Note: Descriptions are shown in the official language in which they were submitted.
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
REDUCING FRICTION IN COMBUSTION ENGINES THROUGH FUEL ADDITIVES
TECHNICAL FIELD
[001] This disclosure relates to fuel additive compositions. More
specifically,
the disclosure relates to friction modifier additives that can be added to
fuel to improve
fuel efficiency of internal combustion engines.
BACKGROUND
[002] There has been considerable effort in recent years to improve the fuel
economy of motor vehicles. In general, the efficiency of automotive engines is
greatly
enhanced by the presence of effective lubrication, particularly at the
interface of
moving parts that are prone to high friction and excessive wear.
[003] Thus, one approach to improving fuel economy has been developing
lubricants and lubricating oil additives that reduce engine friction and thus
reduce
energy requirements. However, improvements in fuel efficiency obtained with
lubricating oil friction reducing additives have been modest and can be
difficult to
ascertain.
[004] Some of these efforts have focused on friction modifiers. Friction
modifiers have been used in limited slip gear oils, automatic transmission
fluids,
slideway lubricants and multipurpose tractor fluids. In particular, with the
desire for
increased fuel economy, friction modifiers have been added to automotive
crankcase
lubricants.
[005] These friction modifiers generally operate at boundary layer conditions
at temperatures where anti-wear and extreme pressure additives are not yet
reactive
by forming a thin mono-molecular layers of physically adsorbed polar oil-
soluble
products or reaction layers which exhibit a significantly lower friction
compared to
typical anti-wear or extreme pressure agents. However, under more severe
conditions
and in mixed lubrication regime these friction modifiers are added with an
anti-wear
or extreme pressure agent. The most common type of anti-wear or extreme
pressure
agent is a zinc dithiophosphate (ZnDTP or ZDDP). ZDDP limit wear by forming a
thick
protective tribofilm on rubbing surfaces.
1
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
[006] Although ZDDP has been widely in use in motor vehicles for many
decades, some recent studies have shown that phosphorus-based antiwear films
can
cause significant increase in friction in thin film, high-pressure, lubricated
contacts.
This, in turn, can have a negative effect on fuel efficiency.
[007] Friction modifiers are known lubricating oil additives that can reduce
boundary friction by adsorbing or reacting on metal surfaces to form thin low-
shear-
strength films.
[008] Since the conditions in an internal combustion chamber are substantially
different from, and much more severe than, those in a crankcase, the fact that
a
particular additive or class of additives has benefited the performance of a
lubricating
oil in an internal combustion engine does not necessarily mean that benefits
will be
gained by using the same types of compounds as additives in the fuel. Thus,
there is
a need to develop fuel additives that can reduce friction and/or improve fuel
economy.
SUMMARY
[009] Provided herein are compositions that can be added to fuel as additives
to provide an enhancement of friction reduction and/or fuel economy of an
internal
combustion engine. These fuel additives include a friction modifier and a
metal
chelating agent that interact synergistically to provide an unexpected level
of
performance.
[010] One example of the present invention includes a fuel composition
comprising greater than 50 wt % of a hydrocarbon fuel boiling in the gasoline
or diesel
range; a minor amount a zinc chelator; and a minor amount of a friction
modifier,
wherein the friction modifier includes at least one polar group.
[011] Another example of the present invention includes a fuel concentrate
composition comprising (1) from 90 to 30 wt % of an organic solvent boiling in
a range
of from 65 C to 205 C and (2) from 10 to 70 wt % of fuel efficiency improver
including
a zinc chelator and a friction modifier having at least one polar group.
[012] Yet another example of the present invention includes a method of
improving fuel efficiency in a spark-ignited combustion engine, the method
2
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
comprising supplying to the engine a fuel composition comprising a zinc
chelator and
a friction modifier having at least one polar group.
BRIEF DESCRIPTION OF DRAWINGS
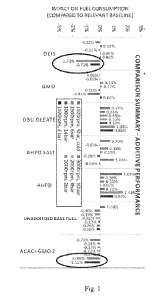
[013] FIG. 1 shows a graph that summarizes the effect of several fuel
additives
on fuel consumption at various engine conditions.
DETAILED DESCRIPTION
[014] To facilitate understanding of the disclosure set forth herein, a number
of terms are defined below. Unless defined otherwise, all technical and
scientific terms
used herein generally have the same meaning as commonly understood by one of
ordinary skill in the art to which this disclosure belongs.
[015] "Gasoline" or "gasoline boiling range components" refers to a
composition containing at least predominantly C4-C12 hydrocarbons. In one
embodiment, gasoline or gasoline boiling range components is further defined
to refer
to a composition containing at least predominantly C4-C12 hydrocarbons and
further
having a boiling range of from about 100 F (37.8 C) to about 400 F (204 C). In
an
alternative embodiment, gasoline or gasoline boiling range components is
defined to
refer to a composition containing at least predominantly C4-C12 hydrocarbons,
having
a boiling range of from about 100 F (37.8 C) to about 400 F (204 C), and
further
defined to meet ASTM D4814.
[016] The term "diesel" refers to middle distillate fuels containing at least
predominantly C10-C25 hydrocarbons. In one embodiment, diesel is further
defined to
refer to a composition containing at least predominantly C10-C25 hydrocarbons,
and
further having a boiling range of from about 165.6 C (330 F) to about 371.1 C
(700 F).
In an alternative embodiment, diesel is as defined above to refer to a
composition
containing at least predominantly C10-C25 hydrocarbons, having a boiling range
of
from about 165.6 C (330 F) to about 371.1 C (700 F), and further defined to
meet
ASTM D975.
3
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
[017] The term "oil soluble" means that for a given additive, the amount
needed to provide the desired level of activity or performance can be
incorporated by
being dissolved, dispersed or suspended in an oil of lubricating viscosity.
Usually, this
means that at least 0.001% by weight of the additive can be incorporated in a
lubricating oil composition. The term "fuel soluble" is an analogous
expression for
additives dissolved, dispersed or suspended in fuel.
[018] A "minor amount" means less than 50 wt % of a composition, expressed
in respect of the stated additive and in respect of the total weight of the
composition,
reckoned as active ingredient of the additive.
[019] An "engine" or a "combustion engine" is a heat engine where the
combustion of fuel occurs in a combustion chamber. An "internal combustion
engine"
is a heat engine where the combustion of fuel occurs in a confined space
("combustion
chamber"). A "spark ignition engine" is a heat engine where the combustion is
ignited
by a spark, usually from a spark plug. This is contrast to a "compression-
ignition
engine," typically a diesel engine, where the heat generated from compression
together with injection of fuel is sufficient to initiate combustion without
an external
spark.
[020] A "zinc chelator" refers to any substance that is able to chelate a zinc
(Zinc2+) ion.
[021] This disclosure describes additive compositions that can be added to
fuel
to enhance friction reduction and/or improve fuel efficiency of internal
combustion
engines. The additive composition ("fuel efficiency improver") comprises at
least two
components: a friction modifier and a zinc chelating agent. When formulated in
accordance with this disclosure, these components take advantage of a
previously
unknown synergy to provide a greater than expected improvement in friction
reduction and/or fuel efficiency in engines.
[022] It is believed that additives added to fuel may be transferred into the
lubricant in the engine piston ring zone where it may reduce friction and wear
and
thus improve economy. However, it may not necessarily be the case that
additives
4
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
added to lubricant are transferred to fuel. Thus, friction modifiers can
provide fuel
economy by reducing friction in the combustion chamber of an internal
combustion
engine.
Zinc Chelator
[023] The chelators employed in the present fuel composition include organic
molecules that can chelate zinc. In general, these chelators can form an inner
complex
with zinc by way of chelate ring formation. The zinc chelator may vary in its
denticity,
that is, the number of atoms of the chelator which binds to zinc. For example,
the zinc
chelator may be bidentate.
[024] Without being limited by theory, it is believed that zinc chelators of
the
present invention can limit the friction caused or induced by ZDDP in an
engine
environment. The limiting effect can be synergistically enhanced when
friction
modifiers of the present invention are also present. While the mechanism is
not fully
understood, It is believed that the friction modifiers of the present
invention can form
a friction-reducing film on a zinc phosphate surface and/or stabilize the zinc
chelator
in an engine environment.
[025] In some embodiments, the zinc chelator may be fuel soluble. In other
embodiments, the zinc chelator may not be oil soluble. In the absence of the
friction
modifier of the present invention, the lack of oil solubility may prevent the
zinc chelator
from chelating with zinc species in a lubricant environment.
[026] The zinc chelators of the present invention include dicarbonyl
compounds, bidentate nitrogen compounds, multidentate nitrogen compounds,
amino acids, citrate esters, carboxylate salts, amine salts, or a suitable
salt thereof. The
metal chelator is present in about 25 to about 5000 ppm of the fuel
composition.
[027] Dicarbonyl compounds can have the structure shown in Formula 1,
wherein Ri and R2 is independently aliphatic, aliphatic branched, cyclic
aliphatic,
aromatic, substituted aromatic, or unsaturated (e.g., olefinic) moiety.
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
>c0 0
Ri R2
Formula 1
[028] A specific example of a dicarbonyl chelator is acetyl acetone. When
acetyl acetone acts as a bidentate ligand, it is often referred to as "acac."
[029] Other dicarbonyl compounds include chelators having the structure
shown in Formula 2, wherein X is 0 or N and has correct valence, R1, R2, and
R3 are
independently aliphatic, aliphatic branched, cyclic aliphatic, aromatic,
substituted
aromatic, or unsaturated (e.g., olefinic) moiety. Additionally, R2 and R3 may
also be H
(enough to satisfy valence of X). Specific examples include ethyl
acetoacetate,
acetoacetic ester, and acetoacetic amide.
0 0
R3
Ri x
I
R2
Formula 2
[030] Some bidentate nitrogen compounds will generally have at least one
nitrogen atom that can directly coordinate to zinc or at least stabilize the
coordination
to zinc to a nearby atom. For example, the bidentate nitrogen compound can
have a
structure shown in Formula 3 below, where Ri and R2 are independently
aliphatic,
aliphatic branched, cyclic aliphatic, aromatic, substituted aromatic,
unsaturated (e.g.,
olefinic) moiety, or H. Both the nitrogen and oxygen can coordinate to zinc to
form a
chelate ring.
6
CA 03152983 2022-03-01
WO 2021/048677 PCT/IB2020/058090
1
-............ ....::::õ..===":"...........,./...õõRi
N
o
R2
Formula 3
[031] Other bidentate nitrogen compounds may also be contemplated.
Specific examples of bidentate nitrogen compounds include hydroxamic acid
(Formula
4), hydrazide (Formula 5), squaric acid (Formula 6), carbamoylphosphonate
(Fomula 7),
oxazoline (Formula 8) and N-hydroxyurea (Formula 9), where R is independently
aliphatic, aliphatic branched, cyclic aliphatic, aromatic, substituted
aromatic,
unsaturated (e.g., olefinic) moiety or H.
R H
HN ____________________
HC( R\ /NNtr/
N
0 H 0
Formula 4 Formula 5
RHN 0
R I 0
R-NH i
I d----1:\
OH OH
Formula 6 Formula 7
HN,..-R
0 HN
HO/ ----?---- I
N R OH -----Nb
Formula 8 Formula 9
7
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
[032] Other specific examples of bidentate nitrogen chelators include amino
methyl compounds, methyl pyridyl compounds, quinolyl compounds, pyrazyl
compounds, 5 membered N-heterocyclic compounds (e.g., pyrrole/ pyrrolidine,
imidazole/imidazoline, triazole) and diethanolisostearamide.
[033] Multidentate nitrogen compounds may also be compatible with the
present invention. Specific examples of these include N,N,N',N'-tetrakis(2-
pyridinylmethyl)-1,2-ethanediamine (Formula 10) and ethylenediaminetetraacetic
acid
(Formula 11).
1
N
I
N N
N N
1
N
I
Formula 10
0
/\
OH HO
0 ___________________ ( /N
0
/ OH HO
\
<
0
Formula 11
8
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
Amino Acids
[034] Amino acids include those that can be represented by the following
general formula:
H
H 0
\
N 1 <
/
H
R OH
Formula 12
wherein R is an "aliphatic" or "aromatic" side chain. Amino acid side chains
can be
broadly classified as aromatic or aliphatic. An aromatic side chain includes
an aromatic
ring. Examples of amino acids with aromatic side chains include for example,
histidine
(Formula 13), phenylalanine (Formula 14), tyrosine (Formula 15), tryptophan
(Formula 16) and the like. Non-aromatic side chains are broadly grouped as
"aliphatic" and include, for example, alanine (Formula 17), glycine (Formula
18),
cysteine (Formula 19), and the like.
[035] The amino acid(s) can be natural and/or non-natural a-amino acids.
Natural amino acids are those encoded by the genetic code, as well as amino
acids
derived therefrom. These include, for example, hydroxyproline (Formula 20), y-
carboxyglutamate (Formula 21), and citrulline (Formula 22). In this
specification, the
term "amino acid" also includes amino acid analogs and mimetics. Analogs are
compounds having the same general structure of a natural amino acid, except
that the
R group is not one found among the natural amino acids.
[036] Representative examples of analogs of naturally occurring amino acids
include homoserine (Formula 23), norleucine (Formula 24), homoproline (Formula
25) and proline (Formula 26). An amino acid mimetic is a compound that has a
structure different from the general chemical structure of an a-amino acid but
functions in a manner similar to one. The amino acid may be an L- or D-amino
acid.
Representative structures are shown below.
9
CA 03152983 2022-03-01
WO 2021/048677 PCT/IB2020/058090
0 0
N_...n)Li
OH OH
HN NH2 NH2
Formula 13 Formula 14
kl
0 0 /
HO
NH2
OH
NH2 OH
0
Formula 15 Formula 16
0
OH 0
=)'L
H2NJL
NH2 OH
Formula 17 Formula 18
0
0
Ki.._11.....
HSYLOH
NH2 OH OH
Formula 19 Formula 20
0 0
0 0
HOHLON
H2N)LNLON
NH2ooH H
NH2
Formula 21 Formula 22
0 0
HOL
OH 1)LON
NH2 NH2
Formula 23 Formula 24
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
0
0
OH
CINFLOH
NH
Formula 25 Formula 26
Miscellaneous Zinc Chelators
[037] Zinc chelators can be made from carboxylate esters with variable non-
polar groups. Zinc chelators can also include multi-functional esters
including citrate
esters. Citrate esters can have the structure shown in Formula 27 wherein R is
alkyl,
alkenyl, cycloalkyl, aromatic, or substituted aromatic moiety.
R
I
c)
0 0
R,... /*'%=,, ,...../\., ,./."..., .,..-R
0" '=''.' -0
oi-i
Formula 27
[038] Specific examples of carboxylate salts include 1,1,3,3-
tetramethylguanidine salt of 2-ethylhexanoic acid (TMG/2-EH), where TMG/2-EH
is
shown in Formula 28.
0
N
.C.)
CH3,NAN-CH3 0
1 1
CH3 CH3
Formula 28
Friction Modifiers
[039] Friction modifiers are additives that can reduce friction and/or wear in
machine components. The friction modifiers of the present invention include
organic
11
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
friction modifiers having at least one polar group. These friction modifiers
are typically
bifunctional in that the friction modifier will also generally have a long
chain and/or
aromatic non-polar group.
[040] The polar group can be an alcohol moiety, amide moiety, amine moiety,
ester moiety, and the like. In some embodiments, the friction modifier can
have more
than one polar moiety (e.g., diol, diester, alkanol amide, etc.).
[041] The friction modifier may be fuel soluble and/or oil soluble. The
friction
modifier of the present invention stabilizes the zinc chelator in a lubricant
environment
thus allowing the zinc chelator to chelate zinc and also adsorb onto a zinc
phosphate
tribofilm to form a friction reducing layer.
[042] Specific friction modifiers with ester moiety include esters of
carboxylic
acid, adipate ester, trimethylolpropane triester, polyol esters (e.g.,
glycerol ester,
sorbitan ester, etc.), polyesters, and esters with high viscosity index (VI)
and/or can
change hydrodynamic friction. In some embodiments, the ester may be borated.
[043] Other compatible friction modifiers include alkanol amides (including
polar group capped fatty amides and polyol amines. Specific alkanol amide
includes
diethanolamide.
[044] Amine friction modifiers include hydrocarbyl amines, fatty acid amines
(e.g., oleylamine), and ethoxylated alkyl amines. Specific amine friction
modifiers
include diethanolamine and diisopropanolamine.
[045] Specific friction modifiers are described in greater detail, in for
example,
U57678747, U58703680, and U59371499 which are hereby incorporated by
reference.
[046] In particular, polyol ester are often used as synthetic basestock oils
that
can be synthesized from a polyol and an acid (e.g., branched acid, linear
saturated acid,
polybasic acid). Examples of polyol ester include glycerol esters, sorbitan
esters, and
the like.
[047] A specific glycerol ester includes a glycerol monooleate (or glyceryl
monooleate), a friction modifier conventionally added to lubricant
compositions. For
example, U.S. Pat. Nos. 5,114,603 and 4,683,069 describe lubricating oil
compositions
12
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
comprising glycerol monooleate, the relevant portions of which are hereby
incorporated by reference.
[048] Examples of commercially available glycerol monooleate include
PriolubeTM 1408 and RadiasurITM 7149 (i.e., esters of fatty acids including
glycerol
trioleate). In a typical commercial product, only about 50-60 mole percent of
the esters
produced are monoesters. The remainder are primarily diesters, with a small
amount
of triester.
[049] Typically, the fuel compositions of this invention contain at least
0.015
wt %, preferably 0.15 to 2.0 wt % of the friction modifier.
[050] The glycerol esters useful for this invention are fuel-soluble and are
preferably prepared from C12 to C22 fatty acids or mixtures thereof such as
are found
in natural products. The fatty acid may be saturated or unsaturated. Certain
compounds found in acids from natural sources may include licanic acid which
contains one keto group. Most preferred C16 to C18 fatty acids are those of
the
formula R¨COOH wherein R is alkyl or alkenyl. Preferred fatty acids are oleic,
stearic,
isostearic, palmitic, myristic, palmitoleic, linoleic, lauric, linolenic, and
eleostearic, and
the acids from the natural products tallow, palm oil, olive oil, peanut oil,
corn oil, Neat's
foot oil and the like. A particularly preferred acid is oleic acid.
[051] The fatty acid monoester of glycerol is preferred, however, mixtures of
mono- and diesters may be used. Preferably any mixture of mono- and diester
contains at least 40% of the monoester. Typically these mixtures of mono- and
diesters
of glycerol contain from 40 to 60 percent by weight of the monoester. For
example,
commercial glycerol monooleate contains a mixture of from 45% to 55% by weight
monoester and from 55% to 45% diester. However, higher mono ester can be
achieved
by distilling the glycerol monoester, diester, triester mixture using
conventional
distillation techniques, with the monoester portion of the distillate product
recovered.
This can result in a product which is essentially all monoester. Thus, the
esters used in
the fuel compositions of this invention may be all monoesters, or a mixture of
mono-
13
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
and diesters in which at least 75 mole percent, preferably at least 90 mole
percent, of
the mixture is the monoester.
Fuel Compositions
[052] The compounds of the present disclosure may be useful as additives in
hydrocarbon fuels to prevent or reduce engine knock or pre-ignition events in
spark-
ignited internal combustion engines.
[053] The compounds of the present disclosure may be formulated as a
concentrate using an inert stable oleophilic (i.e., soluble in hydrocarbon
fuel) organic
solvent boiling in a range of 65 C to 205 C. An aliphatic or an aromatic
hydrocarbon
solvent may be used, such as benzene, toluene, xylene, or higher-boiling
aromatics or
aromatic thinners. Aliphatic alcohols containing 2 to 8 carbon atoms, such as
ethanol,
isopropanol, methyl isobutyl carbinol, n-butanol and the like, in combination
with the
hydrocarbon solvents are also suitable for use with the present additives. In
the
concentrate, the amount of the additive may range from 10 to 70 wt % (e.g., 20
to 40
wt %).
[054] In gasoline fuels, other well-known additives can be employed including
oxygenates (e.g., ethanol, methyl tert-butyl ether), other anti-knock agents,
and
detergents/dispersants (e.g., hydrocarbyl amines, hydrocarbyl
poly(oxyalkylene)
amines, succinimides, Mannich reaction products, aromatic esters of
polyalkylphenoxyalkanols, or polyallylphenoxyaminoalkanes). Additionally,
friction
modifiers, antioxidants, metal deactivators and demulsifiers may be present.
[055] In diesel fuels, other well-known additives can be employed, such as
pour
point depressants, flow improvers, cetane improvers, and the like.
[056] A fuel-soluble, non-volatile carrier fluid or oil may also be used with
compounds of this disclosure. The carrier fluid is a chemically inert
hydrocarbon-
soluble liquid vehicle which substantially increases the non-volatile residue
(NVR), or
solvent-free liquid fraction of the fuel additive composition while not
overwhelmingly
contributing to octane requirement increase. The carrier fluid may be a
natural or
synthetic oil, such as mineral oil, refined petroleum oils, synthetic
polyalkanes and
14
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
alkenes, including hydrogenated and unhydrogenated polyalphaolefins, synthetic
polyoxyalkylene-derived oils, such as those described in U.S. Patent Nos.
3,756,793;
4,191,537; and 5,004,478; and in European Patent Appl. Pub. Nos. 356,726 and
382,159.
[057] The carrier fluids may be employed in amounts ranging from 35 to 5000
ppm by weight of the hydrocarbon fuel (e.g., 50 to 3000 ppm of the fuel). When
employed in a fuel concentrate, carrier fluids may be present in amounts
ranging from
20 to 60 wt % (e.g., 30 to 50 wt %).
[058] The following non-limiting examples have been provided to illustrate
one or more aspects of the present invention.
Example 1
Fuel Consumption Test
[059] Additives were added to fuel to make fuel composition samples. The
samples are summarized in Table 1 below. Table 2 summarizes the various
conditions
of the fuel consumption test.
Table 1
Fuel Sample # Composition
1 Diethanolisostearamide (DEIS)
2 Glycerol Monooleate (GMO)
3 DBU Oleate
4 AHPD salt
AHPD
6 Unadditized base fuel
7 ACAC + GMO
Table 2
Engine RPM (rpm) Engine Pressure (bar)
1100 3 (cold)
2500 6
3000 14
1100 3
3000 10
2000 8
2000 2
CA 03152983 2022-03-01
WO 2021/048677
PCT/IB2020/058090
[060] Figure 1 shows the results of the fuel consumption test on the fuel
samples at the various engine conditions. The engine rpm ranges from 1100 to
3000
rpm while the pressure ranges from 2 to 14 bars.
16