Note: Descriptions are shown in the official language in which they were submitted.
Docket No: Van Gorden ¨ 003CanIPO
OCULAR MEDICATION DELIVERY APPARATUS
FIELD OF THE INVENTION
[001] The present invention generally relates to medication delivery devices,
and more
specifically relates to ocular medication delivery devices. In one particular
embodiment the eye
medication delivery system and apparatus uses a gravity-vector-independent
delivery
mechanism for safe delivery of a metered mist dosage of medication to a
patient's eye and in
particular to the cornea.
BACKGROUND DESCRIPTION OF THE FIELD OF THE INVENTION
[002] There are many reasons and times when eye drops or eye medication need
to be used by
various patients or users. Dry eyes, eye injuries, laser eye surgery, and so
on are just some of
the reasons that eye medications may need to be applied to or placed on the
exterior of the eye
or cornea of a user. The standard or traditional way to apply eye medication
is by allowing
liquid drops to drip or fall onto an open eye from a squeeze dispenser, and
thereby flood the
exterior of the eye.
[003] This action of having a liquid drop fall, by gravity, onto the cornea is
fraught with
problems. If the eye dropper is not located above the eye, the drop falls onto
the patient's cheek
or nose, or forehead. If the patient is not holding his or her eye open, and
if the drop is not
timed appropriately, the drop may simply fall onto the patient's closed eye.
Moreover, even if
the patient can try to hold his or her eye open with one hand, and hold the
medication container
with his or her other hand, by the time the drop releases from the container
tip, the patient may
blink and the medication may only partially be administered. Accordingly, more
often than not,
the application of the drops does not make it into the eye at all. The patient
either blinks,
thereby blocking the medication from getting to the eye, or the patient just
misses in placing the
drops where needed.
[004] Even if the user has the dexterity to position the eye medication device
over his or her
eye, and is able to hold his or her eye open with his or her other hand, and
is able to time the
release of the medication onto his or her cornea, and the user does not
inadvertently blink, then
almost always the result is that the user's eye reacts by tearing to wash the
liquid or medication
out. In each of the former scenarios, the medication is simply wasted, lost,
or substantially
1
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
diluted. Due to mis-administered medication, many patients run out of eye
medication early,
and may not complete their full course of treatment, which often times results
in decreased
prescription effectiveness.
[005] In the dilution scenario, which is known and expected by physicians and
pharmaceutical
manufacturers as a natural reaction to tear up and attempt to flush the
foreign medication out of
the eye. Because of this known effect and reaction, physicians accordingly
either compensate
by increasing the concentration of the medication within the fluid, or
increasing the medication
dosage (number of drops). In either scenario (over prescribing medication, or
increasing
concentration), there is a substantial inefficient use of medication and
increased cost.
[006] Additionally, there is also plenty of room for patients to make an error
and touch the tip
of the eye drop applicator to their eye in order to ensure they deliver the
medication to the eye.
This can lead to the tip being unsanitary and cause and infection, or the
patient can simply
injure their eye by scratching the cornea with the applicator tip. Either
result is very
problematic for the user.
[007] Several devices and systems for eye medication delivery have been
designed and
manufactured for years. Examples of such devices and systems include U.S.
Patent No.
7,524,511 and U.S. Patent No. 8,936,021. While these devices relate to
administering an
aerosolized spray of medication to one's eye, neither provides any means to
ensure that the
medication will make it to the eye surface because there is no means for
holding one's eye open
while the medication is being released. Other patents disclose certain devices
and systems that
have particular uses and appear to address certain issues, but none provides a
comprehensive
solution for the overall objective of being able to effectively and safely
administer eye
medication by an orientation (gravity) independent delivery system using a
metered mist
dosage.
[008] Accordingly, while certain of designs, devices, and apparatus have been
developed and
commercialized to address some of the noted issues relating to the application
and use of eye
medications, none have fully addressed the noted issues and problems. What is
needed is an
eye medical delivery device and system that provides an orientation
independent means for
delivery of a metered mist dosage of ocular medication to a patient's cornea
that safe to use.
Such a device and system has not been created, disclosed, or used in the prior
art.
2
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
SUMMARY OF THE INVENTION
[009] The present invention overcomes the disadvantages of the known prior art
and fulfills
the needs described above by providing an eye medication delivery system and
apparatus using
a gravity-vector-independent safe delivery means to provide a metered mist
dosage of ocular
medication to a patient's eye and specifically to the patient's cornea.
[0010] A preferred aspect of the invention is an ocular medication delivery
apparatus
comprising (a) a main body having an internal cavity shaped and sized to hold
an ocular
medication container; (b) an ocular orbit shaped cup element having a proximal
end shaped and
sized to fit within said main body cavity, and having a distal end sized and
contoured to
comfortably fit around a patient's ocular orbital socket and thereby hold said
patient's eye open
when said ocular orbit shaped cup element is pressed against said patient's
ocular orbital
socket; and (c) an ocular medication container that fits within said main body
internal cavity,
wherein when said ocular orbit shaped cup element proximal end is pressed
against said ocular
medication container, a metered dosage of medication is released as a mist
onto said patient's
eye and cornea.
[0011] A further preferred embodiment of the invention is a method of
administering ocular
medication using an ocular medication delivery apparatus providing an
aerosolized metered
mist dosage of said eye medication, wherein said delivery apparatus comprises
(a) a main body
having an internal cavity shaped and sized to hold an aerosolized medication
container; (b) an
eye orbit shaped cup element having a proximal end shaped and sized to
partially fit within said
main body cavity, and having a distal end sized and contoured to comfortably
fit around a
patient's eye orbital socket and thereby hold said patient's eye open when
said eye orbit shaped
cup element is pressed against said patient's eye orbital socket; and (c) an
aerosolized
medication container that fits within said main body internal cavity, said
method comprising the
steps of (1) engaging said eye orbit shaped cup element within said main body
such that in said
engaged position said eye orbit shaped cup element may be pressed against said
aerosolized
medication container; and (2) pressing said eye orbit shaped cup element
distal end against a
user's eye socket such that said eye orbit shaped cup element proximal end
engages said
aerosolized medication container, wherein with said engaging of said
aerosolized medication
3
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
container, a metered dosage of medication is released as a mist onto said
patient's eye and
cornea.
BRIEF DESCRIPTION OF THE DRAWINGS OF CERTAIN EMBODIMENTS
[0012] To easily identify the discussion of any particular element or act, the
most significant
digit or digits in a reference number refer to the figure number in which that
element is first
introduced.
[0013] FIG. 1 is a perspective view of an embodiment of the ocular medication
delivery device
in its inert state showing the device handle and the eye cup.
[0014] FIG. 2 is a front view of an embodiment of the ocular medication
delivery device in its
inert state showing the device handle and eye cup.
[0015] FIG. 3 is a side view of an embodiment of the ocular medication
delivery device in its
inert state showing the device handle and eye cup.
[0016] FIG. 4 is a top view of an embodiment of the ocular medication delivery
device in its
inert state showing the device handle and eye cup.
[0017] FIG. 5 is perspective view of an embodiment of the ocular medication
delivery device
showing the device main body.
[0018] FIG. 6 is a side view of an embodiment of the ocular medication
delivery device
showing the device main body.
[0019] FIG. 7 is an exploded front view of an embodiment of the ocular
medication delivery
device in its ready state showing the device handle, eye cup, and medication
container.
[0020] FIG. 8 is an exploded perspective view of an embodiment of the ocular
medication
delivery device in its ready state showing the device handle, eye cup, and
medication container.
[0021] FIG. 9 is an exploded side view of an embodiment of the ocular
medication delivery
device in its ready state showing the device handle, eye cup, and medication
container.
[0022] FIG. 10 is a perspective view of an embodiment of the eye cup element
shown in a
cross-sectional view and showing the expansion chamber.
4
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
[0023] FIG. ills a front cross-sectional view of an embodiment of the eye cup
element
showing the internal channels and orifices.
[0024] FIG. 12 is a side view of an embodiment of the eye cup element showing
the cross -
sectional line for the views shown in Figs. 10 and 11.
[0025] FIG. 13 is a perspective view of an embodiment of the eye cup element
of the present
inventive apparatus.
[0026] FIG. 14 is a top view of an embodiment of the eye cup element of the
present inventive
apparatus.
[0027] FIG. 15 is a perspective exploded view of another embodiment of the
present inventive
apparatus.
[0028] FIG. 16 is a front exploded view of another embodiment of the present
inventive
apparatus.
[0029] FIG. 17 is a side exploded view of another embodiment of the present
inventive
apparatus.
[0030] FIG. 18 is a perspective front and back view of an embodiment of the
present inventive
apparatus with a fully enclosed circumference for the eye cup element.
[0031] FIG. 19 is a perspective front and back view of another embodiment of
the present
inventive apparatus with a partially open circumference for the eye cup
element.
[0032] FIG. 20A is a perspective rendering of an embodiment of the ocular
medication delivery
device showing it in a stored or inert configuration.
[0033] FIG. 20B is a perspective rendering of an embodiment of the ocular
medication delivery
device showing it converting from its inert state to its ready state.
[0034] FIG. 20C is a further perspective rendering of an embodiment of the
ocular medication
delivery device showing it converting from its inert state to its ready state.
[0035] FIG. 21 is rendering showing use of an embodiment of the ocular
medication delivery
device with a human patient.
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
[0036] An innovative eye medication delivery device and system is described in
detail in the
following paragraphs, including a description of the several elements of the
device and system,
and the several uses and modes of operation of the device and system.
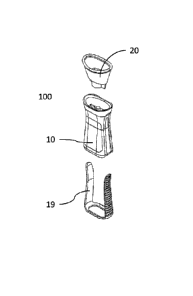
[0037] Illustrated in Fig. 1 is a perspective view of the device 100 shown in
a stored, closed, or
inert configuration. In this embodiment, the device 100 has two primary
elements. A first base
element, or main body 10 having an oblong shape that provides certain
ergonomic features for
improving ease of use of the device 100. The main body 10 has an internal
cavity 11 that is
shaped and sized to accept at least two items. The first item or element is an
eye orbit shaped
cup element 20, shown in Fig. 1 in the main body 10, and also shown in Figs. 5
and 6. The
second item or element is a medication container 30, as described in further
detail below.
[0038] As shown in Fig. 1, only the distal or top end of the eye orbit shaped
cup element 20 is
shown. A front view of the device 100 is further shown in Fig. 2, and further
shows additional
detail of the shape of the distal end of the cup element 20. More
particularly, the cup element
20 has a distal end shape 21 that is oblong or oval so as to encircle a
patient's eye, and to fit
around the patient's eye and within or fitting on the patient's orbit bone
structure. The cup
element distal end shape 21 may also have a softer or flexible lip covering 22
to provide a level
of comfort when the cup element distal end shape 21 is place around the
patient's eye and
against the patient's eye orbital socket. A further view of the device 100 is
shown in Fig. 3
from a side perspective, and from a top view as shown in Fig. 4.
[0039] Additional views of the main body 10 of the device 100 are further
shown in Figs. 5
and 6. As shown in one embodiment, the main body 10 incorporates several
ergonomic features
including over-molded grips 15 on both side sections of the main body 10.
These over-molded
grips 15 provide sensory feedback and assistance to the patient to properly
hold and operate the
device 100, while also providing assistance to the patient to have a firm grip
of the device 100
during operation. A side view of the device 100, shown in Fig.6, provides
another view of the
over-molded grips 15 that may be integrally formed on the exterior of the main
body 10.
[0040] The internal cavity 11 is shown in Fig. 5 from a top perspective view
of the main body
10. In this embodiment, the internal cavity 11 is positioned centered within
both X and Y axes
6
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
of the main body 10 when viewed from above. In another embodiment of the main
body 10, the
internal cavity 11 may incorporate a ledge section that could provide a
physical limit to the
motion of the cup element 20 into the internal cavity.
[0041] The second item or element that fits within the main body 10 is a
medication container
30. Figs. 7 through 9 show, respectively, a front, perspective, and side blown
up views of how
the three elements (the main body 10, the cup element 20, and the medication
container 30) fit
within each other. More particularly, Figs. 7 through 9 present blown-up views
with the
medication container 30 positioned above the internal cavity 11 formed within
the main body
10, and showing the cup element 20 positioned above the medication container
30. In a fully
assembled and ready configuration, the medication container 30 fits snuggly
within the internal
cavity 11 and the cup element 20 similarly fits within the internal cavity 11
and rests upon the
dispensing nozzle 31 of the medication container 30. In operation, when the
cup element 20 is
slightly pressed towards or into the main body 10, further into the internal
cavity 11, and
engages the dispensing nozzle 31 of the medication container 30, a valve 32
within the
medication container 30 is opened, and a metered dosage of the eye medication
is dispensed
through a misting valve 22 in the cup element 20.
[0042] An embodiment of the pressure reduction channel 23 formed within the
eye cup
element 20, as shown in Figs. 10 and 11, is designed to reduce the pressure of
the medication
before the medication is released to and through the atomizer 24. More
particularly, Figs. 10
and 11 show two cutaway views of the cup element, and detailing an embodiment
of the
pressure reduction channel or valve 23 the cup element 20. As shown, the
pressure reduction
channel 23 and atomizer 24 ensure that the medication released into the cup
element 20 and
onto the patient's eye is at a very low pressure, very low speed, and results
in a gentle uniform
application of the medication the patient's eye.
[0043] As described, the cup element 20 has an orifice or cavity 25 into which
the dispensing
nozzle 31 fits when the cup element 20 is placed on top of the medication
container 30. The
cavity 25 shown in Figs. 10 and 11 fits over the dispensing nozzle 31 such
that when the cup
element 20 is pressed slightly into the main body 10 and towards the
medication container 30
(held within the main body internal cavity 11), the dispensing nozzle 31 is
depressed slightly
into the medication container 30 opening medication container valve 32. With
the opening of
the medication container valve 32, a metered dosage of the eye medication is
released through
7
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
the dispensing nozzle 31 and is expelled through the pressure reduction
channel 23 and
atomizer 24 shown in Figs. 10 and 11.
[0044] The design of the pressure reduction channel 23 and atomizer 24 is to
ensure a low
pressure, low velocity mist of the metered dosage of the eye medication is
released into the
wide cup element distal volume 27. As shown, the cup element internal cavity
is designed with
a larger expansion chamber 27 ensuring there is a reduction in the pressure of
the medication
that is released from the medication or aerosol canister 30. This reduction in
pressure of the
dispensed medication before and after the dosage is expelled through the
atomizer further
reduces the medication velocity and accordingly allows the device to be used
in close proximity
to the eye, and provide a gentle misting of the medication to the user's eye.
[0045] In further detail, Figs. 12 through 14 show additional details of an
embodiment of the
cup element 20. More particularly, Figs. 12 through 14 show, respectively a
side, perspective,
and top view of the cup element 20 in isolation. In this embodiment, the shape
of the cup
element 20 with the oval distal end fits around or contoured to fit within and
engage with the
patient's eye or orbital socket, while the proximal end has more of a circular
or cylinder cross-
section to fit within the main body internal cavity 11.
[0046] Also, shown in Figs. 10 through 14 is an example of a softer over
molded lip 22 on the
cup element 20 that provides a comfortable surface that is in contact with the
patient's orbital
socket. The Fig. 13 view, being a top view of the cup element 20 shows a top
view of the
atomizer component 24 of the cup element 20. The section of the cup element 20
onto which
the atomizer 24 fits is shown in the two cutaway views of Figs. 10 and 11.
This small element
24 would fit into the eye cup element 20 to provide consistent, uniform, and
accurate full
atomizing of the metered dosage of medication so as to create a comfortable
and gentle mist
that can evenly coat the patient's eye. An advantage of having the atomizer 24
not being
formed integrally within the eye cup element 20 is that different types of
atomizers 24 and
orifices formed within the atomizers may be used to create different
dispersion patterns for the
metered dosage or simply for different types of medication, including
different medication
viscosities. Figs. 10 and 14 also show views of the eye cup element 20,
illustrating the tapered
bottom or proximal end of the cup element 20 that engages with the medication
container 30
when the cup element 20 is placed within the main body internal cavity 11.
8
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
[0047] Two alternative embodiments and designs for the ocular medication
delivery device 100
are shown in Figs. 15 through 19. Figs. 15 through 17 show perspective, front,
and side
exploded views of an alternative embodiment of the medication delivery device
having three
core elements. Those elements are the cup element 20, the main body 10, and
then a side
element or side band 19 that runs along both sides of the main body 10 and
along the bottom of
the main body.
[0048] A further alternative embodiment of the ocular medication delivery
device 100 is shown
in Figs. 18 and 19 where a full circumference cup element 20 is shown in Fig.
18, and a
partially open cup element 20 is shown in Fig. 19. The partially open cup
element 20 allows for
outside air to intermix with the medication being dispensed from the device
100. Moreover, the
partially open cup element 20 ensures that the pressure within the cup element
20 when the
mediation is dispensed is essentially maintained at the surrounding
atmospheric pressure.
[0049] In a further embodiment of the medication delivery device 100, Figs.
20A through 20C
show a perspective rendering of the device with a pivotable lid 17 affixed to
the main body 10.
The three views shown present how a user may operate the device 100. More
particularly, the
left most image of Fig. 20A shows the device 100 fully closed and inert with
the lid 17 closed.
In the middle image of Fig. 20B, the lid 17 is lifted or pivoted upwards. With
the lid 17 fully
open, the cup element 20 is accessible and may be lifted upwards and rotated
(as shown in Fig.
20C, the rotation is approximately 90 degrees). With the cup element 20
rotated and in position
and active for medication, illustrated in Fig. 20C, the user need only
slightly and gently press
the cup element 20 against his or her orbital socket to engage the cup element
20 against the
medication container 30, which then opens the medication container 31 valve,
resulting in a
gentle misting or aerosolizing of the proper metered dosage eye medication
coating the user's
eye.
[0050] Important in this operation is the engaging of the cup element 20
against the user's
orbital socket. With the slight pressure of the cup element 20 against the
user's orbital socket,
the device 100 maintains the user's eye effectively open, and with the same
pressure activating
the release of the metered dosage from the medication container 30, the user
easily and
comfortably has the proper and correct eye medication administered.
[0051] In another embodiment also having a pivotable lid 17 attached to the
main body 10, the
cup element 20 may be partially spring loaded such that when the pivotable lid
17 is pivoted the
9
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
cup element is urged upwards and automatically rotated such that the
medication delivery
device 100 is fully engaged and ready for usage upon the opening and pivoting
of the lid 17 into
an open configuration.
[0052] Fig. 20A shows the device from a front perspective view, and shows the
eye cup
component fully stored within the device main body, and with the lid fully
engaged and closed,
making it easy to store the device 100 in bag or drawer without fear of it
accidentally
dispensing medication. The over molded soft touch components are also shown in
Figs. 20A
through 20C on the outside of the device along the sides of the main body 10.
These
components could be manufactured as a harder thermoplastic or as soft touch
over molded
components.
[0053] In another embodiment of the delivery device 100, an additional or
alternative release
mechanism may be incorporated into the main body 10 and device 100. More
particularly, as
illustrated in Fig. 20C, one or both of the side elements 19 may be configured
and engageable
such that with a slight compression force to one or both of the side elements,
the dispensing
nozzle of the medication container 30 may be depressed to release the metered
dosage into the
eye cup element 20. Such a configuration may be useful for the administration
of eye
medication to a patient needing assistance, including for example a child, or
an older
individual, or even for non-human patients such as dogs or horses. In another
configuration, a
separate release mechanism may be a push button 33 located on the bottom or
side of the main
body 10, or a push button 33 formed into one or both of the side elements 19.
[0054] Along those lines, the delivery device 100, in particular the eye cup
element 20, may be
configured and sized for different members of the population, including those
with smaller
facial characteristics (e.g., females) or larger facial structure (e.g.,
males). Similarly, the
delivery device 100 may be configured, sized, and shaped for use with non-
human patients,
including as noted above, dogs, cats, horses, or any other animal that may
benefit from having
eye medication administered gently and effectively. An example of use of the
device 100 is
shown in Fig. 21 with a female administering the eye medication.
Date recue/ date received 2021-12-22
Docket No: Van Gorden ¨ 003CanIPO
[0055] While several preferred embodiments and features of the inventive
ocular medication
delivery device 100 have been described and disclosed, in particular with
reference to certain
figures and drawings showing certain exemplary embodiments that relate to
various particular
sized and shaped apparatus, such devices and the disclosed designs as shown
are not to be
construed as limiting the scope of the inventive device or inventive products.
For example, as
described above, the medication delivery device 100 is shown for use with
human patients. The
device has equal utility and application, in potentially different sizes, for
canines, felines,
equines, or other animals. While described herein with the eye cup element and
medication
container being separate elements, such elements could be combined for ease of
manufacturing
and use purposes. All such alternate embodiments are believed to be within the
scope of the
inventive design and the below claims.
[0056] It will be recognized by those skilled in the art that other
modifications, substitutions,
and/or other applications are possible and all such modifications,
substitutions and applications
are within the true scope and spirit of the present invention. It is likewise
understood that the
above disclosure and attached claims are intended to cover all such
modifications, substitutions,
and/or applications.
11
Date recue/ date received 2021-12-22