Note: Descriptions are shown in the official language in which they were submitted.
1
Alloy Having Fine-Scale Eutectic, In Particular Nanoeutectic, Structure And
Production
Of Such An Alloy
The invention relates to an alloy, in particular a light metal alloy, having
an alloy composition with
at least three components and a eutectic structure that is obtained by cooling
the alloy from a liquid
state to a solid state.
The invention furthermore relates to a method for producing an alloy, in
particular a light metal
alloy, with a eutectic structure, wherein the alloy has an alloy composition
with at least three
components and wherein the alloy is cooled, starting from a liquid state, to a
solid state of the alloy
in order to form the eutectic structure.
It is known that it can be advantageous if a portion of a structure of an
alloy is embodied with a
eutectic structure in order to influence casting properties or strength
properties of an alloy. Binary
casting alloys, that is, alloys with two components which have eutectic
microstructures, are often
used as technical application alloys. These alloys are normally characterized
by a eutectic point
in their phase diagram, at which point a liquid phase of the alloy and two
solid phases of the alloy
are in thermodynamic equilibrium with one another, or at which a direct
transition from a liquid
state to a solid state takes place when the alloy is cooled from the liquid
phase, wherein a eutectic
structure is formed. According to Gibbs' phase rule for solids at a constant
pressure f = N ¨ P + 1
with a number of thermodynamic degrees of freedom f, a number of components N,
and a number
of equilibrium phases ID, this corresponds to a number of degrees of freedom
off = D. The direct
transition from liquid phase to solid phase thereby often results in a
formation of a fine and lamellar
structure.
Analogously, in relation to ternary alloy systems, attempts to create alloys
with compositions close
to a ternary eutectic point have also become known, in order to improve
strength properties with
an embodiment of a eutectic structure. According to Gibbs' phase rule f = N ¨
P + 1, this
analogously likewise corresponds, with three components and four phases, to f
= 0 degrees of
freedom. However, high cooling rates are normally required to form alloys of
this type, in order
to create a eutectic structure with pronounced fineness at alloy amounts which
can be used in
CA 03138658 2021- 11- 18
2
applications, and a coordinated additional combination with other elements for
precipitation
hardening is often necessary to increase a strength of the alloy. In most
cases, cooling rates in a
range of 50 Kis to 200 Kis are used for this purpose. However, the requirement
of high cooling
rates in particular limits a technical usability of alloys of this type to
small-scale parts.
This is addressed by the invention. The object of the invention is to specify
an alloy having at
least three components that has high strength and good deformability.
A further goal of the invention is to specify a method for producing an alloy
of this type.
The object is attained according to the invention if, with an alloy of the
type named at the outset,
the eutectic structure is obtained under the condition that a composition of
the alloy lies in a field
around a pseudoeutectic point of a phase diagram of the alloy, so that at
least 85 mol% or at%
eutectic structure is present in the alloy.
The basis of the invention is the finding that, with a composition of an alloy
having at least three
components or elements that are at or in proximity to a pseudoeutectic point
in the phase diagram
of the alloy, a particularly fine-scale or finely structured eutectic
structure can be embodied which,
in particular, can have a finer eutectic structure than an alloy having a
composition chosen that is
at the "usual" eutectic point in the phase diagram. In particular,
characteristic structural spacing
of the eutectic structure in the low micrometer range and specifically in the
nanometer range can
thus be realized, also referred to as a nanoeutectic structure. In addition,
it has been shown that
the eutectic structure thereby formed normally constitutes a principal or
dominant microstructure,
and in particular that often only a small or negligibly small primary
solidification phase and/or
residual solidification phase, or none at all, occurs in proximity to or in a
field around, in particular
at, a eutectic point. This combination of an extraordinarily fine
microstructure of the eutectic
structure and the dominant presence thereof in the alloy enables the alloy to
be embodied with
both high strength, in particular compressive strength, and also pronounced
deformability. In
representations, the pseudoeutectic point is typically denoted such that it is
abbreviated by "e" or
"pE" and the eutectic point abbreviated by "E".
CA 03138658 2021- 11- 18
3
Technically, in a ternary phase diagram, the liquidus line and solidus line
known from the binary
phase diagram typically respectively correspond to curved surface areas and
binary phase areas
correspond to phase volumes. In the ternary phase diagram, the intersecting
lines of liquidus areas
typically form eutectic channels, also referred to as liquidus boundary lines
or monovariant lines,
which end in a ternary eutectic point of the phase diagram. The pseudoeutectic
point thereby
represents a point on the liquidus boundary line that forms a saddle point,
that is, represents a local
extreme along the liquidus boundary line and a minimum perpendicular thereto ¨
in relation to the
bounding single-phase fields.
Sometimes, in representations of two-component boundary systems or content
intersections of, in
particular ternary, phase diagrams, binary eutectics are also inconsistently
referred to as
pseudoeutectic points. However, a terminological designation of this type is
not in the sense of
the present concept, and it is explicitly not intended or signified by, nor is
it comprised by, the
nomenclature "pseudoeutectic point" in this document. In particular, the
pseudoeutectic point is
characterized in that the existence thereof requires an addition or a presence
of at least a third
component or a third element.
In terms of the Gibbs' phase rule, the pseudoeutectic point pE represents in
the ternary alloy system
a local extreme along the liquidus boundary line, which extreme has a number
of degrees of
freedom that is 1 greater than the ternary eutectic E and a number of degrees
of freedom that is 1
less than a single-phase solidification MC. With the Gibbs' phase rule f = N ¨
P + 1 with a number
of thermodynamic degrees of freedom f, a number of components N and a number
of equilibrium
phases P, this corresponds to:
f(E) = 3- 4 + 1 = 0
f(pE) = 3 - 3 + 1 = 1
AMC) = 3 - 2 + 1 = 2
This increased degree of freedom of 1 at the pseudoeutectic point pE compared
to the degree of
freedom of 0 for the ternary eutectic point E is considered to be the cause of
the embodiment of
CA 03138658 2021- 11- 18
4
the finer, often by up to several orders of magnitude, eutectic microstructure
in the region of the
pseudoeutectic point compared to the microstructure formed at the eutectic
point.
Accordingly, for an alloy having four components, the liquidus boundary line
corresponds to a
two-dimensional area and the pseudoeutectic point corresponds to a
pseudoeutectic line. For
respective higher-component alloys having more than four components, a
dimensionality of
associated state regions increases analogously. Within the scope of this
document, the designation
"pseudoeutectic point" is therefore specifically to be understood as a general
term that signifies
both a pseudoeutectic point in a phase diagram of a ternary alloy and also a
corresponding
pseudoeutectic line in a phase diagram of an alloy having four components, or
a corresponding
pseudoeutectic multi-dimensional region in a phase diagram of an alloy having
more than four
components Thus, in this regard, the designations "pseudoeutectic point" and
"pseudoeutectic
region" in particular are used synonymously. It should be understood that a
pseudoeutectic point
of a ternary alloy system thereby constitutes a specific embodiment.
In accordance with this explanation, in particular for the ternary alloy
system, the following thus
holds true for a pseudoeutectic point of a phase diagram of a ternary alloy or
for a pseudoeutectic
point, in particular a pseudoeutectic line or a pseudoeutectic region, of a
phase diagram with more
than three components, according to Gibbs' phase rule:
An < APE) < AMC),
therefore:
0 < f (13E) < N - 1.
The alloy composition at the pseudoeutectic point or the pseudoeutectic point
of the phase diagram
of the alloy having at least three components N is thus in particular
characterized in that, according
to Gibbs' phase rule, the number of degrees of freedom flies between 0 and N-
1.
It has been shown that it is sufficient for both high strength and also for
pronounced deformability
of the alloy if the alloy composition lies in proximity to or in a field
around, in particular at, the
CA 03138658 2021- 11- 18
5
pseudoeutectic point or the saddle point representing said point, so that at
least 85 mol% or at%
(stated in mole percent and atomic percent, respectively) eutectic structure
is present in the alloy.
It is preferable if at least 90 mol% or at%, particularly preferably at least
95 mol% or at%, eutectic
structure is present in the alloy. As a result, the advantageous properties of
high strength with
simultaneously good deformability can be developed in a particularly
pronounced manner.
Specifically, up to 98 mol% or at% can often be attained thereby, so that the
mechanical properties
of the alloy are virtually solely determined by the eutectic microstructure.
The eutectic structure
typically forms in a liquid-solid phase transformation or in a solidification
of the alloy.
High strength and pronounced deformability of the alloy are achievable both if
the alloy is a ternary
alloy and also if the alloy comprises four components or at least five
components. In particular,
the alloy can comprise a plurality of components, for example in the form of
other added
components for mixed crystal hardening and/or precipitation hardening,
depending on the
application objective. It is particularly simple and feasible to embody the
alloy with high strength
and deformability if the alloy is a ternary alloy or quaternary alloy.
The eutectic structure normally has average characteristic structural spacing
or an average spacing
of the phase amounts thereof, in particular lamellae, of less than 3 pm. A
particularly pronounced
strength and deformability can be achieved if the average spacing is thereby
less than 2 pm, in
particular less than 1 pm. This can be achieved, for example, if the alloy
composition of the alloy
is selected such that it is in greater proximity to the stoichiometric
composition of the
pseudoeutectic point. Particularly high strengths can thereby be achieved if
the average spacing
is less than 800 nm, in particular less than 600 nm. Additionally or
alternatively, the average
spacing of the phase amounts can be influenced by varying a cooling speed of
the alloy during the
solidification of the alloy.
It is advantageous if the alloy has a residual solidification at an amount of
maximally 5 mol% or
at%, preferably maximally 3 mol% or at%, in particular preferably maximally 2
mol% or at%. In
this manner, the aforementioned properties advantageously obtained with the
eutectic structure are
influenced only insignificantly or not at all by the amount of residual
solidification. The residual
solidification amount can be set by selecting the alloy composition such that
it is in greater
CA 03138658 2021- 11- 18
6
proximity to the stoichiometric composition of the pseudoeutectic point.
Residual solidification
typically denotes that microstructure amount in which, after the formation of
the eutectic structure,
a residual amount of liquid phase solidifies in the form of a structure that
is no longer eutectic, or
the number or type of the phases being formed changes at the end of the
eutectic solidification.
The amount of residual solidification typically constitutes a factor which
limits the properties
effected by the eutectic structure formed, for which reason it is beneficial
if the residual
solidification is kept as small as possible.
Here, it is particularly
beneficial if the residual
solidification is not embodied in a network-like manner, or with a form of a
network structure, but
rather is preferably embodied, where present, in the form of islands or units
separated from one
another. Normally, the residual solidification is embodied at an amount of at
least 1 mol% or at%,
but can preferably also be smaller.
It is expedient for a pronounced strength and deformability if the alloy has a
primary solidification
at an amount less than 10 mol% or at%, in particular less than 5 mol% or at%,
preferably less than
3 mol% or at%. This enables a very dominant embodiment of the eutectic
structure, or an
embodiment of the eutectic structure at a high structural amount with
aforementioned properties
that can accordingly be advantageously achieved. The primary solidification,
which denotes that
part of the solidified microstructure which, immediately preceding the
formation of the eutectic
structure, does not solidify in the form of a eutectic structure, is, with
regard to a limitation of the
properties that can be attained with the embodiment of the eutectic structure,
less relevant than the
aforementioned residual solidification, but should also preferably be kept as
small as possible.
Normally, the primary solidification is embodied at an amount of at least 1
mol% or at%, but can
preferably also be smaller.
For an embodiment of high strength and particularly pronounced deformability,
it is beneficial if
the primary solidification is formed having or being made of a mixed crystal
phase and, in
particular, not having or not being made of intermetallic phase. This appears
to be an advantageous
criterion that applies to all alloy systems, in order to achieve particularly
application-friendly
strength and deformability properties.
CA 03138658 2021- 11- 18
7
The amount of aforementioned residual solidification and/or primary
solidification can, in a
customary technical manner, be controlled or predetermined using a
thermodynamic calculation
according to Scheil-Gulliver. The Scheil-Gulliver calculation or equation,
sometimes also simply
referred to as the Scheil calculation or equation, describes a distribution of
an alloy amount in an
alloy during a solidification, wherein a local equilibrium on a progressing
solidification front and
a disregarded diffusion in solid phase are normally assumed. A calculation of
this type constitutes
a customary technical tool or textbook knowledge in the field of metallurgy
and is presumed to be
known to a person skilled in the art. This is exemplified in the textbook
"Solidification" by J . A.
Dantzig et al., (ISBN: 978-2-940222-17-9).
It is beneficial if the alloy has a density of less than 8.0 gicm3, in
particular less than 7.5 g/cm3,
preferably less than 6 gicm3. The alloy can thus have a particularly
advantageous strength-to-
weight ratio with regard to an application, especially as a structural part.
It is particularly beneficial
if the alloy is embodied as a light metal alloy. A particularly high
application suitability of the
alloy can thus be achieved. It is advantageous if, for this purpose, the alloy
has less than 5.0 gicm3,
in particular less than 3.0 g/crn3.
Fora feasible use as application material, it is beneficial if the alloy is a
magnesium-based alloy,
aluminum-based alloy, lithium-based alloy, or titanium-based alloy.
It is advantageous if the alloy is a casting alloy. This enables a
particularly feasible production,
specifically of structural parts having aforementioned properties in
particular.
It has proven effective if the alloy is an Al-Mg alloy. Depending on the
precise intended
application, the alloy may comprise other alloy components. In this manner,
application parts
having particular relevance to practical situations, in particular structural
parts, can be produced
having or being made of the alloy. Here, it is particularly beneficial if the
alloy is an Al-Mg-Si
alloy. Advantageously, the alloy can also comprise zinc (Zn), in particular at
an amount greater
than 0.01 wt%, typically greater than 1 wt%. A compressive strength of the
alloy can thus be
optimized. In most cases, the alloy thereby comprises less than 15 wt%, in
particular less than
CA 03138658 2021- 11- 18
8
wt%, preferably between 1.0 wt% and 5.0 wt%, particularly preferably
approximately 3.0 wt%,
zinc.
A high application suitability, to which both strength and also pronounced
deformability are
5 particularly advantageous, can be achieved if the alloy is an Al-Cu-Li
alloy, Al-Cu-Mg alloy, Mg-
Li-Al alloy, Mg-Cu-Zn alloy, Al-Cu-Mg-Zn alloy, or Al-Mg-Si-Zn alloy.
An alloy with high application suitability, specifically in the form of a
structural part, that exhibits
particularly high strength and deformability can be achieved if the alloy is a
magnesium-based
10 alloy comprising, in particular being made of (in at%):
15.0% to 70.0% lithium,
greater than 0.0%, in particular greater than 0.01%, preferably greater than
0.05%, aluminum,
magnesium and production-related impurities as a remainder,
wherein a ratio of aluminum to magnesium (in at%) is 1:6 to 4:6.
An Mg-Li-Al alloy of this type has an alloy composition in a field around or
in proximity to or at
an alloy composition of a pseudoeutectic point in the Mg-Li-Al phase diagram,
so that a finely
structured or micro-scale eutectic microstructure can be attained. The fine-
scale microstructure is
accompanied by a high strength, in particular a high compressive strength,
there being at the same
time a good deformability of the magnesium alloy at corresponding
aforementioned amounts of
lithium in the magnesium alloy. An orientation composition or orientation line
in the phase
diagram is thereby in particular an aluminum-to-magnesium ratio (in atomic
percent, abbreviated
by at%) of approx. 3:6, since a particularly homogeneous fine-scale, or
homogeneous fine lamellar,
microstructure or morphology is found at this ratio. In a range encompassing
this ratio, above all
at an aluminum-to-magnesium ratio (in at%) of 1:6 to 4:6, the fine, in
particular fine lamellar,
microstructure or morphology is also found in a varyingly pronounced degree,
which is
accordingly normally accompanied by varying pronounced magnitudes of strength,
in particular a
magnitude of compressive strength, as well as deformability or ductility of
the magnesium alloy.
Because of these special morphological characteristics in the stated
composition range, a formation
of a magnesium alloy that has both a high strength, in particular compressive
strength, and also a
good deformability is thus enabled. This magnesium alloy and a method for the
production thereof
as well as a realization as a feedstock, semi-finished product or part, and
also specific embodiments
CA 03138658 2021- 11- 18
9
thereof were filed and disclosed in the European Patent Office as part of
European Patent
Application No. 19184999.1 and also as part of International Application No.
PCT/EP2020/058280, the disclosures of which are hereby included in their
entirety in the
disclosure of this document. This applies specifically where, as is stated in
said applications, the
Mg-Al-Li alloy comprises (in at%) 30.0% to 60.0%, in particular 40% bis 50%,
preferably 45%
bis 50%, particularly preferably 45% to 48%, lithium. It is further
advantageous if the Mg-Al-Li
alloy comprises (in at%) greater than 0.05%, in particular greater than 0.1%,
normally greater than
1% aluminum. It has thereby been shown that the Mg-Al-Li alloy can be embodied
with a, in
particular lamellar, microstructure with a high degree of fineness if the
ratio of aluminum to
magnesium (in at%) is 1.2:6 to 4:6, in particular 1.4:6 to 4:6, preferably
1.5:6 to 4:6. It is beneficial
to a pronounced fineness or a fine, in particular lamellar, microstructure if
the ratio of aluminum
to magnesium (in at%) is 1.8:6 to 3.5:6, in particular 2:6 to 3.5:6,
preferably 2.5:6 to 3.5:6. A
particularly high strength, in particular compressive strength, can thus be
achieved. This holds
especially true at an aluminum-to-magnesium ratio (in at%) of 2.8:6 to 3.3:6,
preferably
approximately 3:6, at which a very homogeneous fine morphology or
microstructure is obtainable.
To this end, it is particularly advantageous if the magnesium alloy (in at%)
is 30.0% to 60.0%
lithium and an aluminum-to-magnesium ratio (in at%) is 2.5:6 to 3.5:6, in
particular 2.8:6 to 3.3:6,
preferably approximately 3:6. In this regard, reference is made in particular
to Fig. 1 of the
aforementioned application documents, in which a corresponding arrangement in
an Mg-Li-Al
phase diagram is schematically illustrated, and the disclosure of which as
well as the related
description are accordingly also to be considered part of this document. A
particularly pronounced
homogeneity is also achievable if the magnesium alloy thereby comprises (in
at%) 40.0% to 60.0%
lithium. As stated in the aforementioned applications and also incorporated
accordingly into the
present disclosure, the properties of the Mg-Al-Li alloy can be further
optimized if amounts of
calcium, rare earth metals, in particular yttrium, zinc, and/or silicon are
additionally present
according to the aforementioned applications at corresponding content ranges
stated in the
aforementioned applications. For example, an alloy of this type can be
embodied as Mg-20%Li-
15%A1-1%Ca-0.5%Y (in wt%) or Mg-20%Li-24%AI-1%Ca-0.5%Y (in wt%).
The other object of the invention is attained with a method of the type named
at the outset, under
the condition that the composition is provided such that it lies in a field
around a pseudoeutectic
CA 03138658 2021- 11- 18
10
point of a phase diagram of the alloy, so that the eutectic structure is
embodied at an amount of at
least 85 mol% or at% when the alloy is cooled to the solid phase or
solidifies. As stated above,
the alloy can thus be embodied with high strength and pronounced
deformability. Because the
alloy composition is selected in a field around the pseudoeutectic point, a
eutectic phase reaction
or phase transformation takes place when the alloy is cooled from the liquid
state to the solid state,
or during the liquid-to-solid transition, which phase reaction or
transformation embodies the
eutectic microstructure with a particularly high degree of fineness or fine
structuring as a principal
microstructure amount of the alloy.
It should be understood that the method according to the invention may be
embodied
correspondingly or analogously to the features, advantages, implementations,
and effects that are
described, in particular as described above, within the scope of an alloy
according to the invention.
The same also applies to the alloy according to the invention with regard to a
method according to
the invention.
A feedstock, semi-finished product or part is advantageously realized having,
in particular being
made of, an alloy according to the invention or such that it is obtainable
using a method according
to the invention for producing an alloy according to the invention. In
accordance with the
foregoing explanations, features, and effects of the alloy according to the
invention or of an alloy
produced with a method according to the invention, a feedstock, semi-finished
product or part
formed with an alloy also has an advantageously high strength and good
deformability.
Additional features, advantages, and effects follow from the exemplary
embodiments described
below. In the drawings which are thereby referenced:
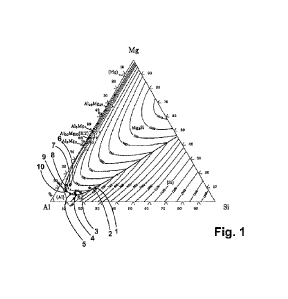
Fig. 1 and Fig. 2 show phase diagram illustrations of an Al-Mg-Si system in
which alloy
compositions of exemplary alloys are indicated;
Fig. 3 through Fig. 12 show optical microscope images of exemplary alloys from
Fig. 1 and Fig. 2;
Fig. 13 through Fig. 20 show yield stress diagrams of exemplary alloys from
Fig. 1 through Fig. 12;
Fig. 21 shows a phase diagram illustration of an Al-Cu-Mg system with an alloy
composition of
an exemplary alloy being drawn;
CA 03138658 2021- 11- 18
11
Fig. 22 shows optical microscope images of the exemplary alloy from Fig. 21;
Fig. 23 shows a yield stress diagram of the exemplary alloy from Fig. 21 and
Fig. 22;
Fig. 24 shows a phase diagram illustration of an Mg-Al-Li system in which
alloy compositions of
exemplary alloys are indicated;
Fig. 25 and Fig. 27 show optical microscope images of exemplary alloys from
Fig. 24;
Fig. 28 and Fig. 29 show yield stress diagrams of exemplary alloys from Fig.
24 through Fig. 27;
Fig. 30 shows a phase diagram illustration of an Mg-Cu-Zn system with an alloy
composition of
an exemplary alloy being drawn;
Fig. 31 and Fig. 32 show optical microscope images of the exemplary alloy from
Fig. 30;
Fig. 33 shows a yield stress diagram of the exemplary alloy from Fig. 30
through Fig. 32;
Fig. 34 shows electron microscope images of an exemplary alloy from an Al-Cu-
Mg-Zn system;
Fig. 35 shows a yield stress diagram of the exemplary alloy from Fig. 34;
Fig. 36 shows a phase amount diagram of an exemplary alloy from an Al-Mg-Si-Zn
system;
Fig. 37 shows a solid amount diagram of a Scheil-Gulliver calculation for the
exemplary alloy
from Fig. 36.
In the course of a development of the alloy according to the invention, series
of tests were
conducted with different alloy compositions of various alloy systems. In each
case, alloys were
thereby chosen with an alloy composition in the field of or around a
pseudoeutectic point of a
respectively related phase diagram, and a eutectic structure was formed by
cooling the alloy from
a liquid state to a solid state. The microstructure was then examined by means
of microscopy. In
addition, various dilatonnetric test series and compression tests were
conducted at room
temperature, approximately 20 C, as a standard, wherein yield curves which
depict a yield stress,
in M Pa, as a function of a degree of deformation, illustrated as the amount
of length change AL
relative to a starting length Lo, that is j'1', and correspondingly
dimensionless, were calculated as a
Lo
result.
Below, test results for exemplary alloys from the alloy systems Al-Mg-Si, Al-
Cu-Mg, Mg-Li-Al,
Mg-Cu-Zn, Al-Cu-Mg-Zn, and Al-Mg-Si-Zn are shown in a representative manner in
order to
illustrate the aforementioned concept on a broad basis.
CA 03138658 2021- 11- 18
12
Al-Mg-Si system:
Fig. 1 and Fig. 2 show illustrations of a ternary phase diagram of an Al-Mg-Si
system, wherein
Fig. 2 is a segment illustration from the phase diagram for the purpose of
showing the relevant
alloy composition range in detail. Ten exemplary alloys from the Al-Mg-Si
system were produced
and examined. The alloy compositions of the exemplary alloys from the Al-Mg-Si
system are
respectively indicated in percentage by weight and atomic percent as exemplary
alloy 1 through
exemplary alloy 10 in Table 1 and correspond to the reference numerals 1
through 10, which in
particular denote the respective alloy composition in the phase diagram from
Fig. 1 and Fig. 2.
Table 1: Ten exemplary alloys from the Al-Mg-Si alloy system.
Al
Mg Si
Exemplary alloy 1 wt% 68.10
9.00 22.90
at% 68.04
9.98 21.98
Exemplary alloy 2 wt% 71.10
9.10 19.80
at% 70.94
10.08 18.98
Exemplary alloy 3 wt% 78.10
6.30 15.60
at% 78.04
6.99 14.97
Exemplary alloy 4 wt% 81.50
5.00 13.50
at% 81.48
5.55 12.97
Exemplary alloy 5 wt% 82.00
6.00 12.00
at% 81.85
6.65 11.51
Exemplary alloy 6 wt% 82.00
10.50 7.50
at% 81.30
11.56 7.14
Exemplary alloy 7 wt% 82.70
10.00 7.30
at% 82.03
11.01 6.96
Exemplary alloy 8 wt% 84.90
10.90 4.20
at% 84.03
11.98 3.99
Exemplary alloy 9 wt% 85.80
9.60 4.60
at% 85.05
10.56 4.38
CA 03138658 2021- 11- 18
13
Exemplary alloy 10 wt% 86.50
7.20 6.30
at% 86.03
7.95 6.02
As can be seen in the phase diagram from Fig. 1 and Fig. 2, the exemplary
alloys 8 through 10
each have compositions which are arranged in a field around a pseudoeutectic
point pE, wherein
the exemplary alloys 8 and 9 are positioned very close to the pseudoeutectic
point and the
exemplary alloy 10 is positioned at a somewhat greater distance from the
pseudoeutectic point pE.
The alloy composition of the exemplary alloy 9 thereby virtually lies at the
pseudoeutectic
point pE. The pseudoeutectic point pE is illustrated in Fig. 2 by a drawn
reference line, wherein
the pseudoeutectic point pE is located at the intersection of the monovariant
line in the direction
of Al3M g2 and the reference line. In Fig. 2, it can also be seen that the
exemplary alloys 3 through
5 are arranged in a field around a eutectic point E of the phase diagram.
Furthermore, the
exemplary alloys 6 and 7 are provided as comparisons, the compositions of
which are located at a
large distance from the pseudoeutectic point pE, evident in Fig. 2, as well as
the exemplary alloys
1 and 2 which, though positioned in direct proximity to a liquidus boundary
line, are positioned at
a greater distance from both the pseudoeutectic point pE and also the eutectic
point E, evident in
Fig. 1.
In Fig. 3 through Fig. 12, optical microscope images of the exemplary alloys 1
through 10 are
shown in order to illustrate a respective microstructure. In Fig. 13 through
Fig. 20, yield stress
diagrams are illustrated as the results of dilatonnetric test series of the Al-
Mg-Si exemplary alloys
which were conducted at room temperature, approximately 20 C. Yield stress
curves are shown,
wherein a yield stress, in M Pa, is illustrated as a function of the degree of
deformation. Each of
the yield stress diagrams shows multiple yield stress curves from alloy
specimens with an alloy
composition corresponding to the alloy composition of one of the exemplary
alloys 1 through 10.
Each yield stress diagram thus represents an alloy composition of one of the
exemplary alloys 1
through 10.
As can be seen in Fig. 10 through Fig. 12, the microscope images of the
exemplary alloys 8 through
10, which have alloy compositions in proximity to or in a field around the
pseudoeutectic point pE,
CA 03138658 2021- 11- 18
14
show a dominant finely structured or fine-scale eutectic structure. By
comparison, microscope
images of the exemplary alloys 4 and 5 can be viewed in Fig. 6 and Fig. 7,
which alloys have an
alloy composition in proximity to the eutectic point E. These show a
pronounced degree of a
eutectic structure which comprises a course structure compared to the
microstructures of the
exemplary alloys 8 and 9. If one compares these with the microscope images of
the exemplary
alloys 1 and 2 shown in Fig. 3 and Fig. 4, the alloy compositions of which are
located at a large
distance from, but in the region of, a liquidus boundary line, it is
discernible that they exhibit an
even courser eutectic microstructure. In Fig. 8 and Fig. 9, microscope images
of the exemplary
alloys 6 and 7 are also shown which have alloy compositions in a distant
region of the
pseudoeutectic point pE or at a great distance therefrom. It can be seen that
a eutectic structure is
already present, but with a relatively course structure and being notably less
dominant and at a
lower amount. In addition, high amounts of residual solidifications are also
evident, identifiable
in Fig. 8 and Fig. 9 in the form of light channels.
Fig. 13 and Fig. 14 show yield stress diagrams of the exemplary alloys 8 and
9, which have alloy
compositions in proximity to or in a field around the pseudoeutectic point pE.
It can be seen that
both exemplary alloy 8 and also exemplary alloy 9 have high strength, in
particular compressive
strength, and pronounced defornnability with yield stresses between 300 MPa
and 400 MPa,
wherein exemplary alloy 8 in particular, illustrated in Fig. 13, exhibits
yield stresses of up to
400 MPa. By comparison, yield stress diagrams of the exemplary alloys 4 and
Scan be viewed in
Fig. 15 and Fig. 16, which alloys have alloy compositions in proximity to the
eutectic point E. The
exemplary alloys 4 and 5 also exhibit high strength and, at least conditional
on individual
specimens, a high deformability, wherein yield stresses lie below those of the
exemplary alloys 8
and 9 at approximately 300 MPa or, in relation to exemplary alloy 5,
illustrated in Fig. 16,
consistently below that. This result correlates with the finding that
exemplary alloys with an alloy
composition in the field of the pseudoeutectic point pE exhibit a particularly
high fine structuring
of the eutectic structure thereof, in particular also compared to the eutectic
structure of exemplary
alloys with alloy compositions in the field of a eutectic point E, which also
explains the higher
strength and pronounced elasticity of alloys in the field of the
pseudoeutectic point.
CA 03138658 2021- 11- 18
15
In Fig. 20, a yield stress diagram of the exemplary alloy 10 is shown, the
alloy composition of
which is arranged at a somewhat greater distance from the pseudoeutectic point
pE. Evident are
slightly lower yield stress values and, in particular, a higher variance
between the individual
measurement results. In Fig. 17 and Fig. 18, it is furthermore shown that, by
comparison,
exemplary alloy 1 and exemplary alloy 2 with alloy compositions in the region
of a liquidus
boundary line, but at a distance from both the alloy composition of the
pseudoeutectic point pE
and also the eutectic point E, have notably poorer strength and deformability
properties. In Fig. 19,
a yield stress diagram corresponding to the alloy composition of the exemplary
alloys 6 and 7 is
additionally shown, the alloy composition of which is positioned at a
relatively large distance from
that of the pseudoeutectic point pE in the phase diagram. The corresponding
yield stress curves
show clearly reduced yield stresses compared to yield stresses of an alloy
composition closer to
the pseudoeutectic point pE, such as that of those shown in Fig. 13 for the
exemplary alloy 8.
It is evident that an alloy composition in a field around a pseudoeutectic
point pE corresponds to
a finely structured eutectic microstructure and an accordingly high strength
and pronounced
deformabi I ity.
In a detailed view, it can be seen that, relative to the monovariant line or
liquidus boundary line in
the direction of Al3Mg2, the exemplary alloy 8 in the phase diagram from Fig.
2 lies above said
line in the Mg2Si region, which is why a solidification begins with an
undesirable formation of
Mg2Si in particular, or a primary solidification is formed with an
intermetallic Mg2Si phase, when
the alloy is cooled from the liquid phase. It has been shown that a primary
solidification formed
with an intermetallic phase has negative effects for an embodiment of both
high strength and also
deformability. To achieve particularly advantageous strength and
deformability, one therefore
generally strives to keep a primary solidification having or being made of
intermetallic phase as
minor as possible, or to prevent it. However, the primary solidification for
exemplary alloy 8 is
so slightly pronounced that it entails virtually no restraint on mechanical
properties. The
microscope images of the exemplary alloy Sin Fig. 10 show extensive regions
with a fine eutectic
structure, in this case formed with Al mixed crystal phase and Mg2Si.
Advantageously, a residual
solidification of Al mixed crystal phase is also only very slightly pronounced
or hardly present.
To keep from undermining the advantageous strength and deformability
properties attainable with
CA 03138658 2021- 11- 18
16
the eutectic structure, one strives to keep a residual solidification as small
as possible or prevent
it. In particular, the residual solidification is not bonded in a network-like
manner, or is embodied
in the form of units separated from one another, which likewise promotes an
advantageous
embodiment of high strength and pronounced deformability. The exemplary alloy
8 thus proves
to be well suited, both with regard to low residual solidification and also
low primary solidification,
to controlling strength properties and deformability on the basis of the fine
eutectic structure. This
can be optimized even further if the alloy composition is chosen such that the
primary solidification
is formed having or being made of a mixed crystal phase and not with an
internnetallic compound
or phase, that is, if the primary solidification is located in the Al mixed
crystal phase region in the
case of the exemplary alloy 8.
This view of the exemplary alloy 8 and also the accompanying explanations
apply analogously to
the exemplary alloy 9. The exemplary alloy 9 has an alloy composition lying
virtually at the
pseudoeutectic point pE. The exemplary alloy 9, as can be seen in Fig. 11,
also shows a fine
eutectic structure with little residual solidification and little primary
solidification. The somewhat
lower strength in comparison with the exemplary alloy 8 is explained by the
lower dissolved
amount of Mg in the Al mixed crystal phase. A strength can be advantageously
achieved by
varying an amount of dissolved elements in the mixed crystal phase, with the
primary solidification
preferably lying, however, in the mixed crystal region and not in the region
of an intermetallic
phase, as stated above.
By comparison, the exemplary alloy 10, as can be seen in Fig. 12, also shows a
fine eutectic
structure, but with a greater amount of residual solidification, in the form
of Al mixed crystal phase
and Si, which residual solidification is also shaped in a network-like manner.
Due to the low Mg
content, most of the Mg is bonded in the form of Mg2Si so that a mixed crystal
hardening of the
Al mixed crystal phase is very slightly pronounced. This corresponds to lower
yield stresses in
the yield stress diagram from Fig. 20.
In a further detailed view of the exemplary alloys arranged at a distance from
the pseudoeutectic
point pE in relation to an alloy composition, it can be seen that the
exemplary alloys 4 and 5, which
lie in the field of the eutectic point E, illustrated in Fig. 6 and Fig. 7,
comprise a low amount of
CA 03138658 2021- 11- 18
17
primary solidification, around which a relatively course eutectic structure,
formed with two phases,
is arranged. A remaining predominant amount of eutectic structure is embodied
as a ternary
eutectic, formed with mixed crystal phase, Al2Si and Si. The mechanical
properties, in particular
strength and deformability, are negatively influenced by the course binary
eutectic structure or
phase in particular. A fine eutectic ternary structure is locally present to
some extent, which
structure transitions into markedly coarsened structures in some locations.
The differences
between the microstructures of exemplary alloys with alloy compositions at, or
in the field of, the
pseudoeutectic point pE compared to those at, or in the field of, the eutectic
point E correlate with
the finding of accordingly improved strength and deformability properties of
alloy compositions
at, or in the field around, the pseudoeutectic point pE.
It can furthermore be seen that the exemplary alloys 6 and 7 comprise course,
polygon-shaped
primary solidifications with the related microscope images shown in Fig. 8 and
Fig. 9. This is
explained by the positioning of the related alloy compositions in the Mg2Si
region of the phase
diagram, as a result of which a pronounced Mg2Si primary solidification forms.
A course eutectic
structure is identifiable therebetween, as well as a high amount of residual
solidification, which is
evident from the light regions or channels in Fig. 8 and Fig. 9. Due to this
structural morphology,
the exemplary alloys 6 and 7 exhibit markedly reduced strengths and yield
stresses, which are
associated in particular with crack initiation and brittle fracture.
In Fig. 2, a particularly advantageous region for the embodiment of an Al-Mg-
Si alloy is drawn as
a gray, planar region. This essentially designates or corresponds to an
aforementioned alloy
composition of the exemplary alloys 8 and 9, but with a variation of the alloy
composition such
that a mixed crystal phase is embodied as primary solidification and, in
particular, no intermetallic
phase is embodied. This enables an embodiment of particularly high strengths
with pronounced
deformability. A particularly advantageous implementation range for an Al-Mg-
Si alloy of this
type is thus ensured if the Al-Mg-Si alloy is arranged in a field around the
pseudoeutectic point in
the Al-Mg-Si phase diagram, wherein the alloy composition in the phase diagram
is arranged
starting from the aforementioned pseudoeutectic point of the phase diagram in
Fig. 2 on a side of
the corresponding nnonovariant line facing an increasing Al amount.
CA 03138658 2021- 11- 18
18
Al-Cu-Mg system:
Fig. 21 shows an illustration of a ternary phase diagram of an Al-Cu-Mg
system. An exemplary
alloy from the Al-Cu-Mg system was produced and examined. The related alloy
composition is
indicated in percentage by weight and atomic percent as exemplary alloy 13 in
Table 2 and
corresponds to reference numeral 13, which in particular denotes the alloy
composition in the
phase diagram from Fig. 21.
Table 2: Exemplary alloy from the Al-Cu-Mg alloy system.
Al
Cu Mg
Exemplary alloy 13 wt% 66.00
24.00 10.00
at% 75.61
11.67 12.72
As can be seen in the phase diagram from Fig. 21, the exemplary alloy 13 has
an alloy composition
which is arranged in a field around a pseudoeutectic point pE. A related
microstructure is
illustrated in Fig. 22 with the aid of optical microscope images. Evident is a
very fine-scale
eutectic microstructure and a low amount of primary solidification formed with
mixed crystal
phase. In Fig. 23, a yield stress diagram is shown as the result of di
latometric test series of the Al-
Cu-Mg exemplary alloy 13, wherein a yield stress, in M Pa, is once again
illustrated as a function
of the degree of deformation. It is evident that very high strengths and yield
stresses are achieved.
The elongation at break also lies in the technologically relevant range for
this alloy system. The
strength and defornnability correspond to the fine eutectic microstructure
and, in particular, to the
low amount of primary solidification.
Mg-Al-Li system:
Fig. 24 shows an illustration of a ternary phase diagram of an Mg-Al-Li
system. Three exemplary
alloys from the Mg-Al-Li system were produced and examined. The alloy
compositions of the
exemplary alloys from the Mg-Al-Li system are respectively indicated in
percentage by weight
and atomic percent as exemplary alloy 14, 15, and 16 in Table 3 and correspond
to the reference
CA 03138658 2021- 11- 18
19
numerals 14, 15, and 16, which in particular denote the respective alloy
composition in the phase
diagram from Fig. 24.
Table 3: Three exemplary alloys from the Mg-Al-Li alloy system.
Mg
Al Li
Exemplary alloy 14 wt% 55.00
29.00 16.00
at% 40.10
19.05 40.85
Exemplary alloy 15 wt% 56.00
24.00 20.00
at% 37.90
14.60 47.40
Exemplary alloy 16 wt% 65.00
15.00 20.00
at% 43.80
9.10 47.10
As can be seen in the phase diagram from Fig. 24, the exemplary alloys 14
through 16 respectively
have an alloy composition which is arranged in a field around a pseudoeutectic
point pE. The
pseudoeutectic point pE is illustrated in Fig. 24 by a drawn reference line,
wherein the
pseudoeutectic point pE is located at the intersection of the monovariant
line, or I iquidus boundary
line, and the reference line. With additions of CaY, in particular
approximately 1 wt% Ca and
approximately 0.5 wt% Y, oxidation properties of the exemplary alloys from the
Mg-Al-Li system
can feasibly be stabilized without negatively influencing how pronounced the
structure is.
In the phase diagram, the exemplary alloys 14 and 15 lie at a somewhat closer
distance in a vicinity
of the pseudoeutectic point and the exemplary alloy 16 somewhat farther away,
wherein the alloy
composition of the exemplary alloy 14 is positioned more or less at the
pseudoeutectic point pE.
According to currently available data, the exemplary alloys 14 through 16 are
present in a mixed
crystal region, in particular such that they form a body-centered cubic
lattice, bcc.
In Fig. 25 through Fig. 27, microstructures are respectively rendered visible
with the aid of
microscope images. The structural morphology from Fig. 25 and Fig. 26
indicates an embodiment
of an extremely fine-scale structure which can no longer be resolved in the
light microscope used.
The grain boundaries which can thereby be recognized are attributable to
oxidic impurities. The
microstructure of exemplary alloy 16 was examined by means of scanning
electron microscopy,
illustrated in Fig. 27. Evident in Fig. 27 are, on the one hand, light grain
boundary phases (in
CA 03138658 2021- 11- 18
20
whitish-gray) that were identified as Al-Ca and, on the other hand, pronounced
fine crystalline
structures or morphologies in a region surrounded by grain boundary phases, in
particular in a
center section of said region, or in the interior of the mixed crystal phase,
clearly visible in
particular in the right-hand image from Fig. 27. In the phase diagram from
Fig. 24, the alloy
composition of the exemplary alloy 16 appears to lie at a relatively far
distance from the
monovariant line and the pseudoeutectic point pE. In this case, however, it
should be noted that,
according to established technical knowledge, the slope in the region of the
body-centered cubic
lattice, bcc, - in which the exemplary alloy 16 is also arranged - in the
phase diagram is very flat,
and that the three elements Mg, Al, and Li also exhibit a high solubility in
one another. It its thus
possible to explain why such an expansive field around the pseudoeutectic
point results, in which
field an advantageous fine-scale eutectic microstructure can be embodied in a
high amount.
Fig. 28 and Fig. 29 show yield stress diagrams of the exemplary alloys 15 and
16 as the results of
dilatometric test series, wherein a yield stress, in M Pa, is once again
illustrated as a function of the
degree of deformation, with Fig. 28 showing yield stress curves relating to
the exemplary alloy 15
and Fig. 29 showing yield stress curves relating to the exemplary alloy 16. It
is evident that both
exemplary alloys have high strengths and yield stresses, as well as pronounced
deformabilities,
corresponding to the fine eutectic microstructures identified. In Fig. 29,
which relates to the
exemplary alloy 16, a possibility of a further property optimization by means
of heat treatment is
also illustrated.
Fig. 29 shows yield curves of alloy specimens immediately after a production
of the exemplary
alloy 16 (as cast), depicted in Fig. 29 as solid lines, denoted by reference
numeral 16-1, and
additionally yield curves of exemplary alloy specimens after a conducted heat
treatment (aged) of
the exemplary alloy 16, depicted in Fig. 29 as dashed lines, denoted by
reference numeral 16-2.
For this purpose, specimens of the exemplary alloy 16 were subjected to a heat
treatment at 330 C
for 3 hours, and yield curves were then calculated by means of compression
tests. A clear influence
of the heat treatment on strength, in particular compressive strength, and
deformability is evident,
as a result of which there is the potential to set compressive strength and
deformability in an
optimized manner using heat treatment, in particular for an eventual intended
application.
CA 03138658 2021- 11- 18
21
As previously explained above in the document, it has proven beneficial to the
realization of an
alloy with high application suitability if the alloy is a magnesium-based
alloy comprising, in
particular being made of, (in at%)
15% to 70.0% lithium,
greater than 0.0%, in particular greater than 0.01%, preferably greater than
0.05%, aluminum,
magnesium and production-related impurities as a remainder,
wherein a ratio of aluminum to magnesium (in at%) is 1:6 to 4:6. The exemplary
alloy 16 can be
viewed as a representative example of this alloy definition, as is shown
within the scope of
European Patent Application Number 19184999.1 and also within the scope of
International
Application Number PCT/EP2020/058280, both of which were filed in the European
Patent
Office. Here, reference is once again made in particular to Fig. 1 from each
of these applications.
In Fig. 24, a corresponding aluminum-to-magnesium ratio (in at%) of 1:6 is
drawn as a dashed
line. The aforementioned aluminum-to-magnesium ratio range (in at% or mol%) of
1:6 to 4:6 is
thereby located to the left of this line in the phase diagram from Fig. 24
and, in particular,
constitutes a specific embodiment in the field around the pseudoeutectic point
pE.
A particularly advantageous implementation range for an Mg-Li-Al alloy that is
usable as an
application alloy, in particular for a structural part, is ensured if the Mg-
Li-Al alloy is arranged in
the Mg-Li-Al phase diagram in a region between the line indicating an aluminum-
to-magnesium
ratio (in at%) of 1:6 and the monovariant line or liquidus boundary line, in
particular with an
aforementioned Li content range. A range of this type is denoted in the phase
diagram from Fig. 24
as a gray, planar region.
It becomes apparent, as was already the case previously within the scope of
the exemplary alloys
from the Al-Si-Mg system, that an alloy composition is preferably chosen such
that the alloy
composition lies in the field of the pseudoeutectic point pE and, moreover,
preferably comprises a
primary solidification having or being made of mixed crystal phase; that is,
that the corresponding
alloy composition is positioned in a mixed crystal region in the phase
diagram.
Mg-Cu-Zn:
CA 03138658 2021- 11- 18
22
Fig. 30 shows an illustration of a ternary phase diagram of an Mg-Cu-Zn
system. An exemplary
alloy from the Mg-Cu-Zn system was produced and examined. The related alloy
composition is
indicated in percentage by weight and atomic percent as exemplary alloy 17 in
Table 4 and
corresponds to reference numeral 17, which in particular denotes the alloy
composition in the
phase diagram from Fig. 30.
Table 4: Exemplary alloy from the Mg-Cu-Zn alloy system.
Al
Cu Zn
Exemplary alloy 17 wt% 58.00
16.5 25.5
As can be seen in the phase diagram from Fig. 30, the exemplary alloy 17 has
an alloy composition
which is arranged in a field around a pseudoeutectic point pE. A related
microstructure is
illustrated in Fig. 31 and Fig. 32 with the aid of optical microscope images.
Evident is a very fine-
scale eutectic microstructure that is at a limit of resolution of a light
microscope. Here, a relatively
large amount of primary solidification can be seen. It is therefore
advantageous for a high strength
and deformability if an alloy composition is selected even closer to the
pseudoeutectic point pE or
closer to the nnonovariant line or liquidus boundary line.
Fig. 33 shows a yield stress diagram as the results of dilatometric test
series of the exemplary
alloy 17, wherein a yield stress, in MPa, is once again illustrated as a
function of the degree of
deformation. It is evident that high strengths and yield stresses are achieved
which, based on the
pronounced amount of primary solidification apparent in the microscope images,
can be further
improved, however, by choosing an alloy composition even closer to the
pseudoeutectic point pE.
In Fig. 33, yield curves of the exemplary alloy 17 immediately following a
production of the
exemplary alloy 17 (as cast) are thereby shown, denoted by reference numeral
17-1, and also yield
curves of the exemplary alloy 17 after a conducted heat treatment, denoted by
reference
numeral 17-2. For this purpose, specimens of the exemplary alloy 17 were
subjected to a heat
treatment at 350 C for 4 hours, and yield curves were then calculated by
means of compression
tests. A clear influence of the heat treatment on strength and deformability
is evident, as a result
of which there is the potential to further optimize strength and deformability
by means of heat
treatment.
CA 03138658 2021- 11- 18
23
Examinations of quaternary alloy systems and quaternary eutectics were then
also carried out. The
alloy systems Al-Cu-Mg-Zn and Al-Mg-Si-Zn in particular were considered for
this purpose.
Al-Cu-Mg-Zn:
In regard to the alloy system Al-Cu-Mg-Zn, an exemplary alloy that lies in the
field of a
pseudoeutectic point pE was produced and examined. The alloy composition is
indicated in
percentage by weight and atomic percent as exemplary alloy 18 in Table 5 and
corresponds to
reference numeral 18.
Table 5: Exemplary alloy from the Al-Cu-Mg-Zn alloy system.
Al
Cu Mg Zn
Exemplary alloy 18 wt% 6.20
75.40 5.40 13.00
at% 12.51
65.00 12.09 10.82
In order to examine the eutectic microstructure, electron microscope images of
the exemplary
alloy 18 were recorded, shown in Fig. 34. Evident is a finely structured
eutectic structure, in
particular with structural dimensions in the nanometer range, clearly visible
in the right-hand
image from Fig. 35 as an expansive grainy region in the center of the picture.
This is a binary eutectic structure in a system with four components or
elements and thus an
increase in the thermodynamic degree of freedom f, explained at the outset,
from 1 to 3 (quaternary
eutectic).
In Fig. 34, substructures are identifiable in the primary regions (in gray),
wherein these are artifacts
of an isostoichionnetric structural transformation (bcc to fcc) in the solid
state. In terms of a direct
influence on strength and defornnability, they are insignificant. Also visible
is a relatively large
amount of primary solidification in the form of a mixed crystal phase (in
light gray to whitish), as
well as an internnetallic secondary phase (in black), in particular in the
form of a Laves phase.
CA 03138658 2021- 11- 18
24
Fig. 35 shows a yield stress diagram as the result of dilatometric test series
with the exemplary
alloy 18. Depicted are yield curves prior to a completed heat treatment,
denoted by reference
numeral 18-1, and yield curves following a completed heat treatment, denoted
by reference
numeral 18-2, wherein a yield stress, in M Pa, is once again illustrated as a
function of the degree
of deformation. It is evident that the exemplary alloy 18 exhibits a very high
strength with a
simultaneously present elongation at break, wherein a deformability can be
varied by means of
heat treatment.
The pronounced primary solidification present as well as the secondary phase
are to be regarded
as brittleness-increasing factors, which is why it would be advantageous to
further reduce these
amounts in order to further improve strength and deformability, for example by
reducing the
distance of the alloy composition from or bringing it even closer to the
pseudoeutectic point pE in
the phase diagram.
Al-Mg-Si-Zn:
In regard to the alloy system Al-Mg-Si-Zn, an exemplary alloy that lies in the
field of a
pseudoeutectic point pE was examined by means of simulation. The alloy
composition is indicated
as exemplary alloy 19 in Table 6 and corresponds to reference numeral 19.
Table 6: Exemplary alloy from the Al-Mg-Si-Zn alloy system.
Al
Mg Si Zn
Exemplary alloy 19 wt% 83.3
9.2 4.5 3.0
As a result of the simulation, phase amounts are illustrated in Fig. 36 as a
function of the
temperature of the exemplary alloy 19. Evident is a direct transition from the
solid to the liquid
phase, corresponding to an embodiment of a eutectic structure. In Fig. 35,
corresponding thereto,
an illustration of the solid amount as a function of the temperature is shown,
determined by means
of a Scheil-Gulliver solidification calculation. The equilibrium and Scheil-
Gulliver solidification
CA 03138658 2021- 11- 18
25
curves shown depict an alloy system which exhibits a binary eutectic
solidification with four
components or elements. Accordingly, there is therefore once again an increase
in the
thermodynamic degree of freedom from 1 to 3. In Fig. 37, the Scheil-Gulliver
calculation shows
a very small amount of primary solidification in the form of a mixed crystal
phase at an amount of
less than 3 mol% or at% and in addition a virtually non-existent residual
solidification.
It is thus analogously apparent that, in addition to a positioning of the
alloy composition in the
field of the pseudoeutectic point pE, an amount of primary solidification
and/or residual
solidification can advantageously also be minimized in order to further
increase or improve
strength properties and deformability properties.
An alloy according to the invention with more than three components having a
eutectic structure
created by a cooling from the liquid state to the solid state can thus
advantageously be embodied
with a finely structured eutectic structure, in particular with a fine
structure in the nanometer range,
which constitutes a dominant or principal phase amount or structure amount in
the alloy if an alloy
composition of the alloy is arranged in the field of or around a
pseudoeutectic point in the phase
diagram. The alloy can thus be embodied with advantageously high strength and
pronounced
deformability. This holds especially true if a primary solidification and/or
residual solidification
is embodied to be very small. Specifically, it is beneficial thereto if the
primary solidification is
formed having or being made of a mixed crystal phase, in particular not having
or being made of
an intermetallic phase, or if the alloy composition is chosen in a
corresponding region in the phase
diagram. An alloy formed in this manner thus offers the potential to realize,
preferably depending
on a specific purpose, robust and resilient components, especially structural
components, in
particular for an intended application in the automotive industry, aircraft
industry, and/or space
industry.
CA 03138658 2021- 11- 18