Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/086878
PCT/1JS2020/057600
ASSAY DEVICES AND METHODS OF MANUFACTURE
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of and
priority to U.S. Prov. Pat. App. No.
62/927,481 filed 29 October 2019, the entirety of which is incorporated herein
by reference.
This Application also references French Patent Application FR1912110 filed 29
October
2019, the entirety of which is incorporated herein by reference.
BACKGROUND
in 1. Technical Field
[0002] Embodiments of the present disclosure relate
generally to test devices, for
example, for amplifying nucleic acids and methods of manufacture of such test
devices.
2. Background
[0003] In the United States, Canada, and Western Europe
infectious disease accounts for
approximately 7% of human mortality, while in developing regions infectious
disease
accounts for over 40% of human mortality. Infectious diseases lead to a
variety of clinical
manifestations. Among common overt manifestations are fever, pneumonia,
meningitis,
diarrhea, and diarrhea containing blood. While the physical manifestations
suggest some
pathogens and eliminate others as the etiological agent, a variety of
potential causative agents
remain, and clear diagnosis often requires a variety of assays be performed.
Traditional
microbiology techniques for diagnosing pathogens can take days or weeks, often
delaying a
proper course of treatment.
[0004] In recent years, the polymerase chain reaction
(PCR) has become a method of
choice for rapid diagnosis of infectious agents. PCR can be a rapid,
sensitive, and specific
t001 to diagnose infectious disease. However, a challenge to using PCR as a
primary means of
diagnosis is the variety of possible causative organisms or viruses and the
low levels of
organism or virus present in some pathological specimens. It is often
impractical to run large
panels of PCR assays, one for each possible causative organism or viruses,
most of which are
expected to be negative. The problem is exacerbated when pathogen nucleic acid
is at low
concentration and requires a large volume of sample to gather adequate
reaction templates_ In
some cases there is inadequate sample to assay for all possible etiological
agents. A solution
is to run "multiplex PCR" wherein the sample is concurrently assayed for
multiple targets in
a single reaction. While multiplex PCR has proved to be valuable in some
systems,
shortcomings exist concerning robustness of high level multiplex reactions and
difficulties
- 1 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
for clear analysis of multiple products. To solve these problems, the assay
may be
subsequently divided into multiple secondary PCRs. Nesting secondary reactions
within the
primary product increases robustness. Closed systems such as the FilmArray
(BioFire
Diagnostics, LLC, Salt Lake City, UT) reduce handling, thereby diminishing
contamination
risk.
[0005] The present invention addresses various
improvements relating to automated or
semi-automated manufacturing of test devices, cost of test devices, and more
rapid sample-to-
answer.
1.43 BRIEF SUMMARY
[0006] Described herein are self-contained reaction
containers (also referred to herein as
'pouches' or `cards'), methods of manufacturing such reaction containers,
instruments,
systems, and methods for rapid amplification of nucleic acids. In an
illustrative embodiment,
a reaction container may be fabricated from a first sheet and a second sheet
of polymeric
material with one or more fluidically connected reaction chambers and reagent
reservoirs
(e.g., aqueous reagent reservoirs) formed between the first and second sheets.
The reaction
chambers and reagent reservoirs may be formed by pressing the first and second
polymeric
sheets between forming plates and propelling a compressed fluid between the
sheets to form
open areas.
[0007] Described herein are:
[0008] Al. A method for forming a reaction
container, comprising:
[0009] providing a polymeric sheet that comprises an
inner planar face and an outer
planar face;
[0010] contacting a first inner planar face of the
polymeric sheet to a second inner planar
face of the polymeric sheet;
[0011] pressing the polymeric sheet between a first
forming plate and a second forming
plate, wherein at least one of the first or second forming plates has one or
more recesses for
forming one or more features selected from the group consisting of a reaction
chamber, a
fluid flow channel, a reagent chamber, or a sample chamber in selected
portions of the
polymeric sheet
[0012] propelling a compressed fluid between the inner
planar faces of the polymeric
sheet while the polymeric sheet is pressed between the forming plates to
reform the selected
portions of the polymeric sheet into a shape defined by the one or more
recesses of the
forming plates;
- 2 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[0013] separating the first forming plate and the
second forming plate so the polymeric
sheet is no longer pressed between the first and second forming plates; and
[0014] removing the reaction container from between the
first forming plate and the
second forming plate.
[0015] A2. The method of clause Al, wherein the provided
polymeric sheet
comprises a first polymeric sheet and a second polymeric sheet, each polymeric
sheet having
an inner planar face and an outer planar face, and the method further
comprising:
[0016] arranging the first and second polymeric sheets
so that the inner planar faces are
arranged adjacent to one another,
in [0017] performing the pressing step, and
[00181 propelling a compressed fluid between the two
polymeric sheets while the two
polymeric sheets are pressed between the forming plates to reform the selected
portions into a
shape defined by the one or more recesses of the forming plates.
[0019] A3. The method of any of clauses Al or
A2 further comprising heating the
polymeric sheet to a softening temperature prior to the pressing step,
performing the pressing
and propelling steps, and cooling the polymeric sheet after the propelling
step to set the shape
defined by the one or more recesses of the forming plates.
[0020] A4. The method of any of clauses Al-A3,
wherein the polymeric sheet
comprises a flexible polymeric material having a thickness in a range of about
0_02 mm to
about 0.1 mm.
[0021] A5. The method of any of clauses Al-A4,
wherein the flexible polymeric
material is selected from the group consisting of polyester, polyethylene,
polyethylene
terephthalate (PET), polycarbonate, polypropylene (PP),
polymethylmethacrylate, mixtures,
combinations thereof
[0022] A6. The method of any of clauses Al-A5, wherein the
flexible polymeric
material comprises a water vapor and/or oxygen barrier material.
[0023] A7. The method of any of clauses Al-A6,
wherein the polymeric material
has a water vapor transmission rate (WVTR) in a range of about 0.01 g/m2/24
hrs to about 3
g/m2/24 hrs, preferably in a range of about 0.05 g/m2/24 hrs to about 2
g/m2/24 hrs, or more
preferably no more than about 1 g/m2/24 hrs, and an oxygen transmission rate
in a range of
about 0.01 cc/m2/24 Ills to about 2 cc/m2/24 hrs, preferably in a range of
about 0.05 cc/m2/24
his to about 2 cc/m2/24 his, or more preferably no more than about 1 cc/m2/24
hrs.
- 3 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[0024] AS. The method of any of clauses Al-A7,
wherein the flexible polymeric
material comprises two or more layers of film material bonded together and the
water vapor
and/or oxygen bather comprises at least one of a metalized or ceramic-coated
film layer.
[0025] A9. The method of any of clauses Al-AS,
wherein the selected portions
comprise one or more of a sample input chamber, a sample preparation chamber,
a sample
reactant recovery/wash chamber, a reaction chamber, or one or more fluid
reagent reservoirs
[0026] Al 0. The method of any of clauses Al-A9,
further comprising heating the
polymeric sheet to a first temperature prior to the pressing step.
[0027] All. The method of any of clauses Al-
A10, wherein separating the first
1.0 forming plate and the second forming plate comprises moving at least
one of the first forming
plate and the second forming plate.
[0028] Al2. The method of any of clauses Al-
All, wherein separating the first
forming plate and the second forming plate comprises moving only one of the
first forming
plate and the second forming plate
[0029] Bl. A method for forming a reaction container, comprising:
[0030] providing a first polymeric sheet and a second
polymeric sheet, wherein the first
and second polymeric sheets each comprise an inner planar face and an outer
planar face,
[0031] contacting the inner planar face of the first
polymeric sheet to the inner planar face
of the second polymeric sheet,
[0032] laminating the first polymeric sheet to the second polymeric sheet;
[0033] making one or more seal lines joining the first
and second polymeric sheets;
[0034] pressing the first and second polymeric sheets
between a first forming plate and a
second forming plate, wherein at least one of the first or second forming
plates has one or
more recesses positioned for reforming the first and second polymeric sheets
to form one or
more openings in a region defined by the one or more seal lines;
[0035] expanding selected areas of the first and second
polymeric sheets into a shape
defined by the one or more recesses of the forming plates by blowing a
compressed gas
between the first and second polymeric sheets while the first and second
polymeric sheets are
pressed between the forming plates;
[0036] separating the first forming plate and the second forming plate,
and
[0037] removing the reaction container from between the
first forming plate and the
second forming plate.
- 4 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[0038] B2. The method of clause Bl, further
comprising cooling the first and
second polymeric sheets subsequent to the expanding step to set the shape
defined by the one
or more recesses of the forming plates.
[0039] B3. The method of any of clauses B1 or
B2, wherein the compressed gas
blown between the first and second polymeric sheets substantially
simultaneously expands
and cools the first and second polymeric sheets
[0040] B4. The method of any of clauses B1-B3,
wherein making the one or more
seal lines comprises defining one or more of a sample input chamber, a sample
preparation
chamber, a sample reactant recovery/wash chamber, at least one reaction
chamber, a one or
more fluid reagent reservoirs, or one or more channels fluidically connecting
the sample input
chamber, the sample preparation chamber, the sample reactant recovery/wash
chamber, the at
least one reaction chamber, and the one or more reagent reservoirs.
[0041] B5. The method of any of clauses B1-B4,
wherein when the first and
second polymeric sheets are pressed between a first forming plate and a second
forming plate
the one or more recesses of the forming plates substantially align with the
one or more areas
defined by the seal lines, and the selected areas expanded by blowing a
compressed gas
between the first and second polymeric sheets comprise one or more of the
sample input
chamber, the sample preparation chamber, the recovery/wash chamber, one or
more reaction
chambers, or one or more reagent reservoirs, and wherein one or more of the
selected areas
expanded by blowing the compressed gas between the first and second polymeric
sheets are
connected by one or more sealed, openable laminated channels.
[0042] B6. The method of any of clauses B1-B5,
wherein the reaction container
comprises sample input chamber fluidically connected to a first reaction
chamber, a second
reaction chamber fluidically connected to the first reaction chamber, and at
least one reagent
reservoir fluidically connected to the sample input chamber, the first
reaction chamber, or the
second reaction chamber, and wherein the method further comprises:
[0043] expanding the sample input chamber and the at
least one reagent reservoir into
shapes defined by the recesses of the forming plates by blowing the compressed
gas between
the first and second polymeric sheets while the first and second polymeric
sheets are pressed
between the forming plates.
1100441 B7. The method of any of clauses B1-B6,
further comprising expanding the
second reaction chamber into a shape defined by the recesses of the forming
plates by
blowing the compressed gas between the first and second polymeric sheets while
the first and
second polymeric sheets are pressed between the forming plates.
- 5 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[0045] B8. The method of any of clauses B1-B7,
further comprising:
[0046] making the one or more seal lines defining a
second reaction chamber,
[0047] expanding the second reaction chamber into a
shape defined by a second reaction
chamber recess of the forming plates,
[0048] providing a reaction card having a plurality of wells formed
therein and spotted
with one or more dried reagents for a second stage reaction,
[0049] inserting the reaction card into the second
reaction chamber via an opening
between the first and second sheets;
[0050] bonding a first planar face of the reaction card
to the first sheet and a second,
opposite planar face of the reaction card to the second sheet, and
[0051] sealing the opening used to insert the reaction
card by sealing the first polymeric
sheet to the second polymeric sheet at the opening.
[0052] B9. The method of any of clauses B1-B8,
further comprising:
[0053] injecting a selected aqueous reagent into the at
least one reagent reservoir via a
reagent reservoir opening between the first and second polymeric sheets,
[0054] sealing the selected aqueous reagent in the at
least one reagent reservoir by sealing
the first polymeric sheet to the second polymeric sheet at the reagent
reservoir opening such
that the reaction container is provided with an aqueous reagent at the time of
manufacture
[0055] B10. The method of any of clauses BL-B9,
further comprising:
[0056] expanding a fluid reservoir and an access channel in the first
reaction chamber
into shapes defined by the recesses of the forming plates by blowing the
compressed gas
between the first and second polymeric sheets while the first and second
polymeric sheets are
pressed between the forming plates,
[0057] injecting an aqueous reagent into the fluid
reservoir in the first reaction chamber
via the access channel, and
[0058] sealing the sample preparation reagent in the
fluid reservoir in the sample
preparation chamber by sealing the first polymeric sheet to the second
polymeric sheet at the
access channel such that the reaction container is provided with the aqueous
reagent in the
first reaction chamber at the time of manufacture.
[0059] B11. The method of any of clauses Bl-B10, wherein the first
and second
polymeric sheets comprise a water vapor and/or oxygen barrier material.
[0060] B12. The method of any of clauses Bl-
B11, wherein the first and second
polymeric sheets have a water vapor transmission rate (WVTR) in a range of
about 0.01
g/m2/24 hrs to about 3 g/m2/24 hrs, preferably in a range of about 0.05
g/m2/24 hrs to about 2
- 6 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
g/m2/24 hrs, or more preferably no more than about 1 g/m2/24 hrs, and/or an
oxygen
transmission rate in a range of about 0.01 cc/m2/24 hrs to about 2 cc/m2/24
hrs, preferably in
a range of about 0.05 cc/m2/24 hrs to about 2 cc/m2/24 hrs, or more preferably
no more than
about 1 cc/m2/24 hrs.
[0061] B13. The method of any of clauses B1-B12, wherein the
water vapor and/or
oxygen bather material comprises at least one of a metalized or ceramic-coated
film layer_
[0062] B14. The method of any of clauses B1-
B13, wherein the first and second
polymeric sheets comprise a material selected from the group consisting of
polyester,
polyethylene, polyethylene terephthalate (PET), polycarbonate, polypropylene
(PP),
polymethylmethacrylate, mixtures, combinations thereof
[0063] B15. The method of any of clauses B1-
B14, further comprising:
[0064] prior to the laminating step, dispensing
droplets of one or more liquid reagents
onto the first polymeric sheet or the second polymeric sheet and drying the
droplets of liquid
reagent dispensed onto the first polymeric sheet or the second polymeric
sheet,
[00651 wherein the droplets of the one or more liquid reagents are
dispensed and dried in
one or more areas to be formed into the sample input chamber, the sample
preparation
chamber, the sample reactant recovery/wash chamber, the at least one reaction
chamber, or
the one or more channels fluidically connecting the sample input chamber, the
sample
preparation chamber, the sample reactant recovery/wash chamber, the at least
one reaction
chamber, and the one or more reagent reservoirs.
[0066] B16. The method of any of clauses B1-
B15, further comprising heating the
first and second polymeric sheets to a temperature sufficient for reforming
first and second
polymeric sheets prior to the pressing step.
[0067] B17. The method of any of clauses Bl-
B16, wherein separating the first
forming plate and the second forming plate comprises moving at least one of
the first forming
plate and the second forming plate.
[0068] B18. The method of any of clauses B1-
B17, wherein separating the first
forming plate and the second forming plate comprises moving only one of the
first forming
plate and the second forming plate.
[0069] Cl.A method for forming a reaction container formed from a first
sheet
and a second sheet and having a first reaction chamber, a reagent reservoir,
and a channel
fluidically connecting the reaction chamber and the reagent reservoir, the
method comprising:
[0070] laminating the first sheet to the second sheet;
- 7 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[0071] making one or more seal lines joining the first
and second sheets to define the first
reaction chamber, the reagent reservoir, and the channel;
[0072] pressing the first and second sheets between a
forming die having a first plate and
a second plate, wherein the forming die comprises recesses having a shape
corresponding to
the reagent reservoir;
[0073] propelling a fluid between the first and second
sheets while the first and second
sheets are clamped in the forming die to reform selected areas of the first
and second sheets
into the shapes of the recesses; and
[0074] removing the reaction container from the forming
die
in [0075] C2. The method of clause Cl, further comprising
injecting an aqueous
reagent into the reagent reservoir via a first reagent reservoir opening
between the first and
second sheets, sealing the aqueous reagent in the reagent reservoir by sealing
the first reagent
reservoir opening such that the reaction container is provided with an aqueous
reagent at the
time of manufacture.
[0076] C3. The method of any of clauses Cl or C2, wherein the
reaction container
further comprises a second reaction chamber fluidly connected to the first
reaction chamber
by a second channel, the method further comprising:
[0077] making the one or more seal lines to join the
first and second sheets to define the
first reaction chamber, the reagent reservoir, the first channel, the second
reaction chamber,
and the second channel, and
[0078] performing the clamping and propelling steps to
selectively to form the reagent
reservoir and the second reaction chamber, wherein the forming die further
comprises a
recess having a shape corresponding to the second reaction chamber.
[0079] C4, The method of any of clauses C1-C3,
further comprising:
[0080] providing a reaction card having a plurality of wells formed
therein and spotted
with one or more dried reagents for a second stage reaction,
[0081] inserting the reaction card into the second
reaction chamber via a second reaction
chamber opening between the first and second sheets;
[0082] bonding a first planar face of the reaction card
to the first sheet and a second,
opposite planar face of the reaction card to The second sheet, and
[0083] sealing the second reaction chamber opening.
[00841 CS. The method of any of clauses Cl-C4,
wherein the reaction container
further comprises a sample input chamber, a sample preparation chamber, and a
sample
reactant recovery/wash chamber, upstream of first reaction chamber and a
plurality of
- 8 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
channels fluidly connecting the sample input chamber, the sample preparation
chamber, and
the sample reactant recovery/wash chamber to the first reaction chamber, the
method further
comprising:
[0085] making the one or more seal lines to join the
first and second sheets to
additionally define each of the sample input chamber, the sample preparation
chamber, the
sample reactant recovery/wash chamber, and the plurality of channels,
[0086] performing the clamping and propelling steps to
additionally form the sample
input chamber, the sample preparation chamber, and the sample reactant
recovery/wash
chamber, wherein the forming die further comprises recesses having shapes
corresponding to
in the sample input chamber and the sample reactant recovery/wash chamber.
[0087] C6. The method of any of clauses Cl-05,
wherein the forming die further
comprises recesses positioned and configured for forming a plurality of
reagent reservoirs
fluidly connected to the sample preparation chamber, the sample reactant
recovery/wash
chamber, and the first reaction chamber, and the method further comprising:
[0088] making the one or more seal lines to join the first and second
sheets to
additionally define each of the plurality of reagent reservoirs and a
plurality of channels
fluidically connecting them to one or more of the sample input chamber, the
sample
preparation chamber, the sample reactant recovery/wash chamber, the first
reaction chamber,
or the second reaction chamber;
[0089] performing the clamping and propelling steps to additionally form
each of the
plurality of reagent reservoirs,
[0090] injecting a selected aqueous reagent into each
of the plurality of reagent reservoirs
via a plurality of reagent reservoir opening between the first and second
sheets,
[0091] sealing the aqueous reagents in each of the
plurality of reagent reservoirs by
sealing the openings such that the reaction container is provided with a
plurality of aqueous
reagents at the time of manufacture.
[0092] CT The method of any of clauses Cl-C6,
wherein the forming die further
comprises a recess having a shape corresponding to a fluid reservoir
positioned in the sample
preparation chamber, and the method further comprising:
[0093] performing the clamping and propelling steps to form the fluid
reservoir in the
sample preparation chamber,
[00941 injecting a sample preparation reagent into the
fluid reservoir via a sample
preparation chamber opening between the first and second sheets, and
- 9 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[0095] sealing the sample preparation reagent in the
fluid reservoir in the sample
preparation chamber by sealing the sample preparation chamber opening such
that the
reaction container is provided with sample preparation reagent at the time of
manufacture.
[0096] C8. The method of any of clauses Cl-C7,
wherein the first sheet to the
second sheet comprise a flexible polymeric material.
[0097] C9 The method of any of clauses Cl-C8,
wherein the flexible polymeric
material comprises a water vapor and/or oxygen bather material.
[0098] C10. The method of any of clauses Cl-C9,
wherein the polymeric material
comprising the water vapor and/or oxygen barrier material has a water vapor
transmission
in rate (WVTR) in a range of about 0.01 g/m2/24 hrs to about 3 g/m2/24 hrs,
preferably in a
range of about 0.05 g/m2/24 hrs to about 2 g/m2/24 hrs, or more preferably no
more than
about 1 g/m2/24 hrs, and an oxygen transmission rate in a range of about 0.01
cc/m2/24 hrs to
about 2 cc/m2/24 hrs, preferably in a range of about 0.05 cc/m2/24 his to
about 2 cc/m2/24
hrs, or more preferably no more than about 1 cc/m2/24 hrs.
[0099] C11. The method of any of clauses Cl-C10, wherein the
polymeric material
is selected from the group consisting of polyester, polyethylene, polyethylene
terephthalate
(PET), polycarbonate, polypropylene (PP), polymethylmethacrylate, mixtures,
combinations
thereof.
[00100] C12. The method of any of clauses Cl-
C11, wherein the water vapor and/or
oxygen bather material comprises at least one of a metalized or ceramic-coated
film layer.
[00101] C13. The method of any of clauses Cl-
C12, wherein making the one or
more seal lines to join the first and second sheets to define the reaction
chamber, the reagent
blister, and the channel comprises one or more of heat sealing, sonic welding,
or laser
welding.
[00102] C14. The method of any of clauses Cl-C13, wherein the first
and second
sheets are heated prior to the clamping step.
[00103] C15. The method of any of clauses Cl-
C14, wherein the heating comprises
selectively heating only regions of the first and second sheets defining the
reaction chamber
and the reagent blister.
[00104] C16. The method of any of clauses C1-C15, wherein
selectively heating
comprises clamping the first and second sheets in between heated plates having
raised areas
corresponding to the reaction chamber and the reagent blister.
[00105] C17. The method of any of clauses Cl-
C16, wherein the fluid propelled
between the first and second sheets is compressed air.
- 10 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00106] C18. The method of any of clauses C1-
C17, further comprising forming one
or more holes in the first sheet or the second sheet and one or more channels
in fluid
communication with the one or more holes, wherein the one or more holes are in
fluid
communication with corresponding conduits in the forming die for propelling
the fluid
between the first and second sheets to form the reaction chamber and the
reagent blister.
[00107] C19 The method of any of clauses C1-
C18, wherein the one or more holes
in the first sheet or the second sheet are clamped in the forming die in fluid
communication
with the conduits for propelling the fluid between the first and second
sheets.
[00108] C20. The method of any of clauses C1-
C19, wherein removing the reaction
in container from the forming die comprises separating the first forming
plate and the second
forming plate so the first and second sheets are no longer clamped in the
forming die.
[00109] C21. The method of any of clauses C1-
C20, wherein separating the first
forming plate and the second forming plate comprises moving at least one of
the first forming
plate and the second forming plate
[00110] C22. The method of any of clauses C1-C21, wherein
separating the first
forming plate and the second forming plate comprises moving only one of the
first forming
plate and the second forming plate.
[00111] C23. The method of any of clauses C1-
C22, wherein the one or more holes
are in the first sheet only and the conduits are not in fluid communication
with any holes in
the second sheet.
[00112] DI. A method for forming a reaction
container formed from a first sheet
and a second sheet and having a reaction chamber, a reagent reservoir, a
channel fluidically
connecting the reaction chamber and the reagent reservoir, and one or more
dried reagents
disposed in the reaction container between the first sheet and the second
sheet, the method
Comprising:
[00113] dispensing one or more liquid reagents onto the first sheet or the
second sheet;
[00114] drying the liquid reagents dispensed onto the first sheet or the
second sheet;
[00115] laminating the first sheet to the second sheet, wherein the laminating
includes
healing the first and second sheets and compressing them, and wherein the
laminated first and
second sheets are reversibly sealed to one another;
[00116] forming one or more seal lines substantially irreversibly bonding the
first and
second sheets together at the seal lines to define the reaction chamber, the
reagent reservoir,
and the channel;
- 11 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00117] clamping the first and second sheets in a forming die having a first
plate and a
second plate, wherein the forming die comprises a recess having a shape
corresponding to the
reagent reservoir;
[00118] propelling a fluid between the first and second sheets while the first
and second
sheets are clamped in the forming die to reform selected areas of the first
and second sheets
into the shapes of the recesses; and
[00119] removing the reaction container from the forming die
[00120] D2. The method of clause D1, wherein
the liquid reagents are dispensed
onto the first or second sheet as droplets.
in [00121] D3. The method of any of clauses D1 or D2, wherein
the liquid reagents
are water-based.
[00122] D4. The method of any of clauses Dl-D3,
wherein the liquid reagents are
air dried on the first or second sheet prior to the laminating.
[00123] D5. The method of any of clauses DI-D4,
wherein the one or more liquid
reagents are dispensed onto the first or second sheet and dried in a region to
be formed into
the reaction chamber.
[00124] D6. The method of any of clauses Dl-D5,
further comprising injecting a an
aqueous reagent into the reagent blister via an opening between the first and
second sheets,
sealing the fluid reagent in the reaction container by sealing the opening
such that the
reaction container is provided with the fluid reagent at the time of
manufacture.
[00125] D7. The method of any of clauses Dl-D6,
wherein the aqueous reagent is
configured for rehydrating the one or more dried reagents disposed in the
reaction container
in preparation for performing an assay using the reaction container.
[00126] D8. The method of any of clauses Dl-D7,
wherein the first sheet to the
second sheet comprise a flexible polymeric material selected from the group
consisting of
polyester, polyethylene, polyethylene terephthalate (PET), polycarbonate,
polypropylene
(PP), polymethylmethacrylate, mixtures, combinations thereof.
[00127] D9. The method of any of clauses Dl-D8,
wherein the flexible polymeric
material comprises a water vapor and/or oxygen barrier material.
[00128] DI O. The method of any of clauses Dl-D9, wherein the
polymeric material
comprising the water vapor and/or oxygen bather material has a water vapor
transmission
rate (WVTR) in a range of about 0.01 g/m2/24 hrs to about 3 g/m2/24 hrs,
preferably in a
range of about 0.05 g/m2/24 hrs to about 2 g/m2/24 hrs, or more preferably no
more than
about I g/m2/24 hrs, and an oxygen transmission rate in a range of about 0.01
cc/m2/24 hrs to
- 12 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
about 2 cc/m2/24 hrs, preferably in a range of about 0.05 cc/m2/24 his to
about 2 cc/m2/24
hrs, or more preferably no more than about 1 cc/1112/24 hrs.
[00129] D11. The method of any of clauses Dl-
D10, wherein the liquid reagents
dispensed onto the first or second sheet comprise an enzyme selected for use
in a molecular
biological or immunological assay (e.g., a reverse transcriptase, a DNA
polymerase, and
combinations thereof).
[00130] D12. The method of any of clauses Dl-
D11, wherein the enzyme regains its
activity following the drying, laminating, and rehydration.
[00131] D13. The method of any of clauses Dl-
D12, wherein removing the reaction
container from the forming die comprises separating the first forming plate
and the second
forming plate so the first and second sheets are no longer clamped in the
forming die.
[00132] D14. The method of any of clauses Dl-
D13, wherein separating the first
forming plate and the second forming plate comprises moving at least one of
the first forming
plate and the second forming plate
[00133] D15. The method of any of clauses Dl-D14, wherein
separating the first
forming plate and the second forming plate comprises moving only one of the
first forming
plate and the second forming plate.
[00134] This summary is provided to introduce a selection of concepts in a
simplified form
that are further described below in the Detailed Description. This Summary is
not intended to
identify key features or essential features of the claimed subject matter, nor
is it intended to
be used as an aid in determining the scope of the claimed subject matter.
[00135] Additional features and advantages will be set forth in the
description that follows,
and in part will be obvious from the description, or may be learned by the
practice of the
invention. The features and advantages may be realized and obtained by means
of the
instruments and combinations particularly pointed out in the appended claims.
These and
other features will become more fully apparent from the following description
and appended
claims, or may be learned by the practice of the invention as set forth
hereinafter.
BRIEF DESCRIPTION OF THE FIGURES
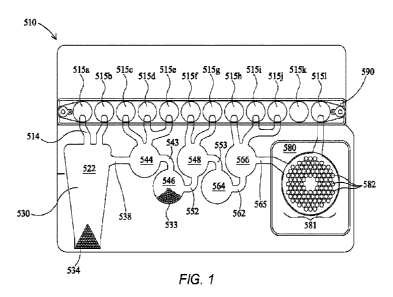
[00136] Fig. 1 shows a flexible pouch useful for self-contained PCR.
[00137] Fig. 2 is an exploded perspective view of an instrument for use with
the pouch of
Fig. 1, including the pouch of Fig. 1.
[00138] Fig. 3 shows the pouch of Fig. 1 along with bladder components of the
instrument
of Fig. 2.
- 13 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00139] Fig. 4 shows a motor used in one illustrative embodiment of the
instrument of Fig.
2.
[00140] Fig. 5A shows another embodiment of a pouch, illustrating the pouch in
an
uncompleted state.
[00141] Fig. 5B shows another view of the pouch of Fig. 5A, illustrating the
pouch in a
completed state
[00142] Fig. 6 shows a separated view of a forming die used during manufacture
of the
pouch shown in Figs. 5A and 5B.
[00143] Fig 7 is a separated perspective of the forming die
[00144] Fig. 8 is a front elevation of a first forming plate of the forming
die of Fig. 6.
[00145] Fig. 9 is a front elevation of a second forming plate of the forming
die of Fig. 6.
[00146] Fig. 10 is a cross sectional perspective of the first forming plate
taken along line
10-10 of Fig. S.
[00147] Fig. 11 is a cross sectional perspective of the first forming plate
taken along line
11-1I of Fig. 8.
[00148] Fig. 12 is a cross section of the first and second forming plates
pressed together.
[00149] Fig.13 is a schematic cross-sectional illustration of polymeric sheets
pressed
between the forming plates to form the pouch.
[00150] Fig. 14 is a schematic cross-sectional illustration of a compressed
fluid being
propelled between the polymeric sheets of Fig. 13 to form chambers in the
pouch.
DETAILED DESCRIPTION
[00151] Example embodiments are described below with reference to the
accompanying
drawings. Many different forms and embodiments are possible without deviating
from the
spirit and teachings of this disclosure and so the disclosure should not be
construed as limited
to the example embodiments set forth herein. Rather, these example embodiments
are
provided so that this disclosure will be thorough and complete, and will
convey the scope of
the disclosure to those skilled in the art. In the drawings, the sizes and
relative sizes of layers
and regions may be exaggerated for clarity. Like reference numbers refer to
like elements
throughout the description.
[00152] Unless defined otherwise, all terms (including technical and
scientific terms) used
herein have the same meaning as commonly understood by one of ordinary skill
in the art to
which the present disclosure pertains. It will be further understood that
terms, such as those
defined in commonly used dictionaries, should be interpreted as having a
meaning that is
- 14 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
consistent with their meaning in the context of the present application and
relevant art and
should not be interpreted in an idealized or overly formal sense unless
expressly so defined
herein. The terminology used in the description of the invention herein is for
the purpose of
describing particular embodiments only and is not intended to be limiting of
the invention.
While a number of methods and materials similar or equivalent to those
described herein can
be used in the practice of the present disclosure, only certain exemplary
materials and
methods are described herein.
[00153] All publications, patent applications, patents or other references
mentioned herein
are incorporated by reference in their entirety. In case of a conflict in
terminology, the present
in specification is controlling.
[00154] Various aspects of the present disclosure, including devices, systems,
methods,
etc., may be illustrated with reference to one or more exemplary
implementations. As used
herein, the terms "exemplary" and "illustrative" mean "serving as an example,
instance, or
illustration," and should not necessarily be construed as preferred or
advantageous over other
implementations disclosed herein. In addition, reference to an
"implementation" or
"embodiment" of the present disclosure or invention includes a specific
reference to one or
more embodiments thereof, and vice versa, and is intended to provide
illustrative examples
without limiting the scope of the invention, which is indicated by the
appended claims rather
than by the following description.
[00155] It will be noted that, as used in this specification and the appended
claims, the
singular forms "a," "an," and "the" include plural referents unless the
content clearly dictates
otherwise. Thus, for example, reference to "a tile" includes one, two, or more
tiles. Similarly,
reference to a plurality of referents should be interpreted as comprising a
single referent
and/or a plurality of referents unless the content and/or context clearly
dictate otherwise.
Thus, reference to "tiles" does not necessarily require a plurality of such
tiles. Instead, it will
be appreciated that independent of conjugation; one or more tiles are
contemplated herein
[00156] As used throughout this application the words "can" and "may" are used
in a
permissive sense (i.e., meaning having the potential to), rather than the
mandatory sense (i.e.,
meaning must). Additionally, the terms "including," "having," "involving,"
"containing,"
"characterized by," variants thereof (e.g., "includes," "has," "involves,"
"contains," etc.), arid
similar terms as used herein, including the claims, shall be inclusive and/or
open-ended, shall
have the same meaning as the word "comprising" and variants thereof (e.g.,
"comprise" and
"comprises"), and do not exclude additional, un-recited elements or method
steps,
illustratively.
- 15 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00157] As used herein, directional and/or arbitrary terms, such as "top,"
"bottom," "left,"
"right," "up," "down," "upper," "lower," "inner," "outer," "internal,"
"external," "interior,"
"exterior," "proximal," "distal," "forward," "reverse," and the like can be
used solely to
indicate relative directions and/or orientations and may not be otherwise
intended to limit the
scope of the disclosure, including the specification, invention, and/or
claims.
[00153] It will be understood that when an element is referred to as being
"coupled,"
"connected," or "responsive" to, or "on," another element, it can be directly
coupled,
connected, or responsive to, or on, the other element, or intervening elements
may also be
presentS In contrast, when an element is referred to as being "directly
coupled," "directly
1.0 connected," or "directly responsive" to, or "directly on," another
element, there are no
intervening elements present.
[00159] Example embodiments of the present inventive concepts are described
herein with
reference to cross-sectional illustrations that are schematic illustrations of
idealized
embodiments (and intermediate structures) of example embodiments As such,
variations
from the shapes of the illustrations as a result, for example, of
manufacturing techniques
and/or tolerances, are to be expected. Thus, example embodiments of the
present inventive
concepts should not be construed as limited to the particular shapes of
regions illustrated
herein but are to include deviations in shapes that result, for example, from
manufacturing.
Accordingly, the regions illustrated in the figures are schematic in nature
and their shapes are
not intended to illustrate the actual shape of a region of a device and are
not intended to limit
the scope of example embodiments.
[00160] It will be understood that although the terms "first," "second," etc.
may be used
herein to describe various elements, these elements should not be limited by
these terms.
These terms are only used to distinguish one element from another. Thus, a
"first" element
could be termed a "second" element without departing from the teachings of the
present
embodiments.
[00161] It is also understood that various implementations described herein
can be utilized
in combination with any other implementation described or disclosed, without
departing from
the scope of the present disclosure. Therefore, products, members, elements,
devices,
apparatuses, systems, methods, processes, compositions, and/or kits according
to certain
implementations of the present disclosure can include, incorporate, or
otherwise comprise
properties, features, components, members, elements, steps, and/or the like
described in other
implementations (including systems, methods, apparatus, and/or the like)
disclosed herein
without departing from the scope of the present disclosure. Thus, reference to
a specific
- 16 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
feature in relation to one implementation should not be construed as being
limited to
applications only within that implementation.
[00162] The headings used herein are for organizational purposes only and are
not meant
to be used to limit the scope of the description or the claims. To facilitate
understanding, like
reference numerals have been used, where possible, to designate like elements
common to the
figures_ Furthermore, where possible, like numbering of elements have been
used in various
figures. Furthermore, alternative configurations of a particular element may
each include
separate letters appended to the element number.
[00163] The term "about" is used herein to mean approximately, in the region
of, roughly,
or around. When the term "about" is used in conjunction with a numerical
range, it modifies
that range by extending the boundaries above and below the numerical values
set forth. In
general, the term "about" is used herein to modify a numerical value above and
below the
stated value by a variance of 5%. When such a range is expressed, another
embodiment
includes from the one particular value and/or to the other particular value.
Similarly, when
values are expressed as approximations, by use of the antecedent "about," it
will be
understood that the particular value forms another embodiment. It will be
further understood
that the endpoints of each of the ranges are significant both in relation to
the other endpoint,
and independently of the other endpoint.
[00164] The word "or" as used herein means any one member of a particular list
and also
includes any combination of members of that list.
[00165] By "sample" is meant an animal; a tissue or organ from an animal,
including, but
not limited to, a human animal; a cell (either within a subject (e.g., a human
or non-human
animal), taken directly from a subject, or a cell maintained in culture or
from a cultured cell
line); a cell lysate (or lysate fraction) or cell extract; a solution
containing one or more
molecules derived from a cell, cellular material, or viral material (e.g. a
polypeptide or
nucleic acid); or a solution containing a non-naturally occurring nucleic
acid, which is
assayed as described herein. A sample may also be any body fluid or excretion
(for example,
but not limited to, blood, urine, stool, saliva, tears, bile, or cerebrospinal
fluid) that may or
may not contain host or pathogen cells, cell components, or nucleic acids.
Samples may also
include environmental samples such as, but not limited to, soil, water (fresh
water, waste
water, etc.), air monitoring system samples (e.g., material captured in an air
filter medium),
surface swabs, and vectors (e.g., mosquitos, ticks, fleas, etc.).
[00166] The phrase "nucleic acid" as used herein refers to a naturally occur-
ring or
synthetic oligonucleotide or polynucleotide, whether DNA or RNA or DNA-RNA
hybrid,
- 17 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
single-stranded or double-stranded, sense or anti sense, which is capable of
hybridization to a
complementary nucleic acid by Watson-Crick base-pairing. Nucleic acids of the
invention
can also include nucleotide analogs (e.g., BrdLT), and non-phosphodiester
intemucleoside
linkages (e.g., peptide nucleic acid (PNA) or thiodiester linkages). In
particular, nucleic acids
can include, without limitation, DNA, RNA, mRNA, rRNA, cDNA, gDNA, ssDNA,
dsDNA,
or any combination thereof
[00167] By "probe?' "primer," or "oligonucleotide" is meant a single-stranded
nucleic acid
molecule of defined sequence that can base-pair to a second nucleic acid
molecule that
contains a complementary sequence (the "target"). The stability of the
resulting hybrid
in depends upon the length, GC content, and the extent of the base-pairing
that occurs. The
extent of base-pairing is affected by parameters such as the degree of
complementarity
between the probe and target molecules and the degree of stringency of the
hybridization
conditions. The degree of hybridization stringency is affected by parameters
such as
temperature, salt concentration, and the concentration of organic molecules
such as
formamide, and is determined by methods known to one skilled in the art.
Probes, primers,
and oligonucleotides may be detectably-labeled, either radioactively,
fluorescently, or non-
radioactively, by methods well-known to those skilled in the art. dsDNA
binding dyes may be
used to detect dsDNA. It is understood that a "prime?' is specifically
configured to be
extended by a polymerase, whereas a "probe" or "oligonucleotide" may or may
not be so
configured.
[00168] By "dsDNA binding dyes" is meant dyes that fluoresce differentially
when bound
to double-stranded DNA than when bound to single-stranded DNA or free in
solution, usually
by fluorescing more strongly. While reference is made to dsDNA binding dyes,
it is
understood that any suitable dye may be used herein, with some non-limiting
illustrative dyes
described in U.S. Pat. No. 7,387,887, herein incorporated by reference. Other
signal
producing substances may be used for detecting nucleic acid amplification and
melting,
illustratively enzymes, antibodies, etc., as are known in the art.
[00169] By "specifically hybridizes" is meant that a probe, primer, or
oligonucleotide
recognizes and physically interacts (that is, base-pairs) with a substantially
complementary
nucleic acid (for example, a sample nucleic acid) under high stringency
conditions, and does
not substantially base pair with other nucleic acids.
[00170] By "high stringency conditions" is meant typically to occur at about a
melting
temperature (Ttn) minus 5 C (i.e. 5 below the Tm of the probe). Functionally,
high
- 18 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
stringency conditions are used to identify nucleic acid sequences having at
least 80%
sequence identity.
[00171] By "lysis particles" is meant various particles or beads for the lysis
of cells,
viruses, spores, and other material that may be present in a sample. Various
examples use
Zirconium ("Zr") silicate or ceramic beads, but other lysis particles are
known and are within
the scope of this term, including glass and sand lysis particles The term
"cell lysis
component" may include lysis particles, but may also include other components,
such as
components for chemical lysis, as are known in the art.
[00172] While PCR is the amplification method used in the examples herein, it
is
in understood that any amplification method that uses a primer may be
suitable. Such suitable
procedures include polymerase chain reaction (PCR); strand displacement
amplification
(SDA); nucleic acid sequence-based amplification (NASBA); cascade rolling
circle
amplification (CRCA), loop-mediated isothermal amplification of DNA (LAMP);
isothermal
and chimeric primer-initiated amplification of nucleic acids (1CAN); target
based-helicase
dependent amplification (MA); transcription-mediated amplification (TMA), and
the like.
Therefore, when the term PCR is used, it should be understood to include other
alternative
amplification methods. For amplification methods without discrete cycles,
reaction time may
be used where measurements are made in cycles, doubling time, or crossing
point (Cp), and
additional reaction time may be added where additional PCR cycles are added in
the
embodiments described herein. It is understood that protocols may need to be
adjusted
accordingly.
[00173] While various examples herein reference human targets and human
pathogens,
these examples are illustrative only. Methods, kits, and devices described
herein may be used
to detect and sequence a wide variety of nucleic acid sequences from a wide
variety of
samples, including, human, veterinary, industrial, and environmental.
[00174] Various embodiments disclosed herein use a self-contained nucleic acid
analysis
pouch to assay a sample for the presence of various biological substances,
illustratively
antigens and nucleic acid sequences, illustratively in a single closed system.
Such systems,
including pouches and instruments for use with the pouches, are disclosed in
more detail in
U.S. Pat. Nos. 8,394,608, 8,895,295, 10,464,060, herein incorporated by
reference in their
entireties. However, it is understood that such pouches are illustrative only,
and the nucleic
acid preparation and amplification reactions discussed herein may be performed
in any of a
variety of open or closed system sample vessels as are known in the art,
including 96-well
plates, plates of other configurations, arrays, carousels, and the like, using
a variety of nucleic
- 19 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
acid purification and amplification systems, as are known in the art. While
the terms "sample
well", "amplification well", "amplification container", or the like are used
herein, these terms
are meant to encompass wells, tubes, and various other reaction containers, as
are used in
these amplification systems. In one embodiment, the pouch is used to assay for
multiple
pathogens. The pouch may include one or more blisters used as sample wells,
illustratively in
a closed system Illustratively, various steps may be performed in the
optionally disposable
pouch, including nucleic acid preparation, primary large volume multiplex PCR,
dilution of
primary amplification product, and secondary PCR, culminating with optional
real-time
detection or post-amplification analysis such as melting-curve analysis.
Further, it is
in understood that while the various steps may be performed in pouches of
the present
invention, one or more of the steps may be omitted for certain uses, and the
pouch
configuration may be altered accordingly. While many embodiments herein use a
multiplex
reaction for the first-stage amplification, it is understood that this is
illustrative only, and that
in some embodiments the first-stage amplification may be singleplex. In one
illustrative
example, the first-stage singleplex amplification targets housekeeping genes,
and the second-
stage amplification uses differences in housekeeping genes for identification.
Thus, while
various embodiments discuss first-stage multiplex amplification, it is
understood that this is
illustrative only.
[00175] Fig. 1 shows an illustrative pouch 510 that may be used in various
embodiments,
or may be reconfigured for various embodiments. Pouch 510 is similar to Fig.
15 of U.S.
Patent No. 8,895,295, with like items numbered the same. Fitment 590 is
provided with entry
channels 515a through 5151, which also serve as reagent reservoirs or waste
reservoirs.
Illustratively, reagents may be freeze dried in fitment 590 and rehydrated
prior to use. Blisters
522, 544, 546, 548, 564, and 566, with their respective channels 514, 538,
543, 552, 553, 562,
and 565 are similar to blisters of the same number of Fig. 15 of U.S. Patent
No. 8,895,295.
Second-stage reaction zone 580 of Fig. 1 is similar to that of U.S. Patent
Application No.
8,895,295, but the second-stage wells 582 of high density array 581 are
arranged in a
somewhat different pattern. The more circular pattern of high density array
581 of Fig. 1
eliminates wells in corners and may result in more uniform filling of second-
stage wells 582.
As shown, the high density array 581 is provided with 102 second-stage wells
582. Pouch
510 is suitable for use in the FilmArray instrument (BioFire Diagnostics,
LLC, Salt Lake
City, UT). However, it is understood that the pouch embodiment is illustrative
only.
[00176] While other containers may be used, illustratively, pouch 510 may be
formed of
two layers of a flexible plastic film or other flexible material such as
polyester, polyethylene
- 20 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
terephthalate (PET), polycarbonate, polypropylene, polymethylmethacrylate,
mixtures,
combinations, and layers thereof that can be made by any process known in the
art, including
extrusion, plasma deposition, and lamination. For instance, each layer can be
composed of
one or more layers of material of a single type or more than one type that are
laminated
together. Metal foils or plastics with aluminum lamination also may be used.
Other bather
materials are known in the art that can be sealed together to form the
blisters and channels If
plastic film is used, the layers may be bonded together, illustratively by
heat sealing.
Illustratively, the material has low nucleic acid binding and low protein
binding capacity.
[00177] For embodiments employing fluorescent monitoring, plastic films that
are
adequately low in absorbance and auto-fluorescence at the operative
wavelengths are
preferred. Such material could be identified by testing different plastics,
different plasticizers,
and composite ratios, as well as different thicknesses of the film. For
plastics with aluminum
or other foil lamination, the portion of the pouch that is to be read by a
fluorescence detection
device can be left without the foil. For example, if fluorescence is monitored
in second-stage
wells 582 of the second-stage reaction zone 580 of pouch 510, then one or both
layers at
wells 582 would be left without the foil (e.g., made from optically
transparent material). In
the example of PCR, film laminates composed of polyester (Mylar, DuPont,
Wilmington DE)
of about 0.0048 inch (0.1219 mm) thick and polypropylene films of 0.001-0.003
inch (0.025-
0.076 mm) thick perform well. Illustratively, pouch 510 may be made of a clear
material
capable of transmitting approximately 80%-90% of incident light.
[00178] In the illustrative embodiment, materials are moved between blisters
by the
application of pressure, illustratively pneumatic pressure, upon the blisters
and channels.
Accordingly, in embodiments employing pressure, the pouch material
illustratively is flexible
enough to allow the pressure to have the desired effect. The term "flexible"
is herein used to
describe a physical characteristic of the material of the pouch. The term
"flexible" is herein
defined as readily deformable by the levels of pressure used herein without
cracking,
breaking, crazing, or the like. For example, thin plastic sheets, such as
Saran' wrap and
Ziploc bags, as well as thin metal foil, such as aluminum foil, are flexible.
However, only
certain regions of the blisters and channels need be flexible, even in
embodiments employing
pneumatic pressure. Further, only one side of the blisters and channels need
to be flexible, as
long as the blisters and channels are readily deformable. Other regions of the
pouch 510 may
be made of a rigid material or may be reinforced with a rigid material. Thus,
it is understood
that when the terms "flexible pouch" or "flexible sample container" or the
like are used, only
portions of the pouch or sample container need be flexible.
- 21 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00179] Illustratively, a plastic film may be used for pouch 510. A sheet of
metal,
illustratively aluminum, or other suitable material, may be milled or
otherwise cut, to create a
die having a pattern of raised surfaces. When fitted into a pneumatic press
(illustratively A-
5302-PDS, Janesville Tool Inc., Milton WI), illustratively regulated at an
operating
temperature of 195 C, the pneumatic press works like a printing press, melting
the sealing
surfaces of plastic film only where the die contacts the film Likewise, the
plastic film(s) used
for pouch 510 may be cut and welded together using a laser cutting and welding
device.
Various components, such as PCR primers (illustratively spotted onto the film
and dried),
antigen binding substrates, magnetic beads, and zirconium silicate beads may
be sealed inside
in various blisters as the pouch 510 is formed. Reagents for sample
processing can be spotted
onto the film prior to sealing, either collectively or separately. In one
embodiment, nucleotide
tri-phosphates (NTPs) are spotted onto the film separately from polymerase and
primers,
essentially eliminating activity of the polymerase until the reaction may he
hydrated by an
aqueous sample. If the aqueous sample has been heated prior to hydration, this
creates the
conditions for a true hot-start PCR and reduces or eliminates the need for
expensive chemical
hot-start components. In another embodiment, components may be provided in
powder or pill
form and are placed into blisters prior to final sealing.
[00180] Pouch 510 may be used in a manner similar to that described in U.S.
Patent No.
8,895,295. In one illustrative embodiment, a 300 pl mixture comprising the
sample to be
tested (100 ill) and lysis buffer (200 pl) may be injected into an injection
port (not shown) in
fitment 590 near entry channel 515a, and the sample mixture may be drawn into
entry
channel 515a. Water may also be injected into a second injection port (not
shown) of the
fitment 590 adjacent entry channel 5151, and is distributed via a channel (not
shown)
provided in fitment 590, thereby hydrating up to eleven different reagents,
each of which
were previously provided in dry form at entry channels 515b through 5151.
Illustrative
methods and devices for injecting sample and hydration fluid (e.g. water or
buffer) are
disclosed in U.S. Pat. No. 10,464,060 , herein incorporated by reference in
its entirety,
although it is understood that these methods and devices are illustrative only
and other ways
of introducing sample and hydration fluid into pouch 510 are within the scope
of this
disclosure. These reagents illustratively may include freeze-dried PCR
reagents, DNA
extraction reagents, wash solutions, immunoassay reagents, or other chemical
entities.
Illustratively, the reagents are for nucleic acid extraction, first-stage
multiplex PCR, dilution
of the multiplex reaction, and preparation of second-stage PCR reagents, as
well as control
reactions. In the embodiment shown in Fig. 1, all that need be injected is the
sample solution
- 22 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
in one injection port and water in the other injection port. After injection,
the two injection
ports may be sealed. For more information on various configurations of pouch
510 and
fitment 590, see U.S. Pat No. 8,895,295, already incorporated by reference.
[00181] After injection, the sample may be moved from injection channel 515a
to lysis
blister 522 via channel 514. Lysis blister 522 is provided with beads or
particles 534, such as
ceramic beads or other abrasive elements, and is configured for vortexing via
impaction using
rotating blades or paddles provided within the FilmArray instrument. Bead-
milling, by
shaking, vortexing, sonicating, and similar treatment of the sample in the
presence of lysis
particles such as zirconium silicate (ZS) beads 534, is an effective method to
form a lysate. It
1.0 is understood that, as used herein, terms such as "lyse," "lysing," and
"lysate" are not limited
to rupturing cells, but that such terms include disruption of non-cellular
particles, such as
viruses. In another embodiment, a paddle beater using reciprocating or
alternating paddles,
such as those described in U.S. Pat. Pub. No. 2019/0344269, herein
incorporated by reference
in its entirety, may be used for lysis in this embodiment, as well as in the
other embodiments
described herein.
[00182] Fig. 4 shows a bead beating motor 819 of instrument 800 of Fig. 2. The
bead
beating motor 819 comprises blades 821 that may be mounted on a first side 811
of support
member 802 of the instrument 800 Blades may extend through slot 804 to contact
pouch
510. It is understood, however, that motor 819 may be mounted on other
structures of
instrument 800. In one illustrative embodiment, motor 819 is a Mabuchi RC-
280SA-2865 DC
Motor (Chiba, Japan), mounted on support member 802. In one illustrative
embodiment, the
motor is turned at 5,000 to 25,000 rpm, more illustratively 10,000 to 20,000
rpm, and still
more illustratively approximately 15,000 to 18,000 rpm. For the Mabuchi motor,
it has been
found that 7.2V provides sufficient rpm for lysis. It is understood, however,
that the actual
speed may be somewhat slower when the blades 821 are impacting pouch 510.
Other
voltages and speeds may be used for lysis depending on the motor and paddles
used.
Optionally, controlled small volumes of air may be provided into the bladder
822 adjacent
lysis blister 522. It has been found that in some embodiments, partially
filling the adjacent
bladder with one or more small volumes of air aids in positioning and
supporting lysis blister
during the lysis process. Alternatively, another structure, illustratively a
rigid or compliant
gasket or other retaining structure around lysis blister 522, can be used to
restrain pouch 510
during lysis. It is also understood that motor 819 is illustrative only, and
other devices may be
used for milling, shaking, or vortexing the sample. In some embodiments,
chemicals or heat
may be used in addition to or instead of mechanical lysis.
- 23 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00183] Once the sample material has been adequately lysed, the sample is
moved to a
nucleic acid extraction zone, illustratively through channel 538, blister 544,
and channel 543,
to blister 546, where the sample is mixed with a nucleic acid-binding
substance, such as
silica-coated magnetic beads 533. Alternatively, magnetic beads 533 may be
rehydrated,
illustratively using fluid provided from one of the entry channel 515c-515e,
and then moved
through channel 543 to blister 544, and then through channel 538 to blister
522 The mixture
is allowed to incubate for an appropriate length of time, illustratively
approximately 10
seconds to 10 minutes. A retractable magnet located within the instrument
adjacent blister
546 (see, e.g, magnet 850, Fig. 2) captures the magnetic beads 533 from the
solution,
forming a pellet against the interior surface of blister 546. If incubation
takes place in blister
522, multiple portions of the solution may need to be moved to blister 546 for
capture. The
liquid is then moved out of blister 546 and back through blister 544 and into
blister 522,
which is now used as a waste receptacle. One or more wash buffers from one or
more of
injection channels 515c to 515e are provided via blister 544 and channel 543
to blister 546.
Optionally, the magnet is retracted and the magnetic beads 533 are washed by
moving the
beads back and forth from blisters 544 and 546 via channel 543. Once the
magnetic beads
533 are washed, the magnetic beads 533 are recaptured in blister 546 by
activation of the
magnet, and the wash solution is then moved to blister 522. This process may
be repeated as
necessary to wash the lysis buffer and sample debris from the nucleic acid-
binding magnetic
beads 533.
[00184] After washing, elution buffer stored at injection channel 515f is
moved to blister
548, and the magnet is retracted. The solution is cycled between blisters 546
and 548 via
channel 552, breaking up the pellet of magnetic beads 533 in blister 546 and
allowing the
captured nucleic acids to dissociate from the beads and come into solution,
The magnet is
once again activated, capturing the magnetic beads 533 in blister 546, and the
eluted nucleic
acid solution is moved into blister 548,
[00185] First-stage PCR master mix from injection channel 515g is mixed with
the nucleic
acid sample in blister 548. Optionally, the mixture is mixed by forcing the
mixture between
blisters 548 and 564 via channel 553. After several cycles of mixing, the
solution is contained
in blister 564, where a pellet of first-stage PCR primers is provided, at
least one set of
primers for each target, and first-stage multiplex PCR is performed. If RNA
targets are
present, a reverse transcription (RT) step may be performed prior to or
simultaneously with
the first-stage multiplex PCR. First-stage multiplex PCR temperature cycling
in the
FilmArray instrument is illustratively performed for 15-20 cycles, although
other levels of
- 24 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
amplification may be desirable, depending on the requirements of the specific
application.
The first-stage PCR master mix may be any of various master mixes, as are
known in the art.
In one illustrative example, the first-stage PCR master mix may be any of the
chemistries
disclosed in U.S. Pat. No. 9,932,634, herein incorporated by reference, for
use with PCR
protocols taking 20 seconds or less per cycle.
[00186] After first-stage PCR has proceeded for the desired number of cycles,
the sample
may be diluted, illustratively by forcing most of the sample back into blister
548, leaving
only a small amount in blister 564, and adding second-stage PCR master mix
from injection
channel 5151. Alternatively, a dilution buffer from 515i may be moved to
blister 566 then
in mixed with the amplified sample in blister 564 by moving the fluids back
and forth between
blisters 564 and 566 via channel 562. If desired, dilution may be repeated
several times, using
dilution buffer from injection channels 515j and 515k, or injection channel
515k may be
reserved, illustratively, for sequencing or for other post-PCR analysis, and
then adding
second-stage PCR master mix from injection channel 515h to some or all of the
diluted
amplified sample. It is understood that the level of dilution may be adjusted
by altering the
number of dilution steps or by altering the percentage of the sample discarded
prior to mixing
with the dilution buffer or second-stage PCR master mix comprising components
for
amplification, illustratively a polymerase, dNTPs, and a suitable buffer,
although other
components may be suitable, particularly for non-PCR amplification methods. If
desired, this
mixture of the sample and second-stage PCR master mix may be pre-heated in
blister 564
prior to movement to second-stage wells 582 for second-stage amplification.
Such preheating
may obviate the need for a hot-start component (antibody, chemical, or
otherwise) in the
second-stage PCR mixture.
[00187] In one embodiment, the illustrative second-stage PCR master mix is
incomplete,
lacking primer pairs, and each of the 102 second-stage wells 582 is pre-loaded
with a specific
PCR primer pair. In other embodiments, the master mix may lack other
components (e.g.,
polymerase, Mg2t, etc.) and the lacking components may be pre-loaded in the
array. If
desired, second-stage PCR master mix may lack other reaction components, and
these
components may be pre-loaded in the second-stage wells 582 as well. Each
primer pair may
be similar to or identical to a first-stage PCR primer pair or may be nested
within the first-
stage primer pair. Movement of the sample from blister 564 to the second-stage
wells 582
completes the PCR reaction mixture. Once high density array 581 is filled, the
individual
second-stage reactions are sealed in their respective second-stage blisters by
any number of
means, as is known in the art. Illustrative ways of filling and sealing the
high density array
- 25 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
581 without cross-contamination are discussed in U.S. Pat. No. 8,895,295,
already
incorporated by reference. Illustratively, the various reactions in wells 582
of high density
array 581 are simultaneously or individually thermal cycled, illustratively
with one or more
Peltier devices, although other means for thermal cycling are known in the
art.
[00188] In certain embodiments, second-stage PCR master mix contains the dsDNA
binding dye LCGireene Plus (BioFire Diagnostics, LLC) to generate a signal
indicative of
amplification. However, it is understood that this dye is illustrative only,
and that other
signals may be used, including other dsDNA binding dyes and probes that are
labeled
fluorescently, radioactively, chemiluminescently, enzymatically, or the like,
as are known in
the art. Alternatively, wells 582 of array 581 may be provided without a
signal, with results
reported through subsequent processing.
[00189] When pneumatic pressure is used to move materials within pouch 510, in
one
embodiment, a "bladder" may be employed. The bladder assembly 810, a portion
of which is
shown in Figs. 2-3, includes a bladder plate 824 housing a plurality of
inflatable bladders
822, 844, 846, 848, 864, and 866, each of which may be individually
inflatable, illustratively
by a compressed gas source. Because the bladder assembly 810 may be subjected
to
compressed gas and used multiple times, the bladder assembly 810 may be made
from
tougher or thicker material than the pouch. Alternatively, bladders 822, 844,
846, 848, 864,
and 866 may be formed from a series of plates fastened together with gaskets,
seals, valves,
and pistons. Other arrangements are within the scope of this invention.
Alternatively, an array
of mechanical actuators and seals may be used to seal channels and direct
movement of fluids
between blisters. A system of mechanical seals and actuators that may be
adapted for the
instruments described herein is described in detail in U.S. Pat. Pub. No.
2019/0344269, the
entirety of which is already incorporated by reference
[00190] Success of the secondary PCR reactions is dependent upon template
generated by
the multiplex first-stage reaction. Typically, PCR is performed using DNA of
high purity.
Methods such as phenol extraction or commercial DNA extraction kits provide
DNA of high
purity. Samples processed through the pouch 510 may require accommodations be
made to
compensate for a less pure preparation. PCR may be inhibited by components of
biological
samples, which is a potential obstacle Illustratively, hot-start PCR, higher
concentration of
Taq polymerase enzyme, adjustments in MgCl2 concentration, adjustments in
primer
concentration, addition of engineered enzymes that are resistant to
inhibitors, and addition of
adjuvants (such as DMSO, TMSO, or glycerol) optionally may be used to
compensate for
lower nucleic acid purity. While purity issues are likely to be more of a
concern with first-
- 26 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
stage amplification, it is understood that similar adjustments may be provided
in the second-
stage amplification as well.
[00191] When pouch 510 is placed within the instrument 800, the bladder
assembly 810 is
pressed against one face of the pouch 510, so that if a particular bladder is
inflated, the
pressure will force the liquid out of the corresponding blister in the pouch
510. In addition to
bladders corresponding to many of the blisters of pouch 510, the bladder
assembly 810 may
have additional pneumatic actuators, such as bladders or pneumatically-driven
pistons,
corresponding to various channels of pouch 510. Figs. 2-3 show an illustrative
plurality of
pistons or hard seals 838, 843, 852, 853, and 865 that correspond to channels
538, 543, 553,
in and 565 of pouch 510, as well as seals 871, 872, 873, 874 that minimize
backflow into
fitment 590. When activated, hard seals 838, 843, 852, 853, and 865 form pinch
valves to
pinch off and close the corresponding channels. To confine liquid within a
particular blister
of pouch 510, the hard seals are activated over the channels leading to and
from the blister,
such that the actuators function as pinch valves to pinch the channels shut.
Illustratively, to
mix two volumes of liquid in different blisters, the pinch valve actuator
sealing the
connecting channel is activated, and the pneumatic bladders over the blisters
are alternately
pressurized, forcing the liquid back and forth through the channel connecting
the blisters to
mix the liquid therein. The pinch valve actuators may be of various shapes and
sizes and may
be configured to pinch off more than one channel at a time. While pneumatic
actuators are
discussed herein, it is understood that other ways of providing pressure to
the pouch are
contemplated, including various electromechanical actuators such as linear
stepper motors,
motor-driven cams, rigid paddles driven by pneumatic, hydraulic or
electromagnetic forces,
rollers, rocker-arms, and in some eases, cocked springs. In addition, there
are a variety of
methods of reversibly or irreversibly dosing channels in addition to applying
pressure normal
to the axis of the channel. These include kinking the bag across the channel,
heat-sealing,
rolling an actuator, and a variety of physical valves sealed into the channel
such as butterfly
valves and ball valves. Additionally, small Peltier devices or other
temperature regulators
may be placed adjacent the channels and set at a temperature sufficient to
freeze the fluid,
effectively forming a seal. Also, while the design of Fig. 1 is adapted for an
automated
instrument featuring actuator elements positioned over each of the blisters
and channels, it is
also contemplated that the actuators could remain stationary, and the pouch
510 could be
transitioned such that a small number of actuators could be used for several
of the processing
stations including sample disruption, nucleic-acid capture, first and second-
stage PCR, and
processing stations for other applications of the pouch 510 such as immuno-
assay and
- 27 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
immuno-PCR. Rollers acting on channels and blisters could prove particularly
useful in a
configuration in which the pouch 510 is translated between stations. Thus,
while pneumatic
actuators are used in the presently disclosed embodiments, when the term
"pneumatic
actuator" is used herein, it is understood that other actuators and other ways
of providing
pressure may be used, depending on the configuration of the pouch and the
instrument.
[00192] Turning back to Fig 2, each pneumatic actuator is connected to
compressed air
source 895 via valves 899. While only several hoses 878 are shown in Fig. 2,
it is understood
that each pneumatic fitting is connected via a hose 878 to the compressed gas
source 895.
Compressed gas source 895 may be a compressor, or, alternatively, compressed
gas source
in 895 may be a compressed gas cylinder, such as a carbon dioxide cylinder.
Compressed gas
cylinders are particularly useful if portability is desired. Other sources of
compressed gas are
within the scope of this invention. Similar pneumatic control may be provided,
for example,
for control of fluid movement in the pouches described herein, or other
actuators, servos, or
the like may be provided.
[00193] Several other components of instrument 810 are also connected to
compressed gas
source 895. A magnet 850, which is mounted on a second side 814 of support
member 802, is
illustratively deployed and retracted using gas from compressed gas source 895
via hose 878,
although other methods of moving magnet 850 are known in the art. Magnet 850
sits in
recess 851 in support member 802. It is understood that recess 851 can be a
passageway
through support member 802, so that magnet 850 can contact blister 546 of
pouch 510.
However, depending on the material of support member 802, it is understood
that recess 851
need not extend all the way through support member 802, as long as when magnet
850 is
deployed, magnet 850 is close enough to provide a sufficient magnetic field at
blister 546,
and when magnet 850 is fully retracted, magnet 850 does not significantly
affect any
magnetic beads 533 present in blister 546. While reference is made to
retracting magnet 850,
it is understood that an electromagnet may be used and the electromagnet may
be activated
and inactivated by controlling flow of electricity through the electromagnet.
Thus, while this
specification discusses withdrawing or retracting the magnet, it is understood
that these terms
are broad enough to incorporate other ways of withdrawing the magnetic field.
It is
understood that the pneumatic connections may be pneumatic hoses or pneumatic
air
manifolds, thus reducing the number of hoses or valves required. It is
understood that similar
magnets and methods for activating the magnets may be used in other
embodiments.
[00194] The various pneumatic pistons 868 of pneumatic piston array 869 are
also
connected to compressed gas source 895 via hoses 878. While only two hoses 878
are shown
- 28 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
connecting pneumatic pistons 868 to compressed gas source 895, it is
understood that each of
the pneumatic pistons 868 are connected to compressed gas source 895. Twelve
pneumatic
pistons 868 are shown, although other configurations are within the scope of
the present
invention.
[00195] A pair of temperature control elements are mounted on a second side
814 of
support 802 As used herein, the term "temperature control element" refers to a
device that
adds heat to or removes heat from a sample. Illustrative examples of a
temperature control
element include, but are not limited to, heaters, coolers, Peltier devices,
resistive heaters,
induction heaters, electromagnetic heaters, thin film heaters, printed element
heaters, positive
in temperature coefficient heaters, and combinations thereof A temperature
control element
may include multiple heaters, coolers, Peltiers, etc. In one aspect, a given
temperature control
element may include more than one type of heater or cooler. For instance, an
illustrative
example of a temperature control element may include a Peltier device with a
separate
resistive heater applied to the top and/or the bottom face of the Peltier.
While the term
"heater" is used throughout the specification, it is understood that other
temperature control
elements may be used to adjust the temperature of the sample.
[00196] As discussed above, first-stage heater 886 may be positioned to heat
and cool the
contents of blister 564 for first-stage PCR. As seen in Fig. 2, second-stage
heater 888 may be
positioned to heat and cool the contents of second-stage blisters 582 of array
581 of pouch
510, for second-stage PCR. It is understood, however, that these heaters could
also be used
for other heating purposes, and that other heaters may be included, as
appropriate for the
particular application.
[00197] As discussed above, while Peltier devices, which thermocycle between
two or
more temperatures, are effective for PCR, it may be desirable in some
embodiments to
maintain heaters at a constant temperature. Illustratively, this can be used
to reduce run time,
by eliminating time needed to transition the heater temperature beyond the
time needed to
transition the sample temperature. Also, such an arrangement can improve the
electrical
efficiency of the system as it is only necessary to thermally cycle the
smaller sample and
sample vessel, not the much larger (more thermal mass) Peltier devices. For
instance, an
instrument may include multiple heaters (La, two or more) at temperatures set
for, for
example, annealing, extension, denaturation that are positioned relative to
the pouch to
accomplish thermal cycling. Two heaters may be sufficient for many
applications. In various
embodiments, the heaters can be moved, the pouch can be moved, or fluids can
be moved
relative to the heaters to accomplish thermal cycling. Illustratively, the
heaters may be
- 29 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
arranged linearly, in a circular arrangement, or the like Types of suitable
heaters have been
discussed above, with reference to first-stage PCR.
[00198] When fluorescent detection is desired, an optical array 890 may be
provided. As
shown in Fig. 2, optical array 890 includes a light source 898, illustratively
a filtered LED
light source, filtered white light, or laser illumination, and a camera 896.
Camera 896
illustratively has a plurality of photodetectors each corresponding to a
second-stage well 582
in pouch 510. Alternatively, camera 896 may take images that contain all of
the second-stage
wells 582, and the image may be divided into separate fields corresponding to
each of the
second-stage wells 582_ Depending on the configuration, optical array 890 may
be stationary,
in or optical array 890 may be placed on movers attached to one or more
motors and moved to
obtain signals from each individual second-stage well 582. It is understood
that other
arrangements are possible. Some embodiments for second-stage heaters provide
the heaters
on the opposite side of pouch 510 from that shown in Fig. 2. Such orientation
is illustrative
only and may be determined by spatial constraints within the instrument.
Provided that
second-stage reaction zone 580 is provided in an optically transparent
material,
photodetectors and heaters may be on either side of array 581.
[00199] As shown, a computer 894 controls valves 899 of compressed air source
895, and
thus controls all of the pneumatics of instrument 800. In addition, many of
the pneumatic
systems in the instrument may be replaced with mechanical actuators, pressure
applying
means, and the like in other embodiments. Computer 894 also controls heaters
886 and 888,
and optical array 890_ Each of these components is connected electrically,
illustratively via
cables 891, although other physical or wireless connections are within the
scope of this
invention. It is understood that computer 894 may be housed within instrument
800 or may be
external to instrument 800. Further, computer 894 may include built-in circuit
boards that
control some or all of the components, and may also include an external
computer, such as a
desktop or laptop PC, to receive and display data from the optical array. An
interface,
illustratively a keyboard interface, may be provided including keys for
inputting information
and variables such as temperatures, cycle times, etc. Illustratively, a
display 892 is also
provided. Display 892 may be an LED, LCD, or other such display, for example.
[00200] Other instruments known in the art teach PCR within a sealed flexible
container.
See, e.g., U.S. Pat. Nos. 6,645,758, 6,780,617, and 9,586,208, herein
incorporated by
reference. However, including the cell lysis within the sealed PCR vessel can
improve ease of
use and safety, particularly if the sample to be tested may contain a
biohazard. In the
embodiments illustrated herein, the waste from cell lysis, as well as that
from all other steps,
- 30 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
remains within the sealed pouch. Still, it is understood that the pouch
contents could be
removed for further testing.
[00201] Turning back to Fig. 2, instrument 800 includes a support member 802
that could
form a wall of a casing or be mounted within a casing. Instrument 800 may also
include a
second support member (not shown) that is optionally movable with respect to
support
member 802, to allow insertion and withdrawal of pouch 510. Illustratively, a
lid may cover
pouch 510 once pouch 510 has been inserted into instrument 800. In another
embodiment,
both support members may be fixed, with pouch 510 held into place by other
mechanical
means or by pneumatic pressure.
1.0 [00202] In the illustrative example, heaters 886 and 888 are mounted on
support member
802. However, it is understood that this arrangement is illustrative only and
that other
arrangements are possible. Illustrative heaters include Peltiers and other
block heaters,
resistive heaters, electromagnetic heaters, and thin film heaters, as are
known in the art, to
thermocycle the contents of blister 864 and second-stage reaction zone 580.
Bladder plate
810, with bladders 822, 844, 846, 848, 864, 866, hard seals 838, 843, 852,
853, and seals 871,
872, 873, 874 form bladder assembly 808, which may illustratively be mounted
on a
moveable support structure that may be moved toward pouch 510, such that the
pneumatic
actuators are placed in contact with pouch 510. When pouch 510 is inserted
into instrument
800 and the movable support member is moved toward support member 802, the
various
blisters of pouch 510 are in a position adjacent to the various bladders of
bladder assembly
810 and the various seals of assembly 808, such that activation of the
pneumatic actuators
may force liquid from one or more of the blisters of pouch 510 or may form
pinch valves
with one or more channels of pouch 510. The relationship between the blisters
and channels
of pouch 510 and the bladders and seals of assembly 808 is illustrated in more
detail in Fig. 3.
SELF-CONTAINED REACTION VESSELS AND METHODS
[00203] Figs. 5A and 5B show another illustrative embodiment of a pouch 100a,
100b
(also referred to herein as a reaction container, a reaction vessel, and/or a
science card) that
may be used in various embodiments, or may be reconfigured for various
embodiments
described herein for PCR, microbial testing, immunologic testing, or for a
variety of other
tests. Fig. 5A shows an example of an uncompleted pouch 100a and Fig. 5B shows
an
illustrative example of a completed pouch 100b with a sample collection swab
103, various
reagents (e.g., lysis buffer 107 and lysis beads 105, magnetic beads 115,
etc.), a second stage
reaction array 109, and other components that may be added to pouch 100b at
the time of
manufacture. Pouch 100a, 100b shown in Figs. 5A and 5B can be manufactured
according to
- 31 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
the methods described herein. Pouch 100a, 100b may be configured for use in an
instrument
described in U.S. Pat. Pub. No. 2019/0046989, herein incorporated by
reference, or in a
variety of other instruments, such as the instrument as described and shown
above with
reference to Fig. 2.
[00204] The illustrative pouch 100a, 100b of Figs. 5A and 5B includes a number
of zones
or blisters where sample preparation, nucleic acid amplification, and
detection can occur The
illustrative pouch 100a, 100b may include a sample input chamber 102, a sample
preparation
chamber 104, a sample wash reagent chamber 106, reactant recovery/wash
chambers 112 and
114, a first reaction chamber 116, and a second reaction chamber 108, and one
or more liquid
1.0 reagent blisters 110a-110g that may be filled with aqueous reagents at
the time of
manufacture (as shown at pouch 100b of Fig, 5B). In some embodiments, one or
more of the
liquid reagents in, for example, blisters 110a-110g may be replaced with dried
reagents that
can be rehydrated at the time of use by liquid sample or other liquid
reagents. Reagents in
chambers and blisters 104, 106, 114, and 110a-110g may be added to pouch 100a,
100b at the
time of manufacture through access channels 243a-243j (Fig. 5A) that are
formed during the
manufacturing process. Likewise, a second-stage reaction array 109 may be
inserted into
pouch 100a via opening 244. After reagents are added, these access openings
243a-243j and
244 may be sealed (e.g., heat sealed) to seal the reagents and second-stage
array in pouch
100b. Because pouch 100a, 100b may be fabricated from bather films that have
very low
rates of water vapor and oxygen transmission, aqueous liquid reagents and/or
dried reagents
in pouch 100b that may be added at the time of manufacture may be stable under
ambient
storage conditions for many months or a year or more (e.g., 3 months, 6
months, 1 year, or
more). Pouch 100a, 100b may have any combination and configuration of
chambers, blisters,
and fluid channels. Below, adaptable methods for manufacturing an assay device
having
formed reaction chambers, blisters, channels, and the like from sheets of film
material are
described. In one example, the methods described herein can be used for
manufacturing the
pouch 100a, 100b. It is understood that such methods of manufacture may also
be applied to
devices having different configurations or different uses, such as any of the
pouches or
reaction containers described above or as described in U.S. Pat Pub. No.
2020/0261914, the
entirety of which is incorporated herein by reference.
[00205] The illustrative pouch 100a, 100b includes a fluidic circuit (i.e,, an
interconnected
series of reaction chambers, channels, and the like in fluid communication)
that can be used
for fluid movement in the pouch 100a, 100b. In the illustrated example, the
fluidic circuit of
pouch 100a, 100b includes a series of fluidically connected chambers (e.g.,
102, 104, 112,
- 32 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
114, 116, 108/109 etc.), blisters (e.g., 106 and 110a-110g) and channels (122,
125a, 125b,
128, 130, 124a-124g, and 129) illustratively for sample input, cell lysis and
nucleic acid
recovery, a first-stage PCR, a second-stage PCR, and detection of
amplification. Channels
122, 125a, 125b, 128, 130, 124a-124g, and 129 may be sealed at the time of
manufacture
(e.g., by lamination of the pouch films), but such channels can be opened in
use (e.g., by
forcing fluid through the channels to peel apart the laminated films) to
permit fluid movement
between blisters and chambers. In an instrument designed to use pouch 100b for
an assay,
channel opening may be selectively controlled and opened channels may be
reclosed with the
use of hard seals that press on the exterior surface of the pouch and pinch
off the channels
(e.g., as described above with reference to Fig. 2).
[00206] The illustrative pouch 100b includes a sample input chamber 102 that
may be
used for inputting a sample into the pouch 100b. In one embodiment, the sample
input
chamber 102 includes and a swab 103. Illustratively, the swab 103 may be used
for collecting
a sample (e.g., from a throat or nasopharyngeal swab site) and then returned
with sample
thereon back into the sample input chamber 102, but this is illustrative only.
In other
embodiments, a variety of liquid, semi-liquid, semi-solid, and solid sample
types may be
introduced directly into the sample input chamber 102. For example, a transfer
pipette or the
like may be used for introducing a liquid sample (e.g., whole blood, positive
blood culture, or
urine) directly into the sample input chamber 102_ Alternatively, swab 103 can
be used to
insert into the pouch 100b a sample previously collected by other means or
devices. Sample
input chamber 102 may be fluidically connected to a sample preparation chamber
104 via
channel 122. Sample preparation chamber 104 may include a lysis buffer 107 and
lysis beads
105 (e.g., zirconium silicate beads) that may be used for lysis of cells in a
sample. In one
embodiment, sample may be washed off of! recovered from swab 103 by flushing
lysis
buffer 107 through channel 122 back and forth between sample input chamber 102
and
sample preparation chamber 104 Cells in the sample may be lysed in chamber 104
by
agitating (e.g., bead beating) the sample, lysis buffer, and lysis beads in
chamber 104 (e.g.,
such as described above with reference to Figs. 1-4). If sample lysis is not
needed, in some
embodiments (not shown) the pouch 100b may include a reconfigured channel 122
that
bypasses the sample preparation chamber 104 and that may be in direct fluid
communication
with chamber 112. In the illustrated embodiment, sample preparation chamber
104 is
fluidically connected to chambers 112, 114, and 102 for, for example, nucleic
acid recovery
from the lysed sample and for waste disposal. In the illustrated embodiment,
chamber 114
includes a quantity of magnetic silicate beads 115. The lysed sample may be
mixed with the
- 33 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
magnetic silicate beads 115 for nucleic acid recovery. For other embodiments,
chambers 112
and 114 may contain other reagents (e.g., an immunologic capture reagent) for
other types of
assays. Following nucleic acid recovery, the magnetic silicate beads 115 may
be recaptured
(e.g., in blister 112 or 114) by a magnet (not shown) external to the pouch
100b and then
washed with a wash buffer (for example, a wash buffer may be provided from
blister 106).
Depending on the assay, one or more washes may be performed_ Following the
wash(es), the
magnetic beads may be recaptured by the magnet and the recovered nucleic acids
may be
eluted into chamber 106 with an elution buffer (for example, an elution buffer
may be
provided from blister 110a)_
[00207] In one embodiment, the eluted nucleic acids in chamber 106 may be
transferred to
chamber 116 for a first-stage nucleic acid amplification reaction (e.g., a PCR
reaction).
Reagents for first-stage PCR may be pre-loaded in pouch 100b in liquid and/or
dried form at
the time of manufacture and may be introduced into chamber 116 from, for
example, one or
more of reagent blisters 1106-110g. In some embodiments, chamber 116 of pouch
100b may
include dried reagents 118 in addition to or instead of the liquid reagents
that may be
provided from reagent blisters 110b-110g. In one example, some reagents may be
more stable
in dried form (e.g., reverse transcriptase, PCR primers, etc.) and may be, as
a result, spotted
onto the film and dried in spots 120 prior to lamination. In another example,
one or more of
the liquid reagents may be more storage-stable in the absence of one or more
reaction
components. In such cases, the reaction component(s) may be spotted onto one
of the layers
of film at the time of manufacture. An illustrative process for spotting,
drying, and
incorporating such dried reagents into an assay device will be described below
in reference to
the manufacturing method(s) described herein. If such spots 120 are present,
they may be
rehydrated when the sample and/or the liquid reagents are introduced into
chamber 116.
[00208] After the first-stage nucleic amplification reaction, a portion of the
product may
be removed (e.g. plunged) from chamber 116 and a dilution solution and second-
stage
nucleic acid amplification reagent (e.g., PCR reagents) may be added to
chamber 116 from,
for example, one or more of reagent blisters 110b-110g. The mix for the second-
stage nucleic
acid amplification reaction (e.g., a PCR reaction) may then be introduced into
the wells of
card 109 of chamber 108 for the second-stage nucleic acid amplification
reaction and
detection of amplified targets. Additional discussion of such pouches or
reaction containers
and their uses described above may be found in U.S. Pat. Pub. No. 2019/0046989
or U.S Pat.
Pub. No. 2020/0261914, the entireties of which were already incorporated
herein by
reference.
- 34 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
[00209] Illustratively, pouch 100a, 100b may be formed of two or more layers
of a flexible
plastic film or other flexible material such as polyester, polyethylene,
polyethylene
terephthalate (PET), polycarbonate, polypropylene (PP),
polymethylmethacrylate, mixtures,
combinations, and layers thereof that can be made by any process known in the
art, including
extrusion, plasma deposition, and lamination. For instance, each layer can be
composed of
one or more layers of material of a single type or more than one type that are
laminated or
fused together. One operative example is a bilayer plastic film that includes
a PET layer and a
PP layer. In one embodiment, flexible polymeric material having a thickness in
a range of
about 0.02 mm to about 0_i mm is used. Metal foils or plastics with aluminum
lamination
in also may be used. Illustratively, the material has low nucleic acid
binding and low protein
binding capacity. If plastic film is used, selected portions of the film
layers may be bonded
together, illustratively by heat sealing or laser welding. If fluorescence
detection is used,
optically transparent material may be used in the appropriate areas of the
pouch (e.g., in the
vicinity of the second-stage array).
[00210] In some embodiments, a bather film may be used in one or more of the
layers
used to form the pouch 100a, 100b_ For instance, bather films may be desirable
for some
applications because they have low water vapor and/or oxygen transmission
rates that may be
lower than conventional plastic films. Because liquid reagents may be provided
in pouch
100b at the time of manufacture, the low water vapor and/or oxygen
transmission rates that
are associated with barrier films can prevent evaporation of water from the
reagents and
prevent oxidation of reagents between the time of manufacture and the time of
use (e.g., up to
three months, up to six months, up to one year, or more). Similarly, because
certain dried
reagents may be provided in pouch 100b at the time of manufacture, the low
water vapor
and/or oxygen transmission rates that are associated with barrier films can
also prevent
degradation of these dried reagents because environmental water and oxygen are
less able to
penetrate the pouch between the time of manufacture and the time of use. In
one example,
typical barrier films may have water vapor transmission rates (WVTR), as
measured, for
example, according to ASTM F1249, as low as 0 g/m2/24 hrs (i.e., the WVTR may
be too
low to be measured by ASTM F1249), or a WVTR in a range of 0 g/m2/24 hrs to
about 3
g/m2/24 hrs (e.g., about 0.01 g/m2/24 hrs to about 3 g/m2/24 hrs), preferably
in a range of
about 0.05 g/m2/24 hrs to about 2 g/m2/24 hrs (e.g., no more than about 1
g/m2/24 hrs) and
oxygen transmission rates, as measured, for example, according to ASTM D3985,
as low as 0
cc/m2/24 hrs (i e., the oxygen transmission rate may be too low to be measured
by ASTM
D3985), or an oxygen transmission rate in a range of 0 cc/m2/24 hrs to about 2
cc/m2/24 hrs
- 35 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
(e.g., about 0.01 cc/m2/24 hrs to about 2 cc/m2/24 hrs), preferably in a range
of about 0.05
cc/m2/24 hrs to about 2 cc/m2/24 hrs (e.g., no more than about 1 cc/m2/24
hrs). Examples of
barrier films include, but are not limited to, films that can be metallized by
vapor deposition
of a metal (e.g., aluminum or another metal) or sputter coated with an oxide
(e.g., A1203 or
Si0x) or another chemical composition. A common example of a metallized film
is
aluminized Mylar, which is metal coated biaxially oriented PET (BoPET) In some
applications, coated barrier films can be laminated with a layer of
polyethylene, PP, or a
similar thermoplastic, which provides sealability and improves puncture
resistance. As with
conventional plastic films, bather films layers used to fabricate a pouch may
be bonded
together, illustratively by heat sealing. Illustratively, the material has low
nucleic acid
binding and low protein binding capacity. Other barrier materials are known in
the art that
can be sealed together to form the blisters and channels.
[00211] In one embodiment, a first polymeric sheet 200 (e.g., a layer of a
flexible plastic
film or other flexible material as described above) includes a first inner
planar face 202 and a
first outer planar face 204 (see, e.g., Fig. 14). A second polymeric sheet 210
(e.g., a layer of a
flexible plastic film or other flexible material as described above) includes
a second inner
planar face 212 and a second outer planar face 214. Illustratively, at least
one of the first
polymeric sheet 200 and the second polymeric sheet 210 can include a barrier
film or other
water vapor and/or oxygen barrier material. In one embodiment, both the first
polymeric
sheet 200 and the second polymeric sheet 210 include a barrier film or other
water vapor
and/or oxygen barrier material. In one embodiment, the first and second
polymeric sheets
200, 210 are formed as one piece, such that the first inner planar face 202
and the second
inner planar face 212 are contiguous, and the first outer planar face 204 and
the second outer
planar face 214 are contiguous. For example, the first and second polymeric
sheets 200, 210
can be formed from one polymeric sheet that is folded onto itself (e.g.,
folded in half). In
another embodiment, the first and second polymeric sheets 200, 210 are
separate polymeric
sheets joined together (e.g., laminated together) and reformed according to
the methods
described herein to form the pouch 100a, 100b. Illustratively, the first and
second polymeric
sheets 200 and 210 may include a PET outer layer, an aluminized bather layer
or a "clear"
barrier layer (e.g., an aluminum oxide or silicon oxide sputter coated coated
barrier) and an
inner PP layer. Preferably, the first polymeric and second polymeric sheets
200 and 210 may
have a WVTR in a range of about 0 g/m2/24 hrs to about 3 g/m2/24 hrs (e.g.,
0.1 g/m2/24 hrs
or less). Typically, aluminized barrier layers offer greater barrier
properties as compared to
sputter coated metal oxide "clear" barrier layers, but both offer
significantly greater barrier
- 36 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
properties that similar films without barrier layers. Illustratively, the
first polymeric sheet 200
may include a polyester (e.g., PET) outer layer, an aluminized barrier layer,
and a PP inner
layer. Illustratively, the second polymeric sheet 210 may include a polyester
(e.g., PET) outer
layer, a sputter coated metal oxide "clear" barrier layer, and a PP inner
layer.
[00212] In one embodiment, one or more polymeric sheets may be used to form a
reaction
container (e g , pouch 100a, 100b). The polymeric sheets include an inner
planar face that
forms the inside of the reaction container and an outer planar face that forms
the outside
surface of the reaction container. In the above examples, the polypropylene
(PP) layer forms
the inside of the reaction container and the polyester layer forms the outside
of the reaction
in container. This is only illustrative, however; the materials that form
the inside and outside
surfaces of the reaction container may be varied depending on the application,
but the
methods described herein may be adapted for fabrication of a reaction
container from any
polymeric sheet material.
[00213] In one embodiment, one polymeric sheet may be used to form a reaction
container
(e.g., pouch 100a, 100b). For example, a polymeric sheet (e.g., polymeric
sheet 200 or
polymeric sheet 210) may be folded onto itself (e.g., folded in half) so that
the inner planar
faces of the two parts of the folded sheet contact each other. In another
embodiment, the first
and second polymeric sheets 200 and 210 may be used to form a reaction
container. In one
embodiment, a method of forming a reaction container may include steps of
providing the
polymeric sheets 200, 210 (i.e., one folded sheet or a first polymeric sheet
and a second
polymeric sheet), contacting the inner planar faces of the polymeric sheets,
pressing the
polymeric sheets between a first forming plate and a second forming plate, and
propelling a
compressed fluid between the inner planar faces of the polymeric sheets while
the polymeric
sheets are pressed between the forming plates to reform selected portions of
the polymeric
sheets into one or more shapes defined by the forming plates. In one
embodiment, at least one
of the first or second forming plates has one or more recesses for forming one
or more
reaction chambers, fluid flow channels, reagent chambers, or sample chambers
in selected
portions of the polymeric sheets. That is, when the compressed fluid is forced
between the
inner planar faces of the polymeric sheets, the polymeric sheets can expand
outward into the
spaces defined by the forming plates. When the pressure is released, the
selected areas of the
polymeric sheets that were expanded into the forming plates retain the
shape(s) (e.g., reaction
chambers, sample chambers, reagent blisters, etc.) defined by the forming
plates.
[00214] In some embodiments, the polymeric sheets (i.e., the folded polymeric
sheet or the
first and second polymeric sheets) may be laminated to one another (i.e.,
reversibly sealed to
- 37 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
one another) prior to the pressing and propelling steps. For example, the
polymeric sheets
may be pressed between hot plates or between heated rollers to reversibly seal
the inner
planar faces to one another. In the example described above, the inner
polypropylene layers
of the sheets may be heated and pressed together such that the opposing
polypropylene layers
are sealed to one another, but sealed in such a way that the sheets can be
peeled apart (i.e., the
sheets are reversibly sealed together) In the specific embodiment where the
opposing
polypropylene layers are reversibly sealed to one another, the lamination may
occur at a
temperature in a range of about 110 C to about 130 C (e.g., 120 C). In a
specific
embodiment, the polymeric sheets may be passed between heated rollers at a
temperature of
about 110 C to about 130 C to reversibly seal the inner planar faces of the
sheets together_ In
one example, the heated rollers exert a pressure of approximately 10 PSI to
100 PSI (-0.07
MN to ¨0.7 MPa) (e.g., about 40-50 PSI) and the sheets are exposed to the
temperature of
about 110 C to about 130 C for approximately 0.05 to 0.5 seconds (e.g., 0.1
seconds). In
some embodiments, the time/temperature/pressure parameters may be adjusted for
different
film materials and/or to create reversible seals having different peel
strengths.
[00215] As discussed herein above, pouch 100b may include dried reagent spots
120. In
one embodiment, liquid reagents may be spotted onto at least one of the first
and second
polymeric sheets 200, 210 and then dried (e.g., air dried) prior to laminating
the first and
second polymeric sheets. In one embodiment, liquid reagents may be added
dropwise onto at
least one of the first and second polymeric sheets 200, 210 and the liquid may
be
subsequently air dried. The liquid may be spotted onto the film manually or
with the aid of a
liquid handling robot. In one embodiment, the liquid reagents may be spotted
onto the films
with a modified ink jet printer head. In one embodiment, the spotted reagents
may be
electrostatically transferred to the film layer as dried powder in a process
similar to laser
printing or photocopying. This would eliminate the need to dry the reagent
prior to
lamination
[00216] In some embodiments, one or more seal lines may be formed on the
polymeric
sheets prior to the pressing and propelling steps. In one embodiment, the one
or more seal
lines define boundaries of a fluidic circuit (i.e., an interconnected flow
path) that can be used
for fluid movement in the reaction container (e.g., pouch 100a, 100b). In one
embodiment,
the fluidic circuit may include one or more reaction chambers, fluid flow
channels, or sample
chambers (e.g., as described above with reference to Figs. SA and 5B). In one
embodiment,
the one or more seal lines may be formed by a heat-sealing apparatus, a laser-
welding
apparatus, a sonic welding apparatus, or other apparatuses known in the art.
Suitable
- 38 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
examples of heat sealing apparatuses include, but are not limited to, heated
plates that have
lines that define the fluidic circuit formed thereon. For example, metal
plates with raised, hot
portions tracing the fluidic circuit may serve to permanently seal the
polymeric sheets to one
another in select areas to define the boundaries of the fluidic circuit. In
one embodiment, such
plates may have cooler regions between the seal lines (cooler regions may be
filled, for
example, with syntactic foam or the like) such that only the seal lines are
sealed Likewise,
examples of laser welding apparatuses are well known in the art. An example of
a laser
welding apparatus may include a laser with a wavelength chosen to weld the
polymeric sheets
together to create permanent seal lines and a computer controller that can be
programmed to
in control the path of the laser. In one embodiment, the first and second
polymeric sheets may
be laminated to one another and then one or more seal lines may be formed to
define the
fluidic circuit prior to the pressing and propelling steps. In some
embodiments, boundaries of
the seal lines may correspond to the shapes in the forming plates such that
the seal lines and
the forming plates define the reformed shapes when the compressed fluid is
propelled
between the polymeric sheets. The polymeric sheets that include openings
formed by the
compressed fluid and the forming plates can be removed from the forming
plates, and the
manufacture of the reaction container can be completed by inserting reagents
(e.g., lysis
components, wash reagents, PCR reagents, etc.) into the correct blisters and
chambers that
have been formed.
[00217] Referring to Figs. 6 and 7, a forming die 218 includes a first forming
plate 220
and a second forming plate 230. The first and second forming plates 220, 230
of the forming
die 218 are configured and dimensioned to be pressed or clamped together to
form the
polymeric sheets 200, 210 into a reaction container (e.g., pouch 100a, bob).
The first
forming plate 220 includes one or more recesses 222. The second forming plate
230 includes
one or more recesses 232. Illustratively, recesses 222 are substantially
aligned with recesses
232 when the first and second forming plates 220, 230 are pressed together so
as to create
forming chambers. For example, as shown in Figs. 8-9, recesses 222 are
substantially a
mirror image of and aligned with recesses 232. As shown in Fig. 13,
illustrative recess 222a
is substantially a mirror image of and aligned with illustrative recess 232a
such that when the
first and second forming plates are pressed together a forming chamber 234 is
defined. The
recesses 222, 232 are shaped so as to reform portions of the first and second
polymeric sheets
200, 210 into desired shapes to form the applicable chambers (e.g., reagent
reservoirs, sample
preparation chambers, etc.). Other configurations are within the scope of the
present
invention, such as recesses that create asymmetrical forming chambers. For
instance, while
- 39 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
recesses 222a and 232a of Fig. 13 are shown as substantial mirror images, they
may have
different shapes or different depths in some embodiments. For example, it may
be preferable
in some embodiments to reform one type of polymeric sheet more than another.
In an
illustrative example, it may be preferable to reform one bather film more than
another
because one barrier may be more or less susceptible to degradation of bather
properties as a
result of the reforming process described herein In another illustrative
example, it may be
preferable to reform one film material more than another because the films
have different
stretch properties ¨ e.g., different abilities to be stretched and reformed.
As such, in one
illustrative example, only one forming plate may include recesses such that
only one of the
in film materials is reformed. Other configurations are within the scope of
the present invention.
[00218] The forming chambers defined between the first and second forming
plates 220,
230 (e.g., such as forming chamber 234 defined by recesses 222a and 232a,
illustrated in Fig.
13) are arranged to form the blisters, chambers, and/or channels in a reaction
container (e.g.,
pouch 100a, 100b). For example, in the illustrated embodiment as best seen in
Figs. 8-9,
recesses 222b, 232b align to create a forming chamber for the sample input
chamber 102.
Recesses 222c, 232c align to create a forming chamber for the sample
preparation chamber
104. Recesses 222d, 232d align to create a forming chamber for the sample
reagent wash
chamber 106. Recesses 222e, 232e align to create a forming chamber for
reaction chamber
108. Recesses 222f, 232f align to create a forming chamber for reagent
blisters 110. In the
illustrated embodiment, each of the recesses 222, 232 includes at least one
vent hole 236 for
venting air expelled from the respective recess during forming of the reaction
container, as
will be described in further detail below. Other configurations are within the
scope of the
invention, such as different recess and forming chambers that may be required
depending on
the configuration of the reaction container or pouch.
[00219] In one embodiment, the first inner planar face 202 and the second
inner planar
face 212 of the film layers 200 and 210 are contacted and pressed between the
first forming
plate 220 and the second forming plate 230. Subsequent to pressing film layers
200 and 210
between forming plates 220 and 230, a compressed fluid (e.g., compressed gas,
compressed
air, compressed liquid, or other suitable compressed fluid) may be propelled
between the first
and second inner planar faces 202, 212. The compressed fluid forces portions
of the first and
second polymeric sheets 200, 210 into the respective recesses 222, 232,
thereby forming an
opening or hollow area 240 between the first and second polymeric sheets (for
example,
reforming the polymeric sheets to include the opening or hollow area between
the sheets)
(see, e.g., Figs. 13 and 14). This opening or hollow area 240 illustratively
can be a blister, a
- 40 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
chamber, and/or a channel in the pouch 100a, 1006. If the polymer sheets have
been
laminated prior to pressing the film layers between the forming plates, the
compressed fluid
peels the sheets apart in the selected portions to form the hollow area
according to the
structure of the forming plates, as described in further detail below.
Alternatively, the films
may not be laminated together prior to pressing and reforming. As the
compressed fluid
forces portions of the polymeric sheets 200, 210 into the recesses 222, 232,
any air that is
displaced from the recesses can exit the forming plates 220, 230 through the
at least one vent
hole 236 in each recess. Each vent hole 236 vents to outside the respective
forming plate 220,
230 (see, e.g, Fig_ 10).
in [00220] In one embodiment, as shown in Figs. 5A, 13, and 14, one or more
holes 260 may
be cut in one of the first and second polymeric sheets 200, 210. In the
illustrated embodiment,
holes 260 are cut in the first polymeric sheet 200 but not in the second
polymeric sheet 210,
although the reverse or having some holes in each of the sheets are possible
and other
configurations are within the scope of the present invention. The holes 260
permit fluid
communication for a compressed fluid source to between the first and second
polymeric
sheets 200, 210. As best seen in Figs. 10-11, the forming die 218 includes one
or more
conduits 262 in fluid communication with the holes 260 (see, e.g., Fig. 11).
As illustrated in
Fig. 13, polymeric sheets 200 and 210 may be compressed between plates 220 and
230 such
that hole(s) 260 are aligned with conduit 262.
[00221] As shown in Fig. 14, compressed fluid (illustrated by the arrows) from
a
compressed fluid source (not shown) enters the forming die 218 and travels
through a conduit
262 to a respective hole 260 in the first polymeric sheet 200 to be propelled
between the first
polymeric sheet and the second polymeric sheet 210. In the illustrated
embodiment, the first
polymeric sheet 200 includes a hole 260 corresponding to each opening or
hollow area 240
that must be formed in the pouch 100a, 100b (e.g., there is a hole 260
corresponding to each
blister, chamber, and/or channel that will be formed by the compressed fluid
and the forming
die). The forming die 218 includes conduits 262 in fluid communication with
each hole 260
so that compressed fluid can be propelled into each hole and between the first
and second
polymeric sheets 200, 210 to each location where an opening 240 must be formed
(e.g., to
opening 240 from hole 260). As shown in Figs. 13 and 14, the forming plates
220 and 230
may include shallow cut-out regions 223a and 233a adjacent to conduit 262 such
that the film
layers 200 and 210 can expand adjacent to conduit 262, as shown at 223 and
233, so that the
compressed fluid can flow between the layers and expand the film to form
opening 240.
Illustratively, the conduit and hole configuration permits all of the openings
to be formed
- 41 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
simultaneously or substantially simultaneously. The channels connecting the
holes 260 to the
reformed openings 240 may subsequently be used to inject fluids (e.g.,
reagents) into the
reaction container (e.g., pouch 100a, 100b). For example, the area that was
expanded around
223 and 233 may form an access channel 243 that can be used to inject fluids
(e.g., reagents)
into opening 240.
[00222] Illustratively, the first and second polymeric sheets 200, 210 may be
laminated
together prior to being pressed or clamped between the forming plates 220,
230.
Illustratively, the lamination may reversibly adhere the sheets 200, 210
together, allowing for
ease of handling but still allowing for formation of the blisters, chamber,
and channels
therebetween. In embodiments where the films are laminated, the compressed
fluid can also
peel apart the lamination so that the compressed fluid can flow between layers
200, 210 and
expand the film to form opening 240_ Furthermore, the first and second
polymeric sheets 200,
210 may be sealed at certain locations prior to being pressed or clamped
between the forming
plates 220, 230. For example, one or more seal lines may be made to join the
first and second
polymeric sheets 200, 210 to define each opening or hollow area 240 that will
be formed
between the sheets. As shown in cross section in Figs. 13 and 14, film layers
200 and 210
may include a seal line 242 (e.g., a laser weld line) that joins film layers
200 and 210 adjacent
to the opening defined by recesses 222a and 232a. In the illustrated cross
section, only one
edge of the seal line 242 is visible but, as explained elsewhere herein, the
seal line may trace
around and define the boundaries of opening 240. In one embodiment, film
layers 200 and
210 may be laminated to one another prior to forming heat seals (e.g., heat
seal 242) and prior
to clamping the film layers 200 and 210 between plates 220 and 230. Suitably,
the seal lines
may be formed by laser welding, heat sealing, sonic welding, or other suitable
sealing
method. Preferably, the seal lines generally align with respective recess
pairs 222, 232 of the
forming plates 220, 230 when the sheets 200, 210 are pressed between the
plates.
[00223] In one embodiment, a laminated polymeric sheet comprising first and
second
polymeric sheets 200, 210 may be joined with the one or more seal lines (e.g.,
laser welds) to
join the first and second polymeric sheets and define a configuration of
blisters, chambers,
and/or channels (i.e., a fluidic circuit). Holes 260 may be cut in the first
and/or second
polymeric sheets 200, 210 during the same laser welding operation or at a
different time. In
addition, one or more alignment holes (130 and 131, Fig. 5B) for aligning the
sheets and laser
weld lines in the forming plates may be cut in the first and/or second
polymeric sheets 200,
210 during the same laser welding operation or at a different time. The
laminated and laser-
welded polymeric sheets 200, 210 may then be pressed or clamped in the forming
die 218
- 42 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
such that the holes 260 (which are also pressed in the forming die) are each
in fluid
communication with respective conduits 262_ In addition, the seal lines
joining the first and
second polymeric sheets 200, 210 may generally align with respective recesses
222, 232 in
the first and second forming plates 220, 230 of the forming die 218. A
compressed fluid is
then propelled through the conduits 262, into the respective holes 260, and
between the first
and second polymeric sheets 200, 210 The compressed fluid is propelled
directly on the
inner planar surfaces 202, 212 of the first and second polymeric sheets 200,
210. The
compressed fluid expands selected areas of the first and second polymeric
sheets 200, 210
into channel formers 223a and 233a and forming chambers 234 formed by aligned
recess
1.0 pairs 222, 232 to form openings 240 between the first and second
polymeric sheets.
Displaced air is vented from the recesses 222, 232 through respective venting
holes 236. The
openings 240 generally correspond to the seal lines previously made to join
the first and
second polymeric sheets together. In one embodiment, films 200 and 210 may be
expanded
into forming chambers 234 at a constant pressure. In another embodiment, films
200 and 210
may be expanded into forming chambers 234 at a variable pressure. For example,
film
expansion may be started at an initial pressure of about 10 PSI (-0.07 MPa)
and them ramp
up linearly over about 1-3 seconds to a pressure of about 100-160 PSI (-0.7
MPa-1.1 MPa);
the pressure may be held at the higher pressure for 1-3 seconds. In one
embodiment, the
pressure may be regulated with a ramping regulator or a solenoid with a small
opening that
acts as a pressure restrictor. Other variable pressure protocols and devices
are within the
scope of the present invention.
[00224] Illustratively, the polymeric sheets 200, 210 are cold formed in the
forming die
218 to create the openings 240. The polymeric sheets 200, 210, which now
include openings
formed by the compressed fluid and the forming plates 220, 230, can be removed
from the
forming plates, and the manufacture of the pouch 100a, 100b can be completed
by inserting a
selection of one or more liquid and/or dried reagents, wash solutions, etc.
into the correct
blisters and chambers that have been formed.
[002251 Although the above-described method does not include heating the
polymeric
sheets 200, 210 prior to forming the sheets between the forming plates 220,
230, it is
understood that the sheets may be heated to a softening temperature (e.g., a
plastic transition
temperature) prior to or during this process to aid formation of the desired
shapes. For
example, the sheets 200, 210 may be pressed between heating plates for heating
prior to
being pressed or clamped in the forming die 218. Illustratively, only selected
portions of the
sheets (e.g., some or all of the portions that will be reformed) may be
heated, for example by
- 43 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
pressing the sheets between heating plates having raised portions
corresponding to the
portions that will be subsequently reformed in the forming plates with the
compressed fluid,
as described above.
[00226] In one embodiment, liquid or aqueous reagents are injected into the
reagent
reservoirs 110 that are formed as described above. The aqueous reagents may be
injected at
the time of manufacture, or may be added later Illustratively, the aqueous
reagents are
injected into the reagent reservoirs 104, 106, and 110a-110g, and the
reservoirs are then
sealed to seal in the aqueous reagents. In some embodiments, one or more of
reservoirs 104,
106, and 110a-110g may include a dried reagent (e g., a freeze-dried reagent
pellet) that can
be rehydrated at the time of use by a liquid reagent or by liquid sample. In
some
embodiments, liquid reagents may be spotted onto at least one of the first and
second
polymeric sheets 200, 210 and then dried prior to laminating the first and
second polymeric
sheets. Illustratively, fluids (e.g., sample preparation fluid in the sample
preparation chamber,
lysis buffer, etc.) may be injected into the corresponding openings (e.g.,
243a-243j of Fig.
5A) that may be formed in the methods described herein.
[00227] Furthermore, a reaction card can be inserted into between the first
and second
polymeric sheets (e.g., sheets 200 and 210), such as is shown and described in
U.S. Pat Pub.
No. 2020/0261914, the entirety of which was already incorporated herein by
reference. For
example, a reaction card 109 having a plurality of wells formed therein and
spotted with one
or more dried reagents for a second stage reaction can be inserted into a
second reaction
chamber 108 formed in the pouch 100a, 100b via an opening 244 between the
first and
second polymeric sheets. A first planar face of the reaction card may be
bonded to the first
polymeric sheet and a second, opposite planar face of the reaction card may be
bonded to the
second polymeric sheet. The opening through which the reaction card was
inserted can be
sealed by sealing the first polymeric sheet to the second polymeric sheet at
the opening 244.
[00228] The present invention may be embodied in other specific forms without
departing
from its spirit or essential characteristics. The described embodiments are to
be considered in
all respects only as illustrative and not restrictive. The scope of the
invention is, therefore,
indicated by the appended claims rather than by the foregoing description.
While certain
embodiments and details have been included herein and in the attached
invention disclosure
for purposes of illustrating the invention, it will be apparent to those
skilled in the art that
various changes in the methods and apparatus disclosed herein may be made
without
departing from the scope of the invention, which is defined in the appended
claims. All
- 44 -
CA 03155527 2022-4-21
WO 2021/086878
PCT/1JS2020/057600
changes which come within the meaning and range of equivalency of the claims
are to be
embraced within their scope.
- 45 -
CA 03155527 2022-4-21