Note: Descriptions are shown in the official language in which they were submitted.
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
HOUSING AND CARTOMISER FOR AN AEROSOL PROVISION SYSTEM
FIELD
The present disclosure relates to a housing for an aerosol provision system.
In addition, the
present disclosure relates to a cartomiser comprising the housing, a
cartomiser for an
aerosol provision system, and an aerosol provision system comprising the
housing.
BACKGROUND
Electronic aerosol provision systems or devices such as electronic cigarettes
(e-cigarettes)
generally contain a cartomiser with a reservoir for an aerosolisable material,
from which
vapour or aerosol is generated for inhalation by a user, for example through
heat
vaporisation. Generally nicotine and often flavourants or flavour agents are
present in the
aerosolisable material of the reservoir, and the vapour or aerosol generating
element is either
downstream of the reservoir or integrated therein so as to vaporise a portion
of the
aerosolisable material. Cartomisers where the reservoir is not refillable with
aerosolisable
material are often referred to in the art as "closed" systems, whereas
cartomisers which
.. facilitate refilling of the aerosolisable material are generally referred
to as "open" systems.
In an open or closed system, the vapour or aerosol generating element is
typically located
downstream of the reservoir, e.g. in an aerosol generation chamber, so that as
a user inhales
on the system and electrical power is supplied to e.g. the heater, air is
drawn into the system
through inlet holes and mixes with the vaporised material in the aerosol
generation chamber.
There is then a flow path connecting the aerosol generation chamber and an
opening in the
mouthpiece of the device, so that incoming air drawn through the aerosol
generation
chamber continues along the flow path, carrying at least some of the aerosol
with it and out
through the mouthpiece opening for inhalation by the user. The term
"downstream" is thus
understood to mean in the direction of aerosol flow, from the reservoir
containing the
aerosolisable material, via the aerosol generation chamber, to the mouthpiece
of the aerosol
provision system where aerosol is inhaled by the user.
In open systems, the aerosol generating element is generally intended to be
replaceable, in
that a user can access the aerosol generating element and replace it when
appropriate. In a
closed system, the vapour or aerosol generating element is generally not
intended for
replacement and can be integrated with the reservoir to form a single unit
along with the
aerosolisable material and an aerosol generation chamber. As a user inhales on
the system
and electrical power is supplied to the element, air is drawn into the system
and mixes with
the vaporised material in the aerosol generation chamber. There is then a flow
path
1
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
connecting the integrated system with an opening in a mouthpiece of the device
so that
generated aerosol or vapour can be inhaled by the user.
Aerosolisable material may also be referred to in the art as aerosol or vapour
precursor
material, and typically includes a solvent along with acids, bases and/or
salts such that the
material is electrically conductive.
SUMMARY
According to some embodiments described herein, there is provided a housing
for an aerosol
provision system comprising a reservoir for an electrically conductive
aerosolisable material,
wherein the potential difference between any two exposed and/or exposable
surfaces of one
or more metal component which is contained in the housing is from 0 mV to
about 35 mV.
The two surfaces are further defined as capable of simultaneously being in
contact with the
aerosolisable material.
The one or more metal component may be part of an aerosol generating element,
and the
aerosol generating element may be integrated with the reservoir. Alternatively
the aerosol
generating element may be downstream of the reservoir. The electrically
conductive
aerosolisable material may be a liquid, and may further contain nicotine or a
salt thereof.
Of the metal components contained in the housing, at least one may be a plated
metal and
the exposable surface of said component may be the metal which is plated. For
instance, the
component may be gold-plated such that the exposable surface is the metal
which is plated
with gold, e.g. brass. The metal components contained in the housing may also
be identical
in the sense that they are composed of a single metal. This metal may be
selected from the
group consisting of nickel, stainless steel, titanium and aluminium.
Alternatively the metal
components may be identical in the sense that they are composed of the same
metals, e.g.
an alloy or a plated-alloy. For example, the metal components contained in the
housing may
include nickel plated with gold or may be composed solely of nickel. The
potential difference
between the exposed and/or exposable surfaces of the one or more metal
components may
be from 0 mV to about 20 mV.
Also provided is a cartomiser comprising the housing described herein, wherein
the
cartomiser is a closed or open system, i.e. non-fillable or refillable with
aerosolisable
material.
In addition there is provided a cartomiser for an aerosol provision system
comprising a
reservoir containing an electrically conductive aerosolisable material and two
exposed and/or
exposable surfaces of one or more metal components which are capable of
simultaneously
2
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
being in contact with the electrically conductive aerosolisable material, and
wherein the
change in dissolved metal content of the electrically conductive aerosolisable
material after
storage of the cartomiser for about 1 to about 8 weeks, e.g. about 2 weeks or
14 days, at
4000 is between 0 and about 20%.
Also provided is an aerosol provision system comprising the housing described
herein or one
of the cartomisers described herein.
Finally there is provided the use of one or more metal components in an
aerosol provision
system to reduce galvanic corrosion, wherein the one or more metal components
have two
surfaces which are simultaneously exposed and/or exposable to an electrically
conductive
aerosolisable material in the aerosol provision system, and said surfaces have
a potential
difference of from 0 mV to about 35 mV.
In the described use the potential difference of the exposed and/or exposable
surfaces may
be from 0 mV to about 20 mV. Further, the electrically conductive
aerosolisable material may
be a liquid and/or may comprise nicotine or a salt thereof.
These embodiments are set out in the appended independent and dependent
claims. It will
be appreciated that features of the dependent claims may be combined with each
other and
with features of the independent claims in combinations other than those
explicitly set out in
the claims. Furthermore the approaches described herein are not restricted to
specific
embodiments such as those set out below, but include and contemplate any
appropriate
combinations of features presented herein. For example, the housing, the
cartomiser
comprising the housing, the cartomiser defined by the dissolved metal content
in the
electrically conductive aerosolisable material after storage, the aerosol
provision system
comprising the housing or the cartomiser, and the use described herein may be
provided in
accordance with approaches described herein which includes any one or more of
the various
features described below as appropriate.
BRIEF DESCRIPTION OF THE DRAWINGS
Embodiments of the invention will now be described, by way of example only,
with reference
to the accompanying drawings, in which:
Figure 1 is a highly schematic drawing of an aerosol generating element in
accordance with
some embodiments of the disclosure. As is discussed in more detail below,
Figure 1 shows a
substrate 1 with a heating surface 4 and two electrical contacts, e.g. wires 2
each
independently connected to an electrical connector 3.
3
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
Figures 2 to 5 are graphs plotted from the ICP-MS values obtained in Example 3
for
dissolved nickel, copper, zinc and gold content following use of each of the
heating elements
analysed in this example, alongside the liquid control. These figures are
discussed in more
detail below.
DETAILED DESCRIPTION
Aspects and features of certain examples and embodiments are discussed and
described
herein. Some aspects and features of certain examples and embodiments may be
implemented conventionally and these are not discussed or described in detail
in the
interests of brevity. It will thus be appreciated that aspects and features of
apparatus and
methods discussed herein which are not described in detail may be implemented
in
accordance with any conventional techniques for implementing such aspects and
features.
As discussed herein, the present disclosure provides a housing for an aerosol
provision
system in which any two exposed and/or exposable surfaces of one or more metal
components thereof have a potential difference of from 0 mV to about 35 mV,
the two
surfaces being capable of simultaneously being in contact with the
aerosolisable material.
Also provided is a cartomiser or aerosol provision system comprising the
housing, along with
a cartomiser which after storage for about 1 to about 8 weeks at 40 C exhibits
a change in
dissolved metal content in the electrically conductive aerosolisable material
between 0% and
about 20%.
In arriving at the present disclosure, the inventors observed discoloration at
the bottom of the
cartomiser, followed by discoloration in the e-liquid and on the aerosol
generating element
(the heater) after use. Without wishing to be bound by theory, the inventors
believed that the
combination of the gold/nickel plated brass electrical connectors and nickel
contact in the
heater was forming a galvanic cell when both metal components were in contact
with the
conductive e-liquid. The galvanic cell was then causing corrosion of the metal
components,
potentially releasing metal ions into solution and thereby leading to the
observed
discolouration.
Following further experiments which are set out in detail in the Examples, the
discolouration
was confirmed as comprising propylene glycol, vegetable glycerin and at least
the following
metals: gold, nickel, copper and zinc. Comparing a discoloured sample with a
control sample
specifically identified an increase in at least the dissolved copper, nickel
and zinc content of
the e-liquid.
As is known in the art, the basis for a galvanic cell is always a redox
reaction which includes
two half-reactions: oxidation at an anode and reduction at a cathode.
Electricity is generated
4
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
due to an electric potential difference between two electrodes which is
created as a result of
the difference between individual potentials of the two metal electrodes with
respect to the
electrolyte. In other words, it is the measure of reducing power of any
element or compound.
More specifically, a galvanic cell involves a spontaneous redox reaction
because the Gibbs
free energy is negative in accordance with the following equation:
6'Gc e11= ¨nFEcc'ell
where n is the number of moles of electrons per mole of products and F is the
Faraday
constant, approximately 96485 C/mol. With a negative Gibbs free energy, a
spontaneous
redox reaction drives the cell to produce an electric potential. It follows
that for a galvanic
cell, E e11 must be > 0 where E .e11 = -Ec athode ¨ Eac'node and Eac'node is
the standard potential at
.. the anode and Ecc'athode is the standard potential at the cathode. Standard
electrode
potentials are known in the art.
For example, the standard electrode potential of zinc is -0.76 V. Thus zinc
will be oxidized by
any electrode whose standard electrode potential is greater than -0.76 V, e.g.
copper (0.34
V) and reduced by any electrode whose standard electrode potential is less
than -0.76 V,
e.g. sodium (-2.71 V).
To identify the possible source of the galvanic cell, the potential difference
between each of
the metal components in the heating element was measured. The results were 101
mV 10
mV for the nickel contact and gold/nickel plated brass electrical connector; 8
mV 10 mV for
the two nickel heater wires; 2 mV 10 mV for the two gold/nickel plated brass
electrical
connectors; and 25 mV 10 mV for the two combinations of nickel contact with
gold/nickel
plated brass electrical connector. In view of these results, it was
hypothesised that a galvanic
cell was arising at least between the nickel contact and the gold/nickel
plated brass electrical
connector when both in contact with the e-liquid, i.e. the two metal surfaces
having the
highest potential difference.
The present disclosure provides a solution to this galvanic corrosion problem
by
incorporating the same or similar metals into the housing, a "similar" metal
being understood
as having a potential difference of from 0 mV to about 35 mV. In particular
the defined
potential difference is between any two exposed and/or exposable surfaces of
one or more
metal component of the housing, the surfaces being capable of simultaneously
being in
contact with the aerosolisable material. Advantageously the present disclosure
is thus able to
reduce the level of metal found in the aerosolisable material and/or aerosol
and thereby
improve user experience and consistency of aerosol delivery.
5
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
For ease of reference, these and further features of the present disclosure
are now
discussed under appropriate section headings. However, the teachings under
each section
are not limited to the section in which they are found.
Housing
The present disclosure provides a housing for an aerosol provision system
comprising a
reservoir for an electrically conductive aerosolisable material. The housing
may be formed of
a plastics material and as well as supporting other components, the housing
may provide a
mechanical interface when incorporated into a cartomiser so that the
cartomiser can be
connected to a control unit of an aerosol provision system as required. The
manner by which
the housing interfaces with the control unit is not significant for the
present disclosure. It may,
for example, comprise a screw thread fitting or any other attachment or
connection means
known to the person skilled in the art. The shape of the housing is also not
limited and may
be any shape known in the art.
The reservoir for the electrically conductive aerosolisable material may be
contained in an
aerosol generation chamber or may be in fluid communication with such a
chamber. By the
term "fluid communication" is meant that the aerosolisable material contained
in the reservoir
is able to flow or move easily from the reservoir towards or in the direction
of the aerosol
generation chamber. When the reservoir is contained in an aerosol generation
chamber, the
reservoir may comprise the majority of the interior volume of the aerosol
generation chamber.
The reservoir may generally conform to the interior of the aerosol generation
chamber.
In some examples, at least an outer wall of the reservoir may be integrally
moulded with the
aerosol generation chamber. In other examples, the reservoir may be a
component which is
formed separately from, but supported in position by, the aerosol generation
chamber. In
examples, the reservoir may have a tapered circular cross-section but have a
flat face
running longitudinally along one side to create a space between an outer wall
of the reservoir
and an inner wall of the aerosol generation chamber to define a flow path
through the
cartomiser through which aerosol generated in the cartomiser is drawn during
use towards
an opening or outlet in the end of the cartomiser. In other examples, the
reservoir may have
an annular shape, with the outer annular surface defined by the aerosol
generation chamber,
and the inner annular surface defining a flow path. It will be appreciated
that there are many
configurations which allow for the provision of a liquid reservoir alongside a
flow path within
the cartomiser.
The reservoir may be formed in accordance with conventional techniques, for
example
comprising a moulded plastics material, machined plastic components, cast
plastic
6
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
components, machined metal components, cast or drawn metal components, metal
components that are formed and subsequently plated with other metal materials,
or mixtures
thereof.
Electrically Conductive Aerosolisable Material
Any reference herein to an "aerosolisable material" is to an electrically
conductive
aerosolisable material. The term "aerosolisable material" may be used
interchangeably with
the terms "aerosol generating material", "vapour generating material",
"aerosol precursor
material" and/or "vapour precursor material". By the term "aerosolisable
material" is meant a
material that is capable of generating aerosol, for example, when heated,
irradiated or
energized in any other way. As appropriate, the aerosolisable material may
comprise one or
more active agents, one or more flavours, one or more aerosol-former
materials, and/or one
or more other functional materials.
Aerosolisable materials may, for example, be in the form of a solid, liquid or
gel which may or
may not contain nicotine and/or flavourants.
By the term "electrically conductive" is meant that the aerosolisable material
is able to
transport an electric charge. As mentioned above, the electrical conductivity
of the
aerosolisable material may arise from the presence of acids, bases and/or
salts. In various
embodiments, the electrical conductivity of the aerosolisable material may
arise from the
presence of salts or other ionic compounds. In various embodiments of the
present
disclosure, the aerosolisable material is therefore an electrolyte because of
these ionic
compounds and/or salts. The skilled person in the art is aware of suitable
techniques to
determine electrical conductivity or ionic content of an aerosolisable
material, and is also able
to provide a suitably electrically conductive aerosolisable material.
Aerosolisable material may, for example, be in the form of a solid, liquid or
gel which may or
may not contain an active agent and/or flavourants. In various embodiments of
the present
disclosure, the electrically conductive aerosolisable material is a liquid. In
other embodiments
of the present disclosure, the aerosolisable material may comprise an
"amorphous solid",
which may alternatively be referred to as a "monolithic solid" (i.e. non-
fibrous). In some
embodiments, the amorphous solid may be a dried gel. The amorphous solid is a
solid
material that may retain some fluid, such as liquid, within it. In some
embodiments, the
aerosolisable material may for example comprise from about 50 wt%, 60 wt%, 70
wt% of
amorphous solid, to about 90 wt%, 95 wt% or 100 wt% of amorphous solid.
In various embodiments of the present disclosure, the aerosolisable material
comprises a
vapour- or aerosol- generating agent; otherwise referred to as an aerosol-
former material.
7
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
The aerosol-former material may comprise one or more constituents capable of
forming an
aerosol. Examples of such agents/constituents are glycerine/glycerol,
propylene glycol,
diethylene glycol, triethylene glycol, tetraethylene glycol, 1,3-butylene
glycol, erythritol, meso-
erythritol, ethyl vanillate, ethyl laurate, a diethyl suberate, triethyl
citrate, triacetin, a diacetin
mixture, benzyl benzoate, benzyl phenyl acetate, tributyrin, lauryl acetate,
lauric acid, myristic
acid, propylene carbonate, and mixtures thereof.
In some embodiments, the aerosol-former material may comprise one or more of
glycerol,
propylene glycol, diethylene glycol, triethylene glycol, tetraethylene glycol,
1,3-butylene
glycol, erythritol, meso-Erythritol, ethyl vanillate, ethyl laurate, a diethyl
suberate, triethyl
citrate, triacetin, a diacetin mixture, benzyl benzoate, benzyl phenyl
acetate, tributyrin, lauryl
acetate, lauric acid, myristic acid, and propylene carbonate.
The one or more other functional materials may comprise one or more of pH
regulators,
colouring agents, preservatives, binders, fillers, stabilizers, and/or
antioxidants.
The aerosolisable material may be present on or in a support, to form a
substrate. The
support may, for example, be or comprise paper, card, paperboard, cardboard,
reconstituted
material, a plastics material, a ceramic material, a composite material,
glass, a metal, or a
metal alloy. In some embodiments, the support comprises a susceptor. In some
embodiments, the susceptor is embedded within the material. In some
alternative
embodiments, the susceptor is on one or either side of the material.
A susceptor is a material that is heatable by penetration with a varying
magnetic field, such
as an alternating magnetic field. The susceptor may be an electrically-
conductive material, so
that penetration thereof with a varying magnetic field causes induction
heating of the heating
material. The heating material may be magnetic material, so that penetration
thereof with a
varying magnetic field causes magnetic hysteresis heating of the heating
material. The
susceptor may be both electrically-conductive and magnetic, so that the
susceptor is
heatable by both heating mechanisms. The device that is configured to generate
the varying
magnetic field is referred to as a magnetic field generator, herein.
The aerosolisable material may also include at least one "flavour",
"flavouring agent" or
"flavourant". The terms "flavour", "flavouring agent" and "flavourant" are
used interchangeably
to refer to materials which, where local regulations permit, are added to a
material to create a
desired taste, aroma or other somatosensorial sensation in a product for adult
consumers.
Reference here to "flavour", "flavouring agent" or "flavourant" include both
singular and multi-
component flavours. They may include naturally occurring flavour materials,
botanicals,
extracts of botanicals, synthetically obtained materials, or combinations
thereof (e.g.,
8
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
tobacco, cannabis, licorice (liquorice), hydrangea, eugenol, Japanese white
bark magnolia
leaf, chamomile, fenugreek, clove, maple, matcha, menthol, Japanese mint,
aniseed (anise),
cinnamon, turmeric, Indian spices, Asian spices, herb, wintergreen, cherry,
berry, red berry,
cranberry, peach, apple, orange, mango, clementine, lemon, lime, tropical
fruit, papaya,
rhubarb, grape, durian, dragon fruit, cucumber, blueberry, mulberry, citrus
fruits, Drambuie,
bourbon, scotch, whiskey, gin, tequila, rum, spearmint, peppermint, lavender,
aloe vera,
cardamom, celery, cascarilla, nutmeg, sandalwood, bergamot, geranium, khat,
naswar, betel,
shisha, pine, honey essence, rose oil, vanilla, lemon oil, orange oil, orange
blossom, cherry
blossom, cassia, caraway, cognac, jasmine, ylang-ylang, sage, fennel, wasabi,
piment,
ginger, coriander, coffee, hemp, a mint oil from any species of the genus
Mentha, eucalyptus,
star anise, cocoa, lemongrass, rooibos, flax, ginkgo biloba, hazel, hibiscus,
laurel, mate,
orange skin, rose, tea such as green tea or black tea, thyme, juniper,
elderflower, basil, bay
leaves, cumin, oregano, paprika, rosemary, saffron, lemon peel, mint,
beefsteak plant,
curcuma, cilantro, myrtle, cassis, valerian, pimento, mace, damien, marjoram,
olive, lemon
balm, lemon basil, chive, carvi, verbena, tarragon, limonene, thymol,
camphene), flavour
enhancers, bitterness receptor site blockers, sensorial receptor site
activators or stimulators,
sugars and/or sugar substitutes (e.g., sucralose, acesulfame potassium,
aspartame,
saccharine, cyclamates, lactose, sucrose, glucose, fructose, sorbitol, or
mannitol), and other
additives such as charcoal, chlorophyll, minerals, botanicals, or breath
freshening agents.
They may be imitation, synthetic or natural ingredients or blends thereof.
They may be in any
suitable form, for example, liquid such as an oil, solid such as a powder, or
gas.
The flavour, flavouring agent or flavourant may be selected from the group
consisting of
extracts, for example liquorice, hydrangea, Japanese white bark magnolia leaf,
tobacco,
chamomile, fenugreek, clove, menthol, Japanese mint, aniseed, cinnamon, herb,
wintergreen, cherry, berry, peach, apple, Drambuie, bourbon, scotch, whiskey,
spearmint,
peppermint, lavender, cardamom, celery, cascarilla, nutmeg, sandalwood,
bergamot,
geranium, honey essence, rose oil, vanilla, lemon oil, orange oil, cassia,
caraway, cognac,
jasmine, ylang-ylang, sage, fennel, pimento, ginger, anise, coriander, coffee,
flavour
enhancers, bitterness receptor site blockers, sensorial receptor site
activators or stimulators,
sugars and/or sugar substitutes (e.g. sucralose, acesulfame potassium,
aspartame,
saccharine, cyclamates, lactose, sucrose, glucose, fructose, sorbitol, or
mannitol), and other
additives such as charcoal, chlorophyll, minerals, botanicals, or breath
freshening agents.
They may be imitation, synthetic or natural ingredients or blends thereof.
They may be in any
suitable form, for example, oil, liquid, or powder.
In some embodiments, the flavour comprises menthol, spearmint and/or
peppermint. In
some embodiments, the flavour comprises flavour components of cucumber,
blueberry, citrus
9
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
fruits and/or redberry. In some embodiments, the flavour comprises eugenol. In
some
embodiments, the flavour comprises flavour components extracted from tobacco.
In some
embodiments, the flavour comprises flavour components extracted from cannabis.
In some embodiments, the flavour may comprise a sensate, which is intended to
achieve a
somatosensorial sensation which are usually chemically induced and perceived
by the
stimulation of the fifth cranial nerve (trigeminal nerve), in addition to or
in place of aroma or
taste nerves, and these may include agents providing heating, cooling,
tingling, numbing
effect. A suitable heat effect agent may be, but is not limited to, vanillyl
ethyl ether and a
suitable cooling agent may be, but not limited to eucolyptol, WS-3.
The aerosolisable material may also comprise other components. Such other
components
may be conventional in the sense that they are typically included in
aerosolisable materials
for e-cigarettes. In various embodiments of the present disclosure, the
aerosolisable material
further comprises an active agent. By the term "active agent" is meant any
agent which has a
biological or physiological effect on a subject when the vapour containing the
active is
inhaled. The active may be a physiologically active material, which is a
material intended to
achieve or enhance a physiological response. The active may, for example, be
selected from
nutraceuticals, nootropics or psychoactives. The one or more active agents may
be selected
from nicotine, botanicals, salts thereof and mixtures thereof. The one or more
active agents
or salts thereof may be of synthetic or natural origin. The active or salt
thereof could be an
extract from a botanical, such as from a plant in the tobacco family. An
example active is
nicotine.
In some embodiments the active agent may be selected from nicotine, caffeine,
taurine,
theine, vitamins such as B6 or B12 or C, melatonin, cannabinoids, or
constituents,
derivatives (e.g. salts) or combinations thereof. The active agent may
comprise one or more
constituents, derivatives or extracts of tobacco, cannabis or another
botanical. Constituents,
derivatives or extracts of cannabis may include one or more cannabinoids or
terpenes.
As noted herein, the active agent may comprise or be derived from one or more
botanicals or
constituents, derivatives or extracts thereof. As used herein, the term
"botanical" includes
any material derived from plants including, but not limited to, extracts,
leaves, bark, fibres,
stems, roots, seeds, flowers, fruits, pollen, husk, shells or the like.
Alternatively, the material
may comprise an active compound naturally existing in a botanical, obtained
synthetically.
The material may be in the form of liquid, gas, solid, powder, dust, crushed
particles,
granules, pellets, shreds, strips, sheets, or the like. Example botanicals are
tobacco,
eucalyptus, star anise, hemp, cocoa, cannabis, fennel, lemongrass, peppermint,
spearmint,
rooibos, chamomile, flax, ginger, ginkgo biloba, hazel, hibiscus, laurel,
licorice (liquorice),
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
matcha, mate, orange skin, papaya, rose, sage, tea such as green tea or black
tea, thyme,
clove, cinnamon, coffee, aniseed (anise), basil, bay leaves, cardamom,
coriander, cumin,
nutmeg, oregano, paprika, rosemary, saffron, lavender, lemon peel, mint,
juniper,
elderflower, vanilla, wintergreen, beefsteak plant, curcuma, turmeric,
sandalwood, cilantro,
bergamot, orange blossom, myrtle, cassis, valerian, pimento, mace, damien,
marjoram, olive,
lemon balm, lemon basil, chive, carvi, verbena, tarragon, geranium, mulberry,
ginseng,
theanine, theacrine, maca, ashwagandha, damiana, guarana, chlorophyll, baobab
or any
combination thereof. The mint may be chosen from the following mint varieties:
Mentha
Arventis, Mentha c.v.,Mentha niliaca, Mentha piperita, Mentha piperita citrata
c.v.,Mentha
piperita c.v, Mentha spicata crispa, Mentha cardifolia, Memtha long ifolia,
Mentha suaveolens
variegata, Mentha pulegium, Mentha spicata c.v. and Mentha suaveolens
In some embodiments, the active agent comprises or is derived from one or more
botanicals
or constituents, derivatives or extracts thereof and the botanical is tobacco.
In some embodiments, the active agent comprises or derived from one or more
botanicals or
constituents, derivatives or extracts thereof and the botanical is selected
from eucalyptus,
star anise, cocoa and hemp.
In some embodiments, the active agent comprises or derived from one or more
botanicals or
constituents, derivatives or extracts thereof and the botanical is selected
from rooibos and
fennel.
In some embodiments, the active agent comprises nicotine and/or a salt
thereof. In some
embodiments, the active agent comprises caffeine, melatonin or vitamin B12.
In various embodiments of the present disclosure, the aerosolisable material
comprises
nicotine and/or a salt thereof. Nicotine may be provided in any suitable
amount depending on
the desired dosage when inhaled by the user. Depending on the other components
of the
aerosolisable material, nicotine may be in a salt form in the aerosolisable
material. If, for
example, an acid is added (e.g. an organic acid) then nicotine will typically
be protonated
leaving the residual anion of the acid in solution. Consequently, it may be
the presence of
nicotine salts which give rise to the electrical conductivity properties of
the aerosolisable
material. The invention is not, however, limited to an aerosolisable material
comprising
nicotine and/or a salt thereof and the skilled person will be aware of other
components (e.g.
other actives or aerosol- generating agents) which result in an electrically
conductive
aerosolisable material because of the formation of ionic compounds and/or
salts.
In various embodiments of the present disclosure, nicotine is present in the
aerosolisable
material in an amount of no greater than about 6 wt% based on the total weight
of the
11
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
aerosolisable material. By the expression "total weight of the aerosolisable
material" is meant
the total weight of the aerosolisable material in which the nicotine is
present.
In various embodiments, nicotine is present in an amount of from about 0.4 to
about 6 wt%
based on the total weight of the aerosolisable material. In various
embodiments, nicotine is
present in an amount of from about 0.8 to about 6 wt% based on the total
weight of the
aerosolisable material. In various embodiments nicotine is present in an
amount of from
about 1 to about 6 wt% based on the total weight of the aerosolisable
material. In various
embodiments, nicotine is present in an amount of from about 1.8 to about 6 wt%
based on
the total weight of the aerosolisable material.
In other embodiments nicotine is present in an amount of no greater than about
3 wt% based
on the total weight of the aerosolisable material. In various embodiments,
nicotine is present
in an amount of from about 0.4 to about 3 wt% based on the total weight of the
aerosolisable
material. In various embodiments, nicotine is present in an amount of from
about 0.8 to about
3 wt% based on the total weight of the aerosolisable material. In various
embodiments
nicotine is present in an amount of from about 1 to about 3 wt% based on the
total weight of
the aerosolisable material. In various embodiments nicotine is present in an
amount of from
about 1.8 to about 3 wt% based on the total weight of the aerosolisable
material.
In other embodiments nicotine is present in an amount of less than about 1.9
wt% based on
the total weight of the aerosolisable material. In various embodiments
nicotine is present in
an amount of less than about 1.8 wt% based on the total weight of the
aerosolisable material.
In various embodiments nicotine is present in an amount of from about 0.4 to
less than about
1.9 wt% based on the total weight of the aerosolisable material. In various
embodiments
nicotine is present in an amount of from about 0.4 to less than about 1.8 wt%
based on the
total weight of the aerosolisable material. In various embodiments nicotine is
present in an
amount of from about 0.5 to less than about 1.9 wt% based on the total weight
of the
aerosolisable material. In various embodiments nicotine is present in an
amount of from
about 0.5 to less than about 1.8 wt% based on the total weight of the
aerosolisable material.
In various embodiments nicotine is present in an amount of from about 0.8 to
less than about
1.9 wt% based on the total weight of the aerosolisable material. In various
embodiments
nicotine is present in an amount of from about 0.8 to less than about 1.8 wt%
based on the
total weight of the aerosolisable material. In various embodiments nicotine is
present in an
amount of from about 1 to less than about 1.9 wt% based on the total weight of
the
aerosolisable material. In various embodiments nicotine is present in an
amount of from
about 1 to less than about 1.8 wt% based on the total weight of the
aerosolisable material.
12
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
In various embodiments of the present disclosure, the aerosolisable material
may contain
one or acids. The aerosolisable material may, for example, contain one or more
acids in
addition to nicotine (as the active agent). The one or more acids may be one
or more organic
acids, e.g. one or more organic acids selected from the group consisting of
benzoic acid,
levulinic acid, malic acid, maleic acid, fumaric acid, citric acid, lactic
acid, acetic acid, succinic
acid, and mixtures thereof. As noted above, when included in the aerosolisable
material in
combination with nicotine, the one or more acids may provide a formulation in
which the
nicotine is at least partially in protonated (such as monoprotonated and/or
diprotonated)
form.
In various embodiments of the present disclosure, the aerosolisable material
comprises
nicotine or another active, optionally a flavourant or flavour agent, and one
or more acids. In
various embodiments of the present disclosure, the aerosolisable material
comprises nicotine
in one of the above described amounts, optionally a flavourant or flavour
agent, and one or
more acids selected from the group consisting of benzoic acid, levulinic acid,
malic acid,
maleic acid, fumaric acid, citric acid, lactic acid, acetic acid, succinic
acid, and mixtures
thereof. In various embodiments, the flavourant or flavour agent is present in
the
aerosolisable material and is defined as above.
Potential Difference
In the present disclosure, the potential difference between any two exposed
and/or
exposable surfaces of one or more metal components contained in the housing is
limited to
being from 0 mV to about 35 mV; the two surfaces being capable of
simultaneously being in
contact with the aerosolisable material.
By the term "potential difference" is meant the difference of electrical
potential between two
points. As is known in the art, a potential difference is measured with a
voltmeter under
atmospheric pressure and at room temperature in a suitable electrolyte. In the
present
disclosure, the potential difference is measured under atmospheric pressure
and room
temperature (approximately 20 C) in the aerosolisable material to be used with
the housing
in the aerosol provision system.
Whether a particular combination of metals is going to give rise to a
potential difference
within the present disclosure can also be estimated based on the galvanic or
electropotential
series. This series ranks metals (and other materials) in order of standard
electrode potential,
and an extract from this series is shown in the following table:
13
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
Metal / Other Reaction Approximate Electrode
Potential (V)
Gold Au + + e- = Au 1.692
Gold Au3+ + 3e- = Au 1.498
Platinum Pt2+ + 2e- = Pt 1.18
Palladium Pd2+ + 2e- = Pd 0.951
Copper Cu + + e- = Cu 0.521
Copper Cu2+ + 2e- = Cu 0.3419
Iron Fe3+ + 3e- = Fe -0.037
Lead Pb2+ + 2e- = Pb -0.1262
Tin Sn2+ + 2e- = Sn -0.1375
Nickel Ni2+ + 2e- = Ni -0.257
Cobalt Co2+ + 2e- = Co -0.28
Cadmium Cd2+ + 2e- = Cd -0.403
Iron Fe2+ + 2e- = Fe -0.447
Chromium Cr3+ + 3e- = Cr -0.744
Zinc Zn2+ + 2e- = Zn -0.7618
Chromium Cr2+ + 2e- = Cr -0.913
Manganese Mn2+ + 2e- = Mn -1.185
Titanium Ti3+ + 3e- = Ti -1.37
Titanium Ti2+ + 2e- = Ti -1.63
Aluminium A/3+ + 3e- = Al -1.662
Magnesium Mg2+ + 2e- = Mg -2.372
Magnesium Mg + + e- = Mg -2.7
Sodium Na + + e- = Na -2.71
Calcium Ca2+ + 2e- = Ca -2.868
Potassium K+ + e- = K -2.931
Lithium Li3+ + 3e- = Li -3.0401
Calcium Ca + + e- = Ca -3.8
A nickel surface and a cobalt surface in contact with an electrically
conductive aerosolisable
material will, for example, have a potential difference of approximately 0.023
V or 23.0 mV. In
contrast, a nickel surface and a gold surface will have a potential difference
of at least
approximately 1.755 V.
The potential difference which is the subject of the present disclosure is
between any two
exposed and/or exposable surfaces of one or more metal components contained in
the
14
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
housing, where the surfaces are capable of simultaneously being in contact
with the
aerosolisable material. By the term "exposed" is meant a surface which is not
covered or
hidden, i.e. the surface is visible in the housing. By the term "exposable" is
meant a surface
which is hidden or covered, e.g. by plating, but can be uncovered or made
visible during use
of the housing in an aerosol provision system. For example, a component made
from a
plated metal will have an exposed surface ¨ the plating ¨ and an exposable
surface ¨ the
metal underneath the plating. During use of the housing in an aerosol
provision system, the
metal underneath the plating may become exposed as the plating degrades.
By the expression "capable of simultaneously being in contact with the
aerosolisable
material" is meant that the aerosolisable material is able to form a contact
junction between
the exposed and/or exposable surfaces of the one or more metal components such
that the
surfaces are in electrical contact and electric charge can flow between the
surfaces. The
location of the one or more metal components in the housing is not therefore
limited; the
metal components must be separate from one another but in electrical contact.
The contact junction may, for example, be formed by the aerosolisable material
in the
reservoir, in an aerosol generating element integrated with the reservoir, in
an aerosol
generating element which is separate from the reservoir, or downstream of the
reservoir
and/or aerosol generating element if the aerosol formed from the aerosolisable
material
forms deposits on two suitable surfaces in the flow path to the mouthpiece of
the device. The
contact junction formed by the aerosolisable material and two
exposed/exposable surfaces of
one or more metal components in the present disclosure does not, however, form
a galvanic
cell because the potential difference between the surfaces is from 0 mV to
about 35 mV.
For example, both the exposed and/or exposable surface of a first metal
component may
have a potential difference of from 0 mV to about 35 mV with respect to any
other exposed
and/or exposable surface of a second metal component contained in the housing
which is
capable of being in contact with the electrically conductive aerosolisable
material at the same
time as said first exposed and/or exposable surface. In this manner, the
housing avoids
metal degradation.
In various embodiments of the present disclosure, the contact junction formed
by the
aerosolisable material and two exposed and/or exposable surfaces is in an
aerosol
generating element which is integrated with the reservoir. In such
embodiments, the exposed
and/or exposable surface of a first metal component of the aerosol generating
element has a
potential difference of from 0 mV to about 35 mV with respect to any other
exposed and/or
exposable surface of a second metal component of the aerosol generating
element. Provided
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
of course that the surfaces are capable of being in contact with the
electrically conductive
aerosolisable material at the same time.
The potential difference between the surfaces is from 0 mV to about 35 mV. In
various
embodiments of the present disclosure, the potential difference is from 0 mV
to about 30 mV.
In various embodiments, the potential difference is from 0 mV to about 25 mV.
In various
embodiments, the potential difference is from 0 mV to about 20 mV. In various
embodiments,
the potential difference is from 0 mV to about 18 mV. In various embodiments,
the potential
difference is from 0 mV to about 15 mV. In various embodiments, the potential
difference is
from 0 mV to about 12 mV. In various embodiments, the potential difference is
from 0 mV to
about 10 mV.
In various embodiments, the potential difference is from 0 mV to about 10 mV.
Aerosol Generating Element
In various embodiments of the present disclosure, the one or more metal
components having
two exposed and/or exposable surfaces are part of an aerosol generating
element. The
aerosol generating element is able to produce aerosol from the aerosolisable
material by any
suitable means, e.g. heat, irradiation or any other method of energizing a
material to form
vapour or aerosol.
In some embodiments, the aerosol generating element is a heater configured to
subject the
aerosol-generating material to heat energy, so as to release one or more
volatiles from the
aerosol-generating material to form an aerosol. In some embodiments, the
aerosol
generator/aerosol generating element is configured to cause an aerosol to be
generated from
the aerosol-generating material without heating. For example, the aerosol
generator may be
configured to subject the aerosol-generating material to one or more of
vibration, increased
pressure, or electrostatic energy.
In various embodiments of the present disclosure, the one or more metal
components are
part of an aerosol generating element which comprises a wick and a heater.
Other known
arrangements may of course be used. The wick and heater are arranged in a
space within
the housing, e.g. in an aerosol generation chamber, such that the wick extends
transversely
across the chamber with its ends extending into the reservoir of aerosolisable
material,
through openings in the inner wall of the reservoir. The openings in the inner
wall of the
reservoir may be sized to broadly match the dimensions of the wick and thereby
provide a
reasonable seal against leakage from the reservoir into the flow path whilst
avoiding unduly
compressing the wick, which may be detrimental to its fluid transfer
performance.
Aerosolisable material, e.g. liquid, may infiltrate the wick through surface
tension or capillary
16
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
action. The heater then comprises the one or more metal components with two
exposed
and/or exposable surfaces which are capable of simultaneously being in contact
with the
aerosolisable material, as described herein.
In other embodiments of the present disclosure, the one or more metal
components are part
of an aerosol generating element as shown in Figure 1. The aerosol generating
element
shown in Figure 1 may be located in the reservoir of the housing or as a
separate component
to the reservoir. When located in the reservoir, the aerosol generating
element may be
integrated therewith and be located in an aerosol generation chamber as
described above,
such that the reservoir and aerosol generation chamber are formed as a single
moulded
component. The aerosol generating element of Figure 1 comprises a porous, wick
substrate
1, e.g a ceramic disc, so that the aerosolisable material within the reservoir
may seep
through the disc to a heating substrate 4 for vaporisation. Attached to the
substrate 1 are
contacts 2 connected to electrical connectors, e.g. electrode pins 3.
With continued reference to Figure 1, the one or more metal components which
have two
exposed and/or exposable surfaces capable of simultaneously contacting the
aerosolisable
material may be the contacts 2, the electrode pins or electrical connectors 3,
the heating
substrate 4 and/or a combination thereof. The contacts 2 may have exposed
surfaces, the
electrical connectors 3 may have exposed and exposable surfaces, being made of
a plated
metal, and/or the heating substrate may include an exposed or exposable
surface.
Alternatively, the contacts 2 may have an exposed surface(s), the electrical
connectors 3
may have an exposed surface(s) and/or the heating substrate 4 may have an
exposed
surface(s). The skilled person will be aware of suitable techniques for making
an aerosol
generating element as shown in Figure 1. For example, the heating substrate
could be
applied to the surface of the wick substrate via known printing techniques for
applying
conductive inks to surfaces etc.
In various embodiments of the present disclosure, the contacts 2, electrical
connectors 3
and/or heating substrate 4 have exposed and/or exposable surfaces which are
capable of
being in simultaneous contact with the electrically conductive aerosolisable
material and
which have a potential difference of from 0 mV to about 35 mV. This potential
difference may
be from 0 my to about 30 mV, from 0 mV to about 25 mV, from 0 mV to about 20
mV, from 0
mV to about 18 mV, from 0 mV to about 15 mV, from 0 mV to about 12 mV or from
0 mV to
about 10 mV.
In various embodiments, the contacts 2, connectors 3 and heating substrate 4
comprise the
same metal or metal alloy, e.g. nickel or a nickel alloy, titanium or a
titanium alloy, or
stainless steel. Exemplary nickel alloys and titanium alloys will be known to
the person skilled
17
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
in the art; the nickel alloy may be NiCrFe or NiCr. In such embodiments, the
aerosol
generating element is composed of a single conductive material, i.e. a single
metal or metal
alloy.
Cartomiser
Also provided by the present disclosure is a cartomiser comprising the housing
as defined
herein. As is known in the art, cartomisers may also be referred to as
cartridges. Throughout
the description herein, the term "cartridge" may therefore be used
interchangeably with
"cartomiser".
The cartomiser of the present disclosure may be a closed or open system as
defined herein.
In various embodiments of the present disclosure, the cartomiser is a closed
system such
that the aerosol generating element is integrated within the reservoir as
already described
herein. The aerosol generating element may be as shown in Figure 1 with the
potential
difference values between metal components having exposed or exposable
surfaces capable
of being in simultaneous contact with the electrically conductive
aerosolisable material, as
described above.
In various embodiments of the present disclosure, the cartomiser comprising
the housing is a
closed system with an aerosol generating in fluid contact with the reservoir,
the one or more
metal components with two exposed and/or exposable surfaces, being part of the
aerosol
generating element. In such embodiments, the two exposed and/or exposable
surfaces are
capable of simultaneously being in contact with the electrically conductive
aerosolisable
material and have a potential difference of from 0 mV to about 35 mV. This
potential
difference may also be from 0 mV to about 30 mV, from 0 mV to about 25 mV,
from 0 mV to
about 20 mV, from 0 mV to about 18 mV, from 0 mV to about 15 mV, or from 0 mV
to about
10 mV.
Additionally the present disclosure provides a cartomiser for an aerosol
provision system
comprising a reservoir containing an electrically conductive aerosolisable
material and two
exposed and/or exposable surfaces of one or more metal components, wherein the
two
surfaces are capable of simultaneously being in contact with the electrically
conductive
aerosolisable material. In such a cartomiser, the change in dissolved metal
content of the
electrically conductive aerosolisable material after storage of the cartomiser
for about 1 to
about 8 weeks at 40 C, e.g. about 2 weeks or 14 days, is between 0 and about
20%.
The features overlapping with the above-described housing are defined
according to the
description already provided. For example, the reservoir, the electrically
conductive
aerosolisable material, the two exposed and/or exposable surfaces of one or
more metal
18
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
components, and the simultaneous contact of these surfaces with the
aerosolisable material.
The cartomiser may also be a closed or open system. In various embodiments,
the
cartomiser may be a closed system.
By the feature "change in dissolved metal content of the electrically
conductive aerosolisable
material after storage of the cartomiser from about 1 to about 8 weeks at 40
C" is meant that
the level of dissolved metals measured in the electrically conductive
aerosolisable material at
the point of filling the cartomiser (T=0) and on removal of the cartomiser
from storage is no
greater than about 20%. In other words, any change or increase is relative to
the background
or baseline level of metals in the electrically conductive aerosolisable
material (e.g. the e-
liquid). Dissolved metal content is determined according to methods known in
the art. In
particular, dissolved metal content is determined by Inductively Coupled
Plasma-Mass
Spectrometry (ICP-MS). Metal content refers to all measurable metals in the e-
liquid, for
example any metals that may be present due to the construction of the product
including
nickel, copper, zinc, gold, titanium, beryllium, silver, aluminium, manganese,
lead, chromium,
arsenic, molybdenum, cobalt, iron and/or tin.
In various embodiments of the present disclosure, the change in dissolved
metal content is
determined after storage of the cartomiser for about 1 to about 6 weeks at 40
C or about 1 to
about 4 weeks, e.g. about 2 weeks or 14 days. In various embodiments of the
present
disclosure, the change in dissolved metal content of the electrically
conductive aerosolisable
material is further between 0 and about 15%. In other embodiments the change
in dissolved
metal content is between 0 and about 10% or between 0% and about 5%. In
various
embodiments of the present disclosure, there is substantially no change in
dissolved metal
content. By the expression "substantially no change" means less than 5%. As
the person
skilled in the art will appreciate, the change in dissolved metal content is
an indication that
galvanic corrosion is not taking place.
Aerosol Provision System
The present disclosure further provides an aerosol provision system comprising
the housing
as described herein or one of the cartomisers as described herein.
As is common in the art, the terms "vapour" and "aerosol", and related terms
such as
"vaporise", "volatilise" and "aerosolise", may be used interchangeably.
Aerosol provision
systems/devices may therefore be referred to herein as "vapour provision
systems/devices",
"aerosol delivery devices/systems", "electronic vapour provision
devices/systems", "electronic
aerosol provision devices/systems", or "e-cigarettes/electronic cigarettes".
These terms may
be used interchangeably and are intended to refer to combustible or non-
combustible aerosol
19
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
provision systems/devices. In some embodiments the aerosol provision system is
a non-
combustible aerosol provision system such as a heating device that releases
compounds
from aerosolisable material(s) without burning or combusting the aerosolisable
materials.
According to the present disclosure, a "combustible" aerosol provision system
is one where a
constituent aerosol-generating material of the aerosol provision system (or
component
thereof) is combusted or burned during use in order to facilitate delivery of
at least one
substance to a user. In some embodiments, the delivery system is a combustible
aerosol
provision system, such as a system selected from the group consisting of a
cigarette, a
cigarillo and a cigar. In some embodiments, the disclosure relates to a
component for use in
a combustible aerosol provision system, such as a filter, a filter rod, a
filter segment, a
tobacco rod, a spill, an aerosol-modifying agent release component such as a
capsule, a
thread, or a bead, or a paper such as a plug wrap, a tipping paper or a
cigarette paper.
The non-combustible aerosol provision system is one where a constituent
aerosol-generating
material (aerosolisable material) of the aerosol provision system (or
component thereof) is
not therefore combusted or burned in order to facilitate delivery of at least
one substance to a
user, and this system can include electronic cigarettes or e-cigarettes that
create aerosol
from aerosol precursor materials by heating or other techniques such as
vibration; and hybrid
systems that provide aerosol via a combination of aerosol precursor materials
and solid
substrate materials, for example hybrid systems containing liquid or gel
precursor materials
and a solid substrate material.
In some embodiments, the aerosol provision system is a non-combustible aerosol
provision
system, such as a powered non-combustible aerosol provision system. In some
embodiments, the non-combustible aerosol provision system, such as a non-
combustible
aerosol provision device thereof, may comprise a power source and a
controller. The power
source may, for example, be an electric power source or an exothermic power
source. In
some embodiments, the exothermic power source comprises a carbon substrate
which may
be energised so as to distribute power in the form of heat to an aerosolisable
material or to a
heat transfer material in proximity to the exothermic power source.
The aerosol provision system can comprise a cartomiser or housing of the
present disclosure
and generally a control unit. The control unit of the aerosol provision system
may generally
comprise an outer housing, an electrical power source (e.g. a battery),
control circuitry for
controlling and monitoring the operation of the aerosol provision system, a
user input button,
and optionally a mouthpiece (which may be detachable). The battery may be
rechargeable
and be of a conventional type, for example of the kind typically used in
electronic cigarettes
and other applications requiring provision of relatively high currents over a
relatively short
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
period. Similarly, a user input button (or other aerosol generation function)
and control
circuity may be conventional. The outer housing may be formed, for example,
from a plastics
or metallic material. Other suitable materials are known in the art. As will
be appreciated, the
aerosol provision system will in general comprise various other elements
associated with its
operating functionality. For example, a port for charging the battery, such as
a USB port or
the like, and these other elements may be conventional.
When a user sucks/inhales on the aerosol provision system of the present
disclosure, air
should be drawn from the environment into the system and at least a portion of
this air enters
the housing or cartomiser. Typically, the incoming air flows past an aerosol
generation
component (e.g. heater) while the heater is receiving electrical power from
the battery in the
control unit so as to generate aerosol from an aerosol precursor material. The
aerosolised
material is then incorporated/entrained into the airflow and drawn through and
out of the
cartomiser for inhalation by a user. The aerosol may be produced or released
in various
ways depending on the nature of the device, system or product. These include
heating to
cause evaporation, heating to release compounds, and vibration of a liquid or
gel to create
droplets.
During normal use, the control circuitry may be configured to monitor various
operational
aspects of the aerosol provision system. For example, the control circuitry
may be configured
to monitor a level of power remaining in the rechargeable battery, and this
may be performed
in accordance with conventional techniques. Additionally the control circuitry
may be
configured to estimate a remaining amount of aerosol precursor material in the
cartomiser, or
substrate material in the consumable, for example based on an accumulated time
of usage
since a new cartomiser or consumable was installed, or based on sensing the
levels in the
cartomiser or consumable. This may be performed in accordance with any
conventional
technique(s). It may, for example, be based on sensing the number of puffs on
the aerosol
provision system in accordance with any conventional technique(s).
If it is determined through monitoring the operational aspects of the aerosol
provision system
that a certain operating condition has arisen, for example, a cartomiser is
approaching
depletion, or a battery level is falling below a predetermined threshold
(which may be
predefined or user set), the aerosol provision system may be configured to
provide a user
notification according to any conventional technique(s). Although described
with reference to
the control circuitry, other user notifications are known in the art and may
be implemented in
the aerosol provision system of the present disclosure. In addition, it will
be appreciated that
there are many other situations in which a user notification might be desired,
the present
disclosure is not limited to providing notification of low levels of liquid or
substrate material or
remaining battery power.
21
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
In one embodiment the aerosol provision system is an electronic non-
combustible aerosol
provision system. In one embodiment, the aerosol provision system is an
electronic cigarette,
also known as a vaping device or electronic nicotine delivery system (END),
although it is
noted that the presence of nicotine in the aerosolisable material is not a
requirement.
In some embodiments, the non-combustible aerosol provision system is an
aerosolisable
material heating system, also known as a heat-not-burn system. An example of
such a
system is a tobacco heating system.
In one embodiment, the aerosol provision system (e.g. the non-combustible
aerosol provision
system) is a hybrid system for providing aerosol by heating, but not burning,
a combination of
aerosolisable materials. In some embodiments, the non-combustible aerosol
provision
system is a hybrid system to generate aerosol using a combination of
aerosolisable
materials, one or a plurality of which may be heated. Each of the
aerosolisable materials may
be, for example, in the form of a solid, liquid or gel and may or may not
contain nicotine. In
some embodiments, the hybrid system comprises a liquid or gel aerosolisable
material and a
solid aerosolisable material. The solid aerosolisable material may comprise,
for example,
tobacco or a non-tobacco product.
Typically, the non-combustible aerosol provision system may comprise a non-
combustible
aerosol provision device and a consumable for use with the non-combustible
aerosol
provision device. In some embodiments, the disclosure relates to consumables
comprising
aerosol-generating material and configured to be used with non-combustible
aerosol
provision devices. These consumables are sometimes referred to as articles
throughout the
disclosure.
In some embodiments, the non-combustible aerosol provision system may comprise
an area
for receiving the consumable, an aerosol generator, an aerosol generation
area, a housing,
a mouthpiece, a filter and/or an aerosol-modifying agent.
In some embodiments, the consumable for use with the non-combustible aerosol
provision
device may comprise aerosol-generating material, an aerosol-generating
material storage
area, an aerosol-generating material transfer component, an aerosol generator,
an aerosol
generation area, a housing, a wrapper, a filter, a mouthpiece, and/or an
aerosol-modifying
agent.
Reduction of galvanic corrosion
The present disclosure also provides the use of one or more metal components
in an aerosol
provision system to reduce galvanic corrosion, wherein the one or more metal
components
22
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
have two surfaces which are simultaneously exposed and/or exposable to an
electrically
conductive aerosolisable material in the aerosol provision system, and said
surfaces have a
potential difference of from 0 mV to about 35 mV.
The features overlapping with the above-described housing are defined
according to the
description already provided. For example, the electrically conductive
aerosolisable material,
the two exposed and/or exposable surfaces of one or more metal components, the
potential
difference and the simultaneous contact of these surfaces with the
aerosolisable material. In
particular, the potential difference values described above are equally
applicable to the use
of the present disclosure as to the housing and cartomiser. Additionally, the
one or more
metal components in the use of the present disclosure may be part of an
aerosol generating
element according to the above description.
The term "galvanic corrosion" is known in the art and may also be referred to
as bimetallic
corrosion. It is an electrochemical process in which one metal corrodes
preferentially when it
is in electrical contact with another, in the presence of an electrolyte. The
reduction of
galvanic corrosion, as provided by the present disclosure, may be measured by
determining
the dissolved metal content in the aerosolisable material after use of the
aerosol provision
system and/or by determining the metal content in the aerosol generated by the
aerosol
provision system.
Measurement of metal content in the aerosolisable material may be carried out
according to
ICP-MS as noted above. Measurement of metal content in the aerosol generated
by the
aerosol provision system may be carried out according to any fully
quantitative method using
calibration standards that are appropriate to the expected values in the test
liquids. Any
increase in metal content of the aerosolisable material relative to the
control value of the
original aerosolisable material indicates transfer of metal(s) and hence
galvanic corrosion.
The present disclosure will now be exemplified with reference to the following
non-limiting
examples.
EXAMPLES: Example 1
Observations were carried out on a set of cartomizers comprising a heating
element having
nickel contacts connected with gold/nickel-plated brass electrical connectors.
In cartomizers
where leakage or seepage of the e-liquid from the reservoir onto the heater
element of the
cartomizer had occurred, discoloration of the liquid contained in the
reservoir (the e-liquid),
after use in an aerosol provision system was observed. As discussed in
Examples 2 and 3
below, this correlated with elevated metal content in the aerosolisable
material.
23
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
It was thus hypothesised that the contact of the metal components of the
heater element ¨
the gold plated and nickel plated brass electrical connector and nickel
contact, e.g. wire - with
the e-liquid was forming a galvanic cell, thereby causing galvanic corrosion
of the heating
element and releasing metal into the system. Corrosion was visually confirmed
by an
observation of cracks in the plating of the electrical connector. Quantitative
confirmation was
then obtained by carrying out an analysis of e-liquid with and without
discoloration using ICP-
MS. The results of this analysis are shown in Table I below:
Table I
ICP-MS of the liquid taken from the No discoloration;
Discoloration;
cartomiser reservoir ug/g ug/g
Cu 2.4 31
Au <0.005 <0.005
Ni 0.25 2.6
Zn 0.16 21
It can be seen from these results that the discoloration of the e-liquid is
attributable to an
increase in copper, nickel and/or zinc content. Given that the heating element
had nickel
contacts and gold/nickel-plated brass electrical connectors, it appears that
the nickel surface
of the contact and/or the nickel plating of the connector, along with the
brass surface of the
connector are being corroded.
Example 2: Potential Difference Experiments
It is known in the art that galvanic corrosion occurs when two dissimilar
metals are in contact
with each other in the presence of an electrolyte. This contact forms a
galvanic cell leading to
H2 formation on the more noble metal and the resulting electrochemical
potential then
develops an electric current that electrolytically dissolves the less noble
material. To confirm
that the corrosion observed in Example 1 was galvanic corrosion and to isolate
the surfaces
forming the galvanic cell, experiments were therefore conducted to measure the
potential
difference between the various components of the heating element. The
combinations
measured were:
(i) nickel contact vs. gold/nickel plated brass electrical connector;
(ii) nickel contact vs. nickel contact;
(iii) gold/nickel plated brass electrical connector and nickel contact vs.
gold/nickel plated
brass electrical connector and nickel contact; and
(iv) gold/nickel plated brass electrical connector vs. gold/nickel plated
brass electrical
connector.
Measurement of the potential difference was with a voltmeter.
24
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
The e-liquid was prepared by diluting commercially available e-liquid with 15
% water wt/wt.
The e-liquid contained flavourant, 5% nicotine, and 1 MeQ lactic acid.
To determine the potential difference between metal surfaces, each of the
combinations (i) to
(iv) were placed into the e-liquid and the potential difference was measured
with the
voltmeter. The results are shown in Table II below.
Table ll
Combination Potential Difference (mV 10
mV)
(i) nickel contact vs. gold/nickel plated 101
brass electrical connector
(ii) nickel contact vs. nickel contact 8
(iii) gold/nickel plated brass electrical 25
connector and nickel contact vs.
gold/nickel plated brass electrical
connector and nickel contact
(iv) gold/nickel plated brass electrical 2
connector vs. gold/nickel plated brass
electrical connector
It can be seen from these values that the highest voltage in e-liquid was
recorded using the
gold/nickel plated brass electrical connector on one probe and nickel contact
on the second
probe. This suggests that the increased copper, nickel and zinc content seen
in Table I was
due to a galvanic cell arising between the exposed nickel contact and the
exposed brass
electrical connector surface. The lowest voltages were seen with the nickel
contact on both
probes and the gold/nickel plated brass electrical connectors on both probes,
i.e. where the
metal components are identical. This suggests that removing the gold/nickel
plated brass
electrical connector from the cartomiser and replacing it with a nickel
electrical connector
should reduce the risk of the galvanic cell effect.
Example 3: Dissolved Metal Content Testing
Example 3 involved the following aerosol generating elements, otherwise
referred to as
heating elements:
Nickel/Copper/Zinc Heating Element:
Wick Substrate - Heating Substrate -
Nickel Contacts - Gold/Nickel Plated
Brass Electrical Connectors
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
Nickel/Nickel Heating Element:
Wick Substrate - Heating Substrate -
Nickel Contacts -
Nickel Electrical
Connectors
Nickel/Gold Heating Element:
Wick Substrate - Heating Substrate -
Nickel Contacts - Nickel/Au Plated
Electrical Connectors
With reference to Figure 1, all elements had a wick substrate 1, a heating
substrate 4, nickel
legs or contacts 2, and either gold/nickel-plated brass electrical connectors
3, nickel electrical
connectors 3 or nickel/Au electrical connectors 3.
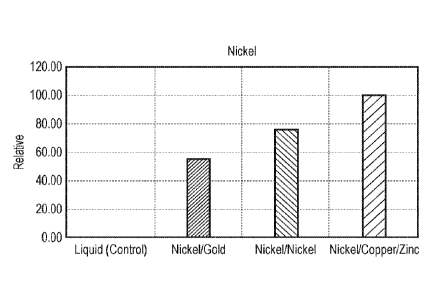
Three samples of each heating element were placed in bottles with 2 ml of e-
liquid and the
bottles gently shaken so that the liquid covered the nickel contacts and
electrical connectors
in their entirety. The bottles containing the heating element and e-liquid
were then stored at
accelerated conditions of 40 C/75% RH for 7, 14, 21 or 28 days. The e-liquid
was the same
as Example 2.
At each time point, the required samples were removed from storage, assessed
visually for
any discoloration, gently shaken to homogenise the liquid, and the liquid then
sampled
directly from the bottles. The samples of liquid were analysed with ICP-MS for
the presence
of various metals. A liquid control, i.e. without any heating element, was
also analysed. The
ICP-MS results for the various metals tested were averaged and then normalised
relative to
the maximum measured value for that particular metal. Figures 2 to 5 are
graphs showing the
results for each of the heating elements analysed alongside the liquid
control.
Figure 2 shows the average dissolved nickel content for each of the heating
elements and
the liquid control relative to the maximum measured value for nickel (set at
100.00). It can be
seen that moving from the nickel/copper/zinc heating element to either the
nickel/nickel or
nickel/gold heating element reduces the level of dissolved nickel in the e-
liquid. This is an
indication that galvanic corrosion is reduced and even eliminated in the
nickel/gold and
nickel/nickel systems. The difference between the nickel/gold and
nickel/nickel heating
elements is simply due to the higher level of nickel in the system. It is not
evidence of an
increase in galvanic corrosion.
Figures 3 and 4 respectively show the average dissolved copper and zinc
content for each of
the heating elements and the liquid control relative to the maximum measured
value for each
metal (set at 100.00). As for the nickel content, it can be seen that moving
from the
nickel/copper/zinc heating element to the nickel/nickel or nickel/gold heating
element reduces
the level of dissolved copper and zinc in the e-liquid. This is further
evidence that galvanic
26
CA 03136787 2021-10-13
WO 2020/212705 PCT/GB2020/050971
corrosion is significantly reduced or even eliminated in the nickel/gold and
nickel/nickel
systems. In summary, by replacing the electrical connector with a component
that has an
exposable surface with a electrode potential which differs from that of the
electrical contact
by 0 mV to about 35 mV, copper and zinc degradation is eliminated.
Finally, Figure 5 shows the average dissolved gold content for each of the
heating elements
and the liquid control relative to the maximum measured value for gold (set at
100.00)
Compared to the nickel/copper/zinc heating element, the average dissolved gold
content can
be seen to decrease with the nickel/nickel heating element and increase with
the nickel/gold
heating element.
On balance it can therefore be concluded that the use of metal components for
the aerosol
generating element which have exposed and/or exposable surfaces whose
potential
difference is from 0 mV to about 35 mV, where the surfaces are capable of
simultaneously
contacting the electrically conductive aerosolisable material, reduces
galvanic corrosion in
the aerosol provision system comprising the aerosol generating element. More
particularly,
when at least the electrical contact and electrical connector of the aerosol
generating
element are made of the same metal (e.g. nickel), galvanic corrosion is
practically eliminated;
notably the nickel, copper, zinc and gold levels in the e-liquid are
significantly reduced
compared to the current heating element.
The various embodiments described herein are presented only to assist in
understanding
and teaching the claimed features. These embodiments are provided as a
representative
sample of embodiments only, and are not exhaustive and/or exclusive. It is to
be understood
that advantages, embodiments, examples, functions, features, structures,
and/or other
aspects described herein are not to be considered limitations on the scope of
the invention
as defined by the claims or limitations on equivalents to the claims, and that
other
embodiments may be utilised and modifications may be made without departing
from the
scope of the claimed invention. Various embodiments of the invention may
suitably comprise,
consist of, or consist essentially of, appropriate combinations of the
disclosed elements,
components, features, parts, steps, means etc. other than those specifically
described
herein. In addition, this disclosure may include other inventions not
presently claimed, but
which may be claimed in future.
The aerosol provision system described herein can be implemented as a
combustible
aerosol provision system, or a non-combustible aerosol provision system as
defined
hereinabove.
27