Note: Descriptions are shown in the official language in which they were submitted.
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
DEVICES AND METHODS FOR PROSTHETIC VALVE DIAMETER
ESTIMATION
FIELD OF THE INVENTION
[001] The present invention relates to devices and methods for measuring
prosthetic valve
expansion diameter.
BACKGROUND OF THE INVENTION
[002] Native heart valves, such as the aortic, pulmonary and mitral valves,
function to assure
adequate directional flow from and to the heart, and between the heart's
chambers, to supply
blood to the whole cardiovascular system. Various valvular diseases can render
the valves
ineffective and require replacement with artificial valves. Surgical
procedures can be
performed to repair or replace a heart valve. Surgeries are prone to an
abundance of clinical
complications, hence alternative less invasive techniques of delivering a
prosthetic heart valve
over a catheter and implanting it over the native malfunctioning valve, have
been developed
over the years.
[003] Different types of prosthetic heart valves are known to date, including
balloon
expandable valve, self-expandable valves and mechanically-expandable valves.
Different
methods of delivery and implantation are also known, and may vary according to
the site of
implantation and the type of prosthetic valve. One exemplary technique
includes utilization of
a delivery assembly for delivering a prosthetic valve in a crimped state, from
an incision which
can be located at the patient's femoral or iliac artery, toward the native
malfunctioning valve.
Once the prosthetic valve is properly positioned at the desired site of
implantation, it can be
expanded against the surrounding anatomy, such as an annulus of a native
valve, and the
delivery assembly can be retrieved thereafter.
[004] Mechanically expandable valves are a category of prosthetic valves that
rely on a
mechanical actuation mechanism for expansion. The actuation mechanism usually
includes a
plurality of actuation/locking assemblies, releasably connected to respective
actuation
members of the valve delivery system, controlled via the handle for actuating
the assemblies
to expand the valve to a desired diameter. The assemblies may optionally lock
the valve's
position to prevent undesired recompression thereof, and disconnection of the
delivery system's
1
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
actuation member from the valve actuation/locking assemblies, to enable
retrieval thereof once
the valve is properly positioned at the desired site of implantation. Various
types of re-
compression assemblies may be utilized to re-compress an expanded prosthetic
valve in order
to allow repositioning or re-crossing procedures to be performed, and to allow
readjustment of
the prosthetic valve expansion diameter.
[005] When implanting an expandable prosthetic valve, it is desirable to
expand the valve to
a maximum size allowed by the patient's anatomical considerations, in order to
avoid
paravalvular leakage or other unfavorable hemodynamic phenomena across the
valve that may
be associated with a mismatch between the valve's expansion diameter and the
surrounding
tissue, while mitigating the risk of annular rupture that may result from over-
expansion. To
ensure optimal implantation size, the diameter of the prosthetic valve should
be monitored in
real-time during the implantation procedure. While real-time monitoring may be
of importance
for all types of prosthetic valves, mechanically expandable may particularly
benefit from such
monitoring, since mechanical actuation mechanisms provides a higher degree of
control over
the rate and extent of valve expansion, enabling the clinician to adjust
expansion diameter in
response to real-time monitored data.
SUMMARY OF THE INVENTION
[006] The present disclosure is directed toward devices, assemblies and
methods for
monitoring radial expansion of a prosthetic valve during prosthetic valve
implantation
procedures. Real-time measurement of the expansion diameter ensures proper
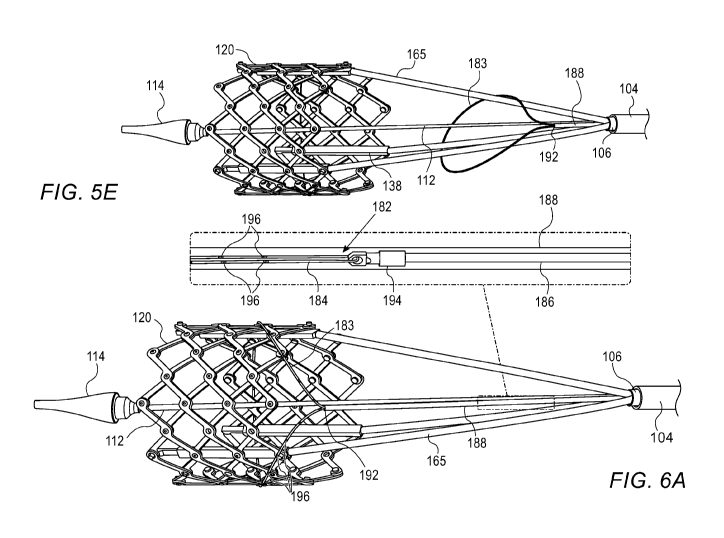
implantation of
the prosthetic valve within a designated site of implantation, such as the
site of malfunctioning
native valve.
[007] According to one aspect of the invention, there is provided a delivery
assembly
comprising a prosthetic valve and a delivery apparatus. The prosthetic valve
is movable
between a radially compressed configuration and a radially expanded
configuration. The
delivery apparatus comprises a handle, a delivery shaft extending distally
from the handle, and
a re-compression assembly. The re-compression assembly comprises a re-
compression shaft
extending through a lumen of the delivery shaft, and a re-compression member
extending
through a lumen of the re-compression shaft. The re-compression member
comprises a loop
portion configured to circumscribe the prosthetic valve, wherein the loop
portion comprises at
2
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
least one radiopaque marker. Relative movement between the re-compression
member and the
re-compression shaft in the axial direction is effective to tighten the loop
portion around the
prosthetic valve, thereby radially compressing the prosthetic valve.
[008] According to some embodiments, the at least one radiopaque marker
comprises a
plurality of radiopaque markers, spaced from each other along at least a
portion of the loop
portion.
[009] According to some embodiments, the radiopaque markers comprise
radiopaque bands.
[010] According to some embodiments, the radiopaque markers span along a
portion of the
loop portion that is at least as long as half of the prosthetic valve
perimeter in the radially
expanded configuration.
[011] According to some embodiments, the at least one radiopaque marker is
disposed along
a minimal marking length, at a position which corresponds to the contact
region between the
loop portion and the perimeter of the prosthetic valve.
[012] According to some embodiments, the minimal marking length is at least as
great as the
perimeter of the prosthetic valve in the radially expanded configuration.
[013] According to some embodiments, the at least one radiopaque marker
comprises
radiopaque coating.
[014] According to some embodiments, the re-compression member further
comprises a
releasable connector. The releasable connector comprises a proximal connector
element and a
distal connector element releasably attached to each other, wherein the re-
compression member
comprises a re-compression member proximal segment coupled to the proximal
connector
element, and wherein the loop portion is coupled to the distal connector
element.
[015] According to some embodiments, the prosthetic valve comprises a guide
member,
wherein at least a portion of the re-compression member extends through a
lumen of the guide
member.
[016] According to some embodiments, the prosthetic valve further comprises a
sleeve
disposed around at least a portion of the circumference of the prosthetic
valve, wherein at least
a portion of the loop portion extends through the sleeve.
3
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[017] According to another aspect of the invention, there is provided a
delivery assembly
comprising a prosthetic valve and a delivery apparatus. The prosthetic valve
is movable
between a radially compressed configuration and a radially expanded
configuration. The
delivery apparatus comprises a handle, a delivery shaft extending distally
from the handle, and
a re-compression assembly. The re-compression assembly comprises a re-
compression shaft
extending through a lumen of the delivery shaft, and a re-compression member
comprising at
least one indicator radiopaque marker. The re-compression shaft comprises at
least one
reference radiopaque marker.
[018] The re-compression member comprises a re-compression member proximal
segment
and loop portion. The re-compression member extends through a lumen of the re-
compression
shaft. The loop potion extends distally from the re-compression shaft.
Relative movement
between the re-compression member and the re-compression shaft in the axial
direction is
effective to tighten the loop portion around the prosthetic valve, thereby
radially compressing
the prosthetic valve. The axial position of the one indicator radiopaque
marker, relative to the
at least one reference radiopaque marker, is indicative of the diameter of the
prosthetic valve.
[019] According to some embodiments, the at least one reference radiopaque
marker
comprises a plurality of reference radiopaque markers, wherein each reference
radiopaque
marker is associated with a different diameter of the prosthetic valve, and
wherein alignment
of the indicator radiopaque marker with any one of the reference radiopaque
markers is
indicative of the diameter associated with the respective reference radiopaque
marker.
[020] According to some embodiments, the re-compression member proximal
segment
comprises the at least one indicator radiopaque marker.
[021] According to some embodiments, the re-compression member further
comprises a
connector, coupled to the re-compression member proximal segment and to the
loop portion.
[022] According to some embodiments, the connector comprises the at least one
indicator
radiopaque marker.
[023] According to some embodiments, the connector is a releasable connector,
comprising
a proximal connector element and a distal connector element releasably
attached to each other,
wherein the re-compression member proximal segment is coupled to the proximal
connector
element, and wherein the loop portion is coupled to the distal connector
element.
4
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[024] According to some embodiments, the prosthetic valve comprises a guide
member,
wherein at least a portion of the re-compression member extends through a
lumen of the guide
member.
[025] According to some embodiments, the prosthetic valve further comprises a
sleeve
disposed around at least a portion of the circumference of the prosthetic
valve, wherein at least
a portion of the loop portion extends through the sleeve.
[026] According to some embodiments, the delivery assembly further comprises a
plurality
of actuation arm assemblies coupled to the prosthetic valve, and configured to
move the
prosthetic valve between the radially compressed and the radially expanded
configurations.
The plurality of actuation arm assemblies comprises a plurality of loop
attachment members,
wherein the loop portion is coupled to, and extends between, the plurality of
loop attachment
members.
[027] According to some embodiments, the handle further comprises a spring
connected to
the re-compression member proximal segment, and configured to apply an axially
oriented
pull-force on the re-compression member proximal segment, wherein the pull-
force is
sufficient to apply a minimal tension magnitude to the loop portion.
[028] According to some embodiments, handle further comprises a pulley
assembly,
comprising a first pulley and a second pulley. The first pulley is attached to
the handle via a
first pin and rotatable around the first pin. The second pulley is attached to
the handle via a
second pin and rotatable around the second pin. The re-compression member
proximal segment
is routed partially around the first pulley and around the second pulley. The
pulley assembly is
configured to apply a minimal tension magnitude to the loop portion.
[029] According to another aspect of the invention, there is provided a
delivery assembly
comprising a prosthetic valve and a delivery apparatus. The prosthetic valve
is movable
between a radially compressed configuration and a radially expanded
configuration. The
delivery apparatus comprises a handle, a delivery shaft extending distally
from the handle, a
re-compression assembly and a diameter gauge.
[030] The re-compression assembly comprises a re-compression shaft extending
through a
lumen of the delivery shaft, and a re-compression member extending through a
lumen of the
re-compression shaft. The re-compression member comprises a re-compression
member
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
proximal segment and a loop portion extending distally from the re-compression
shaft. Relative
movement between the re-compression member and the re-compression shaft in the
axial
direction is effective to apply tension to the loop portion, thereby radially
compressing the
prosthetic valve.
[031] The diameter gauge is coupled to the re-compression assembly at a gauge
coupling
point, and is configured to provide a real-time indication of the diameter of
the prosthetic valve,
based on axial position and/or axial translation of the gauge coupling point.
[032] According to some embodiments, the delivery apparatus further comprises
a plurality
of actuation arm assemblies coupled to the prosthetic valve, and configured to
move the
prosthetic valve between the radially compressed and the radially expanded
configurations.
The plurality of actuation arm assemblies further comprise a plurality of loop
attachment
members. The plurality of actuation arm assemblies comprises a plurality of
loop attachment
members, wherein the loop portion is coupled to, and extends between, the
plurality of loop
attachment members.
[033] According to some embodiments, the loop portion is configured to
circumscribe the
prosthetic valve, such that relative movement between the re-compression
member and the re-
compression shaft in the axial direction is effective to tighten the loop
portion around the
prosthetic valve.
[034] According to some embodiments, the handle further comprises a spring
connected to
the re-compression member proximal segment, and configured to apply an axially
oriented
pull-force on the re-compression member proximal segment, wherein the pull-
force is
sufficient to apply a minimal tension magnitude to the loop portion.
[035] According to some embodiments, the handle further comprises a pulley
assembly. The
pulley assembly comprises a first pulley attached to the handle via a first
pin and rotatable
around the first pin, and a second pulley attached to the handle via a second
pin and rotatable
around the second pin. The re-compression member proximal segment is routed
partially
around the first pulley and around the second pulley. The pulley assembly is
configured to
apply a minimal tension magnitude to the loop portion.
[036] According to some embodiments, the second pulley further comprises a
pole portion
and a gear portion, and the handle further comprises a rack. The rack is
configured to engage
6
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
with the gear portion, such that axial translation of the rack is effective to
rotate the gear
portion. The re-compression member proximal segment is configured to wrap
around the pole
portion.
[037] According to some embodiments, the handle further comprises a display,
wherein the
real time indication is a visual real-time indication, visible via the
display.
[038] According to some embodiments, the diameter gauge comprises indicator
marks,
reflecting the range of the diameters of the prosthetic valve, and a dial. The
dial is coupled to
the re-compression assembly at the gauge coupling point, and configured to
point at the
indicator mark representing the diameter of the prosthetic valve.
[039] According to some embodiments, the dial is attached to the handle via a
dial pivot, and
configured to rotate angularly about the dial pivot when the gauge coupling
point translates in
an axial direction.
[040] According to some embodiments, the dial is orthogonal to a longitudinal
axis of the re-
compression proximal segment, and configured to move along with the re-
compression
assembly when the re-compression proximal segment translates in an axial
direction.
[041] According to some embodiments, the dial is attached, at the gauge
coupling point, to
the re-compression member proximal segment.
[042] According to some embodiments, the diameter gauge comprises a
displacement sensor,
operatively connected to the re-compression assembly, and configured to
generate a signal,
wherein the magnitude of the signal is proportional to the position and/or
axial displacement
gauge coupling point.
[043] According to some embodiments, the displacement sensor comprises a
potentiometer,
and the diameter gauge further comprises a wiper coupled to the re-compression
assembly at
the gauge coupling point. The wiper is configured to contact the potentiometer
at an end of the
wiper opposite to the gauge coupling point.
[044] According to some embodiments, the wiper is attached, at the gauge
coupling point, to
the re-compression member proximal segment.
7
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[045] According to some embodiments, the re-compression assembly further
comprises a
track member extending through the lumen of the re-compression shaft. The
track member
comprises a track member proximal segment and a secondary loop extending
distally from the
re-compression shaft.
[046] According to some embodiments, the dial is attached, at the gauge
coupling point, to
the track member proximal segment.
[047] According to some embodiments, the wiper attached, at the gauge coupling
point, to
the track member proximal segment.
[048] According to some embodiments, the plurality of actuation arm assemblies
further
comprise a plurality of secondary loop attachment members, wherein the
secondary loop is
coupled to, and extends between, the plurality of secondary loop attachment
members.
[049] According to some embodiments, the handle further comprises a track
spring connected
to the track member proximal segment, and configured to apply an axially
oriented pull-force
on the track member proximal segment, wherein the pull-force is sufficient to
apply a minimal
tension magnitude to the secondary loop.
[050] According to another aspect of the invention, there is provided a method
of providing
an indication of the expansion diameter of a prosthetic valve, comprising the
steps: (i) acquiring
at least one image of the frame of a prosthetic valve; (ii) deriving a
dimensionless parameter
from the at least one image; (iii) associating a numerical value of an
expansion diameter of the
prosthetic valve with the dimensionless parameter, and (iv) providing a visual
indication of the
expansion diameter of the prosthetic valve.
[051] According to some embodiments, the step of acquiring at least one image
comprises
acquiring at least one angiogram X-ray image of the frame.
[052] According to some embodiments, the step of acquiring at least one image
comprises
acquiring at least one fluoroscopy image of the frame.
[053] According to some embodiments, the step of associating a numerical value
of an
expansion diameter of the prosthetic valve with the dimensionless parameter is
based on any
of: mathematical formulas, graphs, and/or tables.
8
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[054] According to some embodiments, the step of providing a visual indication
comprises
visualizing the expansion diameter of the prosthetic valve on a digital
screen, as: a numerical
value, a graphical symbol, a textual message, or any combination thereof.
[055] According to some embodiments, the dimensionless parameter is an aspect
ratio of a
length of the frame and a width of the frame.
[056] According to some embodiments, the dimensionless parameter is an opening
angle
between two intersecting struts of the frame.
[057] According to another aspect of the invention, there is provided a
prosthetic valve
comprising a frame and a frame belt. The frame is movable between a radially
compressed
configuration and a radially expanded configuration. The frame belt comprises
at least one
expansion force indicator. At least a portion of the frame belt extends along
at least a portion
of the circumference of the frame in the expanded configuration. The at least
one expansion
force indicator is configured to change a state thereof, when a force
exceeding a specific
magnitude is applied thereto by the frame, during frame expansion.
[058] According to some embodiments, the at least one expansion force
indicator comprises
a radiopaque marker, wherein the change in the state of the at least one
expansion force
indicator is visible under fluoroscopy.
[059] According to some embodiments, the radiodensity of the at least one
expansion force
indicator is higher than a radiodensity of the frame.
[060] According to some embodiments, the at least one expansion force
indicator comprises
a separation zone.
[061] According to some embodiments, the separation zone comprises a frangible
portion.
[062] According to some embodiments, the frangible portion comprises a
plurality of
frangible portions, wherein at least two frangible portions are configured to
disrupt in response
to different tensioning force magnitudes applied thereto.
[063] According to some embodiments, the separation zone comprises a
decouplable portion.
[064] According to some embodiments, the frame belt comprises a plurality of
expandable
portions and a plurality of base portions attached thereto, wherein the at
least one separation
9
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
zone comprises a plurality of separation zone, such that each separation zone
is comprised in a
respective base portion. The expendable portions are configured to
circumferentially expand
along with the frame.
[065] According to some embodiments, the separation zone comprises a
radiopaque marking,
wherein the change in the state of the at least one expansion force indicator
comprises a
transition of the separation zone from an intact state to a separated state.
[066] According to some embodiments, the expandable portions comprise
radiopaque
markings, wherein the change in the state of the at least one expansion force
indicator
comprises a transition of a height of the respective expandable portion from a
first height value
to a second, shorter height value.
[067] According to some embodiments, the at least one expansion force
indicator comprises
a geometrical feature, wherein the geometrical feature has a shape which is
distinguishable
from its neighboring zone along the frame belt, and wherein the change in the
state of the at
least one expansion force indicator comprises translation of the geometrical
feature from a first
zone to a second zone.
[068] According to some embodiments, the prosthetic valve further comprises a
restrictor
configured to allow passage of the at least one geometrical feature there-
through, upon
application of a pulling force on the frame belt, exceeding a predetermined
threshold.
[069] According to some embodiments, the first zone comprises a radiopaque-
covered zone,
configured to mask the geometrical feature when disposed therein, and the
second zone
comprises an exposed zone, in which the geometrical feature is visible under
fluoroscopy when
disposed therein.
[070] According to some embodiments, the first zone comprises a first
orientation of a portion
of the frame belt, and the second zone comprises a second orientation of a
portion of the frame
belt, wherein the second orientation is angled relative to the first
orientation.
[071] According to some embodiments, the prosthetic valve further comprises a
reference
radiopaque marker, wherein the first zone comprises a first spatial position
of the geometrical
feature relative to the reference radiopaque marker, wherein the second zone
comprises a
second spatial position of the geometrical feature relative to the reference
radiopaque marker,
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
and wherein the first spatial position and the second spatial position are on
opposite sides of
the reference radiopaque marker.
[072] According to some embodiments, prosthetic valve further comprises a
sleeve disposed
around at least a portion of the circumference of the prosthetic valve,
wherein at least a portion
of the frame belt extends through the sleeve in at least one configuration of
the prosthetic valve.
[073] According to some embodiments, the at least one geometrical feature
comprises a bead.
[074] According to some embodiments, the at least one geometrical feature
comprises a belt
ratcheting tooth.
[075] According to some embodiments, the restrictor comprises an eyelet.
[076] According to some embodiments, the restrictor comprises sleeve
ratcheting teeth.
[077] According to some embodiments, the frame belt comprises a bio-resorbable
material.
[078] According to some embodiments, there is provided a delivery assembly
comprising the
prosthetic valve and a delivery apparatus. The delivery apparatus comprises a
handle and a belt
pull member, wherein the belt pull member extends distally from the handle,
and is attached to
the frame belt.
[079] According to some embodiments, the delivery assembly further comprises a
belt shaft
extending distally from the handle, wherein at least a portion of the belt
pull member extends
through, and is axially movable relative to, the belt shaft.
[080] According to some embodiments, prosthetic valve further comprises a
guide member,
wherein at least a portion of the frame belt extends through a lumen of the
guide member.
[081] According to some embodiments, the delivery assembly further comprises a
releasable
connector. The releasable connector comprises a proximal connector element and
a distal
connector element releasably attached to each other, wherein the belt pull
member is coupled
to the proximal connector element, and wherein frame belt is coupled to the
distal connector
element.
[082] According to some embodiments, there is provided a delivery assembly
comprising the
prosthetic valve and a delivery apparatus. The delivery apparatus comprises a
handle and a
11
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
transmission line. The transmission line extends distally from the handle, and
is coupled to the
frame belt. The at least one expansion force indicator comprises a stretch
sensor, wherein the
change in the state of the stretch sensor comprises a change of an electrical
property thereof,
when stretched over the prosthetic valve. The transmission line is configured
to conduct electric
signals from the stretch sensor toward the handle.
[083] Certain embodiments of the present invention may include some, all, or
none of the
above advantages. Further advantages may be readily apparent to those skilled
in the art from
the figures, descriptions, and claims included herein. Aspects and embodiments
of the
invention are further described in the specification herein below and in the
appended claims.
[084] Unless otherwise defined, all technical and scientific terms used herein
have the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
pertains. In case of conflict, the patent specification, including
definitions, governs. As used
herein, the indefinite articles "a" and "an" mean "at least one" or "one or
more" unless the
context clearly dictates otherwise.
[085] The following embodiments and aspects thereof are described and
illustrated in
conjunction with systems, tools and methods which are meant to be exemplary
and illustrative,
but not limiting in scope. In various embodiments, one or more of the above-
described
problems have been reduced or eliminated, while other embodiments are directed
to other
advantages or improvements.
BRIEF DESCRIPTION OF THE FIGURES
[086] Some embodiments of the invention are described herein with reference to
the
accompanying figures. The description, together with the figures, makes
apparent to a person
having ordinary skill in the art how some embodiments may be practiced. The
figures are for
the purpose of illustrative description and no attempt is made to show
structural details of an
embodiment in more detail than is necessary for a fundamental understanding of
the invention.
For the sake of clarity, some objects depicted in the figures are not to
scale.
In the Figures:
[087] Fig. 1 shows a view in perspective of a delivery assembly comprising a
delivery
apparatus carrying a prosthetic valve, according to some embodiments.
12
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[088] Fig. 2 shows a view in perspective of a prosthetic valve, according to
some
embodiments.
[089] Fig. 3A shows a view in perspective of an inner member, according to
some
embodiments.
[090] Fig. 3B shows a view in perspective of an actuator assembly, according
to some
embodiments.
[091] Fig. 3C shows a view in perspective of a prosthetic valve including
multiple actuator
assemblies of the type shown in Fig. 3B.
[092] Figs. 4A-4C show an actuator assembly of the type shown in Fig. 3B in
different
operational states thereof.
[093] Figs. 5A-5E show different stages of utilizing a delivery assembly
equipped with a re-
compression assembly, according to some embodiments.
[094] Fig. 6A shows a delivery assembly equipped with a re-compression
assembly having a
plurality of radiopaque markers, according to some embodiments.
[095] Fig. 6B shows a delivery assembly equipped with a re-compression
assembly having a
single continuous radiopaque marker, according to some embodiments.
[096] Figs. 7A-7C show different stages of utilizing a delivery assembly,
equipped with a re-
compression assembly having a releasable connector, according to some
embodiments.
[097] Fig. 8A shows a delivery assembly equipped with a re-compression
assembly having a
distal segment extending between actuation arm assemblies and a proximal
segment coupled
to a dial of a diameter gauge, in a compressed state of the prosthetic valve,
according to some
embodiments.
[098] Fig. 8B shows the delivery assembly of Fig. 8A, in an expanded state of
the prosthetic
valve.
[099] Fig. 8C shows a delivery assembly equipped with a re-compression
assembly having a
distal segment circumscribing the prosthetic valve and a proximal segment
coupled to a dial of
13
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
a diameter gauge, in a compressed state of the prosthetic valve, according to
some
embodiments.
[0100] Fig. 8D shows the delivery assembly of Fig. 8C, in an expanded state of
the prosthetic
valve.
[0101] Fig. 9 shows a delivery assembly equipped with a re-compression
assembly coupled to
a non-pivotable dial of a diameter gauge, according to some embodiments.
[0102] Fig. 10A and 10B shows a delivery assembly equipped with a re-
compression assembly
coupled to a dial of a diameter gauge, and routed through a pulley assembly,
according to some
embodiments.
[0103] Fig. 11 shows a delivery assembly with a re-compression assembly
coupled to a
displacement sensor of a diameter gauge, according to some embodiments.
[0104] Fig. 12 shows a delivery assembly with a track member of a re-
compression assembly
coupled to a diameter gauge, according to some embodiments.
[0105] Figs. 13A-13B show different states a delivery assembly, equipped with
a re-
compression assembly having indicator and reference markers, according to some
embodiments.
[0106] Figs. 14A-14B show different states a delivery assembly, equipped with
a re-
compression assembly having indicator and reference markers, according to
additional
embodiments.
[0107] Figs. 15A-15B show different states a delivery assembly, equipped with
a re-
compression assembly having indicator and reference markers, according to
additional
embodiments.
[0108] Figs. 16A-16B show different states a delivery assembly, equipped with
a re-
compression assembly having indicator and reference markers, according to
additional
embodiments.
[0109] Fig. 17 shows zoomed-in view of a portion of a re-compression assembly,
having a
plurality of reference markers and a plurality of indicator markers, according
to some
embodiments.
14
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0110] Fig. 18 shows a delivery assembly with a track member of a re-
compression assembly,
provided with indicator and reference markers, according to some embodiments.
[0111] Figs. 19A and 19B show a prosthetic valve having a length and diameter
varying
between a crimped and an expanded state, respectively, according to some
embodiments.
[0112] Fig. 20 shows a curve representing the relationship between the aspect
ratio and the
expansion diameter of a prosthetic valve, according to some embodiments.
[0113] Figs. 21A and 21B show a prosthetic valve having opening angles varying
between a
crimped and an expanded state, respectively, according to some embodiments.
[0114] Fig. 22 shows a curve representing the relationship between the opening
angle and the
expansion diameter of a prosthetic valve, according to some embodiments.
[0115] Figs. 23A-23C show different states a prosthetic valve provided with a
frame belt,
according to some embodiments.
[0116] Figs. 24A-24B show different states a portion of a frame belt provided
with frangible
portions, according to some embodiments.
[0117] Figs. 25A-25B show different states a portion of a frame belt provided
with a
decouplable portion, according to some embodiments.
[0118] Figs. 26A-26D show different stages of utilizing a delivery assembly
equipped with a
frame belt having a plurality of geometrical features, according to some
embodiments.
[0119] Fig. 27 shows a prosthetic valve equipped with a frame belt extending
through a guide
member restriction, according to some embodiments.
[0120] Figs. 28A-28B show different states a prosthetic valve provided with a
beaded frame
belt disposed there-around, according to some embodiments.
[0121] Figs. 29A-29B show different states a prosthetic valve provided with a
ratcheting frame
belt disposed there-around, according to some embodiments.
DETAILED DESCRIPTION OF SOME EMBODIMENTS
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0122] In the following description, various aspects of the disclosure will be
described. For the
purpose of explanation, specific configurations and details are set forth in
order to provide a
thorough understanding of the different aspects of the disclosure. However, it
will also be
apparent to one skilled in the art that the disclosure may be practiced
without specific details
being presented herein. Furthermore, well-known features may be omitted or
simplified in
order not to obscure the disclosure. In the figures, like reference numerals
refer to like parts
throughout.
[0123] Throughout the figures of the drawings, different superscripts for the
same reference
numerals are used to denote different embodiments of the same elements.
Embodiments of the
disclosed devices and systems may include any combination of different
embodiments of the
same elements. Specifically, any reference to an element without a superscript
may refer to any
alternative embodiment of the same element denoted with a superscript.
[0124] Fig. 1 constitutes a view in perspective of a delivery assembly 100,
according to some
embodiments. The delivery assembly 100 can include a prosthetic valve 120 and
a delivery
apparatus 102. The prosthetic valve 120 can be on or releasably coupled to the
delivery
apparatus 102. The delivery apparatus can include a handle 110 at a proximal
end thereof, a
nose cone shaft 112 extending distally from the handle 110, a nose cone 114
attached to the
distal end of the nosecone shaft 112, a delivery shaft 106 extending over the
nose cone shaft
112, and optionally an outer shaft 104 extending over the delivery shaft 106.
[0125] The term "proximal", as used herein, generally refers to the side or
end of any device
or a component of a device, which is closer to the handle 110 or an operator
of the handle 110
when in use.
[0126] The term "distal", as used herein, generally refers to the side or end
of any device or a
component of a device, which is farther from the handle 110 or an operator of
the handle 110
when in use.
[0127] The term "prosthetic valve", as used herein, refers to any type of a
prosthetic valve
deliverable to a patient's target site over a catheter, which is radially
expandable and
compressible between a radially compressed, or crimped, state, and a radially
expanded state.
Thus, a prosthetic valve 120 can be crimped or retained by a delivery
apparatus 102 in a
compressed state during delivery, and then expanded to the expanded state once
the prosthetic
valve 120 reaches the implantation site. The expanded state may include a
range of diameters
16
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
to which the valve may expand, between the compressed state and a maximal
diameter reached
at a fully expanded state. Thus, a plurality of partially expanded states may
relate to any
expansion diameter between radially compressed or crimped state, and maximally
expanded
state.
[0128] The term "plurality", as used herein, means more than one.
[0129] A prosthetic valve 120 of the current disclosure may include any
prosthetic valve
configured to be mounted within the native aortic valve, the native mitral
valve, the native
pulmonary valve, and the native tricuspid valve. While a delivery assembly 100
described in
the current disclosure, includes a delivery apparatus 102 and a prosthetic
valve 120, it should
be understood that the delivery apparatus 102 according to any embodiment of
the current
disclosure can be used for implantation of other prosthetic devices aside from
prosthetic valves,
such as stents or grafts.
[0130] According to some embodiments, the prosthetic valve 120 is a
mechanically
expandable valve, and the delivery apparatus 102 further comprises a plurality
of actuation arm
assemblies extending from the handle 110 through the delivery shaft 106. The
actuation arm
assemblies 165 can generally include actuation members 166 (hidden from view
in Fig. 1,
visible in Figs. 4A-4C) releasably coupled at their distal ends to respective
actuator assemblies
138 of the valve 120, and support sleeves 170 (annotated in Fig. 3) disposed
around the
respective actuation members 166. Each actuation member 166 may be axially
movable
relative to the support sleeve 170 covering it.
[0131] The prosthetic valve 120 can be delivered to the site of implantation
via a delivery
assembly 100 carrying the valve 120 in a radially compressed or crimped state,
toward the
target site, to be mounted against the native anatomy, by expanding the valve
120 via a
mechanical expansion mechanism, as will be elaborated below.
[0132] The delivery assembly 100 can be utilized, for example, to deliver a
prosthetic aortic
valve for mounting against the aortic annulus, to deliver a prosthetic mitral
valve for mounting
against the mitral annulus, or to deliver a prosthetic valve for mounting
against any other native
annulus.
[0133] The nosecone 114 can be connected to the distal end of the nosecone
shaft 112. A
guidewire (not shown) can extend through a central lumen of the nosecone shaft
112 and an
17
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
inner lumen of the nosecone 114, so that the delivery apparatus 102 can be
advanced over the
guidewire through the patient's vasculature.
[0134] A distal end portion of the outer shaft 104 can extend over the
prosthetic valve 120 and
contact the nosecone 114 in a delivery configuration of the delivery apparatus
102. Thus, the
distal end portion of the outer shaft 104 can serve as a delivery capsule that
contains, or houses,
the prosthetic valve 120 in a radially compressed or crimped configuration for
delivery through
the patient's vasculature.
[0135] The outer shaft 104 and the delivery shaft 106 can be configured to be
axially movable
relative to each other, such that a proximally oriented movement of the outer
shaft 104 relative
to the delivery shaft 106, or a distally oriented movement of the delivery
shaft 106 relative to
the outer shaft 104, can expose the prosthetic valve from the outer shaft 104.
In alternative
embodiments, the prosthetic valve 120 is not housed within the outer shaft 104
during delivery.
Thus, according to some embodiments, the delivery apparatus 102 does not
include an outer
shaft 104.
[0136] As mentioned above, the proximal ends of the nose cone shaft 112, the
delivery shaft
106, components of the actuation arm assemblies 165, and when present ¨ the
outer shaft 104,
can be coupled to the handle 110. During delivery of the prosthetic valve 120,
the handle 110
can be maneuvered by an operator (e.g., a clinician or a surgeon) to axially
advance or retract
components of the delivery apparatus 102, such as the nosecone shaft 112, the
delivery shaft
106, and/or the outer shaft 104, through the patient's vasculature, as well as
to expand or
contract the prosthetic valve 120, for example by maneuvering the actuation
arm assemblies
165, and to disconnect the prosthetic valve 120 from the delivery apparatus
102, for example
¨ by decoupling the actuation members 166 from the actuator assemblies 138 of
the valve 120,
in order to retract it once the prosthetic valve is mounted in the
implantation site.
[0137] The term "and/or" is inclusive here, meaning "and" as well as "or". For
example,
"delivery shaft 106 and/or outer shaft 104" encompasses, delivery shaft 106,
outer shaft 104,
and delivery shaft 106 with outer shaft 104; and, such "delivery shaft 106
and/or outer shaft
104" may include other elements as well.
[0138] According to some embodiments, the handle 110 can include one or more
operating
interfaces, such as steerable or rotatable adjustment knobs, levers, sliders,
buttons (not shown)
and other actuating mechanisms, which are operatively connected to different
components of
18
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
the delivery apparatus 102 and configured to produce axial movement of the
delivery apparatus
102 in the proximal and distal directions, as well as to expand or contract
the prosthetic valve
120 via various adjustment and activation mechanisms as will be further
described below.
[0139] According to some embodiments, the handle further comprises one or more
visual or
auditory informative elements configured to provide visual or auditory
information and/or
feedback to a user or operator of the delivery apparatus 102, such as a
display 116, LED lights
118, speakers (not shown) and the like.
[0140] Fig. 2 shows an exemplary mechanically expandable prosthetic valve 120
in an
expanded state, according to some embodiments. The prosthetic valve 120 can
comprise an
inflow end portion 124 defining an inflow end 125, and an outflow end portion
122 defining
an outflow end 123. The prosthetic valve 120 can define a longitudinal axis
121 extending
through the inflow end portion 124 and the outflow end portion 122. In some
instances, the
outflow end 123 is the distal end of the prosthetic valve 120, and the inflow
end 125 is the
proximal end of the prosthetic valve 120. Alternatively, depending for example
on the delivery
approach of the valve, the outflow end can be the proximal end of the
prosthetic valve, and the
inflow end can be the distal end of the prosthetic valve.
[0141] The term "outflow", as used herein, refers to a region of the
prosthetic valve through
which the blood flows through and out of the valve 120, for example between
the longitudinal
axis 121 and the outflow end 123.
[0142] The term "inflow", as used herein, refers to a region of the prosthetic
valve through
which the blood flows into the valve 120, for example between inflow end 125
and the
longitudinal axis 121.
[0143] The valve 120 comprises a frame 126 composed of interconnected struts
127, and may
be made of various suitable materials, such as stainless steel, cobalt-chrome
alloy (e.g. MP35N
alloy), or nickel titanium alloy such as Nitinol. According to some
embodiments, the struts 127
are arranged in a lattice-type pattern. In the embodiment illustrated in Fig.
2, the struts 127 are
positioned diagonally, or offset at an angle relative to, and radially offset
from, the longitudinal
axis 121 when the valve 120 is in an expanded position. It will be clear that
the struts 127 can
be offset by other angles than those shown in Fig 2, such as being oriented
substantially parallel
to the longitudinal axis 121.
19
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0144] According to some embodiments, the struts 127 are pivotably coupled to
each other. In
the exemplary embodiment shown in Fig. 2, the end portions of the struts 127
are forming
apices 129 at the outflow end 123 and apices 131 at the inflow end 125. The
struts 127 can be
coupled to each other at additional junctions 130 formed between the outflow
apices 129 and
the inflow apices 131. The junctions 130 can be equally spaced apart from each
other, and/or
from the apices 129, 131 along the length of each strut 127. Frame 126 may
comprise openings
or apertures at the regions of apices 129, 131 and junctions 130 of the struts
127. Respective
hinges can be included at locations where the apertures of struts 127 overlap
each other, via
fasteners, such as rivets or pins, which extend through the apertures. The
hinges can allow the
struts 127 to pivot relative to one another as the frame 126 is radially
expanded or compressed.
[0145] In alternative embodiments, the struts are not coupled to each other
via respective
hinges, but are otherwise pivotable or bendable relative to each other, so as
to permit frame
expansion or compression. For example, the frame can be formed from a single
piece of
material, such as a metal tube, via various processes such as, but not limited
to, laser cutting,
electroforming, and/or physical vapor deposition, while retaining the ability
to collapse/expand
radially in the absence of hinges and like.
[0146] A prosthetic valve 120 further comprises one or more leaflets 128,
e.g., three leaflets,
configured to regulate blood flow through the prosthetic valve 120 from the
inflow end to the
outflow end. While three leaflets 128 arranged to collapse in a tricuspid
arrangement, are
shown in the exemplary embodiment illustrated in Fig. 2, it will be clear that
a prosthetic valve
120 can include any other number of leaflets 128. The leaflets 128 are made of
a flexible
material, derived from biological materials (e.g., bovine pericardium or
pericardium from other
sources), bio-compatible synthetic materials, or other suitable materials. The
leaflets may be
coupled to the frame 126 via commissures 134, either directly or attached to
other structural
elements connected to the frame 126 or embedded therein, such as commissure
posts. Further
details regarding prosthetic valves, including the manner in which leaflets
may be mounted to
their frames, are described in U.S. Patent Nos. 6,730,118, 7,393,360,
7,510,575, 7,993,394 and
8,252,202, and U.S. Patent Application No. 62/614,299, all of which are
incorporated herein
by reference.
[0147] According to some embodiments, the prosthetic valve 120 may further
comprise at least
one skirt or sealing member, such as the inner skirt 136 shown in the
exemplary embodiment
illustrated in Fig. 2. The inner skirt 136 can be mounted on the inner surface
of the frame 126,
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
configured to function, for example, as a sealing member to prevent or
decrease perivalvular
leakage. The inner skirt 136 can further function as an anchoring region for
the leaflets 128 to
the frame 126, and/or function to protect the leaflets 128 against damage
which may be caused
by contact with the frame 126, for example during valve crimping or during
working cycles of
the prosthetic valve 120. Additionally, or alternatively, the prosthetic valve
120 can comprise
an outer skirt 137 (shown for example in Figs. 7A-7C) mounted on the outer
surface of the
frame 126, configure to function, for example, as a sealing member retained
between the frame
126 and the surrounding tissue of the native annulus against which the
prosthetic valve 120 is
mounted, thereby reducing risk of paravalvular leakage past the prosthetic
valve 120. Any of
the inner skirt 136 and/or outer skirt 137 can be made of various suitable
biocompatible
materials, such as, but not limited to, various synthetic materials (e.g.,
PET) or natural tissue
(e.g. pericardial tissue).
[0148] According to some embodiments, a prosthetic valve 120, which can be a
mechanical
prosthetic valve 120, comprises a plurality of actuator assemblies 138,
configured to facilitate
expansion of the valve 120, and in some instances, to lock the valve at an
expanded state,
preventing unintentional recompression thereof, as will be further elaborated
below. Although
Fig. 2 illustrates three actuator assemblies 138, mounted to, and equally
spaced, around an
inner surface of the frame 126, it should be clear that a different number of
actuator assemblies
138 may be utilized, that the actuator assemblies 138 can be mounted to the
frame 126 around
its outer surface, and that the circumferential spacing between actuator
assemblies 138 can be
unequal.
[0149] Figs. 3A-3B show an exemplary embodiment of an actuator assembly 138.
An actuator
assembly 138 may include a hollow outer member 140, secured to a component of
the valve
120, such as the frame 126, at a first location, and an inner member 154
secured to a component
of the valve 120, such as the frame 126, at a second location, axially spaced
from the first
location.
[0150] Fig. 3A constitutes a view in perspective of an exemplary inner member
154, having
an inner member proximal end 156 and an inner member distal end 158. The inner
member
154 comprises an inner member coupling extension 164 proximate to its distal
end 158, which
may be formed as a pin extending radially outward from the inner member 154,
configured to
be received within respective openings or apertures of struts 127 intersecting
at a junction 130
or an apex 129, 131. The inner member 154 may further comprise a linear rack
having a
21
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
plurality of teeth 162 along at least a portion of its length. According to
some embodiments,
one surface of the inner member 154 comprises a plurality of teeth 162.
[0151] The terms "including" and/or "having", as used herein (including the
specification and
the claims), are defined as comprising (i.e., open language).
[0152] Fig. 3B shows the actuation inner member 154 disposed within a lumen
146 of the outer
member 140. The outer member 140 is shown with partial transparency in Fig. 3B
for sake of
clarity. The outer member 140 comprises an outer member proximal end 142
defining a
proximal opening, and an outer member distal end 144 defining a distal
opening. The outer
member 140 can further comprise an outer member coupling extension 148
proximate to its
proximal end 142, which may be formed as a pin extending radially outward from
the external
surface of the outer member 140, configured to be received within respective
openings or
apertures of struts 127 intersecting at a junction 130 or an apex 129, 131.
[0153] The outer member 140 may further comprise a spring biased arm 150,
attached to or
extending from one sidewall of the outer member 140 and having a tooth or pawl
152 at its
opposite end, biased inwards toward the actuation inner member 154 when
disposed within the
outer member lumen 146.
[0154] At least one of the inner or outer member 154 or 140, respectively, is
axially movable
relative to its counterpart. The actuator assembly 138 in the illustrated
embodiments, comprises
a ratchet mechanism or a ratchet assembly, wherein the pawl 152 of the outer
member 140 is
configured to engage with the teeth 162 of the inner member 154. The pawl 152
can have a
shape that is complementary to the shape of the teeth 162, such that the pawl
152 allows a
sliding movement of the inner member 154 in one direction relative to the
outer member 140,
for example in a proximally oriented direction, and resists sliding movement
of the inner
member 154 in the opposite direction, such as a distally oriented direction,
when the pawl 152
is in engagement with the teeth 162 of the inner member 154.
[0155] The arm 150 can be formed of a flexible or resilient portion of the
outer member 140
that extends over and contact, at pawl 152, an opposing side of the outer
surface of the inner
member 154. According to some embodiments, the arm 150 can be in the form of a
leaf spring
that can be integrally formed with the outer member 140 or separately formed
and subsequently
connected to the outer member 140. The arm 150 is configured to apply a
biasing force against
22
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
the outer surface of the inner member 154, so as to ensure that under normal
operation, the
pawl 152 stays engaged with the teeth 162 of the inner member 154.
[0156] According to some embodiments, the inner member 154 further comprises
an inner
member threaded bore 160 extending from its proximal end 156, configured to
receive and
threadedly engage with a threaded portion 168 (shown for example in Figs. 4B-
4C) of a
corresponding actuation member 166. Fig. 3C shows a view in perspective of a
valve 120 in
an expanded state, having its actuator assemblies 138 connected to actuation
members 166
(hidden from view within the support sleeves 170) of the delivery apparatus
102. The leaflets
128 and skirt 136 are omitted from Fig. 3C to expose the actuator assemblies
138 attached to
the frame 126. When actuation members 166 are threaded into the inner members
154, axial
movement of the actuation members 166 causes axial movement of the inner
members 154 in
the same direction.
[0157] According to some embodiments, the actuation arm assemblies 165 are
configured to
releasably couple to the prosthetic valve 120, and to move the prosthetic
valve 120 between
the radially compressed and the radially expanded configurations. Figs. 4A-4C
illustrate a non-
binding configuration representing actuation of the actuator assemblies 138
via the actuation
arm assemblies 165 to expand the prosthetic valve 120 from a radially
compressed state to a
radially expanded state. Fig. 4A shows an actuator assembly 138, having an
outer member 140,
secured to the frame 126 at a first location, and an inner member 154 secured
to the frame 126
at a second location. According to some embodiments, the first location can be
positioned at
an outflow end portion 122, and the second location can be positioned at the
inflow end portion
124. In the illustrated embodiment, the outer member 140 is secured to an
outflow apex 129
via outer member coupling extension 148, and the inner member 154 is secured
to an inflow
apex 131 via inner member coupling extension 164. A proximal portion of the
inner member
154 extends, through the distal opening of the outer member distal end 144,
into the outer
member lumen 146.
[0158] The actuator assembly 138 is shown in Fig. 4A in a radially compressed
state of the
frame valve 120, wherein the outflow and inflow apices 129 and 131,
respectively, are
relatively distanced apart from each other along the axial direction, and the
inner member
proximal end 156 is positioned distal to the outer member proximal end 142.
23
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0159] As further shown in Fig. 4A, the distal portion 168 of the actuation
member 166 is
threadedly engaged with the proximal threaded bore 160 at the proximal end 156
of the inner
member 154. According to some embodiments, as shown in Figs. 4A-4C, the distal
portion 168
of the actuation member 166 includes external threads, configured to engage
with internal
threads of the proximal bore 160 of the inner member 154. According to
alternative
embodiments, an inner member may include a proximal extension provided with
external
threads, configured to be received in and engage with internal threads of a
distal bore formed
within the actuation member (embodiments not shown).
[0160] The support sleeve 170 surrounds the actuation member 166 and may be
connected to
the handle 110. The support sleeve 170 and the outer member 140 are sized such
that the distal
lip 172 of the support sleeve 170 can abut or engage the outer member proximal
end 142, such
that the outer member 140 is prevented from moving proximally beyond the
support sleeve
170.
[0161] In order to radially expand the frame 126, and therefore the valve 120,
the support
sleeve 170 can be held firmly against the outer member 140. The actuation
member 166 can
then be pulled in a proximally oriented direction 14, as shown in Fig. 4B.
Because the support
sleeve 170 is being held against the outer member 140, which is connected to
an outflow apex
129, the outflow end 123 of the frame 126 is prevented from moving relative to
the support
sleeve 170. As such, movement of the actuation member 166 in a proximally
oriented direction
14 can cause movement of the inner member 154 in the same direction, thereby
causing the
frame 126 to foreshorten axially and expand radially.
[0162] More specifically, as shown for example in Fig. 4B, the inner member
coupling
extension 164 extends through openings in two struts 127 interconnected at an
inflow apex
131, while the outer member coupling extension 148 extends through openings in
two struts
127 interconnected at an outflow apex 129. As such, when the inner member 154
is moved
axially, for example in a proximally oriented direction 14, within the outer
member 140, the
inner member coupling extension 164 moves along with the inner member 154,
thereby causing
the portion to which the inner member coupling extension 164 is attached to
move axially as
well, which in turn causes the frame 126 to foreshorten axially and expand
radially.
[0163] The struts 127 to which the inner member coupling extension 164 is
connected are free
to pivot relative to the coupling extension 164 and to one another as the
frame is expanded or
24
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
compressed. In this manner, the inner member coupling extension 164 serves as
a fastener that
forms a pivotable connection between those struts 127. Similarly, struts 127
to which the outer
member coupling extension 148 is connected are also free to pivot relative to
the coupling
extension 148 and to one another as the frame is expanded or compressed. In
this manner, the
outer member coupling extension 148 also serves as a fastener that forms a
pivotable
connection between those struts 127.
[0164] When the pawl 152 is engaged with the teeth 162, the inner member 154
can move in
one axial direction, such as the proximally oriented direction 14, but cannot
move in the
opposite axial direction. This ensures that while the pawl 152 is engaged with
the teeth 162,
the frame 126 can radially expand but cannot be radially compressed. Thus,
after the prosthetic
valve 120 is implanted in the patient, the frame 126 can be expanded to a
desired diameter by
pulling the actuation member 166. In this manner, the actuation mechanism also
serves as a
locking mechanism of the prosthetic valve 120.
[0165] Once the desired diameter of the prosthetic valve 120 is reached, the
actuation member
166 may be rotated in direction 16 to unscrew the actuation member 166 from
the inner member
154, as shown in Fig. 4C. This rotation serves to disengage between the distal
threaded portion
168 of the actuation member 166 and the inner member threaded bore 160,
enabling the
actuation arm assemblies 165 to be pulled away, and retracted, together with
the delivery
apparatus 102, from the patient's body, leaving the prosthetic valve 120
implanted in the
patient. The patient's native anatomy, such as the native aortic annulus in
the case of
transcatheter aortic valve implantation, may exert radial forces against the
prosthetic valve 120
that would strive to compress it. However, the engagement between the pawl 152
and the teeth
162 of the inner member 154 prevents such forces from compressing the frame
126, thereby
ensuring that the frame 126 remains locked in the desired radially expanded
state.
[0166] Thus, the prosthetic valve 120 is radially expandable from the radially
compressed state
shown in Fig. 4A to the radially expanded state shown in Fig. 4B upon
actuating the actuator
assemblies 138, wherein such actuation includes approximating the second
locations to the first
locations of the valve. The prosthetic valve 120 is further releasable from
the delivery
apparatus 102 by decoupling each of the actuation arm assemblies 165 from each
corresponding actuator assemblies 138 that was attached thereto.
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0167] While the inner member 154 and the outer member 140 are shown in the
illustrated
embodiment connected to an inflow apex 131 and an outflow apex 129,
respectively, it should
be understood that they can be connected to other junctions 130 of the frame
126. For example,
the inner member coupling extension 164 can extend through openings formed in
interconnected struts at a junction 130 at the inflow end portion 124,
proximal to the inflow
apices 131. Similarly, the outer member coupling extension 148 can extend
through openings
formed in interconnected struts at a junction 130 at the outflow end portion
122, distal to the
outflow apices 129.
[0168] While the frame is shown above to expand radially outward by axially
moving the inner
member 154 in a proximally oriented direction, relative to the outer member
140, it will be
understood that similar frame expansion may be achieved by axially pushing an
outer member
140 in a distally oriented direction, relative to an inner member 154.
Moreover, while the
illustrated embodiments show the outer member 140 affixed to an outflow end
portion 122 of
the frame 126, and an inner member 154 affixed to an inflow end portion 124 of
the frame 126,
in alternative embodiments, the outer member 140 may be affixed to the inflow
end portion
124 of the frame 126, while the inner member 154 may be affixed to the outflow
end portion
122 of the frame 126.
[0169] According to some embodiments, the handle 110 can comprise control
mechanisms
which may include steerable or rotatable knobs, levers, buttons and such,
which are manually
controllable by an operator to produce axial and/or rotatable movement of
different
components of the delivery apparatus 102. For example, the handle 110 may
comprise one or
more manual control knobs, such as a manually rotatable control knob that is
effective to pull
the actuation members 166 when rotated by the operator.
[0170] According to other embodiments, control mechanisms in handle 110 and/or
other
components of the delivery apparatus 102 can be electrically, pneumatically
and/or
hydraulically controlled. According to some embodiments, the handle 110 can
house one or
more electric motors which can be actuated by an operator, such as by pressing
a button or
switch on the handle 110, to produce movement of components of the delivery
apparatus 102.
For example, the handle 110 may include one or more motors operable to produce
linear
movement of components of the actuation arm assemblies 165, and/or one or more
motors
operable to produce rotational movement of the actuation members 166 to
disconnect the
actuator member distal threaded portion 168 from the actuation inner member
threaded bore
26
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
160. According to some embodiments, one or more manual or electric control
mechanism is
configured to produce simultaneous linear and/or rotational movement of all of
the actuation
members 166.
[0171] While a specific actuation mechanism is described above, utilizing a
ratcheting
mechanism between the inner and the outer members of the actuation assemblies
138, other
mechanisms may be employed to promote relative movement between inner and
outer
members of actuation assemblies, for example via threaded or other engagement
mechanisms.
Further details regarding the structure and operation of mechanically
expandable valves and
delivery system thereof are described in US Patent No. 9,827,093, U.S. Patent
Application
Publication Nos. 2019/0060057, 2018/0153689 and 2018/0344456, and US Patent
Application
Nos. 62/870,372 and 62/776,348, all of which are incorporated herein by
reference.
[0172] Prior to implantation, the prosthetic valve 120 can be crimped onto the
delivery
apparatus 102. This step can include placement of the radially compressed
valve 120 within
the outer shaft 104. Once delivered to the site of implantation, such as a
native annulus, the
valve 120 can be radially expanded within the annulus, for example, by
actuating the actuator
assemblies 138 described herein above. However, during such implantation
procedures, it may
become desirable to re-compress the prosthetic valve 120 in situ in order to
reposition it. Valve
recompression may be achievable, for example, if the mechanical valve 120 has
not yet reached
a locked state, for example by providing a sufficient smooth length (i.e.,
devoid of ratcheting
teeth 162) along the actuator inner member 154, so as to allow axial movement
along a specific
distance prior to pawl 152 engagement with the teeth 162. Alternatively or
additionally, the
delivery assembly 100 can further include release members (not shown),
configured to release
the pawl 152 from the teeth 162 to allow reversible movement that will enable
valve
compression.
[0173] According to some embodiments, the delivery apparatus 102 further
comprises a re-
compression assembly 180, configured to facilitate re-compression of a
prosthetic valve 120
upon expansion thereof.
[0174] Reference is now made to Figs. 5A-5E, showing different optional stages
of utilizing a
delivery assembly 100 equipped with a re-compression assembly 180. Fig. 5A
shows an
enlarged view of a distal portion of the delivery assembly 100, carrying a
prosthetic valve 120
retained in a compressed or crimped state within a distal portion of the outer
shaft 104 during
27
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
delivery to the implantation site. As described above, the distal portion of
the outer shaft 104
can serve as a delivery capsule that covers the crimped prosthetic valve 120.
Upon reaching
the desired site of implantation, the outer shaft 104 can be retracted to
expose the prosthetic
valve 120. Fig. 5A shows partial retraction of the outer shaft 104, exposing a
distal portion of
the valve 120, such as the inflow end portion 124.
[0175] Fig. 5B shows the prosthetic valve 120 exposed (i.e., no longer covered
by the outer
shaft 104). Certain prosthetic valves 120, such as certain mechanically
expandable valves as
described above in conjunction with Figs. 2-4C, may be provided with internal
resiliency
promoting partial expansion thereof when extended out of a capsule or outer
shaft 104.
Furthermore, the mechanically expandable valve 120 may be partially expanded
further to a
larger diameter, prior to being locked in an irreversible manner by the
engagement between the
ratcheting teeth 162 and the pawl 152. For example, a proximal toothless
portion of the actuator
inner member 154 may be provided between the inner member's proximal end 156
and the
ratcheting teeth 162, enabling axial movement between the inner and the outer
members 154
and 140, respectively, along which the axial translation of the inner member
154 is reversible.
[0176] Once the valve 120 is at least partially expanded, either due to its
inherent resiliency or
due to active expansion thereof, prior to being in a locked state, the re-
compression assembly
180 may be utilized to re-compress the valve 120 to a narrower diameter. The
re-compression
assembly 180 may be similarly utilized in combination with a self-expandable
valve in a similar
manner to the following description, once the valve is expanded, for example,
if valve re-
positioning is required. Similarly, as mentioned above, a re-compression
assembly 180 may be
utilized once a mechanically expandable valve 120 is expanded to a locked
state of the actuator
assemblies 138, by utilizing release members that can disengage the pawl 152
from the
ratcheting teeth 162 of the actuator assemble 138.
[0177] According to some embodiments, the re-compression assembly 180
comprises a re-
compression member 182 extending through the lumen of a re-compression shaft
188. The re-
compression shaft 188 extends through the lumen of the delivery shaft 106. The
re-compression
member 182 comprises a flexible re-compression member distal segment 184,
which may be
formed of a flexible wire, cable, suture and the like. The flexible re-
compression member distal
segment 184 is configured to extend distally through an opening formed at the
re-compression
shaft distal end 192, and optionally surround either the valve 120 or
components attached
thereto, such as the support sleeves 170 of actuation arms assemblies 165.
28
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0178] The re-compression member 182 further comprises a re-compression member
proximal
segment 186, which extends through the lumen of the re-compression shaft 188
toward, and
optionally into, the handle 110. In some instances, the re-compression member
proximal
segment 186 may be formed as a continuous extension of the flexible re-
compression member
distal segment 184. Alternatively, the re-compression member proximal segment
186 and the
re-compression member distal segment 184 may be provided as separate
components attached
to each other, wherein either both segments are formed from the same materials
having the
same dimensions, or both are formed from the same materials but each is having
different
dimensions (e.g., one segment being thicker than the other), or each is formed
from different
materials but both are having similar dimension or dissimilar dimensions with
respect to one
another. For example, the re-compression member proximal segment 186 may be
formed from
a stiffer material than the re-compression member distal segment 184.
Additionally or
alternatively, the re-compression member proximal segment 186 may be formed as
a thicker
member than the re-compression member distal segment 184. Any of the re-
compression
member distal segment 184 and/or the re-compression member proximal segment
186 can be
in the form of, for example, a cord, a suture, a wire, a cable or any other
flexible material that
can be tensioned.
[0179] A zoomed-in portion of one exemplary re-compression assembly 180 is
shown in Fig.
5B. In the exemplary embodiment illustrated, the re-compression member 182
comprises re-
compression member proximal segment 186 and re-compression member distal
segment 184
which are separate components, attached to each other via a connector 194.
According to some
embodiments, two proximal ends of the re-compression member distal segment 184
are
attached directly or indirectly to a distal end of the re-compression member
proximal segment
186. In the exemplary embodiments shown in Fig. 5B, the re-compression member
distal
segment 184 is looped through a ring-like portion of a connector 194, having
two parallel
portions thereof extending distally from the connector 194 within the lumen of
the re-
compression shaft 188. The connector 194 can take any other form, configured
to attach to the
re-compression member distal segment 184 and to the re-compression member
proximal
segment 186. Alternatively, the re-compression assembly 180 may be provided
without a
connector 194. For example, the re-compression member distal segment 184 can
be directly
attached to a separate re-compression member proximal segment 186. In another
example, the
re-compression member distal segment 184 and the re-compression member
proximal segment
29
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
186 can be integrally formed, each constituting a different region of a single
continuous re-
compression member 182.
[0180] As further shown in Fig. 5B, a distal portion of the re-compression
member distal
segment 184, extending out of the re-compression shaft distal end 192, may
comprise a loop
portion 183 configured to circumscribe the prosthetic valve 120. The handle
110 may be
maneuvered, for example via knobs, buttons and the like, to adjust the tension
on the loop
portion 183. For example, a re-compression actuation mechanism (not shown) can
be
maneuvered at the handle 110 to either increase tension on the re-compression
member 182, or
release such tension, in order to readjust the diameter of loop portion 183.
[0181] Adjustment of the diameter of the loop portion 183 can be achieved, for
example, by
advancing the re-compression shaft distal end 192 in a distally oriented
direction, relative to
the re-compression shaft distal end 192, thereby reducing the loop portion
diameter. Tensioning
the loop portion 183 to reduce its diameter applies, in turn, an inwardly
directed force on the
valve 120, effective to compress the valve 120. Similarly, retraction of the
re-compression shaft
distal end 192 in a proximally oriented direction relative to the re-
compression member distal
segment 184 releases such tension, allowing the valve 120 to re-expand, either
due to an
internal resiliency of the frame 126, or via activation of expansion
mechanisms, such as the
actuator assemblies 138.
[0182] According to some embodiments, the re-compression shaft 188 is
operatively
connected to a re-compression actuation mechanism in the handle 110, operable
by a knob,
button, switch and the like. The re-compression actuation mechanism can be
used to axially
translate the re-compression shaft 188 in a proximal or distal direction,
relative to the re-
compression member 182.
[0183] It should be noted that the relative movement between the re-
compression shaft 188
and the re-compression member 182 in the axial direction, refers to movement
of the re-
compression shaft 188 relative to the re-compression member 182, and/or
movement of the re-
compression member 182 relative to the re-compression shaft 188. According to
some
embodiments, the re-compression member distal segment 184 can be retracted in
a proximally
oriented direction relative to the re-compression shaft distal end 192, in
order to facilitate valve
compression. Similarly, the re-compression member distal segment 184 can be
advanced in a
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
distally oriented direction relative to the re-compression shaft distal end
192 in order to relieve
tension and allow valve expansion.
[0184] Fig. 5B shows an exemplary state in which the valve 120 is partially
expanded, after
being released from the outer shaft 104. In this state, the loop portion 183
is relatively loose
around the valve 120, e.g., loose enough to allow partial or full valve
expansion. In some
instances, the loop portion 183 may be kept in a tensioned state around a
crimped valve 120
during delivery to the implantation site, thereby providing additional means
by which the valve
120 is kept in a crimped diameter, which may be utilized in addition to, or
instead of, covering
the valve 120 within a capsule or within the distal portion of the outer shaft
104. In such cases,
the diameter of the loop portion 183 may be re-adjusted during the procedure.
For example,
loop portion 183 may be loosened once the prosthetic valve 120 reaches a
desired implantation
site, and/or once the outer shaft 104 is retracted to expose the valve 120.
Partial loosening of
the loop portion 183 may provide control over the valve expansion diameter and
rate of
expansion. Further releasing the loop portion 183 may allow full expansion of
the valve 120.
[0185] Thus, relative movement between the re-compression member 182 and the
re-
compression shaft 188 in the axial direction is effective to tighten the loop
portion 183 around
the prosthetic valve 120, thereby radially compressing the prosthetic valve
120. Specifically, a
tensioned state of the re-compression assembly 180 is defined as a state in
which the tension
of the re-compression member distal segment 184 is sufficient to either
compress the valve
120, or retain it such that the valve 120 cannot expand beyond a maximal
diameter, dictated by
the tension force of the re-compression member 182. A partially tensioned
state refers to any
tensioned state in which the valve 120 is partially expanded, where at any of
the partially
tensioned states, the valve 120 cannot expand beyond a maximal diameter
(determined by the
tension of loop portion 183) and wherein the maximal diameter is higher than
the crimped-state
diameter. A released state of the re-compression assembly 180 is defined as a
state in which
the tension of the re-compression member distal segment 184 does not resist
valve expansion,
thereby allowing free valve expansion.
[0186] While the re-compression member 182 is tightly tensed around the
prosthetic valve 120
in a tensioned state of the re-compression assembly 180, it may loosely
surround the prosthetic
valve 120 when tension is relieved, for example in a released state, or when
the diameter of the
prosthetic valve 120 is lower than the maximal diameter allowable by the loop
portion 183. In
some cases, it may be desirable to keep the loop portion 183 tensed around the
prosthetic valve
31
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
120 at all times, including in a released state, when the prosthetic valve 120
is free to expand
in the radial direction. The constantly tensioned state of the loop portion
183 around the
prosthetic valve 120 can be advantageous, for example, if the loop portion 183
is utilized for
estimation of prosthetic valve diameter 120, as will be elaborated further
below. Under such
configuration, the loop portion is tightly wrapped around the outer surface of
the valve 120
across the entire range of potential valve diameters, between the compressed
state and the fully
expanded state.
[0187] According to some embodiments, a minimal tension magnitude Ts is always
applied to
the re-compression member distal segment 184, and more specifically, to the
loop portion 183.
Such tension is configured to retain the re-compression member distal segment
184 in a
minimally tensed state, even in the absence of external forces acting to
collapse the valve 120.
For example, the minimal tension magnitude Ts may be applied in a released
state of the re-
compression assembly 180. The minimal tension magnitude Ts is selected so as
to apply
sufficient biasing force to keep the loop portion 183 tensed around the valve
120 or other
elements attached thereto, such as actuation arm assemblies 165, yet not high
enough to resist
valve expansion. Thus, the tension applied by the re-compression member distal
segment 184
is higher than Ts in a tensed state of the re-compression assembly 180, and
may be equal to Ts
in a released state of the re-compression assembly 180.
[0188] Fig. 5C shows an exemplary tensioned state of the re-compression
assembly 180,
achieved by pulling the re-compression member distal segment 184 in a
proximally oriented
direction, relative to the re-compression shaft distal end 192, thereby
tensioning the loop
portion 183 so as to apply sufficient radial force to compress the valve 120.
As shown, the
position of the connector 194 in Fig. 5C is proximal relative to its position
in Fig. 5B. Suh
configuration enables re-positioning of the prosthetic valve 120, and/or
prosthetic valve re-
capturing for removal thereof from the patient.
[0189] Fig. 5D shows an exemplary released state of the re-compression
assembly 180, which
may be applicable, for example, upon reaching a desired implantation site
(e.g., after valve
repositioning within the patient's body). In this state, the re-compression
member distal
segment 184 is released, allowing the valve 120 to re-expand, for example to a
fully expanded
diameter. As shown, the position of the connector 194 in Fig. 5D is distal
relative to its position
in Fig. 5C or in Fig. 5B.
32
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0190] According to some embodiments, the re-compression shaft 188 and the re-
compression
member proximal segment 186 may be retracted, as shown in Fig. 5E, for example
by
maneuvering the handle 110 to pull them in a proximally oriented direction,
wherein the loop
portion 183 no longer tightly surrounds the valve 120. Retraction of the re-
compression
assembly 180 may be performed during the implantation process, for example, to
allow
unhindered expansion of the valve 120. Alternatively or additionally, the re-
compression
assembly 180 may be retracted in lieu of delivery apparatus 102 retraction,
for example, after
completing the valve positioning and expansion procedures.
[0191] Prosthetic valve expansion against the surrounding tissue may pose a
variety of risks
associated with a mismatch between the valve expansion diameter and the
surrounding tissue.
One complication is related to valve over-expansion, which may exert excessive
radial forces
on the surrounding anatomy, resulting in potential damage to the tissue or
even annular rupture.
On the other hand, valve under-expansion might increase the risk of aortic
valve or mitral valve
regurgitation. Inappropriate expansion may also result in unfavorable
hemodynamic
performance across the valve 120, such as increased pressure gradients or flow
disturbances
resulting from diameter mismatch, which may be associated with increased risk
of thrombus
formations.
[0192] Thus, in order to avoid the deleterious effects of either annular
rupture, inferior
hemodynamic performance or valve regurgitation, arising due to either over-
expansion or
under-expansion, respectively, of the valve frame 126, a clinician should be
able to control the
degree of frame 126 expansion according to real-time feedback received during
the procedure,
indicating, for example, current valve diameter and/or expansion force.
[0193] According to an aspect of the invention, the re-compression assembly
180 is configured
to provide real-time feedback, such as visual or auditory real-time feedback,
regarding the
radial expansion diameter of the prosthetic valve 120.
[0194] According to some embodiments, the re-compression member distal segment
184
comprises at least one radiopaque marker 196. The at least one, and optionally
a plurality of,
radiopaque markers 196, may span along at least a portion of, and preferably
along the entire
length of, the loop portion 183. According to some embodiments, the at least
one, and
optionally a plurality of, radiopaque markers 196, span along the entire
length of the re-
compression member distal segment 184. A radiopaque marker 196 comprise a
radiopaque
33
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
material, understood to be capable of producing a relatively bright image on a
fluoroscopy
screen or another imaging technique, during the prosthetic valve 120
implantation procedure.
Radiopaque materials can include, but are not limited to, gold, platinum,
tantalum, tungsten
alloy, platinum iridium alloy, palladium, and the like.
[0195] As noted, the loop portion 183 may be configured to be tightly wrapped
around the
external surface of the valve 120 at all times, either during a tensioned
state or during a released
state of the re-compression assembly 180, due to the minimal tension magnitude
Ts applied on
the re-compression member distal segment 184. Thus, the at least one, and
optionally a plurality
of, radiopaque markers 196 disposed along the loop portion 183 wrapped around
the valve 120,
may provide a real-time visually detectable indication of the diameter of the
prosthetic valve
120.
[0196] Figs. 6A-6B show different configurations of radiopaque markers 196
disposed along
at least a portion of the re-compression member distal segment 184, according
to some
embodiments. Fig. 6A shows a variant of the radiopaque markers 196, provided
in the form of
a plurality of radiopaque bands disposed along at least a portion of the
length of the re-
compression member distal segment 184. According to some embodiments, a
plurality of
radiopaque markers 196, such as radiopaque marker bands, may be spaced at
known distances
from each other, for example along a portion the loop portion 183, such that
the radiopaque
marker bands 196 could be used to provide visual estimates of the diameter of
the prosthetic
valve 120. The plurality of radiopaque markers 196 may be spaced from each
other along at
least a portion of the loop portion 183 at any desired pattern. For example,
the plurality of
radiopaque markers 196 may be equally spaced from each other, or may be spaced
at varying
distances from each other.
[0197] According to some embodiments, the plurality of radiopaque markers 196
are disposed
along the loop portion 183 at various positions, thereby providing visual
indication of the valve
diameter. For example, the plurality of radiopaque markers 196 may span along
a portion of
the loop portion 183, which is substantially equal to at least half of the
prosthetic valve
perimeter when expanded to a maximal diameter, so as to ensure detection of
any sub-maximal
diameters. Similarly, the positions of the plurality of radiopaque markers 196
may be set to
cover at least half of the prosthetic valve perimeter when fully expanded, and
circumscribed
by the loop portion 183. In some instances, it may be preferable to cover the
entire loop portion
34
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
183 with the plurality of radiopaque markers 196 in order to compensate for
situations in which
the marked region is not aligned with the angle of view during fluoroscopy.
[0198] According to some embodiments, radiopaque markers 196 can be formed by
means of
radiopaque inks and adhesives, and applied on at least a portion of the re-
compression member
distal segment 184 in a number of ways, such as screen printing, high speed
roller printing,
coating, dipping, etc. According to yet further embodiments, the markers can
provided as
separately formed components, such as annular rings or C-shaped bands that are
mounted on
the re-compression member distal segment 184.
[0199] According to some embodiments, as shown in Fig. 6B, a single radiopaque
marker 196
is disposed along a minimal marking length, at a position which preferably
corresponds to the
contact region between the loop portion 183 and the perimeter of the
prosthetic valve 120. A
minimal marking length may be chosen so as to enable diameter estimation of
the valve 120,
across its entire range of diameters. For example, the minimal marking length
can correspond
to the perimeter of the valve 120, in a range between the minimal crimping
diameter and the
maximal expansion diameter. According to some embodiments, the minimal marking
length is
at least as great as the perimeter of the prosthetic valve 120 when fully
expanded. According
to some embodiments, the entire length of the re-compression member distal
segment 184
comprises a single continuous radiopaque marking 196.
[0200] According to some embodiments, the radiopaque marking is formed as a
radiopaque
coating 196, such that the re-compression member distal segment 184 is coated
by a radiopaque
material along a minimal marking length, which optionally can include its
entire length.
[0201] According to some embodiments, the connector 194 is a releasable
connector,
configured to releasably attach the re-compression member proximal segment 186
to the re-
compression member distal segment 184. Figs. 7A-7C show an exemplary
embodiment of a
delivery assembly 100 equipped with a re-compression assembly 180 having a
releasable
connector 194, according to some embodiments. Fig. 7A shows the re-compression
assembly
180 in a state wherein re-compression member proximal segment 186 is connected
to the re-
compression member distal segment 184 via the releasable connector 194.
According to some
embodiments, the releasable connector 194 comprises a proximal connector
element 193 and
a distal connector element 195, releasably attached to each other. The re-
compression member
proximal segment 186 is coupled to the proximal connector element 193, while
the re-
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
compression member distal segment 184 is coupled to the distal connector
element 195. In the
illustrated example, a re-compression member distal segment 184 may loop
through an eyelet
formed in the distal connector element 195, though any other type of coupling
is contemplated.
[0202] In some applications, the loop portion 183 of the re-compression member
distal
segment 184 may extend through a circumferential sleeve circumscribing the
valve 120. In the
exemplary embodiment illustrated in Figs. 7A-7C, an outer skirt 137 comprises
a sleeve 132
integrally formed therewith, for example along a proximal edge of the outer
skirt 137. The
sleeve 132 can be provided with an opening 133 through which the re-
compression member
distal segment 184 may extend into a lumen of the sleeve 132. While the sleeve
132 is shown
in Figs. 7A-7C as integrally formed with, or attached to (e.g., sewn to), the
outer skirt 137, it
will be clear that in alternative applications, a stand-alone sleeve can be
provided around the
valve 120, such as the circumferential sleeve 830 illustrated (as shown, for
example, in Figs.
15A-16B). Moreover, while shown in Figs. 7A-7C in conjunction with a re-
compression
assembly 180 having a releasable connector 194, it will be clear that the re-
compression
member distal segment 184 looped around the valve 120 according to any other
embodiments
of the invention, such as the embodiments described and illustrated in
conjunction with Figs.
6A-6B, may similarly extend through sleeves 130 or 830.
[0203] The sleeve 132, 830 circumscribing the valve 120 is configured to
retain at least a
portion of the re-compression member distal segment 184 around at least a
portion of the
circumference of the valve 120. In some applications, the sleeve may be
disposed around the
entire circumference, such as shown for sleeve 132 in Figs. 7A-7C and for
sleeve 830 in Figs.
15A-16B. In some applications, the sleeve cab be disposed around a portion of
the
circumference of the valve 120, such as shown for sleeve 830 in Figs. 28A-29B.
In some
applications, a circumferential sleeve circumscribing the valve 120 may
comprise a plurality
of sleeve-portions (not shown), disposed around a circumference of prosthetic
valve 120,
circumferentially spaced from each other.
[0204] According to some embodiments, the valve 120 further comprises a guide
member 840
disposed between the outflow end 123 and the sleeve 132, 830. The guide member
840 is
provided with a guide member lumen 842, defined between a guide member
proximal end 844
and a guide member distal end 846. The guide member proximal end 844 can be
positioned in
alignment with, or distal to, the outflow end 123. The guide member distal end
846 is positioned
36
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
proximal to the guide member sleeve 132, 830, and more specifically, can be
positioned
proximal to the guide member sleeve opening 133, 833.
[0205] At least a portion of the re-compression member 182 extends through the
guide member
lumen 842, and is axially movable there-through. In the illustrated example, a
proximal portion
of the re-compression member distal segment 184, the releasable connector 194,
and a distal
portion of the re-compression member proximal segment 186, may extend through
and be
axially movable within the member 840.
[0206] The re-compression member distal segment 184 can include a plurality of
radiopaque
markers 196 as described and illustrated in conjunction with Fig. 6A, or a
single radiopaque
marker 196 disposed along a minimal marking length thereof, as described and
illustrated in
conjunction with Fig. 6B. The sleeve 132, 830 can comprise a radiolucent
material or include
a cut-out window, so as to allow visibility of radiopaque markers 196 under
fluoroscopy,
thereby enabling the re-compression assembly 180, shown in Figs. 7A-7B, to be
utilized for
providing real-time detectable indications of the diameter of the prosthetic
valve 120 according
to any of the embodiments described and illustrated in conjunction with Figs.
6A-6B.
[0207] Once the desired diameter of the prosthetic valve 120 is reached, at
least a portion of
the re-compression assembly 180 may be released from the valve 120.
Specifically, as shown
in Fig. 7B, the re-compression member proximal segment 186 can be released
from the re-
compression member distal segment 184, which may in turn remain around the
expanded valve
120.
[0208] According to some embodiments, the distal connector element 195 may be
provided
with external threads, which may be engaged with a threaded bore of the
proximal connector
element 193. It will be clear that in alternative applications, the distal
connector element 195
may be provided with a threaded bore, and the proximal connector element 193
may be
provided with matching external threads. In embodiments wherein the distal
connector element
195 and the proximal connector element 193 are threadedly engaged with each
other, the re-
compression member proximal segment 186 may comprises a relatively rigid
material, formed
as a torque transferring wire, cable and the like.
[0209] As shown in Figs. 7B, the proximal connector element 193 can be
released from the
distal connector element 195, and proximally pulled along with the re-
compression member
proximal segment 186, for example through the lumen of the re-compression
shaft 188. Fig.
37
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
7C shows a further step of pulling the re-compression shaft 188 from the guide
member 840.
In some applications, a distal portion of the re-compression shaft 188 is
disposed within the
guide member lumen 842. Additionally or alternatively, a distal portion or a
distal end of the
re-compression shaft 188, is releasably attached to the guide member 840.
[0210] The guide member 840 may be formed as a rigid hollow member, such as a
tube or any
other hollow member with other circular or non-circular cross-sections. The
guide member 840
is rigidly attached to the frame 126, directly or indirectly (e.g., via
another component of the
prosthetic valve 120 attached to the frame 126). According to some
embodiments, the guide
member 840 may be attached a commissure post or a component of the actuator
assembly 138,
such as the actuator outer member 140 as shown in Figs. 7A-7C, wherein
attachment may be
accomplished by welding, gluing, soldering and the like. Alternatively, the
guide member 840
can be attached to the frame 126, such as to at least one junction 130
(alternative embodiments
not shown).
[0211] Another example of a re-compression assembly 180 is shown in Figs. 8A-
8B, wherein
the re-compression member distal segment 184 comprises a distal loop portion
183 which is
wrapped around, or extending between, the support sleeves 170, instead of
circumscribing the
external surface of the prosthetic valve 120. As shown in Fig. 8A, each
support sleeve 170 can
include a loop attachment member 176 in the vicinity of its distal end 172.
The re-compression
member distal segment 184, and more specifically, the loop portion 183, is
connected to, and
extending between, the loop attachment members 176 of the actuator arm
assemblies 165. For
example, the loop portion 183 may be threaded through eyelet-shaped loop
attachment
members 176, as shown in a zoomed-in view of the attachment region between the
actuator
arm assemblies 165 and the prosthetic valve 120 on the top-right of Fig. 8A,
and a zoomed-in
region of the distal portion of a single support sleeve 170 having a loop
attachment member
176 on the top-left of Fig. 8A.
[0212] The loop attachment members 176 can be in the form of eyelets, hooks,
rings, clips,
apertures within the support sleeves 170 and/or the actuation members 166, and
any other
structural elements configured to retain there-between, and enable extension
of, the re-
compression member distal segment 184, and more specifically, the loop portion
183.
[0213] According to some embodiments, relative movement between the re-
compression
member 182 and the re-compression shaft 188 in the axial direction, is
effective to apply
38
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
tension to the loop portion 183 connected to, resulting in radial inward
movement of the
actuation arm assemblies 165, thereby radially compressing the prosthetic
valve 120. Fig. 8A
illustrates the loop portion 183 extending between the support sleeves 170 in
a tensioned state
of the re-compression assembly 180, applying inwardly directed force on the
actuation arm
assemblies 165. As long as the actuation members 166 are attached to the
actuator assemblies
138, the frame 126 of the valve 120 is also proportionally radially
compressed.
[0214] Tensioning of the re-compression assembly 180 shown in Fig. 8A may be
achieved by
maneuvering the handle so as to pull the re-compression member proximal
segment 186 in a
proximally oriented direction relative to the re-compression shaft 188.
Tension release may be
achieved by releasing the pulling force, allowing the re-compression member
proximal
segment 186 to translate in a distally oriented direction as the prosthetic
valve 120 expands.
[0215] Fig. 8B illustrates a released state of the re-compression assembly
180, wherein the
prosthetic valve 120 is allowed to expand relative to its compressed state in
Fig. 8A. As
illustrated, the loop portion 183 is tightly extended between the actuation
arm assemblies 165
both in the expanded state (see Fig. 8B) and the compressed state (see Fig.
8A) of the prosthetic
valve 120, for example due to the minimal tension magnitude Ts applied to the
re-compression
member 182.
[0216] According to some embodiments, the re-compression shaft 188 is
immovable in an
axial direction, such that adjustment of the tension applied to the loop
portion 183 may be
facilitated by either applying, or releasing a pulling force, on the re-
compression member
proximal segment 186. Thus, axial translation of the re-compression member
proximal
segment 186 is proportional to the perimeter of the loop portion 183, which in
turn is
proportional to the diameter of the prosthetic valve 120, as long as the
actuation arm assemblies
165, to which the loop portion 183 is connected, are coupled to the prosthetic
valve 120 (e.g.,
to the valve actuator assemblies 138).
[0217] According to some embodiments, the delivery apparatus 102, and more
specifically the
handle, further comprises a diameter gauge. The diameter gauge is coupled to
the re-
compression assembly at a gauge coupling point, such that expansion or
contraction of the
prosthetic valve 120, when attached to the actuation arm assemblies 165, is
effective to axially
translate the position of the gauge coupling point. The diameter gauge is
configured to provide
39
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
real-time indication of the valve diameter, based on axial position and/or
axial translation of
the gauge coupling point.
[0218] According to some embodiments, the real-time indication provided by the
diameter
gauge is a visual real-time indication. According to some embodiments, the
real-time indication
provided by the diameter gauge is a signal (e.g., an electric signal or an
optic signal) generated
by the diameter gauge. According to some embodiments, the diameter gauge is
coupled to the
re-compression member proximal segment 186 at a gauge coupling point.
[0219] Figs. 8A-8B show an exemplary embodiment of a handle 210, which may be
substantially similar to handle 110. The main difference is that handle 210
further comprises a
diameter gauge 250 coupled to the re-compression member proximal segment 186,
and
configured to provide real-time indication of the diameter of the prosthetic
valve 120, based on
axial position and/or axial translation of the gauge coupling point 270, as
will be elaborated in
further detail below.
[0220] According to some embodiments, the re-compression member proximal
segment 186
extends into the handle 210, for example in order to connect with an internal
mechanism housed
within the handle 210, configured to maneuver the re-compression assembly 180
between a
released state and a tensioned state thereof, including a variety of partially
tensioned states that
may correspond to a variety of respective valve maximal diameters.
[0221] According to some embodiments, the handle 210 may include a user
operable element,
such as steerable or rotatable adjustment knobs, levers, sliders, buttons (not
shown) and the
like, configured to allow a user to adjust the tensioning force applied to the
re-compression
member 182. Additionally or alternatively, the handle 210 may comprise an
automated
mechanism configured to re-adjust such tensioning force according to input
received from a
user interface or from sensors operably coupled to components of the delivery
apparatus 100.
[0222] In some cases, tension applied to the re-compression member 182, for
example by
applying a certain magnitude of a pull-force thereto, may extend length of the
re-compression
member 182 to a certain degree, relative to its length in a released state, or
relative to its length
at other pull force magnitudes that may be applied thereto. Such changes in
the length of the
re-compression member 182 may alter the position of the re-compression member
proximal
segment 186 within the handle. Lengthening of the re-compression member 182
may result in
inaccuracies in valve diameter estimation, as indicated by a diameter gauge,
which is based on
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
the position of the re-compression member proximal segment 186, relative to
the diameter
gauge.
[0223] According to some embodiments, the re-compression member proximal
segment 186
is connected within the handle 210 to a spring 220. The spring 220 may be
affixed to a spring
support member 212 of the handle 210 at a spring first end 222. A spring
second end 224,
opposite to the spring first end 222, may be connected to the re-compression
member proximal
segment 186.
[0224] The spring 220 is configured to apply an axially oriented pull-force on
the re-
compression member proximal segment 186 in a released state. The magnitude of
the force
applied by the spring 220 is sufficient to apply the minimal tension magnitude
Ts to the loop
portion 183, in the absence of other external forces effective to tighten the
loop portion 183
around the actuation arm assemblies 165.
[0225] Figs. 8A-8B schematically illustrate the interior of the handle 210,
wherein the re-
compression member proximal segment 186 is shown to extend all the way into
the handle 210
through the lumen of the delivery shaft 106. The spring 220 is schematically
illustrated, having
the first spring end 222 affixed to spring support member 212 of the handle
210, at a position
proximal to the attachment point between the spring second end 224 and the re-
compression
member proximal segment 186. In such a configuration, the spring 220 is
preferably a coil
compression spring, having a spring coefficient suitable to pull the re-
compression member
proximal segment 186 by a pull force that matches the desired minimal tension
magnitude Ts.
Advantageously, applying the minimal tension magnitude Ts to the loop portion
183 allows
more accurate measurement of prosthetic valve diameter.
[0226] It will be understood that the spring first end 222 and the spring
second end 224 may
be positioned in any number of alternative locations within the handle 210,
which may dictate
the type of spring utilized in conjunction with the re-compression assembly
180. For example,
if the spring first end 222 is positioned distal to the spring second end 224
(configuration not
shown), it may be necessary to implement an extension spring instead of a
compression spring,
such that the spring 220 may extend the re-compression member proximal segment
186
attached to its second end 224 in a proximally oriented direction, to apply
the minimal tension
magnitude Ts on the loop portion 183, as described above.
41
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0227] According to some embodiments, the spring first end 222 comprises a
hook, an eyelet,
a ring and the like, by which it may be coupled to the spring support member
212 of the handle
210. According to some embodiments, the spring's second end 224 comprises a
hook, an eyelet,
a ring and the like, by which it may be coupled to the re-compression member
proximal
segment 186, a dial 254 (described below), or both.
[0228] According to some embodiments, the handle 210 includes a user-operable
mechanism
(not shown in Figs. 8A-8B) connected to the re-compression member proximal
segment 186
and configured to pull the re-compression member proximal segment 186 in a
proximally
oriented direction, to compress the prosthetic valve 120 and/or retain it in a
maximal desired
diameter. Since the tension magnitude applied to the loop portion 183 in a
tensioned state of
the re-compression assembly 180, is higher than the minimal tension magnitude
Ts, the pulling
force applied to the re-compression member proximal segment 186 in a tensioned
state, is
higher than the pulling force applied by the spring 220 in a released state.
[0229] When tension is relieved in a released state of the re-compression
assembly 180, the
only pull force applied to the re-compression member proximal segment 186 is
the pull force
of the spring 220. This, in turn, allows the re-compression member proximal
segment 186 to
translate in a distally oriented direction if the prosthetic valve 120 is
expanded, thereby
extending the spring 220 (in the case of a compression spring) in the same
direction, as shown
in Fig. 8B.
[0230] Various types of springs can be used instead of a coil compression
spring 220, such as
tension springs, torsion springs or leaf springs. Alternatively, the spring
220 can be replaced
and/or additionally accompanied by other biasing members, such as stretchable
and/or elastic
cords, elastomeric bodies (e.g., a silicone of polyurethane component) which
is compressible
under external force application, and returns to its original shape when such
force is removed.
Any such biasing member may replace a coil compression spring 220 as long as
it applies a
biasing force sufficient to apply a minimal tension magnitude to the loop
portion 183.
[0231] According to some embodiments, the handle 210 comprises a diameter
gauge 250
coupled to the re-compression member proximal section 186 at a gauge coupling
point 270.
The diameter gauge 250 is configured to provide a real-time visual indication
of the diameter
of the prosthetic valve 120, based on axial position and/or axial translation
of the gauge
coupling point 270 within the handle 210. The state of the diameter gauge 250
may be visible
42
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
through a visual interface, such as the display 116. In other words, the
diameter gauge 250 may
be configured to provide real-time visual indication of the diameter of the
prosthetic valve 120
via the display 116.
[0232] According to some embodiments, as shown in Figs. 8A-8B, the diameter
gauge 250
comprises a dial 254 coupled, directly or indirectly, to the re-compression
member proximal
segment 186, at a gauge coupling point 270. The dial may be pivotably attached
to a dial
support member 214 of the handle 210 via a dial pivot 256. The dial 254 is
configured to rotate
angularly about the dial pivot 256 when the re-compression member proximal
segment 186
translates in an axial direction.
[0233] According to some embodiments, the diameter gauge 250 comprises a scale
or
indicator marks 258, wherein each indicator mark may be in the form of a
numerical value, or
any other symbol, representative of a specific diameter. The range of the
indicator marks 258
may be chosen to reflect the range of prosthetic valve diameters between the
compressed state
and the expanded state. A dial tip 257, which may be the free end of the dial
254, opposite the
dial pivot 256, points toward the indicator marks 258. The dial tip 257 is
configured to point at
the indicator mark 258 representing of the current diameter of the prosthetic
valve 120.
[0234] According to some embodiments, the display 116 comprises a window,
through which
the indicator marks 258 and the dial tip 257 are visible to an outside viewer
(e.g., an operator
of the delivery assembly 100). According to some embodiments, the indicator
marks 258
comprise color marks, such as green, yellow, red and so on, to provide visual
indication of safe
or dangerous zones, for example. According to some embodiments, the display
116 includes
the indicator marks 258.
[0235] The diameter gauge 250 is configured to translate the axial movement,
and/or axial
position, of the gauge coupling point 270, which move along with the re-
compression member
proximal segment 186, to a corresponding position of the dial 254, pointing at
an indicator
mark representative of the current valve diameter, based on predetermined
mathematical
relationship. For example, application of an axial pull force on the re-
compression member
proximal segment 186, which changes its position within the handle 210, is
translated to
tensioning applied to the loop portion 183. Such tensioning applies radially
inward force on
the actuation arm assemblies 165, resulting in a proportional change in the
perimeter of the
43
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
loop portion 183 wrapped around, and/or extended between, the actuation arm
assemblies 165,
thereby forcing the actuation arm assemblies 165 to move radially inward,
toward each other.
[0236] In the examples of Figs. 8A-8B, the loop portion 183 can be
approximated as a
substantially triangularly shaped loop. Since the prosthetic valve 120 is
coupled to the actuation
arm assemblies 165, the valve diameter proportionally changes in response to
the inwardly
directed movement of the actuation arm assemblies 165. Assuming that the loop
attachment
members 176 are located in the vicinity of the support sleeve distal ends 172,
the loop portion
183 is in close proximity to the valve outflow end 123. In such cases, the
perimeter of the valve
outflow end 123 may be assumed to constitute, in close approximation, a
circular perimeter
encircling the triangularly-shaped loop portion 183. This relationship can be
used to derive the
diameter of the valve 120.
[0237] It will be clear that the above mentioned relationship is described in
a simplified
manner, only to demonstrate the conceptual principles by which the valve
diameter can be
derived from an axial translation or axial position of the re-compression
member proximal
segment 186. Such relationships may be further adjusted to improve the
accuracy of the
measurement. For example, the actual shape of the loop portion 183 may be more
complex,
due to the influence of the position of the re-compression shaft distal end
192 relative to the
loop attachment members 176. Moreover, the number of actuation arm assemblies
165 may be
other than three, resulting in other, potentially more complex, loop portion
contours.
[0238] As indicated above, once the re-compression member proximal segment 186
is
released, the prosthetic valve 120 is free to expand, either due to the
internal resiliency of the
frame 126, or due to active expansion of the prosthetic valve 120, for example
by utilizing the
mechanical expansion mechanism described above. During valve expansion, the
actuation arm
assemblies 165 expand radially outward, thereby enlarging the perimeter of the
loop portion
183, which in turn axially translates the re-compression member proximal
segment 186, along
with the gauge coupling point 270, in a distally oriented direction.
[0239] As shown in Fig. 8B, valve expansion, accompanied by axial translation
of the re-
compression member proximal segment 186, along with the gauge coupling point
270, in a
distally oriented direction, acts to rotate the dial 254 in the appropriate
direction, for example,
counterclockwise as shown in Fig. 8B, relative to Fig. 8A. As a result, the
position of the dial
44
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
tip 257 changes, pointing at the indicator mark 258, representing the valve
diameter or a close
approximation thereof.
[0240] While the gauge coupling point 270 is shown in the exemplary embodiment
of Figs.
8A-8B as an attachment point between the dial 254 and the proximal end of the
re-compression
member proximal segment 186, it will be understood that this is a simplified,
non-binding,
schematic representation of the gauge coupling point 270 position, and that
any other portion
of the re-compression member proximal segment 186 may be coupled, directly or
indirectly,
to the dial 254.
[0241] While the re-compression member proximal segment 186 is shown in the
exemplary
embodiment of Figs. 8A-8B attached at its proximal end to the spring second
end 224, it will
be understood that this is a simplified, non-binding, schematic representation
of the coupling
between the re-compression member proximal segment 186 and the spring 220, and
that any
other portion of the re-compression member proximal segment 186 may be
coupled, directly
or indirectly, to any other portion of the spring 220.
[0242] Figs. 8C and 8D show are-compression assembly 180 having are-
compression member
proximal segment 186 coupled to a diameter gauge 250, in a compressed state
and an expanded
state of the prosthetic valve 120, similar to the views shown in Figs. 8A-8B,
except that the re-
compression assembly 180 is provided with a loop portion 183 configured to
circumscribe the
prosthetic valve 120 in the same manner illustrated and described in
conjunctions with Figs.
5A-5E. All other embodiments described in Figs. 8A-8B are similarly applicable
to the re-
compression assembly 180 shown in Figs. 8C-8D.
[0243] In the examples of Figs. 8C-8D, the loop portion 183 can be
approximated as a
substantially circular loop, having its perimeter changing along with the
perimeter of the
prosthetic valve encircled thereby. This configuration may represent a simple
relationship
between the perimeter of the loop portion 183 and the valve diameter, which
can be utilized to
derive the diameter of the valve 120.
[0244] Fig. 9 shows another configuration of handle 310 equipped with a
diameter gauge 350,
comprising a dial 354 attached to the re-compression proximal segment 186 at a
gauge coupling
point 370. The dial 354 comprises a distal tip 356 pointing at a scale or
indicator marks 358.
The handle 310 is similar to the handle 210, except that it does not
necessarily include a dial
support member. The diameter gauge 350 is similar to diameter gauge 250,
except that the dial
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
354 is not pivotable around a pivot, and is not connected to a dial support
member. The non-
pivotable dial 354 may be oriented substantially orthogonal to a longitudinal
axis of the re-
compression proximal segment 186. Thus, the dial 354 moves along with the re-
compression
proximal segment 186 when it translates in an axial direction, having the dial
tip 357 pointing
at the indicator marks 358 so as to indicate the current diameter of the
prosthetic valve 120,
based on the principles described and illustrated in conjunction with Figs. 8A-
8B.
[0245] An additional embodiment of a handle 410 comprising the diameter gauge
250, and
maneuverable to control the re-compression assembly 180, is shown in Fig. 10A.
Handle 410
may be substantially similar to handle 210, except that it comprises a pulley
assembly 430
having first and second pulleys 432 and 436, respectively. The first pulley
432 is mounted to
any portion of the handle 410. According to some embodiments, the first pulley
432 is
connected via a first pin 434 to a first pulley support member 416 of the
handle 410. The second
pulley 436 can be mounted to any portion of the handle 410, and may be
laterally and/or axially
offset from the first pulley 432. According to some embodiments, the second
pulley 436 is
connected via a second pin 438 to a second pulley support member 418 of the
handle 410. The
first and second pulleys 432 and 436 are freely rotatable about the first and
second pins 234
and 238, respectively.
[0246] In the embodiment illustrated in Fig. 10A, the re-compression member
proximal
segment 186 is routed through the pulley assembly 430 within the handle 410.
For example,
the re-compression member proximal segment 186 can be routed partially around
the first
pulley 432 and around the second pulley 436. According to some embodiments,
the re-
compression member proximal segment 186 can be connected to, and configured to
wrap
around, the second pulley 436. The pulley assembly 430 may be adjusted apply a
minimal
tension magnitude Ts to the re-compression member 182 at all times, including
in a released
state, without the need for a spring 220 attached to the re-compression member
proximal
segment 186. According to other embodiments, a spring 220 is attached to the
re-compression
member proximal segment 186 according to any of the embodiments described
above, in
addition to the re-compression member proximal segment 186 being routed
through the pulley
assembly 430.
[0247] According to some embodiments, the pulley assembly 430 may include one
or more
additional pulleys around which the re-compression member proximal segment 186
may be
routed. According to some embodiments, the second pulley 436 comprises a pole
portion 440,
46
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
around which the re-compression member proximal segment 186 may wrap around,
and a gear
portion 442. The gear portion 442 may be configured to be engaged, for
example, with a
corresponding rack 444. Fig. 10B constitutes a zoomed-in view in perspective
of the second
pulley 436 engaged with a rack 444. The rack 444 may be attached, directly or
indirectly, to a
user controllable element, such as steerable or rotatable adjustment knobs,
levers, sliders,
buttons (not shown) and the like, configured to allow the user to control the
re-compression
assembly 180 by adjusting the tensioning force applied to the re-compression
member 182.
[0248] The user controllable element may be maneuvered to axially translate
the rack 444 in
one direction, thereby rotating the second pulley 436 in a corresponding
direction, for example
to promote further wrapping around of the re-compression member proximal
segment 186
around the pole portion 440. Similarly, the user controllable element may be
maneuvered to
axially translate the rack 444 in an opposite direction, thereby unwrapping
the re-compression
member proximal segment 186 from the pole portion 440, relieving tension from
the loop
portion 183.
[0249] While Figs. 10A-10B illustrate a drive mechanism including a rack 444
and a gear 442,
it will be understood that any other drive mechanism, utilized to control the
direction of rotation
of the second pulley 436, is contemplated.
[0250] According to some embodiments, the handle 410, as illustrated in Figs.
10A-10B,
comprises the diameter gauge 350 instead of the diameter gauge 250, wherein
the re-
compression member proximal segment 186 is coupled to the dial 354 at the
gauge coupling
point 370. In such embodiments, the dial 354 moves along with the re-
compression proximal
segment 186 when it translates in an axial direction, having the dial tip 357
pointing at the
indicator marks 358 so as to indicate the current valve diameter, as described
above in
conjunction with Fig. 9.
[0251] Fig. 11 shows yet another embodiment of a handle 510 comprising a
diameter gauge
450. The handle 510 comprises a pulley assembly 530, which is similar to
pulley assembly 430
with certain differences. The pulley assembly 530 comprises first and second
pulleys 532 and
536, respectively. The first pulley 532 is mounted to any portion of the
handle 510, and can be
connected via a first pin 534 to a first pulley support member 516 of the
handle 510. The second
pulley 536 can be mounted to any portion of the handle 510, and may be
laterally and/or axially
offset from the first pulley 532. According to some embodiments, the second
pulley 536 is
47
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
connected via a second pin 538 to a second pulley support member 518 of the
handle 510. The
first and second pulleys 532 and 536 are freely rotatable about the first and
second pins 534
and 538, respectively.
[0252] The main difference between the pulley assembly 530 and the pulley
assembly 430, is
that the second pulley 536 is devoid of a gear portion, and is therefore not
engaged with a rack.
As shown in Fig. 11, the re-compression member proximal segment 186 can be
routed partially
around the first pulley 532 and partially around the second pulley 536,
extending beyond the
second pulley 536, for example, in a proximal direction, instead of being
configured to wrap
there-around. According to some embodiments, the pulley assembly 530 may
include one or
more additional pulleys around which the re-compression member proximal
segment 186 may
be routed. The re-compression member proximal segment 186 can be attached to a
pulling
mechanism (not shown) at a location proximal to the second pulley 536, wherein
the pulling
mechanism is configured to either pull the re-compression member proximal
segment 186 in a
proximally oriented direction, or release the re-compression member proximal
segment 186.
[0253] The pulley assembly 530 may be adjusted to apply a minimal tension
magnitude Ts to
the re-compression member 182 at all times, including in a released state, in
a similar manner
described above for the pulley assembly 430. According to other embodiments, a
spring 220 is
attached to the re-compression member proximal segment 186 according to any of
the
embodiments described above, in addition to the re-compression member proximal
segment
186 being routed through the pulley assembly 530 (embodiments not shown).
[0254] The diameter gauge 450 comprises a displacement sensor 460, wherein at
least one
component of the diameter gauge 450 is coupled to the re-compression assembly
180 at a gauge
coupling point 470, such that the displacement sensor 460 is operatively
connected to the re-
compression assembly 180.
[0255] The term "operatively connected", as used herein, refers to any type of
interaction
between two components, wherein an action of a first component is effective to
cause a reaction
in the second components. For example, a displacement sensor 460 is
operatively connected to
the re-compression assembly 180 if an axial movement of a component of the re-
compression
assembly 180, such as the component comprising the gauge coupling point 470,
is effective to
cause the displacement sensor 460 to produce a corresponding signal (e.g., an
electric signal or
an optic signal).
48
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0256] According to some embodiments, as shown in Fig. 11, the displacement
sensor 460 is
operatively connected to the re-compression member proximal segment 186 at a
gauge
coupling point 470, and configured to generate a signal, wherein the magnitude
of the signal is
proportional to the position and/or axial displacement of the gauge coupling
point 470.
[0257] According to some embodiments, the displacement sensor 460 comprises a
potentiometer, and the diameter gauge 450 further comprise a wiper 462,
coupled to the re-
compression assembly 180 at the gauge coupling point 470. In the exemplary
embodiment of
Fig. 11, the wiper 462 is coupled to the re-compression member proximal
segment 186 at the
gauge coupling point 470. The wiper 462 is configured to move axially with the
re-compression
member proximal segment 186. The free end of the wiper 462, opposite to the
gauge coupling
point 470, is configured to contact the potentiometer 460, and its position
relative to the
potentiometer 460 affects the electric signal generated by the potentiometer
460. The position
of the wiper 462 and its contact with the potentiometer 460, is directly
proportional to the
perimeter of the loop portion 183, which in turn is proportional to the
perimeter of the prosthetic
valve 120. Therefore, the diameter of the prosthetic valve 120, which can be
derived from said
perimeters, can be determined by measuring the electric signal generated by
the potentiometer
460 which is in contact with the wiper 462.
[0258] As the re-compression member proximal segment 186, along with the gauge
coupling
point 470, is moved axially within the handle 510, the wiper 462 slides
axially across the
surface of the potentiometer 460, and a corresponding voltage may be
transmitted to a control
circuit (not shown). The control circuit may be embedded within the handle
510, and can
include a processor for analyzing the voltage and deriving the valve expansion
diameter
accordingly.
[0259] It should be understood that a displacement sensor 460 is not limited
to a potentiometer,
and other displacement sensors, including linear displacement sensors, may be
utilized.
Exemplary alternative displacement sensors 460 can include a linear variable
differential
transformer (LDVT), an optical linear encoder, an optical sensor, a capacitive
sensor, or any
combination thereof. Angular displacement sensors may also be utilized in the
same manner,
for example to measure the angular or rotational movement of pulleys around
which the re-
compression members 182 extends, based on known correlations between such
rotational
movement and the axial displacement of the re-compression members 182.
49
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0260] According to some embodiments, the displacement sensor 460 is
operatively coupled
to the control unit via one or more wires or cables, or via a wireless
communication link. The
control unit can be configured to receive signals from the displacement
sensor, representative
of the axial movement of the re-compression member proximal segment 186. The
control unit
can be configured to continuously calculate the diameter of the prosthetic
valve 120, based on
measurement inputs provided by the displacement sensor 460.
[0261] According to some embodiments, the displacement sensor 460 is
operatively coupled
to a visual interface, such as a display 116. According to some embodiments,
the displacement
sensor 460 is operatively coupled to a display 116 via the control unit. The
display 116 may
comprise a digital screen, which may present numerical values indicative of
the valve current
diameter, as well as other icons, textual messages or graphical symbols.
Additionally or
alternatively, a visual interface may comprise LED lights 118, lamps or other
visual elements,
configured to provide the user with a visual indication of the current valve
diameter. According
to some embodiments, the control unit is configured to display the diameter of
the prosthetic
valve 120 on the display 116 in real-time, as the prosthetic valve 120 is
expanded and/or
compressed during an implantation procedure.
[0262] According to some embodiments, the control unit further comprises a
memory.
According to some embodiments, selected data, such as raw signal data or
calculated data, may
be stored in the memory. According to some embodiments, the control unit is
configured to log
data during the implantation procedure in the memory. According to some
embodiments, the
control unit is configured to transmit to a remote device, logged data from
the memory, and/or
real-time data.
[0263] According to some embodiments, the control unit is configured to
provide an alert to
an operator in the event of valve over-expansion within a native annulus. The
alert may be an
audible alert, a visual alert, a tactile alert, etc.
[0264] According to some embodiments, the control unit may be further
configured to control
the actuation arm assemblies 165 and/or the re-compression assembly 180, to
expand and/or
contract the prosthetic valve 120, according to pre-programmed
expansion/contraction
algorithms.
[0265] According to some embodiments, the control unit, and/or the display
116, may be
provided as distinct components separated from the delivery apparatus 102,
which can be
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
operatively connected thereto, for example using wires or cables. According to
some
embodiments, the control unit, and/or the display 116, are configured to
communicate
wireles sly with the displacement sensor 460, such as via Bluetooth
communication, radio
waves, infrared signals, or other wireless communication protocols. According
to additional
embodiments, the control unit, and/or the display 116, are integrated within
the handle 510.
For example, a processor and other electrical components of a control unit,
can be located
within the handle 510, and the display 116 may be located on an exterior
surface of the handle
510, such that it can be viewed by a clinician during the implantation
procedure.
[0266] According to some embodiments, the diameter gauge 450 may be similarly
utilized in
conjunction with any other embodiments of handles disclosed hereinabove. For
example, the
diameter gauge 450 may be embedded within the handle 310, having the re-
compression
member proximal segment 186 coupled to the wiper 462 and to a spring 220. In
another
example, the diameter gauge 450 may be embedded within the handle 410, having
the re-
compression member proximal segment 186 routed through the pulley assembly 430
instead
of the pulley assembly 530.
[0267] As mentioned above, tension applied to the re-compression member 182
may
occasionally extend the length of the re-compression member 182 to a certain
degree relative
to a released state, or relative to its length under other pull force
magnitudes that may be applied
thereto. Such changes in the length of the re-compression member 182 may alter
the position
of the gauge coupling point 270, 370 or 470. This may, in turn, result in
inaccuracies in valve
diameter estimation.
[0268] According to some embodiments, the re-compression assembly further
comprises a
track member, extending through the re-compression shaft and attached, via a
secondary loop,
to the actuation arm assemblies 165, in a similar manner to that of the re-
compression member
182. However, unlike the re-compression member 182, the tracking member is not
configured
to displace the actuation arm assemblies 165 in any direction, but rather to
passively follow
their displacements in the radial direction.
[0269] Fig. 12 shows a delivery apparatus 102 equipped with a re-compression
assembly 680
and a handle 610, according to some embodiments. The re-compression assembly
680 is similar
to the re-compression assembly 180, having the re-compression member 182
extending
through a lumen of a re-compression shaft 688. However, the re-compression
assembly 680
51
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
further comprises a track member 682, routed from the handle 610, through the
re-compression
shaft 688, toward the support sleeve distal ends 172.
[0270] The track member 682 may be provided in the form of a wire, a cable, a
string and so
on. According to some embodiments, the track member 682 can be made of the
same materials
as the re-compression member 182, and may be provided in the form of a wire, a
cable, a string,
and so on. According to some embodiments, the track member 682 comprises
material which
resists elongation in an axial direction, to a higher extent relative to the
resistance to elongation
of the re-compression member 182, for example, in a tensioned state of the re-
compression
assembly 680.
[0271] The track member 682 comprises a track member proximal segment 686 and
a track
member distal segment 684, which are the equivalents of the re-compression
member proximal
segment 186 and the re-compression member distal segment 184 described in any
of the
embodiments above. In some instances, the track member proximal segment 686
may be
formed as a continuous extension of the track member distal segment 684.
Alternatively, the
track member proximal segment 686 and the track member distal segment 684 may
be provided
as separate components attached to each other, wherein either both segments
are formed from
the same materials having the same dimensions, or both are formed from the
same materials
but each is having different dimensions (e.g., one segment being thicker than
the other), or each
is formed from different materials but both are having similar dimension or
dissimilar
dimensions with respect to one another..
[0272] According to some embodiments, the track member distal segment 684 can
be attached
to the track member proximal segment 686 via a connector 694, which may be
implemented
according to any of the embodiments related to the connector 194.
[0273] According to some embodiments, each support sleeve 170 can include a
secondary loop
attachment member 177, which can be positioned adjacent the corresponding loop
attachment
member 176 of the same support sleeve 170. The secondary loop attachment
member 177 may
be implemented according to any of the embodiments described for the loop
attachment
member 176. Each secondary loop attachment member 177 may be axially distanced
from the
corresponding loop attachment member 176, either distal or proximal thereto.
Alternatively or
additionally, each secondary loop attachment member 177 may be angularly
offset along the
support sleeve 170, relative to the corresponding loop attachment member 176.
For example,
52
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
the secondary loop attachment member 177 and the loop attachment member 176
can be
positioned at diametrically opposing sides of the respective support sleeve
170.
[0274] The track member 682 extends from the handle 610, shown in Fig. 12,
through a lumen
of the re-compression shaft 688, having a portion of its track member distal
segment 684
extending distally from the re-compression shaft distal end 692, forming a
secondary loop 683
connected to, and extending between, the actuator arm assemblies 165.
[0275] According to some embodiments, the secondary loop 683 is connected to,
and
extending between, the secondary loop attachment members 177, such that the
secondary loop
683 is adjacent the loop portion 183, which extends between the loop
attachment members 176.
According to alternative embodiments, both the loop portion 183 and the
secondary loop 683
may extend through the same loop attachment members 176.
[0276] According to some embodiments, both the track member 682 and the re-
compression
member 182 may extend, side-by-side, through the same lumen of the re-
compression shaft
688. In alternative embodiments, the re-compression shaft 688 is a multi-lumen
shaft, having
each of the track member 682 and the re-compression member 182, extending
through a
separate lumen thereof.
[0277] According to some embodiments, a diameter gauge is attached, at the
gauge coupling
point, to the track member proximal segment 686 (instead of being attached to
the re-
compression member proximal segment 186), and is configured to provide real-
time indication
of the diameter of the prosthetic valve 120, based on axial position and/or
axial translation of
the gauge coupling point.
[0278] The handle 610 shown in Fig. 12 is similar to the handle 510,
comprising a pulley
assembly 630 which may be identical to the pulley assembly 530, with like
numbers referring
to like components, such that the re-compression member proximal segment 186
may be routed
through the pulley assembly 630. Alternatively, the re-compression member
proximal segment
186 may be routed through a pulley assembly 430, or may be connected to a
pulling-
mechanism, configured to apply or release a pull-force thereto, without having
the re-
compression member proximal segment 186 extend between any pulleys within the
handle.
[0279] According to some embodiments, a minimal tension magnitude Ts' is
applied, at all
times, to the track member distal segment 684, and more specifically, to the
secondary loop
53
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
683, configured to retain the secondary loop 683 in a minimally tensed state
between the
actuation arm assemblies 165, while allowing free expansion of the prosthetic
valve 120 in a
radial direction. According to some embodiments, the magnitude of the minimal
tension
magnitude Ts' applied to the secondary loop 683 is substantially identical to
the magnitude of
the minimal tension magnitude Ts applied to the loop portion 183. According to
some
embodiments, the magnitude of the minimal tension magnitude Ts' is different
than the
magnitude of the minimal tension magnitude Ts, for example due to a different
axial position
of the secondary loop 683 relative to the loop portion 183.
[0280] According to some embodiments, the handle 610 may further comprise a
track spring
620, which may be identical in structure and function to the spring 220. The
track spring 620
is attached to a spring support member 612 of the handle 610 via a spring
first end 622, and to
the track member proximal segment 686 via a spring second end 624. Contrary to
the
embodiments described and illustrated for the spring 220 in conjunction with
Figs. 8A-9, the
spring 620 is configured to apply an axially oriented pull-force on the track
member proximal
segment 686 instead of the re-compression member proximal segment 186. The
magnitude of
the force applied by the spring 620 is sufficient to apply the minimal tension
magnitude Ts' to
the secondary loop 683.
[0281] The re-compression member 182 may be utilized to compress the valve
120, having its
re-compression member proximal segment 186 attached to, and controllable by, a
user
controllable element, according to any of the embodiments described herein
above. The track
member 682, on the other hand, is not connected to the user controllable
element, and therefore
is not necessarily utilized to compress the valve 120. Rather, the track
member 682 is
configured to follow the change in valve diameter, having the secondary loop
683 configured
to merely follow expansion or contraction of the prosthetic valve 120 in a
similar manner to
that described for loop portion 183, in any of the embodiments herein above.
[0282] Advantageously, since the maximal tension applied to the track member
682 is the
minimal tension magnitude Ts', which is substantially lower than the tension
applied to the re-
compression member 182 to compress the diameter of the prosthetic valve 120,
the length of
the track member 682 is not extended to the same extent as that of the re-
compression member
182 in a tensioned state of the re-compression assembly 680.
54
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0283] According to some embodiments, the diameter gauge is coupled, at the
gauge coupling
point, to the track member proximal segment 686 (and not to the re-compression
proximal
segment 186).
[0284] In the exemplary embodiment shown in Fig. 12, a diameter gauge 450,
comprising a
displacement sensor 460, such as a potentiometer, is coupled to the track
member proximal
segment 686 at the gauge coupling point 470. More specifically, the wiper 462
is attached to
the track member proximal segment 686 at the gauge coupling point 470, and is
configured to
interact with the potentiometer 460 in the same manner described and
illustrated in conjunction
with Fig. 11. Thus, the valve diameter may be derived from the axial movement
of the gauge
coupling point 470, having a corresponding indication shown, for example, in
the display 116,
in the same manner described and illustrated in conjunction with Fig. 11.
[0285] Advantageously, this configuration separates between the functionality
of the re-
compression member 182 and the functionality of the diameter gauge, such that
while the re-
compression member 182 is utilized to re-compress the prosthetic valve 120 as
necessary, the
diameter gauge follows such changes in diameter, without being affected by
inaccuracies that
may arise from axial elongation of the re-compression member 182 due to the
pull-force
applied thereto during tensioning states.
[0286] According to some embodiments, the track spring 620 may be further
attached to the
re-compression member proximal segment 186, thereby applying a similar basic
tensioning
force on both track member 682 and the re-compression member proximal segment
186
(embodiments not shown). Alternatively or additionally, a spring 220 may be
attached to the
re-compression member proximal segment 186, optionally in addition to the
track spring 620
attached to the track member 682.
[0287] According to some embodiments, the re-compression assembly 680 may be
similarly
utilized with the track member proximal segment 686 attached to a dial
pointing at indicator
marks, in the same manner described and illustrated for the dial 254 and
indicator marks 258
in conjunction with Figs. 8A-8D, or in the same manner described and
illustrated for the dial
354 and indicator marks 358 in conjunction with Fig. 9. In such embodiments,
the track
member proximal segment 686 may be attached to the track spring 620, and/or
routed around
pulleys of a pulley assembly, similar to the pulley assembly 430 or the pulley
assembly 530.
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0288] While Figs. 9-12 are demonstrated for a re-compression assembly 180,
680 having a
loop portion 183 extending between loop attachment members 176, it will be
clear that all of
the configurations and embodiments illustrated and described in conjunction
with Figs. 9-12
can be used in combination with a re-compression assembly 180 having a loop
portion 183
circumscribing the prosthetic valve 120, similar to the configurations
illustrated in Figs. 8C-
8D.
[0289] Specifically, a re-compression assembly 680 comprising a re-compression
member 182
with a loop portion 183 extending between loop attachment members 176, as
illustrated in Fig.
12, can similarly comprise, in certain embodiments, a re-compression assembly
180 having a
loop portion 183 circumscribing the prosthetic valve 120. In such embodiments,
the track
member 682 may be similarly provided with a secondary loop 683 also
circumscribing the
prosthetic valve 120. All other embodiments described in conjunction with Fig.
12 are similarly
applicable with the re-compression assembly 680 having the loops 183 and 683
circumscribing
the prosthetic valve 120.
[0290] While not explicitly illustrated, additional embodiments of a re-
compression assembly
680 may include a re-compression member 182 having a loop portion 183
extending between
loop attachment members 176 and configured to apply sufficient tension so as
to compress the
prosthetic valve 120, used in combination with a track member 682 having a
secondary loop
683 circumscribing the prosthetic valve 120 and configured to merely track the
change in
perimeter of the prosthetic valve 120. Alternatively, embodiments of a re-
compression
assembly 680 may include a re-compression member 182 circumscribing the
prosthetic valve
120 and configured to apply sufficient tension so as to compress the
prosthetic valve 120, used
in combination with a track member 682 having a secondary loop 683 extending
between loop
attachment members 176 and configured to merely track the change in perimeter
of the
prosthetic valve 120.
[0291] According to some embodiments, a diameter gauge according to any of the
embodiments of the current disclosure is operatively coupled to the digital
display 116 or to
the LED lights 118. According to some embodiments, a diameter gauge is
operatively coupled
to the digital display 116 or the LED lights 118 via the control unit. The
digital display 116
may comprise a digital screen, which may present numerical values indicative
of the current
diameter of the prosthetic valve 120. The digital display 116 may similarly
display other icons,
textual messages and/or graphical symbols. Additionally or alternatively, LED
lights 118,
56
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
lamps or other visual elements, can be configured to provide the user with a
visual indication
regarding the diameter of the prosthetic valve 120. According to some
embodiments, the
control unit is configured to display the diameter of the prosthetic valve 120
on the digital
display 116 in real-time, as the prosthetic valve 120 is expanded and/or
compressed during an
implantation procedure.
[0292] According to some embodiments, the control unit further comprises a
memory member,
and selected data, such as raw signal data or calculated data, can be stored
in the memory
member. A memory member may include a suitable memory chip or storage medium
such as,
for example, a PROM, EPROM, EEPROM, ROM, flash memory, solid state memory, or
the
like. A memory member can be integral with the control unit or may be
removably coupled to
the control unit. According to some embodiments, the control unit is
configured to log data
during the implantation procedure in the memory member. According to some
embodiments,
the control unit is configured to transmit logged data from the memory member,
and/or real-
time data, to a remote device.
[0293] According to some embodiments, the control unit is configured to
provide an alert to
an operator in the event that the diameter of the prosthetic valve 120 exceeds
a predefined
threshold. The alert can be an audible alert, a visual alert, a tactile alert,
etc.
[0294] According to some embodiments, the control unit can be further
configured to control
the actuation arm assemblies 165 to expand the prosthetic valve 120 according
to pre-
programmed expansion algorithms.
[0295] According to some embodiments, the control unit and or the display 116
may be
provided as distinct components, separated from the delivery apparatus 102,
and operatively
connected thereto, for example using wires or cables. According to additional
embodiments,
the control unit and/or the display 116 can be formed integrally with the
handle. For example,
a processor and other electrical components of a control unit can be located
within the handle,
and the display 116 can be located on an exterior surface of the handle, as
shown in Fig. 1, such
that it can be viewed by a clinician during the implantation procedure.
[0296] According to some embodiments, an axially stationary component of the
delivery
assembly 100, configured to maintain an affixed axial position relative to the
outflow end 123
during expansion or compression of the prosthetic valve 120, comprises at
least one reference
radiopaque marker 882, and an axially movable component of the re-compression
assembly
57
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
180, configured to be axially movable relative to the outflow end 123 during
expansion or
compression of the prosthetic valve 120, comprises at least one indicator
radiopaque marker
880.
[0297] According to some embodiments, as shown in Figs. 13A-13B, the axially
stationary
component is the re-compression shaft 188, comprising at least one reference
radiopaque
marker 882 around its outer surface, and the axially movable component is the
re-compression
member proximal segment 186, comprising at least one indicator radiopaque
marker 880
around its outer surface. Each reference radiopaque marker 882 and each
indicator radiopaque
marker 880 can be implemented according to any of the embodiments described
herein above
for radiopaque markers 196 in conjunction with Fig. 6A.
[0298] According to some embodiments, the at least one indicator radiopaque
marker 880 is
configured to be visually distinguishable from the at least one reference
radiopaque marker
882, for example by having different dimensions. In the exemplary embodiments
of Fig. 13A,
the indicator radiopaque marker 880 is disposed around the outer surface of
the re-compression
member proximal segment 186, which is thinner than the re-compression shaft
188 it is
disposed in, resulting in an indicator radiopaque marker 880 which is
relatively smaller than
each of the reference radiopaque marker 882. In some applications, the length
of the indicator
radiopaque marker 880 may be different than the length of the reference
radiopaque markers
882.
[0299] According to some embodiments, the re-compression shaft 188 comprises a
radiolucent
material or has a cut-out window, enabling the at least one indicator
radiopaque marker 880 to
be visible there-through under fluoroscopy.
[0300] Figs. 13A shows the prosthetic valve 120 in a compressed state, while
Fig. 13B shows
the valve 120 in an expanded state. The re-compression shaft 188 may be
maintained coupled
to the handle 110 at a predetermined position, having a fixed length thereof
extending toward
the valve 120, such that the position of any portion of the re-compression
shaft 188, including
the reference radiopaque marker 882 disposed around its outer surface,
maintain the same axial
position relative to the outflow end 123 of the valve 120 during expansion
(e.g., for the
compressed state of Fig. 13A to the expanded state of Fig,. 13B) or
compression (e.g., for the
expanded state of Fig. 13B to the compressed state of Fig,. 13A) of the
prosthetic valve 120.
58
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0301] During valve expansion, as shown in Fig. 13B, the loop portion 183 of
the re-
compression member distal segment 184 expands therewith, axially translating
the re-
compression member proximal segment 186, as well as the indicator radiopaque
marker 880
disposed thereon, in a distally oriented direction (i.e., toward the outflow
end 123) relative to
its position in the compressed state of Fig. 13A, and relative to the
reference radiopaque
markers 882.
[0302] As mentioned above, axial translation of the re-compression member
proximal segment
186 is proportional to the perimeter of the loop portion 183, which in turn is
proportional to the
diameter of the prosthetic valve 120. Thus, the at least one reference
radiopaque markers 882
may serve as a "scale", and the indicator radiopaque marker 880 can serve as a
"dial" relative
to the "scale", indicative of the diameter of the prosthetic valve 120.
Accordingly, the re-
compression assembly 180 provided in a delivery assembly 100 to facilitate re-
compression of
a prosthetic valve 120 when required, can be advantageously further utilized
to serve as a real-
time monitoring aid for prosthetic valve diameter during expansion or
compression thereof,
based on the alignment of at least one indicator radiopaque marker 880
relative to reference
radiopaque markers 882 under fluoroscopy. In other words, the axial position
of the one
indicator radiopaque marker 880 relative to the reference radiopaque markers
882 is indicative
of the diameter of the prosthetic valve 120.
[0303] In applications of the re-compression shaft 188 comprising three
reference radiopaque
markers 882 or more, the reference radiopaque markers 882 may be equally
spaced from each
other. Alternatively, at least some of the reference radiopaque markers 882
may be space with
unequal distances.
[0304] The re-compression shaft 188 can include a plurality of reference
radiopaque markers
882, such as the three reference radiopaque markers 882a, 882b and 882 shown
in Figs. 13A-
13B, each corresponding to a specific diameter of the prosthetic valve 120. In
the illustrated
example, the most proximal reference radiopaque marker 882a can correspond to
a first
expanded diameter, such as 27mm. The intermediate reference radiopaque marker
882b can
correspond to a second expanded diameter, larger than the first diameter, such
as 28 mm. The
most distal reference radiopaque marker 882c can correspond to a third
(potentially maximal)
expanded diameter, larger than the second diameter, such as 29 mm. Alignment
of the indicator
radiopaque marker 880 with any of the reference radiopaque markers 882 may be
indicative of
the valve diameter associated with the reference radiopaque marker 882.
Positioning of the
59
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
indicator radiopaque marker 880 between any couple of reference radiopaque
markers 882, as
shown in Fig. 13B, may be indicative of an expansion diameter between the two
diameters
associated with the respective reference radiopaque markers 882. Similarly, an
indicator
radiopaque marker 880 positioned distal to the most distal reference
radiopaque marker 882c
may indicate an expansion diameter exceeding a maximal value.
[0305] While three reference radiopaque markers are shown in the exemplary
embodiment
illustrated in Figs. 13A-13B, it will be clear that any other number of
reference radiopaque
markers 882 is contemplated. For example, more than three reference radiopaque
markers 882
may be utilized to provide a higher resolution of the "scale" provided
thereby. Alternatively, a
single reference radiopaque marker 882 may be provided, for example to serve
only as a
maximal threshold value, such that if the indicator radiopaque marker 880
translates distally to
the single reference radiopaque marker 882, it may be indicative of an
expansion diameter that
exceeds a maximal threshold value (embodiment not shown).
[0306] According to some embodiments, as shown in Figs. 14A-14B, the axially
stationary
component is the re-compression shaft 188, comprising at least one reference
radiopaque
marker 882 around its outer surface, and the axially movable component is the
re-connector
194, comprising at least one indicator radiopaque marker 880 around its outer
surface.
[0307] Figs. 14A and 14B show views similar to those shown in Figs. 13A and
13B
respectively, wherein the re-compression mechanism 180 is identical to any of
the
embodiments described in conjunction with Figs. 13A-13B, except that the
axially movable
component comprising the indicator radiopaque marker 880 is the connector 194.
If the
connector 194 is a releasable connector, the radiopaque marker may be disposed
around either
one of the proximal connector element 193, the distal connector element 195,
or both.
[0308] Figs. 13A-14B show exemplary configurations of a loop portion 183 of
the re-
compression member distal segment 184 threaded through eyelet-shaped loop
attachment
members 176, as elaborated in conjunction with Figs. 8A-8B. These
configurations may be
advantageous as both the connector 194 and the re-compression member proximal
segment
186 are positioned proximal to the outflow end 123 of the valve 120 at all
times, along with
the indicator radiopaque marker 880, disposed on either one of the
aforementioned
components. Thus, the indicator radiopaque marker 880, as well as the
respective reference
radiopaque markers 882, are positioned proximal to the valve outflow end 123,
so as to be
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
visible throughout expansion or compression of the valve 120 under fluoroscopy
without being
potentially obstructed by the frame 126.
[0309] Figs. 15A and 15B show views similar to those shown in Figs. 13A and
13B,
respectively, wherein the re-compression mechanism 180 is identical to any of
the
embodiments described in conjunction with Figs. 13A-14B, except that the loop
portion 183
of the re-compression member distal segment 184 circumscribes the prosthetic
valve 120
instead of being threaded through loop attachment members 176 of the actuator
arm assemblies
165. The frame 126 and other components of the prosthetic valve 120 are
removed from view
in the zoomed-in portions of Figs. 15A-15B for clarity.
[0310] In some applications, the loop portion 183 can enter into a
circumferential sleeve 830
through a circumferential sleeve opening 833. The circumferential sleeve 830
can be disposed
around the frame 126 and attached thereto by gluing, suturing/stitching, and
the like. For
example, the circumferential sleeve 830 can be sutured to a plurality of
junctions 130 and/or
struts 127 of the frame 126. While the loop portion 183 is shown to extend
through a
circumferential sleeve 830 in Figs. 15A-15B, it will be clear that the loop
portion 183 of the
re-compression member distal segment 184 can similarly extend through a sleeve
130 attached
to, or integrally formed with, a skirt such as an outer skirt 137, in the same
manner described
and illustrated in conjunction with Figs. 7A-7C, or it can be looped directly
over the valve 120
without extending through any type of sleeve, in the same manner described an
illustrated in
conjunction with Figs. 5B-6B.
[0311] The configuration shown in Figs. 15A-15B illustrates a re-compression
mechanism 180
equipped with a releasable connector 194, disposed within a guide member 840,
similar to the
embodiments described and illustrated in conjunction with Figs. 7A-7C.
Nevertheless, it will
be clear that the embodiments described in conjunction with Fig. 15A-15B are
similarly
applicable to configurations of a re-compression mechanism 180 which may be
equipped with
a non-releasable connector 194, and does not necessarily extend through a
guide member 840,
in the same manner described an illustrated in conjunction with Figs. 5B-6B.
For configurations
that do include a guide member 840, it is preferable for the indicator
radiopaque marker 880
and the reference radiopaque markers 882 to be positioned throughout the range
of expansion
diameters, proximal to the outflow end 123 of the valve 120. For example, the
indicator
radiopaque marker 880 and the reference radiopaque markers 882 are illustrated
proximal to
the guide member proximal end 844 both in the compressed state and the
expanded state shown
61
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
in Figs. 15A and 15B, respectively, thereby facilitating visibility thereof
under fluoroscopy
without being potentially obstructed by the frame 126.
[0312] In some applications, as shown in Figs. 16A and 16B which are
equivalent to the views
shown in Figs. 15A and 15B, respectively, the reference radiopaque markers
882, such as
markers 882a, 882b and 882c, are disposed over the outer surface of the guide
member 840
instead of the outer surface of the re-compression shaft 188. Since the guide
member 840 is
attached to the frame 126, extending distally from the outflow end 123, the
struts 127 may
mask such reference radiopaque markers 882 and/or the indicator radiopaque
marker 880, due
to their own inherent radiopacity. In some applications, the radiodensity of
the reference
radiopaque markers 882 and/or the indicator radiopaque marker 880 is higher
than that of the
struts 127 or other components of the prosthetic valve 120, so as to enable
the radiopaque
markers 880, 882 to be visibly distinguishable, under fluoroscopy, from the
frame 126 or other
components of the valve 120.
[0313] According to some embodiments, more than one indicator radiopaque
marker 880 may
be utilized. For example, Fig. 17 shows a zoomed in view of a portion of the
re-compression
mechanism 180, which can be implemented in combination with any of the
configurations
described and illustrated in conjunction with Figs. 13A-16B, wherein the re-
compression shaft
188 comprises three reference radiopaque markers 882a, 882b, 882c, and the re-
compression
member proximal segment 186 comprises two indicator radiopaque marker 880a,
880b. The
distance between the plurality of indicator radiopaque markers 880 can be
different than the
distance between equi-spaced (or otherwise spaced) reference radiopaque
markers 882. For
example, setting the distance between indicator radiopaque markers 880a and
880b to be half
of the distance between any two adjacent reference radiopaque markers 882, may
enhance the
potential resolution of the indicated diameter.
[0314] As mentioned above with respect to embodiments described and
illustrated in
conjunction with Figs. 8A-12, tension applied to the re-compression member 182
may
occasionally extend the length of the re-compression member 182 to a certain
degree relative
to a released state, or relative to its length under other pull force
magnitudes that may be applied
thereto. Such changes in the length of the re-compression member 182 may alter
the position
of the indicator radiopaque marker 880. This may, in turn, result in
inaccuracies in valve
diameter estimation.
62
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0315] Thus, any of the embodiments described and illustrated in conjunction
with Figs. 13A-
17 may be used in combination with a handle comprising a spring 220 or
equivalent thereof,
connected to the re-compression member proximal segment 186 according to any
of the
embodiments described and illustrated in conjunction with Figs. 8A-9.
Similarly, any of the
embodiments described and illustrated in conjunction with Figs. 13A-17 may be
used in
combination with a handle comprising a pulley assembly 430 or 530 according to
any of the
embodiments described and illustrated in conjunction with Figs. 10A-10B or 11,
respectively.
[0316] In further applications, any of the embodiments described and
illustrated in conjunction
with Figs. 13A-14B and/or 17 may be used in combination with a delivery
apparatus 102
equipped with a re-compression assembly 680 according to any of the
embodiments described
and illustrated in conjunction with Fig. 12. For example, Fig. 18 illustrates
re-compression
assembly 680 provided with both a re-compression member 182 and a track member
682,
similar to any of the embodiments described and illustrated in conjunction
with Fig. 12. As
illustrated, the re-compression shaft 688 can include the reference radiopaque
markers 882,
and the track member proximal segment 686 (or in alternative embodiments, the
connector
694) can include the indicator radiopaque marker 880. In such embodiments, the
re-
compression member 182 can be utilized to facilitate valve compression when
required, while
the position of the indicator radiopaque marker 880 relative to the reference
radiopaque
markers 882 of the re-compression shaft 688 may provide a real-time indication
of the valve
diameter, as elaborated hereinabove.
[0317] While a plurality of reference radiopaque markers 882, such as three
markers 882a,
882b and 882c, are illustrated, it should be appreciated that a single
reference radiopaque
marker 882 can be similarly utilized in any of the embodiments described in
conjunction with
in Figs. 13A-18. A single reference radiopaque marker 882 can represent a
critical expansion
diameter of interest, such as a maximal allowable expansion diameter, such
that a relative
position of the indicator radiopaque marker 880 relative to the reference
radiopaque marker
882 may be indicative of valve over-expansion.
[0318] According to another aspect of the invention, there is provided a
method of providing
real-time estimation of the expansion diameter of a prosthetic valve 120,
based on a relationship
between the expansion diameter and a dimensionless parameter. The
dimensionless parameter
is either an opening angle of the prosthetic valve 120, or the aspect ratio
between the length
and diameter of the prosthetic valve 120, at each expansion diameter.
63
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0319] Frames of prosthetic valves 120 include a plurality of cells 135,
defined between
sections of the struts 127 intersecting at junctions 130. The shape of each
cell 135, and its
dimensions in different directions, vary during expansion or retraction of the
prosthetic valve
120. The prosthetic valve 120 comprises a plurality of cells, such that the
change in dimensions,
for example, in the longitudinal and lateral directions, of cells 135,
reflects on a change in the
length and diameter of the prosthetic valve 120 as well.
[0320] Figs. 19A-19B show a mechanically expandable prosthetic valve 120 in a
compressed
state and an expanded state, respectively. The exemplary prosthetic valve 120
shown in Figs.
19A-19B comprises struts 127 arranged in a lattice-type pattern,
interconnected at hinged
junctions 130 to form substantially diamond-shaped cells 135. In the crimped
or compressed
state shown in Fig. 19A, the cells 135 have a maximal axial length and minimal
lateral width,
such that the prosthetic valve 120 has a maximal length Li and a minimal
diameter Di. In the
expanded state shown in Fig. 19B, the cells 135 are stretched sideways (e.g.
following rotation
at the hinged junctions 130), forming substantially diamond shaped cells. As a
result, the
prosthetic valve 120 has a length L2 which is shorter than Li, and a minimal
diameter D2 which
is smaller than Di. While a specific type of mechanically expandable
prosthetic valve 120 is
shown in Figs. 19A-19B, other valve types, that may include other cell shapes,
are
contemplated.
[0321] The aspect ratio Rt of the frame 126 can be defined as the ratio of the
frame length L to
the frame diameter D. The aspect ratio Rt is altered during expansion or
compression of the
prosthetic valve 120. For example, the aspect ratio in a compressed state Rti
is defined as Li/Di,
and the aspect ratio in a compressed state Rt2 is defined as L2/D2. Fig. 20
shows an exemplary
curve representing the relationship between aspect ratio Rt and expansion
diameter D for
certain configurations. As shown, the aspect ratio Rt may have a different
value for each
expansion diameter D. While a specific non-linear relationship is shown in
Fig. 20, it will be
clear that other non-linear or linear relationships may be applicable.
[0322] Figs. 21A-21B illustrate a mechanically expandable prosthetic valve 120
in a partially
expanded state and a fully expanded state, respectively, showing exemplary
opening angles of
the frame 126. An opening angle can be defined between any couple of struts
127 that intersect
at a junction 130, wherein the angle varies during expansion or compression of
the valve 120.
Various types of opening angles may be defined, depending on the orientation
of the selected
opening angle. For example, an opening angle a oriented at a longitudinal
direction of the valve
64
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
120 can increase, for example from the acute angle ai shown in Fig. 21A, to a
larger, potentially
obtuse (or at least, less acute) angle a2 shown in Fig. 21B. An exemplary
opening angle a is
shown in Figs. 21A-21B between intersecting struts 127a and 127b.
[0323] Similarly, an opening angle (3 oriented at a circumferential direction
of the valve 120
can decrease, for example from the obtuse angle Pi shown in Fig. 21A, to a
smaller, potentially
acute (or at least, less obtuse) angle (32 shown in Fig. 21B. An exemplary
opening angle 0 is
shown in Figs. 21A-21B between intersecting struts 127b and 127c. In the case
of diamond or
rhombus-shaped cells 135, angles a and (3 are complementary angles, meaning
that each type
of an opening angle can be easily derived from the complementary angle. Thus,
any reference
to a method of acquiring an opening angle a applies with equal force to
acquiring an opening
angle 0.
[0324] Fig. 22 shows an exemplary curve representing the relationship between
an opening
angle a and expansion diameter D for certain configurations. As shown, the
opening angle a
may have a different value for each expansion diameter D. While a specific
relationship of an
opening angle a, increasing along with the expansion diameter D, is shown in
Fig. 22, it will
be clear that other types of relationships, including that of an opening angle
0 decreasing while
the expansion diameter D increases, are contemplated. While a specific type of
mechanically
expandable prosthetic valve 120 is shown in Figs. 21A-21B, other valve types,
that may include
other cell shapes, are contemplated.
[0325] Prosthetic implantation procedures are usually performed under
fluoroscopy, wherein
the frame 126 of the prosthetic valve 120 is radiopaque and visible on an
external monitor. As
disclosed herein, a known relationship between a dimensionless parameter
(i.e., the aspect ratio
Rt or the opening angle a) and expansion diameter D, for a desired range of
expansion
diameters D of a prosthetic valve 120, can be exploited to derive the
expansion diameter D, or
a close approximation thereof, during fluoroscopy imaging of the frame 126.
[0326] According to some embodiments, there is provided a method including the
steps of (1)
acquiring at least one image of the frame 126 of a prosthetic valve 120, (2)
deriving a
dimensionless parameter, from the at least one image, (3) associating a value
of an expansion
diameter D of the prosthetic valve with the dimensionless parameter, and (4)
providing an
indication (e.g., a visual indication) of the expansion diameter D of the
prosthetic valve 120.
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0327] According to some embodiments, the dimensionless parameter in steps (2)
and (3) of
the method is the opening angle a (or 0) between two intersecting struts of
the frame 126.
[0328] According to some embodiments, the dimensionless parameter in steps (2)
and (3) of
the method is the aspect ratio Rt of the length of the frame to the width of
the frame.
[0329] The terms "diameter of the prosthetic valve", "diameter of the frame",
"valve diameter"
and "expansion diameter", as used herein, are interchangeable.
[0330] According to some embodiments, the step of imaging the frame 126
comprises
acquiring at least one angiogram X-ray image of the frame 126. According to
some
embodiments, the step of imaging the frame 126 comprises acquiring at least
one live
fluoroscopy image of the frame 126. According to some embodiment, the at least
one acquired
image of the frame 126 is transmitted to a data control unit, which comprises
a central
processing unit (CPU). The data control unit is configured to identify
information data within
the at least one acquired image, such as to identify or detect the radiopaque
frame 126.
[0331] According to some embodiments, the data control unit is configured to
obtain
parameters representing a length and a width of the frame 126, wherein the
width being
representative of the diameter of the frame 126. The length and width of the
imaged frame 126
may be assigned values in any unit, including number of pixels in the image.
An aspect ratio
Rt is then calculated by the data control unit, dividing the length value by
the width value.
Alternatively or additionally, the angular position of intersecting struts 127
can be used to
derive the angle there-between.
[0332] According to some embodiments, the data control unit further comprises
a memory.
The information of the at least one acquired image, including the measured
length and width
of the frame 126, angular position of intersecting strut 127, and
calculated/derived aspect ratio
Rt or opening angle a, (3, may be stored in the memory.
[0333] According to some embodiments, known relationship between different
aspect ratios
Rt and valve expansion diameters D, and/or between opening angles a, 0 and
valve expansion
diameters D, are stored in the memory. The numerical value of the expansion
diameter D of
the frame 126 may be derived from the aspect ratio Rt and/or the opening angle
a, (3, based on
any of: mathematical formulas, graphs, and/or tables, that may be stored in
the memory.
According to some embodiments, the step of providing a visual indication of
the expansion
66
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
diameter comprises visualization of the expansion diameter on a digital
screen, as: a numerical
value, an icon or other graphical symbol, a textual message, or any
combination thereof.
[0334] Advantageously, the proposed method does not require the use of
calibration
components, such as calibration rulers that include radiopaque markings, to
derive the
expansion diameter. There is no need to directly measure the magnitude (e.g.,
the numerical
value) of the length and/or diameter of the valve 120, as well as specific
dimensions of struts
127, since the method relies on measurement of a dimensionless parameter
(i.e., the aspect ratio
Rt or the opening angle a, (3), from which the expansion diameter may be
derived, based on
known relationships there-between.
[0335] A further advantage conferred by the delivery assemblies and the
methods disclosed
herein, is that they enable continuous real-time diameter monitoring, thereby
providing
valuable feedback to the clinician with respect to the valve expansion within
the native
anatomy. This valuable information may assist in preventing, or at least
reducing, potential
trauma to a tissue (e.g., the annulus). The clinician can continuously
readjust the diameter of
the prosthetic valve 120 as necessary, until the prosthetic valve 120 is
expanded to a diameter
that best fits the native annulus. For example, a diameter which is sufficient
to anchor the
prosthetic valve 120 in place against the surrounding tissue, with little or
no paravalvular
leakage, and without over-expanding the prosthetic valve 120 so as to avoid,
or reduce the risk
of, native annulus rupture.
[0336] According to another aspect of the invention, there is provided a
prosthetic valve which
includes a frame belt circumscribing at least a portion of the frame in an
expanded state,
wherein the frame belt includes at least one expansion force indicator, and
preferably a plurality
of expansion force indicators, configured to provide an indication of the
circumferential force
applied by the prosthetic valve 120, during expansion thereof, to the frame
belt. According to
some embodiments, the expansion force indicators are radiopaque-marked
expansion force
indicators, configured to provide visual indication (for example, under
fluoroscopy) of the
circumferential force applied by the prosthetic valve 120, during expansion
thereof, to the
frame belt.
[0337] As mentioned above, the native anatomy against which the prosthetic
valve 120 is
expanded, may exert responsive radial forces against the prosthetic valve 120
in an opposite
direction. Thus, the diameter of the prosthetic valve 120 is correlated to a
balance between
67
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
outwardly oriented expansion forces applied by the valve 120 to the
surrounding anatomy, and
inwardly oriented responsive forces applied by the surrounding anatomy to the
valve 120.
Valve over-expansion may be defined as a situation in which the valve exerts
excessive radial
forces on the surrounding anatomy, resulting in potential damage to the tissue
or even annular
rupture. Assuming that the relationship between radial force and
circumferential force is
known for the specific valve type, the valve expansion force can be derived
from the
circumferential stress imparted on the frame belt by the valve, detectable by
a change in a state
of the expansion force indicators.
[0338] Figs. 23A, 23B and 23C show a prosthetic valve 120 provided with a
frame belt 860,
in a compressed state, a partially expanded state, and a fully expanded state,
respectively,
according to some embodiments. The frame belt 860 comprises at least one frame
belt diameter
indicator 866, configured to change a state thereof when a force exceeding a
specific magnitude
is applied thereto by the frame 126, during expansion thereof. The change in
the state of the at
least one frame belt diameter indicator 866 is visually distinguishable under
fluoroscopy.
[0339] In some applications, the at least one frame belt diameter indicator
comprises a
separation zone 866, and the visually distinguishable state comprises a
transition of the
separation zone 866 from an intact state to a separated state. Specifically, a
separation zone
866 can comprise a radiopaque marker, according to any embodiment disclosed
above for
radiopaque markers 196 or 880, such that a separated separation zone 866 may
be visible under
fluoroscopy as a discontinuation of a radiopaque-marked region, which was
visible as a
continuous zone prior to separation thereof.
[0340] The term "disrupted" or "separated", as used herein, are
interchangeable, and refer to
being torn, broken, or otherwise disconnected.
[0341] In some applications, the at least one radiopaque expansion force
indicator comprises a
geometrical feature 866 with a shape distinguishable from it neighboring zone,
and the visually
distinguishable state comprises a translation of the geometrical feature 866
from a first zone to
a second zone. Specifically, a geometrical feature 866 can comprise a
radiopaque marker,
according to any embodiment disclosed above for radiopaque markers 196 or 880,
such that
spatial translation thereof from a first zone to a second zone may be visible
under fluoroscopy.
In some variants of the application, the first zone can include a radiopaque
zone, configured to
hide or mask the geometrical features 866 disposed behind it or within a lumen
thereof, from
68
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
visibility under fluoroscopy, and the second zone can include an exposed (or
otherwise
radiolucent) zone, in which the geometrical features 866 may be visible under
fluoroscopy. In
some variants of the application, the first zone can include a first
orientation of a portion of the
frame belt 860, defined between at least two geometrical features 866, and the
second zone can
include a second orientation of a portion of the frame belt 860, defined
between at least two
geometrical features 866, wherein the second orientation is angled, and is
potentially
perpendicular relative to, the first orientation. In some variants of the
application, the first zone
can include a first spatial position of a geometrical features 866 relative to
a reference
radiopaque marker, and the second zone can include a second spatial position
of a geometrical
features 866 relative to the reference radiopaque marker, wherein the first
spatial position and
the second spatial position are defined on opposite sides of the reference
radiopaque marker
(e.g., proximal and distal to the reference radiopaque marker).
[0342] In an exemplary embodiment illustrated in Fig. 23A, a frame belt 860
circumscribes
the valve 120, wherein the frame belt 860 is intact as long as the valve 120
is not expanded to
a diameter that applies a first critical tensioning force thereto. According
to some embodiments,
the frame belt comprises expandable portions 868, configured to
circumferentially expand
along with the frame 126 of the prosthetic valve 120, and base portions 870
provided with
separation zone 866. Disruption of the separation zone 866 results in
separation between
sections of the base portions 870 on both sides thereof. Disruption of a
separation zone 866
may occur upon application of a tensioning force sufficient facilitate such
separation. The
separation facilitating tensioning force may be applied to the separation zone
866 by the
prosthetic valve 120 during expansion thereof.
[0343] The frame belt 860 illustrated in the exemplary embodiment of Figs. 23A-
23C includes
expandable portions 868 in the form of struts connected at junctions to
corresponding base
portions 870. While resulting triangular cells are shown in Figs. 23A-23C, it
will be clear that
any other form is applicable, as long as the expandable portions 868 are
expandable without
being disrupted, when disposed over, or attached to, the frame 126, and as
long as at least one
base portion 870 includes a separation zone 866 that may be disrupted or
separated, upon
application of a sufficient tensioning force applied thereto by the expansion
of the frame 126.
[0344] The frame belt 860 may be disposed around the prosthetic valve 120,
such that upon
application of a tensioning force exceeding a first critical value, as
illustrated for example in
Fig. 23B, at least one separation zone 866 is disrupted, such as by being
broken, torn,
69
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
disconnected, decoupled, and the like. Further expansion of the prosthetic
valve 120 may result
in separation of additional separation zones 866. For example, Fig. 23C shows
a frame belt 860
with disrupted separation zones 866a and 866b, while separation zones 866c and
866d remain
intact.
[0345] In some applications, at least some apices and/or junctions of the
frame belt 860, such
as apices of expandable portions 868, and/or junctions connecting the
expandable portions 868
with base portions 870, may be coupled to junctions 130 of the prosthetic
valve 120, to facilitate
simultaneous expansion of the expandable portions 868 with the frame 126.
[0346] While the frame belt 860 shown in Figs. 23A-23A is disposed over the
entire
circumference of the valve 120, it will be appreciated that in some
applications, the frame belt
860 can be disposed over a portion of the valve's circumference.
[0347] In some implementations, the separation zone 866 comprises a frangible
portion. Figs.
24A-24B show a portion of a frame belt 860a which includes a plurality of
frangible portions
866a. In Fig. 24A, all of the frangible portions 866aa, 866ab, 866ac, 866ad
and 866ae are intact.
Fig. 24B shows a successive state, which may be achieved in a valve partial
expanded
configuration as shown in Fig. 23B for example, in which the frangible zone
866aa is disrupted
(i.e., broken or torn), while other frangible portions, such as 866ab, 866ac,
866ad and 866ae,
remain intact.
[0348] A frangible portion 866a can be provided as a weakened portion along
the frame belt
860a. In applications that include expandable portions 868, the weakened zones
can be provided
along respective base portions 870. The frangible portion 866a can be weakened
by thinning
thereof (relative to other, non-frangible portions of the frame belt 860a), by
inclusion of
weakening features such as perforations, or by having material properties
which are different
than the material properties of other, non-frangible portions of the frame
belt 860a.
[0349] A frame belt 860a can include a plurality of frangible portions 866a,
wherein at least
two of the plurality of frangible portions 866a are configured to disrupt
(i.e., break or tear) in
response to different tensioning force magnitudes applied thereto. Fig. 24A
illustrates an
example of a plurality of frangible portions 866a provided with varying
thickness, wherein
frangible portion 866aa is the thinnest frangible portion, and may therefore
tear or break first,
as shown in Fig. 24B, which may potentially correspond to a valve 120 applying
a first critical
tensioning force of interest, as shown in Fig. 23B. Frangible portion 866aa is
thicker than
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
frangible portion 866aa, frangible portion 866ac is thicker than frangible
portion 866ab,
frangible portion 866ad is thicker than frangible portion 866ac, and frangible
portion 866ae is
thicker than frangible portion 866ad. Thus, further expansion of the
prosthetic valve 120 may
apply increased tensioning forces on the frame belt 860a, which may result in
gradual tearing
or breaking of subsequent frangible portions 866a, as shown for example in
Fig. 23C.
[0350] Each of a plurality of the frangible portions 866a can be configured to
disrupt in
response to a different tensioning force magnitude, which may occur at a
different expansion
diameter of the prosthetic valve 120. In such configurations, the expansion
force can be
assessed by visually inspecting the amount of disrupted zones, represented by
discontinuities
of gapped zones along the radiopaque-marked frame belt 860a under fluoroscopy.
The amount
of frangible portions 866a may be determined according to a desired resolution
of the monitored
expansion forces.
[0351] The base portions 870 can be formed from an extensible material, which
may extend
around the valve 120 while the expandable portions 868 expand, until the point
of their
disruption. Alternatively, the base portions 870 can be provided in a folded
or corrugating
configuration that may unfold or straighten around the valve 120 while the
expandable portions
868 expand, until the point of their disruption. It will be appreciated that
the base portions 870
and the frangible portions 866 can be made of different materials than those
of the expandable
portions 868. For example, the expandable portions 868 may comprise super-
elastic materials
(Nitinol) or non-super-elastic materials (e.g., stainless steel or cobalt
chromium alloys), while
the base portions 870 can be provided with higher flexibility, for example
provided in the form
of a wire, cable, suture, string, or similar materials.
[0352] In some applications, the expandable portions 868 are radiopaque-marked
such that a
change in a geometrical characteristic thereof can be visible under
fluoroscopy, indicative of a
transition from a first state to a second state. For example, a height between
an apex of an
expandable portions 868 and the respective base portion 870 may serve as such
a geometrical
characteristic. As shown in Figs. 24A, the height of the expandable portion
868a may be equal
to the height of all of the other expandable portions 868 in an intact state
thereof. As further
shown in Fig. 24B, the height of the expandable portion 868a, upon disruption
of its respective
frangible portion 860aa, may be shorter that that of expandable portions 868b,
868c, 868d and
868e, whose corresponding frangible portion 860a remain intact.
71
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0353] While the frame belt 860a is shown in Figs. 24A-24B with expandable
portions 868, in
alternative applications, a frame belt 860 can be provided without expandable
portions 868.
For example, a frame belt 860 can be provided as a flexible cable, wire,
string, suture and the
like, having frangible portions 866 disposed there-along, wherein the frame
belt 860 can be
coupled to the frame 120, directly or via intermediate components such as a
skirt 136, 137 or
a sleeve 132, 830 around the valve. The coupling can be facilitated via
biocompatible
adhesives, sutures and the like. Coupling can include a single coupling point,
or a plurality of
coupling points around the valve 120. For example, each frangible portion 866
can be disposed
between two coupling points of the frame belt 860 to the valve 120, such as
coupling to two
adjacent junctions 130 positioned circumferentially on both sides of the
respective frangible
portion 866.
[0354] In some implementations, the separation zone 866 is provided in the
form of a
decouplable portion, configured to decouple when a tension force exceeding a
predetermined
magnitude is applied thereto. Figs. 25A and 25B show a portion of a frame belt
which includes
a decouplable portion 866b in a coupled and decoupled state thereof,
respectively. In the
illustrated example, a "key and lock" type of a decouplable portion 866b can
include a male
part (e.g., a flanged or ball-type head) and a complementary female part
(e.g., a slot or
receptacle configured to tightly fit around the male part), which can be snap-
fit or otherwise
engaged with each other as shown in Fig. 25A. The male and female part can
decouple from
each other, as shown in Fig. 25B, in response to a tensioning force applied
thereto (e.g., on
both ends thereof), exceeding a specific threshold magnitude. Other types of a
decouplable
portion 866b are similarly applicable, wherein a decouplable portion 866b
differs from a
frangible portion 866a only with respect to the type of disruption, which
includes decoupling
or releasing instead of breaking or tearing. Other than the type of
disruption, decouplable
portions 866b may be utilized according to any of the embodiments described
for frangible
portions 866a.
[0355] Since the frame belt is disposed around the frame 126, the struts 127
may mask such
radiopaque-marked expansion force indicators 866, due to their own inherent
radiopacity. In
some applications, the radiodensity of the expansion force indicators 866 is
higher than that of
the struts 127 or other components of the prosthetic valve 120, so as to
enable the radiopaque-
marked expansion force indicators 866 to be visibly distinguishable, under
fluoroscopy, from
the frame 126 or other components of the valve 120.
72
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0356] In an exemplary embodiment illustrated in Fig. 26A, a frame belt 860
with geometrical
features 866 can be configured to extend at least partially around the
prosthetic valve 120, such
that the amount of the geometrical features 866 visible along the portion of
the frame belt 860
circumscribing the valve 120 varies as a function of the valve's expansion
diameter. In some
implementations, the geometrical features 866 are provided in the form of
beads 866a. Each
bead can include a separate indicator radiopaque marker 880, as shown in Fig.
26A.
Alternatively, a continuous portion of the frame belt 860, including
optionally the entire length
thereof, can be marked with a radiopaque marker 196, wherein the geometrical
features 866,
such as the beads, are distinguishable from other radiopaque-marked regions
due to their
distinguishable size and/or shape, as shown for example in Fig. 27.
[0357] According to some embodiments, the delivery assembly 100 further
comprises a
restrictor 848, configured to allow passage of geometrical features 866 there-
through upon
application of a pulling force on the frame belt 860, exceeding a
predetermined threshold.
According to some embodiments, the prosthetic valve 120 comprises the
restrictor 848. In some
implementations, the restrictor 848 is provided in the form of an eyelet 848a,
that may restrict
the passage of beads 866a there-through. Specifically, the inner diameter of
the eyelet 848a can
be slightly smaller than the outer diameter of the beads 866a.
[0358] In the exemplary configuration shown in Figs. 26A-26D, a frame belt
860', provided
with a plurality of beads 866', includes a portion disposed partially around
the circumference
of the valve 120, and a portion which does not necessarily extend around the
valve 120, shown
in Figs. 26A-26B to extend axially, in an orientation that can be
substantially parallel to the
longitudinal axis 121 of the valve 120. The frame belt 860 includes a frame
belt first end 862,
which can be attached directly or indirectly to the frame 126. In some
applications, the portion
of frame belt 860' extending around the frame 126 is disposed in a sleeve,
such as the
circumferential sleeve 830 shown in Figs. 26A-26D. In such cases, the
circumferential sleeve
830 can be coupled to the frame 126, and the frame belt 860' can enter into
the circumferential
sleeve lumen 832 through the circumferential sleeve opening 831. The frame
belt first end 862
can be attached, for example by gluing, suturing, and the like, to the
circumferential sleeve
830, defining the portion of frame belt 860' extending around the frame 126 as
the portion
between the attachment point of the frame belt first end 862 to the
circumferential sleeve 830
(shown in Figs. 26A-26D on the rear side of the frame), and the
circumferential sleeve opening
831.
73
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0359] According to some embodiments, the delivery assembly 120 further
comprises a belt
pull member 886 extending from the handle 110 and attached to, or integrally
formed with, the
frame belt 860. The belt pull member 886 can be provided in the form of a
cable, a suture, a
wire, and the like, and may be coupled to a pulling mechanism at the handle
110, maneuverable
by an operator to retract the belt pull member 886, potentially along with at
least a portion of
the frame belt 860, when desired. According to some embodiments, the delivery
assembly 120
further comprises a belt shaft 888 extending distally from the handle 110,
allowing the belt pull
member 886, potentially along with at least a portion of the frame belt 860,
to extend through
a lumen thereof.
[0360] In some cases, as shown in the configuration illustrated in Figs. 26A-
26B, a portion of
the frame belt 860 can also extend through a guide member 840, that can be
comprised in the
valve 120 and may be utilized in combination with the belt shaft 888,
similarly to any of the
embodiments described for utilization thereof with a re-compression shaft 188.
[0361] In the exemplary configuration illustrated in Fig. 26A, a beaded
portion of the frame
belt 860' can extend axially, through the lumens of the belt shaft 888 and/or
the guide member
840, toward the circumferential sleeve opening 831, and an un-beaded portion
of frame belt
860' can extend circumferentially around the frame 126, within the
circumferential sleeve
lumen 832, in a crimped configuration of the prosthetic valve 120. In some
applications, as
shown in Fig. 26A, the eyelet 848a can be positioned between the guide member
distal end 846
and the circumferential sleeve opening 831, while the frame belt 860' extends
there-through.
For example, the eyelet 848a can be attached to the frame 126 or to the
actuator assembly 138
(e.g., to the actuator outer member 140), distal to the guide member distal
end 846, and
potentially aligned therewith.
[0362] Fig. 26B shows a partially expanded state of the prosthetic valve 120.
In this state, some
of the beads, such as beads 866ca and 8669), are positioned around the
circumference of the
valve 120, within the circumferential sleeve 830, while other beads, such as
beads 866ce, 866cf,
866cg, 866ch, 866ci, 866'j and 866ck, remain out of the circumferential sleeve
830, for example
disposed within the guide member 840 and/or the belt shaft 888.
[0363] The circumferential sleeve 830 can comprise a radiolucent material or
include a cut-out
window, so as to allow visibility of radiopaque marked beads 866' disposed
therein. Thus, the
number of beads 866' circumferentially disposed around the valve 120, which
can be visible
74
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
under fluoroscopy, can be indicative of the valve expansion diameter. For
example, Fig. 26C
shows the valve 120 further expanded, potentially to a final expansion
diameter, wherein a
larger number of beads, such as beads 866ca, 8669), 866cc, 866cd, 866ce,
866cf, 866cg and
866ch, are positioned around the circumference of the valve 120, while a lower
number of
beads, such as beads 866'j and 866ck, remain out of the circumferential sleeve
830.
[0364] The sleeve 830 can advantageously serve as a guide member, directing
the geometrical
features 866 (such as beads 866') around the circumference of the valve 120
upon expansion
thereof. While a circumferential sleeve 830 is described and illustrated for
use in combination
with a frame belt 860 provided with geometrical features 866, it will be clear
that a sleeve 132,
that can be attached to, or integrally formed with, a skirt (e.g., an outer
skirt 137), can be used
instead in the same manner. Moreover, in some applications, the sleeve can be
replaced with
other means of guiding the frame belt 860 circumferentially around the valve
120, such as
suture loops (not shown) looped around the frame 126 (e.g., around struts 127
of junction 130),
through which the frame belt 860 may slide. In yet alternative applications, a
sleeve or other
type of guiding means is used, and the frame belt 860 can be attached, at
least at the frame belt
first end 862, to the frame 126, directly (e.g., to a strut 127 or a junction
130) or indirectly (e.g.,
to a skirt).
[0365] According to some embodiments, the belt pull member 886 may be attached
to the
frame belt 860 via a connector 194, which can be, in some variants of the
embodiments, a
releasable connector 194. For example, once the expansion procedure is
complete, as shown in
Fig. 26C, the proximal connector element 193 can be released from the distal
connector
elements 195, and the belt pull member 886 can be retracted with the proximal
connector
element 193 attached thereto, while the distal connector elements 195,
potentially with a
portion of the frame belt 860 attached thereto, can remain with the expanded
valve 120, for
example disposed within the guide member 840. As shown in Figs. 26D, a
subsequent step can
include retraction of the belt shaft 888 from the valve 120 and the guide
member 840. This
process can be implemented according to any of the embodiments of a re-
compression
assembly 180 having a releasable connector 194, described and illustrated in
conjunction with
Figs. 7A-7C.
[0366] While Fig. 26A shows an un-beaded portion initially disposed
circumferentially around
the valve 120 in the crimped configuration, it will be clear that in
alternative configuration,
beads 866' can be disposed around the valve 126 even in a crimped
configuration. In such
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
cases, the initial amount of beads 866' disposed around the valve 120 in this
configuration,
may be indicative of a crimped diameter, and expansion force can be estimated
by a
corresponding increased number of beads 866.
[0367] In some applications, the guide member 840, and/or at least a portion
(e.g., a distal
portion) of the belt shaft 888, include a radiopaque zone (for example, around
their outer
surfaces), configured to hide or mask the geometrical features 866 disposed
within their
lumens, from visibility under fluoroscopy. Such applications can
advantageously simplify
visual identification of the number of geometrical features 866 (e.g., beads
866') around the
valve 120, as only these beads are visible and unmasked under fluoroscopy.
[0368] While a plurality of beads 866' are illustrated, alternative
configurations can include a
single geometrical feature, such as a single bead 866' which may be hidden
from view, or
otherwise positioned in an initial position, which may change as the valve
expands. Such
configurations may be applicable for diameter detection instead of force
measurement, for
example a maximal expansion diameter, such that exposure of the bead 866, or
alternatively,
positioning thereof in a second distinguishable position, may be indicative of
over-expansion.
[0369] As mentioned above, measurement mechanisms situated at the handle 110,
that rely on
force transmission from a region adjacent the prosthetic valve 120 to the
handle 110, may result
in inaccuracies in valve diameter estimation due to lengthening and/or
multiple bend-regions
or twists that might be formed along the tortuous vasculature of the patient,
and may require
additional means for compensating for such inaccuracies, such as springs or
pulley assemblies,
as described throughout the current disclosure. A frame belt equipped with
expansion force
indicators, including indicators that comprise separation zones and/or
geometrical features
(e.g., beads) according to any of the embodiments disclosed herein, might be
advantageous as
they provide discrete transitions between states of the expansion force
indicators (e.g.,
breaking/tearing, "jumping" through a restrictor, etc.), achieved only once
respective
circumferential force threshold are applied thereto by the expanding frame
126.
[0370] According to some embodiments, frictional forces between any of the
belt pull member
886 and/or the frame belt 860, with any of the belt shaft 888, the guide
member 840 and/or the
sleeve 132, 830, are set to be lower than the estimated circumferential forces
exerted by the
frame 126 on the frame belt 860. This may be achieved by an appropriate
selection of material
76
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
properties, manufacturing such components with desired surface roughness, or
coating with
low-friction layers.
[0371] While not shown explicitly, a frame belt 860 with geometrical features
860 can be
utilized without passing through a guide member 840, and/or without passing
through a belt
shaft 888. Similarly, a frame belt 860 with geometrical features 860 can
extend through a guide
member 840, and/or through a belt shaft 888, which do not mask the radiopaque
markers
around the geometrical features 866. In such configurations, geometrical
features 866, such as
beads 866', may be visible both when positioned around the prosthetic valve
120 (for example,
within a sleeve), or along a portion of the frame belt 860 which does not
circumscribe the valve
120 (e.g., proximal to the sleeve). A viewer (e.g., clinician) can
distinguish, in such cases,
between a circumferential orientation of some beads 866' and a non-
circumferential orientation
of other beads, when viewed under fluoroscopy. For example, with reference
back to Fig. 26B,
it may be possible to identify that the beads 866ca and 8669) define a first
orientation, which is
the circumferential orientation, and that the beads 866ca, 8669), 866cc,
866cd, 866ce, 866cf,
866cg and 866ch, define a second orientation, which is a non-circumferential
orientation, shown
as an axial orientation which is substantially perpendicular to the first
orientation. Once the
circumferential orientation is identified, the number of beads visible in that
orientation can be
indicative of the valve expansion diameter.
[0372] According to some embodiments, the restrictor 848 comprises a reference
radiopaque
marker 882. For example, the eyelet 848a can include a reference radiopaque
marker 882. Since
the eyelet 848a is rigidly attached to one frame component (e.g., the actuator
outer member
140), the reference radiopaque marker 882 remains immovable with respect to
the outflow end
123. A viewer can distinguish, in such cases, between beads 866' positioned on
both sides of
the reference radiopaque marker 882, when viewed under fluoroscopy. For
example, with
reference back to Fig. 26B, it may be possible to identify that the beads
866'a and 8669) are
positioned distal to the radiopaque-marked eyelet 848a, and that the beads
866ca, 8669), 866cc,
866cd, 866ce, 866cf, 866cg and 866ch, are positioned proximal to the
radiopaque-marked eyelet
848a. The number of beads 866' visible on a specific side of the reference
radiopaque marker
882, such as the number of beads 866' distal to the reference radiopaque
marker 882, can be
indicative of the valve expansion diameter.
[0373] In some implementations, the restrictor 848 is implemented as a narrow
opening
through which the frame belt 860 extends. For example, Fig. 27 shows a
configuration which
77
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
is similar to that shown in Fig. 26C, except that the guide member distal end
846 includes a
guide member constriction 848b, which may be formed as an inwardly flanged or
tapering
opening, having an opening diameter which is slightly smaller than the outer
diameter of the
beads 866a, therefore acting as an alternative to the eyelet 848a. While shown
at the guide
member distal end 846, in alternative variations, the guide member
constriction 848b can be
formed as a narrowing at the guide member proximal end 844, or as a local
narrowing anywhere
else along the guide member lumen 842. Similarly, while not shown explicitly,
a narrowing
with an inner diameter slightly smaller than that of the geometrical features
866 can be formed
at the circumferential sleeve opening 831, formed as a local narrowing within
the
circumferential sleeve lumen 832, or as a narrowing at the opening of, or
within the lumen of,
the belt shaft 888.
[0374] According to some embodiments, a frame belt 860 provided with
geometrical features
866, circumscribes the prosthetic valve 120 such that the entire length of its
beaded portion is
disposed around the frame 126. For example, Fig. 28A shows a prosthetic valve
120 in a
crimped configuration, provided with a circumferential sleeve 830
circumscribing a portion of
the circumference of the valve 120. A frame belt 866' is also disposed around
the prosthetic
valve 120, such that a first portion thereof, which is an un-beaded portion
shown in Fig. 28A,
extends out of the circumferential sleeve 830, and another beaded portion is
disposed within
the circumferential sleeve lumen 832. The restrictor 848 can be provided in
the form of a sleeve
constriction 848', at the opening of the sleeve 830 through which the frame
belt 866' extends.
[0375] When the prosthetic valve expands, as shown in Fig. 28B, at least some
of the beads,
such as beads 866ca, 8669), 866cc and 866cd, are pulled out of the
circumferential sleeve 830,
while other beads, such as beads 866ce, 866cf and 866cg, remain within the
sleeve 830.
[0376] The circumferential sleeve 830 includes a circumferential sleeve first
end 834, which
can be an enclosed end portion of the sleeve, and may be affixed to the frame
126 (e.g., to a
junction 130), via an affixing member 850. The affixing member 850 can include
a suture, a
biocompatible adhesive, and the like. The circumferential sleeve 830 further
includes a
circumferential sleeve second end 834, which can be an opening through which
the frame belt
860 may extend. The circumferential sleeve 830 can be coupled to the frame 126
along
additional coupling points thereof via slidable attachment members 852 (see
Fig. 28B), which
can be provided in the form of suture loops, bands, and the like, allowing the
sleeve 830 to
slide there-through while the valve 120 expands.
78
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
[0377] The frame bolt first end 862 can be similarly affixed to the frame 126
(e.g., to a junction
130) via an affixing member 850. The opposite, frame bolt second end 864, can
be a free end
disposed within the circumferential sleeve lumen 832, enabling the frame bolt
860 to slide
through, and partially out of, the sleeve 830, as the valve 120 expands.
[0378] While Fig. 28A shows an un-beaded portion exposed out of the sleeve 830
in the
crimped state, it will be clear that in alternative configuration, beads 866'
can be disposed
around the valve 126 and out of the sleeve 830 in this state. In such
configurations, the initial
amount of beads 866' disposed out of the sleeve 130 in this state, may be
indicative of a crimped
state of the valve, and an increased number of beads 866' that are further
distinguishable due
to repositioning thereof out of the sleeve 830, are indicative of the force
applied by the valve
during expansion thereof, exceeding threshold values required to displace the
beads 866.
[0379] In some applications, the circumferential sleeve 830 comprises a
radiopaque zone, for
example around its outer surface, configured to hide or mask the beads 866'
disposed within
its lumen from visibility under fluoroscopy. Thus, in the state shown in Fig.
28A, all of the
beads 866' shown to be positioned within the circumferential sleeve lumen 832
may be hidden
from view, and only beads pulled out of the sleeve 830, such as beads 866ca,
8669), 866cc and
866'd in Fig. 28B, may be exposed and visible under fluoroscopy, such that the
amount of
visible beads can be indicative of the valve expansion diameter.
[0380] In alternative applications, the beads 866' can be visible through the
sleeve 830 as well.
In such applications, the valve 120 can further comprise a reference
radiopaque marker (not
shown in Figs. 28A-28B). For example, the circumferential sleeve second end
836 can include
a reference radiopaque marker. A viewer can distinguish, in such cases,
between beads 866'
positioned on both sides of the reference radiopaque marker, when viewed under
fluoroscopy.
In a further variation of the applications, the valve 120 can be devoid of a
sleeve 130
(application not shown), but provided with a restrictor 848 such as an eyelet
848a, rigidly
attached to the frame 126 and having a reference radiopaque marker thereon,
wherein the frame
belt 860 extends there-through so as to enable a viewer to distinguish between
beads 866'
positioned on both sides of the reference radiopaque marker in a similar
manner.
[0381] While not explicitly shown, it will be understood that all of the
embodiments described
and illustrated for the beads 866' in conjunction with Figs. 26A-28B, are
similarly applicable
79
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
in general to other geometrical features 866, including, but not limited to:
knots, balls, ribs and
spokes.
[0382] Figs. 29A and 29B show configurations which are similar to the
configurations shown
in Figs. 28A and 28B, respectively, except that the geometrical features 866
are provided in
the form of belt ratcheting teeth 866d, and the restrictor 848 is provided in
the form of
complementary sleeve ratcheting teeth 848d comprised within the
circumferential sleeve lumen
832. Fig. 29B shows a portion of the belt ratcheting teeth 866da exposed out
of the sleeve 830
in an expanded state of the valve 120, and a portion of the belt ratcheting
teeth 866db that may
remain within the sleeve 830. The amount of radiopaque-marked ratcheting teeth
866da
exposed out of the sleeve 130 may be indicative of the valve expansion
diameter. Moreover,
any embodiment that relates to the expansion belt 860' provided with beads
866', as described
and illustrated in conjunction with Figs. 28A-28B, applies with equal force to
the expansion
belt 860d provided with belt ratcheting teeth 866d, as illustrated in Figs.
29A-29B.
[0383] While a plurality of sleeve ratcheting teeth 848d are shown in Figs.
29A-29B, it will be
clear that a restrictor 848 may be similarly implemented as a single tooth or
pawl, configured
to apply a ratcheting mechanism to the belt ratcheting teeth 866d.
[0384] Since the frame belt 860 is required to provide an indication of the
valve's expansion
force during the implantation procedure, and is no longer needed once the
valve is positioned
at the implant site, it can comprise, according to some embodiments, a bio-
resorbable material,
such as a bio-resorbable polymer, configured to dissolve over time. The
resorption rate of the
bio-resorbable frame belt 860 can be controlled by a variety of parameters,
such as the polymer
material, additives, processing and the like. Some examples of polymeric
resorbable materials
include, but are not limited to: Polylactide (PLA), Poly-L-lactide (PLLA),
Polyglycolide
(PGA), Poly-e-Caprolactone (PCL), Trimethylene carbonate (TMC), Poly-DL-
lactide
(PDLLA), Poly-b-hydroxybutyrate (PBA), Poly-p-dioxanone
(PDO) Poly-b-
hydroxypropionate (PHPA) and Poly-b-malic acid (PMLA). Preferably, the bio-
resorbable
material is comprised in the frame belt 860 in a manner that will not
interfere with its
radiopacity, according to some embodiments elaborated herein above.
[0385] According to some embodiments, the frame belt 860 comprises at least
one electrically
conductive expansion force indicator. For example, an expansion force
indicator 866 can
comprise a stretch sensor, configured to change is electrical resistivity when
stretched over the
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
frame 126 during expansion thereof. The stretch sensor can be operatively
coupled, via a
transmission line, to a control unit and display in the handle 110. The
transmission line can be
implemented in a similar manner to that of the belt pull member 886, which may
be releasably
coupled via a releasable electrical connector similar to the releasable
connector 194. The
transmission line and the releasable connector may include various
electrically conductive
materials, such as copper, aluminum, silver, gold, and various alloys such as
tentalum/platinum, MP35N and the like. An insulator can surround the
transmission line and/or
releasable connector. The insulator can include various electrically
insulating materials, such
as electrically insulating polymers.
[0386] In use, expansion of the frame 126 may result in elongation or
stretching of the stretch
sensor, which may generate corresponding electrical signals in the form of
current, voltage,
resistance, or change in the same. The signals may be electrically conducted
to a control
circuitry in the handle 110, potentially via terminals connected to
electrically conductive
releasable connector and transmission line, and may be interpreted and display
on a display
116.
[0387] The transmission line may be releasably attached to the frame belt in a
manner similar
to the configuration exemplified in Figs. 26A-26D, wherein the frame belt may
include a
stretch sensor (not shown) instead of beads 866, wherein the releasable
electrical connector is
represented by the releasable connector 194, and wherein the transmission line
is represented
by the belt pull member 886, which may extend through a transmission line
shaft represented
by the belt shaft 888. According to some embodiments, when the electrically
conductive
proximal connector element 193 is coupled to the electrically conductive
distal connector
element 195 (as shown in Figs. 26A-26B), the transmission line shaft 888 is
hermetically
coupled to the guide member 840, in a manner that seals the electrically
conductive connector
194 from surrounding blood flow. Thus, when the transmission line is decoupled
from the
frame belt, in a configuration similar to that shown in Figs. 26C, the exposed
end of the
proximal connector element 193 remains isolated from the surrounding
environment of the
blood flow, which allows the transmission line to be detached safely pulled
through the shaft
888 while avoiding the risk of exposing the surrounding blood flow or other
tissues to electrical
current thereof.
[0388] The shaft 888 may be threadedly engaged with the guide member 840. Once
the
transmission line is detached from the frame belt and pulled away therefrom,
the shaft 888 may
81
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
be rotated so as to detach from the guide member 840. According to some
embodiments, the
transmission line is pulled along a sufficient distance prior to disengaging
the shaft 888 from
the guide member 840, such that once the shaft 88 is detached and retracted
from the valve
120, in a manner similar to that shown in Fig. 26D, the transmission line
cannot be exposed to
the blood flow flowing through the lumen of the shaft 888.
[0389] Utilization of a frame belt 860 provided with at least one expansion
force indicator in
the form of a stretch sensor, or other types of sensing elements configured to
change an
electrical property thereof (e.g., resistivity or capacitance) in response to
stretch forces
imparted thereon by an expanding valve 120, may be advantageous over visual
detection of
radiopaque-marked expansion force indicators 866, according to any of the
embodiments
described and illustrated in conjunction with Figs. 23A-29B, as it avoids
potential interference
that may result from the inherent radiopacity of the frame 126.
[0390] While the embodiments are described and illustrated throughout the
current disclosure
for use with a mechanically expandable valve 120, it will be clear that
methods for providing
real-time estimation of the valve diameter, based on a relationship between
the expansion
diameter and a dimensionless parameter, as well as methods and devices for
providing real-
time estimation of the valve expansion force based on frame belts 860
according to any of the
embodiments disclosed herein, may be similarly used in combination with other
valve types,
such as balloon expandable valves or self-expandable valves. However,
conventional balloon-
expandable valves and self-expandable valves are typically inflated or
expanded during a short
time period (e.g., in a burst), in a manner which provides limited control of
valve expansion.
In contrast, utilization of the above mentioned imaging methods, or
utilization of frame belts
860, in combination with mechanically expendable valves 120, is advantageous
since the
mechanical expansion mechanism (for example ¨ as described in conjunction with
Figs. 4A-
C) provides a higher degree of control over the rate and extent of valve
expansion, enabling
the clinician to adjust expansion diameter, responsive to real-time feedback
regarding valve
diameter and/or expansion force.
[0391] It is appreciated that certain features of the invention, which are,
for clarity, described
in the context of separate embodiments, may also be provided in combination in
a single
embodiment. Conversely, various features of the invention, which are, for
brevity, described
in the context of a single embodiment, may also be provided separately or in
any suitable sub-
combination or as suitable in any other described embodiment of the invention.
No feature
82
CA 03143501 2021-12-14
WO 2021/086836 PCT/US2020/057502
described in the context of an embodiment is to be considered an essential
feature of that
embodiment, unless explicitly specified as such.
[0392] Although the invention is described in conjunction with specific
embodiments thereof,
it is evident that numerous alternatives, modifications and variations that
are apparent to those
skilled in the art may exist. It is to be understood that the invention is not
necessarily limited
in its application to the details of construction and the arrangement of the
components and/or
methods set forth herein. Other embodiments may be practiced, and an
embodiment may be
carried out in various ways. Accordingly, the invention embraces all such
alternatives,
modifications and variations that fall within the scope of the appended
claims.
83