Note: Descriptions are shown in the official language in which they were submitted.
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
- / ¨
MODIFIED PROSTHETIC HEART VALVE STENT
CROSS REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of U.S. Patent Application No.
62/907,476,
filed September 27, 2019, the entire disclosure which is incorporated by
reference for all
purposes.
TECHNICAL FIELD
[0002] The present disclosure generally relates to controlled expansion of
a
prosthetic heart valve stent and, more particularly, to modifications and/or
asymmetric
expansion of a subvalvular stent to avoid compression and potential mechanical
injury
to the heart's electrical conduction system.
BACKGROUND
[0003] Heart valve disease continues to be a significant cause of morbidity
and
mortality, resulting from a number of ailments including rheumatic fever and
birth
defects. Currently, the primary treatment of aortic valve disease is valve
replacement.
Worldwide, an estimated 300,000 heart valve replacement surgeries are
performed
annually. Many patients receive bioprosthetic heart valve replacements, which
utilize
biologically derived tissues for flexible fluid occluding leaflets. The most
successful
bioprosthetic materials for flexible leaflets are whole porcine valves and
separate
leaflets made from bovine pericardium stitched together to form a tri-leaflet
valve. The
most common flexible leaflet valve construction includes three leaflets
mounted to
commissure posts around a peripheral non-expandable support structure with
free edges
that project toward an outflow direction and meet or coapt in the middle of
the
flowstream. A suture-permeable sewing ring is provided around the inflow end.
[0004] In recent years, advancements in minimally-invasive surgery and
interventional cardiology have encouraged some investigators to pursue
percutaneous
repair and/or replacement of heart valves. One prosthetic valve for use in
such a
procedure can include a radially collapsible and expandable frame to which
leaflets of
the prosthetic valve can be coupled. For example, U.S. Patent Nos. 6,730,118,
7,393,360,
7,510,575, and 7,993,394, which are incorporated herein by reference, describe
exemplary collapsible transcatheter heart valves (THVs). Edwards Lifesciences
of
Irvine, CA, has developed a plastically- or balloon-expandable stent
integrated with a
bioprosthetic valve. The stent/valve device, now called the Edwards Sapien0
Heart
CA 03143382 2021-12-13
WO 2021/061987
PCT/US2020/052496
¨ 2 ¨
Valve, is deployed across the native diseased valve to permanently hold the
valve open,
thereby alleviating a need to excise the native valve.
[0005] Another prior bioprosthetic valve for aortic valve replacement is
provided by
the Edwards Intuity Elite valve system also available from Edwards
Lifesciences.
Aspects of the system are disclosed in U.S. Patent Nos. 8,641,757 and
9,370,418 both to
Pintor, et al. and 8,869,982 to Hodshon, et al. The Edwards Intuity Elite
valve is a
hybrid of a generally non-expandable valve member and an expandable anchoring
stent
that helps secure the valve in place in a shorter amount of time. The implant
process
only requires three sutures which reduces the time-consuming process of tying
knots. A
delivery system advances the Edwards Intuity valve with the stent at the
leading end
until it is located within the left ventricular outflow tract (LVOT), at which
point a
balloon inflates to expand the stent against the left ventricular outflow
tract wall.
[0006] With all expandable prosthetic heart valves, there is the potential
that under
certain conditions the expanding stent could impinge on the conduction system
of the
heart, therefore affecting its function. Solutions are needed.
SUMMARY
[0007] The present application provides a prosthetic heart valve comprising
a
plurality of flexible leaflets arranged to close together along a flow axis
through the
valve to prevent blood flow in one direction, and a support frame surrounding
and
supporting the leaflets. An expandable stent connected to the support frame
defines a
circumference and is convertible from a radially contracted configuration to a
radially
expanded configuration. The stent is defined by a plurality of interconnected
struts,
wherein a pattern of the interconnected struts is consistent around the
circumference
except in a modified region on one circumferential side so that when converted
to the
expanded configuration the modified region of the stent expands radially
outward a
smaller distance than around a remainder of the circumference. Alternatively,
the
modified region when converted to the expanded configuration has larger cells
defined
between the interconnected struts than around a remainder of the circumference
[0008] The support frame may be non-expandable, non-collapsible and the
expandable stent connects to an inflow end of the support frame and is
generally non-
expandable and non-collapsible as a consequence, and wherein the expandable
stent has
an inflow end that converts from the radially contracted configuration to the
radially
expanded configuration. Preferably, the expandable stent is plastically-
expandable.
CA 03143382 2021-12-13
WO 2021/061987
PCT/US2020/052496
¨ 3 ¨
[0009] The plurality of interconnected struts may include a series of
circumferential
row struts between axial column struts, the row struts defining bends between
the
column struts, and wherein at least one row strut in the modified region
defines
shallower bends than around a remainder of the at least one row strut. The
final bend
angles of the at least one row strut in the modified region are preferably
between about
135-1600, while final bend angles around the remainder of the at least one row
strut are
preferably between about 45-900
.
[0010] The heart valve may be configured for implant at an aortic annulus
and
defines three commissure posts at intersections between three of the flexible
leaflets,
and the modified region is centered at one of the three commissure posts and
will
correspond to the location of the membranous interventricular septum and the
conduction system zone. Desirably, the modified region extends
circumferentially
between about 90-1200
.
[0011] In one embodiment, the support frame is expandable and the
expandable
stent forms a portion of the support frame such that the heart valve is fully
expandable.
The support frame in the fully expandable heart valve may be plastically-
expandable or
self-expandable.
[0012] A further understanding of the nature and advantages of the present
invention are set forth in the following description and claims, particularly
when
considered in conjunction with the accompanying drawings in which like parts
bear like
reference numerals.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] The invention will now be explained, and other advantages and
features will
appear with reference to the accompanying schematic drawings wherein:
[0014] Figure 1 illustrates delivery to an aortic annulus of a prior art
heart
valve/holder combination using a valve delivery tube;
[0015] Figure 2 is a partially cutaway perspective view of a prior art
assembled
hybrid prosthetic heart valve;
[0016] Figures 2A and 2B are elevational views of a prior art anchoring
skirt used in
the hybrid prosthetic heart valve and shown in both radially contracted and
expanded
states, respectively;
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 4 ¨
[0017] Figure 3 is a schematic diagram of the conduction system of the
heart with
primary features labeled;
[0018] Figure 4 is a laid-flat image of the aortic valve showing the
general location of
the adjacent conduction system zone;
[0019] Figure 5 is a schematic representation of the outline of a hybrid
prosthetic
heart valve;
[0020] Figure 6 is a laid-flat image of the hybrid prosthetic heart valve
outline of
Figure 5 superimposed over the laid-flat image of the aortic valve of Figure
4;
[0021] Figure 7 is a schematic plan view of an aortic valve indicating the
location of
the adjacent conduction system components;
[0022] Figure 8 is a perspective view of an assembled hybrid prosthetic
heart valve
showing marking on the exterior thereof to indicate rotational placement when
implanting the valve;
[0023] Figures 9A-9C are elevational views of exemplary stent frames of the
present
application for use in an anchoring skirt of a hybrid prosthetic heart valve,
the stent
frames shown radially expanded with struts modified to reduce impact on an
adjacent
heart conduction system;
[0024] Figure 10 is an elevational view of another exemplary stent frame
radially
expanded with struts modified to reduce impact on an adjacent heart conduction
system;
[0025] Figures 11A and 11B are elevational views of a further exemplary
stent frame
shown radially expanded with struts modified to reduce impact on an adjacent
heart
conduction system;
[0026] Figure 12A shows a still further exemplary stent frame from below
prior to
expansion, and Figure 12B shows the stent frame after expansion showing how
one side
does not expand as far as the remainder;
[0027] Figure 13 is a perspective view of a fully-expandable prosthetic
heart valve of
the prior art shown expanded;
[0028] Figure 14 is a perspective view of a modified fully-expandable
prosthetic
heart valve of the present application;
[0029] Figure 15 is an elevational view of another fully-expandable
prosthetic heart
valve of the prior art shown expanded;
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 5 ¨
[0030] Figure 16 illustrates placement of the fully-expandable prosthetic
heart valve
of Figure 15 at an aortic annulus;
[0031] Figures 17A and 17B are elevational views of fully-expandable
prosthetic
heart valves like that shown in Figure 15 with a portion modified to reduce
impact on an
adjacent heart conduction system;
[0032] Figure 18 is a perspective view of a hybrid prosthetic heart
valve/holder
combination on a distal end of a valve delivery system showing expansion of a
distal
skirt using an asymmetric balloon;
[0033] Figure 19 is a perspective view of a fully-expandable prosthetic
heart valve on
a distal end of a valve delivery tube showing expansion thereof using an
asymmetric
balloon;
[0034] Figure 20A is an elevational view of an asymmetric balloon used to
expand
heart valves as modified herein, and Figure 20B is a cross-sectional view
taken along
line 20B-20B in Figure 20A; and
[0035] Figure 21 is an alternative asymmetric balloon used to expand heart
valves
as modified herein.
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
[0036] As mentioned above, one promising prior art technique for heart
valve
replacement is a hybrid valve with a non-expandable valve member and an
expandable
stent thereon which, though still requiring cardiopulmonary bypass, can be
implanted in
a much shorter time frame. The hybrid valve is delivered through direct-access
ports
introduced through the chest.
Hybrid heart valve
[0037] Figure 1 illustrates a snapshot in the process of delivering a prior
art heart
valve 20 to an aortic annulus AA using a valve delivery tube or handle 10. As
will be
seen, the valve delivery handle 10 has a distal coupler 12 and a proximal
coupler 14. For
purpose of orientation, the heart valve 20 has an inflow end down and an
outflow end
up, and the terms proximal and distal are defined from the perspective of the
surgeon
delivering the valve inflow end first. Thus, proximal is synonymous with up or
outflow,
and distal with down or inflow.
[0038] As also illustrated in Figure 2, the prosthetic heart valve 20 is
considered a
hybrid type because it has a non-expandable, non-collapsible valve member 30
and an
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 6 ¨
expandable anchoring skirt 32 attached to and projecting from a distal end of
the valve
member 30. The valve member 30 can take a variety of forms, and may include a
cloth-
covered wireform that follows an undulating path around the periphery of the
valve
with alternating cusps 33 and commissure posts 34. A plurality of flexible
leaflets 36
extend across a generally circular orifice defined within the valve member 30,
each of
which receives peripheral support along the wireform, in particular by two
adjacent
commissure posts 34. An annular, preferably contoured, sewing or sealing ring
38
circumscribes the valve 20 at an axial location approximately between the
valve member
30 and expandable anchoring skirt 32. Three markings 39 are often evenly
spaced
around the cloth-covered sealing ring 38 to delineate to the surgeon the
center of each of
the cusps 33.
[0039] The term "valve member" refers to that component of a heart valve
that
possesses the fluid occluding surfaces to prevent blood flow in one direction
while
permitting it in another. Various constructions of valve members are
available. The
leaflets may be bioprosthetic, synthetic, or other suitable expedients. When
used for
aortic valve replacement, the valve member 30 preferably has three flexible
leaflets 36
which provide the fluid occluding surfaces to replace the function of the
native valve
leaflets. In various preferred embodiments, the valve leaflets may be taken
from another
human heart (cadaver), a cow (bovine), a pig (porcine valve) or a horse
(equine). The
three leaflets are supported by an internal generally tubular frame, which
typically
include a synthetic (metallic and/or polymeric) support structure of one or
more
components covered with cloth for ease of attachment of the leaflets.
[0040] Although the exemplary heart valve 20 is constructed as mentioned,
the
present invention is broader and encompasses any valve member 30 having an
expandable anchoring skirt 32 projecting from an inflow end thereof (for
example, one
without a wireform).
[0041] For definitional purposes, the terms "skirt" or "anchoring skirt"
refer to an
expandable structural component of a heart valve that is capable of attaching
to tissue
of a heart valve annulus. The anchoring skirt 32 described herein may be
tubular or
conical, and have varying shapes or diameters.
[0042] By utilizing an expandable skirt 32 coupled to a non-expandable
valve
member 30, the duration of the implant operation is greatly reduced as
compared with a
conventional sewing procedure utilizing an array of sutures. The expandable
skirt 32
may simply be radially expanded outward into contact with the implantation
site, or
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 7 ¨
may be provided with additional anchoring means, such as barbs. This provides
a rapid
connection means as it does not require the time-consuming process of suturing
the
valve entirely around the annulus. The operation may be carried out using a
conventional open-heart approach and cardiopulmonary bypass. In one
advantageous
feature, the time on bypass is greatly reduced due to the relative speed of
implanting the
expandable stent.
[0043] As a point of further definition, the term "expandable" is used
herein to refer
to a component of the heart valve capable of expanding from a first, delivery
diameter to
a second, implantation diameter. An expandable structure, therefore, does not
mean one
that might undergo slight expansion from a rise in temperature, or other such
incidental
cause such as fluid dynamics acting on leaflets or commissures. Conversely,
"non-
expandable" should not be interpreted to mean completely rigid or
dimensionally stable,
merely that the valve member is not expandable/collapsible like some proposed
minimally-invasively or percutaneously-delivered valves, and some slight
expansion of
conventional "non-expandable" heart valves, for example, may be observed.
[0044] In the description that follows, the term "body channel" is used to
define a
blood conduit or vessel within the body. Of course, the particular application
of the
prosthetic heart valve determines the body channel at issue. An aortic valve
replacement, for example, would be implanted in, or adjacent to, the aortic
annulus.
Likewise, a mitral valve replacement will be implanted at the mitral annulus.
Certain
features of the present invention are particularly advantageous for one
implantation site
or the other, in particular the aortic annulus. However, unless the
combination is
structurally impossible, or excluded by claim language, any of the heart valve
embodiments described herein could be implanted in any body channel.
[0045] In a particularly preferred embodiment, the prosthetic valve 20
comprises a
commercially available, non-expandable prosthetic valve member 30, such as the
Carpentier-Edwards PERIMOUNT Magna Aortic Heart Valve available from Edwards
Lifesciences, while the anchoring skirt 32 includes an inner plastically-
expandable stent
frame covered with fabric. In another embodiment, the valve member 30
comprises a
PERIMOUNT Magna Aortic valve subjected to Resilia0 tissue treatment, which
allows
for dry packaging and sterilization and eliminates the need to rinse the
valves before
implantation. In this sense, a "commercially available" prosthetic heart valve
is an off-
the-shelf (e.g., suitable for stand-alone sale and use) prosthetic heart valve
defining
therein a non-expandable, non-collapsible support structure and having a
sealing ring
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 8 ¨
capable of being implanted using sutures through the sealing ring in an open-
heart,
surgical procedure.
[0046] In the cutaway portion of Figure 2, each of the three leaflets 36
includes
outwardly projecting tabs 40 that pass through inverted U-shaped commissure
posts 42
of an undulating wireform and wrap around cloth-covered upstanding posts 44 of
an
inner polymer band. Tabs 40 from adjacent leaflets converge outside of the
wireform
commissure posts 42 and are sewn together to provide an outer anchor for the
leaflet
free edges 46. In use, fluid forces close the leaflets (coaptation) as seen in
Figure 2 and
exert substantial force on the occluded valve, which translates into inward
force on the
leaflet free edges 46. The assembly of the wrapped leaflet tabs 40 and cloth-
covered
posts 44 sewn together provides a solid anchor that is prevented from inward
movement
by the metallic wireform posts 42. Some flexing is acceptable and even
desirable.
[0047] One feature of the valve member 30 that is often utilized is the
sewing or
sealing ring 38 that surrounds the inflow end thereof. The sealing ring 38
conforms to
an upper end of the anchoring skirt 32 and is located at the junction of the
skirt and the
valve member 30. Moreover, the sealing ring 38 presents an outward flange that
contacts an outflow side of the part of annulus, while the anchoring skirt 32
expands
and contacts the opposite, ventricular side of the annulus, therefore securing
the heart
valve 20 to the annulus from both sides. Furthermore, the presence of the
sealing ring
38 provides an opportunity for the surgeon to use conventional sutures to
secure the
heart valve 20 to the annulus as a contingency.
[0048] The preferred sealing ring 38 defines an undulating upper or outflow
face and
an undulating lower face. Cusps 33 of the valve structure abut valleys in the
sealing
ring 38 upper face opposite locations where the lower face defines peaks.
Conversely, the
valve commissure posts 34 align with locations where the sealing ring 38 lower
face
defines valleys or troughs. The undulating shape of the sealing ring 38
advantageously
matches the anatomical contours of the aortic side of the annulus AA, that is,
the supra-
annular shelf. The ring 38 preferably comprises a suture-permeable material
such as
rolled synthetic fabric or a silicone inner core covered by a synthetic
fabric. In the latter
case, the silicone may be molded to define the undulating contour and the
fabric cover
conforms thereover.
[0049] As seen in Figure 2, the anchoring skirt 32 comprises an inner stent
frame 52
assembled within a tubular section of fabric 54 which is then drawn taut
around the
stent frame, inside and out, and sewn thereto to form the cloth-covered skirt
32. A
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 9 ¨
thicker, more plush fabric flange 56 may also be attached around the fabric 54
for
additional paravalvular sealing benefits. It should be noted that Figure 2
shows the
stent frame 52 in an outwardly expanded state, which occurs during and after
implant
as mentioned.
[0050] In an assembly process, the stent frame 52 may be initially tubular
and then
crimped to a conical shape as see in Figure 2A, for example. Of course, the
frame 52 may
be crimped first and then covered with cloth, or vice versa. Figure 2B shows
the
expanded stent frame 52 isolated and expanded into its implant shape, which is
generally conical and slightly flared out at a lower end.
[0051] With reference again to the implant step of Figure 1, the aortic
annulus AA is
shown schematically isolated and it should be understood that various
anatomical
structures are not shown for clarity. The annulus AA includes a fibrous ring
of tissue
that projects inward from surrounding heart walls. The annulus AA defines an
orifice
between the ascending aorta AO and the left ventricle LV. Although not shown,
native
leaflets project inward at the annulus AA to form a one-way valve at the
orifice. The
leaflets are preferably left in place and outwardly compressed by the
expandable
anchoring skirt 32, or in some cases may be removed prior to the procedure. If
the
leaflets are removed, some of the calcified annulus may also be removed, such
as with a
rongeur. The ascending aorta AO commences at the annulus AA with three outward
bulges or sinuses, two of which are centered at coronary ostia (openings)
leading to
coronary arteries CA. It is important to orient the prosthetic valve 20 so
that the
commissure posts 34 are not aligned with and thus not blocking the coronary
ostia.
[0052] Figure 1 shows a plurality of pre-installed guide sutures 50. The
surgeon
attaches the guide sutures 50 at three evenly spaced locations around the
aortic annulus
AA. In the illustrated embodiment, the guide sutures 50 attach to locations
below or
corresponding to the nadirs of the native cusps or sinuses. The guide sutures
50 are
passed through the annulus AA and back out of the implantation site. Of
course, other
suturing methods or pledgets may be used depending on surgeon preference.
[0053] The guide sutures 50 extend in pairs of free lengths from the
annulus AA and
out of the operating site. The prosthetic heart valve 20 mounts on the distal
end of the
delivery handle 10 and the surgeon advances the valve into position within the
aortic
annulus AA along the guide sutures 50. That is, the surgeon threads the three
pairs of
guide sutures 50 through evenly spaced locations around the suture-permeable
ring 38.
If the guide sutures 50, as illustrated, anchor to the annulus AA below the
aortic
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
- 10 ¨
sinuses, they thread through the ring 38 mid-way between the valve commissure
posts
34, in particular at cusp regions 33 of the sealing ring that may be axially
thicker than
the commissure locations, or uniform all around the circumference.
[0054] Figure 1 illustrates the dual nature of the valve delivery handle 10
in that it
provides both a portion of the handle of the delivery system, as well as a
through lumen
that leads directly through the holder 22 and a leaflet parting member
(described below)
to the space within the anchoring skirt 32. Although not shown, other elements
of the
delivery system mate with the proximal coupler 14 to provide an elongated
access
channel for delivery of an expander such as a balloon to a space within the
anchoring
skirt 32.
[0055] The surgeon advances the heart valve 20 until it rests in a desired
implant
position at the aortic annulus AA. The undulating suture-permeable ring 38
desirably
contacts the ascending aorta AO side of the annulus AA, and is thus said to be
in a
supra-annular position. Such a position enables selection of a larger orifice
prosthetic
valve 20 as opposed to placing the ring 38, which by definition surrounds the
valve
orifice, within the annulus AA, or infra-annularly. Further details of the
delivery
procedure are shown and described in U.S. Patent No. 8,641,757, filed June 23,
2011,
the contents of which are expressly incorporated herein.
[0056] After seating the prosthetic heart valve 20 at the aortic annulus
AA, the
anchoring skirt 32 is expanded into contact with a subvalvular aspect of the
aortic valve
annulus, such as with a balloon, to anchor the valve 20 to the annulus AA and
seal a
concentric space between aortic annulus/LVOT and bio-prosthesis so as to
prevent
paravalvular leaks. The operator then severs any retention sutures (not shown)
between
the holder 22 and valve 20, deflates the balloon and withdraws it along with
the entire
assembly of the leaflet parting member, holder 22 and valve delivery handle
10. Finally,
the guide sutures 50 will be tied off to further secure the valve in place.
[0057] The inner stent frame 52 seen in detail in Figures 2A and 2B may be
similar
to an expandable stainless-steel stent used in the Edwards SAPIENO
Transcatheter
Heart Valve. However, the material is not limited to stainless steel, and
other materials
such as Co-Cr alloys, nitinol, etc., may be used. In one embodiment, the
radial thickness
of the plurality of struts is around 0.4-0.6 mm. In a preferred embodiment,
the material
used should have an elongation at break greater than 33%, and an ultimate
tensile
strength of greater than about 490 MPa. The stent frame 52 may be initially
formed in
several ways. For instance, a tubular portion of suitable metal such as
stainless steel
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
-11 ¨
may be laser cut to length and to form the latticework of chevron-shaped
interconnected
struts. After laser cutting, the stent frame 52 is desirably electro-polished.
Other
methods including wire bending and the like are also possible. Following
manufacture,
and crimping, the inner stent frame 52 assumes a crimped, tapered
configuration that
facilitates insertion through the calcified native aortic valve (see Figure
1).
[0058] It should be noted that the stent frame 52 in Figure 2A commences at
its
upper end 62 in a generally tubular shape and then angles inwardly to be
tapered
toward its lower end 64. That is, the generally tubular portion has a height h
which is
only a portion of the total height H. As shown, the tubular portion has a
height h which
generally corresponds to the height between troughs 60a and the peaks 60b of
an upper
end 62 of the stent frame. The upper end 62 is preferably defined by a thicker
wire for
reinforcement. The upper end 62 follows an undulating path with alternating
arcuate
troughs 60a and pointed peaks 60b that generally corresponds to the undulating
contour
of the underside of the sewing ring 38 (see Figure 3A). Desirably, the height
h of the
peaks 60b above the troughs 60a is between about 25-36% of the total stent
frame
height H, with the ratio gradually increasing for larger valve sizes.
[0059] With reference still to Figure 2A, the constricted stent frame 52 of
the
anchoring skirt 32 has an initial shape following manufacture in a tapered
configuration
with a lower (inflow/leading) end 64 defining a smaller first diameter Di
orifice than
that described by the upper (outflow/trailing) end 62. As mentioned, the
anchoring skirt
32 attaches to an inflow end of the valve member 30, typically via sutures
through the
upper end 62 of the stent frame 52 connected to fabric on the valve member 30
or sewing
ring 38. The particular sewing ring 38 as shown in Figure 3A includes an
undulating
inflow contour that dips down, or in the inflow direction, in the regions of
the valve
cusps 33, and arcs up, in the outflow direction, in the regions of the valve
commissures
34. This undulating shape generally follows the inflow end of the heart valve
member
wireform 50 (see Figure 2) which seats down within the sewing ring 38. The
scalloped
upper end 62 of the stent frame 52 also conforms to this undulating shape,
with peaks
60b aligned with the valve commissures 34 and valleys 60a aligned with the
valve cusps
33.
[0060] The mid-section of the frame 52 has three rows of expandable struts
66 in a
sawtooth pattern between axially-extending struts 68. The axially-extending
struts 68
are in-phase with the peaks 60b and troughs 60a of the upper end 62 of the
stent frame.
The reinforcing ring defined by the thicker wire upper end 62 is continuous
around its
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 12 ¨
periphery and has a substantially constant thickness or wire diameter
interrupted by
eyelets 70, which may be used for attaching sutures between the valve member
30 and
skirt 32. Note that the attachment sutures ensure that the peaks of the upper
end 62 of
the skirt 32 fit closely to the troughs of the sewing ring 38, which are
located under the
commissures of the valve.
[0061] As seen in Figure 2B, the minimum diameter d of the upper end 62 of
the
covered skirt 32 will always be bigger than the ID (which defines the valve
orifice and
corresponding labeled valve size) defined by the prosthetic valve member 30 to
which it
attaches. For instance, if the upper end 62 secures to the underside of the
sewing ring
38, which surrounds the support structure of the valve, it will by definition
be equal to
or larger than the ID or flow orifice of the support structure. Typically,
however, the
upper end 62 attaches via sutures to fabric covering an inner stent structure
(not
shown), one part of which is the inner polymer band 44.
[0062] Figure 2B illustrates the stent frame 52 isolated and in its
expanded
configuration. Balloon inflation is designed to expand only the inflow or
lower end 64 of
the frame, and no expansion loads are exerted on the outflow or upper end 62
to prevent
damage to the supra-annular elements of the valve, and therefore the supra-
annular
valve remains dimensionally unchanged. The inflow end 64 of the prior art
stent frame
52 is designed to expand symmetrically and radially as the balloon inflates.
The lower
end 64 has a diameter D2 which is larger than the diameter of the upper end
62. The
expanded shape of the stent 52 is also preferably slightly flared outward
toward its
lower end 64, as shown, by virtue of expanding with a spherical balloon. This
shape
helps the stent conform to the subvalvular contours of the left ventricle,
below the aortic
valve, and thus helps anchor the valve in place.
Conduction system of the heart
[0063] As mentioned above, it is important to ensure that the expanding
stent frame
52 seals well the space between the implant and the LVOT and it does not
impinge on
the conduction system of the heart, therefore affecting its function. Indeed,
such a
concern is not limited to the hybrid prosthetic heart valve 20 illustrated
herein, but
applies to any expandable valves, in particular those with balloon-expandable
stents.
[0064] As seen in Figure 3, the conduction system of the heart is not
uniformly
distributed around the native heart valves, but instead is concentrated in
several
regions. The cardiac conduction system or impulse conduction system of the
heart
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 13 ¨
generally consists of four structures: 1. The sinoatrial node (SA node) 2. The
atrioventricular node (AV node) 3. The atrioventricular bundle (AV bundle)
bifurcated
into left and right branches, and 4. The Purkinje fibers in the wall of the
heart muscle
(not illustrated). The cardiac muscle fibers that compose these structures are
specialized
for impulse conduction rather than the normal specialization of muscle fibers
for
contraction. The impulses commence at the SA node which is sometime described
as the
heart's pacemaker and is located at the upper portion of the right atrium.
From there,
signals transmit through internodal tracts to the AV node located in the lower
part of
the right atrium, through the AV bundle in the central fibrous tissue between
the
chambers, and to the fibers in the left and right ventricular myocardial
tissue.
[0065] Figure 3 shows the AV node adjacent the aortic valve. A conduction
bundle
(Bundle of His) traverses a membranous septum to an interventricular septum.
During
its course, a Left bundle branch is closer to the Right Coronary annulus and
innervates
the left ventricle through fascicles and Purkinje fibers. The Right bundle
branch exits
from membranous septum, penetrates the upper part of the septum and on to the
right
side of the interventricular septum, leading to the right ventricle and its
fascicles and
Purkinje fibers. Numerous anatomical studies have attempted to map the course
of
these conductive fibers in and around the heart's chambers.
[0066] With reference to laid-flat depiction of the aortic valve in Figure
4, the
conductive pathway adjacent the aortic valve is typically understood to be
located in a
subvalvular region between the right coronary sinus and the non-coronary
sinus. This
conduction system zone is depicted schematically as a triangular area
extending up
between the two sinuses and expanding downward into the left ventricle. The
precise
location, depth and lateral span of the conduction system zone varies between
patients,
though the zone commences at a depth below the annulus where the Bundle of His
emerges, and that depth is believed to decrease in those with aortic stenosis.
Some
clinical results demonstrate that the shorter the depth below which the Bundle
of His
emerges, the higher the risk of conduction abnormalities. A longer depth, on
the other
hand, indicates a longer distance from the annulus to the Bundle of His, which
may
allow longer and wider heart valve implants without necessarily causing
conduction
abnormalities.
[0067] Figure 5 illustrates the outlines of a typical hybrid prosthetic
heart valve,
such as the valve 20 shown in Figure 2. A dashed line 100 indicates the
undulating
shape of the support structure for the three flexible leaflets. The lower
circle 102 is an
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 14 ¨
imaginary line connecting the lower arcuate cusps of the support structure,
which is
intended to be located at the lower ends of the coronary sinuses when
implanted. The
two lines 100, 102 generally describe the outline of a conventional surgical
valve. The
lower conical shape indicated at 104 corresponds to the footprint of an
expanded
subvalvular stent or skirt, such as the skirt 32 shown for the valve 20 in
Figure 2.
[0068] Now with reference to Figure 6, the same general outlines of the
hybrid
prosthetic valve from Figure 5 are superimposed on the laid-flat aortic
annulus as if
implanted. The three upstanding posts of the valve defined by dashed line 100
extend up
between the three sinuses ¨ right, non-coronary, and left. The lower circle
102 extends
just below the sinuses, and the subvalvular skirt shape 104 lies against the
inside of the
left ventricle. This superposition illustrates where possible sources of
interference with
the conduction system zone are located. That is, expansion of the skirt 32
into the
triangular conduction system zone (hatched area) between the right coronary
sinus and
the non-coronary sinus may impact the heart's conduction system.
[0069] Figure 7 is a schematic plan view of an aortic valve indicating the
approximate location of the adjacent conduction system components. Namely, the
Left
bundle branch and Bundle of His are embedded in the cardiac tissue just
outside of the
membranous interventricular septum on the posterior side of the aortic valve.
As stated
above, the normal position of the conduction system components is adjacent the
valve
commissure between the right coronary sinus or cusp (RCS) and the non-coronary
sinus
or cusp (NCS). This location helps inform modifications to prosthetic valves,
as set forth
below.
Hybrid heart valve modifications
[0070] Figure 8 is a perspective view of an assembled hybrid prosthetic
aortic heart
valve 20' modified to avoid interference with the heart's conduction system.
In
particular, the expandable skirt 32' will be modified as explained below. A
preferred
modification involves modification of an inner stent frame of the skirt 32'
around only a
portion of the circumference thereof. The portion modified corresponds to a
portion that
will be implanted adjacent the conduction system, or generally adjacent the
valve
commissure between the right coronary sinus or cusp (RCS) and the non-coronary
sinus
or cusp (NCS), as seen in Figure 7. To guide the surgeon during implant of the
valve 20',
markings on the exterior thereof are provided to indicate rotational
placement. That is,
the surgeon can discern the anatomical features around the aortic valve
visually, but the
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 15 ¨
portion of the stent frame that is modified will not be apparent due to the
outer cloth
coverings 54', 56'.
[0071] Conventional aortic heart valves typically have three distinct
markings
around their periphery that indicates to the surgeon the cusp regions 33, as
seen at 39
in Figure 2. In particular, thick black marker thread is used to form the
markings 39.
The modified valve 20' also has the three cusp markings 39', as well as a
distinct
elongated marking 72 extending between two of the cusp markings 39'. The
elongated
marking 72 thus extends around 1/3 of the way (1200) around the modified valve
20' and
is aligned with a modified arcuate span of the stent frame of the skirt 32'.
When the
surgeon implants the valve 20', he or she rotates the linear marking 72 to
align with
that portion of the anatomy in which is located the conduction system. As
explained
above with reference to Figure 7, the conduction system is expected to be
located
adjacent the valve commissure between the right coronary sinus or cusp (RCS)
and the
non-coronary sinus or cusp (NCS). Thus, the arcuate marking 72 is centered on
the
valve commissure post 42'. The elongated marking 72 may be formed by a printed
indicator, or by sewing one or more lengths of suture along the appropriate
area. The
elongated marking 72 is colored so as to contrast highly with the sealing ring
38', such
as a black marker suture against a white cloth covering. Bright or fluorescent
colors
may also be used to be more visible in dim lighting.
[0072] Figures 9A-9C are elevational views of exemplary stent frames 52a,
52b, 52c
of the present application for use in an anchoring skirt of a hybrid
prosthetic heart
valve, the stent frames are shown radially expanded with struts modified to
reduce
impact on an adjacent heart conduction system. It should be noted that the
stent frames
are constructed generally the same as with the stent frame 52 of Figure 2A,
described
above, aside from the modifications below, and thus like elements will have
like
numbers with the addition of a prime (e.g., 62').
[0073] In Figure 9A, the stent frame 52a is shown with a thicker wire upper
end 62'
having an undulating periphery with alternating troughs 60a' and the peaks
60b'. The
stent frame 52a when constricted has a generally tubular shape at its upper
end 62' and
then angles inwardly to be tapered toward its lower end 64'. When expanded,
the lower
end 64' expands radially outward as shown, with a flared configuration. As
before, a
mid-section of the frame 52a has three circumferential rows of expandable
struts 66' in a
sawtooth pattern with V-shaped bends between axially-extending struts 68'. The
axially-
CA 03143382 2021-12-13
WO 2021/061987
PCT/US2020/052496
¨ 16 ¨
extending struts 68 are in-phase with the peaks 60b' and troughs 60a' of the
upper end
62' of the stent frame.
[0074] In a region 120a (bracketed) of the stent frame 52a centered on one
of the
peaks 60b', the three rows of expandable struts 66' exhibit shallower
(greater) included
angles 0 in the bends of the sawtooth pattern in the expanded state of the
stent frame
52a than in the rest of the frame. More precisely, the bends are shallower in
the region
120a that extends about 1200 between two of the troughs 60a'. Generally, the
region
120a may extend circumferentially between about 90-1200. In an exemplary
embodiment, the included angles of the bends in the region 120a are between
about
135-1600, while the bends in the rows of expandable struts 66' around the rest
of the
stent frame are between about 45-900. The result is that the rows of
expandable struts
66' in the region 120a expand less than around the rest of the stent frame 52a
when
caused to straighten out and lengthen. In other words, they straighten out
faster, as
shown by the final angle 0 of the bends in the expanded frame versus the rest
of the
bends. This produces an asymmetric expansion of the stent frame 52a, with
about 2/3 of
the frame expanding normally and about 1/3 expanding less. The region 120a
forms
something of an arcuate chordal shape when expanded, extending between
circular
adjacent regions, as seen best in Figure 12B.
[0075] It should be noted that the final angle 0 of the bends in the
expanded frame
52a is typically the same bend angle of the stent frame in region 120a when
initially
formed. That is, the frame 52a is fabricated in a tubular shape, then crimped
down to a
smaller diameter prior to packaging and shipping, as the stent frame is
delivered in the
contracted state. Consequently, the final bend angles 0 of the frame 52a are
set at the
time of frame formation. One method of frame construction is laser-cutting the
various
struts from a tubular blank of plastically-expandable material such as
stainless steel or
an elastic material such as nitinol.
[0076] In one embodiment, the majority of the stent frame 52a is configured
to
normally flare outward to a maximum diameter that is several millimeters
greater than
the nominal heart valve size. The "nominal heart valve size" means the labeled
heart
valve size selected for that particular annulus, and generally corresponds in
odd mm
increments to the measured diameter of the naive heart valve orifice. The
"nominal
heart valve size" is also slightly less than the diameter d of the upper end
62' of the
stent frame 52a. For example, the "nominal heart valve size" may be 21 mm, and
the
lower end 64' of the stent frame 52a flares outward to a maximum diameter of
about
CA 03143382 2021-12-13
WO 2021/061987
PCT/US2020/052496
¨ 17-
23.5 mm. However, the region 120a of the stent frame 52a centered on one of
the peaks
60b' is configured to expand outward by between 1-2 mm less, or to a diameter
of
between about 21.5-22.5 mm. This helps reduce the force applied to the
surrounding
subvalvular region where the conduction system is assumed to be.
[0077] In another solution to potential impaction on the conduction system,
Figure
9B shows a stent frame 52b with a lower circumferential row of expandable
struts 66'
removed in the region 120b (bracketed) of the stent frame 52b centered on one
of the
peaks 60b'. In the illustrated embodiment, as with the stent frame 52a, the
region 120b
extends around 1/3 of the periphery of the stent frame between cusps, or about
1200
.
More generally, the region 120b may extend circumferentially between 90-1200.
The
included angles of the bends in the region 120b remain as in the rest of the
frame,
between about 45-900, and thus that portion of the region 120b with
circumferential
struts 66' expands normally. As mentioned above, in some patients the
electrical
conduction system adjacent the aortic valve does not commence until some ways
down
into the left ventricle, in which case expansion of the stent frame 52b may
avoid even
contacting that zone.
[0078] Finally, Figure 9C shows a third alternative stent frame 52c which
also has
the lower circumferential row of expandable struts 66' removed in the region
120c
(bracketed). In addition, the next adjacent circumferential row of expandable
struts 66'
in the region 120c has shallow included bend angles in the expanded state of
the stent
frame 52c, such as in the range stated above for the included angles of the
bends for the
stent frame 52a of Figure 9A. Thus, when the stent frame 52c expands, the
conduction
system zone may be avoided altogether because of the missing lower row, and
the next
adjacent row of struts 66' expands less than the rest of the stent frame
(e.g., asymmetric
radial expansion) which reduces outward pressure on that zone. As before, the
region
120c preferably extends circumferentially between about 90-120 between two of
the
troughs 60a' and is centered on one of the peaks 60b'.
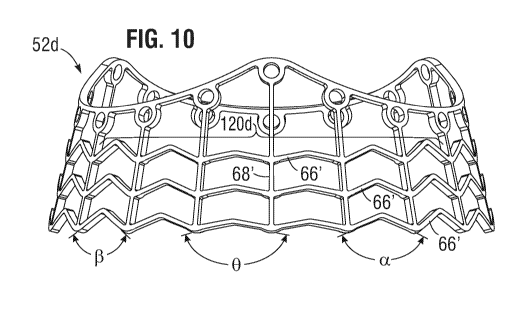
[0079] Figure 10 is an elevational view of another exemplary stent frame
52d
radially expanded with struts modified to produce asymmetric expansion around
the
skirt. In this embodiment, the lower circumferential row of expandable struts
66' in a
region 120d (bracketed) has variable included bend angles, with shallower
angles
toward the center of the region 120d. In particular, there may be eighteen
axially-
extending struts 68' in-phase with the peaks 60b' and troughs 60a' of the
upper end 62'
of the stent frame, which means there are six in each 1/3 dividing the region
120d into
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 18 ¨
six spans across which there are the bends in the expandable struts 66. The
inner two
spans have shallow (large) bend angles, while the next two outward spans have
smaller
bend angles, and the outermost two spans have even smaller bend angles. The
inner two
spans straighten the fastest, as shown by the final angle bend angles 0, the
next two
outward spans straighten less as seen by final bend angles a, and the
outermost two
spans have more room for expansion, as seen by their final bend angles 13.
This alters
the asymmetric expansion such that the reduction in final diameter in the
region 120d is
gradual from the adjacent unaltered regions. More particularly, in comparison
with a
more chordal shape between the adjacent regions, as with the embodiment of
Figure 9A,
the expanded shape of the region 120d is more rounded, closer to the circular
shape of
the rest of the stent frame 52d. This focuses the expansion reduction in the
center of the
region 120d, which again may extend circumferentially between 90-1200. Of
course, the
particular pattern of variance of the included bend angles may differ, and the
illustrated
embodiment is only exemplary.
[0080] Figures 11A and 11B are elevational views of a further exemplary
stent frame
52e shown radially expanded with a middle circumferential row of expandable
struts 66'
removed in a region 120e (bracketed) to reduce impaction on an adjacent native
conduction system zone. Figure 11A shows all of the axially-extending struts
68'
retained to create a plurality of enlarged spaces or cells 122 between struts,
while in
Figure 11B some of them are removed to create a plurality of even larger cells
124. In
both stent frames 52e, the region 120e is desirably centered on one of the
peaks 60b' and
preferably extends circumferentially about 1200, more generally between 90-
1200. These
embodiments thus create larger cells or voids within the region 120e which,
though
expanded normally, reduces direct stent contact with the surrounding native
conduction
system zone. Of course, the included bend angles in the remaining rows of
expandable
struts 66' in the region 120e may also be shallow, as described above, to
produce
asymmetric radial expansion and further reduce the impact on the conduction
system.
[0081] Figure 12A shows the stent frame 52a from below prior to expansion,
and
Figure 12B shows the stent frame 52a after expansion showing how one side does
not
expand as far as the remainder (e.g., asymmetric radial expansion). In
particular, the
region 120a includes the shallower included bend angles 0 than in the rest of
the stent
frame 52a, and thus balloon expansion causes that region 120a to expand more
in an
arcuate chordal shape than circular, as with the remainder of the stent frame
periphery.
The distance AD from an imaginary circle drawn around the maximum diameter
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 19 ¨
expansion is the preferred reduction in expansion diameter in the region 120a.
As
mentioned above, distance AD is preferably between 1-2 mm, and more preferably
about
1.5 mm. Such a small reduction of expanded diameter in the asymmetric region
120a is
believed sufficient to reduce negative impacts on the conduction system.
Fully-expandable heart valve modifications
[0082] Figure 13 is a perspective view of a fully-expandable prosthetic
heart valve
140 of the prior art shown expanded. The heart valve 140 is representative of
a number
of such valves, in particular the Sapien0 line of valves sold by Edwards
Lifesciences of
Irvine, CA. The heart valve 140 includes a structural frame 142 defining a
flow passage
therein and a plurality of flexible leaflets 144 secured within the frame,
typically via
suturing to an intermediate fabric skirt 146. In the illustrated embodiment,
there are
three of the leaflets 144 that meet at commissure posts 148 defined by the
frame 142.
The leaflets 144 extend axially within the frame 142 at the commissure posts
148 and
adjacent leaflets abut each other and are sewn together along the posts. Cusp
edges (not
shown) of the leaflets 144 are also sewn to the frame 142. Free edges 150 of
the leaflets
144 come together or coapt in the flow passage to form the one-way valve.
[0083] The structural frame 142 is fully expandable from a contracted
configuration
to the expanded shape shown. In this way, the contracted valve 140 may be
advanced
through a narrow passage into position at the target annulus, such as through
a
catheter or other delivery, without needing to stop the heart and put the
patient on
cardiopulmonary bypass. The contracted valve 140 is then expelled from the
catheter or
other delivery tube and expanded into contact with the annulus. The frame 142
may be
self-expanding, or as in the case of the Sapien0 line of valves, is balloon-
expandable,
such as being made of stainless steel. The frame 142 typically has a plurality
of
circumferential struts 152 with bends 154 that straighten out when the valve
140
expands. Prior art valves of this type have a tubular frame in both the
contracted and
expanded configurations stemming from a symmetrical distribution and shape of
the
circumferential struts 152.
[0084] Figure 14 is a perspective view of a modified fully-expandable
prosthetic
heart valve 160 of the present application. The valve 160 is in most respects
the same
construction as the representative heart valve 140 of Figure 13, and so like
elements are
given like numbers with the addition of a prime (e.g., 142). As before, the
valve 160
comprises an expandable frame 142' supporting a plurality (e.g., three)
flexible leaflets
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 20 ¨
144'. Once again, adjacent leaflets 144' are secured against each other at
commissure
posts 148'of the frame 142'.
[0085] The frame 142' has a circumferentially-extending region 162
(bracketed) in
which the bends 156' in circumferential struts 152' have a much greater
included angle
then the bends 154' around the remainder of the frame. This modification
reduces the
amount of circumferential and thus radial expansion of the frame 152' in the
region 162.
This reduced or asymmetric expansion helps reduce contact with and thus impact
on the
adjacent conduction system of the heart when the valve 160 expands. If the
heart valve
160 is intended for implant at the aortic annulus, the region 162 is centered
at one of the
commissure posts 148' as the conduction system is believed to be concentrated
near one
of the native commissures. To assist the surgeon in rotationally orienting the
heart
valve 160 during implant, a marker may be placed on either the appropriate
commissure post 148' or on the fabric skirt 146' at that location. Although
not shown,
the marker may be as described above with respect to Figure 8 (e.g., dark
suture marker
spanning 120 ).
[0086] Figure 15 is an elevational view of another fully-expandable
prosthetic heart
valve 170 of the prior art shown expanded. The heart valve 170 generally
comprises a
self-expanding structural frame 172 having a tissue valve 174 sewn thereto. In
one such
embodiment, the EvolutTM TAVR System available from Medtronic Cardiovascular
of
Minneapolis, MN includes a supra-annular, self-expanding nitinol frame, with a
porcine
pericardial tissue valve. The structural frame 172 is somewhat hourglass-
shaped and
defines an enlarged upper region 180, a narrow middle region 182, and an
enlarged
lower region 184.
[0087] The self-expanding nitinol frame 172 may be crimped down to a small
diameter just prior to delivery. As shown in Figure 16, after implantation of
the fully-
expandable prosthetic heart valve 170 at an aortic annulus, the upper region
180
enlarges into the ascending aorta, the narrow middle region 182 registers with
the
aortic annulus AA, and the lower region 184 enlarges into the left ventricle
LV, or in a
subvalvular area. Although the frame 172 is self-expandable and thus exerts
less
outward force on the surrounding tissue, issues may arise from contact with
the
adjacent conduction system of the heart, especially in the subvalvular area.
Moreover,
many surgeons perform a post-implant balloon expansion of the middle region
182 to
help fully expand the frame 172, which may also negatively impact the
conduction
system.
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 21 ¨
[0088] Consequently, Figures 17A and 17B show self-expandable stent frames
for
fully-expandable prosthetic heart valves like that shown in Figure 15 with a
portion
modified to reduce impact on an adjacent heart conduction system. In
particular, the
stent frame 200 in Figure 17A features a region 202 (bracketed) with modified
struts
which cause asymmetric expansion of the frame; namely, less expansion within
the
region 202 as compared to the rest of the circumference. There are a number of
ways to
modify the struts to accomplish this, one of which includes smaller cells 204
between
struts connected by short V-shaped segments 206. The struts 206 that form the
smaller
cells 204 expand somewhat, but not as much as the surrounding struts. If the
valve in
which the stent frame 200 is used is for aortic valve replacement, the region
202 is
preferably centered on one of the valve commissures, and may extend
circumferentially
around the valve by between 90-120 . Additionally, the modified region 202 is
preferably located in the subvalvular area, preferably in the lower region 184
as see in
Figure 15, but also possibly extending up into the middle region 182.
[0089] Figure 17B, on the other hand, illustrates a self-expandable stent
frame 210
with a region 212 (bracketed) modified to reduce the impact on an adjacent
conduction
system by removing a number of struts to form enlarged cells 214. In the
illustrated
embodiment, two enlarged diamond-shaped cells 214 are formed by removing four
intersecting struts in two places, though other patterns are also
contemplated. Removal
of the struts lessens the chance that the expanding frame 210 will contact and
negatively impact the adjacent conduction system. Again, for aortic valve
replacement,
the region 212 is preferably centered on one of the valve commissures, and may
extend
circumferentially around the valve by between 90-120 , and is preferably
located in the
subvalvular area. A combination of enlarged cells as at 214 and asymmetric
expansion
as with stent 200 of Figure 17A is also a possibility.
Modified expansion balloons
[0090] Figure 18 is a perspective view of a valve delivery system 220
similar to that
described above with respect to Figure 1 having a hybrid prosthetic heart
valve 222 on a
distal end thereof. As before, expansion of a distal skirt of the heart valve
222 is
accomplished using a balloon 224 that extends through the middle of the valve
222. In
contrast with the prior system, the balloon 224 is modified to expand
asymmetrically,
with a majority of the circumference at 226 being conventional and an altered
region
228. Specifically, the region 228 is altered so as to expand less than the
larger region
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨ 22 ¨
226. Consequently, the portion of the skirt of the heart valve 222 adjacent
the modified
region 228 expands less as well.
[0091] The region 228 may be modified in a number of ways to undergo a
smaller
radial expansion. One way is to construct the balloon 224 to have the larger
region
formed of compliant (e.g., stretchy) balloon material with the region 228
formed of non-
compliant (e.g., non-stretchy) material. Various balloons of both types of
material are
known, typically formed out of nylon, e.g., polyether block-amide (e.g.,
PEBAXO,
Arkema) blend or nylon/polyether-block-amide blend materials. In one
embodiment, a
mesh of interconnected fibers (not shown) may be embedded within the region
228 of an
otherwise homogenous balloon to create the non-compliant section.
Alternatively, rigid
stiffeners (also not shown) such as nylon cords may be attached to the balloon
224 in the
region 228. In any event, the region 228 is modified to create an asymmetric
expansion
of the balloon 224, which in turn expands the valve skirt asymmetrically.
[0092] Moreover, the balloon 224 may be combined with a modified hybrid
valve as
discussed above, and the region 228 aligned to expand within the region of the
stent
frame that is modified. For instance, the region 228 may extend
circumferentially
between 90-1200, and be aligned within the region 120a of the stent frame 52a
in Figure
9A (or within any of the other modified stent frames). Although the various
modified
stent frames are intended to expand asymmetrically, the modified regions may
simply
pull the remainder of the frames toward that region, resulting in less
asymmetry as
desired. Consequently, using a modified expansion balloon 224 may be needed to
result
in the desired asymmetry.
[0093] Figure 19 is a perspective view of the distal end of a valve
delivery system
230 including a catheter 232 and an asymmetric balloon 234 within a fully-
expandable
prosthetic heart valve 236. The balloon 234 preferably has a majority region
238 that
expands normally and a modified region 240 that expands asymmetrically. The
modified
region 240 may be formed as described above for balloon 224, such as being
formed of a
non-compliant material. When expanded within the heart valve 236, the
asymmetric
expansion causes similar asymmetric expansion of the valve. Further, the
asymmetric
balloon 234 may be used within a fully-expandable prosthetic heart valve 160
modified
as described above with respect to Figure 14. In such a combination, the
modified region
240 is rotationally aligned within the region 162 on the valve 160 modified
for reduced
expansion.
CA 03143382 2021-12-13
WO 2021/061987 PCT/US2020/052496
¨23 ¨
[0094] Figure 20A is an elevational view of the valve delivery system 230
having the
asymmetric balloon 234, and Figure 20B is a cross-sectional view taken along
line 20B-
20B in Figure 20A. As mentioned, the modified region 240 is non-compliant or
stiffened
so as to expand asymmetrically, as seen in Figure 20B.
[0095] Figure 21 shows the asymmetric balloon 234 within the self-
expandable
prosthetic heart valve 170 of the prior art during a procedure of post-implant
expansion
thereof. Preferably, the modified region 240 is rotationally aligned with the
area
adjacent the valve annulus containing the electrical conduction system of the
heart. The
asymmetric balloon 234 thus avoids maximum expansion of the frame of the valve
170
in this area. Further, the valve 170 may be modified to reduce the impacts on
the
conduction system, as with valves 200 and 210 of Figures 17A and 17B. In that
case, the
modified region 240 is rotationally aligned with the modified regions 202,
212,
respectively.
[0096] While this disclosure describes preferred embodiments, it is to be
understood
that the words which have been used are words of description and not of
limitation.
Therefore, changes may be made within the appended claims without departing
from
the true scope of the disclosure.