Note: Descriptions are shown in the official language in which they were submitted.
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
TITLE: COMPOSITIONS AND METHODS OF ENHANCING OPIOID RECEPTOR
ENGAGEMENT BY OPIOID HEXADIENOATES AND OPTIONALLY SUBSTITUTED HEXADIENOATES
[00001] This application is a PCT Application of a Non-Provisional U.S.
Patent Application No.
16/540,058, filed on August 14, 2019 and claiming priority under 35 U.S.C.
119 of prior U.S. Provisional
Patent Serial No. 62/885,311, entitled "COMPOSITIONS AND METHODS OF ENHANCING
OPIOID
RECEPTOR ENGAGEMENT BY OPIOID HEXADIENOATES AND OPTIONALLY SUBSTITUTED
HEXADIENOATES" filed on August 11, 2019.
Field of the Invention
[00002] The present invention relates to opiate derived compositions,
used in therapeutic
areas associated with opioid receptor modulation.
BACKGROUND
[00003] Nalbuphine (Nubain) was launched in 1979 as an analgesic for
moderate to severe
pain and has effectively been used in the clinic since. It is primarily used
in conjunction with anesthetics for
pre- and post-operative analgesia and in labor and delivery for acute and
chronic pain management.
Recently its uses have been expanded to the treatment of locomotive disorders,
dermatological conditions
such as pruritus and addiction management.
[00004] It has also been recently shown that Nalbuphine could prevent
opiate tolerance and
dependence in chronic pain management. It is the only narcotic analgesic of
its type that is not subject to
the Controlled Substances Act, an indication of its safe utility. Nalbuphine
has a low oral bioavailability.
[00005] There are known Nalbuphine prodrugs designed to improve its
pharmacokinetic and
pharmacodynamic properties. Merriam-Webster defines a prodrug as a
pharmacologically inactive
substance that is the modified form of a pharmacologically active drug to
which it is converted
(as by enzymatic action) in the body. Thus, Franklin (WO 2010-GB52211) teaches
that Nalbuphine
1
SUBSTITUTE SHEET (RULE 26)
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
could be modified at phenolic hydroxyl residue.
[00006] Furthermore, Nalbuphine could be coupled to an amino acid or
short peptide (WO
2011007247, Al). Also, Nalbuphine could be modified with dicarboxylic acid
linked amino acid and peptide
(WO 2010112942, Al). Further yet, Nalbuphine could be modified with carbomate
moiety linked amino
acid and peptide (WO 2009092071, A2). Moreover, Jenkins (WO 2007022535, A2)
teaches that
Nalbuphine could be further modified on its phenolic or nitrogen moiety.
[00007] Wang teaches that Nalbuphine could be converted to ester
prodrug (Journal of
Controlled Release, Volume: 115, Issue: 2, Pages: 140-149, Journal, 2006,). Hu
(Jan 11, 2005, TW
226239, B) teaches that delivery systems and Nalbuphine prodrugs which
increase its bioavailability. More
specifically, formulation to increase Nalbuphine's bioavailability includes
vegetable oils, a co-solvent, and
an effective amount of a Nalbuphine ester prodrug or a pharmaceutically
acceptable salt thereof. One
objective of prodrugs is to increase the oral bioavailability of Nalbuphine
and prolong the retention time of
Nalbuphine in a body, thereby maintaining a longer analgesic period of time,
as well as reducing the
analgesic cost.
[00008] Hilfinger (US 20050137141, Al) teaches of Nalbuphine including
a pharmaceutical
species and an amino acid having a covalent bond to the pharmaceutical
species. Huang (International
Journal of Pharmaceutics, Volume: 297, Issue: 1-2, Pages: 162-171, Journal,
2005) teaches of the effects
of iontophoresis and electroporation on transdermal delivery of Nalbuphine
(NA) and its two novel
prodrugs: Nalbuphine benzoate (NAB) and sebacoyl dinalbuphine ester (SDN) from
solutions as well as
from hydrogels.
[00009] Crooks (WO 2005009377, A2) teaches that forming duplex prodrugs
including
Nalbuphine can provide significant increase in the transdermal flux of drugs
across human skin. Uhrich
(WO 2002009768, A2) teaches of therapeutic polyesters and polyamides of
Nalbuphine. Hu (EP 1149836,
Al) teaches of preparation of polynalbuphine derivatives. Pao (Journal of
Chromatography, B: Biomedical
2
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
Sciences and Applications, Volume: 746, Issue: 2, Pages: 241-247, Journal,
2000) teaches of
bioavailability of sebacoyl dinalbuphine ester.
[000010] Han (International Journal of Pharmaceutics, Volume: 177, Issue: 2,
Pages: 201-209,
Journal, 1999) teaches of Mucoadhesive buccal disks for novel Nalbuphine
prodrug controlled delivery:
effect of formulation variables on drug release and mucoadhesive performance.
Sung (International Journal
of Pharmaceutics, Volume: 172, Issue: 1-2, Pages: 17-25, Journal, 1998)
teaches of controlled release of
nalbuphine prodrugs from biodegradable polymeric matrixes: influence of
prodrug hydrophilicity and
polymer composition. Yoa-Pu (US 5750534, A) teaches of Nalbuphine esters
having long-acting analgesic
action.
[000011] Shami (EP 85108258.6) teaches that Nalbuphine can be further modified
into 3-
acetylsalicylate. Additional Nalbuphine prodrugs are disclosed in US 6569449,
Bl; ON 1107333, A; EP
615756, Al; and International Journal of Pharmaceutics, Volume: 38, Issue: 1-
3, Pages: 199-209, Journal,
1987.
[000012] Pharmacokinetic and pharmacodynamic properties of Nalbuphine, its
pharmaceutically
acceptable salt, or ester, or its prodrug could be further modulated by
various delivery systems. Thus Liu
(International Journal of Pharmaceutics, Volume: 257, Issue: 1-2, Pages: 23-
31, Journal, 2003) teaches
that biodegradable polymeric microspheres for Nalbuphine prodrug controlled
delivery. Sung (European
Journal of Pharmaceutical Sciences, Volume: 18, Issue: 1, Pages: 63-70,
Journal, 2003) teaches of
transdermal delivery of nalbuphine and its prodrugs by electroporation. Fang
(Arzneimittel-Forschung,
Volume: 51, Issue: 5, Pages: 408-413, Journal, 2001) teaches of Transdermal
delivery of nalbuphine and
nalbuphine pivalate from hydrogels by passive diffusion and iontophoresis.
[000013] A distinction must be made between an improvement of oral
bioavailability and an
increase in opioid receptor engagement for these opioid derivatives. For
example, esterification of phenoxy
moiety of Nalbuphine (e.g. 3-docosanoate derivative of Nalbuphine) (NB-39) has
been previously claimed
3
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
to have improved oral bioavailability. However, when given orally, the
cumulative analgesia produced by
NB-39 was inferior to the equivalent dose of Nalbuphine in rats and humans.
Furthermore, NB-39 did not
significantly affect pupil dilation (miosis) in humans after oral
administration that is indicative of inferior
opioid receptor engagement.
[000014] Naloxone, sold under the brandname Narcan (and others), is a
medication used
to block the effects of opioids, especially in overdose situations. Naloxone
may also be combined with an
opioid (in the same pill or compound), to decrease the risk of opioid misuse.
For instance, it could be added
to the coating for a sustained release opiate compound, to prevent crushing of
the sustained release
compound, which could lead to an overdose.
[000015] When given intravenously, Naloxone typically works within
two minutes, and when
injected into a muscle, it works within five minutes. It may also be used as a
nasal spray. The effects of
Naloxone typically last for about half an hour to an hour. Thus, multiple
doses and administration of
Naloxone may be required, as the duration of action of most opioids is greater
than that of Naloxone.
[000016] Administration of Naloxone to opioid-dependent individuals
may cause symptoms
of opioid withdrawal, such as, for example, restlessness, agitation, nausea,
vomiting, increased heart rate
and perspiration. To prevent this, small doses of Naloxone can be given every
few minutes until the
desired effect is reached.
[000017] In the individuals with prior history of heart disease or
persons who take
medications that negatively affect the heart, further heart problems have
occurred. Naloxone appears to be
safe in pregnancy, after having been given to and tested on a limited number
of subjects.
[000018] Naloxone is a non-selective and competitive opioid receptor
antagonist. It works
by reversing the depression of the central nervous system and respiratory
system caused by opioids.
Naloxone was originally patented in 1961 and approved for opioid overdose
treatment in the United States
in 1971.
4
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000019] Naloxone, also known as N-allylnoroxymorphone or as 17-allyI-
4,5a-epoxy-3,14-
dihydroxymorphinan-6-one, is a synthetic morphinan derivative and was derived
from oxymorphone (14-
hydroxydihydromorphinone), an opioid analgesic Oxymorphone, in turn, was
derived from morphine, an
opioid analgesic and naturally occurring constituent of the opium poppy.
[000020] Naloxone is a racemic mixture of two enantiomers, (¨)-
naloxone (levonaloxone)
and (-'-)-naloxone (dextronaloxone), only the former of which is active at
opioid receptors. The drug is a
highly lipophilic, allowing it to rapidly penetrate the brain and to achieve a
far greater brain to serum ratio
than that of morphine. Opioid antagonists related to Naloxone include
cyprodime, nalmefene, nalodeine,
naloxol, and naltrexone.
[000021] The chemical half-life of Naloxone is such that injection
and nasal forms have
been marketed with 24-month and 18-month shelf-lives, respectively. A 2018
study noted that the nasal
and injection forms presented as chemically stable to 36- and 28-months,
respectively, which prompted an
as yet incomplete five-year stability study to be initiated. This suggests
that expired caches of material in
community and healthcare settings may still be efficacious substantially
beyond their labeled expiration
dates.
[000022] Certain articles about opioid antagonists emphasize the
shortcomings and
problems with currently known formulations, and the need for an improved and
more stable compound that
may be used safely on patients suffering from opioid addiction.
[000023] An article by Adam Bisaga, entitled "What Should Clinicians
Do As Fentanyl
Replaces Heroin?" (published in Addiction, Vol. 114, pp. 781-86, at
https://onlinelibrary.wiley.com/doi/epdf/10.1111/add.14522) describes that a
high affinity antagonists may
not suffice to block effects of fentanyl and their higher doses that border
concerns over systematic safety
may be required. Furthermore, fentanyl overdose prevention requires higher
doses of naloxone and
repeated dosing that is encumbered by much shorter overdose prevention window
for fentanyl than heroin.
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000024] Roger Chou et al. describes in the article entitled
"Management of Suspected
Opioid Overdose With Naloxone by Emergency Medical Services Personnel"
(published at In Comparative
Effectiveness Review No. 193, at
https://effectivehealthcare.ahrq.govisites/default/files/pdficer-193-
naloxone-finali.pdf) that existing dosing guidelines of naloxone may not be
sufficient to prevent overdose
by fentanyl and fentanyl analogues.
[000025] Rachael Rzasa Lynn et al. describes in the article entitled
"Nalaxone Dosage for
Opioid Reversal: Current Evidence and Clinical Implications" (published in
Therapeutic Advances in Drug
Society Review, Vol. 9(1), pp. 63-88, 2018 at
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5753997/pdf/10.1177_20420986177441
61.pdf) that double
dose of naloxone administered to patient anesthetized with fentanyl produced
no improvement in oxygen
intake, while quadruple dose of naloxone produced significant improvements.
Further he teaches that the
interactions between the opioid agonist and the mu-opioid receptor may be the
greatest determinant of the
speed of recovery from the respiratory effects of many opioids, which may not
markedly accelerate with
increasing doses of naloxone, but rather respond to a minimum effective dose,
while for compounds like
buprenorphine, higher doses of naloxone may even lose efficacy. Then he cites
numerous reports describe
fentanyl overdoses initially unresponsive to IN naloxone and only transiently
reversed with IV naloxone (if at
all), requiring additional IV doses or continuous infusions to prevent
recurrence of toxicity and respiratory
depression.
[000026] IA. Elkiweri et al. describes in the article entitled
"Competitive substrates for P-
glycoprotein and organic anion protein transporters differentially reduce
blood organ transport of fentanyl
and loperamide: pharmacokinetics and pharmacodynamics in Sprague-Dawley rats"
(published online in
2009 at https://www.ncbi.nlm.nih.gov/pubmed/19095843) that naloxone and
fentanyl share a transporter for
cellular influx that becomes saturated by a high plasma concentration of
fentanyl, preventing rapid influx of
naloxone across the BBB regardless of dose.
6
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000027] Rebecca McDonald et al. describes in the article entitled
"Pharmacokinetics of
concentrated naloxone nasal spray for opiod overdose reversal: Phase I healthy
volunteer study"
(published in Addiction, 113, pp. 484-93 at) that high concentration 2 mg
Naloxone intranasal (i.n.) spray
has early absorption rate that is comparable to intramuscular (i.m.) 0.4 mg
injection and could be used as a
take-home antidote. He suggests that high dose i.n. Naloxone could be given
without risk of
"overantagonism".
[000028] Jiten Ranchhodbhai Patel et al. discloses (Publication No.
WO 2013093931 ¨
application PCT/1N20 12/00590, filed Sep. 6, 2012) a novel hydrazide group
containing carbamates of
naloxone.
[000029] Baohua Huang et al. describes in the article entitled "Human
plasma-mediated
hypoxic activation of indolequinone-based naloxone pro-drugs" (published in
Bioorganic & Medicinal
Chemistry Letters in 2009, 19(17), 5016-5020) that indolequinone based
naloxone pro-drug can reverse
opiate induced hypoxia.
[000030] I. Ukrainets et al. discloses in the publication Chemistry
of Heterocyclic
Compounds (2009), 45(4), pp. 405-416) studies of 3-0-acyl derivatives of
naloxone as its potential
prodrugs.
[000031] Xuemei Peng et al. describes in the article "Pharmacological
Properties of Bivalent
Ligands Containing Butorphan Linked to Nalbuphine and Nalaxone at p, 5 and K
Opioid Receptors"
(published in the Journal of Medicinal Chemistry (May 2007), 50(9), 2254-2258)
discloses bivalent ligands
containing butorphan linked to naloxone.
[000032] I. Romanov et al. describes in the Russian Patent
Publication (RU 2221566 ¨
published 01/20/2004) that esters of N-substituted 14-hydroxymorphinans could
be used as highly effective
low toxic an antirelapse agent with prolonged opioprotective effect being
after a single s.c. or i.m. injection.
[000033] I. Romanov et al. describes in the Russian Patent
Publication (RU 2215741 ¨
7
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
published 11/10/2003) the methods of preparation N-substituted 14-
hydroxymorphinane esters.
[000034] Euro-Celtique, S.a., Chevchuk et al. describe in the Patent
Publication No. WO
2003070191 (P0T/US/2003/004999 ¨ published 08/28/2003) the methods of
preventing pain with a
tamper-resistant transdermal device containing 3-acyl- substituted
antagonists.
[000035] Lu Zhengtang discloses in the Chinese Patent No. CN 1204649
(published 01-13-
1999) preparation of naloxone esters.
[000036] S. Lazar et al. describes in the article entitled "Synthesis
and biological activity of
the phosphate and sulfate esters of naloxone and naltrexone" (published in the
European Journal of
Medicinal Chemistry (1994), vol. 29(1), pp. 45-53) the synthesis and
biological activity of the phosphate and
sulfate esters of naloxone.
[000037] Hussein et al. describes (in Pharmaceutical Research (1988),
vol. 5(9), pp. 615-
18) that various prodrugs of naloxone where 3-phenoxy group is esterified
lacked a bitter taste and had
better buccal bioavailability in dogs.
[000038] Elie Gabriel Shami describes in European Patent Publication
No. EP 170090 that
benzoate ester prodrug derivatives of 3-hydroxymorphinans. The aforementioned
publications are
incorporated herein, as part of the specification.
[000039] None of these cited publications describes Naloxone
combination with a
hexadienoate included in the molecule, or indicates that such molecule will
result and provide substantially
more effective and long-lasting neutralizing/sobering effect when administered
to a person.
DESCRIPTION OF INVENTION
[000040] The present invention involves a novel modification of opioids and
their antagonists
that leads to higher opioid receptor engagement when given orally. More
specifically, the present
invention involves modification of the appropriate opiate receptor modulators
(e.g. Nalbuphine,
Buprenorphine, Hydromorphine, Morphine, Pentazocine, Butorphanole, Naloxone,
etc.) or related
8
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
compounds to improve opiates' engagement of the opioid receptors when given
orally.
[000041] The present invention further involves methods of mitigating opiate
low oral
bioavailability when opiates are used, without limitation, for the following
conditions: pain management,
palliative care, anesthesiology (e.g. postoperatively), skin disorders (e.g.
pruritus), addictions (detox or
management), certain locomotive disorders (e.g. levodopa-induced dyskinesias
(LID) in Parkinson's
disease, the dyskinesias associated with Tourette's syndrome, tardive
dyskinesia, Huntington's disease,
etc.
[000042] The present invention involves a novel modification of the opioid
agent (e.g.
Nalbuphine) that provides unexpected results of increasing the engagement of
opioid receptors when given
orally. Thus, this novel modification provides superior quality of care and
allows for a wider range of
therapeutic indications, including chronic conditions that require oral
administration of the opioid.
[000043] The present invention involves a novel modification of the opioid
antagonist, such as
Naloxone combination with a hexadienoate included in the molecule, which
provides substantially more
effective and long-lasting neutralizing/sobering effect when administered to a
person or patient.
[000044] The novel features of the present invention will be further described
with reference to
the following drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[000045] The patent or application file contains at least one drawing
executed in color.
Copies of this patent or patent application publication with color drawing(s)
will be provided by the Office
upon request and payment of the necessary fee,
[000046] FIGURE 1 illustrates NMR 1H spectrum of NB-20 compound,
formulated in
accordance with at least one embodiment of the present invention.
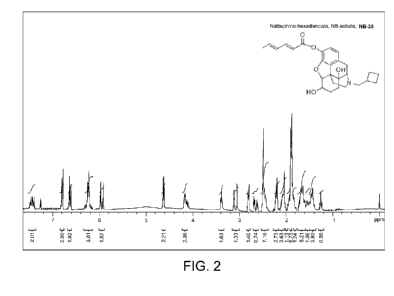
[000047] FIGURE 2 illustrates NMR 1H spectrum of NB-33 compound,
formulated in
accordance with at least one embodiment of the present invention.
9
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000048] FIGURE 3 illustrates NMR 1H spectrum of NB-39 compound,
formulated in
accordance with at least one embodiment of the present invention.
[000049] FIGURE 4 illustrates NMR 1H spectrum of NB-51 compound,
formulated in
accordance with at least one embodiment of the present invention.
[000050] FIGURE 5 illustrates NMR 1H spectrum of NB-52 compound,
formulated in
accordance with at least one embodiment of the present invention.
[000051] FIGURE 6 illustrates NMR 1H spectrum of NB-56 compound,
formulated in
accordance with at least one embodiment of the present invention.
[000052] FIGURE 7 illustrates NMR 1H spectrum of NB-58 compound,
formulated in
accordance with at least one embodiment of the present invention.
[000053] FIGURE 8 illustrates NMR 1H spectrum of NB-78 compound,
formulated in
accordance with at least one embodiment of the present invention.
[000054] FIGURE 9A illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of nalbuphine superposed with co-crystallized
ligand 13-FNA.
[000055] FIGURE 9B illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of naloxone superposed with co-crystallized
ligand p-FNA.
[000056] FIGURE 10A illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of NX-90 in the binding site of 4DKL.
[000057] FIGURE 10B illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of NB-33 in the binding site of 4DKL.
[000058] FIGURE 100 illustrates molecular interaction with Met 151
shown by the
conformer of NB-33 with the binding mode similar to the most energetically
favored conformer.
[000059] FIGURE 10D illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of NB-39 in the binding site of 4DKL.
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000060] FIGURE 11A illustrates the most energetically favored
conformer of nalbuphine (yellow),
naloxone (pink) and co-crystalized p-FNA (white) superposed in the opioid
binding site of 4DKL.
[000061] FIGURE 11B illustrates the most energetically favored
conformers of NX-90 (blue),
NB-33 (red), NB-39 (cyan) and co-crystalized 13-FNA (white) superposed in the
opioid binding site of 4DK.
[000062] FIGS. 12A-C show Hydrophobic (red) and hydrophilic (yellow)
contact preference
areas on the molecular surface of the binding site of 4DKL with the docked
conformer of NX-90, NB-33 and
NB-39, respectively, in accordance with at least one embodiment.
[000063] FIG. 13 shows Graph 1, illustrating superior analgesic
properties of the NB-33,
according to at least one embodiment, in comparison to the equimolar dose of
the parent opioid NB.
DETAILED DESCRIPTON
[000064] The present invention includes formation of an opiate derived
compositions including
hexadienoate and opioid residue in a single molecule, which is used in
therapeutic areas associated with
opioid receptor modulation.
[000065] The various aspects and features of the present invention and the
composition is
described with reference to TABLE 1, which illustrates the selected properties
of compounds NB, NB-20,
NB-28, NB-31, NB-32, NB-33, NB-39, NB-46, NB-51, NB-52, NB-56, NB-58, NB-76,
NB-78.
[000066] Examples of NMR 1H spectrums of selected compounds (examples
including NB-20,
NB-33, NB-39, NB-51, NB-52, NB-56, NB-58, NB-78), formulated in accordance
with at least one
embodiment of the present invention are shown in FIGS 1-8, respectively.
[000067] Surprisingly, 3-hexadienoate derivative of an opioid, created in
accordance with at
least one embodiment of the present invention, produced higher opioid receptor
engagement than the
parent opioid compound. Thus, for example, Nalbuphine 3-hexadienoate (NB-33)
produced superior to the
equivalent dose of both Nalbuphine 3-docosanoate (NB-39) and Nalbuphine (NB)
analgesia in rats and
humans, when given orally. Furthermore, a significant effect of NB-33 on pupil
dilation (miosis) was
11
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
observed in humans, which indicated superior receptor engagement.
[000068] Unexpectedly, when examining the effects of at least one embodiment
of the present
invention, was found that the position and the number of unsaturated sites of
the ester of the phenoxy
moiety is unique for hexadienic backbone and required for a superior
engagement of opioid receptors.
Thus, Nulbuphine 3-alkenoate (e.g. NB-33) produced better analgesia than the
parent opioid, while other
unsaturated acid derivatives of Nalbuphine (e.g. NB-31, NB-32, NB-52, or NB-
78) produced no analgesia in
rats.
[000069] Moreover, evaluating at least one embodiment of the present
invention, it was found
that Nalbuphine 3-hexadienoate has a unique and distinct opiate receptor
signature of its own with human
recombinant opiate receptors expressed in cells.
[000070] In accordance with at least one embodiment, the compounds of
present invention
comprise a general formula I or pharmaceutically acceptable salt of thereof
R2 R4 0
Ri OY
R3 R5
Formula I
wherein R1, R2, R3, R4 or R5 are selected from a group comprising H,
optionally substituted 01-3
and 0A1k), double bonds have E or Z geometry, and Y is an opioid residue.
[000071] In at least one embodiment, the present invention further relates to
methods of
mitigating opiate low oral bioavailability when opiates are used in the
following, but not limited to,
conditions: pain management, palliative care, anesthesiology (e.g.
postoperatively), skin disorders (e.g.
pruritus), addictions (detox or management), certain locomotive disorders
(e.g. levodopa-induced
dyskinesias (LID) in Parkinson's disease, and the dyskinesias associated with
Tourette's syndrome, tardive
dyskinesia and Huntington's disease), etc.
[000072] In at least one embodiment, the present invention is an optionally
substituted
12
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
hexadienoate of a phenoxy moiety modification of the appropriate opiate
receptor modulators or related
compounds to improve opiates' engagement of the opioid receptors when given
orally.
[000073] In another embodiment, the present invention is a is an optionally
substituted
hexadienoate of a 3-phenoxy moiety modification of the appropriate opiate
receptor modulators, including,
but not limited to, Hydromorphine, Morphine, Nalbuphine, Pentazocine,
Butorphanol, Buprenorphine,
Naloxone or related compounds, formulated to improve opiates' engagement of
the opioid receptors when
given orally.
[000074] In at least one further embodiment, the present invention is a 3-
hexadienoate
modification of the appropriate opiate receptor modulators or related
compounds, formulated to improve
opiates' engagement of the opioid receptors when given orally.
[000075] In at least one embodiment, the present invention is a 3-hexadienoate
modification of
Nalbuphine or a pharmaceutically acceptable salt of thereof to improve
engagement of the opioid receptors
when given orally.
[000076] In yet another embodiment, the present invention is a 3-hexadienoate
modification of
Nalbuphine or a pharmaceutically acceptable salt of thereof to improve quality
of pain management when
given orally.
[000077] In a further one or more embodiments, the present invention is a 3-
hexadienoate
modification of Nalbuphine or a pharmaceutically acceptable salt of thereof to
improve quality of pain
management when given intravenously, intranasally, transdermally,
sublingually, rectally, topically,
intramuscularly, subcutaneously or via inhalation.
[000078] The following are further examples of compounds prepared in
accordance with at least
one embodiment of the present invention. The chemical name, composition and
coding name for each of
the compounds in Examples 1 is shown in TABLE 1 below.
13
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
EXAMPLES 1
[000079] (E)-3-(cyclobutylmethyl)-94(3,7-dimethylocta-2,6-dien-1-
yl)oxy)-1,2,3,4,5,6,7,7a-
octahydro-4aH-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7-diol, Nalbuphino-
Geranyl, (NB-20).
Potassium bicarbonate (280 mg, 2.0 mmol) was added to suspension of nalbuphine
hydrochloride (400 mg,
1.0 mmol) in acetone (20 mL) and toluene (20 mL) at room temperature. Geranyl
bromide (320 mg, 1.5
mmol) was added. The reaction mixture was stirred under reflux for 4 h and
overnight at room temperature.
The reaction mixture was evaporated and the residue was purified by column
chromatography (silicagel,
Et0Ac/Heptanes/Me0H, 1:1:0.10). The colorless oil was formed after evaporation
of selected fractions,
yield 45%, purity 91% by HPLC. The structure was confirmed by NMR 1H.
[000080] 3-(cyclobutylmethyl)-9-(((2E,6E)-3,7,11-trimethyldodeca-
2,6,10-trien-1-yl)oxy)-
1,2,3,4,5,6,7,7a-octahydro-4aH-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7-
diol, Nalbuphino-Farnesyl,
(NB-28). This compound was prepared according to the procedure of NB-20, by
substituting geranyl
bromide for farnesyl bromide. The crude material was purified by column
chromatography (silicagel,
Et0Ac/Heptanes, 1:1). The colorless oil was obtained after evaporation of
selected fractions, yield 53%,
purity 93% by HPLC. The structure was confirmed by NMR 1H.
[000081] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-ylundec-10-enoate, Nalbuphino-
undecelenoate, (NB-31). EDCI
(1.04 g, 5.4 mmol) was added to undecylenic acid (1.0 g, 5.4 mmol) in THF (30
mL) at 000 with stirring.
The reaction mixture was stirred for 10 min and Nalbuphine hydrochloride (2.13
g, 5.4 mmol),
trimethylamine (1.1 g, 10.9 mmol) and 4-dimethylaminopyidine (0.22 g, 1.8
mmol) were added at 0 C. The
stirring was continued for 1 h at 000 and at room temperature overnight. The
reaction mixture was filtered,
filtrate was evaporated, and the residue was purified by column chromatography
(silicagel,
Et0Ac/Heptanes, 1:1). The white solid was formed after evaporation of selected
fractions, yield 2.2 g
(78%), purity 95% by HPLC. The structure was confirmed by NMR 1H.
14
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000082] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-yl(E)-3,7-dimethylocta-2,6-dienoate,
Nalbuphino-geranoate, (NB-
32). This compound was prepared according to the procedure of NB-31, by
substituting undecylenic acid
for geranyc acid. The crude material was purified by column chromatography
(silicagel, Et0Ac/Heptanes,
1:1). The white solid was formed after evaporation of selected fractions,
yield 67%, and purity 96% by
HPLC. The structure was confirmed by NMR 1H.
[000083] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-y1(2E,4E)-hexa-2,4-dienoate, Nalbuphino-
sorbate, (NB-33). EDCI
(1.16 g, 6.1 mmol) was added to hexadienoic acid (0.68 g, 6.1 mmol) in THF (30
mL) at 000 with stirring.
The reaction mixture was stirred for 10 min and Nalbuphine hydrochloride (2.39
g, 6.1 mmol),
trimethylamine (1.2 g, 12 mmol) and 4-dimethylaminopyidine (0.25 g, 2 mmol)
were added at 000. The
stirring was continued for 1 h at 000 and at room temperature overnight. The
reaction mixture was filtered,
filtrate was evaporated, and the residue was purified by column chromatography
(silicagel,
Et0Ac/Heptanes, 1:1). The white crystals were formed after evaporation of
selected fractions, yield 2.05 g
(75%), purity 98% by HPLC. The structure was confirmed by NMR 1H.
[000084] 3-(cyclobutylmethyl)-9-(((2E,4E)-hexa-2,4-dienoyl)oxy)-4a,7-
dihydroxy-
2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-3-ium
chloride, Nalbuphino-
sorbate, hydrochloride, (NB-56). HCI (gas) was bubbled into the solution of
nalbuphino-sorbate (NB-33)
(0.4 g, 0.89 mmol) in MTBE (15 mL) at 000. The white precipitate was formed
immediately. The reaction
mixture was stirred for 1 h and the solid was filtered, washed with MTBE and
dried in vacuum. The yield
0.35 g (81%), purity 98% by HPLC. The structure was confirmed by NMR 1H.
[000085] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-yldocosanoate, Nalbuphino-docosanoate,
(NB-39). EDCI (0.56 g,
2.9 mmol) was added to behenic acid (1.0 g, 2.9 mmol) in THF (50 mL) at 000
with stirring. The reaction
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
mixture was stirred for 30 min and Nalbuphine hydrochloride (1.16 g, 2.9
mmol), trimethylamine (0.29 g, 2.9
mmol) and 4-dimethylaminopyidine (0.12 g, 1.0 mmol) were added at 000. The
stirring was continued for 1
h at 000 and at room temperature overnight. The reaction mixture was filtered,
filtrate was evaporated, and
the residue was purified by column chromatography (silicagel, Et0Ac/Heptanes,
1:2). The white solid was
formed after evaporation of selected fractions, yield 1.45 g (73%), purity 97%
by HPLC. The structure was
confirmed by NMR 1H. Synthesis and properties of NB-39 was also described in
US Patent 5750534.
[000086] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-ylisobutyrate, Nalbuphino-isobutyrate, NB-
isovaleroate, (NB-46).
This compound was prepared according to the procedure of NB-31, by
substituting undecylenic acid for
isovaleric acid. The crude material was purified by column chromatography
(silicagel, Et0Ac/Heptanes,
1:1). The white crystals were formed after evaporation of selected fractions,
yield 54%, and purity 95% by
HPLC. The structure was confirmed by NMR 1H.
[000087] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-y1 3-methylbut-2-enoate, Nalbuphino-3,3-
dimethylacrylate, (NB-51).
This compound was prepared according to the procedure of NB-31, by
substituting undecylenic acid for
3,3-dimethyl acrylic acid. The crude material was purified by column
chromatography (silicagel,
Et0Ac/Heptanes, 1:1). The white crystals were formed after evaporation of
selected fractions, yield 77%,
purity 95% by HPLC. The structure was confirmed by NMR 1H.
[000088] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-yl(E)-2-methylbut-2-enoate, Nalbuphino-
2,3-dimethylacrylate, (52).
This compound was prepared according to the procedure of NB-31, by
substituting undecylenic acid for
2,3-dimethyl acrylic acid. The crude material was purified by column
chromatography (silicagel,
Et0Ac/Heptanes, 1:1). The white crystals were formed after evaporation of
selected fractions, yield 75%,
purity 96% by HPLC. The structure was confirmed by NMR 1H.
16
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000089] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-y12-methoxybut-2-enoate, Nalbuphino-2-
methoxycrotonate, (NB-
58). This compound was prepared according to the procedure of NB-31, by
substituting undecylenic acid
for 2-methoxy-crotonyc acid. The crude material was twice purified by column
chromatography (silicagel,
Et0Ac/Heptanes, 1:1). The white oils were formed after evaporation of selected
fractions, yield 27%, purity
94% by HPLC. The structure was confirmed by NMR 1H.
[000090] 7-acetoxy-3-(cyclobutylmethyl)-4a-hydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-y1-(2E,4E)-hexa-2,4-dienoate, Nalbuphino-
hexadienoate-acetate
(NB-76). NB-33 (0.5 g, 1.1 mmol) was stirred in acetic anhydride (7.0 mL) at
40-50 C overnight. Et0H (20
mL) was added and the reaction mixture was evaporated. The residue was twice
purified by column
chromatography (silicagel, Et0Ac/Heptanes, 1:2). The white crystals were
formed after evaporation of
selected fractions, yield 1.45 g (50%), purity 97% by HPLC. The structure was
confirmed by NMR 1H.
[000091] 3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-9-ylcinnamate, Nalbuphino-cynnamate, (NB-
78). This compound was
prepared according to the procedure of NB-31, by substituting undecylenic acid
for 2-trans-cynnamic acid.
The crude material was purified by column chromatography (silicagel,
Et0Ac/Heptanes, 1:1). The white
crystals were formed after evaporation of selected fractions, yield 67%,
purity 94% by HPLC. The structure
was confirmed by NMR 1H.
[000092]
TABLE 1
N Name, Structure Code Stable Stable in
Analgesia
in sGIF plasma (rat)
1 Nalbuphine NB n/a n/a moderate
17
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
HO,
0 OH
N
HO
3-(cyclobutylmethyl)-1,2,3,4,5,6,7,7a-octahydro-4aH-
4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7,9-triol
Chemical Formula: C21H27N04
Molecular Weight: 357.45
2 Nalbuphino-Geranyl, NB-geranyl, NB-20 No No No
IW
0 OH
N---../C7
HO
(E)-3-(cyclobutylmethyl)-94(3,7-dimethylocta-2,6-dien-1-
yl)oxy)-1,2,3,4,5,6,7,7a-octahydro-4aH-4,12-
methanobenzofuro[3,2-e]isoquinoline-4a,7-diol
Chemical Formula: C31H43N04
Molecular Weight: 493.69
3 Nalbuphino-Farnesyl, NB-farnesyl, NB-28 No No No
18
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
\ \ \ 0
IW
0 OH
N
HO
3-(cyclobutylmethyl)-9-(PE,6E)-3,7,11-trimethyldodeca-
2,6,10-trien-1-ypoxy)-1,2,3,4,5,6,7,7a-octahydro-4aH-
4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7-diol
Chemical Formula: C36H51N04
Molecular Weight: 561.81
4 Nalbuphino-undecelenoate NB-31 Yes No Inactive
o
0 i
IW
0 OH
HO
3-(cyclobutylmethyl)-4a,7-di hydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-
9-y1 undec-10-enoate
Chemical Formula: C32H45N05
Molecular Weight: 523.71
Nalbuphino-hexadienoate, NB-sorbate, NB-33 Yes No Excellent
19
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
0
0 OH
HO
3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-
9-y1 (2E,4E)-hexa-2,4-dienoate
Chemical Formula: C27H33N05
Molecular Weight: 451.56
6 Nalbuphino-hexadienoate, hydrochloride; NB-sorbate, NB-56 Yes No No
HCI salt
0
1.--0 lei
0 OH
NIC)0
HO H
CP
3-
(cyclobutylmethyl)-9-(((2E,4E)-hexa-2,4-dienoyl)oxy)-
4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoquinolin-3-ium chloride
Chemical Formula: C27H34CIN05
Molecular Weight: 488.02
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
7 Nalbuphino-geranoate, NB-geranoate, NB-32 Yes No
Inactive
o
//.)-1----o
0
0 OH
HO
3-(cyclobutylmethyl)-4a,7-di hydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoguinolin-
9-y1 (E)-3,7-dimethylocta-2,6-dienoate
Chemical Formula: C31H41N05
Molecular Weight: 507.67
8 Nalbuphino-docosanoate, NB-behenoate, NB-39 Yes No
Inactive
o
o 0
0 OH
N---.):17
HO
3-(cyclobutylmethyl)-4a,7-di hydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoguinolin-
9-y1 docosanoate
Chemical Formula: C43H69N05
21
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
Molecular Weight: 680.03
9 Nalbuphino-3,3-dimethylacrylate, NB-3,3- NB-51 Yes No Inactive
dimethylacrylate, NB-senecioate
o
)1¨.0
IW
0 OH
N---/C7
HO
3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoguinolin-
9-y13-methylbut-2-enoate
Chemical Formula: C26H33N05
Molecular Weight: 439.55
Nalbuphino-2-methoxycrotonate, NB-58 No No No
NB-2-methoxycrotonate
0
,Y1----0
so 401
0 OH
HO
3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
22
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-
9-y12-methoxybut-2-enoate
Chemical Formula: C26H33N06
Molecular Weight: 455.55
11 Nalbuphino-isobutyrate, NB-isovaleroate NB-46 Yes Yes No
0
401
0 OH
HO
3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-
9-y1 isobutyrate
Chemical Formula: C25H33N05
Molecular Weight: 427.54
12 Nalbuphino-2,3-dimethylacrylate, NB-Tiglate NB-52 Yes No
Inactive
0
0 OH
HO
3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
23
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoguinolin-
9-y1 (E)-2-methylbut-2-enoate
Chemical Formula: C26H33N05
Molecular Weight: 439.55
13 Nalbuphino-hexadienoate-acetate, NB-76 Yes No moderate
0
0 co
0
N.--.):3
)LO
7-acetoxy-3-(cyclobutylmethyl)-4a-hydroxy-
2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-
methanobenzofuro[3,2-e]isoguinolin-9-y1 (2E,4E)-hexa-
2,4-dienoate
Chemical Formula: C29H35N06
Molecular Weight: 493.60
14 Nalbuphino-cynnamate NB-78 Yes No Inactive
0
0 is
0 OH
HO
24
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
3-(cyclobutylmethyl)-4a,7-dihydroxy-2,3,4,4a,5,6,7,7a-
octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-
9-ylcinnamate
Chemical Formula: C30H33N05
Molecular Weight: 487.60
EXAMPLE 2 -- Stability in the simulated gastro-intestinal fluid (sGIF).
[000093] Stability of NB-33 in the simulated gastro-intestinal fluid
(sGIF) was evaluated as
below and individual compound data was summarized in Table 1.
[000094] sGIF is 0.5% solution of pepsine(Alfa Aesar, Pepsin, porcine
stomach) in 0.1N
aqueous HCI . Each derivative (50mg) was mixed with sGIF (50 mL) and incubated
at 37 C on a shaker.
The hydrolysis and release of Nalbuphine was monitored by HPLC at T = 0 hr,
[000095] 0.5 hr, 1 hr, 2 hr, and 4 hr. The acceptance criteria was
defined as NLT 80% of the
derivative still intact after 4 hrs.
EXAMPLE 3 - Stability in human plasma.
[000096] Stability of NB-56 in human plasma was evaluated as below
and the individual
compound data was summarized in Table 1.
[000097] NB-56 (1.0 mg) was dissolved in 10 mL of plasma (Plasma
Pooled Normal Human
Plasma, Na-citrate, Innovative Research) with stirring for 10 min at 20 C. The
solution was incubated at 37
C. 1 mL of solution was taken for each test sample. MeCN (0.05 mL) was added
to sample solution.
Shaking for 1 min followed by centrifugation (15 min, 14.000r/m). Supernatant
was filtered off and extracted
with Et0Ac (2x20 mL). The combined extract was dried over MgSO4 and
concentrated in vacuum. The
residue was dissolved in Me0H (20 pL). The solution was used for HPLC
injection.
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[000098] The hydrolysis and release of Nalbuphine was monitored by
HPLC at T = 0 hr,
0.5 hr, 1 hr, 2 hr, and 4 hr. The acceptance criteria was defined as NLT than
20% of hydrolysis after 4 hrs.
EXAMPLE 4
TABLE 2 - Human recombinant opiate receptor data for NB-33
NB-33
Assay
< luM > 1 uM
mu (MOR) (h) (agonist effect)
mu (MOR) (h) (antagonist effect) X X
kappa (KOR) (h) (agonist effect) X
kappa (KOR) (h) (antagonist effect)
delta (DOR) (h) (agonist effect) X
delta (DOR) (h) (antagonist effect)
[000099] Human recombinant opiate receptor (mu, kappa or delta)
expressed in CHO-K1
cells were used. Test compound (NB-33)/or vehicle was incubated with the cells
(4 x 10E5/mL) in modified
HBSS pH 7.4 buffer at 3700 for 30 min. The reaction was evaluated for cAMP
levels by TR-FRET.
Compounds were screened at 0.3, 1 and 3 uM. by Eurofins Pharma Discovery
Services.
[0000100] Data for compound NB-33 is summarized in Table 2.
26
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
EXAMPLE 5
[0000101] Tests on Sprague-Dawley rats were conducted using
Nalbuphine, NB-31, NB-32,
NB-33, NB-33, NB39, NB-51, NB-52, NB-76 and NB-78.
[0000102] Thirty Sprague-Dawley rats (12 week old; male) were randomly
assigned to 10
groups and each group was gavaged with one of the following treatments: 1.
Sesame oil; 2. Nalbuphine (in
sesame oil; 60 uM/kg), 3. NB-31 (in sesame oil; 60 uM/kg), 4. NB-32 (in sesame
oil; 60 uM/kg), 5. NB-33
(in sesame oil; 60 uM/kg), 6. NB-39 (in sesame oil; 60 uM/kg), 7. NB-51 (in
sesame oil; 60 uM/kg), 8. NB-
52 (in sesame oil; 60 uM/kg), 9. NB-76 (in sesame oil; 60 uM/kg), 10. NB-78
(in sesame oil; 60 uM/kg).
Each rat received only one oral dose.
[0000103] The antinociceptive activity was assessed as in Anesth AnaIg
2003; 97; 806-9
using the cold ethanol tail-flick test. The testing temperature was set at -20
C and the cutoff time was 40
seconds. All rats were tested at T= 0 immediately before medication.
Measurements of the antinociceptive
thresholds of saline, nalbuphine and nulbuphine derivatives were done at T = 0
hr, 0.25 hr, 0.5 hr, 1 hr, 1.5
hr, 2 hr, 3 hr and 5 hr followed oral administration.
[0000104] The data, as illustrated in the last column of Table 1
indicates excellent and
superior results for NB-33.
[0000105]
EXAMPLE 6
[0000106] Double-blind, NB hydrochloride and NB-39 controlled, trial
of the antinociceptive
effect of oral NB-33 in healthy volunteers. Each of the three healthy
volunteers was assigned a set of 6
non-transparent gelatin capsules as follows: 2 x NB hydrochloride (MW = 393.4;
39mg), 2 x NB-33 (MW =
451.6; 45mg) and 2 x NB-39 (MW = 680.0; 68mg). Each week a healthy volunteer
would receive a pill from
the assigned set in a random fashion and take it orally. At T = 0 hr, 0.25 hr,
0.5 hr, 1 hr, 1.5 hr, 2 hr, 3 hr
and 5 hr followed oral administration a heat pain threshold was measured (hot
water at 50 C) as well as
27
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
miosis.
[0000107] Following one week of a wash out period, each volunteer
repeated the protocol
until all pills from the assigned set were administered. The individual data
for heat pain threshold as %MPE
= [(test latency - baseline latency)/(baseline latency)] x 100 and for miosis
as %MPE = [(test diameter -
baseline diameter)/(baseline diameter)] x 100 are shown in Table 2 and Table 3
respectively.
[0000108] Tables 3, 4, and 5A-D illustrate that NB-33 resulted in
analgesia and miosis
superior to both the parent opioid NB and the parent opioid prodrug NB-39 when
given orally. The
differences in analgesia and miosis were statistically significant as
indicated in Table 5A-D.
[0000109]
TABLE 3
%MPE (analgesia)
Ohr 0.25 hr 0.5 hr 1 hr 1.5 hr 2 hr 2.5 hr 3
hr 5 hr
33 (45mg) 0.0 0.0 24.0 80.0 140.0 124.0 96.0
60.0 24.0
33 (45mg) 0.0 8.3 66.7 183.3 87.5 191.7 112.5
45.8 20.8
33 (45mg) 0.0 3.7 48.1 59.3 114.8 185.2 133.3
118.5 44.4
33 (45mg) 0.0 31.3 37.5 50.0 68.8 81.3 31.3 25.0
18.8
33 (45mg) 0.0 30.0 37.5 67.5 97.5 157.5 112.5
27.5 22.5
33 (45mg) 0.0 0.0 19.2 100.0 115.4 111.5 119.2
157.7 115.4
NB (35mg) 0.0 0.0 5.6 50.0 100.0 94.4 66.7 22.2
-5.6
NB (35mg) 0.0 29.5 39.3 34.4 54.1 54.1 82.0 32.8
27.9
NB (35mg) 0.0 5.3 10.5 42.1 68.4 94.7 57.9 68.4
5.3
NB (35mg) 0.0 56.3 50.0 87.5 112.5 162.5 106.3
37.5 26.7
NB (35mg) 0.0 5.3 21.1 47.4 73.7 57.9 36.8 57.9
5.3
NB (35mg) 0.0 21.4 35.7 57.1 78.6 50.0 35.7 0.0
0.0
39 (68mg) 0.0 0.0 10.0 10.0 15.0 10.0 5.0 10.0
0.0
39 (68mg) 0.0 17.6 11.8 5.9 11.8 23.5 0.0 11.8
0.0
39 (68mg) 0.0 13.3 40.0 73.3 93.3 93.3 46.7 40.0
6.7
39 (68mg) 0.0 6.2 6.2 10.8 -7.7 33.8 -6.2 9.2
-9.2
39 (68mg) 0.0 8.3 55.6 11.1 19.4 0.0 16.7 0.0
-2.8
39 (68mg) 0.0 0.0 15.0 5.0 5.0 0.0 5.0 0.0
5.0
[0000110]
28
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
TABLE 4
%MPE (miosis)
Ohr 0.25 hr 0.5 hr 1 hr 1.5 hr 2 hr 2.5 hr 3
hr 5 hr
33 (45mg) 0.0 -8.8 3.4 24.0 20.6 37.8 46.4 3.4 3.4
33 (45mg) 0.0 0.3 26.7 40.4 36.5 46.2 63.7 36.5
36.5
33 (45mg) 0.0 20.0 33.3 42.2 42.2 42.2 29.5 15.6
9.3
33 (45mg) 0.0 0.0 -6.3 33.9 33.9 42.9 33.9 25.0
17.2
33 (45mg) 0.0 -5.0 26.7 26.7 36.5 46.2 46.2 36.5
33.7
33 (45mg) 0.0 26.7 26.7 40.4 46.2 55.9 65.7 29.0
32.1
NB (35mg) 0.0 3.1 9.4 21.9 17.2 18.8 18.8 6.2 6.2
NB (35mg) 0.0 9.4 37.5 46.9 65.6 31.3 3.1 6.2 3.1
NB (35mg) 0.0 6.7 0.0 6.7 25.0 55.6 50.0 33.3
22.2
NB (35mg) 0.0 0.0 9.4 21.9 17.2 31.3 25.0 21.9
6.2
NB (35mg) 0.0 3.2 9.7 45.2 45.2 58.1 25.8 16.1
9.7
NB (35mg) 0.0 2.9 8.8 -2.9 29.4 11.8 26.5 20.6
7.8
39 (68mg) 0.0 10.3 14.9 37.9 37.9 14.9 14.9 24.1
3.4
39 (68mg) 0.0 0.0 0.0 6.7 6.7 0.0 6.7 0.0 0.0
39 (68mg) 0.0 25.0 16.7 22.2 33.3 25.0 11.1 11.1
11.1
39 (68mg) 0.0 12.5 9.4 18.8 21.9 17.2 6.2 0.0 0.0
39 (68mg) 0.0 20.0 26.7 36.7 23.3 16.7 13.3 3.3
13.3
39 (68mg) 0.0 3.1 9.4 21.9 25.0 6.2 9.4 0.0 3.1
[0 0 0 0 1 1 1] Independent samples t-test was used to compare the means of
%MPE analgesia
and miosis in the two pairs of samples: NB-33 and NB and NB-33 and NB-39. All
analyses were made
using SPSS (v.25).
* Bold indicates statistical significance at a=0.05
[0000112] Tables 5A-D illustrate comparison of analgesia and miosis
between NB-33 and
NB, NB-39.
TABLE 5A TABLE 5B
29
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
Analgesia, NB-33 vs. NB Analgesia, NB-33 vs. NB-39
his T p-value his t p-value
0.25 -.705 .497 0.25 .700 .506
- -
0.5 1.182 .264 0.5 1.467 .173
1 1.730 .114 1 3.110 .011
.....
1.5 1.699 .120 1.5 4.558 .001
2 2.258 .048 2 5.027 .001
2.5 . 1.982 .076 2.5 5.380 .000
-
3 1.486 .168 3 2.656 .039
1.895 - .087 5 2.642 .025
TABLE 50 TABLE 5D
Miosis, NB-33 vs. NB Miosis, NB-33 vs. NB-39
his t p-value his t p-value
,.,._
0.25 .217 .836 0.25 -.893 .393
..... .....
0.5 .716 .491 0.5 .751 .470
1 1.295 s .224 1 1.843 .095
1.5 .318 .757 1.5 1.985 .075
2 1.328 .232 2 7.253 .000
2.5 2.624 .025 2.5 5.982 .001
3 1.022 .331 3 2.713 .022
5 2.018 .071 5 2.732 .031
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
[0000113]
Table 6A and Graph 1 in FIG. 13 below illustrate additional testing results
for the
NB-33 on Randall-Selitto rats, demonstrating its efficacy and benefits
(including greater stability) in
comparison to base compound.
[0000114]
Table 6A and Graph 1 in FIG. 13 below illustrate additional testing results
for the
NB-33 on Randall-Selitto rats, demonstrating its efficacy and benefits
(including greater stability) in
comparison to base compound.
[0000115]
TABLE 6A
WO# 10656913 AB137003 Species/Strain/Sex: Rate
Randall-Selitto (g)
Treatment Route Dose No. Pre- Post-dose
treatment 0.5 hr lhr 2 hr 4
hr 6 hr
1 92 73 98 92 65 75
2 97 68 88 72 69 62
3 86 68 76 92 97 97
Vehicle 5
(0.9% NaCl) mL/kg
SC 4 91 86 82 77 83 53
83 91 57 69 64 61
Mean 99.9
77.2 80.2 80.4 75.6 69.6
SEM 2.4 4.8 6.8 4.9 6.3
7.7
1 89 78 63 58 64 50
2 92 89 62 71 66 71
PT#1225608 3 100 93 92 103 93 82
3
AFC-2 SC 4 m g/kg 80 107 90 69 93 69
NB.HCI 5 91 134 96 93 61 85
Mean 90.4
100.2 80.6 78.8 75.4 71.4
SEM 3.2 9.6 7.5 8.3 7.2
6.2
1 93 121 98 99 83 79
PT#1225607 2 82 120 103 94 71 64
3.9
AFC-1 SC 3 98 207 199 214 136 101
mg/kg
NB-33.HCI 4 80 161 73 102 63 65
5 96 96 86 85 97 85
31
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
Mean 89.8
141.0* 111.8 118.8 90.0 78.8
SEM 3.7 19.5
22.4 24.0 12.9 6.9
[0000116] The data on the Graph 1 in FIG. 13 shows that NB-33 has
superior analgesic
properties to the equimolar dose of the parent opioid NB. FIG. 13 illustrate
in the graphical form the results
for 1310 NB-33, marked 1310, in comparison to the base NB compound NB, marked
1320 in FIG. 13.
[0000117]
EXAMPLE 7
[0000118] This invention is exemplified by but not limited to the
following compounds,
illustrated below. The following compounds, shown in TABLE 7 below, provide
non-limiting examples of
various opioids, modified by hexadienoate in accordance with at least one
embodiment.
[0000119]
TABLE 7
r Nalbuphino-3-hexadienoate
N
HO 0.
0 :- ''OH
oy j
o
Naloxone-3-hexadienoate
N
Oy
0
32
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
r'A Naltrexone-3-hexadienoate
N
HO 0
O
ol
o
r1:3 Butorphanolo-3-hexadienoate
N
Ir.
w ,
(:)1
0
I Metopon hexadienoate
N
O'sµ
0 0
0
01
0
I Hydromorphone hexadienoate
N>
O's
e =
O
o
33
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
I Levorphanol hexadienoate
,N
.,
1/
0,rH
0
I Morphino-3-hexadienoate
N
.õ.
= OH
d
oy.)
o
Nalorphino-3-hexadienoate
N
d
ol
o
r'A Cyclazocine
hexadienoate
N,
c_O
U 1/
01r)
0
34
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
r'A Ketocyclazocine
hexadienoate
Nõ
O.
0 1/
, I
0--Tr
0
rA Diprenorphine
hexadienoate
N
(:)Fl
7µ
= o
d ,
o,....I
o
I Etorphine
*N
: -
hexadienoate
0 2.
d
oy.)
o
I Levorphanol
,N
.,
hexadienoate
,/
ol
o
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
I Oxymorphone
N
hexadienoate
0 . o
o
o.l.j
o
N Tapentadol
hexadienoate
0
0...1
0
r1:7 Nalbuphino-3-(5-methyl)hexadienoate
N
H 0 0
6 I
(:)
o
Naloxone-3-(5-methyl)hexadienoate
N
IW 6
I
c)
o
36
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
r'A Naltrexone-3-(5-methyl)hexadienoate
N
P. 0O
--wi"¨ d -............---
oy-
o
r Butorphanolo-3-(5-methyl)hexadienoate
N
S
tw
.01
0
I Metopon 5-methylhexadienoate
N
0 0
0
0 1
0
I Hydromorphone 5-methylhexadienoate
N
0 cji
y--_
0..,1
0
37
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
I Levorphanol 5-methylhexadienoate
,N
-,
1
01.r/
0
I Morphino-3-(5-methyl)hexadienoate
N,
.0µ,,....
- OH
(5
0 I
0
Nalorphino-3-(5-methyl)hexadienoate
N
d 1
o
o
r'A Cyclazocine
5-methylhexadienoate
N,
Uc.-0
\/
(301/1
0
38
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
r'A Ketocyclazocine
5-methylhexadienoate
0
Nõ
O.
0
1
0.....r/
0
r'A Diprenorphine
5-methylhexadienoate
N
kOH
f
_ o
d: 1
0-..,.
0
I Etorphine
ON
5-methylhexadienoate
0
0
01
0
I Levorphanol
,N
.,
5-methylhexadienoate
I
0..,
0
39
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
Oxymorphone
. 5-methylhexadienoate
Cr
Jco
o,Trj
NV
Tapentadol
5-methylhexadienoate
0 ,õ=
[0000120]
EXAMPLE 8 - Molecular docking of nalbuphine/naloxone opioid antagonists into p-
opioid receptor.
[0000121] The human m-opioid receptor crystal structures were
downloaded from the RCSB
Protein Data Bank [PDB entry: 4DKL, https://wvvw.rcsb.org/structure/4DKL). The
in silico screening was
carried out with the MOE Dock program, part of the MOE Simulation module
2014.0901. The dissociation
constants (Ki) were calculated from the equation AG=RTIn(K1), where AG
represents binding free energy
which is equivalent to GBVI/WSA dG scoring function, R is the gas constant and
T the temperature. The Ki
was computed starting from the binding free energy values at a fixed
temperature (300 K).
[0000122] Both antagonists nalbuphine and naloxone demonstrate the key
interaction of Asp
147 with their ammonium group. It is known that this bonding to Asp 147 is
typical for the most known
opioid agonists/antagonists. The other duplicate interaction of nalbuphine and
naloxone is bonding of the
hydroxyl group attached to the aryl ring (3-position) to the water molecule,
which contributes in stabilizing
the inactive state of opioid receptors. Differently from nalbuphine, the
hydroxyl group of naloxone attached
to the tertiary carbon atom (14-position) participates in additional hydrogen
bonding to Asp 147.
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
[0000123] FIGURE 9A illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of nalbuphine superposed with co-crystallized
ligand 13-FNA. FIGURE 9B
illustrates the binding mode and molecular interactions of the most
energetically favored conformer of
naloxone superposed with co-crystallized ligand p-FNA.
[0000124] Nalbuphine and naloxone is bonding of the hydroxyl group
attached to the aryl ring
(3-position) to the water molecule, which contributes in stabilizing the
inactive state of opioid receptors.
Differently from nalbuphine, the hydroxyl group of naloxone attached to the
tertiary carbon atom (14-
position) participates in additional hydrogen bonding to Asp 147.
[0000125] FIGURE 10A illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of NX-90 in the binding site of 4DKL.
[0000126] FIGURE 10B illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of NB-33 in the binding site of 4DKL.
[0000127] FIGURE 100 illustrates molecular interaction with Met 151
shown by the
conformer of NB-33 with the binding mode similar to the most energetically
favored conformer.
[0000128] FIGURE 10D illustrates the binding mode and molecular
interactions of the most
energetically favored conformer of NB-39 in the binding site of 4DKL.
[0000129] FIGURE 11A illustrates the most energetically favored
conformer of nalbuphine (yellow),
naloxone (pink) and co-crystalized p-FNA (white) superposed in the opioid
binding site of 4DKL. FIGURE 11B illustrates
the most energetically favored conformers of NX-90 (blue), NB-33 (red), NB-39
(cyan) and co-crystalized [3-
FNA (white) superposed in the opioid binding site of 4DK.
[0000130] Computed dissociation constants (Ki) of NX-90, NB-33, NB-39
established the
higher affinity to the m-receptor (NB-33, NB-39) or a little lower affinity
(NX-90) in comparison to the
affinities of nalbuphine and naloxone. Analogously to the most energetically
favored conformers of
naloxone and nalbuphine, NX-90, NB-33 and NB-39 retain the crucial hydrogen
bonding to the residue of
41
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
Asp147. In this docking mode the "message" attached to the nitrogen atom is
delivered to the correct
"address" sited on the exact area of the binding pocket of the m-receptor.
However, unlike the binding
mode of known m-antagonists (e.g. nalbuphine and naloxone; Figure 11A) the
rigid frames of NX-90, NB-
33 and NB-39 are rotated by 1800 in the binding site (Figure 11B). Conversely,
the binding mode that
describes binding of nalbuphine and naloxone is not possible for all computed
conformers of NB-33, NB-39
and NX-90.
[0000131] Furthermore, both NX-90 and NB-33 have the unique hydrogen
bonding to Met
151 through the hydroxyl group attached to the tertiary carbon atom (14-
position). This interaction makes
both NX-90 and NB-33 different from NB-39 which hydroxyl group at cyclohexane
fragment (6-position)
forms the hydrogen bond with Lys A233 instead. The second differentiating
factor for both NX-90 and NB-
33 is that the rigid conjugated system of the residue of hexadienoic acid has
the extraordinary hydrophobic
cylindrical molecular surface. Simultaneously, the residues Cys217, Thr218,
Asn127, GIn124, Trp133,
Leu219 build the extra complementary hydrophobic molecular surface surrounding
this hexadienyl "tail"
inside the binding pocket (Figure 12A and 12B), whereas no discernible
hydrophobic surface exists in the
areas of binding site surrounding the highly flexible and lacking conjugation
docosanoyl "tail" (Figure 12C).
[0000132] FIGS. 12A-C show Hydrophobic (red) and hydrophilic (yellow)
contact preference
areas on the molecular surface of the binding site of 4DKL with the docked
conformer of NX-90, shown in
FIG. 12A; NB-33, shown in FIG. 12B and NB-39, shown in FIG. 12C.
[0000133] These examples, particularly in FIGURES 9-12 confirm at
least one feature of the
present invention, i.e., that modifying an opioid with a lipophilic moiety
with at least two conjugated double
bonds improves interactions with the opioid receptor.
[0000134] These examples also confirm another feature of the present
invention, i.e., that
modifying an opioid with a lipophilic moiety with at least two conjugated
double bonds improves interactions
with the opioid receptor by rotating the opioid in the active site by 180 C
and creating additional modes of
42
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
interactions with the receptor including a unique hydrophobic pocket.
[0000135] These examples further confirm another feature of the
present invention, i.e., that
modifying an opioid with a lipophilic moiety with at least two conjugated
double bonds changes properties
of the opioid at least in some embodiments of the present invention.
[0000136] These examples confirm yet another feature of the present
invention, i.e., that
modifying an opioid with a lipophilic moiety with at least two conjugated
double bonds improves
antagonistic properties of the opioid at least in some embodiments of the
present invention.
[0000137] Improved Performance of Opioid Receptor Antagonists
[0000138] As described above, in addition to the improved performance
and analgesic
qualities of opiates, the present invention also includes at least one
embodiment where hexadienoate
improves performance of opioid receptor antagonists, such as, for example,
Naloxone.
[0000139] In at least one embodiment, the present invention, and
particularly the
hexadienoate has been combined and tested with at least one specie (or
multiple species) from the
Naloxone group or compound.
[0000140] In at least one embodiment of the present invention, the
Naloxone, having
hexadienoate, is included in the molecule, and provides substantially more
effective and long-lasting
neutralizing/soberingeffect when administered to a subject.
[0000141] In at least one embodiment of the present invention, a
compound NX-90 and NX-
97 having the below formula has been synthesized and analyzed, as shown in
TABLE 8 below.
[0000142]
TABLE 8
43
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
1 0 NX-90
0
0 OH
0
3-ally1-4a-hydroxy-7-oxo-2,3,4,4a,5,6,7,7a-octahydro-
1H-4,12-methanobenzofuro[3,2-e]isoquinolin-9-y1 (2E,4E)-hexa-
2,4-dienoate
Chemical Formula: 025H27N05
Molecular Weight: 421.49
2
o 110I NX-97
o OH HCI
0
3-ally1-4a-hydroxy-7-oxo-2,3,4,4a,5,6,7,7a-octahydro-
1H-4,12-methanobenzofuro[3,2-e]isoquinolin-9-y1 (2E,4E)-hexa-
2,4-dienoate hydrochloride
Chemical Formula: C25H28CIN05
Molecular Weight: 457.95
[0000143] Sobering effect may be noted when administered to a subject.
This compound
may be utilized and synthetized in accordance with at least one embodiment of
the present invention is
referenced and named Naloxone-sorbate, (NX-90). The preparation of the
compound based on at least
one embodiment may proceed as follows.
[0000144] EDCI.HCI (1.36 g, 7.12 mmol) was added to hexadienoic acid
(0.74 g, 6.61 mmol)
in THF (50 mL) at 000 with stirring. Triethylamine (1.39 g, 13.8 mmol) was
added. Stirring for 2 h at 000.
Naloxone hydrochloride (2.00 g, 5.5 mmol and 4-dimethylaminopyidine (0.10 g,
0.82 mmol) were added at
000. The stirring was continued for 1 h at 000 and at room temperature
overnight. The reaction mixture
was filtered, filtrate was evaporated, and the residue was twice purified by
column chromatography
(silicagel, Et0Ac/Heptanes/Triethylamine, 2:1:0.5%). The white crystals were
formed after evaporation of
44
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
selected fractions, yield 0.75 g (32%), purity 98% by HPLC. The structure was
confirmed by NMR 1H.
[0000145] The properties of the NX-90 compound have been studied and
the following
results and specific benefits, including stability data, have been obtained
and confirmed.
[0000146]
TABLE 9¨ NX-90 Stability (GIF) Batch Number Alpha-1-91 (Tested by
Alfacheminvent LLC).
PRODUCT NAME:
NX-90
BATCH NO.: MFG DATE: SAMPLE SIZE: PACKAGING TYPE:
Alpha-1-91 03/21/2019 5.0 MG/2.0 ML Glass vial
(upright)
ASSAY STABILITY TESTING INTERVALS: STATUS:
CONDITIONS: INITIAL, 1, 2, 4, 8, 24 HRS COMPLETED:
GIF, 37 C, 03.22.2019
shaker
TEST Specifications Initial 1 h 2 h 4 h 8 h
24 h
Product
Clear solution Conforms Conf. Conf. Conf. Conf.
Conf.
Appearance
HPLC
Assay: (Area Report results 99.3 99.2 99.3 98.4 95.1
93.0
%)
Single
impurity: Report results 0.6 0.7 0.6 0.6 0.7
0.6
RRT=0.93
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
(Area %)
Single
impurity:
Report results 0.9 2.3
5.5
RRT=0.67
(Area %)
[0000147] Based on the results and observations, shown in Table 9, the
NX-90 has shown
significant improvements over the well-known drug Naloxone.
[0000148] Examples of the combination of NB-33 or similar compounds
with different opiates
and NX-90 with opiate antagonists in accordance with at least one embodiment
of the present invention is
further shown in Table 11 below.
[0000149] It is well documented and commonly known that opioids can be
used for the
treatment of the following medical conditions: pain management, a palliative
care, a postoperative
anesthesiology, a skin disorder, an addiction, a locomotive disorder, a
levodopa-induced dyskinesias (LID)
in Parkinson's disease, a dyskinesias associated with Tourefte's syndrome, a
tardive dyskinesia and a
Huntington's disease and others, The potency and effectiveness of the opioids
used for the treatment of
these medical conditions affects how successful the treatment is.
[0000150] Respectively, the higher engagement of opioid receptors
produces more effective
results for the treating such conditions in accordance with at least one
embodiment of the present
invention. For example, opioids modified with Hexadienoates will be more
effective in treating the
aforementioned conditions, because they have higher engagement of opioid
receptors.
[0000151] Thus, in at least one embodiment of the present invention,
one of the composition
compounds that is formulated based on the present invention, as for example NB-
33 or NX-90 (or others)
46
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
may be utilized for treatment of one of the medical conditions such as a pain
management, a palliative
care, a postoperative anesthesiology, a skin disorder (e.g. pruritus), an
addiction (detox or management),
and/or a locomotive disorder (e.g. levodopa-induced dyskinesias (LID) in
Parkinson's disease, and the
dyskinesias associated with Tourette's syndrome, tardive dyskinesia and
Huntington's disease).
[0000152] EXAMPLE 9
[00001531 TABLE 10 demonstrates human recombinant opiate receptor data
for NX 90.
NX-90
Assay
< luM > 1 uM
mu (MOR) (h) (agonist effect)
mu (MOR) (h) (antagonist effect) X X
kappa (KOR) (h) (agonist effect)
kappa (KOR) (h) (antagonist effect) X X
delta (DOR) (h) (agonist effect)
delta (DOR) (h) (antagonist effect) X
[00001541 Human recombinant opiate receptor (mu, kappa or delta)
expressed in CHO-K1
cells were used. Test compound (NX-90)/or vehicle was incubated with the cells
(4 x 10E5/mL) in modified
HBSS pH 7.4 buffer at 3700 for 30 min. The reaction was evaluated for cAMP
levels (cAMP and/or calcium
flux) by TR-FRET. Compounds were screened at 0.1, 0.3 and 1 uM by Eurofins
Pharma Discovery
Services.
[0000155] Data for compound NX-90 is summarized in Table 10. It shows
that NX-90 is not
47
CA 03149152 2022-01-28
WO 2021/029914 PCT/US2020/021599
a pharmacologically inert compound and has a distinct opioid signature of its
own, similar to the
pharmacological profile of naloxone. Separately, it shown that NB-33 is not a
pharmacologically inert
compound and has a distinct opioid signature of its own, similar to the
pharmacological profile of NB.
[0000156] These results are highly surprising as the prior art suggests
that such
modifications of 3-phenoxy position with fatty acids (e.g. NB-39) are pro-
drugs and by definition are
pharmacologically inert compounds. In accordance with at least one embodiment
of the present invention,
the NX-90 and NB-33 are shown not pharmacologically inert and are not pro-
drugs.
[0000157] In all cases it is understood that the above-described examples
and compounds
are merely illustrative of the many possible specific embodiments which
represent applications of the
present invention. Numerous and varied other arrangements can be readily
devised in accordance with the
principles of the present invention without departing from the spirit and the
scope of the invention.
[0000158] TABLE 11
7!"
11
(c--)
- II--
6a
48
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
I Lista-pm hexatt4rioste.
...14,,
.õ,--- -.....ot
t,
"=-=,,,,L,
ri---.4 ;,-s ,0
kl:,..:..õ)...1._......-;
li
Hydnagtomhone he...o.i4rioate
NI
Nit,--1: ---'"
'T II
1
Lenwphartoi hexadiesate
1 _...i.õ.0
d A
Ni li
II
0
I Morphino-3-hexadiesate
"NN41
k If
0
IIiNakgrihim-3-3-hexadienaat
....-e.¨ ,....-,71
r ' .
Iv--2.1t,H
1
0....,,,,,,-.,.,..._
II
Q
49
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
A
e s Cy...dalo&
./p_¨.s
,
1
N
,,.e.f..... '
ir.---
1,1
If
0
A
..,..-4 ..
i
1 1 .
.0
r
...v.,
.1,sco...B.d.ertaete,
.Ø0.' ' ....,
..
1 r.,
o,...11..--,.."
I Eter,sitne
doN,,,,i-1(4.4,, hexad4neate
..,"---,..
1 0-.
,..,A'' N, ' A''''
n. = ...
1
,
:_,0
1 if
il
,õ..:
,
1 levot:phand
iN
I
d I..,.. ..,,...;,
-,,,....-.-- õ ".
.T.
II
I
(.1)
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
I Ovonafphane
N.
1170 .'
'= .õ4-' ¨0 õ--'
I If
li
N Tapentadd
I _01 hexad, i.lcatR,
,,--
v=
g
11 %
o
Natuphine-3-f5-methyllhexadiemaite
r
Ni
1 0 !-1 ' ,
ry- 7 -oil
T 1
o, ....A......õ
1 '
0
IIN.atm,sim.-3-i54 1...ethiel, -RKatliermate
r--
N
HO
,="&õ,' .,,, .." .0
I
1.1
n
51
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
A Naftirexorte-3-45-meth0)he):adienDan
r
1:=;70 :::::141,
,..,),._,
.11
0
t--- ButoThartdo-3-(5-metiwilbexacr. fwate
(4-4
seNs)
itIVS,aiLj i
Y .,õ,õ..,..
1
0
I kletopen 5-rnet=4fte=xd.:*m..".sate
,:ft=N. -,-= '''474,,,,,-% L,stilt,
r01 i = 4:1
r
if- -
0
I Kvdrzalt-n.phone 5-roeth. .:iheiaderteate
-N
1 11
,,,O, . ---='-."4, ,..--
't
0
i Leveglhartol 5-nrethyttexathaaate
N
. = j
0
...)
I '
II, .
...,
52
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
I Morphino-3-45,-tmethy0exadie:mate
[ P 1
I
Is ...'
(1
IINa(oF:p 5- (5-methyljhexad.,:tricate
i
N.
il A
It --
0
,
,
...,õõ,,..
r5-tnellivt,exzEFterioats
N
,µ
Y il
0
A get;:i,pdazacine
.....i¨
r5-tne,thythexit&enoate
N
.0 ,,,,,<õ,;:i'= \
.,- ir
-,.:. ....,
-.....
1 -
ii
C
53
CA 03149152 2022-01-28
WO 2021/029914
PCT/US2020/021599
1', Dipremxphime
,-," 5.-mthvibexalterizat
I
IV
0
1 Etoi..11ine.
Iiq''' liq 5-mathy:hexackerioate
--" .--..:i!=.,-s..-,,. 4,....,,,
ic,'""i., ..'= N
l' II
0
I Levor.phanol
kili...õ :-:.-rroettlyt cadierioate
-
f's = ,, j'
,
i . .
1 )1'
11-
0
I oxymarphime.
1,4
1 =::;0.' ''''' 11
''Sje. 5-rnetiyOhsxadi.snzate
11 A '
-..,.. ..t0 .-Na
I IT
II
0
NI TapÃA=tad&
L.., ,,,,A, 54.nethy:hExackerroate
==;..S.,,,,
.;.,.
1
. II-
0
54