Note: Descriptions are shown in the official language in which they were submitted.
I
CA 03148955 2022-01-27
DESCRIPTION
[Title of the Invention]
HETEROCYCLIC COMPOUND
[Technical Field]
[0001]
The present invention relates to a novel heterocyclic compound having the
effect of inducing
degradation of interleukin-1 receptor-associated kinase-M (IRAK-M) protein and
expected to be
useful for the prevention/treatment of cancer, fibrosis, infectious diseases,
etc., and a drug
containing the same.
[Background Art]
[0002]
Development of compounds that induce ubiquitination of target proteins and
proteasome
degradation by E3 ligase (referred to as Proteolysis Targeting Chimeras
(PROTAC or Specific
and Nongenetic IAP-dependent Protein Eraser (SNIPER) and the like in some
cases) has been
attempted for the purpose of treatment by reducing disease-related proteins
(Non-Patent
Documents 1 to 9). IRAK-M is a member of the IRAK family of protein kinases
and is a
pseudokinase having no kinase activity (Non-Patent Document 10). IRAK-M is
located
downstream of all Toll-like receptors (TLRs) except TLR3 and a protein which
acts as a negative
feedback regulator of the TLR/interleukin-1 (IL-1) receptor signaling pathway
in vivo (Non-
Patent Document 11). It is located and expressed in some epithelial cells,
including bile duct
epithelial cells, lung epithelial cells and intestinal epithelial cells, and
in immune cells, especially
myeloid cells. IRAK-M plays an important role in maintaining immune
homeostasis such as
induction of endotoxin tolerance by negatively controlling TLR-mediated
induction signals of
inflammatory cytokines in innate immunocompetent cells such as macrophages and
dendritic
cells. (Non-Patent Document 12). IRAK-M has been reported to contribute to
cancer growth by
contributing to immunosuppression by tumor-related macrophages in the tumor
microenvironment, bone marrow-derived immunosuppressive cells, dendritic cells
and so on
(Non-Patent Documents 13 to 15). Furthermore, IRAK-M has been reported to act
on alveolar
macrophages phagocytosis, defense against bacteria and collagen production
promoting ability,
and is involved in fibrosis, asthma, secondary infection after sepsis, and
infectious complications
of hematopoietic stem cell transplantation (Non-Patent Documents 16-18).
Therefore,
compounds that induce the degradation of IRAK-M by linking the X-Linked
Inhibitor of
Apoptosis Protein (XIAP) binder, which is a type of E3 ligase, and the IRAK-M
binder via a
linker can be promising therapeutic agents for cancer, fibrosis, infectious
diseases, and IRAK-M
protein-related diseases.
[0003]
Patent Document 1 reports a compound as an IRAK-M protein degradation inducer.
Patent Documents 2 and 3 report compounds as an IRAK (particularly IRAK-4)
protein
degradation inducer.
Patent Documents 4 to 16 report compounds that induce protein degradation by
using IAP
binders.
Patent Documents 17 to 20 reports compounds containing the structure of N-
(piperidine-4-y1)
thieno [3,2-d] pyrimidin-4-amine or N- (piperidine-4-y1) thieno [3,2-b]
pyridine-7-amine.
[Citation List]
Patent Document
[0004]
Patent Document 1: W02017/211924
Patent Document 2: W02019/099926
Patent Document 3: W02019/133531
Date Recue/Date Received 2022-01-27
2
CA 03148955 2022-01-27
Patent Document 4: W02018/066545
Patent Document 5: Japanese Unexamined Patent Application Publication No. 2013-
056837
Patent Document 6: W02016/169989
Patent Document 7: W02016/172134
Patent Document 8: W02017/011590
Patent Document 9: W02017/182418
Patent Document 10: W02017/201449
Patent Document 11: US2018/0118733 Al
Patent Document 12: US2018/0134688 Al
Patent Document 13: W02018/119448
Patent Document 14: W02018/119357
Patent Document 15: US2019/0119271 Al
Patent Document 16: US2019/0175612 Al
Patent Document 17: W02016/040330
Patent Document 18: W02013/019966
Patent Document 19: US2013/0040957 Al
Patent Document 20: CN103242341 A
Non-Patent Document
[0005]
Non-Patent Document 1: Science. 2017 Mar 17;355(6330) 1163-1167.
Non-Patent Document 2: Cell Chem Biol. 2018 Jan 18;25(1):67-77.e3.
Non-Patent Document 3: Cell Chem. Biol. 2017 Sep 21;24(9) 1181-1190.
Non-Patent Document 4: ACS Chem Biol. 2017 Apr 21;12(4):892-898.
Non-Patent Document 5: Cell Chem Biol. 2018 Jan 18;25(1):78-87.e5.
Non-Patent Document 6: Nat Rev Drug Discov. 2017 Feb;16(2):101-114.
Non-Patent Document 7: Nat Chem Biol. 2015 Aug;11(8):611-7.
Non-Patent Document 8: Chemistry & Biology, 2010, 17(6):551-555
Non-Patent Document 9: Chembiochem, 2005, 6(1):40-46
Non-Patent Document 10: J Biol Chem, 1999 Jul 2; 274(27): 19403-19410
Non-Patent Document 11: Cell, 2002 Jul 26; 110(2): 191-202
Non-Patent Document 12: Infect Dis Rep, 2010 Jan 1; 2(1). pii: e9
Non-Patent Document 13: Oncogene, 2011 May 26; 30(21): 2475-2484
Non-Patent Document 14: J Immunol, 2010 Oct 1; 185(7): 4223-4232
Non-Patent Document 15: Mol Immunol, 2007 Jul; 44(14): 3453-3461
Non-Patent Document 16: J Immunol, 2015 Feb 15; 194(4): 1894-1904
Non-Patent Document 17: J Clin Invest, 2006 Sep; 116(9): 2532-2542, Epub 2006
Aug 17
Non-Patent Document 18: J Immunol, 2010 Jun 1; 184(11): 6299-6308
[Summary of the Invention]
[Technical Problem]
[0006]
An object of the present invention is to provide a novel heterocyclic compound
and a
pharmaceutical compound containing thereof, which have an action of inducing
degradation of
IRAK-M protein and are expected to be useful for prevention and treatment of
cancer, fibrosis,
infectious diseases, etc.
[Solution to Problem]
[0007]
The present inventors have diligently studied to find an IRAK-M protein
degrader and
resultantly found that the compound represented by the following formula
provides an excellent
Date Recue/Date Received 2022-01-27
3
CA 03148955 2022-01-27
degradation-inducing activity of IRAK-M protein, and that the compound may be
useful for
prevention or treatment of cancer, fibrosis, infectious diseases and the like,
leading to completion
of the present invention.
[0008]
The present invention is below.
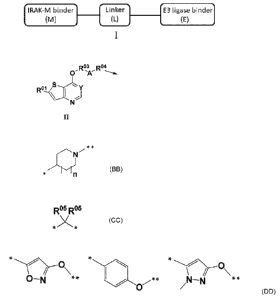
[1] A compound represented by the formula (I):
[0009]
IRAK-M binder Linker E3 ligase binder
(M) (L) (E)
, or a pharmaceutically acceptable salt thereof.
[2] The compound according to the above [1],
wherein the IRAK-M binder (M) is represented by the following formula (II):
[0010]
Rim Rim
0' 'AII
wherein Y represents CH or N, R 1 represents H or Me, R 3 represents a group
represented by the
following structural formula:
[0011]
* *
*
[wherein * represents the binding position to 0, ** represents the binding
position to A, and n is
an integer from 0 to 21, A is represented by the following structural formula:
[0012]
R 5 R 5
*X*
[wherein R 5 each independently represent a hydrogen atom or a C1-6 alkyl
group], or
R 4 is represented by a group selected from the following structural formulae:
[0013]
O-N
**
0
[wherein * represents the binding position to A, and ** represents the binding
position to the
linker], an optionally substituted C1-6 alkylene group, an optionally
substituted C3-10
cycloalkylene group, an optionally substituted C6-14 arylene group, or a bond,
and the arrow
Date Recue/Date Received 2022-01-27
4
CA 03148955 2022-01-27
represent the binding to the linker (L), or a pharmaceutically acceptable salt
thereof.
[3] The compound according to the above [2],
wherein the IRAK-M binder (M) is represented by the following formula (III):
[0014]
A01 ,,--
NI" 'IR"
(20
R -
oi S"---Y
N
III
wherein Y represents CH or N, R 1 represents H or Me, A 1 represents a group
represented by the
following structural formula:
[0015]
R 5 R 5
*x*
[wherein R 5 each independently represent a hydrogen atom or a C1-6 alkyl
group], or
R" ¨
x represents a group selected from the following structural formulae:
[0016]
* * *
kJ /
[wherein * represents the binding position to A 1, and ** represents the
binding position to a
linker], and the arrow represents the binding to the linker (L), or a
pharmaceutically acceptable
salt thereof.
[4] The compound according to the above [2],
wherein the IRAK-M binder (M) is a monovalent group derived from the compound
selected from
the group consisting of (5-((4- (thieno [3,2-b) ] pyridin-7-yloxy) piperidin-1-
y1) methyl)
isoxazol-3-01), 5-(((4-((2) 2) -methylthieno [3,2-b] pyridin-7-y1) oxy)
piperidin-1-y1) methyl)
isoxazol-3-01), 1-methyl-5-((( 4- (thieno [3,2-b] pyridin-7-yloxy) piperidin-1-
y1) methyl) -1H-
pyrazol-3-ol, and 4-((4- (thieno [3,2-d] pyrimidin-4-) yloxy) piperidin-1-y1)
sulfonyl) phenol, or
a pharmaceutically acceptable salt thereof.
[5] The compound according to any one of the above [1] to [4], wherein the
Linker (L) is a group
having 5 to 20 carbon atoms optionally containing a heteroatom, or a
pharmaceutically acceptable
salt thereof.
[6] The compound according to any one of the above [1] to [4], wherein the
Linker (L) is
represented by the following structural formula:
[0017]
Date Recue/Date Received 2022-01-27
5
CA 03148955 2022-01-27
0 * *
* __
[wherein * represents the binding to the IRAK-M binder (M)],
*-(CH2CH20)n(CH2)m(NRCO)s(CH2)t-* (n is a natural number from 1 to 5, m is 0,
1, or 2, and s
is 0 or 1, t is 0 or 1 and R represents a hydrogen atom or C1-6 alkyl group),
or a bond, or a
pharmaceutically acceptable salt thereof.
[7] The compound according to any one of the above [1] to [6], wherein the E3
ligase binder (E)
is represented by the following structural formula (IV):
[0018]
0
= 'L01
N RO2
Ros Ro3
RO6 N RO4
Ro7
R ----E
¨08
0
(IV)
wherein Rol, R02, R03, R04, Ros, Roo, R07 and R08 each independently represent
a hydrogen atom or
a C1-6 alkyl group which may form a ring with each other, D represents the
following formula
(V):
[0019]
W-c_vi
,9)n
( n.,
0 i
j\---NH
HN
H3C/ '-----T
(V)
[wherein m is an integer of 0 to 2, n is an integer of 0 to 2, Wii represents
a methylene group, a
difluoromethylene group, 0, S. SO, SO2, or NR, wherein R represents a hydrogen
atom, a C1-6
alkyl group, a C1-6 alkyl-carbonyl group, a C6-14 aryl-carbonyl group, or a C1-
6 alkylsulfonyl
group, and T represents an optionally halogenated C1-3 alkyl group], or the
following formula
(VI):
[0020]
P
\
Cl¨ __ NH
HN
CH3
(VI)
[wherein Q represents an oxygen atom, formula-NR2i- (wherein R21 represents a
hydrogen atom,
a C1-6 alkyl group, or a C1-6 alkyl group which may form a ring with P), or a
bond, P represents
a hydrogen atom, a C1-6 alkyl group or the binding to the Linker (L)
(including the formation of
Date Recue/Date Received 2022-01-27
6
CA 03148955 2022-01-27
a ring with Q and binding to the linker (L))], E represents the following
formula (VII):
[0021]
R26
R25
R21
1 /N
IR22
R24 R23
VII
[wherein R21, R22, and R23 each independently represent a hydrogen atom, a
halogen atom, a C1-6
alkyl group, a C1-6 alkoxy group, or an optionally substituted carbamoyl
group, and R25 and R26
each independently represent a hydrogen atom, a halogen atom, a C1-6 alkyl
group, a C1-6
alkoxy group, an optionally substituted carbamoyl group, or the binding to the
Linker (L); R24
represents a hydrogen atom, a methyl group, or the binding to the linker (L),
provided that the
binding to the linker (L) is any one of R24, R25, or R261, or the following
formula (VIII):
[0022]
R
R35 \
N R31
I
R32
R34 R33
VIII
[wherein R31, R32, R33, R34, and R35 each independently represent a hydrogen
atom, a halogen
atom, a C1-6 alkyl group, a C1-6 alkoxy group, or an optionally substituted
carbamoyl group, and
R represents a hydrogen atom, C1-6 alkyl group, or the binding to the linker
(L)], either D or E
binds to the linker (L), or a pharmaceutically acceptable salt thereof.
[8] The compound according to any one of the above [1] to [7], wherein the E3
ligase binder (E)
is represented by the following formula (IV):
[0023]
0
D¨ R01
N ___;02
Ro5 Ro3
Ro5
N RO4
R07
R08 ---- E
0
(IV)
wherein Rol, R02, R03, R04, R05, Roo, R07 and R08 each independently represent
a hydrogen atom or
a methyl group, D represents the following formula (V-1):
[0024]
Date Recue/Date Received 2022-01-27
7
CA 03148955 2022-01-27
iµ1_1:______
0
j\---NH
HN
----
H3C
(VA)
[wherein Wii represents a methylene group or difluoromethylene group], or the
following
formula (VI-1)
[0025]
CI NH
HN4
CH3
(VI-1)
[wherein Q represents the binding to the Linker (L)],
E represents the following formula (VII):
[0026]
R26
R25
R21
1 R22
R24 R23
VII
[wherein R21, R22 and R23 each independently represent a hydrogen atom, a
halogen atom, a C1-6
alkyl group, or a C1-6 alkoxy group; R25, and R26 each independently represent
a hydrogen atom,
a halogen atom, a C1-6 alkyl group, or the binding to the Linker (L); and R24
represents a
hydrogen atom, a methyl group, or the binding to the Linker (L), provided that
the binding to the
Linker (L) is any one of R24, R25 or R261, or the following formula (VIII):
[0027]
R
R35 \
N R31
I
R32
R34 R33
VIII
[wherein R31, R32, R33, R34 and R35 each independently represent a hydrogen
atom, a halogen
atom, or a C1-6 alkyl group; R represents a hydrogen atom, C1-6 alkyl group,
or the binding to
the Linker (L)], either D or E binds to the Linker (L), or a pharmaceutically
acceptable salt
thereof.
[9] The compound according to the above [1], wherein the IRAK-M binder (M) is
represented by
the following formula (III):
[0028]
Date Recue/Date Received 2022-01-27
8
CA 03148955 2022-01-27
A01
'R"
y
R .1
III
wherein Y represents CH or N, R01 represents H or Me, A 1 represents *-C1-12-*
or *-S02-*, Rn
represents a group selected from the following structural formula:
[0029]
0
[wherein * represents the binding position to A 1, and ** represents the
binding position to a
linker], and the arrow represent the binding to the linker (L)],
the linker (L) is represented by the following structural formula:
[0030]
0 * *
[wherein * represents the binding to the IRAK-M binder (M)],
*-(CH2C1-120)n(CH2)m(NRCO)s(CH2)t-* (n is a natural number from 1 to 5, m is
0, 1, or 2, and s
is 0 or 1, t is 0 or 1 and R represents a hydrogen atom or C1-6 alkyl group),
or a bond,
E3 ligase binder (E) is represented by the following formula (IV):
[0031]
0
Rol
N---1;02
R05 Rog
Rog RO4
R07
Rog
0
(IV)
[wherein Rol, Roz, R03, R04, Ros, Roo, R07 and R08 each independently
represent a hydrogen atom or
a methyl group; D represents the following formula (V-2):
[0032]
HN NH
-1¨
H3C
(V-2)
, or the following formula (VI-1)
Date Recue/Date Received 2022-01-27
9
CA 03148955 2022-01-27
[0033]
Q NH
HN
CH3
(VI-1)
[wherein Q represents the binding to the Linker (L)], and E represents the
following formula
(VII):
[0034]
R26
R25
R21
1 /N
1 R22
R24 R23
VII
[wherein R21, R22 and R23 each independently represent a hydrogen atom, a
halogen atom, a C1-6
alkyl group, or 1-6 alkoxy group; R25 and R26 each independently represent a
hydrogen atom, a
halogen atom, a C1-6 alkyl group, or the binding to the Linker (L); R24
represents a hydrogen
atom, a methyl group, or the binding to the Linker (L), provided that the
binding to the Linker (L)
is any one of R24, R25 or R261, or the following formula (VIII):
[0035]
R
R35 isi ,t
R31
I
R32
R34 R33
VIII
[wherein R31, R32, R33, R34 and R35 each independently represent a hydrogen
atom, or a C1-6 alkyl
group and R represents a hydrogen atom, or the binding to the Linker (L)],
either D or E binds to
the Linker (L)], or a pharmaceutically acceptable salt thereof.
[0036]
[10] The compound according to the above [1], which compound is selected from
the group
consisting of:
Compound 1: 2-(4-((S)-2-cyclohexy1-2-((S)-2-
(methylamino)propanamido)acetyl)piperazine-l-
carbony1)-5,6-difluoro-N,1-dimethyl-N-(2-(2-(2-45-44-(thieno[3,2-blpyridin-7-
yloxy)piperidin-
1-yOmethyDisoxazol-3-yDoxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide:
[0037]
Date Recue/Date Received 2022-01-27
10
CA 03148955 2022-01-27
\ 0
0---N
N---
0
0 N/Th
\7/40
NH
0
HN
[0038]
Compound 2: 2-(44(S)-2-cyclohexy1-2-((S)-2-
(methylamino)propanamido)acetyl)piperazine-1-
carbony1)-6-methoxy-1-methyl-N-(2-(2-(2-(4-44-(thieno[3,2-dlpyrimidin-4-
yloxy)piperidin-1-
yOsulfonyl)phenoxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide:
[0039]
/N
Nu?-0
S 0
N N
/NH
0 0
/NM
o_ro
errNH
0)
,,1111
HN
[0040]
Compound 3: 14(R)-4-(5,6-difluoro-1-methy1-1H-indole-2-carbony1)-2-
methylpiperazin-1-y1)-2-
42R,5R)-5-methyl-2-42-(2-(2-45-((4-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-
yOmethyDisoxazol-3-y1)oxy)ethoxy)ethoxy)ethoxy)methyDpiperazin-1-yDethan-1-
one:
[0041]
0
N/-
N
LS _______________________________________ 0
/ F
0/ oo
\./\
I
====,\\µµ NH
[0042]
Date Recue/Date Received 2022-01-27
1 1
CA 03148955 2022-01-27
Compound 4: (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-methy1-3-(2-(2-(2-45-
44-(thieno[3,2-
blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-yDoxy)ethoxy)ethoxy)ethoxy)-
1H-indole-2-
carbonyl)piperazin-1-y1)-2-oxoethyl)-2-(methylamino)propanamide:
[0043]
/¨\N
S
N 0-....N
\ _____________
F
F
I
0 N
\
cN,)
co.7NH
)=,,
HN ii/
I
[0044]
Compound 5: (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(3-methy1-2-oxo-14-45-
44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-yDoxy)-6,9,12-
trioxa-3-
azatetradecy1)-1H-indole-2-carbonyl)piperazin-1-y1)-2-oxoethyl)-2-
(methylamino)propanamide:
[0045]
/¨
N ¨C:0
US _________________________________________ F F
N 0_,
\ __________ uNN
o .
O---N--- 0
N"----NO----N.,--N /
111
0 NV''')
NH
\\\
O'µµ
HN
[0046]
Compound 6: (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(2-oxo-24(S)-2-
(((54(4-(thieno[3,2-
blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-1-
yl)ethyl)-1H-
indole-2-carbonyl)piperazin-1-y1)-2-oxoethyl)-2-(methylamino)propanamide:
[0047]
Date Recue/Date Received 2022-01-27
12
CA 03148955 2022-01-27
F
/_
N.,-0_ F II
0
N S I
N 0-, 0 N
\
N
)r--\N---
[0048]
Compound 7: (S)-N-((S)-1-cyclohexy1-2-(4-(5-fluoro-1-(2-oxo-2-((S)-2-(((5-44-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-1-
yl)ethyl)-1H-
indole-2-carbonyl)piperazin-1-y1)-2-oxoethyl)-2-(methylamino)propanamide:
[0049]
F
H 11:
L¨ S I r-NN N
)(N
N 0-, 0)._/ 0
\
... __ITN
\
0---1.11)1
[0050]
Compound 8: (S)-N-((S)-1-cyclohexy1-24(S)-4-(5,6-difluoro-1-(2-oxo-24(S)-2-(45-
44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-
yDoxy)methyl)pyrrolidin-1-
ypethyl)-1H-indole-2-carbony1)-3-methylpiperazin-1-y1)-2-oxoethyl)-2-
(methylamino)propanamide:
[0051]
F
/¨
N? 0_ F 410
0
N S N I0\__/ N N
F
\ ______________
......: N
-f----\
0 HN----
[0052]
Compound 9: (S)-N-((S)-1-cyclohexy1-24(S)-4-(5-fluoro-1-(2-oxo-2-((S)-2-(((5-
44-(thieno[3,2-
blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-1-
yl)ethyl)-1H-
indole-2-carbony1)-3-methylpiperazin-1-y1)-2-oxoethyl)-2-
(methylamino)propanamide:
[0053]
Date Recue/Date Received 2022-01-27
13
CA 03148955 2022-01-27
F
N"
/0
\
. 1 0
I --------\N.-NH F
Ls L
N CL N
0 NN..... j
)r--"\N----
\
Ul
[0054]
Compound 10: (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-4-(2-(2-(2-
(24(54(4-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyl)-2-
(methylamino)propanamide:
[0055]
/¨
N ____ 0
US _____
N ____________ 0-, N F
________ \
.....,.k F
0
N ---
/----\ 0
NH
)----(
HN-
, and
[0056]
Compound 11: (S)-N-((S)-1-cyclohexy1-2-(4-(2-methy1-1-(2-(2-(2-(2-45-44-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethyl)-1H-
indole-5-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide:
[0057]
/
o).\_____31____(-111
N ___ ?-0 ---
US _____________________________________________ N
N _____________ C)07-r 0
\((
0,-N
, or a
pharmaceutically acceptable salt thereof.
[0058]
[11] A medicament containing the compound according to any one of the above
[1] to [10], or a
pharmaceutically acceptable salt thereof.
[12] The medicament according to the above [11], wherein the drug is an IRAK-M
protein
degradation inducer.
Date Recue/Date Received 2022-01-27
14
CA 03148955 2022-01-27
[13] The medicament according to the above [11] or [12], which is a
prophylactic or therapeutic
agent for cancers.
[14] The medicament according to any one of the above [11] to [13], which is
used in
combination with another anticancer agent.
[15] A method for inducing IRAK-M protein degradation, comprising
administering to a patient
in need of treatment an effective amount of the compound or salt thereof
according to any one of
the above [1] to [10].
[16] A method for prevention or treatment of cancers, comprising administering
to a patient in
need of treatment an effective amount of the compound or salt thereof
according to any one of the
above [1] to [10].
[Advantageous Effect of the Invention]
[0059]
The compound of the present invention has an activity of inducing the
degradation of IRAK-M
protein and may be useful as a prophylactic or therapeutic agent for cancer,
fibrosis and
infectious diseases.
[Brief Description of Drawings]
[0060]
FIG. 1 shows the results of the changes in tumor size over time in each group
when
subcutaneously administering the compounds of Examples 1, 6, 7, 8 and 9 three
times every three
days into a Lewis lung cancer cell inoculation model. Each compound was used
as its salt
shown in the figure. The mean standard error is shown in the figure.
[Description of Embodiments]
[0061]
Hereinafter, the present invention as well as compounds of the present
invention and the
method of producing them and their use will be illustrated with reference to
the exemplary
embodiments along with the preferred methods and materials which can be used
in practice of the
present invention. Unless otherwise specified in the sentences, any technical
terms and
scientific terms used in the present specification have the same meaning as
those generally
understood by those of ordinary skill in the art to which the present
invention belongs. Any
materials and methods equivalent or similar to those described in the present
specification can be
used for practicing the present invention. All publications and patents cited
herein in
connection with the present invention described herein are incorporated by
reference, for
example, as indicating methodology, materials, etc. that can be used in the
present invention.
In addition, in the present specification, the description of "A - B"
indicating a numerical range
means a numerical range including A and B which are end points. The same
applies to "A to B".
In the present specification, "Me" means a methyl group unless it has a
distinctly different
meaning depending on the context.
In the present specification, when describing a compound name of a substituent
and so on, a
common name may be used instead of the proper name, but they mean the same
compound.
[0062]
Hereinafter, the definition of each substituent used in the present
specification will be
described in detail. Unless otherwise specified, each substituent has the
following definition.
[0063]
In the present specification, the "halogen atom" includes, e.g., fluorine,
chlorine, bromine and
iodine.
[0064]
In the present specification, the "C1-3 alkyl group" includes e.g., methyl,
ethyl, propyl,
Date Recue/Date Received 2022-01-27
15
CA 03148955 2022-01-27
isopropyl and cyclopropyl.
[0065]
In the present specification, the "optionally halogenated C1-3 alkyl group"
includes, e.g., a Cl-
3 alkyl group optionally having 1 to 5 halogen atoms. Specific examples
thereof include
methyl, chloromethyl, fluoromethyl, dichloromethyl, difluoromethyl,
trichloromethyl,
trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl, tetrafluoroethyl,
pentafluoroethyl, 2-
fluoroethyl, 2,2-difluoroethyl, propyl, 2,2-difluoropropyl, 3,3,3-
trifluoropropyl, isopropyl,
cyclopropyl, 1-fluorocyclopropyl, 2-chlorocyclopropyl, 2-fluorocyclopropyl,
2,2-
difluorocyclopropyl and 2,3-difluorocyclopropyl.
[0066]
In the present specification, the "C1-6 alkyl group" includes, e.g., methyl,
ethyl, propyl,
isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl,
neopentyl, 1-ethylpropyl, hexyl,
isohexyl, 1,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-
ethylbutyl, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl.
[0067]
In the present specification, the "optionally halogenated C1-6 alkyl group"
includes, e.g., a Cl-
6 alkyl group optionally having 1 to 7, preferably 1 to 5 halogen atoms.
Specific examples
thereof include methyl, chloromethyl, fluoromethyl, dichloromethyl,
difluoromethyl,
trichloromethyl, trifluoromethyl, ethyl, 2-bromoethyl, 2,2,2-trifluoroethyl,
tetrafluoroethyl,
pentafluoroethyl, propyl, 2,2-difluoropropyl, 3,3,3-trifluoropropyl,
isopropyl, butyl, 4,4,4-
trifluorobutyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl,
5,5,5-trifluoropentyl,
hexyl and 6,6,6-trifluorohexyl.
[0068]
In the present specification, the "C2-6 alkenyl group" includes, e.g.,
ethenyl, 1-propenyl, 2-
propenyl, 2-methyl-1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 3-methyl-2-
butenyl, 1-pentenyl,
2-pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-pentenyl, 1-hexenyl, 3-hexenyl
and 5-hexenyl.
[0069]
In the present specification, the "C1-6 alkylsulfonyl group" includes, e.g.,
methylsulfonyl,
ethylsulfonyl, propylsulfonyl, isopropylsulfonyl, butylsulfonyl, sec-
butylsulfonyl and tert-
butylsulfonyl.
[0070]
In the present specification, the "C6-14 aryl group" includes, e.g., phenyl, 1-
naphthyl, 2-
naphthy1, 1-anthril, 2-anthril and 9-anthril.
[0071]
In the present specification, the "C6-14 arylene group" include, e.g.,
phenylene, 1,5-
naphthylene, 1,4-naphthylene, 2,3-naphthylene, 1,8-anthrylene and 9,10-
anthrylene.
[0072]
In the present specification, the "C1-6 alkoxy group" includes, e.g., methoxy,
ethoxy, propoxy,
isopropoxy, butoxy, isobutoxy, sec-butoxy, tert-butoxy, pentyloxy and
hexyloxy.
[0073]
In the present specification, examples of the "hydrocarbon group" (including
"hydrocarbon
group" of "optionally substituted hydrocarbon group") include, e. g., a C1-3
alkyl group, a C1-6
alkyl group, a C1-6 alkylene group, a C2-6 alkenyl group, a C6-14 aryl group
and a C6-14
arylene group. In the present specification, examples of the "optionally
substituted hydrocarbon
group" include a hydrocarbon group optionally having substituent(s) selected
from the following
Substituent group A
[0074]
"Substituent group A"
(1) a halogen atom,
(2) a C1-3 alkyl group,
Date Recue/Date Received 2022-01-27
16
CA 03148955 2022-01-27
(3) a C1-6 alkoxy group,
(4) an amino group.
[0075]
The number of the above-described substituent in the "optionally substituted
hydrocarbon
group" is, e.g., 1 to 5, preferably 1 to 3. When the number of the substituent
is two or more,
each substituent may be the same or different.
[0076]
In the present specification, the "optionally substituted C1-6 alkylene group"
or the "optionally
substituted C3-10 cycloalkylene group" includes, e.g., a C1-6 alkylene group
or a C3-10
cycloalkylene group, optionally having a substituent selected from Substituent
Group A described
above (a halogen atom, a C1-3 alkyl group, a C1-6 alkoxy group and an amino
group). The
number of the substituent is, e.g., 1 to 5. When the number of the substituent
is two or more,
each substituent may be the same or different.
[0077]
In the present specification, the "optionally substituted C6-14 aryl group" or
the "optionally
substituted C6-14 arylene group" includes, e.g., a C6-14 aryl group or a C6-14
arylene group,
optionally having a substituent selected from the above-mentioned Substituent
group A (a
halogen atom, a C1-3 alkyl group, a C1-6 alkoxy group and an amino group). The
number of
the substituent is, e.g., 1 to 3. When the number of the substituent is two or
more, each
substituent may be the same or different.
[0078]
In the present specification, the "optionally substituted carbamoyl group"
includes, e.g., a
carbamoyl group optionally having "one or two substituents selected from a Cl -
6 alkyl group, a
C2-6 alkenyl group and a C3-10 cycloalkyl group, each of which optionally
having 1 to 3
substituents selected from the above-mentioned Substituent group A (a halogen
atom, a C1-3
alkyl group, a C1-6 alkoxy group and an amino group)."
[0079]
In the present specification, the "C1-6 alkylene group" includes, e.g.,
methylene, 1,2-ethylene,
1,1-ethylene, 1,2-propylene, 1,3-propylene, 2,2-propylene, 1,4-butylene, 1,2-
butylene, 1,3-
butylene, 2,2-butylene, 1,5-pentylene, 3,3-pentylene and 1,6-hexylene.
[0080]
In the present specification, the "C3-10 cycloalkylene group" includes, e.g.,
1,1-
cyclopropylene, cis-1,2-cyclopropylene, trans-1,2-cyclopropylene, 1,1-
cyclobutylene, cis-1,2-
cyclobutylene, trans-1,2-cyclobutylene, cis-1,3-cyclobutylene, trans-1,3-
cyclobutylene, 1,1-
cyclopentylene, cis-1,2-cyclopentylene, trans-1,2-cyclopentylene, cis-1,3-
cyclopentylene, trans-
1,3-cyclopentylene, 1,1-cyclohexylene, cis-1,2-cyclohexylene, trans-1,2-
cyclohexylene, cis-1,3-
cyclohexylene, trans-1,3-cyclohexylene, cis-1,4-cyclohexylene, trans-1,4-
cyclohexylene, 1,1-
cycloheptynylene, 1,1-cyclooctynylene, 2,2-dimethy1-1,1-cyclopropylene, 2,3-
dimethy1-1,1-
cyclopropylene, 2,2,3,3,4,4-tetramethy1-1,1-cyclobutylene, 7,7-norcaranylene,
7,7-norpinanylene
and 7,7-norbornanylene.
[0081]
In the present specification, the "linker" refers to a chemical moiety
(structure) used to
conjugate a part of a subject compound to another compound. Exemplary linkers
are described
in the present specification. For example, in any compounds described in the
present
specification, a chemical structure used to conjugate a partial structure of a
compound and a
partial structure of another compound can be used as a linker, and corresponds
to the "linker"
referred to in the present specification.
[0082]
In the present specification, the "group having 5-20 carbon atoms optionally
containing a
hetero atom" is a linear or branched alkyl group or alkenyl group, a
cycloalkyl group, an aryl
Date Recue/Date Received 2022-01-27
17
CA 03148955 2022-01-27
group, an arylalkyl group or an alkyl aryl group having 5-20 carbon atoms,
which may contain at
least one hetero atom selected from N or 0, and groups bonded to the same
carbon atom can be
bonded together to form a ring.
[0083]
In the present specification, the term "bond" indicates a state in which two
adjacent groups are
bonded by a single bond. Further, when a plurality of the "single bonds" are
connected, it
indicates a state in which all of them are connected together by a single
bond.
[0084]
Hereinafter, each symbol of the formula (II) will be described.
Y is CH or N, preferably CH.
R 1 is H or Me, preferably H.
The arrow means the binding to the Linker (L).
[0085]
R 3 is a group represented by the following structural formula:
[0086]
* *
*
wherein * represents the binding position to 0, ** represents the binding
position to A, a
nd n is an integer of 0 to 2,
preferably a group represented by the following structural formula:
[0087]
**
N/
wherein * represents the binding position to 0, and ** represents the binding
position to
A.
[0088]
A is a group represented by the following structural formula:
[0089]
R 5 R 5
*x*
wherein R 5 each independently is a hydrogen atom or a C1-6 alkyl group, or
preferably *-CH2 -* or
[0090]
R 4 is any group selected from the following structural formulae:
[0091]
wherein * represents the binding position to A, and ** represents the binding
position to the
Date Recue/Date Received 2022-01-27
18
CA 03148955 2022-01-27
linker, an optionally substituted C1-6 alkylene group, an optionally
substituted C3-10
cycloalkylene group, an optionally substituted C6-14 arylene group, or a bond,
preferably any group represented by the above-mentioned structures.
[0092]
An example of the "substituent" of "an optionally substituted C1-6 alkylene
group" "an
optionally substituted C3-10 cycloalkylene group" or "an optionally
substituted C6-14 arylene
group" shown in R 4 is a substituent selected from the "Substituent group A."
The number of
the substituents is for example 1 to 3. When the number of the substituents is
two or more, the
respective substituents may be the same or different.
[0093]
Hereinafter, each symbol of the formula (IV) will be described.
R01, R02, R03, R04, R05, R06, R07 and Ros each independently represent a
hydrogen atom or a Cl-
6 alkyl group which may form a ring with each other, preferably each
independently represent a
hydrogen atom or a C1-6 alkyl group, more preferably each independently
represent a hydrogen
atom or a C1-3 alkyl group, further preferably each independently represent a
hydrogen atom or a
methyl group.
Either D or E binds to the linker (L).
[0094]
D is represented by the following formula (V):
[0095]
( ln
(
0
HN
H3C/
(V)
wherein m is an integer of 0 to 2, n is an integer of 0 to 2, Wii represents a
methylene group, a
difluoromethylene group, 0, S. SO, SO2, or NR, wherein R represents a hydrogen
atom, a C1-6
alkyl group, a C1-6 alkyl-carbonyl group, a C6-14 aryl-carbonyl group, or a C1-
6 alkylsulfonyl
group, and T represents an optionally halogenated C1-3 alkyl group,
or the following formula (VI):
[0096]
CI) __ NH
HN
CH3
(VI)
wherein Q represents an oxygen atom, formula -NR21- (wherein, R21 represents a
hydrogen atom,
a C1-6 alkyl group, or an alkyl group which may form a ring with P), or a
bond, P represents a
hydrogen atom, a C1-6 alkyl group or the binding to the Linker (L) (including
the formation of a
ring with Q and binding to the linker (L).
[0097]
D is preferably the following formula (V-2):
[0098]
Date Recue/Date Received 2022-01-27
19
CA 03148955 2022-01-27
:::0C-_____I
HWY-
NH
/ ''---
H3C ---
(V-2)
or the following formula (VI-1):
[0099]
Q ¨rs1H
HN
CH3
(VI-1)
wherein Q shows binding to the linker (L).
The structure of D can bind to the linker (L) in the position of P or Q in the
formula
(VI) or Q in the formula (VI-1).
[0100]
E is represented by the following formula (VII):
[0101]
R26
R25
R21
1 /N
1 R22
R24 R23
VII
wherein R21, R22, and R23 each independently represent a hydrogen atom, a
halogen atom, a C1-6
alkyl group, a C1-6 alkoxy group, or an optionally substituted carbamoyl
group, preferably R21,
R22, and R23 each independently represent a hydrogen atom, a halogen atom, a
C1-6 alkyl group,
or a C1-6 alkoxy group; R25 and R26 each independently represent a hydrogen
atom, a halogen
atom, a C1-6 alkyl group, a C1-6 alkoxy group, an optionally substituted
carbamoyl group, or the
binding to the linker (L), preferably R25 and R26 each independently represent
a hydrogen atom, a
halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, or the binding to the
linker (L); and R24
represents a hydrogen atom, a methyl group, or the binding to the linker (L),
provided that the
binding to the linker (L) is any one of R24, R25, or R26,
or the following formula (VIII):
[0102]
R
R35 \
N R31
I
R32
R34 R33
VIII
wherein R31, R32, R33, R34 and R35 each independently represent a hydrogen
atom, a halogen atom,
a C1-6 alkyl group, a C1-6 alkoxy group, or an optionally substituted
carbamoyl group,
Date Recue/Date Received 2022-01-27
20
CA 03148955 2022-01-27
preferably R31, R32, R33, R34 and R35 each independently represent a hydrogen
atom, a halogen
atom, or a C1-6 alkyl group; and R represents a hydrogen atom, C1-6 alkyl
group, or the binding
to the linker (L).
[0103]
Hereinafter, the linker (L) of the formula (I) will be described.
The linker (L) is preferably a group having 5 to 20 carbon atoms which may
contain a
heteroatom, is more preferably a group represented by the following structural
formula:
[0104]
0 *
* __
,
*-(CH2CH20)n(CH2)m(NRCO)s(CH2)t-* (n is a natural number from 1 to 5, m is 0,
1, or 2, s is 0
or 1, t is 0 or 1 and R represents a hydrogen atom or C1-6 alkyl group), or a
bond,
is further preferably a group represented by the following structural formula:
[0105]
0 * *
* __
wherein * represents the binding to the IRAK-M binder (M), or *-(CH2CH20)n-*
(n is a natural
number from 1 to 5).
[0106]
In the present specification, the "compound that adds a function" means a
ligand of any protein
present in a living body, a cell penetrating peptide (CPP), or a kinetophore
that keeps a
compound in the intestinal tract (e.g., polyethylene oxides capped with a
short-chain peptide, a
sugar and quaternary ammonium; etc.).
[0107]
In the present specification, the "IRAK-M protein related disease(s)" is a
disease or an illness
that is described or speculated in association to abnormalities in the IRAK-M
protein itself or its
regulation. Protein abnormalities include, but are not limited to, abnormal
expression or
enhancement of a protein in a living body, and the presence of mutant
proteins.
[0108]
A compound included in compound (I) can be used as a synthetic intermediate in
producing
another compound (I) of the present invention, and is also used as a synthetic
intermediate in
producing an IRAK-M protein degrader other than compound (I).
[0109]
When compound (I) is in a form of a salt, the salt includes, e.g., metal
salts, ammonium salts,
salts with organic base, salts with inorganic acid, salts with organic acid,
salts with basic or
acidic amino acid, and the like. Suitable examples of the metal salt include,
e.g., alkali metal
salts such as sodium salts, potassium salts and the like; alkaline-earth metal
salts such as calcium
salts, magnesium salts, barium salts and the like; aluminum salts, and the
like. Suitable
examples of the salt with organic base include, e.g., salts with
trimethylamine, triethylamine,
pyridine, picoline, 2,6-rutidine, ethanolamine, diethanolamine,
triethanolamine, cyclohexylamine,
dicyclohexylamine, N,N'-dibenzylethylenediamine and the like. Suitable
examples of the salt
with inorganic acid include, e.g., salts with hydrochloric acid, hydrobromic
acid, nitric acid,
sulfuric acid, phosphoric acid and the like. Suitable examples of the salt
with organic acid
Date Recue/Date Received 2022-01-27
21
CA 03148955 2022-01-27
include, e.g., salts with formic acid, acetic acid, trifluoroacetic acid,
phthalic acid, fumaric acid,
oxalic acid, tartaric acid, maleic acid, citric acid, succinic acid, malic
acid, methanesulfonic acid,
benzenesulfonic acid, p-toluenesulfonic acid and the like. Suitable examples
of the salt with
basic amino acid include, e.g., salts with arginine, lysine, ornithine and the
like, and suitable
examples of the salts with acidic amino acids include, e.g., salts with
aspartic acid, glutamic acid
and the like.
[0110]
Among them, pharmaceutically acceptable salts are preferred. For example, when
an acidic
functional group is present in a compound, the salt includes inorganic salts
such as alkali metal
salts (e.g., sodium salts, potassium salts, etc.) and alkaline-earth metal
salts (e.g., calcium salts,
magnesium salts, etc.) and ammonium salts, while when a basic functional group
is present in a
compound, the salt includes salts with inorganic acid such as hydrochloric
acid, hydrobromic
acid, nitric acid, sulfuric acid, phosphoric acid and the like, or salts with
organic acid such as
acetic acid, phthalic acid, fumaric acid, oxalic acid, tartaric acid, maleic
acid, citric acid, succinic
acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid and
the like.
[0111]
The production method of the compound of the present invention is explained in
the
followings. The raw material and reagent used and the compound obtained in
each step in the
following production method may be each in a form of a salt, and examples of
such salt include
those similar to the salts of the compound of the present disclosure.
[0112]
When the compound obtained in each step is in a free form, it can be converted
to the objective
salt according to a method known per se. When the compound obtained in each
step is in a salt
form, it can be converted to the free form or the objective other salt form
according to a method
known per se.
[0113]
The compound obtained in each step can be used directly as the resultant
reaction mixture or as
a resultant crude product for the next reaction. Alternatively, the compound
obtained in each
step can be isolated and/or purified from a reaction mixture according to a
method known per se,
for example, a separation means such as concentration, crystallization,
recrystallization,
distillation, solvent extraction, fractional distillation, column
chromatography and the like.
[0114]
When the raw material compound and reagent used in each step are commercially
available, the
commercially available product can also be used directly.
[0115]
In the reaction in each step, while the reaction time varies depending on the
kind of the reagent
and solvent to be used, it is generally 1 min to 48 hr, preferably 10 min to 8
hr, unless otherwise
specified.
[0116]
In the reaction in each step, while the reaction temperature varies depending
on the kind of the
reagent and solvent to be used, it is generally -78 C to 300 C, preferably -78
C to 150 C, unless
otherwise specified.
[0117]
In the reaction in each step, while the pressure varies depending on the kind
of the reagent and
solvent to be used, it is generally 1 atm to 20 atm, preferably 1 atm to 3
atm, unless otherwise
specified.
[0118]
Microwave synthesizer such as Initiator manufactured by Biotage and the like
may be used for
the reaction in each step. While the reaction temperature varies depending on
the kind of the
reagent and solvent to be used, it is generally room temperature to 300 C,
preferably 50 C to
Date Recue/Date Received 2022-01-27
22
CA 03148955 2022-01-27
250 C, unless otherwise specified. While the reaction time varies depending on
the kind of the
reagent and solvent to be used, it is generally 1 min to 48 hr, preferably 1
min to 8 hr, unless
otherwise specified.
[0119]
In the reaction in each step, the reagent is used in an amount of 0.5
equivalent to 20
equivalents, preferably 0.8 equivalent to 5 equivalents, relative to the
substrate, unless otherwise
specified. When the reagent is used as a catalyst, the reagent is used in an
amount of 0.001
equivalent to 1 equivalent, preferably 0.01 equivalent to 0.2 equivalent,
relative to the substrate.
When the reagent is used as a reaction solvent, the reagent is used in a
solvent amount.
[0120]
Unless otherwise specified, the reaction in each step is carried out without
solvent, or by
dissolving or suspending the raw material compound in a suitable solvent.
Examples of the
solvent include those described in Examples and the following solvents.
alcohols: methanol, ethanol, tert-butyl alcohol, 2-methoxyethanol and the
like;
ethers: diethyl ether, diphenyl ether, tetrahydrofuran, 1,2-dimethoxyethane
and the like;
aromatic hydrocarbons: chlorobenzene, toluene, xylene and the like;
saturated hydrocarbons: cyclohexane, hexane and the like;
amides: N,N-dimethylformamide, N-methylpyrrolidone and the like;
halogenated hydrocarbons: dichloromethane, carbon tetrachloride and the like;
nitriles: acetonitrile and the like;
sulfoxides: dimethyl sulfoxide and the like;
aromatic organic bases: pyridine and the like;
anhydrides: acetic anhydride and the like;
organic acids: formic acid, acetic acid, trifluoroacetic acid and the like;
inorganic acids: hydrochloric acid, sulfuric acid and the like;
esters: ethyl acetate and the like;
ketones: acetone, methyl ethyl ketone and the like;
water.
The above-mentioned solvent can be used in a mixture of two or more kinds
thereof in an
appropriate ratio.
[0121]
When a base is used for the reaction in each step, examples thereof include
those described in
Examples and the following bases.
inorganic bases: sodium hydroxide, magnesium hydroxide and the like;
basic salts: sodium carbonate, calcium carbonate, sodium hydrogencarbonate and
the like;
organic bases: triethylamine, diethylamine, pyridine,
4-dimethylaminopyridine, N,N-dimethylaniline,
1,4-diazabicyclo[2.2.2loctane,1,8-diazabicyclo[5.4.01-7-undecene, imidazole,
piperidine and the
like;
metal alkoxides: sodium ethoxide, potassium tert-butoxide and the like;
alkali metal hydrides: sodium hydride and the like;
metal amides: sodium amide, lithium diisopropylamide, lithium
hexamethyldisilazide and the
like;
organic lithiums: n-butyllithium and the like.
[0122]
When an acid or an acid catalyst is used for the reaction in each step,
examples thereof include
those described in Examples and the following acids and acid catalysts.
inorganic acids: hydrochloric acid, sulfuric acid, nitric acid, hydrobromic
acid, phosphoric acid
and the like;
organic acids: acetic acid, trifluoroacetic acid, citric acid, p-
toluenesulfonic acid, 10-
Date Recue/Date Received 2022-01-27
23
CA 03148955 2022-01-27
camphorsulfonic acid and the like;
Lewis acid: boron trifluoride diethyl ether complex, zinc iodide, anhydrous
aluminum chloride,
anhydrous zinc chloride, anhydrous iron chloride and the like.
[0123]
Unless otherwise specified, the reaction in each step is carried out according
to a method
known per se, for example, the method described in Jikken Kagaku Kouza, 5th
Edition, vol.13-19
(the Chemical Society of Japan ed.); Shin Jikken Kagaku Kouza, vol.14-15 (the
Chemical Society
of Japan ed.); Fine Organic Chemistry, Revised 2nd Edition (L. F. Tietze, Th.
Eicher, Nankodo);
Organic Name Reactions, the Reaction Mechanism and Essence, Revised Edition
(Hideo Togo,
Kodansha); ORGANIC SYNTHESES Collective Volume 1-VII (John Wiley & Sons Inc);
Modern
Organic Synthesis in the Laboratory A Collection of Standard Experimental
Procedures (Jie Jack
Li, OXFORD UNIVERSITY); Comprehensive Heterocyclic Chemistry III, Vol.' -
Vol.14
(Elsevier Japan); Strategic Applications of Named Reactions in Organic
Synthesis (translated by
Kiyoshi Tomioka, Kagakudojin); Comprehensive Organic Transformations (VCH
Publishers
Inc.), 1989, or the like, or the method described in Examples.
[0124]
In each step, the protection or deprotection reaction of an functional group
is carried out
according to a method known per se, for example, the method described in
"Protective Groups in
Organic Synthesis, 4th Ed", Wiley-Interscience, Inc., 2007 (Theodora W.
Greene, Peter G. M.
Wuts); "Protecting Groups 3rd Ed." Thieme, 2004 (P.J.Kocienski), or the like,
or the method
described in Examples.
Examples of the protecting group for a hydroxy group of an alcohol and the
like or a phenolic
hydroxy group include ether-type protecting groups such as methoxymethyl
ether, benzyl ether,
tert-butyldimethylsilyl ether, tetrahydropyranyl ether and the like;
carboxylate ester-type
protecting groups such as acetate ester and the like; sulfonate ester-type
protecting groups such
as methanesulfonate ester and the like; and carbonate ester type protecting
groups such as tert-
butylcarbonate and the like.
Examples of the protecting group for a carbonyl group of an aldehyde include
acetal type
protecting groups such as dimethylacetal and the like; and cyclic acetal-type
protecting groups
such as 1,3-dioxane and the like.
Examples of the protecting group for a carbonyl group of a ketone include
ketal -type
protecting groups such as dimethylketal and the like; cyclic ketal-type
protecting groups such as
1,3-dioxane and the like; oxime-type protecting groups such as 0-methyloxime
and the like; and
hydrazone-type protecting groups such as N,N-dimethylhydrazone and the like.
Examples of the protecting group for a carboxyl group include ester-type
protecting groups
such as methyl ester and the like; and amide-type protecting groups such as
N,N-dimethylamide
and the like.
Examples of the protecting group for a thiol include ether-type protecting
groups such as
benzyl thioether and the like; and ester-type protecting groups such as
thioacetate ester,
thiocarbonate, thiocarbamate and the like.
Examples of the protecting group for an amino group and an aromatic
heterocycle such as
imidazole, pyrrole, indole and the like include carbamate -type protecting
groups such as benzyl
carbamate and the like; amide-type protecting groups such as acetamide and the
like; alkyl
amine-type protecting groups such as N-triphenylmethylamine and the like; and
sulfonamide-type
protecting groups such as methanesulfonamide and the like.
The protecting groups can be removed according to a method known per se, such
as a method
using acid, base, ultraviolet rays, hydrazine, phenylhydrazine, sodium N-
methyldithiocarbamate,
tetrabutylammonium fluoride, palladium acetate or trialkylsilyl halide (e.g.,
trimethylsilyl iodide,
trimethylsilyl bromide), or a reduction method.
[0125]
Date Recue/Date Received 2022-01-27
24
CA 03148955 2022-01-27
When reduction reaction is carried out in each step, examples of the reducing
agent to be used
include metal hydrides such as lithium aluminum hydride, sodium
triacetoxyborohydride, sodium
cyanoborohydride, diisobutylaluminum hydride (DIBAL-H), sodium borohydride,
tetramethylammonium triacetoxyborohydride and the like; boranes such as borane
tetrahydrofuran complex and the like; Raney nickel; Raney cobalt; hydrogen;
formic acid;
triethylsilane and the like. When carbon-carbon double bond or triple bond is
reduced, a method
using a catalyst such as palladium-carbon, Lindlar's catalyst and the like may
be employed.
[0126]
When oxidation reaction is carried out in each step, examples of the oxidizing
agent to be used
include peroxides such as m-chloroperbenzoic acid (mCPBA), hydrogen peroxide,
tert-butyl
hydroperoxide and the like; perchlorates such as tetrabutylammonium
perchlorate and the like;
chlorates such as sodium chlorate and the like; chlorites such as sodium
chlorite and the like;
periodic acids such as sodium periodate and the like; hypervalent iodine
reagents such as
iodosylbenzene and the like; reagents containing manganese such as manganese
dioxide,
potassium permanganate and the like; leads such as lead tetraacetate and the
like; reagents
containing chromium such as pyridinium chlorochromate (PCC), pyridinium
dichromate (PDC),
Jones reagent and the like; halogen compounds such as N-bromosuccinimide (NBS)
and the like;
oxygen; ozone; sulfur trioxido-pyridine complex; osmium tetroxide; selenium
dioxide; 2,3-
dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and the like.
[0127]
When radical cyclization reaction is carried out in each step, examples of the
radical initiator
to be used include azo compounds such as azobisisobutyronitrile (AIBN) and the
like; water-
soluble radical initiators such as 4,4'-azobis-4-cyanopentanoic acid (ACPA)
and the like;
triethylboran in the presence of air or oxygen; benzoyl peroxide and the like.
Examples of the
radical reagent to be used include tributylstannane, tristrimethylsilylsilane,
1,1,2,2-
tetraphenyldisilane, diphenylsilane, samarium iodide and the like.
[0128]
When Wittig reaction is carried out in each step, examples of the Wittig
reagent to be used
include alkylidene phosphoranes and the like. The alkylidene phosphoranes can
be prepared
according to a method known per se, for example, by reacting a phosphonium
salt with a strong
base.
[0129]
When Horner-Emmons reaction is carried out in each step, examples of the
reagent to be used
include phosphonoacetates such as methyl dimethylphosphonoacetate, ethyl
diethylphosphonoacetate and the like; and bases such as alkali metal hydrides,
organic lithiums
and the like.
[0130]
When Friedel-Crafts reaction is carried out in each step, examples of the
reagent to be used
include a combination of a Lewis acid and an acid chloride or a combination of
a Lewis acid and
an alkylating agent (e.g., an alkyl halide, an alcohol, an olefin).
Alternatively, an organic acid or
an inorganic acid can also be used instead of a Lewis acid, and an anhydride
such as acetic
anhydride and the like can also be used instead of an acid chloride.
[0131]
When aromatic nucleophilic substitution reaction is carried out in each step,
a nucleophile
(e.g., an amine, imidazole) and a base (e.g., a basic salt, an organic base)
are used as a reagent.
[0132]
When nucleophilic addition reaction by a carbo anion, nucleophilic 1,4-
addition reaction
(Michael addition reaction) by a carbo anion or nucleophilic substitution
reaction by a carbo
anion is carried out in each step, examples of the base to be used for
generation of the carbo
anion include organic lithiums, metal alkoxides, inorganic bases, organic
bases and the like.
Date Recue/Date Received 2022-01-27
25
CA 03148955 2022-01-27
[0133]
When Grignard reaction is carried out in each step, examples of the Grignard
reagent to be
used include arylmagnesium halides such as phenylmagnesium bromide and the
like;
alkylmagnesium halides such as methylmagnesium bromide and the like. The
Grignard reagent
can be prepared according to a method known per se, for example, by reacting
an alkyl halide or
an aryl halide with metal magnesium in an ether or tetrahydrofuran as a
solvent.
[0134]
When Knoevenagel condensation reaction is carried out in each step, a compound
having an
activated methylene group with two electron withdrawing groups (e.g., malonic
acid, diethyl
malonate, malononitrile etc.) and a base (e.g., an organic base, a metal
alkoxide, an inorganic
base) are used as a reagent.
[0135]
When Vilsmeier-Haack reaction is carried out in each step, phosphoryl chloride
and an amide
derivative (e.g., N,N-dimethylformamide etc.) are used as a reagent.
[0136]
When azidation reaction of an alcohol, an alkyl halide or a sulfonate is
carried out in each step,
examples of the azidating agent to be used include diphenylphosphorylazide
(DPPA),
trimethylsilylazide, sodium azide and the like. For example, for the azidation
reaction of an
alcohol, a method using diphenylphosphorylazide and 1,8-
diazabicyclo[5.4.01undec-7-ene (DBU),
a method using trimethylsilylazide and a Lewis acid, and the like are
employed.
[0137]
When reductive amination reaction is carried out in each step, examples of the
reducing agent
to be used include sodium triacetoxyborohydride, sodium cyanoborohydride,
hydrogen and
formic acid and the like. When the substrate is an amine compound, examples of
the carbonyl
compound to be used include paraformaldehyde, aldehydes such as acetaldehyde
and the like, and
ketones such as cyclohexanone and the like. When the substrate is a carbonyl
compound,
examples of the amine to be used include ammonia, primary amines such as
methylamine and the
like; secondary amines such as dimethylamine and the like, and the like.
[0138]
When Mitsunobu reaction is carried out in each step, an azodicarboxylate
(e.g., diethyl
azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD) etc.) and
triphenylphosphine are
used as a reagent.
[0139]
When esterification reaction, amidation reaction or ureation reaction is
carried out in each step,
examples of the reagent to be used include acyl halides such as acid
chlorides, acid bromides and
the like; activated carboxylic acids such as anhydrides, activated esters,
sulfates and the like.
Examples of the activating agent of the carboxylic acid include carbodiimide
condensing agents
such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (WSCD) and
the like;
triazine condensing agents such as 4-(4,6-dimethoxy 1,3,5-triazin-2-y1)-4-
methylmorpholinium
chloride n-hydrate (DMT-MM) and the like; carbonate condensing agents such as
1,1-
carbonyldiimidazole (CDI) and the like; diphenylphosphoryl azide (DPPA);
benzotriazol-1-yloxy-
trisdimethylaminophosphonium salt (BOP reagent); 2-chloro-l-methyl-pyridinium
iodide
(Mukaiyama reagent); thionyl chloride; lower alkyl haloformates such as ethyl
chloroformate and
the like; 0-(7-azabenzotriazol-1-y1)-N,N,N,N1-tetramethyluronium
hexafluorophosphorate
(HATU); sulfuric acid; 2,4,6-tripropy1-1,3,5,2,4,6-trioxatriphosphorinane-
2,4,6-trioxide (T3P);
combinations thereof and the like. When carbodiimide condensing agent is used,
an additive such
as 1-hydroxybenzotriazole (HOBt), N-hydroxysuccinimide (HOSu),
dimethylaminopyridine
(DMAP) and the like may be added to the reaction system.
[0140]
When coupling reaction is carried out in each step, examples of the metal
catalyst to be used
Date Recue/Date Received 2022-01-27
26
CA 03148955 2022-01-27
include palladium compounds such as palladium(II) acetate,
tetrakis(triphenylphosphine)palladium(0), dichlorobis(triphenylpho
sphine)palladium(II),
dichlorobis(triethylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
1,1'-bis(diphenylphosphino)ferrocene palladium(II) chloride, and the like;
nickel compounds
such as tetrakis(triphenylphosphine)nickel(0) and the like; rhodium compounds
such as
tris(triphenylphosphine)rhodium(III) chloride and the like; cobalt compounds;
copper compounds
such as copper oxide, copper(I) iodide and the like; platinum compounds and
the like. In
addition, a base can be added to the reaction system, and examples thereof
include inorganic
bases, basic salts and the like.
[0141]
When thiocarbonylation reaction is carried out in each step, phosphorus
pentasulfide is
typically used as the thiocarbonylating agent. Alternatively, a reagent having
a 1,3,2,4-
dithiadiphosphetane-2,4-disulfide structure (e.g., 2,4-bis(4-methoxypheny1)-
1,3,2,4-
dithiadiphosphetane-2,4-disulfide (Lawesson's reagent) etc.) can also be used
instead of
phosphorus pentasulfide.
[0142]
When Wohl-Ziegler reaction is carried out in each step, examples of the
halogenating agent to
be used include N-iodosuccinimide, N-bromosuccinimide (NBS), N-
chlorosuccinimide (NCS),
bromine, sulfuryl chloride and the like. In addition, the reaction can be
accelerated by subjecting
a radical initiator such as heat, light, benzoyl peroxide,
azobisisobutyronitrile and the like to the
reaction system.
[0143]
When halogenation reaction of a hydroxy group is carried out in each step,
examples of the
halogenating agent to be used include hydrohalic acids and acid halides of
inorganic acids,
specifically, hydrochloric acid, thionyl chloride, phosphorus oxychloride and
the like for
chlorination, 48% hydrobromic acid and the like for bromination. In addition,
a method of
producing an alkyl halide by reacting an alcohol with triphenylphosphine and
carbon
tetrachloride or carbon tetrabromide or the like can be employed.
Alternatively, a method of
producing an alkyl halide via two-step reaction comprising converting an
alcohol to the
corresponding sulfonate, and then reacting the sulfonate with lithium bromide,
lithium chloride or
sodium iodide can also be employed.
[0144]
When Arbuzov reaction is carried out in each step, examples of the reagent to
be used include
alkyl halides such as ethyl bromoacetate and the like; phosphites such as
triethyl phosphite,
tri(isopropyl) phosphite and the like.
[0145]
When sulfonate esterification reaction is carried out in each step, examples
of the sulfonating
agent to be used include methanesulfonyl chloride, p-toluenesulfonyl chloride,
methanesulfonic
anhydride, p-toluenesulfonic anhydride and the like.
[0146]
When hydrolysis reaction is carried out in each step, an acid or a base is
used as a reagent.
When acid hydrolysis reaction of t-butyl ester is carried out, formic acid,
triethylsilane and the
like may be added to reductively trap t-butyl cation which is by-produced.
[0147]
When dehydration reaction is carried out in each step, examples of the
dehydrating agent to be
used include sulfuric acid, diphosphorus pentoxide, phosphorus oxychloride,
N,N'-
dicyclohexylcarbodiimide, alumina, polyphosphoric acid and the like.
[0148]
When the alkylation reaction of alcohols or amines or aromatic heterocyclics
having an NH
group in the ring (e.g., imidazole, pyrazole) is performed in each step, the
alkylating agent
Date Recue/Date Received 2022-01-27
27
CA 03148955 2022-01-27
includes optionally substituted alkyl halides (e.g., iodomethane), optionally
substituted alkyls
having an optionally substituted C1-6 alkylsulfonyloxy group as a leaving
group, optionally
substituted alkyls having a C6-14 arylsulfonyloxy group optionally substituted
with a Ci_6 alkyl
group, sodium 2-chloro-2,2-difluoroacetate, 2,2-difluoro-2-
(fluorosulfonyl)acetate, and the like.
Further, the base to be used includes organic lithiums, metal alkoxides,
inorganic bases, organic
bases, and the like.
[0149]
When the fluorination reaction is performed in each step, the fluorinating
agent to be used
includes DAST (diethylaminosulfur trifluoride), bis(2-methoxyethyl)
aminosulfur trifluoride, 1-
chloromethy1-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis (tetrafluoroborate)
(Selectfluor), 4-
tert-buty1-2,6-dimethylphenylsulfur trifluoride (FLUOLEAD) and the like.
[0150]
When the Huisgen reaction is performed in each step, an azide compound and an
alkyne
compound are used as the agent used. The catalyst includes monovalent copper
ions, for example,
copper iodide, copper chloride, copper cyanide and the like.
[0151]
When the coupling reaction is performed in each step, the coupling reaction
includes Su
zuki coupling, Stille coupling, Buchwald-Hartwig coupling, Negishi coupling,
Mizoroki-Hec
k reaction, cyanation reaction using copper cyanide or zinc cyanide, and the
like. Reagen
ts such as metal catalysts, phosphine ligands, bases and the like used in the
coupling reac
tion can be used in methods known per se [methods described in, e.g., J. F.
Hartwig, S.
Shekhar, Q. Shen, F. Barrios-Landeros, in The Chemistry of Anilines, Z.
Rappoport, Ed.,
Wiley-Intersicence, New York (2007); L. Jiang, S. L. Buchwald, in Metal-
Catalyzed Cross-
Coupling Reactions, 2nd Ed., A. de Meijere, F. Diederich, Eds., Wiley-VCH,
Weinheim, G
ermany (2004); J. F. Hartwig, in Handbook of Organopalladium Chemistry for
Organic Sy
nthesis, A. de Meijere, F. Diederich, Eds., Wiley, New York (2002); J. F.
Hartwig, in Mo
dern Amination Methods, A. Ricci, Ed., Wiley-VCH, Weinheim, (2000)1 or methods
accord
ing to them, in addition to reagents described above.
[0152]
Hereinafter, a method for producing compound (I) will be described.
Each symbol in the following reaction schemes has the same meaning as
described above
unless otherwise specified. When a specific production method is not
described, a commercially
available raw material compound can be easily obtained, or a raw material
compound can be
produced by a method known per se or an equivalent method thereof, and a
method described in
Examples.
[0153]
When performing a reaction in each step, if there is a reactive site where a
reaction other than
the intended reaction occurs, a protecting group is introduced into the
reactive site in advance by
means known per se as necessary, and the desired reaction is performed, and
thereafter, the
protecting group may be removed also by means known per se.
For example, when the raw material compounds or the intermediates have an
amino group, a
carboxy group or a hydroxyl group as a substituent, these groups may be
protected with a
protecting group generally used in peptide chemistry and the like. In this
case, after the
reaction, the target compound can be obtained by removing the protecting
group(s) as necessary.
[0154]
Compound (I) can be synthesized from the compound (1) which is an IRAK-M
binder or the
compound (4) which is an E3 ligase binder by the method shown in the scheme
below. In each
scheme, compound (I) and each reaction intermediate may independently form
salts.
[0155]
Scheme 1:
Date Recue/Date Received 2022-01-27
28
CA 03148955 2022-01-27
[0156]
(2) (4) (1) (2)
___________ M-L _______ M-L-E _______ L-E
(1) (3) I (5) (4)
A
(2a) M-L1 L2-E (2b)
(3a) (5a)
[0157]
The compound (3) can be produced by subjecting the compound (1) or a reactive
derivative
thereof and the compound (2) which is a linker (L) or a reactive derivative
thereof to an
amidation reaction, a Mitsunobu reaction, an alkylation reaction, a coupling
reaction, and the
like. Compound (I) can be obtained by subjecting the compound (3) or a
reactive derivative
thereof and the compound (4) or a reactive derivative thereof to an amidation
reaction, the
Mitsunobu reaction, an alkylation reaction or a coupling reaction, and the
like.
[0158]
The compound (5) can be produced by subjecting the compound (4) or a reactive
derivative
thereof and compound (2) or a reactive derivative thereof to an amidation
reaction, a Mitsunobu
reaction, an alkylation reaction, a coupling reaction, and the like. Compound
(I) can be
obtained by subjecting the compound (5) or a reactive derivative thereof and
the compound (1) or
a reactive derivative thereof to an amidation reaction, the Mitsunobu
reaction, an alkylation
reaction, a coupling reaction, and the like.
[0159]
The compound (3a) can be produced by subjecting the compound (1) or a reactive
derivative
thereof and compound (2a) or a reactive derivative thereof to an amidation
reaction, a Mitsunobu
reaction, an alkylation reaction, a coupling reaction, and the like. The
compound (5a) can be
produced by subjecting the compound (4) or a reactive derivative thereof and
compound (2b) or a
reactive derivative thereof to an amidation reaction, the Mitsunobu reaction,
an alkylation
reaction, a coupling reaction, and the like. Compound (I) can be obtained by
subjecting the
compound (3a) and the compouynd (5a) or reactive derivatives thereof to an
amidation reaction,
the Mitsunobu reaction, an alkylation reaction, a coupling reaction, the
Huisgen reaction and the
like.
[0160]
The method for producing the IRAK-M binder (M) (compound (1)) represented by
the
following formula (II), which constitutes a part of compound (I), will be
described below.
[0161]
Scheme 2
[0162]
Ro3 Ro4
X1 R 3
0- O'
R 30H (7) A-R 4 (9)
ROL yU
\ I
01
R )
(6) (8) II
[0163]
In the Scheme 2, Xi represents a halogen atom or a leaving group.
[0164]
The compound (8) can be produced by subjecting the compound (6) and the
compound
Date Recue/Date Received 2022-01-27
29
CA 03148955 2022-01-27
(7) to an aromatic nucleophilic substitution reaction, a coupling reaction,
and the like.
The compound (II) can be obtained by subjecting the compound (8) and the
compound (9) or a
reactive derivative thereof to an alkylation reaction, a sulfonylation
reaction, a reductive
amination reaction, and the like.
[0165]
The "L" (the compound (2)) is a linker (L) constituting a part of compound
(I), and "Li" (the
compound (2a)) and the "L2" (the compound (2b)) are a part of the linker (L),
all of which may be
a commercially available product to be used as is or can be produced by a
method known per se
or a method similar thereto.
[0166]
The production method will be described below when the "E" (the compound (4))
is a
compound represented by the following formula (IV-I) and is an XIAP binder
which is one of the
E3 ligase binders constituting a part of compound (I).
[0167]
Scheme 3
[0168]
%Nil( ln
(
,NõCO2H
CO2H (
R01 RO2 )õ
H21\1 0 0
HN 12304 R057c,NH (11)
RoiR02
(13) 0 r RoiR02
4-, H N N NH N
2R05.5<Ro3 __
Ro6 HNYR05 Ro3
R07 ROB RO2 NH R" R06-- NH RO4
R07 R08 07R8
(10) (12) (14)
0 0
HOE HOLE
(15) (15)
W11 Wl1
(
N,CO2H ) ( )n
co2H
0 (o m 0
Hp(
HN R 1Ro2
(11) (13) 02
Ros H N N
2R05. R03 HN---)Hr5 R03
IR06¨t<R04'
Ro7
R08 Ro62--N R 4 T R06¨N RO4
0 R07 R08 R07 R08
0 0
(16) (17) IV-I
[0169]
The compound (12) can be produced by subjecting the compound (10) and the
compound (11)
or a reactive derivative thereof to an amidation reaction and the like. The
compound (14) can be
produced by subjecting the compound (12) and the compound (13) or a reactive
derivative thereof
to an amidation reaction, and the like.
The compound (IV-I) can be produced by subjecting the compound (14) and the
compo
und (15) or a reactive derivative thereof to an amidation reaction, and the
like.
Further, the compound (16) can be produced by subjecting the compound (10) and
the
compound (15) or a reactive derivative thereof to an amidation reaction, and
the like. The
compound (17) can be produced by subjecting the compound (16) and the compound
(11) or a
reactive derivative thereof to an amidation reaction, and the like. The
compound (IV-I) can be
produced by subjecting the compound (17) and the compound (13) or a reactive
derivative thereof
to an amidation reaction, and the like.
[0170]
The production method will be described below when "E" (the compound (4)) is a
compound
represented by the following formula (IV-II):
Date Recue/Date Received 2022-01-27
30
CA 03148955 2022-01-27
[0171]
Scheme 4
[0172]
/4D¨P
Roir,rc Xi 0 Re H3C¨ 2 H3C¨ )
o2 N 0
i NH Roipo
Ros `-`03 (17) N
n (19)
RO6 N R 4 Ros RO3
Ro7
. ,08
R RO6 N RO4
R07 R08 RO6 N RO4
0 R07
R08
0 0
(16) (18) I \/-II
0
HOAE
(22)
X1 0 HN¨\ /Q¨P HN¨\ Q¨P
R9 R02 R01
Xi 0 Fl 3C 2 ___ FloC 2 __ /
1203 NH N 0
HN R04 (17) 1!5<Rizo3 (19) R01Ro2
Ros71( N H R08
\i5<
Ros RO6 NH R 4 Ros R03
R07 R08 R07 rs.08 R06 ¨NH
R04
R07
mos
(10) (20) (21)
[0173]
The compound (18) can be produced by subjecting the compound (16) and the
compound (17)
to an amidation reaction, and the like. The compound (IV-II) can be produced
by subjecting the
compound (18) and the compound (19) to an alkylation reaction, and the like.
Further, the compound (20) can be produced by subjecting the compound (10) and
the
compound (17) to an amidation reaction, and the like. The compound (21) can be
produced by
subjecting the compound (20) and the compound (19) to an alkylation reaction,
and the like. The
compound (IV-II) can be produced by subjecting the compound (21) and the
compound (22) or a
reactive derivative thereof to an amidation reaction, and the like.
[0174]
By converting substituents of compound (I) thus obtained or each intermediate
by means
known per se (that is, introduction of a substituent or conversion of a
functional group), another
compound contained in compound (I) can be obtained. Intermediates
corresponding thereto or
salts thereof can also be obtained.
[0175]
Compound (I) obtained by the above production method can be isolated and
purified by known
means, e.g., solvent extraction, solution pH conversion, phase transfer,
crystallization,
recrystallization or chromatography.
[0176]
When compound (I) contains an optical isomer, a stereoisomer, a regioisomer,
and a rotational
isomer, these are also contained as the compound (I), and each compound can be
obtained as a
single item by a synthesis method and a separation method known per se. For
example, when
compound (I) has an optical isomer, the optical isomer resolved from the
compound is also
included in the compound (I).
Here, the optical isomer can be produced by a method known per se.
[0177]
Compound (I) may be a crystal.
The crystal of compound (I) (hereinafter sometimes abbreviated as crystals of
the present
Date Recue/Date Received 2022-01-27
31
CA 03148955 2022-01-27
invention) can be produced by crystallization of compound (I) by applying a
crystallization
method known per se.
Compound (I) may be a pharmaceutically acceptable co-crystal or a salt
thereof. Here, the
co-crystal or the salt thereof mean a crystalline substance constituted of two
or more special
solids at room temperature, each having different physical properties (e.g.,
structure, melting
point, heat of fusion, hygroscopicity, solubility, and stability). The co-
crystal or the salt thereof
can be produced according to a co-crystallization method known per se.
Compound (I) may be a hydrate, a non-hydrate, a non-solvate, or a solvate.
Furthermore, deuterium converted materials obtained by converting 1H into
2H(D) are also
included in the compound (I).
Compound (I) may be labeled with an isotope (e.g., 3 H, '3 C, '4 C, 1 8 F, 3 5
s, 1 2 5 =
0 and the
like. The compound (I) labeled or substituted with an isotope can be used,
e.g., as a tracer (PET
tracer) for use in positron emission tomography (PET) and is expected to be
useful in fields such
as medical diagnosis and the like.
[0178]
Compound (I) may be used as a prodrug.
A prodrug of compound (I) means a compound which is converted into compound
(I) with a
reaction due to an enzyme, a gastric acid, etc. under the physiological
condition in a living body,
that is, a compound which is enzymatically oxidized, reduced, hydrolyzed, etc.
to be converted
into compound (I), or a compound which is hydrolyzed with gastric acid, etc.,
to be converted
into compound (I).
[0179]
A prodrug of compound (I) includes; a compound in which an amino group of
compound (I) is
acylated, alkylated or phosphorylated (e.g., compounds in which an amino group
of compound (I)
is eicosanoylated, alanylated, pentylaminocarbonylated, (5-methy1-2-oxo-1,3-
dioxolen-4-y1)
methoxycarbonylated, tetrahydrofuranylated, pyrrolidylmethylated,
pivaloyloxymethylated or
tert-butylated); a compound in which a hydroxy group of compound (I) is
acylated, alkylated,
phosphorylated or borated (e.g., compounds in which a hydroxy group of
compound (I) is
acetylated, palmitoylated, propanoylated, pivaloylated, succinylated,
fumarylated, alanylated or
dimethylaminomethylcarbonylated); a compound in which a carboxy group of
compound (I) is
esterified or amidated (e.g., compounds in which a carboxy group of compound
(I) is
ethylesterified, phenylesterified, carboxymethyl esterified,
dimethylaminomethyl esterified,
pivaloyloxymethyl esterified, ethoxycarbonyloxyethyl esterified, phthalidyl
esterified, (5-methyl-
2-oxo-1,3-dioxolen-4-yl)methyl esterified, cyclohexyloxycarbonylethyl
esterified or
methylamidated), and the like. These compounds can be produced from compound
(I) by a
method known per se.
[0180]
Further, a prodrug of compound (I) may be a compound which is converted to
compound (I)
under physiological conditions as described in "JYAKUHIN no KAIHATSU
(Development of
Pharmaceuticals)", Vol. 7, Design of Molecules, p. 163-198, published by
HIROKAWA SHOTEN
(1990).
In the present specification, the prodrug may be in a form of a salt, and
examples of such salt
include those exemplified as the salt of the compound represented by the
formula (I) described
above.
[0181]
Compound (I) may be conjugated with a compound that adds a function, e.g., a
cell penetrating
peptide (CPP), or a kinetophore which keeps a compound in the intestinal tract
(e.g.,
polyethylene oxides capped with a short-chain peptide, sugar and quaternary
ammonium; etc.),
and the compound (I) may bind directly or via a linker to a compound that adds
a function.
[0182]
Date Recue/Date Received 2022-01-27
32
CA 03148955 2022-01-27
Compound (I) can also be used as a payload (the moiety corresponding to the
drug described
above) in an antibody (or peptidic antigen recognition sequence) -drug
conjugate. When
compound (I) is used as a payload, compound (I) may bind to an antibody (or a
peptidic antigen
recognition sequence) directly or via a linker. When compound (I) is used as a
payload, the
linker as described in Chem. Rev., 114, 9154-9218 (2014), Pharma. Res. 32,
3526-3540 (2015),
Bioconjugate Chem. 21, 5-13 (2010), The AAPS journal, 17, 339-351 (2015),
W02011/005761,
and the like, may be used in addition to the linkers exemplified in the
present specification.
[0183]
Compound (I) or a prodrug thereof (herein, these may be collectively
abbreviated as "the
compound of the present invention") has activity of inducing degradation of
IRAK-M and can be
useful as a prophylactic or therapeutic agent for cancer, a cancer growth
inhibitor, a cancer
metastasis inhibitor.
The compound of the present invention exhibits activity of inducing protein
degradation of
IRAK-M, and the compound of the present invention is useful as a medicament,
since it is
superior in the points in terms of drug efficacy, pharmacokinetics (e.g.,
absorption, distribution,
metabolism, excretion), solubility (e.g., water solubility), interaction with
other medicaments
(e.g., drug-metabolizing enzyme inhibitory action), safety (e.g., acute
toxicity, chronic toxicity,
genetic toxicity, reproductive toxicity, cardiotoxicity, carcinogenicity,
central toxicity), and
stability (e.g., chemical stability, stability to an enzyme). Among them, it
is expected to be
effective for the treatment or prophylaxis of cancer, but it is not limited to
this.
The compound of the present invention also has activity of inducing
degradation of IRAK-M
protein for mammals (e.g., mouse, rat, hamster, rabbit, cat, dog, cow, sheep,
monkey, and
human), and in view of the mechanism of action can be used as a medicament of
a prophylaxis or
treatment agent for diseases correlated with IRAK-M protein (herein, these may
be collectively
abbreviated as "IRAK-M associated diseases"): e.g., colon cancers (e.g., colon
cancer, rectal
cancer, anus cancer, familial colorectal cancer, hereditary nonpolyposis
colorectal cancer,
gastrointestinal stromal tumor), lung cancers (e.g., non-small-cell lung
cancer, small-cell lung
cancer, malignant mesothelioma), mesothelioma, pancreatic cancers (e.g.,
pancreatic ductal
adenocarcinoma, pancreatic endocrine tumor), pharyngeal cancer, laryngeal
cancer, esophageal
cancer, stomach cancers (e.g., papillary adenocarcinoma, mucinous
adenocarcinoma,
adenosquamous carcinoma), duodenal cancer, small intestinal cancer, breast
cancers (e.g.,
invasive ductal carcinoma, non-invasive ductal carcinoma, inflammatory breast
cancer), ovarian
cancers (e.g., ovarian epithelial cancer, extragonadal germ cell tumor,
ovarian germ cell tumor,
ovarian low-malignant potential tumors), testis tumors, prostate cancers
(e.g., hormone-dependent
prostate cancer, non-hormone dependent prostate cancer, castration-resistant
prostate cancer),
liver cancers (e.g., hepatocellular cancer, primary liver cancer, extrahepatic
bile duct cancer),
thyroid cancers (e.g., medullary thyroid carcinoma), renal cancers (e.g.,
renal cell carcinomas
(e.g., clear cell renal cell carcinoma), transitional cell cancer of the renal
pelvis and ureter),
uterine cancers (e.g., cervical cancer, uterine body cancer, uterus sarcoma),
gestational
choriocarcinoma, brain tumors (e.g., medulloblastoma, glioma, pineal
astrocytic tumor, pilocytic
astrocytoma, diffuse astrocytoma, anaplastic astrocytoma, pituitary adenoma),
retinoblastoma,
skin cancers (e.g., basalioma, malignant melanoma), sarcomas (e.g.,
rhabdomyosarcoma,
leiomyosarcoma, soft tissue sarcoma, spindle cell sarcoma), malignant bone
tumor, bladder
cancer, blood cancers (e.g., multiple myeloma, leukemias (e.g., acute myeloid
leukemia, chronic
lymphocytic leukemia), malignant lymphoma (B-cell lymphoma, diffuse large B-
cell lymphoma,
MALT lymphoma, follicular lymphoma, mantle cell lymphoma), Hodgkin's disease,
chronic
myeloproliferative disease), cancer of unknown primary; an inhibition agent of
a cancer growth; a
suppression agent of metastasis; a promotion agent of apoptosis; or a
therapeutic agent of
precancerous lesions (e.g., myelodysplastic syndrome).
[0184]
Date Recue/Date Received 2022-01-27
33
CA 03148955 2022-01-27
Further, IRAK-M associated diseases other than cancer include, e.g., asthma,
inflammatory
bone disease, inflammatory lung disease, idiopathic pulmonary fibrosis,
inflammatory bowel
disease (e.g., Crohn's disease, ulcerative colitis, etc.), multiple sclerosis,
systemic inflammatory
response syndrome (SIRS), sepsis, infectious complications of hematopoietic
stem cell
transplantation, influenza infection, acute respiratory syndrome (COVID-19,
MERS, SARS),
acute bacterial meningitis, Helicobacter pylori infection, invasive
staphylococcus aureus
infection, tuberculosis, systemic fungal infection, herpes simplex virus
infection, varicella zoster
virus infection, human papillomavirus infection, acute viral encephalitis,
encephalitis, meningitis,
decreased immune function associated with infection and the like.
[0185]
The compound of the present invention may be administered orally or
parenterally as it is or in
a mixture with a pharmacologically acceptable carrier as a medicament to a
mammal (preferably,
humans).
Hereinafter, the medicament containing the compound of the present invention
(sometimes to
be abbreviated as "the medicament of the present invention") is described in
detail. Examples
of the dosage form of the medicament of the present invention include oral
preparations such as
tablets (e.g., sugar-coated tablets, film-coated tablets, sublingual tablets,
buccal tablets, orally
disintegrating tablets), pills, granules, powders, capsules (e.g., soft
capsules, microcapsule s),
syrups, emulsions, suspensions, films (e.g., orally disintegrating films, oral
mucosa-sticking
films) and the like. Further, examples of the dosage form of the medicament of
the present
invention include also parenteral preparations such as injections, drip
infusions, transdermal
absorption type preparations (e.g., iontophoretic transdermal absorption type
preparations),
suppositories, ointments, nasal preparations, pulmonary preparations, and eye
drops and the like.
Also, the medicament of the present invention may be a release control
preparation such as an
immediate-release preparation or a sustained-release preparation (e.g., a
sustained-release
microcapsule) and the like.
As a dosage form of the medicament of the present invention, a nanoparticle
preparation or a
preparation using a bacterial-derived membrane can also be used.
[0186]
The medicament of the present invention may be prepared by a known preparation
method
generally used in the field of preparation technology (e.g., the method
described in the Japanese
Pharmacopoeia). The medicament of the present invention may contain a suitable
amount of an
additive such as an excipient, a binder, a disintegrant, a lubricant, a
sweeting agent, a surfactant,
a suspending agent, an emulsifier, a colorant, a preservative, an aromatic, a
corrigent, a
stabilizer, a thickening agent and the like generally used in the field of
preparation as necessary.
Examples of the above-mentioned pharmacologically acceptable carriers include
these
additives.
[0187]
For example, tablet may be prepared using an excipient, a binder, a
disintegrant, a lubricant
and the like, and pill and granule may be prepared using an excipient, a
binder and a disintegrant.
Also, powder and capsule may be prepared using an excipient and the like,
syrup may be
prepared using a sweeting agent and the like, and emulsion or suspension may
be prepared using
a suspending agent, a surfactant, an emulsifier and the like.
[0188]
Examples of the excipient include lactose, white sugar, glucose, starch,
sucrose, crystalline
cellulose, powdered glycyrrhiza, mannitol, sodium hydrogencarbonate, calcium
phosphate and
calcium sulfate.
Examples of the binder include 5 to 10 wt% starch liquid paste, 10 to 20 wt%
gum arabic
solution or gelatin solution, 1 to 5 wt% tragacanth solution, carboxymethyl
cellulose solution,
sodium alginate solution and glycerin.
Date Recue/Date Received 2022-01-27
34
CA 03148955 2022-01-27
Examples of the disintegrant include starch and calcium carbonate.
Examples of the lubricant include magnesium stearate, stearic acid, calcium
stearate and
purified talc.
Examples of the sweeting agent include glucose, fructose, invert sugar,
sorbitol, xylitol,
glycerin and simple syrup.
Examples of the surfactant include sodium lauryl sulfate, polysorbate 80,
sorbitan mono-fatty
acid ester and polyoxyl 40 stearate.
Examples of the suspending agent include gum arabic, sodium alginate, sodium
carboxymethyl
cellulose, methyl cellulose and bentonite.
Examples of the emulsifier include gum arabic, tragacanth, gelatin and
polysorbate 80.
[0189]
For example, when the medicament of the present invention is a tablet, the
tablet may be
prepared, e.g., by adding an excipient (e.g., lactose, sucrose, starch), a
disintegrant (e.g., starch,
calcium carbonate), a binder (e.g., starch, gum arabic, carboxymethyl
cellulose, polyvinyl
pyrrolidone, hydroxypropyl cellulose) or a lubricant (e.g., talc, magnesium
stearate, polyethylene
glycol 6000) to the compound of the present invention, compression-molding
according to a
method known per se, and then, if necessary, coating it for the purpose of
taste masking, enteric
property or durability to give a tablet according to a method known per se. As
the coating agent
used for coating, e.g., hydroxypropylmethyl cellulose, ethyl cellulose,
hydroxymethyl cellulose,
hydroxypropyl cellulose, polyoxyethylene glycol, Tween 80, Pluronic F68,
cellulose acetate
phthalate, hydroxypropylmethyl cellulose phthalate, hydroxymethyl cellulose
acetate succinate,
Eudragit (methacrylic acid/acrylic acid copolymer, Rohm, Germany) and pigments
(e.g., iron
oxide red, titanium dioxide) may be used.
[0190]
Examples of the above-described injection include intravenous injection as
well as
subcutaneous injection, intradermal injection, intramuscular injection,
intraperitoneal injection,
drip injection and the like.
[0191]
Such injections are prepared according to a method known per se, that is, by
dissolving,
suspending or emulsifying the compound of the present invention in a
sterilized aqueous liquid or
oily liquid. Examples of the aqueous liquid include physiological saline,
isotonic solutions
containing glucose or other auxiliary drugs (e.g., D-sorbitol, D-mannitol,
sodium chloride) and
the like. The aqueous liquid may contain a suitable solubilizer, e.g., an
alcohol (e.g., ethanol), a
polyalcohol (e.g., propylene glycol, polyethylene glycol), a nonionic
surfactant (e.g., polysorbate
80, HCO-50). Examples of the oily liquid include sesame oil and soybean oil
and the like. The
oily liquid may contain a suitable solubilizing agent. Examples of the
solubilizing agent include
benzyl benzoate, benzyl alcohol and the like. In addition, the injection may
be blended with a
buffer (e.g., phosphate buffer, sodium acetate buffer), a soothing agent
(e.g., benzalkonium
chloride, procaine hydrochloride), a stabilizer (e.g., human serum albumin,
polyethylene glycol),
a preservative (e.g., benzyl alcohol, phenol) and the like. A prepared
injection solution may be
usually filled into an ampoule.
[0192]
The content of the compound of the present invention in the medicament of the
present
invention varies depending on the form of the pharmaceutical preparation, and
is usually about
0.01 to about 100 wt%, preferably about 2 to about 85 wt%, more preferably
about 5 to about 70
wt%, based on the whole preparation.
[0193]
The content of the additive in the medicament of the present invention varies
depending on the
form of the pharmaceutical preparation, and is usually about 1 to about 99.9
wt%, preferably
about 10 to about 90 wt%, based on the whole preparation.
Date Recue/Date Received 2022-01-27
35
CA 03148955 2022-01-27
[0194]
The compound of the present invention is stable and has low toxicity and may
be used safely.
The daily dose of the compound of the present invention varies depending on
the condition and
body weight of a patient, the kind of compound, administration route and the
like, in the case of,
for example, oral administration to patients for the purpose of treating
cancer, the daily dose for
an adult (body weight about 60 kg) is about 1 to about 1000 mg, preferably
about 3 to about 300
mg, and more preferably about 10 to about 200 mg, as the compound of the
present invention,
which may be administered once or in two or three divided doses.
[0195]
When the compound of the present invention is administered parenterally, it is
usually
administered in the form of a liquid (e.g., injection). The dose of the
compound of the present
invention varies depending on the subject of administration, target organ,
symptoms,
administration method and the like, and for example, it is usually about 0.01
to about 100 mg,
preferably about 0.01 to about 50 mg, more preferably about 0.01 to about 20
mg, as the
compound of the present invention, relative to 1 kg of body weight, which is
preferably given by
intravenous injection or subcutaneous injection.
[0196]
The compound of the present invention may be used concurrently with other
drugs.
Specifically, the compound of the present invention may be used together with
a medicament
such as hormonal therapeutic agents, chemotherapeutic agents,
immunotherapeutic agents, or
medicaments inhibiting the action of cell growth factors or their receptors
and the like.
Hereinafter, drugs that can be used in combination or concurrently with the
compound of the
present invention are abbreviated as concomitant drugs.
[0197]
As the "hormonal therapeutic agents" include fosfestrol, diethylstilbestrol,
chlorotrianisene,
medroxyprogesterone acetate, megestrol acetate, chlormadinone acetate,
cyproterone acetate,
danazol, allylestrenol, gestrinone, mepartricin, raloxifene, ormeloxifene,
levormeloxifene, anti-
estrogens (e.g., tamoxifen citrate, toremifene citrate), pill preparations,
mepitiostane,
testololactone, aminoglutethimide, LH-RH agonists (e.g. goserelin acetate,
buserelin, leuprorelin
acetate), droloxifene, epitiostanol, ethinyl estradiol sulfonate, aromatase
inhibitors (e.g.,
fadrozole hydrochloride, anastrozole, letrozole, exemestane, vorozole,
formestane), anti-
androgens (e.g., flutamide, bicalutamide, nilutamide, enzalutamide), 5a-
reductase inhibitors (e.g.,
finasteride, epristeride, dutasteride), adrenal cortex hormone drugs (e.g.,
dexamethasone,
prednisolone, betamethasone, triamcinolone), androgen synthesis inhibitors
(e.g., abiraterone),
retinoids and drugs that retard the metabolism of retinoids (e.g., liarozole),
thyroid hormone, and
their DDS (Drug Delivery System) preparations may be used.
[0198]
As the "chemotherapeutic agents", e.g., alkylating agents, antimetabolites,
anticancer
antibiotics and plant-derived anticancer agents may be used.
[0199]
As the "alkylating agents", e.g., nitrogen mustard, nitrogen mustard N-oxide
hydrochloride,
chlorambucil, cyclophosphamide, ifosfamide, thiotepa, carboquone, improsulfan
tosylate,
busulfan, nimustine hydrochloride, mitobronitol, melphalan, dacarbazine,
ranimustine, sodium
estramustine phosphate, triethylenemelamine, carmustine, lomustine,
streptozocin, pipobroman,
etoglucide, carboplatin, cisplatin, miboplatin, nedaplatin, oxaliplatin,
altretamine, ambamustine,
dibrospidium hydrochloride, fotemustine, prednimustine, pumitepa, ribomustine,
temozolomide,
treosulfan, trofosfamide, zinostatin stimalamer, adozelesin, cystemustine,
bizelesin and their
DDS preparations thereof may be used.
[0200]
As the "antimetabolits", e.g., mercaptopurine, 6-mercaptopurine riboside,
thioinosine,
Date Recue/Date Received 2022-01-27
36
CA 03148955 2022-01-27
methotrexate, pemetrexed, enocitabine, cytarabine, cytarabine ocfosphate,
ancitabine
hydrochloride, 5-FU drugs (e.g., fluorouracil, tegafur, UFT, doxifluridine,
carmofur,
galocitabine, emitefur, capecitabine), aminopterin, nelzarabine, leucovorin
calcium, thioguanine,
butocine, calcium folinate, levofolinate calcium, cladribine, emitefur,
fludarabine, gemcitabine,
hydroxycarbamide, pentostatine, piritrexim, idoxuridine, mitoguazone,
thiazofurin, ambamustine,
bendamustine and their DDS preparations thereof may be used.
[0201]
As the "anticancer antibiotics", e.g., actinomycin-D, actinomycin-C, mitomycin-
C,
chromomycin-A3, bleomycin hydrochloride, bleomycin sulfate, peplomycin
sulfate, daunorubicin
hydrochloride, doxorubicin hydrochloride, aclarubicin hydrochloride,
pirarubicin hydrochloride,
epirubicin hydrochloride, neocarzinostatin, mithramycin, sarkomycin,
carzinophilin, mitotane,
zorubicin hydrochloride, mitoxantrone hydrochloride, idarubicin hydrochloride
and their DDS
preparations (e.g., doxorubicin-encapsulated PEG liposomes) may be used.
[0202]
As the "plant-derived anticancer agents", e.g., etoposide, etoposide
phosphate, vinblastine
sulfate, vincristine sulfate, vindesine sulfate, teniposide, paclitaxel,
docetaxel, cabazitaxel,
vinorelbine and their DDS preparations thereof may be used.
[0203]
As the "immunotherapeutic agents", e.g., picibanil, krestin, schizophyllan,
lentinan, ubenimex,
interferons, interleukins, macrophage colony stimulating factor, granulocyte
colony stimulating
factor, erythropoietin, lymphotoxin, BCG vaccine, Corynebacterium parvum,
levamisole, Toll-
like receptor (TLR) agonist, polysaccharide K, procodazole, anti-CTLA4
antibodies (e.g.,
ipilimumab, tremelimumab), anti-PD-1 antibodies (e.g., nivolumab,
pembrolizumab, cemiplimab,
tislelizmab, sintilimab, toripalimab), anti-PD-Li antibody (e.g.,
atezolizumab, avelumab,
durvalumab), and oncolytic viruses may be used.
[0204]
The "cell growth factors" in the "medicaments inhibiting the action of cell
growth factors or
their receptors" may be any substance that promote cell growth, and usually
include peptides
having a molecular weight of 20,000 or less and exhibiting the action at low
concentrations by
binding to a receptor, and specifically, (1) EGF (epidermal growth factor) or
substances having
substantially the same activity as EGF (e.g., TGFa); (2) insulin or substances
having substantially
the same activity as insulin (e.g., insulin, IGF (insulin-like growth factor)-
1, IGF-2), (3) FGF
(fibroblast growth factor) or substances having substantially the same
activity as FGF (e.g.,
acidic FGF, basic FGF, KGF (keratinocyte growth factor), FGF-10), and (4)other
cell growth
factors [e.g., CSF (colony stimulating factor), EPO (erythropoietin), IL-2
(interleukin-2), NGF
(nerve growth factor), PDGF (platelet-derived growth factor) TGF-I3
(transforming growth factor
13), HGF (hepatocyte growth factor), VEGF (vascular endothelial growth
factor), heregulin,
angiopoietin may be used.
[0205]
The "cell growth factor receptors" may be any receptor as long as it has the
ability to bind to
the above-mentioned cell growth factors, and specifically, EGF receptor,
heregulin receptor (e.g.,
HER3), insulin receptor, IGF receptor-1, IGF receptor-2, FGF receptor-1 or FGF
receptor-2, NGF
receptor, TGFB receptor, HGF receptor, VEGF receptor, angiopoietin receptor
(e.g., Tie2), PDGF
receptor and the like may be used.
[0206]
As the "medicament inhibiting the action of cell growth factors or their
receptors", EGF
inhibitor, TGFa inhibitor, heregulin inhibitor, insulin inhibitor, IGF
inhibitor, FGF inhibitor,
KGF inhibitor, CSF inhibitor, EPO inhibitor, IL-2 inhibitor, NGF inhibitor,
PDGF inhibitor,
TGFI3 inhibitor, HGF inhibitor, VEGF inhibitor, angiopoietin inhibitor, EGF
receptor inhibitor,
HER2 inhibitor, HER3 inhibitor, HER4 inhibitor, insulin receptor inhibitor,
IGF -1 receptor
Date Recue/Date Received 2022-01-27
37
CA 03148955 2022-01-27
inhibitor, IGF-2 receptor inhibitor, FGF receptor-1 inhibitor, FGF receptor-2
inhibitor, FGF
receptor-3 inhibitor, FGF receptor-4 inhibitor, VEGF receptor inhibitor, Tie-2
inhibitor, PDGF
receptor inhibitor, Abl inhibitor, Raf inhibitor, FLT3 inhibitor, c-Kit
inhibitor, Src inhibitor, PKC
inhibitor, Smo inhibitor, ALK inhibitor, ROR1 inhibitor, Trk inhibitor, Ret
inhibitor, mTOR
inhibitor, Aurora inhibitor, PLK inhibitor, MEK(MEK1/2) inhibitor, MET
inhibitor, CDK
inhibitor, Akt inhibitor, ERK inhibitor, PI3K inhibitor and the like may be
used. More
specifically, anti-VEGF antibody (e.g., Bevacizumab, Ramucirumab), anti-HER2
antibody (e.g.,
Trastuzumab, Pertuzumab), anti-EGFR antibody (e.g., Cetuximab, Panitumumab,
Matuzumab,
Nimotuzumab), anti-HGF antibody, Imatinib, Erlotinib, Gefitinib, Sorafenib,
Sunitinib,
Dasatinib, Lapatinib, Vatalanib, Ibrutinib, Bosutinib, Cabozantinib,
Crizotinib, Alectinib,
Vismodegib, Axitinib, Motesanib, Nilotinib, 644-(4-ethy 1piperazin- 1 -y
lmethy Opheny11-N4 l(R)-
pheny lethy11-7H-pyrrolo [2,3-d]pirimidine-4-amine (AEE-788), Vandetanib,
Temsirolimus,
Everolimus, Enzastaurin, Tozasertib, 24N434445-[N-(3-
fluorophenyl)carbamoylmethy11-1H-
pyrazol-3-ylamino]quinazolin-7-yloxy]propy11-N-ethylamino]ethyl phosphate
ester (AZD-1152),
449-chloro-7-(2,6-difluoropheny1)-5H-pyrimido[5,4-d][2]benzazepin-2-
ylamino]benzoic acid, N-
[2-methoxy-5-[(E)-2-(2,4,6-trimethoxyphenyl)vinylsulfonylmethyl]phenyl]glycine
sodium salt
(ON-1910Na), Volasertib, Selumetinib, Trametinib, N42(R),3-dihydroxypropoxy1-
3,4-difluoro-
2-(2-fluoro4-iodophenylamino)benzamide (PD-0325901), Bosutinib, Regorafenib,
Afatinib,
Idelalisib, Ceritinib, Dabrafenib, Ponatinib, Lenvatinib, Midostaurin,
Pazopanib and the like may
be used.
[0207]
In addition to the above-mentioned drugs, L-asparaginase, L-arginase, arginine
deiminases,
aceglaton, procarbazine hydrochloride, protoporphyrin-cobalt complex salt,
mercury
hematoporphyrin-sodium, topoisomerase I inhibitors (e.g., irinotecan,
topotecan, indotecan,
indimitecan), topoisomerase II inhibitors (e.g., sobuzoxane) ),
differentiation-inducing agents
(e.g., retinoid, vitamin D), other angiogenesis inhibitors (e.g., fumagillin,
shark extract, COX-2
inhibitor), a-blockers (e.g., tamsulosin hydrochloride), bisphosphonic acids (
e.g., pamidronate,
zoledronate), thalidomide, lenalidomide, pomalidomide, 5-azacitidine,
decitabine, proteasome
inhibitors (e.g., bortezomib, carfilzomib, ixazomib), NEDD8 inhibitors (e.g.,
Pevonedistat), UAE
inhibitors, PARP inhibitors (e.g., Olaparib, Niraparib, Veliparib), anti-tumor
antibodies such as
anti-CD20 antibodies (e.g., Rituximab, Obinutuzumab), anti-CCR4 antibodies
(e.g.,
Mogamulizumab) and the like, antibody drug conjugates (e.g., Trastuzumab
emtansine,
Brenximab vedotin) and the like may also be used as a concomitant drug.
[0208]
When the compound of the present invention is used for purposes of IRAK-M
associated
diseases other than cancer, besides the above-mentioned concomitants, e.g.,
antibacterial drugs,
antifungal drugs, nonsteroidal anti-inflammatory drugs, steroid drugs,
bronchodilating
preparations, anticoagulants, antiplatelet drugs, thrombolytic drugs,
immunomodulators,
antiprotozoal drugs, antitussives/expectorants, sedatives, anesthetics,
narcotic antagonists, anti-
ulcer drugs, vitamins, vitamins derivatives, antiallergic drugs, anti-
asthmatic drugs, therapeutic
drugs for dermatitis atopic, signal transduction inhibitors, inflammatory
mediator action
suppressants, inflammatory mediator action suppression antibodies,
inflammatory mediator
production suppressants, anti-inflammatory mediator action suppressants, anti-
inflammatory
mediator action suppression antibodies, anti-inflammatory mediator production
suppressants,
antifibrotic drug, al-adrenergic agonist, antiemetic, methemoglobin elevation
inhibitors, and the
like may be used as a concomitant drugs.
[0209]
(1) Antibacterial drugs
(i) Sulfa drugs
Sulfamethizole, sulfisoxazole, sulfamonomethoxine, sulfamethizole,
salazosulfapyridine, silver
Date Recue/Date Received 2022-01-27
38
CA 03148955 2022-01-27
sulfadiazine, etc.
(ii) Quinoline antibacterial drugs
nalidixic acid, pipemidic acid trihydrate, enoxacin, norfloxacin, ofloxacin,
tosufloxacin
tosylate, ciprofloxacin hydrochloride, lomefloxacin hydrochloride,
sparfloxacin, fleroxacin, etc.
(iii) Antiphthisics
isoniazid, ethambutol (ethambutol hydrochloride), para-aminosalicylic acid
(calcium para-
aminosalicylate), pyrazinamide, ethionamide, prothionamide, rifampicin,
streptomycin sulfate,
kanamycin sulfate, cycloserine, etc.
(iv) Antiacidfast bacterium drug
diaminodiphenyl sulfone, rifampicillin, etc.
(v) Antiviral drugs
idoxuridine, aciclovir, vidarabine, ganciclovir, favipiravir, etc.
(vi) Anti-HIV drugs
zidovudine, didanosine, zalcitabine, indinavir sulfate ethanolate, ritonavir,
etc.
(vii) Anti-spirochete drugs
(viii) Antibiotics
tetracycline hydrochloride, ampicillin, piperacillin, gentamicin, dibekacin,
kanendomycin,
lividomycin, tobramycin, amikacin, fradiomycin, sisomicin, tetracycline,
oxytetracycline,
rolitetracycline, doxycycline, ampicillin, piperacillin, ticarcillin,
cefalotin, cephapirin,
cefaloridine, cefaclor, cefalexin, cefroxadine, cefadroxil, cefamandole,
cefuroxime, cefotiam,
cefotiam hexetil, cefuroxime axetil, cefdinir, cefditoren pivoxil,
ceftazidime, cefpiramide,
cefsulodin, cefmenoxime, cefpodoxime proxetil, cefpirome, cefozopran,
cefepime, cefsulodin,
cefmenoxime, cefmetazole, cefminox, cefoxitin, cefbuperazone, latamoxef,
flomoxef, cefazolin,
cefotaxime, cefoperazone, ceftizoxime, moxalactam, thienamycin, sulfazecin,
aztreonam or salts
thereof, griseofulvin, lankacidin, etc.
[0210]
(2) Antifungal drugs
(i) Polyene antibiotics (e.g., amphotericin B, nystatin, trichomycin)
(ii) Griseofulvin, pyrrolnitrin, etc.
(iii) Cytosine antimetabolites (e.g., flucytosine)
(iv) Imidazole derivatives (e.g., econazole, clotrimazole, miconazole nitrate,
bifonazole,
croconazole)
(v) Triazole derivatives (e.g., fluconazole, itraconazole, azole compounds [2-
[(1R, 2R)-2-(2,4-
difluorophenyl) -2-hydroxy-l-methy1-3-(1H-1, 2,4-triazol-1-y1) propy11-444-
(2,2,3,3-
tetrafluoropropoxy) pheny11-3-(2H,4H)-1,2,4-triazolonel
(vi) Thiocarbamic acid derivatives (e.g., tolnaftate)
(vii) Echinocandin derivatives (e.g., caspofungin, micafungin, anidulafungin),
etc.
[0211]
(3) Non-steroidal anti-inflammatory drugs
acetaminophen, phenacetin, ethenzamide, sulpyrine, antipyrine, migrenin,
aspirin, mefenamic
acid, flufenamic acid, diclofenac sodium, loxoprofen sodium, phenylbutazone,
indomethacin,
ibuprofen, ketoprofen, naproxen, oxaprozin, flurbiprofen, fenbufen,
pranoprofen, floctafenine,
epirizole, tiaramide hydrochloride, zaltoprofen, gabexate mesilate, camostat
mesylate, ulinastatin,
colchicine, probenecid, sulfinpyrazone, benzbromarone, allopurinol, gold
sodium thiomalate,
sodium hyaluronate, sodium salicylate, morphine hydrochloride, salicylic acid,
atropine,
scopolamine, morphine, pethidine, levorphanol, ketoprofen, naproxen,
oxymorphone, meloxicam,
celecoxib, rofecoxib, or salts thereof.
[0212]
(4) Steroid drugs
dexamethasone, hexestrol, methimazole, betamethasone, triamcinolone,
triamcinolone
Date Recue/Date Received 2022-01-27
39
CA 03148955 2022-01-27
acetonide, fluocinonide, fluocinolone acetonide, prednisolone,
methylprednisolone, cortisone
acetate, hydrocortisone, fluorometholone, beclometasone propionate, estriol,
etc.
[0213]
(5) Bronchodilating preparations
Metaproterenol, salmeterol, formoterol, carmoterol, etc.
[0214]
(6) Anticoagulants
heparin sodium, sodium citrate, activated protein C, tissue factor pathway
inhibitor,
antithrombin III, dalteparin sodium, warfarin potassium, argatroban, gabexate,
sodium citrate,
etc.
[0215]
(7) Antiplatelet drugs
ozagrel sodium, ethyl icosapentate, beraprost sodium, alprostadil, ticlopidine
hydrochloride,
pentoxifylline, dipyridamole, etc.
[0216]
(8) Thrombolytic drugs
tisokinase, urokinase, streptokinase, etc.
[0217]
(9) Immunomodulators
cyclosporin, tacrolimus, gusperimus, azathioprine, anti-lymphocyte serum,
freeze-dried
sulfonated normal immunoglobulin, erythropoietin, colony stimulating factor,
interleukin,
interferon, etc.
[0218]
(10) Antiprotozoal drugs
metronidazole, tinidazole, diethylcarbamazine citrate, quinine hydrochloride,
quinine sulfate,
etc.
[0219]
(11) Antitussive and expectorant drugs
ephedrine hydrochloride, noscapine hydrochloride, codeine phosphate,
dihydrocodeine
phosphate, isoproterenol hydrochloride, ephedrine hydrochloride,
methylephedrine
hydrochloride, noscapine hydrochloride, alloclamide, chlophedianol, pic
operidamine,
cloperastine, protokylol, isoproterenol, salbutamol, terbutaline,
oxymetebanol, morphine
hydrochloride, dextropetorphan hydrobromide, oxycodone hydrochloride,
dimorphane phosphate,
tipepidine hibenzate, pentoxyverine citrate, clofedanol hydrochloride,
benzonatate, guaifenesin,
bromhexine hydrochloride, ambroxol hydrochloride, acetylcysteine,
ethylcysteine hydrochloride,
carbocysteine, etc.
[0220]
(12) Sedatives
chlorpromazine hydrochloride, atropine sulfate, phenobarbital, barbital,
amobarbital,
pentobarbital, thiopental sodium, thiamylal sodium, nitrazepam, estazolam,
flurazepam,
haloxazolam, triazolam, flunitrazepam, bromvalerylurea, chloral hydrate,
triclofos sodium, etc.
[0221]
(13) Anesthetics
(13-1) Local anesthetics
cocaine hydrochloride, procaine hydrochloride, lidocaine, dibucaine
hydrochloride, tetracaine
hydrochloride, mepivacaine hydrochloride, bupivacaine hydrochloride,
oxybuprocaine
hydrochloride, ethyl aminobenzoate, oxethazaine, etc.
(13-2) General anesthetics
(i) Inhalation anesthetics (e.g., ether, halothane, nitrous oxide, isoflurane,
enflurane),
(ii) Intravenous anesthetics (e.g., ketamine hydrochloride, droperidol,
thiopental sodium,
Date Recue/Date Received 2022-01-27
40
CA 03148955 2022-01-27
thiamylal sodium, pentobarbital), etc.
[0222]
(14) Narcotic antagonists
levallorphan, nalorphine, naloxone or a salt thereof, etc.
[0223]
(15) Anti-ulcer drugs
metoclopramide, histidine hydrochloride, lansoprazole, metoclopramide,
pirenzepine,
cimetidine, ranitidine, famotidine, urogastrone, oxethazaine, proglumide,
omeprazole, sucralfate,
sulpiride, cetraxate, gefarnate, aldioxa, teprenone, prostaglandin, etc.
[0224]
(16) Vitamin drugs
(i) Vitamin A: vitamin Al, vitamin A2 and retinol palmitate
(ii) Vitamin D: Vitamin D1, D2, D3, D4 and D5
(iii) Vitamin E: a-tocopherol, 13-tocopherol, y-tocopherol, 6-tocopherol, dl-a-
tocopherol
nicotinate
(iv) Vitamin K: vitamin Kl, K2, K3 and K4
(v) Folic acid (vitamin M)
(vi) Vitamin B: vitamin Bl, vitamin B2, vitamin B3, vitamin B5, vitamin B6 and
vitamin B12
(vii) Biotin (vitamin H), etc.
[0225]
(17) Vitamin derivatives
various derivatives of vitamins, e.g., ascorbic acid, vitamin D3 derivatives
such as 5,6-trans-
cholecalciferol, 2,5-hydroxycholecalciferol, 1-a-hydroxycholecalciferol, etc.,
vitamin D2
derivatives such as 5,6-trans-ergocalciferol, etc., etc.
[0226]
(18) Antiallergic drugs
diphenhydramine, chlorpheniramine, tripelennamine, clemizole,
diphenylpyraline,
methoxyphenamine, sodium cromoglicate, tranilast, repirinast, amlexanox,
ibudilast, ketotifen,
terfenadine, mequitazine, azelastine, epinastine, ozagrel hydrochloride,
pranlukast hydrate,
seratrodast, etc.
[0227]
(19) Anti-asthmatic drugs
isoprenaline hydrochloride, salbutamol sulfate, procaterol hydrochloride,
terbutaline sulfate,
trimetoquinol hydrochloride, tulobuterol hydrochloride, orciprenaline sulfate,
fenoterol
hydrobromide, ephedrine hydrochloride, ipratropium bromide, oxitropium
bromide, flutropium
bromide, theophylline, aminophylline, sodium cromoglicate, tranilast,
repirinast, ibudilast,
ketotifen, terfenadine, mequitazine, azelastine, epinastine, ozagrel
hydrochloride, pranlukast
hydrate, seratrodast, dexamethasone, prednisolone, hydrocortisone,
beclomethasone propionate,
etc.
[0228]
(20) Therapeutic drugs for Dermatitis atopic
sodium cromoglicate, etc.
[0229]
(21) Antiemetics
phenothiazine derivatives, 5-HT3 receptor antagonists, etc.
[0230]
(22) Methemoglobin elevation inhibitors
methylene blue, ascorbic acid, etc.
[0231]
(23) Integrin inhibitors
Date Recue/Date Received 2022-01-27
41
CA 03148955 2022-01-27
natalizumab, vedolizumab, AJM300, TRK-170, E-6007, etc.
[0232]
(24) Antifibrotic drugs
pirfenidone nintedanib B-aminopropionitrile (BAPN), ursodeoxycholic acid, etc.
[0233]
(25) Others
hydroxycam, diacerein, megestrol acetate, nicergoline, prostaglandins, etc.
[0234]
By combining the compound of the present invention and a concomitant drug, a
superior effect
may be obtained such as (1) the dose of the compound of the present invention
or the concomitant
drug may be reduced as compared with a case where the compound is administered
alone, (2) the
drug to be used in combination with the compound of the present invention may
be selected
depending on the patient's condition (mild case, severe case, etc.), (3) the
treatment period may
be set longer, (4) a therapeutic effect maintaining longer is designed, and
(5) by using the
compound of the present invention in combination with a concomitant drug, a
synergistic effect
may be obtained.
[0235]
Hereinafter, when the compound of the present invention is used in combination
with a
concomitant drug, it is referred to as the "combination drug of the present
invention".
When using the combination drug of the present invention, the administration
time of the
compound of the present invention and the concomitant drug is not limited, and
the compound of
the present invention and the concomitant drug may be administered
simultaneously to a subject
to be administered, or with a time interval. When the administration is
carried out with a time
interval, the time interval varies depending on the effective ingredient to be
administered, dosage
form and administration method, and for example, when the concomitant drug is
administered
first, the compound of the present invention may be administered within 1
minute to 3 days,
preferably within 10 minutes to 1 day, more preferably within 15 minutes to 1
hour after
administration of the concomitant drug. When the compound of the present
invention is
administered first, the concomitant drug may be administered within 1 minute
to 1 day,
preferably within 10 minutes to 6 hours, more preferably within 15 minutes to
1 hour after
administering the compound of the present invention. The dosage of the
concomitant drug may
be in accordance with the dose clinically used, and may be appropriately
selected depending on
the administration subject, administration route, disease, combination, and
the like.
[0236]
Examples of the administration mode when the compound of the present invention
and the
concomitant drug are used concurrently include (1) administration of a single
preparation
obtained by simultaneously preparing the compound of the present invention and
the concomitant
drug, (2) simultaneous administration by the same administration route of two
preparations
obtained by separately preparing the compound of the present invention and a
concomitant drug,
(3) administration with an time interval by the same administration route of
two preparations
obtained by separately preparing the compound of the present invention and a
concomitant drug,
(4) simultaneous administration by the different administration routes of two
preparations
obtained by separately preparing the compound of the present invention and a
concomitant drug,
and (5) administration with a time interval by the different administration
routes of two
preparations obtained by separately preparing the compound of the present
invention and a
concomitant drug (e.g., administration in the order of the compound of the
present invention and
the concomitant drug, or administration in the reverse order).
The dose of the concomitant drug may be appropriately determined based on the
clinically used
dose. The ratio of the compound of the present invention and the concomitant
drug may be
appropriately determined depending on the administration subject,
administration route, target
Date Recue/Date Received 2022-01-27
42
CA 03148955 2022-01-27
disease, symptom, combination and the like. For example, when the
administration subject is a
human, the concomitant drug may be used in an amount of 0.01 to 100 parts by
weight relative to
1 part by weight of the compound of the present invention.
[0237]
Further, the compound of the present invention or the combination drug of the
present
invention may be used in combination with non-drug therapy. Specifically, the
compound of the
present invention or the combination drug of the present invention may be
combined with non-
drug therapy such as (1) surgery, (2) hypertensive chemotherapy using
angiotensin II and the
like, (3) gene therapy, (4) thermotherapy, (5) cryotherapy, (6) laser
cauterization and (7)
radiotherapy.
[0238]
For example, by using the compound of the present invention or the combination
drug of the
present invention before or after the above-mentioned surgery and the like, or
using the
compound or the drug before or after a combined treatment of two or three
kinds thereof, effects
may be obtained such as prevention of the onset of resistance, prolongation of
disease-free
survival, suppression of metastasis or recurrence of cancer, prolongation of
life, and the like.
[0239]
Further, it is possible to combine a treatment with the compound of the
present invention or the
combination drug of the present invention with a supportive therapy [(i)
administration of
antibiotics (e.g., 13-lactam type such as pansporin and the like, macrolide
type such as
clarithromycin and the like) for the complication with various infectious
diseases, (ii)
administration of high-calorie transfusion, amino acid preparation or
multivitamin preparation for
improving malnutrition, (iii) morphine administration for pain relief, (iv)
administration of a drug
for ameliorating side effects such as nausea, vomiting, anorexia, diarrhea,
leukopenia,
thrombocytopenia, decreased hemoglobin concentration, hair loss, liver damage,
renal damage,
DIC, fever and the like, and (v) administration of a drug for suppressing
multiple drug resistance
of cancer, etc..
[EXAMPLES]
[0240]
Further, the present invention is explained in detail by referring to the
following References,
Examples, Experimental Examples and Formulation Examples, which are not to be
construed as
limitative, and the disclosure may be changed within the scope of the present
invention.
In the following Examples, the "room temperature" generally means about 10 C
to about 35
C. The ratios indicated for mixed solvents are volume mixing ratios, unless
otherwise
specified. "%" means "wt%," unless otherwise specified.
In silica gel column chromatography, "NH" means use of aminopropyl silane -
bound silica gel
and "C18" means use of octadecyl-bound silica gel. In HPLC (high-performance
liquid
chromatography), "C18" means use of octadecyl-bonded silica gel. The ratios of
elution
solvents are volume mixing ratios, unless otherwise specified.
In Examples, the following abbreviations are used.
MS: mass spectrum
M: mol concentration
DMSO-do: deuterated dimethyl sulfoxide
11-1 NMR: proton nuclear magnetic resonance
LC/MS: liquid chromatograph mass spectrometer
ESI: Electrospray ionization
APCI: Atmospheric pressure chemical ionization
DCM: dichloromethane
DIEA: diisopropylethylamine
Date Recue/Date Received 2022-01-27
43
CA 03148955 2022-01-27
DMAP: 4-dimethylaminopyridine
DMF: N,N-dimethylformamide
HATU: 2-(7-azabenzotriazol-1-y1)-1,1,3,3-tetrametyluroniumhexafluorophosphate
TEA: triethylamine
THF: tetrahydrofuran
TFA: trifluoroacetate
[0241]
111 NMR was measured by Fourier-transform type NMR. For the analysis,
ACD/SpecManager
(trade name) or MestreNova (trade name) and the like were used. Peaks with
very mild protons
such as a hydroxy group, an amino group and the like are not described.
MS was measured by LC/MS. As an ionization method, ESI method or APCI method
was
used. The data indicates actual measured value (found). Generally, a molecular
ion peak
([M+111 , EM-111-, etc.) is observed. In the case of a compound having a tert-
butoxycarbonyl
group, a peak after elimination of a tert-butoxycarbonyl (Boc) group or a tert-
butyl (tBu) group
may be observed as a fragment ion. In the case of a compound having a carboxyl
group, a peak
of sodium adduct thereof may be observed. In the case of a compound having a
hydroxy group,
a peak after elimination of water may be observed as a fragment ion. In the
case of a salt, a
molecular ion peak or fragment ion peak of free form is generally observed.
Sample concentration (c) used in the optical rotation (MD) is g/100 mL.
Elemental analysis value (Anal.) indicates both calculated value (Calcd) and
measured
value (Found).
[0242]
Example 1
2-(44(S)-2-Cyclohexy1-2-((S)-2-(methylamino)propanamido)acetyl)piperazine-1-
carbony1)-5,6-
difluoro-N,1-dimethyl-N-(2-(2-(2-45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-
l-
yOmethyDisoxazol-3-y1)oxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide
hydrochloride
[0243]
A) 7-(Piperidin-4-yloxy)thieno[3,2-blpyridine dihydrochloride
To a mixture of tert-butyl 4-hydroxypiperidine-1-carboxylate (14.9 g) and DMF
(100 mL) was
added sodium hydride (60%, dispersion in paraffin liquid, 3.0 g) under ice-
cooling. The
reaction mixture was stirred for 10 min, 7-chlorothieno[3,2-blpyridine (10.4
g) was added
thereto, and stirred at 60 C overnight. To the reaction mixture was added
water and extracted
with ethyl acetate. The organic layer was washed with water and brine, dried
over anhydrous
magnesium sulfate, and the solvent was removed under reduced pressure. To a
mixture of tert-
butyl 4-(thieno[3,2-blpyridin-7-yloxy)piperidine-1-carboxylate thus obtained
and ethyl acetate
(100 mL) was added 4 M hydrogen chloride ethyl acetate solution (100 mL). The
reaction
mixture was stirred at room temperature for 2 h, diisopropyl ether (70 mL) was
added thereto,
and the precipitates were collected by filtration to give the title compound
(16.8 g).
MS: [M+111+ 235.2.
[0244]
B) Methyl 3-(allyloxy)isoxazole-5-carboxylate
To a mixture of methyl 3-hydroxyisoxazole-5-carboxylate (15.0 g), potassium
carbonate (17.4 g)
and DMF (200 mL) was added 3-bromoprop-1-ene (13.6 mL), and the reaction
mixture was
stirred at 60 C for 16 h. The reaction mixture was diluted with ethyl acetate
and water, and the
aqueous layer was extraceted with ethyl acetate. The organic layer was washed
with water and
brine, dried over anhydrous magnesium sulfate, and the solvent was removed
under reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane)
to give the title compound (16.6 g).
MS: [M+111+ 184.3.
[0245]
Date Recue/Date Received 2022-01-27
44
CA 03148955 2022-01-27
C) (3-(Allyloxy)isoxazol-5-yOmethanol
To a mixture of methyl 3-(allyloxy)isoxazole-5-carboxylate (16.6 g) and
methanol (300 mL) was
added sodium borohydride (4.80 g). The reaction mixture was stirred at room
temperature for
16 h and the solvent was removed under reduced pressure. The residue was
diluted with ethyl
acetate and water, and the aqueous layer was extraceted with ethyl acetate.
The organic layer
was washed with water and brine, dried over anhydrous magnesium sulfate, and
the solvent was
removed under reduced pressure to give the title compound (13.2 g).
MS: [M+111+ 156.3.
[0246]
D) (3-(Allyloxy)isoxazol-5-yOmethyl methanesulfonate
To a mixture of (3-(allyloxy)isoxazol-5-yOmethanol (13.2 g), TEA (24 mL) and
THF (200 mL)
was added methanesulfonyl chloride (9.94 mL) under ice-cooling. The reaction
mixture was
stirred at room temperature for 1 h, diluted with water, and the aqueous layer
was extracted with
ethyl acetate. The organic layer was washed with brine, dried over anhydrous
magnesium
sulfate, and the solvent was removed under reduced pressure to give the title
compound (18.9 g).
MS: [M+111+ 234.2.
[0247]
E) 3-(Allyloxy)-5-((4-(thieno[3,2-b]pyridin-7-yloxy)piperidin-1-
yl)methyl)isoxazole
A mixture of 7-(piperidin-4-yloxy)thieno[3,2-b]pyridine dihydrochloride (6.16
g), (3-
(allyloxy)isoxazol-5-yl)methyl methanesulfonate (5.14 g), tetrabutylammonium
iodide (4.44 g),
potassium carbonate (9.70 g) and DMF (100 mL) was stirred at room temperature
for 24 h. To
the reaction mixture was added water and the resultant mixture was filtered,
and the filtrate was
extracted with ethyl acetate. The organic layer was washed with water and
brine, dried over
anhydrous magnesium sulfate, and the solvent was removed under reduced
pressure. The
residue was purified by silica gel column chromatography (NH, ethyl
acetate/hexane) to give the
title compound (6.25 g).
MS: [M+111+ 372.2.
[0248]
F) Benzyl (S)-4-(2-((tert-butoxycarbonyl)amino)-2-cyclohexylacetyl)piperazine-
l-carboxylate
To a mixture of (S)-2-((tert-butoxycarbonyl)amino)-2-cyclohexylacetic acid
(2.5 g), benzyl
piperazine-l-carboxylate (2.14 g), DIEA (5.09 mL) and DMF (48.6 mL) was added
HATU (5.54
g) at room temperature. The reaction mixture was stirred at the same
temperature for 6 h. The
reaction mixture was diluted with ethyl acetate and water, and the aqueous
layer was extracted
with ethyl acetate. The organic layer was dried over anhydrous magnesium
sulfate and the
solvent was removed under reduced pressure. The residue was purified by silica
gel column
chromatography (ethyl acetate/hexane) to give the title compound (4.34 g).
MS: [M+111+ 460.2.
[0249]
G) Benzyl (S)-4-(2-amino-2-cyclohexylacetyl)piperazine-1-carboxylate
hydrochloride
To a mixture of benzyl (S)-4-(2-((tert-butoxycarbonyl)amino)-2-
cyclohexylacetyl)piperazine-l-
carboxylate (4.34 g) and ethyl acetate (18.9 mL) was added 4 M hydrogen
chloride ethyl acetate
solution (18.9 mL) at room temperature, and the reaction mixture was stirred
at 45 C for 1 h.
The reaction mixture was concentrated under reduced pressure and the crude
product was
recrystallized from ethyl acetate/hexane to give the title compound (2.96 g).
MS: [M+111+ 360.2.
[0250]
H) Benzyl 4-((S)-2-((S)-2-((tert-butoxycarbonyl)(methyl)amino)propanamido)-2-
cyclohexylacetyl)piperazine-l-carboxylate
To a mixture of (S)-2-((tert-butoxycarbonyl)(methyl)amino)propanoic acid (1.63
g), benzyl (S)-4-
(2-amino-2-cyclohexylacetyl)piperazine-1-carboxylate hydrochloride (2.96 g),
DIEA (5.22 mL)
Date Recue/Date Received 2022-01-27
45
CA 03148955 2022-01-27
and DMF (37.4 mL) was added HATU (4.26 g) at room temperature. The reaction
mixture was
stirred at the same temperature for 6 h. The reaction mixture was diluted with
ethyl acetate and
water, and the aqueous layer was extracted with ethyl acetate. The organic
layer was dried over
anhydrous magnesium sulfate and the solvent was removed under reduced
pressure. The residue
was purified by silica gel column chromatography (ethyl acetate/hexane) to
give the title
compound (3.62 g).
MS: [M+111+ 545.4.
[0251]
I) tert-Butyl ((S)-1-(((S)-1-cy clohexy1-2-oxo-2-(piperazin-l-yDethyDamino)-1-
oxopropan-2-
yl)(methyl)carbamate
To a mixture of benzyl 4-((S)-2-((S)-2-((tert-
butoxycarbonyl)(methyl)amino)propanamido)-2-
cyclohexylacetyl)piperazine-l-carboxylate (3.62 g), 10% palladium on carbon
(362 mg) and ethyl
acetate (67 mL) was stirred under the normal pressure hydrogen atmosphere at
room temperature
for 1 h. The catalysts were filtered off and the filtrate was concentrated
under reduced pressure
to give the title compound (2.46 g).
MS: [M+111+ 411.3.
[0252]
J) Methyl 5,6-difluoro-1-methy1-1H-indole-2-carboxylate
To a mixture of 5,6-difluoro-1H-indole-2-carboxylic acid (20 g) and DMF (200
mL) was added
potassium carbonate (42.0 g) and iodomethane (18.9 mL) at room temperature.
The reaction
mixture was stirred at the same temperature for 18 h, and then stirred at 40
C for 6 h. To the
reaction mixture was added water, and the precipitates were collected by
filtration and washed
with hexane to give the title compound (20 g).
111 NMR (400 MHz, DMSO-do) 6 3.85 (3H, s), 3.99 (3H, s), 7.26 (1H, s), 7.70
(1H, dd, J = 8.24
Hz, 10.84 Hz), 7.78 (1H, dd, J = 6.96 Hz, 11.68 Hz).
[0253]
K) Methyl 5,6-difluoro-3-formy1-1-methy1-1H-indole-2-carboxylate
To a mixture of methyl 5,6-difluoro-l-methyl-1H-indole-2-carboxylate (2 g) and
DCM (20 mL)
was added 1 M titanium tetrachloride DCM solution (17.8 mL) and a mixture of
dichloromethyl
methyl ether (1.7 mL) and DCM (2 mL) at -78 C. The reaction mixture was
stirred at the same
temperature for 2 h. The reaction mixture was diluted with water and
neutralized with saturated
aqueous sodium bicarbonate. The precipitates were filtered off with Celite
and the filtrate was
extracted with DCM. The organic layer was dried over anhydrous sodium sulfate,
and the
solvent was removed under reduced pressure to give the title compound (1.9 g).
111 NMR (400 MHz, DMSO-d6) 6 3.99 (3H, s), 4.02 (3H, s), 8.0 (1H, dd, J = 6.92
Hz, 11.4 Hz),
8.12 (1H, dd, J = 8.24 Hz, 10.76 Hz), 10.34 (1H, s).
[0254]
L) 5,6-Difluoro-3-formy1-1-methy1-1H-indole-2-carboxylic acid
To a mixture of methyl 5,6-difluoro-3-formy1-1-methyl-1H-indole-2-carboxylate
(15 g), THF
(225 mL), methanol (75 mL) and water (75 mL) was added lithium hydroxide
monohydrate (3.73
g), and the reaction mixture was stirred at room temperature for 3 h. The
reaction mixture was
concentrated under reduced pressure and acidified with aqueous potassium
hydrogen sulfate.
The precipitates were collected by filtration to give the title compound (13
g).
MS: [MA41+ 240.1.
[0255]
M) 2-(44(S)-24(S)-2-((tert-Butoxycarbonyl)(methyDamino)propanamido)-2-
cy clohexylac ety Opiperazine -1-carbony1)-5,6-difluoro-l-methyl-1H-indole -3-
carboxylic acid
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-oxo-2-(piperazin-l-
y1)ethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (4.63 g), 5,6-difluoro-3-formy1-1-methy1-1H-
indole-2-
carboxylic acid (2.45 g), DIEA (2.7 mL) and DMF (50 mL) was added HATU (4.67
g). After
Date Recue/Date Received 2022-01-27
46
CA 03148955 2022-01-27
the reaction mixture was stirred at room temperature for 2 h, water was added
thereto and
extracted with ethyl acetate. The organic layer was washed with 0.1 M
hydrochloric acid,
saturated aqueous sodium bicarbonate and brine, respectively and then dried
over anhydrous
magnesium sulfate, and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane).
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-3-
formy1-1-methy1-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate thus
obtained, sodium dihydrogen phosphate (4.90 g), 2-methylbut-2-ene (3.58 g),
tert-butanol (90
mL) and water (30 mL) was added sodium chlorite (2.24 g). The reaction mixture
was stirred at
room temperature overnight, aqueous sodium thiosulfate solution was added
thereto and extracted
with ethyl acetate. The organic layer was washed with water and brine, dried
over anhydrous
magnesium sulfate, and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane and
methanol/ethyl acetate)
to give the title compound (5.46 g).
MS: [M+141+ 648.5.
[0256]
N) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-34(2-(2-(2-
hydroxyethoxy)ethoxy)ethyl)(methyl)carbamoy1)-1-methyl-1H-indole-2-
carbonyl)piperazin-l-y1)-
2-oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of 2-(44(S)-24(S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-
cy clohexylac ety Opiperazine -1-carbony1)-5,6-difluoro-l-methyl-1H-indole -3-
carboxylic acid
(5.46 g), 2-(2-(2-(methylamino)ethoxy)ethoxy)ethan-l-ol (1.65 g), DIEA (2.26
mL) and DMF (8
mL) was added HATU (3.85 g). The reaction mixture was stirred at room
temperature for 3 h,
water was added thereto, and extracted with ethyl acetate. The organic layer
was washed with
saturated aqueous sodium bicarbonate and brine, dried over anhydrous magnesium
sulfate, and
the solvent was removed under reduced pressure. The residue was purified by
silica gel column
chromatography (methanol/ethyl acetate) to give the title compound (3.10 g).
MS: [M+141+ 793.5.
[0257]
0) 5-((4-(Thieno[3,2-blpyridin-7-yloxy)piperidin-l-yl)methyl)isoxazol-3-ol
To a mixture of 3-(allyloxy)-5((4-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-
yOmethyDisoxazole
(5.68 g), triethylsilane (7.3 mL) and THF (100 mL) was added
tetrakis(triphenylphosphine)palladium (884 mg). The reaction mixture was
stirred at room
temperature for 1 h and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (methanol) and the compound thus
obtained was
washed with ethyl acetate to give the title compound (2.50 g).
11-1NMR (300 MHz, DMSO-do) 6 1.68-1.84 (2H, m), 1.95-2.09 (2H, m), 2.35-2.49
(2H, m), 2.63-
2.75 (2H, m), 3.58 (2H, s), 4.74-4.87 (1H, m), 5.93 (1H, s), 7.07 (1H, d, J =
5.5 Hz), 7.50 (1H, d,
J = 5.6 Hz), 8.04 (1H, d, J = 5.4 Hz), 8.50 (1H, d, J = 5.4 Hz), 11.16 (1H,
s).
[0258]
P) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-3-(methyl(2-
(2-(2-45-44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethyl)carbamoy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of 5((4-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-
y1)methyDisoxazol-3-ol (1.50 g),
tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-34(2-(2-(2-
hydroxyethoxy)ethoxy)ethyl)(methyl)carbamoy1)-1-methyl-1H-indole-2-
carbonyl)piperazin-l-y1)-
2-oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (3.59 g),
triphenylphosphine (5.94 g)
and toluene (25 mL) was added di-tert-butyl azodicarboxylate (3.13 g). The
reaction mixture
was stirred at room temperature for 1 h and the solvent was removed under
reduced pressure.
Date Recue/Date Received 2022-01-27
47
CA 03148955 2022-01-27
The residue was purified by silica gel column chromatography (C18,
acetonitrile/5 mM aqueous
ammonium acetate) to give the title compound (2.37 g).
MS: [M+111+ 1106.6.
[0259]
Q) 2-(4-((S)-2-cyclohexy1-2-((S)-2-(methylamino)propanamido)acetyl)piperazine-
l-carbony1)-
5,6-difluoro-N,1-dimethyl-N-(2-(2-(2-45-44-(thieno [3,2-blpyridin-7-
yloxy)piperidin- 1 -
yOmethyDisoxazol-3-yl)oxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide
hydrochloride
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-
methyl-3-(methyl(2-(2-
(2-45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-y1)methyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethyl)carbamoy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (2.37 g) and ethyl acetate
(10 mL) was
added 4 M hydrogen chloride ethyl acetate solution (16.1 mL), and the reaction
mixture was
stirred at room temperature for 1 h. The reaction mixture was concentrated
under reduced
pressure to give the title compound (2.16 g).
1H NMR (300 MHz, DMSO-do ) 60.72-1.26 (7H, m), 1.35 (3H, d, J = 6.8 Hz), 1.50-
1.83 (7H, m),
2.11-2.48 (7H, m), 2.97 (3H, s), 3.20-3.97 (21H, m), 4.13-4.37 (2H, m), 4.39-
4.82 (3H, m), 4.90-
5.59 (2H, m), 6.65 (1H, s), 7.46 (1H, dd, J = 10.9, 7.9 Hz), 7.54-7.96 (4H,
m), 8.53 (1H, d, J =
5.6 Hz), 8.66-9.12 (2H, m), 9.27-9.65 (1H, m), 11.79-12.71 (1H, m).
[0260]
Example 2
2-(4-((S)-2-Cyclohexy1-2-((S)-2-(methylamino)propanamido)acetyl)piperazine-l-
carbony1)-6-
methoxy-1-methyl-N-(2-(2-(2-(4-44-(thieno[3,2-dlpyrimidin-4-yloxy)piperidin-1-
yOsulfonyl)phenoxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide
[0261]
A) Methyl 6-methoxy-1-methy1-1H-indole-2-carboxylate
To a mixture of methyl 6-methoxy-1H-indole-2-carboxylate (11.6 g) and DMF (100
mL) was
added sodium hydride (60%, dispersion in paraffin liquid, 2.93 g) under ice-
cooling. After the
reaction mixture was stirred at the same temperature for 15 min, iodomethane
(3.88 mL) was
added to the reaction mixture and the reaction mixture was stirred at the same
temperature for 1
h. To the reaction mixture was added water (150 mL) and 1 M hydrochloric acid
(250 mL)
under ice-cooling and the aqueous layer was extracted with diethyl ether. The
organic layer was
washed with brine and then dried over anhydrous magnesium sulfate, and the
solvent was
removed under reduced pressure to give the title compound (11.4 g).
MS: [M+111+ 220Ø
[0262]
B) 6-Methoxy-l-methy1-1H-indole-2-carboxylic acid
To a mixture of methyl 6-methoxy-l-methyl-1H-indole-2-carboxylate (11.4 g) and
methanol (100
mL) was added 2 M aqueous sodium hydroxide (52.0 mL) at room temperature and
the reaction
mixture was stirred at 60 C for 1 h. The reaction mixture was cooled to 0 C
and neutralized
with 1 M hydrochloric acid (110 mL), and the precipitates were collected by
filtration to give the
title compound (9.87 g).
MS: [M+111+ 206Ø
[0263]
C) tert-Butyl 4-(6-methoxy-l-methy1-1H-indole-2-carbonyl)piperazine-l-
carboxylate
To a mixture of 6-methoxy-l-methyl-1H-indole-2-carboxylic acid (9.87 g), tert-
butyl piperazine-
l-carboxylate (9.41 g), 1-hydroxy-1H-benzotriazole monohydrate (8.10 g) and
DMF (150 mL)
was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (10.14
g), and the
reaction mixture was stirred at room temperature for 2 h. After the reaction
mixture was cooled
to 0 C, water was added thereto, and the precipitates were collected by
filtration to give the title
compound (16.8 g).
Date Recue/Date Received 2022-01-27
48
CA 03148955 2022-01-27
MS: [M+1-11+ 374.1.
[0264]
D) tert-Butyl 4-(3-formy1-6-methoxy-1-methy1-1H-indole-2-carbonyl)piperazine-1-
carboxylate
To a mixture of tert-butyl 4-(6-methoxy-l-methy1-1H-indole-2-
carbonyl)piperazine-l-carboxylate
(10.4 g) and DMF (100 mL) was added (chloromethylene)dimethylammonium chloride
(7.13 g),
and the reaction mixture was stirred at room temperature for 2 h. To the
reaction mixture was
added water and the resultant mixture was stirred for 30 min, and then the
aqueous layer was
extracted with ethyl acetate. The organic layer was washed with water and
brine, and then dried
over anhydrous magnesium sulfate, and the solvent was removed under reduced
pressure to give
the title compound (10.6 g).
MS: [M+111+ 402.1.
[0265]
E) 6-Methoxy-l-methy1-2-(piperazine -1-carbony1)-1H-indole -3-carbaldehyde
hydrochloride
To a mixture of tert-butyl 4-(3-formy1-6-methoxy-l-methy1-1H-indole-2-
carbonyl)piperazine-1-
carboxylate (10.6 g), dimethyl sulfide (25 mL) and ethyl acetate (100 mL) was
added 4 M
hydrogen chloride ethyl acetate solution (198 mL), and the reaction mixture
was stirred at room
temperature for 1 h. To the reaction mixture was added diisopropyl ether, and
the precipitates
were collected by filtration and washed with diisopropyl ether to give the
title compound (8.1 g).
MS: [M+111+ 302Ø
[0266]
F) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(3-formy1-6-methoxy-l-methy1-1H-
indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of (S)-2-((tert-butoxycarbonyl)amino)-2-cyclohexylacetic acid
(1.96 g), 6-methoxy-
1-methy1-2-(piperazine-l-carbony1)-1H-indole-3-carbaldehyde hydrochloride
(2.57 g), DIEA
(2.66 mL) and DMF (38 mL) was added HATU (3.47 g) at room temperature, and the
reaction
mixture was stirred at the same temperature for 1 h. The reaction mixture was
diluted with ethyl
acetate and water, and the aqueous layer was extracted with ethyl acetate. The
organic layer
was dried over anhydrous magnesium sulfate and the solvent was removed under
reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane).
To a mixture of the product thus obtained and ethyl acetate (38 mL) was added
4 M hydrogen
chloride ethyl acetate solution (38 mL) at room temperature, the reaction
mixture was stirred at
the same temperature for 1 h and the reaction mixture was concentrated under
reduced pressure.
To a mixture of (S)-2-(4-(2-amino-2-cyclohexylacetyl)piperazine-l-carbony1)-6-
methoxy-1-
methy1-1H-indole-3-carbaldehyde (3.35 g) thus obtained, (S)-2-((tert-
butoxycarbonyl)(methyl)amino)propanoic acid (1.55 g), DIEA (6.65 mL) and DMF
(38.1 mL)
was added HATU (4.34 g) at room temperature. The reaction mixture was stirred
at the same
temperature for 1 h. The reaction mixture was diluted with ethyl acetate and
water, and the
aqueous layer was extracted with ethyl acetate. The organic layer was dried
over anhydrous
magnesium sulfate and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to give
the title compound
(2.12 g).
MS: [M+111+ 626.3.
[0267]
G) 2-(44(S)-24(S)-2-((tert-Butoxycarbonyl)(methyDamino)propanamido)-2-
cyclohexylacetyl)piperazine-1-carbonyl)-6-methoxy-1-methyl-1H-indole-3-
carboxylic acid
To a mixture of tert-butyl ((S)-1-(((S)-1-cyclohexy1-2-(4-(3-formy1-6-methoxy-
l-methyl-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate (2.30
g), sodium dihydrogen phosphate (1.76 g), 2-methylbut-2-ene (1.95 mL), tert-
butyl alcohol (29.4
mL) and water (7.4 mL) was added sodium chlorite (665 mg) at room temperature.
The reaction
mixture was stirred at the same temperature for 4 h. The reaction mixture was
diluted with ethyl
Date Recue/Date Received 2022-01-27
49
CA 03148955 2022-01-27
acetate and saturated aqueous sodium thiosulfate, and the aqueous layer was
extracted with ethyl
acetate. The organic layer was washed with water and brine subsequently, dried
over anhydrous
magnesium sulfate and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (methanol/ethyl acetate) to give
the title compound
(770 mg).
MS: [M+111+ 642.4.
[0268]
H) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(34(2-(2-(2-
hydroxyethoxy)ethoxy)ethyl)carbamoy1)-6-methoxy-l-methyl-1H-indole-2-
carbonyl)piperazin-l-
y1)-2-oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
A mixture of 2-(44(S)-24(S)-2-((tert-butoxycarbonyl)(methypamino)propanamido)-
2-
cyclohexylacetyl)piperazine-1-carbonyl)-6-methoxy-1-methyl-1H-indole-3-
carboxylic acid (147
mg), triethylene glycol monoamine (41.0 mg), DIEA (120 L), HATU (131 mg) and
DMF (1.15
mL) was stirred at room temperature for 1 h. The reaction mixture was diluted
with water and
ethyl acetate, and extracted with ethyl acetate. The organic layer was dried
over anhydrous
magnesium sulfate and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (C18, acetonitrile/5 mM aqueous
ammonium
acetate) to give the title compound (133 mg).
MS: [M+111+ 773.5.
[0269]
I) tert-Butyl 4-(thieno[3,2-dlpyrimidin-4-yloxy)piperidine-l-carboxylate
To a mixture of tert-butyl 4-hydroxypiperidine-l-carboxylate (2.42 g) and DMF
(60.1 mL) was
added sodium hydride (60%, dispersion in paraffin liquid, 0.577 g). The
reaction mixture was
stirred for 30 min, 4-chlorothieno[3,2-dlpyrimidine (2.05 g) was added
thereto, and stirred at
room temperature for 1 h, diluted with ethyl acetate and water, and the
aqueous layer was
extracted with ethyl acetate. The organic layer was dried over anhydrous
magnesium sulfate,
and the solvent was removed under reduced pressure. The residue was purified
by silica gel
column chromatography (ethyl acetate/hexane) to give the title compound (3.75
g).
MS: [M+111+ 336Ø
[0270]
J) 4-(Piperidin-4-yloxy)thieno[3,2-dlpyrimidine hydrochloride
To a mixture of tert-butyl 4-(thieno[3,2-dlpyrimidin-4-yloxy)piperidine-l-
carboxylate (3.75 g)
and ethyl acetate (22.4 mL) was added 4 M hydrogen chloride ethyl acetate
solution (55.9 mL).
The reaction mixture was stirred for 30 min, and the solvent was removed under
reduced
pressure. The residue was washed with ethyl acetate to give the title compound
(3.25 g).
1H NMR (300 MHz, DMSO-do ) 62.00-2.12 (2H, m), 2.17-2.33 (2H, m), 3.02-3.37
(4H, m), 5.52-
5.67 (1H, m), 7.62 (1H, d, J = 5.29 Hz), 8.40 (1H, d, J = 5.29 Hz), 8.79 (1H,
s), 8.98-9.34 (2H,
m).
K) 4-((4-(Thieno[3,2-dlpyrimidin-4-yloxy)piperidin-l-y1)sulfonyl)phenol
To a mixture of 4-(piperidin-4-yloxy)thieno[3,2-dlpyrimidine hydrochloride
(215 mg) and
pyridine (2.64 mL) was added 4-hydroxybenzene-l-sulfonyl chloride (152 mg).
The reaction
mixture was stirred for 16 h, and the solvent was removed under reduced
pressure. The residue
was purified by silica gel column chromatography (C18, acetonitrile/5 mM
aqueous ammonium
acetate) to give the title compound (43.5 mg).
1H NMR (300 MHz, DMSO-d6) 6 1.75-1.95 (2H, m), 2.00-2.19 (2H, m), 2.83-3.01
(2H, m), 3.05-
3.21 (2H, m), 5.15-5.51 (1H, m), 6.96 (2H, d, J = 8.69 Hz), 7.46-7.72 (3H, m),
8.32 (1H, d, J =
5.29 Hz), 8.70 (1H, s), 10.10-11.07 (1H, m).
[0272]
L) 2-(4-((S)-2-Cyclohexy1-2-((S)-2-(methylamino)propanamido)acetyl)piperazine -
1-carbony1)-6-
methoxy-l-methyl-N-(2-(2-(2-(44(4-(thieno[3,2-dlpyrimidin-4-yloxy)piperidin-1-
Date Recue/Date Received 2022-01-27
50
CA 03148955 2022-01-27
yOsulfonyl)phenoxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide
To a mixture of 4((4-(thieno[3,2-dlpyrimidin-4-yloxy)piperidin-l-
y1)sulfonyl)phenol (35.5 mg),
tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(34(2-(2-(2-
hydroxyethoxy)ethoxy)ethyl)carbamoy1)-6-
methoxy-l-methyl-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-
oxopropan-2-
y1)(methyl)carbamate (70.0 mg), triphenylphosphine (119 mg) and toluene (0.45
mL) was added
di-tert-butyl azodicarboxylate (62.6 mg). The reaction mixture was stirred at
room temperature
for 2 h and the solvent was removed under reduced pressure. The residue was
purified by silica
gel column chromatography (C18, acetonitrile/5 mM aqueous ammonium acetate).
To the
product thus obtained was added ethyl acetate (0.2 mL) then added 4 M hydrogen
chloride ethyl
acetate solution (679 ttL). After being stirred at room temperature for 1 h,
the solvent was
removed under reduced pressure. The residue was purified by silica gel column
chromatography
(C18, acetonitrile/5 mM aqueous ammonium acetate). The product thus obtained
was dissolved
in methanol, desalted by Amberlyst A21, and the solvent was removed under
reduced pressure to
give the title compound (19.5 mg)
1H NMR (300 MHz, DMSO-d6) 6 0.63-4.78 (54H, m) 5.26-5.43 (1H, m) 6.75-6.95
(1H, m) 7.05-
7.20 (3H, m) 7.37-7.51 (1H, m) 7.55 (1H, d, J = 5.29 Hz) 7.65 (2H, d, J = 9.06
Hz) 7.75-7.85
(1H, m) 7.88-7.99 (1H, m) 8.30 (1H, d, J = 5.29 Hz) 8.69 (1H, s).
[0273]
Example 3
14(R)-4-(5,6-Difluoro-l-methy1-1H-indole-2-carbony1)-2-methylpiperazin-1-y1)-2-
42R,5R)-5-
methyl-2-42-(2-(2-45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-
yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)methyDpiperazin-l-y1)ethan-l-one hydrochloride
[0274]
A) 5,6-Difluoro-l-methy1-1H-indole-2-carboxylic acid
To a mixture of methyl 5,6-difluoro-l-methyl-1H-indole-2-carboxylate (2 g),
THF (14 mL),
methanol (7 mL) and water (7 mL) was added lithium hydroxide monohydrate (1.1
g) and the
reaction mixture was stirred at room temperature for 4 h. The reaction mixture
was concentrated
under reduced pressure and the residue was dissolved in water, acidified by
adding aqueous
potassium hydrogen sulfate, and extracted with ethyl acetate. The organic
layer was washed
with brine, dried over anhydrous sodium sulfate, and the solvent was removed
under reduced
pressure to give the title compound (1.8 g).
MS: [M-11[11 210Ø
[0275]
B) (R)-(5,6-Difluoro-l-methy1-1H-indo1-2-y1)(3-methylpiperazin-1-yOmethanone
To a mixture of 5,6-difluoro-l-methyl-1H-indole-2-carboxylic acid (1.8 g) and
DMF (45 mL) was
added DIEA (4.4 mL), (R)-2-methylpiperazine (1.02 g) and HATU (4.8 g). The
reaction
mixture was stirred at room temperature for 3 h, poured into ice-cold water,
and extracted with
ethyl acetate. The organic layer was washed with brine, dried over anhydrous
sodium sulfate,
and the solvent was removed under reduced pressure. The residue was purified
by silica gel
column chromatography (methanol/DCM) to give the title compound (1.9 g).
MS: [M+111+ 294.4.
[0276]
C) (R)-2-Chloro-1-(4-(5,6-difluoro-l-methy1-1H-indole-2-carbony1)-2-
methylpiperazin-1-
yDethan-1-one
To a mixture of (R)-(5,6-difluoro-l-methy1-1H-indo1-2-y1)(3-methylpiperazin-1-
y1)methanone
(1.9 g) and DCM (25 mL) was added TEA (1.35 mL) and chloroacetyl chloride (0.6
mL) under
ice-cooling. The reaction mixture was stirred at room temperature for 3 h,
diluted with DCM,
and washed with water and brine. The organic layer was dried over anhydrous
sodium sulfate
and the solvent was removed under reduced pressure. The residue was purified
by silica gel
column chromatography (ethyl acetate/hexane) to give the title compound (1.8
g).
Date Recue/Date Received 2022-01-27
51
CA 03148955 2022-01-27
MS: [M+1-11+ 370.2.
[0277]
D) 2-(2-(2-(Benzyloxy)ethoxy)ethoxy)ethyl methanesulfonate
To a mixture of 2-(2-(2-benzyloxyethoxy)ethoxy)ethanol (2 g) and DCM (15 mL)
was added TEA
(1.7 mL) and methanesulfonyl chloride (0.77 mL) under ice-cooling. The
reaction mixture was
stirred at room temperature for 12 h, diluted with DCM, washed with water and
brine, dried over
anhydrous sodium sulfate, and the solvent was removed under reduced pressure
to give the title
compound (2.5 g).
NMR (400 MHz, CDC13) 6 3.02-3.05 (3H, m), 3.61-3.65 (8H, m), 3.74-3.76 (2H,
m), 4.34-
4.36 (2H, m), 4.54 (2H, s), 7.27-7.33 (5H, m).
[0278]
E) tert-Butyl (2R,5R)-4-benzy1-2-methy1-5-(12-phenyl-2,5,8,11-
tetraoxadodecyl)piperazine-1-
carboxylate
To a mixture of tert-butyl (2R,5R)-4-benzy1-5-hydroxymethy1-2-methyl-
piperazine-1-carboxylate
(700 mg) and DMF (10 mL) was added sodium hydride (60%, dispersion in paraffin
liquid, 105
mg). The reaction mixture was stirred for 1 h, 2-(2-(2-
(benzyloxy)ethoxy)ethoxy)ethyl
methanesulfonate (695 mg) was added thereto and additionally stirred at 60 C
for 4 h. The
reaction mixture was cooled to room temperature and 2-(2-(2-
(benzyloxy)ethoxy)ethoxy)ethyl
methanesulfonate (556 mg) was added thereto and additionally stirred at 60 C
for 5 h. To the
reaction mixture was added water and the aqueous layer was extracted with
ethyl acetate. The
organic layer was washed with brine, dried over anhydrous sodium sulfate, and
the solvent was
removed under reduced pressure. The residue was purified by silica gel column
chromatography
(ethyl acetate/hexane) to give the title compound (900 mg).
MS: [M+1-11+ 543.2.
[0279]
F) tert-Butyl (2R,5R)-5-((2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)methyl)-2-
methylpiperazine-1-
carboxylate
To a mixture of tert-butyl (2R,5R)-4-benzy1-2-methy1-5-(12-phenyl-2,5,8,11-
tetraoxadodecyl)piperazine-1-carboxylate (900 mg), acetic acid (0.1 mL) and
ethanol (10 mL)
was added 10% palladium on carbon (200 mg). The reaction mixture was stirred
under the
normal pressure hydrogen atmosphere at room temperature for 16 h and filtered
with Celite , and
the filtrate was concentrated under reduced pressure. To the residue was added
10%
methanol/DCM, and the organic layer was washed with saturated aqueous sodium
bicarbonate
and dried over anhydrous sodium sulfate. The solvent was removed under reduced
pressure to
give the title compound (600 mg).
1H NMR (400 MHz, DMSO-d6) 6 1.12 (3H, d, J = 6.72 Hz), 1.39 (9H, s), 2.41 (1H,
dd, J = 2.74,
12.5 Hz), 2.88-2.94 (2H, m), 3.07 (1H, dd, J = 4.16, 13.5 Hz), 3.31-3.52 (15H,
m), 3.60-3.62 (1H,
m), 3.98 (1H, bs).
[0280]
G) tert-Butyl (2R,5R)-4-(24(R)-4-(5,6-difluoro-1-methy1-1H-indole-2-carbony1)-
2-
methylpiperazin-1-y1)-2-oxoethyl)-5-42-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)methyl)-2-
methylpiperazine-1-carboxylate
To a mixture of tert-butyl (2R,5R)-5-((2-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)methyl)-2-
methylpiperazine-l-carboxylate (592 mg) and THF (15 mL) was added TEA (0.3
mL), (R)-2-
chloro-1-(4-(5,6-difluoro-l-methy1-1H-indole-2-carbony1)-2-methylpiperazin-1-
yDethan-1-one
(550 mg) and tetrabutylammonium iodide (549 mg) and the resultant mixture was
stirred at 60 C
for 24 h. The reaction mixture added ethyl acetate was washed with water and
brine
subsequently, dried over anhydrous sodium sulfate, and the solvent was removed
under reduced
pressure. The residue was purified by silica gel column chromatography
(methanol/ethyl
acetate) to give the title compound (740 mg).
Date Recue/Date Received 2022-01-27
52
CA 03148955 2022-01-27
MS: [M+1-11+ 696.5.
[0281]
H) tert-Butyl (2R,5R)-4-(24(R)-4-(5,6-difluoro-1-methy1-1H-indole-2-carbony1)-
2-
methylpiperazin-1-y1)-2-oxoethyl)-2-methyl-5-42-(2-(2-45-44-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)methyDpiperazine -1-
carboxylate
To a mixture of tert-butyl (2R,5R)-4-(24(R)-4-(5,6-difluoro-l-methy1-1H-indole-
2-carbony1)-2-
methylpiperazin-1-y1)-2-oxoethyl)-5-42-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)methyl)-2-
methylpiperazine-1-carboxylate (30 mg), 54(4-(thieno[3,2-blpyridin-7-
yloxy)piperidin-1-
yOmethyDisoxazol-3-ol (17.1 mg), triphenylphosphine (56.5 mg) and toluene (2
mL) was added
di-tert-butyl azodicarboxylate (29.7 mg). The reaction mixture was stirred at
room temperature
for 16 h and the solvent was removed under reduced pressure. The residue was
purified by
preparative thin layer chromatography to give the title compound (26 mg).
MS: [M+111+ 1008.8.
[0282]
I) 14(R)-4-(5,6-Difluoro-l-methy1-1H-indole-2-carbony1)-2-methylpiperazin-1-
y1)-2-42R,5R)-5-
methyl-2-42-(2-(2-45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-
yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)methyDpiperazin-l-y1)ethan-l-one hydrochloride
To a mixture of tert-butyl (2R,5R)-4-(24(R)-4-(5,6-difluoro-l-methy1-1H-indole-
2-carbony1)-2-
methylpiperazin-1-y1)-2-oxoethyl)-2-methyl-5-42-(2-(2-45-44-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)methyDpiperazine -1-
carboxylate (25 mg) and DCM (1 mL) was added 4 M hydrogen chloride dioxane
solution (0.3
mL) under ice-cooling. The reaction mixture was stirred at room temperature
for 1 h, and the
solvent was removed under reduced pressure. The residue was washed with ether
and pentane to
give the title compound (19 mg).
1H NMR (400 MHz, DMSO-d6, 1 00 C) 6 1.15-1.28 (6H, m), 2.19-2.36 (4H, m),
2.76-2.98
(3H, m), 3.20-4.36 (36H, m), 5.15 (1H, m), 5.66 (1H, s), 6.56 (1H, s), 7.30
(1H, d, J = 8.0 Hz),
7.53-7.59 (2H, m), 7.63 (1H, d, J = 8.0 Hz), 8.22 (1H, d, J = 8.0 Hz), 8.68
(1H, d, J = 8.0 Hz),
9.01-9.40 (1H, m).
[0283]
Example 4
(S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro- 1 -methy1-3-(2-(2-(24(5-44-(thieno
[3,2-blpyridin-7-
yloxy)piperidin- 1 -yOmethyDisoxazol-3-yDoxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-
carbonyl)piperazin- 1 -y1)-2-oxoethyl)-2-(methylamino)propanamide
hydrochloride
[0284]
A) Methyl 5,6-difluoro-3-hy droxy-l-methy1-1H-indole-2-carboxy late
To a mixture of methyl 5,6-difluoro-3-formy1-1-methyl-1H-indole-2-carboxylate
(3.5 g) and
chloroform (50 mL) was added 3-chloroperoxybenzoic acid (5.88 g) and p-
toluenesulfonic acid
(3.15 g) at 5 to 10 C. The reaction mixture was stirred at the same
temperature for 2 h. To
the reaction mixture was added 2 M ammonia methanol solution (30 mL) and the
reaction mixture
was stirred at room temperature for 30 min. The solvent was removed under
reduced pressure
and the residue was diluted with saturated aqueous sodium bicarbonate, and
extracted with DCM.
The organic layer was washed with 10% aqueous sodium thiosulfate, dried over
anhydrous
sodium sulfate, and the solvent was removed under reduced pressure to give the
title compound
(3 g).
1H NMR (400 MHz, DMSO-d6) 63.81 (3H, s), 3.82 (3H, s), 7.56-7.69 (2H, m), 9.36
(1H, s).
[0285]
B) tert-Butyl 4-(5,6-difluoro-3-hydroxy-l-methy1-1H-indole-2-
carbonyl)piperazine-l-carboxylate
To a mixture of methyl 5,6-difluoro-3-hydroxy-l-methyl-1H-indole-2-carboxylate
(4.4 g), tert-
butyl piperazine-l-carboxylate (5.1 g) and toluene (45 mL) was added and 2 M
Date Recue/Date Received 2022-01-27
53
CA 03148955 2022-01-27
trimethylaluminum toluene solution (18.2 mL) under argon atmosphere at room
temperature.
The reaction mixture was stirred at 100 C for 3 h. To the reaction mixture
was added water,
the precipitates were filtered off, and the filtrate was extracted with ethyl
acetate. The organic
layer was dried over anhydrous sodium sulfate and the solvent was removed
under reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane)
to give the title compound (3 g).
MS: [M+H1+ 393.8.
[0286]
C) 2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate
To a mixture of 2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethan-1-ol (5 g) and DCM (100
mL) was
added TEA (4.4 mL), DMAP (1.27 g) and p-toluenesulfonyl chloride (4.8 g) under
ice-cooling,
and the reaction mixture was stirred at room temperature for 2 h. The reaction
mixture was
diluted with DCM and washed with water and brine subsequently. The organic
layer was dried
over anhydrous sodium sulfate and the solvent was removed under reduced
pressure. The
residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to give the title
compound (5.2 g).
MS: [M+H1+ 395Ø
[0287]
D) tert-Butyl 4-(3-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-difluoro-1-
methy1-1H-indole-
2-carbonyl)piperazine-l-carboxylate
To a mixture of tert-butyl 4-(5,6-difluoro-3-hydroxy-l-methy1-1H-indole-2-
carbonyl)piperazine-
l-carboxylate (1 g) and DMF (10 mL) was added cesium carbonate (2.06 g) and 2-
(2-(2-
(benzyloxy)ethoxy)ethoxy)ethyl 4-methylbenzensulfonate (1.49 g), and the
reaction mixture was
stirred at room temperature for 6 h. To the reaction mixture was added water
and the reaction
mixture was extracted with ethyl acetate. The organic layer was washed with
brine, dried over
anhydrous sodium sulfate, and the solvent was removed under reduced pressure.
The residue
was purified by silica gel column chromatography (ethyl acetate/hexane) to
give the title
compound (1.26 g).
MS: [M+H1+ 618Ø
[0288]
E) (3-(2-(2-(2-(Benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-difluoro-l-methy1-1H-indo1-
2-
y1)(piperazin-1-y1)methanone hydrochloride
To a mixture of tert-butyl 4-(3-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-
difluoro-1-
methy1-1H-indole-2-carbonyl)piperazine-l-carboxylate (1.2 g) and DCM (12 mL)
was added 4 M
hydrogen chloride dioxane solution (2 mL) under ice-cooling. The reaction
mixture was stirred
at room temperature for 4 h and the solvent was removed under reduced
pressure. The residue
was washed with diethyl ether to give the title compound (1 g).
MS: [M+H1+ 517.9.
[0289]
F) tert-Butyl (S)-(2-(4-(3-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-
difluoro-l-methy1-1H-
indole-2-carbonyl)piperazin-l-y1)-1-cyclohexyl-2-oxoethyl)carbamate
To a mixture of (3-(2-(2-(2-benzyloxyethoxy)ethoxy)ethoxy)-5,6-difluoro-l-
methy1-1H-indo1-2-
yDpiperazin-l-yl-methanone hydrochloride (1.1 g) and DMF (10 mL) was added
DIEA (0.694
mL). The reaction mixture was stirred at room temperature for 15 min and then
(S)-tert-
butoxycarbonylaminocyclohexylacetic acid (0.512 g), 1-hydroxybenzotriazole
(366 mg) and 1-
ethy1-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (458 mg) were added
thereto. The
reaction mixture was stirred at room temperature for 2 h, poured into ice-cold
water, and
extracted with ethyl acetate. The organic layer was dried over anhydrous
sodium sulfate and the
solvent was removed under reduced pressure to give the title compound (1.1 g).
MS: [M+H1+ 757Ø
Date Recue/Date Received 2022-01-27
54
CA 03148955 2022-01-27
[0290]
G) (S)-2-Amino-1-(4-(3-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-difluoro-
1-methy1-1H-
indole-2-carbonyl)piperazin-l-y1)-2-cyclohexylethan-1-one hydrochloride
To a mixture of tert-butyl (S)-(2-(4-(3-(2-(2-(2-
(benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-difluoro-
l-methy1-1H-indole-2-carbonyl)piperazin-1-y1)-1-cyclohexyl-2-
oxoethyl)carbamate (1.1 g) and
DCM (10 mL) was added 4 M hydrogen chloride dioxane solution (10 mL) under ice-
cooling.
The reaction mixture was stirred at room temperature for 3 h and the solvent
was removed under
reduced pressure to give the title compound (1.1 g).
MS: [M+111+ 657.2.
[0291]
H) tert-Butyl ((S)-1-4(S)-2-(4-(3-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)-
5,6-difluoro-l-
methyl-1H-indole-2-carbonyl)piperazin-l-y1)-1-cyclohexy1-2-oxoethyDamino)-1-
oxopropan-2-
y1)(methyl)carbamate
To a mixture of (S)-2-amino-1-(4-(3-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)-
5,6-difluoro-1-
methy1-1H-indole-2-carbonyl)piperazin-l-y1)-2-cyclohexylethan-l-one
hydrochloride (1.1 g) and
DMF (10 mL) was added DIEA (0.83 mL). The reaction mixture was stirred for 15
min, (S)-2-
(tert-butoxycarbonyl-methyl-amino)-propionic acid (0.323 g) and HATU (0.905 g)
were added
thereto, and the reaction mixture was stirred at room temperature for 16 h.
The reaction mixture
was poured into ice-cold water and extracted with ethyl acetate. The organic
layer was washed
with brine, dried over anhydrous sodium sulfate, and the solvent was removed
under reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane)
to give the title compound (850 mg).
MS: [MA41+ 842.1.
[0292]
I) tert-Butyl ((S)-1-(((S)-1-cyclohexy1-2-(4-(5,6-difluoro-3-(2-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)-1-methy1-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-2-(4-(3-(2-(2-(2-
(benzyloxy)ethoxy)ethoxy)ethoxy)-5,6-
difluoro-l-methyl-1H-indole-2-carbonyl)piperazin-l-y1)-1-cyclohexy1-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (850 mg) and ethanol (10 mL) was added 10%
palladium on
carbon (200 mg) at room temperature and the reaction mixture was stirred under
the normal
pressure hydrogen atmosphere for 3 h. The reaction mixture was filtered with
Celite , rinsed
with ethanol, and the filtrate was concentrated under reduced pressure to give
the title compound
(740 mg).
MS: [M+111+ 752.6.
[0293]
J) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-3-(2-(2-
(24(54(4-(thieno[3,2-
b] pyridin-7-yloxy)piperidin-l-y DmethyDisoxazol-3-y
Doxy)ethoxy)ethoxy)ethoxy)-1H-indole -2-
carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-y1)(methypc arbamate
To a mixture of tert-butyl ((S)-1-(((S)-1-cyclohexy1-2-(4-(5,6-difluoro-3-(2-
(2-(2-
hydroxyethoxy)ethoxy)ethoxy)-1-methy1-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (40 mg), 5-((4-(thieno[3,2-
b]pyridin-7-
yloxy)piperidin-l-yl)methyl)isoxazol-3-ol (21.1 mg), triphenylphosphine (69.7
mg) and toluene
(1.5 mL) was added di-tert-butyl azodicarboxylate (36.7 mg), and the reaction
mixture was stirred
at room temperature for 16 h and concentrated under reduced pressure. The
residue was purified
by preparative thin layer chromatography to give the title compound (36 mg).
MS: [M+111+ 1064.8.
[0294]
K) (S)-N-((S)-1-Cyclohexy1-2-(4-(5,6-difluoro-l-methyl-3-(2-(2-(24(54(4-
(thieno [3,2-b]pyridin-
7-yloxy)piperidin-l-yl)methyDisoxazol-3-y1)oxy)ethoxy)ethoxy)ethoxy)-1H-indole-
2-
Date Recue/Date Received 2022-01-27
55
CA 03148955 2022-01-27
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide hydrochloride
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-
methyl-3-(2-(2-(24(5-
44-(thieno[3,2-b]pyridin-7-yloxy)piperidin-l-yl)methyl)isoxazol-3-
yl)oxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (36 mg) and DCM (1 mL) was added 4 M hydrogen
chloride
dioxane solution (0.3 mL) under ice-cooling and the reaction mixture was
stirred at room
temperature for 2 h. The reaction mixture was concentrated under reduced
pressure and the
residue was washed with diethyl ether and pentane to give the title compound
(24 mg).
1H NMR (400 MHz, DMSO-do) 6 1.03-1.23 (5H, m), 1.33 (3H, d, J = 6.76 Hz), 1.60-
1.68 (4H,
m), 2.16 (2H, m), 2.32 (2H, m), 3.12-3.20 (3H, m), 3.47-3.50 (8H, m), 3.57-
3.74 (15H, m), 3.85-
3.86 (2H, m), 4.14 (2H, s), 4.31 (2H, s), 4.54-4.64 (3H, m), 5.08-5.29 (2H,
m), 6.63 (1H, s), 7.46
(1H, m), 7.65-7.69 (2H, m), 8.35 (1H, m), 8.79-8.80 (2H, m), 9.14 (1H, s),
11.7 (1H, br).
[0295]
Example 5
(S)-N-((S)-1-Cyclohexy1-2-(4-(5,6-difluoro-1-(3-methy1-2-oxo-14-45-44-
(thieno[3,2-b]pyridin-
7-yloxy)piperidin-l-y1)methyDisoxazol-3-y1)oxy)-6,9,12-trioxa-3-azatetradecy1)-
1H-indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide hydrochloride
[0296]
A) tert-Butyl ((S)-1-(((S)-1-cyclohexy1-2-(4-(5,6-difluoro-1H-indole-2-
carbonyl)piperazin-l-y1)-
2-oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-oxo-2-(piperazin-l-
y1)ethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (2.29 g), 5,6-difluoro-1H-indole-2-carboxylic
acid (1.00 g),
DIEA (2.72 mL) and DMF (20 mL) was added HATU (2.89 g) and the reaction
mixture was
stirred at room temperature for 1 h. To the reaction mixture was added water
and ethyl acetate
and the aqueous layer was extracted with ethyl acetate. The organic layer was
dried over
anhydrous magnesium sulfate and the solvent was removed under reduced pressure
to give the
title compound (2.64 g).
MS: [M+111+ 490.3.
[0297]
B) Methyl 2-(2-(44(S)-24(S)-2-((tert-butoxycarbonyl)(methyDamino)propanamido)-
2-
cyclohexylacetyl)piperazine-1-carbonyl)-5,6-difluoro-1H-indo1-1-yDacetate
A mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1H-indole-
2-
carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-y1)(methyl)carbamate
(2.64 g),
potassium carbonate (681 mg), methyl 2-bromoacetate (753 mg) and DMF (20 mL)
was stirred at
room temperature for 16 h. The reaction mixture was diluted with water and
ethyl acetate and
the aqueous layer was extracted with ethyl acetate. The organic layer was
dried over anhydrous
magnesium sulfate and the solvent was removed under reduced pressure. The
residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to give
the title compound
(2.96 g).
MS: [M+111+ 662.4.
[0298]
C) 2-(2-(44(S)-24(S)-2-((tert-Butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacetyl)piperazine-1-carbonyl)-5,6-difluoro-lH-indol-1-yDacetic acid
To a mixture of methyl 2-(2-(44(S)-24(S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-
2-cyclohexylacetyl)piperazine-1-carbonyl)-5,6-difluoro-1H-indo1-1-yDacetate
(2.76 g) and
methanol (20 mL) was added 2 M aqueous sodium hydroxide (10.4 mL). The
reaction mixture
was stirred at room temperature for 2 h, acidified with 1 M hydrochloric acid,
and extracted with
ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate
and the solvent
was removed under reduced pressure to give the title compound (2.16 g).
MS: [M+111+ 648.4.
Date Recue/Date Received 2022-01-27
56
CA 03148955 2022-01-27
[0299]
D) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(14-hydroxy-3-
methy1-2-oxo-6,9,12-
trioxa-3-azatetradecy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-
1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of 2-(2-(44(S)-24(S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-
cyclohexylacetyl)piperazine-1-carbonyl)-5,6-difluoro-1H-indo1-1-yDacetic acid
(1.20 g), 5,8,11-
trioxa-2-azatridecan-13-ol (422 mg), DIEA (0.65 mL) and DMF (9.3 mL) was added
HATU (986
mg) and the reaction mixture was stirred at room temperature for 1 h. The
reaction mixture was
diluted with water and ethyl acetate and extracted with ethyl acetate. The
organic layer was
dried over anhydrous magnesium sulfate and the solvent was removed under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to give the
title compound (695 mg).
MS: [M+111+ 837.5.
[0300]
E) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(3-methy1-2-oxo-14-
45-44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)-6,9,12-
trioxa-3-
azatetradecy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-
oxopropan-2-
y1)(methyl)carbamate
To a mixture of 54(4-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-
y1)methyDisoxazol-3-ol (265
mg), tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(14-hydroxy-3-
methy1-2-oxo-
6,9,12-trioxa-3-azatetradecy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (670 mg), triphenylphosphine (1.05 g) and
toluene (8 mL)
was added di-tert-butyl azodicarboxylate (553 mg) and the reaction mixture was
stirred at room
temperature for 1 h. The reaction mixture was concentrated under reduced
pressure and the
residue was purified by silica gel column chromatography (C18, acetonitrile/5
mM aqueous
ammonium acetate) to give the title compound (575 mg).
MS: [M+111+ 1150.4.
[0301]
F) (S)-N-((S)-1-Cyclohexy1-2-(4-(5,6-difluoro-1-(3-methy1-2-oxo-14-45-44-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)-6,9,12-trioxa-3-
azatetradecy1)-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
hydrochloride
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(3-
methy1-2-oxo-14-45-
44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-y1)oxy)-6,9,12-
trioxa-3-
azatetradecy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-
oxopropan-2-
y1)(methyl)carbamate (575 mg) and ethyl acetate (1 mL) was added 4 M hydrogen
chloride ethyl
acetate solution (2 mL) and the reaction mixture was stirred at room
temperature for 1 h. The
reaction mixture was concentrated under reduced pressure to give the title
compound (549 mg).
1H NMR (400 MHz, DMSO-d6, 1 00 C) 6 0.95-1.32 (5H, m), 1.35-1.40 (3H, m),
1.57-1.78
(6H, m), 1.94-2.08 (2H, m), 2.18-2.27 (2H, m), 3.05-3.78 (32H, m), 3.84-3.95
(1H, m), 3.99-4.21
(2H, m), 4.28-4.33 (2H, m), 4.63-4.70 (1H, m), 4.93-5.03 (1H, m), 5.17-5.26
(2H, m), 6.32-6.40
(1H, m), 6.72 (1H, s), 7.07-7.17 (1H, m), 7.47-7.59 (3H, m), 8.08-8.13 (1H,
m), 8.39-8.48 (1H,
m), 8.53-8.62 (1H, m), 8.63-8.93 (2H, m).
[0302]
Example 6
(S)-N-((S)-1-Cyclohexy1-2-(4-(5,6-difluoro-1-(2-oxo-24(S)-2-(((54(4-
(thieno[3,2-blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-y1)ethyl)-1H-
indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide hydrochloride
[0303]
A) (S)-3-(Pyrrolidin-2-ylmethoxy)-5-((4-(thieno[3,2-blpyridin-7-
yloxy)piperidin-1-
yOmethyDisoxazole
Date Recue/Date Received 2022-01-27
57
CA 03148955 2022-01-27
To a mixture of 54(4-(thieno[3,2-blpyridin-7-y1oxy)piperidin-1-
y1)methyDisoxazol-3-ol (398.8
mg), (9H-fluoren-9-yl)methyl (S)-2-(hydroxymethyl)pyrrolidine-1-carboxylate
(389.2 mg),
triphenylphosphine (378.8 mg) and toluene (5 mL) was added di-tert-butyl
azodicarboxylate
(332.5 mg). The reaction mixture was stirred at room temperature for 30 min
and concentrated
under reduced pressure. The residue was dissolved in DMF (4 mL) and
tetrabutylammonium
fluoride (6 mL). The reaction mixture was stirred at room temperature for 15
min and
concentrated under reduced pressure, and TFA (5 mL) was added to the residue.
The reaction
mixture was stirred at room temperature for 5 min, diluted with water and
washed with ethyl
acetate. The aqueous layer was basified with aqueous 2 M sodium hydroxide
solution and
extracted with ethyl acetate. The organic layer was washed with water and
brine, dried over
anhydrous magnesium sulfate, and the solvent was removed under reduced
pressure. The
residue was purified by silica gel column chromatography (NH, methanol/ethyl
acetate) to give
the title compound (199.4 mg).
MS: [M+111+ 415.2.
[0304]
B) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(2-oxo-24(S)-2-
(((5-44-(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-
y1)ethyl)-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of 2-(2-(44(S)-24(S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-
cyclohexylacetyl)piperazine-1-carbonyl)-5,6-difluoro-1H-indo1-1-yDacetic acid
(199.8 mg), (S)-
3-(pyrrolidin-2-ylmethoxy)-5-((4-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-
yl)methyl)isoxazole
(140.7 mg), DIEA (107 ttL), 1-hydroxybenzotriazole (83.37 mg) and DMF (3 mL)
was added 1-
ethy1-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (118.3 mg). The
reaction mixture
was stirred at room temperature for 2 h, water was added thereto, and the
precipitates were
collected by filtration and washed with water to give the title compound
(320.8 mg).
MS: [MA41+ 1044.3.
[0305]
C) (S)-N-((S)-1-Cyclohexy1-2-(4-(5,6-difluoro-1-(2-oxo-24(S)-2-(((54(4-
(thieno[3,2-blpyridin-
7-yloxy)piperidin-l-yl)methyDisoxazol-3-yl)oxy)methyl)pyrrolidin-l-y1)ethyl)-
1H-indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide hydrochloride
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(2-
oxo-24(S)-2-(((5-
44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
y1)oxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate (380.2 mg) and ethyl acetate (1.5 mL) was added 4 M
hydrogen chloride
ethyl acetate solution (1 mL). The reaction mixture was stirred at room
temperature for 1.5 h
and diisopropyl ether was added thereto. The precipitates were collected by
filtration and
washed with diisopropyl ether to give the title compound (280.3 mg).
MS: [M+111+ 944.2.
The title compound was neutralized with saturated aqueous sodium bicarbonate
and the
precipitates were collected by filtration as free base, subjected to 1H NMR
was measurement.
1H NMR (300 MHz, DMSO-do) 60.78-1.27 (7H, m), 1.45-2.21 (19H, m), 2.41 (3H,
s), 2.58-2.78
(2H, m), 2.86-3.03 (1H, m), 3.18-3.85 (10H, m), 4.02-4.31 (3H, m), 4.53-4.87
(3H, m), 5.04-5.38
(2H, m), 6.10-6.39 (1H, m), 6.76 (1H, s), 7.05 (1H, dd, J = 5.6, 2.7 Hz), 7.49
(1H, dd, J = 5.5, 2.1
Hz), 7.62 (1H, dd, J = 10.9, 8.0 Hz), 7.66-7.84 (1H, m), 7.93 (1H, d, J = 8.6
Hz), 8.02 (1H, dd, J
= 5.4, 3.3 Hz), 8.49 (1H, d, J = 5.4 Hz).
[0306]
Example 7
(S)-N-((S)-1-Cyclohexy1-2-(4-(5-fluoro-1-(2-oxo-2-((S)-2-(((5-44-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-y1)ethyl)-1H-
indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
Date Recue/Date Received 2022-01-27
58
CA 03148955 2022-01-27
[0307]
A) tert-Butyl ((S)-1-(((S)-1-cyclohexy1-2-(4-(5-fluoro-1H-indole-2-
carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-oxo-2-(piperazin-l-
y1)ethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (7 g) and DMF (60 mL) was added 5-fluoro-1H-
indole-2-
carboxylic acid (3.36 g), 1-hydroxybenzotriazole (3.00 g), DIEA (11.9 mL) and
1-ethy1-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (4.25 g). The reaction mixture
was stirred at
room temperature for 16 h, water was added thereto, and extracted with ethyl
acetate. The
organic layer was washed with water, saturated aqueous ammonium chloride,
saturated aqueous
sodium bicarbonate and brine subsequently, and dried over anhydrous sodium
sulfate. The
solvent was removed under reduced pressure to give the title compound (9.5 g).
MS: [M+111+ 572Ø
[0308]
B) Methyl 2-(2-(44(S)-24(S)-2-((tert-butoxycarbonyl)(methyDamino)propanamido)-
2-
cyclohexylacetyl)piperazine-1-carbonyl)-5-fluoro-1H-indo1-1-yl)acetate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5-fluoro-1H-indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-y1)(methyl)carbamate
(9.5 g) and
DMF (50 mL) was added potassium carbonate (6.89 g) and methyl bromoacetate
(4.59 mL) under
ice-cooling, the reaction mixture was stirred at room temperature for 16 h,
and water was added
thereto and extracted with ethyl acetate. The organic layer was washed with
water and brine,
dried over anhydrous sodium sulfate, and the solvent was removed under reduced
pressure. The
residue was purified by silica gel column chromatography (ethyl
acetate/hexane) to give the title
compound (8.6 g).
MS: [MA41+ 644.1.
[0309]
C) 2-(2-(4-((S)-2-((S)-2-((tert-Butoxycarbonyl)(methyl)amino)propanamido)-2-
cyclohexylacetyl)piperazine-l-carbonyl)-5-fluoro-lH-indol-1-y1)acetic acid
To a mixture of methyl 2-(2-(4-((S)-2-((S)-2-((tert-
butoxycarbonyl)(methyl)amino)propanamido)-
2-cyclohexylacetyl)piperazine-l-carbonyl)-5-fluoro-lH-indol-1-yDacetate (8.6
g) and methanol
(25 mL) was added aqueous 2 M sodium hydroxide solution (13.4 mL) and the
reaction mixture
was stirred at room temperature for 20 min. The reaction mixture was diluted
with water and
washed with diethyl ether. The organic layer was extracted with aqueous 0.01 M
sodium
hydroxide solution. The aqueous layer was acidified with 6 M hydrochloric acid
and extracted
with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate
and the solvent
was removed under reduced pressure to give the title compound (8.1 g).
MS: [M+111+ 630.2.
[0310]
D) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5-fluoro-1-(2-oxo-24(S)-2-(((5-44-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-
y1)ethyl)-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of 2-(2-(44(S)-24(S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-
cyclohexylacetyl)piperazine-1-carbonyl)-5-fluoro-1H-indo1-1-yl)acetic acid
(700 mg) and DMF
(10 mL) was added (S)-3-(pyrrolidin-2-ylmethoxy)-54(4-(thieno[3,2-blpyridin-7-
yloxy)piperidin-1-yOmethyDisoxazole (506.8 mg), DIEA (0.78 mL) and HATU (507.8
mg), and
the reaction mixture was stirred at room temperature for 16 h. To the reaction
mixture was
added ice-cooling water and the reaction mixture was extracted with ethyl
acetate. The organic
layer was washed with water and brine, dried over anhydrous magnesium sulfate,
and the solvent
was removed under reduced pressure. The residue was purified by silica gel
column
chromatography (ethyl acetate/hexane) to give the title compound (950 mg).
MS: [M+111+ 1025.8.
Date Recue/Date Received 2022-01-27
59
CA 03148955 2022-01-27
[0311]
E) (S)-N-((S)-1-cyclohexy1-2-(4-(5-fluoro-1-(2-oxo-2-((S)-2-(((5-44-
(thieno[3,2-blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-y1)ethyl)-1H-
indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5-fluoro-1-(2-oxo-2-
((S)-2-(((5-44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate (60 mg) and ethyl acetate (0.2 mL) was added 4 M hydrogen
chloride ethyl
acetate solution (0.043 mL), and the reaction mixture was stirred at room
temperature for 4 h.
The precipitates were collected by filtration. To a mixture of (S)-N-((S)-1-
cyclohexy1-2-(4-(5-
fluoro-1-(2-oxo-2-((S)-2-(((5-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-
yl)methyl)-1,2-
oxazol-3-yl)oxy)methyl)pyrrolidin-1-yDethyl)-1H-indole-2-carbonyl)piperazin-1-
y1)-2-oxoethyl)-
2-(methylamino)propanamide hydrochloride thus obtained and water (0.2 mL) was
added
saturated aqueous sodium bicarbonate (0.043 mL). The reaction mixture was
stirred at room
temperature for 30 min, and the precipitates were collected by filtration to
give the title
compound (46 mg).
1H NMR (400 MHz, DMSO-d6, 1 0 0 C) 61.07-1.25 (m, 6H), 1.55-1.76(m, 6H), 1.76-
1.88(m,
2H), 1.88-2.12 (m, 7H), 2.24 (s, 3H), 2.32 (s, 2H), 2.70-2.79 (m, 2H), 2.95-
3.05 (m, 2H), 3.57-
3.72 (m, 12H), 4.09-4.40 (m, 3H), 4.65 (s, 1H), 4.79 (s, 1H), 5.17-5.23 (m,
2H), 5.99-6.29 (m,
1H), 6.70 (s, 1H), 6.98 (d, J = 5.3 Hz, 1H), 7.01-7.06 (m, 1H), 7.12-7.21 (m,
1H), 7.20-7.29 (m,
1H), 7.34 (d, J = 8.5 Hz, 1H), 7.42-7.55 (m, 2H), 7.67 (s, 1H), 7.95 (d, J =
5.1 Hz, 1H), 8.49 (d, J
= 5.2 Hz, 1H).
[0312]
Example 8
(S)-N-((S)-1-Cyclohexy1-24(S)-4-(5,6-difluoro-1-(2-oxo-24(S)-2-(((5-44-
(thieno[3,2-blpyridin-
7-yloxy)piperidin-l-yl)methyDisoxazol-3-yl)oxy)methyl)pyrrolidin-l-y1)ethyl)-
1H-indole-2-
carbony1)-3-methylpiperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
[0313]
A) (S)-3-(Pyrrolidin-2-ylmethoxy)-5-((4-(thieno[3,2-blpyridin-7-
yloxy)piperidin-1-
yOmethyDisoxazole trihydrochloride
A mixture of methyl 3-hydroxyisoxazole-5-carboxylate (3.79 g), tert-butyl (S)-
2-
(hydroxymethyl)pyrrolidine-1-carboxylate (4.84 g) and triphenylphosphine (6.94
g) was
azeotroped with toluene. To a mixture of the residue and toluene (50 mL) was
added 40%
diethyl azodicarboxylate toluene solution (14 mL) under ice-cooling. The
reaction mixture was
stirred at room temperature for 1 h, the solvent was reduced to half volume
under reduced
pressure, and the reaction mixture was purified by silica gel column
chromatography (ethyl
acetate/hexane). To a mixture of methyl (S)-3-((1-(tert-
butoxycarbonyl)pyrrolidin-2-
yl)methoxy)isoxazole-5-carboxylate thus obtained and methanol (100 mL) was
added sodium
borohydride (1.18 g). The reaction mixture was stirred at room temperature for
4 h, water was
added thereto, and extracted with ethyl acetate. The organic layer was washed
with brine, dried
over anhydrous magnesium sulfate, and the solvent was removed under reduced
pressure. To a
mixture of tert-butyl (S)-2-4(5-(hydroxymethyDisoxazol-3-
yDoxy)methyl)pyrrolidine-1-
carboxylate thus obtained and THF (100 mL) was added methanesulfonyl chloride
(2.10 mL).
The reaction mixture was stirred at room temperature for 2 h, saturated
aqueous sodium
bicarbonate was added thereto, and extracted with ethyl acetate. The organic
layer was washed
with brine, dried over anhydrous magnesium sulfate, and the solvent was
removed under reduced
pressure. A mixture of tert-butyl (S)-2-4(5-
(((methylsulfonyl)oxy)methyDisoxazol-3-
yDoxy)methyl)pyrrolidine-l-carboxylate thus obtained, 7-(piperidin-4-
yloxy)thieno[3,2-
blpyridine dihydrochloride (8.85 g), potassium carbonate (19.9 g),
tetrabutylammonium iodide
(5.32 g) and DMF (50 mL) was stirred at room temperature for 16 h. To the
reaction mixture
Date Recue/Date Received 2022-01-27
60
CA 03148955 2022-01-27
was added water and ethyl acetate, and the reaction mixture was extracted with
ethyl acetate.
The organic layer was washed with water and brine, dried over anhydrous
magnesium sulfate, and
the solvent was removed under reduced pressure. The residue was purified by
silica gel column
chromatography (NH, ethyl acetate/hexane). To a mixture of tert-butyl (S)-2-
4(54(4-
(thieno[3,2-blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-
yDoxy)methyl)pyrrolidine-1-
carboxylate thus obtained and ethyl acetate (20 mL) was added 4 M hydrogen
chloride ethyl
acetate solution (40 mL). The reaction mixture was stirred at room temperature
for 1 h and the
solvent was removed under reduced pressure to give the title compound (8.03
g).
MS: [M+111+ 415.2.
[0314]
B) tert-Butyl (S)-4-(5,6-difluoro-1H-indole-2-carbony1)-3-methylpiperazine-1-
carboxylate
To a mixture of tert-butyl (S)-3-methylpiperazine-1-carboxylate (1.12 g), 5,6-
difluoro-1H-indole-
2-carboxylic acid (1.00 g), DIEA (2.72 mL) and DMF (20 mL) was added HATU
(2.89 g). The
reaction mixture was stirred at room temperature for 1 h, diluted with water
and ethyl acetate,
and extracted with ethyl acetate. The organic layer was dried over anhydrous
magnesium sulfate
and the solvent was removed under reduced pressure to give the title compound
(1.61 g).
MS: [M+H-tBul+ 324.2.
[0315]
C) Methyl 2-(24(S)-44(S)-2-((tert-butoxycarbonyDamino)-2-cyclohexylacety1)-2-
methylpiperazine-1-carbonyl)-5,6-difluoro-lH-indol-1-yDacetate
A mixture of tert-butyl (S)-4-(5,6-difluoro-1H-indole-2-carbony1)-3-
methylpiperazine-1-
carboxylate (1.61 g), potassium carbonate (762 mg), methyl 2-bromoacetate
(0.629 mL) and DMF
(15 mL) was stirred at room temperature for 16 h. To the reaction mixture was
added water and
ethyl acetate, and the reaction mixture was extracted with ethyl acetate. The
organic layer was
dried over anhydrous magnesium sulfate, and the solvent was removed under
reduced pressure.
The residue was purified by silica gel column chromatography (ethyl
acetate/hexane). To a
mixture of tert-butyl (S)-4-(5,6-difluoro-1-(2-methoxy-2-oxoethyl)-1H-indole-2-
carbony1)-3-
methylpiperazine-1-carboxylate thus obtained and ethyl acetate (10 mL) was
added 4 M hydrogen
chloride ethyl acetate solution (21.2 mL). The reaction mixture was stirred at
room temperature
for 1 h and the solvent was removed under reduced pressure. To a mixture of
methyl (S)-2-(5,6-
difluoro-2-(2-methylpiperazine-1-carbony1)-1H-indol-1-y1)acetate hydrochloride
thus obtained,
(S)-2-((tert-butoxycarbonyl)amino)-2-cyclohexylacetic acid (1.20 g), DIEA (3.0
mL) and DMF
(20 mL) was added HATU (3.22 g). The reaction mixture was stirred at room
temperature for 1
h, water and ethyl acetate were added thereto, and extracted with ethyl
acetate. The organic
layer was dried over anhydrous magnesium sulfate, and the solvent was removed
under reduced
pressure to give the title compound (639 mg).
MS: [M+111+ 591.5.
[0316]
D) Methyl 2-(24(S)-44(S)-2-((S)-2-((tert-
butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carbonyl)-5,6-difluoro-lH-indol-1-
y1)acetate
To a mixture of methyl 2-(24(S)-44(S)-2-((tert-butoxycarbonyl)amino)-2-
cyclohexylacety1)-2-
methylpiperazine-l-carbony1)-5,6-difluoro-1H-indo1-1-yDacetate (639 mg) and
ethyl acetate (3
mL) was added 4 M hydrogen chloride ethyl acetate solution (5 mL). The
reaction mixture was
stirred at room temperature for 1 h and the solvent was removed under reduced
pressure. To a
mixture of methyl 2-(24(S)-44(S)-2-amino-2-cyclohexylacety1)-2-
methylpiperazine-l-carbony1)-
5,6-difluoro-1H-indo1-1-yDacetate hydrochloride thus obtained, N-(tert-
butoxycarbony1)-N-
methyl-L-alanine (329 mg), DIEA (0.85 mL) and DMF (5 mL) was added HATU (616
mg). The
reaction mixture was stirred at room temperature for 1 h, water and ethyl
acetate were added
thereto, and extracted with ethyl acetate. The organic layer was dried over
anhydrous
magnesium sulfate and the solvent was removed under reduced pressure. The
residue was
Date Recue/Date Received 2022-01-27
61
CA 03148955 2022-01-27
purified by silica gel column chromatography (ethyl acetate/hexane) to give
the title compound
(450 mg).
MS: [M+111+ 676.5.
[0317]
E) 2-(24(S)-44(S)-2-((S)-2-((tert-Butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carbonyl)-5,6-difluoro-lH-indol-1-
y1)acetic acid
To a mixture of methyl 2-(24(S)-44(S)-2-((S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-cyclohexylacety1)-2-
methylpiperazine-1-
carbonyl)-5,6-difluoro-lH-indol-1-yDacetate (225 mg) and methanol (1.6 mL) was
added 2 M
aqueous sodium hydroxide (0.8 mL). The reaction mixture was stirred at room
temperature for 1
h, acidified with 1 M hydrochloric acid, and extracted with ethyl acetate. The
organic layer was
dried over anhydrous magnesium sulfate, and the solvent was removed under
reduced pressure to
give the title compound (221 mg).
MS: [M+111+ 662.4.
[0318]
F) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-24(S)-4-(5,6-difluoro-1-(2-oxo-24(S)-2-
(45-44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbony1)-3-methylpiperazin-l-y1)-2-oxoethyDamino)-1-
oxopropan-2-
y1)(methyl)carbamate
To a mixture of 2-(24(S)-44(S)-2-((S)-2-((tert-
butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carbonyl)-5,6-difluoro-lH-indol-1-
y1)acetic acid (336
mg), (S)-3-(pyrrolidin-2-ylmethoxy)-5-((4-(thieno[3,2-blpyridin-7-
yloxy)piperidin-1-
yOmethyDisoxazole trihydrochloride (319 mg), DIEA (0.53 mL) and DMF (3 mL) was
added and
HATU (386 mg). The reaction mixture was stirred at room temperature for 2 h,
water was added
thereto, and the precipitates were collected by filtration and purified by
silica gel column
chromatography (C18, acetonitrile/5 mM aqueous ammonium acetate
/acetonitrile). To the
product thus obtained was added ethyl acetate and saturated aqueous sodium
bicarbonate, the
organic layer was dried over anhydrous magnesium sulfate, and the solvent was
removed under
reduced pressure to give the title compound (320 mg).
MS: [M+111+ 1058.5.
[0319]
G) (S)-N-((S)-1-Cyclohexy1-24(S)-4-(5,6-difluoro-1-(2-oxo-24(S)-2-(((5-44-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-
y1)ethyl)-1H-
indole-2-carbony1)-3-methylpiperazin-l-y1)-2-oxoethyl)-2-
(methylamino)propanamide
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-24(S)-4-(5,6-difluoro-1-(2-
oxo-24(S)-2-
(45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yl)methyDisoxazol-3-
yl)oxy)methyl)pyrrolidin-l-yl)ethyl)-1H-indole-2-carbonyl)-3-methylpiperazin-1-
y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (320 mg) and ethyl acetate
(2 mL) was
added 4 M hydrogen chloride ethyl acetate solution (2 mL). The reaction
mixture was stirred at
room temperature for 1.5 h and the solvent was removed under reduced pressure.
To the residue
was added saturated aqueous sodium bicarbonate, and the precipitates were
collected by filtration
to give the title compound (216 mg).
1H NMR (300 MHz, DMSO-d6) 60.77-1.33 (10H, m), 1.40-2.24 (19H, m), 2.31-2.46
(2H, m),
2.58-3.49 (8H, m), 3.53-3.70 (3H, m), 3.82-4.86 (8H, m), 5.01-5.48 (2H, m),
6.07-6.39 (1H, m),
6.67-6.81 (1H, m), 7.05 (1H, dd, J = 5.6, 1.9 Hz), 7.49 (1H, dd, J = 5.6, 3.0
Hz), 7.62 (1H, dd, J =
10.9, 8.1 Hz), 7.68-7.83 (1H, m), 7.89 (1H, t, J = 9.4 Hz), 7.97-8.07 (1H, m),
8.49 (1H, d, J = 5.7
Hz).
[0320]
Example 9
(S)-N-((S)-1-cyclohexy1-24(S)-4-(5-fluoro-1-(2-oxo-24(S)-2-(((5-44-(thieno[3,2-
blpyridin-7-
Date Recue/Date Received 2022-01-27
62
CA 03148955 2022-01-27
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-y1)ethyl)-1H-
indole-2-
carbony1)-3-methylpiperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
[0321]
A) Benzyl (S)-44(S)-2-((tert-butoxycarbonyDamino)-2-cyclohexylacety1)-2-
methylpiperazine-1-
carboxylate
To a mixture of (S)-2-((tert-butoxycarbonyl)amino)-2-cyclohexylacetic acid (5
g), benzyl (S)-2-
methylpiperazine-1-carboxylate hydrochloride (5.26 g), 1-hydroxybenzotriazole
(3.4 g), DIEA
(10.2 mL) and DMF (50 mL) was added 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide
hydrochloride (4.8 g). The reaction mixture was stirred at room temperature
for 2 h, ice-cooling
water was added thereto, and the aqueous layer was extracted with ethyl
acetate. The organic
layer was washed with water, saturated aqueous sodium bicarbonate and brine
subsequently,
dried over anhydrous sodium sulfate, and the solvent was removed under reduced
pressure to give
the title compound (6.7 g).
MS: [M+111+ 474.2.
[0322]
B) Benzyl (S)-44(S)-2-amino-2-cyclohexylacety1)-2-methylpiperazine-1-
carboxylate
hydrochloride
To a mixture of benzyl (S)-44(S)-2-(((tert-butoxy)carbonyDamino)-2-
cyclohexylacety1)-2-
methylpiperazine-1-carboxylate (6.7 g) and DCM (60 mL) was added 4 M hydrogen
chloride
dioxane solution (30 mL). The reaction mixture was stirred at room temperature
for 3 h and the
solvent was removed under reduced pressure. The residue was washed with DCM to
give the
title compound (6 g).
1H NMR (400 MHz, DMSO-do ) 6 1.01-1.25 (8H, m), 1.59-1.70 (6H, m), 2.85-3.18
(3H, m), 3.77-
4.28 (5H, m), 5.10 (2H, s), 7.33-7.37 (5H, m), 8.06-8.04 (3H, m).
[0323]
C) Benzyl (S)-44(S)-24(S)-2-((tert-butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carboxylate
To a mixture of N-(tert-butoxycarbony1)-N-methyl-L-alanine (3 g) and DMF (30
mL) was added
benzyl (S)-44(S)-2-amino-2-cyclohexylacety1)-2-methylpiperazine-1-carboxylate
hydrochloride
(6 g), DIEA (10.3 mL) and HATU (7.3 g). The reaction mixture was stirred at
room temperature
for 16 h, ice-cooling water was added thereto, and the aqueous layer was
extracted with ethyl
acetate. The organic layer was washed with water, saturated aqueous sodium
bicarbonate, and
brine subsequently, dried over anhydrous sodium sulfate, and the solvent was
removed under
reduced pressure. The residue was purified by silica gel column chromatography
(ethyl
acetate/hexane) to give the title compound (6 g).
MS: [M+111+ 559.1.
[0324]
D) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-24(S)-3-methylpiperazin-l-y1)-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate
To a mixture of benzyl (S)-44(S)-24(S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carboxylate (3.8 g) and ethanol (50 mL)
was added 10%
palladium on carbon (1 g). The reaction mixture was stirred under the normal
pressure
hydrogen atmosphere at room temperature for 16 h and filtered with Celite .
The filtrate was
concentrated under reduced pressure to give the title compound (2.3 g).
MS: [M+111+ 425.2.
[0325]
E) Methyl 2-(24(S)-44(S)-2-((S)-2-((tert-
butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carbonyl)-5-fluoro-lH-indol-1-
y1)acetate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-24(S)-3-methylpiperazin-l-
y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (901 mg), 5-fluoro-1H-indole-
2-carboxylic
Date Recue/Date Received 2022-01-27
63
CA 03148955 2022-01-27
acid (380 mg), DIEA (1.14 mL) and DMF (12 mL) was added HATU (1.21 g). The
reaction
mixture was stirred at room temperature for 1 h and poured into water, and the
precipitates were
collected by filtration and azeotroped with toluene. A mixture of tert-butyl
((S)-1-4(S)-1-
cyclohexy1-24(S)-4-(5-fluoro-1H-indole-2-carbony1)-3-methylpiperazin-1-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate thus obtained, potassium
carbonate (322
mg), methyl 2-bromoacetate (356 mg) and DMF (12 mL) was stirred at room
temperature for 16
h. The reaction mixture was diluted with water and ethyl acetate and extracted
with ethyl
acetate. The organic layer was dried over anhydrous magnesium sulfate and the
solvent was
removed under reduced pressure. The residue was purified by silica gel column
chromatography
(ethyl acetate/hexane) to give the title compound (979 mg).
MS: [M+111+ 658.4.
[0326]
F) 2-(24(S)-44(S)-2-((S)-2-((tert-Butoxycarbonyl)(methyDamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carbonyl)-5-fluoro-lH-indol-1-y1)acetic
acid
To a mixture of methyl 2-(24(S)-44(S)-2-((S)-2-((tert-
butoxycarbonyl)(methyDamino)propanamido)-2-cyclohexylacety1)-2-
methylpiperazine-1-
carbonyl)-5-fluoro-lH-indol-1-yDacetate (979 mg) and methanol (8 mL) was added
2 M aqueous
sodium hydroxide (3.72 mL). The reaction mixture was stirred at room
temperature for 1 h,
acidified with 1 M hydrochloric acid, and extracted with ethyl acetate. The
organic layer was
dried over anhydrous magnesium sulfate, and the solvent was removed under
reduced pressure to
give the title compound (915 mg).
MS: [M+111+ 644.4.
[0327]
G) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-24(S)-4-(5-fluoro-1-(2-oxo-24(S)-2-(((5-
44-(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-
y1)ethyl)-1H-
indole-2-carbony1)-3-methylpiperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of 2-(24(S)-44(S)-2-((S)-2-((tert-
butoxycarbonyl)(methypamino)propanamido)-2-
cyclohexylacety1)-2-methylpiperazine-1-carbonyl)-5-fluoro-lH-indol-1-yDacetic
acid (621 mg),
(S)-3-(pyrrolidin-2-ylmethoxy)-5-((4-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-
yOmethyDisoxazole trihydrochloride (606 mg), DIEA (1.0 mL) and DMF (5 mL) was
added
HATU (734 mg). The reaction mixture was stirred at room temperature for 2 h,
water was added
thereto, and the precipitates were collected by filtration and purified by
silica gel column
chromatography (C18, acetonitrile/5 mM aqueous ammonium acetate). The product
thus
obtained was diluted with ethyl acetate and the organic layer was washed with
saturated aqueous
sodium bicarbonate. The organic layer was dried over anhydrous sodium sulfate
and the solvent
was removed under reduced pressure to give the title compound (367 mg).
MS: [M+111+ 1040.5.
[0328]
H) (S)-N-((S)-1-cyclohexy1-24(S)-4-(5-fluoro-1-(2-oxo-24(S)-2-(((5-44-
(thieno[3,2-blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)methyl)pyrrolidin-l-y1)ethyl)-1H-
indole-2-
carbony1)-3-methylpiperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-24(S)-4-(5-fluoro-1-(2-oxo-
24(S)-2-(((5-
44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
y1)oxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbony1)-3-methylpiperazin-l-y1)-2-oxoethyDamino)-1-
oxopropan-2-
y1)(methyl)carbamate (367 mg) and ethyl acetate (2 mL) was added 4 M hydrogen
chloride ethyl
acetate solution (3 mL). The reaction mixture was stirred at room temperature
for 1.5 h and the
solvent was removed under reduced pressure. To the residue was added saturated
aqueous
sodium bicarbonate, and the precipitates were collected by filtration to give
the title compound
(249 mg).
Date Recue/Date Received 2022-01-27
64
CA 03148955 2022-01-27
'H NMR (300 MHz, DMSO-do) 60.74-1.34 (10H, m), 1.38-2.25 (19H, m), 2.33-2.46
(2H, m),
2.60-3.50 (8H, m), 3.55-3.71 (3H, m), 3.79-4.94 (8H, m), 5.00-5.50 (2H, m),
6.03-6.43 (1H, m),
6.61-6.82 (1H, m), 6.98-7.19 (2H, m), 7.26-7.71 (3H, m), 7.89 (1H, t, J = 9.7
Hz), 7.98-8.13 (1H,
m), 8.49 (1H, d, J = 5.5 Hz).
[0329]
Example 10
(S)-N-((S)-1-Cyclohexy1-2-(4-(5,6-difluoro-l-methyl-4-(2-(2-(2-(24(54(4-
(thieno[3,2-b]pyridin-
7-yloxy)piperidin-l-yl)methyDisoxazol-3-yl)oxy)ethoxy)ethoxy)ethoxy)ethoxy)-1H-
indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide hydrochloride
[0330]
A) 2-(Benzyloxy)-3, 4-difluorobenzaldehyde
To a mixture of 3,4-difluoro-2-hydroxy-benzaldehyde (5 g) and acetonitrile (50
mL) was added
potassium carbonate (6.56 g), benzyl bromide (4.51 mL) and sodium iodide (2.37
g). The
reaction mixture was stirred at 60 C for 6 h, filtered with Celite , and the
filtrate was
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography (ethyl acetate/hexane) to give the title compound (7.0 g).
1H NMR (400 MHz, DMSO-do): 6 5.33 (2H, s), 7.30-7.42 (4H, m), 7.45-7.47 (2H,
m), 7.55-7.59
(1H, m), 10.04 (1H, s).
[0331]
B) Methyl (Z)-2-azido-3-(2-(benzyloxy)-3,4-difluorophenyl)acrylate
To a mixture of methyl azidoacetate (3.15 mL), 2-(benzyloxy)-3,4-
difluorobenzaldehyde (2.0 g)
and methanol (10 mL) was added dropwise a mixture of sodium methoxide (1.74 g)
and methanol
(10 mL) at -10 C under argon atmosphere. The reaction mixture was stirred at
the same
temperature for 4 h and stirred at 4 C for 16 h. To the reaction mixture was
added ice-cold water
and the precipitates were collected by filtration to give the title compound
(2.1 g).
1H NMR (400 MHz, DMSO-do): 63.83 (3H, s), 5.14 (2H, s), 6.94 (1H, s), 7.26
(1H, m), 7.38-
7.40 (5H, m), 7.97 (1H, t, J = 6.9 Hz).
[0332]
C) Methyl 4-(benzyloxy)-5,6-difluoro-1H-indole-2-carboxylate
A mixture of methyl (Z)-2-azido-3-(2-(benzyloxy)-3,4-difluorophenyl)acrylate
(2.0 g) and xylene
(30 mL) was stirred at 140 C for 2 h. The reaction mixture was cooled, and
the precipitates
were collected by filtration to give the title compound (700 mg).
1H NMR (400 MHz, DMSO-do): 63.87 (3H, s), 5.41 (2H, s), 7.05 (1H, m), 7.26
(1H, s), 7.32-
7.41 (3H, m), 7.47-7.49 (2H, m), 12.19 (1H, s).
[0333]
D) Methyl 4-(benzyloxy)-5,6-difluoro-1-methy1-1H-indole-2-carboxylate
To a mixture of methyl 4-(benzyloxy)-5,6-difluoro-1H-indole-2-carboxylate (1.7
g) and DMF (20
mL) was added potassium carbonate (1.1 g) and iodomethane (0.4 mL). The
reaction mixture
was stirred at room temperature for 2 h, and ice-cold water was added thereto
and extracted with
ethyl acetate. The organic layer was washed with water and brine, dried over
anhydrous sodium
sulfate, and the solvent was removed under reduced pressure to give the title
compound (1.5 g).
MS: [M+111+ 332.1.
[0334]
E) 4-(Benzy loxy)-5,6-difluoro-l-methy1-1H-indole -2-carboxylic acid
To a mixture of methyl 4-(benzyloxy)-5,6-difluoro-l-methy1-1H-indole-2-
carboxylate (1.5 g) and
THF (30 mL) was added water (6.0 mL) and lithium hydroxide monohydrate (0.285
g). The
reaction mixture was stirred at room temperature for 6 h and the solvent was
removed under
reduced pressure. The residue was acidified with 1 M hydrochloric acid and
extracted with
ethyl acetate. The organic layer was washed with water and brine, dried over
anhydrous sodium
sulfate, and the solvent was removed under reduced pressure to give the title
compound (1.35 g).
Date Recue/Date Received 2022-01-27
65
CA 03148955 2022-01-27
MS: [M+1-11+ 318.1.
[0335]
F) tert-Butyl ((S)-1-4(S)-2-(4-(4-(benzyloxy)-5,6-difluoro-l-methyl-1H-indole-
2-
carbonyl)piperazin-l-y1)-1-cyclohexy1-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of 4-(benzyloxy)-5,6-difluoro-l-methy1-1H-indole-2-carboxylic
acid (1.4 g) and
DMF (20 mL) was added tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-oxo-2-(piperazin-l-
yDethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (1.81 g), HATU (2.52 g) and
DIEA (1.9
mL). The reaction mixture was stirred at room temperature for 2 h, ice-cold
water was added
thereto, and extracted with ethyl acetate. The organic layer was washed with
saturated aqueous
sodium bicarbonate, water, and brine, dried over anhydrous sodium sulfate, and
the solvent was
removed under reduced pressure. The residue was purified by silica gel column
chromatography
(ethyl acetate/hexane) to give the title compound (2.1 g).
MS: [M+111+ 710.1.
[0336]
G) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-4-hydroxy-l-methyl-
1H-indole-2-
carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-2-(4-(4-(benzyloxy)-5,6-difluoro-l-
methyl-1H-indole-2-
carbonyl)piperazin-l-y1)-1-cyclohexy1-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
(2.1 g) and ethanol (50 mL) was added palladium on carbon (400 mg), and the
reaction mixture
was stirred under the normal pressure hydrogen atmosphere at room temperature
for 2 h. The
reaction mixture was filtered with Celite and the filtrate was concentrated
under reduced
pressure to give the title compound (1.7 g).
MS: [M+111+ 620.4.
[0337]
H) 2-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate
To a mixture of 2-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)ethanol (15 g) and
DCM (250 mL)
was added TEA (11.03 mL), DMAP (3.22 g) and p-toluenesulfonyl chloride (12.07
g) under ice-
cooling and the reaction mixture was stirred at room temperature for 2 h. The
reaction mixture
was diluted with DCM and washed with water and brine. The organic layer was
dried over
anhydrous sodium sulfate and the solvent was removed under reduced pressure.
The residue was
purified by silica gel column chromatography (ethyl acetate/hexane) to give
the title compound
(14g).
MS: [M+111+ 439.2.
[0338]
I) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-4-((1-
phenyl-2,5,8,11-
tetraoxatridecan-13-yl)oxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-(((S)-1-cyclohexy1-2-(4-(5,6-difluoro-4-
hydroxy-l-methyl-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate (450
mg) and DMF (5 mL) was added cesium carbonate (591 mg) and 2-(2-(2-(2-
(benzyloxy)ethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate (573 mg). The
reaction
mixture was stirred at room temperature for 16 h, ice-cold water was added
thereto and extracted
with ethyl acetate. The organic layer was washed with water and brine, dried
over anhydrous
sodium sulfate, and the solvent was removed under reduced pressure. The
residue was purified
by silica gel column chromatography (ethyl acetate/hexane) to give the title
compound (490 mg).
MS: [M+111+ 886.4.
[0339]
J) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-4-(2-(2-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)ethoxy)-1-methy1-1H-indole-2-carbonyl)piperazin-l-
y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
Date Recue/Date Received 2022-01-27
66
CA 03148955 2022-01-27
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-
methyl-4-((1-phenyl-
2,5,8,11-tetraoxatridecan-13-yl)oxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (490 mg) and ethanol (15 mL) was added
palladium on carbon
(100 mg). The reaction mixture was stirred under the normal pressure hydrogen
atmosphere at
room temperature for 16 h, filtered with Celite and the filtrate was
concentrated under reduced
pressure to give the title compound (360 mg).
MS: [M+111+ 796.2.
[0340]
K) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-4-(2-(2-(2-
(24(54(4-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-
1-oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-4-(2-(2-
(2-(2-
hydroxyethoxy)ethoxy)ethoxy)ethoxy)-1-methyl-1H-indole-2-carbonyl)piperazin-l-
y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (20 mg), 54(4-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-ol (12.4 mg), triphenylphosphine (32.9
mg) and toluene
(2 mL) was added di-tert-butyl azodicarboxylate (17.3 mg). The reaction
mixture was stirred at
room temperature for 16 h and the solvent was removed under reduced pressure.
The residue
was purified by preparative thin layer chromatography to give the title
compound (25 mg).
MS: [M+111+ 1109.8.
[0341]
L) (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-4-(2-(2-(2-(24(54(4-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethoxy)-1H-
indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
hydrochloride
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-
methyl-4-(2-(2-(2-(2-
45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-
1-oxopropan-2-y1)(methyl)carbamate (25 mg) and DCM (1 mL) was added 4 M
hydrogen chloride
dioxane solution (0.3 mL) at 0 C. The reaction mixture was stirred at room
temperature for 1
h, the solvent was removed under reduced pressure, and the residue was washed
with diethyl
ether to give the title compound (17 mg).
1H NMR (400 MHz, DMSO-d6) 60.83-1.34 (9H, m), 1.60-1.69 (5H, m), 2.07-2.18
(3H, m), 3.17
(3H, s), 3.44-3.74 (25H, m), 3.85-4.66 (9H, m), 5.01-5.27 (2H, m), 6.63 (1H,
bs), 6.81 (1H, s),
7.36-7.44 (2H, m), 7.66 (1H, d, J = 5.32 Hz), 8.35-8.36 (1H, m), 8.74-8.80
(2H, m), 9.10-9.11
(1H, m), 11.36 (1H, brs).
[0342]
Example 11
(S)-N-((S)-1-Cyclohexy1-2-(4-(2-methy1-1-(2-(2-(2-(2-45-44-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)ethoxy)ethoxy)ethoxy)ethyl)-1H-
indole-5-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
[0343]
A) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(2-methy1-1H-indole-5-
carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-oxo-2-(piperazin-l-
y1)ethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (4 g) and DMF (70 mL) was added 2-methy1-1H-
indole-5-
carboxylic acid (1.9 g), DIEA (6.8 mL) and HATU (4.45 g), and the reaction
mixture was stirred
at room temperature for 16 h. The reaction mixture was diluted with water and
the aqueous layer
was extracted with ethyl acetate. The organic layer was washed with water and
brine
subsequently, dried over anhydrous sodium sulfate, and the solvent was removed
under reduced
pressure. The residue was purified by silica gel column chromatography (ethyl
acetate/hexane)
Date Recue/Date Received 2022-01-27
67
CA 03148955 2022-01-27
to give the title compound (4 g).
MS: [M+111+ 568.3.
[0344]
B) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(2-methy1-1-(1-phenyl-2,5,8,11-
tetraoxatridecan-13-
y1)-1H-indole-5-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(2-methy1-1H-indole-5-
carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-y1)(methyl)carbamate
(2 g) and DMF
(30 mL) was added cesium carbonate (2.87 g), the resultant mixture was stirred
at room
temperature for 5 min, 2-(2-(2-(2-(benzyloxy)ethoxy)ethoxy)ethoxy)ethyl 4-
methylbenzensulfonate (2.78 g) was added thereto, and the reaction mixture was
stirred at 80 C
for 16 h. The reaction mixture was diluted with water and the aqueous layer
was extracted with
ethyl acetate. The organic layer was washed with water and brine, dried over
anhydrous sodium
sulfate, and the solvent was removed under reduced pressure. The residue was
purified by silica
gel column chromatography (NH, ethyl acetate/hexane) to give the title
compound (1.3 g).
MS: [M+111+ 834.4.
[0345]
C) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(1-(2-(2-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)ethyl)-
2-methyl-1H-indole-5-carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(2-methy1-1-(1-phenyl-
2,5,8,11-
tetraoxatridecan-13-y1)-1H-indole-5-carbonyl)piperazin-l-y1)-2-oxoethyl)amino)-
1-oxopropan-2-
y1)(methyl)carbamate (1.3 g) and ethanol (50 mL) was added 10% palladium on
carbon (250 mg),
and the reaction mixture was stirred under the normal pressure hydrogen
atmosphere at 25 C for
16 h. The reaction mixture was filtered with Celite and the filtrate was
concentrated under
reduced pressure to give the title compound (1.1 g).
MS: [M+111+ 744.3.
[0346]
D) tert-Butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(2-methy1-1-(2-(2-(2-(2-45-44-
(thieno[3,2-
blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethyl)-1H-
indole-5-carbonyl)piperazin-l-y1)-2-oxoethyDamino)-1-oxopropan-2-
y1)(methyl)carbamate
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(1-(2-(2-(2-(2-
hydroxyethoxy)ethoxy)ethoxy)ethyl)-2-methyl-1H-indole-5-carbonyl)piperazin-l-
y1)-2-
oxoethyDamino)-1-oxopropan-2-y1)(methyl)carbamate (20 mg), 54(4-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-ol (10.6 mg), triphenylphosphine (32.5
mg) and toluene
(2 mL) was added di-tert-butyl azodicarboxylate (24.7 mg), and the reaction
mixture was stirred
at 25 C for 16 h. The reaction mixture was concentrated under reduced
pressure and the
residue was purified by preparative thin layer chromatography to give the
title compound (17
mg).
MS: [M+111+ 1057.3.
[0347]
E) (S)-N-((S)-1-Cyclohexy1-2-(4-(2-methy1-1-(2-(2-(2-(2-45-44-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-l-yOmethyDisoxazol-3-yDoxy)ethoxy)ethoxy)ethoxy)ethyl)-1H-
indole-5-
carbonyl)piperazin-l-y1)-2-oxoethyl)-2-(methylamino)propanamide
To a mixture of tert-butyl ((S)-1-4(S)-1-cyclohexy1-2-(4-(2-methy1-1-(2-(2-(2-
(2-45-44-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyl)isoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethyl)-1H-indole-5-carbonyl)piperazin-l-y1)-2-
oxoethyDamino)-1-
oxopropan-2-y1)(methyl)carbamate (17 mg) and DCM (1 mL) was added TFA (0.01
mL) at 0 C,
and the reaction mixture was stirred at 25 C for 1 h. The reaction mixture
was concentrated
under reduced pressure and the residue was purified by preparative HPLC (C18,
mobile phase:
Date Recue/Date Received 2022-01-27
68
CA 03148955 2022-01-27
acetonitrile/20 mM aqueous ammonium bicarbonate) to give the title compound (4
mg).
MS: [M+1-11+ 957.8.
[0348]
The example compounds 1 to 11 produced are shown below. For each compound, the
compound
name, structural formula, salt type and MS value (MS is the measured value)
are shown.
[0349]
Example No.1 (compound 1): 2-(4-((S)-2-cyclohexy1-2-((S)-2-
(methylamino)propanamido)acetyl)piperazine-l-carbony1)-5,6-difluoro-N,1-
dimethyl-N-(2-(2-(2-
((5-((4-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethyl)-1H-indole-3-carboxamide,
[0350]
N/ \ 0 0 F F
-
S \1 0-N
I
N-
O 0
0 N/"Th
L/N4
NH
0
HN
salt type: HC1; MS value: 1006.6
[0351]
Example No.2 (compound 2): 2-(4-((S)-2-cyclohexy1-2-((S)-2-
(methylamino)propanamido)acetyl)piperazine-l-carbony1)-6-methoxy-1-methyl-N-(2-
(2-(2-(4-
((4-(thieno[3,2-dlpyrimidin-4-yloxy)piperidin-1-
yOsulfonyl)phenoxy)ethoxy)ethoxy)ethyl)-1H-
indole-3-carboxamide,
[0352]
/N 0-..._
N.,?-0_
S 0
N , \ N
\ ,.. \
o'ss..) /-NH
0 0
= Orj / iNTh
- \......-N
0_O
crrNH
0)
"1111
HN
\
salt type: - ; MS value: 1046.5
[0353]
Example No.3 (compound 3): 14(R)-4-(5,6-difluoro-1-methy1-1H-indole-2-
carbony1)-2-
Date Recue/Date Received 2022-01-27
69
CA 03148955 2022-01-27
methylpiperazin-l-y1)-24(2R,5R)-5-methyl-2-42-(2-(2-45-44-(thieno[3,2-
blpyridin-7-
yloxy)piperidin-1-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)methyDpiperazin-l-
yDethan-1-one,
[0354]
0
N/¨
N
LS N 0
________________ 0
F
OOO
I
NH
salt type: HC1; MS value: 909.7
[0355]
Example No.4 (compound 4): (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-methyl-
3-(2-(2-(2-
45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-
(methylamino)propanamide,
[0356]
/¨
N 0
çs
0
/
0
cN
0
coy NH
HN
salt type: HC1; MS value: 966.4
[0357]
Example No.5 (compound 5): (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(3-
methy1-2-oxo-14-
45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-yDoxy)-
6,9,12-trioxa-3-
azatetradecy1)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-
(methylamino)propanamide,
[0358]
Date Recue/Date Received 2022-01-27
70
CA 03148955 2022-01-27
/-
N ___ 0
us L F F
N 0_,
0 41
0----N.---- 0
N------NO---N--- N.N)N /
I
0 NV
NH
\\\
Os's
HN
salt type: HC1; MS value: 1050.3
[0359]
Example No.6 (compound 6): (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-1-(2-oxo-
24(S)-2-(((5-
44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
y1)oxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-
(methylamino)propanamide,
[0360]
F
/¨
N\ ¨C)
F =
0
-:--
N 0,N 0 N N
\ ______________
salt type: HC1; MS value: 944.2
[0361]
Example No.7 (compound 7): (S)-N-((S)-1-cyclohexy1-2-(4-(5-fluoro-1-(2-oxo-
24(S)-2-(((54(4-
(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
yDoxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbonyl)piperazin-l-y1)-2-oxoethyl)-2-
(methylamino)propanamide,
[0362]
F
H f
N ON
H
\ ______________
salt type: - ; MS value: 926.1
[0363]
Example No.8 (compound 8): (S)-N-((S)-1-cyclohexy1-24(S)-4-(5,6-difluoro-1-(2-
oxo-24(S)-2-
(45-44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yl)methyDisoxazol-3-
yl)oxy)methyl)pyrrolidin-l-yl)ethyl)-1H-indole-2-carbonyl)-3-methylpiperazin-1-
y1)-2-
oxoethyl)-2-(methylamino)propanamide,
Date Recue/Date Received 2022-01-27
71
CA 03148955 2022-01-27
[0364]
F
/¨
N? 0_ F\ 0
N S I N H -7---
N =
N 0 0 N
\
ON1 NN__, j
0--151
salt type: - ; MS value: 958.4
[0365]
Example No.9 (compound 9): (S)-N-((S)-1-cyclohexy1-24(S)-4-(5-fluoro-1-(2-oxo-
24(S)-2-(((5-
44-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yOmethyDisoxazol-3-
y1)oxy)methyl)pyrrolidin-l-
yDethyl)-1H-indole-2-carbony1)-3-methylpiperazin-l-y1)-2-oxoethyl)-2-
(methylamino)propanamide,
[0366]
F
/¨
N? 0
N S_ . I 0
-------NN,,- NH 3-:z.--
N 0 c
\ N 0 N
_________________ JIN NN..... j
T------\N ---
0 H
j
salt type: - ; MS value: 940.4
[0367]
Example No.10 (compound 10): (S)-N-((S)-1-cyclohexy1-2-(4-(5,6-difluoro-l-
methyl-4-(2-(2-(2-
(24(54(4-(thieno[3,2-blpyridin-7-yloxy)piperidin-l-yl)methyDisoxazol-3-
yDoxy)ethoxy)ethoxy)ethoxy)ethoxy)-1H-indole-2-carbonyl)piperazin-l-y1)-2-
oxoethyl)-2-
(methylamino)propanamide,
[0368]
N/¨
\
S
________ N 0-...N F
\ __
......k F
0
N----
\____1
Nr---- 04
0
NH
(
)----
- HN---
salt type: HC1; MS value: 1009.7,
and
[0369]
Example No.11 (compound 11): (S)-N-((S)-1-cyclohexy1-2-(4-(2-methy1-1-(2-(2-(2-
(2-45-44-
Date Recue/Date Received 2022-01-27
72
CA 03148955 2022-01-27
(thieno[3,2-blpyridin-7-yloxy)piperidin-1-yOmethyDisoxazol-3-
yl)oxy)ethoxy)ethoxy)ethoxy)ethyl)-1H-indole-5-carbonyl)piperazin-1-y1)-2-
oxoethyl)-2-
(methylamino)propanamide,
[0370]
/
0
H
N 0
S 0 N
0
\¨N 0
\
0¨N
salt type: - ; MS value: 957.8
[0371]
Experimental Example 1: Measurement of XIAP Binding Inhibitory Activity
Human XIAP binding inhibitory activity was measured by the Homogeneous Time
Resolved
Fluorescence (HTRF) method with using commercially available human XIAP_BIR3
domain
purified protein (R & D) and as a ligand a Smac N-terminal peptide (AVPIAQK
(SEQ ID NO: 1))
(hereinafter referred to as "b-Smac"; Peptide Research Laboratories, Inc.)
biotinylated at the C-
terminus according to a conventional method.
The HTRF method will be described in detail below.
The test compound diluted with a reaction buffer (25 mM HEPES Buffer
containing 100 mM
NaCl, 0.1% BSA, and0.1% triton X-100, pH 7.5) was added to a 384-well white
shallow bottom
plate (Greiner 784076) at 1 )(1_, / well and flash centrifuged for 30 seconds.
Subsequently, human
XIAP_BIR3 domain purified protein was diluted with the reaction buffer to
obtain a 90nM
sample diluent, and the resultant sample diluent was added to the above-
mentioned white
superficial plate at 4.5 )(1_, / well and flash centrifuged for 30 seconds.
Subsequently, b-Smac
diluted to 90 nM with the reaction buffer was added to the above-mentioned
white superficial plat
at 4.5 )(1_, / well and flash centrifuged for 30 seconds. A mixed solution of
Anti-6 HIS-Cryptate
(Eu3+ Cryptate-conjugated mouse monoclonal antibody anti-6 Histidine ; cisbio)
and
Streptavidin-XL' (Highgrade XL665-conjugated streptavidin; cisbio) both
diluted 100-fold with
HTRF detection buffer (cisbio) at a volume ratio of 1: 1 was added to the
above white superficial
plate at 10 )(1_, / well. After flash centrifuging the white shallow bottom
plate for 30 seconds, the
white shallow bottom plate was left at room temperature for 4 hours or more in
the dark. The
white superficial plate after being left was subjected to fluorescence
intensity measurement
(excitation wavelength: 320 nm, fluorescence wavelength: 665 nm and 615 nm) by
EnVision
(Perkin Elmer).
The binding inhibition rate (%) was calculated based on the HTRF ratio in the
presence of the
test compound to the HTRF ratio in the absence of the test compound
(fluorescence intensity at
665 nm / fluorescence intensity at 615 nm).
The XIAP binding inhibition rate (%) of test compounds was evaluated. The XIAP
binding
inhibition ratio described as A 75%, 75%> B 50%, 50%> C 25%, D >25% when the
concentration of the test compound is 3 ,M, or 50% inhibitory concentration
(IC50 value)
described as A<0.3 04, 0.3 04 B< 3 04, 3 1\4 C< 30 ,M, is shown in the table
below.
[0372]
[Table 1]
Date Recue/Date Received 2022-01-27
73
CA 03148955 2022-01-27
*Binding inhibition
Compound
rate
Example 1 -/B
Example 2 -/B
Example 3 -/A
Example 4 C/-
Example 5 -/C
Example 6 -/C
Example 7 -/C
Example 8 -/C
Example 9 -/C
Example 10 -/B
Example 11 -/B
*Binding inhibition rate: binding inhibition rate when the concentration of
the test compound is 3
[tM / IC50 value
[0373]
From the above results, it was shown that the compounds of the present
invention have an
excellent TAP (in particular XIAP) binding (inhibition) activities.
[0374]
Experimental Example 2: Measurement of Binding Inhibitory Activity of IRAK-M
IRAK-M binding inhibitory activity was evaluated using active site-dependent
competitive
assay KINOMEscan provided by Eurofins Discover X (Goldstein, D. M. et al.
High-throughput
kinase profiling as a platform for drug discovery. Nat. Rev. Drug Discovery.
7, 391-397 (2008)).
IRAK-M binding inhibitory activity of the test compound was evaluated. The
"%Ctrl" when the
concentration of the test compound is 1 [tM described as A < 25%, 50% > B 25%,
75%>
C 50%, D 75%, or IC50 value described as A <0.03 04, 0.03 [tM B< 0.1 04,
0.1 [tM C
<0.3 [tM, 0.3 [tM D< 1 [tM is shown in the table below. The "%Ctrl" is
calculated by the
following formula.
(test compound signal ¨ positive control compound signal) / (negative control
compound signal ¨
positive control compound signal) x 100
Negative control compound = DMSO (100% Ctrl)
Positive control compound = control compound (0% Ctrl)
[0375]
[Table 2]
Date Recue/Date Received 2022-01-27
74
CA 03148955 2022-01-27
Compound *Binding inhibition activity
Example 1 -/D
Example 2 -/D
Example 3 -/D
Example 4 B/-
Example 5 A/-
Example 6 A/-
Example 7 -/D
Example 8 -/D
Example 9 -/D
Example 10 A/-
Example 11 A/-
*Binding inhibition activity: %Ctrl/IC50 value when the concentration of the
test compound is 1
M
[0376]
Experimental Example 3: Measurement of in vitro Degradation Activity of Target
Protein
In vitro degradation activities of target protein of the example compounds
were evaluated using
Enzyme-linked immuno-sorbent assay (ELISA) by the following assay steps. THP1
cells (ATCC,
TIB-202) were cultured in RPMI-1640 supplemented with 10% FBS, lx sodium
pyruvate, lx
HEPES, D-(+)- glucose and 1% penicillin/streptomycin. THP1 cells were seeded
at a density of
1x106 cells/well in 24-well plate and treated with DMSO control or test
compound, and then
incubated for 24 hours. The cells were collected and lysed on ice for 30
minutes in lysis buffer
(PBS containing 0.1% Triton X-100) containing a protease inhibitor cocktail
(Roche, Cat#
11836170001). The lysates were sonicated for 30sec ON/ 30sec OFF for ten
cycles and
centrifuged for 10 minutes at 13k rpm at 4 C. Protein concentrations were
determined by the
BCA assay (Sigma, Cat # QPBCA-1KT). The ELISA assay was performed using the
Human
IRAK3 / IRAKM /IRAK-M ELISA Kit (LifeSpan BioSciences, Cat # LS-F35271) , and
the
protein levels of IRAK-M were evaluated according to the kit protocol. As to
the IRAK-M
degradation rate of test compounds, protein degradation rate (%) when the
concentration of the
test compound is 1 M described as A75%, 50% B<75%, 25% C<50%, D<25%, or
50% degradation concentration (DC50 value) described as A< 0.03 M, 0.03 M B<
0.1 M
, 0.1 M C< 0.3 M, 0.3 M D< 1 M, is shown in the table below.
[0377]
[Table 3]
Date Recue/Date Received 2022-01-27
75
CA 03148955 2022-01-27
Compound *Degradation rate
Example 1 -/D
Example 2 C/-
Example 3 C/-
Example 4 A/-
Example 5 -/D
Example 6 -/B
Example 7 -/A
Example 8 -/A
Example 9 -/A
Example 10 -/C
Example 11 A/-
*Degradation rate: protein degradation rate when the concentration of the test
compound is 1
M/DC50 value
[0378]
Experimental example 4: Antitumor Effect in a Mouse Lewis Lung Cancer Cell
Inoculation
Model
C57BL/6 mice (Charles River Laboratories Japan, male, 7-8W) were
subcutaneously inoculated
with 2x104 cells/mouse of mouse Lewis lung carcinoma LL/2 (Lewis lung
carcinoma, LLC)
(ATCC, CRL-1642) along with Matrigel on the flank of the mouse. Tumor size was
measured by
electronic calipers 7 days after inoculation, and the groups were divided so
that each group had
an equivalent size, and administration of the compound was started 8 days
later. Tumor size was
calculated by the formula, major diameter of the tumor x minor diameter x
minor diameter 2.
The test compound was suspended in 0.5% methylcellulose or dissolved in saline
and
administered subcutaneously every 3 days for 3 times. Tumor size was measured
periodically
until 16-18 days after the start of the study, and a two-tailed test was
performed for tumor size in
the test compound-treated group and the solvent-treated group on the last day
of the study.
Daily changes of tumor size in each group of example compounds 1, 6, 7, 8, and
9, are shown in
Figure 1. Each compound was used with the salt shown in the figure. Figure
shows the mean
standard error.
From the above results, it was shown that these compounds have suppressive
effects on tumor
growth.
[0379]
Formulation Example 1
A medicament containing the compound of the present invention as an active
ingredient can be
produced, for example, by the following composition.
1. Capsule
(1) Compound obtained in Example 1 40 mg
(2) Lactose 70 mg
(3) Microcrystalline cellulose 9 mg
(4) Magnesium stearate 1 mg
1 capsule 120 mg
After mixing (1), (2), (3) and 1/2 volume of (4), the mixture is granulated.
The remaining (4)
is added to this, and then the whole is encapsulated in a gelatin capsule.
Date Recue/Date Received 2022-01-27
76
CA 03148955 2022-01-27
[0380]
2. Tablet
(1) Compound obtained in Example 1 40 mg
(2) Lactose 58 mg
(3) Corn starch 18 mg
(4) Microcrystalline cellulose 3.5 mg
(5) Magnesium stearate 0.5 mg
1 tablet 120 mg
After mixing (1), (2), (3) and 2/3 volume of (4) and 1/2 volume of (5), the
mixture is
granulated. Then, the remaining (4) and (5) are added to the granules and
pressed-molded into
tablets.
[0381]
Formulation Example 2
After dissolving 50 mg of the compound obtained in Example 1 in 50 mL of
distilled water for
injection (Japanese Pharmacopoeia grade), the distilled water for injection is
added to make 100
mL. The solution is filtered under sterile conditions, then, 1 mL each of
this solution is taken,
filled under sterile conditions into vials for injection, lyophilized and
sealed.
[0382]
The foregoing merely illustrates objects and subjects of the present
invention, and is not
intended to be limiting the accompanying Claims. Without departing from the
accompanying
Claims, various modifications and alterations to the described embodiments
will be apparent to
those skilled in the art in view of the teachings herein.
[Industrial Applicability]
[0383]
The compounds of the present invention are capable of degrading IRAK-M protein
as a
target. Therefore, it is expected to provide effective drugs for the
prevention or treatment
of cancer and other IRAK-M associated diseases.
Date Recue/Date Received 2022-01-27