Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/012055
PCT/CA2020/051021
MOLTEN SALT MEMBRANE ELECTROLYZER
CROSS-REFERENCE TO RELATED APPLICATION
[0001] The present application claims the benefit of United States provisional
patent application
no. 62/878,444 filed on July 25, 2019, the entirety of which is hereby
incorporated by reference_
FIELD OF THE INVENTION.
[0002] In one of its aspects, the present invention relates to molten salt
electrolysis, and in
particular an electrolyzer apparatus for molten salt electrolysis and a
process for producing lithium
metal from lithium carbonate.
INTRODUCTION
[0003] Molten salt electrolysis has been widely practiced for over a hundred
years, including the
Hall-Heroult process for aluminum, the Dow and IG Farben processes for
magnesium, and the
Downs process for alkali metals. The majority of commercial-scale molten salt
electrolytic
processes use chloride or fluoride electrolytes, as these are solvents which
facilitate the
electrowinning of the target metals from their oxides, chlorides, or other
compounds. In many
cases, the electrolyte, or the oxide, chloride, or fluoride of the desired
product metal, have either
physical properties that are undesirable (e.g., toxic, hygroscopic, corrosive,
etc.) or are
disadvantageous for other reasons (cost, availability, security of supply,
competing uses, difficulty
of manufacture, etc.).
[0004] US Patent no. 3,607,684 discloses a process for the manufacture of
alkali metal by
passing an electroyzing current from an anode to a cathode. The anode is in
contact with a fused
metal halide salt comprising ions of the alkali metal and no other monovalent
cations. The cathode
is in the form of liquid alkali metal. Interdisposed between the anode and the
cathode is a
diaphragm. The diaphragm is polycrystalline ceramic material which has ions of
the alkali metal
or ions capable of being replaced by the alkali metal. The diaphragm is
permeable only to
monovalent cations and therefore will pass only the cations of the alkali
metal which is being
manufactured. Halogen can be recovered as the anode product or a halogenated
hydrocarbon
can be recovered as the anode product by introducing a hydrocarbon or
partially halogenated
hydrocarbon into the anode compartment.
1
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0005] US Patent no. 1,501,756 discloses a process of producing alkali metals
and halogens by
electrolysis of fused halide baths, as for example, sodium chloride. An object
of the invention is
to recover halogens containing practically no gaseous impurities.
[0006] US Patent No. 4,988,417 discloses A method of electrolytically
producing lithium includes
providing an electrolytic cell having an anode compartment and a cathode
compartment The
compartments are separated by a porous electrically nonconductive membrane
which will be
wetted by the electrolyte and permit migration of lithium ions therethrough.
Lithium carbonate is
introduced into the anode compartment and produces delivery of lithium ions
from the anode
compartment to the cathode compartment where such ions are converted into
lithium metal. The
membrane is preferably a non-glass oxide membrane such as a magnesium oxide
membrane.
The membrane serves to resist undesired backflow of the lithium from the
cathode compartment
through the membrane into the anode compartment. Undesired communication
between the
anode and cathode is further resisted by separating the air spaces thereover.
This may be
accomplished by applying an inert gas purge and a positive pressure in the
cathode compartment
The apparatus preferably includes an electrolytic cell with an anode
compartment and a cathode
compartment and an electrically nonconductive membrane which is wettable by
the electrolyte
and will permit migration of the lithium ion therethrough while resisting
reverse passage of lithium
therethrough.
IMMARY
[0007] Lithium metal can be produced using a modified Downs cell (see, for
example,
US1501756 and US6063247) from a eutectic mixture of LiCI-KCI using a Lia feed
material. The
Downs cell generally uses bottom-mounted graphite anodes and side-mounted
cathodes in a
refractory-lined cell, typically comprising four connected anode and cathode
assemblies arranged
in a known, "cloverleaf" pattern. Interposed between the anode and cathode is
a metal mesh,
which serves to separate the anode gases from the cathode product, thus
limiting recombination
of the two products.
[0008] Under the influence of sufficient potential, molten lithium metal
plates out onto the cathode,
while chlorine gas evolves at the anode, according to reaction 1 (below). The
metal floats upwards
where it is collected in an annular bell submerged in the electrolyte. The
bell directs the molten
metal out of the cell due to differential metallostatic head produced by the
difference in density
between the electrolyte and the metal. Chlorine gas evolves at the anode as a
result of the
2
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
electrolytic reaction and is captured above the anode. Back-reaction of the
metallic and gaseous
products is prevented by a wire mesh interposed between the two electrodes.
2LiCi(t) = 2Li(() + C12 (g); E = ¨3.609 V
[1]
[0009] One of the drawbacks of the Downs process is that LiCI is hygroscopic,
which makes its
handling challenging and, even when well done, it can act as a source of water
in the electrolytic
cell. Water in a Downs cell has several negative consequences. Firstly, it
reacts with the lithium
chloride, making HCI and Li0H. LiOH has low solubility in the melt, which can
cause it to form
sludge and potentially results in lithium losses. Secondly, water attacks the
graphite anodes,
causing the anodes to oxidize and erode. This is problematic, because the
anode in a Downs cell
cannot be replaced without rebuilding the cell, which can increase direct
costs and downtimeAost
production. Thirdly, while dry chlorine gas can be handled in conventional
materials, wet chlorine
gas is highly corrosive to the interior of the cell and downstream equipment
This means that
these components must be made of special corrosion-resistant steels, and even
these do not
necessarily have long life. This can have serious negative consequences for
equipment
availability. Another drawback of the Downs process may be that the relatively
low quality of the
chlorine gas produced means it has limited value as a by-product, and thus is
generally treated
as a toxic waste gas. This can impose additional costs on the operation of the
Downs, thereby
increasing the cost of the lithium product. In addition, because the LiCI
required by the Downs
process must be of high purity, it is often derived from high purity lithium
carbonate. Conversion
of lithium carbonate to LiCI is a costly process, requiring consumption of HCI
and drying under
vacuum. As a result, LiCI tends to be a costlier source of lithium feed
material than lithium
carbonate (Li2CO3).
[0010] In GB1024689, a method is described that attempts to reduce the
reliance of the Downs
process on LiCI feed. The method proposes feeding a small quantity of Li2CO3
directly into the
anode compartment where it reacts in solid form with the evolved chlorine gas.
This, however,
may not be a practical approach, for a number of reasons. Firstly, because
Li2CO3 is not an easily
flowing bulk solid, it becomes sticky at the typical Downs cell operating
temperatures. This means
that gas permeability can be difficult to maintain, and a crust can form in
due course, resulting in
relatively poor conversion of the Li2CO3. Secondly, 1J2CO3 reacts at the
cathode with lithium metal,
and is directly electrolyzed according to reactions 2 and 3 (below). This may
cause reductions in
current efficiency, elemental carbon sludge formation, and low-solubility Li2O
formation whenever
there is a mismatch between the feeding and consumption of Li2CO3. Such
mismatches can occur
3
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
for operational reasons, such as current setpoint changes, feed system lag,
power fluctuations,
or the aforementioned crusting. These challenges make the proposed method of
GB1024689
difficult to adopt for the commercial production of lithium metal from Li2CO3.
4L1a, + Li2C030, = C(s) + 3L120(1,$)
[2]
3COr (1) = 2CO2(9) + C (s) + 302-(1); E = ¨ ¨25 V
[3]
[0011] US4988417 describes a molten salt LiCI-KCI electrolytic process whereby
lithium
carbonate is fed into a cell with separate anolyte and catholyte compartments.
The cell is
separated by a porous ceramic membrane. According to the disclosure, the cell
is intended to be
operated between 550-770 C, with 5-10% Li2CO3 dissolved in the melt, while
carbon anodes
provide a source of carbon for the reduction reaction. Beneficially, the cell
of the invention is able
to produce relatively high purity lithium metal from lithium carbonate,
according to equation 4,
which has a lower decomposition potential than the conventional chloride
reaction 1, thereby
nominally reducing the energy consumption and cost thereof.
1 3
142CO3(s, I)+2C(s) = 2L1(1) + -2CO2(g); E = ¨2.127 V
[4]
[0012] While it is true that relying on reaction 4 reduces the decomposition
potential of the lithium
metal-producing reaction, there are a number of practical limitations that can
negate this benefit
Porous membranes not only reduce diffusion, they also have substantially
higher resistance to
ionic condition. Typically, this can be 4-10 times higher than the electrolyte
bath, meaning that
membranes of a workable thickness can more than double the resistive losses
due to the anode-
to-cathode gap. Also, because the carbon anodes are consumed by the process,
the electrical
resistance of the anode-to-cathode gap increases over time, leading to further
increases to the
resistive losses as the anode wears.
[0013] One or more of these effects may be mitigated to some extent by
operating at low current
density, which generally lead to a low productivity per unit electrode area.
This can have the effect
of increasing the overall physical size of the electrolysis unit, and/or the
number of cells required
for a given production capacity, which can increase the capital cost and
personnel costs of the
plant.
[0014] Operation at low current density may also require a relatively larger
membrane area, which
may tend to increase carbonate transport between the anolyte and catholyte.
This can result in
4
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
reduced current efficiency and the production of elemental carbon sludge and
Li2O build-up in the
cathode compartment, further reducing the economic performance of the cell.
[0015] Another consequence of operating at low applied potential is that the
process relies
entirely on a carbon-consuming reaction_ This can result in a high carbon
consumption per unit of
metal produced which, given the high cost of graphite, can increase the
operating cost of the
plant.
[0016] In "The Electrowinning of Lithium from Chloride-Carbonate Melts",
Kruesi and Fray
disclose a similar low-potential Li2CO3 electrolysis process to US4988417.
Efforts are made to
reduce carbon costs by employing a durable anode and a preferentially-consumed
bed or slurry
of low-cost carbon. Although most of the carbon anode approaches reported by
Kruesi and Fray
are successful at producing lithium metal, few do so with high current
efficiency, and none achieve
more than a modest reduction in carbon consumption. Also, because the work
continues to use
low applied potentials in an effort to realize energy savings, it is limited
to low current-density
operation.
[0017] Current electrolyzer technology has not been adapted to the membrane
processes
described above at industrially practical scale. With the Downs cell, its non-
contiguous membrane
and bottom-mounted anode make adaptation difficult Replacing the steel mesh
membrane with
a porous membrane is not generally practical, as, for example, it would be
difficult to ensure a
leak-tight seal against the bottom of the vessel, thereby preventing effective
separation of the
anode and cathode compartments. Also, because the anodes are bottom mounted,
the life of the
vessel could be limited to less than a week or two before the anode would have
to be replaced.
[0018] Hall-Heroult cells have been well developed for aluminum electrolysis
with consumable
anodes; however, these are designed for operation with a metal that is denser
than the electrolyte
and so are not suitable for the lithium production processes described herein.
[0019] While the Dow magnesium electrolyzer may be designed for both
consumable anodes
and metal with lower density than the electrolyte, it is generally impractical
to provide feeding
mechanisms that are capable of supplying each individual sub-compartment with
Li2CO3, feed
material while accommodating the anode mechanism, without unduly enlarging the
anode-to-
cathode distance and incurring the attendant resistive losses and heat balance
problems.
Additionally, an arrangement where each anode is in an independent compartment
and the
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
cathodes are in a common compartment leaves the electrolyzer vulnerable to
membrane failure,
as leakage in any single membrane contaminates all cathodes.
[0020] US3607684 discloses a membrane electrolyzer with a beta-alumina
diaphragm and a
solvent metal cathode. This process has drawbacks when used with Li2CO3,
including that the
proposed membranes are located on the bottom of the vessel in a "window pane"
arrangement
Such an arrangement would be difficult to execute without leaks, given the
substantial thermal
expansion of components between assembly and operating temperatures. Also, it
is known that
molten alloys of lithium are relatively aggressive towards alumina, meaning
that the membranes
would be attacked by the flowing metal and any likely gasket materials,
limiting the life of the
electrolyzer substantially.
[0021] Despite the advances made to date in the development molten salt
electrolysis devices,
there is significant room for improvement to address the above-mentioned
problems and
shortcomings of the prior art.
[0022] It may be an object of the present invention to obviate or mitigate at
least one of the above-
mentioned disadvantages of the prior art, and to provide a novel molten salt
electrolyzer
apparatus. For example, the teachings described herein may be related to an
improved process
and apparatus/equipment for the production of lithium metal from lithium
carbonate. To help
facilitate relatively large-scale production of lithium metal, the process and
equipment/apparatus
described herein may preferably have one or more of the following: i)
relatively high specific
productivity, ii) relatively high availability, iii) relatively high metal
recovery, iv) relatively high
lithium metal product purity, v) relatively low operating costs and vi) a
generally acceptable
specific energy use.
101001 In accordance with one broad aspect of the teachings described herein a
process for
producing lithium metal from lithium carbonate using an electrolyzer apparatus
having a
containment vessel defining an anolyte compartment containing a first anode
and a second anode
submerged in a common anolyte bath comprising chloride salts can include: a)
providing a first
cathode housing in the anolyte bath proximate the first anode, the first
cathode housing may
define a first catholyte compartment containing a first cathode and a molten
salt catholyte and
may be at least partially bounded by a first primary transfer portion disposed
between the first
cathode and first anode and comprising a first porous membrane configured to
permit migration
of lithium ions and resist migration of carbonate ions; b) providing a second
cathode housing in
6
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
the anolyte bath proximate the second anode, the second cathode housing may
define a second
catholyte compartment containing a second cathode and the molten salt
catholyte and may be at
least partially bounded by a second primary transfer portion disposed between
the second
cathode and second anode and comprising a second porous membrane configured to
permit
migration of lithium ions and resist migration of carbonate ions; c)
introducing a lithium carbonate
feed material into the anolyte bath; d) applying an electric overpotential
that is sufficient to initiate
electrolysis of lithium carbonate feed material and is greater than an
electric potential required to
initiate electrolysis of lithium chloride (e.g. is substantially greater than
the equilibrium potential of
lithium chloride) between the first anode and the first cathode and between
the second anode
and the second cathode, thereby electrolyzing the lithium carbonate feed
material; d) transferring
lithium ions from the anolyte bath into the first catholyte compartment
through the first primary
transfer portion and resisting the transfer of carbonate ions from the anolyte
bath into the first
catholyte compartment e) transferring lithium ions from the anolyte bath into
the second catholyte
compartment through the second primary transfer portion and resisting the
transfer of carbonate
ions from the anolyte bath into the second catholyte compartment and f)
converting the lithium
ions into lithium metal.
[0101] The process may include introducing chlorine gas into the first
catholyte compartment via
a chlorine delivery system, reacting the chlorine gas with the lithium
carbonate to form lithium
chloride (LiCI) and carbon dioxide, and electrolyzing the lithium chloride.
[0023] A carbonate ion concentration in the catholyte within the first
catholyte compartment may
be less than in the anolyte bath.
[0024] A carbonate ion concentration in first catholyte compartment may be
less than about
100ppm.
[0025] The process may include inhibiting carbon and/or lithium oxide fouling
of the first cathode
by introducing chlorine gas into the catholyte in the first cathode
compartment
[0026] The process may include maintaining a current density of between about
0.75A/cnn2 and
about 4A / crn2 between the first anode and first cathode and between the
second anode and
second cathode.
[0027] The current density may be at least about 1.2 A / cm2.
[0028] The process may include maintaining a concentration of lithium
carbonate of at least 0.1
mol% in the anolyte bath.
7
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0029] The process may include maintaining a concentration of lithium
carbonate of at least 0.5
mol% in the anolyte bath.
[0030] The process may include extracting anode gases generated proximate the
first anode via
an anode gas extraction apparatus and introducing additional lithium carbonate
feed material into
the anolyte bath when a concentration of chlorine gas in the anodes gases
exceeds a
predetermined monitoring threshold.
[0031] A quantity of carbon that is required per unit of lithium metal
produced may be less than
about 0.4kg C / kg Li.
[0032] The process may include maintaining at least one of the anolyte and the
catholyte at a
temperature that is greater than about 375 C and/or about 400 C.
[0033] The process may include maintaining the at least one of the anolyte and
the catholyte at
a temperature that is between about 450 C and about 650 C.
[0034] The anolyte and the catholyte may each include molten LiCI and KCI.
[0035] The electrolyzer apparatus may include a first cathode mounting
apparatus extending over
an open upper end of the containment vessel and supporting at least the first
cathode. The first
cathode mounting apparatus may be removable from the containment vessel and
the first cathode
may be removed with the first cathode mounting apparatus while the anolyte
bath remains
contained within the anolyte compartment.
[0036] The first cathode mounting apparatus may include a first feed port
through which lithium
carbonate is introduced into the anolyte bath. Removing the first cathode
mounting apparatus
may simultaneously remove the first cathode and the first feed port from the
containment vessel.
101001 In accordance with another broad aspect of the teachings described
herein, a molten salt,
membrane electrolyzer apparatus for the production of lithium metal from
lithium carbonate via
an electrolysis process may include a containment vessel defining an anolyte
compartment
containing a molten salt anolyte bath comprising chloride salts and a lithium
carbonate (Li2CO3)
feed material. A first electrode assembly may include a first anode extending
into the anolyte
compartment and in fluid contact with the molten salt anolyte bath and a first
cathode housing
proximate the first anode within the anolyte compartment and in fluid contact
with the molten salt
anolyte bath. The first cathode housing may define a first catholyte
compartment containing a
8
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
molten salt catholyte including chloride salts and being at least partially
bounded by a primary
transfer portion comprising a first porous membrane configured to permit
migration of lithium ions
and resist migration of carbonate ions from the anolyte compartment into the
first catholyte
compartment A first cathode may be provided within the first catholyte
compartment, in fluid
contact with the catholyte and positioned so that the primary transfer portion
is disposed between
the first anode and the first cathode. A second electrode assembly may include
a second anode
extending generally into the anolyte compartment and in fluid contact with the
molten salt anolyte
bath and a second cathode housing proximate the second anode within the
anolyte compartment
and in fluid contact with the molten salt anolyte bath. The second cathode
housing may include a
second catholyte compartment containing a molten salt catholyte including
chloride salts and
being at least partially bounded by a primary transfer portion comprising a
second porous
membrane configured to permit migration of lithium ions and resist migration
of carbonate ions
from the anolyte compartment into the second catholyte compartment. A second
cathode within
the second catholyte compartment may be in fluid contact with the catholyte
and may be
positioned so that the second primary transfer portion is disposed between the
second anode and
the second cathode. A power supply may be configured to apply an electric
potential between at
least the first anode and the first cathode that that is greater than the
electric potential required to
initiate electrolysis of the lithium carbonate feed material and is greater
than the electric potential
required to initiate electrolysis of lithium chloride.
[0037] The containment vessel may include an open upper end and the first
anode, first cathode,
second anode and second cathode may extend downwardly through the open upper
end into the
anolyte bath.
[0038] The electric potential between the first anode and the first cathode
may be at least 4V.
[0039] The electric potential between the first anode and the first cathode
may be at least 7V and
may be about 10V.
[0040] The electric potential between the first anode and the first cathode
may be at least 10V.
[0041] The first electrode assembly may operate at current density of between
about 1 A / cm2
and about 4A / cm2.
[0042] The first electrode assembly may operate at current density of about
1.2 A / cm2.
9
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0043] The first anode may include a generally planar plate having a first
anode active surface
facing the first cathode, and the first cathode may include a generally planar
plate that is
substantially parallel to the first anode and having a first cathode active
surface opposite and
facing the anode active surface.
[0044] The first cathode active surface may be between about 50% and about
200% of the first
anode active surface, and preferably may be between about 80% and about 120%
of the anode
active surface, and more preferably may be substantially the same as the anode
active surface.
[0045] The second electrode assembly may be adjacent the first electrode
assembly such that
the first cathode is disposed between and is generally equally spaced between
the first anode
and the second anode. An electric potential that is sufficient to initiate
electrolysis of lithium
carbonate and is greater than the electric potential required to initiate LiCI
electrolysis may be
applied between the first cathode and the second anode.
[0046] The first cathode housing may include a secondary transfer portion
disposed between the
first cathode and the second anode and may include a porous membrane to permit
migration of
lithium ions from the anolyte compartment into the first catholyte compartment
and resisting the
migration of carbonate ions from the anolyte compartment into the first
catholyte compartment.
[0047] The first cathode housing may be formed at least substantially entirely
from the porous
membrane.
[0048] At least some regions of the first cathode housing outside the primary
transfer portion and
the secondary transfer portion may be treated to inhibit the transmission of
ions through the
regions of the first cathode housing outside the primary transfer portion and
the secondary
transfer portion.
[0049] The least some regions of the first cathode housing outside the primary
transfer portion
and the secondary transfer portion may be coated or impregnated with an ion
blocking material.
[0050] The first cathode housing may be formed at least substantially entirely
from the porous
membrane.
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0051] At least some regions of the first cathode housing outside the primary
transfer portion may
be treated to inhibit the transmission of ions through the regions of the
first cathode housing
outside the primary transfer portion.
[0052] The at least some regions of the first cathode housing outside the
primary transfer portion
may be coated or impregnated with an ion blocking material.
[0053] The porous membrane may be formed from a ceramic material and having an
average
pore size of between about 0_1 and about 100 microns.
[0054] A concentration of carbonate ions within the first catholyte
compartment may be less than
about 100ppnn while the apparatus is in use.
[0055] A concentration of carbonate ions within the first catholyte
compartment may be less than
a concentration of carbonate ions within the anolyte compartment
[0056] The first anode may be removable from the anolyte compartment
independently of the first
cathode housing and the first cathode.
[0057] The first anode may be removable from the anolyte compartment
independently of the
second anode.
[0058] The first anode may be removable from the anolyte compartment without
draining the
molten salt anolyte bath from the anolyte compartment
[0059] A chlorine delivery system may be configured to introduce chlorine gas
into the first
catholyte compartment while the apparatus is in use.
[0060] The chlorine gas may react with Li2CO3 present within the first
catholyte compartment to
produce Lid and carbon dioxide, thereby inhibiting carbon and/or lithium oxide
fouling of the first
cathode.
[0061] The chlorine gas may react with excess lithium within the first
catholyte compartment
thereby inhibiting damage to the membrane.
[0062] A gas extraction apparatus may be configured to capture product gases
formed adjacent
the first anode and convey the product gases away from the containment vessel.
11
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0063] The anolyte bath may be at a temperature of between about 450 C and
about 700 C
degrees Celsius.
[0064] The anolyte bath may be at a temperature of between about 475 C and
about 650 C.
[0065] The first electrode assembly may include an anode mounting apparatus
extending over
the upper end of the containment vessel in a first direction and from which
the first anode is
suspended. The anode mounting apparatus may be detachable from the containment
vessel
whereby the first anode is removed from the anolyte compartment.
[0066] The first anode may be detachably connected to the anode mounting
apparatus.
[0067] The anode mounting apparatus may include an electrical connector that
electrically
connects the first anode to the power supply when the anode mounting apparatus
is attached to
the containment vessel. The electrical connection between the first anode and
the power supply
may be interrupted when the anode mounting apparatus is detached from the
containment vessel.
[0068] The anode mounting apparatus may include an insulating layer disposed
between the
anolyte chamber and the electrical connector to inhibit heat transfer from the
molten salt anolyte
bath to the electrical connector when the apparatus is in use. The insulating
lining may be
removable with the anode mounting apparatus.
[0069] The anode mounting apparatus may include a gas extraction apparatus
having a gas
capture hood positioned above the first anode and configured to capture
product gases formed
adjacent the first anode and bubbling out of the molten salt anolyte bath and
a gas removal conduit
extending from the gas capture hood and configured to convey the product gases
away from the
containment vessel. At least a portion of the gas extraction apparatus may be
removable with the
anode mounting apparatus.
[0070] The gas capture hood may be electrically isolated from the first anode.
[0071] The first electrode assembly may include a cathode mounting apparatus
extending over
the upper end of the containment vessel in the first direction and from which
the first cathode is
suspended. The cathode mounting apparatus may be detachable from the
containment vessel
whereby the first cathode is removed from the containment vessel with the
cathode mounting
apparatus.
12
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0072] The first cathode housing may be suspended from the cathode mounting
apparatus
whereby the first cathode housing is removed from the containment vessel with
the cathode
mounting apparatus.
[0073] The cathode mounting apparatus may include a lithium extraction
assembly that includes
a lithium extraction conduit that extends from an upper end proximate the
cathode mounting
apparatus to a lower end that disposed within the first catholyte compartment
to extract lithium
metal that collects in the catholyte. The lithium extraction conduit may be
removed from the
containment vessel with the cathode mounting apparatus.
[0074] The cathode mounting apparatus may include an electrical connector that
electrically
connects the first cathode to the power supply when the cathode mounting
apparatus is attached
to the containment vessel. The electrical connection between the first cathode
and the power
supply may be interrupted when the cathode mounting apparatus is detached from
the
containment vessel.
[0075] At least one feed port may be provided in the cathode mounting
apparatus through which
the feed material can be introduced into the anolyte compartment.
[0076] The at least one feed port may be removable from the containment vessel
with the cathode
mounting apparatus.
[0077] A plurality of cathode mounting apparatuses and anode mounting
apparatuses may be in
an alternating arrangement Adjacent ones of the cathode mounting apparatuses
and anode
mounting apparatuses may cooperate to cover substantially the entire upper end
of the
containment vessel.
[0078] A filling tube may fluidly conned the anolyte compartment and the first
catholyte
compartment whereby anolyte from the anolyte bath can be drawn into the first
catholyte
compartment when a vacuum is applied to the first catholyte compartment.
[0079] Li2CO3 within the anolyte bath may react with Cl2 produced within the
anolyte
compartment thereby converting it to LiCI, CO2 and 02 and supressing the
emission of Cl2 from
the containment vessel.
[0080] A quantity of carbon required per unit of lithium metal produced may be
less than about
0.4kg C / kg Li.
13
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0081] A concentration of dissolved lithium carbonate in the anolyte within
the anolyte
compartment may be greater than 0.1 mol%.
[0082] The concentration of dissolved lithium carbonate concentration of
dissolved lithium
carbonate in the anolyte within the anolyte compartment may be greater than
0.5 mol% and may
be about 1 mol% or greater.
[0083] A CO2/02 ratio in an off-gas from the anolyte compartment may be
between about 2 and
about 2.5.
[0084] The first porous membrane may have a maximum pore size of about 1
micron and average
pore size less than about 0.5 microns.
[0085] The first porous membrane may have an open porosity of between 10-80%,
and more
preferably between 30-60%.
[0086] The first porous membrane may be substantially rigid.
[0087] The apparatus may include a third electrode assembly including a third
anode extending
into the anolyte compartment and in fluid contact with the molten salt anolyte
bath and a third
cathode housing proximate the third anode within the anolyte compartment and
in fluid contact
with the molten salt anolyte bath. The third cathode housing may define a
third catholyte
compartment containing a molten salt catholyte comprising chloride salts and
being at least
partially bounded by a primary transfer portion comprising a third porous
membrane configured
to permit migration of lithium ions and resist migration of carbonate ions
from the anolyte
compartment into the third catholyte compartment. A third cathode may be
provided within the
third catholyte compartment, in fluid contact with the catholyte and
positioned so that the primary
transfer portion is disposed between the third anode and the third cathode.
[0088] The apparatus may include a fourth electrode assembly including a
fourth anode
extending into the anolyte compartment and in fluid contact with the molten
salt anolyte bath and
a fourth cathode housing proximate the fourth anode within the anolyte
compartment and in fluid
contact with the molten salt anolyte bath. The fourth cathode housing may
define a fourth
catholyte compartment containing a molten salt catholyte comprising chloride
salts and being at
least partially bounded by a primary transfer portion comprising a fourth
porous membrane
configured to permit migration of lithium ions and resist migration of
carbonate ions from the
anolyte compartment into the fourth catholyte compartment. A fourth cathode
may be provided
14
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
within the fourth catholyte compartment, in fluid contact with the catholyte
and positioned so that
the primary transfer portion is disposed between the fourth anode and the
fourth cathode.
[0089] In accordance with yet another broad aspect of the teachings described
herein, a molten
salt, membrane electrolyzer apparatus for the production of lithium metal from
lithium carbonate
via an electrolysis process may include a containment vessel defining an
anolyte compartment
containing a molten salt anolyte bath, the anolyte bath comprising chloride
salts and more than
about 0.1 mol% lithium carbonate (Li2CO3) feed material, and a plurality of
electrode assemblies
spaced apart from each other and extending into the anolyte compartment. Each
electrode
assembly may include: a cathode housing in fluid contact with the molten salt
anolyte bath. The
cathode housing may define fining a catholyte compartment containing a molten
salt catholyte
including chloride salts and being at least partially bounded by a primary
transfer portion including
a porous membrane configured to permit migration of lithium ions and resist
migration of
carbonate ions from the anolyte compartment into the catholyte compartment A
cathode may be
positioned within the catholyte compartment, in fluid contact with the
catholyte and may have an
active surface. An anode may be in contact with the molten salt anolyte bath
and may be
proximate the cathode housing. The anode may have an active surface that is
substantially
equidistant from the active surface of the cathode and may be positioned so
that the primary
transfer portion of the membrane is disposed between the active surface of the
anode and the
active surface of the cathode. A power supply may be configured to apply an
electric potential to
each electrode assembly that is greater than the electric potential required
to initiate electrolysis
of the lithium carbonate feed material.
[0090] The plurality of electrode assemblies may include at least ten
electrode assemblies
arranged in an array within the anolyte compartment
[0091] The anode may include a substantially planar plate and the cathode may
include a
substantially planar plate that is parallel to the anode.
[0092] The primary transfer portion of porous membrane may be substantially
planar and may be
parallel to both the anode and the cathode.
[0093] At least a first and a second anode support apparatus may extend across
an open upper
end of the containment vessel and over the anolyte compartment. The first
anode support
apparatus may support at least a first anode and the second anode support
apparatus may
support at least a second anode.
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[0094] The first anode support apparatus and the first anode supported thereon
may be
removable from the containment vessel independently from the second anode
support apparatus.
[0095] The first anode support apparatus and the first anode supported thereon
may be
removable while the anolyte bath is contained within the anolyte compartment.
[0096] The first anode support apparatus may include an electrical connector
that electrically
connects the first anode to the power supply and wherein the electrical
connection is interrupted
when the first anode support apparatus is removed.
[0097] At least a first cathode support apparatus may be disposed between the
first and second
anode support apparatuses, and a second cathode support apparatus may be on an
opposing
side of the second anode support Each cathode support apparatus may extend
across the open
upper end of the containment vessel and over the anolyte compartment The first
cathode support
apparatus may support at least a first cathode proximate the first anode and
the second cathode
support apparatus may support at least a second cathode proximate the second
anode.
[0098] The first cathode support apparatus and first cathode supported thereon
may be
removable from the containment vessel independently from the first anode
support apparatus.
[0099] The first cathode support apparatus and first cathode supported thereon
may be
removable from the containment vessel while the anolyte bath is contained
within the anolyte
compartment.
[00100] The first cathode support apparatus may include
an electrical connector that
electrically connects the first cathode to the power supply and wherein the
electrical connection
may be interrupted when the first cathode support apparatus is removed.
[00101] A first cathode housing may surround the first
cathode and may be suspended
from the first cathode mounting apparatus whereby the first cathode housing
may be removed
from the containment vessel with the first cathode mounting apparatus.
[00102] The porous membrane may include a non-wetting
ceramic material.
[00103] The non-wetting ceramic material may have an
open porosity of between about
30% and about 60%.
16
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[00104] A ratio of an area of the active surface of the
anode to an area of the active surface
of the cathode may be between about 0.5 and 2, and more preferably is between
0.8 to 1.2.
[00105] A chlorine delivery system may be configured to
introduce chlorine gas into each
catholyte compartment.
[00106] The chlorine gas may react with lithium
carbonate present within the catholyte to
product carbon dioxide and lithium chloride.
[00107] The porous membrane may have a maximum pore size
of about 1 micron and
average pore size less than about 0.5 microns.
[00108] The electric potential between the anode and the
cathode may be at least 4V.
[00109] The electric potential between the anode and the
cathode may be at least 7V and
may be about 10V.
[00110] The electric potential between the anode and the
cathode may be at least 10V.
[00111] Each electrode assembly may operate at a current
density of between about 1 A /
cm2 and about 4A / cm2.
[00112] Each electrode assembly may operate at a current
density of about 1.2 A / cm2.
[00113] The power supply may be configured to apply an
electric potential to each
electrode assembly that is greater than the electric potential required to
initiate electrolysis of
lithium chloride.
[00114] The anolyte bath may be at a temperature of
between about 450 C and about
700 C degrees Celsius.
[00115] Thus, the present inventors have developed a
molten salt electrolyzer apparatus
for the production of alkali metals from suitable feed materials, and in
particular may be useful for
the production of lithium metal from lithium carbonate. The present
electrolyzer apparatus can
help facilitate the relatively large-scale economical production of lithium
metal using Li2CO3 as a
feed source. The use of its repeating, multi-unit arrangement within a single
vessel of the
electrolyzer apparatus described herein may help reduce the number of cells
that need to be built
in order to achieve large-scale production of metal, thereby reducing both the
capital cost and
17
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
maintenance labour burden, as compared to previously reported electrolyzers.
The interleaving
arrangement described herein may also help reduce (and optionally help
minimize) the anode-to-
cathode distance (ACD), which may help reduce the resistive heat losses
incurred by the process,
which may in turn help reduce the operating cost of the plant. Additionally,
the electrolyzer
apparatus described herein may be operated at relatively high current density,
which may help
contribute to achieving a relatively higher specific productivity (for
example, as compared to
previously reported electrolyzers). Furthermore, the top-mounted, self-
contained arrangement of
the anodes and cathodes can help facilitate the relatively simple removal and
frequent
exchange/repair of the electrodes (for example without having to drain the
anolyte bath), thereby
facilitating uninterrupted operation at high throughput. This again can help
reduce the operating
costs of an electrolyzer apparatus.
[00116] To the knowledge of the inventors, a molten salt
electrolyzer apparatus with such
a combination of features is heretofore unknown. Other advantages of the
invention may become
apparent to those of skill in the art upon reviewing the present
specification.
BRIEF DESCRIPTION OF THE DRAWINGS
100117] Embodiments of the present disclosure will be
described with reference to the
accompanying drawings, wherein like reference numerals denote like parts, and
in which:
Figure la is a schematic representation of one example of an electrolyzer;
Figure lb is a schematic representation of one example of an electrode
assembly;
Figure 2 is a schematic representation of another example of an electrolyzer;
Figure 3 is a plan view of another example of an electrolyzer;
Figure 4 is a side elevation view of the electrolyzer of Figure 3;
Figure 5a is a plan section of the electrolyzer of Figure 4, taken along line
A-A;
Figure 5b is an enlarged plan view of an anode mounting apparatus in Figure
5a, through
which line B-B passes;
Figure Sc is an enlarged plan view of a cathode mounting apparatus in Figure
5a, through
which line C-C passes;
18
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
Figure 6 is a split vertical transverse section of the electrolyzer of Figure
5a, taken along
line B-B, illustrating the end-on view of the anode on the left side of the
split, and a section through
the anode on the right side of the split;
Figure 7 is a split vertical transverse section of the electrolyzer of Figure
5a, taken along
line C-C, illustrating the end-on view of the cathode on the left side of the
split, and a section
through the cathode on the right side of the split;
Figure 8a is a split vertical transverse section of a portion of the
electrolyzer of Figure 5b,
taken along line B-B;
Figure 8b is a longitudinal section of the anode beam of Figure 8a, taken
along line 8b-
8b;
Figure 9a is a split vertical transverse section of a portion of the
electrolyzer of Figure 5a,
taken along line C-C;
Figure 9b is a longitudinal section of the cathode beam of Figure 9a, taken
along line 9b-
9b;
Figure 10 is a section of the electrolyzer of Figure 3 through four anode and
cathode
assemblies;
Figure lla is a split vertical transverse section of another example of an
electrolyzer;
Figure llb is a longitudinal section of the anode beam of Figure ha, taken
along line 11 b-
lib; and
Figure 12 is a plot of current density, voltage and gas analysis vs time for
the operation of
one example of an electrolyzer.
DETAILED DESCRIPTION
[00118] Various apparatuses or processes will be
described below to provide an example
of an embodiment of each claimed invention. No embodiment described below
limits any claimed
invention and any claimed invention may cover processes or apparatuses that
differ from those
described below. The claimed inventions are not limited to apparatuses or
processes having all
of the features of any one apparatus or process described below or to features
common to multiple
19
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
or all of the apparatuses described below. It is possible that an apparatus or
process described
below is not an embodiment of any claimed invention. Any invention disclosed
in an apparatus or
process described below that is not claimed in this document may be the
subject matter of another
protective instrument, for example, a continuing patent application, and the
applicants, inventors,
or owners do not intend to abandon, disclaim, or dedicate to the public any
such invention by its
disclosure in this document
[00119] Molten salt electrolysis and electrolyzers can
be used in the production of metals
from oxide, nitrate, sulfate, or carbonate compounds. Novel electrolyzer
apparatuses described
herein include a containment vessel that is configured to contain a molten
salt anolyte (and
function as an anolyte chamber) and to have at least one electrode assembly
and preferably
having at least two electrode assemblies (each having an anode and a
complimentary cathode)
positioned within the containment vessel. Optionally, a single containment
vessel (preferably with
a single anolyte bath) may have 2 or more electrode assemblies (electrode
pairs), and may have
at least 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 ,15 or more electrode
assemblies. In some preferred
embodiments the containment vessel may include at least 10 electrode
assemblies.
[00120] The electrolyzer apparatuses described herein
are preferably configured such that
the anodes are each directly submerged within the common, molten salt anolyte
bath while each
cathode is surrounded by a suitable cathode housing to provide a respective,
discrete catholyte
compartment proximate each cathode. This can provide an apparatus that
includes a common
anolyte compartment in combination with a plurality of discrete catholyte
compartments.
Configuring the apparatus in this manner may help enhance the resilience of
the apparatus and/or
help reduce downtime and/or help facilitate maintenance and repair of the
apparatus. For
example, in this arrangement a failure or fouling of any one membrane or
cathode housing by, for
example, cracking or prolonged lithium metal attack, will only tend to affect
the production from
one electrode pair. This may help provide redundancy for the electrolyzer
apparatus, as it may
continue to be successfully operated even with several compromised membranes
in some
examples. In addition, in the event of failure, or the accumulation of
electrolyzed carbon or other
insoluble build-up, a given membrane can be removed, repaired and/or replaced
without
substantially affecting the operation of the remaining electrodes.
[00121] Each cathode housing can be provided in any
suitable structure, and may be
formed from any suitable material, that can help fluidly isolate the catholyte
from the anolyte while
still allowing a desired level of ion transfer between the anolyte compartment
and catholyte
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
compartments while the apparatus is in use to achieve the desired reactions
and metal formation
(e.g. the housings can be substantially leak-tight). Optionally, at least a
portion of the cathode
housing may be provided by a suitable membrane material, such as a
substantially rigid and
porous ceramic membrane that can maintain separation between the anolyte and
catholyte while
allowing a desired degree of ion transfer. Optionally, the entire cathode
housing or at least
substantially the entire cathode housing may be formed from the porous ceramic
membrane, and
the membrane may be generally continuous such that is covers the front, rear,
side and bottom
faces of the cathode.
[00122] In the described embodiments the portions of
the anodes and cathodes that are
facing each other, and between which at least a majority of the electrolysis
reactions are facilitated
can be described as the respective active surfaces (e.g. the portions between
which the electric
potential is applied). Preferably, the active surfaces of the co-operating
anode and cathode pairs
can be substantially the same shape and size and can have substantially the
same area and
general geometric configuration. For example, the active surfaces of the
anodes and cathodes
can be selected so that a ratio of the areas anode active surface to an area
of the cathode active
surface is between about 0.5 and 2, and more preferably is between 0.8 to 1.2.
In other words,
the active surface of each cathode may be between about 50% and 200% of the
active surface
of the corresponding anode and is preferably between about 80% and 120%. More
preferably,
the active surfaces of the cathodes and anodes are substantially the same
size.
[00123] Optionally, the cathode housing can also define
a respective effective transfer
portion that can be understood to be the portion of the housing that is
disposed between the active
surfaces or portions of a complimentary anode and cathode pair. The transfer
portion of the
housing can preferably include the porous membrane and can be sized so as to
be generally the
same size and shape as the transfer portions of the electrodes. For example,
the transfer portion
of the cathode housing may be sized such that the ratio of the housing
transfer portion area and
the area of the larger of the electrode transfer areas is between 0.5 and 2,
and more preferably,
such that it is between 0.8 to 1.2 relative to the largest electrode.
Maintaining a housing active
transfer area (e.g. where the membrane is located) ratio in the range of about
0.8-1.2 may help
reduce the area open to carbonate ion transport, in contrast to the cells
depicted in the prior art.
That is, the size of the transfer portion of the porous membrane is preferably
between 50% and
200% relative to the active surface of the larger of the associated electrodes
(i.e., relative to the
larger of the anode and cathode in an electrode pairing), and more preferably,
is between 80% to
120% of that active surface_
21
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
100124] Diffusion transport can be generally a function
of active membrane area and may
take place anywhere where a concentration gradient exists. By contrast, the
desired ionic
conduction in the described processes is largely limited to the area
interposed between the
cooperating anode and cathode ¨ i.e. in the region between the transfer
portions. Preferably, the
active membrane area can be selected to generally match the desired transfer
portion
configuration and other portions of the cathode housing and/or membrane can be
treated or
configured to help limited ionic conduction in the non-transfer portion. This
may help limit carbon
and Li2O accumulation resulting from transport of carbonate ions across other
portions of the
membrane and into the cathode compartment, which may be undesirable because it
may reduce
current efficiency and/or may cause elemental carbon sludge or oxide crust
build-up in the
catholyte. The apparatuses described herein may help reduce the negative
effects of this
phenomenon by closely matching the electrode and membrane area ratios as
described, and/or
by operating with relatively low-concentrations of dissolved Li2CO3 in the
electrolyte bath (as
discussed herein).
[00125] This may help reduce the area available for
diffusion, and the driving force for the
transport of carbonate ions across the porous membranes. That is, by limiting
the active area to
the ratios described herein, the ionic conduction may not be adversely
materially affected, while
carbonate ion diffusion transport from the membrane or other housing surfaces
that are not
substantially involved in ionic conduction may be reduced and may optionally
be minimized or
eliminated. This may help reduce the electrolytic production of carbon per
unit of lithium produced,
and thereby may help reduce the formation of the potentially harmful sludge,
and reaction with
the lithium metal to form insoluble lithium oxide. Reducing these unwanted
reactions, may help
reduce the operating cost of the described process and apparatus and may help
extend the useful
life of the membrane compartment, which may contribute to increased apparatus
availability.
[00126] While for simplicity the apparatuses described
herein are shown having their
anodes submerged in a common anolyte bath and the cathodes enclosed by
respective
membranes to provide discrete cathode compartments other embodiments of the
electrolyzer
apparatus may have the opposite configuration. That is, other examples of the
electrolyzer
apparatus may be configured so that the two or more cathodes of the electrode
assemblies are
directly submerged in a common catholyte compartment and each anode may be
enclosed within
a suitable anode housing (such as a ceramic membrane) to provide a plurality
of discrete anolyte
compartments. This may be somewhat less preferable than the primary
embodiments described
herein, (for example it may require introducing feed material separately into
each of the anolyte
22
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
compartments and monitoring their individual parameters rather than being able
to feed and
monitor a common, larger anolyte bath) but could be utilized in some
circumstances. That is,
understood that the discussion of the configuration and operation of the
cathodes and membranes
is also applicable to embodiments in which the anodes are enclosed by the
membrane&
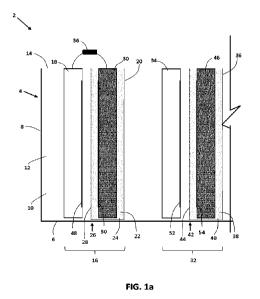
[00127] Referring to Figure 1a, a schematic
representation of features of one example of
a molten salt membrane electrolyzer apparatus 2 is shown. The schematic
drawings illustrate
aspects of some features of the apparatus, and other elements of the apparatus
(such as
conduits, pumps, controllers and the like) have been omitted from this drawing
for
clarity/simplicity. Such components may be provided in some embodiments of the
electrolyser
apparatuses described herein_
[00128] Optionally, the electrolyzer apparatus 2 can be
used for the production of lithium
metal from lithium carbonate via an electrolysis process operating in a
suppressed chlorine
regime (described herein) and in a manner that may consume relatively less
carbon per unit
lithium produced than known apparatuses and processes.
[00129] The electrolyzer apparatus 2 in this example
has a containment vessel 4 that is
configured to define/ provide a compartment for containing liquids, such as a
molten salt, while
the apparatus 2 is in use. The containment vessel 4 may have any suitable
shape and size,
based on the requirements for a given electrolyzer apparatus 2, and may have
any suitable
physical structure/arrangement that can provide a desired level of liquid
containment. For
example ,the containment vessel may be generally rectangular or square in
cross-sectional shape
(i.e. when viewed from above), or may have rounded corners, be hexagonal,
octagonal, cylindrical
or the like. It may include a generally liquid impermeable bottom and side
wall(s) and can be
configured so that corresponding electrode assemblies can be submerged within
the container
liquid/ molten salt.
[00130] The top of the electrolyzer apparatus 2 may be
open (e.g. have an open top or
upper end) as shown, or may at least be openable to help facilitate the
positioning and optionally
installation/extraction of the electrode assemblies, but may also tend to be
generally covered
and/or enclosed when the electrolyzer apparatus 2 is in use to help prevent
the contents of the
electrolyzer apparatus 2 from escaping and/or to help prevent unwanted foreign
debris/objects
from entering the electrolyzer apparatus 2. The electrolyzer apparatus 2 may
also be sized to
23
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
have an internal volume that can accommodate a desired amount of liquid, which
in the case of
the electrolyzers disclosed herein may be a molten salt, or the like.
[00131] In the example illustrated in Figure 1a, the
containment vessel 4 has a generally
rectangular cross-sectional shape and has a bottom wall 6 with an opposing
upper end 14 and at
least one side wall 8 that extends upwardly from the bottom wall 6 toward the
upper end 14.
Together, the bottom wall 6 and side wall 8 co-operate to generally provide
the boundaries of an
anolyte compartment 10 of the electrolyzer apparatus 2. In some embodiments,
the containment
vessel may have two, three, four, or more side walls.
[00132] In the illustrated example, the containment
vessel 4 has an open upper end 14. In
other embodiments, the upper end 14 may be partially or optionally fully
enclosed with a fixed
cover and/or may be reversibly or openably enclosed with a removable cover or
roof. An upper
wall is not shown in this schematic representation but may be included and
positioned to help
enclose the anolyte compartment 10 when the electrolyzer apparatus 2 is in
use.
[00133] Optionally, the containment vessel 4 may be
lined with a liner along at least a
portion of its interior surface to help contain a molten salt and/or to help
protect the bottom and
side walls. The liner can be comprised of any suitable material that can
withstand the expected
operating conditions, including refractory bricks, castable cements, frozen
salt or metal.
[00134] The containment vessel 4 may also optionally
indude a thermal insulating layer
that surrounds at least a portion of the containment vessel 4 and can help
reduce the transfer of
heat or thermal energy between the anolyte compartment 10 and the surrounding
environment
This may help reduce the amount of energy needed to keep the anolyte
compartment at a desired
operating temperature.
[00135] In this embodiment, the walls 6, 8 of the
containment vessel 4 co-operate to at
least partially define an anolyte compartment 10, that preferably has a
generally open upper end
that can be configured to receive the top-mounted (or at least top positioned,
generally
downwardly extending) electrode assemblies described herein. This can help
facilitate the
relatively easier installation and removal of the electrode assemblies (or at
least portions thereof)
into the anolyte compartment 10, as compared to arrangements in which the
electrodes extend
through the sidewalls or bottom wall of the containment vessel. For example,
this may allow the
anodes and/or cathodes to be removable from the anolyte compartment without
having to drain
the molten salt anolyte from the anolyte compartment
24
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[00136] As used herein, references to an open upper end
are intended to describe
structures which that have an open region toward their upper end through which
an electrode
assembly may be inserted in the anolyte compartment in a generally vertical
manner, preferably
without having to pass through the sidewall or bottom wall ¨ and preferably
from a mounting
position that is not submerged within the anolyte while the apparatus is in
use. This can help
reduce the need to provide seals in the sidewall and bottom wall of the
containment vessel, which
may help reduce the chances of leakage and/or wear of the seal components.
This does not
however require that the upper end of the containment vessel 4 remain entirely
"open" or
unobstructed while the apparatus is in use. In fact, it may be preferable to
generally cover and/or
enclose the upper end of the electrolyzer apparatuses when they are in use
(i.e. with the electrode
assemblies already in place) for a variety of reasons (including, for example,
operating efficiency
and safety).
[00137] To help secure and mount the electrodes in their
desired locations the apparatus
2 may include any suitable mounting apparatus, such as an anode mounting
apparatus, a cathode
mounting apparatus and the like. The mounting apparatuses may be the same or
different for the
anodes and cathodes, and optionally a single structural member ¨ such as a
beam, bracket or
the like ¨ may support at least one cathode and at least one anode and may
therefore function
as both an anode mounting apparatus and a cathode mounting apparatus.
[00138] The containment vessel 4 is designed to support
the anolyte 12, any liner lining
the containment vessel 4, and any other component that may be contained within
or on or secured
to the containment vessel 4 and may supply sufficient binding forces to help
ensure leak-tightness
of any lining and withstand the service temperatures without undue
deformation. The containment
vessel 4 may be formed from any suitable structural material, including but
not limited to, steel,
stainless steel, aluminum, or concrete. Optionally, the bottom wall 6 and side
walls 8 of the
containment vessel 4 may be reinforced with ribs or stiffeners to help provide
the desired structural
characteristics.
[00139] The anolyte compartment 10 is configured to
contain a molten salt anolyte 12, that
can include any suitable material for a given use of the electrolyzer
apparatus 2. For example,
the nature of the anolyte selected may vary depending on the type of feed
material to be used
and the corresponding type of metal that is to be produced. Some examples of
suitable anolyte
materials may include primarily chloride salts, such as lithium and/or
potassium chlorides,
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
bromide salts, such as lithium and potassium bromide, and iodide salts, such
as lithium and
potassium iodide.
[00140] If the electrolyzer apparatus 2 is to be
configured to produce lithium metal, then
one example of a suitable anolyte material is a salt that includes LiCI-KCI,
preferably in a molar
concentration ratio of between about 65:35 to 95:5 or to about 100:0, and that
can be maintained
at a temperature of between about 370-660 C (which may be preferably at least
about 10-100 C
higher than the melting point of the anolyte/ electrolyte in some examples).
In the illustrated
example, the anolyte 12 comprises LiCI-KCI and the electrolyzer apparatus 2 is
configured to
utilize lithium carbonate (Li2CO3) as its feed material. Optionally, the feed
material can be
provided as needed while the apparatus is in use to maintain a desired
composition, such as of
between about 0.1-10% Li2CO3M, within the anolyte. Within this concentration
and temperature
range, the solubility of lithium carbonate within the anolyte may be
sufficient to help scrub chlorine
gas from the electrolyte when it is formed at the anode (as described herein),
but not so high as
to encourage unduly fast diffusion of carbonate ions into the cathode
compartment at saturation.
[00141] When the electrolyzer apparatus 2 is in use,
the anolyte material is heated and can
be maintained at any suitable operating temperature that is sufficient to keep
the anolyte in a
sufficiently liquid state, while not being so hot to promote boiling or
excessive evaporation of the
electrolyte components, using any suitable heating apparatus. This can help
facilitate the desired
electrolysis reactions by helping to ensure that the anolyte, and feed
material entrained therein,
can circulate within the common anolyte bath within the apparatus 2 and
between respective pairs
of the anodes and cathodes. The specific operating temperature may vary based
on the anolyte
material, feed material and other factors. Preferably, when the electrolyzer
apparatuses described
herein are in use, the anolyte 12 may be maintained at an operating
temperature that is between
about 375 C and about 750 C degrees Celsius, between about 400 C and about 725
C, and
preferably may be between about 450 C and about 700 C.
[00142] As illustrated in Figure 1a, the electrolyzer
apparatus 2 has a first electrode
assembly 16, which comprises a first anode 18, a first cathode housing 20, and
a first cathode
30, and a second electrode assembly 32, which comprises a second anode 34, a
second cathode
housing 36, and a second cathode 46. In some embodiments, the electrolyzer
apparatus
comprises three, four, five, six, seven, eight, nine, ten or more electrode
assemblies optionally
arranged in an array, grid or the like (see Figures 3-10), while still being
in fluid communication
26
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
with the common anolyte bath, but only two electrode assemblies are shown in
Figure la for
clarity.
[00143] In the illustrated example the first electrode
assembly 16 and second electrode
assembly are generally identical, but in other embodiments may be different
Descriptions of the
operation of the first and second electrode assemblies 16 and 32 can be
applicable to any
electrode assemblies that may be provided in a given example of the
electrolyser apparatus 2. In
the illustrated example, the first anode 18 and second anode 34 both extend
generally
downwardly through the open upper end 14 of the containment vessel 4 into the
common anolyte
compartment 10. In such an arrangement, the first anode 18 and second anode 34
are preferably
in fluid contact with the molten anolyte 12 contained in the common anolyte
compartment 10.
[00144] To help define the multiple catholyte
compartments within the common anolyte
compartment 10, each cathode 30 and 46 is provided within a respective cathode
housing 20 and
36. The cathode housings 20 and 36 are arranged to generally surround their
respective cathodes
30 and 46 in a generally liquid-tight manner, and positioned so that the outer
surfaces of the
cathode housings 20 and 36 are also in fluid contact with the molten anolyte
14 in the common
anolyte compartment 10.
[00145] In this arrangement the interior of each
cathode housing 20 and 36 defines its
respective first and second catholyte compartments 22 and 38, which contain
respective molten
salt catholyte material (identified using characters 24 and 40 respectively).
While liquid
separation between the anolyte and catholyte is preferably maintained while
the apparatus 2 is in
use, the anolyte and catholyte may optionally comprise the same or at least
substantially the
same materials. In this example the catholyte material also includes molten
LiCI-KCI, in
substantially the same ratios as the anolyte 14. Other catholyte materials may
also be used.
[00146] In Figure la, the anodes 18 and 34 are shown in
a side view and are understood
to be generally planar members that extend into the page as shown. Another
schematic
representation of the first electrode assembly 16 is shown in Figure lb in
which the anode 18 and
30 are illustrated as generally planar sheets, with the cathode housing 20
shown as a generally
box-like structure formed from the suitable porous membrane material, the
interior of which forms
the first catholyte compartment 22. The locations of the anode 18, housing 20
and cathode 30
are exaggerated in Figure lb for illustrative purposes, and each of the pieces
is intended to be
positioned within the common anolyte bath. The front, active surface of the
anode 18 faces the
27
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
opposing face of corresponding cathode 30 and forms the anode active surface
48. The opposing
active surface of the cathode 30 forms its reciprocal active surface 50.
Preferably, the active
surface of the anode 18 is configured and positioned to be substantially
equidistant from the active
surface of the cathode 30. If the electrodes 18 and 30 are substantially
planar plates, this can be
achieved by arranging the abode 18 so that it is parallel or at least
substantially parallel to the
cathode 30. Alternatively, the electrodes need not be parallel with each other
to provide
substantially equally spaced active surfaces. For example, the electrode
surfaces may be
concentric circular or cylindrical surfaces or other complimentary-shaped
arcuate surfaces that
are equally spaced from each other along their length/width.
[00147] Likewise, the second anode effective transfer
area 52 is opposite and facing the
second cathode effective transfer area 54. The portions of the membranes 28
and 44 that are
disposed laterally between the opposing active surfaces 48 and 50, and 52 and
54 define the
transfer portions 26 and 42 of the respective membranes 28 and 44.
[00148] The transfer portions 26 and 42 of the first
and second porous membranes 28, 44
are preferably configured to permit the migration of lithium ions from the
anolyte compartment 10
into the first and second catholyte compartments 22, 38, respectively, while
resisting the migration
of carbonate ions from the anolyte compartment 10. Restricting the transport
of carbonate ions
limits the rate of formation of undesirable cathode products, namely carbon
and lithium oxide,
which would otherwise reduce current efficiency through unwanted reaction 3
and may limit the
life of the catholyte by contaminating it with carbon, and potentially reduce
metal recovery by the
formation of low-solubility lithium oxide through unwanted reaction 2.
Relatively better cell
performance may be achieved by minimizing the transport of carbonate ions from
the anolyte to
the catholyte.
[00149] In the illustrated example, the porous
membranes 28,44 help maintain a carbonate
ion concentration gradient between the anolyte 12 and catholyte 24, 40 during
electrolysis, such
that the carbonate ion concentration in the catholyte 24, 40 is substantially
lower than in the
anolyte 12. Preferably, the concentration of carbonate ions within the
catholyte is maintained
between about 50 ppm and about 5000 ppm, and more preferably is less than
about 100 ppm
while the apparatus is in use.
[00150] The porous membranes 28, 44 may be formed of
any suitable material that can
substantially hinder carbonate ion diffusion transport from the anolyte
compartment 10 into a
28
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
catholyte compartment 22, 38. Preferably, the porous membrane is a non-wetting
ceramic, such
as alumina, magnesia, zirconia, lithium-aluminate, petalite, magnesium alumina
spine!, mullite, or
similar, and optionally may have a maximum pore size between 0.1 ¨100 microns
and an average
pore size between 0.05 ¨ 50 microns. More preferably, the porous membrane may
have a
maximum pore size of about 1 micron and average pore size less than about 0.5
microns. Further,
the porous membrane preferably has an open porosity of between 10-80%, and
more preferably
between 30-60%. It is also preferred that the porous membrane is formed in one
piece (e.g. of
integral, one-piece construction) and is substantially free of joints, as this
may help prevent
leakage.
[00151] In some embodiments, the cathode housings may
be formed entirely from a porous
membrane. In other embodiments, as illustrated in Figure 1a, a porous membrane
may form only
a portion, such as the transfer portions 26 and 42, of the cathode housings 20
and 36. Referring
to Figure la, in this example the first cathode 30 is positioned within the
first cathode housing 20
such that the first transfer portion 26 is disposed between the first anode 18
and the first cathode
30 and such that the first cathode 30 is in fluid contact with the catholyte
24 contained within the
first catholyte compartment 22. Likewise, the second cathode 46 is positioned
within the second
cathode housing 36 such that the second transfer portion 42 is disposed
between the second
anode 34 and the second cathode 46 and such that the second cathode 46 is in
fluid contact with
the catholyte 40 contained within the second catholyte compartment 38.
[00152] If the cathode housings 20 and 36 were formed
entirely from the porous membrane
material it may be desirable to treat or otherwise modify some portions of the
membrane to inhibit
ion exchange through the membrane outside the desired transfer portions. For
example,
optionally, portions of the porous membrane and/or cathode housing may be
treated outside of
the transfer portions (whether there is a single transfer portion or multiple
transfer portions on a
given membrane, as discussed herein) to help inhibit the transmission of ions
through regions of
the cathode housing that are outside of the transfer portion. Optionally,
portions of the membrane
outside the transfer portion 26 or 42 may be treated to reduce their porosity
and/or to inhibit ion
transfer therethrough.
[00153] For example, a reduction of available ion
transfer area outside the transfer portion
may achieved by masking pads of the porous membrane (i.e. other than the
transfer portions 26
and 42) using an appropriate coating or glaze, impregnating the porous
membrane with a pore-
filling substance, such as resin or fine particles, or selectively slip-
casting parts using different
29
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
green material formulations. Alternatively, or in addition, the transport of
carbonate ions through
the membrane material may be reduced and/or substantially eliminated by at
least superficially
infiltrating the porous membrane with an ion-selective material, such as a
super-ionic conductor,
such as Li -Al2O3,13
Li5A104, Li2S-P2S5, or
similar super-ionic conductors which are stable at high
temperatures. Super-ionic conductors can have effective conductivity of about
10-1000x lower
than molten salt electrolytes described herein, and accordingly if such
materials are applied to
the transfer portions 26 and 42, they would be applied in relatively thin
layers so as to limit resistive
losses between the electrodes. Such treatment layers may be between about 0.01
microns and
about 100 microns thick. By at least partially infiltrating the pores of the
porous membranes in
the transfer portions 26 and 42, the super-ionic material can be surrounded by
the porous
membrane matrix, which can help provide mechanical support for the coating
material. This may
help enable the use of acceptably thin layers to be formed inside the first
few pores on the outer
surface of the membrane while providing sufficient durability. The super-ionic
conductor thickness
can be controlled using any suitable technique, including by applying a
viscous glaze of super-
ionic conductor material and, if necessary, grinding or otherwise machining
the outer layer of the
porous membrane until the desired thickness of super-ionic conductor material
remains.
100154]
By helping to limit the
rate of carbonate ion transport through the use of porous
membranes with sub-micron average pore size, closely matched in active surface
to the
electrodes, and optionally infiltrated with super-ionic conductor material,
the rate of production of
unwanted products can be substantially reduced. Such a reduction can increase
the current
efficiency and extend the time between catholyte and porous membrane
replacement.
[00155]
Optionally, the anodes and
cathodes can be any suitable shape. In the illustrated
example, the anodes 16,34 have the shape of a generally planar plate that is
substantially parallel
to the respective cathodes 30, 46, which also have the shape of a generally
planar plate.
[00156]
Optionally, some or all of
the electrodes (including the anodes, cathodes or both)
may be removable from the containment vessel 4. For example, the anodes 18 and
34 can be
removable from the anolyte compartment to help facilitate inspection,
maintenance and/or
replacement of each given anode. In the illustrated example, the anodes 18 and
34 (and/or
cathodes 30 and 46) can be removed in the generally upward direction, by
lifting them out of the
anolyte bath. Preferably, at least some of the anodes 18 and 34 and/or
cathodes 30 and 46 can
be removed independently of other ones of the cathode housings, cathodes, and
other anodes.
This can help facilitate the repair of one electrode without requiring removal
of other electrodes.
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
Optionally, at least some of the electrodes can be removable without having to
drain the anolyte
12 from the anolyte compartment 10. This allows for the electrodes to be
replaced (for example,
when worn), without requiring the disassembly of the containment vessel 4.
[00157] The electrolyzer apparatus 2 is preferably
operated at a relatively high current
density of at least 0.75A/cm2 on either electrode in an electrode assembly
(i.e., on either the
anode or cathode), and more preferably 1 to 2A/cm2 or more. In other examples,
the current
density can be between about 0.75 or 1 A / cm2 and about 4A / cm2, and
preferably is at least 1.2
A / cm2 so that electrolysis of both lithium chloride and lithium carbonate
can be achieved in
accordance with the various reactions described herein. The relatively high
current density
operation of the electrolyzer apparatus 2 can help achieve a relatively high
specific productivity,
which may help reduce the capital and/or operating costs for a given
production capacity. For
example, the specific productivity for the illustrated apparatus may be
between about 3 kg Li / m2
electrolyte bath surface / hr and about 15 kg Li / m2 electrolyte bath surface
/ hr, and optionally
may be about 5 ¨ 7.5 kg Li / m2 electrolyte bath surface / hr.
[00158] In some embodiments, the anodes (e.g. 18 and 34)
may be made of any suitable
carbon-based material including, for example, blocks of graphite, semi-
graphite, vitreous carbon,
or another carbonaceous material. When the electrolyzer apparatus 2 is in
operation, carbonate
ions may be oxidized at the anodes according to one or more of reaction 4, 5,
and 6, which may
contribute to the consumption of the anode material.
L12CO3(s, -1-1-2C(s)= 2Li(0+1CO2(g); E = ¨2.127 V
[4]
Li2CO3(s, C(s) = 2Li(01-0O2(9)+ CO(9); E = ¨2.226 V
[5]
Li2CO3(s, 2C(s) = 2Li(0-1-3C0(9); E = ¨2.434 V
[6]
[00159] While not wishing to be bound by any particular
theory or mode of action, under
normal operating conditions, it is expected that anodes will last several days
to several weeks,
after which they will need to be replaced. The self-contained unitary
construction and top-mounted
configuration in the illustrated example allows for removal of the anodes
using an overhead bridge
crane or other lifting device, optionally without needing to shut the cell
down for prolonged periods
of time. This arrangement thus may not only help reduce downtime but the life
of the electrolyzer
apparatus 2 may not be limited by the life of the anodes, which may help
reduce and/or eliminate
31
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
the costs associated with rebuilding the electrolyzer apparatus 2, and/or the
opportunity cost of
ceasing operations for maintenance.
[00160] In other embodiments, the anodes may comprise a
metal, ceramic, cermet, or
composite material that are generally inert or optionally at least semi-inert
to the electrolytic
process and the product gases. Such materials can include metals, ceramics,
cermets,
composites, and are characterized in having very low rates of consumption
during electrolysis.
These inert or semi-inert anodes may reduce the need for removal and
replacement of the
anodes, thereby reducing the maintenance effort required to operate the plant,
and thus may
reduce both the number of personnel and facilities needed for a given
throughput By using inert
or semi-inert anodes, the electrode spacing and/or gap between the anode and
cathode may be
maintained at a substantially constant (subject to less than about 5% change
while in use) and
preferably relatively small value (in the range of 0 ¨ 1%), which may help
reduce the resistive
heating losses incurred in the electrolyzer. Additionally, using an inert or
semi-inert anode may
help reduce carbon dioxide emissions per unit of lithium metal produced, by
forcing the reaction
within the apparatus 2 to the less carbon-intensive variant in reaction 7.
[00161] For example, electrolytic reactions 4, 5 and 6
can produce CO and CO2, while
reaction 7 can produce 02 and CO2. These gases can evolve at the anode surface
(i.e. at the
surface of anode 18 or 34), float to the surface of the anolyte 14, and can
then be captured by the
gas and conducted away from the cell, for example via a suitable gas
extraction apparatus (not
shown in Figure1, but that may contain a gas capture hood connected to a
suitable gas removal
conduit).
L12CO3(s, 1) +12C(s) = 2L1(1)+1CO2(g); E = ¨2.127 V
[4]
L12CO3(s, 1) -I- C(s) = 2141)-1- CO2(9)+ CO(9) ;
= ¨2.226 V [5]
L12CO3(s, 1) + 2C(s) = MG) +3C0(,); ff0 = ¨2.434 V
[6]
L12CO3(s, = 2Li(1)+ CO2(g)+1202; Et' = ¨3.145 V
[7]
[00162] A suitable power supply can also be provided to
apply an electric potential between
respective pairs of electrodes. Preferably, the power supply is relatively
oversized as compared
to known apparatuses for merely completing electrolysis of Li2CO3 and can be
configured to
32
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
provide a relatively high overpotential between the electrodes. As used
herein, overpotential can
be used to refer to an arrangement in which the electric potential applied
between pairs of
electrodes is purposefully higher, and preferably is substantially higher,
than the electric potential
that is required to complete the electrolysis of the lithium carbonate feed
stock material. In
contrast to conventional systems that are configured to reduce/minimize power
consumption, for
example by operating with the lowest electric potential that is sufficient to
process the given feed
stock, the processes and apparatuses described herein can be intentionally
operated at a
seemingly less efficient electric overpotential because it has been discovered
that the
overpotential can provide other advantages for the overall process/apparatus ¨
such as driving
other reactions and helping to control reaction by products and off gases.
[00163] That is, the electric potential that is applied
between the electrodes can be between
at least 1.5V greater than the electric potential that is required to merely
complete electrolysis of
Li2CO3 so that other materials and optionally may be between about 1.5-20V
greater than the
electric potential that is required to merely complete electrolysis of the
Li2CO3feed stock, such as
LiCI may also be subject to electrolysis. While the amount of electrical
potential may vary in a
given apparatus, it is preferably is substantially greater than the
equilibrium potential of lithium
chloride so that it is sufficient to i) initiate electrolysis of lithium
carbonate and ii) be greater than
the electric potential required to initiate LiCI electrolysis. For example,
the electrical potential may
be at least about 4 V, and is preferably at least 7V. In some embodiments, the
electrical potential
may be between about 7V and about 12V, and optionally may be at least 15 V. In
the illustrated
example, the electrolyzer apparatus 2 has a power supply 56, which is
configured to apply an
electrical potential between the first anode 18 and first cathode 30 and can
also provide power to
any of the other electrode pairs within the apparatus 2. The power supply can
be any suitable
type of power supply.
[00164] It is believed that at least partially as a
consequence of the simultaneous
occurrence of these anode reactions, C12, CO2 and CO gasses are evolved at the
surface of the
anodes 18 and 34. The inventors have discovered that relatively small
concentrations of Li2CO3
react readily with Cl2 as it bubbles up through the anolyte 14, converting it
to LiCI, CO2 and 02
according to reaction 8. This can, in effect, act as an inherent chlorine
scrubber. Under these
conditions, the inventors have discovered That there is a near-total
suppression of Cl2 emission
from the apparatus 2, even at high-over potential and anode current densities.
L12CO3(0 + C/20) = 2Li0(,) + CO2Cg) +O2)
[8]
33
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[00165] The apparatuses described herein can utilize
this effect to bias the production of
lithium metal to towards reactions 1 and 7, at the expense of reactions 4, 5
and 6. By doing so,
the quantity of carbon required per unit of metal may be reduced from the
stochiometric -0.5kg C
/ kg U to about 0.4 C 1 kg Li or less, or about 0.3kg C / kg Li or less. To
the extent that carbon for
the reactions is consumed from the anodes 18 and 34, etc. this reduction in
carbon consumption
may be advantageous, as it may help reduce the frequency of anode changes and
may help
reduce the operating costs of the systems.
[00166] While it is generally desirable to avoid the
creation/release of chlorine from
electrolysis reactors as noted herein, the inventors have discovered that the
conditions enabled
by the use of the electrolyzer apparatuses described herein may be
advantageous enough that
in some circumstances it may actually justify the introduction of additional
chlorine gas into the
apparatus while it is in use. It has been discovered that the introduced
chlorine may be largely
consumed as described and, while not wishing to be bound by any particular
theory or mode of
action, the introduction of chlorine gas within the catholyte compartments 22
and 38 may
surprisingly help inhibit carbon and/or lithium oxide fouling of the cathode,
by reacting with the
Li2CO3 present within the catholyte to produce LiCI and carbon dioxide. The
LiCI may be
subsequently electrolyzed under the overpotential operating conditions, which
may help improve
apparatus efficiency and/or lithium production as compared to operating the
apparatus in the
absence of the added chlorine gas. The introduction of chlorine gas in this
manner may also help
inhibit damage to the porous membranes by reading with excess lithium that may
be present in
the catholyte compartment.
[00167] Additionally, chlorine gas may be introduced
into the anolyte, in conjunction with
excess Li2CO3 to convert the latter to Lid. This is advantageous, as it allows
Lid lost from the
electrolyte to evaporation to be replaced without resorting to purchases of
the relatively more
costly LiCI, and thereby reducing the operating cost of the plant, and
simplifying the plant supply
chain.
[00168] Accordingly, the electrolyzer apparatus may
include a chlorine delivery system that
is configured and operable to introduce chlorine gas into at least some of the
catholyte or anolyte
compartments while the apparatus is in use. The chlorine delivery system may
include any
suitable hardware and gas conveying components, including conduits, hoses,
pipes, spargers,
bubblers, valves, pumps/ compressors, filters and the like that are configured
to transport gas
34
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
from the chlorine gas source (e.g. a gas tank or other gas supply) and
introduce it within the
catholyte compartments.
[00169] Optionally, instead of being arranged so that
each cathode only engages a single
anode (and vice versa) as shown in Figure 1a, an electrolyzer apparatus can be
arranged with
multiple electrodes closely-spaced together and so that a given cathode may be
positioned with
and engage two anodes (and vice versa). In such arrangements, each cathode
housing may be
configured to provide two transfer portions on opposing sides of the cathode,
each registered with
the active surface of the opposing anode.
[00170] The apparatuses described herein may be used in
a process for producing lithium
metal from lithium carbonate, and preferably for producing relatively high-
purity lithium metal from
lithium carbonate using a membrane electrolysis process operating in the
suppressed chlorine
regime (SCR). To achieve SCR operation the apparatus described herein can be
operated so
that the desired, applied overpotential and lithium carbonate concentration
are maintained within
the desired ranges while the apparatus is in use, while also reducing and/or
minimizing the
diffusion of carbonate ions into the cathode compartments, and the consumption
of carbon at the
anode.
[00171] In one preferred example, both the anolyte and
catholyte is a LiCL-KCI melt, with
a molar concentration ratio of 65:35 to 95:5, maintained at 475-660 C.
Advantageously, within
this concentration and temperature range, the solubility of lithium carbonate
is sufficient to scrub
chlorine gas from the melt when it is formed at the anode, but not so high as
to encourage unduly
fast diffusion of carbonate ions into the cathode compartment at saturation.
[00172] Electrolysis is carried out with a potential at
least 1-1.5V, or more in excess of that
required for lithium carbonate electrolysis; in other words with potential
sufficient or greater than
that required for lithium chloride electrolysis. Under these conditions,
lithium metal may be
electrolytically produced according to reactions 1, 4, 5, 6, 7. Contrary to
conventional
apparatuses, no attempt is made to operate in the low-voltage operating regime
to realize energy
savings, as this would result in undesired, relatively low current densities
for practical cell
configurations. Instead, energy consumption may be reduced by operating with a
lithium chloride-
rich electrolyte.
[00173] Within the electrolyte concentration range
specified, electrical conductance of the
electrolyte may be 15%-200% higher than the eutectic 60:40 melt of the
conventional processes,
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
which may lead to reduced resistive heat losses. The addition of lithium
carbonate may have a
further effect in enhancing electrical conductance, thereby further reducing
the energy
consumption.
[00174] Counterintuitively, this mode of operation does
not result in the production of
material amounts of chlorine gas, and its many associated drawbacks. While
chlorine gas is
temporarily produced at the anode, the presence of significant quantities of
lithium carbonate in
the melt adjacent to the anode may cause scrubbing of the chlorine gas, and
conversion of the
lithium carbonate into lithium chloride according to reaction 8.
[00175] Advantageously, operation according to the
present teachings may help allow
lithium to be produced from carbonate with substantially reduced carbon
consumption, because
reactions 1 and 7 do not rely on carbon from the anode. As a result, by
establishing electrolytic
conditions favorable to these reactions, the anode carbon consumption per
kilogram of metal
produced can be substantially reduced.
[00176] Table 1 shows experimental results which
indicate the reduction, as compared to
one representative example of a process known in the art.
[00177] Another advantage of the present teachings may
be that, unlike the prior art, the
present invention need not be limited in the current density that can
practically be achieved,
because electrolysis potentials significantly in excess of that theoretically
required for electrolysis
of carbonate can be applied. For example, current densities in excess of
1A/cm2 can be
maintained according to the present teachings, while those of the
representative, conventional
apparatus were limited to 0.25-0.5A/cm2.
[00178] Operation at current densities in excess of 1A /
cm2 helps reduce the specific flux
of carbonate ions across the porous membrane per kilogram of metal produced,
thereby resulting
in reduced relative production of undesirable elemental carbon sludge in the
catholyte
compartment.
[00179] According to an aspect of the present teachings,
SCR operation can be practically
maintained by monitoring the anode gas to determine if the concentration of
chlorine gas exceeds
a monitoring threshold ¨ e.g. if an amount of chlorine gas is present in the
anode gas which would
otherwise be substantially chlorine free. Chlorine gas breakthrough (detection
of chlorine gas in
the anode gas) signals that the lithium carbonate concentration in the anolyte
bath has fallen
36
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
below the target level needed for effective scrubbing and therefore lithium
carbonate should be
added to the anolyte. Chlorine breakthrough can occurs when the dissolved
lithium carbonate
concentration within the anolyte falls below about 0.1 ¨ 0.5 mol%.
Accordingly, the apparatuses
described herein may be operated with a dissolved lithium carbonate
concentration that is at least
0.1 mol%, 0.2 mol%, 0.3 mol%, 0.4 mol%, 0.5 mol%, 0.6 mol%, 0.7 mol%, 0.8
mol%, 0.9 mol%,
1 mol% or greater.
[00180] According to the present teachings, conditions
favouring low anode carbon
consumption can be maintained by monitoring the CO2/02 ratio in the off-gas
collected from the
anolyte compartment and keeping this in the range 2-2.5. This can be done, for
example, by
adjusting the rate of lithium carbonate feed, adjusting the current density or
maintaining an excess
inventory of lithium carbonate in the anolyte and controlling the electrolyte
temperature to maintain
the desired saturation concentration of dissolved lithium carbonate. For
example, by raising the
temperature, additional lithium carbonate can be forced to dissolve into the
anolyte from an
inventory of solid precipitate maintained on the bottom of the electrolyzer.
Table 1- Process According to the Prior Art vs. the Present Invention
Process Conditions
Process Conditions
According to one the
According to one
teachings of a
Example of the Present
conventional process
Teachings
(Kruesi)
Electrode
3-5V
7-12V
Potential
Current Density 0.07 - 0.33 A / cm2
1.0 - 2.0 A / cm2
Carbon
Consumption 0.65 kg C / kg Li
0.25-0.5 kg C / kg Li
LICI-KCI Eutectic
LiCI-KCI
Electrolyte
(60:40 molar)
65:35 to 95:5 molar
CO?- Conc. 0.75 ¨ 5 mol%
0.35-0.75mol%
Operating
522 C ¨ 722 C
475 C - 650 C
Temperature
[00181] Referring to Figure 2, a schematic illustration
of another example an electrolyzer
apparatus 1002 is illustrated. The Apparatus 1002 is generally analogous to
electrolyzer
apparatus 2, and analogous features are identified using like reference
characters indexed by
1000. In this apparatus 1002, the electrodes are closely spaced together such
that an electric
potential is applied on both sides of some of the electrodes.
37
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
100182]
In this apparatus 1002, the
second electrode assembly 1032 is adjacent the first
electrode assembly 1016 such that the first cathode 1030 is disposed between
and is optionally
equally spaced between the first anode 1018 and the second anode 1034 so as to
enable an
electric potential to be applied across both gaps. Arranging the electrodes in
this manner may
help reduce the overall size of the apparatus 1002. In such an arrangement,
the first cathode
housing 1020 comprises a first transfer portion 1026 disposed between and
registered with the
active surfaces 1048 and 1050, and a second transfer portion 1058 disposed
between and
registered with the active surface 1062 on the opposite side of the cathode
1030 and the active
surface 1064 on adjacent anode 1034. This second transfer portion 1058
includes an additional
porous membrane region, which permits migration of lithium ions but resists
the migration of
carbonate ions from the anolyte compartment 1010 into the first catholyte
compartment 1022_
[00183]
Figures 3-10 illustrate an
alternative embodiment of an electrolyzer apparatus
2002 that is generally analogous to electrolyzer apparatus 2, and in which
analogous features are
identified using like reference characters indexed by 2000.
[00184]
In this embodiment, the
containment vessel 2004 is generally square in cross-
sectional shape, and has a bottom wall 2006 with an opposing upper end 2014
(Figure 6) and at
least one side wall 2008 that extends upwardly from the bottom wall 2006
toward the upper end
2014. Together, the bottom wall 2006 and side wall 2008 co-operate to
generally provide the
boundaries of an anolyte compartment 2010 of the electrolyzer apparatus 2002.
In this
embodiment, the electrode assemblies, including assemblies 2016 and 2032
(Figure 10) are
closely spaced and arranged so that the anodes (e.g. 2018 and 2034) and
cathodes (e.g. 2030
and 2046) are alternatingly arranged in two rows (see also Figure 5a).
[00185]
To help define the multiple
catholyte compartments within the common anolyte
compartment 2010, each cathode 2030 and 2046 is provided within a respective
cathode housing
2020 and 2036. The cathode housings 2020 and 2036 are arranged to generally
surround their
respective cathodes 2030 and 2046 in a generally liquid-tight manner, and
positioned so that the
outer surfaces of the cathode housings 2020 and 2036 are also in fluid contact
with the molten
anolyte in the common anolyte compartment.
[00186]
In this arrangement the
interior of each cathode housing 2020 and 2036 defines
its respective first and second catholyte compartments 2022 and 2038, which
contain respective
molten salt catholyte material. While liquid separation between the anolyte
and catholyte is
38
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
preferably maintained while the apparatus 2002 is in use, the anolyte and
catholyte may optionally
comprise the same or at least substantially the same materials. In this
example the catholyte
material also includes molten LiCI-KCl, in substantially the same ratios as
the anolyte. Other
catholyte materials may also be used.
[00187] In Figure 10, the anodes 2018 and 2034 are
shown in a side view and are
understood to be generally planar members that extend into the page as shown.
The front face
of the anode 2018 faces the opposing face of corresponding cathode 2030 and
forms the anode
active surface 2048. The opposing surface of the cathode 2030 forms its
reciprocal active surface
2050. Likewise, the second anode effective transfer area 2052 is opposite and
facing the second
cathode effective transfer area 2054 The portions of the membranes 2028 and
2044 that are
disposed laterally between the opposing active surfaces 2048 and 2050, and
2052 and 2054
define the transfer portions of the respective membranes 2028 and 2044.
[00188] The transfer portions 2026 and 2042 of the
first and second porous membranes
2028, 2044 are preferably configured to permit the migration of lithium ions
from the anolyte
compartment 2010 into the first and second catholyte compartments 2022, 2038,
respectively,
while resisting the migration of carbonate ions from the anolyte compartment
2010. Restricting
the transport of carbonate ions limits the rate of formation of undesirable
cathode products,
namely carbon and lithium oxide, which would otherwise reduce current
efficiency through
unwanted reaction 3 and may limit the life of the catholyte by contaminating
it with carbon, and
potentially reduce metal recovery by the formation of low-solubility lithium
oxide through unwanted
reaction 2. Relatively better cell performance may be achieved by minimizing
the transport of
carbonate ions from the anolyte to the catholyte.
[00189] In the illustrated example, the porous
membranes 2028, 2044 help maintain a
carbonate ion concentration gradient between the anolyte and catholyte during
electrolysis, such
that the carbonate ion concentration in the catholyte is substantially lower
than in the anolyte.
Preferably, the concentration of carbonate ions within the catholyte is
maintained between about
50 ppm and about 5000 ppm, and more preferably is less than about 100 ppm
while the apparatus
is in use.
[00190] In this embodiment, the electrodes are
removable from the containment vessel
2004 and the apparatus 2002 includes an anode mounting apparatus 2066 from
which an anode
(e.g. 2018) can be suspended and extend downwardly into the anolyte
compartment 2014_ In the
39
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
illustrated example (see also Figure 3), the anode mounting apparatus 2066
includes an elongate
anode mounting beam that extends over the upper end 2014 of the containment
vessel 2004 and
the first anode 2018 is suspended from the beam. Another anode is also
supported by the same
beam. Preferably, the anode mounting apparatus 2066 is detachable and
removable from the
containment vessel 2004 which can facilitate access to the anodes mounted
thereon. Optionally,
some or all of the anodes may be detachable from the mounting beam, optionally
via the use of
an intermediary anode mounting stub 2090 (Figure 8), so that the anode 2018
may be detachable
from the anode mounting apparatus 2066, which allows the first anode 2018 to
be removed from
the anolyte compartment 2010 and separated from the mounting beam and replaced
with a new
anode. Optionally, the anodes mounted to a common mounting beam may be
individually
detachable from the beam.
[00191] Similarly (see Figure 9a and 9b), the
electrolyzer apparatus 2002 may have a
cathode mounting apparatus 2078 from which one or more cathodes and optionally
the
associated cathode housings can be suspended and extend downwardly into the
anolyte
compartment 2010. In the illustrated example, the cathode mounting apparatus
2078 includes an
elongate beam that is generally similar to the beam used in the anode mounting
apparatus 2066
and that extends over the upper end 2014 of the containment vessel 2004 and
the first cathode
2030 is suspended from the cathode mounting apparatus 2078. Preferably, the
cathode mounting
apparatus 2078 is detachable from the containment vessel 2004, which allows
the cathode
secured to the cathode mounting apparatus 2078 to be removed from the anolyte
compartment
2010 with the cathode mounting apparatus 2078. Optionally, a cathode housing,
may also be
suspended from the cathode mounting apparatus 2078, such that when the cathode
mounting
apparatus 2078 is removed from the anolyte compartment 2010, the cathode and
its associated
cathode housing is removed. Optionally, the cathode housing may be connected
to the beam or
other support apparatus directly or alternatively, as illustrated in this
example the cathode housing
2020 may be at least partially connected to and supported by the cathode 2030
itself. Optionally,
the cathodes mounted to a common mounting beam may be individually detachable
from the
beam.
[00192] In some embodiments, such as the illustrated
example, the electrolyzer apparatus
2002 may have a plurality of anode mounting apparatuses 2066 and cathode
mounting
apparatuses 2078 in an alternating arrangement. Preferably, each electrode
mounting apparatus
spans or partially spans the electrolyzer apparatus 2002 transversely across
the longitudinal axis.
More preferably, the adjacent anode mounting apparatuses 2066 and cathode
mounting
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
apparatuses 2078, optionally in combination with the use of roof panels and
other such members,
may cooperate to cover all of or substantially all of the upper end 2014 of
the containment vessel
2004 (for example, see Figure 5a) when the apparatus 2002 is in use. The anode
and cathode
mounting apparatuses may be configured in any suitable manner, including but
not limited to
being supported by the containment vessel 2004, being suspended from an
external structure
(walls or roof of an enclosing structure/building), or one type of electrode
mounting apparatus may
support the other.
[00193] Having a plurality of closely-interleaved top-
mounted electrode mounting
apparatuses, may help the present electrolyzer apparatus 2002 have a
relatively larger electrode
area within a given overall apparatus footprint and anode-to-cathode spacing.
This may help
reduce the overall size of the electrolyzer apparatus, which may help
facilitate its use in locations
that could not easily accommodate conventional apparatuses and may help reduce
the overall
cost of a plant of given production capacity.
[00194] Such a repeating electrode configuration may
also may help enable a relatively
large number of electrode pairs to be installed in parallel within the single
electrolyzer apparatus
2002 (and preferably with the anodes being directly submerged in a common
molten salt, anolyte
bath in the common anolyte chamber 2010), thereby allowing relatively higher
capacity production
in the common vessel 2004 of an electrolyzer apparatus 2002 (e.g. while only
having to provide
and maintain a single anolyte bath).
[00195] Optionally, the anode mounting apparatuses 2066
and cathode mounting
apparatuses 2078 may also incorporate other functional components of the
apparatus 2002,
including aspects of the power connection/supply systems, electrolyte supply,
chlorine gas
supply, feed material supply, lithium metal extraction and other such
apparatuses. Incorporating
these subsystems into the structures forming the anode mounting apparatuses
2066 and cathode
mounting apparatuses 2078 may help simplify the design and construction of the
vessel 2004
and/or may help facilitate access to such sub-systems while the apparatus 2002
is in use (for
example, when the anode mounting apparatuses 2066 and cathode mounting
apparatuses 2078
are removed from the vessel 2004). This may also help reduce the number of
separate ancillary
systems (electrical bus components, feed systems, gas supply and off-gas
systems, tapping
system, control systems, instruments, etc.) that are needed for a given metal
production capacity,
thereby reducing the capital expenses required to set-up the present
electrolyzer apparatus 2002,
as compared to known electrolyzer configurations.
41
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[00196] This integration of apparatus 2002 subsystems
may also help make it relatively
easier to operate the described electrolyzer apparatus 2002. For example, as
each of the anode
mounting apparatuses 2066 and cathode mounting apparatuses 2078 span across a
common
anolyte bath the feed material supply apparatus integrated into any one of the
anodes mounting
apparatuses 2066 and cathode mounting apparatuses 2078 may be used to supply
feed material
to multiple electrode pairs. That is, because the operator need not maintain
the balanced of feed
material or other components in multiple, discrete anolyte compartments a
common feed may
support the operation of multiple electrode pairs. Instead, the use of a
common anolyte
compartment 2010 into which at least two or more pairs of electrodes can be
submerged may
help ensure that each anode is exposed to an anolyte having the same, and
preferably the
desired, composition as the present electrolyzer apparatus 2002 is in use. For
example, feed
material that is introduced in one region of the containment vessel may
circulate within the anolyte
and may be available for reaction at a plurality of different electrode
assemblies in the examples
of the electrolyzer apparatuses described herein.
[00197] In some embodiments, the one or more anode
mounting apparatuses are
configured to be self-contained units or substantially self-contained units.
The anode mounting
apparatus 2066 in the illustrated example comprises an anode structural member
2088 (i.e. a
beam in this example). Looking to Figures 8a and 8b, the illustrated anode
mounting apparatus
2066 also comprises an anode electrical connector 2068 (e.g. a bus connection)
that provides
electrical communication between the anodes and the power source, anode stubs
2090 for
helping to attach the anodes, the first anode 2018, an anode gas extraction
apparatus 2072
(including a gas capture hood 2074 in communication with the anode gas removal
conduit 2076
¨ Figure 5b), and insulating roof lining elements 2092, each of which are
directly or indirectly
affixed to the anode structural member 2088 (see Figure 8).
[00198] The anode structural member or members 2088 and
anode stubs 2090 may be
made of any suitable material, such as steel, stainless steel, or other
conductive structural
materials capable of withstanding the process temperature.
[00199] In the illustrated example, the anode electrical
connector 2068 electrically
connects the first anode 2018 to the power supply 2056 when the anode mounting
apparatus
2066 is attached to the containment vessel 2004. When the anode mounting
apparatus 2066 is
detached from the containment vessel 2004, the electrical connection between
the first anode
2018 and the power supply is interrupted. Optionally, the anode mounting
apparatus 2066 may
42
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
have an insulating lining (e.g. roofing elements 2092) disposed between the
anolyte compartment
2010 and the anode electrical connector 2068 to inhibit heat transfer from the
anolyte to the anode
electrical connector 2068 when the electrolyzer apparatus 2002 is in use. The
insulating lining
may be removable with the anode mounting apparatus 2066.
[00200] The anode electrical connector 2068 may be made
of any suitable conductive
material with low electrical resistance, such as copper, brass, aluminum,
silver, bronze, steel or
iron, to help reduce, and optionally minimize the electrical resistive losses
and heating of the
structure. Preferably, anode electrical connectors 2068 are connected to the
anode stubs 2090
via a low electrical resistance method, such as welding, brazing, riveting, or
bolting.
[00201] In some embodiments, the anode mounting
apparatus can include at least some
components of a gas extraction apparatus that can be used to remove product
gases as they
accumulate in the head space toward the upper end 2014 of the vessel 2004 when
it is in use. In
the illustrated example, the gas extraction apparatus 2072 comprises a gas
capture hood 2074
configured to capture product gases formed adjacent an anode and bubbling out
of the anolyte.
The gas capture hood 2074 is positioned above the first anode 2018. The gas
extraction
apparatus 2072 may also include a gas removal conduit 2076. In the illustrated
example, the gas
removal conduit 2076 extends from the gas capture hood 2074 and is configured
convey the
product gases away from the containment vessel 2010.
[00202] In the illustrated example, the gas capture hood
2074 is a gas-tight sheet metal
hood, isolated electrically from the anodes via the anode roof lining elements
2092 (see Figure
10). The bottom lip of each gas capture hood 2074 is immersed below the
minimum level of the
electrolyte bath. Preferably, the gas capture hoods 2074 are sized so that
their width spans
substantially the entire space between the porous membranes 2028, 2044 on
adjacent cathode
housings (see also Figure 10). This arrangement can capture a substantial
portion of the gas that
is evolved at the anode and, because of the immersion, can generate sufficient
pressure to drive
the gas out of the gas removal conduit 2076 and into a gas cleaning system
(not shown). Such
close capture of the gas may allow it to be used as a process input a by-
product stream, or
directed to carbon sequestration, all of which may reduce the carbon footprint
of the process.
[00203] In other embodiments, the gas capture hood 2074
may be formed directly into the
anode roof lining elements 2092, defined by the adjacent cathode mounting
apparatus roof lining
elements (discussed below), or omitted entirely, with anode gas captured by
the gas space 2094
43
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
defined by the anode and cathode mounting apparatuses 2066, 2078, containment
vessel 2004,
and anolyte.
[00204] Preferably, at least a portion of the gas
extraction apparatus 2072 is removable
from the containment vessel 2004, optionally with the anode mounting apparatus
2066. It is also
preferable that the gas capture hood 2074 is electrically isolated from the
anode secured to the
anode mounting apparatus 2066.
[00205] The gas capture hood 2074 and gas removal
conduit 2076 may be made of
stainless steel or any other suitable material that is capable of withstanding
prolonged exposure
to chloride immersion and chloride mist under high temperature, as well as
compatible with
constant exposure to high-temperature carbon dioxide gas, and occasional
contact with chlorine
gas.
[00206] The anode roof lining elements 2092 may be of
sufficient strength to remain
attached to the anode mounting apparatus 2066 during handling, and be
compatible with chloride
melts, while also providing sufficient thermal insulation to protect the anode
structural member
2088 from excessive temperatures. Suitable materials may include fireclay
bricks and castables,
alumina bricks and castables, calcium silicate board, alumina silicate board,
and other similar
refractory materials known to those skilled in the art.
[00207] In some embodiments, the one or more cathode
mounting apparatuses are
configured to be self-contained units or substantially self-contained units.
The cathode mounting
apparatus 2078 in the illustrated example comprises a cathode structural
member 2096. Looking
to Figures 9a and 9b, the illustrated cathode mounting apparatus 2078 also
comprises a cathode
electrical connector 2084, cathode stubs 2098, the first cathode 2030, gas
capture elements
(which in this example are the porous membranes 2028, 2044), shielding gas
conduits 2100
(which can include any required valves, conduits and the like and can be
connectable to be a
suitable source of an inert shield cover gas), a lithium metal extraction
assembly 2080, a lithium
metal extraction conduit 2082, and cathode roof lining elements 2102, each of
which are directly
or indirectly affixed to the cathode structural member 2096.
[00208] It may be desirable to remove and replace
inoperable porous membranes or other
aspects of the cathode housing when the apparatus 2002 is in use or has been
used. For
example, even selective porous membranes may permit some quantity of carbonate
ions to
diffuse into the catholyte chamber These carbonate ions may react either with
the lithium metal,
44
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
or through direct electrolysis, produce insoluble elemental carbon and lithium
oxide according to
reaction 2 or 3 described herein. Over time, the carbon can build up as a
sludge, at least some of
which may accumulate within the porous membranes in the cathode housings,
eventually
rendering the membranes and electrode less effective and potentially
inoperable. As a result, it
may be desirable to service and/or replace the porous membranes and catholyte
periodically over
the life of the apparatus 2002. Affixing the porous membranes to the cathode
mounting apparatus
2078 (and optionally to the cathodes themselves), may help facilitate that
relatively easy removal
and replacement of selected porous membranes by removing the cathode mounting
apparatus
2078 as a unit, optionally using an overhead bridge crane or other lifting
device, without needing
to shut the apparatus 2002 down for prolonged periods of time. This
arrangement may help
reduce downtime and the life of the electrolyzer 2002 may not be limited by
the build-up of
elemental carbon sludge or lithium oxide in the porous membrane or cathode
compartment.
[00209] The cathode structural member 2096, cathode
stubs 2098, and cathode roof lining
element 2102 are analogous to similar components of the anode mounting
apparatus 2066, as
described herein.
[00210] In the illustrated example, the cathode mounting
apparatus 2078 has a cathode
electrical connector 2084 that electrically connects the first cathode 2030 to
the power supply
2056 when the cathode mounting apparatus 2078 is attached to the containment
vessel 2004.
When the cathode mounting apparatus 2078 is detached from the containment
vessel 2004, the
electrical connection between the first cathode 2030 and the power supply is
interrupted.
[00211] The cathode mounting apparatus 2078 may have a
lithium metal extraction
assembly 2080 to extract lithium metal that collects in the catholyte. In the
illustrated example,
the lithium extraction assembly 2080 includes a lithium extraction conduit
2082 that extends from
an upper end proximate the cathode mounting apparatus 2078 to a lower end that
is disposed
within the first catholyte compartment 2022. Optionally, the lithium
extraction conduit 2082 is
removable from the containment vessel 2004 with the cathode mounting apparatus
2078.
[00212] In some embodiments, electrolyzed metal can
accumulate directly in the catholyte
compartments. A vacuum can be applied continuously or intermittently to each
lithium extraction
assembly 2080 to withdraw metal as it is formed. The vacuum draws metal up the
lithium
extraction conduit 2082, towards the lithium extraction assembly 2080 and out
of the cell to a
metal storage system (not shown).
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[00213] In some embodiments, electrolyzed metal is
produced below the level of the
catholyte. Advantageously, the density difference between the metal and salt
bath creates
differential head that pushes the metal into the lithium metal extraction
assembly and supplies the
necessary differential pressure to partially or fully push the metal out of
the electrolyzer.
[00214] Application of a vacuum can lead to
infiltration of the gas space by air or anode
gases, resulting in conversion of the lithium metal to lithium oxide or
lithium carbonate according
to equation 9 or reaction 10, resulting in a reduction of current efficiency.
This may be prevented
by supplying a small flow of inert shielding gas into a gas head space within
each cathode
compartment such as argon, helium, or dry air, through the cathode shielding
gas conduits 2100
to displace the reactive gases_
2Lix0 + 02(9) = Li20 (s)
[9]
41140 CO2(9) = C (s)+2Li 20 (S)
[10]
[00215] In the illustrated example, the electrolyzer
apparatus 2002 has a chlorine delivery
system 2070 configured to introduce chlorine gas into the catholyte
compartments 2022, 2038
when the electrolyzer apparatus 2002 is in use.
[00216] In the illustrated example, the cathode
mounting apparatus 2078 has a feed port
2086 through which feed material can be introduced into the anolyte
compartment 2010. The feed
port 2086 is arranged on the cathode mounting apparatus 2078 such that it is
removed from the
containment vessel 2004 when the cathode mounting apparatus 2078 is removed
from the
containment vessel 2004. Alternatively, or in addition, feed ports may be
provided in the anode
mounting apparatuses, or in areas of the electrolyzer roof that are
independent of anode or
cathode mounting apparatuses.
[00217] Optionally, the catholyte compartments 2022,
2038 may be filled with catholyte
prior to commencing electrolysis. In some embodiments, this may be
accomplished by filling the
individual catholyte compartments 2022, 2038 with solidified salt during
assembly or the catholyte
can be introduced in molten form through suitable separate conduits or the
lithium extraction
conduit prior to installation of the cathode mounting apparatus 2078. In other
embodiments, a
filling tube may be used to allow communication between the anolyte
compartment and the
catholyte compartments 2022, 2038 during apparatus start-up. In the
illustrated example, a filling
tube 2104 is formed in the shape an inverted "J", as shown in Figure 9a and
Figure 7. The filling
46
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
tube 2104 is positioned so that one end is in communication with the anolyte
compartment 2010
and the other end is in the catholyte compartment 2022. The catholyte end of
the filling tube 2104
terminates at the elevation of the minimum bath level. The anolyte end of the
filling tube 2104
extends below the minimum bath level. In the illustrated example, each
catholyte compartment
has a filling tube.
[00218] Through this arrangement, anolyte/ electrolyte
can be drawn into each catholyte
compartment in situ after installing the cathode mounting apparatuses 2078. In
some
embodiments, this can be accomplished by applying a vacuum to the lithium
extraction assembly
2080. The electrolyte flows into the catholyte compartment 2022 through the
filing tube 2104 while
the vacuum is applied. The electrolyte level in the catholyte compartment 2022
rises until it
reaches the lithium extraction conduit 2082. Preferably, the vacuum level can
be controlled such
that it is sufficient to bring electrolyte into the catholyte compartment
2022, but not sufficient to
pull the electrolyte up through the entire lithium extraction conduit 2082 and
into the lithium
extraction assembly 2080. By doing so, the catholyte compartments can be
filled to the desired
level without the need for level sensors or individual filling systems for
each catholyte
compartment While shown as a j-shaped conduit, the filling tube may have other
configurations
in other examples.
[00219] While the electrolyzer apparatuses described
herein are in use carbon may be
formed as a by-product of the desired reactions and may accumulate in certain
regions of the
electrolyzer apparatuses. Such carbon accumulation may be the result of the
transport of
carbonate ions across the membrane and into the cathode compartment such as by
the lithium
carbonate-containing anolyte being drawn into the cathode compartment, despite
the presence
of the cathode housings. This may be undesirable because it may reduce the
current efficiency
and/or may cause elemental carbon sludge and lithium oxide build-up in the
catholyte.
[00220] To help reduce the undesirable carbon formation,
chlorine gas can be bubbled
through the catholyte upon initial filling, and at other suitable times, to
react the lithium carbonate
to carbon dioxide and lithium chloride. This can be accomplished through a
temporary or
permanent tube inserted into the membrane. In some embodiments, the bubbling
can be
continued at a rate sufficient to react with any lithium carbonate diffusing
through the porous
membrane, thereby slowing or eliminating carbon sludge and/or lithium oxide
fouling of the
catholyte and maintaining the concentration of carbonate ions below about 100
ppm, and more
preferably below 50 ppm.
47
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
[00221] Similarly, excess chlorine can be bubbled into
the catholyte or used as a shielding
gas to fill or at least partially fill the headspace within the vessel 2004,
which may help exclude
oxygen from the headspace. This chlorine gas can react with any metal produced
on the exterior
of the gas capture hood 2074 or cathode stub 2098 and convert it back into
lithium chloride. This
may protect the porous membranes 2028, 2044 from attack by lithium metal and
help reduce the
accumulation of lithium metal outside the gas capture hood 2074. This may
allow the cathode
mounting apparatus 2078 to be used for longer before maintenance is required.
[00222] Optionally, the electrolyzer apparatus 2002 can
be equipped with cooling and/or
heating means to adjust the heat balance of the electrolyte. Such cooling or
heating means can
be used to adjust the heat balance in response to various conditions. For
example, as the anode
carbon is consumed, the anode-to-cathode distance increases, which can cause
additional heat
to be generated in the electrolyte, leading to an increase in the temperature
of the electrolyte
bath. This can be balanced by supplying additional cooling, thereby allowing
the electrolyzer
apparatus to tolerate larger changes in anode-to-cathode spacing. It may also
allow larger anodes
to be used, thereby reducing the time between anode replacement.
[00223] Use of cooling and/or heating means may also
allow the temperature of the
electrolyte bath to be set independently of the current and anode-to-cathode
distances_ This
means that the saturation concentration of lithium carbonate in the bath can
be better controlled,
thereby allowing an inventory of undissolved lithium carbonate to be
maintained in the electrolyzer
without affecting the concentration available for electrolysis and, if needed,
allowing it to be rapidly
deployed.
[00224] In some embodiments, cooling elements such as
cast blocks, cast-in conduits, or
channels may be embedded in the lining (walls, floor, roof, or any combination
thereof) of the
electrolyzer apparatus. A cooling medium, for example, water, air, other
gases, molten salts or
ionic fluids, can be made to circulate by forced or natural convection through
the cooling elements,
where it can extract heat and remove it from the electrolyzer apparatus to the
atmosphere or
some other heat sink or heat exchanger.
[00225] In some embodiments, heating elements such as
cast blocks or cast-in conduits
may embedded in the lining (walls, floor, roof, or any combination thereof) of
the electrolyzer
apparatus. A heating medium, for example, steam, air, combustion gases, molten
salts, or ionic
48
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
fluids, can be made to circulate by forced or natural convection through a
heat source, and then
made to pass through heating elements, where it heats the electrolyzer.
[00226] In other embodiments, electrical heating elements may be mounted in
the lining of the
electrolyzer (walls, floor, roof, or any combination thereof) or heating or
cooling elements may be
suspended from the roof or mounted in place of one or more anode or cathode
mounting
apparatuses and immersed in the electrolyte bath.
100227] Alternatively, the electrolyte bath may be removed from the
electrolyzer apparatus and
circulated to a heat exchanger where it can be cooled or heated by another
cooling or heating
element to the desired temperature and returned to the electrolyzer bath.
[00228] Figures 11a and 11 b illustrate a portion of alternative embodiment of
an electrolyzer
apparatus that is generally analogous to electrolyzer apparatus 2000, and in
which analogous
features are identified using like reference characters indexed by 3000. In
this example the
cathode mounting apparatus 3078 includes a cathode structural member 3096
along with a
cathode electrical connector 3084, cathode stubs 3098, the cathode 3030,
membrane 3028
defining the catholyte compartment 3022 and cathode roof lining elements 3102,
each of which
are directly or indirectly affixed to the cathode structural member 3096.
[00229] In this illustrated example, metal can be collected in gas-tight metal
collection elements
3106, in the form of open-bottomed hoods, located in, or forming the top of,
the cathode
compartments 3022 and partially or fully submerged below the level of the
electrolyte. The depth
of submergence can be selected such that the density difference between the
metal and molten
salt electrolyte generates sufficient head to push the metal up and into the
lithium extraction
assembly, including lithium metal extraction conduit 3082. The depth can be
arranged such that
the head is sufficient to drive the metal completely out of the cell, or a
small vacuum may be used
to withdraw the metal. In this arrangement, shielding gas is not required to
protect the metal from
reactions 9 and 10.
100230] A test campaign was completed on one example of a full-scale
electrolyzer according
to the teachings described herein. The cell in the test electrolyzer was
equipped with two electrode
pairs disposed in a common anolyte bath, each having an anode, cathode and
cathode
housing/membrane as described herein, and a power supply capable of delivering
several
thousand amps of current. The cell was fed with lithium carbonate feed
material and operated
49
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
over several hours under conditions described herein, during which period
lithium metal was
produced and collected.
100231] Figure 12 is a plot showing data gathered during this test campaign,
and specifically
shows current density, applied voltage and gas analysis over a span of five
hours, during which
the current was increased in steps, producing periods of operation at 0.2 A /
cm2, 0.6 A / cm2, and
1.25 A / cm2, with the majority of the testing time being spent operating at
the highest current
density.
100232] Despite operating the electrolyzer at different applied voltages
between 10 ¨ 15V for
the majority of the time during testing, no chlorine gas was detected in any
of the gas samples
taken, nor was any chlorine detected in an ambient atmosphere monitor that was
placed near the
roof of the cell, demonstrating that the apparatus constructed in accordance
with the teachings
herein can be operated at relatively high current density, and at a relatively
high process intensity,
with the attendant advantages of having relatively lower capital and operating
costs as compared
to some conventional systems.
[00233] In another example, during a test campaign
completed on a full-scale electrolyzer
apparatus/cell equipped with graphite anodes approximately 23kAh of charge
were passed
between a pair of electrode faces. The anode was removed and carbon loss from
the anode was
measured based on a comparison to the baseline condition of the anode. This
was compared
against the calculated stoichiometric carbon loss expected for the given
charge transfer. The
results of this comparison are tabulated in Table 2 below, and show that the
scale electrolyzer
apparatus based on the teachings described herein reduces consumption of
carbon by
approximately 25% as compared to the stoichiometric value. This shows some
relatively improved
economic characteristics compared to some conventional systems.
Table 2
Total Charge Passed
23.4 kAh
Total Carbon Loss
1.9 kg
Expected Theoretical Carbon
Loss
112 g / kAh
Actual Carbon Loss
82 g / kAh
CA 03145506 2022-1-24
WO 2021/012055
PCT/CA2020/051021
100234] In another example, an electrolyzer according
to the teachings described herein
was operated using a metal oxide semi-inert anode for a period of 12hrs. Metal
product samples
obtained from the apparatus were analyzed using glow discharge mass
spectrometry (GDMS).
The analysis results showing product composition in mass percent are presented
in Table 3.
Despite apparent contamination of the sample with electrolyte (as indicated by
the relatively high
chlorine content), the results indicate that a good quality crude metal was
produced.
Table 3
Li
98.1%
Na
0.7%
Ca
0.0029%
K
0.07%
Fe
0.0035%
Cl
1%
Other
0.1%
Total
100.0%
100235] While this invention has been described with reference to illustrative
embodiments and
examples, the description is not intended to be construed in a limiting sense.
Thus, various
modifications of the illustrative embodiments, as well as other embodiments of
the invention, will
be apparent to persons skilled in the art upon reference to this description.
It is therefore
contemplated that the appended claims will cover any such modifications or
embodiments.
100236] All publications, patents and patent applications referred to herein
are incorporated by
reference in their entirety to the same extent as if each individual
publication, patent, or patent
application was specifically and individually indicated to be incorporated by
reference in its
entirety.
51
CA 03145506 2022-1-24