Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/067856
PCT/US2020/054130
BLOOD GLUCOSE CONTROL SYSTEM
1NCORF'ORATION BY REFERENCE TO ANY PRIORITY APPLICATIONS
100011 This application is being filed on
October 2, 2020, the same date as
International Application No. PCTIU52020/054025, which is titled "BLOOD
GLUCOSE
CONTROL SYS FEW and is hereby expressly incorporated by reference herein in
its entirety
for all purposes. Any and all applications for which a foreign or domestic
priority claim is
identified in the Application Data Sheet as filed with the present application
are hereby
incorporated by reference under 37 CFR 1.57.
TECHNICAL FIELD
100021 The present disclosure relates to
ambulatory medical devices, such as blood
glucose control systems, that provide therapy to a subject.
BACKGROUND
10003) Sustained delivery, pump driven
medicament injection devices generally
include a delivery cannula mounted in a subcutaneous manner through the skin
of the patient
at an infusion site. The pump draws medicine from a reservoir and delivers it
to the patient via
the cannula. The injection device typically includes a channel that transmits
a medicament
from an inlet porno the delivery cannula which results in delivery to the
subcutaneous tissue
layer where the delivery cannula terminates. Some infusion devices are
configured to deliver
one medicament to a patient while others are configured to deliver multiple
medicaments to a
patient.
SUMMARY
[0004] The systems, methods, and devices of
this disclosure each have several
innovative aspects, no single one of which is solely responsible for all the
desirable attributes
disclosed herein. Details of one or more implementations of the subject matter
described in
this specification are set forth in the accompanying drawings and the
description below.
[0005] Certain embodiments disclosed herein
relate to a computer-implemented
method of generating an indication of total carbohydrate therapy over a period
in a subject
-1-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
using a medicament pump configured to deliver at least insulin therapy to the
subject. The
method may be performed by a hardware processor configured to generate dose
control signals
for the medicament pump configured to deliver at least insulin therapy to the
subject. The
method may include receiving a glucose level of the subject and determining
based at least in
part on the glucose level that a triggering event for raising blood glucose
level of the subject
has occurred_ The triggering event may comprise determining that an impending
risk of
hypoglycemia is present in the subject or that an episode of hypoglycemia is
present in the
subject The method may further include determining an amount of a counter-
regulatory agent
to respond to the impending risk of hypoglycemia or the episode of
hypoglycemia. Further, the
method may include determining a dose of carbohydrate therapy based at least
in part on the
amount of the counter-regulatory agent. Additionally, the method may include
tracking, over
a period comprising a plurality of hypoglycemia risk events or hypoglycemia
episodes,
determined doses of carbohydrate therapy to generate the indication of total
carbohydrate
therapy over the period. The method may include outputting the indication of
total
carbohydrate therapy.
[0006] Additional embodiments of the present
disclosure relate to an automated
blood glucose control system configured to generate an indication of total
carbohydrate therapy
over a period in a subject The automated blood glucose control system may
include a
medicament delivery interface configured to operatively connect to a
medicament pump
configured to infuse medicament into the subject. The medicament may comprise
at least
insulin. Further, the automated blood glucose control system may include a
memory
configured to store specific computer-executable instructions, and a hardware
processor in
communication with the memory and configured to execute the specific computer-
executable
instructions to at least: receive a glucose level of the subject; determine
based at least in part
on the glucose level that a triggering event for raising blood glucose level
of the subject has
occurred, wherein the triggering event comprises that an impending risk of
hypoglycemia is
present in the subject or that an episode of hypoglycemia is present in the
subject; determine
an amount of a counter-regulatory agent to respond to the impending risk of
hypoglycemia or
the episode of hypoglycemia; determine a dose of carbohydrate therapy based at
least in part
on the amount of the counter-regulatory agent; track, over a period comprising
a plurality of
hypoglycemia risk events or hypoglycemia episodes, determined doses of
carbohydrate
-2-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
therapy to generate the indication of total carbohydrate therapy over the
period; and output the
indication of total carbohydrate therapy.
(00071 Certain embodiments of the present
disclosure relate to an automated blood
glucose control system configured to generate a backup therapy protocol
comprising insulin
therapy instructions derived from autonomously determined doses of insulin.
The automated
blood glucose control system may include a medicament delivery interface
configured to
operatively connect to a medicament pump for infusing medicament into the
subject. Further,
the automated blood glucose control system may include a memory configured to
store specific
computer-executable instructions, and a hardware processor in communication
with the
memory and configured to execute the specific computer-executable instructions
to at least:
receive a glucose level signal from a sensor operatively configured to
determine glucose levels
in the subject; generate a dose control signal using a control algorithm
configured to
autonomously determine doses of insulin to be infused into the subject for the
purpose of
controlling blood glucose of the subject based at least in part on the glucose
level signal; track
insulin therapy administered to the subject over a tracking period comprising
at least one day
by the automated blood glucose control system, wherein tracking the insulin
therapy comprises
storing an indication of the autonomously determined doses of insulin
delivered to the subject
as basal insulin, correction boluses of insulin, or as mealtime boluses of
insulin; generate at
least one of a backup injection therapy protocol or a backup pump therapy
protocol comprising
insulin therapy instructions based at least in part on the insulin therapy
administered to the
subject over the tracking period; and output the at least one of the backup
injection therapy
protocol or the backup pump therapy protocol on a display enabling therapy to
be maintained
at a rate determined by the automated blood glucose control system when the
automated blood
glucose control system is not providing therapy to the subject.
100081 Additional embodiments of the present
disclosure relate to a computer-
implemented method of generating a backup therapy protocol comprising insulin
therapy
instructions derived from autonomously determined doses of insulin determined
by an
automated blood glucose control system. The method may be performed by a
hardware
processor of the automated blood glucose control system. The method may
include receiving
a glucose level signal from a sensor operatively configured to determine
glucose levels in the
subject and generating a dose control signal using a control algorithm
configured to
-3-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
autonomously determine doses of insulin to be infused into the subject for the
purpose of
controlling blood glucose of the subject based at least in part on the glucose
level signal.
Further, the method may include tracking insulin therapy administered by the
automated blood
glucose control system to the subject over a tracking period comprising at
least one day.
Tracking the insulin therapy may comprise storing an indication of the
autonomously
determined doses of insulin delivered to the subject Further, the method may
include
generating at least one of a backup injection therapy protocol or a backup
pump therapy
protocol comprising insulin therapy instructions based at least in part on the
insulin therapy
administered to the subject over the tracking period. In addition, the method
may include
outputting the at least one of the backup injection therapy protocol or the
backup pump therapy
protocol on a display enabling therapy to be maintained at a rate determined
by the automated
blood glucose control system when the automated blood glucose control system
is not
providing therapy to the subject
100091 Some embodiments of the present
disclosure relate to an automated blood
glucose control system configured to generate a report of therapy protocol
modifications made
by a user of the automated blood glucose control system. The automated blood
glucose control
system may include a medicament delivery interface configured to operatively
connect to a
medicament pump for infusing medicament into a subject. Further, the automated
blood
glucose control system may include a memory configured to store specific
computer-
executable instructions, a stored control parameter value, and a therapy log.
Moreover, the
automated blood glucose control system may include a hardware processor in
communication
with the memory and configured to execute the specific computer-executable
instructions to at
least: receive a glucose level signal from a sensor operatively configured to
determine glucose
levels in the subject: generate a dose control signal using a control
algorithm configured to
autonomously determine doses of insulin to be infused into the subject for the
purpose of
controlling blood glucose of the subject based at least in part on the glucose
level signal and a
control parameter that is modifiable by user interaction with a control
parameter selection
interface element; track user modifications to the control parameter over a
tracking period
comprising at least one day, wherein tracking the user modifications comprises
storing in the
therapy log whether each of the user modifications comprises an increase or a
decrease in the
control parameter from the stored control parameter value and a time during
which each of the
-4-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
user modifications occurred; and generate a report of user modifications to
the control
parameter, wherein the report comprises a measure of frequency of increases
and decreases
from the stored control parameter value.
(0010) Certain embodiments of the present
disclosure relate to a computer-
implemented method of modifying therapy provided to a subject using a blood
glucose control
system. The method may be performed by a hardware processor configured to
generate a dose
control signal for the blood glucose control system. Further, the method may
include receiving
a glucose level signal from a glucose level sensor operatively connected to
the subject.
Moreover, the method may include causing first therapy to be delivered by the
blood glucose
control system to a subject during a first therapy period, wherein the first
therapy is delivered
based at least in part on a first value of a control parameter used by a
control algorithm to
generate the dose control signal. The control parameter may be used by the
control algorithm
to account for accumulation of insulin in the subject, thereby controlling an
insulin dosing
response of the control algorithm to a blood glucose excursion in the subject
as indicated by
the glucose level signal. Further, the method may include determining a first
effect
corresponding at least in part to the first therapy. Determining the first
effect may comprise
analyzing glycemic control of blood glucose in the subject as indicated by the
glucose level
signal. Moreover, the method may include autonomously generating a second
value of the
control parameter. The autonomously generated second value may be determined
as a function
based on the first value and the first effect. In addition, the method may
include modifying the
control parameter from the first value to the second value and causing second
therapy to be
delivered by the blood glucose control system to the subject during a second
therapy period.
The second therapy may be delivered based at least in part on the second value
of the control
parameter. Further, changing the control parameter may modify the therapy
provided to the
subject.
[0011] Additional embodiments of the present
disclosure relate to a computer-
implemented method of modifying therapy provided to a subject using a blood
glucose control
system. The method may be performed by a hardware processor configured to
generate a dose
control signal for the blood glucose control system. The method may include
causing first
therapy to be delivered by the blood glucose control system to a subject
during a first therapy
period. The first therapy may be delivered based at least in part on a first
value of a control
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
parameter used by a control algorithm to generate the dose control signal. The
method may
further include determining a first effect corresponding at least in part to
the first therapy.
Determining the first effect may comprise receiving a glucose level signal
from a glucose level
sensor operatively connected to the subject. Further, the method may include
autonomously
generating a second value of the control parameter based at /east in part on a
baseline value of
the control parameter and an output of a function defined based on glycemic
control of the
subject The glucose level signal may comprise an indication of the glycemic
control of the
subject during the first therapy period. Moreover, the method may include
modifying the
control parameter from the first value to the second value and causing second
therapy to be
delivered by the blood glucose control system to the subject during a second
therapy period
The second therapy may be delivered based at least in part on the second value
of the control
parameter. Changing the control parameter may include modifying the therapy
provided to the
subject
100121 Some embodiments of the present
disclosure relate to a computer-
implemented method of modifying therapy provided to a subject using a blood
glucose control
system. The method may be implemented by a hardware processor configured to
generate a
dose control signal for the blood glucose control system. The method may
include causing first
therapy to be delivered by the blood glucose control system to a subject
during a first therapy
period. The first therapy may be delivered based at least in part on a first
value of a control
parameter used by a control algorithm to generate the dose control signal. The
method may
further include determining a first effect corresponding at least in part to
the first therapy.
Determining the first effect may comprise receiving a glucose level signal
from a glucose level
sensor operatively connected to the subject. Further, the method may include
autonomously
generating a second value of the control parameter. The autonomously generated
second value
may be determined as a function based at least in part on a baseline value.
Moreover, the
method may include modifying the control parameter from the first value to the
second value.
The method may further include causing second therapy to be delivered by the
blood glucose
control system to the subject during a second therapy period. The second
therapy may be
delivered based at least in part on the second value of the control parameter.
Further, changing
the control parameter may include modifying the therapy provided to the
subject The method
may further include determining a second effect corresponding at least in part
to the second
-6-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
therapy and autonomously performing a comparison of the first effect and the
second effect
without action by a human. Further, the method may include selecting one of
the first value of
the control parameter or the second value of the control parameter as an
active control
parameter value based at least in part on the comparison of the first effect
and the second effect.
Moreover, the method may include configuring the blood glucose control system
to provide
therapy to the subject during a third therapy period based at least in part on
the active control
parameter value. The selection of the active control parameter value may
modify the therapy
provided to the subject.
/00131 Moreover, any of the aforementioned
embodiments may be combined. For
example, a single automated blood glucose control system may be configured to
implement
one or more of the aforementioned embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
100141 Throughout the drawings, reference
numbers are re-used to indicate
correspondence between referenced elements. The drawings are provided to
illustrate certain
aspects of the subject matter described herein and not to limit the scope
thereof.
100151 Figure IA illustrates an example blood
glucose control system that provides
blood glucose control via an ambulatory medicament pump.
/00161 Figure 1B illustrates another example
blood glucose control system that
provides blood glucose control via an ambulatory medicament pump.
100171 Figure IC illustrates a further
example blood glucose control system that
provides blood glucose control via an ambulatory medicament pump.
/00181 Figure 2A shows a block diagram of an
example blood glucose control
system.
100191 Figure 2B shows a block diagram of
another example blood glucose control
System.
/00201 Figure 2C shows a block diagram of
another example blood glucose control
system.
100211 Figure 2D shows a block diagram of
another example blood glucose control
system.
-7-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
100221 Figure 3 is a schematic of an example
glucose control system that includes
an electronic communications interface.
[0023] Figure 4A shows a block diagram of an
example blood glucose control
system in online operation mode.
[0024] Figure 4113 shows a block diagram of
an example blood glucose control
system in offline operation mode.
100251 Figure 5 illustrates a block diagram
of a glucose control system in
accordance with certain embodiments.
[0026] Figure 6 illustrates a block diagram
of a controller system in accordance
with certain embodiments.
[0027] Figure 7 presents a flowchart of an
example carbohydrate therapy
equivalence tracking process in accordance with certain embodiments.
100281 Figure 8 presents a flowchart of an
example backup therapy protocol
generation process in accordance with certain embodiments.
[0029] Figure 9 presents a flowchart of an
example control parameter modification
tracking process in accordance with certain embodiments.
[0030] Figure 10 illustrates an example
backup therapy protocol in accordance with
certain embodiments.
[0031] Figure 11 illustrates an example
control parameter modification report in
accordance with certain embodiments.
[0032] Figure 12 illustrates an example meal
selection report that may be included
as part of some implementations of the control parameter modification report
of Figure 11 in
accordance with certain embodiments.
[0033] Figure 13 presents a flowchart of an
example automated blood glucose
control refinement process in accordance with certain embodiments.
[0034] Figure 14A illustrates a simulation of
blood glucose control of a subject
with Tmax set to 65 minutes.
[0035] Figure 14B illustrates a simulation of
blood glucose control of a subject
with Tmax set to 15 minutes.
[0036] Figure MC illustrates a simulation of
blood glucose control of a subject
with Tmax set to 130 minutes.
-8-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
100371 Figure 15 illustrates an example of
blood glucose level signal (CGM trace)
and some of the parameters associated with glycemic control using a blood
glucose control
system.
(0038) Figure 16 presents a flowchart of an
example automated blood glucose
control refinement process based on an adjustment function in accordance with
certain
embodiments.
100391 Figure 17 illustrates some examples of
statistical quantities that may be
generated and utilized by the blood glucose control system as part of
statistical analysis.
100401 Figure 18 presents a flowchart of an
example automated blood glucose
control refinement process in accordance with certain embodiments.
DETAIL ED DESCRIPTION
100411 Some embodiments described herein
pertain to medicament infusion
systems for one or more medicaments and the components of such systems (e.g.,
infusion
pumps, medicament cartridges, cartridge connectors, lumen assemblies, infusion
connectors,
infusion sets, etc.). Some embodiments pertain to methods of manufacturing
infusion systems
and components thereof Some embodiments pertain to methods of using any of the
foregoing
systems or components for infusing one or more medicaments (e.g.,
pharmaceutical, hormone,
etc.) to a patient. As an exemplary illustration, an infusion system may
include an infusion
pump, which can include one or more medicament cartridges or can have an
integrated
reservoir of medicament. An infusion system may include medicament cartridges
and cartridge
connectors, but not a pump. An infusion system may include cartridge
connectors and an
infusion pump, but not medicament cartridges. An infusion system may include
infusion
connectors, a lumen assembly, cartridge connectors, an infusion pump, but not
medicament
cartridges or an infusion set. A blood glucose control system can operate in
conjunction with
an infusion system to infuse one or more medicaments, including at least one
blood glucose
control agent, into a subject. Any feature, structure, component, material,
step, or method that
is described and/or illustrated in any embodiment in this specification can be
used with or
instead of any feature, structure, component, material, step, or method that
is described and/or
illustrated in any other embodiment in this specification. Additionally, any
feature, structure,
-9-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
component, material, step, or method that is described and/or illustrated in
one embodiment
may be absent from another embodiment
Blood Glucose Control System Overview
(00421 Blood glucose control systems are used
to control blood glucose level in a
subject Blood glucose control systems can include a controller configured to
generate dose
control signals for one or more glucose control agents that can be infused
into the subject
Glucose control agents include regulatory agents that tend to decrease blood
glucose level,
such as insulin and insulin analogs, and counter-regulatory agents that tend
to increase blood
glucose level, such as glucagon or dextrose. A blood glucose control system
configured to be
used with two or more glucose control agents can generate a dose control
signal for each of
the agents. In some embodiments, a blood glucose control system can generate a
dose control
signal for an agent even though the agent may not be available for dosing via
a medicament
pump connected to the subject.
[0043] Glucose control agents can be
delivered to a subject via subcutaneous
injection, via intravenous injection, or via another suitable delivery method.
In die case of
blood glucose control therapy via an ambulatory medicament pump, subcutaneous
injection is
most common. An ambulatory medicament pump 100 is a type of ambulatory medical
device,
which is sometimes referred to herein as an ambulatory device, an ambulatory
medicament
device, a mobile ambulatory device, or an ArvID. Ambulatory medical devices
include
ambulatory medicament pumps and other devices configured to be carried by a
subject and to
deliver therapy to the subject.
[0044] In some examples, the ambulatory
medical device (AMD) is an electrical
stimulation device, and therapy delivery includes providing electrical
stimulation to a subject.
An example of an electrical stimulation device is a cardiac pacemaker. A
cardiac pacemaker
generates electrical stimulation of the cardiac muscle to control heart
rhythms. Another
example of an electrical stimulation device is a deep brain stimulator to
treat Parkinson's
disease or movement disorders.
(0045) FIGS. 1A-1C show examples of blood
glucose control systems that provide
blood glucose control via an ambulatory medicament pump connected to a
subject. In FIG. 1A,
the medicament pump 100 is connected to an infusion site 102 using an infusion
set 104. The
-10-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
medicament pump has integrated pump controls 106a that permit a user to view
pump data and
change therapy settings via user interaction with the pump controls 106a. A
glucose level
sensor 110 generates a glucose level signal that is received by the blood
glucose control system.
(0046) In FIG. 1B, the medicament pump 100
communicates with an external
electronic device 108 (such as, for example, a smartphone) via a wireless data
connection. At
least some of the pump controls 106a and 106b can be manipulated via user
interaction with
user interface elements of the external electronic device 108. The glucose
level sensor 110 can
also communicate with the medicament pump 100 via a wireless data connection.
100471 In FIG. IC, the medicament pump 100
includes an integrated c.annula that
inserts into the infusion site 102 without a separate infusion set. At least
some of the pump
controls 106b can be manipulated via user interaction with user interface
elements of an
external electronic device 108. In some instances, pump controls can be
manipulated via user
interaction with user interface elements generated by a remote computing
environment (not
shown), such as, for example, a cloud computing service, that connects to the
medicament
pump 100 via a direct or indirect electronic data connection.
100481 Glucose control systems typically
include a user interface configured to
provide one or more of therapy information, glucose level information, and/or
therapy control
elements capable of changing therapy settings via user interaction with
interface controls. The
user interface can be implemented via an electronic device that includes a
display and one or
more buttons, switches, dials, capacitive touch interfaces, or touchscreen
interfaces. In some
embodiments, at least a portion of the user interface is integrated with an
ambulatory
medicament pump that can be tethered to a body of a subject via an infusion
set configured to
facilitate subcutaneous injection of one or more glucose control agents. In
certain
embodiments, at least a portion of the user interface is implemented via an
electronic device
separate from the ambulatory medicament pump, such as a smartphone.
[0049] FIGS. 2A-2D illustrate block diagrams
showing example configurations of
a glucose control system 20(1 As shown in FIG. 2A, a glucose control system
200a can include
a controller 202a haying an electronic processor 204a and a memory 210a that
stores
instructions 208a executable by the processor 204a. The controller 202a and a
pump 212 can
be integrated with into an ambulatory medical device (AM!)) 100. The AlY1D 100
can include
a transceiver 214a for wireless digital data communications with external
electronic devices.
-11-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
When the instructions 208a stored in memory 210a are executed by the
electronic processor
204a, the controller 202a can implement at least a portion of a control
algorithm that generates
dose control signals for one or more glucose control agents based on time-
varying glucose
levels of the subject and one or more control parameters. The dose control
signals, when
delivered to the pump 212, result in dosing operations that control the blood
glucose of a
subject
100501 As shown in FIG 2B, a glucose control
system 200b can operate at least
partially via execution of instructions 208b by an electronic processor 204b
of an electronic
device 108 separate from the ambulatory medical device 100. The electronic
device 108 can
include a transceiver 214b capable of establishing a wireless digital data
connection to the
AMD 100, and a controller 202b can implement at least a portion of a control
algorithm via
execution of instructions 208b stored in memory 210b. When the instructions
208b stored in
memory 210b are executed by the electronic processor 204b, the controller 202b
can
implement at least a portion of a control algorithm that generates dose
control signals for one
or more glucose control agents based on time-varying glucose levels of the
subject and one or
more control parameters. The dose control signals, when delivered to the pump
212, result in
dosing operations that control the blood glucose of a subject. In some
embodiments, the dose
control signals are transmitted from the device transceiver 214b to the MAD
transceiver 214a
over a short-range wireless data connection 216. The ANID 100 receives the
dose control
signals and passes them to the pump 212 for dosing operations.
100511 As shown in FIG. 2C, a glucose control
system 200c can operate at least
partially via execution of instructions 208c on an electronic processor 204c
integrated with a
remote computer 206, such as, for example, a cloud service. When the
instructions 208c stored
in memory 210c are executed by the electronic processor 204c, the controller
202c can
implement at least a portion of a control algorithm that generates dose
control signals for one
or more glucose control agents based on time-varying glucose levels of the
subject and one or
more control parameter& The dose control siwials, when delivered to the pump
212, result in
dosing operations that control the blood glucose of a subject. In some
embodiments, the dose
control signals are transmitted from the remote computer WAN connection
interface 220c to
the A.MD WAN connection interface 220a over an end-to-end wireless data
connection 218.
-12-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
The AMD 100 receives the dose control signals and passes them to the pump 212
for dosing
operations.
(00521 As shown in FIG 2D, a glucose control
system 200d can have two Of more
controllers 202a, 202b, 202c that cooperate to generate a dose control signal
for dosing
operations by the pump 212. A remote computer 206 can transmit or receive data
or
instructions passed through a WAN connection interface 220c via a WAN wireless
data
connection 218 to a WAN connection interface 220b of an electronic device
1108. The
electronic device 108 can transmit or receive data or instructions passed
through a transceiver
214b via a short-range wireless data connection 216 to a transceiver 214a of
an AMD 100. In
some embodiments, the electronic device can be omitted, and the controllers
202a, 202c of the
AMD 100 and the remote computer 206 cooperate to generate dose control signals
that are
passed to the pump 212. In such embodiments, the AMD 100 may have its own WAN
connection interface 220a to support a direct end-to-end wireless data
connection to the remote
computer 206.
[0053] As shown in FIG. 3, in some
embodiments, the glucose control system 200
includes circuitry that implements an electronic communications interface (Ed)
302
configured to send and receive electronic data from one or more electronic
devices. The ECI
includes a sensor interface 304 configured to receive a glucose level signal
from a sensor 110
such as a continuous glucose monitor (CGM). Some CG/vIs generate the glucose
level signal
at fixed measurement intervals, such as five-minute inteivals. The sensor 110
can be
operatively connected to a subject in order to generate a glucose level signal
that corresponds
to a blood glucose estimate or measurement of the subject The glucose level
signal can be
used by the controller 202 to generate a dose control signal. The dose control
signal can be
provided to a pump 212 via a pump interface 306. In some embodiments, the
sensor interface
304 connects to the sensor 110 via a short-range wireless connection 308. In
some
embodiments, the pump interface 306 connects to the pump 212 via a short-range
wireless
connection 310. In other embodiments, the pump interface 306 connects to the
pump 212 via
a local data bus, such as when the controller 202, the EC/ 306, and the pump
212 are integrated
into an AMD 100.
100541 The controller can be configured to
generate the dose control signal using a
control algorithm that generates at least one of a basal dose, a correction
dose, and/or a meal
-13-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
dose. Examples of control algorithms that can be used to generate these doses
are disclosed in
U.S. Patent Application Publication Nos. 2008/0208113, 2013/0245547,
2016/0331898, and
2018/0220942 (referenced herein as the "Controller Disclosures"), the entire
contents of which
are incorporated by reference herein and made a part of this specification.
The correction dose
can include regulatory or counter-regulatory agent and can be generated using
a model-
predictive control (IVIPC) algorithm such as the one disclosed in the
Controller Disclosures.
The basal dose can include regulatory agent and can be generated using a basal
control
algorithm such as disclosed in the Controller Disclosures. The meal dose can
include
regulatory agent and can be generated using a meal control algorithm such as
disclosed in the
Controller Disclosures. Additional aspects and improvements for at least some
of these
controllers are disclosed herein. The dose control signal can be transmitted
to an infusion motor
306 via the ECI 302 or can be transmitted to the infusion motor 306 via an
electrical conductor
when the controller 202a is integrated in the same housing as the infusion
motor 306.
100551 As shown in FIG. 4A, the controller
400 can be configured to operate in
"online mode" during time periods when the controller receives a glucose level
signal 402 from
a sensor 110. In online mode, the control algorithm generates a dose control
signal 404 that
implements regular correction doses based on values of the glucose level
signal 402 and control
parameters of the control algorithm. The pump 212 is configured to deliver at
least correction
doses and basal doses to the subject without substantial user intervention
while the controller
400 remains in online mode.
100561 As shown in FIG. 4B, the controller
400 can be configured to operate in
"offline mode" during time periods when the controller does not receive a
glucose level signal
402 from a sensor 110, at least during periods when the glucose level signal
402 is expected
but not received. In offline mode, the control algorithm generates a dose
control signal 404
that implements correction doses in response to isolated glucose measurements
406 (such as,
for example, measurements obtained from the subject using glucose test strips)
and based on
control parameters of the control algorithm. The pump 212 is configured to
deliver basal doses
to the subject without substantial user intervention and can deliver
correction doses to the
subject in response to isolated glucose measurements 406 while the controller
400 remains in
offline mode.
-14-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
Example Implementation of Glucose Control System
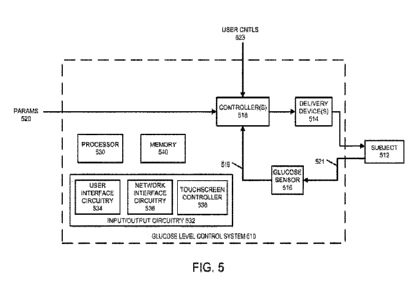
100571 Figure 5 illustrates an automated
glucose control system 510 for regulating
the blood glucose level of an animal subject (subject) 512, which may be a
human. The
automated glucose control system 510 is an example of a medicament infusion
system and
may include any of the embodiments previously described above with respect to
medicament
infusion systems_
100581 The subject 512 may receive doses of
insulin from one or more delivery
devices 514, for example infusion pump(s) coupled by catheter(s) to a
subcutaneous space of
the subject 512. As described below, the delivery devices 514 may also deliver
a counter-
regulatory agent or hyperglycemic anent, such as alucagon or dextrose, for
control of the blood
glucose level under certain circumstances. For the delivery of both insulin
and a counter-
regulatory agent (e.g., glueagon), the delivery devices 514 may be
mechanically driven
infusion mechanisms having dual cartridges for insulin and the counter-
regulatory agent,
respectively, In the present description, reference is made to glucagon
specifically, but it is to
be understood that this is for convenience only and that other counter-
regulatory agents (e.g.,
dextrose) may be used. Similarly, the term "insulin" herein is to be
understood as
encompassing all forms of insulin-like substances including natural human or
animal insulin
as well as synthetic insulin in any of a variety of forms (commonly referred
to as "insulin
analogs").
[0059] For online or autonomous operation, a
glucose sensor 516 is operatively
coupled to the subject 512 to continually sample a glucose level of the
subject 512. In some
cases, the glucose sensor 516 may be referred to as a continuous glucose
monitoring (CGM)
sensor, which may continuously or periodically measure or sense blood glucose
levels of the
subject 512 for at least a period of time. Sensing may be accomplished in a
variety of ways,
generally involving some form of physical coupling 521 between the subject 512
and the
glucose sensor 516. A controller 518 may control operation of the delivery
device(s) 514 as a
function of a glucose level signal 519 from the glucose sensor 516 and subject
to programmed
input parameters (PARAMS) 520 which may be provided by a user such as the
subject 512, a
parent or guardian of the subject 512, or a healthcare provider (e.g., a
clinician or doctor). One
input parameter for automatic operation may include the weight of the subject
512. In some
cases, the glucose control system 510 can provide effective automated control
without
-15-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
receiving explicit information regarding either meals that die subject 512 has
ingested or any
other "feedforward" information, which is achieved in part by an adaptive
aspect to operation
of the controller 518_ In other cases, the glucose control system 510 can use
received
information regarding either meals that the subject ingested, or plans to
ingest, or other
"feedforward" information to modify control of blood glucose andlor delivery
of insulin or
counter-regulatory agent.
100601 The controller 518 is an electrical
device with control circuitry that provides
operating functionality as described herein. In one embodiment, the controller
518 may be
realized as a computerized device (e.g., a hardware processor) having computer
instruction
processing circuitry that executes one or more computer programs each
including respective
sets of computer instructions. In some cases, the processing circuitry will
generally include
one or more processors 530 along with memory 540 and input/output circuitry
532 coupled to
or in communication with the processor(s) 530, where the memory 540 stores
computer
program instructions and data, and the input/output circuitry 532 can provide
interface(s) to
external devices such as the glucose sensor 516 and delivery device(s) 514. In
some cases, the
input/output circuitry 532 may provide a user interface, or may operate with
one or more
processors (a g., the controller 518 or a separate processor 530 included in
the glucose control
system 510 or in a separate computing system, such as a smartphone, a laptop
computer, a
desktop computer, a smartyvatch, and the like) to provide a user interface to
a user (e.g., the
subject 512, a parent or guardian, or a clinician). In some cases, the
input/output circuitry 532
may include a touchscreen and/or a touchscreen controller 538 configured to
control a
touchscreen (not shown).
[0061j In some cases, the controller 518 may
perform all of the functionality of the
glucose level control system 510. In such cases, the processor 530 may be
optional or omitted.
In other cases, the controller 518 may perform at least automated blood
glucose control of the
subject 512, and one or more separate processors 530 may perform one or more
additional
operations of the blood glucose control system 510 (or medicament pump), such
as tracking
occurrences of hyperglycemic or hypoglycemic events or risk events, outputting
data to a user,
controlling or initiating communication with another computing system,
regulating access to
the glucose level control system 510, or other operations unrelated to
operation of a
medicament pump or the delivery devices 514,
-16-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
100621 The input/output circuitry 532 may
control communication with one or
more other computing systems and/or with a user. In some cases, the
input/output circuitry 532
may include one or more separate interface circuits or controllers to
facilitate user interaction
andior communication. For example, the input/output circuitry 532 may include
user interface
circuitry 534, network interface circuitry 536, and/or a touchscreen
controller 538.
100631 The user interface circuitry 534 may
include any circuitry or processors that
may output a user interface to a user and/or receive user input from the user
via the user
interface. The user interface circuitry 534 may receive one or more signals
from a processor
530 corresponding to a user interface. The user interface circuitry 534 may
control a display
to present the user interface to a user based on the one or more signals
received from the
processor 530. Further, the user interface circuitry 534 may include any
circuitry that can
receive a signal corresponding to an interaction by a user with a user
interface and can provide
the signal to the processor 530 and/or controller 518 for further processing.
In some cases, the
user interface circuitry may be replaced by a touchscreen controller 538 that
can control a
touchscreen interface. In other cases, the touchscreen controller 538 may be
in addition to the
user interface circuitry 534,
100641 The network interface circuitry 536
may include any circuitry that enables
communication with a wired or wireless network. The network interface
circuitry 536 may
include one or more network interface cards and/or wireless radios (e.g., a
Bluetooth radio, a
Bluetooth Low Energy (BLE) radio, a 4g LTE radio, a 5G radio, a ND-LTE radio,
and the
like).
[0065] The memory 540 can include non-
volatile memory and/or volatile memory.
The non-volatile memory may include flash memory or solid-state memory.
[0066] The control system 510 is also able to
operate in an offline manner in which
it is used to provide delivery of insulin (and potentially glucagon as well),
independent of or
without receipt of glucose levels reported by the sensor 516. For example, in
cases where the
sensor 516 needs replacing, is not properly connected to the subject 512, or
is defective, the
glucose control system 510 may operate in an offline manner without input from
the sensor
516. Thus, overall operation may be divided between online periods each
including a
succession of sampling intervals when a glucose signal (level) 519 is
available, and offline
periods each including a succession of sampling intervals when the glucose
signal (level) 519
-17-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
is either completely or intermittently unavailable. The description below uses
the terms
"online" and "offline" for these periods. Also, offline operation may be user-
selected for some
reason even when a glucose level signal 519 is available for use.
(0067) User control inputs (USER CNTLs 523)
may be provided via a local or
remote user interface of some type. In some embodiments, the user interface
may resemble
that of conventional insulin pumps or similar devices, e.g., by including
control buttons for
commanding the delivery of a bolus and perhaps a small display. In other
embodiments, the
system may have a wired or wireless interface to a remote device that may
incorporate a fuller-
function user interface, such as a smartphone, smartwatch, laptop computer,
desktop computer,
cloud computing service, or other wearable device or computing device. In some
cases, the
wireless interface may provide access to a local area network, such as a
personal home
network, a company network, or otherwise. Alternatively, or in addition, the
wireless interface
may provide a direct connection between local devices available to a user
(e.g., via Bluetooth
or other near field communication technologies). In some cases, the wireless
interface may
provide access to a wide area network, such as, but not limited to, the
Internet. For example,
the wireless interface may include a cellular interface that permits access to
a network via a
4G or 5G cellular connection. In some cases, the cellular interface may be a
low power
interface, such as narrowband LTE or other Internet of Things (IoT)
interfaces.
(00681 In offline mode, the glucose sensor
516 may be absent, non-functioning, or
not coupled to the subject 512. As such, in offline mode, the blood glucose
signal 519 may not
be available to control automatic operation. In some cases, a user may provide
one or more
blood glucose measurements to the control system 510 to facilitate automatic
operation of the
control system 510. These me-qsurements may be provided over a particular time
period.
Alternatively, or in addition, the glucose control system 510 may use a
therapy history and/or
a history of prior blood glucose control measurements to facilitate automatic
operation of the
control system 510 for at least a particular time period.
100691 The description herein refers to a
"user" as the source of the user control
inputs 523. The "user" as used herein may be the subject 512, a parent or
guardian of the
subject 512, a healthcare provider (e.g., a clinician, doctor, or other person
who may provide
medical care to the subject), or any other user who may be authorized to help
manage therapy
of the subject 512. In certain implementations, the glucose level control
system 510 is a
-18-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
personal device worn by a subject 512 for continual glucose control. In some
such
implementations, the user and subject 512 may be the same person. In other
implementations,
there may be another person involved in the care of the subject 512 and
providing control input,
and in such implementations, that other person has the role of user.
Example Controllers for a Blood Glucose Control System
[0070] Figure 6 shows an example structure of
the controller 518 in accordance
with certain embodiments. The controller 518 illustrated in Figure 6 may
represent a physical
structure with different controllers or processors, or a logical structure
that is implemented by
one or more physical processors. In other words, a single processor may be
used to implement
each of the controllers illustrated in Figure 6, each controller may be
implemented by its own
processor, or certain processors may implement multiple, but not necessarily
all, of the
controllers illustrated in Figure 6 as part of the controller 518. Moreover,
although the
controllers of Figure 6 are illustrated as part of the controller 518, in some
implementations,
one or more of the controllers may be separate from the controller 518.
[0071] The controller 518 may include four
separate controllers, namely a
glucagon (or counter-regulatory agent) controller 622, a basal insulin
controller 624, a
corrective insulin controller 626, and a priming insulin controller 628. The
basal insulin
controller 624 includes a nominal rate controller 630 and a modulating
controller 632. As
shown, the glucagon controller 622 generates a glucagon dose control signal
634 provided to
a glucagon delivery device 514-1. Respective outputs 636-640 from the
controllers 624-628
may be combined to form an overall insulin dose control signal 642 provided to
insulin delivery
device(s) 514-2. As shown, the output signal 636 from the basal insulin
controller 624 may be
formed by a combination of respective outputs of the nominal rate controller
630 and a
modulating controller 632. The insulin delivery device(s) 514-2 may include
devices tailored
to deliver different types and/or quantities of insulin, and the exact
configuration may be
known to and/or under the control of the controllers 624-628. For ease of
description, the
collection of one or more insulin delivery devices 514-2 is referred below to
in the singular as
an insulin delivery device 514-2.
100721 Also shown in Figure 6 are
input/output signals of the various controllers,
including the glucose level signal 519, parameters 520 and user inputs 523 as
well as a set of
-19-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
inter-controller signals 644. The inter-controller signals 644 enable
communication of
information from one controller, where the information is developed or
generated, to another
controller where the information may be used for that controller's control
function.
(0073) The controllers 622-628 may be
operated in either the online/automatic
mode or in the offline mode. In the automated mode, the corrective controller
626 regulates
glucose level using a control scheme such as described in US Patent No.
7,806,854, the
contents of which are hereby incorporated by reference in its entirety herein.
The basal
controller 624 and priming insulin controller 628 may perform adaptive
automated control as
described in International Patent Application Publication WO 2012/058694 A2,
the contents
of which are hereby incorporated by reference in its entirety herein. The
controllers 622-628
generally employ control methods or algorithms that include control parameters
that are
mathematically combined with reported glucose values to generate an output
value that is
converted (either directly or via additional conditioning) into the dose
control signals 634, 642.
For example, the control scheme described in US Patent Na 7,806,854 includes a
generalized
predictive control (GPC) method that incorporates a variety of control
parameters. The control
algorithms are generally adaptive, meaning that control parameters are
dynamically adjusted
during operation to reflect changing operating circumstances and a "learning"
aspect¨by
monitoring its own operation, the algorithm adjusts its operation to be more
specifically
tailored to the individual user, enhancing the algorithm's effectiveness and
reducing or
avoiding a need for additional explicit input information about the user. It
should be noted that
the input parameters 520 may form part of the control parameters used by the
control algorithm.
Other control parameters are internal parameters according to the specifics of
the algorithm,
and selected ones of those internal control parameters are dynamically
adjusted to realize the
adaptation of the control algorithm.
100741 One feature of operation is the
ability of the controllers to learn from recent
past periods of online operation and to use that learning during offline
operation US Patent
No. 10,543,313, the contents of which are hereby incorporated by reference in
its entirety
herein, describes two methods that are usable independently or together in
offline operation.
A first method automatically calculates the correct size of a correction bolus
of insulin at a
time of receiving an isolated glucose measurement, the correction bolus then
being
administered by the system in response to a user control input. A second
method automatically
-20-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
calculates the correct size of a meal bolus of insulin and administers it in
response to a user
control input Both methods utilize information obtained during past periods of
online
operation to automatically calculate correct values, freeing the user of a
need to make the
calculation or provide a correction factor.
Carbohydrate Therapy Equivalence Tracking
[0075] Hyperglycemia is a condition that
occurs when the levels of sugar or
glucose in the blood exceeds a particular level (e.g., 180 mg/dIa). This
condition may occur in
diabetics. To help reduce the occurrence of hyperglycemia, a subject may use
an automated
blood glucose control system,, which may automatically provide insulin to a
subject using a
medicament pump. The administered insulin may help control the blood glucose
level of the
sub ect by consuming glucose in the subject.
100761 Hypoglycemia is a condition that
occurs when the levels of sugar or glucose
in the blood are below a particular level (e.Q., 70 ingidL). This condition
may have adverse
consequences including loss of consciousness, seizures, and death. The levels
of blood sugar
that lead to hyperglycemia and hypoglycemia may vary from patient to patient.
To reduce the
risk of hypoglycemia, a subject may consume carbohydrates to increase blood
sugar. Because
of the severe consequences associated with a hypoglycemic event, subjects
usually consume
carbohydrates that metabolize quickly. These carbohydrates are often unhealthy
but are
preferable to the occurrence of a hypoglycemic event. For example, the
carbohydrates may
include candy bars with a lot of refined sugar.
100771 A bihormonal glucose-control system
may reduce the risk of occurrence of
hypoglycemia by including, in addition to insulin, a counter-regulatory agent
(e.g., Glucagon)
that can be administered to a subject when the blood glucose level drops too
low (e.g., below
50 mWd1). For subjects who do not have a bihormonal glucose-control system, it
may be
useful to understand the reduction in carbohydrate therapy, or the consumption
of
carbohydrates to address hypoglycemic events or potential hypoglycemic events,
that can be
achieved by switching to a bihormonal glucose-control system. Further, it may
be useful for
subjects who do have a bihormonal glucose-control system to understand die
reduction in
carbohydrate therapy obtained by haying the bihormonal glucose-control system.
For example,
understanding the amount of carbohydrate therapy consumed or avoided can be
important in
-21-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
monitoring the subject's nutrition intake. While monitoring nutrition in take
is important for
all people, it is particularly important for diabetics because diabetics must
balance eating
healthy with ensuring that their blood sugar is maintained in a particular
range to avoid both
hyperglycemia and hypoglycemia.
[00781 The present disclosure relates to a
system that can perform a computer-
implemented method of generating an indication of total carbohydrate therapy
over a time
period in a subject using a medicament pump configured to deliver at least
insulin therapy to
the subject. The system may be an automated blood glucose control system
(e.g., the glucose
level control system 510) that includes a hardware processor (e.g.,
controllers 518) for
determining dose control signals to provide the medicament pump (e.g.,
delivery devices 514).
In some cases, the medicament pump may be configured to deliver both insulin
therapy and
counter-regulatory agent (e.g., Glucagon) therapy. Alternatively, the system
may be separate
from the blood glucose control system but may receive blood glucose
information from the
blood glucose control system. For example, the system may be personal
computing system or
a cloud computing system that can received blood glucose information from the
blood glucose
control system.
100791 The system may receive or determine a
glucose level of a subject (e.g.,
subject 512). The glucose level of the subject may be determined based on a
signal (e.g., a
glucose level signal) received from a continuous glucose monitoring (CGM)
sensor (e.g.,
glucose sensor 516) that corresponds to the glucose level of the subject. In
some cases, the
glucose level may be determined from an isolated glucose measurement, such as
may be
obtained using a glucose measurement kit and/or glucose paper.
[0080] Using at least the glucose level of
the subject, the system can determine
whether a triggering event for raising the subject's blood glucose level has
occurred. The
triggering event may include a blood glucose level that indicates an
occurrence of a
hypoglycemic event or a risk of the occurrence of a hypoglycemic event
exceeding a risk
threshold within a particular period of time. A risk of a hypoglycemic event
may be determined
when a glucose level of the subject falls below a glucose threshold. This
glucose threshold may
vary for different subjects and may, in some cases, be specified by the
subject or a caregiver
(e.g., healthcare provider, parent, or guardian). Thus, in some cases,
different triggering events
may be defined based on a risk tolerance of a subject to an occurrence of
hypoglycemia or to
-22-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
possible different preferences for an amount of blood glucose to be present in
the subject.
Different subjects may prefer that blood glucose be maintained, or attempt to
be maintained,
at different levels due, for example, to differences in activity levels or
metabolism by different
subjects. Determining the risk of the occurrence of a hypoglycemic event may
include
receiving an indication of a risk of hypoglycemia from a glucose sensor or a
prediction of a
glucose level at a future time. For example, a determination of an imminent
risk of
hypoglycemia may comprise a determination that the subject's blood glucose
level is expected
to be below 60 mg/d1 within the next 5-15 minutes.
100811 Responsive to the triggering event,
the system may determine an amount of
counter-regulatory agent to administer, or an amount of counter-regulatory
agent that would
be administered if the blood glucose control system included the capability of
administering a
counter-regulatory agent. In some cases, the counter-regulatory agent is
administered by, for
example, the automated blood glucose control system_ In other cases, the
counter-regulatory
agent is not administered, For example, the automated blood glucose control
system may not
be capable of delivering the counter-regulatory agent. As another example, the
automated
blood glucose control system may be capable of delivering the counter-
regulatory agent but
may not have a dose of the counter-regulatory agent available.
100821 The system can use the indication of
the counter-regulatory agent that is
administered or that would be administered to determine a corresponding amount
of
carbohydrates. The corresponding amount of carbohydrates may be indicative of
the amount
of carbohydrates that were consumed to prevent the hypoglycemic event, to
reduce the risk of
the hypoglycemic event, or in response to an occurrence of a hypoglycemic
event.
Alternatively, or in addition, the corresponding amount of carbohydrates may
be indicative of
the amount of carbohydrates that would have been consumed if the counter-
regulatory agent
were not available.
[0083] The corresponding amount of
carbohydrates may be obtained from a
mapping between amounts of a counter-regulatory agent and amounts of
carbohydrates. In
some cases, the mapping may be based on a measured equivalency between
carbohydrates and
a counter-regulatory agent. Alternatively, or in addition, the mapping may be
between a
determined amount of counter-regulatory agent and an amount of carbohydrate a
subject
-23-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
indicates he or she normally consumes when determining that a hypoglycemic
event may
occur
(00841 The mapping may be implemented by a
lookup table that maps different
amounts of counter-regulatory agent to different corresponding amounts of
carbohydrates. In
some cases, a single quantity of counter-regulatory agent may map to different
amounts of
carbohydrates depending on the type of carbohydrate consumed (e.g., simple vs
complex
carbohydrates, or the type of candy bar consumed, etc.). Alternatively, the
mapping may be
based on a formula that converts an amount of counter-regulatory agent to an
amount of
carbohydrates based on a correspondence between the amount of counter-
regulatory agent and
the amount of carbohydrates. The determination of a relationship between the
counter-
regulatory agent and carbohydrates may be based on clinical tests comparing
carbohydrates to
the counter-regulatory agent (e.g., Glucagon, dextrose, etc.). Further, the
mapping may be
based at least in part on a subject's preferred carbohydrate source and/or
characteristics of the
subject (e.g., weight)
[0085] In some cases, the system can track a
number of hypoglycemic events or a
number of occurrences of a trigger indicating an impending risk of a
hypoglycemic event
within a particular time period. The time period may be days, weeks, months,
years, or any
other period of time over which it is desirable to determine a relationship
between
carbohydrates consumed or avoided based on the lack of availability or
availability of a
counter-regulatory agent. In some cases, the tracking of carbohydrate therapy
may be based on
a number of hypoglycemia events or hypoglycemia risk events instead of or in
addition to a
time period.
10086] For each occurrence of a hypoglycemic
event or occurrence of a trigger
indicating an impending risk of a hypoglycemic event. the system can determine
an estimate
of the carbohydrate therapy saved or that would have been saved by having
access to the
counter-therapy agent. The system can generate a report for the time period
that indicates the
total carbohydrate saved or that would have been saved with access to counter-
regulatory
agent. The report may include an aggregate or sum of the carbohydrate therapy
required or
saved during the time period. This time period may be days, weeks, months,
years, or since a
particular time (e.g., since the subject starting using the system). Further,
the report may
indicate the type of carbohydrates typically consumed by the subject when
responding to a
-24-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
hypoglycemic event or a risk of an impending hypoglycemic event, This report
can be
presented to the subject, a healthcare provider, and/or a parent or guardian
of the subject. The
healthcare provider can use this report to help care for the subject. For
example, the healthcare
provider can use the report to generate a nutrition plan for the subject that
accounts for the
carbohydrates consumed to maintain die blood glucose level within a desired or
setpoint range.
100871 The report may include a range of
carbohydrate therapy avoided or likely
consumed to address the risk of hypoglycemia events. Further, the report may
include an
amount of calories saved or not consumed, an amount of sugar avoided, an
amount of food not
consumed, a likely weight gain avoided, etc. based on the use of a counter-
regulatory agent in
place of carbohydrate therapy_
Carbohydrate Therapy Equivalence Tracking Process
100881 Figure 7 presents a flowchart of an
example carbohydrate therapy
equivalence tracking process 700 in accordance with certain embodiments. The
process 700
may be performed by any system that can track the glucose level of a subject
over time and
identify hypoglycemic events, or occurrences when a risk of a hypoglycemic
event satisfies a
threshold (e.g., when the risk of the hypoglycemic event matches Of is above a
particular
probability). For example, the process 700 may be performed by one or more
elements of the
glucose level control system 510. In some cases, at least certain operations
of the process 700
may be performed by a separate computing system that receives indications of
blood glucose
levels of the subject 512 from the glucose level control system 510 and/or
indications of
hypoglycemic events (or identified above threshold hypoglycemic risk events).
Although one
or more different systems may perform one or more operations of the process
700, to simplify
discussions and not to limit the present disclosure, the process 700 is
described with respect to
particular systems.
[0089] The process 700 begins at block 702
where the glucose level control system
510 receives a glucose level of a subject 512. Receiving the glucose level may
include
receiving a glucose level signal corresponding to a glucose level of the
subject The glucose
level signal may be received from the glucose sensor 516 (e.g., a CGM sensor).
Alternatively,
or in addition, the glucose level may be received from a user that provides
the glucose level to
the glucose level control system 510 via a user interface, such as a user
interface generated by
-25-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
the processor 530 that may be output on a touchscreen by the touchscreen
controller 538. The
glucose level received from the user may be a glucose level measured using an
alternative
sensor or measurement mechanism (e.g., diabetes measurement strips) that may
be used in
place of the glucose sensor 516.
(00901 At bock 704, the glucose level control
system 510 determines based at least
in part on the glucose level that a triggering event for raising the blood
glucose level of the
subject 512 has occurred_ The triggering event may include a determination
that a
hypoglycemic event or an episode of hypoglycemia is present or is occurring in
the subject
512. Alternatively, or in addition, the triggering event may include a
determination that there
is an impending risk of hypoglycemia in the subject 512, or an impending risk
that a
hypoglycemic event will occur within a particular amount of time in the
subject 512. The
determination of the hypoglycemic event or the risk of a hypoglycemic event
occurring may
be determined by comparing the glucose level of the subject to a glucose
threshold.
Alternatively, or in addition, the determination of the hypoglycemic event or
the risk of a
hypoglycemic event occurring may be determined by comparing a trend and/or
rate of change
(e.g., rate of decrease) in the glucose level to a threshold. In some cases,
the particular blood
glucose level and the trend in the blood glucose level may be combined to
determine a risk of
hypoglycemia. For example, if the glucose level is low (e.g., below a
particular threshold, such
as 60 mg,"(11), but a determined trend in the glucose level is upwards, then a
risk of
hypoglycemia may be lower than if the glucose level is above the threshold,
but the determined
trend in the glucose level is downwards towards a threshold. In some cases,
the threshold(s)
used to determine whether a hypoglycemic event is occurring or to determine
that there is an
above threshold risk of hypoglycemia occurring may vary based on physiological
characteristics of the subject 512. The physiological characteristics may be
based on
physiological characteristics associated or shared among groups of patients
(e.g., gender, age,
weight) or may be specific to the particular subject 512. For example,
thresholds associated
with a risk of hypoglycemia may be determined based on determined glucose
levels of the
subject 512 during prior occurrences of hypoglycemia as determined by the
glucose level
control system 510 or based on clinical data specific to the subject 512.
100911 In response to the triggering event at
the block 704, the glucose level control
system 510 determines an amount of counter-regulatory agent at block 706. The
glucose level
-26-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
control system 510 may determine the amount of counter-regulatory agent based
at least in
part on the blood glucose level of the subject 512, the amount or percentage
of risk of
hypoglycemia occurring (e.g., a 99% risk or probability of hypoglycemia may
trigger a larger
counter-regulatory agent dose than a 75% risk or probability of hypoglycemia),
physiological
characteristics of the subject 512, a trend in the blood glucose level of the
subject 512, or a
type of counter-regulatory agent.
100921 In some cases, the glucose level
control system 510 may use a delivery
device 514-Ito deliver the determined amount of counter-regulatory agent to
the subject 512.
The counter-regulatory agent may be delivered to the subject 512 in response
to the impending
risk of hypoglycemia or the episode of hypoglycemia, and/or in response to the
glucose level
satisfying or falling below a threshold glucose level. The threshold glucose
level or the
determination of whether to deliver the counter-regulatory agent may be based
on
physiological characteristics of the subject 512 and/or the risk tolerance of
the subject 512 to
a hypoglycemic event. It should be understood that, in the present context,
risk tolerance
generally does not refer to a user's subjective propensity for risk. Instead,
the risk tolerance is
typically an objective determination of how likely the subject 512 is to have
a hypoglycemic
event, or for symptoms of hypoglycemia to occur, when the blood glucose level
of the subject
512 is at a particular level. This risk tolerance may be determined based on a
history of
hypoglycemia, or lack thereof, in the subject 512 at particular blood glucose
levels and/or
based on clinical data obtained for the subject 512.
100931 In other cases, the glucose level
control system 510 may not deliver counter-
regulatory agent to the subject 512 because, for example, the glucose control
system 510 may
not be capable of delivering counter-regulatory agent or because the cartridge
holding the
counter-regulatory agent may be empty or have less than a threshold amount of
counter-
regulatory agent remaining.
[0094] At block 708, the glucose level
control system 510 determines a dose of
carbohydrate therapy based at least in part on the counter-regulatory agent.
The carbohydrate
therapy may refer to carbohydrates consumed to prevent or respond to an
occurrence of
hypoglycemia. The carbohydrates may include any type of carbohydrate that the
subject 512
may consume to prevent or respond to an occurrence of hypoglycemia, and
typically includes
fast-acting carbohydrates, which may include sugary foods that are easily
converted into sugars
-27-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
in the human body. For example, the carbohydrate may be a candy bar, soda,
fruit juice, or
other foods that may have a lot of sugar or refined sugars.
(00951 Determining the dose of carbohydrate
therapy may include accessing a
mapping between the counter-regulatory agent and carbohydrates. This mapping
may be stored
in, and accessed from, the memory 540 and/or may be accessed from another
computing
device. The glucose level control system 510 may determine the dose of
carbohydrate therapy
based at least in part on the mapping and the amount of the counter-regulatory
agent In some
cases, the mapping may vary based on the type of counter-regulatory agent
and/or the type of
carbohydrates. The type of counter-regulatory agent may be identified by a
user or may
automatically be determined based on a medicament cartridge installed or
inserted into the
glucose level control system 5/0. Further, the type of carbohydrates may be
specified by a user
and may include an identity of the type of carbohydrates usually consumed by
the subject 512
when responding to an occurrence or a risk of an occurrence of hypoglycemia.
For example,
the user may specify, via a user interface, whether the subject usually
consumes a candy bar or
fruit juice, or the size of the carbohydrate usually consumed when responding
to an occurrence
or a risk of an occurrence of hypoglycemia.
100961 In some cases, the mapping between the
counter-regulatory agent and
carbohydrates may be generated based on a clinical comparison of the counter-
regulatory agent
to the carbohydrates. Alternatively, or in addition, the mapping may be based
at least in part
on a physiological characteristic of the subject 512.
100971 The mapping may be stored in a lookup
table or other data structure that can
store relationships between different carbohydrates and counter-regulatory
agents. The
mapping may be between different quantities and/or types of carbohydrates and
different
quantities and/or types of counter-regulatory agent. Alternatively, or in
addition, the mapping
may be a formula that relates the carbohydrates to the counter-regulatory
agent or vice versa.
For example, the glucose level control system 510 may use the determined
amount of counter-
regulatory agent as an index to a lookup table to determine a corresponding
quantity of
carbohydrates. Alternatively, the glucose control system 510 may apply the
determined amount
of counter-regulatory agent to a formula to calculate a corresponding quantity
of
carbohydrates. This formula may be generated based on the type of counter-
regulatory agent
and/or carbohydrates, physiological characteristics of the user, and/or
clinical data.
-28-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
100981 In some cases, the mapping may vary
based on the glucose level control
system 510. For example, the glucose level control system 510 may include a
first mapping
when the glucose level control system 510 (or medicament pump thereof) is a bi-
hormonal
pump configured to deliver insulin and counter-regulatory agent therapy to the
subject, and
may include a second mapping when the glucose level control system 510 is not
configured to
deliver the counter-regulatory agent therapy to the subject 512. In some
eases, the glucose level
control system 510 may store both mappings in the memory 54o. For example, the
glucose
level control system 510 may use the first mapping when counter-regulatory
agent is available
and may use the second mapping when counter-regulatory agent is not available.
The mappings
may vary for a number of reasons including because a bi-hormonal glucose level
control
system 510 may more precisely control the occurrence of hypoglycemic events
due to the
availability of counter-regulatory agent, which may therefore alter the
frequency and type of
carbohydrates that a subject may consume.
100991 At block 710, the glucose level
control system 510 outputs an indication of
the dose of carbohydrate therapy. Outputting the indication of the dose of
carbohydrate therapy
may include outputting an indication of the dose of carbohydrate therapy on a
display for
presentation to a user. Further, the indication of the dose of carbohydrate
therapy may be
transmitted to another computing system for display or aggregation with other
therapy data
associated with the subject 512, such as therapy data used by a clinician to
help manager the
subject's 512 care. In some cases, the indication of the dose of carbohydrate
therapy may be
included in a report corresponding to care of the subject 512.
101001 In certain embodiments, the operations
of the process 700 are performed or
repeated over a period of time. For example, the operations associated with
the block 702-708
may be repeated one or more times over the period of time. in such cases, the
determined doses
of carbohydrate therapy may be aggregated for the period of time to determine
a total
carbohydrate therapy for the period of time. Further, the block 710 may
include outputting an
indication of the dose of carbohydrate therapy for each individual time that a
dose of
carbohydrate therapy is determined andlor the aggregated determined doses of
carbohydrate
therapy for the period of time. The period of time may be any time period. For
example, the
period of time may be a day, week, month, year, since the subject 512 began
using the glucose
level control system 510, since a user obtained or ceased obtaining access to
a counter-
-29-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
regulatory agent, or any other period of time. In some cases, the period of
time is defined by
the occurrences of hypoglycemic events or occurrences of the risk of
hypoglycemia satisfying
a threshold. For example, the period of time may be the time associated with
5,10, 15, 100, or
any other number of hypoglycemic events or occurrences of the risk of
hypoglycemia
satisfying a threshold.
101011 The indication of total carbohydrate
therapy may correspond to a reduction
in carbohydrates consumed by the subject 512 because, for example, of the
availability of
counter-regulatory agent to the glucose level control system 510, and
consequently, the subject
514. Thus, the indication of total carbohydrate therapy may correspond to a
reduction in
carbohydrates achievable by an availability to the subject 512 of the counter-
regulatory agent
Further, the indication of total carbohydrate therapy may correspond to an
amount of counter-
regulatory agent provided or that can be provided to the subject as a
substitute for
carbohydrates.
101021 The particular carbohydrates consumed,
or the amount of carbohydrates
consumed by each subject or during each hypoglycemic event, may vary. For
example, a
subject 512 may consume a particular candy bar when the subject's 512 measured
blood sugar
level is too low or when the subject feels that the blood sugar level is
likely low (e.g., begins
to feel some hypoglycemic effects). The subject may consume the whole candy
bar or may
consume a portion. Some of the candy bar may be lost to the subject (e.g.,
fall on the ground).
In other cases, the subject may have different candy bars available, or other
refined sugar
sources, during different hypoglycemic events. Thus, even though there may be
an objective
mapping between carbohydrates and counter-regulatory agent, the amount of
carbohydrates
consumed or avoided due to the availability of counter-regulatory agent may
vary for each
hypoglycemic event. Accordingly, the indication of total carbohydrate therapy
avoided, or that
could be avoided if counter-regulatory agent were available, may indicate a
range of
carbohydrates that may potentially be replaced by the availability of counter-
regulatory agent
101031 In some cases, the indication of
carbohydrate therapy or total carbohydrate
therapy may include one or more of an indication of calories, an indication of
carbohydrates,
an indication of a measure of sugar, an indication of a quantity of food, or
an indication of
weight of the subject attributable to the carbohydrate therapy. The
indications may be
associated with what is consumed due to a lack of counter-regulatory agent, or
what is avoided
-30-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
based on the availability of counter-regulatory agent. For example, the
indication of calories
may be the number of calories not consumed because of the presence of the
counter-regulatory
agent Advantageously, the availability of therapy information relating to the
carbohydrate
therapy or avoided carbohydrate therapy can assist in patient care. For
example, a subject can
reduce refined sugar consumption that can have a number of health
consequences. Further, a
healthcare provider can better help a subject control his or her weight based
on the
carbohydrate information.
101041 The indication of carbohydrate therapy
may be presented to a user in any
presentable form. For example, the indication of carbohydrate therapy may be
presented as a
table, a chart, a graph, a histogram, or other data presentation tool for
indicating the reduction
in carbohydrates over time that is achieved by the presence of counter-
regulatory agent or that
could be achieved by the use of counter-regulatory agent for the particular
subject 512. It
should be understood that the indication of carbohydrate therapy data may vary
for different
users due to differences in physiological characteristics of the users,
differences in the diabetes
of each user, differences in lifestyle of each user, among other factors.
Advantageously, by
using the glucose level control system 510 to track the carbohydrate therapy
of the subject 512
or to determine the carbohydrate therapy avoided or avoidable associated with
counter-
regulatory agent, management of the subject's 512 blood glucose level can be
personalized.
Additional Carbohydrate Therapy Equivalence Tracking Embodiments
101051 People with diabetes often consume
oral carbohydrates for the purpose of
treating or preventing hypoglycemia. Such extra carbohydrates can have
unhealthy
consequences, contributing to weight gain being one of them. Having a
bihormonal glucose-
control system that infuses a counter-regulatory agent (e.g., Glucagon) to
reduce the frequency,
extent, and duration of hypoglycemia can significantly reduce the amount of
oral carbohydrates
that are needed "medicinally" to treat or prevent hypoglycemia
/01061 Certain embodiments of the present
disclosure relate to a method for
translating an amount of online counter-regulatoty dosing (e.g. glucagon)
computed by an
autonomous glucose-control system to an amount of carbohydrates that the user
is estimated
to have been spared from needing by virtue of the counter-regulatory dosing,
or that the user
would be spaced from needing if the user had access to the counter-regulatory
agent In a
-31-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
bihormonal autonomous glucose-control system that infuses both insulin and a
counter-
regulatory agent/hormone, the method may include a mapping between the online
counter-
regulatory dosing, which was delivered to treat or prevent low glucose levels,
and oral
carbohydrates that are estimated to have otherwise been required to achieve a
comparable safe
control situation (had the counter-regulatory dosing not been delivered). In
an insulin-only
autonomous glucose-control system, where doses of a counter-regulatory
agent/hormone are
not delivered, but are still computed online, the method may include a mapping
between the
computed online counter-regulatory dosing and an estimated amount of oral
carbohydrates that
the subject will likely have been spared from needing to consume to treat or
prevent low
glucose levels had the counter-regulatory agent been available and its doses
actually delivered.
[0107] Without loss of generality,
embodiments disclosed herein include an
autonomous glucose-control system where the counter-regulatory agent is
glucagon. However,
other medicaments and/or counter-regulatory agents may be utilized. The method
may include
relating computed online glucagon dosing with consumed oral carbohydrates for
the treatment
or prevention of low glucose levels ("treatment carbs") as observed in real
use (e.g., during
clinical studies) in the insulin-only configuration, and relating the
relationship between the
counter-regulatory agent and carbohydrates to a similar relationship between
delivered online
glucagon doses (or other counter-regulatory agent) and similarly consumed oral
carbohydrates
in the bihormonal (insulin¨glucagon) configuration.
[0108] Using data gathered from real use
(e.g., clinical studies), a relationship
between the consumed treatment carbs in an insulin-only configuration, Cie.,
and the online
computed (but not delivered) glucagon dosing, Ge, can be described by the
relationship Co =
Ray(x) * Ge, where Rio(x) may be a relating factor that can be a function of
several dependencies
that are included in vector x. Such dependencies can include the specific
insulin and/or
glucagon being used (e.g., their clinical properties), and/or the
pharmacokinetic settings
assumed by the control system in relation to insulin and/or glucagon. The
dependencies can
also include the user's body mass and the glucose target used by the glucose-
control system.
In some embodiments, Rio(x) may be a constant, or Rio(x) F. Rio, for a system
exhibiting
limited variation in the relationship between Co and Ge (e.g., due to limited
effect, or limited
or no variation in the associated dependencies).
-32-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
101091 Similar to the insulin-only
configuration, from real-use data, a relationship
between the consumed treatment carbs in a bihormonal (insulin¨glucagon)
configuration, Cbh,
and the online delivered glucagon dosing, Gd, can be described by the
relationship Cbh = Rbh(x)
* Ga., where Rhh(x) may be described in a similar fashion to Rio(x) above. In
some cases, the
quantities C10, (Tie, ebb., and Gd can refer to daily amounts, as averaged
over some period of use
(e_g., a week)_ In some cases, the quantities 00, Ge, Cbb, and Gd can refer to
average daily
amounts per body mass of the user, in which case dependency on body mass can
be eliminated
from x.
101101 In cases where Ge is computed, but no
glucagon is actually delivered in an
insulin-only system, Gc has no effect on glucose insofar as treating or
preventing low glucose
levels, which in turn is generally expected to invoke further computed
glucagon dosing (e.g.,
goes towards increasing the magnitude of Gd for a given situation). By
contrast, since Gd is
delivered in a bihormonal system, it is expected to have an effect in
preventing or reducing the
frequency, extent, or duration of low glucose levels, which in turn is
expected to limit the
overall magnitude of glucagon dosing (e.g., limits Gd for a given situation).
As such, for a
given set of dependencies, it is generally expected that a > Gd between the
two system
configurations. Likewise, since a has no effect in combating low glucose
levels while Gd does
have such an effect, it is expected that treatment carbohydrates Co > Cbh,
when comparing the
two system configurations.
[0111] If one can ideally relate, for a given
real-use case of an insulin-only system
with Cc, what the corresponding Co would have been for the same real-use
scenario, had the
computed online glucagon dosing actually been delivered as Gd, one can project
an estimate
that the user would have required "C0 ¨ Cbh" less treatment carbs (eg., would
have saved that
much), had they instead been using a bihormonal system (with the same insulin
controller),
where glucagon would have been delivered. Conversely, if one can ideally
relate, for a given
real-use case of a bihormonal system with Gd, what the corresponding Cbh would
have been
for the same real-use scenario, had the delivered online glucagon dosing not
been delivered
but only computed as a, one can project an estimate that the user had actually
avoided the
need to take "C0 ¨ Cbh" additional treatment carbs, had they been instead
using an insulin-only
system (with the same insulin controller), where glucagon would not have been
delivered. It
should be understood that the above calculations are an estimate in an ideal
situation as, in
-33-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
practice, it is not possible to have a re-run of a past real-use scenario to
obtain such ideal
relationships.
(01121 For practical implementation, real-use
cases where the insulin-only system
is used can be re-simulated while assuming a bihormonal system is available,
where glucagon
is assumed to be delivered. Since the control system may take delivered doses
into account
when issuing subsequent nearby glucagon doses, the simulated glucagon dosing
may exhibit a
reduction relative to the original Gs of die insulin-only system. With the
glucose profile kept
unaltered in a simulation, the simulation may lack reflecting any resulting
glucose excursions
in response to the assumed delivered glucagon dosing. The simulation in turn
may lack
reflecting the full reduction in glucagon dosing down to Gd that may have been
observed if the
glucose excursions had in fact benefited from glucagon being delivered. Thus,
the reduced
glucagon dosing that is observed in the simulation, pseudo delivered glucagon
ad, may
arguably be exaggerated in magnitude relative to what would have been the
"real Gd". As
described above, based on prior analyses Ge can be mapped to a corresponding
amount Co in
the insulin-only configuration, and ed can be mapped to a corresponding amount
ebh in the
bihormonal configuration. The simulation results, therefore, can map the
reduction "Gs ¨ ad"
to an estimate "Cis ¨ ebh" of treatment carbs that the user would spare had
they been using the
bihormonal system. The estimates may be conservative estimates. Repeating the
simulation
analyses across a variety of real-use cases that span the range of a observed
in practice
provides a global mapping between them and the associated range of (in some
cases,
conservative) estimates "Cis ¨ ebh" of treatment carbs that the user would
likely not need to
consume had they been using the bihormonal system. Conversely, the mapping can
be utilized
when a bihormonal system is being used, where the observed dosing Gd is mapped
back to a
pseudo computed glucagon dc and the resulting associated difference "eio_ Gbh"
provides a
(in some cases, conservative) estimate of the treatment carbs that the user
had likely saved by
virtue of being on the bihormonal system.
101131 Certain embodiments includes a system
that comprises a controller for
automatic control of a blood glucose level of a subject The controller may be
operative to
generate an insulin dose control signal based on time-varying glucose levels
of the subject as
represented by a glucose level signal over time. The glucose level signal can
be generated by
a glucose sensor operative to continually sense a glucose level of the
subject. The insulin dose
-34-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
control signal may control the delivery of doses of insulin by a delivery
device. Further, the
controller can operate at a regular frequency to generate an insulin dose
control signal to
regulate the glucose levels in the subject. During online operation, the
controller can employ a
control algorithm that generates a glucagon dosing signal, which may be mapped
to an
associated amount of oral carbohydrates.
101 NJ The oral carbohydrates may be
associated with the prevention or treatment
of low glucose levels. Further, the mapping between the glucagon dosing signal
and the oral
carbohydrates may be derived from analysis of clinical data. The glucagon
dosing signal may
be computed, but not delivered in an insulin-only system configuration. In
contrast, the
glucagon dosing signal can be computed, and glucagon can be delivered in an
insulin¨glucagon
system configuration. The computed glucagon dosing in an insulin-only system
configuration
can be mapped to an amount of oral carbohydrates that is estimated to have
been saved had
glucagon dosing been delivered if an insulin¨glucagon system configuration had
instead been
used. The delivered glucagon dosing in an insulin¨glucagon system
configuration can be
mapped to an amount of oral carbohydrates that is estimated to have been saved
if an insulin¨
only system configuration had instead been used. The mapping may be dependent
on the
clinical properties of the insulin and glucagon being used, and settings in
the control system
related to the action and effect of insulin and glucagon. Further, the mapping
may be dependent
on the subject's body mass.
Backup Therapy Protocol Generation
101151 An ambulatory medicament device, such
as a blood glucose control system
(e.g., an insulin pump or a combined insulin and counter-regulatory agent
(e.g., Glucagon)
pump), can provide personalized therapy to a subject. In other words, the
ambulatory
medicament device may provide medicament that is specific to a subject's
physiology,
condition, activity, and the like. Further, some ambulatory medicament
device's monitor a
condition of the subject to determine when to provide therapy, what type of
therapy to provide
(e.g., insulin or counter-regulatoty agent therapy), and/or how much therapy
to provide. The
therapy provided by the ambulatory medicament device may be ongoing and, in
some cases,
lifesaving. Thus, it is important that ambulatory medicament device function
uninterrupted.
-35-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
101161 Despite best efforts, sometimes
therapy by the ambulatory medicament
device is interrupted. For example, the ambulatory medicament device may
break, a subject
may run out of or not have access to a necessary disposable (e,g., a
replacement insulin
cartridge, a site kit for changing the site of the ambulatory medicament
device, a replacement
battery, and the like), or the subject may forget to charge a battery of the
ambulatory
medicament device or not be in a location where a power source is available to
charge the
ambulatory medicament device. Thus, there are occasions when the ambulatory
medicament
device may not be available or may need replacing.
101171 When the ambulatory medicament device
is not available, or when a
replacement (temporary or permanent) ambulatory medicament device is being
used, it may
be desirable to have an indication of the therapy settings from the ambulatory
medicament
device. For example, if a user (e.g., a subject, healthcare provider, parent,
or guardian) is
providing alternative therapy (e.g., injection therapy) while the ambulatory
medicament
device, it may be necessary to know the quantity of therapy to provide under
particular
circumstances or at particular times.
[01181 In some cases, a healthcare provider
may have access to therapy information
that may have been previously determined, for example, via clinical testing.
This therapy
information may include any type of information that can be used to determine
therapy to
provide to a subject at a particular time or under particular conditions. For
example, the therapy
information may indicate a setpoint insulin range for the subject, a quantity
of insulin to
provide to the user to adjust glucose levels, an amount of time for insulin to
reach max
concentration in the subject, or any other information that might impact the
timing or amount
of dosing of a medicament.
[0119] The therapy information available to
the healthcare provider may be
insufficient. For example, the subject may not be able to reach the healthcare
provider to obtain
the therapy information at a point in time when the information is needed,
Further, in some
cases the information may be outdated because, for example, the ambulatory
medicament
device may have refined the therapy over time. If the refinements have
occurred recently, it is
possible that the outdated values of the healthcare provider may be sufficient
until a
replacement ambulatory medicament device can repeat the refinement process of
the original
ambulatory medicament device. In other cases, the outdated therapy information
may be
-36-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
insufficient because, for example, the refinements were significant or the
subject may have had
physiological changes (e.g.õ weight gain or weight loss, or metabolism
changes) since the last
time a clinical test was performed. Using outdated therapy information may be
less effective
and may cause discomfort or harm to a subject.
[0120] Certain embodiments of a system
disclosed herein can generate backup
therapy data Using the backup therapy data, a subject (or user) can perform
injection therapy
or configure a replacement ambulatory medicament device if the subject's
current device
malfunctions. By using the backup therapy data, the subject can maintain a
level of therapy
care that matches or more closely matches what was being provided by the
ambulatory
medicament device than clinical data, which may be outdated if available at
all.
[0121] The system can include an automated
blood glucose control system (e.g.,
the glucose level control system 510) configured to generate a backup therapy
protocol
comprising insulin therapy instructions derived from autonomously determined
doses of
insulin, During normal operation, the system may receive glucose level signals
from a sensor
operatively configured to determine glucose levels in a subject. The sensor
can include any
type of sensor that can determine glucose levels. For example, the sensor may
be a Continuous
Glucose Monitoring (CGM) sensor.
101221 Using the determined glucose levels,
the system may autonomously
determine and/or generate a dose control signal using a control algorithm. The
determination
and/or generation of the dose control system may be performed without any user
action or
interaction with the blood glucose control signal. In some cases, the lack of
user action or
interaction with the blood glucose control system refers to conscious action
and may exclude
sensor measurements of physiological characteristics of the subject. The
control algorithm may
autonomously determine doses of insulin to be infused into the subject for the
purpose of
controlling blood glucose of the subject based at least in part on the glucose
level signal. The
control algorithm may include any type of control algorithm.
/01231 For example, the control algorithm may
be a biexponential pharrnacokinetic
(PK) model that models the accumulation of insulin doses in the blood plasma
of the subject
The automated blood glucose system may control delivery or administering of
insulin or a
counter-regulatory agent based on the hi-exponential PK model and one or more
blood glucose
measurements of the subject The bi-exponential PK model may model the
absorption of
-37-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
subcutaneously administered insulin into blood and/or a rate of diminishing
glucose in the
blood. The bi-exponential PK model over time may be represented by the
following equation:
Kt) = KU0(e-att ¨ e-a2t)
(1)
where Uo is the subcutaneous dose in units (U), K is a scaling constant and no
and az are time
constants.
101241 As an alternative example, the control
algorithm may include a linear
algorithm that models diminishing glucose or the accumulation of glucose in
the subject based
on a linear reduction rate. For example, the control algorithm may determine
that a particular
dose, D, of insulin is to be administered to the subject. The control
algorithm may then estimate
that 0.25*D of the insulin is absorbed into the blood plasma per hour over 4
hours. Similarly,
the control algorithm may estimate that the insulin diminishes at a rate of
0.33*D per hour over
three hours upon the insulin reaching maximum concentration within the blood
plasma..
101251 Regardless of the control algorithm
used, the automated blood glucose
control system may administer insulin and, in some cases, a counter-regulatory
agent one or
more times over a particular time period. There may be multiple reasons and/of
triggers that
cause the automated blood glucose control system to supply insulin. For
example, the
automated blood glucose control system may provide a basal does of insulin on
a periodic basis
in an attempt to maintain a steady blood glucose level in the blood plasma of
the subject As
another example, the automated blood glucose control system may supply
mealtime boluses
of insulin to account for an expected amount of glucose to be consumed as part
of a meat The
mealtime bolus may be an amount specified by a user or may be an amount of
insulin
administered in response to an indication of meal size by the subject. This
indication of meal
size may be subjective. In some cases, the size of the bolus of insulin for an
identified meal
size may be a fixed or constant value. In some other cases, the size of the
bolus of insulin for
an identified meal size may vary over time as the automated blood glucose
control system
learns or refines the amount of insulin to administer to a subject to keep the
subject's blood
glucose within a target setpoint The automated blood glucose control system
may learn or
refine the optimal insulin to administer based on a comparison of expected
blood glucose level
measurements to actual blood glucose level measurements when the subject (or
other user)
makes a subjective identification of meal size. In addition to basal and
mealtime boluses of
insulin, the automated blood glucose control system may also supply correction
doses of
-38-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
insulin to the subject based on the glucose level signal. The correction doses
of insulin may be
supplied in response to a model predictive controller (MPC) determining or
estimating that a
user's level of insulin is expected to fall below a threshold in some future
period of time based
on blood glucose level readings. The MPC may execute a control algorithm that
can regulate
glucose concentration to a reference setpoint while simultaneously minimizing
both the control
signal aggressiveness and local insulin accumulation_ A mathematical
formulation describing
the subcutaneous accumulation of administered insulin may be derived based on
nominal
temporal values pertaining to the pharmacokinetics of insulin in the subject
The mathematical
formulation may be in terms of the insulin absorption rate, peak insulin
absorption time, and/or
overall time of action for the insulin (or another medicament). Examples of an
MPC controller
that may be used with embodiments of the present disclosure are described in
U.S. Patent No.
7,806,854, issued on October 5, 2010, the disclosure of which is hereby
incorporated by
reference in its entirety herein for all purposes.
101261 The automated blood glucose control
system may track insulin therapy
administered to the subject over a tracking period. Although the tracking
period is not limited
in length and may generally be any period of time, typically the tracking
period is at least a
minimum period of time sufficient for the automated blood glucose control
system to learn or
refine the amount of medicament (e.g., insulin) to administer to the subject
under particular
conditions (e.g., when particular blood glucose levels are detected or when
particular meal
sizes are identified). For example, the automated blood glucose control system
may initially
administer 6 units of insulin for lunch and 10 units of insulin for dinner.
These initial values
may be set be a healthcare provider and/or a subject based, for example, on
clinical data for
the subject. However, over time (e.g., 3-5 days), the automated blood glucose
control system
may determine that providing 7 units of insulin for lunch and 8 units of
insulin for dinner
maintains the subject's blood glucose level closer to the median of the
setpoint range than did
the initial configuration. Although not limited as such, generally each unit
of insulin is 1/100th
of a milliliter of insulin.
[0127] As indicated, the tracking period can
be any length of time. For example,
the tracking period could be 1 day, 3 days, 5 days, 7 days, anything in
between, or more.
Typically, the tracking period is at lenst long enough to provide sufficient
time to learn or
refine initial settings of the automated blood glucose control system for the
subject In some
-39-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
cases, the tracking period may be 1 or 2 days. In other cases, the tracking
period may be from
a particular time period until a current time period. For example, the
tracking period may be
from the start of therapy until a current point in time. In other cases, the
tracking period may
be a moving or shifting window. For example, the tracking period may be the
least week, two
weeks, month, or year. Further, for non-blood glucose systems, the tracking
period may differ
based on the amount of time sufficient to determine or refine medicament
control values for
the subject. In some cases, the tracking period may a window of a particular
length. This
window may be a moving window. For example, the window may be the previous 7
days. As
time passes, the window moves to continue to encompass the previous 7 days.
101281 Tracking the insulin therapy may
include storing the autonomously
determined doses of insulin delivered to the subject. These autonomously
determined doses of
insulin may include one or more of basal insulin doses, mealtime insulin
boluses, or correction
insulin doses. Moreover, tracking the insulin therapy may including tracking
the type of insulin
used. The type of insulin may include any type of insulin, such as fast-acting
insulin (e.g.,
Lispro, Aspro, or Glulisin), regular or short-acting insulin (e.g., Humulin R,
Novolin R, or
Velosulin R), intermediate-acting insulin (e.g., Hurnulin N, Novolin N.
ReliOn), long-acting
insulin (e.g., detemir (Leyemir), and glargine (Basaglar, Lantus)), or Ultra
long-acting insulin
(e.g., degludec (Tresiba), glargine u-300 (Toujeo)). Further, tracking the
insulin therapy may
include tracking counter-regulatory agent (e.g.. Glucagon) therapy.
[0129] In some cases, tracking the insulin
therapy may include calculating average
therapy provided over a period of time (e.g., over the tracking window). For
example, the
tracking the insulin therapy may include determining a moving average of the
past 7 days of
nominal basal doses during each dosing interval. Assuming basal therapy is
provided every
five minutes, the moving average may be calculated based on the previous 288
doses (e.g.,
over I day) or 2016 doses (e.g., over 7 days). This calculation can be used to
obtain a basal
rate profile for backup therapy. In some cases, the time period may be broken
up into different
time segments that may be associated with different rates of therapy. For
example, there may
be 4 basal therapy periods (e.g., lOpm-4am, 4am-10am, 10am-4pm, and 4pm-10pm).
Thus, a
separate moving average may be calculated for each of the basal therapy
periods over a day,
or over some other time period (e.g., 7 days). The calculated averages may be
used to calculate
a backup basal rate that can be used to program an automated glucose control
system. Further,
-40-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
the basal rate profile may include aggregating the doses across the day to
determine a dose of
long-acting insulin that can be used for injection therapy.
101301 Similar to the basal therapy, a moving
average of correction doses can be
calculated to determine a correction bolus of insulin to supply via a pump or
injection therapy.
Alternatively, or in addition, the moving average of correction doses in
combination with
measurements of blood glucose of the subject over time may be used to
determine a rate of
change of blood glucose from a unit of insulin provided during correction
therapy.
101311 Mealtime boluses may also be
calculated using a moving average. Further,
a separate moving average may be calculated for each meal (e.g., breakfast,
lunch, and dinner)
dose over some period of time (e.g., 7 previous days of mealtimes). In some
cases, each of the
moving averages may be calculated using different windowing functions. For
example, the
moving average may be calculated using a Hann window or a Hamming window. In
some
cases, different levels of dosing may be determined for different meal sizes
and different doses
may be determined for different meals. In some cases, different meals (e.g.,
breakfast vs lunch)
may have different dosing despite similarity in size due, for example, to
differences in the
subject's blood glucose levels when they wake up versus when they usually have
lunch, or
because differences in types of foods consumed at breakfast versus lunch.
Further, in some
cases, differences in metabolisms of different subjects may result in
different mealtime
boluses.
101321 The insulin therapy may be stored in a
therapy log, or any other type of data
structure. Further, the insulin therapy may be stored in a memory of the
automated blood
glucose system, on a companion device, on a computing device of the subject or
user (e.g., a
laptop or desktop), in a cloud computing environment, or in any other storage
system capable
of receiving the insulin therapy information from the automated blood glucose
control system.
101331 Using the therapy log or tracked
insulin data, the automated blood glucose
system, or a computing system with access to the therapy log or tracked
insulin data, may
generate a backup insulin therapy protocol. The backup insulin therapy
protocol may include
a backup injection therapy protocol or a backup pump therapy protocol. The
backup injection
therapy protocol may include one or more amounts of insulin (or other
medicament) to
administer using injection therapy (e.g., manually provided shots) at one or
more times to help
maintain the subject's condition within a normal or desired physiological
range or condition.
-41-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
The backup pump therapy protocol may include data and/or instructions for a
replacement
medicament pump of the same type Of of a different type to supply therapy to
the subject. The
replacement medicament pump may be a permanent replacement or a temporary
replacement
(0134) The backup pump therapy protocol may
be the same as and/or include the
same type of information as the backup injection therapy protocol.
Alternatively, or in addition,
the backup pump therapy protocol may include different values than the backup
injection
therapy protocol. For example, the backup pump therapy protocol may include an
indication
of basal therapy to provide periodically on relatively short increments (e.g.,
every 5 minutes,
every half hour, every hour, etc.). Because an insulin pump may automatically
administer
insulin, it is possible to provide a steady or periodic drip of insulin. It
may be impractical for a
subject using injection therapy to administer insulin manually on similar
short increment&
Instead, a user might administer therapy on a less regular basis (e.g., once
every roughly 4-5
hours or 6-8 hours, prior to mealtimes, after waking, and/or before sleeping,
etc). Accordingly,
the backup therapy protocol for a pump and for injection may differ Further,
the type of insulin
used or identified in the backup protocol may differ. For example, the backup
protocol may
call for use of long-acting insulin, such as, for example, insulin glargine,
or intermediate-acting
insulin, such as, for example human recombinant insulin.
101351 In some cases, the backup pump therapy
protocol may be used to manually
refine pump settings for a replacement blood glucose control system to be used
by the subject.
In other cases, the replacement blood glucose control system may automatically
configure
itself based on the backup therapy protocol. For example, a user may cause the
backup therapy
protocol to be provided to the replacement blood glucose control system, which
may use the
information to self-calibrate.
[0136] Regardless of whether a backup
protocol is generated or needed, collecting
and analyzing therapy data for therapy provided by the automated blood glucose
control system
can be useful for helping to manage a subject's condition. For example,
therapy data may be
useful in determining whether the subject is satisfied with therapy provided
by the automated
blood glucose control system or whether the blood glucose control system is
configured in a
way that best matches the subject's lifestyle or therapy preferences
(subjective or otherwise).
One way to determine whether the blood glucose control system is providing
desired therapy,
or therapy at a desired rate, is to determine the frequency and/or magnitude
of modifications
-42-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
made by the subject, or other user that may help manage a subject's therapy,
to therapy
provided by the automated blood glucose control system.
(01371 The automated blood glucose control
system disclosed herein can track user
modifications to a control parameter over a tracking period. The tracking
period may include
any time period described above for tracking therapy to generate a backup
protocol. Further,
the control parameter may include any type of control parameter that may
affect the
administering of therapy. For example, the control parameter may relate to a
quantity of
therapy, a timing of delivered therapy, a rate that therapy is delivered, or a
trigger of when or
whether to deliver therapy, among other control parameters. Moreover, the
control parameters
may directly affect the delivery of therapy (e.g., specify a time to deliver
the medicament or a
quantity of medicament to deliver) or may indirectly affect therapy (e.g.,
adjust a setpoint range
to maintain blood glucose or a rate of insulin accumulation in the subject,
which may be used
to modify a control algorithm for administering therapy).
101381 The user modifications may include any
change to the control parameter or
settings of the automated blood glucose control system. For example, the
automated blood
glucose control system may track each instance and/or the rate or percentage
of times a user
reduces or increases a control parameter (e.g., an amount of administered
insulin). Further,
tracking changes to the control parameter may including tracking how often a
user pauses
therapy or temporarily adjusted a target blood glucose range, or other control
parameter. In
addition, tracking changes to the control parameter may incl ude tracking when
a user makes
changes to the control parameter. For example, the user may generally modify
the control
parameter at night, but leave the daytime parameter unchanged, or vice versa.
In some cases,
the automated blood glucose control system may track a subject's weight over
time. The weight
may be provided by a user and may affect the blood glucose control (e.g., an
amount of insulin
administered may be related to a subject's weight).
(01391 The automated blood glucose control
system may generate a report that
tracks user modifications to the control parameter. The report may comprise a
measure of the
frequency of increases and decreases from the stored control parameter value.
Further, the
report may include an indicator of a percentage of times a user modified a
control parameter
higher or lower than the stored control parameter value of the automated blood
glucose control
system over the tracking period. In some cases, the report indicates the
number of times that
-43-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
the infusion of insulin is paused over the tracking period, or the speed
(e.g., aggressiveness)
that insulin is delivered to the subject.
[01401 Using this report, a clinician or
other healthcare provider can determine
whether modifications should be made to a control parameter to better manage a
subject's
therapy. For example, if it is determined that a subject is raising a blood
glucose target level 4-
times a week during an evening time or nighttime, the clinician may determine
that the target
setpoint for the evening should be adjusted to reduce the number of
occurrences that a user
manually adjusts therapy, or control parameter settings for therapy, provided
by the automated
blood glucose control system. In some cases, the subject may be adjusted
therapy based on
subjective reasons. In some such cases, the therapy report may enable the
clinician or
healthcare provider to train the subject on controlling his or her disease. In
other cases, the
clinician may determine that the subject has a different tolerance for blood
glucose than
initially determined or than an average subject and may adjust one or more
control parameters
of the automated blood glucose control system accordingly,
[0141] In some implementations, the automated
blood glucose control system may
automatically adjust one or more control parameters over time based on the
report. For
example, if the automated blood glucose control system determines that over a
course of a
month the subject adjusted lower a daytime target glucose range 20 out of 30
days, the
automated blood glucose control system may modify a control parameter to have
a lower
setpoint range. In some cases, the automated blood glucose control system may
communicate
the change to a user, such as the subject, a parent or guardian, or a
healthcare provider.
Example Backup Therapy Protocol Generation Process
[0142] Figure 8 presents a flowchart of an
example backup therapy protocol
generation process 800 in accordance with certain embodiments. The process 800
may be
performed by any system that can track medicament therapy (e.g., insulin
therapy) provided to
a subject over time and generate a backup therapy protocol that may be used if
a glucose level
control system 510 becomes unavailable. For example, the process 800 may be
performed by
one or more elements of the glucose level control system 510. In some cases,
at least certain
operations of the process 800 may be performed by a separate computing system
that receives
indications of medicament therapy provided to the subject 512 from the glucose
level control
-44-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
system 510. Although one or more different systems may perform one or more
operations of
the process 800, to simplify discussions and not to limit the present
disclosure, the process 800
is described with respect to particular systems_
(0143) The process 800 begins at block 802
where the glucose level control system
510 receives a glucose level of a subject 512. Receiving the glucose level may
include
receiving andlor determining a glucose level signal corresponding to a glucose
level of the
subject. The glucose level signal may be received from the glucose sensor 516
(e.g., a CGM
sensor). Alternatively, or in addition, the glucose level may be received from
a user that
provides the glucose level to the glucose level control system 510 via a user
interface, such as
a user interface generated by the processor 530 that may be output on a
touchscreen by the
touchscreen controller 538. The glucose level received from the user may be a
glucose level
measured using an alternative sensor or measurement mechanism (e.g., diabetes
measurement
strips) that may be used in place of the glucose sensor 516.
(0144) At block 804, the glucose level
control system 510 generates an insulin dose
control signal based at least in part on the glucose level signal. In some
cases, the insulin dose
control signal may be a medicament control signal configured to control a
medicament pump
to administer medicament (e.g., insulin, counter-regulatory agent, or other
medicament) to a
subject 512. The dose control signal may be generated using a control
algorithm configured to
autonomously determine doses of insulin to be administered to or infused into
the subject for
the purpose of controlling blood glucose of the subject based at least in part
on the glucose
level or glucose level signal determined at the block 802.
101451 At block 806, the glucose level
control system 510 tracks insulin therapy
administered to the subject 512 over a tracking period. The tracking period is
typically at Ietast
one day and may be longer. For example, the tracking period may be 1 day, 2
days. a week, a
month, several months, a year, any length of time between the foregoing
examples, or even
longer In some cases, the tracking period may be continuous from a point in
time when
tracking begins. For example, the tracking period may encompass the entire
usage lifetime of
the glucose level control system 510 by the subject 512. In cases where the
tracking period is
set for a defined period of time (which may be modified for different
iterations of the process
800), the process 800 may be repeated periodically, upon request, or upon a
triggering event
using a new tracking period, of equal or different length. The triggering
event may include any
-45-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
event that may render a prior generated backup therapy protocol potentially
out-of-date. For
example, the triggering event may include a change in medicament type (e.g.,
different insulin
or counter-regulatory agent formulations), a change in physiological
characteristics of the
subject 512 (e.g., a change in weight, or sensitivity to different glucose
levels or medicament),
or a change in average activity level of the subject 512.
101461 Although the tracking period is
typically at least one day enabling the
glucose level control system 510 to determine a backup protocol based on data
from a full
cycle (e.g., waking and sleeping hours) of glucose level control system 510
use, in some cases,
the tracking period may at least initially be less than a day. For example, an
initial backup
therapy protocol may be generated after a half-day's activity. This initial
backup therapy
protocol may be updated as more data becomes available throughout the day's
(and, in some
cases, subsequent day's) use of the glucose level control system 510.
101471 In some cases, the tracking period may
be defined by or based on a
particular number of insulin administering events, For example, the tracking
period may be
defined by at least ten instances of generating an insulin dose based on a
glucose level signal.
As another example, the tracking period may be defined by a minimum number of
meal events,
correction dose events, and/or basal dose events. For instance, the tacking
period may require
3 meals, or 3 meals of each meal type to occur, 2 correction doses, and/or 100
basal doses. It
should be understood that the aforementioned number of doses is just an
example, and the
tracking period may include more or fewer dose amounts. Moreover, the tracking
period may
be defined or specified as a combination of time and occurrences of a
particular number of
doses of insulin.
101481 In some cases, the tracking period may
be variable, For example, if the
glucose level control system 510 determines that the insulin dose therapy is
inconsistent or
erratic over the tracking period (e.g., due to inconsistent exercise or eating
habits), the tracking
period may be extended.
/01491 Tracking the insulin therapy may
include storing the insulin dose control
signal generated based at least in part on the glucose level signal at the
block 804_ Alternatively,
or in addition, tracking the insulin therapy may include storing an indication
of a quantity of
insulin (or other medicament) corresponding to the insulin (or another
medicament) dose
control signal. The insulin dose control signal and/or the indication of the
quantity of insulin
-46-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
may correspond to a dose of insulin delivered to the subject 512 as a basal
insulin dose, a
correction bobs of insulin, and/or as a mealtime bolus of insulin.
(01501 Storing the insulin dose control
signal and/or the indication of the quantity
of insulin may include storing the insulin dose control signal and/or the
indication of the
quantity of insulin in a therapy log or any other type of data structure in
the memory 540 of
the glucose level control system 510. Alternatively, or in addition, the
glucose level control
system 510 may store the insulin dose control signal and/or the indication of
the quantity of
insulin at a remote data store. This remote data store may be a local
computing system with
which the glucose level control system 510 may communicate (e.g., a laptop,
desktop,
smartphone, or other computing device of the subject 512 or a user). The
glucose control
system 510 may provide the insulin dose control signal data or the indication
of the quantity
of insulin to the local computing system via Bluetoothe or other near field
communication
services, or via a local network. Alternatively, or in addition, the remote
data store may be a
remote computing system that the glucose level control system 510 may
communicate with
over a wide area network, such as a wireless area network, a cellular network
using IoT based
communication technology, cellular communication technology, or any other
communication
network. In some cases, the wide area network may include the Internet. The
glucose level
control system 510 may include a wireless radio that enables it to communicate
with the local
or remote computing system. Further, the remote computing system may be a
computing
system of a data center or a cloud computing environment
[0151] Whether a local or remote computing
system, the glucose level control
system 510 may establish a communication channel with the computing system.
This
communication channel may be an encrypted channel. Further the communication
channel
may be a direct end-to-end connection between the glucose level control system
510 and the
computing system. Once the communication channel is established, the glucose
level control
system 510 may transmit the insulin dose control signal data or the indication
of the quantity
of insulin to the computing system.
(01521 Generally, the operations associated
with the blocks 802-806 may be
repeated multiple times throughout the course of the tracking period. For
example, in some
cases, an insulin dose control system associated with basal insulin may be
generated up to 288
times a day. Accordingly, tracking the insulin therapy may include storing
insulin does control
-47-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
signals and/or corresponding indications of quantities of insulin for a
plurality of autonomously
determined doses of insulin infused into the subject 512 throughout the
tracking period.
(01531 Generally, counter-regulatory agent
therapy includes administering a
counter-regulatory agent (e.g., glucagon) when there is a risk or occurrence
of hypoglycemia.
Usually, the counter-regulatory agent is not supplied on periodic or daily
basis. However, it
can be useful to understand the amount and frequency that counter-regulatory
agent is
administered to the subject 512. For example, it may help a healthcare worker
or user guide or
adjust care for the subject 512. Further, tracking counter-regulatory agent
use may help
determine a minimum quantity of counter-regulatory agent that should be
accessible to the
subject 512, either in a hi-hormonal pump or for use in injection therapy. In
some cases, the
block 806 may include tracking the counter-regulatory agent administered
during the tracking
period. Tracking the counter-regulatory agent therapy may include storing an
indication of
autonomously determined doses of counter-regulatory agent delivered to the
subject 512
responsive to the glucose level signal obtained at the block 802,
[0154] At block 808, the glucose level
control system 510 generates a backup
therapy protocol based at least in part on the tracked insulin therapy. The
backup therapy
protocol may be determined based on an average quantity or rate of insulin
administered to the
user over the tacking period, over different portions (e.g., breakfast, lunch,
and dinner, or
waking and sleeping hours, etc.) of the tracking period, or in response to
particular events (e.g.,
when eating, when blood glucose level exceeds a threshold level, etc.). The
backup therapy
protocol may include a backup injection protocol and/or a backup pump therapy
protocol. The
backup injection protocol may provide a user (e.g., the subject 512_, a parent
or guardian, or
other caretaker for the subject 512) with quantities of insulin that may be
administered to the
subject 512 via injection. Further, the backup injection therapy may indicate
times that the
insulin may be administered. For example, the backup injection therapy may
indicate quantities
of insulin to be administered at particular mealtimes_ Further, the backup
injection therapy may
indicate an effect that a unit of insulin may have no the subject 512 enabling
a user to calculate
how much insulin to administer to the subject 512 when a blood glucose reading
indicates that
the glucose level of the subject 512 is too high (e.g., above a desired
setpoint range).
[01551 Similar to the backup injection
therapy protocol, the backup pump therapy
protocol may provide a user (e.g., the subject 512, a parent or guardian, or
other caretaker for
-48-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
the subject 512) with quantities of insulin that may be administered to the
subject 512 via a
medicament pump. Using the backup pump therapy protocol, a user may configure
the
medicament pump to administer the quantities of insulin identified. The backup
pump therapy
protocol may be used to configure the medicament pump when access to a CGM
sensor is
unavailable (e.g., the subject 512 does not possess a CGM sensor, or the
medicament pump or
CGM sensor has a fault, etc.). Further, the backup pump therapy protocol may
be useful for
providing an initial configuration to a replacement glucose level control
system.
101561 In some cases, the backup injection
therapy protocol and the backup pump
therapy protocol may be the same. However, often at least the recommended
basal therapy
settings may differ. It is generally not practicable for insulin to be
administered to a subject
512 more than a few times a day via injection therapy. Thus, the backup
injection therapy
protocol may identify long acting insulin units or doses that may be
administered on a limited
basis (e.g., once or twice a day). However, the medicament pump may more
easily administer
insulin on a more than limited basis (e.g., every hour, every half hour, every
5 minutes, etc.).
Thus., the backup pump therapy protocol may identify a basal rate of insulin
that may be
administered once every time unit (e.g., once per hour or once per 15 minutes,
or once per five
minutes), or continuously at a particular rate (e.g., 0.5 or 0.6 units) per
time unit (e.g., per
hour). Moreover, the backup pump therapy protocol may identity different rates
for different
portions of a day (e.g., one rate each half of the day, one rate each quarter
of the day, or one
rate during typical waking hours and one rate during typical sleeping hours
for the subject,
etc.).
101571 In some cases, an initial backup
therapy protocol may be generated at the
block SOW The initial backup therapy protocol may be updated over time as
additional insulin
therapy data is obtained.
101581 Generating the backup therapy protocol
may include determining a number
of long acting insulin units based at least in part on an average total basal
insulin provided to
the subject 512 per day over the tracking period. The averaged total basal
insulin provided per
day may be included in a backup injection therapy protocol as a single dose of
long acting
insulin that is configured to help maintain the basal insulin level of the
subject 512 throughout
the day. In some cases, the averaged total basal insulin provided per day may
be included in a
-49-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
backup injection therapy protocol as multiple doses of insulin (e.g., 2 or 3
doses throughout
the day).
(01591 Alternatively, or in addition, the
basal insulin may be included in the backup
therapy protocol, such as in a backup pump therapy protocol, as a dosage rate
that may be
supplied to a pump to provide a rate of basal insulin throughout the day.
Further, in some cases,
each day of the tracking period may be divided into a plurality of sub-
periods. For example,
each day of the tracking period may be divided into two, three, four, or more
time periods, or
equal or different length. In some such cases, generating the backup therapy
protocol may
include determining an hourly basal rate for each sub-period of the plurality
of sub-periods.
This hourly basal rate may be determined by averaging the corresponding sub-
periods for each
day of the tracking period. For example, if each day of the tracking period is
divided into two
sub-periods (e.g., noon to midnight, and midnight to noon), the basal rate
supplied during the
first sub-period throughout the tracking period may be averaged and the basal
rate supplied
during the second sub-period throughout the tracking period may be averaged to
determine
two basal rates for inclusion in the backup therapy protocol. The basal rate
may be determined
on an hourly rate or based on any other time period. Alternatively, the basal
rate may be
determined based on an amount of time that a particular quantity (e.g., one
unit) of insulin is
recommended to be administered to the subject 512 as part of the backup
therapy protocol_ For
example, if the glucose level control system 510 determines that the subject
512 is receiving
one unit of insulin every 1.125 hours, the backup therapy protocol may
indicate the basal rate
to be one unit every 1.125 hours. Alternatively, or in addition, the backup
therapy protocol
may indicate a basal rate of 0.89 units per hour.
[0160] In addition, generating the backup
therapy protocol may include
determining an average correction bolus provided to the subject per day over
the tracking
period. The average correction bolus may be determined by adding the total
amount of
correction doses administered each data and dividing by the number of days in
the tracking
period. The average correction bolus may be included in the backup therapy
protocol as
guidance for the user. However, generally, the correction bolus is supplied in
response to a
determination that a subject's blood glucose level is spiking or exceeding a
threshold, and not
necessarily as a daily dose of insulin. Accordingly, the average correction
bolus may be
included as part of the backup therapy protocol to facilitate the user
understanding an amount
-50-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
of insulin that is likely to be required during an average day, which may be
useful for the user
(e.g., the subject) to determine how much insulin to have accessible to use,
for example, in
injection therapy. In some cases, one or more days, or time periods, of the
tracking period may
be omitted when determining the average correction bolus because, for example,
the one or
more days or time periods may be determined to be outliers. The outliers may
be omitted to
provide a more accurate understanding of average insulin needs or consumption.
101611 In some implementations, the glucose
level control system 510 may
determine an average change in blood glucose at least partially attributable
to a unit of insulin
provided as a correction bolus to the subject during the tracking period. In
some cases, the
glucose level control system 510 may correlate each correction bolus applied
during the
tracking period to a change in the blood glucose level of the subject 512.
101621 Generating the backup therapy protocol
may include determining, for each
mealtime of a plurality of mealtimes per day, an average mealtime bolus of
insulin provided
to the subject over the tracking period. In some cases, the average mealtime
bolus may be
determined for particular meals (e.g., breakfast, lunch, and dinner), while
other periods of food
intake (e.g., snacks or teatime) may be omitted or ignored. Further, the
average mealtime
boluses may be associated with particular meal sizes as identified by a user.
For example, the
glucose level control system 510 may determine an average mealtime bolus for a
small and a
large meal, or for a small, a medium, and a large meal. The average mealtime
bolus may be
determined by averaging an amount of insulin the glucose level control system
510 determines
should be administered to the subject 512 using a control algorithm of the
glucose level control
system 51.0 for each mealtime and identified meal size.
[0163] In some cases, the backup therapy
protocol may include data relating to the
administering of counter-regulatory agent. For example, the backup therapy
protocol may
include an indication of total counter-regulatory agent and/or daily counter-
regulatory agent
provided to the subject over the tracking period.
[01641 At block 810, the glucose level
control system 510 outputs the backup
therapy protocol. Outputting the backup therapy protocol may include
displaying the backup
therapy protocol on a display enabling a user to implement the backup therapy
protocol.
Alternatively, or in addition, outputting the backup therapy protocol may
include transmitting
the backup therapy protocol to a computing device of a user for display and/or
storage. In some
-51-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
cases, the backup therapy protocol may be stored at the glucose level control
system 510 and
may be accessed in response to a user interaction with a user interface of the
glucose level
control system 510.
(0165) In some cases, the process 800 can be
combined at least in part with the
process 900 described below. Thus, in some cases, the backup therapy protocol
may further
include a record of user modifications to one or more control parameters used
by the control
algorithm of the glucose level control system 510 to autonomously determine
doses of insulin
to be infused into or administered to the subject This record of user
modifications may include
an identity of instances of user modification to the control parameter and/or
a percentage of
times a user modified the control parameter during each day of the tracking
period and/or
during the entire tracking period.
101661 Figure 9 presents a flowchart of an
example control parameter modification
tracking process 900 in accordance with certain embodiments. The process 900
may be
performed by any system that can track user interactivity with glucose level
control system
510, and more specifically, occurrences of a user modifying a control
parameter used by the
glucose level control system 510 to help control medicament delivery to the
subject 512. For
example, the process 900 may be performed by one or more elements of the
glucose level
control system 510. In some cases, at least certain operations of the process
900 may be
performed by a separate computing system that receives indications of changes
to control
parameter settings of the glucose level control system 510 from the glucose
level control
system 510 and/or from user interaction with a user interface at the separate
computing system
prior to transmitting the modification to the glucose level control system
51Ø Although one or
more different systems may perform one or more operations of the process 900,
to simplify
discussions and not to limit the present disclosure, the process 900 is
described with respect to
particular systems.
[0167] The process 900 begins at block 902
where the glucose level control system
510 receives a glucose level of a subject 512. The block 902 can include one
or more of the
embodiments previously described with respect to the block 802.
(0168) At block 904, the glucose level
control system 510 generates an insulin dose
control signal based at least in part on the glucose level signal and a
control parameter. The
insulin dose control signal may be generated based on a control algorithm that
enables the
-52-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
glucose level control system 510 to autonomously determine doses of insulin to
be infused into
or administered to the subject to control the blood glucose level of the
subject The control
algorithm may determine the doses of insulin based at least in part on the
control parameter
The control parameter may include any parameter that can affect the operation
or output of the
control algorithm, or the operation of the glucose level control system 510,
and that is
modifiable by a user (e.g., the subject 512 or a user that is at least
partially responsible for care
of the subject 512 (e.g., a parent or guardian)). In some cases, the control
parameter may be,
or may correspond to, a target setpoint for the glucose level of the subject
512. In other cases,
the control parameter may correspond to whether the glucose level control
system 510 is to
generate the insulin dose control signal for at least a period of time. For
example, the control
parameter may relate to whether at least some operation of the glucose level
control system
510 is paused or active. The block 904 can include one or more of the
embodiments previously
described with respect to the block 804.
101691 At block 906, the glucose level
control system 510 tracks one or more user
modifications to the control parameter over a tracking period. The tracking
period may be one
day, less than a day, or it may be longer than one day (e.g., 2 days, 3 days,
a week, a month,
etc.). Further, the tracking period may include one or more periods of time as
previously
described with respect to the process 800. The user may be the subject 512 or
any other user
(e.g., a parent or guardian, or a healthcare provider) that may be permitted
to modify a control
parameter of the glucose level control system 510.
101701 The user may modify the control
parameter using a user interface that may
be generated andior output by the glucose level control system 510.
Alternatively, or in
addition, the user interface may be generated and/or output by a computing
system that can
communicate with andlor modify the control parameter at the glucose level
control system
510. For example, the computing system may be a smartphone, a sin.astwatch, a
laptop, or
desktop computer, or any other type of computing device that may be used to
configure the
glucose level control system 510_ The user interface may be output on a
touchscreen with
which the user may interface to modify the control parameter. The user may
interact with a
control parameter selection element or other user interface element to select
and/or modify the
control parameter. In some rases, the user may provide the control parameter
with any value
supported by the glucose level control system 510. In other cases, the user
may be limited to
-53-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
selecting particular values for the control parameter, which may be less than
the supported
capability of the glucose level control system 510 or less than what other
users are permitted
to select For example, a clinician may be granted a greater modification range
than a parent
for modifying the control parameter.
(01711 Tracking the one or more user
modifications may include storing in the one
or more user modifications in a therapy log, database, or other data
structure. Further, tracking
the one or more user modifications may include tracking or storing whether
each of the user
modifications comprises an increase or a decrease in the control parameter.
The determination
of whether the control parameter has been increased or decreased may be
determined based on
whether a value for the control parameter has been increased or decreased
relative to a
reference value. The reference value may include a current value of the
control parameter, a
default value, a clinical value supplied to the glucose level control system
510, and/or a value
determined by the glucose level control system 510. Further, tracking the one
or more user
modifications may include storing a time and/or one or more conditions under
which the
control parameter is modified. For example, the glucose level control system
510 may store a
time of day, an activity level of the subject 512 as determined from one or
more physiological
sensors and/or as identified by a user, a meal being consumed or not consumed,
and the like.
Moreover, tracking the insulin therapy may include storing an indication of
the autonomously
determined doses of insulin delivered or administered to the subject 512.
[0172] In some cases, the tracking period may
be divided into a plurality of sub-
periods. The sub-periods may correspond to different portions of a day within
the tracking
period. For example, each day of the tracking period may be divided into two
equal halves
corresponding roughly to day and night, or into 3 or 4 different periods
corresponding to a
particular number of hours in the day. The sub-periods may be of equal or
unequal length.
Tracking the one or more user modifications may include tracking the
occurrence of
modifications to the control parameter within the sub-periods of the tracking
period. Further,
the occurrence of modifications within a sub-period of a day within the
tracking period may
be combined with the occurrence of modifications within a corresponding sub-
period of
another day within the tracking period. In other words, each occurrence of a
modification of a
control parameter in a sub-period defined from 9:00-21:00 may be aggregated
across days of
the tracking period.
-54-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
101731 In some cases, a different reference
value may be determined for the control
parameter for each sub-period. In some such cases, tracking the one or more
user modifications
may include tracking modifications to the control parameter value with respect
to the reference
value for the sub-period.
(01741 At block 908, the glucose level
control system 510 generates a report of user
modifications to the control parameter. Alternatively, or in addition, the
repot may be
generated by another computing system, such as a cloud computing system or a
computing
system of a healthcare provider based on data (e.g., occurrences of user
modification of the
control parameter value) received from the glucose level control system 510.
101751 The report may include a measure of
frequency of increases and decreases
from the stored control parameter value. Further, the report may indicate a
number of times
that operation of one or more features of the glucose level control system 510
has been paused
or suspended, or a percentage of the tracking period that operation of one or
more features of
the glucose level control system 510 has been paused or suspended, Moreover,
the report may
indicate a magnitude of the modification to each control parameter for each
occurrence, in
total, and/or on average. In some cases, the report may indicate a percentage
of user
modifications that are higher or lower than the reference value over the
tracking period.
Further, cases where the tracking period, or each day of the tracking period,
is divided into a
sub-period, the report may include a measure of frequency of increases and
decreases from a
reference value for the control parameter for each sub-period of the tracking
period. In some
cases, the report may include an identity of user activity that occurred when,
or within a
threshold time period, of a user modification to a value of the control
parameter. For example,
the report may identify whether a user was exercising (e.g., swimming,
running, dancing, etc.)
when a user modification to the control parameter value was made.
101761 In some embodiments, the block 908 may
include storing the generated
report at the glucose level control system 510 (e.g., in the memory 540)
andlor at a storage of
another computing device. In some cases, the computing device may be a
computing device of
the subject 512 (or parent or guardian). Further, the computing device can be
a computing
device of a healthcare provider. In some cases, the computing device may be a
computing
device of a cloud computing service.
-55-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
101771 The report may be obtained from the
glucose level control system 510 by a
wired connection (e.g., a USB cable). Alternatively, or in addition, the
report may be obtained
via a wireless connection to the glucose level control system 510_ For
example, the glucose
level control system 510 may establish an encrypted connection to a computing
system of a
healthcare provider, which may receive the report from the glucose level
control system 510.
Alternatively, or in addition, the glucose level control system 510 may
establish an encrypted
communication channel with a cloud computing provider, which can receive the
report from
the glucose level control system 510. This report may then be accessed by any
authorized users.
101781 Advantageously, in certain
embodiments, a healthcare provider can use the
report to help manage care of the subject 512. For example, if the healthcare
provider
determines that a user is modifying the control parameter more than a
threshold number of
times or during particular time periods, the healthcare provider may use this
information to
modify the care being provided to the subject 512 and/or to educate the
subject 512 on optimal
care. For example, the rate of therapy may need to be modified or the amount
of insulin may
be too low for the subject's comfort. For example, in some cases, a subject
512 may have a
different tolerance to a blood glucose level than the average user leading the
user to modify a
setpoint range. Understanding this information can help the healthcare
provider manage care
of the subject 512 (e.g., adjusting the initial setpoint range, or modifying a
type of insulin
prescribed).
[0179] Further, as indicated above, the
process 900 may be combined with the
process 800. in other words, a report may be generated that includes both
backup therapy
protocols and a record of the number of times a user may a modification to one
or more control
parameters of the glucose level control system 510. In other cases, the
processes 800 and 900
may be triggered and/or performed independently.
Example Backup Therapy Reports
101801 Figures 10-12 illustrate one non-
limiting example of a backup therapy
report, or a set of reports, that may be generated using one or more of the
embodiments
disclosed herein. In other words, the reports of Figures 10-12 may be portions
of a single report
generated by the glucose level control system 510, or may be separate reports
that are
concurrently generated or that are generated based on different data and/or
over different
-56-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
tracking periods. The report may be generated by the automated blood glucose
control system
510, or by another computing system that may receive therapy data from the
automated blood
glucose control system. Further. Figures 10-12 represent just one non-limiting
example of a
report or set of reports that may be generated. It is possible for other
reports to be generated
that include more or less data. For example, the backup injection therapy
protocol and the
backup pump therapy protocol illustrated in Figure 10 may be separated into
two separate
reports that may be separately generated and/or accessed.
101811 Figure 10 illustrates an example
backup therapy protocol report 1000 in
accordance with certain embodiments. The amount of insulin recommended under
different
ties and/or conditions may be displayed in units. In some cases, the report
1000 may identifr
the quantity of insulin included in a unit andlor the type of insulin.
Further, in some cases, the
report 1000 may be an interactive report that enables a user to modify a type
of insulin or a
unit size of insulin. In some such eases, the table 1002 may update the
recommended number
of units of insulin to administer under particular times or conditions based
on the type of insulin
and or unit size of insulin selected.
101821 The report 1000 may identify the
length of the tracking period 1006 used to
determine the backup therapy protocol. Further, the report 1000 may identify
the time or date
range 1008 during which the tracking period 1006 occurred. Advantageously,
knowing the
tracking period 1006 may help determine an amount of trust to place in the
recommendations
included in the backup therapy protocols. The longer the tracking period, the
more likely that
the recommendations are accurate. A shorter tracking period is more
susceptible to less
accurate recommendations because, for example, the tracking period may
encompass more
days that are outliers for the subject's typical condition or activity level.
For example, a
tracking period of one day that occurs on a day when a subject consumed larger
than normal
meals or exercised significantly more than normal may result in backup therapy
recommendations that do not match the subject's typical lifestyle. Further,
knowing when the
tracking period occurred may be useful to determine how current the
recommendations are and
whether they are a reliable indicator of an amount of insulin a subject should
administer. For
example, if the date range 1008 of the tracking period 1006 is a year old, and
the subject has
gained or lost significant weight over the year, the backup therapy protocol
may no longer be
-57-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
a reliable indication of recommended injection therapy. In such cases, a user
may adjust the
recommendation and/or trigger a new occurrence of the process 800.
(01831 The table 1002 illustrates an example
backup injection therapy protocol,
which may indicate various insulin doses that may be administered to the
subject 512 at various
times or under various conditions using injection therapy. The table 1002
identifies an amount
of insulin the subject 512 may inject when consuming a usual-sized meal for
breakfast, lunch,
or dinner. The usual-sized meal may refer to the size of a meal that the
particular subject 512
usually consumes or has been advised to consume by a healthcare provider. The
units of insulin
specified may refer to an amount of insulin that the automated blood glucose
control system
510 provides the subject 512 on average when the user consumes the identified
usual size meal.
In some cases, the table 1002 may further include recommended insulin doses
for different size
meals. For example, each breakfast may illustrate three different values
(e.g., 5 units, 6 units,
and 8 units) corresponding to light or smaller than usual breakfast, usual
size breakfast, and
heavy or lamer than usual size breakfast.
[0184] It should be understood that the
amount of insulin delivered may vary over
time and/or based on the condition of the patient at a particular time. Thus,
as indicated at the
top of the report 1000, the recommendations in the backup therapy protocols
are suggested for
temporary use for a particular quantity of time (e.g., up to 72 hours in the
illustrated example).
The quantity of time for which the recommendations are valid may vary based on
the subject
512, the amount of historical data collected (e.g., the size of the tracking
period), the amount
of daily variation in the subject's blood glucose level, or any number of
other factors that may
affect the amount of time that the backup therapy protocol can be safely
followed.
[0185] As illustrated by table 1002, the
backup injection therapy protocol may
further identify an amount of long-lasting insulin a subject 512 is
recommended to administer
each day (or at certain times throughout the day). This long-lasting insulin
may be used in
place of the basal insulin that the glucose level control system 510 may
provide on a periodic
basis.
(01861 In addition, the table 1002 identifies
the reduction in glucose level
attributable to one unit of insulin. For example, as illustrated in the table
1002, the automated
blood glucose control system 510 has determined that one unit of insulin
(e.g., 1/ 100th of a
milliliter of insulin) may reduce a subject's 512 blood glucose level by 9
mg/dL. Accordingly,
-58-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
a user implementing injection therapy may measure a subject's 512 blood
glucose level,
determine a difference between the measured blood glucose level and a desired
setpoint or
threshold glucose level, and divide the difference by 9 to determine a number
of units of insulin
to inject in response to a determination that a correction dose is warranted
(e.g., that blood
glucose is outside of a desired setpoint range).
101871 The table 1004 of the report 1000
provides an example of a backup pump
therapy protocol. As illustrated, the backup pump therapy protocol may have
the same therapy
information as the backup injection therapy protocol for mealtimes and for the
correction
factor. However, because a pump may be capable of providing periodic basal
therapy, the long
acting insulin units of the injection therapy may be replaced with a basal
rate indicating a rate
at which the backup or replacement pump should administer insulin to the
subject As
illustrated, the basal rate may vary over time. In the illustrated example, a
basal rate is supplied
for four different time periods constituting a 24-hour day. However, the basal
rate may be
divided into a fewer (e.g.,. 2 twelve-hour blocks) or greater (e, 2., ev-
er3,,, four hours) number of
periods, with each time period potentially having a different basal rate as
determined based on
the historical therapy data provided by an automated blood glucose control
system.
101881 In some cases, the report 1000 may
include additional data that may be
tracked over the tracking period. This additional data may include any data
that may facilitate
care of the subject 512 and/or maintenance of the automated glucose level
control system 510.
Some non-limiting examples of additional data that may be tracked and included
in a report
using, for example, the process 800 or 900 are illustrated in chart 1010 of
the report 1000. For
example, as illustrated in the chart 1010, the report may include the average
blood glucose
level of the subject 512 over the tracking period and/or the corresponding
estimated A 1 C
percentage. Further, the report 1000 may indicate the amount or percentage of
time that the
subject's blood glucose level is within a desired setpoint range and/or is
above the desired
setpoint range. Similarly, the report 1000 may indicate the amount or
percentage of time that
the subject's blood glucose level is below a threshold blood glucose level.
[0189] In addition, the report 1000 may
indicate the average number of meal
announcements per day. As illustrated in the chart 1010, the subject 512 from
which the
example report 1000 was generated made an average of 4_2 meal announcements
indicating
that on average, the subject consumed more than 3 meals a day. In some cases,
the report may
-59-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
further indicate the types of meals announced (e.g., two breakfasts, one
lunch, and one dinner).
The second breakfast may be a large snack that is roughly equivalent in size
to a small breakfast
for the subject. Thus, the subject may have made an additional breakfast meal
announcement.
In some cases, the automated glucose level control system 510 may support a
separate snack
or other meal announcement option.
(01901 The report 1000 may further include
the total amount of insulin
administered to the subject per day, and/or the total amount of counter-
regulatory agent (e.g.,
glucagon) administered to the subject per day. In addition, the report 1000
may indicate the
amount of percentage of time that the automated glucose level control system
510 is able to
connect or communicate with the CGM sensor over the tracking period, which may
correspond
to the amount of time that the automated glucose level control system 510
functions in an
online mode during the tracking period.
101911 Figure 11 illustrates an example
control parameter modification report 1100
in accordance with certain embodiments. As previously stated, the report 1100
may be a
separate report generated using, for example, the process 900. Or the report
1100 may be
included as a second within the report 1000,
101921 The report 1100 may generally provide
an indication of the number or
percentage of times that a user modified one or more control parameters of the
automated
glucose level control system 510 during a tracking period. Further, as with
the report 1000, the
report 1100 may identify the time or date range 1008 during which the tracking
period 1006
occurred. in some cases, a user may interact with the report 1100 to determine
the number of
percentage of times that the user modified one or more control parameters
during a subset of
the tracking period. Similarly, the user may filter or narrow the date range
to view other data
described herein for a subset (e.g., a selected data range) of the tracking
period.
101931 The report 1100 may include a graph
1102 that illustrates the subject's
blood glucose level with respect to the desired target setpoint range over the
course of a day
during the tracking period. This day can be an average of the values obtained
for each day over
the tracking period, or it can illustrate a particular selected day.
(0194) Further, the report 1100 may include a
table 1104 that indicates the
percentage of times that a user modified the blood glucose target during
specific time periods.
In the table 1102 of the non-limiting example report 1100 indicates two time-
periods, daytime
-60-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
and nighttime. However, it should be understood that the table 1104 may
indicate fewer or
more time periods. Further, the time periods may indicate specific times
(e.g., from 9:00 to
21:00 and from 21:00 to 9:00) for the time periods.
(0195) As illustrated, the table 1104 may
indicate the percentage of times that a
user increased or decreased glucose target setpoints. In addition, the report
may indicate the
percentage of times that the user did not modify, or left as usual, the
glucose target setpoint.
This target setpoint indicated in the table 1104 may refer to a single target
value (e.g., 110
mg/dL, 125 mg/dL, 130 mg/dL, etc.), or may refer to a target setpoint range
(e.g., 70-180
mg/dL).
10196) In addition, the report 1100 may
indicate the number of times that a user set
a temporary glucose target during the tracking period (the temporary target
count 1106) or a
selected data range. The report may also indicate a number of times that the
user paused therapy
during the tracking period (e.g., the paused insulin therapy count 1108)
and/or the selected date
range.
[0197] The blood glucose of a subject may be
affected by a subject's weight.
Accordingly, the subject may provide updates of weight to the automated blood
glucose control
system. In some such cases, the report may indicate a change in weight and
when the weight
parameter was modified (e.g., body weight data 1110). In some cases, the
report 1100 may be
filtered to show data before and after a weight change separately. The body
weight data may
be helpful for the healthcare provider to, for example, determine whether
weight change may
at least in part have been a basis for user modifications to target glucose
levels. Generally, the
automated glucose level control system 510 (e.g., using blood glucose
readings) will
automatically account for the effect weight changes may have on blood glucose
control.
However, the subject 512 may feel differently. The ability to collect the
modification data
relating to a user's modification of the automated glucose level control
system 510 and to
correlate the data with weight changes can assist a healthcare provider in
better treating the
subject 512 by, for example, adjusting settings of the automated glucose level
control system
510, changing insulin prescriptions, educating the subject 512, or any other
action that may
improve care of the subject 512.
101981 In some cases, the report may omit
changes to blood glucose target settings
that are below a threshold. In other words, minor changes that may be
statistical noise may be
-61-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
ignored. Further, in some eases, the report may indicate when control
parameters (e.g., at
bedtime, with respect to a particular meal, such as dinner, etc.) are
modified, In some cases,
the report may also indicate the duration of the change to the glucose target
setpoint, or other
control parameter.
[01991 Figure 12 illustrates an example meal
selection report 1200 that may be
included as part of some implementations of the control parameter modification
report 1100
of Figure 11 in accordance with certain embodiments. The report 1200 may
include a table
1202 identifying the average number of times per day that a user (e.g., the
subject 512)
announces each meal type. Typically, a user will announce a meal 0 or I times
a day. However,
in some cases, a user may announce a particular mealtime more than I time to
account, for
example, for large snacks that may be similar in size to a particular meal.
Smaller snacks often
may be handled by the control algorithm of the automated glucose level control
system 510
(e.g., by the corrective insulin controller 626) without a meal announcement.
102001 Further, the table 1202 may identify
the number of times over the tracking
period, or selected time period within the tracking period, that meals of
particular sizes are
announced by a user. For example, the table 102 may indicate the number of
times that a usual
size meal is announced, a smaller than usual size meal is announce, or a
larger than usual size
meal is announced.
Automated Blood Glucose Control Refinement
[0201] An ambulatory medical device (AMD) may
include a control system that
autonomously provides therapy to a subject, for example, based on a health
condition of a
subject (e.g., determined based on one or more measured physiological
indicators or
parameters of the subject). In some examples, the control system may determine
the therapy
time and/or the intensity of the therapy during each therapy delivery based on
one or more
measured physiological parameters (e.g., using one or more subject sensors,
such as a Cal
sensor) and according to a predictive model that may include one or more
control parameters.
In some examples, the predictive model may be used to estimate a physiological
effect of the
therapy in order to adjust the therapy delivery according to an intended
physiological effect.
It is desirable to adaptively adjust the values of the control parameters to
optimize the therapy
delivery to a subject in the presence of time varying and subject specific
factors that may
-62-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
influence the physiological effects of a therapy delivery on the subject. In
some cases, the
AMD may be an ambulatory medicament device that regulates the level of an
analyte in
subject's blood. An example of such ambulatory medicament device is an
automated blood
glucose control system (e.g., the glucose level control system 510) that may
automatically
provide insulin and/or a counter-regulatory agent (e.g., Glucagon) to a
subject 512 to help
control the blood glucose level (BGL) of the subject 512_ Generally, a control
algorithm may
be implemented by the automated blood level glucose control system 510 to
determine when
to deliver insulin and/or how much insulin to provide to the subject 512.
Further, the control
algorithm may control both an ongoing or periodic delivery of insulin (e.g., a
basal dose), and
a correction bolus that may be provided to adjust a subject's blood glucose
level to within a
desired range. The control algorithm may use blood glucose level readings
obtained from a
subject sensor (e.g., a sensor measuring one or more physiological parameters
of the subject in
real time), such as a continuous glucose monitoring (CGM) sensor, that obtains
automated
blood glucose measurements from the subject Moreover, in some cases, the
control algorithm
may deliver a bolus of insulin in response to an indication of a meal to be
consumed or being
consumed by the subject 512.
102021 Insulin may be administered
subcutaneously into blood of a subject 512.
For example, the glucose control system may subcutaneously deliver a
medicament (e.g.,
glucagon) via an infusion set connected to a site on subject's body. There is
often a
delay, referred to as pharmacokinetic (PK) delay, between when the insulin is
provided and
when the amount of insulin in the subject's blood plasma reaches a particular
concentration
level, such as maximum concentration. Th is amount of time may vary based on
the type of
insulin and/or on the physiology of the particular subject For example, with a
fast-acting
insulin, it may take approximately 65 minutes for a bolus of insulin to reach
maximum
concentration in the blood plasma of one subject, but 60, 64, or 70 minutes
for another subject.
For some other types of insulin, it may take anywhere from 3-5 hours to reach
maximum
concentration in the blood plasma of the subject. Additionally, there might be
a delay, referred
to as pharmacodynamic (PD) delay, between variation of the amount of insulin
in the subject's
blood plasma and the resulting variation of glucose level in the subject's
blood. In some
examples, the value of phamiacodynamic (PD) delay may be used to estimate BGL,
based on
an estimated concertation of insulin in patient's blood.
-63-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102031 In some eases, the blood glucose
control system may implement a predictive
algorithm based on a pharmacokinetic (PK) model to estimate the accumulation
of insulin in
the blood plasma of the subject overtime, following the subcutaneous
administration of insulin
to a subject. In some examples, the PK delay may be subject specific and/or
change overtime.
Accordingly, in these examples, the PK model may include one or more
parameters, referred
to as control parameters, that may be subject specific and/or change overtime.
Examples of
factors and parameters that may influence the PK delay and/or the control
parameters of the
PK model may include, type of insulin, blood glucose level (e.g., at the
insulin administration
time), physiological characteristics of the subject, health condition of the
subject, one or more
physiological parameters of the subject time of the administration, location
at which the
infusion set is placed, the amount of insulin administered and the like. The
physiological
characteristics may include characteristics shared among large portions of the
population (e.g.,
weight, gender, age, etc.) as well as characteristics that may be unique or
specific to the subject,
or shared among few people (e.g., characteristics related to genetics).
Differences between the
physiologies of different subjects may result in differences in the optimal
blood glucose range
for each subject, or some subset of subjects. Further, the differences in
physiologies may also
affect the absorption of insulin into the blood plasma. In other words,
different physiologies of
different subjects may result in insulin absorption taking different amounts
of time for different
subjects. Thus, while the maximum concentration of glucose in blood plasma may
occur 65
minutes after delivery of a bolus of fast-acting insulin for one subject, it
may be 60 minutes or
70 minutes for another subject.
102041 Accordingly, in some such examples,
the blood glucose level control system
510 (e.g., the blood glucose control system of an AMD) may implement a method
to adaptively
change the one or more control parameters in of the PK model used in its
control algorithm to
modify its predictions, in order to maintain the BGL within a desired range.
For example, the
blood glucose control system may use readings from one or more subject sensors
(e.g., a CGM)
and/or information received from the subject (e.g., using a user interface of
the AMD), to
modify one or more control parameters.
(0205) As indicated above, a blood glucose
system, such as an automated blood
glucose level control system 510, may control delivery or administering of
insulin, or a
counter-regulatory agent, based on a PK model and one or more blood glucose
level
-64-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
measurements of the subject. In some examples, the PK model can be a bi-
exponential PK
model that may be used to estimate or determine the absorption Of accumulation
of
subcutaneously administered insulin into blood and/or a decay rate of the
insulin level in the
subject's blood for a given value of delivered dose of insulin. In some
examples, the absorption
of insulin over time according to a bi-exponential PK model may be represented
by the
following equation:
p(t) KU0(e-alt ¨ r2t)
(2)
where Uo is the subcutaneous dose in units (U), K is a scaling constant, and
ai and a2 are time
constants that may be used as the control parameters of the model. In some
examples, the peak
time of absorption of insulin, starting from the time that subcutaneous dose
(Uo) is
administered, may be referred to as Tmax and can be determined based on the
following
equation:
log log ¨I¨
(3)
(a2-a1)
In some examples, ai and ta2 can be related (e.g., through an equation such as
az = 1.5 ai or
any other linear or nonlinear mathematical relations). In some such examples,
Tmax alone may
be used as the control parameter of the hi-exponential PK model, In some
cases. Tmax may be
referred to the time at which the concentration of insulin in subject's blood
reaches a maximum
level (et_ g., starting from the time that subcutaneous dose is administered).
In some other
examples, the hi-exponential PK model may be used to estimate or determine the
accumulation
of counter-regulatory agent or hormone (e.g., glucagon) in subject's blood.
Equation 2 may be
used to calculate the pending effect of the accumulated amount of insulin in
the subcutaneously
administered dose, as that can be taken to be the difference between the total
area
, co
( to
p(Odt, which can describe a
measure of the total amount of hormone (e.g., insulin)
that can be absorbed due to a dose Uo) and so p(Odt, which can represent a
measure of the
expended portion of Uo at time.
102061
Often, the blood glucose
control system is configured to maintain a
subject's blood glucose within a particular range (e.g., a normal range). As
blood glucose rises
or falls, the blood glucose control system may administer particular amounts
of insulin or
counter-regulatory agent to the subject to bring the blood glucose level of
the subject back to
within a desired range or closer to a desired setpoint. As explained above, it
may take some
-65-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
non-infinitesimal amount of time for the medicament to be absorbed into the
subject's blood
stream. Thus, a PK model (e.g., the bi-exponential PK model), may be used to
determine how
much insulin or counter-regulatory agent should be provided to the subject in
order to maintain
the subject's blood glucose within a particular range. In some examples, the
PK model (e.g.,
the bi-exponential PK model) may be used to predict the concentration of
insulin blood glucose
level of the subject over time as insulin or counter-regulatory agent is
administered. In some
cases, the control parameter values of the PK model may be set by a healthcare
provider based
on default values obtained through clinical trials andlor based an
individualized treatment plan
for the subject as may be determined based on clinical tests of the subject
andlor on the
healthcare provider's evaluation of the subject, which may be determined based
on tests of the
subject.
102071 However, as previously indicated, the
pharmacokinetic delay and the
control parameters of the PK model, may be subject specific and/or change
overtime due to
various factors. Thus, although clinical data may determine optimal or
recommended values
of the control parameters for an average subject through one or more trials,
the determined data
may not be optimal for a particular subject. Moreover, individualized
treatment plans are
typically based on point-in-time measurements. These point-in-time
measurements may
provide a good guideline for treatment, but the optimal values of the control
parameters for a
subject may vary at different times of day, due to different activities, due
to changes in the
subject over his or her lifetime, or for any other number of reasons.
[0208] The glucose level control system 510
of the present disclosure can
implement a method or process to autonomously and/or automatically modify one
or more
control parameters of a control algorithm, or the model used by the control
algorithm, to
modify therapy provided to the subject using the glucose level control system
510. The method
may be performed by a hardware processor 530 and/or a controller 518 that
controls the
administering of therapy. The system can provide therapy (e.g., insulin) to a
subject in response
to a determination of a blood glucose level of the subject The blood glucose
level may be
determined based at least in part on a glucose level signal obtained from a
glucose level sensor
that is operatively connected to a subject. The determination of the therapy
(e.g., an amount of
insulin or counter-regulatory agent) may be based at least in part on the
blood glucose level
and/or the bi-exponential model. Moreover, the determination of therapy may be
based at least
-66-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
in part on a value or setting of one or more control parameters of the blood
glucose control
system. The one or more control parameters may be, Of may correspond to, one
or more
parameters of the bi-exponential PK model, or any other model or control
algorithm used to
control the administering of therapy to the subject.
[02091 As mentioned above, the system 510 may
provide the therapy based on the
value or setting of the one or more control parameters_ The value or setting
of the one or more
control parameters may be based on an initial configuration of the blood
glucose control system
510 by a healthcare provider, subject, or other user. Further, the initial
configuration may be
based on clinical data or data obtained that is specific to the subject. In
some cases, a control
parameter may be a time constant used by a control algorithm of the blood
glucose control
system (e.g., Tmax in a bi-exponential PK model). This time constant may be
used in a
calculation of an accumulation of insulin in the subject by the control
algorithm. Further, the
control parameter may be used to control an insulin dosing response of the
control algorithm
to a blood glucose excursion in the subject as indicated by a glucose level
signal_ obtained from
a glucose level sensor. In some cases, the control parameter may be, or may be
related to, Tmax
(e.g., defined by equation 2). For example, the control parameter may be an
estimate of Tmax
or a fraction (e.g., 0.5) of Tmax. As previously explained, Tmax may be the
peak time of
absorption of insulin, or the amount of time until the concentration of
insulin from an insulin
dose reaches maximum concentration in the blood of the subject.
[0210] Moreover, the control parameter may be
associated with a setpoint or target
blood glucose level, or a blood glucose range. For example, the control
parameter could relate
to a point in time when an estimated amount of "insulin on board" (e.g., an
amount of insulin
in the subject as determined by a model of insulin accumulation andlor
utilization in the
subject) falls below a threshold value. As another example, the control
parameter can be a
clearance time for insulin boluses (e.g., an estimate of an amount of time for
an administered
bolus of insulin to be utilized by the subject). In some cases, the control
parameter may relate
to T1/2, which corresponds to a time when the concentration of insulin in the
blood plasma
reaches half of the maximum concentration in the blood plasma. In some cases,
the control
parameter may be a parameter that can be used to calculate Tmax or T1/2.
102111 In some examples, the system 510 may
determine an effect of the supplied
therapy (herein referred to as therapy effect or effect). For example, the
therapy effect may be
-67-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
determined by analyzing a glyoemic control of blood glucose (e.g., variation
of BGL or
supplied therapy over a measurement period) in the subject's blood as
indicated by the glucose
level signal received from the glucose sensor (e.g., a CGNI sensor). In some
cases, the control
system may measure or determine the effect of the supplied therapy over time.
In some such
cases, the therapy effect may be determined based on variation of BGL and/or
the amount of
therapy delivered over time. Moreover, in some cases, the system may continue
to supply
therapy to the subject over several therapy delivery times or instances and
may average or
otherwise aggregate the measured or determined effects of the therapy over the
several therapy
delivery times or instances. In some other examples, the system 510 may
determine the therapy
effect based at least in part on an input received from the subject. The input
received from the
subject may include a subjective or objective effect The input received from
the subject may
include manual blood glucose level measurements obtained using, for example,
test strips.
Another example of input may be an indication of light-headedness, difficulty
breathing,
headaches, or any other objective or subjective effect identified by the
subject.
[0212] Based at least in part on the provided
therapy and the measured or
determined effects of the therapy (e.g., the changes in blood glucose level
attributed to the
therapy), the control system 510 may autonomously determine a modification to
one or more
control parameters. For example, the control system may modify Tmax value used
by the
control algorithm (or the PK model used in the control algorithm), for
example, to improve the
effect of a subsequent therapy that may be provided to the subject As such,
the directional
modification (e.g., increase or decrease) of a control parameter value may
depend on the
measured or determined effect of the therapy provided based on the initial or
prior value of a
control parameter. Moreover, the directional modification of the control
parameter value may
depend on a difference between the determined or measured effect of the blood
glucose therapy
and an expected effect of the blood glucose therapy (e.g., calculated based on
PK model). In
some examples, the directional modification of a control parameter may be
determined based
on the amount of therapy doses provided and/or measured BGL of the subject,
during and
between one or more previous therapy deliveries.
(0213) In some examples, the pharmacodynamic
delay for a subject may be a
known value. In these examples, the amount of absorbed insulin in the
subject's blood may
be estimated based on the measured value of BGL received from a glucose
sensor. In some
-68-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
such examples, the directional modification may depend on the difference
between calculated
value of absorbed insulin based on a PK model (e.g., bi-exponential PK model)
with a selected
value of Tmax, and the estimated value of the absorbed insulin based on the
measured value
of BGL received from a glucose sensor.
[0214] Using the modified control parameter,
the system 510 may determine
therapy to deliver to the subject 512 at a therapy delivery time. As with the
initial control
parameter, therapy may be delivered during one or more therapy delivery times
based on the
modified control parameter The system may determine the effect of the therapy
delivered
based on the modified control parameter using one or more of the embodiments
previously
described with respect to the therapy delivered using the initial control
parameter.
(02151 In some examples, the control system
can compare the measured,
determined or reported effects (e.g., physiological effects) from the therapy
delivered using the
initial value of a control parameter and those from the therapy delivered
using the modified
value of the control parameter. Based on the comparison, the control system
may determine
which values of the control parameter is preferable for the subject. In some
examples, the
comparison may be performed in real-time, or substantially in real-time.
Further, the
comparison may be performed by the system 510 without user interaction. The
comparison
may be performed using a comparison method and based on one or more comparison
criteria.
(02161 The comparison method may be based on
finite number of therapy effects
determined or measured at discrete times or based on continuous temporal
variations of an
effect over a period. In some examples the comparison method may involve
statistical analysis
of the measured or determined effects resulting from usage of the initial
value and modified
value of the control parameter. The comparison criterion may be based on the
effects or based
on the temporal variations of the effects over a period. For example, the
preferable control
parameter value can be a value that causes the blood glucose level of the
subject to stay within
a desired range or closer to a setpoint level for the subject Accordingly, the
system can set or
maintain the control parameter to have the value that generated blood glucose
levels that are
closer to the desired range or setpoint for the subject for subsequent
therapy.
(0217) In some cases, the system 510 may
repeat the process for different control
parameter values enabling the system to refine the blood glucose control for
the subject over
time. In subsequent performances of the process, the initial control parameter
value may not
-69-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
be an initial value but may be the most recent selected value for the control
parameter based
on the determined effects of the control parameter_
102181 In some cases, the determination of a
second or modified value for a control
parameter, or the modification of the control parameter may be triggered based
on a glucose
level of the subject not satisfying a threshold. Alternatively, or in
addition, a process of
modifying a control parameter value may be triggered based on a difference
between an
expected glucose value of a subject and an expected glucose value of a subject
after the
administering of therapy exceeding a threshold.
102191 Using the embodiments described
herein, the value of a control parameter
may be autonomously modified without interaction by a subject or user with the
blood glucose
control system. In other words, the blood glucose control system can
automatically adjust
and/or refine a control parameter used by a control algorithm for glycemie
control of the
subject
102201 As previously described, the blood
glucose control system may provide
both insulin therapy and counter-regulatory agent therapy to a subject In some
cases, the blood
glucose control system may only provide insulin therapy. In some such cases,
the blood
glucose control system may output an indication of an amount of counter-
regulatory agent that
may or should be administered to the subject based on a detected condition of
the subject
[02211 The active control parameter value
used by the control parameter may
remain active until a subsequent occurrence of the therapy modification
process. In some cases,
performance of the therapy modification process is continuously performed with
the control
parameter value being modified based at least in part on a determined effect
of the prior control
parameter value. In other cases, the therapy modification process is performed
until the
determined effect of the therapy satisfies a desired threshold (e.g., when the
detected blood
glucose level is within a threshold of a setpoint or median setpoint value).
In some cases, the
therapy modification process is performed a set amount of times and the
control parameter
value that provides the best outcome (e.g., closes to desired blood glucose
level) is set as the
active control parameter for subsequent therapy. In some cases, providing
therapy at different
sites on the subject's body (e.g., back, stomach, leg, or arm) may result in
different blood
glucose absorption rates (associated with different PK delays). Thus, in some
such cases, the
-70-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
therapy modification process may be performed each time the infusion set used
to deliver the
therapy is moved to a different site on the subject
Example Automated Blood Glucose Control Refinement Process
[02221 Figure 13 presents a flowchart of an
example automated blood glucose
control refinement process in accordance with certain embodiments_ The process
1300 may be
performed by any system that can autonomously and/or automatically modify a
control
algorithm and/or a control parameter that affects execution of the control
algorithm based on
feedback (e.g., from a blood glucose signal) relating to therapy administered
to a subject 512.
For example, the process 1300 may be performed by one or more elements of the
glucose level
control system 510. In some cases, at least certain operations of the process
1300 may be
performed by a separate computing system that receives blood glucose data from
the glucose
level control system 510. Although one or more different systems may perform
one or more
operations of the process 1300õ to simplify discussions and not to limit the
present disclosure,
the process 1300 is described with respect to particular systems.
102231 The process 1300 may be performed
automatically and without user
interaction. In some cases, a user may trigger the process 1300 via a command
or interaction
with a user interface. However, once the process 1300 is triggered, the
process 1300 may be
performed automatically. Further, the process 1300 may be performed
continuously,
periodically, or in response to a trigger. The trigger may be time based
and/or based on a
measurement of the glucose level of the subject. For example, the trigger may
correspond to a
determination that a glucose level of a subject differs by more than a
threshold from a predicted
glucose level that is predicted by a glucose level control algorithm based on
the administering
of medicament. Further, the trigger may be based on the activation or first
time use of the
glucose level control system 510 by the subject 512.
[0224] The process 1300 begins at block 1302
where the glucose level control
system 510 receives a glucose level signal corresponding to the glucose level
of a subject 512.
The glucose level signal may be received from a glucose sensor capable of
measuring the level
of glucose in the blood of the subject. For example, the sensor may be a
continuous glucose
monitoring (CGM) sensor. The block 1302 can include one or more of the
embodiments
previously described with respect to the block 802 or 902.
-71-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102251 At block 1304, the glucose level
control system 510 provides a first therapy
during a first therapy period to the subject 512. The first therapy may be
based at least in part
on the glucose level signal and a first value of' a control parameter. The
control parameter may
include any control parameter that affects operation of the glucose level
control system 510
andlor performance of a control algorithm of the glucose level control system
510. The control
algorithm may include any control algorithm used to determine a dose of
medicament (a g.
insulin) to administer to the subject 512. In other words, the controller 518
or the processor
530 may use the control algorithm to generate a dose control signal based at
least in part on a
value (e.g., the first value of the block 1304) of the control parameter to
cause the delivery
device 514 to administer a dose of insulin or other medicament
[0226] In some cases, the control algorithm
may be based on the PK model
(equation 2). Further, in some cases, the control parameter may be Tmax, which
may be
calculated using equation 3. In other rases, the control parameter may be Tu2,
which may relate
to the amount of time for the dose of insulin in the blood stream to drop to
',12 of the maximum
concentration in the blood attributable to the dose administered to the
subject 512. In some
cases, the control parameter corresponds to a time until insulin within blood
plasma of the
subject reaches a particular concentration level subsequent to administration
of an insulin dose.
Moreover, in sonic cases, the control parameter may be a parameter that
affects the
determination of Tmax, such as one or more of the time constants al and a2. In
some
implementations, the control parameter may be used by the control algorithm to
account for
and/or determine an accumulation of insulin (or other medicament) in the
subject 512 andlor a
rate of diminishment of the insulin (or other medicament) in the subject 512.
In some cases,
the control parameter may be used to control an insulin dosing response of the
control
algorithm to a blood glucose excursion in the subject as indicated by the
glucose level signal
received at the block 1302.
[0227] In some instances, the control
parameter may relate to at least one time
constant used in a calculation of an accumulation of insulin in the subject by
the control
algorithm, such as one or more of the time constants al and otz that may be
used in the
calculation of Tmax. In some cases, the control parameter may correspond to a
rate of insulin
diminishment in the subject 512. In some cases, the control parameter may
relate to a target
-72-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
setpoint or a target setpoint range for maintaining or attempting to maintain
the subject's 512
blood glucose level.
(02281 The first therapy may correspond to a
single administering of insulin to the
subject 512. This single administering of insulin may be any type of insulin
administered for
any reason. For example, the insulin dose may be a basal insulin dose, a
priming dose, a dose
supplied in response to a meal announcement, or a correction dose of insulin.
Moreover, the
first therapy may be medicament other than insulin, such as counter-regulatory
agent (e.g.,
glucagon). In some cases, the first therapy may be a plurality of medicament
(e.g., insulin
and/or counter-regulatory agent) doses supplied or administered to the subject
512 over the
first therapy period_ Further, the plurality of medicament doses may include a
variety of types
of medicament doses, such as one or more basal doses, one or more meal doses
associated with
one or more meal announcements, one or more corrective doses, etc.
102291 The first therapy period may be a time
period that corresponds to a single
medicament dose Alternatively, the first therapy period may be a time period
that encompasses
a plurality of medicament doses. Further, the time first therapy period may be
a time period
associated with a defined length of time. Alternatively, or in addition, the
first therapy period
may be defined based on a number of medicament delivery periods. In other
words, the time
period may vary based on the amount of time it takes to deliver or administer
a specified
number of doses of medicament (of any type or of a particular type).
(02301 The first value may be selected based
on a prior therapy or a prior
performance of the process 1300. In some cases, the first value is selected
based on a baseline
value. The baseline value may be associated with clinical data, or it may be
determined based
on initial operation of the glucose level control system 510 for some period
of time before
performance of the process 1300. Alternatively, or in addition, the first
value may be selected
based on clinical data or a particular prescription for the subject 512. In
some cases, the first
value may be based on clinical data for average users or average users that
share certain
physiological data with the subject 512. In some cases, the first value is
determined based on
a healthcare provider's assessment of the subject 512. Further, the first
value may be
determined based on an infusion site (e.g., back, stomach, leg, etc.) for the
glucose level control
system 510. In some cases, the first value may be selected based on
demographics or
-73-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
characteristics of the subject 512. For example, the first value may be based
on the subject's
512 gender, weight, body mass, Of age.
(02311 At block 1306, the glucose level
control system 510 determines a first effect
corresponding, or attributable, at least in part to the first therapy.
Determining the first effect
may include receiving a glucose level signal from the glucose level sensor
operatively
connected to the subject. This glucose level signal may he a subsequent or
updated glucose
reading that is more recent than the glucose level signal received at the
block 1302. The glucose
level signal received at the block 1302 may be used to determine therapy to
administer to the
subject 512 and the glucose level signal received at the block 1306 may be
used to determine
a result of the administered therapy. It should be understood that glucose
level signals may be
received continuously or periodically and can be used to both determine
therapy to administer
and to determine the effect of the administered therapy.
102321 In some cases, determining the first
effect may include analyzing glycemic
control of blood glucose in the subject as indicated by the glucose level
signal. Analyzing the
glycemic control of the blood glucose in the subject may include tracking the
blood glucose
level of the subject 512 over time. Further, analyzing the glycemic control of
the blood glucose
in the subject may include comparing the blood glucose level of the subject
512 over time to a
predicted blood glucose for the subject 512 over time as predicted based on
the PK model used
in the control algorithm using the selected value for the control parameter.
As mentioned
above, in some examples, the measured blood glucose level of the subject 512
over time may
be used to calculate the accumulation and/or diminishment of the insulin level
in subject's
blood. In these examples, analyzing the glycemic control of the blood glucose
in the subject
may include determining whether, or to what degree, the calculated
accumulation and/or
diminishment of insulin (or other medicament) using the PK model (e.g., bi-
exponential PK
model) and the control parameter values used in the control algorithm matches
the
accumulation or diminishment of insulin (or other medicament) estimated based
on the
measured blood glucose level (e.g., obtained from the CGM sensor). In some
cases, the first
effect may, at least partially, be determined by analyzing one or more signals
received from
one or more subject sensors that measure one or more physiological parameters
of the subject
(e.g., heart rate, temperature and the like).
-74-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102331 In yet other examples, the first
effect may be determined based on an input
received from the subject (e.g., using a user interface of the MAD). In some
cases, the first
effect may be determined based at least in part on an assessment or input
provided by the
subject 512 (e.g., using a user interface) with respect to the first value or
the first effect. For
example, if the subject 512 feels woozy, dizzy, lightheaded, nauseous, or
otherwise
uncomfortable during the first therapy period, the subject 512 may, via, for
example, a
touchscreen display of the AMD, indicate how the subject 512 is feeling.
102341 At block 1308, the glucose level
control system 510 obtains a second value
for the control parameter. This second value may be autonomously determined.
Further, in
some cases, the second value may be automatically determined. In some cases,
the second
value is determined based at least in part on a user triggering the blood
glucose control
refinement process 1300. In some such cases the control system may determine
the second
value and present it to the user via a user interface 534 of the control
system 510.
102351 In some other examples, the second
value may be obtained from a user
interface 534 of the blood glucose control system 510 (e.g., in response to a
user interaction
with the user interface). In some examples, the second value may be obtained
from a computing
system that is connected to or otherwise in communication with the glucose
control system.
The communication connection may be a wired or wireless connection. Further,
the wireless
connection may be a direct connection (e.g., via Bluetooth or other near-field
communication
technologies) or a connection over a network (e.g., a local area network, a
wide area network,
a cellular network, etc.).
102361 The second value may be an increase or
decrease of the control parameter
compared to the first value. The second value may be limited to a particular
maximum change
from the first value. Further, the second value may be selected based at least
in part on the first
effect. For example, if the first effect corresponding to the first value
results in blood glucose
being near an upper range of the setpoint range, the second value may be
selected in an attempt
to being the blood glucose level closer to the middle of the setpoint range.
Further, the second
value may be selected based at least in part on characteristics of the subject
512, such as age,
weight, gender, or any other characteristics that may affect blood glucose
management. In
some examples, the second value may be selected based at least in part on the
first effect
-75-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
determined based on an assessment provided by the subject 512, in an attempt
to reduce the
symptoms felt by the subject 512_
(02371 In some cases, the second value of the
control parameter may be generated
based at least in part on a baseline value of the control parameter and an
output of a function
defined based on glycemic control of the subject. In some examples, the
glvcemic control of
the subject may include the measured value of the glucose level in subject's
blood (e.g.,
provided by the CGM) and/or the amount of therapy (e.g., dose of insulin or
counter-regulatory
hormone) provided during the first therapy period. The baseline value of the
control parameter
may correspond to the first value used to provide therapy at the block 1304.
This baseline value
may be a last known optimal value for the subject prior to any changes to the
subject (e.g.,
change in weight, insulin type, or metabolism changes, etc.). Alternatively,
or in addition, the
baseline value may be a value determined by a healthcare provider. In some
cases, the second
value of the control parameter is based at least in part on glycemic control
indicated by the
glucose level signal.
[0238] In some cases, the second value may be
a modification to Tmax or Tin. It
should be understood that Tmax and/or 11,2 may, at least in part, be based on
the physiology
or biochemistry of the subject. 512. Thus, the setting of either Tmax or
Tin.for the setting of the
first value and the second value may refer to setting a parameter of the
control algorithm or the
PK model used by the control algorithm, representative of or corresponding to
Tmax and/or
Tin. For example, the setting of the first value and the second value may
include setting one
or more control parameters that may be used to determined or estimate Tmax
and/or T112 for
the subject 512. However, the set value may differ from the actual value of
Tmax and/or Tin
for the subject 512. Further, as Tmax and/or Tii2 may vary for different
subjects, it is not always
possible to explicitly set or determine Tmax and/or Tin for a subject.
Instead. Tmax and/or Tia
may be estimated or determined by comparing the effects and/or blood glucose
levels
determined for different control parameter values that correspond, at least in
part, to Tmax
and/or Tin. Using the process 1300, the control parameter may iteratively
approach the actual
Tmax and/or 111/2 for the subject 512, or within a threshold of the actual
Tmax and/or Tin for
the subject 512. Alternatively, using the process 1300, the control parameter
(such as one or
more of the time constants ai and a2) may iteratively approach a value that
corresponds to the
actual Tmax and/or T1/2 for the subject 512.
-76-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102391 At block 1310, the glucose level
control system 510 changes the control
parameter to the second value. Changing the control parameter to the second
value causes a
change in the operation or execution of the control algorithm. This change in
the execution of
the control algorithm may result in a change in one or more factors associated
with the
provisioning of therapy to the subject 512. For example, die changing in the
execution of the
control algorithm may result in a change in an amount of medicament delivered,
a timing of
the delivery of the medicament, a rate at which a dose of medicament is
delivered to the subject
512, a target setpoint or target range for the blood glucose of the subject, a
threshold used in
determining whether to deliver medicament (e.g., a threshold difference from
the target
setpoint), or any other factor that may affect therapy delivered to the
subject 511
[0240] At block 1312, the glucose level
control system 510 provides second
therapy during a second therapy period to the subject 512. The second therapy
is based at least
in part on the updated control parameter that is updated to the second value
at the block 1310.
As with the first therapy, the second therapy may refer to one or a plurality
of medicament
doses. Further, the second therapy period may refer to a specific amount of
time, an amount of
time to deliver a particular number of medicament doses, or a particular
number of medicament
doses. In some cases, the block 1312 may include one or more of the
embodiments described
with respect to the block 1304 but using the second value for the control
parameter over the
second therapy period. In some examples, the duration of the second therapy
period may be
equal to the duration of the first period. In some other examples, the number
of therapies
delivered during the second therapy period may be equal to the number of
therapies delivered
during the first second therapy period.
[0241] At block 1314, the glucose level
control system 510 determines a second
effect corresponding at least in part to the second therapy. The block 1314
may include one or
more of the embodiments described with respect to the block 1306, but with
respect to the
second therapy.
[0242] At block 1316, the glucose level
control system 510 selects one of the first
value or the second value based at least in part on a comparison of the first
effect and the
second effect. The comparison of the first effect and the second effect may be
performed
autonomously without action by a user. The glucose level control system 510
may select the
one of the first value or the second value to be a current or active value for
the control parameter
-77-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
based on whether the first effect or the second effect results in improved
care (e.g., closer to a
desired setpoint for a greater period of time, or less volatility in blood
glucose values, or any
other factor that a healthcare provider may use to evaluate the success of
diabetes management)
for the subject 512. In some cases, the glucose level control system 510
selects a third value to
the current or active value for the control parameter. The third value may be
selected based on
the comparison of the first effect and the second effect. For example, if it
is determined that
the first effect is preferable to the second effect, the third value may be
selected based on a
change to the first value in the opposite direction as the change made to the
first value to obtain
the second value. For instance, if in the prior example, where it is
determined that the first
effect is preferable to the second effect, the first value corresponded to a
Tmax of 60 minutes,
and the second value was selected to correspond to a Tmax of a longer time
period (e.g., 65 or
70 minutes), the third value may be selected to correspond to a Tmax of a
shorter time period
(e.g., 50 or 55 minutes).
102431 Comparing the first effect and the
second effect may include determining
whether the first value or the second value brought the subject's 512 glucose
level closer to a
target setpoint and/or maintained the subject's 512 glucose level within a
target range for a
longer period of time. In some cases, comparing the first effect and the
second effect may
include determining whether the first value or the second value resulted in a
more stable blood
glucose level for the subject 512 or less volatility in the blood glucose
level of the subject 512.
In some cases, comparing the first effect and the second effect may include
determining
whether the first value or the second value resulted in more and/or greater
excursions of the
subject's 512 blood glucose level from a target blood glucose range.
[0244] Comparison of the first effect and the
second effect may be performed in
real-time or substantially in real-time accounting for the processing speed of
the hardware
processor 530 or the glucose level control system 510. Thus, in some cases,
the comparison of
the first effect and the second effect may be performed upon determination of
the second effect
[02451 In some embodiments, the comparison of
the first effect and the second
effect may include a statistical comparison or statistical analysis of the
first effect and the
second effect. In some cases, the comparison of the first and second effects
may include
determining whether the second therapy produced a statistically significant
improvement in
therapy (e.g., glycemic control) compared to the first therapy. A
statistically significant
-78-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
improvement may vary depending on the subject or the condition of the subject.
The
comparison can also include a determination of whether there was a
statistically significant
increase in risk factors (e.g., hypoglycemia) during the second therapy period
compared to the
first therapy period. In some embodiments, a statistically significant
improvement may be an
improvement determined based on a first statistical analysis of a set of data
associated with the
first effect and a second statistical analysis associated with the second set
of data associated
with the second effect. For examples, the first and second statistical
analysis may include
calculating the mean and variance of the blood glucose levels measured during
the first and
second therapy periods, respectively. In some examples, an improvement may be
determined
by comparing the mean value and the variance of the blood glucose levels
measured during
the first and second therapy periods. In some examples, an improvement may be
determined
by comparing the mean value and the variance of the blood glucose levels
measured during
the first and second therapy periods with one or more reference values. The
reference values
may be values provided by a health care provider or a user and may be stored
in the memory
540 of the glucose level control system 510. In some examples, the first and
second therapy
Period may be long enough to include a plurality of therapy deliveries (e.g.,
infusion of glucose
and/or glucagon) during each period. In some embodiments, an improvement may
be
determined by comparing by other statistical quantities calculated at least in
part based on the
blood glucose levels measured during the first and second therapy periods. In
some such
embodiments the statistical quantities may be specific statistical quantities
defined for
comparing the effects of a therapy (e.g., medicament delivery for controlling
the blood glucose
level in a subject).
10246] In some cases, the first and/or second
may be output to user (e.g., the subject
or a parent) via a user interface of the glucose control system and/or a
computing system (e.g.,
a smartphone, laptop, personal computer, or the like). In some examples, the
user may use the
determined effect to adjust the value of a control parameter.
[0247] In some cases, the value that better
manages the subject's 512 blood glucose
may be output to a user (e.g., the subject or a parent). The user may then
configure the glucose
level control system 510 based on the selected control parameter value.
Alternatively, or in
addition, the glucose level control system 510 may automatically modify the
value of the
control parameter In some cases, the user may be provided with an opportunity
to confirm the
-79-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
modification. In other cases, the modification may occur automatically without
confirmation.
However, the modification may be presented to the user (e.g., the subject or a
healthcare
provider) and/or logged in a therapy log.
(0248) In some cases, the comparison is
performed by another computing system
that is in communication with the glucose level control system 510. For
example, the glucose
level control system 510 may transmit the glucose level signal, data
determined from the
glucose level signal, and/or the assessment received from the subject,
indicative of the effect
of the blood glucose control, to another computing system, such as a local
computing system,
a smartphone, or a cloud-based computing system. Further, the glucose level
control system
510 may transmit data associated with the control parameters values and the
administering of
medicament to the subject 512 to the computing system. The computing system
may determine
the value of the control parameter that better manages the subject's 512 blood
glucose level.
The computing system may configure the glucose level control system 510 with
the selected
value Alternatively, or in addition, the selected value may be output to a
user who can
configure the glucose level control system 510 with the selected value.
102491 At block 1318, the glucose level
control system 510 provides therapy to the
subject 512 based on the selected value for the control parameter that is
selected at the block
1316. The therapy provided at the block 1318 may be provided during a third
therapy period
that is at some point after the first and second therapy periods. Thus, during
the first two time
periods, the first and second values may be used, respectively, for the
control parameter to
determine the value that results in the better outcome or improved care for
the subject 512.
During subsequent time periods, the value that resulted in the better outcome
for the subject
512 may be used to provide future care for the subject 512. Alternatively, a
new value that is
neither the first or second value may be used to provide subsequent care in an
attempt to find
a value for the control parameter that may provide a better or improved level
of care
closer to a desired target glucose level for a longer period of time) for the
subject 512.
102501 In some examples, providing therapy to
the subject, may include generating
a dose control signal to a delivery devices 514 (e.g., infusion pump coupled
by catheter to a
subcutaneous space of the subject 512) that delivers an amount of a medicament
(e.g., insulin
or a counter-regulatory agent) to the subject wherein the amount may be
determined by the
dose signal.
-80-
CA 03151782 2022-3-18
WO 2021/067856
1PCT/1JS2020/054130
[0251] Providing therapy to the subject 512
based on the selected value may
include configuring the glucose level control system 510 to provide therapy to
the subject 512
during a third therapy period based at least in part on the active control
parameter value. In
some cases, configuring the glucose level control system 510 to provide
therapy to the subject
512 based at least in part on the active control parameter value may end the
process 1300.1n
other cases, the process 1300 may be repeated. Repeating the process 1300 may
include using
the selected value (e.g., the first or second value from a prior iteration of
the process 1300) as
the first value when performing the operations associated with the block 1304.
The second
value generated at the block 1308 may be a new value not used during the prior
iteration of the
process 1300.
[0252] The process 1300 may be repeated until
a difference between the first effect
and the second effect is less than a threshold difference. Alternatively, or
in addition, the
process 1300 may be repeated a particular number of iterations, periodically,
in response to a
command, or in response to determining that the subject's 512 blood glucose
does not satisfy
a particular threshold for a particular amount of time.
[02531 In some examples, the process 1300 may
be used to modify more than one
control parameters of a glucose system (or a control algorithm used by the
control system). In
some such examples, the process 1300 may be used to adjust a first control
parameter during
a first modification period starting from block 1302 and ending at block 1318,
and to adjust a
second control parameter during a second modification period again starting
from block 1302
and ending at block 1318. The second modification period may be immediately
after the first
modification period or delayed by a particular time. In some example, the
control system may
determine when a second control parameter should be modified following the
modification of
a first parameter. In some examples, the delay may be determined at least in
part based on the
measured glycemic control based on the glucose signal (e.g., received from a
CCM sensor). In
some other examples, the delay may be determined based on input received from
a user. In
some examples, the modification of the second control parameter may be at
least partially
determined based on the determined modification of the first control
parameter.
(0254) In some examples, a third control
parameter may be adjusted during a third
time period after adjusting the first and the second control parameters. The
adjustment of the
third control parameter may immediately follow the adjustment of the second
control
-81-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
parameter or may occur after a delay. The delay may be determined at least in
part based on
the glycemic control of the subject after the second control parameter is
adjusted. In some
examples, the glucose control system may be configured to sequentially adjust
the first and
second, or the first, second and third control parameters when the glycemic
control of the
subject satisfies one or more threshold conditions. In some examples, the
duration of the time
period during which a control parameter is adjusted may defer from that of the
other
parameters.
102551 In some embodiments, a modified
version of the process 1300 may be used
to determine a value (e.g., an optimal value) of a control parameter. In some
such examples,
after determining the second effect at block 1314, the control system may skip
block 1316 and
block 1318, and instead obtain a third value for the control parameter. In
some examples, this
third value may be determined at least in part based on the determined second
effect at block
1314. In some examples, this third value may be autonomously determined.
Further, in some
cases, the third value may be automatically determined. In some cases, the
third value is
determined based at least in part on a user triggering the blood glucose
control refinement
process 1300. In some such cases the control system may determine the third
value and present
it to the user via a user interface 534 of the control system 510. In some
examples, the third
value may be provided by a user via a user interface 534 of the control system
510. In some
examples, after obtaining the third value, the system may provide therapy to
the subject based
on the third value. This modified version of process 1300 may be repeated
several times. hi
some examples, this modified version may be repeated until a difference
between the last two
subsequent effects is less than a threshold difference. Alternatively, or in
addition, the modified
version of the process 1300 may be repeated a particular number of iterations,
periodically, in
response to a command, or in response to determining that the subject's 512
blood glucose
does not satisfy a particular threshold for a particular amount of time.
[0256] As described, the process 1300 may be
used to modify one or more control
parameters that affect the delivery of insulin. However, the process 1300 is
not limited as such
and may be used to modify one or more control parameters that affect the
delivery of other
medicaments, such as counter-regulatory agent (e.g., glucauon, dextrose,
etc.). In some cases,
the process 1300 may be used to recommend a change in insulin and/or counter-
regulatory
agent delivery without modifying the delivery. This can be advantageous for
generating
-82-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
recommendations regarding counter-regulatory agent in a single hormone glucose
level control
system 510 that does not support counter-regulatory agent, or that supports
the use of counter-
regulatory agent, but does not have the counter-regulatory agent available.
102571 Moreover, in cases where the process
1300 is used to modify multiple
control parameters, the at least two or more of the control parameters may be
related to each
other_ For example, if the control parameters include the time constants al
and a2, there may
be a relationship between ai and az such that modifying al may cause a
modification to a2.
For instance, CX2 may equal 1.5 times al
102581 The value for the control parameter
set as the active parameter (e.g., the first
value or the second value) at the block 1316 may be used by the control
algorithm to provide
therapy to the subject 512 for a particular period of time or until the
process 1300 is repeated.
As previously explained, in some cases, the process 1300 is repeated
periodically and/or in
response to a trigger, such as a blood glucose value or an average blood
glucose value over a
time period, or an indicate of a site change for the connection of the glucose
level control
system 510 to the subject 512 (e.g., a change in the location of the infusion
set used to provide
the subcutaneous dose).
Hypothetical Example
[02591 As previously described, the peak time
of absorption of insulin may be
referred to as Truax. Different types of insulin may result in different
amounts of time until
peak absorption into the subject's blood or for different subjects. For
example, in one
hypothetical example, the aggregate Tmax among subjects for the fast-acting
insulin lispro and
insulin aspart may be determined to be approximately 65 minutes, while the
aggregate Tmax
among subjects using ultra-fast-acting insulin, such as, for example, the
insulin aspart injection
marketed under the Fiasp brand, which has a formulation to decrease time to
peak absorption,
may be determined to be approximately 40 minutes When using an automated blood
glucose
level control system (such as the glucose level control system 510) with a
control parameter
corresponding to Tmax set to 65 minutes, there may be no statistically
significant improvement
in the average glucose level or the frequency of hypoglycemia when using the
ultra-fast-acting
insulin compared to using the fast-acting insulin. In this comparison, Tmax is
held constant
while varying the type of insulin used.
-83-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102601 When adjusting the value of the
control parameter for the automated blood
glucose level control system to use different Tmax settings, in a hypothetical
example, mean
glucose drops when Tmax is lowered when using the ultra-fast acting insulin.
In this example,
three cohorts of subjects employ control algorithms that use modified Tmax
values when using
a blood glucose control system with ultra-fast-acting insulin such as Fiasp.
The first cohort
uses a blood glucose level control system configured with a Tmax of 65 minutes
for a first
week of therapy and a lower Tmax (such as, for example, 50 minutes) for a
subsequent week
of therapy. The second cohort uses the blood glucose level control system
configured with a
Tmax of 65 minutes for the first week of therapy and an even lower Tmax (such
as, for
example, 40 minutes) for a subsequent week of therapy. The third cohort uses
the blood glucose
level control system configured with a Tmax of 65 minutes for the first week
of therapy and a
sharply lower Tmax (such as, for example, 30 minutes) for a subsequent week of
therapy.
Comparison of the change in Tmax within each cohort and across cohorts
demonstrates that
the mean glucose level drops when Tmax is lowered, and there is no
statistically significant
increase or decrease in hypoglycemia.
[02611 When Tmax is shorter than
physiological insulin absorption peak time,
there is an increased risk of hypoglycemia because the blood glucose level
control system may
stack or administer multiple doses of insulin within a time period. This may
occur because the
blood glucose level control system may incorrectly identify a lower blood
glucose
concentration as a maximum blood glucose level concentration when Tmax is set
below the
actual peak insulin absorption time.
[0262] By using the process 1300 to compare
the effect of different Tmax settings,
it is possible to optimize the Tmax setting for a subject and/or a particular
type of insulin. In
some examples the comparison may be based on one or more statistical methods.
For example,
using the glucose concentration data collected during a therapy period (e.g.,
using a CIG?v1
sensor), the control system may determine whether there is a statistically
significant difference
in mean glucose level during a later period using a different Tmax value
compared to an earlier
evaluation period. If the subsequent or newer value used for Tmax results in
an improved
effect, Tmax or a control parameter of the blood glucose level control system
510
corresponding to Tmax may be set to the newer value, where the change in the
control
parameter value may occur automatically upon determination of a statistically
significant
-84-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
improvement or may occur after generating a notification of the potential
improvement and
receiving confirmation that the change in control parameter value should
occur. After
collecting glucose signals of the subject 512 for a period of' time at a
default or prior value for
Tmax, the value for Tmax may be lowered by a significant amount from the
initial Tmax. For
example, the control algorithm may automatically change Tmax or an associated
time constant
to reflect a Tmax reduction of at least 10 minutes, at least 5 minutes, at
least 2 minutes, no
more than 15 minutes, no more than 20 minutes, no more than 30 minutes, or by
a change
within a range spanning between any two of the preceding values in this
sentence, where the
preceding values are included in the range. The system can perform a
statistical analysis
between the prior data set associated with the higher Tmax, and the current
data set associated
with the lower Tmax. If the controller of the blood glucose level control
system determines
that there is a significant or statistically significant improvement (e.g.,
more than a threshold
improvement) in the mean glucose level for the subject with little or no
increase in
hypoglycemia events or risk events, the system can adopt or recommend the
lower Tmax value
as the preferred Tmax. This process can be repeated using additional
reductions in Tmax. In
some cases, each reduction in Tmax may be smaller than the previous reduction.
Moreover, if
it is determined that there is a not an improvement in the mean glucose level
for the subject
and/or if there is an increase in hypoglycemia or hypoglycemia risk events,
the system may
use the prior Tmax or may select a Tmax between the new Tmax and the prior
Tmax. Thus,
using the process 1300, the system can iteratively modify Tmax to find an
optimal value for
the subject and/or the selected insulin type.
[0263] Moreover, by performing real-time
analysis and optimization of one or
more control parameters, maintenance of the subject's diabetes can be improved
faster and
more accurately compared to delayed analysis that may occur during clinical
testing. Clinical
testing may be less accurate as physiological changes in the subject may not
be captured in real
time.
102641 In some cases, the real-time process
and statistical analysis described above
can be used to analyze other types of biomedical data obtained by one or more
subject sensors
(e.g., measuring one or more physiological parameters). In some such cases,
the additional
biomedical data, such as data may be received from a smartwatch (e.g., blood
pressure, heart
rate), from a weight sensor, or any other type of biomedical sensor. By
adapting the process
-85-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
1300 to perform statistical analysis of the additional biomedical data, it is
possible to perform
a quantitatively objective analysis of biometric data, which can be used by a
healthcare
provider to care for a subject_
(0265) Further, the outcomes of the
comparative analysis described above may be
used to make additional recommendations to the subject. For example, if it is
determined that
the actual Tmax for a particular type of insulin is higher than expected for
the subject it may
be recommended that the subject modify his or her diet in a particular manner
while using that
particular type of insulin.
Example Simulations
[0266] Embodiments of an automated glucose
level control system 510 that can be
adapted for use with embodiments of the present disclosure are described in
International
Publication Na WO 2015/116524, published on August 6, 2015; U.S. Patent No.
9,833,570,
issued on December 5, 2017; and U.S. Patent No. 7,806,854, issued on October
5, 2010, the
disclosures of each of which are hereby incorporated by reference in their
entirety for all
purposes.
102671 The automated glucose level control
system 510 can autonomously
administer insulin doses and account for online accumulation of insulin doses
("insulin on
board") due to the finite rate of utilization of the insulin. The rate the
insulin absorption, and
in turn accumulation, of insulin doses may be modeled by a pharmacokinetic
(PK) model (e.g.,
the bi-exponential PK model represented by equation 2 with preset values of
time constants al
and a2). Of significant clinical significance in relation to the PK model is
the time it takes for
an insulin dose (e.g., administered subcutaneously) to be absorbed in
subject's blood. In some
examples, the peak time for insulin absorption in blood is referred to as
Tmax. In some other
examples. In some other examples, Tmax may be the time at which the
concentration of insulin
reaches its maximum value following the delivery of a specific dose of
insulin. In some such
examples. Tmax may be measured from the time that insulin is provided to the
subject (e.g.,
subcutaneously using an infusion set).
(02681 In some examples, setting the time
constants in the PK model (e.g., at and
a2 in equation 2) may be equivalent to setting Tmax that is inherently assumed
by the model;
conversely, setting Tmax may set the time constants of the PK model. Since the
values of the
-86-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
time constants may be used to determine the online calculation of the
accumulation of insulin
by a control system, the value of the time constants may consequently control
the control
system's insulin dosing response to a given blood glucose level excursion.
Thus, varying Tmax
or time constants associated with Tmax controls the aggressiveness of the
control system's
insulin doses.
[02691 In certain embodiments, the control
system implements a method to adapt
the control system's PK model's Tmax (hence time constants) setting online.
This method may
be performed either by the control system periodically making online
assessments and
calculations that produce recommendations of modifications in Tmax or by the
control system
autonomously modulating Tmax online. In either case, the calculations may be
based on the
control system's performance over some time period. In some cases, adaptations
to Tmax
online, whether autonomously occurring or issued as recommendations can be
based on the
glucose-control performance by the control system over some time interval,
including trends
in glucose level, mean glucose level, or extent and/or duration of low glucose
level
(hypoglycemia) and/or high glucose level (hyperglycemia) occurrence.
Alternatively, the
calculation can be based on the usage of a counter-regulatory agent, the
otherwise intended
usage of a counter-regulatory agent had it been available (e.g., in insulin-
only systems or in
cases where the counter-regulatory agent or its delivery channel are
temporarily unavailable).
The method can impose upper and/or lower (static or dynamic) bounds for the
range over
which the Tmax can vary. The degree of adaptation in Tmax for a given
situation can be
different depending, for example, on the specific insulin being administered
by the control
system.
[0270] In certain embodiments, the described
method may be applicable regardless
of whether the continuous glucose monitor (which can provide the input glucose
signal to the
control system) is online or offline. For example, the method disclosed herein
can be applied
to system described in International Publication No. WO 2015/116524. Fin-ther,
the described
method can coexist with other aspects of the system being activated or not,.
such as, but not
limited to, having a glucose target that is adapted automatically by the
system, e.g., as in the
system described in International Publication No. WO 2017/027459, published on
February
16, 2017, which is hereby incorporated by reference herein for all purposes.
-87-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
[0271] As previously described, the
absorption of subcutaneously administered
insulin into blood may be governed by the bi-exponential PK model of equation
2. Setting the
time constants in the PK model may set a measure of the pending effect of the
accumulated
amount of insulin in the subcutaneously administered dose, as that can be
taken to be the
difference between the total area (J p(Odt, which can describe a measure of
the total
action over time due to a dose U0) and fejt p(Odt, which can represent a
measure of the
expended portion of UO. The peak time, Tmax, of the absorption of insulin
doses into blood
may be given by equation 3. Thus, setting Tmax may set the PK model time
constants, which
can directly govern the magnitude (e.g., aggressive or conservative) of the
control system's
online insulin dosing response to a given glucose profile. Although not
limited as such, for
simplicity, assume that al and a2 are related, e.g. a2 = Li al.
[0272] The bi-exponential PK model may be
used to simulate the relation between
a glucose profile and the medicament (e.g., insulin or gl wagon) doses
delivered to a subject.
Figures 14A-14C illustrate a simulation demonstrating an effect that
increasing or decreasing
the Tmax setting, or value for a control parameter corresponding to Tmax, may
have on the
glucose level control system's 510 online insulin and gluc-agon dosing
response to a given
glucose profile (e.g., temporal variation of blood glucose level over 24
hours).
[0273] Figure 14A illustrates a simulation of
blood glucose control of a subject
with Tmax set to 65 minutes. The graph 1402 illustrates the variation of blood
glucose level
(BGL) of a subject over 24 hours. The range 1404 indicates the desired target
setpoint range
(e.g., between 70 and 120 mgAIL) for the subject's blood glucose level.
Further, the range 1406
indicates the range in glucose level (e.g., below 60 mg/dL) for the subject
that is associated
with hypoglycemia or a risk of hypoglycemia. The graph 1410A illustrates the
administering
of medicament (insulin or glucagon) to the subject over the same 24-hour time
period as graph
1402 based at least in part on the blood glucose level variation illustrated
in the graph 1402.
102741 Figure 14B illustrates a simulation of
blood glucose control of a subject
with Tmax set to 15 minutes. The graph 1410B corresponds to the graph 1410A,
but with Tmax
set to 15 minutes instead of 65 minutes. As illustrated by comparing the graph
1410B to 1410A,
reducing Tmax to 15 minutes may result in an increase in insulin dosing
required to maintain
the given glucose profile 1400.
-88-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102751
Figure 14C illustrates a
simulation of blood glucose control of a subject
with Tmax set to 130 minutes_ The graph and 1410C corresponds to the graph
1410A, but with
Tmax set to 130 minutes instead of 65 minutes. As illustrated by comparing the
graph 1410C
to 1410A, increasing Tmax to 130 minutes may result in a decrease in insulin
dosing required
to maintain the given glucose profile 1400.
102761
Even if the glucose profile
of a subject is unchanged, increasing or
decreasing insulin (or counter-regulatory agent) dosing may affect care of the
subject 512. For
example, the subject may experience different degrees of symptoms (e.g.,
dizziness, nausea,
etc.) attributable to maintenance of the subject's diabetes. Advantageously,
autonomous
optimization of one or more control parameters of a glucose control system,
may reduce the
amount and/or frequency of the medicament doses required to maintain a normal
glucose
profile.
102771
The simulations illustrated
in Figures 14A-14C illustrate one non-limiting
example of the impact of modifying a control parameter of a glucose control
system. In some
cases, different dosing may subsequently lead to different blood glucose
excursions which in
turn may vary the determined insulin¨glucagon doses subsequently. Nonetheless,
the
simulations shown in Figures 142k- MC, demonstrate the correlation between
Tmax as a control
parameter and the determined medicament doses by the glucose level control
system 510 for
each therapy. Further these simulations demonstrate that the determined
therapy doses may be
used as a feedback to adjust Tmax as descried below_
Example Automated Blood Glucose Control Refinement Process
[0278]
In some implementations, the
value of Tmax can be varied automatically
online based on glycemic control in a receding time period. For example, Tmax
can be
described using the following the equation:
Tmax (k) = Trity ax f 07k, Ilk),
(4)
where Tracexis a baseline value of Tmax, f(yk, 9k) is a parameter control
adjustment function
(herein referred to as adjustment function), based on glycemic control of the
glucose signal,
yk, and/or the amount of counter-regulatory dosing, 9k, that is computed by
the control system
(whether delivered or not). Evaluation off (yk, fix) could be over a time
period (e.g., one week,
two weeks, four weeks or other time intervals). For example, f(yk, 9k) =
f(yõ, gn). In
-89-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
some examples, k may represent a current therapy period and N may indicate a
receding time
period that may include one or more therapy periods.
(0279] The parameter control adjustment
function f(y,, 9k) can cause an increase
in Tmaõ(k) relative to Tit, for an increase in hypoglycemia (in severity
and/or duration) or
impending hypoglycemia in gl>,=ceinic control of the glucose signal, yk, over
the receding time
period (that may include one or more therapy periods) and, conversely, can
cause a decrease
in Tmax(k) relative to Tfnax for an increase in hyperglycemia (in severity
and/or duration) in
glycemic control of the glucose signal, yk, over the receding time period.
Moreover, f ic, 9k)
can cause an increase or decrease in Tmax(k) relative to Tat, respectively for
an increase or
decrease in amount of counter-regulatory dosing, 9k, over the receding time
period. The
adjustment f(Yks 9k) to Tmax(k) can be evaluated and effected at discrete
times, which can be
at scheduled periodic intervals (e.g., once every 24 hours, once every three
days, once a week,
etc.), in response to a user command, or based on a physiological measurement
of the subject.
Alternatively, or in addition, adjustments can be evaluated and effected
online when some
metric satisfies a threshold or meets certain criteria within the current
computation window
(e.g., a week, a month, etc.). This criterion can include when hypoglycemia in
yk reaches or
crosses a certain threshold or the level of counter-regulatory dosing in gk
reaches or crosses a
certain threshold_ Alternatively, or in addition, the adjustment can be
effected after some
evaluation related to the glucose signal yk (e.g., mean value) in the current
computation
window has attained a statistically significant difference from its evaluation
in a preceding
computation window (e.g., the week before). These described implementations
allow for
haying dynamic instances that are mathematically determined online as to when
Tmax(k) gets
adjusted arid/or the magnitude by which it is adjusted.
102801 In some examples, therapy periods can
be scheduled regular or periodic
time intervals (e.g., 24 hour periods, two day periods, one week periods,
etc.), based on a user
command, or based on a physiological measurement of the subject. In some other
examples,
therapy periods may be defined as the time interval between two subsequent
therapy deliveries,
and each therapy period may be identified based on the therapy delivery time
that marks the
beginning of the therapy period. In either case, f (yk, gk) may be the
adjustment to flax for the
kw therapy period and gyk, fik) may be evaluated based on the equation f (yk,
9k) =
EZ-N [(y, gn) wherein yn is the glucose signal measured during the lith
therapy period, 9,
-90-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
is the computed dose of a counter-regulatory hormone for the nth therapy
period and N indicates
the receding time period that may include one or more therapy periods. In some
examples, N
may be the number of the therapy periods receding the e therapy period.
(0281) Figure 15 illustrates an example of
blood glucose level signal G(t) 1502
(e.g., a CGM trace received from a CGI'vl sensor) over a therapy period
(starting from Is 1504
and ending at tE 1506) during which one or several doses of insulin and/or a
counter-regulatory
agent (e.g., glucagon) are determined and/or administered by the glucose
control system 510.
For example, an insulin dose of Ui 1508 units may be provided at time tu,t
1510 at a measured
glucose level of a.; 1512 (where i varies from I to die number of insulin
deliveries between ts
1504 and at tT 1506). Similarly the control system may have calculated a dose
of Q 1514 units,
that may have been administered or not, a glucose level aj 1518 at which
glucagon may have
been delivered and the time tc,i 1516, at which glucagon may have been
delivered, (where j
varies from I to the number of glucagon deliveries between ts 1504 and at tE
1506). The control
system may be configured to provide therapy in order to maintain the BGL
within a normal
range defined by an upper bound Gmax 1520 and a lower bound Gut 1522 and close
to a setpoint
Gset 1524. In some examples, the glucose levels above Gtr-ax 1 520 may
indicate hyperglycemia
and glucose levels below Gu1 1522 may be considered hypoglycemia. For example,
during
the therapy period shown in Figure 15, two instances of hyperglycemia 1526 and
two instances
of hypoglycemia 1528 may be identified by the control system. In some
examples, during each
therapy period the control system may store G(t) 1502, to.1510, tej 1516, U
1508 and Q 1514,
for all therapy deliveries (all values of i and j). In some examples, the
value of one or more
control parameters (e.g., T1-flax, (ial) may not change during the therapy
period between ts
1504 and tE 1506.
[0282] Figure 16 presents a flowchart of an
example automated blood glucose
refinement process that may use the above-mentioned modification method to
control Tmax
and/or other control parameters of a glucose control system_ The process 1600
may be
performed by any system that can autonomously and/or automatically modify a
control
algorithm and/or a control parameter that affects execution of the control
algorithm based on
feedback (e.g., from a blood glucose signal) relating to therapy administered
to a subject 512.
For example, the process 1600 may be performed by one or more elements of the
glucose level
control system 510. In some cases, at least certain operations of the process
1600 may be
-91-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
performed by a separate computing system that receives blood glucose data from
the glucose
level control system 510_ Although one or more different systems may perform
one or more
operations of the process 1600, to simplify discussion and not to limit the
present disclosure,
the process 1600 is described with respect to particular systems.
[02831 The process 1600 may be performed
automatically and without user
interaction_ In some cases, a user may trigger the process 1600 via a command
or interaction
with a user interface. However, once the process 1600 is triggered, the
process 1600 may be
performed automatically. Further, the process 1600 may be performed
continuously,
periodically, or in response to a trigger. The trigger may be time based
and/or based on a
measurement of the glucose level of the subject. For example, the trigger may
correspond to a
determination that a glucose level of a subject differs by more than a
threshold from a predicted
glucose level that is predicted by a glucose level control algorithm based on
the administering
of medicament. Further, the trigger may be based on the activation or first
time use of the
glucose level control system 510 by the subject 512.
[0284] The process 1600 begins at block 1602
where a first value is selected for a
control parameter (e.g., a control parameter that may be adaptively modified)
of the glucose
control system 510. For example, the control parameter can be a Tmax value
used in the control
algorithm of the glucose control system 510. In some examples, Tnaax may be
related to one
or more parameters in a PK model used by the control algorithm. As another
example, the
control parameter can be a setpoint (e.g., act 1524 in Figure 15) or the
target value for the
measured value of the blood glucose concentration of a subject 512 (e g.,
measured using a
CGM sensor).
[0285] The first value of the control
parameter may be selected based on a baseline
value. The baseline value may be associated with clinical data, may be
determined based on
operation of the glucose level control system 510 for some period of time
before performance
of the process 1600, or may be determined from a prior performance of the
process 1600.
Alternatively, or in addition, the baseline value may be selected based on
clinical data or a
particular prescription for the subject 512. In some cases, the baseline value
may be based on
clinical data for average users or average users that share certain
physiological data with the
subject 512. In some cases, the baseline value is determined based on a
healthcare provider's
assessment of the subject 512. Further, the baseline value may be determined
based on an
-92-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
infusion site (e.g., back, stomach, leg, etc.) for the glucose level control
system 510. In some
cases, the baseline value may be selected based on demographics or
characteristics of the
subject 512_
(0286) At block 1604, the glucose control
system 510 provides therapy over a time
period to the subject 512. based at least in part on the first value of the
control parameter.
Further, the therapy may be provided based at least in part on one or more
glucose signals
received during the time period. The glucose signals may be received from a
glucose sensor
(e.g., a CGN4) and may correspond to a glucose level of the subject. In some
cases, the time
period may include one or more therapy periods. In some examples, the number
of therapy
periods included in the time period may be equal or unequal therapy periods. A
therapy period
may be a time period that corresponds to a single delivered medicament dose,
which may
include an instantaneous delivery or a delivery of the medicament dose over a
period of time.
Alternatively, a therapy period may be a time period that encompasses a
plurality of
medicament dose deliveries. Further, a therapy period may be a time period
associated with a
defined length of time. Alternatively, or in addition, the therapy period may
be defined based
on a number of medicament periods. In other words, the time period may vary
based on the
amount of time it takes to deliver or administer a specified number of doses
of medicament (of
any type or of a particular type).
(02871 In some examples, the time of delivery
and dose of the plurality of therapies
may be based at least in part on the glucose level signal and the first value
of a control
parameter of the control algorithm used by the glucose control system 510. The
control
parameter may include any control parameter that affects operation of the
glucose level control
system 510 andlor performance of a control algorithm of the glucose level
control system 510.
[0288] For example, the control parameter can
be Tmax, Ti', speed of delivery of
a medicament dose, a setpoint for the glucose level, a blood glucose range, a
threshold value
of blood glucose level (e.g., a maximum or minimum value) and the like. The
control algorithm
may include any control algorithm andfor PK mode/ used to determine a dose of
medicament
(e.g., insulin) to administer to the subject 512. In other words, the
controller 518 or the
processor 530 may use the control algorithm to generate a dose control signal
based at least in
part on a value (e.g., the first value selected at the block 1602) of the
control parameter to cause
the delivery device 514 to administer a dose of insulin or other medicament.
-93-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102891 Each therapy of the plurality of the
therapies provided over the time period,
may correspond to a single administering of insulin to the subject 512_ This
single
administering of insulin may be any type of insulin that may be administered
for any reason
For example, the insulin dose may be a basal insulin dose, a priming dose, a
dose supplied in
response to a meal announcement, or a correction dose of insulin. Moreover,
each therapy
provided may be a medicament other than insulin, such as counter-regulatory
agent (e.g.,
glucagon). In some cases, each therapy delivery may include a plurality of
medicament (e.g.,
insulin and/or counter-regulatory agent) doses supplied or administered to the
subject 512 over
a therapy period. Further, the plurality of medicament doses may include
different types of
medicament doses, such as one or more basal doses, one or more meal doses
associated with
one or more meal announcements, one or more corrective doses, etc.
102901 In some examples, the value of the
control parameter that is being adjusted
may change from one therapy period to another therapy period during the time
window. For
example, the value of the control parameter may change by a given amount in
the beginning
of each therapy period or group of therapy periods. In some other examples,
the value of the
control parameter may change by a given amount after certain number of
therapies. In some
examples, the amount by which the control parameter is changed may be
determined based on
one or more receding therapy periods in the time window. In some cases, the
block 1604 may
include one or more of the embodiments described with respect to the process
1304.
[0291] In some examples, during the therapy
period or one or more therapy periods
of the plurality of therapy periods included in the time period, therapy data
may be obtained
and/or stored. With reference to Figure 15, in some examples, therapy data may
include the
glucose signal, G(t) 1524, the calculated or actual delivery time (t 1516)
1516) and the estimated or
delivered amount of a counter-regulatory agent (Ci 1514). This therapy data
may be stored in
the memory 540 of the glucose level control system 510. Further, the therapy
data may include
a total amount of the counter-regulatory hormone administered during a therapy
period.
Alternatively, or in addition, other parameters and data associated with each
therapy period
may be stored in the memory 540. For example, the total amount of insulin
administered, an
amounts of insulin delivered (Ui 1508), a delivery time On 1510) of the
insulin delivered
during each therapy period, data received from other sensors that may measure
one or more
-94-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
physiological parameters of the subject, data received from the subject or
user (e.g., via a user
interface), and the like.
(02921 At block 1606, the glucose level
control system 510 determines a control
parameter adjustment for the control parameter. The control parameter
adjustment may be
based at least partially on the therapy data. In some embodiments, the
adjustment may be
determined using an adjustment function. For example, the adjustment function
may be the
function f(yk, ilk) for modifying Tmax according to equation 4. In some
examples, the control
parameter adjustment may be determined by analyzing glycemic control of blood
glucose in
the subject as indicated by the glucose level signal (e.g., G(t) 1524 or the
CGM trace).
Analyzing the glycernic control of the blood glucose in the subject may
include tracking the
blood glucose level of the subject 512 over time! Further, analyzing the
glycemic control of
the blood glucose in the subject may include comparing the blood glucose level
of the subject
512 over time to a predicted blood glucose for the subject 512 over time
estimated based on
the PK model and control parameter values used in the control algorithm. In
some examples,
the value of the adjustment function f(vk. g.k) may be calculated at least in
part using the
estimated or actual values of tcõj 1516, (I) 1514, and Ge.i, (where j varies
from I to the number
of counter-regulatory provided during the time period). In some other
examples, determination
of the adjustment function f (yk, 9k) may include a statistical analysis based
on the estimated
or actual values of tsi 1516, Cs 1514, and Go.j, (where j varies from 1 to the
number of counter-
regulatory provided during the time period). In some such examples, the
statistical analysis
may be based on statistical quantities and/or the analytical tools described
below.
102931 In some cases, the adjustment to the
control parameter may be determined
based on the number of hypoglycemia 1528 and/or hyperglycemia 1526 events
and/or duration
of each event. In some examples, the adjustment to the control parameter may
be determined
based on the difference between measured glucose level and the setpoint (Gsef
1524). In some
examples, the adjustment may be determined based on the time intervals during
which the
glucose level stays within a target range (e.g., between Gum 1520 and Gmin
1522). In some
eases, the adjustment may be determined based on the stability of the measured
blood glucose
level for the subject 512 or less volatility in the blood glucose level of the
subject 512. For
example, a statistical analysis may be performed to determine the distribution
rate of change
for G(t) beyond one or more threshold rates.
-95-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
102941 In some cases, the adjustment to the
control parameter may, at least
partially, be determined by analyzing one or more signals received from one or
more subject
sensors that measure one or more physiological parameters of the subject
(e.g., heart rate,
temperature and the like). In yet other examples, the adjustment to the
control parameter may
be determined based on an assessment or input received from the subject 512
(e.g., using a
user interface of the ANID). For example, if the subject 512 feels woozy,
dizzy, lightheaded,
nauseous, or otherwise uncomfortable during one or a plurality of therapy
periods, the subject
512 may, via, for example, a totichscreen user interface or other interface of
the AMD, indicate
how the subject 512 is feeling.
102951 The adjustment may be determined in
real-time or substantially in real-time
accounting for the processing speed of the hardware processor 530, the glucose
level control
system 510, or the time for the subject to provide an assessment of his or her
condition to the
glucose level control system 510. In some eases, the adjustment to the control
parameter may
be determined by a computing system that is in communication with the glucose
level control
system 510. For example, the glucose level control system 510 may transmit the
therapy data,
to another computing system, such as a local computing system, a srnartphone,
or a cloud-
based computing system. Further, the glucose level control system 510 may
transmit the
therapy data and data associated with the control parameters values to the
computing system.
1he computing system may determine the adjustment that better manages the
subject's 512
blood glucose level in the next time period.
10296] At block 1608, the glucose level
control system 510 adjusts the control
parameter using the control parameter adjustment determined at the block 1606.
In some
examples, the adjustment may be performed autonomously or automatically. In
some other
examples, the control parameter adjustment determined at block 1606 may be
presented to the
subject or other user (e.g., parent, guardian, clinician, etc.) via a user
interface (e.g., a
touchscreen display). In some such cases, the subject or other user may be
able to confirm or
modify the control parameter adjustment In other cases, the display of the
control parameter
adjustment may be presented for informational purposes and may not be
adjustable by a user.
In some cases, the control parameter may be adjusted only after receiving the
user confirmation
(e.g., a user interaction with a user interface). In some other examples,
where the adjustment
is determined by a computing system, the adjustment value may be presented to
user via a user
-96-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
interface of the glucose control system or a user interface of the computing
system. In some
cases, the user may adjust the control parameter of the glucose control system
using the
adjustment value received from or presented by the computer system.
(0297) The adjustment at block 1608 may cause
a change in the operation or
execution of the control algorithm. This change in the execution of the
control algorithm may
result in a change in one or more factors associated with the provisioning of
therapy to the
subject 512. For example, the change in the execution of the control algorithm
may result in a
change in an amount of medicament delivered, a timing of the delivery of the
medicament, a
rate at which a dose of medicament is delivered to the subject 512, a target
setpoint or target
range for the blood glucose of the subject, a threshold used in determining
whether to deliver
medicament (e.g., a threshold difference from the target setpoint), or any
other factor that may
affect therapy delivered to the subject 512.
102981 In some cases, the adjusted value of
the control parameter may be output to
a user (e.g., the subject or a parent). The user may then configure the
glucose level control
system 510 based on the selected control parameter value. Alternatively, or in
addition, the
glucose level control system 510 may automatically adjust the value of the
control parameter.
In some cases, the user may he provided with an opportunity to confirm the
adjustment. In
other cases, the adjustment may occur automatically without confirmation_
However, the
adjustment may be presented to the user (e.g., the subject or a healthcare
provider) andfor
logged in a therapy log.
10299] At block 1610, the glucose level
control system 510 provides therapy based
at least in part on the updated control parameter that is updated at the block
1608. The new
value of the control parameter may be maintained during a second time period.
The second
time period may refer to a specific amount of time, an amount of time to
deliver a particular
number of medicament doses, or a particular number of medicament doses.
[03011 The process 1600 may be repeated
during subsequent time periods. In some
examples, the process may be repeated periodically (every 24 hours, every two
days, every
week, or other time intervals). In some cases, the time period may be provided
by the subject
or a user. Further, the process may be repeated in response to a command. In
some cases, the
process may be repeated in response to determining that the subject's 512
blood glucose level
does not satisfy one or more criteria for a particular amount of time. For
example, the process
-97-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
may be repeated when a statistically significant difference between the
measured mean value
of the BGL and a target BGL exceeds a threshold, or a number of hypoglycemia
and/or
hyperglycemia detected exceeds a threshold number during a specific amount of
time.
(0301) In some examples, the process 1600 may
be used to adjust several control
parameters that affect the therapy delivery by the glucose control system. In
some such
examples, the process 1600 may be used to adjust a first control parameter
during a time period
and to adjust a second control parameter during a second time period. The
second time period
may be immediately after the first time period or delayed by a particular
time. In some
implementations, the control system 510 may determine when to adjust the
control parameter.
In some examples, a delay between periods of control parameter adjustment may
be
determined at least in part on the glycemic control of the glucose signal. In
some cases, the
delay may be determined based on input received from a user. Further, the
adjustment of the
second control parameter may be at least partially determined based on the
determined
adjustment for the first control parameter_
[0302] In some embodiments, a third control
parameter may be adjusted during a
third time period. The adjustment of the third control parameter may
immediately follow the
adjustment of the second control parameter or may occur after a delay. The
delay may be
determined at least in part based on the glycemic control of the subject after
the second control
parameter is adjusted. In some cases, the glucose control system may be
configured to
sequentially adjust the first and second, or the first, second, and third
control parameters when
the glvcetnic control of the subject satisfies one or more threshold
conditions. In some
examples, the duration of the time period during which a control parameter is
adjusted may
differ from that of the first and second control parameters.
[0303] As described above, the process 1600
may be used to adjust one or more
control parameters that affect the delivery of insulin. However, the process
1600 is not limited
as such and may be used to modify one or more control parameters that affect
the delivery of
other medicaments, such as a counter-regulatory agent (e.g., glucagon). In
some cases, the
process 1600 may be used to recommend a change in insulin and/or counter-
regulatory agent
delivers' without modifying the delivery. This can be advantageous for
generating
recommendations regarding counter-regulatory agent in a non-hi-hormonal
glucose level
-98-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
control system 510 that does not support counter-regulatory agent, or that
supports the use of
counter-regulatory agent, but does not have the counter-regulatory agent
available.
Implementation of Statistical Analysis in Automated Blood Glucose Control
Refinement
[03041 As described above, a value (e.g., a
baseline value or optimal clinical value)
of one or more control parameters of a PK model and/or control algorithm used
by a glucose
control system 510 may be determined by statistical analysis of therapy data
sets (e.g.,
glycemic control information) collected from multiple cohorts of subjects
(e.g., 20, 50, 100,
200 subjects) during a clinical study. In some examples, the control parameter
(e.g , Tmax)
may be directly measured for the subjects within each cohort (e.g., based on
results of blood
analysis following manual or automated medicament administrations). These
measurements
may be used to determine an optimal value of a control parameter (e.g., Tmax)
to be used in a
glucose control system. In some cases, the blood glucose level (BGL) of the
subjects may be
controlled and recorded for a given period (e.g., one week, two weeks, one
months, or other
periods) using identical or nearly identical glucose control systems. The
subjects in each cohort
may use the same values for a control parameter of the glucose control system
while the
subjects in different cohorts may use different values of the same control
parameter.
Subsequently, the measured therapy data. sets, (e.g., comprising measured
and/or determined
glycemic control information for the subjects) over the given period may be
compared using
statistical analysis to evaluate an optimal value of the control parameter.
For example, the
measured glycemic control of subjects in a first cohort in response to setting
Truax to a first
value, may be compared to the measured glycemic control of subjects in a
second cohort in
response to setting Tmax to a second value. Such comparison may include
various statistical
analysis that can reveal statistically significant differences between
measured glycemic
controls. For example, the mean value, variance and/or standard deviation of
the measured
blood glucose level data obtained from the first and second cohort, may be
compared to a set
of reference values that may be obtained from a third cohort of subjects with
normal blood
glucose level (e.g., nondiabetic subjects). To generate accurate results, such
clinical studies
often require several cohorts each comprising a large number of subjects
(e.g., large enough to
produce enable statistical analysis) and therefore large number of identical
glucose control
systems. For example, in some studies 10, 20, 50, or 100 subjects and glucose
systems may be
-99-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
required. As such, determining the optimal value of one or more control
parameters based on
clinical studies can be expensive and time consuming. Moreover, clinical
studies typically
cannot capture unique physiological characteristics of and real-time
physiological changes of
a subject (even studies include several large cohorts).
[03051 A portable glucose control system that
monitors the BGL in real time and
autonomously or automatically provides medicament to a subject, may collect
and store
therapy data sets that, similar to those collected in clinical studies, may
include sufficient
number data points for a statistical analysis. In some examples, therapy data
may include
glycernic control information (e.g., received from a CGM sensor), other
physiological effects
of the therapy (e.g., obtained from subject sensors or the subject), an amount
and type of
medicament delivered, medicament delivery times, and the like. Advantageously,
these
therapy data sets may be used to determine an optimal value of one or more
control parameters
of the glucose control system or a value for the one or more control
parameters of the glucose
control system that provides improved diabetes management compared to a
default value,
baseline value, or initial clinically determined value. The optimal or
improved values may be
determined based on statistical analysis, including the type of statistical
analysis that may be
used in clinical studies. In some embodiments, the statistical analysis may
include calculating
one or more statistical quantities such as mean, variance, standard deviation,
various statistical
distributions (e.g., those described with respect to FIG. 17 below) and the
like. On board and
real-time (or near real-time) evaluation of values of one or more control
parameters of a
glucose control system based on therapy data collected during one or more
therapy periods
eliminates the need for expensive and time consuming clinical studies and may
improve the
maintenance of a subject's diabetes by, for example, taking into account
unique physiological
characteristics of and real-time physiological changes of a subject Moreover,
on board
evaluation of control parameter values provides for faster and more accurate
diabetes
evaluation and management compared to clinical testing Some of the embodiments
described
herein may be used to determine optimal values of one or more control
parameters that may
be used by a user to adjust the control parameters via a user interface of the
glucose control
system. In some cases, the glucose control system may autonomously adjust one
or more
control parameters using the determined optical values.
-100-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
103061
The therapy data collected by
a glucose control system may include
glycemic control information, information related to medicament delivery
times, doses of
medicament provided, the BGL level at the time of medicament delivery (e.g.,
measured based
on a glucose signal obtained from a CGM sensor), the physiological effects of
the medicament
on a subject (e.g., BGL in a time period after medicament delivery, subjects
assessment and
the like), and any the type of data that may be determined from therapy
provided to the subject
In some embodiments, the glucose control system may collect therapy data
during one or more
therapy periods. With reference to Figure 15, the collected and stored therapy
data during each
therapy period (e.g., a period starting at ts 1504 and ending at tE 1506) may
include, but is not
limited to: a CGM trace Go) 1502, delivered doses
1508) and delivery times
(time to) of
insulin, delivered or determined doses (Ci 1514) and delivery times (to 1516),
of a counter-
regulatory agent (e.g., glucagon) and the like. The therapy data may be stored
in a memory
(e.g., a flash drive, a solid-state drive, a hard disk, or any other type of
non-volatile memory)
of the glucose control system as one or more data sets. Each data set may be
associated with
one or more categories of therapy data or a specific therapy period during
which the therapy
data was collected. In some cases, the value of the one or more control
parameters may change
from one therapy period to another therapy period. For example, the value of
the control
parameter may change by a given amount in the beginning of a therapy period or
a group of
therapy periods. The value of the control parameter may be changed
automatically by the
glucose level control system 510 or by a user via a user interface. In some
cases, the control
parameter may be changed by a given amount after certain number of therapy
periods. The
amount by which the control parameter is changed may be determined based on
therapy data
collected during one or more preceding therapy periods. Alternatively, or in
addition, the
amount by which the control parameter is changed may be provided by a user via
a user
interface. In some cases, the duration of one or more therapy periods is
selected such that the
measured or determined data sets are sufficiently large for statistical
analysis. In some
examples, an uncertainty associated with an optimal or improved value of a
control parameter
determined using statistical analysis may depend on the size of the data set
used for the
analysis.
103071
In some embodiments, the
process 1300 may be used to determine a value
(e.g., an optimal value) of a control parameter using statistical analysis.
For example, statistical
-101-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
analysis may be used to determine the therapy effects at block! 306, block
1314, or to compare
the therapy effects resulting from different control parameter values at step
1316. in some such
examples, at block 1308, the second value of the control parameter may be
provided by the
user (e.g., the subject or the guardian) based at least in part on the first
effect and outcomes of
the statistical analysis performed on the therapy data collected and/or stored
during the first
therapy period (block 1304). In some examples, at step 1316, a statistical
analysis may be
performed based at least in part on the first effect and the second effect to
obtain a comparative
assessment The comparative assessment may be used to determine whether one of
a pair or
set of values of a control parameter results in an improved glycemic control
of the subject
compared to the other values used for the control parameter. In some
embodiments, the
determined value of the control parameter at block 1316 may be provided to the
subject, a
guardian or a healthcare provider via a user interface of the glucose control
system 510 and/or
a computing system (e.g., a smartphone, a notebook a personal computer and the
like)
connected to the glucose control system (e.g., via a wireless link). In some
such embodiments,
the subject, the guardian or the healthcare provider may change the value of
the corresponding
control parameter to the determined value by an interaction with a user
interface before the
next therapy period (e.g., at block 1318). Alternatively, or in addition, the
glucose level control
system 510 may automatically change value of the control parameter to the
determined value
and proceed to block 1318. In some such cases, the user may be provided with
an opportunity
to confirm the modification. In other cases, the modification may occur
automatically without
confirmation. However, the modification may be presented to the user (e.g.,
the subject or a
healthcare provider) and/or logged in a therapy log.
[0308] In some examples, the first and second
therapy provided to the subject
during the first (block 1304) and second (block 1312) therapy periods, may
include a plurality
of therapy deliveries. During the first (block 1304) and second (block 1312)
therapy periods,
a first and second first therapy data may be obtained by the control system
5Ia In some such
cases, the therapy data may comprise glycemic control information that at
least includes the
glucose signal received during the corresponding therapy period. Determining
the first effect
may include calculating statistical characteristics of the therapy data
collected during the
plurality of therapies provided during each period. For example, the control
system 510 may
calculate the mean value, deviation from mean value, and the variance of the
measured BGL.
-102-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
In some cases, the control system 510 may calculate one or more quantities
(e.g., statistical
quantities) to quantify the average blood glucose level and its deviation from
a baseline level.
In some embodiments, the control system 510 may determine one or more
quantities (e.g.,
statistical quantities) to evaluate the variability of glycemic control and
the associated risks
(e.g., risk of hypoglycemia or hyperglycemia) or quantify the average blood
glucose level and
its deviations from a baseline (e.g., normal) level. In some cases, the
duration of the second
period may be equal to the duration of the first period. Alternatively, or in
addition, the duration
of each period may be selected such that each period includes the same number
of therapies
provided to the subject. In some embodiments, the duration of each period may
be selected
such that the number of times therapy is administered during the time period
is large enough
to enable statistically significant assessments. In some cases, at block 1316,
the comparison
between the first effect and the second effect, may include statistical
analysis of statistical data
generated based on the data collected during the first and second period.
103091 In some examples, in addition to the
optimal values of one or more control
parameters, the control system may generate a control parameter optimization
report that may
include the statistical quantities calculated during the optimization process.
Further, the report
may include a graphical representation of the therapy data and related risk
assessments. In
some such examples, this report may be used by the subject or a healthcare
provider to make
decisions related to selecting a determined optimal parameter value.
Additionally, the control
parameter optimization report may include information that may be used by the
subject or a
healthcare provider to modify the overall strategy for managing the subject's
glycemic control.
For example, modifying the mealtime, content or amount of meal consumed by the
subject,
and the like.
[0310] Figure 17 illustrates some examples of
statistical quantities that may be
generated and utilized at blocks 1306 and 1314 of the process 1300, using the
therapy data
1705 during a therapy period, and known parameters of the control system 1703.
In some
embodiments, during the therapy period the value of certain control parameter
may be fixed
and/or selected based on baseline values (e.g., outcomes of previous clinical
studies) or a
previously determined value (e.g., by a different control parameter
modification and/or
optimization process). With reference to Figure 15, in the example shown in
Figure 17, Gain
1722 (lower bound for normal BGL), Guaax 1720 (upper bound for normal BGL) and
Gset 1724
-103-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
(target BGL) are assumed to be known values provided by the subject, the user,
a health care
provider or determined by a computing system based on a set of clinical data.
For example,
Guaa 1722 may between 65mg/dL and 75 mg/dL, Galax 1720 may be between 175
mg/dL and
185 ingldL and Gset 1724 may be between 70 mg/dL and 180 mg/dL. In some
examples, Gt
1724 may be a value (e.g., an optimal) determined by a previous optimization
process (e.g.,
the process 1300). G(t) 1702 (the CGM trance or the measured glycemic
control), Lri 's 1708,
to's 1710, Ci 's 1514 and to 's 1716 may be included in the therapy data
collected during the
therapy period In some examples, the therapy data 1705 may be used to generate
various types
of statistical quantities. For example, the therapy data 1705 may be used to
generate probability
distributions (e.g., discrete or continuous) and/or frequency distributions
(e.g., absolute,
relative, or cumulative) for certain measured or determined values. For
example, the
distributions associated with the glucose concentration 1726 (e.g., portions
of the therapy
period during which the glucose signal was within selected ranges), glucose
change rate 1728
(eg., portions of the therapy period during which the glucose change rate
signal was within
selected ranges rates), insulin dose 1730 (percent of insulin doses provided
within selected
dose ranges), glucagon dose 1732 (percent of glucagon doses provided within
selected dose
ranges), hyperglycemia 1734 (percent of hyperglycemia events detected wherein
the glucose
signal was above Gmax by an amount within selected ranges), hypoglycemia 1736
(percent of
hypoglycemia events detected wherein the glucose signal was below Gaaa by an
amount within
selected ranges) and the like. In some examples, one or more characteristic of
these statistical
distributions (mean, variance, deviation from mean, and the like) or a
specific combination of
some characteristics of these statistical distributions, may be used to
determine (e.g., quantify)
the effect of a therapy. In some examples the therapy data considered to
generate certain
statistical data (e.g., a histogram) may be filtered to exclude the data
points collected during
certain events. For example, during a mealtime, during exercise, and the like.
In some
examples, time bins associated with these events may be specified by a user
through a user
interface.
103111 In some embodiments, the statistical
analysis may comprise analytical
methods and tools that can compare the effect of different control parameter
values. Some
examples of analytical methods and tools that can be used with one or more of
the embodiments
described herein are described in the article "Statistical Mats to Analyze
Continuous Glucose
-104-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
Monitor Data" (W. Clarke et al., Diabetes Technology and Therapeutics, vol.
11. S45-S54,
2009), which is hereby incorporated by reference in its entirety herein.
Examples of methods
and tools that may facilitate extraction of information from complex and
voluminous measured
glycerine control information during therapy periods, are discussed herein. In
some cases, the
therapy data used for statistical analysis includes the glucose trace of the
subject or G(t). In
some examples, G(t) may be a time-stamped series of glycernic data received
from a CGM
sensor (see Figure 17). In some examples, the glucose signal obtained from CGM
may
represent blood glucose level as a discrete time series that approximates G(t)
in steps
determined by the resolution of the particular device (e.g., a reading every 2
min, 5 mm, 10
min and the like). In some examples, statistical analysis may be performed on
the therapy data
(e.g., the glucose signal received from a CUM sensor) to provide an assessment
(e.g., a
comparative assessment) related to: (1) average blood glucose level and
deviations from
normal glycemic control (sometimes referred to as normoglycemia), (2)
variability and risk
assessment, and (3) clinical events, such as post-meal glucose excursions and
hypoglycemic
episodes. In some embodiments, the assessment may be made based on two sets of
therapy
data collected during two time periods. In some such examples, the assessment
may be used
by the control system 510 to determine whether the glycerine control for a
subject has been
improved from a first therapy period to a second therapy period. In some
examples, the
assessment may be used by a health care provider to evaluate the glycemic
control of a subject
during one or more time periods.
103121 In some cases, the blood glucose
control system may determine three values
of average blood glucose: the mean value (e.g., computed for the entire (3(t)
measured during
a therapy period or part of a therapy period), a pre-meal mean value (e.g.,
computed for the
time window of 60-120 min after the meal), and post-meal mean value (e.g.,
computed for the
time window of 0-60 min before meal). Computing of pre- and post-meal averages
and the
difference between the averages can serve as an indication of the overall
effectiveness of pre-
meal bolus timing and bolus amount. In some examples, deviation from target or
normoglycemia may be evaluated by determining percentages of time spent
within, below, or
above preset target limits (e.g., Gain 70 and Gmar----180 mg,./dL). In some
examples, the
percentage of time within each range may be calculated via linear
interpolation between
consecutive glucose readings. In some other examples, percentage of time
within additional
-105-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
ranges can be computed. In some such examples, the probability of occurrence
of extreme
hypoglycemia and hyperglycemia may be also evaluated. To quantify variability
of blood
glucose level, in some examples, standard deviation and variance may be used
to compute
variability of BGL. In some cases, a risk index may be defined that can serve
as a measure of
overall glucose variability when focusing of the relationship between glucose
variability and
risks for hypo- and hyperglycemia. In some examples, an individual function
may be calculated
to split the overall glucose variation into two independent sections related
to excursions into
hypo- and hyperglycemia, and at the same time equalize the amplitude of these
excursions with
respect to the risk they carrv. For example, a BGL transition from 180 to 250
mg/dL may
appear threefold larger than a transition from 70 to 50mg/dL, whereas if
converted into risk,
these fluctuations would appear equal. In some cases, analysis of BGL rate of
change (e.g.,
measured in mgldlimin) may be used to evaluate the dynamics of BGL
fluctuations on the
time scale of minutes. In other words, this is an evaluation of the "local"
properties of the
system as opposed to "global" properties discussed above. In some examples the
local
properties may be assessed at a neighborhood of any point in time by the value
BGL, its first
or, sometimes, second derivatives (acceleration).
103131 In some examples, in addition to
statistical analysis of the therapy data, in
the blocks 1306, 1314, and 1316 of the process 1300, a statistical analysis of
the user inputs
provided during the first or second therapy period may be used in determining
or comparing
the therapy effects. For example, the number of times and time of the day that
the subject has
indicated certain symptoms, may be used to determining therapy effects.
103141 In some cases, in addition to the
statistical analysis of the therapy data in
the blocks 1306, 1314, and 1316 of the process 1300, a statistical analysis of
the biomedical or
physiological data received from one or more subject sensors (e.g., a smart
watch, weight
sensor, etc) may be used in determining or comparing the therapy effects. For
example,
subject's temperature, blood pressure, heart rate), from a weight sensor, or
any other type of
biomedical sensor.
[0315] In some examples, the process 1300 may
be modified to determine the
optimal value of Tmax, or a value of Tmax that provides improved maintenance
of the subject's
diabetes, by reducing Tmax (increasing the aggressiveness of the therapy)
after each therapy
period in a series of therapy periods, until a statistical assessment shows
that further reduction
-106-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
of the Tmax does not improve the mean glucose level without increasing the
probability of
hypoglycemia. Improved maintenance of the subject's diabetes may include
maintaining a
mean glucose level closer to a setpoint glucose level range or reducing
fluctuations in mean
glucose level over time compared to prior control value (e.g., Tmax) settings.
It should be
understood that other metrics may be used to measure an improvement of
maintenance of the
subject's diabetes, such as reduction in hypoglycemia risk events or reduction
in administration
of insulin without increasing diabetic effects or corresponding risks.
103161 Figure 18 presents a flowchart of an
example automated control parameter
refinement process in accordance with certain embodiments. The process 1800
may be
performed by any system that can autonomously andlor automatically modify a
control
algorithm and/or a control parameter that affects execution of the control
algorithm based on
feedback (e.g., from a blood glucose signal) relating to therapy administered
to a subject 512.
For example; the process 1800 may be performed by one or more elements of the
glucose level
control system 510. In some cases, at least certain operations of the process
1800 may be
performed by a separate computing system that receives blood glucose data from
the glucose
level control system 510. Although one or more different systems may perform
one or more
operations of the process 1800, to simplify discussions and not to limit the
present disclosure,
the process 1800 is described with respect to particular systems.
(03171 The process 1800 may be performed
automatically and without user
interaction. In some cases, a user may trigger the process 1800 via a command
or interaction
with a user interface. However, once the process 1800 is triggered, the
process 1800 may be
performed automatically. Further, the process 1800 may be performed
continuously,
periodically, or in response to a trigger. The trigger may be time based
andlor based on a
measurement of the glucose level of the subject. For example, the trigger may
correspond to a
determination that a glucose level of a subject differs by more than a
threshold from a predicted
glucose level that is predicted by a glucose level control algorithm based on
the administering
of medicament. Further, the trigger may be based on the activation or first
time use of the
glucose level control system 510 by the subject 512.
(0318) In some embodiments, the glucose level
control system 510 may perform
the process 1800 in order to adjust one or more control parameters of the
glucose control
system 510 to improve the glycemic control of a subject. The control parameter
may include
-107-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
any control parameter that affects operation of the glucose level control
system 510 andlor
performance of a control algorithm of the glucose level control system 510. In
some such
embodiments, in addition to improving the glycemic control of the subject, the
process 1800
may take into account the risk of hypoglycemia in the subject. In some
embodiments, the
process 1800 may include one or more of the embodiments previously described
with respect
to the process 1300.
103191 The process 1800 begins at block 1802
where an initial value is selected for
a control parameter of the glucose control system (e.g., Tmax or other control
parameters of
the glucose control system selected to be optimized). The control parameter
can be a control
parameter of a pharmacokinetic (PK) model used by a control algorithm PK of
the glucose
control system Sift In some examples, the control parameter may be a time
until insulin within
blood plasma of the subject reaches a particular concentration level
subsequent to
administration of an insulin dose. In some eases, the initial value of the
control parameter may
be based on therapy delivered during a time period prior to the first therapy
period, a clinical
value, or a body mass of the subject.
103201 In some examples, the initial value of
the control parameter may be selected
using one or more of the embodiments described with respect to the block 1304
of the process
1300. In some embodiments, the control parameter may be a control parameter
used by the
control algorithm of the glucose control system to account for accumulation of
insulin in a
subject. In some embodiments the control parameter may be used to control an
insulin dosing
response of the control algorithm to a blood glucose excursion in the subject
based on a glucose
level signal received from a glucose level sensor (e.g., a SGM sensor).
[0321] At block 1804, the control system 510
may provide therapy during a first
therapy period based at least in part on the glucose level signal and the
initial value of the
control parameter. In certain embodiments, the block 1804 can include one or
more of the
embodiments previously described with respect to the block 1304 of the process
1300. In some
embodiments, the first therapy data may include glycemic control information
resulting from
the delivery of the first therapy. In some examples, the system may store all
or some of the
therapy data generated during the first therapy period in a memory of the
control system 510.
In some examples, the therapy provided at block 1804, may comprise a plurality
of
medicament deliveries.
-108-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
103221 At block 1806, the control system 510
may determine the therapy effect of
the therapy provided during the first therapy period using statistical
analysis of the first therapy
data collected and stored at block 1804. In some examples, the statistical
analysis may include
calculating the statistical quantities discussed above and with reference to
Figure 17. In some
cases, the statistical analysis may include regression analysis between
certain mensured and/or
calculated parameters at block 1804 In some such examples the regression
analysis may
include determining an autoregression model. In some examples, the control
system 510 may
determine the therapy effect using one or more of the embodiments described
with respect to
the block 1306 of the process 1300.
103231 At block 1808, the control system 510
may modify the value of the control
parameter compared to the initial value selected at block 1802 or the value
used in the last
therapy period. In some examples, the modified value may be a value that makes
the therapy
more aggressive (e.g., aggressive by a significant amount). For example, when
the control
parameter is Tmax, at block 1808 the value of Tmax may be reduced to an amount
less (e.g.,
5, 10, 15 minutes, or more) than the value used in a previous therapy period
(e.g., the initial
value or the last modified value). In some examples, the modified value of the
control
parameter may be received from a user interface of the blood glucose control
system responsive
to a user interaction with the user interface. The previous therapy period may
be the first
therapy period or any earlier therapy period. In sonic examples, the value for
Tmax may be
lowered by a significant amount (e.g., 10 minutes, 15 minutes, or other
values). Further, the
amount by which Tmax is reduced may be smaller than a previous reduction
during a previous
iteration of the process 1800. In some embodiments, the control parameter may
be modified
automatically without action by a user. In some cases, modifying the control
parameter may
change a timing, a dosage size, or a speed of injection of insulin
administered to the subject.
103241 At block 1810, the control system 510
provides therapy to the subject based
at least in part on the glucose signal and the modified value of the control
parameter received
from block 1808. In some examples, the duration of the therapy period (at
block 1810), may
be equal to the duration of one or more previous therapy periods. In some
other examples, the
duration of the therapy period may be determined based on the determined
therapy effects of
the therapies delivered during one or more previous therapy periods. In some
examples, at
block 1810 the system may store all or some of the therapy data generated
during the therapy
-109-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
period. In some examples, the therapy provided at block 1810, may comprise a
plurality of
medicament deliveries. In some cases, the therapy data may include glycerine
control
information resulting from the delivery of the therapy.
103251 At block 1812, the control system 510
determines the therapy effect of the
therapy provided at block 1810 during the last therapy period. In some
examples, the therapy
effects may be determined based at least in part on the therapy data obtained
and stored at
block 1810. In some examples, the control system 510 may determine the therapy
effect using
one or more of the embodiments described with respect to the block 1306 of the
process 1300.
103261 At block 1814, the control system 510
performs a statistical analysis based
at least in part in the determined therapy effect of the therapies provided
and stored during the
last therapy period and the therapy period before the last therapy period to
obtain a comparative
assessment. In some such examples the comparative assessment may be based on
statistical
analysis of determined effects and the therapy data collected during the
corresponding therapy
periods. In some examples, the statistical analysis may include generating
statistical quantities
(e.g., distributions shown in FIG 17) using the therapy data. In some
examples, the statistical
analysis may include the analytical method described above. In some such
examples, one or
more characteristics of the statistical data may be used to compare the
therapy effects. In some
examples, the statistical analysis may include calculating one or more of a
mean, a median, a
mode, a standard deviation, a rate, a ratio, or a probability based on the
therapy data obtained
in the last two therapy periods or the determined effects of the therapies
provided during the
last two periods.
103271 At the decision block 1816, the
control system 510, based at least in part on
the comparative assessment received from block 1814, the control system 510
may determine
whether the value of the control parameter used during the last therapy period
has improved
the glycernic control for the subject compared to the therapy period before
the last therapy
period_ In some embodiments, the control system 510 may determine whether the
modified
value for the control parameter has resulted in statistically significant
improvement in glycerine
control. In some embodiments, the control system 510 may determine whether the
modified
value for the control parameter has resulted in an improvement of a
physiological parameter
of the subject. In these embodiments, the physiological parameter may be
determined based at
least in part on the glucose level signal received from a glucose level
sensor.
-110-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
103281 If the control system 510 determines
at the decision block 1816 that the
glycemic control for the subject is not improved, the control system 510 may
return to the
block 1810 and continue providing therapy to the subject based on the last
modified value of
the control parameter without any further modification.
[03291 If at the decision block 1816 the
control system 510 determines that the
value of the control parameter used during the last therapy period has
improved the glyceinic
control for the subject compared to the therapy period before the last therapy
period, the control
system 510 proceeds to decision block 1818. In some cases, the improvement in
the glycemic
control should be larger than a threshold level before the system 510 proceeds
to block 1818.
In some cases, the control system proceeds to block 1818 if the modified value
of the control
parameter results in a reduced occurrence of blood glucose excursions compared
to the first
value of the control parameter.
103301 At decision the block 1818 the control
system 510 may determine whether
the frequency and/or severity of hypoglycemia events is incrensed during the
last therapy
period compared to the therapy period before the last therapy period. In some
examples, if the
control system 510 determines that the frequency and/or severity of
hypoglycemia events is
increased (e.g., beyond a threshold number or amount) during the last therapy
period, the
control system 510 may return to the block 1810 and continue providing therapy
to the subject
based on the last modified value of the control parameter without any further
modification. If
at decision block 1818, the control system determines that the change in
frequency and/or
severity of hypoglycemia events is negligible (e.g., below a threshold number
or amount), the
control system may proceed to the block 1808 where the control system 510
modifies the value
of the control parameter. In some examples, the modified value may be a value
that results in
more aggressive therapy (e.g., the value of Tmax may be reduced). In some such
examples,
the amount by which the control parameter is changed may be smaller than a
reduction amount
in one or more previous modifications.
103311 in some examples, at the block 1818
the control system may determine risks
or the frequency and severity of one or more events other than hypoglycemia.
For example,
the control system may determine that in spite of an improvement in ulycemic
control for the
subject, the rate and magnitude of glucose concentration has increased beyond
threshold value.
-111-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
In some such examples, these additional risk determinations may be used to
determine whether
to keep or modify the last value of the control parameter.
(03321 In some embodiments, a modified
version of the process 1800 may be used
by the glucose control system wherein the process stops at block 1816 and the
control system
continues providing therapy based on the last modified value of the control
parameter until a
user input is received. In some such examples, the last value of the control
parameter (modified
at block 1808), the results of the comparative assessment generated based on
the comparison
performed at block 1814 (e.g., whether a statistically significant improvement
in subject's
glycernic control resulted from the last control parameter change), may be
output to the subject,
a guardian or a healthcare provider via a user interface of the glucose
control system 510 and/or
a computing system (e.g., a smartphone, a notebook a personal computer and the
like)
connected to the glucose control system (e.g., via a wireless link). In some
such embodiments,
at least in part based on the outcomes of the comparative assessment, the
subject, the guardian
or the healthcare provider may change the value of the corresponding control
parameter (e.g.,
an interaction with a user interface) before the next therapy period.
103331 In some examples, the statistical
analysis used to determine the therapy
effects (e.g., at blocks 1306 and 1312 in the process 1300, and bock 1806 and
1812 in the
process 1800) or to compare between therapy effects (e.g., at block 1316 in
the process 1300
and block 1814 in the process 1800), may include regression analysis. In some
examples,
regression analysis may be used to find a relation between parameters
calculated andror
measured during the therapy period. For example, with reference to Figure 17,
a regression
analysis may he used to find a relation between lit and the rate of glucose
concentration change
(e.g., using (1(t) near ti) for a plurality of therapies provided during a
therapy period. In some
cases, the outcomes of one or more regression analysis may be used in the
optimization process
to determine a value of the control parameter.
[03341 In some examples, the therapy data
captured and stored during one or more
therapy periods may be divided to equal time intervals wherein each time
interval starts and
ends at substantially the same specific start and end times within a 24
period. In some such
examples, an autoreuression model may be derived for the glycemic control over
the time
interval between the specific start and end times. Subsequently, the resulting
autoregression
model may be used to determine whether the glycemic control has been improved
compared
-112-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
to a previous therapy period. In some cases, the resulting autoregression
model may be used to
make additional adjustments to one or more control parameters in the
subsequent therapy
periods (after therapy periods following the period in which an autoregression
model is
determined).
[03351 In some examples, the outcome of the
statistical analysis of therapy data
may be used to evaluate the accuracy glucose signal generated by a CGM sensor.
103361 As mentioned above in some examples
the glucose control system may
generate a control parameter optimization report that may include some or all
of the statistical
quantities calculated during the optimization process, outcomes of the
statistical analysis and
graphical representation of the therapy data and related risk assessments. In
some such
examples, a Control Variability-Grid Analysis (CVGA) may be included in the
control
parameter optimization report, to visualize the variability of CGM data at a
group level from a
glucose-control point of view. In some examples the graphs may comprise
distinctive groups
of graphs, for example, to visualize average gly-cemia and deviations from
target values,
visualize variability and risk assessment, and event-based clinical
characteristics. In some other
examples, the graphical data may represent average glycemia and deviations
from target
glucose trace and aggregated glucose trace representing the time spent below,
within or above
the preset target range and visualizing the crossing of glycemic thresholds.
In yet other
examples, the control parameter optimization report may include graphs
representing
variability and risk assessment data. For example, a risk trace may be
presented to highlighting
essential variance (e.g., by equalizing the size of glucose deviations towards
hypo- and
hyperglycemia, emphasizing large glucose excursions, and suppress fluctuation
within target
range). In some other examples, histogram of blood glucose rate of change may
be included in
the report to presented, for example, the spread and range of glucose
transitions. In yet other
examples, Poincaret plots may be included in the report to visualize the
stability of the glucose
signal during different therapy periods that may be also associated with
different values of a
control parameter.
Example Embodiments
103371 The following is a list of multiple
sets of example numbered embodiments.
The features recited in the below list of example embodiments can be combined
with additional
-113-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
features disclosed herein. Further, each set of example numbered embodiments
in the
following list can be combined with one or more additional sets of example
numbered
embodiments from the following list Furthermore, additional inventive
combinations of
features are disclosed herein, which are not specifically recited in the below
list of example
embodiments and which do not include the same features as the embodiments
listed below.
For sake of brevity, the below list of example embodiments does not identify
every inventive
aspect of this disclosure. The below list of example embodiments are not
intended to identify
key features or essential features of any subject matter described herein.
I. A computer-implemented method of generating an
indication of total carbohydrate
therapy over a period using a medicament pump configured to deliver at least
insulin therapy
to a subject, the method comprising:
by a hardware processor configured to generate dose control signals for the
medicament pump configured to deliver at least insulin therapy to the subject
receiving a glucose level of the subject;
determining based at least in part on the glucose level that a triggering
event for raising blood glucose level of the subject has occurred, wherein the
triggering event indicates an impending risk of hypoglycemia is present in the
subject or that an episode of hypoglycemia is present in the subject;
determining an amount of a counter-regulatory agent to respond to the
impending risk of hypoglycemia or the episode of hypoglycemia;
determining a dose of carbohydrate therapy based at least in part on the
amount of the counter-regulatory agent;
tracking, over a period comprising a plurality of hypoglycemia risk
events or hypoglycemia episodes, determined doses of carbohydrate therapy to
generate the indication of total carbohydrate therapy over the period: and
outputting the indication of total carbohydrate therapy.
2. The computer-implemented method of Embodiment I, further comprising
providing the amount of the counter-regulatory agent to the subject responsive
to the
impending risk of hypoglycemia or the episode of hypoglycemia.
-114-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
3. The computer-implemented method of Embodiment 1, further comprising
providing the amount of the counter-regulatory agent to the subject responsive
to the glucose
level satisfying Of falling below a threshold glucose level.
4. The computer-implemented method of Embodiment 3, wherein the threshold
glucose level is set based on a risk tolerance of the subject to a
hypoglycemic event.
5. The computer-implemented method of Embodiment 1, wherein the indication of
total carbohydrate therapy corresponds to a reduction in carbohydrates
consumed by the
subject.
6. The computer-implemented method of Embodiment 1, wherein the indication of
total carbohydrate therapy corresponds to a reduction in carbohydrates
achievable by an
availability of the counter-regulatory agent.
7. The computer-implemented method of Embodiment 1, wherein the indication of
total carbohydrate therapy corresponds to an amount of counter-regulatory
agent provided to
the subject as a substitute for carbohydrates.
8. The computer-implemented method of Embodiment 1, wherein the indication of
total carbohydrate therapy comprises an indication of a range of
carbohydrates.
9. The computer-implemented method of Embodiment 1, wherein determining the
dose of carbohydrate therapy comprises:
accessing a mapping between the counter-regulatory agent and carbohydrates;
and
determining the dose of carbohydrate therapy based at least in part on the
mapping and the amount of the counter-regulatory agent.
10. The computer-implemented method of Embodiment 9, wherein the mapping is
based at least in part on a type of the carbohydrates.
11. The computer-implemented method of Embodiment 9, wherein the mapping is
Generated based on a clinical comparison of the counter-regulatory agent to
the carbohydrates.
12. The computer-implemented method of Embodiment 9, wherein the mapping is
based at least in part on a physiological characteristic of the subject.
13. The computer-implemented method of Embodiment 9, wherein the mapping is
based at least in part on a type of the counter-regulatory agent.
-115-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
14. The computer-implemented method of Embodiment 9, wherein the mapping
comprises a formula that relates the comiter-regulatory agent to the
carbohydrates.
15. The computer-implemented method of Embodiment 9, wherein the mapping
comprises a first mapping when the medicament pump comprises a bi-hormonal
pump
configured to deliver counter-regulatory agent therapy to the subject, and
wherein the mapping
comprises a second mapping when the medicament pump is not configured to
deliver the
counter-regulatory agent therapy to the subject
16. The computer-implemented method of Embodiment 1, wherein the indication of
total carbohydrate therapy comprises one or more of an indication of calories,
an indication of
carbohydrates, an indication of a measure of sugar, an indication of a
quantity of food, or an
indication of weight of the subject attributable to the carbohydrate therapy.
17. The computer-implemented method of Embodiment 1, wherein the period
corresponds to a particular time period, to a number of events included in the
plurality of
hypoglycemia risk events, or to a number of episodes included in the plurality
of hypoglycemia
episodes.
18. An automated blood glucose control system configured to generate an
indication of
total carbohydrate therapy over a period in a subject, the automated blood
glucose control
system comprising:
a medicament delivery interface configured to operatively connect to a
medicament pump configured to infuse medicament into the subject, wherein the
medicament comprises at least insulin;
a memory configured to store specific computer-executable instructions; and
a hardware processor in communication with the memory and configured to
execute the specific computer-executable instructions to at least:
receive a glucose level of the subject;
determine based at least in part on the glucose level that a triggering
event for raising blood glucose level of the subject has occurred, wherein the
triggering event indicates that an impending risk of hypoglycemia is present
in
the subject or that an episode of hypoglycemia is present in the subject;
determine an amount of a counter-regulatory agent to respond to the
impending risk of hypoglycemia or the episode of hypoglycemia;
-116-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
determine a dose of carbohydrate therapy based at least in part on the
amount of the counter-regulatory agent;
track, over a period comprising a plurality of hypoglycemia risk events
or hypoglycemia episodes, determined doses of carbohydrate therapy to
generate the indication of total carbohydrate therapy over the period; and
output the indication of total carbohydrate therapy.
19. The automated blood glucose control system of Embodiment 18, wherein the
hardware processor is further configured to operate a control algorithm for
automatic
generation of a counter-regulatory agent dosing signal configured to operate
the medicament
pump to control blood glucose level in the subject based at least in part on a
glucose level
signal received from a glucose level sensor operatively connected to the
subject indicating that
the glucose level does not satisfy a threshold corresponding to the triggering
event.
20. The automated blood glucose control system of Embodiment 18, wherein the
memory is further configured to store a mapping between the counter-regulatory
agent and the
carbohydrates, and wherein the hardware processor is further configured to:
access the mapping from the memory; and
determine the dose of carbohydrate therapy based at least in part on the
mapping
and the amount of the counter-regulatory agent
21. The automated blood glucose control system of Embodiment 20, wherein the
mapping comprises an algorithm that relates the counter-regulatory agent to
the carbohydrates.
22. The automated blood glucose control system of Embodiment 18, wherein the
period
corresponds to a particular time period, to a number of events included in the
plurality of
hypoglycemia risk events, or to a number of episodes included in the plurality
of hypoglycemia
episodes.
[0338]
Additional embodiments of the
present disclosure can be described in view
of the following numbered embodiments:
1.
A computer-implemented method
of modifying therapy provided to a subject using
a blood glucose control system, the method comprising:
by a hardware processor configured to generate a dose control signal for the
blood glucose control system,
-117-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
receiving a glucose level signal from a glucose level sensor operatively
connected to the subject;
causing first therapy to be delivered by the blood glucose control system
to a subject during a first therapy period, wherein the first therapy is
delivered
based at least in part on a first value of a control parameter used by a
control
algorithm to generate the dose control signal, wherein the control parameter
is
used by the control algorithm to account for accumulation of insulin in the
subject, thereby controlling an insulin dosing response of the control
algorithm
to a blood glucose excursion in the subject as indicated by the glucose level
signal;
determining a first effect corresponding at least in part to the first
therapy, wherein determining the first effect comprises analyzing glyc-emic
control of blood glucose in the subject as indicated by the glucose level
signal;
autonomously generating a second value of the control parameter,
wherein the autonomously generated second value is determined as a function
based on the first value and the first effect;
modifying the control parameter from the first value to the second value;
and
causing second therapy to be delivered by the blood glucose control
system to the subject during a second therapy period, wherein the second
therapy is delivered based at least in part on the second value of the control
parameter, and wherein changing the control parameter modifies the therapy
provided to the subject
2. The computer-implemented method of Embodiment I. further comprising:
by the hardware processor,
determining a second effect corresponding at least in part to the second
therapy;
selecting one of the first value of the control parameter or the second
value of the control parameter as an active control parameter value based at
least in part on a comparison of the first effect and the second effect; and
-1 1 8-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
configuring the blood glucose control system to provide therapy to the
subject during a third therapy period based at least in part on the active
control
parameter value, wherein the selection of the active control parameter value
modifies the therapy provided to the subject.
3. The computer-implemented method of Embodiment 1, wherein the control
parameter used by the control algorithm relates to at least one time constant
used in a
calculation of an accumulation of insulin in the subject by the control
algorithm_
4. The computer-implemented method of Embodiment 1, wherein the control
parameter used by the control algorithm corresponds to a rate of insulin
diminishment in the
subject.
5. The computer-implemented method of Embodiment 1, wherein the first therapy
period comprises a time period corresponding to the administering of multiple
instances of
therapy, and wherein the first therapy comprises the multiple instances of
therapy_
6. The computer-implemented method of Embodiment 1, wherein modifying the
control parameter to the second value modifies one or more of a timing, a
dosage size, or an
administration rate of insulin administered during the second therapy period.
7. The computer-implemented method of Embodiment 1, wherein the first value of
the control parameter is based at least in part on one or more of therapy
delivered during a time
period prior to the first therapy period, a clinical value, or a body mass of
the subject
8. The computer-implemented method of Embodiment 1, wherein the control
parameter used by the control algorithm corresponds to a time until insulin
within blood plasma
of the subject reaches a particular concentration level subsequent to
administration of an insulin
dose.
9. A computer-implemented method of modifying therapy provided to a subject
using
a blood glucose control system, the method comprising:
by a hardware processor configured to generate a dose control signal for the
blood glucose control system,
causing first therapy to be delivered by the blood glucose control system
to a subject during a first therapy period, wherein the first therapy is
delivered
based at least in part on a first value of a control parameter used by a
control
algorithm to generate the dose control signal;
-119-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
determining a first effect corresponding at least in part to the first
therapy, wherein determining the first effect comprises receiving a glucose
level signal from a glucose level sensor operatively connected to the subject;
autonomously generating a second value of the control parameter based
at least in part on a baseline value of the control parameter and an output of
a
function defined based on glycemic control of the subject, wherein the glucose
level signal comprises an indication of the glycemic control of the subject
during the first therapy period;
modifying the control parameter from the first value to the second value;
and
causing second therapy to be delivered by the blood glucose control
system to the subject during a second therapy period, wherein the second
therapy is delivered based at least in part on the second value of the control
parameter, and wherein changing the control parameter modifies the therapy
provided to the subject
10. The computer-implemented method of Embodiment 9, further comprising:
by the hardware processor,
determining a second effect corresponding at least in part to the second
therapy;
selecting one of the first value of the control parameter or the second
value of the control parameter as an active control parameter value based at
least in part on a comparison of the first effect and the second effect; and
configuring the blood glucose control system to provide therapy to the
subject during a third therapy period based at least in part on the active
control
parameter value, wherein the selection of the active control parameter value
modifies the therapy provided to the subject.
11. The computer-implemented method of Embodiment 9, wherein the first therapy
comprises multiple instances of therapy administered over the first therapy
period.
12. The computer-implemented method of Embodiment 9, wherein the control
parameter used by the control algorithm corresponds to a time until insulin
within blood of the
subject reaches a particular concentration level attributable to
adininistrafion of an insulin dose.
-120-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
13. A computer-implemented method of modifying therapy provided to a subject
using
a blood glucose control system, the method comprising
by a hardware processor configured to generate a dose control signal for the
blood glucose control system,
causing first therapy to be delivered by the blood glucose control system
to a subject during a first therapy period, wherein the first therapy is
delivered
based at least in part on a first value of a control parameter used by a
control
algorithm to generate the dose control signal;
determining a first effect corresponding at least in part to the first
therapy, wherein determining the first effect comprises receiving a glucose
level signal from a glucose level sensor operatively connected to the subject;
autonomously generating a second value of the control parameter,
wherein the autonomously generated second value is determined as a function
based at least in part on a baseline value;
modifying the control parameter from the first value to the second value;
causing second therapy to be delivered by the blood glucose control
system to the subject during a second therapy period, wherein the second
therapy is delivered based at least in part on the second value of the control
parameter, and wherein changing the control parameter modifies the therapy
provided to the subject;
determining a second effect corresponding at least in part to the second
therapy;
autonomously performing a comparison of the first effect and the
second effect without action by a human;
selecting one of the first value of the control parameter or the second
value of the control parameter as an active control parameter value based at
least in part on the comparison of the first effect and the second effect; and
configuring the blood glucose control system to provide therapy to the
subject during a third therapy period based at least in part on the active
control
parameter value, wherein the selection of the active control parameter value
modifies the therapy provided to the subject.
-121-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
14. The computer-implemented method of Embodiment 13, wherein the second value
of the control parameter is based at least in part on glycemic control
indicated by the glucose
level signal.
15. The computer-implemented method of Embodiment 13, wherein the baseline
value
comprises the first value of the control parameter.
16. The computer-implemented method of Embodiment 13, wherein the first value
of
the control parameter is determined based at least in part on the baseline
value.
17. The computer-implemented method of Embodiment 13, further comprising
performing the comparison of the first effect and the second effect, wherein
the comparison is
performed substantially in real-time in response to determining the second
effect.
18. The computer-implemented method of Embodiment 13, further comprising
performing the comparison of the first effect and the second effect, wherein
the comparison of
the first effect and the second effect comprises performing a statistical
comparison of the first
effect and the second effect.
19. The computer-implemented method of Embodiment 13, further comprising
performing the comparison of the first effect and the second effect, wherein
the comparison of
the first effect and the second effect comprises performing regression
analysis of at least the
first effect and the second effect.
20. The computer-implemented method of Embodiment 13, wherein the second value
of the control parameter may be selected based on performance of a regression
analysis
between a time of absorption of subcutaneously administered insulin into blood
of the subject
and a glycemic control function, wherein the glycemic control function is
based at least in part
on the glucose level signal.
21. An automated blood glucose control system configured to autonomously
modify a
control parameter used by a control algorithm to generate a dose control
signal that causes
therapy to be provided to a subject, the automated blood glucose control
system comprising:
a medicament delivery interface configured to operatively connect to a
medicament pump for infusing medicament into the subject;
a memory configured to store specific computer-executable instructions and
therapy data; and
-122-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
a hardware processor in communication with the memory and configured to
execute the specific computer-executable instructions to at least:
receive a glucose level signal from a glucose level sensor operatively
connected to the subject;
cause first therapy to be delivered to the subject during a first therapy
period, wherein the first therapy is delivered based at least in p2rt on a
first
value of the control parameter used by the control algorithm to generate the
dose control signal, wherein the control parameter is used by the control
algorithm to account for accumulation of insulin in the subject, thereby
controlling an insulin dosing response of the control algorithm to a blood
glucose excursion in the subject as indicated by the glucose level signal;
determine a first effect corresponding at least in part to the first therapy,
wherein determining the first effect comprises analyzing glycemic control of
blood glucose in the subject as indicated by the glucose level signal;
autonomously generate a second value of the control parameter, wherein
the autonomously generated second value is determined as a function based on
the first value and the first effect;
modify the control parameter from the first value to the second value;
and
cause second therapy to be delivered to the subject during a second
therapy period, wherein the second therapy is delivered based at least in part
on
the second value of the control parameter, and wherein changing the control
parameter modifies the therapy provided to the subject.
22. The automated blood glucose control system of Embodiment 21, wherein the
hardware processor is further configured to execute the specific computer-
executable
instructions to at least:
determine a second effect corresponding at least in part to the second
therapy;
select one of the first value of the control parameter or the second value of
the
control parameter as an active control parameter value based at least in part
on a
comparison of the first effect and the second effect; and
-123-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
provide therapy to the subject during a third therapy period based at least in
part
on the active control parameter value, wherein the selection of the active
control
parameter value modifies the therapy provided to the subject
23. The automated blood glucose control system of Embodiment 21, wherein the
control parameter used by the control algorithm relates to at least one time
constant used in a
calculation of an accumulation of insulin in the subject by the control
algorithm.
24. The automated blood glucose control system of Embodiment 21, wherein the
control parameter used by the control algorithm corresponds to a rate of
insulin diminishment
in the subject.
25. The automated blood glucose control system of Embodiment 21, wherein the
first
therapy period comprises a time period corresponding to the administering of
multiple
instances of therapy, and wherein the first therapy comprises the multiple
instances of therapy.
26. The automated blood glucose control system of Embodiment 21, wherein
modifying the control parameter to the second value modifies one or more of a
timing, a dosage
size, or a rate of administration of insulin administered during the second
therapy period.
27. The automated blood glucose control system of Embodiment 211,wherein the
first
value of the control parameter is based at least in part on one or more of
therapy delivered
during a time period prior to the first therapy period, a clinical value, or a
body mass of the
subject.
28. The automated blood glucose control system of Embodiment 21, wherein the
control parameter used by the control algorithm corresponds to a time until
insulin within blood
plasma of the subject reaches a particular concentration level subsequent to
administration of
an insulin dose.
29. The automated blood glucose control system of Embodiment 21, wherein the
control parameter corresponds to Tmax or T1n,
[0339]
Additional embodiments of the
present disclosure can be described in view
of the following numbered embodiments:
1.
A computer-implemented method
of modifying therapy provided to a subject using
a blood glucose control system, the method comprising:
-124-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
by a hardware processor configured to generate a dose control signal for the
blood glucose control system,
receiving a glucose level signal from a glucose level sensor operatively
connected to the subject;
causing first therapy to be delivered by the blood glucose control system
to the subject during a first therapy period, wherein the first therapy is
delivered
based at least in part on a first value of a control parameter used by a
control
algorithm to generate the dose control signal, wherein the control parameter
is
used by the control algorithm to account for accumulation of insulin in the
subject, thereby controlling an insulin dosing response of the control
algorithm
to a blood glucose excursion in the subject as indicated by the glucose level
signal;
obtaining a first therapy data comprising glyceinic control information
resulting from the delivery of the first therapy;
determining a first effect corresponding at least in part to the first
therapy over a first time period, wherein the first effect is determined based
at
least in part on the first therapy data.;
setting the control parameter to a second value that differs from the first
value;
causing second therapy to be delivered by the blood glucose control
system to the subject during a second therapy period, wherein the second
therapy is delivered based at least in part on the second value of the control
parameter, and wherein changing the control parameter modifies the therapy
provided to the subject;
obtaining a second therapy data comprising glycemic control
information resulting from the delivery of the second therapy;
determining a second effect corresponding at least in part to the second
therapy over a second time period, wherein the second effect is determined
based at least in part on the second therapy data;
performing a statistical analysis based at least in part on the first effect
and the second effect to obtain a comparative assessment; and
-125-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
determining based at least in part on the comparative assessment,
whether the second value for the control parameter results in an improvement
in glycemic control for the subject.
2. The computer-implemented method of Embodiment 1, wherein the control
parameter is set to the first value or the second value based at least in part
on a user interaction
with a user interface of the blood glucose control system_
3. The computer-implemented method of Embodiment 1, wherein the control
parameter is automatically set to the first value or the second value without
action by a user,
4. The computer-implemented method of Embodiment 1, wherein the second
value of
the control parameter is autonomously selected_
5. The computer-implemented method of Embodiment 1, wherein, in response to
determining that the second value results in the improvement of glycemic
control for the
subject, the method further comprises selecting the second value of the
control parameter to
cause third therapy to be delivered by the blood glucose control system to the
subject during a
third therapy period.
6. The computer-implemented method of Embodiment 1, wherein determining
whether the second value for the control parameter results in the improvement
in glycemic
control for the subject comprises determining whether the second value for the
control
parameter results in a statistically significant improvement in glycemic
control for the subject.
7. The computer-implemented method of Embodiment 6, wherein the statistically
significant improvement comprises a threshold level of improvement in glycemic
control for
the subject.
8. The computer-implemented method of Embodiment 1, wherein determining
whether the second value for the control parameter results in the improvement
in glycemic
control for the subject comprises determining whether the second value for the
control
parameter results in an improvement of a physiological parameter of the
subject.
9. The computer-implemented method of Embodiment 8, wherein the physiological
parameter is determined based at least in part on the glucose level signal.
10. The computer-implemented method of Embodiment 1, wherein determining
whether the second value for the control parameter results in the improvement
in glycemic
control for the subject comprises determining whether the second value for the
control
-126-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
parameter results in a reduced occurrence of blood glucose excursions compared
to the first
value of the control parameter.
11. The computer-implemented method of Embodiment I. wherein determining
whether the second value for the control parameter results in the improvement
in glycemic
control for the subject comprises determining whether the second value for the
control
parameter results in a reduced risk of an occurrence of a hypoglycemic event
compared to the
first value of the control parameter
12. The computer-implemented method of Embodiment 1, wherein performing the
statistical analysis comprises determining one or more of a mean, a median, a
mode, a standard
deviation, a rate, a ratio, or a probability based on the first therapy data
or the second therapy
data.
13. The computer-implemented method of Embodiment 1, wherein performing the
statistical analysis comprises determining one or more of a mean, a median, a
mode, a standard
deviation, a rate, a ratio, or a probability based at least in part on the
first effect or the second
effect.
14. The computer-implemented method of Embodiment 1, wherein the control
parameter used by the control algorithm corresponds to a time until insulin
within blood plasma
of the subject reaches a particular concentration level subsequent to
administration of an insulin
dose.
15. The computer-implemented method of Embodiment 1, wherein causing the first
therapy to be delivered during the first therapy period comprises causing a
plurality of
instances of therapy to be administered, and wherein at least one of the
plurality of instances
of therapy is administered at a different time period during the first therapy
period than at least
one other instance of therapy.
16. The computer-implemented method of Embodiment 1, wherein the first therapy
period and the second therapy period are of the same duration.
17. The computer-implemented method of Embodiment 1, wherein a first plurality
of
instances of therapy are administered during the first therapy period and a
second plurality of
instances of therapy are administered during the second therapy period.
-127-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
18. The computer-implemented method of Embodiment 1, wherein setting the
control
parameter to the second value causes a modification to one or more of a
timing, a dosage size,
or a speed of injection of insulin administered during the second therapy
period_
19. The computer-implemented method of Embodiment 1, wherein the first value
of
the control parameter is based on one or more of: therapy delivered during a
time period prior
to the first therapy period, a clinical value, or a body mass of the subject
20. The computer-implemented method of Embodiment 1, wherein the control
algorithm is based at least in part on a pharmacokinetic (PK) model.
21. The computer-implemented method of Embodiment 20, wherein the control
parameter comprises a parameter of the pharmacokinetic (PK) model.
22. The computer-implemented method of Embodiment 1, wherein performing the
statistical analysis comprises one or more of performing a regression analysis
or generating an
autoregression model_
23. An automated blood glucose control system configured to autonomously
modify a
control parameter used by a control algorithm to generate a dose control
signal that causes
therapy to be provided to a subject, the automated blood glucose control
system comprising:
a medicament delivery interface configured to operatively connect to a
medicament pump for infusing medicament into the subject;
a memory configured to store specific computer-executable instructions and
therapy data; and
a hardware processor in communication with the memory and configured to
execute the specific computer-executable instructions to at least:
receive a glucose level signal from a glucose level sensor operatively
connected to the subject;
cause first therapy to be delivered to the subject during a first therapy
period, wherein the first therapy is delivered based at least in part on a
first
value of a control parameter used by a control algorithm to generate the dose
control signal, wherein the control parameter is used by the control algorithm
to account for accumulation of insulin in the subject, thereby controlling an
insulin dosing response of the control algorithm to a blood glucose excursion
in the subject as indicated by the glucose level signal;
-128-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
obtain a first therapy data comprising glycemic control information
resulting from the delivery of the first therapy;
determine a first effect corresponding at least in part to the first therapy
over a first time period, wherein the first effect is determined based at
least in
part on the first therapy data;
set the control parameter to a second value that differs from the first
value;
cause second therapy to be delivered by the blood glucose control
system to the subject during a second therapy period, wherein the second
therapy is delivered based at least in part on the second value of the control
parameter, and wherein changing the control parameter modifies the therapy
provided to the subject;
obtain a second therapy data comprising glycemic control information
resulting from the delivery of the second therapy;
determine a second effect corresponding at least in part to the second
therapy over a second time period, wherein the second effect is determined
based at least in part on the second therapy data;
perform a statistical analysis based at least in part on the first effect and
the second effect to obtain a comparative assessment;
determine based at least in part on the comparative assessment, whether
the second value for the control parameter results in an improvement in
glycemic control for the subject.
24. The automated blood glucose control system of Embodiment 23, wherein, in
response to determining that the second value results in the improvement of
&vocalic control
for the subject, the hardware processor is further configured to execute the
specific computer-
executable instructions to at least select the second value of the control
parameter to cause third
therapy to be delivered by the blood glucose control system to the subject
during a third therapy
period.
25. The automated blood glucose control system of Embodiment 23, wherein
determining whether the second value for the control parameter results in the
improvement in
glycemic control for the subject comprises determining whether the second
value for the
-129-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
control parameter results in a threshold level of improvement of at least one
physiological
parameter of the subject
26. The automated blood glucose control system of Embodiment 23, wherein
determining whether the second value for the control parameter results in the
improvement in
glycemic control for the subject comprises determining whether the second
value for the
control parameter results in a reduced occurrence of blood glucose excursions
or a reduced risk
of an occurrence of a hypoglycemic event compared to the first value of the
control parameter.
27. The automated blood glucose control system of Embodiment 23, wherein the
control parameter used by the control algorithm corresponds to a time until
insulin within blood
plasma of the subject reaches a particular concentration level subsequent to
administration of
an insulin dose.
28. The automated blood glucose control system of Embodiment 23, wherein a
length
of the first therapy period is selected to encompass at least a particular
number of instances of
therapy, and wherein a length of the second therapy period is elected to
encompass at least the
particular number of instances of therapy.
29. The automated blood glucose control system of Embodiment 23, wherein
performing the statistical analysis comprises one or more of performing a
regression analysis
or generating an autoregression model.
30. The automated blood glucose control system of Embodiment 23, wherein the
control algorithm is based at least in part on a pharmacokinetic (PK) model,
and wherein the
control parameter comprises a parameter of the pharrnacokinetic (PK) model.
103401 Additional embodiments of the present
disclosure can be described in view
of the following numbered embodiments:
I. An automated blood glucose control system configured to generate a backup
therapy protocol comprising insulin therapy instructions derived from
autonomously
determined doses of insulin, the automated blood glucose control system
comprising:
a medicament delivery interface configured to operatively connect to a
medicament pump for infusing medicament into the subject;
a memory configured to store specific computer-executable instructions; and
-130-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
a hardware processor in communication with the memory and configured to
execute the specific computer-executable instructions to at least
receive a glucose level signal from a sensor operatively configured to
determine glucose levels in the subject;
generate a dose control signal using a control algorithm configured to
autonomously determine doses of insulin to be infused into the subject for the
purpose of controlling blood glucose of the subject based at least in part on
the
glucose level signal;
track insulin therapy administered to the subject over a tracking period
comprising at least one day by the automated blood glucose control system,
wherein tracking the insulin therapy comprises storing an indication of the
autonomously determined doses of insulin delivered to the subject as basal
insulin, as correction boluses of insulin, or as mealtime boluses of insulin;
generate at least one of a backup injection therapy protocol or a backup
pump therapy protocol comprising insulin therapy instructions based at least
in
part on the insulin therapy administered to the subject over the tracking
period;
and
output the at least one of the backup injection therapy protocol or the
backup pump therapy protocol on a display enabling therapy to be maintained
at a rate determined by the automated blood glucose control system when the
automated blood glucose control system is not providing therapy to the
subject.
2. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to execute the specific computer-
executable
instructions to at least store the indication of the autonomously determined
doses of insulin
delivered to the subject in the memory.
3. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to execute the specific computer-
executable
instructions to at least:
establish a communication channel with an external computing system that is
separate from the automated blood glucose control system; and
-131-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
transmit the indication of die autonomously determined doses of insulin
delivered to the subject to the external computing system.
4. The automated blood glucose control system of Embodiment 3, wherein the
external computing system is a computing system of a data center, and the
hardware processor
is further configured to execute the specific computer-executable instructions
to at least control
a radio capable of communicating with the external computing system over a
wide area
network.
5. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to generate the backup injection
therapy protocol by
at least determining a number of long acting insulin units based at least in
part on an average
total basal insulin provided to the subject per day over the tracking period.
6. The automated blood glucose control system of Embodiment 1, wherein each
day
of the tracking period is divided into a plurality of sub-periods, and wherein
the hardware
processor is further configured to generate the backup pump therapy protocol
by at least
determining an hourly basal rate for each sub-period of the plurality of sub-
periods.
7. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to generate the backup injection
therapy protocol or
the backup pump therapy protocol by at least determining an average correction
bolus provided
to the subject per day over the tracking period.
8. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to generate the backup injection
therapy protocol or
the backup pump therapy protocol by at least determining an average correction
bolus provided
to the subject over the tracking period.
9. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to generate the backup injection
therapy protocol or
the backup pump therapy protocol by at least determining an average change in
blood glucose
at least partially attributable to a unit of insulin provided as a correction
bolus to the subject
during the tracking period.
10. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to generate the backup injection
therapy protocol or
the backup pump therapy protocol by at least determining, for each mealtime of
a plurality of
-132-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
mealtimes per day, an average mealtime bolus of insulin provided to the
subject over the
tracking period.
11. The automated blood glucose control system of Embodiment 1, wherein the
hardware processor is further configured to execute the specific computer-
executable
instructions to at least:
track counter-regulatory agent therapy administered to the subject over the
tracking period, wherein tracking the counter-regulatory agent therapy
comprises
storing an indication of autonomously determined doses of counter-regulatory
agent
delivered to the subject responsive to the glucose level signal; and
include, in at least one of the backup injection therapy protocol or the
backup
pump therapy protocol, an indication of total counter-regulatory agent and/or
daily
counter-regulatory agent provided to the subject over the tracking period.
12. The automated blood glucose control system of Embodiment 1, wherein the
control
algorithm is further configured to autonomously determine doses of insulin to
be infused into
the subject for the purpose of controlling blood glucose of the subject based
at least in part on
the glucose level signal and a control parameter that is modifiable by user
interaction with a
control parameter selection interface element, and wherein the hardware
processor is further
configured to execute the specific computer-executable instructions to at
least:
track user modifications to the control parameter over the tracking period,
wherein tracking the user modifications comprises storing in a therapy log
whether
each of the user modifications comprises an increase or a decrease in the
control
parameter from a stored control parameter value and a time during which each
of the
user modifications occurred; and
generate a report of user modifications to the control parameter, wherein the
report comprises a measure of frequency of increases and decreases from the
stored
control parameter value, and wherein the report is included in at least one of
the backup
injection therapy protocol or the backup pump therapy protocol.
13. A computer-implemented method of generating a backup therapy protocol
comprising insulin therapy instructions derived from autonomously determined
doses of
insulin determined by an automated blood glucose control system, the computer-
implemented
method comprising:
-133-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
by a hardware processor of the automated blood glucose control system,
receiving a glucose level signal from a sensor operatively configured to
determine glucose levels in the subject;
generating a dose control signal using a control algorithm configured to
autonomously determine doses of insulin to be infused into the subject for the
purpose of controlling blood glucose of the subject based at least in part on
the
glucose level signal;
tracking insulin therapy administered by the automated blood glucose
control system to the subject over a tracking period comprising at least one
day,
wherein tracking the insulin therapy comprises storing an indication of the
autonomously determined doses of insulin delivered to the subject;
generating at least one of a backup injection therapy protocol or a
backup pump therapy protocol comprising insulin therapy instructions based at
least in part on the insulin therapy administered to the subject over the
tracking
period; and
outputting the at least one of the backup injection therapy protocol or
the backup pump therapy protocol on a display enabling therapy to be
maintained at a rate determined by the automated blood glucose control system
when the automated blood glucose control system is not providing therapy to
the subject
14. The computer-implemented method of Embodiment 13, wherein the autonomously
determined doses of insulin comprise one or more of a basal insulin dose, a
correction bolus
of insulin, or a mealtime bolus of insulin.
15. The computer-implemented method of Embodiment 13, further comprising:
establish a communication channel with an external computing system that is
separate from the automated blood glucose control system; and
transmitting the indication of the autonomously determined doses of insulin
infused into the subject to the external computing system.
16. The computer-implemented method of Embodiment 13, wherein generating the
at
least one of a backup injection therapy protocol or a backup pump therapy
protocol comprises
one or more of the following:
-134-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
determining a number of long acting insulin units based at least in part on an
average total basal insulin provided to the subject per day over the tracking
period;
dividing the tracking period into a plurality of sub-periods and determining
an
hourly basal rate for each sub-period of the plurality of sub-periods;
determining an average correction bolus provided to the subject over the
tracking period;
determining an average change in blood glucose at least partially attributable
to
a unit of insulin provided as a correction bolus to the subject during the
tracking period;
OF
determining, for each mealtime of a plurality of mealtimes per day, an average
mealtime bolus of insulin provided to the subject over the tracking period.
17. The computer-implemented method of Embodiment 13, further comprising
tracking counter-regulatory agent therapy administered to the subject over the
tracking period,
wherein tracking the counter-regulatory agent therapy comprises storing an
indication of
autonomously determined doses of counter-regulatory agent delivered to the
subject
responsive to the glucose level signal, and wherein generating the at least
one of a backup
injection therapy protocol or a backup pump therapy protocol comprises
including, in at least
one of the backup injection therapy protocol or the backup pump therapy
protocol, an
indication of total counter-regulatory agent and/or daily counter-regulatory
agent provided to
the subject over the tracking period.
18. An automated blood glucose control system configured to generate a report
of
therapy protocol modifications made by a user of the automated blood glucose
control system,
the automated blood glucose control system comprising:
a medicament delivery interface configured to operatively connect to a
medicament pump for infusing medicament into a subject;
a memory configured to store specific computer-executable instructions, a
stored control parameter value, and a therapy log; and
a hardware processor in communication with the memory and configured to
execute the specific computer-executable instructions to at least:
receive a glucose level signal from a sensor operatively configured to
determine glucose levels in the subject;
-135-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
generate a dose control signal using a control algorithm configured to
autonomously determine doses of insulin to be infused into the subject for the
purpose of controlling blood glucose of the subject based at least in part on
the
glucose level signal and a control parameter that is modifiable by user
interaction with a control parameter selection interface element;
track user modifications to the control parameter over a tracking period
comprising at least one day, wherein tracking the user modifications comprises
storing in the therapy log whether each of the user modifications comprises an
increase or a decrease in the control parameter from the stored control
parameter value and a time during which each of the user modifications
occurred; and
generate a report of user modifications to the control parameter, wherein
the report comprises a measure of frequency of increases and decreases from
the stored control parameter value.
19. The automated blood glucose control system of Embodiment 18, wherein the
report
further comprises a percentage of user modifications higher or lower than the
stored control
parameter value over the tracking period.
20. The automated blood glucose control system of Embodiment 18, wherein the
report
further comprises a number of times that infusion of insulin is paused over
the tacking period.
21. The automated blood glucose control system of Embodiment 18, wherein the
report
further comprises a percentage of time the stored control parameter is not
modified by a user
over the tracking period
22. The automated blood glucose control system of Embodiment 18, wherein the
tracking period is divided into a plurality of sub-periods, and wherein the
hardware processor
is further configured to track user modifications to the control parameter for
each sub-period
of the tracking period, wherein the report comprises a measure of frequency of
increa..-es and
decreases from the stored control parameter value for each sub-period of the
tracking period.
23. The automated blood glucose control system of Embodiment 22, wherein at
least a
first sub-period of the plurality of sub-periods is associated with a first
value for the control
parameter and at least a first sub-period of the plurality of sub-periods is
associated with a
second value for the control parameter, and wherein the hardware processor is
further
-136-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
configured to track user modifications to the first value of the control
parameter for the first
sub-period and track user modifications to the second value of the control
parameter for the
second sub-period.
24. The automated blood glucose control system of Embodiment 18, wherein the
hardware processor is further configured to track user activity associated
with the user
modifications to the control parameter, and wherein the report of user
modifications to the
control parameter includes an identity of user activity occurring during user
modification to
the control parameter
25. A computer-implemented method of generating a report of therapy protocol
modifications made by a user of an automated blood glucose control system
configured to
infuse medicament into a subject, the computer-implemented method comprising:
by a hardware processor of the automated blood glucose control system,
receiving a glucose level signal from a sensor operatively configured to
determine glucose levels in the subject;
generating a dose control signal using a control algorithm configured to
autonomously determine doses of insulin to be infused into the subject for the
purpose of controlling blood glucose of the subject based at least in part on
the
glucose level signal and a control parameter that is modifiable by user
interaction with a control parameter selection interface element;
tracking user modifications to the control parameter over a tracking
period comprising at least one day, wherein tracking the user modifications
comprises storing in a therapy log whether each of the user modifications
comprises an increase or a decrease in a value of the control parameter from a
stored control parameter value and a time during which each of the user
modifications occurred; and
generating a report of user modifications to the control parameter,
wherein the report comprises a measure of frequency of increases and decreases
from the stored control parameter value.
26. The computer-implemented method of Embodiment 25, wherein the report
further
comprises a percentage of user modifications higher or lower than the stored
control parameter
value over the tracking period.
-137-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
27. The computer-implemented method of Embodiment 25, wherein the report
further
comprises a number of times that infusion of insulin is paused over the
tracking period.
28. The computer-implemented method of Embodiment 25, wherein the tracking
period is divided into a plurality of sub-periods, and wherein the computer-
implemented
method further comprises tracking user modifications to the control parameter
for each sub-
period of the tracking period, and wherein the report comprises a measure of
frequency of
increases and decreases from the stored control parameter value for each sub-
period of the
tracking period.
29. The computer-implemented method of Embodiment 28, wherein at least a first
sub-
period of the plurality of sub-periods is associated with a first value for
the control parameter
and at least a first sub-period of the plurality of sub-periods is associated
with a second value
for the control parameter, and wherein the computer-implemented method further
comprises
tracking user modifications to the first value of the control parameter for
the first sub-period
and tracking user modifications to the second value of the control parameter
for the second
sub-period.
30. The computer-implemented method of Embodiment 25, further comprising
tracking user activity associated with the user modifications to the control
parameter, and
wherein the report of user modifications to the control parameter includes an
identity of user
activity occurring during user modification to the control parameter.
Terminology
[0341] lt is to be understood that not
necessarily all objects or advantages may be
achieved in accordance with any particular embodiment described herein. Thus,
for example,
those skilled in the art will recognize that certain embodiments may be
configured to operate
in a manner that achieves or optimizes one advantage or group of advantages as
taught herein
without necessarily achieving other objects or advantages as may be taught or
suggested
herein.
03421 All of the processes described herein
may be embodied in, and fully
automated via, software code modules executed by a computing system that
includes one or
more computers or processors. The code modules may be stored in any type of
non-transitory
computer-readable medium or other computer storage device. Some or all the
methods may be
-138-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
embodied in specialized computer hardware. Further, the computing system may
include, be
implemented as part of, or communicate with an automated blood glucose system,
an
ambulatory medicament system, or an ambulatory medical device.
(0343) Many other variations than those
described herein will be apparent from this
disclosure. For example, depending on the embodiment, certain acts, events, or
functions of
any of the algorithms described herein can be performed in a different
sequence, can be added,
merged, or left out altogether (for example, not all described acts or events
are necessary for
the practice of the algorithms). Moreover, in certain embodiments, acts or
events can be
performed concurrently, for example, through multi-threaded processing,
interrupt processing,
or multiple processors or processor cores or on other parallel architectures,
rather than
sequentially. In addition, different tasks or processes can be performed by
different machines
and/or computing systems that can function together.
103441 The various illustrative logical
blocks and modules described in connection
with the embodiments disclosed herein can be implemented or performed by a
machine, such
as a processing unit or processor, a digital signal processor (DSP), an
application specific
integrated circuit (ASIC), a field programmable gate array (FPGA) or other
programmable
logic device, discrete gate or transistor logic, discrete hardware components,
or any
combination thereof designed to perform the functions described herein. A
processor can be a
microprocessor, but in the alternative, the processor can be a controller,
microcontroller, or
state machine, combinations of the same, or the like. A processor can include
electrical
circuitry configured to process computer-executable instructions. In another
embodiment, a
processor includes an FPGA or other programmable device that performs logic
operations
without processing computer-executable instructions. A processor can also be
implemented as
a combination of computing devices, for example, a combination of a DSP and a
microprocessor, a plurality of microprocessors, one or more microprocessors in
conjunction
with a DSP core, or any other such configuration. Although described herein
primarily with
respect to digital technology, a processor may also include primarily analog
components. A
computing environment can include any type of computer system, including, but
not limited
to, a computer system based on a microprocessor, a mainframe computer, a
digital signal
processor, a portable computing device, a device controller, or a
computational engine within
an appliance, to name a few.
-139-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
103451 Conditional language such as, among
others, "can," "could," "might" or
"may," unless specifically stated otherwise, are otherwise understood within
the context as
used in general to convey that certain embodiments include, while other
embodiments do not
include, certain features, elements andior steps. Thus, such conditional
language is not
generally intended to imply that features, elements and/or steps are in any
way required for
one or more embodiments or that one or more embodiments necessarily include
logic for
deciding, with or without user input or prompting, whether these features,
elements andlor
steps are included or are to be performed in any particular embodiment.
103461 Disjunctive language such as the
phrase "at least one of X, Y, or Z,." unless
specifically stated otherwise, is otherwise understood with the context as
used in general to
present that an item, term, etc., may be either X, Y, or Z, or any combination
thereof (for
example, X, Y, and/or Z). Thus, such disjunctive language is not generally
intended to, and
should not, imply that certain embodiments require at least one of X. at least
one of Y, or at
least one of Z to each be present
[0347] Any process descriptions, elements or
blocks in the flow diagrams
described herein and/or depicted in the attached figures should be understood
as potentially
representing modules, segments, or portions of code which include one or more
executable
instructions for implementing specific logical functions or elements in the
process. Alternate
implementations are included within the scope of the embodiments described
herein in which
elements or functions may be deleted, executed out of order from that shown,
or discussed,
including substantially concurrently or in reverse order, depending on the
functionality
involved as would be understood by those skilled in the art.
103481 Unless otherwise explicitly stated,
articls such as "a" or l'an" should
generally be interpreted to include one or more described items. Accordingly,
phrases such as
"a device configured to" are intended to include one or more recited devices.
Such one or more
recited devices can also be collectively configured to carry out the stated
recitations. For
example, "a processor configured to carry out recitations A, B and C" can
include a first
processor configured to carry out recitation A working in conjunction with a
second processor
configured to carry out recitations B and C.
103491 It should be emphasized that many
variations and modifications may be
made to the above-described embodiments, the elements of which are to be
understood as being
-140-
CA 03151782 2022-3-18
WO 2021/067856
PCT/US2020/054130
among other acceptable examples. All such modifications and variations are
intended to be
included herein within the scope of this disclosure.
-141-
CA 03151782 2022-3-18