaceutically acceptable salt thereof, wherein:
Ring A is 5-7 membered monocyclic cycloalkyl, 5-7 membered monocyclic
heterocyclyl, 5-6 membered heteroaryl, or phenyl;
X is -S-, -0-, or
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
6
when X is -S- and ring A is phenyl, then R is 3-7 membered monocyclic
heterocyclyl
optionally substituted with one or more groups selected from the group
consisting of halo,
-CN, -OH, -(Ci-C4)alkyl, -(Ci-C4)haloalkyl, -(Ci-C4)hydroxyalkyl, -(Ci-
C4)alkoxy, 4C1-
C4)haloalkoxy, and -NRaRa;
when X is -S- and ring A is 5-7 membered monocyclic cycloalkyl, 5-7 membered
monocyclic heterocyclyl, or 5-6 membered heteroaryl; or when X is -0- or -NRb-
; then R is
-H, -(Ci-C4)alkyl, or -(CH2),3-7 membered monocyclic heterocyclyl, wherein the
4C1-
C4)alkyl or 3-7 membered monocyclic heterocyclyl represented by R is
optionally substituted
with one or more groups selected from the group consisting of halo, -CN, -OH, -
(Ci-C4)alkyl,
-(C i-C4)halo alkyl, -(Ci-C4)hydroxyalkyl, -(Ci-C4)alkoxy, -(Ci-C4)haloalkoxy,
-S 02Ra, and -
NRaRa;
each R1 is independently -H, halogen, -(CH2)kCOOH, -(CH2)kCO(Ci-C4)alkyl,
-(CH2)kCOO(Ci-C4)alkyl, -(CH2)pC(=0)NRaR3, -CH(CF3)NRaR3, -C(=NOH)CF3, or
-CH(CF3)0R3, wherein the (Ci-C4)alkyl in the group represented by R1 is
optionally
substituted with one or more groups selected from the group consisting of
halo, -CN,
-OH, -(Ci-C4)hydroxyalkyl, -(Ci-C4)alkoxy, -(Ci-C4)haloalkoxy, and -NRaRa;
each R2 is independently -H, halo, CN, -
OH, -(Ci-C4)alkoxy,
-COOH, -C(=O)(C -C4)alkyl, -
C(=0)0(C -C4)alkyl, -C(=0)NRa(C -C4)alkyl,
-NRaRa, 3-6 membered monocyclic cycloalkyl, -0(CH2),3-7 membered monocyclic
heterocyclyl, 3-7 membered monocyclic heterocyclyl, or 5-6 membered
heteroaryl, wherein
the -(Ci-C4)alkyl, -(Ci-C4)alkoxy, heterocyclyl, or heteroaryl represented by
R2 or in the
group represented by R2 is optionally substituted with one or more groups
selected from the
group consisting of halo, -CN, -OH, -(Ci-C4)alkyl, -(Ci-C4)alkoxy, -(Ci-
C4)haloalkyl,
-(C1-C4)hydroxyalkyl, -(Ci-C4)haloalkoxy, and -NRaRa;
each R3 is independently H, (Ci-C4)alkyl, (Ci-C4)alkoxy, -(CH2)03-7 membered
monocyclic heterocyclyl, -(CH2)0NH3-7 membered monocyclic heterocyclyl, 5-6
membered
heteroaryl, -0(CH2)p2NRaC(=0)(C -C4)alkyl, -(CH2)p20(CH2)p20(C=0)(C -C4)alkyl,
or
(CH2)p2NRaC(=0)(C1-C4)alkyl, wherein the alkyl, alkoxy, heteroaryl, or
heterocyclyl
represented by R3 or in the group represented by R3 is optionally substituted
with one or more
groups selected from the group consisting of halo, -CN, -OH, -(Ci-C4)alkyl,
-(C 1-C4)halo alkyl, -(C -C4)hydroxyalkyl, -(Ci -C4)alkoxy, -(Ci -C4)halo
alkoxy, -(C 1-
C4)c arbo xyalko xy, -S (0)2(C i-C4)alkyl, -(Ci-C4)hydroxyalkoxy, and -NRaRa;
each Ra is independently -H or -(Ci-C4)alkyl;
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
7
each Rb is independently -H or -(Ci-C4)alkyl; wherein the -(Ci-C4)alkyl
represented
by Rb is optionally substituted with one or more groups selected from halo, -
CN, -OH, -(C3-
C6)cycloalkyl, phenyl, 3-7 membered monocyclic heterocyclyl, and 5-6 membered
hetero aryl;
i is 0 or 1;
k is 0, 1, 2, 3, or 4;
m is 0, 1, 2, 3, or 4;
n is 0, 1, 2, 3, or 4;
p is 0, 1, 2, 3, or 4;
Pi is 0, 1,2, 3, or 4; and
p2 is 2, 3, or 4.
In a second embodiment, the invention provides a compound according to the
previous embodiment, wherein the compound is represented by the following
structural
formula (II):
Ri ID. N,R iii (R2, N or 2
N
A
- N X
I
H (II);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In a third embodiment, the invention provides a compound according to the
first
embodiment, wherein the compound is represented by structural formula (II'):
Ri =N N \
N
õJN NA ) 2)i or 2
X
I
H (if);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In a fourth embodiment, the invention provides a compound according to the
first or
second embodiment, wherein the compound is represented by structural formula
(II-A):
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
8
,R R2
Ri
NJNN Sjr) (II-A);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In a fifth embodiment, the invention provides a compound according to the
first or
second embodiment, wherein the compound is represented by structural formula
(II-B):
= ,R
Ri R2
NNN 1-)
0
(II-B);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In a sixth embodiment, the invention provides a compound according to the
first or
second embodiment, wherein the compound is represented by structural formula
(IT-C):
,R R2
Ri
NJNN I¨)
N
Rb (MC);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In a seventh embodiment, the invention provides a compound according to the
first or
third embodiment, wherein the compound is represented by structural formula
(II'-A):
411 ,R
R1
N/ __ or 1
\
N NA S
(II'-A);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In an eighth embodiment, the invention provides a compound according to the
sixth
embodiment, or a pharmaceutically acceptable salt thereof, wherein Rb is -H or
-(Ci-C4)alkyl,
wherein the -(Ci-C4)alkyl represented by Rb is optionally substituted with one
or more groups
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
9
selected from halo, -CN, and ¨OH. wherein the remainder of the variables are
as defined in
the first embodiment. In a preferred embodiment, Rb is -H.
In a ninth embodiment, the invention provides a compound according to the
first,
second, third, fifth, sixth, seventh, and eighth embodiment, or a
pharmaceutically acceptable
salt thereof, wherein R is -(Ci-C4)alkyl optionally substituted with -(Ci-
C4)alkoxy; or ¨
(CH2),3-6 membered monocyclic heterocyclyl optionally substituted with one or
more groups
selected from the group consisting of halo, -CN, -OH, -(Ci-C4)alkyl, -(Ci-
C4)haloalkyl, -(C1-
C4)hydro xyalkyl, -(Ci-C4)alkoxy, -(Ci-C4)haloalkoxy, and -NRaRa, wherein the
remainder of
the variables are as defined in the first or eighth embodiment.
In a tenth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, or eighth embodiment, or a
pharmaceutically
acceptable salt thereof, wherein R is azetidinyl, azepanyl, morpholinyl,
pyrrolidinyl,
piperidinyl, piperazinyl, oxetanyl, tetrahydrofuranyl, or tetrahydropyranyl,
each of which is
optionally substituted with one or more groups selected from the group
consisting of halo,
-OH, -(Ci-C4)alkyl, and -NH2, wherein the remainder of the variables are as
defined in the
first or eighth embodiment.
In an eleventh embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, or tenth
embodiment, or a
pharmaceutically acceptable salt thereof, wherein R1 is -(CH2)kCOOH, -
(CH2)kCO(Ci-
C4)alkyl optionally substituted with halo, -(CH2)pC(=0)NRaR3, -C(=NOH)CF3, or
-CH(CF3)NRaR3, wherein the remainder of the variables are as defined in the
first, eighth,
ninth or tenth embodiment.
In a twelfth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, or
eleventh embodiment, or a
pharmaceutically acceptable salt thereof, wherein R2 is H; halogen; CN; -(Ci-
C4)alkyl
optionally substituted with halo; -(Ci-C4)alkoxy optionally substituted with
halo, hydroxy,
methoxy, or ethoxy; -C(=0)(Ci-C4)alkyl; 3-4 membered monocyclic cycloalkyl;
¨0(CH2),3-7
membered monocyclic heterocyclyl, 3-7 membered monocyclic heterocyclyl, or 5-6
membered heteroaryl, wherein the heterocyclyl, or heteroaryl represented by R2
or in the
group represented by R2 is optionally substituted with halo, hydroxy, or -(Ci-
C4)alkoxy,
wherein the remainder of the variables are as defined in the first, eighth,
ninth, tenth, or
eleventh embodiment. In a preferred embodiment, R2 is H or -0CF3.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
In a thirteenth embodiment, the invention provides a compound according to the
first,
second, third, fifth, sixth, seventh, eighth, ninth, eleventh, or twelfth
embodiment, or a
pharmaceutically acceptable salt thereof, wherein R is -(Ci-C4)alkyl
optionally substituted
with methoxy or -S02CH3; -(CH2)tetrahydrofuranyl; -(CH2)oxetanyl optionally
substituted
with hydroxy; pyrrolidinyl; piperidinyl; or tetrahydropyranyl; wherein the
pyrrolidinyl or
piperidinyl is optionally substituted with -(Ci-C4)alkyl, wherein the
remainder of the
variables are as defined in the first, eighth, ninth, tenth, eleventh, or
twelfth embodiment. In
a preferred embodiment, the pyrrolidinyl or piperidinyl is optionally
substituted with -CH3.
In a fourteenth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh,
twelfth, or thirteenth
embodiment, or a pharmaceutically acceptable salt thereof, wherein R1 is -
COOH,
-C(=0)CF3, -CH(CF3)(NH2), -C(=NOH)CF3, or -C(=0)NHR3, wherein the remainder of
the
variables are as defined in the first, eighth, ninth, tenth, eleventh,
twelfth, or thirteenth
embodiment.
In a fifteenth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh,
twelfth, thirteenth, or
fourteenth embodiment, or a pharmaceutically acceptable salt thereof, wherein
R3 is
(C 1 -C4)a1kyl, (C 1 -C4)a1koxy, (CH2)0 3-6 membered monocyclic heterocyclyl,
or
(CH2)p2NHC(=0)(Ci-C4)a1kyl, wherein the alkyl, alkoxy, or heterocyclyl
represented by R3
or in the group represented by R3 is optionally substituted with one or more
groups selected
from the group consisting of halo, -OH, -(Ci-C4)alkyl, -(C i-C4)halo alkyl,
-(C 1 -C4)hydroxyalkyl, -(C 1 -C4)alkoxy, -(C
1 -C4)haloalkoxy, -S (0)2(C i-C4)alkyl,
-(Ci-C4)hydroxyalkoxy, and -NRaRa, wherein the remainder of the variables are
as defined in
the first, eighth, ninth, tenth, eleventh, twelfth, thirteenth, or fourteenth
embodiment.
In a sixteenth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh,
twelfth, thirteenth,
fourteenth, or fifteenth embodiment, or a pharmaceutically acceptable salt
thereof, wherein
R2 is H; halogen; CN; -(Ci-C4)alkyl optionally substituted with halo; -(Ci-
C4)alkoxy
optionally substituted with halo, hydroxy, methoxy, or ethoxy; -C(=0)(Ci-
C4)alkyl;
cyclopropyl; 0-tetrahydropyranyl; N-pyrolidinyl; or thiazolyl, wherein the
remainder of the
variables are as defined in the first, eighth, ninth, tenth, eleventh,
twelfth, thirteenth,
fourteenth, or fifteenth embodiment.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
11
In a seventeenth embodiment, the invention provides a compound according to
the
fifth embodiment, wherein the compound is represented by structural formula
(III-B):
R1
= Me N R
N A
- N 0
(III-B);
or a pharmaceutically acceptable salt thereof, wherein the remainder of the
variables are as
defined in the first embodiment.
In an eighteenth embodiment, the invention provides a compound according to
the
first, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth,
eleventh, twelfth,
thirteenth, fourteenth, fifteenth, sixteenth, or seventeenth embodiment, or a
pharmaceutically
acceptable salt thereof, wherein R1 is -COOH or -C(=0)NHR3; R3 is -(Ci-
C4)alkyl,
-hydro xy(C i-C4)alkyl, -
methoxy(Ci-C4)alkyl, - amino (C -C4)alkyl,
-(Ci-C4)hydroxyalkoxy(Ci-C4)alkyl, or -(CH2)p2NHC(=0)(dimethylamino(Ci-
C4)alkY1),
wherein the remainder of the variables are as defined in the first, eighth,
ninth, tenth,
eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, or
seventeenth embodiment.
In a nineteenth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh,
twelfth, thirteenth,
fourteenth, fifteenth, sixteenth, seventeenth, or eighteenth embodiment, or a
pharmaceutically
acceptable salt thereof, wherein R2 is H, halo, -(C i-C4)halo alkyl, -(Ci-
C4)haloalkoxy, -(C 1-
C4)alkoxy optionally substituted with methoxy, or -N-pyrrolidinyl, wherein the
remainder of
the variables are as defined in the first, eighth, ninth, tenth, eleventh,
twelfth, thirteenth,
fourteenth, fifteenth, sixteenth, seventeenth, or eighteenth embodiment. In a
preferred
embodiment, R2 is H, F, CF3, OCHF2, OCF3, OCH2CH2OCH3, or -N-pyrrolidinyl.
In a twentieth embodiment, the invention provides a compound according to the
first,
second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh,
twelfth, thirteenth,
fourteenth, fifteenth, sixteenth, seventeenth, eighteenth, or nineteenth
embodiment, or a
pharmaceutically acceptable salt thereof, wherein
R2 is H, R1 is -C(=0)NH(Ci-C4)alkyl optionally substituted with ¨OH, NH2,
oxetanyl,
or -(Ci-C4)hydroxyalkoxy; or -C(=0)NH(CH2)p2NHC(=0)(dimethylamino(Ci-
C4)alkyl); or
R2 is F, R1 is -C(=0)NH(methoxy(Ci-C4)alkyl); or
R2 is -N-pyrrolidinyl, R1 is -C(=0)NH((Ci-C4)hydroxyalkoxy(Ci-C4)alkY1),
R2 is CF3, OCHF2, or OCF3, R1 is -COOH; or
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
12
R2 is OCH2CH2OCH3, R1 is -C(=0)NH((C -C4)hydroxyalkoxy(C -C4)alkY1),
-C(=0)NH(hydroxy(C -C4)alkY1), -
C(=0)NH(methoxy(Ci-C4)alkY1), or
-C(=0)NH(hydroxy(C -C4)alkoxy) ;
wherein the remainder of the variables are as defined in the first, eighth,
ninth, tenth,
eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, seventeenth,
eighteenth, or
nineteenth embodiment.
In a twentyfirst embodiment, the invention provides a compound according to
the
seventeenth embodiment, or a pharmaceutically acceptable salt thereof, wherein
R1 is -C(=0)NH(Ci-C4)alkyl optionally substituted with ¨OH, NH2, -(Ci-
C4)alkoxy,
or -(Ci-C4)hydroxyalkoxy, and
R2 is H, or OCH2CH2OCH3
In a twentysecond embodiment, the invention provides a compound according to
the
twentyfirst embodiment, or a pharmaceutically acceptable salt thereof, wherein
R2 is H,
wherein the remainder of the variables are as defined in the twentyfirst
embodiment.
In a twentythird embodiment, the invention provides a compound according to
the
twentysecond embodiment, or a pharmaceutically acceptable salt thereof,
wherein
R1 is -C(=0)NH(Ci-C4)alkyl substituted with -(Ci-C4)hydroxyalkoxy,
wherein the remainder of the variables are as defined in the twentysecond
embodiment.
In a twentyfourth embodiment, the invention provides a compound according to
the
twentyfirst embodiment, or a pharmaceutically acceptable salt thereof, wherein
R1 is -C(=0)NH(Ci-C4)alkyl substituted with -(Ci-C4)alkoxy, and
R2 is OCH2CH2OCH3.
In one embodiment, the compound or a pharmaceutically acceptable salt thereof
is
selected from the compounds disclosed in examples and Table 1.
Definitions
The term "pharmaceutically-acceptable salt" refers to a pharmaceutical salt
that is,
within the scope of sound medical judgment, suitable for use in contact with
the tissues of
humans and lower animals without undue toxicity, irritation, and allergic
response, and is
commensurate with a reasonable benefit/risk ratio. Pharmaceutically-acceptable
salts are
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
13
well known in the art. For example, S. M. Berge et al. describes
pharmacologically
acceptable salts in J. Pharm. Sci., 1977, 66, 1-19.
Included in the present teachings are pharmaceutically acceptable salts of the
compounds disclosed herein. Compounds having basic groups can form
pharmaceutically
acceptable salts with pharmaceutically acceptable acid(s).
Suitable pharmaceutically
acceptable acid addition salts of the compounds described herein include salts
of inorganic
acids (such as hydrochloric, hydrobromic, phosphoric, metaphosphoric, nitric,
and sulfuric
acids) and of organic acids (such as acetic, benzenesulfonic, benzoic,
ethanesulfonic,
methanesulfonic, and succinic acids). Compounds of the present teachings with
acidic
groups such as carboxylic acids can form pharmaceutically acceptable salts
with
pharmaceutically acceptable base(s). Suitable pharmaceutically acceptable
basic salts include
ammonium salts, alkali metal salts (such as sodium and potassium salts) and
alkaline earth
metal salts (such as magnesium and calcium salts).
The term "halo" as used herein means halogen and includes chloro, fluoro,
bromo and
iodo.
The term "alkyl" used alone or as part of a larger moiety, such as "alkoxy" or
"haloalkyl" and the like, means saturated aliphatic straight-chain or branched
monovalent
hydrocarbon radical. Unless otherwise specified, an alkyl group typically has
1-5 carbon
atoms, i.e. (Ci-05)alkyl. As used herein, a "(Ci-C4)alkyl" group means a
radical having from
1 to 4 carbon atoms in a linear or branched arrangement. Examples include
methyl, ethyl, n-
propyl, iso-propyl, and the like.
The term "alkoxy" means an alkyl radical attached through an oxygen linking
atom,
represented by ¨0-alkyl. For example, "(Ci-C4)alkoxy" includes methoxy,
ethoxy, propoxy,
and butoxy.
The terms "haloalkyl" and "haloalkoxy" means alkyl or alkoxy, as the case may
be,
substituted with one or more halogen atoms.
The term "cycloalkyl" refers to a monocyclic saturated hydrocarbon ring
system.
Unless otherwise specified, cycloalkyl has from 3-7 carbon atoms. For example,
a C3_C6
cycloalkyl includes cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
Unless otherwise
described, a "cycloalkyl" has from three to six carbon atoms.
The term "heteroaryl", "heteroaromatic", "heteroaryl ring", "heteroaryl
group",
"heteroaromatic ring", or "heteroaromatic group", used alone or as part of a
larger moiety as
in "heteroaralkyl" or "heteroarylalkoxy", refers to monocyclic aromatic ring
groups having
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
14
five or six ring atoms (i.e., "5-6 membered") selected from carbon and at
least one (typically
1 to 4, more typically 1 or 2) heteroatoms (e.g., oxygen, nitrogen, or
sulfur).
Examples of monocyclic heteroaryl groups include furanyl (e.g., 2-furanyl, 3-
furanyl),
imidazolyl (e.g., N-imidazolyl, 2- imidazo lyl, 4-imidazo lyl, 5- imid azo
ly1), isoxazolyl ( e.g., 3 -
iso xazo lyl, 4- iso xazo lyl, 5- iso xazo ly1), oxadiazolyl (e.g., 2-o
xadiazo lyl, 5-o xadiazo ly1),
oxazolyl (e.g., 2-oxazolyl, 4-oxazolyl, 5-oxazoly1), pyrazolyl (e.g., 3-
pyrazolyl, 4-pyrazoly1),
pyrrolyl (e.g., 1-pyrrolyl, 2-pyrrolyl, 3-pyrroly1), pyridyl (e.g., 2-pyridyl,
3-pyridyl, 4-
pyridyl), pyrimidinyl (e.g., 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl),
pyridazinyl (e.g., 3-
pyridazinyl), thiazolyl (e.g., 2-thiazo lyl, 4-thiazo lyl, 5-thiazoly1),
triazolyl (e.g., 2-triazo lyl, 5-
triazolyl), tetrazolyl (e.g., tetrazolyl), thienyl (e.g., 2-thienyl, 3-
thienyl), pyrimidinyl,
pyridinyl and pyridazinyl.
The term "heterocyclyl" refers to a monocyclic non-aromatic ring radical
containing
from 3-7 ring atoms (i.e., "3-7 membered") selected from carbon atom and 1 or
2
heteroatoms. Each heteroatom is independently selected from nitrogen,
quaternary nitrogen,
oxidized nitrogen (e.g., NO); oxygen; and sulfur, including sulfoxide and
sulfone.
Representative heterocyclyl groups include morpholinyl, thiomorpholinyl,
pyrrolidinonyl,
pyrrolidinyl, piperidinyl, piperazinyl, hydantoinyl, valerolactamyl, oxiranyl,
oxetanyl,
tetrahydrofuranyl, tetrahydropyranyl,
tetrahydropyrindinyl, tetrahydropyrimidinyl,
tetrahydrothiophenyl, tetrahydrothiopyranyl, and the like. A "substituted
heterocylyl group"
is substituted at any one or more substitutable ring atom, which is a ring
carbon or ring
nitrogen atom bonded to a hydrogen.
As used herein, many moieties (e.g., alkyl, alkylene, cycloalkyl,
cycloalkylene, aryl,
arylene, heteroaryl, heteroarylene, heterocyclyl or heterocyclylene) are
referred to as being
either "substituted" or "optionally substituted". When a moiety is modified by
one of these
terms, unless otherwise noted, it denotes that any portion of the moiety that
is known to one
skilled in the art as being available for substitution can be substituted,
which includes one or
more substituents. Where if more than one substituent is present, then each
substituent may
be independently selected. Such means for substitution are well-known in the
art and/or
taught by the instant disclosure. The optional substituents can be any
substituents that are
suitable to attach to the moiety.
Suitable substituents are those which do not have a significant adverse effect
on the
ability of the compound to induce HMOX1. Where suitable substituents are not
specifically
enumerated, exemplary substituents include, but are not limited to: (Ci-
05)alkyl, (Ci-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
C5)hydroxyalkyl, (Ci -05)halo alkyl, (C -05) alkoxy, i-
05) haloalkoxy, halogen,
hydroxyl, -CN, -NH2, -NO2, -ORci, _NRalRbl, _s(0)iiRal, _NRals(0)iiRbl,
S(0)1INRaiRbi, -
C(=0)0Ral, -0C(=0)0Ral, -C(=S)0Ral, -0(C=S)Ral,
-C(=0)NRaiRbi, -NRalC(=0)Rbi, -
c(=s)NRalRbl,
NRalc(=s)Rbl,
NRal(C=0)0Rbi, -0(C=0)NRaiRbi, -NRal(C=S)ORbi, -0(C=S)NRalRbl,
_NRal
(C=0)NRaiRbi, -NRal(C=S)NRaiRbi, -C(=S)Ral, -C(=0)Ral, phenyl, or 5-6 membered
heteroaryl. Each Ral and each Rbi are independently selected from ¨H and (Ci-
05)alkyl,
optionally substituted with hydroxyl or (Ci-C3)alkoxy; Rci is ¨H, (Ci-
05)haloalkyl or (C1-
05)alkyl, wherein the (Ci-05)alkyl is optionally substituted with hydroxyl or
(Ci-C3)alkoxy;
and ii is 1 or 2.
Pharmaceutical Compositions
The compounds disclosed herein are HMOX1 inducers. The pharmaceutical
composition of the present invention comprises one or more HMOX1 inducers, or
a
pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable
carrier or diluent.
"Pharmaceutically acceptable carrier" and "pharmaceutically acceptable
diluent" refer
to a substance that aids the formulation and/or administration of an active
agent to and/or
absorption by a subject and can be included in the compositions of the present
disclosure
without causing a significant adverse toxicological effect on the subject. Non-
limiting
examples of pharmaceutically acceptable carriers and/or diluents include
water, NaCl, normal
saline solutions, lactated Ringer's, normal sucrose, normal glucose, binders,
fillers,
disintegrants, lubricants, coatings, sweeteners, flavors, salt solutions (such
as Ringer's
solution), alcohols, oils, gelatins, carbohydrates such as lactose, amylose or
starch,
hydroxymethycellulose, fatty acid esters, polyvinyl pyrrolidine, and colors,
and the like.
Such preparations can be sterilized and, if desired, mixed with auxiliary
agents such as
lubricants, preservatives, stabilizers, wetting agents, emulsifiers, salts for
influencing osmotic
pressure, buffers, coloring, and/or aromatic substances and the like that do
not deleteriously
react with or interfere with the activity of the compounds provided herein.
One of ordinary
skill in the art will recognize that other pharmaceutical excipients are
suitable for use with
disclosed compounds.
The pharmaceutical compositions of the present invention optionally include
one or
more pharmaceutically acceptable carriers and/or diluents therefor, such as
lactose, starch,
cellulose and dextrose. Other excipients, such as flavoring agents,
sweeteners, and
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
16
preservatives, such as methyl, ethyl, propyl and butyl parabens, can also be
included. More
complete listings of suitable excipients can be found in the Handbook of
Pharmaceutical
Excipients (5th Ed., Pharmaceutical Press (2005)). A person skilled in the art
would know
how to prepare formulations suitable for various types of administration
routes.
Conventional procedures and ingredients for the selection and preparation of
suitable
formulations are described, for example, in Remington's Pharmaceutical
Sciences (2003 -
20th edition) and in The United States Pharmacopeia: The National Formulary
(USP 24
NF19) published in 1999. The carriers, diluents and/or excipients are
"acceptable" in the
sense of being compatible with the other ingredients of the pharmaceutical
composition and
not deleterious to the recipient thereof.
Methods of Treatment
In certain embodiments, the invention provides methods of increasing the
activity of
or the amount of HMOX1 in a human subject comprising: administering to a human
subject
an effective amount of the compounds of the invention, or a pharmaceutically
acceptable salt
thereof, or an effective amount of the pharmaceutical composition thereof.
In certain embodiments, the invention provides methods of activating
transcription
factor Nrf2 in a human subject comprising: administering to a human subject an
effective
amount of the compounds of the invention, or a pharmaceutically acceptable
salt thereof, or
an effective amount of the pharmaceutical composition thereof.
In certain embodiments, the invention provides methods of reducing the amount
of
ROS in a human subject comprising: administering to a human subject an
effective amount of
the compounds of the invention, or a pharmaceutically acceptable salt thereof,
or an effective
amount of the pharmaceutical composition thereof.
In certain embodiments, the invention provides methods for using an effective
amount
of the compounds of the invention, or a pharmaceutically acceptable salt
thereof, or an
effective amount of the pharmaceutical composition thereof. The compounds of
the
invention and pharmaceutical compositions thereof may be useful for a variety
of therapeutic
applications including, for example, treating and/or reducing a wide variety
of diseases and
disorders including, for example, fibrotic diseases, neurodegenerative
disease, cardiovascular
disease, renal disease, inflammatory disease, liver disease, eye disease,
thyroid disease, viral
infection, osteoporosis, pregnancy disorders, endometriosis, diabetes,
cancers, skin diseases,
mitochondrial diseases, hematological disorders, and muscle diseases. The
methods
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
17
comprise administering to a subject in need thereof a pharmaceutically
effective amount of
one or more compounds of the invention, a pharmaceutically acceptable salt
thereof, and/or
pharmaceutical compositions thereof.
Compounds that increase levels or activity of HMOX1 are potentially useful in
treating diseases or conditions that may be associated at least in part with
oxidative stress
such as, but not limited to, fibrotic diseases, neurodegenerative disease,
cardiovascular
disease, renal disease, inflammatory disease, liver disease, eye disease,
thyroid disease, viral
infection, osteoporosis, pregnancy disorders, endometriosis, diabetes,
cancers, skin diseases,
mitochondrial diseases, hematological disorders, and muscle diseases. As used
herein, the
diseases or conditions associated with oxidative stress also include chronic
effects (e.g.,
tissue damage, chronic inflammation) associated with persistent or long-term
increases in
oxidative stress due to the diseases or conditions described herein.
Fibrotic diseases associated with oxidative stress include, but are not
limited to,
fibrotic diseases of the lung such as chronic obstructive pulmonary disease
(COPD),
idiopathic pulmonary fibrosis, and sarcoidosis; fibrotic diseases of the liver
including those
caused by alcoholic cirrhosis, steatosis, cholestasis, drug side effect, and
viral infection; and
fibrotic diseases of the skin including autoimmune diseases such as
scleroderma and
psoriasis.
Neurodegenerative diseases associated with oxidative stress include, but are
not
limited to, Friedreich's ataxia, Alzheimer's disease, Parkinson's disease,
amyotrophic lateral
sclerosis, multiple sclerosis, cerebral nerve degenerative disease, and
Charcot-Marie-Tooth
syndrome.
Cardiovascular diseases associated with oxidative stress include, but are not
limited
to, hypertension, heart failure, hypercholesterolaemia, atherosclerosis,
arteriosclerosis,
thrombosis, acute coronary thrombosis, deep vein thrombosis, peripheral
vascular disease,
congestive heart failure, acute coronary syndrome, failure of arterial fistula
for dialysis,
ischemia reperfusion injury, primary pulmonary hypertension, primary pulmonary
arterial
hypertension, and secondary pulmonary arterial hypertension.
Renal diseases associated with oxidative stress include, but are not limited
to, acute
kidney injury, polycystic kidney disease, Alport syndrome, diabetic
nephropathy, glomerular
nephritis, lupus nephritis, sickle cell nephropathy, and acute tubular
necrosis.
Inflammatory diseases associated with oxidative stress include, but are not
limited to,
asthma, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis,
inflammatory
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
18
bowel syndrome, Crohn's disease, celiac disease, ulcerative colitis, chronic
inflammatory
bowel disease, scleroderma, dermatitis, systemic lupus erythematosus,
esophagitis, vasculitis,
pancreatitis, tendonitis, osteoarthritis, rheumatoid arthritis, ankylo sing
spondylitis, and
chronic inflammation of the brain.
Liver diseases associated with oxidative stress include, but are not limited
to, drug
induced liver toxicity, nonalcoholic steatohepatitis, and hepatitis, e.g.,
hepatitis B infection
and hepatitis C infection.
Eye diseases and conditions associated with oxidative stress include, but are
not
limited to, conjunctivitis, glaucoma, uveitis, wound healing (e.g., after
surgery such as
LASIK), eye trauma, corneal grafts, Fuchs' endothelial corneal dystrophy,
macular
degeneration, cataracts, light retinopathy, retinitis pigmentosa, diabetic
retinopathy, and
retinopathy of prematurity, as well as inflammation and tissue damage
associated with these
diseases.
Thyroid diseases associated with oxidative stress include, but are not limited
to,
Graves' disease, follicular adenoma, and papillary and follicular carcinomas.
Lung diseases associated with oxidative stress include, but are not limited
to,
bronchitis, asthma, chronic obstructive pulmonary disease, idiopathic
pulmonary fibrosis,
pulmonary bronchitis, bronchiectasis, pulmonary edema, and emphysema.
Skin diseases associated with oxidative stress include, but are not limited
to,
dermatitis, scleroderma, and psoriasis.
Viral infections associated with oxidative stress include both viral
replication of
viruses, as well as tissue damage (e.g., fibrosis) due to oxidative stress
resulting from chronic
viral infection, for viruses including but are not limited to human
immunodeficiency virus,
hepatitis B, hepatitis C, and herpesvirus.
Diabetic conditions include, but are not limited to, type 1 diabetes mellitus,
type 2
diabetes mellitus, gestational diabetes, pre-diabetes, hyperglycemia, and
metabolic syndrome
as well as secondary conditions resulting from diabetic conditions (e.g.,
congestive heart
failure and nephropathy).
Mitochondrial disease associated with oxidative stress include, but are not
limited to,
mitochondrial myopathies, Leber's hereditary optic neuropathy (LHON),
myoclonic epilepsy
with ragged red fibers (MERFF), mitochondrial encephalomyopathy, lactic
acidosis and
stroke-like episodes (MELAS) or Leigh's Syndrome.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
19
Hematological disorders associated with oxidative stress include, but are not
limited
to, Diamond Blackfan anemia, myelodysplastic syndrome, sickle cell disease and
beta-
thalessemia.
Muscle diseases associated with oxidative stress include, but are not limited
to,
Duchenne muscular dystrophy, limb girdle muscular dystrophy, Becker muscular
dystrophy,
myotonic dystrophy and rhabdomyolysis.
Cancers associated with oxidative stress include, but are not limited to,
breast cancer,
colorectal cancer, lung cancer, ovarian cancer, uterine cancer, prostate
cancer, leukemias,
lymphomas, brain cancer (including glioblastoma multiforme and neuroblastoma),
head and
neck cancer, pancreatic cancer, melanoma, hepatocellular carcinoma, renal
cancer, and soft
tissue sarcomas. In one embodiment, the cancer is breast cancer, colon cancer,
and ovarian
cancer. In one embodiment, the cancer is selected from leukemia, acute myeloid
leukemia,
chronic myelogenous leukemia, breast cancer, brain cancer, colon cancer,
colorectal cancer,
head and neck cancer, hepatocellular carcinoma, lung adenocarcinoma,
metastatic melanoma,
pancreatic cancer, prostate cancer, ovarian cancer and renal cancer. In one
embodiment, the
cancer is lung cancer, colon cancer, brain cancer, neuroblastoma, prostate
cancer, melanoma,
glioblastoma multiforme or ovarian cancer. In another embodiment, the cancer
is lung cancer,
breast cancer, colon cancer, brain cancer, neuroblastoma, prostate cancer,
melanoma,
glioblastoma multiforme or ovarian cancer. In yet another embodiment, the
cancer is breast
cancer, colon cancer and lung cancer. In another embodiment, the cancer is a
breast cancer.
In yet another embodiment, the cancer is a basal sub-type breast cancer or a
luminal B sub-
type breast cancer. In yet another embodiment, the cancer is a basal sub-type
breast cancer.
In yet another embodiment, the basal sub-type breast cancer is ER (estrogen
receptor), HER2
and PR (progesterone receptor) negative breast cancer. In yet another
embodiment, the
cancer is a soft tissue cancer. A "soft tissue cancer" is an art-recognized
term that
encompasses tumors derived from any soft tissue of the body. Such soft tissue
connects,
supports, or surrounds various structures and organs of the body, including,
but not limited to,
smooth muscle, skeletal muscle, tendons, fibrous tissues, fatty tissue, blood
and lymph
vessels, perivascular tissue, nerves, mesenchymal cells and synovial tissues.
Thus, soft tissue
cancers can be of fat tissue, muscle tissue, nerve tissue, joint tissue, blood
vessels, lymph
vessels, and fibrous tissues. Soft tissue cancers can be benign or malignant.
Generally,
malignant soft tissue cancers are referred to as sarcomas, or soft tissue
sarcomas. There are
many types of soft tissue tumors, including lipoma, lipoblastoma, hibernoma,
liposarcoma,
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
leio myo ma, leiomyo sarcoma, rhabdo myo ma, rhabdomyo sarcoma, neuro fibro
ma,
schw anno ma (neurilemo ma) , neuro ma, malignant schw anno ma, neurofibro
sarcoma,
neurogenic sarcoma, nodular tenosynovitis, synovial sarcoma, hemangioma,
glomus tumor,
hemang ioperic yto ma, hemang io endo thelio ma,
angio sarcoma, Kaposi sarcoma,
lymphangioma, fibroma, elastofibroma, superficial fibromatosis, fibrous
histiocytoma,
fibro sarcoma, fibro mato sis, dermatofibro sarcoma protuberans (DFSP),
malignant fibrous
histiocytoma (MFH), myxoma, granular cell tumor, malignant mesenchymomas,
alveolar
soft-part sarcoma, epithelioid sarcoma, clear cell sarcoma, and desmoplastic
small cell tumor.
In a particular embodiment, the soft tissue cancer is a sarcoma selected from
the group
consisting of a fibro sarcoma, a gastrointestinal sarcoma, a leiomyo sarcoma,
a dedifferentiated
liposarcoma, a pleomorphic liposarcoma, a malignant fibrous histiocytoma, a
round cell
sarcoma, and a synovial sarcoma.
Thus the present invention provides a method of treatment comprising
administering
to a subject a compound of Formula (I) or a pharmaceutically acceptable salt
thereof so as to
treat at least one of the diseases or conditions listed above.
A "subject" is a mammal, preferably a human, but can also be an animal in need
of
veterinary treatment, e.g., companion animals (e.g., dogs, cats, and the
like), farm animals
(e.g., cows, sheep, pigs, horses, and the like) and laboratory animals (e.g.,
rats, mice, guinea
pigs, and the like).
Methods of Administration and Dosage Forms
The precise amount of compound administered to provide an "effective amount"
to
the subject will depend on the mode of administration, the type, and severity
of the disease or
condition, and on the characteristics of the subject, such as general health,
age, sex, body
weight, and tolerance to drugs. The skilled artisan will be able to determine
appropriate
dosages depending on these and other factors. When administered in combination
with other
therapeutic agents, e.g., when administered in combination with an anti-cancer
agent, an
"effective amount" of any additional therapeutic agent(s) will depend on the
type of drug
used. Suitable dosages are known for approved therapeutic agents and can be
adjusted by the
skilled artisan according to the condition of the subject, the type of
condition(s) being treated
and the amount of a compound of the invention being used by following, for
example,
dosages reported in the literature and recommended in the Physician's Desk
Reference (57th
Ed., 2003).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
21
The term "effective amount" means an amount when administered to the subject
which results in beneficial or desired results, including clinical results,
e.g., inhibits,
suppresses or reduces the symptoms of the condition being treated in the
subject as compared
to a control. For example, a therapeutically effective amount can be given in
unit dosage
form (e.g., 0.1 mg to about 50 g per day, alternatively from 1 mg to about 5
grams per day;
and in another alternatively from 10 mg to 1 gram per day).
The terms "administer", "administering", "administration", and the like, as
used
herein, refer to methods that may be used to enable delivery of compositions
to the desired
site of biological action. These methods include, but are not limited to,
intraarticular (in the
joints), intravenous, intramuscular, intratumoral, intradermal,
intraperitoneal, subcutaneous,
orally, topically, intrathecally, inhalationally, transdermally, rectally, and
the like.
Administration techniques that can be employed with the agents and methods
described
herein are found in e.g., Goodman and Gilman, The Pharmacological Basis of
Therapeutics,
current ed.; Pergamon; and Remington's, Pharmaceutical Sciences (current
edition), Mack
Publishing Co., Easton, Pa.
In addition, the disclosed HMOX1 inducers can be co-administered with other
therapeutic agents. As used herein, the terms "co-administration",
"administered in
combination with", and their grammatical equivalents, are meant to encompass
administration of two or more therapeutic agents to a single subject, and are
intended to
include treatment regimens in which the agents are administered by the same or
different
route of administration or at the same or different times. In some embodiments
the one or
more compounds described herein will be co-administered with other agents.
These terms
encompass administration of two or more agents to the subject so that both
agents and/or
their metabolites are present in the subject at the same time. They include
simultaneous
administration in separate compositions, administration at different times in
separate
compositions, and/or administration in a composition in which both agents are
present. Thus,
in some embodiments, the compounds described herein and the other agent(s) are
administered in a single composition. In some embodiments, the compounds
described
herein and the other agent(s) are admixed in the composition.
The particular mode of administration and the dosage regimen will be selected
by the
attending clinician, taking into account the particulars of the case (e.g. the
subject, the
disease, the disease state involved, the particular treatment). Treatment can
involve daily or
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
22
multi-daily or less than daily (such as weekly or monthly etc.) doses over a
period of a few
days to months, or even years. However, a person of ordinary skill in the art
would
immediately recognize appropriate and/or equivalent doses looking at dosages
of approved
compositions for treating a disease using the disclosed HMOX1 inducers for
guidance.
The compounds or the corresponding pharmaceutical compositions taught herein
can
be administered to a patient in a variety of forms depending on the selected
route of
administration, as will be understood by those skilled in the art. The
compounds of the
present teachings may be administered, for example, by oral, parenteral,
buccal, sublingual,
nasal, rectal, patch, pump or transdermal administration and the
pharmaceutical compositions
formulated accordingly. Parenteral administration includes intravenous,
intraperitoneal,
subcutaneous, intramuscular, transepithelial, nasal, intrapulmonary,
intrathecal, rectal and
topical modes of administration. Parenteral administration can be by
continuous infusion
over a selected period of time.
The pharmaceutical composition of the invention is formulated to be compatible
with
its intended route of administration. In an embodiment, the composition is
formulated in
accordance with routine procedures as a pharmaceutical composition adapted for
intravenous,
subcutaneous, intramuscular, oral, intranasal, or topical administration to
human beings. In
preferred embodiments, the pharmaceutical composition is formulated for
intravenous
administration.
Typically, for oral therapeutic administration, a compound of the present
teachings
may be incorporated with excipient and used in the form of ingestible tablets,
buccal tablets,
troches, capsules, elixirs, suspensions, syrups, wafers, and the like.
Typically for parenteral administration, solutions of a compound of the
present
teachings can generally be prepared in water suitably mixed with a surfactant
such as
hydroxypropylcellulose. Dispersions can also be prepared in glycerol, liquid
polyethylene
glycols, DMSO and mixtures thereof with or without alcohol, and in oils. Under
ordinary
conditions of storage and use, these preparations contain a preservative to
prevent the growth
of microorganisms.
Typically, for injectable use, sterile aqueous solutions or dispersion of, and
sterile
powders of, a compound described herein for the extemporaneous preparation of
sterile
injectable solutions or dispersions are appropriate.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
23
EXEMPLIFICATION
The abbreviations used in the entire specification may be summarized herein
below with
their particular meaning:
ACN ¨ Acetonitrile;
AcOH ¨ Acetic acid;
Ac20 - Acetic anhydride;
A1C13 - Aluminum chloride;
anh. ¨ Anhydrous;
Aq. ¨ Aqueous;
BOC ¨ tert-Butyloxycarbonyl;
bs ¨ Broad singlet;
Conc. ¨ Concentrated;
C ¨ degree Celsius;
CDI ¨ Carbonyldiimidazole;
CH3MgBr - Methylmagnesium bromide;
CS2 ¨ Carbon disulfide;
d ¨ Doublet;
8 ¨ Delta;
DCM ¨ Dichloromethane;
DIPEA ¨ N,N-Diisopropylethylamine
DMFA ¨ N, N-Dimethylformamide;
DMSO ¨ Dimethylsulfoxide;
DMSO¨d6 ¨ Deuterated dimethylsulfoxide;
D20 ¨ Deuterated water;
DPPA ¨ Diphenylphosphoryl azide;
EDC.HC1¨ 1-(3-Dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride;
Et0H ¨ ethanol;
Et0Ac ¨ Ethyl acetate;
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
24
g ¨ Gram;
h ¨ Hour;
1H ¨ Proton;
H2 ¨ Hydrogen;
HATU N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-l-
ylmethylene]-N-
methylmethanaminium hexafluorophosphate N-oxide;
HBTU - N,N,N',N'-Tetramethy1-0-(1H-benzotriazol-1-y1)uronium
hexafluorophosphate;
1HNMR ¨ Proton nuclear magnetic resonance;
HNO3 ¨ Nitric acid;
H20 ¨ Water;
HC1¨ Hydrochloric acid;
Hz ¨ Hertz;
H2S 04 - Sulfuric acid;
J ¨ Coupling constant;
K2CO3¨ Potassium carbonate;
KOH ¨ Potassium hydroxide;
K3PO4 ¨ Potassium phosphate;
LC ¨ Liquid chromatography;
Li0H. H20 ¨ Lithium hydroxide monohydrate;
M ¨ Molecular ion;
m ¨ Multiplet;
M¨ Molar;
m-CPBA ¨ meta-Chloroperbenzoic acid;
Mel ¨ Methyl iodide;
Me0H ¨ Methanol;
mg ¨ Milligrams;
min ¨ Minutes;
MHz ¨ Mega Hertz (frequency);
mL ¨ Milliters;
CA 03135615 2021-09-29
WO 2020/210339
PCT/US2020/027240
mM - Millimolar;
mmol¨ Millimoles;
MS ¨ Mass spectroscopy;
m/z ¨ Mass to charge ratio;
N ¨ Normality;
NaBH4 ¨ Sodium borohydride;
NaH ¨ Sodium hydride;
NaHCO3 - sodium hydrogencarbonate;
NaNO2 - Sodium nitrite;
NaNO3 - Sodium nitrate;
Na0Et ¨ Sodium ethoxide;
NaOH ¨ Sodium hydroxide;
Na0Me ¨ Sodium methoxide;
NBS ¨ N-Bromosuccinimide
Pd/C ¨ Palladium on Carbon;
Pd(OAc)2 ¨ Palladium (II) acetate;
P0C13 - Phosphorus Oxychloride;
P255 - Phosphorus pentasulfide;
Pt02 ¨ Platinum dioxide:
% ¨ Percentage;
pH ¨ potential of Hydrogen;
psi ¨ Pounds per square inch;
q ¨ Quartet;
RT ¨ Room temperature;
s ¨ Singlet;
50C12 ¨ Thionyl chloride;
t ¨ Triplet;
TBAF ¨ Tetra-n-butylammonium fluoride;
TBDMSC1 - tert-Butyldimethylchlorosilane;
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
26
TEA ¨ Triethyl amine;
TFA ¨ Trifluoroacetic acid;
THF ¨ Tetrahydrofuran;
TLC ¨ Thin layer chromatography.
Examples lh and li. Synthesis of 2-
(benzo[d]oxazol-2-ylamino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid and Synthesis of 2-(benzo[d]oxazol-2-
ylamino)-N-
(2-methoxyethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide:
is OH
a ¨SH 101 C) 1) )1.-- 101 101
/ 2 e
NH2
la 1 b 1 c
Me
CI N H Me
N H Me
101 NH2
EtO2C NO2 EtO2C NO2 EtO2C N H2 EtO2C
1 d le 1 f
Met H
Me H Me H N1\1,0
N,:0 HO N,N, HN r!1
=
1 g
EtO2C Me
0
Conditions: a) Carbon disulfide, KOH, Ethanol, Reflux, 16 h; b) K2CO3, Methyl
iodide, Acetonitrile, 0 C - RT, 5
h; c) m-CPBA, DCM, 0 C - RT, 4 h; d) Methyl amine, DMF, 60 C, 16 h; e) 10%
Pd/C, Me0H, H2, RT, 5 h; 0
Cyanogen bromide, THF, H20, 50 C, 16 h; g) NaH, 2-
(methylsulfonyl)benzo[d]oxazole, 1,4-Dioxane, RT, 16 h;
h) Li0H.H20, THF, Ethanol, Water, 60 C, 16 h; i) 2-methoxyethylamine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of benzo[d]oxazole-2-thiol (la)
To a solution of 2-aminophenol (4.0 g, 36.7 mmol) in ethanol (80 mL) at RT was
added
powdered potassium hydroxide (3.59 g, 64.2 mmol) and carbon disulfide (20 mL,
330.3
mmol) and the reaction mixture was refluxed for 16 h. The reaction mixture was
concentrated, diluted with water (100 mL) and acidified with 1 N HC1. The
solid obtained
was filtered and dried under vacuum to afford the title compound (4.0 g, 72%);
1H NMR (400
MHz, DMSO-d6): 8 13.85 (bs, 1H), 7.51-7.49 (m, 1H), 7.32-7.23 (m, 3H); LC-MS:
m/z 152.0
(M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
27
Step-b: Synthesis of 2-(methylthio)benzo[d]oxazole (lb)
To a solution of benzo[d]oxazole-2-thiol (3.0 g, 19.9 mmol) in acetonitrile
(50 mL) at 0 C
was added potassium carbonate (3.01 g, 21.9 mmol) and methyl iodide (1.36 mL,
21.9 mmol)
and the reaction mixture was stirred at RT for 5 h. The reaction mixture was
diluted with
water (60 mL), extracted with Et0Ac (2 X 100 mL). The combined organic layer
was washed
with water (50 mL), brine (50 mL), dried over anhydrous sodium sulfate and
concentrated
under vacuum to afford the product which was used in the next step without any
further
purification (2.5 g, 76%); 1H NMR (400 MHz, DMSO-d6): 8 7.65-7.62 (m, 2H),
7.34-7.30
(m, 2H), 2.76 (s, 3H); LC-MS: m/z 166.0 (M+1) .
Step-c: Synthesis of 2-(methylsulfonyl)benzo [d] oxazole (1c)
To a solution of 2-(methylthio)benzo[d]oxazole (500 mg, 3.0 mmol) in DCM (20
mL) at 0 C
was added meta-chloroperbenzoic acid (938 mg, 9.1 mmol) and the reaction
mixture was
stirred at RT for 4 h. The reaction mixture was diluted with water (40 mL) and
aqueous layer
was extracted with DCM (2X100 mL). The combined organic layer was washed with
saturated sodium bicarbonate solution (6 X 150 mL), water (50 mL), brine (50
mL), dried
over anhydrous sodium sulfate and concentrated under vacuum to afford the
product which
was immediately used in the next step without any further purification (300
mg); LC-MS:
m/z 198.0 (M-F1) .
Step-d: Synthesis of ethyl 4-(methylamino)-3-nitrobenzoate (1d)
To a solution of ethyl 4-chloro-3-nitrobenzoate (30.0 g, 131 mmol) in DMFA
(100 mL) at RT
was added methyl amine (26.8 mL (40% aqueous solution), 262 mmol) and the
reaction
mixture was stirred at 60 C for 16 h. The reaction mixture was cooled to RT,
diluted with
cold water (1000 mL) and stirred at RT for 1 h. The solid obtained was
filtered and dried
under vacuum to afford the product as a yellow solid (26.0 g, 89%); 1H NMR
(400 MHz,
DMSO-d6): 8 8.62 (d, J = 2.0 Hz, 1H), 8.59 (bs, 1H), 7.99 (dd, J = 2.0 Hz, J =
8.8 Hz, 1H),
7.07 (d, J = 8.8 Hz, 1H), 4.29 (q, J = 6.8 Hz, 2H), 3.01 (d, J = 5.2 Hz, 3H),
1.31 (t, J = 6.8
Hz, 3H); LC-MS: m/z 225.0 (M+1) .
Step-e: Synthesis of ethyl 3-amino-4-(methylamino)benzoate (le)
To a solution of ethyl 4-(methylamino)-3-nitrobenzoate (13.0 g, 58 mmol) in
methanol (180
mL) was added slurry of 10% Pd/C (1.3 g in 10 mL of ethanol) under nitrogen
atmosphere.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
28
The flask was kept in a Parr shaker at RT under hydrogen pressure (60 psi) for
5h. The
reaction mixture was filtered through celite bed and concentrated under vacuum
to afford the
title compound (10.0 g, 88%); 1H NMR (400 MHz, DMSO-d6): 8 7.23 (dd, J = 1.6
Hz, J =
8.4 Hz 1H), 7.16 (d, J = 1.6 Hz, 1H), 6.39 (d, J = 8.4 Hz, 1H), 5.38-5.37 (m,
1H), 4.66 (s,
2H), 4.18 (q, J= 7.2 Hz, 2H), 2.77 (d, J= 5.2 Hz, 3H), 1.26 (t, J= 7.2 Hz,
3H); LC-MS: m/z
195.1 (M+1) .
Step-f: Synthesis of ethyl 2-amino-1-methyl-1H-benzo[d]imidazole-5-carboxylate
(1f)
To a solution of ethyl 3-amino-4-(methylamino) benzoate (5.6 g, 28.8 mmol) in
THF (23 mL)
and water (56 mL) at RT was added cyanogen bromide (3.7 g, 34.6 mmol) and the
reaction
mixture was stirred at 50 C for 16 h. The reaction mixture was cooled to RT,
diluted with
water (50 mL) and ethyl acetate (50 mL). The aqueous layer was basified with
saturated
sodium bicarbonate solution and extracted with Et0Ac (2 X 250 mL). Combined
organic
layer was washed with water (100 mL), brine (100 mL), dried over anhydrous
sodium sulfate
and concentrated under vacuum to give the product which was used in the next
step without
further purification (5.2 g, 82%); 1H NMR (400 MHz, DMSO-d6): 8 7.70 (d, J =
1.2 Hz, 1H),
7.59 (dd, J = 1.2 Hz, J = 8.4 Hz, 1H), 7.20 (d, J = 8.4 Hz, 1H), 6.64 (s, 2H),
4.27 (q, J = 6.8
Hz, 2H), 3.53 (s, 3H), 1.32 (t, J = 6.8 Hz, 3H).
Step-g: Synthesis of ethyl 2-
(benzo [d] oxazol-2-ylamino)-1-methyl-/H-
benzo [d] imidazole-5-carboxylate (1g)
To a solution of ethyl 2-amino-l-methyl-1H-benzo [d] imidazole-5-carboxylate
(150 mg, 0.68
mmol) in 1,4-dioxane (4 mL) at RT was added sodium hydride (96 mg, 2.4 mmol)
and the
reaction mixture was stirred for 15 min followed by the addition of 2-
(methylsulfonyl)benzo[d]oxazole (202 mg, 1.0 mmol). The reaction mixture was
stirred at
RT for 16 h and concentrated, diluted with water (20 mL) and acidified (pH -
6) with 1 N
HC1. The solid obtained was filtered, dried under vacuum and purified by
combiflash column
chromatography using 100% DCM as an eluent to afford the title compound (50
mg, 22%);
1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.24 (s, 1H), 7.88 (d, J = 8.4
Hz, 1H),
7.53-7.43 (m, 3H), 7.22 (t, J = 7.2 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 4.33
(q, J = 7.2 Hz, 2H),
3.64 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H); LC-MS: m/z 337.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
29
Step-h: Synthesis of 2-(benzo[d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (1h)
To a stirred solution of ethyl 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-
benzo [d] imidazole-
5-carboxylate (50 mg, 0.15 mmol) in a mixture of solvent of THF (1 mL),
ethanol (1 mL) and
water (0.5 mL), was added lithium hydroxide monohydrate (19 mg, 0.45 mmol).
The
reaction mixture was heated at 60 C for 16 h. The reaction mixture was cooled
to RT and
concentrated under reduced pressure. The residue was dissolved in water,
acidified with 1 N
HC1 to obtain the solid which was filtered and dried under vacuum to afford
the title
compound (35 mg, 76%); 1H NMR (400 MHz, DMSO-d6): 8 12.60 (bs, 2H), 8.20 (d, J
= 1.6
Hz, 1H), 7.87 (dd, J = 1.6 Hz, J = 8.0 Hz, 1H), 7.51-7.48 (m, 2H), 7.44 (d, J
= 7.2 Hz, 1H),
7.23-7.20 (m, 1H), 7.15-7.11 (m, 1H), 3.64 (s, 3H); LC-MS: m/z 308.6 (M+1) .
Step-i: Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2-methoxyethyl)-1-methyl-
1H-
benzo [d] imidazole-5-carboxamide (1i)
To a stirred solution of 2-(benzo [d]oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (30 mg, 0.1 mmol) in DMFA (1.0 mL) at 0 C was added N-
ethyldiisopropyl
amine (0.02 mL, 0.1 mmol) and diphenylphosphoryl azide (0.02 mL, 0.1 mmol).
The reaction
mixture was stirred for 30 min, followed by the addition of 2-
methoxyethylamine (8 mg, 0.1
mmol). The reaction mixture was then stirred at RT for 16 h. Once the reaction
was
completed (monitored by TLC), the reaction mixture was diluted with cold water
(15 mL)
and stirred for 15 min. The solid obtained was filtered, washed with diethyl
ether and dried
under vacuum to afford the title compound (22 mg, 53%) as white solid; 1H NMR
(400 MHz,
DMSO-d6): 8 12.2 (bs, 1H), 8.45 (t, J = 4.9 Hz, 1H), 8.08 (s, 1H), 7.76 (dd, J
= 1.5 Hz, J =
8.3 Hz, 1H), 7.47 (d, J = 8.3 Hz, 1H), 7.44-7.39 (m, 2H), 7.25-7.19 (m, 1H),
7.13-7.10 (m,
1H), 3.63 (s, 3H), 3.48-3.44 (m, 4H), 3.29 (s, 3H); LC-MS: m/z 364.0 (M-1).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Examples 2d and 2e. Synthesis of 1-
(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylic acid
and Synthesis of N-
(2-methoxyethyl)-1-(tetrahydro-2H-pyran-4-y1)-2-46-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide:
0 0 co)
CI
a NH
NH NO2 =EtO2C NO2 E102C 2a EtO2C = NH, EtO2C
2b 2c 40
ocF,
r
N H
N,H
N
HO2C N M ea N
0
2d
401 OCF3 2e
OCF3
Conditions: a) tetrahydro-2H-pyran-4-amine , K2CO3, DMSO, 70 C, 16 h; b) 10%
Pd/C, Me0H, H2, RT, 5 h; c) 2-
Amino-(trifluoromethoMbenzothiazole, 1,1-Thiocarbonyldiimidazole, EDC.HCI,
DMF, 100 C, 16 h; d) Li0H.H20,
THF, Me0H, Water, 60 C, 16 h; e) 2-methoxyethylamine, DPPA, DIPEA, DMF, 0 C -
RT, 16 h
Step-a: Synthesis of ethyl 3-nitro-4-((tetrahydro-2H-pyran-4-yl)amino)benzoate
(2a)
To a stirred solution of ethyl 4-chloro-3-nitrobenzoate (1.0 g, 4.4 mmol) in
DMSO (10 mL)
was added potassium carbonate (1.2 g, 8.7 mmol) and tetrahydro-2H-pyran-4-
amine (0.53 g,
5.2 mmol) at RT. Then the reaction mixture was heated to 70 C and stirred for
16 h and then
cooled to RT. The reaction mixture was diluted with cold water and the solid
obtained was
filtered and dried under vacuum to afford the title compound (1.1 g, 86%); 1H
NMR (400
MHz, DMSO-d6): 8 8.62 (d, J = 2.0 Hz, 1H), 8.23 (d, J = 7.8 Hz, 1H), 7.97 (dd,
J = 1.9 Hz, J
= 8.8 Hz, 1H), 7.29 (d, J = 9.3 Hz, 1H), 4.29 (q, J = 7.3 Hz, 2H), 3.96-3.94
(m, 1H), 3.90-
3.83 (m, 2H), 3.51-3.45 (m, 2H), 1.96-1.93 (m, 2H), 1.66-1.61 (m, 2H), 1.31
(t, J = 7.3 Hz,
3H); LC-MS: m/z 294.8 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4-((tetrahydro-2H-pyran-4-yl)amino)benzoate
(2b)
The title compound was synthesized using the same procedure which was followed
for
intermediate le using ethyl 3-nitro-4-((tetrahydro-2H-pyran-4-
yl)amino)benzoate as starting
material (Yield:100%); 1H NMR (400 MHz, DMSO-d6): 8 7.19 (d, J = 8.0 Hz, 1H),
7.17 (s,
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
31
1H), 6.52 (d, J= 8.3 Hz, 1H), 4.99 (d, J= 7.3 Hz, 1H), 4.97 (s, 2H), 4.18 (q,
J= 7.3 Hz, 2H),
3.89-3.87 (m, 2H), 3.57-3.53 (m, 1H), 3.45-3.40 (m, 2H), 1.92-1.89 (m, 2H),
1.48-1.38 (m,
2H), 1.25 (t, J = 7.4 Hz, 3H); LC-MS: m/z 264.8 (M+1) .
Step-c: Synthesis of ethyl 1-
(tetrahydro-2H-pyran-4-y1)-24(6-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylate (2c)
To a stirred solution of 2-amino-6-(trifluoromethoxy)benzothiazole (177 mg,
0.76 mmol) in
DMFA (55 mL) was added 1,1'-thiocarbonyldiimidazole (270 mg, 1.5 mmol) and the
solution was heated at 100 C for 4 h. The reaction mixture was cooled to RT.
EDC.HC1 (291
mg, 1.5 mmol) was added and the reaction mixture was heated at 60 C for 10
min, followed
by the addition of ethyl 3-amino-4-((tetrahydro-2H-pyran-4-yl)amino)benzoate
(200 mg, 0.76
mmol). Then the reaction mixture was heated at 100 C for 16 h. It was cooled
to RT, diluted
with water (50 mL) and extracted with Et0Ac (2 X 50 mL). The combined organic
layers
were washed with water (30 mL), brine (30 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum. The
residue was purified by combiflash column
chromatography using 100% DCM as an eluent to afford the title compound (120
mg, 31%);
LC-MS: m/z 505.0 (M-1).
Step-d: Synthesis of 1-
(tetrahydro-2H-pyran-4-y1)-24(6-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylic acid
(2d)
The title compound was synthesized using the same procedure which was followed
for
intermediate lh, using ethyl 1-
(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo [d] thiazol-2- yl) amino)- 1H-benzo [d] imidazo le-5-c
arbo xylate as
starting material (Yield:53%); 1H NMR (400 MHz, DMSO-d6): 8 12.91 (bs, 1H),
12.57 (bs,
1H), 8.24 (s, 1H), 8.02-7.64 (m, 4H), 7.37 (s, 1H), 4.96-4.94 (m, 1H), 4.20-
4.05 (m, 2H),
3.59-3.52 (m, 2H), 2.60-2.44 (m, 2H), 1.79-1.76 (m, 2H); LC-MS: m/z 477.0 (M-
1).
Step-e: Synthesis of N-(2-methoxyethyl)-1-(tetrahydro-2H-pyran-4-y1)-24(6-
(trifluoromethoxy)benzo [d] thiazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
(2e)
The title compound was synthesized using the same procedure which was followed
for
intermediate li using 1-(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo [d] thiazol-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
32
2-yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid as starting material
(Yield:18%); 1H
NMR (400 MHz, DMSO-d6): 8 8.25 (t, J = 5.4 Hz, 1H), 7.90 (d, J = 1.5 Hz, 1H),
7.71-7.64
(m, 2H), 7.33 (d, J = 0.9 Hz, 1H), 7.16-7.13 (m, 1H), 6.87 (d, J = 8.8 Hz,
1H), 5.23-5.17 (m,
1H), 4.11-4.10 (m, 2H), 3.65 (t, J= 11.2 Hz, 2H), 3.35-3.32 (m, 4H), 3.28 (s,
3H), 2.66-2.60
(m, 2H), 1.92-1.90 (m, 2H); LC-MS: m/z 536.0 (M+1) .
Example 3d and 3e. Synthesis of 1-(1-methylpiperidin-4-y1)-24(6-
(trifluoromethoxy)benzoldlthiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylic acid
and Synthesis of N-(2-methoxyethyl)-1-(1-methylpiperidin-4-y1)-
24(6-
(trifluoromethoxy)benzo [d] thiazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
Me
Me Me r
N¨N,H
i& CI a NH b Ai NH c
EtO2C N
EtO2C NO2 EtO2C NO2 EtO2C NH2 7 N
3a 3b 3c
OCF3
Me Me
r
H \ 1--1\11
HOC N MeON N
N N
3d =
0
3e
OCF3 = OCF3
Conditions: a) 1-methylpiperidin-4-amine , DIPEA, DMF, 70 C, 16 h; b) 10%
Pd/C, Me0H, H2, RT,
2 h; c) 2-Amino-6-(trifluoromethoxy)benzothiazole, 1,1'-
Thiocarbonyldiimidazole, EDC.HCI, DMF,
100 C,16 h; d) Li0H.H20, THF, Et0H, Water, 60 C, 16 h; e) 2-methoxyethylamine,
DPPA, DIPEA,
DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 4-((1-methylpiperidin-4-yl)amino)-3-nitrobenzoate
(3a)
To a stirred solution of ethyl 4-chloro-3-nitrobenzoate (500 mg, 2.2 mmol) in
DMFA (5 mL)
was added 1-methylpiperidin-4-amine (0.30 mL, 2.6 mmol) and N-ethyldiisopropyl
amine
(0.8 mL, 5.0 mmol). The reaction mixture was heated to 70 C and continued
stirring for 16 h
at the same temperature. Reaction mixture was cooled to RT, diluted with water
(50 mL) and
extracted with Et0Ac (2 X 100 mL). The combined organic layer was washed with
water (50
mL), brine (30 mL), dried over anhydrous sodium sulfate and concentrated under
vacuum to
afford the title compound (620 mg, 92%); 1H NMR (400 MHz, DMSO-d6): 8 8.62 (s,
1H),
8.25 (d, J = 8.0 Hz, 1H), 7.97 (d, J = 9.2 Hz, 1H), 7.23 (d, J = 9.2 Hz, 1H),
4.30 (q, J = 7.6
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
33
Hz, 2H), 3.71 (bs, 1H), 2.69-2.66 (m, 2H), 2.18 (s, 3H), 2.16-2.13 (m, 2H),
1.96-1.93 (m,
2H), 1.67-1.58 (m, 2H), 1.33 (t, J= 7.6 Hz, 3H); LC-MS: m/z 307.7 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4-((1-methylpiperidin-4-yl)amino)benzoate
(3b)
The title compound was synthesized using the same procedure which was followed
for
intermediate le using ethyl 4((1-methylpiperidin-4-yflamino)-3-nitrobenzoate
as the starting
material and stirring for 2 h (Yield:100%); 1H NMR (400 MHz, DMSO-d6): 8 7.19-
7.16 (m,
2H), 6.47 (d, J = 8.4 Hz, 1H), 4.96 (d, J = 7.2 Hz, 1H), 4.78 (bs, 2H), 4.18
(q, J = 7.2 Hz,
2H), 3.37-3.31 (m, 1H), 2.86-2.83 (m, 2H), 2.25 (s, 3H), 2.19-2.13 (m, 2H),
1.94-1.91 (m,
2H), 1.54-1.45 (m, 2H), 1.26 (t, J= 6.8 Hz, 3H);
LC-MS: m/z 278.2 (M+1) .
Step-c: Synthesis of ethyl 1-
(1-methylpiperidin-4-y1)-2-((6-
(trifluoromethoxy)benzo [d] thiazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate (3c)
The title compound was synthesized using the same procedure which was followed
for
intermediate 2c using ethyl 3-amino-44(1-methylpiperidin-4-yflamino)benzoate
as starting
material (Yield:15%); 1H NMR (400 MHz, DMSO-d6): 8 12.6 (bs, 1H), 8.14 (s,
1H), 7.91 (s,
1H), 7.79 (d, J = 8.3 Hz, 1H), 7.62 (d, J = 8.3 Hz, 1H), 7.55 (s, 1H), 7.35
(d, J = 8.8 Hz, 1H),
4.74-4.68 (m 1H), 4.34 (q, J = 6.8 Hz, 2H), 3.01-2.98 (m, 2H), 2.67-2.56 (m,
2H), 2.30 (s,
3H), 2.20-2.15 (m, 2H), 1.79-1.76 (m, 2H), 1.35 (t, J = 6.9 Hz, 3H); LC-MS:
m/z 520.1
(M+1) .
Step-d: Synthesis of 1-(1-methylpiperidin-4-y1)-2-((6-(trifluoromethoxy)benzo
[d] thiazol-
2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid (3d)
The title compound was synthesized using the same procedure which was followed
for
intermediate lh using ethyl 1-
(1-methylpiperidin-4-y1)-2-((6-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylate as
starting material (Yield:56%); 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H),
8.12 (s,
1H), 7.91 (s, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.60 (d, J = 8.3 Hz, 1H), 7.58
(bs, 1H), 7.34 (d, J
= 8.9 Hz, 1H), 4.79-4.68 (m 1H), 3.02-2.99 (m, 2H), 2.57-2.54 (m, 2H), 2.30
(s, 3H), 2.21-
2.15 (m, 2H), 1.79-1.77 (m, 2H); LC-MS: m/z 491.6 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
34
Step-e: Synthesis of N-
(2-methoxyethyl)-1-(1-methylpiperidin-4-y1)-24(6-
(trifluoromethoxy)benzo[d]thiazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
(3e)
The title compound was synthesized using the same procedure which was followed
for
intermediate li using 1-(1-methylpiperidin-4-y1)-2-((6-
(trifluoromethoxy)benzo[d]thiazol-2-
yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid as starting material
(Yield:40%); 1H
NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.44 (s, 1H), 8.09 (s, 1H), 7.91 (s,
1H), 7.70-
7.58 (m, 3H), 7.35 (d, J = 6.8 Hz, 1H), 4.75-4.65 (m, 1H), 3.50-3.43 (m, 4H),
3.28 (s, 3H),
3.02-2.99 (m, 2H), 2.60-2.54 (m, 2H), 2.31 (s, 3H), 2.20-2.15 (m, 2H), 1.79-
1.76 (m, 2H);
LC-MS: m/z 549.2 (M+1) .
Example 4d and 4e. Synthesis of 1-
(1-methylpyrrolidin-3-y1)-24(6-
(trifluoromethoxy)benzo[cuthiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylic acid
and Synthesis of N-
(2-methoxyethyl)-1-(1-methylpyrrolidin-3-y1)-24(6-
(trifluoromethoxy)benzo[d]thiazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide:
Me Me ,Me
9 PH
CI NH NH
a
EtO2C =N )r-S
EtO2C NO2 EtO2C NO2 EtO2C NH2 N
4a 4b 4c
OCF3
,Me ,Me
N H
H ¨11'
r-s MeO HOC N )N N )r-S
N
0
4d N
4e
OCF3 OCF3
Conditions: a) 1-methylpyrrolidin-3-amine, DIPEA, DMF, 70 C, 16 h; b) 10%
Pd/C, Me0H, H2, 2 h;
c) 2-Amino-6-(trifluoromethoxy)benzothiazole, 1,1'-Thiocarbonyldiimidazole,
EDC.HCI, DMF, 100 C,
16 h; d) Li0H.H20, THF, Me0H, Water, 60 C,16 h; e) 2-methoxyethylamine, DPPA,
DIPEA, DMF,
0 C - RT, 16
Step-a: Synthesis of ethyl 4-((1-methylpyrrolidin-3-yl)amino)-3-nitrobenzoate
(4a)
The title compound was synthesized using the same procedure which was followed
for
intermediate 3a using ethyl 4-chloro-3-nitrobenzoate and 1-methylpyrrolidin-3-
amine as
starting materials (Yield:79%); 1H NMR (400 MHz, DMSO-d6): 8 8.62 (d, J = 1.9
Hz, 1H),
8.39 (d, J= 6.8 Hz, 1H), 7.98 (dd, J= 2.0 Hz, J= 8.8 Hz, 1H), 7.15 (d, J= 9.3
Hz, 1H), 4.32-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
4.36 (m, 3H), 2.80-2.75 (m, 1H), 2.70-2.59 (m, 2H), 2.42-2.30 (m, 2H), 2.29
(s, 3H), 1.73-
1.65 (m, 1H), 1.31 (t, J= 7.4 Hz, 3H); LC-MS: m/z 294.1 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4((1-methylpyrrolidin-3-yl)amino)benzoate
(4b)
The title compound was synthesized using the same procedure which was followed
for
intermediate le using ethyl 4-((1-methylpyrrolidin-3-yl)amino)-3-nitrobenzoate
as starting
material and stirring for 2h (Yield: 78%); 1H NMR (400 MHz, DMSO-d6): 6 7.19-
7.15 (m,
2H), 6.38 (d, J= 8.3 Hz, 1H), 5.16 (d, J= 6.9 Hz, 1H), 4.81 (s, 2H), 4.18 (q,
J= 7.3 Hz, 2H),
3.95 (bs, 1H), 2.74-2.70 (m, 1H), 2.44-2.34 (m, 2H), 2.28-2.22 (m, 1H), 2.25
(s, 3H), 1.67-
1.63 (m, 1H), 1.25 (t, J= 7.3 Hz, 3H); LC-MS: m/z 264.1 (M+1) .
Step-c: Synthesis of ethyl 1-
(1-methylpyrrolidin-3-y1)-2-46-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylate (4c)
The title compound was synthesized using the same procedure which was followed
for
intermediate 2c using ethyl 3-amino-4-((1-methylpyrrolidin-3-yl)amino)benzoate
as starting
material. Product obtained was used in the next step without further
purification; LC-MS: m/z
506.1 (M+1) .
Step-d: Synthesis of 1-
(1-methylpyrrolidin-3-y1)-2-((6-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylic acid
(4d)
The title compound was synthesized using the same procedure which was followed
for
intermediate lh using ethyl 1-
(1-methylpyrrolidin-3-y1)-2-((6-
(trifluoromethoxy)benzo [d] thiazol-2- yl) amino)- 1H-benzo [d] imidazo le-5-c
arbo xylate as
starting material, using THF, Me0H and water (2:2:1) as solvent (Yield:13%);
1H NMR (400
MHz, DMSO-d6+D20): 8 8.03 (s, 1H), 7.83 (s, 1H), 7.78 (d, J = 8.3 Hz, 1H),
7.59-7.54 (m,
2H), 7.32 (d, J = 8.8 Hz, 1H), 5.50 (bs, 1H), 3.86-3.75 (m, 2H), 3.35 (bs,
1H), 3.07-3.05 (m,
1H), 2.91 (s, 3H), 2.28-2.0 (m, 2H); LC-MS: m/z 478.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
36
Step-e: Synthesis of N-(2-methoxyethyl)-1-(1-methylpyrrolidin-
3-y1)-2-46-
(trifluoromethoxy)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
(4e)
The title compound was synthesized using the same procedure which was followed
for
intermediate li using 1-(1-methylpyrrolidin-3-y1)-2-((6-
(trifluoromethoxy)benzo [d] thiazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid as starting material
(Yield: 30%); 1H
NMR (400 MHz, DMSO-d6): 8 8.10 (bs, 1H), 8.04 (s, 1H), 7.85 (s, 1H), 7.76 (bs,
1H), 7.68
(d, J = 8.3 Hz, 1H), 7.62 (bs, 1H), 7.30 (d, J = 8.8 Hz, 1H), 5.50 (bs, 1H),
3.52-3.44 (m, 4H),
3.30 (s, 3H), 2.76-2.65 (m, 2H), 2.50 (s, 3H, merged with DMSO peak), 2.31-
2.30 (m, 2H),
2.15-2.13 (m, 2H); LC-MS: m/z 535.0 (M-F1) .
Example 5. Synthesis of N-
(2-methoxyethyl)-1-methyl-2-44,5,6,7-
tetrahydrobenzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide:
Mei H Mei H Mei H
N NN
NHMe A b A qii) *N
NS
a
5c
Me02C NI-12 5a 5b HN
Me0 HO
0 0 /--/ 0
Me0
Conditions: a) 4,5,6,7-tetrahydrobenzo[d]thiazol-2-amine, 1,1'-
Thiocarbonyldiimidazole, EDC.HC1,
100 C, 16 h; b) Li0H.H20, THE, Me0H, Water, 60 C, 16 h; c) 2-
methoxyethylamine, DPPA, D1PEA,
MT, 0 C - RT, 16 h
Step-a: Synthesis of methyl 1-methyl-2-44,5,6,7-tetrahydrobenzo[d]thiazol-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxylate (5a)
To a stirred solution of 4,5,6,7-tetrahydrobenzo [d] thiazol-2-amine (216 mg,
1.4 mmol) in
DMFA (5 mL) was added 1,1'-thiocarbonyldiimidazole (494 mg, 2.8 mmol) and the
solution
was heated at 100 C for 2 h. The reaction mixture was cooled to RT. EDC.HC1
(533 mg, 2.8
mmol) was added and the reaction mixture was heated at 60 C for 10 min,
followed by the
addition of methyl 3-amino-4-(methylamino)benzoate (250 mg, 1.4 mmol). The
reaction
mixture was heated at 100 C for 16 h. It was cooled to RT, diluted with water
(50 mL) and
extracted with Et0Ac (2 X 50 mL). The combined organic layers were washed with
water
(30 mL), brine (30 mL), dried over anhydrous sodium sulfate and concentrated
under
vacuum. The residue was purified by combi flash column chromatography using
100% DCM
as an eluent to afford the title compound (110 mg, 23%); 1H NMR (400 MHz, DMSO-
d6): 8
12.01 (bs, 1H), 7.97 (s, 1H), 7.73 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.37 (d,
J = 8.4 Hz, 1H),
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
37
3.84 (s, 3H), 3.61 (s, 3H), 2.50 (bs, 4H, merged with DMSO peak), 1.78 (bs,
4H); LC-MS:
m/z 341.0 (M-1).
Step-b: Synthesis of 1-methyl-2((4,5,6,7-tetrahydrobenzo[d] thiazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid (5b)
The title compound was synthesized using the same procedure which was followed
for
intermediate lh using methyl 1-methyl-2-((4,5,6,7-tetrahydrobenzo [d] thiazol-
2-yl)amino)-
1H-benzo[d]imidazole-5-carboxylate as starting material, using THF, Me0H and
water
(2:2:1) as solvent (Yield: 83%); 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 2H),
7.95 (s,
1H), 7.73 (dd, J = 0.8 Hz, J = 8.4 Hz, 1H), 7.34 (d, J = 8.4 Hz, 1H), 3.61 (s,
3H), 2.50 (bs,
4H, merged with DMSO peak), 1.78 (bs, 4H); LC-MS: m/z 327.0 (M-1).
Step-c: Synthesis of N-(2-methoxyethyl)-1-methy1-2-44,5,6,7-tetrahydrobenzo[d]
thiazol-
2-yl)amino)-1H-benzo[d] imidazole-5-carboxamide (5c)
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methyl-2-((4,5,6,7-tetrahydrobenzo [d] thiazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid as starting material (Yield: 30%); 1H
NMR (400 MHz,
DMSO-d6): 8 12.0 (bs, 1H), 8.34 (bs, 1H), 7.95 (s, 1H), 7.64 (d, J = 8.4 Hz,
1H), 7.32 (d, J =
8.4 Hz, 1H), 3.60 (s, 3H), 3.48-3.42 (m, 4H), 3.28 (s, 3H), 2.50 (bs, 4H,
merged with DMSO
peak), 1.78 (bs, 4H); LC-MS: m/z 384.0 (M-1).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
38
Example 6. Synthesis of 24(6,6-dimethy1-4,5,6,7-tetrahydrobenzo[d]thiazol-
2-
yl)amino)-N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-1H-benzo[d]imidazole-5-
carboxamide
Me Me
Me Me Me Me Me Me NI NI'
a b C __ = - EtO2C
NI)I-S
\i z- z
d HO2O
NI)I\i-õ.S_
0 0, ,Me Nr.<
NH2
I Me _ Me Me
Me Me Me
Me
_ NI
H ip ¨NH
.... HO e õ,......",õ õ,--,õN
0 N /---S
0 N \Me
Me
Conditions: a) Trimethylsilyl trifluoromethanesulfonate, TEA, DCM, 0 C, 1.5 h;
b) NBS, Sodium acetate, THF, water, RT, 2
h; Thiourea, 80 C, 6 h; RT, 16 h; c) ethyl 3-amino-4-(methylamino)benzoate,
1,1'-Thiocarbonyldiimidazole, EDC.HCI, DMF,
100 C, 18 h; d) Li0H.H20, THF, Et0H, water, 80 C, 16 h; e) 2-(2-
Aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT,
16 h
Step-a: Synthesis of ((4,4-dimethylcyclohex-1-en-1-ypoxy)trimethylsilane
To a solution of 4,4-dimethylcyclohexan-1-one (3.0 g, 23.8 mmol) in DCM (180
mL) at 0 C
was added triethylamine (9.95 mL, 71.42 mmol). The reaction mixture was
stirred for 5 min
followed by the addition of trimethylsilyl trifluoromethanesulfonate (6.5 g,
29.28 mmol in 54
mL of DCM). The reaction mixture was stirred at 0 C for 1.5 h and then
quenched with
saturated sodium bicarbonate solution (30 mL) and water (100 mL). The organic
layer was
separated and washed with water (2 X 50 mL), brine solution (50 mL), dried
over anhydrous
sodium sulfate and concentrated under vacuum to afford the titled compound
(3.8 g, 81%)
which was used in the next step without further purification; 1H NMR (400 MHz,
CDC13): 8
4.74 (t, J = 4.0 Hz, 1H), 1.99-1.97 (m, 2H), 1.80 (d, J = 2.0 Hz, 2H), 1.39
(t, J = 6.8 Hz, 2H),
1.16 (s, 6H), 0.15 (s, 9H).
Step-b: Synthesis of 6,6-dimethy1-4,5,6,7-tetrahydrobenzo[d]thiazol-2-amine
To a solution of ((4,4-dimethylcyclohex-1-en-l-y1)oxy)trimethylsilane (3.8 g,
19.16 mmol) in
THF (40 mL) and water (40 mL) at RT was added N-bromosuccinimide (4.09 g, 23.0
mmol)
and sodium acetate (0.22 g, 2.7 mmol). The reaction mixture was then stirred
for 2 h.
Thiourea (1.43 g, 18.8 mmol) was added and the reaction mixture was heated at
80 C for 6 h
with stirring. Then it was cooled to RT and continued stirring for 16 h. The
reaction mixture
was quenched with water (30 mL) and extracted with DCM (2 X 50 mL). The
aqueous layer
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
39
was basified (pH _9) with 2 M sodium hydroxide and stirred for 2 h at RT. The
solid
precipitated was filtered and dried under vacuum to afford the titled compound
(1.3 g, 37%)
which was used in the next step without further purification; 1H NMR (400 MHz,
DMSO-d6):
8 6.55 (bs, 2H), 2.35 (t, J = 6.4 Hz, 2H), 2.26 (s, 2H), 1.46 (t, J = 6.0 Hz,
2H), 0.95 (s, 6H);
LC-MS: m/z 183.1 (M+1) .
Step-c: Synthesis of ethyl 24(6,6-dimethy1-4,5,6,7-tetrahydrobenzo [d] thiazol-
2-
yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxylate
To a stirred solution of 6,6-dimethy1-4,5,6,7-tetrahydrobenzo [d] thiazol-2-
amine (809 mg,
4.44 mmol) in DMFA (10 mL) was added 1,1'-thiocarbonyldiimidazole (1.58 g,
8.88 mmol)
and the solution was heated at 100 C for 2 h. The reaction mixture was cooled
to RT and
EDC.HC1 (1.70 g, 8.88 mmol) was added. The reaction mixture was heated at 50 C
for 10
min, followed by the addition of ethyl 3-amino-4-(methylamino) benzoate (800
mg, 4.44
mmol). It was then heated at 100 C for 16 h and cooled to RT. The reaction
mixture was
diluted with water (50 mL) and extracted with Et0Ac (2 X 100 mL). The combined
organic
layers were washed with water (30 mL), brine (30 mL), dried over anhydrous
sodium sulfate
and concentrated under vacuum. The residue was purified by combi flash column
chromatography using 100% DCM as an eluent to afford the titled compound (500
mg, 32%);
1H NMR (400 MHz, DMSO-d6): 8 12.10 (bs, 1H), 7.98 (s, 1H), 7.74 (d, J= 8.4 Hz,
1H), 7.37
(d, J = 8.4 Hz, 1H), 4.30 (q, J = 7.2 Hz, 2H), 3.61 (s, 3H), 2.50 (2H merged
with DMSO
peak), 2.33 (s, 2H), 1.56 (t, J = 6.0 Hz, 2H), 1.33 (t, J = 7.2 Hz, 3H), 1.00
(s, 6H); LC-MS:
m/z 385.1 (M+1) .
Step-d: Synthesis of 2((6,6-dimethy1-4,5,6,7-tetrahydrobenzo [d] thiazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylic acid
To a stirred solution of ethyl 24(6,6-dimethy1-4,5,6,7-
tetrahydrobenzo[d]thiazol-2-yDamino)-
1-methyl-1H-benzo[d]imidazole-5-carboxylate (500 mg, 1.30 mmol) in a mixture
of THF (4
mL), ethanol (4 mL) and water (2 mL) was added lithium hydroxide monohydrate
(136 mg,
3.25 mmol). The reaction mixture was heated at 80 C for 16 h and then cooled
to RT and
concentrated under reduced pressure. The residue was dissolved in water,
acidified with 1 N
HC1 to obtain the solid which was filtered and dried under vacuum to afford
the titled
compound (300 mg, 65%); 1H NMR (400 MHz, DMSO-d6): 8 8.00 (s, 1H), 7.86 (d, J
= 8.4
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 3.64 (s, 3H), 2.50 (2H merged with DMSO
peak), 2.38 (s,
2H), 1.58 (t, J= 6.0 Hz, 2H), 1.01 (s, 6H); LC-MS: m/z 357.1 (M+1) .
Step-e: Synthesis of 24(6,6-dimethy1-4,5,6,7-tetrahydrobenzo [d] thiazol-2-
yl)amino)-N-
(2-(2-hydroxyethoxy)ethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
To a stirred solution of 2-((6,6-dimethy1-4,5,6,7-tetrahydrobenzo [d] thiazol-
2-yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylic acid (100 mg, 0.28 mmol) in DMFA (2
mL) at
0 C was added N-ethyldiisopropyl amine (0.04 mL, 0.28 mmol) and HBTU (106 mg,
0.28
mmol). The reaction mixture was stirred for 30 min, followed by the addition
of 2-(2-
aminoethoxy)ethan-1-ol (0.02 mL, 0.28 mmol) and stirring was continued at RT
for 16 h.
Once the reaction was completed (monitored by TLC), the reaction mixture was
diluted with
cold water (20 mL) and stirred for 15 min. The solid obtained was filtered and
dried under
vacuum. The residue was purified by combi flash column chromatography using 2%
Me0H
in DCM as an eluent to afford the titled compound (50 mg, 40%); 1H NMR (400
MHz,
DMSO-d6): 8 12.0 (bs, 1H), 8.34 (s, 1H), 7.95 (s, 1H), 7.64 (d, J = 8.4 Hz,
1H), 7.32 (d, J =
8.4 Hz, 1H), 4.60 (bs, 1H), 3.60 (s, 3H), 3.56-3.42 (m, 8H), 2.50 (2H merged
with DMSO
peak), 2.32 (m, 2H), 1.55 (t, J= 6.0 Hz, 2H), 1.00 (s, 6H); LC-MS: m/z 444.1
(M+1) .
Example 7. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-methyl-
4,5,6,7-
tetrahydrobenzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
Me
Me Me Me
a j*, I:..., 0\ ¨).--c EtO2C 4110 Nr\l---NH d
¨).-
S Nt
0 O. .Me N=---(
Si. NH2
I Me
Me Me
Me Me
io NI -NH H 0 1\1-1 NH
HO2C HOoN N \
j---S
Nt 0 N\aL
Me Me
Conditions: a) Trimethylsilyl trifluoromethanesulfonate, TEA, DCM, 0 C, 1.5 h;
b) NBS,
Sodium acetate, Et0H, water, RT, 2 h; Thiourea, 80 C, 6 h; RT, 16 h; c) ethyl
3-amino-4-
(methylamino)benzoate, 1X-Thiocarbonyldiimidazole, EDC.HC1, DMF, 100 C, 16 h;
d)
Li0H.1120, THF, Et0H, water, 60 C, 16 h; e) 2-(2-Aminoethoxy)ethan-1-ol, HBTU,
DIPEA, DMF, 0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
41
Step-a: Synthesis of trimethyl((4-methylcyclohex-1-en-1-yl)oxy)silane
To a solution of 4-methylcyclohexan-1-one (2.0 g, 17.8 mmol) in DCM (50 mL) at
0 C was
added triethylamine (7.5 mL, 53.4 mmol) and stirred for 5 min followed by the
addition of
trimethylsilyl trifluoromethanesulfonate (4.95 g, 22.3 mmol in 30 mL of DCM).
The reaction
mixture was stirred at 0 C for 1.5 h and then quenched with saturated sodium
hydrogen
carbonate solution (20 mL) and water (100 mL). The organic layer was separated
and washed
with water (2 X 100 mL), brine solution (50 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum to afford the titled compound (3.4 g, 100%); 1H NMR
(400 MHz,
DMSO-d6): 8 4.74-4.71 (m, 1H), 2.66-2.49 (m, 1H), 2.16-1.99 (m, 2H), 1.89-1.88
(m, 1H),
1.62-1.57 (m, 2H), 1.25-1.22 (m, 1H), 0.89 (d, J = 2.4 Hz, 3H), 0.27 (s, 9H).
Step-b: Synthesis of 6-methyl-4,5,6,7-tetrahydrobenzo[d]thiazol-2-amine
To a solution of trimethyl((4-methylcyclohex-1-en-1-y1)oxy)silane (3.3 g, 17.9
mmol) in
THF (25 mL) and water (25 mL) at RT was added N-bromosuccinimide (3.83 g, 21.5
mmol)
and sodium acetate (0.20 g, 2.5 mmol) and then stirred for 2 h. Thiourea (1.43
g, 18.8 mmol)
was added and the reaction mixture was heated at 80 C for 6 h with stirring.
The reaction
mixture was then cooled to RT and continued stirring for 16 h. The reaction
mixture was
quenched with water (30 mL) and extracted with DCM (2 X 100 mL). The aqueous
layer was
basified (pH ..9) with 2 M sodium hydroxide and extracted with DCM (3 X 100
mL). The
combined organic layers were washed with brine solution (50 mL), dried over
anhydrous
sodium sulfate and concentrated under vacuum to afford the titled compound
(1.9 g, 63%);
1H NMR (400 MHz, DMSO-d6): 8 6.55 (bs, 2H), 2.57-2.51 (m, 1H), 2.40-2.36 (m,
2H),
2.12-2.05 (m, 1H), 1.79-1.74 (m, 2H), 1.35-1.34 (m, 1H), 1.00 (d, J= 6.4 Hz,
3H); LC-MS:
m/z 169.2 (M+1) .
Step-c: Synthesis of ethyl 1-methyl-24(6-methyl-4,5,6,7-
tetrahydrobenzo[d]thiazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylate
To a stirred solution of 6-methyl-4,5,6,7-tetrahydrobenzo[d]thiazol-2-amine
(400 mg, 2.38
mmol) in DMFA (10 mL) was added 1,1'-thiocarbonyldiimidazole (848 mg, 4.76
mmol) and
the solution was heated at 100 C for 2 h. The reaction mixture was cooled to
RT and
EDC.HC1 (914 mg, 4.76 mmol) was added. The reaction mixture was heated at 50 C
for 10
min, followed by the addition of ethyl 3-amino-4-(methylamino)benzoate (462
mg, 2.38
mmol). The reaction mixture was heated at 100 C for 16 h. It was cooled to RT,
diluted with
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
42
water (50 mL) and extracted with Et0Ac (2 X 50 mL). The combined organic
layers were
washed with water (30 mL), brine (30 mL), dried over anhydrous sodium sulfate
and
concentrated under vacuum. The residue was purified by combi flash column
chromatography using 100% DCM as an eluent to afford the title compound (400
mg, 45%);
1H NMR (400 MHz, DMSO-d6): 8 12.03 (bs, 1H), 7.98 (s, 1H), 7.73 (dd, J = 1.0
Hz, J = 8.3
Hz, 1H), 7.37 (d, J = 8.3 Hz, 1H), 4.30 (q, J = 7.2 Hz, 2H), 3.62 (s, 3H),
2.67-2.60 (m, 1H),
2.50 (2H merged with DMSO peak), 2.18-2.11 (m, 1H), 1.87-1.84 (m, 2H), 1.46-
1.42 (m,
1H), 1.34 (t, J= 7.1 Hz, 3H), 1.05 (d, J= 6.3 Hz, 3H); LC-MS: m/z 371.05 (M+1)
.
Step-d: Synthesis of 1-methyl-24(6-methyl-4,5,6,7-tetrahydrobenzo [d]
thiazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh, using ethyl 1-methyl-2-((6-methyl-4,5,6,7-tetrahydrobenzo [d]
thiazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylate as starting material (Yield:
100%); 1H NMR
(400 MHz, DMSO-d6): 8 13.01 (bs, 1H), 12.83 (bs, 1H), 8.01 (s, 1H), 7.87 (d, J
= 8.4 Hz,
1H), 7.56 (d, J = 8.4 Hz, 1H), 3.67 (s, 3H), 2.67-2.66 (m, 1H), 2.52 (2H
merged with DMSO
peak), 2.33-2.21 (m, 1H), 1.91-1.86 (m, 2H), 1.48-1.43 (m, 1H), 1.06 (d, J =
6.4 Hz, 3H);
LC-MS: m/z 343.05 (M+1) .
Step-e: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-methyl-4,5,6,7-
tetrahydrobenzo [d] thiazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-methyl-4,5,6,7-tetrahydrobenzo [d]
thiazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-
1-ol as
starting materials (Yield: 48%); 1H NMR (400 MHz, DMSO-d6): 8 11.99 (bs, 1H),
8.35 (t, J
= 5.6 Hz, 1H), 7.95 (s, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.32 (d, J = 8.4 Hz,
1H), 4.62 (t, J = 5.0
Hz, 1H), 3.61 (s, 3H), 3.55-3.50 (m, 4H), 3.47-3.42 (m, 4H), 2.67-2.66 (m,
1H), 2.52 (2H
merged with DMSO peak), 2.17-2.16 (m, 1H), 1.90-1.88 (m, 2H), 1.45-1.40 (m,
1H), 1.05
(d, J= 6.4 Hz, 3H); LC-MS: m/z 430.10 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
43
Example 8. Synthesis of 2-((5,6-dihydro-4H-cyclopenta [d] thiazol-2-yl)amino)-
N-(2-
methoxyethyl)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
Me S/9
N
CX 0 -AP" E
H 2N S t0
=0
Me
Me S/9
S/9
d
NI N
H ¨NH
HOS N> MeON N
0
0
Conditions: a) Iodine, Thiourea, 110 C, 12 h; b) ethyl 3-amino-4-(methylamino)
benzoate, 1,11-Thiocarbonyl diimidazole, EDC.HC1, DMF, 100 C, 16 h; c)
Li0H.H20, THF, Ethanol, Water, 60 C, 16 h; h) 2-methoxyethyl amine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 5,6-dihydro-4H-cyclopenta[d]thiazol-2-amine
A mixture of cyclopentanone (1.0 g, 11.9 mmol), thiourea (1.8 g, 23.8 mmol)
and iodine (3.0
g, 11.9 mmol) was stirred in a sealed tube at 110 C for 12 h. The reaction
mixture was cooled
to RT and hot water (30 mL) was added and stirred for 30 min. The reaction
mixture was
extracted with diethyl ether (2 X 20 mL), aqueous layer was basified (pH ¨ 8)
with solid
sodium bicarbonate and extracted with DCM (3 X 30 mL). The combined organic
layer was
washed with brine (20 mL), dried over anhydrous sodium sulfate and
concentrated under
vacuum. The resulted residue was purified by combiflash chromatography using
10%
methanol in DCM and 0.1% of aqueous ammonia as eluent to afford the title
compound (160
mg, 10%); 1H NMR (400 MHz, CDC13): 8 4.77 (bs, 2H), 2.79-2.74 (m, 2H), 2.68-
2.64 (m,
2H), 2.41-2.34 (m, 2H); LC-MS: m/z 141.1 (M+1) .
Step-b: Synthesis of ethyl 2-((5,6-dihydro-4H-cyclopenta[d] thiazol-2-
yl)amino)-1-
methyl-1H-benzo[d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 5a using ethyl 3-amino-4-(methylamino)benzoate and 5,6-dihydro-4H-
cyclopenta[d]thiazol-2-amine as starting materials (Yield: 23%); 1H NMR (400
MHz,
CA 03135615 2021-09-29
WO 2020/210339
PCT/US2020/027240
44
DMSO-d6): 8 12.0 (bs, 1H), 8.05 (s, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.38 (d, J
= 8.4 Hz, 1H),
4.30 (q, J = 6.8 Hz, 2H), 3.56 (s, 3H), 2.80-2.72 (m, 4H), 2.36-2.33 (m, 2H),
1.33 (t, J = 6.8
Hz, 3H); LC-MS: m/z 343.0 (M+1) .
Step-c: Synthesis of 24(5,6-dihydro-4H-cyclopenta[d]thiazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh, using ethyl 24(5,6-dihydro-4H-cyclopenta[d]thiazol-2-yl)amino)-1-
methyl-
1H-benzo [d]imidazole-5-carboxylate as starting material (Yield: 55%); LC-MS:
m/z 315.1
(M+1) .
Step-d: Synthesis of 2-
((5,6-dihydro-4H-cyclopenta[d] thiazol-2-yl)amino)-N-(2-
methoxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 24(5,6-dihydro-4H-cyclopenta[d]thiazol-2-yl)amino)-1-methyl-
1H-
benzo [d] imidazole-5-carboxylic acid as starting material (Yield: 13%); 1H
NMR (400 MHz,
DMSO-d6): 8 12.0 (bs, 1H), 8.36 (bs, 1H), 7.95 (s, 1H), 7.67 (d, J = 7.6 Hz,
1H), 7.33 (d, J =
8.4 Hz, 1H), 3.56 (s, 3H), 3.47-3.42 (m, 4H), 3.28 (s, 3H), 2.77-2.67 (m, 4H),
2.36-2.33 (m,
2H); LC-MS: m/z 372.0 (M+1) .
Example 9.
Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(4,5,6,7-
tetrahydrothiazolo[5,4-c]pyridin-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
hydrochloride
Me Me
Boc H
0
N /
r d
N N
Y\S
EtO2C
N-Boc HO2C -
t\N-Boc
Boc
NH2
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Me Me
)
H 101 r\l/>¨NH H 40 N¨NH
e HOoN r-S
TBSON
0 0
N,Boc NH
HCI
Conditions: a) i) Pyrrolidone hydrobromide, THF, reflux, 10 min ii) Thiourea,
Et0H, reflux, 3 h; b)
ethyl 3-amino-4-(methylamino)benzoate, 1,1'-thiocarbonyldiimidazole, EDC, DMF,
100 C, 18 h;
c) Li0H.H20, THF, Me0H, H20, 50 C, 16 h; d) 2-(2-((tert-
butyldimethylsilyhoxy)ethoxy)ethan-1-
amine, HBTU, DIPEA, DMF, 0 C - RT, 16 h; e) 4 M HCI in 1,4-Dioxane, 1,4-
Dioxane, 0 C - RT,
16 h
Step-a: Synthesis of tert-butyl 2-amino-6,7-dihydrothiazolo[5,4-c]pyridine-
5(4H)-
carboxylate
To a stirred solution of tert-butyl 4-oxopiperidine-1-carboxylate (3.0 g,
15.07 mmol) in THF
(70 mL) at RT was added pyrrolidone hydrobromide (7.47 g, 15.07 mmol) and the
reaction
mixture was refluxed for 10 min. The reaction mixture was cooled to RT, the
precipitated
solid was removed by filtration and the filtrate was concentrated. The residue
obtained was
dissolved in ethanol (80 mL) followed by the addition of thiourea (1.53 g,
20.14 mmol) and
the reaction mixture was refluxed for 3 h. The reaction mixture was cooled to
RT,
concentrated under vacuum. The residue was purified by combiflash
chromatography using
5% methanol in DCM as an eluent to afford the title compound (450 mg, 12%); 1H
NMR
(400 MHz, DMSO-d6): 8 6.80 (s, 2H), 4.29 (s, 2H), 3.56 (t, J = 5.6 Hz, 2H),
2.43 (bs, 2H),
1.41 (s, 9H); LC-MS: m/z 256.2 (M+1) . .
Step-b: Synthesis of tert-butyl 2((5-(ethoxycarbony1)-1-methyl-1H-benzo [d]
imidazol-2-
yl)amino)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate
To a stirred solution of tert-butyl 2-amino-6,7-dihydrothiazolo[5,4-c]pyridine-
5(4H)-
carboxylate (424 mg, 1.66 mmol) in DMFA (6 mL) was added 1,1'-
thiocarbonyldiimidazole
(593 mg, 3.33 mmol) and the solution was heated at 100 C for 2 h. The reaction
mixture was
cooled to RT and EDCBC1 (639 mg, 3.33 mmol) was added. The reaction mixture
was
heated at 50 C for 10 min, followed by the addition of ethyl 3-amino-4-
(methylamino)benzoate (300 mg, 1.66 mmol). The reaction mixture was heated at
100 C for
16 h. It was cooled to RT, diluted with water (50 mL) and extracted with Et0Ac
(2 X 50
mL). The combined organic layers were washed with water (30 mL), brine (30
mL), dried
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
46
over anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combiflash chromatography using 0.4% methanol in DCM as an eluent to afford
the titled
compound (130 mg, 18%); 1H NMR (400 MHz, DMSO-d6): 8 12.10 (bs, 1H), 8.03 (bs,
1H),
7.77 (d, J = 8.4 Hz, 1H), 7.39 (d, J = 8.4 Hz, 1H), 4.39 (bs, 2H), 4.30 (q, J
= 6.8 Hz, 2H),
3.67-3.59 (m, 5H), 2.50 (2H merged with DMSO peak), 1.43 (s, 9H), 1.34 (t, J =
6.8 Hz, 3H);
LC-MS: m/z 458.2 (M+1) .
Step-c: Synthesis of 2-05-(tert-butoxycarbony1)-4,5,6,7-tetrahydrothiazolo[5,4-
c]pyridin-2-y1)amino)-1-methyl-1H-benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using tert-butyl 2-((5-(etho xyc arbo ny1)- 1-methyl- 1H-benzo
[d]imidazol-2-
yl)amino)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate as starting
material and
THF, methnol, water (2:2:1) as solvent. (Yield: 70%); 1H NMR (400 MHz, DMSO-
d6): 8
12.50 (bs, 1H), 8.00 (s, 1H), 7.78 (d, J = 8.4 Hz, 1H), 7.40 (d, J = 8.4 Hz,
1H), 4.40 (s, 2H),
3.66 (t, J = 4.8 Hz, 2H), 3.60 (s, 3H), 2.66-2.63 (m, 2H), 1.43 (s, 9H); LC-
MS: m/z 430.1
(M+1) .
Step-d: Synthesis of tert-butyl
24(54(242-((tert-
butyldimethylsilypoxy)ethoxy)ethyl)carbamoy1)-1-methyl-1H-benzo[d]imidazol-2-
yl)amino)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e
using 2-((5-(tert-butoxycarbony1)-4,5,6,7-tetrahydrothiazolo [5,4-
c]p yridin-2- yl)amino)-1-methy1-1H-benzo [d] imidazole-5-c arbo xylic acid
and 2-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)ethan-1-amine as starting materials (Yield:
40%); LC-MS:
m/z 631.2 (M+1) .
Step-e: Synthesis of N-
(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(4,5,6,7-
tetrahydrothiazolo-[5,4-c]pyridin-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
hydrochloride
To a stirred solution of tert-butyl 2-
((5-((2-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)ethyl)carbamoy1)-1-methyl-1H-benzo [d]imidazol-2-
yl)amino)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate (50 mg, 0.08
mmol) in 1,4-
dioxane (0.8 mL) at 0 C was added 4 M HC1 in 1,4-dioxane (0.3 mL) and stirred
at RT for
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
47
16 h. The reaction mixture was concentrated under reduced pressure. The
residue was
triturated with diethyl ether and solvent was decanted. The solid obtained was
dried under
vacuum to afford the title compound (12 mg, 33%); 1H NMR (400 MHz, CD30D): 8
8.01 (s,
1H), 7.87 (d, J = 8.0 Hz, 1H), 7.55 (d, J = 8.4 Hz, 1H), 4.35 (s, 2H), 3.74
(s, 3H), 3.71-3.53
(m, 10H), 3.05 (t, J= 6.0 Hz, 2H); LC-MS: m/z 417.1 (M+1) .
Example 10. Synthesis of 24(5-acetyl-4,5,6,7-tetrahydrothiazolo[5,4-
c]pyridin-2-
yl)amino)-N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-1H-benzo[d]imidazole-5-
carboxamide
Me Me
N H ye
N,
N,N
Jr N us
1110 N &,t\ a N FS I")
HOC HO2C
HO2C N"--13oc NH TFA
hMe
Me
io,N N )FS
o
NNo
0
Conditions: a) TFA, DCM, 0 C - RT, 16 h; b) Ac20, Pyridine, 0 C - RT, 16 h; c)
2-(2-aminoethoxy)ethan-
1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 1-methyl-2-04,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxylic acid trifluoroacetic acid salt
To a stirred solution of 2-((5-(tert-butoxycarbony1)-4,5,6,7 -
tetrahydrothiazolo [5,4-c]pyridin-
2-yl)amino)-1-methy1-1H-benzo [d] imidazole-5-carboxylic acid (170 mg, 0.39
mmol) in
DCM (5 mL) at 0 C was added TFA (0.1 mL, 1.19 mmol) and stirred at RT for 16
h. The
reaction mixture was concentrated under reduced pressure and the residue
obtained was
triturated with diethyl ether and solvent was decanted. The residue was dried
under vacuum
to afford the title compound (180 mg, 100%); 1H NMR (400 MHz, DMSO-d6): 8 9.22
(s,
2H), 8.04 (s, 1H), 7.81 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.42 (d, J = 8.4 Hz,
1H), 4.23 (s,
2H), 3.60 (s, 3H), 3.48-3.46 (m, 2H), 2.89 (bs, 2H); LC-MS: m/z 330.1(M+1) .
Step-b: Synthesis of 24(5-acetyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylic acid
To a stirred solution of 1-methy1-2-((4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-
2-yl)amino)-
1H-benzo [d] imidazole-5-carboxylic acid trifluoroacetic acid salt (180 mg,
0.55 mmol) in
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
48
pyridine (2 mL) at 0 C was added acetic anhydride (0.06 mL, 0.6 mmol) and
stirred at RT
for 16 h. The reaction mixture was diluted with water (15 mL), and extracted
with Et0Ac (2
X 50 mL). The combined organic layers were washed with 1 N HC1 (30 mL), water
(20 mL),
brine (20 mL), dried over anhydrous sodium sulfate and concentrated under
vacuum and the
residue obtained was triturated with diethyl ether and solvent was decanted.
The obtained
solid was dried under vacuum to afford the title compound (70 mg, 46%); LC-MS:
m/z 372.1
(M+1) .
Step-c: Synthesis of 24(5-acetyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-
yl)amino)-N-
(2-(2-hydroxyethoxy)ethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 24(5- acety1-4,5,6,7-tetrahydrothiazo lo [5,4- c]
pyridin-2- yl)amino)- 1-
methy1-1H-benzo [d] imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-
ol as starting
materials (Yield: 29%); 1H NMR (400 MHz, DMSO-d6): 8 12.0 (bs, 1H), 8.38 (s,
1H), 7.96
(d, J = 3.6 Hz, 1H), 7.67 (d, J = 7.6 Hz, 1H), 7.35 (d, J = 8.4 Hz, 1H), 4.62-
4.49 (m, 3H),
3.78-3.73 (m, 2H), 3.60-3.41 (m, 10H), 3.30 (2H merged with DMSO moisture
peak), 2.09
(s, 3H); LC-MS: m/z 459.1 (M+1) .
Example 11. Synthesis of 24(6,7-dihydro-4H-pyrano[4,3-d]thiazol-2-yl)amino)-N-
(2-
methoxyethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
Me Me Me
N H * NrH,s
b
N )rS
Me0 Nt7 HO Mea -
OraN>--N H2 ""
0 0 0 0 0
0
Conditions: a) Methyl 3-amino-4-(methylamino)benzoate, 1,1'-
thiocarbonyldiimidazole , EDC, DMF, 100 C, 16 h; b)
Li0H.H20, THF, Me0H, H20, 60 C, 16 h; c) 2-methoxyethan-1-amine , DPPA, DIPEA,
DMF, 0 C - RT, 16 h
Step-a: Synthesis of methyl 24(6,7-dihydro-4H-pyrano[4,3-dithiazol-2-yl)amino)-
1-
methyl-1H-benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 5a using methyl 3-amino-4-(methylamino)benzoate and 6,7-dihydro-4H-
pyrano[4,3-d]thiazol-2-amine as starting materials and stirring 16 h (Yield:
20%); 1H NMR
(400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.02 (s, 1H), 7.77 (d, J = 8.0 Hz, 1H),
7.40 (d, J =
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
49
8.4 Hz, 1H), 4.58 (s, 2H), 3.93 (t, J = 5.6 Hz, 2H), 3.84 (s, 3H), 3.60 (s,
3H), 2.50 (2H,
merged with DMSO peak); LC-MS: m/z 343.0 (M-1).
Step-b: Synthesis of 24(6,7-dihydro-4H-pyrano[4,3-dithiazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using methyl 2-((6,7-dihydro-4H-pyrano[4,3-d]thiazol-2-yDamino)-1-
methyl-
1H-benzo14]imidazole-5-carboxylate as starting material and using THF,
methanol and water
(2:2:1) as solvent (Yield: 52%); 8 12.40 (bs, 2H), 7.98 (s, 1H), 7.75 (d, J=
8.0 Hz, 1H), 7.36
(d, J = 8.0 Hz, 1H), 4.58 (s, 2H), 3.92 (t, J = 4.8 Hz, 2H), 3.59 (s, 3H),
2.50 (2H, merged with
DMSO peak); LC-MS: m/z 330.7 (M+1) .
Step-c: Synthesis of 24(6,7-dihydro-4H-pyrano[4,3-d]thiazol-2-yl)amino)-N-(2-
methoxyethyl)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2((6,7-dihydro-4H-pyrano [4,3 -d]thiazol-2-yflamino)-1-
methy1-1H-
benzo[d]imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials. The
crude product was purified by combiflash chromatography using 1.2% methanol in
DCM as
an eluent (Yield: 34%); 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.40 (bs,
1H), 7.96
(s, 1H), 7.66 (bs, 1H), 7.35 (d, J= 8.4 Hz, 1H), 4.58 (bs, 2H), 3.93 (s, 2H),
3.58 (s, 3H), 3.48-
3.42 (m, 4H), 3.27 (s, 3H), 2.50 (2H, merged with DMSO peak); LC-MS: m/z 387.6
(M+1) .
Example 12. Synthesis of 2((1H-benzo[d] imidazol-2-yl)amino)-N-(2-
methoxyethyl)-1-
methyl-1H-benzo[d] imidazole-5-carboxamide:
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Me
IN-11
1101,)¨NH 2 ¨NH
b Me0 =
N )r-NH
12a S 0 12b
Me Me
H N H
HO
* d
NNH 110
N
N
N Me0
0 12c 0 Example 12
Conditions: a) 1,1'-Thiocarbonyldiimidazole , Acetonitrile, RT, 16 h; b)
methyl 3-amino-4-
(methylamino)benzoate ,EDC.HCI, DMF, 100 C, 16 h; d) Li0H.H20, THF, Me0H,
Water, 60 C, 16 h;
e) 2-methoxyethan-1-amine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of N-(1H-benzo [d] imidazol-2-y1)-1H-imidazole-l-
carbothioamide
To a solution of 1H-benzo[d]imidazol-2-amine (1.0 g, 7.5 mmol) in acetonitrile
(10 mL) at
RT was added 1,1'-thiocarbonyldiimidazole (1.34 g, 7.5 mmol) and it was
stirred at RT for 16
h. The reaction mixture was filtered and the solid obtained was dried under
vacuum to afford
the title compound (1.1 g, 60%); 1H NMR (400 MHz, DMSO-d6): 8 13.18 (bs, 2H),
8.55 (s,
1H), 7.93 (d, J = 1.2 Hz, 1H), 7.63-7.59 (m, 2H), 7.36-7.32 (m, 2H), 6.99 (d,
J = 1.2 Hz, 1H).
Step-b: Synthesis of methyl 2-((1H-benzo [d] imidazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
To a stirred solution of N-(1H-benzo[d]imidazol-2-y1)-1H-imidazole-l-
carbothioamide (188
mg, 0.77 mmol) in DMFA (5 mL) was added EDC.HC1 (297 mg, 1.5 mmol) and heated
to
C for 10 min. The reaction mixture was cooled to RT, followed by the addition
of methyl
3-amino-4-(methylamino)benzoate (150 mg, 0.77 mmol) and it was heated at 100 C
for 16 h.
The reaction mixture was cooled to RT, diluted with water (30 mL) and
extracted with
Et0Ac (2 X 50 mL). The combined organic layer was washed with water (30 mL),
brine (30
mL), dried over anhydrous sodium sulfate and concentrated under vacuum. The
residue was
purified by combi flash column chromatography using 2% methanol in DCM as
eluent to
afford the title compound (40 mg, 16%); 1H NMR (400 MHz, DMSO-d6): 8 12.12
(bs, 2H),
8.05 (d, J = 1.6 Hz, 1H), 7.73 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.37-7.34 (m,
3H), 7.12-7.08
(m, 2H), 3.85 (s, 3H), 3.65 (s, 3H); LC-MS: m/z 322.0 (M+1) .
Step-c: Synthesis of 2-01H-benzo [d] imidazol-2-yl)amino)-1-methyl-
1H-
benzo[d]imidazole-5-carboxylic acid
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
51
The title compound was synthesized using the same procedure which was followed
for
intermediate lh, using methyl 2-((1H-benzo [d] imidazol-2-yl)amino)-1-methyl-
1H-
benzo[d]imidazole-5-carboxylate as starting material and methanol, THF and
water as
solvent (Yield: 92%); 1H NMR (400 MHz, DMSO-d6): 8 13.0 (bs, 3H), 7.92 (s,
1H), 7.89 (d,
J = 1.6 Hz, 1H), 7.60 (d, J = 8.4 Hz, 1H), 7.45-7.41 (m, 2H), 7.29-7.26 (m,
2H), 3.68 (s, 3H);
LC-MS: m/z 308.1 (M+1) .
Step-d: Synthesis of 24(1H-benzo[d]imidazol-2-yl)amino)-N-(2-methoxyethyl)-1-
methyl-
1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-((1H-benzo [d] imidazol-2-yl)amino)-1-methyl-1H-benzo [d]
imidazo le-
5-carboxylic acid as starting material (Yield: 14%); 1H NMR (400 MHz, DMSO-
d6): 8 12.10
(bs, 2H), 8.33 (t, J = 4.8 Hz, 1H), 7.98 (d, J = 0.8 Hz, 1H), 7.62 (dd, J =
1.2 Hz, J = 8.0 Hz,
1H), 7.35-7.30 (m, 3H), 7.11-7.07 (m, 2H), 3.64 (s, 3H), 3.50-3.42 (m, 4H),
3.29 (s, 3H); LC-
MS: m/z 365.0 (M-F1) .
Example 13 and 14. Synthesis of 1-methyl-24(5-
(trifluoromethoxy)-1H-
benzo[d]imidazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid and
Synthesis of
N-(2-methoxyethyl)-1-methyl-24(5-(trifluoromethoxy)-1H-benzo[d]imidazol-2-
y1)amino)-1H-benzo[d]imidazole-5-carboxamide:
NO2
a NH2
F3C0 NH2 F3C0 NH2
Me
Me Me S
Nj
N C Et0 40
110 b
Et0 2 -A.- Et0 N
0
0 0
Me
Me OCF3
=
,¨NH Nj¨NH
¨A-- HO -Ab- Me S N)NH
)7¨NH
0 N
0 13 N 14
OCF3 OCF3
Conditions: a) 10% Pd/C, Me0H, H2, RT, 16 h; b) 1,1'-Thiocarbonyldiinnidazole,
Acetonitrile,
60 C, 16 h; c) 4-(trifluoronnethoxy)benzene-1,2-diannine, EDC.HCI, DMF, 100 C,
16 h; d)
Li0H.H20, THF, Et0H, Water, 60 C, 16 h; e) 2-nnethoxyethan-1-amine, DPPA,
DIPEA,
DMF, 0 C - RT, 16 h
Step-a: Synthesis of 4-(trifluoromethoxy)benzene-1,2-diamine
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
52
To a solution of methyl 2-nitro-5-(trifluoromethoxy)aniline (2.0 g, 9.0 mmol)
in methanol (40
mL) was added 10% Pd/C (1.0 g) under nitrogen atmosphere. Then the reaction
mixture was
stirred under hydrogen gas for 16 h. The reaction mixture was filtered through
a bed of celite
and concentrated under vacuum to afford the title compound (1.6 g, 93%); 1H
NMR (400
MHz, DMSO-d6): 8 6.48 (d, J = 8.0 Hz, 1H), 6.45 (d, J = 1.6 Hz, 1H), 6.29 (dd,
J = 2.0 Hz, J
= 8.0 Hz, 1H), 4.80 (s, 2H), 4.58 (s, 2H); LC-MS: m/z 193.0 (M+1) .
Step-b: Synthesis of ethyl 2-(1H-imidazole-1-carbothioamido)-1-methyl-1H-
benzo[d]imidazole-5-carboxylate
To a solution of ethyl 2-amino-1-methy1-1H-benzo [d] imidazole-5-carboxylate
(500 mg, 2.28
mmol) in acetonitrile (10 mL) at RT was added 1,1'-thiocarbonyldiimidazole
(529 mg, 2.97
mmol) and reaction mixture was stirred at 60 C for 16 h. It was cooled to RT
and stirred for
30 min. The solid obtained was filtered and dried under vacuum to afford the
title compound
(500 mg, 66%); 1H NMR (400 MHz, DMSO-d6): 8 13.40 (bs, 1H), 8.68 (s, 1H), 8.40
(d, J =
1.2 Hz, 1H), 8.03 (s, 1H), 7.99 (dd, J= 1.2 Hz, J= 8.4 Hz, 1H), 7.75 (d, J=
8.4 Hz, 1H), 7.01
(s, 1H), 4.35 (q, J = 7.2 Hz, 2H), 3.79 (s, 3H), 1.36 (t, J = 7.2 Hz, 3H); LC-
MS: m/z 328.2
(M-1).
Step-c: Synthesis of ethyl 1-methyl-2-((5-(trifluoromethoxy)-1H-
benzo[d]imidazol-2-
yl)amino)-1H-benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 12 Step-b, using ethyl 2-(1H- imidazo le- 1-c arbothio amido)- 1-
methyl- 1H-
benzo [d] imidazole-5-carboxylate and 4-(trifluoromethoxy)benzene-1,2-diamine
as starting
materials (Yield: 27%); 1H NMR-VT at 90 C (400 MHz, DMSO-d6): 8 12.0 (bs, 2H),
8.09 (s,
1H), 7.77 (d, J = 8.4 Hz, 1H), 7.35 (d, J = 8.4 Hz, 2H), 7.28 (s, 1H), 7.0 (d,
J = 8.4 Hz, 1H),
4.35 (q, J= 6.8 Hz, 2H), 3.63 (s, 3H), 1.35 (t, J= 6.8 Hz, 3H); LC-MS: m/z
420.0 (M+1) .
Step-d: Synthesis of 1-methyl-2-((5-(trifluoromethoxy)-1H-benzo[d]imidazol-2-
yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
intermediate lh, using ethyl 1-methy1-2-((5-(trifluoromethoxy)-1H-benzo [d]
imidazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylate (Yield: 77%); 1H NMR (400 MHz,
DMSO-
d6): 8 12.60 (bs, 3H), 8.05 (d, J = 1.6 Hz, 1H), 7.82 (dd, J = 1.6 Hz, J = 8.4
Hz, 1H), 7.45 (d,
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
53
J = 8.4 Hz, 1H), 7.40 (d, J = 8.8 Hz, 1H), 7.34 (s, 1H), 7.09 (dd, J = 1.2 Hz,
J = 8.8 Hz, 1H),
3.65 (s, 3H); LC-MS: m/z 392.0 (M+1) .
Step-e: Synthesis of N-(2-methoxyethyl)-1-methyl-24(5-(trifluoromethoxy)-1H-
benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methy1-2-((5-(trifluoromethoxy)-1H-benzo [d] imidazol-2-
yl)amino)-
1H-benzo [d] imidazole-5-carboxylic acid as starting material (Yield: 43%); 1H
NMR (400
MHz, DMSO-d6): 8 12.20 (bs, 2H), 8.37 (bs, 1H), 8.0 (d, J = 1.2 Hz, 1H), 7.68
(dd, J = 1.2
Hz, J = 8.4 Hz, 1H), 7.36 (d, J = 8.4 Hz, 2H), 7.30 (s, 1H), 7.01 (d, J = 8.4
Hz, 1H), 3.63 (s,
3H), 3.59-3.43 (m, 4H), 3.29 (s, 3H); LC-MS: m/z 449.0 (M+1) .
Example 15. Synthesis of 1-methyl-24(1-methyl-5-(trifluoromethoxy)-1H-
benzo[d]imidazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid
da NH2 da NH Me al NH Me
a
11111111"
F300 1111111" NO2 F300 4111" NO
F3C0 NH2
- 2
Me ye
N H
N me ye
Et0
NVie c Et0 rrN
HO N ')FN
/>¨NH N N *
0 N
0
0
00F3 ocF3
Conditions: a) NaH, Methyl iodide, DMF, 0 C - RT, 1 h; b) 10% Pd/C, Me0H, H2,
RT, 6 h; c) N1-methyl-4-
(trifluoromethoxy)benzene-1,2-diamine, EDC.HCI, DMF, 100 C, 16 h; d) Li0H.H20,
THF, Et0H, H20, 60 C, 16 h
Step-a: Synthesis of N-methyl-2-nitro-4-(trifluoromethoxy)aniline
To a stirred solution of 2-nitro-4-(trifluoromethoxy)aniline (2.0 mg, 9.0
mmol) in DMFA (20
mL) at 0 C was added sodium hydride (60% dispersion in mineral oil) (0.4 g,
10.0 mmol) and
the reaction mixture was stirred for 10 min. Methyl iodide (0.56 mL, 9.0 mmol)
was added to
the reaction mixture and stirred at RT for 1 h. The reaction mixture was
quenched with
saturated ammonium chloride solution and extracted with ethyl acetate (2 X 100
mL). The
combined organic layer was washed with water (50 mL), brine (50 mL), dried
over
anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combiflash chromatography using 5% ethyl acetate in hexane as an eluent to
afford the title
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
54
compound (1.8 g, 85%); 1H NMR (400 MHz, DMSO-d6): 8 8.28 (d, J = 4.0 Hz, 1H),
8.00 (d,
J = 2.4 Hz, 1H), 7.61 (dd, J = 2.4 Hz, J = 9.2 Hz, 1H), 7.10 (d, J = 9.2 Hz,
1H), 2.98 (d, J =
5.2 Hz, 3H); LC-MS: m/z 237.0 (M+1) .
Step-b: Synthesis of AT1-methyl-4-(trifluoromethoxy)benzene-1,2-diamine
The title compound was synthesized using the same procedure which was followed
for
compound le using N-methyl-2-nitro-4-(trifluoromethoxy)aniline as starting
material and
stirring for 6 h (Yield: 92%); 1H NMR (400 MHz, DMSO-d6): 8 6.44 (s, 1H), 6.42
(d, J =
10.4 Hz, 1H), 6.33 (d, J = 8.4 Hz, 1H), 4.84 (bs, 2H), 2.70 (s, 3H); LC-MS:
m/z 207.0
(M+1) .
Step-c: Synthesis of ethyl 1-methyl-24(1-methyl-5-(trifluoromethoxy)-1H-
benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 12 Step-b using ethyl 2-(1H- imidazo le- 1-c arbothio amido)- 1-methyl-
1H-
benzo [d] imidazole-5-carboxylate and N1-methy1-4-(trifluoromethoxy)benzene-
1,2-diamine
as starting materials (Yield: 37%); LC-MS: m/z 434.2 (M+1) .
Step-d: Synthesis of 1-methyl-2-01-methyl-5-(trifluoromethoxy)-1H-benzo [d]
imidazol-
2-yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methy1-2-((1-methyl-5-(trifluoromethoxy)-1H-benzo
[d] imidazol-
2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate as starting material (Yield:
53%); 1H NMR
(400 MHz, DMSO-d6): 8 12.5 (bs, 2H), 8.10 (d, J= 1.2 Hz, 1H), 7.79 (dd, J= 1.6
Hz, J= 8.4
Hz, 1H), 7.44 (s, 1H), 7.41-7.37 (m, 2H), 7.08 (d, J = 8.8 Hz, 1H), 3.70 (s,
3H), 3.68 (s, 3H);
LC-MS: m/z 406.1 (M+1) .
Example 16. Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-hydroxyethyl)-1-
methyl-
1H-benzo[d]imidazole-5-carboxamide
Me Me
I I
N H N H
1104 N a
HO
N )T-- -1"-- H
N 104 N
--0
N a
0 N .
HOr----"
0 N ts
Conditions: a) 2-aminoethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
To a stirred solution of 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (50 mg, 0.16 mmol) in DMFA (1.0 mL) at 0 C was added N-
ethyldiisopropyl
amine (0.03 mL, 0.16 mmol) and HBTU (62 mg, 0.16 mmol). The reaction mixture
was
stirred for 30 min, followed by the addition of 2-aminoethan-1-ol (10 mg, 0.16
mmol) and
stirring was continued at RT for 16 h. Once the reaction was completed
(monitored by TLC),
the reaction mixture was diluted with cold water (20 mL) and stirred for 15
min. The solid
obtained was filtered, washed with diethyl ether and dried under vacuum to
afford the title
compound (25 mg, 44%) as white solid; 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs,
1H),
8.36 (t, J = 5.4 Hz, 1H), 8.08 (d, J = 0.9 Hz, 1H), 7.78-7.76 (m, 1H), 7.48
(d, J = 8.3 Hz,
2H), 7.43 (d, J = 7.8 Hz, 1H), 7.21 (t, J = 7.3 Hz, 1H), 7.12 (t, J = 7.3 Hz,
1H), 4.72 (bs, 1H),
3.63 (s, 3H), 3.54 (t, J = 6.4 Hz, 2H), 3.37-3.34 (m, 2H); LC-MS: m/z 352.2
(M+1) .
Example 17. Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2-(2-
hydroxyethoxy)ethyl)-
1-methyl-1H-benzo [d] imidazole-5-carboxamide
Me ye
i& CI
H
io N.Me b
EtO2C W.-- NO2 a¨"" EtO2C EtO2C NH EtO2C ¨/NH 2 /10
= N \r
NO2
EtO2C N *
ye
Me
N H
H Ns
* 140
L N
HO N 110
0 N
0
Conditions: a) Methyl amine, DMF, 60 C, 16 h; b) 10% Pd/C, Me0H, H2, RT, 3 h;
c) Cyanogen bromide, THF, H20, 60 C, 16 h;
d) NaH, 2-chlorobenzo[ ci]oxazole , 1,4-Dioxane, RT, 16 h; e) Li0H.H20, THF,
Ethanol, Water, 60 C, 16 h; f) 2-(2-
aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 4-(methylamino)-3-nitrobenzoate
To a solution of ethyl 4-chloro-3-nitrobenzoate (30.0 g, 131 mmol) in DMFA
(100 mL) at RT
was added methyl amine (26.8 mL (40% aqueous solution), 262 mmol) and the
reaction
mixture was stirred at 60 C for 16 h. The reaction mixture was cooled to RT,
diluted with
cold water (1000 mL) and stirred for 1 h. The solid obtained was filtered and
dried under
vacuum to afford the product as a yellow solid (28.0 g, 96%); 1H NMR (400 MHz,
DMSO-
d6): 8 8.62 (d, J = 2.0 Hz, 1H), 8.59 (bs, 1H), 7.99 (dd, J = 2.0 Hz, J = 8.8
Hz, 1H), 7.07 (d, J
= 8.8 Hz, 1H), 4.29 (q, J = 6.8 Hz, 2H), 3.01 (d, J = 5.2 Hz, 3H), 1.31 (t, J
= 6.8 Hz, 3H);
LC-MS: m/z 225.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
56
Step-b: Synthesis of ethyl 3-amino-4-(methylamino)benzoate
To a solution of ethyl 4-(methylamino)-3-nitrobenzoate (27.0 g, 120 mmol) in
methanol (300
mL) was added slurry of 10% Pd/C (2.8 g in 15 mL of ethanol) under nitrogen
atmosphere.
The flask was kept in a Parr shaker at RT under hydrogen (60 psi) for 3 h. The
reaction
mixture was filtered through celite bed and concentrated under vacuum to
afford the title
compound (19.0 g, 82%); 1H NMR (400 MHz, DMSO-d6): 8 7.23 (dd, J = 1.6 Hz, J =
8.4 Hz
1H), 7.16 (d, J= 1.6 Hz, 1H), 6.39 (d, J= 8.4 Hz, 1H), 5.38-5.37 (m, 1H), 4.66
(s, 2H), 4.18
(q, J = 7.2 Hz, 2H), 2.77 (d, J = 5.2 Hz, 3H), 1.26 (t, J = 7.2 Hz, 3H); LC-
MS: m/z 195.1
(M+1) .
Step-c: Synthesis of ethyl 2-amino-1-methyl-1H-benzo [d] imidazole-5-
carboxylate
To a solution of ethyl 3-amino-4-(methylamino)benzoate (17.0 g, 87.6 mmol) in
THF (68
mL) and water (170 mL) at RT was added cyanogen bromide (11.13 g, 105.2 mmol)
and the
reaction mixture was stirred at 60 C for 16 h. The reaction mixture was cooled
to RT and
basified with saturated sodium bicarbonate solution. The solid obtained was
filtered and dried
under vacuum to afford the title compound which was used in the next step
without further
purification (18.0 g, 94%); 1H NMR (400 MHz, DMSO-d6): 8 7.70 (d, J = 1.2 Hz,
1H), 7.59
(dd, J = 1.2 Hz, J = 8.4 Hz, 1H), 7.20 (d, J = 8.4 Hz, 1H), 6.64 (s, 2H), 4.27
(q, J = 6.8 Hz,
2H), 3.53 (s, 3H), 1.32 (t, J= 6.8 Hz, 3H); LC-MS: m/z 220.1 (M+1) .
Step-d: Synthesis of ethyl 2-(benzo [d] oxazol-2-ylamino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
To a solution of ethyl 2-amino-l-methyl-1H-benzo [di imidazole-5-carboxylate
(4.0 g, 18.3
mmol) in 1,4-dioxane (40 mL) at 0 C was added sodium hydride (60% dispersion
in mineral
oil) (1.09 g, 27.4 mmol) portion wise and stirred for 10 min. 2-
chlorobenzo[d]oxazole (2.79
g, 18.3 mmol) was added to the reaction mixture and stirred at RT for 16 h.
The reaction
mixture was poured over cold water (100 mL) and stirred at RT for 5 to 10 min.
The obtained
solid was filtered, dried under vacuum and purified by combiflash column
chromatography
using 100% DCM as an eluent to afford the title compound (4.0 g, 60%); 1H NMR
(400
MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.24 (s, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.53-
7.43 (m, 3H),
7.22 (t, J = 7.2 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 4.33 (q, J = 7.2 Hz, 2H),
3.64 (s, 3H), 1.35
(t, J= 7.2 Hz, 3H); LC-MS: m/z 337.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
57
Step-e: Synthesis of 2-(benzo [d] oxazol-2-ylamino)-1-methyl-1H-benzo [d]
imidazole-5-
carboxylic acid
To a stirred solution of ethyl 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-
benzo [d] imidazole-
5-carboxylate (4.0 g, 11.90 mmol) in a mixture of solvent of THF (20 mL),
ethanol (20 mL)
and water (10 mL) was added lithium hydroxide monohydrate (1.25 g, 29.76
mmol). The
reaction mixture was heated at 60 C with stirring for 16 h. The reaction
mixture was cooled
to RT and concentrated under reduced pressure. The residue was dissolved in
water (60 mL),
acidified with 1 N HC1 to obtain the solid which was filtered and dried under
vacuum to
afford the title compound (3.4 g, 93%); 1H NMR (400 MHz, DMSO-d6): 8 12.60
(bs, 2H),
8.20 (d, J = 1.6 Hz, 1H), 7.87 (dd, J = 1.6 Hz, J = 8.0 Hz, 1H), 7.51-7.48 (m,
2H), 7.44 (d, J
= 7.2 Hz, 1H), 7.23-7.20 (m, 1H), 7.15-7.11 (m, 1H), 3.64 (s, 3H); LC-MS: m/z
309.1
(M+1) .
Step-f: Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-(2-
hydroxyethoxy)ethyl)-1-
methyl-1H-benzo [d] imidazole-5-carboxamide
To a stirred solution of 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (3.0 g, 9.74 mmol) in DMFA (30 mL) at 0 C was added N-
ethyldiisopropyl
amine (4.36 mL, 24.25 mmol) and HBTU (4.06 g, 10.71 mmol). The reaction
mixture was
stirred for 30 min, followed by the addition of 2-(2-aminoethoxy)ethan-1-ol
(1.02 g, 9.74
mmol) and stirring was continued at RT for 16 h. Once the reaction was
completed
(monitored by TLC), the reaction mixture was diluted with cold water (300 mL)
and stirred
for 30 min. The solid obtained was filtered, dried under vacuum and purified
by combiflash
column chromatography using 5% methanol in DCM as an eluent to afford the
title
compound (2.9 g, 75%) as white solid; 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs,
1H),
8.43 (t, J = 5.3 Hz, 1H), 8.07 (s, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.48 (d, J =
7.9 Hz, 2H), 7.43
(d, J= 7.8 Hz, 1H), 7.21 (t, J= 7.3 Hz, 1H), 7.11 (t, J= 7.3 Hz, 1H), 4.60
(bs, 1H), 3.63 (s,
3H), 3.57-3.42 (m, 8H); LC-MS: m/z 396.2 (M+1) .
Example 18. Synthesis of N-(2-aminoethyl)-2-(benzo[d]oxazol-2-ylamino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxamide hydrochloride
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
58
=Me Me
Me
Ni Ni
H ¨NH
r\i/>¨NH a BocH ,
HO = N b 1-12N
=
0 N
N
N HCI N
N 0 0
Conditions: a) tert-butyl (2-aminoethyl)carbamate, HBTU, DIPEA, DMF, 0 C - RT,
16 h; b) 4 N HCI in 1,4-Dioxane, THF,
0 C - RT, 3 h
Step-a: Synthesis of tert-butyl (2-(2-(benzo[d] oxazol-2-ylamino)-1-methyl-1H-
benzo [d] imidazole-5-carboxamido)ethyl)carbamate
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-(benzo [d] oxazol-2- ylamino)- 1-methyl- 1H-benzo [d]
imidazo le-5-
carboxylic acid and tert-butyl (2-aminoethyl)carbamate as starting materials
(Yield: 51%); 1H
NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.41 (t, J= 5.1 Hz, 1H), 8.08 (s,
1H), 7.75 (d,
J= 8.3 Hz, 1H), 7.48 (d, J= 8.4 Hz, 2H), 7.43 (d, J= 7.8 Hz, 1H), 7.23-7.19
(m, 1H), 7.14-
7.09 (m, 1H), 6.92 (d, J = 5.4 Hz, 1H), 3.64 (s, 3H), 3.34-3.33 (m, 2H merged
with DMSO
moisture peak), 3.15-3.10 (m, 2H), 1.38 (s, 9H); LC-MS: m/z 451.55 (M+1) .
Step-b: Synthesis of N-(2-aminoethyl)-2-(benzo[d]oxazol-2-ylamino)-1-methyl-1H-
benzo[d]imidazole-5-carboxamide hydrochloride
To a solution of tert-butyl (2-(2-(benzo[d]oxazol-2-ylamino)-1-methy1-1H-
benzo[d]imidazole-5-carboxamido)ethyl)carbamate (50 mg, 0.11 mmol) in THF (2
mL) at
0 C was added 4 N HC1 in 1,4-dioxane (0.5 mL) and reaction mixture was stirred
at RT for 3
h. The reaction mixture was concentrated on rotary evaporator and stirred in
diethyl ether (10
mL). The solid obtained was filtered and dried under vacuum to afford the
titled compound
(35 mg, 81%); 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.70 (s, 1H), 8.13
(s, 1H),
7.96 (bs, 3H), 7.86 (d, J = 8.3 Hz, 1H), 7.56 (d, J = 8.3 Hz, 1H), 7.48 (d, J
= 7.9 Hz, 2H),
7.25 (t, J= 7.6 Hz, 1H), 7.17 (t, J= 7.8 Hz, 1H), 3.67 (s, 3H), 3.55 (q, J=
5.7 Hz, 2H), 3.03-
2.99 (m, 2H); LC-MS: m/z 351.10 (M+1) .
Example 19. Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2-(2-
(dimethylamino)acetamido)ethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
59
0 Me
Thr,OH a me2N-ThiN A )(Me b
Me2N 0 Me Me2NTh(NNH2 TFA
0 0 0
Me Me
NH , me N, I H 101
HO N -)"-- -2 N N
N 0 N
0
Conditions: a) tert-butyl (2-aminoethyl)carbamate, HBTU, DIPEA, DMF, 0 C - RT,
16 h; b) TFA,
DCM, 0 C - RT, 16 h; c) N-(2-aminoethyl)-2-(dimethylamino)acetamide
trifluoroacetate, HBTU,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of tert-butyl (2-(2-(dimethylamino)acetamido)ethyl)carbamate
To a stirred solution of dimethylglycine (1.0 g, 9.71 mmol) in DMFA (10 mL) at
0 C was
added N-ethyldiisopropyl amine (1.69 mL, 9.71 mmol) and HBTU (3.68 g, 9.71
mmol). The
reaction mixture was stirred for 30 min, followed by the addition of tert-
butyl (2-
aminoethyl)carbamate (1.55 g, 9.71 mmol). The reaction mixture was stirred at
RT for 16 h.
Once the reaction was completed (monitored by TLC), the reaction mixture was
diluted with
cold water (50 mL) and extracted with Et0Ac (2 X 100 mL). The combined organic
layers
were washed with water (3 X 30 mL), brine solution (50 mL), dried over
anhydrous sodium
sulfate and concentrated under vacuum. The solid obtained was stirred in
diethyl ether (20
mL) for 5 min, filtered and dried under vacuum to afford the title compound
(600 mg, 25%);
1H NMR (400 MHz, DMSO-d6): 8 7.77 (t, J = 5.6 Hz, 1H), 6.83 (t, J = 5.2 Hz,
1H), 3.11 (q, J
= 6.2 Hz, 2H), 2.99 (q, J= 6.1 Hz, 2H), 2.82 (s, 2H), 2.18 (s, 6H), 1.37 (s,
9H); LC-MS: m/z
246.15 (M+1) .
Step-b: Synthesis of N-(2-aminoethyl)-2-(dimethylamino)acetamide
trifluoroacetate
To a solution of tert-butyl (2-(2-(dimethylamino)acetamido)ethyl)carbamate
(600 mg, 2.45
mmol) in DCM (15 mL) at 0 C was added TFA (0.56 mL, 7.34 mmol) and reaction
mixture
was stirred at RT for 16 h. The reaction mixture was concentrated on rotary
evaporator and
dried under vacuum to afford the titled compound (600 mg, 100%); 1H NMR (400
MHz,
DMSO-d6): 8 9.75 (bs, 1H), 8.75 (t, J = 5.6 Hz, 1H), 7.90 (bs, 2H), 3.88 (s,
2H), 3.38 (q, J =
6.2 Hz, 2H), 2.91 (q, J= 5.9 Hz, 2H), 2.82 (s, 6H) ; LC-MS: m/z 146.20 (M+1) .
Step-c: Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-(2-
(dimethylamino)acetamido)ethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
To a stirred solution of 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (100 mg, 0.32 mmol) in DMFA (4 mL) at 0 C was added N-
ethyldiisopropyl
amine (0.17 mL, 0.97 mmol) and HBTU (123 mg, 0.32 mmol). The reaction mixture
was
stirred for 30 min, followed by the addition of N-(2-aminoethyl)-2-
(dimethylamino)acetamide
trifluoroacetate (145 mg, 0.39 mmol). The reaction mixture was then stirred at
RT for 16 h.
Once the reaction was completed (monitored by TLC), the reaction mixture was
diluted with
cold water (15 mL). The solid obtained was filtered, washed with diethyl ether
and dried
under vacuum to afford the title compound (40 mg, 28%) as a white solid; 1H
NMR (400
MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.44 (t, J = 4.9 Hz, 1H), 8.07 (s, 1H), 7.92
(bs, 1H), 7.74
(d, J= 7.8 Hz, 1H), 7.49-7.47 (m, 2H), 7.43 (d, J= 7.8 Hz, 1H), 7.21 (t, J=
7.6 Hz, 1H), 7.12
(t, J = 7.6 Hz, 1H), 3.63 (s, 3H), 3.37 (t, J = 5.2 Hz, 2H), 3.35 (2H merged
with DMSO
moisture peak), 2.86 (s, 2H), 2.19 (s, 6H); LC-MS: m/z 436.0 (M+1) .
Example 20. Synthesis of 2-(benzo[d]oxazol-2-ylamino)-1-methyl-
N-(2-
morpholinoethyl)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-morpholinoethan-1-amine and 2-(benzo[d]oxazol-2-ylamino)-1-
methy1-
1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H NMR (400
MHz, DMSO-
d6): 8 12.21 (bs, 1H), 8.33 (s, 1H), 8.06 (s, 1H), 7.73 (d, J = 8.4 Hz, 1H),
7.48 (d, J = 8.4 Hz,
1H), 7.43 (d, J = 8.0 Hz, 2H), 7.21 (t, J = 7.2 Hz, 1H), 7.11 (t, J = 8.0 Hz,
1H), 3.63 (s, 3H),
3.58 (t, J = 3.6 Hz, 4H), 3.41 (q, J = 6.4 Hz, 2H), 3.30 (2H merged with DMSO
moisture
peak), 2.43 (bs, 4H); LC-MS: m/z 421.2 (M+1) .
Example 21. Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2-
(dimethylamino)ethyl)-1-
methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using N,N-dimethylethane-1,2-diamine and 2-(benzo[d]oxazol-2-
ylamino)-1-
methy1-1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H NMR
(400 MHz,
DMSO-d6): 8 12.21 (bs, 1H), 8.29 (t, J = 5.2 Hz, 1H), 8.07 (s, 1H), 7.74 (d, J
= 8.4 Hz, 1H),
7.48-7.42 (m, 3H), 7.21 (t, J= 7.6 Hz, 1H), 7.11 (t, J= 8.0 Hz, 1H), 3.63 (s,
3H), 3.39-3.35
(m, 2H), 2.50-2.42 (m, 2H), 2.19 (s, 6H); LC-MS: m/z 379.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
61
Example 22. Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2-((4,5-dihydro-1H-
imidazol-
2-yl)amino)ethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using N1-(4,5-dihydro-1H-imidazol-2-yl)ethane-1,2-diamine and 2-
(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d] imidazole-5-carboxylic acid
as starting
materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 2H), 8.52 (bs, 1H), 8.20
(bs, 1H),
8.09 (s, 1H), 7.75 (d, J= 8.4 Hz, 1H), 7.52-7.43 (m, 3H), 7.21 (t, J= 7.2 Hz,
1H), 7.12 (t, J=
8.0 Hz, 1H), 3.64 (s, 3H), 3.59 (s, 2H), 3.45-3.43 (m, 2H), 3.36-3.34 (m, 4H);
LC-MS: m/z
417.05 (M-1)-.
Example 23. Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2-hydroxypropy1)-1-
methyl-
lH-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-aminopropan-2-ol and 2-(benzo[d]oxazol-2-ylamino)-1-methy1-
1H-
benzo[d]imidazole-5-carboxylic acid as starting materials. 1H NMR (400 MHz,
DMSO-d6):
8 12.30 (bs, 1H), 8.36 (t, J = 5.6 Hz, 1H), 8.19 (d, J = 1.2 Hz, 1H), 7.78
(dd, J = 1.2 Hz, J =
8.4 Hz, 1H), 7.52-7.36 (m, 3H), 7.21-7.19 (m, 1H), 7.14-7.09 (m, 1H), 4.87-
4.78 (m, 1H),
3.81 (q, J = 6.0 Hz, 1H), 3.64 (s, 3H), 3.24-3.20 (m, 2H), 1.14 (d, J = 6.8
Hz, 3H); LC-MS:
m/z 366.2 (M+1) .
Example 24. Synthesis of 2-(benzo[d] oxazol-2-ylamino)-N-(2,3-dihydroxypropy1)-
1-
methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 3-aminopropane-1,2-diol and 2-(benzo[d]oxazol-2-ylamino)-1-
methy1-
1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H NMR (400
MHz, DMSO-
d6): 8 12.30 (bs, 1H), 8.31 (t, J= 5.2 Hz, 1H), 8.08 (s, 1H), 7.77 (dd, J= 1.6
Hz, J= 8.4 Hz,
1H), 7.49-7.42 (m, 3H), 7.21 (t, J = 8.0 Hz, 1H), 7.11 (t, J = 7.2 Hz, 1H),
4.82 (d, J = 4.4 Hz,
1H), 4.58 (bs, 1H), 3.64 (s, 3H), 3.45-3.37 (m, 3H), 3.26-3.19 (m, 2H); LC-MS:
m/z 382.1
(M+1) .
Example 25. Synthesis of 2-(benzo[d]oxazol-2-ylamino)-N-(2-(2-
hydroxypropoxy)ethyl)-
1,6-dimethyl-1H-benzo[d]imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
62
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-aminoethoxy)propan-2-ol and 2-(benzo [d]o xazol-2-
ylamino)- 1-
methy1-1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H NMR
(400 MHz,
DMSO-d6): 8 12.30 (bs, 1H), 8.42 (t, J = 5.2 Hz, 1H), 8.07 (s, 1H), 7.75 (dd,
J = 1.2 Hz, J =
8.4 Hz, 1H), 7.49-7.42 (m, 3H), 7.21 (t, J= 7.6 Hz, 1H), 7.11 (t, J= 8.0 Hz,
1H), 4.57 (bs,
1H), 3.75 (q, J = 5.6 Hz, 1H), 3.63 (s, 3H), 3.57-3.54 (m, 2H), 3.46-3.43 (m,
2H), 3.29-3.23
(m, 2H), 1.03 (d, J= 6.4 Hz, 3H); LC-MS: m/z 410.2 (M+1) .
Example 26. Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-((3-hydroxyoxetan-3-
yl)methyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 3-(aminomethyl)oxetan-3-ol and 2-(benzo[d]oxazol-2-ylamino)-
1-
methy1-1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H NMR
(400 MHz,
DMSO-d6): 8 12.30 (bs, 1H), 8.55 (t, J = 5.2 Hz, 1H), 8.10 (d, J = 1.2 Hz,
1H), 7.79 (dd, J =
1.6 Hz, J = 8.4 Hz, 1H), 7.51 -7.42 (m, 3H), 7.21 (t, J = 7.6 Hz, 1H), 7.14
(t, J = 7.6 Hz, 1H),
5.90 (s, 1H), 4.52 (d, J = 6.8 Hz, 2H), 4.41 (d, J = 6.4 Hz, 2H), 3.64 (s,
3H), 3.59 (d, J = 6.0
Hz, 2H); LC-MS: m/z 394.2 (M+1) .
Example 27. Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-(2-
hydroxy-2-
methylpropoxy)ethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-aminoethoxy)-2-methylpropan-2-ol and 2-(benzo[d]oxazol-
2-
ylamino)-1-methy1-1H-benzo [d] imidazole-5-carboxylic acid as starting
materials. 1H NMR
(400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.43 (t, J = 5.2 Hz, 1H), 8.07 (d, J =
1.2 Hz, 1H),
7.75 (dd, J = 2.4 Hz, J = 8.4 Hz, 1H), 7.49 -7.42 (m, 3H), 7.21 (dd, J = 1.2
Hz, J = 7.6 Hz,
1H), 7.12 (dd, J = 1.2 Hz, J = 7.6 Hz, 1H), 4.34 (bs, 1H), 3.63 (s, 3H), 3.57
(t, J = 6.4 Hz,
2H), 3.47-3.43 (m, 2H), 3.20 (s, 2H), 1.09 (s, 6H); LC-MS: m/z 424.2 (M+1) .
Example 28. Synthesis of 2-(benzo[d]oxazol-2-ylamino)-1-methyl-N-(2-
(pyrrolidin-1-
ypethyl)-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-(pyrrolidin-1-yl)ethan-1-amine and 2-(benzo[d]oxazol-2-
ylamino)-1-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
63
methyl-1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H NMR
(400 MHz,
DMSO-d6): 8 12.24 (bs, 1H), 8.38 (t, J = 5.6 Hz, 1H), 8.07 (s, 1H), 7.74 (d, J
= 8.4 Hz, 1H),
7.49-7.42 (m, 3H), 7.21 (t, J = 6.8 Hz, 1H), 7.11 (t, J = 7.2 Hz, 1H) 3.69 (s,
3H), 3.48-3.37
(m, 2H), 2.64-2.57 (m, 2H), 2.50 (4H merged in DMSO peak), 1.66 (bs, 4H); LC-
MS: m/z
405.2 (M+1) .
Example 29. Synthesis of 2-(2-(2-(benzo[d] oxazol-2-ylamino)-1-methyl-1H-
benzo[d]imidazole-5-carboxamido)ethoxy)acetic acid
H Me H Me
HO yOMe
a b
0 Me 0 0 Me 0
Me Me
N Me
HO,N
HO =N-NH C EtO , / 0
N A N
0 N 0 0
0 N
Conditions: a) ethyl 2-bromoacetate, NaH, KI, THF, 0 C - RT, 2 h; b) TFA, DCM,
RT, 5 h; c) ethyl 2-(2-aminoethoxy)acetate
trifluoro acetate, HBTU, DIPEA, DMF, 0 C - RT, 16 h; d) Li0H.H20, Ethanol,
THF, Water, RT, 3 h
Step-a: Synthesis of ethyl 2-(2-((tert-butoxycarbonyl)amino)ethoxy)acetate
To a solution of tert-butyl (2-hydroxyethyl)carbamate (1.0 g, 6.21 mmol) in
THF (10 mL) at
0 C was added sodium hydride (60% dispersion in mineral oil) (397 mg, 9.93
mmol) by
portions followed by the addition of potassium iodide (164 mg, 0.99 mmol) and
ethylbromo
acetate (2.07 g, 12.42 mmol). The reaction mixture was stirred at RT for 2 h.
The reaction
mixture was diluted with cold water (60 mL) and extracted with ethyl acetate
(3 X 60 mL).
The combined organic layers were washed with water (30 mL), brine solution (30
mL), dried
over anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combiflash column chromatography using 1% methanol in dichloromethane as an
eluent to
afford the titled compound (1.0 g, 65%); 1H NMR (400 MHz, DMSO-d6): 8 6.74
(bs, 1H),
4.14-4.08 (m, 4H), 3.45 (t, J= 5.6 Hz, 2H), 3.08 (q, J= 6.2 Hz, 2H), 1.37 (s,
9H), 1.19 (t, J=
6.8 Hz, 3H); LC-MS: m/z 148.1 (M-Boc) .
Step-b: Synthesis of ethyl 2-(2-aminoethoxy)acetate trifluoroacetic acid salt
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
64
To a stirred solution of ethyl 2-(2-((tert-butoxycarbonyl)amino)ethoxy)acetate
(1.0 g, 4.05
mmol) in DCM (5 mL) at RT was added trifluoroacetic acid (1 mL) and stirred at
RT for 5 h.
The reaction mixture was concentrated under reduced pressure to afford the
titled compound
(1.1 g, 100%) which was used in the next step without any further
purification; 1H NMR (400
MHz, DMSO-d6): 8 7.88 (bs, 3H), 4.17 (s, 2H), 4.14 (q, J = 6.8 Hz, 2H), 3.68
(t, J = 5.0 Hz,
2H), 3.01 (q, J = 5.2 Hz, 2H), 1.21 (t, J = 7.0 Hz, 3H); LC-MS: m/z 148.2
(M+1) .
Step-c: Synthesis of ethyl 2-(2-(2-(benzo[d]oxazol-2-ylamino)-1-methyl-1H-
benzo [d] imidazole-5-carboxamido)ethoxy)acetate
To a stirred solution of 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (150 mg, 0.48 mmol) in DMFA (2 mL) at 0 C was added N-
ethyldiisopropyl
amine (0.26 mL, 1.46 mmol) and HBTU (184 mg, 0.48 mmol). The reaction mixture
was
stirred for 30 min, followed by the addition of ethyl 2-(2-aminoethoxy)acetate
trifluoroacetate
(129 mg, 0.53 mmol). The reaction mixture was then stirred at RT for 16 h.
Once the reaction
was completed (monitored by TLC), the reaction mixture was diluted with cold
water (6 mL)
and stirred for 5 min. The solid precipitated was filtered and dried under
vacuum to afford the
titled compound (125 mg, 59%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H),
8.46 (bs,
1H), 8.08 (s, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.49-7.47 (m, 2H), 7.43 (d, J =
7.6 Hz, 1H), 7.21
(t, J = 8.0 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 4.15 (s, 2H), 4.12 (q, J = 7.0
Hz, 2H), 3.65-3.62
(m, 5H), 3.47 (q, J= 5.8 Hz, 2H), 1.19 (t, J= 7.0 Hz, 3H); LC-MS: m/z 438.1
(M+1) .
Step-d: Synthesis of 2-(2-(2-(benzo [d] oxazol-2-ylamino)-1-methyl-
1H-
benzo[d]imidazole-5-carboxamido)ethoxy)acetic acid
To a solution of ethyl 2-(2-(2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo
[d] imidazole-
5-carboxamido)ethoxy)acetate (110 mg, 0.25 mmol) in a mixture of solvents of
THF (1 mL),
ethanol (1 mL) and water (0.5 mL) was added lithium hydroxide monohydrate (26
mg, 0.63
mmol). The reaction mixture was stirred at RT for 3 h. The reaction mixture
was
concentrated under reduced pressure, the residue was dissolved in water (5 mL)
and acidified
with 1 N HC1 to obtain the solid which was filtered and dried under vacuum to
afford the
titled compound (80 mg, 78%); 1H NMR (400 MHz, DMSO-d6): 8 12.60 (bs, 1H),
8.47 (s,
1H), 8.08 (s, 1H), 7.77 (d, J = 8.4 Hz, 1H), 7.50-7.43 (m, 3H), 7.22 (t, J =
7.6 Hz, 1H), 7.13
(t, J = 7.2 Hz, 1H), 4.06 (s, 2H), 3.64 (bs, 5H), 3.45 (q, J = 5.2 Hz, 2H); LC-
MS: m/z 410.4
(M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Example 30. 2-(2-(2-(benzo[d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxamido)ethoxy)ethyl DL-valinate hydrochloride
Boo
H2N HN
Me )y)H a me )((:)H
Me 0 Me 0
Me
Me
,
H b HNBoc H
HO c) N
/ NH
Me y.(0.7'N N ¨)1"-
/\. N
N 0 Me 0 0
Me
CIH H2N H
N )1-0
Me 0 0 N
Conditions: a) Di-tert-butyl dicarbonate, Sodium hydroxide, THF, Water, RT, 16
h; b) (tert-
butoxycarbonyl)valine, HATU, DIPEA, DMF, 0 C - RT, 16 h; c) 4 N HC1 in 1,4-
dioxane, 1,4-dioxane, 10 C -
RT, 16 h
Step-a: Synthesis of (tert-butoxycarbony1)-DL-valine
To a stirred solution of DL-valine (2.0 g, 17.07 mmol) in THF (25 mL) and
water (20 mL) at
RT was added sodium hydroxide (0.82 g, 20.5 mmol) and di-tert-butyl
dicarbonate (4.09 g,
18.77 mmol) and the reaction mixture was stirred for 16 h. The mixture was
cooled to 0 C,
acidified with 1 N HC1 and extracted with Et0Ac (2 X 50 mL). The combined
organic layers
were washed with brine solution (30 mL), dried over anhydrous sodium sulfate
and
concentrated under vacuum. Crude product (1.8 g) was used in the next step
without any
further purification,
Step-b: Synthesis of 2-(2-(2-(benzo[d]oxazol-2-ylamino)-1-methy1-1H-
benzo[d]imidazole-5-carboxamido)ethoxy)ethyl (tert-butoxycarbonyl) -DL-
valinate
To a stirred solution of 2-(benzo [d]oxazol-2-ylamino)-N - (2- (2-hy dr oxy
ethoxy)ethyl)-1-
methy1-1H-benzo[d]imidazole-5-carboxamide (70 mg, 0.18 mmol) and (tert-
butoxycarbonyl)
-DL-valine (42 mg, 0.19 mmol) in DMFA (5 mL) at 0 C was added N-
ethyldiisopropyl amine
(0.04 mL, 0.21 mmol) and HATU (80 mg, 0.21 mmol). The reaction mixture was
stirred at
RT for 16 h. Once the reaction was completed (monitored by TLC), the reaction
mixture was
diluted with cold water (20 mL) and extracted with ethyl acetate (2 X 30 mL).
The combined
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
66
organic layers were washed with water (20 mL), brine solution (20 mL), dried
over
anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combiflash column chromatography using 4.1% methanol in dichloromethane as an
eluent to
afford the titled compound (40 mg, 38%); LC-MS: m/z 595.7 (M+1) .
Step-c: Synthesis of 2-(2-(2-(benzo[d] oxazol-2-ylamino)-1-methyl-1H-benzo [d]
imidazole-
5-carboxamido)ethoxy)ethyl DL-valinate hydrochloride
To a stirred solution of 2-(2-(2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-
benzo [d] imidazole-
5-carboxamido)ethoxy)ethyl (tert-butoxycarbonyl) -DL-valinate (40 mg, 0.07
mmol) in 1,4-
dioxane (5 mL) at 10 C was added 4 N HC1 in 1,4-dioxane (0.03 mL, 0.13 mmol)
and the
reaction mixture was stirred at RT for 16 h. Once the reaction was completed
(monitored by
TLC), the reaction mixture was concentrated under reduced pressure and
lyophilized for 18 h
to afford the titled compound (19 mg, 53%) as hydrochloride salt; 1H NMR (400
MHz,
DMSO-d6): 8 12.50 (s, 1H), 8.52 (bs, 1H), 8.40 (bs, 3H), 8.09 (s, 1H), 7.80
(d, J = 8.4 Hz,
1H), 7.54 (d, J = 7.6 Hz, 1H), 7.48 (d, J = 7.6 Hz, 2H), 7.25 (t, J = 7.2 Hz,
1H), 7.17 (t, J =
8.0 Hz, 1H), 4.43-4.40 (m, 2H), 4.28-4.25 (m, 2H), 3.95 (bs, 1H), 3.70-3.67
(s, 3H), 3.61-
3.57 (m, 2H), 3.45-3.44 (m, 2H), 2.15-2.11 (m, 1H), 0.97 (d, J= 6.8 Hz, 3H),
0.92 (d, J= 6.8
Hz, 3H); LC-MS: m/z 495.1 (M+1) .
Examples 31 and 32. Synthesis of 1-methyl-24(6-(trifluoromethyl)benzoldloxazol-
2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid and Synthesis of N-(2-
methoxyethyl)-
1-methyl-24(6-(trifluoromethyl)benzo [d] oxazol-2-yl)amino)-1H-benzo [d]
imidazole-5-
carboxamide
Fõ so OH a F3C so OH b F3C 411 so OH
c F3C al 0 d F3C so 0
õ... µ _),.. ¨SH ¨CI "
N N
NO2 NH2
M
Me e
,
Ain Ni
Me
NNNI);1 0 HO VI NNFil-_/ 0
0 Ni ¨NH2 e EtO2C i
N
EtO2C N N
W 0
WI
CF3
CF3
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
67
Me
H N
MeON N
r.c
3
Conditions: a) HNO3, Acetic acid, 0 C- RT, 16 h; b) 10% Pd/C, Me0H, H2, RT, 16
h; c) Potassium ethyl xanthate, Ethanol,
Reflux, 16 h; d) S0Cl2, Cat.DMF , reflux, 3 h; e) NaH, 2-chloro-6-
(trifluoromethyl)benzo[ c]oxazole , 1,4-Dioxane, RT, 16 h; f)
Li0H.H20, THF, Ethanol, Water, 60 C, 16 h; g) 2-methoxyethylamine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-nitro-5-(trifluoromethyl)phenol
To a solution of 3-(trifluoromethyl)phenol (5.0 g, 30.86 mmol) in acetic acid
(50 mL) was
added 60% aqueous nitric acid (3.5 mL) dropwise at 0 C. Then the mixture was
stirred at this
temperature for 1.5 h and stirring was continued at RT for 16 h. The mixture
was poured into
ice water (80 mL) and extracted with Et0Ac (2 X 100 mL). The combined organic
layers
were washed with brine solution (50 mL), dried over anhydrous sodium sulfate
and
concentrated in vacuum. The residue was purified by combiflash column
chromatography
using 5% ethyl acetate in hexane as eluent to afford the titled compound (1.0
g, 16%); 1H
NMR (400 MHz, DMSO-d6): 8 11.75 (bs, 1H), 8.06 (d, J= 8.3 Hz, 1H), 7.41 (d, J=
1.4 Hz,
1H), 7.32 (dd, J= 1.4 Hz, J= 8.3 Hz, 1H); LC-MS: m/z 206.0 (M-1)-.
Step-b: Synthesis of 2-amino-5-(trifluoromethyl)phenol
To a solution of 2-nitro-5-(trifluoromethyl)phenol (1.0 g, 4.83 mmol) in
methanol (15 mL)
was added 10% Pd/C (100 mg) under nitrogen atmosphere. The reaction mixture
was stirred
under hydrogen balloon for 16 h. The reaction mixture was filtered through a
celite bed and
the filtrate was concentrated under vacuum to afford the title compound (850
mg, 99%); 1H
NMR (400 MHz, DMSO-d6): 8 9.58 (bs, 1H), 6.88 (s, 1H), 6.86 (s, 1H), 6.66 (d,
J = 7.8 Hz,
1H), 5.18 (s, 2H); LC-MS: m/z 178.0 (M+1) .
Step-c: Synthesis of 6-(trifluoromethyl)benzo[d]oxazole-2-thiol
To a solution of 2-amino-5-(trifluoromethyl)phenol (850 mg, 4.8 mmol) in
ethanol (10 mL)
at RT was added potassium ethyl-xanthate (1.69 g, 10.56 mmol) and the reaction
mixture was
refluxed for 16 h. The reaction mixture was concentrated under vacuum and
diluted with cold
water (50 mL), acidified with 1 N HC1. The solid obtained was filtered and
dried under
vacuum to afford the title compound (1.0 g, 95%); 1H NMR (400 MHz, DMSO-d6): 8
14.2
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
68
(bs, 1H), 7.98 (s, 1H), 7.66 (d, J = 8.4 Hz, 1H), 7.41 (d, J = 7.8 Hz, 1H); LC-
MS: m/z 217.9
(M-1)-.
Step-d: Synthesis of 2-chloro-6-(trifluoromethyl)benzo[d]oxazole
To a solution of 6-(trifluoromethyl)benzo[d]oxazole-2-thiol (500 mg, 2.28
mmol) in thionyl
chloride (4 mL) at RT was added dimethyl formamide (catalytic amount) and the
reaction
mixture was refluxed for 3 h. The reaction mixture concentrated, diluted with
cold water (30
mL) and extracted with Et0Ac (2 X 30 mL). The combined organic layers were
washed with
brine solution (20 mL), dried over anhydrous sodium sulfate and concentrated
under vacuum
to afford the title compound (290 mg, 57%); 1H NMR (400 MHz, DMSO-d6): 8 8.31
(s, 1H),
7.97 (d, J= 8.3 Hz, 1H), 7.80 (d, J= 8.3 Hz, 1H).
Step-e: Synthesis of ethyl 1-methyl-24(6-(trifluoromethyl)benzo[d]oxazol-2-
y1)amino)-
1H-benzo[d]imidazole-5-carboxylate
To a solution of ethyl 2-amino-l-methyl-1H-benzo [d] imidazole-5-carboxylate
(240 mg, 1.09
mmol) in 1,4-dioxane (8 mL) at 0 C was added sodium hydride (60% dispersion in
mineral
oil) (109 mg, 2.74 mmol) followed by the addition of 2-chloro-6-
(trifluoromethyl)benzo[d]oxazole (290 mg, 1.31 mmol). The reaction mixture was
stirred at
RT for 16 h. The reaction mixture was concentrated, diluted with cold water
(15 mL) and
stirred at RT for 30 min. The solid obtained was filtered, dried under vacuum
and purified by
combiflash column chromatography using 100% DCM as an eluent to afford the
title
compound (150 mg, 34%); 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.25 (d,
J = 1.5
Hz, 1H), 7.89 (dd, J = 1.5 Hz, J = 8.3 Hz, 1H), 7.83 (s, 1H), 7.61-7.51 (m,
3H), 4.33 (q, J =
6.9 Hz, 2H), 3.66 (s, 3H), 1.36 (t, J = 6.9 Hz, 3H); LC-MS: m/z 405.30 (M+1) .
Step-f: Synthesis of 1-methyl-2((6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid (Example 31)
To a stirred solution of ethyl 1-methy1-2-((6-(trifluoromethyl)benzo[d]oxazol-
2-yl)amino)-
1H-benzo[d]imidazole-5-carboxylate (80 mg, 0.20 mmol) in a mixture of solvent
of THF (0.5
mL), ethanol (0.5 mL) and water (0.25 mL) was added lithium hydroxide
monohydrate (20
mg, 0.50 mmol). The reaction mixture was heated at 60 C for 16 h. The reaction
mixture was
cooled to RT and concentrated under reduced pressure. The residue was
dissolved in water,
acidified with 1 N HC1 to obtain the solid which was filtered and dried under
vacuum to
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
69
afford the title compound (55 mg, 81%); 1H NMR (400 MHz, DMSO-d6): 8 12.80
(bs, 1H),
12.40 (bs, 1H), 8.22 (s, 1H), 7.89 (d, J = 8.3 Hz, 1H), 7.84 (s, 1H), 7.63-
7.53 (m, 3H), 3.67
(s, 3H); LC-MS: m/z 377.05 (M+1) .
Step-g: Synthesis of N-(2-methoxyethyl)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
(Example 32)
To a stirred solution of 1-methy1-24(6-(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid (50 mg, 0.13 mmol) in DMFA (1.5 mL) at 0 C
was
added N-ethyldiisopropyl amine (0.02 mL, 0.13 mmol) and diphenylphosphoryl
azide (0.02
mL, 0.13 mmol). The reaction mixture was stirred for 30 min, followed by the
addition of 2-
methoxyethylamine (0.01 mL, 0.13 mmol) and the reaction mixture was then
stirred at RT for
16 h. Once the reaction was completed (monitored by TLC), the reaction mixture
was diluted
with cold water (15 mL) and stirred for 15 min. The solid obtained was
filtered, washed with
diethyl ether and dried under vacuum to afford the title compound (28 mg,
49%); 1H NMR
(400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.48 (s, 1H), 8.10 (s, 1H), 7.83 (s,
1H), 7.79 (d, J=
8.4 Hz, 1H), 7.61-7.51 (m, 3H), 3.67 (s, 3H), 3.48-3.44 (m, 4H), 3.30 (s, 3H);
LC-MS: m/z
434.1 (M+1) .
Example 33. Synthesis of N-(2-hydroxyethyl)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
M
Me e
N
H r\j¨NH
¨NH a
HO VI N --(:) ¨> HON 0 N ir-0
# N
N 0 0
WI CF3 WI
CF3
Conditions: a) 2-aminoethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid as starting materials (Yield: 34%); 1H
NMR (400 MHz,
DMSO-d6): 8 12.40 (bs, 1H), 8.39 (t, J= 5.3 Hz, 1H), 8.10 (s, 1H), 7.82 (s,
1H), 7.80 (d, J=
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
8.3 Hz, 1H), 7.61-7.51 (m, 3H), 4.72 (t, J = 5.2 Hz, 1H), 3.67 (s, 3H), 3.54-
3.51 (m, 2H),
3.37-3.34 (m, 2H); LC-MS: m/z 420.0 (M+1) .
Example 34. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
Me
Me
=
N H
r,0
a H N)T-0
HO =
0 N
CF
0
CF3
LIP F
_ 3
Conditions: a) 2-(2-aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-(trifluoromethyl)benzo [d]oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-ol as
starting materials
(Yield: 50%); 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.46 (bs, 1H), 8.10
(s, 1H),
7.83 (s, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.62-7.52 (m, 3H), 4.60 (bs, 1H), 3.67
(s, 3H), 3.58-
3.44 (m, 8H); LC-MS: m/z 464.20 (M+1) .
Example 35. Synthesis of N-
(2-hydroxypropy1)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-aminopropan-2-ol and 1-methy1-2-((6-
(trifluoromethyl)benzo[d]oxazol-
2-yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H
NMR (400
MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.35 (t, J = 1.2 Hz, 1H), 8.10 (d, J = 1.2
Hz, 1H), 7.82-
7.79 (m, 2H), 7.61-7.51 (m, 3H), 4.76 (d, J = 4.0 Hz, 1H), 3.84-3.80 (m, 1H),
3.67 (s, 3H),
3.26-3.21 (m, 2H), 1.09 (d, J= 6.4 Hz, 3H); LC-MS: m/z 434.2 (M+1) .
Example 36. Synthesis of 1-
methyl-N-(2-(pyrrolidin-1-ypethyl)-2-((6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-(pyrrolidin-1-yl)ethan-1-amine and
1-methy1-2-((6-
(trifluoromethyl)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
71
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.39 (t, J =
5.2 Hz,
1H), 8.09 (s, 1H), 7.82 (s, 1H), 7.77 (d, J = 8.4 Hz, 1H), 7.61-7.51 (m, 3H),
3.66 (s, 3H),
3.43-3.38 (m, 2H), 2.61 (t, J = 7.2 Hz, 2H), 2.5 (m, 4H Merged in DMSO peak),
1.69 (bs,
4H); LC-MS: m/z 473.1 (M+1) .
Example 37. Synthesis of 1-
methyl-N-(2-(piperidin-1-ypethyl)-2-06-
(trifluoromethyl)benzo[d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-(piperidin-1-yl)ethan-1-
amine and 1-methy1-2-((6-
(trifluoromethyl)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.32 (t, J =
5.6 Hz,
1H), 8.07 (s, 1H), 7.82 (s, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.61-7.51 (m, 3H),
3.66 (s, 3H),
3.41-3.36 (m, 2H), 2.43-2.32 (m, 6H), 1.53-1.38 (m, 6H); LC-MS: m/z 487.15
(M+1) .
Example 38. Synthesis of N-(2-(2-hydroxypropoxy)ethyl)-1-methyl-2-((6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-aminoethoxy)propan-2-
ol and 1-methy1-2-((6-
(trifluoromethyl)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.46 (t, J =
5.6 Hz,
1H), 8.09 (s, 1H), 7.83 (s, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.62-7.52 (m, 3H),
4.58 (d, J = 4.4
Hz, 1H), 3.77-3.73 (m, 1H), 3.66 (s, 3H), 3.56 (t, J = 6.0 Hz, 2H), 3.45 (t, J
= 5.2 Hz, 2H),
2.29-2.23 (m, 2H), 1.04 (d, J= 6.4 Hz, 3H); LC-MS: m/z 478.2 (M+1) .
Example 39. Synthesis of N-
(2,3-dihydroxypropy1)-1-methyl-2-((6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 3-aminopropane-1,2-diol and 1-
methy1-2-((6-
(trifluoromethyl)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.39 (t, J =
5.2 Hz,
1H), 8.10 (s, 1H), 7.84 (s, 1H), 7.80 (d, J = 8.4 Hz, 1H), 7.62-7.53 (m, 3H),
4.87 (d, J = 4.4
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
72
Hz, 1H), 4.62 (bs, 1H), 3.67 (s, 4H), 3.45-3.40 (m, 1H), 3.30 (2H merged with
DMSO
moisture peak), 3.25-3.19 (m, 1H); LC-MS: m/z 450.15 (M+1) .
Example 40. Synthesis of N-(2-(2-(dimethylamino)acetamido)ethyl)-1-methyl-24(6-
(trifluoromethyl)benzo[d] oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 19 Step-c using N-(2-aminoethyl)-2-(dimethylamino)acetamide
trifluoroacetate and
1-methyl-2-((6-(trifluoromethyl)benzo [d] o xazol-2-yl)amino)-1H-benzo [d]
imidazo le-5-
carboxylic acid as starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs,
1H), 8.47
(bs, 1H), 8.09 (s, 1H), 7.92 (bs, 1H), 7.83 (s, 1H), 7.76 (d, J = 8.4 Hz, 1H),
7.61-7.52 (m,
3H), 3.66 (s, 3H), 3.38-3.35 (m, 4H), 2.86 (s, 2H), 2.19 (s, 6H); LC-MS: m/z
504.2 (M+1) .
Example 41. Synthesis of 2-(2-(1-methyl-24(6-(trifluoromethyl)benzo[d] oxazol-
2-
yl)amino)-1H-benzo[d] imidazole-5-carboxamido)ethoxy)acetic acid
Me Me
Me N
H N¨NH b
Ho 40 ,NH a Et0y..,0.---,N N HO tir Ni
¨NE);_o
N N
0 N N 0
0 0 0
CF3 CF3
CF3
Conditions: a) ethyl 2-(2-aminoethoxy)acetate trifluoraro acetate, HBTU,
DIPEA, DMF, 0 C - RT, 16 h; b) Li0H.H20,
Ethanol, THF, Water, RT, 3 h
Step-a: Synthesis of ethyl 2-(2-(1-methyl-24(6-(trifluoromethyl)benzo[d]
oxazol-2-
yl)amino)-1H-benzo[d] imidazole-5-carboxamido)ethoxy)acetate
The title compound was synthesized using the same procedure which was followed
for
Example 29 Step-c using 1-methyl-2-((6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid and ethyl 2-(2-aminoethoxy)acetate
trifluoraro aetate as
starting materials (Yield: 65%); 1H NMR (400 MHz, DMSO-d6): 8 12.38 (bs, 1H),
8.49 (bs,
1H), 8.10 (s, 1H), 7.82 (s, 1H), 7.79 (d, J = 8.8 Hz, 1H), 7.61-7.52 (m, 3H),
4.15-4.09 (m,
4H), 3.66-3.62 (m, 5H), 3.48-3.46 (m, 2H), 1.19 (t, J = 7.0 Hz, 3H); LC-MS:
m/z 506.1
(M+1) . .
Step-b: Synthesis of 2-(2-(1-methyl-24(6-(trifluoromethyl)benzo[d] oxazol-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxamido)ethoxy)acetic acid
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
73
The title compound was synthesized using the same procedure which was followed
for
Example 29 Step-d using ethyl 2-(2-(1-methy1-2-((6-(trifluoromethyl)benzo [d]
oxazol-2-
yl)amino)- 1H-benzo [d] imidazo le-5-c arbo xamido)etho xy)acetate as
starting material
(Yield:75%); 1H NMR (400 MHz, DMSO-d6): 8 12.70 (bs, 1H), 12.30 (bs, 1H), 8.49
(bs,
1H), 8.10 (s, 1H), 7.82-7.78 (m, 2H), 7.61-7.51 (m, 3H), 4.06 (s, 2H), 3.66-
3.62 (m, 5H),
3.48-3.41 (m, 2H); LC-MS: m/z 478.2 (M+1) .
Example 42. Synthesis of N-(2-(2-hydroxy-2-methylpropoxy)ethyl)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-aminoethoxy)-2-methylpropan-2-ol and 1-methy1-2-((6-
(trifluoromethyl)benzo [d]oxazol-2-yl)amino)-1H-benzo [d] imid azo le-5-c arbo
xylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.83 (s, 1H),
8.08 (s,
1H), 7.81 (s, 1H), 7.77 (d, J = 8.4 Hz, 1H), 7.58-7.52 (m, 3H), 4.32 (bs, 1H),
3.66 (s, 3H),
3.58-3.56 (m, 2H), 3.46-3.44 (m, 2H), 3.21 (s, 2H), 1.08 (s, 6H); LC-MS: m/z
492.1 (M+1) .
Example 43.
Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(5-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
40 CI OH OH
a b
0µ
N
F3C NO2 F3C NO2 F3C NH2 F3C F3C
Me Me Me
M
arah N e
f Ho=,>-NH H / -
NH
N hiC)0N N
N/>¨NH2 EtO2C N N N
0
EtO2C /-0
140
C F 3 C F 3
C F 3
Conditions: a) NaOH, DMSO, 60 C, 16 h; b) 10% Pd/C, Me0H, H2, RT, 16 h; c)
Potassium ethyl xanthate, Ethanol,
Reflux, 16 h; d) S0Cl2, Cat.DMF, reflux, 1 h; e) NaH, 2-chloro-5-
(trifluoromethyl)benzo[d]oxazole, 1,4-Dioxane, RT, 16 h; f)
Li0H.H20, THF, Methanol, Water, 50 C, 16 h; g) 2-(2-aminoethoxy)ethan-1-ol ,
HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-nitro-4-(trifluoromethyl)phenol
To a solution of 1-chloro-2-nitro-4-(trifluoromethyl)benzene (10.0 g, 44.3
mmol) in DMSO
(100 mL) at RT was added sodium hydroxide (4.44 g, 110.7 mmol) and the mixture
was
stirred at 60 C for 16 h. The reaction mixture was cooled to RT, diluted with
water (250 mL)
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
74
and extracted with Et0Ac (200 mL). The aqueous layer was acidified to pH-1
with 3 N HC1
and extracted with Et0Ac (2 X 250 mL), organic layer was washed with brine
solution (50
mL), dried over anhydrous sodium sulfate and concentrated under vacuum to
afford the titled
compound (7.0 g, 81%); 1H NMR (400 MHz, DMSO-d6): 8 11.99 (bs, 1H), 8.22 (d, J
= 1.6
Hz, 1H), 7.88 (dd, J = 2.0 Hz, J = 8.8 Hz, 1H), 7.30 (d, J = 8.8 Hz, 1H).
Step-b: Synthesis of 2-amino-4-(trifluoromethyl)phenol
To a solution of 2-nitro-4-(trifluoromethyl)phenol (5.0 g, 24.15 mmol) in
methanol (50 mL)
was added 10% Pd/C (2.5 g) under nitrogen atmosphere. The reaction mixture was
stirred
under hydrogen balloon for 16 h. The reaction mixture was filtered through a
celite bed and
the filtrate was concentrated under vacuum to afford the title compound (3.0
g, 70%); 1H
NMR (400 MHz, DMSO-d6): 8 9.85 (bs, 1H), 6.87 (d, J = 2.0 Hz, 1H), 6.77-6.70
(m, 2H),
5.0 (bs, 2H); LC-MS: m/z 178.1 (M+1) .
Step-c: Synthesis of 5-(trifluoromethyl)benzo[d]oxazole-2-thiol
To a solution of 2-amino-4-(trifluoromethyl)phenol (3.0 g, 16.9 mmol) in
ethanol (45 mL) at
RT was added potassium ethyl-xanthate (6.78 g, 42.4 mmol) and the reaction
mixture was
refluxed for 16 h. The reaction mixture was concentrated under vacuum and
diluted with cold
water (100 mL), acidified with 3 N HC1. The precipitated solid was filtered
and dried under
vacuum to afford the title compound (3.0 g, 81%); 1H NMR (400 MHz, DMSO-d6): 8
14.2
(bs, 1H), 7.72 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 8.4 Hz, 1H), 7.51 (s, 1H); LC-
MS: m/z 220.0
(M+1) .
Step-d: Synthesis of 2-chloro-5-(trifluoromethyl)benzo [d] oxazole
To a solution of 5-(trifluoromethyl)benzo[d]oxazole-2-thiol (2.0 g, 9.1 mmol)
in thionyl
chloride (10 mL) at RT was added N,N-dimethyl formamide (catalytic amount) and
the
reaction mixture was refluxed for 1 h. The reaction mixture concentrated,
diluted with cold
water (60 mL) and extracted with Et0Ac (2 X 100 mL). The combined organic
layers were
washed with brine solution (50 mL), dried over anhydrous sodium sulfate and
concentrated
under vacuum to afford the title compound (1.3 g, 65%); 1H NMR (400 MHz, DMSO-
d6): 8
8.23 (s, 1H), 8.02 (d, J = 8.8 Hz, 1H), 7.85 (d, J = 8.8 Hz, 1H).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Step-e: Synthesis of ethyl 1-methyl-24(5-(trifluoromethyl)benzo[d] oxazol-2-
yl)amino)-
1H-benzo[d] imidazole-5-carboxylate
To a solution of ethyl 2-amino-l-methyl-1H-benzo [d] imidazole-5-carboxylate
(200 mg, 0.91
mmol) in 1,4-dioxane (10 mL) at 0 C was added sodium hydride (60% dispersion
in mineral
oil) (91 mg, 2.27 mmol) and stirred for 15 min followed by the addition of 2-
chloro-5-
(trifluoromethyl)benzo[d]oxazole (222 mg, 1.0 mmol) and stirring was continued
at RT for
16 h. The reaction mixture was concentrated, diluted with cold water (50 mL)
and acidified
with 3 N HC1 and extracted with ethyl acetate (2 X 50 mL). The combined
organic layers
were washed with water (30 mL), brine solution (30 mL), dried over anhydrous
sodium
sulfate and concentrated under vacuum. The residue was purified by combiflash
column
chromatography using 100% dichloromethane as an eluent to afford the titled
compound (150
mg, 40%); 1H NMR (400 MHz, DMSO-d6): 8 12.41 (bs, 1H), 8.25 (s, 1H), 7.90 (dd,
J = 1.2
Hz, J = 8.4 Hz, 1H), 7.67-7.64 (m, 2H), 7.57 (d, J = 8.4 Hz, 1H), 7.49 (d, J =
8.4 Hz, 1H),
4.33 (q, J= 7.2 Hz, 2H), 3.66 (s, 3H), 1.35 (t, J= 7.2 Hz, 3H); LC-MS: m/z
405.00 (M+1) .
Step-f: Synthesis of 1-methyl-2((5-(trifluoromethyl)benzo[d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid
To a stirred solution ethyl 1-methyl-2-((5-(trifluoromethyl)benzo [d]oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylate (150 mg, 0.37 mmol) in a mixture of solvents
of THF (2
mL), methanol (2 mL) and water (2 mL) was added lithium hydroxide monohydrate
(78 mg,
1.85 mmol). The reaction mixture was heated at 50 C for 16 h. The reaction
mixture was
cooled to RT and concentrated under reduced pressure. The residue was
dissolved in water
(30 mL), acidified to pH -2 with 3 N HC1 and stirred for 1 h. The solid
precipitated was
filtered and dried under vacuum to afford the title compound (140 mg, 100%);
1H NMR (400
MHz, DMSO-d6): 8 12.85 (bs, 1H), 12.40 (bs, 1H), 8.18 (s, 1H), 7.87 (d, J =
8.4 Hz, 1H),
7.67 (s, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.46 (d, J =
8.0 Hz, 1H), 3.64
(s, 3H); LC-MS: m/z 377.1 (M+1) .
Step-g: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(5-
(trifluoromethyl)benzo[d] oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-
carboxamide
To a stirred solution of 1-methy1-24(5-(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid (80 mg, 0.21 mmol) in DMFA (5 mL) at 0 C
was added
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
76
N-ethyldiisopropyl amine (0.04 mL, 0.21 mmol) and HBTU (81 mg, 0.21 mmol). The
reaction mixture was stirred for 15 min, followed by the addition of 2-(2-
aminoethoxy)ethan-
1-ol (22 mg, 0.21 mmol). The reaction mixture was then stirred at RT for 16 h.
Once the
reaction was completed (monitored by TLC), the reaction mixture was diluted
with cold
water (30 mL) and stirred for 1 h. The solid precipitated was filtered and
dried under vacuum.
The solid was stirred in diethyl ether (15 mL), filtered and dried under
vacuum to afford the
titled compound (80 mg, 85%); 1H NMR (400 MHz, DMSO-d6): 8 12.33 (bs, 1H),
8.46 (t, J =
5.4 Hz, 1H), 8.09 (d, J = 1.2 Hz, 1H), 7.78 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H),
7.68 (s, 1H), 7.64
(d, J = 8.4 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 8.4 Hz, 1H), 4.60
(t, J = 5.2 Hz,
1H), 3.66 (s, 3H), 3.58-3.43 (m, 8H); LC-MS: m/z 464.2 (M+1) .
Examples 44 and 45. Synthesis of 1-methyl-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-
yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid and Synthesis of N-(2-
methoxyethyl)-
1-methyl-24(6-(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo [d]
imidazole-5-
carboxamide:
F,co 110 OH F3C0 110 OH . F3C0 01-1c F3c0 400 Q
a
NO2 NH2
F3C0 0
F3CO 0, 0µ, Me
/ ¨SMe
N
Me Me Me
Me so IS¨NH
HO so 1\i'>¨NH Li h
¨NH
NJ Et0 N g
N - N
¨0- 0 N 0 N
EtO2C
OCF3 OCF3 OCF3
Conditions: a) HNO3, Acetic acid, 15 C- RT, 16 h; b) 10% Pd/C, Me0H, H2, RT, 5
h; c) Carbon disulfide,
KOH, Ethanol, Reflux, 6 h; d) K2CO3, Methyl iodide, Acetonitrile, RT, 16 h; e)
m-CPBA, DCM, 0 C - RT, 6
h; f) NaH, 2-(methylsulfonyI)-6-(trifluoromethoxy) benzo[d]oxazole, 1,4-
Dioxane, RT, 16 h; g) Li0H.H20,
THF, Ethanol, Water, 60 C, 16 h; h) 2-methoxyethyl amine, DPPA, DIPEA, DMF, 0
C - RT, 16 h
Step-a: Synthesis of 2-nitro-5-(trifluoromethoxy)phenol
To a solution of 3-(trifluoromethoxy)phenol (1 g, 5.6 mmol) in acetic acid (10
mL) was
added 60% aqueous nitric acid (1 mL) dropwise at 10-15 C. The mixture was
stirred at this
temperature for 1.5 h and continued to stir at RT for 16 h. The mixture was
poured into ice
water (20 mL) and extracted with Et0Ac (2 X 25 mL). The combined organic
layers were
washed with brine solution (25 mL), dried over anhydrous sodium sulfate and
concentrated in
vacuum. The residue was purified by combiflash chromatography using 5% Et0Ac
in
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
77
hexanes as eluent to afford the title compound (300 mg, 24%); 1H NMR (400 MHz,
CDC13):
8 10.73 (s, 1H), 8.18 (d, J= 9.6 Hz, 1H), 6.99 (s, 1H), 6.83 (d, J= 9.2 Hz,
1H); LC-MS: m/z
222.0 (M-1).
Step-b: Synthesis of 2-amino-5-(trifluoromethoxy)phenol
To a solution of 2-nitro-5-(trifluoromethoxy)phenol (300 mg, 1.35 mmol) in
methanol (6 mL)
was added 10% Pd/C (60 mg) under nitrogen atmosphere. The reaction mixture was
stirred
under hydrogen gas for 5 h. The reaction mixture was filtered through a bed of
celite and
concentrated under vacuum to afford the title compound (250 mg, 96%); 1H NMR
(400 MHz,
DMSO-d6): 8 9.60 (s, 1H), 6.59-6.57 (m, 2H), 6.53-6.50 (m, 1H), 4.68 (bs, 2H);
LC-MS: m/z
194.0 (M+1) .
Step-c: Synthesis of 6-(trifluoromethoxy)benzo [d] oxazole-2-thiol
To a solution of 2-amino-5-(trifluoromethoxy)phenol (250 mg, 1.3 mmol) in
ethanol (5 mL)
at RT was added powdered potassium hydroxide (127 mg, 2.3 mmol) and carbon
disulfide (1
mL, 17.0 mmol). The reaction mixture was refluxed for 6 h. After cooling to
RT, the mixture
was concentrated in vacuo. The residue was diluted with cold water (100 mL),
acidified with
1 N HC1. The solid obtained was filtered and dried under vacuum to afford the
title
compound (200 mg, 66%); 1H NMR (400 MHz, DMSO-d6): 8 14.10 (bs, 1H), 7.73 (s,
1H),
7.32 (s, 2H); LC-MS: m/z 235.9 (M+1) .
Step-d: Synthesis of 2-(methylthio)-6-(trifluoromethoxy)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lb using 6-(trifluoromethoxy)benzo[d]oxazole-2-thiol as starting
material and
stirring at RT for 16 h (Yield: 94%); 1H NMR (400 MHz, DMSO-d6): 8 7.85 (d, J
= 1.6 Hz,
1H), 7.73 (d, J= 8.0 Hz, 1H), 7.37-7.34 (m, 1H), 2.77 (s, 3H); LC-MS: m/z
250.1 (M+1) .
Step-e: Synthesis of 2-(methylsulfony1)-6-(trifluoromethoxy)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lc using 2-(methylthio)-6-(trifluoromethoxy)benzo[d]oxazole as
starting material
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
78
and stirring at RT for 6 h (Yield: 94%); 1H NMR (400 MHz, DMSO-d6): 8 8.22 (s,
1H), 8.15
(d, J= 8.8 Hz, 1H), 7.62 (d, J= 8.8 Hz, 1H), 3.67 (s, 3H).
Step-f: Synthesis of ethyl 1-methyl-2-((6-(trifluoromethoxy)benzo[d] oxazol-2-
yl)amino)-
1H-benzo[d] imidazole-5-carboxylate
To a solution of ethyl 2-amino-l-methy1-1H-benzo [d] imidazole-5-carboxylate
(100 mg, 0.46
mmol) in 1,4-dioxane (2 mL) at RT was added sodium hydride (60% dispersion in
mineral
oil) (64 mg, 1.6 mmol) followed by the addition of 2-(methylsulfony1)-6-
(trifluoromethoxy)benzo[d]oxazole (167 mg, 0.6 mmol). The reaction mixture was
stirred at
RT for 16 h. It was then concentrated, diluted with cold water (15 mL) and
acidified with 1 N
HC1. The solid obtained was filtered, dried under vacuum and purified by
combiflash column
chromatography using 100% DCM as an eluent to afford the title compound (30
mg, 16%);
1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.24 (s, 1H), 7.89 (dd, J = 1.6
Hz, J = 8.4
Hz, 1H), 7.58-7.50 (m, 3H), 7.22 (d, J = 8.0 Hz, 1H), 4.33 (q, J = 7.6 Hz,
2H), 3.65 (s, 3H),
1.35 (t, J= 7.2 Hz, 3H); LC-MS: m/z 421.0 (M+1) .
Step-g: Synthesis of 1-methyl-2-((6-(trifluoromethoxy)benzo[d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh, using ethyl 1-methyl-2-((6-(trifluoromethoxy)benzo [d] oxazol-2-
yl)amino)-
1H-benzo [d] imidazole-5-carboxylate as starting material (Yield: 72%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.80 (bs, 1H), 12.30 (bs, 1H), 8.20 (s, 1H), 7.88 (d, J = 8.4 Hz,
1H), 7.58 (s,
1H), 7.52 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.0 Hz, 1H), 3.65 (s, 3H); LC-MS:
m/z 393.0
(M+1) .
Step-h: Synthesis of N-(2-methoxyethyl)-1-methyl-24(6-
(trifluoromethoxy)
benzo[d] oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methy1-2-((6-(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-
1H-
benzo[d]imidazole-5-carboxylic acid as starting material (Yield: 23%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.30 (bs, 1H), 8.47 (t, J = 5.2 Hz, 1H), 8.08 (s, 1H), 7.78 (d, J
= 8.4 Hz, 1H),
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
79
7.57 (s, 1H), 7.52-7.49 (m, 2H), 7.21 (d, J = 8.4 Hz, 1H), 3.64 (s, 3H), 3.48-
3.44 (m, 4H),
3.28 (s, 3H); LC-MS: m/z 450.0 (M+1) .
Example 46. Synthesis of 1-
methyl-N-(1H-pyrazol-4-y1)-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1H-pyrazol-4-amine and 1-
methy1-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.65 (bs, 1H), 12.30 (bs,
1H), 10.40
(s, 1H), 8.17 (d, J = 1.2 Hz, 1H), 8.02 (bs, 1H), 7.89 (dd, J = 1.2 Hz, J =
8.4 Hz, 1H), 7.69
(bs, 1H), 7.58-7.49 (m, 3H), 7.21 (d, J = 8.4 Hz, 1H), 3.67 (s, 3H); LC-MS:
m/z 458.0
(M+1) .
Example 47. Synthesis of N-(1,3-dihydroxy-2-(hydroxymethyl)propan-2-y1)-1-
methy1-2-
((6-(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-amino-2-(hydroxymethyl)propane-1,3-diol and 1-methy1-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.05 (s, 1H),
7.43 (d, J
= 8.4 Hz, 1H), 7.57 (s, 1H), 7.50 (d, J = 5.8 Hz, 2H), 7.26 (s, 1H), 7.21 (d,
J = 8.4 Hz, 1H),
4.83 (bs, 3H), 3.71 (bs, 6H), 3.65 (s, 3H); LC-MS: m/z 496.3 (M+1) .
Example 48. Synthesis of
N- (1,3-dihydroxyp ropan-2- y1)- 1 -methyl-24(6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-amino-propane-1,3-diol and 1-
methy1-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.09 (s, 1H),
7.88 (d, J
= 7.6 Hz, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.57 (d, J = 1.2 Hz, 1H), 7.51 (d, J
= 8.0 Hz, 2H),
7.21 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 4.66 (t, J = 6.0 Hz, 2H), 4.00-3.95 (m,
1H), 3.65 (s,
3H), 3.54 (t, J = 6.0 Hz, 4H); LC-MS: m/z 466.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Example 49. Synthesis of N-(2-(2-(dimethylamino)acetamido)ethyl)-1-methy1-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 19 Step-c using N-(2-aminoethyl)-2-(dimethylamino)acetamide
trifluoroacetate and
1-methyl-2-((6-(trifluoromethoxy)benzo [d] o xazol- 2- yl)amino)- 1H-benzo [d]
imid azo le-5-
carboxylic acid as starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.0 (bs,
1H), 8.45
(t, J = 4.4 Hz, 1H), 8.07 (s, 1H), 7.91 (t, J = 5.6 Hz, 1H), 7.75 (d, J = 8.4
Hz, 1H), 7.56 (s,
1H), 7.50 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.0 Hz, 1H), 3.64 (s, 3H), 3.36
(t, J = 5.6 Hz, 2H),
3.30 (2H merged with DMSO moisture peak), 2.85 (s, 2H), 2.18 (s, 6H); LC-MS:
m/z 520.5
(M+1) .
Example 50. Synthesis of 1-
methyl-N-(1-methy1-1H-pyrazol-4-y1)-2-46-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methyl-1H-pyrazol-4-amine and
1-methy1-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 10.40 (s,
1H), 8.17 (s,
1H), 8.03 (s, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.58-7.51 (m, 4H), 7.22 (d, J =
7.6 Hz, 1H), 3.83
(s, 3H), 3.66 (s, 3H); LC-MS: m/z 472.0 (M+1) .
Example 51. Synthesis of
1-methyl-N-(oxetan-3-y1)-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using oxetan-3-amine and 1-methy1-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid as starting materials. 1H
NMR (400
MHz, DMSO-d6): 8 12.30 (bs, 1H), 9.07 (d, J= 6.0 Hz, 1H), 8.10 (s, 1H),
7.85(d, J= 8.4 Hz,
1H), 7.57 (s, 1H), 7.52 (t, J = 8.8 Hz, 2H), 7.21 (d, J = 8.4 Hz, 1H), 5.05-
5.00 (m, 1H), 4.78
(t, J= 6.4 Hz, 2H), 4.62 (t, J= 6.4 Hz, 2H), 3.65 (s, 3H); LC-MS: m/z 448.0
(M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
81
Example 52. Synthesis of N-((3-hydroxyoxetan-3-yl)methyl)-1-methyl-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 3-(aminomethyl)oxetan-3-ol and 1-
methy1-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.54 (t, J =
6.0 Hz,
1H), 8.10 (d, J = 1.2 Hz, 1H), 7.80 (dd, J = 1.2 Hz, J = 8.8 Hz, 1H), 7.57 (d,
J = 1.6 Hz, 1H),
7.53-7.49 (m, 2H), 7.21 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H), 5.88 (s, 1H), 4.52
(d, J = 6.4 Hz,
2H), 4.41 (d, J = 6.4 Hz, 2H), 3.65 (s, 3H), 3.59 (d, J = 6.0 Hz, 2H); LC-MS:
m/z 478.2
(M+1) .
Example 53. Synthesis of N-
(2-hydroxyethyl)-1-methyl-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-aminoethan-1-ol and 1-
methy1-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.38 (t, J =
5.2 Hz,
1H), 8.09 (d, J = 1.2 Hz, 1H), 7.78 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H), 7.57 (s,
1H), 7.52-7.49
(m, 2H), 7.22 (d, J = 8.0 Hz, 1H), 4.72 (t, J = 5.2 Hz, 1H), 3.64 (s, 3H),
3.54 (q, J = 6.0 Hz,
2H), 3.37-3.30 (m, 2H); LC-MS: m/z 436.0 (M+1) .
Example 54. Synthesis of 1-
methyl-N-(2-(methylsulfonypethyl)-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-(methylsulfonyl)ethan-1-amine hydrochloride and 1-methy1-2-
((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid as
starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.68 (t, J =
5.2 Hz,
1H), 8.09 (d, J = 1.6 Hz, 1H), 7.76 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.57 (s,
1H), 7.53-7.50
(m, 2H), 7.27-7.20 (m, 1H), 3.70 (q, J = 6.0 Hz, 2H), 3.65 (s, 3H), 3.40 (t, J
= 6.8 Hz, 2H),
3.05 (s, 3H); LC-MS: m/z 497.9 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
82
Examples 55 and 56. Synthesis of 2-46-(2-methoxyethoxy)benzoldloxazol-2-
yl)amino)-1-
methyl-1H-benzoldlimidazole-5-carboxylic acid and Synthesis of 24(642-
methoxyethoxy)benzo [d] oxazol-2-yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-
benzo [d] imidazole-5-carboxamide
OH OH Me0 401 OH
a MeOC) C) c C) =
C)\ SH
NO2 = NO2 NH2 MeO
NMe
cõ.1Me0C) R
Et0 =
--NH
N
/2¨CI
0
o,oMe
Ire Me
HO N )T-0 N Me0
N
0 0
o0Me o0Me
Conditions: a) 2-methoxyethan-1-ol, Sodium, 100 C, 16 h; b) 10% Pd/C, Me0H,
H2, RT, 16 h; c)
Potassium ethyl xanthate, Ethanol, Reflux, 16 h; d) 50Cl2, Cat.DMF, reflux, 2
h; e) NaH, ethyl 2-
amino-1-methyl-1H-benzo[d]imidazole-5-carboxylate, 1,4-Dioxane, RT, 16 h; f)
Li0H.H20, THF,
Ethanol, Water, 60 C, 8 h; g) 2-methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT,
16 h
Step-a: Synthesis of 5-(2-methoxyethoxy)-2-nitrophenol
To a stirred 2-methoxyethan-1-ol (50 mL) at 0 C was added sodium metal (2.29
g, 95.46
mmol) and the reaction mixture was allowed to stir at RT for 30 min, followed
by the
addition of 5-fluoro-2-nitrophenol (5.0 g, 31.82 mmol). The reaction mixture
was heat to
100 C for 16 h. Once the reaction was completed (monitored by TLC), the
reaction mixture
was cooled to 0 C, diluted with cold water (200 mL) and acidified with 1 N
HC1. The solid
obtained was filtered and dried under vacuum to afford the title compound (4.0
g, 59%) as a
yellow solid; 1H NMR (400 MHz, DMSO-d6): 8 10.93 (s, 1H), 7.96 (d, J = 9.3 Hz,
1H), 6.64-
6.58 (m, 2H), 4.19-4.17 (m, 2H), 3.67-3.65 (m, 2H), 3.30 (s, 3H).
Step-b: Synthesis of 2-amino-5-(2-methoxyethoxy)phenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 5-(2-methoxyethoxy)-2-nitrophenol as starting material
(Yield: 96%);
LC-MS: m/z 184.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
83
Step-c: Synthesis of 6-(2-methoxyethoxy)benzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-(2-methoxyethoxy)phenol as starting material
(Yield:
76%); 1H NMR (400 MHz, DMSO-d6): 8 13.78 (s, 1H), 7.29 (d, J= 2.4 Hz, 1H),
7.19 (d, J=
8.8 Hz, 1H), 6.96 (dd, J = 2.4 Hz, J = 8.8 Hz, 1H), 4.18-4.16 (m, 2H), 3.73-
3.71 (m, 2H),
3.36 (s, 3H); LC-MS: m/z 226.0 (M+1) .
Step-d: Synthesis of 2-chloro-6-(2-methoxyethoxy)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-(2-methoxyethoxy)benzo[d]oxazole-2-thiol as starting
material
and stirred for 2 h (Yield: 69%); LC-MS: m/z 228.05 (M+1) .
Step-e: Synthesis of ethyl 24(6-(2-methoxyethoxy)benzo [d] oxazol-2-yl)amino)-
1-methyl-
1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and 2-chloro-6-(2-methoxyethoxy)benzo[d]oxazole as starting
materials (Yield:
24%); 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.21 (d, J = 1.5 Hz, 1H),
7.86 (dd, J
= 1.5 Hz, J= 8.3 Hz, 1H), 7.49 (d, J= 8.3 Hz, 1H), 7.35 (d, J= 8.8 Hz, 1H),
7.14 (d, J= 2.4
Hz, 1H), 6.83 (dd, J = 2.4 Hz, J = 8.8 Hz, 1H), 4.33 (q, J = 7.2 Hz, 2H), 4.12
(t, J = 4.6 Hz,
2H), 3.67 (t, J= 4.6 Hz, 2H), 3.62 (s, 3H), 3.32 (s, 3H), 1.35 (t, J= 7.1 Hz,
3H); LC-MS: m/z
411.0 (M+1) .
Step-f: Synthesis of 24(6-(2-methoxyethoxy)benzo [d] oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh, using ethyl 24(6-(2-methoxyethoxy)benzo[d]oxazol-2-yl)amino)-1-
methyl-
1H-benzo[d]imidazole-5-carboxylate as starting material (Yield: 77%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.15 (bs, 2H), 8.17 (d, J= 1.5 Hz, 1H), 7.85 (dd, J= 2.0 Hz, J=
8.8 Hz, 1H),
7.47 (d, J = 8.3 Hz, 1H), 7.36 (d, J = 8.3 Hz, 1H), 7.13 (d, J = 2.4 Hz, 1H),
6.82 (dd, J = 2.4
Hz, J = 8.3 Hz, 1H), 4.12 (t, J = 4.6 Hz, 2H), 3.67 (t, J = 4.6 Hz, 2H), 3.62
(s, 3H), 3.32 (s,
3H); LC-MS: m/z 383.0 (M-F1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
84
Step-g: Synthesis of 24(6-(2-methoxyethoxy)benzo[d]oxazol-2-yl)amino)-N-(2-
methoxyethyl)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-((6-(2-methoxyethoxy)benzo[d]oxazol-2-yl)amino)-1-methyl-
1H-
benzo [d]imidazole-5-carboxylic acid as starting material (Yield: 29%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.15 (bs, 1H), 8.44 (t, J= 5.4 Hz, 1H), 8.05 (d, J= 1.5 Hz, 1H),
7.75 (dd, J=
1.4 Hz, J = 8.3 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.35 (d, J = 8.3 Hz, 1H),
7.13 (d, J = 2.4
Hz, 1H), 6.82 (dd, J= 2.4 Hz, J= 8.3 Hz, 1H), 4.12-4.10 (m, 2H), 3.68-3.66 (m,
2H), 3.62 (s,
3H), 3.49-3.43 (m, 4H), 3.32 (s, 3H), 3.28 (s, 3H); LC-MS: m/z 440.60 (M+1) .
Example 57. Synthesis of N-(2-hydroxyethyl)-24(6-(2-
methoxyethoxy)benzo[d]oxazol-
2-yl)amino)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
Me Me
NI
HO I. N-1\11--0 a HO N
N
0 0 9a 0
W o0Me o0Me
Conditions: a) 2-aminoethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 24(6-(2-methoxyethoxy)benzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d]imidazole-5-carboxylic acid as starting material (Yield: 27%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.16 (bs, 1H), 8.35 (t, J = 5.4 Hz, 1H), 8.05 (d, J = 1.0 Hz,
1H), 7.75 (dd, J =
1.5 Hz, J = 8.3 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.35 (d, J = 8.8 Hz, 1H),
7.12 (d, J = 2.4
Hz, 1H), 6.81 (dd, J= 2.5 Hz, J= 8.4 Hz, 1H), 4.72 (bs, 1H), 4.11 (t, J= 4.6
Hz, 2H), 3.67 (t,
J = 4.6 Hz, 2H), 3.61 (s, 3H), 3.53 (t, J = 5.3 Hz, 2H), 3.35 (t, J = 6.0 Hz,
2H), 3.32 (s, 3H);
LC-MS: m/z 426.45 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Example 58. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-24(6-(2-
methoxyethoxy)benzo [d] oxazol-2-yl)amino)-1-methyl-1H-benzo [d] imidazole-5-
carboxamide
fyle NiMe
/)---NH H
HO N a HO .7*()\. N N )1-0
0 0 10a
o 0 M e o M e
Conditions: a) 2-(2-aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-(2-methoxyethoxy)benzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d] imidazole-5-carboxylic acid and 2-(2-arninoethoxy)ethan-1-ol as
starting materials
(Yield: 61%); 1H NMR (400 MHz, DMSO-d6): 8 12.15 (bs, 1H), 8.41 (t, J = 5.4
Hz, 1H),
8.05 (d, J= 0.9 Hz, 1H), 7.74 (dd, J= 1.5 Hz, J= 8.3 Hz, 1H), 7.45 (d, J= 8.3
Hz, 1H), 7.35
(d, J = 8.8 Hz, 1H), 7.12 (d, J = 1.9 Hz, 1H), 6.82 (dd, J = 2.4 Hz, J = 8.8
Hz, 1H), 4.60 (bs,
1H), 4.13 (t, J = 4.6 Hz, 2H), 3.67 (t, J = 4.6 Hz, 2H), 3.62 (s, 3H), 3.57-
3.42 (m, 8H), 3.32
(s, 3H); LC-MS: m/z 470.25 (M+1) .
Examples 59 and 60. Synthesis of 24(6-isopropylbenzo[d]oxazol-2-yl)amino)-1-
methyl-
1H-benzo[d]imidazole-5-carboxylic acid and Synthesis of 2-((6-
isopropylbenzo[d]oxazol-
2-yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
Me Me Me Me Me
OH a OH b OH c 0,
Me (01 ¨0- Me= M e= ¨a- me so 0,/\,
/¨SH ¨I.- Me
NO2 NH2 =
Me
Me
Me
diki/40 N
,
>---NH
e Et0 101 N 0 f HO
1111, N >F0 Me0
0 N
N alb
N N 0
0
iv 1410 Me Me Me
Me
Me Me
Conditions: a) NaNO3, NaNO2, 3 M H2SO4, DCM, RT, 24 h; b) 10% Pd/C, Me0H, H2,
RT, 16 h; c) Potassium ethyl
xanthate, ethanol, reflux, 16 h; d) S0Cl2, Cat.DMF, reflux, 2 h; e) NaH, ethyl
2-amino-1-methyl-1 H-benzo[c]imidazole-5-
carboxylate , 1,4-Dioxane, RT, 16 h; f) Li0H.H20, THF, ethanol, water, 60 C, 5
h; g) 2-methoxyethylamine, DPPA, DIPEA,
DMF, 0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
86
Step-a: Synthesis of 5-isopropyl-2-nitrophenol
To a solution of 3-isopropylphenol (2.0 g, 14.7 mmol) in DCM (30 mL) and 3 M
sulfuric acid
(25 mL) was added sodium nitrate (1.37 g, 16.18 mmol) and sodium nitrite(10
mg, 0.14
mmol) at RT. Then the mixture was stirred for 24 h. The mixture was poured
into ice water
(100 mL) and extracted with DCM (2 X 50 mL). The combined organic layers were
washed
with brine solution (50 mL), dried over anhydrous sodium sulfate and
concentrated in
vacuum. The residue was purified by combiflash chromatography using 5% ethyl
acetate in
hexane as eluent to afford the titled compound (500 mg, 19%); 1H NMR (400 MHz,
DMSO-
d6): 8 10.76 (s, 1H), 7.86 (d, J = 8.8 Hz, 1H), 6.98 (d, J = 1.5 Hz, 1H), 6.89
(dd, J = 1.5 Hz, J
= 8.3 Hz, 1H), 2.92-2.89 (m, 1H), 1.19 (d, J= 6.9 Hz, 6H); LC-MS: m/z 180.0 (M-
1).
Step-b: Synthesis of 2-amino-5-isopropylphenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 5-isopropyl-2-nitrophenol as starting material (Yield: 86%);
1H NMR
(400 MHz, DMSO-d6): 8 8.88 (bs, 1H), 6.50 (dd, J = 1.9 Hz, J = 10.7 Hz, 1H),
6.41 (d, J =
2.0 Hz, 1H), 6.39 (d, J = 2.0 Hz, 1H), 4.25 (s, 2H), 2.68-2.61 (m, 1H), 1.10
(d, J = 6.8 Hz,
6H); LC-MS: m/z 152.15 (M+1) .
Step-c: Synthesis of 6-isopropylbenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-isopropylphenol as starting material (Yield:
68%); 1H
NMR (400 MHz, DMSO-d6): 8 13.74 (s, 1H), 7.41 (s, 1H), 7.20-7.13 (m, 2H), 3.0-
2.93 (m,
1H), 1.21 (d, J= 6.9 Hz, 6H); LC-MS: m/z 194.15 (M+1) .
Step-d: Synthesis of 2-chloro-6-isopropylbenzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-isopropylbenzo[d]oxazole-2-thiol as starting
material and stirred
for 2 h (Yield: 85%); 1H NMR (400 MHz, DMSO-d6): 8 7.17 (s, 1H), 7.02-6.97 (m,
2H),
2.96-2.86 (m, 1H), 1.19 (d, J= 6.8 Hz, 6H); LC-MS: m/z 196.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
87
Step-e: Synthesis of ethyl 24(6-isopropylbenzo[d]oxazol-2-yl)amino)-1-methyl-
1H-
benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-isopropylbenzo[d]oxazole as starting materials (Yield: 77%); 11-1
NMR (400 MHz,
DMSO-d6): 8 12.15 (bs, 1H), 8.22 (d, J= 1.0 Hz, 1H), 7.86 (dd, J= 1.0 Hz, J=
8.3 Hz, 1H),
7.50 (d, J= 8.3 Hz, 1H), 7.38 (d, J= 8.3 Hz, 1H), 7.33 (s, 1H), 7.11 (d, J=
7.8 Hz, 1H), 4.33
(q, J = 7.0 Hz, 2H), 3.63 (s, 3H), 3.01-2.95 (m, 1H), 1.35 (t, J = 7.0 Hz,
3H), 1.25 (d, J = 6.8
Hz, 6H); LC-MS: m/z 379.1 (M+1) .
Step-f: Synthesis of 24(6-isopropylbenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-isopropylbenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylate as starting material and stirring at 60 C for
5 h (Yield:
92%); 11-1 NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 2H), 8.18 (s, 1H), 7.86 (d, J
= 8.3 Hz,
1H), 7.49 (d, J= 8.3 Hz, 1H), 7.39 (d, J= 7.8 Hz, 1H), 7.33 (s, 1H), 7.10 (d,
J= 7.8 Hz, 1H),
3.63 (s, 3H), 2.98-2.97 (m, 1H), 1.25 (d, J= 6.8 Hz, 6H); LC-MS: m/z 351.20
(M+1) .
Step-g: Synthesis of 24(6-isopropylbenzo[d]oxazol-2-yl)amino)-N-(2-
methoxyethyl)-1-
methyl-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-
((6-isopropylbenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylic acid as starting material (Yield: 69%); 11-1
NMR (400 MHz,
DMSO-d6): 8 12.15 (bs, 1H), 8.44 (s, 1H), 8.07 (s, 1H), 7.75 (d, J= 8.3 Hz,
1H), 7.46 (d, J=
8.3 Hz, 1H), 7.37 (d, J = 8.3 Hz, 1H), 7.32 (s, 1H), 7.09 (d, J = 7.8 Hz, 1H),
3.63 (s, 3H),
3.48-3.44 (m, 4H), 3.28 (s, 3H), 2.98-2.97 (m, 1H), 1.25 (d, J = 6.8 Hz, 6H);
LC-MS: m/z
408.0 (M+1) .
Example 61.
Synthesis of N-(2-hydroxyethyl)-24(6-isopropylbenzo[d]oxazol-2-
yl)amino)-1-methyl-1H-benzo[d]imidazole-5-carboxamide.
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-isopropylbenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
88
benzo[d]imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials. 1H NMR
(400 MHz, DMSO-d6): 8 12.50 (bs, 1H), 8.43 (bs, 1H), 8.08 (s, 1H), 7.82 (d, J
= 8.0 Hz, 1H),
7.55 (d, J= 8.2 Hz, 1H), 7.38-7.36 (m, 2H), 7.16 (d, J= 8.0 Hz, 1H), 3.67 (s,
3H), 3.54 (d, J
= 5.6 Hz, 2H), 3.36 (q, J = 6.0 Hz, 2H), 3.02-2.99 (m, 1H), 1.25 (d, J = 7.2
Hz, 6H); LC-MS:
m/z 394.0 (M+1) .
Example 62. Synthesis of 2-46-(difluoromethoxy)benzo [d] oxazol-2-yl)amino)-1-
methyl-
1H-benzoldlimidazole-5-carboxylic acid
F OH F OBn HO io OBn F2HCO OBn d F2HCO
OH
a
-lb-
NO2 NO2 NO2 NO2 NH
OCHF2 ocHF2
Me N\
fyle N \
F2HCO Et0 AI 0, F2HCO
=1\i/>¨NH h HO = />¨NH
so 5_c,
glr N 0
0
Conditions: a) K2CO3, Benzyl bromide, DMF, RT, 16 h; b) KOH, water, 100 C, 30
h; c) Diethyl (bromodifluoromethyl)
phosphonate, KOH, ACN and water (1:1), RT, 1 h; d) 10% Pd/C, Me0H, H2, RT, 16
h, e) Potassium ethyl xanthate,
ethanol, reflux, 16 h; 0 SOC12, CatDMF, reflux, 2 h; g) NaH, ethyl 2-amino-1-
methy1-1H-benzoidlimidazole-5-
carboxylate, 1,4-dioxane, RT, 16 h; 0 Li0H.H20, THF, ethanol, water, 60 C, 16
h
Step-a: Synthesis of 2-(benzyloxy)-4-fluoro-1-nitrobenzene
To a stirred solution of 5-fluoro-2-nitrophenol (10 g, 63.7 mmol) in DMFA (100
mL) at RT
was added potassium carbonate (10.54 g, 76.43 mmol) and benzyl bromide (7.56
mL, 63.7
mmol). The reaction mixture was then stirred at RT for 16 h. Once the reaction
was
completed (monitored by TLC), the reaction mixture was diluted with water (300
mL) and
extracted with ethylacetate (2 X 100 mL). The combined organic layers were
washed with
cold water (2 X 200 mL), brine solution (100 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum. The residue was purified by combiflash column
chromatography
using 5% Et0Ac in hexane as eluent to afford the titled compound (12.0 g,
76%); 1H NMR
(400 MHz, DMSO-d6): 8 8.04 (dd, J = 5.8 Hz, J = 9.3 Hz, 1H), 7.48-7.34 (m,
6H), 7.02-6.97
(m, 1H), 5.33 (s, 2H).
Step-b: Synthesis of 3-(benzyloxy)-4-nitrophenol
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
89
To a stirred solution of KOH (7.93 g, 141.7 mmol) in water (70 mL) at RT was
added 2-
(benzyloxy)-4-fluoro- 1-nitrobenzene (7.0 g, 28.3 mmol) and the reaction
mixture was stirred
at 100 C for 30 h. The reaction mixture was cooled to RT, diluted with water
(100 mL),
acidified with 3N HC1 and extracted with ethyl acetate (3 X 50 mL). The
combined organic
layers were washed with brine solution (50 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum. The residue was purified by combiflash column
chromatography
using 20% Et0Ac in hexane as eluent to afford the titled compound (2.0 g,
29%); 1H NMR
(400 MHz, DMSO-d6): 8 10.88 (s, 1H), 7.90 (d, J = 8.8 Hz, 1H), 7.47 (t, J =
7.4 Hz, 2H),
7.41-7.39 (m, 2H), 7.36-7.32 (m, 1H), 6.69 (d, J = 2.0 Hz, 1H), 6.49 (dd, J =
2.4 Hz, J = 9.3
Hz, 1H), 5.25 (s, 2H); LC-MS: m/z 244.0 (M-1).
Step-c: Synthesis of 2-(benzyloxy)-4-(difluoromethoxy)-1-nitrobenzene
To a stirred solution of 3-(benzyloxy)-4-nitrophenol (1.1 g, 4.49 mmol) in
acetonitrile (6 mL)
at 0 C was added potassium hydroxide (5.02 g, 89.79 mmol) in water (6 mL) and
diethyl
(bromodifluoromethyl) phosphonate (1.58 mL, 8.98 mmol). The reaction mixture
was stirred
at RT for 1 h and diluted with water (100 mL). The reaction mixture was
extracted with ethyl
acetate (3 X 60 mL). The combined organic layers were washed with brine
solution (50 mL),
dried over anhydrous sodium sulfate and concentrated under vacuum. The residue
was
purified by combiflash column chromatography using 10% Et0Ac in hexane as
eluent to
afford the titled compound (1.2 g, 91%); 1H NMR (400 MHz, DMSO-d6): 8 8.04 (d,
J = 9.3
Hz, 1H), 7.64-7.27 (m, 6H), 7.26 (d, J = 2.5 Hz, 1H), 6.93 (dd, J = 2.5 Hz, J
= 8.8 Hz, 1H),
5.34 (s, 2H).
Step-d: Synthesis of 2-amino-5-(difluoromethoxy)phenol
To a solution of 2-(benzyloxy)-4-(difluoromethoxy)-1-nitrobenzene (1.2 g, 4.07
mmol) in
methanol (20 mL) was added 10% Pd/C (300 mg) under nitrogen atmosphere. The
reaction
mixture was stirred under hydrogen balloon for 16 h. The reaction mixture was
filtered
through a bed of celite and concentrated under vacuum to afford the title
compound (600 mg,
84%); LC-MS: m/z 176.0 (M+1) .
Step-e: Synthesis of 6-(difluoromethoxy)benzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-(difluoromethoxy)phenol as starting material
(Yield:
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
81%); 1H NMR (400 MHz, DMSO-d6): 8 13.96 (bs, 1H), 7.49 (d, J = 2.0 Hz, 1H),
7.26 (d, J
= 8.3 Hz, 1H), 7.21 (t, J = 73.2 Hz, 1H), 7.13 (dd, J = 2.4 Hz, J = 8.8 Hz,
1H); LC-MS: m/z
218.0 (M+1) .
Step-f: Synthesis of 2-chloro-6-(difluoromethoxy)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-(difluoromethoxy)benzo[d]oxazole-2-thiol as starting
material
and stirred for 2 h (Yield: 82%); 1H NMR (400 MHz, DMSO-d6): 8 7.80 (d, J =
8.8 Hz, 1H),
7.74 (d, J= 2.5 Hz, 1H), 7.29-7.27 (m, 2H).
Step-g: Synthesis of ethyl 2((6-(difluoromethoxy)benzo [d] oxazol-2-yl)amino)-
1-methyl-
1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-(difluoromethoxy)benzo [d] oxazole as starting materials (Yield:
27%); 1H NMR
(400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.23 (d, J = 1.0 Hz, 1H), 7.88 (dd, J =
1.5 Hz, J =
8.4 Hz, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H), 7.38-7.37 (m,
1H), 7.19 (s,
1H), 7.07-7.00 (m, 1H), 4.33 (q, J = 7.0 Hz, 2H), 3.64 (s, 3H), 1.35 (t, J =
7.1 Hz, 3H); LC-
MS: m/z 403.0 (M+1) .
Step-h: Synthesis of 2((6-(difluoromethoxy)benzo [d] oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-(difluoromethoxy)benzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylate as starting material (Yield: 93%); 1H NMR (400
MHz,
DMSO-d6): 8 12.80 (bs, 1H), 12.40 (bs, 1H), 8.19 (d, J= 1.0 Hz, 1H), 7.87 (dd,
J= 1.4 Hz, J
= 8.3 Hz, 1H), 7.52-7.46 (m, 2H), 7.38-7.00 (m, 3H), 3.64 (s, 3H); LC-MS: m/z
375.0
(M+1) .
Example 63. Synthesis of 24(6-(difluoromethoxy)benzo[d]oxazol-2-yl)amino)-N-(2-
methoxyethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-((6-(difluoromethoxy)benzo[d]oxazol-2-yl)amino)-1-methyl-
1H-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
91
benzo [d] imidazole-5-carboxylic acid and 2-methoxyethan-1-amine as starting
materials; 1H
NMR (400 MHz, DMSO-d6): 8 12.25 (bs, 1H), 8.45 (t, J = 5.2 Hz, 1H), 8.07 (s,
1H), 7.77 (d,
J = 8.4 Hz, 1H), 7.49-7.45 (m, 2H), 7.37 (s, 1H), 7.18 (s, 1H), 7.06-7.00 (m,
1H), 3.63 (s,
3H), 3.48-3.44 (m, 4H), 3.28 (s, 3H); LC-MS: m/z 432.0 (M+1) .
Example 64. Synthesis of 2-((6-(difluoromethoxy)benzo[d] oxazol-2-yl)amino)-N-
(2-
hydroxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2((6-(difluoromethoxy)benzo [d] o xazol-2- yl)amino)- 1-
methyl- 1H-
benzo [d] imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials; 1H NMR
(400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.38 (t, J = 5.6 Hz, 1H), 8.08 (s, 1H),
7.78 (d, J =
8.4 Hz, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.38-7.00 (m,
3H), 3.64 (s,
3H), 3.53 (t, J = 6.4 Hz, 2H), 3.35 (q, J = 6.0 Hz, 2H); LC-MS: m/z 418.0
(M+1) .
Example 65. Synthesis of N-(2-methoxyethyl)-1-methyl-24(5-methylbenzo[d]
oxazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxamide
di OH a OH b 0,/>_sH c 0/>_sme d
0/>_so2me
Me 411" NO2 Me NH2 me 4Ir N Me N Me
00 ci io ci ci ye
H
H NH
HO CI
NO2 NO2 Me0 NO2
MeON NO 2
0 0 0 0
Me
Me Vie
NH
H -NH
N/>--NH2 j N
MeON N
Me0 N "-----N NH2 0
Me0
0 0
Me
Conditions: a) 10% Pd/C, Me0H, H2, RT, 16 h; b) Potassium ethyl xanthate,
Et0H, Reflux, 16 h; c) K2CO3, Mel, ACN, 0 C -
RT, 3 h; d) m-CPBA, DCM, 0 C - RT, 3 h; e) S0Cl2, Cat.DMF, DCM, Reflux, 4 h;
f) 2-methoxyethan-1-amine, TEA, DCM,
0 C - RT, 16 h; g) 2 M methyl amine in THF, DIPEA, DMF, 70 C, 24 h; h) 10%
Pd/C, Me0H, H2, RT, 16 h; i) Cyanogen
bromide, THF, H20, 50 C to 60 C, 16 h; j) NaH, 5-methyl-2-
(methylsulfonyl)benzo[ c]oxazole , 1,4-Dioxane, RT, 16 h
Step-a: Synthesis of 2-amino-4-methylphenol
To a solution of 4-methyl-2-nitrophenol (1.75 g, 11.4 mmol) in methanol (15
mL) was added
a slurry of 10% Pd/C (500 mg in 5 mL methanol) under nitrogen atmosphere. Then
the
reaction mixture was stirred under hydrogen gas balloon for 16 h. The reaction
mixture was
filtered through a bed of celite and concentrated under vacuum to afford the
title compound
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
92
(1.3 g, 93%); 1H NMR (400 MHz, DMSO-d6): 8 8.62 (bs, 1H), 6.49 (d, J = 8.0 Hz,
1H), 6.38
(s, 1H), 6.17 (d, J= 8.0 Hz, 1H), 4.36 (bs, 2H), 2.07 (s, 3H); LC-MS: m/z
124.2 (M+1) .
Step-b: Synthesis of 5-methylbenzo[d]oxazole-2-thiol
To a solution of 2-amino-4-methylphenol (1.3 g, 10.5 mmol) in ethanol (15 mL)
at RT was
added potassium ethyl-xanthate (3.7 g, 22.2 mmol) and the reaction mixture was
refluxed for
16 h. The reaction mixture was concentrated under vacuum and diluted with cold
water (30
mL), acidified with 1 N HC1. The solid obtained was filtered and dried under
vacuum to
afford the title compound (1.3 g, 75%); 1H NMR (400 MHz, DMSO-d6): 8 13.78 (s,
1H),
7.37 (d, J= 8.0 Hz, 1H), 7.07-7.05 (m, 2H), 2.36 (s, 3H); LC-MS: m/z 166.1
(M+1) .
Step-c: Synthesis of 5-methyl-2-(methylthio)benzo[d]oxazole
To a solution of 5-methylbenzo[d]oxazole-2-thiol (1.3 g, 7.9 mmol) in
acetonitrile (15 mL) at
0 C was added potassium carbonate (1.3 g, 9.4 mmol) and methyl iodide (0.53
mL, 8.7
mmol) and the reaction mixture was stirred at RT for 3 h. The reaction mixture
was
concentrated under vacuum and diluted with water (30 mL), acidified with 1 N
HC1. The
solid obtained was filtered and dried under vacuum to afford the title
compound (1.4 g, 99%);
1H NMR (400 MHz, DMSO-d6): 8 7.50 (d, J = 8.0 Hz, 1H), 7.43 (s, 1H), 7.11 (d,
J = 8.4 Hz,
1H), 2.74 (s, 3H), 2.40 (s, 3H); LC-MS: m/z 180.1 (M+1) .
Step-d: Synthesis of 5-methyl-2-(methylsulfonyl)benzo[d]oxazole
To a solution of 5-methyl-2-(methylthio)benzo [d] oxazole (350 mg, 1.95 mmol)
in DCM (10
mL) at 0 C was added meta-chloroperbenzoic acid (2.14 g, 6.8 mmol) and the
reaction
mixture was stirred at RT for 3 h. The reaction mixture was diluted with DCM
(100 mL),
washed with saturated sodium bicarbonate solution (2 X 30 mL), aqueous 1N
sodium
hydroxide (30 mL), water (30 mL), brine (30 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum to afford the product which was immediately used in
the next
step without any further purification (340 mg); 1H NMR (400 MHz, DMSO-d6): 8
7.84-7.79
(m, 2H), 7.49 (d, J = 8.4 Hz, 1H), 3.65 (s, 3H), 2.48 (s, 3H).
Step-e: Synthesis of 4-chloro-3-nitrobenzoyl chloride
To a solution of 4-chloro-3-nitrobenzoic acid (15.0 g, 74.6 mmol) in DCM (150
mL) at 10 C
was added thionyl chloride (15.9 mL, 223.8 mmol) and DMFA (1 mL) and the
reaction
mixture was refluxed for 4 h. The reaction mixture was concentrated under
vacuum to afford
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
93
the product which was immediately used in the next step without any further
purification
(15.0 g).
Step-f: Synthesis of 4-chloro-N-(2-methoxyethyl)-3-nitrobenzamide
To a solution of 2-methoxyethan- 1-amine (5.1 g, 68.2 mmol) in DCM (60 mL) at
0 C was
added triethylamine (20 mL, 138.6 mmol) and stirred for 10 min. Then a
solution of 4-chloro-
3-nitrobenzoyl chloride (15.0 g, 68.2 mmol) in DCM (50 mL) was added to the
reaction
mixture and stirred the contents at RT for 16 h. The reaction mixture was
diluted with DCM
(150 mL) and water (100 mL). Organic layer was separated and washed with water
(150 mL),
brine (100 mL), dried over anhydrous sodium sulfate and concentrated under
vacuum to
afford the title compound (18.5 g, 100%); 1H NMR (400 MHz, DMSO-d6): 8 8.88
(bs, 1H),
8.51 (d, J= 2.0 Hz, 1H), 8.14 (dd, J= 2.0 Hz, J= 8.4 Hz, 1H), 7.90 (d, J= 8.4
Hz, 1H), 3.49-
3.42 (m, 4H), 3.30 (s, 3H).
Step-g: Synthesis of N-(2-methoxyethyl)-4-(methylamino)-3-nitrobenzamide
To a solution of 4-chloro-N-(2-methoxyethyl)-3-nitrobenzamide (18.0 g, 69.8
mmol) in
DMFA (180 mL) at 0 C was added DIPEA (12.5 mL, 69.8 mmol) and 2 M methyl amine
in
THF (70 mL, 139.3 mmol). The reaction mixture was stirred at 70 C for 24 h.
The reaction
mixture was cooled to RT, diluted with water (200 mL) and extracted with Et0Ac
(2 X 250
mL). The combined organic layers were washed with water (100 mL), brine (100
mL), dried
over anhydrous sodium sulfate and concentrated under vacuum to give the
product which was
used in the next step without further purification (17.0 g, 96%); 1H NMR (400
MHz, DMSO-
d6): 8 8.65 (d, J = 2.0 Hz, 1H), 8.54 (t, J = 5.2 Hz, 1H), 8.42 (d, J = 4.8
Hz, 1H), 8.02 (dd, J =
1.6 Hz, J = 9.2 Hz, 1H), 7.03 (d, J = 9.2 Hz, 1H), 3.46-3.40 (m, 4H), 2.98 (d,
J = 3.2 Hz, 3H),
3.26 (s, 3H); LC-MS: m/z 254.1 (M+1) .
Step-h: Synthesis of 3-amino-N-(2-methoxyethyl)-4-(methylamino)benzamide
To a solution of N-(2-methoxyethyl)-4-(methylamino)-3-nitrobenzamide (6.0 g,
23.7 mmol)
in methanol (100 mL) was added a slurry of 10% Pd/C (1.2 g in 20 mL methanol)
under
nitrogen atmosphere. The reaction mixture was stirred under hydrogen gas
balloon for 16 h.
The reaction mixture was filtered through a bed of celite and concentrated
under vacuum to
afford the title compound (4.0 g, 75%); 1H NMR (400 MHz, DMSO-d6): 8 7.88 (t,
J = 5.2
Hz, 1H), 7.11-7.06 (m, 2H), 6.34 (d, J= 8.0 Hz, 1H), 5.07 (bs, 1H), 4.54 (bs,
2H), 3.42-3.34
(m, 4H) 3.27 (s, 3H), 2.75 (d, J = 2.8 Hz, 3H); LC-MS: m/z 224.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
94
Step-i: Synthesis of 2-amino-N-(2-methoxyethyl)-1-methy1-1H-benzo[d]imidazole-
5-
carboxamide
To a solution of 3-amino-N-(2-methoxyethyl)-4-(methylamino)benzamide (2.0 g,
8.92 mmol)
in THF (10 mL) and water (20 mL) at RT was added cyanogen bromide (1.04 g,
9.82 mmol)
and the reaction mixture was stirred at 50 C to 60 C for 16 h. The reaction
mixture was
cooled to RT and basified with saturated sodium bicarbonate solution,
extracted with ethyl
acetate (2 X 100 mL). The combined organic layers were washed with water (50
mL), brine
(50 mL), dried over anhydrous sodium sulfate and concentrated under vacuum to
afford the
crude product which was purified by combiflash chromatography using 5%
methanol in
DCM as an eluent to afford the title compound (1.3 g, 59%); 1H NMR (400 MHz,
DMSO-
d6): 8 8.26 (bs, 1H), 7.66 (s, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.14 (d, J = 8.0
Hz, 1H), 6.52 (bs,
2H), 3.51 (s, 3H), 3.45-3.41 (m, 4H), 3.26 (s, 3H); LC-MS: m/z 249.2 (M+1) .
Step-j: Synthesis of N-(2-methoxyethyl)-1-methy1-2-((5-methylbenzo[d]oxazol-2-
yl)amino)-1H-benzo[d]imidazole-5-carboxamide
To a solution of 2-amino-N-(2-methoxyethyl)-1-methy1-1H-benzo [d] imidazole-
5-
carboxamide (300 mg, 1.2 mmol) in 1,4-dioxane (5 mL) at 10 C was added sodium
hydride
(60% dispersion in mineral oil) (96 mg, 2.4 mmol) and stirred for 10 min
followed by the
addition of 5-methyl-2-(methylsulfonyl)benzo [d] oxazole (306 mg, 1.45 mmol)
and it was
stirred at RT for 16 h. The reaction mixture was quenched with cold water (20
mL) and
extracted with ethyl acetate (2 X 50 mL). The combined organic layers were
washed with
water (30 mL), brine (30 mL), dried over anhydrous sodium sulfate and
concentrated under
vacuum to obtain the crude product which was purified by combiflash
chromatography using
2% methanol in DCM as an eluent to afford the title compound (6 mg, 1%); 1H
NMR (400
MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.45 (t, J = 5.2 Hz, 1H), 8.08 (d, J = 1.6 Hz,
1H), 7.76 (dd,
J = 1.2 Hz, J = 8.0 Hz, 1H), 7.47 (d, J = 8.4 Hz, 1H), 7.30 (d, J = 8.4 Hz,
1H), 7.26 (s, 1H),
6.93 (d, J = 7.6 Hz, 1H), 3.62 (s, 3H), 3.50-3.42 (m, 4H), 3.29 (s, 3H), 2.49
(s, 3H); LC-MS:
m/z 380.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
Examples 66 and 67. Synthesis of 24(5-fluorobenzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid and Synthesis of 24(5-fluorobenzo[d]oxazol-
2-
yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
OH OH
a b (D¨S02Me
NO2 NH2 F N
fyle
Me Me
Et0 =
¨NH
N HO =
¨NH 1\i---NH
N g N
N
0 N 0 N
0
111
Conditions: a) 10% Pd/C, Me0H, H2, RT, 16 h; b) Potassium ethyl xanthate,
Et0H, Reflux, 16 h; c) K2CO3, Mel, ACN, RT,
16 h; d) m-CPBA, DCM, 0 C - RT, 4 h; e) NaH, ethyl 2-amino-1-methyl-1H-
benzo[c]imidazole-5-carboxylate , 1,4-Dioxane,
RT, 16 h; f) Li0H.H20, THF, Ethanol, Water, 60 C, 16 h; g) 2-
methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-amino-4-fluorophenol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-b using 4-fluoro-2-nitrophenol as starting material (Yield:
91%); 1H NMR
(400 MHz, DMSO-d6): 8 8.88 (bs, 1H), 6.56-6.53 (m, 1H), 6.35 (dd, J = 2.0 Hz,
J = 10.4 Hz,
1H), 6.13-6.08 (m, 1H), 4.78 (bs, 2H); LC-MS: m/z 128.1 (M+1) .
Step-b: Synthesis of 5-fluorobenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-4-fluorophenol as starting material (Yield:
86%); 1H
NMR (400 MHz, DMSO-d6): 8 14.01 (bs, 1H), 7.55-7.51 (m, 1H), 7.15-7.08 (m,
2H); LC-
MS: m/z 170.0 (M-F1) .
Step-c: Synthesis of 5-fluoro-2-(methylthio)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Compound lb using 5-fluorobenzo[d]oxazole-2-thiol as starting material
(stirring for 16 h)
(Yield: 92%); 1H NMR (400 MHz, DMSO-d6): 8 7.69-7.65 (m, 1H), 7.53 (dd, J =
2.4 Hz, J =
8.8 Hz, 1H), 7.19-7.11 (m, 1H), 2.76 (s, 3H); LC-MS: m/z 184.1 (M-F1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
96
Step-d: Synthesis of 5-fluoro-2-(methylsulfonyl)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lc using 5-fluoro-2-(methylthio)benzo[d]oxazole as starting material
(stirring for
4 h) (Yield: 99%); 1H NMR (400 MHz, DMSO-d6): 8 8.05-8.01 (m, 1H), 7.94 (dd,
J= 2.4
Hz, J = 8.0 Hz, 1H), 7.57-7.54 (m, 1H), 3.67 (s, 3H); LC-MS: m/z 216.0 (M+1) .
Step-e: Synthesis of ethyl 2((5-fluorobenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-l-methyl-1H-benzo [d] imidazole-5-carboxylate
and 5-
fluoro-2-(methylsulfonyl)benzo[d]oxazole as starting materials (Yield: 26%);
1H NMR (400
MHz, DMSO-d6): 8 12.34 (bs, 1H), 8.23 (s, 1H), 7.88 (dd, J= 1.6 Hz, J= 8.4 Hz,
1H), 7.49
(d, J = 8.0 Hz, 1H), 7.46-7.42 (m, 1H), 7.23 (dd, J = 2.4 Hz, J = 9.2 Hz, 1H),
6.97-6.91 (m,
1H), 4.34 (q, J = 6.4 Hz, 2H), 3.64 (s, 3H), 1.36 (t, J = 4.4 Hz, 3H); LC-MS:
m/z 355.2
(M+1) .
Step-f: Synthesis of 2((5-fluorobenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((5-fluorobenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate as starting material (Yield: 58%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.3 (bs, 1H), 8.19 (s, 1H), 7.87 (d, J= 8.4 Hz,
1H), 7.51 (d, J=
8.8 Hz, 1H), 7.45-7.42 (m, 1H), 7.24 (dd, J = 2.4 Hz, J = 8.8 Hz, 1H), 6.96-
6.91 (m, 1H),
3.64 (s, 3H); LC-MS: m/z 327.2 (M+1) .
Step-g: Synthesis of 2((5-fluorobenzo [d] oxazol-2-yl)amino)-N-(2-
methoxyethyl)-1-
methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 24(5-fluorobenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials (Yield:
62%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.20 (s, 1H), 8.06 (s, 1H),
7.76 (d, J
= 8.4 Hz, 1H), 7.44 (d, J = 8.4 Hz, 1H), 7.40-7.37 (m, 1H), 7.20 (d, J = 8.8
Hz, 1H), 6.89 (t, J
= 10.0 Hz, 1H), 3.63 (s, 3H), 3.50-3.45 (m, 4H), 3.09 (s, 3H); LC-MS: m/z
384.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
97
Examples 68 and 69. Synthesis of 24(6-fluorobenzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid and Synthesis of 24(6-fluorobenzo[d]oxazol-
2-
yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
OH
a
OH
b F os c F 0, d F os
¨SMe ¨S02Me
NO2 NH2
Vie Vie MeN
Et0 N HO vies N )ro MeO 40N N
0
0 N N N
0
Conditions: a) 10% Pd/C, Me0H, H2, RT, 16 h; b) Potassium ethyl xanthate,
Et0H, Reflux, 16 h; c) K2CO3, Mel, ACN, 0 C -
RT, 5 h; d) m-CPBA, DCM, 0 C - RT, 4 h; e) NaH, ethyl 2-amino-1-methyl-1H-
benzo[c]imidazole-5-carboxylate , 1,4-
Dioxane, RT, 16 h; f) Li0H.H20, THF, Ethanol, Water, 60 C, 16 h; g) 2-
methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT,
16 h
Step-a: Synthesis of 2-amino-5-fluorophenol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-b using 5-fluoro-2-nitrophenol as starting material (Yield:
92%); 1H NMR
(400 MHz, DMSO-d6): 8 9.5 (bs, 1H), 6.54-6.51 (m, 1H), 6.46 (dd, J = 2.8 Hz, J
= 10.0 Hz,
1H), 6.37-6.32 (m, 1H); LC-MS: m/z 128.2 (M+1) .
Step-b: Synthesis of 6-fluorobenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-fluorophenol as starting material (Yield:
89%); 1H
NMR (400 MHz, DMSO-d6): 8 13.94 (bs, 1H), 7.57 (dd, J = 2.0 Hz, J = 8.4 Hz,
1H), 7.25-
7.22 (m, 1H), 7.19-7.14 (m, 1H); LC-MS: m/z 170.0 (M-F1) .
Step-c: Synthesis of 6-fluoro-2-(methylthio)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lb using 6-fluorobenzo[d]oxazole-2-thiol as starting material
(stirring for 5 h)
(Yield: 99%); 1H NMR (400 MHz, DMSO-d6): 8 7.67-7.63 (m, 2H), 7.24-7.18 (m,
1H), 2.75
(s, 3H); LC-MS: m/z 184.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
98
Step-d: Synthesis of 6-fluoro-2-(methylsulfonyl)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lc using 6-fluoro-2-(methylthio)benzo[d]oxazole as starting material
(stirring for
4 h) (Yield: 95%); 1H NMR (400 MHz, DMSO-d6): 8 8.08-8.02 (m, 1H), 8.01-7.99
(m, 1H),
7.56-7.46 (m, 1H), 3.67 (s, 3H).
Step-e: Synthesis of ethyl 2((6-fluorobenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-carboxylate
and 6-
fluoro-2-(methylsulfonyl)benzo[d]oxazole as starting materials (Yield: 28%);
1H NMR (400
MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.23 (d, J = 1.6 Hz, 1H), 7.88 (dd, J = 2.0
Hz, J = 8.4 Hz,
1H), 7.52 (d, J= 8.4 Hz, 1H), 7.45-7.41 (m, 2H), 7.10-7.04 (m, 1H), 4.33 (q,
J= 6.8 Hz, 2H),
3.64 (s, 3H), 1.35(t, J = 7.2 Hz, 3H); LC-MS: m/z 355.1 (M+1) .
Step-f: Synthesis of 2((6-fluorobenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
To a stirred solution of ethyl 2-((6-fluorobenzo [d] oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d] imidazole-5-carboxylate (45 mg, 0.13 mmol) in a mixture of solvent
of THF (1 mL),
ethanol (1 mL) and water (0.5 mL) was added lithium hydroxide monohydrate (10
mg, 0.25
mmol). The reaction mixture was heated at 60 C for 16 h with stirring. The
reaction mixture
was cooled to RT and concentrated under reduced pressure. The residue was
dissolved in
water (15 mL), acidified with 1 N HC1 to obtain the solid which was filtered
and dried under
vacuum to afford the title compound (30 mg, 72%); 1H NMR (400 MHz, DMSO-d6): 8
12.8
(bs, 1H), 12.3 (bs, 1H) 8.19 (d, J = 1.6 Hz, 1H), 7.87 (dd, J = 1.6 Hz, J =
8.4 Hz, 1H), 7.50
(d, J= 8.8 Hz, 1H), 7.46-7.41 (m, 2H), 7.09-7.03 (m, 1H), 3.63 (s, 3H); LC-MS:
m/z 327.05
(M+1) .
Step-g: Synthesis of 2((6-fluorobenzo [d] oxazol-2-yl)amino)-N-(2-
methoxyethyl)-1-
methyl-1H-benzo [d] imidazole-5-carboxamide
To a stirred solution of 24(6-fluorobenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid (30 mg, 0.09 mmol) in DMFA (2 mL) at 0 C
was added
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
99
N-ethyldiisopropyl amine (0.03 mL, 0.18 mmol) and diphenylphosphoryl azide
(0.02 mL,
0.09 mmol). The reaction mixture was stirred for 30 min, followed by the
addition of 2-
methoxyethylamine (6 mg, 0.09 mmol) and the reaction mixture was then stirred
at RT for 16
h. Once the reaction was completed (monitored by TLC), the reaction mixture
was diluted
with cold water (6 mL) and stirred for 15 min. The solid obtained was
filtered, washed with
diethyl ether and dried under vacuum to afford the title compound (20 mg, 58%)
as white
solid; 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.45 (t, J = 5.2 Hz, 1H),
8.07 (d, J =
1.2 Hz, 1H), 7.75 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H), 7.49-7.40 (m, 3H), 7.08-
7.03 (m, 1H),
3.63 (s, 3H), 3.50-3.43 (m, 4H), 3.39 (s, 3H); LC-MS: m/z 384.1 (M+1) .
Examples 70 and 71. Synthesis of 6-fluoro-1-methyl-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic
acid
and Synthesis of 6-fluoro-N-(2-methoxyethyl)-1-methyl-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
Me
Me Me
NH F F
Me0 a
Me0 =1--NH2 b Me0
N
NH2 N
0
0 0
OCF3
Me Me
HOO d N
Me0
N N
0 0
OCF3 OCF3
Conditions: a) Cyanogen bromide, THF, H20, 60 C, 16 h; b) NaH, 2-
(methylsulfonyI)-6-
(trifluoromethoxy)benzo[d]oxazole, 1,4-Dioxane, RT, 16 h; c) Li0H.H20, THF,
Me0H, H20,
60 C, 16 h; d) 2-methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of methyl 2-amino-6-fluoro-1-methyl-1H-benzo[d] imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using methyl 5-amino-2-fluoro-4-(methylamino)benzoate as starting
material
and heating to 60 C for 16h. (Yield: 89%); 1H NMR (400 MHz, DMSO-d6): 8 7.54
(d, J =
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
100
6.4 Hz, 1H), 7.16 (d, J= 11.6 Hz, 1H), 6.69 (bs, 2H), 3.80 (s, 3H), 3.51 (s,
3H); LC-MS: m/z
224.1 (M+1) .
Step-b: Synthesis of methyl 6-fluoro-1-methyl-24(6-
(trifluoromethoxy)benzoldloxazol-
2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using methyl 2-amino-6-fluoro-1-methy1-1H-benzo [d] imidazole-5-
carboxylate
and 2-(methylsulfony1)-6-(trifluoromethoxy)benzo [d] oxazole as starting
materials (Yield:
18%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.10 (d, J= 6.0 Hz, 1H),
7.95-7.51
(m, 3H), 7.22 (d, J= 8.4 Hz, 1H), 3.87 (s, 3H), 3.61 (s, 3H); LC-MS: m/z 425.1
(M-F1) .
Step-c: Synthesis of 6-fluoro-1-methyl-24(6-(trifluoromethoxy)benzo [d] oxazol-
2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using methyl 6-fluoro-1-methy1-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-
y1)amino)-1H-benzo [d] imidazole-5-carboxylate as starting material and THF,
methanol and
water as solvents (2:2:1) (Yield: 66%); 1H NMR (400 MHz, DMSO-d6): 8 13.0 (bs,
1H), 12.3
(bs, 1H), 8.08 (d, J = 6.0 Hz, 1H), 7.59 (s, 1H), 7.53-7.49 (m, 2H), 7.22 (d,
J = 7.2 Hz, 1H),
3.61 (s, 3H); LC-MS: m/z 411.1 (M+1) .
Step-d: Synthesis of 6-fluoro-N-(2-methoxyethyl)-1-methyl-24(6-
(trifluoromethoxy)-
benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 6-fluoro-1-methy1-2-((6-(trifluoromethoxy)benzo[d]oxazol-2-
y1)amino)-
1H-benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials
(Yield: 48%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.18 (d, J= 4.0 Hz,
1H), 7.86
(d, J= 6.4 Hz, 1H), 7.58 (s, 1H), 7.52-7.49 (m, 2H), 7.21 (d, J= 9.2 Hz, 1H),
3.61 (s, 3H),
3.47-3.44 (m, 4H), 3.29 (s, 3H); LC-MS: m/z 468.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
101
Example 72. Synthesis of 2-(benzo [d] oxazol-2-ylamino)-6-fluoro-N-(2-(2-
hydroxyethoxy)ethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
Me Me
Me NI NI
Me0 N ¨)1.- HO N
=
Me0 N N N
0
0
0
Me
H F
HO ./0/\N N )-/-0
N
0
Conditions: a) NaH, 2-chlorobenzo[d]oxazole, 1,4-Dioxane, RT, 16 h; f)
Li0H.H20, THF,
Me0H, H20, 60 C, 16 h; g) 2-(2-aminoethoxy)ethan-1-ol , HBTU, DIPEA, DMF, 0 C -
RT, 16 h
Step-a: Synthesis of methyl 2-(benzo [d] oxazol-2-ylamino)-6-fluoro-l-methyl-
1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using methyl 2-amino-6-fluoro-1-methy1-1H-benzo [d]
imidazole-5-
carboxylate and 2-chlorobenzo[d]oxazole as starting materials (Yield: 66%); 1H
NMR (400
MHz, DMSO-d6): 8 12.25 (bs, 1H), 8.09 (d, J = 6.0 Hz, 1H), 7.55-7.44 (m, 3H),
7.24-7.22
(m, 1H), 7.17-7.12 (m, 1H), 3.86 (s, 3H), 3.60 (s, 3H); LC-MS: m/z 341.0 (M+1)
.
Step-b: Synthesis of 2-(benzo[d]oxazol-2-ylamino)-6-fluoro-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using methyl 2-(benzo[d]oxazol-2-ylamino)-6-fluoro-1-methyl-1H-
benzo [d]imidazole-5-carboxylate as starting material and methanol, THF and
water (2:2:1) as
solvent (Yield: 75%); 1H NMR (400 MHz, DMSO-d6): 8 12.80 (bs, 2H), 8.07 (d, J
= 6.8 Hz,
1H), 7.50-7.44 (m, 3H), 7.22 (t, J= 6.8 Hz, 1H), 7.15-7.11 (m, 1H), 3.60 (s,
3H); LC-MS:
m/z 327.0 (M+1) .
Step-c: Synthesis of 2-(benzo [d] oxazol-2-ylamino)-6-fluoro-N-(2-(2-
hydroxyethoxy)ethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-(benzo[d]oxazol-2-ylamino)-6-fluoro-1-methyl-1H-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
102
benzo[d]imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-01 as
starting materials
(Yield: 49%); 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.15 (bs, 1H), 7.86
(d, J =
6.4 Hz, 1H), 7.50-7.47 (m, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.23-7.19 (m, 1H),
7.14-7.10 (m,
1H), 4.60 (bs, 1H), 3.60 (s, 3H), 3.57-3.51 (m, 4H), 3.48-3.31 (m, 4H); LC-MS:
m/z 414.05
(M+1) .
Example 73. Synthesis of 24(5-fluoro-1H-benzo[d]imidazol-2-yl)amino)-1-methyl-
1H-
benzo[d]imidazole-5-carboxylic acid
Me Me
Me =Me s s\ NH N H
N b H
Et0 40 Nri Et0 ¨NH2 ,>_NH Et0
101 N )FNH HO N AIR
N
0 0
0 0
Conditions: a) 1,1'-thiocarbonyldiimidazole, ACN, 60 C, 16 h; b) 4-
fluorobenzene-1,2-diamine, EDC.HCI, DMF, 100 C, 16 h; c)
Li0H.H20, THF, Me0H, H20, 60 C, 16 h
Step-a: Synthesis of ethyl 2-(1H-imidazole-1-carbothioamido)-1-methyl-1H-
benzo[d]imidazole-5-carboxylate
To a solution of ethyl 2-amino-l-methy1-1H-benzo [d] imidazole-5-carboxylate
(1.0 g, 4.5
mmol) in acetonitrile (20 mL) at RT was added 1,1'-thiocarbonyldiimidazole
(1.06 g, 5.9
mmol) and stirred at 60 C for 16 h. The reaction mixture was cooled to RT and
stirred for 30
min. The solid obtained was filtered and dried under vacuum to afford the
titled compound
which was used in the next step without further purification (1.1 g, 73%); 1H
NMR (400
MHz, DMSO-d6): 8 13.4 (bs, 1H), 8.68 (s, 1H), 8.41 (d, J = 1.6 Hz, 1H), 8.03
(s, 1H), 7.99
(dd, J = 1.2 Hz, J = 8.8 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.01 (d, J = 1.2
Hz, 1H), 4.35 (q, J
= 7.2 Hz, 2H), 3.79 (s, 3H), 1.36 (t, J = 6.8 Hz, 3H).
Step-b: Synthesis of ethyl 2-05-fluoro-1H-benzo[d]imidazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylate
To a stirred solution of ethyl 2-(1H-imidazole-l-carbothioamido)-1-methy1-1H-
benzo[d]imidazole-5-carboxylate (900 mg, 2.74 mmol) in DMFA (15 mL) was added
EDCBC1 (1.05 g, 5.47 mmol) and heated to 60 C for 10 min. The reaction mixture
was
cooled to RT, followed by the addition of 4-fluorobenzene-1,2-diamine (345 mg,
2.74 mmol)
and it was heated at 100 C for 16 h. The reaction mixture was cooled to RT,
diluted with
water (30 mL) and extracted with Et0Ac (2 X 50 mL). The combined organic layer
was
washed with water (30 mL), brine (30 mL), dried over anhydrous sodium sulfate
and
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
103
concentrated under vacuum to afford a residue which was purified by combiflash
chromatography using 0.5% methanol in DCM as an eluent to afford the title
compound (200
mg, 21%); LC-MS: m/z 354.2 (M+1) .
Step-c: Synthesis of 2-45-fluoro-1H-benzo[d]imidazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
To a stirred solution of ethyl 2-((5-fluoro-1H-benzo [d] imidazol-2-yl)amino)-
1-methyl-1H-
benzo[d]imidazole-5-carboxylate (200 mg, 0.57 mmol) in a mixture of solvent of
THF (5
mL), methanol (5 mL) and water (5 mL) was added lithium hydroxide monohydrate
(119 mg,
2.83 mmol). The reaction mixture was heated at 60 C for 16 h with stirring.
The reaction
mixture was cooled to RT and concentrated under reduced pressure. The residue
was
dissolved in water (15 mL), acidified with 1 N HC1 to obtain the solid which
was filtered and
dried under vacuum to afford the title compound (150 mg, 81%); 1H NMR (400
MHz,
DMSO-d6): 8 12.2 (bs, 2H), 8.04 (s, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.32-7.26
(m, 2H), 7.13 (d,
J = 8.4 Hz, 1H), 6.88 (t, J = 8.0 Hz, 1H), 3.62 (s, 3H); LC-MS: m/z 326.05
(M+1) .
Example 74. Synthesis of 1-(2-(dimethylamino)ethyl)-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxylic
acid
NMe2
NMe2 NMe2
di CI lith NH b ith NH c
Et0 110
E102C 411114-IF NO2 E1020 111111111" NO2- E1020 NH2 0
NMe2 NMe2
N,>_NH N/>¨NH
EtO2C N HO2C N )r-0
N
OCF3
OCF3 N
Conditions: a) N1,N1-dimethylethane-1,2-diamine , DIPEA, DMF, 60 C, 16 h; b)
10% Pd/C, Me0H,
H2, RT, 16 h; c) Cyanogen bromide, THF, H20, 60 C, 16 h; d) NaH, 2-
(methylsulfonyI)-6-
(trifluoromethoxy)benzo[d]oxazole , 1,4-Dioxane, RT, 16 h; e) Li0H.H20, THF,
Et0H, H20, 60 C, 16 h
Step-a: Synthesis of ethyl 4-((2-(dimethylamino)ethyl)amino)-3-nitrobenzoate
To a solution of ethyl 4-chloro-3-nitrobenzoate (5.0 g, 21.8 mmol) in DMFA (40
mL) at RT
was added DIPEA (9.5 mL, 54.4 mmol) and Ni,Ni-dimethylethane-1,2-diamine (2.87
g, 32.7
mmol) and the reaction mixture was stirred at 60 C for 16 h. The reaction
mixture was
cooled to RT, diluted with cold water (250 mL) and stirred for 10 min. The
precipitated solid
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
104
was filtered and dried under vacuum to afford the title compound (4.8 g, 92%);
1H NMR (400
MHz, DMSO-d6): 8 8.65 (t, J = 4.4 Hz, 1H), 8.62 (d, J = 2.0 Hz, 1H), 7.79 (dd,
J = 2.0 Hz, J
= 8.8 Hz, 1H), 7.13 (d, J= 9.2 Hz, 1H), 4.32-4.26 (m, 2H), 3.47-3.43 (m, 2H),
2.57-2.52 (m,
2H), 2.22 (s, 6H), 1.33-1.28 (m, 3H); LC-MS: m/z 282.2 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4-((2-(dimethylamino)ethyl)amino)benzoate
The title compound was synthesized using the same procedure which was followed
for
compound le using ethyl 4-((2-(dimethylamino)ethyl)amino)-3-nitrobenzoate as
starting
material and stirring for 16 h. (Yield: 67%); 1H NMR (400 MHz, DMSO-d6): 8
7.23 (dd, J =
2.0 Hz, J= 8.0 Hz, 1H), 7.18 (d, J= 2.0 Hz, 1H), 6.45 (d, J= 8.4 Hz, 1H), 5.09
(bs, 1H), 4.68
(s, 2H), 4.18 (q, J= 6.8 Hz, 2H), 3.19-3.15 (m, 2H), 2.49-2.46 (m, 2H), 2.18
(s, 6H), 1.26 (t,
J = 6.8 Hz, 3H); LC-MS: m/z 252.2 (M+1) .
Step-c: Synthesis of ethyl 2-amino-1-(2-(dimethylamino)ethyl)-1H-benzo [d]
imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-((2-(dimethylamino)ethyl)amino)benzoate as
starting
material and heating to 60 C for 16 h. (Yield: 26%); LC-MS: m/z 277.2 (M+1) .
Step-d: Synthesis of ethyl 1-(2-(dimethylamino)ethyl)-24(6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-1-(2-(dimethylamino)ethyl)-1H-benzo [d]
imidazole-5-
carboxylate and 2-(methylsulfony1)-6-(trifluoromethoxy)benzo [d] oxazole as
starting
materials (Yield: 26%); 1H NMR (400 MHz, DMSO-d6): 8 12.4 (bs, 1H), 8.25 (d, J
= 1.2 Hz,
1H), 7.87 (dd, J= 1.2 Hz, J= 8.4 Hz, 1H), 7.59-7.57 (m, 2H), 7.51 (d, J= 8.4
Hz, 1H), 7.22
(d, J= 8.8 Hz, 1H), 4.36-4.26 (m, 4H), 2.68 (q, J= 6.8 Hz, 2H), 2.22 (s, 6H),
1.35 (t, J= 6.8
Hz, 3H); LC-MS: m/z 478.1 (M+1) .
Step-e: Synthesis of 1-(2-(dimethylamino)ethyl)-24(6-
(trifluoromethoxy)benzoldloxazol-
2-y1)amino)-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-(2-(dimethylamino)ethyl)-24(6-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
105
(trifluoromethoxy)benzo [d] o xazol-2- yl)amino)- 1H-benzo [d] imidazo le-5-c
arbo xylate as
starting material (Yield: 18%); 1H NMR (400 MHz, DMSO-d6): 8 12.8 (bs, 1H),
12.3 (bs,
1H), 8.20 (d, J= 1.6 Hz, 1H), 7.86 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.59-7.51
(m, 3H), 7.21
(d, J= 8.4 Hz, 1H), 4.27 (t, J= 6.4 Hz, 2H), 2.79-2.66 (m, 2H), 2.21 (s, 6H);
LC-MS: m/z
450.1 (M+1) .
Examples 75 and 76. Synthesis of 1-(2-methoxyethyl)-24(5-(trifluoromethoxy)-1H-
benzo[d]imidazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxylic acid and
Synthesis of
N,1-bis(2-methoxyethyl)-2-45-(trifluoromethoxy)-1H-benzo[d]imidazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxamide
OMe OMe OMe OMe
CI
=N />¨NH
EtO2C NO2 N
EtO2C 41111" NO2 EtO2C 11111" NH2 ,>_NH2
Et0 Et0
0 0
OMe OMe
rs7le
1
N> H
1)¨NH g H = 11¨NH 11#H HO =N
MeON N
Et0 N 0 N 0 N
0 *
OCF3 OCF3 OCF3
Conditions: a) 2-methoxyethan-1-amine, DMSO, 60 C, 16 h; b) 10% Pd/C, Me0H,
H2, RT, 16 h; c) Cyanogen bromide, THF, H20,
60 C, 16 h; d) 1,1'-thiocarbonyldiimidazole, ACN, 60 C, 16 h; e) 4-
(trifluoromethoxy)benzene-1,2-diamine, EDC.HCI, DMF, 100 C, 16
h; f) Li0H.H20, THF, Me0H, H20, 60 C, 16 h; g) 2-methoxyethylamine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 4-((2-methoxyethyl)amino)-3-nitrobenzoate
To a solution of ethyl 4-chloro-3-nitrobenzoate (10 g, 43.7 mmol) in DMSO (100
mL) at RT
was added 2-methoxyethan-1-amine (7.5 mL g, 87.3 mmol) and the reaction
mixture was
stirred at 60 C for 16 h. The reaction mixture was cooled to RT, diluted with
cold water (250
mL) and stirred for 1 h. The precipitated solid was filtered and dried under
vacuum to afford
the title compound (12 g, 100%); 1H NMR (400 MHz, DMSO-d6): 8 8.61 (d, J = 2.4
Hz, 1H),
8.54 (s, 1H), 7.97 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H), 7.18 (d, J = 9.2 Hz, 1H),
4.29 (q, J = 6.8
Hz, 2H), 3.60 (bs, 4H), 3.31 (s, 3H), 1.31 (t, J= 6.8 Hz, 3H); LC-MS: m/z
269.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
106
Step-b: Synthesis of ethyl 3-amino-4-((2-methoxyethyl)amino)benzoate
The title compound was synthesized using the same procedure which was followed
for
compound le using ethyl 4-((2-methoxyethyl)amino)-3-nitrobenzoate as starting
material
(Yield: 75%); 1H NMR (400 MHz, DMSO-d6): 8 7.22-7.17 (m, 2H), 6.46 (d, J = 8.4
Hz, 1H),
5.20 (t, J= 7.6 Hz, 1H), 4.74 (bs, 2H), 4.18 (q, J= 7.2 Hz, 2H), 3.53 (t, J=
5.6 Hz, 2H), 3.30-
3.26 (m, 5H), 1.26 (t, J= 7.6 Hz, 3H); LC-MS: m/z 239.1 (M+1) .
Step-c: Synthesis of ethyl 2-amino-1-(2-methoxyethyl)-1H-benzo[d]imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-((2-methoxyethyl)amino)benzoate as starting
material
and heating to 60 C for 16 h. (Yield: 73%); LC-MS: m/z 264.1 (M+1) .
Step-d: Synthesis of ethyl 2-(1H-imidazole-l-carbothioamido)-1-(2-
methoxyethyl)-1H-
benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 12a using ethyl 2-amino-1-(2-methoxyethyl)-1H-benzo [d] imidazole-5-
carboxylate
and 1,1'-thiocarbonyldiimidazole as starting materials (Yield: 60%); 1H NMR
(400 MHz,
DMSO-d6): 8 13.5 (bs, 1H), 8.66 (s, 1H), 8.42 (s, 1H), 8.01-7.97 (m, 2H), 7.78
(d, J= 8.8 Hz,
1H), 7.01 (s, 1H), 4.54 (t, J = 4.8 Hz, 2H), 4.35 (q, J = 7.2 Hz, 2H), 3.74
(t, J = 4.8 Hz, 2H),
3.20 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H).
Step-e: Synthesis of ethyl 1-(2-methoxyethyl)-2-45-(trifluoromethoxy)-1H-
benzo[d]imidazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 12b using ethyl 2-(1H-imidazole-1-carbothioamido)-1-(2-methoxyethyl)-
1H-
benzo [d] imidazole-5-carboxylate and 4-(trifluoromethoxy)benzene-1,2-diamine
as starting
materials (Yield: 38%); LC-MS: m/z 464.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
107
Step-f: Synthesis of 1-(2-methoxyethyl)-24(5-(trifluoromethoxy)-1H-
benzo[d]imidazol-
2-yl)amino)-1H-benzo[d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-(2-methoxyethyl)-24(5-(trifluoromethoxy)-1H-
benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate as
starting material and
THF, methanol and water as solvents. (Yield: 65%); 1H NMR (400 MHz, DMSO-d6):
8 12.3
(bs, 3H), 8.08 (d, J= 1.2 Hz, 1H), 7.74 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.41
(d, J= 8.4 Hz,
1H), 7.36-7.31 (m, 2H), 7.02 (d, J= 8.0 Hz, 1H), 4.32 (t, J= 5.2 Hz, 2H), 3.71
(t, J= 6.0 Hz,
2H), 3.25 (s, 3H); LC-MS: m/z 436.0 (M+1) .
Step-g: Synthesis of N,1-bis(2-methoxyethyl)-2-05-(trifluoromethoxy)-1H-
benzo[d]imidazol-2-y1)amino)-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-methoxyethyl)-24(5-(trifluoromethoxy)-1H-benzo [d]
imidazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as
starting
materials (Yield: 2%); 1H NMR (400 MHz, DMSO-d6): 8 12.23 (bs, 1H), 8.26-8.23
(m, 1H),
7.58-7.53 (m, 1H), 7.43-7.38 (m, 1H), 7.30-6.37 (m, 5H), 4.50-4.48 (m, 2H),
3.86-3.80 (m,
2H), 3.32 (s, 3H), 3.27-3.25 (m, 4H), 3.16 (s, 3H); LC-MS: m/z 493.1 (M+1) .
Examples 77 and 78. Synthesis of 1-(2-methoxyethyl)-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic
acid
and Synthesis of N,1-bis(2-methoxyethyl)-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-
y1)amino)-1H-benzo[d]imidazole-5-carboxamide
OMe OMe
OMe
16
=N NI,
/1¨NH H
_NH2 Ho2c N meoN N
Et0 N N
0
OCF3 0 OCF3
Conditions: a) NaH, 2-chloro-6-(trifluoromethoxy)be nzo[d]oxa zole, 1,4-
Dioxane, 0 C - 90 C, 16 h;
b) 2-methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
108
Step-a: Synthesis of 1-(2-methoxyethyl)-24(6-(trifluoromethoxy)benzo [d]
oxazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid
To a solution of ethyl 2-amino-1-(2-methoxyethyl)-1H-benzo [d] imidazole-5-
carboxylate
(200 mg, 0.76 mmol) in 1,4-dioxane (10 mL) at 0 C was added sodium hydride
(60%
dispersion in mineral oil) (107 mg, 2.66 mmol) and stirred for 10 min. 2-
chloro-6-
(trifluoromethoxy)benzo[d]oxazole (270 mg, 1.14 mmol) was added to the
reaction mixture
and stirred at 90 C for 16 h. The reaction mixture was cooled to RT. Solvent
was evaporated
and the residue was diluted with cold water (20 mL), acidified with 1N HC1.
The reaction
mixture was extracted with ethyl acetate (2 x 50 mL). Combined organic layer
was washed
with water (30 mL), brine (30 mL), dried over anhydrous sodium sulfate and
concentrated
under vacuum. The residue was purified by combiflash chromatography using 2%
methanol
in DCM as an eluent to afford the title compound (70 mg, 21%); 1H NMR (400
MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.3 (bs, 1H), 8.20 (d, J= 1.6 Hz, 1H), 7.86 (dd,
J= 1.2 Hz, J=
8.0 Hz, 1H), 7.58-7.52 (m, 3H), 7.22 (d, J = 7.2 Hz, 1H), 4.36 (t, J = 4.8 Hz,
2H), 3.74 (t, J =
5.6 Hz, 2H), 3.24 (s, 3H); LC-MS: m/z 437.05 (M+1) .
Step-b: Synthesis of N,1-bis(2-methoxyethyl)-2-((6-(trifluoromethoxy)benzo [d]
oxazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-methoxyethyl)-24(6-(trifluoromethoxy)benzo[d]oxazol-2-
yl)amino)-
1H-benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials
(Yield: 2%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.45 (bs, 1H), 8.08
(s, 1H),
7.75 (d, J = 8.4 Hz, 1H), 7.56 (s, 1H), 7.53-7.50 (m, 2H), 7.21 (d, J = 8.4
Hz, 1H), 4.35 (t, J =
4.8 Hz, 2H), 3.73 (t, J= 5.2 Hz, 2H), 3.48-3.43 (m, 4H), 3.28 (s, 3H), 3.24
(s, 3H); LC-MS:
m/z 494.15 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
109
Example 79 and 80. Synthesis of 1-(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid
and Synthesis of N-(2-methoxyethyl)-1-(tetrahydro-2H-pyran-4-y1)-24(6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
r(:)
coj
ro,
N H
N
NH
N )r
Et0 =N/)¨NH2 EtO2C H020
EtO2C NH2 am
0 OCF3
OCF3cD
Me0 N
0 N
OCF3
Conditions: a) Cyanogen bromide, THF, H20, 60 C, 16 h; b) NaH, 2-chloro-6-
(trifluoromethoxy)benzo[ d]oxazole , 1,4-Dioxane, RT, 16
h; c) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; d) 2-methoxyethylamine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 2-amino-1-(tetrahydro-2H-pyran-4-y1)-1H-benzo [d]
imidazole-
5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-((tetrahydro-2H-pyran-4-yflamino)benzoate as
starting
material and heating to 60 C. (Yield: 92%); 1H NMR (400 MHz, DMSO-d6): 8 7.71
(s, 1H),
7.55 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.41 (d, J= 8.0 Hz, 1H), 6.65 (bs, 2H),
4.50-4.48 (m,
1H), 4.28 (q, J= 7.2 Hz, 2H), 4.05-4.01 (m, 2H), 3.47 (t, J= 11.2 Hz, 2H),
2.36-2.32 (m,
2H), 1.72 (d, J= 10.4 Hz, 2H), 1.31 (t, J= 6.8 Hz, 3H); LC-MS: m/z 290.1 (M+1)
.
Step-b: Synthesis of ethyl 1-(tetrahydro-2H-pyran-4-y1)-2-06-
(trifluoromethoxy)-
benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate
To a solution of ethyl 2-amino-1-(tetrahydro-2H-pyran-4-y1)-1H-benzo [d]
imidazole-5-
carboxylate (100 mg, 0.35 mmol) in 1,4-dioxane (2 mL) at RT was added sodium
hydride
(60% dispersion in mineral oil) (34 mg, 0.86 mmol) and stirred for 10 min. 2-
chloro-6-
(trifluoromethoxy)benzo[d]oxazole (99 g, 0.41 mmol) was added to the reaction
mixture and
stirred at RT for 16 h. The reaction mixture was concentrated and diluted with
cold water (30
mL) and stirred at RT for 5 to 10 min. The solid obtained was filtered, dried
under vacuum
and purified by combiflash chromatography using 100% DCM as an eluent to
afford the title
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
110
compound (60 mg, 35%); 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.29 (d, J
= 1.6
Hz, 1H), 7.86 (dd, J = 1.2 Hz, J = 8.0 Hz, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.58
(d, J = 1.6 Hz,
1H), 7.52 (d, J = 8.4 Hz, 1H), 7.21 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H), 4.97-
4.93 (m, 1H), 4.33
(q, J= 7.6 Hz, 2H), 4.07-4.03 (m, 2H), 3.56 (t, J= 9.2 Hz, 2H), 2.63-2.55 (m,
2H), 1.79 (d, J
= 9.2 Hz, 2H), 1.35 (t, J= 6.8 Hz, 3H); LC-MS: m/z 491.1 (M+1) .
Step-e: Synthesis of 1-(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate as
starting material (Yield: 71%); 1H NMR (400 MHz, DMSO-d6): 8 12.8 (bs, 1H),
12.50 (bs,
1H), 8.24 (d, J= 1.6 Hz, 1H), 7.86 (dd, J= 1.2 Hz, J= 8.8 Hz, 1H), 7.71 (d, J=
8.0 Hz, 1H),
7.58 (d, J= 1.2 Hz, 1H), 7.53 (d, J= 8.8 Hz, 1H), 7.22 (d, J= 10.4 Hz, 1H),
5.01-4.92 (m,
1H), 4.05 (dd, J= 4.0 Hz, J= 11.2 Hz, 2H), 3.58-3.53 (m, 2H), 2.67-2.57 (m,
2H), 1.78 (d, J
= 10.0 Hz, 2H); LC-MS: m/z 463.1 (M+1) .
Step-f: Synthesis of N-(2-methoxyethyl)-1-(tetrahydro-2H-pyran-4-y1)-24(6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(tetrahydro-2H-pyran-4-y1)-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-
yDamino)-1H-benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as
starting
materials (Yield: 76%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.48 (t, J
= 5.2 Hz,
1H), 8.13 (d, J= 1.2 Hz, 1H), 7.76 (dd, J= 1.6 Hz, J= 8.8 Hz, 1H), 7.68 (d, J=
8.0 Hz, 1H),
7.57 (s, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.21 (d, J = 8.8 Hz, 1H), 4.98-4.92
(m, 1H), 4.07-4.04
(m, 2H), 3.56 (t, J= 1.2 Hz, 2H), 3.49-3.44 (m, 4H), 3.28 (s, 3H), 2.67-2.58
(m, 2H), 1.78 (d,
J = 9.6 Hz, 2H); LC-MS: m/z 520.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339
PCT/US2020/027240
111
Example 81. Synthesis of N-(2-methoxyethyl)-1-(tetrahydro-2H-pyran-4-y1)-24(5-
(trifluoromethoxy)-1H-benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
co) ro,
r_c)
ro,
N
=N,)_N, b Et0 )=.- HO
4111111)-P
Et0 Et0 N N
0
0 S N
0
OCF3 OCF3
coj
N/)¨NH
d
Me0 N NH
0 N
OCF3
Conditions: a) 1,1'-thiocarbonyldiimidazole, ACN, 60 C, 16 h; b) 4-
(trifluoromethoxy)benzene-1,2-diamine , EDC.HCI , DMF, 100 C, 16
h; c) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; d) 2-methoxyethylamine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 2-(1H-imidazole-1-carbothioamido)-1-(tetrahydro-2H-
pyran-
4-y1)-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 12a using ethyl 2-amino-1-(tetrahydro-2H-pyran-4-y1)-1H-benzo [d]
imidazole-5-
carboxylate and 1,1'-thiocarbonyldiimidazole as starting materials (Yield:
72%); 1H NMR
(400 MHz, DMSO-d6): 8 13.50 (bs, 1H), 8.64 (s, 1H), 8.43 (s, 1H), 7.98 (d, J =
4.4 Hz, 1H),
7.96-7.94 (m, 1H), 7.09 (s, 1H), 6.72 (bs, 1H), 5.05-5.00 (m, 1H), 4.36-4.26
(m, 2H), 4.06-
4.02 (m, 2H), 3.60 (t, J= 11.6 Hz, 2H), 2.54-2.50 (m, 2H), 1.86 (d, J= 9.6 Hz,
2H), 1.37-
1.33 (m, 3H).
Step-b: Synthesis of ethyl 1-(tetrahydro-2H-pyran-4-y1)-2-05-
(trifluoromethoxy)-1H-
benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 12b using ethyl 2-(1H-imidazole-1-carbothioamido)-1-(tetrahydro-2H-
pyran-4-
y1)-1H-benzo [d] imidazole-5-carboxylate and 4-(trifluoromethoxy)benzene-1,2-
diamine as
starting materials (Yield: 46%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H),
8.12 (d, J=
6.4 Hz, 1H), 7.95 (s, 1H), 7.74 (dd, J = 2.0 Hz, J = 8.4 Hz, 1H), 7.57 (d, J =
8.4 Hz, 1H),
7.37-7.32 (m, 2H), 7.06 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 4.97-4.90 (m, 1H),
4.32(q, J= 6.8
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
112
Hz, 2H), 4.08-4.05 (m, 2H), 3.51 (t, J= 11.6 Hz, 2H), 2.67-2.55 (m, 2H), 1.75
(d, J= 9.6 Hz,
2H), 1.07 (t, J = 8.4 Hz, 3H); LC-MS: m/z 490.0 (M+1) .
Step-c: Synthesis of 1-(tetrahydro-2H-pyran-4-y1)-24(5-(trifluoromethoxy)-1H-
benzo[d]imidazol-2-yl)amino)-1H-benzoldlimidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-(tetrahydro-2H-pyran-4-y1)-2-((5-(trifluoromethoxy)-
1H-
benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylate as
starting material
(Yield: 55%); 1H NMR (400 MHz, DMSO-d6): 8 12.7 (bs, 3H), 8.04 (s, 1H), 7.80
(d, J = 8.4
Hz, 1H), 7.65 (d, J = 8.8 Hz, 1H), 7.42-7.35 (m, 2H), 7.12 (d, J = 8.0 Hz,
1H), 4.93 (bs, 1H),
4.08-4.05 (m, 2H), 3.54-3.48 (m, 2H), 2.61-2.58 (m, 2H), 1.77 (d, J= 10.0 Hz,
2H); LC-MS:
m/z 462.15 (M+1) .
Step-d: Synthesis of N-(2-methoxyethyl)-1-(tetrahydro-2H-pyran-4-y1)-24(5-
(trifluoromethoxy)-1H-benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(tetrahydro-2H-pyran-4-y1)-2-((5-(trifluoromethoxy)- 1H-
benzo [d] imidazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid and 2-
methoxyethylamine as starting materials (Yield: 42%); 1H NMR (400 MHz, DMSO-
d6): 8
12.2 (bs, 2H), 8.37 (t, J= 5.6 Hz, 1H), 8.02 (d, J= 1.6 Hz, 1H), 7.64 (dd, J=
1.2 Hz, J= 8.0
Hz, 1H), 7.52 (d, J = 8.8 Hz, 1H), 7.53-7.30 (m, 2H), 7.03 (d, J = 8.0 Hz,
1H), 4.98-4.89 (m,
1H), 4.08-4.05 (m, 2H), 3.51-3.43 (m, 6H), 3.28 (s, 3H), 2.67-2.58 (m, 2H),
1.75-1.73 (m,
2H); LC-MS: m/z 519.1 (M+1) .
Examples 82 and 83. Synthesis of 24(5-chloro-1H-benzo[d]imidazol-2-yl)amino)-1-
methyl-1H-benzoldlimidazole-5-carboxylic acid and Synthesis of 2-05-chloro-1H-
benzo[d] imidazol-2-yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-benzo [d]
imidazole-5-
carboxamide
Me Me 1)11eN
Me S
a b Ho 40 meo."-JI
Et0 is N,NH ,N Et0 N N 0 N
0
0
0
CI CI CI
Conditions: a) 4-chlorobenzene-1,2-diamine, EDC.HCI, DMF, 100 C, 16 h; b)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h;
c) 2-methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
113
Step-a: Synthesis of ethyl 24(5-chloro-1H-benzo[d]imidazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 12b using ethyl 2-(1H-imidazole-1-carbothioamido)-1-methy1-1H-
benzo[d]imidazole-5-carboxylate and 4-chlorobenzene-1,2-diamine as starting
materials
(Yield: 24%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 2H), 8.10 (s, 1H), 7.76
(d, J= 8.4
Hz, 1H), 7.40-7.29 (m, 3H), 7.09 (dd, J= 1.2 Hz, J= 8.4 Hz, 1H), 4.31 (q, J=
6.8 Hz, 2H),
3.64 (s, 3H), 1.35 (t, J = 6.8 Hz, 3H); LC-MS: m/z 370.05 (M+1) .
Step-b: Synthesis of 24(5-chloro-1H-benzo[d]imidazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((5-chloro-1H-benzo [d] imidazol-2-yl)amino)-1-
methyl-1H-
benzo [d] imidazole-5-carboxylate as starting material (Yield: 84%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.3 (bs, 3H), 8.07 (s, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.37-7.29
(m, 3H), 7.07 (d,
J = 7.6 Hz, 1H), 3.63 (s, 3H); LC-MS: m/z 342.0 (M+1) .
Step-c: Synthesis of 24(5-chloro-1H-benzo[d]imidazol-2-yl)amino)-N-(2-
methoxyethyl)-
1-methyl-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-((5-chloro-1H-benzo [d] imidazol-2-yl)amino)-1-methyl-1H-
benzo[d] imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials (Yield:
49%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 2H), 8.36 (s, 1H), 7.99 (s, 1H),
7.66 (d, J
= 7.2 Hz, 1H), 7.35 (d, J = 8.4 Hz, 1H), 7.30-7.28 (m, 2H), 7.06 (d, J = 8.4
Hz, 1H), 3.62 (s,
3H), 3.48-3.43 (m, 4H), 3.17 (s, 3H); LC-MS: m/z 399.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
114
Examples 84 and 85. Synthesis of 1-ethyl-2-46-(trifluoromethoxy)benzo[d]oxazol-
2-
yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid and Synthesis of 1-ethyl-N-(2-
methoxyethyl)-24(6-(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-
benzo [d] imidazole-5-carboxamide
Me Me
N N
CI N Me =
a NMe b Et0 =N¨I\II-12 Et0
EtO2C NO2 EtO2C NO2 EtO2C NH2 0 0 N
OCF3
Mel Me
N
NH NH
e HO N )FC) MeON
0 N
= OCF3 0 N
OCF3
Conditions: a) Aq.Ethyl amine, DMF, 60 C, 16 h; b) 10% Pd/C, Me0H, H2, RT, 16
h; c) Cyanogen bromide, THF, H20, 60 C, 16 h; d)
NaH, 2-chloro-6-(trifluoromethoxy)benzo[d]oxazole, 1,4-Dioxane, RT, 16 h; e)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h; f) 2-
methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 4-(ethylamino)-3-nitrobenzoate
To a solution of ethyl 4-chloro-3-nitrobenzoate (4.0 g, 17.5 mmol) in DMFA
(100 mL) at RT
was added ethylamine (2.24 mL (70% aqueous solution), 34.9 mmol) and the
reaction
mixture was stirred at 60 C for 16 h. The reaction mixture was cooled to RT,
diluted with
cold water (200 mL) and stirred for 15 min. The precipitated solid was
filtered and dried
under vacuum to afford the title product (3.0 g, 63%); 1H NMR (400 MHz, DMSO-
d6): 8
8.62 (d, J= 2.0 Hz, 1H), 8.51 (t, J= 5.2 Hz, 1H), 7.97 (dd, J= 2.0 Hz, J= 9.2
Hz, 1H), 7.14
(d, J = 9.2 Hz, 1H), 4.29 (q, J = 6.8 Hz, 2H), 3.49-3.42 (m, 2H), 1.33 (t, J =
5.2 Hz, 3H), 1.23
(t, J= 7.2 Hz, 3H); LC-MS: m/z 239.0 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4-(ethylamino)benzoate
The title compound was synthesized using the same procedure which was followed
for
compound le using ethyl 4-(ethylamino)-3-nitrobenzoate as starting material
(Yield: 79%)
and the crude compound was used in the next step without any purification.
Step-c: Synthesis of ethyl 2-amino-1-ethyl-1H-benzo [d] imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-(ethylamino)benzoate as starting material
(Yield: 74%);
1H NMR (400 MHz, DMSO-d6): 8 7.70 (s, 1H), 7.58 (dd, J = 1.6 Hz, J = 8.4 Hz,
1H), 7.23
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
115
(d, J= 8.4 Hz, 1H), 6.66 (bs, 2H), 4.27 (q, J= 7.2 Hz, 2H), 4.05 (q, J= 6.8
Hz, 2H), 1.31 (t, J
= 6.8 Hz, 3H), 1.20 (t, J= 7.6 Hz, 3H); LC-MS: m/z 234.1 (M+1) .
Step-d: Synthesis of ethyl 1-ethyl-24(6-(trifluoromethoxy)benzo[d]oxazol-2-
yl)amino)-
1H-benzo[d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-l-ethy1-1H-benzo14]imidazole-5-carboxylate and
2-
chloro-6-(trifluoromethoxy)benzo [d] oxazole as starting materials (Yield:
54%); 1H NMR
(400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.25 (d, J= 1.6 Hz, 1H), 7.88 (dd, J= 2.0
Hz, J= 8.8
Hz, 1H), 7.60-7.57 (m, 2H), 7.51 (d, J= 8.8 Hz, 1H), 7.22 (dd, J= 1.6 Hz, J=
8.8 Hz, 1H),
4.33 (q, J= 7.2 Hz, 2H), 4.22 (q, J= 7.6 Hz, 2H), 1.37-1.31 (m, 6H); LC-MS:
m/z 435.05
(M+1) .
Step-e: Synthesis of 1-ethyl-24(6-(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-
1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-ethy1-2-((6-(trifluoromethoxy)benzo[d]oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylate as starting material (Yield: 75%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.3 (bs, 1H), 8.21 (s, 1H), 7.88 (d, J = 8.0 Hz,
1H), 7.58-7.57
(m, 2H), 7.52 (d, J= 8.4 Hz, 1H), 7.20 (d, J= 9.6 Hz, 1H), 4.22 (q, J= 7.6 Hz,
2H), 1.33 (t, J
= 6.8 Hz, 3H); LC-MS: m/z 407.0 (M+1) .
Step-f: Synthesis of 1-ethyl-N-(2-methoxyethyl)-24(6-
(trifluoromethoxy)benzo[d]oxazol-
2-yl)amino)-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-ethy1-2-((6-(trifluoromethoxy)benzo[d]oxazol-2-yDamino)-1H-
benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials (Yield:
58%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.46 (s, 1H), 8.09 (s, 1H),
7.77 (d, J
= 8.4 Hz, 1H), 7.56-7.50 (m, 3H), 7.21 (d, J = 8.0 Hz, 1H), 4.22 (q, J = 7.6
Hz, 2H), 3.48-
3.44 (m, 4H), 3.28 (s, 3H), 1.32 (t, J= 7.6 Hz, 3H); LC-MS: m/z 464.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
116
Example 86. Synthesis of 1-ethyl-N-(2-hydroxyethyl)-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
Me) Me
N/).¨NH a N,>_NH
>F0 HO
=
HO
0 N
OCF3 0 N
OCF3
Conditions: a) 2-aminoethan-1-ol , HBTU, DIPEA, DMF, 0 C - RT, 16 h
To a stirred solution of 1-ethyl-2-((6-(trifluoromethoxy)benzo [d]oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid (60 mg, 0.14 mmol) in DMFA (1 mL) at 0 C
was
added N-ethyldiisopropyl amine (0.02 mL, 0.14 mmol) and HBTU (52 mg, 0.14
mmol). The
reaction mixture was stirred for 30 min, followed by the addition of 2-
aminoethan-1-ol (8 mg,
0.14 mmol) and stirring was continued at RT for 16 h. Once the reaction was
completed
(monitored by TLC), the reaction mixture was diluted with cold water (15 mL)
and stirred for
15 min. The solid obtained was filtered, washed with diethyl ether and dried
under vacuum to
afford the title compound (20 mg, 30%) as white solid; 1H NMR (400 MHz, DMSO-
d6): 8
12.3 (bs, 1H), 8.37 (t, J = 4.8 Hz, 1H), 8.09 (s, 1H), 7.78 (d, J = 8.4 Hz,
1H), 7.56-7.49 (m,
3H), 7.21 (d, J = 8.0 Hz, 1H), 4.72 (t, J = 5.6 Hz, 1H), 4.22 (q, J = 7.2 Hz,
2H), 3.53 (q, J =
5.2 Hz, 2H), 3.39-3.37 (m, 2H), 1.32 (t, J= 8.0 Hz, 3H); LC-MS: m/z 450.1
(M+1) .
Examples 87 and 88. Synthesis of 1-((tetrahydrofuran-3-yl)methyl)-2-((6-
(trifluoromethoxy)-benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxylic acid
Synthesis of N-(2-methoxyethyl)-1-((tetrahydrofuran-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
di CI
a
N
JOo
NO2 ditih E
EtO2C NO21
Et0 =N,>_NH2
_ 2 Et02,-, =-=2 EtO2C NH2
0
00 00
\Th
\Th N
/>¨NH
i& NI;1¨NH />¨NH f __ MeON N
Et0 =¨s HO = N 0 N
0 N
0 N
OCF3
OCF3 OCF3
Conditions: a) (tetrahydrofuran-3-yl)methanamine , DIPEA, THF, 60 C, 16 h; b)
10% Pd/C, Me0H, H2, RT, 16 h;
c) Cyanogen bromide, THF, H20, 50 C, 16 h; d) NaH, 2-chloro-6-
(trifluoromethoxy)benzo[ c]oxazole , 1,4-
Dioxane, RT, 16 h; e) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; f) 2-
methoxyethylamine, DPPA, DIPEA, DMF,
0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
117
Step-a: Synthesis of ethyl 3-nitro-4-(((tetrahydrofuran-3-
yl)methyl)amino)benzoate
To a solution of ethyl 4-chloro-3-nitrobenzoate (2.0 g, 8.7 mmol) in THF (40
mL) at RT was
added DIPEA (4.6 mL, 26.1 mmol) and (tetrahydrofuran-3-yl)methanamine (1.06 g,
10.5
mmol) then the reaction mixture was stirred at 60 C for 16 h. The reaction
mixture was
cooled to RT, diluted with cold water (200 mL) and stirred for 1 h. The solid
precipitated was
filtered and dried under vacuum to afford the title compound (2.4 g, 94%); 1H
NMR (400
MHz, DMSO-d6): 8 8.62-8.59 (m, 2H), 7.97 (dd, J = 2.0 Hz, J = 9.2 Hz, 1H),
7.20 (d, J = 8.8
Hz, 1H), 4.29 (q, J= 7.6 Hz, 2H), 3.82-3.77 (m, 1H), 3.73-3.69 (m, 1H), 3.66-
3.60 (m, 1H),
3.53-3.50 (m, 1H), 3.45-3.40 (m, 2H), 2.67-2.58 (m, 1H), 2.03-1.95 (m, 1H),
1.69-1.63 (m,
1H), 1.31 (t, J= 7.2 Hz, 3H); LC-MS: m/z 295.0 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4-(((tetrahydrofuran-3-
yl)methyl)amino)benzoate
The title compound was synthesized using the same procedure which was followed
for
compound le using ethyl 3-nitro-4-(((tetrahydrofuran-3-
yl)methyl)amino)benzoate as starting
material (Yield: 86%); 1H NMR (400 MHz, DMSO-d6): 8 7.20 (dd, J = 2.0 Hz, J =
8.0 Hz,
1H), 7.16 (d, J= 2.0 Hz, 1H), 6.46 (d, J= 8.0 Hz, 1H), 5.26 (bs, 1H), 4.75 (s,
2H), 4.18 (q, J
= 7.2 Hz, 2H), 3.77-3.73 (m, 2H), 3.64-3.62 (m, 1H), 3.49-3.47 (m, 1H), 3.07
(t, J= 6.4 Hz,
2H), 2.50-2.49 (m, 1H), 2.11-1.98 (m, 1H), 1.63-1.48 (m, 1H), 1.26 (t, J= 7.2
Hz, 3H); LC-
MS: m/z 265.1 (M+1) .
Step-c: Synthesis of ethyl 2-amino-1-((tetrahydrofuran-3-yl)methyl)-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-(((tetrahydrofuran-3-
yl)methyl)amino)benzoate as
starting material (Yield: 79%); 1H NMR (400 MHz, DMSO-d6): 8 7.70 (s, 1H),
7.58 (dd, J =
1.6 Hz, J= 8.4 Hz, 1H), 7.27 (d, J= 8.4 Hz, 1H), 6.71 (s, 2H), 4.27 (q, J= 6.8
Hz, 2H), 4.03
(d, J= 8.0 Hz, 2H), 3.78-3.71 (m, 1H), 3.63-3.59 (m, 2H), 3.48-3.41 (m, 1H),
2.72-2.68 (m,
1H), 1.85-1.79 (m, 1H), 1.65-1.58 (m, 1H), 1.31 (t, J= 7.2 Hz, 3H); LC-MS: m/z
290.1
(M+1) .
Step-d: Synthesis of ethyl 1-((tetrahydrofuran-3-yl)methyl)-2-46-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
118
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-ethy1-1H-benzo[d]imidazole-5-
carboxylate and 2-
chloro-6-(trifluoromethoxy)benzo [d] oxazole as starting materials (Yield:
44%); 1H NMR
(400 MHz, DMSO-d6): 8 12.4 (bs, 1H), 8.26 (d, J= 1.6 Hz, 1H), 7.88 (dd, J= 1.6
Hz, J= 8.4
Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.59 (s, 1H), 7.52 (d, J = 8.4 Hz, 1H),
7.22 (d, J = 7.6 Hz,
1H), 4.33 (q, J= 7.2 Hz, 2H), 4.20-4.18 (m, 2H), 3.88-3.86 (m, 1H), 3.70-3.56
(m, 3H), 3.80-
3.78 (m, 1H), 1.94-1.91 (m, 1H), 1.73-1.71 (m, 1H), 1.35 (t, J= 7.2 Hz, 3H);
LC-MS: m/z
491.15 (M+1) .
Step-e: Synthesis of 1-((tetrahydrofuran-3-yl)methyl)-2-((6-(trifluoromethoxy)-
benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-((tetrahydrofuran-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate as
starting material (Yield: 76%); 1H NMR (400 MHz, DMSO-d6): 8 12.8 (bs, 1H),
12.3 (bs,
1H), 8.21 (s, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.62-7.58 (m, 2H), 7.53 (d, J =
8.8 Hz, 1H), 7.21
(d, J= 8.4 Hz, 1H), 4.19 (d, J= 7.6 Hz, 2H), 3.89-3.84 (m, 1H), 3.70-3.58 (m,
3H), 2.89-2.85
(m, 1H), 1.98-1.90 (m, 1H), 1.76-1.68 (m, 1H); LC-MS: m/z 463.1 (M+1) .
Step-f: Synthesis of N-(2-methoxyethyl)-1-((tetrahydrofuran-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-((tetrahydrofuran-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid and
2-methoxyethylamine as starting materials (Yield: 18%); 1H NMR (400 MHz, DMSO-
d6): 8
12.3 (bs, 1H), 8.47 (s, 1H), 8.09 (s, 1H), 7.77 (d, J= 7.6 Hz, 1H), 7.60-7.50
(m, 3H), 7.21 (d,
J= 8.0 Hz, 1H), 4.18 (bs, 2H), 3.87 (bs, 1H), 3.67-3.57 (m, 3H), 3.47 (bs,
4H), 3.28 (s, 3H),
2.88 (bs, 1H), 1.92 (bs, 1H), 1.73 (bs, 1H); LC-MS: m/z 520.15 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
119
Examples 89 and 90. Synthesis of 1-(2-(methylsulfonypethyl)-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic
acid
and Synthesis of N-(2-methoxyethyl)-1-(2-(methylsulfonypethyl)-2-46-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
SO2Me SO2Me SO2Me
CI a NH NH
40 N,>_NH2
Eto2c qr. NO2e
_ NO2 EtO2O NH2 Et0
0
SO2Me SO2Me SO2Me
N,
e HO =f H 101 /1¨NH
Et0 N 0 N
N
0 N
OCF3 0 N 0 N
OCF3 OCF3
Conditions: a) 2-(methylsulfonyl)ethan-1-amine hydrochloride, DIPEA, DMF, 60
C, 16 h; b) 10% Pd/C, Me0H, H2, RT, 16 h;
c) Cyanogen bromide, THF, H20, 50 C, 16 h; d) NaH, 2-chloro-6-
(trifluoromethoMbenzo[ c]oxazole , 1,4-Dioxane, RT, 16 h;
e) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; f) 2-methoxyethylamine, DPPA, DIPEA,
DMF, 0 C - RT, 16 h
Step-a: Synthesis of ethyl 4-((2-(methylsulfonyl)ethyl)amino)-3-nitrobenzoate
The title compound was synthesized using the same procedure which was followed
for
compound 3a using ethyl 4-chloro-3-nitrobenzoate and 2-(methylsulfonyl)ethan-1-
amine
hydrochloride as starting materials and heating to 60 C. (Yield: 89%); 1H NMR
(400 MHz,
DMSO-d6): 8 8.69 (t, J = 6.0 Hz, 1H), 8.63 (d, J = 2.0 Hz, 1H), 8.02 (dd, J =
2.0 Hz, J = 8.8
Hz, 1H), 7.20 (d, J= 9.2 Hz, 1H), 4.30 (q, J= 6.8 Hz, 2H), 3.89 (q, J= 5.6 Hz,
2H), 3.52 (t, J
= 6.8 Hz, 2H), 3.08 (s, 3H), 1.30 (t, J= 6.8 Hz, 3H); LC-MS: m/z 317.0 (M+1) .
Step-b: Synthesis of ethyl 3-amino-4-((2-(methylsulfonyl)ethyl)amino)benzoate
The title compound was synthesized using the same procedure which was followed
for
compound le using ethyl 4-((2-(methylsulfonyl)ethyl)amino)-3-nitrobenzoate as
starting
material (Yield: 86%); 1H NMR (400 MHz, DMSO-d6): 8 7.24-7.20 (m, 2H), 6.52
(d, J = 8.4
Hz, 1H), 5.45 (t, J= 5.2 Hz, 1H), 4.72 (s, 2H), 4.19 (q, J= 7.2 Hz, 2H), 3.58
(q, J= 6.4 Hz,
2H), 3.38 (t, J = 6.8 Hz, 2H), 3.03 (s, 3H), 1.26 (t, J = 6.8 Hz, 3H); LC-MS:
m/z 287.0
(M+1) .
Step-c: Synthesis of ethyl 2-amino-1-(2-(methylsulfonypethyl)-1H-benzo [d]
imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-((2-(methylsulfonyl)ethyl)amino)benzoate as
starting
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
120
material (Yield: 86%); 1H NMR (400 MHz, DMSO-d6): 8 7.71 (d, J = 1.2 Hz, 1H),
7.61 (dd,
J= 1.6 Hz, J= 8.4 Hz, 1H), 7.28 (d, J= 8.4 Hz, 1H), 6.75 (s, 2H), 4.47-4.40(m,
2H), 4.30 (q,
J= 7.6 Hz, 2H), 3.55 (t, J= 6.8 Hz, 2H), 3.03 (s, 3H), 1.31 (t, J= 6.8 Hz,
3H).
Step-d: Synthesis of ethyl 1-(2-(methylsulfonypethyl)-24(6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-1-(2-(methylsulfonyl)ethyl)-1H-benzo [d]
imidazole-5-
carboxylate and 2-chloro-6-(trifluoromethoxy)benzo [d] oxazole as starting
materials (Yield:
24%); 1H NMR (400 MHz, DMSO-d6): 8 13.4 (bs, 1H), 8.25 (d, J = 1.6 Hz, 1H),
7.89 (dd, J
= 1.2 Hz, J = 8.0 Hz, 1H), 7.63-7.61 (m, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.56-
7.51 (m, 1H),
4.61 (t, J= 6.8 Hz, 2H), 4.34 (q, J= 7.2 Hz, 2H), 3.73 (d), J= 6.8 Hz, 2H),
3.14 (s, 3H), 1.35
(t, J= 7.6 Hz, 3H); LC-MS: m/z 513.05 (M-F1) .
Step-e: Synthesis of 1-(2-(methylsulfonypethyl)-24(6-(trifluoromethoxy)benzo
[d] oxazol-
2-yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-(2-(methylsulfonyl)ethyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate as
starting material (Yield: 17%); 1H NMR (400 MHz, DMSO-d6): 8 12.8 (bs, 1H),
12.4 (bs,
1H), 8.20 (d, J = 1.2 Hz, 1H), 7.88 (dd, J = 1.2 Hz, J = 8.0 Hz, 1H), 7.61-
7.59 (m, 2H), 7.54
(d, J= 8.4 Hz, 1H), 7.22 (d, J= 8.8 Hz, 1H), 4.61 (t, J= 6.8 Hz, 2H), 3.72 (t,
J= 6.8 Hz, 2H),
3.14 (s, 3H); LC-MS: m/z 484.9 (M-F1) .
Step-f: Synthesis of N-(2-methoxyethyl)-1-(2-(methylsulfonypethyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-(2-(methylsulfonyl)ethyl)-2-((6-
(trifluoromethoxy)benzo[d]oxazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as
starting
materials (Yield: 32%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.48 (t,
J = 5.2
Hz, 1H), 8.08 (s, 1H), 7.77 (d, J = 8.4 Hz, 1H), 7.67-7.54 (m, 3H), 7.21 (d, J
= 9.6 Hz, 1H),
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
121
4.60 (t, J = 6.8 Hz, 2H), 3.74 (t, J = 10.8 confirm coupling constant Hz, 2H),
3.48-3.44 (m,
4H), 3.28 (s, 3H), 3.14 (s, 3H); LC-MS: m/z 542.0 (M+1) .
Example 91. Synthesis of 2-06-bromobenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
Me
Br al OH 40
NO2 IW Br dal OH NH2 =sEl Br Br ,
d Et0 N a O c
N
N 0
Br
Me
HO 111111" N
N
0
Br
Conditions: a) SnC122H20, Et0H, Reflux, 4 h; b) Potassium ethyl xanthate,
Et0H, Reflux, 16 h; c) SOCl2, Cat.DMF, Reflux,
3 h; d) NaH, ethyl 2-amino-1-methyl-1 H-benzo[c]imidazole-5-carboxylate , 1,4-
Dioxane, RT, 16 h; e) Li0H.H20, THF, Et0H,
H20, 60 C, 16 h
Step-a: Synthesis of 2-amino-5-bromophenol
To a solution of 5-bromo-2-nitrophenol (4.0 g, 18.35 mmol) in ethanol (60 mL)
at RT was
added SnC12.2H20 (20.6 g, 91.74 mmol) and the reaction mixture was refluxed
for 4 h. The
reaction mixture was cooled to RT, concentrated under reduced pressure. The
residue was
diluted with water (20 mL) and basified with saturated aqueous sodium
bicarbonate solution,
extracted with ethyl acetate (2 X 100 mL). The combined organic layer was
washed with
water (50 mL), brine (30 mL), dried over anhydrous sodium sulfate and
concentrated under
vacuum to afford the title compound (2.4 g, 70%); 1H NMR (400 MHz, DMSO-d6): 8
9.44
(bs, 1H), 6.75 (d, J= 2.4 Hz, 1H), 6.67 (dd, J= 2.0 Hz, J= 8.4 Hz, 1H), 6.51
(d, J= 8.4 Hz,
1H), 4.65 (bs, 2H); LC-MS: m/z 190.0 (M+1) .
Step-b: Synthesis of 6-bromobenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-bromophenol as starting material (Yield:
98%); 1H
NMR (400 MHz, DMSO-d6): 8 14.0 (bs, 1H), 7.84 (d, J = 1.6 Hz, 1H), 7.47 (dd, J
= 1.6 Hz, J
= 8.4 Hz, 1H), 7.18 (d, J= 8.4 Hz, 1H); LC-MS: m/z 227.9 (M-1)-.
Step-c: Synthesis of 6-bromo-2-chlorobenzo[d]oxazole
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
122
To a solution of 6-bromobenzo [d] oxazole-2-thiol (2.89 g, 12.56 mmol) in
thionyl chloride
(20 mL) at RT was added N,N-dimethylformamide (0.2 mL) and the reaction
mixture was
refluxed for 3 h. The reaction mixture was concentrated, diluted with cold
water (50 mL) and
extracted with Et0Ac (2 X 50 mL). The combined organic layers were washed with
saturated
aqueous sodium bicarbonate solution (50 mL), brine solution (40 mL), dried
over anhydrous
sodium sulfate and concentrated under vacuum to afford the title compound (2.8
g, 96%); 1H
NMR (400 MHz, DMSO-d6): 8 8.14 (s, 1H), 7.72 (d, J = 8.8 Hz, 1H), 7.62 (dd, J
= 2.0 Hz, J
= 8.8 Hz, 1H).
Step-d: Synthesis of ethyl 2((6-bromobenzo[d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino- 1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
6-bromo-2-chlorobenzo[d]oxazole as starting materials (Yield: 20%); 1H NMR
(400 MHz,
DMSO-d6): 8 11.56 (bs, 1H), 8.23 (d, J= 1.6 Hz, 1H), 7.88 (dd, J= 1.6 Hz, J=
8.4 Hz, 1H),
7.72 (d, J = 1.6 Hz, 1H), 7.54 (d, J = 8.8 Hz, 1H), 7.38-7.36 (m, 2H), 4.33
(q, J = 7.2 Hz,
2H), 3.64 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H); LC-MS: m/z 416.9 (M-F1) .
Step-e: Synthesis of 2((6-bromobenzo[d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-bromobenzo [d]oxazol-2- yl)amino)- 1-methyl- 1H-
benzo [d] imidazole-5-carboxylate as starting material (Yield: 75%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.3 (bs, 1H), 8.19 (s, 1H), 7.87 (d, J= 8.4 Hz,
1H), 7.71 (d, J=
1.6 Hz, 1H), 7.51 (d, J= 8.8 Hz, 1H), 7.42-7.35 (m, 2H), 3.64 (s, 3H); LC-MS:
m/z 388.9
(M+1) .
Example 92. Synthesis of N-(2-hydroxyethyl)-24(6-methoxybenzo[d] oxazol-2-
yl)amino)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
123
F io OH
a NO2 NO2 Me0 OH b Me0 NH2
OH Me0 = -SH d Me0 0,
=õ0,
Me =Me Me
--NH
Et0 N f HO N )T-0 HO =No N
N
0
0
N N
OMe OMe OMe
Conditions: a) Na0Me, Me0H, 60 C, 40 h; b) 10% Pd/C, Me0H, H2, RT, 16 h; c)
Potassium ethyl xanthate, Et0H, Reflux,
16 h; d) S0Cl2, Cat.DMF, Reflux, 2 h; e) NaH, ethyl 2-amino-1-methyl-1H-
benzo[c]imidazole-5-carboxylate , 1,4-Dioxane,
RT, 16 h; f) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; g) 2-aminoethan-1-ol, HBTU,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 5-methoxy-2-nitrophenol
To a stirred suspension of sodium methoxide (0.86 g, 18.35 mmol) in methanol
(50 mL) at
RT was added a solution of 5-fluoro-2-nitrophenol (3.0 g, 19.1 mmol) in
methanol (40 mL)
through a syringe and the reaction mixture was heated at 60 C for 40 h. The
reaction mixture
was cooled to RT, quenched with cold water (50 mL), acidified with 1 N HC1 and
extracted
with ethyl acetate (2 X 100 mL). The combined organic layer was washed with
water (50
mL), brine (50 mL), dried over anhydrous sodium sulfate and concentrated under
vacuum.
The residue was purified by combiflash chromatography using 3% ethyl acetate
in hexane as
an eluent to afford the title compound (2.0 g, 62%); 1H NMR (400 MHz, DMSO-
d6): 8 10.94
(s, 1H), 7.97 (d, J = 9.6 Hz, 1H), 6.63 (d, J = 2.4 Hz, 1H), 6.59 (dd, J = 3.0
Hz, J = 9.2 Hz,
1H), 3.83 (s, 3H); LC-MS: m/z 167.95 (M -1)-.
Step-b: Synthesis of 2-amino-5-methoxyphenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 5-methoxy-2-nitrophenol as starting material (Yield: 96%);
1H NMR
(400 MHz, DMSO-d6): 8 8.8 (bs, 1H), 6.49 (d, J= 8.4 Hz, 1H), 6.30 (d, J= 2.1
Hz, 1H),
6.18-6.14 (m, 1H), 3.58 (s, 3H); LC-MS: m/z 140.15 (M+1) .
Step-c: Synthesis of 6-methoxybenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-methoxyphenol as starting material (Yield:
72%); 1H
NMR (400 MHz, DMSO-d6): 8 13.7 (s, 1H), 7.21 (d, J = 2.4 Hz, 1H), 7.13 (d, J =
8.4 Hz,
1H), 6.88 (dd, J = 2.0 Hz, J = 8.4 Hz, 1H), 3.77 (s, 3H); LC-MS: m/z 182.0
(M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
124
Step-d: Synthesis of 2-chloro-6-methoxybenzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-methoxybenzo[d]oxazole-2-thiol as starting material
and stirred
for 2 h (Yield: 91%); LC-MS: m/z 184.0 (M+1) .
Step-e: Synthesis of ethyl 24(6-methoxybenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-methoxybenzo [d]oxazole as starting materials (Yield: 18%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.3 (bs, 1H), 8.21 (d, J = 1.4 Hz, 1H), 7.87 (dd, J = 1.2 Hz, J =
8.4 Hz, 1H),
7.50 (d, J= 8.0 Hz, 1H), 7.36 (d, J= 8.4 Hz, 1H), 7.13 (d, J= 2.0 Hz, 1H),
6.83 (dd, J= 2.4
Hz, J = 8.4 Hz, 1H), 4.33 (q, J = 6.8 Hz, 2H), 3.82 (s, 3H), 3.62 (s, 3H),
1.35 (t, J = 7.2 Hz,
3H); LC-MS: m/z 367.0 (M-F1) .
Step-f: Synthesis of 24(6-methoxybenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-methoxybenzo [d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylate as starting material (Yield: 79%); 1H NMR (400
MHz,
DMSO-d6): 8 12.5 (bs, 2H), 8.17 (s, 1H), 7.85 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H),
7.47 (d, J=
8.4 Hz, 1H), 7.37 (d, J= 8.8 Hz, 1H), 7.12 (d, J= 2.0 Hz, 1H), 6.82 (dd, J=
2.4 Hz, J = 8.8
Hz, 1H), 3.79 (s, 3H), 3.62 (s, 3H); LC-MS: m/z 339.0 (M+1) .
Step-g: Synthesis of N-(2-hydroxyethyl)-24(6-methoxybenzo[d]oxazol-2-yl)amino)-
1-
methyl-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-methoxybenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid and 2-aminoethan-l-ol as starting
materials (Yield:
59%); 1H NMR (400 MHz, DMSO-d6): 8 12.18 (bs, 1H), 8.35 (t, J= 5.2 Hz, 1H),
8.05 (d, J=
1.2 Hz, 1H), 7.75 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.45 (d, J= 8.4 Hz, 1H),
7.35 (d, J= 8.4
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
125
Hz, 1H), 7.11 (d, J= 2.4 Hz, 1H), 6.81 (dd, J= 2.4 Hz, J= 8.8 Hz, 1H), 4.72
(bs, 1H), 3.78
(s, 3H), 3.61 (s, 3H), 3.53 (t, J = 6.0 Hz, 2H), 3.35-3.30 (m, 2H); LC-MS: m/z
382.0 (M+1) .
Examples 93 and 94. Synthesis of 1-((3-hydroxyoxetan-3-yl)methyl)-2-46-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic
acid
and Synthesis of 1-((3-hydroxyoxetan-3-yl)methyl)-N-(2-methoxyethyl)-2-46-
(trifluoromethoxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
HO
r-e0 1-1,247-NO2 b ,__\("NH2
61
Hr..30)
HOr>C? HOr>C. OH
f
CI c NH d NH e
N/>-NH2 _______________________________________________
=EtO2C
/ ¨NH
Et0
EtO2C NO2 EtO2C NO2 EtO2C NH2 N0
OCF3
Ns
40 NI/>¨NH h H 40 ,,,NH
MeON N
HO2C )r-0 N
N 0
OCF3
OCF3
Conditions: a) Nitromethane, TEA, RT, 16 h; b) 10% Pd/C, Me0H, H2, RT, 3 h; c)
3-(aminomethyl)oxetan-3-ol , DIPEA, DMF, 60 C, 16
h; d) 10% Pd/C, Me0H, H2, RT, 16 h; e) Cyanogen bromide, THF, H20, 50 C, 16 h;
f) NaH, 2-chloro-6-
(trifluoromethoMbenzo[ c]oxazole , 1,4-Dioxane, RT, 16 h; g) Li0H.H20, THF,
Et0H, H20, 60 C, 16 h; h) 2-methoxyethylamine, DPPA,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 3-(nitromethyl)oxetan-3-ol
To a solution of oxetan-3-one (10.0 g, 138.9 mmol) in nitromethane (25 mL) at
0 C was
added triethylamine (5 mL, 347.2 mmol) and the reaction mixture was stirred at
RT for 16 h.
The reaction mixture was concentrated under reduced pressure. The residue was
purified by
combi flash column chromatography using 40% ethyl acetate in hexane as an
eluent to afford
the title compound (14.0 g, 77%) as pale yellow liquid; 1H NMR (400 MHz,
CDC13): 8 4.82
(s, 2H), 4.71 (d, J= 8.0 Hz, 2H), 4.61 (d, J= 8.0 Hz, 2H), 3.53 (s, 1H).
Step-b: Synthesis of 3-(aminomethyl)oxetan-3-ol
To a solution of 3-(nitromethyl)oxetan-3-ol (5.0 g, 37.6 mmol) in methanol (80
mL) was
added a slurry of 10% Pd/C (2.0 g in 20 mL methanol) under nitrogen
atmosphere. Then the
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
126
reaction mixture was stirred under hydrogen gas balloon for 3 h. The reaction
mixture was
filtered through a bed of celite and concentrated under vacuum to afford the
title compound
(3.8 g, 98%); LC-MS: m/z 104.2 (M+1) .
Step-c: Synthesis of ethyl 4-(((3-hydroxyoxetan-3-yl)methyl)amino)-3-
nitrobenzoate
The title compound was synthesized using the same procedure which was followed
for
compound 3a using ethyl 4-chloro-3-nitrobenzoate and 3-(aminomethyl)oxetan-3-
ol as
starting materials heating to 60 C (Yield: 54%); 1H NMR (400 MHz, DMSO-d6): 8
8.62 (d, J
= 1.6 Hz, 1H), 8.53 (bs, 1H), 7.99 (dd, J = 2.0 Hz, J = 9.2 Hz, 1H), 7.29 (d,
J = 9.2 Hz, 1H),
6.35 (s, 1H), 4.51 (d, J= 6.8 Hz, 2H), 4.44 (d, J= 6.8 Hz, 2H), 4.29 (q, J=
7.2 Hz, 2H), 3.75
(d, J= 5.2 Hz, 2H), 1.31 (t, J= 6.8 Hz, 3H); LC-MS: m/z 297.1(M+1) .
Step-d: Synthesis of ethyl 3-amino-4-(((3-hydroxyoxetan-3-
yl)methyl)amino)benzoate
The title compound was synthesized using the same procedure which was followed
for
compound le using ethyl 4-(((3-hydroxyoxetan-3-yl)methyl)amino)-3-
nitrobenzoate as
starting material (Yield: 95%); 1H NMR (400 MHz, DMSO-d6): 8 7.23-7.20 (m,
2H), 6.57 (d,
J = 8.4 Hz, 1H), 5.96 (bs, 1H), 5.04 (bs, 1H), 4.75 (s, 2H), 4.46-4.43 (m,
4H), 4.19 (q, J = 7.2
Hz, 2H), 3.40 (d, J= 5.6 Hz, 2H), 1.26 (t, J= 6.8 Hz, 3H); LC-MS: m/z 267.1
(M+1) .
Step-e: Synthesis of ethyl 2-amino-14(3-hydroxyoxetan-3-yl)methyl)-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound if using ethyl 3-amino-4-(((3-hydroxyoxetan-3-
yl)methyl)amino)benzoate as
starting material (Yield: 70%); 1H NMR (400 MHz, DMSO-d6): 8 7.72 (s, 1H),
7.59 (dd, J =
1.6 Hz, J= 8.0 Hz, 1H), 7.35 (d, J= 8.0 Hz, 1H), 6.56 (s, 2H), 6.45 (s, 1H),
4.55 (d, J= 6.4
Hz, 2H), 4.43 (d, J = 6.0 Hz, 2H), 4.34 (s, 2H), 4.28 (q, J = 6.8 Hz, 2H),
1.32 (t, J = 5.2 Hz,
3H); LC-MS: m/z 292.1 (M+1) .
Step-f: Synthesis of ethyl 1-((3-hydroxyoxetan-3-yl)methyl)-2-46-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-14(3-hydroxyoxetan-3-yl)methyl)-1H-
benzo [d] imidazole-5-carboxylate and 2-chloro-6-(trifluoromethoxy)benzo [d]
oxazole as
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
127
starting materials (Yield: 18%); 1H NMR (400 MHz, DMSO-d6): 8 12.4 (bs, 1H),
8.24 (d, J=
1.6 Hz, 1H), 7.85 (dd, J= 1.6 Hz, J = 8.4 Hz, 1H), 7.60-7.58 (m, 2H), 7.53 (d,
J = 8.4 Hz,
1H), 7.23 (dd, J= 1.2 Hz, J= 8.8 Hz, 1H), 6.18 (s, 1H), 4.79 (d, J= 6.8 Hz,
2H), 4.50 (bs,
2H), 4.46 (d, J = 6.8 Hz, 2H), 4.33 (q, J = 6.8 Hz, 2H), 1.35 (t, J = 7.6 Hz,
3H); LC-MS: m/z
493.0 (M+1) .
Step-g: Synthesis of 14(3-hydroxyoxetan-3-yOmethyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-((3-hydroxyoxetan-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylate as
starting material (Yield: 49%); 1H NMR (400 MHz, DMSO-d6): 8 12.8 (bs, 1H),
12.3 (bs,
1H), 8.19 (d, J= 1.2 Hz, 1H), 7.84 (dd, J= 1.2 Hz, J= 8.0 Hz, 1H),7.60-7.52
(m, 3H), 7.22
(dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 6.18 (s, 1H), 4.79 (d, J= 6.4 Hz, 2H), 4.51
(s, 2H), 4.46 (d,
J= 6.4 Hz, 2H); LC-MS: m/z 465.1 (M+1) .
Step-h: Synthesis of 14(3-hydroxyoxetan-3-yOmethyl)-N-(2-methoxyethyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-((3-hydroxyoxetan-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid and
2-methoxyethylamine as starting materials. Crude product was purified by
combiflash
chromatography using 2% methanol in DCM as an eluent to afford the title
compound (Yield:
28%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.43 (t, J = 5.2 Hz, 1H),
8.07 (s, 1H),
7.73 (d, J= 8.8 Hz, 1H), 7.58 (s, 1H), 7.55-7.51 (m, 2H), 7.21 (d, J= 8.4 Hz,
1H), 6.17 (s,
1H), 4.79 (d, J = 6.8 Hz, 2H), 4.50 (s, 2H), 4.46 (d, J = 6.4 Hz, 2H), 3.49-
3.43 (m, 4H), 3.28
(s, 3H); LC-MS: m/z 522.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
128
Example 95. Synthesis of N-(2-hydroxyethyl)-14(3-hydroxyoxetan-3-yl)methyl)-2-
((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
Hiso,
HX0
NI/>¨N H a H is / ¨NH
HO2C N HO N )r-0
N
N 0
O
OCF3 CF3
Conditions: a) 2-aminoethan-1-ol , HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-((3-hydroxyoxetan-3-yl)methyl)-2-((6-
(trifluoromethoxy)benzo [d] oxazol-2-yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid and
2-aminoethan-1-ol as starting materials. Crude product was purified by
combiflash
chromatography using 3% methanol in DCM as an eluent to afford the title
compound (Yield:
15%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.35 (t, J = 5.6 Hz, 1H),
8.07(d, J =
1.2 Hz, 1H), 7.73 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.58 (s, 1H), 7.58-7.51 (m,
2H), 7.22 (dd,
J = 1.2 Hz, J= 10.0 Hz, 1H), 6.17 (s, 1H), 4.80 (d, J = 6.8 Hz, 2H), 4.71 (t,
J = 5.2 Hz, 1H),
4.51 (s, 2H), 4.46 (d, J= 6.4 Hz, 2H), 3.55-3.51 (m, 2H), 3.37-27 (m, 2H); LC-
MS: m/z
508.45 (M+1) .
Examples 96 and 97. Synthesis of 24(6-chlorobenzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid and Synthesis of 24(6-chlorobenzo[d]oxazol-
2-
yl)amino)-N-(2-hydroxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
fyle
CI OH NH2 />¨SH N CI =0 CI io 0/
CI c Et0 1101
a >7-0
N
0
fyle fyle
/>--NH H 11101
HO 1110 N
0 HON
N =
0
CI 0 N
=
ci
Conditions: a) Potassium ethyl xanthate, Et0H, Reflux, 16 h; b) SOCl2,
Cat.DMF, Reflux, 3 h; c)
NaH, ethyl 2-amino-1-methyl-1 H-benzo[ c]imidazole-5-carboxylate , 1,4-
Dioxane, RT, 16 h; d)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h; e) 2-aminoethan-1-ol , HBTU, DIPEA, DMF,
0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
129
Step-a: Synthesis of 6-chlorobenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-chlorophenol as starting material (Yield:
69%); 1H
NMR (400 MHz, DMSO-d6): 8 14.0 (bs, 1H), 7.73 (d, J = 2.0 Hz, 1H), 7.35 (dd, J
= 1.6 Hz, J
= 8.0 Hz, 1H), 7.23 (d, J= 8.8 Hz, 1H); LC-MS: m/z 184.0 (M-1)-.
Step-b: Synthesis of 2,6-dichlorobenzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-chlorobenzo[d]oxazole-2-thiol as starting material
(Yield: 69%);
1H NMR (400 MHz, DMSO-d6): 8 8.01 (d, J= 1.6 Hz, 1H), 7.77 (d, J= 8.4 Hz, 1H),
7.51
(dd, J = 2.0 Hz, J = 8.4 Hz, 1H).
Step-c: Synthesis of ethyl 2-((6-chlorobenzo[d] oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
2,6-dichlorobenzo[d]oxazole as starting materials (Yield: 24%); 1H NMR (400
MHz, DMSO-
d6): 8 12.3 (bs, 1H), 8.23 (d, J = 1.6 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.60
(d, J = 2.0 Hz,
1H), 7.53 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.25 (dd, J = 2.0 Hz,
J = 8.0 Hz, 1H),
4.33 (q, J= 7.2 Hz, 2H), 3.64 (s, 3H), 1.35 (t, J= 7.2 Hz, 3H); LC-MS: m/z
369.0 (M-1)-.
Step-d: Synthesis of 2-((6-chlorobenzo[d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-chlorobenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo [d]imidazole-5-carboxylate as starting material (Yield: 81%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.3 (bs, 1H), 8.19 (d, J= 1.2 Hz, 1H), 7.87 (dd,
J= 1.6 Hz, J=
8.4 Hz, 1H), 7.59 (d, J= 2.0 Hz, 1H), 7.51 (d, J= 8.4 Hz, 1H), 7.45 (d, J= 8.4
Hz, 1H), 7.24
(dd, J = 2.0 Hz, J = 8.4 Hz, 1H), 3.64 (s, 3H); LC-MS: m/z 342.9 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
130
Step-e: Synthesis of 2-46-chlorobenzo[d]oxazol-2-yl)amino)-N-(2-hydroxyethyl)-
1-
methyl-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-chlorobenzo [d]oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials (Yield:
53%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.37 (t, J = 5.6 Hz, 1H),
8.08 (s, 1H),
7.78 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 2.0 Hz, 1H), 7.49 (d, J = 8.8 Hz, 1H),
7.44 (d, J = 8.4
Hz, 1H), 7.24 (dd, J = 2.0 Hz, J = 8.4 Hz, 1H), 4.72 (t, J = 5.2 Hz, 1H), 3.64
(s, 3H), 3.55-
3.51 (m, 2H), 3.37-3.33 (m, 2H); LC-MS: m/z 386.1 (M+1) .
Examples 98 and 99. Synthesis of 1-methyl-24(6-(2,2,2-
trifluoroethoxy)benzo[d]oxazol-
2-yl)amino)-1H-benzo[d]imidazole-5-carboxylic acid and Synthesis of N-(2-
methoxyethyl)-1-methyl-24(6-(2,2,2-trifluoroethoxy)benzo[d] oxazol-2-yl)amino)-
1H-
benzo[d] imidazole-5-carboxamide
=F3c.,0 0
F NO2 OH F so b OBn F3 OBn F3C 30 ith OH F C 0
a d R
NO2
NO2 41" NH2
=
0cF3 0cF3 0CF3
Me N Me N Me N
HO
NI
Nt-0 h
NJ
Et0 11111r N M ,N
e0 ¨ N
=
0 0 0
Conditions: a) Benzyl bromide, K2CO3, DMF, 0 C - RT, 16 h; b)
trifluoroethanol, NaH, THF, 0 C - RT, 3 h; c) 10% Pd/C,
Me0H, H2, RT, 16 h; d) Potassium ethyl xanthate, Et0H, Reflux, 16 h; e) S0Cl2,
Cat.DMF, Reflux, 2 h; f) NaH, ethyl 2-
amino-1-methyl-1H-benzo[c]imidazole-5-carboxylate, 1,4-Dioxane, RT, 16 h; g)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h; h)
2-methoxyethylamine, DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-(benzyloxy)-4-fluoro-1-nitrobenzene
To a stirred solution of 5-fluoro-2-nitrophenol (8.0 g, 50.9 mmol) in DMFA (80
mL) at 0 C
was added potassium carbonate (14.0 g, 101.8 mmol) and benzyl bromide (5.4 mL,
45.8
mmol) and the reaction mixture was stirred at RT for 16 h. The reaction
mixture was diluted
with water (200 mL) and extracted with ethyl acetate (2 X 200 mL). The
combined organic
layer was washed with water (2 X 200 mL), brine (50 mL), dried over anhydrous
sodium
sulfate and concentrated under vacuum. The residue was purified by combiflash
chromatography using 10% ethyl acetate in hexane as an eluent to afford the
title compound
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
131
(8.0 g, 64%); 1H NMR (400 MHz, DMSO-d6): 8 8.06-8.02 (m, 1H), 7.47-7.36 (m,
6H), 7.05-
6.95 (m, 1H), 5.33 (s, 2H).
Step-b: Synthesis of 2-(benzyloxy)-1-nitro-4-(2,2,2-trifluoroethoxy)benzene
To a solution of trifluoroethanol (0.23 mL, 3.24 mmol) in THF (10 mL) at 0 C
was added
sodium hydride (60% dispersion in mineral oil) (194 mg, 4.86 mmol) by portions
and stirred
for 1 h. Then a solution of 2-(benzyloxy)-4-fluoro-1-nitrobenzene (400 mg,
1.62 mmol) was
added to the reaction mixture and stirred at RT for 2 h. The reaction mixture
was
concentrated and diluted with cold water (20 mL). Solid precipitated was
filtered and dried
under vacuum to afford the title compound (350 mg, 66%); 1H NMR (400 MHz, DMSO-
d6):
8 8.01 (d, J = 9.2 Hz, 1H), 7.48-7.33 (m, 5H), 7. 11(d, J = 2.8 Hz, 1H), 6.82
(dd, J = 2.4 Hz, J
= 8.8 Hz, 1H), 5.32 (s, 2H), 4.94 (q, J = 8.8 Hz, 2H).
Step-c: Synthesis of 2-amino-5-(2,2,2-trifluoroethoxy)phenol
To a solution of 2-(benzyloxy)-1-nitro-4-(2,2,2-trifluoroethoxy)benzene (350
mg, 1.07 mmol)
in methanol (10 mL) was added 10% Pd/C (50 mg) under nitrogen atmosphere. The
reaction
mixture was stirred under hydrogen gas balloon for 16 h. The reaction mixture
was filtered
through a bed of celite and concentrated under vacuum to afford the title
compound (210 mg,
95%); 1H NMR (400 MHz, DMSO-d6): 8 6.51 (d, J = 8.8 Hz, 1H), 6.38 (d, J = 2.4
Hz, 1H),
6.28 (dd, J= 2.8 Hz, J = 8.8 Hz, 1H), 4.48 (q, J = 8.8 Hz, 2H); LC-MS: m/z
208.0 (M+1) .
Step-d: Synthesis of 6-(2,2,2-trifluoroethoxy)benzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-(2,2,2-trifluoroethoxy)phenol as starting
material
(Yield: 79%); 1H NMR (400 MHz, DMSO-d6): 8 13.8 (bs, 1H), 7.38 (d, J= 2.4 Hz,
1H), 7.18
(d, J= 8.8 Hz, 1H), 7.01 (dd, J= 2.4 Hz, J= 8.8 Hz, 1H), 4.79 (q, J= 8.8 Hz,
2H); LC-MS:
m/z 250.0 (M+1) .
Step-e: Synthesis of 2-chloro-6-(2,2,2-trifluoroethoxy)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-(2,2,2-trifluoroethoxy)benzo[d]oxazole-2-thiol as
starting
material and stirring for 2 h. (Yield: 87%); 1H NMR (400 MHz, DMSO-d6): 8 7.69
(d, J = 8.8
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
132
Hz, 1H), 7.60 (d, J= 2.4 Hz, 1H), 6.15 (dd, J= 2.4 Hz, J= 8.8 Hz, 1H), 4.85
(q, J= 8.8 Hz,
2H).
Step-f: Synthesis of ethyl 1-methyl-2-46-(2,2,2-trifluoroethoxy)benzo[d]oxazol-
2-
yl)amino)-1H-benzo[d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-(2,2,2-trifluoroethoxy)benzo [d] oxazole starting materials (Yield:
21%); 1H NMR
(400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.22 (d, J= 1.6 Hz, 1H), 7.88 (dd, J= 1.2
Hz, J= 8.0
Hz, 1H), 7.51 (d, J= 8.4 Hz, 1H), 7.39 (d, J= 8.4 Hz, 1H), 7.29 (d, J= 2.4 Hz,
1H), 6.94 (dd,
J = 2.4 Hz, J = 8.4 Hz, 1H), 4.77 (q, J = 8.8 Hz, 2H), 4.33 (q, J = 7.2 Hz,
2H), 3.63 (s, 3H),
1.35 (t, J= 7.2 Hz, 3H); LC-MS: m/z 435.35 (M+1) .
Step-g: Synthesis of 1-methyl-24(6-(2,2,2-trifluoroethoxy)benzo[d] oxazol-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methy1-24(6-(2,2,2-trifluoroethoxy)benzo[d]oxazol-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxylate as starting material (Yield: 62%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.5 (bs, 2H), 8.18 (d, J= 1.2 Hz, 1H), 7.86 (dd, J= 1.6 Hz, J=
8.4 Hz, 1H),
7.48 (d, J= 8.8 Hz, 1H), 7.40 (d, J= 8.8 Hz, 1H), 7.29 (d, J= 2.4 Hz, 1H),
6.93 (dd, J= 2.4
Hz, J = 8.8 Hz, 1H), 4.77 (q, J = 9.2 Hz, 2H), 3.62 (s, 3H); LC-MS: m/z 407.0
(M+1) .
Step-h: Synthesis of N-(2-methoxyethyl)-1-methyl-24(6-(2,2,2-
trifluoroethoxy)benzo[d] oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methy1-2-((6-(2,2,2-trifluoroethoxy)benzo[d]oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials (Yield:
70%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.4 (t, J = 5.6 Hz, 1H),
8.06 (s, 1H),
7.75 (d, J= 8.0 Hz, 1H), 7.46 (d, J= 8.0 Hz, 1H), 7.39 (d, J= 8.0 Hz, 1H),
7.28 (d, J= 1.6
Hz, 1H), 6.93 (dd, J= 2.4 Hz, J= 8.4 Hz, 1H), 4.77 (q, J= 8.8 Hz, 2H), 3.62
(s, 3H), 3.48-
3.43 (m, 4H), 3.28 (s, 3H); LC-MS: m/z 464.0 (M-F1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
133
Example 100. Synthesis of N-(2-hydroxyethy1)-1-methyl-2-46-(2,2,2-
trifluoroethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
o oF, o CF3
Me N, Me N
N
N =H ¨NH
HO ¨NH
HO
0 0
Conditions: a) 2-aminoethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-(2,2,2-trifluoroethoxy)benzo [d] oxazol-
2-yl)amino)-
1H-benzo[d]imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials (Yield:
63%); 1H NMR (400 MHz, DMSO-d6): 8 12.5 (bs, 1H), 8.42 (t, J = 5.2 Hz, 1H),
8.07 (s,
1H), 7.81 (d, J= 8.4 Hz, 1H), 7.53 (d, J= 8.4 Hz, 1H), 7.40 (d, J= 8.8 Hz,
1H), 7.32 (d, J=
2.4 Hz, 1H), 6.98 (dd, J= 2.4 Hz, J= 8.8 Hz, 1H), 4.80 (q, J= 8.8 Hz, 2H),
3.66 (s, 3H), 3.54
(t, J= 6.4 Hz, 2H), 3.36 (q, J= 6.0 Hz, 2H); LC-MS: m/z 450.0 (M+1) .
Examples 101 and 102. Synthesis of 2-46-(2-hydroxyethoxy)benzo[d]oxazol-2-
yl)amino)-1-methy1-1H-benzo [d] imidazole-5-carboxylic acid and Synthesis of 2-
4642-
hydroxyethoxy)benzo [d] oxazol-2-yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-
benzo [d] imidazole-5-carboxamide
F 40 OBn HO 11101 O Bn =OBn _ 40 OH d
a
TBSO TBSO TBSO =5_
SH
NO2 NO2 NO2 NH2
Vie
Vie
/>¨NH ¨S0 Me N/
so 0 TBSO''' 40 to N >ro
h HO N
¨SMe ¨)"" ) g E N",
2 0 N db. 0
Me
H is N,>_NH
meo^ NO
N db.
0
RIP
Conditions: a) KOH, water, 100 C, 30 h; b) (2-bromoethoxy)(tert-
butyhdimethylsilane, K2CO3, DMF, 130 C, 4 h; c) 10%
Pd/C, Me0H, H2, RT, 16 h; d) Potassium ethyl xanthate, Et0H, Reflux, 16 h; e)
K2CO3, Mel, ACN, RT, 16 h; f) m-CPBA,
DCM, 0 C - RT, 4 h; g) NaH, ethyl 2-amino-1-methyl-1H-benzo[d]imidazole-5-
carboxylate , 1,4-Dioxane, RT, 16 h; h)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h; h) 2-methoxyethylamine, DPPA, DIPEA,
DMF, 0 C - RT, 16 h
Step-a: Synthesis of 3-(benzyloxy)-4-nitrophenol
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
134
To a stirred suspension of 2-(benzyloxy)-4-fluoro-1-nitrobenzene (4.0 g, 16.2
mmol) in water
(70 mL) at RT was added potassium hydroxide (4.53 g, 80.9 mmol) and the
reaction mixture
was stirred at 100 C for 30 h. The reaction mixture was cooled to RT, diluted
with water (50
mL) and acidified with 1 N HC1 and extracted with ethyl acetate (3 X 100 mL).
The
combined organic layer was washed with water (50 mL), brine (50 mL), dried
over
anhydrous sodium sulfate and concentrated under vacuum to obtain the residue
which was
purified by combiflash chromatography using 20% ethyl acetate in hexane as an
eluent to
afford the title compound (1.5 g, 38%); 1H NMR (400 MHz, DMSO-d6): 8 10.88 (s,
1H),
7.90 (d, J = 8.8 Hz, 1H), 7.49-7.47 (m, 2H), 7.43-7.40 (m, 2H), 7.35 (d, J =
6.8 Hz, 1H), 6.69
(d, J = 2.4 Hz, 1H), 6.49 (dd, J = 2.4 Hz, J = 9.2 Hz, 1H), 5.25 (s, 2H).
Step-b: Synthesis of (2-(3-(benzyloxy)-4-nitrophenoxy)ethoxy)(tert-
butyl)dimethylsilane
To a stirred solution of 3-(benzyloxy)-4-nitrophenol (1.5 g, 6.1 mmol) in DMFA
(15 mL) at
RT was added potassium carbonate (2.1 g, 15.3 mmol) and (2-bromoethoxy)(tert-
butyl)dimethylsilane (1.75 g, 7.3 mmol) in a seal tube and heated at 130 C for
4 h. The
reaction mixture was cooled to RT, diluted with water (50 mL) and extracted
with ethyl
acetate (2 X 50 mL). The combined organic layer was washed with water (2 X 500
mL),
brine (30 mL), dried over anhydrous sodium sulfate and concentrated under
vacuum to obtain
the residue which was purified by combiflash chromatography using 5% ethyl
acetate in
hexane as an eluent to afford the title compound (8.0 g, 64%); 1H NMR (400
MHz, DMSO-
d6): 8 7.96 (d, J = 8.8 Hz, 1H), 7.49-7.47 (m, 2H), 7.43-7.39 (m, 2H), 7.36-
7.34 (m, 1H), 6.89
(d, J = 2.4 Hz, 1H), 6.67 (dd, J = 2.4 Hz, J = 9.2 Hz, 1H), 5.32 (s, 2H), 4.17
(t, J = 4.4 Hz,
2H), 3.92 (t, J = 4.8 Hz, 2H), 0.86 (s, 9H), 0.02 (s, 6H).
Step-c: Synthesis of 2-amino-5-(2-((tert-butyldimethylsilyl)oxy)ethoxy)phenol
The title compound was synthesized using the same procedure which was followed
for
compound le using (2-(3-(benzyloxy)-4-nitrophenoxy)ethoxy)(tert-
butyl)dimethylsilane as
starting material (Yield: 87%); 1H NMR (400 MHz, DMSO-d6): 8 6.47 (d, J = 8.0
Hz, 1H),
6.30 (d, J= 2.8 Hz, 1H), 6.15 (dd, J= 2.4 Hz, J= 8.4 Hz, 1H), 3.84-3.80 (m,
4H), 0.87 (s,
9H), 0.02 (s, 6H); LC-MS: m/z 284.1 (M+1) .
Step-d: Synthesis of 6-(2-((tert-butyldimethylsilypoxy)ethoxy)benzo [d]
oxazole-2-thiol
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
135
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)phenol as
starting material (Yield: 77%). The crude compound was used in the next step
without any
analytical data.
Step-e: Synthesis of 6-(2-((tert-butyldimethylsilypoxy)ethoxy)-2-
(methylthio)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lb using 6-(2-((tert-butyldimethylsilyl)oxy)ethoxy)benzo[d]oxazole-2-
thiol as
starting material and stirring for 16 h. (Yield: 80%); 1H NMR (400 MHz, DMSO-
d6): 8 7.50
(d, J = 8.8 Hz, 1H), 7.28 (d, J = 2.4 Hz, 1H), 6.91 (dd, J = 2.8 Hz, J = 8.8
Hz, 1H), 4.06 (t, J
= 4.4 Hz, 2H), 3.92 (t, J = 5.2 Hz, 2H), 2.27 (s, 3H), 0.86 (s, 9H), 0.2 (s,
6H); LC-MS: m/z
340.0 (M+1) .
Step-f: Synthesis of 6-(2-((tert-butyldimethylsilypoxy)ethoxy)-2-
(methylsulfony1)-
benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lc using 6-(2-((tert-butyldimethylsilyl)oxy)ethoxy)-2-
(methylthio)benzo[d]oxazole as starting material and stirring for 4 h (Yield:
100%); 1H NMR
(400 MHz, DMSO-d6): 8 7.90-7.89 (m, 1H), 7.55 (d, J = 8.0 Hz, 1H), 6.99-6.97
(m, 1H), 4.10
(t, J = 4.8 Hz, 2H), 3.95 (t, J = 5.2 Hz, 2H), 3.62 (s, 3H), 0.85 (s, 9H), 0.2
(s, 6H).
Step-g: Synthesis of ethyl 24(6-(2-((tert-
butyldimethylsilypoxy)ethoxy)benzo[d]oxazol-2-
yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-l-methy1-1H-benzo [d] imidazole-5-carboxylate
and 6-(2-
((tert-butyldimethylsilyl)oxy)ethoxy)-2-(methylsulfonyl)benzo [d] oxazole as
starting
materials (Yield: 17%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.21 (d,
J= 1.6 Hz,
1H), 7.86 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.49 (d, J= 8.0 Hz, 1H), 7.35 (d, J=
8.4 Hz, 1H),
7.12 (d, J= 2.4 Hz, 1H), 6.81 (dd, J= 1.6 Hz, J= 8.0 Hz, 1H), 4.32 (q, J= 7.6
Hz, 2H), 4.06-
4.04 (m, 2H), 3.94-3.92 (m, 2H), 3.62 (s, 3H), 1.35 (t, J = 6.8 Hz, 3H), 0.88
(s, 9H), 0.2 (s,
6H); LC-MS: m/z 511.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
136
Step-h: Synthesis of 24(6-(2-hydroxyethoxy)benzo[d] oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid
To a stirred solution of ethyl 24(6-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)benzo [d] oxazol-2-
yl)amino)-1-methy1-1H-benzo [d] imidazole-5-carboxylate (80 mg, 0.15 mmol) in
a mixture of
solvent of THF (2 mL), ethanol (2 mL) and water (1 mL) was added lithium
hydroxide
monohydrate (32 mg, 0.78 mmol). The reaction mixture was heated at 60 C for 16
h with
stirring. The reaction mixture was cooled to RT and concentrated under reduced
pressure.
The residue was dissolved in water (20 mL) and acidified with 1 N HC1. The
solid obtained
was filtered and dried under vacuum to afford the title compound (50 mg, 87%);
1H NMR
(400 MHz, DMSO-d6): 8 12.8 (bs, 1H), 12.3 (bs, 1H), 8.16 (d, J= 1.6 Hz, 1H),
7.85 (dd, J=
1.6 Hz, J = 8.4 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H),
7.12 (d, J = 2.4
Hz, 1H), 6.82 (dd, J = 2.4 Hz, J = 8.4 Hz, 1H), 4.85 (bs, 1H), 4.01 (t, J =
4.8 Hz, 2H), 3.75-
3.73 (m, 2H), 3.66 (s, 3H); LC-MS: m/z 369.0 (M+1) .
Step-i: Synthesis of 24(6-(2-hydroxyethoxy)benzo[d] oxazol-2-
yl)amino)-N-(2-
methoxyethyl)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-((6-(2-hydroxyethoxy)benzo [d] oxazol-2- yl) amino)- 1-
methyl- 1H-
benzo [d] imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials. The
crude product was purified by combiflash chromatography using 4% methanol in
DCM as an
eluent (Yield: 17%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.43 (t, J =
5.2 Hz,
1H), 8.05 (d, J= 1.2 Hz, 1H), 7.75 (dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.45 (d, J=
8.8 Hz, 1H),
7.35 (d, J= 8.4 Hz, 1H), 7.12 (d, J= 2.4 Hz, 1H), 6.82 (dd, J= 2.8 Hz, J= 8.8
Hz, 1H), 4.11
(t, J= 4.4 Hz, 2H), 3.66 (t, J= 4.4 Hz, 2H), 3.61 (s, 3H), 3.49-3.41 (m, 4H),
3.28 (bs, 1H),
3.24 (s, 3H); LC-MS: m/z 426.4 (M+1) .
Example 103. Synthesis of 2-06-(2-hydroxyethoxy)benzo[d] oxazol-2-yl)amino)-N-
(2-
hydroxyethyl)-1-methyl-1H-benzo[d] imidazole-5-carboxamide
Me
N H
N
NNA I V =
a HO
HOZ---/N
0 N
OH
$0 0 N
0
Conditions a) 2-aminoethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
137
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-(2-hydroxyethoxy)benzo [d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d]imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials. The crude
product was purified by combiflash chromatography using 6% methanol in DCM as
an eluent
to afford the title compound (Yield: 18%); 1H NMR (400 MHz, DMSO-d6): 8 12.05
(bs, 1H),
8.35 (t, J = 5.2 Hz, 1H), 8.05 (s, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.45 (d, J =
8.4 Hz, 1H), 7.35
(d, J = 8.4 Hz, 1H), 7.11 (d, J = 2.4 Hz, 1H), 6.82 (dd, J = 2.8 Hz, J = 8.8
Hz, 1H), 4.85 (bs,
1H), 4.72 (t, J = 4.8 Hz, 1H), 4.01 (t, J = 4.8 Hz, 2H), 3.72 (q, J = 4.4 Hz,
2H), 3.61 (s, 3H),
3.53 (q, J= 5.6 Hz, 2H), 3.37-3.33 (m, 2H); LC-MS: m/z 412.0 (M+1) .
Example 104. Synthesis of 2-06-(2-hydroxyethoxy)benzo [d] oxazol-2-yl)amino)-N-
(2-(2-
hydroxyethoxy)ethyl)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
Me ye
ip I\I_A
, = N H
a
---N
HO N
0 N ar
kir 0
/..,....20H
HO-...../..-0/----/ 0 N ar
Conditions: a) 2-(2-aminoethoxy)ethan-1-ol , HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-(2-hydroxyethoxy)benzo [d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d]imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-ol as
starting materials.
The crude product was purified by combiflash chromatography using 10% methanol
in DCM
as an eluent to afford the title compound (Yield: 18%); 1H NMR (400 MHz, DMSO-
d6): 8
12.05 (bs, 1H), 8.41 (bs, 1H), 8.04 (s, 1H), 7.74 (d, J= 8.4 Hz, 1H), 7.45 (d,
J= 8.4 Hz, 1H),
7.35 (d, J= 8.8 Hz, 1H), 7.11(s, 1H), 6.82 (d, J= 6.8 Hz, 1H), 4.61 (bs, 2H),
4.01 (t, J= 4.4
Hz, 2H), 3.72 (t, J= 4.8 Hz, 2H), 3.66 (s, 3H), 3.57-3.43 (m, 8H); LC-MS: m/z
456.3
(M+1) .
Example 105. Synthesis of N-(2-aminoethyl)-2-06-(2-
hydroxyethoxy)benzo[d]oxazol-2-
y1)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxamide hydrochloride
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
138
Me Me
N H
1110 irNvo
0 H Nr'V
HO
\MeL
0 N
MP 0
HO
H 0 N
OH
Me
N H
H nr-0
N
CIH H2N OH
0
Conditions: a) tert-butyl (2-aminoethyl)carbamate, HBTU, DIPEA, DMF, 0 C - RT,
16 h; b) HCI, 1,4-
Dioxane, 0 C - RT, 3 h
Step-a: Synthesis of tert-butyl (2-(24(6-(2-hydroxyethoxy)benzo [d] oxazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxamido)ethyl)carbamate
To a stirred solution of 24(6-(2-hydroxyethoxy)benzo [d] oxazol-2-yDamino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid (100 mg, 0.27 mmol) in DMFA (2 mL) at 0 C
was
added N-ethyldiisopropyl amine (0.09 mL, 0.54 mmol) and HBTU (102 mg, 0.27
mmol). The
reaction mixture was stirred for 30 min, followed by the addition of tert-
butyl (2-
aminoethyl)carbamate (48 mg, 0.30 mmol) and stirring was continued at RT for
16 h. Once
the reaction was complete, the reaction mixture was diluted with water (15 mL)
and stirred
for 15 min. The obtained solid was filtered, dried under vacuum to get the
crude product
which was purified by combiflash chromatography using 3% methanol in DCM as an
eluent
to afford the title compound (70 mg, 50%); 1H NMR (400 MHz, DMSO-d6): 8 12.20
(bs,
1H), 8.39 (bs, 1H), 8.05 (s, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.45 (d, J = 8.4
Hz, 1H), 7.35 (d, J
= 8.0 Hz, 1H), 7.11 (d, J= 2.0 Hz, 1H), 6.91 (bs, 1H), 6.82 (dd, J= 2.0 Hz, J=
8.4 Hz, 1H),
4.85 (bs, 1H), 4.01 (t, J = 4.8 Hz, 2H), 3.72 (bs, 2H), 3.61 (s, 3H), 3.30 (2H
merged with
DMSO moisture peak), 3.12 (d, J= 6.0 Hz, 2H), 1.38 (s, 9H); LC-MS: rniz 511.3
(M+1) .
Step-b: Synthesis of N-(2-aminoethyl)-24(6-(2-hydroxyethoxy)benzo [d] oxazol-2-
yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxamide hydrochloride
To a stirred solution of tert-butyl (2-(2-((6-(2-hydroxyethoxy)benzo [d]
oxazol-2-yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxamido)ethyl)carbamate (70 mg, 0.14 mmol)
in 1,4-
dioxane (3 mL) at 0 C was added 4 M HC1 in 1,4-dioxane (1 mL) and stirred at
RT 3 h. The
reaction mixture was concentrated under reduced pressure and the residue was
triturated with
diethyl ether and solvent was decanted. The obtained solid was dried under
vacuum to afford
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
139
the title compound (35 mg, 57%); 1H NMR (400 MHz, DMSO-d6): 8 12.5 (bs, 1H),
8.78 (s,
1H), 8.12-8.07 (m, 4H), 7.90 (d, J = 8.0 Hz, 1H), 7.56 (d, J = 8.4 Hz, 1H),
7.36 (d, J = 8.4
Hz, 1H), 7.18 (s, 1H), 6.88 (d, J = 8.0 Hz, 1H), 4.02 (bs, 2H), 3.73 (bs, 2H),
3.66 (s, 3H),
3.56 (q, J= 5.2 Hz, 2H), 3.02 (q, J= 5.6 Hz, 2H); LC-MS: m/z 411.2 (M+1) .
Example 106. Synthesis of N-(2-((4,5-dihydro-1H-imidazol-2-yl)amino)ethyl)-2-
((6-(2-
hydroxyethoxy)benzo [d] oxazol-2-yl)amino)-1-methyl-1H-benzo [d] imidazole-5-
carboxamide
Me fyle
tau 1\1___NH
01,1\1 40 N
---NH
N >F0
HO le
0 N Air
111P 0"----/OH H H 0 N
VI o0H
Conditions: a) N1-(4,5-dihydro-1 H-imidazol-2-yhethane-1,2-diarnine , HBTU,
DIPEA, DMF, 0 C - RT,
16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-(2-hydroxyethoxy)benzo [d] oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d] imidazole-5-carboxylic acid and N1-(4,5-dihydro-1H-imidazol-2-
yl)ethane-1,2-
diamine as starting materials (Yield: 58%); 1H NMR (400 MHz, DMSO-d6): 8 8.73
(bs, 1H),
8.49 (s, 1H), 8.05 (s, 1H), 7.75 (d, J= 8.4 Hz, 1H), 7.41 (d, J= 8.0 Hz, 1H),
7.31 (d, J= 8.4
Hz, 1H), 7.08 (d, J= 2.0 Hz, 1H), 6.79 (dd, J= 1.6 Hz, J= 8.0 Hz, 1H), 4.86
(bs, 1H), 4.00
(t, J= 4.8 Hz, 2H), 3.72 (bs, 2H), 3.60-3.55 (m, 7H), 3.43 (bs, 2H), 3.30 (2H
merged with
DMSO moisture peak); LC-MS: m/z 479.2 (M+1) .
Examples 107 and 108. Synthesis of 24(5-fluoro-6-
(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxylic acid and Synthesis of
24(5-
fluoro-6-(trifluoromethyl)benzo [d] oxazol-2-yl)amino)-N-(2-hydroxyethyl)-1-
methyl-1H-
benzo [d] imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
140
F3. 40 OH F3C 40 OH
ab
F3C OH
F3C 40 0 F30 0)_
NO2 NH2
Me
Me Me N
Et0 IIP
Atm N H N H
PI IV )r
N
N
HO
HON
CF3
0 N
CF3 0 N
CF3 0
Conditions: a) HNO3 (65-70%), AcOH, 0 C - RT, 2 h; b) 10% Pd/C, Me0H, H2, RT,
16 h; c) Potassium ethyl xanthate,
Et0H, Reflux, 16 h; d) S0Cl2, Cat.DMF, Reflux, 2 h; e) NaH, ethyl 2-amino-1-
methyl-1H-benzo[cl]imidazole-5-carboxylate ,
1,4-Dioxane, RT, 16 h; f) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; g) 2-
aminoethan-1-ol , HBTU, DIPEA, DMF, 0 C - RT, 16
Step-a: Synthesis of 4-fluoro-2-nitro-5-(trifluoromethyl)phenol
To a solution of 4-fluoro-3-(trifluoromethyl)phenol (1 g, 5.6 mmol) in acetic
acid (20 mL)
was added 60% aqueous nitric acid (3 mL) in acetic acid (5 mL) dropwise at 10-
15 C. The
reaction mixture was stirred at room temperature for 2 h. Then the mixture was
poured into
ice water (50 mL) and extracted with Et0Ac (3 X 50 mL). The combined organic
layers were
washed with water (2 X 50 mL), brine solution (30 mL), dried over anhydrous
sodium sulfate
and concentrated under vacuum to obtain the crude product which was purified
by
combiflash chromatography using 5% Et0Ac in hexanes as eluent to afford the
title
compound (700 mg, 22%); 1H NMR (400 MHz, DMSO-d6): 8 11.71 (bs, 1H), 8.14 (d,
J =
10.4 Hz, 1H), 7.44 (d, J= 5.6 Hz, 1H); LC-MS: m/z 224.0 (M-1)-.
Step-b: Synthesis of 2-amino-4-fluoro-5-(trifluoromethyl)phenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 4-fluoro-2-nitro-5-(trifluoromethyl)phenol as starting
material (Yield:
82%); 1H NMR (400 MHz, DMSO-d6): 8 9.54 (s, 1H), 6.78 (d, J = 7.2 Hz, 1H),
6.48 (d, J =
13.2 Hz, 1H), 5.5 (bs, 2H).
Step-c: Synthesis of 5-fluoro-6-(trifluoromethyl)benzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-4-fluoro-5-(trifluoromethyl)phenol as starting
material
(Yield: 82%); 1H NMR (400 MHz, DMSO-d6): 8 14.4 (bs, 1H), 8.10 (d, J= 5.6 Hz,
1H), 7.43
(d, J= 9.6 Hz, 1H); LC-MS: m/z 236.0 (M-1)-.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
141
Step-d: Synthesis of 2-chloro-5-fluoro-6-(trifluoromethyl)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 5-fluoro-6-(trifluoromethyl)benzo [d] oxazole-2-thiol
as starting
material and stirring for 2 h (Yield: 99%); 1H NMR (400 MHz, DMSO-d6): 8 7.74
(d, J = 6.0
Hz, 1H), 7.30 (d, J = 10.0 Hz, 1H).
Step-e: Synthesis of ethyl 2((5-fluoro-6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo [d] imidazole-5-
carboxylate and
2-chloro-5-fluoro-6-(trifluoromethyl)benzo [d] oxazole as starting materials
(Yield: 52%); 1H
NMR (400 MHz, DMSO-d6): 8 12.43 (bs, 1H), 8.22 (d, J = 1.6 Hz, 1H), 7.88 (dd,
J = 1.6 Hz,
J= 8.4 Hz, 1H), 7.83 (d, J= 6.0 Hz, 1H), 7.75 (d, J= 8.4 Hz, 1H), 7.41 (d, J=
11.2 Hz, 1H),
4.33 (q, J= 7.2 Hz, 2H), 3.66 (s, 3H), 1.35 (t, J= 7.2 Hz, 3H); LC-MS: m/z
423.0 (M+1) .
Step-f: Synthesis of 2((5-fluoro-6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 24(5-fluoro-6-(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylate as starting material (Yield: 86%);
1H NMR (400
MHz, DMSO-d6): 8 12.4 (bs, 2H), 8.20 (s, 1H), 7.89 (d, J = 8.4 Hz, 1H), 7.84
(d, J = 6.4 Hz,
1H), 7.55 (d, J= 8.8 Hz, 1H), 7.44 (d, J= 11.2 Hz, 1H), 3.67 (s, 3H); LC-MS:
m/z 395.1
(M+1) .
Step-g: Synthesis of 24(5-fluoro-6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-N-(2-
hydroxyethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((5-fluoro-6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylic acid and 2-aminoethan-1-ol as
starting materials
(Yield: 72%); 1H NMR (400 MHz, DMSO-d6): 8 12.35 (bs, 1H), 8.40 (bs, 1H), 8.09
(s, 1H),
7.84-7.79 (m, 2H), 7.54 (d, J= 8.4 Hz, 1H), 7.44 (d, J= 11.6 Hz, 1H), 4.50
(bs, 1H), 3.67 (s,
3H), 3.54 (t, J = 6.4 Hz, 2H), 3.38-3.33 (m, 2H); LC-MS: m/z 438.0 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
142
Example 109. Synthesis of N-(2-methoxyethyl)-1-methyl-2-(oxazolo[5,4-b]pyridin-
2-
ylamino)-1H-benzo[d]imidazole-5-carboxamide
Me
N OH
a NOH b c (p
¨v" I
I I Et0
N )F0
NO2 NH2
Me Me
0
is
HO 40
N )T-0 f N
N
0 N Me0
0
Conditions: a) 10% Pd/C, Me0H, H2, RT, 16 h; b) Thiophosgene, THF, RT, 16 h;
c) SOCl2, Cat.DMF, Reflux, 2 h; d) NaH,
ethyl 2-amino-1-methyl-1 H-benzo[c]imidazole-5-carboxylate , 1,4-Dioxane, RT,
16 h; e) Li0H.H20, THF, Et0H, H20, 60 C,
16 h; f) 2-methoxyethan-1-amine , DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 3-aminopyridin-2-ol
The title compound was synthesized using the same procedure which was followed
for
compound le using 3-nitropyridin-2-ol as starting material (Yield: 85%); 1H
NMR (400
MHz, DMSO-d6): 8 11.3 (bs, 1H), 6.59 (dd, J= 2.0 Hz, J= 6.8 Hz, 1H), 6.43 (dd,
J= 2.4 Hz,
J= 6.8 Hz, 1H), 5.98 (t, J= 6.0 Hz, 1H), 4.97 (bs, 2H); LC-MS: m/z 111.25
(M+1) .
Step-b: Synthesis of oxazolo[5,4-b]pyridine-2-thiol
To a solution of 3-aminopyridin-2-ol (500 mg, 4.5 mmol) in THF (15 mL) at RT
was added
thiophosgene (0.41 mL, 5.4 mmol) slowly over a period of 15 min and stirred at
RT for 16 h.
The reaction mixture was quenched with saturated aqueous ammonium chloride
solution (9
mL) and concentrated. Aqueous layer was basified with 10 N sodium hydroxide
solution (5
mL) and extracted with ethyl acetate (2 x 30 mL), aqueous layer was acidified
with 1 N HC1
and extracted with ethyl acetate (2 X 100 mL). The organic layer was dried
over anhydrous
sodium sulfate and concentrated under vacuum to get the residue which was
purified by
combiflash chromatography using 2% methanol in DCM as an eluent to afford the
title
compound (200 mg, 29%); LC-MS: m/z 153.15 (M+1) .
Step-c: Synthesis of 2-chlorooxazolo[5,4-b]pyridine
To a solution of oxazolo[5,4-b]pyridine-2-thiol (200 mg, 1.31 mmol) in thionyl
chloride (5
mL) at RT was added /V,N-dimethylformamide (1 drop) and the reaction mixture
was
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
143
refluxed for 2 h. The reaction mixture was concentrated under reduced pressure
and the crude
compound was used in the next step without any further purification (220 mg);
1H NMR (400
MHz, DMSO-d6): 8 8.40 (dd, J = 1.6 Hz, J = 4.8 Hz, 1H), 8.26 (dd, J = 1.2 Hz,
J = 7.6 Hz,
1H), 7.56-7.53 (m, 1H); LC-MS: m/z 155.1 (M+1) .
Step-d: Synthesis of ethyl 1-methyl-2-(oxazolo[5,4-b]pyridin-2-ylamino)-1H-
benzo[d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-
carboxylate and
2-chlorooxazolo[5,4-b]pyridine as starting materials (Yield: 20%); 1H NMR (400
MHz,
DMSO-d6): 8 8.24 (s, 1H), 8.02 (d, J = 5.2 Hz, 1H), 7.89 (dd, J = 1.6 Hz, J =
8.8 Hz, 1H),
7.79 (d, J = 7.2 Hz, 1H), 7.56 (d, J = 8.4 Hz, 1H), 7.29-7.26 (m, 1H), 4.33
(q, J = 7.2 Hz,
2H), 3.67 (s, 3H), 1.35 (t, J= 6.8 Hz, 3H); LC-MS: m/z 338.15 (M+1) .
Step-e: Synthesis of 1-methyl-2-(oxazolo[5,4-b]pyridin-2-ylamino)-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methyl-2-(oxazolo [5,4-b]pyridin-2-
ylamino)-1H-
benzo[d] imidazole-5-carboxylate as starting material (Yield: 54%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.4 (bs, 1H), 8.20 (s, 1H), 8.01 (d, J = 5.2 Hz,
1H), 7.89 (d, J =
8.0 Hz, 1H), 7.79 (d, J = 7.2 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.28-7.25 (m,
1H), 3.67 (s,
3H); LC-MS: m/z 310.1(M+1) .
Step-f: Synthesis of N-(2-methoxyethyl)-1-methyl-2-(oxazolo[5,4-b]pyridin-2-
ylamino)-
1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methyl-2-(oxazolo [5,4-b]pyridin-2-ylamino)-1H-benzo [d]
imidazo le-5-
carboxylic acid and 2-methoxyethylamine as starting materials (Yield: 38%); 1H
NMR (400
MHz, DMSO-d6): 8 12.38 (bs, 1H), 8.47 (s, 1H), 8.09 (s, 1H), 8.0 (d, J = 3.6
Hz, 1H), 7.79-
7.77 (m, 2H), 7.52 (d, J = 8.4 Hz, 1H), 7.27-7.24 (m, 1H), 3.66 (s, 3H), 3.48-
3.44 (m, 4H),
3.28 (s, 3H); LC-MS: m/z 367.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
144
Example 110. Synthesis of N-(2-hydroxyethyl)-1-methyl-2-(oxazolo[5,4-b]pyridin-
2-
ylamino)-1H-benzo[d]imidazole-5-carboxamide
Me ye
N H
lipN0 a H NrHvO
HO
N
HO
0 0
Conditions: a) 2-aminoethan-1-ol , HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methy1-2-(oxazolo[5,4-b]pyridin-2-ylamino)-1H-
benzo [d]imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials (Yield:
51%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.38 (bs, 1H), 8.09 (s, 1H),
8.01 (d, J
= 4.4 Hz, 1H), 7.80-7.76 (m, 2H), 7.52 (d, J = 8.4 Hz, 1H), 7.25 (t, J = 6.8
Hz, 1H), 4.72 (bs,
1H), 3.66 (s, 3H), 3.53 (bs, 2H), 3.35 (q, J = 6.0 Hz, 2H); LC-MS: m/z 353.0
(M+1) .
Examples 111 and 112. Synthesis of 1-methyl-2-(oxazolo[4,5-b]pyridin-2-
ylamino)-1H-
benzo[d]imidazole-5-carboxylic acid and Synthesis of N-(2-methoxyethyl)-1-
methyl-2-
(oxazolo[4,5-b]pyridin-2-ylamino)-1H-benzo[d]imidazole-5-carboxamide
Me
b-O l 1110 d
Et0 INNH2 N
0
Me Me
HO =ILO
N e
Me0
0 0 Nr
Conditions: a) CS2, Et0H, Reflux, 12 h; b) SOCl2, Cat.DMF, Reflux, 2 h; c)
NaH, ethyl 2-amino-1-
methyl-1H-benzo[c]imidazole-5-carboxylate , 1,4-Dioxane, RT, 16 h; d)
Li0H.H20, THF, Et0H, H20,
60 C, 16 h; e) 2-methoxyethan-1-amine , DPPA, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of oxazolo[4,5-b]pyridine-2-thiol
To a solution of 2-aminopyridin-3-ol (2.2 g, 20 mmol) in ethanol (40mL) at RT
was added
potassium hydroxide (1.68 g, 30 mmol) and carbondisulfide (15 mL). The
reaction mixture
was refluxed for 12 h, then cooled to RT, diluted with water (75 mL) and
neutralized with
glacial acetic acid. A solid precipitated and was filtered and dried under
vacuum to afford the
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
145
title compound (1.8 g, 59%); 1H NMR (400 MHz, DMSO-d6): 8 14.5 (s, 1H), 8.23
(dd, J =
1.2 Hz, J = 5.2 Hz, 1H), 7.88 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.29-7.26 (m,
1H); LC-MS:
m/z 153.1 (M+1) .
Step-b: Synthesis of 2-chlorooxazolo[4,5-b]pyridine
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using oxazolo[4,5-b]pyridine-2-thiol as starting material
and heating for
2 h. (Yield: 100%); 1H NMR (400 MHz, DMSO-d6): 8 8.01 (dd, J = 1.6 Hz, J = 5.6
Hz, 1H),
7.61 (dd, J= 1.2 Hz, J= 8.0 Hz, 1H), 7.10-7.06 (m, 1H); LC-MS: m/z 155.01 (M-
F1) .
Step-c: Synthesis of ethyl 1-methyl-2-(oxazolo[4,5-b]pyridin-2-ylamino)-1H-
benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-
carboxylate and
2-chlorooxazolo[4,5-b]pyridine as starting materials (Yield: 32%); 1H NMR (400
MHz,
DMSO-d6): 8 7.98 (s, 1H), 7.94 (d, J = 4.4 Hz, 1H), 7.62 (d, J = 9.2 Hz, 1H),
7.36 (d, J = 7.2
Hz, 1H), 7.21-7.18 (m, 1H), 6.77-6.74 (m, 1H), 4.32-4.26 (m, 2H), 3.57 (s,
3H), 1.38 (t, J =
6.8 Hz, 3H); LC-MS: m/z 338.15 (M+1) .
Step-d: Synthesis of 1-methyl-2-(oxazolo[4,5-b]pyridin-2-ylamino)-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methyl-2-(oxazolo [4,5-b]pyridin-2-
ylamino)-1H-
benzo[d]imidazole-5-carboxylate as starting material (Yield: 73%); 1H NMR (400
MHz,
DMSO-d6): 8 12.83 (bs, 1H), 12.44 (bs, 1H), 8.26-8.23 (m, 2H), 7.89 (d, J =
8.4 Hz, 1H),
7.78 (d, J = 7.6 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.12-7.09 (m, 1H), 3.72
(s, 3H); LC-MS:
m/z 310.15 (M+1) .
Step-e: Synthesis of N-(2-methoxyethyl)-1-methyl-2-(oxazolo[4,5-b]pyridin-2-
ylamino)-
1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methyl-2-(oxazolo [4,5-b]pyridin-2-ylamino)-1H-benzo [d]
imidazo le-5-
carboxylic acid and 2-methoxyethylamine as starting materials (Yield: 53%); 1H
NMR (400
CA 03135615 2021-09-29
WO 2020/210339
PCT/US2020/027240
146
MHz, DMSO-d6): 8 12.4 (bs, 1H), 8.48 (bs, 1H), 8.23 (d, J= 4.4 Hz, 1H), 8.13
(s, 1H), 7.79-
7.75 (s, 2H), 7.53 (d, J = 8.4 Hz, 1H), 7.11-7.08 (m, 1H), 3.67 (s, 3H), 3.48-
3.44 (m, 4H),
3.29 (s, 3H); LC-MS: m/z 367.2 (M+1) .
Example 113. Synthesis of N-(2-hydroxyethyl)-1-methyl-24(5-
(trifluoromethypoxazolo[5,4-b]pyridin-2-y1)amino)-1H-benzo [d] imidazole-5-
carboxamide
02NH2
F3C NOH F3C NOH c
a
¨SH ¨F3C0"
N
NH2
0
Me Me Me
Ni Ni
Et0 N /> ))--0 HO
NI N)F-C) HON N
0 \=N 0 \N 0 \=N
CF3 CF3 CF3
Conditions: a) ethyl (E)-5,5,5-trifluoro-4-oxopent-2-enoate, Na0Et, Et0H, 90
C, 2 h; b)10% Pd/C, Me0H, H2, RT,
16 h; c) Thiophosgene, THF, RT, 16 h; d) S0Cl2, Cat.DMF, reflux, 3 h; e) NaH,
ethyl 2-amino-1-methyl-1H-
benzo[d]imidazole-5-carboxylate , 1,4-Dioxane, RT, 16 h; f) Li0H.H20, THF,
Et0H, H20, 60 C, 16 h; g) 2-
aminoethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 3-nitro-6-(trifluoromethyl)pyridin-2-ol
To a solution of 2-nitroacetamide (5.0 g, 47.8 mmol) in ethanol (25 mL) at RT
was added
ethyl (E)-5,5,5-trifluoro-4-oxopent-2-enoate (11.24 g, 57.3 mmol) and 25%
sodium ethoxide
in ethanol (25.9 mL, 95.3 mmol). The reaction mixture was stirred at 90 C for
2 h. Reaction
mixture was cooled to RT, diluted with water (50 mL), acidified with 1N HC1
and extracted
with Et0Ac (2 X 150 mL). The combined organic layers were washed brine
solution (50
mL), dried over anhydrous sodium sulfate and concentrated under vacuum to get
the residue
which was purified by combiflash chromatography using 50% Et0Ac in hexanes as
eluent to
afford the title compound (1.5 g); 1H NMR (400 MHz, DMSO-d6): 8 8.58 (d, J =
8.4 Hz, 1H),
7.84 (bs, 1H), 7.51 (d, J= 7.6 Hz, 1H).
Step-b: Synthesis of 3-amino-6-(trifluoromethyl)pyridin-2-ol
The title compound was synthesized using the same procedure which was followed
for
compound le using 3-nitro-6-(trifluoromethyl)pyridin-2-ol as starting material
(Yield: 82%);
1H NMR (400 MHz, DMSO-d6): 8 11.7 (bs, 1H), 6.88 (bs, 1H), 6.69 (bs, 1H), 5.60
(s, 2H);
LC-MS: m/z 179.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
147
Step-c: Synthesis of 5-(trifluoromethypoxazolo[5,4-b]pyridine-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 109 Step-b using 3-amino-6-(trifluoromethyl)pyridin-2-ol as starting
material
(Yield: 65%); 1H NMR (400 MHz, DMSO-d6): 8 7.81-7.75 (m, 2H); LC-MS: m/z 219.0
(M-
1).
Step-d: Synthesis of 2-chloro-5-(trifluoromethypoxazolo[5,4-b]pyridine
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 5-(trifluoromethyl)oxazolo[5,4-b]pyridine-2-thiol as
starting
material (Yield: 74%). The crude compound was used in the next step without
further
purification.
Step-e: Synthesis of ethyl 1-methyl-2-45-(trifluoromethypoxazolo[5,4-b]pyridin-
2-
yl)amino)-1H-benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-5-(trifluoromethyl)oxazolo[5,4-b]pyridine as starting materials
(Yield: 43%); 1H
NMR (400 MHz, DMSO-d6): 8 12.5 (bs, 1H), 8.27 (s, 1H), 7.93-7.89 (m, 2H), 7.75
(d, J =
12.4 Hz, 1H), 7.62 (d, J= 8.4 Hz, 1H), 4.34 (q, J= 6.8 Hz, 2H), 3.71 (s, 3H),
1.35 (t, J= 7.2
Hz, 3H); LC-MS: m/z 406.1 (M+1) .
Step-f: Synthesis of 1-methyl-24(5-(trifluoromethypoxazolo[5,4-13]pyridin-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methyl-2-((5-(trifluoromethyl)oxazolo [5 ,4- b]
pyridin-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylate as starting material (Yield:
75%); 1H NMR
(400 MHz, DMSO-d6): 8 8.20 (bs, 1H), 7.74-7.70 (m, 2H), 7.55 (d, J = 6.8 Hz,
1H), 7.09 (d,
J= 8.4 Hz, 1H), 3.62 (s, 3H); LC-MS: m/z 378.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
148
Step-g: Synthesis of N-(2-hydroxyethyl)-1-methyl-24(5-
(trifluoromethypoxazolo[5,4-
13] pyridin-2-yl)amino)-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methy1-2-((5-(trifluoromethyl)oxazolo[5,4-b]pyridin-2-
yDamino)-
1H-benzo [d] imidazole-5-carboxylic acid and 2-aminoethan-1-ol as starting
materials (Yield:
28%); 1H NMR (400 MHz, DMSO-d6): 8 8.22 (bs, 1H), 7.78 (bs, 1H), 7.60 (d, J =
8.0 Hz,
1H), 7.51 (bs, 1H), 7.31 (d, J= 8.4 Hz, 2H), 4.70 (t, J= 5.2 Hz, 1H), 3.67 (s,
3H), 3.50 (q, J=
5.2 Hz, 2H), 3.36 (q, J= 6.0 Hz, 2H); LC-MS: m/z 421.1 (M+1) .
Example 114. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-2-
(thiazolo[4,5-
13] pyrazin-2-ylamino)-1H-benzo [d] imidazole-5-carboxamide
N, 0 N s Me
a ( ji,) b
OEtc"-- =N>---NH
N NH2 N N N OEt N r-S
H H EtO2C
114a
N
Me
Me N H
N
110 nrs
1\1)1-S N
H020 0 N
Conditions: a) 0-ethyl carbonisothiocyanatidate, 1,4-Dioxane, RT, 16 h; b) 6 M
HCI, 1,4-Dioxane, 50 C, 4 h; c) NaOH,
H20, 120 C, 16 h; d) ethyl 3-amino-4-(methylamino)benzoate, 1,1'-
thiocarbonyldiimidazole , EDC, DMF, 100 C, 18 h;
e) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; f) 2-(2-aminoethoxy)ethan-1-ol ,
HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 114a
To a stirred solution of pyrazin-2-amine (2.0 mg, 21.0 mmol) in 1,4-dioxane
(20 mL) at RT
was added 0-ethyl carbonisothiocyanatidate (3.03 g, 23.1 mmol) and stirred at
RT for 16 h.
The reaction mixture was concentrated under vacuum. To the residue ethyl
acetate was added
and the precipitated solid was filtered and dried under vacuum to afford the
title compound
(2.0 g, 42%); 1H NMR (400 MHz, DMSO-d6): 8 12.07 (s, 1H), 11.76 (s, 1H), 9.67
(s, 1H),
8.50 (s, 2H), 4.24 (q, J = 7.2 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H); LC-MS: m/z
227.0 (M+1) .
Step-b: Synthesis of ethyl thiazolo[4,5-b]pyrazin-2-ylcarbamate
To a stirred solution of 114a (1.8 g, 7.96 mmol) in 1,4-dioxane (9 mL) was
added 6 M HC1
(18 mL) and stirred at 50 C for 4 h. The reaction mixture was cooled to RT and
solid
obtained was filtered and dried under vacuum to afford the title compound (1.5
g, 84%); 1H
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
149
NMR (400 MHz, DMSO-d6): 8 12.60 (bs, 1H), 8.58 (d, J = 3.2 Hz, 1H), 8.44 (d, J
= 2.4 Hz,
1H), 4.29 (q, J = 6.8 Hz, 2H), 1.30 (t, J = 7.6 Hz, 3H); LC-MS: m/z 225.0
(M+1) .
Step-c: Synthesis of thiazolo[4,5-b]pyrazin-2-amine
To a solution of ethyl thiazolo[4,5-b]pyrazin-2-ylcarbamate (500 mg, 2.23
mmol) in water (5
mL) was added sodium hydroxide (267 mg, 6.69 mmol) and heated at 120 C for 16
h. The
reaction mixture was cooled to RT, acidified with 1 N HC1. The obtained solid
was filtered
and dried under vacuum to afford the title compound (300 mg, 88%); 1H NMR (400
MHz,
DMSO-d6): 8 8.42 (s, 2H), 8.23 (d, J = 2.8 Hz, 1H), 8.03 (d, J = 2.8 Hz, 1H);
LC-MS: m/z
153.1 (M+1) .
Step-d: Synthesis of ethyl 1-methyl-2-(thiazolo[4,5-b]pyrazin-2-ylamino)-1H-
benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound 2c using ethyl 3-amino-4-(methylamino)benzoate and thiazolo[4,5-
b]pyrazin-2-
amine as starting materials (Yield: 9%); 1H NMR (400 MHz, DMSO-d6): 8 12.63
(bs, 1H),
8.44 (d, J= 2.4 Hz, 1H), 8.33 (s, 1H), 8.23 (d, J= 2.8 Hz, 1H), 7.91 (dd, J=
1.2 Hz, J= 8.4
Hz, 1H), 7.60 (d, J = 8.4 Hz, 1H), 4.33 (q, J = 7.2 Hz, 2H), 3.67 (s, 3H),
1.35 (d, J = 7.2 Hz,
3H); LC-MS: m/z 355.0 (M+1) .
Step-e: Synthesis of 1-methyl-2-(thiazolo[4,5-b]pyrazin-2-ylamino)-1H-
benzo[d]imidazole-5-carboxylic acid
To a stirred solution of ethyl 1-methy1-2-(thiazolo[4,5-b]pyrazin-2-ylamino)-
1H-
benzo [d] imidazole-5-carboxylate (60 mg, 0.17 mmol) in a mixture of solvent
of THF (1 mL),
ethanol (1 mL) and water (0.5 mL) was added lithium hydroxide monohydrate (35
mg, 0.85
mmol). The reaction mixture was heated at 60 C for 16 h with stirring. The
reaction mixture
was cooled to RT and concentrated under reduced pressure. The crude material
was directly
used in the next step (40 mg); LC-MS: m/z 326.95 (M+1) .
Step-f: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-2-(thiazolo[4,5-
b]pyrazin-2-
ylamino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methy1-2-(thiazolo[4,5-b]pyrazin-2-ylamino)-1H-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
150
benzo [d] imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-01 as
starting materials
(Yield: 59%); 1H NMR (400 MHz, DMSO-d6): 8 12.60 (bs, 1H), 8.46-8.41 (m, 2H),
8.20-
8.14 (m, 2H), 7.78 (s, 1H), 7.56 (s, 1H), 4.60 (t, J= 4.8 Hz, 1H), 3.70 (s,
3H), 3.59-3.43 (m,
8H); LC-MS: m/z 414.05 (M+1) .
Examples 115 and 116. Synthesis of 2-06-cyclopropylbenzo [d] oxazo1-2-
yl)amino)-1-
methyl-1H-benzo[d]imidazole-5-carboxylic acid and Synthesis of 24(6-
cyclop ropylbenzo[d] oxazol-2-yl)amino)-N-(2-methoxyethyl)-1-methyl-1H-
benzo [d] imidazole-5-carboxamide
Br OH
a A OH A OH
(3-s H d 1110 e
NO2 NO2 NH2
Me
Vie fyle
H
Et0 io
NH io
N >F0 f HO NH
N >F0 )=-- Me0N 110 N ro
N
N 0 0 N 0
V
Conditions: a) Cyclopropyl boronic acid, Pd(OAc)2, Tricyclohexyl phosphine,
K3PO4, Toluene, H20, 100 C, 16 h; b) Pt02,
Et0H, THF, H2, RT, 4 h; c) Potassium ethyl xanthate, Et0H, Reflux, 16 h; d)
S0Cl2, Cat.DMF, Reflux, 3 h; e) NaH, ethyl 2-
amino-1-methyl-1H-benzo[cl]imidazole-5-carboxylate , 1,4-Dioxane, RT, 16 h; f)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h; g)
2-methoxyethan-1-amine , DPPA, DIPEA, DMF, 0 C - RT, 16h
Step-a: Synthesis of 5-cyclopropy1-2-nitrophenol
To a stirred solution of 5-bromo-2-nitrophenol (2.0 g, 9.17 mmol) and
cyclopropyl boronic
acid (1.02 g, 11.92 mmol) in toluene (40 mL) was added potassium phosphate
(6.81 g, 32.1
mmol), tricyclohexyl phosphine (0.25 g, 0.91 mmol) and water (2 mL). The
reaction mixture
was purged with nitrogen gas for 10 min and then palladium diacetate (0.1 g,
0.45 mmol) was
added to the reaction mixture and it was heated at 100 C for 16 h. The
reaction mixture was
cooled to RT, filtered through a celite bed and washed with ethyl acetate (150
mL). The
organic layer was washed brine solution (50 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum to get the residue which was purified by combiflash
chromatography using 100% hexane as an eluent to afford the title compound
(0.9 g, 55%);
1H NMR (400 MHz, DMSO-d6): 8 10.72 (bs, 1H), 7.82 (d, J = 8.8 Hz, 1H), 6.82
(d, J = 2.0
Hz, 1H), 6.68 (dd, J = 2.0 Hz, J = 8.8 Hz, 1H), 1.99-1.94 (m, 1H), 1.08-1.03
(m, 2H), 0.78-
0.74 (m, 2H).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
151
Step-b: Synthesis of 2-amino-5-cyclopropylphenol
To a solution of 5-cyclopropy1-2-nitrophenol (1.75 g, 11.4 mmol) in ethanol (4
mL) and THF
(4 mL) was added a Pt02 (12 mg). The reaction mixture was stirred under
hydrogen gas
balloon for 4 h. It was filtered through a celite bed and concentrated under
vacuum to afford
the title compound (250 mg, 100%); 1H NMR (400 MHz, DMSO-d6): 8 8.99 (bs, 1H),
6.61-
6.59 (m, 1H), 6.45 (d, J = 2.0 Hz, 1H), 6.43-6.34 (m, 1H), 5.35 (bs, 2H), 1.74-
1.65 (m, 1H),
0.80-0.75 (m, 2H), 0.48-0.44 (m, 2H).
Step-c: Synthesis of 6-cyclopropylbenzo[d]oxazole-2-thiol
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-cyclopropylphenol as starting material
(Yield: 90%); 1H
NMR (400 MHz, DMSO-d6): 8 13.72 (bs, 1H), 7.22 (s, 1H), 7.11-7.04 (m, 2H),
2.02-1.98 (m,
1H), 0.98-0.94 (m, 2H), 0.71-0.67 (m, 2H); LC-MS: m/z 190.05 (M-1).
Step-d: Synthesis of 2-chloro-6-cyclopropylbenzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-cyclopropylbenzo[d]oxazole-2-thiol as starting
material (Yield:
98%); 1H NMR (400 MHz, DMSO-d6): 8 7.59 (d, J = 8.0 Hz, 1H), 7.46 (s, 1H),
7.18 (d, J =
8.4 Hz, 1H), 2.09-2.01 (m, 1H), 1.02-0.99 (m, 2H), 0.75-0.74 (m, 2H); LC-MS:
m/z 194.0
(M+1) .
Step-e: Synthesis of ethyl 24(6-cyclopropylbenzo[d]oxazol-2-yl)amino)-1-methyl-
1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-l-methyl-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-cyclopropylbenzo[d]oxazole as starting materials (Yield: 27%); 1H
NMR (400
MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.22 (s, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.50
(d, J = 8.4 Hz,
1H), 7.33 (d, J= 8.4 Hz, 1H), 7.15 (s, 1H), 6.97 (d, J= 6.8 Hz, 1H), 4.35-4.27
(m, 2H), 3.62
(s, 3H), 2.02-1.98 (m, 1H), 1.36-1.30 (m, 3H), 0.97-0.92 (m, 2H), 0.71-0.67
(m, 2H); LC-
MS: m/z 377.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
152
Step-f: Synthesis of 2-((6-cyclopropylbenzo[d] oxazol-2-yl)amino)-1-methyl-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-cyclopropylbenzo [d]oxazol-2-yl)amino)-1-methyl-
1H-
benzo[d]imidazole-5-carboxylate as starting material (Yield: 81%); 1H NMR (400
MHz,
DMSO-d6): 8 13.5-11.2 (bs, 2H), 8.18 (s, 1H), 7.91 (d, J= 8.4 Hz, 1H), 7.58
(d, J= 8.4 Hz,
1H), 7.34 (d, J = 8.4 Hz, 1H), 7.22 (s, 1H), 7.04 (d, J = 8.4 Hz, 1H), 3.67
(s, 3H), 2.06-2.02
(m, 1H), 1.00-0.95 (m, 2H), 0.72-0.68 (m, 2H); LC-MS: m/z 349.0 (M+1) .
Step-g: Synthesis of 2-((6-cyclopropylbenzo[d] oxazol-2-yl)amino)-N-(2-
methoxyethyl)-1-
methyl-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-((6-cyclopropylbenzo[d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid and 2-methoxyethylamine as starting
materials (Yield:
46%); 1H NMR (400 MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.44 (t, J = 5.2 Hz, 1H),
8.06 (d, J =
1.6 Hz, 1H), 7.75 (d, J= 8.4 Hz, 1H), 7.46 (d, J= 8.4 Hz, 1H), 7.33 (d, J= 8.0
Hz, 1H), 7.14
(s, 1H), 6.96 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 3.62 (s, 3H), 3.48-3.43 (m,
4H), 3.28 (s, 3H),
2.02-1.98 (m, 1H), 0.96-0.92 (m, 2H), 0.70-0.66 (m, 2H); LC-MS: m/z 406.2
(M+1) .
Example 117. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(7-
(trifluoromethyl)benzo[d] oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-
carboxamide
Vie
NO2 NH2 CF3 CF3
HO
a HO
HO la c Os
(:)ss ¨ad ,_c, N )r-0
N
CF3
F3C F3C F3C 0
fyle fyle
NH
f HO N N
0 N Abi CF3 ¨)" 0
1.1 0 N CF3
Conditions: a) NaNO3, NaNO2, H2SO4, DCM, RT, 16 h; b)10% Pd/C, Me0H, H2, RT, 4
h; c) Potassium ethyl xanthate,
Et0H, reflux, 16 h; d) S0Cl2, Cat.DMF, reflux, 2 h; e) NaH, ethyl 2-amino-1-
methyl-1 H-benzo[c]imidazole-5-carboxylate ,
1,4-Dioxane, RT, 16 h; f) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; g) 2-(2-
aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF,
0 C - RT, 16 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
153
Step-a: Synthesis of 2-nitro-6-(trifluoromethyl)phenol
To 3 M sulfuric acid (25 mL) stirring at RT was added sodium nitrate (1.73 g,
20.37 mmol)
and sodium nitrite (100 mg) followed by the addition of a solution of 2-
(trifluoromethyl)phenol (3.0 g, 18.52 mmol) in DCM (40 mL) and stirred for 16
h. The
mixture was poured into ice water (50 mL) and extracted with DCM (2 X 100 mL).
The
combined organic layers were washed with water (50 mL), brine solution (50
mL), dried over
anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combiflash chromatography using 5% ethyl acetate in hexane as eluent to afford
the titled
compound (1.3 g, 40%); 1H NMR (400 MHz, DMSO-d6): 8 11.34 (bs, 1H), 8.27 (dd,
J= 1.6
Hz, J = 8.4 Hz, 1H), 8.00 (dd, J = 1.2 Hz, J = 7.6 Hz, 1H), 7.21-7.17 (m, 1H).
Step-b: Synthesis of 2-amino-6-(trifluoromethyl)phenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 2-nitro-6-(trifluoromethyl)phenol as starting material and
stirring for 4 h
(Yield: 90%); 1H NMR (400 MHz, DMSO-d6): 8 6.88-6.84 (m, 1H), 6.75-6.70 (m,
2H), 6.50
(bs, 2H); LC-MS: m/z 178.05 (M+1) .
Step-c: Synthesis of 7-(trifluoromethyl)benzo[d]oxazole-2(3H)-thione
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-6-(trifluoromethyl)phenol as starting material
(Yield:
65%); 1H NMR (400 MHz, DMSO-d6): 8 14.28 (bs, 1H), 7.59-7.53 (m, 2H), 7.49-
7.45 (m,
1H); LC-MS: m/z 218.1 (M-1)-.
Step-d: Synthesis of 2-chloro-7-(trifluoromethyl)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 7-(trifluoromethyl)benzo [d] oxazole-2(3H)-thione as
starting
material and stirring for 2 h (Yield: 84%); 1H NMR (400 MHz, DMSO-d6): 8 8.11
(d, J = 8.0
Hz, 1H), 7.84 (d, J= 7.6 Hz, 1H), 7.64 (t, J= 7.2 Hz, 1H).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
154
Step-e: Synthesis of ethyl 1-methyl-2-07-(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-
1H-benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-7-(trifluoromethyl)benzo [d]oxazole as starting materials (Yield:
36%); 1H NMR
(400 MHz, DMSO-d6): 8 12.41 (s, 1H), 8.26 (d, J= 1.6 Hz, 1H), 7.90 (dd, J= 1.6
Hz, J= 8.4
Hz, 1H), 7.75 (d, J = 6.4 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 7.44-7.38 (m,
2H), 4.33 (q, J =
6.8 Hz, 2H), 3.67 (s, 3H), 1.35 (d, J = 6.8 Hz, 3H); LC-MS: m/z 405.0 (M+1) .
Step-f: Synthesis of 1-methyl-24(7-(trifluoromethyl)benzo[d]oxazol-2-yl)amino)-
1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methy1-2-((7-(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-1H-
benzo [d]imidazole-5-carboxylate as starting material (Yield: 75%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.83 (bs, 1H), 12.40 (bs, 1H), 8.22 (d, J = 1.6 Hz, 1H), 7.89
(dd, J= 1.2 Hz, J
= 8.4 Hz, 1H), 7.75 (d, J= 8.4 Hz, 1H), 7.55 (d, J= 8.0 Hz, 1H), 7.43-7.37 (m,
2H), 3.67 (s,
3H); LC-MS: m/z 377.0 (M+1) .
Step-g: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(7-
(trifluoromethyl)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((7-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-ol as
starting materials
(Yield: 61%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.46 (t, J = 5.2
Hz, 1H),
8.10 (d, J= 1.2 Hz, 1H), 7.80-7.73 (m, 2H), 7.53 (d, J= 8.4 Hz, 1H), 7.42-7.38
(m, 2H), 4.60
(bs, 1H), 3.67 (s, 3H), 3.58-3.43 (m, 8H); LC-MS: m/z 464.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
155
Example 118. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-24(1-methyl-1H-
benzo[d] imidazol-2-yl)amino)benzo [d] oxazole-5-carboxamide
0 H me
00 F 00 NHMe b 40 NHMe Me p
a c =/>¨NH 2 Et0 N N
)T¨NI
NO2 NO2 NH2 N
0
0 H me 0,
irN f
/i¨NH ,Me
110 N )T¨NI
HO
N * HO
0 N
0
Conditions: a) Aq.Methyl amine, DMF, 60 C, 16 h; b) 10% Pd/C, Me0H, H2, RT, 16
h; c) Cyanogen bromide, THF, H20, 50 C, 16 h; d)
NaH, ethyl 2-chlorobenzo[d]oxazole-5-carbo4ate , 1,4-Dioxane, RT, 16 h; e)
Li0H.H20, THF, Et0H, H20, 60 C, 16 h; f) 2-(2-
aminoethoMethan-1-ol , HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of N-methyl-2-nitroaniline
To a solution of 1-fluoro-2-nitrobenzene (1.0 g, 7.08 mmol) in DMFA (3 mL) at
RT was
added methyl amine (1 mL, 40% aqueous solution, 35.4 mmol) and the reaction
mixture was
stirred at 60 C for 16 h. The reaction mixture was cooled to RT, diluted with
cold water (50
mL) and stirred for 2 h. The solid obtained was filtered and dried under
vacuum to afford the
product as a yellow solid (700 mg, 75%); 1H NMR (400 MHz, DMSO-d6): 8 8.14
(bs, 1H),
8.06 (dd, J = 1.2 Hz, J = 8.8 Hz, 1H), 7.57-7.53 (m, 1H), 6.98 (d, J = 8.8 Hz,
1H), 6.69-6.66
(m, 1H), 2.96 (d, J = 4.8 Hz, 3H); LC-MS: m/z 153.0 (M+1) .
Step-b: Synthesis of N1-methylbenzene-1,2-diamine
The title compound was synthesized using the same procedure which was followed
for
compound le using N-methyl-2-nitroaniline as starting material (Yield: 98%);
1H NMR (400
MHz, DMSO-d6): 8 6.52-6.48 (m, 2H), 6.41-6.34 (m, 2H), 4.45 (bs, 2H), 2.68 (s,
3H); LC-
MS: m/z 123.2 (M+1) .
Step-c: Synthesis of 1-methyl-1H-benzo[d] imidazol-2-amine
The title compound was synthesized using the same procedure which was followed
for
compound if using N1-methylbenzene-1,2-diamine as starting material (Yield:
83%); 1H
NMR (400 MHz, DMSO-d6): 8 7.11-7.09 (m, 2H), 6.94-6.87 (m, 2H), 6.37 (s, 2H),
3.78 (s,
3H).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
156
Step-d: Synthesis of ethyl 24(1-methyl-1H-benzo [d] imidazol-2-
yl)amino)benzo [d] oxazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using 1-methyl-1H-benzo [d] imidazol-2-amine and ethyl 2-
chlorobenzo [d] oxazole-5-carboxylate as starting materials (Yield: 31%); 1H
NMR (400 MHz,
DMSO-d6): 8 12.20 (bs, 1H), 7.99 (d, J = 1.6 Hz, 1H), 7.76-7.74 (m, 1H), 7.64-
7.62 (m, 1H),
7.52 (d, J = 8.0 Hz, 1H), 7.45-7.43 (m, 1H), 7.26-7.23 (m, 2H), 4.32 (q, J =
7.6 Hz, 2H), 3.63
(s, 3H), 1.35 (t, J = 6.8 Hz, 3H); LC-MS: m/z 336.95 (M+1) .
Step-e: Synthesis of 24(1-methyl-1H-benzo [d] imidazol-2-yl)amino)benzo [d]
oxazole-5-
carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((1-methy1-1H-benzo [d] imidazol-2-yl)amino)benzo
[d] oxazole-5-
carboxylate as starting material (Yield: 78%); 1H NMR (400 MHz, DMSO-d6): 8
12.80 (bs,
1H), 12.22 (bs, 1H), 7.98 (d, J = 1.6 Hz, 1H), 7.76 (dd, J = 2.0 Hz, J = 8.4
Hz, 1H), 7.67-7.63
(m, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.47-7.45 (m, 1H), 7.28-7.21 (m, 2H), 3.68
(s, 3H); LC-
MS: m/z 309.1 (M-F1) .
Step-f: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-24(1-methyl-1H-benzo [d]
imidazol-2-
yl)amino)benzo [d] oxazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((1-methy1-1H-benzo[d] imidazol-2-y1) amino)benzo [d]
o xazole-5-
carboxylic acid and 2-(2-aminoethoxy)ethan-1-ol as starting materials. The
crude product
was purified by combiflash chromatography using 9% methanol in DCM as an
eluent (Yield:
64%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.48 (t, J = 5.2 Hz, 1H),
7.92 (s,
1H), 7.65-7.62 (m, 2H), 7.48-7.45 (m, 2H), 7.28-7.22 (m, 2H), 3.63 (s, 3H),
3.57-3.35 (m,
8H); LC-MS: m/z 396.2 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
157
Example 119. Synthesis of 2-(benzo[d]oxazol-2-ylamino)-N-(2-
methoxyethoxy)-1-
methyl-1H-benzo[d]imidazole-5-carboxamide
0
N¨OH a N¨O H2N,00Me
\--\
0 0 OMe
=Me
Me
HO
Ni
401 --NH
Me0(:) ¨NH,N N
N
0
0
Conditions: a) 1-bromo-2-methoxyethane, TEA, DMF, 60 C, 16 h; b) Hydrazine
hydrate, Me0H,
reflux, 4 h; c) 0-(2-methoxyethyl)hydroxylamine, DPPA, DIPEA, DMF, 0 C - RT,
16 h
Step-a: Synthesis of 2-(2-methoxyethoxy)isoindoline-1,3-dione
To a stirred solution of N-hydroxyphthalimide (4.6 g, 28.2 mmol) in DMFA (15
mL) at RT
was added triethyl amine (8.0 mL, 56.4 mmol) and 1-bromo-2-methoxyethane (4.0
mL, 42.3
mmol). The reaction mixture was then stirred at 60 C for 16 h. Once the
reaction was
completed (monitored by TLC), the reaction mixture was cooled to RT, diluted
with cold
water (100 mL) and extracted with ethyl acetate (2 X 100 mL). The combined
organic layers
were washed with cold water (2 X 200 mL), brine solution (50 mL), dried over
anhydrous
sodium sulfate and concentrated under vacuum to afford the titled compound
(2.8 g, 45%);
1H NMR (400 MHz, DMSO-d6): 8 7.86 (s, 4H), 4.26 (t, J = 4.4 Hz, 2H), 3.65-3.63
(m, 2H),
3.25 (s, 3H); LC-MS: m/z 222.20 (M+1) .
Step-b: Synthesis of 0-(2-methoxyethyl)hydroxylamine
To a stirred solution of 2-(2-methoxyethoxy)isoindoline-1,3-dione (2.8 g, 12.6
mmol) in
methanol (75 mL) at RT was added hydrazine hydrate (0.95 g, 19.0 mmol) and
refluxed for 4
h. Then the reaction mixture was cooled to RT, filtered and the filtrate was
concentrated. The
residue obtained was stirred in diethyl ether (30 mL) and filtered. The
filtrate was
concentrated under vacuum to afford the titled compound (550 mg, 48%); 1H NMR
(400
MHz, DMSO-d6): 8 5.50 (s, 2H), 3.84-3.82 (m, 2H), 3.58-3.56 (m, 2H), 3.39 (s,
3H).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
158
Step-c: Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-methoxyethoxy)-1-
methyl-1H-
benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
compound li using 2-(benzo [d]oxazo 1-2- ylamino)- 1-methyl- 1H-benzo [d] imid
azo le-5-
carboxylic acid and 0-(2-methoxyethyl)hydroxylamine as starting materials
(Yield: 20%); 1H
NMR (400 MHz, DMSO-d6): 8 12.28 (s, 1H), 11.73 (s, 1H), 8.01 (s, 1H), 7.64 (d,
J= 8.3 Hz,
1H), 7.48 (d, J = 7.8 Hz, 2H), 7.44 (d, J = 7.8 Hz, 1H), 7.22 (t, J = 7.8 Hz,
1H), 7.12 (t, J =
7.9 Hz, 1H), 4.04 (t, J = 4.2 Hz, 2H), 3.63 (s, 3H), 3.59 (t, J = 4.2 Hz, 2H),
3.31 (s, 3H); LC-
MS: m/z 382.0 (M+1) .
Example 120. Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-hydroxypropoxy)-
1-
methyl-1H-benzo [d] imidazole-5-carboxamide
o 0
OH
101 N-OH N-OOH
1-121\ifChile
0 0 Me
Me Me
-NH
HO = N,NH c Me 110 r.0-N N/>
0 N OH 0 N
Conditions: a) 2-methyloxirane , DIPEA, Tetrabutylammonium bromide, Toluene,
Reflux, 5 h; b) 6
N Aq.HCI, RT, 16 h; c) 1-(aminooxy)propan-2-ol hydrochloride salt, HBTU,
DIPEA, DMF, 0 C -
RT, 16h
Step-a: Synthesis of 2-(2-hydroxypropoxy)isoindoline-1,3-dione
To a stirred solution of 2-hydroxyisoindoline-1,3-dione (5.0 g, 30.65 mmol)
and 2-
methyloxirane (4.1 mL, 61.3 mmol) in toluene (50 mL) at RT was added DIPEA
(0.6 mL, 3.1
mmol) and tetrabutylammonium bromide (0.99 g, 3.1 mmol). The reaction mixture
was then
refluxed for 5 h. The mixture was cooled to RT, concentrated under reduced
pressure and
extracted with Et0Ac (2 X 100 mL) and water (50 mL). The combined organic
layers were
washed with water (30 mL), brine solution (30 mL), dried over anhydrous sodium
sulfate and
concentrated under vacuum. The residue was purified by combiflash column
chromatography
using 100% dichloromethane as an eluent to afford the titled compound (4.0 g,
59%); 1H
NMR (400 MHz, DMSO-d6): 8 7.85 (s, 4H), 4.80 (d, J = 4.8 Hz, 1H), 4.03-4.00
(m, 1H),
3.96-3.93 (m, 2H), 1.14 (d, J= 6.0 Hz, 3H); LC-MS: m/z 222.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
159
Step-b: Synthesis of 1-(aminooxy)propan-2-ol hydrochloride
A solution of 2-(2-hydroxypropoxy)isoindoline-1,3-dione (2.0 g, 9.0 mmol) in 6
N aqueous
hydrochloric acid (15 mL) was stirred at RT for 16 h. The solid precipitated
in the reaction
mixture was removed by filtration and filtrate was concentrated under reduced
pressure to
afford the titled compound as hydrochloride salt (1.0 g), crude compound was
used in the
next step without further purification.
Step-c: Synthesis of 2-(benzo[d]oxazol-2-ylamino)-N-(2-hydroxypropoxy)-1-
methyl-1H-
benzo[d]imidazole-5-carboxamide
To a stirred solution of 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (100 mg, 0.32 mmol) in DMFA (5 mL) at 0 C was added N-
ethyldiisopropyl
amine (0.06 mL, 0.32 mmol) and HBTU (122 mg, 0.32 mmol). The reaction mixture
was
stirred for 30 min, followed by the addition of 1-(aminooxy)propan-2-ol
hydrochloride (41
mg, 0.32 mmol) and stirring was continued at RT for 16 h. Once the reaction
was completed
(monitored by TLC), the reaction mixture was diluted with cold water (20 mL)
and extracted
with ethyl acetate (2 X 50 mL). The combined organic layers were washed with
water (30
mL), brine solution (30 mL), dried over anhydrous sodium sulfate and
concentrated under
vacuum. The residue was purified by preparative HPLC method to afford the
titled compound
(35 mg, 28%).
Preparative HPLC purification method details:
DILUTION : THF: ACN: WATER
MOBILE PHASE A: 100% Water
MOBILE PHASE B: 100% ACETONITRILE
GRADIENT : 0/10, 6/20, 10/80
FLOW : 15m1/min
COLUMN : Luna (250 x 21.1 x 5i.tm)
1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 11.7 (bs, 1H), 8.01 (d, J = 1.2
Hz, 1H),
7.63 (dd, J = 1.2 Hz, J = 8.0 Hz, 1H), 7.48 (d, J = 8.8 Hz, 2H), 7.43 (d, J =
7.6 Hz, 1H), 7.21
(t, J = 7.6 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 4.90 (bs, 1H), 3.92-3.89 (m,
1H), 3.78-3.74 (m,
2H), 3.63 (s, 3H), 1.10 (d, J= 6.4 Hz, 3H); LC-MS: m/z 382.05 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
160
Example 121. Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-
(2-
(dimethylamino)acetamido)ethoxy)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
Nme,
0
0 0
0 0
/111 N¨O c _(1,, H2NN
N¨OH a 1111 N¨C)\¨\
NH2 TFA 1111 N-0 0
HN¨Boc 0 0
0 NMe2
0
Me
Me
110 --NH
HO
1110 N,¨NH Me2N
N N Ahh
0 0
0 N
Conditions: a) tert-butyl (2-hydroxyethyl)carbamate , DIAD, Triphenyl
phosphine, THF, 0 C - RT, 16 h; b) TFA, DCM, 0 C - RT, 3
h; c) dimethylglycine , HATU, DIPEA, DMF, 0 C - RT, 16 h; d) Hydrazine
hydrate, Methanol, reflux, 4 h; e) N-(2-(aminooxy)ethyl)-
2-(dimethylamino)acetamide, HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of tert-butyl (2-((1,3-dioxoisoindolin-2-
yl)oxy)ethyl)carbamate
To a stirred solution of 2-hydroxyisoindoline-1,3-dione (5.0 g, 30.67 mmol) in
THF (150
mL) at 0 C was added tert-butyl (2-hydroxyethyl)carbamate (4.93 g, 30.67 mmol)
and
triphenylphosphine (8.03 g, 30.67 mmol). The reaction mixture was stirred for
10 min
followed by the addition of diisopropyl azodicarboxylate (6.19 g, 30.67 mmol)
and continued
stirring at RT for 16 h. The reaction mixture was diluted with water (50 mL)
and extracted
with ethyl acetate (2 X 100 mL). The combined organic layers were washed with
water (50
mL), brine solution (50 mL), dried over anhydrous sodium sulfate and
concentrated under
vacuum. The residue was purified by combiflash column chromatography using 10%
ethyl
acetate in hexane as an eluent to afford the titled compound (12.0 g, semi
pure) which was
used in the next step without any further purification; 1H NMR (400 MHz, DMSO-
d6): 8 7.86
(s, 4H), 6.82 (bs, 1H), 4.13 (t, J= 5.6 Hz, 2H), 3.28-3.27 (m, 2H), 1.37 (s,
9H); LC-MS: m/z
207.0 (M-Boc) .
Step-b: Synthesis of 2-(2-aminoethoxy)isoindoline-1,3-dione trifluoroacetic
acid salt
To a stirred solution of tert-butyl (2-((1,3-dioxoisoindolin-2-
yl)oxy)ethyl)carbamate (12.0 g,
39.08 mmol) in DCM (150 mL) at 0 C was added trifluoroacetic acid (20 mL) and
stirred at
RT for 3 h. The reaction mixture was concentrated under vacuum, the residue
was stirred in
diethyl ether (50 mL) and the solid precipitated was filtered and dried under
vacuum to afford
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
161
the titled compound (3.8 g, 32%); 1H NMR (400 MHz, DMSO-d6): 8 8.05 (bs, 3H),
7.90 (s,
4H), 4.34 (t, J = 5.2 Hz, 2H), 3.38 (q, J = 7.2 Hz, 2H).
Step-c: Synthesis of 2-
(dimethylamino)-N-(2-((1,3-dioxoisoindolin-2-
yl)oxy)ethyl)acetamide
To a stirred solution of 2-(2-aminoethoxy)isoindoline-1,3-dione
trifluoroacetate (1.0 g, 4.85
mmol) in DMFA (15 mL) at 0 C was added dimethylglycine (499 mg, 4.85 mmol), N-
ethyldiisopropyl amine (1.69 mL, 9.7 mmol) and HATU (2.02 g, 5.34 mmol) and
the reaction
mixture was stirred at RT for 16 h. Once the reaction was completed (monitored
by TLC), the
reaction mixture was diluted with water (50 mL) and extracted with ethyl
acetate (2 X 50
mL). The combined organic layers were washed with water (30 mL), brine
solution (30 mL),
dried over anhydrous sodium sulfate and concentrated under vacuum. The residue
was
purified by combiflash column chromatography using 2% methanol in
dichloromethane as an
eluent to afford the titled compound (280 mg, 29%); LC-MS: m/z 292.1 (M+1) .
Step-d: Synthesis of N-(2-(aminooxy)ethyl)-2-(dimethylamino)acetamide
To a stirred solution of 2-
(dimethylamino)-N-(2-((1,3-dioxoisoindolin-2-
yl)oxy)ethyl)acetamide (280 mg, 0.96 mmol) in methanol (5 mL) at RT was added
hydrazine
hydrate (0.3 mL) and refluxed for 4 h. The reaction mixture was cooled to RT
and
concentrated on rotary evaporator. The residue obtained was stirred in diethyl
ether (30 mL)
and filtered. The filtrate was concentrated under vacuum to afford the titled
compound (120
mg, 77%); 1H NMR (400 MHz, CDC13): 8 7.39 (bs, 1H), 5.50 (bs, 2H), 3.73 (t, J
= 5.2 Hz,
2H), 3.57-3.47 (m, 2H), 2.96 (s, 2H), 2.29 (s, 6H).
Step-e: Synthesis of 2-(benzo [d] oxazol-2-ylamino)-N-(2-(2-
(dimethylamino)acetamido)ethoxy)-1-methy1-1H-benzo [d] imidazole-5-carboxamide
To a stirred solution of 2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazole-5-
carboxylic acid (80 mg, 0.26 mmol) in DMFA (2 mL) at 0 C was added N-
ethyldiisopropyl
amine (0.04 mL, 0.26 mmol) and HBTU (98 mg, 0.26 mmol). The reaction mixture
was
stirred for 30 min, followed by the addition of N-(2-(aminooxy)ethyl)-2-
(dimethylamino)acetamide (41 mg, 0.26 mmol). The reaction mixture was then
stirred at RT
for 16 h. Once the reaction was completed (monitored by TLC), the reaction
mixture was
diluted with cold water (15 mL) and extracted with ethyl acetate (2 X 20 mL).
The aqueous
layer was concentrated under vacuum. The residue obtained was purified by
preparative
HPLC method to afford the titled compound (10 mg, 9%).
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
162
Preparative HPLC purification method details:
DILUTION : THF: ACN: WATER
MOBILE PHASE A: 10 mM aqueous ammonium acetatae
MOBILE PHASE B: 100% ACETONITRILE
GRADIENT : T/%B: 0/10, 10/50, 17/65, 19/90
FLOW : 15m1/min
COLUMN : Phenomenex Luna (250 x 21.2 x 5i.tm)
1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 11.60 (bs, 1H), 8.01 (bs, 2H),
7.64 (d, J=
8.8 Hz, 1H), 7.49-7.42 (m, 3H), 7.20 (t, J = 8.0 Hz, 1H), 7.12 (t, J = 8.0 Hz,
1H), 3.90 (s,
2H), 3.63 (s, 3H), 3.41 (q, J = 6.0 Hz, 2H), 2.89 (s, 2H), 2.32 (s, 6H); LC-
MS: m/z 452.2
(M+1) .
Example 122. Synthesis of N-(2-(dimethylamino)ethoxy)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
0
a
N-0 H SI N-0\ \b 112 IN
,
NMe2
0 0 NMe2
Me =Nre
N
H 1)--NH
HO WI N
N
0
0 N
W
3
CF3
Conditions: a) 1-Chloro-2-dimethylaminoethane hydrochloride, TEA, DMF, 60 C,
16 h; b)
Hydrazine hydrate, Me0H, reflux, 4 h; c) 2-(aminooxy)- N,N-dimethylethan-1-
amine , HBTU,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-(2-(dimethylamino)ethoxy)isoindoline-1,3-dione
The title compound was synthesized using the same procedure which was followed
for
Example 119 Step-a using N-hydroxyphthalimide and 1-chloro-2-
dimethylaminoethane
hydrochloride as starting materials and 4 equivalents of triethyl amine
(Yield: 17%); 1H
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
163
NMR (400 MHz, DMSO-d6): 8 7.86 (s, 4H), 4.21 (t, J = 5.4 Hz, 2H), 2.61 (t, J =
5.4 Hz, 2H),
2.18 (s, 6H); LC-MS: m/z 235.1 (M+1) .
Step-b: Synthesis of 2-(aminooxy)-N,N-dimethylethan-1-amine
The title compound was synthesized using the same procedure which was followed
for
Example 119 Step-b using 2-(2-(dimethylamino)ethoxy)isoindoline-1,3-dione as
starting
material (Yield: 34%); 1H NMR (400 MHz, DMSO-d6): 8 5.88 (bs, 2H), 3.58 (t, J
= 5.9 Hz,
2H), 2.38 (t, J= 5.9 Hz, 2H), 2.13 (s, 6H); LC-MS: m/z 105.20 (M+1) .
Step-c: Synthesis of N-(2-(dimethylamino)ethoxy)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid and 2-
(aminooxy)-N,N-dimethylethan-1-amine as
starting materials (Yield: 15%); 1H NMR (400 MHz, DMSO-d6): 8 12.40 (bs, 2H),
8.01 (s,
1H), 7.82 (s, 1H), 7.66 (d, J = 1.5 Hz, 1H), 7.64 (d, J = 1.5 Hz, 1H), 7.61-
7.51 (m, 2H), 3.99
(t, J = 5.4 Hz, 2H), 3.66 (s, 3H), 2.55 (t, J = 5.3 Hz, 2H), 2.22 (s, 6H); LC-
MS: m/z 463.50
(M+1) .
Example 123. Synthesis of 2-(benzo[d]oxazol-2-ylamino)-N-(2-
(dimethylamino)ethoxy)-
1-methyl-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-(aminooxy)-N,N-dimethylethan-1-amine and 2-(benzo [d]
oxazol-
2-ylamino)-1-methy1-1H-benzo [d] imidazole-5-carboxylic acid as starting
materials.
1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 8.00 (s, 1H), 7.63 (d, J = 8.4
Hz, 1H), 7.48
(d, J = 8.4 Hz, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.21 (t, J = 7.2 Hz, 1H), 7.12
(t, J = 7.6 Hz, 1H),
3.99 (t, J= 6.0 Hz, 2H), 3.82 (s, 3H), 2.56 (t, J= 5.2 Hz, 2H), 2.32 (s, 6H);
LC-MS: m/z
395.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
164
Example 124. Synthesis of N-(2-methoxyethoxy)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
Me Me
a H
HO = Me00,N1 =
N
0
0 N
cp CF3
3
Conditions: a) 0-(2-methoxyethyl)hydroxylamine, DPPA, DIPEA, DMF, 0 C - RT, 16
h
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methyl-2-((6-(trifluoromethyl)benzo [d]oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid and 0-(2-methoxyethyl)hydroxylamine as
starting
materials (Yield: 28%); 1H NMR (400 MHz, DMSO-d6): 8 12.39 (bs, 1H), 11.72 (s,
1H),
8.03 (s, 1H), 7.83 (s, 1H), 7.67 (d, J = 8.3 Hz, 1H), 7.61 (d, J = 8.3 Hz,
1H), 7.57-7.52 (m,
2H), 4.04 (t, J = 4.4 Hz, 2H), 3.66 (s, 3H), 3.59 (t, J = 4.4 Hz, 2H), 3.30
(s, 3H merged in
DMS0); LC-MS: m/z 450.15 (M+1) .
Example 125. Synthesis of N-(2-hydroxyethoxy)-1-methyl-24(6-
(trifluoromethyl)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
M
Me e
¨NH =
HO )7_0 H0(:),N N 0
N N
0 0
CF3 CF3
Conditions: a) 2-(aminooxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid and 2-(aminooxy)ethan-1-ol as starting
materials
(Yield: 51%); 1H NMR (400 MHz, DMSO-d6): 8 12.39 (bs, 1H), 11.73 (s, 1H), 8.04
(s, 1H),
7.84 (s, 1H), 7.68 (d, J = 8.3 Hz, 1H), 7.61 (d, J = 8.3 Hz, 1H), 7.57-7.53
(m, 2H), 4.78 (s,
1H), 3.94 (t, J= 4.9 Hz, 2H), 3.67 (s, 3H), 3.66-3.63 (m, 2H); LC-MS: m/z
436.15 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
165
Example 126. Synthesis of N-(2-hydroxyethoxy)-1-methyl-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
fyle fyle
N
N
¨NH N 0 --NH
HO 40 ¨ipa - HO.,7 -N
/ 0 N/
N 0
0
W 10 0 C F3
0 C F3
Conditions: a) 2-(aminooxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-(trifluoromethoxy)benzo [d]oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylic acid and 2-(aminooxy)ethan-1-ol as starting
materials
(Yield: 58%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 11.72 (s, 1H), 8.02
(d, J =
1.0 Hz, 1H), 7.66 (d, J = 6.9 Hz, 1H), 7.58 (s, 1H), 7.53-7.51 (m, 2H), 7.22
(d, J = 8.3 Hz,
1H), 4.78 (t, J = 5.6 Hz, 1H), 3.94 (t, J = 4.7 Hz, 2H), 3.64-3.62 (m, 5H); LC-
MS: m/z 451.9
(M+1) .
Example 127. Synthesis of N-(2-methoxyethoxy)-1-methyl-24(6-
(trifluoromethoxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
0
Me
Me
IV IV
HO 40/>---NH
N ,.._0 .. Me00.11
N
0
0 N, WI OCF3
OCF3
Conditions: a) 0-(2-methoxyethyphydro4amine , DPPA, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
compound li using 1-methyl-2-((6-(trifluoromethoxy)benzo [d]oxazol-2- yl)
amino)- 1H-
benzo [d] imidazole-5-carboxylic acid and 0-(2-methoxyethyl)hydroxylamine as
starting
materials (Yield: 28%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H), 11.70 (s,
1H),
8.01 (d, J= 1.4 Hz, 1H), 7.65 (dd, J= 1.5 Hz, J= 8.3 Hz, 1H), 7.58 (d, J= 1.5
Hz, 1H), 7.53-
7.49 (m, 2H), 7.22 (dd, J = 1.5 Hz, J = 8.3 Hz, 1H), 4.05-4.02 (m, 2H), 3.64
(s, 3H), 3.61-
3.58 (m, 2H), 3.31 (s, 3H); LC-MS: m/z 466.0 (M-F1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
166
Example 128. Synthesis of N-(2-hydroxyethoxy)-2-06-(2-
methoxyethoxy)benzo[d]oxazol-2-yl)amino)-1-methyl-1H-benzo[d] imidazole-5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-(aminooxy)ethan-1-ol and 2-((6-(2-
methoxyethoxy)benzo [d]oxazol-2-yl)amino)-1-methyl-lH-benzo [d] imidazo le-5-
carboxylic
acid as starting materials. 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 11.68
(s, 1H),
7.98 (s, 1H), 7.63 (d, J= 8.0 Hz, 1H), 7.46 (d, J= 8.4 Hz, 1H), 7.35 (d, J=
8.8 Hz, 1H), 7.12
(s, 1H), 6.82 (d, J= 8.8 Hz, 1H), 4.78 (s, 1H), 4.11 (bs, 2H), 3.93 (bs, 2H),
3.67-3.61 (m,
7H), 3.32 (s, 3H merged with DMSO moisture peak); LC-MS: m/z 442.2 (M+1) .
Example 129. Synthesis of N-(2-hydroxyethoxy)-2-06-(2-
hydroxyethoxy)benzo[d]oxazol-2-yl)amino)-1-methyl-1H-benzo[d] imidazole-5-
carboxamide
Me
Me Atm.
Atm, N
NI( N -/--C) a
N NI( -/--C)
HO OH HO/ O' =
0
0
WI 0
0
Conditions: a) 2-(aminooxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-(2-hydroxyethoxy)benzo [d] oxazol-2-yl)amino)-1-
methyl-1H-
benzo[d]imidazole-5-carboxylic acid and 2-(aminooxy)ethan-l-ol as starting
materials. The
crude product was purified by combiflash chromatography using 10% methanol in
DCM as
an eluent to afford the title compound (Yield: 14%); 1H NMR (400 MHz, DMSO-
d6): 8 12.15
(bs, 1H), 11.67 (s, 1H), 7.98 (s, 1H), 7.63 (d, J = 7.6 Hz, 1H), 7.45 (d, J =
8.8 Hz, 1H), 7.35
(d, J= 8.4 Hz, 1H), 7.11(s, 1H), 6.82 (d, J= 8.8 Hz, 1H), 4.83 (bs, 1H), 4.77
(bs, 1H), 4.01 (t,
J = 4.4 Hz, 2H), 3.93 (t, J = 4.4 Hz, 2H), 3.72 (bs, 2H), 3.61 (bs, 5H); LC-
MS: m/z 428.2
(M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
167
Example 130. Synthesis of 2-05-fluoro-6-(trifluoromethyl)benzo[d]oxazol-2-
yl)amino)-
N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-5-carboxamide
Me ye
* .
NrNi%_0
HO a ,N
N dr
1111-11, HO-.../--0
N
0 CF3 0 Air
W CF3
F F
Conditions: a) 2-(aminooxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((5-fluoro-6-(trifluoromethyl)benzo [d] oxazol-2-
yl)amino)-1-
methyl-1H-benzo [d] imidazole-5-carboxylic acid and 2-(aminooxy)ethan-1-ol as
starting
materials (Yield: 61%); 1H NMR (400 MHz, DMSO-d6): 8 12.45 (bs, 1H), 11.74 (s,
1H),
8.03 (s, 1H), 7.85 (d, J = 5.6 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.55 (d, J =
8.0 Hz, 1H), 7.45
(d, J= 11.2 Hz, 1H), 4.50 (bs, 1H), 3.94 (t, J= 4.4 Hz, 2H), 3.66 (s, 3H),
3.64-3.63 (m, 2H);
LC-MS: m/z 454.1 (M+1) .
Example 131 Synthesis of N-(2-hydroxyethoxy)-1-methyl-2-((6-methyl-4,5,6,7-
tetrahydrobenzo[d] thiazol-2-yl)amino)-1H-benzo [d] imidazole-5-carboxamide
Me Me
N
NH --- 0 N-1 NH
a HO_NH
1110 N '0- N >----s
HO2C Nic\:),. 0 Nt
Me
M e
Conditions: a) 2-(aminooxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methy1-2-((6-methyl-4,5,6,7-tetrahydrobenzo [d]
thiazol-2-
yl)amino)-1H-benzo [d] imidazo le-5-carboxylic acid and 2-(aminooxy)ethan-l-ol
as starting
materials (Yield: 43%); 1H NMR (400 MHz, DMSO-d6): 8 12.05 (bs, 1H), 11.56 (s,
1H),
7.83 (s, 1H), 7.55 (dd, J = 1.0 Hz, J = 8.3 Hz, 1H), 7.35 (d, J = 8.3 Hz, 1H),
4.78 (bs, 1H),
3.92 (t, J = 4.9 Hz, 2H), 3.63-3.61 (m, 5H), 2.67-2.59 (m, 1H), 2.50 ( 2H
merged with
CA 03135615 2021-09-29
WO 2020/210339
PCT/US2020/027240
168
DMSO peak), 2.18-2.11 (m, 1H), 1.87-1.84 (m, 2H), 1.46-1.43 (m, 1H), 1.05 (d,
J= 6.3 Hz,
3H); LC-MS: m/z 402.2 (M+1) .
Example 132. Synthesis of 2-46-acetylbenzo [d] oxazol-2-yl)amino)-N-(2-
methoxyethoxy)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
0 0 0 0 OH
Me laao me OH b me = 0/)_sH c me niti 0/)_sme d me 0/>_sme e
ON
NH N N N
Me Me
OTBS OTBS sr NH 0 NH
Me = />¨SMe f Me = / ¨S02Me g N = rC)
Et0 N Et0 N me
0 OTBS 0 OH
Me
Me Me
../r NH 0
N ,N
Et0 N = me
HO N me 0
0 N
0 0 0 0 eip Me
0
Conditions: a) 10% Aq. NaOH Solution, Reflux, 4 h; b) Potassium ethyl
xanthate, Et0H, Reflux, 16 h; c) K2CO3, Mel, ACN, RT, 3
h; d) NaBH4, Me0H, 0 C - RT, 1 h; e) TBDMSCI, Imidazole, DCM, 0 C - RT, 2 h;
f) m-CPBA, DCM, 0 C - RT, 4 h; g) NaH, ethyl
2-amino-1-methyl-1H-benzo[d]imidazole-5-carboxylate, 1,4-Dioxane, RT, 16 h; h)
TBAF in THF, THF, 0 C - RT,16 h; 0 Dess-
martin periodinane, DCM, 0 C - RT, 1 h; j) LiOH H20, THF, Et0H, H20, 60 C, 16
h; k) 0-(2-methoxyethyl)hydroxylamine, HBTU,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 1-(4-amino-3-hydroxyphenyl)ethan-1-one
A mixture of 6-acetylbenzo [d] oxazol-2(3H)-one (5.0 g, 28.2 mmol) in 10%
aqueous sodium
hydroxide (50 mL) was refluxed for 4 h. The reaction mixture was cooled to RT,
acidified
with 3 N HC1 followed by basification to pH -8 with saturated sodium carbonate
solution.
The precipitated solid was filtered and dried under vacuum afford the title
compound (4.1 g,
98%); 1H NMR (400 MHz, DMSO-d6): 8 9.34 (s, 1H), 7.28 (dd, J = 2.0 Hz, J = 8.4
Hz, 1H),
7.22 (d, J= 2.0 Hz, 1H), 6.58 (d, J= 8.4 Hz, 1H), 5.44 (s, 2H), 2.36 (s, 3H).
Step-b: Synthesis of 1-(2-mercaptobenzo [d] oxazol-6-ypethan-1-one
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 1-(4-amino-3-hydroxyphenyl)ethan-1-one (Yield: 100%);
1H NMR
(400 MHz, DMSO-d6): 8 14.2 (bs, 1H), 8.04 (s, 1H), 7.94 (d, J = 8.0 Hz, 1H),
7.33 (d, J = 8.0
Hz, 1H), 2.60 (s, 3H); LC-MS: m/z 192.02 (M-1)-.
Step-c: Synthesis of 1-(2-(methylthio)benzo [d] oxazol-6-ypethan-1-one
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
169
The title compound was synthesized using the same procedure which was followed
for
compound lb using 1-(2-mercaptobenzo[d]oxazol-6-yl)ethan-1-one as starting
material
(Yield: 78%); 1H NMR (400 MHz, DMSO-d6): 8 8.22 (s, 1H), 7.98 (d, J = 8.4 Hz,
1H), 7.73
(d, J = 8.0 Hz, 1H), 2.80 (s, 3H), 2.67 (s, 3H).
Step-d: Synthesis of l-(2-(methylthio)benzo [d] oxazol-6-yDethan-1-ol
To a stirred solution of 1-(2-(methylthio)benzo [d] oxazol-6-yl)ethan-1-one
(4.1 g, 19.8 mmol)
in methanol (80 mL) at 0 C was added sodium borohydride (1.12 g, 29.7 mmol)
and the
reaction mixture was stirred for 1 h. The reaction mixture was quenched with
cold water (100
mL) and extracted with Et0Ac (2 X 150 mL). The combined organic layers were
washed
with water (50 mL), brine (50 mL), dried over anhydrous sodium sulfate and
concentrated
under vacuum to afford the titled compound (4.1 g, 100%); 1H NMR (400 MHz,
DMSO-d6):
8 7.57-7.53 (m, 2H), 7.31 (dd, J = 1.2 Hz, J = 8.0 Hz, 1H), 5.28 (d, J = 3.6
Hz, 1H), 4.85-
4.80 (m, 1H), 2.74 (s, 3H), 1.35 (d, J= 6.4 Hz, 3H).
Step-e: Synthesis of 6-(l-((tert-butyldimethylsilyl)oxy)ethyl)-2-
(methylthio)benzo [d] oxazole
To a stirred solution of 1-(2-(methylthio)benzo [d] oxazol-6-yl)ethan-1-ol
(4.1 g, 19.6 mmol)
in DCM (80 mL) at 0 C was added imidazole (2.0 g, 29.4 mmol) and stirred for 5
min
followed by the addition of TBDMSC1 (3.5 g, 23.5 mmol) and it was stirred at
RT for 2 h.
The reaction mixture was diluted with water (100 mL) and extracted with DCM (2
X 150
mL). The combined organic layers were washed with water (50 mL), brine (50
mL), dried
over anhydrous sodium sulfate and concentrated under vacuum to afford the
titled compound
(6.6 g, 100%); 1H NMR (400 MHz, DMSO-d6): 8 7.63-7.61 (m, 2H), 7.36 (d, J =
8.0 Hz,
1H), 5.08 (q, J = 6.4 Hz, 1H), 2.80 (s, 3H), 1.42 (d, J = 6.0 Hz, 3H), 0.91
(s, 9H), 0.2 (s, 6H);
LC-MS: m/z 324.1 (M+1) .
Step-f: Synthesis of 6-(l-((tert-butyldimethylsilyl)oxy)ethyl)-2-
(methylsulfony1)-
benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lc using 6-(1-((tert-butyldimethylsilyl)oxy)ethyl)-2-
(methylthio)benzo[d]oxazole
as starting material and stirred for 4 h (Yield: 100%); 1H NMR (400 MHz, DMSO-
d6): 8
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
170
7.96-7.49 (m, 3H), 5.14-5.11 (m, 1H), 3.66 (s, 3H), 1.40 (d, J= 6.0 Hz, 3H),
0.88 (s, 9H), 0.2
(s, 3H), -0.2 (s, 3H).
Step-g: Synthesis of ethyl 24(6-(1-((tert-butyldimethylsilypoxy)ethyl)benzo
[d] oxazol-2-
yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-l-methy1-1H-benzo [d] imidazole-5-carboxylate
and 6-(1-
((tert-butyldimethylsilyl)oxy)ethyl)-2-(methylsulfonyl)benzo [d] oxazole as
starting materials
(Yield: 31%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.23 (d, J= 1.6 Hz,
1H), 7.87
(dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.51 (d, J = 8.8 Hz, 1H), 7.42-7.39 (m, 2H),
7.20 (d, J = 8.0
Hz, 1H), 5.0 (d, J = 6.4 Hz, 1H), 4.32 (q, J = 7.6 Hz, 2H), 3.63 (s, 3H), 1.40-
1.33 (m, 6H),
0.87 (s, 9H), 0.001 (s, 3H), -0.036 (s, 3H).
Step-h: Synthesis of ethyl 24(6-(1-hydroxyethyl)benzo [d] oxazol-2-yl)amino)-1-
methyl-
1H-benzo [d] imidazole-5-carboxylate
To a stirred solution of ethyl 2-((6-(1-((tert-
butyldimethylsilyl)oxy)ethyl)benzo [d] oxazol-2-
yl)amino)-1-methy1-1H-benzo [d] imidazole-5-carboxylate (1.4 g, 2.8 mmol) in
THF (30 mL)
at 0 C was added 1 M TBAF in THF (5.7 mL, 5.7 mmol) and the reaction mixture
was stirred
at RT for 16 h. The reaction mixture was quenched with saturated ammonium
chloride
solution (50 mL) and extracted with Et0Ac (2 X 100 mL). The combined organic
layers were
washed with water (30 mL), brine (30 mL), dried over anhydrous sodium sulfate
and
concentrated under vacuum. The residue was stirred in pentane for 15 min and
solid obtained
was filtered and dried under vacuum to afford the titled compound (1.1 g,
100%); 1H NMR
(400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 8.23 (s, 1H), 7.87 (d, J= 8.4 Hz, 1H),
7.51 (d, J= 8.4
Hz, 1H), 7.41-7.39 (m, 2H), 7.19 (d, J = 7.6 Hz, 1H), 5.16 (bs, 1H), 4.80 (bs,
1H), 4.33 (q, J
= 7.2 Hz, 2H), 3.63 (s, 3H), 1.40-1.35 (m, 6H); LC-MS: m/z 381.15 (M+1) .
Step-i: Synthesis of ethyl 2-06-acetylbenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
To a stirred solution of ethyl 2-((6-(1-hydroxyethyl)benzo [d] oxazol-2-
yl)amino)-1-methyl-
1H-benzo [d] imidazole-5-carboxylate (1.1 g, 2.9 mmol) in DCM (30 mL) at 0 C
was added
Dess-martin periodinane (1.47 g, 3.5 mmol) and the reaction mixture was
stirred for 1 h. The
reaction mixture was diluted with DCM (100 mL), water (50 mL). The organic
layer was
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
171
washed with saturated sodium bicarbonate solution (2 X 50 mL), water (50),
brine (30 mL),
dried over anhydrous sodium sulfate and concentrated under vacuum to afford
the titled
compound (540 mg, 49%); 1H NMR (400 MHz, DMSO-d6): 8 12.4 (bs, 1H), 8.26 (s,
1H),
8.00 (s, 1H), 7.91-7.89 (m, 2H), 7.58-7.51 (m, 2H), 4.34 (q, J = 6.8 Hz, 2H),
3.87 (s, 3H),
2.66 (s, 3H), 1.35 (t, J= 6.8 Hz, 3H); LC-MS: m/z 379.15 (M-F1) .
Step-j: Synthesis of 2-((6-acetylbenzo [d] oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] -
imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 2-((6-acetylbenzo [d]oxazol-2-yl)amino)-1-
methyl-1H-
benzo [d] imidazole-5-carboxylate as starting material (Yield: 68%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.8 (bs, 1H), 12.4 (s, 1H), 8.21 (s, 1H), 7.99 (s, 1H), 7.82-7.79
(m, 2H), 7.59-
7.52 (m, 2H), 3.67 (s, 3H), 2.66 (s, 3H); LC-MS: m/z 351.1 (M+1) .
Step-k: Synthesis of 2-((6-acetylbenzo [d] oxazol-2-yl)amino)-N-(2-
methoxyethoxy)-1-
methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-((6-acetylbenzo[d]oxazol-2-yl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylic acid and 0-(2-methoxyethyl)hydroxylamine as
starting
materials (Yield: 42%); 1H NMR (400 MHz, DMSO-d6): 8 12.4 (bs, 1H), 11.7 (s,
1H), 8.02-
7.99 (s, 2H), 7.99 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.68-7.63 (m, 1H), 7.54-
7.51 (m, 2H),
4.04 (t, J = 4.4 Hz, 2H), 3.66 (s, 3H), 3.60 (t, J = 4.4 Hz, 2H), 3.22 (s,
3H), 2.60 (s, 3H); LC-
MS: m/z 424.15 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
172
Example 133. Synthesis of N-(2-hydroxyethyl)-24(6-(2-hydroxypropan-2-
yl)benzo [d] oxazol-2-yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
Me Me Me
NI 1
sir rE,,_0
N H * Nr' ')F
)F0
H Nr0
HO N a ap
Me
TBSO"---/N
Me TBSO"----"N 0 N
Me
0 0
0 OH
0 Me
Me
H NrNEI)F0
HO"----"N
0 N
Me
OH
Me
Conditions: a) 2-((tert-butyldimethylsilypoxy)ethan-1-amine, HBTU, DIPEA, DMF,
0 C - RI, 16 h; b) CH3MgBr in diethyl ether,
THF, 0 C, 30 min; c) TBAF in THF, THF, 0 C - RI, 4 h
Step-a: Synthesis of 2-((6-acetylbenzo [d] oxazol-2-yl)amino)-N-(2-((tert-
butyldimethylsilypoxy)ethyl)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e
using 2-((6- acetylbenzo [d] o xazol-2- yl)amino )- 1-methyl- 1H-
benzo [d] imidazole-5-carboxylic acid and 2-((tert-
butyldimethylsilyl)oxy)ethan-1-amine as
starting materials (Yield: 48%); LC-MS: m/z 508.25 (M+1) .
Step-b: Synthesis of N-(2-((tert-butyldimethylsilypoxy)ethyl)-2-((6-(2-
hydroxypropan-2-
yl)benzo [d] oxazol-2-yl)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
To a stirred solution of
2-((6-acetylbenzo [d] oxazol-2-yl)amino)-N-(2-((te rt-
butyldimethyls ilyl)o xy)ethyl)- 1-methyl- 1H-benzo [d] imidazo le-5-c arbo
xamide (200 mg, 0.39
mmol) in THF (5 mL) at 0 C was added 3 M methyl magnesium bromide in diethyl
ether
(0.13 mL, 0.39 mmol) and the reaction mixture was stirred for 30 min. The
reaction mixture
was quenched with saturated aqueous ammonium chloride solution (20 mL) and
extracted
with ethyl acetate (2 X 50 mL). The combined organic layers were washed with
brine (30
mL), dried over anhydrous sodium sulfate and concentrated under vacuum to
afford the titled
compound (100 mg, crude); LC-MS: m/z 524.3 (M+1) .
Step-c: Synthesis of N-(2-hydroxyethyl)-24(6-(2-hydroxypropan-2-
yl)benzo[d]oxazol-2-
y1)amino)-1-methyl-1H-benzo [d] imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
173
The title compound was synthesized using the same procedure which was followed
for
Example 132 Step-h using N-(2-((tert-butyldimethylsilyl)oxy)ethyl)-2-((6-(2-
hydroxypropan-2-y1)benzo [d] oxazol-2-yl)amino)-1-methyl-1H-benzo [d]
imidazole-5-
carboxamide as starting material and stirring for 4 h. Crude product was
purified by
preparative HPLC method (Yield: 6%).
Preparative HPLC purification method details:
DILUTION : ACN: WATER
MOBILE PHASE A: 0.1% formic acid in Water
MOBILE PHASE B: 100% ACETONITRILE
GRADIENT : 0/10,2/10,6/100,10/100, 11/10, 12/10
FLOW : 1.0m1/min
COLUMN : Kinetex C-18 (250 x 21.1 x 5i.tm)
1H NMR (400 MHz, DMSO-d6): 8 12.23 (bs, 1H), 8.35 (bs, 1H), 8.06 (s, 1H), 7.76
(d, J = 6.8
Hz, 1H), 7.51 (s, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H),
7.30 (d, J = 8.4 Hz,
1H), 5.03 (bs, 1H), 4.73 (bs, 1H), 3.62 (s, 3H), 3.53 (bs, 2H), 3.35-3.34 (m,
2H), 1.47 (s, 6H);
LC-MS: m/z 410.2 (M+1) .
Example 134. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methy1-2-((6-(thiazol-
4-
yObenzo[d]oxazol-2-yDamino)-1H-benzo[d]imidazole-5-carboxamide
0= f=--N
0c) a Br s s s
,0 0 la OH d OsEl
N
NH2 4110" N
Me Me
Et0 40 Ni ¨NH
S 7 0 f s = N,¨so2me N )7-0 h HO 10 N )7-0
= N¨SMe 0 N 0 N
N
I I
Asa N H
H irN)r
0 N
Conditions: a) AlC13,DMF, 2-bromoacetyl chloride, 0 C - 75 C, 2.5 h; b)
Formamide, P2S5, 1,4-Dioxane, Reflux, 18 h; c) NaOH, H20,
Reflux, 16 h; d) Potassium ethyl xanthate, Et0H, Reflux, 16 h; c) K2CO3, Mel,
ACN, RT, 4 h; f) m-CPBA, DCM, 0 C - RT, 4 h; g) NaH,
ethyl 2-amino-1-methyl-1H-benzo[d]imidazole-5-carboxylate, 1,4-Dioxane, RT, 16
h; h) LiON H20, THF, Et0H, H20, 60 C, 16 h; k) 2-
(2-aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 6-(2-bromoacetyl)benzo[d]oxazol-2(3H)-one
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
174
DMFA (5.75 mL, 74 mmol) was added to dropwise to A1C13 (34.5 g, 259.3 mmol)
and the
mixture was stirred at 45 C for 30 min. Then benzo[d]oxazol-2(3H)-one (5.0 g,
37 mmol)
and 2-bromoacetyl chloride (4.6 mL, 55.5 mmol) was added to the reaction
mixture and
stirred at 75 C for 2 h. The reaction mixture was cooled to RT and poured over
ice and stirred
for 10 min. The precipitated solid was filtered and dried under vacuum to
afford the titled
compound (4.0 g, 42%); 1H NMR (400 MHz, DMSO-d6): 8 12.13 (bs, 1H), 7.90-7.87
(m,
2H), 7.23 (d, J = 7.6 Hz, 1H), 4.89 (s, 2H); LC-MS: m/z 253.9 (M-1)-.
Step-b: Synthesis of 6-(thiazol-4-yl)benzo [d] oxazol-2(3H)-one
To a stirred solution of formamide (5.49 mL, 137.7 mmol) in 1,4-dioxane (100
mL) at RT
was added phosphorus pentasulfide (6.1 g, 27.5 mmol) and heated at 100 C for 2
h. The solid
precipitated was removed by filtration and the filtrate was added to 6-(2-
bromoacetyl)benzo [d] oxazol-2(3H)-one (13.5, 13.7 mmol). The reaction mixture
was stirred
at 100 C for 16 h and concentrated on rotary evaporator to remove 1,4-dioxane.
The residue
was diluted with ethyl acetate (150 mL), water (100 mL) and extracted. Organic
layer was
washed with water (50 mL), brine (50 mL), dried over anhydrous sodium sulfate
and
concentrated under vacuum. The residue was purified by combiflash
chromatography using
30% ethyl acetate in hexane as an eluent to afford the title compound (1.2 g,
40%); 1H NMR
(400 MHz, DMSO-d6): 8 11.72 (s, 1H), 9.18 (d, J = 1.6 Hz, 1H), 8.12 (d, J =
1.2 Hz, 1H),
7.88 (s, 1H), 7.83 (d, J= 8.0 Hz, 1H), 7.15 (d, J= 8.0 Hz, 1H); LC-MS: m/z
219.0 (M+1) .
Step-c: Synthesis of 2-amino-5-(thiazol-4-yl)phenol
To a stirred solution of 6-(thiazol-4-yl)benzo [d] oxazol-2(3H)-one (600 mg,
2.75 mmol) in
water (6 mL) at RT was added sodium hydroxide (1.09 g, 27.5 mmol) and heated
at 100 C
for 16 h. The reaction mixture was cooled to RT, quenched with saturated
ammonium
chloride solution and extracted with ethyl acetate (100 mL). Organic layer was
washed with
water brine (50 mL), dried over anhydrous sodium sulfate and concentrated
under vacuum to
afford the title compound (400 mg, 76%); 1H NMR (400 MHz, DMSO-d6): 8 9.13
(bs, 1H),
9.06 (d, J = 1.2 Hz, 1H), 7.65 (d, J = 1.6 Hz, 1H), 7.31 (d, J = 1.2 Hz, 1H),
7.19 (d, J = 7.6
Hz, 1H), 6.61 (d, J= 8.4 Hz, 1H), 4.71 (bs, 2H); LC-MS: m/z 193.0 (M+1) .
Step-d: Synthesis of 6-(thiazol-4-yl)benzo [d] oxazole-2(3H)-thione
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
175
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-(thiazol-4-yl)phenol (Yield: 92%); 1H NMR
(400 MHz,
DMSO-d6): 8 13.99 (s, 1H), 9.22 (d, J = 1.6 Hz, 1H), 8.23 (d, J = 1.6 Hz, 1H),
8.10 (d, J =
1.2 Hz, 1H), 7.99 (dd, J = 1.6 Hz, J = 8.4 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H);
LC-MS: m/z
234.95 (M+1) .
Step-e: Synthesis of 2-(methylthio)-6-(thiazol-4-yl)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lb using 6-(thiazol-4-yl)benzo [d] oxazole-2(3H)-thione as starting
material stirring
for 4 hours (Yield: 84%); 1H NMR (400 MHz, DMSO-d6): 8 9.21 (bs, 1H), 8.24-
8.23 (m,
2H), 8.01 (d, J = 8.4 Hz, 1H), 8.69 (d, J = 8.4 Hz, 1H), 2.78 (s, 3H); LC-MS:
m/z 249.0
(M+1) .
Step-f: Synthesis of 2-(methylsulfony1)-6-(thiazol-4-y1)benzo [d] oxazole
The title compound was synthesized using the same procedure which was followed
for
compound lc using 2-(methylthio)-6-(thiazol-4-yl)benzo[d]oxazole as starting
material and
stirring for 4 h. Crude compound was used in the next step without any
analytical data.
Step-g: Synthesis of ethyl l-methyl-2-46-(thiazol-4-yl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
compound lg using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-carboxylate
and 2-
(methylsulfony1)-6-(thiazol-4-y1)benzo [d] oxazole as starting materials
(Yield: 26%); 1H
NMR (400 MHz, DMSO-d6): 8 12.4 (bs, 1H), 9.20 (d, J = 4.4 Hz, 1H), 8.25 (s,
1H), 8.22 (s,
1H), 8.08 (s, 1H), 7.92-7.87 (m, 2H), 7.54-7.50 (m, 2H), 4.33 (q, J = 7.6 Hz,
2H), 3.66 (s,
3H), 1.35 (t, J = 6.8 Hz, 3H); LC-MS: m/z 420.05 (M+1) .
Step-h: Synthesis of l-methyl-24(6-(thiazol-4-yl)benzo[d]oxazol-2-y1)amino)-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methy1-24(6-(thiazol-4-yl)benzo[d]oxazol-2-y1)amino)-
1H-
benzo[d]imidazole-5-carboxylate as starting material and stirring for 5 h
(Yield: 71%); 1H
CA 03135615 2021-09-29
WO 2020/210339
PCT/US2020/027240
176
NMR (400 MHz, DMSO-d6): 8 9.16 (s, 1H), 8.00 (s, 1H), 7.95 (s, 1H), 7.81 (s,
1H), 7.77 (d,
J = 8.0 Hz, 1H), 7.66 (d, J = 8.4 Hz, 1H), 7.20 (d, J = 7.6 Hz, 1H), 7.06 (d,
J = 8.4 Hz, 1H),
3.59 (s, 3H); LC-MS: m/z 392.1 (M+1) .
Step-i: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-(thiazol-4-
yl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2((6-(thiazol-4-yl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-ol as
starting materials.
Crude product was purified by combiflash chromatography using 20% Methanol in
DCM as
an eluent (Yield: 28%); 1H NMR (400 MHz, DMSO-d6): 8 12.3 (bs, 1H), 9.19 (s,
1H), 8.45
(bs, 1H), 8.13-8.04 (m, 3H), 7.91 (d, J = 7.6 Hz, 1H), 7.77 (d, J = 8.4 Hz,
1H), 7.51 (d, J =
8.0 Hz, 2H), 3.66 (s, 3H), 3.56-3.44 (m, 8H); LC-MS: m/z 479.05 (M+1) .
Example 135. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-(3-methyl-
2-
oxooxazolidin-5-yl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
0 OH OH
Br 0 Br 0c) b MeHN c Me- d
so
Me Me Me Me
N Cbz Cbz N Cbz
¨1\11-1
Et0 =NN¨NH2 Et0 6110¨N:cbz ¨'f"- HO lir H =
N
0 0 0 0
Me
Me N H
Me N 0
is ,¨NH2 N
TBDivisoo,õ.; 11P N
0 0 0
0 1411I
0
Nr- Me
Me
Conditions: a) NaBH4, Me0H, 0 C - RT, 1 h; b) 40% Aq. methylamine, RT, 30 min;
c) CD, DMF, 100 C, 16 h; d) POCI3, TEA, reflux, 5
h; e) NaHCO3, Benzyl chloformate in toluene, acetone, H20, 0 C - RT, 16 h; f)
Li0H.H20, THF, Et0H, H20, 80 C, 16 h; g) 2-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)ethan-1-amine, HBTU, DIPEA, DMF, 0 C - RT, 16 h;
h) Pd/C, H2, Me0H, RT, 6 h; i) 5-(2-
chlorobenzo[d]oxazol 6 yl) 3 methyloxazolidin-2-one , NaH, 1,4-Dioxane, RT, 3
h; j)TBAF, THF, RT 16 h
Step-a: Synthesis of 6-(2-bromo-1-hydroxyethyl)benzo[d]oxazol-2(3H)-one
To a stirred solution of 6-(2-bromoacetyl)benzo[d]oxazol-2(3H)-one (4.0 g,
15.62 mmol) in
methanol (50 mL) at 0 C was added sodium borohydride (587 mg, 15.62 mmol)
portion wise
and the reaction mixture was stirred for 1 h. The reaction mixture was
quenched with water
(100 mL) and concentrated under reduced pressure to remove methanol. The
aqueous layer
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
177
was extracted with Et0Ac (3 X 100 mL). Combined organic layers were washed
with brine
(30 mL), dried over anhydrous sodium sulfate and concentrated under vacuum to
afford the
titled compound (4.0 g, 99%); LC-MS: m/z 257.9 (M+1) .
Step-b: Synthesis of 6-(1-hydroxy-2-(methylamino)ethyl)benzo [d] oxazol-2(3H)-
one
A mixture of 6-(2-bromo-1-hydroxyethyl)benzo [d] oxazol-2(3H)-one (4.0 g, 15.5
mmol) in
40% aqueous methylamine (20 mL) was stirred at RT for 30 min. The reaction
mixture was
concentrated under vacuum and the residue was purified by combiflash
chromatography
using 5% methanol in DCM as an eluent to afford the title compound (1.8 g,
56%); 1H NMR
(400 MHz, DMSO-d6): 8 11.8 (bs, 1H), 8.9 (bs, 1H), 7.47 (d, J = 1.2 Hz, 1H),
7.28 (dd, J =
1.2 Hz, J= 8.0 Hz, 1H), 7.17 (d, J= 8.0 Hz, 1H), 5.68 (t, J= 4.8 Hz, 1H), 4.29
(t, J= 6.4 Hz,
1H), 3.81-3.74 (m, 2H), 1.24 (s, 3H); LC-MS: m/z 209.05 (M+1) .
Step-c: Synthesis of 6-(3-methyl-2-oxooxazolidin-5-yObenzo [d] oxazol-2(3H)-
one
To a stirred solution of 6-(1-hydroxy-2-(methylamino)ethyl)benzo [d] oxazol-
2(3H)-one (1.8
g, 8.65 mmol) in DMFA (20 mL) at RT was added 1,1'-carbonyldiimidazole (1.54
g, 9.52
mmol). The reaction mixture was heated to 100 C with stirring for 16 h. The
reaction mixture
was cooled to RT, diluted with water (100 mL) and extracted with ethyl acetate
(3 X 100
mL). The combined organic layer was washed brine solution (50 mL), dried over
anhydrous
sodium sulfate and concentrated under vacuum. The residue was purified by
combiflash
chromatography using 1% methanol in DCM as an eluent to afford the title
compound (500
mg, 25%); 1H NMR (400 MHz, DMSO-d6): 8 7.22 (s, 1H), 7.09 (d, J = 8.0 Hz, 1H),
7.01 (d,
J= 8.0 Hz, 1H), 3.57-3.54 (m, 1H), 3.45-3.41 (m, 1H), 3.34-3.30 (m, 1H), 2.16
(s, 3H).
Step-d: Synthesis of 5-(2-chlorobenzo [d] oxazol-6-y1)-3-methyloxazolidin-2-
one
A 50 mL round bottom flask was charged with 6-(3-methy1-2-oxooxazolidin-5-
yl)benzo[d]oxazol-2(3H)-one (500 mg, 2.14 mmol) and P0C13 (0.98 mL, 10.68
mmol)
followed by slow addition of triethyl amine (1.51 mL, 10.68 mmol) at 0 C. The
reaction
mixture was heated to reflux for 5 h. The reaction mixture was cooled to RT,
poured over ice
and extracted with ethyl acetate (2 X 50 mL). The combined organic layer was
washed with
saturated sodium bicarbonate solution (2 x 50 mL), water (50 mL), brine
solution (30 mL),
dried over anhydrous sodium sulfate and concentrated under vacuum. The residue
was
purified by combiflash chromatography using 50% Et0Ac in hexane as an eluent
to afford
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
178
the title compound (120 mg, 22%); 1H NMR (400 MHz, DMSO-d6): 8 7.58 (s, 1H),
7.35 (dd,
J= 1.6 Hz, J= 8.0 Hz, 1H), 7.19 (d, J= 7.6 Hz, 1H), 4.64 (bs, 1H), 4.23-4.19
(m, 1H), 4.16-
4.11 (m, 1H), 2.46 (s, 3H).
Step-e: Synthesis of ethyl 2-(bis((benzyloxy)carbonyl)amino)-1-methyl-1H-
benzo [d] imidazole-5-carboxylate
To a stirred solution of ethyl 2-amino-1-methy1-1H-benzo [d]imidazole-5-
carboxylate (1.0 g,
3.24 mmol) in acetone (10 mL) and water (10 mL) at 0 C was added sodium
bicarbonate
(722 mg, 6.88 mmol) and benzyl chloroformate (1.2 mL, 4.22 mmol, 50% in
toluene). The
reaction mixture was stirred at RT for 16 h. The reaction mixture was diluted
with water (100
mL) and extracted with ethyl acetate (2 X 100 mL). The combined organic layer
was washed
brine solution (50 mL), dried over anhydrous sodium sulfate and concentrated
under vacuum.
The crude compound was used in the next step without any further purification
(2.2 g, 99%);
LC-MS: m/z 488.2 (M+1) .
Step-f: Synthesis of 2-0(benzyloxy)carbonyl)amino)-1-methyl-1H-benzo [d]
imidazole-5-
carboxylic acid
To a stirred solution of ethyl 2-(bis((benzyloxy)carbonyl)amino)-1-methy1-1H-
benzo [d] imidazole-5-carboxylate (2.2 g, 4.52 mmol) in a mixture of solvent
of THF (15 mL),
ethanol (15 mL) and water (10 mL) was added lithium hydroxide monohydrate (948
mg, 22.6
mmol). The reaction mixture was heated at 80 C for 16 h with stirring. The
reaction mixture
was cooled to RT and concentrated under reduced pressure. The residue was
dissolved in
water (30 mL), acidified with 1 N HC1 and extracted with ethyl acetate (2 X
150 mL). The
combined organic layer was washed brine solution (50 mL), dried over anhydrous
sodium
sulfate and concentrated under vacuum to give the crude compound which was
used in the
next step without any further purification (1.4 g, 95%); LC-MS: m/z 326.1
(M+1) .
Step-g: Synthesis of benzyl
(54(242-((tert-
butyldimethylsilypoxy)ethoxy)ethyl)carbamoy1)-1-methyl-1H-benzo [d] imidazol-2-
yl)carbamate
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 2-(((benzyloxy)carbonyl)amino)-1-methy1-1H-benzo [d]
imidazo le-5-
carboxylic acid and 2-(2-((tert-butyldimethylsilyl)oxy)ethoxy)ethan-l-amine as
starting
materials (Yield: 66%); 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.42 (t,
J = 5.2
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
179
Hz, 1H), 7.85 (s, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.41-7.33 (m, 6H), 5.08 (s,
2H), 3.66 (t, J =
5.2 Hz, 2H), 3.54-3.38 (m, 9H), 0.81 (s, 9H), 0.03 (s, 6H); LC-MS: m/z 527.25
(M+1) .
Step-h: Synthesis of 2-amino-N-(2-(2-((tert-
butyldimethylsilypoxy)ethoxy)ethyl)-1-
methyl-1H-benzo[d] imidazole-5-carboxamide
To a solution of benzyl (54(2-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)ethyl)carbamoy1)-1-
methyl-1H-benzo [d] imidazol-2-yl)carbamate (320 mg, 0.61 mmol) in methanol (8
mL) was
added 10% Pd/C (40 mg) under nitrogen atmosphere. Then the reaction mixture
was stirred
under hydrogen gas balloon for 6 h. The reaction mixture was filtered through
a bed of celite
and concentrated under vacuum to afford the title compound (170 mg, 71%); 1H
NMR (400
MHz, DMSO-d6): 8 8.22 (t, J = 4.8 Hz, 1H), 7.65 (s, 1H)), 7.45 (d, J = 8.0 Hz,
1H), 7.14 (d, J
= 8.0 Hz, 1H), 6.52 (s, 2H), 3.69 (t, J = 4.8 Hz, 2H), 3.55-3.46 (m, 7H), 3.41-
3.37 (m, 2H),
0.84 (s, 9H), 0.01 (s, 6H); LC-MS: m/z 393.65 (M+1) .
Step-i: Synthesis of N-(2-(2-((tert-butyldimethylsilypoxy)ethoxy)ethyl)-1-
methyl-24(6-
(3-methyl-2-oxooxazolidin-5-yl)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-
5-
carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using 2-amino-N-(2-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)ethyl)-1-
methyl-1H-benzo [d] imidazole-5-carboxamide and 5-(2-chlorobenzo [d] oxazol-6-
y1)-3-
methyloxazolidin-2-one as starting materials stirring for 3 h (Yield: 74%); 1H
NMR (400
MHz, DMSO-d6): 8 11.74 (bs, 1H), 8.43 (t, J = 5.2 Hz, 1H), 8.05 (d, J = 1.2
Hz, 1H), 7.77
(dd, J= 1.6 Hz, J= 8.4 Hz, 1H), 7.56 (d, J= 8.4 Hz, 1H), 7.42 (d, J= 1.2 Hz,
1H), 7.25 (dd,
J = 1.6 Hz, J= 8.4 Hz, 1H), 7.15 (d, J= 8.0 Hz, 1H), 4.87 (t, J = 7.2 Hz, 1H),
4.35 (t, J = 9.2
Hz, 1H), 3.82-3.76 (m, 5H), 3.68 (t, J= 5.2 Hz, 2H), 3.54-3.52 (m, 2H), 3.48-
3.31 (m, 2H),
2.62 (s, 3H), 0.84 (s, 9H), -0.01 (s, 3H), -0.02 (s, 3H); LC-MS: m/z 609.3
(M+1) .
Step-j: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-(3-methyl-2-
oxooxazolidin-5-yl)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-
carboxamide
To a stirred solution of N-(2-(2-((tert-butyldimethylsilyl)oxy)ethoxy)ethyl)-1-
methyl-2-((6-
(3-methyl-2-oxooxazolidin-5-y1)benzo [d] oxazol-2-yl)amino)-1H-benzo [d]
imidazole-5-
carboxamide (30 mg, 0.05 mmol) in THF (2 mL) at 0 C was added 1 M TBAF in THF
(0.1
mL) and the reaction mixture was stirred at RT for 16 h. The reaction mixture
was quenched
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
180
with saturated ammonium chloride solution (10 mL) and extracted with Et0Ac (2
X 30 mL).
The combined organic layers were washed with water (20 mL), brine (20 mL),
dried over
anhydrous sodium sulfate and concentrated under vacuum to obtain a residue
which was
purified by combiflash chromatography using 5% methanol in DCM as an eluent to
afford the
title compound (14 mg, 58%); 1H NMR (400 MHz, DMSO-d6): 8 11.8 (bs, 1H), 8.43
(t, J =
5.2 Hz, 1H), 8.06 (s, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H),
7.38 (s, 1H),
7.21 (d, J= 8.0 Hz, 1H), 7.13 (d, J= 7.6 Hz, 1H), 4.87 (t, J= 8.0 Hz, 1H),
4.59 (s, 1H), 4.35
(t, J = 9.2 Hz, 1H), 3.82 (t, J = 9.2 Hz, 1H), 3.78 (s, 3H), 3.56-3.41 (m,
8H), 2.63 (s, 3H);
LC-MS: m/z 495.2 (M+1) .
Example 136. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-
morpholinobenzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
O
F WI 01-1 a , 41111" c
Os 0,
/1¨CI
Me
NO2 NO2 NH2 N
fyle N Me
=N ¨NH HO Mr H=,>_NH
N ,u,.
Et0 N
0 N
0 N
Conditions: a) Morpholine, ACN, 60 C, 2 h; b) 10% Pd/C, Me0H, H2, RT, 3 h; c)
Potassium ethyl xanthate, Et0H, Reflux,
16h; d) S0Cl2, Cat.DMF, DCM, RT, 1 h; e) NaH, ethyl 2-amino-1-methyl-1 H-
benzo[c]imidazole-5-carboxylate , 1,4-
Dioxane, RT, 16 h; f) Li0H.H20, THF, Et0H, H20, 60 C, 16 h; g) 2-(2-
aminoethoxy)ethan-1-ol, HBTU, DIPEA, DMF, 0 C -
RT, 16 h
Step-a: Synthesis of 5-morpholino-2-nitrophenol
To a stirred solution of 5-fluoro-2-nitrophenol (5.0 g, 31.84 mmol) in
acetonitrile (50 mL) at
RT was added morpholine (8.31 g, 95.54 mmol) and the reaction mixture was
heated at 60 C
for 2 h. The reaction mixture was cooled to RT, diluted with cold water (300
mL) and the
precipitated solid was filtered and dried under vacuum to afford the title
compound (5.0 g,
70%); 1H NMR (400 MHz, DMSO-d6): 8 10.9 (bs, 1H), 7.88 (d, J = 9.2 Hz, 1H),
6.65 (dd, J
= 2.4 Hz, J = 9.6 Hz, 1H), 6.44 (d, J = 2.8 Hz, 1H), 3.70 (t, J = 5.2 Hz, 4H),
3.41 (t, J = 4.8
Hz, 4H); LC-MS: m/z 225.1 (M+1) .
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
181
Step-b: Synthesis of 2-amino-5-morpholinophenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 5-morpholino-2-nitrophenol as starting material and stirred
for 3 h
(Yield: 81%); 1H NMR (400 MHz, DMSO-d6): 8 8.8 (bs, 1H), 6.48 (d, J = 8.0 Hz,
1H), 6.33
(d, J = 2.4 Hz, 1H), 6.19 (dd, J = 2.8 Hz, J = 8.8 Hz, 1H), 3.68 (t, J = 5.2
Hz, 4H), 2.84 (t, J =
4.8 Hz, 4H); LC-MS: m/z 195.1 (M+1) .
Step-c: Synthesis of 6-morpholinobenzo [d] oxazole-2(3H)-thione
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-morpholinophenol as starting material
(Yield: 75%); 1H
NMR (400 MHz, DMSO-d6): 8 13.6 (s, 1H), 7.14 (d, J = 2.4 Hz, 1H), 7.09 (d, J =
8.8 Hz,
1H), 6.91 (dd, J= 2.4 Hz, J= 8.8 Hz, 1H), 3.73 (t, J= 4.4 Hz, 4H), 3.10 (t, J=
4.8 Hz, 4H);
LC-MS: m/z 237.1 (M+1) .
Step-d: Synthesis of 2-chloro-6-morpholinobenzo[d]oxazole
To a solution of 6-morpholinobenzo[d]oxazole-2(3H)-thione (500 mg, 1.12 mmol)
in DCM
(10 mL) at RT was added thionyl chloride (0.79 mL, 10.6 mmol) and
dimethylformamide
(0.2 mL) and the reaction mixture was stirred at RT for 1 h. The reaction
mixture was poured
on cold water (50 mL), basified with saturated sodium bicarbonate solution and
extracted
with DCM (2 X 50 mL). The combined organic layers were washed with brine
solution (30
mL), dried over anhydrous sodium sulfate and concentrated under vacuum. The
residue was
purified by combiflash chromatography using 30% Et0Ac in hexane as eluent to
afford the
title compound (300 mg, 60%); 1H NMR (400 MHz, DMSO-d6): 8 7.56 (d, J = 8.8
Hz, 1H),
7.29 (d, J= 2.4 Hz, 1H), 7.09 (dd, J= 2.4 Hz, J= 8.8 Hz, 1H), 3.75 (t, J= 4.8
Hz, 4H), 3.16
(t, J= 4.8 Hz, 4H); LC-MS: m/z 239.0 (M+1) .
Step-e: Synthesis of ethyl 1-methyl-2((6-morpholinobenzo [d] oxazol-2-
yl)amino)-1H-
benzo [d] imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-morpholinobenzo [d] oxazole as starting materials (Yield: 37%); 1H
NMR (400
MHz, DMSO-d6): 8 12.2 (bs, 1H), 8.20 (d, J= 1.2 Hz, 1H), 7.86 (dd, J= 1.6 Hz,
J= 8.4 Hz,
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
182
1H), 7.49 (d, J = 8.4 Hz, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.09 (d, J = 2.4 Hz,
1H), 6.87 (dd, J =
2.4 Hz, J= 8.8 Hz, 1H), 4.32 (q, J= 8.4 Hz, 2H), 3.76 (t, J= 4.4 Hz, 4H), 3.61
(s, 3H), 3.09
(t, J= 4.8 Hz, 4H), 1.35 (t, J= 7.2 Hz, 3H); LC-MS: m/z 422.1 (M+1) .
Step-f: Synthesis of 1-methyl-2-((6-morpholinobenzo[d] oxazol-2-yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methyl-2-((6-morpholinobenzo [d]oxazol-2-yl)amino)-
1H-
benzo [d] imidazole-5-carboxylate as starting material (Yield: 71%); 1H NMR
(400 MHz,
DMSO-d6): 8 12.5 (bs, 1H), 8.16 (d, J= 1.2 Hz, 1H), 7.85 (d, J= 8.4 Hz, 1H),
7.46 (d, J=
8.4 Hz, 1H), 7.34 (d, J = 8.8 Hz, 1H), 7.08 (d, J = 2.0 Hz, 1H), 6.86 (dd, J =
2.0 Hz, J = 8.8
Hz, 1H), 3.76 (t, J = 4.4 Hz, 4H), 3.61 (s, 3H), 3.09 (t, J = 4.4 Hz, 4H); LC-
MS: m/z 394.0
(M+1) .
Step-g: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-
morpholinobenzo[d]-
oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methyl-2-((6-morpholinobenzo [d]oxazol-2-yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-1-ol as
starting materials
(Yield: 98%); 1H NMR (400 MHz, DMSO-d6): 8 12.4 (bs, 1H), 8.49 (t, J = 4.8 Hz,
1H), 8.05
(d, J= 1.2 Hz, 1H), 7.78 (d, J= 7.2 Hz, 1H), 7.52 (d, J = 7.2 Hz, 1H), 7.34
(d, J = 8.4 Hz,
1H), 7.15 (s, 1H), 6.94 (d, J= 7.2 Hz, 1H), 3.86 (bs, 4H), 3.64 (s, 3H), 3.56-
3.54 (m, 4H),
3.47-3.45 (m, 4H), 3.14 (bs, 4H); LC-MS: m/z 481.2 (M+1) .
Example 137. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-
(pyrrolidin-1-
yl)benzo[d] oxazol-2-yl)amino)-1H-benzo[d] imidazole-5-carboxamide
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
183
=0 d 0
c
/ ¨C1
F
ir OH a C1N
OH b OH =
NO2 NO2 NH2
Me Me Me
N'NH _NH H \I-NH
f HO
e Et0 = i>¨ N 0 N )F0
0 N 0 N
0 N
Conditions: a) Pyrrolidine, Acetonitrile, 100 C, 12 h; b) 10% Pd/C, Me0H, H2,
RT, 4 h; c) Potassium ethyl xanthate,
Ethanol, Reflux, 16h; d) SOCl2, DCM, 0 C, 1 h; e) NaH, ethyl 2-amino-1-methyl-
1H-benzo[d]imidazole-5-carboxylate, 1,4-
Dioxane, 0 C - RT, 4 h; f) Li0H.H20, THF, Ethanol, Water, 60 C, 16 h; g) 2-(2-
Aminoethoxy)ethan-1-ol, HBTU, DIPEA,
DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-nitro-5-(pyrrolidin-1-yl)phenol
To a solution of 5-fluoro-2-nitrophenol (2.0 g, 12.73 mmol) in acetonitrile 20
mL) at RT was
added pyrrolidine (3.15 mL, 38.21 mmol) and stirred at 100 C for 12 h. The
reaction mixture
was concentrated under vacuum and the residue was purified by combi-flash
column
chromatography using 30% ethyl acetate in hexane as an eluent to afford the
titled compound
(1.5 g, 57%); 1H NMR (400 MHz, DMSO-d6): 8 7.85 (d, J = 9.2 Hz, 1H), 6.28 (dd,
J = 2.4
Hz, J= 9.2 Hz, 1H), 6.02 (d, J= 2.4 Hz, 1H), 3.39-3.36 (m, 4H), 1.98-1.94 (m,
4H).
Step-b: Synthesis of 2-amino-5-(pyrrolidin-1-yl)phenol
To a solution of 2-nitro-5-(pyrrolidin-1-yl)phenol (1.5 g, 7.20 mmol) in
methanol (300 mL)
was added 10% Pd/C (300 mg) under nitrogen atmosphere. The reaction mixture
was stirred
under hydrogen balloon for 4 h. The reaction mixture was filtered through a
celite bed and
the filtrate was concentrated under vacuum to afford the crude compound (1.3
g) which was
used in the next step without any further purification and structural
confirmation by analytical
technique.
Step-c: Synthesis of 6-(pyrrolidin-1-yl)benzo [d] oxazole-2(3H)-thione
To a solution of 2-amino-5-(pyrrolidin-1-yl)phenol (1.3 g, 7.30 mmol) in
ethanol (13 mL) at
RT was added potassium ethyl-xanthate (2.3 g, 14.6 mmol) and the reaction
mixture was
refluxed for 16 h. The reaction mixture was concentrated under vacuum and
diluted with cold
water (50 mL), acidified with 1 N HC1. The solid obtained was filtered and
dried under
vacuum to afford the titled compound (0.8 g, 50%) which was used in the next
step without
further purification; 1H NMR (400 MHz, DMSO-d6): 8 13.50 (bs, 1H), 7.03 (d, J
= 8.4 Hz,
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
184
1H), 6.65 (d, J= 2.0 Hz, 1H), 6.46 (dd, J= 2.0 Hz, J= 8.4 Hz, 1H), 3.22-3.19
(m, 4H), 1.97-
1.94 (m, 4H); LC-MS: m/z 220.9 (M+1) .
Step-d: Synthesis of 2-chloro-6-(pyrrolidin-1-yl)benzo [d] oxazole
To a solution of 6-(pyrrolidin-1-yl)benzo [d] oxazole-2(3H)-thione (400 mg,
1.82 mmol) in
DCM (10 mL) at 0 C was added thionyl chloride (0.66 mL, 9.09 mmol) and the
reaction
mixture was stirred at 0 C for 1 h. The reaction mixture was quenched with
water (20 mL),
extracted with Et0Ac (2 X 50 mL). The combined organic layers were washed with
saturated
sodium bicarbonate solution (30 mL), brine solution (30 mL), dried over
anhydrous sodium
sulfate and concentrated under vacuum to afford the titled compound (300 mg,
74%) which
was used in the next step without further purification; 1H NMR (400 MHz,
CD30D): 8 6.91
(d, J= 8.4 Hz, 1H), 6.48 (d, J= 2.0 Hz, 1H), 6.42 (dd, J= 2.4 Hz, J= 8.8 Hz,
1H), 3.21-3.16
(m, 4H), 1.96-1.91 (m, 4H).
Step-e: Synthesis of ethyl 1-methyl-24(6-(pyrrolidin-1-yl)benzo [d] oxazol-2-
yl)amino)-
1H-benzo [d] imidazole-5-carboxylate
To a stirred solution of ethyl 2-amino-1-methy1-1H-benzo [d] imidazole-5-
carboxylate (200
mg, 0.91 mmol) in 1,4-dioxane (5 mL) at 0 C was added sodium hydride (60%
dispersion in
mineral oil) (73 mg, 1.82 mmol) and stirred for 30 min followed by the
addition of 2-chloro-
6-(pyrrolidin-1-yl)benzo [d] oxazole (243 mg, 1.09 mmol). The reaction mixture
was stirred at
RT for 4 h and then quenched with cold water (20 mL) and extracted with Et0Ac
(2 X 50
mL). Combined organic layers were washed with water (30 mL), brine (30 mL),
dried over
anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combi-flash column chromatography using 3% methanol in DCM as an eluent to
afford the
titled compound (150 mg, 40%); 1H NMR (400 MHz, DMSO-d6): 8 12.10 (bs, 1H),
8.18 (d, J
= 1.6 Hz, 1H), 7.85 (dd, J= 1.2 Hz, J= 8.4 Hz, 1H), 7.46 (d, J= 8.4 Hz, 1H),
7.29 (d, J= 8.8
Hz, 1H), 6.64 (d, J = 2.0 Hz, 1H), 6.46 (dd, J = 2.0 Hz, J = 8.4 Hz, 1H), 4.32
(q, J = 6.8 Hz,
2H), 3.60 (s, 3H), 3.26-3.23 (m, 4H), 1.99-1.95 (m, 4H), 1.35 (t, J = 6.8 Hz,
3H); LC-MS:
m/z 406.2 (M+1) .
Step-f: Synthesis of 1-methyl-24(6-(pyrrolidin-1-yl)benzo [d] oxazol-2-
yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
185
To a stirred solution of ethyl 1-methy1-24(6-(pyrrolidin-1-yl)benzo[d[oxazol-2-
yDamino)-
1H-benzo[d[imidazole-5-carboxylate (150 mg, 0.37 mmol) in a mixture of solvent
of [THF (2
mL), ethanol (2 mL) and water (1 mL)] was added lithium hydroxide monohydrate
(77 mg,
1.85 mmol). The reaction mixture was heated at 60 C for 16 h. The reaction
mixture was
cooled to RT and concentrated under reduced pressure. The residue was
dissolved in water,
acidified with 1 N HC1 to obtain the solid which was filtered and dried under
vacuum to
afford the titled compound (120 mg, 86%); 1H NMR (400 MHz, DMSO-d6): 8 7.87
(s, 1H),
7.61 (d, J = 7.6 Hz, 1H), 7.00 (d, J = 8.4 Hz, 1H), 6.95 (d, J = 7.6 Hz, 1H),
6.50 (s, 1H), 6.31
(d, J = 7.2 Hz, 1H), 3.52 (s, 3H), 3.25-3.21 (m, 4H), 1.97-1.93 (m, 4H); LC-
MS: m/z 376.0
(M-1).
Step-g: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methy1-24(6-(pyrrolidin-1-
yObenzo[d]oxazol-2-yDamino)-1H-benzo[d]imidazole-5-carboxamide
To a stirred solution of 1-methy1-2-((6-(pyrrolidin-1-y1)benzo [d] oxazol-2-
yl)amino)-1H-
benzo[d[imidazole-5-carboxylic acid (120 mg, 0.32 mmol) in DMFA (5 mL) at 0 C
was
added N-ethyldiisopropyl amine (0.08 mL, 0.48 mmol) and HBTU (180 mg, 0.48
mmol). The
reaction mixture was stirred for 30 min, followed by the addition of 2-(2-
aminoethoxy)ethan-
1-ol (80 mg, 0.48 mmol) and stirring was continued at RT for 16 h. Once the
reaction was
completed (monitored by TLC), the reaction mixture was diluted with cold water
(20 mL)
and extracted with Et0Ac (2 X 50 mL). Combined organic layers were washed with
water
(25 mL), brine (25 mL), dried over anhydrous sodium sulfate and concentrated
under
vacuum. The residue was purified by preparative HPLC purification method to
afford the
titled compound (15 mg, 10%);
Preparative HPLC purification method details:
DILUTION : THF +Acetonitrile: Water (50:50)
MOBILE PHASE A: 0.1% Formic acid in water
MOBILE PHASE B: Acetonitrile (100%)
GRADIENT : T/%B: 0/15, 10/35
FLOW RATE : 15mL/min
COLUMN : Agilent ZORBAX XDB C18 (150 x 21.2 x 5i.tm)
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
186
1H NMR (400 MHz, DMSO-d6): 8 12.0 (bs, 1H), 8.40 (t, J = 5.2 Hz, 1H), 8.03 (s,
1H), 7.73
(d, J = 8.4 Hz, 1H), 7.43 (d, J = 8.4 Hz, 1H), 7.28 (d, J = 8.4 Hz, 1H), 6.63
(bs, 1H), 6.46 (bs,
1H), 4.63 (s, 1H), 3.59 (s, 3H), 3.57-3.39 (m, 8H), 3.24 (bs, 4H), 1.97 (bs,
4H); LC-MS: m/z
465.20 (M+1) .
Example 138. Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-
((tetrahydro-
2H-pyran-4-ypoxy)benzo[d]oxazol-2-y1)amino)-1H-benzoldlimidazole-5-carboxamide
s do 0
OH
F OH a r",..-"o (D
dith, OH
NO2
NO2 gr NH2
N
41111) N
NMee fyle Mee
Et0 N
f HO =N
N
/ ¨NH
N
N /F-0
0
0
0
Conditions: a) Tetrahydro-2H-pyran-4-ol, NaH, DMF, 60 C, 16 h; b)10% Pd/C,
Me0H, H2, RT, 6 h; c) Potassium ethyl
xanthate, Et0H, Reflux, 16 h; d) SOCl2, Cat.DMF, Reflux, 3 h; e) NaH, ethyl 2-
amino-1-methyl-1H-benzo[cl]imidazole-5-
carboxylate , 1,4-Dioxane, RT, 16 h; f) Li0H.H20, THF, Et0H, H20, 60 C, 16 h;
g) 2-(2-aminoethoxy)ethan-1-ol, HBTU,
DIPEA, DMF, 0 C - RT, 16 h
Step-a: Synthesis of 2-nitro-5-((tetrahydro-2H-pyran-4-yl)oxy)phenol
To a solution of tetrahydro-2H-pyran-4-ol (2.27 mL, 22.29 mmol) in DMFA (15
mL) at 10 C
was added sodium hydride (60% dispersion in mineral oil) (1.27 g, 31.84 mmol)
and stirred
for 5 min, followed by the addition of 5-fluoro-2-nitrophenol (1.0 g, 6.37
mmol) to the
reaction mixture and it was stirred at 60 C for 16 h. The reaction mixture was
cooled to RT,
quenched with cold water (100 mL) and neutralized with 1 N HC1. The
precipitated solid was
filtered and dried under vacuum to afford the title compound (1.0 g, 33%); 1H
NMR (400
MHz, DMSO-d6): 8 10.90 (bs, 1H), 7.95 (d, J = 9.2 Hz, 1H), 6.68 (d, J = 2.0
Hz, 1H), 6.63
(dd, J = 2.4 Hz, J = 9.2 Hz, 1H), 4.71-4.68 (m, 1H), 3.86-3.82 (m, 2H), 3.52-
3.49 (m, 2H),
1.99-1.97 (m, 2H), 1.64-1.56 (m, 2H).
Step-b: Synthesis of 2-amino-5-((tetrahydro-2H-pyran-4-yl)oxy)phenol
The title compound was synthesized using the same procedure which was followed
for
compound le using 2-nitro-5-((tetrahydro-2H-pyran-4-yl)oxy)phenol as starting
material and
stirring for 6 h (Yield: 87%); 1H NMR (400 MHz, DMSO-d6): 8 9.0 (bs, 1H), 6.47
(d, J = 8.4
Hz, 1H), 6.32 (d, J = 2.8 Hz, 1H), 6.21 (dd, J = 2.4 Hz, J = 8.4 Hz, 1H), 4.22-
4.17 (m, 1H),
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
187
3.83-3.78 (m, 2H), 3.44-3.40 (m, 2H), 1.89-1.85 (m, 2H), 1.54-1.45 (m, 2H); LC-
MS: m/z
209.95 (M+1) .
Step-c: Synthesis of 6-((tetrahydro-2H-pyran-4-yl)oxy)benzo[d]oxazole-2(3H)-
thione
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-c using 2-amino-5-((tetrahydro-2H-pyran-4-yl)oxy)phenol as
starting
material (Yield: 75%); 1H NMR (400 MHz, DMSO-d6): 8 13.74 (bs, 1H), 7.30 (d, J
= 2.4 Hz,
1H), 7.12 (d, J= 8.8 Hz, 1H), 6.92 (dd, J= 2.4 Hz, J= 8.4 Hz, 1H), 4.59-4.53
(m, 1H), 3.87-
3.82 (m, 2H), 3.47-3.40 (m, 2H), 1.98-1.93 (m, 2H), 1.61-1.52 (m, 2H); LC-MS:
m/z 252.1
(M+1) .
Step-d: Synthesis of 2-chloro-6-((tetrahydro-2H-pyran-4-yl)oxy)benzo[d]oxazole
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-d using 6-((tetrahydro-2H-pyran-4-yl)oxy)benzo [d] oxazole-
2(3H)-thione
as starting material (Yield: 25%); 1H NMR (400 MHz, DMSO-d6): 8 7.59 (d, J =
8.8 Hz, 1H),
7.47 (d, J = 2.0 Hz, 1H), 7.02 (dd, J = 2.0 Hz, J = 8.8 Hz, 1H), 4.62-4.59 (m,
1H), 3.85-3.77
(m, 2H), 3.49-3.43 (m, 2H), 1.97-1.94 (m, 2H), 1.61-1.52 (m, 2H); LC-MS: m/z
254.1
(M+1) .
Step-e: Synthesis of ethyl 1-methyl-2-06-((tetrahydro-2H-pyran-4-
yl)oxy)benzo[d]oxazol-2-y1)amino)-1H-benzo[d]imidazole-5-carboxylate
The title compound was synthesized using the same procedure which was followed
for
Example 32 Step-e using ethyl 2-amino-1-methy1-1H-benzo[d]imidazole-5-
carboxylate and
2-chloro-6-((tetrahydro-2H-pyran-4-yl)oxy)benzo [d] oxazole as starting
materials (Yield:
23%); 1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 8.21 (d, J = 1.2 Hz, 1H),
7.85 (dd, J
= 1.6 Hz, J= 8.4 Hz, 1H), 7.49 (d, J= 8.4 Hz, 1H), 7.35 (d, J= 8.8 Hz, 1H),
7.19 (d, J= 2.4
Hz, 1H), 6.85 (dd, J = 2.0 Hz, J = 8.8 Hz, 1H), 4.56-4.54 (m, 1H), 4.32 (q, J
= 7.2 Hz, 2H),
3.88-3.85 (m, 2H), 3.62 (s, 3H), 3.51-3.44 (m, 2H), 2.00-1.98 (m, 2H), 1.61-
1.58 (m, 2H),
1.35 (q, J= 6.8 Hz, 3H); LC-MS: m/z 437.2 (M+1) .
Step-f: Synthesis of 1-methyl-24(6-((tetrahydro-2H-pyran-4-
yl)oxy)benzo[d]oxazol-2-
y1)amino)-1H-benzo[d]imidazole-5-carboxylic acid
The title compound was synthesized using the same procedure which was followed
for
compound lh using ethyl 1-methy1-2-((6-((tetrahydro-2H-pyran-4-
yl)oxy)benzo[d]oxazol-2-
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
188
yl)amino)-1H-benzo [d]imidazole-5-carboxylate as starting material (Yield:
67%); 1H NMR
(400 MHz, DMSO-d6): 8 12.40 (bs, 1H), 8.17 (d, J = 1.2 Hz, 1H), 7.86 (dd, J =
1.2 Hz, J =
8.4 Hz, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 5.6 Hz, 1H), 7.20 (d, J =
2.4 Hz, 1H), 6.86
(dd, J = 2.4 Hz, J = 8.8 Hz, 1H), 4.57-4.53 (m, 1H), 3.88-3.84 (m, 2H), 3.62
(s, 3H), 3.51-
3.44 (m, 2H), 2.00-1.95 (m, 2H), 1.61-1.58 (m, 2H); LC-MS: m/z 409.1 (M+1) .
Step-g: Synthesis of N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-24(6-((tetrahydro-
2H-
pyran-4-yl)oxy)benzo[d]oxazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide
The title compound was synthesized using the same procedure which was followed
for
Example 6 Step-e using 1-methy1-24(6-((tetrahydro-2H-pyran-4-
yl)oxy)benzo[d]oxazol-2-
yl)amino)-1H-benzo [d] imidazole-5-carboxylic acid and 2-(2-aminoethoxy)ethan-
1-ol as
starting materials. The crude product was purified by combiflash
chromatography using 4%
methanol in DCM as an eluent (Yield: 40%); 1H NMR (400 MHz, DMSO-d6): 8 12.17
(bs,
1H), 8.44 (d, J = 5.6 Hz, 1H), 8.05 (d, J = 1.2 Hz, 1H), 7.74 (dd, J = 1.6 Hz,
J = 8.4 Hz, 1H),
7.46 (d, J= 8.4 Hz, 1H), 7.34 (d, J= 8.4 Hz, 1H), 7.18 (d, J= 2.0 Hz, 1H),
6.84 (dd, J= 2.4
Hz, J = 8.8 Hz, 1H), 4.62 (bs, 1H), 4.57-4.51 (m, 1H), 3.89-3.84 (m, 2H), 3.61
(s, 3H), 3.55-
3.33 (m, 10H), 1.98-1.95 (m, 2H), 1.61-1.57 (m, 2H); LC-MS: m/z 496.2 (M+1)
Examples 139, 140 and 141. Synthesis of 1-(2-(benzo[d]oxazol-2-ylamino)-1-
methyl-1H-
benzo[d]imidazol-5-y1)-2,2,2-trifluoroethan-1-one, Synthesis of (Z)-1-(2-
(benzo[d]oxazol-
2-ylamino)-1-methy1-1H-benzo[d]imidazol-5-y1)-2,2,2-trifluoroethan-1-one oxime
and
Synthesis of N-(5-(1-amino-2,2,2-trifluoroethyl)-1-methyl-1H-benzo[d]imidazol-
2-
yObenzo[d]oxazol-2-amine
F F
dth c N, Me
F3C F3C F3 N,me Me d F3C = F3C
N¨NH2
NO2NO2 NH2
0 0 0 0 0
Me Me Me
N mH
1\i¨NH 1\i¨NH
f F3C = N g F3C N
0
F3C 0 *
HON 0 NH2
0
Conditions: a) Conc.H2SO4, Fuming nitric acid, 0 C - RT, 2 h; b) Aq.Methyl
amine, DMF, 0 C - RT, 2 h; c) Iron, Conc.HCI,
Me0H, 0 C - RT, 1 h, 60 C, 1 h; d) Cyanogen bromide, THF, Water, 60 C, 16 h;
e) 2-Chlorobenzoxazole, Sodiumhydride,
1,4-Dioxane, 0 C - RT, 16 h; f) hydroxylamine hydrochloride, Potassium
acetate, Ethanol, reflux, 16 h; g) LiAIH4 (1M
solution in THF), THF, 10 C, 4 h
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
189
Step-a: Synthesis of 2,2,2-trifluoro-1-(4-fluoro-3-nitrophenypethan-1-one
To a solution of 2,2,2-trifluoro-1-(4-fluorophenyl)ethan- 1-one (3.0 g, 15.62
mmol) in conc.
sulfuric acid (12 mL) at 0 C was added fuming nitric acid (0.9 mL) and stirred
at RT for 2 h.
The reaction mixture was quenched with cold water (100 mL) and extracted with
ethyl
acetate (200 mL). Organic layer was washed with cold water (2 X 50 mL), brine
solution (50
mL), dried over anhydrous sodium sulfate and concentrated under vacuum to
afford the titled
compound (3.5 g, 94%) which was used in the next step without further
purification; 1H
NMR (400 MHz, DMSO-d6): 8 8.80 (d, J = 6.8 Hz, 1H), 8.37 (d, J = 8.8 Hz, 1H),
7.54 (t, J =
9.4 Hz, 1H).
Step-b: Synthesis of 2,2,2-trifluoro-1-(4-(methylamino)-3-nitrophenypethan-1-
one
To a stirred solution of 2,2,2-trifluoro-1-(4-fluoro-3-nitrophenyl)ethan-1-one
(3.5 g, 14.7
mmol) in DMFA (17.5 mL) at 0 C was added 40% aqueous methyl amine (3.5 mL) and
the
reaction mixture was stirred at RT for 2 h. The reaction mixture was diluted
with cold water
(100 mL) and extracted with ethyl acetate (2 X 100 mL). Combined organic
layers were
washed with water (2 X 50 mL), brine solution (50 mL), dried over anhydrous
sodium sulfate
and concentrated under vacuum to afford the titled compound (3.3 g, 89%) which
was used in
the next step without further purification; 1H NMR (400 MHz, DMSO-d6): 8 8.99
(bs, 1H),
8.69 (s, 1H), 8.05 (d, J = 9.2 Hz, 1H), 7.19 (d, J = 9.2 Hz, 1H), 3.06 (d, J =
4.8 Hz, 3H); LC-
MS: m/z 247.1 (M-1).
Step-c: Synthesis of 1-(3-amino-4-(methylamino)pheny1)-2,2,2-trifluoroethan-1-
one
To a stirred solution of 2,2,2-trifluoro-1-(4-(methylamino)-3-
nitrophenyl)ethan- 1-one (2.0 g,
8.1 mmol) in methanol (20 mL) at 0 C was added iron (2.25 g, 40.3 mmol) and
conc.
hydrochloric acid (5.0 mL, 40.3 mmol). The reaction mixture was stirred at RT
for 1 h and
then heated at 60 C with stirring for another 1 h. The reaction mixture was
cooled to RT,
diluted with methanol (30 mL) and filtered through a celite pad. The filtrate
was concentrated
and diluted with water (50 mL), basified with saturated sodium bicarbonate
solution and
extracted with Et0Ac (2 X 50 mL). The combined organic layers were washed with
water
(30 mL), brine (30 mL), dried over anhydrous sodium sulfate and concentrated
under vacuum
to afford the titled compound (1.5 g, 84%) which was used in the next step
without further
purification; 1H NMR (400 MHz, DMSO-d6): 8 7.35 (d, J = 8.4 Hz, 1H), 7.21 (s,
1H), 6.50
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
190
(d, J = 8.8 Hz, 1H), 6.30 (bs, 1H), 4.96 (s, 2H), 2.85 (d, J = 4.8 Hz, 3H); LC-
MS: m/z 218.90
(M+1) .
Step-d: Synthesis of 1-(2-amino-1-methy1-1H-benzo[d]imidazol-5-y1)-
2,2,2-
trifluoroethan-1-one
To a stirred solution of 1-(3-amino-4-(methylamino)pheny1)-2,2,2-
trifluoroethan-1-one (1.0
g, 4.6 mmol) in THF (10 mL) and water (10 mL) at RT was added cyanogen bromide
(0.58 g,
5.5 mmol) and the reaction mixture was stirred at 60 C for 16 h. The reaction
mixture was
concentrated under reduced pressure and the residue was diluted with water (50
mL), basified
with saturated sodium bicarbonate solution and extracted with Et0Ac (2 X 100
mL).
Combined organic layers were washed with water (50 mL), brine (500 mL), dried
over
anhydrous sodium sulfate and concentrated under vacuum to afford the titled
compound
which was used in the next step without further purification (900 mg, 81%); 1H
NMR (400
MHz, DMSO-d6): 8 7.75 (s, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.38 (d, J = 8.4 Hz,
1H), 6.96 (s,
2H), 3.58 (s, 3H); LC-MS: m/z 244.1 (M+1) .
Step-e: Synthesis of 1-(2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo [d]
imidazol-5-
y1)-2,2,2-trifluoroethan-l-one
To a stirred solution of 1-(2-amino-1-methy1-1H-benzo [d] imidazol-5-y1)-2,2,2-
trifluoroethan-
1-one (900 mg, 3.70 mmol) in 1,4-dioxane (20 mL) at 0 C was added sodium
hydride (60%
dispersion in mineral oil) (518 mg, 12.96 mmol) and stirred for 15 min
followed by the
addition of 2-chlorobenzoxazole (567 mg, 3.70 mmol). The reaction mixture was
stirred at
RT for 16 h. The reaction mixture was then quenched with cold water (30 mL)
and extracted
with Et0Ac (2 X 50 mL). Combined organic layers were washed with water (30
mL), brine
(30 mL), dried over anhydrous sodium sulfate and concentrated under vacuum.
The residue
was purified by combi flash column chromatography using 100% DCM as an eluent
to afford
the titled compound (700 mg, 52%); 1H NMR (400 MHz, DMSO-d6): 8 12.50 (bs,
1H), 8.35
(s, 1H), 7.97 (d, J = 8.8 Hz, 1H), 7.67 (d, J = 8.8 Hz, 1H), 7.52-7.46 (m,
2H), 7.25 (t, J = 7.8
Hz, 1H), 7.16 (t, J= 7.8 Hz, 1H), 3.67 (s, 3H); LC-MS: m/z 361.1 (M+1) .
Step-f: Synthesis of (Z)-1-(2-(benzo[d] oxazol-2-ylamino)-1-methy1-1H-benzo
[d] imidazol-
5-y1)-2,2,2-trifluoroethan-1-one oxime
To a stirred solution of 1-(2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo
[d] imidazol-5-
y1)-2,2,2-trifluoroethan- 1-one (700 mg, 1.94 mmol) in ethanol (20 mL) at RT
was added
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
191
hydroxylamine hydochloride (405 mg, 5.83 mmol) and potassium acetate (572 mg,
5.83
mmol). The reaction mixture was stirred at 80 C for 16 h. Once the reaction
was completed
(monitored by TLC), the reaction mixture was concentrated under reduced
pressure and
diluted with water (50 mL) and extracted with Et0Ac (2 X 100 mL). Combined
organic
layers were washed with water (50 mL), brine (30 mL), dried over anhydrous
sodium sulfate
and concentrated under vacuum. The residue was purified by combi flash column
chromatography using 2% methanol in DCM as an eluent to afford the titled
compound (500
mg, 68%); 1H NMR (400 MHz, DMSO-d6): 8 13.05 (bs, 1H), 12.40 (bs, 1H), 7.81-
7.76 (m,
1H), 7.59-7.53 (m, 1H), 7.48-7.46 (m, 2H), 7.41-7.37 (m, 1H), 7.24 (t, J = 7.4
Hz, 1H), 7.15
(t, J= 7.6 Hz, 1H), 3.65 (s, 3H); LC-MS: m/z 376.1 (M+1) .
Step-g: Synthesis of N-(5-(1-amino-2,2,2-trifluoroethyl)-1-methyl-1H-benzo[d]
imidazol-
2-yl)benzo [d] oxazol-2-amine
To a stirred solution of (Z)-1-(2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-
benzo [d] imidazol-
5-y1)-2,2,2-trifluoroethan-1-one oxime (300 mg, 0.80 mmol) in THF (5 mL) at 10
C was
added 1 M solution of lithium aluminium hydride (1.6 mL, 1.60 mmol). The
reaction mixture
was stirred at 10 C for 4 h. Once the reaction was completed (monitored by
TLC), the
reaction mixture was quenched with 1N sodium hydroxide solution (5 mL),
diluted with
water (10 mL) and extracted with Et0Ac (2 X 30 mL). Combined organic layers
were
washed with water (20 mL), brine (20 mL), dried over anhydrous sodium sulfate
and
concentrated under vacuum. The residue was purified by preparative HPLC method
to afford
the titled compound (100 mg, 35%).
Preparative HPLC purification method details:
DILUTION : THF + ACN: WATER (50:50)
MOBILE PHASE A: 0.1% formic acid in Water
MOBILE PHASE B: 100% ACETONITRILE
GRADIENT : T/%B: 0/15, 10/40
FLOW RATE : 15mL/min
COLUMN : Kinetex C-18 (250 x 21.1 x 5i.t.m)
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
192
1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 7.73 (s, 1H), 7.46-7.36 (m, 4H),
7.20 (t, J
= 7.2 Hz, 1H), 7.10 (t, J= 7.2 Hz, 1H), 4.59-4.57 (m, 1H), 3.61 (s, 3H); LC-
MS: m/z 362.3
(M+1) .
Two isomers of this compound were separated by chiral HPLC purification.
Chiral HPLC purification method details:
DILUTION : IPA: DCM (90:10)
MOBILE PHASE A: 0.1 % DEA in Hexane
MOBILE PHASE B: IPA: DCM (90:10) %
ISOCRATIC : A: B (40:60)
FLOW RATE : 15mL/min
Peak-1 analytical data:
1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 7.73 (s, 1H), 7.46-7.36 (m, 4H),
7.20 (t, J
= 7.2 Hz, 1H), 7.10 (t, J= 7.2 Hz, 1H), 4.59-4.57 (m, 1H), 3.61 (s, 3H); LC-
MS: m/z 362.05
(M+1) .
Peak-2 analytical data:
1H NMR (400 MHz, DMSO-d6): 8 12.20 (bs, 1H), 7.73 (s, 1H), 7.46-7.36 (m, 4H),
7.20 (t, J
= 7.2 Hz, 1H), 7.10 (t, J= 7.2 Hz, 1H), 4.60-4.58 (m, 1H), 3.61 (s, 3H); LC-
MS: m/z 362.05
(M+1) .
Example 142. Synthesis of 1-(2-(benzo[d] oxazol-2-ylamino)-1-methyl-1H-
benzo [d] imidazol-5-y1)-2,2,2-trifluoroethan-1-ol
Me
N H
1p . rcrN ,... F3c 0 N¨NH
N X,---N
F3C OH 0
0
Conditions: a) Sodium borohydride, Me0H, 0 C - RT, 1 h
To a stirred solution of 1-(2-(benzo [d] oxazol-2-ylamino)-1-methy1-1H-benzo
[d] imidazol-5-
y1)-2,2,2-trifluoroethan- 1-one (50 mg, 0.14 mmol) in methanol (3 mL) at 0 C
was added
sodium borohydride (6 mg, 0.15 mmol) and the reaction mixture was stirred at
RT for 1 h.
The reaction mixture was quenched with cold water (10 mL) and extracted with
Et0Ac (2 X
20 mL). The combined organic layers were washed with water (15 mL), brine (15
mL), dried
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
193
over anhydrous sodium sulfate and concentrated under vacuum. The residue was
purified by
combi flash column chromatography using 2% methanol in DCM as an eluent to
afford the
titled compound (40 mg, 80%); 1H NMR (400 MHz, DMSO-d6): 8 12.30 (bs, 1H),
7.77 (s,
1H), 7.46-7.34 (m, 4H), 7.20 (t, J= 7.6 Hz, 1H), 7.10 (t, J= 7.6 Hz, 1H), 6.90
(bs, 1H), 5.24-
5.22 (m, 1H), 3.62 (s, 3H); LC-MS: m/z 363.30 (M+1) .
Example 143. ELISA assay to measure human Heme Oxygenase-1 (HMOX-1) in
HepG2 lysate
Reagents and technical notes:
ELISA capture antibody, standard HO-1, detection antibody and streptavidin HRP
were
supplied in the DuoSet IC Human total HO-1 ELISA from R&D systems (DYC3776-2).
The
substrate cocktail was KPL LumiGLO reserve Chemiluminescent Substrate (54-71-
00). PBS
was Corning Cellgro Cell Culture (21-040-CV). 10x PBS+0.05% tween20 (PBST) was
from
KPL.
All incubations of the plate were at room temperature (20 C) in a closed
drawer.
The ELISA plate was processed manually. All loadings were with a multichannel
pipet.
Plates were emptied by shaking into the sink and tapping onto paper towels to
remove any
remaining reagent. Plate washing was accomplished by loading all wells with
PBS+0.05%
tween20 (PBST) using a squirt bottle, shaking the PBST out and tapping the
plate onto paper
towels.
Preparation of the ELISA plate:
1. anti-HO-1 capture antibody was 1440 i.t.g/m1 after reconstitution in PBS.
This antibody
was diluted to 8 i.t.g/m1 in PBS and 50 ill per well was added to a 384 well
greiner
"lumitrac 200" plate. Incubated overnight.
2. The plate was emptied and 100 ill PBS+1% BSA added to all wells. Incubated
90
minutes at room temperature.
Preparation of HepG2 lysate:
1. Cells were cultured in a 96-well tissue culture plate washed with PBS and
then the plate
frozen at -70 C overnight. Plates were allowed to warm to ice temperature in
an ice
bucket. 20 ill of PBS, 0.5% Triton X100, 1 mM EDTA with lx HALTI'm protease
inhibitors was added to each well and the plates incubated on ice for 1 hour.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
194
2. The lysates were frozen at -20 C overnight.
Measurement of HMOX1 ("H0-1")
1. In a polypropylene 384 well plate, human HO-1(after reconstitution in
PBS+0.5%
Triton+1 mM EDTA) was serially diluted from 20 ng/ml 2-fold in PBS+0.5% triton-
X100, 1 mM EDTA to make a 12-point standard curve including a zero point.
2. In a polypropylene 96 well plate, HepG2 lysate was diluted 1 to 20 in
Diluent # 4 from
DUOSET kit in which the assay standard is also diluted.
3. The PBS+1% BSA was emptied from the ELISA plate and 30 ill of all samples
and
standards were added (duplicates recommended). The plate was incubated 90
minutes.
4. Emptied plate and washed 4 times with PBST.
5. Diluted HO-1 detection antibody to 200ng/m1 in PBS+1%BSA and added 30 ill
to all
wells. Incubated 90 minutes.
6. Emptied plate and washed 4 times with PBST.
7. Added 30 ill streptavidin HRP at 1/200 in PBS+1%BSA (stock streptavidin
concentration
not specified) to all wells. Incubated 30 minutes.
8. Emptied plate and washed 4 times with PBST.
9. Diluted Lumiglo reserve reagent two parts buffer to one part lumiglo
substrate. Added 30
ill to all wells. Incubated 5 minutes.
10. Measured chemiluminescence on spectramax M5 using 150 ms integration.
Table 1 below lists EC50 and fold changes of human HMOX1 protein levels
relative to
DMSO control upon treatment with representative compounds.
Example 144. Kinetic Solubility
[IL of 10 mM DMSO stock solution were aliquotted to 490 [IL of DMSO solution
and
separately 490 [IL of Dulbecco's phosphate buffer saline, DPBS, pH 7.4
solution in 1.2 mL
96-well plates in duplicate. Final concentration was 200 11M of the test
compound. Samples
were incubated at 25 C with shaking at 200 rpm for 16 h. Samples were
centrifuged at 3500
rpm for 20 min at 25 C, and supernatant was submitted for HPLC analysis.
Kinetic solubility was measured using the following equation:
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
195
Kinetic Solubility in [EM Test Conc. [EM x Peak area of compound in DPBS
sample (2% DMSO solution)
Peak area of compound in 100% DMSO
The kinetic solubility data of representative compounds of the present
invention are provided
in Table 1 below.
Table 1
Maximum
Example
EC50 Solubility 0
Structure IUPAC name
Fold t..)
No.
(PM) (PM) =
induction
t..)
o
F 0
F>r so
F 1-methy1-2-(6-trifluoromethoxy-
,-,
Comparator N o benzothiazol-2-ylamino)-1H-
,.tD
1 s-2(4 0 m...-.....õo 0
H benzoimidazole-5-carboxylic acid [2- 0.9* ND
H N P
N (2-hydroxy-ethoxy)-ethyl]-
amide
/
Me
1 H
N N 0
=N i 1 410 2-(benzo [d]oxazol-2-ylamino)-1-
lh methy1-1H-benzo[d]imidazole-5-
4.4* 7-38 ND
P
carboxylic acid
.
HO
,
0
1¨
.
t4
,,
Mel H
2
,
.
NN 0
,
. " 2-
(benzo[d]oxazol-2-ylamino)-N-(2- ,,'
li N N it
methoxyethyl)-1-methyl-1H-
0.8* 6-15 ND
HN benzo [d] imidazole-5-carboxamide
r--/ 0
Me0
1-d
n
1-i
cp
t..)
o
t..)
o
O-
t..)
-4
t..)
.6.
o
1-(tetrahydro-2H-pyran-4-y1)-2-((6- 0
0 N H
t..)
(trifluoromethoxy)benzo [d] thiazol-2- =
--N
N/A 10 (n =2) ND t..)
o
2d HO2C N )----S
yl)amino)-1H-benzo [d] imidazole-5-
N/
,-,
carboxylic acid
=
,o
OCF3
co_.) N-(2-methoxyethyl)-1-(tetrahydro-2H-
H 0N H
pyran-4-y1)-2-((6-
1
¨1\1 (trifluoromethoxy)benzo [d]
thiazol-2- 3.8* 20-33 ND
NneON
N)---./s yl)amino)-1H-benzo [d]
imidazole-5-
2e
P
0
VI carboxamide
2
N
u,
1¨
.
OCF3
t4
--4
Me
"
I\1
,,0
,
p1-(1-methylpiperidin-4-y1)-24(6-
0 N ,H
(trifluoromethoxy)benzo [d] thiazol-2-
3d
---N
yl)amino)-1H-benzo [d] imidazole-5-
9.2*
13 ND
HO2C N )---S
N/ carboxylic acid
1. OCF3
IV
n
1-i
cp
t..)
=
t..)
=
t..)
-4
t..)
.6.
=
Me
c51
N-(2-methoxyethyl)-1-(1-
0
methylpiperidin-4-y1)-2-((6-
t..)
o
N H
w
H 0 N (trifluoromethoxy)benzo [d] thiazol-2- 1.1*
6-46 ND
yl)amino)-1H-benzo [d] imidazole-5-
o
3e MeON N N)---./ S
WI
carboxamide
c,.)
0
OCF3
,Me
91
1-(1-methylpyrrolidin-3-y1)-2-((6-
0 N ,H
(trifluoromethoxy)benzo [d] thiazol-2-
---N
4.7* 29-33 ND
HO2C N )---S
N/ yl)amino)-1H-benzo [d] imidazole-5-
P
4d carboxylic acid
,-,
.
,4z
t4
cio
OCF3
0"
"
,
Me
'
.
N-(2-methoxyethyl)-1-(1-
'
methylpyrrolidin-3-y1)-2-((6-
H N/>¨ NIP
I" (trifluoromethoxy)benzo [d] thiazol-
2- N/A 19 ND
meoN N )7--s yl)amino)-1H-benzo [d] imidazole-5-
4e 0 N
WI carboxamide
ocF3
1-d
n
1-i
cp
t..)
=
t..)
=
t..)
-4
t..)
.6.
=
Me
1 H
N N s
N-(2-methoxyethyl)-1-methy1-2-
411 N N i ((4,5,6,7-tetrahydrobenzo [d]
thiazol-2- 0
1.3*
7-12 ND t..)
yl)amino)-1H-benzo [d] imidazole-5-
=
HN carboxamide
t..)
i-J
7---/ 0
o
Me0
c,.)
vD
Me
Me 2-((6,6-dimethy1-4,5,6,7-
tetrahydrobenzo [d] thiazol-2-
o 8.1
6 s-AN N ..-----,,..-0OH yl)amino)-N-(2-(2-
3.0 41
HN¨<" 0 H hydroxyethoxy)ethyl)-1-methy1-1H-
Me benzo [d] imidazole-5-carboxamide
Me
NI N-(2-(2-hydroxyethoxy)ethyl)-1-
H w ¨NH methyl-2-((6-methyl-4,5,6,7-
HO .oN
7.2 P
7 N )7--s tetrahydrobenzo [d] thiazol-2-
2.7* 27 2
o N \o, yl)amino)-1H-benzo [d]
imidazole-5-
,-,
.
Me
carboxamide
,4z t4
vD
,,
.
/9
'i
Me
2-((5,6-dihydro-4H-
,
S
,)
NI )=--"N cyclopenta[d]thiazol-2-yl)amino)-N-
2.5*
5-8 6.9
8 H 101 ¨NH (2-methoxyethyl)-1-methy1-1H-
Me0 N N benzo [d] imidazole-5-carboxamide
0
--.....
HN N-(2-(2-hydroxyethoxy)ethyl)-1-
HCI o methy1-2-((4,5,6,7-
1-d
2.8
n
9 tetrahydrothiazolo[5,4-c]pyridin-2-
ND 2
s-AHNN4 0 H--0,--
.
N yl)amino)-1H-benzo [d] imidazole-5-
cp
/
t..)
Me carboxamide hydrochloride
o
t..)
o
'a
t..)
-4
t..)
.6.
o
0
Me)N\ 24(5-acety1-4,5,6,7-
tetrahydrothiazolo[5,4-c]pyridin-2-
YNN o yl)amino)-N-(2-(2- ND 2
8.6
0
sIN4 Ai H,0,...
w
N OH hydroxyethoxy)ethyl)-1-methy1-1H-
=
w
N WI benzo [d] imidazole-5-carboxamide
o
i-J
Mei
1-,
0----....,
o
w
2-((6,7-dihydro-4H-pyrano[4,3-
c,.)
o
YNN 0 d] thiazol-2-yl)amino)-N-(2-
11 s-1( N 0 N.---...õ0.me
6.6 5 ND
HN¨ H methoxyethyl)-1-methy1-1H-
N benzo [d] imidazole-5-carboxamide
Me/
Me
i
N H
. N
N 2-((1H-benzo [d]imidazol-2-yl)amino)-
H V-NH
N-(2-methoxyethyl)-1-methyl-1H-
4.5 5.5 ND
12 N
Me0
/.-----/ benzo [d] imidazole-5-carboxamide
p N .
, 2A
0
Me
= tnA
1\1
o ,,
2
,
I 40 I N
'
HO
H 1-methyl-2-((5-(trifluoromethoxy)-
0
N )7--NH 1H-benzo [d]imidazol-2-yl)amino)-
-
0 N
1.9* 6-27 ND
13
I. 1H-benzo [d]imidazole-5-carboxylic
acid
OCF3
IV
n
1-i
cp
t..)
o
t..)
o
O-
t..)
-4
t..)
.6.
o
Me
N
I-1 401 ¨NH N-(2-methoxyethyl)-1-methyl-2-((5-
N
N
0
Me0 )7--NH (trifluoromethoxy)-1H-
4.2 t..)
0 N
2.3* 6-25 =
benzo[d]imidazol-2-yflamino)-1H-
t..)
14
WI benzo [d] imidazole-5-carboxamide
o
i-J
,-,
o
OCF3
,.tD
MR
N
HN-
101 1-methyl-2-((1-methyl-5-
15 N--=--( N
OCF3 5.3* 11-13 ND
101 N-me benzo [d] imidazol-2-yl)amino)-1H-
benzo[d]imidazole-5-carboxylic acid
HO2C
P
Me
2
i
N H
u,
16 H 0 -.-.N
\--
2-(benzo[d]oxazol-2-ylamino)-N-(2-
11.7
hydroxyethyl)-1-methyl-1H-
0.7* 8-27 t..) .
o t4
,-,
N)0
2
HO/----.,N
benzo [d] imidazole-5-carboxamide
0 N to
'0:1
'
IV
l0
Me
$
N H 2-(benzo [d] oxazol-2-ylamino)-N-(2-
17 * ----N (2-hydroxyethoxy)ethyl)-1-methyl-
11.6
H --0 1H-benzo [d]imidazole-5-carboxamide
1.4* 11-40
,N N a
HO /0' ¨ N *
0
Me
Iv
N N-(2-aminoethyl)-2-(benzo [d]
oxazol- n
1-i
I-1 101 -NH 2-ylamino)-1-methyl-1H- 12.4
cp
18 H2NN N )7-0
0.7* 41-52 n.)
HCI N
0 benzo [d] imidazole-5-carboxamide
t..)
WI hydrochloride
=
'a
t..)
-4
t..)
.6.
o
Me
1\1
H ¨NH
02-(benzo [d]oxazol-2-ylamino)-N-(2-
Me2NNN -- N 7---.0
(2-(dimethylamino)acetamido)ethyl)-
0
19 H
1.8* 14-26 ND t..)
0 N 0 1-
methy1-1H-benzo [d] imidazole-5- =
t..)
o
carboxamide
,-,
o
0 C,>-NH
N ).-,-._-N
2-(benzo[d]oxazol-2-ylamino)-1-
20 Me-N 0 methyl-
N-(2-morpholinoethyl)-1H- 1.0 8 7.3
H benzo
[d] imidazole-5-carboxamide
N N
0 0
P
.
Me 0 0 lei 2-(benzo[d]oxazol-2-ylamino)-N-(2-
t..)
.
21 I
o t4
t..)
Me' N N s N )--='N (dimethylamino)ethyl)-1-methyl-1H-
1.1 14 N/A ,,
,,0
H
¨NH benzo
[d] imidazole-5-carboxamide ,
N
Me
.
40 N 2-(benzo [d]oxazol-2-ylamino)-N-(2-
0
H ((4,5-dihydro-1H-imidazol-2- 3.5
22 -.,N N
0 INN Ai [\il \
HN----/ yl)amino)ethyl)-1-methy1-1H-
ND 18
MeiN WI benzo
[d] imidazole-5-carboxamide
0
1-d
n
N o 2-(benzo[d]oxazol-2-ylamino)-N-(2-
>20
23 0 --/ISNIN Ai HI,Me hydroxypropy1)-1-methyl-1H-
2.1* 25-61
cp
N Wi OH
benzo[d]imidazole-5-carboxamide t..)
o
t..)
Mei
=
O'
w
-4
w
.6.
o
0
N o 2-(benzo [d] oxazol-2-ylamino)-N-
(2,3-
12.4
24 A ,,N 0 N OH dihydroxypropy1)-1-methyl-1H-
ND 49
0
HN¨ H
OH benzo[d]imidazole-5-carboxamide
t..)
o
Me'
N
N
o
0
N 0 2-(benzo[d]oxazol-2-ylamino)-N-(2-
,-,
o
Me (2-hydroxypropoxy)ethyl)-1,6- 6.1
c,.)
25 OA N
2.2 26 yD
HN¨ dimethy1-1H-benzo[d]imidazole-5-
N
Me carboxamide
0
2-(benzo [d] oxazol-2-ylamino)-N-((3-
HN II Me NI ' 0 Ill hydroxyoxetan-3-yl)methyl)-1-
8.6
26 1-1y 1\1---. -4N N methy1-1H-benzo[d]imidazole-5-
2.0 26
H carboxamide
0
P
0
2
. Me
u,
HN NI - 0 lik 2-(benzo[d]oxazol-2-ylamino)-
N-(2-
27 N---; N ---(
N
(2-hydroxy-2-methylpropoxy)ethyl)-
ND
17 10.6
1-methyl- [d] imidazole-5-
H
,,
,,0
,
,
o
0
S4M- e carboxamide
:'
OH
Me
0
HN ==,
NMeI 0 IP 2-(benzo[d]oxazol-2-ylamino)-1-
28
NNN
H methyl-N-(2-(pyrrolidin-1-yl)ethyl)-
1.6* 41
1H-benzo [d] imidazole-5-carboxamide
11.3
1-d
/ ¨IN
\--)
n
1-i
cp
t..)
=
t..)
=
'a
t..)
-4
t..)
.6.
=
0
* Me
HN Nr 0 lip
19.3
2-(2-(2-(benzo [d] oxazol-2-ylamino)-
-A
N
H 1-methyl-1H-benzo [d] imidazole-5-
ND 12 0
29 N--- N
t..)
=
<0 carboxamido)ethoxy)acetic acid
t..)
o
i-J
,-,
o
co2H
c,.)
0 2-(2-(2-(benzo [d] oxazol-2-
ylamino)-
30 Me 0 0 0 1-
methyl-1H-benzo [d] imidazole-5- 3.2
nne'Coc)N 0 N X---N
3.5* 25-53
H ¨NH carboxamido)ethoxy)ethyl DL-
NH2 HCI N valinate hydrochloride
Me
Me
N 1-methyl-2-((6-
31 ¨NH
HO =
(trifluoromethyl)benzo [d] oxazol-2- 14.9 P
31 N )7-0
1.1* 25-29
N yl)amino)-1H-benzo [d] imidazole-
5- 2
0
lei carboxylic acid
t..)
.
o t4
cF3
.6. "
Me
2
,
H 0 IV;
2¨NH N-(2-
methoxyethyl)-1-methyl-2-((6- 2,1
N).
32 MeON N )7-0
(trifluoromethyl)benzo [d] oxazol-2-
0.5*
14-50 1.4
N
yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
0 lei
cF3
Me
i
N H N-(2-
hydroxyethyl)-1-methy1-2-((6-
0 --N
33 H ---0
(trifluoromethyl)benzo [d] oxazol-2-
0.3*
32-45 0.9 1-d
n
yl)amino)-1H-benzo [d] imidazole-5- 1-i
HO/----./N N to
0 carboxamide
cp
t..)
cF3
o
t..)
o
O-
t..)
-4
t..)
.6.
o
Me N-(2-(2-hydroxyethoxy)ethyl)-1-
Ni H
N methyl-24(6-
34 H *
N )F (trifluoromethyl)benzo [d] oxazol-
2- 0.8* 31-36 8.0
,N
0
HO,/---0' -' 0 N4
yl)amino)-1H-benzo [d] imidazole-5-
CF3
carboxamide
t..)
o
t..)
o
40, ,Me CF3 N-
(2-hydroxypropy1)-1-methy1-24(6- o
HN N 0 fi (trifluoromethyl)benzo [d] oxazol-
2- <2 ,.tD
35
1.2* 37
N"-L -A yl)amino)-1H-benzo [d] imidazole-5-
HO.-- N N
H carboxamide
Me
0 cF3
sit ,Me 1-methyl-N-(2-(pyrrolidin-l-
yl)ethyfl-
HN y 0 lip 2-((6-
36
N"-- --4
N N
H (trifluoromethyl)benzo [d] oxazol-2-
1.3*
42
yl)amino)-1H-benzo [d] imidazole-5-
2.8
P
\ - - = ) carboxamide
2
u,
0 C F 3
vi
it Me 1-methyl-N-(2-(piperidin-l-
yflethyl)- ,,
2
HN y- 0 se 2-((6-
37
N"--N -A
N
H (trifluoromethyl)benzo [d] oxazol-2- ND 28
yl)amino)-1H-benzo [d] imidazole-5-
<2 :'
-
(\l)
carboxamide
F3c 0N-(2-(2-hydroxypropoxy)ethyl)-1-
N 0 Me methyl-24(6-
<2
38 0-A N
N -) (trifluoromethyl)benzo [d] oxazol-
2- 1.3* 30 1-d
HN¨ 0 H OH yl)amino)-1H-benzo [d] imidazole-5-
n
1-i
N
i carboxamide
Me
ci)
n.)
o
n.)
o
n.)
-4
n.)
.6.
o
0 CF3
. ,Me N-(2,3-dihydroxypropy1)-1-methyl-2-
HN N 0 /11 ((6-(trifluoromethyl)benzo [d]
oxazol- 2.1
39
H0/4 -JN
N
H 2-yl)amino)-1H-benzo [d] imidazole-
5-
OH N N
carboxamide
0.95* 56-82 0
t..)
o
n.)
=
i-J
0 CF3
o
410 ,Me N-(2-(2-
(...)
(...)
HN NI 0 IIIP
(dimethylamino)acetamido)ethyl)-1-
40 N.--N---N
H methyl-24(6-
0.69* 56-93 0.5
NH (trifluoromethyl)benzo [d] oxazol-
2-
7---- yl)amino)-1H-benzo [d] imidazole-5-
Me2N 0 carboxamide
0 CF3
40, me
P
HN N - 0 IP 2-(2-(1-methy1-24(6-
.
41
NI"'-' --4
N N
(trifluoromethyl)benzo [d] oxazol-2-
4.8* 35-39 13.8
yl)amino)-1H-benzo [d] imidazole-5- H
,
t..)
.
o 6-
o,
,,
(0 carboxamido)ethoxy)acetic acid
,,0
,
,
.
co2H
-
F3c 0N-(2-(2-hydroxy-2-
N 0 Me methylpropoxy)ethyl)-1-methy1-2-((6-
3.6
42 1,Nne
c)-1( ,N 0 N(:)/' (trifluoromethyl)benzo [d] oxazol-
2- 2.3* 29
HN¨K H yl)amino)-1H-benzo [d] imidazole-5-
N
i carboxamide
Me
F3C r& N-(2-(2-hydroxyethoxy)ethyl)-1-
1-d
o o methyl-24(5-
8.0
n
1-i
43 N---=( N N(:)0H
(trifluoromethyl)benzo [d] oxazol-2- 1.3* 56
HN¨ 0 H yl)amino)-1H-benzo [d] imidazole-5-
cp
t..)
N
=
i carboxamide
t..)
Me
O'
n.)
-4
n.)
.6.
o
1\Me
HO 01 ¨NH -- 1-methyl-2-((6-
N )i-0 (trifluoromethoxy)benzo [d]oxazol-2-
0
44
1.3* 9-14 ND
0 N yl)amino)-1H-benzo [d] imidazole-5-
t..)
I. OCF3 carboxylic acid
=
i-J
,-,
c'
Me
c,.)
yD
H 110N
1 ¨NH
N-(2-methoxyethyl)-1-methy1-2-((6-
MeON
N ii--0 (trifluoromethoxy)benzo [d]oxazol-2- 0.2
0.64* 10 - 28
45 0 N
101 yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
OCF3
O OCF3
Me 1-methyl-N-(1H-pyrazol-4-y1)-24(6-
46 HN . y" 0 Sto (trifluoromethoxy)benzo
[d]oxazol-2-
2.2* 14-20 <5 P
2
.-z-----.. N----N-AN oxam
yl)amino)-1H-benzo [d] imidazole-5-
N
\-NH H
carbide t..) .
o t4
--4
,,
0 N-(1,3-dihydroxy-2-
)`"
OCF3
.
Me (hydroxymethyl)propan-2-y1)-1-
HN IF y' 0 lip methyl-24(6-
<2 )
'
47 HO
N--- - (trifluoromethoxy)benzo [d] oxazol-
2- 1.6* 13-16
\---e¨OH N A N
H yl)amino)-1H-benzo [d] imidazole-5-
OH carboxamide
O OCF3 N-(1,3-dihydroxypropan-2-
y1)-1-
II ,Me methyl-24(6-
0 /11 HN N
3.9
48 e -M N"--L -A (trifluoromethoxy)benzo [d]oxazol-2-
2.0* 13-20 1-d
n
OH N N
H yl)amino)-1H-benzo [d] imidazole-5-
OH carboxamide
cp
t..)
=
t..)
O-
t..)
-4
t..)
.6.
o
0 OCF3
= ,Me N-(2-(2-
HN N 0 111, (dimethylamino)acetamido)ethyl)-1-
49 NL N N
H methyl-24(6-
0.8* 14-23 <2 0
t..)
=
w
NH (trifluoromethoxy)benzo [d] oxazol-
2-
c¨i yl)amino)-1H-benzo [d] imidazole-5-
,-,
o
Me2N 0 carboxamide
(...)
(...)
,z
O OCF3 1-methyl-N-(1-methyl-1H-pyrazol-4-
Me
HN 104 y" 0 I* y1)-24(6-
4.0
50 (trifluoromethoxy)benzo [d] oxazol-
2- ND 12-18
-----...---. N--- .-A
N N yl)amino)-1H-benzo [d] imidazole-5-
\ H
N-NsMe carboxamide
O
OCF3 p
1-methyl-N-(oxetan-3-y1)-2-((6-
.
51 HN 104 NMe (trifluoromethoxy)benzo [d] oxazol-
2-
1.4* 30-57 <2 ,
6 '
H yl)amino)-1H-benzo [d] imidazole-5-
NNN carboxamide
t..) .
o ril
cee
,,
.
,,
0
,
,
0
0 OCF3 N-((3-hydroxyoxetan-3-yl)methyl)-1-
' ,
,,
HN 410 IN'IVie 0 11* methyl-24(6-
<2
52 (trifluoromethoxy)benzo [d] oxazol-
2- 1.0 27
H(12...y N---- --4
N N yl)amino)-1H-benzo [d] imidazole-5-
H
0 carboxamide
O OCF3
410, ,Me N-(2-hydroxyethyl)-1-methy1-2-((6-
HN N 0 . (trifluoromethoxy)benzo [d] oxazol-
2- 7.3 1-d
53
N'L
N N
H yl)amino)-1H-benzo [d] imidazole-5-
carboxamide
1.1* 29-38 n
1-i
cp
OH
w
o
w
o
O-
w
-4
w
.6.
o
0 ocF3 1-methyl-N-(2-
(methylsulfonyl)ethyl)-
. ,Me 2-((6-
HN y 0 Ilip
54
N--- --A
N
H (trifluoromethoxy)benzo[d]oxazol-2- 3.2*
N
yl)amino)-1H-benzo[d]imidazole-5- 31-37 ND 0
t..)
=
t..)
SO2Me carboxamide
=
0
,-,
H0
2C . Me ---\
2-((6-(2-
o
N' 0 . Ls'OMe
methoxyethoxy)benzo[d]oxazol-2- ,.tD
NL yl)amino)-1-methyl-1H- 4.5 15 ND
N N
H benzo
[d] imidazole-5-carboxylic acid
Me 2-((6-(2-
ni
i-i 101 ¨NH methoxyethoxy)benzo[d]oxazol-2-
9.7
56 Me0 N N )T-0 yl)amino)-N-(2-methoxyethyl)-1-
1.4* 21-38
N P o
methy1-1H-benzo[d]imidazole-5-
W ooMe carboxamide
2
Me
ni N-(2-hydroxyethyl)-24(6-(2-
o t4
,)
H 0 _NE, mth th)bn[d] 2
,,.
eoxyeoxyezooxazol-2- 11. ,
57 HON N )ro
1.2* 34-40
N yl)amino)-1-methy1-1H-
o
'
VI -oMe
benzo[d]imidazole-5-carboxamide
o
Me
ni N-(2-(2-hydroxyethoxy)ethyl)-
24(6-
H 0 ---NH (2-methoxyethoxy)benzo [d] oxazol-2-
7.7
58 Ha.õ----Ø-",-N N )ro
1.7* 41-60
N yl)amino)-1-methy1-1H-
0
ig o,-,onne benzo[d]imidazole-5-carboxamide
1-d
Me
n
1-i
Me 2-((6-isopropylbenzo[d]oxazol-2-
HOC . N ,Me .
c(I)59 0 yl)amino)-1-methyl-1H- 3.3 30 ND t..)
o
t..)
NL benzo[d]imidazole-5-
carboxylic acid c'
-::--,
N N
w
H
-4
t..)
.6.
o
Me
H 0 N ---NH 2-((6-isopropylbenzo[d]oxazol-2-
,,N N )7-.0 yl)amino)-N-(2-methoxyethyl)-1-
0.9 0
60 Me0
N
0.7* 42-56 t..)
c:=
0 methyl-1H-benzo [d] imidazo le-5-
t..)
gl Me carboxamide
o
i-J
,-,
o
Me
vD
Me
0 Me N-(2-hydroxyethyl)-24
. (6-
0 ,Me isopropylbenzo[d]oxazol-2-yl)amino)-
1.2
61 HN NI 0 .
1.5 60
N"-- -4
N H 1-methyl-1H-benzo[d]imidazole-5-
N carboxamide
OH
0 OCHF2
P
.
2-((6-
Me N'
u,
(difluoromethoxy)benzo[d]oxazol-2-
16.2 t..) .
62 0 NI "--0
yl)amino)-1-methy1-1H-
5 .1* 15-24 ,-, 6-
-NH
,,0
HO = benzo [d] imidazo le-5-carboxylic
acid ,
N,I,
0
r,
0 OCHF2 2-((6-
e 411 ,M (difluoromethoxy)benzo[d]oxazol-2-
HN y 0 lip
0.8
63
---N--4
H yl)amino)-N-(2-methoxyethyl)-1-
1.3* 11-15
methyl-1H-benzo [d] imidazo le-5-
N N
OMe carboxamide
0 0CHF2 2-((6-
1-d
411 ,Me (difluoromethoxy)benzo [d] oxazol-2-
n
1-i
HN NI 0 IIP
2.0
64 N
N ----N--4
H yl)amino)-N-(2-hydroxyethyl)-1-
methyl-1H-benzo [d] imidazo le-5-
1.8* 11-18
cp
t..)
=
t..)
o
OH carboxamide
O-
t..)
-4
t..)
.6.
o
Mel H
N N
40 = N-(2-methoxyethyl)-1-methyl-2-((5-
N N
0
65 methylbenzo[d]oxazol-2-yl)amino)-
1.2* 15-42 ND t..)
o
HN 1H-benzo [d] imidazole-5-carboxamide
t..)
o
Me
o
Me-0
c,.)
vD
0
HO el
24(5-fluorobenzo [d] oxazol-2-
66
N-Me yl)amino)-1-methyl-1H-
5.4* 19-22 ND
N--(HN4 1.1 F benzo[d]imidazole-5-carboxylic acid
'----
0
Mel H
P
24(5-fluorobenzo [d] oxazol-2-
N N _O.
,
11 li li
,..,
.
,
67 N N . yl)amino)-N-(2-methoxyethyl)-1-
<5
0.91* 18-56 ,-,
methy1-1H-benzo[d]imidazole-5-
N).
"
HN carboxamide
,
,
7---/ 0
F ,cr'
"
Me-0
.
F
68 HO2C . Me ilo
N, 0 24(6-fluorobenzo[d]oxazol-2-
yl)amino)-1-methyl-1H-
2.0* 16-21 ND
N ---L --- N benzo [d] imidazole-5-carboxylic acid
N
H
MI H
1-d
N N 0
n
=
24(6-((6-2-
69 N N
1-i
. "
yl)amino)-N-(2-methoxyethyl)-1-
<5 cp
F
0.66* 13-36 t..)
methy1-1H-benzo[d]imidazole-5-
=
t..)
HN carboxamide
c'
O-
-4
Me-0
w
.6.
o
Me
F 0 Nj H
6-fluoro-l-methy1-2-((6-
-Ni
70 HO2C N )----O
N/ (trifluoromethoxy)benzo[d]oxazol-2-
3.2* 8-11 ND 0
t..)
yl)amino)-1H-benzo [d] imidazole-5-
=
t..)
Si OCF3 carboxylic acid
o
i-J
,-,
o
Me
F 6-fluoro-N-(2-methoxyethyl)-1-
H 1401 I\I¨NH methyl-24(6-
71 Me,oN N ),---o (trifluoromethoxy)benzo[d]oxazol-2-
0.73* 8-12 0.3
o N
0 yl)amino)-1H-benzo[d]imidazole-5-
carboxamide
ocF3
40 2-(benzo[d]oxazol-2-ylamino)-6-
N o fluoro-N-(2-(2-hydroxyethoxy)ethyl)-
2.7 P
72 A ,,N 0 N'C)'=OH
1.1* 31-72 -
HN¨K H 1-methy1-1H-benzo [d] imidazole-5-
,
N F carboxamide
,
Me,
1-, õ
n.)
N,
Me
0
"
i
,
N H
}-N
,
N)73 11110 1\ii )T-NH
2((5-fluoro-1H-benzo[d]imidazol-2-
-
HO2C N . yl)amino)-1-methy1-1H-
2.9* 8-12 ND
benzo[d]imidazole-5-carboxylic acid
F
HO2C 0
rNMe2 1-(2-(dimethylamino)ethyl)-24(6-
1-d
n
N-I (trifluoromethoxy)benzo[d]oxazol-2-
1-i
74
N-- N
--=< s yl)amino)-1H-benzo[d]imidazole-5-
3.6* 17-28 ND
cp
t..)
HN- carboxylic acid
c'
t..)
0 OCF3
c'
'a
w
--4
w
.6.
o
HO2C 0
rOMe 1-(2-
methoxyethyl)-24(5-((5
75 N-1
(trifluoromethoxy)-1H- 0 11.6
0
H
.37* 15-25
benzo[d]imidazol-2-yl)amino)-1H-
t..)
o
N¨HN4 1.1
t..)
benzo[d]imidazole-5-carboxylic acid
=
i-J
N OCF3
o
c..)
OMe
c..)
rj
N N,1-bis(2-methoxyethyl)-2-((5-
H 101 ¨NH
(trifluoromethoxy)-1H-
2.7*
18-20 ND
76 MeON N )7z---N benzo[d]imidazol-2-yflamino)-1H-
o HN
S' benzo[d]imidazole-5-carboxamide
OCF3
OMe
P
rd
.
w
,
N 1-(2-methoxyethyl)-24(6-
t..)
.
,-,
6-
77 1.1 ¨NH (trifluoromethoxy)benzo[d]oxazol-2-
HO2C N )7-0 yl)amino)-1H-benzo[d]imidazole-5-
c,"
,
N carboxylic acid
0
OCF3
OMe
rj
N N,1-bis(2-methoxyethyl)-2-((6-
101 ¨NH (trifluoromethoxy)benzo [d]oxazol-2-
78 2.2* 21-23 ND
N
MeONH )7--0 yl)amino)-1H-benzo[d]imidazole-5-
e
1-d
o N
lei OCF3 carboxamid
n
1-i
cp
t..)
=
t..)
=
'a
t..)
-4
t..)
.6.
=
Ho2c A
0
1-(tetrahydro-2H-pyran-4-y1)-2-((6-
N
(trifluoromethoxy)benzo [d] oxazol-2-
79
4.0* 27-51 12.4 0
N¨ N
yl)amino)-1H-benzo[d]imidazole-5- t..)
o
HN¨ 01 carboxylic acid
t..)
=
i-J
0 OCF3
,-,
o
N N-(2-methoxyethyl)-1-(tetrahydro-2H-
N pyran-4-y1)-2-((6-
80 1 N I e 11 I 01 ¨NH
(trifluoromethoxy)benzo[d]oxazol-2- 1.9* 22-23 ND
)i--0
yl)amino)-1H-benzo [d] imidazole-5-
0 N
elcarboxamide
OCF3
P
,0:
w -
1-,
.6.
,,
N-(2-methoxyethyl)-1-(tetrahydro-2H-
2
N ,
,
OMe pyran-4-y1)-2-((5-(trifluoromethoxy)- . 81
NE I 0 ¨NH 2.3* 19-30 ND .. ,
N )-_-:--__N
1H-benzo [d] imidazol-2-yl)amino)-
."
0 HN
101 1H-benzo [d] imidazole-5-carboxamide
OCF3
Me
01 N
¨NH
2-((5-chloro-1H-benzo[d]imidazol-2 1-d
n
)
1-i
82 HO2C N N ¨NH -
yl)amino)-1-methyl-1H-
2.4* 29-33 ND
101
benzo[d]imidazole-5-carboxylic acid cp
t..)
o
t..)
o
O-
t..)
CI
-4
t..)
.6.
o
Me
I-1 01 N¨NH 2-((5-chloro-1H-benzo [d] imidazol-
2-
N
Me0 ¨ 0 N i----NH
yl)amino)-N-(2-methoxyethyl)-1- t..)
83 N
1.8* 16-21 ND =
t..)
methyl-1H-benzo [d] imidazole-5-
o
0
IS carboxamide
,-,
o
CI
rMe
N 1-ethyl-2-((6-
84(trifluoromethoxy)benzo [d] oxazol-2-
HO2C N )i---0
5.4 17 ND
N yl)amino)-1H-benzo [d] imidazole-5-
carboxylic acid
OCF3
P
rMe
Nu,
H SI ¨NH 1-ethyl-N-(2-methoxyethyl)-2((6-
t..) .
u,
85 MeON N )7--0 (trifluoromethoxy)benzo [d] oxazol-
2-
1.3* 13-36 3.3 "
"0
carboxamide
:
0 N
WI yl)amino)-1H-benzo [d] imidazole-5-
'
N)
OCF3
rMe
N
H SI ¨NH 1-ethyl-N-(2-hydroxyethyl)-24(6-
86 HON N )/----0 (trifluoromethoxy)benzo [d] oxazol-
2-
1.3* 15-32 7.1
0 N
WI yl)amino)-1H-benzo [d] imidazole-5-
ocF3 carboxamide
1-d
n
1-i
cp
t..)
=
t..)
=
'a
t..)
-4
t..)
.6.
=
ro)
N 1-((tetrahydrofuran-3-yl)methyl)-2-
0 ¨NH ((6-(trifluoromethoxy)benzo [d]
oxazol- 0
t..)
87
6.8 21 ND
N
=
HO2C =)7-0 2-yl)amino)-1H-benzo [d] imidazole-5-
t..)
o
N
i-J
SO carboxylic OCF3 acid
o
ro N-(2-methoxyethyl)-1-
N
H 401 -NH ((tetrahydrofuran-3-yl)methyl)-2-((6-
88 MeON N )T-0 (trifluoromethoxy)benzo [d] oxazol-
2- 1.5* 13-37 3.9
o N
lei yl)amino)-1H-benzo [d] imidazole-5-
ocF3
carboxamide
P
SO2Me
rd
. õ
,,
N 1-(2-(methylsulfonyl)ethyl)-24(6-
"0
,
101 ¨NH (trifluoromethoxy)benzo [d] oxazol-
2- '
89 N
ND 10 ND ' ,
HO2C =)7-0 yl)amino)-1H-benzo [d] imidazole-5-
" N carboxylic acid
0 OCF3
SO2Me
rj N-(2-methoxyethyl)-1-(2-
N H (methylsulfonyl)ethyl)-24(6-
1-d
10I -NH
n
90 MeON N )/---0 (trifluoromethoxy)benzo [d] oxazol-
2- 4.8 22 ND
o N
el yl)amino)-1H-benzo [d] imidazole-5-
ocF3
carboxamide
cp
t..)
o
t..)
o
O-
t..)
-4
t..)
.6.
o
Br
H0
2C 40 ,Me ilo 2-((6-bromobenzo [d] oxazol-2-
91 N
2.6
0
N"-LN -AN benzo
[d] imidazole-5-carboxylic acid t..)
o
o
O
OMe 4''J
1-
411 ,Me N-(2-hydroxyethyl)-24(6-
=
4,4
HN N 0 =
methoxybenzo[d]oxazol-2-yl)amino)- 3.5 vD
--IN -4
N N
H 1-methy1-1H-benzo [d] imidazole-5-
92 N
carboxamide
1.4* 12-20
OH
Hlo
N 1-((3-
hydroxyoxetan-3-yflmethyl)-2-
1. ¨NH ((6-(trifluoromethoxy)benzo [d]
oxazol-
93
ND 22 ND
N 70 H 02C )-
2-yflamino)-1H-benzo[d]imidazole-5-
P
101 carboxylic acid
2
N
u,
OCF3
-4 ,,
/0\
,,0
,
O \OH 0CF3
1-((3-hydroxyoxetan-3-y0methyl)-N-
,I,
7,
(2-methoxyethyl)-24(6-
'
94 HN =
NI r 0 N '1(trifluoromethoxy)benzo [d] oxazol-2- 1.3 28 0.3
--4 "
N
H
yl)amino)-1H-benzo[d]imidazole-5-
1\1 carboxamide
OMe
/0\
O \OH OCF3 N-(2-hydroxyethyl)-
14(3-
hydroxyoxetan-3-yl)methyl)-2-((6-
1-d
n
1-i
95 HN = NI r 0 =
(trifluoromethoxy)benzo[d]oxazol-2- 1.6 21 ND
cp
N---
"--
N
H
yl)amino)-1H-benzo[d]imidazole-5-
NI carboxamide
t..)
=
t..)
=
O-
OH
4,.)
--.4
.6.
o
CI
HO2C ito ,Me sp N 24(6-((6-2-
96 0 yl)amino)-1-methyl-1H-
2.8* 39-56 ND
N---L -4 benzo[d]imidazole-5-carboxylic acid
0
w
N N
o
H
w
o
0 CI
1¨
11 ,Me 2-((6-chlorobenzo [d] oxazol-2-
o
HN N 0 if yl)amino)-N-(2-hydroxyethyl)-1-
c,.)
97
N'LN -A
N
H methy1-1H-benzo [d] imidazole-5-
carboxamide
1.4* 33-51 <5
OH
OCH2CF3 1-methy1-24(6-(2,2,2-
HO2C 1110 ,Me .
98 N 0 trifluoroethoxy)benzo[d]oxazol-2-
4.1*
36-56 ND
N---L -A yl)amino)-1H-benzo [d] imidazole-5-
N N carboxylic acid
H
p
o ocH2c F3
N-(2-methoxyethyl)-1-methy1-2-((6-
.. 2
410 ,Me (2,2,2-
t..)
.
HN N 0 leo
0.4 ,-, 6-
99
¨.-L.--
H trifluoroethoxy)benzo[d]oxazol-2-
0.92* 25-31
yl)amino)-1H-benzo [d] imidazole-5-
cio ,,
,,0
N N N
,
,I,
OMe carboxamide
7,
0 OC H2C F3 N-(2-hydroxyethyl)-1-methyl-2-
((6-
= ,Me (2,2,2-
HN N 0 leo
5.3
--L-A
H trifluoroethoxy)benzo [d] oxazol-
2-
1.2*
24-35
yl)amino)-1H-benzo [d] imidazole-5-
100 N N N
OH carboxamide
0 2-((6-(2-
111,
1-d
HO2C ,Me
n
101 N 0
i 11 H 0 hydroxyethoxy)benzo[d]oxazol-2-
ND
21 ND
N''L -- yl)amino)-1-methy1-1H-
cp
N N benzo [d] imidazole-5-carboxylic
acid t..)
o
H
w
o
'a
w
--4
w
.6.
o
0 0---\ N 2-((6-(2-
HN
IF ,Me `---OH
hydroxyethoxy)benzo yl)amino)-N-(2-methoxyethyl)-1-
)benzo [d] oxazol-2-
0 .
5.3
102
NN)'"N
H
methy1-1H-benzo[d]imidazole-5- 1.3* 26-40 0
t..)
o
t..)
OMe carboxamide
=
i-J
0 0---\ 2-((6-(2-
o
11, , `---OH
hydroxyethoxy)benzo [d] oxazol-2- c,.)
vD
HN =NMe o .
yl)ano)-N-(2-hydroxyethyl)-1-
3.1*
37-39
>20
103
NN '"N
H mi
methy1-1H-benzo [d] imidazole-5-
OH carboxamide
HO 02-((6-(2-
N o
hydroxyethoxy)benzo [d] oxazol-2-
104 OA N N (:)H yl)amino)-N-(2-(2-
3.7 23 6.6
HN- H el
N hydroxyethoxy)ethyl)-1-methy1-1H-
P
Mei
2
benzo [d] imidazole-5-carboxamide
,-,
õ
HO o ---.
VD n,
0 N-(2-aminoethyl)-2-((6-(2-
N)0
,
0--1 N
N NH2
hydroxyethoxy)benzo [d] oxazol-2-
H N sp H Ha
N,
105 N yl)amino)-1-methyl-1H-
4.1 36 ND
Rid
benzo [d] imidazole-5-carboxamide
hydrochloride
HO 0 N-(2-((4,5-dihydro-1H-imidazol-2-
N 0
H yl)amino)ethyl)-24(6-(2-
1-d
106
hydroxyethoxy)benzo[d]oxazol-2- ND 6 ND n
0-1 iN aiii N.........õ.N.õsõNõ,
1-i
H HN--7 yl)amino)-1-methy1-1H-
N 114111111
Mei benzo [d] imidazole-5-
carboxamide cp
t..)
o
t..)
o
O-
t..)
-4
t..)
.6.
o
CF3 2((5-fluoro-6-
107
HO2C 0 Me sp
(trifluoromethyl)benzo[d]oxazol-2-
N- 0
F 4.5 45 ND
N-JN yl)amino)-1-methy1-1H-
0
w
N N benzo [d] imidazole-5-carboxylic acid
=
H
w
o
0 CF3 2((5-fluoro-6-
,-,
'S Me (trifluoromethyl)benzo[d]oxazol-
2- o
HN N 0 .
F 0.3
,.tD
yl)amino)-N-(2-hydroxyethyl)-1-
1.5 29 108
N---L -4
N N
H methy1-1H-benzo [d] imidazole-5-
OH carboxamide
0
. me /N----_:\ N-(2-methoxyethyl)-1-methy1-2-
HN N- 0
7.4
NN ----)--' (oxazolo[5,4-19]pyridin-2-
ylamino)- 6.1 26
109
N N
H 1H-benzo [d] imidazole-5-carboxamide
P
OMe
.
0
u,
w -
HN . N-Me 0.-) N-) N-(2-hydroxyethyl)-1-
methyl-2-
18.9
o N)N /
H xazolo[5,4-19]pyridin-2-ylamino)-
7.6 60
1H-benzo [d] imidazole-5-carboxamide "0 110
N N N
(o
,
,
'
,
OH
."
HO2C 10. 1-methyl-2-(oxazolo[4,5-19]pyridin-
2-
ylamino)-1H-benzo [d] imidazole-5- ND 3 ND
NN N
N carboxylic acid
H
0
HN
4 N1 ,Me ¨ N-(2-methoxyethyl)-1-methyl-2-
1-d
n
112
N-- N 0 N H (oxazolo[4,5-
b]pyridin-2-ylamino)- ND 15 ND
1H-benzo [d] imidazole-5-carboxamide N
cp
t..)
w
OMe
'a
w
--4
w
.6.
o
0 CF3
N
41 ,Me _si ]PYridin-2- 1 ) N-
(2-hydroxyethyl)-1-methyl-2-((5-
HN I 0 \ / (trifluoromethyl)oxazolo[5,4-
9.1
113
N"-N-AN
H b -1H-
Y )amino
benzo [d] imidazole-5-carboxamide
ND 20 0
t..)
o
n.)
OH
0
1-,
o
. Me N--r----\
c,.)
vD
HN =N 114 N-(2-(2-hydroxyethoxy)ethyl)-1-
N-:NN--'(N N
H methyl-2-(thiazolo[4,5-b]pyrazin-
2-
ND
33 12.2
ylamino)-1H-benzo [d] imidazole-5-
0
Scarboxamide
HO
24(6-((6-2- p
Apo ,Me
115 HO2C N 0 yl)amino)-1-methyl-1H- 6.2
71 ND 2
Nr-L -A benzo [d] imidazole-5-carboxylic
acid
t..)
.
N N
H
,,
,,0
,
0
.
7,
24(6-((6-2- '
,
.
yl)amino)-N-(2-methoxyethyl)-1- 116 HN =NI
Me 0 3.0* 32-38 ND
A
H methy1-1H-benzo [d] imidazole-5-
N--.N.-N carboxamide
OMe
= , 40 N-(2-(2-hydroxyethoxy)ethyl)-1-
r3s, N o methyl-24(7-
1.2
117 o---1( N 0 N,0,.0õ, (trifluoromethyl)benzo [d] oxazol-2-
1.8* 38 1-d
HN¨ H
yl)amino)-1H-benzo [d] imidazole-5- n
N
1-3
i
Me carboxamide
cp
t..)
o
t..)
o
t..)
-4
t..)
.6.
o
0 N-(2-(2-hydroxyethoxy)ethyl)-24(1-
118 N o methy1-1H-benzo [d] imidazol-2-
8.0 24 ND
N--1( N N -OH yl)amino)benzo[d]oxazole-5-
0
Me/ HN¨ 0 H
w
0 carboxamide
=
t..)
o
Me
I
1-,
o
H 0 --NH 2-(benzo[d]oxazol-2-ylamino)-N-(2-
c,.)
0.5
119 Me00,N N )7---0 methoxyethoxy)-1-methyl-1H-
0.6* 47-65
0 N benzo[d]imidazole-5-carboxamide
1.1
0
= ,Me
HN y 0 lip 2-(benzo [d]oxazol-2-ylamino)-N-(2-
120 b N--N -A hydroxypropoxy)-1-methy1-1H-
2.0 26 6.5
N N
P
H benzo[d]imidazole-5-carboxamide
.
0H
5¨
,
Me
40 2-(benzo[d]oxazol-2-ylamino)-N-(2-
t..) ,,
.
o o 0 Me (2-
9.0
"
'7
0
121 N--=-( N 0 N-ON)N-me (dimethylamino)acetamido)ethoxy)-1-
1.4* 22-23 7,
HN¨ H H methyl-1H-benzo[d]imidazole-5-
.
N
Me/ carboxamide
Me
Ni N-(2-(dimethylamino)ethoxy)-1-
H 1.1 ¨NH
methyl-24(6-
me2N ,70.-N N
)i-0 5.1
122 N (trifluoromethyl)benzo[d]oxazol-2-
0.4* 51-67
0
ISI yl)amino)-1H-benzo[d]imidazole-5-
1-d
n
cF3 carboxamide
cp
t..)
o
t..)
o
O-
t..)
-4
t..)
.6.
o
0
HN
. N ,Me
I 0 11* 2-(benzo [d] oxazol-2-ylamino)-N-
(2-
123 b N -- \ N --"N (dimethylamino)ethoxy)-1-
methyl- ND 22 10.5
0
H 1H-benzo [d] imidazole-5-
carboxamide t..)
=
t..)
o
M e2N
t''J
1-,
o
Me
c,.)
Ni N-(2-methoxyethoxy)-1-methy1-24(6-
c,.)
124 H 101 =¨=1\1H
(trifluoromethyl)benzo [d] oxazol-2-
N )i- 0
0.2* 31-52 <2
N yl)amino)-1H-benzo [d] imidazole-5-
o
WI carboxamide
c F3
Me
Ni
H N-(2-hydroxyethoxy)-1-methyl-24(6-
((6
125 (trifluoromethyl)benzo [d] oxazol-
2- 3.5 p
N 0 N / ))--- N H i- 0
0.3* 32-59
N yl)amino)-1H-benzo [d] imidazole-5-
2
carboxamide
0 =cF3
t..)
.
,,
Me
,,0
Ni N-(2-hydroxyethoxy)-1-methy1-24(6-
:'
126 H I. ,)¨NH
H 0 (:), N N )i-0 (trifluoromethoxy)benzo [d] oxazol-
2-
0.5*
33-37 2.1 ,,'
'
N yl)amino)-1H-benzo [d] imidazole-5-
o
WI carboxamide
oc F3
Me
Ni N-(2-methoxyethoxy)-1-methy1-24(6-
127 10 --NH
(trifluoromethoxy)benzo [d] oxazol-2-
<2
Me0 (:). N )7- o
0.5* 27-36 1-d
N yl)amino)-1H-benzo [d] imidazole-5-
n
o
VI carboxamide
cp
oc F3
N
0
N
0
7a
N
--4
N
.6.
0
0 0---\
* ,Me lik µ---.0Me N-(2-hydroxyethoxy)-2-((6-(2-
HN N 0
4.7
128 b N-----'L methoxyethoxy)benzo[d]oxazol-2-
1.2* 48-75 0
N N yl)amino)-1-methy1-1H-
t..)
S H
benzo [d] imidazole-5-carboxamide
=
t..)
o
i-J
HO
o
0
0 --\
c,.)
sli ,Me µ"-OH N-(2-hydroxyethoxy)-2-((6-(2-
vD
HN N 0 ilk
14.1
129 b N-::--L hydroxyethoxy)benzo[d]oxazol-2-
3.4* 54-58
N N yl)amino)-1-methy1-1H-
S H
benzo [d] imidazole-5-carboxamide
HO
0 CF3
. ,Me 24(5-fluoro-6-
HN NI 0 . F (trifluoromethyl)benzo[d]oxazol-2-
<2
p
130 yl)amino)-N-(2-hydroxyethoxy)-1- 0.74* 42
N
) H methy1-1H-benzo [d] imidazole-5-
carboxamide
2
t..)
.
HO
.6. ,,
,,0
Me
N N-(2-hydroxyethoxy)-1-methy1-24(6-
:'
'
H 40
,,
¨NH methyl-4,5,6,7-
'
H00,N N
6.7
131 )-i-S tetrahydrobenzo[d]thiazol-2-
1.4* 35-62
0 Nt yl)amino)-1H-benzo [d] imidazole-
5-
carboxamide
Me
Me
0 0
Iv
Illo ,Me 2-((6-acetylbenzo [d] oxazol-2-
n
1-i
HN NI 0 le= yl)amino)-N-(2-methoxyethoxy)-1-
3.1
132
b N---\N -A methy1-1H-benzo [d] imidazole-5-
1.5 39
cp
S N
H carboxamide
t..)
o
t..)
o
O-
Me0
n.)
--4
n.)
.6.
o
Me
Me
HO 0 N-(2-hydro xyethyl)-24(6-(2-
133 N o hydro xypropan-2- yl)benzo [d] o
xazol-
5.3
17 9.2
0
o-A N el N OF1
2- yl) amino)-1-methy1-1H- t..)
o
HN¨ H
w
benzo [d] imidazole-5-carboxamide
MeIN
o
1-,
=
si------N
w
w
..--
IW N-(2-(2-hydroxyethoxy)ethyl)-1-
,.tD
134 N o methyl-2((6-(thiazol-4-
1.4*
42-70 <2
0A N 0 N 00Fi yl)benzo kilo xazol-2- yl)amino)-
1H-
HN H benzo [d] imidazole-5-carboxamide
MN
0
----0
Me -N N-(2-(2-hydroxyethoxy)ethyl)-1-
methyl-2-((6-(3-methyl-2-
135 N 0
0 xoo xazolidin-5- yl)benzo [d]oxazol-2-
NA 1 ND 2
,
O-A N N OH
Lnu'
HN 0 H yl)amino)-1H-benzo [d] imidazo le-5-
t..)
.
N carboxamide
u, "
Mel
2
'7
S'
(--0\
r:,
0 N¨"
HN =,Me
N'N/le o 00 methyl-24(6-
N---L -A
N
H morpholinobenzo [d] o xazol-2-
2.9*
36
yl)amino)-1H-benzo [d] imidazo le-5-
136 N
10.6
0 carboxamide
od
)
n
,-i
HO
cp
w
o
w
o
O-
w
--4
w
.6.
o
0 NO
410 ,Me
N-(2-(2-hydroxyethoxy)ethyl)-1-
0
HN y a lip
,..,
137 H methyl-
24(6-((6-l-
1.3*
24-70 6.9
y1)benzo [d]oxazol-2-yl)amino)-1H-
t..)
o
i-J
,-,
0 benzo [d] imidazole-5-carboxamide
=
vD
HO
N N-(2-(2-hydroxyethoxy)ethyl)-1-
o methyl-24(6-((6-2H-pyran-4-
0
138 OA N N (:)0 H
yl)oxy)benzo[d]oxazol-2- 2.0* 20 ND
HN¨ 140 H yl)amino)benzo [d] oxazole-5-
N
Me/ carboxamide
40
P
2
N 0 1-(2-(benzo[d]oxazol-2-ylamino)-1-
<2
139 methy1-1H-benzo [d] imidazol-5-y1)-
2.6 21 t..) .
o-i(FIN¨N op C F3 C:
2,2,2-trifluoroethan-l-one
N
2
Me/
'0Al
40 N N_OH (Z)-1-(2-(benzo[d]oxazol-2-ylamino)-
.
-
140 1 1-methy1-1H-benzo[d]imidazol-5-y1)-
ND 19 ND
cF3
o---/(HN¨N 0 N 2,2,2-trifluoroethan-1-one oxime
Me/
40
N NH2 N-(5-(1-amino-2,2,2-trifluoroethyl)-
1-
9.0
o---/( N 141
methyl-1H-benzo[d]imidazol-2- 3.6* 22 1-d
HN¨ 0 C F3
n
N yl)benzo
[d]oxazol-2-amine
Me/
CP
w
o
w
o
O'
w
-4
w
.6.
o
0
N OH 1-(2-(benzo[d]oxazol-2-ylamino)-1-
142 N
O methyl-1H-benzo[d]imidazol-5-y1)- 3.3* 27 ND
HN¨ 0 cF3
2,2,2-trifluoroethan-l-ol
0
t..)
N
o
Me/
w
o
i.i.
w
w
o
*Average of 2 or more EC50 determinations
ND = Not Determined
NA = Not Active
P
2
u,
--4
r.,
N)
'7
2
N)
,-o
n
,-i
cp
t..)
=
t..)
=
-c=-::.--,
t..)
-4
t..)
.6.
=
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
228
Example 145. hERG Assay
Cell lines and cell culture
HEK 293 cell line stably expressing hERG channel (Cat# K1236) was purchased
from
Invitrogen. The cells were cultured in 85% DMEM, 10% dialyzed FBS, 0.1 mM
NEAA, 25 mM
HEPES, 100 U/mL Penicillin-Streptomycin and 5 pg/mL Blasticidin and 400 pg/mL
Geneticin.
Cells were split using TrypLETm Express about three times a week, and
maintained between
¨40% to ¨80% confluence. Before the assay, the cells were transferred onto the
coverslips at 5 x
105 cells /per 6 cm cell culture dish and induced with doxycycline at 1 pg/mL
for 48 hours.
Solution preparations
1) External solution (in mM): 132 NaCl, 4 KC1, 3 CaCl2, 0.5 MgCl2, 11.1
glucose, and 10
HEPES (pH adjusted to 7.35 with NaOH)
2) Internal solution (in mM): 140 KC1, 2 MgCl2, 10 EGTA, 10 HEPES and 5
MgATP (pH
adjusted to 7.35 with KOH)
3) hERG currents were tested in presence of 10 pM compound concentration
with final
concentration of DMSO of 0.1%.
Experimental procedure
1) Removed the coverslip from the cell culture dish and placed it on the
microscope stage in
bath chamber.
2) Located a desirable cell using the 10x objective. Located the tip of the
electrode under the
microscope using the 10x objective by focusing above the plane of the cells.
Once the tip was in
focus, advanced the electrode downwards towards the cell using the coarse
controls of the
manipulator, while simultaneously moving the objective to keep the tip in
focus.
3) When directly over the cell, switched to the 40x objective and used the
fine controls of
the manipulator to approach the surface of the cell in small steps.
4) Applied gentle suction through the side-port of the electrode holder to
form a gigaohm
seal.
5) Used the Cfast to remove the capacity current that was in coincidence
with the voltage
step. Obtained the whole cell configuration by applying repetitive, brief,
strong suction until the
membrane patch ruptured.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
229
6) Set membrane potential to -60 mV at this point to ensure that hERG
channels were not
open. The spikes of capacity current should then be cancelled using the Cslow
on the amplifier.
7) Set holding potential to -90 mV for 500 ms; recorded current at 50 kHz
and filtered at 10
kHz. Leaking current was tested at -80 mV for 500 ms.
8) The hERG current was elicited by depolarizing at +30 mV for 4.8 seconds
and then the
voltage was taken back to -50 mV for 5.2 seconds to remove the inactivation
and observe the
deactivating tail current. The maximum amount of tail current size was used to
determine hERG
current amplitude.
9) Recorded current for 120 seconds to assess the current stability. Only
stable cells with
recording parameters above threshold were applied for the compound
administrations.
10) Firstly vehicle control was applied to the cells to establish the
baseline. Once the hERG
current was found to be stabilized for 5 minutes, test compound was applied.
hERG current in
the presence of test compound was recorded for approximately 5 minutes to
reach steady state
and then 5 sweeps were captured. In order to ensure the good performance of
cultured cells and
operations, the positive control, Dofetilide, with 5 dose concentration was
also used to test the
same batch of cells.
Data analysis
The following criteria were used to determine data acceptability.
1) Initial seal resistance > 1 GQ;
2) Leak currents < 50% of the control peak tail currents at any time;
3) The peak tail amplitude >250 pA;
4) Membrane resistance Rm > 500 MQ;
5) Access resistance (Ra) < 10 MQ;
6) Apparent run-down of peak current < 2.5% per mm.
Data that met the above criteria for hERG current quality were further
analyzed as the following
steps.
1) Percent hERG current inhibition was calculated using the following
equation.
Note: PatchMaster or Clampfit software was used to extract the peak current
from the
original data.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
230
Peak current inhibition = (1- Peak tail current compound x100
Peak tail current vehicle
2) The dose response curve of test compounds was plotted with percentage of
hERG current
inhibition against the concentration of test compounds using Graphpad Prism
6.0, and fit to a
sigmoid dose-response curve with a variable slope.
hERG data for selected compounds in manual patch clamp assay are provided in
Table 2 below.
Notably, hERG values were measured only for compounds that showed >5 M
solubility.
Accurate measurement of hERG activity was not possible for compounds with
solubility below
M.
N ,H
N X.=N
X
R2 R: Methyl
Table 2
hERG
ID# X R1 R2 inhibition
@ 10 uM
Comparator 1 S -CONHCH2CH2OCH2CH2OH -0CF3 66%
Comparator 2 S -CONHCH2CH2NHCOCH2N(Me)2 -0CF3 93%
Compound A S -CONHCH2CH2OCH2CH2OH -H 20%
Ex 71 S -CONHCH2CH2OCH2CH2OH -CH3 6%
Ex 34 0 -CONHCH2CH2OCH2CH2OH -CF3 61%
Ex 122 0 -CONHOCH2CH2N(Me)2 -CF3 87%
Ex 31 0 -COOH -CF3 0%
Ex 137 0 -CONHCH2CH2OCH2CH2OH -N-pyrrolidine 18%
Ex 16 0 -CONHCH2CH2OH H 35%
Ex 17 0 -CONHCH2CH2OCH2CH2OH H 16%
Ex 18 0 -CONHCH2CH2NH2 H 12%
Ex 19 0 -CONHCH2CH2NHCOCH2N(Me)2 H 29%
Ex 23 0 -CONHCH2CH(OH)CH3 H 24%
Ex 25 0 -CONHCH2CH2OCH2CH(OH)CH3 H 8%
Ex 26 0 -CONHCH2C(0H)(-CH2OCH2-) H 18%
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
231
Ex 20 0 -CONHCH2CH2-N-morpholine H 59%
Ex 123 0 -CONHOCH2CH2N(Me)2 H 54%
Ex 28 0 -CONHCH2CH2-N-pyrrolidine H 64%
Ex 121 0 -CONHOCH2CH2NHCOCH2N(Me)2 H 66%
Ex 69 0 -00NHCH2CH20Me F 38%
Ex 56 0 -00NHCH2CH20Me -0CH2CH20Me 35%
Ex 57 0 -CONHCH2CH2OH -0CH2CH20Me 15%
Ex 58 0 -CONHCH2CH2OCH2CH2OH -0CH2CH20Me 11%
Ex 128 0 -CONHOCH2CH2OH -0CH2CH20Me 18%
Ex 62 0 -COOH -OCHF2 13%
Ex 44 0 -COOH -0CF3 15%
Ex 862 0 -CONHCH2CH2OH -0CF3 64%
Ex 53 0 -CONHCH2CH2OH -0CF3 70%
Ex 13 NH -COOH -0CF3 34%
Ex 753 NH -COOH -0CF3 9%
Ex 73 NH -COOH -F 5%
Ex 14 NH -00NHCH2CH20Me -0CF3 87%
1. Example 7 is a tetrahydrobenzo[d]thiazole compound instead of a
benzothiazole compound.
2. R is ethyl.
3. R is CH2CH20Me.
Example 146. Microscale Thermophoresis (MST) Assay/Bach 1 Binding Assay
Outline of protein expression / production
Human BACH1 (construct-ID: 10xHIS-GP-BACH1(aa179-736)-Thrombin-FLAG) was
expressed in Sf9 insect cells. Protein purification was performed using
affinity chromatography,
followed by size-exclusion chromatography. Quality of resulting protein was
judged using SDS-
PAGE analysis.
Assay principle
The MST technology is based on measurement of protein movement along a
temperature
gradient. Thereby, fluorescently labeled protein is loaded into capillaries,
where an infrared laser
heats a small volume. Before and during heating, fluorescent intensity is
measured at the site of
irradiation and the loss of fluorescence in the IR laser focus is quantified.
Two major factors contribute to change of fluorescence signal. First of all,
the so-called
TRIC (Temperature Related Intensity Change) effect is caused by the
temperature-dependency
of fluorophore's quantum yield. Herein, the extent of the temperature
dependence depends on the
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
232
chemical environment, which may be changed by the binding of a ligand (e.g.
compound,
peptide) to the target. Moreover, Kd value between ligand and target can
depend on temperature.
Second, thermophoresis, which is defined as movement of fluorescent molecules
along
temperature gradient, is a major contributor to change of fluorescence.
Hereby, protein
movement along the gradient depends on hydrodynamic radius, charge and
hydration shell.
These properties can change upon association of the labeled protein with
another species, e.g.
compound. Thereby, MST detects the differences in protein movement with
increasing ligand
concentration: from this, the fraction of ligand bound protein and a Kd value
can be determined.
MST assay protocol
For Kd determination of compounds binding BACH1, the following protocol was
applied: 10xHIS-GP-BACH1(aa179-736)-Thrombin-FLAG (expressed and purified at
Proteros)
was fluorescently labeled via its lysine-residues (2nd gen NHS-NT.647 dye).
Experiments were performed using the experimental device Monolith NT.115 Pico,
NanoTemper Technologies, with MST power set to medium and excitation power set
to 2 % at a
reaction temperature of 25 C.
lOnM fluorescently labeled BACH1 was applied to reaction buffer containing 50
mM
HEPES pH 8.0, 100 mM NaCl, 5 % glycerol, 0.05% Tween20 and 1mM DTT in a
reaction
volume of 8 pL using Monolith NT.115 Premium Capillaries. Compounds were
applied at a
maximal concentration of 103pM with 15 subsequent factor 2 dilutions.
Kd data for selected compounds in MST assay are provided in Table 3 below.
Table 3.
Example Number Kd (pM) Kd error (pM)
17 0.25 0.051
33 0.26 0.051
56 0.25 0.15
112 0.56 0.13
124 0.20 0.089
From the data provided in Table 3 above, it can be inferred the compounds of
the present
invention also bind to Bachl.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
233
Example 147. HMOX1 Inducers of the Invention Strongly Increase in vitro
Expression of
HMOX1
RNA isolation and quantification of gene expression
Inducing HMOX1 expression has been shown to reduce vaso-occlusive crises
(VOCs), in
particular, heme-induced vaso-occlusion in Sickle cell disease (SCD) mice
(Belcher JD, et al.
Antioxid Redox Signal 2017, 26:748-762; Krishnamoorthy S, et al. JCI Insight
2017, 2:e96409).
HepG2 cells were seeded at 250k/well in 12-well tissue culture plates coated
with collagen
(collagen I, rat tail (thin plate coating); ENZO Life Sciences) for
approximately
24 hours. The seeded cells were then treated with dose titration of the
compound of Example 17
or dimethyl fumarate (DMF) (a Nrf2 activator; Fisher Scientific) via media
change, with
biological triplicates performed for each treatment. After approximately 24
hours of treatment,
cells were visually inspected to confirm that no toxicity occurred with
compound treatment. The
treated cells were washed with PBS, aspirated, parafilmed and frozen at -80 C
prior to RNA
isolation. RNA was isolated using Machery-Nagel isolation kit (NucleoSpin
RNA: catalog
740955.250) and then quantitated using ThermoFisher Nanodrop.
RNA was diluted for Nanostring gene expression analysis. The diluted RNA at an
input
of 200ng RNA was run on a Nanostring instrument, which was supported with
nCounter
SPRINT Profiler System and n5o1ver4.0 Software, nCounter SPRINT Cartridge,
nCounter
SPRINT Reagent Pack, and nCounter SPRINT Hybridization Buffer. Analysis was
done
manually using raw data obtained from n5o1ver4.0 Software. CLTC, POLR2A, RPL27
and TBP
were used as reference genes for normalization. Data was graphed as fold
change compared to
DMSO treatment.
The relative mRNA levels of the HMOX1 gene in HepG2 cells treated with the
compound of Example 17 or DMF were measured according to the methods described
above.
Note that for gene expression comparative analysis between the compound of
Example 17 and
DMF, HepG2 cells were treated with the test compound for 24 hours (results
shown in FIG. 1).
It was found that the compound of Example 17 induced HMOX1 expression much
more
strongly than another Nrf2 activator DMF (FIG. 1A). For example, 30 M of the
compound of
Example 17 induced HMOX1 expression 4 times higher than 250 M DMF.
The relative mRNA levels were also compared for several other Bachl-responsive
genes
in HepG2 cells treated with the compound of Example 17 versus Nrf2 activator
DMF.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
234
Additionally, the compound of Example 17 and DMF induced FTH1 expressions with
comparable fold increases (FIG. 1B).
Examples 148. HMOX Inducers of the Invention Reduce Vaso-occlusion in SCD Mice
Increase Fetal Hemoglobin (HbF) in SCD Mice
Dosing scheme of HMOX Inducers to HbSS Townes sickle mice
HbSS Townes sickle mice were oral gavaged once daily with Vehicle or the
compound of
Example 17 listed below for 8 days. To minimize experimental inconsistency,
fresh formulation
was prepared every day and vortexed prior to drawing test article into syringe
for each dosing,
and dosing was performed by the same individual throughout the day. The last-
day dose was
administered by oral gavage 4 hours prior to hemin infusion described below.
1. Vehicle (0.5% w/v Tween 80 in 0.45% w/v methyl cellulose)
2. the compound of Example 17 (10 mg/kg PO dosing)
3. the compound of Example 17 (25 mg/kg PO dosing)
4. the compound of Example 17 (50 mg/kg PO dosing)
Measurement of vaso-occlusion (stasis)
Male and female sickle mice, approximately 12 weeks of age, were weighed
before
surgery. Animals were anesthetized with a mixture of ketamine (106 mg/kg) and
xylazine
(7.2mg/kg) and dorsal skin-fold chambers (DSFCs) were surgically implanted. On
the same day
anesthetized mice were placed on a special intravital microscopy stage, and 20-
22 flowing
subcutaneous venules in the DSFC window were selected and mapped. After venule
selection
and mapping, hemin chloride (2.677 mM; Frontier Scientific), dissolved in
sterile saline
containing sodium carbonate (11.36 mM; Sigma-Aldrich) and D-sorbitol (9.59 mM;
Sigma-
Aldrich), was filtered (0.22 pm), diluted 1:10 in sterile saline (267.7 M
hemin, final) and
infused into the tail veins of mice (.012 mug, 3.2 mols heme/kg body weight).
All of the
selected venules were re-examined at 1 h after hemin infusion, and the number
of static (no flow)
venules was counted and expressed as percent stasis. Four hours after hemin
infusion, mice were
euthanized in a CO2 atmosphere and the liver, spleen and kidneys were removed,
flash frozen,
and stored at -85 C.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
235
Measurement of F-cells
Heparinized whole blood was collected 4 hours after hemin infusion from the
inferior
vena cava of HbSS Townes mice administered Vehicle or the compound of Example
17. F-cells
were stained on whole blood smears by the Kleihauer¨Betke method using a fetal
cell stain kit
(Simmler) according to the manufacturer's instructions. F-cells and total
erythrocytes were
counted in 4 separate microscopic fields at 100X magnification for each mouse.
The F-cells were
expressed as a percentage of total erythrocytes (i.e., red blood cells). Human
fetal cord blood was
used as a positive control.
Vaso-occlusion is a hallmark of SCD. To evaluate whether the disclosed
compounds can
effectively reduce vaso-occlusion, heme-induced vaso-occlusion (stasis) was
measured in the
subcutaneous venules of HbSS-Townes sickle mice with implanted dorsal skinfold
chambers
(DSFCs) according to the methods described above. As shown in FIG. 2,
microvascular stasis
was significantly reduced in HbSS-Townes mice administered with the compound
of
Example 17 as compared with those administered with the Vehicle at one hour
after heme
infusion. Furthermore, the compound of Example 17 dose responsively inhibited
microvascular
stasis (FIG. 2). For the compound of Example 17, stasis was reduced to ¨17%
with 10 mg/kg
dosage, to ¨12% with 25 mg/kg dosage, and to ¨5% with 50 mg/kg dosage.
At high enough concentrations, HbF can inhibit hemoglobin S (HbS)
polymerization and
subsequent hemolysis and vaso-occlusion (Krishnamoorthy S, et al. JCI Insight
2017, 2: e96409).
To evaluate whether HMOX Inducers of the Invention can effectively increase
HbF, F-cells (i.e.,
HbF-containing red blood cells) were measured as a percentage of total red
blood cells according
to the methods described above. As shown in FIG. 3, percent F-cells were
significantly
increased in HbSS-Townes mice administered the compound of Example 17 as
compared with
those administered with the vehicle. Specifically, percent F-cells were
increased to ¨55-70% at
all doses tested for the compound of Example 17, more than doubled than the
mice administered
with the vehicle. The increase in percent F-cells did not appear to be dose
responsive.
The results as shown above demonstrate that the compounds of the present
invention are
HMOX inducers and Bach 1 binders/inhibitors, and can be used to treat SCD at
least through
binding Bach 1, increasing HMOX1 activity, increasing HbF, and reducing vaso-
occlusion.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
236
Example 149. Glutathione (GSH) Levels in Primary Human Endothelial Cells
Primary human pulmonary artery endothelial cells (HPAEC) (Lonza # CC-2530)
cultured
in endothelial cell growth medium-2 (EGM2 media, Lonza # CC-3162) were seeded
in white 96-
well plate (Corning # 3610) at 12.5k cells per well and placed at 37 C in 5%
CO2. 18-24hr post
seeding, cells were treated with the compound of Example 17 via media change.
24hr post
treatment with the compound, cells were treated using a freshly prepared hemin
stock (Sigma-
Aldrich #51280 in 0.1N NaOH) alone or with the compound of Example 17. After
30min of
hemin stress, cells were visually inspected for toxicity, with no toxicity
being noted. Media was
aspirated off and GSHGloTM Glutathione Assay (Promega # V6911) was run
following the
manufacturer's protocol. White bottom plate seal (PerkinElmer #6005199) was
used during read.
Analysis was done using Softmax Pro.
The results are shown in Figure 4. While hemin induces oxidative stress in
primary
human pulmonary arterial endothelial cells, thereby reducing GSH levels, pre-
incubation with a
HMOX inducer/Bach 1 inhibitor (e.g., the compound of Example 17) protects
these cells from
hemin mediated oxidative stress.
Example 150. Gene Expression in Primary Human Endothelial Cells
Inflammatory conditions in endothelial cells increase expression of adhesion
molecules,
such as VCAM-1, ICAM-1 and E-selectin through NF-kB signaling. HMOX1 -/-
endothelial
cells show increased expression of VCAM-1 in response to TNF stimulation, as
compared to
HMOX +/+ endothelial cells. See Seldon et al., J Immunol December 1, 2007, 179
(11) 7840-7851.
Primary human pulmonary artery endothelial cells (HPAEC) (Lonza # CC-2530)
were
seeded in 12-well plates (TrueLine #TR5001) at 200k cells per well and placed
at 37 C in 5%
CO2. 18-24hr post seeding, cells were treated with the compound of Example 17
via media
change. 24hr post treatment with the compound, cells were treated with lOng/mL
TNFa
(Invitrogen #PHC3015 in H20) alone or with the compound of Example 17. After 4
hours of
treatment, cells were visually inspected for toxicity, with no toxicity being
noted. Media was
aspirated off, cells were washed once with lx PBS (Corning #21-040-CV),
aspirated dry,
parafilmed and placed at -80 C until RNA isolation. RNA was isolated with the
NucleoSpin
kits (MACHEREY-NAGEL # 740955.250, USA) following kit protocol. RNA was used
to
generate cDNA using the High-Capacity cDNA Reverse Transcription Kit
(ThermoFisher
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
237
#4368814, USA) in 20 pL reactions. After reverse transcription was completed,
cDNA was
diluted 1:10 with water. 20ng cDNA was mixed with water, iQTM SYBR@ Green
Supermix
(BioRad # 170-8886), and 300nM primer (IDT #NM_001078) for a total lOul
reaction and
loaded into 384-well white qPCR plates and subsequently analyzed using BioRad
CFX384. Raw
data was exported from software (Biorad CFX Manager, USA) and imported into a
spreadsheet
(Microsoft Excel, USA). To calculate fold change, the ACt on a per sample
basis was calculated
as Ct (Gene of interest) - Ct (Average of reference genes). The MCt was then
calculated as ACt
(experimental sample) - Average ACt (control group). Fold change was
calculated as 2-AACT.
The results are shown in Figure 5. Specifically, human primary endothelial
cells were
activated using TNF-a stimulation. For example, TNF-a induces expression of
vascular cell
adhesion molecule (VCAM) in primary human pulmonary arterial endothelial
cells. Yet, pre-
treatment with a HMOX inducer/Bach 1 inhibitor (e.g., the compound of Example
17) reduced
this TNF-a mediated endothelial cell activation as evidenced by the reduction
of the expression
of the adhesion molecule VCAM-1.
Example 151. PK Study
9-10 week old male C57BL/6 mice were dosed orally with 50 mg/kg of the test
compound formulated in 5% w/v tween 80 and 0.5% methyl cellulose. At 0.5 and 4
h post
dosing, whole blood was collected, animals were sacrificed and brain tissues
were collected.
The blood samples were immediately placed on ice, and centrifuged within 60 mm
at 4 C for 3
mm at 14000 rpm to obtain plasma. Plasma was transferred by pipette to pre-
labeled Eppendorf
tubes, stored at -80 C until analyzed.
Brain tissue samples were collected and rinsed with fresh ice cold 0.9% NaCl
solution,
dried quickly and thereafter frozen on dry ice/liquid nitrogen and stored at -
70 10 C until
analyzed. Homogenization was done using phosphate buffer saline and used for
analysis.
Bioanalysis was performed using LC-MS/MS (API 4000).
The test results are provided in Table 4 below. The compound of Example 56
compound
showed good brain penetration.
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
238
Table 4
Example 56
Example 17
me0', 40/
0
01 0 Ho-Th 0ANN4
os N.,.....õ,0Me Comparator 1**
Matrix INN _(; ,,,, N 4 rir",,,,0
Mil
MI
0.5 hr 4 hr 0.5 hr 4 hr 1 hr 4 hr
Plasma
4235 771 72 38 4239 428 281 318 2073 137 288
124
(ng/ml)
Brain (ng/g) 188 83 N/A* 4227 600 122 153 N/A N/A
* Not available
** The compound of Example 17 and the compound of Example 56 were measured in
the same
study. Comparator 1 was measured in a previous study.
Example 152. PD Study
9-10 week old male C57BL/6 mice were dosed orally with 50 mg/kg of vehicle or
compound formulated in 5% w/v tween 80 and 0.5% methyl cellulose. At indicated
time points
(3 and 6 hours or or 4 and 8 hours post-dosing as shown in the results below),
whole blood was
collected, animal was sacrificed and liver was collected. Blood samples were
transferred to vials
containing 4 pL of 10% w/v EDTA, centrifuged at 6000 rpm for 8 in below 10 C
to obtain
plasma. Samples were frozen and later processed for HMOX1 protein levels as
indicated below:
HMOX 1 protein levels: mouse liver
Preparation of Homogenization/Lysis Buffer
The homogenization buffer was prepared according Table A.
Table A. Preparation of homogenization Buffer
Stock Final Volume
Hepes (Ameresco #J848-100m1) 1M 25mM 2.5m1
NaCl(BostonBioProducts #A28Q12R) 5M 300mM 6m1
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
239
MgCl2 (Sigma #7786-30-3) 1M 1.5mM 150u1
EDTA (Ameresco #E177-500m1) 0.5M 20uM 4u1
Triton X-100 (Sigma #T8787-250m1) 100% 0.10% 100u1
MiliQ H20 91.246m1
Final Volume 100mL
Immediately prior to use, add DTT (ThermoScientific #R0861) to a final
concentration of
5mM and 100X Halt Protease & Phosphatase Inhibitor Cocktail (ThermoScientific
#78440) to a
final concentration of 1X.
Preparation of mouse liver samples
Liver samples were crushed by mortar and pestle under freezing conditions.
Pulverized
tissue was transferred into pre-chilled bead ruptor OMNI tubes (OMNI
International, Cat#19-
628). lmL of homogenization buffer (+DTT and HALT) was added to pulverized
tissue and
tubes were placed on ice. Samples were loaded and run through bead ruptor at
speed 5.64m/s, for
two 20sec cycles with lOsec dwell between cycles and samples were immediately
returned to
ice. All material was transferred to a new chilled 2mL Eppendorf tube. Samples
were
centrifuged for 5 minutes at 2,000 rpm at 4 C. Supernatant was transferred to
a fresh chilled 1.7
nit Eppendorf tube. Samples were centrifuged for 5 minutes at 2,000 rpm at 4
C. The
supernatant, consisting of cytosol and microsomes, was transferred to a fresh
chilled 1.7mL
Eppendorf tube. Samples were stored at -80 C. Microsome samples were
quantitated using
Pierce 660 Assay (ThermoFisher Cat#22660) with pre-diluted BSA standards
(ThermoFisher
Cat#23208) ranging from 125 ¨ 2000 ug/mL. Each sample was diluted to lOng/u1
for an ELISA
input of 0.5ug in 50u1.
Preparation of assay reagents
All assay reagents were provided in the Heme Oxygenase 1 (H01) Mouse
SimpleStep
ELISA Kit (Abcam #ab204524). Prior to use, all reagents were equilibrated to
room temperature.
1X Wash Buffer PT was prepared by diluting 10X Wash Buffer PT 1:10 with
deionized water.
Antibody cocktail was prepared by diluting the 10X Capture Antibody and 10X
Detector
Antibody to 1X in Antibody Diluent 5BI. Mouse heme oxygenase 1 protein
standard was
reconstituted using 500u1 homogenization buffer (Table A), mixed and held at
room temperature
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
240
for 10 minutes prior to dilution. An eight point standard curve was generated
by diluting the
Stock Standard 1:2 for standard curve ranging from 10,000 ¨ 156.3 pg/mL.
Heme Oxygenase 1 (H01) Mouse SimpleStep ELISA
50u1 of all samples and standards were added to appropriate wells in
duplicate. 50u1 of
1X Antibody Cocktail was added to each well. The plate was sealed with
provided seal and
incubated at room temperature on a plate shaker at 400rpm. After one hour
incubation, the
Antibody Cocktail was aspirated and the wells were washed three times with
350u1 1X Wash
Buffer PT, aspirating completely between each step. After the last wash was
aspirated dry, 100u1
TMB Substrate was added to each well and the plate was incubated in the dark
at room
temperature for 10 minutes on a plate shaker at 400rpm. After the 10 minute
incubation, 100u1 of
Stop Solution was added to each well. Plate was shaken at 400rpm for 1 minute
to mix. Softmax
Pro 7Ø3 was used to read OD at 450nm as well as for analysis.
Heme Oxygenase 1 protein levels in mouse plasma
Preparation of assay reagents
All assay reagents were provided in the Heme Oxygenase 1 (H01) Mouse
SimpleStep
ELISA Kit (Abcam #ab204524). 1X Wash Buffer PT was prepared by diluting 10X
Wash Buffer
PT 1:10 with deionized water. Antibody cocktail was prepared by diluting the
10X Capture
Antibody and 10X Detector Antibody to 1X in Antibody Diluent 5BI. Mouse heme
oxygenase 1
protein standard was reconstituted using 500u1 Sample Diluent NS, mixed and
held at room
temperature for 10 minutes prior to dilution. An eight point standard curve
was generated by
diluting the Stock Standard 1:2 for standard curve ranging from 5,000 ¨ 78.1
pg/mL.
Preparation of plasma sample
Prior to ELISA, frozen plasma was thawed on ice. Plasma samples were diluted
1:10 in
Sample Diluent NS for a final concentration of 10% plasma.
Heme Oxygenase 1 (H01) Mouse SimpleStep ELISA
50u1 of samples and standards were added to appropriate wells in duplicate.
50u1 of 1X
Antibody Cocktail was added to each well. The plate was sealed with provided
seal and
incubated at room temperature on a plate shaker at 400rpm. After one hour
incubation, the
CA 03135615 2021-09-29
WO 2020/210339 PCT/US2020/027240
241
Antibody Cocktail was aspirated and the wells were washed three times with
350u1 1X Wash
Buffer PT, aspirating completely between each step. After the last wash was
aspirated dry, 100u1
TMB Substrate was added to each well and the plate was incubated in the dark
at room
temperature for 10 minutes on a plate shaker at 400rpm. After the 10 minute
incubation, 100u1 of
Stop Solution was added to each well. Plate was shaken at 400rpm for 1 minute
to mix. Softmax
Pro 7Ø3 was used to read OD at 450nm as well as for analysis.
Representative compounds of the present disclosure were tested and the
aresults are
provided in Table 5 blow. Plasma and liver protein levels assessed by mouse
HMOX1 ELISA
assay (kit) and were compared to time-matched vehicle control mice to give
fold induction.
Carboxylic acids showed modest increase in HMOX1 protein, although
concentrations were well
above EC5() (30-100x).
Table 5
HMOX1 Plasma Liver Liver
EC50 h Protein Cone uM Protein
(uM) (3h, 6h) (3h, 6h) (3h, 6h)
Comparator 1 1.1x 12.2 1.6x
50 mpk 0.9
Study 667 1.3x 1.3 3.2x
Ex. 13 1.1x 63 1.8x
Carboxylic
50 mpk A ci d 1.9
Study 667 0.8x 5.4 1.2x
Ex. 44 C b 1.0 217 1.6x
50 mpk aroxylic2.0
ci Ad
Study 667 1.1x 162 2.1x
Ex. 17 1.7x* 11 3.3x*
50 mpk 1.4
Study 702* 1.1x* 4.9 3.1x*
* Time points were 4 and 8 hours instead of 3 and 6 hours.