Note: Descriptions are shown in the official language in which they were submitted.
WO 2020/264260
PCT/US2020/039770
LABORATORY AUTOMATION SYSTEM IMPLEMENTING EFFICIENT PATH FOR
MATERIAL AND LABWARE TRANSFERS
CROSS-REFERENCE TO RELATED APPLICATIONS
100011 This application claims the benefit of priority to
US Application No. 62/867,447,
filed June 27, 2019, and incorporated by reference herein in its entirety.
100021 This application is related to: International
Application No. PCT/U52017/029725
(U.S. Patent Pub. No. US 2017/0316353), filed on April 26, 2017, which claims
the benefit
of priority to U.S. Application No. 15/140,296, filed on April 27, 2016; U.S.
Patent No.
9,988,624; and International Application No. PCT/US2018/057583 (Pub. No.
WO/2019/084315), which claims priority to U.S. Application No. 62/577,615,
filed October
26, 2017, all which are hereby incorporated by reference herein in their
entirety.
BACKGROUND
Field of the Disclosure
100031 The present disclosure is generally directed to high-
throughput genomic engineering
of organisms, and, more particularly, to reducing the travel cost (e.g., path
distance) of
multiple liquid transfers between source and destination points on source and
destination
arrays, respectively.
Description of Related Art
100041 The subject matter discussed in the background section should not be
assumed to be prior
art merely as a result of its mention in the background section. Similarly, a
problem
mentioned in the background section or associated with the subject matter of
the background
section should not be assumed to have been previously recognized in the prior
art. The
subject matter in the background section merely represents different
approaches, which in
and of themselves may also correspond to implementations of the claimed
technology.
100051 Microbe engineering enables the generation of novel chemicals, advanced
materials, and
pharmaceuticals. A strain design company, on behalf of itself or third
parties, may modify a
previously described DNA segment to enhance the metabolic production of a
microbial host
by improving output properties such as yield, productivity, growth rate, and
titer.
100061 One approach to optimizing the performance of an incompletely
understood system, such
as a living cell, is to test many different genetic modifications and
empirically determine
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
which perform best. To explore high throughput screening in this large
combinatorial space,
laboratory systems may employ robotic liquid handlers to transfer nucleotides,
enzymes (e.g.,
DNA polymerases), and other reagents into wells of a microplate (otherwise
referred to
herein as a "plate") that contain microorganisms, such as microbes, having
genomes to be
modified. The engineered organisms may then be fermented to produce a product
of interest.
100071 One type of liquid handler is an acoustic liquid handler, such as those
in the ECHO
series provided by LABCYTE, INC. Acoustic drop ejection is a technology which
uses
highly focused sound energy to cause an ultra-small (e.g., 2.5 nL, 25nL)
droplet to dislocate
from a source well and be deposited into a destination well. To transfer a
higher volume of
liquid (an example of an "object," as used herein), multiple droplets may be
transferred from
the source well to the destination well. The ECHO 550, for example, can
transfer liquids
from either 384 or 1536 well source plates to 96, 384, 1536 and 3456 well
destination plates.
The handler can also transfer liquids to other arrays, such as slides.
100081 A commercially available path determination software tool for acoustic
liquid handling
transfers is the LABCYTE ECHO Plate Reformat application. This software can
substantially reduce the total runtime for an example set of transfers by
modifying the order
in which the transfers occur.
100091 Others have considered the issue of developing an efficient path for
dispensing liquids_
For example, A. Peddi, et al., Efficient and Effective Path for Automated
Dispensing of Bio-
Precipitant Solutions, Proceedings of the 2005 IEEE International Conference
on
Automation Science and Engineering, August 1-2, 2005 ('Peddi"), explores
reducing the
path length for a dispensing tip of a robotic system that delivers solutions
into wells. The
paper concludes that a technique for solving the traveling salesman problem is
the best
heuristic.
100101 Compared to conventional approaches, it is desired to reduce the cost
(e.g., time) for a set
of array-to-array transfers in a liquid handling system, such as acoustic
liquid handling
system.
SUMMARY OF THE DISCLOSURE
100111 Liquid handlers form a critical part of an automated robotic system
used in the high
throughput fabrication and analysis of synthetic microorganisms. Liquid
handlers may be
2
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
used to transfer volumes of liquid from wells in a source plate to wells in a
destination plate_
In particular, acoustic liquid handlers employ an inverted destination plate
positioned over a
source plate, and then use an acoustic transducer to induce the formation of
droplet of liquid
that "jumps" up from the source plate to the destination plate.
100121 In a typical application, a liquid handler is given a list of transfers
that outlines the source
and destination array points (e.g., plate wells) for each transfer. Based on
the layout of this
transfer map (pick list), the order in which transfers occur can greatly
affect the total amount
of time it takes to execute all the transfers. Embodiments of the disclosure
determine the
order for series of liquid transfers to substantially reduce the total
transfer cost (e.g., time) as
compared to conventional techniques, or even minimize the total transfer cost
if the
algorithm is given enough computation time.
100131 Embodiments of the disclosure provide cost functions that are used to
determine a low-
cost sequential ordering of transfers. To determine such an ordering,
embodiments of the
disclosure provide the costs as inputs to a commercially available path
determination
software tool, such as a Traveling Salesman Problem ("TSP") solver software
tool.
According to embodiments of the disclosure, the cost functions are designed
for acoustic
liquid handlers in which there are two moving components, such as the acoustic
transducer
and the destination plate. In other acoustic liquid handlers, such as the EDC
BIOSYSTEMSTm ATS GEN5, the source and destination plates move while the
transducer
remains fixed.
100141 According to embodiments of the disclosure, a liquid transfer occurs
when source and
destination points are aligned at a liquid transfer position (also referred to
as a "node"
herein). According to embodiments of the disclosure, for an acoustic liquid
transfer, the
acoustic transducer is also aligned with the source well position. According
to embodiments
of the disclosure, the first and second components (e.g., the transducer and
the destination
plate) move along separate paths to the new liquid transfer position.
100151 A "tour" refers to a sequential ordering of the liquid transfers. Each
transition from one
liquid transfer position to another liquid transfer position incurs a cost
(e.g., distance).
According to embodiments of the disclosure, separate costs are incurred for
the separate first
and second component paths. According to embodiments of the disclosure, the
cost for a
transition from one liquid transfer position to the next comprises the maximum
of the travel
3
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
costs for the first and second components. According to embodiments of the
disclosure, the
cost for a tour is the sum of the costs for each transition between liquid
transfer positions
along the tour. Embodiments of the disclosure select the tour with the lowest
cost.
100161 More generally, embodiments of the disclosure compute cost functions
for a laboratory
automation system with acoustic transducers or other actuators in which two
components
move while a third component remains fixed, where the two movable components
may, for
example, be any two of the following three components: a source array, a
destination array,
or an actuator. Each array (e.g., plate, tube rack) may be an arrangement of
points (e.g.,
volumes or areas in the x-y plane) from or to which liquid volumes or other
"objects" are
transferred, such as a plate, tube rack, or other labware having wells, tubes,
or other points,
respectively.
100171 Embodiments of the disclosure provide computer-implemented methods,
systems, and
non-transitory computer-readable media for determining a sequential ordering
of a plurality
of predefined transfers for transferring an object (e.g., liquid volume, tube,
powder) from a
plurality of source points (e.g., wells, tubes) of a source array (e.g.,
plate, tube rack) to a
plurality of destination points of a destination array, wherein a laboratory
automation system
(e.g., liquid handler, tube sorter, powder handler) includes at least a first
component and a
second component of the following three components: a source array, a
destination array, or
an actuator (e.g., acoustic, pneumatic, vibratory).
100181 As described herein, (a) for each unique transition to a next transfer
of the plurality of
predefined transfers, wherein each transfer corresponds to a transfer
position, Embodiments
of the disclosure (i) determine a first component travel cost of the first
component between a
current transfer position and a next transfer position corresponding to the
next transfer,
wherein the current transfer position corresponds to positioning of a current
source point with
respect to a current destination point for a current transfer of the plurality
of predefined
transfers, the next transfer position corresponds to positioning of a next
source point with
respect to a next destination point for the next transfer; (ii) determine a
second component
travel cost of the second component between the current transfer position and
the next
transfer position; and (iii) determine a transition travel cost, of a
plurality of transition travel
costs, for the transition as the maximum of the first component travel cost
and the second
component travel cost. Embodiments of the disclosure (b) determine a resolved
sequential
4
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
ordering of the plurality of predefined transfers based at least in part upon
the plurality of
transition travel costs.
100191 According to embodiments of the disclosure, each tour of a plurality of
tours comprises a
unique sequential ordering of the plurality of predefined transfers, the
method further
comprising determining the cost of each tour based at least in part upon an
aggregate of the
transition travel costs for the tour. According to embodiments of the
disclosure, the plurality
of tours corresponds to all combinations of sequential ordering of the
plurality of predefined
transfers. According to embodiments of the disclosure, the cost of each tour
comprises a sum
of the transition travel costs for the tour. According to embodiments of the
disclosure,
determining a resolved sequential ordering is based at least in part upon the
tour that has the
lowest cost. According to embodiments of the disclosure, determining the
resolved
sequential ordering of the plurality of predefined transfers comprises solving
the traveling
salesman problem.
100201 According to embodiments of the disclosure, the laboratory automation
system comprises
a liquid handler, the object is a liquid, and the first and second components
travel according
to the resolved sequential ordering. According to embodiments of the
disclosure, the actuator
is an acoustic transducer.
100211 According to embodiments of the disclosure, the first and second
components are
movable and a third component of the three components remains fixed. According
to
embodiments of the disclosure, a third component of the three components is
also positioned
at the next transfer position for the next transfer. According to embodiments
of the
disclosure, positioning comprises alignment. According to embodiments of the
disclosure,
each transition travel cost is a distance, or a function of distance and
travel time.
100221 According to embodiments of the disclosure, the destination array
resides in a plane
parallel to a plane in which the source array resides. According to
embodiments of the
disclosure, the source and destination arrays are plates and the source and
destination points
are wells. According to embodiments of the disclosure, the first and second
components are
the actuator and the destination plate, respectively.
100231 Embodiments of the disclosure further comprise moving the first and
second components
according to the resolved sequential ordering. Embodiments of the disclosure
further
comprise assembling at least one nucleotide sequence based at least in part
upon moving the
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
first and second components according to the resolved sequential ordering.
Embodiments of
the disclosure further comprise manufacturing a product of interest based at
least in part upon
at least one nucleotide sequence assembled by moving the first and second
components
according to the resolved sequential ordering.
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] Figs. 1A and 1B each illustrate a source plate and a destination plate
in a liquid handler,
according to embodiments of the disclosure.
[0025] Fig. 2 illustrates a distributed system including a liquid handler,
according to
embodiments of the disclosure.
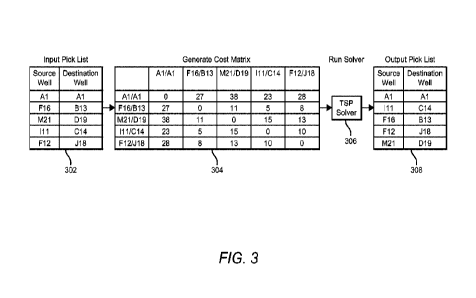
[0026] Fig. 3 illustrates a process for determining an improved, low-cost
sequential liquid
transfer ordering according to embodiments of the disclosure.
[0027] Figs. 4A and 4B depict steps for DNA assembly, transformation, and
strain screening,
according to embodiments of the disclosure.
[0028] Fig. 5 illustrates a cloud computing environment according to
embodiments of the
disclosure.
[0029] Fig. 6 illustrates an example of a computer system that may be used to
execute program
code stored in a non-transitory computer readable medium (e.g., memory) in
accordance with
embodiments of the disclosure.
DETAILED DESCRIPTION
[0030] The present description is made with reference to the accompanying
drawings, in which
various example embodiments are shown. However, many different example
embodiments
may be used, and thus the description should not be construed as limited to
the example
embodiments set forth herein. Rather, these example embodiments are provided
so that this
disclosure will be thorough and complete. Various modifications to the
exemplary
embodiments will be readily apparent to those skilled in the art, and the
generic principles
defined herein may be applied to other embodiments and applications without
departing from
the spirit and scope of the disclosure. Thus, this disclosure is not intended
to be limited to
the embodiments shown, but is to be accorded the widest scope consistent with
the principles
and features disclosed herein.
6
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
10031] As used herein the terms "organism" "microorganism" or "microbe" should
be taken
broadly. These terms are used interchangeably and include, but are not limited
to, the two
prokaryotic domains, Bacteria and Archaea, as well as certain eukaryotic fungi
and protists,
as well as yeasts.
100321 Embodiments of the disclosure provide systems, methods, and computer-
readable media
storing instructions for determining a sequential ordering of liquid transfers
(e.g., acoustic
liquid transfers) that is more efficient than the ordering methodology
employed in
conventional high throughput genomic engineering systems. Embodiments of the
disclosure
provide cost functions from which an efficient sequential ordering may be
computed.
100331 Figs. 1A and 1B each illustrate a source plate 102 and a destination
plate 104 in a liquid
handler, such as the ECHO 550, according to embodiments of the disclosure.
Referring to
Fig. 1A, respective source and destination wells are aligned at a liquid
transfer position for a
liquid transfer from a source well 106 to a destination well 108. According to
embodiments
of the disclosure, an acoustic transducer 110 is aligned below the source well
position (at the
liquid transfer position for this transfer) to cause liquid droplets to jump
up from the source
well 106 to the destination well 108.
100341 Fig. 2 illustrates a distributed system 200 of embodiments of the
disclosure including a
liquid handler 202 of embodiments of the disclosure. The liquid handler 202
includes a
controller 109, arrays 204 (e.g., plates 102, 104), and at least one actuator
206 (e_g., motors)
for moving the arrays 204 in at least the x-y plane and for achieving other
liquid handler
operations, such as liquid transfers from source array points (e.g., source
wells) to destination
array points (e.g., destination wells).
100351 A user interface 208 includes a client-side interface such as a text
editor or a graphical
user interface (GUI). The user interface 208 may reside at a client-side
computing device
210, such as a laptop or desktop computer. The client-side computing device
210 is coupled
to one or more servers 212 through a network 214, such as the Internet. The
client device 210
may be integrated into, or otherwise reside locally with the liquid handler
202, or reside
remotely from the liquid handler 202. In the example shown in the figure, the
client device
210 acts as the interface between the liquid handler 202 and server(s) 212.
100361 The server(s) 212 are coupled locally or remotely to one or more
databases 216, which
may include one or more corpora of libraries including data such as pick
lists, software
7
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
applications such as tour optimization applications, and data concerning the
assembly of
nucleotide sequences. The database(s) 216 may include public databases, as
well as custom
databases generated by the user or others.
100371 In embodiments, the server(s) 212 include at least one processor and at
least one memory
storing instructions that, when executed by the processor(s), perform
operations disclosed
herein, including computing costs or determining a sequential ordering of
liquid transfers (or
both), thereby acting as a tour determination engine 107, according to
embodiments of the
disclosure.
100381 According to embodiments of the disclosure, the software and associated
hardware for all
the system 200 elements (e.g., tour determination engine 107, controller 109,
liquid handler
202) may reside locally with the client 210 instead of at the server(s) 212,
or be distributed
among client 210, server(s) 212, and liquid handler 202 in any combination. In
the example
shown in the figure, firmware in the controller 109 may receive an output pick
list from the
engine 107 to cause actuators 206 to move one or more arrays 204. (In
embodiments, the
controller 109 may also cause a transducer or other actuator to execute a
transfer between
source and destination arrays 204.) In embodiments, all or parts of these
computing elements
may run as a cloud-based service, depicted further in Fig. 5. The controller
109 may reside,
for example, at the server(s) 212 or at the liquid handler 202. The
database(s) 216 may be
local or remote with respect to the client 210 or distributed locally or
remotely.
100391 After the current transfer, the controller 109 uses the output pick
list provided by the
engine 107 to determine the next transfer. The controller 109 causes the
actuators 206 to
move the transducer 110 and the destination plate 104 to enable the next
transfer at the next
liquid transfer position, according to embodiments of the disclosure. For this
transfer, the
destination well 158 is moved into alignment over source well 156 at a next
liquid transfer
position, and transducer 119 is moved into alignment under source well 156, as
shown in Fig.
1B.
100401 Fig. 3 illustrates a process for determining an improved, low-cost
sequential liquid
transfer ordering according to embodiments of the disclosure. A pick list 302
specifies each
transfer to be made from a source point (e.g., source well) to a destination
point (e.g.,
destination well). This simplified pick list shows five transfers. In a
laboratory, a typical pick
list may specify a total of 300-1500 transfers between, for example, two 384-
well plates.
8
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
10041] Each entry in the pick list 302 specifies an identification of a source
point or a destination
point. In the example of this pick list, each point is denoted by its x-y
coordinates, where
letters specify rows and numbers specify columns (e.g., Al is the origin in
standard plate
terminology). Those skilled in the art will recognize that other
identification indicia (e.g.,
standard x-y numerical coordinates) may be used to specify array point
position. According
to embodiments of the disclosure, the coordinates are selected so that both
source and
destination arrays reside in the same frame of reference. For example, the
coordinates may be
chosen so that from a perspective looking down on the arrays, the origin in
the x-y plane is
located at the lower left corner of the source array (which is facing up).
100421 In the same example, because the destination array is mirrored about
the y axis, the origin
of the destination array in this frame of reference would reside at the lower
right corner of the
destination array when it is facing up. The lower left corner corresponds to
(23, 0) in a 16-
row x 24-column, 384-well plate, for example. If the destination array were
instead mirrored
about the x axis, the origin of the destination array in the exemplary frame
of reference
would reside at the upper left corner of the destination array when it is
facing up.
100431 According to embodiments of the disclosure in which an acoustic
transducer and a
destination plate are the first and second movable components, to move from
one source well
to the next, the transducer 110 (corresponding to an actuator 206) travels one
path (denoted
here as a "transducer path" or "source path") from the current source well 106
to the next
source well 156. The destination plate 104 travels another path (denoted here
as a
"destination path") from its current position where the current destination
well 108 is aligned
with the current source well 106 to its next position where the next
destination well 158 is
aligned with the next source well 156.
100441 A cost matrix 304 shows an example of costs computed by the tour
determination engine
107 for every transition to a new liquid transfer position, according to
embodiments of the
disclosure. Each cell in the cost matrix 304 represents the transition travel
cost (e.g.,
distance) to move from a liquid transfer position represented by the matrix
row header to a
liquid transfer position represented by a matrix column header. Assuming that
the cost is
bidirectional (e.g., the distance between a first node to second node is the
same as from the
second node to the first node), the matrix is symmetric about its diagonal.
Under this
assumption and looking at only the entries above the diagonal we see that five
liquid
9
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
transfers can be sequentially ordered in ten unique combinations (5C2= 10)
having ten unique
costs.
100451 According to embodiments of the disclosure, the individual costs are
used by the engine
107 itself to determine a sequential ordering of the liquid transfers ("tour")
that minimizes
the total cost over all tours, given applicable constraints such as available
computation time.
According to embodiments of the disclosure, the engine 107 determines the
costs of all the
unique tours based upon an aggregate (e.g., sum) of the transition travel
costs for each tour.
According to embodiments of the disclosure, the engine 107 determines the low-
cost
sequential ordering of the predefined liquid transfers as the tour having the
lowest cost.
According to embodiments of the disclosure, the engine 107 may call externally
provided
software, such as a commercially available TSP solver 306, to perform this
function.
100461 One such low-cost sequential ordering is shown in output pick list 308,
where, unlike
input pick list 302, the order of the rows from top to bottom represents a low-
cost ordering of
liquid transfers computed by embodiments of the disclosure. According to
embodiments of
the disclosure, due to constraints such as available computation time, the
algorithm may not
be able to compute the absolute minimum-cost tour over all combinations of
liquid transfer
ordering, and thus may use a different, sub-optimal algorithm, such as the
Nearest Neighbor
heuristic or other heuristic described in Peddi.
100471 To perform all the transfers specified in the pick list, first and
second components (e.g.,
the transducer 110 and the destination plate 104) travel respective first and
second
component tours through all the liquid transfer positions comprising their
respective paths,
according to embodiments of the disclosure. (These component tours are
distinguished from
a tour representing the overall sequential ordering of transitions between
liquid transfers.)
100481 An objective is to minimize, within constraints, the total cost of
moving the components
(e.g., destination plate 104, transducer 110) of the system to perform all the
liquid transfer
operations specified by the pick list. According to embodiments of the
disclosure, the cost for
one sequential ordering (tour) of liquid transfers is the total time to
perform all the liquid
transfers within one tour over all the nodes on the tour. Some factors that
contribute to the
cost are:
= distance between source positions for each transition between nodes
= distance between destination positions for each transition between nodes
= speeds of the two moving components
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
100491 According to embodiments of the disclosure, each path between nodes
requires
movement of two components (e.g., the transducer and the destination plate). A
challenge is
to compute the cost of each transition between nodes based upon the movement
of the
component parts of the liquid handling system.
100501 According to embodiments of the disclosure, the respective speeds of
the first and second
components are the same, so the cost for each transfer may be represented in
terms of
distance.
100511 According to embodiments of the disclosure in which the source array
102 is fixed and
the actuator 206 (e.g., acoustic transducer 110) and the destination array 104
are the first and
second movable components, the engine 107 may compute the cost according to
Equation 1
below. For simplicity, the equation assumes that the actuator 206 and
destination array 104
speeds are the same. Under these assumptions, the cost function may be
computed according
to the Euclidean distances traveled by the actuator 206 and the destination
plate 104.
Cost = max (Did, D
- Destination)
Eq. 1
where
N
Did = j(S Xi ¨ SXJ)2 (Syi¨Syj)2
DDestination = 0 1 S.Xi ¨ SXJ) ¨ (DXE ¨ DXJ))2 ((Syt ¨ Syj) ¨ (Dyi¨ Dy1))2
Dza is the cost (e.g., distance) incurred by the actuator (e.g., transducer),
Doestination is the cost incurred by the destination array,
Sri is the x coordinate of the source point (e.g., well) in the reference
frame during the ith
transfer,
Syi is the y coordinate of the source point during the id' transfer,
Dxt is the x coordinate of the destination point (e.g., well) during the id'
transfer, and
Dyi is the y coordinate of the destination point during the ith transfer, and
11
CA 03140458 2021- 12-2
WO 2020/264260
PCT/US2020/039770
index/ is the index for another transfer, e.g., the transfer immediately
following the ith
transfer.
100521 The cost for a transition between consecutive liquid transfer positions
is the maximum of
the two cost functions because the liquid handler 202 must wait for the
slowest one of the
moving components (e.g., the transducer or the destination plate) to complete
its positioning
before the handler can execute the liquid transfer.
100531 According to embodiments of the disclosure, after the engine 107
computes the costs for
every combination of transitions between nodes for the source and destination
points (e.g.,
wells), the engine 107 then may compute a low-cost sequential ordering of
transfers using a
known solution. For example, the engine 107 may employ a publicly available
software tool
for solving the traveling salesman problem, such as that found in the GOOGLE
OR-tools
software suite for combinatorial optimization.
100541 The output pick list 308 represents the tour having the lowest cost.
The transfers may be
used for a number of objectives, such as assembly of nucleotide sequences. The
engine 107
may provide the output pick list 308 to the controller 109. In response, the
controller 109
signals the actuators 206 to move two out of the following three system
components: source
array, actuator, destination array, in the prescribed order to achieve the
desired objective_
100551 According to embodiments of the disclosure in which the movable
components (e.g.,
transducer and destination plate) move in the x and y axes at speeds dependent
upon each
other, the engine 107 may compute the cost function according to Equation 2
below.
According to embodiments of the disclosure, the handler 202 includes separate
actuators for
x and y motion of the movable components (e.g., transducer and destination
plate).
According to embodiments of the disclosure, the controller 109 actuates the x
and y motion
at different speeds to allow for a smooth, simultaneous arrival of the two
components at the
liquid transfer position (node) along a straight-line path. Cost here may be
measured in time,
instead of distance.
100561 For example, assume that to move the transducer 110 along a path from
source well A to
source well B for a liquid transfer, the transducer 110 must traverse 3 cm in
the x direction
and 4 cm in the y direction. The controller 109 commands the x and y actuators
to move
simultaneously at different speeds so the transducer 110 arrives at well B in
a straight-line
12
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
path. The cost (e.g., time) would be determined by the longest path (the
Chebyshev distance)
¨4 cm,
100571 Equation 2 reflects this scenario:
Cost = max (Dar& DDestination)
Eq. 2
where
Dxd = max (ISxt ¨ Sxj I), I SYt SY' I)
DDõtination = max (I(Sxi ¨ Sxj) ¨ (Dxi¨ Dxj)l,
(Dyi¨ Dyi)l)
100581 In sum, the transducer and destination plate costs computed by Eq. 2
are determined by
the longest path (which is the rate-limiting path under the applicable
assumptions) in the x or
y dimension.
100591 According to embodiments of the disclosure, the algorithm for computing
cost is
generalized to apply to any liquid handling system in which two out of the
three components
of (source array, destination array, actuator) move into alignment at liquid
transfer positions,
while the remaining component remains fixed.
100601 Equations 3 and 4 are generalized formulas analogous to Equations 1 and
2, respectively.
Cost = max (D1,D,2)
Eq. 3
Dc1 = 10.3xi ¨ Sxj) ¨ KrKs(Dxt ¨ Dxj))2 + ((Syi¨Syi)¨ KTKs(Dyi¨ Dy1))2
Dc2 = j((DXi ¨ DX)) ¨ KTKD(SXE ¨ SX .0)2 ((Dyi¨
KTKD(Syi¨Syi))2
Cost= max (iic1,D)
Eq. 4
Dci =max (I(Sxi ¨ Sxj) ¨ KrKs(Dxi ¨ Dxj)l, (SYi SY') ¨ KTKADYi DYAI)
Dc2= max (I(Dxi ¨ Dxj) ¨ KTKD(Sxi Sx.i)I, I(DYi
KTKD(SY1¨SY1)1)
If the actuator (e.g., transducer) moves, KT = 1, else KT = 0
If the source array moves, Ks = 1, else Ks = 0
If the destination array moves, Kr) = 1, else KA) = 0
13
CA 03140458 2021- 12- 2
WO 2020/264260
PCT/US2020/039770
where
Dci is the cost incurred by the first component,
Dc2 is the cost incurred by the second component,
100611 According to other embodiments of the disclosure, the first and second
components (e.g.,
transducer and destination plate) speeds differ. In that case, cost (e.g.,
time) is a function of
both the path lengths and the speeds. Accordingly, in Eqs. 1-4, each component
cost would
be divided by the respective travel speeds of the component.
100621 This disclosure provides different approaches to computing cost
functions for transitions
between nodes. Regardless of which solution is employed, embodiments of the
disclosure
provide an inventive way to determine cost functions for node transitions in a
pick list or
other transfer list in a laboratory automation system, provided the system has
source and
destination arrays and an actuator to effect a transfer at the node. Further,
of the three
components (source array, destination array, actuator), two must be moveable
and one must
be fixed.
100631 According to embodiments of the disclosure, the laboratory automation
system is a liquid
handler, such as an acoustic liquid handler, where liquids are transferred
from multiple points
on a source array to multiple points on a destination array using an acoustic
actuator.
100641 According to other embodiments of the disclosure, the laboratory
automation system is a
tube sorter, such as a pneumatic tube sorter, where tubes are transferred from
multiple points
on a source array to multiple points on a destination array using a pneumatic
actuator.
100651 According to other embodiments of the disclosure, the laboratory
automation system is a
powder handler, such as a vibratory powder dispenser, where powders are
transferred from
multiple points on a source array to multiple points on a destination array
using a vibratory
actuator.
100661 Nucleotide sequence assembly and production of product of interest
100671 Nucleotide sequence assembly
100681 Performing liquid transfers with liquid handling robots to assemble
nucleotide sequences
is known in the art. For example, the transfers performed in the sequential
order determined
by embodiments of the disclosure may be used to transfer templates and primers
into
14
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
destination wells to achieve PCR reactions. The liquid handler 202 may then
mix the PCR-
amplified nucleotide parts with reagents, such as enzymes, to assemble the
parts into
nucleotide sequences. Embodiments of the disclosure may employ the Gibson or
Golden
Gate assembly protocols, for example. The sequences may then be circularized
into plasmid
form. The plasmids may then be inserted (via, e.g., electroporation) into
microbes such as
bacteria, which are fermented in a bioreactor to produce a product of
interest, such as a
desired protein.
100691 As an example, Figs. 4A and 4B depict general steps for DNA assembly,
transformation,
and strain screening, according to embodiments of the disclosure. Fig. 4A
depicts steps for
building DNA fragments, cloning DNA fragments into vectors, transforming the
vectors into
host strains, and removing selection markers. Fig. 4B depicts steps for high-
throughput
culturing, screening, and evaluation of selected host strains. This figure
also depicts optional
steps of culturing, screening, and evaluating selected strains in culture
tanks.
100701 As a particular example, the use of acoustic liquid transfers for
assembling nucleotides is
described in, e.g., Kanigowska, et al., Smart DNA Fabrication Using Sound
Waves:
Applying Acoustic Dispensing Technologies to Synthetic Biology, Journal of
Laboratory
Automation, 2016, Vol. 21(1)49-56, incorporated by reference herein in its
entirety. In the
exemplary process described below, the authors used the ECHO 550 handler for
DNA
synthesis and assembly at the nanoliter scale.
100711 Gibson DNA Assembly
100721 The Gibson assembly method is one of the most used in synthetic
biology, and it can
assemble DNA sequences up to small genome sizes from overlapping DNA fragments
in an
isothermal one-pot reaction. The advantage of Gibson assembly is that it is
sequence-
independent and generates scarless final assembled DNA products. Typically,
the Gibson
assembly requires about a 40 bp homologous region between two adjacent DNA
fragments,
and these homologous regions are usually added to the fragments by a high-
fidelity PCR.
According to embodiments of the disclosure, the assembly reaction takes place
in a cocktail
of enzymes (the "Gibson master mix") at 50 C for 60 min: (1) First, T5
exonulease chews
back the DNA in a 5` to 3' direction from the homologous terminal ends to
reveal reverse
complementary single-stranded sequences between two adjacent fragments. (2)
While the 5
to 3' DNA digestion proceeds, a high-fidelity DNA polymerase fills in the
single-
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
stranded DNA region. (3) Finally, Taq DNA ligase seals the nicked DNA strands,
which
yields the final assembled product.
100731 Gibson Reaction Setup by ECHO 550. Two pairs of primers (YCp2391 and
YCp2392
for fragment 1, and YCp2393 and YCp2394 for fragment 2 amplify two fragments
with 40
bp end homology from a red fluorescent protein (RFP)-containing plasmid
pPCO25, thus
allowing subsequent Gibson reassembly of the plasmid. Gibson master mix (40
pl.) is added
to source plate 1, such as an ECHO '384 polypropylene plate. Each DNA fragment
(10 pL)
is added to source plate 2, such as an ECHO 384-well low-dead-volume plate.
One-pot
Gibson assembly is incubated at 50 C for 60 minutes in a preheated PCR thermal
cycler.
100741 Golden Gate Assembly
100751 According to embodiments of the disclosure, the Golden Gate DNA
assembly method
uses a combination of a TypeIIS restriction enzyme and a ligase to assemble
the DNA
fragments. TypenS enzymes (e.g., Bsal and BsmBI enzymes) are endonucleases
that cut
outside their recognition sites, creating 4 bp DNA overhangs. The Golden Gate
DNA
assembly reaction starts with a given TypellS endonuclease DNA digestion,
leaving behind
staggered cuts in the backbone and the fragment DNA. The design-imposed DNA
complementarity allows annealing of the resulting "sticky ends: creating the
desired plasmid
construct. In the final reaction step, the T4 DNA ligase repairs the nicks to
complete the
DNA construction phase.
100761 Golden Gate Reaction Setup by Echo 550. The HcKan_P plasmid (2.8 kb,
diluted to 10
ng/pl) may be used as the acceptor vector. This plasmid carries a KanR
selectable marker,
along with an RFP cassette flanked by a pair of outward-facing BsaI sites. The
promoter
pMBP1 (500 bp) is amplified directly from yeast BY4741 (MATa, leu2A0 met15A0
ura3A0
his3A1) genomic DNA with primers YCp2395 and YCp2396, and a pair of inward-
facing
Bsal sites is added to flank the promoter part. The PCR product is purified
using a PureLink
PCR purification kit (Life Technologies) and diluted to 20 ng/pl. The 4 bp
overhangs are
designed in such a way that the promoter can be efficiently assembled into the
acceptor
vector. The Golden Gate master mix is made of 35 'IL T4 ligase (2000 U/ 1, New
England
Biolabs, NEB), 35 p.L BsaI-HF (NEB), 52.5 RL lox T4 buffer (NEB), and 25
p1.200 x BSA
(NEB). Golden Gate assembly reactions are set up using the following cycling
conditions: 15
cycles of 5 minutes at 37 C and 10 minutes at 16 C, 5 minutes at 50 C, 10
minutes at 80 C,
16
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
and hold at 4 C. Five reaction volumes arranging from 50 to 1000 nL are set
up, and each
reaction performed in triplicate. Golden Gate master mix (30 pL) is added to
source plate 1,
such as an Echo* 384 polypropylene plate. pM1BP1 PCR product (10 pL) and HcKan
P
vector (10 pL) are added to source plate 2, such as an Echo 384 low-dead-
volume plate.
100771 Bacterial Transformation
100781 According to embodiments of the disclosure, bacterial competent cells
are added to each
well containing an assembled product. For example, competent E. coli (20 pL;
MAX
Efficiency DH5a, Life Technologies) is added to each well of the reaction
plate. According
to embodiments of the disclosure, the PCR plate is incubated on ice for 20
minutes and then
placed in a heat block at 42 C for 45 s. The plate is placed back on ice to
incubate for 5
minutes, before adding 200 pL of room temperate Super Optimal Catabolite
repression
(SOC) medium to each well. The plate is incubated at 37 C with shaking at 200
rpm for 1 h.
A multichannel pipet is used to slowly drip 40 pL of each transformation
mixture onto a
mermFisherTM OmniTrayTm containing selective solid agar medium (LB¨Kan).
Alternatively, 100 pi. of transformation mixture is plated on individual petri
dishes with
selective solid agar medium (Golden Gate assembly, LB¨Kan; Gibson assembly,
LB¨
Amp). Plates are incubated overnight at 37 C until colonies appear.
100791 Fermentation
100801 Using known techniques, the synthesized bacteria may be transferred to
a bioreactor
containing feedstock for fermentation_ Under controlled conditions, the
bacteria ferment to
produce a desired product of interest (e.g., small molecule, peptide,
synthetic compound,
fuel, alcohol) based upon the assembled DNA.
100811 Other types of microbes can function as platform organisms in
industrial biotechnology,
including yeasts fermenting sugar compounds into end-products, as well as
microalgae via
photosynthesis (phototrophic algae) or fermentation (heterotrophic algae).
100821 The bacteria or other cells can be cultured in conventional nutrient
media modified as
appropriate for desired biosynthetic reactions or selections. Culture
conditions, such as
temperature, pH and the like, are those suitable for use with the host cell
selected for
expression, and will be apparent to those skilled in the art. Many references
are available for
the culture and production of cells, including cells of bacterial, plant,
animal (including
mammalian) and archaebacterial origin. See e.g., Sambrook, Ausubel (all
supra), as well as
17
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
Berger, Guide to Molecular Cloning Techniques, Methods in Enzymology volume
152
Academic Press, Inc., San Diego, CA; and Freshney (1994) Culture of Animal
Cells, a
Manual of Basic Technique, third edition, Wiley-Liss, New York and the
references cited
therein; Doyle and Griffiths (1997) Mammalian Cell Culture: Essential
Techniques John
Wiley and Sons, NY; Humason (1979) Animal Tissue Techniques, fourth edition
W.H.
Freeman and Company; and Ricciardelle et al., (1989) In Vitro Cell Dev. Biol.
25:1016-1024,
all of which are incorporated herein by reference. For plant cell culture and
regeneration,
Payne et al (1992)Plant Cell and Tissue Culture in Liquid Systems John Wiley &
Sons, Inc.
New York, N.Y.; Gamborg and Phillips (eds) (1995) Plant Cell, Tissue and Organ
Culture;
Fundamental Methods Springer Lab Manual, Springer-Verlag (Berlin Heidelberg
N.Y.);
Jones, ed. (1984) Plant Gene Transfer ant/Expression Protocols, Humana Press,
Totowa,
N.J. and Plant Molecular Biology (1993) R. R. D. Croy, Ed. Bios Scientific
Publishers,
Oxford, U.K. ISBN 0 12 198370 6, all of which are incorporated herein by
reference. Cell
culture media in general are set forth in Atlas and Parks (eds.) The Handbook
of
Microbiological Media (1993) CRC Press, Boca Raton, Fla., which is
incorporated herein by
reference. Additional information for cell culture is found in available
commercial literature
such as the Life Science Research Cell Culture Catalogue from Sigma-Aldrich,
Inc (St Louis,
Mo.) ("Sigma-LSRCCC") and, for example, The Plant Culture Catalogue and
supplement
also from Sigma-Aldrich, Inc (St Louis, Mo.) ("Sigma-PCCS"), all of which are
incorporated
herein by reference.
100831 The culture medium lobe used should in a suitable manner satisfy the
demands of the
respective strains. Descriptions of culture media for various microorganisms
are present in
the "Manual of Methods for General Bacteriology" of the American Society for
Bacteriology
(Washington D.C., USA, 1981), incorporated by reference herein.
100841 The synthesized cells may be cultured continuously, or discontinuously
in a batch process
(batch cultivation) or in a fed-batch or repeated fed-batch process for the
purpose of
producing the desired organic compound. A summary of a general nature about
known
cultivation methods is available in the textbook by Chmiel (BioprozeStechnik.
1: Einfiihrung
in die Bioverfahrenstechnik (Gustav Fischer Verlag, Stuttgart, 1991)) or in
the textbook by
Storhas (Biorealctoren and periphere Einrichtungen (Vieweg Verlag,
Braunschweig/Wiesbaden, 1994)), all of which are incorporated by reference
herein.
18
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
100851 Classical batch fermentation is a closed system, wherein the
composition of the medium
is set at the beginning of the fermentation and is not subject to artificial
alterations during the
fermentation. A variation of the batch system is a fed-batch fermentation. In
this variation,
the substrate is added in increments as the fermentation progresses. Fed-batch
systems are
useful when catabolite repression is likely to inhibit the metabolism of the
cells and where it
is desirable to have limited amounts of substrate in the medium. Batch and fed-
batch
fermentations are common and well known in the art.
100861 Continuous fermentation is a system where a defined fermentation medium
is added
continuously to a bioreactor and an equal amount of conditioned medium is
removed
simultaneously for processing and harvesting of desired biomolecule products
of interest.
Continuous fermentation generally maintains the cultures at a constant high
density where
cells are primarily in log phase growth. Continuous fermentation generally
maintains the
cultures at a stationary or late log/stationary, phase growth. Continuous
fermentation systems
strive to maintain steady state growth conditions.
100871 Methods for modulating nutrients and growth factors for continuous
fermentation
processes as well as techniques for maximizing the rate of product formation
are well known
in the art of industrial microbiology.
100881 For example, a non-limiting list of carbon sources for cellular
cultures include, sugars
and carbohydrates such as, for example, glucose, sucrose, lactose, fructose,
maltose,
molasses, sucrose-containing solutions from sugar beet or sugar cane
processing, starch,
starch hydrolysate, and cellulose; oils and fats such as, for example, soybean
oil, sunflower
oil, groundnut oil and coconut fat; fatty acids such as, for example, palmitic
acid, stearic acid,
and linoleic acid; alcohols such as, for example, glycerol, methanol, and
ethanol; and organic
acids such as, for example, acetic acid or lactic acid.
100891 A non-limiting list of the nitrogen sources include, organic nitrogen-
containing
compounds such as peptones, yeast extract, meat extract, malt extract, corn
steep liquor,
soybean flour, and urea; or inorganic compounds such as ammonium sulfate,
ammonium
chloride, ammonium phosphate, ammonium carbonate, and ammonium nitrate. The
nitrogen
sources can be used individually or as a mixture.
19
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
100901 A non-limiting list of the possible phosphorus sources include,
phosphoric acid,
potassium dihydrogen phosphate or dipotassium hydrogen phosphate or the
corresponding
sodium-containing salts.
100911 The culture medium may additionally comprise salts, for example in the
form of
chlorides or sulfates of metals such as, for example, sodium, potassium,
magnesium, calcium
and iron, such as, for example, magnesium sulfate or iron sulfate.
100921 Finally, essential growth factors such as amino acids, for example
homoserine and
vitamins, for example thiamine, biotin or pantothenic acid, may be employed in
addition to
the abovementioned substances.
100931 In some embodiments, the pH of the culture can be controlled by any
acid or base, or
buffer salt, including, but not limited to sodium hydroxide, potassium
hydroxide, ammonia,
or aqueous ammonia; or acidic compounds such as phosphoric acid or sulfuric
acid in a
suitable manner. In some embodiments, the pH is generally adjusted to a value
of from 6.0 to
8.5, preferably 6.5 to 8.
100941 The cultures may include an anti-foaming agent such as, for example,
fatty acid
polyglycol esters. The cultures may be modified to stabilize the plasmids of
the cultures by
adding suitable selective substances such as, for example, antibiotics.
100951 The cultures may be carried out under aerobic or anaerobic conditions.
In order to
maintain aerobic conditions, oxygen or oxygen-containing gas mixtures such as,
for example,
air, are introduced into the culture. It is likewise possible to use liquids
enriched with
hydrogen peroxide. The fermentation is carried out, where appropriate, at
elevated pressure,
for example at an elevated pressure of from 0.03 to 0.2 MPa. The temperature
of the culture
is normally from 20 C to 45 C and preferably from 25 C to 40 C, particularly
preferably
from 30 C to 37 C. In batch or fed-batch processes, the cultivation may be
continued until an
amount of the desired product of interest (e.g. an organic-chemical compound)
sufficient for
recovery has formed. This aim can normally be achieved within 10 hours to 160
hours. In
continuous processes, longer cultivation times are possible. The activity of
the
microorganisms results in a concentration (accumulation) of the product of
interest in the
fermentation medium and/or in the cells of said microorganisms.
100961 Computing environment
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
[0097] Fig. 5 illustrates a cloud computing environment according to
embodiments of the
disclosure. In embodiments of the disclosure, software 1010 may be implemented
for the tour
determination engine or other computer operations disclosed herein in a cloud
computing
system 1002, to enable users to determine the low-cost sequential ordering of
liquid transfers,
control the liquid transfers performed by the liquid handler, and perform
other computer-
implemented operations according to embodiments of the disclosure. Client
computers 1006,
such as those illustrated in Fig. 6, access the system via a network 1008,
such as the Internet.
The system may employ one or more computing systems using one or more
processors, of
the type illustrated in Fig. 6. The cloud computing system itself includes a
network interface
1012 to interface the software 1010 to the client computers 1006 via the
network 1008. The
network interface 1012 may include an application programming interface (API)
to enable
client applications at the client computers 1006 to access the system software
1010.
[0098] A software as a service (SaaS) software module 1014 offers the system
software 1010 as
a service to the client computers 1006. A cloud management module 1016 manages
access to
the system 1010 by the client computers 1006. The cloud management module 1016
may
enable a cloud architecture that employs multitenant applications,
virtualization or other
architectures known in the art to serve multiple users.
[0099] Fig. 6 illustrates an example of a computer system 1100 that may be
used to execute
program code stored in a non-transitory computer readable medium (e.g.,
memory) in
accordance with embodiments of the disclosure. The computer system includes an
input/output subsystem 1102, which may be used to interface with human users
and/or other
computer systems depending upon the application. The I/0 subsystem 1102 may
include,
e.g., a keyboard, mouse, graphical user interface, touchscreen, or other
interfaces for input,
and, e.g., an LED or other flat screen display, or other interfaces for
output, including
application program interfaces (APIs). Elements of embodiments of the
disclosure, such as
the tour determination engine 107, client device 210, the controller 109, and
the liquid
handler 202 may be implemented with a computer system like that of computer
system 1100.
[00100] Program code may be stored in non-transitory
media such as persistent storage in
secondary memory 1110 or main memory 1108 or both. Main memory 1108 may
include
volatile memory such as random access memory (RAM) or non-volatile memory such
as
read only memory (ROM), as well as different levels of cache memory for faster
access to
21
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
instructions and data. Secondary memory may include persistent storage such as
solid state
drives, hard disk drives or optical disks. One or more processors 1104 reads
program code
from one or more non-transitory media and executes the code to enable the
computer system
to accomplish the methods performed by the embodiments herein. Those skilled
in the art
will understand that the processor(s) may ingest source code, and interpret or
compile the
source code into machine code that is understandable at the hardware gate
level of the
processor(s) 1104. The processor(s) 1104 may include graphics processing units
(GPUs) for
handling computationally intensive tasks.
[00101] The processor(s) 1104 may communicate with
external networks via one or more
communications interfaces 1107, such as a network interface card, WiFi
transceiver, etc. A
bus 1105 communicatively couples the I/0 subsystem 1102, the processor(s)
1104,
peripheral devices 1106, communications interfaces 1107, memory 1108, and
persistent
storage 1110. Embodiments of the disclosure are not limited to this
representative
architecture. Alternative embodiments may employ different arrangements and
types of
components, e.g., separate buses for input-output components and memory
subsystems
[00102] Those skilled in the art will understand that
some or all of the elements of
embodiments of the disclosure, and their accompanying operations, may be
implemented
wholly or partially by one or more computer systems including one or more
processors and
one or more memory systems like those of computer system 1100. In particular,
elements of
the tour determination engine 107, the client device 210, the controller 109,
the liquid
handler 202, and any other automated systems or devices described herein may
be computer-
implemented. Some elements and functionality may be implemented locally and
others may
be implemented in a distributed fashion over a network through different
servers, e.g., in
client-server fashion, for example. In particular, server-side operations may
be made
available to multiple clients in a software as a service (SaaS) fashion, as
shown in Figure 5.
[00103] Those skilled in the art will recognize that, in
some embodiments, some of the
operations described herein (e.g., moving lab equipment) that do not involve
data processing
may be performed by human implementation, or through a combination of
automated and
manual means.
[00104] Although the disclosure may not expressly
disclose that some embodiments or
features described herein (such as those recited in the claims) may be
combined with other
22
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
embodiments or features described herein, this disclosure should be read to
describe any such
combinations that would be practicable by one of ordinary skill in the art.
Unless otherwise
indicated herein, the term "include" shall mean "include, without limitation,"
and the term
"or" shall mean non-exclusive "or" in the manner of "and/or."
[00105] All references cited herein, including, without
limitation, articles, publications,
patents, patent publications, and patent applications, are incorporated by
reference in their
entireties for all purposes, except that any portion of any such reference is
not incorporated
by reference herein if it: (1) is inconsistent with embodiments of the
disclosure expressly
described herein; (2) limits the scope of any embodiments described herein; or
(3) limits the
scope of any terms of any claims recited herein. Mention of any reference,
article,
publication, patent, patent publication, or patent application cited herein is
not, and should
not be taken as an acknowledgment or any form of suggestion that it
constitutes relevant
prior art or forms part of the common general knowledge in any country in the
world, or that
it discloses essential matter.
[00106] In the claims below, a claim n reciting "any one
of the preceding claims starting
with claim x," shall refer to any one of the claims starting with claim x and
ending with the
immediately preceding claim (claim n-1) For example, claim 35 reciting "The
system of any
one of the preceding claims starting with claim 28" refers to the system of
any one of claims
28-34.
[00107] SELECTED EMBODIMENTS OF THE DISCLOSURE
Each embodiment below corresponds to one or more embodiments of the
disclosure.
Dependencies below are understood to refer back to embodiments within the same
set.
Method embodiments
Set 1
1. A computer-implemented method for determining a
sequential ordering of a plurality of
predefined transfers for transferring an object from a plurality of source
points of a source
array to a plurality of destination points of a destination array, wherein a
laboratory
23
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
automation system includes at least a first component and a second component
of the
following three components: a source array, a destination array, or an
actuator, the
method comprising:
a, for each unique transition to a next transfer of
the plurality of predefined transfers,
wherein each transfer corresponds to a transfer position:
i. determining a first component travel cost of the first component between
a
current transfer position and a next transfer position corresponding to the
next transfer, wherein
1. the current transfer position corresponds to positioning of a current
source point with respect to a current destination point for a current
transfer of the plurality of predefined transfers,
2, the next transfer position
corresponds to positioning of a next
source point with respect to a next destination point for the next
transfer;
ii. determining a second component travel cost of the second component
between the current transfer position and the next transfer position; and
iii. determining a transition travel cost, of a plurality of transition travel
costs,
for the transition as the maximum of the first component travel cost and
the second component travel cost; and
b. determining a resolved sequential ordering of
the plurality of predefined transfers
based at least in part upon the plurality of transition travel costs.
2. The method of embodiment 1, wherein each tour of a plurality of tours
comprises a
unique sequential ordering of the plurality of predefined transfers, the
method further
comprising determining a cost of each tour based at least in part upon an
aggregate of the
transition travel costs for the tour.
3. The method of embodiment 2, wherein the plurality of tours corresponds
to all
combinations of sequential ordering of the plurality of predefined transfers.
4. The method of any one of the preceding embodiments starting with embodiment
2,
wherein the cost of each tour comprises a sum of the transition travel costs
for the tour.
24
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
5. The method of any one of the preceding embodiments starting with embodiment
2,
wherein determining a resolved sequential ordering is based at least in part
upon the tour
that has the lowest cost.
6. The method of embodiment 1, wherein the first and second components are
movable and
a third component of the three components remains fixed.
7. The method of any one of the preceding embodiments, wherein each transition
travel cost
is a distance.
8. The method of any one of the preceding embodiments, wherein each transition
travel cost
is a function of distance and travel time.
9. The method of any one of the preceding embodiments, wherein the laboratory
automation
system comprises a liquid handler, and the object is a volume of liquid.
10. The method of any one of the preceding embodiments, wherein positioning
comprises
alignment.
11. The method of any one of the preceding embodiments, wherein a third
component of the
three components is also positioned at the next transfer position for the next
transfer.
12. The method of any one of the preceding embodiments, wherein determining
the resolved
sequential ordering of the plurality of predefined transfers comprises solving
the traveling
salesman problem.
13. The method of any one of the preceding embodiments, wherein the source and
destination arrays are plates and the source and destination points are wells.
14. The method of any one of the preceding embodiments, wherein the first and
second
components are the actuator and the destination plate, respectively.
15. The method of any one of the preceding embodiments, wherein the actuator
is an acoustic
transducer.
16. The method of any one of the preceding embodiments, further comprising
moving the
first and second components according to the resolved sequential ordering.
17. The method of any one of the preceding embodiments, wherein the
destination array
resides in a plane parallel to a plane in which the source array resides.
18. The method of any one of the preceding embodiments, further comprising
assembling at
least one nucleotide sequence based at least in part upon moving the first and
second
components according to the resolved sequential ordering.
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
19. The method of any one of the preceding embodiments, further comprising
manufacturing
a product of interest based at least in part upon at least one nucleotide
sequence
assembled by moving the first and second components according to the resolved
sequential ordering.
20. A liquid hander of any one of the preceding embodiments in which the first
and second
components travel according to the resolved sequential ordering.
System embodiments
Set 1
1. A system for determining a sequential ordering of a plurality of
predefined
transfers for transferring an object from a plurality of source points of a
source
array to a plurality of destination points of a destination array, wherein a
laboratory automation system includes at least a first component and a second
component of the following three components: a source array, a destination
array,
or an actuator, the system comprising:
one or more processors; and
one or more memories operatively coupled to the one or more processors and
storing
instructions, that when executed by at least one of the one or more
processors, cause
the system to:
a. for each unique transition to a next transfer of
the plurality of predefined transfers,
wherein each transfer corresponds to a transfer position:
i. determine a first component travel cost of the first component between a
current transfer position and a next transfer position corresponding to the
next transfer, wherein
1. the current transfer position corresponds to positioning of a current
source point with respect to a current destination point for a current
transfer of the plurality of predefined transfers,
2. the next transfer position corresponds to positioning of a next
source point with respect to a next destination point for the next
transfer;
ii. determine a second component travel cost of the second component
between the current transfer position and the next transfer position; and
26
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
iii. determine a transition travel cost, of a plurality of transition travel
costs,
for the transition as the maximum of the first component travel cost and
the second component travel cost; and
b. determine a resolved sequential ordering of the
plurality of predefined transfers
based at least in part upon the plurality of transition travel costs.
2. The system of embodiment 1, wherein each tour of a plurality of tours
comprises a
unique sequential ordering of the plurality of predefined transfers, and a
cost of each tour
is based at least in part upon an aggregate of the transition travel costs for
the tour.
3. The system of embodiment 2, wherein the plurality of tours corresponds
to all
combinations of sequential ordering of the plurality of predefined transfers.
4. The system of any one of the preceding embodiments starting with embodiment
2,
wherein the cost of each tour comprises a sum of the transition travel costs
for the
tour.
5. The system of any one of the preceding embodiments starting with
embodiment 2,
wherein determining a resolved sequential ordering is based at least in part
upon the tour
that has the lowest cost.
6. The system of embodiment 1, wherein the first and second components are
movable and
a third component of the three components remains fixed.
7. The system of any one of the preceding embodiments, wherein each
transition travel cost
is a distance.
8. The system of any one of the preceding embodiments, wherein each
transition travel cost
is a function of distance and travel time.
9. The system of any one of the preceding embodiments, wherein the
laboratory automation
system comprises a liquid handler, and the object is a volume of liquid.
10. The system of any one of the preceding embodiments, wherein positioning
comprises
alignment.
11. The system of any one of the preceding embodiments, wherein a third
component of the
three components is also positioned at the next transfer position for the next
transfer.
27
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
12. The system of any one of the preceding embodiments, wherein determining
the resolved
sequential ordering of the plurality of predefined transfers comprises solving
the traveling
salesman problem.
13. The system of any one of the preceding embodiments, wherein the source and
destination
arrays are plates and the source and destination points are wells.
14. The system of any one of the preceding embodiments, wherein the first and
second
components are the actuator and the destination plate, respectively.
15. The system of any one of the preceding embodiments, wherein the actuator
is an acoustic
transducer.
16. The system of any one of the preceding embodiments, wherein the one or
more memories
store further instructions that, when executed, cause the system to move the
first and
second components according to the resolved sequential ordering.
17. The system of any one of the preceding embodiments, wherein the
destination array
resides in a plane parallel to a plane in which the source array resides.
18. The system of any one of the preceding embodiments, wherein at least one
nucleotide
sequence is assembled based at least in part upon moving the first and second
components according to the resolved sequential ordering.
19. The system of any one of the preceding embodiments, wherein a product of
interest is
manufactured based at least in part upon at least one nucleotide sequence
assembled by
moving the first and second components according to the resolved sequential
ordering.
20. A liquid hander of any one of the preceding embodiments in which the first
and second
components travel according to the resolved sequential ordering.
Computer-readable medium embodiments
Set 1
1. One or more non-transitory computer-readable media storing instructions for
determining a sequential ordering of a plurality of predefined transfers for
transferring an object from a plurality of source points of a source array to
a
plurality of destination points of a destination array, wherein a laboratory
automation system includes at least a first component and a second component
of
the following three components: a source array, a destination array, or an
actuator,
28
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
wherein the instructions, when executed by one or more computing devices,
cause
at least one of the one or more computing devices to:
a. for each unique transition to a next transfer of
the plurality of predefined transfers,
wherein each transfer corresponds to a transfer position:
i. determine a first component travel cost of the first component between a
current transfer position and a next transfer position corresponding to the
next transfer, wherein
1. the current transfer position corresponds to positioning of a current
source point with respect to a current destination point for a current
transfer of the plurality of predefined transfers,
2. the next transfer position corresponds to positioning of a next
source point with respect to a next destination point for the next
transfer;
ii. determine a second component travel cost of the second component
between the current transfer position and the next transfer position; and
determine a transition travel cost, of a plurality of transition travel costs,
for the transition as the maximum of the first component travel cost and
the second component travel cost; and
Ii determine a resolved sequential ordering of the
plurality of predefined transfers
based at least in part upon the plurality of transition travel costs.
2. The one or more non-transitory computer-readable media of
embodiment 1, wherein each
tour of a plurality of tours comprises a unique sequential ordering of the
plurality of
predefined transfers, and a cost of each tour is based at least in part upon
an aggregate of
the transition travel costs for the tour.
3. The one or more non-transitory computer-readable media of embodiment 2,
wherein the plurality of tours corresponds to all combinations of sequential
ordering of the plurality of predefined transfers.
4. The one or more non-transitory computer-readable media of any one of the
preceding embodiments starting with embodiment 2, wherein the cost of each
tour
comprises a sum of the transition travel costs for the tour.
29
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
5. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments starting with embodiment 2, wherein determining a resolved
sequential
ordering is based at least in part upon the tour that has the lowest cost.
6. The one or more non-transitory computer-readable media of embodiment 1,
wherein the
first and second components are movable and a third component of the three
components
remains fixed.
7. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein each transition travel cost is a distance.
8. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein each transition travel cost is a function of distance and
travel time.
9. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein the laboratory automation system comprises a liquid
handler, and
the object is a volume of liquid.
10. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein positioning comprises alignment.
11. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein a third component of the three components is also
positioned at
the next transfer position for the next transfer.
12. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein determining the resolved sequential ordering of the
plurality of
predefined transfers comprises solving the traveling salesman problem.
13. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein the source and destination arrays are plates and the
source and
destination points are wells.
14. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein the first and second components are the actuator and the
destination plate, respectively.
15. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein the actuator is an acoustic transducer.
16. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein the computer-readable media store further instructions
that, when
CA 03140458 2021-12-2
WO 2020/264260
PCT/US2020/039770
executed, cause the first and second components to move according to the
resolved
sequential ordering.
17. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein the destination array resides in a plane parallel to a
plane in which
the source array resides.
18. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein at least one nucleotide sequence is assembled based at
least in part
upon moving the first and second components according to the resolved
sequential
ordering.
19. The one or more non-transitory computer-readable media of any one of the
preceding
embodiments, wherein product of interest is manufactured based at least in
part upon at
least one nucleotide sequence assembled by moving the first and second
components
according to the resolved sequential ordering.
20. A liquid hander of any one of the preceding embodiments in which the first
and second
components travel according to the resolved sequential ordering.
31
CA 03140458 2021-12-2