Note: Descriptions are shown in the official language in which they were submitted.
ALUMINUM-BASED WELDING ELECTRODES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of priority to U.S. Non-
Provisional
Patent Application Number 17/446,778, filed September 2, 2021, entitled
"ALUMINUM-
BASED WELDING ELECTRODES," , to U.S. Non-Provisional Patent Application Number
17/464,535, filed September 1, 2021, entitled "ALUMINUM-BASED WELDING
ELECTRODES," and to U.S. Provisional Patent Application Number 63/090,867,
filed
October 13, 2020, entitled "ALUMINUM-BASED WELDING ELECTRODES," the
contents of which are hereby incorporated by reference herein in their
entireties.
BACKGROUND
Field
[0002] The disclosed technology relates generally to welding, and
more
particularly to consumable electrodes based on aluminum and methods of welding
using the
same.
Description of the Related Art
[0003] The engineering use of aluminum and alloys thereof continues
to increase
because of the various advantageous properties of this unique material. The
advantageous
features of aluminum and its alloys include light weight, a relatively wide
range of tunable
strength properties, excellent corrosion resistance, thermal conductivity,
reflectivity and
widely available shapes and compositions, to name a few. Owing to these and
other
properties, aluminum can be an excellent choice for many applications from
aerospace to heat
exchangers, trailer fabrication and, most recently, automotive body panels and
frames.
However, welding aluminum can pose unique challenges including suppressing
weld defects
and improving the performance of the weld metal.
SUMMARY
[0004] In one aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum and a fluidity-
enhancing metal
-1-
Date Recue/Date Received 2021-10-08
capable of forming a binary eutectic with aluminum, wherein the binary
eutectic undergoes a
binary eutectic solidification at a eutectic temperature of 595-660 C. The
fluidity-enhancing
metal is present in form and a hypoeutectic concentration of 0.05-0.5 weight %
such that a
solidification temperature range of a molten weld metal formed by melting the
consumable
welding electrode is less than 65 C.
[0005] In another aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum and a fluidity-
enhancing metal
capable of forming a binary eutectic with aluminum, wherein the binary
eutectic undergoes a
binary eutectic solidification at a eutectic temperature of 595-660 C. The
fluidity-enhancing
metal is present in the form of a compound selected from an oxide, a halide, a
hydroxide, a
sulfide, a sulfate, a carbonate, a phosphate, a nitride, a nitrite, a nitride,
a carbide, a boride, an
aluminide, a telluride or a combination thereof.
[0006] In another aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum and a fluidity-
enhancing metal
capable of forming a binary eutectic with aluminum, wherein the binary
eutectic undergoes a
binary eutectic solidification at a eutectic temperature of 595-660 C. The
fluidity-enhancing
metal is present in form and a hypoeutectic concentration such that a molten
weld metal
formed from the consumable welding electrode has a fluidity that is higher by
at least 5%
relative to a molten weld metal formed under substantially the same welding
conditions using
a consumable welding electrode that has the same base metal composition
without the
fluidity-enhancing metal.
[0007] In another aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum, and a fluidity-
enhancing metal
capable of forming a binary eutectic composition with aluminum, wherein the
binary eutectic
composition undergoes a binary eutectic solidification at a eutectic
temperature lower than a
melting temperature of pure aluminum by less than 90 C, wherein the fluidity-
enhancing
metal is present in form and an amount such that a weld metal formed from the
consumable
welding electrode has one or more of the following, relative to a weld metal
formed under
substantially the same welding conditions using the consumable welding
electrode without
the fluidity-enhancing metal:
-2-
Date Recue/Date Received 2021-10-08
a weld metal height (H) that is lower by at least 5%,
a weld metal width (W) that is higher by at least 5%,
a H/W ratio that is lower by at least 5%,
a penetration (P) that is lower by at least 5%, and
a weld toe angle (q) that is lower by at least 5%.
[0008] In one aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum and a fluidity-
enhancing metal
capable of forming a binary eutectic composition with aluminum, wherein the
binary eutectic
composition undergoes a binary eutectic solidification at a eutectic
temperature lower than a
melting temperature of pure aluminum by less than 90 C. The fluidity-
enhancing metal is
present in form and an amount such that a molten weld metal formed from the
consumable
welding electrode has a fluidity that is higher by at least 5% relative to a
molten weld metal
formed under substantially the same welding conditions using the consumable
welding
electrode without the fluidity-enhancing metal.
[0009] In another aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum and a fluidity-
enhancing metal
selected from the group consisting of nickel (Ni), gold (Au), calcium (Ca),
strontium (Sr),
scandium (Sc), yttrium (Y), terbium (Tb), europium (Eu), cerium (Ce),
praseodymium (Pr),
ytterbium (Yb), holmium (Ho), erbium (Er), lanthanum (La), dysprosium (Dy),
samarium
(Sm), lutetium (Lu), thulium (Tm), neodymium (Nd), gadolinium (Gd), lithium
(Li), iron
(Fe), cadmium (Cd) or a combination thereof. The fluidity-enhancing metal is
present in an
amount greater than 0.05% and less than or equal to a binary eutectic
composition by weight
on the basis of a combined weight of aluminum and the fluidity enhancing
metal.
[0010] In another aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum, and a fluidity-
enhancing metal
selected from the group consisting of nickel (Ni), gold (Au), calcium (Ca),
strontium (Sr),
scandium (Sc), yttrium (Y), terbium (Tb), europium (Eu), cerium (Ce),
praseodymium (Pr),
ytterbium (Yb), holmium (Ho), erbium (Er), lanthanum (La), dysprosium (Dy),
samarium
(Sm), lutetium (Lu), thulium (Tm), neodymium (Nd), gadolinium (Gd), lithium
(Li), iron
(Fe), cadmium (Cd) or a combination thereof. The fluidity-enhancing metal is
present in
-3-
Date Recue/Date Received 2021-10-08
form and an amount such that a molten weld metal formed from the consumable
welding
electrode has a fluidity that is higher by at least 5% relative to a molten
weld metal formed
under substantially the same welding conditions using the consumable welding
electrode
without the fluidity-enhancing metal.
[0011] In another aspect, a consumable welding electrode comprises a
base metal
composition comprising at least 70% by weight of aluminum, and a fluidity-
enhancing metal
selected from the group consisting of nickel (Ni), gold (Au), calcium (Ca),
strontium (Sr),
scandium (Sc), yttrium (Y), terbium (Tb), europium (Eu), cerium (Ce),
praseodymium (Pr),
ytterbium (Yb), holmium (Ho), erbium (Er), lanthanum (La), dysprosium (Dy),
samarium
(Sm), lutetium (Lu), thulium (Tm), neodymium (Nd), gadolinium (Gd), lithium
(Li), iron
(Fe), cadmium (Cd) or a combination thereof, wherein the fluidity-enhancing
metal is present
in form and an amount such that a weld metal formed from the consumable
welding electrode
has one or more of the following relative to a weld metal formed under
substantially the same
welding conditions using the consumable welding electrode without the fluidity-
enhancing
metal:
a weld metal height (H) that is lower by at least 5%,
a weld metal width (W) that is higher by at least 5%,
a H/W ratio that is lower by at least 5%,
a penetration (P) that is lower by at least 5%, and
a weld toe angle (q) that is lower by at least 5%.
[0012] In yet another aspect, a method of welding an aluminum
workpiece,
comprising providing a consumable welding electrode according to any
comprising an
aluminum-based base metal composition and a fluidity-enhancing metal selected
from the
group consisting of nickel (Ni), gold (Au), calcium (Ca), strontium (Sr),
scandium (Sc),
yttrium (Y), terbium (Tb), europium (Eu), cerium (Ce), praseodymium (Pr),
ytterbium (Yb),
holmium (Ho), erbium (Er), lanthanum (La), dysprosium (Dy), samarium (Sm),
lutetium
(Lu), thulium (Tm), neodymium (Nd), gadolinium (Gd), lithium (Li), iron (Fe),
cadmium
(Cd) or a combination thereof; and generating an arc to form a weld metal
using the
consumable welding electrode at a weld travel speed of 10-50 inches per
minute.
-4-
Date Recue/Date Received 2021-10-08
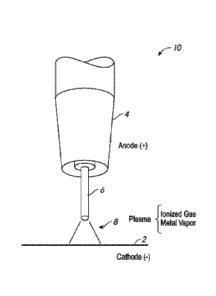
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] FIG. 1 is a schematic illustration of a metal arc welding
process.
[0014] FIG. 2 is a schematic cross-sectional view of a weld bead.
[0015] FIG. 3A is an idealized binary phase diagram of an alloy for
illustration of
a solidification temperature range of an alloy having a binary eutectic.
[0016] FIG. 3B is a binary phase diagram of the Al-Ce alloy system.
[0017] FIG. 4A is a schematic illustration of a solid welding wire
having a fluid-
enhancing metal alloyed therein to enhance molten weld metal fluidity,
according to
embodiments.
[0018] FIG. 4B is a schematic illustration of a solid welding wire
having a fluid-
enhancing metal compound mixed therein to enhance molten weld metal fluidity,
according
to embodiments.
[0019] FIG. 4C is a schematic illustration of a coated solid welding
wire
configured to enhance molten weld metal fluidity, according to embodiments.
[0020] FIG. 4D is a schematic illustration of a cored welding wire
configured to
enhance molten weld metal fluidity, according to embodiments.
[0021] FIG. 5 is a flow chart illustrating a method of enhancing
molten weld
metal fluidity during aluminum welding, according to embodiments.
[0022] FIG. 6 illustrates a gas metal arc welding (GMAW) system
adapted for
welding aluminum using a welding wire configured to enhance molten weld metal
fluidity,
according to embodiments.
DETAILED DESCRIPTION
[0023] The weight of aluminum is about one third that of steel. A
cubic inch of
aluminum weighs 0.098 lbslin3 compared to steel, which weighs 0.283 lbslin3.
Aluminum
has a wide range of strength properties that vary from 13,000 psi tensile
strength for pure
aluminum up to 90,000 psi tensile strength for the strongest heat-treatable
aluminum alloys.
Aluminum provides excellent corrosion resistance in many environments. The
thin refractory
oxide that forms on the surface of aluminum provides a protective barrier.
Aluminum is up
to five times more thermally conductive than steel. Aluminum is reflective of
radiant heat,
-5-
Date Recue/Date Received 2021-10-08
and the surface finish of aluminum is frequently used to take advantage of
this feature. Due
to these and other advantageous properties of aluminum, engineering
applications of
aluminum continue to grow in number and complexity. Correspondingly,
challenges of
welding aluminum continues to grow, including suppressing weld defects and
improving the
properties of the weld metal. In general, aluminum is considered to have
comparatively
lower weldability than steels due to various reasons, including higher
affinity of aluminum
towards atmospheric gases, higher thermal expansion coefficient, higher
thermal and
electrical conductivity, lower rigidity and higher solidification temperature
range, among
other reasons. These characteristics of aluminum alloys in general can render
welding
aluminum be more prone to defect formation in the weld metal.
[0024] Many aluminum-based welding electrodes show poor molten weld
metal
fluidity. Among the various reasons that lower the weldability of Al, the
relatively low
fluidity of the molten weld metal formed from some aluminum-based welding
electrodes can
cause particular types of defects in the weld metal. For example, lower molten
weld metal
fluidity can lead to undercuts at relatively high travel speeds, poor wetting
at the weld toes,
higher porosity and lower penetration. The lower molten weld metal fluidity
can also lead to
higher porosity in the weld metal due to inter-dendritic porosity formation.
In addition, the
lower molten weld metal fluidity can result in taller weld beads, which can in
turn increase
the likelihood of to stress concentration at the weld toes and lead to failure
in fatigue mode.
In addition to causing susceptibility to these weld metal defects, because the
relatively low
molten weld metal fluidity can restrict controllability of the weld pool, it
can in turn limit
welding to slower travel speeds, which lowers productivity.
[0025] In addition, while some aluminum-based welding electrodes
provide
higher molten weld fluidity compared to others, e.g., 4XXX alloys, they pose a
different set
of challenges. For example, some elements are known to provide relatively
higher molten
weld fluidity, e.g., silicon. However, the weld metals formed from Al-based
welding
electrodes containing Si can have compromised weld shear strength. A such,
electrodes
based on 4XXX alloys may not be suitable for welding work pieces formed of
5XXX alloys
for some applications, as brittle phases such as Mg2Si phase may reduce the
ductility of the
weld.
-6-
Date Recue/Date Received 2021-10-08
[0026] Without limitation, the disclosed technology addresses these
and other
aspects of aluminum-based welding electrodes. In particular, the disclosed
welding
electrodes according to various embodiments disclosed herein include alloying
elements that
can increase the molten weld metal fluidity without substantially compromising
some of the
desirable attributes, e.g., shear strength.
Arc Welding Using Aluminum-Based Welding Wires
[0027] FIG. 1 is a schematic illustration of a configuration of an
Al-based welding
wire or electrode in metal arc welding processes, according to embodiments.
The Al-based
welding wire 6 can be configured for lower fluidity of the molten weld metal
according to
embodiments. In the illustrated metal arc welding, e.g., gas-metal arc welding
(GMAW), an
electric arc is created between a consumable Al-based welding wire 6, which is
electrically
connected to one electrode 4 (e.g., anode (+)), and a workpiece 2, which
serves as another
electrode (e.g., cathode (-)). Thereafter, a plasma 8 is sustained, which
contains neutral and
ionized gas molecules, as well as neutral and charged clusters or droplets of
the material of
the Al-based welding wire 6 that have been vaporized by the arc. During
welding, the
consumable welding wire 6 is advanced toward the workpiece 2, and the
resulting molten
weld metal droplets formed from the Al-based welding wire 6 deposit onto the
workpiece,
thereby forming a weld metal or bead.
[0028] The Al-based welding wire 6 can be used in various arc
welding processes,
including gas-metal arc welding processes, which may employ either solid
electrode wires
(GMAW) or metal-cored wires (GMAW-C). The Al-based welding wire 6 can also be
used
in flux-cored arc welding processes (FCAW), which can be gas shielded flux-
cored arc
welding (FCAW-G) or self-shielded flux-cored arc welding (FCAW-S). The Al-
based
welding wire 6 can further be used in shielded metal arc welding (SMAW)
processes and
submerged arc welding (SAW) processes, among others.
Aluminum-Based Welding Wire with Enhanced Fluidity of the Molten Weld Metal
[0029] To address the above noted and other challenges of aluminum
welding, the
welding wires according to embodiments are configured to substantially enhance
the fluidity
-7-
Date Recue/Date Received 2021-10-08
of the molten weld metal. To enhance fluidity in molten weld metal, the
welding wire 6
(FIG. 1) according to embodiments comprises an Al-based base metal composition
comprising at least 70% by weight of aluminum and a fluidity-enhancing metal.
The base
metal composition may additionally include any other element that may serve to
provide the
desired characteristics of the final weld metal, including elements that may
overlap those
present in the work piece. As discussed more infra, the inventors have
discovered that
effective fluidity-enhancing metals include metals capable forming a binary
eutectic with
aluminum, wherein the binary eutectic undergoes a binary eutectic
solidification at a eutectic
temperature lower than a melting temperature of pure aluminum by less than 90
C. The
fluidity-enhancing metal is present in form and an amount such that a molten
weld metal
formed from the consumable welding electrode has a fluidity that is higher by
at least 5%
relative to a molten weld metal formed using the base metal composition
without the fluidity-
enhancing metal. Enhancing the fluidity of the molten weld metal enhances
controllability of
the weld pool, which can in turn enable welding at faster travel speed,
thereby improving
productivity. The enhanced fluidity can also lead to improvements in the
performance of the
resulting weld metal, e.g., through reduction of various undesirable weld
characteristics
described above, including undercuts, poor wetting at the weld toes, higher
porosity, lower
penetration and taller weld beads.
Fluidity of Molten Weld Metal & Weld Metal Shape
[0030] As described herein and in the technology of welding, without
limitation,
the term fluidity as it relates to molten metal refers to metallurgical
fluidity, which is a
measure of the distance a molten metal can flow in a mold of a constant cross-
sectional area
before it solidifies. It will be appreciated that this definition is different
from the definition
presented in physics which describes fluidity as the inverse of viscosity,
which is a
fundamental temperature-related property of a liquid.
[0031] As described herein, unless the description of molten weld
metal as
disclosed herein is inconsistent under either of the definitions, the term
fluidity shall
encompass both metallurgical and physics definitions. However, if the
description of molten
weld metal as disclosed herein is inconsistent under either of the
definitions, the term fluidity
-8-
Date Recue/Date Received 2021-10-08
shall refer to one of the metallurgical and physics definitions that does not
render the
description inconsistent.
[0032] A number of methods can be employed to measure the fluidity
of molten
metals. Common to many measurement techniques is flowing the molten metal into
a narrow
channel. Fluidity is reported as a measure of the length or volume of the mold
filled by the
metal stream before it freezes. Fluidity testing can be carried out in
different ways. Among
the most popular fluidity tests are the spiral-shaped mold test and the vacuum
fluidity test.
The former test measures the length the molten metal flows inside a spiral-
shaped mold. The
latter test measures the length the metal flows inside a narrow channel when
sucked from a
crucible by using a vacuum pump. These and other methods are disclosed in M.
Di Sabatino,
"Fluidity of Aluminium Foundry Alloys," Ph.D. Thesis submitted to Norwegian
University
of Science and Technology (2005) and "On Fluidity of Aluminum Alloys," La
Metallurgia
Italiana 100 (3): 17-22 (2008), the content of each of which is incorporated
by reference it its
entirety.
[0033] As described herein, unless the description of the fluidity of molten
weld
metal as disclosed herein is inconsistent when measured using any of the tests
described
above, the described fluidity shall refer to that which is measured using any
and all of the
tests described above. However, if the description of the fluidity of molten
weld metal as
disclosed herein is inconsistent under any of test methods described above,
the described
fluidity shall refer to that which is measured using any of the tests
described above that gives
rise to results that are consistent with the description.
[0034] The fluidity of the weld metal is affected to multiple
factors, including
thermodynamic parameters such as the chemical composition of the molten weld
metal, the
solidification range and heat of fusion, as well as physical parameters such
as viscosity and
surface tension, to name a few. In particular, the dynamics of solidification
of the weld plays
an important role in determining the fluidity of the weld. The solidification
of the weld is in
turn governed to a large extent by the weld composition and the thermodynamics
resulting
therefrom, as described further infra.
[0035] The weld fluidity can directly affect the shape of the
resulting weld metal,
as schematically illustrated in FIG. 2. FIG. 2 is a schematic cross-sectional
view of a weld
-9-
Date Recue/Date Received 2021-10-08
metal bead 26 formed on a workpiece or substrate 22, and a heat affected zone
or a
penetration zone 24 having a depth into the workpiece 22. As described herein,
the weld
metal 26 can be characterized by a bead height (H), a bead width (W) a depth
of penetration
(P) and a toe angle (0). The H and P are measured in a vertical direction away
from the plane
of the major surface of the workpiece 22. The W is measured in a lateral
direction along the
plane of the major surface of the workpiece 22. The q is measured between the
plane of the
major surface of the workpiece 22 and a line of tangent at the base of the
weld metal 26. As
discussed above, for many applications, a higher fluidity of the molten weld
metal may be
desired, which in turn results one more of a lower H, higher W, lower H/L
ratio, a higher P
and a smaller 0.
[0036] It will be appreciated that, by varying several process
parameters, different
bead profiles can be achieved for a given composition. For example, an
increase in the H, W
and/or H/W may be obtained with increasing wire feed speed (WFS) at a
particular arc
voltage and contact tip to workpiece distance (CTWD). At constant WFS, the H
may
increase and/or the W may decrease with decrease in arc voltage. In addition
to the process
parameters, because the shape of the weld metal depends on many extrinsic
factors such as
the composition, shape and surface condition of the workpiece, a person having
ordinary skill
in the art will appreciate that the most meaningful measurement of an
improvement in the
fluidity of a molten weld metal formed from a welding electrode having a
fluidity-enhancing
metal present therein can be made when the thus formed weld metal is compared
to a weld
metal formed under substantially the same welding conditions using a
consumable welding
electrode that has the same base metal composition but without the fluidity-
enhancing metal.
Composition and Thermodynamic Characteristics of Fluidity-Enhanced Weld Metal
[0037] For a pure metal or a eutectic alloy, the solidification
takes place at a
single temperature. In the case of an alloy at a composition other than a
Eutectic temperature,
the solidification of the liquid mixture can take place over a range of
temperatures. Over this
range of temperatures, precipitation of one or more phases can occur. The
inventors have
discovered that the precipitation can result in formation of a "mushy" zone
including a slurry-
like mixture of liquid and precipitates between the solidifying weld metal and
the fusion line.
-10-
Date Recue/Date Received 2021-10-08
Without being bound to any theory, the precipitates formed during
solidification can serve as
nucleation sites for new grains, which can limit the fluidity of the molten
weld metal. The
inventors have discovered that, by adding certain fluidity-enhancing metal
having a relatively
small range of temperatures over which the "mushy" zone can form as alloying
elements, the
fluidity of the molten weld metal can be substantially enhanced, as described
herein.
[0038] FIG. 3A is a schematic idealized binary phase diagram, for
illustrative
purposes only, of a hypothetical alloy that undergoes solidification. It will
be appreciated
that, while solidification of a weld metal may deviate significantly from
equilibrium
conditions, an equilibrium phase diagram nevertheless provides valuable
insight into the
solidification process. The x and y axes represent the concentration of an
alloying element or
solute and temperature, respectively. It will be appreciated that, while the
illustrated phase
diagram has been idealized by assuming that the solidus and liquidus are
straight lines, actual
alloy systems can have curved solidus and liquidus. The composition Xi.ax
denotes the
maximum content of the alloying element or solute for solidification of the
binary alloy as a
single phase alloy. A partition coefficient k can be defined by Xs/XL, where
XS and XL are
mole fractions of the solute in the solid and liquid is equilibrium at a given
temperature. The
solidification process depends in rather a complex way on various factors such
as temperature
gradients, cooling rates and growth rates. Under equilibrium conditions, the
alloy having a
composition Xo, begins to solidify at the temperature Ti, with the formation
of a small
amount of a solid precipitate, with a composition kX. As the temperature is
lowered, e.g., at
T2, more solid forms, and provided cooling is slow enough to allow extensive
solid state
diffusion, the solid and liquid have compositions XS, XL, following the
solidus and liquidus
lines. The relative amounts of solid and liquid at any temperature are given
by the lever rule.
At T2, the last drop of liquid will have a composition X/k and the solidified
metal will have a
composition X.
[0039] Referring back to FIG. 2, the solidification generally starts
at the fusion
line defining the depth of the penetration region 24, and the base-metal
grains serve as the
nucleation sites. Depending on whether the base alloy of the base metal (BM)
and the filler
alloy are the same or different, the grain growth near the fusion line can
occur by epitaxial or
non-epitaxial mechanisms, respectively. The rest of the weld metal away from
the fusion line
- 1 1 -
Date Recue/Date Received 2021-10-08
solidifies through a competitive growth mechanism, which can depend on the
direction of
maximum heat extraction. For a pure element, due to the absence of impurities
in the weld,
the weld can flow relatively freely. However, in alloys, solidification takes
place over a
range of temperatures, as schematically illustrated in FIG. 3A at nonzero
solute
concentrations. This leads to the formation of the "mushy" zone including a
slurry-like
mixture of liquid and precipitates between the solidifying weld metal and the
fusion line that
contains a mixture of liquid and solid precipitates. The precipitates formed
during
solidification can in turn serve as nucleation sites for new grains which can
obstruct the flow
of the weld. For example, relatively poor weld fluidity observed in weld
metals formed from
welding electrodes formed of Al-Mg alloys can be attributed in part to a large
solidification
range. The inventors have discovered that, by adding the fluidity-enhancing
elements that
have a relatively small range of temperatures over which the "mushy" zone can
form, the
formation of the precipitates that impede the flow of the molten weld metal
can be reduced,
thereby substantially enhancing the fluidity of the molten weld metal.
[0040] Once a pure element is alloyed with another element, the
fluidity initially
decreases up to a point. Then the fluidity starts increasing until the
eutectic composition is
reached and then again starts decreasing beyond the eutectic composition. Al-
Si alloys are an
exception, in which the fluidity increases beyond the eutectic composition
(12.5 wt. % Si). Si
has 4.5 times higher heat of fusion than Al; this extra heat can keep the weld
fluid. In the
case of Al-Mg alloys, fluidity drops drastically from pure Al levels with the
introduction of
Mg until 2 wt. % Mg; then, it increases to till the eutectic composition (-33
wt. % Mg).
[0041] In recognition of these attributes of weld metal fluidity,
the inventors have
discovered that addition of certain fluidity-enhancing elements in certain
effective amount as
part of the welding wire can substantially increase the fluidity. According to
various
embodiments, the consumable welding electrode comprises a base metal
composition
comprising at least 70% by weight of aluminum and a fluidity-enhancing metal.
[0042] The base metal composition can have a composition that is
similar to the
workpiece to be welded. The base metal composition can include any composition
that is
known in the art according to a system of four-digit numbers that have been
developed by the
-12-
Date Recue/Date Received 2021-10-08
Aluminum Association, Inc., to designate the various wrought aluminum alloy
types. The
base metal composition can include one or more of, e.g.:
[0043] 1XXX series: These are aluminums of 99 percent or higher
purity which
are used primarily in the electrical and chemical industries. These alloys are
usually used for
their electrical conductivity and/or corrosion resistance. Their sensitivity
to hot cracking is
very low.
[0044] 2XXXseries. Copper is the principal alloy in this group,
which provides
extremely high strength when properly heat treated. These alloys may not
produce as good
corrosion resistance and are often clad with pure aluminum or special-alloy
aluminum.
These alloys are used in the aircraft industry.
[0045] 3XXX series. Manganese is the major alloying element in this
group,
which is non-heat-treatable. Manganese content can be less than about 2.0
percent. These
alloys have moderate strength and can be easily worked. These moderate
strength
aluminum¨manganese alloys are relatively crack resistant.
[0046] 4XXX series. Silicon is the major alloying element in this
group. It can
be added in sufficient quantities to substantially reduce the melting point
and is used for
brazing alloys and welding electrodes. Most of the alloys in this group are
non-heat-
treatable.
[0047] 5XXX series. Magnesium is the major alloying element of this
group,
which are alloys of medium strength. They possess good welding characteristics
and good
resistance to corrosion, but the amount of cold work should be limited. These
higher strength
aluminum¨magnesium alloys are the most common structural aluminum sheet and
plate
alloys. This series has the highest strength of the non heat-treatable
aluminum alloys. They
are used in chemical storage tanks and pressure vessels as well as structural
applications,
railway cars, dump trucks and bridges, because of its superior corrosion
resistance.
[0048] 6XXX series. Alloys in this group contain silicon and
magnesium, which
make them heat treatable. These alloys possess medium strength and good
corrosion
resistance. This medium strength, heat-treatable series is primarily used in
automotive, pipe,
railings and structural extrusion applications.
-13-
Date Recue/Date Received 2021-10-08
[0049] 7XXX series. Zinc is the major alloying element in this
group.
Magnesium is also included in most of these alloys. Together, they form a heat-
treatable alloy
of very high strength, which is used for aircraft frames. It is primarily used
in the aircraft
industry. The weldability of the 7XXX series may be compromised in higher
copper grades,
as many of these grades are crack sensitive due to wide melting ranges and low
solidus
melting temperatures. They are widely used for bicycle frames and other
extruded
application.
[0050] The base metal composition of the welding wires according to
various
embodiments disclosed herein can include Mn in a weight percentage of, on the
basis the
total weight of the welding wire, 0.01-0.02%, 0.02-0.05%, 0.05-0.10%, 0.1-
0.2%, 0.2-0.5%,
0.5-1.0%, 1.0-1.5%, 1.5-2.0%, or a value in a range defined by any of these
values; Si in a
weight percentage of, on the basis the total weight of the welding wire, 0.1-
0.2%, 0.2-0.5%,
0.5-1.0%, 1.0-2.0%, 2.0-5.0%, 5.0-10%, 10-15%, 15-20%, or a value in a range
defined by
any of these values; Fe in a weight percentage of, on the basis of the total
weight of the
welding wire, 0.02-0.05%, 0.05-0.10 %, 0.1-0.2%, 0.2-0.5%, 0.5-1.0%, or a
value in a range
defined by any of these values; Mg in a weight percentage of, on the basis the
total weight of
the welding wire, 0.1-0.2%, 0.2-0.5%, 0.5-1.0%, 1.0-2.0%, 2.0-5.0%, 5.0-10%,
or a value in
a range defined by any of these values; Cr in a weight percentage of, on the
basis the total
weight of the welding wire, 0.01-0.02%, 0.02-0.05%, 0.05-0.10%, 0.1-0.2%, 0.2-
0.5%, 0.5-
1.0%, or a value in a range defined by any of these values; Cu in a weight
percentage of, on
the basis the total weight of the welding wire, 0.01-0.02%, 0.02-0.05%, 0.05-
0.10%, 0.1-
0.2%, 0.2-0.5%, 0.5-1.0%, 1.0-2.0%, 2.0-5.0%, 5.0-10%, or a value in a range
defined by any
of these values; Ti in a weight percentage of, on the basis of the total
weight of the welding
wire, 0.02-0.05%, 0.05-0.10 %, 0.1-0.2%, 0.2-0.5%, 0.5-1.0%, or a value in a
range defined
by any of these values; Zn in a weight percentage of, on the basis of the
total weight of the
welding wire, 0.05-0.10%, 0.1-0.2%, 0.2-0.5%, 0.5-1.0%, or a value in a range
defined by
any of these values; and Al in a weight percentage of, on the basis of the
total weight of the
welding wire, 70-75 %, 70-75%, 75-80%, 80-85%, 85-90%, 90-95%, 95-99.9%, or a
value in
a range defined by any of these values, which can be the balance of the
welding wire or the
base metal composition.
-14-
Date Recue/Date Received 2021-10-08
[0051] According to various embodiments, the consumable welding
electrode
comprises a the fluidity-enhancing metal that is present in form and an amount
such that a
molten weld metal formed from the consumable welding electrode has a fluidity
that is
higher by at least 5%, 10%, 20%, 50%, 100%, 200%, 500%, 1000%, or a value in
range
defined by any of these values, relative to a molten weld metal formed under
substantially the
same welding conditions using a consumable welding electrode having the same
composition
except for the fluidity-enhancing metal.
[0052] As described above, post-deposition characterization of the
solidified weld
metal can also provide indications of the fluidity of the molten weld metal.
Referring back to
FIG. 2, weld metal fluidity can be inferred based on the weld metal shape
factors, such as the
height, width, height/width ratio and/or weld toe angle. According to various
embodiments,
the fluidity-enhancing metal is present in form and an amount such that a weld
metal formed
from the consumable welding electrode has one or more of the following
characteristics
relative to a weld metal formed under substantially the same welding
conditions using a
consumable welding electrode having the same composition except for the
fluidity-enhancing
metal: a weld metal height (H) that is lower by at least 5%, 50%, 100%, 150%,
200%, 250%,
300% or a value in a range defined by any of these values; a weld metal width
(W) that is
higher by at least 5%, 20%, 40%, 60%, 80%, 100% or a value in a range defined
by any of
these values; a H/W ratio that is lower by at least 5%, 50%, 100%, 150%, 200%,
250%,
300% or a value in a range defined by any of these values; a penetration (P)
that is lower by
at least 5%, 20%, 40%, 60%, 80%, 100% or a value in a range defined by any of
these values;
and a weld toe angle (0) that is lower by at least 5%, 20%, 40%, 60%, 80%,
100% or a value
in a range defined by any of these values.
[0053] As discussed above, the inventors have discovered that a
property of an
effective fluidity-enhancing element is the capability of forming a binary
eutectic
composition with aluminum with a relatively low temperature range within which
a "mushy"
zone is formed, as described above. A physical parameter that is indicative of
this
temperature range is the solidification temperature range. Thus, the inventors
have
discovered that one of the desirable physical attributes of an effective
fluidity-enhancing
element is a relatively narrow solidification temperature range within a
relevant composition
-15-
Date Recue/Date Received 2021-10-08
range. The solidification temperature range can be defined as the temperature
range between
the liquidus and solidus. Referring back to FIG. 3, the solidification
temperature range for
the composition Xo is T3-Ti. The inventors have further realized that an alloy
system having
a relatively narrow solidification temperature range according to embodiments
forms a binary
eutectic composition that undergoes a binary eutectic solidification at a
eutectic temperature
that is within a relatively close proximity to the melting temperature of pure
aluminum.
[0054] Referring back to FIG. 3A, for the idealized binary alloy
system, it will be
appreciated that the maximum value of the solidification temperature range
does not exceed
the difference between the melting temperature the pure metal and the eutectic
temperature
TE. As such, the eutectic temperature can be a selection criterion for the
fluidity-enhancing
metal. According to various embodiments, the fluidity-enhancing metal forms a
binary
eutectic composition at a temperature lower than a melting temperature of pure
aluminum by
less than 90 C, 80 C, 70 C, 60 C, 50 C, 40 C, 30 C, 20 C, 10 C, or
less than a value in
range defined by any of these values. For a condition under which the melting
temperature of
aluminum is 660 C, the binary eutectic composition melts at a melting
temperature of less
than 570 C, 580 C, 590 C, 600 C, 610 C, 620 C, 630 C, 640 C, 650 C,
660 C, or less
than a value in range defined by any of these values. For instance, a fluidity-
enhancing metal
capable of forming a binary eutectic with aluminum according to embodiments
undergoes a
binary eutectic solidification at a eutectic temperature of 595-660 C. For
illustrative
purposes, one example alloy system having these attributed is the Al-Ce alloy
system, a
binary phase diagram of which is illustrated in FIG. 3B. As illustrated, the
Eutectic
temperature of 621 C is within the range of 595-660 C.
[0055] TABLE 1 below shows approximate maximum solidification
temperature
ranges for some example fluidity-enhancing elements, according to embodiments.
TABLE 2
below shows eutectic temperatures and eutectic compositions and composition
ranges for
some example fluidity-enhancing elements within which the fluidity of the
molten weld
metal can be enhanced.
TABLE 1
Relevant Alloy Systems Max Solidification/Freezing Range
in
-16-
Date Recue/Date Received 2021-10-08
Hypoeutectic Alloys ( C)
Al-Ni system ¨20
Al-Au system ¨10
Al-Ca system ¨50
Al-Ce system ¨40
Al-Er system ¨10
Al-Dy system ¨10
Al-Eu system ¨30
Al-Lu system ¨40
Al-Nd system ¨30
Al-Pr system ¨20
Al-Sm system ¨40
Al-Sr system ¨10
Al-Ni system ¨65
Al-Fe-system ¨5
Al-Cd system ¨10
TABLE 2
Relevant Alloy Systems Eutectic Eutectic Relevant
Temperature
Composition (%wt. Composition Range
( C) solute) (%wt. solute)
Al-Ni system 642 ¨9% 0.05 ¨ 9%
Al-Au system 650 7.5% 0.05 - 7.5%
Al-Ca system 613 ¨9% 0.05 ¨ 9%
Al-Ce system 621 ¨10% 0.05 - 10%
Al-Er system 649 ¨10% 0.05 - 10%
Al-Dy system 635 ¨10% 0.05 ¨ 10%
Al-Eu system 630 ¨6% 0.05 ¨ 6%
Al-Lu system 620 ¨14% 0.05 ¨ 14%
Al-Nd system 632 ¨10% 0.05 ¨ 10%
Al-Pr system 640 ¨10% 0.05 ¨ 10%
Al-Sm system 635 ¨10% 0.05 ¨ 10%
Al-Sr system 650 ¨2% 0.05 ¨ 2%
Al-La system 640 ¨10% 0.05 ¨ 10%
Al-Y system 637 ¨10% 0.05 ¨ 10%
Al-Tb system 634 ¨10% 0.05 ¨ 10%
Al-Sc system ¨660 0.47% 0.05 ¨ 0.47%
Al-Gd system 650 ¨20% 0.05 ¨ 20%
Al-Tm system 645 ¨16% 0.05 ¨ 16%
Al-Yb system 625 20% 0.05 ¨ 20%
Al-Ho system 635 ¨10% 0.05 ¨ 10%
-17-
Date Recue/Date Received 2021-10-08
Al-Ni system 596 8% 0.05 - 8%
Al-Fe-system 655 1.7% 0.05 - 1.7%
Al-Cd system 650 6.95% 0.05 ¨ 6.96%
[0056] According to one embodiment, a consumable welding electrode
comprises
a base metal composition comprising at least 70% by weight of aluminum and a
fluidity-
enhancing metal capable of forming a binary eutectic with aluminum, wherein
the binary
eutectic undergoes a binary eutectic solidification at a eutectic temperature
of 595-660 C.
The fluidity-enhancing metal according to embodiments is selected from the
group consisting
of nickel (Ni), gold (Au), calcium (Ca), strontium (Sr), scandium (Sc),
yttrium (Y), terbium
(Tb), europium (Eu), cerium (Ce), praseodymium (Pr), ytterbium (Yb), holmium
(Ho),
erbium (Er), lanthanum (La), dysprosium (Dy), samarium (Sm), lutetium (Lu),
thulium (Tm),
neodymium (Nd), gadolinium (Gd), lithium (Li), iron (Fe), cadmium (Cd) or a
combination
thereof.
[0057] According to a more particular embodiment, the binary
eutectic undergoes
a binary eutectic solidification at a eutectic temperature > 595 and < 630 C.
According to
this embodiment, the fluidity-enhancing metal is selected from the group
consisting of
calcium (Ca), cerium (Ce), lutetium (Lu), ytterbium (Yb), lithium (Li) or a
combination
thereof.
[0058] According to another more particular embodiment, the binary
eutectic
undergoes a binary eutectic solidification at a eutectic temperature > 630 and
< 645 C. In
this embodiment, the fluidity-enhancing metal is selected from the group
consisting of nickel
(Ni), dysprosium (Dy), europium (Eu), yttrium (Y), terbium (Tb), holmium (Ho),
lanthanum
(La), praseodymium (Pr), samarium (Sm), neodymium (Nd), or a combination
thereof.
[0059] According to a more particular embodiment, the binary
eutectic undergoes
a binary eutectic solidification at a eutectic temperature > 645 and < 660 C.
According to
this embodiment, the fluidity-enhancing metal is selected from the group
consisting of gold
(Au), strontium (Sr), scandium (Sc), erbium (Er), gadolinium (Gd), thulium
(Tm), iron (Fe),
cadmium (Cd) or a combination thereof.
[0060] According to various embodiments, the fluidity-enhancing
metal is present
at a hypoeutectic concentration. Referring back to FIG. 3A, the composition X.
denotes the
-18-
Date Recue/Date Received 2021-10-08
maximum content of the fluidity-enhancing metal at which the binary alloy
solidifies as a
single phase alloy.
[0061] According to some embodiments, the hypoeutectic concentration
is such
that the molten weld metal formed from the consumable welding electrode
solidifies into a
single phase having the aluminum (face-centered cubic) crystal structure.
However,
embodiments are not so limited and in other embodiments, the molten weld metal
formed
from the consumable welding electrode solidifies into multiple phases
including the
aluminum crystal structure and at least another phase including the fluidity-
enhancing metal.
[0062] The welding wire can include one or more of these elements,
on the basis
the total weight of the welding wire, 0.01-0.02%, 0.02-0.05%, 0.05-0.10 %, 0.1-
0.2%, 0.2-
0.5%, 0.5-1.0%, 1.0-1.5%. 1.5-2.0%, 2.0-2.5%, 2.5-3.0%, 3.0-3.5%, 3.5-4.0%,
4.0-4.5%, 4.5-
5.0%, or a value in a range defined by any of these values. In a particular
embodiment, the
fluidity-enhancing metal is present in form and a hypoeutectic concentration
of 0.05-0.5
weight % such that a solidification temperature range of a molten weld metal
formed by
melting the consumable welding electrode is less than 65 C.
[0063] In some of these embodiments, the fluidity-enhancing metal
may be
present in elemental metal form. In some other of these embodiments, the
fluidity-enhancing
metal may be present in the form of an oxide, halide, hydroxide, sulfide,
sulfate, carbonate,
phosphate, nitride, nitrite, nitride, carbide, boride, aluminide, telluride or
a combination
thereof.
Structure of Fluidity-Enhanced Welding Electrode
[0064] FIG. 4A is a schematic illustrations of a solid welding wire
40A
configured to enhance weld metal fluidity, according to embodiments. In the
illustrated
embodiment, the fluidity-enhancing metal maybe alloyed with the base metal
composition,
e.g., to form a solid solution, such that the fluidity-enhancing metal as
present may form
metallic bonds with aluminum and other metal elements of the base metal
composition as
described above. In these embodiments, the consumable welding electrode is a
solid wire
comprising a homogenous solution or mixture, e.g., an alloy, formed by the
base metal
composition and the fluidity-enhancing metal.
-19-
Date Recue/Date Received 2021-10-08
[0065] FIG. 4B is a schematic illustrations of a solid welding wire
40B configured
to enhance weld metal fluidity, according to some other embodiments. Unlike
the solid
welding wire 40A (FIG. 4A), in the embodiment illustrated in FIG. 4B, the
fluidity-enhancing
metal maybe be present in the form of a compound such as an oxide, halide,
hydroxide,
sulfide, sulfate, carbonate, phosphate, nitride, nitrite, nitride, carbide,
boride, aluminide,
telluride or a combination thereof. In these embodiments, the consumable
welding electrode
is a solid wire comprising a heterogenous mixture formed by the base metal
composition and
the compound of the fluidity-enhancing metal. The compound of the fluidity-
enhancing
metal may be present, e.g., in powder form that is dispersed within a matrix
of the base metal
composition.
[0066] FIG. 4C is a schematic illustration of a coated solid welding
wire 42
configured to enhance fluidity of the weld metal, according to embodiments.
FIG. 4D is a
schematic illustration of a cored welding wire 46 configured to enhance weld
fluidity,
according to embodiments. In these embodiments, the fluidity-enhancing metal
may be
chemically and/or physically separated from the base metal composition. For
example, in the
welding wire 42 (FIG. 4C), the fluidity-enhancing metal may be present as a
coating 44
formed on the outer surface of a core wire 43 formed of the base metal
composition. The
coating 44 can include the fluid-enhancing metal in elemental, alloy or
compound in a
suitable form, e.g., a powder form. Alternatively, in the illustrated
embodiment of FIG. 4D,
the consumable welding wire 46 may be a cored wire comprising a core 48 and a
sheath 49,
wherein the core 48 comprises the fluidity-enhancing metal, e.g., in powder
form 47, and the
sheath 49 comprises the base metal composition.
Method of Enhancing Fluidity in Aluminum-Based Weld Metals
[0067] FIG. 5 is a flow chart illustrating a method of enhancing
fluidity of the
weld metal during aluminum welding, according to embodiments. The method
includes
providing 54 providing a consumable welding electrode comprising an aluminum-
based base
metal composition and a fluidity-enhancing metal selected from the group
consisting of
nickel (Ni), gold (Au), calcium (Ca), strontium (Sr), scandium (Sc), yttrium
(Y), terbium
(Tb), europium (Eu), cerium (Ce), praseodymium (Pr), ytterbium (Yb), holmium
(Ho),
-20-
Date Recue/Date Received 2021-10-08
erbium (Er), lanthanum (La), dysprosium (Dy), samarium (Sm), lutetium (Lu),
thulium (Tm),
neodymium (Nd), gadolinium (Gd) or a combination thereof. The consumable
welding
electrode can be according to any one of the above-described embodiments. The
method
additionally includes generating 58 an arc to form a molten weld bead using
the consumable
welding electrode at a weld travel speed of 10-50 inches per minute. The
fluidity-enhancing
metal is present in form and an amount such that the molten weld metal has a
fluidity that is
higher relative to a molten weld metal formed under substantially the same
welding
conditions using the consumable welding electrode without the fluidity-
enhancing metal.
The method illustrated in FIG. 5 can be implemented in any suitable welding
process,
including gas-metal arc welding processes described below by way of example.
[0068] In gas-metal arc welding using solid (GMAW) or metal-cored
electrodes
(GMAW-C), a shielding gas is used to provide protection for the weld pool and
the weld
bead against atmospheric contamination during welding. When solid electrodes
are used,
they are appropriately alloyed with active ingredients that, in combination
with the shielding
gas, may be designed to enhance the weld metal fluidity as described above
while also
providing low porosity or porosity-free welds with the desired physical and
mechanical
properties of the resulting weld metal. When metal-cored electrodes are used,
some of the
active ingredients including a fluidity-enhancing metal may be added in the
core of the cored
wire, and designed to provide a similar function as in the case of solid
electrodes.
[0069] Solid and metal-cored electrodes are designed to provide,
under
appropriate gas shielding, a solid, substantially porosity-free weld metal
with yield strength,
tensile strength, ductility and impact toughness to perform satisfactorily in
the final
applications. These electrodes may also be designed to minimize the quantity
of slag
generated during welding. For some applications, metal-cored electrodes can be
used as an
alternative to solid wires to increase productivity. As described herein,
metal-cored
electrodes refer to composite electrodes having a core that is at least
partially filled and
surrounded by a metallic outer sheath. The core can include metal powder and
active
ingredients to help with arc stability, weld wetting and appearance and
desired physical and
mechanical properties. The metal-cored electrodes are manufactured by mixing
the
ingredients of the core material and depositing them inside a formed strip,
and then closing
-21-
Date Recue/Date Received 2021-10-08
and drawing the strip to the final diameter. For some applications, cored
electrodes can
provide increased deposition rates and a wider, relatively consistent weld
penetration profile
compared to solid electrodes. As described herein, metal-cored electrodes
(GMAW-C) refer
to electrodes having a core whose ingredients are primarily metallic. When
present,
nonmetallic components in the core have a combined concentration less than 5%,
3% or 1%,
on the basis of the total weight of each electrode. The relatively low
nonmetallic components
may distinguish GMAW-C electrodes from flux-cored arc welding electrodes
described in
more detail, infra. The GMAW-C electrodes can be characterized by a spray arc
and high
quality weld metal.
[0070] Similar to gas-metal arc welding using metal-cored
electrodes (GMAW-
C), electrodes used in flux-cored arc welding (FCAW, FCAW-S, FCAW-G) also
include a
core surrounded by a shell. That is, the cored electrodes used in flux-cored
arc welding have
a core that is at least partially filled and surrounded by a metallic outer
sheath, similar to
metal-cored electrodes described above. However, unlike metal-cored electrodes
(GMAW-
C), the cored electrodes used in flux-cored arc welding (FCAW) additionally
includes fluxing
agents designed to provide protection for the weld pool and the weld bead
against
atmospheric contamination during welding, at least partially in lieu of a
shielding gas. The
cored electrodes used in flux-cored arc can additionally include other active
ingredients to
help with arc stability, weld wetting and appearance and desired physical and
mechanical
properties. In one aspect, flux-cored arc electrodes may be distinguished from
metal-cored
electrodes by the amount of nonmetallic components present in the core, whose
combined
concentration can be less than 5%, 3% or 1%, on the basis of the total weight
of each
electrode.
[0071] A large number of fluxing agent compositions for flux-cored
electrodes
have been developed to control the arc stability, modify the weld metal
composition, and to
provide protection from atmospheric contamination. In flux-cored electrodes,
arc stability
may be controlled by modifying the composition of the flux. As a result, it
may be desirable
to have substances which serve well as plasma charge carriers in the flux
mixture. In some
applications, fluxes can also modify the weld metal composition by rendering
impurities in
the metal more easily fusible and providing substances with which these
impurities may
-22-
Date Recue/Date Received 2021-10-08
combine. Other materials are sometimes added to lower the slag melting point,
to improve
slag fluidity, and to serve as binders for the flux particles. Various wires
used in FCAW may
share some similar characteristics, e.g., forming a protective slag over the
weld, using a drag
angle technique, having the ability to weld out-of-position or flat and
horizontal at higher
deposition rates, having the ability to handle relatively higher amount of
contaminants on the
plate, etc. On the other hand, different types of flux-cored arc welding
processes exist,
namely: self-shielded flux-cored arc welding (FCAW-S) and gas-shielded flux-
cored arc
welding (FCAW-G).
[0072]
FIG. 6 schematically illustrates an example gas metal arc welding
(GMAW) system 110 configured for aluminum¨based welding wires according to
embodiments. The GMAW system 110 includes an electrical power source 112, a
wire drive
assembly 114, a shielding gas supply system 116, and a cable assembly 118 for
delivering
electrical power, a welding wire in a spool 124 and a shielding gas in a
shield gas source 128
configured to be delivered to a workpiece 120 to be welded. The wire drive
assembly 114
typically includes a reel stand 122 for carrying the spool 124 including a
continuous
consumable wire electrode as well as a drive mechanism 126 including one or
more drive
wheels (not shown) for driving the welding wire from the spool 124 through the
cable
assembly 118 to the workpiece 120. The shielding gas supply system 116
normally includes
a shielding gas source 128 and a gas supply conduit 130 in fluid communication
with cable
assembly 118. As illustrated in FIG. 6, the cable assembly 118 typically
includes an
elongated flexible cable 132 attached on one end to the power source 112, the
wire drive
assembly 114 and the gas supply system 116, and on the other end to a weld gun
134.
Additional Examples
1. A consumable welding electrode, comprising:
a base metal composition comprising at least 70% by weight of aluminum;
and
a fluidity-enhancing metal capable of forming a binary eutectic composition
with aluminum, wherein the binary eutectic composition undergoes a binary
eutectic
-23-
Date Recue/Date Received 2021-10-08
solidification at a eutectic temperature lower than a melting temperature of
pure
aluminum by less than 90 C,
wherein the fluidity-enhancing metal is present in form and an amount such
that a weld metal formed from the consumable welding electrode has one or more
of
the following, relative to a weld metal formed under substantially the same
welding
conditions using the consumable welding electrode without the fluidity-
enhancing
metal:
a weld metal height (H) that is lower by at least 5%,
a weld metal width (W) that is higher by at least 5%,
a H/W ratio that is lower by at least 5%,
a penetration (P) that is lower by at least 5%, and
a weld toe angle (q) that is lower by at least 5%.
2. A consumable welding electrode, comprising:
a base metal composition comprising at least 70% by weight of aluminum;
and
a fluidity-enhancing metal capable of forming a binary eutectic composition
with aluminum, wherein the binary eutectic composition undergoes a binary
eutectic
solidification at a eutectic temperature lower than a melting temperature of
pure
aluminum by less than 90 C,
wherein the fluidity-enhancing metal is present in form and an amount such
that a molten weld metal formed from the consumable welding electrode has a
fluidity
that is higher by at least 5% relative to a molten weld metal formed under
substantially the same welding conditions using the consumable welding
electrode
without the fluidity-enhancing metal.
3. A consumable welding electrode, comprising:
a base metal composition comprising at least 70% by weight of aluminum;
and
a fluidity-enhancing metal selected from the group consisting of nickel (Ni),
gold (Au), calcium (Ca), strontium (Sr), scandium (Sc), yttrium (Y), terbium
(Tb),
europium (Eu), cerium (Ce), praseodymium (Pr), ytterbium (Yb), holmium (Ho),
-24-
Date Recue/Date Received 2021-10-08
erbium (Er), lanthanum (La), dysprosium (Dy), samarium (Sm), lutetium (Lu),
thulium (Tm), neodymium (Nd), gadolinium (Gd) or a combination thereof,
wherein the fluidity-enhancing metal is present in an amount greater than
0.05% and less than or equal to a binary eutectic composition by weight on the
basis
of a combined weight of aluminum and the fluidity enhancing metal.
4. A consumable welding electrode, comprising:
a base metal composition comprising at least 70% by weight of aluminum;
and
a fluidity-enhancing metal selected from the group consisting of nickel (Ni),
gold (Au), calcium (Ca), strontium (Sr), scandium (Sc), yttrium (Y), terbium
(Tb),
europium (Eu), cerium (Ce), praseodymium (Pr), ytterbium (Yb), holmium (Ho),
erbium (Er), lanthanum (La), dysprosium (Dy), samarium (Sm), lutetium (Lu),
thulium (Tm), neodymium (Nd), gadolinium (Gd) or a combination thereof,
wherein the fluidity-enhancing metal is present in form and an amount such
that a molten weld metal formed from the consumable welding electrode has a
fluidity
that is higher by at least 5% relative to a molten weld metal formed under
substantially the same welding conditions using the consumable welding
electrode
without the fluidity-enhancing metal.
5. A consumable welding electrode, comprising:
a base metal composition comprising at least 70% by weight of aluminum;
and
a fluidity-enhancing metal selected from the group consisting of nickel (Ni),
gold (Au), calcium (Ca), strontium (Sr), scandium (Sc), yttrium (Y), terbium
(Tb),
europium (Eu), cerium (Ce), praseodymium (Pr), ytterbium (Yb), holmium (Ho),
erbium (Er), lanthanum (La), dysprosium (Dy), samarium (Sm), lutetium (Lu),
thulium (Tm), neodymium (Nd), gadolinium (Gd) or a combination thereof,
wherein the fluidity-enhancing metal is present in form and an amount such
that a weld metal formed from the consumable welding electrode has one or more
of
the following, relative to a weld metal formed under substantially the same
welding
-25-
Date Recue/Date Received 2021-10-08
conditions using the consumable welding electrode without the fluidity-
enhancing
metal:
a weld metal height (H) that is lower by at least 5%,
a weld metal width (W) that is higher by at least 5%,
a H/W ratio that is lower by at least 5%,
a penetration (P) that is lower by at least 5%, and
a weld toe angle (q) that is lower by at least 5%.
6. The consumable welding electrode of any of the above Embodiments, wherein
the
fluidity-enhancing metal selected from the group consisting of nickel (Ni),
gold (Au),
calcium (Ca), strontium (Sr), scandium (Sc), yttrium (Y), terbium (Tb),
europium (Eu),
cerium (Ce), praseodymium (Pr), ytterbium (Yb), holmium (Ho), erbium (Er),
lanthanum
(La), dysprosium (Dy), samarium (Sm), lutetium (Lu), thulium (Tm), neodymium
(Nd),
gadolinium (Gd) or a combination thereof is present in an amount greater than
0.1% and less
than or equal to the eutectic composition by weight on the basis of a combined
weight of
aluminum and the fluidity enhancing metal.
7. The consumable welding electrode of any of the Embodiments, wherein the
fluidity-enhancing metal is capable forming a binary eutectic composition with
aluminum,
wherein the binary eutectic composition undergoes a binary eutectic
solidification at a
eutectic temperature of 570-660 C.
8. The consumable welding electrode of any of the above Embodiments, wherein
the
fluidity-enhancing metal is present in form and an amount such that a molten
weld metal
formed from the consumable welding electrode has a fluidity that is higher by
at least 10%
relative to a molten weld metal formed using the base metal composition
without the fluidity-
enhancing metal.
9.
The consumable welding electrode of any one of the above embodiments,
wherein the base metal composition further comprises one or both of silicon
(Si) and
magnesium (Mg) as alloying elements for alloying with aluminum in the weld
metal bead
formed using the consumable welding electrode.
10. The consumable welding electrode of any of the above Embodiments, wherein
a
solidified weld metal formed from the consumable welding electrode has a yield
strength
-26-
Date Recue/Date Received 2021-10-08
and/or tensile strength that is higher or within 10% of a yield strength
and/or tensile strength
of a solidified weld metal formed using the base metal composition without the
fluidity-
enhancing metal.
11. The consumable welding electrode of any one of the above Embodiments,
wherein the fluidity-enhancing metal is present in elemental metal form.
12. The consumable welding electrode of any one of the above Embodiments,
wherein the fluidity-enhancing metal is present in a compound selected from an
oxide, a
halide, a hydroxide, a sulfide, a sulfate, a carbonate, a phosphate, a
nitride, a nitrite, a nitride,
a carbide, a boride, an aluminide, a telluride or a combination thereof.
13. The consumable welding electrode of any one of the above Embodiments,
wherein the welding electrode is configured for welding at a weld travel speed
of 10-50
inches per minute under a welding condition.
14. The consumable welding electrode of any one of the above Embodiments,
wherein the welding electrode is configured for a gas metal arc welding
(GMAW).
15. The consumable welding electrode of any one of the above Embodiments,
wherein the consumable welding electrode comprises a core wire comprising the
base metal
composition and a coating comprising the fluidity-enhancing metal surrounding
the core
wire.
16. The consumable welding electrode of any one of Embodiments 1-14, wherein
the
consumable welding electrode is a cored wire comprising a core and a sheath,
wherein the
core comprises the fluidity-enhancing metal and the sheath comprises the base
metal
composition.
17. The consumable welding electrode of any one of Embodiments 1-14, wherein
the
consumable welding electrode is solid wire comprising a homogenous mixture of
the base
metal composition and the fluidity-enhancing metal.
18. A method of welding an aluminum workpiece, comprising:
providing a consumable welding electrode comprising an aluminum-based
base metal composition and a fluidity-enhancing metal selected from the group
consisting of nickel (Ni), gold (Au), calcium (Ca), strontium (Sr), scandium
(Sc),
yttrium (Y), terbium (Tb), europium (Eu), cerium (Ce), praseodymium (Pr),
ytterbium
-27-
Date Recue/Date Received 2021-10-08
(Yb), holmium (Ho), erbium (Er), lanthanum (La), dysprosium (Dy), samarium
(Sm),
lutetium (Lu), thulium (Tm), neodymium (Nd), gadolinium (Gd) or a combination
thereof; and
generating an arc to form a molten weld metal using the consumable welding
electrode at a weld travel speed of 10-50 inches per minute,
wherein the fluidity-enhancing metal is present in form and an amount such
that the molten weld metal has a fluidity that is higher relative to a molten
weld metal
formed under substantially the same welding conditions using the consumable
welding electrode without the fluidity-enhancing metal.
19. The method of welding according to Embodiment 18, wherein the consumable
welding wire is according to any one of Embodiments 1-17.
[0073] Unless the context clearly requires otherwise, throughout the
description
and the claims, the words "comprise," "comprising," "include," "including" and
the like are
to be construed in an inclusive sense, as opposed to an exclusive or
exhaustive sense; that is
to say, in the sense of "including, but not limited to." The word "coupled",
as generally used
herein, refers to two or more elements that may be either directly connected,
or connected by
way of one or more intermediate elements. Likewise, the word "connected", as
generally
used herein, refers to two or more elements that may be either directly
connected, or
connected by way of one or more intermediate elements. Additionally, the words
"herein,"
"above," "below," and words of similar import, when used in this application,
shall refer to
this application as a whole and not to any particular portions of this
application. Where the
context permits, words in the above Detailed Description using the singular or
plural number
may also include the plural or singular number, respectively. The word "or" in
reference to a
list of two or more items, that word covers all of the following
interpretations of the word:
any of the items in the list, all of the items in the list, and any
combination of the items in the
list.
[0074] Moreover, conditional language used herein, such as, among
others, "can,"
"could," "might," "may," "e.g.," "for example," "such as" and the like, unless
specifically
stated otherwise, or otherwise understood within the context as used, is
generally intended to
convey that certain embodiments include, while other embodiments do not
include, certain
-28-
Date Recue/Date Received 2021-10-08
features, elements and/or states. Thus, such conditional language is not
generally intended to
imply that features, elements and/or states are in any way required for one or
more
embodiments or whether these features, elements and/or states are included or
are to be
performed in any particular embodiment.
[0075]
While certain embodiments have been described, these embodiments have
been presented by way of example only, and are not intended to limit the scope
of the
disclosure. Indeed, the novel apparatus, methods, and systems described herein
may be
embodied in a variety of other forms; furthermore, various omissions,
substitutions and
changes in the form of the methods and systems described herein may be made
without
departing from the spirit of the disclosure. For example, while blocks are
presented in a
given arrangement, alternative embodiments may perform similar functionalities
with
different components and/or circuit topologies, and some blocks may be
deleted, moved,
added, subdivided, combined, and/or modified. Each of these blocks may be
implemented in
a variety of different ways. Any suitable combination of the elements and acts
of the various
embodiments described above can be combined to provide further embodiments.
The
various features and processes described above may be implemented
independently of one
another, or may be combined in various ways. All possible combinations and
subcombinations of features of this disclosure are intended to fall within the
scope of this
disclosure.
-29-
Date Recue/Date Received 2021-10-08