Note: Descriptions are shown in the official language in which they were submitted.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
1
USE OF AN RXR AGONIST IN TREATING HERZ CANCERS
BACKGROUND
[0001] Compounds which have retinoid-like biological activity are well known
in the art and
are described in numerous United States patents including, but not limited to,
U.S. Pat. Nos.
5,466,861; 5,675,033 and 5,917,082, all of which are herein incorporated by
reference.
Preclinical studies with rexinoids suggest that selective activation of
retinoid X receptors
(RXR), which modulate functions associated with differentiation, inhibition of
cell growth,
apoptosis and metastasis, may be useful in treating a variety of diseases
associated with the
biochemical functions modulated by RXR.
[0002] For example, TARGRETIN (bexarotene), which is a retinoid X receptor
(RXR)
agonist with retinoic acid receptor (RAR) agonist activity as well, was
approved by the U.S.
Food and Drug Administration for the treatment, both oral and topical, of
cutaneous
manifestations of cutaneous T cell lymphoma in patients who are refractory to
at least one
prior systemic therapy. Encouraging results were obtained with TARGRETIN in
several
Phase ll studies in NSCLC. However, the pivotal Phase Ill clinical study did
not show
increased survival. One possible explanation for the limited success of
bexarotene is that its
activation of RAR decreases its efficacy as an anticancer agent. Thus, more
selective RXR
agonists may hold greater promise.
[0003] Agents targeting human epidermal growth factor receptor 2 (Her2), both
anti-Her2
antibodies and inhibitors of the tyrosine kinase activity of Her2, have had
significant, but not
universal success in treating Her2 + cancers, particularly Her2 + breast
cancers.
[0004] Treatments for cancer are ever evolving, gaining in specificity and
sophistication.
Early non-surgical cancer treatments generally targeted rapidly dividing cells
which were more
sensitive to radiological and chemical assault. Over time, more specific and
less generally
toxic treatments have been developed. Some treatments appear to have broad
applicability,
for example immune checkpoint inhibitors or rexinoids. Others are targeted to
cancers that
express a particular antigen or other biomarker involved in the regulation of
proliferation or
differentiation; including many monoclonal antibodies and kinase inhibitors.
Yet as the variety
of cancer treatments has grown, it has become ever harder to determine which
treatments
might be productively combined and for what indications.
SUMMARY
[0005] Herein disclosed are improved methods of treatment of Her2 + cancers
comprising
treating a patient having a Her2 + tumor with a combination of a Her2-
targeting therapeutic
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
2
agent and a RXR agonist capable of inhibiting cancer growth. In some
embodiments the
treatment combination further comprises thyroid hormone.
[0006] In some embodiments, the RXR agonist is capable of activating RXR/Nurr1
heterodimeric receptors. In some embodiments the RXR agonist is a compound of
Formula I
as disclosed herein below, or a pharmaceutically-acceptable salt thereof. In
some
embodiments the RXR agonist is a compound of Formula ll as disclosed herein
below, or a
pharmaceutically-acceptable salt thereof. In some embodiments, compounds of
Formula I and
Formula II, and their pharmaceutically-acceptable salts, are referred to as
means for activating
RXR/Nurr1 heterodimeric receptors or rexinoid means for inhibiting tumor
growth.
[0007] In some embodiments, Her2-targeting therapeutic agent is an inhibitor
of Her2 kinase
activity or Her2-mediated signaling. In some embodiments Her2-targeting
therapeutic agent
is therapeutic anti-Her2 antibody. Therapeutic antibodies may mediate antibody-
dependent
cellular cytotoxicity (ADCC) instead of, or in addition to, inhibiting
signaling (kinase activity).
Trastuzumab and pertuzumab are examples of therapeutic anti-Her2 antibodies,
as disclosed
herein below. In some embodiments such antibodies are referred to as
immunoglobulin means
for inhibiting Her2 + tumor cell proliferation, means for mediating ADCC of
Her2 + tumor cells,
or immunoglobulin means for inhibiting Her2 signaling.
[0008] In some embodiments, a Her2-targeting therapeutic agent is an antibody-
drug
conjugate comprising an anti-Her2 antibody. In some embodiments, the anti-Her2
antibody
has therapeutic activity alone, while in other embodiments it does not, merely
serving to deliver
a cytotoxic agent to Her2 + cells. Ado-trastuzumab emtansine is an example of
a Her2-targeting
antibody-drug conjugate, as disclosed herein below. In some embodiments such
antibody-
drug conjugates are referred to as means for delivering a cytotoxic agent to
Her2 + cells.
[0009] In some embodiments, the inhibitor of Her2 kinase activity or Her2-
mediated
signaling is a small organic molecule (small drug) inhibitor of Her2 kinase
activity. Lapatinib
and neratinib are examples of Her2 kinase inhibitors as disclosed herein
below. In some
embodiments such small drug inhibitor of Her2 kinase activity are referred to
as small molecule
means for inhibiting Her2 kinase activity.
[0010] In some
embodiments, the Her2 + cancer is a Her2 + breast cancer. In some
embodiments the Her2 + cancer is a Her2 + ovarian cancer, stomach cancer,
adenocarcinoma
of the lung, uterine cancer (such as serous endometrial carcinoma), gastric
cancer, or salivary
duct carcinoma.
[0011] In some embodiments, the herein disclosed treatments are carried out
concurrently
with other pharmaceutical therapies or radiotherapies. In alternative
embodiments, the herein
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
3
disclosed treatments are the exclusive therapy in the time interval in which
they are conducted.
In some embodiments, the herein disclosed treatments serve as a debulking
treatment in
preparation for subsequent surgical removal of tumor. In some embodiments, the
herein
disclosed treatments are applied as adjuvant therapy subsequent to surgical
removal of tumor
to address any residual disease or potential recurrent disease.
[0012] Other embodiments include combination drug compositions or formulations
comprising at least one Her2-targeting therapeutic agent and at least one RXR
agonist
capable of inhibiting cancer growth. In other embodiments the combination
(used for
treatment) may include more than one agent in one or the other classification
(Her2 targeting
agent and/or RXR agonist). In still other embodiments the combination may
further include
thyroid hormone. In some embodiments the thyroid hormone is thyroxine.
BRIEF DESCRIPTION OF DRAWINGS
[0013] FIGURE 1 is a three-dimensional plot depicting the growth inhibitory
effects of
IRX4204 and trastuzumab, alone and in combination, on breast cancer cell
lines: MCF7 cells
(Figure 1 A) and SkBr3 cells (Figure 1B).
[0014] FIGURE 2 is a three-dimensional plot depicting the growth inhibitory
effects of
IRX4204 and lapatinib, alone and in combination, on breast cancer cell lines:
MCF7 cells
(Figure 2A), SkBr3 cells (Figure 2B), BT474 cells (Figure 2C), and MDA-MB-361
cells (Figure
2D).
[0015] FIGURE 3 is a three-dimensional plot depicting the growth inhibitory
effect of
IRX4204 and neratinib, alone and in combination, on breast cancer cell lines:
MCF7 cells
(Figure 3A), SkBr3 cells (Figure 3B), BT474 cells (Figure 3C), and MDA-MB-361
cells (Figure
3D).
DESCRIPTION
[0016] The herein disclosed embodiments include methods of treating Her2+
cancers, such
as Her2+ carcinoma of the breast, with a combination a retinoid X (rexinoid)
receptor (RXR)
agonist and a Her2-targeted anticancer agent. Some embodiment further comprise
administration of thyroid hormone in conjunction with the RXR agonist.
Embodiments include
a RXR agonist for use in combination with a Her2-targeted anticancer agent, or
a thyroid
hormone and a Her2-targeted anticancer agent, in the treatment of Her2+
cancers. In some
embodiments the Her2+ cancer is Her2+ breast cancer.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
4
[0017] The methods of treatment involve the administration of a combination of
two, three,
or more therapeutic agents. Moreover, the administration of one of these
agents may be
described as being done in coordination or conjunction with another of these
agents. By such
combination, or administration of in coordination or in conjunction with, it
is meant that the
manner of administration of each of these agents is such that the physiologic
effects of the
agents overlap in time. This does not require that the agents be contained in
the same
composition or formulation, or that they be administered as separate
compositions at the same
time, by the same route of administration, or on the same schedule, though in
some
embodiments any of the foregoing may be the case. Indeed, while it is possible
to administer
RXR agonists, thyroid hormone, and Her2 kinase inhibitors on a daily schedule,
antibodies
are more typically administered at intervals measured in weeks.
[0018] Among the earlier successful targeted cancer therapies are those
targeting human
epidermal growth factor receptor 2 (Her2). Her2-targeted treatments include
monoclonal
antibodies (mAbs), such as trastuzumab and pertuzumab, and inhibitors of Her2
kinase
activity, such as lapatinib, neratinib, and afatinib (which all also inhibit
epidermal growth factor
receptor (EGFR)). The rexinoid IRX4204 has shown activity against a variety of
cancers in
model systems (see for example, U.S. Patent Pub. 2008-0300312, which is
incorporated
herein by reference in its entirety for all that it teaches about the use of
RXR agonists for the
treatment of cancer) especially when used in combination with thyroid hormone
(see for
example, U.S. Patent Pub. 2008-0300312, which is incorporated herein by
reference in its
entirety for all that it teaches about the use of RXR agonists for the
treatment of cancer). It is
disclosed herein that the combination of IRX4204 and a Her2-targeted
therapeutic agent are
particularly effective against Her2 + cancers, such as Her2 + breast cancer.
Thus various
embodiments are combinations of IRX4204 and a Her2-targeted therapeutic agent,
and
methods of treatment involving administration of both IRX4204 and a Her2-
targeted
therapeutic agent. Some embodiments further include thyroid hormone or the
administration
of thyroid hormone. Other embodiments exclude thyroid hormone or the
administration of
thyroid hormone.
[0019] A Her2-targeted therapeutic agent as used herein is a therapeutic agent
that inhibits
growth of cancer cells by inhibiting Her2 function. Such agents include mAbs
that bind to Her2,
antibody-drug conjugates comprising such mAbs, and inhibitors of Her2 tyrosine
kinase
activity. Collectively, such agents can be referred to as means for
therapeutically targeting
Her2. Some embodiments specifically include or are limited to one or more of
these classes
of agent, or one or more species within one or more of these classes of agent.
Some
embodiments specifically exclude one or more of these classes of agent, or one
or more
snecies within nne nr mnre nf these Is nf anent Thus means for therapeutically
targeting
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
Her2 are mAbs that bind to Her2, antibody-drug conjugates comprising mAbs that
bind to
Her2, and inhibitors or Her2 tyrosine kinase activity. In some embodiments,
the mAb in an
antibody-drug conjugate has therapeutic activity by itself, in other
embodiments it does not.
[0020] Many embodiments comprise administration of a single anti-Her2 mAb, but
some
embodiments comprise administration of multiple anti-Her2 mAbs, for example,
trastuzumab
and pertuzumab. While the disclosed embodiments are generally described as
using anti-Her2
mAbs, further embodiments can substitute anti-Her2 polyclonal antiserum for
anti-Her2 mAb.
Some embodiments can comprise administration of additional mAbs targeting
other antigens,
some embodiments specifically exclude administration of other antibodies.
Anti-Her2 antibodies
[0021] Her2 is a growth factor receptor found on, and implicated in, a variety
of cancers,
especially breast cancer but also, for example, gastroesophageal cancer,
ovarian cancer,
stomach cancer, adenocarcinoma of the lung, uterine cancer (such as serous
endometrial
carcinoma), or salivary duct carcinoma. Anti-Her2 antibodies are believed to
work as
anticancer agents through a combination of mechanisms: inhibition of signaling
through Her2,
antibody-dependent cellular cytotoxicity (ADCC), and mediating presentation of
tumor antigen
by antigen presenting cells (APC), such as macrophages; additional mechanisms
may also
exist. The best understood and most clinically advanced anti-Her2 antibodies
are
trastuzumab, pertuzumab, and margetuximab.
[0022] In some embodiments, these antibodies are administered by intravenous
infusion. In
exemplary embodiments infusions may be done over 30-90 minutes and may occur
at
intervals of 1-3 weeks for as long as a year. In some such embodiments, the
antibody is
administered at an initial higher dose and a subsequent lower dose. In some
such
embodiments, the initial higher dosage is twice the subsequent lower dosage.
In some such
embodiments, the initial dose is single administration. In other such
embodiments the initial
dosage is administered multiple times before switching to the lower subsequent
dosage.
Specific examples of dosages and dose regimens can be found in the prescribing
information
for HERCEPTINO and PERJETAO, which are incorporated herein by reference in
their
entireties.
[0023] Trastuzumab (sold as HERCEPTINO) binds to domain IV of the
extracellular
segment of Her2. Trastuzumab inhibits the proliferation of cells that
overexpress Her2 and
mediates ADCC. Biosimilar antibodies to trastuzumab have been developed and
are marketed
in some jurisdictions.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
6
[0024] Pertuzumab (sold as PERJETAO) recognizes the extracellular dimerization
domain
(domain II) of Her2, a different epitope than trastuzumab. By preventing
ligand dependent
dimerization, it inhibits signaling through Her2, leading to cell growth
arrest and apoptosis.
Pertuzumab also mediates ADCC. Pertuzumab augmented the activity of
trastuzumab in
tumor xenograft models that over express Her2.
[0025] Margetuximab (currently still in clinical development) recognizes the
same epitope
as trastuzumab, but possesses an engineered Fc region designed to increase Fc-
dependent
mechanisms of immune attack, such as ADCC. The engineered Fc region confers
increased
binding to activating Fc-y receptors (CD16A) and reduced binding to inhibitory
Fc-y receptors
(CD16B) on immune effector cells, including monocytes, macrophages, dendritic
cells and
natural killer (NK) cells.
[0026] Further anti-Her2 mAbs include TrasGEXO, HM2, hertuzumab, and HT-19.
TrasGEXO and HM2 are being developed as "biobefters" of trastuzumab, while the
other two
are independently derived. In HM2 a metal-binding motif has been incorporated
into
trastuzurnab to aid conjugation. TrasGEXO is a glycosylation-optirnized
version of
trastuzumab. Hertuzumab had a higher ELISA-based affinity for Her2 than
trastuzumab. HT
19 is an igG1 antibody that is non-competitive for HER2 binding with
trastuzumab and
pertuzumab; that is, it binds a different epitope than either of those two
mAbs.
[0027] Many embodiments comprise administration of a single anti-Her2 mAb, but
some
embodiments comprise administration of multiple anti-Her2 mAbs, for example,
trastuzumab
and pertuzumab. While the disclosed embodiments are generally described as
using anti-Her2
mAbs, further embodiments can substitute anti-Her2 polyclonal antiserum for
anti-Her2 mAb.
Antibody-Drug conjugates
[0028] In addition to use of anti-Her2 mAbs themselves as therapeutic agents,
anti-Her2
mAbs have also been incorporated into antibody-drug conjugates. One example is
ado-
trastuzumab emtansine (sold as KADCYLA0). Further examples include A166, ALT-
P7
(trastuzumab biobetter HM2 conjugated in a site-specific manner to monomethyl
auristatin E),
ARX788 (a monoclonal HER2 targeting antibody site-specifically conjugated, via
a non-natural
amino acid linker para-acetyl-phenylalanine (pAcF), to monomethyl auristatin
F), DHES0815A
(a monoclonal HER-2 targeting antibody linked to pyrrolo[2,1-
c][1,4]benzodiazepine
monoamide), DS-8201a (trastuzumab deruxtecan; trastuzumab, an enzymatically
cleavable
maleimide glycynglycyn-phenylalanyn-glycyn (GGFG) peptide linker and a
topoisomerase I
inhibitor), RC48 (humanized anti-HER2 antibody hertuzumab conjugated with
monomethyl
auristatin E (MMAE) via a cleavable linker), SYD985 (avicdtrastuzumab
duocarmazine;
traRtimimah linkari via a riaavahla nantiria
to the synthetic duocarmycin
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
7
analog seco
DUocarnnycin hydroxyBenzannide Azaindole), MEDI4276 (HER2-bispecific
antibody targeting two different epitopes on HER2, site-specifically
conjugated via a
maleimidocaproyl linker to the potent tubulysin-based microtubule inhibitor
AZ13599185) and
XMT-1522 (TAK-522; HT-19 conjugated with the DOLAFLEXIN platform to
auristatin F-
hydroxypropylamide).
[0029] In some embodiments, the Her2-targeting component comprises, or is, an
antibody-
drug conjugate comprising an anti-Her2 antibody.
Her2 Kinase Inhibitors
[0030] Her2 and EGFR are closely related protein tyrosine kinases and many
drugs
developed as an inhibitor of one also inhibit the other. At least four drugs
that are irreversible
inhibitors of these kinases are now marketed as a cancer treatment, though the
indications
vary: lapatinib, neratinib, afatinib, and dacomitinib. It should be noted that
not all EGFR
inhibitors are irreversible inhibitors or are known to cross-inhibit Her2.
EGFR inhibitors that do
not - or are not known to - inhibit Her2 should not be considered Her2
inhibitors as the term is
used herein. In some embodiments, the Her2 inhibitor is an irreversible
inhibitor. In some
embodiments, the Her2 inhibitor is not a reversible inhibitor.
[0031] Lapatinib (sold as TYKERBO) can be administered, according to its
prescribing
instructions which are incorporated herein by reference in their entirety, on
a 21 day treatment
cycle. Lapatinib plus capecitabine Ãs taken on days 1 to 14. Lapatinib one is
taken on days
15 to 21. At the end of the 21 days, the treatment cycle should be repeated
until disease
progression or unacceptabie toxicity occurs Capecitabine is administered
orally in two doses
approximately 12 hours apart at a dosage of 2000 mg/m2iday.
[0032] Neratinib (sold as NERLYNX0) can be administered, according to its
prescribing
instructions which are incorporated herein by reference in their entirety,
with food at an initial
dose of 240 mg/day and taken daily for a year. If toxicity exceeds grade 1,
the dose can be
reduced by 40 mg/day in stepwise fashion until toxicity is grade 1 or less. If
the dose has been
reduced to 120 mg/day and toxicity remains greater than grade 1, treatment
with Neratinib
should be discontinued.
[0033] Afatinib (sold as GILOTRIFG)) can be administered, according to its
prescribing
instructions which are incorporated herein by reference in their entirety,
orally without food
once daily at 40 mg/day until disease progression or no longer tolerated by
the patient.
[0034] Dacomitinib (sold as VIZIMPROO) can be administered, according to its
prescribing
instructions which are incorporated herein by reference in their entirety,
orally with or without
food, once daily at 45 mg/day until disease progression or unacceptable
toxicity occurs. Upon
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
8
occurrence of unacceptable toxicity, the dosage can be reduced in stepwise
fashion to 30 or
15 mg/day.
[0035] The above dosing information, in addition to disclosing specific
embodiments in
which these Her2 kinase inhibitors may be used in combination with an RXR
agonist, provides
general guidance as to dosing practices with such drugs. The disclosed
embodiments are not
necessarily limited to these specific dosing regimens and it is within the
skill of the physician
to modify these regimens for individual patients. Due to the improved and
synergistic effect of
these drugs when used in combination with a RXR agonist, unacceptable toxicity
may be
avoided by use of lower dosages of the kinase inhibitor while still achieve
beneficial
therapeutic effect.
RXR agonists
[0036] Preclinical studies with rexinoids suggest that selective activation of
Retinoid X
Receptors (RXR), which modulate functions associated with differentiation,
inhibition of cell
growth, apoptosis and metastasis, may be useful in treating a variety of
diseases associated
with the biochemical functions modulated by RXR.
[0037] The Retinoic Acid Receptors (RARs) and RXRs and their cognate ligands
function
by distinct mechanisms. The term "RAR" as used herein refers to one or more of
RARa,
RAR, or RARy. The term "RXR" as used herein refers to one or more of RXRa,
RXR, or
RXRy. A RAR biomarker is a distinctive biological, biochemical or biologically
derived
indicator that signifies patient RAR activity. RAR biomarkers include, but are
not limited to,
CYP26 levels, CRBPI levels, and the like, and combinations thereof.
[0038] In some embodiments, the RAR activation threshold means one or more of
a CYP26
level which is 25% increased over baseline and a CRBPI level 25% increased
over baseline.
The RARs form heterodimers with RXRs and these RAR/RXR heterodimers bind to
specific
response elements in the promoter regions of target genes. The binding of RAR
agonists to
the RAR receptor of the heterodimer results in activation of transcription of
target genes
leading to retinoid effects. On the other hand, the disclosed RXR agonists do
not activate
RAR/RXR heterodimers. RXR heterodimer complexes like RAR/RXR can be referred
to as
non-permissive RXR heterodimers as activation of transcription due to ligand-
binding occurs
only at the non-RXR protein (e.g., RAR); activation of transcription does not
occur due to
ligand binding at the RXR.
[0039] RXRs also interact with nuclear receptors other than RARs and RXR
agonists may
elicit some of its biological effects by binding to such RXR/receptor
complexes. These
RXR/receptor complexes can be referred to as permissive RXR heterodimers as
activation of
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
9
transcription due to ligand-binding could occur at the RXR, the other
receptor, or both
receptors. Examples of permissive RXR heterodimers include, without
limitation, peroxisome
proliferator activated receptor/RXR (PPAR/RXR), farnesyl X receptor/RXR
(FXR/RXR),
nuclear receptor related-1 protein (Nurr1/RXR) and liver X receptor/RXR
(LXR/RXR).
Alternately, RXRs may form RXR/RXR homodimers which can be activated by RXR
agonists
leading to rexinoid effects. Also, RXRs interact with proteins other than
nuclear receptors and
ligand binding to an RXR within such protein complexes can also lead to
rexinoid effects. Due
to these differences in mechanisms of action, RXR agonists and RAR agonists
elicit distinct
biological outcomes and even in the instances where they mediate similar
biological effects,
they do so by different mechanisms. Moreover, the unwanted side effects of
retinoids, such
as pro-inflammatory responses or mucocutaneous toxicity, are mediated by
activation of one
or more of the RAR receptor subtypes. Stated another way, biological effects
mediated via
RXR pathways would not induce pro-inflammatory responses, and thus, would not
result in
unwanted side effects.
[0040] Thus, aspects of the present specification provide, in part, a RXR
agonist. As used
herein, the term "RXR agonist", is synonymous with "selective RXR agonist" and
refers to a
compound that selectively binds to one or more RXR receptors like a RXRa, a
RXR, or a
RXRy in a manner that elicits gene transcription via an RXR response element.
As used
herein, the term "selectively binds," when made in reference to a RXR agonist,
refers to the
discriminatory binding of a RXR agonist to the indicated target receptor like
a RXRa, a RXR,
or a RXRy such that the RXR agonist does not substantially bind with non-
target receptors
like a RARa, a RAR 6 or a RARy. In some embodiments, the term "RXR agonist"
includes
esters of RXR agonist.
[0041] For each of the herein disclosed embodiments the RXR agonists can be
compounds
having the structure of Formula I
A \ H
/
(Th
CO2R.
, (Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or the agonist is a
pharmaceutically acceptable
salt of the compounds.
[0042] Also disclosed herein are esters of RXR agonists. An ester may be
derived from a
carboxylic acid of C1, or an ester may be derived from a carboxylic acid
functional group on
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
another part of the molecule, such as on a phenyl ring. While not intending to
be limiting, an
ester may be an alkyl ester, an aryl ester, or a heteroaryl ester. The term
alkyl has the meaning
generally understood by those skilled in the art and refers to linear,
branched, or cyclic alkyl
moieties. C1_6 alkyl esters are particularly useful, where alkyl part of the
ester has from 1 to 6
carbon atoms and includes, but is not limited to, methyl, ethyl, propyl,
isopropyl, n-butyl, sec-
butyl, iso-butyl, t-butyl, pentyl isomers, hexyl isomers, cyclopropyl,
cyclobutyl, cyclopentyl,
cyclohexyl, and combinations thereof having from 1-6 carbon atoms, etc. In
some
embodiments the RXR agonist is the ethyl ester of formula I.
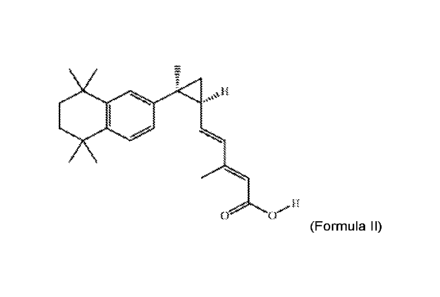
[0043] In some embodiments the RXR agonist is 3,7-dimethy1-6(S),7(S)-methano,7-
[1,1,4,4-tetramethy1-1,2,3,4-tetrahydron-aphth-7-y1]2(E), 4(E) heptadienoic
acid, also known
as IRX4204, and has the following chemical structure:
,t
(Formula II)
[0044] Pharmaceutically acceptable salts of RXR agonists can also be used in
the disclosed
embodiments. Compounds disclosed herein which possess a sufficiently acidic, a
sufficiently
basic, or both functional groups, and accordingly can react with any of a
number of organic or
inorganic bases, and inorganic and organic acids, to form a salt.
[0045] Acids commonly employed to form acid addition salts from RXR agonists
with basic
groups are inorganic acids such as hydrochloric acid, hydrobromic acid,
hydroiodic acid,
sulfuric acid, phosphoric acid, and the like, and organic acids such as p-
toluenesulfonic acid,
methanesulfonic acid, oxalic acid, p-bromophenyl-sulfonic acid, carbonic acid,
succinic acid,
citric acid, benzoic acid, acetic acid, and the like. Examples of such salts
include the sulfate,
pyrosulfate, bisulfate, sulfite,
bisulfite, phosphate, monohydrogenphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide,
acetate,
propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caproate,
heptanoate,
propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate,
maleate, butyne-1,4-
dioate, hexpe-1,6-dioate, benzoate, chlorobenzoate, methylbenzoate,
dinitrobenzoate,
hydroxybenzoate, methoxybenzoate, phthalate, sulfonate, xylenesulfonate,
phenylacetate,
phenylpropionate, phenylbutyrate, citrate, lactate, gamma-hydroxybutyrate,
glycolate, tartrate,
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
11
methanesulfonate, propanesulfonate, naphthalene-1-sulfonate, naphthalene-2-
sulfonate,
mandelate, and the like.
[0046] Bases commonly employed to form base addition salts from RXR agonists
with acidic
groups include, but are not limited to, hydroxides of alkali metals such as
sodium, potassium,
and lithium; hydroxides of alkaline earth metal such as calcium and magnesium;
hydroxides
of other metals, such as aluminum and zinc; ammonia, and organic amines, such
as
unsubstituted or hydroxy-substituted mono-, di-, or trialkylamines;
dicyclohexylamine; tributyl
amine; pyridine; N-methyl,N-ethylamine; diethylamine; triethylamine; mono-,
bis-, or tris-(2-
hydroxy-lower alkyl amines), such as mono-, bis-, or tris-(2-
hydroxyethyl)amine, 2-hydroxy-
tert-butylamine, or tris-(hydroxymethyhmethylamine, N,N-di-lower alkyl-N-
(hydroxy lower
alkyl)-amines, such as N,N-dimethyl-N-(2-hydroxyethyhamine, or tri-(2-
hydroxyethyl)amine;
N-methyl-D-glucamine; and amino acids such as arginine, lysine, and the like.
[0047] IRX4204, like some other RXR ligands, does not activate non-permissive
heterodimers such as RAR/RXR. However, IRX4204, is unique in that it
specifically activates
the Nurr1/RXR heterodimer and does not activate other permissive RXR
heterodimers such
as PPAR/RXR, FXR/RXR, and LXR/RXR. Other RXR ligands generally activate these
permissive RXR heterodimers. Thus, all RXR ligands cannot be classified as
belonging to
one class. IRX4204 belongs to a unique class of RXR ligands which specifically
activate RXR
homodimers and only one of the permissive RXR heterodimers, namely the
Nurr1/RXR
heterodimer.
[0048] In one embodiment, the selective RXR agonist does not activate to any
appreciable
degree the permissive heterodimers PPAR/RXR, FXR/RXR, and LXR/RXR. In another
embodiment, the selective RXR agonist, activates the permissive heterodimer
Nurr1/RXR.
One example of such a selective RXR agonist is 3,7-dimethy1-6(S),7(S)-
methano,7-[1,1,4,4-
tetramethyl-1,2,3,4-tetrahydronaphth-7-y1]2(E),4(E) heptadienoic acid
(IRX4204) disclosed
herein, the structure of which is shown in Formula II. In other aspects of
this embodiment, the
RXR agonists activates the permissive heterodimers PPAR/RXR, FXR/RXR, or
LXR/RXR by
1% or less, 2% or less, 3% or less, 4% or less, 5% or less, 6% or less, 7% or
less, 8% or less,
9% or less, or 10% or less relative to the ability of activating agonists to
the non-RXR receptor
to activate the same permissive heterodimer. Examples of RXR agonists, which
activates one
or more of PPAR/RXR, FXR/RXR, or LXR/RXR include LGD1069 (bexarotene) and
LGD268.
[0049] Binding specificity is the ability of a RXR agonist to discriminate
between a RXR
receptor and a receptor that does not contain its binding site, such as a RAR
receptor.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
12
[0050] Particular embodiments provide methods of treating cancer comprising
administering to a patient in need of such treatment a RXR agonist at a level
below an RAR
activating threshold and at or above an RXR activating threshold.
[0051] For IRX4204, the RAR ECio (the concentration effective to cause a 10%
of maximal
activation of the RAR) is 300 nM for the OC isoform and 200 nM for the f3 and
y isoforms. Thus,
in some embodiments, concentrations not exceeding 200 nM are considered to be
below an
RAR activating concentration. For IRX4204, the RXR EC90 (the concentration
effective to
cause a 90% of maximal activation of the RXR) is 0.1 nM for the OC and y
isoforms and 1 nM
for the f3 isoform. Thus, in some embodiments concentrations of at least 0.1
nM are considered
to be above an RXR activating threshold. Based on studies in humans, oral
dosages of
IRX4204 of 20 mg/m2/day will produce systemic concentrations that remain below
200 nM.
Similarly, it is estimated that an oral dosage in the range of 0.01 to 0.02
mg/m2/day will produce
systemic concentrations of 0.1nM or greater. Thus, in various individual
embodiments a
dosage of IRX4204 is at least 0.01, 0.02, 0.03, 0.05, 0.1, 0.3, 0.5, 1, 3 or 5
mg/m2/day and
does not exceed 150, 200, or 300 mg/m2/day, or any range bound by a pair of
these values.
[0052] In other embodiments, the dosage for a human adult of the RXR agonist,
for example
IRX4204, is from 0.2 to 300 mg/day, such as in individual embodiments, from
0.5, 1, 2, 3, 4,
5, 6, 7, 8, 9, or 10 mg/day, but not to exceed 10, 15, 20, 50, or 100 mg/day,
or any range
bound by a pair of these values.
[0053] The RXR agonist can be administered to a mammal using standard
administration
techniques, including parenteral, oral, intravenous, intraperitoneal,
subcutaneous, pulmonary,
transdermal, intramuscular, intranasal, buccal, sublingual, or suppository
administration. The
term "parenteral," as used herein, includes intravenous, intramuscular,
subcutaneous, rectal,
vaginal, and intraperitoneal administration. The RXR agonist preferably is
suitable for oral
administration, for example as a pill, tablet or capsule. Administration may
be continuous or
intermittent. In certain embodiments, the total daily dosage of RXR agonist
can be
administered as a single dose or as two doses administered with a 24 hour
period spaced 8
to 16, or 10 to 14, hours apart.
Thyroid hormone
[0054] Both biologically sourced and synthetic thyroid hormones have been used
in
medicine. The major forms of thyroid hormone are referred to a T3
(triiodothyronine) and T4
(thyroxine). Thyroxine is less active but has a longer half-life and is
sometimes considered a
prohormone of triiodothyronine. As used herein, the term "thyroid hormone"
refers to thyroxine
and triiodothyronine. Thyroxine (thyroid hormone T4, levothyroxine sodium) is
a tyrosine-
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
13
based hormone produced by the thyroid gland and is primarily responsible for
regulation of
metabolism. Both have wide commercial availability and are suitable for use in
the herein
disclosed embodiments. However, the synthetic form of T4, levothyroxine, is
much more
commonly utilized (except in patients unable to convert T4 into T3) as its
longer half-life in the
body facilitates once-daily administration. In some embodiments, the
administered thyroid
hormone is specifically thyroxine. In some embodiments, the administered
thyroid hormone is
triiodothyronine.
[0055] Administration of RXR agonists, or esters thereof, may lead to the
suppression of
serum thyroid hormones and possibly to clinical hypothyroidism and related
conditions.
However, in some embodiments thyroid hormone is not co-administered (or is not
primarily
co-administered) to remediate a suppression of serum thyroid hormone levels.
Co-
administration of thyroid hormone with an RXR agonist improves the RXR
agonist's anti-
cancer efficacy, as compared to the effect of the RXR agonist alone, likely
through multiple
mechanisms of action. The co-administered thyroid hormone can also mitigate
the
hypothyroid-inducing effects of the RXR agonist, thereby improving the
clinical safety and
tolerability of the treatment. Thus, in preferred embodiments, thyroid hormone
is co-
administered with an RXR agonist to improve the efficacy of the treatment,
whether or not
administration of the RXR agonist has caused, or is expected to cause,
clinical
hypothyroidism. By administration of thyroid hormone in coordination or in
conjunction with
the RXR agonist it is meant that the manner of administration of each of these
two agents is
such that the physiologic effects of the two agents overlap in time. This does
not require that
the RXR agonist and thyroid hormone be contained in the same composition or
formulation,
or that they be administered as separate compositions at the same time, by the
same route of
administration, or on the same schedule, though in some embodiments any of the
foregoing
may be the case.
[0056] Suitable thyroxine doses are generally from about 5 pg/day to about 250
pg/day
orally initially with an increase in dose every 2-4 weeks as needed. In other
embodiments,
the suitable thyroxine dose is from about 5 pg/day to about 225 pg/day, from
about 7.5 pg/day
to about 200 pg/day, from about 10 pg/day to about 175 pg/day, from about 12.5
pg/day to
about 150 pg/day, from about 15 pg/day to about 125 pg/day, from about 17.5
pg/day to about
100 pg/day, from about 20 pg/day to about 100 pg/day, from about 22.5 pg/day
to about 100
pg/day, from about 25 pg/day to about 100 pg/day, from about 5 pg/day to about
200 pg/day,
from about 5 pg/day to about 100 pg/day, from about 7.5 pg/day to about 90
pg/day, from
about 10 pg/day to about 80 pg/day, from about 12.5 pg/day to about 60 pg/day,
or from about
15 pg/day to about 50 pg/day. Increases in dose are generally made in
increments of about
in-Vriav ahrmt 7 5 ini/riav hrmt 11) un/dav ahrmt 12 5 Uri/day, about 15
pg/day, about 20
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
14
pg/day, or about 25 pg/day. In certain embodiments, the suitable thyroid
hormone dose is a
dose able to produce serum levels of T4 in the top 50%, the top 60%, the top
70%, the top
80%, or the top 90% of the normal range for the testing laboratory. As the
normal range of T4
levels may vary by testing laboratory, the target T4 levels are based on
normal ranges
determined for each particular testing laboratory.
[0057] For each embodiment involving a combination of RXR agonist and Her2-
targeting
therapeutic agent, there is a parallel embodiment in which the combination
further comprises
thyroid hormone.
Pharmaceutical compositions and formulations
[0058] The various component active agents used in the herein described
treatments will
typically exist as pharmaceutical compositions or formulations. Such
compositions or
formulations may be a liquid formulation, semi-solid formulation, or a solid
formulation. A
formulation disclosed herein can be produced in a manner to form one phase,
such as, e.g.,
an oil or a solid. Alternatively, a formulation disclosed herein can be
produced in a manner to
form two phases, such as, e.g., an emulsion. A pharmaceutical composition
disclosed herein
intended for such administration may be prepared according to any method known
to the art
for the manufacture of pharmaceutical compositions.
[0059] Liquid formulations suitable for parenteral injection or for nasal
sprays may comprise
physiologically acceptable sterile aqueous or nonaqueous solutions,
dispersions, suspensions
or emulsions and sterile powders for reconstitution into sterile injectable
solutions or
dispersions. Formulations suitable for nasal administration may comprise
physiologically
acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions
or emulsions.
Examples of suitable aqueous and nonaqueous carriers, diluents, solvents or
vehicles include
water, ethanol, polyols (propylene glycol, polyethyleneglycol (PEG), glycerol,
and the like),
suitable mixtures thereof, vegetable oils (such as olive oil) and injectable
organic esters such
as ethyl oleate. Proper fluidity can be maintained, for example, by the use of
a coating such
as lecithin, by the maintenance of the required particle size in the case of
dispersions and by
the use of surfactants.
[0060] A pharmaceutical composition disclosed herein can optionally include a
pharmaceutically acceptable carrier that facilitates processing of an active
compound into
pharmaceutically acceptable compositions. As used herein, the term
"pharmaceutically
acceptable" refers to those compounds, materials, compositions, and/or dosage
forms which
are, within the scope of sound medical judgment, suitable for contact with the
tissues of human
beings and animals without excessive toxicity, irritation, allergic response,
or other problem
complications commensurate with a reasonable benefit/risk ratio. (This
definition is also
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
application to the phrase "pharmaceutically-acceptable salts"). As used
herein, the term
"pharmacologically acceptable carrier" is synonymous with "pharmacological
carrier" and
refers to any carrier that has substantially no long term or permanent
detrimental effect when
administered and encompasses terms such as "pharmacologically acceptable
vehicle,
stabilizer, diluent, additive, auxiliary, or excipient." Such a carrier
generally is mixed with an
active compound or permitted to dilute or enclose the active compound and can
be a solid,
semi-solid, or liquid agent. It is understood that the active compounds can be
soluble or can
be delivered as a suspension in the desired carrier or diluent. Any of a
variety of
pharmaceutically acceptable carriers can be used including, without
limitation, aqueous media
such as, e.g., water, saline, glycine, hyaluronic acid and the like; solid
carriers such as, e.g.,
starch, magnesium stearate, mannitol, sodium saccharin, talcum, cellulose,
glucose, sucrose,
lactose, trehalose, magnesium carbonate, and the like; solvents; dispersion
media; coatings;
antibacterial and antifungal agents; isotonic and absorption delaying agents;
or any other
inactive ingredient. Selection of a pharmacologically acceptable carrier can
depend on the
mode of administration. Except insofar as any pharmacologically acceptable
carrier is
incompatible with the active compound, its use in pharmaceutically acceptable
compositions
is contemplated. Non-limiting examples of specific uses of such pharmaceutical
carriers can
be found in Pharmaceutical Dosage Forms and Drug Delivery Systems (Howard C.
Ansel et
al., eds., Lippincott Williams & Wilkins Publishers, 7th ed. 1999); Remington:
The Science and
Practice of Pharmacy (Alfonso R. Gennaro ed., Lippincott, Williams & Wilkins,
20th ed. 2000);
Goodman & Gilman's The Pharmacological Basis of Therapeutics (Joel G. Hardman
et al.,
eds., McGraw-Hill Professional, 10th ed. 2001); and Handbook of Pharmaceutical
Excipients
(Raymond C. Rowe et al., APhA Publications, 4th edition 2003). These protocols
are routine
and any modifications are well within the scope of one skilled in the art and
from the teaching
herein.
[0061] A pharmaceutical composition disclosed herein can optionally include,
without
limitation, other pharmaceutically acceptable components (or pharmaceutical
components),
including, without limitation, buffers, preservatives, tonicity adjusters,
salts, antioxidants,
osmolality adjusting agents, physiological substances, pharmacological
substances, bulking
agents, emulsifying agents, wetting agents, sweetening or flavoring agents,
and the like.
Various buffers and means for adjusting pH can be used to prepare a
pharmaceutical
composition disclosed herein, provided that the resulting preparation is
pharmaceutically
acceptable. Such buffers include, without limitation, acetate buffers, borate
buffers, citrate
buffers, phosphate buffers, neutral buffered saline, and phosphate buffered
saline. It is
understood that acids or bases can be used to adjust the pH of a composition
as needed.
Pharmaceutically acceptable antioxidants include, without limitation, sodium
metabisulfite,
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
16
sodium thiosulfate, acetylcysteine, butylated hydroxyanisole, and butylated
hydroxytoluene.
Useful preservatives include, without limitation, benzalkonium chloride,
chlorobutanol,
thimerosal, phenylmercuric acetate, phenylmercuric nitrate, a stabilized oxy
chloro
composition, such as, e.g., sodium chlorite and chelants, such as, e.g., DTPA
or DTPA-
bisamide, calcium DTPA, and CaNaDTPA-bisamide. Tonicity adjustors useful in a
pharmaceutical composition include, without limitation, salts such as, e.g.,
sodium chloride,
potassium chloride, mannitol or glycerin and other pharmaceutically acceptable
tonicity
adjustor. The pharmaceutical composition may be provided as a salt and can be
formed with
many acids, including but not limited to, hydrochloric, sulfuric, acetic,
lactic, tartaric, malic,
succinic, etc. Salts tend to be more soluble in aqueous or other protonic
solvents than are the
corresponding free base forms. It is understood that these and other
substances known in
the art of pharmacology can be included in a pharmaceutical composition useful
in the
invention.
[0062] Pharmaceutical formulations suitable for administration by inhalation
include fine
particle dusts or mists, which may be generated by means of various types of
metered, dose
pressurized aerosols, nebulizers, or insufflators.
[0063] Semi-solid formulations suitable for topical administration include,
without limitation,
ointments, creams, salves, and gels. In such solid formulations, the active
compound may be
admixed with at least one inert customary excipient (or carrier) such as, a
lipid and/or
polyethylene glycol.
[0064] Solid formulations suitable for oral administration include capsules,
tablets, pills,
powders and granules. In such solid formulations, the active compound may be
admixed with
at least one inert customary excipient (or carrier) such as sodium citrate or
dicalcium
phosphate or (a) fillers or extenders, as for example, starches, lactose,
sucrose, glucose,
mannitol and silicic acid, (b) binders, as for example, carboxmethylcellulose,
alignates,
gelatin, polyvinylpyrrolidone, sucrose and acacia, (c) humectants, as for
example, glycerol, (d)
disintegrating agents, as for example, agar-agar, calcium carbonate, potato or
tapioca starch,
alginic acid, certain complex silicates and sodium carbonate, (e) solution
retarders, as for
example, paraffin, (f) absorption accelerators, as for example, quaternary
ammonium
compounds, (g) wetting agents, as for example, cetyl alcohol and glycerol
monostearate, (h)
adsorbents, as for example, kaolin and bentonite, and (i) lubricants, as for
example, talc,
calcium stearate, magnesium stearate, solid polyethylene glycols, sodium
lauryl sulfate or
mixtures thereof. In the case of capsules, tablets and pills, the dosage forms
may also
comprise buffering agents.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
17
[0065] The small molecule components of the various embodiments, that is, the
RXR
agonist, thyroid hormone, and Her2 kinase inhibitors are capable of being
formulated in solid,
oral dosage forms. The antibody components are generally formulated as liquids
typically for
intravenous infusion. In alternative embodiments the antibody components may
be supplied
in lyophilized form for reconstitution as a liquid locally at the site of
treatment, where they are
also typically infused intravenously into the patient. While intravenous
infusion is typical, in
alternative embodiments the antibody may be administered by another route of
administration,
such as subcutaneous injection or infusion.
Treatment
[0066] As used herein, the terms "treatment," "treating," and the like refer
to obtaining a
desired pharmacologic and/or physiologic effect. This may be observed directly
as a slowing
of tumor growth, stabilization of disease, or a partial or complete response
(that is, tumor
regression or elimination of tumors), or extended overall or disease-free
survival. Treatment
may also be observed as an amelioration or reduction of symptoms related to
the underlying
cancer. However, as cancer treatment, the disclosed embodiments' aim and
mechanism is
directed to inhibiting, stabilizing, or reducing tumor growth (including
metastases), or partially
or completely eliminating tumors, or extending overall or disease-free
survival; effects on other
cancer symptoms are secondary. Direct treatment of such other symptoms (for
example, pain,
nausea, loss of appetite, etc.) is not within the scope of treating cancer as
used herein. That
is, treating a symptom, for example, cachexia in a cancer patient is not
treating cancer.
However, an agent that treats cancer (e.g., has an impact on the growth and/or
spread of
cancer) may also ameliorate a symptom, such as cachexia, either indirectly,
through its effect
on the cancer, or directly, through a pleiotropic effect. A "therapeutically
effective amount"
refers to an amount effective, at dosages and for periods of time necessary,
to achieve a
desired therapeutic result. The therapeutically effective amount may vary
according to factors
such as the disease state, age, sex, and weight of the individual, and the
ability of the Her2-
targeted therapy, RXR agonist, and if used, thyroid hormone, to elicit a
desired response in
the individual.
[0067] However, the dose administered to a mammal, particularly a human, in
the context
of the present methods, should be sufficient to effect a therapeutic response
in the mammal
over a reasonable timeframe. One skilled in the art will recognize that the
selection of the
exact dose and composition and the most appropriate delivery regimen will also
be influenced
by inter alia the pharmacological properties of the formulation, the nature
and severity of the
condition being treated, and the physical condition and mental acuity of the
recipient, as well
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
18
as the potency of the specific compound, the age, condition, body weight, sex
and response
of the patient to be treated, and the stage/severity of the disease.
[0068] Treatment activity includes the administration of the medicaments,
dosage forms,
and pharmaceutical compositions described herein to a patient, especially
according to the
various methods of treatment disclosed herein, whether by a healthcare
professional, the
patient his/herself, or any other person. Treatment activities include the
orders, instructions,
and advice of healthcare professionals such as physicians, physician's
assistants, nurse
practitioners, and the like that are then acted upon by any other person
including other
healthcare professionals or the patient his/herself. In some embodiments,
treatment activity
can also include encouraging, inducing, or mandating that a particular
medicament, or
combination thereof, be chosen for treatment of a condition - and the
medicament is actually
used - by approving insurance coverage for the medicament, denying coverage
for an
alternative medicament, including the medicament on, or excluding an
alternative
medicament, from a drug formulary, or offering a financial incentive to use
the medicament,
as might be done by an insurance company or a pharmacy benefits management
company,
and the like. In some embodiments, treatment activity can also include
encouraging, inducing,
or mandating that a particular medicament be chosen for treatment of a
condition - and the
medicament is actually used - by a policy or practice standard as might be
established by a
hospital, clinic, health maintenance organization, medical practice or
physicians group, and
the like.
[0069] To benefit from the combined effect of a RXR agonist of Formula I (or
pharmaceutically acceptable salt thereof) and Her2-targeted therapeutics,
embodiments
include methods of treatment comprising or consisting of administering RXR
agonist of
Formula I (or pharmaceutically acceptable salt thereof) and a Her2-targeted
therapeutic to a
patient having a Her2+ cancer. Some embodiments further comprise
administration of thyroid
hormone in coordination with administration of the RXR agonist. In some
embodiments the
Her2+ cancer is a Her2+ breast cancer. In some embodiments the Her2+ cancer is
a Her2+
gastroesophageal cancer, ovarian cancer, stomach cancer, adenocarcinoma of the
lung,
uterine cancer (such as serous endometrial carcinoma), or salivary duct
carcinoma. Some
embodiments specifically include one or more of these cancers. Other
embodiments
specifically exclude one or more of these cancers.
[0070] In various embodiments the herein disclosed treatments may be applied
as a primary
therapy, as a debulking therapy prior to surgical removal of tumor, or as an
adjuvant therapy
subsequent to any mode of primary therapy (especially surgery) to address
residual disease
and/or lower the risk of recurrent cancer.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
19
[0071] In some embodiments the patient having a Her2+ cancer has not been
previously
treated with either RXR agonist of Formula I (or pharmaceutically acceptable
salt thereof) or
a Her2-targeted therapeutic. In some embodiments the patient has been
previously treated
with RXR agonist of Formula I (or pharmaceutically acceptable salt thereof)
and has achieved
stable disease or a partial response (in some embodiments, as defined by
RECIST or iRECIST
criteria) - that is, the cancer is sensitive to RXR agonist of Formula I (or
pharmaceutically
acceptable salt thereof) - and a Her2-targeted therapeutic is added to the
treatment regimen.
In some embodiments the patient has been previously treated with a Her2-
targeted
therapeutic and has achieved stable disease or a partial response (in some
embodiments, as
defined by RECIST or iRECIST criteria) - that is, the cancer is sensitive to a
Her2-targeted
therapeutic - and RXR agonist of Formula I (or pharmaceutically acceptable
salt thereof) is
added to the treatment regimen.
[0072] Thus some embodiments entail administration of an RXR agonist to a
patient with a
Her2+ tumor who has received, is receiving, or is scheduled to receive, a Her2-
targeted
therapeutic agent. Some embodiments entail administration of an RXR agonist to
a patient in
whom a Her2-targeted therapeutic agent has had some therapeutic effect (less
than a
complete response), that is administration of the RXR agonist is added to the
therapeutic
regimen for the Her2-targeted therapeutic agent. Some embodiments entail
administration of
a Her2-targeted therapeutic agent to a patient in whom an RXR agonist (or RXR
agonist in
conjunction with thyroid hormone) has had some therapeutic effect (less than a
complete
response), that is administration of the Her2-targeted therapeutic agent is
added to the
therapeutic regimen for the RXR agonist.
[0073] Therapeutic efficacy can be monitored by periodic assessment of treated
patients.
For repeated administrations over several days or longer, the treatment can be
repeated until
a desired suppression of disease or disease symptoms occurs. However, other
dosage
regimens may be useful and are within the scope of the present disclosure.
Antibodies typically
have a much longer half-life in the body than the other active agents used in
these methods
and therefore there will typically be substantially longer intervals (measured
in weeks) between
administrations.
[0074] The effectiveness of cancer therapy is typically measured in terms of
"response."
The techniques to monitor responses can be similar to the tests used to
diagnose cancer such
as, but not limited to:
= A lump or tumor involving some lymph nodes can be felt and measured
externally
by physical examination.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
= Some internal cancer tumors will show up on an x-ray or CT scan and can
be
measured with a ruler.
= Blood tests, including those that measure organ function can be
performed.
= A tumor marker test can be done for certain cancers.
[0075] Regardless of the test used, whether blood test, cell count, or tumor
marker test, it
is repeated at specific intervals so that the results can be compared to
earlier tests of the same
type.
[0076] Response to cancer treatment is defined several ways:
= Complete response - all of the cancer or tumor disappears; there is no
evidence
of disease. Expression level of tumor marker (if applicable) may fall within
the
normal range.
= Partial response - the cancer has shrunk by a percentage but disease
remains.
Levels of a tumor marker (if applicable) may have fallen (or increased, based
on
the tumor marker, as an indication of decreased tumor burden) but evidence of
disease remains.
= Stable disease - the cancer has neither grown nor shrunk; the amount of
disease has not changed. A tumor marker (if applicable) has not changed
significantly.
= Disease progression - the cancer has grown; there is more disease now
than
before treatment. A tumor marker test (if applicable) shows that a tumor
marker
has risen.
[0077] Other measures of the efficacy of cancer treatment include intervals of
overall
survival (that is time to death from any cause, measured from diagnosis or
from initiation of
the treatment being evaluated)), cancer-free survival (that is, the length of
time after a
complete response cancer remains undetectable), and progression-free survival
(that is, the
length of time after disease stabilization or partial response that resumed
tumor growth is not
detectable).
[0078] There are two standard methods for the evaluation of solid cancer
treatment
response with regard to tumor size (tumor burden), the WHO and RECIST
standards. These
methods measure a solid tumor to compare a current tumor with past
measurements or to
compare changes with future measurements and to make changes in a treatment
regimen. In
the WHO method, the solid tumor's long and short axes are measured with the
product of
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
21
these two measurements is then calculated; if there are multiple solid tumors,
the sum of all
the products is calculated. In the RECIST method, only the long axis is
measured. If there
are multiple solid tumors, the sum of all the long axes measurements is
calculated. However,
with lymph nodes, the short axis is measured instead of the long axis. There
is also a variation
of the RECIST method for immunotherapies (iRECIST) which takes into account
distinctive
behaviors linked to these types of therapeutics, such as delayed responses
after
pseudoprogression. Both the RECIST 1.1 guidelines and the iRecist guidelines
are
incorporated by reference herein in their entirety.
[0079] In some embodiments of the herein disclosed methods, the tumor burden
of a treated
patient is reduced by about 5%, about 10%, about 15%, about 20%, about 25%,
about 30%,
about 35%, about 40%, about 45%, about 50%, about 55% about 60%, about 65%,
about
70%, about 75%, about 80%, about 90%, about 95%, about 100%, or any range
bound by
these values.
[0080] In other embodiments, the 1-year survival rate of treated subjects is
increased by
about 5 /0, about 10%, about 15%, about 20 /o, about 25 /o, about 30%, about
35 /o, about 40%,
about 45%, about 50%, about 55% about 60%, about 65%, about 70%, about 75%,
about
80%, about 90%, about 95%, about 100%, or any range bound by these values.
[0081] In other embodiments, the 5-year survival rate of treated subjects is
increased by
about 5 /0, about 10%, about 15%, about 20 /o, about 25 /o, about 30%, about
35 /o, about 40%,
about 45%, about 50%, about 55% about 60%, about 65%, about 70%, about 75%,
about
80%, about 90%, about 95%, about 100%, or any range bound by these values.
[0082] In other embodiments, the 10-year survival rate of treated subjects is
increased by
about 5 /0, about 10%, about 15%, about 20 /o, about 25 /o, about 30%, about
35 /o, about 40%,
about 45%, about 50%, about 55% about 60%, about 65%, about 70%, about 75%,
about
80%, about 90%, about 95%, about 100%, or any range bound by these values.
[0083] In yet other embodiments, the subject has a sustained remission of at
least 6 months,
at least 7 months, at least 8 months, at least 9 months, at least 10 months,
at least 11 months,
at least 12 months, at least 14 months, at least 16 months, at least 18
months, at least 20
months, at least 22 months, at least 24 months, at least 27 months, at least
30 months, at
least 33 months, at least 36 months, at least 42 months, at least 48 months,
at least 54 months,
or at least 60 months or more.
[0084] In other embodiments, the methods may additionally help to treat or
alleviate
conditions, symptoms, or disorders related to cancer. In some embodiments,
these conditions
or symptoms may include, but are not limited to, anemia, asthenia, cachexia,
Cushing's
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
22
Syndrome, fatigue, gout, gum disease, hematuria, hypercalcemia,
hypothyroidism, internal
bleeding, hair loss, mesothelioma, nausea, night sweats, neutropenia,
paraneoplastic
syndromes, pleuritis, polymyalgia rheumatica, rhabdomyolysis, stress, swollen
lymph nodes,
thrombocytopenia, Vitamin D deficiency, or weight loss. While a cancer
treatment may reduce
or treat associated symptoms, treating symptoms associated with cancer, is not
treating
cancer if there is no expectation that tumor will be reduced or eliminated or
their growth or
spread will be inhibited.
[0085] Toxicities and adverse events are sometimes graded according to a 5
point scale. A
grade 1 or mild toxicity is asymptomatic or induces only mild symptoms; may be
characterized
by clinical or diagnostic observations only; and intervention is not
indicated. A grade 2 or
moderate toxicity may impair activities of daily living (such as preparing
meals, shopping,
managing money, using the telephone, etc.) but only minimal, local, or non-
invasive
interventions are indicated. Grade 3 toxicities are medically significant but
not immediately life-
threatening; hospitalization or prolongation of hospitalization is indicated;
activities of daily
living related to self-care (such as bathing, dressing and undressing, feeding
oneself, using
the toilet, taking medications, and not being bedridden) may be impaired.
Grade 4 toxicities
are life-threatening and urgent intervention is indicated. Grade 5 toxicity
produces an adverse
event-related death. Thus in various embodiments, use of an RXR agonist, or
RXR agonist
and thyroid hormone, reduces the grade of a toxicity that would otherwise be
associated with
use of the Her2-targered therapy, by allowing a lower dose to be used without
substantial
sacrifice of efficacy. In some embodiments, use of an RXR agonist, or RXR
agonist and thyroid
hormone, in combination with the Her2-targeted therapeutic agent limits a
toxicity to grade 1
or less, or produces no observation of the toxicity, without substantial
reduction of efficacy as
would be expected from the Her2-targeted therapeutic agent alone. In some
embodiments,
the combined use of the Her2-targeted therapeutic agent and the RXR agonist,
or RXR agonist
and thyroid hormone, allows continued use of the Her2-targeted therapeutic
agent at a lower
dosage with therapeutic effect in instances where treatment with the Her2-
targeted therapeutic
agent would have had to have been discontinued due to unacceptable toxicity.
In some of
these embodiments, the Her2-targeted therapeutic agent comprises a Her2 kinase
inhibitor.
[0086] The combination of the disclosed RXR agonists and the Her2 targeted
therapeutics
are synergistic in effect. That is, they interact in a positive manner to
produce a greater
inhibition of tumor cell growth, than would be expected from the independent
(non-interacting)
effects of the two. Thus, some embodiments produce improved efficacy. Other
embodiments
allow for the reduction of dosage in order to reduce toxicity while still
achieving at least similar
efficacy as provided by an individual therapeutic agent. In some embodiments,
both reduced
tnxicitv and imnmved efficarm ( mnared tn the mnre tnxic single agent) is
achieved.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
23
[0087] For each method of treatment there are further parallel embodiments
related to the
foregoing methods directed to use of the RXR agonist in conjunction with a
Her2-targeted
therapeutic agent, or a Her2-targeted therapeutic agent and thyroid hormone,
to treat Her2+
cancer; directed to use of the RXR agonist in the manufacture of a medicament
for use in
combination with a Her2-targeted therapeutic agent, or a Her2-targeted
therapeutic agent and
thyroid hormone, to treat Her2+ cancer.
[0088] Further embodiments include a combination comprising a RXR agonist as
herein
described and a Her2-targeted therapeutic agent. Some embodiments further
comprise a
thyroid hormone. In some embodiments, the Her2-targeted therapeutic agent is
an anti-Her2
antibody. In some embodiments the Her2-targeted therapeutic agent is a Her2
kinase inhibitor.
[0089] Further embodiments include kits comprising the above combinations. The
kits may
additionally comprise solvents, diluents, injectors, and the like that may
facilitate
administration of one or more of the therapeutic agents. The kits may further
comprise
instructions for the coordinated use of the therapeutic agents utilized in the
disclosed methods,
whether or not any particular agent is supplied in the kit.
LIST OF PARTICULAR EMBODIMENTS
[0090] The following listing of embodiments is illustrative of the variety of
embodiments with
respect to breadth, combinations and sub-combinations, class of invention,
etc., elucidated
herein, but is not intended to be an exhaustive enumeration of all embodiments
finding support
herein.
[0091] Embodiment 1. A method of treating a patient with Her2+ cancer
comprising
administering a RXR agonist of Formula I,
A
N,
f
co2R.
,..--
N (Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, to the patient,
wherein the patient has received, is receiving, or is scheduled to receive a
Her2-
targeted therapeutic agent.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
24
[0092] Embodiment 2. A method
of treating a patient with Her2+ cancer comprising
administering a RXR agonist of Formula I,
A A 1i
-
(Th
õ
CO2R
-A\ (Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, and a Her2-targeted therapeutic agent.
[0093] Embodiment 3. A method
of treating a patient with Her2+ cancer undergoing
treatment with a RXR agonist of Formula I,
';=A
., }-1
(
\--e
co-A
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof,
wherein there is evidence of therapeutic effect that is less than a complete
response,
comprising continuing treatment with the RXR agonist and initiating treatment
with a Her2-
targeted therapeutic agent.
[0094] Embodiment 4. A method
of treating a patient with Her2+ cancer undergoing
treatment with a Her2-targeted therapeutic agent, wherein there is evidence of
therapeutic
effect that is less than a complete response, comprising continuing treatment
with the Her2-
targeted therapeutic agent and initiating treatment with a RXR agonist of
Formula I,
,.,
I
I
CO21-1.
\
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof.
[0095] Embodiment 5. A method
of treating a patient with Her2+ cancer comprising
administering a RXR agonist of Formula I,
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
4*> A ,04
t\--)
COA
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, to the patient,
wherein the patient has received, is receiving, or is scheduled to receive
means for
therapeutically targeting Her2.
[0096] Embodiment 6. The
method of Embodiment 5, wherein the means for
therapeutically targeting Her2 are:
means for inhibiting Her2 + tumor cell proliferation,
means for mediating ADCC of Her2 + tumor cells,
immunoglobulin means for inhibiting Her2 signaling,
means for delivering a cytotoxic agent to Her2 + cells, or
small molecule means for inhibiting Her2 kinase activity.
[0097] Embodiment 7. A method
of treating a patient with Her2 + cancer comprising
administering means for activating RXR/Nurr1 heterodimeric receptors or
rexinoid means for
inhibiting tumor growth, wherein the patient has received, is receiving, is
scheduled to receive
a Her2-targeted therapeutic agent.
[0098] Embodiment 8. The
method of any one of Embodiments 1-7, further comprising
administering thyroid hormone in conjunction with the RXR agonist.
[0099] Embodiment 9. The
method of Embodiment 8, wherein the thyroid hormone is
thyroxine.
[0100] Embodiment 10. The
method of any one of Embodiments 1-9, wherein the RXR
agonist, the means for activating RXR/Nurr1 heterodimeric receptors, or the
rexinoid means
for inhibiting tumor growth, is a compound of Formula I.
[0101] Embodiment 11. The
method of any one of Embodiments 1-9, wherein the RXR
agonist, the means for activating RXR/Nurr1 heterodimeric receptors, or the
rexinoid means
for inhibiting tumor growth, is a pharmaceutically-acceptable salt of a
compound of Formula I.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
26
[0102] Embodiment 12. The method of any one of Embodiments 1-9, wherein the
RXR
agonist, the means for activating RXR/Nurr1 heterodimeric receptors, or the
rexinoid means
for inhibiting tumor growth, is a compound of Formula ll
(Formula II).
[0103] Embodiment 13. The method of any one of Embodiments 1-12, wherein:
the Her2-targeted therapeutic agent,
the means for inhibiting Her2 + tumor cell proliferation,
the means for mediating ADCC of Her2 + tumor cells,
the immunoglobulin means for inhibiting Her2 signaling, or
the means for delivering a cytotoxic agent to Her2 + cells,
comprises an anti-Her2 therapeutic antibody.
[0104] Embodiment 14. The method of Embodiment 13, wherein the therapeutic
antibody is trastuzumab or pertuzumab.
[0105] Embodiment 15. The method of Embodiment 13, wherein the therapeutic
antibody is margetuximab, TrasGEX, HM2, hertuzumab, or HT-19
[0106] Embodiment 16. The method of any one of Embodiments 1-13, wherein
the Her2-
targeted therapeutic agent, the means for inhibiting Her2 + tumor cell
proliferation, or the
immunoglobulin means for inhibiting Her2 signaling, comprises an antibody-drug
conjugate
wherein the antibody is an anti-Her2 antibody.
[0107] Embodiment 17. The method of Embodiment 16, wherein the antibody-
drug
conjugate or the means for delivering a cytotoxic agent to Her2 + cells, is
ado-trastuzumab
emtansine.
[0108] Embodiment 18. The method of Embodiment 16, wherein the antibody-
drug
conjugate or the means for delivering a cytotoxic agent to Her2 + cells, is
A166, ALT-P7,
ARX788, DHES0815A, DS-8201a, RC48, SYD985, MEDI4276, or XMT-1522.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
27
[0109] Embodiment 19. The
method of any one of Embodiments 1-12, wherein the Her2-
targeted therapeutic agent, or the small molecule means for inhibiting Her2
kinase activity,
comprises a Her2 kinase inhibitor.
[0110] Embodiment 20. The
method of Embodiment 19, wherein the Her2 kinase
inhibitor is lapatinib or neratinib.
[0111] Embodiment 21. The
method of Embodiment 19, wherein the Her2 kinase
inhibitor is afatimb or dacomitinib.
[0112] Embodiment 22. The
method of any one of Embodiments 1-21, wherein the
treatment is applied as a debulking therapy.
[0113] Embodiment 23. The
method of any one of Embodiments 1-21, wherein the
treatment is applied as adjuvant therapy.
[0114] Embodiment 24. The
method of any one of Embodiments 1-23, wherein the Her2+
cancer is Her2 + breast cancer.
[0115] Embodiment 25. The
method of any one of Embodiments 1-23, wherein the Her2+
cancer is Her2 + gastroesophageal cancer, ovarian cancer, stomach cancer,
adenocarcinoma
of the lung, uterine cancer (such as serous endometrial carcinoma), or
salivary duct
carcinoma.
[0116] Embodiment 26. The
method of any one of Embodiments 1-25, wherein a
therapeutic response to the RXR agonist and the Her2-targeted therapeutic
agent is greater
than to the response to either of the agents alone.
[0117] Embodiment 27. The
method of Embodiment 26, wherein the greater therapeutic
response is a slowing of tumor growth, stabilization of disease, a partial
response, a complete
response, extended overall survival, or disease-free survival.
[0118] Embodiment 28. The
method of Embodiment 26 or 27, wherein response is
evaluated according to RECIST or iRECIST criteria.
[0119] Embodiment 29. The
method of any one of Embodiments 26-28, comprising a
reduction or amelioration of secondary symptoms.
[0120] Embodiment 30. A
combination comprising a Her2-targeted therapeutic agent, or
means for therapeutically targeting Her2, and a RXR agonist of Formula I,
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
28
4*> A ,04
t\--)
COA
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof.
[0121] Embodiment 31. The combination of Embodiment 30, wherein the Her2-
targeted
therapeutic agent, or means for therapeutically targeting Her2, comprises:
means for inhibiting Her2 + tumor cell proliferation,
means for mediating ADCC of Her2 + tumor cells,
immunoglobulin means for inhibiting Her2 signaling,
means for delivering a cytotoxic agent to Her2 + cells, or
small molecule means for inhibiting Her2 kinase activity.
[0122] Embodiment 32. The combination of Embodiment 30 or 31, further
comprising
thyroid hormone.
[0123] Embodiment 33. The combination of any one of Embodiments 30-32,
wherein the
RXR agonist is a compound of Formula ll
, t
0 0
(Formula II).
[0124] Embodiment 34. The combination of any one of Embodiments 30-33,
wherein:
the Her2-targeted therapeutic agent,
the means for inhibiting Her2 + tumor cell proliferation,
the means for mediating ADCC of Her2 + tumor cells,
the immunoglobulin means for inhibiting Her2 signaling, or
the means for delivering a cytotoxic agent to Her2 + cells,
rnmnriec,c nn nnfi_I-1=e) fh=rnr1=1 nnfihnt-h:
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
29
[0125] Embodiment 35. The combination of Embodiment 34, wherein the
therapeutic
antibody is trastuzumab or pertuzumab.
[0126] Embodiment 36. The combination of any one of Embodiments 30-34,
wherein the
Her2-targeted therapeutic agent, the means for inhibiting Her2 + tumor cell
proliferation, or the
immunoglobulin means for inhibiting Her2 signaling, comprises an antibody-drug
conjugate
wherein the antibody is an anti-Her2 antibody.
[0127] Embodiment 37. The combination of Embodiment 36, wherein the
antibody-drug
conjugate is ado-trastuzumab emtansine.
[0128] Embodiment 38. The combination of any one of Embodiments 30-33,
wherein the
Her2-targeted therapeutic agent, or the small molecule means for inhibiting
Her2 kinase
activity, comprises a Her2 kinase inhibitor.
[0129] Embodiment 39. The combination of Embodiment 38, wherein the Her2
kinase
inhibitor is lapatinib or neratinib.
[0130] Embodiment 40. A kit comprising the combination of any one of
Embodiments
30-39.
[0131] Embodiment 41. A RXR agonist of Formula I,
I %
f
aka
?==".
N
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, for use in treating a patient with Her2 + cancer, wherein the patient
has received, is
receiving, or is scheduled to receive a Her2-targeted therapeutic agent.
[0132] Embodiment 42. A RXR agonist of Formula I,
<1,
\;;.;=
,,==" "Ny." --.`" 'NZ= =
ZN, _____________________________________
k`==>*,/ /
CO=IR
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, and a Her2-targeted therapeutic agent for use in combination in
treating a patient with
Her2 + cancer.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
[0133] Embodiment 43. A combination of a RXR agonist of Formula I,
-:;,, A H
,..--',,,, s ',.el \s,
.-- .,=-...,- _...---
,,-- --,,
t ,
.....-1
.--''''== ,--' 7"¨".--\
\C=0=ZR
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, and a Her2-targeted therapeutic agent for use in treating a patient
with Her2+ cancer.
[0134] Embodiment 44. A pharmaceutical combination for treating Her2+
cancer
comprising a RXR agonist of Formula I,
A
__,.."'-4........._,`
esU,...¨
\ ,.?--...,...,
\,,, / \
N>Kr '-'-
.... -... (Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, and a Her2-targeted therapeutic agent.
[0135] Embodiment 45. A RXR agonist of Formula I,
{ ,.-,,..õ.. "
'\=:,,,..
, i \
i \s,..õ-/ / \
µ-... ./'-.. .-''' CO2R,
"õ.
...,,,,,,
N'N (Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, for use in manufacturing a medicament for treating Her2+ cancer, in a
patient who has
received, is receiving, or is scheduled to receive a Her2-targeted therapeutic
agent.
[0136] Embodiment 46. A RXR agonist of Formula I,
A
---.,
,õ,-.' -,..,.,-=.._ N. ,---- ---- --..,
( i ---N __
,.õ....,,, i --, /,,.._\ ,.....-- -,... ,...--- coR.,
, ,.
,-.>N N- (Formula I)
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
31
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, and a Her2-targeted therapeutic agent, for use in manufacturing
medicaments for use
together to treat Her2+ cancer.
[0137] Embodiment 47. A Her2-targeted therapeutic agent for treating Her2+
cancer in
a patient with Her2+ cancer, with a therapeutic response less than a complete
response to
treatment with a RXR agonist of Formula I,
/'"\=
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof,
in combination with the RXR agonist.
[0138] Embodiment 48. A RXR agonist of Formula I,
A ..}1
()
co,R
(Formula I)
wherein R is H, or lower alkyl of 1 to 6 carbons; or a pharmaceutically-
acceptable salt
thereof, for treating Her2+ cancer in a patient with Her2+ cancer, with a
therapeutic response
less than a complete response to treatment with a Her2-targeted therapeutic
agent,
in combination with the Her2-targeted therapeutic agent.
[0139] It should be manifest that each or Embodiments 41-48 can be modified in
a manner
similar to the modification of Embodiments 1-5 and 7 by Embodiments 5 and 8-
29.
EXAMPLES
[0140] The following non-limiting examples are provided for illustrative
purposes only in
order to facilitate a more complete understanding of representative
embodiments now
contemplated. These examples should not be construed to limit any of the
embodiments
described in the present specification,
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
32
Example 1
Inhibition of Breast Cancer Cell Growth by IRX4204 plus Trastuzumab
[0141] Two breast cancer cell lines, MCF7 and SkBr3 were cultured in the
presence of 0,
10, 100, or 1000 nM IRX4204 and 0, 0.1, 1, or 10 pg/ml trastuzumab. MCF7 is an
ER + PR+
Her2- cell line. SkBr3 is an ER- PR- Her2 + cell line. Cells were plated in 96
well optical plates
and IRX-4204 and trastuzumab were added 24 hours after cell plating. After a
further 6 days,
the cells were fixed with 4% paraformaldehyde in phosphate buffered saline
(PBS). Nuclei
were then stained with DAPI and imaged with the MetaXpresse microscope
(Molecular
Devices, San Jose, CA). Cell nuclei were segmented then counted by defining
pixel intensity
over background and object size, using the algorithm of the MetaXpresse image
analysis
software. Experimental data points were performed at a minimum of
quadruplicate, and results
were reported as average cell count standard deviation (SD). As seen in
Figure 1A, neither
agent had more than marginal effect on the Her2- MCF7 cell line. As seen in
Figure 1B, both
agents individually have a moderate growth inhibitory effect on the Her2 +
SkBr3 cell line, and
had a very substantial, growth inhibitory effect when used in combination,
even at the lowest
concentrations tested.
[0142] To assess if this improved growth inhibition effect was synergistic,
the percent
inhibition observed for the highest concentrations of the combined therapeutic
agents was
compared to the percent inhibition that would be expected for the combination
based on the
observed inhibition of the therapeutic agents used alone, if the agents acting
independently,
that is, without interaction (see Table 1). That is, PAE = (FE1 + ((1 - FE1) x
FE2)) x 100, where
PAE is the predicted additive effect, FE1 is the observed fractional effect of
a 1st treatment,
and FE2 is the observed fractional effect of a 2nd treatment.
Table 1. Synergistic Effect of the Combination of IRX4204 and Trastuzumab on
the
Inhibition of the Growth of SkBr3 Cells.
Treatment DAPI Nuclei Count % Inhibition
Control 25958
IRX4204 1000 nM 18274 29.4
If Additive = 68.5%
Herceptin 1000 ng 11582 55.4
IRX4204 + Herceptin 2946 88.7
[0143] Whereas the combined inhibitory effect of IRX4204 and trastuzumab would
be
predicted to be 68.5% if there was no interaction between the effects of the
two agents, in fact
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
33
the observed degree of inhibition was 88.7%, clearly indicating that IRX4204
and trastuzumab
interact in a synergistic manner.
Example 2
Inhibition of Breast Cancer Cell Growth by IRX4204 plus Lapatinib or Neratinib
[0144] The experiment of Example 1 was repeated using the Her2 kinase
inhibitors lapatinib
or neratinib instead of trastuzumab at 0, 0.1, 1, or 10 nM. Additionally the
panel of breast
cancer cell lines was expanded to also include the ER + PR + Her2 + cell line
BT474 and the ER+
PR- Her2 + cell line MDA-MB-361. The general pattern seen above, that the Her2-
MCF7 cell
line showed generally marginal response to the treatments and that the Her
cell lines
exhibited a greater degree of inhibition to the combination than either agent
alone, was again
observed (see Figures 2 and 3).
[0145] To assess if this improved growth inhibition effect was synergistic,
the percent
inhibition observed for the highest concentrations of the combined therapeutic
agents was
compared to the percent inhibition that would be expected for the combination
based on the
observed inhibition of the therapeutic agents used alone, if the agents acting
independently,
that is, without interaction (see Tables 2-4).
Table 2. Synergistic Effect of the Combination of IRX4204 and Lapatinib or
Neratinib on
the Inhibition of the Growth of SkBr3 Cells.
Treatment DAPI Nuclei Count % Inhibition
Control 19855
1RX4204 1000 nM 18906 4.8
If Additive = 89.4%
Lapatinib 1000 nM 2211 88.9
IRX4204 + Lapatinib 743 96.3
Control 6696
IRX4204 1000 nM 5218 22.1
If Additive = 89.5%
Neratinib 1000 nM 903 86.5
IRX4204 + Neratinib 329 95.1
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
34
Table 3. Synergistic Effect of the Combination of IRX4204 and Lapatinib or
Neratinib on
the Inhibition of the Growth of BT474 Cells.
Treatment DAPI Nuclei Count % Inhibition
Control 2937
IRX4204 1000 nM 3399 -15.7*
If Additive = 58.8%
Lapatinib 1000 nM 1211 58.8
IRX4204 + Lapatinib 813 72.3
Control 2192
IRX4204 1000 nM 2043 6.8
If Additive = 92.4%
Neratinib 1000 nM 180 91.8
IRX4204 + Neratinib 130 94.1
*Zero inhibition used in calculation of predicted additive effect
Table 4. Synergistic Effect of the Combination of IRX4204 and Lapatinib or
Neratinib on
the Inhibition of the Growth of MDA-MB-361 Cells.
Treatment DAPI Nuclei Count % Inhibition
Control 6696
IRX4204 1000 nM 5218 22.1
If Additive = 89.5%
Lapatinib 1000 nM 903 86.5
IRX4204 + Lapatinib 329 95.2
Control 5297
IRX4204 1000 nM 2459 43.6
If Additive = 97.3%
Neratinib 1000 nM 249 95.3
IRX4204 + Neratinib 57 98.9
[0146] In each case the observed degree of inhibition exceeded that predicted
if there was
no interaction between the effects of the two agents, even though in some
cases the individual
therapeutic agents were quite effective alone, leaving little room for synergy
to be observed.
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
These data also clearly indicate that IRX4204 and the small molecule Her2
kinase inhibitors
interact in a synergistic manner.
[0147] In closing, it is to be understood that although aspects of the present
specification
are highlighted by referring to specific embodiments, one skilled in the art
will readily
appreciate that these disclosed embodiments are only illustrative of the
principles of the
subject matter disclosed herein. Therefore, it should be understood that the
disclosed subject
matter is in no way limited to a particular methodology, protocol, and/or
reagent, etc.,
described herein. As such, various modifications or changes to or alternative
configurations
of the disclosed subject matter can be made in accordance with the teachings
herein without
departing from the spirit of the present specification. Lastly, the
terminology used herein is for
the purpose of describing particular embodiments only, and is not intended to
limit the scope
of the present invention, which is defined solely by the claims. Accordingly,
the present
invention is not limited to that precisely as shown and described.
[0148] Certain embodiments of the present invention are described herein,
including the
best mode known to the inventors for carrying out the invention. Of course,
variations on these
described embodiments will become apparent to those of ordinary skill in the
art upon reading
the foregoing description. The inventor expects skilled artisans to employ
such variations as
appropriate, and the inventors intend for the present invention to be
practiced otherwise than
specifically described herein. Accordingly, this invention includes all
modifications and
equivalents of the subject matter recited in the claims appended hereto as
permitted by
applicable law. Moreover, any combination of the above-described embodiments
in all
possible variations thereof is encompassed by the invention unless otherwise
indicated herein
or otherwise clearly contradicted by context.
[0149] Groupings of alternative embodiments, elements, or steps of the present
invention
are not to be construed as limitations. Each group member may be referred to
and claimed
individually or in any combination with other group members disclosed herein.
It is anticipated
that one or more members of a group may be included in, or deleted from, a
group for reasons
of convenience and/or patentability. When any such inclusion or deletion
occurs, the
specification is deemed to contain the group as modified thus fulfilling the
written description
of all Markush groups used in the appended claims.
[0150] Unless otherwise indicated, all numbers expressing a characteristic,
item, quantity,
parameter, property, term, and so forth used in the present specification and
claims are to be
understood as being modified in all instances by the term "about." As used
herein, the term
"about" means that the characteristic, item, quantity, parameter, property, or
term so qualified
encompasses a range of plus or minus ten percent above and below the value of
the stated
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
36
characteristic, item, quantity, parameter, property, or term. Accordingly,
unless indicated to
the contrary, the numerical parameters set forth in the specification and
attached claims are
approximations that may vary. At the very least, and not as an attempt to
limit the application
of the doctrine of equivalents to the scope of the claims, each numerical
indication should at
least be construed in light of the number of reported significant digits and
by applying ordinary
rounding techniques. Notwithstanding that the numerical ranges and values
setting forth the
broad scope of the invention are approximations, the numerical ranges and
values set forth in
the specific examples are reported as precisely as possible. Any numerical
range or value,
however, inherently contains certain errors necessarily resulting from the
standard deviation
found in their respective testing measurements. Recitation of numerical ranges
of values
herein is merely intended to serve as a shorthand method of referring
individually to each
separate numerical value falling within the range. Unless otherwise indicated
herein, each
individual value of a numerical range is incorporated into the present
specification as if it were
individually recited herein.
[0151] The terms "a," "an," "the" and similar referents used in the context of
describing the
present invention (especially in the context of the following claims) are to
be construed to cover
both the singular and the plural, unless otherwise indicated herein or clearly
contradicted by
context. All methods described herein can be performed in any suitable order
unless
otherwise indicated herein or otherwise clearly contradicted by context. The
use of any and
all examples, or exemplary language (e.g., "such as") provided herein is
intended merely to
better illuminate the present invention and does not pose a limitation on the
scope of the
invention otherwise claimed. No language in the present specification should
be construed
as indicating any non-claimed element essential to the practice of the
invention.
[0152] Specific embodiments disclosed herein may be further limited in the
claims using
consisting of or consisting essentially of language. When used in the claims,
whether as filed
or added per amendment, the transition term "consisting of" excludes any
element, step, or
ingredient not specified in the claims. The transition term "consisting
essentially of" limits the
scope of a claim to the specified materials or steps and those that do not
materially affect the
basic and novel characteristic(s). Embodiments of the present invention so
claimed are
inherently or expressly described and enabled herein.
[0153] All patents, patent publications, and other publications referenced and
identified in
the present specification are individually and expressly incorporated herein
by reference in
their entirety for the purpose of describing and disclosing, for example, the
compositions and
methodologies described in such publications that might be used in connection
with the
present invention. These publications are provided solely for their disclosure
prior to the filing
CA 03142076 2021-11-25
WO 2020/251556
PCT/US2019/036594
37
date of the present application. Nothing in this regard should be construed as
an admission
that the inventors are not entitled to antedate such disclosure by virtue of
prior invention or for
any other reason. All statements as to the date or representation as to the
contents of these
documents is based on the information available to the applicants and does not
constitute any
admission as to the correctness of the dates or contents of these documents.