Note: Descriptions are shown in the official language in which they were submitted.
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
ELECTRODE PARTICLES SUITABLE FOR BATTERIES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This is
a PCT International application which claims benefit and priority to U.S.
Provisional Patent Application Serial Numbers 62/875,299, 62/875,315 and
62/875,318 all
filed July 17, 2019 and U.S. Patent Application Serial Numbers 16/929,222,
16/929,233
and 16/929,248 all filed on July 15, 2020 and each entitled "ELECTRODE
PARTICLES
SUITABLE FOR BATTERIES," which are all incorporated herein in their entirety.
FIELD OF THE INVENTION
[0002] This
invention relates to batteries and particularly to materials useful for making
the anode for batteries and more particularly useful for the anode in metal
ion batteries.
BACKGROUND OF THE INVENTION
[0003]
Rechargeable lithium-ion batteries have been extensively adopted in many
portable systems and devices such as cell phones, tablets, computers, handheld
portable
tools and new devices that are being developed relying on the power and weight
advantages
of lithium ion batteries. The
advantages are light weight, high voltage, high
electrochemical equivalence and good conductivity. The broad uses and
acceptance of
lithium ion batteries has come through many advances and developments. One
area of
development for lithium-ion batteries has been focused on the anode or
negative electrode
of lithium-ion batteries where much has been accomplished.
[0004] The key
considerations for anodes for lithium ion batteries, especially for
portable devices is high volume and weight specific capacity and long battery
life over
many multiple charge and discharge cycles. In prior work, anode materials were
produced
with initial coulombic efficiency approaching 95% with long life through
coated and
graphitized carbon precursor materials. This is described in US 7,323,120 to
Mao et al.
where petroleum coke is ground to a preferred size, subjected to a solvent
coating process,
having the coating oxidatively stabilized at an elevated temperature and then
the whole
particle carbonized and graphitized at even higher temperature in an inert
environment.
The particles formed highly graphitic structures with a protective coating on
the surface
that protected the underlying graphite sheets from the electrolyte of the
battery. The
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
protective coating protects the edges of graphite sheets which are believed to
be
catalytically active for the electrolytes in batteries. The electrolytes
thereby decompose
the graphite sheets during the charging cycle and thereby quickly and
drastically reduce
the efficiency and storage capacity for lithium ions in the anode. The coating
created on
anode particles comprised a layer of poorly graphitizable material that when
graphitized
with the rest of the particle formed a more stable graphite with respect to
catalytic
decomposition from the electrolyte, but not suitable for itself intercalating
lithium ions.
But lithium ions are able to easily pass through the coating and intercalate
into the more
organized graphite sheets. Indeed, this is very good material with good
properties and good
cycle life. However, its production requires the use of substantial volumes of
solvent along
with multiple successive separate heat treatments in different atmospheres,
all of which
add up to be expensive. But, for high value uses where high specific capacity
is needed in
a compact space and minimal weight are important, this anode is currently most
advantageous.
[0005] The
most important parameters of graphite negative electrode materials for
lithium-ion batteries are the initial coulombic efficiency and specific
capacity. It has been
well known that highly crystalline graphite powders have high specific
capacity and very
poor initial coulombic efficiency and are not usable as negative electrode
material for
lithium-ion batteries. Through many years of extensive research and
development,
sophisticated processes have been developed to mitigate the problems related
to specific
capacity and the initial coulombic efficiency; the major solutions concentrate
on high
temperature graphitization and coating the particles with poorly graphitizable
carbon
before graphitization to provide protection from the electrolyte for the
underlying graphite
sheets in the particles. Because the mean average particle size of graphite
negative
electrode materials is smaller than 30 microns and individual particles must
be uniformly
coated with poorly graphitizable carbon, graphite negative electrode materials
are currently
manufactured through complicated processing steps. As a result, the production
cost is
high and for some coating processes, product yield is low.
[0006] With
all materials, higher performance at lower cost are continuous drivers and
any progress in either performance or cost would be very desirable.
2
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
BRIEF SUMMARY OF THE DISCLOSURE
[0007] The invention relates to a graphitic carbon powder comprising
particles having
a mean average particle size of between 1 p.m and 50 p.m where the particles
comprise at
least 99% by weight of carbon graphite with a modified surface comprising
carbide
compounds that comprise at least 5 ppm and no more than 1% by weight of the
particles.
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] A more complete understanding of the present invention and benefits
thereof
may be acquired by referring to the follow description taken in conjunction
with the
accompanying drawings in which:
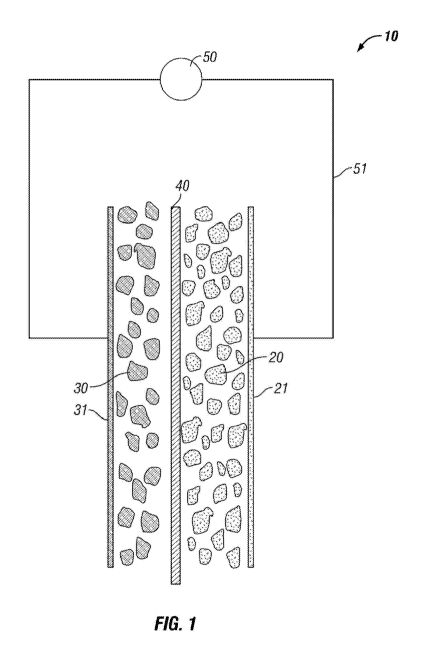
[0009] Figure 1 is schematic view of a battery cell in a hypothetical
circuit showing
the anode, cathode, electrolyte and a circuit.
DETAILED DESCRIPTION
[0010] Turning now to the detailed description of the preferred arrangement
or
arrangements of the present invention, it should be understood that the
inventive features
and concepts may be manifested in other arrangements and that the scope of the
invention
is not limited to the embodiments described or illustrated. The scope of the
invention is
intended only to be limited by the scope of the claims that follow.
[0011] First, turning to Figure 1, a schematic battery is indicated by the
arrow 10. The
battery includes multiple particles of cathode material 20 and multiple
particles of anode
material on the opposite side of an electrolyte separator 40. Each of the
particles of cathode
20 and anode 30 are held in an electrically conductive paste (not specifically
shown) to a
respective metal electrode. An electric load, indicated at 50, such as a light
or electric
motor may be attached to the battery 10 with wiring shown at 51. When battery
10 is
charged, positive ions are stored in the anode particles 30. Due to the
electro-chemical
natures of the cathode and anode materials, the positive ions are urged
(attracted and
repelled, respectively) to move from the anode 30 through the electrolyte
separator 40 and
into the cathode. While the ions move through the electrolyte, electrons pass
around
through the metal electrode 31 and through the wiring 51 and load 50 to the
cathode to
balance the electrical charge. The process of passing the electrons through
the load causes
electrical work to be accomplished such as illuminating a light bulb or
turning an electric
3
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
motor. For lithium-ion batteries, the cathode is generally formed of a lithium
bearing
chemical structure that forms lithium ions during charging of the battery that
transit across
the separator 40 and intercalate into the anode. Anode materials are less
chemically
complex and high performing anode materials may densely store the lithium ions
in a
manner where they are easily liberated fully back to the cathode without
permanent
bonding into the anode. This invention focuses on the makeup of the anode
material used
in batteries like that shown in Figure 1.
[0012] While
carbon-coated anode materials have proven to be very attractive
properties for high value batteries where low weight and compact size are
important, in
contrast, there are lower value uses for batteries where weight and size are
not as critical.
Such lower value uses include fixed location energy storage devices where very
high
energy capacity is needed such as, for example, standby power for a power
distribution
grid.
[0013] In
looking at batteries to meet those needs, studies have been undertaken to
develop battery designs that use a larger volume of uncoated, graphitized
petroleum coke
materials to offset the expected decay of anode performance over multiple
cycles of
charging and discharging. In the process of exploring optimal graphitization
levels for
batteries, some graphite nucleating agents were added to accelerate the
formation of
graphitic structure at lower temperatures. However, the end result was a
rather high
performing anode material and further developmental work quickly turned to
understanding the nature of the new anode product and why it performed at a
higher level
than expected.
[0014] What is
believed to have occurred in these tests is that rather than nucleating
graphite formation, the nucleating agents have reacted with the carbon surface
of the
particles forming a carbide compounds or reacted with nitrogen gas to form
nitride
compounds at the surfaces of the particles. The carbide and nitride compounds
do not
appear to form deep into the particle thereby preserving the bulk of the
particle as
crystalline graphite for ion intercalation. The carbides and nitrides
apparently protect the
graphite structure from the electrolyte in the metal ion batteries thereby
preventing the
electrolyte from interacting with the graphite. It is commonly known that
electrolytes
4
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
break down the graphite in the anode and yet the small thickness of this
modified surface
has preserved the graphite sheets in the present invention.
[0015] Initial
studies began with boron as the nucleating agent. Since graphitizing
must be conducted in a non-oxygen environment or the carbon with burn-off
principally
forming carbon dioxide, graphitizing is typically performed under a non-oxygen
blanket
gas. Under a nitrogen blanketing gas, nitrides may also be formed on the
surface.
However, for some nucleating agents, the nitride form may boil off and not
remain on the
surface. The stable nitrides and/or carbides are best seen to form when the
graphitizing
temperature is above the melting point of the stable carbide or nitride
molecules but below
their boiling points. For nucleating agents that form stable nitrides that
boil below the
graphitizing temperature, other blanketing gases may be chosen that are inert.
Argon has
been successfully used in those circumstances. Referring to Table 1, potential
nucleating
agents are shown with representative carbides and nitrides that may be formed
during
graphitizing. Referring to Table 2, the respective coulombic efficiency and
specific
capacity are shown for representative batteries made with boron used as a
carbide or nitride
forming agent.
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
Table 1
Melting Point Boiling Melting
Point
Element Carbide Nitride
( C) Point ( C) ( C)
B4C 2350 >3500 BN 2967
Ce CeC2 2250 CeN
La LaC2 LaN
Mn Mn3C 1520 Mn2N3, MnN >1800
MoC 2577 MoN 2577
Mo
Mo2C 2687 Mo2N 2687
NbC 3608 4300 NbN 2050
Nb
Nb2C 3080
Si SiC 2830 Si3N4 1900
Ti TiC 3067 TiN 2947
VC 2810 VN 2050
V
V2C 2167
YC2 2400 YN
Zr ZrC 3532 ZrN 2952
[0016] The
carbide and nitride forming materials are blended with the powdered coke
at about 0.1 wt% to about 5 wt%. It is believed that the carbides and nitrides
form on the
surface of the particles as the underlying carbon forms the graphitic
structures within.
Thus, the inventive process for making the anode powder includes preparing the
graphite
precursor to the desired size by milling or other known process and adding a
suitable
amount of the carbide or nitride forming elements by blending together and
then subjecting
the blended mixture to graphite forming temperature for a time duration
sufficient to form
the surface chemistry and the underlying graphite structure. For some coke
materials, it
may be preferred to carbonize them to drive off hetero-atoms and other non-
carbon atoms
prior to graphitizing by calcining. Carbonizing is typically a heat-treating
process that is
6
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
below graphite temperatures but well elevated such as between 900 C and 1500 C
and
typically in a calciner to increase the carbon content of the coke to at least
92% or a higher
content such as 95% or at least 97%.
[0017]
Preferably, the surfaces of the particles are continuous in either carbides or
a
mixture of carbides and nitrides leaving no graphite exposed to the
electrolyte. It is also
preferred that the surface would be preferably smoother versus coarse or
jagged to the
extent that would be obtainable. Most graphite materials have a jagged surface
where the
graphite sheets are more prone to fracturing as the particles are sized. A
smooth surface is
believed to be much more resistant to electrolyte attack on the bulk graphite
structure and
that is achieved in the prior art by coating. The carbide surface can range
from a few atoms
thick, resulting in a modified surface that is a few nanometers thick and may
be thicker
depending on the selected carbide forming compound or compounds, but does not
alter the
jagged surface to the more desirable smooth surface. The weight content of
such a
carbide-forming surface or elements in the graphitized powders can range from
about 50
ppm up to about 5000 ppm, also depending on the selected compound or
compounds.
[0018] The
types of cokes and carbide-forming compounds were discovered to also
play important roles in forming desirable graphite anode materials. The
selected cokes are
preferably calcined or at least partially calcined at a temperature between
500 and 2000 C
before graphitization. Green cokes, particularly those with high volatile
matter may react
with the selected carbide-forming compound to form volatile compounds,
resulting in
evaporation of such elements before forming stable carbide at graphitization
temperature.
On the other hand, cokes that are carbonized or graphitized at a temperature
above 2000 C
are more chemically stable and do not have the chemical reactivity with the
selected
carbide-forming compounds such as salts and oxides, resulting in evaporation
of such
added salts or oxides during temperature ramping on graphitization.
[0019] The
atmosphere under which the mixtures of coke and carbide forming
compounds are graphitized is a factor in selecting such carbide forming
compounds.
Non-oxidizing gases such as argon, helium, and nitrogen are preferred for
graphitization.
However, in the case of nitrogen gas atmosphere, some carbide-forming elements
may also
react with nitrogen to form undesirable nitride compounds, particularly those
volatile
nitride compounds that dilute or diminish carbide contents. Thus, the
selection of the
7
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
carbide-forming compounds is limited to those elements that form high
temperature
carbides and/or nitrides. For graphitization in argon or other non-reactive
gases, the
preferred carbide-forming elements form stable carbides at temperatures above
2000 C.
In other words, the best results are where the melting point of the resulting
carbides is
above 2500 C, and preferably above 2700 C.
[0020]
Moreover, this form of anode material is not coated with a graphite precursor
(or carbon layer that is different from the bulk). The invention alters the
existing surface
to have carbide compounds or carbide and nitride compounds formed on the
surface that
protects the core of the particles through many charging and discharging
cycles. So,
without the highly graphitic crystallinity at the surface which is chemically
reactive with
the electrolyte the nitride or carbide or both at the surface cause the decay
of the bulk
graphite material to be substantially reduced or eliminated thereby reducing
one mode of
battery deactivation.
[0021] This
would suggest that anode material comprising coke whether from
petroleum or coal tar could be sized by any of a number of methods to get a
mean average
particle size so that most of the particles are between about 3 microns and up
to about 30
microns which could then be graphitized in an inert atmosphere up to about
3100 C.
[0022]
Measuring particle size is subject to many viewpoints. In the preferred
invention, particle size may be tailored to the battery use or to a battery
manufacturer's
specifications. Ideally, the particles are substantially similar size
considering variabilities
of milling, sieving and other sizing technology. And the fact that the
particles are not likely
to be spherical adds an additional level of complexity. Fortunately, particle
size
measurement does not need to be complicated. In general, using laser
diffraction or
imaging systems made by Malvern or Horiba using volume-based calculations
provides
reasonable accuracy for purposes of providing such anode powders for use in
lithium ion
batteries. And by these measurements, the mean average particle size within
the useful
powders are typically between 1 and 50 microns and more typically within a
narrower
range.
[0023] So,
this invention provides a new graphite electrode material for lithium-ion
batteries and also provides a simpler process for manufacturing such electrode
materials.
In one embodiment related to the graphite anode materials, the graphite
particles contain
8
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
metal or non-metal carbide and nitride components on particle surfaces, such a
carbide or
nitride content ranges between 5 ppm and 1% by weight, preferably between 50
ppm and
2000 ppm, but more preferably less than about 1500 ppm and even more
preferably
between about 100 ppm and about 1000 ppm. The carbide and nitride may be
single
element or a mixture of different elements. The amount that is blended into
with the carbon
precursor is between about 500 ppm and 10 weight percent, but more preferably
between
1000 ppm and 3 weight percent. The mean average particle size for the anode
particles
ranges between 3 and 30 microns and preferably between 3 and 25 microns.
[0024] The
process for producing the graphite materials includes two primary steps:
milling graphitizable carbon precursors to specified particle sizes and then
graphitizing the
resulting powders with the carbide and nitride forming materials at a specific
temperature
range. In a little more detail, the carbon precursors are selected from
petroleum and coal
tar cokes. Green cokes are preferred. The selected carbon precursors are
milled to a
powder having a mean particle size of less than 30 [tm, depending on specific
battery
requirements by any mechanical milling method such as ball-milling, knife-
milling,
impact-milling, and jet-milling. Typical mean particle sizes range from 3 [tm
to 25 [tm.
Optionally, the milled powders are carbonized in a non-oxidizing environment
to eliminate
non-carbon elements. It should be noted that sizing is preferred before
graphitizing as
graphitizing makes the particles more brittle yielding more jagged and
irregular shaped
particles which are more vulnerable to catalytic decomposition of the graphite
sheet
structures.
[0025] The
milled powders (carbonized or green) are combined with carbide and
nitride forming compounds and graphitized in an inert environment such as
nitrogen,
argon, helium or combinations thereof at the temperature higher than 2650 C,
preferably
between 2800 C and 3000 C. The carbide and nitride forming compounds may be
transition metals, non-metals, rare earth metals and combinations thereof. The
quantity of
the carbide or nitride forming compounds used is between 100 ppm and 10% by
weight of
the total mass, preferably between 0.05 wt% and 2 wt%.
9
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
Explanation of Examples
[0026] The
usefulness of such produced materials is assessed as the negative electrode
material (lithium intercalation) in coin cells with lithium metal as the
counter electrode.
The preparation procedure is described below:
[0027]
Electrode preparation -- Each electrode was fabricated with the following
steps:
Step 1) About 2 g of the graphitized powder and 0.043 g of carbon black, 0.13
g of
polyvinylidene difluoride (PVDF) (in 10 wt% solution (in N-methyl
pyrrolidinone (NMP))
were placed in a 25-ml plastic vial and shaken with about 3 g of 1/8" steel
balls for 10 min
in a mill to form uniform paste. Additional NMP was added to make the mixture
more
flowable as needed. Step 2) A thin film of the resulting paste was cast on a
copper foil or
aluminum foil with a doctor-blade coater. The resulting film was dried on a
hot plate at
120 C for at least 2 hours. Step 3) The dried film was trimmed to a 5-cm wide
strip and
densified through a roller press. Step 4) Three disks (1.5 cm in diameter) of
each film were
punched out with a die cutter as electrodes. The electrode weight was
determined by
subtracting the total weight of each disk by the weight of the disk substrate.
The electrode
composition was 92 wt% graphite, 6 wt% PVDF, and 2 wt% carbon black, and the
mass
loading was about 10 mg/cm2.
[0028] Each
coin cell was subjected to electrochemical tests. The coins each consists
of bottom can, lithium metal as the counter electrode, separator, disk
electrode, stainless
steel disk spacer, wave spring, and top can. These components were
sequentially placed
in the bottom can. The electrolyte was added to the separator before the disk
electrode was
stacked. An electrolyte of 1 M LiPF6 in 40 vol% ethylene carbonate, 30 vol%
dimethyl
carbonate, and 30 vol% diethylene carbonate mixture was used. After the top
can was
dropped onto the stack, the assembly was transferred to the coin cell crimper
and crimped
together.
[0029] The
electrochemical tests were performed on an electrochemical test station
with the different charge/discharge test programs for negative electrode and
positive
electrode materials, respectively, as follows:
[0030] As
negative electrode material for lithium-ion batteries - A) charging at a
constant current of -1.0 mA to 0.0 V, B) further charging at 0.0 volt for one
hour, C)
discharging at 1 mA until the voltage reached 2.0 volt, and D) repeating steps
A through C
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
times or for 5 cycles. The electrical charge passed during charging and
discharging on
each cycle was recorded and used to calculate the specific capacity and
coulombic
efficiency. All the tests were conducted at ambient temperature and the cells
were tested
in a glove box where oxygen and moisture levels were below 3 ppm.
Analysis of carbide and nitride forming element contents
[0031] After
graphitization, the powders are dissolved in acid solution and analyzed
for the elemental contents by standard inductively coupled plasma mass
spectrometry.
Example Set 1
[0032] Two
petroleum green coke samples were acquired from different sources and
dried, crushed, and milled to a mean average particle size of 5 p.m. The first
sample was
from a Phillips 66 refinery in Ponca City, Oklahoma and the second sample was
LXP from
a second Phillips 66 refinery in Lake Charles, Louisiana. Each of the powders
were
blended with 1 wt% and 2 wt% elemental boron (<1 p.m mean particle size) and
compared
to a sample of powder without boron. The mixtures were graphitized in argon
environment
at 2900 C and were subsequently assessed as a negative electrode material for
a lithium-
ion battery. For comparison, these anode powders were graphitized under same
conditions.
Table 2 lists the discharge specific capacities and initial coulombic
efficiencies for such
graphitized powders. Without boron, the initial coulombic efficiencies are
very low
(<40%) and the discharge capacities are also low (-300 mAh/g). Such materials
are not
suitable for use as a negative electrode material for lithium-ion batteries.
With boron, the
graphitized powders exhibit excellent properties as negative electrode
material for lithium-
ion batteries (high capacity> 350 mAh/g and initial coulombic efficiency>
91%).
Table 2
Initial Coulombic Efficiency (%) Specific Capacity (mAh/g)
Boron (wt%)
Coke Sample 1 Coke Sample 2 Coke Sample 1 Coke Sample 2
0 37 40 298 301
1 90.3 92.2 354.0 359.6
2 92.7 89.8 355.3 357.0
11
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
Example Set 2
[0033]
Additional coke sample powder of Coke Sample 1 from Example Set 1 was
graphitized with several blends of Boron and other carbide and nitride forming
elements.
Six examples were created each with 1.5 wt% of a blend. The blends comprised
boron and
cerium at three different ratios of boron to cerium of 1:10, 10:1, and 1:1.
These carbide
and nitride forming compounds were selected from metal and non-metal chemicals
and
graphitized in a nitrogen atmosphere at 2900 C. The graphitized powders were
evaluated
in the same way as those in Example Set 1. Table 3 lists the discharge
specific capacities
and initial coulombic efficiencies for such graphitized powders. The fourth
and fifth
columns show the elemental contents of the carbide and nitride forming
elements in the
powders after graphitization. The first three samples exhibited an initial
coulombic
efficiency greater than 91% and specific capacity greater than 335 mAh/g,
which
demonstrates that high performance anode graphite powders can be produced
economically
according to this invention.
[0034]
Referring to Table 3 below, it should be quite apparent that at 2900 C
graphitization temperature, the carbide forming element causes a physical
difference in the
resulting electrode that provides a huge boost to the initial coulombic
efficiency. The
carbide forming elements have high melting points and seem to cause the carbon
at the
surface to form carbide crystals or accept (accommodate) nitride crystals at
the surface that
both allow ions to pass easily in and out of the graphite while at the same
time protecting
the graphite from the electrolyte.
Table 3
Mixture of Carbide and Specific Coulombic
Cerium
Nitride forming capacity efficiency Boron (ppm)
elements (mAh/g) (%) (PPm)
B and Ce (1:10) 336.2 93.6 474 248
B and Ce (10:1) 342.3 91.9 3060 165
B and Ce (1:1) 340.0 94.0 185 174
Example Set 3
[0035] A
sample of green, anode grade petroleum coke that is typically used in making
anodes for aluminum smelting was dried at 100 C, crushed in a roller mill,
and pulverized
12
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
with a laboratory jet mill to a mean average particle size of 5 [tm. This
sample of coke has
a volatile content of 12 weight percent and was divided into six separate
samples. The first
three samples were blends of boron and cerium and the last three were silicon,
manganese
and yttrium at about 1.5 weight percent. Each group in separate small
crucibles was placed
in a large graphite container and graphitized at 2900 C for 15 minutes in an
argon gas
environment.
[0036] The graphitized powders were evaluated as anode material for lithium-
ion
batteries in coin cells, as described above. The critical parameters are the
specific discharge
capacity and initial coulombic efficiency, and the results were listed in
Table 4. The
contents of the carbide forming elements in the graphitized samples are listed
in Table 9.
The graphitized samples with a significant content of carbide forming elements
yielded an
excellent initial coulombic efficiency (>92%) and specific capacity, and those
with an
undetectable content of carbide forming element showed poor initial coulombic
efficiency
(<60%) and low specific capacity.
Table 4
Initial
Specific
o. C ulombic
Graphite sample capacity
efficiency
(mAh/g)
(%)
Example Set 3
Boron and Cerium (10:1) 352.7 91.7
Boron and Cerium (1:10) 339.1 93.7
Boron and Cerium (1:1) 335.8 93.7
Silicon 320.0 62.4
Manganese 323.8 66.3
Yttrium 315.0 93.9
Example Set 4
[0037] The same set of the mixtures as those in Example Set 3 was
graphitized in the
same way at a temperature of 2900 C but in a nitrogen gas environment. The
resulting
graphite powders were evaluated in the same way as Example Set 3. The
resulting specific
capacities and initial coulombic efficiencies for these samples are listed in
Table 5 below.
The measured properties are similar to those in Example Set 3 except that
yttrium that
showed diminished performance in the initial coulombic efficiency. The carbide
forming
13
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
material also forms nitrides with the nitrogen gas that evaporates at a
temperature lower
than the graphitization temperature and it is believed that the surface
treatment did not stay
on the particles rendering them unsuitable as anode material in a metal ion
battery.
Table 5
Initial
Specific
Coulombic
Graphite sample capacity
efficiency
(mAh/g)
(A)
Example Set 4
Boron and Cerium (10:1) 342.3 91.9
Boron and Cerium (1:10) 340.0 94.0
Boron and Cerium (1:1) 336.2 93.6
Silicon 319.1 56.9
Manganese 322.1 57.2
Yttrium 308.0 54.1
[0038] These examples show that the graphitized powders with the presence
of
carbide-forming elements exhibit excellent properties as anode material for
lithium-ion
batteries, the ones without a content of such carbide-forming element do not
have the
desirable property (low coulombic efficiency).
Example Set 5
[0039] For the Set 5 of the Examples, three grades of green petroleum coke
were dried
at 100 C, crushed in a roller mill, and pulverized with a laboratory jet mill
to a mean
average particle size of 5, 8, 11 and 15 p.m, respectively. The resulting coke
powders were
heated in nitrogen gas at 950 C for two hours to remove the volatile matter.
These coke
powders are labeled as A, B, and C in the examples described below where A is
an
aluminum anode grade petroleum coke, B is a premium petroleum coke of the type
that is
used for anodes in electric arc furnaces for making recycled steel, and C is a
lower grade
premium petroleum coke which has been used as a precursor for making anodes in
metal
ion batteries having elevated volatile content.
[0040] A sample of various coke particles are blended including 11 p.m
powder of coke
A, 5 and 8 p.m powders of coke B, and a 15 p.m powder of coke C along with two
carbide-
forming compounds (element boron and cerium oxide) with the weight content of
0.5%
and 1.5%. The resulting mixtures were graphitized under the same conditions as
Example
14
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
Set 4 and tested as anode material for lithium-ion batteries. The graphitized
samples are
labeled as A5, B5, B8, and C15, respectively in this example. The test results
were listed
in Table 6 below.
Table 6
Initial
Specific
Graphite o. C ulombic
capacity
sample efficiency
(mAh/g)
(%)
Example Set 5
All 348.2 94.4
B5 355.7 92.7
B8 356.2 93.6
C15 356.3 94.6
Comparative Example Set 1
[0041] The 5
um powder of coke A and the 5 and 8-micrometer powders of coke B
were graphitized in nitrogen gas environment without any carbide forming
elements under
the same condition as Example Set 4. The graphitized powders were evaluated as
anode
material for lithium-ion batteries in the same way as the above examples.
These samples
were labeled as AS, B5, and B8, respectively in this example. The test results
are also
listed in Table 7 under Comparative Example 1 below.
Table 7
Specific
Graphite Initial Coulombic
capacity
sample (mAh/g) efficiency (%)
Comparative Example Set 1
AS 313.9 53.6
B5 312.5 41.7
B8 309.9 38.2
Comparative Example Set 2
[0042] The 5
and 8 micrometer powders of coke B were coated with 8 wt% and 6 wt%
pitch using the solution phase precipitation method as described in US patent
7,323,120.
The pitch coating process involves several steps including a) dispersing the
coke powder
in an organic solvent, b) dissolving the selected pitch in the organic
solvent, c) heating both
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
the coke and pitch solution to an elevated temperature, d) mixing the two
solutions and
cooling the mixture under continuous agitation so that a certain heavy portion
of the
dissolved pitch precipitates out as solid film on coke particles, e)
separating the pitch-
coated coke particles from the solution by filtration, f) washing out the
residual pitch
solution on the coated coke particles using extra organic solvent and finally
drying the
pitch-coated particles. The pitch-coated powders were further processed by
oxidation in
air at an elevated temperature (below 350 C) so that the resulting particles
become
infusible and the coated pitch becomes less graphitizable than the bulk coke
core. This
process is typically named as stabilization. After pitch-coating and
stabilization, the
powders were graphitized under the same condition as Example Set 4. The
graphitized
powders were evaluated as anode material for lithium-ion batteries in the same
way as
before and the results are posted in Table 8 under Comparative Example 2
below.
Table 8
Initial
Specific
Graphite C ulombic
capacity
sample efficiency
(mAh/g)
(A)
Comparative Example 2
B5 325.4 95.0
B8 328.7 95.4
[0043] The
Sample Sets 3 and 4 were subjected to analytical testing to determine its
constituents after graphitization. The amounts of carbide and nitride forming
elements in
the anode material after testing are shown in in Table 9. Not all elements
could be
measured considering the intrinsically low levels and the capabilities of
inhouse testing
equipment.
16
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
Table 9
Graphite Element content (ppm)
sample B Ce Mn Si
Example Set 3
A 1670 14.6
493 3060
320 248 belowNot Not
detectable
63.5 tested tested
level (BDL)
72.9 BDL
12.2
Example Set 4
A 3060 165
185 174
474 248 belowNot Not
detectable
79.8 level (BDL) tested tested
51.1 BDL
8.36
[0044] In Sample
Set 5, an anode sample was made with 8 p.m premium coke by
graphitizing in nitrogen gas at 2900 and for fifteen minutes in a nitrogen
environment, with
a combination of Boron and another Carbide or Nitride forming element at a
ratio 1:3. The
weights are measured before graphitization. The results are shown in Table 10.
Table 10
Specific Initial
Carbide Element capacity Coulombic
(mAh/g) efficiency (%)
Example Set 5
B and Ce (0.5%, 1.5% by wt) 348.9 92.5
B and La (0.5%, 1.5% by wt) 343.9 92.6
B and Mo (0.5%, 1.5% by wt) 332.5 93.1
B and Nb (0.5%, 1.5% by wt) 332.6 92.5
B and Ti (0.5%, 1.5% by wt) 349.2 93.4
B and F (0.5%, 1.5% by wt) 334.4 92.1
[0045] The above
examples demonstrate that the graphite powders produced according
to this invention exhibit superior specific capacity and excellent initial
coulombic
efficiency compared to those made through the state-of-art processes and that
the process
17
CA 03147591 2022-01-14
WO 2021/011650
PCT/US2020/042133
is simple and the resulting graphite powders have different chemical
compositions on either
particle surface or bulk from those made with prior art processes.
[0046] In
closing, it should be noted that the discussion of any reference is not an
admission that it is prior art to the present invention, especially any
reference that may have
a publication date after the priority date of this application. At the same
time, each and
every claim below is hereby incorporated into this detailed description or
specification as
an additional embodiment of the present invention.
[0047]
Although the systems and processes described herein have been described in
detail, it should be understood that various changes, substitutions, and
alterations can be
made without departing from the spirit and scope of the invention as defined
by the following
claims. Those skilled in the art may be able to study the preferred
embodiments and
identify other ways to practice the invention that are not exactly as
described herein. It is
the intent of the inventors that variations and equivalents of the invention
are within the
scope of the claims while the description, abstract and drawings are not to be
used to limit
the scope of the invention. The invention is specifically intended to be as
broad as the
claims below and their equivalents.
18